CHAPTER 92
Hidradenitis Suppurativa
Nemesha Desai1, Hessel H. van der Zee2 and Gregor B. E. Jemec3
1St John's Institute of Dermatology, Guy's and St Thomas' NHS Foundation Trust, London, UK
2Department of Dermatology, Erasmus Medical Center, Rotterdam, the Netherlands
3Department of Dermatology, Roskilde Hospital; Health Sciences Faculty, University of Copenhagen, Copenhagen, Denmark
Definition and nomenclature
Hidradenitis suppurativa is a chronic, inflammatory, recurrent, debilitating, follicular disease that usually presents after puberty. There are painful, deep-seated inflamed lesions in the apocrine gland-bearing areas of the body, most commonly the axillary, inguinal and ano-genital regions. This definition is based on the San Francisco modification of the Dessau criteria [1].
The following three criteria must be met for the diagnosis to be made:
- Typical lesions, that is deep-seated, painful nodules: ‘blind boils’ in early lesions; abscesses, draining sinuses, bridged scars and paired or multiheaded open pseudocomedones in secondary lesions.
- Typical topography: axillae, groin, perineal and perianal region, buttocks and infra- and intermammary folds.
- Chronicity and recurrence of lesions.
Introduction and general description
Velpeau was the first to describe the condition of recurrent, painful, inflammatory abscesses of the axillae and groin in 1839 [2]. Verneuil later coined the term hidradenitis suppurativa (HS), a misnomer derived from the historical hypothesis that the disorder related to inflammation of the sweat glands [3]. Current evidence demonstrates that HS is a primary disorder of the hair follicle.
HS is a chronic, inflammatory, follicular disorder characterized by recurrent painful nodules, abscesses, draining sinus tracts and scarring. It localizes to areas of apocrine gland-bearing skin, predominantly the axillae, groin and ano-genital sites. Severity is defined by Hurley stage, which is often used to direct therapy [4]. Management is challenging and requires a combined medical and surgical approach. Complications of severe disease include contractures, anaemia, lymphoedema and squamous cell carcinoma. The psychosocial impact is significant [5]. The impact on quality of life is higher on average than for other inflammatory dermatoses [6].
Epidemiology
Incidence and prevalence
An annual incidence of six per 100 000 person-years is reported in a US insurance-based study; the incidence was highest amongst young women aged 20–29 years (18.4 per 100 000). The incidence may be rising [7].
The estimated prevalence in large European population studies is 1–2% [8]. Reported rates vary from 0.00033% to 4%, relating to differences in study population, diagnostic criteria and use of self-reporting [7, 8, 9, 10, 11]. A prevalence of 4% was observed in young adults attending a sexually transmitted disease clinic [9].
Age
The average age of sufferers is 24.2 years (± 12 years) [12]. HS typically presents after puberty; the average age of onset is between the second and third decade, with a sharp decline after the fifth decade. Onset after menopause is uncommon. There are rare cases of prepubertal onset associated with premature adrenarche.
Sex
Women are more frequently affected than men, in a ratio of 2.7 : 1 [12]. Topographical distribution of lesions can vary between sexes. For example, perianal and gluteal disease more commonly affects males; females are more likely to have genito-femoral and submammary lesions [13].
Ethnicity
There are no formal studies of ethnic variation.
Associated diseases
Diseases associated with HS include disorders of the follicular occlusion tetrad, systemic inflammatory disorders and genodermatoses [14].
Follicular occlusion tetrad. Acne conglobata (see chapter 90), dissecting cellulitis of the scalp (see Chapter 107) and pilonidal sinus (see Chapter 113) can coexist with HS; together they comprise the ‘follicular occlusion tetrad’. Typical acne vulgaris occurs at a rate equivalent to the general population, although severe acne at atypical sites is more common in male patients with HS [12].
Inflammatory disorders. One inflammatory disorder associated with HS is Crohn disease (see Chapter 97), which shares epidemiological, histological and therapeutic features with HS. Seventeen per cent of patients with Crohn disease were considered likely to have had coexistent HS in one study [15]. An inflammatory spondyloarthropathy of axial and appendicular joints, which isHLA-B27- and rheumatoid factor-negative, is well described with HS. A temporal relationship is seen between flares of joint and skin disease [14]. Other associated inflammatory disorders include pyoderma gangrenosum and the syndromes SAPHO (synovitis, acne, pustulosis, hyperostosis and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum and acne), PASH (pyoderma gangrenosum, acne conglobata and suppurative hidradenitis) and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne and suppurative hidradenitis) (see Chapters 45, 49 and 90) [14]. Furthermore, peripheral ulcerative keratitis (Mooren-type corneal ulceration) has been described in patients with HS [16].
Genodermatoses. Genodermatoses associated with HS or HS-like lesions include keratosis ichthyosis deafness syndrome, pachyonychia congenita (see Chapter 69), steatocystoma multiplex (see Chapter 134) and Dowling–Degos disease (see Chapter 70) [14].
Pathophysiology
Early studies implicated apocrine gland occlusion as a primary pathogenic event. However, histopathological observations have since demonstrated that follicular involvement is central to pathogenesis. The following sequence of events has been suggested: infundibular hyperkeratosis, follicular dilatation/cyst formation, follicular rupture with subsequent inflammation, and fistula formation by epidermal strands [17].
Predisposing factors
Obesity and smoking
Obesity and smoking are the two main factors associated with HS. Obesity is implicated as a risk factor and correlates with disease severity: 69–77% of patients are either overweight or obese [18]. An increase of 1 kg/m2 body mass index was associated with a mean increase of 0.84 Sartorius score units in one study [19]. In addition to cases describing the beneficial effect on HS of weight loss, bariatric surgery and weight loss reduces self-reported HS in the morbidly obese [20]. Increased pro-inflammatory cytokine release from visceral fat, physical occlusion and epidermal barrier stress at intertriginous skin sites may all play a role [17]. There is an increased prevalence of smoking amongst patients with HS compared with controls (89% versus 46% in one study) [21]; 63–93.7% of patients are current or ex-smokers [18]. A temporal or dose effect of smoking is not consistently demonstrated. It is not known if smoking cessation improves the course of disease.
Hormonal influences
Hormonal influences are documented and supported by a female preponderance, observed perimenstrual disease flares and improvement during pregnancy [13]. Clinical signs of virilization are, however, usually absent, circulating androgen levels are typically normal and no differences in androgen metabolism have been observed in large case series [22].
Host defence
Alterations of the innate immune system are thought to underlie disease pathogenesis. No major immune abnormalities have been found. In one study no abnormalities in granulocyte function or serum immunoglobulin levels could be detected [23]. However, some aberrant immune functions have been reported in small studies. These include an enhanced production of free oxygen radicals by stimulated neutrophil granulocytes; an impaired secretion of tumour necrosis factor a (TNF-α) and interleukin 6 (IL-6) upon stimulation of monocytes by bacterial compounds; and a diminished percentage of natural killer cells in the blood [24].
In addition, several inflammatory and anti-inflammatory cytokines levels are elevated in HS lesions. Highly up-regulated cytokines include IL-1β, TNF-α and IL-10. On the other hand, IL-2, IL-4, IL-5 and interferon γ (INF-γ) are hardly detectable in HS lesions [25, 26]. The IL-23/Th17 pathway seems to be activated, reflected by enhanced expression of IL-17A, IL-12 and IL-23 in HS skin [27].
There are also changes in the expression of innate immune system peptides. Significantly decreased expression of Toll-like receptors and a relative deficiency of a range of antimicrobial peptides have been reported in HS lesions [28, 29, 30, 31, 32, 33]. In addition, the mRNA expression levels of regulators and inducers of antimicrobial peptides (AMPs) such as IL-22 and IL-20 have been found to be reduced compared with psoriasis and atopic eczema [30]. Further details of all these changes are given in Table 92.1.
Table 92.1 Changes in protein levels of cytokines, Toll-like receptors and antimicrobial peptides and/or their mRNA in hidradenitis suppurativa.
| Molecule | Change compared with healty skin | Change compared with psoriasis | Protein or mRNA | Reference |
| Cytokines | ||||
| IL-1β | Increased | Increased | Protein and mRNA | 25,26,30,32 |
| TNF-α | Increased | Increased | Protein | 25,26,29 |
| IL-6 | Decreased/increased | Protein and mRNA | 29,32 | |
| IL-8 | Increased | mRNA | 32 | |
| IL-10 | Increased | Increased | Protein and mRNA | 25,26,29,32 |
| TGF-β | Decreased | Protein | 29 | |
| CXCL9 | Increased | Protein | 26 | |
| Monokine induced by IFN-γ (MIG) | Increased | Protein | 26 | |
| IL-11 | Increased | Protein | 26 | |
| B-lymphocyte chemoattractant (BLC) | Increased | Protein | 26 | |
| IL-17A | Increased | Protein and mRNA | 26,27 | |
| IL-12 | Increased | Protein and mRNA | 27 | |
| IL-23 | Increased | Protein and mRNA | 27 | |
| IL-20 | Increased | Decreased | Protein and mRNA | 30 |
| IL-22 | Increased | Decreased | Protein and mRNA | 30 |
| IL-24 | Increased | Increased | mRNA | 30 |
| IL-26 | Increased | mRNA | 30 | |
| ICAM-1 | Decreased | Protein | 29 | |
| α-MSH | Decreased | Protein | 29 | |
| IGF-1 | Decreased | Protein | 29 | |
| IFN-γ | Normal/Increased | mRNA | 25,30 | |
| Matrix metalloproteinase 1 (MMP-1) | Increased | mRNA | 32 | |
| Toll-like receptors (TLRs) | ||||
| TLR-2 | Increased/decreased | Protein and mRNA | 28,29 | |
| TLR-3 | Decreased | Protein | 29 | |
| TLR-4 | Decreased | Protein | 29 | |
| TLR-7 | Decreased | Protein | 29 | |
| TLR-9 | Decreased | Protein | 29 | |
| Antimicrobial peptides (AMPs) | ||||
| Human β-defensin-1 (HBD1) | Decreased | 30 | ||
| Human β-defensin-2 (HBD2) | Increased | Decreased | Protein and mRNA | 29,30,31,32 |
| Human β-defensin-3 (HBD3) | Increased | Decreased | Protein and mRNA | 30,33 |
| Ribonuclease 7 | Decreased | 33 | ||
| S100A7 (psoriasin) | Increased/no change | Decreased | Protein and mRNA | 30,31,33 |
| S100A8 | Increased | Decreased | mRNA | 30 |
| S100A9 | Increased | Decreased | mRNA | 30 |
| Cathelicidin (LL-37) | Increased | mRNA | 32 |
CXCL, chemokine(C-X-C motif); ICAM, intercellular adhesion molecule; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; MSH, melanocyte-stimulating hormone; TNF, tumour necrosis factor.
Medications
An exacerbation or onset of disease has been reported following lithium and sirolimus therapy [34, 35].
Pathology
Histopathological changes vary with disease stage. Early changes, which precede clinically evident lesions, are characterized by a sparse lymphocytic infiltrate of the terminal follicular unit and sebaceous gland atrophy [36, 37]. Follicular hyperplasia, perifollicular lymphocytic inflammatory infiltration, interfollicular psoriasiform hyperplasia and dilatation of the follicular lumen follow in developed lesions [36]. Cysts lined by stratified squamous epithelium containing lammellated keratin and free hair shafts appear [36]. During flares, abscess formation and ruptured follicular units are seen, associated with a dense, dermal, mixed, inflammatory infiltrate including histiocytes and giant cells that extends to interfollicular apocrine and eccrine structures and deep into the subcutis. Sinus tract formation and fibrosis follows [36].
Histopathological variations less frequently seen include isolated inflammation of the apocrine gland (apocrinitis in 5%), sebaceous gland necrosis, epithelioid granulomas and B-cell pseudofollicles [36].
Causative organisms
The role of bacteria remains to be clarified. HS is currently regarded as a primary inflammatory disorder without a defined infectious trigger. Microbiology from superficial and deep sampling often demonstrates negative culture or only normal skin flora with multiple non-pathogenic bacterial species in the majority of cultures. The most common bacterial isolates are Staphylococcus epidermidis and S. aureus, followed by Peptostreptococcus species and Propionibacterium acnes [38, 39, 40]. Streptococcal antibodies are usually not found.
Genetics
Approximately one-third of patients have a family member with HS [12]. HS does not appear to be linked to specific human leukocyte antigen (HLA) types [41]. In some cases an autosomal pattern of inheritance can be found [42]. Loss-of-function mutations in the γ-secretase genes Nicastrin, Presenilin-1 and Presenilin enhancer-2 are probably responsible for a small number of familial cases. Gamma-secretase regulates notch signalling, which plays a role in epidermal and terminal hair follicle differentiation, immune cell development and immune functions. Deficient notch signalling in mice has been shown to be associated with the conversion of hair follicles to keratin-enriched epidermal cysts as a result of changes to the outer root sheath cells [43, 44].
Environmental factors
Mechanical irritation and shear forces are potential contributory factors. There is no evidence that poor hygiene, variation in routine depilatory techniques or use of antiperspirant are relevant to HS.
Clinical features
All three diagnostic criteria shown in Box 92.1 must be met for a diagnosis of HS to be made.
History
A history of typical lesions at typical sites with recurrence and chronicity is required for diagnosis (Box 92.1). Typical index lesions consist of painful subcutaneous nodules or abscesses that persist for a mean duration of 7–15 days. This is followed by spontaneous regression, partial regression (to form non-inflammatory, asymptomatic nodules) or progression to abscess formation with the rupture and release of purulent malodorous discharges. Typical sites are the axillae; the inguino-genital, perineal, perianal and gluteal areas; and infra- and inter-mammary skin.
Recurrence takes the form of acute intermittent or continuous disease, involving new skin sites or pre-existing non-inflammatory nodules. Acute intermittent flares consist of solitary or multiple lesions, which are localized or disseminated across regions. Periods of remission (characterized by normal skin or persistent non-inflammatory nodules) may last for weeks to months. Continuous active disease can lead to the formation of coalescing nodules and sinus tracts associated with chronic, daily, purulent discharge and pain.
Presentation
Index lesions include inflamed and non-inflamed dermal and subcutaneous nodules (Figure 92.1), which may be evident on palpation alone; rounded (as opposed to ‘pointing’) abscesses (Figure 92.2); and draining or non-draining sinus tracts (Figures 92.3 and 92.4). Scarring is typically bridged or ‘rope-like’, it can be hypertrophic or atrophic, producing depressions especially on the buttocks, and may be associated with contractures (Figure 92.5). Pseudo- (secondary) comedones are often seen, typically paired, polyporous and grouped (Figure 92.6). Closed comedones are not seen.

Figure 92.1 Multiple non-inflamed nodules in a patient with Hurley stage II disease of the genital area.

Figure 92.2 Classical axillary abscess as seen in hidradenitis suppurativa.

Figure 92.3 Non-draining sinus, recognized by the palpable oblong shape.
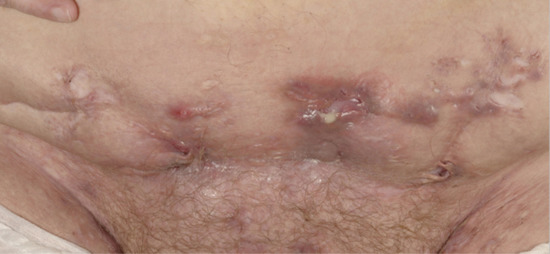
Figure 92.4 Multiple inflamed nodules and draining sinuses in active Hurley stage II disease of the mons pubis.

Figure 92.5 Scar: rope-like band formed by fibrotic tissue, most often seen in the axillae.

Figure 92.6 Tombstone comedones in the axilla.
Associated lesions include follicular papules and pustules, pyogenic granulomas at sinus tract openings and indurated plaques. Epidermoid cysts are seen in some patients on external genitalia, the face and the thorax. Regional lymphadenopathy is not routinely seen.
Lesions are localized to inverse (flexural) areas. The commonest sites are the axillae and inguinal and ano-genital regions, including the external genitalia and the perineal, perianal and gluteal skin (Figures 92.7, 92.8 and 92.9). Sub- and intermammary skin can also be affected, as can, less commonly, retroauricular, preauricular and occipital skin (Figure 92.10). Truncal variants are also reported (Figure 92.11).

Figure 92.7 Follicular pattern involving the genito-femoral area showing non-active disease.

Figure 92.8 Follicular pattern involving the buttocks.

Figure 92.9 Nodules and sinus tracts involving the buttocks.

Figure 92.10 Ectopic disease with retroauricular involvement.

Figure 92.11 Ectopic plaque on the chest of a patient. Notice the classical involvement seen in the axillae.
The clinical presentation varies with respect to the number of anatomical regions affected, the extent of the lesions and the types of lesions within a single region. The spectrum ranges from mild disease consisting of solitary nodules to severe disease comprising extensive inflamed confluent nodules, sinus tracts and scarring filling an entire anatomical region.
Clinical variants
Hidradenitis suppurativa is a heterogeneous disorder. Epidemiological studies indicate that multiple phenotypic subtypes are likely to exist, grouped by topographical predilection and lesion subtype. Three clinical variants have been suggested, although their clinical significance has not been studied [45].
- The classical variant, characterized by scarring lesions in the axillae and under the breasts, occurs most often in women and often includes lesions in the ano-genital area.
- A second variant is characterized by the additional involvement of the ears, chest, back or legs, with pilonidal sinuses, comedones, severe acne and a family history of HS, which may be a part of the follicular occlusion triad (see Chapter 90).
- A third variant is characterized by gluteal involvement, papules and folliculitis and may be more common in men.
Differential diagnosis
The differential diagnosis of HS is extensive. Index lesions should be differentiated from abscesses, carbuncles or furunculosis associated with primary cutaneous bacterial infection (typically staphylococcal or streptococcal) or secondary infection of cystic structures (e.g. epidermoid cysts and Bartholin glands). Crohn disease can result in inflammatory abscesses and sinus tracts at ano-genital sites. However, in Crohn disease, fistulae are usually intersphincteric, whereas in HS they do not involve the gastrointestinal tract. Clinical and histological overlap results in diagnostic challenge. Rare infections, including tuberculosis, sporotrichosis, actinomycosis and lymphogranuloma venereum, can present with both abscesses and sinus tracts. Steatocystoma multiplex and neoplastic diseases such as Langerhans cell histiocytosis should also be considered in the differential diagnosis.
Classification of severity
The Hurley staging system refers to three stages based on the presence and extent of sinus tracts and scarring (Table 92.2) [4]. Hurley staging allows stratification of therapy but is not useful for assessing response to therapy.
Table 92.2 Definition of the three Hurley stages in hidradenitis suppurativa.
| Stage | Features |
| I (Figure 92.12) | Recurrent abscess formation without sinus tracts and cicatrization |
| II (Figure 92.13) | Recurrent abscesses with widely separated sinus tracts and cicatrization |
| III (Figure 92.14) | Multiple interconnected abscesses, sinus tracts and cicatrization diffusely involving an entire region |

Figure 92.12 Hurley stage I disease of the genito-femoral area showing a solitary nodule. Notice the normal-looking surrounding skin and perilesional halo of discoloration indicating a recent episode of inflammation.

Figure 92.13 Hurley stage II disease of the genito-femoral area. (a) Hurley stage II is a broad category which may involve more severe disease as shown here. (b) Inactive, mild disease. (c) Severe, active, multifocal disease. Notice the areas of normal-looking skin separating the lesions.

Figure 92.14 Hurley stage III disease. (a) Non-draining, confluent, chronic lesions involving the entire axilla. (b) Active draining lesions in the axilla.
The hidradenitis severity score (HSS; or modified Sartorius score) is a more detailed, validated and dynamic score, which can be used to assess both severity and treatment response [46]. The HSS was originally developed to assess the results of surgery, but has been modified to include inflammatory elements. It correlates with the Hurley score but is more precisely defined and responsive to change [46]. The HSS incorporates assessment of the number of anatomical regions affected, lesions (counts of inflamed and non-inflamed nodules and sinus tracts), the extent of area involved and the presence of normal skin between lesions to give a regional and total score. The utility of the score is limited in severe disease where the presence of confluent nodules and sinus tracts reduces the accuracy of individual lesion counts.
The hidradenitis suppurativa clinical response (HiSCR) has been suggested as an outcome measure when studying treatments [47]. It is defined as a ≥50% reduction from baseline in the number of inflamed lesions (abscesses or inflamed nodules), without a concomitant increase in the number of draining fistulae. The HiSCR has been validated against Hurley stage, the HSS, the HS physician's global assessment (PGA) system and patient-reported outcomes (visual analogue pain scale, dermatology life quality index and work productivity and activity impairment questionnaire) [47]. Patients who achieve an HiSCR appear to have meaningful improvement of their disease.
Other quantitative outcomes used to support the assessment of disease severity include the HS PGA, pain scales (visual analogue or numerical) and validated quality of life questionnaires (e.g. DLQI, Skindex). The frequency of flares may play a role in the assessment of intermittent disease, and should be assessed over a minimum period of 3 months.
Complications and co-morbidities
Superinfection constitutes a curable complication, and should be suspected when flares are preceded by stinging or smarting pain or associated with the development of pustules and other superficial lesions. Structural complications of longstanding disease include lymphatic obstruction leading to clinical lymphoedema. The ano-genital sites are most severely affected and progression to scrotal elephantiasis can occur. Fistula formation to the gastrointestinal tract (anal canal and rectum), genito-urinary tract (urethra, bladder and vagina) and peritoneum are extremely rarely described, and when seen should trigger examination for Crohn disease. Cutaneous squamous cell carcinoma (SCC) can complicate chronic ano-genital disease, most commonly the gluteal skin of male patients, and carries a poor prognosis; it has been reported more often in men than in women [18]. There are no reports of SCC complicating axillary disease to date. Other complications of chronic disease include anaemia (multifactorial), hypoalbuminaemia, hypergammaglobulinaemia and rarely amyloidosis and sacral bacterial osteomyelitis [18].
A profound impact on quality of life complicates disease at all stages of severity. Significant psychological, social and economic impact appears more commonly than in many other chronic inflammatory dermatoses [6]. Depression is frequent, with one study suggesting 40% of patients have a concomitant diagnosis of depression [48].
Disease course and prognosis
Chronicity is the hallmark. The mean duration is 18.8 years [49]. Milder forms (Hurley stage I) are more frequent and reported to affect approximately two-thirds of patients, with intermediate disease (Hurley stage II) affecting one-quarter and severe disease (Hurley stage III) about one-fifth of patients [49]. Both intermittent and continuous disease can be seen at each stage. The natural course of progression between stages is not defined. In patients older than 50 years, the disease is progressively rarer with increasing age suggesting that spontaneous remission may occur over time. A long-term follow-up study has reported that this is seen in approximately 40% of HS patients after a median follow-up period of 22 years [50].
Investigations
Microbiology (swabs, purulent exudate and tissue) and histopathology are indicated for refractory or atypical cases to exclude flare secondary to superinfection and to consider relevant differentials. Imaging (both ultrasound and magnetic resonance imaging) defines subclinical extension, complications of severe disease and informs preoperative planning. Routine bloods in severe disease (Hurley stage III) may reveal anaemia (multifactorial), hypoalbuminaemia, polyclonal hypergammaglobulinaemia and elevated C-reactive protein or other less commonly used inflammatory markers that can serve as an adjunct for monitoring severe disease.
Management
Treatment strategy should be individually based and take the following into account:
- The need for patient empowerment.
- The recognition that the disease is multifocal from the onset and that generalized medical treatment is therefore as appropriate as in other inflammatory dermatoses.
- The recognition that once scarring occurs, surgical removal of affected areas is the only curative therapeutic option.
-
A management flow chart for HS according to the Hurley stage is shown in Figure 92.15.

Figure 92.15 Management flow chart for the initial treatment of hidradenitis suppurativa according to the Hurley stage. Arrows indicate the recommended sequence of initial choice but, on failure to respond, secondary choices can be made freely from any treatment. Colour coding reflects the evidence level supporting the recommendations: blue, expert opinion; green, case series with 30+ patients or randomized controlled trial. TNF, tumour necrosis factor.
Adjuvant treatment
Hidradenitis suppurativa is a disease that significantly impacts on quality of life; self-care may help patients cope with day-to-day routine as well as with the disease itself. Patients should be encouraged and supported to lose weight. Tobacco abstinence should be encouraged. Patients should also be shown how to bandage suppurating lesions and recommended to wear loose fitting clothes [1].
Analgesics
Hidradenitis suppurativa is painful and patients should be offered appropriate analgesic therapy, including non-steroidal anti-inflammatory therapy and paracetamol. In selected cases centrally acting analgesics are indicated [1].
Topical therapy
Topical clindamycin lotion 0.1% may be beneficial and appears to offer control of milder lesions [51]. For patients with localized, recalcitrant, established lesions (Hurley stage II), topical resorcinol 15% in a suitable ointment appears to offer improved disease control [52]. The benefits of antiseptics such as chlorhexidine washes or benzoyl peroxide remain unproven.
Systemic antibiotics
Tetracycline and the combination of clindamycin and rifampicin have been used in the largest reported series. Oral tetracycline 500 mg b.d. for 3 months appears to be useful in mild but widespread disease [53]. Longer term treatment may be considered if the treatment is effective. For more advanced cases, combined treatment with clindamycin 300 mg b.d. and rifampicin 300 mg b.d. given for 10 weeks appears to be effective in a large proportion of cases [54, 55]. This regimen reduced disease severity by an average of 50% as measured by HSS in one case series [54]. There are no formal studies of longer term treatment, but responders may require repeated treatments for recurrences.
A range of other antibiotics have been reported to be effective in smaller series, including the combination of rifampicin, metronidazole and moxifloxacin [56]. On suspicion of superinfection, antibiotic therapy should be started based on prior microbial susceptibility testing.
Anti-inflammatory treatment
For single lesions, intralesional triamcinolone (3–5 mg) often ameliorates symptoms rapidly. For more severe disease, systemic therapy is often necessary. A variety of drugs may be used for this; short-term systemic prednisolone (0.5–1.0 mg/kg body weight) or ciclosporin (3–5 mg/kg body weight), or longer term treatment using dapsone (100 mg daily), have all been reported to be useful for disease control [57].
Biological agents
Tumour necrosis factor-α antibodies appear to have a beneficial effect in moderate to severe HS [57]. Three randomized controlled trials (RCTs) have been conducted with adalimumab. They indicated that approximately twice as many patients treated actively achieved a significant effect (as defined by HiSCR) using a dosing regimen similar to that used for inflammatory bowel disease (a loading dose of 160 mg at week 0 and 80 mg at week 2, followed at week 4 by 40 mg every week, for a total of 12 weeks) as compared with patients not treated actively (58.9% versus 27.6% respectively) [58, 59]. An RCT of standard infliximab failed to reach its primary end point, but a post hoc analysis of the results indicated significant improvement in actively treated patients [60]. For etanercept, an RCT failed to confirm the initially promising results of open studies [61]. There have been anecdotal reports of benefit from ustekinumab and from anti-IL-1 agents but these have not as yet been systematically evaluated.
Retinoids
Isotretinoin is rarely effective in HS and its use is not encouraged. In contrast, case series suggest that acitretin (50 mg or more daily) may be effective in a significant proportion of patients and in some cases offers long-term remissions [57].
Experimental therapies
Zinc gluconate (90 mg/day) has been advocated as maintenance therapy or in combination with other therapies [62]. Other treatments that have been reported to be effective in cases or small series include intramuscular immunoglobulin, metformin and botulinum toxin [63, 64, 65].
Surgery
Several lesion-directed therapies have been described as useful in the management of HS.
Incision and drainage. Classical incision and drainage is useful only when frank fluctuating abscesses appear. The inflamed nodules of HS only respond with additional scarring. Incisions carry a 100% recurrence rate and should only be used if manifest fluctuation is found [66].
Deroofing. Deroofing is a tissue-saving technique, whereby the ‘roof’ of an abscess or sinus tract is surgically removed either through electrosurgery (using a loop) or conventional surgery [67]. A blunt probe is inserted in sinus openings discharging purulent exudate. If an opening cannot be identified, a small incision can be made to introduce a probe into the lesions. The full extent of the lesion is explored systematically with the probe, and the roof of the lesion surgically removed using the probe as a guide, leaving the partly epithelialized/granulating floor of the lesion exposed. The ensuing defect is left open to heal by secondary intention.
Localized surgery. Single lesions can be surgically excised; this results in a lower recurrence rate than after incision and drainage [68]. Generally, complete surgical removal of the lesion is required, suggesting that wider excisions have a better result than more limited exision. Whether or not the defect should be closed is a matter of debate. Patients with few, clinically stable, non-inflamed lesions (nodules or sinus tracts) are most suitable for localized surgery.
Ablative lasers and electrosurgery. Removal of all involved tissue appears to be necessary for successful surgical treatment of HS. CO2 laser evaporation provides a method whereby all visibly affected tissue can be vaporized using a scanner in a manner akin to macroscopic Mohs’ surgery (i.e. systematically evaporating abnormal tissue under visual guidance until healthy tissue is reached everywhere) [69]. The technique offers radical treatment whilst still being tissue-sparing. Postsurgical defects are usually left to heal by secondary intention. They normally heal sufficiently for resumption of work after 2–3 weeks, but it can take 8–10 weeks for the tissues to re-epithelialize fully. Patient satisfaction with the technique is high [70].
Extensive surgery. In severe disease when entire sites are involved with multiple interconnecting sinus tracts, the only curative method is excision of the entire area involved. The margins of excision in the reported case series range from 1 cm up to the excision of all hair-bearing skin of the affected region. For extensive ano-genital disease, this may therefore require multidisciplinary collaboration with plastic surgery and, where a temporary colostomy may be required, with colorectal surgery. The often large defects can be left to heal by secondary intent. Particular attention should, however, be paid to mobilization of the affected areas in order to avoid the development of postoperative strictures, especially in the axillae. The defects can also be closed by split-skin graft or flaps [71].
Non-ablative lasers/intense pulsed light
Hair removal using light-based therapies appears to have a beneficial effect in HS. Studies have found significant improvement following monthly treatments with both neodymium:yttrium-aluminium-garnet (Nd:YAG) laser as well as intense pulsed light (IPL) [72, 73].
Radiotherapy
In selected patients with particularly recalcitrant disease, radiotherapy may be considered. Fractionated radiotherapy has been described as effective in the older literature. Total doses of up to 12 Gy have been administered as single doses of 0.5–1.0 Gy given 4–12 times [72].
References
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619.
- Velpeau AA. In: Bechet JZ, ed. Dictionnaire de Médecine, un Repertoire Générale des Sciences Medicals sous le Rapport Theorique et Pratique, Vol. 2. Paris, 1839:1839–91.
- Verneuil A. De L'hidrosadenite phlegmoneuse et des abces sudoripares. Arch Gen Med 1864;2:537–57.
- Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigh R, Roenigh H, eds. Dermatologic Surgery. New York: Marcel Dekker, 1989:729–39.
- Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011;91:328–32.
- Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010;90:264–8.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol 2013;133:97–103.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol 2014;170:884–9.
- Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996;35:191–4.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol 2008;59:596–601.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013;68:412–19.
- Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol 2014;71:460–7.
- Jemec GB. The symptomatology of hidradenitis suppurativa in women. Br J Dermatol 1988;119:345–50.
- Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinology 2010;2:9–16.
- Van der Zee HH, van der Woude CJ, Florencia EF, et al. Hidradenitis suppurativa and inflammatory bowel disease: are they associated? Results of a pilot study. Br J Dermatol 2010;162:195–7.
- Meskin SW, Carlson EM. Mooren's-type ulceration associated with severe hidradenitis suppurativa: a case report and literature review. Ocul Immunol Inflamm 2011;19:340–2.
- Van der Zee HH, Laman JD, Boer J, et al. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol 2012;21:735–9.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009;60:539–61.
- Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009;161:831–9.
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol 2014;94:553–7.
- Konig A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology 1999;198:261–4.
- Barth JH, Layton AM, Cunliffe WJ. Endocrine factors in pre- and postmenopausal women with hidradenitis suppurativa. Br J Dermatol 1996;134:1057–9.
- Dvorak VC, Root RK, MacGregor RR. Host-defense mechanisms in hidradenitis suppurativa. Arch Dermatol 1977;113:450–3.
- Giamarellos-Bourboulis EJ, Antonopoulou A, Petropoulou C, et al. Altered innate and adaptive immune responses in patients with hidradenitis suppurativa. Br J Dermatol 2007;156:51–6.
- Van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol 2011;164:1292–8.
- Van der Zee HH, Laman JD, de Ruiter L, et al. Adalimumab (antitumour necrosis factor-alpha) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol 2012;166:298–305.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol 2011;65:790–8.
- Dreno B, Khammari A, Brocard A, et al. Hidradenitis suppurativa: the role of deficient cutaneous innate immunity. Arch Dermatol 2012;148:182–6.
- Hunger RE, Surovy AM, Hassan AS, et al. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br J Dermatol 2008;158:691–7.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol 2011;186:1228–39.
- Schlapbach C, Yawalkar N, Hunger RE. Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. J Am Acad Dermatol 2009;61:58–65.
- Bechara FG, Sand M, Skrygan M, et al. Acne inversa: evaluating antimicrobial peptides and proteins. Ann Dermatol 2012;24:393–7.
- Hofmann SC, Saborowski V, Lange S, et al. Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol 2012;66:966–74.
- Marinella MA. Lithium therapy associated with hidradenitis suppurativa. Acta Derm Venereol 1997;77:483.
- Scheinfeld N. Diseases associated with hidranitis suppurativa: part 2 of a series on hidradenitis. Dermatol Online J 2013;19:18558.
- Van der Zee HH, de Ruiter L, Boer J, et al. Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br J Dermatol 2012;166:98–106.
- Kamp S, Fiehn AM, Stenderup K, et al. Sebaceous gland number and volume is significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br J Dermatol 2011;164:1017–22.
- Jemec GB, Faber M, Gutschik E, et al. The bacteriology of hidradenitis suppurativa. Dermatology 1996;193:203–6.
- Lapins J, Jarstrand C, Emtestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol 1999;140:90–5.
- Sartorius K, Killasli H, Oprica C, et al. Bacteriology of hidradenitis suppurativa exacerbations and deep tissue cultures obtained during carbon dioxide laser treatment. Br J Dermatol 2012;166:879–83.
- Lapins J, Olerup O, Emtestam L. No human leukocyte antigen-A, -B or -DR association in Swedish patients with hidradenitis suppurativa. Acta Derm Venereol 2001;81:28–30.
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science 2010;330:1065.
- Pink AE, Simpson MA, Desai N, et al. Gamma-secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Invest Dermatol 2013;133:601–7.
- Melnik BC, Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol 2013;22:172–7.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol 2013;133:1506–11.
- Sartorius K, Killasli H, Heilborn J, et al. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol 2010;162:1261–8.
- Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the hidradenitis suppurativa clinical response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014;171:1434–42.
- Onderdijk AJ, van der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2013;27:473–8.
- Von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2000;14:389–92.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol 2014;171:819–24.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983;22:325–8.
- Boer J, Jemec GB. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol 2010;35:36–40.
- Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998;39:971–4.
- Van der Zee HH, Boer J, Prens EP, et al. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology 2009;219:143–7.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology 2009;219:148–54.
- Join-Lambert O, Coignard H, Jais JP, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology 2011;222:49–58.
- Blok JL, van Hattem S, Jonkman MF, et al. Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. Br J Dermatol 2013;168:243–52.
- Jemec GB, Sundaram M, Pinsky B, Gu Y, Williams D, Bao Y. Adalimumab improves treatment satisfaction with medication (TS-M) in patients with moderate to severe hidradenitis suppurativa (HS) in a 12-week randomised controlled trial (PIONEER I). J Invest Dermatol 2014;134:S30–8.
- Jemec G, Gottlieb A, Forman S, et al. Efficacy and safety of adalimumab in patients with moderate to severe hidradenitis suppurativa: results from PIONEER II, a Phase 3 randomized placebo-controlled trial [Abstract FC08.2]. In: 23rd EADV Congress: Building Bridges. Amsterdam: European Academy of Dermatology and Venereology, 2014. http://eadvamsterdam2014-m.poken.com/event/92466382/product/100251716/description (last accessed June 2015).
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol 2010;62:205–17.
- Lee RA, Dommasch E, Treat J, et al. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol 2009;60:565–73.
- Brocard A, Knol AC, Khammari A, et al. Hidradenitis suppurativa and zinc: a new therapeutic approach. A pilot study. Dermatology 2007;214:325–7.
- Goo B, Chung HJ, Chung WG, et al. Intramuscular immunoglobulin for recalcitrant suppurative diseases of the skin: a retrospective review of 63 cases. Br J Dermatol 2007;157:563–8.
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol 2013;27:1101–8.
- O'Reilly DJ, Pleat JM, Richards AM. Treatment of hidradenitis suppurativa with botulinum toxin A. Plast Reconstr Surg 2005;116:1575–6.
- Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis 1998;13:164–8.
- Van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol 2010;63:475–80.
- Van Rappard DC, Mooij JE, Mekkes JR. Mild to moderate hidradenitis suppurativa treated with local excision and primary closure. J Eur Acad Dermatol Venereol 2012;26:898–902.
- Lapins J, Marcusson JA, Emtestam L. Surgical treatment of chronic hidradenitis suppurativa: CO2 laser stripping-secondary intention technique. Br J Dermatol 1994;131:551–6.
- Mikkelsen PR, Dufour DN, Zarchi K, et al. Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: a retrospective study. Dermatol Surg 2015;41:255–60.
- Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg 2012;38:517–36.
- Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg 2009;35:1188–98.
- Highton L, Chan WY, Khwaja N, et al. Treatment of hidradenitis suppurativa with intense pulsed light: a prospective study. Plast Reconstr Surg 2011;128:459–65.
- Trombetta M, Werts ED, Parda D. The role of radiotherapy in the treatment of hidradenitis suppurativa: case report and review of the literature. Dermatol Online J 2010;16:16.