CHAPTER 94
Disorders of the Sweat Glands
Ian H. Coulson1 and Niall J. E. Wilson2
1Burnley General Hospital, East Lancashire NHS Trust IHC, UK
2Royal Liverpool and Broadgreen University Hospitals, UK
Introduction
In this chapter the anatomy, physiology and diseases of eccrine and apocrine sweat glands are described. The clinical patterns, causes and associations of excessive sweating on the one hand and of reduced or absent sweating on the other are addressed in detail. Guidance is given on the management of hyperhidrosis and on the choice of appropriate therapy, including topical and systemic agents and surgery. The presentation and management of occlusive and inflammatory disorders of eccrine sweat glands is covered fully, as are the clinical features and management of abnormal sweat odour and colour and of apocrine miliaria. Brief reference is made to conditions associated with sweat gland inclusions; discussion of the latter and of neoplasms derived from sweat gland elements is to be found elsewhere in the book.
ECCRINE GLANDS
Anatomy and physiology of eccrine glands
Human eccrine sweat glands have two distinct functions [1–3, 4]. They allow body cooling by evaporation, and have thus contributed in a major way to adaptation to a hot environment by humans; they also moisten the skin on the palms and soles at times of activity, and thus improve their grip.
Eccrine sweat glands are distributed over the whole skin surface including the glans penis and foreskin, but not on the lips, external ear canal, clitoris or labia minora. The number varies greatly with site, from 620/cm2 on the soles, about 120/cm2 on the thighs to 60/cm2 on the back [5]. The total number on the body surface is between 2 and 5 million, and is the similar in different ethnic groups. It has been calculated that the weight of the eccrine glands totals 100 g. The glands vary in size from person to person by a factor of five and this probably accounts for individual as well as regional differences in sweat rate (maximal individual gland secretion rates ranging from 2 to 20 nL/min/gland).
Embryologically, sweat glands are derived from a specialized down-growth of the epidermis at about the third month of intrauterine life on the palms and soles and at about 5 months elsewhere; they resemble adult glands by 8 months. Sweat glands are morphologically normal at birth but may not function fully until about 2 years of age. No new eccrine glands develop after birth. Unlike the apocrine glands they have no developmental relationship with the pilosebaceous follicle, although some glands may eventually come to open into the follicular neck. The gland consists of a secretory coil in the lower dermis (Figure 94.1a) and subcutaneous tissue, and a duct leading through the dermis to the intraepidermal sweat duct unit (Figure 94.1b). Apoeccrine glands have features of both eccrine and apocrine glands, but seem to be nearer to eccrine in function. They open onto the surface, and produce a copious watery fluid. They may account for 10–45% of adult axillary glands [6].
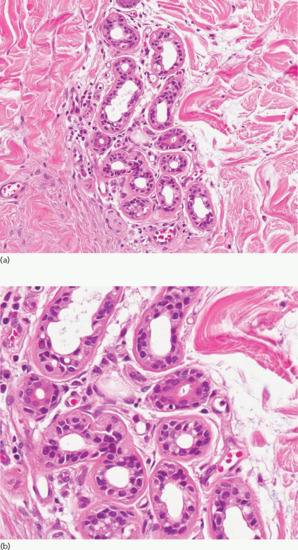
Figure 94.1 (a) A normal eccrine unit composed of secretory glands and ducts. Magnification 10× (H&E). (b) Closer view of eccrine glands showing the double layer of lining epithelial cells. Magnification 40× (H&E). (Courtesy of Dr Arti Bakshi, Royal Liverpool University Hospital, Liverpool, UK.)
The secretory coil contains three types of cell: large clear cells, which are the main secretory cells, small dark cells, which resemble mucus-secreting cells of other organs but whose function is not known, and myoepithelial cells [7]. The large and small cells of the secretory coil, unlike those of the duct, are attached to the basement membrane, although individual sections may at times suggest a double layer. Outside the basement membrane are the longitudinally arranged myoepithelial cells, whose function is probably to support the gland, but they may also help propel the sweat towards the surface. They respond to cholinergic stimuli. The function of the coil is to produce from plasma a watery isotonic secretion which can subsequently be modified by the duct. Ultrastructurally, the large clear cells are characterized by the presence of many mitochondria and by both intricate basal infoldings and intercellular canaliculi. Para-nitrophenyl phosphatase activity, which reflects catalytic activity of Na-K-ATPase is evident in the basal infoldings but not the intercellular canaliculi, suggesting that the basal areas are the sites of active ion transport requisite for sweat secretion. The classic theory suggests that acetylcholine passively increases entry of sodium into the cell, and this is then pumped out by the sodium pump into the intercellular canaliculi rather than directly through the luminal margin. However, there are other theories [4]. Fluid secretion is believed to be mediated osmotically, but the mechanism by which water moves has long been obscure. The discovery of aquaporins (AQPs) may challenge this theory. AQPs are a group of intercellular membrane water channel proteins, which allow movement of large amounts of fluid. In animal models, sweat secretion in AQP5 null mice was markedly decreased [8]. AQP5 has been identified in the dark cells of human eccrine sweat glands but its role in human sweating is still not clear [9, 10]. Many different monoclonal antibodies can be shown to react with different portions of the sweat glands, allowing distinction of the gland from other components of the skin [11].
The duct consists of two or more layers of relatively uniform cuboidal cells. About one-third of the coil has this histology, as well as the uncoiled part passing up to the epidermis. The basal cells are rich in mitochondria and their entire membranes are rich in Na-K-ATPase activity, suggesting sodium pumping occurs along the entire duct membrane, and performs an active part in modifying the secretion produced by the coil.
It has been suggested that sweat glands do not cool the skin only by evaporation of heat from the surface, but that they also act as heat pipes. According to this theory, evaporation of the fluid at the base of the duct allows water vapour to pass up the duct and condense nearer the surface, and thence return to the deeper parts by capillary action. Such systems are a very effective way of transferring heat quickly [12].
The intraepidermal sweat unit is lined by a layer of specialized cells that often may be distinguished only with difficulty from the surrounding epidermis. On the palms and soles, it has a well-developed coil structure that is not so apparent in other sites.
The techniques for studying the function of the eccrine sweat glands [12, 13, 14] include the following:
- Collection of sweat in bags or pads at rest, after exposure to heat, or after injection or iontophoresis of pilocarpine or other cholinergic agonists.
- Direct measurement of water loss.
- Microcannulation of the duct or coil [15].
- Measurement of electrical potentials and electrical resistance of the skin, which depends on both the sweat present on the epidermis and the column present within the duct [16, 17].
- Visualization of the individual sweat droplets. This may be achieved by direct microscopy, by in vivo staining, by forming plastic impressions [18] or by indicators that become coloured on contact with water, such as the starch/iodine technique [19], bromophenol blue [13], quinizarin [20] and the food dye Edicol ponceau. The plastic or silicone impression techniques are probably the most reliable, and can produce a permanent record. A simple modification of the starch/iodine test is to dry the skin, paint it with 2% iodine in alcohol, allow it to dry, and then press the skin against a good-quality paper. The starch in the paper reacts with iodine in the presence of water, so that each sweat droplet shows up as a minute dark spot. Alternatively, the starch may be suspended in castor oil (50 g in 100 mL) and painted onto the iodine-treated skin (Figure 94.2). Special dry starch/iodine powders can be dusted directly onto the skin [21].
- Isolated glands. It is possible to isolate single eccrine glands (and also hair follicles, sebaceous glands and apocrine sweat glands) by the relatively simple technique of shearing tissues with scissors [22, 23]. This allows the physiology, biochemistry and tissue culture behaviour to be studied in vitro.
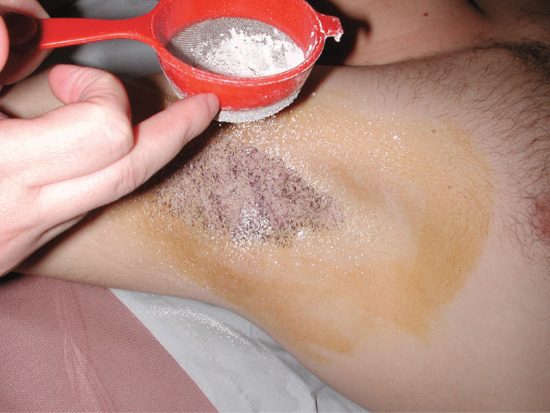
Figure 94.2 Identifying the extent of axillary hyperhidrosis – the skin has been cleaned with povidobe iodine solution and then sprinkled with corn starch powder from a fine culinary sieve. The hyperhidrotic areas are blue-black.
Control of eccrine sweating [1–3, 4]
Eccrine gland secretion is influenced by a number of stimuli including thermal, osmotic, mental and gustatory factors, mediated by a complex of central and local control mechanisms. As a result, the quantity and composition of sweat is highly variable from minimal basal activity to a maximum of 3 L in 1 h.
Central control
Thermoregulatory sweating is primarily controlled in response to internal body temperature and secondarily influenced by skin temperature [24]. The effect of a rise in core temperature is nine times more efficient than the same rise in skin temperature in stimulating sweating. Central and peripheral changes in temperature influence the thermal receptors in the preoptic area and anterior hypothalamus. An increase in core temperature activates cooling mechanisms including sweating, panting and vasodilatation. Conversely, cooling promotes heat-preservation mechanisms such as vasoconstriction and shivering [25].
Although the precise neural pathway that mediates eccrine sweating in humans is still unclear, evidence from animal studies suggest that the efferent pathway from the hypothalamus includes the medulla, lateral horn of the spinal cord and sympathetic ganglia [26].
Osmotic factors also influence the rate of sweat production. Both hyperosmolality and hypovolaemia decrease sweat production, presumably in an attempt to prevent further loss of body fluid [27, 28].
Centres and pathways controlling mental sweating are not fully known but areas within the frontal region of the brain have been identified. Functional magnetic resonance studies indicate that neural pathways for thermal and mental sweating are similar [29]. Mental stimuli enhance sweat production particularly from the palms and soles, potentially improving grip at times of stress.
Local control
From the sympathetic ganglia non-myelinated C fibres pass to eccrine sweat glands ending at many cholinergic terminals and a few adrenergic terminals [30]. Although stimulation of adrenergic nerves increases sweating this is much less marked than the response to cholinergic stimulation [31, 32, 33]. The adrenergic nerve supply seems to play little part in the normal modulation of eccrine sweating in humans. In addition, vasoactive intestinal polypeptide, calcitonin gene-related peptide and nitric oxide may play some role in the control of eccrine sweating [24].
Other factors may modify the quantity and quality of sweat in the presence of an intact sympathetic nerve supply including hormones, circulatory changes and axon and spinal reflexes. Sweat coils contain androgen receptors [34], and androgens may be at least partly responsible for the increase in sweating around puberty and for the greater sweat activity in males.
The composition of sweat [4, 33] varies greatly from person to person, time to time and site to site. It has a basic similarity to the plasma from which it is derived. The sweat duct is largely responsible for the modification in sweat constituent concentration that occurs, and this will therefore vary according to how rapidly the sweat is passing through the duct. The most important constituents are sodium, chloride, potassium, urea and lactate. Sweat is hypotonic and this is largely due to reabsorption of sodium in the duct. At increased sweat rates the sodium concentration rises, presumably because there is reduced time for ductal reabsorption. The normal sodium concentration is between 10 and 20 mmol/L at low sweat rates, and up to 100 mmol/L at high rates. Aldosterone can increase ductal sodium reabsorption and in Addison disease high sweat sodium can be demonstrated (70–80 mmol/L). Antidiuretic hormone may reduce sweat rates in humans, but it also induces local vasoconstriction.
An increase in sweat electrolytes occurs in cystic fibrosis and forms the basis of the sweat chloride test [35]. Mutations in the CFTR gene in cystic fibrosis result in abnormalities of chloride transport across epithelial cells on mucosal surfaces [36]. A raised level of chloride in sweat (above 60 mmol/L) is considered consistent with a diagnosis of cystic fibrosis although it is recommended that the test is repeated on two occasions [35].
Lactate is found in a concentration of 4–40 mmol/L, which greatly exceeds the concentration found in plasma. It is formed in the gland from glucose from the blood. It is interesting to speculate whether urea and lactate can act to moisturize the stratum corneum.
Glucose is present in small quantities only (usually 0–0.17 mmol/L, although levels up to 0.3 mmol/L may be found). High sweat glucose may be found in uncontrolled diabetes and this may create a favourable environment for skin infections. The pH is 4–6.8.
A variety of other substances may be found in sweat, including pharmacologically active substances and inhibitors, antigens, antibodies and drugs [4]. Some of these seem to be excreted, and have no special function; others may have a definite function, for example a urokinase-type plasminogen activator may play a part in the digestion of glycoprotein plugs in sweat pores [37]. Active excretion or secretion of drugs such as griseofulvin and ketoconazole may contribute to their efficacy.
DISORDERS OF ECCRINE SWEAT GLANDS
Hyperhidrosis
Definition and nomenclature
Hyperhidrosis is defined as excessive production of sweat, that is, more than is required for thermoregulation [1]. It can be defined gravimetrically [2] as greater than 2 standard deviations above mean values of sweat secretion for a normal population in various sites (palmar 50 mg/min/m2, plantar 50 mg/min/m2, axillary 150 mg/min/m2 and facial 50 mg/min/m2).
Introduction and general description
Hyperhidrosis can be a major inconvenience and embarrassment to sufferers. In theory, when there is overproduction of sweat it should be possible to determine whether this is due to abnormal sweat glands, pharmacologically active agents acting on the glands, abnormal stimulation of the sympathetic pathway between the hypothalamus and the nerve ending, or to overactivity of one of the three different ‘centres’ responsible for thermoregulatory, mental and gustatory sweating. Any difficult case should be approached from first principles in this way.
Most cases of hyperhidrosis can be classified as one of the following:
- Generalized.
- Focal – palmar, plantar, axillary and cranio-facial.
- Localized naevoid.
- Compensatory.
- Hyperhidrosis with extensive anhidrosis (Ross syndrome).
Epidemiology
In a series of Polish medical students, 16% admitted to perceived hyperhidrosis. Less than half of these, however, were determined to have gravimetrically measured sweat secretion rates defined as greater than 2 standard deviations above the reference range for a given site [2]. Generalized and focal naevoid hyperhidrosis are relatively rare. There is no gender or racial preponderance.
Generalized hyperhidrosis
Pathophysiology
There is marked physiological variation in thermoregulatory sweating from person to person in the absence of disease. An increase in the temperature of blood bathing the hypothalamus increases heat loss by sweating and vasodilatation. Some instability of the sweat regulating centre is caused by many febrile conditions, so that sweating may occur at times when there is no fever. This instability may persist for days, or even months, after the fever has subsided, and in some cases is such a prominent feature that the term ‘sweating sickness’ has been used [3]. Generalized sweating may occur in disorders of unknown aetiology that alter the setting of the thermoregulatory centre and may be associated with episodic hypothermia [1].
For a list of disorders associated with generalized hyperhidrosis see Box 94.1.
Thermoregulatory sweating occurs during or after many infective processes, and may be the presenting manifestation of malaria, tuberculosis, brucellosis, lymphoma, subacute bacterial endocarditis, etc. Night sweats are often part of the clinical picture. A similar mechanism may account for the hyperhidrosis associated with alcohol intoxication or gout, and after vomiting. The mechanism of generalized hyperhidrosis that may be associated with diabetic autonomic neuropathy, hyperthyroidism, hyperpituitarism, hypoglycaemia, obesity, the menopause and malignant disease is unknown. Increased sweating has been documented in some patients with Parkinson disease, but others have noted the combination of patchy anhidrosis and compensatory hyperhidrosis, suggesting autonomic dysfunction. Paroxysmal sweating, tachycardia and headaches strongly suggest a phaeochromocytoma. Hypertension is noted during attacks. Cases have been reported of patients who develop generalized sweating in a thermal pattern, but induced by cold [3].
Hyperhidrosis is seen in association with peripheral neuropathies, as in familial dysautonomia, or Riley–Day syndrome, a recessively inherited disorder of Ashkenazi Jews comprising an absent axon reflex flare after histamine injection, pupillary meiosis, diminished tendon reflexes, diminished pain sensation and absent fungiform papillae of the tongue (see Chapter 85). Excess sweating is thought to be due to sweat centre excitability. Congenital autonomic dysfunction with universal pain loss is similar, but individuals are not Ashkenazi Jews, have a complete absence of pain sensation with accidental self-mutilation, corneal opacities and episodic fever.
Generalized hyperhidrosis may be associated with brain lesions (diencephalic lesions, malformations of the corpus callosum, microgyria) and may be accompanied by episodic hypothermia. Some drugs – for example, fluoxetine – are able to cause generalized hyperhidrosis. In many cases of generalized hyperhidrosis of the thermal type, but with no obvious underlying disease, the aetiology remains unknown, even after extensive investigation.
Focal hyperhidrosis
Pathophysiology
Focal hyperhidrosis includes palmoplantar, axillary and cranio-facial (‘emotional’) hyperhidrosis [4]. Emotional or mental activity increases sweating, especially on the palms, soles, axillae and, to a lesser extent, groin, face and scalp. It should be emphasized that mental activity devoid of any clear emotional content may provoke sweating. Thermal stimuli and physical effort increase this effect in many cases. Most cases of hyperhidrosis presenting to the dermatologist are of this type. Although mental or emotional factors are the usual trigger for this type of sweating, and in some patients deep-seated emotional disturbances may be found, in many there seems to be some facilitation of the nervous pathways causing physiological mental sweating.
Clinical features
Focal hyperhidrosis may be a significant disability and embarrassment, particularly if sweat drips from the hands onto the floor; rusting of metal objects may be an occupational problem, or clothing may be saturated. Patients with axillary hyperhidrosis often wear only black or white garments as these show the wetness less obviously than coloured clothes (Figure 94.3).

Figure 94.3 Axillary hyperhidrosis: patients often wear white or black garments as the wetness is not as visibly obvious as with coloured clothes.
Hyperhidrosis may be associated with Raynaud phenomenon and reflex sympathetic dystrophy, or may follow cold injury. Frequently there is a family history of excessive sweating.
Palmoplantar hyperhidrosis. Palmoplantar hyperhidrosis (Figure 94.4) occurs in both sexes, and commonly begins in childhood or around puberty. The sweating of the palms and soles may be either continuous or phasic [4]. When continuous, it is worse in the summer, and not so clearly precipitated by mental factors. When phasic, it is usually precipitated by minor emotional or mental activity, and is not markedly different in summer and winter. The hands may be cold and show a tendency to acrocyanosis. Hyperhidrosis may affect the hands, feet and axillae in any combination, but only a minority of patients with axillary hyperhidrosis also have involvement of the palms and soles. Troublesome hyperhidrosis of the feet occurs especially in young adult men. When this is associated with vasomotor changes, so that the sodden skin is also cold and cyanotic, the name ‘symmetrical lividity’ is sometimes applied. Palmoplantar hyperhidrosis is one component of various syndromes in which palmoplantar keratoderma occurs. It also occurs with the nail–patella syndrome (see Chapter 69).
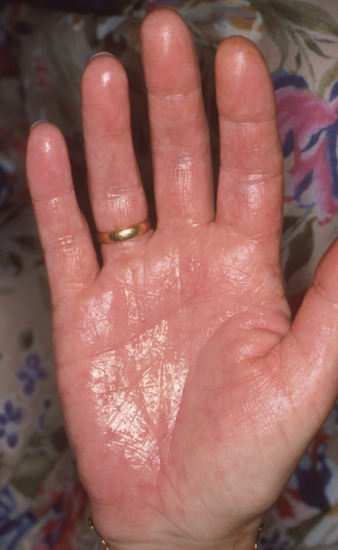
Figure 94.4 Palmar hyperhidrosis.
Axillary hyperhidrosis. This may be continuous, or more commonly phasic, and may or may not be aggravated by heat or mental activity. It is uncommon before puberty. Axillary sweating on undressing is very common. Axillary hyperhidrosis is due to overactivity of the eccrine glands, unlike axillary odour, which is mainly apocrine in origin.
Cranio-facial hyperhidrosis. Cranio-facial hyperhidrosis (Figure 94.5) is often phasic, occurs in middle age and may be exacerbated by heat, exercise and eating but, unlike true gustatory hyperhidrosis, not exclusively so. It may be more persistent and usually presents at a later age than palmoplantar hyperhidrosis. The entire face and scalp may be affected; sweating sufficient to soak the hair is an additional embarrassment.
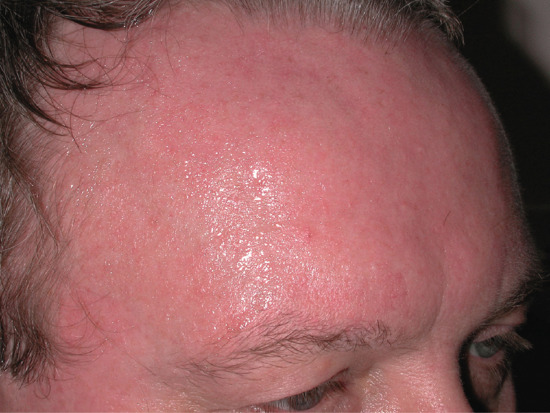
Figure 94.5 Cranio-facial hyperhidrosis. It may be sufficiently profuse to drip off the face and wet the hair.
Complications and co-morbidities
Palmoplantar hyperhidrosis predisposes to vesicular eczema (pompholyx) and allergic sensitization to footwear constituents; control of sweating may thus reduce the risk of contact dermatitis to footwear materials. Maceration of the skin, particularly in the toe clefts, is common and may predispose to both dermatophyte and bacterial infection (see Chapters 26 and 32). Pitted keratolysis of the feet, due to infection with Kytococcus sedentarius, is strongly associated with hyperhidrosis (Figure 94.6).
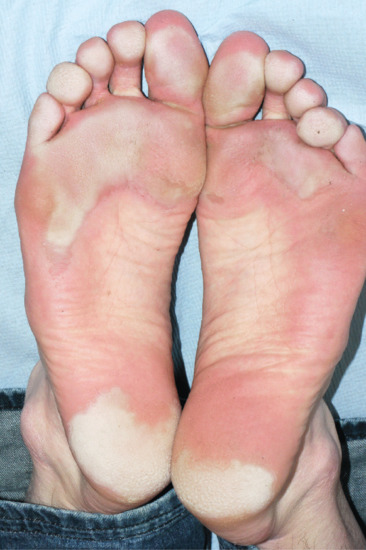
Figure 94.6 94.6Plantar hyperhidrosis showing maceration of the plantar keratin and secondary pitted keratolysis due to infection with Kytococcus sedentarius.
Disease course and prognosis
Hyperhidrosis may persist for some years, but there is a tendency to spontaneous improvement of axillary and palmar hyperhidrosis after the age of 25 years.
Investigations
Thyroid function and gravimetric determination of sweat rate estimation are seldom contributory.
Localized circumscribed and asymmetrical hyperhidrosis
Pathophysiology
The causes of localized hyperhidrosis are outlined in Box 94.2. Excessive sweating may be due to neurological lesions involving any part of the sympathetic pathway from the brain to the nerve ending. It may be the presenting symptom but it is quite exceptional for this to occur as an isolated phenomenon in the absence of other neurological symptoms or signs. Such lesions may be within the central nervous system (cortex, basal ganglia or spinal cord), the sympathetic pathway and ganglia, or in the peripheral nerves [1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. It must be remembered that the distribution of the sympathetic nerves does not exactly correspond with sensory dermatomes. One sympathetic grey ramus may supply 10 or more sensory segments, and one white ramus extends over at least five. Asymmetrical sweating may also occur reflexively from visceral disturbances, adjacent to an area of anhidrosis or due to axon reflex stimulation, around a leg ulcer, for example, or around glomus tumours, blue rubber bleb naevi or a sudoriparous angioma.
Compensatory hyperhidrosis occurs in normal sweat glands when those elsewhere are not functioning because of neurological or skin disease, diabetes or after sympathectomy. It is also a component of Ross syndrome (see below).
Functional sweat gland naevi have been reported [13], but must be distinguished from sweat gland hypertrophy associated with local hyperhidrosis of some other aetiology. Areas of skin may be localized (Figure 94.7) [14], termed idiopathic circumscribed hyperhidrosis, or as extensive as one-half of the body [15] and may sweat continuously or, more commonly, with mental activity. They may represent functional naevi, where the eccrine glands show increased sensitivity to cholinergic neurotransmitters. In the absence of other neurological symptoms or signs, they are seldom a manifestation of a progressive neurological lesion.
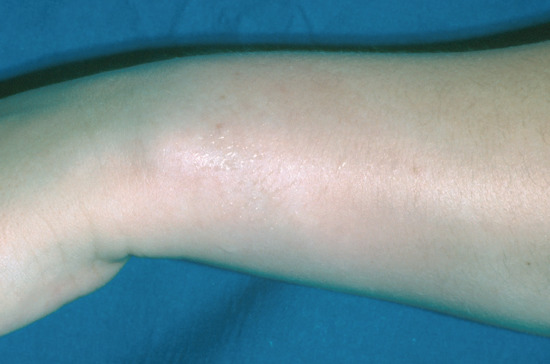
Figure 94.7 Circumscribed (naevoid) hyperhidrosis on the wrist. There is a solitary area of hyperhidrosis with normal sweating elsewhere on the rest of the skin.
Cold-induced sweating syndrome
This rare condition presents in infancy with poor feeding and difficulty in suckling, followed in adulthood by paradoxically cold-induced hyperhidrosis and anhidrosis in heat [1]. There is an associated mild neuropathy, kyphoscoliosis, valgus deformity of the elbows, high arched palate and digital syndactyly. Inactivation of a cardiotropin-like cytokine, a second ligand for ciliary neurotrophic factor receptor, has been identified in this syndrome.
Gustatory hyperhidrosis
Pathophysiology
Hyperhidrosis precipitated by eating specific foods can occur physiologically in many people [1]. Hot spicy foods are the most likely cause. The central connections of this reflex are not fully known.
Gustatory hyperhidrosis also occurs in pathological conditions involving the autonomic nervous system. Localized areas of intense hyperhidrosis may occur on the face, and even on the knees [2]. These disorders are very rare, usually start in childhood and are not progressive. Their nature is little understood.
Much the commonest cause, however, is damage to the sympathetic nerves around the head and neck [3, 4, 5]. After such an injury, regeneration occurs not only from the proximal ends of the damaged sympathetic nerves, but also from damaged or undamaged parasympathetic nerves. In this way, abnormal connections are made. Thus, the reflex arcs that normally allow chewing or taste stimulation to cause parotid or gastric secretion may cause sweating in a localized zone corresponding to the area of skin in which the sympathetic innervation has been damaged. The commonest site is within the distribution of the auriculotemporal nerve, which may be injured by trauma, abscess or surgery in the parotid region (auriculotemporal or von Frey syndrome). Submental gustatory sweating follows injuries involving the chorda tympani, and sweating in the distribution of the greater auricular nerve commonly follows radical neck surgery [6]. Injury to fibres from the vagus may cause gustatory sweating localized to the upper arm after cervical sympathectomy.
Gustatory sweating may occur in diabetes as part of a widespread autonomic neuropathy [7]. It has also followed herpes zoster [8].
Clinical features
Gustatory sweating is by no means uncommon, and occurs in 50–80% of patients subjected to operations on the parotid gland. Usually the symptoms appear 4–7 months after the operation, and either persist indefinitely or wane after 3–5 years. The stimuli required to initiate the reflex vary, as does the severity. Sometimes chewing, without taste sensation, is the most important stimulus. In many cases it is merely a curiosity, but in others it can be a significant disability. As well as sweating there is usually vasodilatation, which in rare instances may occur by itself in the absence of visible sweating. For a classification and list of causes of gustatory hyperhidrosis see Box 94.3. Olfactory hyperhidrosis, in which the trigger stimulus is smell, has also been recorded [9].
Management
Treatment of severe cases may require surgical interruption of the parasympathetic pathway – for example, section of the glossopharyngeal nerve within the skull, or tympanic neurectomy [4]. Excision of the auriculotemporal nerve is usually followed by recurrence. Topical therapy with aluminium chloride [10], topical glycopyrronium bromide [11] or botulinum toxin injections may be helpful [7].
Management of hyperhidrosis
Topical drug treatment
Topical anticholinergics. Atropine-like drugs may be absorbed sufficiently to produce a beneficial local effect without associated systemic side effects, but none of those at present available can be relied upon to do so [1]. Poldine methosulphate, 1–4% in alcohol, suppresses experimentally induced sweating, but unfortunately has not proved to be useful in clinical practice [2]. Topical 0.5% glycopyrronium bromide cream has been successfully used in gustatory hyperhidrosis in diabetics and at 0.5–2.0% for axillary hyperhidrosis [3]. A 2.0% aqueous solution has been used in scalp hyperhidrosis, and may be used to treat axillae.
Eccrine duct-blocking agents. These drugs act by impeding the delivery of sweat to the skin surface. Formalin (40% aqueous solution of formaldehyde) 1% soaks have long been used for treatment of hyperhidrosis of the feet, but are unsuitable for the hands and axillae. Glutaraldehyde 10% in a buffered solution, pH 7.5, swabbed onto the feet three times weekly, has helped some patients [4], but stains the skin so that it is suitable only for the feet. There is a small risk of allergic sensitization both to formaldehyde and to glutaraldehyde.
For axillary hyperhidrosis (as opposed to bromhidrosis), the most commonly used topical applications are aluminium or zirconium salts. Aluminium chloride, the first to be introduced, is in many ways the best, but may be irritant to the skin and damage clothes. Many other salts – for example, the chlorhydrates – are in use in cosmetic preparations [5]. Improved results can be achieved by applying 20% aluminium chloride in absolute ethanol at night after drying the axilla, with or without polythene occlusion. It should initially be applied nightly but may later be required only once every 1–4 weeks [6, 7]. Commercial preparations are available. Mild irritation of the skin from such therapy may be helped by a weak topical corticosteroid. The same treatment can also be tried on the hands and feet, or other localized areas of hyperhidrosis, but usually with rather less success. The mode of action of aluminium salts is uncertain, but they can be shown to affect both the duct and the secretory coil [8].
Iontophoresis. One of the more satisfactory methods of controlling hyperhidrosis of the hands and feet is by iontophoresis, using either tap water or anticholinergic drugs such as 0.05% glycopyrronium bromide solution [9–11, 12]. The mode of action of tapwater iontophoresis is not known. In very soft water areas, adding sodium bicarbonate to the iontophoresis solution is reported to improve efficacy. Direct current is usually used, with each palm or sole being treated for 30 min with 20 mA, initially three times a week. Once euhidrosis is established, monthly maintenance treatment may be sufficient. Alternating current is less effective, but may usefully be combined with direct current (alternating current offset) to produce a safer, more comfortable treatment [13]. Once control has been achieved, a single treatment may prove effective for some weeks. Minor systemic side effects due to absorption of anticholinergic agents, such as dry mouth and eye symptoms, are not uncommon, and can be avoided if tap water alone is used. The authors’ practice is to initiate thrice-weekly treatment on a hospital out-patient basis, and, if this is successful, a small battery-operated home unit can be purchased for maintenance therapy [14]. Less frequent treatment will then be required. When the sweating is controlled, the associated lividity, coolness and oedema improve. Similar treatment has also been used for the axilla, but is less often needed because topical applications or injections of botulinum toxin are more effective in this site. Devices have been designed to deliver iontophoresis to the chest and back affected by post-sympathectomy compensatory hyperhidrosis.
Botulinum toxin A injection. This compound produces prolonged blockade of neuronal acetylcholine release at the neuromuscular junction and in cholinergic autonomic neurons: it has been used to treat dystonic conditions for many years. In recent years, intradermal injection has been used to produce a marked reduction of sweating in hyperhidrotic areas associated with a variety of conditions [15, 16–18]. Different preparations of botulinum A toxin have different activities, and dose schedules differ for each product; 0.1 mL of appropriately diluted botulinum toxin administered by high intradermal injection can be given to 1 cm2 areas of skin appropriately anaesthetized – topical eutectic lignocaine/prilocaine is sufficient for axillary skin, but palms and soles require regional nerve blockade. Each axilla usually requires 12 injections, hands 20 and each foot 24–36. A reduction in sweating is apparent within 48 h and the benefit will normally last for up to 8 months in axillary and 6 months in palmar and cranio-facial hyperhidrosis [19]. Reinjection seems to be effective, and to date resistance has not been seen in hyperhidrosis (although it eventually occurs in 5% of patients treated intramuscularly for dystonia). Botulinum toxin has been used for idiopathic circumscribed and gustatory hyperhidrosis including Frey syndrome, the hyperhidrotic areas in Ross syndrome, and frontal and cranio-facial hyperhidrosis. A sight transient reduction of thenar and hypothenar muscle power is a minor problem after palmar injections [18]. The use of botulinum toxin on the forehead may produce short-lived frown reduction [19].
Systemic drug treatment
Atropine-like drugs have been used to block the effect of acetylcholine on the sweat glands, but their side effects are often more troublesome than the hyperhidrosis itself. These include dryness of the mouth, constipation, urinary retention and disturbances of vision, due to paralysis of accommodation. More serious side effects, for example glaucoma, hyperthermia and convulsions, can occur. Atropine itself is seldom employed. Propantheline may be prescribed in doses of 15 mg three times daily, increasing, if tolerated, to as much as 150 mg daily [20], but overall the results are disappointing. Methantheline at a dose of 50 mg three times a day has recently been advocated [21]. The oral antimuscarinic agent oxybutynin, usually used to treat bladder instability, has been reported to be effective for generalized and focal hyperhidrosis. The dose is escalated from 2.5 mg daily to 5 mg twice a day as tolerated, with an improvement in quality of life in 65% of those treated [22]. Glycopyrollate at a dose of 1–2 mg once to four times a day often gives useful sweat reduction without other marked anticholinergic effects [23]. Clonidine at a dose of 0.1 mg twice daily may be useful, but hypotension may limit its use. Ganglion-blocking drugs can inhibit sweating, but side effects from hypotension are usually too troublesome. Calcium-channel blockers, such as diltiazem [24], have helped some patients. In cases with a pronounced emotional factor, sedative or tranquillizing drugs are often useful, but psychiatric treatment may be necessary. Both clonazepam [25] and amitriptyline have helped isolated cases of unusual localized hyperhidrosis.
Surgical treatment
Sympathectomy. Sympathectomy, whether cervical, transaxillary or endoscopic, causes anhidrosis if complete [26, 27]. Sweating may return after a period of some years, due either to regeneration of sympathetic fibres or to fibres that do not pass through the sympathetic ganglia [28]. The former open approach has been largely replaced by an endoscopic procedure, which may be successful in treating palmar, axillary and cranio-facial hyperhidrosis. A pneumothorax is induced, and an operating endoscope inserted into the thorax via a small axillary incision, allowing visualization of the sympathetic trunk. Interruption of the sympathetic fibres between the second and fourth thoracic ganglia can be achieved by surgical transection, radiofrequency ablation, phenol destruction, cautery, clamping or clipping [29]. The latter technique has the potential advantage of partial reversibility. Most surgeons treat both sides at a single session.
With both the open and endoscopic approaches, satisfactory reduction of palmar hyperhidrosis is achieved in over 95% of cases; it is a little less successful for axillary hyperhidrosis. A recent consensus guideline [30] suggested that an international nomenclature should be adopted that refers to the rib levels (R) instead of the vertebral level at which the nerve is interrupted. It states that the highest success rates occur when interruption is performed at the top of R3 or the top of R4 for hyperhidrosis limited to the palms. R4 may offer a lower incidence of compensatory hyperhidrosis but moister hands. For hyperhidrosis involving the upper limbs and/or the axillae, interruptions at R4 and R5 are recommended. The top of R3 is best for cranio-facial hyperhidrosis.
In a series of 1731 patients who underwent endoscopic sympathectomy, the initial failure rate was 9%, and there was recurrence in 2%; overall, 98% of those treated were satisfied with the result. Compensatory hyperhidrosis occurred in 88% of patients, and was severe in 27%. Only 2.5% of patients experienced regret for having the operation [31]. Large case series using endoscopic techniques in children show it to be an acceptable option, with a low recurrence rate [32].
Complications of sympathectomy include haemothorax, pneumothorax, chylothorax, nipple sensitivity and Horner syndrome. There are rare instances of transient or permanent bradycardia complicating the technique. Other disadvantages are that the palms or soles may become excessively dry, and irritant eczema after sympathectomy has been reported. In five patients who had undergone a clipping procedure and subsequently developed compensatory hyperhidrosis, removal of the clips resulted in a return of the palmar sweating and abolition of the compensatory hyperhidrosis [33]. It has been suggested that ablation at the level of the third thoracic ganglion does not produce this side effect. Abolition of severe facial blushing may be a desirable consequence, and resolution of palmar eczema has been reported after endoscopic sympathectomy.
In general, only those patients with severe disability from hyperhidrosis of the hands or axillae warrant surgery, and in these selected cases the results can be very gratifying. Endoscopic sympathectomy has been used successfully in the treatment of severe cranio-facial hyperhidrosis [34]. Pedal sympathetic denervation requires lumbar sympathectomy; if more cranial lumbar ganglia are removed, ejaculatory impotence may occur. A selective retroperitoneal approach has recently been advocated that has no effect on sexual function in either men or women [35]. An endoscopic technique employing clips may also be employed with high patient satisfaction and minimal surgical morbidity.
Excision of the axillary vault. Axillary hyperhidrosis may be greatly helped by local excision of the axillary vault [36, 37]. Variations of this technique include subcutaneous curettage of the axillary skin [38], directly trimming the eccrine glands after reflection of the axillary skin using only a short incision [39], and tumescent liposuction of the axillae [40]. Microwave devices that ablate eccrine glands in the axillae are under development [41].
Granulosis rubra nasi
Definition
Granulosis rubra nasi is a rare disorder characterized by hyperhidrosis of the nose and associated skin changes [1].
Epidemiology and pathophysiology
The condition was first described by Jadassohn. The pathogenesis remains obscure but in some cases there is evidence of autosomal dominant transmission [2]. Symptoms develop from as early as 6 months but can occur at any age in childhood and occasionally in adults.
Clinical features
Initial presentation is with excess sweating localized over the tip of the nose. Droplets of sweat are usually visible. With time erythema, papules, vesicles and telangiectasia may develop over the nose, cheeks and upper lips (Figure 94.8) [3]. In the vast majority of cases, resolution occurs around puberty but persistent cases are recognized. Patients may also report peripheral acrocyanosis and palmoplantar hyperhidrosis. A single case has been described in association with phaeochromocytoma [4]. Skin biopsy demonstrates a chronic inflammatory cell infiltrate with dilatation of vascular spaces [3].
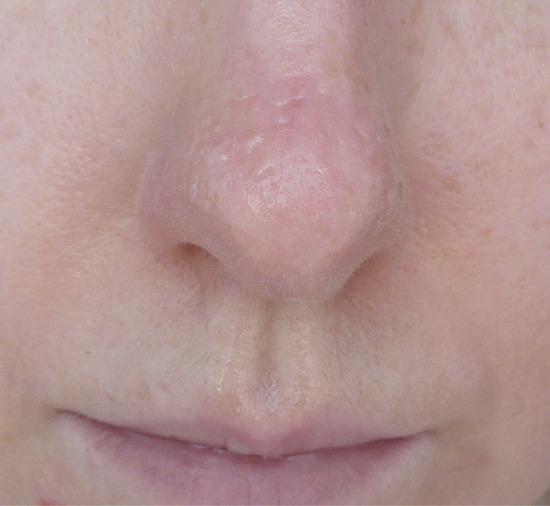
Figure 94.8 Granulosis rubra nasi in young adult female showing localised hyperhidrosis with beads of sweat on nose and philtrum together with multiple vesicles and mild erythema on dorsum of nose. Courtesy of Dr E.P. Burova, Bedford Hospital.
Investigations
These are not usually needed.
Management
Reassurance, given the natural history of the condition, is sufficient in most cases. Botulinum toxin has been reported as effective [5].
Anhidrosis and hypohidrosis
Diminished sweat production may be partial (hypohidrosis) or complete (anhidrosis) [1]. Disturbance of any part of the physiological pathway of sweat production may decrease sweating. Causes may be broadly classified as being either of neurological (Box 94.4) or eccrine gland origin (Box 94.5).
Impairment of sweat production interferes with the body's temperature control mechanisms. Symptoms include heat intolerance, fatigue, drowsiness and pyrexia. In severe cases death may result.
Examination of patients with hypo- and anhidrosis is often unremarkable. Autonomic function tests, in particular the quantitative sudomotor axon reflex test and the thermoregulatory sweat test (essentially a heat stress in a warmed room), may help delineate the distribution of anhidrosis and point towards a cause [2]. Skin biopsy is also helpful to identify eccrine sweat gland abnormalities.
Ross syndrome
This rare syndrome is characterized by a triad of segmental anhidrosis, tonic pupils (Figure 94.9a) and absent deep tendon reflexes [3]. It is a progressive degenerative disorder of sensory and autonomic nerves [4, 5]. Involvement of the cardiac sympathetic nerve supply has also been reported [6].
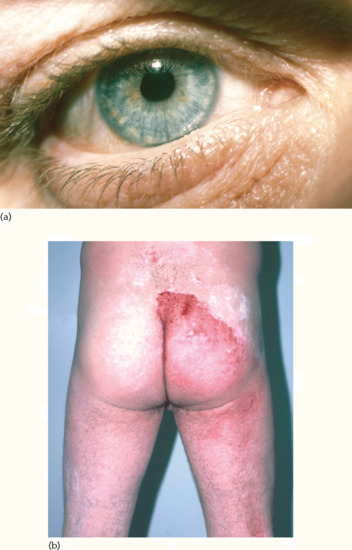
Figure 94.9 Ross syndrome. (a) The pupils are tonic, asymmetrical and irregular in outline. (b) Most of the skin is anhidrotic but the remaining areas of enervated eccrine glands demonstrate compensatory hyperhidrosis (demonstrated by edicol Ponceau powder, which turns red on hydration).
The main symptoms are those of heat intolerance and socially disabling compensatory hyperhidrosis (Figure 94.9b), which may be asymmetrical, patchy or unilateral. Hyperhidrosis arising in this situation has been successfully treated with iontophoresis and botulinum toxin [4, 7].
Acquired idiopathic generalized anhidrosis
This term describes a heterogeneous group of very rare disorders characterized by progressive loss of sweating and heat intolerance [8]. Three subtypes have been proposed: idiopathic pure sudomotor failure, sudomotor neuropathy and sweat gland failure [9]. In a proportion of patients, lymphocytic infiltration of the eccrine glands is seen and the presence of such inflammation may explain the response to oral corticosteroids observed in this condition [10, 11].
Miliaria
Definition and nomenclature
This is a common acute or subacute skin condition that arises due to the occlusion or disruption of eccrine sweat ducts in hot humid conditions, resulting in a leakage of sweat into the epidermis (miliaria crystallina and miliaria rubra) or dermis (miliaria profunda) [1–3].
Introduction and general description
The three forms of miliaria – miliaria crystallina (sudamina), miliaria rubra (prickly heat) and miliaria profunda – occur as a result of either occlusion or disruption of the eccrine sweat ducts. They differ in clinical form due to the different levels at which occlusion occurs, although some authorities have suggested that disruption of the duct rather than occlusion is responsible [4]. In miliaria crystallina, the obstruction is very superficial, within the stratum corneum, and the vesicle is subcorneal, producing a vesicle containing clear fluid. In miliaria rubra, the later changes include keratinization of the intraepidermal part of the sweat duct, with leakage and then formation of a vesicle around the duct. In miliaria profunda, there is rupture of the duct at the level of or below the dermal–epidermal junction.
Miliaria crystallina can easily be produced experimentally by minimal, non-specific epidermal injury and profuse sweating [5]. It is often seen in febrile illnesses associated with profuse sweating. It occurs commonly in infants due to a delay in patency developing in the sweat ducts.
Miliaria rubra may be produced experimentally in susceptible subjects by epidermal injury [6]. It can be reproduced regularly by occlusion of the skin under polythene for 3–4 days, following which anhidrosis lasts for about 3 weeks. Prolonged exposure of the skin to sweat achieves the same effect. The first event may be an increase in the skin flora, perhaps with Staphylococcus epidermidis being responsible for producing an extracellular polysaccharide substance or slime that blocks the lumen of the sweat duct [1, 7]. The parakeratotic plugs, which are a notable feature of the later stages of the disease, are not the primary cause of the obstruction, but arise in the repair process, and may further aggravate the obstruction. Leakage of sweat into the epidermis is responsible for the final production of the lesions, and for their further aggravation.
Miliaria profunda is due to more severe damage to the sweat ducts, and usually follows repeated attacks of miliaria rubra. It may be reproduced by experimental injury.
Rarely, miliaria may be associated with pseudohypoaldosteronism; high sweat sodium levels produce damage of the eccrine ducts, causing lesions similar to those seen in miliaria rubra. It can be precipitated by drugs (bethanechol, isotretinoin and doxorubicin).
Epidemiology
Miliaria crystallina occurs commonly in infants due to a delay in patency developing in the sweat ducts. In a large Japanese study, it was identified in 4.5% of babies, with a peak frequency at 1 week [8]. The incidence of miliaria rubra, and particularly miliaria profunda, is highest in hot, humid conditions, but it may occur in desert regions, affecting up to 30% of people exposed to these climatic conditions. It may begin within a few days of arrival in a tropical climate, but is maximal after 2–5 months. There is a striking variation in individual susceptibility. Infants are especially prone.
Miliaria rubra is common on the trunk in hospitalized patients who have to be nursed on their backs on bedding that has waterproof occlusive membranes below the sheets. It may also commonly be seen after occlusive therapy with polythene. Outbreaks on the legs in miners working in tropical climates have been reported [9].
Clinical features
Clinical features of the three types of miliaria are as follow.
Miliaria crystallina. Clear, thin-walled vesicles, 1–2 mm in diameter without an inflammatory areola, are usually symptomless and develop in crops, mainly on the trunk. In persistent febrile illnesses, recurrent crops may occur. The vesicles soon rupture, and are followed by superficial, branny desquamation.
Miliaria rubra. Typical lesions develop on the body, especially in areas of friction with clothing, and in flexures. The lesions are uniformly minute erythematous papules, which may be present in very large numbers (Figure 94.10). Characteristically, the lesions produce intense discomfort in the form of an unbearable pricking sensation. Relief is often instantaneous when the stimulus to sweating is abolished by a cool shower. In infants, lesions commonly appear on the occluded skin of the neck, groins and axillae, but also occur elsewhere.
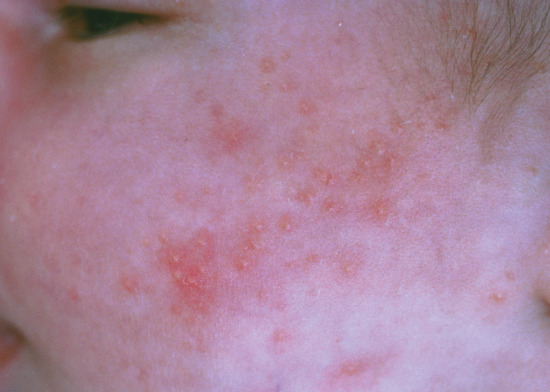
Figure 94.10 Miliaria rubra affecting the cheeks of an infant. (Courtesy of Dr Richard Logan, Bridgend, UK.)
Miliaria profunda. This nearly always follows repeated attacks of miliaria rubra, and is uncommon except in the tropics. The lesions are easily missed. The affected skin is covered with pale, firm papules 1–3 mm across, especially on the body, but sometimes also on the limbs. There is no itching or discomfort from the lesions.
Patients often wrongly refer to polymorphic light eruption as ‘prickly heat’; here the relationship of the rash to light, particularly on newly exposed sites, is usually straightforward.
Disease course and prognosis
This depends mainly on environmental factors. If continued sweating occurs, recurrent episodes lasting a few days are usual, but discomfort may be continuous. However, after a few months some degree of acclimatization occurs, and the disorder becomes less prevalent.
The most important complications of miliaria are secondary infection and disturbance of heat regulation. Secondary bacterial infection is common and sometimes serious. This may present as impetigo. In other cases, the pustules are more clearly related to sweat ducts, although in pustular miliaria factors other than bacterial infection are implicated [10]. Miliaria rubra in young infants may predispose to multiple superimposed staphylococcal abscesses [11]. In most cases of miliaria rubra the changes are reversible if further sweating is avoided, but permanent damage to the sweat duct may occur, especially after miliaria profunda.
Management
The only really effective prevention or treatment for miliaria is avoidance of further sweating. Even if this is achieved only for a few hours a day, as in an air-conditioned office or bedroom, considerable relief is experienced. For the very susceptible person, a move away from tropical climates may be essential. Avoidance of excessive clothing, friction from clothing, excessive use of soap and contact of the skin with irritants will reduce the incidence. The large number of treatments advocated for prickly heat is the best indication of their relative ineffectiveness if sweating is not reduced. In the absence of gross secondary sepsis, the effect of topical or systemic antibiotics or other antibacterial preparations on established miliaria is disappointing, but they may have some role in prophylaxis [1]. Oral ascorbic acid 500 mg twice daily was found to diminish the severity of miliaria, as was the degree of subsequent anhidrosis in experimentally induced disease [12]. Calamine lotion is probably as effective as anything for the relief of discomfort, but because of its drying effect, a bland emollient (e.g. oily cream or menthol in aqueous cream) may subsequently be required to prevent further epidermal damage. Isotretinoin was reported to help a recalcitrant case of miliaria profunda [13].
Neutrophilic eccrine hidradenitis
Definition and nomenclature
Neutrophilic eccrine hidradenitis refers to a rare clinical condition with non-specific features but characteristic acute inflammation of the eccrine sweat glands, seen on skin biopsy.
Introduction and general description
Neutrophilic eccrine hidradenitis (NEH) may arise in a variety of very difficult clinical situations, producing an eruption with very distinct pathological features. It can be classified into the following types:
- Chemotherapy-induced neutrophilic eccrine hidradenitis.
- Infectious neutrophilic eccrine hidradenitis.
- Palmoplantar neutrophilic eccrine hidradenitis.
- Neutrophilic eccrine hidradenitis with HIV infection .
- Neutrophilic eccrine hidradenitis with Behçet disease.
Epidemiology
This is a rare condition that may be considered a reaction pattern to a variety of stimuli [1, 2, 3]. Drug-induced NEH is primarily seen in patients receiving cytotoxic chemotherapy, whilst childhood NEH affects otherwise well children [4, 5]. Attacks of childhood NEH are reported to be more frequent in the spring and summer. Disease-associated NEH and infectious NEH are both very rare.
Pathophysiology
The key pathological feature of NEH is necrosis of the eccrine epithelium in association with a dense neutrophilic infiltrate. In drug-induced NEH this is most commonly reported with the use of cytotoxic chemotherapeutic agents. Typical histological changes can be induced experimentally by the local injection of bleomycin into human skin, suggesting that the eccrine glands are subject to direct toxicity [6]. Other drugs reported in association with NEH include carbamazepine, tumour necrosis factor α antagonists and cetuximab [7, 8, 9].
Childhood NEH is not associated with underlying disease. However, it may follow physical exertion and exposure to damp footwear [10].
Infectious NEH is most frequently encountered in immunosuppressed individuals. Causative organisms implicated include Serratia, staphylococci, streptococci and Nocardia [11, 12, 13]. NEH has also been reported in HIV infection, Behçet disease and as a paraneoplastic phenomenon in both haematological and solid organ malignancies [12, 13, 14, 15].
Clinical features
Drug-associated NEH typically occurs 8–10 days after starting chemotherapy. Painful erythematous papules and plaques develop on the limbs, neck and face [2]. Facial erythema and swelling may be severe enough to mimic cellulitis [16]. The condition typically resolves within 2 weeks of treatment ending. Recurrence, however, may occur with subsequent courses of chemotherapy [17].
Childhood NEH has a particular predilection for the soles and less frequently the palms. Typically, tender plaques and nodules are seen. Attacks resolve spontaneously in 3 weeks but the condition may be recurrent [4, 5, 18].
Investigations
In drug-induced NEH skin biopsy is diagnostic. Associated neutropenia is also common. Further investigations may be necessary to exclude sepsis. Investigation of childhood NEH is not ususally necessary.
Management
In the majority of cases the condition resolves without any treatment. In adult NEH systemic corticosteroids, dapsone and colchicine have all been recommended [15, 19]. Dapsone may also be helpful in preventing recurrent disease [20].
Eccrine syringosquamous metaplasia
Definition
Eccrine syringosquamous metaplasia is used to describe both histological change, seen within eccrine sweat glands, and also a distinct skin eruption associated with the use of chemotherapeutic agents [1].
Pathophysiology
Predisposing factors
Eccrine syringosquamous metaplasia is characterized by the transformation of cuboidal ductal epithelial cells into areas of squamous differentiation [2]. This process has been reported in a wide variety of different settings including pyoderma gangrenosum, panniculitis and infection [3, 4]. It is considered a non-specific marker of eccrine duct damage and may be confused histologically with squamous cell carcinoma. Similar histological changes are seen in patients undergoing chemotherapy, presumably forming part of a spectrum of cytotoxic eccrine damage, which includes neutrophilic eccrine hidradenitis [5].
Clinical features
In patients undergoing chemotherapy, eccrine syringosquamous metaplasia has been observed following the use of a range of drugs, most notably cytarabine and protein kinase inhibitors. The eruption develops during or shortly after chemotherapy and slowly resolves spontaneously. Widespread papulovesicular lesions, acral erythema and an intertriginous eruption may all be seen.
Investigations
Skin biopsy is usually diagnostic.
Management
The condition resolves spontaneously but symptom control may be required.
Drugs and eccrine glands
A number of drugs are concentrated and secreted by eccrine glands. This may partially account both for their therapeutic effect and cutaneous toxicity. Drugs known to be secreted include sulfaguanidine, sulfadiazine, amphetamines, arsenicals, iodides, phenytoin, phenobarbitone, carbamazepine, griseofulvin, ketoconazole, fluconazole, ciprofloxacin, diamorphine, cocaine and nicotine [1].
Sweat testing may be used as an alternative to urine testing in the setting of substance abuse [2].
Disorders with sweat gland cellular inclusions
The accumulation of substances within eccrine secretory cells occurs in a number of metabolic conditions summarized in Table 94.1 [1–6].
Table 94.1 Disorders with characteristic eccrine gland inclusion.
| Microscopic changes | Disorder |
| Membrane-bound vacuoles in secretory cells | Mucopolysaccharidoses (Hurler, Hunter and Sanfillipo types) |
| Intracytoplasmic lipid inclusions | Sphingolipidoses |
| Secretory cell inclusions | Fabry disease, fucosidosis, Kanzaki disease, adrenoleukodystrophy and maltase deficiency |
| PAS-positive granules in outer duct cells | Lafora disease |
PAS, periodic acid–Schiff stain.
APOCRINE GLANDS
Anatomy and physiology of apocrine glands
Apocrine sweat glands derive their name from the way their secretion appears, on light microscopy, to be derived by pinching off parts of the cytoplasm (from the Greek apo- ‘away’ and krinein ‘to separate’). They are epidermal appendages, and develop as part of the pilosebaceous follicle in the fourth to fifth month of intrauterine life. In the embryo they are present over the entire skin surface, but most glands subsequently disappear, so that in the adult the characteristic distribution in the axillae, perianal region and areolae of the breasts is found [1–3, 4]. So-called ectopic glands may be found elsewhere. The mammary glands and ceruminous glands in the external auditory meati are modified apocrine glands. Apocrine glands are poorly developed in childhood, and begin to enlarge with the approach of puberty. The activity of the glands is androgen dependent, and the glands show marked testosterone 5α-reductase activity [5].
The glands are larger than eccrine glands, and in the dissected specimen are visible to the naked eye. They are situated in the subcutaneous tissue. Each consists of a tubule and a duct. The latter is often quite short, and opens into the neck of the hair follicle above the sebaceous gland. Despite their embryological origin from the hair follicle, some apocrine glands eventually come to open on the surface of the skin. The secretory coil is a simple convoluted tube. It is lined by a single layer of columnar or cuboidal cells resting on a basement membrane. The free edge of the cells may show the appearance of apocrine secretion (Figure 94.11). Electron microscopy shows that this may be partly an artefact, but eccrine, apocrine and even holocrine secretion may all be found in places [3, 6, 7]. The apocrine duct closely resembles the eccrine duct, and consists of a double layer of cuboidal cells. Outside the basement membrane of the gland and duct is a longitudinal layer of myoepithelial cells. Their function is to support the duct and to propel the secretion to the surface, and waves of peristalsis have been seen in them [8]. Where the duct opens into the neck of the hair follicle there is the equivalent of the acrosyringium of the eccrine duct, although it is less obvious [9]. Apocrine sweat glands have an adrenergic sympathetic supply but neural control appears to be unimportant [10].
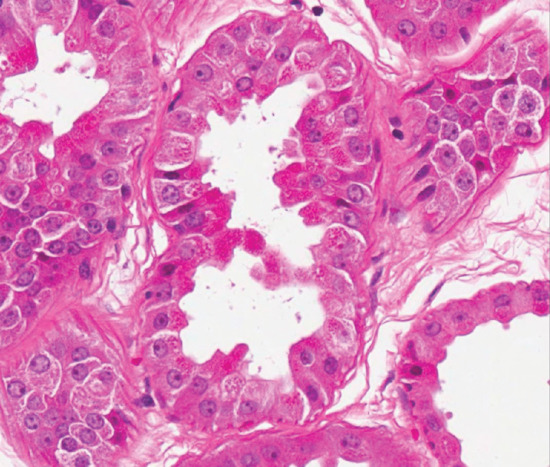
Figure 94.11 Normal apocrine glands lined by cells with abundant eosinophilic cytoplasm and decapitation secretions. Magnification 40× (H&E). (Courtesy of Dr Arti Bakshi, Royal Liverpool University Hospital, Liverpool, UK.)
Apocrine glands secrete small quantities of an oily fluid. This secretion is odourless on reaching the surface of the skin. Bacterial decomposition is responsible for the production of odiferous compounds. In particular, corynebacterial aminoacylase is responsible for the production of 3-methyl-2-hexenoic and 3-hydroxy-3-methylhexenoic acids [11]. Smaller quantities of odiferous sulphanylalkanols and steroids have also been identified [12, 13]. The production of these metabolites is under the genetic control of the ABCC11 gene, which encodes an ATP-driven efflux pump. Individuals homozygotic for a mutation in this gene produce much less odour. Mutation in this gene is predominant in South-East Asians, explaining racial variations in body odour [14].
There is evidence that products of apocrine sweat glands may act as human pheromones but the specific chemicals and pathways involved have not yet been elucidated [15].
DISORDERS OF APOCRINE SWEAT GLANDS
Abnormal sweat odour (bromhidrosis)
Definition and nomenclature
Human skin odour is a matter of concern both to those anxious about emitting it and to those who have to endure it when it emanates from others. It is largely due to the production of volatile chemicals by the actions of bacteria on secreted apocrine sweat.
Epidemiology
Human skin odour is largely determined by alteration or degradation of odourless substances secreted by apocrine glands by bacteria, especially Corynebacterium species, in the presence of hyperhidrosis [1]. Eccrine secretion is usually odourless but can contain odour-producing substances that are excreted in it, such as drugs, arsenic and garlic. Sebaceous secretion has some odour, as do the decomposition products of keratin, which can cause malodour in some hyperkeratanizing disorders such as keratodermas and Darier disease. On occasion, malodour appreciated only by the patient may be a symptom of monosymptomatic delusion of malodour (olfactory reference disorder) which requires psychiatric intervention [2], or from an organic lesion of the central nervous system.
Incidence
There is marked individual and racial variation in body odour, and what is socially acceptable varies greatly with race and social upbringing.
Age
As apocrine secretion increases under androgen control, malodour usually developes at or after puberty. Cases of generalized bromhidrosis in children have been reported due to chronic retention of nasal foreign bodies, and as a result of precocious puberty.
Sex
Malodour is more evident in men, but men and women seek medical management with equal frequency [3].
Ethnicity
There is anecdotal evidence that axillary malodour is more prevalent in European and African individuals, and less so in East Asians, Chinese and Koreans.
Associated diseases
Recent studies have associated axillary malodour with wet-type ear wax, and this is the result of a single nucleotide polymorphism in the ABCC11 gene [4]. This gene is responsible for the function of the apical efflux pump and the conjugation of some of the odour-producing thioalcohols. Dry ear wax individuals do not produce these substances.
Pathophysiology
In the axillae, high humidity due to eccrine sweating and sebaceous secretion results in a rich microflora, including bacteria of the genera Staphylococcus, Micrococcus, Corynebacterium and Propionobacterium. An increased axillary pH may facilitate the overgrowth of these bacteria. Apocrine secretions are largely odourless, but biotransformation by bacteria, particularly corynebacteria, results in the liberation of short- and medium-chain volatile fatty acids (C2 to C10 branch length), 16-androstene steroids and thioalcohols, each of which may produce its own odour signature [1]. Studies have demonstrated histological differences between normal and bromhidrotic apocrine glands; in the bromhidrotics, the apocrine glands were larger and more numerous [5].
Clinical features
Presentation may be due to the patient being conscious of emitting odour, or as the result of comments from friends and relatives. Sniff testing may result in the appreciation of the character of the malodour, such as ‘onion-like and beefy’ or a ‘lighter fruity’ note.
Management
The treatment of axillary bromhidrosis includes the omission of foodstuffs such as garlic from the diet, frequent washing of the axillary regions and local antibacterial substances. There is no evidence that measures used to control axillary eccrine hyperhidrosis – for example, aluminium salts and anticholinergic drugs – have much effect on the apocrine glands, although excessive eccrine excretion may favour the spread of apocrine secretion and facilitate proliferation of the odour-producing microflora.
Deodorants are the mainstay of therapy as the fragrances disguise the undesired odour. A topical glycine-soya sterocomplex agent has shown encouraging improvement on both the intensity and quality of odour in patients with bromhidrosis [6]. A silver-zeolite powder has been shown to have strong antibacterial effects on axillary microflora and to diminish axillary malodour [7]. Botulinum toxin A has been used to treat axillary [8] and genital malodour [9] with good effect: the impact on eccrine sweating and rendering the area anhidrotic may prevent bacterial activity and thus have an effect on odour. Surgical excision of axillary subcutaneous tissue by a variety of surgical techniques (axillary shave and subsection of subcutaneous glands, laser ablation, ultrasound ablation, intradermal alcohol injection and liposuction), which removes both eccrine and apocrine glands, has been performed with good effect in those dissatisfied with conservative measures [10, 11, 12].
Trimethylaminuria
Definition and nomenclature
This disorder results from excessive amounts of the offensively smelling tertiary amine trimethylamine appearing in eccrine and apocrine sweat, breath and urine, and imparting an unpleasant rotting fish smell to sufferers [1].
Epidemiology
Incidence
The ability to N-oxidize trimethylamine into trimethylamine oxide (which has no odour) is distributed polymorphically, and sufferers are homozygous for an allele that determines this impaired reaction. One per cent of the population are heterozygous carriers of the allele.
Age
Most cases present in their late teens or early twenties.
Sex
The incidence does not vary by gender.
Pathophysiology
Affected individuals are unable to oxidize trimethylamine, which is produced by the intestinal bacterial degradation of choline and carnitine in food, to the odourless trimethylamine N-oxide. This can occur as a primary problem, as a result of a mutation in the flavin-containing mono-oxygenase3 (FMO3) gene [2]. Secondary trimethylaminuria can occur when there is an increased burden of trimethylamine, and is seen when there is an increased production of it from its precursors by gut bacteria in conditions such as blind loop syndrome, uraemia and liver disease.
Clinical features
The unpleasant odour, which is often worse after eating seafood, during periods of stress or during menstruation, can be the source of much distress, rejection and resentment. Sufferers are sometimes unaware of their smell, which may be intermittent and may not be detected by physicians when consulted. Trimethylaminuria was found in 7% of a series of individuals who perceived themselves to be malodorous [3].
Investigations
The condition can be diagnosed by direct estimation of trimethylamine in the urine after a marine fish meal. Both affected individuals and heterozygous carriers have abnormally elevated excretion of trimethylamine after such an oral challenge.
Management
A diet low in carnitine and choline may help. Egg yolks, legumes, red meats, fish and beans should be avoided. Short courses of metronidazole or neomycin may temporarily reduce the bacteria that degrade the carnitine and choline in the gut. Charcoal and copper chlorophyllin have been shown to reduce urinary trimethylamine concentrations to normal levels in sufferers [4].
Chromhidrosis
Definition
Chromhidrosis is the secretion of vividly coloured apocrine sweat [1, 2]. It is most commonly a blue, yellow or green colour and is usually of apocrine origin, and seen in the axilla, areola of the nipple or face.
Clinical features
Apocrine sweat may be tinged with a yellow, green or blue hue in up to 10% of the population. Only rarely does it occur to the striking degree that merits the term chromhidrosis. It results from the secretion of lipofuscins in apocrine sweat, and may be associated with the secretion of coloured breast milk. The more oxidized lipofuscins appear deeper in colour; the lighter-coloured pigments may fluoresce. The diagnosis can be confirmed by finding lipofuscin pigment granules that may fluoresce on fluorescence microscopy in the apocrine secretory cells. Affected individuals’ clothes may also fluoresce on Wood's light illumination. The secretion of coloured sweat starts at puberty and persists until there is a gradual regression of apocrine function in old age. Coloured sweat may be discharged from the glands in response to exercise and emotional stimuli, and after manipulation of the skin. The axillae are the most frequently affected sites, although facial [3] and areolar [4] chromhidrosis are recorded.
Management
Pseudochromohidrosis
Pseudochromohidrosis is the secretion of clear sweat that changes to a coloured secretion after it exits the sweat duct. It may occur due to chromogenic or porphyrin-producing bacteria on the skin [7]. A case of facial red chromhidrosis in a child responded to erythromycin, the antibiotic eradicating a chromogenic bacterium which was felt to be the cause of the abnormal colour [8].
Exogenous chromogens can affect eccrine sweat. Occupational exposure to copper salts has been reported to produce blue eccrine sweat; excessive consumption of a red food dye resulted in red sweat staining of underwear in one reported case [9].
Alkaptonuria (ochronosis) may result in dark perspiration.
Apocrine miliaria
Definition and nomenclature
Apocrine miliaria is a disorder of the apocrine glands comparable to prickly heat of the eccrine glands, and caused by obliteration of the apocrine duct at the infundibulum [1]. It usually presents with an itchy papular eruption in the axillae, ano-genital area or on the areolae of the nipple.
Epidemiology
Incidence and prevalence
It is uncommon.
Sex
It predominates in females. Familial twin and male cases are reported [2].
Pathophysiology
The condition occurs as a result of apocrine sweat duct occlusion by aggregates of epithelial cells of the apocrine or apoeccrine secretory cells [3]. The earliest pathological sign is a small vesicle in the apocrine duct. Later, the apocrine glands are seen to be enlarged, and as a consequence of repeated inflammatory events, perifollicular xanthomatosis with perifollicular foam cells expressing CD68 may develop [4].
Clinical features
The disease occurs mainly in women soon after puberty, but can be postmenopausal [1]. It can occur in males or in children, and has been reported in females with Turner syndrome and in identical twins. In recent years it has been reported after laser axillary hair epilation [5]. Itching, which may be intense, occurs in the axillae, and to a lesser extent in the ano-genital region and around the breasts. Objectively there may be little to see at first, but later skin-coloured or slightly pigmented, dome-shaped, follicular papules develop (Figure 94.12). Hair loss in the axillae usually ensues. The itching is often provoked by those emotional stimuli that normally cause apocrine secretion. The disease runs a very prolonged course, and may persist until the menopause. Some remission may occur in pregnancy.
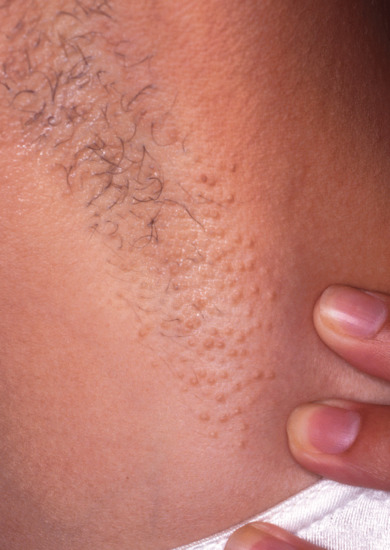
Figure 94.12 Axillary apocrine miliaria (Fox–Fordyce disease).
Differential diagnosis
It is distinctive so is usually easy to diagnose.
Management
Response to treatment is unsatisfactory. Topical and intralesional steroids provide some benefit, but their use is limited by atrophy. Topical clindamycin is reported to have been of help [6]. Treatment with 4–6-weekly doses of ultraviolet radiation, sufficient to cause exfoliation, helps some patients [7]. Topical retinoic acid may also be helpful, as may oral contraceptive agents and oral retinoids [8]. Other cases are sufficiently severe to require electrocautery [9], surgical excision of the affected skin or subcutaneous removal of the apocrine glands [10].
References
Anatomy and physiology of eccrine glands
- Montagna W, Parakkal PF. The Structure and Function of Skin, 3rd edn. London: Academic Press, 1974.
- Rothman S. Physiology and Biochemistry of the Skin. Chicago: University of Chicago Press, 1954.
- Sato K. The physiology and pharmacology of the eccrine sweat gland. In: Goldsmith LA, ed. Biochemistry and Physiology of the Skin. Oxford: Oxford University Press, 1983.
- Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. J Am Acad Dermatol 1989;20:537–63, 713–26.
- Szabo G. The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Philos Trans R Soc Lond Biol 1967;252:447–85.
- Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol 1987;252:R166–80, 181–7.
- Breathnach AS. An Atlas of the Ultrastructure of Human Skin. London: Churchill Livingstone, 1971.
- Ncjsum LN, Kwon TH, Jensen UB, et al. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci USA 2002;99:511–56.
- Kabashima K, Simauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol 2008;59:S28–32.
- Nakahigashi K, Namura T, Miyachi Y, et al. Normal immunostaining for aquaporin-5 in the lesions of palmoplantar hyperhidrosis. Case Rep Dermatol 2013;5:61–3.
- Cotton DWK. Immunohistochemical staining of normal sweat glands. Br J Dermatol 1986;113:441–9.
- Thiele FAJ, Mier PD, Reay DA. Heat transfer across the skin. The role of the ‘resting’ sweat gland. Thermography, Proceedings of the 1st European Congress, Amsterdam. Bibl Radiol 1975;6:140–3.
- Sulzberger MB, Hermann F. The Clinical Significance of Disturbances in the Delivery of Sweat. Springfield, IL: Thomas, 1954.
- Collins KJ. Measurement of sweating and sweat gland function. In: Greaves MW, Shuster S, eds. The Pharmacology of the Skin. Berlin: Springer-Verlag, 1989.
- Schulz IJ. Micropuncture studies of the sweat formation in cystic fibrosis patients. J Clin Invest 1969;48:1470–7.
- Montagna W, Ellis RA, Silver AF. Advances in Biology of Skin, Vol. 3. Eccrine Sweat Glands and Eccrine Sweating. Oxford: Pergamon, 1962.
- Christie MJ. Electrodermal activity in the 1980s: a review. J R Soc Med 1981;74:616–17.
- Harris DR, Polk BF, Willis I. Evaluating sweat gland activity with imprint techniques. J Invest Dermatol 1972;58:78–84.
- Muller SA, Kierland RR. The use of a modified starch-iodine test for investigating local sweating responses to intradermal injection of methacholine. J Invest Dermatol 1959;32:126–8.
- Guttman I. A demonstration of the study of sweat secretion by the quinizarin method. Proc R Soc Med 1941;35:77–89.
- Sato KT, Richardson A, Sato K. One step iodine starch method for direct investigation of sweating. Am J Med Sci 1988;295:528–31.
- Kealey T, Philpott MP. Human pilosebaceous culture: the background. In: Leigh I, Lane B, Watt F, eds. The Keratinocyte Handbook. Cambridge: Cambridge University Press, 1994:109–29.
- Kealey T, Philpott M, Guy R, Dove N. Skin gland and appendage epithelial cells. In: Harris A, ed. Practical Cell Biology Series. Cambridge: Cambridge University Press, 1996:147–77.
- Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans. Front Biosci (Schol Ed) 2010;2:685–96.
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104.
- Shibasaki M, Wilson TE, Crandall G. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol 2006;100:1692–701.
- Fortney SM, Wenger CB, Bove JR, et al. Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol 1984;57:1688–95.
- Fortney SM, Nadel ER, Wenger CB, et al. Effect of blood volume on sweating rate and body fluids in exercising humans. J Appl Physiol 1981;51:1594–600.
- Farrell MJ, Trevaks D, Taylor NA, et al. Brain stem representation of thermal and psychogenic sweating in humans. Am J Physiol Integr Comp Physiol 2013;304:R810–17.
- Uno H. Sympathetic innervation of the sweat glands and piloarrector muscles of macaques and human beings. J Invest Dermatol 1977;69:112–20.
- Randall WC, Kimura KK. The pharmacology of sweating. Pharmacol Rev 1955;7:365–97.
- Warndorff JA, Hamer M. The response of the sweat glands to alpha-adrenergic stimulation with isoprenaline. Br J Dermatol 1974;90:263–8.
- Wolf JE, Maibach HI. Palmar eccrine sweating – the role of adrenergic and cholinergic mediators. Br J Dermatol 1974;91:439–46.
- Choudry R, Hodgins MB, Van der Kwast TH, et al. Localization of androgen receptors in human skin by immunohistochemistry. J Endocrinol 1992;133:467–75.
- Le Grys VA, Yankaskas JR, Quittell LM, et al. Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr 2007;151:85–9.
- Rowe SM, Clancy JP. Advances in cystic fibrosis therapies. Curr Opin Pediatr 2006;18:604–13.
- Takemura T, Hibinoo T, Sato K. Urokinase-type plasminogen activator in human eccrine sweat. Br J Dermatol 1993;128:178–83.
Disorders of eccrine sweat glands
Hyperhidrosis
- Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. J Am Acad Dermatol 1989;20:537–63, 713–26.
- Stefaniak TJ, Proczko M. Gravimetry in sweating assessment in primary hyperhidrosis and healthy individuals. Clin Auton Res 2013;23:197–200.
- Hahn AF, Waaler PE, Kvistad PH, Bamforth JS, et al. Cold-induced sweating syndrome: CISS1 and CISS2: manifestations from infancy to adulthood. Four new cases. J Neurol Sci 2010;15(293):68–75.
- Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol 2012;26:1–8.
- Iseri PK, Bayramgurler D, Koc K. Unilateral localized hyperhidrosis associated with frontal lobe meningioma. Neurology 2004;63:1753–4.
- Chatterjee S, Ghosh K, Banerjee T. An intramedullary tumor presenting with hyperhidrosis. Neurol India 2004;52:39.
- Korr IM. Skin resistance patterns associated with visceral disease. Fed Proc Am Soc Exp Biol 1949;8:87–8.
- Schliack H, Schiffter R. JadassohnsHandbuch der Haut und Geschlechts-krankheiten. Berlin: Springer, 1979.
- McCoy BP. Apical pulmonary adenocarcinoma with contralateral hyperhidrosis. Arch Dermatol 1981;117:659–61.
- Dua J, Grabczynska S. Eccrine nevus affecting the forearm of an 11-year-old girl successfully controlled with topical glycopyrrolate. Pediatr Dermatol 2013;28:10.
- Salama H, Shwayder T. Eccrine nevus presenting as a hypopigmented patch. Pediatr Dermatol 2008;25:613–15.
- Srinivas CR, Rao PLNG. Sudoriparous angioma: regression following intravascular aethoxysclerol, a sclerosing agent. Br J Dermatol 1988;119:111–13.
- Lapiere S. A propos d'une observation de naevus sudoripare avec hyperidrose. Dermatologica 1957 ;115 :293–7.
- Mellinkoff SM. Localized paroxysmal hyperhidrosis. Am J Med Sci 1951;221:86–8.
- Champion RH, Herxheimer A. Unilateral hyperhidrosis of the trunk. Acta Med Scand 1960;168:17–20.
Cold-induced sweating syndrome
- Hahn AF, Waaler PE, Kvistad PH, et al. Cold-induced sweating syndrome: CISS1 and CISS2: manifestations from infancy to adulthood. Four new cases. J Neurol Sci 2010;15(293):68–75.
Gustatory hyperhidrosis
- Lee TS. Physiological gustation sweating in a warm climate. J Physiol 1954;124:528–42.
- Mellinkoff SM, Mellinkoff MJ. Gustatory hyperhidrosis of the left knee. JAMA 1950;142:901–2.
- Drummond PD. Mechanism of gustatory flushing in Frey's syndrome. Clin Auton Res 2002;12:144–6.
- Kurchin A, Adar R, Zweig A, Mozes M. Gustatory phenomena after upper dorsal sympathectomy. Arch Neurol 1977;34:619–23.
- Bloor K. Gustatory sweating and other responses after cervicothoracic sympathectomy. Brain 1969;92:137–46.
- McGibbon BM, Paletta FX. Further concepts in gustatory sweating. Plast Reconstr Surg 1972;49:639–42.
- Restivo DA, Lanza S, Patti F, et al. Improvement of diabetic autonomic gustatory sweating by botulinum toxin type A. Neurology 2002;59:1971–3.
- Drummond PD, Boyce GM, Lance JW. Postherpetic gustatory flushing and sweating. Ann Neurol 1987;21:559.
- Eedy DJ, Corbett JR. Olfactory facial hyperhidrosis responding to amitriptyline. Clin Exp Dermatol 1987;12:298–9.
- Black MJM, Gunn A. The management of Frey's syndrome with aluminium chloride hexahydrate antiperspirant. Ann R Coll Surg Engl 1990;72:49–52.
- Shaw JE, Abbott CA, Tindle K, et al. A randomised controlled trial of topical glycopyrrolate, the first specific treatment for diabetic gustatory sweating. Diabetologia 1997;40:299–301.
Management of hyperhidrosis
- McMillan FSK, Reller HH, Snyder FH. Antiperspirant action of topically applied anticholinergics. J Invest Dermatol 1964;43:363–7.
- Grice KA, Bettley FR. Inhibition of sweating by poldine methosulphate (Nacton). Br J Dermatol 1966;78:458–64.
- Mackenzie A, Burns C, Kavanagh G. Topical glycopyrrolate for axillary hyperhidrosis. Br J Dermatol 2013;169:483–4.
- Juhlin L, Hansson H. Topical glutaraldehyde for plantar hyperhidrosis. Arch Dermatol 1968;97:327–30.
- Jass HE. Rationale of formulations of deodorants and antiperspirants. In: Frost P, Horwitz SN, eds. Principles of Cosmetics for the Dermatologist. St Louis: Mosby, 1982:98–104.
- Anon. Aluminium chloride for hyperhidrosis. Drug Ther Bull 1981;19:101–2.
- Shelley WB, Hurley HJ. Studies on topical antiperspirant control of axillary hyperhidrosis. Acta Derm Venereol (Stockh) 1975;95:241–60.
- McWilliams SA, Montgomery I, Jenkinson DM, et al. Effects of topically applied antiperspirant on sweat gland function. Br J Dermatol 1987;117:617–26.
- Levit F. Simple device for treatment of hyperhidrosis by iontophoresis. Arch Dermatol 1968;98:505–7.
- Abell E, Morgan K. The treatment of idiopathic hyperhidrosis with glycopyrronium bromide and tapwateriontophoresis. Br J Dermatol 1974;91:87–91.
- Hölzle E, Alberta N. Long-term efficacy and side-effects of tap water iontophoresis of palmo-plantar hyperhidrosis: the usefulness of home therapy. Dermatologica 1987;175:126–35.
- Stolman LP. Treatment of excessive sweating of the palms by iontophoresis. Arch Dermatol 1987;123:895–6.
- Reinauer S, Neusser A, Schauf G, Holzle E. Iontophoresis with alternating current and direct current offset (AC/DC iontophoresis): a new approach for the treatment of hyperhidrosis. Br J Dermatol 1993;129:166–9.
- Akins DL, Meisenheimer JL, Dobson RL. Efficacy of the Drionic unit in the treatment of hyperhidrosis. J Am Acad Dermatol 1987;16:828–32.
- Heckmann M, Ceballos-Baumann AO, Plewig G. Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med 2001;344:488–93.
- Kinkelin I, Hund M, Naumann M, Hamm H. Effective treatment of frontal hyperhidrosis with botulinum toxin A. Br J Dermatol 2000;143:824–7.
- Schnider P, Moraru E, Kittler H, et al. Treatment of focal hyperhidrosis with botulinumtoxin type A: long-term follow-up in 61 patients. Br J Dermatol 2001;145:289–93.
- Swartling C, Farnstrand C, Abt G, et al. Side-effects of intradermal injections of botulinumA toxin in the treatment of palmar hyperhidrosis: a neurophysiological study. Eur J Neurol 2001:8;451–6.
- Boger A, Herath H, Rompel R, Ferbert A. Botulinum toxin for treatment of craniofacial hyperhidrosis. J Neurol 2000;247:857–61.
- Canaday BR, Stanford RH. Propantheline bromide in the management of hyperhidrosis associated with spinal cord injury. Ann Pharmacother 1995;29:489–92.
- Müller C, Berensmeier A, Hamm H, Dirschka T, et al. Efficacy and safety of methantheline bromide (Vagantin®) in axillary and palmar hyperhidrosis: results from a multicenter, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol 2012;2:1468–3083.
- Tupker RA, Harmsze AM, Deneer VH. Oxybutynin therapy for generalized hyperhidrosis. Arch Dermatol 2006;142:1065–6.
- Walling HW. Systemic therapy for primary hyperhidrosis: a retrospective study of 59 patients treated with glycopyrrolate or clonidine. J Am Acad Dermatol 2012;66:387–92.
- James WD, Schoomaker EB, Rodman OG. Emotional eccrine sweating. A heritable disorder. Arch Dermatol 1987;23:925–9.
- Takase Y, Tsushimi K, Yamamoto K, et al. Unilateral localized hyperhidrosis responding to treatment with clonazepam. Br J Dermatol 1992;126:416.
- Gjerris F, Oleson HP. Palmar hyperhidrosis: long-term results following high thoracic sympathectomy. Acta Neurol Scand 1975;51:167–72.
- Drott C, Gothberg G, Claes G. Endoscopic transthoracic sympathectomy: an efficient and safe method for the treatment of hyperhidrosis. J Am Acad Dermatol 1995;33:78–81.
- Orteu CH, McGregor JM, Almeyda JR, Rustin MHA. Recurrence of hyperhidrosis after endoscopic transthoracic sympathectomy: case report and review of the literature. Clin Exp Dermatol 1995;20:230–3
- Lin CC, Mo LR, Lee LS, et al. Thoracoscopic T2-sympathetic block by clipping: a better and reversible operation for treatment of hyperhidrosis palmaris: experience with 326 cases. Eur J Surg 1998;580(Suppl.):13–18.
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642–8.
- De Andrade Filho LO, Kuzniec S, Wolosker N, et al. Technical difficulties and complications of sympathectomy in the treatment of hyperhidrosis: an analysis of 1731 cases. Ann Vasc Surg 2013;27:447–53.
- Imhof M, Zacherl J, Plas EG, et al. Long-term results of 45 thoracoscopic sympathectomies for primary hyperhidrosis in children. J Pediatr Surg 1999;34:1839–42.
- Lin CC, Mo LR, Lee LS, et al. Thoracoscopic T2-sympathetic block by clipping: a better and reversible operation for treatment of hyperhidrosis palmaris: experience with 326 cases. Eur J Surg 1998;580(Suppl.):13–18.
- Lin TS, Fang HY. Transthoracic endoscopic sympathectomy for craniofacial hyperhidrosis: analysis of 46 cases. J Laparoendosc Adv Surg Tech 2000;10:243–7.
- Reisfeld R. Endoscopic lumbar sympathectomy for focal plantar hyperhidrosis using the clamping method. Surg Laparosc Endosc Percutan Tech 2010;20:231–6.
- Hurley HJ, Shelley WB. Axillary hyperhidrosis. Br J Dermatol 1966;78:127–40.
- Munro DD, Verbov J, Gorman DJ. Axillary hyperhidrosis. Br J Dermatol 1974;90:325–9.
- Rompel R, Scholz S. Subcutaneous curettage vs. injection of botulinum toxin A for treatment of axillary hyperhidrosis. J Eur Acad Dermatol Venereol 2001;15:207–11.
- Lawrence CM, Lonsdale Eccles AA. Selective sweat gland removal with minimal skin excision in the treatment of axillary hyperhidrosis: a retrospective clinical and histological review of 15 patients. Br J Dermatol 2006;155:115–18.
- Swinehart JM. Treatment of axillary hyperhidrosis: combination of the starch-iodine test with the tumescent liposuction technique. Dermatol Surg 2000;26:392–6.
- Hong HC, Lupin M, O'Shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg 2012;38:728–35.
Granulosis rubra nasi
- Aram H, Mohaghedi AP. Granulosis rubra nasi. Cutis 1972;10:463.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J 1937;2:1068.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J 2013;4:208–9.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and phaeochromocytoma. Ann Dermatol Venereol 1996;123:106–8.
- Grazziotin TC, Buffon RB, da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin type A. Dermatol Surg 2009;35:1298–9.
Anhidrosis and hypohidrosis, Ross syndrome, Acquired idiopathic generalized anhidrosis
- Shelley WB, Horvath PN, Pilsbury DM. Anhidrosis. Medicine 1950;29:194–224.
- Low PA, Tomalia VA, Park KJ. Autonomic function tests: some clinical applications. J Clin Neurol 2013;9:1–8.
- Ross AT. Progressive selective sudomotor denervation; a case with coexisting Adie's syndrome. Neurology 1958;8:809–17.
- Sommer C. Selective degeneration of sudomotor fibers in Ross syndrome and successful treatment of compensatory hyperhidrosis with botulinum toxin. Muscle Nerve 1998;21:1790–3.
- Nolano M, Provitera V, Perretti A, et al. Ross syndrome: a rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain 2006;129:2119–31.
- Druschky K, Hilz MJ, Koelsch C, et al. Cardiac sympathetic denervation in Ross syndrome demonstrated by MIBG-SPECT. J Auton Nerv Syst 1999;28:184–7.
- Reinauer S, Schauf G, Holzle E. Ross syndrome: treatment of segmental compensatory hyperhidrosis with a modified iontophoretic device. J Am Acad Dermatol 1993;28:308–11.
- Murakami K, Sobue G, Terao S, et al. Acquired idiopathic generalized anhidrosis: a distinctive clinical syndrome. J Neurol 1988;235;428–31.
- Chen YC, Wu CS, Chen GS, et al. Identification of subgroups of acquired idiopathic generalized anhidrosis. Neurologist 2008;14:318–20.
- Ando Y, Fujii S, Sakashita N, et al. Acquired idiopathic generalized anhidrosis; clinical manifestations and histochemical studies. J Neurol Sci 1995;132:80–3.
- Palm F, Löser C, Gronau W, et al. Successful treatment of acquired idiopathic generalized anhidrosis. Neurology 2007;68:532–3.
Miliaria
- Holzle E, Kligman AM. The pathogenesis of miliaria rubra. Br J Dermatol 1978;99:117–37.
- Leithead CS, Lind AR. Heat Stress and Heat Disorders. London: Cassell, 1964.
- Sargent F, Slutsky HL. The natural history of the eccrine miliarias. N Engl J Med 1957;256:401–8, 451.
- Shuster S. Duct disruption, a new explanation of miliaria. Acta Derm Venereol (Stockh) 1997;77:1–3.
- Shelley WB, Horvath PN. Experimental miliaria in man, 3. J Invest Dermatol 1950;14:193–204.
- Lyons RE, Levine R, Auld D. Miliaria rubra. Arch Dermatol 1962;86:282–6.
- Mowad CM, McGinley KJ, Foglia A, Leyden JJ. A role of extracellular polysaccharide substance produced by Staphylococcus epidermidis in miliaria. J Am Acad Dermatol 1995;33:729–33.
- Hidano A, Purwoko R, Jutsukawa K. Statistical survey of skin changes in Japanese neonates. Pediatr Dermatol1986;3:130–4.
- Donoghue AM, Sinclair MJ. Miliariarubra of the lower limbs in underground miners. Occup Med (Lond) 2000;50:430–3.
- Lobitz WC. Pustular miliaria. JAMA 1952;148:1097–100.
- Lubowe II, Perlman HH. Periporitis staphylogenes and other complications of miliaria in infants and children. Arch Dermatol Syphilol 1954;69:543–53.
- Hindson TC, Worsley DE. The effects of administration of ascorbic acid in experimentally induced miliaria and hypohidrosis in volunteers. Br J Dermatol 1969;81:226–7.
- Kirk JF, Wilson BB, Chun W, Cooper PH. Miliaria profunda. J Am Acad Dermatol 1996;35:54–6.
Neutrophilic eccrine hidradenitis
- Wenzel FG, Horn T. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol 1998;38:1–17.
- Harris T, Fine JD, Berman RS, et al. Neutrophilic eccrine hidradenitis. Arch Dermatol 1982;118:268.
- Bailey DL, Barron D, Lucky AW. Neutrophilic eccrine hidradenitis. Case report and review of the literature. Pediatr Dermatol 1989;6:33–8.
- Stahr BJ, Cooper PH, Caputo RV. Idiopathic plantar hidradenitis occurring primarily in children. J Cutan Pathol 1994;21:289–96.
- Shih IH, Huang YH, Yang CH, et al. Childhood neutrophilic eccrine hidradenitis: a clinicopathologic and immunohistochemical study of 10 patients. J Am Acad Dermatol 2005;52:963–6.
- Templeton S, Solomon AR, Swerlick RA. Intradermal bleomycin injections into normal human skin. A histopathlogic and immunopathologic study. Arch Dermatol 1994;130:577–83.
- Bhanu P, Santosh KV, Gondi S, et al. Neutrophilic eccrine hidradenitis: a new culprit – carbamazepine. Indian J Pharmacol 2013;45:91–2.
- Harwryluk EB, Linskey KR, Duncan LM, et al. Broad range of adverse cutaneous eruptions in patients on TNF-alpha antagonists. J Cutan Pathol 2012;39:481–92.
- Turan H, Kaya E, Gurlevik Z, et al. Neutrophilic eccrine hidradenitis induced by cetuximab. Cutan Ocul Toxicol 2012;31:148–50.
- Naimer SA, Zvulunov A, Ben-Amitai D, Landau M. Plantar hidradenitis in children induced by exposure to wet footwear. Pediatr Emerg Care 2000;16:182–3.
- Vuong CH, Walters R, Stein JA. Infectious eccrine hidradenitis associated with intense sun exposure. Cutis 2013;92:40–5.
- Takai T, Matsunaga A. A case of neutrophilic eccrine hidradenitis associated with streptococcal infectious endocarditis. Dermatology 2006;212:203–5.
- Antonovich DD, Berke A, Grant-Kels JM, et al. Infectious eccrine hidradenitis caused by nocardia. J Am Acad Dermatol 2004;50:315–18.
- Gómez Vázquez M, Peteiro C, Toribio J. Neutrophilic eccrine hidradenitis heralding the onset of chronic myelogenous leukaemia. J Eur Acad Dermatol Venereol 2003;17:328–30.
- Lee SG, In SG, Shin JH, et al. Neutrophilic eccrine hidradenitis in non-small cell lung cancer. Int J Dermatol 2007;46:59–60.
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol 2007;56:693–6.
- Bernstein EF, Spielvogel RL, Topolsky DL. Recurrent neutrophilic eccrine hidradenitis. Br J Dermatol 1992;127:529–33.
- Simon M, Jr, Cremer H, von den Driesch P. Idiopathic recurrent palmoplantar hidradenitis in children. Report of 22 cases. Arch Dermatol 1998;134:76–9.
- Belot V, Perrinaud A, Corven C, et al. Adult idiopathic neutrophilic eccrine hidradenitis trested with colchicine. Presse Med 2006;35:1475–8.
- Shear NH, Knowles SR, Shapiro L, Poldre P. Dapsone in prevention of recurrent neutrophilic eccrine hidradenitis. J Am Acad Dermatol 1996;35:819–22.
Eccrine syringosquamous metaplasia
- Wenzel FG, Horn DT. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol 1998;38:1–17.
- King DT, Barr DJ. Syringosquamous metaplasia: mucinous and squamous variants. J Cutan Pathol 1979;6:284–91.
- Metcalf JS, Maize JC. Squamous syringometaplasia in lobular panniculitis and pyoderma gangrenosum. Am J Dermatopathol 1990;12:141–9.
- Chetty R, Bramdev A, Govender D. Cytomegalovirus-induced syringosquamous metaplasia. Am J Dermatopathol 1999;21:487–90.
- Bhawan J, Malhotra R. Syringosquamous metaplasia: a distinctive eruption in patients receiving chemotherapy. Am J Dermatopathol 1990;12:1–6.
Drugs and eccrine glands
- Johnson HL, Maibach HI. Drug excretion in human sweat. J Inv Dermatol 1971;56:182–8.
- Huestis MA, Cone EJ, Wong CJ, et al. Monitoring opiate use in substance abuse patients with sweat and urine testing. J Anal Toxicol 2000;24:509–21
Disorders with sweat gland cellular inclusions
- Belchin RW. Ultrastructure and function of eccrine glands in the mucopolysaccharidoses. Arch Pathol 1973;96:339–44.
- Drut R. Eccrine sweat gland involvement in GM gangliosidosis. J Cutan Pathol 1978;5:35–40.
- Nakamura T, Kaneko H, Nishino I. Angiokeratoma corporis diffusum (Fabry disease): ultrastructural studies of the skin. Acta Derm Venereol (Stockh) 1981;61:37–41.
- Martin JJ, Cruterich C. Morphologic study of skin biopsy specimens: a contribution to the diagnosis of metabolic disorders with involvement of the nervous system. J Neurol Neurosurg Psychiatry 1978;41:232–6.
- Carpenter S, Kaepati G. Sweat gland cells in Lafora disease: diagnosis by skin biopsy. Neurology 1981;31:1564.
Anatomy and physiology of apocrine glands
- Hurley HJ, Shelley WB. The Human Apocrine Sweat Gland in Health and Disease. Springfield, IL: Thomas, 1960.
- Ebling FJG. Apocrine glands in health and disease. Int J Dermatol 1989;28:508–11.
- Montagna W, Parakkal PF. The Structure and Function of Skin, 3rd edn. London: Academic Press, 1974.
- Montagna W. Histology and cytochemistry of human skin XXIV. Further observations on the axillary organ. J Invest Dermatol 1964;42:119–29.
- Takayasu S, Wakimoto H, Itami S, et al. Activity of testosterone 5μ-reductase in various tissues of human skin. J Invest Dermatol 1980;74:187–91.
- Hashimoto K, Gross BG, Lever WF. Electronmicroscopic study of apocrine secretion. J Invest Dermatol 1966;46:378–90.
- Schaumburg-Lever G, Lever WF. Secretion from human apocrine glands. J Invest Dermatol 1975;64:38–41.
- Hurley HJ, Shelley WB. The role of the myoepithelium of the human apocrine sweat gland. J Invest Dermatol 1954;22:143–56.
- Tani M, Yamamoto K, Mishima Y. Apocrine acrosyringeal complex in the human skin. J Invest Dermatol 1980;75:431–5.
- Robertshaw D. Neural and humoral control of apocrine glands. J Invest Dermatol 1974;63:160–7.
- Natsch A, Gfeller H, Gygax P, et al. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J Biol Chem 2003;278:5718–27.
- Natsch A, Schmid J, Flachsmann F. Identification of odoriferous sulfanylalkanols in human axilla secretions and their formation through cleavage of cysteine precursors by a C-S lysase isolated from axilla bacteria. Chem Biodiversity 2004;1:1058–72.
- Bird S, Gower DB. The validation and use of a radioimmunoassay for 5 alpha-androst-16-en-3-one in human axillary collections. J Steroid Biochem 1981;14:213–19.
- Martin A, Saathoff M, Kuhn F, et al. A functional ABCC11 allele is essential in the biochemical formation of human axillary odor. J Invest Dermatol 2010;130:529–40.
- Wysocki CJ, Preti G. Facts, fallacies, fears, and frustrations with human pheromones. Anat Rec A Discov Mol Cell Evol Biol 2004;281:1201–11.
Disorders of apocrine sweat glands
Abnormal sweat odour (bromhidrosis)
- James AG, Austin C, Cox D, Taylor D, Calvert R. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol Ecol 2013;83:527–40.
- Phillips KA, Menard W. Olfactory reference syndrome: demographic and clinical features of imagined body odor. Gen Hosp Psychiatry 2011;33:398–406.
- Morioka D, Ohkubo F, Amikura Y. Clinical features of axillary osmidrosis: a retrospective chart review of 723 Japanese patients. J Dermatol 2013;40:384–8.
- Nakano M, Miwa N, Hirano A, Yoshiura K, Niikawa N. A strong association of axillary osmidrosis with the wet earwax type determined by genotyping of the ABCC11 gene. BMC Genet 2009;4(10):42.
- Bang YH, Kim JH, Paik SW, et al. Histopathology of apocrine bromhidrosis. Plast Reconstr Surg 1996;98:288–92.
- Gregoriou S, Rigopoulos D, Chiolou Z, et al. Treatment of bromhidrosis with a glycine-soya sterocomplex topical product. J Cosmet Dermatol 2011;10:74–7.
- Nakane T, Gomyo H, Sasaki I, et al. New antiaxillary odour deodorant made with antimicrobial Ag-zeolite (silver-exchanged zeolite). Int J Cosmet Sci 2006;28:299–309.
- He J, Wang T, Dong J. A close positive correlation between malodor and sweating as a marker for the treatment of axillary bromhidrosis with Botulinum toxin A. J Dermatolog Treat 2012;23:461–4.
- Lee JB, Kim BS, Kim MB, et al. A case of foul genital odor treated with botulinum toxin A. Dermatol Surg 2004;30:1233–5.
- Han X, Li F. Percutaneous ethanol injection for the treatment of axillary osmidrosis. Clin Exp Dermatol 2013;38:484–8.
- Huang YH, Yang CH, Chen YH, Chen CH, Lee SH. Reduction in osmidrosis using a suction-assisted cartilage shaver improves the quality of life. Dermatol Surg 2010;36:1573–7.
- Qian JG, Wang XJ. Effectiveness and complications of subdermal excision of apocrine glands in 206 cases with axillary osmidrosis. J Plast Reconstr Aesthet Surg 2010;63:1003–7.
Trimethylaminuria
- Humbert JR, Hammond KB, Hathaway WE, et al. Trimethylaminuria: the fish odour syndrome. Lancet 1970;i: 770–1.
- Dolphin CT, Janmohamed A, Smith RL, et al. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat Genet 1997;17:491–4.
- Ayesh R, Mitchell SC, Zhang A, Smith RL. The fish odour syndrome: biochemical, familial and clinical aspects. BMJ 1993;307:655–7.
- Yamazaki H, Fujieda M, Togashi M, et al. Effects of the dietary supplements, activated charcoal and copper chlorophyllin, on urinary excretion of trimethylamine in Japanese trimethylaminuria patients. Life Sci 2004;16(74):2739–47.
Chromhidrosis
- Shelley WB, Hurley HJ. Localized chromhidrosis: a survey. Arch Dermatol Syphilol 1954;69:449–71.
- Hurley HJ, Shelley WB. The Human Apocrine Sweat Gland in Health and Disease. Springfield, IL: Thomas, 1960.
- Marks JG, Jr. Treatment of apocrine chromhidrosis with topical capsaicin. J Am Acad Dermatol 1989;21:418–20.
- Saff DM, Owens R, Kahn TA. Apocrine chromhidrosis involving the areolae in a 15-year-old amateur figure skater. Pediatr Dermatol 1995;12:48–50.
- Wu JM, Mamelak AJ, Nussbaum R, McElgunn PS. Botulinum toxin A in the treatment of chromhidrosis. Dermatol Surg 2005;31:963–5.
- Matarasso SL. Treatment of facial chromhidrosis with botulinum toxin type A. J Am Acad Dermatol 2005;52:89–91.
- Poh-Fitzpatrick MB. ‘Red sweat.’ J Am Acad Dermatol 1981;4:481–26.
- Thami GP, Kanwar AJ. Red facial pseudochromhidrosis. Br J Dermatol 2000;142:1219–20.
- Cilliers J, de Beer C. The case of red lingerie: chromhidrosis revisited. Dermatology 1999;199:149–52.
Apocrine miliaria
- Hurley HJ, Shelley WB. The Human Apocrine Sweat Gland in Health and Disease. Springfield, IL: Thomas, 1960.
- Graham JH, Shafer JC, Helwig EB. Fox–Fordyce disease in male identical twins. Arch Dermatol 1960;82:212–21.
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol 2003;48:453–5.
- Bormate AB, Jr, Leboit PE, McCalmont TH. Perifollicular xanthomatosis as the hallmark of axillary Fox–Fordyce disease: an evaluation of histopathologic features of 7 cases. Arch Dermatol 2003;144:1020–4.
- Yazganoğlu KD, Yazici S, Büyükbabani N, Ozkaya E. Axillary Fox–Fordyce-like disease induced by laser hair removal therapy. J Am Acad Dermatol 2012;67:e139–140.
- Miller ML, Harford RR, Yeager JK. Fox–Fordyce disease treated with topical clindamycin solution. Arch Dermatol 1995;131:1112–13.
- Leyh F. Morbus Fox–Fordyce: Überlegungen zur Pathogenese. Hautarzt 1973;24:482–5.
- Effendy I, Ossowski B, Happle R. Fox–Fordyce disease in a male patient: response to oral retinoid treatment. Br J Dermatol 1994;19:67–9.
- Pasricha JS, Nayyar KC. Fox–Fordyce disease in the postmenopausal period treated successfully with electrocoagulation. Dermatologica 1973;147:271–3.
- Chavoin JP, Charasson T, Barnard JD. Surgical treatment of areolar hidradenitis suppurativa and Fox–Fordyce disease. Ann Chir Plast Esthet 1994;39:233–8.