3
Enzyme Inhibitors—Topoisomerase I Inhibitors
CAMPTOTHECANS
What are the chemotherapy agents in the topoisomerase I inhibitor class?
• Irinotecan (Camptosar®) and topotecan (Hycamtin®)
What malignancies are the topoisomerase I inhibitors approved for by the U.S. Food and Drug Administration (FDA)?
FDA-Approved Uses of Topoisomerase I Inhibitors
Agent |
FDA Approval |
Irinotecan |
Metastatic colorectal cancer |
Topotecan |
Cervical cancer, metastatic ovarian cancer, small cell lung cancer |
Abbreviation: FDA, U.S. Food and Drug Administration.
How do the topoisomerase I inhibitors work?
• Topoisomerase I is an enzyme that relieves tension and supercoiling of DNA by binding to DNA and creating transient single-strand breaks, forming what is known as the cleavable complex
• Topoisomerase I inhibitors bind to the enzyme and stabilize the normally transient cleavable complex and prevent religation of DNA
• Collision of the DNA replication fork with the cleavable complex prevents DNA replication and produces DNA strand breaks, leading to apoptosis. Accumulation of supercoils ahead of the replication fork also contributes to cytotoxicity
• Irinotecan is a prodrug that must be converted to its active metabolite, SN-38, via a carboxylesterase enzyme (Figure 3.1)
• Cell cycle phase specific (S phase)
What are the common mechanisms of resistance to topoisomerase inhibitors?
• Point mutations in topoisomerase I that prevent camptothecin binding
• Alterations in carboxylesterase enzyme concentrations may reduce activation of irinotecan to its active metabolite, SN38 (Figure 3.1)
• Decreased expression of topoisomerase I within tumor cells
• Active efflux and reduced accumulation in tumor cells secondary to active transport mechanisms (P-glycoprotein [P-gp], organic aniontransporting polypeptides [OATPs])
• Altered intracellular localization of topoisomerase I (nucleolus vs. nucleus/cytoplasm), reducing its interaction with DNA and subsequent cellular cytotoxicity in the presence of camptothecins
• Reduced cell death in response to camptothecin/topoisomerase I/DNA complex formation secondary to alterations in DNA damage checkpoint and apoptotic pathways (eg, ERK, BCL-2, Chk-1/Chk-2, MAPK)
What are the common dosing ranges for the topoisomerase I inhibitors?
• Irinotecan (several dosing strategies exist, the most common are in the following text)
• 65 to 125 mg/m2 intravenous (IV) weekly × 4 weeks (6-week cycle)
• 165 to 180 mg/m2 IV every 2 weeks (FOLFIRINOX)
• 250 to 350 mg/m2 IV every 3 weeks
• Topotecan
– 0.75 to 1.5 mg/m2 IV × 3 to 5 days every 21 days
– 2.3 mg/m2 orally (PO) × 5 days every 21 days
– 4 mg/m2 days 1, 8, and 15 every 28 days
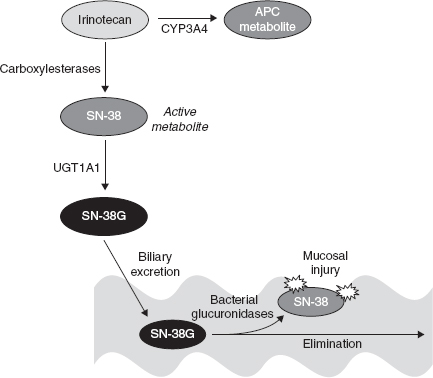
FIGURE 3.1. Irinotecan metabolism and elimination: Irinotecan is converted via carboxylesterases (primarily carboxylesterase 2 in humans) to an active metabolite, SN-38. It also undergoes hepatic oxidation, primarily via CYP3A4, to a 100-fold less-active metabolite, APC. The SN-38 metabolite is responsible for the majority of cytotoxicity of irinotecan. This agent undergoes glucuronidation via several UGT1A isoforms, with UGT1A1 being the most predominant. Polymorphisms exist in the UGT1A1 enzyme, and patients with UGT1A1*28 alleles have reduced glucuronidation of active SN-38 and are at increased risk for irinotecan toxicities (neutropenia and diarrhea). SN-38G is excreted via the biliary route. Bacterial glucuronidases in the gastrointestinal (GI) tract convert SN-38G back to the active SN-38, and this may be responsible for the late-onset diarrhea of irinotecan. Several studies have utilized cephalosporin prophylaxis to reduce bacterial conversion of SN-38G to active SN-38 to reduce diarrhea and improve compliance, primarily in pediatric patients.
Are the topoisomerase I inhibitors metabolized/eliminated renally or hepatically?
• Irinotecan—Primarily eliminated hepatically (Figure 3.1) and excreted via biliary routes; dose adjust for hepatic dysfunction
– Patients homozygous for the UGT1A1*28 allele are at higher risk for severe neutropenia and may require reduced initial doses of irinotecan
• Topotecan—Primarily renally eliminated (undergoes glucuronidation and minor hepatic metabolism) and requires dose adjustment for moderate/severe renal impairment
Are there drug interactions with any of the topoisomerase I inhibitors?
• Irinotecan—valproic acid (reports of increased/decreased SN-38 concentrations), drugs that interfere with glucuronidation via UGT1A1 (inhibitors decrease SN-38 concentrations/efficacy, inducers increase SN-38 concentrations/toxicity), drugs that affect CYP3A4
• Topotecan—P-gp inhibitors/inducers
What are the class adverse effects of the topoisomerase I inhibitors?
• Myelosuppression (neutropenia predominates), alopecia, mucositis
What are the most common adverse effects of each topoisomerase I inhibitor?
• Irinotecan—early-onset diarrhea (during infusion or within first 12–24 hours), associated with flushing, diaphoresis, and cramping (due to inhibition of acetylcholinesterase activity by irinotecan; treat with atropine), late-onset diarrhea (manage with antidiarrheals; consider cephalosporin use—see Figure 3.1), myelosuppression (primarily neutropenia, less common with weekly administration), nausea/vomiting, increased liver function tests (LFTs), fatigue
• Topotecan—late-onset diarrhea (does not produce cholinergic reaction like irinotecan), mucositis, myelosuppression, fatigue, nausea/vomiting, rash, increased LFTs
What are the premedications required?
• No routine premedications (other than standard antiemetics) required
• Can consider premedication with atropine in patients who experience cholinergic symptoms with irinotecan
What is the emetogenicity level of the topoisomerase I inhibitors?
• Irinotecan and topotecan have a low emetogenic potential
Are the topoisomerase I inhibitors vesicants or irritants?
• Irinotecan and topotecan are irritants