CHAPTER ONE
MASHING THE PLEASURE BUTTON
Montréal, 1953. Fortunately, Peter Milner and James Olds didn’t have perfect aim. While postdoctoral fellows at McGill University, under the direction of the renowned psychologist Donald Hebb, Olds and Milner were conducting experiments that involved implanting electrodes deep in the brains of rats. The implanting surgery, conducted while the animals were anesthetized, involved cementing a pair of electrodes half a millimeter apart to their skulls. After a few days of recovery from the surgery, the rats were fine. Long, flexible wires were then attached to the electrodes at one end and to an electrical stimulator at the other, to allow for activation of the specific brain region where the tips of the electrodes had come to rest.
One fall day Olds and Milner were testing a rat in which they had attempted to target a structure called the midbrain reticular system. Located at the midline of the brain, at the point where its base tapers to form the brain stem, this region had previously been shown by another lab to control sleeping and waking cycles. In this particular surgery, however, the electrodes had gone astray and come to rest still at the midline, but at a somewhat more forward position in the brain, in a region called the septum.
The rat in question was placed in a large rectangular box with corners labeled A, B, C, and D and was allowed to explore freely. Whenever the rat went to corner A, Olds pressed a button that delivered a brief, mild electrical shock through the implanted electrodes. (Unlike the rest of the body, brain tissue does not have the receptors that allow for pain detection, so such shocks don’t produce a painful sensation within the skull.) After a few jolts, the rat kept returning to corner A and finally fell asleep in a different location. The next day, however, the rat seemed even more interested in corner A than the others. Olds and Milner were excited: They believed that they had found a brain region that, when stimulated, provoked general curiosity. However, further experiments on this same rat soon proved that not to be the case. By this time, the rat had acquired a habit of returning often to corner A to be stimulated. The researchers then tried to coax the rat away from corner A by administering a shock every time the rat made a step in the direction of corner B. This worked all too well—within five minutes, the rat relocated to corner B. Further investigation revealed that this rat could be directed to any location within the box with well-timed brain shocks—brief ones to guide the rat to the target location and then more sustained ones once it arrived there.
Many years earlier the psychologist B. F. Skinner had devised the operant conditioning chamber, or “Skinner box,” in which a lever press by an animal triggered either a reinforcing stimulus, such as delivery of food or water, or a punishing stimulus, such as a painful foot shock. Rats placed in a Skinner box will rapidly learn to press a lever for a food reward and to avoid pressing a lever that delivers the shock. Olds and Milner now modified the chamber so that a lever press would deliver direct brain stimulation through the implanted electrodes. What resulted was perhaps the most dramatic experiment in the history of behavioral neuroscience: Rats would press the lever as many as seven thousand times per hour to stimulate their brains. They weren’t stimulating a “curiosity center” at all—this was a pleasure center, a reward circuit, the activation of which was much more powerful than any natural stimulus. A series of subsequent experiments revealed that rats preferred pleasure circuit stimulation to food (even when they were hungry) and water (even when they were thirsty). Self-stimulating male rats would ignore a female in heat and would repeatedly cross foot-shock-delivering floor grids to reach the lever. Female rats would abandon their newborn nursing pups to continually press the lever. Some rats would self-stimulate as often as two thousand times per hour for twenty-four hours, to the exclusion of all other activities. They had to be unhooked from the apparatus to prevent death by self-starvation. Pressing that lever became their entire world (Figure 1.1).
Further work was done to systematically vary the placement of the electrode tips and thereby map the reward circuits of the brain. These experiments revealed that stimulation of the outer (and upper) surface of the brain, the neocortex, where sensory and motor processing mostly reside, produced no reward—the rats continued to press the lever at chance levels. However, deep in the brain, there was not just a single discrete location underlying reward. Rather, a group of interconnected structures, all located near the base of the brain and distributed along the midline, constituted the reward circuit. These included the ventral tegmental area, the nucleus accumbens, the medial forebrain bundle, and the septum, as well as portions of the thalamus and hypothalamus (more on these various regions later). Not all these areas were equally rewarding. Stimulation in some parts of this medial forebrain pleasure circuit could support self-stimulation rates of seven thousand lever presses per hour, while others elicited only two hundred per hour.
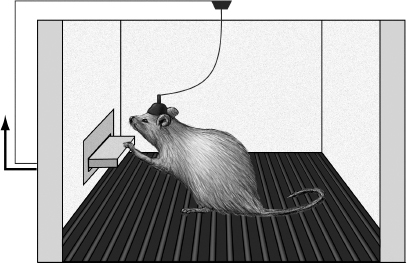
Figure 1.1 Self-stimulation of the pleasure circuit in a rat. When the rat presses the lever, it causes brief electrical stimulation to travel down the wire and activate the electrodes implanted deep in the rat’s brain, in various portions of the medial forebrain pleasure circuit. This setup can be modified in several useful ways. For example, the electronics can be configured so that a rat must make many lever presses to get a single stimulation. In addition, a hollow needle can be implanted together with the stimulating electrodes to inject drugs directly into the pleasure circuit. Illustration by Joan M. K. Tycko.
It’s hard to imagine now, but in 1953 the notion that motivational or pleasure/reward mechanisms could be localized to certain brain regions or circuits was highly controversial. The dominant theory, which had held sway for many years, was that excitation of the brain was always punishing and that learning and the development of behavior could be explained solely by punishment avoidance. This was called the drive-reduction hypothesis. In Olds’s characterization of the theory, “pain supplies the push and learning based on pain reduction supplies the direction.” There was no need for reward or pleasure: This model was all stick, no carrot. The pioneering experiments of Olds and Milner clearly demolished the punishment-only model in favor of a more comprehensive, hedonistic view that “behavior is pulled forward by pleasure as well as pushed forward by pain.”1
I know what you’re thinking: What does it feel like for a human to have his or her medial forebrain reward circuitry stimulated with an electrode? Does it produce a crazy pleasure that’s better than food or sex or sleep or even Seinfeld reruns? We do in fact know the answer to that question. The bad news is that that answer comes, in part, from some deeply unethical experiments.
Dr. Robert Galbraith Heath was the founder and chairman of the Department of Psychiatry and Neurology at Tulane University in New Orleans. He served from 1949 to 1980, and during that time the major focus of his work involved stimulation of the brains of institutionalized psychiatric patients, often African Americans, using surgically implanted electrodes. His main goal—to use brain stimulation to relieve the symptoms of psychiatric disorders such as major depression and schizophrenia—was laudable. However, he did not obtain proper informed consent from his patients and took decisions in experimental design that would never be approved by modern human-subjects ethical review boards.
Perhaps the most egregious example was reported in a paper entitled “Septal stimulation for the initiation of heterosexual behavior in a homosexual male,” published in the Journal of Behavioral Therapy and Experimental Psychiatry in 1972.2 The rationale behind this experiment was that because stimulation of the septal area evoked pleasure, if it was combined with heterosexual imagery it could “bring about heterosexual behavior in a fixed, overt homosexual male.” And so Patient B-19, a twenty-four-year-old male homosexual of average intelligence who suffered from depression and obsessive-compulsive tendencies, was wheeled into the operating room. Electrodes were implanted at nine different sites in deep regions of his brain, and three months were allowed to pass after the surgery to allow for healing (Figure 1.2). Initially stimulation was delivered to all nine electrodes in turn. However, only the electrode implanted in the septum produced pleasurable sensations. When Patient B-19 was finally allowed free access to the stimulator, he quickly began mashing the button like an eight-year-old playing Donkey Kong. According to the paper,
During these sessions, B-19 stimulated himself to a point that, both behaviorally and introspectively, he was experiencing an almost overwhelming euphoria and elation and had to be disconnected despite his vigorous protests.
So, not to put too fine a point on it, Heath’s patient responded just as Olds and Milner’s rats had. Given the chance, he would stimulate his pleasure circuit to the exclusion of all else.
Lest anyone think that it is only men—creatures of inherently base urges—who would respond in this manner, another recorded case, performed by a different group, involved a woman who received an electrode implant in her thalamus, an adjacent deep brain structure, to control chronic pain. This technique has proven effective for some patients whose severe pain is not well-controlled by drugs. However, in this patient the stimulation spread to nearby brain structures, producing an intense pleasurable and sexual feeling:
At its most frequent, the patient self-stimulated through out the day, neglecting her personal hygiene and family commitments. A chronic ulceration developed at the tip of the finger used to adjust the amplitude dial and she frequently tampered with the device in an effort to increase the stimulation amplitude. At times she implored her family to limit her access to the stimulator, each time demanding its return after a short hiatus.3
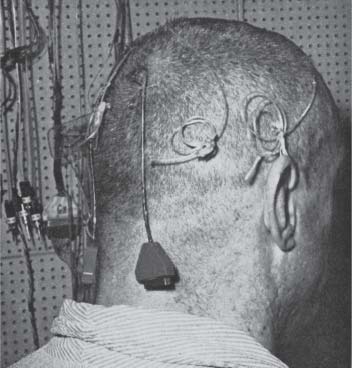
Figure 1.2 A patient of Dr. Robert Galbraith Heath with chronically implanted electrodes, one of which activated the medial forebrain bundle passing through the septum, a key part of the pleasure circuit. From Robert G. Heath, “Depth recording and stimulation studies in patients,” in Arthur Winter, ed., The Surgical Control of Behavior (Springfield, Il.: Charles C. Thomas, 1971), 24. Reprinted with permission from Charles C. Thomas.
Back to Patient B-19: Before his brain stimulation began, he was shown a “15 min long ‘stag’ film featuring sexual intercourse and related activities between a male and female.” Not surprisingly, he was sexually indifferent to this material and even a bit angry about being made to view it. Following pleasure circuit self-stimulation, however, he readily agreed to re-view the film “… and during its showing became sexually aroused, had an erection and masturbated to orgasm.” All this in the decidedly unsexy environment of the lab. So, with the patient starting to exhibit heterosexual tendencies, what were the experimenters to do? Would he ever have an actual sexual relationship with a woman? After careful consideration of all the options and with the well-being of the patient foremost in their minds, Drs. Heath and Charles E. Moan made a sober medical and scientific decision: Upon getting approval from the attorney general of the state of Louisiana, they hired a hooker to come to the lab at Tulane and attempt to seduce him. She succeeded—they had sexual intercourse. The concluding sentence to the lengthy, overly descriptive paragraph describing their two-hour-long sexual encounter reads, “Then, despite the milieu and the encumbrance of the electrode wires [poor B-19 was attached to an EEG machine the whole time], he successfully ejaculated [in her vagina].”
Did Patient B-19 actually become heterosexual? Following discharge from the hospital, he had a sexual relationship with a married woman for several months, much to the delight of Drs. Moan and Heath, who found this development quite encouraging. His homosexual activity was reduced during this period, but did not stop completely: He still liked to turn tricks with men to earn money. Long-term follow-up information was not available. Writing in the discussion section of their scientific report, Moan and Heath were enthusiastic about the prospects for this therapy: “Of central interest in the case of B-19 was the effectiveness of pleasurable stimulation of new and more adaptive sexual behavior.” While it’s clear that Patient B-19 found the brain stimulation to be intensely pleasurable, I’m not convinced that he truly became heterosexual, even temporarily. It should also be cautioned that this report concerns only a single individual, not a population (with a control group).
This study is morally repugnant on many different levels—the profound arrogance of attempting to “correct” someone’s sexual orientation, the medical risk of unjustified brain surgery, the gross violations of privacy and human dignity. Fortunately, homosexual conversion therapy with brain surgery and pleasure center stimulation was soon abandoned. Stepping back a bit, what we are left with, from this and a handful of other studies, is an appreciation of the immense power of direct electrical stimulation of the brain’s pleasure circuitry to influence human behavior, at least in the near term.
Let’s now take a minute to consider some important details of the pleasure circuit. I hesitate to burden you with neuroanatomy, but just a smidgen will go a long way in explaining how we experience pleasure. We’ll use the rat as an example, which is appropriate because the anatomy of the rat’s pleasure circuit is very similar to that of our own (Figure 1.3). When neurons in the region called the ventral tegmental area (VTA) are active, brief electrical impulses (called spikes) race from their cell bodies (located in the VTA proper) along long, thin information-sending fibers called axons. The axons have specialized structures at their endpoints called axon terminals. Some of the axon terminals of the VTA neurons are located some distance away in a region called the nucleus accumbens. When the traveling electrical spikes reach the axon terminals, they trigger the release of the neurotransmitter dopamine, which is stored in the terminals in tiny membrane-bound blobs called vesicles. When a spike enters the axon terminal, it initiates a complex series of electrical and chemical events that result in the fusion of the vesicle membrane with the membrane of the axon terminal, thereby causing the contents of the vesicle, including the dopamine neurons, to be released into a narrow fluid-filled space surrounding the axon terminal called the synaptic cleft. The dopamine molecules then diffuse and bind to specialized dopamine receptors on their target neurons, initiating a series of chemical signals therein (Figure 1.4).
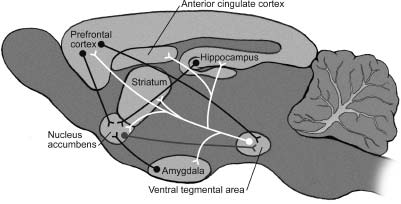
Figure 1.3 The pleasure circuit in the brain of a rat. This view shows a section through the middle of the rat brain, oriented so that the nose is at the left and the tail at the right. The central axis of the pleasure circuit is the dopamine-containing neurons of the ventral tegmental area (VTA) and their axons, drawn in white, which project to the nucleus accumbens. These VTA neurons also send their dopamine-releasing axons to the prefrontal cortex, the dorsal striatum, the amygdala, and the hippocampus. The VTA neurons receive excitatory drive from the prefrontal cortex and inhibitory drive from the nucleus accumbens. Illustration by Joan M. K. Tycko.
Neurons of the VTA also send dopamine-releasing axons to other brain regions, including the amygdala and the anterior cingulate cortex, which are emotion centers; the dorsal striatum, involved in some forms of habit learning; the hippocampus, involved in memory for facts and events; and the prefrontal cortex, a region that controls judgment and planning (and that is particularly expanded in humans as compared with other mammals).
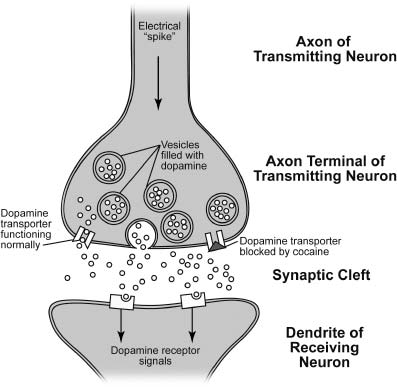
Figure 1.4 A synapse that uses the neurotransmitter dopamine. Dopamine is packed into membrane-bound vesicles in the presynaptic (information-transmitting) neuron. When electrical spikes traveling down the axon reach the axon terminal, they trigger the fusion of dopamine-containing vesicles with the presynaptic membrane, causing dopamine to spill out into the fluid-filled synaptic cleft. Dopamine released into the cleft can bind dopamine receptors on the dendrite of the postsynaptic (information-receiving) neuron, thereby exerting its effects, or undergo reuptake into the axon terminal through a dopamine transporter, where it will be recycled for later use. Cocaine and amphetamines block this reuptake process, causing dopamine to linger in the cleft and thereby activate dopamine receptors more effectively. Illustration by Joan M. K. Tycko.
In addition to sending out signals, the neurons of the VTA also receive electrochemical information from other brain regions—most notably from a group of axons, called the medial forebrain bundle, that run to it from the prefrontal cortex and other areas (passing through the septum and the thalamus). The medial forebrain bundle axons release the excitatory neurotransmitter glutamate in the VTA. This causes the VTA neurons to fire spikes that propagate down their axons and then release dopamine onto their targets. The VTA dopamine neurons also receive signals from neurons in the nucleus accumbens. However, the nucleus accumbens axons release the inhibitory neurotransmitter GABA (gamma-aminobutyric acid), which silences VTA neurons, preventing dopamine release.4 The neurons in the nucleus accumbens, in addition to receiving dopamine axons from the VTA, also receive excitatory glutamate-containing fibers directly from the prefrontal cortex, the amygdala, and the hippocampus.
So what does this brain circuit diagram mean for the feeling of pleasure? Here’s the central insight: Experiences that cause the dopamine-containing neurons of the VTA to be active and thereby release dopamine in their targets (the nucleus accumbens, the prefrontal cortex, the dorsal striatum, and the amygdala) will be felt as pleasurable, and the sensory cues and actions that preceded and overlapped with those pleasurable experiences will be remembered and associated with positive feelings. When Olds and Milner implanted electrodes to directly activate the pleasure circuit of rats, they placed them in positions that effectively stimulated the medial forebrain bundle, the axons that excite the dopamine neurons of the VTA. In fact, the electrode locations that produced the strongest pleasure (as determined by the frequency and duration of lever pressing the rats would perform) were those that most effectively activated the dopamine neurons of the VTA. Likewise, Moan and Heath’s Patient B-19 and the handful of other humans who have received pleasure from direct brain stimulation all had electrode placements that produced excitation of the VTA dopamine neurons.5
How does activation of VTA dopamine neurons produce pleasure? Several lines of evidence indicate that catching a buzz from direct activation of the brain’s pleasure circuitry with electrodes depends specifically upon release of dopamine in the target regions of the VTA neurons. Dopamine is released in these areas and diffuses across the synaptic cleft (the fluid-filled gap between neurons) before it binds and activates receptors on the target cells to exert its effects (Figure 1.4). After it has been released, dopamine doesn’t just diffuse away; most of it is taken back up into axon terminals through the action of a protein called the dopamine transporter. The recycled dopamine is then repackaged into storage vesicles to be released again later. This means that drugs that block dopamine transporters will augment and extend the natural action of dopamine on the dopamine receptors of the target neurons, producing a more intense and longer-lasting signal.
When moderate doses of drugs that block the dopamine transporter, like amphetamines and cocaine, are given to rats, they increase the amounts of lever-pressing for medial forebrain bundle stimulation. This effect is most easily observed if the strength of the electrical stimulator is turned down a bit so it is not already producing maximum pleasure. Conversely, either drugs that block dopamine receptors (which are called dopamine receptor antagonists or neuroleptics) or surgical destruction of the dopamine cells of the VTA will cause a rat that has been engaging in vigorous lever-pressing for pleasurable stimulation to stop.
Some psychoactive drugs work, at least in part, by hijacking the pleasure circuit—they artificially increase the effects of dopamine release from VTA neurons. (We’ll get into the details of this in the next chapter.) Like humans, rats will self-administer certain drugs if given the chance. If you hook up a rat to a Skinner box in which lever presses deliver cocaine or amphetamines (either intravenously or directly into the brain), it will press the lever repeatedly. It will do so even if it has to work very hard to achieve the desired results—that is, even if a hundred lever presses are required to deliver a single tiny injection of drug. Just like Olds and Milner’s rats with electrodes implanted in their pleasure circuits, and just like humans with implanted electrodes given unlimited access to brain pleasure circuit stimulation, rats allowed to self-administer cocaine and amphetamines will ignore food, water, sex, their personal hygiene, and even their young offspring in favor of the drug. With such behavior these “rat addicts” are a horrifyingly accurate reflection of the ruined lives of human drug addicts.
We’ve discussed what follows artificial activation of the dopamine-using pleasure circuit using electrodes or certain psychoactive drugs: intense pleasure that can, in some cases, become the basis for a profound and self-destructive addiction. But what happens under the opposite circumstances, when the dopamine neurons in the pleasure circuit are damaged or destroyed, thereby reducing the amount of dopamine released and thus dialing down the rheostat of pleasure?
Parkinson’s disease is a neurological disorder that typically afflicts people over fifty and is more common in men than women. Its most striking symptoms involve the control of movement, and include tremor (in the hands, legs, jaw, and face), stiffness, slowness, and problems with balance and coordination. Parkinson’s is chronic and progressive, usually starting with a subtle tremor but often progressing in a downward slide, resulting in difficulties with basic functions such as walking, talking, and the physical actions of eating (swallowing and chewing). Some cases run in families, but most do not. While the ultimate cause of Parkinson’s disease is unknown, the proximate cause is well described: It results from a loss of dopamine-containing cells in the brain, particularly in two regions, the substantia nigra and the VTA—an integral part of the pleasure circuit. At present, there is no cure for Parkinson’s disease, but a number of therapies are available to treat the symptoms by increasing dopamine action to compensate for the loss of dopamine-containing neurons. One is a drug called levodopa, a chemical precursor of dopamine. When levodopa is taken up into the surviving dopamine neurons, it causes them to make more dopamine and then to boost their release of dopamine. Obviously, this depends upon there being a sufficient number of surviving dopamine neurons—if they are all dead, then levodopa therapy will fail. Another therapy is a class of drug called dopamine receptor agonists. These drugs bind to dopamine receptors and activate them, thereby mimicking the actions of dopamine.6 Levodopa and dopamine receptor agonists are often given together, and in many cases can significantly relieve the tremor and other movement problems, providing years of symptomatic relief. Unfortunately, however, as Parkinson’s progresses, more and more dopamine neurons die, and as a consequence these drugs have to be given at higher and higher doses to control the symptoms.
In his original 1817 description of the “shaking palsy” that came to bear his name, James Parkinson classified it as a pure movement disorder that left “the senses and intellect …uninjured.” However, that assessment has turned out not to be true, and likely resulted from the surprising fact that Parkinson examined only a single shaking palsy patient in his medical office—the other five cases that formed the basis of his essay were merely observed and sometimes interviewed as he walked around the streets of London.7 As early as 1913 it had become clear to some neurologists that Parkinson’s disease also involved symptoms of cognition and mood and that these symptoms often preceded the onset of movement disorders. On average, Parkinson’s patients tend to be introverted, rigid, stoic, slow to anger, and generally uninterested in seeking out novel experiences. They use alcohol, tobacco, and other psychoactive drugs at a much lower rate than the general population. Their personalities and actions are, in fact, the polar opposite of typical drug addicts, who are more likely to be impulsive, quick-tempered novelty-seekers. Given that profile, it was quite unusual when case reports started appearing showing an unusually high incidence of pathological gambling among Parkinson’s patients.
In January 2001 a sixty-four-year-old man was admitted to an outpatient treatment facility for problem gamblers in northern Italy. He had lost the equivalent of $5,000 playing slot machines over a three-year period, causing him to become estranged from his wife and to move in with his elderly mother. He had been diagnosed with Parkinson’s disease twelve years earlier and had been placed on dopamine replacement therapy, consisting of a combination of levodopa and dopamine receptor agonists. The psychiatrists at the clinic performed cognitive-behavioral therapy (which is often effective for pathological gamblers) and prescribed antidepressants (serotonin-specific reuptake inhibitors, SSRIs). The treatment was unsuccessful, and the patient soon dropped out of the program and resumed his compulsive gambling. When he finally returned to the clinic in September 2002, the psychiatrists in charge had an insight—they asked the patient’s daughter to surreptitiously monitor her father’s intake of drugs. What they found was that, acting on his own, he had significantly increased his prescribed dose of both levodopa and dopamine receptor agonists. When confronted, he readily admitted increasing the dose and stated that he enjoyed the euphoric mood that accompanied it. (He liked the gambling too—he was just concerned about losing all his money.) When the drug doses were reduced to the prescribed level, his gambling stopped within a few days. In recent years, there have been a flood of similar case reports in the medical literature.8
Analysis of these reports revealed an interesting finding. Among patients treated solely with levodopa, the incidence of compulsive gambling was very low. (It’s about 1 percent in the general population.) However, among Parkinson’s patients treated with dopamine receptor agonists, there was a remarkable 8 percent incidence of pathological gambling. Gambling was in fact only the most common manifestation of a broad range of impulse control disorders. A small but significant population of these patients started compulsively eating, shopping (or shoplifting), and having risky sex—all behaviors that are highly atypical for the normal Parkinson’s sufferer. In almost all cases, the impulse control disorder began shortly after an increase in the dose of dopamine receptor agonist and could be terminated by reducing the dose.
The best explanation for these findings is that in untreated Parkinson’s disease, chronically low levels of dopamine result in a dialing down of the pleasure/reward circuit and a disinclination to seek novel experiences, and are associated with a reduced risk of addiction. In contrast, in some Parkinson’s patients treated with high doses of dopamine receptor agonists, the level of dopamine action, in both the pleasure circuit and associated structures, is high, thereby dialing up the function of the pleasure circuit. This confers increased vulnerability to impulse control disorders and addiction.
To this point we’ve considered how the pleasure circuit can be artificially activated—hijacked by drugs or zapped by implanted electrodes. We’ve also examined the opposite case, in which the function of the pleasure circuit (and related structures) is attenuated in Parkinson’s disease. While these stories are illuminating and help establish the existence of the pleasure circuit, we must now consider how the pleasure circuit functions naturally, in the healthy state, in the absence of artificial manipulations. Of course, the pleasure circuits of the brain have not evolved just to be activated by implanted electrodes or drugs. We must experience basic behaviors such as eating, drinking, and mating as pleasurable (rewarding) in order to survive and procreate. This consideration is not unique to humans. Indeed, rudimentary pleasure pathways appear quite early in evolutionary history. Even the soil-dwelling roundworm C. elegans, which is a millimeter long and has only 302 neurons in its entire body, has some basic pleasure circuitry. These worms typically feed on bacteria and are very good at following odor cues to find clumps of them. However, when a group of eight key neurons containing dopamine are silenced, the worms are mostly indifferent to this favorite food source (even though they can still detect odors). To anthropomorphize, the worms just don’t seem to find eating bacteria to be that much fun anymore. This indicates that some aspects of the biochemistry of pleasure appear to have been conserved through hundreds of millions of years of evolution. In both modern roundworms like C. elegans (which follow an ancient body plan) and humans, dopamine-containing neurons occupy a central position in the pleasure circuit. This evolutionary conservation, from worms to humans, speaks to the central role of pleasure in the development of behavior.
In humans, rats, and other mammals, the reward circuit is much more complex, as it is interwoven with brain centers involved in decision-making, planning, emotion, and memory storage. When we find an experience pleasurable, it sets in motion several processes with different time courses: (a) We like the experience (the immediate sensation of pleasure); (b) we associate both external sensory cues (sights, sounds, odors, etc.) and internal cues (our own thoughts and feelings at the time) with the experience, and these associations allow us to predict how we should behave to repeat it; and (c) we assign a value to the pleasurable experience (from a little to a lot), so that in the future we can choose among several pleasurable experiences and determine how much effort we are willing to expend and risk we are willing to take in order to get them. (As my old friend Sharon expressed it, “I never met a man who excited me as much as a baked potato with sour cream.”)
Human societies strictly regulate pleasurable activities, and most have a concept of vice that’s applied to unregulated indulgence in food, sex, drugs, or gambling. Using a brain scanner, it has now become possible to observe activation of the brain’s pleasure circuitry in humans. Not surprisingly, this circuit is activated by “vice” stimuli: orgasm, sweet and fatty foods, monetary reward, and some psychoactive drugs. What’s surprising is that many behaviors that we consider virtuous have similar effects. Voluntary exercise, certain forms of meditation or prayer, receiving social approval, and even donating to charity can all activate the human pleasure circuit. There’s a neural unity of virtue and vice—pleasure is our compass, no matter the path we take.
As we shall discover, the evidence for a general neurobiological model of pleasure is compelling and is only growing stronger as more research is done. How, then, should we think about the pleasures—both virtues and vices—that animate our lives? That wonderful meal, the blissful feeling of connectedness during prayer, that night of great sex, the “runner’s high” from a Saturday morning workout, a hilarious tipsy night at the bar with friends—are those all reducible to medial forebrain circuit activity and dopamine surges? Well, yes and no. Yes, in the sense that there seems to be a neural rheostat of reward involving the medial forebrain dopamine circuit that’s engaged by almost everything we find pleasurable.9 No, in the sense that the activity of the pleasure circuit in isolation results in a lifeless pleasure lacking color and depth. What makes pleasure so compelling is that, through the interconnection of the pleasure circuit with other brain regions, we adorn it with memory, with associations and emotions and social meaning, with sights, sounds, and smells. A circuit-level model of pleasure shows us what is necessary but not sufficient. The transcendence and the texture emerge from the web of associated sensations and emotions that the pleasure circuit engages. Our task now shall be to explore how that all plays out in the brain.