CHAPTER 39
Eczematous Disorders
John R. Ingram
Department of Dermatology & Wound HealingCardiff University, UK
ASSESSMENT, INVESTIGATION AND MANAGEMENT OF ECZEMATOUS DISORDERS
Eczema
Definition and nomenclature
Eczema, a term derived from the Greek word ‘ε′κζεμα’ meaning ‘to boil’, is a clinical and histological pattern of inflammation of the skin seen in a variety of dermatoses with widely diverse aetiologies. Clinically, eczematous dermatoses are characterized by variable intensity of itching and soreness, and, in variable degrees, a range of signs including dryness, erythema, excoriation, exudation, fissuring, hyperkeratosis, lichenification, papulation, scaling and vesiculation. Histologically, the clinical signs are reflected by a range of epidermal changes including spongiosis (epidermal oedema) with varying degrees of acanthosis and hyperkeratosis, accompanied by a lymphohistiocytic infiltrate in the dermis.
Introduction and general description
In order to structure our understanding of the large number of eczematous dermatoses, a number of classification systems have been advanced. Classification based on aetiology has been proposed and is a useful system where the cause is clear, for example patch test-proven allergic contact dermatitis. However, frequently the aetiology is unclear or, for example in hand eczema, there are a number of probable aetiologies in an individual patient. Another option is to use the morphology of the condition as a classification, for example in the case of nummular dermatitis where ‘nummular’ is derived from the Latin word ‘nummus’, which means ‘coin’. However, one morphological pattern of eczema can have a variety of causes, for example pompholyx eczema can be due to irritant or allergic contact dermatitis. In addition, the morphology of an acute presentation, which tends to be oedematous and exudative (Figure 39.1a), may differ from chronic eczema, in which the predominant features are dryness, hyperkeratosis and lichenification (Figure 39.1b).

Figure 39.1 (a) Acute eczema of the arm, with erythema and marked exudation. (b) Chronic eczema of the arm.
More recently, the International Statistical Classification of Diseases and Related Health Problems 11th Revision (ICD-11) is moving towards a composite system based on aetiology, where known, and skin site. Box 39.1 provides a list of the eczematous dermatoses split into those with a known exogenous trigger and those that are thought to be more endogenous. Management of exogenous eczemas is to remove the cause if possible, whereas endogenous eczemas more often require pharmacological intervention. All of the conditions listed are defined and described in further detail in this and subsequent chapters. Some disorders in which the predominant feature is lichenification are generally regarded as eczematous dermatoses and these are also included in this chapter. Eczema is the commonest cause of erythroderma and so this clinical presentation is described here.
There remain cases of eczema that do not fit any of the described patterns [3]. These are not uncommon and have been termed ‘unclassified eczema’ [4]. These patients may have a poor prognosis, with a tendency for the disease to become chronic. It is not unusual to see generalized, apparently endogenous, but otherwise unclassified eczema in the elderly [5].
Epidemiology
Incidence and prevalence
Eczematous dermatoses account for a large proportion of all skin disease. There have been numerous studies of the prevalence of atopic eczema (see Chapter 41), but fewer in other types of eczema. A survey of the prevalence of skin disease was carried out in the USA on a sample of over 20 000 people who were representative of the whole population, and who were examined meticulously for skin disease by trained observers [6]. Nearly one-third had some significant skin pathology. The point prevalence of all forms of eczema was 18 per 1000, seven of whom had atopic eczema. Hand eczema, dyshidrotic eczema and nummular eczema each accounted for about two per 1000. In a population-based survey of public health issues in Stockholm, Sweden, a postal questionnaire was sent to 15 000 adult inhabitants. Fifteen per cent of respondents reported a history of childhood eczema. The 1-year prevalence of hand eczema was 8%, skin symptoms on the face were present in 14% and allergy to nickel was reported in 15% of females [7]. Population-based studies of contact allergic dermatitis suggest 40% of subjects demonstrate at least one contact allergic reaction [8].
The Royal College of General Practitioners Birmingham, UK Research Unit Weekly Returns Unit data for 2006 reported the 1-year period prevalence of non-atopic eczema to be 25 per 1000 population [9]. Consultations for eczema are a major part of the workload in primary care. Horn [10] recorded the details of 6819 dermatological consultations in a UK general practice of 3000–4000 patients from 1958 to 1985. Eczema patients formed the largest group (19% of the consultations). In a general practice in Belfast, 8% of patients seen during an 8-week period had a dermatological condition. Eczema accounted for 25% of these, of which 63% were considered to be exogenous in origin [11].
In addition, many cases of eczema are referred to hospital. In the UK, eczema consultations account for 10% of the total secondary care dermatology caseload [9]. A national tertiary referral centre in Singapore described the profile of eczema referred between 1989 and 1990 [12]; 25 448 new cases were analysed. These represented 34% of new cases seen at the centre. Sixty-seven per cent of eczema cases were classified as endogenous and 13.7% were contact dermatitis. Exfoliative dermatitis comprised 0.5% of all eczemas. The authors commented on the increase in the proportion of endogenous eczema seen in 1989–90 compared with that reported in 1973.
Age and sex
Certain subtypes of eczema can be seen more commonly in particular age groups. Most cases of eczema in infants and young children are atopic. In the National Health and Nutrition Examination Survey (NHANES) epidemiological survey in the USA [13], atopic eczema was by far the most common form found up to the age of 11 years; discoid (nummular) and ‘dyshidrotic’ eczema were recorded, but were much less frequent. Perioral eczema is common in children with atopic eczema (see Chapter 41), but it can also occur in non-atopic children. Hand eczema is common in atopic children and uncommon in non-atopic children. Several specific patterns of eczematous change are predominantly found in children; for example, juvenile plantar dermatosis and napkin dermatitis.
Pompholyx and atopic eczema are less common in elderly people, while other forms of eczema assume greater importance. Nummular dermatitis occurs particularly in elderly males in winter, and asteatotic eczema of the legs is also common. In older factory workers, irritant hand eczema can be very troublesome, although allergic contact dermatitis becomes less common with advancing age. The subject is discussed more fully in Chapter 128.
Ethnicity
Eczematous dermatoses are reported in all ethnic groups but data are limited regarding ethnic differences for non-atopic eczema.
Associated diseases
Atopic eczema is associated with some forms of non-atopic eczematous dermatoses, in particular hand eczema (see Chapter 41).
Pathophysiology
There has been considerable research on the pathogenesis of some types of eczema, particularly allergic contact dermatitis, primary irritant contact dermatitis and atopic eczema. One particular challenge in this research field has been distinguishing non-specific common pathways from specific mechanisms. The interaction of trigger factors, keratinocytes and T lymphocytes seems particularly important in most eczema types.
Allergic contact dermatitis represents a reproducible model of eczema development [14], as discussed in Chapter 128. Eczema may also be provoked in a non-allergic manner, as in irritant contact dermatitis. Three predominant processes occurring in irritant dermatitis are disturbed barrier function, epidermal cell change and release of inflammatory mediators and cytokines.
Certain irritants may provoke a chronic reaction in which an effect on epidermal cell turnover predominates, leading to lichenification; whereas in acute irritant reactions inflammatory mediator and cytokine release is similar to that seen in acute allergic contact dermatitis [15]. There is debate whether the cytokine profiles of the two reactions differ [15–17]. Indeed, it has been proposed that irritant reactions to haptens may be required to facilitate contact sensitization [18].
After activation of the immune pathway by cytokine release, the accumulation of inflammatory cells progresses, leading to the morphological changes that are apparent histologically and clinically. The histological changes of primary irritant dermatitis are similar to those seen in allergic contact dermatitis, but they appear to proceed more quickly, depending on the concentration of the irritant used. Both intracellular and intercellular oedema are visible throughout the epidermis at 3–6 h, and within 24 h there may be epidermal necrosis, with cellular vacuolation and nuclear pyknosis. In severe forms, the primary epidermal damage may progress to subepidermal blister formation.
As discussed later in this chapter and in Chapter 41, genetic studies have demonstrated the importance of filaggrin, a 37 kDa intracellular protein that binds to and condenses the keratin cytoskeleton, resulting in squame formation [19]. Loss-of-function mutations of the fillaggrin gene result in impaired skin barrier function and are linked to atopic eczema and some subtypes of non-atopic eczema.
Predisposing factors
These will be discussed in relation to each subtype of eczema in the relevant sections of this chapter.
Pathology
The histopathological features of eczema, in common with the clinical features, are variable, and depend particularly on whether the disease is acute or chronic [20–23].
In the acute phase (Figure 39.2a), the histological picture is dominated by spongiosis, an intercellular epidermal oedema that leads to stretching and eventual rupture of the intercellular attachments, with the formation of vesicles. The epidermal vesicles commonly occur in discrete foci, but on the palms and soles they tend to become large by coalescence. There is variable infiltration of the epidermis by lymphocytes. Increased epidermal mitotic activity leads to acanthosis but, if spongiosis is intense, disintegration of the suprapapillary epidermis may cause clefts to form. The intercellular oedema may be diffuse, but is commonly most intense in the mid-epidermal region. Loosening and disruption of the individual cells occurs and some intracellular vacuolation may be found, with displacement of the nucleus from the centre of the cell. Loose, shrunken epidermal cells may resemble histiocytes. Vesicles and the oedematous epidermis may be permeated by mononuclear cells, chiefly monocytes.

Figure 39.2 (a) Acute eczema. The epidermis shows distinct vesicle formation. The vesicle contains serum, and a moderate number of inflammatory cells. Magnification 100× (H&E). (b) Subacute eczema. There is irregular acanthosis and patchy spongiosis, with the formation of incipient microvesicles. A few lymphocytes are migrating up from the dermis into the epidermis. Magnification 100× (H&E). (c) Chronic lichenified eczema. There is compact hyperkeratosis, some patchy parakeratosis and irregular acanthosis. Mild spongiosis is seen throughout much of the epidermis, and there is a lymphocytic infiltrate in the upper dermis. Magnification 100× (H&E).
(Courtesy of Dr M. J. Cook, Guildford, Surrey, UK.)
In the subacute stage (Figure 39.2b), spongiosis and vesiculation diminish and increasing acanthosis is associated with formation of a parakeratotic horny layer. This often contains layers of coagulated plasma and pyknotic nuclei of inflammatory cells.
In chronic eczema (Figure 39.2c), hyperkeratosis gradually replaces parakeratosis. Acanthosis is more prominent than spongiosis. Inflammatory cells are less evident in the epidermis, but dermal changes become more prominent. The rete ridges become elongated and broadened, changes are then those of lichenification.
Vascular dilatation in the dermis is marked in all stages. The papillary vessels are particularly involved, and in lichenification they may become tortuous. The infiltrate is predominantly lymphohistiocytic, although polymorphs and eosinophils may be present in very acute eczema and eosinophils are particularly common in eczematous drug eruptions. In the presence of infection, polymorphs may invade the epidermis. In grossly lichenified eczema, prurigo and exfoliative dermatitis, the infiltrate is mixed and may be so dense that it simulates a granuloma.
The trauma of rubbing or scratching may cause superficial erosions, haemorrhage or subepidermal fibrinoid changes. Although some degree of lichenification is always present during a prolonged attack of eczema, it is particularly prominent in atopic eczema. At times, extreme hyperkeratosis and papillomatosis develop.
With secondary infection, the formation of follicular or subcorneal pustules can simulate the appearance of impetigo, although typical eczematous changes are still visible at the edges of the lesion.
Although spongiosis and a dermal lymphohistiocytic infiltrate are always present at some stage in eczema, the dynamic nature of the changes and their modification by secondary events may make histological diagnosis difficult. All the changes mentioned, with the exception of the spongiotic vesicle, may also be found in burns or simple traumatic lesions of the skin. The distinction between eczema and psoriasis can be especially difficult, particularly on the palms and soles. Seborrhoeic dermatitis is particularly difficult to distinguish from psoriasis, but the finding of Munro microabscesses is suggestive of the latter. It is generally true that cases which cause diagnostic difficulty clinically often have equivocal histological appearance. The histological features of pityriasis rosea are those of eczema, but the clinical features, particularly the distribution, are characteristic.
Other modifications of the histopathological pattern in relation to different clinical subtypes of eczema are described later in this chapter.
Causative organisms
Most eczema subtypes are not thought to be primarily due to infection although they may be complicated by secondary bacterial or viral infection. The exception is infective dermatitis, which is discussed in a separate section later in this chapter. The issue of staphylococcal colonization of the skin in atopic eczema and possible eczema exacerbation by staphylococcal toxin superantigen production is discussed in more detail in Chapter 41.
Genetics
Filaggrin mutations are linked to hand eczema, irritant contact dermatitis and allergic contact dermatitis due to nickel and possibly other allergens [24–26], as discussed in more detail in the relevant sections.
Environmental factors
Irritants and allergens may act as triggers for irritant contact dermatitis and allergic contact dermatitis, respectively.
Clinical features
History
The hallmark of eczema in terms of symptoms is pruritus, which may disturb sleep and other elements of quality of life and can affect other family members profoundly as well. When taking the history, it is important to note occupation and recreational activities because these may be relevant in terms of irritant and allergen exposures.
Presentation
Acute eczema presents as an eruption that is typically oedematous, vesicular and may be exudative. In chronic eczema, these features give way to a more stable picture of erythema, scaling, excoriation and lichenification. The specific presentation of different subtypes of non-atopic eczema will be discussed in later sections of this chapter.
Secondary dissemination
A characteristic feature of eczematous inflammation is its tendency to spread far from its point of origin and to become generalized [27]. This phenomenon is often termed autosensitization or, more specifically, autoeczematization [28]. Generalized spread is especially likely when the primary site of the eczema is on the legs or the feet. There is also a tendency for an eruption beginning on the feet to extend to the hands and vice versa. The eczema may have been present for only a few days, or for many years, before dissemination occurs. The dissemination, which is often preceded by an exacerbation at the primary site, often occurs rapidly. The secondary eruption may at first consist of small oedematous papules, but these soon become obviously eczematous and grouped papulovesicles may become confluent in small plaques. Occasionally, the lesions take the form of red macules or weals. The distribution is usually symmetrical.
The course of the secondary eruption depends largely on the progress of the primary lesion. If the primary lesion remains acutely inflamed, the eruption increases in severity and may become generalized. If the patient is rested and the local lesion is allowed to settle, the secondary eruption will subside, but will often recur very readily if the local lesion relapses. In a small proportion of patients, the generalized secondary eruption evolves into erythroderma, which may become self-perpetuating.
Conditioned hyperirritability
This refers to the phenomenon whereby an area of inflamed skin on one part of the body results in a generalized hyperirritability of the skin at distant sites. There is considerable evidence that eczematous patients are more vulnerable to mild primary irritants than are unaffected people. Conditioned hyperirritability seems to be associated with any focal inflammation of the skin, and it may explain some clinical phenomena such as the tendency for patients suffering from eczema, especially acute eczema, to have a higher proportion of false positive ‘irritant’ reactions to patch tests. The ‘angry back’ syndrome, in which a strongly positive patch test response can increase the percentage of false positive reactions on the back at the same time, is an example of this.
There may be several mechanisms underlying the dissemination of eczema and conditioned hyperirritability. Circulating activated T lymphocytes are increased in number [29, 30]. In addition, peripheral blood mononuclear cells show increased proliferation in the presence of an autologous skin homogenate as compared with control subjects. This suggests that an abnormal cell-mediated immune response against autologous skin antigens could be occurring [31, 32].
Differential diagnosis
This will be discussed in relation to each non-atopic eczema subtype covered later in this chapter.
Classification of severity
While a large number of severity measurement scales exist for atopic eczema, there are relatively few available for non-atopic eczema.
Complications and co-morbidities
A reduction in skin barrier function increases the risk of both bacterial and viral secondary skin infection. However, eczema herpeticum is linked primarily to atopic eczema and filaggrin mutations (see Chapter 41).
Disease course and prognosis
Eczema tends to follow a chronic relapsing remitting course. Issues specific to each non-atopic eczema subtype will be covered later in this chapter.
Investigations
Most cases of eczema can be diagnosed clinically. It can sometimes be helpful to measure the total IgE level in order to determine whether an individual is atopic, particularly when the distribution of eczema is atypical and there is no background of other atopic illness. Secondary infection can be confirmed by taking swabs for culture and sensitivity to identify the causative organism and any bacterial resistance, such as meticillin-resistant Staphylococcus aureus (MRSA). When dermatophyte infection is suspected, a potassium hydroxide preparation should be examined or scrapings sent to a laboratory for microscopy and culture. Microscopy, or dermoscopic examination of the skin, can also be helpful to confirm a diagnosis of scabies, which is easy to miss in a patient with pre-existing eczema. Biopsy can occasionally be helpful in confirming the eczematous nature of the eruption, and immunofluorescence can help identify less common conditions such as dermatitis herpetiformis or, in older patients, a non-bullous presentation of bullous pemphigoid.
Patch testing in eczema [33–35]
Routine use of patch testing is not indicated for typical presentations of endogenous eczema such as atopic eczema, pityriasis alba and seborrhoeic eczema. This investigation is much more important in atypical or asymmetrical eruptions, and especially in dermatitis affecting the face, hands and feet [35].
In apparently endogenous eczema the threshold for patch testing should still be low. Sensitization commonly develops to topical medicaments, prescribed or self-administered, and this may exacerbate the eruption. Sometimes, topical remedies are concealed or forgotten by the patient, or the reaction they cause is partially suppressed by the concomitant use of topical corticosteroids. Sometimes, an unexpected positive test points to a ‘hidden’ or obscure cause, for example fragrances, preservatives, vehicles, epoxy or rubber chemicals. Such substances are commonly encountered in the environment and in topical medication.
When a strong suspicion of a contact allergic cause for the eczema exists, it may be important to test with other potential allergens in addition to the routine battery – household plants, material extracted from footwear, phosphorus sesquisulphide, acrylates, etc. The observer must be wary of false positive, irritant reactions, especially in the context of conditioned hyperirritability [36]. For this reason it is always wise to allow the acute phase of eczema to subside before patch testing is carried out, or to repeat any positive tests when it has done so. If a contact urticaria is thought to be occurring, the patch tests should be read 1 h after application [37].
Even if a positive patch test is judged to be relevant, it does not necessarily follow that exclusion of the substance from the patient's environment will result in a cure. The allergen may be only one of several contributory factors.
Although patch tests are designed to detect allergens, many substances give an irritant reaction when tested, and it is often difficult to be sure of the relevance of such a reaction to the patient's eczema. It must also be borne in mind that many topical medicaments can produce an irritant reaction, for example alcohol-based preparations and propylene glycol. The choice of concentration of test substance and suitable vehicle are very important [34] (see also Chapter 128 for details of patch testing).
Management
During history taking it is important to explore previous treatment experiences and gauge how much time and effort the patient will be able to devote to the care of their (or their child's) skin. An additional issue is the attitude of the patient to the risks and potential adverse effects associated with any treatment that may be required. A particularly common problem is an inappropriate level of anxiety about the use of topical corticosteroids.
A considerable variety of effective treatment modalities is available. These range from conservative and extremely safe approaches such as rest and the application of emollients, to more hazardous treatments such as phototherapy and systemic immunosuppressants. Some frequently used treatments are listed in Table 39.1. When an extrinsic cause is identified or suspected this should be removed. In all cases, exposure to irritants should be carefully avoided and the skin should be protected using emollients and appropriate dressings. Psychological support is an important aspect of management at all stages.
Table 39.1 Indications for therapeutic agents in eczema
| Therapeutic agent | Acute | Subacute | Chronic |
| Rest, sedation | ++ | + | ± |
| Wet dressings and soaks | ++ | ± | − |
| Wet wrap bandaging | ++ | + | ± |
| Paste bandages | ± | + | ++ |
| Sedative antihistamines | ++ | ++ | + |
| Emollients | ++ | ++ | ++ |
| Corticosteroids, local | + | ++ | + |
| Pimecrolimus (topical) | + | ++ | ++ |
| Tacrolimus (topical) | + | ++ | ++ |
| Tar, ichthammol, etc. | ± | + | ++ |
| Polythene occlusion | ± | + | + |
| Intralesional steroids | − | ± | + |
| Habit reversal therapy | − | ± | + |
| X-ray therapy | − | − | ± |
| UVB phototherapy | − | + | + |
| PUVA phototherapy | − | + | + |
| UVA1 phototherapy | − | + | + |
| Systemic corticosteroids | + | + | ± |
| Ciclosporin | + | + | ± |
| Azathioprine | − | + | + |
| Methotrexate | − | + | + |
| Alitretinoin (hand eczema) | − | + | + |
PUVA, psoralen and UVA; UV, ultraviolet.
Acute eczema
Acute eruptions and exacerbations of eczema cause great alarm and anxiety, and the stress of the situation is usually aggravated by loss of sleep caused by intense pruritus and soreness. Adequate rest is essential, and support from a dermatology day unit is very helpful. An affected leg should be elevated and affected hands should be used as little as possible.
Highly oedematous, vesicular and exudative eruptions such as pompholyx benefit from soaks in an astringent such as a 1 : 10 000 solution of potassium permanganate, or alternatively Burow's solution BP, which contains the astringent aluminium acetate. Liberal applications of bland emollients are soothing. Moderate or potent topical corticosteroids are generally used, at least for a few days, to speed resolution of acute episodes. Topical calcineurin inhibitors such as tacrolimus and pimecrolimus may also have a role. Tacrolimus ointment 0.1% is probably similar in potency to potent topical corticosteroids [38], while pimecrolimus has a lower potency [39]. When practical, tubular bandaging can be used to help keep topical medications in place. The wet wrapping technique, in which a layer of wet tubular bandage (e.g. Tubifast®) is covered with a dry layer, can be particularly useful. Hazards include a risk of hypothermia, although, in moderation, the cooling effect is highly beneficial. Emollients and other medications can be applied under the bandaging as required. Penetration of topical corticosteroids can be significantly increased by this form of occlusion, enhancing both beneficial and adverse effects. Mild or moderate corticosteroids should be used for the face and genital areas. Potent or very potent corticosteroids are required, at least initially, for acute pompholyx on the hands or feet.
When secondary infection is present, or staphylococcal contamination of the skin is thought to be an aggravating factor, oral antibiotics may be required. Topical preparations containing antibiotics or antiseptics in combination with steroids can also be helpful. These compound formulations should only be used when there is an indication for each constituent, in order to avoid unnecessary exposure to the risks of sensitization and emergence of bacterial resistance.
Excoriation can be reduced further by a sedative antihistamine such as hydroxyzine, and additional hypnotics may be needed for a few days to ensure sleep.
Subacute eczema
If an acute eczema has failed to clear almost completely in 3–4 weeks, perpetuating factors such as exposure to a sensitizing agent and concordance with treatment should be considered. Dermatology day unit review or admission to hospital can often be helpful in these circumstances. Paste bandages are of particular value in occluding areas that are frequently excoriated, as in many lower leg eczemas. These must be applied carefully to ensure that they are firm but not tight enough to cause discomfort or to restrict blood flow. Corticosteroids under polythene occlusion for a few days may be helpful at this stage to reduce pruritus. Topical immunomodulators such as tacrolimus and pimecrolimus are alternatives.
Chronic eczema
First line therapy for chronic eczema is the frequent application of emollients and avoidance of irritants such as soap and relevant allergens if identified. Daily application of topical corticosteroids is required to treat flares, the choice of potency being determined by eczema severity, skin site and, in children, patient age. Once a flare has been treated successfully, the frequency of application of topical corticosteroids should be reduced gradually to prevent rebound flares. A subsequent period of twice-weekly application may be needed to maintain remission in flare-prone individuals [40]. Topical calcineurin inhibitors are applied in a similar way [40] and may be needed to avoid skin atrophy for individuals who have had prolonged exposure to corticosteroids in areas such as the face.
Coal tar or shale tar (ichthammol), usually applied as creams containing tar extracts or bandages impregnated with tar paste, can be soothing and reduce pruritus. Occlusive dressings and bandages may be useful.
Various modalities of phototherapy have been used successfully in chronic eczema resistant to topical therapy, especially for atopic eczema. These include narrow-band UVB [41], psoralen and UVA (PUVA) [41] and UVA1 [42].
In the most severe cases, oral immunosuppressive therapy may be required. Systemic corticosteroids act rapidly, improving symptoms within a day or two, but there is the risk of eczema relapse when treatment is discontinued [43]. Longer term treatment is provided by ciclosporin [43], azathioprine [44] and methotrexate [45]. Mycophenolate mofetil has also been used, but evidence is limited to pilot studies [46]. All of these systemic modalities require careful monitoring. Alitretinoin, an oral retinoid, has recently been licensed for chronic hand eczema following a large randomized controlled trial [47]. However, the evidence for use of phototherapy and systemic immunosuppressants for non-atopic eczema is largely extrapolated from trials for atopic eczema (see Chapter 41).
Nummular dermatitis
Definition and nomenclature
Nummular dermatitis is characterized by a single, non-specific morphological feature, namely circular or oval plaques of eczema with a clearly demarcated edge. It should be distinguished from an irregular, patchy form of eczema in which the lesions are not clearly demarcated. The condition is poorly defined, however, because many eczema patients have one or two circular or oval lesions and few patients with nummular dermatitis have circular lesions alone [1, 2].
Introduction and general description
Nummular dermatitis is often referred to as discoid eczema. It tends to be a chronic problem, undergoing relapses and remissions. The evidence base for treatment is largely based on studies of atopic eczema, due to a paucity of studies specifically investigating this subtype of non-atopic eczema.
Epidemiology
Incidence and prevalence
A study of over 20 000 people, from 1 to 74 years of age, in the USA, who were examined for skin disease by trained observers, found the prevalence of nummular dermatitis to be two per 1000 [3].
Age
Nummular dermatitis tends to affect women in early adulthood, whereas in men onset is commonest in the older age groups.
Associated diseases
Some authors have found a high incidence of atopy in patients with nummular dermatitis [4], but others have not [5], and levels of immunoglobulin E (IgE) are often within the normal range [6]. However, lesions of similar morphology may undoubtedly occur as part of atopic eczema.
Pathophysiology
Predisposing factors
See the section on associated diseases.
Pathology [7, 8]
Histology usually shows a subacute dermatitis indistinguishable from other forms of eczema, with spongiotic vesicles and a predominantly lymphohistiocytic infiltrate. Eosinophils may also be present in the upper dermis.
Electron microscopic studies have shown that the intense intercellular oedema leads to a reduction in the number of desmosomes between the cells of the basal layer, whereas those in the stratum spinosum are mostly preserved.
Causative organisms
Some authors have stressed the role of infection [9, 10]. As in other forms of eczema, heavy colonization of the lesions by staphylococci may increase their severity, even in the absence of clinical evidence of infection [9, 10]. However, allergic sensitivity to staphylococci or micrococci may be responsible at least for secondary dissemination [11].
Genetics
There may be an indirect link via atopy (see the section on associated diseases above).
Environmental factors
There may be a clinically relevant underlying allergic contact dermatitis in about one-third of patients. In one case series 33% of patients were considered to have clinically relevant positive results, reacting to rubber chemicals, formaldehyde, neomycin, chrome and nickel [12]. On being re-contacted by telephone, eight of 13 patients reported improvement in their nummular dermatitis as a result of avoidance of allergens identified by patch testing [12].
Dry skin due to low environmental humidity is sometimes associated with nummular dermatitis [13], particularly in the elderly [14]. An association between excessive alcohol intake and nummular dermatitis has been reported [15].
Nummular dermatitis has occurred rarely as a result of sensitivity to aloe [16], depilating creams [17] and mercury [18], and in patients taking methyldopa [19]. In addition, oral gold therapy in the form of sodium aurothiomalate and auranofin has been associated with nummular dermatitis in a dose-dependent manner, with resolution on discontinuing the drug and recurrence after rechallenge [20].
Clinical features
Presentation
The diagnostic lesion of nummular dermatitis is a coin-shaped plaque of closely set, thin-walled vesicles on an erythematous base. This arises, quite rapidly, from the confluence of tiny papules and papulovesicles. These may occur, in the phase of very acute dissemination, as individual lesions on the trunk or limbs at the same time as localized plaques are being formed. In the acute phase the lesions are dull red, very exudative or crusted and highly pruritic (Figure 39.3). They progress towards a less vesicular and more scaly stage, often with central clearing, and peripheral extension, causing ring-shaped or annular lesions. As they fade, they leave dry, scaly patches.

Figure 39.3 Nummular dermatitis of the lower leg.
(Courtesy of Dr W. A. D. Griffiths, Epsom Hospital, Surrey, UK.)
After any period of between 10 days and several months, secondary lesions occur, often in a mirror-image configuration on the opposite side of the body. It is very characteristic of this disease that patches which have apparently become dormant may become active again, particularly if treatment is discontinued prematurely.
Clinical variants
There are a number of variants:
- Nummular dermatitis: exudative type.
- Nummular dermatitis: dry type.
- Nummular dermatitis of the hands.
- Exudative discoid and lichenoid chronic dermatosis.
In ‘exudative’ nummular dermatitis the skin lesions resemble the acute phase of the more typical form of the condition, with leakage of serous fluid and crust formation. This variant usually represents the more severe end of the disease spectrum and may require oral antibiotic treatment.
‘Dry’ nummular dermatitis is an uncommon variant, consisting of multiple, dry, scaly, round or oval discs on the arms or legs, but also with scattered microvesicles on an erythematous base on the palms and soles [21]. Itching is minimal, in contrast with other forms of nummular dermatitis, and the condition persists for several years, with fluctuation or remission. It is notably resistant to treatment.
Nummular dermatitis of the hands affects the dorsa of the hands or the backs or sides of individual fingers. It often develops as a single plaque, which may occur at the site of a burn or a local chemical or irritant reaction. Secondary lesions may occur on the hands, fingers or forearms, but generalized spread is uncommon. It is a not uncommon form of irritant occupational dermatitis, but may also occur without occupational exposure. An atopic history appears to be more frequent in young women with discoid hand eczema than in other forms of the disease.
Exudative discoid and lichenoid chronic dermatosis [22] has no rigid criteria, but it is a widespread, extremely pruritic eruption, characterized by discoid lesions with ‘lichenoid’ and exudative phases, which either coexist or alternate rapidly with each other (Figure 39.4). After a chronic course of months or years the condition ends in spontaneous cure. It occurs predominantly in adult male Jews, usually between the ages of 40 and 60 years. More than 100 cases have been reported [23], but some authors deny its existence as a distinct entity and consider it to be a variant of nummular dermatitis [24]. The scarcity of reports of this entity over the last three decades lends support to this contention.

Figure 39.4 Exudative discoid and lichenoid chronic dermatitis.
(Courtesy of Dr A. Warin, Royal Devon and Exeter Hospital, Exeter, UK.)
Differential diagnosis
When lesions of nummular dermatitis clear in the centre they may simulate tinea infection. If there is any doubt, skin scrapings should be examined for the presence of mycelia. In psoriasis, the lesions are dry, the scaling is more prominent and the irritation milder. Pityriasis rosea may transiently resemble nummular dermatitis, but usually presents with the history of a herald patch and characteristic oval patches with long axes parallel to the ribs.
Table 39.2 gives the diagnostic features of a number of types of discoid lesions that should be considered in the differential diagnosis.
Table 39.2 Diagnosis of some discoid skin lesions
| Disease | Distribution | Features | Histology | Course and evolution |
| Tinea corporis | Limbs or trunk | Oval or round, itchy Scraping produces scale for mycology | PAS stain shows fungus | Progresses and spreads steadily until treated |
| Nummular dermatitis | Limbs more than trunk | Oval or round, very itchy | Eczema, often intense changes | Variable, fluctuant or intermittent |
| Pityriasis alba | Face, proximal limbs | Depigmentation | Very mild eczema | Spontaneous remission after 1 or more years |
| Chronic superficial dermatitis | Limbs more than trunk | Oval or round, no infiltration | Epidermal eczematous | Very chronic, benign, no fluctuations |
| Prelymphomatous eruption | Flank, trunk, proximal limbs | Angular, bizarre, infiltrated, itchy | Dermal infiltrate | Persistent, may change to lymphoma |
Disease course and prognosis
All forms of nummular dermatitis are chronic, with partial remission during which plaques tend to clear in their centres (Table 39.2). Relapse may occur at variable intervals and most are worse during the colder months of the year. A review of 325 cases showed that most either cleared within a year or persisted for many years [25].
Investigations
Exogenous contact dermatitis should be suspected if the condition is unusually severe and persistent or if patches are few, asymmetrical or of unusual configuration. Irritants and, occasionally, sensitizers, may provoke this discoid type of response. When the history suggests this, patch tests should be performed. In a series reported from India [26], positive patch tests were detected in 56% of patients, of which the commonest were to chromate, nickel, cobalt or fragrance. A recent Italian study found highest sensitization rates with nickel sulphate (10.2%), potassium dichromate (7.3%) and cobalt chloride (6.1%) [27].
Management
General considerations such as the avoidance of irritants apply, as with other forms of eczema. Bed rest and removal from a stressful environment can be helpful. Ambient conditions of low humidity should be corrected.
Emollients and topical corticosteroids are useful. In the early stages, a potent or very potent steroid may be needed and combination with a topical antiseptic or antibiotic can be considered. Traditionally, a range of coal tar pastes or ointments were used in the less acute stages, and sometimes a combination of tar and dilute corticosteroid proved useful in long-term management.
A course of a broad-spectrum oral antibiotic, such as oxytetracycline or clarithromyin, is often helpful in severe exudative cases.
Resources
Patient resources
- British Association of Dermatology: www.bad.org.uk/leaflets.
- DermNet NZ, Discoid eczema: http://dermnetnz.org/dermatitis/nummular-dermatitis.html.
- Medscape, Nummular dermatitis: http://emedicine.medscape.com/article/1123605-overview.
- NHS Choices, Discoid eczema: http://www.nhs.uk/conditions/Eczema-(discoid)/Pages/Introduction.aspx. (All last accessed June 2015.)
Asteatotic eczema
Definition and nomenclature
This is eczema developing in very dry skin, usually in the elderly.
Introduction and general description
Asteatotic eczema usually affects the legs, arms and hands of elderly people in the context of dry skin. A characteristic ‘crazy-paving’ pattern is observed on the legs in particular, resulting in the synonym of eczéma craquelé. It may flare during winter due to a reduction in humidity associated with central heating. Management centres on the restoration of skin hydration.
Epidemiology
Incidence and prevalence
A recent study of elderly Japanese care home residents found an asteatotic eczema prevalence of 16.4% for those undergoing rehabilitation and 41.2% for those in long-term residential care, with a mean age in the two groups of 85 years and 87 years respectively [1].
Age
Elderly people are predominantly affected and prevalence increases with increasing age.
Sex
There is insufficient evidence to comment on whether gender affects the likelihood of asteatotic eczema.
Ethnicity
All ethnicities can be affected.
Associated diseases
Asteatotic eczema may be a presenting sign of myxoedema [2]. It can also be due to zinc deficiency [3].
Pathophysiology
Predisposing factors
At present, the relevant factors in the production of asteatotic eczema can be considered to be: (i) a naturally ‘dry’ skin and a lifelong tendency to chapping; (ii) a further reduction in lipid with age, illness, malnutrition or hormonal decline; (iii) increased transpiration relative to the environmental water content; (iv) loss of integrity of the water reservoir of the horny layer; (v) chapping and degreasing (and perhaps cell damage) by industrial or domestic cleansers or solvents; (vi) low environmental humidity and dry, cold winds increasing convection loss; and (vii) repeated minor trauma leading to inflammation and further disorganization of the surface aqueous/lipid balance. Percutaneous absorption through the degreased and damaged epidermis is increased, and contact irritants and sensitizers may further damage and irritate the skin.
Pathology
Although the condition is thought to be due to a decrease in skin surface lipid, the exact pathogenesis of the skin changes is obscure. The amino acid content of the skin is lower in more severe cases [4]. A decrease in the keratohyaline-derived natural moisturizers may also be important [5].
Hyposteatosis occurs in many conditions of maldevelopment, malnutrition and atrophy of the skin, but does not necessarily lead to eczema. The part played by the loss of fluid from the skin has been underrated in the past. The relationship between the transpiration rate and the lipid layer has been the subject of many studies [6]. Using excised skin it was shown that removal of these lipids increased water loss by 75 fold, and that this returned to normal when they were restored. The implications for treatment are obvious.
The histopathological features are those of a mild, subacute eczema, with a varying amount of dermal infiltrate. When vesicular or nummular dermatitis supervenes, the changes are more marked, and are as seen in the latter disease.
Genetics
No specific genetic mutations have been documented in the literature to date.
Environmental factors
A patient will often ascribe the onset to an event or change in life that is quite trivial, for example the installation of central heating or a particularly cold, dry winter [7]. In industry, years of contact with degreasing agents may be tolerated until, usually in the 50–60-year age group, some small additional hazard precipitates a disabling dermatitis.
Diuretics sometimes appear to be an important contributory factor in elderly people [8]. Cimetidine has also been reported to cause asteatotic dermatitis [9], as have topical corticosteroids [10].
Clinical features
History
Irritation in this form of eczema is often intense, and worse with changes of temperature, particularly on undressing at night.
Presentation
The condition occurs particularly on the legs, arms and hands. The asteatotic skin is dry and slightly scaly (Figure 39.5). The surface of the backs of the hands is marked in a criss-cross fashion. The finger pulps are dry and cracked, producing distorted prints and retaining a prolonged depression after pressure (‘parchment pulps’). On the legs the pattern of superficial markings is more marked and deeper (‘crazy-paving’ pattern or eczéma craquelé). In some patients the fissures may become haemorrhagic. The borders of this irregular reticulation become erythematous and slightly raised, and frank eczematous changes finally develop. Similarly, on the hands, localized areas become chapped or itchy, and eventually form eczematous patches.

Figure 39.5 Asteatotic eczema.
Clinical variants
Extensive or generalized forms involving the trunk as well as the legs are rare but should raise the suspicion of malignancy. Cases have been reported in association with malignant lymphoma [11], angioimmunoblastic lymphadenopathy [12], anaplastic gastric adenocarcinoma [13] and spheroidal cell carcinoma of the breast [14].
Differential diagnosis
Asteatotic eczema has a characteristic appearance thus the differential diagnosis is limited to other forms of eczema.
Classification of severity
There are no specific severity classification systems.
Complications and co-morbidities
As with all forms of eczema, secondary infection is possible due to a reduction in skin barrier function. Nummular dermatitis can also occur on a background of asteatotic eczema, although the relationship between the two conditions is uncertain.
Disease course and prognosis
Without treatment, the condition is usually chronic, relapsing each winter and clearing in the summer, but eventually becoming permanent. Scratching, rubbing or contact irritants and sensitizers cause further eczematous changes or spread; or a more diffuse vesiculosquamous eruption occurs.
Investigations
There are no specific invesigations unless generalized asteatotic eczema is present.
Management
The patient's immediate environment may need to be adjusted. Central heating should be humidified where possible, and abrupt temperature changes should be avoided. Wool is usually poorly tolerated and possibly damaging due to irritation. Baths are best restricted and should not be hot. Bath oils or oatmeal packs may be helpful, although it is important to remember that bath oils are potentially hazardous in the elderly as the bath may be made slippery. Emollients should be used after bathing and during the day. Emollient creams such as those based on lanolin or paraffins are generally helpful. Soap substitutes should also be prescribed to reduce irritation from soap.
Weak topical corticosteroids are often prescribed, and those contained in a urea base (see Chapter 18) are very appropriate in this situation as urea encourages hydration. Among the older remedies, ichthammol is of value.
Resources
Patient resources
- DermNet NZ, Eczema craquelé: http://www.dermnetnz.org/dermatitis/eczema-craquele.html (last accessed June 2015).
Dermatitis and eczema of the hands
Definition and nomenclature
The term hand eczema implies that the dermatitis is largely confined to the hands, with only minor involvement of other areas. If the eczema is widespread and the hands appear to be involved only coincidentally, it is preferable to refer to hand involvement.
Introduction and general description
Hand eczema is a common and distressing condition, and has a particular impact on quality of life due to its effects on dexterity, appearance and social functioning [1]. Up to 30% of occupational medical practice relates to hand eczema, with important issues regarding medical litigation, worker's compensation and disability. A quarter of the patients referred to a specialized contact dermatitis clinic suffered from hand eczema [2].
No single classification of hand eczema is completely satisfactory. As with eczematous dermatoses in general, classification is based partly on aetiology and partly on morphology (Boxes 39.2 and 39.3, respectively). Several different morphological forms are seen clinically as fairly consistent entities, but some of these entities can have several different causes. Conversely, a single cause can sometimes produce several different morphological patterns.
Most cases of hand eczema have a multifactorial aetiology. This not only makes treatment difficult, but it can cause considerable problems in medicolegal cases, for example occupational dermatitis in which negligence is alleged against an employer.
Epidemiology
Incidence and prevalence
Minor degrees of hand eczema are very common, and virtually everyone suffers from mild dryness and chapping at some time or another. Approximately 25% of hand eczema patients may never seek medical advice [3]. The perception of whether these changes amount to hand dermatitis can influence the findings of epidemiological studies [4].
The crude incidence rate of self-reported hand eczema was 5.5 cases per 1000 person-years in a population-based retrospective study from Sweden [5]. A review of studies performed between 1964 and 2007 found a hand eczema point prevalence of about 4%, a one-year prevalence of nearly 10% and a lifetime prevalence of 15% [6].
In high-risk groups the figures are even higher. In Finland, 44% of 617 hospital personnel engaged in ‘wet work’, including nurses, cleaners and kitchen staff, had a past or present history of hand eczema and 28% had had at least two attacks [7]. This is confirmed by a study demonstrating a significantly greater risk of hand dermatitis among hairdressers compared with office workers [8].
Age
In women, the prevalence of self-reported hand eczema peaks in young adults and appears to decline with increasing age. This is demonstrated by a Swedish health survey involving 10 950 participants in which the one-year prevalence of hand eczema decreased from 12% in women aged 19–29 years to less than 6% in women aged 70–80 years [9]. In men, there are conflicting data regarding the effect of age, with some surveys reporting no change and others finding a reduction in frequency with increasing age [6].
Sex
Hand eczema is more than twice as common in women compared with men. Based on seven studies, the median incidence rate of hand eczema was 9.6 cases/1000 person-years in women and 4.0 in men [6].
Ethnicity
Hand eczema is a common problem in all ethnic groups.
Associated diseases
Hand eczema is more common in people with a previous history of atopic eczema elsewhere [10]. The most common site of atopic eczema in the adult is the hands, and in some patients the hands alone may be involved. The atopic state may first become apparent with the development of hand eczema in an adolescent or young adult when they are exposed to school, hobby or occupational irritants. There is no specific topographical pattern, although the feet may also be involved [11, 12]. The atopic diathesis may also predispose to a discoid pattern of hand eczema in young adults.
Pathophysiology
Predisposing factors
Atopy, a naturally dry skin, or a superadded contact allergic or irritant dermatitis, are all predisposing factors. The common link is now known to be filaggrin gene mutations, as discussed in the Genetics section below.
The role of stress in aggravating hand eczema is difficult to evaluate, and the disease itself is very stressful [13]. Many patients give a convincing account of exacerbations at times of acute anxiety, frustration or grief. The role of hormonal factors is also difficult to assess. Occasionally, there is a history of premenstrual exacerbation, or deterioration during pregnancy.
Pathology
In general, the differences between the various forms of hand eczema are clinical rather than histological, but the considerably thickened horny layer and the presence of numerous sweat glands modify the histological features of eczema on the hands.
Causative organisms
This is not applicable, except for secondary bacterial infection.
Genetics
Twin studies suggest hereditary factors play a role in the development of hand eczema [14], with the atopic diathesis as the commonest endogenous cause [15]. Loss-of-function mutations of the filaggrin gene underpin the link with atopy and are associated with early onset and persistence of hand eczema in atopic subjects [16]. Carriers of filaggrin gene mutations are more likely to develop digital fissures (chapping) than those who do not carry the mutation [17]. In a 3-year prospective observational cohort study of 459 patients with occupational irritant contact dermatitis on the hands, those with atopy and filaggrin mutations had more sick leave and a threefold higher rate of job loss compared with controls [18]. Filaggrin gene mutations have also been linked with an increased susceptibility to chronic irritant contact dermatitis in case–control studies [19] and chronic hand eczema characterized by combined allergic and irritant contact dermatitis [20].
Environmental factors
Contact irritants are the commonest exogenous cause of hand eczema [21], but contact allergens including chromate, epoxy glues and rubber are also important (see Chapter 128). All patterns of hand eczema are possible in contact allergy. Rubber dermatitis usually affects the dorsa of the hands (Figure 39.6), but so can contact irritant and atopic eczema [22]. Certain occupations are particularly likely to provoke hand eczema. The problem of occupational eczema in hairdressers, fish industry workers, farmers, construction workers, dental and medical personnel, metal workers and caterers has provoked many studies to determine its prevalence and to develop programmes for the prevention of hand dermatitis.

Figure 39.6 Bullous eczema due to contact allergy to rubber gloves.
Type 1 allergic reactions to certain proteins may also give rise to hand eczema. In mild cases they provoke a vesicular eczema of the fingers, particularly amongst those who prepare seafood. In a Scandinavian study, one-third of restaurant food handlers with hand dermatitis had a seafood contact urticaria [23]. Amongst health care workers, reactions to natural rubber latex protein found in latex gloves are a particular problem. The reactions range from contact urticaria to rhinitis, asthma and anaphylaxis [24].
Oral ingestion of allergens such as nickel, chromium or balsam of Peru has been reported to provoke or aggravate hand eczema in sensitized subjects, although the importance of this phenomenon is controversial [26]. Cold and dry air may play some part [27].
Clinical features
History
Particular attention should be given to the patient's occupational and recreational activities because these may indicate potential contact allergies. Occupational involvement is suggested by improvement associated with leave from work. Current hand-protection strategies should also be elicited.
Hand eczema often has a severe impact on quality of life, due to pruritus, painful fissuring, loss of dexterity and impairment of social functioning. Median dermatology life quality index (DLQI) scores [28] in patients attending patch test clinics were found to be 7.0 and 8.0 in males and females, respectively [29].
Presentation
This will be discussed in relation to each clinical variant below.
Clinical variants
Hyperkeratotic palmar eczema
This condition is a distinct form of hand eczema that is characterized by highly irritable, scaly, fissured, hyperkeratotic patches on the palms and palmar surfaces of the fingers (Figure 39.7) [21]. It is common, and 2–5% of all applications for permanent disability pensions in some western European countries are due to hyperkeratotic hand eczema.
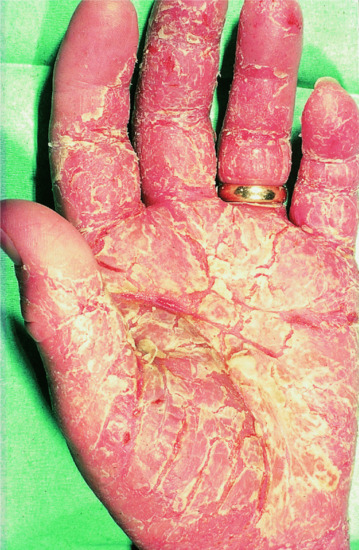
Figure 39.7 Hyperkeratotic palmar eczema.
Pompholyx
Pompholyx is a form of eczema of the palms and soles in which oedema fluid accumulates to form visible vesicles or bullae (Figure 39.8). As a result of the thick epidermis in these sites, the blisters tend to become relatively large before they burst. Pompholyx probably accounts for about 5–20% of all cases of hand eczema [21]. There is some debate whether the term pompholyx should be reserved for typical cases in which the attacks resolve and recur. Chronic, recurrent vesiculation without periods of remission may be termed chronic vesicular dermatitis.

Figure 39.8 Pompholyx eczema. (a) Small vesicles coalescing into blisters on the lateral aspect of a finger. (b) Confluent vesicles of the palm.
Apron eczema
This condition is a type of hand eczema that involves the proximal palmar aspect of two or more adjacent fingers and the contiguous palmar skin over the metacarpophalangeal joints, thus resembling an apron (Figure 39.9) [3]. This pattern of hand eczema may be irritant, allergic or endogenous.

Figure 39.9 Apron eczema, showing the characteristic distribution.
Chronic acral dermatitis
This is a distinctive syndrome affecting patients in middle age. A chronic, intensely pruritic, hyperkeratotic, papulovesicular eczema of the hands and feet, is associated with grossly elevated IgE levels in subjects with no personal or family history of atopy. The condition responds to oral corticosteroids, but the response to topical therapy is poor [21].
Nummular dermatitis
This is also known as discoid eczema (see section on nummular dermatitis earlier in this chapter).
Fingertip eczema
This condition presents a characteristic pattern, involving the palmar surface of the tips of some or all of the fingers. The skin is dry, cracked and sometimes breaks down into painful fissures (Figure 39.10). Usually remaining localized, it may occasionally extend along the palmar surfaces of the fingers to merge with palmar eczema. Two patterns may be distinguished. The first and most common involves most or all of the fingers, mainly those of the dominant hand, and particularly the thumb and forefinger. The condition is usually worse in the winter and generally improves on holiday. Fingertip eczema is usually a cumulative irritant dermatitis in which degreasing agents combine with trauma as causative factors; patch tests are typically negative or not relevant. The second pattern involves preferentially the thumb, forefinger and third finger of one hand. This is usually occupational and may be either irritant (e.g. in newspaper delivery employees) or allergic (e.g. to colophony in polish). The condition usually involves the dominant hand, but there may be allergy to onions, garlic [30] and other kitchen products held in the non-dominant hand when being cut. In these cases, patch testing (and 20 min contact tests) may be rewarding.

Figure 39.10 Fingertip eczema in a patient with wear and tear eczema.
(Courtesy of Dr D. A. Burns, Leicester Royal Infirmary, Leicester, UK.)
‘Gut’/slaughterhouse eczema
Workers who eviscerate and clean pig carcasses are at risk of developing vesicular eczema which starts in the finger webs and spreads to the sides of the fingers. This is a mild, self-limiting condition, which clears in a week or two, even if the patient remains at work, but it can recur at intervals. Workers in Danish bacon factories call this ‘fat eczema’, although there is little evidence that it is due to fat, and prick tests to pig fat extracts are negative [31]. The pathogenesis is unknown, but some slaughter workers have developed contact urticaria from exposure to animal blood [32].
Patchy vesiculosquamous eczema
In a large group of cases, a mixture of irregular, patchy, vesiculosquamous lesions occur on both hands, usually asymmetrically. In contrast to the lesions of discoid hand eczema, the degree of activity and distribution of the lesions vary. Nail changes are common if the nail folds are affected.
Recurrent focal palmar peeling
The condition is sometimes a mild form of pompholyx; it is also known as desquamation en aires, keratolysis exfoliativa or ringed keratolysis of the palms. During the summer months, small areas of superficial, white desquamation develop on the sides of the fingers and on the palms or on the feet (Figure 39.11). They appear abruptly, and expand before peeling off. There is little or no irritation, and vesicles as such are not seen. The condition is probably not rare, but because it is relatively asymptomatic it often does not reach the dermatologist. Some patients subsequently develop true pompholyx.

Figure 39.11 Recurrent focal palmar peeling. (a) Well-established lesions on the hands
(Courtesy of Dr A. Marsden, St George's Hospital, London, UK). (b) Lesions on the feet.
Ring eczema
This characteristic pattern particularly affects young women, rarely men. The condition usually starts soon after marriage or childbirth. An irritable patch of eczema begins under a ring – usually a broad wedding ring – and typically spreads to involve the adjacent side of the middle finger and the adjacent area of the palm. It may remain confined to these sites, but is occasionally followed by the appearance of discoid patches elsewhere; or a more diffuse vesicular eczema may develop. Despite the clearly defined demarcation of the initial eruption, these patients are not sensitive to gold or copper although nickel, cobalt and even chromium sensitivity are more commonly found on patch testing than might be expected; only rarely can ‘white gold’ alloys be implicated. Ring dermatitis has been described as the clinical presentation of fragrance sensitization [33]. Transference of the ring to the other hand is often rapidly followed by the appearance of eczema at the new site and, once affected, patients may find that wearing of the ring for only a few minutes, even without washing, causes irritation. This type of hand eczema is probably due primarily to concentrations of soap and detergent beneath rings (which may tighten on fingers immersed in hot water), but microtrauma, especially friction, may also play a role. Very rarely, radioactive gold in a ring may cause radiation dermatitis that mimics this type of wedding ring eczema [34].
Differential diagnosis
The diagnosis of hand eczema is usually self-evident, but the distinction from psoriasis can be very difficult and may only become apparent over time with development of psoriasis at other sites. In some cases even biopsy does not allow a clear distinction to be made. In most cases of psoriasis on the hands, however, the silvery nature of the scale, involvement of the knuckles, sharply demarcated ‘scalloped’ edges to the erythema along the borders of the hands and fingers, and the relative absence of pruritus are helpful pointers. A family history of psoriasis and the presence of nail pits in the absence of nail fold lesions are also suggestive.
Tinea manuum can be missed, particularly when it is extensive (Figure 39.12) or secondarily infected. Unilateral scaling of the palm should always suggest a possible Trichophyton infection, and a discoid plaque due to Tricophyton verrucosum is sometimes seen in farmers.

Figure 39.12 Trichophyton infection of the hands that failed to respond to topical steroids. Note the nail involvement.
Lichen planus (Figure 39.13) and pityriasis rubra pilaris may resemble eczema on the hands, but usually present with typical lesions at other sites.

Figure 39.13 Lichen planus mimicking hyperkeratotic hand eczema, but the margins are well demarcated and the lesions on the left wrist are characteristic of lichen planus.
Pompholyx eczema can resemble palmoplantar pustulosis. Clinically, the two conditions are distinguished by the presence of vesicles in the former and sterile pustules that resolve with characteristic brown marks in the latter. A pustular bacteride secondary to bacterial infection elsewhere in the body is a less common differential. Repeated attacks of pompholyx may produce hyperkeratotic lesions that mimic psoriasis vulgaris. Pemphigoid, linear IgA disease and pemphigoid gestationis occasionally present with blisters on the palms that mimic pompholyx.
It must be emphasized that the whole skin should be examined in any case of hand eczema in which the diagnosis is in doubt. There may, for example, be evidence of nickel allergy or tinea pedis, or small patches of psoriasis of which the patient is unaware.
Classification of severity
A photographic guide for assessing the severity of chronic hand dermatitis has been developed and validated, mainly for use in clinical trial settings [35]. In daily practice, measurement of quality of life using a dermatology-specific index can be used by clinicians to quantify disease severity. UK National Institute for Health and Care Excellence (NICE) guidelines for alitretinoin for chronic hand eczema require a DLQI score [29] to be measured [36, 37].
Complications and co-morbidities
Secondary bacterial infection of hand eczema may occur, usually associated with a sudden deterioration, pain and/or exudate from the affected areas. In pompholyx eczema, infection may also be indicated by the new onset of pustules rather than vesicles, usually in association with pain.
Disease course and prognosis
Unless a responsible allergen can be identified and removed, the prognosis of hand eczema for an individual is uncertain. Even if a relevant allergen is identified, it may be difficult to avoid all contact with the hands for allergens such as nickel and fragrance. Atopic hand eczema probably has the worst prognosis of all types of hand eczema [21]. In general, eczema on the dorsa of the hands clears more readily, and is less likely to recur than palmar eczema.
Following an acute attack of pompholyx, about one-third of patients experience no further episodes, one-third suffer from recurrent episodes and in the remainder the condition develops into a chronic, possibly hyperkeratotic phase. Those forms of hand eczema that are due in part or wholly to the effects of irritants carry a particularly poor prognosis unless these irritants can be completely removed. Patients who have suffered from severe hand eczema will often remain vulnerable to mild irritants for several months after the eczema has apparently cleared. Interdigital dermatitis has been shown to be a potential precursor to more severe hand dermatitis in hairdressers. Recognition of this sign by the patient may allow early intervention to prevent progression of the disease [38].
A questionnaire follow-up answered by 868 of 1115 persons with hand eczema identified 15 years previously in a population-based study found that 44% had experienced hand eczema in the previous year [39]. For about 5%, the hand eczema had resulted in a major change such as long sick leave periods, early retirement or a change of occupation.
Investigations
A circumscribed and asymmetrical area of scaling and vesiculation of the palm or sole should suggest the possibility of dermatophytosis, and scrapings should be examined for fungus. If the erythema is limited to one or two interdigital clefts, or is asymmetrical, or involves the dorsal skin to any extent, the possibility of a contact dermatitis must be considered and investigated by patch testing. Patch testing should be guided by the patient's occupational and other allergen exposures. If there are immediate symptoms on wearing latex gloves, then type 1 latex hypersensitivity should be excluded with latex prick testing.
Management
Chronic hand eczema
Management of chronic hand eczema, defined as persisting for at least 6 weeks, is similar to eczema affecting other skin sites and involves the avoidance of irritants, frequent application of emollients and use of topical corticosteroids when indicated.
Avoidance of irritants is particularly difficult for patients with hand eczema because they are so ubiquitous. Patient education is of paramount importance and this can be reinforced by printed or online advice sheets. Gloves usually provide the best protection against irritants and advice should be given regarding the optimal choice of glove in terms of the material, size and weight depending on the individual's particular needs. Rubber gloves generally give good protection for housework. In patients with a rubber allergy, polyvinyl chloride household gloves should be worn instead. It should be noted that some allergens, such as acrylates and epoxy resins, can penetrate vinyl or rubber gloves [40]. Gloves that develop holes should be discarded immediately and, if sweating makes the condition worse, it may be helpful to wear cotton gloves beneath the protective ones.
Barrier creams are used in an attempt to prevent hand eczema of occupational origin, however in practice they may not be applied effectively [41], and the debate continues about their actual benefit [42]. A recent Cochrane review of interventions such as emollients and barrier creams for preventing occupational irritant hand dermatitis found generally positive results but no statistical significance was reached [43].
Emollients should be applied frequently, and containers should be left at convenient locations at home and at work so that they are readily available. In general, choice of emollient is directed by the patient to ensure maximal compliance. Soap substitutes should be used in place of soap for all hand washing. Patients should be warned that some topical preparations sold over the counter by pharmacists as antipruritics or emollients can contain irritants such as alcohol or propylene glycol.
Topical corticosteroids are required for all but the mildest cases of hand eczema. For severe hand eczema, potent or very potent topical corticosteroids may be needed. Painful fissures of the fingertips are a particular therapeutic problem and these can be treated with corticosteroid-impregnated adhesive tape, which provides both physical protection and local delivery of topical corticosteroid.
In difficult, unresponsive cases the use of a topical corticosteroid under occlusion may be considered. The steroid is applied at bedtime, and polythene gloves, sealed at the wrist with sticky tape, are worn overnight. This can be an effective treatment, but it greatly increases the risk of atrophy and secondary bacterial infection, and should be discontinued as soon as the eczema shows satisfactory improvement. After improvement with daily corticosteroid use, the intermittent use of a potent corticosteroid cream can be used safely to prevent relapse [44].
If hand eczema does not respond to topical corticosteroid therapy, the diagnosis should be reviewed, particularly with regard to the possibility of tinea. The patient should be asked again about exposure to irritants or allergens, and the possibility of contact sensitization to medicament bases, preservatives or the corticosteroid itself should be considered, with patch testing if necessary.
Topical calcineurin inhibitors provide a further option for treating hand eczema. In a left/right comparative trial with 16 subjects, topical tacrolimus 0.1% proved similar in efficacy to mometasone furoate [45]. The response to tacrolimus was better on the palms than soles.
Tar pastes have been used for chronic unresponsive cases and salicylic acid ointment is also sometimes helpful for hyperkeratosis and persistent scaling [46]. Intradermal injection of triamcinolone (10 mg/mL) into recalcitrant, localized patches of hand eczema may also be beneficial [46]. Other treatments include Grenz rays [47] and, for pompholyx eczema, iontophoresis has been used successfully [48].
At present, the retinoid alitretinoin (9-cis-retinoic acid) is the only licensed systemic therapy for chronic hand eczema. It interacts with both retinoid X and retinoic acid receptors and has been shown to improve chronic hand eczema in a large placebo-controlled trial with over 1000 subjects [49]. After 24 weeks of alitretinoin 30 mg daily, 48% of patients were clear or almost clear of their hand eczema, corresponding to a number needed to treat (NNT) of about 4 (95% confidence interval 2.63–4.25) [50]. Mucocutaneous adverse effects are less common than for other systemic retinoids and the commonest issue reported was headache, which occurred in one-fifth of patients. In the UK, NICE has approved alitretinoin for patients who have severe chronic hand eczema, defined by the physician's global assessment and a DLQI score of 15 or more, and who have failed potent topical corticosteroid therapy [37].
There are only limited randomized controlled trial (RCT) data regarding the use of other systemic agents for chronic hand eczema. A small RCT involving 29 patients with hyperkeratotic hand eczema suggested some benefit from acitretin [51], however this is unsuitable for women of child-bearing age due to its long half-life and teratogenicity. An RCT of 41 patients compared ciclosporin 3 mg/kg daily with a potent topical corticosteroid and found no significant difference between the two groups [52]. A brief report of an RCT comparing azathioprine 50 mg daily and a very potent topical corticosteroid with the very potent topical corticosteroid alone suggests possible additive benefit from low-dose azathioprine [53].
Oral psoralen and UVA (PUVA) phototherapy and UVB therapy have been used to treat chronic hand eczema and limited RCT evidence suggests that oral PUVA may be more effective than UVB [54]. Topical hand PUVA soaks are frequently used in clinical practice although there is only limited published evidence for hand eczema. Case series evidence suggests that hand PUVA soaks are less effective for hyperkeratotic hand eczema [55].
Acute hand eczema
For hand eczema that presents acutely, it is important to eliminate any precipitant, for example a contact allergen. Emollients should be applied copiously and, if the eruption involves exudate or pompholyx vesicles, then dilute potassium permanganate soaks are helpful. Large bullae may be aspirated using a sterile syringe. Systemic antibiotics will be required if secondary bacterial infection develops and empirical therapy with flucloxacillin to provide cover for staphylococci is generally effective, pending culture and sensitivity results from swabs.
Resources
Further information
- Diepgen TL, Elsner P, Schliemann S, et al. Guideline on the management of hand eczema ICD-10 code: L20. L23. L24. L25. L30. J Dtsch Dermatol Ges 2009;7(Suppl. 3):S1–16.
- National Institute for Health and Care Excellence, Alitretinoin for the treatment of severe chronic hand eczema: http://guidance.nice.org.uk/ta177 (last accessed June 2015).
Patient resources
- British Association of Dermatologists: www.bad.org.uk/leaflets.
- DermNet NZ, Hand dermatitis: www.dermnet.org.nz/dermatitis/hand-dermatitis.html.
- National Institute for Health and Care Excellence patient information regarding alitretinoin for chronic hand eczema: www.nice.org.uk/guidance/ta177.
(All last accessed June 2015.)
Dermatitis and eczema of the lower legs
Definition and nomenclature
Dermatitis and eczema of the lower legs is subclassified by ICD-11 as venous eczema, stasis dermatitis and allergic contact dermatitis. Lower limb venous eczema encompasses the skin changes that result from venous hypertension. Stasis dermatitis relates to the skin changes that result from reduced lower leg venous flow.
Introduction and general description
Venous eczema and stasis dermatitis both result from dysfunctional venous drainage of the lower legs. There is a great deal of overlap between the two skin conditions, which share the same clinical features, and they are distinguished by the presence or absence of venous hypertension. Allergic contact dermatitis of the lower legs is a common complication of both skin conditions.
Epidemiology
Incidence and prevalence
The combined prevalence of venous eczema and venous stasis is estimated to be between 3% and 11% of the population [1, 2].
Age
Venous eczema patients are usually middle-aged or elderly.
Sex
There is an increased incidence in females, which may be due to hormonal effects and the tendency for deep-vein thrombosis (DVT) to occur during pregnancy.
Ethnicity
Venous eczema affects all ethnicities.
Associated diseases
As discussed previously, there is an association between venous eczema and allergic contact dermatitis.
Pathophysiology
Predisposing factors
Venous eczema is more likely after a previous DVT and in the presence of venous stasis, which is itself linked to obesity, immobility, and previous cellulitis [2].
Pathology [37–6]
In venous eczema, the oxygen content in the femoral venous blood of the leg affected by venous hypertension is increased, and the venous blood in such limbs has a faster circulation time than normal [7, 8]. These observations could be explained by the development of arteriovenous shunts in the affected areas, but the use of radioactively labelled macroaggregates or microspheres has failed to provide any evidence for such shunts.
An alternative explanation for these findings has been provided by Browse and Burnand [4], who suggested that the high ambulatory venous pressure within the calf muscle pump is transmitted to the capillary circulation in the skin and subcutaneous tissues of the calf. This distends the local capillary bed and widens the endothelial pores, thus allowing fibrinogen molecules to escape into the interstitial fluid, where they form a fibrin sheath around the capillaries. This layer of fibrin presumably forms a pericapillary barrier to the diffusion of oxygen and other nutrients that are essential for the normal vitality of the skin. The hypothesis that pericapillary fibrin impedes oxygen diffusion has been supported by a study using positron emission tomography [9].
It has also been suggested that cutaneous inflammation in venous hypertension may result from increased sequestration of white cells in the venules, with a consequent release of proteolytic enzymes and free radicals which produce tissue damage [5]. In normal subjects, white cells are sequestered in the limb when venous pressure is elevated, and in patients with venous insufficiency the effect is enhanced, with increased endothelial contact and adhesion of white cells [10]. This effect may be related to an increase in expression of adhesion molecules intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on the vascular endothelium in affected skin [11].
Causative organisms
This is not applicable, except in cases of secondary infection, in which standard organisms such as Staphylococcus and Streptococcus are implicated.
Genetics
No strong genetic links have been identified to date.
Clinical features
History
Venous eczema may develop suddenly or insidiously. It may occur as a late result of DVT.
Presentation
Venous eczema and stasis dermatitis are both erythematous, scaly and often exudative eruptions usually seen around the ankle and lower leg (Figure 39.14). Occasionally, similar changes occur at other sites of venous hypertension such as the pendulous skin over an obese abdomen or in association with an ateriovenous fistula in the upper limb [12]. The eczema is often accompanied by other manifestations of venous hypertension, including dilatation or varicosity of the superficial veins, oedema, purpura, haemosiderosis and ulceration (Figure 39.15), or small patches of white, atrophic, telangiectatic scarring (‘atrophie blanche’). These changes, which occur in various combinations, are discussed in more detail in Chapter 103. Leashes of dilated venules around the dorsum of the foot or ankle are particularly common. There may be a subepidermal vascular proliferation producing purple papules around the ankle, which may resemble Kaposi sarcoma [13].

Figure 39.14 Venous (gravitational) eczema.

Figure 39.15 Venous eczema of the ankle with ulceration at the medial malleolus.
Clinical variants
Secondary patches of eczema may develop on the other leg, even when it is not affected by obvious venous insufficiency. Generalized secondary dissemination may occur, and occasionally this can progress to erythroderma.
Differential diagnosis
Although most cases of eczema of the lower leg are secondary to venous hypertension or stasis dermatitis, it must be remembered that many other types of eczema may affect this region, and in many cases there are multiple causative factors. In children or young adults, atopic eczema may manifest as lichenified patches around the ankle or behind the knees. Allergic contact dermatitis of the lower legs is usually due to topical medicaments (Figure 39.16). Patch testing is often indicated. An infected ulcer may be complicated by infective eczema spreading from the edge of the ulcer, with response to appropriate antibiotic therapy. Nummular dermatitis is common on the lower leg, usually on the anterior or anterolateral aspect. Asteatotic eczema commonly affects the legs of elderly patients.

Figure 39.16 Contact eczema of the lower legs due to allergy to paste bandages.
Although the exact cause of eczema on the lower legs can be difficult to elucidate, other conditions can usually be readily identified. Psoriasis may present as a single, irritating plaque on the leg, but is usually more scaly and clearly marginated. Hypertrophic lichen planus of the lower leg may occasionally be mistaken for eczema if there are no characteristic lesions elsewhere. Dermatophyte infection may present as diffuse erythema and scaling and may be difficult to recognize, particularly if it has been treated with topical steroids. Profuse actinic keratoses may cause red, irritable patches on the lower legs in sunny climates. In the late stage of borreliosis, the leg can feel heavy, with thick cyanotic itchy skin that may mimic the changes of venous hypertension [15].
Classification of severity
No specific venous eczema or stasis dermatitis severity scores are currently available.
Complications and co-morbidities
Allergic contact dermatitis
Clinically this may present as an eczematous eruption with a sharp, linear cut-off matching to the application of a topical therapy, wound dressing, or compression hosiery. Allergic contact dermatitis is a common complication of venous eczema, possibly because of the large number of antigen-presenting cells in the inflamed skin [14] and also because of prolonged contact with topical therapies and compression hosiery. Allergens include topical antibiotics, topical steroids, preservatives, fragrances and rubber accelerators. Allergic contact dermatitis of the lower leg is covered in more detail in Chapter 128.
Secondary infection
This usually presents as a sudden worsening of eczema. If cellulitis ensues, the patient may experience pain and increased skin temperature and swelling of the affected area, as well as malaise and rigors if infection becomes systemic. The mode of antibiotic administration depends on the severity of the infection, ranging from topical antibiotics for mild infection, oral for moderate and intravenous for severe infection.
Lipodermatosclerosis
Chronic venous insufficiency may result in lipodermatosclerosis, described in more detail in Chapter 103.
Venous ulceration
There is an association between venous eczema and venous ulceration because these are both the result of venous hypertension. Ulcer healing is inhibited by both venous eczema and stasis dermatitis, in part due to chronic lower leg swelling.
Disease course and prognosis
Venous eczema is a chronic condition that undergoes relapses and remissions. Long-term improvement may be provided by effective lower limb compression, if tolerated, or in some cases by varicose vein surgery.
Investigations
Ankle brachial pressure index (ABPI) measurement is required prior to consideration of compression therapy. An ABPI of more than 0.8 indicates suitability for graduated compression bandages, in the absence of vessel calcification due to diabetes or atherosclerosis, which can give a falsely high reading (see Chapter 104).
Management
Any underlying venous hypertension and/or pedal oedema should be controlled. Obese patients should be urged to lose weight. Well-fitted support stockings or firm bandages can be helpful if worn regularly and care is taken to avoid the formation of a band at the top of the leg. The legs should be elevated as effectively as possible.
Mild topical steroids may be used to relieve irritation, but the use of potent corticosteroids should be limited to short periods of a few days as they may cause cutaneous atrophy and increase the risk of ulceration. Topical tacrolimus has been reported to be effective [16]. Bacterial infection must be treated where appropriate, but the risk of sensitization to topical antibiotics and antiseptics should be borne in mind, and systemic antibiotics may be preferable. Bacteria cultured from a swab are not necessarily playing a pathogenic role. If trauma is thought to be playing a part, and the patient cannot resist scratching, a paste bandage may be helpful.
Resources
Patient resources
- British Association of Dermatology: www.bad.org.uk/leaflets.
- DermNet NZ, Venous eczema: www.dermnetnz.org/dermatitis/venous-eczema.html. Patient, Varicose eczema: www.patient.co.uk/doctor/Varicose-Eczema.htm.
- National Institute for Health and Care Exellence, Clinical Knowledge Summaries, Venous eczema and lipodermatosclerosis: http://cks.nice.org.uk/venous-eczema-and-lipodermatosclerosis.
(All last accessed June 2015.)
Dermatitis and eczema of the eyelids
Definition
This is eczema affecting predominantly the eyelids.
Pathophysiology [1, 2]
This is a common clinical presentation. Eyelid involvement is a very common feature of atopic eczema (Figure 39.17) and it is likely that many cases represent mild atopic eczema without other manifestations. Seborrhoeic dermatitis may also underlie the presentation. Contact allergy to various components of eye makeup, nail varnish, fragrance, rubber [3] or ophthalmic medicaments [4] are responsible for some cases. Allergy to nickel in spectacle frames may cause eczema near the lower eyelids. In some cases the cause remains obscure.

Figure 39.17 Eyelid atopic eczema (note the infra-orbital Dennie–Morgan fold).
Management
When the condition is not amenable to removal of the cause, treatment with hydrocortisone cream is often effective. Topical immunosuppressants (tacrolimus, pimecrolimus) can also be particularly useful in this setting, and avoid the risks of inducing atrophy, rosacea or raised intraocular pressure associated with prolonged use of topical corticosteroids.
Juvenile plantar dermatosis
Definition and nomenclature
This condition is characterized by shiny, dry, fissured dermatitis of the plantar surface of the forefoot.
Introduction and general description
The first record of this condition appeared in 1968, and since then it has been described under a variety of names depending on the authors’ beliefs concerning pathogenesis and the possible association with atopy. The name ‘juvenile plantar dermatosis’ has the merit of making no presumptions about cause.
Epidemiology
Age
It occurs mainly in children aged 3–14 years. Only occasional cases are seen in adults or infants.
Sex
There is a slight preponderance of male children.
Associated diseases
An association with atopy has been proposed but the evidence for this is not convincing. In a controlled study, a personal or family history of eczema or other atopic illness was not more common in cases than in controls [1].
Pathophysiology [2, 3]
The histology shows a mild, non-specific eczema. A blockage of sweat ducts can sometimes be identified.
Genetics
Juvenile plantar dermatosis has been reported in identical twins [4].
Environmental factors [1, 2, 5–8]
It seems likely that changes in fashion, and the resulting changes in the composition of children's socks and shoes, may have been responsible for the emergence of this disease. Waxing and waning fashions for the use of synthetic materials such as nylon and plastics, compared with more porous natural materials such as cotton, wool and leather, may have been responsible. However the exact pathogenesis of the disease remains uncertain [5]. Occasional cases have occurred in children wearing open leather sandals and cotton socks. Many of the affected children are keen on dancing or sports, and this suggests that friction and enhanced sweating may be playing some part.
Clinical features
The presenting features of juvenile plantar dermatosis are redness and soreness on the plantar surface of the forefoot, which assumes a shiny, ‘glazed’ and cracked appearance (Figure 39.18). The condition is most severe on the ball of the foot and toe pads, and tends to spare the non-weight-bearing instep. The toe clefts are normal and this helps to distinguish the condition from tinea pedis. The symmetry of the lesions is a striking feature. Occasionally, the disease can affect the hands, resulting in sore, shiny, fissured palms or fingertips. This is more likely in atopic subjects [9].
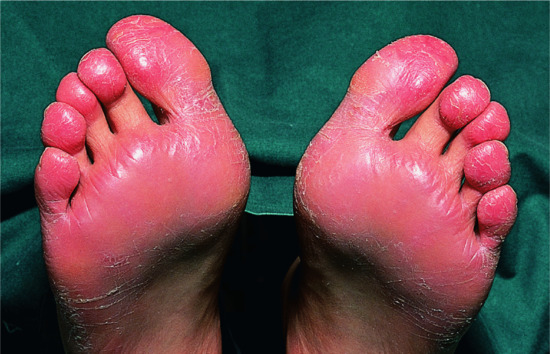
Figure 39.18 Juvenile plantar dermatosis, showing the characteristic glazed appearance of the forefoot skin.
Most cases will clear spontaneously during childhood or adolescence, but the condition may persist into adulthood [10].
Investigations
The diagnosis is clinical, although skin scrapings to exclude fungus and patch tests to exclude footwear allergy may be helpful if there is any doubt. Consultation with the manufacturer of the shoes may help to identify potential allergens.
Management
Patients are usually advised to change from non-porous footwear to 100% cotton socks, and leather shoes or sandals, although this strategy may not resolve the problem [1, 8, 10, 11]. A variety of topical preparations may help, including urea preparations, Lassar's paste, white soft paraffin, tar or tacrolimus ointment, but no single preparation is always effective [2, 12–14].
MISCELLANEOUS SPECIFIED ECZEMATOUS DERMATOSES
Infective dermatitis
Definition and nomenclature
Infective eczema is eczema that is caused by microorganisms or their products, and that by definition clears when the organisms are eradicated (Figure 39.19). This should be distinguished from infected eczema in which eczema due to some other cause is complicated by secondary bacterial or viral invasion of the skin (Figure 39.20). In practice, however, the two conditions can coexist, and the distinction may be difficult.

Figure 39.19 Infective eczema in a non-atopic man. Histology of this localized rash showed eczema, and Staphylococcus aureus was repeatedly isolated. There was no response to topical corticosteroid therapy, but the condition cleared rapidly with oral flucloxacillin.
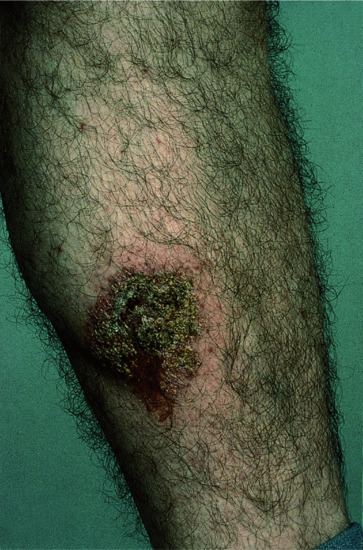
Figure 39.20 Infected dermatitis. This man had a patch of nummular dermatitis that became secondarily infected with Staphylococcus aureus.
Introduction and general description
Infective dermatitis is a controversial entity and some dermatologists never make the diagnosis. This is because the bacterial flora of an eczematous lesion differs quantitatively from that of normal skin [1] and the demonstration that organisms are present does not establish that they are modifying the lesion. The distinction between colonization and infection can be very difficult, but the presence of an increased venous C-reactive protein level may offer a useful clue [2].
Nevertheless, cases are seen occasionally in which bacterial or viral invasion of the skin seems to occur as the primary event and is followed by secondary eczematization which can spread for some centimetres beyond the area of obvious infection. The patches of eczema that occasionally develop around lesions of molluscum contagiosum provide a good example (Figure 39.21). The pearly papules of molluscum are the initiating event and eczema can develop in the surrounding skin some days later, even when the lesions have not been scratched or traumatized. The eczema generally clears when the molluscum lesions subside. Similarly, one occasionally sees eczematous skin around infected wounds, this clears with antibiotic treatment alone.

Figure 39.21 An area of eczematization developing around lesions of molluscum contagiosum. The skin had previously appeared normal, and it returned to normal when the molluscum infection cleared.
(Courtesy of Dr D. A. Burns, Leicester Royal Infirmary, Leicester, UK.)
Pathophysiology
The mechanism by which microorganisms cause eczema is not understood. Bacterial antigens can promote a cytotoxic reaction in the skin, but this is perhaps more likely to aggravate or perpetuate than to initiate the eczematous process [3–6]. Bacterial superantigens such as staphylococcal protein A and enterotoxin B [7] may be profound immune stimulants and may aggravate atopic eczema (see Chapter 41). Bacterial antigens may play this role in a variety of syndromes, including nummular dermatitis, and not merely in infective dermatitis. Cultured staphylococci applied topically to human skin can also provoke an eczematous delayed hypersensitivity reaction [7, 8].
The possibility that bacterial antigens from systemic foci of infection may cause eczema has not been fully established. It does seem to be accepted, however, that eczematous reactions can occur as an allergic reaction to a fungal infection elsewhere in the skin (see the dermatophytide section later in this chapter).
Pathology
The histological picture of infective eczema is in general that of subacute or chronic eczema, in which spongiosis is combined with acanthosis, hyperkeratosis and patchy parakeratosis. The dermis shows inflammatory changes, with polymorphonuclear and lymphocytic infiltration that invades the epidermis to a variable extent. In some stages, subcorneal pustulation may be conspicuous.
Clinical features
As mentioned earlier, the distinction between infective and infected eczema can be difficult.
Infected eczema
Infected eczema shows erythema, exudation and crusting. The exudation may be profuse, generating crusting, or slight, with the accumulation of layers of somewhat greasy, moist scale, beneath which the surface is raw and red. The margin is characteristically sharply defined, and the horny layer is often split to form an encircling collarette. There may be small pustules in the advancing edge and, where a flexure is involved, it is often the site of a deep and persistent fissure.
Infective eczema
Infective eczema usually presents as an area of advancing erythema, sometimes with microvesicles. It is seen predominantly around discharging wounds or ulcers, or moist skin lesions of other types. Infective dermatitis is relatively common in patients with venous leg ulcers, but care must be taken to distinguish it from contact dermatitis due to the application of topical medicaments.
Tinea pedis may also become eczematous due to the overgrowth of Gram-negative organisms [9]. Infective dermatitis may also complicate chronic threadworm infestation, pediculosis or scabies. It is not always clear how much of the eczematous change is due to: repeated scratching; secondary impetigo; or a direct response to the infestation.
Clinical variants
Infective dermatitis of the forefeet is a distinctive pattern of eczema that mainly affects the interdigital spaces on the dorsum of the medial toes. Staphylococci or streptococci can be cultured, and the lesions respond to antiseptic or antibiotic therapy [10]. This condition seems to occur particularly in patients with poor standards of hygiene, and is predisposed by hyperhidrosis and heavy footwear. In children, the condition must be distinguished from juvenile plantar dermatosis.
Management
Factors predisposing to infection should be sought, and eliminated when possible. Although topical antibacterial agents are effective in mild forms of infective eczema due to bacteria, systemic antibiotics may be needed. In acute exudative lesions, potassium permanganate soaks are helpful for the first 2 or 3 days, in combination with a systemic antibiotic.
Infective dermatitis of children associated with human T-cell leukaemia virus 1 infection
Sweet, in 1966 [1], used the term infective dermatitis to describe a pattern of dermatitis observed in Jamaican children. Subsequently, Walshe documented the clinical features in 25 Jamaican children [2]. They included severe, exudative eczema with crusting involving the scalp, eyelid margins, perinasal skin, retroauricular areas, axillae and groins. There was a generalized, fine papular rash, a chronic nasal discharge and positive cultures for Staphylococcus aureus or β-haemolytic streptococci from the nose and/or skin. The rash responded to oral antibiotic therapy, but relapsed on its cessation.
LaGrenade et al., in 1990 [3], found human T-cell leukaemia virus 1 (HTLV-1) infection in all of 14 children with this pattern of dermatitis. A later case report identified one of the children originally described by Sweet and Walshe who, 17 years later, had developed adult T-cell leukaemia [4]. Some of these children may also go on to develop tropical spastic paraparesis [5]. This pattern of dermatitis may be an important early marker of HTLV-1 infection [6].
Post-traumatic eczema
It is rare for eczematous dermatoses to demonstrate the Koebner (isomorphic) phenomenon. However, this may occasionally occur, and eczema may also develop at sites of trauma in individuals who have no past history of eczematous dermatosis. Eczema can also occur in burn scars [1].
There have been several case reports of dermatitis occurring in the saphenous vein graft donor site for coronary artery bypass surgery [2, 3]. The rash comprises reddish brown, slightly crusted and scaly patches, with occasional papulovesicles. Histology shows subacute spongiotic dermatitis. The condition responds to topical corticosteroids, but tends to relapse when the treatment is stopped. Initially, postoperative venous stasis was suggested as the cause. Subsequently, two cases have been reported associated with sensory neuropathy in the distribution of the saphenous nerve [4]. The dermatitis was located in the same area. Otherwise, the cases were identical to those previously reported. The dermatitis and the sensory neuropathy resolved in tandem over a 2-year period.
Pityriasis alba
Definition
This is a pattern of dermatitis in which hypopigmentation is the most conspicuous feature. Some erythema and scaling usually precede the development of hypopigmentation but these are often relatively mild.
Epidemiology
Age
Pityriasis alba occurs predominantly in children between the ages of 3 and 16 years.
Sex
The sexes are equally susceptible.
Associated diseases
Pityriasis alba is often a manifestation of atopic eczema but it is not confined to atopic individuals.
Pathophysiology
The histological changes are unimpressive – acanthosis and mild spongiosis, with moderate hyperkeratosis and patchy parakeratosis. There may be follicular plugging, spongiosis and sebaceous gland atrophy [1–4]. On electron microscopy there are reduced numbers of active melanocytes and a decrease in the number and size of melanosomes in affected skin [3]. Although pigment is reduced, melanocyte numbers are not and may even be increased relative to healthy skin [5].
Clinical features [2, 5–7]
The individual lesion is a rounded, oval or irregular hypopigmented patch that is usually not well marginated. Lesions are often slightly erythematous and have fine scaling. Initially, the erythema may be conspicuous and there may even be minimal serous crusting. Later, the erythema subsides completely and, at the stage at which the lesions are commonly seen by a physician, they show only persistent fine scaling and hypopigmentation. It is this that usually induces the patient to seek advice. The hypopigmentation is most conspicuous in pigmented skin, and in lighter skins may become more evident after sun tanning (Figure 39.22).

Figure 39.22 In pityriasis alba the failure of the affected patches to tan may first bring them to the patient's notice.
(Courtesy of Dr A. Marsden, St George's Hospital, London, UK.)
There are usually several patches ranging from 0.5 to 2 cm in diameter, but they may be larger, especially on the trunk. In children the lesions are often confined to the face, and are most common on the cheeks and around the mouth and chin. In 20% of affected children the neck, arms and shoulders are involved as well as the face. Less commonly the face is spared and there are scattered lesions on the trunk and limbs.
Differential diagnosis
The age incidence, fine scaling and distribution of the lesions usually suggest the diagnosis. Conspicuous hypopigmentation may lead to a misdiagnosis of vitiligo. Naevus depigmentosus most commonly presents at birth or before 3 years of age and most often causes single, well-marginated lesions on the trunk [8]. However, this condition may be difficult to distinguish from pityriasis alba when it occurs on the face and in cases of later onset. Nummular dermatitis in an atopic child is intensely pruritic, and the lesions are larger and more oedematous. In older children and adults, early trunk lesions may be mistaken for psoriasis but the distribution and the relatively mild scaling should exclude this diagnosis. Mycosis fungoides, although relatively rare, may present with lesions clinically resembling pityriasis alba [9]. This condition may also be difficult to distinguish histologically and so follow up and repeated biopsies are sometimes required.
Disease course and prognosis
The course is extremely variable. Most cases persist for some months, and some may still show hypopigmentation for a year or more after all scaling subsides. Recurrent crops of new lesions may develop at intervals. The average duration of the common facial form in childhood is a year or more.
Management
Response to treatment is often disappointing, mainly because the pigmentation takes a long time to recover. The scaling may be reduced by a bland emollient cream, and for chronic lesions on the trunk a mild tar paste may be helpful. Mild topical corticosteroids are helpful if inflammation persists. Topical tacrolimus and pimecrolimus are effective in facial atopic eczema and seem likely to prove helpful, if required, in pityriasis alba [10].
Chronic superficial scaly dermatitis (small plaque parapsoriasis)
Definition and nomenclature
This is a chronic condition characterized by the presence of round or oval erythematous, slightly scaly patches on the limbs and trunk, which histologically show mild eczematous changes with little or no dermal infiltrate.
Introduction and general description
This condition was formerly included with various potentially prelymphomatous eruptions under the general term of ‘parapsoriasis’ [1–3]. The term chronic superficial scaly dermatitis was introduced by Calnan and Meara [4] to distinguish a subgroup of patients who did not progress to frank lymphoma, and the features that they used to distinguish this subgroup are shown in Table 39.3. The nomenclature has been discussed in detail by Lambert and Everett [2].
Table 39.3 Features that distinguish between a prelymphomatous (prereticulotic) eruption and chronic superficial scaly dermatitis
| Prelymphomatous eruption | Chronic superficial scaly dermatitis |
| Bizarre or angulated shape | Regular, round or oval shape |
| Fine scale | Coarser scale |
| May be irritable | Little or no irritation |
| Progresses to cutaneous lymphoma | Does not become malignant |
| Histology | |
| Absence of epidermal eczema | May be eczematous changes |
| Dermal infiltrate | Little or no dermal infiltrate |
The condition is clinically benign by definition [4]. However, in some cases clonality of the lymphocytic infiltrate can be demonstrated [5], a feature suggesting that this may be regarded as an abortive form of cutaneous T-cell lymphoma [6]. Ackerman has taken this argument further and expressed the view that even an abortive T-cell lymphoma is still a T-cell lymphoma, so this condition is a clinical presentation of mycosis fungoides [7].
Epidemiology
Age
In most cases the onset is in middle life.
Sex
It is much commoner in men than in women.
Ethnicity
The disease occurs in all races, although it is probably rare in people with darker skin types.
Pathophysiology
The aetiology of the condition is unknown.
Pathology
The histology is not characteristic. It usually shows the changes of a very mild eczematous eruption, consisting of patchy parakeratosis, mild spongiosis and a slight, mainly perivascular, infiltrate in the dermis, chiefly composed of lymphocytes [3].
Clinical features
The disease begins insidiously with one or more erythematous, slightly scaly patches. The legs, trunk and arms are most often affected (Figure 39.23). It seldom involves the face, palms or soles. The patches are generally round or oval, but finger-like processes are also common, especially on the trunk, giving rise to the alternative name ‘digitate dermatosis’. The patches are usually about 2.5 cm across, although much larger areas occur at times, especially on the legs. The colour is pink, brown or slightly yellow. The individual patches are often slightly wrinkled and appear like cigarette paper. Symptoms are usually minimal, but some itching may occur.

Figure 39.23 Chronic superficial scaly dermatitis.
Differential diagnosis
The main differentials are nummular dermatitis, eczematides, poikiloderma in its early phase, and the early stages of the classic (Alibert) form of mycosis fungoides (see Table 39.3 and Chapter 140). The history, clinical appearance, response to treatment and histology will usually establish the diagnosis. Some cases originally diagnosed as chronic superficial scaly dermatitis later develop reticulate pigmentation or atrophy, and these cases may then need to be reclassified as prelymphomatous poikiloderma.
Disease course and prognosis
The patches are more prominent in winter than in summer, and may clear temporarily with natural or artificial sunlight. They will also clear for a time with suitable topical medications but recur in the same, or adjacent, areas when treatment is stopped. After extending they then usually remain static and, with minor fluctuations, persist throughout life. In a few patients the condition clears permanently.
Management
Only symptomatic treatment is required to allay irritation. Mild steroid ointments are generally beneficial. Natural sunlight, narrow-band UVB and PUVA can be helpful when required, but relapse often occurs soon. Narrow-band UVB may also be effective in inducing remission, which can last from a few weeks to many months [8].
Dermatophytide
Definition
This is a reaction, at a remote site, to a dermatophyte infection [1–3].
Introduction and general description
This diagnosis should be suspected when the presence of a dermatophyte infection has been established and no fungus can be demonstrated in the dermatophytide lesions. The diagnosis is supported further by clearing of the dermatophytide after the dermatophyte has been eradicated. A dermatophytide is thus a secondary, distant, aseptic skin lesion. The terminology implies analogy to the cutaneous tuberculides associated with tuberculosis [1], although the pathomechanisms involved may be different.
Epidemiology
Incidence and prevalence
One study indicated that this condition is rare, as only 10 cases were confirmed in 1500 dermatophyte infections [2]. Others have suggested that dermatophytide may be more common. In one retrospective review of cases of dermatophytide, 37 cases were seen in a dermatology department in a 2-year period [4].
Clinical features
Various clinical patterns of dermatophytide can occur. On the hands, eczematous vesicles may occur symmetrically on the sides of the fingers, usually as a reaction to tinea pedis. An eczematous dermatophytide can also mimic pityriasis rosea [1]. Other dermatophytides have been described, often as single case reports, including erysipelas-like dermatitis, erythema nodosum, erythema annulare centrifugum, urticaria and erythroderma. A dermatophytide is probably more likely to develop with inflammatory dermatophytes, such as Trichophyton mentagrophytes of the zoophilic type [2]. Treatment of the dermatophyte leads to resolution of the secondary rash [4]. A similar allergic reaction to a yeast infection may be termed a candidide (levuride). In this case the eczematous reaction may be localized to the hands or groin [1].
A dermatophytide can be mimicked by bacterial infection. In one study, Staphylococcus aureus and β-haemolytic streptococci were commonly isolated from lesions that were thought clinically to be examples of a dermatophytide [2].
Halo dermatitis
Definition and nomenclature
Halo dermatitis is the occurrence of an eczematous ring surrounding a melanocytic naevus.
Introduction and general description
Meyerson [1] described two patients with multiple, pruritic, papulosquamous lesions surrounding melanocytic naevi (Figure 39.24). More than 20 similar cases have since been described [2–4], mainly in young adults. Histology shows a benign naevus surrounded by a dermal lymphocytic and eosinophilic infiltrate, with overlying acanthosis, spongiosis and parakeratosis. One case developed during treatment with interferon α-2b in a patient with dysplastic naevus syndrome and Behçet disease [5]. The condition usually resolves spontaneously within a few months, without involution of the naevus. It differs from Sutton halo depigmentation, although the two conditions have been reported to coexist in the same patient and in one case progression to Sutton naevus occurred [6]. Similar changes are not infrequently seen around seborrhoeic keratoses [7] and other elevated skin lesions [8] and are termed the Meyerson phenomenon.

Figure 39.24 Halo dermatitis showing eczema around a mole.
Murray Williams warts
Multiple seborrhoeic keratoses occurring in areas of resolved eczema have only occasionally been reported since the phenomenon was described by Williams in 1956 [1, 2]. Multiple seborrhoeic keratoses arise in the few months following resolution of the eczema and tend to gradually resolve by 5–6 months.
OTHER RELATED DERMATOSES
Lichen simplex and lichenification
Definition and nomenclature
Lichen simplex is an eczematous dermatosis characterized by a small number of heavily lichenified plaques or, very often, a single lesion.
Introduction and general description
Lichenification is a change in appearance and texture of the skin associated particularly with pruritic dermatoses. Mild or early lichenification presents as a rather subtle coarsening of the skin surface markings on a background of dry and usually erythematous skin. As the condition progresses, the skin becomes markedly thickened and hyperkeratotic (Figure 39.25). Lichenification may occur spontaneously, when it is known as lichen simplex, or may occur as a secondary consequence of eczema and other inflammatory dermatoses.

Figure 39.25 Lichenification of the arm in a patient with atopic eczema.
Epidemiology
Age
Lichen simplex is uncommon in childhood. The peak incidence is between 30 and 50 years of age, but it is seen at any age from adolescence onwards. Secondary lichenification of atopic eczema can occur in any age group and broadly mirrors the prevalence of atopic eczema in the particular age group.
Sex
Women are affected by lichen simplex more often than men.
Ethnicity
There is no conclusive evidence of a link with particular ethnicities.
Associated diseases
A dermatomal pattern of lichen simplex chronicus has been described as the initial presentation of an intramedullary neoplasm with syringomyelia [1].
Pathophysiology
Predisposing factors
Patients with lichen simplex are more readily conditioned to scratch following an itch stimulus than are control subjects [2]. Lichenification frequently occurs as a manifestation of atopic eczema [3] but is not universally present and lichen simplex occurs in many individuals who show no stigmata of atopy. In the predisposed subject, emotional tensions play an important role in favouring the development of lichen simplex and ensuring its perpetuation [4].
Pathology [5, 6]
The histological changes of lichen simplex vary with site and duration. Acanthosis and variable degrees of hyperkeratosis are usually observed. The rete ridges are lengthened. Spongiosis is sometimes present, and small areas of parakeratosis are occasionally seen. There is hyperplasia of all components of the epidermis [7]. The labelling index has been shown autoradiographically to be over 25%, but the transit time is longer than in psoriasis [8].
The dermis contains a chronic inflammatory infiltrate, and in very chronic lesions there may be some fibrosis. Silver impregnation techniques show proliferation of the Schwann cells, which may make up an appreciable proportion of the cellular infiltrate.
In very chronic lesions, especially in giant lichenification, the acanthosis and hyperkeratosis are gross, and the rete ridges are irregularly but strikingly elongated and widened.
Genetics
There are no specific genetic links reported for lichen simplex. Lichenified atopic eczema is linked with filaggrin gene mutations.
Environmental factors
Lichenification can arise as a result of allergic contact dermatitis. Lichen simplex has been linked in a case series to allergic contact dermatitis to hair dye [9].
Clinical features
History
In all forms of lichenification, pruritus is a prominent symptom, and is often out of proportion to the extent of the objective changes. It may develop in paroxysms of great intensity. Scratching tends to give initial satisfaction, but is then continued until the skin is sore.
Presentation
In lichen simplex, single and multiple sites are involved with about equal frequency. Almost any area may be affected, but the commonest sites are those that are conveniently reached. The usual sites are the nape of the neck, lower legs (Figure 39.26a) and ankles (Figure 39.26b), sides of the neck, scalp, upper thighs, vulva, pubis or scrotum, and extensor forearms. In lichenified eczema and secondary lichenification, the sites are those affected by the primary dermatosis, for example limb flexural sites in atopic eczema.
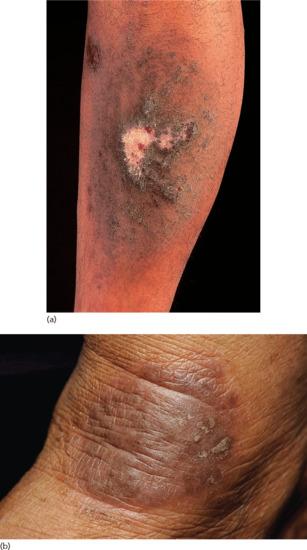
Figure 39.26 Lichen simplex. (a) On the lower leg
(Courtesy of Dr D. A. Burns, Leicester Royal Infirmary, Leicester, UK). (b) On the ankles.
During the early stages of lichen simplex, the skin is erythematous and slightly oedematous, and normal skin markings are exaggerated. The erythema and oedema subside and the central area becomes scaly and thickened and sometimes pigmented. Surrounding this central plaque is a zone of lichenoid papules and beyond this an indefinite zone of slight thickening and pigmentation merging with normal skin. These features may be greatly modified by the site and duration of the lesion. In mild cases, follicular eczematous papules may be seen, particularly on the forearms and elbow regions of children (Figure 39.27).

Figure 39.27 Follicular papules of lichenification adjacent to the elbow.
Clinical variants
Lichen simplex of the nape of the neck (lichen nuchae) is usually confined to women. The plaque may be limited to a small area around the midline of the nape or may extend some distance into the scalp and over the neck. Scaling is often profuse and psoriasiform, and episodes of secondary infection are frequent. The fold behind the ear may also be involved. Scaling, crusting and fissuring are more evident than the usual changes of lichenification. Other regions of the scalp are less often affected. The presenting manifestation is an area of scaling, with twisted, broken hairs. The epidermal thickening may be great enough to form a nodule.
If lichenification occurs at sites where the subcutaneous tissues are lax and excoriation continues for many years, solid tumour-like plaques may be formed, with a warty, cribriform surface. This variant is known as giant lichenification of Pautrier [10] and occurs mainly in the genito-crural region.
The descriptive term pebbly lichenification has been applied to a distinctive clinical variant, consisting of discrete, smooth nodules, seen occasionally in atopic and seborrhoeic subjects, and in photodermatitis. Clinically it may simulate lichen planus.
Differential diagnosis
The morphological diagnosis of lichenification is usually characteristic. However, lichen planus, lichen amyloidosus and psoriasis are differential diagnoses that may be elicited by checking other anatomical sites. Sometimes, however, no conclusive diagnosis is possible on either clinical or histological grounds. A patient with psoriasis may develop lichen simplex that combines the histological features of both conditions.
Once the diagnosis of lichenification has been established its causation must be carefully investigated. Secondary lichenification complicates persistent skin lesions of many types. It occurs on the lower leg in the presence of venous insufficiency, in atopic eczema, in asteatotic eczema, in low-grade chronic contact dermatitis and in some chronic infections with Trichophyton rubrum. Symmetrical lesions may suggest secondary lichenification from a contact dermatitis.
Complications and co-morbidities
An association has been reported between lichen simplex and depression and dissociative experiences [11].
Disease course and prognosis
Lichen simplex tends to follow a chronic course unless the itch–scratch cycle can be broken.
Investigations
Skin scrapings for mycological investigation may be indicated. Suspicion of allergic contact dermatitis should be investigated by patch testing.
Management
A careful psychological history should be taken to elucidate any underlying problems. The nature of lichen simplex and the need to break the scratching habit must be explained. Sedative antihistamines may be helpful and antibiotics may be necessary if secondary infection is present. A potent or very potent topical corticosteroid can be very helpful in some cases, but seems to have no effect in others.
On an arm or leg it is useful to apply an occlusive zinc paste bandage, if tolerated, which prevents scratching and improves skin hydration. Self-adhesive, steroid-impregnated tape (e.g. Haelan® tape) can often be effective for localized lesions. Alternatively, a potent steroid ointment under polythene occlusion, for short periods, may also be considered. Modest improvement has also been shown with 5% doxepin cream [12].
For solitary, circumscribed, chronic lesions, dermal infiltration with triamcinolone (10 mg/mL) can be effective.
Response has also been reported to capsaicin cream [13].
Resources
Patient resources
- DermNet NZ, Lichen simplex: http://www.dermnet.org.nz/dermatitis/lichen-simplex.html (last accessed June 2015).
Erythroderma
Definition and nomenclature
Erythroderma is the term applied to any inflammatory skin disease that affects more than 90% of the body surface.
Introduction and general description
Erythroderma is included in this chapter because eczema is the commonest underlying cause. Erythroderma may be the initial presentation of eczema in an individual or, more commonly, arises in the context of longstanding eczema.
Epidemiology
Incidence and prevalence
A survey of dermatologists from the Netherlands estimated the annual incidence at 0.9 per 100 000 population [1].
Age and sex
In a case series of 97 patients from Iran, the mean age at presentation was 46 years [2]. The male to female ratio was 1.85 : 1.
Ethnicity
There have been no specific ethnic variations reported.
Associated diseases
This will vary depending on the underlying cause of the erythroderma.
Pathophysiology
Predisposing factors [3–7]
The main causes of erythroderma in adults are listed in Table 39.4 and are discussed below. The figures vary somewhat with the age of the population, and are based on several published studies. In younger people, for example military personnel, there will be a larger proportion due to drug allergies [7]. Drugs commonly causing erythroderma are listed in Chapter 119. In some communities, the incidence of erythroderma may be higher because of self-medication and use of herbal remedies such as St John's wort [9]. The causes of erythroderma in the newborn are considered in Chapter 116.
Table 39.4 Causes of erythroderma and relative prevalence in adults [3–7]
| Condition causing erythroderma | Relative prevalence (%) |
| Eczema of various subtypes | 40.0 |
| Psoriasis | 25.0 |
| Lymphoma and leukaemias | 15.0 |
| Drugs (including phenylbutazone, phenytoin, carbamazepine, gold salts, lithium, cimetidine) | 10.0 |
| Unknown | 8.0 |
| Hereditary disorders (ichthyosiform erythroderma, pityriasis rubra pilaris) | 1.0 |
| Pemphigus foliaceus | 0.5 |
| Other skin diseases (lichen planus, dermatophytosis, crusted scabies, dermatomyositis) | 0.5 |
Other rare causes of erythroderma include sarcoidosis [10], Hailey–Hailey disease [11], pemphigoid [12], toxic shock syndrome [13], lupus erythematosus [14], angioimmunoblastic lymphadenopathy [15] and dermatomyositis [16]. Graft-versus-host disease may progress to erythroderma in some cases. A related disorder was reported in Japan, where cases of fatal erythroderma occurred following major surgery. It was suggested that these cases were examples of post-transfusion graft-versus-host disease [17].
Erythroderma has occasionally been reported with seroconversion following HIV infection [18]. In established AIDS, erythroderma may arise from a variety of causes including seborrhoeic dermatitis, lymphoma or an unknown cause [19]. However, it should be noted that CD4+ T lymphocytopenia has been associated with erythroderma in the absence of HIV infection [20].
Pathology
Histopathology can help identify the cause of erythroderma in up to 50% of cases, particularly if multiple skin biopsies are examined [21]. The histological appearances vary depending upon the severity and duration of the inflammatory process. In the acute stage, spongiosis and parakeratosis are prominent, and a non-specific inflammatory infiltrate permeates a grossly oedematous dermis to a variable depth. In the chronic stage, acanthosis and elongation of the rete ridges become more prominent.
In erythroderma due to lymphoma the infiltrate may become increasingly pleomorphic and eventually acquire specific diagnostic features such as a band-like lymphoid infiltrate at the dermal–epidermal junction, with atypical cerebriform mononuclear cells and Pautrier microabscesses [22]. In other cases, however, it remains non-specific throughout its course, and the distinction can be difficult. Patients with Sézary syndrome often show some features of chronic dermatitis and benign erythroderma may occasionally show some features suggestive of lymphoma.
Immunophenotyping of the lymphoid infiltrate may not solve the problem as it generally shows features of mature T cells in both benign and malignant erythroderma [23].
In psoriasis, papillomatosis and clubbing of the papillary zones may be seen, and in pemphigus foliaceus, superficial acantholysis will be present. In ichthyosiform erythroderma and pityriasis rubra pilaris, repeated biopsies from carefully selected sites may reveal their characteristic features.
Genetics
This will depend on the underlying condition.
Clinical features
History and presentation
Erythroderma developing in primary eczema or associated with a lymphoma is often of sudden onset. Patchy erythema, which rapidly generalizes, may be accompanied by fever, shivering and malaise. Hypothermia may develop.
The erythema extends rapidly, and may be universal in 12–48 h. Scaling appears after 2–6 days, often first in the flexures, but it varies greatly in degree and character from case to case. The scales may be large or fine and bran-like. At this stage the skin is bright red, hot and dry, and palpably thickened. The intensity of the erythema may fluctuate over periods of a few days or even a few hours. Irritation is sometimes severe, but a sensation of tightness is more characteristic. Many patients complain of feeling cold, especially when the erythema is increasing.
When the erythroderma has been present for some weeks, the scalp and body hair may be shed and the nails become ridged and thickened and they may also be shed. The periorbital skin is inflamed and oedematous, resulting in ectropion, with consequent epiphora. In very chronic cases there may be pigmentary disturbances, especially in black people, in whom patchy or widespread loss of pigment is often seen.
The degree of enlargement of the lymph nodes in the absence of an underlying malignant lymphoma is variable. They are usually slightly or moderately enlarged and of rubbery consistency, but in some cases the enlargement may be gross. It is important that this dermatopathic lymphadenopathy is not mistaken for lymphoma. In difficult cases, lymph node biopsy may be advisable, but the pathologist must be told that the patient is erythrodermic for a reliable histological interpretation to be made.
The general picture is modified according to the nature of any underlying disease and the patient's age and general physical condition.
Clinical variants
Clinical variants are considered in terms of the underlying cause of the erythroderma. Papuloerythroderma of Ofuji is also discussed as a distinct clinical variant.
Eczematous dermatoses
Generalization of an eczema occurs most frequently in the sixth and seventh decades when venous eczema is a common precedent. However, atopic erythroderma may occur at any age. Exacerbation of existing lesions usually precedes the generalization, which follows the usual pattern. Pruritus is often intense. Some elderly patients have increased serum IgE and lactic dehydrogenase levels, with eosinophilia [24].
Psoriasis
In erythrodermic psoriasis (Figure 39.28) the clinical picture may be highly desquamative but when the erythroderma is fully developed the specific features of psoriasis are often lost. In some cases, crops of miliary pustules may develop at intervals, and transition to generalized pustular psoriasis may occur, especially in cases treated with potent topical corticosteroids or systemic steroids [25]. Emotional stress, intercurrent illness and phototherapy overdosage may also precipitate erythroderma.

Figure 39.28 Erythrodermic psoriasis.
Lymphoma, leukaemia and other malignancy
Cutaneous T-cell lymphoma is the commonest malignancy to cause erythroderma (Figure 39.29), followed by Hodgkin disease. Non-Hodgkin lymphoma, leukaemias and myelodysplasia have also been reported as causes. Association with other internal malignancies has been observed less often [6].

Figure 39.29 Erythroderma in Sézary syndrome.
(Courtesy of Dr B. Dharma, University Hospitals Coventry and Warwickshire, UK.)
The clinical picture follows the pattern already described, but the accentuation of certain features should arouse suspicion that a lymphoma is associated, even if repeated investigations over a period of months fail to provide convincing confirmatory evidence. In many such cases the underlying disease will eventually be detected. Pruritus is often very severe. The erythroderma is universal, and infiltration of the skin may be so severe that the patient's features are deformed. Rubbing and scratching may produce secondary lichenification. Enlargement of the lymph nodes may be considerable, even if histologically they are not infiltrated by the lymphoma.
A biopsy of involved skin may show only non-specific features initially, and may need to be repeated several times before infiltration with atypical lymphocytes becomes evident. A skin biopsy for the analysis of T-cell receptor gene rearrangement, to determine whether clonality is present in the infiltrate, may be helpful [26, 27]. Lymph node biopsy may be diagnostic of lymphoma but often shows only the features of dermatopathic lymphadenopathy. There may be hepatosplenomegaly. A differential white blood cell count should be performed and the blood examined for abnormal cells. Eosinophilia may suggest Hodgkin disease. Atypical lymphocytes with cerebriform nuclei (Sézary cells) are often observed in erythroderma regardless of cause. When they constitute more than 20% of the circulating peripheral blood mononuclear cells they become diagnostic of the leukaemic variant of cutaneous T-cell lymphoma known as Sézary syndrome. Large Sézary cells (15–20 μm in diameter) are diagnostic even in small numbers [28]. The demonstration of a clonal T-cell population in the peripheral blood by analysis of T-cell receptor genes, using polymerase chain reaction, appears to offer high diagnostic specificity for Sézary syndrome. The sensitivity also appears high, but on occasions the test may need to be repeated if initial results are negative and this diagnosis is still suspected [28].
Drugs
A wide range of drugs can cause erythroderma. Among the more commonly implicated are phenylbutazone, phenytoin, carbamazepine, cimetidine, gold salts and lithium [29]. The eruption may start as a generalized eczema, or scarlatiniform or morbilliform erythema, often accompanied by some irritation, which increases steadily in severity. Erythema may first appear in the flexures, or over the whole skin (Figure 39.30). This group has the best prognosis of all the causes of erythroderma [3, 30], often resolving in 2–6 weeks [31]. However, it is important to remember that the cutaneous manifestations of drug hypersensitivity may be accompanied by the involvement of other organs, for example haematological abnormalities, hepatitis, nephritis or pneumonitis. An example is the DRESS syndrome (drug reaction with eosinophilia and systemic symptoms; see Chapter 119).
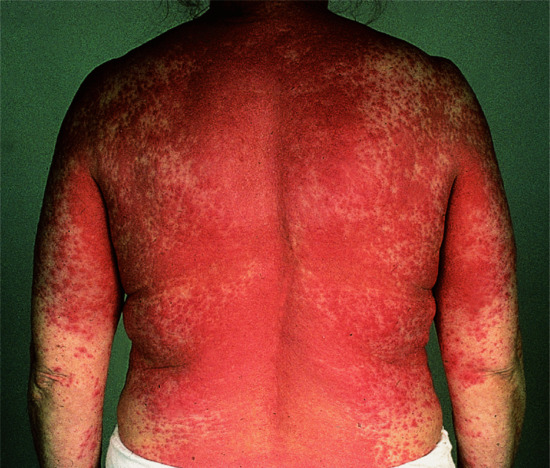
Figure 39.30 Widespread drug rash. This will progress rapidly to erythroderma if the drug is continued.
Erythroderma of unknown origin
The percentage of cases in which no underlying disease is demonstrable diminishes with the thoroughness of investigation and the duration of observation, but in any series of cases it is rarely below 10% [4, 5, 31]. The cutaneous changes may precede any other evidence of a lymphoma by many months or years. If these cases are excluded, the remaining patients with chronic erythrodermas of unknown origin consist mainly of elderly men, in whom the condition runs a very long course with partial and temporary remissions, sometimes known as the ‘red man syndrome’. It is characterized by marked palmoplantar keratoderma, dermatopathic lymphadenopathy and a raised serum IgE [24, 32]. It is important to note that this conditionis not established erythrodermic cutaneous T-cell lymphoma, which is occasionally referred to as ‘l'homme rouge’.
The three commonest causes of idiopathic protracted erythroderma are probably atopic eczema of the elderly, intake of drugs overlooked by the patient and prelymphomatous eruptions [4].
Ichthyosiform erythroderma
This condition is usually present from birth or early infancy.
Pityriasis rubra pilaris
The erythrodermic forms may begin in childhood or adult life. The presence of follicular, horny plugs on the knees and elbows, and on the backs of the fingers and toes, is distinctive. In many cases, scattered islands of normal skin persist in the erythrodermic regions, and horny plugs may be evident around their margins. These normal pale ‘islands’ are very suggestive of the diagnosis, and the skin on the palms and soles often has an orange discoloration.
Pemphigus foliaceus
Moist, crusted lesions on the face and upper trunk often precede the development of the erythroderma. Scaling is conspicuous, moist and adherent. Crops of thin-walled bullae may erupt, especially on the limbs.
Lichen planus
Erythrodermic lichen planus is very rare but lichenoid reactions to gold, quinine and other drugs have resulted in erythroderma. As the initial erythema and oedema subside, individual violaceous papules may be revealed. The buccal mucous membrane may show typical lacy, bluish white streaks.
Dermatophytosis
Generalized erythroderma has very rarely resulted from chronic infection with organisms such as Trichophyton violaceum.
Norwegian scabies
The heavily crusted hands and feet, with thickened nails, characteristic of Norwegian scabies, may occasionally be accompanied by generalized erythema and scaling. The condition is often mistaken for erythrodermic psoriasis. The occurrence of scabies in others in the same environment, or in the medical or nursing staff caring for the patient, may reveal the diagnosis.
Papuloerythroderma of Ofuji [33–37]
This distinctive pattern of erythroderma was described by Ofuji in 1984 [33]. It differs from ordinary erythroderma in that papulation is prominent, it tends to spare the face and flexures, and is often intensely pruritic. It is not yet clear whether this represents a distinct disease or a reaction pattern. Although most cases were found to be idiopathic, several have been reported in association with other diseases. Papuloerythroderma occurs in later life, with ages at diagnosis ranging from 57 to 100 years. Many cases occur in the eighth or ninth decades. Males are predominantly affected and the male : female ratio is estimated at 4.7 : 1 [34].
Histological features are usually non-specific. In the epidermis there are generally mild degrees of acanthosis, spongiosis, hyperkeratosis and focal parakeratosis. There is marked lymphohistiocytic infiltration of the dermis, predominantly perivascular in distribution, and eosinophils are often conspicuous. A mild degree of epidermotropism has occasionally been observed and, rarely, plasma cells and multinucleate giant cells may be present. Immunofluorescence is negative.
The erythroderma typically begins with an eruption of brownish red, flat-topped papules that become confluent (Figure 39.31a). The limbs and trunk are affected and the face and flexures tend to be spared. A characteristic and distinctive pattern of sparing of the abdominal flexures has been termed the ‘deck chair sign’ [35], indicating a similarity to the distribution of sunburn in one who has been sitting out in a deck chair for too long (Figure 39.31b). The lesions sometimes develop along scratch marks. Pruritus is a consistent feature and ranges from moderate to very severe. Additional features often observed include hyperkeratosis and fissuring of the palms and soles, and benign lymphadenopathy. There is usually circulating eosinophilia and a raised IgE, as well as a mild degree of absolute or relative lymphocytopenia. In terms of prognosis, papuloerythroderma typically persists for many years, although some cases have remitted.

Figure 39.31 Papuloerythroderma of Ofuji. (a) The papules. (b) The ‘deck-chair sign’ (sparing of the body folds).
(Courtesy of Dr M. J. Tidman, Edinburgh Royal Infirmary, Edinburgh, UK.)
Reports of papuloerythroderma occurring in association with malignancies, which have included T-cell [36, 37] and B-cell lymphomas [37], and gastric [37], lung [37], colon [38], prostate [34] and hepatocellular [39] carcinomas, would suggest that this eruption may sometimes occur as a paraneoplastic phenomenon. There are also several reports that papuloerythroderma may progress into mycosis fungoides [34, 40, 41], in one case 11 years after the onset of symptoms [42]. In some cases, papuloerythroderma therefore seems to be a presentation of cutaneous T-cell lymphoma. One case developed into psoriasis [34]. Papuloerythroderma has also been reported in association with HIV infection [43, 44] and, in one case, biliary sepsis [45].
In terms of management, emollients, topical corticosteroids and antihistamines have produced a slow response in some cases. The condition can respond well to oral prednisolone, although high doses are sometimes required. PUVA, including bath PUVA, has proved effective in several reports. Azathioprine [46], ciclosporin [47] and etretinate [48] may be effective. Retinoids or phototherapy may be preferable to immunosuppressants because of the possible progression to cutaneous T-cell lymphoma and association with malignancy. However, papuloerythroderma is sometimes very refractory to treatment.
Differential diagnosis
See Table 39.4 for possible underlying causes.
Classification of severity
By definition, erythroderma involves more than 90% of the body surface.
Complications and co-morbidities
The main complications of erythroderma are haemodynamic and metabolic disturbances. Blood flow through the skin is markedly increased and this can result in high-output cardiac failure, especially in elderly patients [49, 50]. The increased skin perfusion may lead to hypothermia [51]. Fluid loss by transpiration is much increased and is roughly proportional to the basal metabolic rate. Hypoalbuminaemia is in part due to increased protein loss from exfoliated scale, which may reach 9 g/m2 of body surface or more each day [52]. This in turn results in peripheral oedema. Immune responses may become altered, reflected by an increase in γ-globulins or CD4+ T lymphocytopenia in the absence of HIV infection [20].
Disease course and prognosis
Erythroderma is a potentially fatal condition. Current mortality rates are probably lower than historically reported, varying from 18% to 64% [3, 5, 7], but erythroderma remains particularly dangerous in elderly people. Cutaneous, subcutaneous and respiratory infections are common and pneumonia remains the commonest cause of death [25]. Complications resulting from systemic treatment of the erythroderma also contribute to mortality rates.
The more frequent forms of erythroderma, including eczematous, psoriatic or of unknown origin, may continue for months or years and often follow a relapsing-remitting course [25].
Investigations
The greatest diagnostic yield to determine an underlying cause is obtained from multiple skin biopsies, however characteristic histological features are often lacking despite this [53]. A detection of T-cell clonality in the skin or peripheral blood should be sought if lymphoma is suspected, as described above.
Management
Treatment in hospital is frequently necessary, especially in acute and fulminant cases, because some patients may develop profound loss of homeostasis. In these cases the protein and electrolyte balance, circulatory status and body temperature require close monitoring. Appropriate fluid intake should be maintained and cardiac failure must be treated if it develops. All non-essential drugs should be withdrawn if they might be responsible for the erythroderma. Patients should be monitored for infection to ensure timely instigation of antibiotics if needed.
The cutaneous inflammation should be treated in the first instance with greasy emollients in order to restore skin barrier function. Other topical treatments should be used with caution because systemic absorption is greatly increased. The majority of patients will improve over a week or two with this regimen, during which time the diagnosis of the underlying condition will probably be established. Systemic therapy is usually necessary and will depend on the underlying cause identified. Systemic corticosteroids should be avoided for erythroderma due to psoriasis to avoid pustular transformation [25].
References
Patient resources
- British Association of Dermatology: www.bad.org.uk/leaflets.
- DermNet NZ, Discoid eczema: http://dermnetnz.org/dermatitis/nummular-dermatitis.html.
- Medscape, Nummular dermatitis: http://emedicine.medscape.com/article/1123605-overview.
- NHS Choices, Discoid eczema: http://www.nhs.uk/conditions/Eczema-(discoid)/Pages/Introduction.aspx. (All last accessed June 2015.)
Patient resources
- DermNet NZ, Eczema craquelé: http://www.dermnetnz.org/dermatitis/eczema-craquele.html (last accessed June 2015).
Further information
- Diepgen TL, Elsner P, Schliemann S, et al. Guideline on the management of hand eczema ICD-10 code: L20. L23. L24. L25. L30. J Dtsch Dermatol Ges 2009;7(Suppl. 3):S1–16.
- National Institute for Health and Care Excellence, Alitretinoin for the treatment of severe chronic hand eczema: http://guidance.nice.org.uk/ta177 (last accessed June 2015).
Patient resources
- British Association of Dermatologists: www.bad.org.uk/leaflets.
- DermNet NZ, Hand dermatitis: www.dermnet.org.nz/dermatitis/hand-dermatitis.html.
- National Institute for Health and Care Excellence patient information regarding alitretinoin for chronic hand eczema: www.nice.org.uk/guidance/ta177. (All last accessed June 2015.)
Patient resources
- British Association of Dermatology: www.bad.org.uk/leaflets.
- DermNet NZ, Venous eczema: www.dermnetnz.org/dermatitis/venous-eczema.html. Patient, Varicose eczema: www.patient.co.uk/doctor/Varicose-Eczema.htm.
- National Institute for Health and Care Exellence, Clinical Knowledge Summaries, Venous eczema and lipodermatosclerosis: http://cks.nice.org.uk/venous-eczema-and-lipodermatosclerosis. (All last accessed June 2015.)
Patient resources
- DermNet NZ, Lichen simplex: http://www.dermnet.org.nz/dermatitis/lichen-simplex.html (last accessed June 2015).
References
Assessment, investigation and management of eczematous disorders
- Altekrueger I, Ackerman AB. ‘Eczema’ revisited. A status report based on current textbooks of dermatology. Am J Dermatopathol 1994;16:517–22.
- Johansson SG, Bieber T. New diagnostic classification of allergic skin disorders. Curr Opin Allergy Clin Immunol 2002;2:403–6.
- MacKenzie-Wood AR, Freeman S. Unclassified endogenous eczema. Contact Dermatitis 1999;41:18–21.
- Li LF, Liu G, Wang J. Prognosis of unclassified eczema. Arch Dermatol 2008;144:160–4.
- Shaffrali FC, Colver GB, Messenger AG, Gawkrodger DJ. Experience with low-dose methotrexate for the treatment of eczema in the elderly. J Am Acad Dermatol 2003;48:417–19.
- Johnson M-LT, Roberts J. Prevalence of Dermatological Disease Among Persons 1–74 Years of Age. No. PHS 79–1660. Washington DC: US Department of Health Education, National Center for Health Statistics, 1978.
- Meding B, Liden C, Berglind N. Self-diagnosed dermatitis in adults. Results from a population survey in Stockholm. Contact Dermatitis 2001;45:341–5.
- Schafer T, Böhler S, Ruhdorfer S, et al. Epidemiology of contact allergy in adults. Allergy 2001;56:1192–6.
- Schofield J, Grindlay D, Williams H. Skin Conditions in the UK: a Health Care Needs Assessment. Nottingham: Centre of Evidence Based Dermatology, University of Nottingham, 2009.
- Horn R. The pattern of skin disease in general practice. Dermatol Pract 1986;2:14–19.
- Steele K. Primary dermatological care in general practice. J R Coll Gen Pract 1984;34:22–4.
- Goh CL, Chua-Ty C, Koh SL. A descriptive profile of eczema in a tertiary referral centre in Singapore. Ann Acad Med Singapore 1993;22:307–15.
- Johnson MLT, Roberts R. Skin Conditions and Related Need for Medical Care Among Persons 1–74 Years of Age. Series II, No. 212. Washington, DC: US Department of Health Education, National Center for Health Statistics 1978.
- Girolomoni G, Sebastiani S, Albanesi C, Cavani A. T-cell populations in the development of atopic and contact allergy. Curr Opin Immunol 2001;13:733–7.
- Berardesca E, Distante F. The modulation of skin irritation. Contact Dermatitis 1994;31:281–7.
- Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol Lett 1998;102–103:277–82.
- Effendy I, Loffler H, Maibach HI. Epidermal cytokines in murine irritant responses. J Appl Toxicol 2000;20:335–41.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol 2002;27:138–46.
- Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci 2009;122:1285–94.
- Baer RL, Rosenthal SA, Sims CF. The allergic eczema-like reaction and the primary irritant reaction. Arch Dermatol 1957;76:549–60.
- Komura J, Ofuji S. Ultrastructural studies of allergic contact dermatitis in man. Arch Dermatol Res 1980;267:275–82.
- Russell Jones R. The histogenesis of eczema. Clin Exp Dermatol 1983;8:213–25.
- Russell Jones R, MacDonald DM. Eczema: immunopathogenesis and histogenesis. Am J Dermatopathol 1982;4:335–6.
- Thyssen JP, Carlsen BC, Menné T, et al. Filaggrin null mutations increase the risk and persistence of hand eczema in subjects with atopic dermatitis: results from a general population study. Br J Dermatol 2010;163:115–20.
- De Jongh CM, Khrenova L, Verberk MM, et al. Loss-of-function polymorphisms in the filaggrin gene are associated with an increased susceptibility to chronic irritant contact dermatitis: a case–control study. Br J Dermatol 2008;159:621–7.
- Novak N, Baurecht H, Schafer T, et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol 2008;128:1430–5.
- Calnan CD. Eczema for me. Trans St John's Hosp Dermatol Soc 1968;54:54–64.
- Young AW. Dynamics of autosensitisation dermatitis. A clinical and microscopic concept of autoeczematisation. Arch Dermatol 1958;77:495–9.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with auto-eczematisation. J Am Acad Dermatol 1986;13:1039–41.
- Kasteler JS, Peterson MJ, Vance JE, Zone JJ. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol 1992;128:795–8.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Auto-eczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol 1993;28:56–60.
- Fehr BS, Takashima A, Bergstresser PR, Cruz PD, Jr. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. A model for autoeczematization in humans? Am J Contact Dermatitis 2000;11:145–54.
- Cronin E. Contact dermatitis. VII: Reactions to contact allergens given orally and systemically. Br J Dermatol 1972;86:104–7.
- De Groot AC, ed. Patch Testing. Test concentrations and vehicles for 2800 allergens. Amsterdam: Elsevier, 1986.
- Wilkinson DS. The role of contact allergy in hand eczema. Trans St John's Hosp Dermatol Soc 1970;56:19–27.
- Bruynzeel DP, Maibach HI. Excited skin syndrome (angry back). Arch Dermatol 1986;122:323–8.
- Hjorth N, Roed-Peterson J. Occupational protein contact dermatitis in food handlers. Contact Dermatitis 1976;2:28–42.
- Reitamo S, Rustin M, Ruzicka T, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol 2002;109:547–55.
- Ashcroft DM, Chen LC, Garside R, et al. Topical pimecrolimus for eczema. Cochrane Database Syst Rev 2007;Issue 4:CD005500.
- Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2011;164:415–28.
- Reynolds NJ, Franklin V, Gray JC. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001;357:2012–16.
- Gambichler T, Othlinghaus N, Tomi NS, et al. Medium-dose ultraviolet (UV) A1 vs. narrowband UVB phototherapy in atopic eczema: a randomized crossover study. Br J Dermatol 2009;160:652–8.
- Schmitt J, Schäkel K, Fölster-Holst R, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol 2010;162:661–8.
- Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet 2006;367:839–46.
- Schram ME, Roekevisch E, Leeflang MM, et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol 2011;128:353–9.
- Neuber K, Schwartz I, Itschert G, Dieck AT. Treatment of atopic eczema with oral mycophenolate mofetil. Br J Dermatol 2000;143:385–91.
- Ruzicka T, Lynde CW, Jemec GB, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema (CHE) refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol 2008;158:808–17.
Nummular dermatitis
- Bendl BJ. Nummular eczema of stasis origin: a morphological pattern of diverse etiology. Int J Dermatol 1979;18:129–35.
- Shelley WB, ed. Consultations in Dermatology, Vol II. Philadelphia: WB Saunders, 1974;172–5.
- Johnson M-LT, Roberts J. Prevalence of Dermatological Disease Among Persons 1–74 Years of Age. No. PHS 79–1660. Washington, DC: US Department of Health Education, National Center for Health Statistics, 1978.
- Carr R, Berke M, Becker SW. Incidence of atopy in patients with various neurodermatoses. Arch Dermatol 1964;89:20–6.
- Hellgren L, Mobacken H. Nummular eczema. Clinical and statistical data. Acta Derm Venereol (Stockh) 1969;49:189–96.
- Kreuger GG. IgE levels in nummular eczema and ichthyosis. Arch Dermatol 1973;107:56–8.
- Ackerman AB, ed. Histologic Diagnosis of Inflammatory Skin Diseases. Philadelphia: Lea and Febiger, 1978:499–506.
- Elder D, ed. Lever's Histopathology of the Skin, 8th edn. Philadelphia: Lippincott, 1997:209–16.
- Leyden JJ, Kligman AM. The case for steroid–antibiotic combinations. Br J Dermatol 1977;96:179–87.
- Wachs GN, Maibach H. Co-operative double blind trial of an antibiotic–corticoid combination in impetiginized atopic dermatitis. Br J Dermatol 1976;95:323–8.
- Parish WE, Welbourn E, Champion RH. Hypersensitivity to bacteria in eczema. IV: Cytotoxic effect of antibacterial antibody on skin cells acquiring bacterial antigens. Br J Dermatol 1976;95:493–500.
- Fleming C, Parry E, Forsyth A, Kemmett D. Patch testing in discoid eczema. Contact Dermatitis 1997;36:261–4.
- Rollins TG. From xerosis to nummular dermatitis. JAMA 1968;206:637.
- Aoyama H, Tanaka M, Hara M, et al. Nummular eczema: an addition of senile xerosis and unique cutaneous reactivities to enviromental aeroallergens. Dermatology 1999;199:135–9.
- Higgins EM, DuVivier AW. Cutaneous disease and alcohol misuse. Br Med Bull 1994;50:85–98.
- Morrow DM, Rapaport MJ, Strick RA. Hypersensitivity to aloe. Arch Dermatol 1980;116:1064–5.
- Le Coz CJ. Contact nummular (discoid) eczema from depilating cream. Contact Dermatitis 2002;42:111–12.
- Adachi A, Horikawa T, Takashima T, Ichihashi M. Mercury-induced nummular dermatitis. J Am Acad Dermatol 2000;43:383–5.
- Church R. Eczema provoked by methyldopa. Br J Dermatol 1974;91:373–8.
- Wilkinson SM, Smith AG, Davis MJ, et al. Pityriasis rosea and discoid eczema: dose related reactions to treatment with gold. Ann Rheum Dis 1992;51:881–4.
- Calnan CD, Meara RH. Discoid eczema – dry type. Trans St John's Hosp Dermatol Soc 1956;37:26–8.
- Sulzberger MB, Garbe W. Nine cases of distinctive exudative discoid and lichenoid chronic dermatosis. Arch Dermatol Syphilol 1937;36:247–72.
- Sulzbeger MB. Distinctive exudative discoid and lichenoid chronic dermatosis (Sulzberger and Garbe) re-examined – 1978. Br J Dermatol 1979;100:13–20.
- Rongioletti F, Corbella L, Rebora A. Exudative discoid and lichenoid chronic dermatosis (Sulzberger–Garbe). A fictional disease? Int J Dermatol 1989;28:40–3.
- Cowan MA. Nummular eczema – a review, follow-up and analysis of 325 cases. Acta Derm Venereol (Stockh) 1961;41:453–60.
- Khurana S, Jain VK, Aggarwal K, Gupta S. Patch testing in discoid eczema. J Dermatol 2002;29:763–7.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis 2012;23:153–7.
- Roberts H, Orchard D. Methotrexate is a safe and effective treatment for paediatric discoid (nummular) eczema: a case series of 25 children. Australas J Dermatol 2010;51:128–30.
Asteatotic eczema
- Kimura N, Nakagami G, Takehara K, et al. Prevalence of asteatosis and asteatotic eczema among elderly residents in facilities covered by long-term care insurance. J Dermatol 2013;40:770–1.
- Warin AP. Eczéma craquelé as the presenting feature of myxoedema. Br J Dermatol 1973;89:289–91.
- Weismann K, Wadskov S, Mikkelson HI. Acquired zinc deficiency dermatosis in man. Arch Dermatol 1978;114:1509–11.
- Horri I, Nakayama Y, Obata M, et al. Stratum corneum hydration and amino-acid content in xerotic skin. Br J Dermatol 1989;121:587–92.
- Tezuka T. Electron microscopic changes in xerosis senilis epidermis. Dermatologica 1983;166:59–61.
- Onken HD, Moyer CA. The water barrier in human epidermis. Arch Dermatol 1963;87:584–90.
- Anonymous. Winter skin. Lancet 1990;335:226.
- Caplan RM. Superficial haemorrhagic fissures of the skin. Arch Dermatol 1970;101:442–51.
- Greist MC, Epinette WW. Cimetidine-induced xerosis and asteatotic dermatitis. Arch Dermatol 1982;118:253–4.
- Björnberg J. Erythema craquelé provoked by corticosteroids on normal skin. Acta Derm Venereol (Stockh) 1982;62:147–51.
- Barker DJ, Cotterill JA. Generalised eczéma craquelé as a presenting feature of lymphoma. Br J Dermatol 1977;97:323–6.
- Van Voorst Vader PC, Folkers E, van Rhenen DJ. Craquelé-like eruption in angioimmunoblastic lymphadenopathy. Arch Dermatol 1979;115:370.
- Greenwood R. Generalised eczéma craquelé as a presenting feature of adenocarcinoma. Br J Dermatol 1983;109:277–8.
- Ridley CM. Eczéma craquelé and systemic carcinoma. Br J Dermatol 1984;110:246.
- Schulz P, Bunselmeyer B, Bräutigam M, Luger TA. Pimecrolimus cream 1% is effective in asteatotic eczema: results of a randomized, double-blind, vehicle-controlled study in 40 patients. J Eur Acad Dermatol Venereol 2007;21:90–4.
Dermatitis and eczema of the hands
- Moberg C, Alderling M, Meding B. Hand eczema and quality of life: a population-based study. Br J Dermatol 2009;161:397–403.
- Smith HR, Armstrong DK, Wakelin SH, et al. Descriptive epidemiology of hand dermatitis at the St John's contact dermatitis clinic 1983–97. Br J Dermatol 2000;142:284–7.
- Agrup G. Hand eczema with other dermatoses in South Sweden. Acta Derm Venereol (Stockh) 1969;49(Suppl. 61):28–37.
- Vermeulen R, Kromhout H, Bruynzeel DP, de Boer EM. Ascertainment of hand dermatitis using a symptom-based questionnaire; applicability in an industrial population. Contact Dermatitis 2000;42:202–6.
- Meding B, Jarvholm B. Incidence of hand eczema – a population-based retrospective study. J Invest Dermatol 2004;122:873–7.
- Thyssen JP, Johansen JD, Linneberg A, Menne T. The epidemiology of hand eczema in the general population – prevalence and main findings. Contact Dermatitis 2010;62:75–87.
- Lammintausta T, Kalimo K, Havu VK. Occurrence of contact allergy and hand eczema in hospital ‘wet work’. Contact Dermatitis 1982;8:84–90.
- Uter W, Pfhalberg A, Gefeller O, Schwanitz HJ. Risk of hand dermatitis among hairdressers versus office workers. Scand J Work Environ Health 1999;25:450–6.
- Meding B, Liden C, Berglind N. Self-diagnosed dermatitis in adults. Results from a population survey in Stockholm. Contact Dermatitis 2001:45:341–5.
- Sinha A, Harrison PV. Latex glove allergy among hospital employees: a study in the north-west of England. Occup Med (Oxf) 1998;48:405–10.
- Cronin E. Clinical patterns of hand eczema in women. Contact Dermatitis 1985;13:153–61.
- Lee HJ, Ha SJ, Ahn WK, et al. Clinical evaluation of atopic hand dermatitis. Pediatr Dermatol 2001;18:102–6.
- Miller RM, Coger RW. Skin conductance conditioning with dyshidrotic eczema patients. Br J Dermatol 1979;101:435–40.
- Bryld LE, Agner T, Kyvik KO, et al. Hand eczema in twins: a questionnaire investigation. Br J Dermatol 2000;142:298–305.
- Forsbeck M, Skog E, Asbrink E. Atopic hand dermatitis. Acta Derm Venereol (Stockh) 1983;63:9–13.
- Thyssen JP, Carlsen BC, Menné T, et al. Filaggrin null mutations increase the risk and persistence of hand eczema in subjects with atopic dermatitis: results from a general population study. Br J Dermatol 2010;163:115–20.
- Thyssen JP, Ross-Hansen K, Johansen JD, et al. Filaggrin loss-of function mutation R501X and 2282del4 carrier status is associated with fissured skin on the hands: results from a cross-sectional population study. Br J Dermatol 2011;166:46–53.
- Landeck L, Visser M, Skudlik C, et al. Clinical course of occupational irritant contact eczema of the hands in relation to filaggrin genotype status and atopy. Br J Dermatol 2012;167:1302–9.
- De Jongh CM, Khrenova L, Verberk MM, et al. Loss-of-function polymorphisms in the filaggrin gene are associated with an increased susceptibility to chronic irritant contact dermatitis: a case–control study. Br J Dermatol 2008;159:621–7.
- Molin S, Vollmer S, Weiss EH, et al. Filaggrin mutations may confer susceptibility to chronic hand eczema characterized by combined allergic and irritant contact dermatitis. Br J Dermatol 2009;161:801–7.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol (Stockh) 1989;69:227–33.
- Duarte I, Terumi Nakano J, Lazzarini R. Hand eczema: evaluation of 250 patients. Am J Contact Dermatitis 1998;9:216–23.
- Hjorth N, Roed Petersen J. Occupational protein contact dermatitis in food handlers. Contact Dermatitis 1976;2:28–42.
- Hamann CP. Natural rubber latex protein sensitivity: a review. Am J Contact Dermatitis 1993;4:4–21.
- Christensen OB, Möller H. External and internal exposure to the antigen in the hand eczema of nickel allergy. Contact Dermatitis 1975;1:136–41.
- Fisher AA. Possible role of diet in pompholyx and nickel dermatitis – a critical survey. Cutis 1978;22:412–14.
- Uter W, Gefeller O, Schwanitz HJ. An epidemiological study of the influence of season (cold and dry air) on the occurrence of irritant skin changes of the hands. Br J Dermatol 1998;138:266–72.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–16.
- Agner T, Andersen KE, Brandao FM, et al. Hand eczema severity and quality of life: a cross-sectional, multicentre study of hand eczema patients. Contact Dermatitis 2008;59:43–7.
- Bleumink KE. Contact dermatitis to garlic. Arch Dermatol Forsch 1973;247:117–24.
- Hjorth N. Gut eczema in slaughterhouse workers. Contact Dermatitis 1978;4:49–52.
- Goransson K. Occupational contact urticaria to fresh cow and pig blood in slaughtermen. Contact Dermatitis 1982;7:281–2.
- Cordoba S, Sanchez-Perez J, Garcia-Diez A. Ring dermatitis as a clinical presentation of fragrance sensitization. Contact Dermatitis 2000;42:242.
- Gerwig T, Winer MN. Radioactive jewelry as a cause of cutaneous tumor. JAMA 1968;205:595–6.
- Coenraads PJ, Van Der Walle H, Thestrup-Pedersen K, et al. Construction and validation of a photographic guide for assessing severity of chronic hand dermatitis. Br J Dermatol 2005;152:296–301.
- Paulden M, Rodgers M, Griffin S, et al. Alitretinoin for the treatment of severe chronic hand eczema. Health Technol Assess 2010;14(Suppl. 1):39–46.
- National Institute for Health and Care Excellence. Alitretinoin for the treatment of severe chronic hand eczema. NICE technology appraisal guidance TA177. http://guidance.nice.org.uk/ta177 (last accessed June 2015).
- Schwanitz HJ, Uter W. Interdigital dermatitis: sentinel skin damage in hair dressers. Br J Dermatol 2000;142:1011–12.
- Meding B, Wrangsjo K, Jarvholm B. Fifteen-year follow-up of hand eczema: persistence and consequences. Br J Dermatol 2005;152:975–980.
- Mourisden HIT, Faber O. Penetration of protective gloves by allergens and irritants. Trans St John's Hosp Dermatol Soc 1973;57:230.
- Wigger-Alberti W, Maraffio B, Wernli M, Elsner P. Self-application of a protective cream. Pitfalls of occupational skin protection. Arch Dermatol 1997;133:861–4.
- Wigger-Alberti W, Elsner P. Do barrier creams and gloves prevent or provoke contact dermatitis. Am J Contact Dermatitis 1998;9:100–6.
- Bauer A, Schmitt J, Bennett C, et al. Interventions for preventing occupational irritant hand dermatitis. Cochrane Database Syst Rev 2010;Issue 6:CD004414.
- Veien NK, Olholm Larsen P, Thestrup-Pederson K, Schou G. Long term intermittent treatment of chronic hand eczema with mometasone furoate. Br J Dermatol 1999;140:882–6.
- Schnopp C, Remling R, Möhrenschlager M, et al. Topical tacrolimus (FK506) and mometasone furoate in treatment of dyshidrotic palmar eczema: a randomized, observer-blinded trial. J Am Acad Dermatol 2002;46:73–7.
- Epstein E. Hand dermatitis: practical management and current concepts. J Am Acad Dermatol 1984;10:395–424.
- Lindelof B, Wrangsjo K, Liden S. A double-blind study of Grenz ray therapy in chronic eczema of the hands. Br J Dermatol 1987;117:77–80.
- Odia S, Vocks E, Rakoski J, Ring J. Successful treatment of dyshidrotic hand eczema using tap water iontophoresis with pulsed direct current. Acta Derm Venereol 1996;76:472–4.
- Ruzicka T, Lynde CW, Jemec GB, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema (CHE) refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol 2008;158:808–17.
- Ingram JR, Batchelor JM, Williams HC. Alitretinoin: a potential advance in the management of severe chronic hand eczema. Arch Dermatol 2009;145:314–15.
- Thestrup-Pedersen K, Andersen KE, Menne T, Veien NK. Treatment of hyperkeratotic dermatitis of the palms (eczema keratoticum) with oral acitretin. A single-blind placebo-controlled study. Acta Derm Venereol 2001;81:353–5.
- Granlund H, Erkko P, Eriksson E, et al. Comparison of cyclosporine and topical betamethasone-17,21-dipropionate in the treatment of severe chronic hand eczema. Acta Derm Venereol 1996;76:371–6.
- Agarwal US, Besarwal RK. Topical clobetasol propionate 0.05% cream alone and in combination with azathioprine in patients with chronic hand eczema: an observer blinded randomized comparative trial. Indian J Dermatol Venereol Leprol 2013;79:101–3.
- Rosen K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands; a comparison of PUVA and UVB treatment. Acta Derm Venereol (Stockh) 1987;67:48–54.
- Behrens S, von Kobyletzki G, Gruss C, et al. PUVA-bath photochemotherapy (PUVA-soak therapy) of recalcitrant dermatoses of the palms and soles. Photodermatol Photoimmunol Photomed 1999;15:47–51.
Dermatitis and eczema of the lower legs
- Coon MM, Willis PW, Keller JB. Venous thromboembolism and other venous disease in the Tecumseh Community Health Study. Circulation 1973;48:839–46.
- Da Silva A, Widmer LK, Martin H, et al. Varicose veins and chronic venous insufficiency: prevalence and risk factors in 4376 subjects in the Basel Study II. Vasa 1974;3:118–25.
- Burton JL. Venous hypertension, fibrin and leg ulcers. Br J Dermatol 1983;109:229–31.
- Browse NL, Burnand KC. The cause of venous ulceration. Lancet 1982;ii:243–5.
- Coleridge Smith PD, Thomas P, Scurr JH, et al. Causes of venous ulceration: a new hypothesis. BMJ 1988;296:1726–7.
- Heng MC. The post-phlebitic syndrome. Int J Dermatol 1987;26:14–20.
- Fontaine R. Remarks concerning venous thrombosis and its sequelae. Surgery 1957;41:6–25.
- Piulachs P, Vidal-Barraquerr F. Pathogenic study of varicose veins. Angiology 1953;4:59–100.
- Hopkins NFG. Positron emission tomography in venous ulceration and liposclerosis: a study of regional tissue function. BMJ 1983;286:333–6.
- Thomas PRS, Nash GB, Dormandy JA. White cell accumulation in dependent legs of patients with venous hypertension: a possible mechanism for trophic changes in the skin. BMJ 1988;296:1693–5.
- Peschen M, Lahaye T, Henning B, et al. Expression of the adhesion molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the skin is modulated in progressing stages of chronic venous insufficiency. Acta Derm Venereol (Stockh) 1999;79:27–32.
- Bilen N, Apaydin R, Harova G, et al. Stasis dermatitis of the hand associated with an iatrogenic arteriovenous fistula. Clin Exp Dermatol 1998;23:208–10.
- Boyle J, Burton JL. Pseudo-Kaposi sarcoma. Lancet 1986;ii:921.
- Bahmer FZ. Immunohistologische Charakterisierung stauungsdermatotisch veränderter Unterschenkelhaut. Z Hautkr 1987;62:1056–63.
- Fagrell B, Steirnstedt G, Ostergen J. Acrodermatitis chronica atrophicans (Herxheimer) can often mimic a peripheral vascular disorder. Acta Med Scand 1986;220:485–8.
- Dissemond J, Knab J, Lehnen M, et al. Successful treatment of stasis dermatitis with topical tacrolimus. Vasa 2004;33:260–2.
Dermatitis and eczema of specified sites
Dermatitis and eczema of the eyelids
- Guin JD. Eyelid dermatitis: a report of 215 patients. Contact Dermatitis 2004;50:87–90.
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis 2006;55:280–5.
- Guin JD. Clinical presentations of patients sensitive to natural rubber latex. Dermatitis 2004;15:192–6.
- Dewachter P, Mouton-Faivre C. Anaesthetists should be aware of delayed hypersensitivity to phenylephrine. Acta Anaesthesiol Scand 2007;51:637–9.
Juvenile plantar dermatosis
- Ashton RE, Griffiths WA. Juvenile plantar dermatosis – atopy or footwear? Clin Exp Dermatol 1986;11:529–34.
- Mackie RM. Juvenile plantar dermatosis. Semin Dermatol 1982;1:67–75.
- Neering H, Van Dijk E. Juvenile plantar dermatosis. Acta Derm Venereol (Stockh) 1978;58:531.
- Stankler L. Juvenile plantar dermatosis in identical twins. Br J Dermatol 1978;99:585–6.
- Shrank AB. Aetiology of juvenile plantar dermatosis. Br J Dermatol 1979;100:641–6.
- Young E. Forefoot eczema – further studies and a review. Clin Exp Dermatol 1986;11:523–8.
- Schultz H, Zachariae H. The Trafuril test in recurrent juvenile eczema of hands and feet. Acta Derm Venereol (Stockh) 1972;52:398–400.
- Graham RM, Verbov JL, Vickers CFH. Juvenile plantar dermatosis. Clin Exp Dermatol 1987;12:468.
- Lim KB, Tan T, Rajan VS. Dermatitis palmaris sicca – a distinctive pattern of hand eczema. Clin Exp Dermatol 1986;11:553–9.
- Jones SK, English JSC, Forsyth A, et al. Juvenile plantar dermatosis – an eight year follow-up of 102 patients. Clin Exp Dermatol 1987;12:5–7.
- Kint A. Dermatitis plantaris sicca. Dermatologica 1982;165:500–9.
- Millard LG, Gould DG. Juvenile plantar dermatosis. Clin Exp Dermatol 1977;2:186–7.
- Möller H. Atopic winter feet in children. Acta Derm Venereol (Stockh) 1972;52:401–5.
- Shipley DR, Kennedy CT. Juvenile plantar dermatosis responding to topical tacrolimus ointment. Clin Exp Dermatol 2006;31:453–4.
Miscellaneous specified eczematous dermatoses
Infective dermatitis
- Nilsson E, Henning C, Hjoreitsson M-L. Density of the microflora in hand eczema before and after topical treatment with a potent corticosteroid. J Am Acad Dermatol 1986;15:192–7.
- Goodfield M. C-reactive protein levels in venous ulceration: an indication of infection? J Am Acad Dermatol 1988;18:1048–52.
- Parish WE, Welbourn E, Champion RH. Hypersensitivity to bacteria in eczema. Titre and immunoglobulin class of antibodies to staphylococci and micrococci. Br J Dermatol 1976;95:285–93.
- Parish WE, Welbourn E, Champion RH. Hypersensitivity to bacteria in eczema. IV: cytotoxic effect of antibacterial antibody on skin cells acquiring bacterial antigens. Br J Dermatol 1976;95:493–506.
- Welbourn E, Champion RH, Parish WE. Hypersensitivity to bacteria in generalized eczema. I: bacterial culture, skin tests, and immunofluorescent detection of immunoglobulins and bacterial antigens. Br J Dermatol 1976;94:619–25.
- Welbourn E, Champion RH, Parish WE. Hypersensitivity to bacteria in eczema. III: Arthus-like responses in bacterial antigens in the absence of specific antibody. Br J Dermatol 1976;95:379–87.
- Skov L, Olsen JV, Giornd R, et al. Application of staphylococcal enterotoxin B on normal and atopic skin induces upregulation of T cells by a superantigen-mediated mechanism. J Allergy Clin Immunol 2000;105:820–6.
- Rockl H. Mikrobiele Genese von Ekzemen. Hautarzt 1964;15:398–9.
- Leyden JJ, Kligman AM. Interdigital athlete's foot. The interaction of dermatophytes and resident bacteria. Arch Dermatol 1978;114:1466–9.
- Weismann K, Hjorth N. Microbial eczema of the feet. Br J Dermatol 1982;107:330–7.
Infective dermatitis of children associated with human T-cell leukaemia virus 1 infection
- Sweet RD. A pattern of eczema in Jamaica. Br J Dermatol 1966;78:93–100.
- Walshe M. Infective dermatitis in Jamaican children. Br J Dermatol 1967;79:229–36.
- LaGrenade L, Hanchard B, Fletcher V, et al. Infective dermatitis of Jamaican children: a marker for HTLV-1 infection. Lancet 1990;336:1345–7.
- Hanchard B, LaGrenade L, Carberry C, et al. Childhood infective dermatitis evolving into adult T-cell leukaemia after 17 years. Lancet 1991;338:1593–4.
- LaGrenade L. HTLV-1, infective dermatitis, and tropical spastic paraparesis. Mol Neurobiol 1994;8:147–53.
- LaGrenade L, Manns A, Fletcher V, et al. Clinical pathologic and immunologic features of human T-lymphotrophic virus type-1 associated infective dermatitis in children. Arch Dermatol 1998;134:439–44.
Post-traumatic eczema
- Mathias CG. Post-traumatic eczema. Dermatol Clin 1988;6:35–42.
- Carr RD, Rau RC. Dermatitis at vein graft site in coronary artery bypass patients. Arch Dermatol 1981;117:814–15.
- Bart RS. Dermatitis at vein graft site. Arch Dermatol 1983;119:97.
- Hruza LL, Hruza GJ. Saphenous vein graft donor site dermatitis: case reports and literature review. Arch Dermatol 1993;129:609–12.
Pityriasis alba
- O'Farrell N. Pityriasis alba. Arch Dermatol 1956;73:376–7.
- Wells BT, Whyte BT, Kierland R. Pityriasis alba. A ten-year survey and review of the literature. Arch Dermatol 1960;82:183–9.
- Zaynoun ST, Aftimos BG, Tenekjian KK. Extensive pityriasis alba: a histological, histochemical and ultrastructural study. Br J Dermatol 1983;108:83–90.
- Vargos-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol 1993;32:870–3.
- In S-I, Yi SW, Kang HY, et al. Clinical and histopathologic characteristics of pityriasis alba. Clin Exp Dermatol 2009;34:591–7.
- Adamson HG. On a form of chronic superficial dermatitis in circumscribed patches with symmetrical distribution occurring in children. Br J Dermatol 1908;120:109–22.
- Bassaly M, Miale A, Prasad AS. Studies on pityriasis alba. Arch Dermatol 1963;88:272–3.
- Kim SK, Kang HY, Lee ES, et al. Clinical and histopathologic characteristics of nevus depigmentosus. J Am Acad Dermatol 2006;55:423–8.
- Whitmore SE, Simmons-O'Brien E, Rotter FS. Hypopigmented mycosis fungoides. Arch Dermatol 1994;130:476–80.
- Fujita WH, McCormick CL, Parneix-Spake A. An exploratory study to evaluate the efficacy of pimecrolimus cream 1% for the treatment of pityriasis alba. Int J Dermatol 2007;46:700–5.
Chronic superficial scaly dermatitis
- Hu CH, Winklemann RK. Digitate dermatosis. A new look at symmetrical small plaque parapsoriasis. Arch Dermatol 1973;107:65–9.
- Lambert WE, Everett MA. The nosology of parapsoriasis. J Am Acad Dermatol 1981;5:731–45.
- Samman PD. The natural history of parapsoriasis en plaques (chronic superficial dermatitis) and prereticulotic poikiloderma. Br J Dermatol 1972;87:405–11.
- Calnan CD, Meara RH. Chronic superficial scaly dermatitis. Trans St John's Hosp Dermatol Soc 1956;37:12–13.
- Haeffner AC, Smoller BR, Zepter K, Wood GS. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol 1995;131:321–4.
- Burg G, Dummer R. Small plaque (digitate) parapsoriasis is an ‘abortive cutaneous T-cell lymphoma’ and is not mycosis fungoides. Arch Dermatol 1995;131:336–8.
- Ackerman AB. If small plaque (digitate) parapsoriasis is a cutaneous T-cell lymphoma, even an ‘abortive’ one, it must be mycosis fungoides. Arch Dermatol 1996;132:562–6.
- Hofer A, Cerroni L, Kerl H, Wolf P. Narrowband (311 nm) UV-B therapy for small plaque parapsoriasis and early-stage mycosis fungoides. Arch Dermatol 1999;135:1377–80.
Dermatophytide
- Jillson OF. Dermatophytids and candidids. Semin Dermatol 1983;2:60.
- Kaaman T, Torssander J. Dermatophytide – a misdiagnosed entity. Acta Derm Venereol (Stockh) 1983;63:404–8.
- Peck SM. Fungus antigens and their importance as sensitizers in the general population. Ann NY Acad Sci 1950;50:1362–75.
- Veien NK, Hattel T, Laurberg G. Plantar Trichophyton rubrum infections may cause dermatophytids on the hands. Acta Derm Venereol (Stockh) 1994;74:403–4.
Halo dermatitis
- Meyerson LB. A peculiar papulosquamous eruption involving pigmented naevi. Arch Dermatol 1971;103:510–12.
- Brenan J, Kossard S, Krivanek J. Halo eczema around melanocytic naevi. Int J Dermatol 1985;24:226–9.
- Nicholls DSH, Mason GH. Halo dermatitis around a melanocytic naevus: Meyerson's naevus. Br J Dermatol 1988;118:125–9.
- Weedon D, Farnsworth J. Spongiotic changes in melanocytic naevi. Am J Dermatopathol 1984;6:257–9.
- Krischer J, Pechere M, Salomon D, et al. Interferon alfa-2b-induced Meyerson's nevi in a patient with dysplastic nevus syndrome. J Am Acad Dermatol 1999;40:105–6.
- Ramon R, Silvestre JF, Betlloch I, et al. Progression of Meyerson's naevus to Sutton's naevus. Dermatology 2000;200:337–8.
- Rosen R, Paver K, Kossard S. Halo eczema surrounding seborrheic keratosis: an example of perilesional dermatitis. Australas J Dermatol 1990;31:73–6.
- Gallais V, Lacour JP, Perrin C, et al. Halo eczema around a histiocytofibroma: the Meyerson phenomenon. Ann Dermatol Vénéréol 1993;120:617–20.
Murray Williams' warts
- Williams MG. Acanthomata appearing after eczema. Br J Dermatol 1956;68:268–71.
- Horiuchi Y. Multiple seborrhoeic verrucae following eczema. J Dermatol 1989;16:505–7.
Lichen simplex and lichenification
- Kinsella LJ, Carney-Godley K, Feldman E. Lichen simplex chronicus as the initial manifestation of intramedullary neoplasm and syringomyelia. Neurosurgery 1992;30:418–21.
- Robertson IM, Jordan JM, Whitlock FA. Emotions and skin (III) – the conditioning of scratch responses in cases of lichen simplex. Br J Dermatol 1975;92:407–12.
- Singh G. Atopy in lichen simplex (neurodermatitis circumscripta). Br J Dermatol 1973;88:625–7.
- Cormia FE. Basic concepts in the production and management of the psychosomatic dermatoses – II. Br J Dermatol 1951;63:129–51.
- Cowan MA. Neurohistological changes in lichen simplex chronicus. Arch Dermatol 1964;89:562–8.
- Shaffer B, Beerman H. Lichen simplex chronica and its variants. A discussion of certain psychodynamic mechanisms and clinical and histopathologic correlation. Arch Dermatol Syphilol 1951;64:340–51.
- Marks R, Wells GC. Lichen simplex: morphodynamic correlates. Br J Dermatol 1973;88:249–56.
- Marks R, Wells GC. A histochemical profile of lichen simplex. Br J Dermatol 1973;88:557–62.
- Chey WY, Kim KL, Yoo TY, Lee AY. Allergic contact dermatitis from hair dye and development of lichen simplex chronicus. Contact Dermatitis 2004;51:5–8.
- Berlin C. Lichenificatio gigantea. (Lichenification géante of Brocq and Pautrier). Arch Dermatol Syphilol 1939;39:1012–20.
- Konuk N, Koca R, Atik L, et al. Psychopathology, depression and dissociative experiences in patients with lichen simplex chronicus. Gen Hosp Psychiatry 2007;29:232–5.
- Drake LA, Millikan LE. The antipruritic effect of 5% doxepin cream in patients with eczematous dermatitis. Arch Dermatol 1995;131:1403–8.
- Tupker RA, Coenraads PJ, van der Meer JB. Treatment of prurigo nodularis, chronic prurigo and neurodermatitis circumscripta with topical capsaicin. Acta Derm Venereol (Stockh) 1992;72:463.
Erythroderma
- Sigurdsson V, Steegmans PHA, van Vloten WA. The incidence of erythroderma: a survey among all dermatologists in the Netherlands. J Am Acad Dermatol 2001;45:675–8.
- Akhyani M, Ghodsi ZS, Toosi S, Dabbaghian H. Erythroderma: a clinical study of 97 cases. BMC Dermatol 2005;5:5.
- Abrahams I, McCarthy JT, Sanders SL. 101 cases of exfoliative dermatitis. Arch Dermatol 1963;87:96–101.
- Botella-Estrada R, Sanmartin O, Oliver V, et al. Erythroderma: a clinico-pathological study of 56 cases. Arch Dermatol 1994;130:1503–7.
- Hasan T, Jansen CT. Erythroderma: a follow-up of 50 cases. J Am Acad Dermatol 1983;8:836–40.
- Rosen T, Chappell R, Drucker C. Exfoliative dermatitis: presenting sign of internal malignancy. South Med J 1979;72:652–3.
- Nicolis GD, Helwig WB. Exfoliative dermatitis. A clinicopathological study of 135 cases. Arch Dermatol 1973;108:788–97.
- Wong KS, Wong SN, Tham SN, et al. Generalised exfoliative dermatitis: a clinical study of 108 patients. Ann Acad Med Singapore 1988;17:520–3.
- Holme SA, Roberts DL. Erythroderma associated with St John's wort. Br J Dermatol 2000;143:1127–8.
- Morrison JG. Sarcoidosis in a child presenting as erythroderma with keratotic spines. Br J Dermatol 1976;95:93–7.
- Marsch WC, Stuttgen G. Generalized Hailey–Hailey disease. Br J Dermatol 1978;99:553.
- Tappeiner G, Konrad K, Holubar K. Erythrodermic bullous pemphigoid. J Am Acad Dermatol 1982;6:489–92.
- Bach M. Dermatological signs in the toxic shock syndrome. J Am Acad Dermatol 1983;8:343–7.
- De Spain J, Clark DP. Subacute cutaneous lupus erythematosus presenting as erythroderma. J Am Acad Dermatol 1988;19:388–92.
- Bernengo MG, Levi L, Zina G. Skin lesions in angio-immunoblastic lymphadenopathy. Br J Dermatol 1981;104:131–9.
- Pierson JC, Taylor JS. Erythrodermic dermatomyositis. J Am Acad Dermatol 1993;28:136.
- Sakakibara T, Ida T, Mannouji E, et al. Post-transfusion graft-versus-host disease following open heart surgery: report of six cases. J Cardiovasc Surg 1989;30:687–91.
- Janniger CK, Gascon P, Schwartz RA, et al. Erythroderma as the initial presentation of the acquired immunodeficiency syndrome. Dermatologica 1991;183:143–5.
- Sadick NS, McNutt NS, Kaplan MH. Papulosquamous dermatoses of AIDS. J Am Acad Dermatol 1990;22:1270–7.
- Griffiths TW, Stevens SR, Cooper KD. Acute erythroderma as an exclusion criterion for idiopathic CD4+ T lymphocytopenia. Arch Dermatol 1994;130:1530–3.
- Walsh NMG, Prokopetz R, Tron VA, et al. Histopathology in erythroderma: review of a series of cases by multiple observers. J Cutan Pathol 1994;21:419–23.
- Sentis HJ, Willemze R, Scheffer E. Histopathologic studies of Sézary syndrome and erythrodermic mycosis fungoides: a comparison with benign forms of erythroderma. J Am Acad Dermatol 1986;15:1217–26.
- Abel EA, Lindae ML, Hoppe TR, et al. Benign and malignant forms of cutaneous erythroderma: cutaneous immunophenotypic characteristics. J Am Acad Dermatol 1988;19:1089–95.
- Asai T, Horiuchi Y. Senile erythroderma with serum hyper-IgE. Int J Dermatol 1989;28:225–8.
- Boyd AS, Menter A. Erythrodermic psoriasis. J Am Acad Dermatol 1989;21:985–91.
- Cherny S, Mraz S, Su L, et al. Heteroduplex analysis of T-cell receptor gamma gene rearrangement as an adjuvant diagnostic tool in skin biopsies for erythroderma. J Cutan Pathol 2001;28:351–5.
- Cordel N, Lenormand B, Courville P, et al. Detection of clonal T-cell receptor gamma gene rearrangement with the use of PCR-DGGE for diagnosis of erythroderma. Ann Dermatol Vénéréol 2001;128:220–3.
- Russell-Jones R, Whittaker S. T-cell receptor gene analysis in the diagnosis of Sézary syndrome. J Am Acad Dermatol 1999;41:254–9.
- Wilson DC, Jester JD, King LE. Erythroderma and exfoliative dermatitis. Clin Dermatol 1993;11:67–72.
- King LE. Erythroderma. Who, where, when, why and how? Arch Dermatol 1994;130:1545–7.
- King LE, Dufresne RG, Lovett G, Rosin MA. Erythroderma: review of 82 cases. South Med J 1986;79:1210–15.
- Thestrup-Pederson K, Halkier-Sorenson L, Sogaard H, Zacharie H. The red man syndrome: exfoliative dermatitis of unknown aetiology. A description and follow-up of 38 patients. J Am Acad Dermatol 1988;18:1307–12.
- Ofuji S, Furukawa F, Miyachi Y, et al. Papuloerythroderma. Dermatologica 1984;16:125–30.
- Bech-Thomsen N, Thomsen K. Ofuji's papuloerythroderma: a study of 17 cases. Clin Exp Dermatol 1998;23:79–83.
- Farthing CF, Staughton RCD, Harper JI, Rowland Payne CME, Copeman PWM. Papuloerythroderma – a further case with the ‘deck chair sign’. Dermatologica 1986;172:65–6.
- Grobb JJ, Collet-Villette AM, Horchowski N, et al. Ofuji papuloerythroderma. Report of a case with T-cell skin lymphoma and discussion of the nature of this disease. J Am Acad Dermatol 1989;20:927–31.
- Ofuji S. Papuloerythroderma. J Am Acad Dermatol 1990;22:697.
- Schepers C, Malvehy J, Azon-Masoliver A, et al. Papuloerythroderma of Ofuji: a report of two cases including the first European case associated with visceral carcinoma. Dermatology 1996;193:131–5.
- Nishijima S. Papuloerythroderma associated with hepatocellular carcinoma. Br J Dermatol 1998;139:1115–16.
- Dwer CM, Chapman RS, Smith GD. Papuloerythroderma and cutaneous T-cell lymphoma. Dermatology 1994;188:326–8.
- Shah M, Reid WA, Layton AM. Cutaneous T-cell lymphoma presenting as papuloerythroderma – a case and review of the literature. Clin Exp Dermatol 1995;20:161–3.
- Tan YK, Tan KC, Ong BH. Papuloerythroderma of Ofuji and cutaneous T-cell lymphoma. Br J Dermatol 1997;137:160–1.
- Garcia-Patos V, Repiso T, Rodriguez-Cano L, Castells A. Ofuji papuloerythroderma in a patient with the acquired immunodeficiency syndrome. Dermatology 1996;192:164–6.
- Lonnee ER, Toonstra J, van der Putte SCJ, et al. Papuloerythroderma of Ofuji in a HIV-infected patient. Br J Dermatol 1996;135:489–504.
- Azon-Masoliver A, Casado J, Brunet J, et al. Ofuji's papuloerythroderma following choledocholithiasis with secondary sepsis: complete resolution with surgery. Clin Exp Dermatol 1998:23:84–6.
- Quemeneur T, Ghislain PD, Morant C, et al. Ofuji's papuloerythroderma: two cases treated with azathioprine. Ann Dermatol Vénéréol 2002;129:213–15.
- Sommer S, Henderson CA. Papuloerythroderma of Ofuji responding to treatment with cyclosporin. Clin Exp Dermatol 2000;25:293–5.
- Fujii K, Kanno Y, Ohgo N. Etretinate therapy for papuloerythroderma. Eur J Dermatol 1999;9:610–13.
- Fox RH. Temperature regulation in erythroderma. J R Coll Physicians Lond 1967;1:372–9.
- Leading Article. Haemodynamics of extensive skin disease. Lancet 1983;i:1144.
- Krook G. Hypothermia in patients with exfoliative dermatitis. Acta Derm Venereol (Stockh) 1960;40:142.
- Freedberg IM, Baden HP. The metabolic response to exfoliation. J Invest Dermatol 1962;38:277–84.
- Zip C, Murray S, Walsh NMG. The specificity of histopathology in erythroderma. J Cutan Pathol 1993;20:393–8.