CHAPTER 5
Epidemiology of Skin Disease
Hywel C. Williams
Centre of Evidence-Based Dermatology, Nottingham University Hospitals NHS Trust, Nottingham, UK
What is epidemiology and why is it relevant to dermatology?
Epidemiology is the simplest and most direct method of studying the causes of diseases in humans and many contributions have been made by studies that have demanded nothing more than an ability to count, to think logically and to have an imaginative idea.
(Sir Richard Doll 1987 [1])
Many dermatologists still think of epidemiology in terms of describing the prevalence and the age, sex and geographical characteristics of a particular skin disease. Whilst it is true that epidemiology is often used in this fashion to describe the burden of disease in human populations, as Sir Richard Doll points out above, epidemiology also offers one of the most powerful and direct methods of evaluating the causes of skin diseases in human populations. One definition of epidemiology is therefore ‘the study of the distribution and causes of diseases in human populations'. In addition to describing the burden and causes of skin diseases in populations, clinical epidemiology is concerned with describing the natural history and prognosis of diseases and with evaluating interventions that seek to prevent or treat diseases [2]. The term dermatoepidemiology refers to the study of the epidemiology of dermatological disorders [3]. Because epidemiological studies are often concerned with making observations about highly complex natural experiments, methods for minimizing bias or adjusting for confounding factors (see glossary) have had to be developed. These new methods, along with the high scientific rigour necessary for designing and interpreting epidemiological studies, are aspects from which all dermatological research can benefit. Epidemiology is therefore relevant to dermatology for the six reasons shown in Box 5.1.
Although epidemiology is still sometimes perceived as a novelty in dermatology, the first epidemiological discoveries in dermatology can be traced back to 1746, when James Lind [4] concluded that scurvy in sailors was related to dietary factors. He then showed, by means of a controlled study, that the disease readily responded to the addition of fresh oranges and lemons in the sailors' diet. The principles developed by Lind have resulted in the establishment of a library documenting the development of fair tests in treatments in health care [5]. In 1914, Joseph Goldberger [6] observed that 8% of 418 patients admitted to the Georgia State Sanatorium developed pellagra, compared with none of the 293 Sanatorium employees. He suggested that pellagra was due to an absence of ‘essential vitamins', today recognized as nicotinic acid, and proceeded to test his suggestion in a community trial. Thus, dermatoepidemiology is not such a new subject, and with over 2000 skin disease reaction patterns described, the scope of the topic is vast. This chapter will therefore not deal with the epidemiology of specific skin diseases, which will be described where possible under the relevant disease sections. It will instead attempt to illustrate the relevance of modern epidemiology to dermatology by using specific examples. The principles of evidence-based dermatology, which relies heavily on methods developed from clinical epidemiology, are discussed in more detail in Chapter 17 by Bigby and Williams. A glossary of commonly used epidemiological terms and further reading sources are to be found at the end of this chapter.
Thinking in terms of populations rather than individuals
Community diagnosis
One of the first hurdles to overcome when considering the epidemiology of a skin disease is to think in terms of populations rather than individuals. Many physicians find this conceptual jump quite difficult, as they are used to dealing with individual patients on a daily basis, whereas epidemiological studies refer to groups of individuals. Just as molecules, genes and individuals exhibit various aggregate characteristics, entire groups or populations exhibit their own unique characteristics and problems that enable a community diagnosis to be achieved [1].
Interesting patterns can occur when one explores the potential implications of treating an entire community (the public health approach) rather than sick individuals who present themselves to doctors (the high-risk approach). Rose [2], for example, showed that a 10 mm lowering of blood pressure distribution as a whole (e.g. from reducing salt intake) would correspond to about a 30% reduction in the total attributable mortality, simply because of the shape of the change conferred on the distribution curve in relation to specific ‘disease' cut-off points. In other words, a little bit of benefit spread across the entire population distribution may result in large population benefits in absolute terms.
During the scabies epidemics that occurred on the islands near Panama in the 1980s [3], it was found that even the best topical treatments when administered properly to individuals had no sustainable impact on the overall prevalence of scabies (which was very high in this population and which was associated with considerable morbidity from secondary pyoderma). When a population approach was adopted, that is treating all individuals with a programme of continuing surveillance, the prevalence of scabies fell dramatically to less than 2%, as shown in Table 5.1, and was sustained at that level until the US invasion of Panama interrupted these efforts. Thus, just as individuals become ‘diseased', entire populations can become sick [2]. In these situations, whether it be an infestation such as scabies or a disease of modern society such as obesity, tackling the problem based on a population diagnosis is usually beneficial, cost-effective and appropriate [4].
Table 5.1 Prevalence of scabies among 756 Kuna Indians on the island of Ticantiki, Panama [3]
| Date | Community treatment status | Prevalence of scabies (%) |
| July 1986 | Conventional treatment | 33.0 |
| October 1986 | Community control and surveillance instituted | 0.7 |
| July 1987 | Breakdown due to supply problem | 3.6 |
| December 1988 | Programme running again | 1.5 |
| March 1990 | US invasion of Panama | 12.0 |
From Taplin 1991 [3]. Reproduced with permission of Elsevier.
Skin diseases as ‘entities' in the population
The concept of considering the health of entire populations also applies to the classification of skin diseases. Typically, dermatologists are preoccupied with deviants who present themselves to secondary and tertiary care because of troublesome symptoms associated with more severe or chronic disease and who are at the extreme end of the normal distribution curve of variations in skin health and disease. Such individuals usually have well-defined physical signs that prompt those studying them to declare them as discrete ‘entities' [5, 6]. Such distinctions often become blurred when community surveys are undertaken. In a community survey of atopic eczema, for instance [7], it was noticed that indeterminate or borderline cases who had limited areas of dry skin or a single patch of eczematous inflammation were quite common. In these circumstances, perhaps the more relevant question is not ‘Has the person got eczema yes/no?' but ‘To what extent does this person have eczema?'
The concept of a distribution of disease severity at a population level may also be helpful in evaluating different treatment policies. For example, it has been estimated that a small change in the treatment threshold of isotretinoin from severe to moderate cases of acne could result in a 15-fold increase in prescriptions in absolute terms, simply because moderate cases outnumber severe cases by so much [8].
Making comparisons and drawing inferences
Epidemiological reasoning usually progresses in an ordered fashion, starting with a hypothesis that has been suggested by good clinical observations (e.g. palmoplantar psoriasis seems to be commoner in smokers) or descriptive studies of populations. The hypothesis (that smoking is a risk factor for palmoplantar psoriasis) is then tested in an epidemiological study, which gathers and analyses data in a systematic fashion in relation to an appropriate comparison group to see if a statistical association exists. Such a study was carried out, which confirmed a strong association between smoking prior to the onset of palmoplantar psoriasis [9]. Analytical epidemiology is therefore concerned with making comparisons. These comparisons rely upon the uneven distribution of disease and risk factors within and between populations to shed light on possible causes of ill health. Thus, previous case–control studies [10] showed that smoking was far more common in patients with lung cancer – a finding that led to more sophisticated studies to establish disease causality [11]. If everyone had smoked, it is unlikely that smoking would have been identified as a cause of lung cancer.
In the simplest form of epidemiological study, a count is made of the number of cases with a particular skin disease (the numerator) within a catchment population (the denominator). The probability or frequency of disease occurrence may then be compared in two or more populations – for example, one exposed to a putative causative agent compared with another that is not. Inferences are then drawn based on the magnitude of the differences of disease frequency between the populations in light of possible alternative explanations such as chance, bias and confounding (see glossary). If such associations are genuine, further evidence is usually needed to determine whether they are causal in nature [12]. The whole process brings us one step nearer to the dermatoepidemiologist's ultimate goal – that of preventing skin disease, providing such causes are amenable to individual/public health manipulation. Prevention of skin disease is clearly more desirable than treating diseased individuals (Figure 5.1).
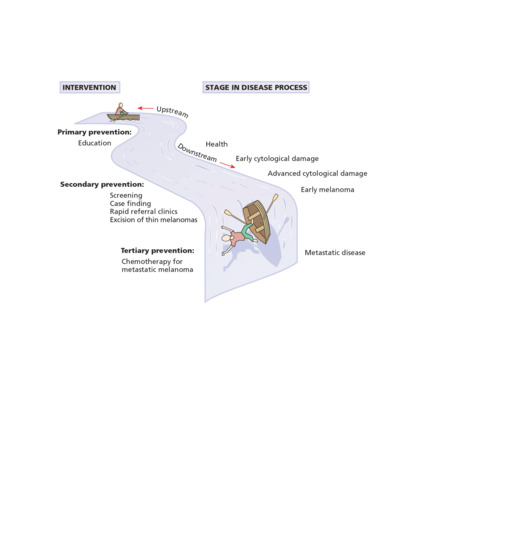
Figure 5.1 Disease prevention in a serious condition such as melanoma is much more sensible than treating sick individuals with expensive drugs at the end of a long chain of irreversible pathological events.
When referring to an epidemiological study, it is important to make a distinction between the study population chosen for a particular study (e.g. those attending a hospital out-patient clinic) and the base population about whom one wishes to make inferences, as shown in Figure 5.2 [13].
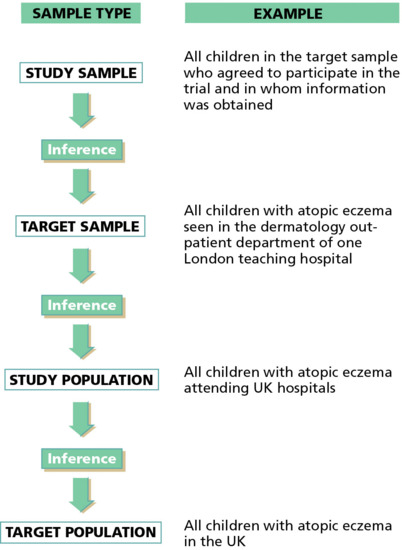
Figure 5.2 Generalizing the results of a clinical trial of a new treatment for atopic eczema from a study of children attending one hospital department to all children with atopic eczema in the UK requires several jumps of inference.
The prevention paradox
Interventions that confer large population health gains may not confer much benefit as perceived by individuals. Thus, in the example of scabies in Panama [3], although the population's health as a whole benefited greatly, many apparently healthy individuals may have not appreciated being treated for scabies, as it was not known who would have developed scabies in the absence of the prevention programme. Similarly, it is difficult to say which child will benefit from being immunized for tuberculosis in a BCG immunization programme, because events have not yet occurred. This conflict between large gains in the health of entire populations versus small gains in the health of individuals has been termed the ‘prevention paradox' [4]. In the field of contact dermatitis, for example, eradication of a rare but potent contact sensitizer may have a great impact on affected individuals but little impact on the overall total burden of contact dermatitis in the general population. On the other hand, reduction in the amount of contact with formaldehyde, a less potent but far more common sensitizer in the general population, will result in a much larger reduction in the burden of contact dermatitis in that population, simply because far more people are exposed to formaldehyde [14]. As Table 5.2 illustrates, a little bit of harm affecting a lot of people can therefore add up to more than a lot of harm affecting a few people, in population terms. The first step when considering the epidemiology of skin disease is therefore to think about populations rather than individuals.
Table 5.2 The prevention paradox: a little bit of harm affecting a lot of people can add up to more than a lot of harm affecting a few people [14]
| Eradication of exposure in: | ||
| The population | The individual | |
| Rare: Exposure with high relative risk | Small benefit | Large benefit |
| (e.g. contact sensitization to diphencyprone) | ||
| Common: Exposure with low relative risk (e.g. smoking and psoriasis) | Large benefit | Small benefit |
From Williams [14]. Reproduced with permission from Karger.
More than one disease?
Many of the conditions that are considered one disease today, such as atopic eczema and vitiligo, may turn out to be composed of several different diseases over time. Whilst it is easy to divide diseases into subtypes as a result of combinations of tests such as the division of atopic eczema into four subtypes on the basis of patch tests and IgE tests [15], the key issue when considering a change in nomenclature is that it should increase one's predictive ability – also known as progressive nosology. For example, the division of pemphigus, which formally referred to several diseases in which blistering was a feature, into pemphigus, pemphigoid, linear IgA disease and other variants on the basis of immunological discoveries, has been a key aspect to guiding the strength and duration of immunosuppressive therapy [16]. Techniques such as latent class analysis have been used with skin diseases such as hidradenitis suppurativa that suggest that three main types may exist, which may require different treatment approaches [17]. The concept of stratified medicine based on exo-phenotypic features has been somewhat understudied and overshadowed by the concept of stratified or personalized medicine based on genetic or serological tests (the endo-phenotype).
How much of a public health problem is skin disease?
The need for a clear disease definition in epidemiological studies
A glance at the dermatological journals makes it clear that the subject matter of most current research is defined – wholly or in part – by diagnostic labels or criteria. But if these criteria are not explicitly stated, are prone to vary from one patient to the next in unpredictable ways, and vary systematically from place to place and time to time, the usefulness of such research is gravely impaired. Although phrases such as ‘diagnosed by dermatologists' or ‘diagnosed independently by two experienced physicians' and ‘all with typical symptoms' may be adequate for dealing with individual patients, they are hopelessly inadequate when describing groups of individuals in epidemiological studies [1]. For instance, it has been shown that even experienced physicians are perfectly capable of disagreeing with each other over the classic signs of atopic eczema [2], and when two agree, a third is capable of disagreeing. What is regarded as ‘typical' in London may be nothing of the sort in Lampeter, Lima, Lhasa or Lusaka.
Diagnostic criteria that work well in hospital studies may perform poorly in community studies because of the effect of low disease prevalence on positive predictive value (see glossary) and an increase in borderline cases. The properties of good diagnostic criteria for use in epidemiological studies are summarized in Box 5.2 [3].
Not only is it important to use diagnostic criteria of known validity and repeatability in epidemiological studies, but it is sometimes important to qualify cases identified by such criteria by some measure of disease severity (Box 5.3). For example, prevalence surveys of acne in the absence of severity measures are not very helpful in quantifying the disease problem, since physiological acne (non-inflammatory lesions) affects over 90% of adolescents [4]. Again, severity grading systems used in a hospital setting (which are usually non-linear and tend to favour severe and currently active disease) may not be so helpful in describing disease severity in milder community cases, where intermittent disease may be more common.
Impairment, disability and handicap caused by skin disease
The burden of skin disease can refer to disease occurrence using terms such as prevalence or incidence (epidemiological burden), the effects of skin disease on a person's well-being (quality of life burden) or the direct and indirect costs associated with skin disease (economic burden). A working group affiliated to the US National Institute of Arthritis and Musculoskeletal Diseases (NIAMS) examined such conceptual frameworks in more detail, supplemented by thorough reviews of current data [5]. They concluded that further research is needed to reach consensus on how skin diseases and their associated burden should be defined.
In addition to the concept of burden, consideration of the three concepts of impairment, disability and handicap may be helpful in separating those effects that result from disordered function from those that are conferred on individuals by society. Impairment refers to the organic lesion produced by a disease, for example a broken limb; disability is the dysfunction that results from that impairment, for example not being able to walk; and handicap is the disadvantage that society confers upon the individual as a result of the impairment, for example unemployment. Handicap in skin disease may not be as explicit as that associated with a broken limb, but the psychological consequences of skin disease, which include ‘failure of display' [6, 7], may be just as important. It has been shown that relatively minor skin complaints often cause more anguish to people than other more serious medical problems [6]. Also, because skin disease is so common, a little bit of morbidity affecting a lot of people can add up to far more than a lot of morbidity affecting only a few people. It is this product of high prevalence times moderate morbidity that makes skin disease very important from the public health point of view (Figure 5.3). Small changes in health policy can have large financial implications, simply because they affect so many people.
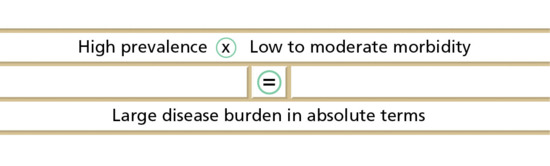
Figure 5.3 Skin disease is a major public health problem: in public health terms, a little bit of misery affecting a lot of people can add up to more than serious illnesses that affect only a few people.
One population-based, cross-sectional study conducted in the USA on a random sample of 20 479 people examined by dermatologists in 1971–1974 [8] has pointed to the magnitude of disability and handicap from skin diseases; skin conditions were reported to limit activity in 10.5 per 1000 of the population aged 1–74 years, or 9% of those persons with such skin conditions. About 10% of those with skin complaints considered the condition to be a handicap to their employment or housework, and 1% considered themselves to be severely handicapped. About one-third of those persons with skin conditions indicated that the condition(s) were a handicap in their social relations. The dermatological examiners rated more than two-thirds of those with skin complaints as disfigured to some extent from the condition, and about one-fifth of those were rated moderately or severely disfigured. More than half of those with skin complaints reported some overall discomfort from the condition, such as itching or burning. An estimated 62.8 per 1000 US civilians (or 56% of those with skin complaints) indicated that the conditions were recurrent, with 49% active in the preceding 7–12 months.
The 1989 UK General Household Survey estimated that 16 per 1000 persons were affected by a longstanding skin disorder sufficiently severe to limit their activities [9]. Another survey of disability, amongst 14 000 adults conducted in the mid-1980s, found that 1% of complaints causing disability in private households and 2% in communal establishments were due to skin disease [10]. A survey of self-reported skin problems in 8000 adults in Uppland, Sweden, found that 20.5% reported skin problems [11]. Those reporting skin problems scored lower on all eight dimensions of the SF-36, a generic quality of life instrument.
In addition to disability and handicap, some chronic skin diseases such as atopic eczema also incur considerable additional direct costs to families, such as that needed to purchase moisturizers, special soaps, extra laundry expenses, cotton clothing and bedding. The Lothian Atopic Dermatitis Study estimated that the mean cost to the patient was £25.90 per 2 months, while the mean cost to the UK National Health Service was £16.20 in 1994 [12]. A systematic review of cost studies of atopic eczema in the USA found very wide ranging estimates of 364 million to 3.8 billion US dollars per year, with very little information on indirect costs [13]. Another study estimated the direct cost of care for patients with psoriasis and psoriatic arthritis in the USA [14]. An ambitious project in the USA tried to estimate the direct and indirect costs of people with 22 of the commonest skin disease categories in 2004 and found that they accounted for around $29 billion in direct costs (medical care and products), $10 billion in lost productivity costs and a further $56 billion for loss of quality of life [15].
Global Burden of Disease 2010 study
In previous editions of this book, this author attempted to describe the various key population-based studies that have occurred across the world over the last 40 years. Achieving the goal of estimating the global burden of skin disease in a standardized way remained elusive until the publication of the Global Burden of Disease 2010 (GBD 2010) study data [16]. Previous attempts at summarizing world health in general had significant methodological shortcomings [17], many of which have been overcome by the GBD 2010 survey. The GBD 2010 survey covers all human disease and has used sophisticated Bayesian methods for estimating prevalence, incidence, mortality and disability for over 200 human diseases in 187 countries, broken down by age and sex, from the period 1990–2010. Data on disability-adjusted life years and years lived with disability for all diseases and injuries have been published elsewhere [18, 19]. GBD 2010 also contains a wealth of skin data that have been assembled as a result of an exhaustive search for relevant studies, such as those included and described in detail in the last edition of this book. The skin data for GBD 2010 has been published in full in the Journal of Investigative Dermatology [16] and includes a description of disability caused by 16 common non-fatal skin groups: eczema, psoriasis, acne, alopecia areata, pruritus, pressure ulcers, urticaria, scabies, fungal skin diseases, impetigo, abscess and other bacterial skin diseases, cellulitis, viral warts, molluscum contagiosum/warts, non-melanoma skin cancer and other. These are described by country, age and sex in 187 countries. The key measure of disability used to describe the data includes years lost due to disability (YLD) and the disability-adjusted life year (DALY). One DALY can be thought of as a year of lost healthy life, and is calculated by adding the sum of the years of life lost (YLL) due to premature mortality combined with the YLD for people living with the health condition or its consequences [20]. Assumptions used to calculate these estimates are described fully elsewhere [16].
Figure 5.4 shows the YLD due to the main 16 skin condition groupings by region in 2010 as a proportion of total YLD and as YLD per 100 000 of the population. Higher rates of disability due to infectious skin diseases are seen in regions such as Africa, whereas disorders such as pruritus and eczema are higher in Europe. Figure 5.5 shows YLD for skin disease according to age, and reveals a striking bimodal distribution with one peak in early life largely due to eczema and acne and a steady rise in a range of skin diseases in older life. Eczema as a group emerges as the commonest reason for YLD across the regions and over the different age groups. Figure 5.6 attempts to show the global distribution of DALYs for skin disease and illustrates the disproportionate burden of skin disease in mainly sub-Saharan Africa and lower rates across large parts of Russia, Mongolia and China.
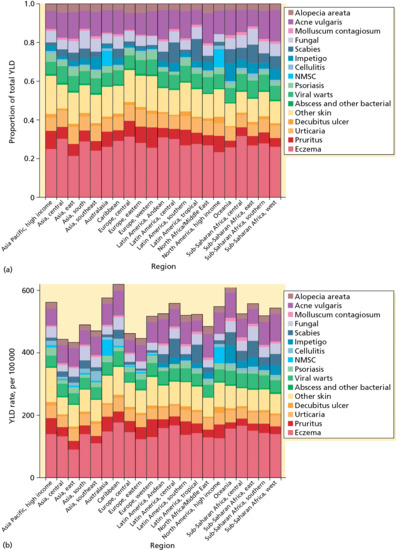
Figure 5.4 Years lost due to disability (YLD) for the main 16 skin condition groupings by region in 2010 as a proportion of total YLD and as YLD per 100 000 of the population. NMSC, non-melanoma skin cancer.
(From Hay et al. 2013 [16]. Reproduced with permission of Nature Publishing Group.)

Figure 5.5 Years lost due to disability (YLD) for skin disease according to age from the 2010 Global Burden of Disease study showing a peak in younger life and a steady increase in burden in later life. NMSC, non-melanoma skin cancer. (From Hay et al. 2013 [16].
Reproduced with permission of Nature Publishing Group.)
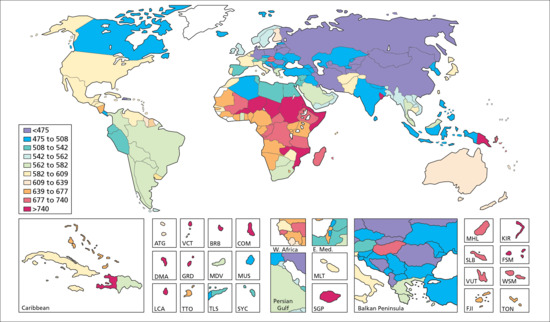
Figure 5.6 Global distribution of disability-adjusted life years (DALYs) for skin disease from the 2010 Global Burden of Disease study illustrating the disproportionate burden of skin disease in sub-Saharan Africa. (From Hay et al. 2013 [16].
Reproduced with permission of Nature Publishing Group.)
When compared with other diseases, skin conditions in 2010 ranked as the fourth leading cause of non-fatal burden expressed as YLDs and as the 18th cause of DALYs. Three skin diseases (fungal infections, other skin and subcutaneous diseases, and acne) were amongst the 10 most common diseases globally, and another five (eczema, pruritus, molluscum/warts, impetigo and scabies) appeared in the top 50.
Although the GBD 2010 data have limitations, such as missed studies and sparse data from some parts of the world, it is nevertheless the first comprehensive attempt to collate data and model missing data and disfigurement and sequelae from skin disease data using a robust and explicit approach that will serve as a benchmark for future planned annual GBD surveys. The surveys provide essential data for public health planning and suggest that skin disease is a priority for most countries across the world.
It is important to point out that all of the above data refer to non-fatal skin disease, and that data on all diseases with significant impacts on mortality (including diseases treated by dermatologists such as melanoma and infectious diseases) have been collated in another key publication [21]. Not surprisingly, infectious diseases such as dengue fever and measles appear as the top causes of mortality for skin disorders. Although the number of melanoma increase steadily in older age, it is one of those cancers that seems to have a disproportionate mortality impact in younger, economically active people. Non-melanoma skin cancer, although rarely associated with mortality, shows mortality rates approaching melanoma, probably due to the much larger number of tumours (especially squamous cell carcinoma) involved.
What determines the frequency of skin disease in populations?
Risk factors, association and causation
In the first instance, epidemiological studies seek to establish risk factors for diseases, that is, factors that are associated with an increased frequency of disease. When associations between skin diseases and risk factors are discovered (e.g. by demonstrating an increased risk of palmoplantar psoriasis in smokers [1]), it should be understood that such associations do not necessarily imply causation. The association between smoking and psoriasis may simply be a chance finding (around one in 20 studies with a P value of less than 0.05 in favour of rejecting the null hypothesis of no association will be wrong due to chance alone), or it could be due to confounding (i.e. a third factor such as alcohol or social class, which is independently associated with both smoking and psoriasis [2]). The association could be due to a bias – for example, people with psoriasis in hospital may be more likely to recall antecedent events or seek reasons for explaining their illness in comparison with healthy controls [3]. Further analyses or new studies are usually needed to establish whether risk factors are causative – for example, by evaluating the strength of the association, biological gradient, relationship in time, consistency between different studies, biological plausibility, coherence of evidence with external sources, experimental evidence and specificity of findings as suggested by the Bradford-Hill criteria of causality [4].
The causes of some skin diseases are already established – for example, the herpes simplex virus causes cold sores – but for most dermatological conditions the causes are unknown. Nevertheless, epidemiological research has already established many risk factors for skin diseases which may help to serve as pointers to specific causes. Direct manipulation of these risk factors may help in preventing or reducing disease even before the specific cause is found. For example, in the London cholera epidemics of the 1850s, John Snow [5] postulated that the disease was spread by some ‘morbid matter' in the water supply and proceeded to intervene by removing the pump handle in Broad Street. This resulted in a dramatic fall in incident cholera cases. All of this occurred some 20 years before germ theory had become established in Europe. Snow's work illustrates one of the beauties of epidemiological research, which is that knowledge of pathophysiology is not a prerequisite for determining aetiology.
Even when a causative agent is discovered, for example Vibrio cholerae, exposure to this agent does not necessarily imply disease. Of those exposed to cholera during an outbreak, some will die from the disease, some will be very ill, some will be slightly unwell, some will be apparently healthy (but still carry the organism) and some will not be affected at all. The absence of disease in some individuals following exposure is probably due to a whole range of factors such as chance, infecting dose, genetic heterogeneity and other constitutional and environmental factors that interact together to produce the final clinical picture. This phenomenon of apparent health in the presence of an established harmful exposure has been exploited by individuals in order to avoid modifying their behaviour. One often hears statements such as ‘my grandfather smoked 40 cigarettes a day all his life and he did not get lung cancer' in order to justify their habits. In order to explain such phenomena, we return to the epidemiological concept of groups of people or populations and probability of disease [6]. On average, groups of people who smoke cigarettes are 10 times more likely to develop lung cancer when compared with those who do not smoke.
It is also important to separate risk factors associated with disease incidence, that is the number of new cases in a given population occurring over a defined period, from those that determine disease chronicity, that is the determinants of how long a particular disease will last once an individual has it, as the risk factors for each of these aspects may be different. Many dermatoepidemiology surveys have measured the prevalence of skin disease when examining risk factors [7], but because prevalence is a function of incidence times chronicity, it is often difficult to say whether these risk factors are important in people developing a disease for the first time, or whether they maintain the disease once established.
Risk factors for skin disease may operate at many different levels. Some may predispose to disease (e.g. a mother with atopic eczema genetically predisposes her child to atopic eczema), some may precipitate disease (e.g. exposure to high levels of house-dust mite may precipitate atopic eczema for the first time) and some may be important in perpetuating that disease (e.g. failure to use prescribed treatments may worsen the course of atopic eczema). Some of the commonest risk factors for skin disease are discussed here.
Genetics
In addition to a few rare diseases such as epidermolysis bullosa, where specific chromosomal mutations have been closely correlated with different disease phenotypes, many genes may be important in many of the major inflammatory skin diseases. Thus in atopic eczema, genes such as filaggrin mutations that play a key role in skin barrier function, as well as several other genes that code for inflammatory responses, may be important in explaining the variation in disease phenotype [8]. Genes such as those that predispose for melanoma may only express their beneficial or deleterious effects when individuals who carry them are additionally exposed to key environmental risk factors such as ultraviolet light [9]. Some genes may be responsible for disease predisposition and some may be responsible for disease severity and chronicity, as exemplified by molecular subsets in the gene expression signatures in the skin in scleroderma [10].
Early environment
There is evidence to suggest that the experience of the fetus in utero (e.g. in terms of nutrition) is critical in ‘programming' adult diseases such as hypertension and diabetes [11]; in utero programming may well operate for many skin diseases such as atopic eczema [12]. Epigenetics is another interesting field whereby environmental exposures such as tobacco smoke or dietary exposures may induce a persistent genetic state through gene transcription [13].
Later environment
Age and sex are often included in the descriptive epidemiology of many skin diseases and may point to further risk factors. The marked female preponderance of lichen sclerosus, for example, suggests that hormonal factors may be important in this disease.
Ethnic group may account for some variations in disease rates. Thus, it has been shown that atopic eczema is twice as common in black Caribbean children in comparison with similar white children in the UK [14] and, conversely, that mortality from most cancers is less common in black ethnic groups in the UK [15]. Ethnic group, which refers to a way of life encompassing a whole range of dietary and cultural factors, must be distinguished from racial factors [16], which are often more difficult to define because of the considerable mixing of modern populations. Care also has to be taken in lumping many distinct ethnic groups together – for example, combining the diverse cultures of black Africans and black Caribbeans into ‘blacks' may be totally inappropriate, both in terms of respecting the identity of the separate cultures and because such lumping together may obscure important epidemiological associations [17]. The term ‘race' should not be used in epidemiological studies, as it has no scientific meaning [18]. Migration itself may be an important factor in determining skin diseases; for example, individuals who migrated from China (where atopic eczema is not very common) developed much higher rates of disease (similar to the rates in the local population) after migration to Hawaii [19]. Migrants may not be totally representative of their indigenous peoples, but they may nevertheless show the effect of the environment in determining the frequency of skin disease.
Secular factors may reflect changes in the natural history of skin disease or of transient environmental exposures. Thus, the epidemic of melanoma skin cancer has been attributed by some to increased exposure to sunlight over the last 50 years [20]. There is now clear evidence from the International Study of Asthma and Allergies in Childhood that the prevalence of atopic eczema has increased in most countries across the world over a 10-year period, but the reasons for this change are less clear [21].
Socioeconomic factors may also be crucial in accounting for the distribution of skin disease. In many poorer countries where overcrowding and poor sanitation may occur, infectious or ectoparasitic skin diseases such as secondarily infected scabies or pediculosis are commoner [22, 23]. In wealthier countries, where such infectious dermatoses are less common, new ‘diseases' such as concern regarding the cosmetic appearance of sun-damaged skin or thread veins may preoccupy the population in their quest for a perfect skin. Some skin diseases, such as atopic eczema, also demonstrate a genuine positive social class trend: that is higher prevalences in more wealthy groups [7]. Some of this increase in reported eczema may have been due to differences in reporting between socioeconomic groups, but other genuine environmental factors such as hygiene, carpets, central heating, family size or differences in treatment and other health-seeking behaviours also probably play a part.
Geography and climate are important considerations in describing the frequency of skin disease. Thus, consideration of the marked latitude gradient of melanoma in white-skinned peoples has supported the concept that exposure to sunlight is an important risk factor for this disease [24]. Paul [25] has drawn attention to the concepts of macroclimate, which in the ordinary geographical sense refers to temperature, rainfall and humidity, and microclimate, which refers to the immediate domestic and occupational environment a given individual finds himself or herself in. These are discussed further by Marshall [26] and Canizares [27]. The combination of temperature, rainfall and humidity may be crucial to sustain certain infectious disease vectors such as the Simulium fly in onchocerciasis, and may for example account for seasonal fluctuations in pyoderma secondary to scabies during the wet season in Lilongwe in Malawi [28]. An elegant study by Silverberg et al. merged data on the self-report of eczema from 91 642 children aged up to 17 years with national climatic data and found a lower prevalence of eczema to be associated with higher outdoor humidity, high UV exposure and higher temperatures when analysed across the USA [29].
Occupational factors are occasionally a very important factor for skin disease. Thus, exposure to irritants and contact sensitizers in light and heavy industry accounts for a very large burden of hand dermatitis and lost revenue for both individuals and the state. Certain occupations, for example mining, where workers are constantly exposed to damp conditions, may predispose to fungal infections. Some diseases may occasionally occur in outbreaks from work-related substances, for example chloracne due to dioxins, vinyl chloride disease and hydroquinone-induced leukomelanoderma. The reader is referred to standard texts of occupational dermatoses and to Chapter 130 for further reading [30, 31].
Infective agents may directly cause or be suspected to cause many skin diseases. Thus, for a long time, it was suspected that fifth disease was caused by an infectious agent, but it was not until 1983 that human parvovirus B19 was identified as the causative organism [32]. Similarly, there is reasonable circumstantial evidence to suggest that diseases such as pityriasis rosea are caused by infectious agents, even though no specific agents have yet been consistently isolated [33, 34].
Dietary factors may be crucial in some skin diseases. As the examples of Lind and Goldberger in the opening section of this chapter illustrated, vitamin deficiency states may directly cause skin diseases. Other deficiency diseases with skin manifestations, such as acrodermatitis enteropathica, are completely reversible with the administration of the appropriate agent, in this case zinc. Some diseases, such as phenylketonuria and dermatitis herpetiformis, may be transformed by restricting substances that affected individuals cannot handle – for example, phenylalanine and gluten, respectively. Some skin diseases, such as atopic eczema and acute urticaria, may be modified by the avoidance of dietary allergens in a proportion of cases. Leisure activities such as gardening or habits such as smoking cigarettes and drinking alcohol may be important risk factors for many skin diseases including contact dermatitis, psoriasis and porphyria cutanea tarda. Medicines, although intended to alleviate human disease, are a very common cause of cutaneous eruptions, some of which (e.g. toxic epidermal necrolysis) can be fatal.
Describing the natural history and associations of specific skin diseases
Patients with a skin disease often ask ‘How long will it last?' and ‘Will it come back again?', yet reliable answers to such questions are scarce. Special studies are required to answer these questions, which ideally involve following, over many years, individuals with typical and well-defined disease in terms of morphology and severity [1]. Such prospective studies are rare in dermatology [2]. Another approach is to identify cases with a specific skin disease from old hospital records and then to trace them in order to find out what has happened to them since they were seen [3]. Studies on the natural history of disease are often difficult to interpret because of incomplete follow-up, the intermittent nature of many skin diseases, which can make the distinction between ‘real' and ‘apparent' clearance rates difficult [4], and because the treatment of many diseases can improve over time, rendering some with mild disease into the non-diseased category when they would previously have been classified as diseased. Guidelines regarding the attributes of what makes a good follow-up study are summarized elsewhere [5].
Disease associations of specific skin diseases may also give insight into possible causative factors. Thus, the high incidence of laryngeal carcinoma in psoriasis patients might be evidence for the possible role of cigarette smoking in psoriasis [6]. Many publications have emerged over the last 7 years linking psoriasis with the metabolic syndrome, suggesting that it is a systemic disease and that patients with psoriasis should be screened for cardiovascular risk factors [7, 8]. Establishing disease co-occurrence, for example atopic eczema and psoriasis, may also shed light on shared or opposing immunopathological mechanisms [9, 10]. Great care has to be exercised in interpreting disease associations generated from hospital sources because, in the absence of an appropriate denominator, many types of bias may occur [11, 12]. Data linkage from large representative general practitioner databases have yielded some valuable insights into disease associations such as an increased risk of stroke following chickenpox [13], a threefold increase in mortality for patients with pyoderma gangrenosum [14] and a decreased risk of brain tumours and increased risk of attention deficit hyperactivity disorder in children with atopic eczema [15].
Health services research in dermatology
Broadly defined, dermatological health services research is concerned with studying how dermatological health care is delivered with the ultimate aim of benefiting patients. Dermatological health services research thus covers a wide variety of service aspects, such as determinants of referrals to hospital departments, evaluation of cost-effectiveness of alternative treatment strategies, quantifying the dermatological needs of the community, evaluating the role of teledermatology and dermatological nurses and exploring economic aspects of screening and other prevention strategies. These diverse studies require a range of quantitative and qualitative research methods, such as focus groups, surveys, analysis of routinely collected referral and outcome data, before and after studies, comparative studies, interrupted time series, action research, randomized controlled trials, decision analysis and health economic modelling alongside evidence syntheses. Research establishes which treatments or services should be used, whereas audit seeks to establish whether health care providers perform these services to a required standard [1]. As in any other branch of epidemiology, health services research requires meticulous attention to be given to aspects of study design. A useful introduction to health services research in dermatology is given elsewhere [2].
Needs assessments in dermatology
When evaluating dermatological health services, certain steps need to be followed [3]:
- Establish the size and nature of the dermatological need based on epidemiological data.
- Summarize the currently available supply of services for that problem.
- Appraise the evidence for effectiveness of those services.
- Propose models of care that best fit the epidemiological data and evidence of effectiveness of care within current resources.
- Propose outcome measures and targets that can be monitored after implementation.
Such an assessment has been attempted for UK dermatological health services by this author [4], and a comprehensive update of the assessment was undertaken by Schofield et al. [5].
Services available for people with skin diseases
People with skin problems obtain help from various sources, including self-help, advice from pharmacists, advice and treatment from the primary care team and specialist services. Little research has been conducted to clarify the relative health gain and appropriateness of the various health care settings for different subgroups of skin disease. The estimated number of people using current dermatology health services in the UK at various entry points, for a population of 100 000 over a 1-year period, is summarized in Table 5.3 [6, 7, 8, 9, 10, 11].
Table 5.3 A guide to the number of persons per 100 000 per year using dermatology services
| Group | Number using service |
| Number with a skin complaint | 25 000 (at least 25% of total population) |
| Number who will self-treat | 7500 (30% of those with skin complaint) |
| Number who will seek advice from their GP | 14 550a (15% of total population or 19% of all GP consultations) |
| Number referred to dermatologist | 1162 (8% of those attending their GP for skin problems, or 1.2% of the total population) |
| Number admitted to hospital | 24–31 (2–3% of all new dermatology referrals) |
| Number of deaths due to skin disease | 5b (0.4% of all new dermatology referrals) |
From Williams 1997 [4] with permission.
aExcludes skin neoplasms, viral warts, herpes simplex and scabies.
bIncludes people dying from cellulitis, chronic ulcer of the skin and severe drug reactions who might not have been admitted under a dermatologist's care.
Self-help
Although self-help/medication is not traditionally regarded as a health service, the range and availability of over-the-counter skin products is an important element in the equation of balancing need, supply and demand. Around 30% of those with a skin complaint decide to self-medicate, and this proportion is similar for trivial and for moderate to severe disease [6]. Many effective skin treatments are available over the counter in the UK, such as 1% hydrocortisone for mild eczema, topical aciclovir cream for cold sores, topical benzoyl peroxide for acne, and numerous antifungal preparations and wart removers. Pharmacists occupy a key role in advising the public on the use of these products, but whether this advice is beneficial or whether it simply delays appropriate medical consultation has not been studied adequately in the UK [12]. Self-help groups are often a useful source of advice to those with chronic skin diseases [13].
Primary care
The majority of those with a skin complaint who seek medical help in the UK and some other countries such as Canada and the Netherlands are treated by their family practitioner (general practitioner or GP). Skin conditions were the most frequent reason for people in England and Wales to consult their GP with a new problem in 2006, which equates to around 6.1% (0.8 million) of the population (12.9 million people). The most common reasons were skin infections and eczema [5]. In the USA, it has been estimated that around 36.5% of patients attending their family practitioner over a 2-year period had a skin complaint [14]. The range of skin disorders seen in general practice is similar to that in the general population, with relatively few subcategories accounting for the majority of consultations. As one would anticipate, proportionally more incident diseases such as skin infections (e.g. impetigo, herpes simplex and viral exanthems) are seen in general practice than in secondary care [8].
Secondary care
Although dermatology covers around 2000 disease–reaction patterns, over 70% of specialist activity is concerned with fewer than 10 main disease categories, as shown in Box 5.4 [4]. Age-specific attendance rates are more common in female patients and also rise with increasing age. Around 12% of referrals were considered inappropriate by dermatologists in one UK study [14]. There is considerable variation in referral rates to specialist care within the UK and there is some evidence to suggest that much of the regional variation in referral rates may be governed by established patterns of care and the number of available consultants, rather than by any objective dermatological need [4]. Roland and Morris [15] showed no relationship between referral rates for dermatology services and medical need as suggested by standardized mortality ratios or mean number of prescriptions issued by GPs (standardized regression coefficient of 0.1). It should be emphasized, though, that mortality ratios are not a suitable surrogate measure for dermatological need. A strong relationship between dermatology referral rates and the number of dermatology consultants per 100 000 population was present, however, in their study (standardized regression coefficient of 0.82, P <0.001).
Relationship between need, supply and demand for dermatological care
Unlike commerce, which aims to balance supply with demand, caring for sick human beings requires consideration of a third factor – that of medical need. Medical need may be defined as the ability to benefit from medical care, demand as that which people ask for, and supply as what the service does or could provide [3, 4]. Not all dermatological need is demanded (e.g. a person may be unaware that he or she has an early melanoma), not all that is demanded is needed (e.g. cosmetic removal of all moles), although all that is supplied is usually needed or demanded. The division between what constitutes reasonable need (e.g. somebody worried that a mole may be cancerous) and demand (e.g. somebody requesting removal of an ‘ugly' mole) is especially blurred in dermatology. Defining ‘need' in dermatology is therefore quite difficult, and is a process that requires the participation of society so that appropriate policies can be set in the light of finite resources.
Two population surveys conducted in the 1970s have produced useful data on the relationship between the need, supply and demand for dermatological care. In a study of 2180 adults in Lambeth who were examined for skin disease [7], it was shown that for those with moderate/severe skin disease, only 24% had made use of any medical service in the previous 6 months. A further 30% had used self-medication. Medical usage was still considerable for those with trivial skin disease, with 10% using medical services and 33% self-medicating.
In the US HANES-1 study [16], there was a considerable mismatch between what the dermatologists considered to represent medical need and what the population were concerned about. Only one-third (31%) of persons with significant skin pathology diagnosed by the dermatologists expressed concern about these specific skin conditions, whereas nearly 18% of those who complained about their skin conditions were not considered to have serious conditions by the dermatologists. Thus, both of these population studies suggest that, at any one time, around one-quarter to one-third of the population have a skin problem that could benefit from medical care, yet around 80% do not seek medical help.
The relationship between need, supply and demand for dermatology services in developing countries may be very different from those in developed countries. Many surveys have shown a high prevalence of need, mainly due to infectious dermatoses. There is marked maldistribution of care for people with skin diseases throughout the world, with meagre to absent dermatological services in many countries. Leprosy, onchocerciasis and leishmaniasis are probably the commonest skin diseases worldwide, but the epidemiological research afforded to these diseases is usually scanty. Groups such as the International Foundation for Dermatology work to remedy such inequalities, with the ultimate aim of a healthy skin for all [17]. Getting the right people to the right services is a major challenge. In the state of Guerrero in Mexico, for instance, skin complaints represent the second commonest reason for referral to rural clinics, resulting in a detrimental effect on other important activities such as immunization programmes and antenatal care [18]. In addition to such opportunity costs, this study also showed how much family income is wasted on ineffective treatments for skin infections and scabies.
Conclusions
This chapter has demonstrated the fundamental importance that the discipline of epidemiology plays in understanding skin diseases in context, from the clinic to the population. Not only is epidemiology concerned with issues such as describing the incidence, prevalence and human and financial cost of skin disease, but it is also one of the most direct ways of finding out the causes of skin diseases. Finding out causes is important because it may lead to the prevention of skin disease on a massive scale. For example, it has been found through a number of epidemiological studies that atopic eczema is less common in large, less economically advantaged families [1, 2]. This observation gave rise to the hygiene hypothesis, which postulated that increased exposure to microbes and infections in early life might protect against atopy [1, 3]. The hygiene hypothesis led to a full-scale, randomized controlled trial of Lactobacilli cultures given to pregnant mothers and infants, a study that suggested that around 50% of atopic eczema could be prevented by such a measure in infants [4]. Even though this particular study had some potential flaws [5], it nevertheless demonstrates the potential power of prevention.
Epidemiological principles have been key to developing the principles of evidence-based medicine described in Chapter 17 and of health services research in relation to dermatology. Although traditional epidemiology may be superseded by genetic epidemiology and other new hybrids as biomedical knowledge develops, there will always be a need for a thorough understanding of the principles of assessing risk and the roles that the ‘big three' factors of chance, bias and confounding may play in any study. Similarly, the principles of critically appraising published literature using a framework derived from epidemiology are as basic to dermatological clinical practice as diagnosing skin rashes [6]. Nijsten and Stern nicely summarize how modern epidemiology has led to better understanding of skin diseases and their treatments such as in Lyme disease, severe cutaneous reactions, long-term safety of PUVA (psoralen–UVA), co-morbidities in psoriasis and teratogenicity of isotretinoin [7]. The importance of clear reporting of the various types of epidemiological studies using the Strengthening the Reporting of Observational Studies in Epidemiology guidance (STROBE; http://www.strobe-statement.org/, last accessed May 2014) cannot be overemphasized [8, 9].
Glossary of epidemiological terms
Measures of disease frequency
- Prevalence. The proportion of people with a disease at any one time. Point prevalence refers to prevalence at one point in time. Period prevalence refers to the proportion with a disease (existing and new cases) over a longer period, for example 1 year.
- Incidence. The rate of new cases developing over a specified time period, for example the incidence of melanoma in the USA in men in 1983–1987 was 6.9 per 100 000 per year [1].
Measures of disease associations
- Risk factor. A factor that increases the risk of disease. This could be a specific exposure, for example asbestos giving rise to mesothelioma, or an attribute such as gender or social class which is indirectly associated with an increased frequency of disease.
- Relative risk. This is the ratio of the risk of disease occurring in those exposed to the agent under investigation divided by the risk of those not exposed. It is a measure of the strength of the risk factor.
- Attributable risk. This is the difference between the incidence rate in those exposed to a factor and the incidence rate in those unexposed. It is a measure of the absolute effect of the exposure.
- Odds ratio. An approximation of relative risk used in case–control studies. It is the ratio of the odds of exposure in cases to the odds of exposure in controls.
- Hazard ratio. This a special form of relative risk that is used in studies that examine survival to express the relative risk of an end point or ‘hazard' occurring at any given time.
Interpreting results
- Sampling error. This refers to the variation in values that a given sample could be expected to show by chance alone.
- P value. When referring to the association of a disease with a particular exposure, a P value of <0.05 means that a value as extreme as that obtained by the study would be expected to be observed by chance in less than one in 20 such studies (or <5% of the time). It is convention at this level of significance to reject the null hypothesis of no difference between the compared groups.
- Confidence intervals. This refers to the range of plausible values for a main study finding. It is based on the size of the sample and the size of the difference between compared groups. For example, the 95% bounds of a relative risk of 2.0 for smoking in a sample of psoriasis sufferers was 1.5–2.5. If the association is a genuine one, this means that the reader can be 95% confident that the true population relative risk resides between the values of 1.5 and 2.5.
- Bias. Bias is a systematic error resulting in an incorrect conclusion about the association between an exposure and an outcome. Over 30 types of bias have been described [2], but they fall into two main groups: (i) selection, that is the two groups to be compared are not comparable in terms of factors in addition to the exposure of interest; and (ii) information, that is collection of information about the disease and exposure in a fashion that could bias response, for example, those evaluating a new drug were aware of the treatment allocation when assessing patients' response to treatment. An excellent description of the main forms of bias in epidemiological research is to be found elsewhere [3].
- Confounding. This is where the association between an exposure and disease is mixed up with a third factor that is independently associated with both the exposure and the disease, for example the protective effect of prolonged breastfeeding (the exposure) on the development of atopic eczema (the disease) may be due to confounding by parental atopy. The risk of atopic eczema is increased in children born to atopic parents, and atopic parents are more likely to practise prolonged breastfeeding in their infants because they may be more aware of a possible protective effect of breastfeeding.
- Association and causation. Association between an exposure and disease does not necessarily imply causation. Other factors such as chance, bias and confounding may explain that association.
Validity and repeatability
- Internal validity. Internal validity, for example of a diagnostic test, refers to the extent to which the test measures what it is meant to measure. This is normally measured in terms of sensitivity (proportion of true cases correctly identified) and specificity (proportion of non-cases correctly identified).
- External validity. This refers to the extent to which findings from one particular study (the study population) can be generalized to the target population – for example, to what extent are the favourable results of a clinical trial to test a new oral agent for children with severe atopic eczema attending hospital applicable to children with milder eczema in the community?
- Predictive value. When comparing the performance of a test or diagnostic criterion with a gold standard (e.g. clinical diagnosis of melanoma against histological diagnosis), the positive predictive value refers to the probability that someone is a genuine case given a positive test result. Negative predictive value refers to the probability that a person does not have that disease given a negative test result. In addition to sensitivity and specificity, predictive value is dependent on the overall prevalence of the disease being studied [4].
- Repeatability. This refers to the extent to which two observations agree with each other. This may be between two observers (inter-observer agreement) or between replicate measurements in one observer (within-observer agreement). Repeatability is measured by chance-corrected agreement measures such as the κ statistic [5], or by differences between two observers plotted against corresponding means of observations and not correlation coefficients [6].
Types of epidemiological study
- Observational or descriptive studies. These are studies where the frequency of a disorder is described in terms of its association with various background attributes such as age, sex and ethnicity.
- Analytical studies. These set out to test specific hypotheses on the relationship between a potential exposure and disease. These may be cross-sectional (e.g. ‘Is atopic eczema more common in black Caribbean children in London compared with white children?'), case–control (e.g. ‘Is a history of preceding infection more common in people with pityriasis rosea than in controls?') or cohort (e.g. ‘Are people who are exposed to diesel fumes more likely to develop asthma than those who are not?').
- Intervention studies. These are studies in which groups of individuals are allocated to an experimental treatment prospectively. Clinical trials are the commonest examples. Occasionally, such trials are conducted at a community level, for example vaccine trials.
- Screening. This refers to the examination of healthy people who would not otherwise have sought medical help for the presence or absence of disease. Principles for evaluating the usefulness of screening are described elsewhere [7].
Checklist for reading ‘epidemiological studies' in dermatology
This checklist represents a general approach to exploring key elements that should have been included in an epidemiological study. For recommendations on what to include when reporting case–control, cohort and prevalence studies, please refer to the STROBE checklist (STrengthening the Reporting of OBservational studies in Epidemiology) at http://www.strobe-statement.org (last accessed May 2014).
- Is there a clear objective(s)?
- Is the study design appropriate and efficient for the question posed?
- Have cases (numerators) been clearly defined?
- Is there a population denominator?
- Have the main hypotheses and outcome measures been stated a priori?
- Is there a rationale for the study's sample size?
- Have potential confounders been considered and measured?
- Has the study attempted to minimize selection and information biases?
- Have the data been analysed appropriately?
- Are the main results clearly presented with confidence intervals?
- Have subgroups or post hoc findings been treated appropriately?
- Have the authors discussed alternative explanations such as chance, bias and confounding?
- Are the study's conclusions supported by the main results?
- Who sponsored the study? Could sponsorship have affected the choice of data and the way they were presented?
Recommended further reading and useful dermatoepidemiology resources
General epidemiology
Gordis L. Epidemiology, 3rd edn. Philadelphia: Elsevier Saunders, 2004.
Hennekens CH, Buring JE. Epidemiology in Medicine. Boston: Little, Brown, 1987.
Holmes L, Jr. Concise Guide to Epidemiology and Biostatistics for Clinicians. Boston: Jones and Bartlett Publishers, 2011.
Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd edn. London: Lippincott, Williams and Wilkins, 2008.
Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine, 2nd edn. Boston: Little, Brown, 1991.
Schlesselman JJ. Case Control Studies: Design, Conduct, Analysis. Oxford: Oxford University Press, 1982.
Biostatistics
Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall, 1992.
Gilmore SJ. Evaluating statistics in clinical trials: making the unintelligible intelligible. Aust J Dermatol 2008;49:177–84; quiz 185–6.
Gore SM, Altman DG. Statistics in Practice. London: British Medical Association, 1991.
Kahn HA. Statistical Methods in Epidemiology. Oxford: Oxford University Press, 1989.
Kirkwood BR. Essentials of Medical Statistics. Oxford: Blackwell Scientific Publications, 1988.
Systematic reviews
Chalmers I, Altman D. Systematic Reviews. London: British Medical Journal Publishing Group, 1995.
Cochrane Library: www.thecochranelibrary.com (last accessed May 2014).
Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). Cochrane Collaboration, 2011. www.cochrane-handbook.org (last accessed May 2014).
Reddi A, Prescott L, Doney E, et al. The Cochrane Skin Group: a vanguard for developing and promoting evidence-based dermatology. J Evid Based Med 2013;6:236-42.
Dermatoepidemiology reports
Barzilai DA, Mikkilineni R, Davis BR, Stevens SR, Mostow EN. Implementation of dermato-epidemiology curriculum at Case Western Reserve University dermatology program. Dermatol Online J 2004;10:1.
Chuang TY. Dermatoepidemiology. 1: Epidemiologic methods. Int J Dermatol 1993;32:251–6.
Grobb JJ, MacKie RM, Stern R, Weinstock MA. Epidemiology, Causes and Prevention of Skin Diseases. Oxford: Blackwell Science, 1997.
Langan SM, Bouwes Bavinck JN, Coenraads PJ, et al.; European Dermato-Epidemiology Network. Update on the activities of the European Dermato-Epidemiology Network (EDEN). Dermatology 2006;213:1–2.
Marks R. Dermatoepidemiology: wherefore art thou in this perilous time of need? Int J Dermatol 2001;40:167–8.
Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease. J Invest Dermatol 2012;132:994–1002.
VanBeek M, Beach S, Braslow L, et al. Highlights from the report of the working group on ‘Core measures of the burden of skin diseases’. J Invest Dermatol 2007;127:2701–6.
Weinstock MA. Dermatoepidemiology. Dermatol Clin 1995;13:505–716.
Williams H, Svensson A, Diepgen T, et al.; European Dermato-Epidemiology Network (EDEN). Epidemiology of skin diseases in Europe. Eur J Dermatol 2006;16:212–18.
Dermatoepidemiology textbooks
Williams HC. Atopic Dermatitis. Cambridge: Cambridge University Press, 2000. (A textbook dedicated to the epidemiology of atopic eczema.)
Williams HC, Strachan DP. The Challenge of Dermato-epidemiology. Boca Raton, FL: CRC Press, 1997. (This contains a comprehensive ‘toolbox’ section at the start of the book, followed by a summary of the epidemiology of specific skin diseases.)
Epidemiology computer software
Dean AD, Dean JA, Burton JH, Dicker RC. Epi Info, Version 3.5.1: a Word Processing, Database and Statistics Programme for Epidemiology on Microcomputers. Atlanta, GA: Centers for Disease Control, 2008. Available as a free download from http://www.cdc.gov/epiinfo/ (last accessed May 2014).
Gardner MJ, Gardner SB, Winter PD. Confidence Interval Analysis (CIA), Version 1.2. London: British Medical Journal Publishing Group, 1991.
Evidence-based dermatology
Bigby M. Evidence-based medicine in a nutshell: a guide to finding and using the best evidence in caring for patients. Arch Dermatol 1998;134:1609–18.
Bigby M. Snake oil for the 21st century. Arch Dermatol 1998;134:1512–14.
Rees J. Evidence-based medicine: the epistemology that isn't. J Am Acad Dermatol 2000;43:727–9.
Williams H. Dowling Oration 2001. Evidence-based dermatology – a bridge too far? Clin Exp Dermatol 2001;26:714–24.
Williams HC. Why is the center of evidence-based dermatology relevant to Indian dermatology? Indian J Dermatol 2009:54(2):118–23.
Williams HC, Bigby M, Herheimer A, et al. Evidence-Based Dermatology, 3rd edn. London: BMJ Books/Wiley Blackwell, 2014.
Williams HC, Dellavalle RP. The growth of clinical trials and systematic reviews in informing dermatological patient care. J Invest Dermatol 2012;132:1008–17.
Interest groups/societies
American Dermatoepidemiology Network (ADEN): http://adenet.us/.
British Epidermo-Epidemiology Society (BEES). BEES runs an annual meeting, annual 3-day course on understanding epidemiological principles in evidence-based dermatology and a summer school. http://www.bees.org.uk/about/.
European Dermato-Epidemiology Network (EDEN). Website containing details of current epidemiological projects and meetings. http://eden.dermis.net/content/e02eden/e01aims/e01programm/index_ger.html.
International Dermatoepidemiology Association (IDEA). Normally accessed via EDEN or ADEN. (All last accessed May 2014.)
Useful websites and online resources
Centre for Evidence-Based Medicine (CEBM). The Centre is based at Oxford, UK. The website contains a list of useful teaching resources for evidence-based medicine, along with a glossary of terms and Palm resources. http://www.cebm.net/.
Centre of Evidence Based Dermatology. This author's team, which includes the Cochrane Skin Group and the UK Dermatology Clinical Trials Network. The Centre website has a large resource section that includes databases of all randomized controlled trials for eczema, the UK Diagnostic Criteria for Atopic Dermatitis manual, annual evidence updates for common skin diseases and details about the international Harmonising Outcomes for Eczema (HOME) project. http://www.nottingham.ac.uk/dermatology/.
Cochrane Library. Widely acknowledged as the most reliable source of evidence, it contains the largest collection of systematic reviews and clinical trials in the world. It is free to any user in the UK with an IP address, and also in many other countries such as India, Finland, Ireland and Norway. http://www.thecochranelibrary.com.
Guidelines on reporting various study designs such as epidemiological studies, randomized controlled clinical trials, diagnostic test studies, etc. are all to be found at the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) website. http://www.equator-network.org/.
ProQolid. Patient-reported outcome and quality of life instruments database. http://www.qolid.org/.
Trainees' curriculum for dermato-epidemiology. A list of guided reading and questions developed by dermatoepidemiologists from the International Dermato Epidemiology Association (IDEA) in conjunction with the American Academy of Dermatology (AAD). http://www.nottingham.ac.uk/~muzidea/aadcurric.htm.
(All last accessed May 2014.)
References
What is epidemiology and why is it relevant to dermatology?
- Doll R. Foreword. In: Hennekens CH, Buring JE, Mayrent SL, eds. Epidemiology in Medicine. Toronto: Little, Brown, 1987:xi–xii.
- Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology. Toronto: Little, Brown, 1985.
- Chuang T-Y, Reizner GT. Dermatoepidemiology. Part 1: Epidemiologic methods. Int J Dermatol 1993;32:251–6.
- Lind J. A Treatise of the Scurvy in Three Parts, Containing an Inquiry into the Nature, Causes and Cure of That Disease, together with a Critical and Chronological View of What Has Been Published on the Subject. Edinburgh: Sands, Murray and Cochran, 1753.
- Chalmers I. The James Lind Library. Illustrating the development of fair tests of treatments in health care. Royal College of Physicians of Edinburgh. http://www.jameslindlibrary.org/ (last accessed May 2014)
- Goldberger J. The etiology of pellagra. Public Health Rep 1914;29:1683–6.
Thinking in terms of populations rather than individuals
- Barker DJP, Rose G. Epidemiology in Medical Practice, 2nd edn. Edinburgh: Churchill Livingstone, 1979.
- Rose G. Sick individuals and sick populations. In: Buck C, Llopis A, Nájera E, Terris M, eds. The Challenge of Epidemiology. Washington: Pan American Health Organization, 1988:829–37.
- Taplin D, Porcelain SL, Meinking TL, et al. Community control of scabies: a model based on the use of permethrin cream. Lancet 1991;337:1016–18.
- Rose G. Strategy of prevention: lessons from cardiovascular disease. BMJ 1978;40:1069–118.
- Kendell RE. The Role of Diagnosis in Psychiatry. Oxford: Blackwell Scientific Publications, 1975.
- Burton JL. The logic of dermatological diagnosis. Clin Exp Dermatol 1981;6:1–21.
- Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol 1995;32:212–17.
- Williams HC. Dermatology. In: Stevens A, Raftery J, eds. Health Care Needs Assessment, Series II. Oxford: Radcliffe Medical Press, 1997:261–348.
- Mills CM, Srivastava ED, Harvey IM, et al. Smoking habits in psoriasis: a case control study. Br J Dermatol 1992;127:18–21.
- Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchogenic carcinoma: a study of six hundred and eighty-four proved cases. JAMA 1950;143:329–36.
- Doll R, Bradford-Hill A. Mortality in relation to smoking: ten years' observations of British doctors. BMJ 1964;i:1460–7.
- Bradford-Hill A. The environment and disease: association or causation? J R Soc Med 1965;58:295–300.
- Miettinen OS. Commentary on the paper by Zhang et al. Lack of evolution of epidemiologic methods and concepts. In: Morabia A, ed. A History of Epidemiological Methods and Concepts. Basel: Birkhäser Verlag, 2004,365–6.
- Williams HC. Relative and attributable risk and its relevance to the prevention of contact dermatitis. In: Elsner P, Lachapelle JM, Wahlberg J, Maibach HI, eds. Current Problems in Dermatology, Vol. 25. Basel: Karger, 1996:10–17.
- Imayama S, Hashizume T, Miyahara H, et al. Combination of patch test and IgE for dust mite antigens differentiates 130 patients with atopic dermatitis into four groups. J Am Acad Dermatol 1992;27:531–8.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev 2010;10:84–9.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol 2013;133:1506–11.
How much of a public health problem is skin disease?
- Williams HC. The derivation and validation of diagnostic criteria for atopic dermatitis for use in epidemiological studies [Doctoral dissertation]. London: University of London, 1994.
- Williams HC, Burney PGJ, Strachan D, Hay RJ. The UK Working Party's diagnostic criteria for atopic dermatitis. 2: Observer variation of clinical diagnosis and signs of atopic dermatitis. Br J Dermatol 1994;131:397–405.
- Williams HC. Defining cases. In: Williams HC, Strachan DP, eds. The Challenge of Dermato-Epidemiology. Boca Raton, FL: CRC Press, 1997:13–23.
- Rademaker M, Garioch JJ, Simpson NB. Acne in schoolchildren: no longer a concern for dermatologist. BMJ 1989;298:1217–20.
- VanBeek M, Beach S, Braslow L, et al. Highlights from the report of the working group on ‘Core measures of the burden of skin diseases’. J Invest Dermatol 2007;127:2701–6.
- Ryan TJ. Disability in dermatology. Br J Hosp Med 1991;46:33–6.
- Ryan TJ. Healthy skin for all. Int J Dermatol 1994;33:829–35.
- Johnson MLT. Skin Conditions and Related Need for Medical Care Among Persons 1–74 years, United States, 1971–4. Vital and Health Statistics, Series 11, No. 212; Publication No. PHS 79-1660. Washington: US Department of Health, Education and Welfare/National Center for Health Statistics, 1978:1–72.
- Breeze E, Trevor G, Wilmot A. The 1989 General Household Survey. London: HMSO, 1991.
- Martin J, Meltzer H, Elliot D. The Prevalence of Disability Among Adults. London: HMSO, 1988.
- Bibgefors K, Lindberg M, Isacson D. Self-reported dermatological problems and use of prescribed topical drugs correlate with decreased quality of life: an epidemiological survey. Br J Dermatol 2002;147:285–90.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JAA. The cost of atopic eczema. Br J Dermatol 1996;135:20–3.
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol 2008;25:1–6.
- Javitz HS, Ward MM, Farber E, et al. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol 2002;46:850–60.
- Bickers DR, Lim HW, Margolis D, et al; American Academy of Dermatology Association; Society for Investigative Dermatology. The burden of skin diseases: 2004, a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006;55:490–500.
- Hay RJ, Johns NE, Williams HC, et al. The Global Burden of Skin Disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014;134:1527–34
- Murray CJL, Lopez A, eds. Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard University Press, 1996.
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2197–223.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2163–96.
- World Health Organization. Metrics: disability-adjusted life year (DALY). http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ (last accessed May 2014).
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128.
What determines the frequency of skin disease in populations?
- O'Doherty CJ, MacIntyre C. Palmoplantar pustulosis and smoking. BMJ 1985;291:861–4.
- Williams HC. Smoking and psoriasis. BMJ 1994;308:428–9.
- Naldi L, Parazzini F, Peserico A, et al. Family history, smoking habits, alcohol consumption and risk of psoriasis. Br J Dermatol 1992;127:212–17.
- Bradford-Hill A. The environment and disease: association or causation? J R Soc Med 1965;58:295–300.
- Snow J. On the Mode of Communication of Cholera, 2nd edn. London: Churchill Livingstone, 1854.
- Rose G. Sick individuals and sick populations. In: Buck C, Llopis A, Nájera E, Terris M, eds. The Challenge of Epidemiology. Washington: Pan American Health Organization, 1988:829–37.
- Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged? BMJ 1994;308:1132–5.
- McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol 2013;131:280–91.
- Stratigos AJ, Dimisianos G, Nikolaou V, et al. Melanocortin receptor-1 gene polymorphisms and the risk of cutaneous melanoma in a low-risk southern European population. J Invest Dermatol 2006;126:1842–9.
- Milano A, Pendergrass SA, Sargent JL, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLOS ONE 2008;3:e2696.
- Barker DJP. Fetal and Infant Origins of Adult Disease. London: British Medical Journal Publishing Group, 1992.
- Godfrey KM, Barker DJP, Osmond C. Disproportionate fetal growth and raised IgE concentration in adult life. Clin Exp Allergy 1994;24:641–8.
- Tezza G, Mazzei F, Boner A. Epigenetics of allergy. Early Hum Dev 2013;89(Suppl. 1):S20–1.
- Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol 1995;32:212–17.
- Marmot MG, Adelstein AM, Bulusu L. Immigrant Mortality in England and Wales 1978. London: HMSO, 1984.
- Silver SE. Melanocytic nevus density in Asian, Indo-Pakistani, and white children. J Am Acad Dermatol 1992;27:277–88.
- Bhopal R. Needs of black and ethnic minorities. BMJ 1992;305:1156–7.
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol 2002;138:673–4.
- Worth RM. Atopic dermatitis among Chinese infants in Honolulu and San Francisco. Hawaiian Med J 1962;22:31–6.
- Polednak AP. Trends in cancer incidence in Connecticut, 1935–91. Cancer 1994;74:2863–72.
- Williams H, Stewart A, von Mutius E, Cookson B, Anderson HR and the International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study groups. Is eczema really on the increase worldwide? Journal Allergy and Clinical Immunology 2008;121:947–54.
- Gbakima AA, Lebbie AR. The head louse in Sierra Leone: an epidemiological study among school children, in the Njala area. West Afr J Med 1992;11:165–71.
- Harris MD, Nako T, Hopkins DM, et al. Skin infections in Tanna, Vanuatu in 1989. Papua New Guinea Med J 1992;35:906–7.
- Weinstock MA. Melanoma and nevi. In: Williams HC, Strachan DP, eds. The Challenge of Dermato-Epidemiology. Boca Raton, FL: CRC Press, 1997:191–207.
- Paul JR. Clinical Epidemiology. Chicago: University of Chicago Press, 1958.
- Marshall J. Epidemiology of skin diseases. In: Simons RDG, Marshall J, eds. Essays on Tropical Dermatology. Amsterdam: Excerpta Medica Foundation, 1969:17–23.
- Canizares O. Epidemiology and ecology of skin diseases in the tropics and subtropics. In: Canizares O, ed. A Manual of Dermatology for Developing Countries, 2nd edn. Oxford: Oxford University Press, 1993:22–35.
- Kristensen JK. Scabies and pyoderma in Lilongwe, Malawi: prevalence and seasonal fluctuation. Int J Dermatol 1991;30:699–702.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol 2013;133:1752–9.
- Adams RM. Occupational Skin Disease, 2nd edn. London: Saunders, 1990.
- Rycroft RJG, Menné T, Frosch PJ. Textbook of Contact Dermatitis, 2nd edn. Berlin: Springer, 1995.
- Anderson LJ. Human parvovirus B19. Pediatr Ann 1990;19:509–16.
- Chuang T-Y, Perry HO, Ilstrup DM, Kurkland LT. Recent upper respiratory tract infection and pityriasis rosea: a case–control study of 249 matched pairs. Br J Dermatol 1983;108:587–91.
- Rebora A, Drago F, Broccolo F. Pityriasis rosea and herpesviruses: facts and controversies. Clin Dermatol 2010;28:497–501.
Describing the natural history and associations of specific skin diseases
- Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology. Toronto: Little, Brown, 1985:173–85.
- Burr ML, Dunstan FD, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol 2013;168:1339–42.
- Rystedt I. Long term follow-up in atopic dermatitis. Acta Derm Venereol (Stockh) 1985;114(Suppl.):117–21.
- Williams HC, Strachan DP, Hay RJ. The natural history of childhood eczema. Br J Dermatol 1993;129(Suppl. 42):26.
- Williams HC, Wuthrich B. The natural history of atopic dermatitis. In: Williams HC, ed. Atopic Dermatitis: the Epidemiology, Causes and Prevention of Eczema. Cambridge: Cambridge University Press, 2000:41–59.
- Jorgen HO, Moller H, Frentz G. Malignant tumors in patients with psoriasis. J Am Acad Dermatol 1992;27:716–22.
- Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol 2008;20:416–22.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol 2013;68:654–62.
- Henseler T, Ye B, Christophers E. Coincidence of psoriasis and other diseases. Arch Dermatol 1992;284:19.
- Beer WE, Smith AE, Kassab JY, et al. Concomitance of psoriasis and atopic dermatitis. Dermatology 1992;184:265–70.
- Williams HC, Strachan DP. Psoriasis and eczema are not mutually exclusive diseases. Dermatology 1994;189:238–40.
- Gerber LM, Wolf AM, Braham RL, Alderman MH. Effects of sample selection on the coincidence of hypertension and diabetes. JAMA 1982;247:43–6.
- Thomas SL, Minassian C, Ganesan V, Langan SM, Smeeth L. Chickenpox and risk of stroke: a self-controlled case series analysis. Clin Infect Dis 2014;58:61–8.
- Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol 2012;132:2166–70.
- Deckert S, Kopkow C, Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy 2014;69:37–45.
Health services research in dermatology
- Smith R. Audit and research. BMJ 1992;305:905–9.
- Chren MM. Dermatologic health services research. Dermatol Clin 1995;13:689–95.
- Stevens A, Raftery J. Introduction. In: Stevens A, Raftery J, eds. Health Care Needs Assessment, Series I. Oxford: Radcliffe Medical Press, 1994:11–30.
- Williams HC. Dermatology. In: Stevens A, Raftery J, eds. Health Care Needs Assessment, Series II. Oxford: Radcliffe Medical Press, 1997:261–348.
- Schofield J, Grindlay D, Williams H. Skin Conditions in the UK: a Health Care Needs Assessment. Nottingham: Centre of Evidence-Based Dermatology, University of Nottingham, 2009.
- British Market Research Bureau. Everyday Health Care. A Consumer Study of Self-Medication in Great Britain. London: Proprietary Association of Great Britain, 1987.
- Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth: a community study of prevalence and use of medical care. Br J Prev Soc Med 1976;30:107–14.
- Royal College of General Practitioners. Morbidity Statistics from General Practice: Fourth National Study, 1991–2. London: HMSO, 1995.
- Carmichael AJ. Achieving an accessible dermatology service. Dermatol Pract 1995;3:13–16.
- Ferguson JA, Goldacre MJ, Newton JN, Dawber RPR. An epidemiological profile of inpatient workload in dermatology. Clin Exp Dermatol 1992;17:407–12.
- Office of Population Censuses and Surveys. 1992 Mortality Statistics. London: HMSO, 1994.
- Williams HC. Extended role of pharmacists in dermatology. J Clin Pharm Ther 1996;20:310–12.
- Weber MB, Fontes Neto, Pde T, Prati C, et al. Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. J Eur Acad Dermatol Venereol 2008;22:992–7.
- Lowell BA, Froelich CW, Federman DG, Kirsner RS. Dermatology in primary care: prevalence and patient disposition. J Am Acad Dermatol 2001;45:250–5.
- Roland M, Morris R. Are referrals by general practitioners influenced by the availability of consultants? BMJ 1988;297:599–600.
- Johnson MLT. Skin Conditions and Related Need for Medical Care Among Persons 1–74 years, United States, 1971–4. Vital and Health Statistics, Series 11, No. 212; Publication No. (PHS) 79–1660. Washington: US Department of Health, Education and Welfare/National Center for Health Statistics, 1978:1–72.
- Ryan TJ. Healthy skin for all. Int J Dermatol 1994;33:829–35.
- Hay RJ, Hernandez HA, Lopez GC, et al. Wastage of family income on skin disease in Mexico. BMJ 1994;309:848.
Conclusions
- Strachan DP. Hay fever, hygiene, and household size. BMJ 1989;299:1259–60.
- Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged? BMJ 1994;308:1132–5.
- Sherriff A, Golding J. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool infants. Arch Dis Child 2002;87:26–9.
- Kalliomaki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076–9.
- Foisy M, Boyle, RJ, Chalmers JR, Simpson EL, Williams HC. The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid Based Child Health 2011;6:1322–39.
- Williams HC. Beyond the year 2000: how may epidemiology influence future clinical practice in dermatology? Clin Dermatol 2001;19:55–8.
- Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease. J Invest Dermatol 2012;132:994–1002.
- Langan S, Schmitt J, Coenraads PJ, Svensson A, von Elm E, Williams H; European Dermato-Epidemiology Network (EDEN). The reporting of observational research studies in dermatology journals: a literature-based study. Arch Dermatol 2010;146:534–41.
- Bastuji-Garin S, Sbidian E, Gaudy-Marqueste C, et al.; European Dermatology Network (EDEN). Impact of STROBE statement publication on quality of observational study reporting: interrupted time series versus before-after analysis. PLOS ONE 2013;8(8):e64733.
Glossary of epidemiological terms
- Parkin DM, Muir CS, Whelan SL, et al., eds. Cancer Incidence in Five Continents, Vol. 6. Lyon: International Agency for Research on Cancer, 1992.
- Sackett DL. Bias in analytical research. J Chron Dis 1979;32:51–63.
- Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health 2004;58:635–41.
- Armitage P, Berry G. Statistical Methods in Medical Research, 2nd edn. Oxford: Blackwell Scientific Publications, 1991.
- Williams HC, Burney PGJ, Strachan D, Hay RJ. The UK Working Party's diagnostic criteria for atopic dermatitis. 2: Observer variation of clinical diagnosis and signs of atopic dermatitis. Br J Dermatol 1994;131:397–405.
- Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ 1992;304:1491–4.
- Barker DJP, Rose G. Epidemiology in Medical Practice, 2nd edn. Edinburgh: Churchill Livingstone, 1979:116–24.