CHAPTER 22
Principles of Photodynamic Therapy
Sally Ibbotson1 and Kevin McKenna2
1Photobiology Unit, University of Dundee, Ninewells Hospital and Medical School, Dundee, UK
2Dermatology Department, Belfast Trust, Belfast, UK
What is photodynamic therapy?
The aim of all dermatology treatments is to clear diseased tissue, whilst sparing normal healthy skin. Photodynamic therapy (PDT) has three key components: tissue-localized photosensitizer, photochemical activation by light of appropriate wavelength(s) and oxygen, which together result in oxidative stress, inflammation and cell death [1]. Treatment for diseased tissue alone is achieved by photosensitizer localization and targeted light delivery, and it is this relative selectivity that has led to considerable interest in the clinical application of PDT. Systemic or topical photosensitization can be used but as most dermatological PDT is topical, this will be the focus of this chapter.
Interestingly, although many studies have investigated the mechanisms of effect of PDT in vitro, there is little known about what happens following topical PDT to human skin in vivo. The effects of PDT depend on the subcellular localization and concentration of photosensitizer, accessibility of light of the appropriate wavelength and oxygen availability, which will in turn be influenced by the treatment parameters used.
During PDT, the photosensitizer absorbs light, is photochemically activated and is converted to a higher energy singlet state; when it returns to the ground state, fluorescence occurs. Alternatively, the singlet state may transfer to the more stable triplet state. Both type I and type II photo-oxidative reactions occur: type I involves direct hydrogen and electron transfer from the triplet state of the photosensitizer to a substrate; type II occurs when electrons or energy are transferred to molecular oxygen in the ground state and singlet oxygen is generated. The generation of reactive oxygen species, in particular singlet oxygen, is thought to be important in terms of causing nuclear, mitochondrial and membrane damage and in initiating signalling pathways with subsequent gene transcription, pro-inflammatory changes, cell cycle arrest, apoptosis and necrosis.
In vivo studies have shown that prostaglandin E2, histamine and nitric oxide are released following topical PDT [2]. Activation of the tumour suppressor gene, p53, is thought to be less important following PDT than following UV irradiation [3]. Topical PDT is immunosuppressive in humans and reduces epidermal Langerhans cell numbers after exposure; paradoxically, despite its immunosuppressive effects, it is widely and effectively used for the treatment of skin malignancies and dysplasia [4, 5, 6]. Effects on the vasculature, including endothelial cell damage, vasoconstriction and vessel occlusion, are considered important for systemic PDT, where photosensitizer delivery is principally via the vasculature, but are less important for topical PDT, where there is local cutaneous uptake and metabolism of photosensitizer pro-drugs. The effects of PDT are considered to be mainly membrane-mediated, but DNA damage, strand breaks and mutagenesis can occur. To date, however, there is no evidence for a carcinogenic effect. Interestingly, topical cutaneous PDT may have an important role in delaying the development of actinic keratosis and invasive squamous cell carcinoma in organ transplant recipients.
History and background
The term ‘photodynamic reaction’ was first coined at the beginning of the 20th century following a series of experiments by a medical student of von Tappeiner, Oscar Raab, in which he observed amplification of the cytotoxic effects of acridine orange on protozoa in the presence of daylight. In 1903, the therapeutic effects of this photodynamic reaction were explored by Jesionek and von Tappeiner and they demonstrated the beneficial effects of topical eosin and sunlight or arc lamp exposure for a range of skin diseases, including skin cancers and lupus vulgaris.
The effects of systemic photosensitizers were investigated by Meyer-Betz in 1912. He injected himself with haematoporphyrin and, when subsequently exposed to daylight during a tram journey, he experienced an extreme cutaneous phototoxic reaction. Subsequent clinical studies using intramuscular haematoporphyrin and UV exposure showed some therapeutic effect in psoriasis. However, it was not until Dougherty and colleagues undertook key early clinical studies in the 1970s using purified haematoporphyrin derivative for PDT in patients with a range of skin tumours that the potential of PDT, with its selective phototoxic effects, was recognized as suitable for development as an anticancer treatment [7]. A purified haematoporphyrin preparation is now approved for use in systemic PDT for several internal malignancies, although not for skin cancers.
An ideal photosensitizer needs to accumulate in the target tissue, absorb light at clinically relevant wavelengths, be photochemically activated efficiently and have rapid clearance and minimal dark toxicity. Several chemicals have been investigated for their potential in systemic PDT: these include porphyrins, chlorins, porphines, phthalocyanines and texapyrins. The principal systemic photosensitizers in current use for PDT are porfimer-sodium (Photofrin®) for lung and oesophageal cancer and temoporfin (tetrakis(hydroxymethyl)phosphonium chloride; Foscan®) for head and neck squamous cell carcinoma. Both are associated with prolonged photosensitivity to visible light over several weeks and there is also a risk of phototoxicity occurring at sites of injection if extravasation occurs. The chlorin-derived photosensitizer, BPD-MA (Verteporfin®), can be used for systemic PDT for wet macular degeneration.
One of the main factors limiting the use of systemic PDT in dermatology is that patients are generally well and the prolonged visible light photosensitivity associated with systemic drug use is not desirable. Furthermore, systemic 5-aminolaevulinic acid (ALA) may cause gastrointestinal upset and hepatotoxicity, and so is rarely used in dermatology. Systemic PDT has, however, been used in Gorlin naevoid basal cell carcinoma (BCC) syndrome, where there are multiple lesions requiring simultaneous treatment, and for treating larger areas, for example for extensive vulval intraepithelial neoplasia [8, 9].
The accessibility of skin led to further investigation of the use of topical photosensitization for PDT. In 1990, Kennedy reported the successful use of ALA as a pro-drug for PDT for malignant and pre-cancerous skin lesions [10, 11]. Topical ‘porphyrin’ PDT is now widely incorporated into dermatology services such that dermatologists involved in the management of patients with skin cancer and pre-cancers should have access to a PDT service [12, 13, 14–17, 18].
Photosensitizers used for photodynamic therapy in dermatology
Topical ‘porphyrin’ PDT employs the principles of the haem cycle (Figure 22.1). A non-photosensitizing pro-drug is used. The licensed pro-drugs in current use are ALA (Levulan® Kerastick®, Ameluz® and Alacare®) and the methyl ester of ALA, methyl aminolevulinate (MAL) (Metvix®/Metvixia®), which penetrate the epidermis due to their low molecular weight. Following the application of pro-drugs to a skin lesion (usually a superficial non-melanoma skin cancer or dysplastic lesion) and uptake, the rate-limited step of ALA synthase is bypassed and, due to a second rate-limited step at ferrochelatase, protoporphyrin IX (PpIX) accumulates in the target tissue. PpIX is a potent photosensitizer expressed in all mammalian nucleated cells at very low sub-photosensitizing levels. When it is present in increased amounts, however, it is a potent and efficient photosensitizer, which can be activated by light of the appropriate wavelengths. It has a short half-life and is cleared with 24–48 h.
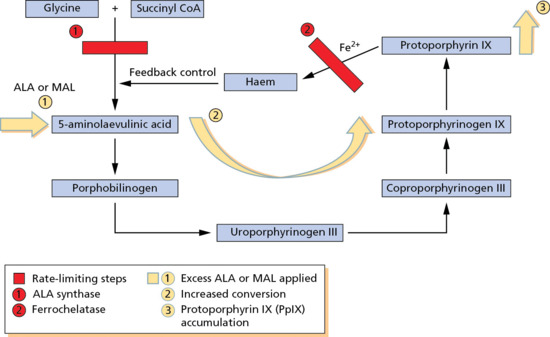
Figure 22.1 ‘Porphyrin’ photodynamic therapy uses the haem cycle. ALA, 5-aminolaevulinic acid; CoA, co-enzyme A; MAL, methyl aminolevulinate.
PpIX has a characteristic crimson red fluorescence when exposed to UVA (Wood's light), a phenomenon that can be used diagnostically, for example in the delineation of skin tumour margins (Figure 22.2) [19, 20, 21], or, when ALA is administered systemically, for the diagnosis of gastrointestinal malignancies or dysplasia. When ALA and MAL are applied topically the processes involved in the preferential uptake of PpIX by diseased skin are poorly understood. Surface measurements 6 h after topical ALA application show that PpIX fluorescence in non-melanoma skin cancer is up to 15 times more intense than in adjacent normal skin. Although this does not necessarily fully reflect deep accumulation of the photosensitizer, PpIX does in general reach higher concentrations in tumour tissue than in the surrounding skin (Figure 22.2) [22]. It is likely that altered permeability of the stratum corneum overlying a skin cancer or pre-cancer facilitates the uptake and conversion of these small molecules. Whereas ALA is hydrophilic, MAL is lipophilic and was introduced with the aim of increasing uptake and specificity for diseased skin. Esterases are, however, required to remove the methyl chain from MAL prior to conversion to PpIX and overall there is no convincing evidence to support any major differences in efficacy between ALA PDT and MAL PDT in clinical studies in humans.
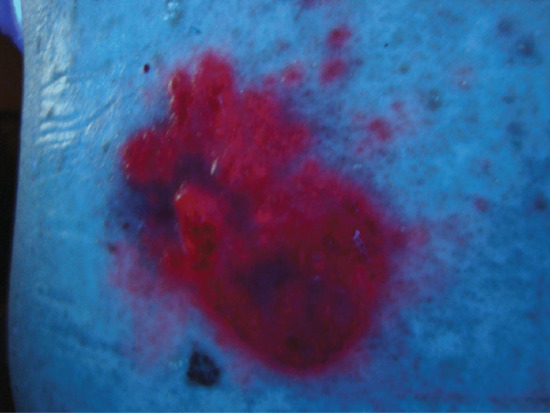
Figure 22.2 Crimson red protoporphyrin IX fluorescence in a superficial basal cell carcinoma 3 h after methyl aminolevulinate application, showing the tumour specificity and adjacent tumour islands that need to be included in the irradiation field.
There may be other factors involved in determining the relative specificity of PpIX accumulation in diseased skin. In vitro studies have led investigators to propose that relative iron deficiency, altered haem cycle enzyme expression, change in pH and state of cell differentiation in diseased tissue may each influence this, although the actual mechanisms in vivo are unknown and, importantly, are not specific to tumour cells. This is demonstrated by relative PpIX accumulation after pro-drug application in benign hyperproliferative conditions, such as psoriasis and viral warts.
Many of the earlier published studies of topical PDT used non-licensed proprietary preparations of ALA. However, approved licensed preparations of both ALA and MAL are now available. MAL is available as Metvix/Metvixia (16%, Galderma) for PDT and is used for actinic keratoses (AKs) on the face and scalp, Bowen disease and superficial BCC (and also for nodular BCCs when other available treatments are considered less suitable). The use of MAL for these indications has been approved by the licensing authorities of most European countries and a significant number of countries elsewhere (e.g. Australia, South Africa, Canada, Brazil), though not in the USA.
A preparation of ALA in solution (Levulan Kerastick; 20%, DUSA Pharmaceuticals) is approved by the US Food and Drug Administration (FDA) for PDT of mild to moderate AKs on the face and scalp [23]. More recently, ALA in a nanocolloid emulsion (Ameluz; 8%, Biofrontera) has been approved: this has been shown to result in an increased depth of PpIX accumulation after pro-drug application when compared with MAL in a porcine skin model ex vivo [24]. Ameluz PDT is licensed for use in thin to moderately thick AKs of the face and scalp [25]. In addition, an ALA patch (Alacare; 8 mg, 2 mg/cm2, Spirig HC) is licensed for use in several European countries for PDT for thin AKs on the face and scalp [26]. Other developments in the formulations for pro-drug delivery include the use of penetration enhancers or iron chelators, such as dimethyl sulfoxide (DMSO), ethylenediaminetetra-acetic acid (EDTA), desferrioxamine or the hydroxypyridinone, CP94. There is, however, no convincing evidence of improved PDT efficacy to date and these are mainly still at the experimental stage [27, 28, 29, 30]. Physical methods to improve pro-drug uptake are also under investigation and include laser pre-treatment or microneedling [31, 32, 33].
Light sources
The majority of photosensitizers used in PDT absorb maximally in the visible waveband. The absorption of porphyrins is broad, with peak absorption in the Soret band around 410 nm and several other smaller peaks between 500 and 650 nm (Figure 22.3). For PDT to be effective the photosensitizer needs to be activated within the target tissue. Although short wavelengths in the blue/violet range of the visible spectrum are the most potent at activating PpIX in vitro, tissue penetration in vivo is limited to 1–2 mm. Photochemical activation of PpIX by red light at 630–635 nm is, however, still significant and red light has the advantage of penetrating tissue to a depth of 6–7 mm. For this reason these longer wavelengths are the ones most commonly used in PpIX-based PDT. The exception to this is the approved regimen for PDT for AKs in the USA using Levulan Kerastick and a blue light-emitting diode (LED) light source (BLU-U®). This provides sufficient efficacy for these superficial lesions but would be unlikely to offer adequate depth of effect for superficial BCC or Bowen disease [23].
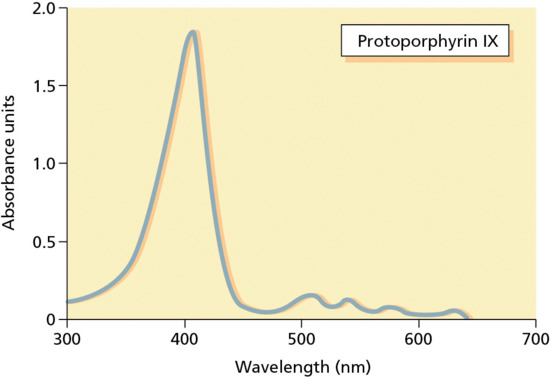
Figure 22.3 Absorption spectrum of protoporphyrin IX.
Historically, either filtered photographic slide projectors or lasers were used as light sources. In the limited studies that have been undertaken, there is no evidence that the efficacy of topical PDT is superior if a laser rather than a polychromatic light source is used [30, 34]. Indeed, there are theoretical grounds for supposing that polychromatic sources might be more effective given that there are additional PpIX photoproducts that have absorption peaks around 670 nm.
A range of polychromatic light sources has been used in PDT, including filtered tungsten filament, metal halide and short arc xenon sources with peak emission in the 630–635 nm waveband [35, 36, 37]. The field size, irradiance, uniformity of irradiation and treatment times vary considerably between the different types. Laser sources, including dye lasers and more recently the more compact and cheaper diode lasers, again using the 630–635 nm wavelengths, have also been employed. Intense pulsed light sources may also be successfully used for PDT.
In recent years, the emergence of LEDs for PDT has resulted in the availability of compact, cheap and easy to maintain sources of relatively uniform irradiance, which can be used to treat field diameters of up to approximately 20 cm. These LED sources have more therapeutically weighted emission spectra that are narrower than other polychromatic sources and thus deliver light more efficiently. In order to avoid thermal effects, light energy no greater than 50 mW/cm2 should be delivered to the target. There is indeed some evidence from ex vivo studies that very low irradiance may improve PDT efficiency. The efficiency of different light sources for PDT can be considered in the concept of total effective fluence, which takes into account incident spectral irradiance, optical transmission through tissue and photosensitizer absorption [38].
It is difficult to compare the results of published studies as there is wide variation in the treatment parameters used in PDT: these include variables in light delivery, such as the light source, irradiance and dose used. Although high irradiance light delivery reduces treatment times, this may reduce PDT efficiency as a result of rapid oxygen depletion. On the other hand, fractionation of light delivery as an alternative to low irradiance light delivery might also be therapeutically beneficial [39, 40, 41, 42, 43]. The timing of light delivery to coincide with peak photosensitizer accumulation is also important and, for topical PDT, most regimens typically include irradiation 3–6 h after pro-drug application. Blue or green light may be effective for AKs but the depth of effect will limit its use for most other conditions. Regular calibration of light sources is essential for light delivery during PDT, as lack of uniformity of irradiation may lead to inaccuracy in dose delivery and consequently suboptimal treatment.
Developments in light delivery include the use of very low irradiance LED sources in portable ambulatory PDT, and early clinical data are encouraging. Furthermore, daylight can be used for PDT and this is a convenient way for patients to treat themselves at home, although the depth of PDT effect may be limited by the fact that most of the therapeutically effective wavelengths in daylight are in the blue light spectrum. Other developments in light delivery include the use of conformable light-emitting polymers and fabrics for lesions on curved body regions, and even use of red light LEDs originally designed for road traffic controls [44].
Indications
The main indications for topical PDT are AKs, intraepithelial carcinoma (Bowen disease) and superficial basal cell carcinoma (sBCC); its role in other skin diseases is not yet fully established [12, 14–17, 45, 46, 47, 48]. There is good evidence to support the use of topical PDT in the management of AK, Bowen disease and sBCC. It is highly effective in selected cases and, because of its relative sparing of normal tissue, it can be used for multiple large ‘field’ areas and at sites of poor healing such as below the knee. Topical PDT is non-invasive, undertaken on an out-patient basis and treatment can be repeated without cumulative risk [13].
Actinic keratosis
Topical PDT is effective for thin- and moderate-thickness AKs on the face and scalp using either MAL (Metvix/Metvixia) or ALA (Levulan Kerastick, Ameluz or non-licensed ALA preparations). Reported clearance rates (as assessed at 3 months after treatment) are in the region of 90% – whilst these are at least as good as those with liquid nitrogen cryotherapy or topical 5-fluorouracil, the cosmetic outcome is superior (Figure 22.4) [12, 14, 15, 17, 49, 50]. There is, however, a relatively high recurrence rate of up to 19% at 1 year [51].
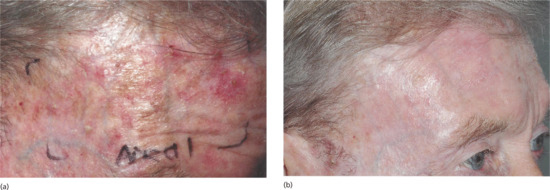
Figure 22.4 Field treatment of actinic keratoses on the forehead and temple (a) before and (b) 3 months after a single treatment of topical photodynamic therapy.
A head-to-head comparison of Ameluz, Metvix and placebo PDT for thin- to moderate-thickness facial and scalp AKs showed superior efficacy of Ameluz PDT, particularly if a red light LED source was used, with sustained responses at 1 year [52, 53]. Three months following completion of treatment with Ameluz or Metvix PDT, lesion clearance rates were 90% and 83% respectively, with sustained clearance of 53.1% and 40.8% at 1 year reported. There were no significant differences in adverse effects [52, 53].
In an intra-subject comparison study, ALA PDT was more effective than imiquimod for moderate-thickness AKs and was equivalent to imiquimod for thin AKs on the upper limbs, although patient preference favoured PDT [54]. In a second similar study in patients with AKs, in which patient tolerance and satisfaction with treatment were the main end points, both imiquimod and PDT were considered to be acceptable treatments for AK but patient satisfaction was higher with PDT [55].
Patch ALA PDT (Alacare; Spirig HC) has been shown to be superior to cryotherapy for mild AK of the face and scalp but is limited to a maximum of six single AKs and is not suitable for diffuse actinic damage (‘field change’) [26, 56].
A single PDT treatment may be insufficient and repeat treatment has been shown to improve response rates, particularly for moderate-thickness AKs [57]. The conventional topical PDT treatment regimen for AK thus involves a single treatment, and if this does not achieve clearance at 3 months then a double treatment cycle with treatments 1 week apart would be administered, although this is not usually required for thin AK [57]. It should be noted that the response to PDT of thick hyperkeratotic AKs, particularly those on acral sites, is disappointing and may be less than 50%. In one study, MAL PDT was less effective than cryotherapy for acral AKs [58].
PDT is ideally suited for field treatment of actinically damaged skin; it allows large areas of subclinical disease to be treated and can be particularly advantageous at sites of poor healing, such as the lower leg, or where cosmetic outcome is a significant concern. Furthermore, PDT has an important role in the management of patients at high risk of invasive squamous cell carcinoma (SCC) – including those who are heavily photodamaged or have a history of multiple SCCs – or patients who require immunosuppressive medication (e.g. organ transplant recipients) or who are otherwise immunosuppressed. In addition to clearing existing AKs, PDT can reduce the rate of development of new AKs; there may also be a reduction in the risk of developing SCC, although this needs further study [59, 60, 61, 62, 63, 64, 65, 66, 67]. PDT is more effective than 5-fluorouracil for treating AKs in organ transplant recipients [68].
PDT has been used for actinic cheilitis and can be useful at this difficult treatment site, although recurrence is common: a combined approach using topical imiquimod in addition has been proposed for resistant cases [69, 70]. PDT has a particular role for multiple, superficial lesions and for lesions occurring at sites of poor healing and should also be considered if other treatments have failed.
Topical PDT is thus ideal for widespread non-hyperkeratotic AKs on the face and scalp. MAL (Metvix/Metvixia) and ALA (Ameluz) PDT are licensed for this indication (see Figure 22.4). There are wide variations in the treatment protocols that have been used, although a 3–4 h interval between pro-drug application and irradiation is most commonly advocated. The Metvix/Metvixia and Ameluz PDT regimens specify a 3 h interval. The Alacare patch is applied for 4 h and does not need prior surface preparation or additional occlusion.
Red, blue and green light PDT may be effective for superficial AK. Blue light PDT using a topical solution of ALA (Levulan Kerastick) applied for 14–18 h without occlusion and a light dose of 10 J/cm2 is approved in the USA for the treatment of thin- to moderate-thickness AKs [23]. However, most approved topical PDT regimens for AK using MAL or ALA employ red light at a dose of 37.5 J/cm2.
Bowen disease
Topical MAL (Metvix/Metvixia) PDT using red light LED irradiation at 37.5 J/cm2 is licensed for Bowen disease with two treatments 1 week apart, repeated at 3 months if there is only partial response. Red light irradiation is required to achieve sufficient penetration of light for PDT: green light PDT has been shown to be of inferior efficacy for Bowen disease. Based on clearance assessed 3 months after the last treatment, response rates of 86–93% can be expected, with sustained remission of 68–71% at 2 years (Figure 22.5) [12, 14, 15, 17]. Topical PDT has been shown in two separate studies to be at least as effective for Bowen disease as cryotherapy and topical 5-fluorouracil but to be associated with fewer adverse effects and improved cosmetic outcome [71, 72].

Figure 22.5 Bowen disease on the lower leg (a) before and (b) 1 year after photodynamic therapy (PDT). Stasis change, oedema and xerosis were prominent and although the risk of poor healing was significant, this patient had no problems after PDT.
PDT is the treatment of choice for large or multiple areas of Bowen disease, particularly on poor healing sites such as the lower legs (Figure 22.5). It can also be used to treat other difficult sites such as the digits or genitalia. Response rates are reduced if there is evidence of microinvasive SCC or a high degree of cellular atypia. PDT is not indicated for invasive SCC because of the high risk of recurrence and the potential for metastasis.
Basal cell carcinoma
The same regimen of MAL PDT as used for Bowen disease is licensed for treating sBCC, using a double treatment cycle of two treatments 1 week apart, with repeat treatment at 3 months if necessary (Figure 22.6) [12, 14, 15, 17]. Clearance rates of 92–97% for sBCC treated with MAL PDT are reported at 3 months follow-up after one or two treatments [17, 73, 74]. Weighted clearance rates of 87% for sBCC and 53% for nodular BCC at follow-up intervals of up to 36 months following PDT were reported in one review of 12 studies [75].
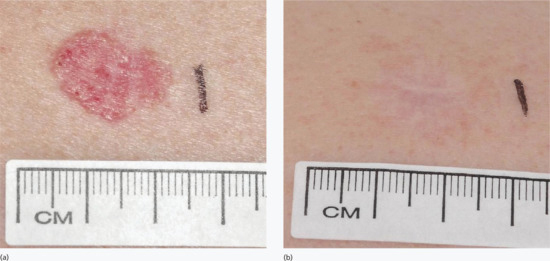
Figure 22.6 Superficial basal cell carcinoma on the back (a) before and (b) 1 year after photodynamic therapy. Note the excellent cosmetic outcome, with only the scar of the initial diagnostic biopsy evident.
In comparisons between cryotherapy and PDT, there were no differences in response rates for sBCC but the cosmetic outcome was superior with PDT [76]; similar 5-year recurrence rates of 22% for PDT and 20% for cryotherapy were observed in one study [73]. In another study comparing topical MAL PDT with 5-fluorouracil or imiquimod for sBCC (n = 601), PDT was inferior to imiquimod as assessed by tumour clearance at 12-month follow-up but equivalent in efficacy to topical 5-fluorouracil [77]. However, only patients who would be able to comply with the topical cream regimens were included and only a single cycle of PDT was used, which is suboptimal and not as described in its licensed protocol. More local adverse effects were seen with imiquimod and 5-fluorouracil, and three patients randomized to the topical creams developed local wound infection. Thus, although the results of this study are important, in practice many patients are unable to use or tolerate imiquimod or 5-fluorouracil and we do not know whether the same outcome would have been seen if a second treatment cycle of PDT had been undertaken. Each patient therefore needs to be evaluated on an individual basis in terms of what treatment approach is likely to suit best.
Equivalent efficacy has been shown for PDT and surgery for sBCC at 1 year, although with improved cosmetic outcome with PDT [74, 78]. Topical PDT is inferior to surgery for nodular BCC, with increased recurrence rates at 5-year follow-up [79, 80, 81, 82, 83]. This is likely to be due to a combination of inadequate PpIX accumulation in deeper tumour cells and poor light penetration. PDT should be reserved for thin BCCs with histological thickness <2 mm [84]. Only if surgery is contraindicated should PDT be considered for nodular BCC, as is reflected in the licensed approval for MAL (Metvix/Metvixia) PDT [85]. If topical PDT is used for nodular BCC, then prior debulking of the nodular component by curettage would be advised. Use of penetration enhancers, microneedling, laser pre-treatment, iron chelators and fractionated regimens have been investigated in an attempt to improve efficacy, but there is no doubt that PDT is inferior to surgery for the treatment of nodular BCC.
There is poor uptake of PpIX by morphoeic (sclerosing) BCCs after ALA or MAL pro-drug application. They have been shown not to be responsive to topical PDT and the presence of significant morphoeic (sclerosing) changes on histology would represent a contraindication to PDT.
Systemic PDT can be considered for high-risk patients such as those with the naevoid BCC (Gorlin) syndrome, although patients must be counselled about the prolonged visible light photosensitivity associated with systemic PDT.
Other indications
Acne.
Increasing evidence supports the value of topical PDT for inflammatory acne vulgaris in patients for whom more conventional therapies are ineffective or not possible [16, 46, 86, 87, 88, 89, 90]. Response rates of 54–68% reduction in inflammatory lesions may be expected but the adverse effects from treatment, including florid phototoxicity and the development of multiple sterile pustules, can be significant.
Viral warts and HPV-related neoplasia.
Topical PDT may also be effective for recalcitrant viral warts with response rates of approximately 50%, although treatment is often painful and can be difficult to tolerate [91]. Topical PDT has also been reported to be effective for genital warts [16, 92, 93, 94, 95, 96], Bowenoid papulosis [97], vulval intraepithelial neoplasia [15, 93, 98, 99, 100] and penile intraepithelial neoplasia (erythroplasia of Queyrat) [15, 101, 102, 103, 104] although its role in these conditions has not been established [15].
Other forms of cutaneous neoplasia.
There are individual case reports of response to PDT in extramammary Paget disease [105, 106, 107, 108] and localized cutaneous T-cell [109, 110, 111] and B-cell lymphoma [112].
Aesthetic dermatology.
PDT has been advocated for photorejuvenation, although further work is required to establish its place in this arena [16, 113].
Miscellaneous.
There are also reports of efficacy of topical PDT in several other inflammatory and infective skin conditions including cutaneous leishmaniasis, onychomycosis, leg ulcers, localized scleroderma, cutaneous sarcoid, lichen sclerosus, lichen planus, necrobiosis lipoidica and granuloma annulare (Box 22.1). Further studies are required to establish the role of PDT in these conditions [16, 46, 114, 115, 116, 117, 118, 119, 120]. On the other hand there is evidence to suggest that PDT is ineffective for psoriasis [121, 122, 123] and for porokeratosis [124, 125].
Contraindications
Topical PDT is contraindicated for disease with metastatic potential such as invasive SCC or melanoma. Importantly, in highly pigmented lesions such as melanoma or heavily pigmented BCC, red light absorption by melanin may reduce efficacy of treatment. Topical PDT is not advisable for thick tumours such as thick nodular BCC or for morphoeic (sclerosing) BCC. Patients with porphyria or xeroderma pigmentosum should not be treated with PDT, and there is evidence in the latter condition of prolonged phototoxicity with PDT. Tumours at high-risk sites such as the mid-face should also not be considered for topical PDT unless a more conventional surgical approach is contraindicated. Under those circumstances, PDT may prove efficacious for those difficult-to-treat lesions, although it would not be the treatment of choice.
Methodology and regimens
Diagnosis and patient selection
A number of factors need to be taken into account when deciding whether to use PDT to treat a specific disorder in a particular patient. PDT is ideally suited for elderly, frail patients who are able to attend for treatment as opposed to those who would prefer to be involved in the administration of their own treatment with, for example, topical 5-fluorouracil or imiquimod. PDT is a particularly good treatment choice for multiple and/or large low-risk (and therefore generally thin) lesions, diffuse field change at sites where healing may be problematic, such as the lower leg, or where cosmetic outcome is important (Figures 22.7 and 22.8).
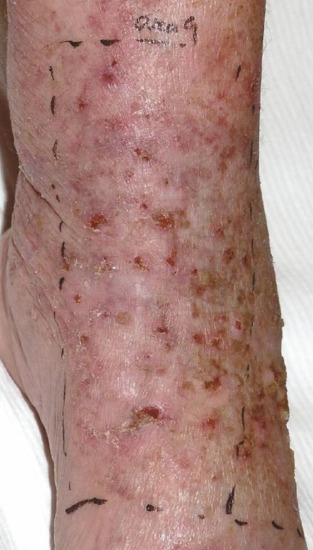
Figure 22.7 Photodynamic therapy (PDT) is ideal for multiple and/or large lesions and field change. This patient had extensive actinic keratosis, Bowen disease and field carcinogenesis following chronic sun exposure and was keen to obtain good healing and cosmetic outcome. She was therefore an ideal candidate for PDT.
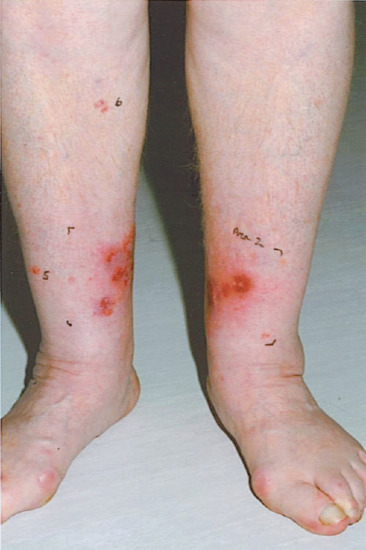
Figure 22.8 Photodynamic therapy (PDT) is ideal for lower leg sites as the risk of poor healing and ulceration is reduced. This patient has lower leg oedema, stasis changes and multiple areas of Bowen disease. PDT would be the treatment of choice.
Detailed history taking and clinical examination are required to establish patient co-morbidities that may be relevant: these include diabetes, immunosuppression, peripheral venous insufficiency and dependent oedema, all of which may impair healing following PDT. Immunosuppression may also increase the risk of recurrence. If there is significant dependent oedema, compression stockings may be advised in order to facilitate healing following PDT at lower leg sites.
A representative confirmatory biopsy is usually undertaken. If a BCC has a histological thickness of >2 mm, PDT is generally contraindicated. The site and size of the lesion should be assessed and baseline photography undertaken, as cosmetic outcome following clearance can be such that it may be difficult to identify where the lesion was.
It is important that patients are provided with verbal and written information about treatment: some centres obtain written consent. Topical PDT generally takes place in dermatology departments and involves the patient in a half-day visit. It is usually undertaken by a nurse or technician, who must be provided with adequate training [126].
Lesion preparation
Lesion preparation is important if there is marked hyperkeratosis or crusting. The experimental use of laser pre-treatment, microneedling and penetration enhancers such as DMSO and iron chelators has been investigated with the intention of improving pro-drug uptake and PpIX accumulation. In practice, many centres simply advise the application of white soft paraffin (Vaseline®) to lesions for 1–3 days prior to treatment in order to soften hyperkeratosis and crusting and to facilitate subsequent lesion preparation immediately prior to PDT [27, 28, 29, 30, 31, 32, 33]. This involves using either a wooden spatula or a disposable ring curette to remove surface keratinous débris (Figure 22.9) and does not usually require local anaesthetic.
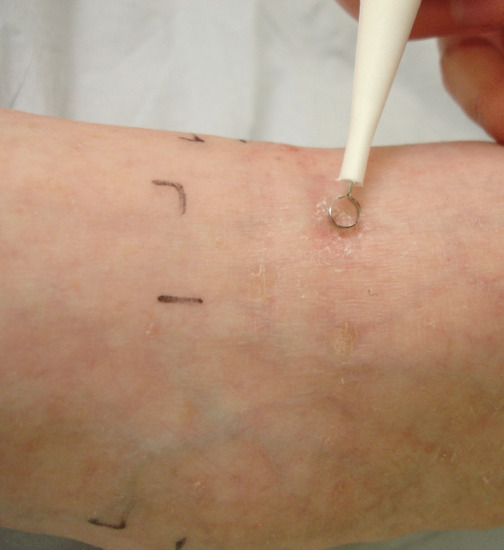
Figure 22.9 Lesion preparation using a disposable ring curette.
Pro-drug application
The pro-drug is applied after surface preparation, and in practice is usually MAL (Metvix/Metvixia 16%), ALA (Ameluz 8%) or other non-licensed proprietary ALA preparations (usually 20% w/v) (Figure 22.10). The pro-drug is applied under plastic film occlusion (e.g. Tegaderm®) as a thin (c. 1 mm thick) even application to the lesion including a rim of at least 5–10 mm of normal appearing skin (Figure 22.11). Metvix and Ameluz should be occluded for 3 h prior to irradiation; it is usually recommended that other preparations of ALA be applied 4–6 h in advance, although regimens with application times ranging from 15 min to 18 h are reported [127].
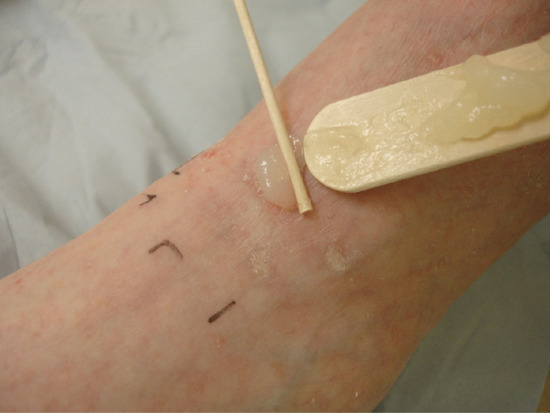
Figure 22.10 Pro-drug application.
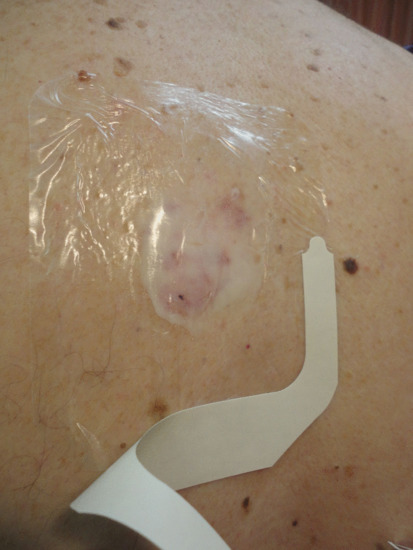
Figure 22.11 Pro-drug occlusion under Tegaderm. An additional UV/visible light opaque dressing would also be applied if the lesion is on an exposed site.
If the lesion to be treated is on a light-exposed site, a UV and visible light opaque dressing (e.g. Mepore®) should be applied on top of the occlusive film dressing so that PpIX photobleaching and the PDT reaction do not commence prematurely. The ALA patch (Alacare, Spirig HC) PDT regimen does not require surface preparation or additional occlusion. PDT using Levulan Kerastick does not involve occlusion but the treatment site should be protected from sun and bright light.
Following the pro-drug incubation period, the dressings should be removed and any residual cream wiped from the surface. Wood's light can be used to identify PpIX fluorescence, which is usually largely limited to the lesional site but can help reveal adjacent areas of subclinical disease which should then be included in the treatment field. A rim of 5 mm of clinically normal skin should be included in the irradiation field (Figure 22.12).
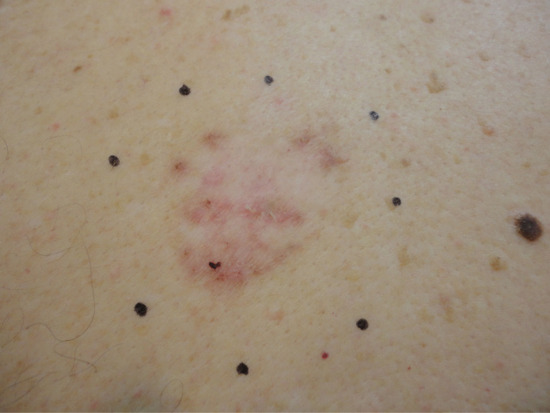
Figure 22.12 Mapping out the irradiation field to include a 5 mm rim of clinically uninvolved skin.
Irradiation
Irradiation may be performed using a variety of light sources, though the most commonly used are red light LED sources with peak emissions around 632–635 nm (e.g. Aktilite® or RhodoLED®) (Figure 22.13). The irradiance emitted by the source must be monitored and for LEDs this is usually approximately 80 mW/cm2. The approved dose for use in MAL and ALA (Ameluz) PDT is 37.5 J/cm2, although some centres use a higher dose (e.g. 75 J/cm2), as there are experimental data to indicate that increased PpIX photobleaching may continue if higher doses are used.
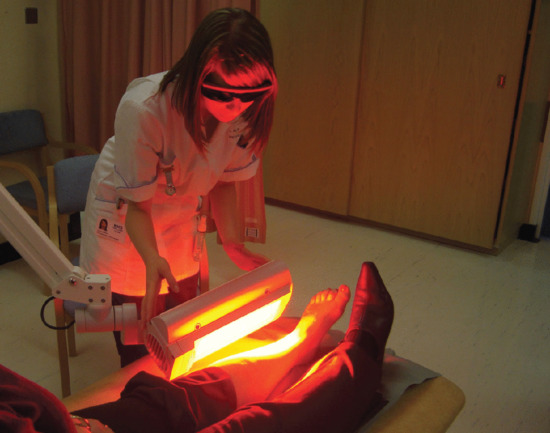
Figure 22.13 Photodynamic therapy to the lower leg using a red LED source.
Historically, when polychromatic or laser light sources were used, irradiation doses of 125–200 J/cm2 or more could be utilized, although it is important that irradiance is kept below 150 mW/cm2 to avoid additional thermal effects. There is no evidence that these higher light doses are associated with improved outcomes. Likewise, as only low levels of PpIX accumulate in adjacent normal skin, red light toxicity to the surrounding skin is not a concern.
The time for irradiation using the LED sources is generally 8–17 min dependent on the dose and irradiance. A lesional field diameter of up to 20 cm2 can be treated with standard PDT using either LED or metal halide sources (e.g. Waldmann 1200®).
Ambulatory photodynamic therapy
There is good evidence ex vivo, and increasing evidence in vivo, that light delivery at lower irradiance results in more efficient PDT and reduced pain during irradiation [128, 129, 130, 131]. Ambulatory PDT employs this principle of very low irradiance light delivery over a prolonged time period [129, 132, 133]. A small lightweight portable red LED array (peak emission 633 nm (lower–upper full width at half maximum 624–639 nm) at very low irradiance (approximately 7 mW/cm2) is used for irradiation (Ambulight®, Ambicare Health Ltd).
In the ambulatory PDT regimen the lesions are assessed and prepared as for conventional PDT. The pro-drug, which is MAL in studies to date, is applied to the lesion and occluded (Tegaderm) and the LED device (Ambulight) is immediately secured in place using adhesive over the lesion site. The device is programmed to remain switched off for 3 h whilst PpIX accumulates and the patient can go home as soon as the device is secured in place. It is operated by a battery pack, which can be held in the pocket or attached to a belt so the patient can go about their normal daily activities. After 3 h the device will switch on and a total dose of 75 J/cm2 is delivered over a 3 h period (Figure 22.14). The patient then removes the device, wipes the residual cream from the surface and returns the battery pack and disposable head to the clinic. At present the devices are of a size that permits treatment of lesions up to 2.4 cm diameter, but larger devices are likely to become available in the future.
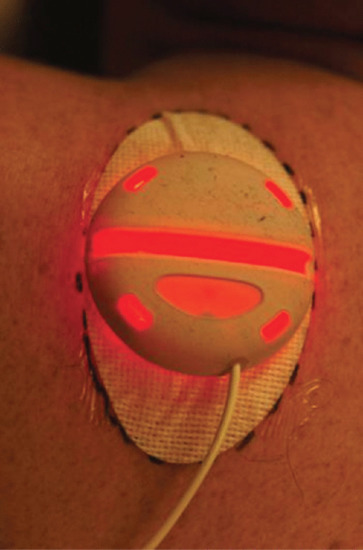
Figure 22.14 Ambulatory photodynamic therapy. Irradiation is underway, as indicated by the red light emission from the portable inorganic LED source (Ambulight).
To date, early studies with ambulatory PDT are encouraging in terms of low pain scores during treatment and 1-year efficacy rates of approximately 84% for Bowen disease and sBCC [134]. However, the evidence base is limited at present, and the results of a prospective randomized comparative study are awaited. The UK's National Institute for Health and Care Excellence (NICE) has evaluated the procedure at this early stage in its development and, whilst not giving formal approval, did state that it was an option for PDT.
Daylight photodynamic therapy
Daylight PDT is an alternative way to use low irradiance light delivery during PDT. This has the advantage of convenience for the patient and larger areas can be treated in one session (Figure 22.15). This has been explored for treatment of AKs on the face and scalp [135, 136, 137, 138, 139]. The therapeutically effective wavelengths of daylight are mainly in the blue light part of the spectrum and daylight PDT may not have sufficient depth of effect to treat lesions of Bowen disease or BCC. However, preliminary work has indicated that it may be possible to treat these with daylight PDT [140]. Formal prospective studies are required to judge whether indeed this is the case.
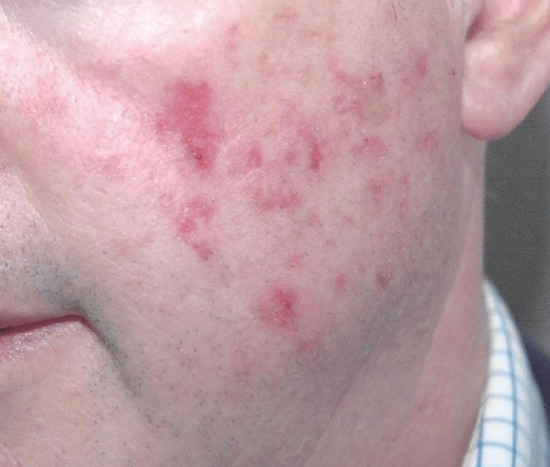
Figure 22.15 Daylight photodynamic therapy (PDT) allows field areas of superficial actinic keratosis on exposed sites to be treated in one session. This man had daylight PDT to the face and scalp and this shows the typical reaction 3 days after treatment with subclinical areas of disease becoming more apparent.
In the daylight PDT regimen for facial and scalp AKs, SPF50 absorbent sunscreen is applied to all sites, both the lesional areas to be treated and the unaffected normal skin that will be exposed to daylight, in order to prevent the development of UV erythema (sunburn). Fifteen minutes after sunscreen application, the AKs are prepared using a disposable ring curette. The photosensitizer pro-drug (MAL in the published studies) is then applied without occlusion to the areas to be treated and the patient is advised to expose the affected areas to continuous daylight for 2 h, preferably starting no later than half an hour after application of the photosensitizer. If it is impracticable to be out of doors because of inclement weather or patient frailty, then sitting indoors beside a window would be a reasonable alternative. In northern latitudes, treatment can be undertaken between April and September, whereas elsewhere, such as in Australia, year-round treatment may be feasible.
Studies have shown that there is no difference in efficacy or adverse effects between a regimen involving 1.5 or 2.5 h of continuous daylight exposure, with no advantage to longer exposure times; 2 h continuous exposure is generally accepted as being sufficient. It is the visible component of daylight that is important therapeutically and studies have been undertaken that indicate that on any dry day, including cloudy days, effective daylight PDT can be used in northern latitudes between April and September, although it is likely that it could be used for more of the year in the southern hemisphere. No difference in the clearance rates of AKs (c. 75–79%) was seen between daylight or conventional PDT, but daylight PDT was associated with lower pain scores [135, 136, 137, 138, 139].
Treatment schedules, aftercare and follow-up
Most topical PDT regimens call for a single PDT treatment for AKs and two treatments a week apart for Bowen disease and sBCC.
Following treatment, a dry dressing should be applied to treated areas. It is advisable to keep the areas out of direct sunlight for 24–48 h as photosensitivity can persist for this time period due to further PpIX accumulation following PDT. The degree of phototoxic inflammation seen after ambulatory and daylight PDT differs little from that following standard clinic-based PDT.
A review should be undertaken at 3 months and if there is no, or only partial, clinical response then a second treatment cycle should be undertaken with two treatments a week apart, including for AKs. Further review should be undertaken at 6 months and, given that Bowen disease and BCC recur in up to a quarter of patients at 5-year follow-up, patients should ideally be followed up by a dermatologist at least annually to ensure that clearance is maintained and that new lesions are detected. The same principles of review times and treatment regimens also apply to ambulatory and daylight PDT. Careful records of all treatments and attendances must be kept.
Adverse effects
Acute effects
The adverse effects of topical PDT (Box 22.2) include the expected acute phototoxic effects of pain, itch, discomfort, erythema, oedema, exudation and crusting [141]. The degree of erythema and phototoxicity is dependent on the size of the field treated and the degree of subclinical photodamage. Erythema peaks about 1 h after PDT but persists for about 7–10 days. Exudation and crusting are common (Figures 22.16 and 22.17). Fair-skinned photodamaged patients are most at risk of extensive erythema and phototoxicity (Figure 22.18). For this reason an initial test area of up to 5 × 5 cm may be advisable before more widespread treatment of patients with markedly photodamaged facial and scalp skin. Periocular oedema may occur and patients should be warned of this and advised that sleeping with an additional pillow for 48 h after treatment may minimize the problem. Urticaria occurs at the treatment site in the minority of patients during or immediately after PDT (Figure 22.19) [142].
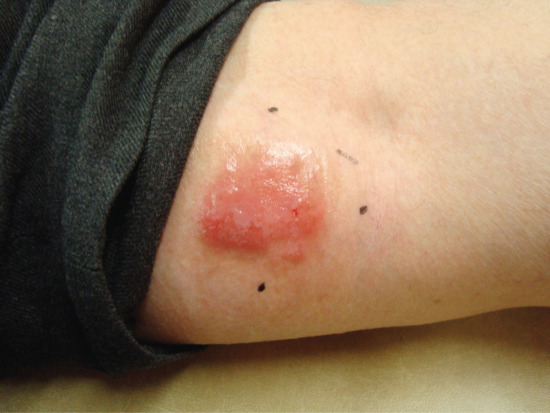
Figure 22.16 Erythema and oedema immediately after photodynamic therapy.
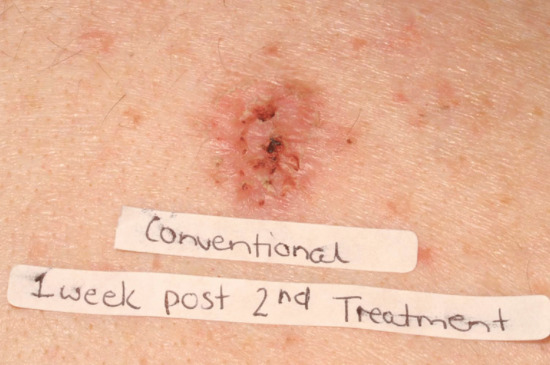
Figure 22.17 Expected residual erythema and crusting 1 week after photodynamic therapy.
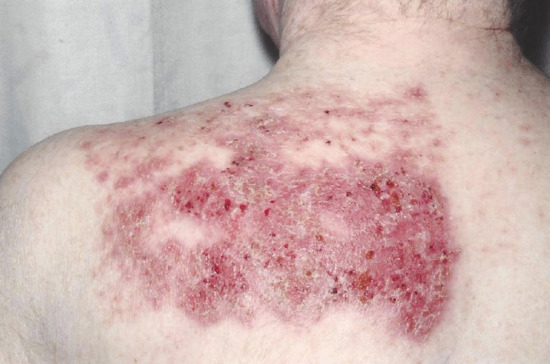
Figure 22.18 Severe phototoxic reaction persisting 1 week after photodynamic therapy in a fair-skinned subject with marked field change photodamage.
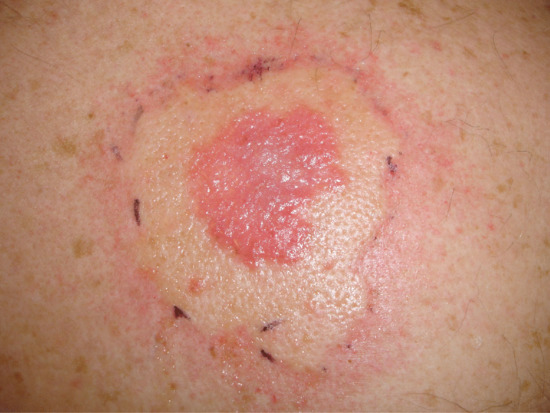
Figure 22.19 Urticaria at the treatment site immediately after photodynamic therapy.
Infection and cellulitis occur infrequently. Topical PDT has bactericidal and bacteriostatic effects and therefore infection occurs less commonly than, for example, with cryotherapy. The development of sterile pustules following PDT for acne vulgaris is common and patients should be warned of this adverse effect. Purpura and bruising are uncommon. Ulceration is more likely at lower leg sites, and if dependent oedema is present, the use of compression stockings throughout the PDT treatment course may assist with healing and reduce the risk of ulceration.
Dermatitis and allergy
Dermatitis may occur at the treatment site and may be secondary to phototoxicity or be irritant in nature. However, the possibility of allergic contact dermatitis to the pro-drug needs to be considered. Patients who have had multiple treatments and treatments to large areas are particularly at risk (Figure 22.20) [143, 144, 145]. There may be cross-reaction between the pro-drugs, although allergy to MAL does not necessarily imply allergy to ALA or vice versa. Patch testing should be organized if contact allergy is suspected, as further PDT in allergic patients may result in a generalized dermatitis (Figure 22.21) [146]. In addition, given that both the MAL and Ameluz preparations contain arachis oil, PDT presents a potential, albeit unlikely, hazard for patients with extreme peanut allergy. Bullous pemphigoid has been reported in a single case localized to sites of PDT [147].
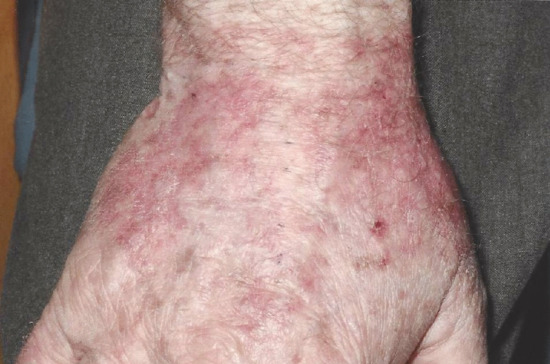
Figure 22.20 Dermatitis arising at the photodynamic therapy (PDT) treatment site in a patient who had received multiple treatments with topical PDT to field areas. This was confirmed to be contact allergic in nature by patch testing.
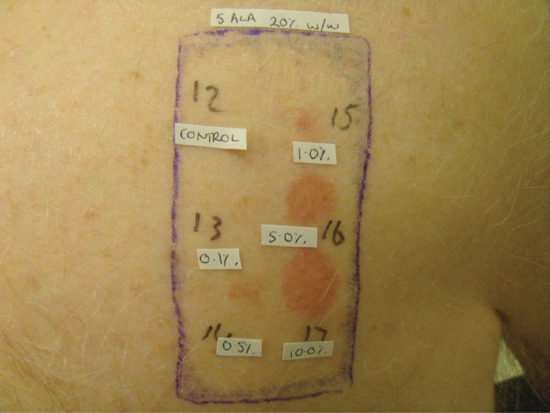
Figure 22.21 Positive patch tests confirming contact allergy to a proprietary preparation of 5-aminolaevulinic acid (ALA). Contact allergy to both ALA and methyl aminolevulinate may uncommonly occur, particularly after multiple treatments, often to large areas.
Pain
The main acute adverse effect of PDT is pain and most patients experience some discomfort, with severe pain in 16–20% [148, 149, 150]. The mechanism of PDT-induced pain is unknown although it occurs maximally in the first few minutes of treatment and is typically of a burning, prickling, stabbing nature. Pain usually rapidly subsides as soon as irradiation stops. It is not known if there are patient-dependent factors, such as genetic susceptibility, which influence pain. PDT treatment of large lesions and lesions on the head, neck and genital sites is more likely to be associated with significant pain. There is also some evidence that lesions with the strongest fluorescence may be associated with more severe pain [151, 152, 153]. PDT treatment of warts appears to be associated with particular discomfort during treatment.
In one study of 983 PDT treatments, 44% of patients required pain-reducing interventions [151]. However, methods of pain relief for PDT are generally not particularly effective. Cold water sprays and pausing irradiation may provide some relief, as may a fan or forced air cooling [154]. Distracting the patient and engaging in conversation is anecdotally of some help, although this has not been formally studied. Pre-treatment with topical tetracaine gel, EMLA (eutectic mixture of local anaesthetics), capsaicin or morphine does not significantly alleviate pain [152, 155, 156, 157]. The role of transepidermal nerve stimulation has been investigated but is of limited use and can only be applied to certain body sites [158]. Subcutaneous anaesthetic and nerve blockade can be used and the latter has been shown to be more effective than forced air cooling. These interventions are, however, invasive and are limited to use at certain sites [159, 160, 161].
It has been claimed that MAL PDT is of equivalent efficacy but less painful than ALA PDT, although in the studies in which this has been investigated there have been other variables such as time of application of the pro-drugs which may have influenced the development of pain [162, 163, 164, 165] . In a recent study in which treatment of AKs with ALA (Ameluz) and MAL PDT were compared, no significant differences in pain experienced were detected [52].
Irradiation parameters influence the severity of PDT-induced pain. Variable pulse light delivery may be associated with less pain than LED sources. The latter appears to be comparable with broadband and laser sources in this regard [166]. Green light is associated with less pain than red light when used for PDT of AK but not in a study using PDT for Bowen disease; as green light has limited penetration into the skin it would not normally be recommended for treating Bowen disease [167, 168]. The total light dose used during irradiation does not seem to influence pain but studies with low irradiance LEDs and daylight PDT indicate that reduced rate of light delivery (irradiance) is associated with reduced pain although, of course, irradiation times are longer.
The risk of scarring following PDT is low (<1%) and when it does occur this is more likely to be atrophic than hypertrophic. If the dermal–epidermal junction is disrupted milia may appear, although this is rarely seen. Photo-onycholysis, hypo/hyperpigmentation and increased/reduced hair growth are also possible medium- to long-term risks of topical PDT, although again these are rare.
There is no evidence of long-term carcinogenic risk of PDT in humans. There have been anecdotal reports of, for example, an invasive SCC arising in an area of penile intraepithelial neoplasia following PDT and of melanoma occurring in actinically damaged skin previously treated with PDT. Whether such events are casually related is unproven [103, 169, 170] but there is some evidence to suggest that they are not. PDT has been shown in animal models to delay UV-induced carcinogenesis. There is also evidence that PDT may retard AK development and that repeated PDT field treatment might possibly reduce the risk of SCC in organ transplant recipients. Its potential preventative role is currently being explored.
Clinical governance
Despite the good evidence base for topical PDT in dermatology and the availability of British and European guidelines and NICE interventional procedures guidance, there remain significant areas where a consensus on best practice has yet to be reached. This is particularly true in determining how a PDT service should be organized, managed and audited and, crucially, agreeing the criteria for offering patients PDT rather than an alternative treatment modality [171]. Are we undertaking safe and effective PDT?
One study highlighted the widespread use of topical PDT but the relative lack of data on treatment parameters and outcomes in everyday clinical practice. Detailed documentation should be kept on patient and lesion characteristics, treatment parameters and outcomes including efficacy, adverse effects, particularly pain, and recurrence rates. This will enable regular audit to be conducted. The British Association of Dermatologists has produced guidelines that define specific audit standards in terms of efficacy, recurrence rates and proportion of patients experiencing severe pain. Audit will enable each department offering PDT to determine whether these standards are being met in practice.
How to set up a PDT service
A business case to set up a new PDT service will need to explain in detail why the service is required, including an exposition of the advantages of PDT over existing therapies and the cost implications for using PDT rather than alternative treatment modalities. A full assessment of expected patient numbers, staffing requirements (including both nurse and clinician time), drug costs and consumables, equipment and training should be undertaken. Clinician input is required as appropriate patient and lesion selection is essential. Input from a medical physicist will also be needed for calibrating and measuring the irradiance of light sources. A dermatologist should be identified to oversee the service. Adequate staff training is critical and protocols, guidelines and governance arrangements must be put in place.
What's new?
Increasingly, studies ex vivo are being undertaken in an attempt to understand in greater detail the molecular mechanisms involved in PDT. The use of fluorescence imaging, mathematical modelling and in vivo monitoring of oxygen saturation and blood perfusion are informing our understanding of the effects of PDT on human skin and may thereby help to optimize treatment regimens [20, 21, 172].
The field of fluorescence diagnosis has largely been experimental but the use of PpIX to demarcate disease margins with detailed fluorescence analysis by spectroscopy and charge-coupled device (CCD) imaging has the potential to improve sensitivity and specificity. In the future it may be incorporated into clinical practice for diagnostic purposes.
The continued development of new drugs and formulations for PDT and mechanisms to enhance drug uptake and accumulation, together with advances in light delivery (e.g. using conformable light-emitting fabrics), demonstrate that PDT is an evolving treatment modality. In consequence, continuing scientific study will be required to ensure that technical advances in drugs and equipment are properly evaluated in order to be able to better understand their role in the management of skin disease. Optimization of treatment regimens and reduction of pain are essential for the wider acceptance of PDT although there is good evidence to support its use in superficial BCC, Bowen disease and AK, and a growing body of evidence to support its use in other conditions such as acne vulgaris [173, 174].
In conclusion, PDT has many advantages and few adverse effects. The development of improved mechanisms for drug delivery and irradiation will help refine the clinical practice of PDT. The use of non-invasive monitoring may improve efficacy and tolerance of therapy. There is great potential for the application of PDT in other diverse cutaneous diseases and this requires further investigation.
References
- Henderson BW, Dougherty TJ. How does photodynamic therapy work. Photochem Photobiol 1992;55(1):145–57.
- Brooke RCC, Sinka A, Sidhu UK, et al. Histamine is released following aminolevulinic acid-photodynamic therapy of human skin and mediates an aminolevulinic acid dose-related immediate inflammatory response. J Invest Dermatol 2006;126(10):2296–301.
- Finlan LE, Kernohan NM, Thomson G, et al. Differential effects of 5-aminolaevulinic acid photodynamic therapy and psoralen plus ultraviolet A therapy on p53 phosphorylation in normal human skin in vivo. Br J Dermatol 2005;153(5):1001–10.
- Evangelou G, Farrar MD, White RD, et al. Topical aminolaevulinic acid-photodynamic therapy produces an inflammatory infiltrate but reduces Langerhans cells in healthy human skin in vivo. Br J Dermatol 2011;165(3):513–19.
- Matthews YJ, Damian DL. Topical photodynamic therapy is immunosuppressive in humans. Br J Dermatol 2010;162(3):637–41.
- Evangelou G, Farrar MD, Cotterell L, et al. Topical photodynamic therapy significantly reduces epidermal Langerhans cells during clinical treatment of basal cell carcinoma. Br J Dermatol 2012;166(5):1112–15.
- Dougherty TJ, Kaurfman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res 1978;38:2628–35.
- Ibbotson S. Photodynamic therapy. In: FergusonJ, DoverJ, eds. Photodermatology. London: Manson Publishing, 2006:125–34.
- Ibbotson SH, Szeimies RM. Photodynamic therapy. In: LimHW, HonigsmannH, HawkJLM, eds. Photodermatology. London: Informa Healthcare, 2007:369–88.
- Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B Biol 1990;6(1–2):143–8.
- Peng Q, Berg K, Moan J, et al. 5-aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol 1997;65(2):235–51.
- Braathen LR, Szeimies RM, Bassett-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. J Am Acad Dermatol 2007;56(1):125–43.
- Ibbotson SH, Moseley H, Brancaleon L. Photodynamic therapy in dermatology: Dundee clinical and research experience. Photodiag Photodynam Ther 2004;1:211–23.
- Morton CA, Brown SB, Collins S, et al. Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group. Br J Dermatol 2002;146(4):552–67.
- Morton CA, McKenna KE, Rhodes LE, et al. Guidelines for topical photodynamic therapy: update. Br J Dermatol 2008;159(6):1245–66.
- Morton CA, Szeimies RM, Sidoroff A, et al. European guidelines for topical photodynamic therapy, part 2: emerging indications – field cancerization, photorejuvenation and inflammatory/infective dermatoses. J Eur Acad Dermatol Venereol 2013;27(6):672–9.
- Morton CA, Szeimies RM, Sidoroff A, et al. European guidelines for topical photodynamic therapy, part 1: treatment delivery and current indications – actinic keratoses, Bowen's disease, basal cell carcinoma. J Eur Acad Dermatol Venereol 2013;27(5):536–44.
- Moseley H, Ibbotson S, Woods J, et al. Clinical and research applications of photodynamic therapy in dermatology: Experience of the Scottish PDT Centre. Laser Surg Med 2006;38(5):403–16.
- Smits T, Kleinpenning MM, Blokx WAM, et al. Fluorescence diagnosis in keratinocytic intraepidermal neoplasias. J Am Acad Dermatol 2007;57:824–31.
- Tyrrell J, Campbell S, Curnow A. Validation of a non-invasive fluorescence imaging system to monitor dermatological PDT. Photodiag Photodynam Ther 2010;7(2):86–97.
- Tyrrell JS, Campbell SM, Curnow A. The relationship between protoporphyrin ix photobleaching during real-time dermatological methyl-aminolevulinate photodynamic therapy (MAL-PDT) and subsequent clinical outcome. Laser Surg Med 2010;42(7):613–19.
- Svanberg K, Andersson T, Killander D, et al. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol 1994;130:743–51.
- Piacquadio D, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple acintic keratoses of the face and scalp. Arch Dermatol 2004;140:41–6.
- Maisch T, Santarelli F, Schreml S, et al. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp Dermatol 2010;19:e302–5.
- Szeimies RM, Radny P, Sebastian M, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br J Dermatol 2010;163(2):386–94.
- Hauschild A, Stockfleth E, Popp G, et al. Optimization of photodynamic therapy with a novel self-adhesive 5-aminolaevulinic acid patch: results of two randomized controlled phase III studies. Br J Dermatol 2009;160(5):1066–74.
- Christensen E, Skogvolle E, Viset T, et al. Photodynamic therapy with 5-aminolaevulinic acid, dimethylsulfoxide and curettage in basal cell carcinoma: a 6-year clinical and histological follow-up. J Eur Acad Dermatol Venereol 2009;23:58–66.
- Pye A, Campbell S, Curnow A. Enhancement of methyl-aminolevulinate photodynamic therapy by iron chelation with CP94: an in vitro investigation and clinical dose-escalating safety study for the treatment of nodular basal cell carcinoma. J Cancer Res Clin Oncol 2008;134(8):841–9.
- Pye A, Curnow A. Direct comparison of delta-aminolevulinic acid and methyl-aminolevulinate-derived protoporphyrin IX accumulations potentiated by desferrioxamine or the novel hydroxypyridinone iron chelator CP94 in cultured human cells. Photochem Photobiol 2007;83:766–73.
- Soler AM, Angell-Peterson E, Warloe T, et al. Photodynamic therapy of superficial basal cell carcinoma with 5-aminolevulinic acid with dimethylsulfoxide and ethylendiaminetetraacetic acid: a comparison of two light sources. Photochem Photobiol 2000;71(6):724–9.
- Mikolajewska P, Donnelly RF, Garland MJ, et al. Microneedle pre-treatment of human skin improves 5-aminolevulinic acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res 2010;27(10):2213–20.
- Moseley H, Brancaleon L, Lesar AE, et al. Does surface preparation alter ALA uptake in superficial non-melanoma skin cancer in vivo? Photoderm Photoimmunol Photomed 2008;24(2):72–5.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol 2012;166(6):1262–9.
- Clark C, Bryden A, Dawe R, et al. Topical 5-aminolaevulinic acid photodynamic therapy for cutaneous lesions: outcome and comparison of light sources. Photoderm Photoimmunol Photomed 2003;19(3):134–41.
- Brancaleon L, Moseley H. Laser and non-laser light sources for photodynamic therapy. Laser Med Sci 2002;17(3):173–86.
- Morton CA, Whitehurst C, Moseley H, et al. Development of an alternative light source to lasers for photodynamic therapy: 3. Clinical evaluation in the treatment of pre-malignant non-melanoma skin cancer. Laser Med Sci 1995;10:165–71.
- Szeimies RM, Hein R, Baumler W, et al. A possible new incoherent lamp for photodynamic treatment of superficial skin lesions. Acta Derm Venereol (Stockh) 1994;74:117–19.
- Moseley H. Total effective fluence: a useful concept in photodynamic therapy. Laser Med Sci 1996;11(2):139–43.
- De Haas ERM, de Vijlder HC, Sterenborg HJCM, et al. Fractionated aminolevulinic acid-photodynamic therapy provides additional evidence for the use of PDT for non-melanoma skin cancer. J Eur Acad Dermatol Venereol 2008;22(4):426–30.
- De Haas ERM, Kruijt B, Sterenborg HJCM, et al. Fractionated illumination significantly improves the response of superficial basal cell carcinoma to aminolevulinic acid photodynamic therapy. J Invest Dermatol 2006;126(12):2679–86.
- De Haas ERM, Sterenborg HJCM, Neumann HAM, et al. Response of Bowen disease to ALA-PDT using a single and a 2-fold illumination scheme. Arch Dermatol 2007;143(2):264–5.
- De Vijlder H, Sterenborg HJCM, Neumann HA, et al. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: five-year follow-up of a randomized, prospective trial. Acta Dermato Venerol 2012;92(6):641–7.
- Sotiriou E, Apalla Z, Chovarda E, et al. Single vs. fractionated photodynamic therapy for face and scalp actinic keratoses: a randomized, intraindividual comparison trial with 12-month follow-up. J Eur Acad Dermatol Venereol 2012;26(1):36–40.
- Enk CD, Levi A. Low-irradiance red LED traffic lamps as light source in PDT for actinic keratoses. Photodermatol Photoimmunol Photomed 2012;28(6):332–4.
- Fritsch C, Goerz G, Ruzicka T. Photodynamic therapy in dermatology. Arch Dermatol 1998;134(2):207–14.
- Ibbotson SH. Topical 5-aminolaevulinic acid photodynamic therapy for the treatment of skin conditions other than non-melanoma skin cancer. Br J Dermatol 2002;146(2):178–88.
- Kurwa HA, Barlow RJ. The role of photodynamic therapy in dermatology. Clin Exp Dermatol 1999;24:143–8.
- Lehmann P. Methyl aminolaevulinate-photodynamic therapy: a review of clinical trials in the treatment of actinic keratoses and nonmelanoma skin cancer. Br J Dermatol 2007;156:793–801.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database of Systematic Reviews 2012, Issue12, Art No.: CD004415, doi: 10.1002/14651858.CD00415.pub2.
- Morton CA, Campbell S, Gupta G, et al. Intra-individual, right-left comparison of methyl aminolaevulinate photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized, controlled study. Br J Dermatol 2006;155(5):1029–36.
- Tschen EH, Wong DS, Pariser DM, et al. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: phase IV multicentre clinical trial with 12-month follow up. Br J Dermatol 2006;155(6):1262–9.
- Dirschka T, Radny P, Dominicus R, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison to a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol 2012;166(1):137–46.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol 2013;168(4):825–36.
- Sotiriou E, Apalla Z, Maliamani F, et al. Intraindividual, right-left comparison of topical 5-aminolevulinic acid photodynamic therapy vs. 5% imiquimod cream for actinic keratoses on the upper extremities. J Eur Acad Dermatol Venereol 2009;23(9):1061–5.
- Serra-Guillen C, Nagore E, Hueso L, et al. A randomized comparative study of tolerance and satisfaction in the treatment of actinic keratosis of the face and scalp between 5% imiquimod cream and photodynamic therapy with methyl aminolaevulinate. Br J Dermatol 2011;164(2):429–33.
- Szeimies RM, Stockfleth E, Popp G, et al. Long-term follow-up of photodynamic therapy with a self-adhesive 5-aminolaevulinic acid patch: 12 months data. Br J Dermatol 2010;162(2):410–14.
- Tarstedt M, Rosdahl I, Berne B, et al. A randomized multicenter study to compare two treatment regimens of topical methyl aminolevulinate (Metvix (R))-PDT in actinic keratosis of the face and scalp. Acta Derm Venereol 2005;85(5):424–8.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol 2008;158(5):994–9.
- Apalla Z, Sotiriov E, Chovarda E, et al. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br J Dermatol 2010;162(1):171–5.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol 2012;26(9):1063–6.
- De Graaf YGL, Kennedy C, Wolterbeek R, et al. Photodynamic therapy does not prevent cutaneous squamous-cell carcinoma in organ-transplant recipients: results of a randomized-controlled trial. J Invest Dermatol 2006;126:569–74.
- Dragieva G, Hafner J, Dummer R, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen's disease in transplant recipients. Transplantation 2004;77(1):115–21.
- Dragieva G, Prinz BM, Hofner J, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients. Br J Dermatol 2004;151(1):196–200.
- Piaserico S, Fortina AB, Rigotti P, et al. Topical photodynamic therapy of actinic keratosis in renal transplant recipients. Transplant Proc 2007;39(6):1847–50.
- Wennberg AM, Stenquist B, Stockfleth E, et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation 2008;86:423–9.
- Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg 2010;36(5):652–8.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol 2006;86:25–8.
- Perrett CM, McGregor JM, Warwick J et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol 2007;156(2):320–8.
- Sotiriou E, Apalla Z, Chovarda E, et al. Photodynamic therapy with 5-aminolevulinic acid in actinic cheilitis: an 18-month clinical and histological follow-up. J Eur Acad Dermatol Venereol 2010;24(8):916–20.
- Sotiriou E, Lallas A, Goussi C, et al. Sequential use of photodynamic therapy and imiquimod 5% cream for the treatment of actinic cheilitis: a 12-month follow-up study. Br J Dermatol 2011;165(4):888–92.
- Morton C, Horn M, Leman J, et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or fluorouracil for treatment of squamous cell carcinoma in situ – results of a multicenter randomized trial. Arch Dermatol 2006;142(6):729–35.
- Morton CA, Whitehurst C, Moseley H, et al. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen's disease. Br J Dermatol 1996;135(5):766–71.
- Basset-Seguin N, Ibbotson SH, Emstestam L, et al. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol 2008;18(5):547–53.
- Szeimies RM, Ibbotson S, Murrell DF, et al. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J Eur Acad Dermatol Venereol 2008;22(11):1302–11.
- Peng Q, Warloe T, Berg K, et al. 5-aminolevulinic acid-based photodynamic therapy – clinical research and future challenges. Cancer 1997;79(12):2282–308.
- Wang I, Bendsoe N, Klinteberg CAF, et al. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol 2001;144:832–40.
- Arits AHMM, Mosterd K, Essors BAB, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol 2013;14(7):647–54.
- Sebaratnam DF, Venugopal SS, Murrell DF. A comparison in real clinical practice of methyl aminolevulinate photodynamic therapy and surgery for small superficial basal cell carcinoma: 3-year recurrence rates and cosmetic outcomes. J Eur Acad Dermatol Venereol 2011;25(1):117–18.
- Berroeta L, Clark C, Dawe R, et al. A randomized study of minimal curettage followed by topical photodynamic therapy compared with surgical excision for low-risk nodular basal cell carcinoma. Br J Dermatol 2007;157(2):401–3.
- Lindberg-Larsen R, Solvsten H, Kragballe K. Evaluation of recurrence after photodynamic therapy with topical methylaminolaevulinate for 157 basal cell carcinomas in 90 patients. Acta Derm Venereol 2011;92:144–7.
- Mosterd K, Thissen MRTM, Nelemans P, et al. Fractionated 5-aminolaevulinic acid-photodynamic therapy vs. surgical excision in the treatment of nodular basal cell carcinoma: results of a randomized controlled trial. Br J Dermatol 2008;159(4):864–70.
- Rhodes LE, di Rie M, Enstram Y, et al. Photodynamic therapy using topical methyl aminolevulinate vs surgery for nodular basal cell carcinoma. Arch Dermatol 2004;140:17–23.
- Rhodes LE, di Rie MA, Leifsdottir R, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol 2007;143(9):1131–6.
- Morton CA, Mackie RM, Whitehurst C, et al. Photodynamic therapy for basal cell carcinoma: effect of tumor thickness and duration of photosensitizer application on response. Arch Dermatol 1998;134:248–9.
- Horn M, Hulf HC, Warloe T, et al. Topical methyl aminolaevulinic photodynamic therapy in patients with basal cell carcinoma prone to complications and poor cosmetic outcome with conventional treatment. Br J Dermatol 2003;149(6):1242–9.
- De Leeuw J, Van Der Beek N, Bjerring P, et al. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid 0.5% liposomal spray and intense pulsed light in combination with topical keratolytic agents. J Eur Acad Dermatol Venereol 2010;24(4):460–9.
- Horfelt C, Funk J, Frohm-Nilsson M, et al. Topical methyl aminolaevulinate photodynamic therapy for treatment of facial acne vulgaris: results of a randomized, controlled study. Br J Dermatol 2006;155(3):608–13.
- Mavilia L, Malara G, Moretti G, et al. Photodynamic therapy of acne using methyl aminolaevulinate diluted to 4% together with low doses of red light. Br J Dermatol 2007;157:810–11.
- Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using methyl aminolaevulinate: a blinded, randomized, controlled trial. Br J Dermatol 2006;154:969–76.
- Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid versus methyl aminolevulinate. J Am Acad Dermatol 2006;64:647–51.
- Stender IM, Na RH, Fogh H, et al. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial. Lancet 2000;355(9208):963–6.
- Chen K, Chang BZ, Ju M, et al. Comparative study of photodynamic therapy vs. CO2 laser vaporization in treatment of condylomata acuminata, a randomized clinical trial. Br J Dermatol 2007;156(3):516–20.
- Fehr MK, Hornung R, Degen A, et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Laser Surg Med 2002;30(4):273–9.
- Liang J, Lu XN, Tang H, et al. Evaluation of photodynamic therapy using topical aminolevulinic acid hydrochloride in the treatment of condylomata acuminata: a comparative, randomized clinical trial. Photoderm Photoimmunol Photomed 2009;25(6):293–7.
- Stefanaki IM, Georgiou S, Themelis GC, et al. In vivo fluorescence kinetics and photodynamic therapy in condylomata acuminata. Br J Dermatol 2003;149:972–6.
- Wang XL, Wang HW, Wang HS, et al. Topical 5-aminolaevulinic acid-photodynamic therapy for the treatment of urethral condylomata acuminata. Br J Dermatol 2004;151:880–5.
- Yang CH, Hui CY, Lee JC, et al. Photodynamic therapy for bowenoid paplulosis using a novel incoherent light emission diode device. Br J Dermatol 2003;149(Suppl. 64):91.
- Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res 2001;61:192–6.
- Fehr MK, Hornung, R, Schwarz VA, et al. Photodynamic therapy of vulvar intraepithelial neoplasia III using topically applied 5-aminolevulinic acid. Gynecol Oncol 2001;80(1):62–6.
- Hillemanns P, Wang X, Staehle S, et al. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol 2006;100(2):271–5.
- Lee MR, Ryman W. Erythroplasia of Queyrat treated with topical methyl animolevulinate photodynamic therapy. Australas J Dermatol 2005;46(3):196–8.
- Stables GI, Stringer MB, Robinson DJ, et al. Erythroplasia of Queyrat treated by topical aminolaevulinic acid photodynamic therapy. Br J Dermatol 1999;140(3):514–17.
- Varma S, Holt PJA, Anstey AV. Erythroplasia of Queyrat treated by topical aminolaevulinic acid photodynamic therapy: a cautionary tale. Br J Dermatol 2000;142(4):825–6.
- Paoli JTB, Ternesten Bratel A, Lowhagen GB, et al. Penile intraepithelial neoplasia: results of photodynamic therapy. Acta Derm Venereol 2006;86:418–21.
- Mikasa K, Watanabe D, Kondo C, et al. 5-aminolevulinic acid-based photodynamic therapy for the treatment of two patients with extramammary Paget's disease. J Dermatol 2005;32(2):97–101.
- Raspagliesi F, Fontanelli R, Rossi G, et al. Photodynamic therapy using a methyl ester of 5-aminolevulinic acid in recurrent Paget's disease of the vulva: a pilot study. Gynecol Oncol 2006;103(2):581–6.
- Shieh S, Dee AS, Cheney RT, et al. Photodynamic therapy for the treatment of extramammary Paget's disease. Br J Dermatol 2002;146(6):1000–5.
- Zawislak AA, McCarron PA, McCluggage WG, et al. Successful photodynamic therapy of vulval Paget's disease using a novel patch-based delivery system containing 5-aminolevulinic acid. Br J Obstet Gynaecol 2004;111(10):1143–5.
- Debu A, Girard C, Kluger N, et al. Topical methyl aminolaevulinate–photodynamic therapy in erosive facial mycosis fungoides. Br J Dermatol 2010;163(4):884–5.
- Edstrom DW, Porwit A, Ros AM. Photodynamic therapy with topical 5-aminolaevulinic acid for mycosis fungoides: clinical and histological response. Acta Derm Venereol 2001;81:184–8.
- Zane C, Venturini M, Sala R, et al. Photodynamic therapy with methylaminolevulinate as a valuable treatment option for unilesional cutaneous T-cell lymphoma. Photoderm Photoimmunol Photomed 2006;22(5):254–8.
- Mori M, Campolmi P, Mavilia L, et al. Topical photodynamic therapy for primary cutaneous B-cell lymphoma: a pilot study. J Am Acad Dermatol 2006;54(3):524–6.
- Karrer S, Kohl E, Feise K, et al. Photodynamic therapy for skin rejuvenation: review and summary of the literature – results of a consensus conference of an expert group for aesthetic photodynamic therapy. J Deut Dermatol Ges 2013;11(2):137–48.
- Asilian A, Davami M. Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial. Clin Exp Dermatol 2006;31(5):634–7.
- Hillemanns P, Untch M, Prove F, et al. Photodynamic therapy of vulvar lichen sclerosus with 5-aminolevulinic acid. Obstet Gynecol 1999;93(1):71–4.
- Horn M, Wolf P. Topical methyl aminolevulinate photodynamic therapy for the treatment of folliculitis. Photoderm Photoimmunol Photomed 2007;23(4):145–7.
- Morley S, Griffiths J, Philips G, et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol 2013;168(3):617–24.
- Szeimies RM, Schleyer V, Moll I, et al. Adjuvant photodynamic therapy does not prevent recurrence of condylomata acuminata after carbon dioxide laser ablation-a phase III, prospective, randomized, bicentric, double-blind study. Dermatol Surg 2009;35(5):757–64.
- Van der Snoek EM, Robinson DJ, van Hellemond JJ, et al. A review of photodynamic therapy in cutaneous leishmaniasis, in J Eur Acad Dermatol Venereol 2008;22:918–22.
- Evangelou G, Krasagakis K, Giannikaki E, et al. Successful treatment of cutaneous leishmaniasis with intralesional aminolevulinic acid photodynamic therapy. Photodermatol Photoimmunol Photomed 2011;27(5):254–6.
- Beattie PE, Dawe RS, Ferguson J, et al. Lack of efficacy and tolerability of topical PDT for psoriasis in comparison with narrowband UVB phototherapy. Clin Exp Dermatol 2004;29(5):560–2.
- Radakovic-Fijan S, Belcha-Thalhammer U, Schleyer V, et al. Topical aminolaevulinic acid-based photodynamic therapy as a treatment option for psoriasis? Results of a randomized, observer-blinded study. Br J Dermatol 2005;152:279–83.
- Schleyer V, Radakovic-Fijan S, Karrer S, et al. Disappointing results and low tolerability of photodynamic therapy with topical 5-aminolaevulinic acid in psoriasis. A randomized, double-blind phase I/II study. J Eur Acad Dermatol Venereol 2006;20(7):823–8.
- Boiy A., de Witte PAM, Roelandts R. Topical treatment of disseminated superficial actinic porokeratosis with hypericin–photodynamic therapy: a case report. Photodiag Photodyn Ther 2010;7(2):123–5.
- Nayeemuddin FA, Wong M, Yell J, et al. Topical photodynamic therapy in disseminated superficial actinic porokeratosis. Clin Exp Dermatol 2002;27(8):703–6.
- Attili SK, Ibbotson SH. How we treat Bowen's disease with topical photodynamic therapy in Dundee. Photodiag Photodyn Ther 2009;6(1):41–5.
- Braathen LR, Paredes BE, Saksela O, et al. Short incubation with methyl aminolevulinate for photodynamic therapy of actinic keratoses. J Eur Acad Dermatol Venereol 2009;23(5):550–5.
- Cottrell WJ, Paquette AD, Keymel KR, et al. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin Cancer Res 2008;14(14):4475–83.
- Ibbotson SH. Irradiance is an important determinant of pain experienced during topical photodynamic therapy. J Am Acad Dermatol 2011;65(1):201–2.
- Langmack K, Mehta R, Twyman P, et al. Topical photodynamic therapy at low fluence rates – theory and practice. J Photochem Photobiol B Biol 2001;60(1):37–43.
- Middelburg TA, van Zaane F, de Bruijn HS, et al. Fractionated illumination at low fluence rate photodynamic therapy in mice. Photochem Photobiol 2010;86:1140–6.
- Attili SK, Lesar A, McNeill A, et al. An open pilot study of ambulatory photodynamic therapy using a wearable low-irradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer. Br J Dermatol 2009;161(1):170–3.
- Moseley H, Allen JW, Ibbotson S, et al. Ambulatory photodynamic therapy: a new concept in delivering photodynamic therapy. Br J Dermatol 2006;154(4):747–50.
- Ibbotson S, Ferguson J. Ambulatory photodynamic therapy using low irradiance inorganic light-emitting diodes for the treatment of non-melanoma skin cancer: an open study. Photoderm Photoimmunol Photomed 2012;28(5):235–9.
- Wiegell SR, Fabricius S, Gniadecka M, et al. Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomized multicentre study. Br J Dermatol 2012;166(6):1327–32.
- Wiegell SR, Wulf HC, Szeimies RM, et al. Daylight photodynamic therapy for actinic keratosis: an international consensus. J Eur Acad Dermatol Venereol 2012;26:673–9.
- Wiegell SR, Fabricius S, Stender IM, et al. A randomized, multicentre study of directed daylight exposure times of 11/2 vs. 21/2 h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br J Dermatol 2011;164(5):1083–90.
- Wiegell SR, Haedersdal M, Eriksen P, et al. Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home-based daylight exposure: a double-blinded randomized clinical trial. Br J Dermatol 2009;160(6):1308–14.
- Wiegell SR, Haedersdal M, Philipsen PA, et al. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol 2008;158:740–6.
- Wiegell SR, Skødt V, Wulf HC. Daylight-mediated photodynamic therapy of basal cell carcinomas – an explorative study. J Eur Acad Dermatol Venereol 2014;28:169–74.
- Ibbotson SH. Adverse effects of topical photodynamic therapy. Photodermatol Photoimmunol Photomed 2011;27(3):116–30.
- Kerr AC, Ferguson J, Ibbotson SH. Acute phototoxicity with urticarial features during topical 5-aminolaevulinic acid photodynamic therapy. Clin Exp Dermatol 2007;32(2):201–2.
- Harries MJ, Street G, Gilmour E, et al. Allergic contact dermatitis to methyl aminolevulinate (Metvix) cream used in photodynamic therapy. Photodermatol Photoimmunol Photomed 2007;23:35–6.
- Hohwy T, Andersen KE, Solvsten H, et al. Allergic contact dermatitis to methyl aminolevulinate after photodynamic therapy in 9 patients. Contact Dermatitis 2007;57:321–3.
- Korshoj S, Solvsten H, Erlandsen M, et al. Frequency of sensitization to methyl aminolaevulinate after photodynamic therapy. Contact Dermatitis 2009;60(6):320–4.
- Wulf HC, Philipsen P. Allergic contact dermatitis to 5-aminolaevulinic acid methylester but not to 5-aminolaevulinic acid after photodynamic therapy. Br J Dermatol 2004;150:143–5.
- Rakvit P, Kerr AC, Ibbotson SH. Localized bullous pemphigoid induced by photodynamic therapy. Photodermatol Photoimmunol Photomed 2011;27(5):251–3.
- Arits A, Van De Weert MM, Nelemans PJ, et al. Pain during topical photodynamic therapy: uncomfortable and unpredictable. J Eur Acad Dermatol Venereol 2010;24(12):1452–7.
- Grapengiesser S, Gudmundsson O, Larko O, et al. Pain caused by photodynamic therapy of skin cancer. Clin Exp Dermatol 2002;27:493–7.
- Warren CB, Karai LJ, Vidimos A, et al. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol 2009;61(6):1033–43.
- Miller IM, Nielsen JS, Lophaven S, et al. Factors related to pain during routine photodynamic therapy: a descriptive study of 301 patients. J Eur Acad Dermatol Venereol 2011;25(11):1275–81.
- Sandberg C, Stenquist B, Rosdahl I, et al. Important factors for pain during photodynamic therapy for actinic keratosis. Acta Derm Venereol 2006;86:404–8.
- Wiegell SR, Skiveren J, Philipsen PA, et al. Pain during photodynamic therapy is associated with protoporphyrin IX fluorescence and fluence rate. Br J Dermatol 2008;158(4):727–33.
- Pagliaro J, Elliott T, Bulsara M, et al. Cold air analgesia in photodynamic therapy of basal cell carcinomas as Bowen's disease: an effective addition to treatment: a pilot study. Dermatol Surg 2004;30(1):63–6.
- Holmes MV, Dawe RS, Ferguson J, et al. A randomized, double-blind, placebo-controlled study of the efficacy of tetracaine gel (Ametop((R)) for pain relief during topical photodynamic therapy. Br J Dermatol 2004;150(2):337–40.
- Langan SM, Collins P. Randomized, double-blind, placebo-controlled prospective study of the efficacy of topical anaesthesia with a eutetic mixture of lignocaine 2.5% and prilocaine 2.5% for topical 5-aminolaevulinic acid-photodynamic therapy for extensive scalp actinic keratoses. Br J Dermatol 2006;154(1):146–9.
- Skiveren J, Haedersdal M, Philipsen PA, et al. Morphine gel 0.3% does not relieve pain during topical photodynamic therapy: a randomized, double-blind, placebo-controlled study. Acta Derm Venereol 2006;86:409–11.
- Halldin CB, Paoli J, Sandberg C, et al. Transcutaneous electrical nerve stimulation for pain relief during photodynamic therapy of actinic keratoses. Acta Derm Venereol 2008;88(3):311–13.
- Halldin CB, Paoli J, Sandberg C, et al. Nerve blocks enable adequate pain relief during topical photodynamic therapy of field cancerization on the forehead and scalp. Br J Dermatol 2009;160:795–800.
- Paoli J, Halldin C, Ericson MB, et al. Nerve blocks provide effective pain relief during topical photodynamic therapy for extensive facial actinic keratoses. Clin Exp Dermatol 2008;33(5):559–64.
- Serra-Guillen C, Hueso L, Nagor E, et al. Comparative study between cold air analgesia and supraorbital and supratrochlear nerve block for the management of pain during photodynamic therapy for actinic keratoses of the frontotemporal zone. Br J Dermatol 2009;161(2):353–6.
- Kasche A, Luderschmidt S, Ring J, et al. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J Drug Dermatol 2006;5:353–6.
- Kuijpers DI, Thissen MR, Thissen CA, et al. Similar effectiveness of methyl aminolevulinate and 5-aminolevulinate in topical photodynamic therapy for nodular basal cell carcinoma. J Drug Dermatol 2006;5:642–5.
- Moloney FJ, Collins P. Randomized, double-blind, prospective study to compare topical 5-aminolaevulinic acid methylester with topical 5-aminolaevulinic acid photodynamic therapy for extensive scalp actinic keratosis. Br J Dermatol 2007;157:87–91.
- Wiegell SR, Stender IM, Na R, et al. Pain associated with photodynamic therapy using 5-aminolevulinic acid or 5-aminolevulinic acid methylester on tape-stripped normal skin. Arch Dermatol 2003;139(9):1173–7.
- Babilas P, Knobler R, Hummel S, et al. Variable pulsed light is less painful than light-emitting diodes for topical photodyamic therapy of actinic keratosis: a prospective randomized controlled trial. Br J Dermatol 2007;157(1):111–17.
- Fritsch C, Stege H, Saalmann G, et al. Green light is effective and less painful than red light in photodynamic therapy of facial solar keratoses. Photodermatol Photoimmunol Photomed 1997;13(5–6):181–5.
- Morton CA, Whitehurst C, Moore JV, et al. Comparison of red and green light in the treatment of Bowen's disease by photodynamic therapy. Br J Dermatol 2000;143:767–72.
- Schreml S, Gantner S, Steinbauer J, et al. Melanoma promotion after photodynamic therapy of a suspected Bowen's disease lesion. Dermatology 2009;219:279–81.
- Wolf P, Fink-Puches R, Reimann-Weber A, et al. Development of malignant melanoma after repeated topical photodynamic therapy with 5-aminolevulinic acid at the exposed site. Dermatology 2006;194(1):53–4.
- Ibbotson SH, Dawe RS, Morton CA. A survey of photodynamic therapy services in dermatology departments across Scotland. Clin Exp Dermatol 2013;38(5):511–16.
- Valentine RM, Ibbotson SH, Wood K, et al. Modelling fluorescence in clinical photodynamic therapy. Photochem Photobiol Sci 2013;12(1):203–13.
- Calzavara-Pinton PG, Rossi MT, Aronson E, et al. A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 2: Oncologic and infectious indications. Photochem Photobiol Sci 2013;12(1):158–65.
- Calzavara-Pinton PG, Rossi MT, Aronson E, et al. A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 1: Inflammatory and aesthetic indications. Photochem Photobiol Sci 2013;12(1):148–57.