CHAPTER 24
Principles of Radiotherapy
Charles G. Kelly and John Frew
Northern Centre for Cancer Care, Freeman Hospital, Newcastle upon Tyne, UK
Introduction
The clinical effects of ionizing radiation on the skin have been known since the discovery of X-rays in 1895 [1, 2]. Initially both benign and malignant skin conditions were irradiated and dose and clinical indications were chosen empirically with little knowledge of the late effects of radiation on skin and subcutaneous tissue. Indications for treating benign disease by irradiation have declined since the advent of topical steroids.It is in the best interest of patients with skin tumours to be seen in a clinic where the expertise of specialists in radiotherapy and oncology, plastic surgery and micrographic surgery as well as dermatology are present, and this is being achieved with the mandatory development of multidisciplinary team meetings and clinics in the UK as described in improving outcomes guidance [3].
Ionizing radiation in the treatment of skin cancer
X-ray photon beams: megavoltage and kilovoltage X-ray therapy
X-rays are part of the electromagnetic spectrum, of shorter wavelength and more energetic than UV light. The most commonly used form of therapeutic radiation is megavoltage radiotherapy using energies of greater than 1 million electron volts (>1 MeV), delivered by linear accelerators in the treatment of deep-seated tumours. Previously, these beams have usually not been suitable for the treatment of skin cancers because of their greater penetration and skin sparing properties, but that has changed in the last few years, with advances in beam delivery technology, although megavoltage beams are still only used for a small minority of skin cancer patients and in particular clinical situations. X-rays in the kilovoltage range (50–140 kV) are very suitable for treating skin cancer as their maximum energy is deposited on the surface. Fall off subsequently is exponential (Figure 24.1), and some radiation dose does reach underlying subcutaneous structures. Higher kilovoltage beams can be used for thicker tumours.

Figure 24.1 Approximate per cent depth dose values for 90 kV superficial X-ray beam.
Electron beams
Beta rays are electrons and can be derived from radioactive isotopes, such as strontium-90 or be produced by a linear accelerator. An electron beam differs from a superficial X-ray beam or other photon beams, as the energy does not fall off exponentially as the beam goes through tissue but builds up to a peak and then falls off rapidly (Figure 24.2). The depth of penetration is proportional to the energy of the beam. A useful treatment depth in centimetres is approximately one third of the energy of the beam, so a 4.5 MeV electron beam will be useful for treating to a depth of 1.5 cm, and a 12 MeV beam to 4 cm. The width at the peak of the beam also increases with electron energy giving more of the plateau at the peak at higher energies, as shown by the representation of the 9 MeV beam shape in Figure 24.2. This can be useful when trying to enclose all of a thick tumour within the highest energy part of the beam. To avoid skin sparing, tissue equivalent material such as wax or paraffin gauze is applied over the tumour so that energy is deposited in this ‘build-up’ material, moving the beam peak left towards the skin surface in Figure 24.2.

Figure 24.2 Approximate per cent depth dose curves for 4.5 and 9 MeV electron beams.
Superficial X-ray therapy is indicated for the majority of skin cancer treatments. However, there is a slightly higher risk of late bone and cartilage necrosis with this modality, as X-rays are absorbed proportionally more in high-molecular-weight tissues than in soft tissue. Electrons are absorbed similarly in all tissues, and have the advantage of sparing sensitive underlying tissues. These may be the modality of choice for tumours on the pinna, scalp, dorsum of the hand and tibia. Superficial X-rays have been used successfully in the past in these sites [1], but electrons are now preferred [2].
Unfortunately in practice, electrons have a limited use in the treatment of common skin cancers. As an electron beam is produced by a linear accelerator, which is much larger than a superficial X-ray machine, the treatment applicator is unwieldy and the minimum applicator size on the skin is 4 cm2 for a reliable dose calculation. Air cavities can give unacceptable dose inhomogeneity with electrons and eye shielding is not possible for treatment of periorbital tumours. Setting up treatment fields with electrons is often more time-consuming than with superficial X-ray therapy.
It is possible to irradiate the whole skin area with an electron beam [3, 4, 5, 6, 7, 8]. The minimal depth dose characteristics that may be achieved avoid the irradiation of subcutaneous structures, which would occur if X-ray therapy that is absorbed exponentially was used. This technique is used in the treatment of mycosis fungoides [4]. Multiple radiation fields are combined to give a homogeneous dose to the whole skin down to a depth of approximately 1 cm.
Moulds, applicators and implants
Other treatment modalities available for the irradiation of skin cancer, but in less common use, are using a ‘mould’, which is a cast loaded with radioactive material applied to the surface of the tumour and secured in place for the required treatment time, or using interstitial brachytherapy as described below for the treatment of keloids [1, 2]. The use of these techniques has seen a slight renaissance in some countries in the last decade with the introduction of more modern applicators [3]. Cosmetic results are good but there is a need for hospital admission for radiation protection reasons and results depend on local expertise.
Superficial radiotherapy treatment technique
The superficial X-ray tube is manoeuvrable, and the patient can be made comfortable on a treatment couch with an applicator resting on the skin to be treated (Figure 24.3). The tumour and a margin of normal tissue are treated with a standard applicator. If the tumour is an irregular shape in two dimensions, a piece of lead shielding 1.5 mm thick can be ‘cut out’ in the shape of the skin to be treated, and the applicator rests on this (Figure 24.4). The individual surface area for treatment is measured and the treatment time adjusted by calculation, giving the number of machine units which have to be delivered for each treatment.

Figure 24.3 Superficial X-ray machine.

Figure 24.4 (a) Lead mask with area for treatment ‘cut-out’. (b) BCC to be treated in this manner.
Once the applicator is in position, the radiographer leaves the treatment room. Treatments last for a few minutes on each occasion, and the patient is watched throughout using a remote camera system or special glass window.
Megavoltage X-ray therapy technique
While kilovoltage beams remain the most common modality to treat skin tumours, developments in radiotherapy technology over the last decade have increased interest in the use of megavoltage X-ray therapy in treating superficial skin lesions. The development of intensity modulated radiotherapy (IMRT) has enabled techniques where energy from megavoltage beams can be delivered separately and distinctly into individual small tissue volumes, building up complex 3D photon distributions with different doses delivered to specific areas adjacent to each other and with no or very little dose been given to areas containing organs at risk (OAR).
These advances in technology have allowed developments such as a ‘bathing cap’ distribution as seen in Figure 24.5, which allows homogeneous dose distribution across the skin of the scalp with rapid dose fall-off below the surface tissues resulting in much less dose being delivered to underlying brain.
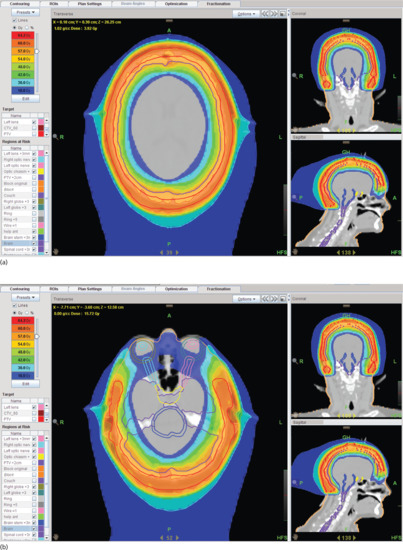
Figure 24.5 (a) ‘Bathing cap’ distribution showing minimal radiotherapy dose to the eyes, optic nerves and chiasm. (b) Bathing cap showing high radiotherapy dose to the skin of the scalp and reducing dose to the brain.
An example of how this can be achieved is with a TomoTherapy® linear accelerator, where the machine head revolves around the patient in a spiral movement with the beam being delivered by the machine head divided up into multiple tiny beamlets, which can all be adjusted individually to give dynamically a different dose to each small defined volume, allowing widely varying dose distributions to be built up in a 3D pattern within the patient.
These new megavoltage IMRT techniques are also useful whenever a relatively high homogeneous surface dose is required with sparing of underlying or neighbouring structures.
A further example of a ‘stocking’ distribution is shown in Figure 24.6a,b where an appropriate dose could be delivered homogeneously to the foot and ankle of a patient with skin lymphoma, sparing underlying tissues. The benefit of using this method of treatment can be seen in Figure 24.6c,d before and after treatment. The foot and lower leg were held in position for treatment using the immobilization device in Figure 24.6e.
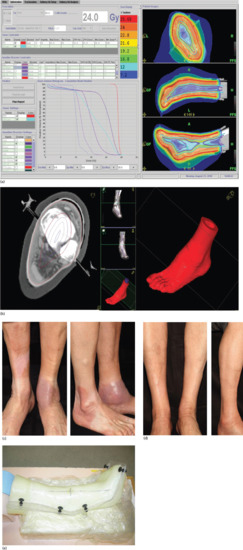
Figure 24.6 (a) Homogeneous dose distribution over the skin of the foot, ankle and lower leg using TomoTherapy. (b) Dose distribution for TomoTherapy treatment of the foot, ankle and lower leg showing high-dose delivery to the skin with reducing dose to the centre of the limb. (c) Before and (d) after treatment for skin lymphoma lesions using TomoTherapy. (e) Immobilization device for set-up in (c) and (d) to enable very accurate dose to be given with no limb movement.
(© Newcastle upon Tyne Hospitals NHS Foundation Trust.)
Radiosensitivity
All radiation is destructive. The basis of radiotherapy is that abnormal cells are more radio-responsive than differentiated cells. Radio-responsiveness and radio-curability are dissimilar and are functions of differences in cell population kinetics, so a rapidly resolving cancer may recur and a slowly resolving tumour may be completely cured. Radiotherapy is usually given as a fractionated course, as the intervals between doses allow for the recovery of normal cells. The effect of a dose of radiation is reduced by increasing the number of fractions in which it is given or by lengthening the total overall treatment time. The effect of irradiation can be modified by anoxia, infection, oedema, trauma and any inborn genetic susceptibility. The face tolerates irradiation well and radical dosages may be accompanied by good cosmetic results [1].
When irradiating benign conditions, the minimum dose and the lowest voltage appropriate to achieve the desired effect should be chosen. The threat of radiation carcinogenesis must clearly be seen in the context of the clinical indication for treatment. There is a threshold for somatic radiation changes which need not be breached in the treatment of benign conditions. The late radiation sequelae of treatment given many years ago are inexcusable with modern standards of dosimetry and equipment, and should not be seen in the treatment of benign diseases [2, 3].
Indications for radiotherapy
Benign disease
Some of the misconceptions about the role of radiotherapy have arisen because of its widespread use in the past for the treatment of benign conditions. X-ray production was discovered in 1895, and soon afterwards it was noted that the antiproliferative effect of low-energy X-rays was advantageous in the short term in a variety of visible tumours and benign dermatoses, and sometimes multiple re-treatments were offered. There was, however, no dose measurement apart from the biological effect of ‘skin erythema’, no radiation protection for workers or patients, and no appreciation of the late effects of radiodermatitis, leukaemia and other malignancies, and of skin cancer induction after a very long latent period.
Radiation epilation for tinea capitis, a treatment offered widely in Europe, well demonstrates radiation carcinogenesis. In Israel, between 1946 and 1960 over 20 000 immigrant children were treated with radiation soon after their arrival, by government order, with twice the subsequent incidence of cancer, usually of the brain, head and neck [1]. Skin cancers induced by this treatment have a very long latent period, often over 30 years, and cases treated in the 1930s and 1940s have been seen until recently in cancer treatment centres in the UK (Figure 24.7). For a fascinating and well-illustrated discussion of these early treatments and their consequences see Mould [2].
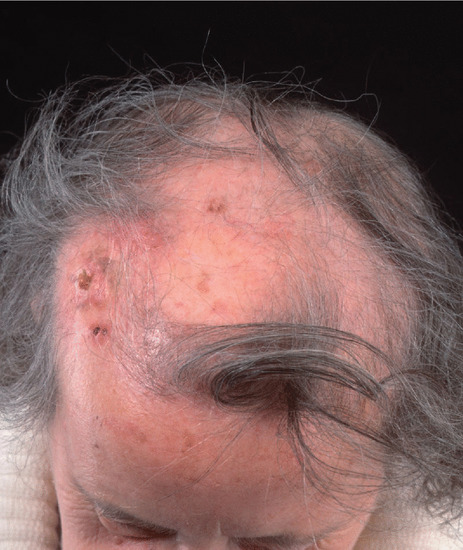
Figure 24.7 Late sequelae after radiation treatment for ringworm – atrophy, pigmentation change, alopecia, telangiectasia and multiple BCCs.
Radiotherapy is used much less now in the management of benign skin conditions than formerly and then usually only after other treatment modalities have failed or are contraindicated. In some conditions, such as psoriasis and eczema, there is a response to radiotherapy but often the benefit is only temporary [3, 4, 5, 6, 7]. This coupled with the risk of late radiation-induced malignancy has led to the decline in its use in all but the most refractory of cases. It is also still used occasionally in the management of keratoacanthoma where the differentiation from squamous cell carcinoma (SCC) cannot be made with complete confidence [8, 9]. Other rare uses are in Darier disease [7], familial benign chronic pemphigus [7] and acropustulosis of Hallopeau.
Recent National Institute for Health and Care Excellence (NICE) guidance on the use of Grenz rays, which are very low kilovolt X-rays absorbed chiefly in the epidermis, advises that the evidence for their safety and efficiency is very limited, and that their use in inflammatory skin conditions be limited to carefully selected patients who are refractory to other treatments, and preferably under trial conditions or at least with national data collections [3, 7, 10].
Keloids (see Chapter 96)
These represent the most common benign condition now treated with radiotherapy. Intractable keloids resistant to intralesional steroids or other conventional treatment may respond well to radiation. Excision of the keloid with early irradiation of the scar and stitch marks is more successful, but in some sites, for example the tip of the shoulder or upper middle chest where surgery is inadvisable, good response of pain, itch and redness can be achieved with some regression of the keloid itself. Relatively high doses are necessary; these will cause temporary pigmentation, which will remain for many months in pigmented skin. Doornbos et al. [1] noted that 17 of 18 unexcised keloids that were less than a year old regressed with 1500 cGy given in three treatments over 6 days at 120 kV. Older keloids respond less well to irradiation. The most satisfactory management of keloids is post-excision irradiation where a dose–response relationship can be seen. Total doses less than 900 cGy, irrespective of fractionation and postsurgical interval, did not prevent recurrence. Three doses of 400cGy were given by Kovalic and Perez [2] with a 73% success rate. Using the commonly employed dose of 900 cGy, Lo et al. [3] described an 85% success rate and Borok et al. [4] a 96% response rate. No late sequelae or carcinogenesis was described by any of the previously quoted authors with follow-up in excess of 30 years.
As well as using superficial X-rays or electron beam radiotherapy [5], treatment can be given using a radioactive iridium wire implant. With the latter technique, at the time of excision a small plastic tube is inserted below the incision with both ends of the tube exposed. The patient is then transferred to the radiotherapy department within 24 h and the tube is loaded with iridium wire and the scar irradiated to a dose of 20 Gy at 2 mm from the wire over 2 days [6]. Escarmant et al. [7] describes the results of treating 783 keloid scars in 544 patients with interstitial iridium implants. Skin applicators using either high-dose rate or low-dose rate brachytherapy have also been used [8].
Malignant skin disease
Basal cell carcinoma and squamous cell carcinoma of the skin (see Chapters 141 and 142)
For most small basal cell carcinomas (BCC) or SCC without nodal involvement, surgical excision or radiotherapy will give excellent cure rates [1, 2, 3, 4, 5].
Superficial radiotherapy has a long history in the successful treatment of BCC and SCC skin. 1695 such patients received treatment in 1945 at the Queensland Radium Institute [6]. A number of mature series have been reported, dated from 1947 onwards [7], and shown cure rates of 90% or over.
Treatment of choice
If cure is the intention, then surgery or radiotherapy are the treatment options, although a variety of other modalities including cryotherapy, curettage, photodynamic therapy and topical therapies (e.g. imiquimod) may be appropriate for certain tumours [8, 9, 10].
The decision as to which is the most appropriate modality will depend on several factors. These include the size and location of the tumour, any involvement of underlying tissues and the likely functional and cosmetic outcome. The majority of low-risk BCCs can be managed satisfactorily by simple surgical techniques with good cosmesis and low recurrence rates. For larger BCCs and SCCs in critical sites, particularly around the eyes, ears and nose, Mohs micrographic surgery followed by appropriate surgical reconstruction is now widely used (see Chapter 20). Radiotherapy should, however, still be considered as a valuable treatment option, and in some clinical situations as useful as surgery [11]. The patient's overall performance status and co-morbidities are as important as the complexity of any intervention under consideration. In reaching a decision, social factors such as accessibility of treatment centres, ability to cooperate with treatment and patient preference all need to be taken into account. The regular skin cancer multidisciplinary team meetings held throughout the UK are very helpful in guiding such decisions. Cure is not always a realistic proposition and radiotherapy can offer very worthwhile palliation (Figures 24.8 and 24.9).
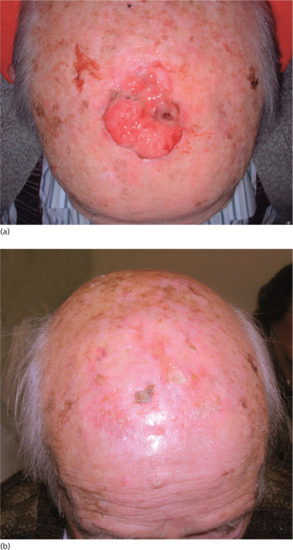
Figure 24.8 (a) SCC too large to expect long-term cure. (b) Satisfactory control at 6 months.
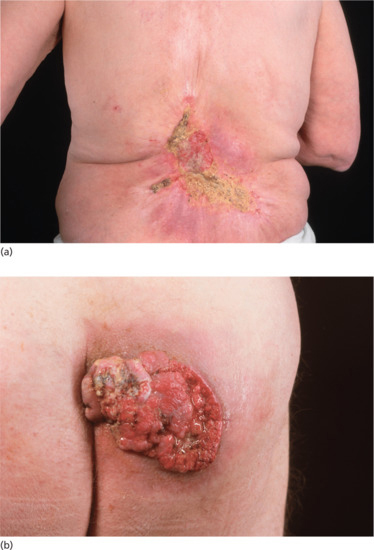
Figure 24.9 (a) SCC on the back, unsuitable for radiotherapy. (b) SCC on the buttock not suitable for radiotherapy. The sites shown in (a) and (b) tolerate radiotherapy less well and are prone to painful acute reactions, infection and slow healing.
Radiotherapy has a role to play in the treatment of almost all malignant skin conditions but, because there is considerable regional and national variation in access to radiotherapy and awareness of its value, its current use is patchy [8]. Although radiation damage with skin atrophy, telangiectasia, necrosis and ulceration occurred in the past, better dosimetery, a wider range of radiotherapy modalities [12] and more careful fractionation have considerably reduced this risk. In the UK, multidisciplinary skin cancer teams are now helping to ensure that the most appropriate modality is offered to every patient with skin cancer. This is in contrast to previous practice where only 2% of patients with BCC were referred to joint clinics [13] or a radiotherapeutic option was needlessly dismissed [14]. UK National Guidance [15] recommends that all health professionals treating skin cancer should be members of such a team and should work to locally approved protocols based upon nationally accepted guidelines, such as those of the British Association of Dermatologists [16]. A similar approach has been recommended in American guidelines [17].
Surgery or radiotherapy
It is not disputed that surgery offers a better cosmetic result with the passage of time, nor that radiotherapy is able to preserve existing structure and function, although not of course able to replace tissue destroyed by tumour.
Few trials have compared surgery directly with radiotherapy. Avril et al. [18] showed that in 347 patients with facial tumours less than 4 cm in diameter, a surgical recurrence rate within 3–5 years was 1/174 in the surgery group compared to 11/173 for radiotherapy. The cosmetic result was better with surgery. However, the radiotherapy regimens were heterogeneous, and the surgical results exceptionally good, better even than for Mohs microsurgery, where one large series had a 30-month recurrence of 3/160 tumours, compared to 5/171 for conventional surgery [19]. Published studies of radiotherapy for skin cancer are, however, remarkably consistent in reporting high long-term control rates of well over 90% [20, 21]. Advantages and disadvantages of each modality are summarized in Table 24.1.
Table 24.1 Patient factors influencing choice of skin cancer treatment.
| Surgery generally preferred | Radiotherapy may be preferred |
| Younger patients | Older patients |
| Multiple tumours | If surgery would result in poor cosmetic result or loss of function, particularly if micrographic surgery not available (e.g. nasal tip, lips, periocular, ears) |
| Gorlin syndrome [22] | |
| Infiltrative basal cell carcinomas | |
| Bulky tumours >6 cm | To avoid complex surgical reconstruction in the frail elderly |
| If there is erosion of bone or cartilage | |
| Tumours located on the trunk | To avoid general anaesthesia in patients who would not tolerate surgery under local anaesthesia |
| Ano-genital tumours | |
| Tumours below the knee [23] | To avoid general anaesthesia in patients who would otherwise require this for surgical management |
There is little difference in outcome between external beam radiotherapy using superficial X-rays or an electron beam [24, 25]. Locally placed moulds or applicators have also been used for malignant skin tumours, placing a radioactive source over the tumour and leaving this in position for a predetermined period [12, 13], or implanting radioactive wire into the tumour-bearing tissue. As described earlier, megavoltage radiotherapy using IMRT is now starting to be used for treating larger skin areas, often when surgery would not be possible, such as treating large areas of the scalp or peripheral limbs.
The areas which have traditionally been considered as not suitable for radiotherapy, such as over the nose, pinna, dorsum of the hand or anterior lower leg can be treated if careful consideration is given to the volume treated, the total dose and the fractionation. It is also important to consider the general fitness of the patient and the condition and physiological reserve of the skin in the site to be treated [26, 27, 28, 29].
There are some patients who, for practical reasons, cannot have radiotherapy. If patients are confused or unable to lie still because of confusion or neurological disease, then it can be impossible to deliver radiotherapy effectively and safely.
Adjuvant and salvage radiotherapy for skin cancer
Although ideally the patient should receive a single curative modality of treatment, sometimes patients are presented who have had a macroscopic excision of an SCC or a BCC but involved margins histopathologically.
Adjuvant radiotherapy has been advocated by some authors [30, 31, 32] where there has been inadequate excision and further surgery is either not possible or not recommended; if the tumour shows prognostic indicators of poor outcome such as positive lymph nodes, especially with extracapsular spread; with a large primary SCC; or in higher risk primary tumours of the head and neck. Adjuvant radiotherapy may improve outcome in SCC, especially if there is perineural invasion, when there is an increased risk of incomplete clearance even after Mohs surgery [31]; there is also some evidence that delaying radiotherapy may result in early tumour recurrence [33].
Because of the risk of nodal spread or perineural invasion from an incompletely excised SCC, it is sensible to offer ‘postoperative’ radiotherapy to the scar and tumour bed using a full normal dose and fractionation regimen. With a well-healed scar or skin graft, the radiation reaction is often very mild.
The situation is different for BCC, where Lui et al. [34] reported a series of 186 incompletely excised BCCs: 119 had immediate radiotherapy postoperatively, and 67 ‘wait and see’ with radiotherapy for clinical recurrence. The predicted cure rates at 5 years were 91% versus 61%, respectively, but 10-year cure rates of 90–92% were observed for both immediate and delayed treatment. When surgical re-excision is inappropriate, it may thus be reasonable, particulary in the elderly, merely to observe and to offer adjuvant radiotherapy only for clinical recurrence.
Radiotherapy dose fractionation and treatment regimens
Radiotherapy regimens have evolved empirically over a long period of time and a wide range is in current use. Generally, the greater the fractionation of the total dose, i.e. the smaller the individual dose administered at each session, the better the cosmetic outcome. This is obviously very important to some patients, but others are content to accept a poorer cosmetic outcome in exchange for fewer hospital visits (Table 24.2).
Table 24.2 Commonly used superficial radiotherapy dosage regimens for basal cell carcinomas and cutaneous squamous cell carcinomas. These fractionation regimens are only examples. Many centres will have other similar but locally derived dose fraction regimens.
| Total dose (Gy) | No. of fractions | Fractionation interval |
| 18 | 1 | – |
| 28 | 2 | 7 weeks apart |
| 35 | 5 | Daily (for tumours less than 4 cm in diameter) |
| 45 | 10 | Daily (for tumours more than 4 cm in diameter) |
The SI unit of absorbed dose is called the Gray (Gy), and is 1 Joule/kg. This unit has replaced the rad. Note that 100 rad = 1 J/kg = 1 Gy; 1 rad = 1 cGy (centigray). The dose prescription is defined by the total dose given, the energy of the beam, the number of fractions given, the total number of days over which treatment is given and the volume or area treated. For example, a typical prescription for the treatment of a small BCC might be ‘35 Gy using 90 kV superficial X-ray beam given in five fractions over 5 days to a 3 cm diameter circular area’. This time–dose relationship is crucial to understanding the biological effect.
Dose and fractionation regimens are similar for BCC and SCC. There is a small therapeutic ratio between tumour cure and normal tissue necrosis, and even a slight alteration of dose or fractionation can be critical; in a retrospective study of 1005 BCCs and SCCs treated by superficial X-rays in 1976 at the Christie Hospital, Manchester, Chan et al. found that for a field size of 3 cm2 or less, a single fraction of 20 Gy gave a 10-year disease-free rate of over 90%, with late necrosis rate of 6%. An increase of dose to 22.5 Gy did not improve tumour control rates, but did significantly increase late necrosis [35].
Fractionated radiotherapy is usually preferred for larger tumours, as cure rates are higher and better long-term cosmetic results are achieved [36, 37, 38].
Radiotherapy for particular skin sites: basal cell and squamous cell carcinoma
Eyelid (Figure 24.10)
The upper eyelid, a rare site for skin cancer, is best treated surgically, as keratinization of the tarsal conjunctiva may cause subsequent traumatic damage to the cornea. Tumours of the lower eyelid, however, are often suitable for treatment with superficial X-rays. A protective metal internal eyeshield is placed like a large contact lens over the globe of the eye after the application of local anaesthetic eye drops, with an applicator resting on this and on the lower lid tumour with an appropriate margin. The whole lower eyelid can be successfully treated in this manner if required.
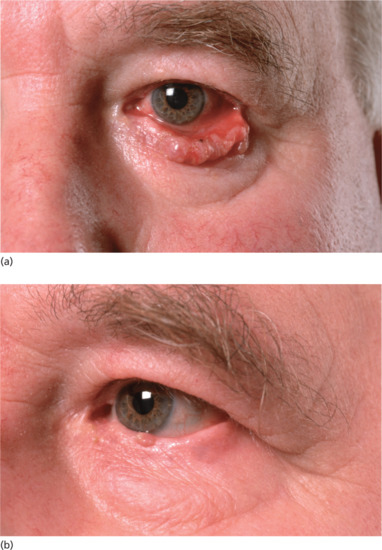
Figure 24.10 (a) BCC of the lower eyelid. (b) The same patient 6 months after superficial X-ray treatment.
Inner canthus (Figure 24.11)
These tumours, close to the orbit, have a high cure rate if there is meticulous attention to dose calculation. An individual thin lead mask is made for the patient by mould technicians, with the treatment area cut out. This provides a seating for the applicator, avoids the possibility of trauma on the treated side and protects the contralateral eye from the exit beam. If the naso-lacrimal duct has been damaged by tumour and the patient has epiphora for this reason, then radiotherapy is not likely to reverse this symptom. However, it is uncommon for radiation per se to cause a new stenosis of the ductal system. Different authors advocate either radiotherapy or Mohs micrographic surgery depending on the size of the tumour, potential for disfigurement or loss of function [1, 2].
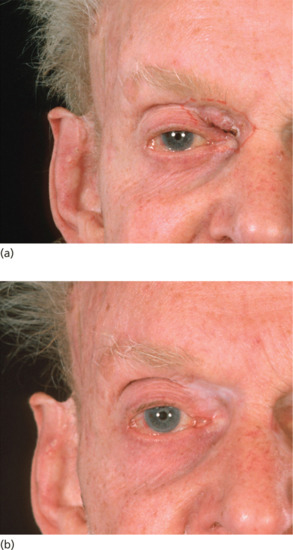
Figure 24.11 (a) BCC of the upper inner canthus. (b) The same patient 5 years after superficial X-ray treatment.
Ear (Figures 24.12 and 24.13)
Because tumours of the pinna and outer ear canal have close proximity to cartilage, electrons are preferred for treatment (see earlier). In a study of 62 patients treated by orthovoltage techniques, however, local control was achieved in 97% of cases and only one of the six cases with clinically suspected cartilage necrosis failed to heal spontaneously [3]. In a large Canadian study, in which 93% of patients remained tumour free at 10 years [4], persistent ulceration was observed in only 3%, and did not relate to beam energy but to dose and fractionation. However, electron treatment does give very good results for tumours covered by a 4 or 5 cm diameter applicator [5] and most departments will offer electrons unless for particular reasons the patient is unable to tolerate the Linac procedures. The visible tumour is delineated, with a margin of at least 1 cm to account for the depth dose characteristics of the electron beam, and the fact that the smallest reliable treatment applicator size is 4 cm diameter. The patient lies on the treatment couch with the ear for treatment uppermost, and wax or paraffin gauze is applied to make a level treatment surface, to fill in air gaps of sufficient thickness to avoid skin sparing. If the tumour is on the posterior surface of the pinna, the ear itself can act as a build-up zone, with wax as a posterior filler to ensure an even dose. A lead shield behind this protects the scalp. Once this ‘sandwich’ is in place, the applicator is brought down firmly to rest on the surface, and treatment delivered using 5 or 6 MeV electrons.
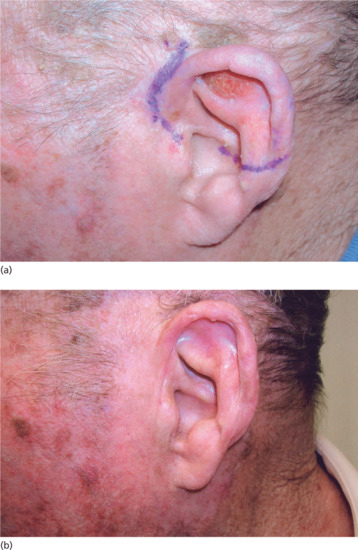
Figure 24.12 (a) BCC of the upper pinna. (b) The same patient 6 weeks after electron beam therapy.

Figure 24.13 (a) BCC of the posterior aspect of the ear. (b) The same patient 6 months after electron beam treatment.
Nose
Although skin cancers here are very closely applied to cartilage, in practice superficial X-rays give excellent control rates, and often a better cosmetic result than tumours irradiated at other sites. A lead plug is inserted inside the nostril to protect nasal mucosa from the exit beam. At the naso-labial fold, the depth to be treated needs careful consideration.
Other areas of the face (Figure 24.14)
Even large BCCs can do well with radiotherapy [6].
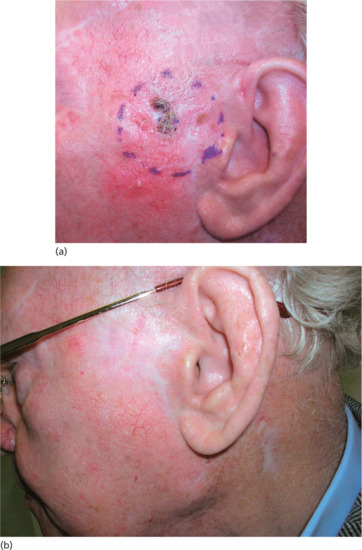
Figure 24.14 (a) Large BCC pre-auricular skin. (b) The same patient 6 months after superficial X-ray treatment.
Lip (Figure 24.15)
Squamous carcinoma of the lip has a high local cure rate with radiotherapy using either electrons or kilovoltage X-rays [7]. Treatment margins should be generous, because of the tendency for the tumours to spread mucosally, usually at least 1 cm of apparently normal tissue. The whole thickness of the lip is treated, choosing appropriate electron energy and build-up wax, or higher energy kilovoltage X-rays (300 kV). A wax-coated lead gum shield protects the inner mouth from the exit beam, but a patch of mucositis within the mouth is inevitable, and will interfere with normal eating and drinking for a few days after treatment.
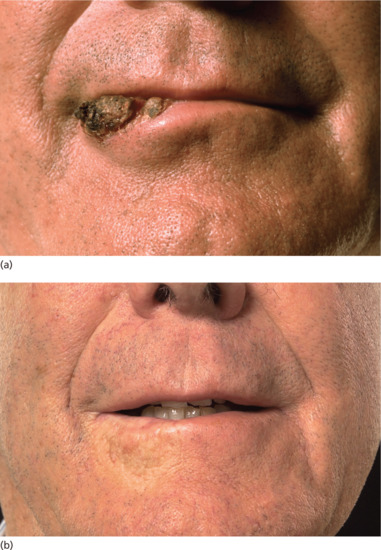
Figure 24.15 (a) SCC of the lip. (b) The same patient 8 years after higher energy kilovoltage treatment.
The patient is usually able to tolerate treatment to most of the upper or lower lip at one time, but not both areas simultaneously. The final cosmetic result is usually excellent.
As well as electron treatment, local brachytherapy can also be used for lip carcinoma [8].
Parotid and cervical lymph node involvement from cutaneous squamous cell carcinoma
A subset of patients who develop cutaneous SCC of the skin of the head and neck risk developing regional disease within parotid or cervical nodes, with worse outcomes [9]. Several studies have now shown that combination treatment with surgery and adjuvant radiotherapy gives higher rates of local control of parotid and cervical node metastasis than either treatment given alone [9, 10, 11, 12, 13].
Radiotherapy for particular skin tumours
Basal cell carcinoma (see Chapter 141)
Radiotherapy is useful for the primary treatment of small basal cell carcinomas or larger lesions where surgery will leave a poor functional or cosmetic result (see earlier) [1, 2, 3]. It should not be used for re-treating BCCs which have recurred after previous radiotherapy as they may then behave in a more aggressive manner [4]. The infiltrating (morphoeic) BCC subtype or the presence of underlying bone or cartilage involvement are relative contraindications to radiation treatment [5].
Squamous cell carcinoma of the skin (see Chapter 142)
As with BCC, radiotherapy can be used as either the primary modality of treatment or as adjuvant treatment after surgery if there is a narrow surgical margin of clearance, the margin often being determined by the particular anatomical site of the tumour. The technique and dose is the same as that for treating a basal cell cancer, but a wider margin is taken around the tumour and the patient is subjected to a more frequent and longer follow-up, as suggested in the British Association of Dermatologists guidelines [6]. If the SCC has developed on the face, it is mandatory to check for cervical lymphadenopathy. Radiotherapy may be especially useful for SCC on or around the nose where resection may be incomplete or the potential for reconstruction suboptimal [7].
The size of the SCC is also important with one study showing radiotherapy giving local control in over 90% of SCCs less than 1 cm, but in only 56% of tumours larger than 5 cm [8].
Adjuvant radiotherapy may reduce the development of nodal disease in higher risk cutaneous SCC as described by Veness [9].
Radiotherapy can also be useful in palliation of advanced skin tumours and in some patients give long-term survival [10].
Bowen disease (see Chapter 142)
Radiotherapy can be an effective treatment for Bowen disease [3], but it is not commonly used, particularly since the introduction of photodynamic therapy (see Chapter 22). Lesions on the leg have to be treated with caution [11, 12] as there is a danger of ulceration and very slow healing if large areas are treated. A recent retrospective review of 44 cases by VanderSpek et al. where radiotherapy was used as a primary treatment or for managing residual disease showed a crude local control rate of 93% [13].
Melanoma (see Chapter 143)
Melanoma cells are not insensitive to radiotherapy as is sometimes stated (Figure 24.16), but they do require higher dose per fraction regimens to overcome their higher radio-resistance. Patients may thus be given larger individual doses in less frequent fractions (‘hypofractionation’) [14]. The main indication for the use of radiotherapy in melanoma is for palliation of cutaneous and visceral metastases [15]. There have been uncontrolled studies suggesting that adjuvant radiotherapy may improve local control [16, 17], and recently the Trans Tasman Radiation Oncology Group (TROG) reported that patients at risk of regional failure (as defined by having multiple nodes, large nodes or extracapsular spread) randomized to receive adjuvant radiotherapy had a decreased risk of such failure at a median follow-up of 6 years compared with those who were not; there was, however, a high (40%) incidence of severe lymphoedema in those receiving treatment of the groin and no relapse-free or overall survival benefit [18, 19, 20]. The impact of adjuvant radiotherapy on survival is unknown but may be minimal even if local control is improved [21].

Figure 24.16 (a) Malignant melanoma, pre-radiotherapy. (b) Malignant melanoma, post-radiotherapy.
Lentigo maligna and lentigo maligna melanoma (see Chapter 143)
Radiotherapy has been used successfully in both of these conditions. In one German study, there was no recurrence in any of 42 patients with lentigo maligna, with a mean follow-up of 15 months, and only two of 22 patients with lentigo maligna melanoma showed local recurrence. Cosmetic results were also reported as good or excellent in the majority of patients [22]. Similar complete responses with good cosmetic outcomes have been reported from Australia [23] and Canada [24].
Merkel cell carcinoma (see Chapter 145)
These tumours of neuroendocrine origin are most common on the head and neck, but can occur elsewhere on the skin. They have a tendency to recur locally after conventional surgical removal and improved local control has now been shown in a large number of studies with a combination of wide excision and adjuvant postoperative radiotherapy to the primary tumour site and local nodal drainage [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]; some studies have also shown improved survival [29]. Mohs surgery is now widely used for removal of Merkel cell carcinoma and, if complete excision is achieved, adjuvant radiotherapy may not add benefit in terms of improved local control or survival [36]. These are radio-sensitive tumours and radiotherapy has been used as a primary treatment, with local control rates of over 75% [37, 38, 39, 40, 41].
Kaposi sarcoma (see Chapter 137)
It is well known that these tumours are sensitive to low-dose radiotherapy. Either superficial X-rays or electron beams can be used depending on the thickness of the lesion(s) and consequently the depth of treatment required. Treatment is used cautiously as patients are prone to develop severe local reactions and mucositis. Radiotherapy is also used to palliate systemic disease such as pulmonary masses [42, 43].
Dermatofibrosarcoma protuberans (see Chapter 137)
This low-grade sarcoma has a propensity to recur after surgery alone and local control is improved by giving adjuvant radiotherapy [44, 45].
Carcinoma metastatic to the skin from other primaries
Radiotherapy can provide useful palliation for cutaneous metastases from visceral tumours such as breast, colon and lung (Chapter 147).
Squamous cell and basal cell carcinoma in transplant patients (see Chapter 146)
Skin tumours developing after organ transplant are known to have more aggressive characteristics and carry a poorer prognosis [46]. These patients develop more SCCs than BCCs, reversing the normal ratio, and often develop multiple skin cancers at an earlier age. There is no contraindication to using radiotherapy if surgery is considered inappropriate and it may be used as adjuvant therapy for patients with more aggressive tumours [47].
Cutaneous lymphoma
Radiotherapy represents the single most effective treatment strategy for cutaneous lymphomas. In contrast to other skin cancers, lymphomas are extremely sensitive to radiotherapy with doses as low as 4 Gy sometimes sufficient for local control. Surgery is therefore rarely indicated unless there is a solitary nodule where excision biopsy can be both diagnostic and provide definitive local treatment. The low radiotherapy doses are almost always within the constraints of normal tissue tolerance and therefore radiotherapy can be safely delivered with minimal risk of long-term complications.
An overview of the classification and management of lymphomas is described in Chapter 139. The following summarizes the technical aspects of radiotherapy with a focus on mycosis fungoides, which accounts for 50% of cutaneous lymphomas.
Mycosis fungoides (see Chapter 139)
Mycosis fungoides evolves slowly with the majority of patients falling into a favourable prognostic group with a median survival of more than 35 years from presentation [1]. It is essential to involve specialist histopathologists, dermatologists, oncologists and haematologists in a multidisciplinary approach to management.
Individual patches, plaques and tumours
Whilst mycosis fungoides is normally not isolated to one area, local radiotherapy is frequently utilized for troublesome lesions with a local control rate in excess of 90% [2]. The recurrence rates are dependent on the dose given with a dose–response relationship observed up to doses of 30–40 Gy [3]. In practice, lower doses are preferred, as they still convey a high chance of local control, are more convenient for patients and permit overlap of adjacent fields or re-treatment if necessary [4]. The current recommended UK doses are 8 Gy in two fractions for patches and plaques, and 12 Gy in three fractions for tumours [5].
Radiotherapy can be delivered with X-rays (low-energy superficial or orthovoltage), or electrons as described earlier with a directly applied field. The target volume is the clinically defined lesion with a margin of 0.5–1 cm laterally. Patches and plaques are typically treated to a depth of 7–9 mm with careful assessment of the thickness of tumours to determine the appropriate beam energy and thickness of bolus when using electrons [6].
More extensive tumours, particularly when extending around curved areas require careful radiotherapy planning. Patient-specific immobilization devices such as thermoplastic shells for the head and neck region are essential to ensure accurate delivery of the planned radiotherapy treatment. More complex techniques include matched field electron plans and IMRT plans using modern machines such as a TomoTherapy unit (see Figures 24.5 and 24.6). When delivering radiotherapy to large tumours, treatment is normally fractionated with typical doses between 20 and 24 Gy in 10–12 fractions [6].
Total skin electron beam therapy
A significant number of patients with mycosis fungoides develop generalized skin involvement. Total skin electron beam therapy (TSEBT) can provide excellent palliation particularly when other skin-directed therapies including psoralens and ultraviolet A (PUVA) fail. The response rates and duration of response are dependent on the stage of the disease. A systematic review of TSEBT as monotherapy in 952 patients reported complete response rates of 96% in stage IA, IB and IIA disease. Despite this, the 5-year relapse-free survival rates were only 10–23% [7].
The standard dose for TSEBT is 30–36 Gy as endorsed by the European Organisation for Research and Treatment of Cancer (EORTC) and the American Association of Physicists in Medicine guidelines [8, 9]. The original Stanford schedule delivered the treatment over a 9–10-week period although this has been truncated in the more commonly used UK schedule of 30 Gy in 20 fractions over a 5-week period [6]. In recognition of the palliative nature of TSEBT, there has been growing interest in reduced dose schedules with favourable toxicity profiles. Whilst doses as low as 4 Gy in four fractions have been investigated, the duration of remission is short [10]. In a recent report, however, patients treated with 10 to <20 Gy and 20 to <30 Gy were found to have similar overall response rates (>50% improvement) and relapse-free survival rates to patients treated with conventional doses of 30–36 Gy [11].
The aim of TSEBT is to treat the entire target volume, which comprises the epidermis, adnexal structures and dermis to a uniform dose. The EORTC recommendations are that the 80% isodose should be at least 4 mm deep to the surface of the skin, and that the dose at 20 mm depth should be less than 20% of the maximum dose at the skin surface [8]. There are a large number of different treatment techniques used to deliver TSEBT, the most common of which is the modified Stanford technique. This involves treating the patient standing in six positions holding onto a frame for stability (Figure 24.17). The distance between the linear accelerator and the patient needs to be at least 3 m. At this distance, the beams are angled approximately 20° above and below the central axis to deliver a reasonably uniform dose distribution. With this technique, the typical variation in dose at the skin surface is ±10% dropping to ±5% at 3 mm depth [9].
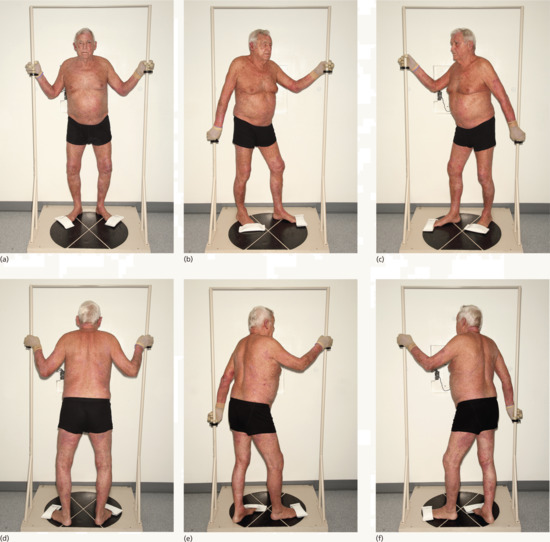
Figure 24.17 (a–f) The modified Stanford technique. Patient positions to optimize a uniform dose to all the skin when using total skin electron beam therapy (TSEBT).
Despite this being described as the ‘optimal’ technique, there is inherent shielding of the scalp, perineum and soles of the feet which may require additional treatment. Conversely, some areas such as the wrists, ankles and ears may require shielding due to higher doses being received; shielding may also be required in previously treated areas. Sensitive areas which are spared from involvement with mycosis fungoides such as the nail beds and testicles can be shielded with lead fingernail shields and a cricket box, respectively. Custom-made lead-lined goggles are also used to shield the eyes which can be treated afterwards with superficial X-rays if there is involvement of the eyelids.
The side effects are related to the dose given, with an increased chance of toxicity with standard doses of 30–36 Gy [12]. The most common acute complications of standard dose TSEBT are erythema and moist desquamation which can be severe in patients with erythroderma. Temporary loss of fingernails and toenails also occurs if these are not shielded. Long-term side effects include xerosis and an inability to sweat properly for 6–12 months. Due to concern over possible skin atrophy and potential necrosis there is a reluctance to offer more than two conventional courses of TSEBT in a patient's lifetime.
Other lymphomas (see Chapter 139)
The technical approach to radiotherapy planning for other types of skin lymphoma is similar to that outlined earlier for individual patches, plaques and tumours of mycosis fungoides. A brief commentary on radiotherapy for specific histological subtypes is provided below.
Other cutaneous T-cell lymphomas
The approach to radiotherapy for primary cutaneous anaplastic large cell lymphoma, and subcutaneous panniculitis-like T-cell lymphoma is similar. Patients presenting with solitary or localized disease can be treated with radical radiotherapy with a recommended dose of 40 Gy in 20 fractions [13]. Lower doses may be equally effective but there are no published data. Systemic therapies should be considered for patients with multiple lesions, although short palliative radiotherapy schedules can be useful. Primary cutaneous CD4+ small or medium-sized pleomorphic T-cell lymphoma typically presents with a solitary nodule on the head and neck region which can be treated with surgery or radiotherapy.
Other cutaneous T-cell lymphomas (CTCL) including cutaneous γ-δ T-cell lymphoma, aggressive epidermotropic CD8-positive T-cell lymphoma, and extranodal natural killer T-cell lymphoma, nasal-type, which can occasionally present in the skin, have in common an aggressive clinical course with poor survival despite multiagent chemotherapy.
Cutaneous B-cell lymphoma
The EORTC published consensus recommendations for the management of cutaneous B-cell lymphoma (CBCL) in 2008 which were largely based on expert opinion due to lack of available evidence [14]. In the World Health Organization (WHO)–EORTC classification, three main types of CBCL are described. Primary cutaneous follicle centre lymphoma (PCFCL) and primary cutaneous marginal zone lymphoma (PCMZL) are indolent conditions with 10-year survival rates in excess of 90%. The EORTC recommend a dose of 30 Gy with a margin of 1–1.5 cm of uninvolved skin. In contrast, primary cutaneous diffuse large cell lymphoma, leg type, is an aggressive lymphoma typically affecting the elderly with frequent involvement of extracutaneous sites. As a result, systemic chemotherapy is indicated followed by radiotherapy to the lesion with generous margins of 2–5 cm.
Acute radiation reaction (acute radiodermatitis) (Figures 24.18a–c and 24.19a–c)
Radiation works by damaging clonogenic cells, both benign and malignant, and the damage is expressed at the next cell division, when the cell either dies or repairs sublethal damage. Tissues with a rapid turnover such as the epidermis and mucosal endothelium therefore show radiation damage soon after treatment, and this is the ‘acute reaction’. For the treatment to result in cure, the tumour must be unable to repair this damage [1,2].

Figure 24.18 (a) Large BCC of the cheek. (b) The same patient showing acute radiation reaction 6 weeks after superficial X-ray treatment. (c) The same patient showing settled reaction 6 months after superficial X-ray treatment.
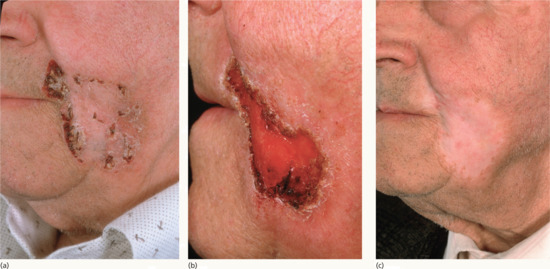
Figure 24.19 (a) Large BCC of the jaw. (b) The same patient showing acute radiation reaction 4 weeks after superficial X-ray treatment. (c) The same patient showing settled reaction 6 months after superficial X-ray treatment.
In acute radiodermatitis, histopathology shows oedema and sparseness of connective tissue beneath the epidermis. There may be flattening and loss of epidermal rete ridges with separation of the elastic tissue from the basal layer. Capillary endothelium may be hypertrophic and congested capillaries a feature. Haemorrhage and thrombosis are often observed. Special stains may show subtle changes in the DNA–RNA structure of epithelial cells as early as the third day [1, 2]. During the healing phase, the patchiness of the pathology is a striking feature. Atrophy may be bordered by epidermal hyperplasia, pigmentation is very irregular, and blood vessels are of variable size and shape; deeper vessels may be fibrosed. Normal tissue repair occurs when normal clonogenic cells on the basement membrane, which have either not received damage or have repaired sublethal damage, repopulate the basement membrane and re-cover the epithelium [3].
In a commonly used regimen, where the patient attends at daily intervals for five doses of treatment, the irradiated skin starts to redden 5–7 days after the first treatment, because of increased vascularity and inflammation associated with cell damage. Subsequent doses add to the reaction, which proceeds through dry and sometimes moist desquamation and scabbing, usually reaching a height of discomfort at 3–4 weeks after treatment finishes; it then settles over the next 2–3 weeks with re-epithelialization, which begins in the perifollicular areas and proceeds as cell colonies coalesce to cover the denuded surface.
If the skin is previously unbroken or the tumour nodular, treatment may be followed by no more than a very mild erythematous reaction with little discomfort for the patient. The acute reaction is more painful if there is ulceration and scabbing before treatment commences. The larger the surface area of the tumour and therefore the treatment field, the longer the healing takes; even so, healing is commonly virtually complete by 6 weeks after the end of the course. The brisker the treatment, i.e. the larger the dose and the shorter the time over which it is given, the more marked the acute reaction is likely to be, as there is less opportunity for normal tissue repair between treatments. Individual skin reactions vary, and those who burn in the sun tend to have a brisker reaction to X-ray treatment. Occasionally, healing may be complicated by secondary impetiginization, especially on the face.
If the exit beam passes through mucosa, a patch of mucositis of similar size to the treatment field will ensue. This reaction usually occurs earlier and is of much shorter duration than the skin reaction, and heals completely without long-term sequelae. The nasal mucosa may swell and occasionally bleed, with the feeling of a blocked nose; in the mouth, a sore patch may make eating uncomfortable for a few days. Although protective lead can be used to protect structures beyond the inner mucosa, such as the gums and the nasal septum, the mucosa just beneath the cancer cannot be protected.
It is unusual to see acute radiation reactions with diagnostic imaging but it can occur with fluoroscopy. Both dose and length of the diagnostic procedure are important causal factors. Balter et al. have comprehensively reviewed this [4].
Management
The natural history of the acute reaction cannot be modified, but the patient can avoid making the reaction worse. The area should be left open to the air as far as possible and protected from trauma, extremes of heat or cold, irritation with medicated creams or ointments, physical rubbing or vigorous and repeated washing, all of which can damage the regenerating clonogenic cells and result in ulceration. Steroid creams are also best avoided. Patients are warned to seek advice if signs of infection develop. Once the treated area is dry and scabbed, normal washing can resume. The mucosal reaction must be endured, although soft paraffin and/or a simple mouthwash helpful it may provide relief and if the patient is having excessive nasal crusting, simple saline nasal douches may make the patient more comfortable.
Late radiation reaction (chronic radiodermatitis) (Figures 24.20 and 24.21)
Tissues with a slow rate of cell division such as subcutaneous fat, fibrous tissue and small blood vessels will not show radiation effects until months or years after treatment. At 6 months, treated skin is often slightly paler or pinker than adjacent untreated skin; with the passage of time, sequelae usually appear, with late appearances established at 5–10 years. The irradiated skin may show pallor or increased pigmentation, with variable atrophy, telangiectasia, fibrosis and loss of appendageal structures. With time, the blood supply of the treated area may become compromised, resulting in ulceration either spontaneously or after trauma; such ulcers often heal spontaneously, however [5].

Figure 24.20 (a) Extensive erosive BCC of the ear. (b) The same patient 2 years after superficial X-ray treatment.

Figure 24.21 Telangiectasia after treatment for a small BCC of the nose.
The fundamental histopathology of chronic radiodermatitis is fibrosis and occlusion of the cutaneous vasculature with varying degrees of homogenization of dermal connective tissue. Residual vessels may be enormously dilated. Bizarre, large, stellate fibroblasts may be seen in the dermis. Fibrosis of the deep dermis and subcutaneous tissue may occasionally occur after megavoltage radiotherapy [6]. The changes in the epidermis vary from simple atrophy to acanthosis and extreme dyskeratosis. There is usually loss of adnexa such as hair follicles.
The late cosmetic result varies and may reflect the underlying damage caused initially by the tumour as well as the patient's skin type. In one study of 339 patients, telangiectasia, pigmentation and fibrosis were directly related to increasing size of field needed for treatment rather than the treatment regimen used [7]. The late cosmetic result is also less good if the patient has had repeated cryotherapy to the area (authors’ personal observation) or been left with a poor surgical scar. Chronic radiation change may sometimes be difficult to distinguish from tumour recurrence.
Ischaemic necrosis following radiotherapy should rarely be seen when modern techniques of dose fractionation are used. Radionecrosis typically occurs 1–2 years after treatment and is often precipitated by trauma or infection. Excision and grafting may provide the only satisfactory treatment of extensive radionecrosis, although small areas may slowly heal with conservative management [5].
Induction of skin tumours within irradiated fields is very rare indeed, as would be expected with the latency for this and the average age of the patient receiving radiotherapy for skin cancers.
Tumour recurrence after radiotherapy
BCC and SCC are not always cured by radiotherapy [1, 2, 3], especially if the patient has had prior treatment with multiple modalities [4]. Ulceration lasting more than 6 months after therapy usually means persistent tumour. Some authorities suggest that one reason for avoiding primary radiotherapy is that surgical salvage is more difficult after treatment failure, but if the patient is referred on at an early stage, excision of the irradiated skin and grafting is usually possible. Mohs surgery may give the best results in these cases [4]. As with surgical treatment, radiotherapy for recurrent BCC has a higher relapse rate. Age, immunosuppression and treatment modality are also important as well as size of tumour for recurrence [5].
Re-treatment with radiation is not usually offered, because the primary is by definition relatively radio-resistant. There is an increased risk of radionecrosis if normal tissues, which may have already received a ‘tolerance dose’, are exposed to further radiation. However, in practice, radiotherapy has been given successfully in these circumstances with acceptable cosmetic results, especially if there has been a sufficient time interval between the two radiotherapy treatments [6]. Occasionally, it is reasonable to offer treatment to persistent tumour at the edge of a previous radiotherapy field, when a small degree of overlap may be acceptable; although the risk of a small area of necrosis will be higher, this strategy is often acceptable to the patient, and successful.
Radiation-induced tumours
There is evidence that the risk both of BCC and of SCC is increased in areas of skin previously irradiated for benign or malignant disease including Hodgkin disease, breast cancer, acne, ankylosing spondylitis and tinea capitis [1, 2].
BCCs of the scalp may not present until 50 years after X-ray epilation for ringworm. Such tumours are much more rarely seen since the virtual abandonment of radiotherapy for benign diseases, the development of more sophisticated radiotherapy machinery and advances in the understanding of radiobiology. It has not, however, been possible to demonstrate any precise quantitative relationship between the development of skin cancers and the amount of radiation received on the skin surface, nor is it known what total dose or fractionation regimen would be most carcinogenic.
Although a greater knowledge of the limitations and effectiveness of radiation should prevent the occurrence of late radiation damage including carcinogenicity, the long latent periods involved warn against early complacency [3, 4, 5].
As the numbers of childhood cancer survivors increases, there is some evidence for an increased incidence of BCC developing within irradiated skin sites [6], but this is not confirmed by all studies [7]. As most of these children will also have had treatment with chemotherapy, the aetiological factors causing their second malignancy cannot be easily disentangled.
Management
Most radiation-induced tumours should be excised. However, where there is no radiation damage evident on the skin, a subsequent radical dose of radiotherapy can normally be tolerated [8].
Rare tumours associated with previous irradiation
Atypical fibroxanthoma (see Chapter 137) (syn. pseudosarcoma of the skin)
This tumour, seen particularly in fair-skinned males who have suffered actinic damage, may also follow radiation damage [9, 10, 11].
Radiation-induced sarcoma (see Chapter 137)
Radiotherapy-induced sarcomas make up an estimated 5% of all sarcomas and occur in less than 0.2% of patients who have had radiotherapy [12, 13]. Children are more susceptible to developing radiation-induced cancer [14]. There can be a long latent period with sarcomas appearing 5–15 years or even longer post-treatment [15, 16].
Post-irradiation sarcomas of both soft tissue and bone are characteristically high grade and include undifferentiated pleomorphic sarcoma (previously known as malignant fibrous histiocytoma), spindle cell sarcoma, osteosarcoma, leiomyosarcoma and angiosarcoma, the latter being especially associated with radiotherapy for breast cancer [15].
Development of sarcoma appears to be related to exposure to kilovoltage rather than megavoltage radiotherapy and to receiving higher doses of radiotherapy. It is also associated with a range of genetic syndromes which may predispose to cancer development such as Li–Fraumeni syndrome.
Diagnosis and investigation are as for primary sarcomas, including a carefully planned biopsy.
There is evidence that prognosis is worse for radiation-induced sarcomas compared to primary sarcomas [16]. Surgery and chemotherapy are the primary treatment modalities with re-irradiation usually avoided.
References
X-ray photon beams: megavoltage and kilovoltage X-ray therapy
- Caccialanza M, Piccinno R, Kolesnikova L. Radiotherapy of skin carcinomas of the pinna: a study of 115 lesions in 108 patients. Int J Dermatol 2005;44:513–17.
- Miller RA, Spittle MF. Electron beam therapy for difficult cutaneous basal and squamous cell carcinoma. Br J Dermatol 1982;106:4429–36.
- Spittle MF. Mycosis fungoides. Electron beam therapy in England. Cancer Treat Rep 1979;63:639–41.
- Freiman A, Sasseville D. Treatment of mycosis fungoides: overview. J Cutan Med Surg 2006;10:228–33.
- Floyd SR, Pantanowitz L, McDermott DF et al. Plasma cell problems: Case 1. Disseminated cutaneous plasmacytomas treated with total skin electron radiotherapy. J Clin Oncol 2005;23:3138–40.
- Knobler E. Current management strategies for cutaneous T-cell lymphoma. Clin Dermatol 2004;22:197–208.
- Lundin J, Osterborg A. Therapy for mycosis fungoides. Curr Treat Options Oncol 2004;5:203–14.
- Fuks A, Bagshaw MA. Total-skin electron treatment of mycosis fungoides. Radiology 1971;100:145–50.
Moulds, applicators and implants
- Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys 2000;47:95–102.
- Berridge JK, Morgan DAL. A comparison of late cosmetic results following two different radiotherapy techniques for treating basal cell carcinoma. Clin Oncol 1997;9:400–2.
- Celada F, Rodriguez S, Botella R, et al. Non-malignant skin cancer treated with HDR Valencia applicator: clinical outcomes. J Contemp Brachy 2014;6:167–72.
Radiosensitivity
- Fitzpatrick PJ, Thompson GA, Easterbrook WM, et al. Basal and squamous cell carcinoma of the eyelids and their treatment by radiotherapy. Int J Rad Oncol Biol Phys 1984;10:449–54.
- Shore RE. Radiation-induced skin cancer in humans. Med Pediatr Oncol 2001;36:549–54.
- Traenkle HL. X-ray induced skin cancer in man. Natl Cancer Inst Monogr 1963;10:423–32.
Benign disease
- Shore RE, Albert RE, Reed M, et al. Skin cancer incidence among children irradiated for ringworm of the scalp. Radiat Res 1984;100:192–204.
- Mould R. A Century of X-rays and Radioactivity in Medicine, with Emphasis on Photographic Records of the Early Years. Bristol: Institute of Physics Publishing, 1993.
- Mortensen AC, Kjeldsen H. Carcinomas following Grenz ray treatment of benign dermatoses. Acta Derm Venereol (Stockh) 1987;67:523–5.
- Bleehen NM. Insights from radiation treatment for benign disease. BMJ 1987;295:512–13.
- Rowell NR. A follow-up study of superficial radiotherapy for benign dermatoses: recommendations for the use of X-rays in dermatology. Br J Dermatol 1973;88:583–90.
- Rowell NR. Ionizing radiation in benign dermatoses. In: Rook AJ, ed. Recent Advances in Dermatology, Vol. 4. Edinburgh: Churchill Livingstone, 1977:329–50.
- Fairris GM, Jones DH, Mack DP, et al. Conventional superficial X-ray versus Grenz ray therapy in the treatment of constitutional eczema of the hands. Br J Dermatol 1985;112:339–41.
- Caccialanza M, Sopelana N. Radiation therapy of keratoacanthomas: results in 55 patients. Int J Rad Oncol Biol Phys 1988;16:475–7.
- Donahue B, Cooper JS, Rush S. Treatment of aggressive keratoacanthomas by radiotherapy. J Am Acad Dermatol 1990;23:489–93.
- National Institute for Clinical Excellence. Interventional Procedure Overview of Grenz Rays Therapy for Inflammatory Skin Conditions, March 2007 www.nice.org.uk/IPG236 (last accessed April 2015).
Keloids
- Doornbos JF, Stoffel SJ, Hass AC, et al. The role of kilovoltage irradiation in the treatment of keloids. Int J Rad Oncol Biol Phys 1990;18:833–9.
- Kovalic JJ, Perez C. Radiation therapy following keloidectomy: a 20-year experience. Int J Rad Oncol Biol Phys 1989;17:77–80.
- Lo TCM, Seckel BR, Salzman FA, et al. Single-dose electron beam irradiation in treatment and prevention of keloids and hypertrophic scars. J Radiother Oncol 1990;19:267–72.
- Borok TL, Bray M, Sinclair I, et al. Role of ionizing irradiation for 393 keloids. Int J Rad Oncol Biol Phys 1988;15:865–70.
- Bischof M, Krempien R, Debus J, Treiber M. Postoperative electron beam radiotherapy for keloids: objective findings and patient satisfaction in self-assessment. Int J Dermatol 2007;46:971–5.
- Rio E, Bardet E, Ferron C. Interstitial brachytherapy of perioral facial skin carcinomas of the face: A retrospective study of 97 cases Int J Rad Oncol Biol Phys 2005;63:753–57.
- Escarmant P, Zimmernann S, Amar A, et al. The treatment of 783 keloid scars by iridium 192 interstitial irradiation after surgical excision. Int J Rad Oncol Biol Phys 1993;26:245–51.
- De Cicco L, Vischioni B, Vavassori A, et al. Post-operative management of keloids: low dose rate and high-dose rate brachytherapy. Brachytherapy 2014;13:508–13.
Basal cell carcinoma and squamous cell carcinoma of the skin
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys 2001;51:748–55.
- Morrison WH, Garden AS, Ang KK. Radiation therapy for nonmelanoma skin carcinomas. Clin Plast Surg 1997;24:719–29.
- Goldschmidt H, Breneman JC, Breneman DL. Ionizing radiation therapy in dermatology. J Am Acad Dermatol1994;30:157–82.
- Ashby MA, McEwan L. Treatment of non-melanoma skin cancer: a review of recent trends with special reference to the Australian scene. Clin Oncol (R Coll Radiol)1990;2:284–94.
- Cho M, Gordon L, Rembielak A, Woo TC. Utility of radiotherapy for treatment of basa cell carcinoma: a review. Br J Dermatol 2014;171:5:968–73.
- Kearsley H, Harris TJ, Bourne RG. Radiotherapy for superficial skin cancer at the Queensland Radium Institute: famine in the land of plenty. Int J Radiat Oncol Biol Phys 1988;15:995–9.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow up. J Dermatol Surg Oncol 1989;15:315–28.
- Bath-Hextall FJ, Perkins W, Bong J, et al. Interventions for basal cell carcinoma of the skin. Cochrane Collaboration Review, 2007. Cochrane Database Syst Rev 2007;Oct(4):CD005414.
- Dubin N, Kopf AW. Multivariate risk score for the recurrence of cutaneous basal cell carcinomas. Arch Dermatol 1983;119:373–7.
- McPartlin AJ, Slevin Nj, Sykes AJ, et al. Radiotherapy treatment of nonmelanoma skin cancer: a survey of current UK practice and commentary Br J Radiol 2014 Nov;87(1043):20140501.
- Barrett-Lee P. Radiotherapy for skin cancer: as good as surgery? Br J Dermatol 2014;171:930.
- Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys 2000;47:95–102.
- Motley RJ, Gould DJ, Douglas WS, Simpson NB. Treatment of basal cell carcinoma by dermatologists in the United Kingdom. British Association of Dermatologists Audit Subcommittee and the British Society for Dermatological Surgery. Br J Dermatol 1995;132:437–40.
- Kuijpers DI, Thissen MR, Neumann MH. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol 2002;3:247–59.
- National Institute for Health and Clinical Excellence (NICE). Improving Outcomes for People with Skin Tumours including Melanoma: The Manual, February 2006 http://www.nice.org.uk/guidance/csgstim/evidence/improving-outcomes-for-people-with-skin-tumours-including-melanoma-the-manual-2006-guidance2 (last accessed July 2015).
- British Association of Dermatologists Clinical Guidelines http://www.bad.org.uk/healthcare-professionals/clinical-standards/clinical-guidelines (last accessed July 2015).
- National Comprehensive Cancer Network (NCCN). Skin Cancer Guidelines www.nccn.org/professionals/physician_gls/PDF/nmsc.pdf (last accessed April 2015).
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomised study. Br J Cancer 1997;76:100–6.
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs' micrographic surgery for basal cell carcinoma of the face: randomised controlled trial. Lancet 2004;364:1766–72.
- Olschewski T, Bajor K, Lang B, et al. Radiotherapy of basal cell carcinoma of the face and head: Importance of low dose per fraction on long-term outcome. J German Soc Dermatol 2006;4:124–30.
- Schulte KW, Lippold A, Auras C, et al. Soft X-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol 2005;53:993–1001.
- National Institute for Health and Clinical Excellence (NICE). Improving Outcomes for People with Skin Tumours including Melanoma: The Manual, February 2006:118 http://www.nice.org.uk/guidance/csgstim/evidence/improving-outcomes-for-people-with-skin-tumours-including-melanoma-the-manual-2006-guidance2 (last accessed July 2015).
- Podd TJ. treatment of lower limb basal cell and squamous cell carcinomas with radiotherapy. Clin Oncol 1992;4:44–5.
- Petrovich Z, Kuisk H, Langholz B, et al. Results and patterns of failure in 646 patients with carcinoma of the eyelids, pinna, and nose. Am J Surg 1987;154:147–50.
- Griep C, Davelaar J, Scholten AN, et al. Electron beam therapy is not inferior to superficial x-ray therapy in the treatment of skin carcinoma. Int J Radiat Oncol Biol Phys1995;32:1347–50.
- Silva JJ, Tsang RW, Panzarella T, et al. Results of radiotherapy for epithelial skin cancer of the pinna: the Princess Margaret Hospital experience, 1982–1993. Int J Radiat Oncol Biol Phys 2000, 47:451–9.
- Avila J, Bosch A, Aristizabal S, et al. Carcinoma of the pinna. Cancer 1977;40:2891–5.
- Lim JT. Irradiation of the pinna with superficial kilovoltage radiotherapy. Clin Oncol (R Coll Radiol) 1992;4:236–9.
- Bertelsen K, Gadeberg C. Carcinoma of the eyelid. Acta Radiol Oncol Radiat Phys Biol 1978;17:58–64.
- Geohas J, Roholt NS, Robinson JK. Adjuvant radiotherapy after excision of cutaneous squamous cell carcinoma. J Am Acad Dermatol 1994;30:633–6.
- Han A, Ratner D. What is the role of adjuvant radiotherapy in the treatment of cutaneous squamous cell carcinoma with perineural invasion? Cancer 2007;109:1053–9.
- Garcia-Serra A, Hinerman RW, Mendenhall WM. Carcinoma of the skin with peri-neural invasion. Head & Neck 2003;12:1027–33.
- Fogarty GB, Burt J, Ainslie J. Delay of postoperative radiotherapy can be associated with recurrence. J Plast Reconstr Aesthet Surg 2006;59:203–5.
- Lui FF, Maki R, Warde P, Payne D, Fitzpatrick P. A management approach to incompletely excised basal cell carcinomas of skin. Int J Radiat Oncol Biol Phys 1991;20:423–8.
- Chan S, Dhadda AS, Swindell R. Single fraction radiotherapy for small superficial carcinoma of the skin. Clin Oncol 2007;19:256–9.
- Hliniak A, Maciejewski B, Trott K. The influence of the number of fractions, overall treatment time and field size on the local control of cancer of the skin Br J Radiol 1983;56:596–8.
- Berridge JK, Morgan DA. A comparison of late cosmetic results following two different radiotherapy techniques for treating basal cell carcinoma. Clin Oncol (R Coll Radiol) 1997;9:400–2.
- Rowe DE, Carroll RJ, Day CL. Longterm recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient followup. J Dermatol Surg Oncol 1989;15:315–28.
Radiotherapy for particular skin sites: basal cell carcinoma and squamous cell carcinoma
- Leshin B, Yeatts P, Anscher M, et al. Management of periocular basal cell carcinoma: Mohs' micrographic surgery versus radiotherapy Surv Ophthalmol 1993;38:193–212.
- Malhotra R, Huilgol SC, Huynh NT, et al. The Australian Mohs' database, Part II: Periocular basal cell carcinoma outcome at 5 year follow up Ophthalmology 2004;111:631–6.
- Lim JTW. Irradiation of the pinna with superficial kilovoltage radiotherapy. Clin Oncol 1992;4:236–9.
- Hayter CRR, Lee KHY, Groome PA, et al. Necrosis following radiotherapy for carcinoma of the pinna. Int J Radiat Oncol Biol Phys 1996;36:1033–7.
- Hunter RD, Pereira DTM, Pointon RCS. Megavoltage electron beam therapy in the treatment of basal and squamous cell carcinomata of the pinna. Clin Radiol 1982;33:341–5.
- Matthiesen C, Thompson JS, Forest C, et al. The role of radiotherapy for T4 nonmelanoma skin carcinoma. J Med Imaging Rad Oncol 2011;55:407–16.
- Sykes AJ, Allan E, Irwin C. Squamous cell carcinoma of the lip: the role of electron treatment. Clin Oncol 1996;8:384–6.
- Guibert M, David I, Vergez S, et al. Brachytherapy in lip carcinoma: long-term results. Int J Radiat Oncol Biol Phys 2011;81;e839–43.
- Veness MJ, Porceddu S, Palme CE. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head & Neck 2007;29:621–31.
- Jol JA, Van Velthuysen ML, Hilgers FJ. Treatment results of regional metastasis from cutaneous head and neck squamous cell carcinoma. EJSO 2002;29:81–6.
- Audet N, Palme CE, Gullane PJ. Cutaneous metastatic squamous cell carcinoma to the parotid gland: Analysis and outcome. Head & Neck 2004;26:727–32.
- Hong TS, Kriesel KJ, Hartig GK. Parotid area lymph node metastases from cutaneous squamous cell carcinoma: Implications from diagnosis, treatment, and prognosis. Head & Neck 2005;27:851–6.
- Ch'ng S, Maitra A, Lea R. Parotid metastasis – An independent prognostic factor for head and neck cutaneous squamous cell carcinoma. J Plas Reconst & Aesthet Surg 2006;59:1288–93.
Radiotherapy for particular skin tumours
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. Part 4: X-ray therapy. J Dermatol Surg Oncol 1992;18:549.
- Cho M, Gordon L, Rembielak A, et al. Utility of radiotherapy for treatment of basal cell carcinoma: a review. Br J Dermatol 2014;171:968–73.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys 2001;51:748.
- Fleming ID, Amonette R, Monaghan T, Fleming MD. Principles of management of basal and squamous cell carcinoma of the skin. Cancer 1995;75:699.
- Petrovich Z, Kuisk H, Langholz B, et al. Treatment of carcinoma of the skin with bone and/or cartilage involvement. Am J Clin Oncol 1988;11:110–13.
- Motley R, Kersey P, Lawrence C. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol 2002;146:18–25.
- Tsao MN, Tsang RW, Liu FF. Radiotherapy management for squamous cell carcinoma of the nasal skin: The Princess Margaret hospital experience. Int J Rad Onc Biol Phys 2002;52:973–9.
- Lovett RD, Perez CA, Shapiro SJ, Garcia DM. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys 1990;19:235.
- Veness MJ. Treatment recommendations in patients diagnosed with high-risk cutaneous squamous cell carcinoma. Australas Radiol 2005;49:365–76.
- Lee WR, Mendenhall WM, Parsons JT, Million RR. Radical radiotherapy for T4 carcinoma of the skin of the head and neck: a multivariate analysis. Head & Neck 1993;15:320–4.
- Dupree ML, Kiteley RA, Weismantle K, et al. Radiation therapy for Bowen's disease: lessons for lesions of the lower extremity. J Am Acad Dermatol 2001;45:401–4.
- Cox NH, Dyson P. Wound healing on the lower leg after radiotherapy or cryotherapy of Bowen's disease and other malignant skin lesions. Br J Dermatol 1995;133:60–5.
- VanderSpek LA, Pond GR, Wells W. Radiation therapy for Bowen's disease of the skin. Int J Rad Oncol Biol Phys 2005 ;63:505–10.
- Chang DT, Amdur RJ, Morris CG. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys 2006;66:1051–5.
- Seegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, et al. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. Int J Radiat Oncol Biol Phys 1999;44:607–18.
- Burmeister BH, Smithers BM, Davis S. Radiation therapy following nodal surgery for melanoma: an analysis of late toxicity. Aust NZ J Surg 2002;72:344–8.
- O'Brien CJ, Petersen-Schafer K, Papadopoulos T, et al. Evaluation of 107 therapeutic and elective parotidectomies for cutaneous melanoma. Am J Surg 1994;168,5:400–3.
- Burmeister BH, Mark SB, Burmeister E. A prospective phase II study of adjuvant post operative radiation therapy following nodal surgery in malignant melanoma Trans Tasman Radiation Oncology Group (TROG) study 96.06 Radiother Oncol 2006;8:136–42.
- Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol 2012;13:589.
- Henderson M. Adjuvant radiotherapy after lymphadenectomy in melanoma patients: Final results of an intergroup randomized trial (ANZMTG 0.1.02/TROG 02.01). American Society of Clinical Oncology (ASCO), abstract 9001 of a presentation at the 2013 ASCO meeting, 2013; can also be found at J Clin Oncol 2013;31(15) Suppl. May 20:9001.
- Mendenhall WM, Amdur RJ, Grobmeyer SR. Adjuvant radiotherapy for cutaneous melanoma. Cancer 2008;112:1189–96.
- Schmid-Wendtner MH, Brunner B, Konz B, et al. Fractionated radiotherapy of lentigo maligna and lentigo maligna melanoma in 64 patients. J Am Acad Dermatol 2000;43:477–82.
- Harwood AR. Conventional radiotherapy in treatment of lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol 1982;6:310–16.
- Tsang RW, Liu FF, Wells W, Payne DG. Lentigo maligna of the head and neck. Results of treatment by radiotherapy. Arch Dermatol 1994;130:1008–12.
- Jabbour J, Cumming R, Scolyer RA. Merkel cell carcinoma: Assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in the early stage disease – results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol 2007;14:1943–52.
- Veness MJ. Merkel cell carcinoma (primary cutaneous neuroendocrine carcinoma): An overview on management. Aust J Dermatol 2006;47:160–5.
- Veness MJ, Perera l, McCourt J. Merkel cell carcinoma: Improved outcome with adjuvant radiotherapy. ANZ J Surg 2005;75: 275–81.
- Clark JR, Veness MJ, Gilbert R. Merkel cell carcinoma of the head and neck: Is adjuvant radiotherapy necessary? Head & Neck 2007;29:249– 57.
- Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol 2007;25:1043–7.
- Eich HT, Eich D, Staar S, et al. Role of postoperative radiotherapy in the management of Merkel cell carcinoma. Am J Clin Oncol 2002;25:50–6.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol 2001;8:204–8.
- Fenig E, Brenner B, Katz A, et al. The role of radiation therapy and chemotherapy in the treatment of Merkel cell carcinoma. Cancer 1997;80:881–5.
- Kokoska ER, Kokoska MS, Collins BT, et al. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg 1997;174:688–93.
- al-Ghazal SK, Hong A. Merkel cell carcinoma of the skin treated by primary radiotherapy. Br J Dermatol 1997;136:640–1.
- Suntharalingam M, Rudoltz MS, Mendenhall WM. Radiotherapy for Merkel cell carcinoma of the skin of the head and neck. Head & Neck 1995;17:96–101.
- Boyer JD, Zitelli JA, Brodland DG et al. Local control of primary Merkel cell carcinoma: Review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiation. J Am Acad Dermatol 2002;47:885–91.
- Morrison WH, Peters LJ, Silva EG. The essential role of radiation therapy in securing loco-regional control of Merkel cell carcinoma. Int J Rad Oncol Biol Phys 1990;19:583–91.
- Mortier L, Mirabel X, Fournier C. Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol 2003;139:1587–90.
- Veness M, Foote M, Gebski V, Poulsen M. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys 2010;78:703.
- Pape E, Rezvoy N, Penel N, et al. Radiotherapy alone for Merkel cell carcinoma: a comparative and retrospective study of 25 patients. J Am Acad Dermatol 2011;65:983.
- Sundaresan P, Hruby G, Hamilton A, et al. Definitive radiotherapy or chemoradiotherapy in the treatment of Merkel cell carcinoma. Clin Oncol (R Coll Radiol) 2012;24:e131.
- Kirova YM, Belembaogo E, Frikha H. Radiotherapy in the management of epidemic Kaposi's sarcoma: a retrospective study of 643 cases. Radiother Oncol 1998;46:19–22.
- Harrison M, Harrington KJ, Tomlinson DR, Stewart JS. Response and cosmetic outcome of two fractionation regimens for AIDS-related Kaposi's sarcoma. Radiother Oncol 1998;46:23–8.
- Ballo MT, Zagars GK, Pisters P, Pollack A. The role of radiation therapy in the management of dermatofibrosarcoma protuberans. Int J Radiat Oncol Biol Phys 1998;40:823–7.
- Haas RL, Keus RB, Loftus BM, The role of radiotherapy in the local management of dermatofibrosarcoma protuberans. Soft Tissue Tumours Working Group. Eur J Cancer 1997;33:1055–60.
- Veness MJ, Quinn DI, Ong CS. Aggressive cutaneous malignancies following cardiothoracic transplantation, the Australian experience. Cancer 1999;85:1758–64.
- Veness MJ, Harris D. Role of radiotherapy in the management of organ transplant recipients diagnosed with non-melanoma skin cancers. Australas Radiol 2007;51:12–20.
Mycosis fungoides and other lymphomas
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010;28(31):4730–9.
- Hoppe RT. Mycosis fungoides: radiation therapy. Dermatol Ther 2003;16(4):347–54.
- Cotter GW, Baglan RJ, Wasserman TH, et al. Palliative radiation treatment of cutaneous mycosis fungoides – a dose response. Int J Radiat Oncol Biol Phys 1983;9(10):1477–80.
- Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys 2009;74(1):154–8.
- Whittaker SJ, Marsden JR, Spittle M, et al. Joint British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. Br J Dermatol 2003;149(6):1095–107.
- Morris SL. Skin lymphoma. Clin Oncol (R Coll Radiol) 2012;24(5):371–85.
- Jones GW, Hoppe RT, Glatstein E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol Oncol Clin North Am 1995;9(5):1057–76.
- Jones GW, Kacinski BM, Wilson LD, et al. Total skin electron radiation in the management of mycosis fungoides: Consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J Am Acad Dermatol 2002;47(3):364–70.
- Karzmark CJ, Anderson J, Fessenden P, et al. Total Skin Electron Therapy: Technique and Dosimetry. Report of Task Group 30 Radiation Therapy Committee, American Association of Physicists in Medicine, 1988, AAPM report No. 23 http://www.aapm.org (last accessed July 2015).
- Kamstrup MR, Specht L, Skovgaard GL, et al. A prospective, open-label study of low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys 2008;71(4):1204–7.
- Harrison C, Young J, Navi D, et al. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys 2011;15:81(4):e651–7.
- Hoppe RT, Fuks Z, Bagshaw MA. Radiation therapy in the management of cutaneous T-cell lymphomas. Cancer Treat Rep 1979;63(4):625–32.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl. 6:vi149–54.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 2008;112(5):1600–9.
Acute radiation reaction (acute radiodermatitis)
- Kurban AK, Farah FS. Effects of X-irradiation of the skin. Acta Derm Venereol (Stockh) 1969;49:64–71.
- Panizzon RG, Cooper JS, eds. Radiation Treatment and Radiation Reactions in Dermatology. Berlin: Springer, 2004.
- Harrington K, Jankowska P, Hingorani, M. Overview: molecular biology for the radiation oncologist: the 5Rs of radiobiology meet the hallmarks of cancer. Clin Oncol 2007;19:561–71.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients' skin and hair. Radiology 2010;254:326–40.
- Chan S, Dhadda AS, Swindell R. Single fraction radiotherapy for small superficial carcinoma of the skin. Clin Oncol 2007;19:256–9.
- James WD, Odom RB. Late subcutaneous fibrosis following megavoltage radiotherapy. J Am Acad Dermatol 1980;3:616–18.
- Lovett RD, Perez CA, Shapiro SJ, et al. External irradiation of epithelial skin cancer. Int J Rad Onc Biol Phys 1990;19:235–42.
Tumour recurrence after radiotherapy
- Shore RE. Radiation-induced skin cancer in humans. Med Ped Oncol 2001;36:549–54.
- Smith SP, Grande DJ. Basal cell carcinoma recurring after radiotherapy: a unique, difficult treatment subclass of recurrent basal cell carcinoma J Dermatol Surg Oncol 1991;17:26–30.
- Lovett RD, Perez CA, Shapiro SJ, Garcia DM. External irradiation of epithelial skin cancer. Int J Rad Oncol Biol Phys 1990;19:235–42.
- Smith SP, Grande DJ. Basal cell carcinoma recurring after radiotherapy: a unique, difficult treatment subclass of recurrent basal cell carcinoma. J Dermatol Surg Oncol 1991;17:26–30.
- Khan L, Breen D, Zhang L, et al. Predictors of recurrence after radiotherapy for nonmelanoma skin cancer. Curr Oncol 2014;21:e326–9.
- Chao KS, Gerber RM, Perez CA. Re-radiation of recurrent skin cancer of the face. Cancer 1995;75:2351–5.
Radiation-induced tumours and rare tumours associated with previous irradiation
- Lichter MD, Karagas MR, Mott LA, Spencer SK, Stukel TA, Greenberg ER. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. The New Hampshire Skin Cancer Study Group. Arch Dermatol 2000;136:1007–11.
- Meara RH. Superficial basal-cell epitheliomata following radiotherapy. Br J Dermatol 1964;76:294–6.
- Fuks A, Bagshaw MA. Total-skin electron treatment of mycosis fungoides. Radiology 1971;100:145–50.
- Martin H, Strong E, Spiro RH. Radiation induced skin cancer of the head and neck. Cancer 1970;25:61–71.
- Shore RE. Radiation-induced skin cancer in humans. Med Pediatr Oncol 2001;36:549–54.
- Levi F, Moeckli R, Randimbison L. Skin cancer in survivors of childhood and adolescent cancer. Eur J Cancer 2006;42:656–9.
- Paulino AC, Fowler BZ. Secondary neoplasms after radiotherapy for a childhood solid tumour. Ped Hem Oncol 2005;22:89–101.
- Short KA, Calman FM, Ross DA. Can radiotherapy cure radiation induced skin cancer? Clin Exper Dermatol 2006;32:109–11.
- Hudson AW, Winkelmann RK. Atypical fibroxanthoma of the skin: a reappraisal of 19 cases in which the original diagnosis was spindle-cell squamous carcinoma. Cancer 1972;29:413–22.
- Kemmett D, Gawkrodger DJ, McLaren KM, et al. Two atypical fibroxanthomas arising separately in X-irradiated skin. Clin Exp Dermatol 1988;13:382–4.
- Kempson RL, McGavran MH. Atypical fibroxanthomas of the skin. Cancer 1964;17:1463–71.
- Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer 1993;72:887.
- Amendola BE, Amendola MA, McClatchey KD, Miller CH Jr. Radiation-associated sarcoma: a review of 23 patients with postradiation sarcoma over a 50-year period. Am J Clin Oncol 1989;12:411.
- Hawkins MM, Wilson LM, Burton HS, et al. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst 1996;88:270.
- Fletcher CDM, Chibon F, Mertens F. Undifferentiated/unclassified sarcomas. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classifiction of Tumours of Soft Tissue and Bone, 4th edn. Lyon: IARC, 2013:236.
- Gladdy RA, Qin LX, Moraco N, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol 2010;28:2064.