CHAPTER 34
Arthropods
Gentiane Monsel1, Pascal Delaunay2, and Olivier Chosidow1
1UPEC-Université Paris-Est Créteil Val de Marne, Department of Dermatology, Hôpital Henri Mondor, Créteil, France
2Department of Parasitology-Mycology, Centre Hospitalier Universitaire de Nice, Hôpital de l'Ardet, Nice, France
SKIN DISEASE DUE TO ARTHROPODS
Definition
Arthropods are among the oldest animals. They are characterized by segmented bodies; paired, jointed appendages (legs and antennae); an exoskeleton; and a bilateral symmetry. They can grow only by moulting. Usually, arthropods go through the following life stages: egg, larva or nymph and finally mature adult (male or female).
The arthropod phylum is the most diverse of all the animal phyla, most of the species being in the class Insecta.
Arthropods can be divided into two groups: mandibulates with antennae and chelicerates without antennae. The mandibulates include insects, Chilopoda and Diplopoda. The chelicerates include scorpions, spiders and mites.
Pathophysiology
Arthropods produce their effects on the skin by a variety of mechanisms [1, 2, 3, 4], more than one of which may be implicated simultaneously.
Mechanical trauma
The puncture wound or laceration produced by the penetration of the skin seldom causes serious disturbance to the host. The nature of the trauma inflicted depends upon the structure of the mouthparts, which show wide variation between different species. There are two methods of feeding on blood: ‘vessel feeders’ insert the tip of their mouthparts into a capillary, and ‘pool feeders’ lacerate the skin, damage blood vessels and feed on the extravasated blood. Vessel feeders include sucking lice (Anoplura) and most mosquitoes, and pool feeders include stable flies and tsetse flies.
Injection of irritant, cytotoxic or pharmacologically active substances
An injected substance may contain pharmacologically active agents that produce local or, if in sufficient quantity, systemic effects. Salivary secretions and sting venoms may contain various enzymes such as: hyaluronidase, proteases, peptidases and phospholipases; kinins; histamine-liberating agents; histamine; 5-hydroxytryptamine; or acetylcholine.
Injection of potential allergens
The vast majority of reactions to arthropod bites or stings depend upon the presence in the host, of specific antibodies to antigenic substances in the arthropod saliva or venom. Investigation of extracts of venom sacs and salivary glands from many species, using modern immunological techniques, has demonstrated the presence of numerous antigens, some specific for a single species, and others common to several related species or even to related genera [5].
The type of reaction provoked by an arthropod bite or sting in an individual patient largely depends on previous exposure to the same or related species. When an individual is bitten for the first time by a species whose salivary secretions contain no directly injurious substance there is commonly no reaction. After repeated bites, sensitivity starts to develop, manifest by an itchy papule developing about 24 h after each bite, and persisting for several days. With prolonged exposure, an immediate weal reaction occurs, to be followed by the delayed papular reaction. After a further period of exposure, the delayed reaction no longer occurs, and eventually there is no reaction at all. The patient is then said to be immune. Mellanby [6] demonstrated this sequence of events with mosquito bites, and a similar response is seen with the bites of many other arthropods.
Some patients show a severe systemic hypersensitivity to arthropod allergens, manifested by anaphylaxis. The antigenic substances in the venoms of Hymenoptera (bees, wasps, hornets) are more likely to induce severe systemic hypersensitivity reactions than are the antigens of most other insects.
The capacity of a patient to respond to an antigenic stimulus is also an important factor in determining the reaction to an arthropod. The reactions of patients who are immunosuppressed, as a result of either disease or therapy, are modified. Examples of this include the occurrence of crusted scabies in immunosuppressed individuals, and the response to bites in patients with chronic lymphocytic leukaemia, HIV infection and Epstein–Barr virus associated natural killer cell leukaemia/lymphoma. In HIV-infected individuals, pruritic papular eruption may be a reaction to arthropod bites. The most accepted hypothesis is that this condition reflects an altered and exaggerated immune response to arthropod antigens in a subset of susceptible HIV-infected patients [7].
‘Exaggerated reaction of insect bite’, also called ‘insect-bite-like reaction’ or ‘eosinophilic eruption of haematoproliferative disease’, is a relatively common and disturbing skin reaction in chronic lymphocytic leukaemia patients [8]. It may be related to the immune dysregulation accompanying chronic lymphocytic leukaemia and further exacerbated by external factors, including actual insect bites, chemoimmunotherapy and pyogenic infection. The diagnosis is based on the clinical characteristics and findings of dermatitis with an eosinophil-rich infiltrate on biopsy [9].
Secondary infection
Bacterial infection may be introduced at the time of the bite, but commonly gains entry as a result of scratching, and may confuse the clinical picture. Compartment syndrome caused by streptococcal cellulitis complicating an insect bite has been described [10].
Invasion of the host's tissues
Certain flies cause myiasis, in which the host's tissues are invaded by larvae.
Contact reactions
Simple contact with the secretions of certain arthropods, or with their living or dead bodies, may provoke irritant or allergic contact reactions. For example, the secretions of blister beetles produce a severe irritant reaction, and repeated handling of cockroaches may induce contact urticaria and dermatitis.
Reactions to retained mouthparts
Persistent granulomatous papules or nodules may be provoked by retained mouthparts, for example those of ticks.
Transmission of disease
Many diseases have arthropod vectors, for example malaria (mosquitoes), leishmaniasis (sandflies) and typhus (lice) (Table 34.1).
Table 34.1 Main arthropod-transmitted diseases
| Arthropods | Transmitted disease |
| Insecta | |
| Mosquitoes and flies | Malaria, yellow fever, dengue fever, viral encephalitis, onchocerciasis, leishmaniasis, sleeping sickness, West Nile fever |
| Fleas | Typhus, bubonic plague |
| Lice | Typhus, trench fever, relapsing fever |
| Reduviid bugs | Chagas disease |
| Cockroaches | Bacteria? |
| Arachnida | |
| Ticks | Lyme borreliosis, Rocky Mountain spotted fever, tick paralysis, Colorado tick fever, babesiosis, ehrlichiosis, Q fever, tularaemia |
| Mites | Scrub typhus |
Environmental factors
There are a number of environmental and social factors that determine the range of arthropod species to which an individual is exposed.
Persons living and working in tropical climates tend to wear fewer clothes, and therefore expose larger areas of the body to bites and stings. Clothing itself is essential to the existence of the body louse, and areas of constriction of clothing affect the distribution of the skin lesions caused by certain mites (e.g. harvest mites).
Certain occupations carry an increased risk of reactions to arthropods [11]. Forestry workers, for example, may be exposed to the urticating hairs of the caterpillars of certain species of Lepidoptera, and dockworkers handling foodstuffs may be attacked by mites infesting the cargo.
In some societies, humans are exposed to attack by the parasites of the domestic animals with which they cohabit.
Housing can influence exposure to arthropod attack in a number of ways. Overcrowded homes favour transmission of ectoparasites, such as lice and the scabies mite, and dilapidated housing provides an ideal habitat for bedbugs. Spiders and scorpions will take up residence in garages, outhouses and woodpiles.
The methods by which an arthropod is attracted to its host species include body heat, carbon dioxide in exhaled air (e.g. mosquitoes, ticks, fleas, bedbugs), the view (e.g. tsetse fly) and displacement of air or vibrations caused by the host (e.g. fleas) [12]. Human sweat contains mosquito attractants, and anhidrotic subjects are unattractive to mosquitoes [13, 14]. The human skin microflora may be responsible for producing compounds that attract mosquitoes and, as there is variation in the microflora between individuals, body odour probably contributes to susceptibility to biting [15, 16]. Recently, it was shown that malaria-infected mosquitoes express enhanced attraction to human odour [17]. Human odour also appears to play a part in attracting sandflies [18]. Pregnant women appear to be more attractive to mosquitoes than the non-pregnant [19, 20].
There is also a suggestion of increased susceptibility to mosquito bites in patients with HIV infection receiving antiretroviral therapy and suffering from lipoatrophy [21].
Alcohol ingestion also seems to promote mosquito attraction [22].
Certain species of flies are attracted to skin ulcers and purulent material, in which they lay their eggs.
Insect pheromones play a part in attacks by large numbers of Hymenoptera. Honeybees, when stinging, emit an alarm pheromone from glands in their sting chambers, and this guides other bees to attack an intruder.
Pathology [23-25]
The histopathological changes associated with arthropod bites depend upon a number of factors, including the arthropod involved, the type of immunological reaction provoked and the duration of the lesion.
In the acute phase, there is a superficial and deep, perivascular and interstitial inflammatory infiltrate, which is characteristically wedge shaped. The infiltrate is usually mixed in composition with an abundance of lymphocytes and eosinophils, although neutrophils and histiocytes can also be seen. Neutrophils may predominate in reactions to fleas, mosquitoes, fire ants and brown recluse spiders. Sweet-like reaction to arthropod bites have also been reported [26]. Over the most prominent superficial infiltrates, spongiosis can be seen, sometimes with progression to vesicle formation or epidermal necrosis.
In papular urticaria there is prominent papillary dermal oedema and a perivascular chronic inflammatory infiltrate with a significant admixture of eosinophils.
Bullous reactions develop beneath a more or less intact epidermis, and may be multilocular.
In older lesions, excoriated areas may be altered by the scratching, resulting in parakeratosis and a dermal infiltrate with neutrophils and lymphocytes.
Chronic reactions often have a pseudolymphomatous appearance. The dermis contains a dense inflammatory infiltrate of lymphoid cells and histiocytes, with an admixture of eosinophils and plasma cells, and the presence of atypical mononuclear cells with hyperchromatic nuclei. Secondary lymphoid follicles with germinal centres are sometimes formed. Multinucleated cells may also occur. If retained mouthparts are present, there may also be giant cells of foreign-body type.
Additional histopathological features associated with particular arthropods are noted in the relevant sections of this chapter.
Clinical features
The very large number of species of biting and stinging arthropods, their different feeding habits and the variation in individual patients’ responses to the various irritants and allergens injected determine the diversity of clinical features. Any arthropod bite can be totally asymptomatic. The type and distribution of lesions produced by individual arthropods are discussed in the relevant sections throughout this chapter. Table 34.2 shows the clinical and epidemiological features of the main arthropod bites. Clinical features of arthropod bites are not specific, so diagnosis relies on an array of arguments, none of which is specific by itself; it is the association of elements that is suggestive.
Table 34.2 Arthropod bites: main clinical and epidemiological features
| Arthropod | Clinical feature on examination | Location | Timing of pruritus | Context |
| Bedbugs | 3–4 bites in a line or curve | Uncovered areas | Morning | Travelling |
| Fleas | 3–4 bites in a line or curve | Potentially anywhere | Daytime | Pet owners or rural living |
| Mosquitoes | Non-specific papules | Potentially anywhere | Anopheles spp. night; Culex spp. night; Aedes spp. Day | Worldwide distribution |
| Head lice [42] | Eggs attached to hairs. Live lice on the head associated with itchy, excoriated lesions | Scalp, ears, and neck | Any | Children, parents, or contact with children |
| Body lice [42] | Excoriated papules and hyperpigmentation; live lice inside clothes | Back | Any | Homeless people, developing countries |
| Scabies [42] | Vesicles, burrows, nodules and non-specific secondary lesions | Interdigital spaces, forearms, breasts, genitalia | Night | Sexually transmitted, households or institutions |
| Ticks | Erythema migrans or ulcer | Potentially anywhere | Asymptomatic | Pet owners or hikers |
| Pyemotes ventricosus [43] | Comet sign, a linear erythematous macular tract | Under clothes | Any time when inside habitat | People exposed to woodworm contaminated furniture (P. ventricosus is a woodworm parasite) |
| Spiders | Necrosis (uncommon) | Face and arms | Immediate pain, no itching | Rural living |
Adapted from Bernardeschi et al. [41]. Source and copyright holder: BMJ Publishing Group Ltd.
The most frequently encountered response is papular urticaria (Figure 34.1). Initially, an extremely itchy urticarial weal develops at the site of the bite, and this is succeeded by a firm pruritic papule, which usually persists for several days. The weal and papule may show a central haemorrhagic punctum, and the papule may be surmounted by a tiny vesicle. Lesions are often grouped in clusters, and develop in crops at irregular intervals.
Figure 34.1 Typical papular urticaria. In this case, in response to flea bites.
The number and distribution of skin lesions produced by the bites depend upon the type of exposure and the feeding habits of the arthropod involved. New bites by the same species will often cause a recrudescence of activity in existing lesions.
Bullous reactions are common on the lower legs (Figure 34.2), but may occur in other sites, especially in children. In the presence of lower limb venous hypertension, haemorrhagic or ulcerated lesions may develop. More severe local changes are sometimes found, with cellulitis and lymphangitis in the apparent absence of secondary infection. Eruptive pseudoangiomatosis-like lesions have also been reported as a response to arthropod bites [27, 28].
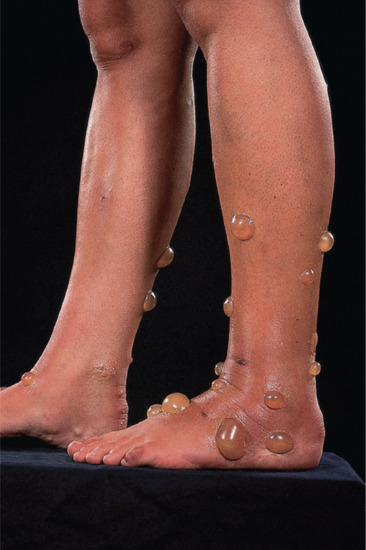
Figure 34.2 Bullous lesions in response to arthropod bites.
(Courtesy of Dr F.A. Ive, Durham, UK.)
Irritation is an almost constant symptom, and rubbing and scratching may increase the inflammatory changes, and induce eczematization. When the bites are very numerous, or if the local reaction is severe, there may be fever and malaise.
Secondary infection is a common complication, and may manifest as impetigo, folliculitis, cellulitis and lymphangitis.
Anaphylactic shock is unusual except after Hymenoptera stings, but is occasionally seen with some other arthropods.
Bite reactions may persist for months. Tick attachment sites, in which the mouthparts may be retained, are the most likely to persist, but so may bites of mosquitoes and other arthropods.
Investigations
The diagnosis of arthropod bites is often self-evident, for example when the patient has spent the afternoon in the garden on a hot day in summer and subsequently develops typical lesions on exposed areas of skin. However, difficulty arises when the source of the bites is not immediately obvious. Only good clinical observation and specific questions will suggest a particular insect and collection of it is necessary for subsequent examination.
The distribution of the bites may provide a clue to their origin (see Table 34.2), for example localization to the abdomen and thighs in cheyletiellosis or contact with sarcoptic mange in dogs, and involvement of the legs below the knees when the lesions are produced by cat or dog fleas. Patients should be asked about domestic pets; not only their own, but also those in the homes of close relatives who are visited regularly, as ectoparasites associated with pet animals are often the source of persistent arthropod bites. If the bites are not localized, but scattered all over the body, consider reactions to arthropods biting in the patient's bedroom, such as bird fleas, bird mites or bedbugs. Enquire if the patient has recently moved house. It may be that the previous owners of the new home kept pet animals, and have left a legacy of domestic flea infestation. Even if the house remained empty for a considerable time before the new owners took up residence, the flea population will be waiting in cocoons to emerge when the new occupants arrive. Adult fleas can survive starvation for variable lengths of time depending upon species and environmental conditions [12, 29] – a newly emerged and unfed dog flea, Ctenocephalides canis, will survive for approximately 60 days. In the absence of their natural hosts, such animal flea populations will not usually survive for more than a few months.
If the history and examination do not suggest a possible source for the problem, or if the dermatologist wishes to confirm a suspected source, the following procedures may be useful [30, 31].
- The patient's pet animals should be examined, if possible, for signs of skin disease. Cheyletiellosis and canine scabies produce characteristic changes on an affected animal [32]. Skin scrapings will confirm sarcoptic mange, and vigorous combing of scale from the coat of a dog suffering from cheyletiellosis (Figure 34.3) will provide material in which Cheyletiella mites may be identified. If the animal cannot be examined, the patient should be provided with a sheet of black paper, and asked to collect brushings or combings from the animal's coat for subsequent examination.
- If domestic infestation with cat or dog fleas is suspected, this can often be confirmed by examination of debris from the pet's bedding. The patient is supplied with a large polythene bag and instructed to place the pet's bedding in the bag and shake it vigorously for a few minutes. The bedding is then removed, the bag sealed, and delivered to the dermatologist for microscopy of the debris. Macroscopically, flea eggs and faeces have a ‘pepper and salt’ appearance (Figure 34.4), and the larvae are grub-like. For identification, adult fleas should be ‘cleared’ in 10% potassium hydroxide for 24 h so that the majority of the pigment is removed and the anatomical details revealed. Cat and dog fleas are readily identified, but if unfamiliar species are encountered, the help of an entomologist with an interest in Siphonaptera should be sought. Correct identification of fleas is important so that proper control measures may be carried out [33].
- If problems from bird fleas or bird mites are suspected, it is often of value to examine dust obtained with a vacuum cleaner from bedrooms. This is, however, time consuming and requires some expertise.
- It may be necessary to visit the patient's home to establish whether there are birds’ nests under the eaves, which might be a source of fleas or mites, or to take specimens from household pets.
- Mites that might have relevance to human dermatoses may be isolated from clothing, furnishings or bedding by the techniques described by Hewitt et al. [34].

Figure 34.3 Typical heavy scale in the coat of a dog suffering from Cheyletiella infestation.

Figure 34.4 Typical ‘pepper and salt’ appearance of flea eggs and faeces in the debris from a cat's bedding.
An entomologist is often valuable in these situations, not only for identification of arthropods, but also to advise about their relevance to the situation. An arthropod discovered at the scene of the crime may only be an innocent bystander.
In some cases, in spite of extensive efforts, the source of the bites remains unknown, and the dermatologist can then only treat the problem symptomatically with oral antihistamines, topical antipruritics and insect repellents.
Management
Prevention: insect repellents [35, 36, 37, 38]
There are several strategies that can be employed in attempts to avoid arthropod bites/stings and arthropod-related disease transmission, including protective clothing, insecticide-impregnated netting and repellents. With regard to the latter, there are two principal categories of commercially available insect repellents—plant-derived essential oils and synthetic chemicals. The former group includes citronella, oil of eucalyptus, peppermint, tea-tree oil, lavender, soybean oil and neem oil. DEET (N,N-diethyl-m-toluamide [or N,N-diethyl-3-methylbenzamide]), the most widely used repellent, is an example of the latter.
Recently introduced repellents include DEPA (N,N-diethyl phenylacetamide), PMD (para-menthane-3,8-diol) and picaridin, a synthetic derivative of pepper.
Unfortunately, with many of these agents, their volatility means that the repellent effect is transient (between 4 and 8 h) for the more efficient and benefit can only be sustained by repeated application. In addition, effectiveness is often limited to a narrow spectrum of susceptible arthropods detecting carbon dioxide (e.g. mosquitoes, ticks, sandflies).
General management
Species-specific treatment will be discussed in the relevant sections throughout this chapter.
General treatment principle includes [39]:
- Local wound care by cleansing, removing of remaining arthropod parts.
- Management of pain and patient discomfort, by using ice packs, application of topical corticosteroid, systemic antihistamine, injection of local anaesthetics or sometimes the use of systemic analgesic.
- Institution of supportive measures in case of allergic (anaphylaxis) or toxic reaction.
- Antibiotic therapy in case of secondary infection.
- Antivenom administration in case of envenomation from particular species.
- Tetanus prophylaxis if necessary.
- Desensitization with venom immunotherapy using extracted insect venom. It may be an effective therapy for preventing further allergic reactions to insect stings, which can improve quality of life [40].
CLASS INSECTA
Mosquitoes, gnats, midges and flies (Diptera)
Definition
The order Diptera is one of the largest of the insect orders. Diptera are two-winged flies with a single pair of membranous forewings, and with hindwings modified as balancing organs (halteres). Most feed on nectar, plant exudates or decaying animal and vegetable matter, but some are blood-sucking, and some have larvae parasitic on humans. To the dermatologist, the Diptera are important as biting insects and as the cause of myiasis, in addition to their capacity to transmit disease (Table 34.3).
Table 34.3 Main transmitted diseases by the insect of the order Diptera
| Suborder | Family | Species | Transmitted diseases |
| Nematocera | Culicidae (mosquitoes) | Anopheles, Culex, Aedes | Malaria, yellow fever, filariasis, dengue fever, chikungunya, West Nile virus, Rift valley fever, Japanese encephalitis |
| Psychodidae (sandflies) | Phlebotomus | Cutaneous and visceral leishmaniaisis, Toscana fever | |
| Lutzomyia | Cutaneous and visceral leishmaniaisis, bartonellosis in New World (Carrion disease) | ||
| Simuliidae (blackflies) | Simulium | Onchocerciasis, tularaemia | |
| Brachycera | Glossinidae | Glossina | Sleeping sickness |
| Tabanidae | Tabanus (horse flies) | Tularaemia | |
| Chrysops (deer flies) | Loiasis, tularaemia | ||
| Muscidae | Fannia | Myiasis | |
| Musca | |||
| Calliphoridae | Cochliomyia | Myiasis | |
| Sarcophagidae | Sarcophaga | Myiasis | |
| Oestridae | Dermatobia | Myiasis | |
| Gasterophilus | |||
| Oestrus | |||
| Hypoderma |
The Diptera are currently usually classified in two suborders based on characteristics shown by larvae, pupae and adults – the Nematocera and the Brachycera. Detailed information on the morphology, biology and medical importance of Diptera is provided in comprehensive texts by Kettle [1], and Lane and Crosskey [2].
Classification
Suborder Nematocera (long-horned flies)
The Nematocera are small flies with long many-segmented filamentous antennae. With a few exceptions the medically important species are blood-suckers.
Family Culicidae (mosquitoes)
Mosquitoes have worldwide distribution, and are responsible for the transmission of a number of human diseases, including malaria (Chapter 33), filariasis, yellow fever, West Nile virus, chikungunya and dengue fever. Three mosquito genera are responsible: Anopheles, Culex and Aedes (Ochlerotatus). Human malaria is transmitted exclusively by Anopheles species. Both male and female mosquitoes will imbibe sweet juices from flowers or ripe fruit, but only the females pierce the skin and suck the blood of vertebrate animals for production of eggs. Most mosquitoes are nocturnal feeders, but species from the genus order Aedes (Ochlerotatus) are diurnal. The eggs of mosquitoes are deposited on or near water, and adults develop via aquatic larval and pupal stages.
Family Psychodidae (sandflies)
These are tiny (2–3 mm long) hairy flies with lanceolate wings and long legs. They are widely distributed, especially in the tropics and subtropics.
Genus Phlebotomus
Species of Phlebotomus are vectors of cutaneous and visceral leishmaniasis (Chapter 33) in the Old World. Phlebotomus species are also vectors of Toscana fever (TOSV), identified in 1971 [3]. Phlebotomus bites cause a condition known as harara (urticaria multiformis endemica) in Israel and the surrounding countries.
Genus Lutzomyia
Lutzomyia species are vectors of cutaneous and visceral leishmaniasis (Chapter 33) and bartonellosis in the New World.
Family Simuliidae [4]
Popularly known as blackflies, and with a worldwide distribution, these are small (2–6 mm) flies with a characteristic humped thorax, and short broad wings. They breed only in areas of fast-flowing water, and bite during the day.
Over large parts of the tropics, several species of blackfly are responsible for transmission of onchocerciasis (Chapter 33)—principally the Simulium damnosum complex (several closely related species) in West Africa, S. neavei in East Africa, S. metallicum in Venezuela and S. ochraceum in Guatemala. In temperate regions, the greatest problem caused by simuliids is their painful bites, and some species are such a persistent nuisance at certain times of the year that they may make large areas unpleasant to live or work in. In Yugoslavia, the notorious Golubatz fly, S. columbaschense (S. columbaczense), which bred in the Danube at Golubatz, caused both mortality among livestock and human misery until environmental changes eliminated it. In North America, the most troublesome biting species are S. venustum, which is in the Holarctic and occurs from Alaska to Greenland and south to Texas and South Carolina, and Prosimulium mixtum, which occurs in the north-eastern USA and eastern Canada. Simulium posticatum (the Blandford fly), formerly named S. austeni Edwards, is widely distributed throughout Europe and European Russia. In England, it is found in an arc running from East Anglia through Oxfordshire into Dorset. In the Stour valley area of Dorset, particularly in the region of Blandford Forum, the fly is notorious for the severity of the reaction to its bites [5, 6]. It had not been known as a pest in the UK prior to the 1960s. The eggs are laid in cracks in vertical river banks, a short distance above the water [7]. The larvae are concentrated in stretches of fast-flowing water immediately downstream of barrages and weirs, where they attach themselves to weeds or stones and feed on phytoplankton. Adults hatch in May, and are on the wing in May, June and early July. Females require a blood meal before oviposition, and although they will bite various wild and domestic animals, they appear to prefer humans and dogs. In the 1990s, biological control, using a bacterium (Bacillus thuringiensis var. israelensis) that selectively targeted the fly larvae, significantly reduced the severity of the problem.
Family Ceratopogonidae (biting midges; ‘punkies’; ‘no-see-ums’)
These small flies (1–3 mm in length) have a worldwide distribution, and are notorious as biting pests. The biting midges of the West Highlands of Scotland (the commonest species of which is Culicoides impunctatus), for example, are an intolerable nuisance, and pose a problem to the Scottish tourist industry [8]. Males and females feed on nectar, but most females require a blood meal for maturation of the ovaries and egg production. There are four genera that suck blood: Culicoides, Leptoconops, Austroconops and Forcipomyia (subgenus Lasiohelea). They breed in rivers, swamps and marshes; they often occur in swarms and will readily attack any mammal in their vicinity. A few species enter homes and bite at night.
The genus Culicoides is widely distributed. Leptoconops species are largely restricted to the warmer parts of the Old and New World. Austroconops contains only one species, which is restricted to western Australia. Lasiohelea species are principally associated with tropical and subtropical rainforests.
Suborder Brachycera (circular-seamed flies, muscoid flies and short-horned flies)
The Brachycera are stout-bodied flies with short antennae, often composed of three segments, and never more than six.
Family Tabanidae
Many species of three genera of this family will attack humans – Tabanus (horse flies), Chrysops (deer flies) and Haematopota (clegs). They are large flies, and have a worldwide distribution. Only females suck blood. Tabanid flies act as vectors for loiasis (Chapter 33) and tularaemia (Chapter 26), and some species may transmit anthrax mechanically [9].
Family Rhagionidae (snipe flies)
Species of Symphoromyia occurring in the Palaearctic and Nearctic regions are vicious biters. Atherix is another blood-sucking genus in the Nearctic and Neotropical regions, and Spaniopsis is troublesome in Australia.
Family Chloropidae (eye flies; frit flies)
These flies are about 2 mm in length. The adults of some species are attracted to open sores, body secretions and the eyes, particularly eyes with a copious discharge. Hippelates and Siphunculina species are associated with humans and can act as mechanical vectors of yaws, conjunctivitis and streptococcal skin infection.
Family Muscidae (house flies; stable flies; tsetse flies)
This family includes the familiar house fly Musca domestica and the lesser house fly Fannia canicularis. These do not bite, but may act as mechanical vectors of disease. The muscids Stomoxys calcitrans (stable fly) and Haematobia species (horn flies) have mouthparts modified for sucking blood. They usually feed on large quadrupeds, but can inflict painful bites on humans. Tsetse flies are vectors of trypanosomiasis (Chapter 33). They are confined to Africa south of the Sahara.
Family Hippoboscidae (flat flies; louse flies; keds)
Members of this family are blood-sucking ectoparasites of birds and animals. Several species of ked have been recorded as biting humans [10, 11].
Members of several other families of Diptera are important in that their larvae may cause myiasis.
Pathology
Diagnosis of mosquito bites is rarely performed in the acute phase, but histopathology will show an upper dermal perivascular infiltrate consisting of lymphocytes with histiocytes, eosinophils and mast cells. There may be mild oedema and a slight general increase in mast cells and eosinophils in the dermis. The overlying epidermis may show spongiosis sometimes amounting to vesiculation. In older lesions, excoriation often results in epidermal necrosis and crusting with a dermal infiltrate of lymphocytes and neutrophils. In addition, it is shown that saliva has a major role in the transmission of pathogens to the host agent [12].
Clinical features [13, 14]
The clinical features of the bites of insects of this large and diverse order are variable. The nature of the pharmacologically active substances injected, and the degree of acquired allergic sensitivity to the antigenic substances in the saliva, are the main factors that determine the reaction. For most of the Diptera, the allergic component is by far the more important. The nature of any injected toxins is usually unknown and the effects attributable to them are usually slight. The clinical picture will also be influenced by the biting habits of the species concerned.
The reaction to mosquito bites is determined by previous exposure, and the sequence of events following multiple bites was elucidated by Mellanby [14]. In an individual not previously exposed, the bites produce no response. With subsequent bites, a delayed reaction occurs, consisting of pruritic papules, which develop approximately 24 h after the bites and persist for several days. After repeated bites for several weeks, the response changes, with the appearance of an immediate weal at the bite site. This resolves after about 2 h, to be replaced by the delayed reaction. Further exposure provokes the immediate reaction, but not the delayed response. Eventually, tolerance is acquired, and no reaction occurs. Studies of the bite reaction in relation to age have shown an increase in immediate reactions from early childhood to adolescence, and a decrease thereafter. The appearance and intensity of delayed reactions decrease with age [15]. It has been proved conclusively that the mosquito salivary glands are the source of the antigens responsible for the bite reactions [16].
Anaphylactic reactions to mosquito bites are rare [17]. Gaig et al. reported a patient with a serum sickness-like illness associated with mosquito bites [18]. Severe local reactions are not uncommon, and in highly sensitive subjects bullae, cellulitis and eczematization are often seen, especially on the legs. Gravitational factors probably play a role in the development of bullae on the legs [19]. Exaggerated hypersensitivity responses to mosquito bites have been reported in patients suffering from chronic lymphatic leukaemia [20, 21, 22, 23]. Although the lesions frequently appear months after the diagnosis of leukaemia and are unrelated to its course and therapy, they can also herald development or recurrence of leukaemia or lymphoma [24]. However, although the clinical picture and histological features are typical of arthropod bites, in many cases patients do not recall being bitten [22, 25]. Exaggerated responses to mosquito bites have also been described in patients with HIV infection [26, 27, 28], and a chronic pruritic eruption in patients with AIDS in South Florida has been attributed to mosquito bites [29].
In recent years, there have been a number of reports from Japan of severe hypersensitivity to mosquito bites preceding the development of malignant histiocytosis [30, 31]. This has now been characterized as a disease in which there is a triad of hypersensitivity to mosquito bites, chronic Epstein–Barr virus infection and natural killer cell leukaemia/lymphoma [32, 33, 34, 35]. It affects predominantly Japanese in the first two decades of life. The skin lesions are bullae, which develop at mosquito bite sites, undergo necrosis, and heal with residual scarring [36]. Accompanying the skin lesions are systemic features, principally high fever and general malaise. Affected individuals die of haemophagocytic syndrome (malignant histiocytosis). Screening for haematological malignancies, latent Epstein–Barr virus infection and natural killer cell lymphocytosis should be considered in patients with unsual arthropod bites reaction [34, 36].
The bites of Simuliidae, which may be very numerous, are on exposed skin. The sites of the bites are often marked by a small blood crust with surrounding ecchymosis. Within a few hours, small pruritic papules develop, and these resolve after several days [37]. However, severe reactions with marked oedema of the limbs and constitutional upset occasionally occur, and in some cases nodules and discoid eczematous areas persist at the sites of the bites for several months [38]. The bites of the Blandford fly occur most frequently on the legs, and women are principally affected [5, 6]. The bites often produce a severe local reaction, with oedema and blistering, and may be accompanied by systemic manifestations, including pyrexia, arthralgia and meningism.
The biting midges of the family Ceratopogonidae generally cause small, papular lesions on exposed parts of the skin, but wealing and bulla formation may occur in sensitized individuals. Weal-like lesions, papules and persistent nodules have been described following bites from Leptoconops torrens in California [39].
Midges of the family Chironomidae are closely related to ceratopogonids. These midges do not bite, but hypersensitivity to their larvae, used as aquarium fish food and as bait, is well recognized [40, 41], and includes contact urticaria [42] and protein contact dermatitis [43]. Recently, one study suggested that occupational exposure to chironomids may cause sensitization with circulating immunoglobulin E (IgE) antibodies in sewage workers [44].
The bites of keds may be followed by the development of persistent pruritic papules [11].
The bites of horse flies and stable flies are often very painful and frequently become secondarily infected. Anaphylactic reaction to horse flies has also been reported in two patients already known to be allergic to stinging Hymenoptera venom, suggesting a cross allergen, between the Hymenoptera venom and the mosquito saliva [45].
Eruptive pseudoangiomatosis-like lesions have also been reported as a response to mosquito bites [46, 47] and a relationship to Culex pipiens bites has been demonstrated [48].
Management [49, 50, 51]
Diptera bites should be cleansed thoroughly with soap and water to avoid secondary infection. A short course of topical steroids and systemic antihistamines may be used to control pruritus. Rare allergic reactions should be treated aggressively. Antihistamines taken prophylactically have been demonstrated in studies to decrease weal formations and subsequent pruritus following mosquito bites [52, 53].
Prevention of mosquito and sandfly bites requires the use of protective clothing and chemical repellents, and methods to reduce the numbers of flies and mosquitoes in a given area. As the Anopheles species that carry malaria bite mostly at night, retiring to the indoors in the evening plays a major role in disease prevention. Transmission is also prevented by repellents and pyrethroid-impregnated mosquito netting. All travelers to malaria-endemic areas should take the recommended chemoprophylaxis. In contrast to Anopheles mosquitoes, Aedes mosquitoes that carry dengue tend to bite during the day. Repellents and protective clothing must be used to prevent transmission in dengue-endemic areas.
The hierarchy of measures against bites depends on the travel or the stay (e.g. place, season, length, modalities) and the persons (e.g. age, pregnancy, other pathology). The use of skin insect repellents is recommended, using an active ingredient, which has been evaluated as innocuous (low toxicity, genotoxicity, ecotoxicity). Active ingredients currently being evaluated are DEET, picaridin (icaridin or KBR3023), 3-(N-acetyl-N-butyl)aminopropionic acid ethyl ester (IR35/35) and PMDRBO (mixture of cis- and trans-para-menthan-3,8-diol) [54].
Myiasis
Definition
Myiasis is the infestation of body tissues of animals by the larvae (maggots) of Diptera [1, 2, 3, 4]. Humans are sometimes infested depending of their behaviour, environment or clinical status. Parasitologically, flies may be classified into two main myiasis-producing groups: obligatory and facultative. Obligatory myiasis-producers always pass their larval stage parasitically in the body of an animal. Larvae of facultative myiasis-producers usually develop on decaying flesh or vegetable matter, but may infest wounds.
Clinically, myiasis can be classified according to the part of the body affected. Cutaneous myiasis includes wound myiasis, furuncular myiasis, and migratory myiasis, in which larvae penetrate and develop within the skin. The second form is cavitary myiasis. In nasopharyngeal myiasis, the nose, sinuses and pharynx are affected, and ophthalmomyiasis involves the eye, orbit and periorbital tissues. Intestinal and uro-genital myiasis involve invasion of the alimentary tract or uro-genital system.
Classification
All species of specific myiasis and most of facultative myiasis are classified within Calyptratae. Taxonomic division of the Calyptratae is presented in Table 34.4.
Table 34.4 Taxonomic division of the Calypratae
| Superfamily | Family | Subfamily or tribes | Species |
| Muscoidae | Muscidae | Muscina spp. | |
| Musca domestica (house fly) | |||
| Fanniidae | Fannia scalaris | ||
| Fannia canicularis | |||
| Oestroidea | Oestridae | Cuterebrinae | Dermatobia hominis (human botfly) |
| Cuterebra spp. | |||
| Alouattalyia baeri | |||
| Gasterophilinae | Gasterophilus spp. (horse botflies) | ||
| Hypodermatinaedae | Hypoderma bovis (cattle botfly) | ||
| Hypoderma lineatum | |||
| Hypoderma tarandi | |||
| Oestrinae | Oestrus spp. (sheep nasal botfly) | ||
| Sarcophagidae | Wohlfahrtia magnifica (spotted flesh fly) | ||
| Wohlfahrtia vigil | |||
| Wohlfahrtia opaca | |||
| Sarcodexia lambens | |||
| Calliphoridae | Phormia regina | ||
| Protophormia terranovae | |||
| Chrysomya bezziana | |||
| Chrysomya megacephala | |||
| Chrysomya albiceps | |||
| Chrysomya rufifacies | |||
| Cochliomyia hominivorax (New World screwworm) | |||
| Auchmeromyiinae | Auchmeromyia senegalensis (Congo floor maggot) | ||
| Luciliinae | Lucilia spp. | ||
| Calliphorinae | Calliphora spp. | ||
| Calliphorini | Cordylobia anthropophaga (tumbu fly) | ||
| Cordylobia rodhaini (Lund's fly) |
Adapted from Francesconi and Lupi [54]. Copyright holder of original artwork from which this table was adapted: American Society for Microbiology.
Family Muscidae
Eggs of Fannia canicularis (lesser house fly) and Musca domestica (house fly) may be deposited on ulcers and give rise to wound myiasis [5].
Family Calliphoridae (blowflies)
Genus Cochliomyia (Callitroga)
These New World screwworms are distributed in the Americas, but are no longer established in North America, following intensive eradication efforts involving the release of a huge number of sterile male flies. Cases of myiasis involve the larvae of only two species of Cochliomyia: C. macellaria and C. hominivorax (americana). The larva of C. macellaria is a facultative parasite, which may be responsible for secondary infestation of wounds. Larvae of C. hominivorax are obligatory parasites, which feed on living tissue and can penetrate unbroken skin [6, 7, 8], but they may also infest wounds.
Genus Chrysomya
The Old World equivalent of Cochliomyia, Chrysomya bezziana, the Old World screwworm, is important medically as the larvae are obligate parasites in wounds.
Genus Cordylobia
Cordylobia anthropophaga, the ‘tumbu’ fly, is widespread in tropical Africa south of the Sahara [9], and most reported cases of tumbu fly myiasis are acquired in Africa [10, 11]. There are, however, reports of myiasis acquired elsewhere, including Spain [12], Portugal [13] and Saudi Arabia [14]. Tumbu fly myiasis occurring in two boys who had never been to Africa might have been acquired as a result of their father, who made frequent visits to Africa, bringing tumbu fly eggs back amongst his possessions [15]. Cordylobia (Stasisia) rodhaini, the only other species of Cordylobia known to infest humans, has a more limited distribution in tropical Africa, principally the rainforest areas. Extensive furuncular myiasis due to C. rodhaini has been reported in an Italian man who acquired the problem while working in Ethiopia [16]. Eggs are not laid on the host, but on sand or soil, especially if contaminated by urine or faeces. People are most commonly parasitized during the rainy season. Tourists can be infested by dressing with their humid clothes that were lying on the ground, because adult flies tend to oviposit on soiled or humid clothing. After hatching, the larva raises its cephalic end searching for a suitable host. In the wild, rats are the usual host, but around human habitation, dogs and humans are common hosts. The larva attaches itself by means of its oral hooks, and rapidly penetrates the skin. When development is complete, usually in 14–16 days, it drops to the ground to pupate. Some of the factors that affect the distribution include unhygienic situations, high humidity, poverty and the use of soiled clothes [17].
Genus Auchmeromyia
Although strictly not a cause of myiasis, the larva of the fly Auchmeromyia senegalensis, the Congo floor maggot, is a blood-sucking parasite of humans. This fly occurs throughout tropical Africa, where it lives in native huts and lays its eggs in the soil of the floor. The larvae lie buried in the soil during the day, but emerge at night to feed on the sleeping occupants of the huts. Once engorged, they drop off the host and burrow back into the soil.
Other genera
Larvae of members of the genera Phormia (black blowflies) [18, 19, 20], Lucilia (greenbottle) and Calliphora (bluebottle) may also be secondary invaders of wounds in man. In a study of wound myiasis in urban and suburban USA (in which homelessness, alcoholism and peripheral vascular disease were frequent co-factors) the majority of species identified were blowflies, the most common being Lucilia sericata [21].
There has been a recent resurgence of interest in the use of maggots (usually those of the greenbottle, Lucilia sericata) for wound debridement, an added bonus of which is their ingestion of meticillin-resistant Staphylococcus aureus [22]. The larvae must be prepared and maintained in sterile conditions before clinical use [23]. Maggot therapy has the following three core beneficial effects on a wound: debridement, disinfection and enhanced healing.
Family Sarcophagidae (flesh flies)
Genus Sarcophaga
There are occasional reports of members of this genus infesting wounds [24].
Genus Wohlfahrtia
These flies are similar to Sarcophaga and are important myiasis-causing flies in camels and sheep. The larvae of Wohlfahrtia magnifica may be deposited in the ear, eye or nose, and cause extensive destruction of healthy tissue. Delir et al. reported an Iranian woman with a cavity in the left labium majus occupied by a number of W. magnifica larvae [25]. W. magnifica occurs in south-eastern Europe, southern and Asiatic Russia, the Middle East and North Africa. Wohlfahrtia vigil and W. opaca are North American species whose females deposit larvae on the skin of young animals, resulting in furuncular myiasis. Lesions are identical to those of Dermatobia. Human furuncular myiasis occurs only in young babies, as the larvae are unable to penetrate adult skin [26].
Family Oestridae
Genus Cuterebra (rodent or rabbit botfly)
Rabbits and rodents are the natural hosts for the larvae of these flies, which are sometimes responsible for human furuncular myiasis [27, 28, 29]. Baird et al. [30] reviewed 54 cases of North American cuterebrid myiasis.
Genus Dermatobia (human botfly)
Dermatobia hominis is the only species in the genus. It is a bluebottle-like fly found in the neotropical areas of the New World, extending from southern Mexico to northern Argentina. It occurs in areas where temperature and humidity are relatively high, principally lowland forests. Dermatobia hominis causes cutaneous myiasis in a wide range of mammalian hosts, including humans, and is particularly important as a parasite of cattle.
The female fly does not deposit her eggs directly, but uses other insects, such as day-flying mosquitoes and blood-sucking flies, as vectors to carry her eggs to the host. This phenomenon is called ‘phoresia’ and explains the preferential localization of lesions in non-covered areas of the body unlike African myiasis caused by Cordylobia anthropophaga which contaminates covered area. The female fly grasps the insect vector in midair and deposits a number of eggs on its abdomen. When the vector subsequently feeds on a potential host, the eggs hatch and the larvae rapidly burrow into the skin (Figure 34.5). Larval development lasts approximately 50–60 days, following which the larva emerges, drops to the ground and pupates. Human botfly myiasis should always be considered as a cause of boil-like lesions in patients who have recently returned from endemic areas [31, 32, 33, 34, 35, 36, 37].

Figure 34.5 Third instar larva of Dermatobia hominis (the human botfly). Note the rows of backward-pointing spines.
Genus Gasterophilus (horse botfly)
A form of migratory cutaneous myiasis known as ‘creeping eruption’ is caused by Gasterophilus larvae. The Gasterophilinae are mainly parasites of the alimentary tract of horses, but occasionally larvae of certain species of Gasterophilus, including G. haemorrhoidalis and G. pecorum, penetrate human skin.
Genus Oestrus (sheep nostril fly)
Oestrus ovis, which develops in the nasopharyngeal passages of sheep and goats, and Rhinoestrus purpureus, which parasitizes horses, are occasionally responsible for human myiasis, especially ophthalmomyiasis. The fly leaves its larvae (not eggs) directly in the eye and patients complain of mild or severe foreign-body sensation, redness, watery eyes or lid swelling. Larvae on the external surface of the eye can be detected and removed under dim light. Larvae can hide in the ocular fornices [38, 39].
Genus Hypoderma (warble flies)
The larvae of Hypoderma species are obligate parasites of cattle. Man is an abnormal host for Hypoderma, and the larvae do not mature fully. After penetrating the skin, the larvae produce migratory subcutaneous swellings [40, 41]. They may also invade the eye (ophthalmomyiasis), producing severe damage [41, 42]. Marked eosinophilia may accompany infestation, and Starr et al. [43] reported a cattle rancher in whom an illness marked by pleuritis, pericarditis and myositis, and due to infestation with H. lineatum, mimicked the hypereosinophilic syndrome. A recent epidemic of ophthalmomyiasis with ocular injury has been reported in five children who had visited reindeer (also called caribou) herding areas in Norway or Sweden, due to Hypoderma tarandi, a bumblebee-like fly that is common in subarctic regions [41]. Imported cases of human disease have also been reported [44].
Clinical features [1, 2, 3, 4, 32]
The habits of the flies and their larvae determine the variations in the clinical manifestations for which they are responsible.
Cutaneous myiasis
Traumatic or wound myiasis has been a serious complication of war wounds in tropical areas, and is sometimes seen in neglected ulcers or wounds in most parts of the world [45]. Cochliomyia hominivorax, Chrysomya bezziana and W. magnifica are the most common flies, worldwide, that cause obligatory human wound myiasis. Wound myiasis is most often initiated when flies oviposit in necrotic, haemorrhaging or pus-filled lesions [46]. In the presence of an open wound, the most important predisposing factors for wound myiasis are a lack of hygiene and poor socioeconomic status [46].
Obligatory cutaneous myiasis occurs in two main clinical forms (furuncular and migratory myiasis); in both there may be mild constitutional symptoms and eosinophilia. Both occur mainly on exposed skin – often the face, scalp, arms or legs [47]. In the furuncular form, boil-like lesions develop gradually over a few days. Each lesion has a central punctum, which discharges serosanguinous fluid. The posterior end of the larva, equipped with a group of spiracles, is usually visible in the punctum, and its movements may be noticed by the patient (Figure 34.6). The lesions are often extremely painful but sometimes not. The inflammatory reaction around the lesions may be accompanied by lymphangitis and regional lymphadenopathy and/or systemic symptoms [48]. Secondary bacterial infection is a possible complication. Once the larva has emerged, or has been removed, the lesions rapidly resolve. The flies causing furuncular myiasis in humans are Dermatobia hominis, Cuterebra, Cordylobia anthropophaga, Cordylobia (Stasisia) rodhaini, Wohlfahrtia species and Hypoderma species.
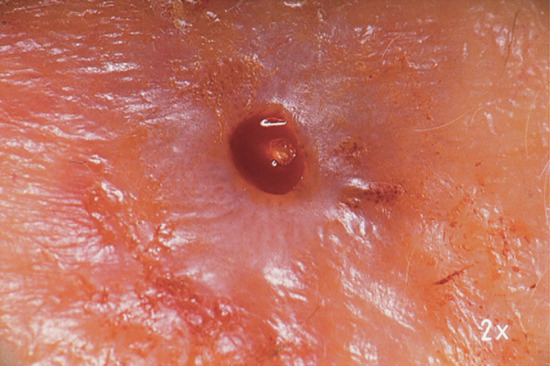
Figure 34.6 Furuncle-like lesion produced by Dermatobia hominis. The tail of the larva is visible in the centre of the lesion.
The second principal clinical form (migratory myiasis) is a creeping eruption, resembling cutaneous larva migrans (see Chapter 33), in which a tortuous thread-like red line with a terminal vesicle marks the passage of the larva through the skin. The larva lies ahead of the vesicle in apparently normal skin [49]. The larva may live for months in human skin and may migrate 1–30 cm/day. Infestation may present with pustules, nodules or recurrent swelling [50]. This form of myiasis is produced by Gasterophilus larvae. The inflammatory nodular lesions produced by Hypoderma species are migratory.
Cavitary myiasis
The infestation of natural body cavities is called cavitary myiasis. Cavitary myiasis receive specific names, depending on the anatomic region affected. Internal organs may also be affected. Eristalis tenax, a fly called ‘drone fly’, can cause intestinal, gastric or urinary myiasis. It is an accidental myiasis related to ingestion of contaminated uncooked food or water containing fly larvae. Rare cases are mainly reported from India, Africa or Europe [51, 52, 53].
Investigations [54]
Furuncular myiasis
Clinical features are often sufficient to diagnose furuncular myiasis, especially in endemic regions. Dermoscopy may be helpful in difficult cases, showing a yellowish structure with black barb-like spines [55, 56]. Ultrasound has also been used to confirm furuncular myiasis and may be useful to remove the larvae [57, 58]. When ultrasound failed to detect larvae, colour Doppler sonography was able to visualize the continuous movement of internal fluids of the larva and to confirm the diagnosis [59].
Migratory myiasis
The diagnosis relies on the identification of a dipteran larva. Magnification is used to visualize the parasite. In Hypoderma furuncular lesion, ultrasound may be helpful to detect the larva [44].
Wound myiasis
Diagnosis is easily made by the clinical inspection of the wound.
Management
Furuncular myiasis
The larva of Cordylobia can often be expressed by firm pressure around the edges of the lesion, but sometimes the punctum may require enlarging surgically.
The larva of Dermatobia hominis has a bulbous anterior end equipped with rows of spines (Figure 34.7) that help to anchor it in the skin and make its removal by manual pressure difficult [48]. Traditional methods of treatment include occluding the punctum with pork fat [60, 61], blocking the spiracles of the larva and stimulating premature extrusion. A similar result may be obtained with mineral oil, petrolatum (Vaseline) or butter. Surgical management is most frequently recommended: the punctum is enlarged by cruciate incisions, and this enables removal of an intact larva [62]. The injection of lignocaine (lidocaine) underneath the nodule may be sufficient to push the larva out [63], and Loong et al. [64] also found that injection of 2 ml of 2% lidocaine into the blind end of the cavity facilitated non-surgical removal of the larva.

Figure 34.7 Scanning electron micrograph of the spines of a Dermatobia hominis larva.
Migratory myiasis [54]
After identification of its position, the larvae may be removed with a needle. The extraction of Hypoderma larvae may require a cruciform incision, or only expression in case of furuncular lesions. However, in most cases, a surgical excision is necessary. Oral ivermectin or albendazole may be helpful, if extraction is not possible because the larva is too deep into the tissue [44].
Wound myiasis
Wound myiasis requires debridement and irrigation to remove larvae, and treatment of secondary infection.
Ophthalmomyiasis
The treatment is simply removal of all larvae. Antihistamine drops and/or topical antibiotics may also be used, as needed.
Ivermectin
Ivermectin has been used both topically and orally in the management of myiasis [33, 41, 42, 65, 66, 67, 68, 69, 70]. Oral treatment of human myiasis is based on anecdotal reports, and most of the experience comes from veterinary medicine. Different therapeutic schemes have been adopted for ivermectin use in the treatment of myiasis. Ivermectin is not recommended for furuncular myiasis, because it may kill the larva inside the lesion, with a consequent inflammatory reaction.
Fleas (Siphonaptera)
Definition
Fleas are small (1–8 mm long) wingless insects, laterally compressed to facilitate moving between the animal hairs. Male and female adults are blood-sucking ectoparasites of mammals and birds. Approximately 2000 species and subspecies are known. Egg, larvae and cocoon stages occur on the ground. The larvae of fleas are not parasitic, but feed on organic material that they find in the nest or dwelling place of the host [1, 2].
Classification
The order Siphonaptera contains three families of medical importance.
Family Tungidae
This family contains tropical species that burrow in human skin (see Tungiasis).
Family Pulicidae
Members of this family occur throughout the world, and some species transmit plague (Yersinia pestis) (see Chapter 26) and murine typhus (Rickettsia typhi) (see Chapter 26) [3, 4, 5]. Fleas play also a role in the transmission of rural epidemic typhus (Rickettsia prowazekii) in the USA [6]. In recent years, the flea-borne spotted fever agent Rickettsia felis has emerged and can be found throughout the world [7]. Cat fleas have been shown to be vectors of Bartonella henselae, the pathogen responsible for cat scratch disease and bacillary angiomatosis [8, 9]. Bartonella quintana has been detected in cat fleas [10] and in Pulex irritans [11], although its main vector is the body louse. Infections from Bartonella quintana have re-emerged, predominantly among the homeless populations in cities in both Europe and the USA [12]. The rabbit flea (Spilopsyllus cuniculi) may be a potential vector of Bartonella alsatica, which has been responsible for endocarditis and lymphadenitis in humans [13, 14]. Many species are important only for the irritability of their bites. The species most frequently parasitizing humans are the human flea, Pulex irritans (mainly in tropical areas), and the cat and dog fleas, Ctenocephalides felis and Ctenocephalides canis (mainly in occidental areas), but other species will bite humans in the absence of their normal host. The tropical rat flea, Xenopsylla cheopis, is the vector of bubonic plague.
The adult female flea lays her eggs during feeding on the host, and the eggs fall to the ground, where an important food source for the larvae is the faeces of the adult flea. The larvae subsequently form cocoons, and under suitable conditions of temperature and humidity the life cycle may be completed in a few weeks. However, the cocoon stage can sometimes last as long as a year, and the flea may emerge only in response to vibrations produced by the movement of possible hosts.
In a household occupied by infested pet dogs or cats, fleas in various stages of development are found in the animals’ bedding, and on carpets and soft furnishings. In a survey carried out in the UK in 2005, the prevalence of flea infestation in domestic cats was 21.1% (98.9% of fleas were C. felis) and in dogs 6.8% (93.1% of fleas were C. felis) [15]. To date, no clinical case of murine typhus has been described in Spain, while the presence of R. typhi in cats and fleas has been demonstrated [16].
Family Ceratophyllidae
Species in this family are mainly parasitic on rodents and birds. Bird fleas overwinter in cocoons in birds’ nests, and emerge in spring. At this time they can become household pests, as they may gain access to bedrooms from nests under the eaves.
Epidemiology
Infestation with the human flea, Pulex irritans, occurs mainly in congested communities with low standards of hygiene. It is now rare in developed countries. Cat and dog flea infestation in the home is, however, common.
Animal fleas are common throughout the world, and persons in contact with domestic animals are frequently bitten. Severe attacks are sometimes experienced by individuals moving into premises long empty, but previously occupied by pet cats or dogs. The vibration caused by footsteps triggers the emergence of fleas from their cocoons. Attacks are more likely to occur when the fleas do not have access to their usual host. Household infestations with bird fleas may occur from nests or nest boxes on or near the house [17], and similar problems may occur in the workplace [18]. An outbreak of papular urticaria in a nursery school was traced to an infestation with dog fleas from a fox's burrow beneath the building [19]. Similar problems were caused by Ctenocephalides felis entering houses from raccoons which had bred in the cavity between two houses [20].
Clinical features [6, 21]
Flea bites usually provoke typical papular urticaria in a sensitized individual. Occasionally, the reaction is more severe, and bullae may occur. The lesions may be grouped in lines or irregular clusters. Cat and dog flea bites occur predominantly on the legs or buttocks, and are most profuse around the ankles (Figure 34.8), but they can also occur on the forearms. They are much more common in women than men, as trousers and socks protect the legs. Bites from bird fleas tend to be more extensive, as the sleeping occupants of bedrooms usually provide larger areas of exposed flesh.
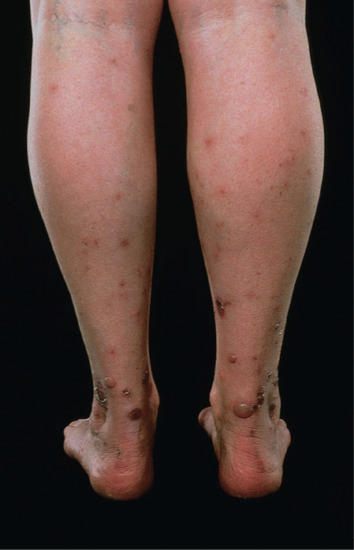
Figure 34.8 Typical distribution of cat or dog flea bites on the legs.
Investigations [22]
If flea infestation from pet animals is suspected, this can be confirmed by microscopical examination of debris from the animals’ bedding material.
The principal sign of flea infestation in an affected animal is the presence of dried concretions of flea faeces on the animal's coat. Some animals will also have signs of flea allergy dermatitis, with areas of crusting and alopecia, most frequently on the lower back and the base of the tail.
If fleas from another source are suspected of causing bites, it may be necessary to examine samples taken with a vacuum cleaner from rooms, or to visit the suspect premises. It is important to identify the flea species responsible for an infestation so that efforts at eradication may be accurately directed at the source [23]. Cat fleas (Figure 34.9), dog fleas and common bird fleas may be readily identified after ‘clearing’ in 10% potassium hydroxide for 48 h [4], but the help of an entomologist should be sought.

Figure 34.9 Ctenocephalides felis, the cat flea.
Management [24]
The development of topical and oral agents such as fipronil, imidacloprid, lufenuron and selamectin has revolutionized domestic cat and dog flea control [25]. More recently, newer flea products have been marketed such as dinotefuran, metaflumizone, spinetoram and spinosad [26, 27]. However, flea control may remain difficult. Flea control programmes must also take into account the age, lifestyle and allergy status of the animal, the presence of other companions in the household and owner ability and resources.
Pest-control companies will deal with flea infestation from other sources. In private homes, personal action can be taken to investigate by meticulous cleaning with a vacuum and a floorcloth on resting places of the cat or dog (bed, sofa, carpet) to remove eggs, larvae and cocoons.
Tungiasis
Introduction and general description
Tungiasis is caused by the sand flea Tunga penetrans, also known as the jigger or chigoe.
Epidemiology
Originally a native of South America, it subsequently spread to Africa [28, 29], the Caribbean and India. Tungiasis has reappeared in Mexico [30], where it was previously last recorded in 1948. The ease of world travel has contributed to tungiasis being encountered in non-endemic areas [28, 29, 31, 32, 33, 34, 35, 36, 37, 38].
Pathophysiology
Tunga penetrans is the smallest known flea (1 mm long). Its larvae develop in dry sandy soil, and development from egg to adult takes about 3 weeks in favourable conditions. The impregnated female flea burrows into the feet of mammals, preferring humans and pigs. In humans, the fleas establish themselves between the toes, under the nails and on the soles, but other parts of the body may be affected. Once embedded in the skin, the flea's abdomen enlarges to the size of a pea, and large numbers of eggs are produced. The eggs are subsequently gradually extruded over a period of 2 weeks, and the female flea dies and is sloughed from the skin [39].
Pathology
Anatomical components of the flea are sufficiently distinctive to enable a diagnosis of tungiasis to be made histologically [40]. The distinguishing features are an eosinophilic cuticle with tracheal rings and adjacent eggs. The adjacent tissue exhibits basal epidermal hyperplasia [41].
Clinical features [28, 42]
The presence of the fleas causes intense irritation. The typical appearance of an individual lesion is initially a black dot surrounded by a halo of erythema, followed by enlargement to form a mother of pearl-coloured papule with a central dark punctum, produced by the enlarging flea abdomen (Figure 34.10). Secondary infection is common, and tetanus has often complicated tungiasis in the past [43]. In severe cases, the feet may be honeycombed by multiple lesions, causing serious discomfort and disability [44]. Tungiasis can be a serious health problem in resource-poor communities, where children can be teased and stigmatized [45]. The differential diagnosis of tungiasis includes myiasis, verruca vulgaris, ingrowing toe nail, acute paronychia, mycotic granuloma, malignant melanoma, and arthropod bites [46]. Use of the dermoscope aids diagnosis by demonstrating the surface features [47, 48, 49]. Tungiasis may act as a portal of entry of bacterial superinfection, e.g. necrotizing fasciitis [45].
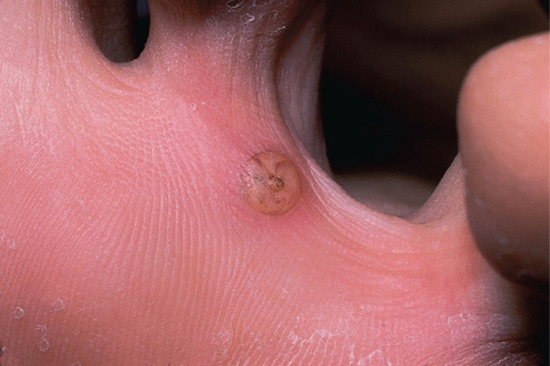
Figure 34.10 Tungiasis, showing a characteristic lesion on the sole of the foot.
(Courtesy of Dr N.H. Cox, Cumberland Infirmary, Carlisle, UK.)
Management [50, 51]
The best procedure for dealing with tungiasis is blunt dissection of the intact parasite. Local people where the infection is endemic are very skilled at doing this, usually with a pin or splinter of wood. This can also be accomplished by enlarging the surface punctum and extracting the flea with tweezers, curettage and cautery, or excision.
A plant-based repellent, assessed in Brazil, proved to be extremely effective in preventing infestation with T. penetrans [52].
The unwary traveller may well acquire tungiasis, and those visiting endemic areas should be warned to wear stout shoes, and not to sit on the ground.
Bees, wasps and ants (Hymenoptera)
Definition
The adults of many species in this large order of insects have evolved a sting apparatus. The sting may or may not be barbed. Some use the sting in defence and others use it offensively in hunting for food. Males have no sting apparatus. Humans are frequently stung by these insects, with reactions varying from local discomfort to fatal anaphylaxis.
The Hymenoptera are readily recognized by the narrow waist (isthmus) connecting the abdomen to the thorax. Some of the more important families are described below.
Classification
Superfamily Apoidea (bees)
Honeybees (Apis mellifera)
Honeybees possess a barbed sting. When humans are stung, the bee is frequently unable to remove the sting. The sting and venom apparatus are avulsed from the bee's abdomen in its struggles, but the venom apparatus continues to function and pump in more venom.
‘Africanized’ honeybees, the product of interbreeding between bees from southern Africa and European species, have caused significant problems [1]. These aggressive (‘killer’) bees, which have characteristics of their African antecedents, including strong colony defensive behaviour, have migrated northward from Brazil to the southern USA and more recently several North American states [2]. These Africanized bees may have negative impacts such as swarming, aggressive behaviour, and the ability to mass attack, resulting in serious and fatal envenomation with humans and animals [2].
Humblebees; bumblebees (Bombus spp.)
The sting is not barbed, and the bumblebee is therefore able to sting repeatedly. Most species are inoffensive, and only sting defensively when severely provoked.
Superfamily Vespoidea
Family Vespidae (social wasps)
This family includes wasps, yellow-jackets and hornets. Species of Vespa, Vespula and Polistes inflict painful stings. Wasps can also sting repeatedly, as they either have small barbs or none at all on their stings.
In Europe, Vespa velutina is a predator for honeybees, spreading rapidly from south-west of France since 2006 with increasing stings on humans [3].
Superfamily Bethyloidea
These are small solitary wasps. They sometimes become abundant in houses infested by woodworm; indeed they are parasitic on the larvae of Lepidoptera and Coleoptera. Scleroderma domesticum (Figure 34.11), Epyris californicus and Cephalonomia gallicola may inflict troublesome stings. Bites of Scleroderma dosmesticum have no typical localization on the body. This house pest occurs usually in summer time. Management must be to treat furniture which has woodworm. This is rarely recommended because these insects are confused with ants [4].

Figure 34.11 Scleroderma domesticum.
Superfamily Scolioidea
Family Formicidae (ants)
Many ant species are equipped with powerful stings, including the Australian jumper and bull ants [5, 6], and Solenopsis, the fire ant. Fire ants, so called because of the burning pain of their stings, have been particularly problematic in recent years in the USA. There are several native species of fire ant in the USA, but it is the red and black imported fire ants S. invicta and S. richteri, inadvertently brought to the USA from South America, that have become troublesome pests [4, 7, 8, 9, 10]. Solenopsis invicta is also well established in two locations of the Brisbane area of Australia [11]. Wood imported from South America was the source of fire ants responsible for anaphylaxis in a woman in Málaga, Spain [12], and more recently one case of imported fire ant causing anaphylaxis in Canada [13]. A child who had multiple fire ant stings presented with anaphylaxis and evolving signs of systemic envenomation, including rhabdomyolysis and renal failure, thus illustrating the potential for a significant Hymenoptera toxidrome [14].
The fire ant first uses its powerful mandibles to grip its victim, and drives its non-barbed sting into the skin. It then rotates about the point of attachment of the mandibles and inflicts further stings in a circular pattern [8, 9, 10, 15]. Although largely outdoor insects, fire ants may move into dwellings, causing problems for the inhabitants [16].
Species of Pogonomyrmex (harvester ants) may inflict multiple painful stings [4].
Pathophysiology
Venoms [17-28, 29, 30, 31]
The composition of venoms is complex. Pharmacologically active and antigenic substances are both present, and an individual's reaction to the sting is determined partly by the quantity of the former, and partly by the degree of acquired hypersensitivity to the latter. Hymenoptera venom contains vasoactive amines, small polypeptides and larger protein molecules. The components of vespid (wasps, yellow-jackets and hornets) venoms include histamine, serotonin, mast cell degranulating peptide, wasp kinin, phospholipases, hyaluronidase and antigen 5. The three major allergens in vespid venoms are phospholipases, hyaluronidase and antigen 5. The venom of the honeybee contains histamine, mast cell degranulating peptide, melittin, phospholipase A2, hyaluronidase and acid phosphatase. The three proteins in honeybee venom which are important allergens are phospholipase A2, hyaluronidase and acid phosphatase. In addition, the polypeptide melittin is also antigenic. Bumblebee venom appears to be chemically and antigenically related to honeybee venom [26].
Study of fire ant venom was impeded for many years by the extreme difficulty in obtaining sufficient amounts. The venom is composed of 90–95% water-insoluble piperidine alkaloids [27], which are not allergenic but are responsible for the immediate hive formation and the development of the sterile pustule at the sting site [32]. In recent studies, the alkaloid compositions have been reinvestigated and found to be much more complex than previously thought [33, 34]. Alkaloid compositions vary among species of fire ants. When commercial grade venom became available, several potent allergenic proteins were identified [9, 24]. Antigenic similarity between fire ant venom, bee and wasp venoms and scorpion venom has been demonstrated [25, 28].
Allergy to Hymenoptera venom is mediated by IgE antibodies. The antigenic substances in the venom of many Hymenoptera are more liable to induce high degrees of hypersensitivity of the immediate type than are the antigens of most other insects. Several risk factors are associated with the occurrence of severe systemic anaphylactic sting reactions [35]: vespid stings, older age [36], elevated tryptase concentration [36], male patient [36], specific medication (angiotensin-converting inhibitors) [36] and mastocytosis [37, 38]. Preceding less severe systemic reaction may also predispose the patient to more severe reactions [36].
Clinical features [17, 18, 29, 30]
Reactions to bee and wasp stings may be classified as local and systemic. Both may have a toxic or a hypersensitive mechanism. The typical local toxic reaction produced by pharmacologically active components of the venom is burning pain, which may be very severe, followed by erythema and oedema. This local reaction subsides in a few hours. The systemic effects of multiple stings include hypotension, generalized vasodilatation, severe headache, vomiting, diarrhoea and shock, and the cumulative effect of a large number of stings may be fatal, particularly in children.
In some cases, hypersensitivity produces only a more intense local reaction manifest as increased oedema, usually developing within the first half hour, but occasionally delayed for several hours. If a generalized anaphylactic reaction occurs, this is usually within a few minutes of the sting. The manifestations of a generalized reaction may be classified as cutaneous (pruritus, erythema, urticaria and angio-oedema), respiratory (laryngeal oedema, bronchospasm) or vascular (tachycardia, hypotension, shock). These features may occur separately or in combination, and in varying degrees of severity.
Occasionally, late-onset reactions to stings occur [19]. In some patients, an urticarial reaction develops several hours after the sting, and in others a serum sickness-like reaction occurs, with urticaria, joint swelling and arthralgia.
A patient with a foreign-body granuloma and IgE pseudolymphoma following multiple bee stings has been reported [39], and another with an eosinophilic foreign-body granuloma after multiple self-administered bee stings as treatment in traditional Korean medicine [40].
Skin lesions produced by fire ants typically occur in clusters [8, 9, 10]. The site of attachment of the mandibles may be marked by two minute haemorrhagic puncta. The initial reaction to the sting is the development of a weal, followed within a few hours by a vesicle. The fluid in the vesicle gradually becomes cloudy, and after 8–10 h the typical lesion is an umbilicated pustule on a red oedematous base. The pustule subsequently ruptures, forming a crust, and after several days the lesions heal, frequently leaving small scars. Hand–foot syndrome associated with multiple fire ant stings has been described [41].
Systemic hypersensitivity reactions may also occur, and feature generalized urticaria and angio-oedema, wheezing, nausea and vomiting, and hypotension [8, 42, 43]. These manifestations may increase in severity with successive attacks, and fatal anaphylaxis can occur [10, 13, 14].
Management [30, 35, 44, 45]
Management of large local reactions
Conventional advice with regard to honeybee stings is that the sting should be immediately scraped off, never pinched because the remaining venom is inoculated; but a study by Visscher et al. [46] suggests that the method of removal does not affect the quantity of venom received and is therefore unimportant; the sting should simply be removed as rapidly as possible. The application of a potent topical corticosteroid to the sting site before the area is cooled with wet dressings or cool packs is usually recommended [35]. Oral antihistamine and/or systemic corticosteroids may be recommended even if there are no controlled studies; it is considered that this therapy should be initiated as soon as possible after the sting [35].
Management of systemic anaphylactic reactions
The treatment of choice for anaphylaxis is intramuscular epinephrine (adrenaline) (in adults, a dose of 0.5 mL 1 : 1000 solution should be administered, and repeated after about 5 min in the absence of clinical improvement or if deterioration occurs after the initial treatment), followed by chlorpheniramine (10–20 mg, intramuscular or slow intravenous) and hydrocortisone (100–500 mg, intramuscular or slow intravenous) [45]. Patients at risk of an anaphylactic response to Hymenoptera stings should wear a device such as a MedicAlert warning bracelet, in case they are discovered unconscious following a sting. They should also carry a sting emergency kit containing an antihistamine and corticoid for oral administration and most importantly an auto-injectable adrenaline to all patients with a history of systemic anaphylactic reactions. They should therefore receive instruction in self-administration of adrenaline.
Venom immunotherapy
The introduction of venom immunotherapy has reduced the risk of anaphylaxis in Hymenoptera-sensitive patients. It is thought to exert its beneficial effect by stimulating the development of IgG (blocking) antibodies against the venom allergens. This prophylactic measure is indicated in patients with a history of life-threatening reactions to stings, positive skin tests and the presence of venom-specific serum IgE. However, such therapy should only be carried out in specialized units.
According to current European guidelines, venom immunotherapy is recommended only for patients with a history of moderate to severe reactions [47]. In cases of mild reactions, which are limited to the skin, venom immunotherapy is not considered mandatory. Golden et al. [48] suggested expanding the clinical indications for venom immunotherapy to include large local reactors where insect stings cause significant morbidity and impair the quality of life.
Lice (Phthiraptera)
Definition and nomenclature
Lice are members of the order Phthiraptera. They are wingless, dorsoventrally flattened insects, which are obligate ectoparasites of birds and mammals. The Phthiraptera are highly host specific and spend their entire lives on the host. Members of the suborder Anoplura are blood-sucking ectoparasites of mammals.
Classification
Humans are parasitized by two species of Anoplura: Pediculus humanus, divided into Pediculus humanus capitis (the head louse) and Pediculus humanus humanus (the clothing or body louse), and Pthirus pubis, the pubic or crab louse. Head lice and clothing lice are morphologically and biologically similar but have distinct ecologies. They have almost the same basic genetic content, i.e. being ecotypes of the same species (Pediculus humanus) [1], even if they differentially express certain genes. They are capable of interbreeding, but on the host they maintain their territorial preferences. Pthirus pubis is morphologically quite distinct from Pediculus. It is a louse that has only one close relative in the insect world, a species living on gorillas (Pthirus gorillae) and the ancestor of human pubic lice. It is thought that our human ancestors acquired pubic lice from gorillas, perhaps by sharing their bedding material or by humans feeding on gorillas. Interestingly, chimpanzees, our closest primate relative, are parasitized by Pediculus species (P. schaeffi) but not by Pthirus, and orangutans do not have lice.
Pthirus is the correct zoological name for the crab louse—the name should have been Phthirus, but a misprint was inadvertently accepted by the International Committee on Zoological Nomenclature [2].
The Anoplura are vessel feeders (solenophages), introducing their mouthparts directly into a blood vessel to withdraw blood [3, 4]. The components responsible for probing the skin and piercing a blood vessel are a group of stylets, which are kept withdrawn within the head unless the insect is feeding. In the front of the head is a small snout-like tube, the haustellum, which is soft, eversible and armed with teeth. When the louse is about to feed, the haustellum is everted and the buccal teeth rotated outwards (Figure 34.12a). The teeth cut into the epidermis and the haustellum is driven into the skin. It eventually comes to rest with the buccal teeth fully everted, anchoring the mouthparts (Figure 34.12b). Once fixed in the skin, a bundle of stylets is pushed forward through the opening in the haustellum by protractor muscles within the head of the louse (Figure 34.12c). The stylets are advanced into the dermis as a single bundle or fascicle, and probe for a small blood vessel.
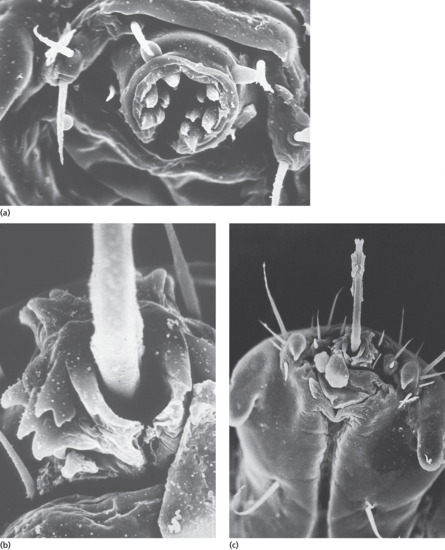
Figure 34.12 Scanning electron micrograph of a crab louse showing: (a) haustellum with buccal teeth; (b) everted buccal teeth; and (c) the protruded stylet bundle.
Head lice (pediculosis capitis)
Epidemiology
Incidence and prevalence [5, 6, 7]
The head louse has a worldwide distribution, and head louse infestation (pediculosis capitis) is common both in developed and developing countries. However, precise data on current prevalence are relatively sparse. The traditional perception of head lice as a parasitosis exclusively associated with schoolchildren of low socioeconomic status must now be challenged [8].
High rates of head louse infection have been reported from the USA, Canada and several other countries; a review by Gratz [5] provides a comprehensive survey of published information relating to prevalence.
In the UK, prevalence is 2%, with an annual incidence of 37% [9]. In Australia, the reported prevalence in schoolchildren is 13%; in Brazil, a prevalence of 43% in a slum and 28% in a fishing village was shown in 2005 [10]; and in China, the prevalence is 14% (range of 0–52%) [11].
Age/sex
Head lice are more common on children, particularly in the age range 3–11 years, than on adults, and most surveys have shown that girls are more frequently infected than boys. Behaviour patterns in girls and boys at different ages probably influence rates of infection [12]. For example, in primary schools, children are organized into small groups around desks, and head to head contact is frequent. In addition, hair contact is probably more likely between girls than boys. Older children tend to be more independent, and more separated from their peers. The contribution of hair length to infection is contentious. Some studies have not shown any correlation between hair length and louse infection rates, but in others children with longer hair have had higher infection rates. A survey from Israel, in which detection of infection was by means of a louse comb, rather than direct visual inspection, found a significantly higher infection rate in children with long and medium length hair than in those with short hair [13].
Ethnicity
Several authors have noted a low incidence of head louse infection in black Americans [14, 15], although infection rates in black Africans are high [5]. It has been suggested that the use of pomades by black Americans provides an environment unsuited to establishment of infestation [14], but head lice are quite common in the Indian subcontinent, where hair oils and creams are frequently used [16]. A survey in Brazil found the same prevalence in black as in white people [17].
Associated diseases
Head lice are not known to transmit any human pathogens. However, Bartonella quintana has been detected in the head lice of homeless individuals and Nepalese slum children [18, 19]. In a study in Paris in 2008–09, Acinetobacter baumanii, but not B. quintana, was detected in 95 samples (33%) from a total of 288 DNA samples from adult head lice collected from the heads of schoolchildren. The significance of the finding of A. baumanii in head lice is uncertain [20].
Pathophysiology [2, 7, 21, 22, 23]
The adult female is a greyish white insect 3–4 mm long (Figure 34.13). The male is slightly smaller. The claws on the legs are adapted for clinging to hair. During her lifespan of approximately 40 days, the female lays an average of about seven eggs daily. The eggs are cemented to hair shafts with a chitinous cement material secreted by the female's accessory glands [23] (Figure 34.14a). In temperate climates, in order to provide a suitable temperature for incubation, the eggs are attached to hair close to the surface of the scalp. They are oval, flesh coloured and have a lid (operculum) capping the free end of the egg (Figure 34.14b). The operculum is pushed off by the emerging louse nymph. Once the louse has emerged, the empty egg case or ‘nit’ appears white, and is easier to see than the intact eggs close to the scalp surface. Eggs hatch in about 8 days and, following three moults, the louse nymph reaches maturity in approximately 10 days.

Figure 34.13 Pediculus capitis, the head louse.
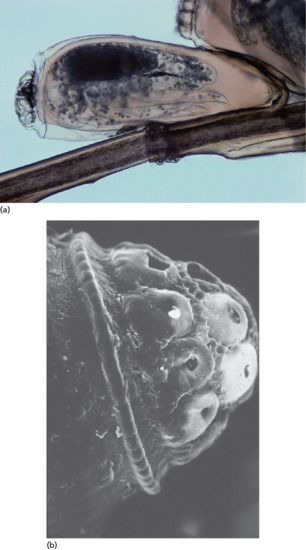
Figure 34.14 (a) Head louse eggs cemented to a hair shaft. (b) Scanning electron micrograph of the operculum on a head louse egg.
It is thought that the majority of head louse infections are acquired by direct head to head contact, optimal conditions for transfer being when hairs are parallel and slow moving [24]. In developing countries, spread of lice is encouraged by poverty, poor hygiene and overcrowding. Lack of hygiene alone does not encourage head louse infection.
There are conflicting opinions about the importance of fomites in transmission of head lice [25], and in practice the putative role of caps, scarves, combs and brushes is difficult to confirm or refute.
In Australia, an examination, employing a vacuum cleaner fitted with a filter, of classroom floors in schools in which there was an overall prevalence of head lice of 20.9%, did not reveal any lice on the floors [26], indicating that there is no requirement for anti-louse measures on carpets and floors.
Clinical features
Although many individuals are asymptomatic [27], scalp pruritus is the characteristic manifestation of head louse infection. Secondary bacterial infection may occur as a result of scratching, and concomitant head louse infection must always be considered in cases of scalp impetigo. Pruritic papular lesions may occur on the nape of the neck, and occasionally a generalized non-specific pruritic eruption develops [28]. In severe neglected cases, pus and exudate may produce matting of the hair – a state that has been termed ‘plica polonica’, from its prevalence in Poland in the early part of the 20th century. However, matting of the hair can occur in the absence of louse infection, and it has been suggested that this term should be discarded [29].
The empty egg cases (nits) occur in greatest density on the parietal and occipital regions (Figure 34.15). However, on naked-eye inspection, they may be confused with peripilar keratin casts (‘pseudonits’; hair muffs) [30, 31] or dried globules of cheap hair lacquer.

Figure 34.15 Numerous head louse eggs and empty egg cases.
Investigations
Detection of adult lice and nymphs provides evidence of an ‘active’ infestation, whereas the presence of eggs and egg cases alone merely indicates that infection has occurred at some time. The most reliable method of diagnosing current active infestation is by detection combing, which has been shown to be superior to direct visual examination of the hair and scalp [13, 32, 33, 34]. This is an important criterion for several reasons:
- Individuals who do not have evidence of active infestation should not receive chemical treatment [34].
- Participants in clinical trials of pediculicides should have live lice present on the head before enrolment, not just eggs alone.
- Children who do not have evidence of active infestation may be inappropriately excluded from school [34, 35, 36].
Management
Principles of management [6, 7, 22, 34, 37, 38–40]
Management of head louse infestation is difficult because good comparative effectiveness research is still lacking and louse resistance to pyrethroid has emerged. An initial Cochrane systematic review concerning pediculicides was withdrawn in 2007 [41] but a new one is in process [42].
The ideal treatment should be completely safe, free of harmful chemicals, readily available, easy to use and inexpensive. It should also be effective where there are local variations in resistance.
General guidelines for the use of chemical pediculicides have included advice to repeat treatment after 7–10 days [43] because of limited ovicidal activity, and that lotion and liquid formulations are preferable to shampoos as the latter expose the insects to relatively low concentrations of insecticide with subsequent poor efficacy and which, in the long term, might favour the development of resistance. Preparations with an aqueous basis are less likely to irritate an excoriated scalp than alcoholic solutions, do not irritate the bronchi of asthmatics and are not flammable. Sprays are not suitable for people with asthma.
Family members should be examined, and treated only if they show evidence of active infestation by the presence of live lice. Nits may be removed with a fine-toothed comb. Treatment has most chance of success if it is applied or undertaken correctly and if all affected individuals in the household are treated simultaneously. People should be advised to check whether treatment was successful by detection combing on day 2 after completing a course of treatment, and again after an interval of 7 days (http://cks.nice.org.uk/head-lice last accessed October 2014).
All materials that touched the heads of infested persons, such as hats, scarves, bedding and cushions, must be thoroughly washed in hot water (50°C at least) [44]. Any infested materials kept in plastic bags for 3 days may be safely used. Hair grooming aids, such as brushes, combs and curlers, should be discarded or decontaminated with an insecticidal powder.
Acquired resistance to insecticides
Classical insecticides like dichlorodiphenyltrichloroethane (DDT), lindane, carbaryl and malathion have been progressively replaced since the 1980s by pyrethrin and pyrethroid insecticides. These insectides are efficient, safe, convenient and cost-effective. Available formulations include 1% permethrin and pyrethrins plus piperonyl butoxide. However, lice with pyrethroid-resistant phenotype emerged in the early 1990s [45]. There is now evidence of widespread pyrethroid resistance, and less frequently malathion [45, 46, 47, 48, 49, 50, 51, 52].
Pyrethroids are neurotoxins that modify louse voltage-gated sodium channel (VGSC), causing spastic paralysis and death. Permethrin resistance in head lice is mostly conferred by the knockdown resistance (kdr) trait, conferred by three point mutations (M815I–T917I–L920F) in the VGSC α-subunit gene [53]. The prevalence of kdr-like louse alleles is not exactly known, but seems to be extremely variable, depending on the geographical area [54, 55].
Alternative treatment
Resistance of head lice to insecticides led researchers to look for alternatives.
Physical treatment is an alternative to the use of chemical agents and, in the UK, the ‘Bug Busting’ (Community Hygiene Concern, London, UK) wet-combing method has been promoted as a treatment for head lice. The technique involves ordinary shampooing of the hair, followed by the application of generous amounts of conditioner, and combing using a fine-toothed comb to remove lice. This procedure is repeated every 4 days for 2 weeks [56, 57, 58, 59, 60]. Shaving the head is usually not acceptable because of psychosocial impact.
Dimethicone lotion (4% long-chain linear silicone in a volatile silicone base) [61, 62, 63] blocks the outer respiratory tract of lice and therefore water excretion, which causes physiological stress and death [64]. Coconut-derived emulsion shampoo, benzyl alcohol lotion 5% and spinosad cream rinse may be used too [65, 66, 67], as well as isopropyl myristate and cyclomethicone together [68].
Other readily available occlusive substances such as oils and margarine have been suggested but information on effectiveness is anecdotal. In one study that examined vinegar, isopropyl alcohol, olive oil, mayonnaise, melted butter and petroleum jelly, the use of petroleum jelly caused the greatest egg mortality, allowing only 6% to hatch [69].
Essential oils have been widely used in traditional medicine for the eradication of head lice, but because of the variability of their constitution, the effects may not be reproducible [70]. Several products including lavender and tea-tree oils are marketed for the treatment of head lice and are in wide use. Although many plants naturally produce insecticides for their own protection that may be synthesized for use by humans, such as pyrethroids, some of these insecticidal chemicals produce toxic effects as well.
Ivermectin is an antiparasitic drug used for onchocerciasis and lymphatic filariasis (Chapter 33). It induces arthropod and nematode paralysis and death by interrupting neurotransmission, acting on glutamate-gated or γ-aminobutyric acid–gated chloride channels. In a recent cluster-randomized controlled trial of patients with head lice refractory to insecticides, a single oral dose of ivermectin 400 μg/kg repeated within 7 days achieved higher louse-free rates on day 15 than 0.5% malathion (95.2% versus 85.0%) and their household members (92.4% versus 79.1%) [71]. This is an ‘off-label’ use of such dosage of ivermectin and its safety in patients with head louse infestation remains unknown. Topical ivermectin has shown greater efficacy than placebo in a recent randomized control trial and has been approved by the Food and Drug Administration in 2012 [72, 73]. The lotion is convenient (i.e. applied to dry hair, left for 10 min, then rinsed with water), which should increase compliance.
First line
In the UK, the choice of a particular treatment strategy will depend on age, individual or parent preference, cost and success or failure with previous treatments. There is limited evidence to support the effectiveness of each treatment option recommended. No option is clearly superior or inferior to the others in terms of effectiveness and there are advantages and disadvantages for each method, and no method can guarantee success. Treatment options include: dimethicone, bug busting, isopropyl meristate and cyclomethicone, coconut or malathion (http://cks.nice.org.uk/head-lice last accessed October 2014). The 2015 American Academy of Pediatrics recommend the use of 1% permethrin or pyrethrin insecticide as first line therapy [74]. In case of therapeutic failure, before considering acquired resistance to insecticides other options should be discarded, e.g. low compliance, wrong formulation, insufficient regimen, reinfestation (Box 34.1). If resistance in the community has been proven or live lice are present 1 day after the completion of treatment, a switch to malathion may be necessary.
Second line
Second line treatment depends on the first line used in the UK. In the USA, second line options include wet combing or treatment with dimethicone or other topical agents, depending on the availability of the agents in the country.
Third or fourth line
Ivermectin if available (depending on the country) should be the last choice, whether topical (for still-infested persons) or oral (especially for mass treatment, off label).
Resources
Further information
www.cdc.gov/parasites/lice/head/
http://cks.nice.org.uk/head-lice (Both last accessed October 2014.)
Clothing/body lice (pediculosis corporis)
Epidemiology [22]
Incidence and prevalence
Because body lice are associated with poor socioeconomic conditions, with infestation occurring only when clothes are not changed or washed regularly (pediculosis vestimenti), indigent, homeless and refugee-camp populations are predominantly affected. Body louse prevalence is underestimated in more developed countries and, as the number of homeless people increases, louse-borne infectious diseases are also on the rise. Molecular approaches are now convenient tools for epidemiological studies of louse-borne bacteria [75].
Associated diseases
Body lice may transmit several important diseases. The first is the epidemic typhus, caused by Rickettsia prowazekii (see Chapter 26). Symptoms include headache, fever, confusion and rash. The disease usually becomes epidemic in populations living in poor crowded conditions. A huge outbreak of epidemic typhus (and trench fever but not relapsing fever) occurred in 1995–97 in Burundi, first affecting prison inmates, then more than 45 000 camp refugees [76]. Relapsing fever is caused by a spirochete, Borrelia recurrentis. Recent outbreaks have occurred in Ethiopia and Southern Sudan [77]. Trench fever is related to Bartonella quintana. The disease was first recognized during World War I. Symptoms include fever, myalgias, headache, meningoencephalitis, chronic adenopathies and transient maculopapular exanthema, but may be asymptomatic. Endocarditis may sometimes occur [78]. Homeless people with chronic alcoholism are at risk.
Pathophysiology
This louse is almost identical in appearance to the head louse, except that it is usually slightly larger, and its development is similar. Its natural habitat is the clothing of its host, and it only visits the skin to feed. Its eggs are cemented to clothing fibres, with a preference for clothing close to the skin. Seams are a favoured site for oviposition. It thrives in situations where normal hygiene is lacking. The clothing louse and its eggs will not survive high-temperature washing and ironing, and it is intolerant of temperature changes in its environment. It is therefore a parasite of individuals whose clothing is rarely changed or washed. The number of lice and eggs on the clothing varies greatly. In most infected individuals, the population is small, but in some there may be thousands of lice.
Clinical features [22, 79]
In most infected persons, itching is the principal complaint. Pruritus is the result of sensitization to louse salivary antigens. Others, who have not become sensitized or have acquired tolerance to the bites, are asymptomatic. The body is often covered in excoriations, and there may be secondary bacterial infection. In those who have harboured clothing lice for long periods of time the skin is often hyperpigmented (so-called ‘vagabonds’ disease’; morbus errorum), and this is probably a post-inflammatory phenomenon.
Lice and eggs should be sought in the clothing (Figure 34.16).

Figure 34.16 Clothing lice and eggs.
Management [6]
Unlike the other forms of louse infestation, the lesions caused by body lice are the main focus of treatment. Antibiotics are needed to treat louse-borne infectious disease. Bed linens and clothes should be systematically decontaminated, and this action suffices for some physicians. Others recommend thorough washing of the body with soap followed by application of pyrethrins/pyrethroids or malathion for 8–24 h. Recently, ivermectin (3 doses of 12 mg each, given at 7-day intervals) was shown to reduce the number of body lice infesting a population of homeless men. Such treatment may be effective in limiting the viability of body lice in patients living in an institution or routinely returning to a treatment centre or shelter [80]. Depending on the geographical location of the infested individual and his or her contact with other similarly infested individuals, the physician should consider the possibility of louse-borne disease. Infested furniture, mattresses and box springs should be discarded or fumigated to destroy lice and nits. Infested materials sealed in plastic bags may be used safely after 3 days.
Resources
Further information
www.cdc.gov/parasites/lice/body/ (last accessed October 2014).
Crab lice (phthiriasis pubis)
Epidemiology [22]
Crab lice are transmitted by close physical contact, usually sexual, and infection with these lice occurs most frequently among sexually active young adults. It is standard practice in genito-urinary medicine (GUM) clinics to monitor prevalence rates of sexually transmitted infections, including crab lice. A marked decline in the number of female cases in one department in the UK led to the suggestion that waxing of pubic hair, particularly the fashion known as the ‘Brazilian’, was an important factor responsible for the decline [81]!
Because many patients with crab louse infection who attend GUM clinics are found to be suffering from other sexually transmitted infections [82, 83], screening for these is indicated.
Pathophysiology
The crab louse is quite distinctive in appearance (Figure 34.17) and habits from Pediculus. Its body is squat, and the second and third pairs of legs carry heavy, pincer-like claws. When static, the crab louse uses these huge claws to grip adjacent hairs close to the skin surface (Figure 34.18). Its eggs are squat and bulging compare to Pediculus. They are light brown in colour and, like those of the head louse, are cemented to the hair of the host (Figure 34.19). It is adapted to living in hair of a particular density. Scalp hair, except at the scalp margins, is too dense, but the crab louse will colonize axillary hair, eyebrows, eyelashes, beard hair and hair on the trunk and limbs, in addition to pubic hair. It is mainly sedentary, but becomes active at night when the host is sleeping [84]. It moves by transferring its grip from one hair to another. The crab louse has difficulty moving when taken from its host, whereas head and clothing lice are quite mobile off the host.

Figure 34.17 Pthirus pubis, the crab louse.
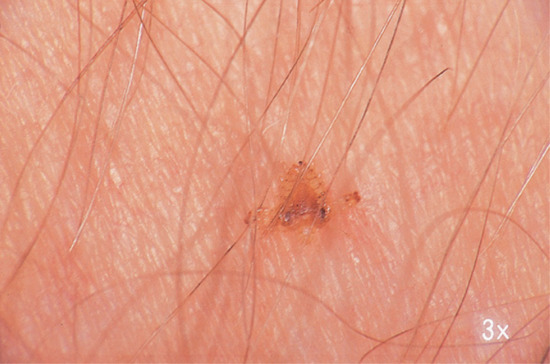
Figure 34.18 Crab louse clinging to hairs on the abdomen.
Figure 34.19 Crab louse eggs attached to abdominal hair.
Clinical features
Itching, mainly in the evening and at night, is the principal symptom. Close inspection of affected areas will reveal lice grasping hairs close to the skin surface, and louse eggs attached to the hair shafts. Louse faeces are often visible as rust-coloured speckles on the skin and hair, and the underclothes may be spotted with altered blood.
When crab lice are discovered on the pubic area, other hairy areas of the body should be examined, as these lice may colonize eyebrows, eyelashes (Figure 34.20), beard, axillae, areolar hair and the scalp margins [85, 86]. In heavy infections in men, the hair on the trunk and limbs may be extensively colonized. A case has been reported in which the presence of an enormous population of lice was attributed to inappropriate use of topical steroids [84].
Figure 34.20 Crab louse eggs on the eyelashes.
Blue-grey macules (maculae caeruleae) are occasionally seen on the skin [87], but their precise pathogenesis is unknown. Bullous lesions attributed to crab lice have been reported [88, 89].
In children, crab lice may colonize the eyelashes and scalp [90]. Infection in children is usually acquired by close physical contact with infected parents. As an isolated finding, it is not indicative of sexual abuse, although this may occasionally occur [91].
Management [92, 93]
Pubic lice are treated with the same insecticidal creams or lotions as pediculosis capitis, with a second application after 7–10 days as the products have a poor ovicidal activity. Resistance to pyrethrins has been shown [94]. All hairy areas of the body should be treated at the same time. Shaving is sometimes necessary when nits are plentiful. Infestations of the eyelashes should be treated with permethrin 5% cream (washed off after 10 min) or only with petrolatum (applied twice a day for 8–10 days), followed by mechanical removal of the nits [95, 96, 97]. Oral ivermectin has been used by some authors [98].
As in other louse infestations, all sexual contacts should be examined and treated when necessary. Bedding and clothes should be washed in hot water (50°C). Prepubertal children presenting with pubic louse infestations should be evaluated with regard to possible child abuse [91]. Treatment failure is usually a result of an untreated hairy area or reinfestation from an untreated sexual contact. In addition, patients should also be screened for associated sexually transmitted disease.
Resources
Further information
www.cdc.gov/parasites/lice/pubic/
http://www.cdc.gov/mmwr/pdf/rr/rr5912.pdf
Department of Health, UK: http://cks.nice.org.uk/pubic-lice (All last accessed October 2014.)
Bugs (Hemiptera)
Definition
Hemiptera comes from the Greek hemi (half) and pteron (wing) because their wings are hardened near the base and membranous near the ends.
Classification
The suborder Heteroptera, the true bugs, has two families which are haematophagous and ectoparasitic for humans and three families with health impact in case of contact or bite:
- Cimicidae family (including bedbugs) with four major genera: Cimex, Oeciacus, Leptocimex and Haematosiphon.
- Reduviidae family (including kissing and assassin bugs) with three major genera: Triatoma, Rhodnius and Panstrongylus.
- Anthocoridae family commonly called minute pirate bugs or flower bugs.
- Pentatomidae family commonly called stink bugs.
- Belostomatidae family commonly called giant water bugs.
Hematophagous bugs (bedbugs and kissing bugs) are a minority compared to the high number of Hemiptera but they have a considerable impact on human health. A global resurgence of bedbugs has been observed since the 1990s, with clinical and pest control implications. The kissing bugs are vectors of Trypanosoma cruzi, the parasite of Chagas disease.
Family Cimicidae, including bedbugs
Classification [1, 2, 3]
This family includes 24 genera and 110 known species [4]. Cimicidae is a cosmopolitan family. Species are all ectoparasites of homeothermic vertebrates. All the stages of the life cycle are haematophagous. The host is often very specific and includes mainly birds and bats. It has been suggested that Cimicidae became adapted to feeding on humans when cave dwellers took up residence alongside bats. Bedbugs (Cimex spp.) is a small part of the family of Cimicidae, and of the genus Cimex.
Genus Cimex
Cimex lectularius (the common bedbug) is the species of bedbug that is cosmopolitan. This one will be discussed here.
Cimex hemipterus (the tropical bedbug) is less tolerant of low temperatures than C. lectularius. This bug is confined to tropical and subtropical regions, including India, Burma, Malaysia, South China and Central Africa but infestation is possible in occidental countries because of world trade.
Cimex pipistrelli (the batbug), originating in a bat roost in a house, was responsible for itchy skin lesions in one of the house occupants [5].
Genus Leptocimex
Leptocimex boueti has a limited distribution in West Africa, where it parasitizes humans and bats.
Genus Oeciacus
Several species are usually found on birds and in their nests, for example Oeciacus hirundinis, the martin bug, and O. vicarius, the swallow bug. They may invade houses from nests under the eaves, and will bite humans readily, but it is unlikely that they can complete their life cycles on human blood, or take up residence in houses as bedbugs do.
Genus Haematosiphon
Haematosiphon inodorus, the only species in this genus, is also known as the Mexican chicken bug. As the name suggests, its major host is the chicken, but it can be a serious pest in human domiciles if these are close to chicken roosts.
Introduction and general description
Bedbugs are resurging in developed countries [6], possibly due to international travel and changes in pest control practices [7]. Diagnosis of bedbug infestation relies on clinical manifestations of bites and direct observation of the arthropod, which is rarely recognized by those who are bitten [8]. Evidence is lacking on the bedbug's capacity to transmit disease, management of eradication and the economic impact of infestations.
Epidemiology
Australian and European observational studies have recently shown increases in pest manager interventions. There was also a growing number of bedbugs identified in Australian laboratories between 2001 and 2004 [9, 10, 11]. The reasons for this resurgence are not clearly understood. Different contributing factors may have played a role: first, the increased number of domestic and international travel [12]. Second, the emergence of pyrethroid insecticides resistance [7, 13], due to multiple mutations, may also have participated to the dissemination [14]. Finally, using new techniques like bait to control other insects such as cockroaches may have permitted the growth of bedbug populations [15].
Bedbugs may be able to transmit diseases. However, their vectorial role has not been clearly demonstrated [6]. The pathogens which have been detected in or on bedbugs are hepatitis C virus [16], Aspergillus spp., meticillin-resistant Staphylococcus aureus [17], hepatitis B virus and Trypanosoma cruzi [6].
Pathophysiology [3]
Bedbugs are 4–5 mm in length, with dorsoventrally flattened, oval bodies, the forewings reduced to scale-like pads, and the hindwings absent. The mouthparts are modified into a proboscis adapted for piercing and sucking.
Female bedbugs deposit their pearly white, flask-shaped eggs in the crevices of floors and walls, in furniture, bedframes and mattresses. Each female lays about 300 eggs in her lifetime. The eggs hatch after about 10 days: the nymphal stage lasts approximately 6 weeks, during which time the bug moults five times (Figure 34.21).
Figure 34.21 The life cycle of the bedbug. (From Bernadeschi et al. 2013 [21]. Source and copyright holder: BMJ Publishing Group Ltd.)
Bedbugs normally feed at night, usually about an hour before dawn, but they may feed during the day if circumstances are favourable [18]. They usually fear light and avoid glossy surfaces. During the day, they hide in dark places such as crevices in furniture and mattresses or peeling wallpaper. Searching for a food source is erratic, and is probably at random at distances greater than a few centimetres, but in the final approach to the host, both temperature and odour play a part in guiding the bug. Feeding time is relatively short (3–12 min). During feeding, the bedbug injects saliva containing an anticoagulant and anaesthetic. Recently, a study demonstrates that allergens causing itching and skin lesions are most likely contained in the saliva of bedbugs. Interestingly, bedbugs without salivary glands attempted to feed but were unable to do so, indicating that saliva is necessary for the feeding process. Furthermore, bedbug saliva was potent enough to cause pruritus and lesion development in a human volunteer by topical application alone, without breaking the skin [19].
In the absence of a suitable food supply, however, adult bedbugs can survive starvation, in ideal circumstances, for a year or more.
In the absence of its usual host, C. lectularius will attack other animals, and Cimicidae normally parasitic on other hosts are similarly prepared to attack humans, invading houses from birds’ nests or chicken runs.
The transmission of bedbugs may be passive, usually in clothing, luggage and furniture. Active dissemination also occur using electric wiring or ventilation ducts. Recently, the number of bedbugs increased drastically in specific places like low budget hotels, bed and breakfasts, night trains, cruise ships and even nursing homes [6, 20, 21]. A recent survey conducted in the UK underlined that most people are not able to recognize bedbugs (Figure 34.22) [8].

Figure 34.22 Bedbug adult (5–7 mm): Cimex lectularius.
Clinical features
Presentation
The initial bite is usually not felt because of the anaesthetic contained in the saliva of bedbugs. Allergic reactions to saliva may sometimes occur late. Although most patients do not have any symptoms after the first bites, reactions may occur 10 days later [22]. The most common reactions to bedbugs are pruritic maculopapular lesions centred by the bite, visible as a haemorrhagic punctum [7]. The distribution of the lesions may be evocative if the lesions are grouped on a line or a curve (‘breakfast-lunch-dinner alignment’, Figure 34.23a). However, this pattern of distribution is not specific for bedbugs and can be found in other arthropod infestations. Most of the lesions are distributed on uncovered areas of the body. Nodules (Figure 34.23b), bullous lesions (Figure 34.23c) or urticaria (Figure 34.23d), are less commonly found [7, 23]. Few cases of systemic symptoms or anaphylaxis have been reported [7]. Bedbug infestation may also present as isolated pruritus. Patient's questioning usually highlights similar symptoms in people who share the same bed or travel. Furthermore, lesions classically disappear when changing sleeping place. Bedbug infestation is confirmed by the identification of the insect, not requiring the assistance of an entomologist in most cases.
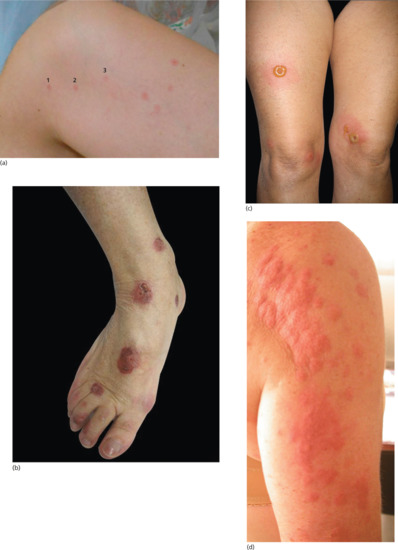
Figure 34.23 Clinical manifestations of bedbug bites: (a) three or four skin lesions are often seen in a ‘breakfast (1), lunch (2), dinner (3)’ distribution or (b) ‘wheel’ distribution. (c) Atypical bullous lesions and (d) urticarial. (From Bernadeschi et al. 2013 [21].)
The bites of other Cimicidae are essentially similar, but their distribution depends on the method of exposure. Haematosiphoniasis is the name given to the cutaneous lesions caused by the bites of Haematosiphon inodorus (the Mexican chicken bug). Polymorphic lesions, consisting of weals, papules, vesicles, pustules and scabs, occur predominantly on exposed parts of the body [24].
Differential diagnosis
Various differential diagnoses can be made. The first is other arthropod bites [25], mainly scabies. However, the distribution of the lesions is not the same, as covered areas are commonly involved in scabies. Furthermore, a central punctum corresponding to the bite site is not visible as in bedbug infestation. Flea infestation can be confounding, because of the same pattern of distribution of lesions, located on a line or curve.
Other dermatological differential diagnoses may require skin biopsy. They include Sweet syndrome, erythema multiforme or bullous dermatitis.
Complications and co-morbidities
Secondary bacterial infections (Staphylococcus or Streptococcus) of the cutaneous lesions are the main complications [26]. The psychological consequences may be important, leading sometimes to parasitophobia, but these have not been evaluated.
Management
Management of bedbug bites
Management of bedbug bites is based on expert opinion. Topical steroids are used to relieve symptoms [27]. Systemic antihistamines may be helpful in cases of severe pruritus [27]. Secondary bacterial infections may require topical or systemic antibiotics, depending on the severity [28].
Management of bedbug infestation
Patient education
Education of patients by professionals is fundamental. Detection and identification of the arthropod (Figure 34.24a) or its faecal traces (Figure 34.24b) in suspected areas should be explained to patients [29].

Figure 34.24 To educate patients to the ‘search and destroy’ strategy, general practitioners should show them pictures of (a) bedbugs and their typical hideouts (e.g. a fabric couch) and (b) bedbug faecal traces on the bedframe.
Non-chemical intervention
Bedbugs may be able to survive without feeding for 1 year, consequently, keeping infested places vacant cannot be recommended [6]. All life stages of bedbugs may be killed by washing at 60°C, dry cleaning or tumble drying at 40°C [30]. Even if there is no scientific evidence of their efficacy, pest managers recommend the disposal of infested furniture, the physical removal of bedbugs and vacuuming [31]. Silicates may be helpful in the management of bedbugs, but need further investigation [32].
Chemical treatment
Bedbug infestation should be treated by insecticides, mainly pyrethroids [33], carbamates and insect growth regulators. Organophosphates are not used anymore in Europe [34]. Bedbug resistance to all insecticides has been demonstrated [13, 35]. In this situation, a combination of available products may be preferred. A second treatment is often necessary because of the low efficiency of insecticides against eggs. The delay between the two treatments is not clearly known, ranging from 4 to 20 days, depending on the temperature of the infested site.
Prevention of infestation [31]
The best way to prevent the spread of bedbugs is by early detection. However, the effectiveness of prevention procedures has not been evaluated. It is classically recommended to wash mattress covers and bed linen at 60°C. Buying secondhand mattresses and furniture must be strictly forbidden. The use of bedbugs traps may also be an interesting way to prevent infestation [36].
Resources
Further information
University of Kentucky (www.ca.uky.edu/entomology/entfacts/entfactpdf/ef636.pdf)—comprehensive lesson on bedbugs.
Centers for Disease Control and Prevention (www.cdc.gov/nceh/ehs/topics/bedbugs.htm)—link to various articles on bedbugs.
NHS Choices (http://nhs.uk/conditions/bites-insect/pages/introduction.aspx)—clinical knowledge summary about insect bites. (All last accessed October 2014.)
Patient resources
Up to Date (www.uptodate.com/contents/bedbugs?source=search_result&search=bedbugs&selectedTitle=1˜10)—provides accurate general knowledge about bedbugs.
Bed-Bugs.co.uk (http://bed-bugs.co.uk/)—provides an interesting picture gallery of bedbugs and their bites, together with practical tips for eradication.
Pest Control UK (www.pestcontrol-uk.org/pests/bed-bugs)—DIY control of bedbugs.
Pest Control Australia (http://medent.usyd.edu.au/bedbug/) (All last accessed October 2014.)
Family Reduviidae (kissing bugs, assassin bugs and cone-nosed bugs)
Epidemiology
The majority of species of Reduviidae are predators on other insects, and are commonly called assassin bugs for this reason, but some attack humans and other animals. Most species are encountered in North, Central and South America, but some occur in Africa, the Middle and Far East and in Australia. The subfamily Triatominae is the most important medically and includes the three major genuses Triatoma, Rhodnius and Panstrongylus. These genuses feed exclusively by sucking the blood of vertebrate animals. Adult Triatominae are large insects, commonly measuring 20–28 mm in length. They have an elongated head with a prominent proboscis and long four-jointed antennae.
The Triatominae are largely confined to the western hemisphere, with the majority of species being distributed in North, Central and South America. In the USA, Triatoma sanguisuga has the widest distribution, extending from the southeastern and mid-Atlantic states westwards, including Texas. Triatomines feed on a wide range of hosts, and domestic species feed on humans and domestic animals. They are of medical importance as vectors of Trypanosoma cruzi in Chagas disease [1, 2]. This disease has been traditionally restricted to Latin America, but cases have been reported in the USA. A recent study in Arizona showed that 41.5% of collected kissing bugs (n = 164) were infected with T. cruzi, indicating that the risk for infection in this region may be higher than previously thought [3].
Pathophysiology
In nature, triatomine bugs form colonies in the habitat of their host, for example a small mammal's nest or animal lair. In the south-western USA, infestations are often found in the nests of wood rats. Some species, however, have become totally domesticated, and live and breed in human dwellings, laying their eggs in cracks and crevices in the floors and walls. The young hatch as nymphs, which are miniature versions of the adults. Nymphs and adults hide in crevices during the day and emerge at night to feed.
Clinical features [4, 5]
The bites of the predatory species of reduviid bugs (assassin bugs) are purely defensive, and are usually extremely painful [6, 7]. Triatome reduviids are known as kissing bugs because of their tendency to bite the face, especially around the lips. The bites of the blood-sucking Triatominae, however, are painless – this is essential to the parasite if it is to feed undisturbed. In an individual not previously exposed to the bites, there may be little reaction, but anaphylactic reactions are also a major concern [8]. With repeated exposure, hypersensitivity develops, and reactions ranging from pruritic papules to haemorrhagic nodules and bullae may occur. A door-to-door survey showed that living in triatomine-exposed areas may be associated with a high prevalence of self-reported allergies [9].
Family Anthocoridae, commonly called minute pirate bugs or flower bugs
The Anthocoridae are related to the Cimicidae. Bugs of this family are mostly predacious on other insects, but are known to bite humans occasionally. Lyctocoris campestris is a cosmopolitan species closely associated with humans, found for example in haystacks and granaries [1]. Anthocoris kingi and Anthocoris nemorum will also bite humans [2]. Another anthocorid bug, Dufouriellus ater, attacked many workers in a clothing factory in north-east England [3]. Human-biting potential of the predatory flower bug Orius majusculus has also been reported [4].
Family Pentatomidae, commonly called stink bugs
These bugs have glands in the thorax that emit a foul-smelling compound. This defence helps them to repel potential predators. Some insects of the family Pentatomidae, commonly known as marias-fedidas (‘stink Mary’) can cause serious irritation to human skin, very similar to that produced by vesicant beetles. Areas of erythema and vesiculation are accompanied by burning and pruritus [1]. There is no information on the pharmacological properties of substances secreted by the Pentatomidae but treatment is similar to that used after contact with Paederus or cantharidin.
Palomena prasina (the green shield bug), a member of this family, has been reported as the cause of perioral blistering in a small child [2].
Family Belostomatidae (giant water bugs)
The giant water bugs are rare cosmopolitan insects. They are typically encountered in freshwaters streams and ponds. Alternate names include ‘toe biters’ because they can deliver an unpleasant sting and ‘electric light bug’ because they are attracted to lights. Giant water bugs may produce very painful lesions on humans and may carry infections [1, 2]. Belostomatidae and another family (the Naucoridae) are carnivorous insects and are suspected to play a role in the transmission of Buruli ulcer and in the ecological expansion of the Mycobacterium ulcerans niche. The majority of cases of bites and suspected transmission of infection are localized in Africa occurring mainly in poor local communities. Other cases have been reported in Asia, Australia and South America [3, 4, 5, 6].
Thrips (Thysanoptera)
Definition
Thrips (‘thunder flies’) are tiny winged insects, 1–2 mm in length, and usually yellowish brown or black in colour. The name thrips is derived from the Greek word meaning ‘wood louse’. The order Thysanoptera (‘fringe wing’) comprises about 5000 species with a worldwide distribution. The majority feed on plant juices and some are important agricultural pests [1].
Pathophysiology
Some species are predatory on other arthropods. A few species appear able to suck blood, and there are a number of reports that thrips can be responsible for skin lesions [2, 3, 4, 5, 6]. Most thrips, however, are unable to penetrate the human epidermis, and probably cause itching and prickling sensations only by their movement on the skin surface and their efforts to obtain water from perspiration.
Clinical features
Thrip bites, which occur on exposed skin, produce tiny puncta and small pink macules or papules [3]. Large numbers of American soldiers in Hawaii developed hypoanaesthetic papular lesions surrounded by blanched halos, which, it was suggested, were caused by Cuban laurel thrips [7]. Thrips infestation may sometimes be confused with delusional disorders because houseplants or ornamental plants can be infested by thrips. While individuals often present to dermatologists with bizarre stories and plastic bags of ‘bugs’, it is important to take a thorough history and perform careful examination of the specimens, the house or its environment, so as not to lead to a wrong conclusion of ‘delusory syndrome’ (Ekbom syndrome) [8, 9]. Thrips have also been mistaken for headlice [10].
Beetles (Coleoptera)
Definition
Beetles are insects whose forewings are modified to form hard wing cases for the membranous or reduced hindwings. There are over 370 000 known species, but it is likely that many more await discovery. They are mainly terrestrial and the majority feed on decaying animal or vegetable matter, but some are predaceous on other insects.
Classification
Vesicating and allergenic species are the main species of interest to dermatologists.
Vesicating species [1, 2, 3]
Family Meloidae (oil beetles; blister beetles)
Most of the beetles in this group only cause problems when crushed on the skin, but some may emit their vesicating fluid without being crushed. The family is large and widely distributed. The beetles of this family feed on leaves of crops, such as tomatoes and potatoes, making interaction with humans likely. Many species contain the irritant cantharidin, which commonly is called ‘Spanish fly’. Cantharidin is a vesicant that comes from more than 1500 species of ‘blister’ beetles. It is absorbed into the lipid component of keratinocyte membranes, where it activates neutral serine proteases, leading to the degeneration of desmosomes and resulting in vesicles and bullae [4]. Cantharidin has an undeserved reputation as an aphrodisiac, which is unfortunate for a chemical capable of producing severe toxicity. It has been used in blistering plasters and hair restorers, and in the treatment of warts and molluscum contagiosum.
Lytta vesicatoria (Figure 34.25) is perhaps the best known of the blister beetles. It is a large bright metallic green beetle, which lives mainly in the Mediterranean region, mainly on ash trees, but is sporadically found further north, occasionally as far as England.

Figure 34.25 Lytta vesicatoria on an ash tree, the best known of the blister beetles.
(Courtesy of Dr M. Cornet, Nice, France.)
Other vesicating species include Epicauta spp. (USA, Mexico, India, Sudan, Senegal) [5] and Mylabris spp. (Nigeria, India) [6, 7].
Family Staphylinidae (rove beetles) [8, 9, 10]
The genus Paederus, found worldwide, includes many species containing a vesicant, pederin, which is chemically distinct from cantharidin. Pederin is released when the beetles are crushed, provoking an acute irritant contact dermatitis. Lesions may be plaque-like, linear (when a beetle has been brushed off the skin, leaving a streak of pederin on the skin surface) or ‘kissing’ (when a beetle has been crushed between two flexural surfaces). A major outbreak of vesicular dermatitis on Okinawa in 1966 was traced to contact with the beetle Paederus fuscipes [11], and a number of other reports have documented Paederus dermatitis from several parts of the world [12, 13, 14, 15, 16, 17, 18, 19], including outbreaks which occurred in a military unit training in the Arizona desert during heavy rain and flooding [20], and in a military base in Iraq [21]. A plague of whiplash rove beetles (Paederus australis) forced evacuation of an aboriginal community in the Northern Territory of Australia [22]. Paederus sabaeus has been responsible for several outbreaks of dermatitis in Africa at the end of the rainy season [23, 24, 25, 26].
You et al. [27] reported a case of bullous contact dermatitis following the use of crushed Paederus beetles for the treatment of vitiligo.
Histopathological changes of Paederus dermatitis include intraepidermal and subepidermal blistering, epidermal necrosis and acantholysis [28]. Mitotic figures and apoptotic changes such as chromatin condensation and DNA fragmentation have also been identified in the basal and suprabasal layers of the epidermis [19].
It has been proposed that the biblical third, fourth and sixth plagues of Egypt might have been related to rove beetles and the bullous lesions they cause [29].
Family Oedemeridae [2]
Oxycopis vittata has been reported as causing a blistering dermatitis in Puerto Rico [30]. Sessinia species (coconut beetles) have caused blistering in the Gilbert Islands [7].
Thelyphassa lineata produced a bullous dermatosis in a large number of New Zealand army personnel [31], and there is a report of blister beetle dermatosis in Hawaii caused by Thelyphassa apicata [32].
Family Tenebrionidae (darkling beetles)
Many species inhabit wood, flour and grain stores. Tribolium castaneum (the ‘rust-red flour beetle’) has caused a pruritic eruption in workers handling infested jute packing bags [33]. The secretion of Tribolium species is mainly composed of quinones.
Species of Blaps can eject defensive secretions, which are irritant and cause blistering.
Clinical features [1, 2, 3]
Usually, lesions are produced only when the beetle is crushed on the skin. A weal forms rapidly and is followed by a blister after 12–24 h. The blisters are sometimes linear ‘whiplash dermatitis’. A characteristic feature is the development of kissing lesions, where a blister comes into contact with another area. Blisters induced in a small child by Mylabris bifasciata were associated with severe systemic manifestations of cantharidin poisoning [6]. In Paederus dermatitis, vesicles generally appear toward the centre of the plaque and frequently become pustular [18]. This is in contrast to cantharidin dermatitis, which is characterized by non-inflammatory vesicles and bullae [17]. Paederus-induced keratitis has also been reported [34]. The clinical differential diagnosis includes acute allergic or irritant contact dermatitis, thermal burns, chemical burns, herpes zoster, herpes simplex and bullous impetigo [17, 21]. An important differential diagnosis to consider is phytophotodermatitis as there are many similarities between the two conditions including linear asymmetrical erythema, blister formation and depigmentation [35].
Management
Experts agree that affected patients should be managed as irritant contact dermatitis, with removal of the toxin by immediate washing with soap and water. Primary prevention by increasing public awareness during outbreaks, decreasing the use of artificial lights at night and using mosquito nets is advocated by several authors [18, 21].
Allergenic species
Family Dermestidae [36]
The beetles in this cosmopolitan family feed on hides, woollen materials and stored food. The adult beetles are not known to be directly injurious to humans, but their larvae are covered with hairs, which may cause skin lesions.
Clinical features
The skin lesions are not distinctive. Dermatitis, urticaria and papular urticaria may occur. Papular urticaria in a child, caused by the larvae of Dermestes maculatus DeGeer, has been reported, but it was uncertain whether the reaction to the hairs was irritant or allergic [37]. Dermestes peruvianus was responsible for dermatitis, vasculitis, cervical lymphadenopathy and pulmonary nodular interstitial infiltrates in a man whose bed was colonized by the beetles [38].
The irritating hairs from the larvae of carpet beetles (Anthrenus spp.) may also cause skin lesions [39, 40, 41]. There is also a report of the damaging effect of Anthrenus larvae on paraffin-embedded tissue specimens, especially the sectioned surface of hyperkeratotic lesions [42].
Cockroaches (Dictyoptera)
Definition
Cockroaches are members of the order Dictyoptera, suborder Blattaria. They belong to one of the primitive orders of insects, being allied to crickets, grasshoppers, preying mantids and stick insects. Cockroaches were originally adapted to hot climates, but a number of species have established themselves in cool climates by living inside warm human habitations. They are active nocturnally, and are attracted to any organic material that may serve as food. This theoretically makes them potential mechanical vectors (by transportation, also called phoresy) of pathogenic organisms [1, 2, 3]. The main pest species are Periplaneta americana, P. australasiae, Blatta orientalis and Blatella germanica.
Clinical features [4, 5]
Contact urticaria and dermatitis have been described in laboratory workers and others handling cockroaches constantly [6, 7, 8], and urticated papules developed in a medical records clerk exposed to copious insect debris containing fragments of B. germanica when clearing old case notes from a derelict hut [9]. Chronic urticaria in a child has been attributed to cockroach hypersensitivity [10].
Locusts (Orthoptera)
Definition
Sensitivity reactions, manifest as asthma and allergic rhinitis, are a recognized occupational hazard in those working with laboratory colonies of locusts [1, 2, 3]. The principal allergen appears to derive from the peritrophic membrane, which is present in the gut and surrounds faeces [2].
Clinical features
Contact urticaria to locusts has been reported by Monk [4] in a laboratory research worker who handled a large number of locusts. The patient produced a positive reaction to locust antigen on prick testing and a wealing reaction at the site of contact with a live locust. Similarly, worsening of asthma, and urticaria in an atopic research laboratory worker, on exposure to grasshoppers, has been described [5].
Butterflies and moths (Lepidoptera)
Definition and nomenclature
Many members of this large order are of importance to the dermatologist because of the irritant properties of the hairs or spines of the caterpillars and sometimes of the adults. Skin lesions in the majority of cases are produced by a combination of mechanical and pharmacological effects [1, 2]. The offending caterpillars are distributed through many different families [1, 3].
Classification
The Lepidoptera order is one of the largest orders of insects. Caterpillars are the worm-like larval forms of Lepidoptera. The Lepidoptera contain probably between 125 000 and 150 000 different species of caterpillars, moths and butterflies [4].
Epidemiology
The true number of cases of caterpillar and moth reactions remains unknown, because few cases are reported in the literature, and probably only the ones with severe reaction.
Epidemics of caterpillar dermatitis are frequent, depending on the seasonal abundance of the different species. For example, there are periodic outbreaks of gypsy moth caterpillars (Lymantria dispar) [5, 6], Douglas fir tussock moth caterpillar (Orgyia pseudotsugata) [7], puss caterpillars (Megalopyge opercularis), buck moth caterpillars (Hemileuca maia) [8], several Euproctis species [9] and several species of processionary caterpillars (Thaumetopoea) (Figure 34.26). Due to climate changes in recent years and a tendancy towards warmer summers, an ongoing broadening from the southwest to the northeast of the UK is probable [10, 11].

Figure 34.26 Thaumetopoea pityocampa.
(Courtesy of Dr M. Dutheil, Nice, France.)
Some species have the capacity to be disseminated widely by wind dispersal. This phenomenon is also called ‘ballooning’ [12].
Winds can also disperse caterpillar setae, which may cause dermatitis or ophthalmia nodosa [13, 14]. A large outbreak of dermatitis caused by setae of the Asian mulberry tussock moth (E. flava) has been described in China, using airborne dissemination [9].
Dermatitis caused by adult moths is less frequent. Caripito itch was described by Dinehart et al. in crewmen who docked their ship in Caripito, Venezuela. The dermatitis was secondary to the setae from female moths (Hylesia) [15]. Outbreaks of Hylesia metabus are sometimes notified in French Guiana [16].
Caterpillar dermatitis is quite frequent in children, but the reasons for this elevated frequency remain unknown, and may be due to parental concern and increased reporting [12, 17].
Pathophysiology
Lepidopetera undergo four life stages: egg, caterpillar, pupa or chrysalis, and adult. The term ‘lepidopterism’ is applied to the ill effects on humans of a structure or product of some part of a moth or butterfly at any stage of its life history. Some authors apply the term ‘erucism’ to injurious effects from caterpillars, and ‘lepidopterism’ to ill effects from adults. In the majority of cases, damage to human skin and mucosae occurs as a result of epithelial penetration by the ‘hairs’ (setae) of caterpillars. In addition to a foreign-body reaction, there is often an effect from venom. Setae develop from trichogen cells of the epidermis. They are hollow, and may function as sensory receptors or communicate with a poison gland cell and contain venom. They commonly have barbs, which hold them in place when they have penetrated the skin. In some families of moths, the caterpillars have clumps of much smaller setae known as ‘dart hairs’ or ‘spicules’, which are pointed at both ends and carry fine barbs. The point of attachment to the caterpillar is very narrow and easily fractured; hence, contact with the caterpillar may release huge numbers of these tiny darts. Such dart hairs are present in a number of species, including the brown tail moth (Euproctis crysorrhoea) and the pine processionary caterpillar (Thaumetopoea pityocampa) (see Figure 34.26). Setae are also woven into cocoons, and into the webs of the silk-spinning caterpillars.
Spines are an extension of the cuticle of the caterpillar and contain venom. The spines either have a terminal plug of inspissated material at their open ends, which is released by pressure, or a weak point at which the spine fractures to allow the venom to escape. Poisonous spines occur particularly on the caterpillars of the moth families Cochlididae (Eucleidae; Limacodidae), Saturniidae and Megalopygidae.
The venoms present in the setae and spines of caterpillars of a number of families of Lepidoptera have been studied, but not fully elucidated. Some contain histamine, histamine liberators, serotonin and proteases. A protein, the thaumetopoein, has been isolated from pine processionary caterpillar hairs [18, 19]. This has a direct effect on mast cells, leading to degranulation, and explains the urticating properties of these caterpillars. However, IgE-mediated hypersensitivity also appears to be responsible for some reactions to Thaumetopoea [20, 21, 22]. Moneo et al. [23] have demonstrated a 15 kDa IgE-binding protein in a larval extract. Immediate and delayed-type reactions to Euproctis pseudoconspersa caterpillar venom extracts have also been demonstrated [24].
In some species – for example, moths of the genus Hylesia (family Saturniidae) – irritating setae are carried by the adults. This genus is notorious for causing outbreaks of ‘butterfly itch’, ‘moth dermatitis’ or ‘Caripito itch’ [15] in tropical South America.
Pathology
The pathology of caterpillar dermatitis is usually non-specific [25, 26]. Histology may be more specific when embedded spines are found [15]. Granulomas have been demonstrated in cases of ophthalmia nodosa [14], dendrolimiasis and pararamose.
Clinical features [1, 2, 27]
Presentation
Clinical features induced by caterpillars and moths are wide, ranging from localized stinging reactions, papular urticaria, urticarial weals, haemorrhagic diathesis, ophthalmia nodosa, dendrolimiasis, pararamose and oral exposure [27].
Localized stinging reactions consist in immediate mild to severe pain that lasts hours to days. Systemic symptoms are sometimes associated. Contact with Megalopyge caterpillars [28] produces immediate intense burning local pain accompanied by a spreading erythema around the puncture sites. The affected area becomes oedematous, and there is often lymphangitis and regional lymphadenopathy. The local changes may be accompanied by pyrexia, headache, nausea and vomiting, particularly in children [29].
In papular urticaria, there are mild to moderate localized pruritic papules or eczematous lesions, predominantly in exposed area. Lesions are caused by the setae from hairy or bristly caterpillars or from adult moths.
Urticarial weals and angio-oedema are seen with three species of processionary caterpillars (all belong to the genus Thaumetopoea). These lesions are secondary to type I hypersensitivity reactions [22, 30, 31, 32]. Systemic symptoms have sometimes been reported [33, 34, 35].
The term ‘lonomism’ refers to a severe bleeding diathesis with intracranial haemorrhage, secondary to caterpillars found in Brazil and Venezuela (L. obliqua and L. achelous) [32].
Dendrolimiasis combines dermatitis and rheumatological involvement (arthralgia and arthritis) and is caused by contact with the Masson pine caterpillar (genus Dendrolimus) found in China [36]. Pararamose is quite similar, with skin eruption and arthritis, caused by contact with the Brazilian moth Premolis semirufa [10, 37].
In the eye, caterpillar setae may cause a variety of changes ranging from conjunctivitis to ophthalmia nodosa [13, 14, 38] and even panophthalmitis.
Oral exposure is rare and the most common sites of exposure are the tongue and lips. Most cases occur in children [27].
Differential diagnosis
Differential diagnosis may be broad as cutaneous lesions and histology are not specific. Questioning of the patient should elicit a history of caterpillar exposure. This is key to the diagnosis.
Management [27]
Management of lepidopterism is mainly based on expert opinion and is largely symptomatic. Immediate washing with soap and water is classically recommended. Topical steroids and oral antihistamines should be used for mild reaction to control pruritus. Embedded setae should be removed, sometimes with the help of adhesive tape. Systemic steroids may be necessary in severe reactions. Opioid analgesia may be required in puss caterpillar stings.
Surgical removal of granuloma formation may be necessary in ophthalmia nodosa.
Specific antivenom against potentially fatal Lonomia genus envenomation is available [39].
CLASS ARACHNIDA
Arachnida are readily distinguished from insects, as the adults have no wings or antennae and possess four pairs of legs. Unlike insects, where the body is divided into three segments (head, thorax and abdomen), arachnids have only two, the cephalothorax, from which the legs arise, and the abdomen.
The Arachnida are classified into seven orders, only three of which are of medical importance as follows.
- Araneae (spiders).
- Scorpiones (scorpions).
- Acari (ticks and mites).
Spiders (Araneae)
Introduction and general description
The appearance of many of the larger spiders inspires terror or disgust, but very few of the many thousands of species are dangerous to humans [1, 2]. That is why, when observing a large skin bite, many people suspect a spider bite with no scientific, clinical or entomological evidence. The myth that bites from various species cause necrotic ulceration may not be completely true [2]. In fact, bites by spiders from the genus Loxosceles can result in necrotic arachnidism and sometimes systemic illness, but many cases of necrotic arachnidism are only suspected and not proven [3]. Spiders are mostly shy and avoid contact with humans. Almost all are venomous and bite, but only a few have chelicerae strong enough to penetrate human skin, and in most cases the bites are trivial. A recent Australian study describing 750 cases of spider bite, involving 26 spider families, showed that most of the time there were only a few symptoms [4]. The European tarantula, Lycosa tarantula, which inspired the tarantella in Italy in the Middle Ages, inflicts a temporarily painful but harmless bite. Some lycosid spiders in South America, for example Lycosa antibucana, cause severe swelling and lymphangitis. In the USA, the term ‘tarantula’ is erroneously applied to the large ‘bird’ or ‘crab’ spiders of the family Theraphosidae, which attack only when vigorously provoked, and whose bite may be painful but not dangerous. Some colourful species kept as pets, for example Brachypelma smithi, are among several that have urticating hairs capable of causing prolonged pruritus. Many spiders whose bites are dangerous, and sometimes fatal, are small, inconspicuous and unimpressive.
Clinical features
The clinical syndrome following the bite of a spider is known as arachnidism [5, 6, 7, 8]. The form of arachnidism caused by species of the family Loxoscelidae is known as loxoscelism, and that by widow spiders (Latrodectus species) latrodectism [9].
Air transport of crates of fruit and other materials may introduce exotic species to countries in which they are unable to multiply but can survive long enough to attack humans.
Diagnosis [2]
The diagnosis of spider bite is based on a clear history of a spider biting, ideally with collection and correct identification of the spider responsible, sometimes requiring the assistance of an entomologist. In areas where the spiders are recognized, the general population may identify a few spiders, such as widow spiders. The identification of spider venom in human tissue is not possible.
Differential diagnosis
Differential diagnosis may be broad including pyoderma gangrenosum, herpes simplex and zoster, staphylococcal or streptococcal infection, lymphomatoid papulosis, chemical burn and squamous cell carcinoma.
Family Theridiidae
Genus Latrodectus (widow spiders)
Epidemiology
Spiders of this genus are widely distributed throughout the world. Latrodectus mactans, the black widow spider, occurs throughout subtropical and tropical regions. Other species have a similar, but more limited, range, although some extend to the temperate regions of Russia and Canada. It is the adult female spiders that produce the most damaging bites in humans, but bites by male spiders have been reported in Australia [10].
Pathophysiology
The female of L. mactans is glossy black, with a body length of 1.5 cm and a leg span of up to 5 cm. She normally spins her web in empty burrows or under stones, but may be found in dark corners of barns, garages, store rooms or outdoor lavatories. She bites humans only in self-defence. Latrodectus venom is considered to be one of the most potent toxins, exceeding that of snake venoms, but the dose injected is minute in relation to the body weight of a human victim. The toxins of all species of Latrodectus that have been studied appear to be closely related, and the symptoms from envenomation are similar.
Latrodectus hasselti, the red-back spider, is common in Australia [11, 12]. Latrodectus geometricus, the brown widow spider, bites reluctantly, but is occasionally troublesome to vineyard workers in South Africa.
Clinical features (latrodectism) [5, 6, 7, 13, 14, 15]
In the days of the outdoor lavatory, Latrodectus webs were often spun across the toilet seat, and this led to the frequent occurrence of bites on the buttocks and genitalia. The bite of Latrodectus species is fairly painless, but within a few minutes increasingly severe pain develops, usually at the site of the bite but also spreading to the adjacent region or even to the back, chest or abdomen. Cramp-like or colicky abdominal pain is particularly common. Puncta may be visible at the site of the bite, and there is local erythema and oedema. There is frequently profuse sweating, and neuromuscular involvement causes paraesthesiae, incoordination and paralysis. The pain begins to subside within 24 h, and other symptoms resolve within 2–3 days, although weakness and lethargy may persist for longer. Myocardial damage, occasionally fatal, has been reported, but only from some species of widow spiders such as Latrodectus tredecimguttatus (Figure 34.27) [16, 17].

Figure 34.27 Latrodectus tredecimguttatus the female, with the small male on her abdomen.
(Courtesy of Dr J.J. Peres, Peillon, France.)
Management [2]
There is no consensus concerning the management of latrodectism as evidence to support therapies is scarce, and there are no controlled trials. Treatments that have been used include general measures such as analgesics and benzodiazepines, and more specific measures such as antivenom [18, 19], calcium and magnesium. The effectiveness of widow spider antivenoms remains to be assessed [18, 19]. Furthermore, there are some concerns regarding the tolerance of these antivenoms with reported cases of anaphylaxis following administration [20, 21, 22].
Family Hexathelidae
Genera Atrax/Hadronyche (Australia and south Pacific) and Macrothele (Taiwan and parts of eastern Asia) – funnel web spiders
Perhaps the best known of these is the Sydney funnel web spider, Atrax robustus [11], a large aggressive spider that is nocturnal and predominantly insectivorous. It normally lives under rocks and logs, but the spread of the Sydney suburbs into its habitat provided similar hiding places under houses. Funnel web spiders are the most deadly spiders worldwide.
Clinical features [8, 23]
The bite of funnel web spiders is invariably painful. From the majority of bites, especially those of female spiders, no general symptoms follow, and recovery is uneventful. However, the large amount of venom from male spiders may cause severe systemic symptoms. Nausea and vomiting are early features, accompanied by abdominal pain, profuse sweating, piloerection, muscle fasciculation, lacrimation, excess salivation, dyspnoea and pulmonary oedema. Several fatalities were recorded prior to the development of an antivenom.
Management
The compression bandage-splinting method of first aid is effective in delaying onset of envenomation and may enhance local inactivation of venom. Severe reactions will require hospital admission and full supportive measures. Funnel web spider antivenom should be given urgently to any patient with severe envenomation, because it probably reduces the risk of death and the length of hospital stay [24].
Family Sicariidae (formerly Loxoscelidae)
Genus Loxosceles (‘fiddleback’ spider; ‘violin’ spider; ‘brown recluse’ spider)
Over 100 species of Loxosceles are found in a worldwide distribution, but the majority are in North and South America, where loxoscelism is a major health problem.
Identification of a Loxosceles spider is based on six eyes in a curved row on the upper part of the body (the prosoma). It has recently been suggested that under future climate change scenarios, the spider's distribution may expand northward, invading previously unaffected regions of the USA [25]. Several species are known to induce human skin necrosis: L. reclusa, L. laeta, L. deserta, L. arizonica and L. rufescens [3, 8]. The most notorious is L. reclusa, the brown recluse spider, which is tan to brown in colour, with a dark-brown, violin-shaped marking on the dorsum of the cephalothorax – hence the names ‘fiddleback’ and ‘violin’ spider. Loxosceles reclusa is active mainly at night and most bites occur when the spider is trapped against the person [3]. Its natural habitat is in dark areas beneath rocks and in holes and caves. It is also found in homes, in areas that are dark, dirty and undisturbed, such as attics, cupboards and garages.
Loxosceles laeta also occurs widely in South America. Loxosceles rufescens is widespread in southern Australia or in Mediterranean regions [26].
Clinical features (loxoscelism) [5, 6, 7, 8, 14, 27]
There are two distinct clinical forms of loxoscelism: necrotic cutaneous loxoscelism, and the much less frequent viscerocutaneous loxoscelism. The clinical manifestations depend upon the age and health of the victim, the amount of venom injected and the site of the bite – fatty areas such as the proximal thigh and the buttocks show more cutaneous reaction and extensive involvement of the entire subcutaneous layer.
In necrotic cutaneous loxoscelism, there is local damage to the skin and subcutaneous tissues, but systemic symptoms are mild. The bite of the spider is usually relatively painless. However, after an interval of minutes or hours, severe pain develops at the site, accompanied by erythema, oedema and a central bulla. In severe envenomation, a ‘target’ lesion is seen – central blue/purple discoloration surrounded by an ischaemic halo and an outer ring of erythema (the ‘red, white and blue’ sign). After 3 or 4 days, the central area becomes necrotic, and an eschar develops. The eschar is eventually shed, leaving an ulcer, which may take a considerable time to heal. The size of maximum necrosis appears to be predictive of time to complete healing [27]. Robb et al. [28] described a patient with a generalized vasculitic exanthem following a brown recluse spider bite. Acute generalized exanthematous pustulosis following Loxosceles reclusa envenomation has also been reported [29].
In viscerocutaneous or systemic loxoscelism, which is more common in children than adults, general symptoms of pyrexia, severe malaise, restlessness and headache are marked. Within 24 h of the onset of general symptoms, ecchymoses, jaundice, haematuria and haemoglobinuria indicate massive intravascular haemolysis, which may result in acute renal failure and death [30, 31, 32, 33, 34, 35].
Management [3, 8, 14]
Rest, application of Ice Compresses and Elevation (RICE therapy) help to reduce inflammation and pain. Other treatments have been tried for loxoscelism, including antivenom, corticosteroids, dapsone, antihistamines, antibiotics, analgesics, hyperbaric oxygen therapy, electric shock, curettage and surgical excision [36, 37, 38]. However, there is no consensus concerning the efficacy of any of these treatments because they are not supported by controlled randomized trials. In this setting, the efficacy of antivenom and the timing of its use has not been clearly demonstrated [39, 40]. In severe cutaneous loxoscelism with ulceration, negative pressure wound therapy may be helpful [41].
Family Lycosidae (wolf spiders)
There are a few reports of bites by members of the genus Lycosa [8, 42, 43]. They usually cause only local pain, swelling and erythema, without cutaneous necrosis or significant systemic symptoms.
Other venomous species [7, 8, 11, 44, 45, 46, 47]
Spiders of several other families may cause unpleasant bites. Long-legged sac spiders of the genus Chiracanthium (family Miturgidae), which are found in many parts of the world, may cause local pain, oedema and small areas of necrosis [8, 46]. Tegenaria agrestis (family Agelenidae), the hobo spider (previously known as the aggressive house spider), is a cause of necrotic arachnidism in the north-west USA [8, 45, 46]. Cases of bites from Hololena spiders, associated with headache and vomiting, have been recently reported [48]. Members of the families Gnaphosidae, Salticidae (jumping spiders), Sparassidae (huntsmen spiders) and Oxyopidae (lynx spiders) all occasionally bite humans, but the effects are usually mild, unless there is secondary bacterial infection.
Public concern with regard to the toxic effects of white-tailed spiders (Lampona cylindrata and L. murina) is not supported by studies which have shown that these spiders are unlikely to cause necrotic arachnidism [49, 50].
Scorpions (Scorpiones)
Definition
Scorpions are arachnids of the order Scorpiones. They are widely distributed in the tropics and subtropics. Approximately 1500 species of scorpions are described worldwide [1]. Only a few species are potentially dangerous for humans. The dangerous scorpions all belong, except one Scorpionidae, to the family of Buthidae, distributed in both the Old and New Worlds [1, 2, 3, 4, 5]. The venom is carried in the curved sting at the tip of the tail, which is swung over the scorpion's head to strike its prey. The principal components are neurotoxins [5, 6], but some venoms also contain 5-hydroxytryptamine, histamine and kinins.
Epidemiology
Many scorpions are quite harmless, and their stings of little consequence. There are areas of the world however, where the risks from a scorpion bite are high and these include Africa (north-Saharan, sub-Saharan, South Africa), the Near and Middle East, southern India and the Americas (in Mexico and southern, eastern South America). Worldwide, the estimate of serious scorpion bites is over 1.2 million with 3250 deaths [1]. Species of Androctonus and Buthus (Figure 34.28) are important in the Middle East and North Africa, and Centruroides species cause problems in the southern USA and Mexico [7].

Figure 34.28 Buthus occitanus.
(Courtesy of Dr J.J. Peres, Peillon, France.)
Pathophysiology
Tityus species are responsible for numerous episodes of envenomation in Brazil and Venezuela [8]. The venom of Tityus serrulatus is the most potent and results from tityustoxin. This toxin acts by binding to voltage-dependent sodium and potassium ion channels, leading to sialorrhoea, lacrimation and rhinorrhoea [9]. American troops stationed in Iraq and Afghanistan have suffered scorpion stings [10]. In this region, the sting of Hemiscorpius lepturus produces most cutaneous injury including purpura, necrosis and formation of bullae or ulcers [11]. Hemiscorpius lepturus also produces hemicalcin, which is a neurotoxin acting on ryanodine-sensitive calcium channels [12].
The incidence of scorpionism is low in Australia [13], where there are no dangerous species of scorpion [14].
Although adults are more often bitten, children experience more severe envenomations and their mortality is higher [1].
Clinical features [1, 15, 16, 17, 18, 19]
The effects of scorpion stings may be local or systemic, and they vary according to the species responsible. The local effects are usually immediate severe burning pain and hyperaesthesia, and there may be marked swelling. Pain remains often the only symptom. Appearance of digestive symptoms within the first few hours (in 5% of the cases) marks the entry of the patient into a serious stage of envenomation. Systemic effects include restlessness, profuse sweating, muscle spasms, difficulty with speech, marked increase in salivary and lacrimal secretion, nausea, vomiting, convulsions, hypertension, cardiac arrhythmias, myocarditis and pulmonary oedema. Death is usually due to respiratory or cardiac failure.
Management [1, 14, 16, 18, 20-23]
Prevention is important and is based first on individual precautions. People must check clothes and shoes while getting dressed. Collective measures are also important: house walls should be constructed with smooth coating to inhibit access of scorpions and doorways of houses should be checked and cleaned regularly. The benefit of insecticide has not been proven.
Once a bite has occurred, early treatment includes neutralizing the circulating toxin as quickly as possible, combating the symptoms of envenomation and general supportive measures. Ice packs should be applied, and the injection of local anaesthetic without vasoconstrictors around the sting site will help to reduce the pain. Specific antivenoms are available and indicated in all severe cases. Specific and symptomatic treatments may not be sufficient to prevent fatal outcome. However, in countries where scorpionism is a serious public health problem, antivenom and supportive treatments have significantly decreased mortality.
Ticks (Acari)
Definition [1]
Ticks are large acarines, which are blood-sucking ectoparasites of vertebrates. They are important vectors of diseases such as tick-borne relapsing fever, and in a number of viral, rickettsial (Chapter 26) and Borrelia (Chapter 26) infections (Lyme disease).
Classification
Ticks are typical arachnids, possessing mouthparts referred to as the capitulum, an unsegmented body and four pairs of legs in the adult. Larval ticks have three pairs of legs.
There are two major families: the Ixodidae (hard ticks) and the Argasidae (soft ticks). The term ‘hard’ refers to the dorsal chitinous shield or scutum, which is present in the Ixodidae but not in the Argasidae (Figures 34.29 and 34.30). In Ixodidae, the scutum covers the whole dorsum in the male but only a small anterior part in the female. In argasids there is little difference between the sexes. The mouthparts of ixodid ticks (capitulum) project forwards and are easily visible from above, whereas those of the argasid ticks can only be seen from below. A conspicuous component of the mouthparts is the toothed hypostome (Figures 34.31 and 34.32).

Figure 34.29 Ixodidae: hard tick. The term ‘hard’ refers to the dorsal chitinous shield or scutum. The mouthparts (capitulum) of ixodid ticks project forwards and are easily visible from above. (Adapted from Rodhain and Perez 1985 [36]. Copyright holder of original artwork: Maloine.)
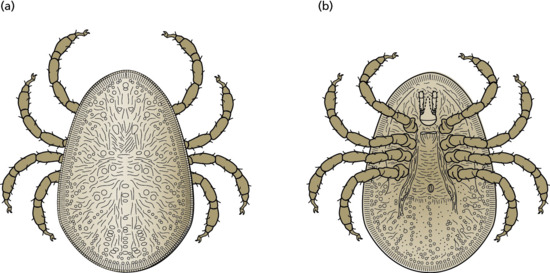
Figure 34.30 Argasid: soft tick. The term soft refers to the absence of scutum. The mouthparts (capitulum) can only be seen from below. (Adapted from Rodhain and Perez 1985 [36]. Copyright holder of original artwork: Maloine.)
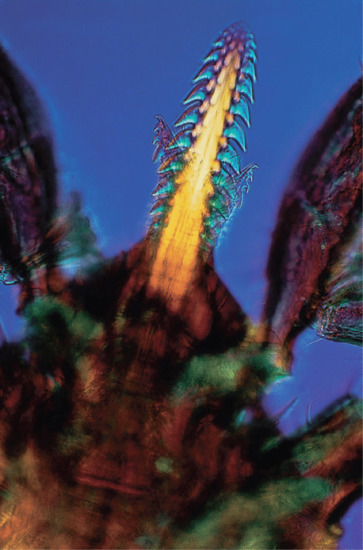
Figure 34.31 Mouthparts of Ixodes ricinus nymph to show the toothed hypostome (interference contrast microscopy).
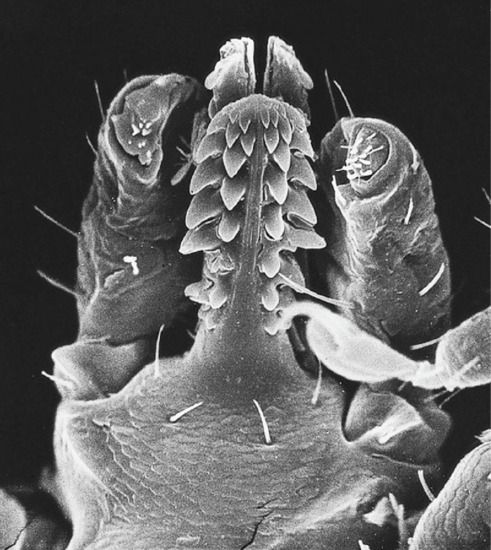
Figure 34.32 Scanning electron micrograph of tick mouthparts.
Introduction and general description
Ixodid ticks have four stages in their life cycle: egg, larva, nymph and adult. The larva and nymph require blood meals before further development can occur, and the adult female (Figure 34.33) also requires a blood meal before egg laying. The female lays one large batch of eggs and then dies. Some ixodids use one host for larval, nymphal and adult stages (one-host ticks), whereas others require two or, more usually, three separate hosts.

Figure 34.33 Ixodes ricinus, the sheep tick (engorged female). (a) Dorsal view. (b) Ventral view.
To find suitable hosts, the larvae, nymphs and adults climb low vegetation and raise the first pair of legs (‘questing’), which carry sense organs (Haller's organ). These organs are sensitive to a number of stimuli, including carbon dioxide in the exhalations of a potential host. If the host brushes past the vegetation, the tick will immediately grasp the animal's coat. Argasidae undergo several nymphal stages, and the adult female feeds a number of times during her lifetime, laying several batches of eggs. Ixodid ticks feed on the host varying from several days (usually 2–4 days) to weeks, depending on such factors as life stage, host type and species of tick. Whereas argasids visit their hosts nocturnally to feed for short periods of time on their hosts, varying from several minutes (usually 1–2 h) to days, depending on the same factors (stage, host, tick). Argasids are mainly parasites of birds, bats and humans. Most ticks are essentially parasites of wild animals, and humans are incidental hosts.
When attaching itself to the host, the tick uses its toothed chelicerae to cut into the epidermis, before thrusting the hypostome into the opening and gradually penetrating the dermis. The hypostome becomes anchored by a protein cement, produced by the salivary glands, which forms a cone around the hypostome and interlocks with its teeth [1, 2]. Argasids, being rapid feeders, do not attach themselves as securely as ixodid ticks.
Ticks as vectors of disease [1, 3, 4] (Table 34.5)
Table 34.5 Important tick-borne diseases
| Disease | Agent | Vectors | Distribution |
| African tick bite fever | Rickettsia africae | Amblyomma hebraeum and A. variegatum | Sub-saharan Africa, West Indies |
| Australian spotted fever | Rickettsia marmionii spp. | Haemaphysalis novaeguineae | Australia |
| Babesiosis | Babesia microti, Babesia strain WA-1 | Ixodes scapularis | Eastern, midwestern, western USA, Europe |
| Crimean–Congo haemorrhagic fever | Nairovirus | Hyalomma marginatum | Asia, Africa and Europe |
| Human monocytic ehrlichiosis | Ehrlichia chaffeensis | Amblyomma americanum | Eastern, southern, midwestern USA, Europe and Africa |
| Human granulocytic ehrlichiosis | E. ewingii related to E. equi, E. phagocytophila | Ixodes spp. | Eastern, midwestern, western USA, Europe |
| Japanese spotted fever | Rickettsia japonica | Ixodes ovatus, Dermacentor taiwanensis, Haemaphysalis longicornis and H. flava | Japan |
| Lyme disease | Borrelia burgdorferi, B. garinii, B. afzelii | Ixodes scapularis | Northeastern, Pacific coast, midwestern, upper north central USA, northern Eurasia |
| Mediterranean fever | Rickettsia conorii | Rhipicephalus sanguineus | Mediterranean region and Africa to Indian subcontinent |
| Queensland tick typhus | Rickettsia australis | Ixodes holocyclus and Ixodes tasmani | Australia, Tasmania |
| Rocky Mountain spotted fever | Rickettsia rickettsii | Dermacentor variabilis, Dermacentor andersoni, Amblyomma spp. and Rhipicephalus sp. | North, Central and South America |
| Siberian tick typhus | Rickettsia sibirica | Dermacentor nuttalli, D. marginatus, D. silvarum and Haemaphysalis concinna | Broadly distributed through north Asia |
| Southern tick associated rash illness | Borrelia lonestari | Amblyomma americanum | Southeastern, south central USA |
| Tick-borne relapsing fever | B. hermsii, B. Turicatae, B. parkeri | Ornithodoros spp. | Western USA |
| Tick-borne lymphadenopathy (TIBOLA) | Rickettsia slovaca, R. raoultii and R. rioja | Dermacentor marginatus | Southern and eastern Europe, Asia |
| Tularaemia | Francisella tularensis | Amblyomma americanum | Throughout USA, Europe and Asia |
| Dermacentor andersoni | |||
| Dermacentor variabilis |
Tick-borne diseases are highly regional and new diseases and geographical areas of prevalence continue to emerge [5]. Migratory birds have been implicated in the spread of diseases to new regions [6]. Within the large family of ixodid ticks, there are several genera of medical importance, including Dermacentor, Haemaphysalis, Rhipicephalus, Amblyomma, Hyalomma and Ixodes. Removal and identification of the tick can be a major step in the management of infectious disease (Figure 34.34).
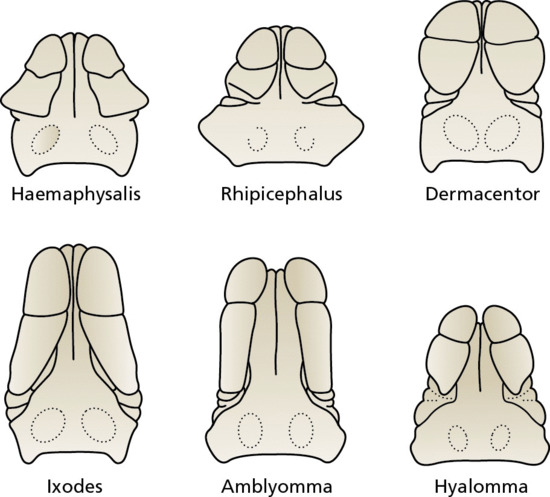
Figure 34.34 Morphology of capitulum and genera of Ixodidae hard tick. Within the large family of ixodid ticks, there are several genera of medical importance, including Haemaphysalis, Rhipicephalus, Dermacentor, Ixodes, Amblyomma and Hyalomma. (Adapted from Rodhain and Perez 1985 [36]. Copyright holder of original artwork: Maloine.)
Dermacentor species act as vectors for a number of diseases, including Rocky Mountain spotted fever (Chapter 26), tick-borne lymphadenopathy (TIBOLA), also called Dermacentor-borne necrosis erythema lymphadenopathy (DEBONEL) [7], Siberian tick typhus, Colorado tick fever and several types of viral encephalitis.
Haemaphysalis species may also carry Rocky Mountain spotted fever, Siberian tick typhus and Colorado tick fever.
Rhipicephalus sanguineus (the brown dog tick) transmits Rickettsia conorii, the causative organism of boutonneuse fever (Mediterranean spotted fever). Rhipicephalus sanguineus is normally confined to the tropics, but it may be encountered in temperate climates in centrally heated houses [8]. Ticks of the genus Amblyomma transmit Rickettsia africae, the organism responsible for African tick bite fever, tularaemia and human granulocytic anaplasmosis (ehrlichiosis) [9, 10, 11]. Amblyomma americanum (the lone star tick) has been recognized as a vector for Borrelia lonestari, the organism thought to be responsible for southern tick-associated rash illness (STARI), a Lyme disease-like infection [12], which is still an enigma despite years of research [13].
Ixodes species are important vectors of certain haemorrhagic fevers and viral encephalitis, and also of Lyme disease (Chapters 25 and 26). They may also transmit babesiosis to humans [14]. The principal vectors of Lyme disease are the sheep tick Ixodes ricinus in Europe, and Ixodes dammini (East coast) and Ixodes pacificus (West coast) in the USA.
Various species of Argasidae may also act as vectors of disease, the most important being Ornithodoros species, which transmit tick-borne relapsing fever. In Israel, O. tholozani (the cave tick), which is endemic in the Middle East, is the vector of Borrelia persica, a causative agent of relapsing fever [15].
Pathophysiology
The pathophysiology of acute tick bite lesions remains unclear. Tick biting parts can reach into the deep dermis, exposing these tissue layers to mouthpart and salivary antigens. Reactions may vary, depending on the interaction between host and a particular tick species.
Pathology [3]
At the point of penetration of the tick mouthparts there is coagulation necrosis of the epidermis and papillary dermis [16]. Surrounding the hypostome is the homogeneous cement [17]. The punctured epidermis shows parakeratosis, spongiosis and frequently pseudoepitheliomatous hyperplasia. There is marked dilatation of upper dermal blood vessels, and a dense perivascular infiltrate of neutrophils and lymphocytes. Histology of the bite site several weeks after removal of the tick shows a perivascular and periadnexal infiltrate of lymphocytes, plasma cells and histiocytes [18, 19]. Foreign-body giant cells may also be present in the infiltrate. If the hypostome has been damaged during removal of the tick, fragments of the mouthparts may be seen.
Clinical features [3, 5, 20]
In the case of ixodid ticks, it is usually the parasite itself that attracts the patient's attention. Larvae, nymphs or adults may be discovered attached to the skin, and humans usually become accidental hosts when walking through, or sitting in, an area that contains ticks [21]. Larval ticks, sometimes referred to as ‘seed ticks’, are very small, and may go unnoticed unless present in large numbers [22, 23, 24]. Bites from soft ticks may be particularly painful [25], perhaps because of their fast feeding or unique salivary contents. The colour of engorged ticks has led patients to suspect they had melanoma [26].
Several factors may be responsible for the type and intensity of tick bite reactions. They include: feeding duration, mouthpart size, tick species, previous exposure and individual sensitivity. Dermatoses may be acute or chronic and may occur away from the site of the initial tick bite.
Acute lesions include erythematous macules, papules or nodules, tissue necrosis and ulcers. Erythematous plaques may be difficult to differentiate from erythema migrans, as they can expand to several centimetres. However, these erythematous plaques do not have a tendency to clear in the centre like erythema migrans [16]. There may be focal necrosis leading to necrotic ulcers [25] but more commonly, the reaction at the site of the bite is mild oedema, vesiculation or bullae formation [27, 28]. Pruritic papules were a prominent feature of larval Amblyomma tick bites in a case reported by Fisher et al. [29]. A papular urticarial response to ticks has been reported in berry pickers [30], and papular urticaria has been observed developing within a few days of contact with numerous larval ticks of I. ricinus. The bites of the cave tick, Ornithodoros tholozani, produce characteristic deep red crusted papules or nodules, with a central punctum [15]. Post-inflammatory hyperpigmentation may persist several months after acute lesions.
Acute lesions may sometimes persist and become chronic. Chronic lesions include plaques, papules and nodules [25]. The formation of tick bite granuloma are probably responsible of these lesions, which may persist for months or years [21]. The pathophysiology of the granuloma formation remains unclear, but may be due to the persistence of tick mouthparts or cuticular fragments in the deep dermis [17].
Auto-eczematization has been reported in association with a tick bite granuloma [31]. Temporary alopecia may develop around the sites of tick attachment to the scalp [19, 32]. The aetiology of the alopecia remains unclear.
The main complication of tick bites is secondary infection (Staphylococcus aureus and group A Streptococcus), such as impetigo, ecthyma, erysipelas, cellulitis and superinfected necrotic ulcers [21, 25]. These secondary infections may result from the persistence of tick material in the dermis or from host scratching.
Finally, tick bites can cause non-dermatological disease such as anaphylaxis, paralysis and other systemic symptoms [33]. Tick paralysis is an ascending flaccid paralysis probably caused by a neurotoxin injected by the feeding tick [34, 35]. Occasionally, bulbar paralysis, respiratory failure and death occur. The site of action of the toxin appears to be in the region of the neuromuscular synapse. If the tick is removed, all the signs usually resolve rapidly, but sometimes recovery is slow. Children are more frequently affected than adults.
Tick paralysis occurs in particular localities in association with specific ticks [3]. Offending species include Dermacentor andersoni and D. variabilis (USA); Ixodes holocyclus (Australia); I. pilosus (South Africa); I. ricinus, I. hexagonus and Rhipicephalus sanguineus (Europe).
Management [5]
Tick removal
Removal of multiple ticks may be difficult. Ticks should not be removed by a sudden forcible movement, as this will often leave the mouthparts embedded in the skin. The best is to remove the tick intact. This can be due with the help of tweezers or other special device. The tick must be gripped as close to the skin as possible, and gentle traction usually succeeds. The risk of vector-borne disease transmission is minor if the tick is removed within 24 h. Applications of fingernails, hot matches or isopropyl alcohol have never demonstrated efficiency.
Treatment
Except for viral fevers and babesiosis, tetracycline is the antibiotic of choice for most tick-borne diseases. Delayed antibiotherapy can be fatal especially in Rocky Mountain spotted fever, so therapy should be started quickly in case of fever and headache in an endeminc area, without awaiting laboratory confirmation.
Prevention
Avoidance of tick-infested areas, use of repellents and rapid tick removal are the key points of primary prevention. Secondary prevention is based on prophylactic antibiotics or rapid institution of antibiotics if symptoms appear.
Mites (Acari)
Family Sarcoptidae: human classical scabies
Definition
Scabies in humans and other animals is caused by mites of the family Sarcoptidae, which includes Sarcoptes scabiei, the scabies mite, and Notoedres cati, a mange mite of cats.
The Sarcoptes causing scabies in humans and sarcoptic mange in many other animals are physiological variants of a single species, S. scabiei. Their host specificity is not complete, but they usually survive for only a short period on another host.
Introduction and general description [1-7, 8, 9-11]
Scabies is an ectoparasitic infection caused in humans by the Sarcoptes scabiei var. hominis. The adult female measures approximately 0.4 mm long by 0.3 mm broad, and the smaller male 0.2 mm long by 0.15 mm broad. The body is creamy white and is marked by transverse corrugations, and on its dorsal surface by bristles and spines. There are four pairs of short legs; the anterior two pairs end in elongated peduncles tipped with small suckers. In the female, the rear two pairs of legs end in long bristles (setae) (Figure 34.35), whereas in the male bristles are present on the third pair and peduncles with suckers on the fourth.

Figure 34.35 Sarcoptes scabiei, the scabies mites. Female with eggs.
Copulation occurs in a small burrow excavated by the female. The burrow is not confined to the stratum corneum, but is inclined downwards into the epidermis. Approximately 40–50 eggs are laid by each female during a lifespan of 4–6 weeks. Eggs hatch after 3–4 days into larvae, which dig new burrows closer to the skin surface. There, the larvae mature into adult mites in about 4 days. The adults may then either stay in that host or be scratched off and transmitted to a new host. Adult females can live in the host for up to a month. The life cycle lasts around 14–21 days. The mites show a preference for certain sites in which to burrow, and appear to avoid areas with a high density of pilosebaceous follicles. The average number of adult female mites on an individual suffering from the common form of scabies is about 12 [10, 11]. Only in crusted scabies are large numbers of mites present. Individually, pruritus represents a nuisance. The risk of contagiousness, impetiginization, psychosocial impact and potential associated sexually transmitted diseases constitute a concern.
Human scabies has played a modest, but not insignificant role in history; the story of scabies has been related in detail by Hebra [1], Beeson [2], Heilesen [3], Friedman [4] and Parish [5].
Epidemiology [8, 12, 13, 14, 15, 16]
Incidence and prevalence
Scabies affects around 100–300 million people worldwide, but accurate figures of its prevalence are difficult to obtain [12]. A study by Downs et al. [17] on data collected in the UK between 1967 and 1996 showed a high incidence of scabies in the late 1960s and early 1970s, a drop during the 1980s and a rise throughout the 1990s. A Danish study, which assessed data collected between 1900 and 1975 [18], showed incidence rates in the 1960s and 1970s similar to those in the UK study, and high incidence rates during the two World Wars. However, a rise in incidence started 2 years prior to hostilities on both occasions. Data from records kept in Edinburgh, UK, also showed rises in incidence associated with the World Wars, but coinciding precisely with their onset [19].
Several other studies have noted a seasonal variation [20, 21]. A proposed explanation for the cyclical fluctuations in prevalence in developed countries is the ‘herd immunity’ theory [13, 22]. This suggests that an epidemic of scabies confers a degree of immunity, so that a further epidemic will not occur until a new, susceptible population has arisen. However, the persistent high levels of scabies in many underdeveloped countries, without any marked cyclical variation, are evidence against this view. Many factors have been suggested to determine the epidemiology of scabies in impoverished communities, including social attitudes, population movements, malnutrition, lack of access to health care, inadequate treatment and deficient hygiene but so far these assumptions have not been substantiated [23].
Age
Scabies occurs in all age groups. However, it becomes frequent in the elderly in residential and nursing home environments. In a questionnaire survey of dermatologists in UK hospitals, reported in 2000 [24], respondents estimated that approximately 30% of all cases of scabies they encountered occurred in institutions such as care homes and hospitals. Although some outbreaks are related to cases of crusted scabies, others appear to originate from residents who have ordinary scabies with many burrows, and therefore a large mite population, or from infected careers. A recent work highlighted that in some countries, the prevalence of at least one case of scabies in health care settings could reach 45% in open studies [25].
Sex ratio
The overall sex incidence is probably equal, even if mothers are more frequently affected than fathers in families.
Ethnicity
Whereas all ethnic groups are susceptible, there are some differences which are probably related to customs and social factors rather than inherent susceptibility. Some reports suggested that black Americans appeared less susceptible [26, 27], but this was disputed [28]. In a study of a multiracial population in Hawaii [29], scabies was far more frequent in white people and Hawaiians than in Japanese and Filipinos, and this was thought to be related to family size and social customs.
Pathophysiology [6, 8, 9, 16, 30, 31]
Scabies is usually transmitted by close physical contact, such as prolonged hand-holding or the sharing of a bed. It is often suggested that fertilized female mites are responsible for transmission, although there is no firm evidence to support this contention, but it seems unlikely in view of their relatively small numbers and inclination to remain within their burrows. Away from the host, scabies mites survive for 24–36 h at room conditions (21°C and 40–80% relative humidity) [32], and live mites have been demonstrated in dust samples collected in the homes of infected patients [33].
Allergic sensitivity to the mite or its products appears to play an important role in determining the development of lesions other than burrows, and in producing pruritus. However, the sequence of immunological events is unclear and requires further elucidation.
Evidence suggests that both immediate and delayed-type hypersensitivity are involved. Skin tests with mite extracts have given equivocal results, although positive immediate-type reactions to intradermal tests have frequently been obtained in patients within a few months of scabies infection. Normal IgE levels were reported in one series of scabies patients [34], but later studies have shown significantly elevated levels in many individuals [35, 36, 37].
Susceptibility or resistance to S. scabiei infection shows some genetic predisposition. This is hypothesized to correlate susceptibility to sever disease with the dominance of an IgE-driven Th2 response or resistance to the infestation by an interferon-γ dominated Th1 response. This may be modulated by cytokine regulation in the skin and other immunological control mechanisms [38]. Recent developments in scabies mite biology have shown that scabies can now be considered to be a complex interaction between host, parasite and their associated microbiomes. Animal and in vivo models of infestation should facilitate a better understanding of these host–parasite interactions, which is critical to improving the treatment of scabies [39, 40].
Clinical features [12, 14]
Presentation
Itching is the most obvious manifestation of scabies, usually sparing the face in adult classic scabies. It is generally worst at night and when the patient is warm. The onset occurs 3–4 weeks after the infection is acquired. Reinfection of a previously cured individual, however, may provoke immediate symptoms [14]. Typical locations of lesions are the finger webs (Figure 34.36a), the flexor surfaces of the wrists, the elbows, the axillae, the buttocks and genitalia (Figure 34.36b) and the breasts of women (Figure 34.36c). The typical lesions of scabies are burrows (Figure 34.36d,e), which appear as slightly raised brownish tortuous lesions. Inflammatory pruritic papules or nodules, sometimes surmounted by burrows, on the male genitalia are characteristic. The genitalia of males should be therefore systematically examined once scabies is suspected as these lesions may provide an important diagnostic clue if burrows are absent or difficult to find. Nodules are intensely itchy, and may persist for weeks or months after the scabies has been effectively treated [14]. Secondary lesions are not specific. They include excoriations, eczematization (Figure 34.36f) and impetiginization, and may occur anywhere.
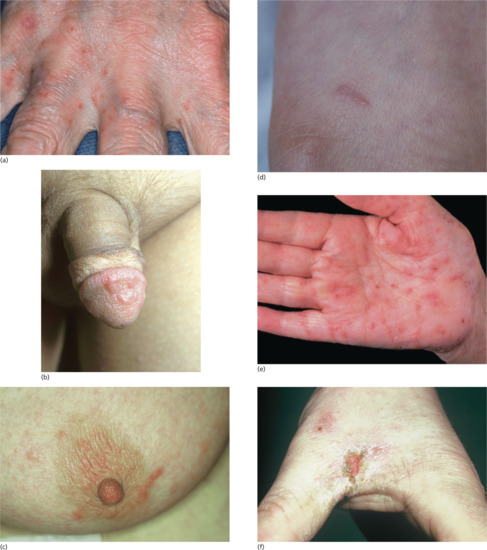
Figure 34.36 (a) Typical scabies in the finger webs. (b) Pruritic papules and nodules on the penis in scabies infestation. The genitalia should be examined in all instances of suspected scabies infestation, especially when the patient reports itching. (c) Papular lesions on the nipples and areolae are a common location for scabies in women. Given this woman's history of pruritus, scabies was easily identified by the finding of scabies in this location. (d) A typical linear burrow with a tiny vesicle at the distal end. (e) Numerous scabies burrows on the palm. Such obvious lesions are rarely seen, as they are usually obscured by eczema, impetigo or both. The more common presentation of scabies with eczematization of the scratched lesion is shown in (f). The chronic pruritus of scabies rapidly leads to scratching and explains why eczema is frequently observed. (From New England Journal of Medicine, Chosidow O, 2006;354:1718–27 Copyright
© Massachusetts Medical Society. Reprinted with permission.)
In sub-Saharan Africa, where prevalence of scabies is high, a report found that association of diffuse itching with cutaneous lesions and two typical locations or a household member with itching was highly sensitive (100%) and specific (97%) for a diagnosis of scabies [41].

Figure 34.37 Scabies: clinical variants. (a) Scabies in an infant. Localization on the sole is not atypical in this form of scabies, nor is involvement of the face, scalp and palms. (b) The foot of an infant with scabies superinfection presenting as impetigo. In such patients, the risk of glomerulonephritis associated with nephritogenic strains of Streptococcus is of concern in developing countries. (c) Atypical papular scabies in an elderly woman who also had similar lesions on her back. Frequently, scabies goes unrecognized in such patients because itching is attributed to senile pruritus. (From New England Journal of Medicine, Chosidow O, 2006;354:1718–27 Copyright
© Massachusetts Medical Society. Reprinted with permission.)
Clinical variants (Figure 34.37a–c)
Atypical forms of scabies [42] (Table 34.6) may occur and be very difficult to diagnose. The clinical features of scabies in infants and young children differ in certain respects from those in older children and adults. In addition to the more extensive distribution of burrows mentioned above, vesicular and vesiculopustular lesions on the hands and feet are frequent, extensive eczematization is often present, and there may be multiple crusted nodules on the trunk and limbs. In the elderly, burrows commonly occur on the palms and soles, and may be very numerous. Truncal papulosquamous lesions, often surmounted by burrows, are common. Secondary eczematization is often troublesome. Crusted scabies is discussed separately (see below).
Table 34.6 Special forms of scabies
| Variable | Major clinical features |
| Involved subpopulation | |
| Infants and young children [88] | Lesions are vesicles, pustules and nodules, but their distribution may be atypical. Eczematization and impetigo are common; scabies may be confused with atopic eczema or acropustulosis. Pruritus may be so severe that infants can be irritable and eat poorly |
| Homeless people | Eczematization and impetigo are common. Extensive excoriated lesions are not necessarily indicative of scabies in homeless people but pruritus in a homeless shelter should suggest a diagnosis of scabies |
| The elderly | Atypical presentation is common. Scabies epidemics are reported frequently in nursing homes, where a single patient with crusted scabies may be the index patient leading to infection of other residents, as well as health care workers and their families |
| Immunocompromised patients | Severe scabies (i.e. atypical papular scabies or crusted scabies) develops predominantly in patients receiving topical or systemic corticosteroids, those with HIV infection, organ-transplant recipients and patients of advanced age. Pruritus may be mild or absent (i.e. scabies incognito) |
| Indigenous communitiesb | Scabies, whether crusted or not, may be endemic (e.g. risk factors include poor nutritional status, inadequate medical facilities, and overcrowding). The burden of the disease may be very high among Aboriginal people in northern Australia, children in Africa or the Solomon Islands, and resettlement colonies in New Delhi, India, for example. Because of the high rate of scabies superinfection, Australian Aboriginal communities have the highest rate of poststreptococcal glomerulonephritis in the world |
| Atypical presentation | |
| Scabies of the scalp | Scabies may accompany or simulate seborrhoeic dermatitis or dermatomyositis on the scalp; infants, children, the elderly, patients with AIDS and patients with crusted scabies may be affected |
| Nodular scabies | A few violaceous, pruritic nodules are often localized on the groin, axillae and male genitalia; they represent a hypersensitivity reaction to mite antigens and persist weeks or months after treatment |
| Scabies mimicking immunologically mediated diseases | Bullous pemphigoid , urticaria, chronic lymphocytic leukaemia, B-cell lymphoma with monoclonal infiltrate, CD30+ lymphoid proliferations, necrotizing vasculitis [89, 90] and lupus erythematosus can all mimic scabies |
Adapted from Chosidow, N Engl J Med, 2006 [12] with permission. Copyright holder of original: Massachusetts Medical Society.
aScabies has been added to the list of neglected tropical diseases by the WHO (http://www.who.int/neglected_diseases/diseases/scabies/en/)
Complications
In addition to these primary manifestations, secondary features may occur, and can confuse the clinical picture. Eczematous changes are common, and may be widespread and severe. The inappropriate use of topical steroids may further modify the clinical picture to mimic other dermatoses (see section on crusted scabies below) – so-called ‘scabies incognito’ [16]. Secondary infection, manifest as folliculitis or impetigo, may also be severe and extensive. In the tropics and subtropics, where nephritogenic strains of β-haemolytic streptococci may be responsible for secondary impetigo, haematuria-related glomerulonephritis occurs as a complication of scabies [43].
Investigations [12, 16, 44, 45, 46]
The typical history of pruritus with nocturnal exacerbations, the presence of contact cases within the family and the distribution of the eruption of inflammatory papules, should suggest the diagnosis. The presence of genital lesions in men or breast nodules in women is strongly suggestive. Absolute confirmation can only be made by the discovery of burrows and/or microscopic examination. A burrow is gently scraped off the skin with a blunt scalpel, and the material placed in mineral oil on a microscope slide. The presence of mites, eggs, fragments of egg shells or scybala confirms the diagnosis. Failure to find mites is common and does not rule out scabies [12, 46]. Parasitological confirmation of the diagnosis should be made in all cases if possible. It is essential in cases of crusted scabies or scabies in health care settings.
Dermoscopy is useful for detecting burrows and visualizing their contents, the mite in its burrow resembling a ‘jet-with-contrail’ (40× magnification) [47]. More recently, it was shown that low-magnification (10×) standard handheld dermoscopy could be a valuable tool for diagnosing common scabies [48]. At low magnification, the circumflex accent-like image (as the French letter ‘ô’) represents the head and the two pairs of front legs of the mite. It has been shown that sensitivity of this technique was 91% and specificity 86% [49]. However, the external validity of this monocentric study performed with trained investigators needs to be confirmed in other centres. Dermoscopy is less time-consuming than skin scraping procedure because it allows a quick screening of a large number of sites. It causes less discomfort for the patient, so it is also better accepted [49]. However, the use of dermoscopy is limited by the cost of the dermoscope and the sensitivity may decrease in inexperienced hands.
Adhesive tape test may be useful in particular situations. After firmly applying the adhesive side of the tape onto an appropriate skin lesion of patients, the tape is pulled off and transferred directly onto a slide for microscopy, affixing the adhered separated part of the corneal skin. This tape method is simple and may be useful for diagnosis of severe scabies infestation in long-term nursing units [50]. However, its sensitivity is low [51].
These new methods (dermoscopy and adhesive tape test) may increase the sensitivity of skin scraping tests and limit false negative results [49, 51]. However, comparing the accuracy of different tests for diagnosing scabies remains elusive without a criterion standard [52, 53].
A skin biopsy may confirm the diagnosis of scabies if a mite or parts can be identified. However, in most cases, the histology shows non-specific features, with epidermal spongiosis, papillary oedema, and superficial and deep perivascular inflammatory cell infiltrates with numerous eosinophils [54].
Confocal microscopy was recently employed for the confirmation of the clinical diagnosis of scabies [55, 56, 57] but its performance should be evaluated in larger diagnosis studies [52]. The use is also limited by its cost.
In the absence of confirmed mites, diagnosis is currently based entirely on clinical and epidemiological findings. Given the extensive differential diagnoses, the specificity of clinical diagnosis is poor, especially for those inexperienced regarding scabies [46]. Furthermore, there are the difficulties in distinguishing between active infestation, residual skin reaction and reinfestation. Despite the relatively low sensitivity of diagnostic testing, empirical treatment is not recommended for patients presenting with generalized itching [12].
Management [8, 12, 16, 44, 58, 59, 60, 61, 62, 63]
Indication for therapy
Treatment should be prescribed to the patient and close physical contacts, even without pruritus or cutaneous lesions.
Patient education
Patients should be advised to avoid close physical contact until they and their household members and sexual partners have been treated. A detailed verbal and written information about scabies infestation should be given to the patient [64].
Treatment options
Topical and oral products are available although rigorous studies to guide their use are lacking. Topical treatment includes permethrin, lindane, benzyl benzoate, esdepallethrine (bioallethrin), crotamiton and precipitated sulphur. Topical scabicides have neurotoxic effects on mites. Table 34.7 summarizes the doses and side effects of common agents used in scabies management.
Table 34.7 Drugs commonly used to treat scabies
| Treatment | Dosage | Treatment regimen | Contraindication | Advantages | Disadvantages | Comments |
| Permethrin | 5% cream | Rinsed off after 8–12 h | – | Effective, well tolerated, safe | Itching and stinging on application | Second application often routinely prescribed 1 week after the first |
| Lindane | 1% lotion or cream | Rinsed off after 6 h | Pregnant women, infants, seizure disorders | Effective, inexpensive | Cramps, dizziness, seizures in children | Withdrawn in the European Union because of neurotoxicity concerns |
| Benzyl benzoate | 25% ointment | Rinsed off after 24 h (one or several times) | Pregnant women and infants (only 12-h application) | Effective, inexpensive | Can cause severe skin irritation | Not currently available in Canada, approved in Europe |
| Esdepalletrin (bioallethrin) | 0,6% aerosol | Rinsed off after 12 h | People with asthma | – | – | – |
| Crotamiton | 10% ointment | Rinsed off after 24 h and then reapplied for an additional 24 h | – | Well tolerated, safe for infants | Questionable efficacy | Often used on scabies nodules in children |
| Precipitated sulphur | 2–10% precipitate in petroleum base | Rinsed off after 24 h and then reapplied every 24 h for the next 2 days (with a bath taken between each application) | – | Safe for infants, pregnant and breastfeeding women | Questionable efficacy, skin irritation | – |
| Ivermectin | Pills | 200 μg/kg repeated on day 7–14 | Children <15 kg; pregnant or breastfeeding women | Good patient compliance | Expensive | Not approved in many countries |
Adapted from Chosidow, N Engl J Med, 2006 [12] with permission. Copyright holder of original: Massachusetts Medical Society.
It is not known if oral or topical treatment is more advantageous for improved efficacy, tolerance or convenience [61, 65, 66, 67]. Despite the varied methodological quality of trials, a recent meta-analysis suggested that topical permethrin is the most effective [61]. In one recent trial in which two doses of permethrin were compared with a single dose of ivermectin, only a small and non-significant advantage was observed with permethrin (93 versus 86%) [68]. These results are in contrast to other trials in which day 14 efficacy was reported to range from 26 to 100% [69, 70, 71]. Chhaiya et al. [70] also examined topical application of 1% ivermectin, and found it to be more effective than a single dose of oral ivermectin.
Oral ivermectin interrupts the γ-aminobutyric acid induced neurotransmission of many parasites including mites, but is not licensed for use in scabies in most countries. It is given at 200 μg/kg as a single dose in patients >2 years of age and >15 kg. A second dose is necessary 7–14 days later due to the lack of ovicidal action of the drug [12, 63]. Because ingestion of food increases the bioavailability of ivermectin by a factor of 2 [72], taking it with food might enhance the penetration of the drug into the epidermis. Ivermectin is apparently a safe drug with a low incidence of adverse effects. Many of the reported adverse effects have occurred in individuals given ivermectin for the treatment of filariasis, in whom serious reactions were thought to be related to death of the parasites [73]. A report suggesting a pattern of excess deaths in elderly people in a residential unit, who were given ivermectin to control a scabies outbreak, raised concerns about its safety [74]. However, the conclusions of this report were challenged [75, 76], and other authors’ findings regarding its safety are reassuring [77, 78]. It has been suggested that conditions disturbing the blood–brain barrier integrity such as young mammals may allow the drug enter the central nervous system [79, 80, 81]. Therefore some authors consider the drug must be contraindicated in children less than 5 years of age or under 15 kg, and during lactation. However, it appears to be safe in children [82, 83, 84] and its use in pregnant women is discouraged in the USA but possible in France [85, 86].
Finally, permethrin or ivermectin may be used for the treatment of classical scabies. If permethrin is not available, benzyl benzoate may be used. Oral ivermectin is more expensive and not licensed in most countries; however, this agent may be preferred for patients who cannot tolerate topical therapy or are unlikely to adhere to a therapeutic regimen [12, 63]. In classical scabies, the combination of topical therapy and oral ivermectin has never been compared with either treatment alone. Table 34.8 presents strategies of treatment according to the clinical picture.
Table 34.8 Treatment of scabies by clinical features or situation
| Purpose of therapy | Recommended therapy | Alternative therapy | Specific associated measures | Comments |
| Classical scabies | Two applications of permethrin, 5%, or benzyl benzoate | Two doses of oral ivermectin, 200 μg/kg (at days 1 and 7–14) | – | People in close physical contact, even without symptoms should receive treatment at the same time |
| Children <2 years old | Permethrin or benzyl benzoate (only 12-h application) | Ivermectin is contraindicated in children <15 kg | Treat the face, except mouth and eyes | Treat scabies nodules with crotamiton |
| Pregnancy | Permethrin, benzyl benzoate (only 12-h application) and sulphur | Ivermectin is contraindicated in the USA, but not in France | – | – |
| Superinfected scabies | Prefer oral ivermectin if skin is affected | Antibiotherapy before topical treatment | – | Risk of poststreptococcal glomerulonephritis and systemic sepsis |
| Institutional outbreak of scabies | Treat clinical cases as for classical and crusted scabies | – | Simultaneously treat all cases and all exposed people | Formation of an outbreak management team |
Adapted from Monsel and Chosidow 2012 [58].
Additional measures
Examination and laboratory investigation to search for sexually transmitted infection should be performed as scabies is considered to be a sexually transmitted disease [60].
Topical treatment must be applied to the entire skin surface, including the scalp, all folds, groin, navel and external genitalia, as well as the skin under the nails. Treating the face of babies is essential because transmission may occur by breastfeeding. This may be not necessary in adults with classical scabies. Hands should not be washed during therapy, otherwise the treatment should be reapplied. If topical treatment is applied by another person, it is recommended that this person wears protective gloves.
All clothes and bedding must be washed at high temperature (>50°C) or must be kept in a plastic bag for up to 72 h. Materials or fomites that cannot be washed should be treated with insecticidal products. The time course for the eradication of parasites after treatment is unknown. However, it is possible that patients receiving oral ivermectin remain contagious longer than those receiving topical therapies [12, 63].
Special treatment considerations
Table 34.8 presents the treatment of scabies according to the clinical feature or situation.
Children
Benzyl benzoate, esdepallethrin and permethrin may be used in infants. Benzyl benzoate and esdepallethrine are safe in children <2 years of age, but duration of use should be limited to 12 h. Ivermectin is contraindicated in children <15 kg.
Impetigo
Oral ivermectin should be preferred in this situation. If topical treatment is chosen, antibiotherapy against Streptococcus pyogenes and Staphylococcus aureus should be performed before.
Pregnancy or breastfeeding
Permethrin, benzyl benzoate and sulphur appear to be safe in pregnancy, although the evidence is limited [87]. Oral ivermectin is not approved in the USA but is permitted in France [85, 86].
Institutional outbreaks
The management of institutional outbreaks is mainly based on consensus expert opinion. It requires coordination and adequate education of all involved personnel and a sustained effort to rapidly control the outbreak. Prompt recognition of the index case, formation of an outbreak management team, determining the extent of the outbreak and risk factors for transmission, immediate implementation of infection control practices, simultaneous treatment of cases and all exposed people, and concomitant environmental disinfection are key factors for controlling a scabies epidemic in health care settings [25].
Follow-up
Itching may persist several weeks after scabies, and this should be clearly explained to the patient. Emollients may be helpful in cases of cutaneous irritation. The persistence of itching after 4 weeks should be reinvestigated (Table 34.9).
Table 34.9 Causes of persistant itching after scabicide therapy and management
| Causes | Management | |
| Cutaneous irritation | Overtreatment Eczematization Contact dermatitis |
Intensive use of emollient Intensive use of emollient Topical steroid |
| Treatment failure | Poor compliance: inappropriate or insufficient treatment Resistance to scabicide Reinfestation or relapse |
Further scabicide application Change scabicide Further scabicide application |
| Psychogenic pruritus | Delusions of parasitosis Non-parasitic dermatosis |
Antipsychotic drugs (prescribed by dermatologists and/or psychiatrists) Treat the underlying cause |
Adapted from Chosidow 2000 [14].
Family Sarcoptidae: human crusted scabies
Introduction and general description
The appellation ‘Norwegian’ derives from the description in Norway by Danielssen and Boeck [1] of a type of scabies in which huge numbers of mites were present in lepers. von Hebra referred to this as ‘scabies Norvegica Boecki’ [2]. As others, we strongly recommend that ‘Norwegian’ should be discarded and replaced by ‘crusted’ or hyperkeratotic [3, 4].
Crusted scabies is a rare and severely debilitating form of the disease, characterized by the infestation of up to millions of mites and the development of hyperkeratotic skin crust. An undiagnosed case of crusted scabies may be the source of an outbreak of common scabies.
Pathophysiology [5, 6]
In common scabies, there are few mites, probably because scratching destroys the burrows. Crusted scabies occurs in people with an inadequate immune response to the mite, allowing them to multiply. It is a severe disease with a significantly higher morbidity than ordinary scabies.
Patients who are mentally retarded or suffer from dementia may develop crusted scabies [7], and Down syndrome is a frequent association [3, 8]. The reason for this association with mental abnormality is not completely understood, but lack of appreciation of pruritus may be important.
Crusted scabies may develop in patients who are immunosuppressed, either as a result of disease [9, 10, 11] or therapy [11, 12, 13, 14], including with infliximab and tocilizumab [15, 16]. In recent years, there have been numerous reports of its occurrence in patients with HIV infection, and it has been reported in immune reconstitution inflammatory syndrome [17] and it is also an indicator of human T-cell lymphotrophic virus (HTLV-1) infection [18, 19, 20]. Crusted scabies has also resulted from the use of topical steroids [21] and pimecrolimus [22].
Crusted scabies sometimes occurs in otherwise healthy individuals [23, 24], and in northern Australia, where crusted scabies is a problem in the Aboriginal population, 42% of a series of 78 patients had no identifiable risk factors [25].
Clinical features [25, 26, 27, 28]
Large warty crusts form on the hands (Figure 34.38a) and feet (Figure 34.38b,c), and the palms and soles may be irregularly thickened and fissured. The nail apparatus is frequently affected, with masses of horny debris accumulating beneath thickened and discoloured nails (Figure 34.38d). Erythema and scaling occur on the face, neck, scalp (Figure 34.38e) and trunk, and may generalize. The extent of the erythroderma and the warty plaques varies greatly, and either may predominate. Crusted scabies may be localized, affecting only the scalp, face, fingers, toenails or soles [28]. Itching is often absent or slight, but may occasionally be severe. Generalized lymphadenopathy is present in some cases, and blood eosinophilia and elevated IgE levels are common.
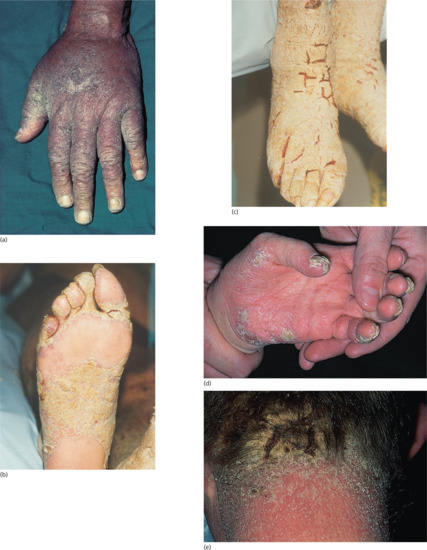
Figure 34.38 Crusted (Norwegian) scabies of the hand (a) and foot (b, c). (d) Grossly dystrophic nails in crusted scabies. (e) Severe scalp involvement in crusted scabies.
Crusted scabies may masquerade as hyperkeratotic eczema, psoriasis, Darier disease [29], contact dermatitis [30] and Langerhans cell histiocytosis [31].
The diagnosis is readily confirmed by examination of scrapings, which will be teeming with mites and eggs.
Management [32, 33, 34, 35, 36, 37, 38, 39]
General principles
Hospitalization and isolation of the patient are required to manage crusted scabies because of the risk of transmission to people in physical contact. All individuals in contact should be treated. A keratolytic agent such as a salicylic acid preparation should be used to treat hyperkeratosis. Nails should be cut short and brushed with a scabicidal agent [34]. Expert consensus recommends combining topical and oral therapy [35], although this has never been evaluated. Topical scabicide application should be repeated until two parasitological tests 3 days apart become negative. The administration schedule of ivermectin should be based on the severity of infection [40]; between three and seven doses have been proposed [27]. However, Currie et al. [41] reported evidence of resistance to ivermectin in two patients who had received multiple doses for recurrences of crusted scabies. Recently, a simple clinical grading scale to aid in the management of patients with crusted scabies has been proposed and may be useful [42].
Family Sarcoptidae: animal scabies
Introduction and general description
Transmission of animal scabies to humans is probably rare, because of the relative host specificity of the mites [1]. However, recurrent exposure to animal scabies mites can produce troublesome and diagnostically puzzling lesions.
Many varieties of Sarcoptes scabiei have been incriminated, including the following.
- The mites causing sarcoptic mange in horses, cattle, buffalo, pigs, camels, monkeys, sheep and goats [2, 3, 4, 5, 6, 7].
- Sarcoptes scabiei var. canis commonly causes transient skin lesions in those in contact with infested dogs [8, 9, 10, 11, 12, 13, 14]. Exceptionally, scrapings from human skin have shown mites and eggs, and symptoms have persisted after contact with the animal has ceased [15]. Canine scabies has been experimentally transferred to humans [16]. Affected animals have areas of scaling and hair loss on the ears, face and limbs [17].
- Notoedres cati, the cause of sarcoptic mange in cats, is almost unknown in the UK, but where it is endemic in the cat population, as in India [18] and Japan [19], human skin lesions may occur.
Clinical features
Skin lesions resulting from contact with animal scabies vary in extent and distribution, according to the mode of exposure. The eruption is usually composed of small pruritic weals or papules, which are frequently excoriated, and resemble human scabies, but without burrows. Lesions from exposure to sarcoptic mange in dogs and notoedric mange in cats usually occur at sites of contact with the animal, principally the chest, abdomen, thighs and forearms.
Management
If contact with animal scabies is suspected, the diagnosis can only be confirmed by examining and taking scrapings from the suspect animal. Affected animals should be treated by a veterinary practitioner.
Human skin lesions are self-limiting, and will resolve once exposure to the affected animal has ceased, or it has been treated. Despite being self-limited, the skin eruption may be uncomfortable, and topical treatment such as 5% permethrin cream will hasten recovery [20]. Oral ivermectin (200 μg/kg single dose) has been used [20, 21], as well as topical corticosteroids, menthol preparations, and oral antihistamines for symptomatic relief [22].
Family Knemidokoptidae
Knemidokoptes mutans causes scaly leg in domestic poultry, and Mesoknemidokoptes laevis is a closely related mite which causes depluming itch in poultry; both have caused skin lesions in humans [1].
Family Psoroptidae
Mites of the family Psoroptidae cause mange in domestic animals. Species of Chorioptes and Psoroptes from cattle, horses and sheep have occasionally affected humans [1, 2]. Otodectes cynotis is a common parasite in the ears of cats and dogs, and has been discovered in the ears of a patient suffering from otitis externa [3, 4]. It was also considered to be responsible for a pruritic dermatosis in a patient whose dog was infested. Psoroptic skin disease resembles scabies infestation. The distribution of the dermatitis is dependent upon the areas that come in close contact with the animals [5].
Family Listrophoridae
Listrophorus gibbus, a common parasite of the domestic rabbit [6], has been reported as causing papular urticaria in a child [7].
Mites of stored products [1,2]
Introduction and general description
Storage mite allergy is a well-recognized problem in certain occupations, including farmers, grain elevator workers and bakers. In addition to respiratory allergy, skin lesions can occur, secondary to bites or contact with allergens. These mites proliferate in a warm humid environment with relative humidity of 80% and a temperature between 25 and 30°C. Herbivorous and fungivorous, they subsist on fungi and are pests of stored food products with high moisture content.
Classification
Family Acaridae
These mites attack flour, grain, dried meat, cheese and dried fruit.
Acarus siro is the most important pest of storage premises, and is found on flour, grain and, occasionally, cheese. It may cause skin lesions on those who handle these products.
Tyrophagus putrescentiae [3, 4, 5, 6] is mostly found in stored food with a high fat and protein content such as dried eggs, ham, herring meal, cheese, nuts and copra. Tyrophagus longior is found on cheese, grain, hay and copra [7].
Suidasia nesbitti is particularly associated with wheat pollards and bran, and has been recorded as causing dermatitis in humans [8].
Rhizoglyphus species occur on flower bulbs and have caused dermatitis in persons handling stored bulbs.
Family Carpoglyphidae
Carpoglyphus passularum (lactis) is found on all kinds of dried fruit, and may cause dermatitis [9, 10].
Family Glycyphagidae
Glycyphagus domesticus is a widely distributed species, often found in large numbers on plant and animal remains in houses and stables. It has also been found in flour, wheat, hay, tobacco, cheese and ham. Glycyphagus destructor is often abundant in hay, straw and grain.
Pathophysiology
It has been suggested that the dermatitis caused by these mites, which are not haematophagous, results from irritation by mite products, either faecal or secretory [5]. Extracts from storage mites include endotoxins which may modulate cell adhesion and secretion of cytokines by microvascular endothelial cells. These modulating properties vary among mite species [11].
Dockers and warehouse workers handling stored products are most at risk, but shopkeepers and domestic workers are occasionally affected.
Clinical features [12]
The eruption provoked by these mites is sometimes called ‘copra itch’ or ‘grocer's itch’, and is often composed of minute intensely pruritic papules or papulovesicles on exposed parts of the body, principally on the head and neck, and forearms, but occasionally more widespread. The appearance of the eruption on the face may suggest an acute contact dermatitis.
Mites, house?dust: cutaneous reactions to
Introduction and general description
Dermatophagoides pteronyssinus, the house-dust mite, was first discovered by Trouessart in dust shaken from tanned mammal skins [1]. It was subsequently established that it is widely distributed in the human environment in house dust and beds [2, 3].
Epidemiology
It occurs worldwide, and has been reported from all inhabited continents [4]. It is commonly associated with Euroglyphus maynei and Dermatophagoides farinae, which are related species in the same family, the Pyroglyphidae. In the USA, D. farinae appears to be more plentiful in house dust than D. pteronyssinus [5].
Pathophysiology
The largest numbers of mites are found in houses that are damp and inadequately heated [4]. Numbers vary seasonally, increasing in early summer to reach a maximum by early autumn. In the UK, numbers are low in winter and increase in spring, when temperature and relative humidity rise [6].
The main food of D. pteronyssinus is human skin scales [7]. Xerophylic moulds, especially Aspergillus penicilloides, are essential for the growth and survival of D. pteronyssinus. The moulds digest lipid in the scales which is toxic to the mites.
The major house-dust mite allergens (Der p1 and Der f1) are present in the faecal pellets.
Clinical features
The role of the house-dust mite in the pathogenesis of atopic eczema remains controversial [8, 9, 10, 11, 12, 13] (Chapter 41). The allergens of Euroglyphus maynei are thought to play a role in the sensitization and induction of clinical symptoms of atopic eczema [14, 15] The mites that most frequently induce atopic eczema are D. pteronyssinus and D. farinae [16].
Several studies have indicated that, in many individuals, the condition can be improved by techniques designed to reduce exposure to house-dust mite allergen [17, 18, 19, 20, 21, 22], although the benefits on clinical status appear to be greater in children than in adults [14], and it is not possible to predict which patients will benefit. One study demonstrated that the houses of patients with moderate to severe atopic eczema had more house-dust mites than controls [23]. A recent critically appraised article concluded that there is unsufficient evidence to support house-dust mite reduction in the management of atopic eczema [24]. A systematic Cochrane review is in process [25].
Management
Measures employed to reduce the house-dust mite allergen load include regular vacuum cleaning of carpets, or their removal, bedding covers made of material such as microporous Goretex®, and the use of acaricides, including benzyl benzoate and permethrin [26].
Pyemotes mites
Introduction and general description
Pyemotes mites are all primarily parasites of insects or their larvae. They only affect humans when the latter come into contact with the food of their natural hosts.
The mite P. tritici preys on the larvae of many species of insect, infesting grain, straw or hay, and stored foodstuffs. Another species, P. ventricosus, preys on the larvae of wood-boring beetles, including the common furniture beetle Anobium punctatum.
Epidemiology
Pyemotes mites have been responsible for attacks of dermatitis in those shovelling grain or coming into contact with infested straw [1] and husk rice [2]. The dermatitis has been referred to by a number of terms, including ‘barley itch’, ‘grain-shovellers’ itch’, ‘grain itch’, ‘straw itch’, ‘cotton-seed dermatitis’ and ‘acarodermatitis urticarioides’. Pyemotes dermatitis has been reported in shop workers coming into contact with wheat used for decorative purposes [3, 4, 5]. Dermatitis in workers in a food mixing shed at a piggery was attributed to P. herfsi [6], and P. zwoelferi was incriminated in dermatitis acquired by contact with a package of everlasting flowers [7]. Dermatitis in a fisherman handling crab pots made of cherry wood was probably caused by P. beckeri [8].
An outbreak of dermatitis in a small hospital in Queensland, Australia, was attributed to Pyemotes mites originating in an adjacent grain storage facility [9]. Rodriguez Casado et al. described a Pyemotes dermatitis outbreak associated with A. punctatum-infested wood desks in a school [10]. Recently, an outbreak of P. ventricosus (Figure 34.39) associated with A. punctatum was reported in south-eastern France [11].

Figure 34.39 Pyemotes ventricosus.
Since the beginning of the 20th century, Pyemotes spp. have been recognized to cause dermatitis. In previously recorded outbreaks, ectoparasites of insect larvae feeding on plants were responsible for dermatitis in workers exposed to agricultural products [1, 8, 9]. Dermatitis caused by Pyemotes spp. is very rarely reported and has been associated with home interior infestations of P. ventricosus associated with A. punctatum [11].
Clinical features [12, 13]
The lesions are erythematous pruritic macules or urticated papules surmounted by vesicles; occasionally they may be bullous. These lesions are sometimes associated with a specific linear erythematous macular tract, called the ‘comet sign’ [11, 14, 15] (Figure 34.40). This sign might represent the onset of specific lymphangitis but usually it is not. Whether this sign is specific to Pyemotes spp. or P. ventricosus remains unknown [11]. The lesions are often very numerous, their distribution are on covered places of the body and depend upon the mode of exposure. In grain handlers, they are usually on the forearms and neck, but they may be profuse around the waist and in the groin area. If the source of the mites is removed, the eruption is self-limiting and should resolve in 1–3 weeks. Systemic signs are rare but may include fever, chills, malaise and diarrhoea [4].
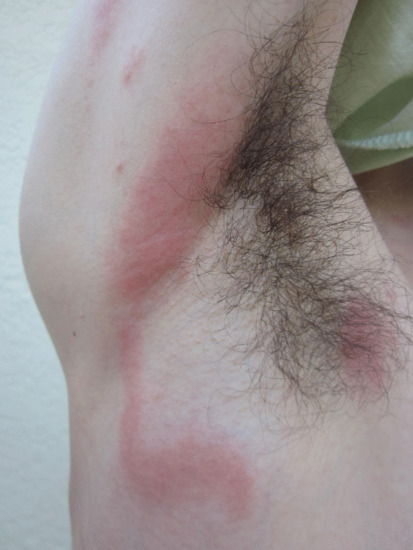
Figure 34.40 The ‘comet sign’.
Management
Treatment is symptomatic with topical corticosteroids and antihistamines and resolution depends on the elimination of the source of the infesting mites [2]. To eliminate Pyemotes mites, infested areas may be treated with acaricides and/or the removal of infested furniture.
Mites, Tydeidae: cutaneous reactions to
Dermatitis in eight woodworkers in Perugia, Italy, was attributed to contact with Pronematus davisi mites on wood imported from North America [1]. This mite has a worldwide distribution, and is widespread in North America, where it usually lives under bark.
Mites, plant: cutaneous reactions to
Some mites of the family Tetranychidae (‘spider mites’) cause cutaneous irritation or urtication in humans [1, 2, 3, 4]. These mites are phytophagous and occur on every type of crop and ornamental plant [5]. The name ‘spider mites’ is derived from the silk webbing they produce from palpal glands.
Mites, Cheyletiella: cutaneous reactions to
Introduction and general description
Species of Cheyletiella mites are non-burrowing, obligatory parasites of certain mammals, predominantly dogs, cats and rabbits. The entire life cycle is completed on the host. Each egg is attached to a hair shaft by means of a fine thread, which is woven around it into a cocoon-like structure by the female mite. The adult mite develops via a larval and two nymphal stages. Adult mites move rapidly over the skin surface in pseudotunnels in keratinous debris. They use their hook-like palpi to attach themselves to the host while feeding on tissue fluids [1, 2].
Epidemiology
Cheyletiella mites were first reported as attacking humans by Lomholt [3] of Copenhagen, and in 1938 a case was reported from England [4]. It gradually became apparent that Cheyletiella infestation of dogs, cats and rabbits was common in most European countries, in the USA [5], in Canada [6] and in Australasia [7, 8]. The distribution of these mites is likely to be worldwide. Many earlier reports incorrectly identified the species as C. parasitivorax when it was probably C. yasguri. It is now clear that C. parasitivorax is predominantly a parasite of rabbits, C. yasguri of dogs and C. blakei of cats [9, 10]. The three species are morphologically very similar, but distinguishable by the shape of a special sensory organ on the dorsal surface of genu I [10, 11, 12].
It is not clear from the limited information available whether the incidence of these mites is increasing, or whether infestation is becoming more frequently recognized. An investigation in the Netherlands [13] of 41 households in which two or more cats were kept showed Cheyletiella infestation of the animals in 27, with 20% of the human contacts having skin lesions. Any age, breed or sex of animal may be affected. In dogs, cheyletiellosis is particularly common in boxers.
Most affected animals are asymptomatic, but some may suffer from pruritus. The most obvious sign of infestation is excessive dandruff, especially on the back, which is often known as ‘walking dandruff’ [14] or ‘mobile dandruff’ by veterinary dermatologists.
Clinical features in humans [15, 16, 17]
The typical clinical picture is of large numbers of intensely itchy papules (Figure 34.41). Surmounting the papules there may be tiny vesicles, and older lesions may show small areas of necrosis. Bullous lesions may occur [18, 19]. The distribution of lesions corresponds to areas of contact with an infested animal, the abdomen and thighs being frequently involved as a result of an animal sitting on its owner's lap. The chest and arms may also be affected from carrying the animal. Dobrosavljevic et al. [20] reported a woman whose skin lesions were accompanied by eosinophilia, ‘increased immune complexes’ and rheumatological symptoms.
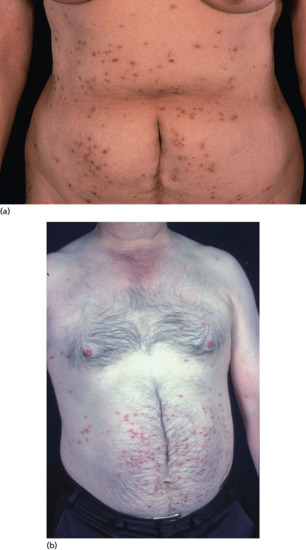
Figure 34.41 (a, b) Abdominal lesions in cheyletiellosis.
In a case with an extensive eruption, intradermal skin testing with an extract of Cheyletiella mites produced both immediate and delayed hypersensitivity responses [21].
Investigations [22]
The diagnosis may be confirmed by examination of combings from the animal's coat for the presence of mites. The suspect animal should be placed on a sheet of black paper, and the coat, particularly along the back, vigorously combed, preferably with a fine-toothed comb. The debris collected can then be examined microscopically (Figure 34.42).
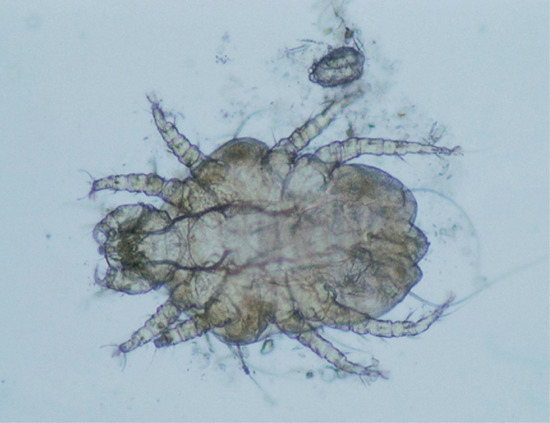
Figure 34.42 Cheyletiella yasguri.
Management
The affected animal should be treated by a veterinary practitioner, using antiectoparasitic shampoos and dips. With reports of mites occasionally being found off the host animal, a thorough cleaning of the pets’ sleeping area would be wise to prevent possible reinfection. Human skin lesions may be treated with a topical antipruritic. Once an animal has been treated effectively, there will be no further lesions on its owner within 3 weeks [23].
Harvest mites
Introduction and general description
Harvest mites belong to the family Trombiculidae. More than 1200 species of trombiculids have been described, and many may attack human beings or livestock [1].
Harvest mites are parasitic as larvae, but free living as nymphs and adults. The larvae may cause troublesome dermatitis (trombidiosis; scrub itch), and some are important vectors of rickettsial disease. They have many common names throughout the world, for example orange tawny (Ireland), chigger or red bug (USA).
The eggs are laid in soil. The six-legged larvae which emerge climb onto low vegetation to wait for suitable vertebrate hosts. On the host, the larvae move to areas where the skin is thin, such as the ears, axillae, groins and genitalia. There they pierce the skin with their cheliceral claws and inject saliva, which has cytolytic properties, into the epidermis [2]. This action forms a tube-like canal (stylosome) through which the mites feed on tissue fluids and cell debris. Once engorged, they fall to the ground and develop into eight-legged adults via a nymphal stage. Nymphs and adults feed on vegetable debris and the eggs of insects and other arthropods.
Epidemiology
Neotrombicula autumnalis, the European harvest mite, is widely distributed throughout Europe. In the UK [3], the larval mites are most numerous from May to October, with a peak in September. The most favoured natural host is the rabbit. Neotrombicula autumnalis is not known to transmit disease.
Eutrombicula alfreddugesi and E. splendens are the most common chiggers attacking humans in the USA, and E. batatas is an important dermatitis-producing species in South America.
In South-East Asia, Australia and the Pacific Islands, trombidiosis is commonly caused by E. wichmanni, and species of Odontacarus and Schoengastia [4, 5].
Species of Leptotrombidium including L. akamushi, L. pallidum and L. deliense are important vectors of scrub typhus (tsutsugamushi disease), caused by Orientia tsutsugamushi [6]. L. akamushi has a wide distribution, ranging from Japan and China southwards through South-East Asia to Indonesia and eastwards throughout the Philippines to New Guinea. Leptotrombidium deliense occurs in China, the Indian subcontinent, Malaya, Indonesia, the Philippines, New Guinea and Australia. The natural hosts of L. akamushi and L. deliense are rodents and insectivores. Leptotrombidium subquadratum has been reported as a cause of pruritus and dermatitis in dogs and humans in South Africa [7].
Clinical features [4, 8, 9]
Humans are infested while working in or walking through grass or low vegetation. The response to the bites of harvest mites appears to be determined by the irritant effect of the mites’ saliva and an acquired hypersensitivity to salivary antigens. Within a few hours, erythematous macules appear at the sites of the bites, and these gradually develop into extremely itchy papules or papulovesicles.
The distribution of lesions is determined by the preference of mites for thin skin, and the clothing of the host. Lesions commonly occur around the feet and ankles, the groins and genitalia, the axillae, the wrists and antecubital fossae, and areas constricted by clothing, such as the waistline. In heavy infestations, the whole body may be covered in lesions.
Chigger bites on the penis in children are responsible for a seasonal acute hypersensitivity reaction in the USA known as the ‘summer penile syndrome’ [10].
Trombiculid mite bites have provided evidence to implicate a suspect in a murder investigation [11].
Management [12, 13]
Treatment of trombiculid mite bites is aimed at relief of the symptoms. Topical anaesthetics, topical antipruritics, topical or intralesional corticosteroid, oral antihistamines or counterirritants may be used.
Preventive methods include avoidance of high-risk areas and the use of insect repellent on both clothing and skin.
Bird, rodent and reptile mites (Gamasida)
Introduction and general description
Family Dermanyssidae [1, 2]
Dermanyssid mites are haematophagous parasites of birds and mammals. Dermanyssus gallinae (chicken mite) (Figure 34.43), the red poultry mite, is a common parasite of domestic and wild birds. Poultry keepers, veterinary practitioners and others in direct contact with birds are sometimes attacked. Other dermanyssid mites responsible for dermatitis include D. hirundinis and D. americanus. Avian mites greatly increase during the hot season. They leave feathers or nests to walk away. In this case, they may enter buildings via windows, ventilation grills or air conditioners, causing skin lesions on the occupants who observe hundreds of mites on the edges of windows or on furniture [3, 4, 5, 6, 7, 8]. Lucky et al. [9] reported itchy papular lesions related to contact with pet gerbils infested with D. gallinae and Ornythonyssus sylviarum, and reviewed other reported cases of avian mite bites. Dermanyssus gallinae may be a public health problem because of its potential role as a vector of disease (zoonosis): Chlamydia psittaci, Erysipelotrix rhusiopathiae, Escherichia coli and Salmonella, Shigella and Staphylococcus species have been isolated from the blood of mites, and it has been linked to cases of spirochaetosis, chicken pox, Newcastle disease, typhoid fever, fowl cholera and equine encephalitis, with transmission occurring between birds. No cases of transmission to humans have yet been reported [10, 11].

Figure 34.43 Dermanyssus gallinae: comparison in size with the head of a match.
Liponyssoides sanguineus, the house-mouse mite, is an ectoparasite of small rodents. It is of medical importance because it is the vector of Rickettsia akari, the agent causing rickettsial pox.
Family Macronyssidae [1]
Members of the Macronyssidae family are haematophagous ectoparasites of birds, mammals and reptiles.
Ornithonyssus sylviarum (the northern fowl mite) and O. bursa (the tropical fowl mite) are pests of domestic and wild birds, and occasionally attack humans [12, 13, 14]. Ornithonyssus bacoti, although known as the tropical rat mite, is cosmopolitan, occurring in both tropical and temperate areas of the world. There are a number of reports of its effects on humans in close proximity to infested rats, mice, hamsters and gerbils [15, 16, 17, 18, 19, 20, 21].
Ophionyssus natricis, a snake mite, caused skin lesions in a family owning a pet python [22].
Clinical features [2, 17, 23, 24]
The clinical effects vary according to the route and severity of infestation and the degree of the host's response. Most commonly, there is a profuse eruption of small intensely itchy weals or papules, sometimes grouped, and often asymmetrical. The lesions may have a central punctum, and vesicles occasionally occur in the centre of the papules, especially in children. Because of the intense pruritus, excoriations are common, and secondary infection may occur. Upon close inspection, the mite may be identified as a small red dot in the centre of the lesion.
There is no characteristic distribution, as this is determined by the situation in which the bites are acquired. Those handling infested poultry tend to have lesions on the hands and forearms, whereas persons attacked by mites in bedding have more extensive bites. Occasionally, lesions are grouped adjacent to areas of tight clothing around the waistline. Rossiter [25] reported otitis externa associated with D. gallinae in two poultry catchers.
Investigations
In heavy infestations, the causative mites are often noticed by those affected, and any specimens obtained should be sent for identification to an entomologist familiar with Acari. When mite infestation is suspected, but no specimens are available, it may be necessary to visit the patient's home or workplace to determine the source of the problem.
Management
Avian mite dermatitis may be treated topically with corticosteroids, and the itching may subside with oral antihistamines [3, 8, 9, 14, 24]. The use of topical acaricides is controversial, because the mite does not live or reproduce on humans, but despite this, some authors recommend applying 1–5% topical permethrin [26].
Follicle Mites (Demodicidae)
Demodex folliculorum (Simon), the follicle mite, is an obligate parasite of the human pilosebaceous follicle. It was first discovered in cerumen by the anatomist Jakob Henle in 1841, but it was the dermatologist Gustav Simon who provided the first complete description of the parasite, under the name Acarus folliculorum, in 1842 [1, 2]. The generic name Demodex was created for it in 1843 by the zoologist Richard Owen.
Introduction and general description [1, 3]
Demodex folliculorum measures 0.3–0.4 mm in length, and has an elongated striated abdomen, giving it a worm-like appearance (Figure 34.44). A morphologically distinct species, D. brevis Akbulatova, has been recognized [4]. Demodex folliculorum occupies the hair follicle, and the smaller D. brevis the sebaceous and meibomian glands.
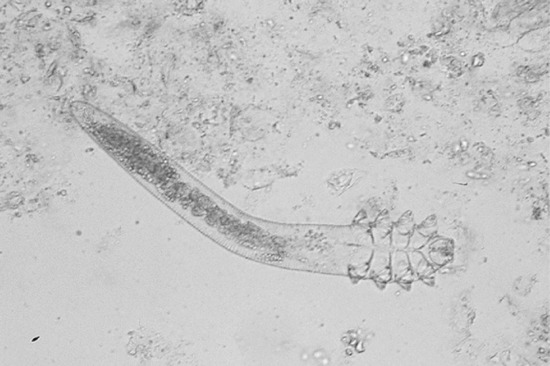
Figure 34.44 Demodex folliculorum, the follicle mite.
The lifespan of D. folliculorum is thought to be approximately 2 weeks. The heart-shaped eggs hatch to produce hexapod larvae, and the eight-legged adults develop via two nymphal stages.
Follicle mites show a predilection for areas of high sebum production [5] and they have been shown to contain lipase [6]. They are most numerous on the forehead, cheeks, nose and nasolabial folds, but they are also found on the scalp, in the external ear, in eyelash follicles and meibomian glands, and on the upper chest and nipples. They have also been discovered on the penis, mons veneris, buttocks and in ectopic sebaceous glands in the buccal mucosa [7]. Demodex folliculorum assumes a head-down position in the follicle, often with the tip of the abdomen protruding from the follicular orifice. Follicle mites are quite motile, and migrate from follicle to follicle. Most infested follicles contain two to six mites, but occasionally they are much more numerous. The prevalence of both D. folliculorum and D. brevis increases with age [8, 9] and it is likely that with adequate sampling techniques mites could be discovered in some follicles in the entire adult population. The skin surface biopsy technique is a useful method of assessing the population density and distribution of Demodex mites [10, 11]. New imaging techniques such as high-definition optical coherence tomography or confocal laser scanning microscopy may be helpful for the in vivo detection of Demodex mites. It enables fast and non-invasive recognition of Demodex mites and might become a useful tool in the diagnosis and treatment monitoring of Demodex-related skin diseases [12, 13, 14].
Pathophysiology
Demodex folliculorum has been implicated in the pathogenesis of a condition named pityriasis folliculorum [15, 16]. This was originally described as occurring predominantly in middle-aged or older women who rarely washed their faces, but used large quantities of make-up and cleansing creams. The lack of washing and use of facial cosmetics were considered to be aetiological factors, but a similar appearance has subsequently been described in women who washed their faces regularly [16, 17]. This dermatosis is characterized by diffuse facial erythema, follicular plugs and vesiculopustules, which impart a ‘nutmeg grater’ appearance to the skin. These mites populate facial areas such as the forehead, eyebrows, eyelids, chin and nose. Occasionally, they may be found on the perioral mucosa, ear canal, chest and other body regions. In reported cases, skin scrapings have contained unusually large numbers of Demodex, and the condition has responded to treatment with topical acaricides.
The question of the pathogenic role of Demodex in rosacea (Chapter 91) has always been debated [18, 19, 20]. Although studies employing skin surface biopsy have shown statistically significant increases in the density of Demodex mites in the facial skin of patients with rosacea compared with controls [10, 11, 21, 22], it is still not clear whether rosacea merely provides a suitable environment for multiplication of the mites, or whether the mites play a role in initiating the disease. Skin biopsies taken from patients with rosacea, following topical therapy with sulphur, failed to show any correlation between clinical improvement and reduction in mite population [23]. In large studies of the histopathology of rosacea [24, 25], Demodex was conspicuously absent from areas of inflammation in sections in which it was present elsewhere. A recent report using meta-analysis of previous studies seemed to show a statistically significant association between Demodex species infestation and the development of rosacea [26].
It has been suggested that a local delayed hypersensitivity response to Demodex antigens might be partly responsible for the inflammatory component of rosacea [24], and the observation that most T cells in the granulomatous infiltrate surrounding extrafollicular Demodex are helper inducer T cells [27] lends support to the hypothesis that the pathogenesis of rosacea involves a cell-mediated immune response. A report has also noted that bacterial antigens associated with Bacillus oleronius, isolated from D. folliculorum, have the potential to stimulate an inflammatory response in patients with papulopustular rosacea [28].
Although extrafollicular Demodex or fragments of Demodex may be found in the granulomatous lesions of rosacea [29], its role in their induction has not been established. The mite may simply be displaced because the hair follicles have been destroyed by the inflammatory process.
A view that the beneficial effects of metronidazole in rosacea might be mediated through an action against Demodex [30] was not supported by the finding that mites can survive high concentrations of this drug in vitro [31].
Clinical features
Rosacea-like eruptions in which large numbers of mites could be demonstrated and which responded to therapy with acaricides [15, 32, 33, 34, 35] or metronidazole [36] have been described. The effectiveness of acaricidal treatment can be evaluated by skin surface biopsy [37].
Demodex has been implicated in the causation of papular and papulopustular lesions in immunosuppressed individuals, including children with leukaemia [38, 39, 40, 41], a patient with tumour stage mycosis fungoides [42] and patients with HIV infection [43, 44, 45, 46].
Facial lesions attributed to Demodex have been described in immunocompetent children, in the form of a localized scaly patch [47], rosacea-like and pityriasis folliculorum-like lesions [48] and scalp lesions mimicking favus in another child [49].
A study of D. folliculorum density in perioral dermatitis suggested that increased mite density was a secondary phenomenon related to topical steroids, and also showed that mite density increased significantly in relation to the length of treatment [50].
Demodex is present in eyelash follicles, and has been implicated in the pathogenesis of blepharitis in some patients, although its importance as a cause is disputed [51]. Oral ivermectin may be useful as a complement in the treatment of D. folliculorum infestation with ocular manifestation [52, 53]. The value of topical ivermectin should be confirmed in a large real life population.
CLASS CHILOPODA (CENTIPEDES) AND DIPLOPODA (MILLIPEDES)
Species within the classes Chilopoda and Diplopoda are classified in the mandibulate subphylum of the phylum Arthropoda.
Centipedes
Introduction and general description
Chilopoda is a class with approximately 2500 known species of chilopods. The common chilopod is the centipede, which is terrestrial, nocturnally active and carnivorous against other arthropods or worms. Some of the giant species also feed on small mice and birds. Centipedes are elongated arthropods, with bodies composed of many segments, each bearing one pair of legs. The first pair of legs is modified, and provided with powerful hollow claws, which are used to grip prey and inject venom from poison glands in the basal segments of the legs (Figure 34.45).

Figure 34.45 Scolopendra cingulata Latreille 1829, the most common scolopendromorph species in the Mediterranean area.
(Courtesy of Dr Botterman and Dr Melhem, Nice, France.)
Clinical features
The claws of smaller species of centipedes are unable to penetrate human skin, but some tropical and subtropical species, principally members of the orders Scutigeromorpha and the giant Scolopendromorpha, can inflict painful ‘bites’ [1, 2, 3, 4, 5]. The bites cause local pain, erythema and oedema, which may persist for several hours. The bites may sometimes lead to necrosis or a delayed hypersensitive reaction [6]. Systemic symptoms include nausea, dizziness and pyrexia. There are rare reports of acute myocardial infarction following a centipede bite [7, 8] and even human deaths [8, 9, 10]. Secondary infection is a major complicating factor of the envenomation.
Management
The site should be washed with soap and water to reduce the risk of wound contamination. Cold compresses and analgesia will help with pain control [11].
Millipedes
Introduction and general description
Diplopoda is a class with approximately 10 000 known species of diplopods. The common diplopod is the millipede which is terrestrial on the forest floor. Millipedes also have multisegmented bodies, and most segments bear two pairs of legs. They feed mainly on decaying vegetable matter, and are generally regarded as harmless, but some large tropical species can cause injury to humans when acting defensively. The injurious effects of the defensive secretions of the giant Spirobolida millipedes of tropical and subtropical zones are well known to the indigenous populations of these areas.
Millipedes have numerous ‘repugnatorial’ glands distributed along the body segments, which provide a chemical defence system, and it is the corrosive secretions of these glands which may cause burns or allergy on the skin. In the majority of species, these secretions ooze out and form droplets around the foramina of the glands, but a few species are capable of squirting the fluid for some distance [12]. Millipede secretions contain benzoquinones and hydroquinones.
Clinical features [1, 12, 13, 14, 15]
Children often try to pick up millipedes, and are therefore at most risk of burns from the corrosive defensive secretions. If millipede secretions enter the eye, they produce a severe irritant conjunctivitis. Contact with the skin produces a local burning sensation and a yellowish brown stain, which gradually darkens to deep mahogany or purple-brown [16]. This colour is produced by oxidation of the quinones in the secretions. The lesions blister within a day or two, but in the absence of secondary infection, will heal and desquamate in 10–14 days. The discoloration may persist for months [17]. In dark-skinned individuals, persistent hypopigmentation is a common sequel.
Management [12]
Skin lesions should be washed with copious amounts of water to remove any remaining secretions, and the area cleaned with alcohol (a solvent of benzoquinones) if available. Blisters should be treated with a topical antiseptic. Ocular injuries should be dealt with by an ophthalmologist, because severe envenomation can result in blindness [18].
References
Skin disease due to arthropods
- Alexander JO'D. General considerations. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:3–9.
- Bagnall B, Rook A. Arthropods and the skin. In: Rook A, ed. Recent Advances in Dermatology, Vol. 4. Edinburgh: Churchill Livingstone, 1977:59–90.
- Stawiski MA. Insect bites and stings. Emerg Med Clin North Am 1985;3:785–808.
- Walton GS. Cutaneous responses to arthropods. In: Verbov J, ed. New Clinical Applications in Dermatology. Talking Points in Dermatology III. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1988:103–24.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol Chic Ill 2013;149:372–3.
- Mellanby K. Man's reaction to mosquito bites. Nature 1946;158:554.
- Resneck JS Jr, Van Beek M, Furmanski L, et al. Etiology of pruritic papular eruption with HIV infection in Uganda. J Am Med Assoc 2004;292:2614–21.
- Barzilai A, Shpiro D, Goldberg I, et al. Insect bite-like reaction in patients with hematologic malignant neoplasms. Arch Dermatol 1999;135:1503–7.
- Bairey O, Goldschmidt N, Ruchlemer R, et al. Insect-bite-like reaction in patients with chronic lymphocytic leukemia: a study from the Israeli Chronic Lymphocytic Leukemia Study Group. Eur J Haematol 2012;89:491–6.
- Evans AV, Darvay A, Jenkins IH, et al. Compartment syndrome following an insect bite. Br J Dermatol 2001;144:636–8.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol 1983;22:75–91.
- Marshall AG. The Ecology of Ectoparasitic Insects. London: Academic Press, 1981.
- Khan AA, Maibach HI, Strauss WG, et al. Increased attractiveness of man to mosquitoes with induced eccrine sweating. Nature 1969;223:859–60.
- Maibach HI, Khan AA, Strauss WG, et al. Attraction of anhidrotic subjects to mosquitoes. Arch Dermatol 1966;94:215–17.
- Keystone JS. Of bites and body odour. Lancet 1996;347:1423.
- Lu T, Qiu YT, Wang G, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol 2007;17:1533–44.
- Smallegange RC, van Gemert G-J, van de Vegte-Bolmer M, et al. Malaria infected mosquitoes express enhanced attraction to human odor. PLOS One 2013;8:e63602.
- Hamilton JG, Ramsoondar TM. Attraction of Lutzomyia longipalpis to human skin odours. Med Vet Entomol 1994;8:375–80.
- Lindsay S, Ansell J, Selman C, et al. Effect of pregnancy on exposure to malaria mosquitoes. Lancet 2000;355:1972.
- Martinez Espinosa F, Alecrim WD, Daniel-Ribeiro CT. Attraction of mosquitoes to pregnant women. Lancet 2000;356:685.
- Greub G, Fellay J, Telenti A. HIV lipoatrophy and mosquito bites. Clin Infect Dis 2002;34:288–9.
- Shirai O, Tsuda T, Kitagawa S, et al. Alcohol ingestion stimulates mosquito attraction. J Am Mosq Control Assoc 2002;18:91–6.
- Weedon D. Skin Pathology, 2nd edn. Edinburgh: Churchill Livingstone, 2005:738–49.
- Calnan CD. Persistent insect bites. Trans St Johns Hosp Dermatol Soc 1969;55:198–201.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998;38:877–95;quiz 896–7.
- Battistella M, Bourrat E, Fardet L, et al. Sweet-like reaction due to arthropod bites: a histopathologic pitfall. Am J Dermatopathol 2012;34:442–5.
- Restano L, Cavalli R, Colonna C, et al. Eruptive pseudoangiomatosis caused by an insect bite. J Am Acad Dermatol 2005;52:174–5.
- Ban M, Ichiki Y, Kitajima Y. An outbreak of eruptive pseudoangiomatosis-like lesions due to mosquito bites: erythema punctatum Higuchi. Dermatol Basel Switz 2004;208:356–9.
- Busvine JR. Insects and Hygiene. London: Chapman and Hall, 1980.
- Burns DA. The investigation and management of arthropod bite reactions acquired in the home. Clin Exp Dermatol 1987;12:114–20.
- Hewitt M, Walton GS, Waterhouse M. Pet animal infestations and human skin lesions. Br J Dermatol 1971;85:215–25.
- Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology, 6th edn. Philadelphia: WB Saunders, 2000.
- Hosie G. Observations on the occurrence of Ceratophyllus gallinae around new housing estates in the west of Scotland. In: Traub R, Starcke H, eds. Fleas. Rotterdam: AA Balkema, 1980:415–20.
- Hewitt M, Barrow GI, Miller DC, et al. Mites in the personal environment and their role in skin disorders. Br J Dermatol 1973;89:401–9.
- Brown M, Hebert AA. Insect repellents: an overview. J Am Acad Dermatol 1997;36:243–9.
- Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med 2002;347:13–18.
- Elston DM. Prevention of arthropod-related disease. J Am Acad Dermatol 2004;51:947–54.
- Debboun M, Frances SP, Strickman DA. Insect Repellents: Principles, Methods and Uses. Boca Raton: CRC Press, 2006.
- Steen CJ, Carbonaro PA, Schwartz RA. Arthropods in dermatology. J Am Acad Dermatol 2004;50:819–42;quiz 842–4.
- Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev 2012;10:CD008838.
- Bernardeschi C, Le Cleach L, Delaunay P, et al. Bed bug infestation. BMJ 2013;346:f138.
- Chosidow O. Scabies and pediculosis. Lancet 2000;355:819–26.
- Del Giudice P, Blanc-Amrane V, Bahadoran P, et al. Pyemotes ventricosus dermatitis, southeastern France. Emerg Infect Dis 2008;14:1759–61.
Mosquitoes, gnats, midges and flies (Diptera)
- Kettle DS. Medical and Veterinary Entomology, 2nd edn, 1995.
- Lane RP, Crosskey RW, eds. Medical Insects and Arachnids. London: Chapman and Hall, 2012.
- Charrel RN, Bichaud L, de Lamballerie X. Emergence of Toscana virus in the Mediterranean area. World J Virol 2012;1:135–41.
- Laird M, ed. Blackflies. London: Academic Press, 1981.
- Healing TD, Dlugolecka MD, Morgan DTJ, et al. The Blandford fly. Commun Dis Rep 1 July 1988.
- Inskip H, Campbell L, Godfrey K, et al. A survey of the prevalence of biting by the Blandford fly during 1993. Br J Dermatol 1996;134:696–9.
- Welton JS, Bass JAB, Ladle M, et al. Distribution of oviposition sites and, characteristics of egg development in the “Blandford fly” Simulium posticatum (Diptera: Simuliidae). J Appl Ecol 1987;24:865–79.
- Stuart AE, Evans A, Brooks C, et al. The biting midge of the West Highlands: fifty years of research. Scott Med J 1996;41:143–6.
- McKendrick DR. Anthrax and its transmission to humans. Cent Afr J Med 1980;26:126–9.
- Alexander JO'D. Reactions to Dipterous biting flies. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:115–33.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol 1982;62:307–11.
- Surasombatpattana P, Ekchariyawat P, Hamel R, et al. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J Invest Dermatol 2013;134:281–4.
- Allen JR. Mosquitoes and other biting flies. In: Parish CL, Nutting WB, Schwartzman RM, eds. Cutaneous Infestations of Man and Animal. New York: Praeger, 1983:344–55.
- Melanby K. Man's reaction to mosquito bites. Nature 1946;158:554.
- Oka K, Ohtaki N. Clinical observations of mosquito bite reactions in man: a survey of the relationship between age and bite reaction. J Dermatol 1989;16:212–19.
- Hudson A, Bowman L, Orr CW. Effects of absence of saliva on blood feeding by mosquitoes. Science 1960;131:1730–1.
- Galindo PA, Gómez E, Borja J, et al. Mosquito bite hypersensitivity. Allergol Immunopathol (Madr) 1998;26:251–4.
- Gaig P, García-Ortega P, Enrique E, et al. Serum sickness-like syndrome due to mosquito bite. J Investig Allergol Clin Immunol 1999;9:190–2.
- Walker GB, Harrison PV. Seasonal bullous eruption due to mosquitoes. Clin Exp Dermatol 1985;10:127–32.
- Lidén S, Bäck O, Tärnvik A. Chronic lymphatic leukaemia, malignant melanomas and mosquito hypersensitivity. Acta Derm Venereol 1977;57:81–92.
- Weed RI. Exagerrated delayed hypersensitivity to mosquito bites in chronic lymphocytic leukaemia. Blood 1965;26:257–68.
- Davis MD, Perniciaro C, Dahl PR, et al. Exaggerated arthropod-bite lesions in patients with chronic lymphocytic leukemia: a clinical, histopathologic, and immunopathologic study of eight patients. J Am Acad Dermatol 1998;39:27–35.
- Rongioletti F, Rebora A. Follicular mucinosis in exaggerated arthropod-bite reactions of patients with chronic lymphocytic leukemia. J Am Acad Dermatol 1999;41:500.
- Benmously R, Hammami H, Rouatbi M, et al. Insect bite-like reaction associated with the relapse of non-Hodgkin B cell-lymphoma. Eur J Dermatol 2009;19:406–7.
- Barzilai A, Shpiro D, Goldberg I, et al. Insect bite-like reaction in patients with hematologic malignant neoplasms. Arch Dermatol 1999;135:1503–7.
- Diven DG, Newton RC, Ramsey KM. Heightened cutaneous reactions to mosquito bites in patients with acquired immunodeficiency syndrome receiving zidovudine. Arch Intern Med 1988;148:2296.
- Smith KJ, Skelton HG 3rd, Vogel P, et al. Exaggerated insect bite reactions in patients positive for HIV. Military Medical Consortium for the Advancement of Retroviral Research. J Am Acad Dermatol 1993;29:269–72.
- Resneck JS Jr, Van Beek M, Furmanski L, et al. Etiology of pruritic papular eruption with HIV infection in Uganda. JAMA 2004;292:2614–21.
- Penneys NS, Nayar JK, Bernstein H, et al. Chronic pruritic eruption in patients with acquired immunodeficiency syndrome associated with increased antibody titers to mosquito salivary gland antigens. J Am Acad Dermatol 1989;21:421–5.
- Hidano A, Kawakami M, Yago A. Hypersensitivity to mosquito bite and malignant histocytosis. Jpn J Exp Med 1982;52:303–6.
- Mohri S, Kawashima Y, Uchigata Y, et al. A case of mosquito hypersensitivity terminating as malignant histiocytosis. J Dermatol 1982;9:437–43.
- Ohsawa T, Morimura T, Hagari Y, et al. A case of exaggerated mosquito-bite hypersensitivity with Epstein-Barr virus-positive inflammatory cells in the bite lesion. Acta Derm Venereol 2001;81:360–3.
- Ishihara S, Yabuta R, Tokura Y, et al. Hypersensitivity to mosquito bites is not an allergic disease, but an Epstein-Barr virus-associated lymphoproliferative disease. Int J Hematol 2000;72:223–8.
- Tokura Y, Ishihara S, Tagawa S, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol 2001;45:569–78.
- Asada H. Hypersensitivity to mosquito bites: a unique pathogenic mechanism linking Epstein-Barr virus infection, allergy and oncogenesis. J Dermatol Sci 2007;45:153–60.
- Roh EJ, Chung EH, Chang YP, et al. A case of hypersensitivity to mosquito bite associated with Epstein-Barr viral infection and natural killer cell lymphocytosis. J Korean Med Sci 2010;25:321–3.
- Borah S, Goswami S, Agarwal M, et al. Clinical and histopathological study of Simulium (blackfly) dermatitis from North-Eastern India – a report. Int J Dermatol 2012;51:63–6.
- Gudgel EF, Grauer FH. Acute and chronic reactions to black fly bites; Simulium fly. AMA Arch Derm Syphilol 1954;70:609–15.
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol 1981;117:785–7.
- Galindo PA, Feo F, Gómez E, et al. Hypersensitivity to chironomid larvae. J Investig Allergol Clin Immunol 1998;8:219–25.
- Cabrerizo Ballesteros S, de Barrio M, Baeza ML, et al. Allergy to chironomid larvae (red migde larvae) in non professional handlers of fish food. J Investig Allergol Clin Immunol 2006;16:63–8.
- Galindo PA, Melero R, García R, et al. Contact urticaria from chironomids. Contact Dermatitis 1996;34:297.
- De Jaegher C, Goossens A. Protein contact dermatitis from midge larvae (Chironomus thummi thummi). Contact Dermatitis 1999;41:173.
- Seldén AI, Calo A, Mölleby G, et al. Chironomid midge sensitization in sewage workers: case study. Med Vet Entomol 2013;27:346–8.
- Quercia O, Emiliani F, Foschi FG, et al. The wasp-horsefly syndrome. Eur Ann Allergy Clin Immunol 2008;40:61–3.
- Restano L, Cavalli R, Colonna C, et al. Eruptive pseudoangiomatosis caused by an insect bite. J Am Acad Dermatol 2005;52:174–5.
- Ban M, Ichiki Y, Kitajima Y. An outbreak of eruptive pseudoangiomatosis-like lesions due to mosquito bites: erythema punctatum Higuchi. Dermatology (Basel) 2004;208:356–9.
- Oka K, Ohtaki N, Kasai S, et al. Two cases of eruptive pseudoangiomatosis induced by mosquito bites. J Dermatol 2012;39:301–5.
- Elston DM. Prevention of arthropod-related disease. J Am Acad Dermatol 2004;51:947–54.
- Torpy JM. JAMA patient page. Insect bites and stings. JAMA 2013;310:110.
- Management of simple insect bites: where's the evidence? Drug Ther Bull 2012;50:45–8.
- Karppinen A, Brummer-Korvenkontio H, Reunala T, et al. Rupatadine 10 mg in the treatment of immediate mosquito-bite allergy. J Eur Acad Dermatol Venereol 2012;26:919–22.
- Karppinen A, Brummer-Korvenkontio H, Petman L, et al. Levocetirizine for treatment of immediate and delayed mosquito bite reactions. Acta Derm Venereol 2006;86:329–31.
- PPAV Working Groups. Personal protection against biting insects and ticks. Parasite 2011;18:93–111.
Myasis
- Alexander JO'D. Cutaneous myiasis. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:87–113.
- Hall M, Wall R. Myiasis of humans and domestic animals. Adv Parasitol 1995;35:257–334.
- Kettle DS. Medical and Veterinary Entomology, 2nd edn. Wallingford: CAB International, 1995:268–91.
- Burns DA. Myiasis. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. Maarssen: Elsevier, 2006:251–8.
- Logan JC, Walkey M. A case of endemic cutaneous myiasis. Br J Dermatol 1964;76:218–22.
- Macias EG, Graham AJ, Green M, et al. Cutaneous myiasis in South Texas. N Engl J Med 1973;289:1239–41.
- Poindexter HA. Cutaneous myiasis. Arch Dermatol 1979;115:235.
- Schreiber MM, Schuckmell N, Sampsel J. Human myiasis. JAMA 1964;188:828–9.
- Günther S. Clinical and epidemiological aspects of the dermal Tumbu-fly-myiasis in Equatorial-Africa. Br J Dermatol 1971;85:226–31.
- Hasegawa M, Harada T, Kojima Y, et al. An imported case of furuncular myiasis due to Cordylobia anthropophaga which emerged in Japan. Br J Dermatol 2000;143:912–14.
- Lodi A, Bruscagin C, Gianni C, et al. Myiasis due to Cordylobia anthropophaga (Tumbu-fly). Int J Dermatol 1994;33:127–8.
- Laurence BR, Herman FG. Letter: Tumbu fly (Cordylobia) infection outside Africa. Trans R Soc Trop Med Hyg 1973;67:888.
- Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J 2006;23:236–7.
- Omar MS, Abdalla RE. Cutaneous myiasis caused by tumbu fly larvae, Cordylobia anthropophaga in southwestern Saudi Arabia. Trop Med Parasitol 1992;43:128–9.
- Baily GG, Moody AH. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga acquired in Europe. BMJ (Clin Res Ed) 1985;290:1473–4.
- Pampiglione S, Schiavon S, Fioravanti ML. Extensive furuncular myiasis due to Cordylobia rodhaini larvae. Br J Dermatol 1992;126:418–19.
- Ogbalu OK, Achufusi TGO, Orlu EE. Epidemiology of human furuncular myiasis of Cordylobia anthropophaga (Grunberg) in Nigeria. Int J Dermatol 2013;52:331–6.
- Alexis JB, Mittleman RE. An unusual case of Phormia regina myiasis of the scalp. Am J Clin Pathol 1988;90:734–7.
- Hall RD, Anderson PC, Clark DP. A case of human myiasis caused by Phormia regina (Diptera: Calliphoridae) in Missouri, USA. J Med Entomol 1986;23:578–9.
- Reames MK, Christensen C, Luce EA. The use of maggots in wound debridement. Ann Plast Surg 1988;21:388–91.
- Sherman RA. Wound myiasis in urban and suburban United States. Arch Intern Med 2000;160:2004–14.
- Sherman RA, Hall MJ, Thomas S. Medicinal maggots: an ancient remedy for some contemporary afflictions. Annu Rev Entomol 2000;45:55–81.
- Nigam Y, Bexfield A, Thomas S, et al. Maggot therapy: the science and implication for CAM Part I – History and bacterial resistance. Evid Based Complement Alternat Med 2006;3:223–7.
- Arbit E, Varon RE, Brem SS. Myiatic scalp and skull infection with diptera Sarcophaga: case report. Neurosurgery 1986;18:361–2.
- Delir S, Handjani F, Emad M, et al. Vulvar myiasis due to Wohlfahrtia magnifica. Clin Exp Dermatol 1999;24:279–80.
- Smith FD, Shaffer KL, Gasseling PA, et al. Furuncular myiasis caused by Wohlfahrtia vigil (Walker). First case reported in Nebraska. Arch Dermatol 1981;117:119–20.
- Goddard J. Human infestation with rodent botfly larvae: a new route of entry? South Med J 1997;90:254–5.
- Keth AC. Three incidents of human myiasis by rodent Cuterebra (Diptera: Cuterebridae) larvae in a localized region of western Pennsylvania. J Med Entomol 1999;36:831–2.
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261–3.
- Baird JK, Baird CR, Sabrosky CW. North American cuterebrid myiasis. Report of seventeen new infections of human beings and review of the disease. J Am Acad Dermatol 1989;21:763–72.
- Keech JP. Dermatobia hominis – in Belize. J R Army Med Corps 1981;127:131–3.
- Lane RP, Lowell CR, Griffiths WA, et al. Human cutaneous myiasis – a review and report of three cases due to Dermatobia hominis. Clin Exp Dermatol 1987;12:40–5.
- Jelinek T, Nothdurft HD, Rieder N, et al. Cutaneous myiasis: review of 13 cases in travelers returning from tropical countries. Int J Dermatol 1995;34:624–6.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol 1995;132:811–14.
- Gewirtzman A, Rabinovitz H. Botfly infestation (myiasis) masquerading as furunculosis. Cutis 1999;63:71–2.
- Veraldi S, Gorani A, Süss L, et al. Cutaneous myiasis caused by Dermatobia hominis. Pediatr Dermatol 1998;15:116–18.
- Maier H, Hönigsmann H. Furuncular myiasis caused by Dermatobia hominis, the human botfly. J Am Acad Dermatol 2004;50:S26–30.
- Omar MS, Das AB, Osman NI. External ophthalmomyiasis due to the sheep nostril botfly larva Oestrus ovis in Saudi Arabia. Ann Trop Med Parasitol 1988;82:221–3.
- Fekry AA, el Serougi AO, Ayoub SA. Oestrus ovis (sheep nasal fly) infesting the eyes and the nose of a camel keeper family. J Egypt Soc Parasitol 1997;27:493–6.
- Morgan RJ, Moss HB, Honska WL. Muiasis; Hypoderma myiasis, occurrence in Oklahoma. Arch Dermatol 1964;90:180–5.
- Kan B, Otranto D, Fossen K, et al. Dermal swellings and ocular injury after exposure to reindeer. N Engl J Med 2012;367:2456–7.
- Kan B, Asbakk K, Fossen K, et al. Reindeer warble fly-associated human myiasis, Scandinavia. Emerging Infect Dis 2013;19:830–2.
- Starr J, Pruett JH, Yunginger JW, et al. Myiasis due to Hypoderma lineatum infection mimicking the hypereosinophilic syndrome. Mayo Clin Proc 2000;75:755–9.
- Puente S, Otranto D, Panadero R, et al. First diagnosis of an imported human myiasis caused by Hypoderma sinense (Diptera: Oestridae), detected in a European traveler returning from India. J Travel Med 2010;17:419–23.
- Spigel GT. Opportunistic cutaneous myiasis. Arch Dermatol 1988;124:1014–15.
- Fernandes LF, Pimenta FC, Fernandes FF. First report of human myiasis in GoiáS state, Brazil: frequency of different types of myiasis, their various etiological agents, and associated factors. J Parasitol 2009;95:32–8.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med 2012;43:618–21.
- Möhrenschlager M, Mempel M, Weichenmeier I, et al. Scanning electron microscopy of Dermatobia hominis reveals cutaneous anchoring features. J Am Acad Dermatol 2007;57:716–18.
- McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol 2008;58:907–26;quiz 927–9.
- Fassler C, Lanco S, Denis A, et al. [Subcutaneous myiasis. A case report.] Arch Pediatr 2000;7:840–3.
- Mumcuoglu I, Akarsu GA, Balaban N, et al. Eristalis tenax as a cause of urinary myiasis. Scand J Infect Dis 2005;37:942–3.
- Dubois E, Durieux M, Franchimont MM, et al. [An unusual case in Belgium of intestinal myiasis due to Eristalis tenax.] Acta Clin Belg 2004;59:168–70.
- Aguilera A, Cid A, Regueiro BJ, et al. Intestinal myiasis caused by Eristalis tenax. J Clin Microbiol 1999;37:3082.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev 2012;25:79–105.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol 2007;143:123–4.
- Abraham LS, Azulay-Abulafia L, Aguiar D de P, et al. Dermoscopy features for the diagnosis of furuncular myiasis. An Bras Dermatol 2011;86:160–2.
- Bowry R, Cottingham RL. Use of ultrasound to aid management of late presentation of Dermatobia hominis larva infestation. J Accid Emerg Med 1997;14:177–8.
- Schechter E, Lazar J, Nix ME, et al. Identification of subcutaneous myiasis using bedside emergency physician performed ultrasound. J Emerg Med 2011;40:e1–3.
- Richter J, Schmitt M, Müller-Stöver I, et al. Sonographic detection of subcutaneous fly larvae in human myiasis. J Clin Ultrasound 2008;36:169–73.
- Ruch DM. Botfly myiasis. Arch Dermatol 1967;96:677–80.
- Sauder DN, Hall RP 3rd, Wurster CF. Dermal myiasis: the porcine lipid cure. Arch Dermatol 1981;117:681–2.
- Richards KA, Brieva J. Myiasis in a pregnant woman and an effective, sterile method of surgical extraction. Dermatol Surg 2000;26:955–7.
- Nunzi E, Rongioletti F, Rebora A. Removal of Dermatobia hominis larvae. Arch Dermatol 1986;122:140.
- Loong PT, Lui H, Buck HW. Cutaneous myiasis: a simple and effective technique for extraction of Dermatobia hominis larvae. Int J Dermatol 1992;31:657–9.
- Denion E, Dalens P-H, Couppié P, et al. External ophthalmomyiasis caused by Dermatobia hominis. A retrospective study of nine cases and a review of the literature. Acta Ophthalmol Scand 2004;82:576–84.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol 2005;44:981–8.
- Osorio J, Moncada L, Molano A, et al. Role of ivermectin in the treatment of severe orbital myiasis due to Cochliomyia hominivorax. Clin Infect Dis 2006;43:e57–9.
- Clyti E, Nacher M, Merrien L, et al. Myiasis owing to Dermatobia hominis in a HIV-infected subject: Treatment by topical ivermectin. Int J Dermatol 2007;46:52–4.
- Wakamatsu TH, Pierre-Filho PTP. Ophthalmomyiasis externa caused by Dermatobia hominis: a successful treatment with oral ivermectin. Eye (Lond) 2006;20:1088–90.
- Gealh WC, Ferreira GM, Farah GJ, et al. Treatment of oral myiasis caused by Cochliomyia hominivorax: two cases treated with ivermectin. Br J Oral Maxillofac Surg 2009;47:23–6.
Fleas
- Busvine JR. Insects and Hygiene. London: Chapman and Hall, 1980:245–56.
- Kettle DS. Medical and Veterinary Entomology, 2nd edn. Wallingford: CAB International, 1995:323–43.
- Stenseth NC, Atshabar BB, Begon M, et al. Plague: past, present, and future. PLOS Med 2008;5:e3.
- Bibikova VA. Contemporary views on the interrelationships between fleas and the pathogens of human and animal diseases. Annu Rev Entomol 1977;22:23–32.
- Raoult D, Mouffok N, Bitam I, et al. Plague: history and contemporary analysis. J Infect 2013;66:18–26.
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis 2010;14:e667–76.
- Pérez-Osorio CE, Zavala-Velázquez JE, Arias León JJ, et al. Rickettsia felis as emergent global threat for humans. Emerging Infect Dis 2008;14:1019–23.
- Flexman JP, Lavis NJ, Kay ID, et al. Bartonella henselae is a causative agent of cat scratch disease in Australia. J Infect 1995;31:241–5.
- Chomel BB, Kasten RW, Floyd-Hawkins K, et al. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 1996;34:1952–6.
- Rolain J-M, Franc M, Davoust B, et al. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerging Infect Dis 2003;9:338–42.
- Rolain J-M, Bourry O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerging Infect Dis 2005;11:1742–4.
- Foucault C, Barrau K, Brouqui P, et al. Bartonella quintana bacteremia among homeless people. Clin Infect Dis 2002;35:684–9.
- Jeanclaude D, Godmer P, Leveiller D, et al. Bartonella alsatica endocarditis in a French patient in close contact with rabbits. Clin Microbiol Infect 2009;15(Suppl. 2):110–11.
- Angelakis E, Lepidi H, Canel A, et al. Human case of Bartonella alsatica lymphadenitis. Emerging Infect Dis 2008;14:1951–3.
- Bond R, Riddle A, Mottram L, et al. Survey of flea infestation in dogs and cats in the United Kingdom during 2005. Vet Rec 2007;160:503–6.
- Nogueras MM, Pons I, Ortuño A, et al. Molecular detection of Rickettsia typhi in cats and fleas. PLOS One 2013;8:e71386.
- Wolff K. [Fleas of birds as facultative ectoparasites of man (author's transl.).] Schweiz Rundsch Med Prax 1975;64:1173–5.
- Chua EC, Goh KT. A flea-borne outbreak of dermatitis. Ann Acad Med Singap 1987;16:648–50.
- Rothenborg HW. Letter: of fleas and foxes. Arch Dermatol 1975;111:1215–16.
- Hunter KW Jr, Campbell AR, Sayles PC. Human infestation by cat fleas, Ctenocephalides felis (Siphonaptera: Pulicidae), from suburban raccoons. J Med Entomol 1979;16:547.
- Alexander JO'D. Flea bites and other diseases caused by fleas. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:159–71.
- Burns DA. The investigation and management of arthropod bite reactions acquired in the home. Clin Exp Dermatol 1987;12:114–20.
- Hosie G. Observations on the occurrence of Ceratophyllus gallinae around new housing estates in the West of Scotland. In: Traub R, Starcke H, eds. Fleas. Rotterdam: AA Balkema, 1980:415–20.
- Siak M, Burrows M. Flea control in cats: new concepts and the current armoury. J Feline Med Surg 2013;15:31–40.
- Rust MK. Advances in the control of Ctenocephalides felis (cat flea) on cats and dogs. Trends Parasitol 2005;21:232–6.
- Dryden M, Payne P, Lowe A, et al. Efficacy of a topically applied formulation of metaflumizone on cats against the adult cat flea, flea egg production and hatch, and adult flea emergence. Vet Parasitol 2007;150:263–7.
- Blagburn BL, Young DR, Moran C, et al. Effects of orally administered spinosad (Comfortis) in dogs on adult and immature stages of the cat flea (Ctenocephalides felis). Vet Parasitol 2010;168:312–17.
- Feldmeier H, Heukelbach J. Tungiasis. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Disease. Maarssen: Elsevier, 2006:233–41.
- Douglas-Jones AG, Llewelyn MB, Mills CM. Cutaneous infection with Tunga penetrans. Br J Dermatol 1995;133:125–7.
- Ibáñez-Bernal S, Velasco-Castrejón O. New records of human tungiasis in Mexico Siphonaptera: Tungidae). J Med Entomol 1996;33:988–9.
- Yotsu RR, Tamaki T, Ujiie M, et al. Imported tungiasis in a Japanese student returning from East Africa. J Dermatol 2011;38:185–9.
- Grupper M, Potasman I. Outbreak of tungiasis following a trip to Ethiopia. Travel Med Infect Dis 2012;10:220–3.
- Sanusi ID, Brown EB, Shepard TG, et al. Tungiasis: report of one case and review of the 14 reported cases in the United States. J Am Acad Dermatol 1989;20:941–4.
- Spradbery JP, Bromley J, Dixon R, et al. Tungiasis in Australia: an exotic disease threat. Med J Aust 1994;161:173.
- Wardhaugh AD, Norris JF. A case of imported tungiasis in Scotland initially mimicking verrucae vulgaris. Scott Med J 1994;39:146–7.
- Gelmetti C, Carrera C, Veraldi S. Tungiasis in a 3-year-old child. Pediatr Dermatol 2000;17:293–5.
- Fein H, Naseem S, Witte DP, et al. Tungiasis in North America: a report of 2 cases in internationally adopted children. J Pediatr 2001;139:744–6.
- Grunwald MH, Shai A, Mosovich B, et al. Tungiasis. Australas J Dermatol 2000;41:46–7.
- Smit FGAM. Siphonaptera. In: Smith KGV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973:327–8.
- Smith MD, Procop GW. Typical histologic features of Tunga penetrans in skin biopsies. Arch Pathol Lab Med 2002;126:714–16.
- Maco V, Maco VP, Tantalean ME, et al. Histopathological features of tungiasis in Peru. Am J Trop Med Hyg 2013;88:1212–16.
- Alexander JO'D. Tungiasis. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:171–6.
- Feldmeier H, Heukelbach J, Eisele M, et al. Bacterial superinfection in human tungiasis. Trop Med Int Health 2002;7:559–64.
- Vallarelli AFA, SouzaEM de. Disseminated tungiasis. An Bras Dermatol 2011;86:1027–8.
- Feldmeier H, Sentongo E, Krantz I. Tungiasis (sand flea disease): a parasitic disease with particular challenges for public health. Eur J Clin Microbiol Infect Dis 2013;32:19–26.
- Muehlen M, Heukelbach J, Wilcke T, et al. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil. II. Prevalence, parasite load and topographic distribution of lesions in the population of a traditional fishing village. Parasitol Res 2003;90:449–55.
- Bauer J, Forschner A, Garbe C, et al. Dermoscopy of tungiasis. Arch Dermatol 2004;140:761–3.
- Di Stefani A, Rudolph CM, Hofmann-Wellenhof R, et al. An additional dermoscopic feature of tungiasis. Arch Dermatol 2005;141:1045–6.
- Dunn R, Asher R, Bowling J. Dermoscopy: Ex vivo visualization of fleas head and bag of eggs confirms the diagnosis of Tungiasis. Australas J Dermatol 2012;53:120–2.
- Heukelbach J. Revision on tungiasis: treatment options and prevention. Exp Rev Anti Infect Ther 2006;4:151–7.
- Gibbs SS. The diagnosis and treatment of tungiasis. Br J Dermatol 2008;159:981.
- Feldmeier H, Kehr JD, Heukelbach J. A plant-based repellent protects against Tunga penetrans infestation and sand flea disease. Acta Trop 2006;99:126–36.
Bees, wasps and ants
- Schumacher MJ, Egen NB. Significance of Africanized bees for public health. A review. Arch Intern Med 1995;155:2038–43.
- Ferreira RS Jr, Almeida RAMB, Barraviera SRCS, et al. Historical perspective and human consequences of Africanized bee stings in the Americas. J Toxicol Environ Health B Crit Rev 2012;15:97–108.
- Rome Q, Dambrine L, Onate C, et al. Spread of the invasive hornet Vespa velutina Lepeletier, 1836, in Europe in 2012 (Hym., Vespidae). Bull Soc Entomol France 2013;118:21–2.
- James MT, Harwood RF, eds. Herms's Medical Entomology, 6th edn. London: Macmillan, 1976.
- Sutherland SK. Venomous Creatures of Australia. Melbourne: Oxford University Press, 1982.
- Trinca JC. Insect allergy in Australia: results of a five-year survey. Med J Aust 1964;2:659–63.
- Kemp SF, deShazo RD, Moffitt JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol 2000;105:683–91.
- Hoffman DR. Fire ant venom allergy. Allergy 1995;50:535–44.
- Stafford CT. Hypersensitivity to fire ant venom. Ann Allergy Asthma Immunol 1996;77:87–95;quiz 96–9.
- Prahlow JA, Barnard JJ. Fatal anaphylaxis due to fire ant stings. Am J Forensic Med Pathol 1998;19:137–42.
- Solley GO, Vanderwoude C, Knight GK. Anaphylaxis due to red imported fire ant sting. Med J Aust 2002;176:521–3.
- Fernández-Meléndez S, Miranda A, García-González JJ, et al. Anaphylaxis caused by imported red fire ant stings in Málaga, Spain. J Investig Allergol Clin Immunol 2007;17:48–9.
- Lee JK, Betschel SD. A case of the first documented fire ant anaphylaxis in Canada. Allergy Asthma Clin Immunol 2013;9:25.
- Cochran J, McSwain SD, Evans M, et al. Anaphylaxis and delayed hymenoptera in a child with fire ant envenomation. Am J Emerg Med 2013;31:632.e1–3.
- Smith JD, Smith EB. Multiple fire ant stings. A complication of alcoholism. Arch Dermatol 1971;103:438–41.
- deShazo RD, Williams DF. Multiple fire ant stings indoors. South Med J 1995;88:712–15.
- Alexander JO'D. Hymenoptera stings. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:135–58.
- Schmidt JO. Allergy to venomous insects. In: Graham JM, ed. The Hive and the Honey Bee. Hamilton, Illinois: Dadant, 1992:1209–69.
- Reisman RE, Livingston A. Late-onset allergic reactions, including serum sickness, after insect stings. J Allergy Clin Immunol 1989;84:331–7.
- Valentine MD. Insect venom allergy: diagnosis and treatment. J Allergy Clin Immunol 1984;73:299–304.
- King TP, Kochoumian L, Joslyn A. Wasp venom proteins: phospholipase A1 and B. Arch Biochem Biophys 1984;230:1–12.
- Hoffman DR. Allergens in Hymenoptera venom XIII: Isolation and purification of protein components from three species of vespid venoms. J Allergy Clin Immunol 1985;75:599–605.
- Hoffman DR. Allergens in bee venom. III. Identification of allergen B of bee venom as an acid phosphatase. J Allergy Clin Immunol 1977;59:364–6.
- Hoffman DR. Allergens in Hymenoptera venom XXIV: the amino acid sequences of imported fire ant venom allergens Sol i II, Sol i III, and Sol i IV. J Allergy Clin Immunol 1993;91:71–8.
- Hoffman DR, Dove DE, Moffitt JE, et al. Allergens in Hymenoptera venom. XXI. Cross-reactivity and multiple reactivity between fire ant venom and bee and wasp venoms. J Allergy Clin Immunol 1988;82:828–34.
- Hoffman DR, Jacobson RS. Allergens in Hymenoptera venom. XXVII: bumblebee venom allergy and allergens. J Allergy Clin Immunol 1996;97:812–21.
- Brand JM, Blum MS, Fales HM, et al. Fire ant venoms: comparative analyses of alkaloidal components. Toxicon 1972;10:259–71.
- Nugent JS, More DR, Hagan LL, et al. Cross-reactivity between allergens in the venom of the common striped scorpion and the imported fire ant. J Allergy Clin Immunol 2004;114:383–6.
- Reisman RE. Insect stings. N Engl J Med 1994;331:523–7.
- Ewan PW. Venom allergy. BMJ 1998;316:1365–8.
- Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol 2002;2:341–6.
- Hoffman DR. Ant venoms. Curr Opin Allergy Clin Immunol 2010;10:342–6.
- Chen L, Fadamiro HY. Re-investigation of venom chemistry of Solenopsis fire ants. I. Identification of novel alkaloids in S. richteri. Toxicon 2009;53:469–78.
- Chen L, Fadamiro HY. Re-investigation of venom chemistry of Solenopsis fire ants. II. Identification of novel alkaloids in S. invicta. Toxicon 2009;53:479–86.
- Ruëff F, Chatelain R, Przybilla B. Management of occupational Hymenoptera allergy. Curr Opin Allergy Clin Immunol 2011;11:69–74.
- Ruëff F, Przybilla B, Biló MB, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase – a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol 2009;124:1047–54.
- Ruëff F, Dugas-Breit S, Przybilla B. Stinging Hymenoptera and mastocytosis.Curr Opin Allergy Clin Immunol 2009;9:338–42.
- Niedoszytko M, de Monchy J, van Doormaal JJ, et al. Mastocytosis and insect venom allergy: diagnosis, safety and efficacy of venom immunotherapy. Allergy 2009;64:1237–45.
- Hermes B, Haas N, Grabbe J, et al. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994;130:780–4.
- Park JH, Kim JG, Cha SH, et al. Eosinophilic foreign body granuloma after multiple self-administered bee stings. Br J Dermatol 1998;139:1102–5.
- Carr ME. Hand-foot syndrome in a patient with multiple fire ant stings. South Med J 2004;97:707–9.
- Schmid WH. Medical implications: imported fire ants, Solenopsis invicta. Cutis 1977;19:794–7.
- Lockey RF. Systemic reactions to stinging ants. J Allergy Clin Immunol 1974;54:132–46.
- Reisman RE. Venom hypersensitivity. J Allergy Clin Immunol 1994;94:651–8;quiz 659–61.
- Chamberlain D. Emergency medical treatment of anaphylactic reactions. Project Team of the Resuscitation Council (UK). J Accid Emerg Med 1999;16:243–7.
- Visscher PK, Vetter RS, Camazine S. Removing bee stings. Lancet 1996;348:301–2.
- Bonifazi F, Jutel M, Biló BM, et al. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy 2005;60:1459–70.
- Golden DBK, Kelly D, Hamilton RG, et al. Venom immunotherapy reduces large local reactions to insect stings. J Allergy Clin Immunol 2009;123:1371–5.
Lice (Phthiraptera)
- Veracx A, Rivet R, McCoy KD, et al. Evidence that head and body lice on homeless persons have the same genotype. PLOS One 2012;7:e45903.
- Nuttall GHF. The biology of Pediculus humanus. Parasitology 1917;10:80–185.
- Lavoipierre MM. Feeding mechanism of blood-sucking arthropods. Nature 1965;208:302–3.
- Lavoipierre MM. Feeding mechanism of Haematopinus suis, on the transilluminated mouse ear. Exp Parasitol 1967;20:303–11.
- Gratz NG. Human Lice. Their Prevalence, Control and Resistance to Insecticides. A Review 1985–1997. Geneva: World Health Organization, 1997. WHO/CTD/WHOPES/97.8.
- Chosidow O. Scabies and pediculosis. Lancet 2000;355:819–26.
- Burgess IF. Human lice and their control. Annu Rev Entomol 2004;49:457–81.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerging Infect Dis 2008;14:1493–4.
- Harris J, Crawshaw JG, Millership S. Incidence and prevalence of head lice in a district health authority area. Commun Dis Public Health 2003;6:246–9.
- Heukelbach J, Wilcke T, Winter B, et al. Epidemiology and morbidity of scabies and pediculosis capitis in resource-poor communities in Brazil. Br J Dermatol 2005;153:150–6.
- Fan C-K, Liao C-W, Wu M-S, et al. Prevalence of Pediculus capitis infestation among school children of Chinese refugees residing in mountanous areas of northern Thailand. Kaohsiung J Med Sci 2004;20:183–7.
- Downs AM, Stafford KA, Stewart GH, et al. Factors that may be influencing the prevalence of head lice in British school children. Pediatr Dermatol 2000;17:72–4.
- Mumcuoglu KY, Friger M, Ioffe-Uspensky I, et al. Louse comb versus direct visual examination for the diagnosis of head louse infestations. Pediatr Dermatol 2001;18:9–12.
- Litt JZ. The quiddity of the head louse. Arch Dermatol 1978;114:1099.
- Slonka GF, McKinley TW, McCroan JE, et al. Epidemiology of an outbreak of head lice in Georgia. Am J Trop Med Hyg 1976;25:739–43.
- Bhutani LK. Pediculosis capitis. Arch Dermatol 1979;115:675.
- De Madureira PR. Pediculosis and ethnic groups. Int J Dermatol 1991;30:524.
- Bonilla DL, Kabeya H, Henn J, et al. Bartonella quintana in body lice and head lice from homeless persons, San Francisco, California, USA. Emerging Infect Dis 2009;15:912–15.
- Sasaki T, Poudel SKS, Isawa H, et al. First molecular evidence of Bartonella quintana in Pediculus humanus capitis (Phthiraptera: Pediculidae), collected from Nepalese children. J Med Entomol 2006;43:110–12.
- Bouvresse S, Socolovshi C, Berdjane Z, et al. No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp Immunol Microbiol Infect Dis 2011;34:475–7.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol 2004;50:1–12;quiz 13–14.
- Burgess IF. Human lice and their management. Adv Parasitol 1995;36:271–342.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol 2005;53:129–33.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol 2002;119:629–31.
- Fine BC. Controversy about pediculosis capitis. N Engl J Med 1984;311:801.
- Speare R, Thomas G, Cahill C. Head lice are not found on floors in primary school classrooms. Aust N Z J Public Health 2002;26:208–11.
- Mumcuoglu KY, Klaus S, Kafka D, et al. Clinical observations related to head lice infestation. J Am Acad Dermatol 1991;25:248–51.
- Ronchese F. Generalized dermatitis from pediculosis capitis. N Engl J Med 1946;234:665.
- Parish LC. Plica polonica. In: Parish LC, Nutting WB, Schwartzman RM, eds. Cutaneous Infestations of Man and Animal. New York: Praeger, 1983:43–9.
- Held JL, Bernstein RM. Hair casts or pseudonits acquired following psychological trauma. Cutis 1989;43:380–1.
- Scott MJ Jr, Scott MJ Sr. Nits or not? Pseudonits – simple office diagnosis. JAMA 1980;243:2325–6.
- De Maeseneer J, Blokland I, Willems S, et al. Wet combing versus traditional scalp inspection to detect head lice in schoolchildren: observational study. BMJ 2000;321:1187–8.
- Bingham P, Kirk S, Hill N, et al. The methodology and operation of a pilot randomized control trial of the effectiveness of the Bug Busting method against a single application insecticide product for head louse treatment. Public Health 2000;114:265–8.
- Mumcuoglu KY, Barker SC, Burgess IE, et al. International guidelines for effective control of head louse infestations. J Drugs Dermatol 2007;6:409–14.
- Pollack RJ, Kiszewski AE, Spielman A. Overdiagnosis and consequent mismanagement of head louse infestations in North America. Pediatr Infect Dis J 2000;19:689–93;discussion 694.
- Williams LK, Reichert A, MacKenzie WR, et al. Lice, nits, and school policy. Pediatrics 2001;107:1011–15.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med 2002;346:1645–50.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol 2005;4:207–11.
- Elston DM. Nit picking. J Am Acad Dermatol 2005;53:164–7.
- Dodd CS. Interventions for treating head lice. Cochrane Database Syst Rev 2001:CD001165.
- Dodd CS. WITHDRAWN: interventions for treating headlice. Cochrane Database Syst Rev 2006:CD001165.
- Do G, Van der Wouden JC, Klootwijk T, et al. Interventions for treating head lice. Cochrane Database Syst Rev 2011;Issue 10;Art. No.:CD009321.
- Mumcuoglu KY. Effective treatment of head louse with pediculicides. J Drugs Dermatol 2006;5:451–2.
- Izri A, Chosidow O. Efficacy of machine laundering to eradicate head lice: recommendations to decontaminate washable clothes, linens, and fomites. Clin Infect Dis 2006;42:e9–10.
- Chosidow O, Chastang C, Brue C, et al. Controlled study of malathion and d-phenothrin lotions for Pediculus humanus var capitis-infested schoolchildren. Lancet 1994;344:1724–7.
- Burgess IF, Brown CM, Peock S, et al. Head lice resistant to pyrethroid insecticides in Britain. BMJ 1995;311:752.
- Downs AM, Stafford KA, Harvey I, et al. Evidence for double resistance to permethrin and malathion in head lice. Br J Dermatol 1999;141:508–11.
- Downs AMR, Stafford KA, Hunt LP, et al. Widespread insecticide resistance in head lice to the over-the-counter pediculocides in England, and the emergence of carbaryl resistance. Br J Dermatol 2002;146:88–93.
- Izri MA, Brière C. First cases of resistance of Pediculus capitis Linné 1758 to malathion in France.Presse Med 1995;24:1444.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse Pediculus capitis from Israel. Med Vet Entomol 1995;9:427–32,447.
- Picollo MI, Vassena CV, Mougabure Cueto GA, et al. Resistance to insecticides and effect of synergists on permethrin toxicity in Pediculus capitis (Anoplura: Pediculidae) from Buenos Aires. J Med Entomol 2000;37:721–5.
- Burkhart CG, Burkhart CN. Clinical evidence of lice resistance to over-the-counter products. J Cutan Med Surg 2000;4:199–201.
- SupYoon K, Symington SB, Hyeock Lee S, et al. Three mutations identified in the voltage-sensitive sodium channel alpha-subunit gene of permethrin-resistant human head lice reduce the permethrin sensitivity of house fly Vssc1 sodium channels expressed in Xenopus oocytes. Insect Biochem Mol Biol 2008;38:296–306.
- Hodgdon HE, Yoon KS, Previte DJ, et al. Determination of knockdown resistance allele frequencies in global human head louse populations using the serial invasive signal amplification reaction. Pest Manag Sci 2010;66:1031–40.
- Bouvresse S, Berdjane Z, Durand R, et al. Permethrin and malathion resistance in head lice: results of ex vivo and molecular assays. J Am Acad Dermatol 2012;67:1143–50.
- Ibarra J, Hall DM. Head lice in schoolchildren. Arch Dis Child 1996;75:471–3.
- Roberts RJ, Casey D, Morgan DA, et al. Comparison of wet combing with malathion for treatment of head lice in the UK: a pragmatic randomised controlled trial. Lancet 2000;356:540–4.
- Hill N, Moor G, Cameron MM, et al. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ 2005;331:384–7.
- Dawes M. Combing and combating head lice. BMJ 2005;331:362–3.
- Chosidow O. Bug Buster for head lice: is it effective? Arch Dermatol 2006;142:1635–7.
- Burgess IF, Brown CM, Lee PN. Treatment of head louse infestation with 4% dimeticone lotion: randomised controlled equivalence trial. BMJ 2005;330:1423.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimeticone: randomized observer blinded comparative trial. BMC Infect Dis 2008;8:115.
- Burkhart CG, Burkhart CN. Asphyxiation of lice with topical agents, not a reality…yet. J Am Acad Dermatol 2006;54:721–2.
- Burgess IF. The mode of action of dimeticone 4% lotion against head lice, Pediculus capitis. BMC Pharmacol 2009;9:3.
- Connolly M, Stafford KA, Coles GC, et al. Control of head lice with a coconut-derived emulsion shampoo. J Eur Acad Dermatol Venereol 2009;23:67–9.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol 2010;27:19–24.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics 2009;124:e389–95.
- Kaul N, Palma KG, Silagy SS, et al. North American efficacy and safety of a novel pediculicide rinse, isopropyl myristate 50% (Resultz). J Cutan Med Surg 2007;11:161–7.
- Takano-Lee M, Edman JD, Mullens BA, et al. Home remedies to control head lice: assessment of home remedies to control the human head louse, Pediculus humanus capitis (Anoplura: Pediculidae).J Pediatr Nurs 2004;19:393–8.
- Priestley CM, Burgess IF, Williamson EM. Lethality of essential oil constituents towards the human louse, Pediculus humanus, and its eggs. Fitoterapia 2006;77:303–9.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med 2010;362:896–905.
- Chosidow O, Giraudeau B. Topical ivermectin – a step toward making head lice dead lice? N Engl J Med 2012;367:1750–2.
- Pariser DM, Meinking TL, Bell M, et al. Topical 0.5% ivermectin lotion for treatment of head lice. N Engl J Med 2012;367:1687–93.
- Frankowski BL, Bocchini JA Jr, Council on School Health and Committee on Infectious Diseases. Head lice. Pediatrics 2010;126:392–403.
- Roux V, Raoult D. Body lice as tools for diagnosis and surveillance of reemerging diseases. J Clin Microbiol 1999;37:596–9.
- Raoult D, Ndihokubwayo JB, Tissot-Dupont H, et al. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 1998;352:353–8.
- Sundnes KO, Haimanot AT. Epidemic of louse-borne relapsing fever in Ethiopia. Lancet 1993;342:1213–15.
- Drancourt M, Mainardi JL, Brouqui P, et al. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med 1995;332:419–23.
- Alexander JO'D. Infestation with Anoplura – lice. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:29–55.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis 2006;193:474–6.
- Armstrong NR, Wilson JD. Did the “Brazilian” kill the pubic louse? Sex Transm Infect 2006;82:265–6.
- Opaneye AA, Jayaweera DT, Walzman M, et al. Pediculosis pubis: a surrogate marker for sexually transmitted diseases. J R Soc Health 1993;113:6–7.
- Pierzchalski JL, Bretl DA, Matson SC. Phthirus pubis as a predictor for chlamydia infections in adolescents. Sex Transm Dis 2002;29:331–4.
- Nielsen AO, Secher L. Pediculosis pubis in a patient treated with topical steroids. Cutis 1980;25:655,658.
- Chitchang S, Yodmani B. Phthiriasis capitis. J Med Assoc Thai 1983;66:727–9.
- Signore RJ, Love J, Boucree MC. Scalp infestation with Phthirus pubis. Arch Dermatol 1989;125:133.
- Safdi SA, Farrington J. Constitutional reactions and maculae ceruleae attending phthiriasis pubis. Am J Med Sci 1947;214:308–11.
- Kern AB. Bullous eruption due to pediculosis pubis. AMA Arch Derm Syphilol 1952;65:334–9.
- Brenner S, Yust I. Bullous eruption in a case of bullous pediculid. Cutis 1988;41:281.
- Goldman L. Phthirus pubis infestation of the scalp and cilia in young children;report of five additional cases. Arch Derm Syphilol 1948;57:274.
- Scott MJ, Esterly NB. Eyelash infestation by Phthirus pubis as a manifestation of child abuse. Pediatr Dermatol 1983;1:179.
- Scott GR, Chosidow O, IUSTI/WHO. European guideline for the management of pediculosis pubis, 2010. Int J STD AIDS 2011;22:304–5.
- Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010;59:1–110.
- Speare R, Koehler JM. A case of pubic lice resistant to pyrethrins.Aust Fam Physician 2001;30:572–4.
- Burns DA. The treatment of Pthirus pubis infestation of the eyelashes. Br J Dermatol 1987;117:741–3.
- Couch JM, Green WR, Hirst LW, et al. Diagnosing and treating Phthirus pubis palpebrarum. Surv Ophthalmol 1982;26:219–25.
- Ronchese F. Treatment of pediculosis ciliorum in an infant. N Engl J Med 1953;249:897–8.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol 2000;118:134–5.
Bugs (Hemiptera)
- British Museum (Natural History). The Bed Bug. London: the Trustees of the British Museum (Natural History), 1973 (Economic series, no. 5).
- Kettle DS. Medical and Veterinary Entomology, 2nd edn. Wallingford: CAB International, 1995:344–60.
- Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae). Annu Rev Entomol 2007;52:351–74.
- Usinger RL. Monograph of Cimicidae (Hemiptera–Heteroptera), Vol. 7. College Park, MD: Entomological Society of America, 1966:50.
- Whyte AS, Garnett PA, Whittington AE. Bats in the belfry, bugs in the bed? Lancet 2001;357:604.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011;52:200–10.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009;301:1358–66.
- Reinhardt K, Harder A, Holland S, et al. Who knows the bed bug? Knowledge of adult bed bug appearance increases with people's age in three counties of Great Britain. J Med Entomol 2008;45:956–8.
- Doggett S, Greary M, Russell R. The resurgence of bed bugs in Australia. J Environ Health 2004;4:30–8.
- Richards L, Boase CJ, Gezan S, Cameron MM. Are bed bug infestations on the increase within Greater London? J Environ Health Res 2009;9:17–22.
- Kilpinen O, Vagn Jensen KM, Kristensen M. Bed bug problems in Denmark, with a European perspective. In Robinson WH, Bajomi D, eds. Proceedings of the 6th International Conference on Urban Pests. OOK-Press Kft, 2008:395–9.
- Delaunay P. Human travel and traveling bedbugs. J Travel Med 2012;19:373–9.
- Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol 2011;48:1023–30.
- Zhu F, Wigginton J, Romero A, et al. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch Insect Biochem Physiol 2010;73:245–57.
- Doggett SL, Dwyer DE, Peñas PF, et al. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012;25:164–92.
- Silverman AL, Qu LH, Blow J, et al. Assessment of hepatitis B virus DNA and hepatitis C virus RNA in the common bedbug (Cimex lectularius L.) and kissing bug (Rodnius prolixus). Am J Gastroenterol 2001;96:2194–8.
- Lowe CF, Romney MG. Bedbugs as vectors for drug-resistant bacteria. Emerging Infect Dis 2011;17:1132–4.
- Kinnear J. Epidemic of bullous erythema on legs due to bed-bugs. Lancet 1948;2:55.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013;149:372–3.
- Delaunay P, Blanc V, Dandine M, et al. Bedbugs and healthcare-associated dermatitis, France. Emerging Infect Dis 2009;15:989–90.
- Bernardeschi C, Le Cleach L, Delaunay P, et al. Bed bug infestation. BMJ 2013;346:f138.
- Reinhardt K, Kempke D, Naylor RA, et al. Sensitivity to bites by the bedbug, Cimex lectularius. Med Vet Entomol 2009;23:163–6.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, et al. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012;125:688–94.
- Andrade RN. Haematosiphoniasis. In: Simons RDC, ed. Handbook of Tropical Dermatology, Vol. 2. Amsterdam: Elsevier, 1953:905–7.
- Steen CJ, Carbonaro PA, Schwartz RA. Arthropods in dermatology. J Am Acad Dermatol 2004;50:819–42;quiz 842–4.
- Heukelbach J, Hengge UR. Bed bugs, leeches and hookworm larvae in the skin. Clin Dermatol 2009;27:285–90.
- Management of simple insect bites: where's the evidence? Drug Ther Bull 2012;50:45–8.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev 2012;1:CD003261.
- Seidel C, Reinhardt K. Bugging forecast: unknown, disliked, occasionally intimate. Bed bugs in Germany meet unprepared people. PLOS One 2013;8:e51083.
- Naylor RA, Boase CJ. Practical solutions for treating laundry infested with Cimex lectularius (Hemiptera: Cimicidae). J Econ Entomol 2010;103:136–9.
- Doggett SL; Australian Environmental Pest Managers Association. A Code of Practice for the Control of Bed Bug Infestations in Australia. Westmead Hospital, 4th edn, 2011 http://medent.usyd.edu.au/bedbug/cop_ed4_draft.pdf (last accessed October 2014).
- Wang C, Gibb T, Bennett GW. Evaluation of two least toxic integrated pest management programs for managing bed bugs (Heteroptera: Cimicidae) with discussion of a bed bug intercepting device. J Med Entomol 2009;46:566–71.
- Davies TGE, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012;26:241–54.
- Lehnert MP, Pereira RM, Koehler PG, et al. Control of Cimex lectularius using heat combined with dichlorvos resin strips. Med Vet Entomol 2011;25:460–4.
- Kilpinen O, Kristensen M, Jensen K-MV. Resistance differences between chlorpyrifos and synthetic pyrethroids in Cimex lectularius population from Denmark. Parasitol Res 2011;109:1461–4.
- Wang C, Gibb T, Bennett GW, et al. Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J Econ Entomol 2009;102:1580–5.
Family Reduviidae
- Ghauri MSK. Reduviidae (including Triatominae) (cone nose-bugs, kissing-bugs and assassin-bugs). In: Smith KCV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973:378–85.
- Cook ML, Lee DJ. Effects on humans of bites of Australian non-bloodsucking Reduviid bugs. Med J Aust 1977;ii:833–5.
- Reisenman CE, Lawrence G, Guerenstein PG, et al. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona, USA. Emerg Infect Dis 2010;16:400–5.
- Alexander JO'D. Infestation by Hemiptera. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:57–74.
- Shields TS, Walsh EN. ‘Kissing bug’ bite. Arch Dermatol 1956;74:14–21.
- Kettle DS. Medical and Veterinary Entomology, 2nd edn. Wallingford: CAB International, 1995:344–60.
- Smith FD, Miller NC, Camazzo SJ, et al. Insect bite by Argilus cristatus, a North American Reduviid. Arch Dermatol 1958;77:324–30.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629–34.
- Walter J, Fletcher E, Moussaoui R, et al. Do bites of kissing bugs cause unexplained allergies? Results from a survey in triatomine-exposed and unexposed areas in southern california. PLOS One 2012;7:e44016.
Family Anthocoridae
- Woodward TE. A case of persistent attacks on a human by Lyctocoris campestris (F.) (Hem., Anthocoridae). Entomol Month Mag 1951;87:44.
- Ghauri MSK. Anthocoridae. In: Smith KGV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973:389.
- Dolling WR. Dufouriellus ater (Dufour) (Hemiptera: Anthocoridae) biting industrial workers in Britain. Trans R Soc Trop Med Hyg 1977;71:355.
- Kampen H, Werner D. Human-biting potential of the predatory flower bug Orius majusculus (Hemiptera: Anthocoridae). Parasitol Res 2011;108:1579–81.
Family Pentatomidae
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Hemiptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med 2002;13:48–50.
- Jones SK, Strong L, Burton JL. Perioral blisters in a bug-biting baby. Br J Dermatol 1988;119:121–5.
Family Belostomatidae
- Haddad V, Cardoso JL, Lupi O, Tyring SK. Tropical dermatology: venomous arthropods and human skin. Part I. Insecta. J Am Acad Dermatol 2012;67:339.e1–14.
- Haddad V Jr, Schwartz EM, Schwartz CA, Carvalho LN. Report of seven human envenomations caused by giant water bugs of Belostomatidae family (Hemiptera, Heteroptera). Wilderness Environ Med 2010;21:130–3.
- Ebong SM, Eyangoh S, Marion E, et al. Survey of water bugs in bankim, a new buruli ulcer endemic area in Cameroon. J Trop Med 2012;2012:123843
- Doannio JM, Konan KL, Dosso FN, et al. Micronecta sp (Corixidae) and Diplonychus sp (Belostomatidae), two aquatic Hemiptera hosts and/or potential vectors of Mycobacterium ulcerans (pathogenic agent of Buruli ulcer) in Cote d'Ivoire. Med Trop 2011;71:53–7.
- Benbow ME, Williamson H, Kimbirauskas R, et al. Aquatic invertebrates as unlikely vectors of Buruli ulcer disease. Emerg Infect Dis 2008;14:1247–54.
- Asiedu K, Scherpbier R, Raviglione M. Buruli ulcer: Mycobacterium ulcerans infection. Geneva, Switzerland: World Health Organization, 2000.
Thrips (Thysanoptera)
- Lewis T. Thrips: their biology, ecology and economic importance. London: Academic Press, 1973.
- Herms WB, James MT. Medical Entomology, 5th edn. New York: Macmillan, 1961:538–9.
- Fishman HC. Thrips. Arch Dermatol 1987;123:993–4.
- Mumcuoglu KY, Volman Y. Thrips stings in Israel: a case report. Isr J Med Sci 1988;24:715.
- Leigheb G, Tiberio R, Filosa G, et al. Thysanoptera dermatitis. J Eur Acad Dermatol Venereol 2005;19:722–4.
- Cooper RG. Dermatitis and conjunctivitis in workers on an ostrich farm following thrips infestation. Indian J Med Res 2007;125:588–9.
- Goldstein N, Skipworth GB. Papular eruption secondary to thrips bites. Halos in Hawaii. JAMA 1968;203:53–5.
- Martin J 4th, Richmond A, Davis BM, et al. Thysanoptera dermatitis presenting as folie à deux. Arch Dermatol 2012;148:864–5.
- Guarneri F, Guarneri C, Mento G, et al. Pseudo-delusory syndrome caused by Limothrips cerealium. Int J Dermatol 2006;45:197–9.
- Meinking TL, Elgart G, Eyerdam DH, et al. Thrips mistaken for headlice or Ekban syndrome. Int J Dermatol 2006;45:327–8.
Beetles (Coleoptera)
- Alexander JO'D. Arthropods and Human Skin. Berlin: Springer-Verlag, 1984;75–85.
- Nicholls DS, Christmas TI, Greig DE. Oedemerid blister beetle dermatosis: a review. J Am Acad Dermatol 1990;22:815–19.
- Southcott RV. Injuries from Coleoptera. Med J Aust 1989;151:654–9.
- Moed L, Shwayder TA, Chang MW. Cantharidin revisited: a blistering defense of an ancient medicine. Arch Dermatol 2001;137:1357–60.
- Lehmann CF, Pipkin JL, Ressmann AC. Blister beetle dermatosis. AMA Arch Derm 1955;71:36–8.
- Browne SG. Cantharidin Poisoning Due to a “Blister Beetle.” BMJ 1960;2:1290–1.
- Smith KGV. Coleoptera and other insects. In: Smith KGV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973:413–15.
- Kerdel-Vegas F, Goihman-Yahr M. Paederus dermatitis. Arch Dermatol 1966;94:175–85.
- George AO. Paederus dermatitis – a mimic. Contact Dermatitis 1993;29:212–13.
- Brazzelli V, Martinoli S, Prestinari F, et al. Staphylinid blister beetle dermatitis. Contact Dermatitis 2002;46:183–4.
- Armstrong RK, Winfield JL. Staphylinidae dermatitis on Okinawa. J Med Entomol 1968;5:362.
- Kamaladasa SD, Perera WD, Weeratunge L. An outbreak of paederus dermatitis in a suburban hospital in Sri Lanka. Int J Dermatol 1997;36:34–6.
- Sendur N, Savk E, Karaman G. Paederus dermatitis: a report of 46 cases in Aydin, Turkey. Dermatology (Basel) 1999;199:353–5.
- Uslular C, Kavukçu H, Alptekïn D, et al. An epidemicity of Paederus species in Cukurova region. Cutis 2002;69:277–9.
- Zargari O, Kimyai-Asadi A, Fathalikhani F, et al. Paederus dermatitis in northern Iran: a report of 156 cases. Int J Dermatol 2003;42:608–12.
- Qadir SNR, Raza N, Rahman SB. Paederus dermatitis in Sierra Leone. Dermatol Online J 2006;12:9.
- Gnanaraj P, Venugopal V, Mozhi MK, et al. An outbreak of Paederus dermatitis in a suburban hospital in South India: a report of 123 cases and review of literature. J Am Acad Dermatol 2007;57:297–300.
- Huang C, Liu Y, Yang J, et al. An outbreak of 268 cases of Paederus dermatitis in a toy-building factory in central China. Int J Dermatol 2009;48:128–31.
- Assaf M, Nofal E, Nofal A, et al. Paederus dermatitis in Egypt: a clinicopathological and ultrastructural study. J Eur Acad Dermatol Venereol 2010;24:1197–201.
- Claborn DM, Polo JM, Olson PE, et al. Staphylinid (rove) beetle dermatitis outbreak in the American southwest? Mil Med 1999;164:209–13.
- Davidson SA, Norton SA, Carder MC, et al. Outbreak of dermatitis linearis caused by Paederus ilsae and Paederus iliensis (Coleoptera: Staphylinidae) at a military base in Iraq. US Army Med Dep J 2009:6–15.
- Todd RE, Guthridge SL, Montgomery BL. Evacuation of an Aboriginal community in response to an outbreak of blistering dermatitis induced by a beetle (Paederus australis). Med J Aust 1996;164:238–40.
- Penchenier L, Mouchet J, Cros B, et al. [Invasions of Paederus sabaeus (Coleoptera Staphylinidae) in central Africa. 1. Entomological and epidemiological aspects.] Bull Soc Pathol Exot 1994;87:45–8.
- Chandenier J, Quézédé P, Chandenier B, et al. [Invasions of Paederus sabaeus (Coleoptera Staphylinidae) in central Africa. 2. Clinical and therapeutic aspects in Brazzaville.] Bull Soc Pathol Exot 1994;87:49–51.
- Okiwelu SN, Umeozor OC, Akpan AJ. An outbreak of the vesicating beetle Paederus sabaeus Er. (Coleoptera: Staphylinidae) in Rivers State, Nigeria. Ann Trop Med Parasitol 1996;90:345–6.
- Vanhecke C, Malvy D, Guevart E, et al. [Paederus dermatitis: a retrospective study of 74 cases occurring in 2008 in Guinea-Conakry.] Ann Dermatol Venereol 2010;137:189–93.
- You D-O, Kang J-D, Youn N-H, et al. Bullous contact dermatitis caused by self-applied crushed Paederus fuscipes for the treatment of vitiligo. Cutis 2003;72:385–8.
- Borroni G, Brazzelli V, Rosso R, et al. Paederus fuscipes dermatitis. A histopathological study. Am J Dermatopathol 1991;13:467–74.
- Norton SA, Lyons C. Blister beetles and the ten plagues. Lancet 2002;359:1950.
- Fleisher TL, Fox I. Oedemerid beetle dermatitis. Arch Dermatol 1970;101:601–5.
- Christmas TI, Nicholls D, Holloway BA, et al. Blister beetle dermatosis in New Zealand. N Z Med J 1987;100:515–17.
- Samlaska CP, Samuelson GA, Faran ME, et al. Blister beetle dermatosis in Hawaii caused by Thelyphassa apicata (Fairmaire). Pediatr Dermatol 1992;9:246–50.
- Williamson DM. Itching eruption in miners caused by a rare beetle (Triboleum castaneum). Br J Dermatol 1964;76:388–9.
- Huang F-C, Chen W-J, Shih M-H. Paederus-induced keratitis.Cornea 2010;29:941–3.
- Mammino JJ. Paederus dermatitis: an outbreak on a medical mission boat in the Amazon. J Clin Aesthet Dermatol 2011;4:44–6.
- Freeman P. Dermestidae. In: Freeman P, ed. Common Insect Pests of Stored Food Products. A guide to their identification. London: the Trustees of the British Museum (Natural History), 1980:27–32.
- Rustin MH, Munro DD. Papular urticaria caused by Dermestes maculatus Degeer. Clin Exp Dermatol 1984;9:317–21.
- Ramachandran S, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol 1997;136:943–5.
- Cormia FE, Lewis GM. Contact dermatitis from beetles, with a report of a case due to the carpet beetle, Anthrenus scrophulariae. N Y State J Med 1948;48:2037–9.
- Ahmed AR, Moy R, Barr AR, et al. Carpet beetle dermatitis. J Am Acad Dermatol 1981;5:428–32.
- Horster S, Prinz JC, Holm N, et al. [Anthrenus dermatitis.]Hautarzt 2002;53:328–31.
- Jurecka W, Gebhart W, Mainitz M. Anthrenus sp. The paraffin block eater bug. Am J Dermatopathol 1987;9:204–7.
Cockroaches (Dictyoptera)
- García F, Notario MJ, Cabanás JM, et al. Incidence of bacteria of public health interest carried by cockroaches in different food-related environments. J Med Entomol 2012;49:1481–4.
- Pai H-H. Multidrug resistant bacteria isolated from cockroaches in long-term care facilities and nursing homes. Acta Trop 2013;125:18–22.
- Tilahun B, Worku B, Tachbele E, et al. High load of multi-drug resistant nosocomial neonatal pathogens carried by cockroaches in a neonatal intensive care unit at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Antimicrob Resist Infect Control 2012;1:12.
- Alexander JO'D. Thysanoptera and Dictyoptera, suborder Blattaria. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:22–7.
- Roth LM, Willis ER.The Medical and Veterinary Importance of Cockroaches. Smithsonian Miscellaneous Collections 134 (No. 10), 1957:1–147.
- Bernton HS, Brown H. Insect allergy: the allergenic potentials of the cockroach. South Med J 1969;62:1207–10.
- Zschunke E. Contact urticaria, dermatitis and asthma from cockroaches (Periplaneta americana). Contact Dermatitis 1978;4:313–14.
- Zschunke E. Contact urticaria, contact dermatitis, and asthma from cockroaches. Arch Dermatol 1978;114:1715–16.
- Monk BE, Pembroke AC. Cockroach dermatitis: an occupational hazard. BMJ (Clin Res Ed) 1987;294:935.
- Tahan F. Chronic urticaria with cockroach hypersensitivity. Pediatr Dermatol 2006;23:300–1.
Locusts (Orthoptera)
- Frankland AW. Locust sensitivity. Ann Allergy 1953;11:445–53.
- Tee RD, Gordon DJ, Newman Taylor AJ. Allergy to locusts (Schistocerca gregaria and Locusta migratoria). J Allergy Clin Immunol 1985;75:122.
- Lopata AL, Fenemore B, Jeebhay MF, et al. Occupational allergy in laboratory workers caused by the African migratory grasshopper Locusta migratoria. Allergy 2005;60:200–5.
- Monk BE. Contact urticaria to locusts. Br J Dermatol 1988;118:707–8.
- Soparkar GR, Patel PC, Cockroft DW. Inhalant atopic sensitivity to grasshoppers in research laboratories. J Allergy Clin Immunol 1993;92:61–5.
Butterflies and moths (Lepidoptera)
- Alexander JO'D. Reactions to Lepidoptera. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:177–97.
- Southcott RV. Lepidoptera and skin infestation. In: Parish CL, Nutting WB, Schwartzman RM, eds. Cutaneous Infestations of Man and Animal. New York: Praeger, 1983:304–43.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol 1983;8:77–93.
- Gullan PJ, Cranston P. The Insects: an outline of entomology, 3rd ed. London: Wiley-Blackwell, 2004.
- Tuthill RW, Canada AT, Wilcock K, et al. An epidemiologic study of gypsy moth rash. Am J Public Health 1984;74:799–803.
- Kikuchi T, Kobayashi K, Sakata K, et al. Gypsy moth-induced dermatitis: a hospital review and community survey. Eur J Dermatol 2012;22:384–90.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol 2007;56:952–5.
- Walker RB, Thomas T, Cupit D, et al. An epidemic of caterpillar sting dermatitis in a rural West Virginia community. W V Med J 1993;89:58–60.
- De-Long S. Mulberry tussock moth dermatitis. A study of an epidemic of unknown origin. J Epidemiol Community Health 1981;35:1–4.
- Diaz JH. The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming. Am J Trop Med Hyg 2005;72:347–57.
- Mindlin MJ, le Polain de Waroux O, Case S, et al. The arrival of oak processionary moth, a novel cause of itchy dermatitis, in the UK: experience, lessons and recommendations. Public Health 2012;126:778–81.
- Hossler EW. Caterpillars and moths: Part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol 2010;62:1–10;quiz 11–12.
- Haluska FG, Puliafito CA, Henriquez A, et al. Experimental gypsy moth (Lymantria dispar) ophthalmia nodosa. Arch Ophthalmol 1983;101:799–801.
- Watson PG, Sevel D. Ophthalmia nodosa. Br J Ophthalmol 1966;50:209–17.
- Dinehart SM, Archer ME, Wolf JE Jr, et al. Caripito itch: dermatitis from contact with Hylesia moths. J Am Acad Dermatol 1985;13:743–7.
- Jourdain F, Girod R, Vassal JM, et al. The moth Hylesia metabus and French Guiana lepidopterism: centenary of a public health concern. Parasite Paris Fr 2012;19:117–28.
- Müller CSL, Tilgen W, Pföhler C. Caterpillar dermatitis revisited: lepidopterism after contact with oak processionary caterpillar. BMJ Case Rep 2011;2011.
- Lamy M, Pastureaud MH, Novak F, et al. Thaumetopoein: an urticating protein from the hairs and integument of the pine processionary caterpillar (Thaumetopoea pityocampa Schiff., Lepidoptera, Thaumetopoeidae). Toxicon 1986;24:347–56.
- Rodriguez-Mahillo AI, Gonzalez-Muñoz M, Vega JM, et al. Setae from the pine processionary moth (Thaumetopoea pityocampa) contain several relevant allergens. Contact Dermatitis 2012;67:367–74.
- Werno J, Lamy M, Vincendeau P. Caterpillar hairs as allergens. Lancet 1993;342:936–7.
- Fuentes Aparicio V, Zapatero Remón L, Martínez Molero MI, et al. Allergy to pine processionary caterpillar (Thaumetopoea pityocampa) in children. Allergol Immunopathol (Madr) 2006;34:59–63.
- Fuentes Aparicio V, de Barrio Fernández M, Rubio Sotés M, et al. Non-occupational allergy caused by the pine processionary caterpillar (Thaumetopoea pityocampa). Allergol Immunopathol (Madr) 2004;32:69–75.
- Moneo I, Vega JM, Caballero ML, et al. Isolation and characterization of Tha p 1, a major allergen from the pine processionary caterpillar Thaumetopoea pityocampa. Allergy 2003;58:34–7.
- Natsuaki M. Immediate and delayed-type reactions in caterpillar dermatitis. J Dermatol 2002;29:471–6.
- De Jong MC, Hoedemaeker J, Jongebloed WL, et al. Investigative studies of the dermatitis caused by the larva of the brown-tail moth (Euproctis chrysorrhoea Linn.). II. Histopathology of skin lesions and scanning electron microscopy of their causative setae. Arch Dermatol Res Arch Für Dermatol Forsch 1976;255:177–91.
- Edwards EK Jr, Edwards EK, Kowalczyk AP. Contact urticaria and allergic contact dermatitis to the saddleback caterpillar with histologic correlation. Int J Dermatol 1986;25:467.
- Hossler EW. Caterpillars and moths: Part II. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol 2010;62:13–28;quiz 29–30.
- Gardner TL, Elston DM. Painful papulovesicles produced by the puss caterpillar. Cutis 1997;60:125–6.
- Finkelstein Y, Raikhlin-Eisenkraft B, Taitelman U. Systemic manifestations of erucism: a case report. Vet Hum Toxicol 1988;30:573–4.
- Vega JM, Moneo I, Armentia A, et al. Allergy to the pine processionary caterpillar (Thaumetopoea pityocampa). Clin Exp Allergy J 1999;29:1418–23.
- Vega JM, Moneo I, Armentia A, et al. Pine processionary caterpillar as a new cause of immunologic contact urticaria. Contact Dermatitis 2000;43:129–32.
- Veiga AB, Blochtein B, Guimarães JA. Structures involved in production, secretion and injection of the venom produced by the caterpillar Lonomia obliqua (Lepidoptera, Saturniidae). Toxicon 2001;39:1343–51.
- Vega JM, Moneo I, Armentia A, et al. Anaphylaxis to a pine caterpillar. Allergy 1997;52:1244–5.
- Inal A, Altintaş DU, Güvenmez HK, et al. Life-threatening facial edema due to pine caterpillar mimicking an allergic event. Allergol Immunopathol (Madr) 2006;34:171–3.
- Gottschling S, Meyer S, Dill-Mueller D, et al. Outbreak report of airborne caterpillar dermatitis in a kindergarten. Dermatol Basel Switz 2007;215:5–9.
- Huang DZ. Dendrolimiasis: an analysis of 58 cases. J Trop Med Hyg 1991;94:79–87.
- Costa RM, Atra E, Ferraz MB, et al. “Pararamose”: an occupational arthritis caused by lepidoptera (Premolis semirufa). An epidemiological study. Rev Paul Med 1993;111:462–5.
- Portero A, Carreño E, Galarreta D, et al. Corneal inflammation from pine processionary caterpillar hairs. Cornea 2013;32:161–4.
- Rocha-Campos AC, Gonçalves LR, Higashi HG, et al. Specific heterologous F(ab')2 antibodies revert blood incoagulability resulting from envenoming by Lonomia obliqua caterpillars. Am J Trop Med Hyg 2001;64:283–9.
Spiders
- Isbister GK. Necrotic arachnidism: the mythology of a modern plague. Lancet 2004;364:549–53.
- Isbister GK, Fan HW. Spider bite. Lancet 2011;378:2039–47.
- Swanson DL, Vetter RS. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med 2005;352:700–7.
- Isbister GK, Gray MR. A prospective study of 750 definite spider bites, with expert spider identification. QJM 2002;95:723–31.
- Alexander JO'D. Spider bites. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:209–26.
- King LE Jr. Spider bites. Arch Dermatol 1987;123:41–3.
- Wong RC, Hughes SE, Voorhees JJ. Spider bites. Arch Dermatol 1987;123:98–104.
- Sams HH, Dunnick CA, Smith ML, et al. Necrotic arachnidism. J Am Acad Dermatol 2001;44:561–73;quiz 573–6.
- Vetter RS, Isbister GK. Medical aspects of spider bites. Annu Rev Entomol 2008;53:409–29.
- Isbister GK, Gray MR. Latrodectism: a prospective cohort study of bites by formally identified redback spiders. Med J Aust 2003;179:88–91.
- Southcott RV. Australian Harmful Arachnids and their Allies. Mitcham: RV Southcott, 1978.
- Jelinek GA, Banham ND, Dunjey SJ. Red-back spider-bites at Fremantle Hospital, 1982-1987. Med J Aust 1989;150:693–5.
- Maretić Z. Latrodectism: variations in clinical manifestations provoked by Latrodectus species of spiders. Toxicon 1983;21:457–66.
- Stawiski MA. Insect bites and stings. Emerg Med Clin North Am 1985;3:785–808.
- Jelinek GA. Widow spider envenomation (latrodectism): a worldwide problem. Wilderness Environ Med 1997;8:226–31.
- Sari I, Zengin S, Davutoglu V, et al. Myocarditis after black widow spider envenomation. Am J Emerg Med 2008;26:630.e1–3.
- Pneumatikos IA, Galiatsou E, Goe D, et al. Acute fatal toxic myocarditis after black widow spider envenomation. Ann Emerg Med 2003;41:158.
- Isbister GK, Brown SGA, Miller M, et al. A randomised controlled trial of intramuscular vs. intravenous antivenom for latrodectism – the RAVE study. QJM 2008;101:557–65.
- Ellis RM, Sprivulis PC, Jelinek GA, et al. A double-blind, randomized trial of intravenous versus intramuscular antivenom for red-back spider envenoming. Emerg Med Australas 2005;17:152–6.
- Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med 1992;21:782–7.
- Murphy CM, Hong JJ, Beuhler MC. Anaphylaxis with Latrodectus antivenin resulting in cardiac arrest. J Med Toxicol 2011;7:317–21.
- Hoyte CO, Cushing TA, Heard KJ. Anaphylaxis to black widow spider antivenom. Am J Emerg Med 2012;30:836.e1–2.
- Miller MK, Whyte IM, White J, et al. Clinical features and management of Hadronyche envenomation in man. Toxicon 2000;38:409–27.
- Isbister GK, Gray MR, Balit CR, et al. Funnel-web spider bite: a systematic review of recorded clinical cases. Med J Aust 2005;182:407–11.
- Saupe EE, Papes M, Selden PA, et al. Tracking a medically important spider: climate change, ecological niche modeling, and the brown recluse (Loxosceles reclusa). PLOS One 2011;6:e17731.
- Hubiche T, Delaunay P, del Giudice P. A case of loxoscelism in southern France. Am J Trop Med Hyg 2013;88:807–8.
- Sams HH, Hearth SB, Long LL, et al. Nineteen documented cases of Loxosceles reclusa envenomation. J Am Acad Dermatol 2001;44:603–8.
- Robb CW, Hayes BB, Boyd AS. Generalized vasculitic exanthem following Loxosceles reclusa envenomation. J Cutan Pathol 2007;34:513–14.
- Lane L, McCoppin HH, Dyer J. Acute generalized exanthematous pustulosis and Coombs-positive hemolytic anemia in a child following Loxosceles reclusa envenomation. Pediatr Dermatol 2011;28:685–8.
- Williams ST, Khare VK, Johnston GA, et al. Severe intravascular hemolysis associated with brown recluse spider envenomation. A report of two cases and review of the literature. Am J Clin Pathol 1995;104:463–7.
- De Souza AL, Malaque CM, Sztajnbok J, et al. Loxosceles venom-induced cytokine activation, hemolysis, and acute kidney injury. Toxicon 2008;51:151–6.
- Rosen JL, Dumitru JK, Langley EW, et al. Emergency department death from systemic loxoscelism. Ann Emerg Med 2012;60:439–41.
- Elbahlawan LM, Stidham GL, Bugnitz MC, et al. Severe systemic reaction to Loxosceles reclusa spider bites in a pediatric population. Pediatr Emerg Care 2005;21:177–80.
- McDade J, Aygun B, Ware RE. Brown recluse spider (Loxosceles reclusa) envenomation leading to acute hemolytic anemia in six adolescents. J Pediatr 2010;156:155–7.
- Lane DR, Youse JS. Coombs-positive hemolytic anemia secondary to brown recluse spider bite: a review of the literature and discussion of treatment. Cutis 2004;74:341–7.
- Elston DM, Miller SD, Young RJ 3rd, et al. Comparison of colchicine, dapsone, triamcinolone, and diphenhydramine therapy for the treatment of brown recluse spider envenomation: a double-blind, controlled study in a rabbit model. Arch Dermatol 2005;141:595–7.
- Barrett SM, Romine-Jenkins M, Fisher DE. Dapsone or electric shock therapy of brown recluse spider envenomation? Ann Emerg Med 1994;24:21–5.
- Phillips S, Kohn M, Baker D, et al. Therapy of brown spider envenomation: a controlled trial of hyperbaric oxygen, dapsone, and cyproheptadine. Ann Emerg Med 1995;25:363–8.
- Manríquez JJ, Silva S. [Cutaneous and visceral loxoscelism: a systematic review.] Rev Chilena Infectol 2009;26:420–32.
- Tambourgi DV, Gonçalves-de-Andrade RM, van den Berg CW. Loxoscelism: from basic research to the proposal of new therapies. Toxicon 2010;56:1113–19.
- Wong SL, Defranzo AJ, Morykwas MJ, et al. Loxoscelism and negative pressure wound therapy (vacuum-assisted closure): a clinical case series. Am Surg 2009;75:1128–31.
- Campbell DS, Rees RS, King LE. Wolf spider bites. Cutis 1987;39:113–14.
- Redman JF. Human envenomation by a lycosid. Arch Dermatol 1974;110:111–12.
- Krinsky WL. Envenomation by the sac spider Chiracanthium mildei. Cutis 1987;40:127–9.
- Vest DK. Necrotic arachnidism in the northwest United States and its probable relationship to Tegenaria agrestis (Walckenaer) spiders. Toxicon 1987;25:175–84.
- Centers for Disease Control and Prevention (CDC). Necrotic arachnidism – Pacific Northwest, 1988-1996. Morb Mortal Wkly Rep 1996;45:433–6.
- White J, Hirst D, Hender E. 36 cases of bites by spiders, including the white-tailed spider, Lampona cylindrata. Med J Aust 1989;150:401–3.
- Vetter RS. Envenomation by spiders of the genus Hololena (Araneae: Agelenidae). Toxicon 2012;60:312–14.
- Isbister GK, Gray MR. White-tail spider bite: a prospective study of 130 definite bites by Lampona species. Med J Aust 2003;179:199–202.
- Banks J, Sirvid P, Vink C. White-tailed spider bites – arachnophobic fallout? N Z Med J 2004;117:U748.
Scorpions (Scorpiones)
- Chippaux J-P, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop 2008;107:71–9.
- Sheals JG. Arachnids (scorpions, spiders, ticks, etc.). In: Smith KGV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973:417–72.
- Mesquita MBS, Moraes-Santos T, Moraes MFD. Centrally injected tityustoxin produces the systemic manifestations observed in severe scorpion poisoning. Toxicol Appl Pharmacol 2003;187:58–66.
- Stahnke HL. Arizona's lethal scorpion. Ariz Med 1972;29:490–3.
- Balozet L. Scorpionism in the Old World. In: Bucherl W, Buckley EE, eds. Venomous Animals and their Venom, Vol. III. Venomous Invertebrates. New York: Academic, 1971:349–71.
- Allen C. Arachnid envenomations. Emerg Med Clin North Am 1992;10:269–98.
- Forrester MB, Stanley SK. Epidemiology of scorpion envenomations in Texas. Vet Hum Toxicol 2004;46:219–21.
- Freire-Maia L, Campos JA, Amaral CF. Approaches to the treatment of scorpion envenoming. Toxicon 1994;32:1009–14.
- Rogowski RS, Krueger BK, Collins JH, et al. Tityustoxin K alpha blocks voltage-gated noninactivating K+ channels and unblocks inactivating K+ channels blocked by alpha-dendrotoxin in synaptosomes. Proc Natl Acad Sci USA 1994;91:1475–9.
- Shiau DT, Sanders JW, Putnam SD, et al. Self-reported incidence of snake, spider, and scorpion encounters among deployed U.S. military in Iraq and Afghanistan. Mil Med 2007;172:1099–102.
- Radmanesh M. Cutaneous manifestations of the Hemiscorpius lepturus sting: a clinical study. Int J Dermatol 1998;37:500–7.
- Shahbazzadeh D, Srairi-Abid N, Feng W, et al. Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem J 2007;404:89–96.
- Isbister GK, Volschenk ES, Balit CR, et al. Australian scorpion stings: a prospective study of definite stings. Toxicon 2003;41:877–83.
- Southcott RV. Australian Harmful Arachnids and their Allies. Mitcham: RV Southcott, 1978.
- Alexander JO'D. Scorpion stings. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:199–207.
- Gueron M, Yaron R. Cardiovascular manifestations of severe scorpion sting. Clinicopathologic correlations. Chest 1970;57:156–62.
- Carbonaro PA, Janniger CK, Schwartz RA. Scorpion sting reactions. Cutis 1996;57:139–41.
- Amitai Y. Clinical manifestations and management of scorpion envenomation. Public Health Rev 1998;26:257–63.
- Rahav G, Weiss AT. Scorpion sting-induced pulmonary edema. Scintigraphic evidence of cardiac dysfunction. Chest 1990;97:1478–80.
- Hiller K, Jarrod MM, Franke HA, et al. Scorpion antivenom administered by alternative infusions. Ann Emerg Med 2010;56:309–10.
- Brown N, Landon J. Antivenom: the most cost-effective treatment in the world? Toxicon 2010;55:1405–7.
- Tuuri RE, Reynolds S. Scorpion envenomation and antivenom therapy. Pediatr Emerg Care 2011;27:667–72;quiz 673–5.
- Haddad V Jr, Cardoso JLC, Lupi O, et al. Tropical dermatology: Venomous arthropods and human skin: Part II. Diplopoda, Chilopoda, and Arachnida. J Am Acad Dermatol 2012;67:347.e1–9;quiz 355.
Ticks (Acari)
- Arthur DR. Ticks and Disease. Oxford: Pergamon, 1961.
- Chinery WA. The nature and origin of the “cement” substance at the site of attachment and feeding of adult Haemaphysalis spinigera (Ixodidae). J Med Entomol 1973;10:355–62.
- Alexander JO'D. The effects of tick bites. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:363–82.
- Sheals JG. Arachnids (scorpions, spiders, ticks, etc.). In: Smith KGV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973:417–72.
- Elston DM. Tick bites and skin rashes. Curr Opin Infect Dis 2010;23:132–8.
- Jordan BE, Onks KR, Hamilton SW, et al. Detection of Borrelia burgdorferi and Borrelia lonestari in birds in Tennessee. J Med Entomol 2009;46:131–8.
- Raoult D, Berbis P, Roux V, et al. A new tick-transmitted disease due to Rickettsia slovaca. Lancet 1997;350:112–13.
- Fox MT, Sykes TJ. Establishment of the tropical dog tick, Rhipicephalus sanguineus, in a house in London. Vet Rec 1985;116:661–2.
- Roux O, Desruelles F, Delaunay P, et al. Ticks and photo safari in South Africa. Br J Dermatol 2000;143:1109–10.
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol 2003;48:307–37.
- Elston DM. Human infestation by larval Amblyomma ticks. Arch Dermatol 2006;142:497–500.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol 2004;42:1163–9.
- Herman-Giddens ME. Southern tick-associated rash illness: further considerations. Clin Infect Dis 2012;54:887–8;author reply 888–9.
- Reubush TK 2nd, Cassaday PB, Marsh HJ, et al. Human babesiosis on Nantucket Island. Clinical features. Ann Intern Med 1977;86:6–9.
- Leker RR, Felsenstein I, Raveh D, et al. Ornithodoros tholozani bites: a unique clinical picture. J Am Acad Dermatol 1992;27:1025–6.
- Patterson JW, Fitzwater JE, Connell J. Localized tick bite reaction. Cutis 1979;24:168–9,172.
- Aoki K, Kamata H, Iida T, et al. Tick bite: two cases studied by scanning electron microscopy. Br J Dermatol 1984;110:233–40.
- Goldman L. Tick bite granuloma: failure of prevention of lesion by excision of tick bite area. Am J Trop Med Hyg 1963;12:246–8.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol 1982;7:537–42.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol 2003;49:363–92;quiz 393–6.
- Pearce RL, Grove DI. Tick infestation in soldiers who were bivouacked in the Perth region. Med J Aust 1987;146:238–40.
- Fujiwara K, Ono T, Kawashima K. Multiple larval tick infestation of man. A case of infesting larvae seeming to be pustules with a red halo. J Dermatol 1981;8:157–9.
- Jones BE. Human “seed tick” infestation. Amblyomma americanum larvae. Arch Dermatol 1981;117:812–14.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J 1985;78:751–3.
- Kain KC. Skin lesions in returned travelers. Med Clin North Am 1999;83:1077–102.
- Halpern SM, Munro DD. Tickborne melanoma? BMJ 1994;309:1693.
- Hoogstraal H, Gallagher MD. Blisters, pruritus, and fever after bites by the Arabian tick Ornithodoros (Alectorobius) muesebecki. Lancet 1982;2:288–9.
- Condy JB, Norval RA, Blackburn NK, et al. The effects of the bites of Argas brumpti (Acarina: Argasidae) on humans. Cent Afr J Med 1980;26:212–13.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol 2006;142:491–4.
- Wakkerman CT, Van Rijnj. Strophulus arthropodicus caused by ticks. Hautarzt 1965;16:37–8.
- Shasky DR. Tick bite granuloma with autoeczematization. Arch Dermatol 1972;106:916.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm 1955;71:524–5.
- Fernández-Soto P, Dávila I, Laffond E, et al. Tick-bite-induced anaphylaxis in Spain. Ann Trop Med Parasitol 2001;95:97–103.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946–1996. Clin Infect Dis 1999;29:1435–9.
- Greenstein P. Tick paralysis. Med Clin North Am 2002;86:441–6.
- Rodhain F, Perez C. Précis d'entomologie Médicale et Vétérinaire. Paris: Maloine, 1985.
Scabies
- Hebra F von. On Diseases of the Skin including the Exanthemata, Vol. II. London: the New Sydenham Society, 1868: 164–252.
- Beeson BB. Acarus scabiei. Study of its history. Arch Dermatol Syphilol 1927;16:294–307.
- Heilesen B. Studies on Acarus scabiei and scabies. Acta Derm Venereol Suppl (Stockh) 1946;14:1–370.
- Friedman R. The Story of Scabies. New York: Froben, 1948.
- Parish LC. History of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York: Marcel Dekker 1985:3–8.
- Alexander JO'D. Scabies. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:227–92.
- Van Neste D, Mrena E, Marchal G. Life cycle of scabies mite (Sarcoptes scabiei var. hominis) studied by scanning electron microscopy (author's transl.). Ann Dermatol Vénéréol 1981;108:355–61.
- Burgess I. Sarcoptes scabiei and scabies. Adv Parasitol 1994;33:235–92.
- Van Neste DJ. Human scabies in perspective. Int J Dermatol 1988;27:10–15.
- Bartley WC, Mellanby K. The parasitology of human scabies (women and children). Parasitology 1944;35:207–8.
- Mellanby K. Scabies. Hampton: EW Classey, 1972.
- Chosidow O. Clinical practices. Scabies. N Engl J Med 2006;354:1718–27.
- Fuller LC. Epidemiology of scabies. Curr Opin Infect Dis 2013;26:123–6.
- Chosidow O. Scabies and pediculosis. Lancet 2000;355:819–26.
- Burkhart CG. Scabies: an epidemiologic reassessment. Ann Intern Med 1983;98:498–503.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis 2006;6:769–79.
- Downs AM, Harvey I, Kennedy CT. The epidemiology of head lice and scabies in the UK. Epidemiol Infect 1999;122:471–7.
- Christophersen J. The epidemiology of scabies in Denmark, 1900 to 1975. Arch Dermatol 1978;114:747–50.
- Savin JA. Scabies in Edinburgh from 1815 to 2000. J R Soc Med 2005;98:124–9.
- Mimouni D, Ankol OE, Davidovitch N, et al. Seasonality trends of scabies in a young adult population: a 20-year follow-up. Br J Dermatol 2003;149:157–9.
- Downs AMR. Seasonal variation in scabies. Br J Dermatol 2004;150:602–3;author reply 603.
- Shrank AB, Alexander SL. Scabies: another epidemic? BMJ 1967;1:669–71.
- Feldmeier H, Jackson A, Ariza L, et al. The epidemiology of scabies in an impoverished community in rural Brazil: presence and severity of disease are associated with poor living conditions and illiteracy. J Am Acad Dermatol 2009;60:436–43.
- Bennett CE, Keefe M, Reynolds JC. Perceptions of the incidence of scabies and efficacy of treatment in U.K. hospitals. Br J Dermatol 2000;143:1337–8.
- Bouvresse S, Chosidow O. Scabies in healthcare settings. Curr Opin Infect Dis 2010;23:111–18.
- Alexander AM. Role of race in scabies infestation. Arch Dermatol 1978;114:627.
- Kelly AP. Scabies in blacks. Arch Dermatol 1978;114:1245.
- Rietschel RL, Lewis CW, Jones HE, et al. Scabies and role of race. Arch Dermatol 1979;115:109–10.
- Funaki B, Elpern DJ. Scabies epidemiology, Kauai, Hawaii, 1981-1985. Int J Dermatol 1987;26:590–2.
- Dahl MV. The immune system in scabies. In: Orkin M, Maibach HI, eds, Cutaneous Infestations and Insect Bites. New York: Marcel Dekker, 1985:75–83.
- Falk ES, Bolle R. In vitro demonstration of specific immunological hypersensitivity to scabies mite. Br J Dermatol 1980;103:367–73.
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol 1984;11:210–15.
- Arlian LG, Estes SA, Vyszenski-Moher DL. Prevalence of Sarcoptes scabiei in the homes and nursing homes of scabietic patients. J Am Acad Dermatol 1988;19:806–11.
- Hancock BW, Ward AM. Serum immunoglobulin in scabies. J Invest Dermatol 1974;63:482–4.
- Chevrant-Breton J, Desrues E, Auvray E, et al. [Serum IgE values in human scabies: study of 79 cases (author's transl.).] Ann Dermatol Vénéréol 1981;108:979–83.
- Falk ES. Serum immunoglobulin values in patients with scabies. Br J Dermatol 1980;102:57–61.
- Dahl JC, Scwartz B, Graudal C, et al. Serum IgE antibodies to the scabies mite. Int J Dermatol 1985;24:313–15.
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasite Immunol 2010;32:532–40.
- Walton SF, Oprescu FI. Immunology of scabies and translational outcomes: identifying the missing links. Curr Opin Infect Dis 2013;26:116–22.
- Holt DC, Fischer K. Novel insights into an old disease: recent developments in scabies mite biology. Curr Opin Infect Dis 2013;26:110–15.
- Mahé A, Faye O, N'Diaye HT, et al. Definition of an algorithm for the management of common skin diseases at primary health care level in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2005;99:39–47.
- Orkin M. Scabies: what's new? Curr Probl Dermatol 1995;22:105–11.
- Reid HF, Birju B, Holder Y, et al. Epidemic scabies in four Caribbean islands, 1981–1988. Trans R Soc Trop Med Hyg 1990;84:298–300.
- Walton SF, Holt DC, Currie BJ, et al. Scabies: new future for a neglected disease. Adv Parasitol 2004;57:309–76.
- Heukelbach J, Feldmeier H. Scabies. Lancet 2006;367:1767–74.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev 2007;20:268–79.
- Argenziano G, Fabbrocini G, Delfino M. Epiluminescence microscopy. A new approach to in vivo detection of Sarcoptes scabiei. Arch Dermatol 1997;133:751–3.
- Prins C, Stucki L, French L, et al. Dermoscopy for the in vivo detection of sarcoptes scabiei. Dermatol Basel Switz 2004;208:241–3.
- Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol 2007;56:53–62.
- Katsumata K, Katsumata K. Simple method of detecting sarcoptes scabiei var hominis mites among bedridden elderly patients suffering from severe scabies infestation using an adhesive-tape. Intern Med Tokyo Jpn 2006;45:857–9.
- Walter B, Heukelbach J, Fengler G, et al. Comparison of dermoscopy, skin scraping, and the adhesive tape test for the diagnosis of scabies in a resource-poor setting. Arch Dermatol 2011;147:468–73.
- Chosidow O, Sbidian E. Scabies finally getting the attention it merits! Ann Dermatol Venereol 2012;139:425–7.
- Albrecht J, Bigby M. Testing a test: critical appraisal of tests for diagnosing scabies. Arch Dermatol 2011;147:494–7.
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol 1981;20:600–5.
- Perrot J-L, Cinotti E, Labeille B, et al. Rapid diagnosis of scabies by manual confocal reflectance microscopy. Ann Dermatol Venereol 2012;139:502–5.
- Cinotti E, Perrot J-L, Labeille B, et al. On the feasibility of confocal microscopy for the diagnosis of scabies. Ann Dermatol Vénéréol 2013;140:215–16.
- Levi A, Mumcuoglu KY, Ingber A, et al. Detection of living Sarcoptes scabiei larvae by reflectance mode confocal microscopy in the skin of a patient with crusted scabies. J Biomed Opt 2012;17:060503.
- Monsel G, Chosidow O. Management of scabies. Skin Ther Lett 2012;17:1–4.
- Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. Morb Mortal Wkly Rep 2010;59:1–110.
- Scott GR, Chosidow O, IUSTI/WHO. European guideline for the management of scabies, 2010. Int J STD AIDS 2011;22:301–3.
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev 2007:CD000320.
- Mounsey KE, McCarthy JS. Treatment and control of scabies. Curr Opin Infect Dis 2013;26:133–9.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med 2010;362:717–25.
- Centers for Disease Control and Prevention (CDC). Scabies fact sheet. Atlanta: CDC, 2005. http://www.cdc.gov/ncidod/dpd/parasites/scabies/factsht_scabies.htm (last accessed October 2014).
- Hu S, Bigby M. Treating scabies: results from an updated Cochrane review. Arch Dermatol 2008;144:1638–40;discussion 1640–1.
- Le Cleach L, Chosidow O. Commentary on “Interventions for treating scabies”. Evidence-Based Child Health Cochrane Rev J 2011;6:1865-6.
- Steer AC, Kearns T, Andrews RM, et al. Ivermectin worthy of further investigation. Bull WHO 2009;87:A;author reply B.
- Goldust M, Rezaee E, Hemayat S. Treatment of scabies: comparison of permethrin 5% versus ivermectin. J Dermatol 2012;39:545–7.
- Ly F, Caumes E, Ndaw CAT, et al. Ivermectin versus benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: a randomized controlled trial. Bull WHO 2009;87:424–30.
- Chhaiya SB, Patel VJ, Dave JN, et al. Comparative efficacy and safety of topical permethrin, topical ivermectin, and oral ivermectin in patients of uncomplicated scabies. Indian J Dermatol Venereol Leprol 2012;78:605–10.
- Bachewar NP, Thawani VR, Mali SN, et al. Comparison of safety, efficacy, and cost effectiveness of benzyl benzoate, permethrin, and ivermectin in patients of scabies. Indian J Pharmacol 2009;41:9–14.
- Guzzo CA, Furtek CI, Porras AG, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 2002;42:1122–33.
- Gardon J, Gardon-Wendel N, Demanga-Ngangue, et al. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997;350:18–22.
- Barkwell R, Shields S. Deaths associated with ivermectin treatment of scabies. Lancet 1997;349:1144–5.
- Reintjes R, Hoek C. Deaths associated with ivermectin for scabies. Lancet 1997;350:215;author reply 216.
- Coyne PE, Addiss DG. Deaths associated with ivermectin for scabies. Lancet 1997;350:215–16;author reply 216.
- Alexander ND, Bockarie MJ, Kastens WA, et al. Absence of ivermectin-associated excess deaths. Trans R Soc Trop Med Hyg 1998;92:342.
- Del Giudice P, Marty P, Gari-Toussaint M, et al. Ivermectin in elderly patients. Arch Dermatol 1999;135:351–2.
- Burkhart CN, Burkhart CG. Before using ivermectin therapy for scabies. Pediatr Dermatol 1999;16:478–9;discussion 480.
- Burkhart CN, Burkhart CG. Ivermectin: a few caveats are warranted before initiating therapy for scabies. Arch Dermatol 1999;135:1549–50.
- Bredal WP. Deaths associated with ivermectin for scabies. Lancet 1997;350:216.
- Del Mar Sáez-De-Ocariz M, McKinster CD, Orozco-Covarrubias L, et al. Treatment of 18 children with scabies or cutaneous larva migrans using ivermectin. Clin Exp Dermatol 2002;27:264–7.
- Brooks PA, Grace RF. Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. J Paediatr Child Health 2002;38:401–4.
- Bécourt C, Marguet C, Balguerie X, et al. Treatment of scabies with oral ivermectin in 15 infants: a retrospective study on tolerance and efficacy. Br J Dermatol 2013;169:931–3.
- Centre de Reference sur les Agents Teratogenes (CRAT). http://www.lecrat.org/. (last accessed October 2014).
- US Food and Drug Administration (FDA). http://www.fda.gov. (last accessed October 2014).
- Mytton OT, McGready R, Lee SJ, et al. Safety of benzyl benzoate lotion and permethrin in pregnancy: a retrospective matched cohort study. Int J Obstet Gynaecol 2007;114:582–7.
- Burns BR, Lampe RM, Hansen GH. Neonatal scabies. Am J Dis Child 1979;133:1031–4.
- Jarrett P, Snow J. Scabies presenting as a necrotizing vasculitis in the presence of lupus anticoagulant. Br J Dermatol 1998;139:701–3.
- Valks R, Buezo GF, Daudén E. Scabies and leukocytoclastic vasculitis in an HIV-seropositive man. Int J Dermatol 1996;35:605–6.
Crusted scabies
- Danielssen DC, Boeck W. Traite de la Spedalsked ou Elephantiasis des Grecs. Paris: JB Baillière, 1848.
- Hebra F von S. Norvegica. In: On Diseases of the Skin including the Exanthemata, Vol. II. London: the New Sydenham Society, 1868:213–16.
- Calnan CD. Crusted scabies. Br J Dermatol 1950;62:71–8.
- Parish LC, Lomholt G. Crusted scabies: alias Norwegian scabies. Int J Dermatol 1976;15:747–8.
- Alexander JO'D. Arthropods and Human Skin. Berlin: Springer-Verlag, 1984.
- Burgess I. Sarcoptes scabiei and scabies. Adv Parasitol 1994;33:235–92.
- Herridge CF. Norwegian scabies (crusted scabies). BMJ 1963;1:239–40.
- Burks JW Jr, Jung R, George WM. Norwegian scabies. Arch Dermatol 1956;74:131–40.
- Logan JCP, Grant PW, Keczkes K. Norwegian scabies and lymphatic leukaemia. Br J Dermatol 1967;79:303–5.
- Suzumiya J, Sumiyoshi A, Kuroki Y, et al. Crusted (Norwegian) scabies with adult T-cell leukemia. Arch Dermatol 1985;121:903–4.
- Yonekura K, Kanekura T, Kanzaki T, et al. Crusted scabies in an adult T-cell leukaemia/lymphoma patient successfully treated with oral ivermectin. J Dermatol 2006;33:139–41.
- Barnes L, McCallister RE, Lucky AW. Crusted (Norwegian) scabies. Occurrence in a child undergoing bone marrow transplant. Arch Dermatol 1987;123:95–7.
- Espy PD, Jolly HW Jr. Norwegian scabies. Occurrence in a patient undergoing immunosuppression. Arch Dermatol 1976;112:193–6.
- Paterson WD, Allen BR, Beveridge CW. Norwegian scabies during immunosuppressive therapy. BMJ 1973;4:211–12.
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol 2005;52:719–20.
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine 2011;78:402–4.
- Fernández-Sánchez M, Saeb-Lima M, Alvarado-de la Barrera C, et al. Crusted scabies-associated immune reconstitution inflammatory syndrome. BMC Infect Dis 2012;12:323.
- Brites C, Weyll M, Pedroso C, Badaró R. Severe and Norwegian scabies are strongly associated with retroviral (HIV-1/HTLV-1) infection in Bahia, Brazil. AIDS 2002;16:1292–3.
- Bergman JN, Dodd WA, Trotter MJ, et al. Crusted scabies in association with human T-cell lymphotropic virus 1. J Cutan Med Surg 1999;3:148–52.
- Blas M, Bravo F, Castillo W, et al. Norwegian scabies in Peru: the impact of human T cell lymphotropic virus type 1 infection. Am J Trop Med Hyg 2005;72:855–7.
- Clayton R, Farrow S. Norwegian scabies following topical steroid therapy. Postgrad Med J 1975;51:657–9.
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol 2006;23:299–300.
- Judge MR, Kobza-Black A. Crusted scabies in pregnancy. Br J Dermatol 1995;132:116–19.
- Baysal V, Yildirim M, Türkman C, et al. Crusted scabies in a healthy infant. J Eur Acad Dermatol Venereol 2004;18:188–90.
- Roberts LJ, Huffam SE, Walton SF, Currie BJ. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect 2005;50:375–81.
- Guldbakke KK, Khachemoune A. Crusted scabies: a clinical review. J Drugs Dermatol 2006;5:221–7.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med 2010;362:717–25.
- Chosidow O. Clinical practices. Scabies. N Engl J Med 2006;354:1718–27.
- Anolik MA, Rudolph RI. Scabies simulating Darier disease in an immunosuppressed host. Arch Dermatol 1976;112:73–4.
- Wolf R, Wolf D, Viskoper RJ, et al. Norwegian-type scabies mimicking contact dermatitis. Postgrad Med 1985;78:228–30.
- Kartono F, Lee EW, Lanum D, et al. Crusted Norwegian scabies in an adult with Langerhans cell histiocytosis. Arch Dermatol 2007;143:626–8.
- Meinking TL, Taplin D, Hermida JL, et al. The treatment of scabies with ivermectin. N Engl J Med 1995;333:26–30.
- Huffam SE, Currie BJ. Ivermectin for Sarcoptes scabiei hyperinfestation. Int J Infect Dis 1998;2:152–4.
- Chosidow O. Scabies and pediculosis. Lancet 2000;355:819–26.
- Alberici F, Pagani L, Ratti G, Viale P. Ivermectin alone or in combination with benzyl benzoate in the treatment of human immunodeficiency virus-associated scabies. Br J Dermatol 2000;142:969–72.
- Del Giudice P. Ivermectin in scabies. Curr Opin Infect Dis 2002;15:123–6.
- Santoro AF, Rezac MA, Lee JB. Current trends in ivermectin usage for scabies. J Drugs Dermatol 2003;2:397–401.
- Monsel G, Chosidow O. Management of scabies. Skin Therapy Lett. 2012;17:1–4.
- Scott GR, Chosidow O. European guideline for the management of scabies. Int J STD AIDS 2010;22:301–3.
- Ortega-Loayza AG, McCall CO, Nunley JR. Crusted scabies and multiple dosages of ivermectin. J Drugs Dermatol 2013;12:584–5.
- Currie BJ, Harumal P, McKinnon M, et al. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis 2004;39:e8–12.
- Davis JS, McGloughlin S, Tong SY, et al. A novel clinical grading scale to guide the management of crusted scabies. PLOS Negl Trop Dis 2013;7:e2387.
Animal scabies
- Arlian LG, Runyan RA, Estes SA. Cross infestivity of Sarcoptes scabiei. J Am Acad Dermatol 1984;10:979–86.
- Chakrabarti A, Chatterjee A, Chakrabarti K, et al. Human scabies from contact with water buffaloes infested with Sarcoptes scabiei var. bubalis. Ann Trop Med Parasitol 1981;75:353–7.
- Chakravorty AN, Ghosh S, Banerjee AK. Case notes of scabies in a family transmitted from goats. Indian Med Gaz 1953;88:153–4.
- Fain A. Epidemiological problems of scabies. Int J Dermatol 1978;17:20–30.
- Goldman L, Feldman MD. Human infestation with scabies of monkeys. Arch Dermatol Syphilol 1949;59:175–8.
- Macdonald RAS. Observations on an extensive human infection by sarcoptic mange of the horse. Lancet 1922;i:738–9.
- Toomey N. Scabies of animal origin. Urol Cutan Rev 1922;26:473–89.
- Beck AL Jr. Animal scabies affecting man. Arch Dermatol 1965;91:54–5.
- Charlesworth EN, Johnson JL. An epidemic of canine scabies in man. Arch Dermatol 1974;110:572–4.
- Emde RN. Sarcoptic mange in the human. Arch Dermatol 1961;84:633–6.
- Ruiz-Maldonado R, Tamayo L, Dominguez J. Norwegian scabies due to Sarcoptes scabiei var. canis. Arch Dermatol 1977;113:1733.
- Smith EB, Claypoole TF. Canine scabies in dogs and humans. JAMA 1967;199:59–64.
- Tannenbaum MH. Canine scabies in man: a report of human mange. JAMA 1965;193:141–2.
- Thomsett LR. Mite infestations of man contracted from dogs and cats. BMJ 1968;3:93–5.
- Norins AL. Canine scabies in children. ‘Puppy dog’ dermatitis. Am J Dis Child 1969;117:239–42.
- Estes SA, Kummel B, Arlian L. Experimental canine scabies in humans. J Am Acad Dermatol 1983;9:397–401.
- Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology. Philadelphia: WB Saunders, 2000.
- Chakrabarti A. Human notoedric scabies from contact with cats infested with Notoedres cati. Int J Dermatol 1986;25:646–8.
- Ito K, Ito Y, Kondo S, et al. Animal scabies in humans. Bull Pharmacol Res Inst 1968;77:1–8.
- Bandi KM, Saikumar C. Sarcoptic mange: a zoonotic ectoparasitic skin disease. J Clin Diagn Res 2013;7:156–7.
- Burroughs RF, Elston DM. What's eating you? Canine scabies. Cutis 2003;72:107–9.
- McClain D, Dana AN, Goldenberg G. Mite Infestations. Dermatol Ther 2009;22:327–46.
Family Knemidokoptidae, family Psoroptidae and family Listrophoridae
- Toomey N. Scabies of animal origin. Urol Cutan Rev 1922;26:473–89.
- Elston DM. What's eating you? Psoroptes mites. Cutis 2006;77:283–4.
- Van de Heyning J, Thienpont D. Otitis externa caused by the mite Otodectes cynotis. Laryngoscope 1977;87:1938–41.
- Herwick RP. Lesions caused by canine ear mites. Arch Dermatol 1978;114:130.
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther 2009;22:327-46.
- Owen D. Common Parasites of Laboratory Rodents and Lagomorphs. Medical Research Council Laboratory Animals Centre Handbook, No. 1. London: HMSO, 1972.
- Burns DA. Papular urticaria produced by the mite Listrophorus gibbus. Clin Exp Dermatol 1987;12:200–1.
Mites of stored products
- Hughes AM. The Mites of Stored Food and Houses, 2nd edn. Ministry of Agriculture, Fisheries and Food Technical Bulletin, No. 9. London: HMSO, 1976.
- Tee RD. Allergy to storage mites. Clin Exp Allergy 1994;24:636–40.
- Estévez DQ. Occupational contact urticaria-dermatitis by Tyrophagus putrescentiae. Contact Dermatitis 2006;55:308–9.
- Fields JP, Hoke AW, Cronce PC. Cheese mite dermatitis. Arch Dermatol 1968;98:669–70.
- Vidal C, Rial A. Airborne contact dermatitis from Tyrophagus putrescentiae. Contact Dermatitis 1998;33:181.
- Armentia A, Fernández A, Pérez-Santos C, et al. Occupational allergy to mites in salty ham, chorizo and cheese. Allergol Immunopathol Madr 1994;22:152–4.
- Thomas EWP. Dermatitis due to Tyroglyphus longior Gerv. var Castellani, Hirst in cheese dust. Br J Dermatol 1942;54:313–19.
- Kilpiö O, Pirilä V. A new tyroglyphid mite causing dermatitis. Acta Derm Venereol Suppl. (Stockh) 1952;29:197–200.
- Pirilä V. On cheese and fig mite dermatitis. Acta Derm Venereol (Stockh) 1951;31:630–7.
- Pirilä V, Kilpiö O. Occupational mite dermatitis. Acta Derm Venereol (Stockh) 1954;34:368–71.
- Elder BL, Morgan MS, Arlian LG. Effect of stored product mite extracts on human dermal microvascular endothelial cells. J Med Entomol. 2012;49:1411–18.
- Alexander JO'D. Skin eruptions caused by mites from stored food. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:345–52.
House-dust mites
- Hughes AM. The Mites of Stored Food and Houses. Ministry of Agriculture, Fisheries and Food Technical Bulletin 9. London: HMSO, 1976.
- Maunsell K, Wraith DG, Cunnington AM. Mites and house-dust allergy in bronchial asthma. Lancet 1968;1:1267–70.
- Sesay HR, Dobson RM. Studies on the mite fauna of house dust in Scotland with special reference to that of bedding. Acarologia 1972;14:384–92.
- Spieksma FT, Spieksma-Boezeman MI. The mite fauna of house dust with particular reference to the house-dust mite Dermatophagoides pteronyssinus (Trouessart, 1897) (Psoroptidae: Sarcoptiformes). Acarologia 1967;9:226–41.
- Wharton GW. Mites and commercial extracts of house dust. Science 1970;167:1382–3.
- Hughes AM, Maunsell K. A study of a population of house dust mite in its natural environment. Clin Allergy 1973;3:127–31.
- Voorhorst R, Spieksma FThM, Varekamp H. House-dust Atopy and the House-dust Mite Dermatophagoides pteronyssinus (Trouessart 1897). Leiden: Stafleu's Scientific Publishing, 1969.
- Platts-Mills TA, Mitchell EB, Rowntree S, et al. The role of dust mite allergens in atopic dermatitis. Clin Exp Dermatol 1983;8:233–47.
- Tupker RA, De Monchy JG, Coenraads PJ, et al. Induction of atopic dermatitis by inhalation of house dust mite. J Allergy Clin Immunol 1996;97:1064–70.
- Reitamo S, Visa K, Kähönen K, et al. Eczematous reactions in atopic patients caused by epicutaneous testing with inhalant allergens. Br J Dermatol 1986;114:303–9.
- Norris PG, Schofield O, Camp RD. A study of the role of house dust mite in atopic dermatitis. Br J Dermatol 1988;118:435–40.
- Shah D, Hales J, Cooper D, et al. Recognition of pathogenically relevant house dust mite hypersensitivity in adults with atopic dermatitis: a new approach? J Allergy Clin Immunol 2002;109:1012–18.
- Beltrani VS. The role of house dust mites and other aeroallergens in atopic dermatitis.Clin Dermatol 2003;21:177–82.
- Gutgesell C, Heise S, Seubert S, et al. Double-blind placebo-controlled house dust mite control measures in adult patients with atopic dermatitis. Br J Dermatol 2001;145:70–4.
- Oosting A-J, de Bruin-Weller MS, Terreehorst I, et al. Effect of mattress encasings on atopic dermatitis outcome measures in a double-blind, placebo-controlled study: the Dutch mite avoidance study. J Allergy Clin Immunol 2002;110:500–6.
- Fuiano N, Fusilli S, Incorvaia C. House dust mite-related allergic diseases: role of skin prick test, atopy patch test, and RAST in the diagnosis of different manifestations of allergy. Eur J Pediatr 2010;169:819–24.
- Casimir GJ, Duchateau J, Gossart B, et al. Atopic dermatitis: role of food and house dust mite allergens. Pediatrics 1993;92:252–6.
- Roberts DL. House dust mite avoidance and atopic dermatitis. Br J Dermatol 1984;110:735–6.
- Tan BB, Weald D, Strickland I, et al. Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet 1996;347:15–18.
- Ricci G, Patrizi A, Specchia F, et al. Effect of house dust mite avoidance measures in children with atopic dermatitis. Br J Dermatol 2000;143:379–84.
- Friedmann PS. Dust mite avoidance in atopic dermatitis. Clin Exp Dermatol 1999;24:433–7.
- Arshad SH, Bateman B, Sadeghnejad A, et al. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol 2007;119:307–13.
- Beck HI, Korsgaard J. Atopic dermatitis and house dust mites. Br J Dermatol 1989;120:245–51.
- Garritsen FM, ter Haar NM, Spuls PI. House dust mite reduction in the management of atopic dermatitis. A critically appraised topic. Br J Dermatol 2013;168:688–91.
- Nankervis H, Smith EV, Boyle RJ et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Library Protocol. http://www.thecochranelibrary.com. (last accessed October 2014).
- Cameron MM. Can house dust mite-triggered atopic dermatitis be alleviated using acaricides? Br J Dermatol 1997;137:1–8.
Pyemotes mites
- Booth BH, Jones RW. Epidemiological and clinical study of grain itch. J Am Med Assoc 1952;150:1575–9.
- Uenotsuchi T, Satoh E, Kiryu H, et al. Pyemotes dermatitis caused by indirect contact with husk rice. Br J Dermatol 2000;143:680–2.
- Rycroft RJ, Kennedy C. Pyemotes dermatitis in display artists. Clin Exp Dermatol 1981;6:629–34.
- Betz TG, Davis BL, Fournier PV, et al. Occupational dermatitis associated with straw itch mites (Pyemotes ventricosus). JAMA 1982;247:2821–3.
- Grob M, Dorn K, Lautenschlager S. [Grain mites. A small epidemic caused by Pyemotes species.] Hautarzt 1998;49:838–43.
- Samsinák K, Chmela J, Vobrázková E. Pyemotes herfsi (Oudemans, 1936) as causative agent of another mass dermatitis in Europe (Acari, Pyemotidae). Folia Parasitol 1979;26:51–4.
- Le Fichoux Y, Rack G, Motte P, et al. [Pruriginous dermatitis due to Pyemotes zwoelferi Krczal, 1963. About several cases in the Alpes-Maritimes (France) (author's transl.).] Acta Trop 1980;37:83–9.
- Hewitt M, Barrow GI, Miller DC, et al. A case of Pyemotes dermatitis. With a note on the role of these mites in skin disease. Br J Dermatol 1976;94:423–30.
- Letchford J, Strungs I, Farrell D. Pyemotes species strongly implicated in an outbreak of dermatitis in a Queensland country hospital. Pathology 1994;26:330–2.
- Rodríguez-Casado MJ, Cerro-González R, Martín-Blázquez JL, et al. [Outbreak of Pyemotes dermatitis in an elementary school.] Enferm Infecc Microbiol Clin 2004;22:370–1.
- Del Giudice P, Blanc-Amrane V, Bahadoran P, et al. Pyemotes ventricosus dermatitis, southeastern France. Emerging Infect Dis 2008;14:1759–61.
- Alexander JO'D. Pyemotes infestation. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:317–25.
- Fine RM, Scott HG. Straw itch mite dermatitis caused by Pyemotes ventricosus: comparative aspects. South Med J 1965;58:416–20.
- Del Giudice P, Caumes E, Boissy C, et al. An outbreak of creeping eruption in southern France. Br J Dermatol 2007;157:824–5.
- Bellido-Blasco JB, Arnedo-Pena A, González-Morán F, et al. [Dermatitis outbreaks caused by Pyemotes.] Med Clin (Barc) 2000;114:294–6.
Family Tydeidae
- Stingeni L, Principato M. Epidemic occupational dermatitis caused by Pronematus davisi (Acari: Tydeidae). Br J Dermatol 2002;146:929–30.
Plant mites
- Derrick EH. A tetranychid mite which may attack man. Aust J Sci 1954;17:67–8.
- Desch CE Jr. Mites causing or transmitting human disease. In: Parish LC, Nutting WB, Schwartzman RM, eds. Cutaneous Infestations of Man and Animal. New York: Praeger, 1983:261–83.
- Manson DCM. The spider mite family Tetranychidae in New Zealand V — Tetranychus (Tetranychus) moutensis. A new species of spider mite from flax (Phormium tenax Forst). N Z J Sci 1970;13:323–7.
- Southcott RV. Australian Harmful Arachnids and their Allies. Mitcham: Southcott RV, 1978.
- Jeebhay MF, Baatjies R, Chang YS, et al. Risk factors for allergy due to the two-spotted spider mite (Tetranychus urticae) among table grape farm workers. Int Arch Allergy Immunol 2007;144:143–9.
Cheyletiella mites
- Fox TS, Ewing SA. Morphologic features, behavior, and life history of Cheyletiella yasguri. Am J Vet Res 1969;30:269–85.
- Miller WH Jr. Cheyletiella infestation. In: Parish LC, Nutting WB, Schwartzman RM, eds. Cutaneous Infestations of Man and Animal. New York: Praeger, 1983:255–60.
- Lomholt S. To Tilfaelde af Dyrefnat hos Mennesket (Cheiletiella parasitivorax). Hospitalstidende 1918;61:1098–9.
- Davies JHT. Another acarine disease. Br J Dermatol 1938;50:243–4.
- Keh B. Intense pruritus in man and concurrent infestation of Cheyletiella blakei Smiley (Acari: Cheyletiellidae) on cats in a home in California. Calif Vector Views 1975;22:1–4.
- Ayalew L, Vaillancourt M. Observations on an outbreak of infestation of dogs with Cheyletiella yasguri and its public health implications. Can Vet J 1976;17:184–91.
- Moxham JW, Goldfinch TT, Heath ACG. Cheyletiella parasitivorax infestation of cats associated with skin lesions of man. NZ Vet J 1968;16:50–2.
- Taylor RM. Cheyletiella parasitivorax infestation of a cat and associated skin lesions of man. Aust Vet J 1969;45:435.
- Gething MA, Walton GS. Possible host specificity of Cheyletiella mites. Vet Rec 1972;88:512.
- Smiley RL. A review of the family Cheyletiellidae (Acarina). Ann Entomol Soc Am 1970;63:1056–78.
- Hewitt M, Turk SM. Cheyletiella sp. in the personal environment. Br J Dermatol 1974;90:679–83.
- Van Bronswijk JEMH, de Kreek EJ. Cheyletiella (Acari: Cheyletiellidae) of dog, cat and domesticated rabbit: a review. J Med Entomol 1976;13:315–27.
- Ottenschot TRF, Gil D. Cheyletiellosis in long-haired cats. Tijdschrg Diergeneesk 1978;103:1104–8.
- Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology. Philadelphia: WB Saunders, 2000.
- Alexander JO'D. Infestation with cheyletiellid mites. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:327–35.
- Hewitt M, Walton GS, Waterhouse M. Pet animal infestations and human skin lesions. Br J Dermatol 1971;85:215–25.
- Wagner R, Stallmeister N. Cheyletiella dermatitis in humans, dogs and cats. Br J Dermatol 2000;143:1110–12.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol 1997;37:265–7.
- Tsianakas P, Polack B, Pinquier L et al. La cheyletellose: une étiologie inhabituelle d'éruption vesiculobulleuse. Ann Dermatol Venereol 2000;127:826–9.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol 2007;46:397–9.
- Maurice PDL, Schofield O, Griffiths WAD. Cheyletiella dermatitis: a case report and the role of specific immunological hypersensitivity in its pathogenesis. Clin Exp Dermatol 1987;12:381–4.
- Burns DA. The investigation and management of arthropod bite reactions acquired in the home. Clin Exp Dermatol 1987;12:114–20.
- Elston DM. What's eating you? Cheyletiella mites. Cutis 2004;74:23–4.
Harvest mites
- Kettle DS. Acari – Prostigmata and Gamasida (chiggers, blood-sucking mites). In: Medical and Veterinary Entomology. London: Croom Helm, 1984:380–405.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology 1950;40:247–60.
- Richards WS. The distribution and biology of the harvest mite in Great Britain (Trombiculidae, Acarina). Parasitology 1950;40:118–26.
- Alexander JO'D. Infestation with trombiculid mite larvae. In: Arthropods and Human Skin. Berlin: Springer-Verlag 1984:353–62.
- Sheals JG. Arachnida (scorpions, spiders, ticks etc.). In: Smith KGV, ed. Insects and other Arthropods of Medical Importance. London: the Trustees of the British Museum (Natural History), 1973: 417–72.
- Uchikawa K, Kumada N. Endemic outbreaks of tsutsugamushi disease in Japan and vector chiggers (Trombidiformes: Trombiculidae). In: Channabasavanna GP, Viraktamath CA, eds. Progress in Acarology, Vol. 1. New Delhi: Oxford and IBH, 1988:103–6.
- Heyne H, Ueckermann EA, Coetzee L. First report of a parasitic mite, Leptotrombidium (Hypotrombidium) subquadratum (Lawrence) (Acari: Trombiculidae: Trombiculinae), from dogs and children in the Bloemfontein area, South Africa. J S Afr Vet Assoc 2001;72:105–6.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol 1983;22:75–91.
- Poulson PA. Cutaneous reactions to some parasitic arthropods with special reference to the harvest mite (Trombicula autumnalis Shaw). Acta Derm Venereol Suppl. (Stockh) 1952;29:290–3.
- Smith GA, Sharma V, Knapp JF, Shields BJ. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care 1998;14:116–18.
- Pritchard JG, Kossoris PD, Leibovitch RA, et al. Implications of trombiculid mite bites: report of a case and submission of evidence in a murder trial. J Forensic Sci 1986;32:301–6.
- Elston DM. What's eating you? Chiggers. Cutis 2006;77:350–2.
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther 2009;22:327–46.
Bird, rodent and reptile mites (Gamasida)
- Kettle DS. Medical and Veterinary Entomology. London: Croom Helm, 1984:391–9.
- Alexander JO'D. Infestation with gamasid mites. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:303–15.
- Auger P, Nantel J, Meunier N, et al. Skin acariasis caused by Dermanyssus gallinae (de Geer): an in-hospital outbreak. Can Med Assoc J 1979;120:700–3.
- Naltsas S, Hodge SJ, Gataky GJ Jr, et al. Eczematous dermatitis caused by Dermanyssus americanus. Cutis 1980;25:429–31.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med 1987;147:2185–7.
- Bellanger AP, Bories C, Foulet F, et al. Nosocomial dermatitis caused by Dermanyssus gallinae. Infect Control Hosp Epidemiol 2008;29:282–3.
- Sexton DJ, Haynes B. Bird-mite infestation in a university hospital. Lancet 1975;1:445.
- Uesugi Y, Aiba S, Suetake T, et al. Multiple infestations with avian mites within a family. Int J Dermatol 1994;33:566–7.
- Lucky AW, Sayers C, Argus JD, et al. Avian mite bites acquired from a new source – pet gerbils: report of 2 cases and review of the literature. Arch Dermatol 2001;137:167–70.
- Circella E, Pugliese N, Todisco G, et al. Chlamydia psittaci infection in canaries heavily infested by Dermanyssus gallinae. Exp Appl Acarol 2011;55:329–38.
- Valiente Moro C, De Luna CJ, Tod A, et al. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol 2009;48:93–104.
- Hidano A, Asanuma K. Letter: Acariasis caused by bird mites. Arch Dermatol 1976;112:881–2.
- Lodha KR. The occurrence of tropica fowl mite, Ornithonyssus (Bdellonyssus, Liponyssus) bursa on man in Rajasthan (India). Vet Rec 1969;84:363–5.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol 2000;25:129–31.
- Charlesworth EN, Clegern RW. Tropical rat mite dermatitis. Arch Dermatol 1977;113:937–8.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol 1982;118:676–8.
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis 1998;27:1465–9.
- Chung SL, Hwang SJ, Kwon SB, et al. Outbreak of rat mite dermatitis in medical students. Int J Dermatol 1998;37:591–4.
- Creel NB, Crowe MA, Mullen GR. Pet hamsters as a source of rat mite dermatitis. Cutis 2003;71:457–61.
- Beck W, Pfister K. [Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings in Munich: 3 case reports.] Wien Klin Wochenschr 2004;116(Suppl. 4):65–8.
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology (Basel) 2007;215:66–8.
- Schultz H. Human infestation by Ophionyssus natricis snake mite. Br J Dermatol 1975;93:695–7.
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol 2013;38:374–7.
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther 2009;22:327–46.
- Rossiter A. Occupational otitis externa in chicken catchers. J Laryngol Otol 1997;111:366–7.
- Dogramaci AC, Culha G, Ozçelik S. Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatol Treat 2010;21:319–21.
Follicle mites (Demodicidae)
- Hirst S. Studies on Acari, No. 1. The Genus Demodex, Owen. London: the British Museum (Natural History), 1919.
- King DF, King LA, Rabson SM. Demodex folliculorum of Simon. J Am Acad Dermatol 1983;8:907–8.
- Nutting WB. Hair follicle mites (Acari: Demodicidae) of man. Int J Dermatol 1976;15:79–98.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol 1972;58:169–77.
- Riechers R, Kopf AW. Cutaneous infestation with Demodex folliculorum in man. J Invest Dermatol 1969;52:103–6.
- Jimenez-Acosta F, Planas L, Penneys N. Demodex mites contain immunoreactive lipase. Arch Dermatol 1989;125:1436–7.
- Franklin CD, Underwood JC. Demodex infestation of oral mucosal sebaceous glands. Oral Surg Oral Med Oral Pathol 1986;61:80–2.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol 1982;7:583–9.
- Sengbusch HG, Hauswirth JW. Prevalence of hair follicle mites, Demodex folliculorum and d. brevis (Acari: Demodicidae), in a selected human population in western New York, USA. J Med Entomol 1986;23:384–8.
- Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol 1993;128:650–9.
- Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol 1993;28:443–8.
- Maier T, Sattler E, Braun-Falco M, et al. High-definition optical coherence tomography for the in vivo detection of demodex mites. Dermatology (Basel) 2012;225:271–6.
- Sattler EC, Maier T, Hoffmann VS, et al. Noninvasive in vivo detection and quantification of Demodex mites by confocal laser scanning microscopy. Br J Dermatol 2012;167:1042–7.
- Lacey N, Forton FMN, Powell FC. Demodex quantification methods: limitations of confocal laser scanning microscopy. Br J Dermatol 2013;169:212–13.
- Ayres S. Rosacea-like demodicidosis . Calif Med 1963;98:328–30.
- Dominey A, Tschen J, Rosen T, et al. Pityriasis folliculorum revisited. J Am Acad Dermatol 1989;21:81–4.
- Fariña MC, Requena L, Sarasa JL, et al. Spinulosis of the face as a manifestation of demodicidosis. Br J Dermatol 1998;138:901–3.
- Burns DA. Follicle mites and their role in disease. Clin Exp Dermatol 1992;17:152–5.
- Elston DM. Demodex mites: facts and controversies. Clin Dermatol 2010;28:502–4.
- Lacey N, Ní Raghallaigh S, Powell FC. Demodex mites – commensals, parasites or mutualistic organisms? Dermatology (Basel) 2011;222:128–30.
- Diaz-Perez JL. Demodex mites in rosacea. J Am Acad Dermatol 1994;30:812–13.
- Erbağci Z, Ozgöztaşi O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol 1998;37:421–5.
- Robinson TW. Demodex folliculorum and rosacea. A clinical and histological study. Arch Dermatol 1965;92:542–4.
- Marks R, Harcourt-Webster JN. Histopathology of rosacea. Arch Dermatol 1969;100:683–91.
- Ramelet AA, Perroulaz G. [Rosacea: histopathologic study of 75 cases.] Ann Dermatol Venereol 1988;115:801–6.
- Zhao YE, Wu LP, Peng Y, et al. Retrospective analysis of the association between Demodex infestation and rosacea. Arch Dermatol 2010;146:896–902.
- Rufli T, Büchner SA. T-cell subsets in acne rosacea lesions and the possible role of Demodex folliculorum. Dermatologica 1984;169:1–5.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol 2007;157:474–81.
- Grosshans EM, Kremer M, Maleville J. [Demodex folliculorum and the histogenesis of granulomatous rosacea.] Hautarzt 1974;25:166–77.
- Kürkçüoğlu N, Atakan N. Metronidazole in the treatment of rosacea. Arch Dermatol 1984;120:837.
- Persi A, Rebora A. Metronidazole and Demodex folliculorum. Acta Derm Venereol 1981;61:182–3.
- Ayres S Jr, Ayres S 3rd. Demodectic eruptions (demodicidosis) in the human. 30 years' experience with 2 commonly unrecognized entities: pityriasis folliculorum (Demodex) and acne rosacea (Demodex type). Arch Dermatol 1961;83:816–27.
- Lindmaier A, Jurecka W, Lindemayr H. Demodicidosis mimicking granulomatous rosacea and transient acantholytic dermatosis (Grover's disease). Dermatologica 1987;175:200–4.
- Shelley WB, Shelley ED, Burmeister V. Unilateral demodectic rosacea. J Am Acad Dermatol 1989;20:915–17.
- Forstinger C, Kittler H, Binder M. Treatment of rosacea-like demodicidosis with oral ivermectin and topical permethrin cream. J Am Acad Dermatol 1999;41:775–7.
- Hoekzema R, Hulsebosch HJ, Bos JD. Demodicidosis or rosacea: what did we treat? Br J Dermatol 1995;133:294–9.
- Forton F, Seys B, Marchal JL, et al. Demodex folliculorum and topical treatment: acaricidal action evaluated by standardized skin surface biopsy. Br J Dermatol 1998;138:461–6.
- Sahn EE, Sheridan DM. Demodicidosis in a child with leukemia. J Am Acad Dermatol 1992;27:799–801.
- Ivy SP, Mackall CL, Gore L, et al. Demodicidosis in childhood acute lymphoblastic leukemia;an opportunistic infection occurring with immunosuppression. J Pediatr 1995;127:751–4.
- Castanet J, Monpoux F, Mariani R, et al. Demodicidosis in an immunodeficient child. Pediatr Dermatol 1997;14:219–20.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol 2003;42:724–6.
- Nakagawa T, Sasaki M, Fujita K, et al. Demodex folliculitis on the trunk of a patient with mycosis fungoides. Clin Exp Dermatol 1996;21:148–50.
- Ashack RJ, Frost ML, Norins AL. Papular pruritic eruption of Demodex folliculitis in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol 1989;21:306–7.
- Dominey A, Rosen T, Tschen J. Papulonodular demodicidosis associated with acquired immunodeficiency syndrome. J Am Acad Dermatol 1989;20:197–201.
- Sanchez-Viera M, Hernanz JM, Sampelayo T, et al. Granulomatous rosacea in a child infected with the human immunodeficiency virus. J Am Acad Dermatol 1992;27:1010–11.
- Jansen T, Kastner U, Kreuter A, et al. Rosacea-like demodicidosis associated with acquired immunodeficiency syndrome. Br J Dermatol 2001;144:139–42.
- Won JH, Ahn SK, Lee SH. Unusual manifestation of demodicidosis in a child. Int J Dermatol 1993;32:822.
- Patrizi A, Neri I, Chieregato C, et al. Demodicidosis in immunocompetent young children: report of eight cases. Dermatology (Basel) 1997;195:239–42.
- García-Vargas A, Mayorga-Rodríguez JA, Sandoval-Tress C. Scalp demodicidosis mimicking favus in a 6-year-old boy. J Am Acad Dermatol 2007;57:S19–21.
- Dolenc-Voljc M, Pohar M, Lunder T. Density of Demodex folliculorum in perioral dermatitis. Acta Derm Venereol 2005;85:211–15.
- Norn MS. Demodex folliculorum. Incidence and possible pathogenic role in the human eyelid. Acta Ophthalmol Suppl 1970;108:7–85.
- Holzchuh FG, Hida RY, Moscovici BK, et al. Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. Am J Ophthalmol 2011;151:1030–4.e1.
- Filho PAN, Hazarbassanov RM, Grisolia ABD, et al. The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex spp. Br J Ophthalmol 2011;95:893–5.
Centipedes and millipedes
- Alexander JO'D. Centipede bites and millipede burns. In: Arthropods and Human Skin. Berlin: Springer-Verlag, 1984:383–9.
- Burnett JW, Calton GJ, Morgan RJ. Centipedes. Cutis 1986;37:241.
- Lin TJ, Yang CC, Yang GY, et al. Features of centipede bites in Taiwan. Trop Geogr Med 1995;47:300–2.
- Elston DM. What's eating you? Centipedes (Chilopoda). Cutis 1999;64:83.
- Bush SP, King BO, Norris RL, et al. Centipede envenomation. Wilderness Environ Med 2001;12:93–9.
- Fung HT, Lam SK, Wong OF. Centipede bite victims: a review of patients presenting to two emergency departments in Hong Kong. Hong Kong Med J 2011;17:381–5.
- Senthilkumaran S, Meenakshisundaram R, Michaels AD, et al. Acute ST-segment elevation myocardial infarction from a centipede bite. J Cardiovasc Dis Res 2011;2:244–6.
- Yildiz A, Biçeroglu S, Yakut N, et al. Acute myocardial infarction in a young man caused by centipede sting. Emerg Med J 2006;23:e30.
- Undheim EAB, King GF. On the venom system of centipedes (Chilopoda), a neglected group of venomous animals. Toxicon 2011;57:512–24.
- Malta MB, Lira MS, Soares SL, et al. Toxic activities of Brazilian centipede venoms. Toxicon 2008;52:255–63.
- Chaou C-H, Chen C-K, Chen J-C, et al. Comparisons of ice packs, hot water immersion, and analgesia injection for the treatment of centipede envenomations in Taiwan. Clin Toxicol (Phila) 2009;47:659–62.
- Radford AJ. Millipede burns in man. Trop Geogr Med 1975;27:279–87.
- Shpall S, Frieden I. Mahogany discoloration of the skin due to the defensive secretion of a millipede. Pediatr Dermatol 1991;8:25–7.
- Mason GH, Thomson HD, Fergin P, et al. Spot diagnosis. The burning millipede. Med J Aust 1994;160:718;726.
- Elston DM. What's eating you? Millipedes (Diplopoda). Cutis 2001;67:452.
- De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila) 2011;49:187–90.
- Lima CAJ, Cardoso JLC, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda Class (“millipedes”). An Bras Dermatol 2010;85:391–2.
- Hudson BJ, Parsons GA. Giant millipede “burns” and the eye. Trans R Soc Trop Med Hyg 1997;91:183–5.