Appendix C
Material properties and length scales
Contents
Introduction
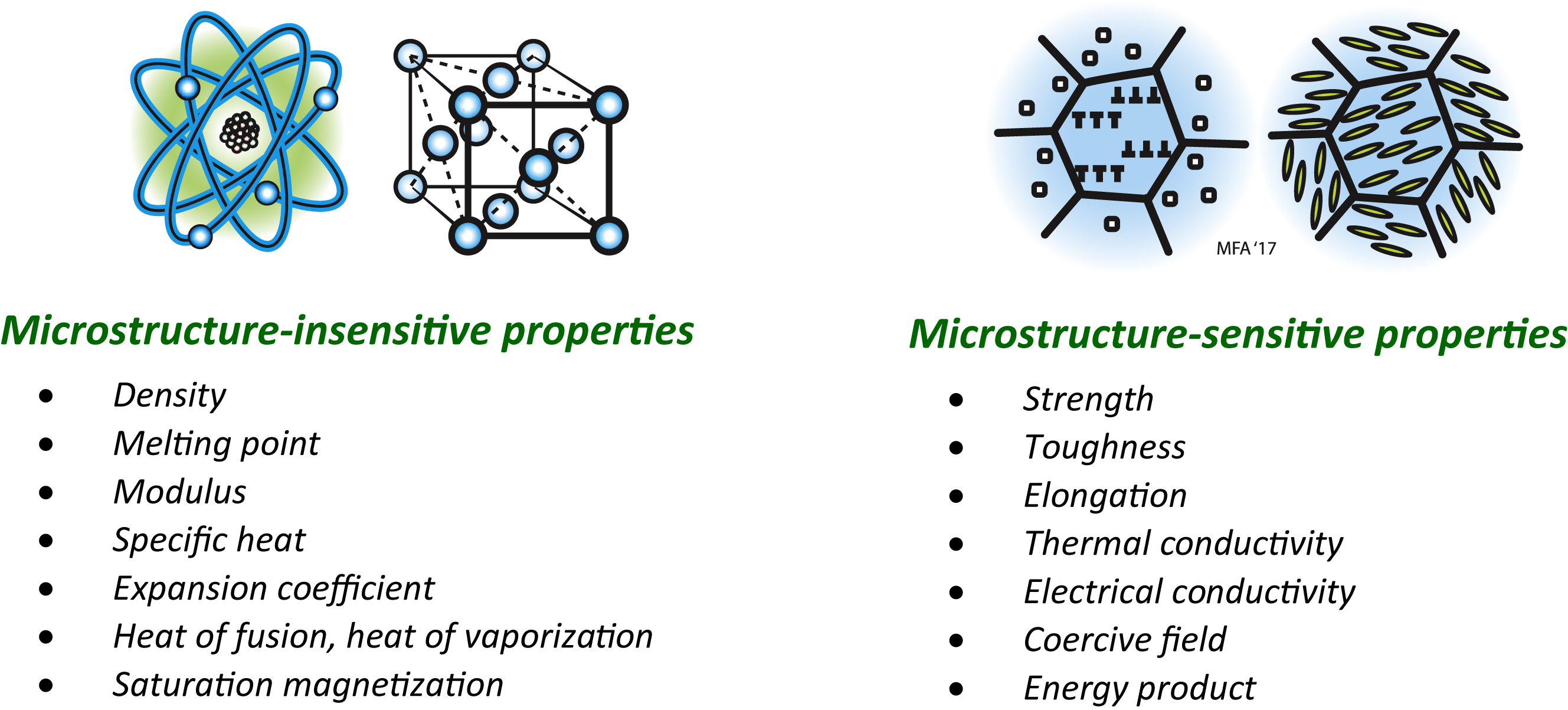
Material properties were classified in Chapter 4, and in the figure opening this Appendix, as:
Throughout the book, the underlying science of all the material properties has been explored by drilling down to the relevant length scale of atoms, molecules, and microstructures. The length scales in materials – from atoms to precipitates to grains, surface roughness, and cracks – span many orders of magnitude. A key skill in mastering materials science and engineering is to understand and appreciate the relationships between properties and the relevant features and their length scales. So here, for reference, we draw together all of the internal material features introduced in the book, and the range of length scale behind each property.
C1. Metals
Figure C1 summarises the main microstructural features in metals. Starting at the bottom with atoms, we have crystalline packing (with the exception of the unusual amorphous metals) – responsible for elastic moduli and density (Chapter 4). Atom-scale defects—vacancies and solute atoms – were introduced in Chapter 6. Thermal, electrical, optical, and magnetic behaviours are most directly influenced by this atomic scale of microstructure (Chapters 12, 14–16). Yield strength, toughness, fatigue, friction, and wear depend firmly on the microstructural length scales associated with microscopy, from dislocations and the obstacles to their motion (precipitates and grain boundaries), through to grains, surface roughness, porosity, and cracks (Chapters 6, 8, 9, 11). Fatigue cracks start at grain scale but grow to the dimensions of the component itself at fracture. Diffusion is fundamentally an atomic-scale phenomenon, but its effects are governed by the larger length scales over which it operates – those of precipitation, dislocation motion at temperature, grains, porosity, and electrochemical reactions; i.e. the microstructural phenomena behind a host of service and processing behaviours – corrosion, oxidation, creep, sintering, and heat treatments (Chapters 13, 17, 19). Metal products themselves span a huge range: kitchen foil is around 10 μm thick, automotive panels a millimetre or so, and ship propellers are several metres in diameter, while bridges and buildings reach the kilometre scale.
C2. Ceramic and glasses
Ceramics are crystalline, and glasses are amorphous. Figure C2 shows both structures near the bottom of our materials length scale. Many of the properties directly reflect the atomic layout and the intrinsically strong nature of the covalent or ionic bonding – from elastic modulus and density to electrical insulation. Atomic scales also govern viscosity in molten glass and diffusion in compacted ceramic powders (though again diffusion operates at the scale of porosity during processing, Chapter 19). Cracks in ceramics are closely related to grain size, and it is cracks and surface finish that dominate failure in both ceramics and glasses. So these are the key features governing fracture strength, toughness, friction, and wear (Chapters 8, 9, 11).
C3. Polymers and elastomers
Polymers and elastomers are inherently molecular rather than atomic. Figure C3 shows the wide diversity at this scale; depending on the chemistry, polymer molecules arrange into amorphous, crystalline, or cross-linked structures, and some can be aligned by drawing. Density, modulus, and the functional properties again reflect behaviour at this scale (Chapters 4, 14, 16); strength and toughness bring in larger features such as crazes and cracks (Chapters 6, 8). Polymer foams and fibre-reinforced polymer composites have additional length scales relating to their architecture—for example, cell size, fibre diameter, and ply thickness (Chapters 4, 19).


