CHAPTER 21
Principles of Phototherapy
Kevin McKenna1 and Sally Ibbotson2
1Dermatology Department, Belfast Trust, Belfast, UK
2Photobiology Unit, University of Dundee, Ninewells Hospital and Medical School, Dundee, UK
Introduction
Phototherapy (or ‘light therapy’) is a form of treatment for skin conditions involving the administration of non-ionizing radiation (most commonly within the ultraviolet part of the electromagnetic spectrum) in a controlled manner to the skin. Photodynamic therapy and laser treatment, which may be regarded as specialized forms of phototherapy, are discussed elsewhere (see Chapters 22 and 23) but a discussion of extracorporeal photochemotherapy (ECP), in which the target is white blood cells rather than skin, is included here. A wide variety of dermatoses are responsive to phototherapy, which is used most commonly for psoriasis and eczema.
The UVB part of the spectrum is usually defined as that between 280 and 320 nm (though the International Commission on Illumination (CIE) has set the upper limit of the range at 315 nm). It can be delivered with the ‘full spectrum’ (broad-band UVB lamps 270–350 nm, which include shorter wavelengths from the UVA spectrum) or with just a small part (narrow-band UVB [TL-01R] lamps 311–313 nm) (Figure 21.1). In recent years narrow-band UVB has largely replaced the use of broad-band UVB [1, 2].
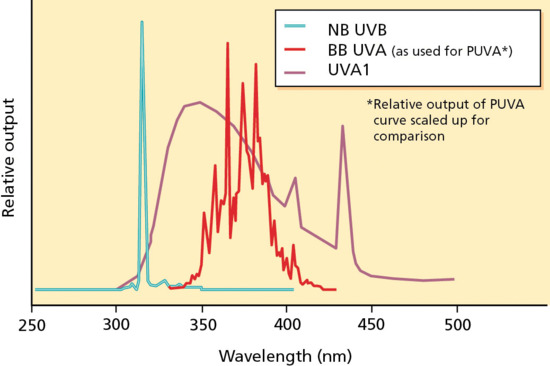
Figure 21.1 Spectra of narrow-band UVB (NB-UVB), broad-band UVA (BB-UVA) and UVA-1. PUVA, psoralen and UVA.
The UVA spectrum is commonly defined as that between 320 and 400 nm and is usually used in combination with a psoralen photosensitizer, either orally or topically, when it is known as photochemotherapy (PUVA) [3, 4]. Skin diseases responsive to PUVA are similar to those responsive to UVB phototherapy, particularly psoriasis and eczema. ECP (photopheresis) involves the addition of psoralen to the patient's white blood cells after they have been separated from whole blood ex vivo.
The photoactivated white blood cells are then irradiated with UVA and reinfused back into the patient. Photopheresis is used to treat the erythrodermic stage of cutaneous T-cell lymphoma, graft-versus-host-disease and other conditions [5, 6]. The longer wavelengths of UVA are known as UVA-1 (340–400 nm) and have been shown to be beneficial in a number of chronic dermatoses including atopic eczema and sclerosing skin disorders [7, 8].
UVB phototherapy has anti-inflammatory, immunosuppressive and cytotoxic properties. The mechanisms of its action are unclear, but include the induction of cis-urocanic acid, Langerhans cell depletion, altered antigen presentation, decreased activity of natural killer (NK) cells and apoptosis of T lymphocytes and keratinocytes [9, 10, 11].
The mechanisms of action of PUVA include the cross-linking of DNA by psoralen photoadducts, the inhibition of DNA replication, Langerhans cell depletion, immunosuppressive effects on T-lymphocyte function and migration and the restoration of Th17/regulatory T-cell imbalance in psoriasis [12, 13, 14]. Photopheresis results in dendritic cells acquiring antigen from apoptotic lymphocytes, which elicit a specific immune response without causing systemic immunosuppression [15, 16, 17]. UVA-1 phototherapy penetrates deeper into the dermis and induces interstitial collagenase and several cytokines, resulting in a softening of sclerotic skin [18, 19]. In addition, UVA-1 phototherapy causes a reduction in tumour necrosis factor α (TNF-α) in the skin, amongst other mediator effects; it is also cytotoxic with T-cell apoptosis being a prominent feature [20].
History and background
The beneficial effects of sunlight or heliotherapy on the skin have been known since antiquity. The treatment of vitiligo with psoralen extract from seeds in combination with sunlight is recorded in ancient Egyptian, Indian and Chinese manuscripts [3, 21, 22]. The primary therapeutic component of sunlight, ultraviolet radiation, would not be discovered until 1801 by Johann Ritter [23]. The lethality of UV radiation to bacteria was demonstrated by Downes and Blunt in 1877. The father of modern phototherapy, Niels Finsen, demonstrated the effectiveness of UV therapy for lupus vulgaris at the end of the 19th century [24, 25]. Finsen went on to be awarded the Nobel Prize for his work in 1903.
It was suggested by Alderson in 1923 that a mercury quartz lamp be used to treat psoriasis [26]. The combination of tar and UVB therapy for psoriasis was promoted by Goeckerman in the USA in 1925, and the combination of UVB and dithranol (anthralin) for the same by Ingram in England in 1953 [27, 28]. A broad-band fluorescent UVB system for the treatment of psoriasis was developed by Wiskemann in the 1960s. These lamps had a much higher output than the mercury quartz lamp. The action spectrum for the clearance of psoriasis with a peak at 313 nm was defined in 1976 by Parrish and Jaenicke in the USA [29]. This work paved the way for the development of the more specific and efficient narrow-band UVB phototherapy for psoriasis in the 1980s [30, 31].
Photochemotherapy was revived in 1947 with the isolation of 8-methoxypsoralen and 5-methoxypsoralen from plant extracts [32, 33]. El Mofty et al. demonstrated the clinical benefit of these psoralens with natural sunlight or with UV lamps for the treatment of vitiligo [34]. Photochemotherapy or PUVA was revolutionized by Parrish et al. in 1974 with the introduction of a high-intensity fluorescent UVA tube for the treatment of psoriasis [35]. A decade later, extracorporeal photochemotherapy or photopheresis was introduced by Edelson for the treatment of cutaneous T-cell lymphoma (CTCL) [5]. ECP is recommended as first line treatment for erythrodermic CTCL. In 1981, Mutzhas and colleagues first reported the development of an irradiation device that emitted long-wave UV radiation (UVA-1; 340–400 nm) [36]. It was noted that it readily provoked pigmentation and polymorphic light eruption (PLE). It was thus initially used for PLE provocation testing. The therapeutic potential of UVA-1 was not to be recognized until the early 1990s when it was reported to be beneficial for atopic eczema [7].
Ultraviolet radiation
What is ultraviolet radiation?
Ultraviolet radiation (UVR) is that part of the solar electromagnetic spectrum that lies between X-rays and visible light. UVR is normally defined as UVA (320–400 nm), UVB (280–320 nm) and UVC (<280 nm), although the International Commission on Illumination uses 315 nm rather than 320 nm as the boundary between UVA and UVB [37].
UVC radiation is absorbed by the earth's atmosphere before it reaches the surface of the earth. UVB radiation is significantly more effective (100–1000 times more potent) than UVA at producing erythema, cellular effects and DNA damage. UVA radiation is less energetic but penetrates more deeply into the skin, particularly the longer UVA-1 wavelengths, and as such has been implicated in chronic skin photoageing [38].
Artificial sources
The most common means of producing UVR artificially is by the passage of an electric current through mercury vapour enclosed in a fluorescent tube. The excited electrons of the mercury are absorbed by a phosphor coating on the inside of the tube, which results in re-emission of radiation of longer wavelengths by the process of fluorescence (Figure 21.2). By changing the phosphors used, a variety of different spectra in the UVA or UVB regions can be produced [39]. Tubes commonly used include narrow-band UVB: TL-01 (Waldmann/Cosmedico/Hybec/Lumenis); broad-band UVB: TL-12 (Philips) or the more selective UV-6 (Sylvania); and UVA: TL-09 (Philips) lamps for PUVA. UVA-1 can be produced either by low output fluorescent tubes, such as the TL-10 (Philips) or by the higher output metal halide UVA-1 sources (Sellamed, Dr Hönle, Dermalight), which require significant cooling [40].

Figure 21.2 Whole body narrow-band UVB cabinet.
Equipment for the delivery of phototherapy
A wide variety of narrow-band (TL-01) UVB units are available, including whole body cabins, whole body panels, small panel irradiators and point sources (Figure 21.3) [1]. Whole body cabins contain 1800 mm long fluorescent tubes that line the walls in front of reflective metal surfaces which enable greater dose uniformity and greater treatment efficiency to be achieved. Whole body panels necessitate rotation of the patient to provide uniform irradiation: careful attention to position and posture is required to avoid under- or overdosing. Furthermore, these units are a significant UV hazard to others, who must avoid passing in front of the panel. They may, however, be useful for home phototherapy. Small panel irradiators are used for the treatment of palmar and plantar skin. Point source devices avoid unnecessary irradiation of unaffected skin but care is needed to avoid under- or overdosing at overlap areas.

Figure 21.3 Narrow-band UVB unit for the treatment of hands and feet.
PUVA units are either whole body cabins or small panel irradiators for the treatment of the hands and feet or other localized areas of the body, such as the lower legs or scalp [37].
Space and expense may be saved by using a cabin with a combination of UVB and UVA tubes. However, as only half the number of tubes of each type can be accommodated, longer treatment times are required. They also pose a significant hazard if the wrong tubes are selected and so in general are not recommended.
Ultraviolet calibration and dosimetry
To provide consistency and repeatability of treatment doses it is essential that the irradiance of the UV unit being used is determined at regular intervals. The dose is calculated from the formula below:
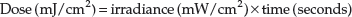
It is important that this is performed using calibrated radiometers that are traceable back to a national reference. This ensures that treatment delivered at one phototherapy centre is the same as that given at any other phototherapy centre. Some whole body cabins have built-in radiometers, but these are unreliable. They may give inaccurate readings when tubes are changed, if the radiometer is not cleaned or if shielding occurs with very obese patients.
To determine the mean UV irradiance to which a patient will be exposed in a whole body cabin, a designated patient irradiance (DPI) is determined [37]. This is for a subject of average height and build at chest, waist and knee level. The irradiance should be measured at the anterior, posterior and both lateral positions, that is, at 12 body sites. This can be determined in one of two ways, the direct or the indirect methods. In the direct method the irradiance at the specified sites must be measured while the investigator (usually from the nearest medical physics department) is standing in the unit. The skin and eyes must be protected with suitable protective clothing and goggles. In the indirect method the irradiance is measured whilst the unit is unoccupied. This can be done with the radiometer clamped to a stand whose position can be adjusted up or down (Figure 21.4). The mean value of the DPI is multiplied by a correction factor (typically 0.8–0.9) to take into account the occupancy effect of the patient in the cabin.

Figure 21.4 Irradiance of UV tubes being measured by a radiometer clamped in position at a fixed distance.
Indications for phototherapy
UVB phototherapy and PUVA
Psoriasis
Psoriasis is the most frequent indication for both UVB and PUVA [2, 41, 42, 43]. Narrow-band (TL-01) UVB (NB-UVB) can be used to treat all variants except generalized pustular and erythrodermic psoriasis, for which PUVA can, however, be considered [44]. NB-UVB is more effective than broad-band UVB (BB-UVB) and is on average of similar efficacy to PUVA for the treatment of psoriasis (Figure 21.5). Thin plaques as seen in guttate and seborrhoeic psoriasis respond readily to UVB therapy. UVB is generally used in preference to PUVA: it has a lesser skin cancer risk than PUVA, is easier to administer and, with its shorter exposure times, facilitates a greater throughput of patients. PUVA should be considered in those patients who have failed to respond to UVB or whose duration of remission following UVB is consistently of short duration.
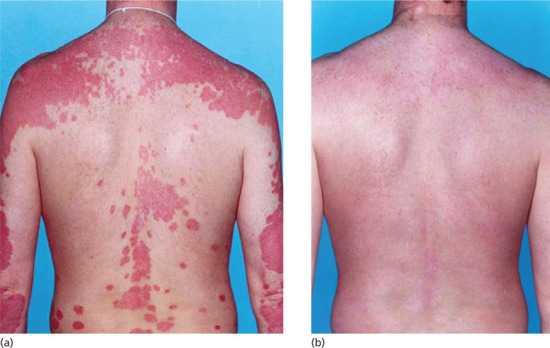
Figure 21.5 Psoriasis affecting a patient's back (a) before and (b) after narrow-band (TL-01) UVB therapy showing the effectiveness of the treatment.
(Reproduced with permission of British Dermatological Nursing Group.)
Atopic eczema
Atopic eczema has been treated effectively with BB-UVB [45], combined UVB and UVA [46], UVA-1 [7], NB-UVB [47] and PUVA (Figure 21.6) [48]. It is commonly stated that it is preferable that UVA-1, if available, should be used for acute flares and NB-UVB should be used for chronic disease, although there is no evidence to support this. A flare of disease is often seen in the early stages of treatment, usually due to the heat generated in the cabinets. Lower dose increments and more prolonged treatment courses than used for psoriasis are often required [49]. Clinical impression is that relapse also tends to be more frequent than in psoriasis, although there is no firm objective evidence for this. PUVA can be considered if UVB has failed or in severe atopic eczema in children associated with growth retardation.
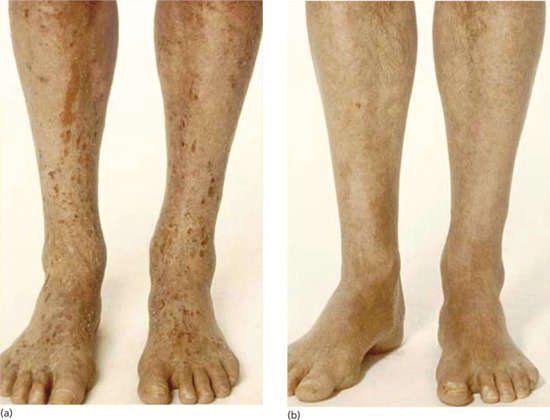
Figure 21.6 Atopic eczema affecting a patient's legs (a) before and (b) after narrow-band UVB therapy.
Cutaneous T-cell lymphoma
Narrow-band UVB and PUVA have been used effectively for the treatment of CTCL (mycosis fungoides) [50, 51, 52]. UVB is particularly useful for patch stage CTCL, but PUVA is preferable for the plaque stage. Increased inflammation may be seen in the early stages of treatment but usually settles without stopping therapy. ‘Sanctuary sites’ such as the flexures, which are less accessible to phototherapy, may be areas where disease persists.
Vitiligo
Vitiligo may respond both to NB-UVB and PUVA [53, 54, 55]. NB-UVB is more effective than PUVA but response is variable and treatment usually has to be prolonged, often over many months to a year or more. Affected areas that tend to be more photoresponsive include those on the face and those of recent onset, of limited extent, containing pigmented hairs or involving non-acral sites. Acral areas typically respond less well but are often the sites that bother the patient most. Tanning of normal skin, which exaggerates the contrast with vitiliginous skin, is more obvious in patients with darker skin types. Patients of skin type I and II are more at risk of burning. Patients should be carefully selected and the risks and benefits clearly discussed.
Polymorphic light eruption
Narrow-band UVB and PUVA are equally effective for desensitization of PLE but the former is used more frequently nowadays as it has a better side effect profile [56, 57]. It is usually administered in spring time or before travel to sunny locations. Provocation of the rash, which occurs in approximately 50% of patients, can be managed with topical corticosteroid creams and reduction of dose. Patients are typically given 15 treatments (three times per week for 5 weeks) for three consecutive years prior to a year without treatment to assess whether there is any change in disease expression. Evidence as to the optimal regimen to use is lacking.
Other conditions
Many other skin conditions have been treated with UVB and PUVA therapy with variable efficacy. Representative examples of such conditions are listed in Table 21.1.
Table 21.1 Other conditions that may respond to UVB phototherapy or PUVA.
| Condition | Comment |
| Alopecia areata [58, 59] | Efficacy of PUVA is not clearly established; relapse is common |
| Graft-versus-host disease [60, 61] | PUVA is helpful in extensive lichenoid disease |
| Granuloma annulare [62] | Can be beneficial |
| Lichen planus [63, 64] | Response is variable; PUVA is useful for palmar disease |
| Necrobiosis lipoidica [65] | PUVA can be beneficial – in ulcer healing and camouflage effect |
| Nodular prurigo [66, 67] | Can be beneficial; relapse is common |
| Other photodermatoses (actinic prurigo, chronic actinic dermatitis, erythropoietic protoporphyria, solar urticaria) [68, 69, 70] | Can be effective but close supervision is required; consider referral to a centre with special interest |
| Palmo-plantar pustulosis [71] | PUVA is more effective |
| Pityriasis lichenoides chronica [72] | Can be beneficial |
| Pityriasis rosea [73] | Rarely needed; used in severe symptomatic disease |
| Pityriasis rubra pilaris [74, 75] | PUVA only |
| Pompholyx eczema [76, 77] | PUVA is more effective |
| Pruritus [78, 79] | Can be beneficial; UVB is effective for itch of renal failure or liver disease |
| Seborrhoeic dermatitis [80] | Responsive to UVB but rapid relapse is common |
| Subcorneal pustular dermatosis [81, 82] | Can be beneficial |
| Systemic sclerosis/morphoea [83] | PUVA is beneficial |
| Urticaria [84] | Good evidence to support a role for UVB |
| Urticaria pigmentosa [85] | Can be beneficial; relapse is common |
Choice of phototherapy modality: UVB versus PUVA
Narrow-band UVB therapy is now much more commonly used than PUVA. This is primarily due to the well-documented cumulative risk of skin cancer associated with PUVA [86, 87]. In addition, many studies have shown the efficacy of NB-UVB for psoriasis and other dermatoses to be comparable with that of PUVA [1]. It should not be forgotten that PUVA penetrates more deeply into the skin than UVB radiation and is thus better suited for thick plaque disease and for treating the palms and soles (Box 21.1). The relapse rate of psoriasis following PUVA therapy is less than that of NB-UVB therapy. PUVA can also be highly effective for generalized pustular or erythrodermic psoriasis.
Contraindications to UVB and PUVA
Patients should be assessed for their suitability for either UVB or PUVA therapy prior to starting therapy (Box 21.2). Individual risk and benefit must always be assessed.
UVA-1 phototherapy
The evidence base for using UVA-1 relates mainly to its use in atopic eczema, the sclerosing skin conditions, in particular morphoea and scleroderma, and various subtypes of lupus erythematosus. The application of UVA-1 has also been investigated for several other skin diseases (e.g. urticaria pigmentosa, disseminated granuloma annulare, CTCL and psoriasis in HIV-infected patients), although the evidence base is largely restricted to case series and case reports [40, 88]. In general, UVA-1 is probably most useful as an option to be considered for some of the rarer skin diseases where other treatment options are limited. It is not usually first line phototherapy and its availability at present is limited to specialist centres.
Atopic eczema
UVA-1 phototherapy can be of benefit, particularly for atopic prurigo, and, if it is available, should be considered for the patient who has failed to respond to NB-UVB or PUVA [7, 89, 90] Studies have shown that high- and medium-dose UVA-1 are of equivalent efficacy although medium-dose treatment is more effective than low-dose. UVA-1 has been shown to be superior to potent topical corticosteroid and broadband UVA/B. UVA-1 is of equivalent efficacy or possibly slightly less effective than NB-UVB and is less effective than PUVA.
Sclerosing skin conditions
There is evidence of utility of UVA-1 for the treatment of cutaneous sclerosis in morphoea and systemic sclerosis, particularly if the disease is progressive, symptomatic and/or restricting movement [91]. There is also limited evidence to support its use in the treatment of cutaneous graft-versus-host disease [92], nephrogenic fibrosing dermopathy [93] and both extragenital and genital lichen sclerosus [94, 95]. UVA-1 at medium dose has been shown to be superior to NB-UVB and even low-dose treatment can be effective, although prolonged treatment courses may be needed.
Lupus erythematosus
Paradoxically there is good evidence for the use of very low-dose (about 6 J/cm2 per individual dose) UVA-1 for systemic lupus erythematosus (LE) with demonstrable reduction in systemic disease activity [96, 97]. UVA-1 has also been used successfully for subacute cutaneous LE, tumid LE and, although generally less responsive, for chronic cutaneous (discoid) LE.
Extracorporeal photochemotherapy
Extracorporeal photochemotherapy, or photopheresis, has evidence of benefit for the treatment of erythrodermic cutaneous T-cell lymphoma [5, 98, 99, 100] and for graft-versus-host disease [98, 101, 102, 103]. It has also been used for a wide range of skin diseases such as systemic sclerosis [104], some immunobullous disorders [105], psoriasis [106], atopic eczema [107], lichen planus [108], LE [109], scleredema [110] and dermatomyositis [111]. Evidence of benefit for any of these conditions is generally weak. It is of interest that the best evidence for the efficacy of ECP is in the prophylaxis of cardiac transplant rejection [112].
Cutaneous T-cell lymphoma
Extracorporeal photochemotherapy is licensed for the treatment of CTCL, particularly in patients with erythrodermic disease, including those with Sézary syndrome. Patients who respond best have a disease of shorter duration, have near normal CD8+ cell counts [113], are immunocompetent [114] and have circulating Sézary cells [115, 116]. Evidence for ECP for the treatment of non-erythrodermic mycosis fungoides is poor [117–119]. Added benefit has been claimed when ECP is combined with interferon [115, 120], bexarotene [121] or electron beam therapy [122].
Graft-versus-host disease
Graft-versus-host disease complicating allogeneic bone marrow transplantation can be subdivided into acute and chronic phases. There is evidence that ECP is of benefit for the chronic phase, particularly for cutaneous or mucosal involvement. There is little evidence of benefit for associated hepatic disease [6, 98, 101, 102, 103].
How different therapies are administered
UVB phototherapy
Phototherapy is most frequently referred to as UVB therapy and can be delivered using either of two different sources: broad-band or narrow-band. It has been shown that NB-UVB is more effective than BB-UVB, and thus BB-UVB is no longer commonly used. It is recommended that the starting dose of UVB is based on the minimal erythema dose in order to reduce the risks of burning on the one hand or under-treatment on the other, and to detect unsuspected photosensitivity [1].
Minimal erythema dose
The minimal erythema dose (MED) is defined in the UK as the dose of radiation that produces minimal, just perceptible, erythema at 24 h post irradiation (in other countries it is defined as the dose that produces well-defined erythema) [123]. There is a poor correlation between MED and skin type [124]. The UVB MED gives an objective measure of a patient's cutaneous reactivity to UVB.
The MED can be determined by the use of a homemade template or by using automatic or semiautomatic UV exposure devices. The homemade template can be made of any UV-opaque material in which there are a number of apertures (typically eight) through which different doses of UVB can be delivered to the skin. The range of doses used are selected based on skin phototype and are a geometric series [125] such as: 25, 50, 70, 100, 140, 200, 280, 390, 550, 650 and 770 mJ/cm2 for NB-UVB (Figure 21.7). The usual site for testing with the template is on the mid-back, avoiding the midline. The UVB testing source uses a bank of UVB tubes identical to those used in the irradiation cabinet and whose irradiance is regularly monitored. In some units the actual irradiation cabinet is used with the patient standing or sitting within it, but this requires careful covering of the patient to avoid unnecessary erythema and to some patients this can be very claustrophobic. In addition to being time consuming it also means that this cabinet is not available for treatment whilst the procedure is undertaken. Alternatively a hand-held semiautomated UV device can be used which is placed directly on the skin being tested. The plate in contact with the skin is perforated with a number of apertures fitted with UV-attenuating mesh foil. Essentially, the finer the mesh, the more UV it blocks. One such device delivers 10 doses with an attenuation factor of 1.26 between apertures allowing a sequence of doses to be delivered (Figure 21.8) [126].
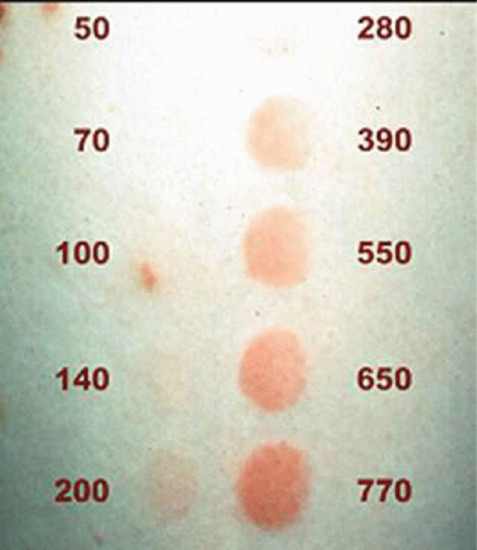
Figure 21.7 Narrow-band UVB minimal erythema dose (MED) reading 24 h after the irradiation of phototest sites. Doses are in mJ/cm2.

Figure 21.8 Hand-held Hybec ‘Durham’ phototesting unit.
Regimen variables
Starting dose.
The evidence for optimal dose regimens mainly relates to psoriasis. The action spectrum for the clearance of psoriasis peaks in the UVB region around 311–313 nm and it was with this in mind that NB-UVB lamps were designed by Philips. To achieve a clearance of psoriasis it is not necessary to use erythemogenic dose regimens. The starting dose is commonly initiated at a percentage of the MED (usually 70% or 50%) to minimize the risk of developing significant erythema. Interestingly, one study showed increased erythemal episodes with a 50% MED starting dose regimen when compared with 70% MED or arbitrary skin phototype-based starting dose regimens, and showed no difference in efficacy between any of the three regimens [127]. However, undertaking a MED or a small area test dose is important to detect unsuspected abnormal photosensitivity.
Increments.
As the skin acclimatizes to UVB therapy by epidermal thickening and pigmentation, it is necessary to increase the dose incrementally. Comparison of a low increment regimen (20%) with a high increment regimen (40%) showed the former to result in 50% fewer episodes of erythema requiring postponement of treatment [128]. A low-dose regimen is advocated with a reduction to 10% increments if significant erythema develops.
Frequency.
Treatment three times a week is preferred to a five times per week schedule for patients of skin phototypes I–III. This is because the advantage of a marginally more rapid clearance of psoriasis with the latter regimen is outweighed by the inconvenience to the patient of a higher frequency of treatment, and the higher cumulative UVB dose received [129]. Both twice and three times weekly regimens are effective for psoriasis, although three times a week treatment results in faster clearance.
Number of exposures.
Clearance of psoriasis can usually be achieved with 20–25 treatments, although more prolonged courses may be needed, particularly with stubborn disease. Atopic eczema also usually requires more prolonged treatment courses than those used for achieving clearance in psoriasis. A course of 15 treatments is usually used for PLE desensitization, although optimal treatment parameters have not been established.
Ways to deliver phototherapy
UVB phototherapy is most commonly delivered in a cabinet in which the patient is surrounded by TL-01 fluorescent tubes (Figure 21.9). Hand and foot units can also be used for localized hand and foot disease.

Figure 21.9 Patient with psoriasis receiving narrow-band UVB in a whole body cabinet.
Involved psoriatic skin has a much higher tolerance of UVB than the surrounding uninvolved skin. With UVB devices that can be targeted directly at individual psoriatic plaques, higher doses of UVB can therefore be used [130, 131]. Treatment with 2–6 multiples of MED can clear localized plaques of psoriasis after five or six treatments. Coherent UVB in the form of the 308 nm excimer laser can also be used for stubborn localized psoriatic plaques, although availability is limited [132].
Home phototherapy has been particularly advocated for patients who cannot attend hospital because of geographical, work, economic or other reasons [133, 134]. Such treatment has raised concerns of suboptimal therapy, greater risks and medicolegal implications, which have not been confirmed [135]. However, a recent study from the Netherlands, in which home phototherapy was compared with out-patient UVB, found that the treatments had similar efficacy and that the cumulative doses and rates of short-term side effects were comparable [136]. Cost analysis of home phototherapy versus hospital UVB therapy in Scotland concluded that home phototherapy was cost effective and that the development of this means of treatment delivery should be encouraged [134, 137].
PUVA
Psoralens
The most frequently used psoralens for oral use are 8-methoxypsoralen (8-MOP) and 5-methoxypsoralen (5-MOP) [3, 49, 138]. The former is used preferentially as it is considered to be more effective and is less expensive. Nausea is a common side effect of 8-MOP and, if troublesome, switching to 5-MOP is helpful. The dose of psoralen used is most commonly determined on the basis of body weight: 0.6 mg/kg for 8-MOP and 1.2 mg/kg for 5-MOP. Some authorities advocate calculating the dose according to body surface area: 25 mg/m2 for 8-MOP and 50 mg/m2 for 5-MOP [139]. The latter method of dosing has been shown to improve the therapeutic effect of PUVA in psoriasis.
Oral 8-MOP is taken 2 h before treatment with a light meal and 5-MOP should be taken at 2–2.5 h before treatment.
Topical psoralens have been used for centuries for the treatment of vitiligo. Psoralens currently used for topical therapy include 8-MOP and trimethylpsoralen (TMP) [140]. In equivalent concentrations TMP is up to 30 times more phototoxic, although it is now rarely used [141]. Psoralen can be applied topically in a variety of ways: a bath solution for whole body treatment and soak, paint, cream or gel for hands and feet, scalp and other localized areas.
Topical therapy is preferable to oral therapy in patients with hepatic dysfunction, gastrointestinal disease, cataracts, poor compliance with eye protection and risk of drug interactions (e.g. warfarin), and to allow shorter irradiation times (e.g. for children, the elderly or those with claustrophobia).
A frequently used bath PUVA regimen in the UK involves dissolving 30 mL of a 1.2% 8-MOP lotion in 140 litres of water (final concentration 2.6 mg/L) [140]. The patient bathes in this for 15 min, followed by immediate exposure to UVA. When treating hands and feet with topical PUVA there should be a 30 min delay prior to irradiation to allow psoralen absorption into palmar and plantar skin [142].
Minimal phototoxic dose
The minimal photoxic dose (MPD) is determined in a similar way to MED except that it is measured 2 h (or 2.5 h for 5-MOP) after the patient has ingested a standard dose of psoralen (8-MOP or 5-MOP). Typical UVA test doses used include 0.5, 0.7, 1.0, 1.4, 2.0, 2.8, 3.7, 5.5, 7.7 and 10.8 J/cm2 but may vary between centres. The test sites are read after 72–96 h [143].
MPD testing allows the determination of the optimal starting dose, allows identification of under-dosage or poor absorption and may identify patients with abnormal photosensitivity.
Regimen variables
Starting dose.
The starting dose should ideally be based on the patient's MPD, and between 40% (topical PUVA) and 70% (oral PUVA) of the MPD is recommended [144, 145]. If MPD testing is unavailable or the patient's skin is too extensively involved to measure the MPD, the starting dose is based on skin phototype: I, 0.5 J/cm2; II, 1.0 J/cm2; III, 1.5 J/cm2; IV, 2.0 J/cm2. The starting dose of bath PUVA should always be based on MPD testing because of the risk of severe photosensitivity.
Increments.
Doses are normally increased in 20–40% increments; lower increments may be indicated if significant erythema develops. If barely perceptible asymptomatic erythema develops, the dose can be kept the same until the erythema settles.
Frequency.
PUVA therapy is usually administered twice weekly as PUVA erythema is delayed [146]. It had been assumed that topical PUVA erythema was maximal at 72 h but more recent studies have shown the peak to occur at between 96 and 144 h [147, 148]. Some centres still use PUVA three times per week but this regimen can result in an increased incidence of burning and is therefore not advised, particularly given the cumulative carcinogenic risk of PUVA.
Number of exposures.
The number of exposures required for PUVA therapy of dermatoses is similar to that of TL-01 UVB therapy (see earlier in the chapter). As there is a significantly increased risk of skin cancer with increasing cumulative exposure to PUVA, the number of treatments should be kept to a minimum and maintenance therapy avoided.
Ways to deliver PUVA
As with UVB phototherapy, PUVA is usually delivered in a cabin with fluorescent tubes that surround the patient. PUVA can also be delivered locally with units that can be used to irradiate the hands and feet (Figure 21.10). Bath PUVA and localized PUVA are also available for patients when systemic PUVA is not recommended or is less appropriate.
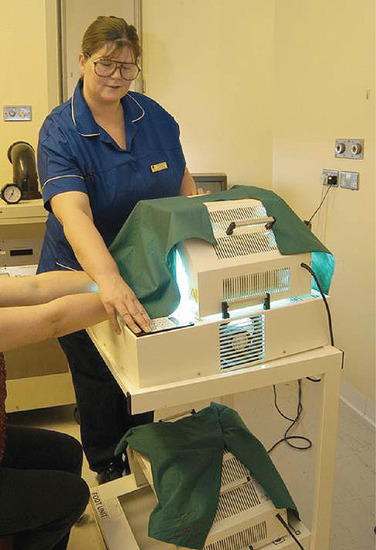
Figure 21.10 Patient's hands being irradiated with UVA following a topical psoralen soak.
Eye protection
Following the ingestion of psoralen tablets, patients are required to wear UVA-absorbing glasses before therapy and for at least 12 h after therapy (24 h for children and patients with pre-existing cataracts or atopic eczema) [49]. During therapy UV-blocking goggles must be worn. Eye protection with bath PUVA is only necessary in patients with very extensive disease when the risk of systemic absorption becomes significant.
Combination therapy
Combination therapy (i.e. UVB or PUVA combined with topical or systemic therapy) is used to increase the clinical response of psoriasis and to decrease the number of phototherapy exposures and thus the cumulative dose.
Topical agents
A variety of topical agents, including emollients [149], tar [150], dithranol (anthralin) [151], tazarotene [152] and vitamin D analogues such as calcipotriol [153, 154], have been used in combination with phototherapy. Evidence for significant adjuvant or UV sparing effects is limited. Studies of both calcipotriol and tazarotene have shown faster clearance of psoriasis with significantly lower median cumulative UV exposure. Vitamin D analogues are best used after UV exposure as they are unstable if exposed to UV radiation [155]. Topical immunomodulatory agents such as tacrolimus and pimecrolimus should be avoided. A multicentre study has shown significant benefit when patients are treated with TL-01 UVB in the presence of Dead Sea salt solution [156]. In practice, if phototherapy is used optimally, the role of adjunctive therapies is less clear.
Systemic agents
There is good evidence that the combination of PUVA or UVB with an oral retinoid (so-called Re-PUVA or Re-UVB, respectively) increases efficacy and has a significant dose sparing effect [157]. Retinoids are also beneficial due to their protective effects against skin cancer development [158]. Concomitant systemic immunosuppressive agents should be avoided in combination with phototherapy in order to avoid an augmented risk of skin cancer. Eruptive skin cancers have been reported when ciclosporin is used in patients who have received high cumulative doses of PUVA [159]. NB-UVB enhances the efficacy of a variety of biological agents including alefacept [160], etanercept [161], adalimumab [162] and ustekinumab [163]. However, most studies did not include a phototherapy-alone control group. The potential for enhanced skin carcinogenesis is a significant concern with such combinations and they should, in general, be avoided.
UVA-1 phototherapy
UVA-1 phototherapy uses long wavelength UVA radiation (340–400 nm), filtering out the erythemogenic UVA2 and UVB wavelengths (290–340 nm). UVA-1 phototherapy has been used at a wide variety of doses, typically described as high (>60 J/cm2), medium (30–60 J/cm2), low (10–20 J/cm2) and very low (<10 J/cm2). There is very little evidence to define what the optimal UVA-1 treatment regimen should be, although common practice is stated below [40, 86].
Minimal erythema dose
This is determined using a range of doses which reflect whether a low, medium or very high-dose schedule is being used.
Regimen variables
Starting dose.
The starting dose is based on the MED and is usually 50% of the latter.
Increments.
Ten to 20% increments can be used at each treatment depending on whether or not erythema develops.
Frequency.
Treatment is administered 3–6 times per week.
Number of exposures.
In general, a trial of a minimum of 15 treatments would be recommended before deciding on whether to continue treatment or not.
Ways to deliver UVA-1
UVA-1 can be delivered either via low irradiance lamps (such as Philips TL-10) or from metal halide portable or whole body UVA-1 units. The latter provide a much higher irradiance thus allowing increased dose delivery.
Extracorporeal photochemotherapy
The photopheresis procedure takes place in three stages: leukapheresis, photoactivation and reinfusion [6, 164]. Whole blood is removed from the patient and then centrifuged to separate the red blood cells (RBCs) from the white blood cells (WBCs). The WBCs (along with some plasma and RBCs) form the buffy coat. The latter is then mixed with saline and 8-MOP (UVADEX®) and photoactivated by being passed through a plastic film which is irradiated by UVA lamps (Figure 21.11). The irradiated buffy coat is then reinfused into the patient.
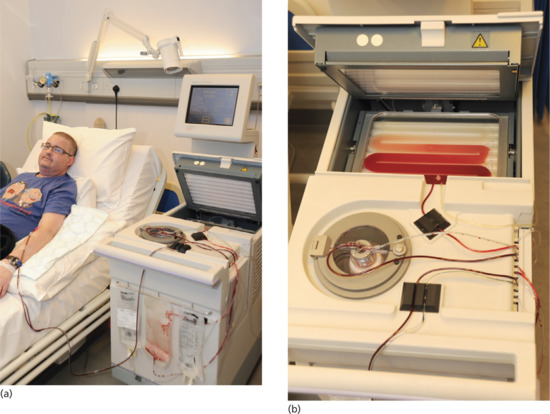
Figure 21.11 (a) Patient receiving extracorporeal photopheresis. (b) Close up of the upper part of photopheresis equipment showing the patient's buffy coat containing white blood cells being circulated between UVA tubes.
Phototesting
Minimal photoxic dose testing is not necessary as irradiation occurs outside the body in a machine.
Regimen variables
Standard dose.
The dose of UVA delivered to the patient's lymphocytes is 1–2 J/cm2.
Increments.
None, the above standard dose is used for each treatment.
Frequency.
It is administered on 2–3 consecutive days once per month (for graft-versus-host disease every 2–3 weeks). The frequency of treatment can be increased in non-responders.
Number of treatments.
Treatment is generally continued for 6 months before declaring treatment failure if there is a lack of response. In patients who respond to therapy this can be continued at a decreased frequency to provide maintenance control if necessary.
Ways to deliver ECP
Extracorporeal photochemotherapy is most commonly delivered using the UVAR XTSR system (Therakos) as described previously. Although 8-MOP may be administered orally, plasma levels can be erratic, side effects such as nausea, vomiting and diarrhoea may be troublesome, and there is a risk of burning from exposure to ambient UV [165, 166].
Adverse effects
UVB phototherapy
Acute effects
Erythema
Erythema and burning are the most common side effects of treatment. Erythema peaks at 12–24 h and can be associated with pain, swelling and blistering (Figure 21.12) [1, 167]. Burning is more likely in patients who are of skin phototype I/II, are obese, have inadvertent exposure of previously covered skin (e.g. after a hair cut), are taking phototoxic drugs or herbal medication or have unknown photosensitivity (e.g. lupus erythematosus). Burning may also be due to operator error in programming the cabinet timer or selecting the correct dose for the correct patient. Mild erythema is managed using topical corticosteroid creams and emollients. Further treatment is withheld until the erythema settles, after which a lower incremental dose regimen should be introduced. More severe reactions may require a short course of oral corticosteroids and/or non-steroidal anti-inflammatory drugs (NSAIDs).
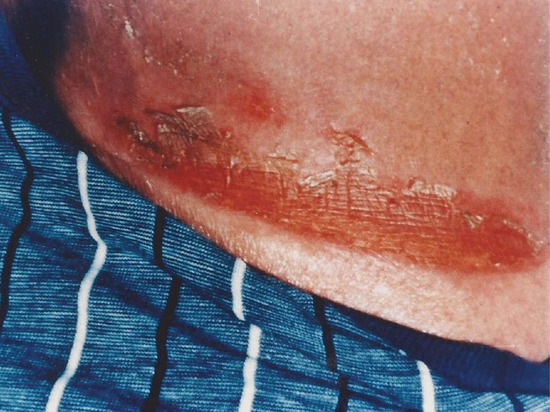
Figure 21.12 Burn sustained to the lower trunk during narrow-band UVB therapy due to slipping of the patient's underpants.
Pruritus
This is usually due either to phototherapy-induced dryness of the skin or to the underlying disorder being treated. It can usually be controlled by emollients [168].
Herpes simplex virus reactivation
This most commonly affects the lip and occurs more frequently in those with a past history of such outbreaks [169]. The areas in question can be protected by a sunscreen or visor if necessary.
Lesional blistering
Blistering of psoriatic plaques is an uncommon side effect of TL-01 phototherapy and is usually asymptomatic [170]. It typically occurs mid-way through a treatment course and usually settles spontaneously or following dose reduction. It can occur in the absence of erythema or burning.
Flare of polymorphic light eruption
The precipitation of PLE is a not uncommon occurrence during phototherapy in those who are predisposed to it (Figure 21.13) [168]. This is managed by the use of topical corticosteroid creams and adjustment of the treatment regimen.
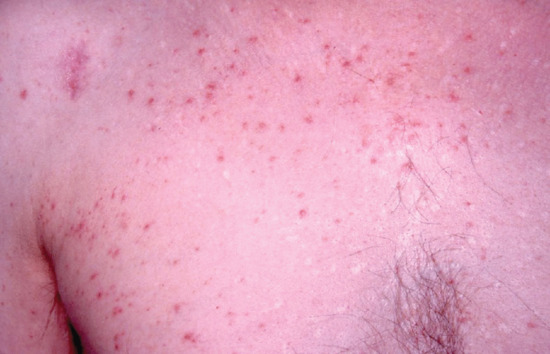
Figure 21.13 Flare of polymorphic light eruption on the chest induced by UVB therapy.
Chronic effects
Photoageing
Premature cutaneous ageing may be induced or exacerbated by UVB phototherapy. The skin develops a leathery appearance, xerosis, wrinkling, pigmentary changes, loss of elasticity and increased fragility [2].
Carcinogenesis
Broad-band UVB has a carcinogenic risk that is not well defined due to the many confounding variables; these include recreational sun exposure, exposure to sunbeds or PUVA, skin type and exposure to systemic immunosuppressive therapies. A meta-analysis of studies using BB-UVB estimated an excess risk of non-melanoma skin cancer of about 2% per year [171]. Stern's long-term follow-up study of PUVA recipients in the USA showed that the risk of developing genital squamous cell carcinoma was 4.6 times greater in men with psoriasis and a history of significant UVB exposure (>300 treatments) than in men with similar PUVA exposure but without UVB exposure [172]. Genital protection during phototherapy continues to be recommended to minimize this risk.
The carcinogenic risk of NB-UVB, which is being increasingly used for delivering phototherapy, is not fully defined in humans but is less than that of PUVA. Extrapolation from animal studies would suggest that NB-UVB is 2–3 times per MED more carcinogenic than BB-UVB [173]. It has been suggested that this risk is abrogated in clinical practice, as the number of MEDs to clear psoriasis with NB-UVB is less than a third that required with BB-UVB. Clinical studies to date have not identified a significant risk of skin cancer in patients treated with NB-UVB, but further long-term follow-up studies are required [174, 175, 176]. As the risk of skin cancer with NB-UVB is not yet quantified, this therapy should continue to be used with a degree of caution.
PUVA
Acute effects
Acute phototoxicity
The erythema induced by PUVA is delayed, thus incurring a risk of cumulative injury if treatments are more frequent than twice per week. Peak erythema following the administration of oral and topical PUVA occurs between 72 and 120 h and between 96 and 144 h, respectively [177, 178]. Acute PUVA phototoxicity can range from mild erythema to severe pain with oedema, blistering and systemic upset including malaise and fever. PUVA erythema not only appears later than UVB erythema but lasts longer. A rarer form of phototoxic reaction to PUVA is nail damage with photo-onycholysis [179] or subungual haemorrhage [180]. Episodes of burning are more common with topical PUVA than with oral PUVA due to greater epidermal concentrations of psoralen and uneven absorption into the skin. The smaller risk of burning from oral PUVA can be lessened further by using 5-MOP rather than 8-MOP [181]. Predisposing factors for the development of erythema are similar to those discussed for UVB- induced erythema.
Management of phototoxic reactions depend on their severity and can range from dose increment adjustments to withdrawal of treatment. Mild to moderate erythema can be managed with moderate to potent topical corticosteroid creams and emollients. Severe reactions with blistering may require a potent topical corticosteroid such as clobetasol propionate supplemented by a short course of systemic corticosteroids.
Pruritus and pain
Pruritus occurs in up to 25% of patients and is associated with dryness of the skin [180, 182]. This can usually be controlled with emollients. A rare but severe idiosyncratic side effect is PUVA pain that can last for weeks or months and does not appear to correlate with PUVA phototoxicity [183, 184]. PUVA pain is important to identify as management is difficult. Low-frequency electrotherapy [185], topical capsaicin [186] and oral gabapentin [187] or phenytoin [188] have all been advocated. The continuation of PUVA or further courses in the future are contraindicated as recurrence of pain is common.
Nausea
Nausea is a common side effect in patients treated with oral 8-MOP PUVA. This can be lessened by taking the psoralen with a light meal or by using an antiemetic. Alternatively switching to 5-MOP or topical PUVA may help [49].
Blistering
Subepidermal bullae have been reported to be induced by PUVA [3, 189]. Phototoxic reactions to PUVA can result in bullae that occur most commonly acrally. Induction of bullous pemphigoid or a flaring of this in a patient with pre-existing disease is recognized.
Provocation of photodermatoses
Polymorphic light eruption can be precipitated by PUVA as can undiagnosed lupus erythematosus [168].
Herpes simplex reactivation
As with UVB phototherapy, outbreaks of herpes can be precipitated by PUVA [190]. Management is no different.
Other side effects
Less commonly observed side effects include transient nail pigmentation, a facial dermatitis resembling seborrhoeic dermatitis, folliculitis, facial hypertrichosis [168, 180], disseminated superficial actinic porokeratosis [191], lichenoid eruptions [192], headaches, insomnia [193] and allergic reactions to 8-MOP [194, 195].
Chronic effects
Photoageing
PUVA induces features resembling photoageing (dermatoheliosis) except that changes are not confined to sun-exposed sites. The clinical features are similar to those described for UVB therapy (see UVB chronic effects on the skin) but pigmentary changes and xerosis are more prominent. These changes are cumulative and dose related [168, 196].
PUVA keratoses
These manifest as multiple hyperkeratotic papules, most commonly located on non-sun-exposed sites, for example the legs, trunk and sides of the hands, feet and digits [197]. They occur most frequently in those patients exposed to high cumulative doses and are associated with an increased risk of non-melanoma skin cancer.
PUVA lentigines
These are large, irregular, stellate, darkly pigmented macular lesions (Figure 21.14) that histopathologically consist of large and atypical melanocytes. They occur most frequently in patients of skin type I/II who have received high numbers of PUVA treatments [198, 199]. There is no proven link between PUVA lentigines and the development of melanoma. Interestingly, however, a recent study has found that the T1799A BRAF mutation is commonly found in PUVA lentigines, suggesting premalignant potential [200].
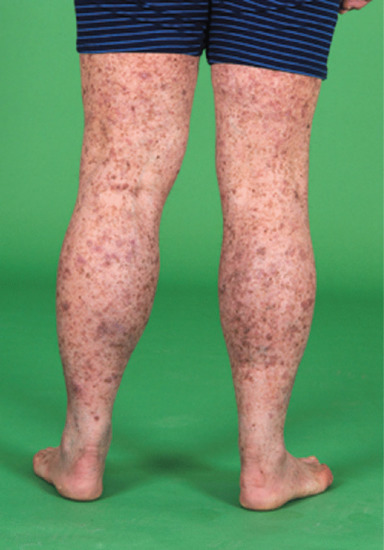
Figure 21.14 PUVA lentigines affecting a patient's legs.
Non-melanoma skin cancer
There is now substantial evidence for the carcinogenic risk of PUVA therapy in large cohorts of patients followed up for prolonged periods of time, particularly from the USA and Sweden [201, 202, 203, 204, 205, 206, 207]. There is a marked and dose-dependent increased risk of squamous cell carcinoma (SCC): in the most recent report from the American cohort, the risk of developing one or more SCCs in a year was strongly associated with the total number of PUVA treatments (350–450 versus <50 treatments; incidence rate ratio (IRR) 6.01) [208]. The overall excess risk of SCC in the high exposure group was significantly greater than this (IRR = 20.92). A 16-fold increase in incidence of genital SCC was observed in males from the same cohort whose exposure to PUVA was high (>240 treatments) rather than low (<140 treatments) [172]. It is recommended that lifetime exposure should be limited to 150–200 treatment sessions or a cumulative dose of 1000–1500 J/cm.2 Maintenance therapy should be avoided [49].
Important risk factors for PUVA- induced skin cancer include skin types I/II, a previous history of skin cancer, previous exposure to radiotherapy or arsenic [209, 210, 211, 212], immunosuppressive therapy, especially ciclosporin [159, 204, 213, 214], UVB therapy [215] and the presence of PUVA keratosis [197] or lentigines [198].
PUVA is mutagenic [216, 217], immunosuppressive [218, 219, 220] and carcinogenic [221, 222]. p53 mutations have been detected in 65% of PUVA- induced SCCs. The mutational pattern consists not only of PUVA-specific mutations (T to A transversions) but also ‘fingerprint’ mutations, as seen following solar exposure and UVB therapy (i.e. C to T or CC to TT mutations) [223, 224, 225]. PUVA has various effects on the immune system including decreasing the number of CD3+, CD4+ and CD8+ T lymphocytes in both the epidermis and dermis and affecting Langerhans cell immune expression [218, 219]. In addition, PUVA has also been reported to reduce a variety of circulating lymphocyte subsets [220]. Decreased immune surveillance may contribute to the carcinogenic effects of PUVA. To date there is no evidence to support an increased risk of skin cancer occurring in patients who have 227received bath or topical PUVA [226].
Melanoma
PUVA can stimulate the growth of melanoma cells and induce melanocytic tumours in animal experiments [227, 228]. A fivefold increase over the expected incidence of melanoma 15 years after first treatment was reported from a US 16-centre PUVA cohort [229]. Follow-up of the same cohort (an average of 2.25 years later) has showed a further increase to nine times the expected incidence [230]. The melanomas were more common in patients of skin type I/II and in those who had received at least 250 PUVA exposures. European study groups, on the other hand, have not found an increased risk of melanoma with PUVA [207, 211, 231, 232]. In particular, the study of 4799 Swedish patients treated with PUVA with an average follow-up of 16 years did not detect an increased incidence of melanoma [207]. Due to the long latent period of melanoma, further follow-up of these cohorts will be required to clarify melanoma risk in patients treated by PUVA.
Internal malignancy
It is known that patients with psoriasis have a threefold increased risk of lymphoma compared with the general population [233]. As PUVA alters immune function and surveillance there is a concern that the risk of internal malignancy, particularly of lymphoproliferative neoplasms, may be increased. To date studies have not demonstrated a significant association of PUVA with the development of lymphoma [234], nor a consistent relationship with other internal malignancies [205, 207, 235].
Ophthalmological effects
Psoralens can penetrate the ocular lens, where 8-MOP has been detected in humans at 12 h and in rats at 12–24 h after systemic administration [236, 237]. Following exposure to UVA, psoralens can bind to proteins in the lens, where they can accumulate as a result of the lack of cell turnover [238].
Cataract development following PUVA has been reported in some animal experiments [3]. Several studies of patients who have received PUVA have not shown an association between PUVA and cataract development [239, 240, 241]. A study of 82 patients who refused to wear protective sunglasses after PUVA did not observe any lens abnormalities. Decreased lacrimation and conjunctival hyperaemia occurred in some cases, however [242]. It is recommended that protective eyewear be worn for 12 h after the ingestion of psoralen and for 24 h in individuals with pre-existing cataracts or who may be at increased risk of cataract (e.g. children and patients with atopic eczema).
UVA-1 phototherapy
Acute effects
UVA-1 is generally well tolerated. Expected acute adverse effects include erythema, pigmentation, induction of PLE and recrudescence of herpes simplex virus infection [40]. Erythema induced by UVA-1 has a biphasic time course, with an early peak at 15–60 min and a delayed peak that may be maximal as early as 8 h after exposure, although this latter peak may be broad and plateau between 8 and 24 h [243].
Chronic effects
UVA-1 phototherapy, in common with NB-UVB and PUVA, is photogenotoxic, photomutagenic and carcinogenic in a mouse model. There is no evidence as yet of increased risk of skin cancer in humans. There are, however, concerns about its potential carcinogenic risk as it has been shown to induce cyclobutane pyrimidine dimers, particularly thymine dimers, and the basal layer is particularly vulnerable [244]. Oxidative DNA damage occurs at both genomic and nucleotide levels and there is also altered calcineurin signalling, which may impair tumour suppression [245].
Extracorporeal photochemotherapy
Adverse events occurring with ECP are uncommon providing psoralen is added to the treatment bag and not given orally [246]. Side effects reported include transient hypotension, low-grade pyrexia, an increase in erythema of the skin and anaemia with long-term use [164, 247].
Patient selection, assessment and education
Patient selection and assessment
Patients requiring phototherapy should be appraised for factors relating to skin cancer risk (see Box 21.2) [49]. A referral form completed in the clinic should document the patient's risk factor profile and should include documentation of skin type, previous natural sunlight/UVB/PUVA/sunbed use, previous skin cancer or precursors, previous systemic immunosuppressive drug therapy and exposure to radiotherapy (Figure 21.15). In addition, a full skin examination should be performed to assess solar damage and to look for the presence of multiple freckles/moles, premalignant skin lesions and skin cancers.

Figure 21.15 UVB referral form.
It is also important to document all medication, especially any oral photoactive drug or herbal product being taken such as a thiazide diuretic or St John's wort (Hypericum perforatum). Most photoactive drugs absorb maximally in the UVA region and only some (including thiazides and quinine) have extension of their action spectrum into the UVB region. In one study, patients taking NSAIDs, calcium channel antagonists and phenothiazines were reported to have a lower NB-UVB MED, a factor which may need to be taken into consideration in patients being assessed for phototherapy [248]. Photosensitivity with UVA-1 phototherapy is more likely in patients taking photoactive drugs [40]. With PUVA, the overwhelming photosensitization by psoralen usually means that other photoactive drugs are unlikely to cause any additional clinically important photosensitization.
Topical therapies should also be assessed. Calcineurin inhibitors such as tacrolimus should be discontinued [249]. Patients with epilepsy should be asked whether seizures can be induced by light exposure. As PUVA therapy is mutagenic this treatment should be avoided in pregnancy. Oral PUVA should be used with caution if the patient has significant liver dysfunction.
Patient education
Patients who are commencing phototherapy or PUVA should be counselled regarding both the acute and chronic side effects of treatment. This should be reinforced by providing a patient information leaflet. Patients should be advised regarding the appropriate use of emollients. The importance of reporting of any new medication and of avoiding fragrance-containing products, sunbathing and sunbeds during the course of treatment should be emphasized. Also, it is important to advise patients to maintain a consistent hairstyle and to explain the rationale for this advice. Males should be advised of the need to protect the genitalia. The importance of wearing protective eyewear should be stressed. The treatment cabin should be shown to the patient and the protocol explained, stressing the importance of regular attendance. A consent form should be signed by the patient before treatment is commenced.
Patient and staff safety
Both patient and staff safety should be ensured. For patient safety, see Box 21.3.
Individuals who may be exposed to UV radiation include nurses and nursing assistants, medical physicists, doctors or, less commonly, physiotherapists. The most important risk to staff is exposure to UVB. Staff who conduct dosimetry should wear goggles, face shields, appropriate clothing and a sunscreen (SPF>30) if they have to enter the cabinet with the lamps on. When UV cabinets are being used, the lamps should be switched off before opening or entering the cabinet.
Patient follow-up: skin cancer surveillance
It is well recognized that systemic PUVA therapy is associated with a dose-related increased risk of non-melanoma skin cancer, particularly squamous cell carcinoma. It has been recommended that the maximum lifetime dose should not exceed 1000–1500 J/cm2 or 150–200 exposures [49]. The increased carcinogenic risk seen with systemic PUVA has not been seen with bath PUVA. Bath PUVA involves lower UVA doses but results in a much higher epidermal psoralen concentration than oral PUVA. Thus the potential for induction of mutagenic DNA lesions remains and ongoing vigilance in these patients is required. It is important to take into account total UVA dose in addition to treatment numbers when considering the carcinogenic risk of PUVA. It is recommended that patients who exceed these maximum thresholds of exposure should have an annual skin examination carried out to detect pre-malignant and malignant skin lesions. This is particularly important for those patients with other risk factors, such as those of skin type I/II.
Because NB-UVB was introduced only relatively recently, long-term follow-up data equivalent to those available for PUVA are as yet lacking. The preliminary data have not, however, suggested a significant increase in skin cancer risk from NB-UVB [174, 175, 176]. Further long-term data are required from follow-up of large cohorts of patients.
Mathematical models have been created in attempts to quantify the risk of skin cancer resulting from NB-UVB but the methodologies used are very variable. One group, on the premise that an increased risk of non-melanoma skin cancer of no more than 50% is ‘acceptable’, has recommended limiting lifetime exposure to 450 treatments [253]. However, there are concerns about defining a ceiling number of treatments in the absence of robust clinical data to support this and, more importantly, without assessing risk/benefit on an individual basis.
Clinical governance
A phototherapy service should have a designated responsible person or lead clinician (usually a consultant dermatologist). This individual should ensure that high quality standards are maintained. Guidelines and protocols should be maintained and updated. Staff should be suitably trained and their knowledge should be updated through formal clinical professional development (CPD). The service should be delivered by a multidisciplinary team which should consist of the lead clinician, lead phototherapy nurse, medical physicist and other relevant assistant staff. Areas that require being audited regularly include documentation, adverse events, equipment maintenance and risk management.
Documentation
In most of Europe and North America there is a legal requirement for documentation that should include patient records, dosimetry and equipment maintenance, adverse events and summary records of departmental activity.
Patient records
Referral forms for phototherapy or PUVA must be completed by a dermatologist or authorized dermatology practitioner. The content of this form should include patient identity, the disease being treated, type of phototherapy requested, current systemic medications, previous phototherapy and details about the absence or presence of any contraindications or risk factors for phototherapy. A record of informed consent must be obtained and patients must receive a patient information leaflet. A formal nursing assessment should be recorded. Phototest results, treatment type (including details of psoralen dose), incremental regimen prescribed, all treatment visits, UV doses administered, response to treatment and adverse incidents must all be systematically documented.
Dosimetry and equipment maintenance
The UV equipment used must be ‘CE’ marked as a medical device and be appropriately maintained by an approved engineer or medical physicist. Assessment of the electrical safety and inspection of the integrity of all UV units should form part of a regular maintenance schedule, careful records of which must be kept.
Records must be kept of all UV calibration readings, which must be carried out by an approved medical physicist. Any radiometer used must be calibrated with traceability to the UK National Physics Laboratory or equivalent authority elsewhere [37]. A policy for the replacement of low output or failed UV lamps should be in place.
Adverse incidents
All adverse incidents should be recorded. The circumstances should be clearly described and action taken. These incidents should be discussed at department meetings to minimize the risk of recurrence of such events.
Performance indicators
These reflect the workload of the department and should include the total number of patients treated, total number of treatment sessions with a breakdown by treatment modality, number of patients on the waiting list and average waiting time from referral to treatment.
Risk management
A formal risk assessment of potential hazards in the phototherapy department, especially from inadvertent exposure to UV radiation, should be undertaken at least annually. The waiting areas must be separate from treatment areas. Warning signs should be displayed on entering areas where UV is being used. An infection control and hygiene policy should be established, particularly with regard to cleaning the phototherapy equipment.
Audit
The phototherapy service should be regularly audited to ensure it is maintaining standards and adhering to guidelines [1]. Many aspects of the service can be audited, including: quality of phototherapy records; clinical outcome including adverse events; workload and activity statistics; waiting list management and access to the service; and discharge and follow-up procedures.
How to set up a phototherapy unit
The setting up of a phototherapy service involves the participation of different groups – including medical and nursing staff, the medical physics service, local health service management and hospital engineers (with regard both to electrical supply and safety). The involvement of patient organizations may help persuade administrators and funders of the desirability of providing such a service.
It is vital that adequate space is provided. For a modest district-based service serving a population of 200 000 people there should be sufficient room to accommodate one NB-UVB cabinet, one PUVA cabinet, a set of UV units for the local treatment of hands and feet and UV units for phototesting. If topical bath PUVA is to be offered, bathing facilities are necessary. In addition to the treatment area, which requires adequate ventilation to cope with heat emitted by the UV equipment, space is also required for a waiting area with access to toilet facilities and for reception and nursing administration areas with appropriate IT facilities and document storage space.
Policies need to be established for ongoing UV cabinet maintenance, lamp replacement and radiometry. Staff should have a background of experience and training in dermatology and should have attended a core phototherapy course followed by a period of supervised practice in a unit where UV therapy is already provided. Protocols and evidence-based guidelines must be in place and staff training completed before the service is opened.
What's new: developments
Phototherapy is a highly effective and safe treatment option for many skin diseases and continued awareness of the availability of this form of treatment is important.
One of the factors limiting further therapeutic advances in phototherapy sources has been our relative lack of understanding of the action spectra for the clearance of diseases other than psoriasis. Most of the developmental work relating to optimizing phototherapy regimens has been with psoriasis in mind. Future work to define the optimal wavelengths for the treatment of diseases such as eczema, mycosis fungoides and vitiligo is required in order to promote the development of light sources and treatment regimens that are disease-specific.
Advances in technology are enabling the development of compact, portable, efficient and inexpensive UV and visible light-emitting diode (LED) light sources. Taken together with modern methods of instant communication, these may facilitate the development of patient self-administered home phototherapy services with ready access to expert supervision and assistance coordinated from a central phototherapy department. Clinical governance is essential and the development of national networks to centralize this is ongoing. The future of phototherapy is encouraging. As new insights into skin biology are gained, there will be opportunities to refine treatment further to improve its already good efficacy/risk profile.
References
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol 2004;151:283–297.
- Berneburg M, Rocken M, Benedix F. Phototherapy with narrowband UVB. Acta Derm Venereol 2005;85:1–11.
- Gupta AK, Anderson TF. Psoralen photochemotherapy. J Am Acad Dermatol 1987;17:703–34.
- Honigsmann H. Phototherapy for psoriasis. Clin Exp Dermatol 2001;26:343–50.
- Edelson RL, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy: preliminary results. N Engl J Med 1987:316:297–303.
- McKenna KE, Whittaker S, Rhodes LE, et al. Evidence based practice of photopheresis 1987–2001: a report of a workshop of the British Photodermatology Group and UK Skin Lymphoma Group. Br J Dermatol 2006;154:7–20.
- Krutmann J, Czech W, Diepgen T, et al. High-dose UVA1 therapy in the treatment of patients with atopic dermatitis. J Am Acad Dermatol 1992;26:225–30.
- Kerscher M, Volkenandt M, Gruss C, et al. Low-dose UVA1 phototherapy for treatment of localized scleroderma. J Am Acad Dermatol 1998;38:21–6.
- El-Ghorr AA, Norval M. Biological effects of narrow-band (311 nm TL01) UVB irradiation: a review. J Photochem Photobiol B Biol 1997;38:99–106.
- Ozawa M, Ferenczi K, Kikuchi T, et al. 312-nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med 1999;189:711–18.
- Aufiero BM, Talwar H, Young C, et al. Narrow band UVB induces apoptosis in human keratinocytes. J Photochem Photobiol 2006;82:132–9.
- Pathak MA. Mechanisms of psoralen photosensitization reactions. Natl Cancer Inst Monogr 1984;66:41–6.
- Okamoto H, Takigawa M, Horio T. Alteration of lymphocyte functions by 8-methoxypsoralen and long-wave ultraviolet radiation. I. Suppressive effects of PUVA on T-lymphocyte migration in vitro. J Invest Dermatol 1985;84:203–5.
- Furuhashi T, Saito C, Torii K, et al. Photo(chemo)therapy reduces circulatory Th17 cells and restores circulating regulatory T cells in psoriasis. PLOS One 2013;8:e54895.
- Berger CL, Xu A-L, Hanlon D. Induction of tumor loaded dendritic cells. Int J Cancer 2001;91:438–77.
- Di Renzo M, Rubegni P, De Aloe G, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T cell lymphoma. Immunology 1997;92:99–103.
- Vowels BR, Cassin M, Boufal MH, et al. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha in monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol 1992;98:686–92.
- Gruss C, Reed JA, Altmeyer P, et al. Induction of interstitial collagenase (MMP-1) by UVA-1 phototherapy in morphea fibroblasts. Lancet 1997;350:1295–6.
- Gambichler T, Kreuter A, Tomi NS, et al. Gene expression of cytokines in atopic eczema before and after ultraviolet A1 phototherapy. Br J Dermatol 2008;158:1117–20.
- Godar DE. UVA1 radiation triggers two different final apoptotic pathways. J Invest Dermatol 1999;112:3–12.
- Roelandts R. The history of phototherapy: something new under the sun? J Am Acad Dermatol 2002;46:926–30.
- Honigsmann H. History of phototherapy in dermatology. J Photochem Photobiol Sci 2013;12:16–21.
- Roelandts R. Bicentenary of the discovery of the ultraviolet rays. Photoderm Photoimmunol Photomed 2002;18:208.
- Downes AH, Blunt TP. Researches on the effect of light upon bacteria and other organisms. Proc R Soc London 1877;26:488–500.
- Moller KI, Kongshoi B, Philipsen PA, Thomsen VO, Wulf HC. How Finsen's light cured lupus vulgaris. Photodermatol Photoimmunol Photomed 2005;21:118–24.
- Alderson HE. Heliotherapy in psoriasis. Arch Dermatol 1923;8:79–80.
- Goeckerman WH. The treatment of psoriasis. Northwest Med 1925;24:229–31.
- Ingram JT. The approach to psoriasis. BMJ 1953;12:591–4.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol 1981;76:359–62.
- Van Weelden H, Baart de La Faille H, Young E, van der Leun JC. A new development in UVB phototherapy of psoriasis. Br J Dermatol 1988;119:11–19.
- Green C, Ferguson J, Lakshmipathi T, Johnson BE. 311 nm UVB phototherapy – an effective treatment for psoriasis. Br J Dermatol 1988;119:691–6.
- Fahmy IR, Abu-Shady H, Schonberg AA. Crystalline principle from Ammi majus. Nature 1947;160:468.
- Fahmy IR, Abu-Shady H. Ammi majus Linn: the isolation and properties of ammoidin, ammidin and majudin, and their effect in the treatment of leukoderma. Q J Pharm Pharmacol 1948;21:499–503.
- El-Mofty AM. A preliminary clinical report on the treatment of leukoderma with Ammi majus Linn. J Egypt Med Assoc 1948;31:651–65.
- Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med 1974;291:1207–11.
- Mutzhas MF, Hofmann C, Plewig G. A new apparatus with high radiation energy between 320–460 nm – physical description and dermatological applications. J Invest Dermatol 1981;76:42–47.
- Taylor DK, Anstey AV, Coleman AJ, et al. Guidelines for dosimetry and calibration in ultraviolet radiation therapy: a report of a British Photodermatology Group workshop. Br J Dermatol 2002;146:755–63.
- Young AR. Cumulative effects of ultraviolet radiation on the skin: cancer and photoaging. Semin Dermatol 1990;9:25–31.
- Diffey BL, Hart GC. Ultraviolet and Blue-light Phototherapy – Principles, Sources, Dosimetry and Safety. Report No. 76. York: Institute of Physics and Engineering in Medicine, 1997:27.
- Kerr AC, Ferguson J, Attili SK, et al. Ultraviolet A1 phototherapy: a British Photodermatology Group workshop report. Clin Exp Dermatol 2012;37 (3):219–26.
- Dawe RS. A quantitative review of studies comparing the efficacy of narrow-band and broad-band ultraviolet B for psoriasis. Br J Dermatol 2003;149:669–72.
- Markham T, Rogers S, Collins P. Narrowband UV-B (TL-01) phototherapy vs oral 8-methoxypsoralen psoralen-UV-A for the treatment of chronic plaque psoriasis. Arch Dermatol 2003;139:325–8.
- Yones SS, Palmer RA, Garibaldinos TT, Hawk JL. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol 2006;142:836–42.
- Robinson A, Van Voohees AS, Hsu S, et al. Treatment of pustular psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 2012;67:279–88.
- Jekler J, Larko O. UVA solarium versus UVB phototherapy of atopic dermatitis: a paired comparison study. Br J Dermatol 1991;125:569–72.
- Midelfart K, Stenvold S-E, Volden G. Combined UVB and UVA phototherapy of atopic eczema. Dermatologica 1985;171:95–8.
- Hudson-Peacock MJ, Diffey BL, Farr PM. Narrowband UV-B phototherapy for severe atopic dermatitis. Br J Dermatol 1995;133:653–67.
- Sheehan MP, Atherton DJ, Norris P, Hawk JLM. Oral psoralen photochemotherapy in severe childhood atopic eczema: an update. Br J Dermatol 1993;129:431–7.
- British Photodermatology Group. British Photodermatology Group guidelines for PUVA. Br J Dermatol 1993;130:246–55.
- Gathers RC, Scherschun L, Malick F, Fivenson DP, Lim HW. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol 2002;47:191–7.
- Honigsmann H, Tanew A, Wolff K. Treatment of mycosis fungoides with PUVA. Photodermatol 1987;4:55–8.
- Herrmann JJ, Roenigk HH, Jr, Hurria A, et al. Treatment of mycosis fungoides with photochemotherapy (PUVA): long-term follow-up. J Am Acad Dermatol 1995;33:234–42.
- Tjioe M, Gerritsen MJ, Juhlin L, van de Kerkhof PC. Treatment of vitiligo vulgaris with narrow band UVB (311 nm) for one year and the effect of addition of folic acid and vitamin B12. Acta Derm Venereol 2002;82:369–72.
- Grimes PE, Minus HR, Chakrabarti SG, et al. Determination of optimal photochemotherapy for vitiligo. J Am Acad Dermatol 1982;7:771–8.
- Pacificio A, Leone G. Photo(chemo)therapy for vitiligo. Photodermatol Photoimmunol Photomed 2011;27:261–77.
- Bilsland D, George SA, Gibbs NK, Aitchison T, Johnson BE, Ferguson J. A comparison of narrow band phototherapy (TL-01) and photochemotherapy (PUVA) in the management of polymorphic light eruption. Br J Dermatol 1993;129:708–12.
- Dummer R, Ivanova K, Scheidegger EP, Burg G. Clinical and therapeutic aspects of polymorphous light eruption. Dermatology 2003;207:93–5.
- Van der Schaar WW, Sillevis Smitt JH. Evaluation of PUVA-therapy for alopecia areata. Dermatologica 1984;168:250–2.
- Assouly P. Alopecia areata: update on therapy. Ann Dermatol Venereol 2002;129:831–6.
- Grundmann-Kollmann M, Martin H, Ludwig R, et al. Narrowband UV-B phototherapy in the treatment of cutaneous graft versus host disease. Transplantation 2002;74:1631–4.
- Jampel RM, Farmer ER, Vogelsang BB, et al. PUVA therapy for chronic cutaneous graft-vs-host disease. Arch Dermatol 1991;127:1673–8.
- Browne F, Turner D, Goulden V. Psoralen and ultraviolet A in the treatment of granuloma annulare. Photoderm Photoimmunol Photomed 2011;27:81–4.
- Saricaoglu H, Karadogan SK, Baskan EB, Tunali S. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed 2003;19:265–7.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs UVB-311nm for the treatment of lichen planus. Photoderm Photoimmunol Photomed 2007;23:15–19.
- McKenna DB, Cooper EJ, Tidman MJ. Topical psoralen plus ultraviolet A treatment for necrobiosis lipoidica. Br J Dermatol 2000;143:1333–4.
- Tan E, Lim D, Rademaker M. Narrowband UVB phototherapy in children. A New Zealand experience. Australas J Dermatol 2010;51:268–73.
- Streit V, Thiede R, Wiedow O, Christophers E. Foil bath PUVA in the treatment of prurigo simplex subacuta. Acta Derm Venereol (Stockh) 1987;82:317–20.
- Collins P, Ferguson J. Narrow-band UVB (TL-01) UVB phototherapy: an effective preventative treatment for the photodermatoses. Br J Dermatol 1995;132:956–63.
- Roelandts R. Phototherapy of photodermatoses. J Dermatol Treat 2002;13(4):157–60.
- Honigsmann H. Mechanisms of phototherapy and photochemotherapy for photodermatoses. Dermatol Ther 2003;16:23–7.
- Rosen K, Mobacken H, Swanback G. PUVA, etretinate, and PUVA-etretinate therapy for pustulosis palmoplantaris. Arch Dermatol 1987;123:885–9.
- Farnaghi F, Seirafi H, Ehsani AH, Aghari ME, Noormohammadpour P. Comparison of the therapeutic effects of narrowband UVB vs PUVA in patients with pityriasis lichenoides. J Eur Acad Dermatol Venereol 2011;25:913–16.
- Chuh A. Narrow band UVB therapy and oral acyclovir for piyriasis rosea. Photoderm Photoimmunol Photomed 2004;20:64–5.
- Vergilis-Kalner IJ, Mann DJ, Petronic-Rosic V, Tsoukas MM. Pityriasis rubra pilaris sensitive to narrow band ultraviolet light. J Drugs Dermatol 2009;8:270–3.
- Kaskel P, Grundmann-Kollmann M, Schiller PI, et al. Bath-PUVA is a treatment for pityriasis rubra pilaris provoked by ultraviolet B. Br J Dermatol 1999;140:709–70.
- Rosen K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands. A comparison of PUVA and UVB treatment. Acta Derm Venereol 1987;67(1):48–54.
- Schempp CM, Muller H, Czech W, et al. Treatment of chronic palmoplantar eczema with local bath-PUVA therapy. J Am Acad Dermatol 1997;36:733–7.
- Hsu MM, Yang CC. Uraemic pruritus responsive to broadband ultraviolet (UV) B therapy does not readily respond to narrowband UVB therapy. Br J Dermatol 2003;149:888–9.
- Baldo A, Sammarco E, Plaitano R, et al. Narrowband (TL01) ultraviolet B phototherapy for pruritus in polycythaemia vera. Br J Dermatol 2002;147:979–81.
- Pirkhammer D, Seeber A, Honigsmann H, Tanew A. Narrow-band ultraviolet B (ATL-01) phototherapy is an effective and safe treatment option for patients with severe seborrhoeic dermatitis. Br J Dermatol 2000;143:964–8.
- Cameron H, Dawe RS. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) treated with narrowband (TL01) UVB phototherapy. Br J Dermatol 1997;137:150–1.
- Bauwens M, de Coinck A, Roseeuw D. Subcorneal pustular dermatosis treated with PUVA. Dermatology 1999;198:203–5.
- Ridge CA, Moktar A, Barry J, Murphy GM. Photochemotherapy and methotrexate used to treat generalised cutaneous scleroderma. J Eur Acad Dermatol Venereol 2007;21:692–3.
- Aydogan K, Karadogan SK, Tunali S, Saricaoglu H. Narrowband ultraviolet B (311nm, TL01) phototherapy in chronic ordinary urticaria. Int J Dermatol 2012;51(1):98–103.
- Vella Briffa D, Eady RAJ, James MP, et al. Photochemotherapy (PUVA) in the treatment of urticaria pigmentosa. Br J Dermatol 1983;109:67–75.
- Dawe RS. Ultraviolet A1 phototherapy. Br J Dermatol 2003;148:626–37.
- Tzaneva S, Seeber A, Schwaiger M, et al. High-dose versus medium-dose UVA1 phototherapy for patients with severe generalised atopic dermatitis. J Am Acad Dermatol 2001;45:503–7.
- Kowalzick L. UVA1 For atopic dermatitis: medium dose superior to low dose. J Am Acad Dermatol 2001;44:548.
- Morita A, Kobayashi K, Isomura I, et al. Ultraviolet A1 (340–400nm) phototherapy for scleroderma in systemic sclerosis. J Am Acad Dermatol 2000;43:670–4.
- Grundmann-Kollman M, Behrens S, Gruss C, et al. Chronic sclerodermic graft-versus-host disease refractory to immunosuppressive treatment responds to UVA1 phototherapy. J Am Acad Dermatol 2000;42:134–6.
- Tran KT, Prather HB, Cockerell CJ, Jacobe H. UV-A1 therapy for nephrogenic systemic fibrosis. Arch Dermatol 2009;145:1170–4.
- Kreuter A, Gambichler T, Avermaete A, et al. Low-dose ultraviolet A1 phototherapy for extragenital lichen sclerosus: results of a preliminary study. J Am Acad Dermatol 2002;46:251–5.
- Beattie PE, Dawe RS, Ferguson J, Ibbotson SH. UVA1 phototherapy for genital lichen sclerosus. Clin Exp Dermatol 2000;31:343–7.
- McGrath H, Jr. Ultraviolet-A1 irradiation decreases clinical disease activity and autoantibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol 1994;12:129–35.
- Polderman MC, Huizinga TW, Le Cessie S, Pavel S. UVA-1 cold light treatment of SLE: a double blind, placebo controlled crossover trial. Ann Rheum Dis 2001;60:112–15.
- Scarisbrick JJ, Taylor P, Holtick U, et al. UK consensus statement on the use of extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br J Dermatol 2008;158:1365–2133.
- Kim YH, Varghese A, Hoppe RT. Prognostic factors in erythrodermic mycosis fungoides and the Sezary syndrome. Arch Dermatol 1995;131:1003–8.
- Fraser-Andrew E, Seed P, Whittaker S, Rusell-Jones R. Extracorporeal photopheresis in Sezary syndrome: no significant effect in the survival of 44 patients with a peripheral blood T-cell clone. Arch Dermatol 1998;134:1001–5.
- Child FJ, Ratnavel R, Watkins P et al. Extracorporeal photopheresis (ECP) in the treatment of chronic graft-versus-host disease (GVHD). Bone Marrow Transplant 1999;23:881–7.
- Rossetti D, Dall'Amico R, Crovetti G, et al. Extracorporeal photochemotherapy for the treatment of graft-versus-host disease. Bone Marrow Transplant 1996;18:175–81.
- Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood 1998;92:3098–104.
- Rook AH, Freundlich B, Jegasothy BV, et al. Treatment of systemic sclerosis with extracorporeal photochemotherapy. Arch Dermatol 1992;128:337–46.
- Wollina U, Lange D, Looks A. Short-time extracorporeal photochemotherapy in the treatment of drug-resistant autoimmune bullous diseases. Dermatology 1999;198:140–4.
- Vonderheid EC, Bigler RD, Rogers TJ, Kadin ME, Griffin TD. Effect of extracorporeal photopheresis on selected immunologic parameters in psoriasis vulgaris. Yale J Biol Med 1989;62:653–64.
- Prinz B, Nachbar F, Plewig G. Treatment of severe atopic dermatitis with extracorporeal photopheresis. Arch Dermatol Res 1994;287:48–52.
- Bécherel PA, Bussel A, Chosidow O, Rabian C, Piette JC, Francès C. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet 1998;351:805.
- Knobler RM, Graninger W, Graninger W, Lindmaier A, Trautinger F, Smolen JS. Extracorporeal photochemotherapy for the treatment of systemic lupus erythematosus. Arthritis Rheum 1992;35:319–24.
- Stables GI, Taylor PC, Highet AS. Scleredema associated with paraproteinaemia treated by extracorporeal photopheresis. Br J Dermatol 2000;142:781–3.
- De Wilde A, DiSpaltro FX, Geller A, Szer R, Klainer AS, Bisaccia E. Extracorporeal photochemotherapy as adjunctive treatment in juvenile dermatomyositis: a case report. Arch Dermatol 1992;128:1656–7.
- Barr Ml, Meiser BM, Eisen HJ, et al. Photopheresis for the prevention of rejection in cardiac transplantation. Photopheresis Transplantation Study Group. N Engl J Med 1998;339:1744–51.
- Heald PW, Perez MI, Christensen I, et al. Photopheresis therapy of cutaneous T-cell lymphoma: the Yale–New Haven Hospital experience. Yale Biol Med 1989;62:629–38.
- Wolf JT, Lessin SR, Singh AH, Rook AH. Review of immunomodulation by photopheresis: treatment of cutaneous T-cell lymphoma, autoimmune disease, and allograft rejection. Artif Organs 1994;18:888–97.
- Gottleib SL, Wolfe JT, Foc FE, et al. Treatment of cutaneous T-cell lymphoma with extracorporeal photophoresis monotherapy and in combination with recombinant interferon-alpha: a 10 year experience at a single institution. J Am Acad Dermatol 1996;35:946–57.
- Evans AV, Wood BP, Scarisbrick JJ, et al. Extracorporeal photopheresis in Sezary syndrome: hematologic parameters as predictors of response. Blood 2001;98:1298–301.
- Heald PW, Rook A, Perez MI, et al. Treatment of erythrodermic cutaneous T-cell lymphoma with extracorporeal photochemotherapy. J Am Acad Dermatol 1992;27:427–33.
- Armus S, Keyes B, Cahill C, et al. Photopheresis for the treatment of cutaneous T-cell lymphoma. J Am Acad Dermatol 1990;23:898–902.
- Rubegni P, De Aloe G, Fimiani M. Extracorporeal photochemotherapy in long-term treatment of early stage cutaneous T-cell lymphoma. Br J Dermatol 2000;143:894–6.
- Fimiani M, Rubegni P, D'Ascenzo G, Andreassi L. Extracorporeal photochemotherapy in the early treatment of cutaneous T-cell lymphoma. J Am Acad Dermatol 1994;31:828–9.
- Dippel E, Schrag ED, Goerdt S, Orfanos CE. Extracorporeal photopheresis and interferon-α in advanced cutaneous T-cell lymphoma. Lancet 1997;350:32–3.
- Tsirigotis P, Pappa V, Papageorgiou S, et al. Extracorporeal photopheresis in combination with bexarotene in the treatment of mycosis fungoides and sezary syndrome. Br J Dermatol 2007;156(6):1379–81.
- Wilson LD, Jones GW, Kim D, et al. Experience with total skin electron beam therapy in combination with extracorporeal photopheresis in the management of patients with erythrodermic (T4) mycosis fungoides. J Am Acad Dermatol 2000;43:54–60.
- Studniberg HM, Weller P. PUVA, UVB, psoriasis, and nonmelanoma skin cancer. J Am Acad Dermatol 1993;29:1013–22.
- Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol 1998;134:595–8.
- Farr PM, Diffey BL. Quantitative studies on cutaneous erythema induced by ultraviolet radiation. Br J Dermatol 1984;111:673–82.
- Gordon PM, Saunders PJ, Diffey BL, Farr PM. Phototesting prior to narrowband (TL-01) UV-B phototherapy. Br J Dermatol 1998;139:811–14.
- Farr PM. Irradiation testing of the skin. In: Hawk JLM, ed. Photodermatology. London: Arnold, 1999:261–5.
- Otman SG, Edwards C, Gambles B, Anstey AV. Validation of a semiautomated method of minimal erythema dose testing for narrowband ultraviolet B phototherapy. Br J Dermatol 2006;155(2):416–21.
- Dawe RS, Cameron HM, Yule S, et al. A randomized comparison of methods of selecting narrowband UV-B starting dose to treat chronic psoriasis. Arch Dermatol 2011;147(2):168–74.
- Wainwright NJ, Dawe RS, Ferguson J. Narrowband ultraviolet B (TL-01) phototherapy for psoriasis: which incremental regimen? Br J Dermatol 1998;139:410–14.
- Dawe RS, Wainwright NJ, Cameron H, Ferguson J. Narrow-band (TL-01) ultraviolet B phototherapy for chronic plaque psoriasis: three times or five times weekly treatment? Br J Dermatol 1998;138:833–9.
- Asawanonda P, Chingchai A, Torranin P. Targeted UV-B phototherapy for plaque-type psoriasis. Arch Dermatol 2005;141:1542–6.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol 2012;66(4):664–72.
- Trehan M, Taylor C. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol 2002;46:732–7.
- Jordan WP, Jr, Clarke AM, Hale RK. Long-term modified Goekerman regimen for psoriasis using an ultraviolet B light source in the home. J Am Acad Dermatol 1981;4:584–91.
- Cameron H, Yule S, Moseley H, Dawe RS, Ferguson J. Taking treatment to the patient: development of a home ultraviolet B phototherapy service. Br J Dermatol 2002;147:957–65.
- Sarkany RPE, Anstey A, Diffey BL, et al. Home phototherapy: report on a workshop of the British Photodermatology Group, December 1996. Br J Dermatol 1999;140:195–9.
- Koek MBG, Buskens E, van Weeelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ 2009;338:1181–6.
- Anstey A. Home UVB phototherapy for psoriasis: is as safe and effective as outpatient treatment, but provision is poor. BMJ 2009;338:1153–4.
- Stern RS. Psoralen and ultraviolet light therapy for psoriasis. N Engl J Med 2007;357:682–90.
- Sakuntabhai A, Diffey BL, Farr PM. Calculation of 8-methoxypsoralen dose according to body surface area in PUVA treatment. Br J Dermatol 1995;133:919–23.
- Halpern SM, Anstey AV, Dawe RS, et al. Guidelines for topical PUVA: a report of a Workshop of the British Photodermatology Group. Br J Dermatol 2000;142:22–31.
- Koulu LM, Jansen CT. Skin photosensitizing and Langerhans' cell depleting activity of topical (bath) PUVA therapy: comparison of trimethylpsoralen and 8-methoxypsoralen. Acta Derm Venereol (Stockh) 1983;63:137–41.
- Konya J, Diffey BL, Hindson TC. Time course of activity of topical 8-methoxypsoralen on palmoplantar skin. Br J Dermatol 1992;127:654–5.
- Ibbotson SH, Farr PM. The time-course of psoralen ultraviolet A (PUVA) erythema. J Invest Dermatol 1999;113:346–50.
- Buckley DA, Healy E, Rogers S. A comparison of twice-weekly MPD-PUVA and three times-weekly skin typing-PUVA regimens for the treatment of psoriasis. Br J Dermatol 1995;133:417–22.
- Collins P, Wainwright NJ, Amorim I, et al. 8-MOP PUVA for psoriasis: a comparison of a minimal phototoxic dose-based regimen with a skin-type approach. Br J Dermatol 1996;135:248–54.
- Sakuntabhai A, Sharpe GR, Farr PM. Response of psoriasis to twice weekly PUVA. Br J Dermatol 1993;128:166–71.
- Behrens-Williams S, Gruss C, Grundmann-Kollmann M, et al. Assessment of minimal phototoxic dose following 8-methoxypsoralen bath: maximal reaction on average after 5 days. Br J Dermatol 2000;142:112–15.
- Grundmann-Kollmann M, Leiter U, Behrens S, et al. The time course of phototoxicity of topical PUVA: 8-methoxypsoralen cream-PUVA vs. 8-methoxypsoralen gel-PUVA. Br J Dermatol 1999;140:988–90.
- Berne B, Blom I, Spangberg S. Enhanced response of psoriasis to UVB therapy after pretreatment with a lubricating base. A single-blind controlled study. Acta Derm Venereol (Stockh) 1990;70:474–7.
- Menkes A, Stern RS, Arndt KA. Psoriasis treatment with suberythemogenic ultraviolet B radiation and coal tar extract. J Am Acad Dermatol 1985;12:21–5.
- Carrozza P, Hausermann P, Nestle FO, et al. Clinical efficacy of narrow-band UVB (311nm) combined with dithranol in psoriasis. An open pilot study. Dermatology 2000;200:35–9.
- Koo JY. Tazarotene in combination with phototherapy. J Am Acad Dermatol 1998;39(4 Pt 2):S144–8.
- Woo WK, McKenna KE. Combination TL01 ultraviolet B phototherapy and topical calcipotriol for psoriasis: a prospective randomized placebo-controlled clinical trial. Br J Dermatol 2003;149:146–50.
- Speight EL, Farr PM. Calciotriol improves the response of psoriasis to PUVA. Br J Dermatol 1994;130:79–82.
- Lebwohl M, Quijije J, Gilliard J, et al. Topical calcitriol is degraded by ultraviolet light. J Invest Dermatol 2003;121:594–5.
- Schiffner R, Schiffner-Rohe J, Wolf G, et al. Evaluation of a multicentre study of synchronous application of narrowband ultraviolet B phototherapy (TL01) and bathing in Dead Sea salt solution for psoriasis vulgaris. Br J Dermatol 2000;142:740–7.
- Green C, Lakshmipathi T, Johnson BE, Ferguson J. A comparison of the efficacy and relapse rates of narrow-band UVB (TL01) monotherapy vs. etretinate (re-TL01) vs. etretinate-PUVA (re-PUVA) in the treatment of psoriasis patients. Br J Dermatol 1992;127:5–9.
- Nijsten TEC, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol 2003;49:644–50.
- Marcil I, Stern RS. Squamous cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort study. Lancet 2001;358:1042–5.
- Legat FJ, Hofer A, Wackernagel A, et al. Narrowband UV-B phototherapy, alefacept, and clearance of psoriasis. Arch Dermatol 2007;143:1016–22.
- Gambichler T, Tigges C, Scola N, et al. Etanercept plus narrowband ultraviolet B phototherapy of psoriasis is more effective than etanercept monotherapy at 6 weeks. Br J Dermatol 2011;164:1383–6.
- Wolf P, Hofer A, Weger W, et al. 311nm ultraviolet B-accelerated response of psoriatic lesions in adalimumab-treated patients. Photodermatol Photoimmunol Photomed 2011;27:186–9.
- Wolf P, Weger W, Legat FJ, et al. Treatment of 311-nm ultraviolet B-enhanced response of psoriatic lesions in ustekinumab-treated patients: a randomized intraindividual trial. Br J Dermatol 2012;166:147–53.
- Christensen I, Heald P. Photopheresis in the 1990's. J Clin Apheresis 1991;6:216–20.
- Heald P, Perez M, Gasparro F. Dosage guidelines: extracorporeal photochemotherapy (photopheresis). Arch Dermatol 1990;126:1369.
- Shephard SE, Nestle FO, Panizzon R. Pharmacokinetics of 8-methoxypsoralen during extracorporeal photopheresis. Photodermatol Photoimmunol Photomed 1999;15:64–74.
- Man I, Dawe RS, Ferguson J, Ibbotson SH. An intraindividual study of the characteristics of the characteristics of erythema induced by bath and oral methoxsalen photochemotherapy and narrowband ultraviolet B. Photochem Photobiol 2003;78:55–60.
- Laube S, George SA. Adverse effects with PUVA and UVB. J Dermatol Treat 2001;12:101–5.
- Perna JJ, Mannix ML, Rooney JF, et al. Reactivation of latent herpes simplex virus infection by ultraviolet light: a human model. J Am Acad Dermatol 1987;17:473–8.
- George SA, Ferguson J. Lesional blistering following narrow-band (TL-01) UVB phototherapy for psoriasis: a report of four cases. Br J Dermatol 1992;127:445–6.
- Pasker-de Jong PCM, Wielink G, van der Valk PGM, van der Wilt G-J. Treatment with UV-B for psoriasis and non-melanoma skin cancer. Arch Dermatol 1999;135:834–40.
- Stern RS and Members of the Photochemotherapy Follow-up Study. Genital tumours among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. N Engl J Med 1990;322:1093–7.
- Young AR. Carcinogenicity of UVB phototherapy assessed. Lancet 1995;345:1431–2.
- Weischer M, Blum A, Eberhard F, et al. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB photherapy: a first retrospective study. Acta Derm Venereol 2004;84:370–4.
- Man I, Crombie IK, Dawe RS, et al. The photocarcinogenic risk of narrowband UVB (TL01) phototherapy: early follow-up data. Br J Dermatol 2005;152:755–7.
- Hearn RMR, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol 2008;159:931–5.
- Ibbotson SH, Farr PM. The time-course of psoralen ultraviolet A (PUVA) erythema. J Invest Dermatol 1999;113:346–50.
- Behrens-Williams S, Gruss C, Grundmann-Kollmann M, et al. Assessment of minimal phototoxic dose following 8-methoxypsoralen bath: maximal reaction on average after 5 days. Br J Dermatol 2000;142:112–15.
- Morgan JM, Weller R, Adams SJ. Onycholysis in a case of atopic eczema treated with PUVA photochemotherapy. Clin Exp Dermatol 1992;17:65–6.
- Wolff K. Side-effects of psoralen photochemotherapy (PUVA). Br J Dermatol 1990;122:S117–25.
- Brickl R, Schmid J, Koss FW. Clinical pharmacology of oral psoralen drugs. Photodermatology 1984;1:174–86.
- Melski JW, Tanenbaum L, Parrish JA, et al. Oral methoxsalen photochemotherapy for the treatment of psoriasis: a cooperative clinical trial. J Invest Dermatol 1977;68:328–35.
- Tegner E. Severe skin pain after PUVA treatment. Acta Derm Venereol 1979;59:467–70.
- Van Praag MCG, Tseng LNI, Mommaas AM, et al. Minimising the risks of PUVA treatment. Drug Safety 1993;8:340–9.
- Roelandts R, Stevens A. PUVA-induced itching and skin pain. Photodermatol Photoimmunol Photomed 1990;7:141–2.
- Burrows NP, Norris PG. Treatment of PUVA-induced skin pain with capsaicin. Br J Dermatol 1994;131:584–5.
- Zamiri M, Bilsland D. Treatment of bath PUVA-induced skin pain with gabapentin. Br J Dermatol 2004;151:516–17.
- Norris PG, Maurice PD, Schott GD,, et al. Persistent skin pain after PUVA. Clin Exp Dermatol 1987;12:403–5.
- Kao CL, Krathen RA, Wolf JE, et al. Psoralen plus ultraviolet A-induced bullous pemphigoid. J Drugs Dermatol 2008;7:695–6.
- Jeanmougin M, Manciet JR, Castelneau JP, et al. [Systemic photoallergy induced by methoxalen.] Ann Dermatol Venereol 1992;119:277–80.
- Allen AL, Glaser DA. Disseminated superficial actinic porokeratoses associated with topical PUVA. J Am Acad Dermatol 2000;43:720–2.
- Nanda S, Grover C, Reddy BS. PUVA-induced lichen planus. J Dermatol 2003;30:151–3.
- Vernassiere C, Petitpain N, Trechot P, et al. 8-methoxypsoralen and neurological disorders: from dysosmia to migraine. Photodermatol Photoimmunol Photomed 2006;22:217–18.
- Takashima A, Yamamoto K, Kimura S, et al. Allergic contact and photocontact dermatitis due to psoralens in patients with psoriasis treated with topical PUVA. Br J Dermatol 1991;124:37–42.
- Park JY, Rim JH, Choe YB, et al. Anaphylaxis to 8-methoxypsoralen during photochemotherapy. Photodermatol Photoimmunol Photomed 2003;19:37–8.
- Matz H. Phototherapy for psoriasis: what to choose and how to use: facts and controversies. Clin Dermatol 2010;28:73–80.
- Van Praag MCG, Bouwes Bavinck JN, Bergman W, et al. PUVA keratosis. A clinical and histopathologic entity associated with an increased risk of nonmelanoma skin cancer. J Am Acad Dermatol 1993;28:412–17.
- Rhodes AR, Stern RS, Melski JW. The PUVA lentigo: an analysis of predisposing factors. J Invest Dermatol 1983;81:459–63.
- Rhodes AR, Harrist TJ, Momtaz TK. The PUVA induced pigmented macule: a lentiginous proliferation of large, sometimes cytologically atypical, melanocytes. J Am Acad Dermatol 1983;9:47–58.
- Lassacher A, Worda M, Kaddu S, et al. T1799A BRAF mutation is common in PUVA lentigines. J Invest Dermatol 2006;126:1915–18.
- Stern RS, Thibodeau LA, Kleinerman RA, et al. Risks of cutaneous carcinoma in patients treated with oral methoxsalen photochemotherapy for psoriasis. N Engl J Med 1979;300:800–13.
- Stern RS, Laird N, Melski J, et al. Cutaneous squamous cell carcinoma in patients treated with PUVA. N Engl J Med 1984;310:1156–61.
- Stern RS, Lange R. Non-melanoma skin cancer occurring in patients treated with PUVA five to ten years after first treatment. J Invest Dermatol 1988;91:120–4.
- Stern RS, Lange R. The carcinogenic risk of treatments for severe psoriasis. Cancer 1994;73:2759–64.
- Lindelof B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer: a large-scale epidemiological study. Lancet 1991;338:91–3.
- Lindelof B, Sigurgeirsson B, Tegner E, et al. Comparison of the carcinogenic potential of Trioxsalen bath PUVA and oral methoxsalen PUVA. Arch Dermatol 1992;128:1341–4.
- Lindelof B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol 1999;141:108–12.
- Stern RS; PUVA Follow-up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol 2012;66:553–62.
- Honigsmann H, Wolff K, Gschnait F, et al. Keratoses and non-melanoma skin tumours in long-term photochemotherapy (PUVA). J Am Acad Dermatol 1980;3:406–14.
- Tanew A, Honigsmann H, Ortel B, et al. Non-melanoma skin tumours in long-term photochemotherapy treatment of psoriasis. J Am Acad Dermatol 1986;15:960–5.
- Henseler T, Christophers E, Honigsmann H, et al. Skin tumours in the European PUVA study. J Am Acad Dermatol 1987;16:108–16.
- Mali-gerrits MG, Gaasbeek D, Boezeman J, et al. Psoriasis therapy and the risk of skin cancers. Clin Exp Dermatol 1991;16:85–9.
- Fitzsimons CP, Long J, MacKie RM. Synergistic carcinogenic potential of methotrexate and PUVA in psoriasis. Lancet 1983;29:235–6.
- Van De Kerkhof PCM, De Rooij MJM. Multiple squamous cell carcinomas in a psoriatic patient following high-dose photochemotherapy and cyclosporin treatment: response to long-term acitretin maintenance. Br J Dermatol 1997;136:275–8.
- Lim JL, Stern RS. High levels of ultraviolet B exposure increase the risk of non-melanoma skin cancer in psoralen and ultraviolet A-treated patients. J Invest Dermatol 2005;124:505–13.
- Sage E, Bredberg A. Damage distribution and mutation spectrum: the case of 8-methoxy-psoralen and UVA in mammalian cells. Mutat Res 1991;263:217–22.
- Peritz AE, Gasparro FP. Psoriasis, PUVA and skin cancer – molecular epidemiology the curious question of T–A transversions. J Invest Dermatol Symp Proc 1999;4:11–16.
- Friedmann P. Disappearance of epidermal Langerhans cells during PUVA therapy. Br J Dermatol 1981;105:219–21.
- Vella Briffa D, Parker D, Tosca N, et al. The effect of photochemotherapy (PUVA) on cell mediated immunity in the guinea pig. J Invest Dermatol 1981;77:377–80.
- Viander M, Jansen CT, Eskola J. Influence of whole body PUVA treatments on human peripheral blood NK cell activity. Photodermatology 1984;1:23–9.
- Roelandts R. Mutagenicity and carcinogenicity of methoxypsoralen plus UV-A. Arch Dermatol 1984;120:662–9.
- Young AR. Photocarcinogenicity of psoralens used in PUVA treatment: present status in mouse and man. J Photochem Photobiol Biol 1990;6:237–47.
- Wang XM, McNiff JM, Klump V, et al. An unexpected spectrum of p53 mutations from squamous cell carcinomas in patients treated with PUVA. Photochem Photobiol 1997;66:294–9.
- Seidl H, Kreimer-Erlacher H, Back B, et al. Ultraviolet exposure as the main initiator of p53 mutations in basal cell carcinomas from psoralen and ultraviolet A-treated patients with psoriasis. J Invest Dermatol 2001;117:365–70.
- Stern RS, Bolshakov S, Nataraj AJ, Ananthaswamy HN. p53 mutation in non-melanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol 2002;119:522–6.
- Hannuksela-Svahn A, Sigurgeirsson B, Pukkala E, et al. Trioxsalen bath PUVA did not increase the risk of squamous cell carcinoma and cutaneous malignant melanoma in a joint analysis of 944 Swedish and Finnish patients with psoriasis. Br J Dermatol 1999;141:497–501.
- Aubin F, Donawho CK, Kripke ML. Effect of psoralen plus ultraviolet A radiation on in vivo growth of melanoma cells. Cancer Res 1991;51:5893–7.
- Alcalay J, Bucana C, Kripke ML. Cutaneous pigmented melanocytic tumour in a mouse treated with psoralen plus ultraviolet A radiation. Photodermatol Photoimmunol Photomed 1990;7:28–31.
- Stern RS, Khanh TN, Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet radiation (PUVA). N Engl J Med 1997;336:1041–5.
- Stern RS and the PUVA Follow up Study. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol 2001;44:755–61.
- Bruynzeel I, Bergmann W, Hartevelt HM, et al. ‘High single-dose’ European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol 1991;124:49–55.
- McKenna KE, Patterson CC, Handley J, McGinn S, Allen G. Cutaneous neoplasia following PUVA therapy for psoriasis. Br J Dermatol 1996;134:639–42.
- Gelfand JM, Belin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol 2003;139:1425–9.
- Stern RS. Lymphoma risk in psoriasis. Results of the PUVA Follow-up Study. Arch Dermatol 2006;142:1132–5.
- Stern RS, Vakeva LH. Noncutaneous malignant tumors in the PUVA follow-up study: 1975–1996. J Invest Dermatol 1997;108:897–900.
- Lerman S, Megaw J, Willis I. Potential ocular complications from PUVA therapy and their prevention. J Invest Dermatol 1980;74:197–9.
- Lerman S, Jocoy M, Borkman RF. Photosensitization of the lens by 8-methoxypsoralen. Invest Ophthalmol Vis Sci 1977;16:1065–8.
- Van Praag MCG, Tseng LNL, Mommaas AM, Boom BW, Vermeer BJ. Minimising the risks of PUVA treatment. Drug Safety 1993;8:340–9.
- Stern RS, Parrish JA, Fitzpatrick TB. Ocular findings in patients treated with PUVA. J Invest Dermatol 1985;85:269–73.
- Stern RS. Ocular lens findings in patients treated with PUVA. Photochemotherapy Follow-up Study. J Invest Dermatol 1994;103:534–8.
- Malanos D, Stern RS. Psoralen plus ultraviolet A does not increase the risk of cataract: a 25-year prospective study. J Invest Dermatol 2007;57:231–7.
- Calzavara-Pinton PG, Carlino A, Manfredi E, et al. Ocular side effects of PUVA-treated patients refusing eye sun protection. Acta Derm Venereol Suppl 1994;186:164–5.
- Beattie PE, Dawe RS, Ferguson J, Ibbotson SH. Dose-response and time-course characteristics of UV-A1 erythema. Arch Dermatol 2005;141:1549–55.
- Mouret S, Philippe C, Gracia-Chantegrel J, et al. UVA- induced cyclobutane pyrimidine dimmers in DNA: a direct photochemical mechanism? Org Biomol Chem 2010;8:1706–11.
- Kielbassa C, Roza L, Epe B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997;18:811–16.
- Knobler RM, Trautinger F, Graninger W, et al. Parenteral administration of 8-methoxypsoralen in photopheresis. J Am Acad Dermatol 1993;28:580–4.
- Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev 2001;15:103–8.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol 2000;142:389–90.
- Niwa Y, Terashima T, Sumi H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. Br J Dermatol 2003;149:960–7.
- Deleu H, Roelandts R. Protecting the eye from ultraviolet A radiation during photochemotherapy. Photodermatol Photoimmunol Photomed 1990;7:233–6.
- Moseley H, Jones SK. Clear ultraviolet blocking lenses for use by PUVA patients. Br J Dermatol 1990;123:775–81.
- Moseley H, Perkins W. Clear eyewear for PUVA patients. Br J Dermatol 1992;127:657–78.
- Diffey BL. Factors affecting the choice of a ceiling on the number of exposures with TL-01 ultraviolet B phototherapy. Br J Dermatol 2003;149:428–30.