CHAPTER 95
Acquired Disorders of the Nails and Nail Unit
David A. R. de Berker1, Bertrand Richert2 and Robert Baran3
1Bristol Dermatology Centre, Bristol Royal Infirmary, Bristol, UK
2Brugmann – St Pierre and Children's University Hospitals, Université Libre de Bruxelles, Brussels, Belgium
3University of Franche-Comté, Nail Disease Centre, Cannes, France
ANATOMY AND BIOLOGY OF THE NAIL UNIT
Structure
Gross anatomy [1, 2–5]
The component parts of the nail apparatus are shown in Figure 95.1. The nail is firmly attached to the nail bed; it is less adherent proximally, apart from the posterolateral corners. Approximately one-quarter of the nail is covered by the proximal nail fold, and a narrow margin of the sides of the nail plate is often occluded by the lateral nail folds. Underlying the proximal part of the nail is the white lunula (half-moon lunule); this area represents the most distal region of the matrix [6]. It is most prominent on the thumb and great toe and may be partly or completely concealed by the proximal nail fold in other digits. The reason for the white colour is not known [7, 8, 9]. The natural shape of the free margin of the nail is the same as the contour of the distal border of the lunula. The nail plate distal to the lunula usually appears pink, due to its translucency, which allows the redness of the vascular nail bed to be seen through it. The proximal nail fold has two epithelial surfaces, dorsal and ventral; at the junction of the two, the cuticle projects distally onto the nail surface. The lateral nail folds are in continuity with the skin on the sides of the digit laterally, and medially they are joined by the nail bed. Some authorities term the lateral nail fold and adjacent tissue lateral to the nail fold the nail wall.
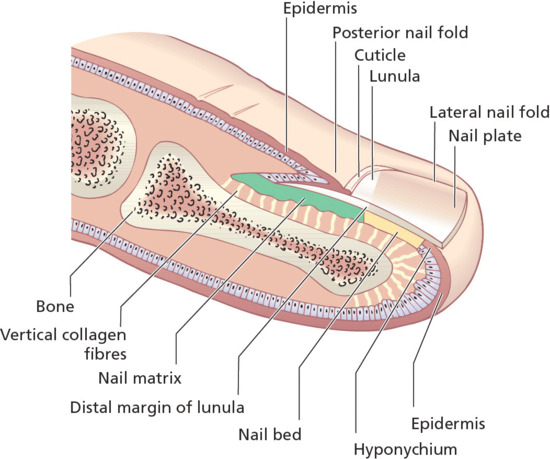
Figure 95.1 Longitudinal section of a digit showing the dorsal nail apparatus.
The definition of the nail matrix is controversial [10]. There is common acceptance that there is a localized region beneath the proximal nail which produces the major part of the normal nail plate. For those who consider this the sole source of nail it is termed simply the matrix, or germinal matrix. However, there is some evidence that other epithelial parts of the nail unit also contribute to the nail plate, and these are then also attributed matrix status (Figure 95.2). The matrix can be subdivided into dorsal (the ventral aspect of the proximal nail fold), intermediate (germinal matrix or matrix) and ventral (nail bed) sections. The nail bed is also termed the sterile matrix and its role in the production of the nail is unclear. Although it appears that the nail plate may thicken by up to 30% as it passes from the distal margin of the lunula to the end of the nail bed [3], this is not associated with an increase in cell numbers and may represent compaction of the nail from distal tip trauma rather than nail bed or nail plate production [11]. The situation may change in disease, where the nail bed changes its histological appearance to gain a granular layer [12] and may contribute a false nail of cornified epithelium to the undersurface of the nail [5]. The gap beneath the free edge is known as the hyponychium.
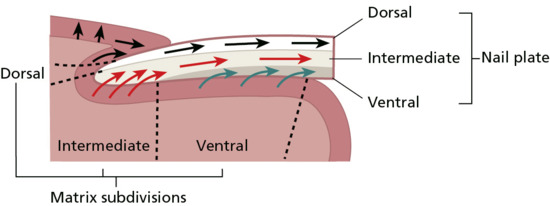
Figure 95.2 Direction of differentiation and cell movement within the nail apparatus.
When the attached nail plate is viewed from above, two distinct areas may be visible: the lunula proximally and the larger distal pink zone. On close examination, two further distal zones can often be identified: the distal yellowish-white margin and immediately proximal to this the onychodermal band [13]. Histologically, it is defined as the most distal attachment of cornified epithelium to the undersurface of the nail and has been termed the nail isthmus [14]. As such, it is structurally significant for the adherence of the nail plate to the nail bed. Once breached, as in conditions such as psoriasis, separation of the nail bed from the nail plate can be progressive.
Microscopic anatomy [15]
Nail folds
The proximal nail folds are similar in structure to the adjacent skin but are normally devoid of dermatoglyphic markings and pilosebaceous glands. There is a normal granular layer. From the distal area of the proximal nail folds, the cuticle adheres to the upper surface of the nail plate; it is composed of modified stratum corneum and serves to protect the structures at the base of the nail, particularly the germinal matrix, from environmental insults such as irritants, allergens and bacterial and fungal pathogens.
Nail matrix (intermediate matrix)
The nail matrix produces the nail plate in the absence of disease (see Figure 95.2). The basal compartment of the matrix is broader than the same region in normal epithelium or in other parts of the nail unit, such as the nail bed [10]. There is no granular layer, and cells differentiate with the expression of trichocyte ‘hard’ keratin (K31–40 and K81–86) as they become incorporated into the nail plate, alongside normal epithelial keratins [15, 16, 17]. During this process, they may retain their nuclei until more distal in the nail plate. These retained nuclei are called pertinax bodies. Apart from this, the detailed cytological changes seen in the matrix epithelium under the electron microscope are essentially the same as in the epidermis [18, 19].
The nail matrix contains melanocytes in the lowest three cell layers and these donate pigment to the keratinocytes. The presence of 6.5 melanocytes per millimetre of matrix basement membrane can be used as a guide to a normal matrix melanocyte population [20]. The appearance of melanocytes separate from the basement membrane distinguishes them from those found in the nail folds, which are primarily basal [21]. Unlike melanocytes in the proximal nail fold and most other sites, nail matrix melanocytes do not express human leukocyte antigen (HLA)-A/B/C antigens [21]. Matrix melanocytes are further distinguished from those elsewhere by their failure to produce melanin in normal circumstances in white people. This can change, with melanotic streaks presenting in local inflammatory, naevoid or neoplastic disease. In non-white people, brown streaks are common and are almost universal in Afro-Caribbeans by the age of 60 years.
Langerhans cells are detectable in the matrix by CD1a staining, and the matrix appears to contain basement membrane components indistinguishable from normal skin [22].
Nail bed
The nail bed consists of epidermis with underlying connective tissue closely apposed to the periosteum of the distal phalanx. There is no subcutaneous fat in the nail bed, although scattered dermal fat cells may be visible microscopically.
The nail bed epidermis is usually no more than two or three cells thick, although there may be tongues of epithelium that extend obliquely down. The transitional zone from living keratinocyte to dead ventral nail plate cell is abrupt, occurring in the space of one horizontal cell layer; in this regard it closely resembles the Henle layer of the internal root sheath of the epidermis [23]. Nail bed cells do not have any independent movement, and it is yet to be clearly demonstrated whether they are incorporated into an overlying nail plate as it grows distally [24]. The process of nail bed keratinization has been likened to that seen in rat tail epidermis, possibly being affected by pressure changes. The loss of the overlying nail results in the development of a granular layer, which is otherwise present only in disease states [11, 25, 26].
The nail bed dermal collagen is mainly orientated vertically, being directly attached to the phalangeal periosteum and the epidermal basal lamina. Within the connective tissue network lie blood vessels, lymphatics, a fine network of elastic fibres and scattered fat cells; at the distal margin, eccrine sweat glands have been seen [1].
Nail plate
The nail plate comprises three horizontal layers: a thin dorsal lamina, the thicker intermediate lamina and a ventral layer from the nail bed [4]. This is not always apparent with normal light microscopy using routine stains, where the nail demonstrates a transition between flattened cells dorsally and thicker cells on the ventral aspect. Electron microscopy shows squamous cells with tortuous interlocking plasma membranes [18, 19]. At high magnification, the contents of each cell show a uniform fine granularity similar to the hair cuticle [23]. Nail plate thickness can be measured in health and disease using ultrasound or optical coherence tomography [27].
The nail plate contains significant amounts of phospholipid, mainly in the dorsal and intermediate layers, which contribute to its flexibility. The detectable free fats and long-chain fatty acids may be of extrinsic origin. For further details of these and other histochemical changes in the components of the nail apparatus, see these more detailed texts [8].
Nail biology
Genes influencing the presence or absence or malformation of nails have been sought in connection with inherited abnormalities of the nail unit. The role of these genes in normal nail embryogenesis or production are difficult to determine, but it is clear that when there are mutations in the R-spondin genes and others influencing the Wnt signalling pathway, nail loss or reduction occurs. This first became evident from mutations seen in the R-spondin4 gene in a family with an autosomal recessive pattern of anonychia [28]. More subtle forms of nail dysplasia can be attributed to defects of Frizzled6 which in common with R-spondin4, enhances the Wnt signalling pathway and is found in inherited nail dysplasia [29]. In claw differentiation in knock-out mice, it is associated with expression of keratins K86, K81, K34 and K31; two epithelial keratins, K6a and K6b: all keratins with significance in nail formation and biology. Primary abnormalities in the Wnt signalling itself are also associated with inherited nail dysplasias such as Schöpf–Schulz–Passarge syndrome (Wnt10a) [30]. Mutations in other genes such as LMX1B are associated with multisystem disease such as nail–patella syndrome [31] which may have overlap with elements of Wnt signalling.
Keratin represents 80% of nail mass and its distribution and differentiation is pivotal. One classification of keratins is to divide them into ‘soft’ epithelial keratins or ‘hard’ trichocyte keratins (K31–40 and K81–86). The latter are characteristic of hair and nail differentiation, where their high sulphur content is responsible for their rugged physical qualities. This is matched by the resistance of trichocyte keratins to dissolution in strong solvent.
Keratin distribution in the nail and associated epithelium has been studied in adult [14, 15, 16], infant [17] and embryonic [32] digits. Immunohistochemistry of the epithelial structures of the normal nail demonstrates that the suprabasal keratin pair K1/K10 is found on both aspects of the proximal nail fold and to a lesser degree in the matrix. However, it is absent from the nail bed. This is reversed when there is nail bed disease, such as onychomycosis or psoriasis, where a granular layer develops and K1/K10 becomes expressed at corresponding sites [23]. The nail bed contains keratin synthesized in normal basal layer epithelium, K5/K14, which is also found in nail matrix. An antibody marking the epitope characteristically associated with keratin expressed in the basal layer is found throughout the thickness of the nail bed, but only basally in the matrix [26].
K6/K16 is identified in the nail bed but not the germinal matrix [16]. This is as proliferation is not a prominent feature. The nail bed has very low rates of proliferation [10, 33], and it may be that K6/K16 more precisely illustrates a loss of differentiation, often associated with proliferation in skin but representing the resting state of nail bed epithelium.
The location of K6/K16 is reflected in the localization of the features of pachyonychia congenita. In this group of autosomal dominant disorders, there is thickening of the nail plate attributed to disease of the nail bed in variants of the disease attributed to abnormalities in each of these keratins [34, 35].
Trichocyte keratins 31, 34, 81, 85 and 86 have all been demonstrated immunohistochemically in the nail unit [15, 16]. Proximally, these do not extend onto the ventral aspect of the proximal nail fold, sometimes described as the dorsal matrix and distally their expression is limited to a margin taken as corresponding to the lunula. Their distribution appears to define a matrix consistent with the classic description of the germinal matrix.
Blood supply [1]
There is a rich arterial blood supply to the nail bed and matrix derived from paired digital arteries, a large palmar and small dorsal digital artery on either side. The palmar arteries are supplied from the large superficial and deep palmar arcades [2]. The main supply passes into the pulp space of the distal phalanx before reaching the dorsum of the digit (Figure 95.3). Distally, the arteries are extremely tortuous and coiled, which allows them to be distorted without kinking to occlude supply. There are two main arterial arches (proximal and distal) supplying the nail bed and matrix, formed from anastomoses of the branches of the digital arteries. In the event of damage to the main supply in the pulp space, such as may occur with infection or scleroderma, there may be sufficient blood from the accessory vessels to permit normal growth of the nail. At a microvascular level, there are three patterns. Within the matrix, vessels are longitudinal with helical twisting. The axis becomes more longitudinal in the nail bed without the tortuosity – a pattern that is also seen in the distal proximal nail fold. This orientation is reflected in the appearance of splinter haemorrhages. In the digit pulp, vessels follow the pattern of the dermatoglyphics [3]. Nail vessel videomicroscopy can be used as part of a dynamic and anatomical modelling process establishing the parameters of blood flow and vessel anatomy [4].
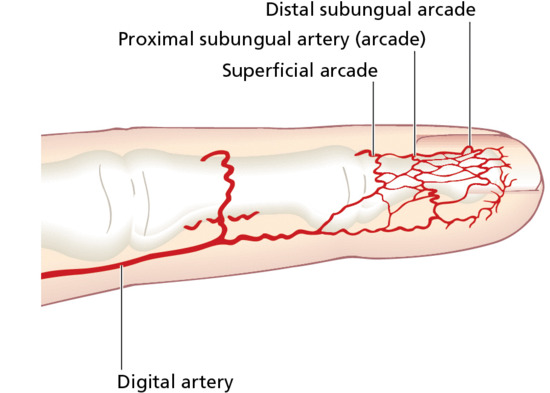
Figure 95.3 Arterial supply of the distal finger.
There are many arteriovenous anastomoses beneath the nail – glomus bodies – which are concerned with heat regulation. Glomus bodies are important in maintaining acral circulation under cold conditions: arterioles constrict with cold but glomus bodies dilate [5]. These occupy the subdermal tissues and increase in number in a gradient towards the distal nail bed [6].
Nail growth and morphology
Clinicians experienced in observing the slow rate of growth of diseased or damaged nails are apt to view the nail apparatus as inert, although it is biochemically and kinetically active throughout life. In this respect, it differs from most hair follicles, which undergo periods of quiescence as part of the follicular cycle.
Cell kinetics
The kinetic activity of the matrix has been examined using many techniques. These include immunohistochemistry, autoradiography and direct measurement of matrix product (i.e. nail plate) by ultrasound [1], micrometer or histology.
There is a broad basal compartment of proliferating cells in the matrix, which can be detected immunohistochemically with antibodies to proliferating cell nuclear antigen and Ki-67 (Figure 95.4); both antigens are associated with proliferating cells [2]. The matrix is also the site of maximal inclusion of tritiated thymidine if injected into the peritoneum of squirrel monkeys and followed subsequently by autoradiography [3]. Although there was some inclusion of thymidine into the nail bed, Zaias and Alvarez [3] interpreted the findings as indicating that the nail bed had no role in the creation of the nail plate. Norton [4] drew a similar conclusion from work with live human subjects where labelled thymidine and glycine were injected locally to act as markers of proliferating and metabolically active keratinocytes, and both primarily labelled the matrix.
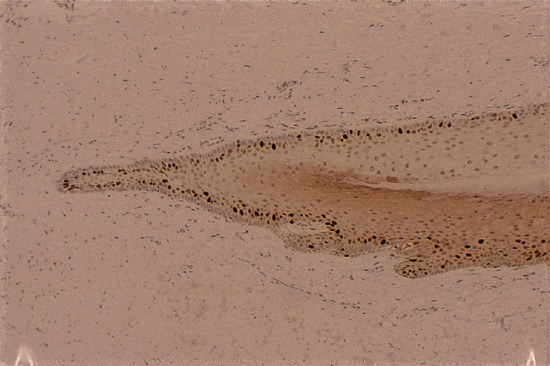
Figure 95.4 Proliferating epithelial cells of the matrix and ventral aspect of the proximal nail fold, staining with the antibody MIB-1.
However, the earlier work of Lewis [5] suggested on histological grounds that the nail plate is a trilaminar structure originating from three separate matrix zones: the dorsal matrix (ventral aspect of proximal nail fold), intermediate matrix (germinal matrix) and ventral matrix (nail bed). In support of this, Johnson et al. [6, 7] demonstrated that 21% of the nail thickness is gained as it passes over the nail bed, implying that the nail bed is generating this fraction of the nail plate. De Berker et al. [2] noted that the increase in nail thickness did not coincide with corresponding increases of nail plate cells. This challenges the interpretation that nail thickens over the nail bed because of a contribution from underlying structures. An alternative explanation may be appropriate, such as compaction arising from repetitive distal trauma. Others have also debated this issue [8] and, although the nail bed may have a significant contribution to make in disease [9], the evidence for its contribution at other times is conflicting.
Nail morphology
Why the nail grows flat, rather than as a heaped-up keratinous mass, has generated much thought and discussion [10, 11–14]. Several factors probably combine to produce a relatively flat nail plate: the orientation of the matrix rete pegs and papillae; adherence to the nail bed; the direction of cell differentiation [15]; and moulding of the direction of nail growth between the proximal nail fold and distal phalanx [16]. Containment laterally within the lateral nail folds assists this orientation, and the adherent nature of the nail bed is likely to be important. In diseases such as psoriasis, the nail bed can lose its adherent properties, exhibiting onycholysis. In addition, there may be subungual hyperkeratosis. These combined factors make psoriasis the most common pathology in which up-growing nails are seen. Onychogryphosis is characterized by upward growth of thickened nail. In this condition, the nail matrix may become bucket-shaped and the effect of the overlying proximal nail fold is lost.
Linear nail growth [17, 18, 19]
During the 20th century, many studies were carried out on the linear growth of the nail plate in health and disease; these have been reviewed [20, 21] and are listed in Tables 95.1 and 95.2 [22]. Most of these studies have been performed by observing the distal movement of a reference mark etched on the nail plate over a fixed period of time; this may well correlate with matrix germinative cell kinetics but there is no direct proof that it does. However, studies on nail growth in psoriasis, and its inhibition by cytostatic drugs [23, 24], suggest that cell kinetics and linear growth rate do have a direct correlation.
Table 95.1 Physiological and environmental factors affecting the rate of nail growth.
| Faster | Slower |
| Daytime | Night |
| Pregnancy [25] | |
| Right-hand nails | Left-hand nails [27] |
| Youth, increasing age | Old age [18, 27, 30] |
| Fingers | Toes [31] |
| Summer [18] | Winter or cold environment [32, 33] |
| Middle, ring and index fingers | Thumb and little finger [28, 31, 34, 35] |
| Male gender | Female gender [27, 35] |
| Minor trauma/nail biting [26, 27] |
Table 95.2 Pathological factors affecting the rate of nail growth.
| Faster | Slower |
| Psoriasis [36] Normal nails [23] Pitting Onycholysis [37] Pityriasis rubra pilaris [21, 38] Etretinate, rarely [39] Idiopathic onycholysis of women [37] Bullous ichthyosiform erythroderma [13] Hyperthyroidism [28] Levodopa [40] Arteriovenous shunts [28] |
Finger immobilization [41] Fever [42] Beau's lines [43] Methotrexate [24], azathioprine [24], etretinate [39] Denervation [44] Poor nutritionKwashiorkor [45] Hypothyroidism [28] Yellow nail syndrome [13] Relapsing polychondritis [46] |
Fingernails grow approximately 1 cm every 3 months and toenails at one-third of this rate.
NAIL SIGNS AND THEIR SIGNIFICANCE
It is important for clinicians to understand and accurately describe nail findings if they are to communicate effectively with their colleagues. Signs fall into categories of shape, surface and colour.
Nails: abnormalities of shape
Clubbing
In clubbing, there is increased transverse and longitudinal nail curvature with hypertrophy of the soft-tissue components of the digit pulp. The nail can be ‘rocked’ and in causes associated with cardiopulmonary disease there may be local cyanosis.
There are three forms of geometric assessment that can be performed. Lovibond's angle is found at the junction between the nail plate and the proximal nail fold, and is normally less than 160°. This is altered to over 180° in clubbing (Figure 95.5). Curth's angle at the distal interphalangeal joint is normally about 180°. This is diminished to less than 160° in clubbing (Figure 95.6). Schamroth's window is seen when the dorsal aspects of two fingers from opposite hands are apposed, revealing a window of light, bordered laterally by the Lovibond angles (Figure 95.7). As this angle is obliterated in clubbing, the window closes [1]. Assessment of clubbing at the bedside shows poor agreement between examiners [2] in milder cases and there are problems in using firm morphometric analyses that do not lend themselves to routine clinical practice [3]. Ultrasound criteria for diagnosis can also be used [4].
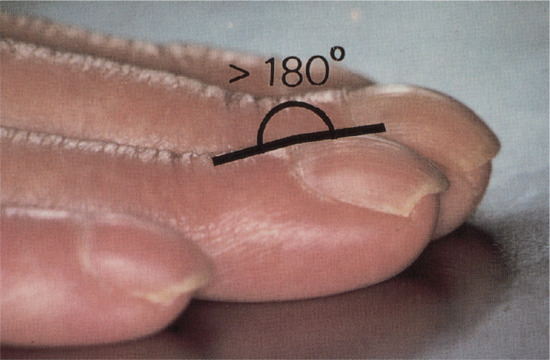
Figure 95.5 Clubbing: Lovibond's profile sign. The angle is normally less than 160° but exceeds 180° in clubbing.
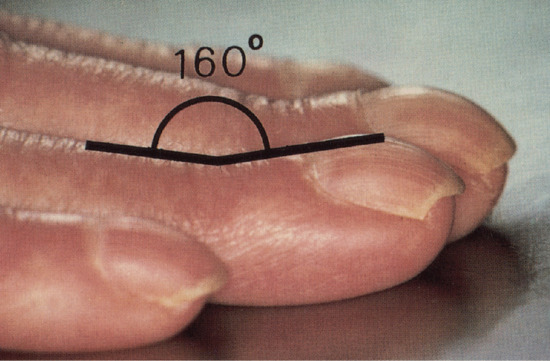
Figure 95.6 Clubbing: Curth's modified profile sign.
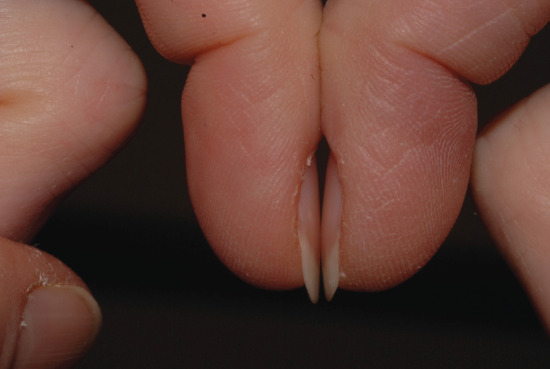
Figure 95.7 Schamroth's window is seen clearly in this image of normal nails.
Clubbing appears to be related more to increased blood flow through the vasodilated plexus of nail unit vasculature than to vessel hyperplasia, although MRI studies have also implicated hypervascularity [5]. Altered vagal tone and microvascular infarcts have also been implicated [6, 7]. Mutations in the HPGD [8] and SLCO2A1 [9] genes have each been linked to pachydermoperiostosis (primary hypertrophic osteoarthropathy), of which clubbing is a component (see below and chapter 154): their gene products are involved in prostaglandin metabolism and prostaglandin transmembrane transport, respectively, suggesting that prostaglandins may be important. Other factors such as bradykinin and serotonin or reactive factors associated with hypoxia could have relevance.
The list of diseases associated with clubbing has a pattern where chronic inflammation of the bowel and lung are seen with or without precipitating infection. Some of these diseases can be clustered, with tuberculosis associated with underlying fibrotic lung disease or HIV, all of which are found to have independent associations [10]. Vascular causes can be associated with central cyanotic ischaemia, as in heart disease, or local factors such as the unilateral soft-tissue changes of hemiplegia [11]. An isolated subungual tumour located within the mid-proximal zone of the subungual space can displace the nail unit upwards in a form similar to clubbing. However, some of the other features are typically lacking, such as the fluctuant quality of the proximal nail and nail fold [12]. This can be included in the category of pseudoclubbing which arises from local pathology such as osteolysis of the tip of the digit seen in systemic sclerosis.
Clubbing is a component of secondary hypertrophic osteoarthropathy as well as of pachydermoperiostosis: in both, a subungual lymphocytic infiltrate may be found and, with this, some associated fibrosis which may ultimately create reactive bone changes and osteoarthropathy. In the primary genetic form, pachydermoperiostosis, there is arthritis and subperiosteal new bone formation affecting the long bones. The secondary form has many of the same benign associations as isolated digital clubbing but is much more strongly associated with malignancy, particularly bronchial carcinoma.
A list of conditions associated with nail clubbing is given in Table 95.3.
Table 95.3 Causes of nail clubbing.
| Cause | Comment |
| Asbestosis | Clubbing is found in about 40% of those with asbestosis |
| Thoracic carcinoma | Includes carcinoma of bronchus, pleura, lymphosarcoma, mediastinal lymphoma and metastatic disease in the lung arising outside the thorax |
| Cystic fibrosis | This is acquired in adolescence or early adulthood. It can be used as a predictive factor for clinical progression of disease |
| Cryptogenic fibrosing alveolitis | Clubbing is an indicator of disease morbidity |
| Mesothelioma | Clubbing is found in about a third of those with mesothelioma and may in some instances be associated with the asbestosis which is a predisposing factor |
| Nasopharyngeal carcinoma | Clubbing can be an association with nasopharyngeal carcinoma in both children and adults |
| Pulmonary arteriovenous malformation | Can be found associated with hereditary haemorrhagic telangiectasia |
| Sarcoidosis | Clubbing can be a local manifestation of sarcoid within the distal digit or a feature of pulmonary involvement |
| Cyanotic heart disease | Typically a patent ductus arteriosus or septal defect |
| Infective endocarditis | Clubbing can reverse when the infection is resolved |
| Hepatopulmonary syndrome | Associated with a shunt that gives rise to breathlessness and cyanosis |
| Carcinoma of the oesophagus | Usually associated with the pattern seen with hypertrophic osteoarthropathy |
| Inflammatory bowel disease | May be seen with hypertrophic osteoarthropathy |
| Laxative abuse | It is not clear whether clubbing resolves if laxative abuse stops |
| Liver disease | A range of liver diseases is implicated. When treatment is a liver transplant, the clubbing has been seen to reverse |
| Chronic parasitic infestation | Examples include dysentery caused by Trichuris trichiuria |
| HIV | In one observational study, 37% of HIV patients had clubbing. The mean duration of the HIV was 4 years |
| Tuberculosis | Pulmonary tuberculosis is often associated with other diseases in turn associated with clubbing, such as HIV or coexisting lung disease |
| Thyroid disease | The distinction between thyroid acropachy, pachydermoperiostitis and clubbing is not always clear in reports |
| Lupus erythematosus | A rare association |
| POEMS syndrome | Found in 70% of patients with this rare syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes (see chapter 148) |
| Hemiplegia | Typically associated with other soft-tissue changes in the hemiplegic hand |
| Subungual tumour | An isolated subungual tumour can create the shape of a clubbed digit, although the rocking of the proximal nail may be absent |
Koilonychia
In koilonychia (Greek: koilos, hollow; onyx, nail), there is reverse curvature in the transverse and longitudinal axes giving a concave dorsal aspect to the nail (Figure 95.8) [1]. Fingers and toes may be affected, with signs most prominent in the thumb or great toe.
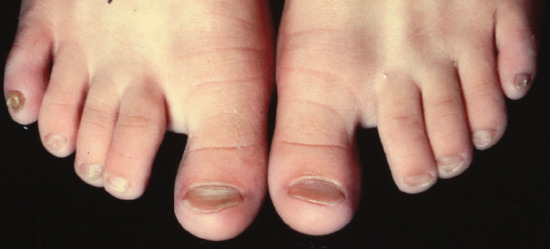
Figure 95.8 Koilonychia. This image is of congenital koilonychia in a young girl.
Koilonychia is common in infancy as a benign feature of the great toenail, although in some infants its persistence may be associated with a deficiency of cysteine-rich keratin [2] in trichothiodystrophy. The most common systemic association is with iron deficiency [3] and haemochromatosis, although the majority of adults with koilonychia demonstrate a familial pattern, which may be autosomal dominant [4]. In dermatoses such as psoriasis and dermatophyte infection, nail bed hyperkeratosis may push the nail up distally to produce a spoon-shaped nail. In mechanics, softening of the nail from contact with oil may be a factor [5], and in hairdressers, permanent wave solutions may be causal [6].
Pincer nail
Pincer nail describes a dystrophy where nail growth is pitched towards the midline, combined with increased transverse curvature. It presents in three patterns [1, 2, 3]. Probably the most common is in association with psoriasis, where the thumbs and big toes are the most likely to be affected, although the pattern is not as organized and symmetrical as that seen in the inherited version. In the latter, there is often a gradient of involvement, radiating from the thumbs and big toes outwards, which progresses with time (Figure 95.9). The third variant is the individual nail which develops a pincer deformity. In this instance, careful imaging and surgical exploration should be undertaken to exclude an isolated space-occupying lesion beneath the matrix [4, 5, 6].
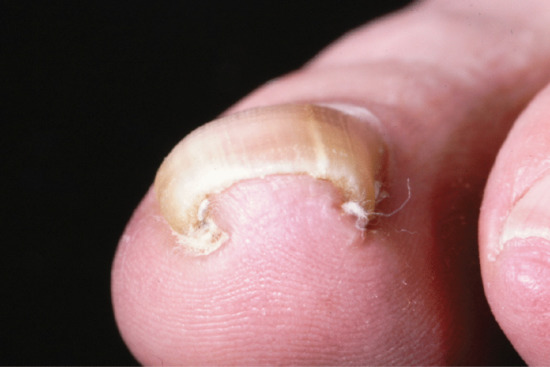
Figure 95.9 Pincer nail.
Pain may arise due to embedding of the pincer nail in the lateral nail folds and nail bed, which becomes most pronounced distally. Imaging can be helpful. Treatment is usually by surgery to relieve the pain. In the toes, it is usually best to perform a lateral ablation of the most embedded margin. This will sometimes lead to a shift of the nail such that the other side no longer embeds. If both sides require ablation, the dimensions of the toenail may mean that it is better to ablate the entire matrix rather than to leave a central zone of nail. The alternative of corrective surgery in toes has less chance of success, although successful case series are reported. When treating the thumbs or fingers, the chance of success with corrective surgery is higher and the cosmetic and functional handicap of ablation may not be acceptable. Again, a lateral ablation may be adequate, but more complex procedures entail altering the alignment of the matrix [2, 7, 8], level of the nail bed [9] and addressing any midline hypertrophy of the distal phalanx. Some surgeons advocate a combination of reconstruction and ablation [10]. Nail braces rarely produce long-term benefit, although promising outcomes have been reported [11].
Anonychia
Anonychia is the absence of all or part of one or several nails [1]. It may be congenital, acquired or transient. The underlying genetic abnormality of the congenital form has recently been identified as a mutation in the R-spondin4, Frizzled6 or Wnt10a genes (see above: nail biology), which play a part in Wnt signalling within the cell [2]. There may be a biological interaction with the underlying phalanx in embryogenesis (see Chapter 69) [3].
Acquired forms are due to scarring of the nail matrix. This can arise through burns, surgery or trauma, or be due to inflammatory dermatoses such as lichen planus where the entire nail matrix is scarred and lost [4]. Similar scarring can occur in variants of epidermolysis bullosa, with irreversible nail loss (Figure 95.10). The transient variant is due to nail shedding. This can occur due to an intense physiological or local inflammatory process, in the absence of scarring.
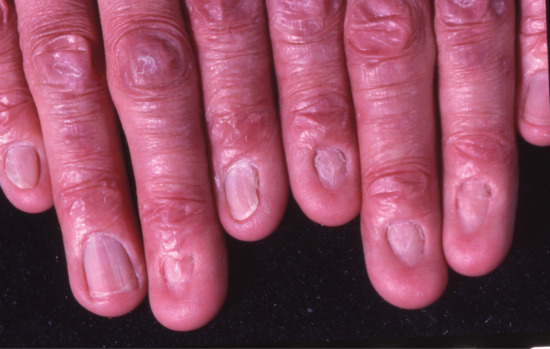
Figure 95.10 Anonychia: nail loss in a 50-year-old man with dominant dystrophic epidermolysis bullosa.
Nails: abnormalities of nail attachment
Nail shedding
Nails can be lost through different mechanisms as follows:
- Complete loss of the nail plate due to proximal nail separation extending distally [1] is called onychomadesis and is a progression of profound Beau's lines. This may reflect local or systemic disease and in the latter may result in temporary loss of all nails.
- Local dermatoses such as the bullous disorders and paronychia may cause nail loss. Generalized dermatoses may be manifest, for example toxic epidermal necrolysis (TEN) and severe/rapid onset of pustular psoriasis. Scarring of the nail unit is seen in lichen planus and following TEN, which may both provoke nail loss.
- Trauma is a common cause of recurrent loss and may reflect the nature of the activity, such as football, or some underlying abnormality of footwear [2] or foot mechanics. It is often associated with subungual haemorrhage [3]. In the long term, athletes often develop thickened dystrophic nails matching a history of recurrent shedding. More severe trauma can result in a degloving event removing all tissue from the end of the phalanx.
- Temporary loss has also been described due to retinoids [4], and to large doses of cloxacillin and cephaloridine during the treatment of two anephric patients [5].
- Onychoptosis defluvium or alopecia unguium describes atraumatic, familial, non-inflammatory nail loss [6]. It may be periodic and associated with dental amelogenesis imperfecta.
- Nail shedding can be part of an inherited structural defect, most obviously in epidermolysis bullosa [7], although at times the diagnosis may be occult [8].
Onycholysis
Onycholysis is the distal and/or lateral separation of the nail from the nail bed [1] and can be graded [2]. Psoriatic onycholysis can be considered the reference point for other forms of onycholysis and is typically distal, with variable lateral involvement. Isolated islands of onycholysis present as ‘oil spots’ or ‘salmon patches’ in the nail bed: at the border of onycholysis, the nail bed is usually reddish-brown, reflecting the underlying psoriatic inflammatory changes. All the common causes are associated with diminished adherence of the nail to the nail bed as a primary (idiopathic) or secondary event: the latter include trauma, fungal infection, eczema, drug reactions and photo-onycholysis [3].
Idiopathic onycholysis
This is a painless separation of the nail from its bed which occurs without apparent cause. Overzealous manicure, frequent wetting and cosmetic ‘solvents’ may be the cause but may not be admitted by the patient. There may, however, be a minor traumatic element, as the condition occurs rather more often in persons who keep their nails abnormally long. Maceration with water may also be a factor [3]. It must be distinguished from other causes of onycholysis (see later). The affected nails grow very quickly [4].
The condition usually starts at the tip of one or more nails and extends to involve the distal third of the nail bed (Figure 95.11). Persistent manicure is attempted to remove the debris which accumulates within the onycholytic space, and this can result in a crescentic margin of onycholysis matching the onychocorneal band and appearing similar in all involved digits. Pain occurs only if there is further extension as a result of trauma or if active infection supervenes. More often there is microbial colonization of a mixed nature, including Candida albicans and several bacteria, of which Pseudomonas aeruginosa is the most common. If the condition persists for several months, the nail bed becomes dark and irregularly thickened. The condition is mostly seen in women and many cases return to normal after a few months. The longer it lasts, the less likely is the nail to become reattached, due to keratinization of the exposed nail bed.
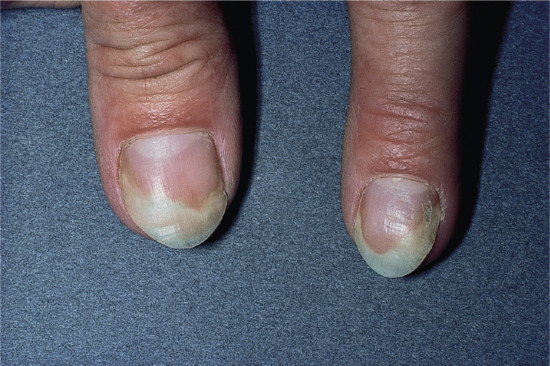
Figure 95.11 Onycholysis: idiopathic type.
Management [5]
Cut away as much as possible of the loosened nail and apply a topical steroid preparation containing broad spectrum antimicrobials effective against both yeasts and bacteria. Reattachment is slow, and the loosened nail should be recut several times if necessary. Some authorities still recommend 4% thymol in chloroform (not available in the US) as a means of preventing infection and further maceration of the nail bed; however, 2% thymol is often as strong as the patient can tolerate and is usually effective. Where antimicrobial therapy is needed for Pseudomonas, gentamicin eye drops can be useful. Drying under the onycholytic nails with a hair-dryer has been advocated in order to desiccate the environment in which Pseudomonas would otherwise grow. Soaking the fingertips several nights a week in vinegar or sodium hypochlorite solution (Milton) for 5 min can be useful to prevent recurrence. Domestic vinegar is between 3 and 9% acetic (synonym: ethanoic) acid. At the higher strength it can be irritant, especially if the area being treated is already sore. A dilution of 4 parts water to 1 part vinegar is likely to avoid risk of irritancy. Milton is 1% sodium hypochlorite. A 0.25% solution is suitable for wound care, which means a dilution with 4 parts water to 1 part Milton.
Secondary onycholysis
There are many causes of onycholysis [5–8]. Psoriasis, fungal infection, dermatitis and trauma are amongst the most common. Thirty per cent of psoriatics with nail involvement will have onycholysis, with toenail involvement more common than fingernails [9]. Onycholysis occurs in general medical conditions, including impaired peripheral circulation, hypothyroidism [8], hyperthyroidism [9], hyperhidrosis, yellow nail syndrome and shell nail syndrome. Minor trauma is a common cause, and many occupational cases are due to trauma [10]. Immersion of the hands in soap and water may be considered traumatic, as also may the use of certain nail cosmetics. It has also been described after the application of 5% 5-fluorouracil to the fingertips where it can be used therapeutically for warts [11]. There is a condition of hereditary partial onycholysis associated with hard nails [12]. Photo-onycholysis (Figure 95.12) may occur during treatment with psoralens, demethylchlortetracycline and doxycycline [13, 14], and rarely other antibiotics. This is sometimes associated with cutaneous photosensitivity (see Chapter 24). Drugs such as retinoids [15] and cancer chemotherapy can also be implicated, with taxanes eliciting nail changes in between 19 and 44% of patients, depending on the chemotherapy regimen [16]; cooling the hand with a specialized glove has been demonstrated to help diminish or delay onset of these adverse effects [17, 18].
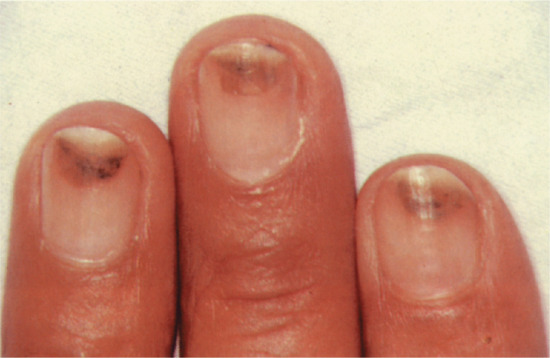
Figure 95.12 Photo-onycholysis with a uniform pattern of discoloured onycholysis in the midline.
Pterygium [1]
The term pterygium describes the winged appearance achieved when a central fibrotic band divides a nail proximally in two (Figure 95.13). However, the fibrotic tissue may not always grossly alter the nail and can extend from the lateral nail fold as well as the more typical proximal nail fold. A large pterygium may destroy the whole nail.
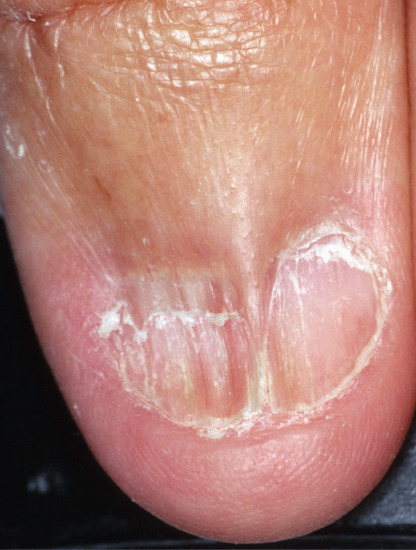
Figure 95.13 Nail pterygium due to lichen planus.
An inflammatory destructive process precedes pterygium formation. There is fusion between the nail fold and underlying nail bed and matrix. The fibrotic band then obstructs normal nail growth. Superficial abnormal vessels may be seen and there are no skin markings. It most typically develops in trauma or lichen planus and its variants, including idiopathic atrophy of the nail [2] and graft-versus-host disease [3]. It can also occur in leprosy, where it may represent scarring secondary to neuropathic damage and secondary purulent infection [4].
Ventral pterygium
Ventral pterygium (Figure 95.14) or pterygium inversum unguis [1, 2] occurs on the distal undersurface of the nail, with forward extension of the nail bed epithelium dislocating the hyponychium and obscuring the distal groove. Causes include trauma, systemic sclerosis [2, 3], Raynaud phenomenon, lupus erythematosus, familial subungual pterygium [4] and infections [5]. The overlying nail may be normal, but adjacent soft tissues can be painful.
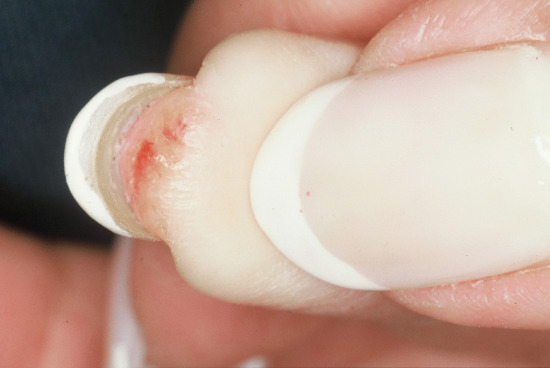
Figure 95.14 Ventral pterygium due to allergy to formaldehyde nail hardener.
Changes in nail surface
Longitudinal grooves
Longitudinal grooves may run all or part of the length of the nail in the longitudinal axis, and need to be distinguished from ridges which are proud of the nail surface [1]. Grooves may be full or partial thickness.
The median canaliform dystrophy of Heller [2] is the most distinctive form (Figure 95.15) [3]. The author has seen it in children under 10, but the literature is potentially misleading due to the confusion between midline transverse ridging of habit tic and true canaliform dystrophy [4]. The nail is split, usually in the midline, with a fir-tree-like appearance of ridges angled backwards. The thumbs are most commonly affected and the involvement may be symmetrical. The cuticle may be normal, as distinct from the cuticle in habit tic deformity (‘washboard nails’). After a period of months or years the nails often return to normal, but relapse may occur [5] and a ridge may replace the original defect. Some patients give a definite history of trauma [1] and rarely the disorder can be attributed to oral retinoids [6]. Although familial cases have been recorded, the majority of cases are sporadic and of unknown cause [7].
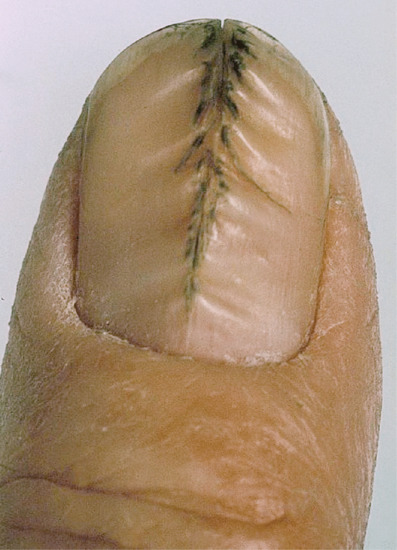
Figure 95.15 Median canaliform dystrophy of Heller.
Tumours (e.g. viral warts, myxoid cysts, periungual fibromas) pressing on the matrix, or a proximal nail fold pterygium, may produce a longitudinal groove.
Transverse grooves and Beau's lines [1, 2]
Transverse grooves may be full or partial thickness through the nail. When they are endogenous they have an arcuate margin matching the lunula. If exogenous, such as those due to manicure, the margin may match the proximal nail fold and the grooves may be multiple as in washboard nails associated with a habit tic [3]. When multiple, it may be difficult to distinguish a habit tic from psoriasis. Transverse grooves may occur on isolated diseased digits (trauma, inflammation or neurological events) [4] or may be generalized, reflecting an acute systemic event such as a drug reaction [5], myocardial infarction, measles, mumps or pneumonia. If there is a systemic cause, they are usually referred to as Beau's lines [2]. They arise through temporary interference with nail formation and become visible on the nail surface (Figure 95.16) some weeks after the precipitating event. The distance of the groove from the nail fold is related to the time since the onset of growth disturbance. The depth and width of the groove may be related to the severity and duration of disturbance, respectively. In many cases, grooves are seen on all 20 nails but are most prominent on the thumb and great toenail, and are deeper in the midline of the nail. Full-thickness grooves can be associated with distal extension of the plane of separation of the nail plate. This can lead to nail loss, termed onychomadesis.
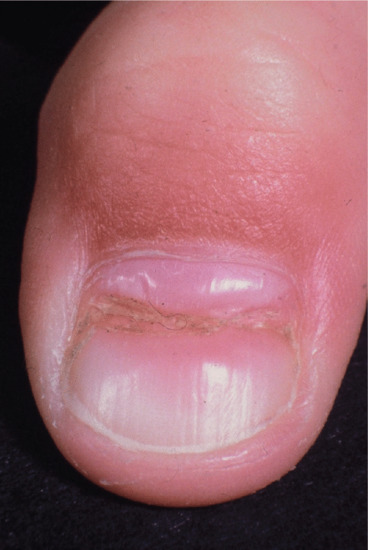
Figure 95.16 Beau's lines present as transverse grooves in the nail matching the proximal margin of the nail matrix and lunula.
Nail pitting
Nail pitting presents as punctate erosions in the nail surface. Individual pits may be shallow or deep, with a regular or irregular outline. The individual pits of psoriasis are said to be less regular in form and in overall pattern than those of alopecia areata, but this is not always the case. When numerous, they appear randomly distributed upon the nail surface or have a geometric pattern. The latter may cause rippling or create a grid of pits. Mild pitting may also occur in association with different patterns of eczema, but is usually more subtle or localized than psoriatic pitting. Extensive pitting combined with other surface irregularities results in the appearance of trachyonychia. An isolated large pit may produce a localized full-thickness defect in the nail plate termed elkonyxis, which is found in reactive arthritis, psoriasis and following trauma.
Histologically, pits represent foci of parakeratosis, reflecting isolated nail malformation [1, 2] and are present in the fingernails of about half of psoriatics with nail involvement.
Trachyonychia
Trachyonychia presents as a rough surface affecting all of the nail plate and up to 20 nails (20-nail dystrophy) [1, 2]. The original French term was ‘sand-blasted nails’, which evokes the main clinical feature of a grey roughened surface (Figure 95.17). It is mainly associated with alopecia areata [3], psoriasis and lichen planus, although the most common presentation is as an isolated nail abnormality. In the isolated form, histology shows spongiosis and a lymphocytic infiltrate [4] of the nail matrix. It may present at birth, as a self-limiting condition in childhood or as a more chronic problem in adulthood. There is some response to potent topical, locally injected and systemic steroids, but this may be temporary.
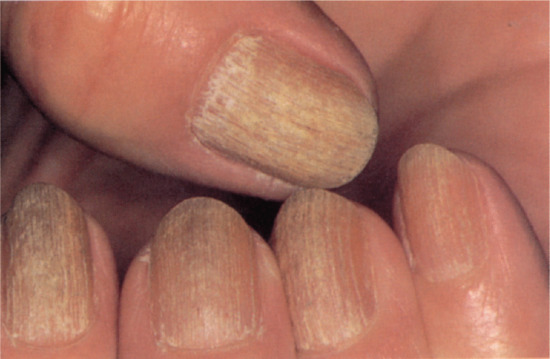
Figure 95.17 Trachyonychia: roughened surface of up to 20 nails.
Onychoschizia (lamellar dystrophy)
Onychoschizia is also known as lamellar nail dystrophy and is characterized by transverse splitting into layers at or near the free edge (Figure 95.18) in fingers and toes, especially in infants [1]. There is a subtle distinction between the static features, such as types of split, and the subjective experience of having brittle nails. Usually these characteristics coincide, although clinicians and patients may prefer to use one term over the other. The different features can be assessed within a scoring system [2]. Variants include splitting at the lateral margins alone and multiple crenellated splits at the free edge. It is seldom associated with any systemic disorder, although it has been reported with polycythaemia [3], HIV infection [4] and glucagonoma [5] and has been referred to as a ‘syndrome’ [2].
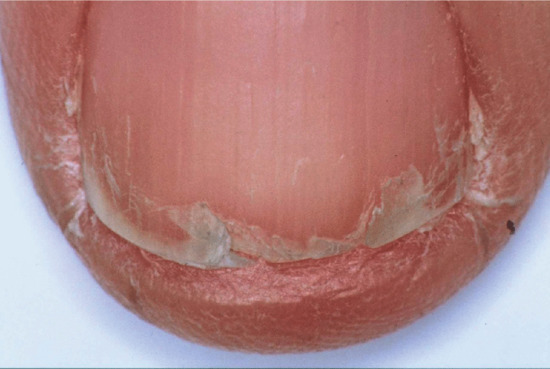
Figure 95.18 Onychoschizia (lamellar splitting).
Scanning electron microscopy illustrates the tendency of the lamellar structure of the nail to separate after repeated immersion in water [6], although case–control studies show that occupation is not a major determinant of the condition [7]. However, efforts at retaining hydration (gloves, emollient and base coat with nail varnish) may help reverse clinical changes. Biotin has been used as systemic therapy, but the evidence for its efficacy is weak [8].
Beading and ridging
Beading and longitudinal ridging of the nails are common minor nail surface abnormalities which become more prominent with age (Figure 95.19). They are not an indication of disease.
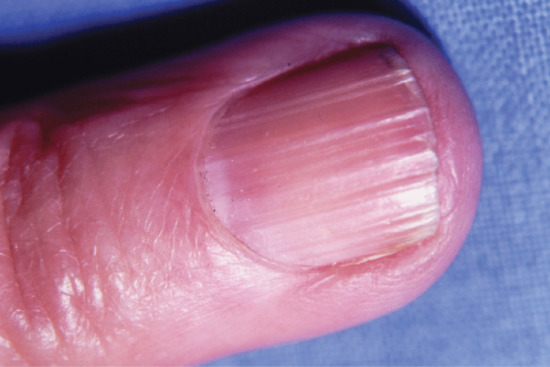
Figure 95.19 Longitudinal ridging of the nail.
Changes in nail colour [1–7]
Alteration in nail colour may occur because of changes affecting the dorsal nail surface, the substance of the nail plate, the undersurface of the nail or the nail bed.
Nail plate pigmentation
Exogenous pigment on the upper surface is easy to demonstrate by scraping the nail. If the proximal margin of the pigment is an arc matching the proximal nail fold, this is a further clue confirming an exogenous source. Nicotine is a typical pigment with the ‘quitters’ nail, which demonstrates the cessation of smoking and nicotine-free fingers for 2 months. Henna and spray tan are other common causes. Where there is onycholysis, the ventral surface of the nail can also become pigmented (Figure 95.20) and the most common instance is the green colour seen from colonization with Pseudomonas (Figure 95.21).
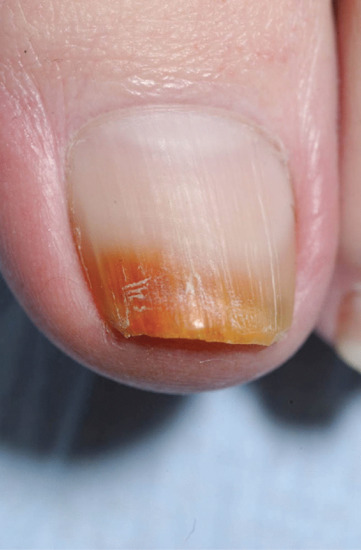
Figure 95.20 Orange pigmentation of onycholytic toenail due to orange dye from work-boots.
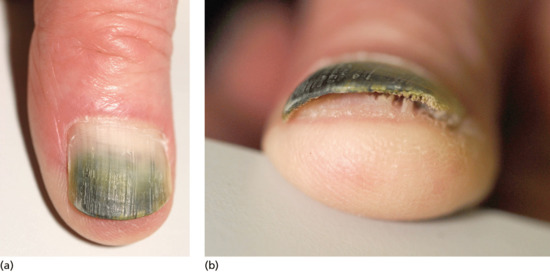
Figure 95.21 Green pigmentation of onycholytic fingernail due to Pseudomonas.
Nail colour can be changed by the incorporation of pigment into the nail plate, most commonly in the form of melanin produced by matrix melanocytes during nail formation. This produces a brown longitudinal streak the entire length of the nail. In white people this is abnormal and requires thorough assessment and, in some instances, biopsy. In darker-skinned people it is a common normal variant. The incorporation of heavy metals and some drugs into the nail plate via the matrix can also alter nail colour, such as the grey colour associated with silver or the grey-blue discoloration due to antimalarials or phenothiazines.
Loss of nail plate lucency
Nail colour may also be affected by alterations in the normal cellular and intercellular organization, such that there is loss of normal lucency. The disruption of normal nail plate formation by disease, chemotherapy, poisons or trauma can result in waves of parakeratotic nail cells or small splits between cells within the nail. Both make the nail less lucent and produce the white marks of true leukonychia (see later). Transmission electron microscopy suggests that there is a change in keratin fibre organization, which might provide an intracellular basis for altered diffractive properties. This disruption may occur at nail formation or subsequently in the case of fungal nail infection, where discoloration may start distolaterally rather than via the matrix.
Subungual disturbances
Subungual hyperkeratosis as from dermatophyte infection or psoriasis may also change the apparent colour of the nail.
Subungual haemorrhage produces a variety of colour changes ranging from bright red to black. Splinter haemorrhages result from leakage of blood from nail bed capillaries and may be due to local trauma or to microemboli, classically from infective endocarditis.
Nail bed changes
Vascular abnormalities can affect apparent nail colour as in blue nails from cyanosis and bright red nails from carbon monoxide poisoning. In addition to such generalized vascular changes there can be localized changes, as seen with nail bed tumours. The increased vascularity of a glomus tumour in comparison with the surrounding nail bed may be the sole method of determining its location.
Dermoscopy can be very helpful in the assessment of nail plate pigmentation and underlying nail bed changes [7].
Leukonychia
True leukonychia
This is a white discoloration of the nail attributable to matrix dysfunction; it occurs in a variety of patterns [1, 2].
True hereditary leukonychia
In this rare condition, the nails are milky porcelain white. If the whole of the nail plate is affected it is called total leukonychia (Figure 95.22) [3]. In subtotal leukonychia, the proximal two-thirds are white, becoming pink distally. This is attributed to a delay in keratin maturation, and the nail may still appear white at the distal overhang.
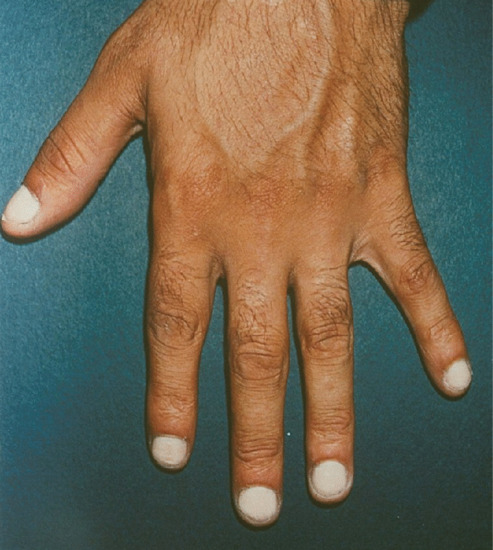
Figure 95.22 Total leukonychia.
Transverse leukonychia (Mees’ lines) reflects a systemic disorder, such as chemotherapy or poisoning [4], or systemic infection [5] affecting matrix function. The 1–2 mm wide transverse band is in the arcuate form of the lunula and is analogous to a Beau's line, with which it is occasionally found.
Punctate leukonychia comprises white spots of 1–3 mm diameter attributed to minor matrix trauma (e.g. manicure) (Figure 95.23); it is also seen in alopecia areata. The pattern and number of spots may change as the nail grows. With longitudinal leukonychia, there is a parakeratotic focus in the matrix, sometimes attributable to Darier disease or a small tumour. Striate leukonychia is a term used in different settings. It could be argued to occupy the middle ground between the marks of Mees’ lines and punctate leukonychia being reported in both alopecia areata and chemotherapy.
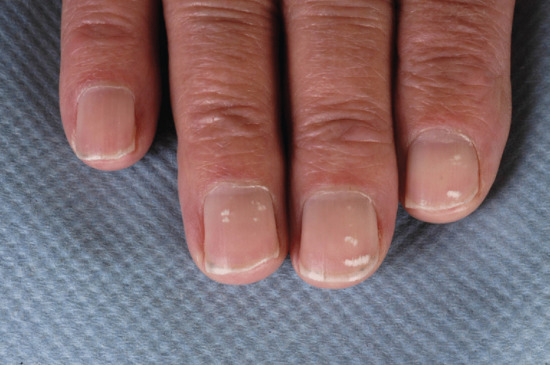
Figure 95.23 Punctate leukonychia.
Apparent leukonychia
Here, changes in the nail bed are responsible for the white appearance [1, 2]. Nail bed pallor may be a non-specific sign of anaemia, oedema or vascular impairment. It may occur in particular patterns which have become associated with certain conditions.
Terry's nail is a term used to describe nails which are white proximally and normal distally and is attributed to cirrhosis, congestive cardiac failure or diabetes [3]. Nail bed biopsy reveals only mild changes of increased vascularity.
Half-and-half nails describes nails where there is a proximal white zone and distal (20–60%) brownish sharp demarcation, the histology of which suggests an increase of vessel wall thickness and melanin deposition. It is seen in 9–50% of patients with chronic renal failure and after chemotherapy (Figure 95.3).
It is unclear whether the variant Neapolitan nails, where there are bands of white, brown and red, is a version of half-and-half or Terry's nails, or a feature of old age.
Muehrcke's paired white bands are parallel to the lunula in the nail bed, with pink between two white lines. They are commonly associated with hypoalbuminaemia, the correction of which by albumin infusion can reverse the sign. They have also recently been reported following placement of a left ventricular assist device in a patient with congestive heart failure [4].
Colour changes due to drugs and chemicals [1]
There are a number of colour changes which can be caused by drugs. Yellowing of the nail is a rare occurrence in prolonged tetracycline therapy, which can also produce a pattern of dark distal photo-onycholysis, Topical 5-fluorouracil may also cause yellow nails: the whole nail is affected and returns to normal when the drug is discontinued [2, 3]. A bluish colour, is seen with mepacrine (quinacrine) [4], the nails fluorescing yellow-green or white when viewed under Wood's light. Normal nails show slight fluorescence of violet-blue colour. Hydroxyurea has been reported to result in blue lunulae [5]. Chloroquine may produce blue-black pigmentation of the nail bed [6]. Other antimalarials may produce longitudinal or vertical bands of pigmentation on the nail bed or in the nail [7].
Hyperpigmentation due to increased melanin in the nail and nail bed has been noted in children after 6 weeks of treatment with doxorubicin (adriamycin) [9, 10]. Other similar cytotoxic drugs may cause a variety of patterns of increased pigmentation [1]. However, in AIDS, longitudinal melanonychia may be seen in untreated cases [11, 12] as well as in those receiving zidovudine [9, 13].
Argyria may discolour the nails slate blue [8], and inorganic arsenic may produce longitudinal bands of pigment or transverse white (Mees' lines).
Yellow nail syndrome
The nails in yellow nail syndrome are yellow due to thickening, sometimes with a tinge of green possibly due to secondary infection with Candida or Pseudomonas. The lunula is obscured and there is increased transverse and longitudinal curvature of the nail plate with and loss of cuticle (Figure 95.24). Occasionally, there is chronic paronychia with onycholysis and transverse ridging [1]. The condition usually presents in adults, but may occur as early as the age of 8 years [2]. It does not appear to run in families [3]. Some of the clinical features may overlap with lichen planus [4], although the latter does not have the other systemic features normally seen in this syndrome.
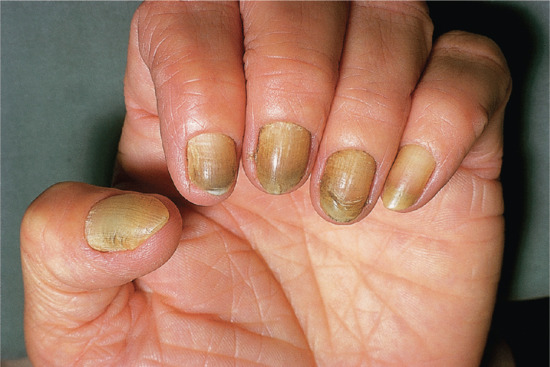
Figure 95.24 Yellow nail syndrome.
The feature nail changes are usually accompanied by lympho-edema [5] at one or more sites and by respiratory or nasal sinus disease. The nails grow at a greatly reduced rate: 0.1–0.25 mm/week for fingernails compared with the lowest normal rate of 0.5 mm/week. All 20 nails may be involved, although often a few are spared. Histologically, in the nail bed and matrix, dense fibrous tissue is found replacing subungual stroma, with numerous ectatic endothelium-lined vessels [6]. A foreign-body reaction has been noted [7]. It has been suggested that obstruction of lymphatics by this dense stroma leads to the abnormal lymphatic function found in the affected digits in some [8] but not all [9] cases.
The oedema is variable and may affect the legs, face or hands and occasionally it is universal. In some instances, the oedema has been shown to be due to abnormalities of the lymphatics, either atresia or, in some cases, varicosity [10]. Other cases have normal lymphatics, suggesting that a functional rather than an anatomical defect may be present [11], or that perhaps only the smallest lymph vessels are defective. Although the nail changes may draw attention to the underlying lymphatic abnormality, they are found only in a minority of patients with congenital abnormality of the lymphatics. Recurrent pleural effusions have been noted [12, 13]. Chronic bronchitis and bronchiectasis may also occur [12]. The condition may be associated with an increased incidence of malignant neoplasms [10, 14, 15]. Other associations include d-penicillamine therapy [5] and nephrotic syndrome [16].
In hypothyroidism and AIDS [17] there may be yellow nails, but it is debatable whether these represent yellow nail syndrome or simply the discoloration of nail associated with retarded growth [18]. There does not appear to be an inherited element in spite of original reports [19, 20].
Nail features can fluctuate enormously over time. Attempted treatments include oral and topical vitamin E, oral zinc, prednisolone and the treatment of chronic infection at other sites [20, 21, 22, 23, 24, 25]. There is debate as to whether itraconazole is of value as treatment. The drug has been demonstrated to increase the rate of longitudinal growth, but an open trial in eight patients demonstrated that half gained no benefit with respect to nail changes [26]. It is reported that results are better when itraconazole or fluconazole are combined with oral vitamin E [27]. Many authorities achieve about a 50% resolution rate, but it is not clear how much of this is part of the natural time disease course [28].
Red lunulae
Erythema of all or part of the lunula may affect all digits, but is usually most prominent in the thumb. Duration of the change will depend on the cause. When associated with cardiac failure, it may follow the course of management of the cardiac disease. When due to a subungual tumour such as a myxoid cyst or glomus tumour, it will remain until the tumour is removed. Inflammatory connective tissue causes may also result in a fluctuating course. Erythema is less intense in the distal lunula, where it can merge with the nail bed or be demarcated by a pale line, and can be obliterated by pressure on the nail plate. The appearance can fade over a few days. A single report of histological features failed to reveal vascular or epidermal changes [1]. Dotted red lunulae have been reported in psoriasis and alopecia areata, but otherwise the list of associations is so broad that it is unconvincing [2].
The exception to this is a red lunula seen in a single digit. In this setting, it often indicates a local disturbance of vascular flow, which is most likely to be a benign tumour. Glomus tumours and subungual myxoid cysts are the most common [3] and the colour may vary between blue and red.
Longitudinal erythronychia (Figure 95.25)
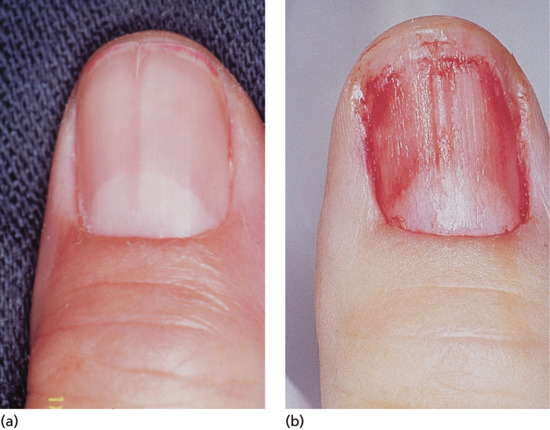
Figure 95.25 (a) Longitudinal erythronychia. (b) The longitudinal ridge in the nail bed corresponds to the groove on the undersurface of the nail plate.
A longitudinal red streak in the nail can have several causes [1, 2]. All will have a corresponding band of thinned nail plate as part of the defect. The effect of this is a strip where blood in the underlying nail bed is seen more easily not only because the nail plate is thinner but also because blood pools in the underlying nail bed capillaries as a result of reduced compression by the overlying nail. Splinter haemorrhages may lie longitudinally within the strip. Such strips of thinned nail arise because of focally reduced proliferation within the matrix. This can be due directly to matrix pathology or may be secondary to focal pressure on the matrix with secondary loss of function.
The matrix pathology includes a spectrum of epidermal disorders. The most common are lichen planus and Darier disease, where thin longitudinal red streaks may terminate at the free edge with a split. In Darier disease, there may be a small subungual keratosis [3]. Acantholytic dyskeratotic naevus and warty subungual dyskeratoma [4] may both represent localized forms of Darier disease. Longitudinal erythronychia is also a component of acrokeratosis verruciformis of Hopf in which there is both clinical and pathological overlap with Darier disease.
Pressure on the matrix may be exerted by any of the full range of dermal tumours as well as tumours of the bone and cartilage that arise from the distal phalanx.
For instances where no primary disease can be identified to explain erythronychia affecting multiple nails the descriptive term ‘idiopathic polydactylous erythronychia’ has been proposed [5].
Baran and Perrin have coined the term ‘onychopapilloma’ to describe the isolated benign warty distal nail bed lesions found in association with longitudinal erythronychia for which no underlying cause can be identified [6]. The papilloma is a secondary element, given that it is found distally in the nail bed while the cause lies proximally within the matrix. However, there is a category of this disease where the matrix disease remains unclear and the distal papilloma represents the identifiable entity. Isolated longitudinal erythronychia needs careful assessment, however, as a similar clinical presentation can be due to conditions such as Bowen disease [6] or basal cell carcinoma [7] of the matrix. Biopsy may be warranted if the erythronychia is observed to change.
Not all causes of longitudinal erythronychia conform to these rules. This is particularly the case where there are multiple red streaks associated with a dermatosis and additional nail changes. It can be a feature of lichenoid diseases of the nail unit, discoid lupus erythematosus, psoriasis, Langerhans cell histiocytosis and a number of other diseases where there is patchy nail atrophy. It can be difficult to decide whether histology is needed to ensure a benign diagnosis or one with no systemic implications. A narrow band (approximately 2 mm) that is not changing over 12 months or more with no other elements of the history or examination to cause concern would normally mean that continued monitoring alone would be sufficient management.
Splinter haemorrhages
Splinter haemorrhages represent longitudinal haemorrhages in the nail bed conforming to the pattern of subungual vessels [1–4]. They are most frequently seen in the distal nail bed and on the fingers of the dominant hand, reflecting trauma as the cause. In dermatological practice, they are often found in association with psoriasis, dermatitis and fungal infection of the nails. As they occur under so many conditions, their importance as a sign of disease is often exaggerated. Focal pathology may also represent a cause, as in longitudinal erythronychia and onychomatricoma (see above).
Large numbers of proximal haemorrhages with no obvious traumatic origin may indicate a systemic cause [5], such as bacterial endocarditis, antiphospholipid syndrome [6] or medication [7]. Nail bed psoriasis may dispose to splinter haemorrhages in the most used digits. Unilateral splinter haemorrhages may arise after arterial catheterization on the involved side. Examination under oil with a dermoscope may reveal greater detail.
TRAUMATIC NAIL DISORDERS
Nails may show signs of acute trauma, scars following acute trauma or chronic repetitive trauma.
Acute trauma
Acute trauma is classified with respect to severity, ranging from a small haematoma to digit amputation (Table 95.4) [1].
Table 95.4 Classification of acute nail trauma. (From Van Beek et al. [1].)
| Type | Effect | Therapy |
| I | Small haematoma associated with a small break in the nail bed | Fenestration of nail over the haematoma |
| II | Large haematoma with significant nail bed injury | Remove nail in order to identify site and nature of subungual damage |
| III | Large haematoma, nail plate displaced | X-ray may reveal fracture of terminal phalanx, usually in association with nail bed laceration which requires resorbable 6/0 suture |
| IV | Severe crush injury | Avulsion needed to reveal matrix, with multiple lacerations requiring careful reconstruction |
| V | Amputation of tip of digit, may include parts of matrix | If tip can be retrieved, it should be used as a graft. Otherwise nail bed from other sites may provide autologous grafts |
Subungual haematoma [1, 2]
Subungual bleeding is a common sign. It may present as a feature of acute trauma, with pain due to the recent event in combination with pain arising from the pressure exerted by the subungual accumulation of blood. A haematoma arising within the matrix will be incorporated into the nail plate [3]. Where the haematoma is associated with acute trauma, there is usually pain and the diagnosis is obvious. However, with less extreme trauma, a haematoma may not develop immediately and may be painless. This is most common in the toes and may give rise to clinical uncertainty as to whether it represents early subungual melanoma. A history of traumatic sporting hobbies is useful, and signs of symmetrical nail trauma and inappropriate footwear all indicate trauma as the cause of the appearance. Dermoscopy will nearly always resolve the situation [2, 4], but if it does not, making a small punch in the surface of the nail may reveal old blood as the source of pigment. Malignancies can bleed and so confirmation of blood does not refute the possibility of a tumour; however, as an isolated finding in the absence of other clues, this test should be sufficient to obviate the need for surgical exploration. An alternative is to score a transverse groove in the nail at the proximal margin of the pigment and observe over a few weeks as the discoloration grows out. If pigment continues to spread proximal to the groove, surgical exploration is warranted.
The only treatment that can be offered is to relieve the pressure, and if dealt with soon after the injury this can be done by puncturing the nail, for instance with a hot pointed implement, cautery, small drill or punch biopsy. This procedure will relieve pain and may save the nail. The possibility of an underlying fracture must be considered for larger haematomas [1].
It is stated that if more than 50% of the visible nail is affected, the nail plate should be removed. However, there is evidence to challenge this rule. A comparison between two groups of children having exploration and repair or trephination alone showed fewer complications in the latter group and considerably less investment in medical time [5]. A literature review failed to find clear evidence for avulsion [6].
Nail bed laceration
The nail bed may be lacerated by incisions, crush and avulsion injuries. In simple injuries there is displacement of the nail plate. Initially, the nail bed damage should be assessed by avulsion, and then the nail can be replaced after any necessary nail bed repair has been performed. The nail plate can be used as a useful splint [1]; a small window for drainage of blood and exudate is made in the nail [2]. More complicated injuries may require flap or graft reconstructions and, in some instances, vascularized composite nail grafts are used with microvascular anastomoses. When the wounds arise from crush injury, fracture is relatively common. If the distal tuft has been fractured to leave fragments of bone dispersed in the soft tissues, long-term morbidity may be prevented if these are removed [3].
Delayed trauma
The most common kind of chronic deformity following an acute injury is a split nail or reduction in the length of the nail bed with consequent overcurvature of the tip of the nail.
Cure of a split nail deformity is difficult, with only a modest chance of success [1]. Sometimes, there is an associated pterygium. Treatment entails excision of the nail bed and matrix scar and, in the case of a pterygium, a split-skin graft or part of the nail plate may be placed on the ventral aspect of the proximal nail fold to help prevent recurrence of the pterygium. It is important to keep the wounded aspects of nail bed or matrix separate from the overlying nail fold after surgery, and this is often best done by returning the nail plate after soaking it in antiseptic during the procedure.
If treatment is required for a shortened distal phalanx with nail bed changes, there are two choices [2]: the entire nail can be phenolized, or a V–Y advancement flap can be performed based on two neurovascular pedicles.
Chronic repetitive trauma
Chronic repetitive trauma may take several forms. Some have been considered in other sections detailing transverse ridges produced by a habit tic (Figure 95.26), the canaliform dystrophy of Heller (Figure 95.27) and chronic paronychia (Figure 95.28).
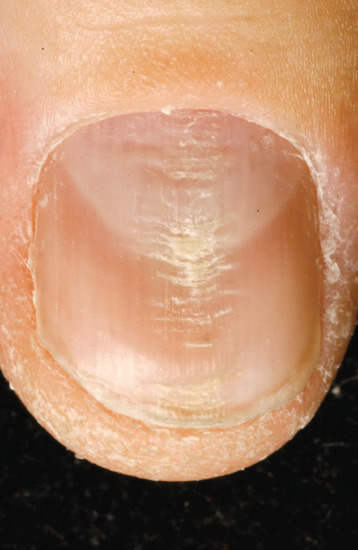
Figure 95.26 Transverse ridges resulting from habit tic.
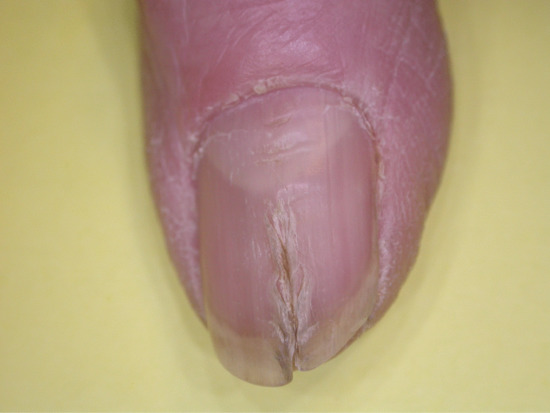
Figure 95.27 Median canaliform dystrophy of Heller affecting distal portion of nail plate; the enlarged lunula and transverse ridging seen proximally reflect chronic trauma.
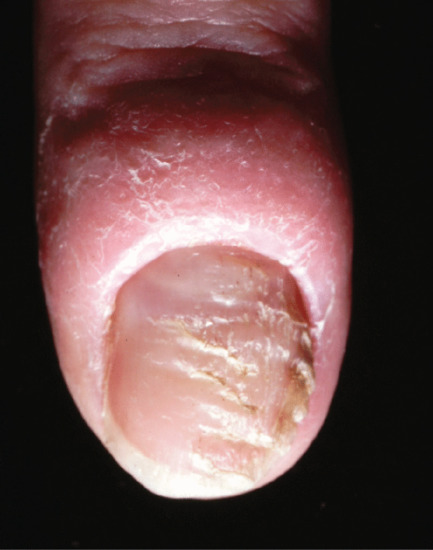
Figure 95.28 Chronic paronychia.
Nail biting
The nail plate, periunguium and nail bed are all subject to nail biting and picking. Although fingers are most commonly involved, rarely toenails are also bitten [1]. Nail biting produces distinctive features, which are found in 60% of children, 45% of adolescents and 10% of adults [2]. The majority of moderate nail biters have no associated psychiatric disorder [3]. Focal abnormalities, such as viral warts, are often a complication, whether as a cause or as a result of the Koebner effect after biting. Severe damage may be associated with self-mutilating disorders such as Lesch–Nyhan syndrome. Dental problems can arise due to nail embedded in gums or between teeth [4].
The nails are typically short, with up to 50% of the nail bed exposed. The free edge may be even or ragged. Surface change may include splitting of the nail into layers or a sand-papered effect, and the nail may acquire a brown longitudinal streak [5]. The most aggressive nail biting (onychotillomania/onychophagia) can produce subungual haemorrhage, strips of nail loss, with residual spurs or loss of the entire nail (Figure 95.29). Onychotillomania may be allied to parasitophobia when the patient picks off pieces claiming that they contain parasites [6]. A rough and irregular nail and nail fold may result with haemorrhage in the nail fold also. Many fingernails are involved. Oral pimozide may be beneficial [7].
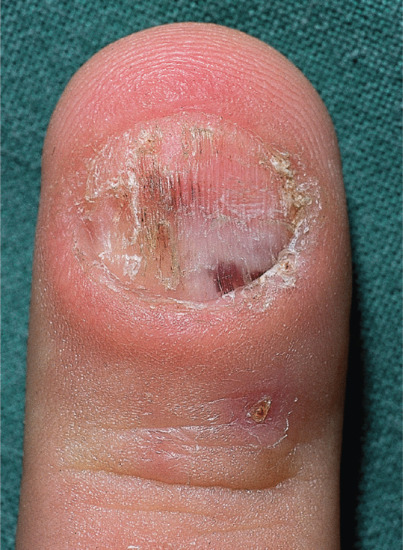
Figure 95.29 Nail biting can be extensive, with damage to the nail folds and nail plate causing subungual haemorrhage.
Trauma followed by secondary infection involving the matrix may make nail loss permanent or result in pterygium formation. The nail folds are sometimes bitten in addition to, or as a substitute for, the nail. This can lead to bleeding and chronic paronychia with acute infective exacerbations. This in turn may lead to nail plate damage or ridging and nail fold scarring. In cases associated with infection, osteomyelitis of the terminal phalanx can develop [8, 9]. Subjects will sometimes deny nail biting and attribute the appearance to a disease that stops nail growth. Transverse grooves scored proximally in the nail plate will confirm that the nail is growing by moving distally with time. In aggressive nail biting, the groove may be eroded from the surface.
Trauma is sometimes inflicted by other nails, with pushing back of the proximal nail fold as part of a habit tic (see above). This results in serial transverse ridges and depressions running up the midline of the nail, associated with loss of the cuticle (see Figure 95.29). In more conscious forms of self-damage, sharp instruments are used to produce dermatitis artefacta of the nail unit, and the nail fold is commonly preserved [10].
Management
Treatment is often unsuccessful and cure relies largely on the motivation of the patient. Where the patient acknowledges an element of self damage, they may comply with the use of paper surgical tape as a dressing over the tip of the digit 24 h a day for 2–3 months. This needs to be replaced several times a day in some instances. In the first month, it may be helpful to combine the tape with moderate potency topical steroid to suppress any inflammation. Ensure there is no infection prior to this. Local antiseptics and antimicrobial ointments may help settle the infection secondary to nail unit damage. Antiseptics or treatments with the most bitter taste are often prescribed in the belief that this will discourage biting. This is seldom the case. Antidepressants [11] and behavioural therapy [12] have been used with some success in limited studies.
Damage from nail manicure instruments
Metal instruments, such as a nail file or scissors, wooden or plastic orange sticks, or nail whitener pencils may create acute or chronic injuries in the nail area. Onycholysis may result from using the sharp point for cleaning under the nail plate. Nails, however, are best cleaned with a nail brush and soap, because overzealous manicure, pushing back the cuticles, may result in white streaks across several nails. Cleaning around the nail with contaminated instruments may lead to acute or chronic paronychia. According to Brauer and Baran [1], it is not advisable to cut or clip the nail plate, as this produces a shearing action that weakens the natural layered structure and promotes fracturing and splitting. An emery board is preferred for shaping the fingernail by filing from the sides of the nail towards the centre.
Trauma from footwear
Onychogryphosis and nail hypertrophy [1–5]
Onychogryphosis is an acquired dystrophy usually affecting the great toenail, which is thickened, yellow and twisted. It is most commonly seen in the elderly often made worse because of difficulties in self care of the feet [1, 2, 4]. Trauma and biomechanical foot problems may, however, precipitate similar changes in middle age or earlier.
At one time, onychogryphosis was known as ostler's nail, because some cases could be traced to injury caused by a horse trampling on the foot of the ostler. Competitive sport is a more contemporary cause. The injury, once sustained, is aggravated by footwear. As the nail becomes longer and thicker, damage from footwear becomes progressively more important. Nail hypertrophy implies thickening and increase in length, whereas onychogryphosis implies curvature also.
Some cases of nail hypertrophy are intrinsic, and this applies especially to toenails other than the nail of the great toe. The nail becomes thick and circular in cross section instead of flat, and thus comes to resemble a claw.
In onychogryphosis, one or more nails become greatly thickened (Figure 95.30) and, with neglect, increase in length, becoming curved like a ram's horn (Figure 95.31). The nails of the great toes are most often involved, but no toenail is exempt. It is possible that the nail plate distortion produced by chronic untreated onychomycosis may be partly responsible for onychogryphosis at a later stage. In extreme cases, the free edge may press on or even re-enter the soft tissues of the foot.
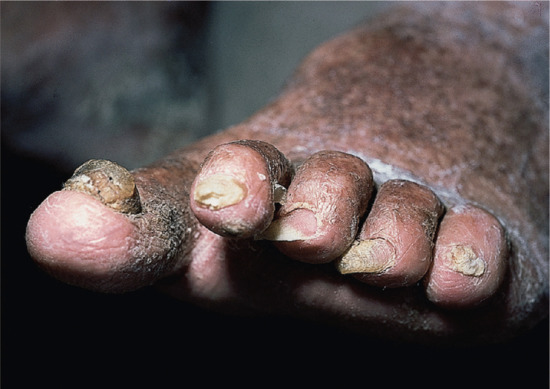
Figure 95.30 Early onychogryphosis of the left great toenail.
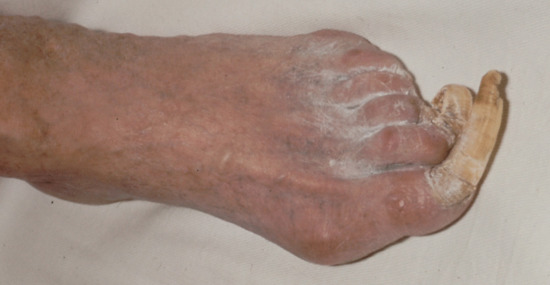
Figure 95.31 Severe onychogryphosis resulting from neglect.
Treatment of onychogryphosis and nail hypertrophy may be either radical or palliative. Radical treatment consists of surgical removal of the nail and matrix and is recommended in those with good circulation (Figure 95.32). Palliative treatment requires regular paring and trimming of the affected nails, usually by a podiatrist using nail clippers and a file or mechanical burr. The thickened nails are extremely hard and trimming is difficult. Other causes of thickened nails include psoriasis, pityriasis rubra pilaris, Darier disease, fungal infections, pachyonychia congenita, congenital ectodermal defects and congenital malalignment of the great toenails [6].

Figure 95.32 (a–c) Onychogryphosis is often best treated with ablation of the nail matrix.
Ingrowing toenail [1, 2, 3]
The nail can ingrow on any of its four margins, although lateral ingrowing is the most common pattern and is usually found on the big toe. The soft tissue at the side of the nail (lateral nail fold) is penetrated by the edge of the nail plate, resulting in pain, inflammation and, later, the formation of granulation tissue [4]. Infection is not typically associated, although the combination of pain, redness and swelling with ooze will dispose to treatment with antibiotics. Penetration of the nail fold is often caused by spicules of nail at the edge of the nail plate which have been separated from the main portion of the nail. The great toes are those most often affected. The main cause for the deformity is lateral compression of the toe due to ill-fitting footwear, and the main contributory cause is cutting the toenails in a half-circle instead of straight across. Anatomical features, such as an abnormally long great toe and prominent lateral nail folds, are important in some cases. Sport, with the toe impacting on the inside of the shoe through kicking or other movements, can be a contributory factor.
Nail can embed in the proximal nail fold when there is disturbance of nail growth, usually through trauma. This results in dislodging of the nail upwards with a new nail growing beneath. The proximal aspect of the old nail then impacts on the ventral aspect of the proximal nail fold and this creates the same features of inflammation, ooze, swelling, redness and pain as seen when the lateral nail fold is affected. Proximal nail ingrowing is known as retronychia and is self-limiting over a matter of several months as eventually the older nail is shed. During that time, nothing effectively relieves the problem and avulsion is the treatment of choice. The replacement nail usually grows back without any problem [5].
In infancy, ingrowing toenail most commonly occurs before shoes are worn and is associated with crawling, ‘pedalling’ or wearing undersized ‘jumpsuits’ [6]; acute paronychia may be associated. Rarely, it is congenital [7] and even familial [2]. In children, ingrowing is commonly distal rather than lateral. Management is conservative in most instances, with topical steroid and antiseptic preparations. Surgery is occasionally required [8].
Clinical features
The first symptoms are pain and redness, shortly followed by swelling and pus formation. Granulation tissue then forms and adds to the swelling and discharge. More severe infection may follow (Figure 95.33a,b). There is seldom any difficulty with diagnosis. Excess nail fold granulation tissue can also be a feature of amelanotic melanoma and reactions to medications such as retinoids, ciclosporin, antiretroviral drugs and chemotherapy [9, 10, 11, 12, 13, 14, 15].
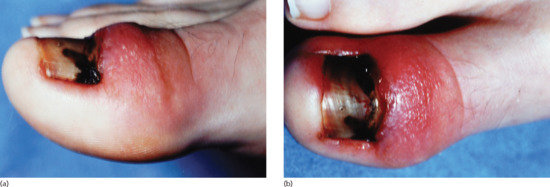
Figure 95.33 (a,b) Ingrowing great toenail complicated by proximal ingrowing (retronychia).
Management (see Nail surgery section)
Treatment may be difficult and prolonged. The first essential is to insist that the patient wear shoes sufficiently wide, high and pliable to remove lateral pressure [16]. Any abnormality of foot/toe function should be corrected. The patient must also be instructed to cut the nail straight across instead of in a semicircle. The nail must be allowed to grow until its edges are clear of the end of the toe before it is cut; this prevents the further formation of marginal spicules. In the early stages, the infection may be overcome by the application of antiseptics and by inserting a pledget of cotton-wool under the edge of the nail. Taping the toe or applying plastic gutters between nail edge and nail fold are alternatives [17]. These can be supplemented with acrylic nail to build up a smooth surface able to push the nail fold away and relieve ingrowing [18].
Twice-daily warm water baths followed by careful drying and powdering are helpful. Potent topical corticosteroids can help to diminish inflammation and suppress granulation tissue. They should, however, only be used after infection has been ruled out or is being actively managed. If the infection is more severe with local cellulitis, an appropriate systemic antibiotic should be administered. When granulation tissue forms this should be destroyed by cauterization with a silver nitrate stick. It is important that an amelanotic melanoma is not missed [19], and if there are atypical features a biopsy should be performed.
If conservative measures fail, operative intervention will be necessary. Removing the nail alone is likely to result in recurrence of ingrowing when the nail returns [20] and so should be combined with a curative procedure such as phenolization of the relevant part of the matrix [4, 21]. Although surgical excision of the matrix can provide an excellent result, it is more dependent than phenolization on the skill of the practitioner. In large studies, phenol treatment results in a greater cure rate and less morbidity (see Nail surgery section) [21].
TUMOURS UNDER OR ADJACENT TO THE NAIL
Benign tumours
Lobular capillary haemangioma (pyogenic granuloma) of nail apparatus
Definition and nomenclature
Pyogenic granuloma (PG) is a common acquired benign vascular tumour frequently encountered at the nail apparatus (nail bed and folds).
Introduction and general description
Although lobular capillary haemangiomas (PGs) may occur at many different sites (see Chapter 137), they have a particular predilection for the soft tissues around the nail.
Pathophysiology
Nail PGs are due to a range of causes that act through different pathogenetic mechanisms which are as yet not clearly understood.
Predisposing factors
Nail PGs are secondary to four main causes as follows:
- 1Trauma: local trauma is the most common cause of PGs involving the nail apparatus. Ingrowing nail, retronychia [1], friction from footwear [2], a range of self-induced disorders (onychotillomania, onychophagia and aggressive manicure) and accidental penetration of a foreign body may also promote the development of PG [3].
- Drugs: the main characteristic of drug-induced nail PGs is the involvement of multiple digits, both fingers and toes. Several drugs have been implicated including retinoids (systemic and topical) [4, 5–8], antiretroviral therapies (indinavir, lamivudine) [4, 9–11], mitozanthrones [12], ciclosporin [13] and systemic 5-fluorouracil [14]. A number of new targeted therapies have become an increasingly important cause of PG. The antineoplastic therapies which are very commonly associated with multiple PGs are epidermal growth factor receptor (EGFR) inhibitors (cetuximab, gefitinib) [4], agents of the fluoropyrimidine family (capecitabine) [15, 16, 17] and agents of the taxan family (docetaxel, paclitaxel) [4, 18, 19]. Multiple eruptive PGs have also been reported in association with anti-CD20 antibody treatment for severe rheumatoid arthritis [20].
- Peripheral nerve injury: different conditions, all having in common injury to the peripheral nerves, have been reported to be associated with nail changes and PGs of the proximal nail fold. Plaster cast immobilization is one such condition, where poor application technique may result in peripheral nerve damage from mechanical compression [21]. Patients will often complain of paraesthesiae and pain. A few days after cast removal, the nail plate detaches proximally from the bed (onychomadesis) and is associated with periungual swelling and PG formation under the proximal nail fold. Similar nail changes have been observed in reflex sympathetic dystrophy [22]. Periungual PGs have also been reported after Guillain–Barré syndrome [23], in patients with hemiplegia [24] and after multiple episodes of hypoxia [25].
- PGs due to inflammatory systemic diseases: periungual PGs involving multiple fingernails and toenails have been reported in cutaneous sarcoidosis, psoriasis and seronegative spondyloarthritis [4].
Pathology
Histopathology shows the characteristic features of PG, irrespective of cause and location (see chapter 137).
Clinical features
History
The patient's history usually identifies the cause of the PG.
Presentation
A PG starts as a minute red papule that rapidly grows to the size of a pea or even a cherry. It bleeds easily, and the surface may become eroded by necrosis of the overlying epidermis.
Clinical variants
PGs are commonly located at the proximal nail fold, but may develop distally in the hyponychium or on the nail bed. In the latter instance, which often results from prolonged frictional trauma, the PG is associated with onycholysis (Figure 95.34).
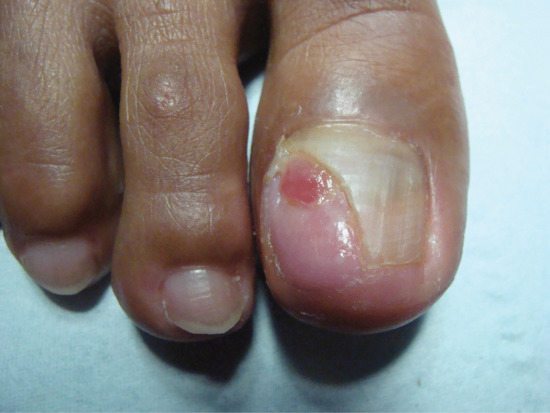
Figure 95.34 Pyogenic granuloma resulting from friction of the overlapping second toe against the lateral aspect of the great toenail.
Differential diagnosis
When a PG is single, especially if it involves the nail bed, histological examination is necessary to rule out melanoma and squamous carcinoma.
Complications and co-morbidities
In some instances PG may promote local infection.
Disease course and prognosis
If local trauma is suspected, the cause should be addressed (nail spur in ingrowing toenail, surgical removal of foreign body, stopping nail manipulation in onychotillomania, etc.). For drug-induced PGs, conservative treatment is recommended as they are likely to recur until the responsible drug is discontinued or replaced if necessary with a different agent. PG due to cast immobilization usually heals with topical corticosteroids. PGs due to reflex sympathetic dystrophy or to systemic diseases are more difficult to treat and often need several cycles of topical therapy or surgical removal [4].
Investigations
Histological examination should always be undertaken to rule out amelanotic melanoma or squamous cell carcinoma when faced with a single PG without a clear aetiology.
Glomus tumour
Definition and nomenclature
Glomus tumour, a benign tumour of the myoarterial glomus (see chapter 137), is an important cause of severe pain under the nail [26].
Epidemiology
Incidence and prevalence
This is an uncommon neoplasm which represents about 1–2% of all hand tumours [26].
Age
Glomus tumour occurs mainly in patients in their forties.
Sex
It affects predominantly women (up to 90% of cases) [27].
Pathophysiology
Glomus tumours arise principally in the pulp or nail bed or matrix of the distal phalanx, where the glomus bodies of Masson are numerous.
Pathology
A solid glomus tumour is composed of clusters of glomus cells surrounding capillaries. Glomus cells are uniform and round with pale eosinophilic cytoplasm, and a centrally located round nucleus. A basal lamina, highlighted by periodic acid–Schiff (PAS), surrounds each cell [28].
Presentation
Pain is the predominant symptom of a subungual glomus tumour. The pain may be pulsating, spontaneous or provoked by the slightest trauma. Variations in temperature, especially cold, may trigger pain radiating to the shoulder. Pain is sometimes described as worse at night. One case reports that even polishing the nail was unbearable [29].
Clinical variants
There are two main clinical presentations of subungual glomus tumour as follows:
- A small reddish or bluish spot (<1 cm) seen through the nail plate (Figure 95.35).
- A longitudinal erythronychia with distal notching or overlying longitudinal fissure.
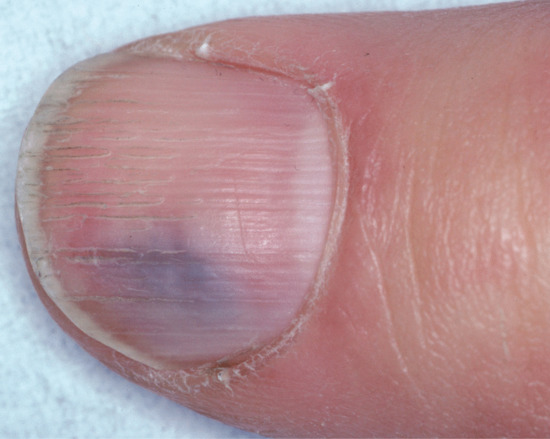
Figure 95.35 Painful glomus tumour of the nail bed. Note the bluish hue.
Differential diagnosis
Differential diagnosis includes all causes of nail pain (Box 95.1). Exceptionally, glomus tumour might be totally painless.
Complications and co-morbidities
Pressure of the glomus tumour on the underlying phalanx may induce bone erosion in 50% of cases [27, 30].
Disease course and prognosis
Patients have been wrongly referred to psychiatrists due to misdiagnosed glomus tumour where no nail alteration was visible and no proper work-up performed.
Investigations
Glomus tumour is the main indication for MRI of the nail unit [31]. It offers the highest sensitivity and best assessment of the extent of the tumour. The signal behaviour varies with the histological nature (vascular, cellular, myxoid) of the lesion [32]. MRI accurately determines the spatial location of the tumour, enabling a precise and radical surgical resection to be carried out [33]. Recurrent symptoms can usually be attributed to small synchronous satellite lesions [34].
Management
Treatment consists of surgical removal of the tumour. Two approaches are possible: the direct approach after nail plate avulsion through the nail bed or the matrix followed by meticulous repair [35, 36] or the lateral approach on the volar aspect of the lateral nail fold. The latter gives a more restricted view of the tumour with a higher chance of incomplete excision compared with the transungual approach [27, 37]. It should be recommended only for lesions that are proximal and deep seated [38].
Subungual exostosis
Definition
Subungual exostosis is an isolated slow-growing benign osteochondral outgrowth from the distal phalanx.
Most authors consider it to be a distinct clinicopathological entity [39], but some classify them with osteochondromas [40].
Epidemiology
Incidence and prevalence
Subungual exostosis is probably considerably underreported. The prevalence is unknown.
Age
Patients within their twenties are mostly affected [41, 42, 43].
Sex
The sex ratio varies from series to series but is most probably 1 : 1.
Pathophysiology
Subungual exostosis was previously thought to be a reactive process. It is now considered a true neoplasm harbouring a pathognomonic translocation t(X;6)(q22;q13-14) [44].
Predisposing factors
Trauma seems to be the most important aetiological factor [41].
Pathology
Histopathology shows a bony tumour with a hyaline cartilaginous cap.
Clinical features
History
The association of nail deformity and pain is highly suggestive, but pain is often not present.
Presentation
All large series (n = 19–45) have shown that the great toenail is affected in three quarters of cases [41, 42, 45]. Subungual exostosis usually elevates the nail plate as it emerges from the hyponychium (Figure 95.36a) or from a lateral sulcus. In its early stages, the tumour may have a porcelain white hue with superficial telangiectases and a collarette surrounding its base. As the tumour enlarges, it develops a thick hyperkeratotic surface.
Figure 95.36 Subungual exostosis: exophytic growth of bone emerging from under the nail plate through collarette of skin (note the telangiectases) (a); exostosis from the dorsal surface of the terminal phalanx presenting as painful onycholysis (b,c).
Clinical variants
Dorsal subungual exostoses may present as a nondescript erythematous patch seen through the nail plate, with or without onycholysis (Figure 95.36b,c).
Differential diagnosis
Differential diagnosis includes verruca vulgaris, fibrokeratoma, PG, ingrowing toenail, squamous cell carcinoma and amelanotic melanoma.
Complications and co-morbidities
Erosion and secondary infection of the nail bed may give rise to a subungual PG-like outgrowth [46] (Figure 95.37).

Figure 95.37 This lesion was mistaken for an ingrowing toenail. X-rays confirmed the presence of subungual exostosis.
Investigations
Radiographic examination is the cornerstone in the diagnosis of subungual exostosis. Early lesions, mostly formed from cartilage, may not be visible.
Management
Treatment is resection of the outgrowth under full aseptic conditions.
Digital myxoid pseudocyst
Definition and nomenclature
Digital myxoid pseudocysts are the second most common benign tumours of the digits.
Epidemiology
Incidence and prevalence
The exact incidence and prevalence are not known.
Age
Over 50 years old.
Sex
It is estimated that women are affected more than twice as often as men [47].
Associated diseases
Osteoarthritis.
Pathophysiology
It is now believed that digital myxoid pseudocysts result from leakage of synovial fluid through a breach in the joint capsule of the distal interphalangeal joint [48], as could be demonstrated in more than 85% of cases in a study using MRI [49].
Predisposing factors
The presence of osteophytes and reduction of the joint space from osteoarthritis or repetitive occupational trauma [50] promote leakage of joint fluid.
Pathology
Digital myxoid pseudocysts manifest as well-circumscribed but unencapsulated cyst-like dermal swellings, devoid of any lining. They consist of large mucin-filled spaces containing spindle-shaped and stellate fibroblasts without atypia [28].
Presentation
The clinical features depend upon their location in relation to the nail apparatus. De Berker et al. classified them into three subtypes [51, 52] as follows:
- Type A: the most common presentation, the digital myxoid pseudocyst presents as a nodule between the distal interphalangeal joint and the proximal nail fold (Figure 95.38).
- Type B: the digital myxoid pseudocysts is in the proximal nail fold and presses on the underlying matrix resulting in a longitudinal groove in the nail plate (Figure 95.39a,b). The groove often varies in depth according to the fluctuating volume of the cyst (Figure 95.39c). A small keratotic tip protruding from under the proximal nail fold may also be observed.
- Type C: the digital myxoid pseudocyst exerts pressure from under the matrix, giving rise to a reddish or bluish lunula (Figure 95.40).
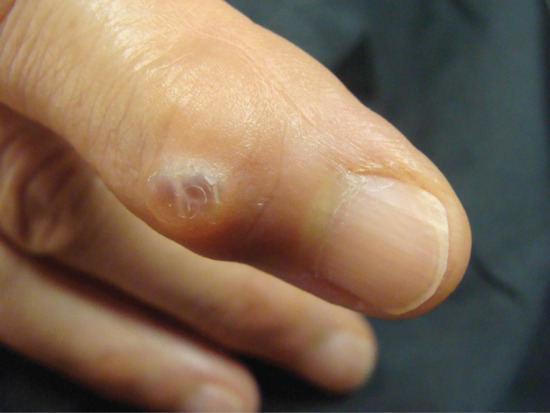
Figure 95.38 Digital myxoid pseudocyst type A.
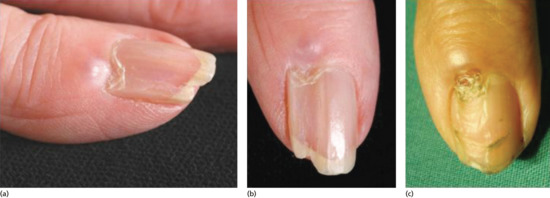
Figure 95.39 Digital myxoid pseudocyst type B. Note the longitudinal groove arising from underneath the proximal nail fold where the matrix is compressed by the overlying pseudocyst and extending to the free edge of the nail (a–c); any tumour compressing the matrix may give rise to a longitudinal gutter as shown in (a) and (b) but only a myxoid pseudocyst, which commonly fluctuates in size and therefore the pressure exerted on the matrix, may give rise to the irregular guttering as seen in (c).

Figure 95.40 Digital myxoid pseudocyst type C. Note the red macule within the lunula.
Differential diagnosis
Main differential diagnosis is fibrokeratoma in subtype B.
Disease course and prognosis
In most instances, digital myxoid pseudocysts are asymptomatic but unsightly and therefore may bother the patient. Increased pressure within the joint may be responsible for pain.
Investigations
None are necessary except for type C, for which ultrasound or MRI may be needed.
Management
Numerous treatments have been recommended for this condition. Their aim is to obliterate the leakage from the joint, by inducing fibrosis around the capsule.
Acquired ungual fibrokeratoma
Definition and nomenclature
Acquired ungual fibrokeratoma is a solitary benign asymptomatic nodule with a hyperkeratotic tip that forms in the periungual area or, rarely, within or under the nail plate.
Pathophysiology
Trauma is thought to be the major causative factor.
Pathology
Acquired ungual fibrokeratomas are pedunculated fibroepithelial lesions. The epidermis is hyperkeratotic and acanthotic, with thickened, often branching, rete ridges. The core of the lesions is composed of fibroblasts and dense collagen fibres. The vascular component is sometimes prominent [28]. No histogical difference has been found between isolated acquired ungual fibrokeratomas and the Koenen tumours of tuberous sclerosis [53].
Presentation
Most of them emerge from under the proximal nail fold and lie in a longitudinal groove which extends to the free edge of the plate. Their size varies considerably from tiny to prominent (Figure 95.41); they may sometimes be bifid.
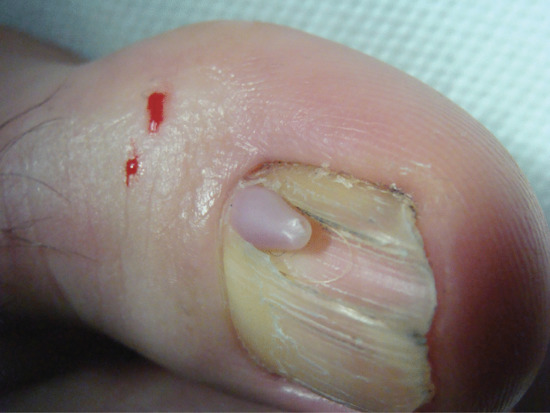
Figure 95.41 Submatricial fibrokeratoma pressing onto the underlying matrix with subsequent longitudinal smooth groove.
Clinical variants
Rarely, an acquired ungual fibrokeratoma may originate from the matrix and grow into the nail plate (intraungual fibrokeratoma) (Figure 95.42) to eventually emerge in the middle of the nail. Subungual fibrokeratomas arising from the nail bed are also rare.
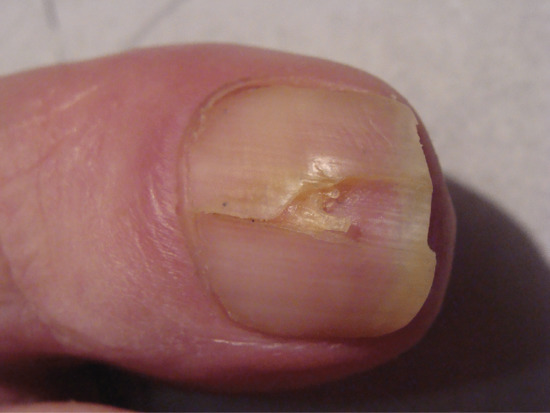
Figure 95.42 Intraungual (dissecting) fibrokeratoma. The lesion grows within the nail plate and emerges at its distal half.
Differential diagnosis
Fibroma, keloid, Koenen tumours, recurring digital fibrous tumour of childhood, cutaneous horn, exostosis.
Investigations
Histology is mandatory as Bowen disease may present as a pseudofibrokeratoma [54, 55].
When lesions are present on several digits, tuberous sclerosis should be ruled out. The lesions are then called Koenen tumours (Figure 95.43). They develop most commonly on the toes around puberty and their number increases with age.
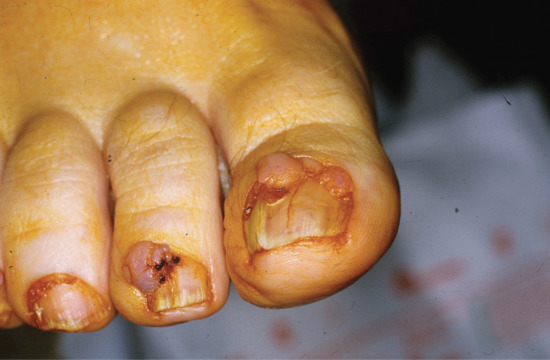
Figure 95.43 Multiple soft fibrokeratomas in tuberous sclerosis.
Management
Surgical removal.
Subungual keratoacanthoma
Definition
Subungual keratoacanthoma is a rare benign but rapidly growing and aggressive tumour that is usually situated in the most distal portion of the nail bed.
Epidemiology
Incidence and prevalence
Unknown.
Sex
Subungual keratoacanthoma occurs predominantly in males (75% of cases) [56].
Pathophysiology
The pathogenesis not understood.
Predisposing factors
Trauma [56], oncogenic human papillomavirus [57] and, in one case of steel wool [58], have each been suggested as contributory factors.
Pathology
Microscopic examination shows a squamoproliferative lesion with a focal crateriform pattern and overlying hyperkeratosis with ortho- and parakeratosis. Lobules of squamous epithelium are often well differentiated, composed of large keratinocytes with copious ‘glassy’ eosinophilic cytoplasm. Dyskeratotic cells are numerous, but atypia and mitotic figures are rare. Tumour protein p53 and proliferation marker Ki-67 can help distinguish subungual keratoacanthomas from subungual squamous cell carcinomas [28].
Genetics
In women, the development of multiple subungual keratoacanthomas may represent a late manifestation of incontinentia pigmenti.
Clinical features
History
Subungual keratoacanthomas are rapidly growing tumours (within weeks) which are always painful and are most often located on the distal part of the nail bed. They are most commonly located on the thumb but the index and middle fingers are also well-recognized sites [56].
Presentation
The tumour may start as a small and painful keratotic nodule just under the free edge of the nail, rapidly growing to 1–2 cm in diameter within 4–8 weeks. After clipping of the overlying nail plate, the typical gross appearance resembles that of keratoacanthoma at other sites as a dome-shaped nodule with a central crater plugged by keratinous material (Figure 95.44). The tumour may rapidly plunge deeper and erode the underlying bony phalanx.

Figure 95.44 Subungual distal keratoacanthoma. Note the keratotic plug on the distal bed.
Clinical variants
If located more proximally under the nail fold, subungual keratoacanthomas may present as a painful chronic paronychia [56, 59].
Differential diagnosis
The three main differential diagnoses are: epidermoid implantation cyst, subungual wart and squamous cell carcinoma.
Complications and co-morbidities
Lonlasting lesions may lead to complete destruction of the distal bony phalanx.
Investigations
Standard X-rays consistently demonstrate a well-defined cup-shaped erosion of the underlying bone (Figure 95.45). The margins of the defect show no evidence of sclerosis or any sign of periosteal reaction [60]. This lytic effect is attributed to the very rapid compression from the tumour rather than tumour invasion [61]. Long-term radiological follow-up data following subungual keratoacanthomas are sparse, but failure to reossify [58], partial repair of the bony defect [62] and spontaneous regression with full reossification [63] have all been reported.
Figure 95.45 X-rays showing massive osteolysis of the distal bony phalanx associated with subungual keratoacanthoma.
Onychomatricoma
Definition and nomenclature
Onychomatricoma is a rare benign tumour of the matrix, with peculiar clinical and pathological features, first described in 1992 by Baran and Kint [64].
Epidemiology
Incidence and prevalence
Unknown.
Age
All published cases were adults except one in a child, in whom the diagnosis was purely clinical and without histopathological verification [67].
Ethnicity
The overwhelming majority of cases are reported in white people, and only exceptionally in non-Europeans [68].
Pathophysiology
The origin of the tumour remains obscure. It most probably stems from a disturbed differentiation of nail matrix cells. The tumour digitations are onychogenic and responsible for the thickening of the nail plate.
Pathology
Histopathology is unique.
Two different zones may be observed as follows:
- The distal zone is characterized by multiple ‘glove finger’ papillary projections covered by a matrix-type epithelium devoid of stratum granulosum that keratinizes through an eosinophilic keratogenous zone.
- The proximal zone is dome shaped in transverse sections and lined by a papillomatous matrix-type epithelium, with vertically oriented deep invaginations into the stroma. These invaginations surround optically empty cavities in a characteristic V-shaped configuration [28].
Clinical features
History
The condition is indolent and patients mostly seek medical advice for cosmetic purposes, when the tumour has been evolving for several years.
Presentation
Single fingers are most commonly affected (75%), mainly the middle finger [69]. Few reports mention involvement of the lesser toes [70] or exceptionally of several digits [71].
Several clinical signs are striking enough to either make the diagnosis or at least to arouse suspicion (Figure 95.46) as follows:
- Thickening of the nail plate, of various width, often sparing a part of normal pinkish nail.
- Transverse and longitudinal overcurvature of the affected portion of the nail.
- Xanthonychia of the affected part of the nail.
- Longitudinal ridging, sometimes quite prominent on the surface of the nail.
- Splinter haemorrhages, mostly proximal but sometimes distal.
- Woodworm cavities at the free edge of the thickened nail plate (Figure 95.47).
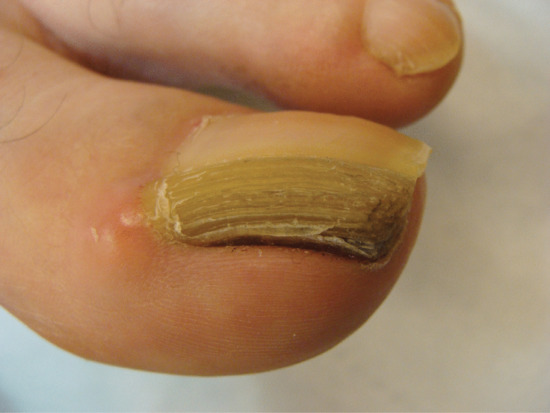
Figure 95.46 Onychomatricoma, pigmented variant: note the very well-delimited longitudinal thickening of the plate.
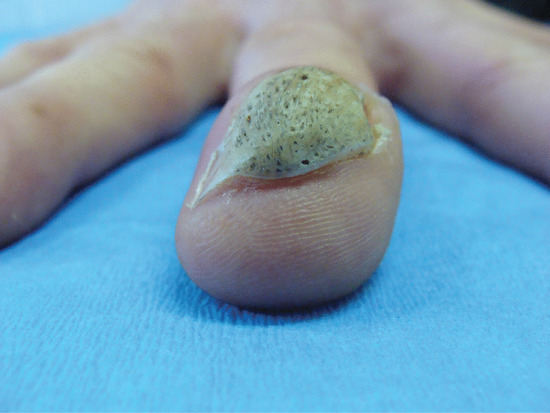
Figure 95.47 Onychomatricoma: ‘woodworm’ cavities in the nail plate are especially visible in this longstanding case (>40 years).
Clinical variants
Some unusual clinical variants have been reported: giant form [72], association with dorsal pterygium [73, 74] or associated with onychomycosis and longitudinal melanonychia [75]. Only rarely is the length of the digitations such that clipping of the free edge of the nail induces bleeding [76].
Differential diagnosis
Clinical presentation is characteristic but onychomycosis and Bowen disease [77] should be ruled out.
Disease course and prognosis
Excellent prognosis if skilled surgeons perform the surgery. Longlasting lesions may end in complete destruction of the nail plate.
Investigations
Dermoscopy confirms diagnosis in showing the woodworm perforations at the distal edge of the nail. Recently, nail clipping of the diseased part of the nail has been shown to be a minimally invasive method to achieve the correct diagnosis of onychomatricoma [78]. MRI is typical and reveals a tumour emerging from the nail matrix [79]. Ultrasonic examination seems promising [80]. Nail avulsion is diagnostic as it exposes a villous tumour, reminiscent of a sea anemone, emerging from the matrix while the nail appears as a thickened funnel, storing filamentous digitations of matrix fitting into the holes of the proximal nail extremity (Figure 95.48).
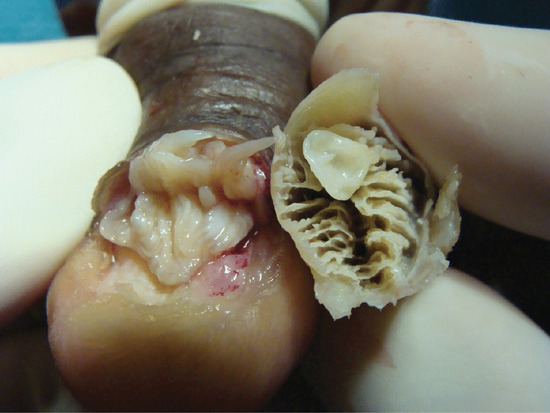
Figure 95.48 Onychomatricoma: showing the sea anemone-like matrix tumour and the cavities in the avulsed nail plate into which digitate projections from the tumour had infiltrated.
Management
Surgical removal of the tumour is the only option. The tumour should only be shaved from the underlying matrix [35].
Superficial acral fibromyxoma
Definition and nomenclature
Superfical acral fibromyxoma is a rare slow-growing soft-tissue tumour which has a predilection for the subungual and periungual regions of the fingers and toes in adults [81]. It is a distinct clinicopathological entity, recognized by Fetsch et al. in 2001 [82].
Epidemiology
Incidence and prevalence
Unknown.
Age
Middle-aged adults.
Sex
Males are more commonly affected than women (male to female ratio: 1.6 : 1).
Pathology
It presents as a relatively well-circumscribed but unencapsulated dermal tumour composed of spindle-shaped cells integrated in a fibromyxoid matrix, sometimes invading the subcutis, often with accentuated vasculature and increased numbers of mast cells. Nuclear atypia is slight or absent and mitotic figures are infrequent. Immunohistochemically, more than 90% of cases are positive for CD34. CD99 and epithelial membrane antigen (EMA) are often focally positive [28, 83].
Presentation
Superfical acral fibromyxoma is normally diagnosed on histology and its clinical presentation has not been well characterized. The tumour is located in the nail bed or nail folds. Some reports describe a dome-shaped, well-circumscribed, whitish to pink firm tumour, sometimes surrounded by a basal collarette, lifting up the plate (Figure 95.49) and covered with very thin fissured keratin; if located deep in the lateral nail fold, it presents as a swollen fold covered with normal skin. It may or may not be painful [84, 85].
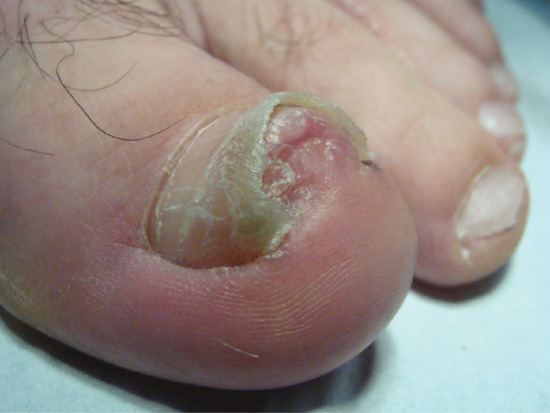
Figure 95.49 Superficial fibromyxoma of the nail bed elevating the distal plate.
Clinical variants
Exceptionally, superfical acral fibromyxoma may be located beneath the matrix [86].
Differential diagnosis
Lipoma, schwannoma and neurofibroma.
Complications and co-morbidities
Bony involvement occurs in one third of cases [86].
Disease course and prognosis
No metastases were observed in a recent series of 124 cases with a mean follow-up of 35 months [87].
Investigations
Radiological imaging should be performed to rule out bony involvement.
Management
Complete surgical resection, as it has a propensity for local recurrence if incompletely excised [87].
Onychopapilloma
Definition and nomenclature
Onychopapilloma is a benign longitudinally oriented subungual tumour of unknown aetiology.
Pathophysiology
Pathology
Onychopapilloma is characterized by: acanthosis and papillomatosis, mostly of the distal part of the nail bed; matrix metaplasia of the nail bed with an onychogenous zone; canaliform deformation of the ventral part of the nail plate; and a keratinous mass under the distal nail plate [28].
Clinical features
History
Patients seek medical advice either because of pain or because they catch the fissured free edge of the nail.
Presentation
Onychopapilloma usually presents as an isolated pink longitudinal nail streak (erythronychia) extending from the distal matrix to the free edge, from under which emerges a fine filiform subungual keratosis. It may be accompanied by distal onycholysis or a fissure. Distal splinter haemorrhages are also common (Figure 95.50).
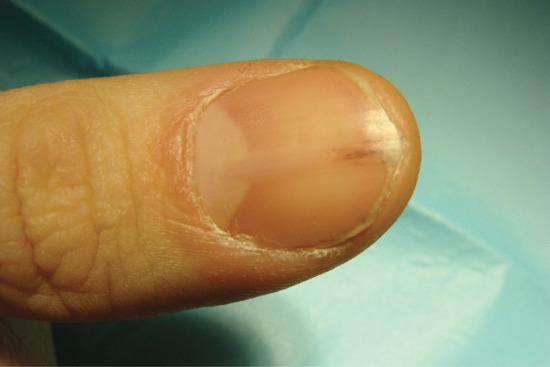
Figure 95.50 Onychopapilloma: note the longitudinal erythronychia starting in the distal matrix, the distal splinter haemorrhages and the onycholysis at its distal end. The nail was longer at that place because the papilloma impaired nail clipping (pain).
Clinical variants
Onychopapilloma may, albeit rarely, present as longitudinal melanonychia [90] or leukonychia [91].
Differential diagnosis
Bowen disease [89] and nail lichen planus [92] may present in rare instances as an onychopapilloma.
Management
The onychopapilloma is usually excised only if it bothers the patient or to rule out other tumours.
Malignant tumours
Squamous cell carcinoma
Definition
Squamous cell carcinoma is the most frequent malignant tumour of the nail apparatus, where presentation as in situ squamous cell carcinoma (Bowen disease) is more common than invasive squamous cell carcinoma.
Epidemiology
Age
The mean age at presentation is 60 years [93, 94, 95, 96).
Sex
Three quarters of cases occur in males [93, 95, 96].
Pathophysiology
Predisposing factors
One third of patients with squamous cell carcinoma of the nail apparatus have a personal history of human papillomavirus-associated genital disease (genital warts, dysplasia or cancer of the cervix) or a similar history in a sexual partner. The average time between the onset of the genital disease and the appearance of the nail tumour is around 12 years [95]. It is estimated that genito-digital transmission of human papillomavirus is responsible for up to 60% of cases of squamous cell carcinoma of the nail apparatus (both in situ and invasive forms). Prolonged unprotected contact with chemical mutagens is another possible predisposing factor.
Pathology
The picture is identical to that of Bowen disease in other skin areas [97]. The most important feature to look for is the intact basement membrane defining the in situ form.
Causative organisms
Human papillomavirus, especially serotype 16, which is isolated in three quarters of cases [95], but also serotypes 2, 6, 11, 18, 26, 31, 34, 35, 56, 58 and 73 [98–101].
Environmental factors
Ionizing radiation, arsenic and pesticides have been suggested as potential causative factors [95].
Clinical features
History
Patients are often not bothered by this indolent and painless condition and therefore tend to seek medical advice very late (mean delay 6 years) [93, 102].
Presentation
The clinical presentation is protean, accounting for the delay in diagnosis. The largest published series identifies the right index and middle fingers as the most commonly affected. This finding is in agreement with the postulated genito-digital transmission of human papillomavirus [93]. The condition is usually solitary but, uncommonly, tumours may arise in more than one digit [103, 104, 105]. The most common clinical findings are, in decreasing order of frequency, subungual hyperkeratosis (Figure 95.51), onycholysis (Figure 95.52), oozing and nail plate destruction. Oozing is an underrecognized sign and is only occasionally reported in the literature [93, 106].
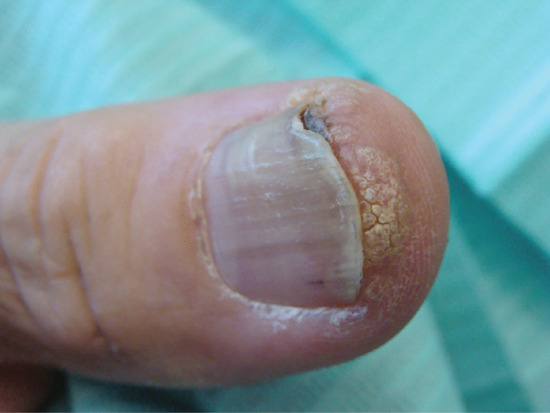
Figure 95.51 Bowen disease: warty lesion of the distal bed and hyponychium. The lesion was treated for several years as a wart.

Figure 95.52 Onycholysis and oozing of the great toenail bed due to invasive squamous cell carcinoma.
Clinical variants
Squamous cell carcinoma may also present as longitudinal melanonychia [107], an onychopapilloma [89], a fibrokeratoma [54] or may simulate an onychomatricoma [77].
Verrucous carcinoma (carcinoma cuniculatum) of the nail apparatus is a rare low-grade variant of squamous cell carcinoma, characterized by a local aggressiveness but a low potential for metastasis. Only 13 cases have been reported in the literature [108, 109]. It presents clinically as a slowly enlarging warty papillomatous plaque and, of reported cases, were most commonly located on the thumb, the hallux or the fifth toe.
Differential diagnosis
The main differential diagnosis is a wart [93].
Disease course and prognosis
Prognosis both of in situ and of invasive forms is good: metastases are exceptional [98, 110] and only three deaths have been reported [96, 111, 112].
Investigations
Radiological imaging should be performed to rule out bony involvement, which was however detected in only 2% of cases in the largest reported series of squamous cell carcinoma of the nail unit (n = 58) [93].
Management
The goal of treatment is eradication of the tumour. However, even with sophisticated surgical techniques, recurrences are not uncommon, probably because human papillomavirus is difficult to eradicate [93].
Basal cell carcinoma
Definition
Basal cell carcinoma very rarely involves the nail apparatus: 20 cases only have been reported in the literature.
Epidemiology
Age
The average age at diagnosis is 65 years.
Pathology
The pathological features are identical to those observed on the skin (see Chapter 141).
Environmental factors
One basal cell carcinoma of the proximal nail fold was reported in a respiratory specialist using radioscopy for 30 years [119] and another in a worker dealing with azo dyes [120].
Clinical features
History
In the published cases, diagnosis was delayed by an average of about 10 years.
Presentation
The classical clinical features as observed on the skin are very rarely encountered. There is no typical clinical presentation (see Differential diagnosis). Two cases presented as longitudinal melanonychia. Diagnosis was histological in all cases. The thumb is most frequently involved, followed by the hallux [121].
Clinical variants
Longitudinal melanonychia is a rare presentation [121, 122].
Differential diagnosis
Chronic paronychia, PG, amelanotic melanoma, squamous cell carcinoma, bacterial or a mycotic infection and habit tic [123].
Investigations
Radiographic imaging to rule out bony involvement.
Management
Surgical removal.
Melanoma (see Chapter 143)
Definition
Melanoma of the nail apparatus is rare but associated with poor prognosis.
Very early recognition and excision provides the best chance of survival.
Epidemiology
Incidence and prevalence
The prevalence ranges from 0.18 to 2.8% of all cutaneous melanomas [125]. The incidence has been estimated at 0.1/100 000/year [126].
Age
Average age of onset is between the sixth and seventh decade. Melanoma of the nail apparatus is exceptional in children, with only 13 reported cases to date [127].
Ethnicity
The proportion of melanomas involving the nail apparatus is much higher in populations of African and East Asian ethnicity than in white people: about 25% of melanomas are located at the nail apparatus in Japanese and African Americans. However, the absolute incidence may well be similar in all racial groups [125].
Pathophysiology
Trauma is often mentioned as a potential causative factor, but no clear link can be established with certainty [128]. UV radiation is not responsible as the nail plate acts as a barrier to penetration of UV [129]; furthermore, the similar frequency of melanoma of the nail apparatus in dark- and fair-skinned peoples suggests that pigmentation is not protective [125].
Pathology
Most cases are acral lentiginous melanoma. In melanoma of the nail apparatus, the histological subtype, the Clark's level, and the Breslow thickness are difficult to assess because of the peculiar nail anatomy [28]. Immunochemistry is particularly helpful for the diagnosis of early disease and for the determination of excision margins: HMB-45 is more sensitive than Mart-1 for detecting intraepithelial melanocytes and the latter is in turn more sensitive than S-100 protein. In invasive melanoma of the nail apparatus, however, S-100 protein is the most sensitive and was the only positive marker in cases of desmoplastic melanoma and in areas with chondroid differentiation [130].
Clinical features
History
Diagnosis is very often delayed and associated with poor prognosis. Patients do not suspect cancer at that site and by the time they consult the melanoma is already advanced with a thick Breslow index [131]. It has been shown that only one third of patients with longitudinal melanonychia seek medical advice [125].
Presentation
In three quarters of cases, melanoma of the nail apparatus starts in the matrix and presents as longitudinal melanonychia [132] (Figure 95.53). In the remainder, it arises from the nail bed and presents as a pigmented or amelanotic nodule, ulceration with bleeding, nail fold pigmentation, unexplained paronychia and/or partial destruction of the nail plate [133] (Figure 95.54).
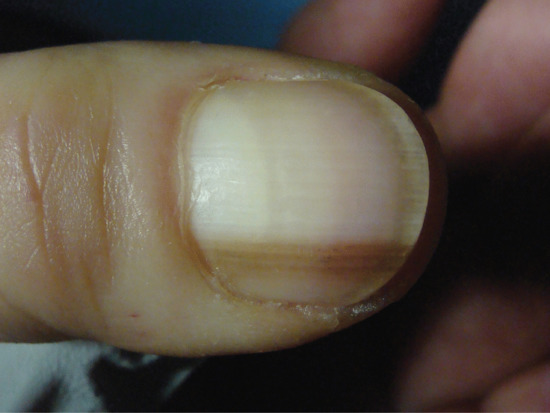
Figure 95.53 Narrow longitudinal melanonychia on a thumb. Dermoscopy showed loss of parallelism that prompted excisional biopsy. Histological examination revealed melanoma in situ.

Figure 95.54 Friable granulation tissue under the plate of the great toenail in an old lady wearing sandals all year round. Pyogenic granuloma was suspected but histology revealed an amelanotic melanoma. (Courtesy of M. Caucanas, Toulouse, France.)
Clinical variants
As many as 20–30% of melanomas of the nail apparatus are amelanotic [125]. Melanoma of the nail apparatus is even more treacherous when it manifests as isolated onychorrhexis [134] or as a fissure in the nail [135].
Hutchinson's sign describes the presence of pigment on the proximal, lateral or distal nail fold. It represents the radial growth phase of subungual melanoma. Although this sign is highly suggestive of melanoma it is not pathognomonic.
Differential diagnosis
All causes of longitudinal melanonychia and tumours of the nail bed (squamous cell carcinoma, PG, etc.).
Complications and co-morbidities
Metastasis.
Disease course and prognosis
Survival rate for in situ melanoma is reported as 100%. The 5-year survival rate was 88% for a Breslow thickness of less than 2.5 mm but only 40% for a thickness greater than 2.5 mm [136].
Investigations
The dermoscopic pattern is well established (brown background with brown to black lines which are unevenly pigmented, irregularly spaced, of variable thickness and with or without interruption of parallelism) [137, 138, 139]. Some authors have performed matrix dermoscopy after nail avulsion and identified four dermoscopic patterns which showed high sensitivity and specificity [140]. The recent development of intraoperative reflection confocal microscopy examination of the nail matrix has enabled one-step surgical management [141].
Incisional biopsy is not recommended, as it does not allow complete histological examination of the pigmented lesion. Several excisional biopsy techniques are available [142, 143]. Sentinel lymph node biopsy for melanomas of the nail apparatus greater than 1 mm in thickness is probably warranted but firm evidence of its benefit in this situation is lacking.
Management
Studies have demonstrated that amputation confers no survival advantage as long as the tumour is fully excised [125, 143, 144]. Only one study compared local excision to amputation in patients with melanoma of the nail apparatus. In this study of 62 patients with melanoma of the nail apparatus of mean thickness 1.68 mm, no significant differences in recurrence or survival rates were detected. Overall disease-free survival at 5 years was 92% [145].
Excision margins for melanoma of the nail apparatus remain controversial. Surgery poses a challenge because of the lack of surrounding soft tissue [146]. As the matrix is fixed to bone, it is difficult to achieve deep excision margins, without amputation or removal of a layer of bone [147]. Many publications report that treatment of in situ melanoma of the nail apparatus by en bloc removal of the nail unit with 5–10 mm margins followed by a full-thickness skin graft [146, 148, 149, 150, 151, 152] results in excellent survival rates with optimal cosmetic and functional results. As there is no evidence that aggressive amputation is associated with higher survival rates, amputation should be aimed at retaining the greatest function possible [153].
Adjuvant systemic chemotherapy and isolated limb perfusion have been used, but no survival benefit has been demonstrated.
PERIONYCHIAL DISORDERS
Nail fold infections
Infections of the nail fold are represented by inflammation, swelling and abscess formation. They can be acute or chronic, isolated or associated with PG.
Acute paronychia
Most patients are children and adolescents.
Acute paronychia is a common complaint usually due to staphylococcal infection, but herpes virus, orf virus and some fungi as well as pemphigus can cause acute paronychia. Cytology (Tzanck smear) may be useful in distinguishing non-bacterial from bacterial paronychia [1]. The latter may result from local injuries, a prick from a thorn in a lateral nail groove, a splinter, torn hangnails or nail biting, the two latter being the most common predisposing factors. It also occurs frequently as an episode during the course of chronic paronychia, when other organisms may be involved including streptococci, Pseudomonas aeruginosa, coliform organisms and Proteus vulgaris. Bacterial paronychia may also present as a subacute infection.
Acute paronychia presents as a painful red swelling of the lateral paronychial area (Figure 95.55). If superficial it may point close to the nail and can easily be drained by incision with a pointed (no. 11) scalpel without anaesthesia. Sometimes a bullous pyoderma brings to light a narrow sinus. This may be a part of a ‘collar-stud’ abscess that may communicate with a deeper necrotic inoculation zone. This must be laid open and excised. Deeper lesions should be treated with penicillinase-resistant antibiotics initially. If there is no clear sign of response within 2 days, surgical intervention under local anaesthesia is required, particularly in children. We recommend the removal of the proximal third of the nail plate cut transversally with nail-splitting scissors without initial incisional drainage. This gives more rapid relief and more sustained drainage. In associated subungual infection probing will determine the most painful area and provide an indication of where the nail plate should be cut away. Soaking the finger twice a day in an antiseptic solution such as chlorhexidine results in rapid healing.

Figure 95.55 Acute bacterial paronychia (whitlow).
Complications of acute paronychia may include osteitis and amputation. Acquired periungual fibrokeratoma after staphylococcal paronychia has been reported [2].
As trauma and terminal phalanx fractures can mimic acute paronychia, radiography is advised when the latter occurs after trauma.
Herpetic paronychia
This uncommon condition appears mostly in children under 2 years old. It is due to primary inoculation of the herpes simplex virus from herpes stomatitis or herpes labialis and presents as single or grouped blisters close to the nail; it may give a honeycomb appearance. Clear at first, the blisters soon become purulent and may rupture and be replaced by crusts (Figure 95.56). The infection is usually very painful and takes about 3 weeks to resolve, with pain for half that time. Lymphangitis sometimes occurs and may precede vesiculation. Diagnosis may be established by recovering the virus from a recent blister and by cytological examination of the blister floor (Tzanck smear) [3]. Transmission to contacts may occur, explaining the appearance of herpetic whitlow in dental workers or nurses who do not wear gloves and come into contact with herpes labialis.
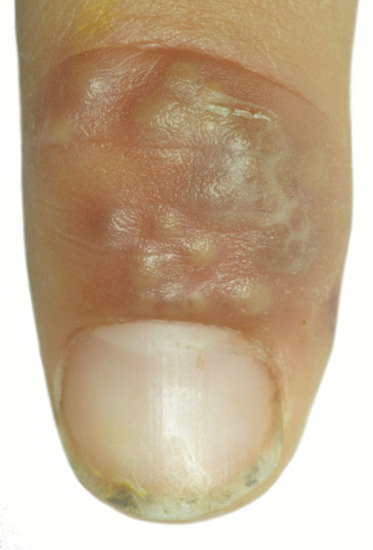
Figure 95.56 Herpetic whitlow.
Treatment probably does little to shorten the course of the disorder, but cleaning with chlorhexidine followed by application of a bland cream is recommended. Relapse may occur as with other primary herpetic infections. Long-term treatment with thymidine analogues, such as oral aciclovir, famciclovir and valaciclovir, may be useful if recurrences are frequent.
Numbness of the finger has been reported following infection, as well as persistent lymphoedema. Herpetic paronychia may cause complete destruction of the nail, bacterial superinfection and systemic spread that may cause meningitis [4]. Longstanding cases, particularly in patients with HIV infection, may have an atypical, often verrucous appearance.
Orf paronychia
Orf virus has been reported in subjects who have had a history of contact with animals.
Erythema multiforme secondary to viral infections
In this condition, erythema and oedema of the proximal nail fold often occur. It can be observed in patients with paronychial infection caused by orf or herpes viruses [5].
Paronychia of the great toe of infants
Undersized infant jumpsuits can be responsible for paronychia of the great toe. The undersized garments most probably produce primary trauma with subsequent infection or possible focal ischaemia, increasing the risks of infection after minor trauma [6].
Chronic paronychia
Chronic paronychia is an inflammatory dermatosis of the nail folds which causes retraction of the periungual tissues with resultant secondary effects on the nail matrix, nail growth and soft-tissue attachments (Figure 95.57a–c). It may be associated with infection secondary to an underlying chronic dermatosis, for instance an irritant contact dermatitis from wet work or exposure to caustic materials. Alternatively, it may be secondary to atopic eczema or psoriasis, where minor provocation can result in active disease [7].
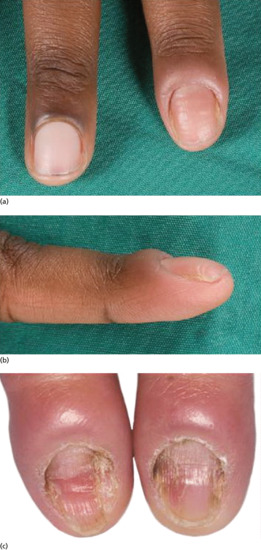
Figure 95.57 (a–c) Chronic paronychia: paronychial swelling, loss of cuticle and mildly dystrophic nail in early disease (a,b); severe nail dystrophy in more advanced disease (c).
Cold wet hands are predisposed to chronic paronychia. Handling of wet foods represents a particular hazard, as these often combine several predisposing factors including wet working conditions, a cold environment and irritation from the food itself. Chronic paronychia is predominantly a disease of domestic and catering workers, bar staff and fishmongers. The majority of cases are in patients of working age, although it is also seen in children, especially as a result of finger or thumb sucking.
Any finger may be involved, although it is most frequently the index and middle fingers of the right hand and the middle finger of the left. These fingers may be more subject to minor trauma than the others. The condition begins as a slight erythematous swelling of the paronychial tissues. It may be painless but, if tender, is much less so than in acute paronychia, except when pressed. The cuticle is lost and pus may form below the nail fold. Inflammation adjacent to the nail matrix disturbs nail growth, resulting in irregular transverse ridges and other surface irregularities, which may be combined with discoloration.
There is some evidence that the darkening of the lateral edges of the nail plate may be due to the pigment of Candida spp. though it is sometimes associated with Pseudomonas infection of the nail [8]. The lateral discoloured edges of the nail plate become cross-ridged when the disease mainly affects the lateral nail fold. Repeated acute exacerbations produce numerous irregular transverse ridges or waves on the nail surface, which often becomes rough. Yeast fungi may cause chronic or acute paronychia. Candida paronychia can be observed in children who have oral candidosis or a habit of thumb sucking. Neoscytalidium dimidiatum may also produce darkening of the lateral edges of the nail plate; by contrast, paronychia due to moulds such as Fusarium spp. is often associated with proximal leukonychia. Dermatophytic paronychia is rare.
In longstanding cases, the size of the nail may be reduced, and this reduction is exaggerated by the bolstering of the fold all around the nail. Most of the nail deformity is due to inflammation, which interferes with the formation of the nail, but a true Candida infection of the nail plate is occasionally seen, especially in patients with immunodeficiency.
Much of the chronic inflammation seen in this disorder probably arises from an irritant reaction to material sequestered beneath the proximal nail fold. The loss of the cuticle means that detergent and other solvents may gain access to this tight space and act like a prolonged irritant patch test. Acute exacerbations occur from time to time and are due to secondary bacterial infection. Various organisms may be found, including Staphylococcus aureus or Staph. epidermidis, Proteus vulgaris, Escherichia coli and Pseudomonas aeruginosa.
Besides this most common type of chronic paronychia, a long list of causes of paronychia is provided (see Table 95.5 online at www.rooksdermatology.com).
Table 95.5 Causes of paronychia.
| Bacterial | ||
| Classical organisms | ||
| Erysipeloid | ||
| Leprosy | ||
| Milker's nodules | ||
| Mycobacterium marinum infection | ||
| Prosector's tuberculosis verrucosa cutis | ||
| Pseudomonas | ||
| Staphylococci | ||
| Streptococci | ||
| Syphilis | ||
| Tularaemia | ||
| Unusual organisms | ||
| Actinobacillus actinomycetemcomitans | ||
| Bartonella henselae | ||
| Corynebacterium spp. (10% of patients affected by pitted keratolysis) | ||
| Eikenella corrodens | ||
| Klebsiella pneumoniae | ||
| Serratia marcescens | ||
| Torulopsis maris | ||
| Fungal | ||
| Aspergillus niger | ||
| Blastoschizomyces capitatus | ||
| Candida spp. | ||
| Fusarium spp. | ||
| Microsporum gypseum | ||
| Neoscytalydium spp. | ||
| Scopulariopsis brevicaulis | ||
| Trichosporum beigelli | ||
| Curvularia lunata | ||
| Parasitic | ||
| Tungiasis | ||
| Leishmaniasis | ||
| Viral | ||
| Herpetic whitlow | ||
| Milker's nodules | ||
| Orf | ||
| Subungual warts | ||
| Occupational | ||
| Agricultural workers | ||
| Animal origin (bristle, sea urchin, oyster shell) | ||
| Automotive workers (sulphuric acid exposure | ||
| from batteries) | ||
| Bakers and pastry cook | ||
| Barbers ans hairdressers (onycholysis) | ||
| Bar-tenders | ||
| Bean shellers | ||
| Book binders (paste) | ||
| Bricklayers (limes, cement, mortar) | ||
| Builders and carpenters (including glass fibre) | ||
| Button makers | ||
| Cement workers | ||
| Chemist and laboratory workers | ||
| Chicken factory workers | ||
| Confectioners | ||
| Cooks | ||
| Cosmetic workers | ||
| Dentists | ||
| Dinitro-salicylic acid | ||
| Dyers (aniline dyes, producing stains | ||
| and necrosis) | ||
| Engravers (brittle nails) | ||
| Etchers, glass etchers (brittle nails) | ||
| Fishermen | ||
| Fishmongers | ||
| Florists & gardeners (onycholysis) (hyacinth, daffodil and narcissus bulbs, tulip fingers) | ||
| Glaziers (brittle nails) | ||
| Groundskeepers | ||
| Harpists | ||
| Housewives/husbands and house cleaners | ||
| Janitorial and domestic workers | ||
| Manicurists (artificial nails) | ||
| Meat handlers | ||
| Mechanics | ||
| Milkers (onycholysis from bristle) | ||
| Oil rig workers | ||
| Painters | ||
| Pathologists | ||
| Photographic developers (brittle nails, discoloration) | ||
| Pianists | ||
| Physicians, dentists, nurses | ||
| Potato peelers | ||
| Prosector's paronychia | ||
| Radio workers (methanol, causing pigmentation and nail loss) | ||
| Salt plant workers (ulcers) | ||
| Shoe workers (brittle nails) | ||
| Swimming pool granuloma | ||
| Tanners (whitlow) | ||
| Textile workers (threads of fabric) | ||
| Violonist (nail dystrophy) | ||
| Wood-workers (brittle nails, stains) | ||
| Wool-workers (wool threads) | ||
| Dermatological diseases | ||
| Artificial nails | ||
| Atopic dermatitis | ||
| Contact dermatitis | ||
| Darier disease | ||
| Dyskeratosis congenita | ||
| Erythema multiforme | ||
| Finger sucking (children) | ||
| Frostbite | ||
| Granulomas | ||
| Hidrotic ectodermal dysplasia | ||
| Ingrowing toenails | ||
| Leukaemia cutis | ||
| Lichen planus | ||
| Pachyonychia congenita | ||
| Parakeratosis pustulosa | ||
| Pemphigoid, pemphigus | ||
| Pernio | ||
| Psoriasis | ||
| Radiodermatitis (chronic) | ||
| Reactive arthritis | ||
| Repeated microtrauma | ||
| Retronychia | ||
| Rubinstein-Taybi syndrome | ||
| Stevens-Johnson syndrome | ||
| Toxic epidermal necrolysis | ||
| Systemic disease | ||
| Acrodermatitis enteropathica | ||
| Acrodermatosis paraneoplastica | ||
| Chronic mucocutaneous candidiasis | ||
| Cushing syndrome | ||
| Diabetes | ||
| Digital ischaemia | ||
| Epidemic encephalitis | ||
| Glioma | ||
| Glucagonoma syndrome | ||
| Graft-versus-host disease | ||
| Hypoparathyroidism | ||
| Immunosuppression | ||
| Job syndrome | ||
| Langerhans cell histiocytosis | ||
| Multiple mucosal neuroma syndrome | ||
| Myeloma-associated systemic amyloidosis | ||
| Neurofibroma | ||
| Neuropathies (sensory or autonomic) | ||
| Primary systemic amyloidosis | ||
| Raynaud syndrome | ||
| Sarcoidosis | ||
| Schwannoma | ||
| Systematized mutiple fibrillar neuroma | ||
| Systemic lupus erythematosus | ||
| Systemic sclerosis | ||
| Tricho-oculo-vertebral syndrome | ||
| Thromboangiitis obliterans | ||
| Wiskott-Aldrich syndrome | ||
| Yellow nail syndrome | ||
| Zinc deficiency | ||
| Drugs | ||
| Acitretin | ||
| Docetaxel | ||
| Cephalexin | ||
| 5-Fluorouracil | ||
| Cyclophosphamide/vincristine | ||
| Ciclosporin | ||
| Indinavir | ||
| Isotretinoin | ||
| Lamivudine | ||
| Methotrexate | ||
| Sulphonamides | ||
| Zudovidine | ||
| Tumours (primary or secondary of the nail unit) | ||
| Bizarre parosteal osteochondromatous proliferation of tubular bones | ||
| Bowen disease | ||
| Enchondroma | ||
| Kerathoacanthoma | ||
| Melanoma | ||
| Metastases | ||
| Myxoid pseudocyst | ||
| Neurofibroma | ||
| Osteoid osteoma | ||
| Squamous cell carcinoma |
Management
Treatment is a combination of avoidance of precipitants, hand care and medication. Perhaps the most important part of the treatment, but the one most difficult to achieve, is keeping the hands dry. Patients involved in wet work should be advised to wear cotton gloves under rubber or plastic gloves and avoid manicure of the proximal nail fold. General hand care with emollients and protection from trauma and irritants is helpful. If these precautions are not followed, the condition is unlikely to settle whatever medical treatment is given.
Topical therapy requires a combination of steroid and antimicrobial. A potent steroid may be used for short periods if there is adequate antimicrobial cover. Injected triamcinolone (2.5 mg/mL) is very useful. Topical imidazoles are usually sufficient to treat Candida and may provide modest activity against some bacteria. More potent topical antibacterials may occasionally be needed. Twice a day application of Dakin solution (sodium hypochlorite) is very effective against Pseudomonas infection.
When significant nail dystrophy ensues and medical therapy has been unsuccessful, chronic paronychia can be treated surgically with good results and resolution of the dystrophic nail. In patients who experience repeated acute flares associated with chronic paronychia, additional removal of the base of the nail plate is useful.
Primary syphilis on the finger
The finger accounts for 5–14% of extragenital primary syphilitic chancres. It may present as a deep painful horseshoe-shaped whitlow with diffuse induration of paronychial tissues and associated regional lymphadenopathy. Pain and tenderness of the fingertips with swelling and serous discharge may also be observed [9].
Periungual toe infections in neutropenic patients
In neutropenic patients with haematological or solid-organ malignancy, the most common causes of paronychia with cellulitis of the toe are Fusarium and, less frequently, Aspergillus. The key to successful management is early removal of the infected nail for diagnosis and institution of appropriate therapy [10].
Subungual abscess
In this uncommon form of nail infection, pockets of pus form directly beneath the nail plate without coexisting paronychia (Figure 95.58). There is a yellow discoloration of the nail and onycholysis may affect the distal third of the nail. The severe throbbing pain is similar to that associated with a subungual haematoma and is caused by pressure.

Figure 95.58 Painful dorsolateral fissure of the fingertip.
The treatment is simple: a red hot wire (e.g. from a heated paper clip) applied to the nail allows release of the pus and bacterial culture. Partial avulsion of the abnormal nail area allows the nail bed to be treated with chlorhexidine, mupirocin or fusidic acid [11].
Other disorders of the perionychium
Drug-induced paronychia
Paronychia may be caused by certain drugs such as isotretinoin and can involve multiple digits [1].
Periungual tissues subject to trauma – onychotillomania
Hangnails are small portions of the horny epidermis that have split away from the lateral nail fold. They are often triangular in shape, with a hard pointed distal end and an adherent base (Figure 95.59). Hangnails are common in people who handle irritants or who work primarily with their hands. Inflammation is usually present causing pain, particularly if the hangnails are interfered with (onychotillomania). Attempts to remove them may be complicated by acute or chronic paronychia. In addition to classical hangnails, scaling of the nail folds with scattered small haemorrhages and focal erosions or necrosis may be observed, typically involving the toes. Hangnails should be snipped off using sharp-pointed scissors. Mupirocin or fusidic acid ointment may prevent or clear low-grade infection.
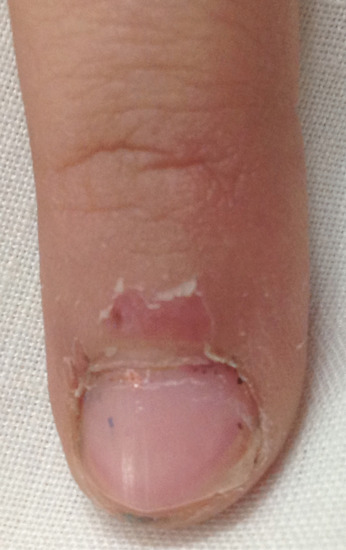
Figure 95.59 Hangnail.
Painful dorsolateral fissure of the fingertip
This condition is not uncommon and occurs particularly in manual workers involved in wet work or in contact with irritants or solvents; it is also seen in patients receiving chemotherapy or targeted therapies where the fissures, often painful, are associated with xerosis and become infected [12]. Interestingly, the fissures are distal to and in line with the lateral nail groove (Figure 95.60). The discomfort experienced by the subjects may render many subtle tasks difficult and even impossible. There appears to be no anatomical basis for the site of fissure, though it could reflect some structural weakness distal to the lateral nail grooves.

Figure 95.60 Subungual abscess in neutropenic patient receiving cancer chemotherapy. (Courtesy of B. Fouilloux, France.)
Hypertrophy of the lateral nail fold
This condition is usually the result of longstanding ingrown nails in adults. Inflammation may range from the subclinical to severe. It is also seen as a congenital condition appearing as overgrowths of the lateral nail folds of both halluces shortly after birth. Hypertrophy of the lateral nail fold resolves spontaneously during the first year of life [13].
DERMATOSES AFFECTING THE NAILS
Nail psoriasis (see also Chapter 35)
Psoriasis is probably the most common disorder affecting fingernails, with consequent dystrophy. Between 1.5 and 3% of the population have psoriasis, and up to 50% of psoriatics have nail involvement [1]: over a lifetime, this proportion may cumulatively increase to 80–90% [2]. De Jong et al. [3] reported that 93% of people with nail psoriasis considered it a significant cosmetic handicap, 58% found that it interfered with their job and 52% described pain as a symptom.
In children with psoriasis, the reported prevalence of nail involvement ranges from 7% [4] to 39% [5]; pitting has been observed in the first week of life of a neonate whose mother had severe psoriasis [6].
Psoriatic nail changes are prominent in childhood nail disease, parakeratosis pustulosa: approximately one third of affected children will develop manifest psoriasis over time, a smaller fraction will have variants of eczema and half of the total will get better [7].
Pathophysiology
Nail unit psoriasis is a localized form of the process active elsewhere in the body and the features represent a combination of local skin changes and secondary effects on nail plate growth. The occlusion of the nail bed by the nail plate means that scale produced by the latter cannot be shed in the normal way, resulting in subungual hyperkeratosis, loss of nail plate adhesion (onycholysis) and oil spots: the latter are thought to arise from a combination of focal onycholysis and exudation.
High-resolution MRI illustrates the close relationship between the soft-tissue attachments of the distal interphalangeal joint and the proximal element of the nail unit. Consequently, inflammation of the joint seen in psoriatic arthritis has a close association with inflammation of the nail matrix. The extensor tendon of this joint is the main relevant soft-tissue attachment in this area and its inflammation is termed an enthesopathy. There is good evidence of a correlation between the presence of an enthesopathy and changes in the nearby nail [8, 9].
Clinical features
In order of reducing frequency, nail signs of psoriasis include pits, onycholysis, subungual hyperkeratosis, nail plate discoloration, uneven nail surface, splinter haemorrhages, acute and chronic paronychia, and transverse midline depressions in the thumbnails [10]. These features can be recorded using the Nail Psoriasis Severity Index (NAPSI), an instrument for precise documentation of nail abnormalities for use in trials and, more generally, for assessing response to interventions [11]. An alternative instrument is Baran's Nail Psoriasis Severity Index, which has been validated by a Polish team [12, 13].
Pits
Pits more commonly affect fingers than toes (Figure 95.61). They represent punctate surface depressions arising from proximal matrix disease (Table 95.6). Zaias [1, 14] has demonstrated small columns of pathological parakeratotic nail falling off the upper surface of the nail plate to produce a pit. Some authorities advocate nail plate histology as a means of diagnosing nail psoriasis [15]. This can be useful for the exclusion of fungal infection, although the specificity of nail plate changes in psoriasis is yet to be established. The origin of pits means that they can be influenced by disease in the proximal nail fold and it is thought that injection of triamcinolone into the nail fold alone can suppress this clinical feature. The pattern of pitting may be disorganized or occur in transverse/longitudinal rows as seen in alopecia areata [2]. Pits may be shallow or large [14], to the point of leaving a punched-out hole in the nail plate (elkonyxis).
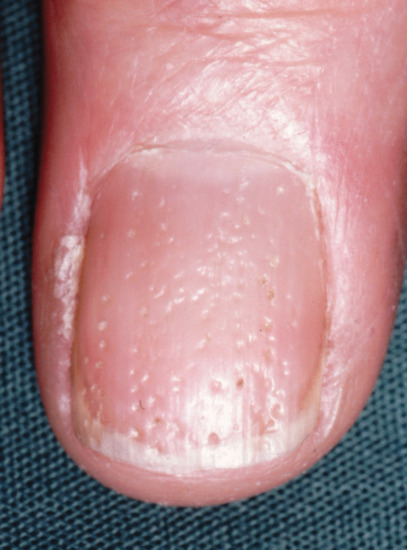
Figure 95.61 Psoriasis: pitting.
Table 95.6 Relationship between clinical features and site of disease activity in psoriasis of the nail. (From Zaias [1].)
| Clinical feature | Area of disease | Duration of disease |
| Changes in nail plate | Matrix | |
| Pits | Proximal matrix | Episodic: short |
| Transverse furrows | Proximal matrix; distal extension depends on depth of furrow | 1–2 weeks |
| Crumbling nail plate | Entire matrix | Prolonged |
| Leukonychia with rough surface | Proximal matrix; leukonychia may involve distal matrix | Variable |
| Changes in nail bed and hyponychium | Nail bed | |
| Splinter haemorrhages | Nail bed dermal ridge haemorrhage | Short |
| Oily spot/onycholysis | Nail bed psoriasis | Prolonged |
| False nail following onychomadesis | Nail bed psoriasis | Prolonged |
| Subungual hyperkeratosis | Nail bed psoriasis | Prolonged |
| Yellow/green discoloration of nail bed | Secondary infection by yeasts or Pseudomonas | Prolonged |
Onycholysis
Focal nail bed parakeratosis produces an ‘oil spot’ or ‘salmon patch’. Extension of this area to the free edge results in onycholysis, which typically has a reddish-brown proximal margin (Figure 95.62). Alternatively, onycholysis may commence at the distal edge (Figure 95.63), representing disruption of the onychocorneal band [16]. Once this band of firm attachment has been breached, the process is often progressive. Minor manicure, wet work and leverage from long nails exacerbate the problem.
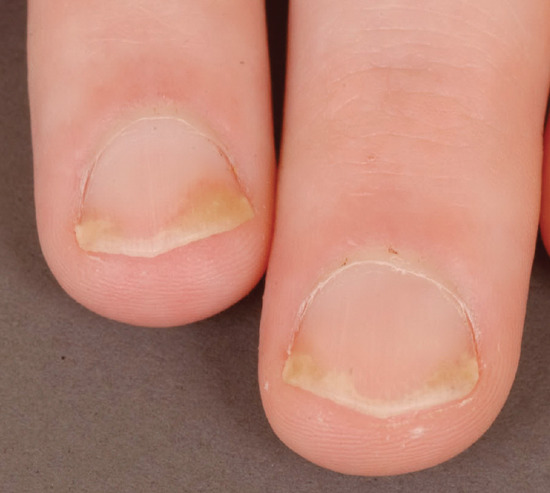
Figure 95.62 Psoriasis: salmon patches progressing to onycholysis.

Figure 95.63 Psoriasis: distal onycholysis.
Discoloration
Discoloration in psoriasis is multifactorial. The major factors are nail thickening and subungual hyperkeratosis. Both of these contribute to a yellow appearance, particularly common in the toenails. It is possible that repeated trauma at this site elicits the isomorphic reaction, with local exacerbation of psoriasis. The coincidence of onychomycosis and psoriasis is also commoner in the toenails [17] and can modify the clinical appearances. Candida spp. and Pseudomonas infection can result in green discoloration. While non-dermatophytes and bacteria are common, dermatophyte infection is rare [1].
Subungual hyperkeratosis
Subungual hyperkeratosis represents nail bed disease (Figure 95.64). Substantial nail plate thickening may result: it is most marked distally and extends proximally. The fingertip may become very tender where there is gross thickening, as the nail plate attachment is greatly reduced and the nail can easily be caught and tug on the matrix attachment. Subungual hyperkeratosis is a prominent feature of pityriasis rubra pilaris affecting the nails and is often associated with splinter haemorrhages [17, 18].
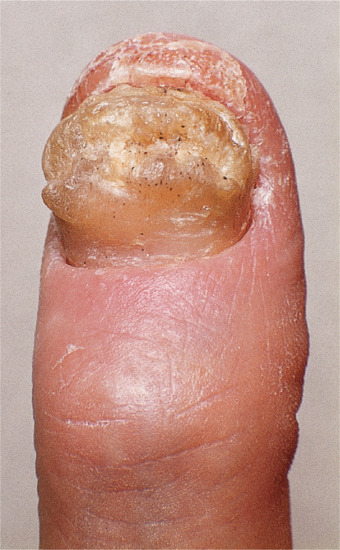
Figure 95.64 Psoriasis: subungual hyperkeratosis.
Nail plate abnormalities
Splits, atrophy and fragility may be seen. The nail may also thicken, independently of subungual hyperkeratosis. Transverse midline depressions resembling the nail changes seen in ‘washboard nails’ [19] are also seen. The latter are normally attributed to the habit tic of disrupting the cuticle (Figure 95.65) and although this may play a part in psoriasis, it appears that there is a lower threshold for their development in the presence of psoriasis.
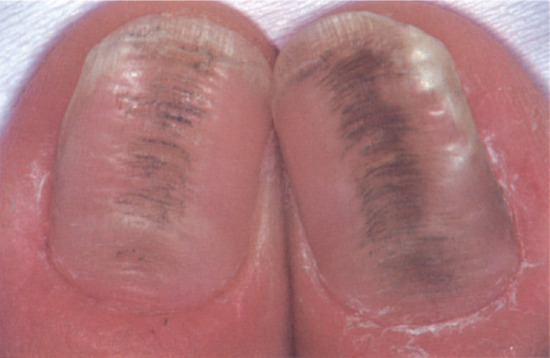
Figure 95.65 Multiple transverse grooves of the thumbnails.
Splinter haemorrhages
Splinter haemorrhages are seen in the nail bed of 42% of fingernails and 6% of toenails [20]. This may be due to the increased capillary prominence and fragility in nail bed dermis in psoriasis and to the presence of dystrophy. Where transverse overcurvature occurs for reasons other than psoriasis, splinter haemorrhages are also common, suggesting that mechanical factors may be important.
Subacute and chronic paronychia
Periungual involvement may be dramatic and inflammatory, giving rise to gross disruption of the nail matrix. Loss of the nail may follow, with scaling of the nail bed or a deep transverse furrow. Chronic psoriatic paronychia causes loss of the cuticle. The nail plate can become thin [21], although this may be offset by matrix disease, which can result in thickened nail. The nail fold may be scaly, as in psoriasis elsewhere.
Acropustulosis
This form of psoriasis involves destructive pustulation of the nail unit. It may present as a component of pustular psoriasis, palmoplantar pustulosis [22], acrodermatitis continua of Hallopeau23 or, on isolated digits, as parakeratosis pustulosa [23], which is seen typically in young girls. The nail plate may be lifted off by sterile pustules in the nail bed and matrix (Figure 95.66). There is associated erythema and discomfort of the end of the digit. There may be long-term nail loss, except in parakeratosis pustulosa, which usually resolves spontaneously.
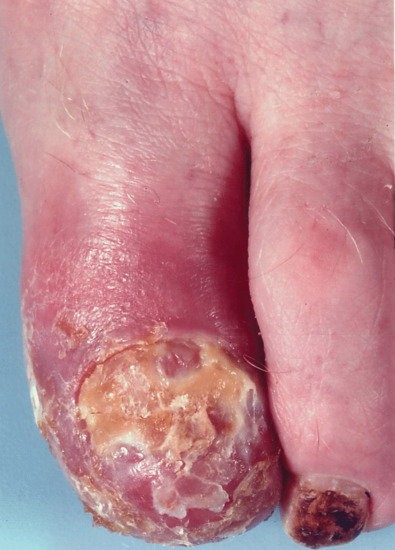
Figure 95.66 Acropustulosis: nail plate has been destroyed by intense pustular inflammation.
Parakeratosis pustulosa may affect only part of one digit. There is pitting and ridging combined with fine scaling erythema of the periunguium and only very rarely pustules. It is usually interpreted as a form of psoriasis [6], although it shares histological features with eczema [24], and some consider it a variant of eczema [25].
Acrodermatitis continua of Hallopeau (see Chapter 35) can be very aggressive and result in resorptive osteolysis [26] or loss of the toes and distal parts of the fingers [27]. In a study of 20 patients with the condition, seven were male and 13 female, with a mean age of 46 years, and all had involvement of only one digit, with no features of psoriasis elsewhere [28]. Clinical experience suggests that occurrence on multiple digits is also a common pattern.
Differential diagnosis
When the diagnosis of psoriatic nail dystrophy is in doubt, the main differential diagnoses are onychomycosis and lichen planus, and less commonly eczema or reactive arthritis (Reiter syndrome) where pitting may be seen [28]. Onychomycosis more commonly affects the toenails, whereas the fingernails are more commonly involved in psoriasis. Equally, there are often changes on the nail surface alone in psoriasis, whereas in onychomycosis there are usually visible abnormalities within or beneath the nail plate. Onychomycosis of the fingernails tends to involve only one or a minority of digits, in contrast with psoriasis where multiple digits are often affected.
Some forms of fingernail lichen planus are very difficult to distinguish from psoriasis. Both may result in roughened nails (trachyonychia) with subungual hyperkeratosis. If pits are prominent the diagnosis of psoriasis can be made, but if they are subtle and difficult to distinguish from other surface changes, they may be part of lichen planus. The nails in reactive arthritis and pityriasis rubra pilaris can also be difficult to differentiate from psoriasis [29, 30], where distal subungual hyperkeratosis and splinter haemorrhages are common [8]. Aggressive forms of atypical nail psoriasis presenting in later life may represent acrokeratosis paraneoplastica of Bazex (see Chapter 147). The patient is usually male, with subungual hyperkeratosis and scaling of the periunguium, ears and nose associated with malignancies of the upper gastrointestinal or respiratory tract [31, 32, 33].
Arthritis of the distal interphalangeal joint suggests a psoriatic cause of any associated dystrophy [34], with the exception of changes due to a myxoid pseudocyst associated with adjacent osteoarthritis. Baker et al. [34] found that there was no strict relationship between which joints are arthritic and which nails are dystrophic, although Jones et al. [35] noted that in a group of 100 psoriasis patients with arthritis and nail involvement there was a significantly greater chance of joint disease in the adjacent distal interphalangeal joint. There was also a significant correlation between the PASI (Psoriasis Area and Severity Index) score and the NAPSI score, and between the latter and duration of psoriasis. A variant of nail psoriasis presents with pain and soft-tissue swelling of the distal digit associated with psoriatic nail changes and underlying bone erosion and periosteal reaction. This can develop in the absence of joint involvement and has been given the unwieldy term ‘psoriatic onychopachydermoperiostitis’ [36].
Histopathology
Histopathology varies according to the clinical focus of the disease [1, 37]. The matrix and nail bed develop a granular layer. Conversely, the hyponychium, where a granular layer is normally present, no longer has one [1]. Where there is subungual hyperkeratosis, there are mounds of parakeratotic keratinocytes beneath the nail plate. Neutrophils may be found throughout these mounds and Munro microabscesses may form. Similar features are seen in acrodermatitis continua of Hallopeau [25]. Amorphous material interpreted as glycoprotein may accumulate within the keratotic mass [1]. Acanthosis and elongation of the rete ridges is present, with increased dilatation and tortuosity of the capillaries of the dermal papillae. Where the nail is lost, the nail bed may form a false nail of compacted hyperkeratosis [38]. The matrix can become quiescent, which can be demonstrated immunohistochemically by the absence of synthesis of the hard keratin 31, which is normally a major constituent of the nail [39].
The nail plate may show faults, clinically manifest as transverse splits and pits, which are lined with parakeratotic cells. These probably originate from the most proximal part of the matrix, or the ventral aspect of the proximal nail fold [1].
Management [40–43]
General hand care is important to avoid provocation of the isomorphic (Koebner) response, whereby minor trauma may elicit psoriasis. These measures include avoiding manicure, keeping the nails short, wearing gloves for wet work and heavy or greasy manual work, avoiding direct exposure to solvents and encouraging emollient usage. Concealment with nail lacquer is a reasonable approach to milder forms of psoriasis, and surface irregularities can be smoothed by the use of nail gel. This is a polymer, applied by a beautician and hardened by exposure to a table-top UVA source. The gel can then be shaped and buffed. Gel or other forms of sculptured or adherent artificial nails have the potential for aggravating onycholysis and are not usually recommended if this is a prominent feature.
Active treatments are mainly directed at the more dystrophic forms of nail involvement and may sometimes help with onycholysis. Often the focus of therapy is the proximal nail fold, where active psoriasis is disturbing the underlying matrix and lack of cuticle is promoting chronic paronychia. Medical treatments include the following.
Local steroids. Clobetasol propionate ointment may be used without occlusion, rubbed into the nail fold. Duration of treatment is limited by local atrophy. It is useful for psoriatic paronychia where there are secondary nail plate changes. Onycholysis may benefit if the nail is clipped back to the point of nail plate attachment and the nail bed treated topically. Candida is a frequent colonizer of this space and warrants treatment at the same time. Triamcinolone acetonide may be used by injection into the nail fold or nail bed with regional or digital ring block. Using 0.1 mL injections of 10 mg/mL triamcinolone acetonide at matrix and nail bed sites on no more than two or three occasions, de Berker and Lawrence reported a good response in subungual hyperkeratosis, nail plate thickening and ridging [44]. However, onycholysis and pitting improved in only 50% of nails. Alternative regimens employ more dilute triamcinolone (2.5–5 mg/mL) and are routinely used more than two or three times per digit, infiltrating the proximal nail fold alone and making a ring block optional. The Dermojet® may also be used to inject corticosteroid directly into the skin of the nail fold under pressure but there are risks of blood splash back and, in one report, of bone damage.
Topical vitamin D analogues. Calcipotriol can be useful where there is subungual hyperkeratosis and nail thickening [45]. It has also been used in combination with topical steroid on an alternating basis (a.m./p.m.) [46] and it is can be used as a combined steroid and calcipotriol ointment or gel. Calcipotriol has the advantage of avoiding the risk of atrophy with long-term use when used without combined steroid, but it is not as effective at treating the nail fold inflammation and consequent changes in proximal matrix function, which manifest as ridging and pitting.
Maintenance treatment with calcipotriol may also be one of the most effective topical therapies for pustular nail psoriasis [47].
Photochemotherapy. Nails may improve in response to general psoralen and UVA (PUVA) therapy or with local PUVA to the nail unit. The latter can be administered using either topical or systemic psoralen. Specially designed high-dose UVA handsets have been advocated. Eighteen of 26 patients showed a greater than 50% improvement in nail changes following whole-body PUVA, although pitting was unresponsive [48]. Four of five patients improved with local therapy: onycholysis was more responsive than pitting, but one patient with severe pitting showed improvement [49].
Retinoids. The nail plate is thinned by acitretin. This reduces subungual hyperkeratosis and good clinical results have been reported [50, 51]. Pustulation may be improved. Topical tazarotene 0.1% gel can be helpful for onycholysis and pitting when applied under occlusion [52].
Others. A Cochrane review of therapies for nail psoriasis concluded that quality of data was in general poor and that, although the systemic agents including biological agents appeared to be beneficial, the length of follow-up was insufficient to provide adequate safety data [53]. Systemic methotrexate and ciclosporin may both help the nail unit but would not usually be advocated as therapy for nail disease alone [52, 54]. Biological agents can also be effective, although they are usually given in the context of severe disease elsewhere [55, 56, 57]. Paradoxically, there are reports of psoriasis precipitated by biological agents given for rheumatological diseases [58]. Acrodermatitis continua of Hallopeau and psoriatic onychopachydermoperiostitis [59] may cause sufficient distress to warrant systemic therapy in the absence of disease elsewhere: they may respond to methotrexate.
There is very anecdotal evidence of benefit from topical ciclosporin [60] and from topical 1% 5-fluorouracil 1% in either 20% urea [61] or in propylene glycol [62]. Pitting and subungual hyperkeratosis were thought to respond well to the urea formulation. 5-fluorouracil should not be used in the presence of onycholysis. Superficial radiotherapy [63] and electron beam therapy [64] have been shown to be of only temporary benefit and are not usually recommended. Treatment of coincident fungal infection may provide clinical benefit, although it is seldom a dermatophyte and positive cultures may merely represent colonization.
Darier disease of the nails [1, 2, 3–5] (see Chapter 66)
Nail involvement is common in Darier disease; 96% of patients are reported to have acral changes of which nail changes are the most common [2]. These include red and/or white longitudinal streaks in the nail, often terminating in a V-shaped nick (Figure 95.67). The streak may represent a zone of fragile or thinned nail, which makes it prone to fragmentation at the tip with the consequent nick. In severe cases, the nails are almost lost by extension of the fragmentation process to involve the entire matrix. Subungual hyperkeratotic papules can be found in the hyponychium. Histologically, matrix and nail bed changes resemble the acantholysis seen in involved skin, with the addition of multinucleate giant cells and epithelial hyperplasia in the nail bed [5]. These histological features make it possible to diagnose Darier disease when it is confined to the nail [1]. Excess ridging and a rough nail surface may also be found, as may total leukonychia. Occasionally, marked thickening of the nail plate occurs. It is probable that the nail is sometimes affected in the absence of disease elsewhere [1].
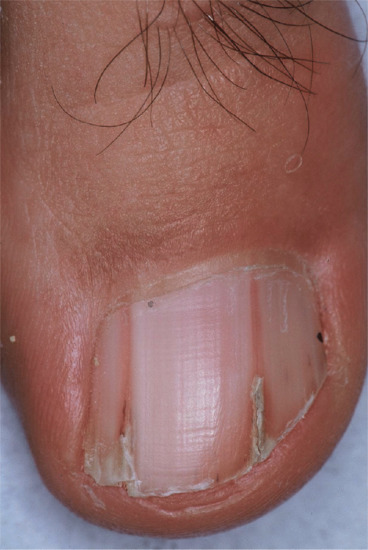
Figure 95.67 Darier disease: white and red longitudinal lines and distal notching.
Hailey–Hailey disease has some histological similarities and may also present with longitudinal white streaks [2]. However, the disease does not have the same destructive effect and is not associated with hyperkeratoses or pain and loss of function associated with the nail splits and disintegration sometimes seen in Darier disease.
A case of squamous cell carcinoma developing in a nail bed with chronic changes of Darier disease has been reported [6]. Pain or conspicuous uncharacteristic features in a nail apparatus affected by Darier disease may therefore be indications for biopsy.
Eczema involving the nails
Nail changes in eczema may be seen in the context of eczema elsewhere, with hand eczema, or as an isolated finding with periungual and subungual features. Endogenous and exogenous factors may contribute. The nail changes may reflect this division, in that they may be in response to a systemic atopic disposition, with pitting in the absence of inflammation, or may demonstrate the effects of local eczema in the nail unit influencing nail formation.
The common allergens such as nickel, fragrance and medicaments rarely have particular bearing on nail abnormalities. However, rubber, chrome and irritant dermatitis are significant factors in hand dermatitis. These materials, and hand dermatitis in general, are associated with particular occupations. Selective exposure to such allergens or strong irritants is as important as chronic low-grade irritation from milder irritants such as water and detergents seen in catering workers. High concentrations of and prolonged exposure to allergens and irritants can result from sequestration beneath the free edge of the nail.
Cyanoacrylates used in prosthetic nails can provoke local and distant allergic reactions. Formaldehyde, occasionally used as a nail hardener, can provoke painful onycholysis if the patient becomes sensitized, or sometimes when acting solely as an irritant. Some allergens may cause nail dystrophy without associated inflammation.
A combination of atopy and an exogenous irritant or allergic contact reaction is common.
Clinical features
Nail matrix disturbance is reflected in thickening, pits, nail loss, transverse ridges, Beau's lines and furrows in a pattern similar to psoriatic nail disease [1] (Table 95.7).
Table 95.7 Differential diagnosis between four common nail disorders: fungal infections, psoriasis, chronic paronychia and dermatitis.
| Fungal infections | Psoriasis | Chronic paronychia | Dermatitis | |
| Colour | Often yellow or brown; part or whole of nail | May be normal or yellow or brown | Edge of nail often discoloured brown or black | May be normal |
| Onycholysis | Frequent | Frequent | Usually absent | Confined to tip or absent |
| Pitting | Infrequent | Often present and fine | Uncommon | Coarse pits frequent |
| Filaments or spores in potash preparations | Filaments, usually abundant | Absent | May be spores in edge of nail; filaments and spores in scrapings from nail fold | Absent |
| Cross-ridging | Absent | Uncommon | Frequent | Frequent |
| Other | Associated fungal infections elsewhere | Associated psoriasis elsewhere or family history of psoriasis | Predominantly women; wet work and cold hands cause predisposition | Recent history of dermatitis on hands |
Nail bed disease can manifest as subungual hyperkeratosis, splinter haemorrhages, onycholysis or pain.
Nail changes may betray eczema elsewhere and the nails may be buffed smooth and shiny, indicating their use as a tool for rubbing.
Associated hand dermatitis may show vesicles, scaling, erythema, cracks and swollen fingers, although the presence of vesicles will not always distinguish the condition from psoriasis, which should be sought at other sites. The distribution on the hand or foot may give some clues as to possible local causes, such as gloves, shoes, prosthetic nails or nail varnish. Hands and feet should always be examined together, as the presence of disease in both diminishes the likelihood of a contact dermatitis. Associated disease can present as periorbital eczema in contact allergy to nail cosmetics [2], though often there may be no evidence of inflammation on or around the nails themselves.
Defining the presence of atopy or patch testing can be useful even in the absence of active eczema as subungual hyperkeratosis and discomfort may be disproportionate to the cutaneous features [3].
Management
General hand care is important, with the avoidance of soap, irritants, wet work and any identified cause. Protective gloves should be used, with copious emollient application. Barrier creams are not usually adequate protection once features have developed. Potent topical steroids may be needed, sometimes with additional topical or systemic antimicrobial therapy. These should be rubbed in around the nail folds. In the young, steroids may precipitate premature closure of the phalangeal epiphyses if too potent or used for too long [4]. Osteomyelitis has also been reported in children using potent topical steroids in this area.
Hand or foot PUVA can help.
Lichen planus of the nails and related conditions (see also chapter 37)
Nails are involved in about 10% of cases of disseminated lichen planus [1]. In a study of 24 adults with nail lichen planus, nail changes were the sole manifestation of the disease in 75% [2, 3], and the proportion may be higher in children (Figure 95.68) [4, 5], in whom lichen planus of all types is rare. This suggests only a modest degree of overlap between the disease process in the nail unit and at other sites. Although the skin lesions may itch intensely, nail disease may be relatively asymptomatic except when nails are shed.
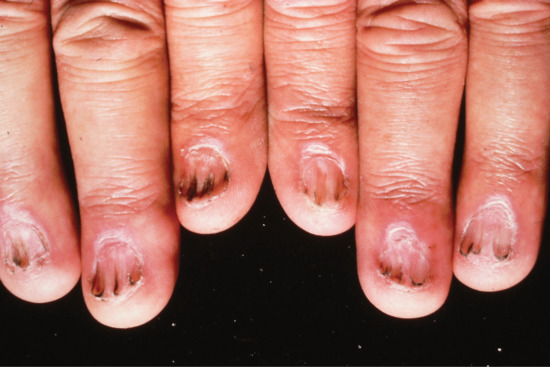
Figure 95.68 Severe onychatrophy from juvenile onset lichen planus of nails.
Clinical features
The disease can involve the proximal nail folds with bluish-red discoloration. Nail plate changes include thinning or thickening, onychorrhexis, brittleness, crumbling or fragmentation, and accentuation of surface longitudinal ridging. All these features are secondary to disease affecting the matrix, which can also produce transient or permanent longitudinal melanonychia [6], longitudinal erythronychia [7] or leukonychia as a post-inflammatory phenomenon (Figure 95.69). When inflammation is intense and widespread within the nail apparatus, nails may be shed. Single longitudinal depressions in the nail, with a distal notch or entire split, may arise from a pterygium: this is a fibrotic band of tissue fusing the proximal nail fold with the nail bed and matrix following destructive local inflammation. Surviving proximal matrix is unable to push growing nail through the scar tissue, with a consequent split. Thickening, with features resembling yellow nail syndrome, is a less common pattern of presentation [7]. Where this occurs there is usually little difficulty in making the distinction in the fingernails as the prominent surface changes and/or atrophy of lichen planus are seen. However, these changes are less obvious in the toes, where yellow discoloration due to thickening can be marked [8].
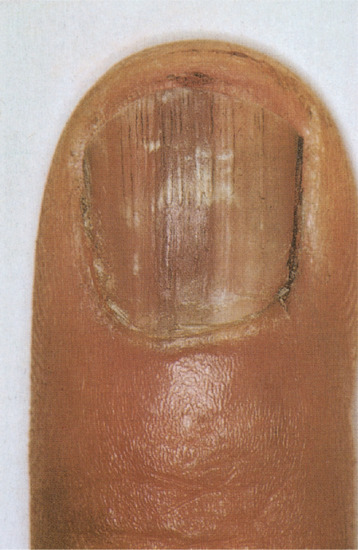
Figure 95.69 Lichen planus with longitudinal melanonychia.
Nail bed disease can produce subungual hyperkeratosis and onycholysis. Ulcerative lichen planus may affect the soles of the feet but may also involve the toenails: permanent anonychia may follow [9] (Figure 95.70).
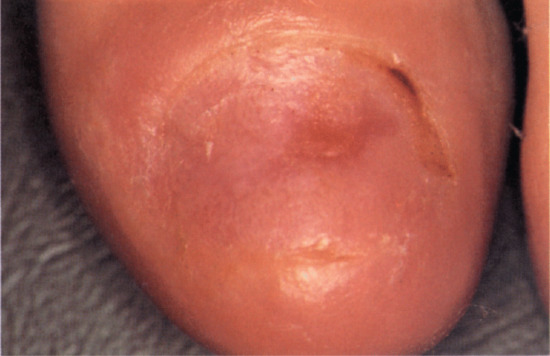
Figure 95.70 Anonychia following lichen planus.
Clinical variants
Twenty-nail dystrophy, in which there is stippling of the nail plate (trachyonychia: see Figure 95.17), may involve all 20 nails but may affect as few as four or five. It is seen in a range of autoimmune diseases [10], especially in alopecia areata [3] but also in primary biliary cirrhosis and possibly in pemphigus [11]. In itself, it does not indicate the diagnosis of lichen planus, but is one of the recognized forms of the disease. It is one of the more common childhood patterns of presentation in which the nails feel rough and lose their lustre [12]. It has a reasonably good prognosis, in contrast with idiopathic atrophy of the nails, which may also occur in children. In this form, the surface change is less marked and the change in overall nail morphology greater, with thinning and disintegration of the nail plate. Although nail biopsy is seldom undertaken in children, it may be warranted where the diagnosis of lichen planus needs to be explored. If destructive lichen planus is not treated in childhood, there will be lifelong loss of nails. In the related disorder, lichen nitidus, numerous pits giving a fine rippling effect have been reported [13]. Longitudinal ridging, beads and thickening may occur and the nails may become brittle.
In keratosis lichenoides chronica, although the skin condition may resemble hyperkeratotic lichen planus, the nail changes may mimic psoriasis; 30% have nail involvement, with hyperkeratotic hypertrophy of periungual tissues. Lichen planus nail changes are seen in graft-versus-host disease [14] and in the disseminated lichenoid papular dermatosis of AIDS. There can be an overlap between lichen planus and discoid lupus erythematosus, both in the skin and nails. Coexistence of skin and nail lichen sclerosus has been reported [15]. Lichen striatus may extend down a limb to the nails [16].
The differential diagnosis for the range of appearances of lichen planus in the nail unit includes Stevens–Johnson syndrome, infection, peripheral vascular disease, trauma and radiodermatitis. Scarring inherited abnormalities such as dyskeratosis congenita, Schöpf–Schulz–Passarge syndrome, Darier disease and variants of epidermolysis bullosa can also present with nail atrophy and scarring with overlap with the appearance of lichen planus.
Histology
In twenty-nail dystrophy, there is a granular layer in the nail bed and matrix with marked spongiosis [3]. The hypergranulosis is believed to reflect the disordered keratinization that causes both subungual hyperkeratosis and the poor nail plate formation. In other forms of nail lichen planus, in addition to hypergranulosis, there is occasionally saw-toothing of the rete pattern but colloid bodies are rarely seen [2, 17, 18].
In twenty-nail dystrophy, it may be useful to perform a screen for organ-specific antibodies because of the association with alopecia areata and the related autoimmune diathesis [3].
Management
Treatment needs to be commenced early and at sufficient potency to ensure that the disease does not progress whilst treated (Table 95.8). Once scarring has progressed sufficiently to cause a pterygium, there will be an irreversible component. In mild forms, where there is just nail fold redness and subtle nail surface changes, potent topical corticosteroids rubbed into the nail folds for 2–3 months may be adequate. Triamcinolone acetonide may be injected into the proximal nail fold under local anaesthetic. In children, potent systemic steroid therapy puts them at risk of premature closure of the phalangeal epiphyses and prolonged courses should be administered with the collaboration of a paediatrician. Oral steroids at up to 60 mg/day have been used to arrest severe scarring nail lichen planus [2]. Triamcinolone acetonide can be given intramuscularly at a dose of 0.5–1 mg/kg per month for 3–6 months [12]. There are reports of moderate success in the treatment of severe disease in children with systemic agents including oral prednisolone, dapsone and acitretin usually combined with topical therapy [19]. Ciclosporin can also be of benefit and azathioprine has been used to good effect in erosive disease [20]. Methotrexate is mentioned in review articles [21] and alitretinoin [22] in case reports. Ulcerative lichen planus of the nail unit may benefit from grafting the nail bed.
Table 95.8 Systemic therapies in common dermatological diseases affecting the nail.
| Ciclosporin | Methotrexate | Prednisolone | Acitretin | Fumaric acid esters | Azathioprine | Biologicals | |
| Psoriasis | ++ | + | – | + | + | – | ++ |
| Lichen planus | + | – | ++ | + | – | + | – |
| 20-nail dystrophy | + | – | ++ | – | – | – | – |
| Eczema | ++ | + | + | – | – | + | – |
Justification for all systemic treatments in nail disease may be based on the combined presentation of skin and nails. It is less common to prescribe on the basis of nail disease alone. Course duration can usually be limited to pulses of 3 months in a 9–12-month period, repeated if needed. Doses are as for the cutaneous disease.
++Good choice, with moderate evidence supporting its use.
+Reasonable choice, with case reports or small series supporting use.
–Little or no published evidence.
NAILS IN CHILDHOOD AND OLD AGE
Childhood [1]
In early childhood, the nail plate is relatively thin and may show temporary koilonychia. This is particularly prominent on the great toes. Under the age of 5 years, nails are also prone to terminal onychoschizia (lamellar splitting). This can be most prominent on the sucked thumb, but is also seen on the toes. Sucking may also lead to paronychia, which can be a troublesome condition in childhood, with pain and nail dystrophy (Figure 95.71).
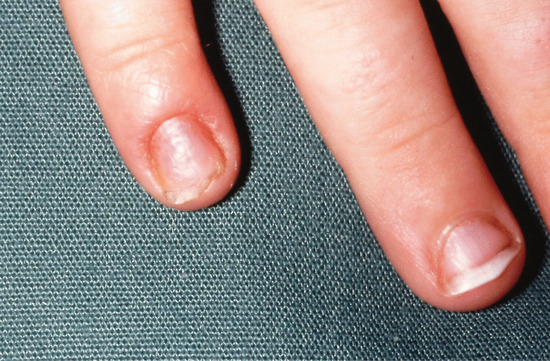
Figure 95.71 Paronychia of the little finger in a 2-year-old child.
Ingrowing can also cause pain and may present in different forms. At birth, there is often a degree of distal ingrowing, particularly in the great toe, as the nail has not surmounted the tip of the digit in its development [2]. In a more gross form, this may present as congenital hypertrophic lip of the hallux, where soft-tissue overgrowth may resemble fibrous tumours of the digit before spontaneously disappearing [3]. Painful distal embedding can lead to infection, but as long as the toenail is properly orientated with respect to the underlying phalanx, the condition usually subsides. In one series of seven children, two needed surgery due to painful persistence of the problem [4]. The changes associated with congenital malalignment of the great toe may also subside within 5–10 years in about 50% of children. In this condition, there is deviation of the tip of the great toenail laterally, rotating on the distal phalanx. The nail is yellow, triangular, thickened and has transverse ridges [5].
Fungal infection is relatively uncommon in children, with a prevalence of 0.3% [6] to 0.44% [7]. Terbinafine is not licensed for use in children in most countries, although there is evidence of its efficacy and it is sometimes used [7].
Beau's lines can be seen in up to 92% of normal infants between 8 and 9 weeks of age [8]. Normal surface markings of the nail can differ in children from those seen in adults. A herringbone pattern is common and gradually diminishes with time [9], which may reflect a gradual change in the pattern of matrix maturation.
Old age
With age, altered arterial and venous supply and cumulative trauma affect the feet more than most other body sites [10]. Elastic tissue changes diffusely affecting the nail bed epidermis are often seen histologically. The whole subungual area in old age may show thickening of blood vessel walls with vascular elastic tissue fragmentation [11]. Nail growth is inversely proportional to age [12].
The nail plate becomes paler, dull and opaque with advancing years, and white nails similar to those seen in cirrhosis, uraemia and hypoalbuminaemia may be seen in normal subjects. Longitudinal ridging is present to some degree in most people after 50 years of age and this may give a ‘sausage links’ or beaded appearance.
For details of the common traumatic abnormalities and changes due to inadequate pedicure or neglect, detailed texts should be consulted [1, 12]. Nail problems in the elderly are often associated with more widespread mechanical changes of the foot, and it is often more important to direct treatment at maintaining mobility rather than the restoration of normal nails [13, 14].
Onychomycosis is one of the most common nail diseases of the elderly, and is often combined with an element of traumatic dystrophy that will predispose to relapse after treatment. There are concerns that drug interactions in this group might make systemic therapy a poor choice [15]. This is not borne out by one large study designed to examine the effects of terbinafine. But the study also revealed that complete cure at the conclusion of the trial occurred in only 28% of cases, a factor which should be considered before instigating therapy [16].
IMAGING OF THE NAIL
X-ray examination
Under normal circumstances, X-ray examination reveals little of the soft structures of the nail unit. It can, however, be useful in identifying a range of pathologies including underlying exostoses, bone cysts, acroosteolysis, psoriatic arthropathy and reactive changes; it has also been used to measure nail bed thickness in a study of finger clubbing. Pincer nail deformity or trauma, including nail biting, can be associated with radiologically detectable osteomyelitis.
Most isolated nail dystrophies should be X-rayed prior to surgical exploration. Benign space-occupying lesions may compress the underlying bone with corresponding upward convexity in the nail. Osteoid osteoma may be manifest through a characteristic nidus, although X-ray is not sufficient to rule out this pathology and may need to be supplemented with bone scan or MRI. Chondroid tumours may be located externally to the bone, but may be detected by X-ray as a lucency within the bone. Similarly, X-ray may reveal bony invasion by locally invasive or metastatic malignancy: in invasive subungual squamous cell carcinoma, up to 55% of patients will have radiological evidence of involvement of the underlying phalanx.
Acquired acro-osteolysis, acronecrosis and distal phalangeal erosive lesions
Acro-osteolysis in adults is a predominantly bilateral lysis of the distal phalanges of the digits. Radiographs are poor at differentiating longitudinal from transverse acro-osteolysis [1]. Acquired varieties (Box 95.2) are by far the most frequent and usually secondary to a medical condition, trauma or toxins. Investigation of the cause is based more on clinical and laboratory data than on imaging. Idiopathic acro-osteolysis in adulthood may be sporadic or familial.
Occupational acro-osteolysis
Workers involved in the polymerization of vinyl chloride have developed acro-osteolysis. The other features of this occupational condition are Raynaud phenomenon and scleroderma-like skin changes (see Chapter 130). Exposure to vapours of synthetic materials used in the production of other plastic products may occasionally produce similar abnormalities. The acro-osteolysis begins as small cortical erosions which enlarge to produce transverse defects in the terminal phalanges (Figure 95.72a). The isolated phalangeal tuft may then fragment and resorb. If exposure is eliminated, healing may occur with coalescence of phalangeal fragments resulting in a pseudoclubbing, the thumb being more commonly affected than other digits.
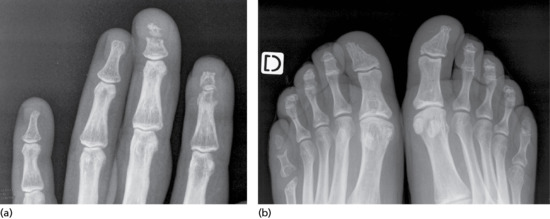
Figure 95.72 Transverse acro-osteolysis of the fingernail (a); acro-osteolysis of the toenail (b). (Courtesy of J. L. Drapé, France.)
Connective tissue diseases
Transverse acro-osteolysis, is rarely associated with Raynaud phenomenon, rheumatoid vasculitis, psoriasis or scleroderma. When present, acro-osteolysis is almost certainly secondary to vascular compromise.
Bony erosions of the phalanges occur in 40–80% of patients with systemic sclerosis. Gradual resorption of the tuft leads to ‘pencilling’ of the phalanx and in some patients all of the distal phalanx may be destroyed. The presence of sclerodactyly and/or calcinosis cutis helps indicate the correct diagnosis. ‘Whittling’ or ‘pencilling’ of the tufts also occurs in psoriasis and can result in a peg-shaped phalanx (Figure 95.72b) [1]. In acronecrosis, the final stage of acro-osteolysis, the soft tissues in the fingertip telescope around the shortened tuft resulting in pseudoclubbing.
Thermal/biomechemical/neuropathic injuries
Thermal injuries (i.e. frostbite [2], electrical and chemical burns) can result in acronecrosis long after the initial insult and may be due to a combination of mechanical and vascular injury. Phalangeal microgeodic syndrome is an uncommon benign condition firstly described by Maroteaux in 1970 [3]. Clinical manifestations include swelling and redness of one or more phalanges of one or both hands. Radiological signs encompass multiple small osteolytic areas and sclerosis compatible with acro-osteolysis. A relation to cold exposure has been suggested since patients often present this during the colder months of the year [4]. Acro-osteolysis has been reported in young guitar players [5] probably related to persistent mechanical injury resulting in vascular compromise and avascular necrosis.
Hyperparathyroidism
In hyperparathyroidism and renal osteodystrophy, increased levels of parathyroid hormone produce excessive bone resorption and altered bone formation. The earliest radiological sign of this disease is cortical resorption of the phalangeal tuft [6].
Many other diseases can cause distal phalangeal destruction.
Soft-tissue lesions
Epidermoid implantation cyst. This appears after a crush or a penetrating injury. On radiographic examination, it is characterized by a well-defined cystic lucency in the distal phalanx. It occurs more commonly in the phalangeal tuft, rather than at its base.
Glomus tumour. This hamartoma of hypertrophied elements of the normal glomus body is usually a well-encapsulated, soft pink or purple mass, smaller than 1 cm in diameter. When there is osseous involvement, it is characteristically an extrinsic pressure erosion, although occasionally, a more ‘punched-out’ appearance develops. Glomus tumour may demonstrate particular radiological features. Mathis and Schulz [7] reviewed 15 such tumours on the digit and found that nine had characteristic changes of bony erosion. This was smooth and concave in most cases, but occasionally had a punched-out appearance on the phalangeal tuft. Van Geertruyden et al. [8] noted bone erosion or alteration in 36% of 51 cases of subungual glomus tumour. Arteriography may reveal a star-shaped telangiectatic zone but generally, ultrasound or MRI is thought to be more useful in delineating and characterizing the tumour.
Keratoacanthoma. Bony destruction of the distal phalanx is present in virtually all cases of subungual keratoacanthoma and may be seen on radiographs even when examined shortly after clinical presentation. The destruction is characteristically well-defined, smooth, circular and limited to the tip of the phalanx [9].
Osseous neoplasms
Enchondroma is a benign tumour arising from mature hyaline cartilage. It is a small well-defined cystic lucency in the phalanx, sometimes having scalloped margins or a sclerotic rim, and is most commonly located centrally in the bone. In the distal phalanx, the enchondroma is typically located at the base of the phalanx, abutting the articular surface.
Osteoid osteoma is a benign osteoblastic lesion consisting of a small oval or round mass, called a nidus, usually smaller than 1 cm. All those affecting the terminal phalanx have a similar appearance characterized by a sclerotic nidus with a radiolucent halo (‘ring sequestrum’) [10].
Aneurysmal bone cysts and giant cell tumours rarely occur in the distal phalanges. Both may have similar radiological features characterized by lytic expansive lesions involving the entire phalanx [11].
Haemangiomas may arise in the bone or soft tissue of the distal phalanx. When primary in the bone, they have a characteristic radiographic appearance of linear striations parallel to the shaft of the bone. Soft-tissue haemangiomas are more common and may manifest as local soft-tissue masses, localized bony overgrowth, phleboliths in the soft tissue and pressure erosion of the underlying bone.
Ultrasound imaging
Ideally, a compact linear and variable-frequency probe that works in the range of frequencies from 7 to 22 MHz is used for performing the examination (Figure 95.73a). The machines are capable of detecting the blood flow of the nail bed in real time. Three-dimensinal ultrasound reconstructions may also provide valuable information concerning tumour size, location, shape and internal characteristics (Figure 95.73b). The nail unit is comprised of three main areas: the nail plate, the nail bed and the paronychial tissues. The dorsal and ventral plates present a bilaminar hyperechoic structure (two parallel lines) separated by a very thin hypoechoic layer (interplate space). Low-velocity arterial and venous vessels are usually detectable within the nail bed (colour Doppler with spectral curve analysis) (Figure 95.73c). The distal insertion of the lateral bands of the extensor tendon in the distal phalanx shows a fibrillar hyperechoic pattern, typical of tendinous structures. The bony margin of the distal phalanx shows a continuous hyperechoic line following the contour of the cortex of the bone that is only interrupted by the anechogenicity of the distal interphalangeal joint space, which contains fluid and cartilage (Figure 95.73d) [12].

Figure 95.73 Ultrasound: grey scale ultrasound (longitudinal view) demonstrates the normal sonographic anatomy of the nail (a); 3D ultrasound reconstruction of the nail (longitudinal view) (b); power Doppler ultrasound (longitudinal view) shows the blood flow within the nail bed (c); extended field of view of the nail and periungual structures (longitudinal view) (d).
Optical coherence tomography
Optical coherence tomography (OCT) is an optical analogue of ultrasound, using infrared instead of acoustic waves [13] The reflection of infrared light from the tissue is measured by interferometry, and 2D grey scale images are generated. Images reflecting the different layers may be either horizontal or vertical (similar to ultrasound). Functional aspects such as speckle variation and vascular flow may be included in some equipment. Three-dimensional images can be generated. The axial resolution is <5 and lateral resolution <7 μm; the scanning depth is up to 2 mm, limiting its use to very superficial tissues. It has been widely investigated in dermatology, particularly in non-melanoma skin cancers.
It has been claimed that OCT can differentiate morphological details and nail thickness better than high-resolution ultrasound and thus it has been advocated as an assessment tool for onychomycosis. Furthermore, OCT imaging is consistent with both physical and ultrasound findings in patients with symptomatic psoriatic nail disease. Surprisingly, OCT of diffuse psoriatic nail dystrophy as assessed on clinical examination has shown relative normality of the superficial nail but abnormalities at the nail plate anchorage to the nail bed. Given that OCT can also measure nail plate thickness, OCT has the potential to provide more objective and informative quantitative data for use in outcome measures for interventional trials in psoriatic nail disease. In one study, OCT detected subtle abnormalities in 12 clinically normal nails and in 41 nails with normal ultrasound findings [14].
Confocal microscopy
Reflectance confocal microscopy (RCM) focuses infrared light in a specific focal plane, so that structures above and below this plane do not interfere with the image. RCM give horizontal images of the analysed tissue with an axial resolution of <1.25 μm and a lateral resolution of <5 μm, i.e. cellular level, to a depth of 350 μm. No stains are required for RCM imaging. Skin and nails are ideal locations for exploration by RCM, because they are easily accessible sites. The unique properties of RCM make it possible to explore the capillary nail fold at a cellular level.
The nail plate can be scanned from the surface to the lower part adjacent to the underlying nail bed. Three different layers can be differentiated by RCM according to the intensity of the reflection. The superficial layer shows a brighter reflection, followed by a zone with a poorer signal, followed again by a brighter zone in the deepest part.
Two main applications have interested dermatologists so far as follows:
- Confocal microscopy and onychomycosis. As attempts to document fungal infection by potassium hydroxide (KOH) preparations and fungal culture are sometimes unsatisfactory, Hongcharu et al. [15] first reported the possible application of in vivo RCM for the diagnosis of onychomycosis. The author correlated RCM findings with the results from routine KOH preparations. RCM was found to be faster and more accurate than the conventional microscopy used with KOH preparations in the diagnosis of onychomycosis [16].
-
Confocal microscopy and melanonychia. Intraoperative dermoscopy and RCM have recently been advocated for better visualization of nail matrix pigmentation during exploratory nail surgery for melanonychia (Figure 95.74) [17]. In most cases, the RCM images obtained either ex vivo/in vivo were sufficiently reliable to make a diagnosis; the slight loss of quality sometimes observed in vivo because of subtle movements, did not adversely affect their diagnostic value. Intraoperative RCM revealed sufficiently atypical cytological and architectural features to accurately indicate the correct final diagnosis of melanoma. A good correlation between RCM and histopathology was found: subungual melanoma was diagnosed intraoperatively and in seven out of eight cases proved to be melanomas by histological examination [18].
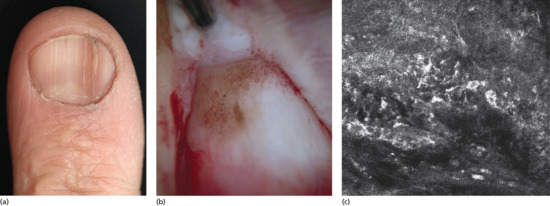
Figure 95.74 Nail melanoma: clinical presentation (a); dermoscopy (b) and in vivo reflectance confocal microscopy (c) of the nail matrix in the same patient. (Courtesy of L. Thomas, France.)
In skin and nails, RCM combines the advantages of dermoscopy (non-invasive examination of the whole area of interest without alteration of the epithelial surface) and histopathology (resolution at a cellular level). RCM enables melanocytes to be distinguished readily from adjacent structures and permits the visualization of the architectural and cytological features of the melanocytic proliferation. Ex vivo examination can be used as a complementary technique if the data provided by in vivo examination are not diagnostically valid.
Magnetic resonance imaging
MRI is a medical imaging technique used in radiology to investigate the anatomy and physiology of the body. MRI scanners use strong magnetic fields and radio waves to form images of the body. Small surface coils dedicated to digit imaging are now available in all high-field MRI units [19]. MRI can detect tumours as little as 1 mm in diameter; it also defines their location and their tissue characteristics, all of which facilitates surgical management. T1-weighted sequence allows morphological evaluation of lesion contour and anatomical extent; T2-weighted sequence defines tissue characterization from signal intensity emitted by the tumour; gadolinium highlights the vascularization of tumours and may improve definition of lesion contours. The signals obtained in the various sequences are characteristic and can distinguish the most common tumours encountered in the nail region.
MRI can be helpful for investigating a range of periungual neoplasms and cysts. It is the gold standard for assessing glomus tumours of the nail unit and is particularly useful for myxoid (synovial) cysts, where even normal soft tissues can be distinguished and an in vivo anatomical assessment made (Figure 95.75a–c and Figure 95.76). MRI can help identify the inflammatory changes of psoriatic arthritis, where it has consequences for the soft-tissue element of the nail unit resulting in abnormal nail growth and appearance.
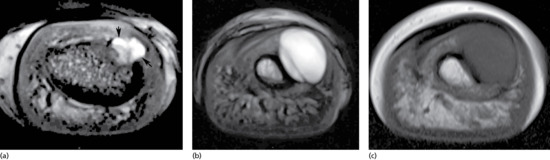
Figure 95.75 Axial T2-weighted image at the level of the distal interphalangeal joint (arrows): pedicle of the myxoid pseudocyst connected with the joint (arrows) (a). Axial T2 (b) and axial T1 (c) at the level of the nail cul-de-sac: lifting of the matrix and the root of the nail plate due to the cyst; bone scalloping of the dorsolateral aspect of the distal phalanx. (Courtesy of J. L. Drapé, France.)
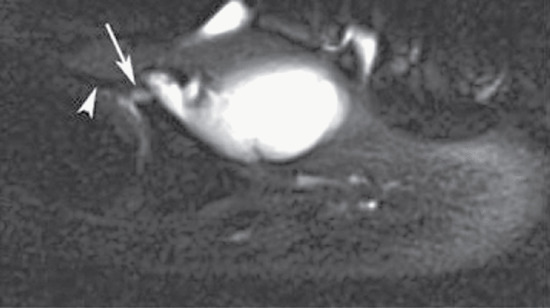
Figure 95.76 Sagittal section T2 fat saturated image: pedicle connecting with the joint (arrow) under the extensor tendon (arrow heads). (Courtesy of the copyright holder J. L. Drapé, France.)
Proximal nail fold capillaroscopy
Proximal nail fold capillaroscopy is a simple in vivo non-invasive and reliable technique used for evaluating superficial microvascular structures. The capillaroscope is composed of an optical microscope with a 50× to 200× magnification. A cold light source is used in order to avoid vasodilatation. It is of particular value for examining the microvasculature of the proximal nail fold, with its special arrangement of vascular loops parallel to the skin surface, which cannot be well visualized by the naked eye.
Skin is ‘prepared’ with an ointment of cedar oil or liquid petrolatum in order to improve optical transmission. Second, third, fourth and fifth fingernails of both hands are examined consecutively. Some authors have suggested that a dermoscope (or an ophthalmoscope) can be used for capillaroscopic examination of the proximal nail fold. The lower magnification permits visualization of megacapillaries, but is insufficient to explore other components of the bloodstream in detail.
Useful information can be obtained by an overall examination of the microvascular structure of the proximal nail folds. Capillary loops physiologically have a hairpin shape and are arranged in two parallel longitudinal rows: usually 12–18 loops are found per millimetre (Figure 95.77a). Proximal to and arranged perpendicularly to them, the subpapillary veins can be visualized. It is important to recognize a number of different patterns of no pathological significance: these include a glomerular conformation and elongated and tortuous loops. Pathological changes include ramified vessels, elongated loops, megacapillaries, loss of the normal parallel arrangement of loops and microaneurysms. Under the highest magnification, blood flow can be directly observed: either a normal continuous flow or a pathological intermittent flow with irregular interruptions occurring over time. Vasomotor tone can be evaluated subjectively by detailed observation of a vascular field. It is overactive in idiopathic Raynaud phenomenon and decreased in acrocyanosis. In lupus erythematosus, rapid and marked changes in vasomotor tone can be observed within the same capillary loop. It should be noted that deep epidermal pigmentation may reduce the visibility of the capillary loops.
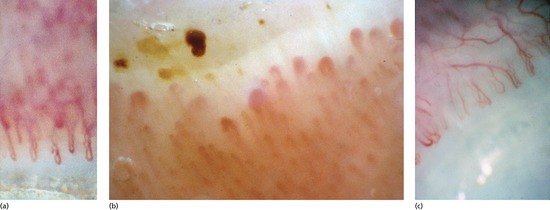
Figure 95.77 Normal nail fold capillaries (×60) (a); acrocyanosis showing dilatation of the nail fold capillaries, stasis and thrombosis of many vessels (b); rheumatoid arthritis showing rather elongated capillary loops (c).
Examination of the background may also yield useful information. In lightly pigmented skin, the background colour is pinkish, but can be orange if there is venous stasis. A pericapillary halo can be observed in inflammatory conditions and in cases of vascular stasis. A ‘hazy’ appearance may be seen in systemic sclerosis. Haemorrhages are observed in cases of evolving microangiopathy: a ‘pearl necklace’ appearance differentiates these from trauma-induced haemorrhages where the appearance is blotchy.
Acrosyndromes
Raynaud phenomenon
Raynaud phenomenon may be either idiopathic, Raynaud disease, or associated with a connective tissue disease (Raynaud sign or symptom). The most important indication for capillaroscopy is in the differential diagnosis between these two situations with very different prognoses. In Raynaud disease capillaroscopy is normal. Rarely, efferent capillaries can be found to be somewhat enlarged and some subtle haemorrhages can be observed. During the vasoconstrictive phase, a whitish background and a reduction in the number of visible capillaries can be observed. The appearances in connective tissue disease are discussed below.
Acrocyanosis
Acrocyanosis is clinically characterized by a painless distal cyanosis often with hyperhidrosis and cold extremities. This disease is worsened by exposure to cold. Capillaroscopy shows a normal or slightly increased number of capillaries of slightly enlarged diameter (especially in the efferent part of the loop). The loops are often tortuous over a cyanotic background. The blood flow is slowed and often has a granular appearance (Figure 95.77b).
Livedo
In livedo reticularis capillaroscopy shows enlarged efferent loops intermixed with normal loops and the blood flow is slowed. In livedo racemosa (‘broken livedo’) microvasculitis can sometimes be detected.
Chiblains (perniosis)
In chilblains affecting the fingers, homogeneously dilated vascular loops are observed in association with normal loops.
Systemic autoimmune diseases
Rheumatoid arthritis
Signs of dermal vasculitis are observed with tortuous and ramified capillary loops (Figure 95.77c). In some cases, short parallel loops in a ‘fish shoal’-like pattern are observed. Background is not hazy and the blood flow is granular and fast.
Dermatomyositis
The clinically visible telangiectases of the cuticle correspond to megacapillaries as seen under the capillaroscope (Figure 95.78). However, in contrast with systemic sclerosis, there are no avascular areas.
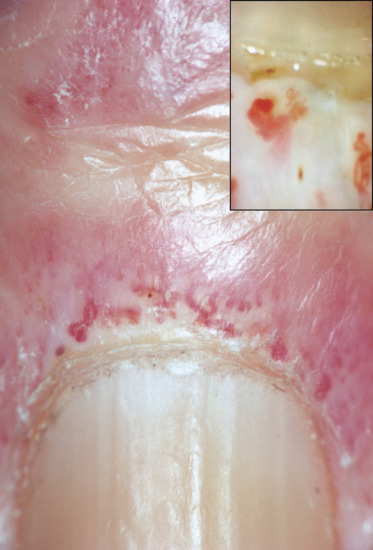
Figure 95.78 Dermatomyositis showing dilated nail folds capillaries and obstructed and thrombosed capillaries (×60) (inset).
Lupus erythematosus
In lupus erythematosus, observed abnormalities are morphological and rheological. Elongated loops, irregular enlargement of the afferent and/or efferent vessels, ramified and in some cases tortuous loops, and microaneurysms are the most common morphological changes (Figure 95.79a).

Figure 95.79 Lupus erythematosus (a) and systemic sclerosis (b). (Courtesy of the copyright holder C. Mathis, Belgium.)
Systemic sclerosis
Typical changes are observed in systemic sclerosis (Figure 95.79b) and nail fold video-capillaroscopy [20] is at present the most valuable tool for allowing an early diagnosis as follows:
- Rarefaction of the capillary loops with avascular areas.
- Megacapillaries of different size and shape often corresponding to the clinically visible telangiectases of the cuticle.
- Granular blood flow.
- Few haemorrhages.
Four stages are usually recognized as follows:
- Stage I: appearance of a few ‘open’ loops, ‘U-shaped’ and a few slightly enlarged capillaries.
- Stage II: slight decrease in the number of capillary loops, a few megacapillaries among many normal loops, hazy background.
- Stage III: marked decrease in the number of capillary loops, many megacapillaries, atrophic capillary loops, very hazy background.
- Stage IV: many avascular areas, reduced number of megacapillaries, very hazy background.
Psoriasis
Cutaneous microcirculation is different in psoriatic patients and normal individuals.
Vascular changes have been reported in nail folds. For this reason, Ribeiro et al. [21] studied psoriatic nail fold video-capillaroscopy in 46 patients (mean age 50.5, median disease duration 10 years) and 50 healthy controls and found lower capillary density, increase of avascular areas and morphologically abnormal capillaries in psoriatic subjects. No association between changes in capillary density and duration, extent or severity of the disease was noted. However, the presence of avascular areas was more common in patients whose nails were affected by the disease (pitting or dystrophy).
Toxic diseases
Vinyl chloride poisoning produces sclerodactyly. Capillaroscopy shows megacapillaries without decreased vascular density or avascular areas. The background is not hazy.
NAIL SURGERY
Introduction and general description
Patients often fear nail surgery because of anticipated pain, both during anaesthesia and postoperatively. The potential for causing permanent postoperative nail dystrophy frightens the practitioner.
A good knowledge of anaesthetic techniques, nail anatomy and surgical procedures is a prerequisite for a successful nail surgery with almost no pain and minimal scarring. It is also mandatory to involve a dermatopathologist who is familiar with the histological idiosyncrasies of the nail unit.
Anaesthesia
Premedication
Premedication may be useful in anxious patients. Short action molecules should be preferred: hydroxyzine, diazepines, orally or sublingually, the latter acting more rapidly. The combination of hydroxyzine 25 mg the night before the operation with 0.5 mg lorazepam sublingually 1 h prior to surgery is very effective [1]. Midazolam is favoured by some surgeons as it has short-acting hypnotic, anxiolytic and retrograde amnesic properties [2]. The use of EMLA under occlusive dressing prior to surgery will alleviate only the pain caused by needle insertion but not that due to injection of local anaesthetic and distension of tissues [3].
Equipment
Injections into the nail apparatus encounter high resistance and the use of a Luer lock syringe is mandatory. Using very thin needles (30 G) will decrease pain from puncture and limit the anaesthetic flow and rate of distension of the soft tissues. It is common for the physician to spend more time administering the anaesthesia than performing the surgical procedure.
Anaesthetics
Plain lidocaine 1 or 2% is the reference local anaesthetic. It acts for 60 min. As it is acid, pain during infusion may be reduced by prior alkalinization [4]. Warming the anaesthetic to 37°C also reduces the pain associated with infusion [5].
Lidocaine with epinephrine (adrenaline) is safe in the digits [6, 7, 8, 9] except in patients with vasospastic, thrombotic or severe medical conditions. However, the use of epinephrine is of little interest in nail surgery as, to achieve a bloodless field, a tourniquet must be placed at the base of the digit in almost all procedures. Bupivacaine 0.5% acts after 45 min for up to 480 min [10]. It may be added to lidocaine to lengthen the postoperative analgesia. An alternative is to inject 0.5–1 mL of bupivacaine immediately postoperatively into the lateral aspect of the digit: this will act as a ‘volumetric’ tourniquet and prevent further bleeding [11]. Ropivacaine has the same quick onset as lidocaine, provides better postoperative pain relief [12, 13, 14] and is less cardiotoxic than bupivacaine [15]. Pain at infiltration depends on concentration. For routine use, a 2 mg/mL concentration provides a very comfortable anaesthesia with full sensation restored by 7 h. Ropivacaine produces slight vasoconstriction at low dosages [16].
Procedures
The so-called ‘ring block’ is still very popular but it should not be recommended any more. Its main drawbacks are the ‘late’ anaesthetic effect, requiring up to 20 min to develop, and the potential hazard of compression and trauma to neurovascular bundles with subsequent postoperative oedema and prolonged pain [17].
The distal digital block is the technique of choice in nail surgery. The injection site is 1 cm proximal and lateral to the junction of the proximal nail fold and the lateral nail fold. The needle is pushed at a 45° angle directed distally, down to the bone. 0.5 mL of anaesthetic will anaesthetize the branches of the dorsal nerve. The needle is then partially withdrawn and pushed down vertically skimming the lateral aspect of the phalanx towards the pulp where another 0.5 mL are deposited to block the branches of the palmar nerves. For complete anaesthesia, the procedure should be repeated on the opposite side. Anaesthesia takes effect immediately.
Instrumentation
Basic nail surgery requires only very few specific instruments. The classical tray should include an elevator to detach the plate from its attachments (e.g. Freer or Locke elevator, or a dental spatula), a nail splitter, straight haemostat, fine iris or Gradle scissors, no. 15 surgical blade, fine-toothed Adson forceps, a fine-needle holder, 3/0 and 4/0 non-absorbable sutures.
Diagnostic surgery
Proximal nail fold biopsy
Three techniques are available for biopsying this area as follows:
- When the indication is similar to a biopsy elsewhere on the skin, a punch biopsy (not over 3 mm) may be taken on the proximal nail fold, taking care that its distal margin is always preserved.
- The shave biopsy technique is also very useful for this area. Haemostasis can be obtained with aluminium chloride solution.
- When more tissue is required, as in collagen diseases, a crescent-shaped excision, 2–3 mm wide, is carried out from one side to the other. This amount of tissue allows histology, immunohistology and electron microscopy to be performed [18]. The procedure is the same as the surgical treatment of chronic paronychia (see later).
Nail bed biopsy
Indications for nail bed biopsies are diseases of the nail bed presenting as onycholysis, subungual hyperkeratosis or tumour. In the absence of onycholysis, a partial or total nail avulsion (see later) should be performed to expose the area to be biopsied. As for skin, incisional biopsy is performed with a punch and excisional biopsy with a blade. The punch should be pushed down to the bone. No suture is required, as a defect up to 4 mm across will heal by secondary intention without dystrophy.
Elliptical biopsy of the nail bed is indicated to remove larger specimens (e.g. small tumours). The elliptical excision should always be orientated in a longitudinal axis. The nail bed is very fragile and tightly adherent to the bone so that reapproximation of the margins may be difficult. To overcome this, lateral undermining of the edges should be generous. Suturing may leave a gap that will heal by secondary intention.
Nail matrix biopsy
Matrix biopsies are most useful for longitudinal melanonychia. An accurate histological diagnosis requires examination of the entire pigmented lesion and therefore incisional biopsies are not recommended and only excisional biopsies should be performed. Dystrophic sequelae are unlikely if the pigment is confined to the distal matrix, as the latter synthesizes the ventral part of the nail plate. The only consequence will be a nail plate thinned from below. Fortunately, in the majority of cases longitudinal melanonychia originates in the distal matrix [19]. If the pigment is located within or extends to the proximal matrix, a nail plate dystrophy is highly probable, as this part of the matrix generates the upper third of the nail plate.
Several techniques are available according to the width and shape of the band. Each of the following procedures starts identically in order to expose the nail matrix. Using an elevator, the proximal nail fold is detached from the nail plate; two lateral incisions at 45° enable it to be reflected. Then, a lateral avulsion (‘sardine tin’ avulsion) of the proximal third of the nail plate exposes the whole matrix and the most proximal part of the nail bed.
- For a well-circumscribed round pigmented lesion of <3 mm in diameter located in the distal matrix, the punch biopsy technique is best [20]. A 3 mm punch encompassing the whole pigmented macule is pushed vertically into the matrix down to the bone (Figure 95.80a–d). The defect is left open and the nail plate is laid back in place and sutured to the lateral nail fold. Punching through the nail plate at the origin of the longitudinal melanonychia before avulsing is very useful when dealing with lightly pigmented bands: the process of avulsion often detaches the superficial layers of the matrix epithelium and the origin of the band may then be difficult to identify. By performing a punch in this manner, the area to biopsy can be clearly seen once the nail plate has been avulsed [21].
- If the source of pigment is oriented longitudinally, it should be excised using a longitudinal ellipse with minimal margins. The edges of the incision are widely undermined and reapproximated with 5/0 or 6/0 absorbable sutures (Figure 95.81a–d). The nail is laid back in place and sutured to the lateral nail fold. The proximal nail fold is returned to its anatomical position and the lateral incisions are sutured [21].
- If the source of pigment is oriented horizontally, a crescent-shaped excision should be performed with the convex portion of the incision matching the contour of the lunula. The borders of the defects are generously undermined and the edges are gently reapproximated with 5/0 or 6/0 absorbable sutures. The avulsed nail plate and proximal nail fold are then replaced as described above.
- For very extensive pigmentation (>6 mm in diameter) the tangential excision is recommended. A shallow incision is carried out around the pigmented zone. The scalpel is then held horizontally and with sawing motions the lesion is removed from the deep dermis. It should not be thicker than 0.5 mm. The specimen is placed on filter paper and properly oriented for the pathologist. The avulsed nail is put back and secured to the lateral fold. This technique has proven sufficient to allow adequate diagnosis in all cases. Its main drawback is a recurrence of the pigmentation in about three quarters of cases [22]. This technique should be restricted to large pigmented bands that were formerly an indication for immediate total ablation of the nail unit (Figure 95.82a–d). This technique avoids mutilating surgery in cases where the pigment derives from a large benign lesion. If histopathology shows that the lesion is malignant, further surgery is required.
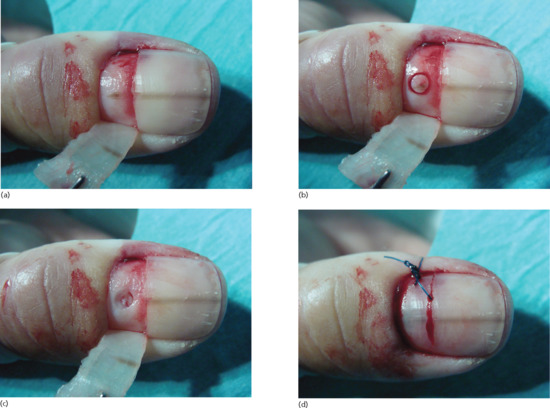
Figure 95.80 (a) Avulsion of the proximal third of the plate exposes the pigment area responsible for the longitudinal pigmentation. (b) A 3 mm punch is performed around the whole pigmented area. (c) The specimen is removed down to the bone. (d) The plate is put back in place and secured to the lateral fold.
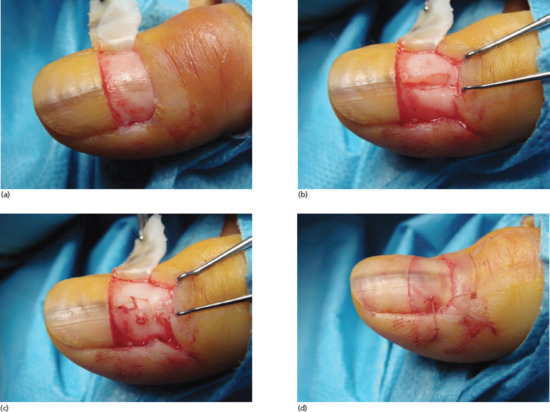
Figure 95.81 (a) Avulsion of the proximal third of the plate demonstrates that the pigment area responsible for the pigmentation extends longitudinally on the matrix. (b) The whole pigmented area is removed in a longitudinal elliptical excision. (c) After undermining the wedges, the defect is reapproximated with absorbable sutures. (d) The plate is put back in place and secured to the lateral nail fold.
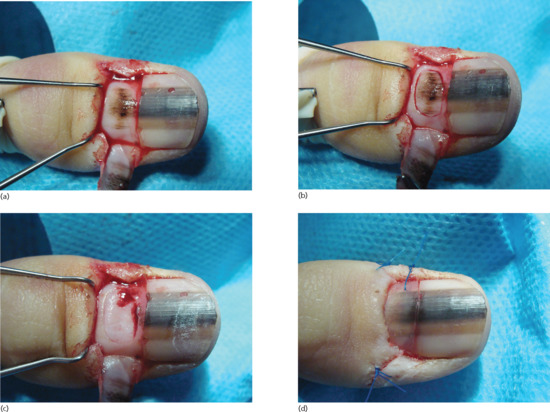
Figure 95.82 (a) Avulsion of the proximal third of the plate exposes the wide pigment area responsible for the longitudinal pigmentation. (b) An incision is carried out all around the whole pigmented area. (c) The specimen is tangentially removed as a whole. (d) The plate is put back in place and secured to the lateral nail fold.
Biopsy of whole structures of nail apparatus
The lateral longitudinal biopsy permits study of all components of the nail unit: proximal nail fold, matrix, nail bed, nail plate and hyponychium. This is the most rewarding biopsy technique when dealing with a disease presenting as alterations of the nail plate surface. This will narrow the nail permanently due to the partial amputation of the lateral horn of the matrix. In order to avoid any postoperative lateral deviation, the specimen should not exceed 3 mm in width [23]. The incision starts half way between the cuticle and the crease of the distal interphalangeal joint and runs distally through the proximal nail fold, the nail plate and its bed to the hyponychium. At the junction of the lateral and proximal nail fold, the incision should follow a laterally curved direction extending halfway down the lateral aspect of the finger as far as the distal interphalangeal joint, in order to ensure removal of the lateral horn of the matrix. A second incision, starting from the distal extremity of the previous one, runs from the hyponychium into the lateral sulcus and joins the proximal end of the previous incision. The resulting sigmoid biopsy specimen (Figure 95.83) is then carefully detached from the bone with fine scissors. At the proximal end of the biopsy, care must be taken to include the matrix by avoiding lifting the scissors too soon and thus foreshortening the specimen. The defect is reapproximated with horizontal mattress sutures in order to recreate a lateral nail fold [24].
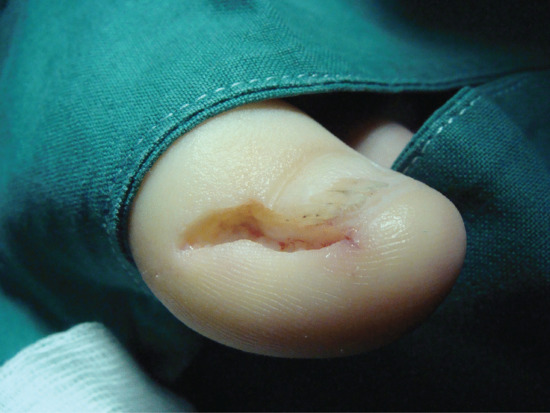
Figure 95.83 Lateral longitudinal biopsy. Note the sigmoid shape of the defect that can be easily closed.
Excisional surgery
Nail avulsion
Nail avulsion is a core procedure in nail surgery: it allows inspection of and access to a subungual lesion in the nail bed or matrix for biopsy or excision (Figure 95.84); it is an adjuvant treatment in onychomycosis as it reduces the fungal mass; it is part of the treatment of an acute paronychia and of ingrowing toenail. Total surgical removal should be discouraged: the distal nail bed may shrink and become distorted dorsally. In addition, the loss of counterpressure from the nail plate allows dorsal expansion of the distal pulp, promoting distal embedding. Partial nail avulsion should always be favoured. However, in some instances (e.g. prominent dystrophic total onychomycosis) total avulsion is unavoidable.
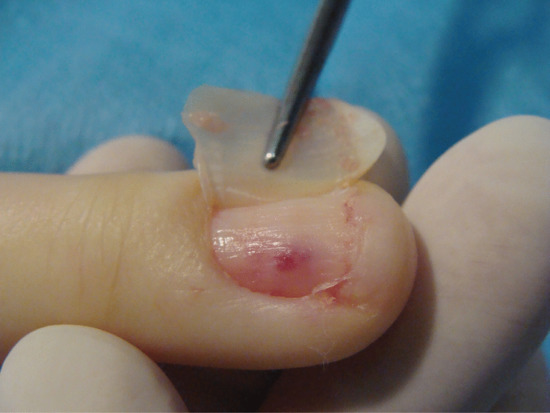
Figure 95.84 Lateral avulsion (‘sardine tin’ avulsion) allows exposure of the complete nail bed and excisional biopsy of the nail bed tumour.
Total surgical nail avulsion
This may be carried out using either a distal or a proximal approach.
Distal approach
An elevator is gently slid under the proximal nail fold in a back-and-forth motion from side to side, so avoiding injuring the fragile longitudinal nail bed ridges, until the proximal nail fold is freed from the nail plate. The elevator is then pushed under the nail plate from the distal free edge until the elevator gives way (meaning the elevator has reached the matrix area to which the nail plate is loosely attached). Caution must be taken to detach the lateral horns of the nail plate fully. A jaw of a sturdy haemostat is slid under the whole length of a lateral portion of the nail plate and grasped firmly. In an upward rotating motion, the nail plate is avulsed [25].
Proximal approach
The proximal approach is advised when the distal subungual area strongly adheres to the nail plate (e.g. thick hyperkeratosis) and it is then difficult to find a cleavage plane between the plate and the bed. The proximal nail fold is detached as described above. The elevator then reflects the proximal nail fold and is delicately inserted under the base of the nail plate where the adherence to the matrix is weak. The procedure is repeated along the whole width of the nail root. The avulsion progresses distally following the natural cleavage plane up to the hyponychium [25].
Partial surgical nail avulsion
The considerable advantage of this technique is that it leaves a large portion of normal nail plate that still exerts a pressure on the underlying soft tissues, reducing the risk of distal embedding. It is a must in the treatment of some types of onychomycosis (longitudinal streaks, lateral disease, dermatophytoma, onychomycosis due to moulds) [26] (Figure 95.85a,b). Partial nail avulsion is part of many surgical procedures: chemical cautery of a part of the matrix in ingrowing toenails, treatment of acute paronychia, surgical exploration of any nail bed or matrix tumour. It is performed in the same way as the distal approach method of total surgical nail avulsion, but restricted to a limited portion of the nail plate. For exposure of the matrix area, avulsion of the proximal third of the nail plate is best. It starts with two lateral incisions on the proximal nail fold at 45° enabling it to be reflected. A jaw of a nail splitter is inserted under the lateral border of the nail plate, approximately 5 mm distal to the lunula. The plate is cut horizontally to the other side. A haemostat grasps the lateral portion of the plate and lifts it up laterally, as for a sardine tin, exposing the whole matrix area (see Figures 95.80a, 95.81a and 95.82a). After surgery, the plate is laid back in place and sutured to the lateral fold.
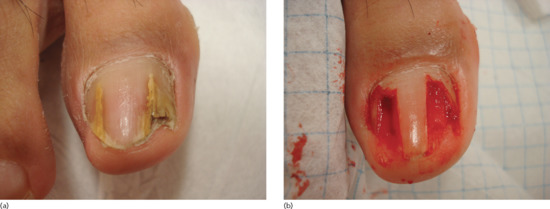
Figure 95.85 (a) Dermatophyte onychomycosis with longitudinal spikes. (b) After surgical removal of the yellow streaks.
Acute paronychia
Acute paronychia generates a lot of pain and prolonged pressure from the swollen paronychial soft tissues onto the matrix may impair the normal regrowth of the nail plate. Incision of the proximal nail fold is discouraged as it may result in a deformed eponychium. If the pus collection is located under the proximal nail fold, the best treatment is avulsion of the proximal third of the nail plate (see Partial nail avulsion earlier) to allow drainage of the pus. If the collection is in the lateral nail fold, avulsion of a lateral strip of nail plate should be carried out. Systemic antibiotics are prescribed empirically and adapted if necessary once culture results are known [27].
Chronic paronychia
Surgical treatment is indicated when medical treatment has failed. An elevator is inserted into the proximal nail groove under the proximal nail fold in order to protect the matrix and the extensor tendon. With a no. 15 surgical blade, a crescent-shaped excision of the proximal nail fold is performed: the incision should run from one side to the other, reaching its maximum width (5 mm) in the midline of the proximal nail fold. The incision should include the five most proximal millimetres of the lateral nail folds. The blade should be held obliquely at 45° down to the nail plate (Figure 95.86a,b). Complete healing by secondary intention will restore the proximal nail fold with its cuticle in less than 5 weeks (Figure 95.86c). However, the nail plate will appear a bit longer with a larger lunula [28]. This technique is also adequate for small very distal type B myxoid pseudocysts.

Figure 95.86 (a) Chronic paronychia resistant to topicals and steroid injections. (b) Crescent-shaped excision of a part of the proximal nail fold. (c) Complete re-epithelialization at day 8. Note that the nail is now apparently longer.
Fibrokeratoma resection
For a fibrokeratoma arising from the ventral surface of the proximal nail fold, reflection of the latter with two oblique incisions at 45°, exposes the whole nail pocket (Figure 95.87). In most instances, the fibrokeratoma originates from the most proximal part of the ventral proximal nail fold. The tumour should be delicately dissected up to its base using fine iris scissors and then severed. Injuring the matrix is impossible as the nail plate is still in place. The proximal nail fold is then laid back and secured with 5/0 stitches or adhesive strips [29].
Figure 95.87 Two lateral incisions at 45° allow reflection of the proximal nail fold; visualization of the tumour and its removal.
For a fibrokeratoma arising from the nail bed, a nail avulsion is required in order to expose the nail bed (Figure 95.88). The tumour is excised in the same manner as an elliptical biopsy of the bed (see Nail bed biopsy earlier). In both forms, incomplete resection leads to recurrence.
Figure 95.88 Trap door avulsion permits access to the nail bed tumour. The latter will be removed in a longitudinal excision.
Chemical cautery for ingrowing toenails
The therapeutic aim of this procedure is a selective cautery of the lateral horns of the matrix to obtain a permanently narrowed nail plate that will solve the ‘nail plate–lateral nail fold conflict’ once and for all. This technique may be performed on both sides of pincer nails and suppresses immediately the ‘pincer’ effect of the lateral edge of the nail plate on the soft tissues. Chemical cautery with phenol is easy to learn and is very effective (<3% recurrence rate). It is the recommended procedure in several Cochrane reviews as it has a high success rate with low morbidity [30, 31]. After a distal digital block, a 3–5 mm wide lateral strip of nail is avulsed up to its most proximal part. This partial nail avulsion exposes the lateral horns of the matrix at the proximal part of the cavity. Chemical cautery of the lateral horns of the matrix is carried out with a cotton-tipped applicator dipped into 88% phenol and then pushed into the cavity (Figure 95.89a–c). The applicator is left in place for 4 min [32]. To ensure the effect of the phenol, it is essential to work in a bloodless field using a tourniquet. Spillage of phenol onto the periungual tissues should be avoided as this causes unnecessary burns. For beginners, application of a greasy ointment onto the perionychium prior to cauterization may protect the tissues. After the procedure, applying alcohol will not ‘neutralize’ the phenol but only dilute it [33]. Phenol induces coagulation of proteins from the matrix epithelium. Once coagulated, the epithelium becomes impermeable to any liquid. Release of the tourniquet will allow the blood proteins to inactivate any residual phenol [21]. Patients have little postoperative pain as phenol has, apart from its caustic effect, important antiseptic and anaesthetic properties. The major drawback of this technique is the prolonged oozing from the phenolic burn. This may last up to 5 weeks. Daily home care (soakings and antiseptic ointment) are required until the wounds are completely dry. The ooze should not be mistaken for infection. Very high success rates can be achieved with other caustics including 10% KOH [34] and 100% trichloracetic acid [35].
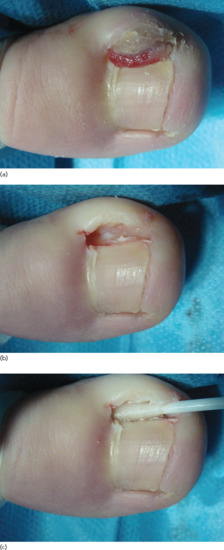
Figure 95.89 (a) Ingrowing toenail with pyogenic granuloma. (b) After curettage of the pyogenic granuloma, a lateral strip of nail is avulsed. (c) A cotton-tipped applicator, dipped into 88% phenol is pushed into the cavity. Note the bloodless surgical field.
Longitudinal melanonychia removal
See matrix biopsies.
Postoperative care
Dressings should include generous amounts of antiseptic ointment covered with petrolatum gauze to avoid adhesion to the wound and to facilitate removal of the dressing. Two to three fine-mesh gauze squares and either tubular elastic net or elastic bandage complete it. A narrow bandage (4 cm) is a more flexible form of dressing which enables pressure to be applied more precisely over the wound. The dressing should provide no more than light compression in order not to compromise the blood flow. This bulky dressing will enable postoperative bleeding to be absorbed and will provide some protection against trauma. The limb should be kept elevated for 48 h to ease throbbing and facilitate healing. The patient should wear a sling if the surgery involves a finger, or keep the foot elevated for 2 days for toe surgery. This also means that the patient should not plan to drive home following surgery. Painkillers should be prescribed for 3 days. The dressing should be removed on day 2, if necessary after soaking. Further care includes twice-daily dressings with antiseptic ointment covered by a plaster until complete healing has been achieved.
THE NAIL AND COSMETICS
Nail coatings represent an attractive nail enhancement. They may harden upon evaporation (nail varnish) or polymerize (sculptured nails, gels, preformed artificial nails) [1].
Coatings that harden upon evaporation
Nail varnish
The term ‘nail lacquer’ is sometimes used to include enamels, top coats and base coats, either as separate entities or combined in one product. Although chemically similar, they contain different ratios of the same constituents to lend different characteristics. The base coat is used to improve the adhesion or bonding of enamel to the nail. A top coat improves the depth and lustre of the enamel and increases its resistance to chipping and abrasion. Nail polishes consist of solids and solvent ingredients, the former representing about 30%, the latter 70% of the product. The ingredients can be divided into six principal groups (Box 95.3).
The base coat is formulated in a manner similar to standard lacquer, but it has a lower non-volatile content (less nitrocellulose) and lower viscosity, because a thinner film is desirable; it may also contain hydrolysed gelatine. In the top coat, the nitrocellulose content is increased and the resin reduced. A slight increase in plasticizer content improves the elasticity of the film. There is no pigment. The top coat often has an added sunscreen.
Reactions such as an allergic contact dermatitis to nail varnish frequently appear on any part of the body accessible to the nails, with paradoxically no signs in or around the nail apparatus [2]. The most commonly involved areas are the eyelids (Figure 95.90), the lower half of the face, the sides of the neck and the upper chest. Sometimes the use of nail polish on stockings to stop ‘runs’ or on nickel-plated costume jewellery to prevent nickel dermatitis may induce nail polish dermatitis on the legs or at the site of the metal contact. Connubial or transfer nail polish dermatitis may occur in the user's partner or other close contacts. Although any ingredient may account for distant allergic contact dermatitis, tosylamide formaldehyde resin is the most common culprit. After the nail polish is removed, the dermatitis usually clears rapidly unless secondary infection or lichenification has occurred. Eluate from uncoated metal pellets present in some bottles to keep the varnish in a liquid state may cause nickel reactions and onycholysis.
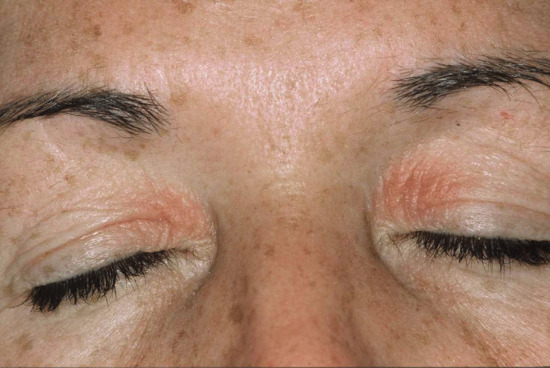
Figure 95.90 Allergy to nail varnish presenting as an eyelid dermatitis.
Nail plate staining from the use of polish is most commonly yellow-orange in colour (Figure 95.91). It typically starts near the cuticle, extends to the nail tip and becomes progressively darker from base to tip. With time, the dyes penetrate the nail too deeply to be removed. Injury to the nail plate from nail lacquers is rare. However, ‘granulation’ of nail keratin, a superficial friability, can be observed in some instances where individuals leave nail lacquer on for many weeks or where there is poor formulation of the product.
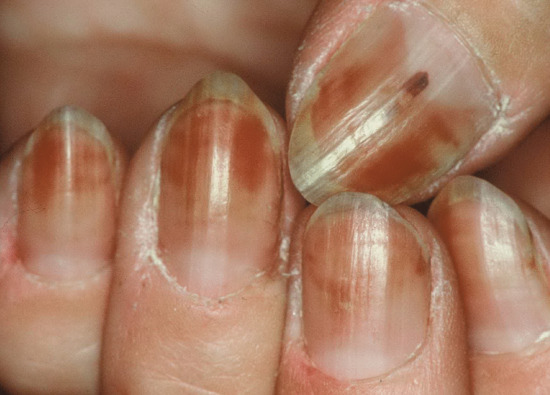
Figure 95.91 Staining of the nail plates from nail varnish.

Figure 95.92 Complication of nail extensions: allergy to acrylate adhesive presenting as onycholysis.
For patch testing, several nail lacquers should be used and tested ‘as is’ using occlusive chambers (e.g. Finn Chambers® or IQ Ultra Chambers®); the lacquers should be allowed to dry for 15 min before application of the patches, because the solvents and diluents may cause false-positive reactions.
The substances listed in Box 95.4 should be included in the test battery.
Various cosmetic companies now make varnishes that are formulated without the sensitizing resin and are toluene free. The presence of nickel in any product can be detected using the dimethylglyoxime spot test, which is highly specific.
Nail polish removers. These are composed of various solvents such as acetone. Occasionally, nail polish removers cause trouble by excessive drying of the nail plate, and may be responsible for some inflammation of the nail folds.
Coatings that polymerize
Sculptured nails
The nail is first thoroughly cleansed and painted with antiseptic and antifungal solutions. The nail is frequently dried with a diethyl ether based nail dehydrating agent and sometimes ‘primed’ with methacrylic acid/solvent adhesion promoter which works like a double-sided adhesive tape, sticking to both the nail and to the acrylic.
Self-curing acrylic resins are obtained by blending a methyl, ethyl or isobutyl methacrylate monomer which comes in a liquid form and a polymethyl or ethyl methacrylate polymer, which is a powder. The monomer also contains a stabilizer such as hydroquinone and N,n-dimethyl-p-toluidine as an accelerator. The polymer contains benzoyl peroxide as a polymerization initiator. Liquid monomer and powder polymer are mixed and the compound has to be moulded on the natural nail. Self-curing acrylic resins harden at room temperature. When hardened, the compound produces a prosthetic nail that is enlarged and elongated by repeated applications. The prosthesis can be filed and manicured to shape, as the plate grows out, further applications of acrylic can be made to maintain a regular contour.
Allergic reactions
Allergic reactions due to sculptured nails may occur 2–4 months, or even as long as 16 months, after the first application. The first indication is an itch in the nail bed. Paronychia, which is usually present in allergic reactions, is associated with excruciating pain in the nail area, and sometimes with paraesthesia. The nail bed is dry and thickened, and there is usually onycholysis. The natural nail plate becomes thinner, splits and is sometimes discoloured. It takes several months for the nails to return to normal. Permanent nail loss is exceptional, as is intractable prolonged paraesthesia [3].
Irritant reactions
Irritant reactions to monomers occur. These manifest as a thickening of the nail bed's keratin layer, which can sometimes cause the entire nail bed to thicken with or without onycholysis. Nonetheless, the overwhelming majority of cases result from physical trauma or abuse.
Damage to the natural nail is not unusual after 2–4 months of wear of a sculptured nail. If it becomes yellow or crumbly, this means that the product was applied and maintained incorrectly. The patient should find a better-qualified nail technician. The problem may well not be the acrylic nail materials but rather the thinning of the nail due to excessive filing with heavy abrasives.
Primer (methacrylic acid) is a strong irritant, which may produce third-degree burns. It is hazardous if the cuticles are flooded or spills are not washed out immediately. Primer can permeate the plate and soak into the nail bed if the nails are too thin. Soap or baking soda dissolved in water are excellent neutralizers. If primer gets into the eye, it should be rinsed with water for at least 15 min and a Poisons Information Centre should be contacted.
Light-cured gels
Gel system products are a premixed variant of sculptured nails in a semi-liquid form, either acrylic based (14% of the market) or cyanoacrylate based (1% or less of the market). Their virtual lack of odour makes gels popular in full-service beauty salons. UV light-cured gels are the best known of the different gel technologies. These gels contain urethanes and (meth)acrylate compounds, a photoinitiator and cellulose, which necessitates anti-yellowing agents and a UV light unit. The proportion of resins to monomers determines the gel consistency. When the gel is exposed to light of an appropriate wavelength, polymerization occurs, resulting in hardening of the gel. UV gels never involve catalysts and often do not require primers. Depending upon their composition, the gels can be used for different purposes as follows:
- Instead of the sculptured nail technique. However, they do not permit the formation of nails which are as long and resistant as those from the classical liquid–powder technique.
- Over preformed plastic tips: the nail surface is buffed. After disinfection, the preformed plastic tip is simply fixed with cyanoacrylate glue on the distal half of the nail.
- To protect a natural or varnished nail: this procedure is known as ‘nail capping’.
- Capping with fabric (silk, linen or fibreglass fixed with cyanoacrylate glue) adds strength and is known as ‘nail wrapping’.
The gel nails are useful in patients seeking treatment for cosmetically disfigured nails with the exception of psoriasis, where the risk of the Koebner phenomenon is high [4].
Gel enhancement products shrink by up to 20%, which may result in lifting and tip cracking. As an effect of excessive shrinkage, clients may comment that the enhancement feels tight on the nail bed. Other symptoms include throbbing or warmth below the nail plate. This may lead to tender and sore fingertips. Photobonded acrylate has been observed to cause nail reactions, sometimes with nail loss and paraesthesia. Hemmer et al. [4] have patch tested ‘hypoallergenic’ commercial products in patients wearing photobonded acrylic nails who had perionychial and subungual eczema. Triethyleneglycol dimethacrylate, hydroxyfunctional methacrylates, and (meth)-acrylated urethanes proved to be relevant allergens in photobonded nail preparations. Methacrylated epoxy resin sensitization was not observed. The omission of irritant methacrylic acid in UV-curable gels does not reduce the high sensitizing potential of new acrylates. Contrary to the manufacturers’ declarations, all ‘hypoallergenic’ products continue to include functional acrylate monomers, and therefore retain the potential for allergic sensitization. Gels and acrylics, being chemically distinct entities, will not necessarily cross-react.
Unreacted UV gel in the dusts and filings may produce distant allergic reactions. Although sensitization to butyl-hydroxytoluene is possible, gels usually contain acrylated oligomers and monomers. Acrylates are far more likely to cause sensitization than methacrylates or stabilizers.
Finally, thick and ornately painted gel false nails that may be difficult to remove present a real challenge to pulse oximetry. It appears to be the polish more than the sculpted nail that interferes with the readings.
Gel polish
This is a new manicure system applied in a salon by a nail technician. The application involves a base coat that is cured under a UV lamp, two layers of a proprietary nail varnish, and a top coat. During the curing process with a UV lamp, the manufacturer states that solvents evaporate and leave tiny ‘tunnels’ in the layer of varnish, connected by acetone-dissolvable polymers.
Preformed plastic nails
Preformed plastic nails are packaged in several shapes and sizes to conform to the normal nail plate configuration. Such nails are trimmed to fit the fingertip and are fixed with cyanoacrylate adhesive supplied with the kit. The usefulness of these prosthetic nails is limited by the need for some normal nail to be present for attachment. Normal physical and chemical insults to the nails cause the preformed plastic nails to loosen. If the preformed nails remain in place for more than 2–3 days, they may cause onycholysis and nail surface damage. Eczematous painful paronychia due to cyanoacrylate nail preparations may be observed after about 3 months. Dystrophy and discoloration of the nails may become apparent and last for several months. In some cases, distant contact dermatitis of the face and eyelids occurs. On patch testing, the patients react far more often to the adhesive than to the prosthetic nails (Figure 95.92). Suggested test substances are p-tertiary butylphenol resin (1% petrolatum); tricresyl ethyl phthalate (5% petrolatum); cyanoacrylates and other glues (5% in methylethylketone).
Nail-mending kits
These include paper strips of a basic film-forming product to create a ‘splint’ for the partially fractured nail plate. The split is first bonded with cyanoacrylate glue, then the nail is painted with fibred clear nail polish. A piece of wrap fabric is cut and shaped to fit over the nail surface. This is then embedded in varnish of high solid content, and several coats are applied.
Removal of nail coatings that polymerize
The most commonly used solvent for removal of self-curing acrylic is acetone. Warming the solvent with great care can cut product removal time in half. However, most gels are difficult to remove because they are highly cross-linked and resistant to many solvents. Therefore, if gel enhancements have to be removed, they should be slowly filed (not drilled) with a medium-grit file, leaving a very thin layer of product. They should then be soaked in warm product remover and, once softened, the remaining product may be scraped away with a wooden pusher stick.
Cuticle removers
These are lotions or gels containing approximately 0.4% sodium or KOH. The lotion is left in place for 1–3 min and then washed off. Creams containing 1–5% lactic acid (pH 3–3.7) are also used.
Nail hardeners
There are two main groups of products that make nail-hardening claims.
Products in the first group provide a protective coating, therefore the implied benefits come from the added strength and durability of the coating itself, rather than changes to the physical properties of the nail plate. Some consist of nail polish modified by the addition of extra ingredients including nylon fibres, acrylate resin and hydrolysed proteins: they function either as a base coat for nail polish or as a stand-alone treatment. Others applied as a base coat are essentially a modification of clear nail polish with different solvents and combinations of polyester, acrylic and polyamide resins designed to provide better adhesion of the coloured nail coating.
The second type of hardener chemically alters the structure of the nail. These products may contain up to 5% formaldehyde tissue fixative, but are designed to be applied only to the free edge of the nail while the skin is shielded. Most products never exceed 3% formaldehyde and the more widely sold brands contain less than 1%. Higher concentrations of formaldehyde can adversely affect both the nail plate and the surrounding tissue.
Nail changes due to hardeners may include pain, subungual haemorrhage and bluish discoloration of the nail. Formaldehyde nail hardeners have also been reported as causing onycholysis and both irritant and allergic contact dermatitis. Patch testing should be performed with formaldehyde (1 or 2% aqueous).
Silicone rubber nail prosthesis
For a wide variety of nail problems, ranging from deformed nail to complete loss of the terminal phalanx, a silicone rubber thimble- shaped finger-cover may be indicated. This prosthesis is easily fitted onto the finger stump, encasing the entire distal phalanx; it must be fine and flexible to maintain pulp sensitivity and have the same marking and colouring as the finger. The fixation is excellent and the nail form accepts nail varnish well. The most well known are Pillet Hand Prostheses® (PHPs), which are available in the US and some European countries. When there has been loss of tissue from the distal phalangeal pulp, a ‘sub-mini’ digital prosthesis is also available.
Nail cream
This is an ordinary water-in-oil moisturising cream, with low water (30%) and high lipid content. It is applied, after cleaning the hands, to prevent or diminish brittleness.
Nail buffing
Weekly buffing may be indicated for removing small particles of nail debris, thus enhancing the lustre and smoothness of the nail plate. Buffing creams, which contain waxes and finely ground pumice, and buffing powders are abrasive and should not be overused on thin nails.
Nail whitener
This is a pencil-like device with a white clay (kaolin) core used to deposit colour on the undersurface of the free edge of the nail.
Infection risks
Medical staff with artificial nails or nail extensions may put patients at risk through carriage of pathogens. The UK guidelines now require medical staff not to wear such embellishment. Nail varnish is also thought to be associated with bacterial carriage when it becomes chipped, although the evidence for this is less strong. Infection through nail salons and the manicuring process is a further factor that adds to the risks for those with artificial nails.
Conclusion
Nail beauty therapy is a flourishing and innovative industry with low overall risks of serious adverse events. It may certainly produce an attractive enhancement of normal nails and can be very valuable for disguising unsightly nail conditions: it is not recommended for psoriatic nails as it may provoke the Koebner phenomenon. Potential hazards include damage from instrumentation and allergic contact dermatitis.
References
Structure
- Zook EG. Anatomy and physiology of the perionychium. Hand Clin 2002;18:553–9.
- Haneke E. Surgical anatomy of the nail apparatus. Dermatol Clin 2006;24:291–6.
- Johnson M, Comaish JS, Shuster S. Nail is produced by the normal nail bed: a controversy resolved. Br J Dermatol 1991;125:27–9.
- Lewin K. The normal fingernail. Br J Dermatol 1965;77:421–4.
- Samman PD. Anatomy and physiology. In: Samman PD, Fenton D, eds. The Nails in Disease. London: Heinemann, 1986:1–20.
- Cohen PR. The lunula. J Am Acad Dermatol 1996;34:943–53.
- Achten G. L'ongle normal et pathologique. Dermatologica 1963;126:229–34.
- Dawber RPR, de Berker D, Baran R. Science of the nail apparatus. In: Baran R, Dawber RPR, eds. Diseases of the Nails and Their Management, 4th edn. Oxford: Blackwell Science, 2010:1–34.
- Burrows MT. The significance of the lunula of the nail. Johns Hopkins Hosp Rep 1919;18:357–61.
- de Berker D, Angus B. Markers of epidermal proliferation are limited to nail matrix in normal nail. Br J Dermatol 1996;135:555–9.
- de Berker DAR, MaWhinney B, Sviland L. Quantification of regional matrix nail production. Br J Dermatol 1996;134:1083–6.
- Fanti PA, Tosti A, Cameli N, Varotti C. Nail matrix hypergranulosis. Am J Dermatopathol 1994;16:607–10.
- Terry RB. The onychodermal band in health and disease. Lancet 1955;i:179–81.
- Perrin C. Peculiar zone of the distal nail unit: the nail isthmus. Am J Dermatopathol 2007;29:108–9.
- de Berker D, Wojnarowska F, Sviland L, et al. Keratin expression in the normal nail unit: markers of regional differentiation. Br J Dermatol 2000;142:89–96.
- Perrin C, Langbein L, Schweizer J. Expression of hair keratins in the adult nail unit: an immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. Br J Dermatol 2004;151:362–71.
- Hashimoto K. Ultrastructure of the human toenail. 1. Proximal nail matrix. J Invest Dermatol 1971;56:235–46.
- Hashimoto K. Ultrastructure of the human toenail. Ultrastruct Res 1971;36:391–410.
- Tosti A, Cameli N, Piraccini BM, et al. Characterisation of nail matrix melanocytes with anti-PEP1, anti-PEP8, TMH-1 and HMB-45 antibodies. J Am Acad Dermatol 1994;31:193–6.
- de Berker D, Graham A, Dawber RPR, Thody A. Melanocytes are absent from the normal nail bed: the basis of a clinical dictum. Br J Dermatol 1996;134:564.
- Ito T, Ito N, Saathoff M, et al. Immunology of the human nail apparatus: the nail matrix is a site of relative immune privilege. J Invest Dermatol 2005;125:1139–48.
- Sinclair RD, Wojnarowska F, Leigh IM, Dawber RPR. The basement membrane zone of the nail. Br J Dermatol 1994;131:499–505.
- Achten G. L'ongle normal. J Med Esth Chir Dermatol 1988;XV:193–200.
- Zaias N. The movement of the nail bed. J Invest Dermatol 1967;48:402–3.
- Zaias N. The Nail in Health and Disease. New York: Spectrum Press, 1990:6–7.
- de Berker D, Sviland L, Angus BA. Suprabasal keratin expression in the nail bed: a marker of dystrophic nail bed differentiation. Br J Dermatol 1995;133(Suppl. 45):16.
- Mogensen M, Thomsen JB, Skovgaard LT, Jemec GB. Nail thickness measurements using optical coherence tomography and 20-MHz ultrasonography. Br J Dermatol 2007;157:894–900.
- Blaydon DC, Ishii Y, O'Toole EA, et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 2006;38:1245–7.
- Cui CY, Klar J, Georgii-Heming P, et al. Frizzled6 deficiency disrupts the differentiation process of nail development. J Invest Dermatol 2013;133:1990–7.
- Nagy N, Wedgeworth E, Hamada T, White JM, Hashimoto T, McGrath JA. Schöpf–Schulz–Passarge syndrome resulting from a homozygous nonsense mutation in WNT10A. J Dermatol Sci 2010;58:220–2.
- Dai JX, Johnson RL, Ding YQ. Manifold functions of the nail–patella syndrome gene Lmx1b in vertebrate development. Dev Growth Differ 2009;51:241–50.
- Moll I, Heid HW, Franke WW, Moll R. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. Differentiation 1988;39:167–84.
- Zaias N, Alvarez J. The formation of the primate nail plate. An autoradiographic study in the squirrel monkey. J Invest Dermatol 1968;51:120–36.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol 2012;166:875–8.
- McLean WH, Epithelial Genetics Group. Genetic disorders of palm skin and nail. J Anat 2003;202:133–41.
Blood supply
- Dawber RPR, de Berker DAR, Baran R. Science of the nail apparatus. In: Baran R, Dawber RPR, de Berker DAR, Haneke E, Tosti A, eds. Diseases of the Nails and Their Management, 3rd edn. Oxford: Blackwell Science, 2001:1–47.
- Smith DO, Oura C, Kimura C, Toshimuri K. Arterial anatomy and tortuosity in the distal finger. J Hand Surg 1991;16A:297–302.
- Sangiorgi S, Manelli A, Congiu T, et al. Microvascularization of the human digit as studied by corrosion casting. J Anat 2004;204:123–31.
- Lo LC, Lin KC, Hsu YN, et al. Pseudo three-dimensional vision-based nail-fold morphological and hemodynamic analysis. Comput Biol Med 2012;42:873–84.
- Ryan TJ. The arteriovenous anastomoses. In: Jarrett A, ed. The Physiology and Pathophysiology of the Skin, Vol. 2. London: Academic Press, 1973:612–14.
- Wolfram-Gabel R, Sick H. Vascular networks of the periphery of the fingernail. J Hand Surg 1995;20B:488–92.
Nail growth and morphology
- Finlay AY, Moseley H, Duggan TC. Ultrasound transmission time: an in vivo guide to nail thickness. Br J Dermatol 1987;117:765–70.
- de Berker D, MaWhinney B, Sviland L. Quantification of regional matrix nail production. Br J Dermatol 1996;134:1083–6.
- Zaias N, Alvarez J. The formation of the primate nail plate. An autoradiographic study in squirrel monkeys. J Invest Dermatol 1968;51:120–36.
- Norton LA. Incorporation of thymidine 3H and glycine-2 3H in the nail matrix and bed of humans. J Invest Dermatol 1971;56:61–8.
- Lewis BL. Microscopic studies of foetal and mature nail and surrounding soft tissue. Arch Dermatol 1954;70:732–7.
- Johnson M, Comaish JS, Shuster S. Nail is produced by the normal bed: a controversy resolved. Br J Dermatol 1991;125:27–9.
- Johnson M, Shuster S. Continuous formation of nail along the bed. Br J Dermatol 1993;128:277–80.
- Pinkus F. In: Jasassohn J, eds. Handbuch der Haut und Geschlechtskrankheiten. Berlin: Springer, 1927:267–89.
- Samman PD. The ventral nail. Arch Dermatol 1961;84:192–5.
- Baran R. Nail growth direction revisited. Why do nails grow out instead of up? J Am Acad Dermatol 1981;4:78–84.
- Kligman AM. Nail growth direction revisited. Why do nails grow out instead of up? Response. J Am Acad Dermatol 1981;4:78–84.
- Kligman AM. Why do nails grow out instead of up? Arch Dermatol 1961;84:181–3.
- Samman PD. The Nails in Disease, 3rd edn. London: Heinemann, 1978:14.
- Schmiegelow P, Lindner J, Puschmann M. Autoradiographische Quantifizierung dosisabhängiger 35S-Cystin- bzw 35S-Methionin-Applikationen und des Einflusses unmarkierter Thioaminosäuren auf den 3H-Thymidin-Markierungsindex der Keimzellen von Nägeln und Haaren im Tierexperiment. Akt Dermatol 1983;9:62–6.
- Hashimoto K. Ultrastructure of the human toenail. Arch Dermatol Forsch 1971;240:1–22.
- Kelikian H. Congenital Deformities of the Hand and the Forearm. Philadelphia: Saunders, 1974:210–12.
- Baran R, Dawber RPR, eds. Guide Médico-Chirurgical des Onychopathies. Paris: Arnette, 1990:12.
- Bean WB. Nail growth: 30 years of observations. Arch Intern Med 1974;134:497–502.
- Dawber R, Baran R. Nail growth. Cutis 1987;39:99–102.
- Dawber RPR, de Berker D, Baran R. Science of the nail apparatus. In: Baran R, Dawber RPR, eds. Diseases of the Nails and Their Management, 2nd edn. Oxford: Blackwell Science, 1994:1–34.
- Runne U, Orfanos CE. The human nail. Curr Probl Dermatol 1981;9:102–49.
- de Doncker P, Pierard GE. Acquired nail beading in patients receiving itraconazole: an indicator of faster nail growth? A study using optical profilometry. Clin Exp Dermatol 1994;19:404–6.
- Dawber RPR. Fingernail growth in normal and psoriatic subjects. Br J Dermatol 1970;82:454–7.
- Dawber RPR. The effect of methotrexate, corticosteroids and azathioprine on fingernail growth in psoriasis. Br J Dermatol 1970;83:680–8.
- Hewitt D, Hillman RW. Relation between rate of nail growth in pregnant women and estimated previous general growth rate. Am J Clin Nutr 1966;19:436–9.
- Gilchrist ML, Buxton LHD. The relation of fingernail growth to nutritional status. J Anat 1939;73:575–81.
- Hamilton JB, Tereda H, Mestler GE. Studies of growth throughout the lifespan in Japanese. Gerontology 1955;10:401–10.
- Orentreich N, Markofsky J, Vogelman JH. The effect of ageing on the rate of linear nail growth. J Invest Dermatol 1979;73:126–30.
- Pfister R. Das normale Onychodiagramm. Z Haut Geschlechtskr 1955;18:132–7.
- Lavelle CE. Nail growth. Curr Probl Dermatol 1981;9:102–4.
- Pfister R, Henera J. Wachstum und Gestaltung der Zehennagel bei Gesunden. Arch Klin Exp Dermatol 1965;223:263–74.
- Donovan KM. Antarctic environment and nail growth. Br J Dermatol 1977;96:507–10.
- Roberts DF, Sandford MR. A possible climatic effect on nail growth. Appl Physiol 1958;13:135–7.
- Knobloch VH. Das normale Wachstum der Fingernagel. Dtsch Med Wochenschr 1953;78:743–5.
- Le Gros-Clark WE, Buxton LHD. Studies in nail growth. Br J Dermatol 1938;50:221–9.
- Landherr G, Braun-Falco O, Hofmann C, et al. Fingernagel Wachstum bei Psoriatitern unter PUVA-Therapie. Hautarzt 1982;33:210–13.
- Dawber RPR, Samman PD, Bottoms E. Fingernail growth in idiopathic and psoriatic onycholysis. Br J Dermatol 1971;85:558–67.
- Dawber RPR. The ultrastructure and growth of human nails. Arch Dermatol Res 1980;269:197–204.
- Baran R. Action thérapeutique et complications du rétinoid aromatique sur l'appareil unguéal. Ann Dermatol Vénéréol 1982;109:367–70.
- Miller E. Levodopa and nail growth. N Engl J Med 1973;288:916–19.
- Dawber RPR. Effects of immobilisation on fingernail growth. Clin Exp Dermatol 1981;6:1–4.
- Sibinger MS. Observations on growth of fingernails in health and disease. Pediatrics 1959;24:225–33.
- Weismann K. Beau and his description of transverse depressions on nails. Br J Dermatol 1977;97:571–2.
- Head H, Sherrin J. The consequence of injury to peripheral nerves in man. Brain 1905;28:116–18.
- Babcock MJ. Methods of measuring fingernail growth in nutritional studies. J Nutr 1955;55:323–38.
- Estes SA. Relapsing polychondritis. Cutis 1983;32:471–6.
Clubbing
- Lampe RM, Kagan A. Detection of clubbing: Schamroth's sign. Clin Pediatr 1983;22:125.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001;286:341–7.
- Goyal S, Griffiths AD, Omarouayache S, Mohammedi R. An improved method of studying fingernail morphometry: application to the early detection of fingernail clubbing. J Am Acad Dermatol 1998;39: 640–2.
- Roy HS, Wang Z, Ran H, Song W, Zheng Y. Diagnosis of digital clubbing by high-frequency ultrasound imaging. Int J Dermatol 2013;52:1–5.
- Nakamura J, Halliday NA, Fukuba E, et al. The microanatomic basis of finger clubbing: a high-resolution magnetic resonance imaging study. J Rheumatol 2014;41:523–7.
- Currie AE, Gallagher PJ. The pathology of clubbing: vascular changes in the nail bed. Br J Dis Chest 1988;82:382–5.
- Silveri F, Carlino G, Cervini C. The endothelium/platelet unit in hypertrophic osteoarthropathy. Clin Exp Rheumatol 1992;10(Suppl. 7):61–6.
- Bergmann C, Wobser M, Morbach H, et al. Primary hypertrophic osteoarthropathy with digital clubbing and palmoplantar hyperhidrosis caused by 15-PGHD/HPGD loss-of-function mutations. Exp Dermatol 2001;20:531–3.
- Busch J, Frank V, Bachmann N, et al. Mutations in the prostaglandin transporter SLCO2A1 cause primary hypertrophic osteoarthropathy with digital clubbing. J Invest Dermatol 2012;132:2473–6.
- Dever LL, Matta JS. Digital clubbing in HIV-infected patients: an observational study. AIDS Patient Care STDS 2009;23:19–22.
- Siragusa M, Schepis C, Cosentino FI, et al. Nail pathology in patients with hemiplegia. Br J Dermatol 2001;144:557–60.
- Baran R, Perrin C. Subungual perineurioma: a peculiar location. Br J Dermatol 2002;146:125–8.
Koilonychia
- Stone OJ. Spoon nails and clubbing: significance and mechanisms. Cutis 1975;16:235–41.
- Jalili MA, Al-Kassab S. Koilonychia and cystine content of nails. Lancet 1959;ii:108–10.
- Hogan GR, Jones B. The relationship of koilonychia and iron deficiency in infants. J Pediatr 1970;77:1054.
- Bergeron JR, Stone OJ. Koilonychia. A report of familial spoon nails. Arch Dermatol 1967;95:351.
- Dawber RPR. Occupational koilonychia. Br J Dermatol 1974;91(Suppl. 10):11.
- Alanko K, Kanerva L, Estlander T, et al. Hairdresser's koilonychia. Am J Contact Dermatitis 1997;8:177–8.
Pincer nail
- Baran R, Haneke E, Richert B. Pincer nails: definition and surgical treatment. Dermatol Surg 2001;27:261–6.
- Haneke E. Ingrown and pincer nails: evaluation and treatment. Dermatol Ther 2002;15:148–58.
- Mimouni D, Ben-Amitai D. Hereditary pincer nail. Cutis 2002;69:51–3.
- Hwang SM, Lee SH, Ahn SK. Pincer nail deformity and pseudo-Kaposi's sarcoma: complications of an artificial arteriovenous fistula for haemodialysis. Br J Dermatol 1999;141:1129–32.
- Vanderhooft SL, Vanderhooft JE. Pincer nail deformity after Kawasaki's disease. J Am Acad Dermatol 1999;41:341–2.
- Jemec GB, Thomsen K. Pincer nails and alopecia as markers of gastrointestinal malignancy. J Dermatol 1997;24:479–81.
- Plusje LG. Pincer nails: a new surgical treatment. Dermatol Surg 2001;27:41–3.
- Brown RE, Zook EG, Williams J. Correction of pincer-nail deformity using dermal grafting. Plast Reconstr Surg 2000;105:1658–61.
- Mutaf M, Sunay M, Isk D. A new surgical technique for the correction of pincer nail deformity. Ann Plast Surg 2007;58:496–500.
- Aksakal AB, Akar A, Erbil H, Onder M. A new surgical therapeutic approach to pincer nail deformity. Dermatol Surg 2001;27:55–7.
- Ozawa T, Yabe T, Ohashi N, Harada T, Muraoka M, Ishii M. A splint for pincer nail surgery: a convenient splinting device made of an aspiration tube. Dermatol Surg 2005;31:94–8.
Anonychia
- Solammadivi SV. Simple anonychia. South Med J 1981;74:1555.
- Blaydon DC, Ishii Y, O'Toole EA, et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 2006;38:1245–7.
- Baran R, Juhlin L. Bone dependent nail formation. Br J Dermatol 1986;114:371–5.
- Pall A, Gupta RR, Gulati B, Goyal P. Twenty nail anonychia due to lichen planus. J Dermatol 2004;31:146–7.
Nail shedding
- Runne U, Orfanos CE. The human nail. Curr Probl Dermatol 1981;9:102–49.
- Almeyda J. Platform nails. BMJ 1973;i:176.
- Baran R, Barth J, Dawber RPR. Nail Disorders. London: Dunitz, 1991:84–8.
- Baran R. Retinoids and the nails. J Dermatol Treat 1990;1:151–4.
- Eastwood JB, Curtin JR, Smith EKM, et al. Shedding of the nails apparently induced by large amounts of cephaloridine and cloxacillin in 2 anephric patients. Br J Dermatol 1969;81:750–2.
- Oliver WJ. Recurrent onychoptosis occurring as a family disorder. Br J Dermatol 1927;26:59–68.
- Bruckner-Tuderman L, Schnyder UW, Baran R. Nail changes in epidermolysis bullosa: clinical and pathogenetic considerations. Br J Dermatol 1995;132:339–44.
- Dharma B, Moss C, McGrath JA, Mellerio JE, Ilchyshyn A. Dominant dystrophic epidermolysis bullosa presenting as familial nail dystrophy. Clin Exp Dermatol 2001;26:93–6.
Onycholysis
- Ray L. Onycholysis: a classification and study. Arch Dermatol 1963;88:181–5.
- Daniel CR III, Iorizzo M, Piraccini BM, Tosti A. Grading simple chronic paronychia and onycholysis. Int J Dermatol 2006;45:1447–8.
- Baran R, Juhlin L. Drug-induced photo-onycholysis. Three subtypes identified in a study of 15 cases. J Am Acad Dermatol 1987;17:1012–16.
- Dawber RPR, Samman PD, Bottoms E. Fingernail growth in idiopathic and psoriatic onycholysis. Br J Dermatol 1971;85:558–60.
- Wilson JW. Paronychia and onycholysis: aetiology and therapy. Arch Dermatol 1965;92:726–30.
- Baran R, Dawber RPR, Richert B. Physical signs. In: Baran R, Dawber RPR, eds. Diseases of the Nails and Their Management. Oxford: Blackwell Science, 2001:76–7.
- Kechijian P. Onycholysis of the fingernails: evaluation and management. J Am Acad Dermatol 1985;12:552–60.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Dermatol Syphilol 1940;44:426–8.
- Luria MN, Asper SP. Onycholysis in hyperthyroidism. Ann Intern Med 1958;42:102–8.
- Brazzelli V, Carugno A, Alborghetti A, et al. Prevalence, severity and clinical features of psoriasis in fingernails and toenails in adult patients: Italian experience. J Eur Acad Dermatol Venereol 2012;26:1354–9.
- Heinmann H, Silverberg MG. Onycholysis in fur workers. Arch Dermatol Syphilol 1941;44:426–8.
- Shelley WB. Onycholysis due to 5-fluorouracil. Acta Derm Venereol (Stockh) 1972;52:320–2.
- Schultz HD. Hereditary partial onycholysis and hard nails. Dermatol Wochenschr 1966;152:766–8.
- Franks SB, Coton HJ, Mirkin W. Photo-onycholysis due to tetracycline. Arch Dermatol 1971;103:520.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed 2002;18:202–7.
- Onder M, Oztas MO, Oztas P. Isotretinoin-induced nail fragility and onycholysis. J Dermatol Treat 2001;12:115–16.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol 2003;14:333–7.
- Scotte F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol 2005;23:4424–9.
Pterygium
- Richert BJ, Patki A, Baran RL. Pterygium of the nail. Cutis 2000;66:343–6.
- Samman PD. Idiopathic atrophy of the nails. Br J Dermatol 1969;81:746–9.
- Little BJ, Cowan MA. Lichen planus-like eruption and nail changes in a patient with graft-versus-host disease. Br J Dermatol 1990;122:841–3.
- Patki AH, Mehta JM. Pterygium unguis in a patient with recurrent type 2 lepra reaction. Cutis 1989;44:311–12.
Ventral pterygium
- Drake L. Pterygium inversum unguis. Arch Dermatol 1976;112:255–6.
- Caputo R, Cappio F, Rigoni C, et al. Pterygium inversum unguis. Report of 19 cases and review of the literature. Arch Dermatol 1993;129:1307–9.
- Patterson JW. Pterygium inversum unguis-like changes in scleroderma. Arch Dermatol 1977;113:1429–30.
- Amblard P, Reymond JL. Familial subungual pterygium. Ann Dermatol Vénéréol 1980;107:949–50.
- Patki AH. Pterygium inversum unguis in a patient with leprosy. Arch Dermatol 1990;126:1110.
Longitudinal grooves
- Ronchese F. Peculiar nail anomalies. Arch Dermatol 1951;63:565–9.
- Heller J. Dystrophia unguium mediana canaliformis. Dermatol Z 1928;51:416–17.
- Zelger J, Wohlfarth P, Putz R. Dystrophia unguium mediana canaliformis Heller. Hautarzt 1974;25:629.
- Avhad G, Ghuge P. Median canaliform dystrophy of Heller. Indian Pediatr 2013;50:1073.
- Sweet RD. Dystrophia unguium mediana canaliformis. Arch Dermatol Syphilol 1951;64:61–2.
- Bottomley W, Cunliffe W. Median canaliform dystrophy associated with isotretinoin therapy. Br J Dermatol 1992;127:447.
- Sweeney SA, Cohen PR, Schulze KE, Nelson BR. Familial median canaliform nail dystrophy. Cutis 2005;75:161–5.
Transverse grooves and Beau's lines
- de Berker DAR. What is a Beau's line? Int J Dermatol 1994;33:545–6.
- Beau JHS. Note sur certain caractères de séméiologie rétrospective présentés par les ongles. Arch Gén Méd 1846;11:447–9.
- Macaulay WL. Transverse ridging of the thumbnails. Arch Dermatol 1966;93:421–3.
- Lee YJ, Yun SK. Unilateral Beau's lines associatd with a fingertip crushing injury. J Dermatol 2005;32:914–16.
- Piraccini BM, Iorizzo M, Starace M, Tosti A. Drug-induced nail diseases. Dermatol Clin 2006;24:387–91.
Nail pitting
- Zaias N. Psoriasis of the nail. A clinicopathologic study. Arch Dermatol 1969;99:567–79.
- Brazzelli V, Carugno A, Alborghetti A, et al. Prevalence, severity and clinical features of psoriasis in fingernails and toenails in adult patients: Italian experience. J Eur Acad Dermatol Venereol 2012;26:1354–9.
Trachyonychia
- Samman PD. Trachyonychia (rough nails). Br J Dermatol 1979;101:701–5.
- Gordon KA, Vega JM, Tosti A. Trachyonychia: a comprehensive review. Indian J Dermatol Venereol Leprol 2011;77:640–5.
- Baran R. Twenty nail dystrophy of alopecia areata [Letter]. Arch Dermatol 1981;117:1.
- Tosti A, Fanti PA, Morelli R, et al. Spongiotic trachyonychia. Arch Dermatol 1991;127:584–5.
Onychoschizia
- Scher RK. Brittle nails. Int J Dermatol 1989;28:515–16.
- van de Kerkhof PC, Pasch MC, Scher RK, et al. Brittle nail syndrome: a pathogenesis-based approach with a proposed grading system. J Am Acad Dermatol 2005;53:644–51.
- Graham-Brown RAC, Holmes R. Lamellar nail dystrophy with polycythaemia. Clin Exp Dermatol 1980;5:209–12.
- Cribier B, Mena ML, Rey D, et al. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol 1998;134:1216–20.
- Chao SC, Lee JY. Brittle nails and dyspareunia as first clues to recurrences of malignant glucagonoma. Br J Dermatol 2002;146:1071–4.
- Wallis MS, Bowen WR, Guin JR. Pathogenesis of onychoschizia (lamellar dystrophy). J Am Acad Dermatol 1991;24:44–8.
- Lubach D, Beckers P. Wet working conditions increase brittleness of nails but do not cause it. Dermatology 1992;185:120–2.
- Colombo VE, Gerber F, Bronhofer M, et al. Treatment of brittle fingernails and onychoschizia with biotin: scanning electron microscopy. J Am Acad Dermatol 1990;23:1127–32.
Changes in nail colour
- Baran R. Longitudinal melanotic streaks as a clue to Laugier–Hunziker syndrome. Arch Dermatol 1979;115:1448–9.
- Daniel CR. Nail pigmentation abnormalities. Dermatol Clin 1985;3:431–43.
- Daniel CR, Zaias N. Pigmentary abnormalities of the nails with emphasis on systemic diseases. Dermatol Clin 1988;6:305–13.
- Higashi N. Melanonychia due to tinea unguium. Hifu 1990;32:379–80.
- Lovemann AB, Fliegelman MT. Discoloration of the nails. Arch Dermatol 1955;72:153–6.
- Shellow WVR, Koplon BS. Green striped nails: chromonychia due to Pseudomonas aeruginosa. Arch Dermatol 1963;97:149–53.
- Thomas L, Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol Ther 2007;20:3–10.
True leukonychia
- Norgett EE, Wolf F, Balme B, et al. Hereditary ‘white nails’: a genetic and structural study. Br J Dermatol 2004;151:65–72.
- Grossman M, Scher RK. Leukonychia: review and classification. Int J Dermatol 1990;29:535–41.
- Baran R, Dawber RPR. Physical signs. In: Baran R, Dawber RPR, eds. Diseases of the Nails and Their Management, 2nd edn. Oxford: Blackwell Science, 1994:72.
- Marino MT. Mees' lines. Arch Dermatol 1990;126:827–8.
- Mautner GH, Lu I, Ort RJ, Grossman ME. Transverse leukonychia with systemic infection. Cutis 2000;65:318–20.
Apparent leukonychia
- Albright SD, Wheeler CE. Leukonychia: total and partial leukonychia in a single family with review of the literature. Arch Dermatol 1964;90:392–9.
- Grossman M, Scher RK. Leukonychia: review and classification. Int J Dermatol 1990;29:535–41.
- Holzberg M, Walker HK. Terry's nails: revised definition and new correlations. Lancet 1984;i:896–9.
- Alam M, Scher RK, Bickers DR. Muehrcke's lines in a heart transplant recipient. J Am Acad Dermatol 2001;44:316–17.
Colour changes due to drugs and chemicals
- Baran R, Dawber RPR, Richert B. Physical signs. In: Baran R, Dawber RPR, de Berker DAR, Haneke E, Tosti A, eds. Diseases of the Nails and Their Management, 3rd edn. Oxford: Blackwell Science, 2001:86–103.
- Fiallo P. Yellow nails as an adverse reaction to the topical use of 5-fluorouracil for the treatment of nail psoriasis. J Dermatol Treat 2009;20:299–301.
- Orentreich N, Harber LC, Tromovitch TH. Photosensitivity and photoonycholysis due to demethylchlortetracycline. Arch Dermatol 1961;83:730–7.
- Mallon E, Dawber RPR. Longitudinal melanonychia induced by minocycline. Br J Dermatol 1994;130:794–5.
- Jeevankumar B, Thappa DM. Blue lunula due to hydroxyurea. J Dermatol 2003;30:628–30.
- Tuffanelli D, Abraham RK, Dubois E. Pigmentation from antimalarial drugs. Arch Dermatol 1963;88:419–26.
- Maguire A. Antimalarial nail pigmentation. Lancet 1963;i:667–71.
- Ramelli G. Argyria. Cutis 1972;10:155–9.
- Goark SP, Hood AF, Nelson K. Nail pigmentation associated with zidovudine. J Am Acad Dermatol 1984;5:1032–3.
- Pratt CB, Shanks EC. Hyperpigmentation of the nails due to doxorubicin. JAMA 1974;228:460.
- Fisher BK, Warner LC. Cutaneous manifestations of AIDS. Int J Dermatol 1987;16:615–30.
- Panwalker A. Nail pigmentation in AIDS. Ann Intern Med 1987;107:943–4.
- Gallais V, Lacour JPh, Perrin C, et al. Acral hyperpigmented macules and longitudinal melanonychia in AIDS patients. Br J Dermatol 1992;126:387–91.
Yellow nail syndrome
- Samman PD, White WF. The ‘yellow nail’ syndrome. Br J Dermatol 1964;76:153–7.
- Magid M, Esterly NB, Prendiville J, Fujisaki C. The yellow nail syndrome in an 8 year old girl. Pediatr Dermatol 1987;4:90–3.
- Hoque SR, Mansour S, Mortimer PS. Yellow nail syndrome: not a genetic disorder? Eleven new cases and a review of the literature. Br J Dermatol 2007;156:1230–4.
- Baran R. Lichen planus of the nails mimicking the yellow nail syndrome. Br J Dermatol 2000;143:1117–18.
- Ilchyshin A, Vickers CFH. Yellow nail syndrome associated with penicillamine therapy. Acta Derm Venereol (Stockh) 1983;63:554–5.
- De Coste SD, Imber MJ, Baden HP. Yellow nail syndrome. J Am Acad Dermatol 1990;22:608–11.
- Mallon E, Dawber RPR. Nail unit histopathology in the yellow nail syndrome. Br J Dermatol 1995;133(Suppl. 45):55.
- Fenton DA, Bull R, Gane J, et al. Abnormal lymphatic function assessed by quantitative lymphoscintigraphy in the yellow nail syndrome. Br J Dermatol 1990;123(Suppl. 37):32.
- Ellis JP, Marks R, Pery BJ. Lymphatic function: the disappearance rate of 131-I albumin from the dermis. Br J Dermatol 1970;82:593–9.
- Miller E, Rosenow EC, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest 1972;61:452–8.
- Bull RH, Fenton DA, Mortimer PS. Lymphatic function in the yellow nail syndrome. Br J Dermatol 1996;134:307–12.
- Emerson PA. Yellow nails, lymphoedema and pleural effusions. Thorax 1966;21:247–53.
- Dilley JJ, Kierland RR, Randall RV, Schick RM. Primary lymphoedema associated with yellow nails and pleural effusions. JAMA 1968;203:670–3.
- Burrows NP, Russell Jones R. Yellow nail syndrome in association with carcinoma of the gall bladder. Clin Exp Dermatol 1991;16:471–3.
- Stosiek N, Peters KP, Hiller D, et al. Yellow nail syndrome in a patient with mycosis fungoides. J Am Acad Dermatol 1993;28:792–4.
- Cockram CS, Richards P. Yellow nails and nephrotic syndrome. Br J Dermatol 1979;101:707–9.
- Chernosky ME, Finley VK. Yellow nail syndrome in patients with AIDS. J Am Acad Dermatol 1985;13:731–6.
- Scher RK. Acquired immunodeficiency syndrome and yellow nails. J Am Acad Dermatol 1988;18:758–9.
- Hoque SR, Mansour S, Mortimer PS. Yellow nail syndrome: not a genetic disorder? Eleven new cases and a review of the literature. Br J Dermatol 2007;156:1230–4.
- Maldonado F, Tazelaar HD, Wang CW, Ryu JH. Yellow nail syndrome: analysis of 41 consecutive patients. Chest 2008;134:375–81.
- Baran R, Thomas L. Combination of fluconazole and alpha-tocopherol in the treatment of yellow nail syndrome. J Drugs Dermatol 2009;8:276–8.
- Ayres S, Mihan R. Yellow nail syndrome. Response to vitamin E. Arch Dermatol 1973;108:267–8.
- Williams HC, Buffham R, du Vivier A. Successful use of topical vitamin E solution in the treatment of nail changes in yellow nail syndrome. Arch Dermatol 1991;127:1023–8.
- Arroyo JF, Cohen ML. Yellow nail syndrome cured by zinc supplementation. Clin Exp Dermatol 1993;18:62–4.
- Pang SM. Yellow nail syndrome resolution following treatment of pulmonary tuberculosis. Int J Dermatol 1993;32:605–6.
- Tosti A, Piraccini BM, Iorizzo M. Systemic itraconazole in the yellow nail syndrome. Br J Dermatol 2002;146:1064–7.
- Baran R. The new oral antifungal drugs in the treatment of the yellow nail syndrome. Br J Dermatol 2002;147:189–91.
- Piraccini BM, Urciuoli B, Starace M, Tosti A, Balestri R. Yellow nail syndrome: clinical experience in a series of 21 patients. J Dtsch Dermatol Ges 2014;12:131–7.
Red lunulae
- Cohen PR. Red lunulae: case report and review of the literature. J Am Acad Dermatol 1992;26:292–4.
- Wilkerson MG, Wilkin JK. Red lunulae revisited: a clinical and histopathologic examination. J Am Acad Dermatol 1989;20:453–7.
- de Berker D, Goettmann S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol 2002;46:394–8.
Longitudinal erythronychia
- de Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol 2004;140:1253–7.
- Baran R, Perrin C. Localized multinucleate distal subungual keratosis. Br J Dermatol 1995;133:77–82.
- Zaias N, Ackerman AB. The nail in Darier–White disease. Arch Dermatol 1973;107:193–9.
- Baran R, Perrin C. Focal subungual warty dyskeratoma. Dermatology 1997;195:278–80.
- Baran R, Dawber RP, Perrin C, Drape JL. Idiopathic polydactylous longitudinal erythronychia: a newly described entity. Br J Dermatol 2006;155:219–21.
- Baran R, Perrin C. Longitudinal erythronychia with distal subungual keratosis: onychopapilloma of the nail bed and Bowen's disease. Br J Dermatol 2000;143:132–5.
- Gee BC, Millard PR, Dawber RP. Onychopapilloma is not a distinct clinicopathological entity. Br J Dermatol 2002;146:156–7.
Splinter haemorrhages
- Heath D, Williams DR. Nail haemorrhages. Br Heart J 1978;40:1300–5.
- Kilpatrick ZM, Greenberg PA, Sanford JP. Splinter haemorrhages, their clinical significance. Arch Intern Med 1965;115:730–5.
- Monk BE. The prevalence of splinter haemorrhages. Br J Dermatol 1980;103:183–5.
- Young JB, Will EJ, Mulley GP. Splinter haemorrhages: facts and fiction. J R Coll Phys Lond 1988;22:240–3.
- Gross NJ, Tall R. Clinical significance of splinter haemorrhages. BMJ 1963;ii:1496–8.
- Ames DE, Asherson RA, Aynes B, et al. Bilateral adrenal infarction, hypoadrenalism and splinter haemorrhages in the primary antiphospholipid syndrome. Br J Rheumatol 1994;31:117–20.
- Nakamura M, Miyachi Y. Sunitinib-induced subungual splinter haemorrhage and acral erythema. Eur J Dermatol 2008;18:344–5.
Acute trauma
- Van Beek AL, Kassan MA, Adson MH, Dale V. Management of acute fingernail injuries. Hand Clin 1990 Feb;6(1):23–35.
Subungual haematoma
- Dean B, Becker G, Little C. The management of the acute traumatic subungual haematoma: a systematic review. Hand Surg 2012;17:151–4.
- Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol 2007;56:835–47.
- Oztas MO. Clinical and dermoscopic progression of subungual hematomas. Int Surg 2010;95:239–41.
- Stone OJ, Mullins JF. The distal course of nail haemorrhage. Arch Dermatol 1963;88:186–7.
- Roser SE, Gellman H. Comparison of nail bed repair versus nail trephination for subungual hematomas in children. J Hand Surg 1999;24A:1166–70.
- Batrick N, Hashemi K, Freij R. Treatment of uncomplicated subungual haematoma. Emerg Med J 2003;20:65.
Nail bed laceration
- Brown RE. Acute nail bed injuries. Hand Clin 2002;18:561–75.
- Tos P, Titolo P, Chirila NL, Catalano F, Artiaco S. Surgical treatment of acute fingernail injuries. J Orthop Traumatol 2012;13:57–62.
- Zook EG. Understanding the perionychium. J Hand Ther 2000;13:269–75.
Delayed trauma
- Hoffman S. Correction of a split nail deformity. Arch Dermatol 1973;108:568–9.
- Zook EG, Baran R, Haneke E, Dawber RPR. Nail surgery and traumatic abnormalities. In: Baran R, Dawber RPR, de Berker DAR, Haneke E, Tosti A, eds. Diseases of the Nails and Their Management, 3rd edn. Oxford: Blackwell Science, 2001:425–514.
Nail biting
- Hurley PT, Balu V. Self-inflicted anonychia. Arch Dermatol 1982;118:956–7.
- Malone AJ, Massler M. Index of nail biting in children. J Abnorm Social Psychol 1952;47:193–202.
- Pacan P, Grzesiak M, Reich A, Kantorska-Janiec M, Szepietowski JC. Onychophagia and onychotillomania: prevalence, clinical picture and comorbidities. Acta Derm Venereol 2014;94:67–71.
- Alessandri Bonetti G, Incerti Parenti S, Zucchelli G. Onychophagia and postorthodontic isolated gingival recession: diagnosis and treatment. Am J Orthod Dentofacial Orthop 2012;142:872–8.
- Baran R. Nail biting and picking as a possible cause of longitudinal melanonychia. Dermatologica 1990;181:126–8.
- Combes FC, Scott MJ. Onychotillomania. Arch Dermatol Syphilol 1951;63:778–80.
- Hamann K. Onychotillomania treated with pimozide (Orap). Acta Derm Venereol (Stockh) 1982;62:364–7.
- Tosti A, Peluso AM, Bardazzi F, et al. Phalangeal osteomyelitis due to nail biting. Acta Derm Venereol (Stockh) 1994;74:206–7.
- Waldmann BA. Osteomyelitis caused by nail biting. Pediatr Dermatol 1991;7:189–90.
- Norton L. Self-induced trauma to the nails. Cutis 1987;40:223–7.
- Leonard HL, Lenane MC, Swedo SC, et al. A double blind comparison of clomipramine and desipramine treatment of severe onychophagia (nail biting). Arch Gen Psychiatry 1991;48:821–7.
- Silber KP, Haynes CE. Treating nailbiting: a comparative analysis of mild aversion and competing response therapies. Behav Res Ther 1992;30:15–22.
Damage from nail manicure instruments
- Brauer E, Baran R. Cosmetics: the care and adornment of the nail. In: Baran R, Dawber RPR, de Berker DAR, Haneke E, Tosti A, eds. Diseases of the Nails and Their Management, 3nd edn. Oxford: Blackwell Science, 2001:358–369.
Onychogryphosis and nail hypertrophy
- Cohen PR, Scher RK. Geriatric nail disorders: diagnosis and management. J Am Acad Dermatol 1992;26:521–31.
- Dawber RPR, Bristow I, Mooney J. The Foot: Problems in Podiatry and Dermatology. London: Dunitz, 1996.
- Douglas MA, Krull EA. Diseases of the nails. In: Conn WB, ed. Current Therapy. Philadelphia: Saunders, 1981:712.
- Gilchrist AK. Common foot problems in the elderly. Geriatrics 1979;34:67–70.
- Lubach D. Erbliche onychogryphosis. Hautarzt 1982;33:331–3.
- Baran R, Bureau H. Congenital malalignment of the great toenail as a cause of ingrowing toenail in infancy. Clin Exp Dermatol 1983;6:619–23.
Ingrowing toenail
- Baran R, Bureau H. Congenital malalignment of the great toenail as a cause of ingrowing toenail in infancy. Clin Exp Dermatol 1983;6:619–23.
- Cambiaghi S, Pistritto G, Gelmeti C. Congenital hypertrophy of the lateral nail folds of the hallux in twins. Br J Dermatol 1997;136:635–6.
- Samman PD. Nail deformities due to trauma. In: Samman PD, Fenton DA, eds. The Nails in Disease. London: Heinemann, 1986:148–9.
- Baran R, Haneke E, Richert B. Pincer nails: definition and surgical treatment. Dermatol Surg 2001;27:261–6.
- de Berker DA, Richert B, Duhard E, Piraccini BM, André J, Baran R. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol 2008;58:978–83.
- Verbov J. Ingrowing toenails in infancy. BMJ 1978;ii:1087.
- Katz A. Congenital ingrown toenails. J Am Acad Dermatol 1996;34:519–20.
- Piraccini BM, Parente GL, Varotti E, Tosti A. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol 2000;17:348–51.
- Baran R. Retinoids and the nails. J Dermatol Treat 1990;1:151–4.
- Higgins EM, Hughes JR, Snowden S, Pembroke AC. Cyclosporin-induced periungual granulation tissue. Br J Dermatol 1995;132:829–30.
- Nicolopoulos J, Howard A. Docetaxel-induced nail dystrophy. Australas J Dermatol 2002;43:293–6.
- Wasner G, Hilpert F, Schattschneider J, et al. Docetaxel-induced nail changes: a neurogenic mechanism. A case report. J Neurooncol 2002;58:167–74.
- Stemmler HJ, Gutschow K, Sommer H, et al. Weekly docetaxel (Taxotere) in patients with metastatic breast cancer. Ann Oncol 2001;12:1393–8.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002;46:284–93.
- Heim M, Schapiro J, Wershavski M, Martinowitz U. Drug-induced and traumatic nail problems in the haemophilias. Haemophilia 2000;6:191–4.
- Wernick J, Gibbs RC. Pedal biomechanics and toenail disease. In: Scher RK, Daniel CR, eds. Nails: Therapy, Diagnosis, Surgery. Philadelphia: Saunders, 1990:244–9.
- Schulte KW, Neumann NJ, Ruzicka T. Surgical pearl: nail splinting by flexible tube. A new noninvasive treatment for ingrown toenails. J Am Acad Dermatol 1998;39:629–30.
- Arai H, Arai T, Nakajima H, Haneke E. Formable acrylic treatment for ingrowing nail with gutter splint and sculptured nail. Int J Dermatol 2004;43:759–65.
- Lemont H, Brady J. Amelanotic melanoma masquerading as an ingrown toenail. J Am Podiatr Med Assoc 2002;92:306–7.
- Rounding C, Hulm S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev 2005;(2):CD001541.
- Richert B. Surgical management of ingrown toenails: an update overdue. Dermatol Ther 2012;25:498–509.
Tumours under or adjacent to the nail
- de Berker DA, Richert B, Duhard E, Piraccini BM, André J, Baran R. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol 2008;58:978–83.
- Richert B. Frictional pyogenic granuloma of the nail bed. Dermatology 2001;202:80–1.
- Colver GB. Onychotillomania. Br J Dermatol 1987;117:397–9.
- Piraccini BM, Bellavista S, Misciali C, Tosti A, de Berker D, Richert B. Periungual and subungual pyogenic granuloma. Br J Dermatol 2010;163:941–53.
- Piraccini BM, Iorizzo M. Drug reactions affecting the nail unit: diagnosis and management. Dermatol Clin 2007;25:215–21, vii.
- Campbell JP, Grekin RC, Ellis CN, Matsuda-John SS, Swanson NA, Voorhees JJ. Retinoid therapy is associated with excess granulation tissue responses. J Am Acad Dermatol 1983;9:708–13.
- Teknetzis A, Ioannides D, Vakali G, Lefaki I, Minas A. Pyogenic granulomas following topical application of tretinoin. J Eur Acad Dermatol Venereol 2004;18:337–9.
- Dawkins MA, Clark AR, Feldman SR. Pyogenic granuloma-like lesion associated with topical tazarotene therapy. J Am Acad Dermatol 2000;43:154–5.
- Bouscarat F, Bouchard C, Bouhour D. Paronychia and pyogenic granuloma of the great toes in patients treated with indinavir. N Engl J Med 1998;338:1776–7.
- Tosti A, Piraccini BM, D'Antuono A, Marzaduri S, Bettoli V. Paronychia associated with antiretroviral therapy. Br J Dermatol 1999;140:1165–8.
- Calista D, Boschini A. Cutaneous side effects induced by indinavir. Eur J Dermatol 2000;10:292–6.
- Freiman A, Bouganim N, O'Brien EA. Case reports: mitozantrone-induced onycholysis associated with subungual abscesses, paronychia, and pyogenic granuloma. J Drugs Dermatol 2005;4:490–2.
- Higgins EM, Hughes JR, Snowden S, Pembroke AC. Cyclosporin-induced periungual granulation tissue. Br J Dermatol 1995;132:829–30.
- Curr N, Saunders H, Murugasu A, Cooray P, Schwarz M, Gin D. Multiple periungual pyogenic granulomas following systemic 5-fluorouracil. Australas J Dermatol 2006;47:130–3.
- Piguet V, Borradori L. Pyogenic granuloma-like lesions during capecitabine therapy. Br J Dermatol 2002;147:1270–2.
- Piqué-Duran E, Pérez-Díaz MJ, Pérez-Cejudo JA. Pyogenic granuloma-like lesions caused by capecitabine therapy. Clin Exp Dermatol 2008;33:652–3.
- Vaccaro M, Barbuzza O, Guarneri F, Guarneri B. Nail and periungual toxicity following capecitabine therapy. Br J Clin Pharmacol 2008;66:325–6.
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol 2012;11:262–8.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol 2003;14:333–7.
- Wollina U. Multiple eruptive periungual pyogenic granulomas during anti-CD20 monoclonal antibody therapy for rheumatoid arthritis. J Dermatol Case Rep 2010;4:44–6.
- Tosti A, Piraccini BM, Camacho-Martinez F. Onychomadesis and pyogenic granuloma following cast immobilization. Arch Dermatol 2001;137:231–2.
- Tosti A, Baran R, Peluso AM, Fanti PA, Liguori R. Reflex sympathetic dystrophy with prominent involvement of the nail apparatus. J Am Acad Dermatol 1993;29:865–8.
- Mazereeuw-Hautier J, Bonafé J-L. Bilateral Beau's lines and pyogenic granulomas following Guillain-Barré syndrome. Dermatology 2004;209:237–8.
- Siragusa M, Schepis C, Cosentino FI, Spada RS, Toscano G, Ferri R. Nail pathology in patients with hemiplegia. Br J Dermatol 2001;144:557–60.
- Guhl G, Torrelo A, Hernández A, Zambrano A. Beau's lines and multiple periungueal pyogenic granulomas after long stay in an intensive care unit. Pediatr Dermatol 2008;25:278–9.
- Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg 1977;2A:261–5.
- Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg 1996;21B:257–60.
- André J, Sass U, Richert B, Theunis A. Nail pathology. Clin Dermatol 2013;31:526–39.
- Anakwe RE, McEachan JE. A glomus tumour beneath the painful unpolished nail. Can Med Assoc J 2010;182:1329.
- Foucher G, Le Viet D, Dailiana Z, Pajardi G. Glomus tumor of the nail area. A propos of a series of 55 patients. Rev Chir Orthop Reparatrice Appar Mot 1999;85:362–6.
- Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg 1990;15A:507–9.
- Drapé JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology 1995;195:507–15.
- Richert B, Baghaie M. Medical imaging and MRI in nail disorders: report of 119 cases and review. Dermatol Ther 2002;15:159–64.
- Gandhi J, Yang SS, Hurd J. The anatomic location of digital glomus tumor recurrences. J Hand Surg 2010;35A:986–9.
- di Chiacchio N, Richert B, Haneke E. Surgery of the matrix. In: Richert B, di Chiacchio N, Haneke E, eds. Nail Surgery. London: Informa, 2011:103–32.
- Lee D-W, Yang J-H, Chang S, et al. Clinical and pathological characteristics of extradigital and digital glomus tumours: a retrospective comparative study. J Eur Acad Dermatol Venereol 2011;25:1392–7.
- Heim U, Hänggi W. Subungual glomus tumors. Value of the direct dorsal approach. Ann Chir Main 1985;4:51–4.
- Hazani R, Houle JM, Kasdan ML, Wilhelmi BJ. Glomus tumors of the hand. Eplasty 2008;8:e48.
- Lee SK, Jung MS, Lee YH, Gong HS, Kim JK, Baek GH. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int 2007;28:595–601.
- Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologic–pathologic correlation. Radiographics 2000;20:1407–34.
- Landon GC, Johnson KA, Dahlin DC. Subungual exostoses. J Bone Joint Surg Am 1979;61:256–9.
- De Berker DA, Langtry J. Treatment of subungual exostoses by elective day case surgery. Br J Dermatol 1999;140:915–18.
- Davis DA, Cohen PR. Subungual exostosis: case report and review of the literature. Pediatr Dermatol 1996;13:212–18.
- Storlazzi CT, Wozniak A, Panagopoulos I, et al. Rearrangement of the COL12A1 and COL4A5 genes in subungual exostosis: molecular cytogenetic delineation of the tumor-specific translocation t(X;6)(q13–14;q22). Int J Cancer 2006;118:1972–6.
- Fleegler EJ, Zeinowicz RJ. Tumors of the perionychium. Hand Clin 1990;6:113–33; discussion 135–6.
- Dumontier CA, Abimelec P. Nail unit enchondromas and osteochondromas: a surgical approach. Dermatol Surg 2001;27:274–9.
- Sonnex TS. Digital myxoid cysts: a review. Cutis 1986;37:89–94.
- de Berker D, Lawrence C. Ganglion of the distal interphalangeal joint (myxoid cyst): therapy by identification and repair of the leak of joint fluid. Arch Dermatol 2001;137:607–10.
- Drapé JL, Idy-Peretti I, Goettmann S, et al. MR imaging of digital mucoid cysts. Radiology 1996;200:531–6.
- Pietrzak A, Wojnowska D, Chodorowska G, Dybiec E, Gorzelak M, Urban J. Multiple myxoid cysts of both hands in a cashier: a case report. Ann Univ Mariae Curie Sklodowska Med 2003;58:478–81.
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol 2002;46:394–8.
- de Berker DA, Lawrence CM. Treatment of myxoid cysts. Dermatol Surg 2001;27:296–9.
- Kint A, Baran R. Histopathologic study of Koenen tumors. Are they different from acquired digital fibrokeratoma? J Am Acad Dermatol 1988;18:369–72.
- Baran R, Perrin C. Pseudo-fibrokeratoma of the nail apparatus with melanocytic pigmentation: a clue for diagnosing Bowen's disease. Acta Derm Venereol 1994;74:449–50.
- Haneke E. Epidermoid carcinoma (Bowen's disease) of the nail apparatus simulating acquired ungual fibrokeratoma. Skin Cancer 1991;6:217–21.
- Baran R, Mikhail G, Costini B, Tosti A, Goettmann-Bonvallot S. Distal digital keratoacanthoma: two cases with a review of the literature. Dermatol Surg 2001;27:575–9.
- Baran R, Tosti A, De Berker D. Periungual keratoacanthoma preceded by a wart and followed by a verrucous carcinoma at the same site. Acta Derm Venereol 2003;83:232–3.
- Oliwiecki S, Peachey RD, Bradfield JW, Ellis J, Lovell CR. Subungual keratoacanthoma: a report of four cases and review of the literature. Clin Exp Dermatol 1994;19:230–5.
- Lovett JE, Haines TA, Bentz ML, Shestak KC, Flynn KJ, Kapadia SB. Subungual keratoacanthoma masquerading as a chronic paronychia. Ann Plast Surg 1995;34:84–7.
- Allen CA, Stephens M, Steel WM. Subungual keratoacanthoma. Histopathology 1994;25:181–3.
- Levy DW, Bonakdarpour A, Putong PB, Mesgarzadeh M, Betz RR. Subungual keratoacanthoma. Skeletal Radiol 1985;13:287–90.
- Pellegrini VD Jr, Tompkins A. Management of subungual keratoacanthoma. J Hand Surg 1986;11A:718–24.
- Sinha A, Marsh R, Langtry J. Spontaneous regression of subungual keratoacanthoma with reossification of underlying distal lytic phalynx. Clin Exp Dermatol 2005;30:20–2.
- Baran R, Kint A. Onychomatrixoma. Filamentous tufted tumour in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol 1992;126:510–15.
- Misciali C, Iorizzo M, Fanti PA, et al. Onychoblastoma (hamartoma of the nail unit): a new entity? Br J Dermatol 2005;152:1077–8.
- Ko CJ, Shi L, Barr RJ, Mölne L, Ternesten-Bratel A, Headington JT. Unguioblastoma and unguioblastic fibroma: an expanded spectrum of onychomatricoma. J Cutan Pathol 2004;31:307–11.
- Piraccini BM, Antonucci A, Rech G, Starace M, Misciali C, Tosti A. Onychomatricoma: first description in a child. Pediatr Dermatol 2007;24:46–8.
- Tosti A, Piraccini BM, Calderoni O, Fanti PA, Cameli N, Varotti E. Onychomatricoma: report of three cases, including the first recognized in a colored man. Eur J Dermatol 2000;10:604–6.
- Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J 2006;12(6):4.
- Becerro de Bengoa R, Gates JR, Losa Iglesias ME, Alija Martinez B. Rare toenail onychomatricoma: surgical resolution of five cases. Dermatol Surg 2011;37:709–11.
- Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol 1998;39:560–4.
- Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, Rangel L, Dominguez-Cherit J. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol 2007;46:634–6.
- Perrin C, Baran R. Onychomatricoma with dorsal pterygium: pathogenic mechanisms in 3 cases. J Am Acad Dermatol 2008;59:990–4.
- Goettmann S, Zaraa I, Moulonguet I. Onychomatricoma with pterygium aspect: unusual clinical presentation. Acta Derm Venereol 2006;86:369–70.
- Fayol J, Baran R, Perrin C, Labrousse F. Onychomatricoma with misleading features. Acta Derm Venereol 2000;80:370–2.
- Raison-Peyron N, Alirezai M, Meunier L, Barneon G, Meynadier J. Onychomatricoma: an unusual cause of nail bleeding. Clin Exp Dermatol 1998;23:138.
- Baran R, Perrin C. Bowen's disease clinically simulating an onychomatricoma. J Am Acad Dermatol 2002;47:947–9.
- Miteva M, de Farias DC, Zaiac M, Romanelli P, Tosti A. Nail clipping diagnosis of onychomatricoma. Arch Dermatol 2011;147:1117–18.
- Goettmann S, Drape JL, Idy-Peretti I, et al. Magnetic resonance imaging: a new tool in the diagnosis of tumours of the nail apparatus. Br J Dermatol 1994;130:701–10.
- Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol 2009;145:1461–2.
- Ashby-Richardson H, Rogers GS, Stadecker MJ. Superficial acral fibromyxoma: an overview. Arch Pathol Lab Med 2011;135:1064–6.
- Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol 2001;32:704–14.
- André J, Sass U, Theunis A. Diseases of the nails. In: Calonje E, Brenn T, Lazar A, McKee PH, eds. McKee's Pathology of the Skin, 4th edn. Philadelphia: Saunders Elsevier, 2012:1051–75.
- André J, Theunis A, Richert B, de Saint-Aubain N. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol 2004;26:472–4.
- Cogrel O, Stanislas S, Coindre J-M, et al. Superficial acral fibromyxoma: three cases. Ann Dermatol Vénéréol 2010;137:789–93.
- Chabbab F, Metz T, Saez Beltran L, Theunis A, Richert B. Superficial acral fibromyxoma in a sub-matricial location: an unusual variant. Ann Dermatol Venereol 2014;141:94–105.
- Hollmann TJ, Bovée JVMG, Fletcher CDM. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol 2012;36:789–98.
- Baran R, Perrin C. Localized multinucleate distal subungual keratosis. Br J Dermatol 1995;133:77–82.
- Baran R, Perrin C. Longitudinal erythronychia with distal subungual keratosis: onychopapilloma of the nail bed and Bowen's disease. Br J Dermatol 2000;143:132–5.
- Miteva M, Fanti PA, Romanelli P, Zaiac M, Tosti A. Onychopapilloma presenting as longitudinal melanonychia. J Am Acad Dermatol 2012;66:e242–3.
- Criscione V, Telang G, Jellinek NJ. Onychopapilloma presenting as longitudinal leukonychia. J Am Acad Dermatol 2010;63:541–2.
- Richert B, Iorizzo M, Tosti A, André J. Nail bed lichen planus associated with onychopapilloma. Br J Dermatol 2007;156:1071–2.
- Lecerf P, Richert B, Theunis A, André J. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol 2013;69:253–61.
- Mikhail GR. Subungual epidermoid carcinoma. J Am Acad Dermatol 1984;11:291–8.
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol 2011;64:1147–53.
- Dalle S, Depape L, Phan A, Balme B, Ronger-Savle S, Thomas L. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol 2007;156:871–4.
- André J, Sass U, Theunis A. Diseases of the nail. In: Calonje E, Brenn T, Lazar A, McKee PH, eds. McKee's Pathology of the Skin, 4th edn. Philadelphia: Saunders Elsevier, 2012:1068–9.
- McHugh RW, Hazen P, Eliezri YD, Nuovo GJ. Metastatic periungual squamous cell carcinoma: detection of human papillomavirus type 35 RNA in the digital tumor and axillary lymph node metastases. J Am Acad Dermatol 1996;34:1080–2.
- Shimizu A, Tamura A, Abe M, et al. Detection of human papillomavirus type 56 in Bowen's disease involving the nail matrix. Br J Dermatol 2008;158:1273–9.
- Forslund O, Nordin P, Andersson K, Stenquist B, Hansson BG. DNA analysis indicates patient-specific human papillomavirus type 16 strains in Bowen's disease on fingers and in archival samples from genital dysplasia. Br J Dermatol 1997;136:678–82.
- Kawashima M, Jablonska S, Favre M, Obalek S, Croissant O, Orth G. Characterization of a new type of human papillomavirus found in a lesion of Bowen's disease of the skin. J Virol 1986;57:688–92.
- de Berker DA, Dahl MG, Malcolm AJ, Lawrence CM. Micrographic surgery for subungual squamous cell carcinoma. Br J Plast Surg 1996;49:414–19.
- Baran RL, Gormley DE. Polydactylous Bowen's disease of the nail. J Am Acad Dermatol 1987;17:201–4.
- Goodman G, Mason G, O'Brien T. Polydactylous Bowen's disease of the nail bed. Australas J Dermatol 1995;36:164–5.
- Strong ML. Bowen's disease in multiple nail beds: case report. J Hand Surg 1983;8A:329–30.
- Ongenae K, Van De Kerckhove M, Naeyaert J-M. Bowen's disease of the nail. Dermatology 2002;204:348–50.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen's disease. J Am Acad Dermatol 1988;18:1359–60.
- Matoso A, Jellinek N, Telang GH. Verrucous carcinoma of the nail unit. Am J Dermatopathol 2012;34:e106–10.
- Tosti A, Morelli R, Fanti PA, Morselli PG, Catrani S, Landi G. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology 1993;186:217–21.
- Canovas F, Dereure O, Bonnel F. A propos of a case of epidermoid carcinoma of the nail bed with intraneural metastasis to the median nerve. Chir Main 1998;17:232–5.
- Kouskoukis CE, Scher RK, Kopf AW. Squamous-cell carcinoma of the nail bed. J Dermatol Surg Oncol 1982;8:853–5.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol 2003;48:385–93.
- Laffitte E, Saurat J-H. Recurrent Bowen's disease of the nail: treatment by topical imiquimod. Ann Dermatol Vénéréol 2003;130:211–13.
- Sturm HM. Bowen's disease and 5-fluorouracil. J Am Acad Dermatol 1979;1:513–22.
- Sau P, McMarlin SL, Sperling LC, Katz R. Bowen's disease of the nail bed and periungual area. A clinicopathologic analysis of seven cases. Arch Dermatol 1994;130:204–9.
- Tan B, Sinclair R, Foley P. Photodynamic therapy for subungual Bowen's disease. Australas J Dermatol 2004;45:172–4.
- Usmani N, Stables GI, Telfer NR, Stringer MR. Subungual Bowen's disease treated by topical aminolevulinic acid-photodynamic therapy. J Am Acad Dermatol 2005;53(5 Suppl. 1):S273–6.
- Sheen Y-S, Sheen M-C, Sheu H-M, Yang S-F, Wang Y-W. Squamous cell carcinoma of the big toe successfully treated by intra-arterial infusion with methotrexate. Dermatol Surg 2003;29:982–3.
- Serrano-Ortega S, Fernández-Angel I, Dulanto-Campos E, Rodríguez-Archilla A, Linares-Solano J. Basal cell carcinoma arising in professional radiodermatitis of the nail. Br J Dermatol 2002;147:628–9.
- Engel E, Ulrich H, Vasold R, et al. Azo pigments and a basal cell carcinoma at the thumb. Dermatology 2008;216:76–80.
- Forman SB, Ferringer TC, Garrett AB. Basal cell carcinoma of the nail unit. J Am Acad Dermatol 2007;56:811–14.
- Kim HJ, Kim YS, Suhr KB, Yoon TY, Lee JH, Park JK. Basal cell carcinoma of the nail bed in a Korean woman. Int J Dermatol 2000;39:397–8.
- Shah D, Leopold G, Sowden J. Basal cell carcinoma masquerading as habit tic. Clin Exp Dermatol 2011;36:920.
- Brasie RA, Patel AR, Nouri K. Basal cell carcinoma of the nail unit treated with Mohs micrographic surgery: superficial multicentric BCC with jagged borders: a histopathological hallmark for nail unit BCC. J Drugs Dermatol 2006;5:660–3.
- Thai KE, Young R, Sinclair RD. Nail apparatus melanoma. Australas J Dermatol 2001;42:71–81; quiz 82–3.
- Martin DE, English JC, Goitz RJ. Subungual malignant melanoma. J Hand Surg 2011;36A:704–7.
- Tosti A, Piraccini BM, Cagalli A, Haneke E. In situ melanoma of the nail unit in children: report of two cases in fair-skinned Caucasian children. Pediatr Dermatol 2012;29:79–83.
- Möhrle M, Häfner HM. Is subungual melanoma related to trauma? Dermatology 2002;204:259–61.
- Stern DK, Creasey AA, Quijije J, Lebwohl MG. UV-A and UV-B penetration of normal human cadaveric fingernail plate. Arch Dermatol 2011;147:439–41.
- Theunis A, Richert B, Sass U, Lateur N, Sales F, André J. Immunohistochemical study of 40 cases of longitudinal melanonychia. Am J Dermatopathol 2011;33:27–34.
- Bristow IR, de Berker DA, Acland KM, Turner RJ, Bowling J. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res 2010;3:25.
- Ishihara Y, Matsumoto K, Kawachi S, Saida T. Detection of early lesions of ‘ungual’ malignant melanoma. Int J Dermatol 1993;32:44–7.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg 2009;28:49–54.
- André J, Moulonguet I, Goettmann-Bonvallot S. In situ amelanotic melanoma of the nail unit mimicking lichen planus: report of 3 cases. Arch Dermatol 2010;146:418–21.
- Vassallo C, Derlino F, Torti S, et al. Longitudinal deep fissure and distal onycholysis of the right thumb. Amelanotic subungual melanoma. Arch Dermatol 2012;148:947–52.
- Banfield CC, Dawber RP. Nail melanoma: a review of the literature with recommendations to improve patient management. Br J Dermatol 1999;141:628–32.
- Koga H, Saida T, Uhara H. Key point in dermoscopic differentiation between early nail apparatus melanoma and benign longitudinal melanonychia. J Dermatol 2011;38:45–52.
- Tosti A, Argenziano G. Dermoscopy allows better management of nail pigmentation. Arch Dermatol 2002;138:1369–70.
- Ronger S, Touzet S, Ligeron C, et al. Dermoscopic examination of nail pigmentation. Arch Dermatol 2002;138:1327–33.
- Hirata SH, Yamada S, Enokihara MY, et al. Patterns of nail matrix and bed of longitudinal melanonychia by intraoperative dermatoscopy. J Am Acad Dermatol 2011;65:297–303.
- Debarbieux S, Hospod V, Depaepe L, Balme B, Poulalhon N, Thomas L. Perioperative confocal microscopy of the nail matrix in the management of in situ or minimally invasive subungual melanomas. Br J Dermatol 2012;167:828–36.
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol 2007;56:803–10.
- Richert B, Theunis A, Norrenberg S, André J. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol 2013;69:96–104.
- Finley RK III, Driscoll DL, Blumenson LE, Karakousis CP. Subungual melanoma: an eighteen-year review. Surgery 1994;116:96–100.
- Moehrle M, Metzger S, Schippert W, Garbe C, Rassner G, Breuninger H. ‘Functional’ surgery in subungual melanoma. Dermatol Surg 2003;29:366–74.
- Duarte AF, Correia O, Barros AM, Azevedo R, Haneke E. Nail matrix melanoma in situ: conservative surgical management. Dermatology 2010;220:173–5.
- Chow WTH, Bhat W, Magdub S, Orlando A. In situ subungual melanoma: digit salvaging clearance. J Plast Reconstr Aesthet Surg 2012;66:274–6.
- Lazar A, Abimelec P, Dumontier C. Full thickness skin graft for nail unit reconstruction. J Hand Surg 2005;30B:194–8.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg 2007;60:635–8.
- Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. Br J Dermatol 2011;165:852–8.
- High WA, Quirey RA, Guillén DR, Munõz G, Taylor RS. Presentation, histopathologic findings, and clinical outcomes in 7 cases of melanoma in situ of the nail unit. Arch Dermatol 2004;140:1102–6.
- Neczyporenko F, André J, Torosian K, Theunis A, Richert B. Management of in situ melanoma of the nail apparatus with functional surgery: report of 11 cases and review of the literature. J Eur Acad Dermatol Venereol 2014;28:550–7.
- Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol 2007;56:835–47.
Nail fold infections
- Dardu M, Ruocco V. Clinical cytologic features of antibiotic resistant acute paronychia. J Am Acad Dermatol 2014;70:120–6.
- Sezer E, Bridges AE, Koseoglu D, Yuksek J. Acquired periungual fibrokeratoma developing after acute staphylococcal paronychia. Eur J Dermatol 2009;19:636–7.
- Ruocco V, Ruocco E. Tzanck smear, an old test for the new millennium: when and how. Int J Dermatol 1999;38:830–4.
- Karpathios T, Moustaki M, Yiallouros P, et al. HSV-2 meningitis disseminated from a herpetic whitlow. Paediatr Int Child Health 2012;32:121–2.
- Huff JC. Erythema multiforme. Dermatol Clin 1985;3:141–52.
- Walker S. Paronychia of the great toenail of infants. Clin Pediatr 1979;18:247.
- Baran R. Nail alterations in hand eczema. In: Alikhan A, Lachapelle JM, Maibach HI, eds. Textbook of Hand Eczema. Berlin: Springer-Verlag, 2014:37–47.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification. J Am Acad Dermatol 2011;65:1219–27.
- Dawber RPR, Baran R. Painful dorso-lateral fissure of the fingertip: an extension of the lateral nail groove. Clin Exp Dermatol 1984;9:419–20.
- Palfi, Ponai K, Varkonyi V, et al. Primary syphilis of the finger. Dermatology 2008;217:252–3.
- Velez AP, Greene JN, Vincent AL, Elko-Simms LM. Impact and etiology of periungual toe infections in neutropenic patients. Infect Dis Clin Pract 2012;20:e31–4.
- Baran R. Treatment of ingrowing toenails in infancy. J Dermatol Treat 1989;1:55–7.
- Fleming TE, Brodell RT. Subungual abscess: a bacterial infection of the nail bed. J Am Acad Dermatol 1997;37:486–7.
Nail psoriasis
- Zaias N. Psoriasis of the nail. A clinical–pathologic study. Arch Dermatol 1969;99:567–79.
- Samman PD. The Nails in Disease, 3rd edn. London: Heinemann, 1978.
- de Jong EM, Seegers BA, Gulinck MK, Boezeman JB, van de Kerkhof PC. Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patients. Dermatology 1996;193:300–3.
- Puissant A. Psoriasis in children under the age of 10: a study of 100 observations. Gaz Sanita 1970;19:191.
- Nanda A, Kaur S, Kaur I, et al. Childhood psoriasis: an epidemiologic survey of 112 patients. Pediatr Dermatol 1990;7:19–21.
- Stankler L. Foetal psoriasis. Br J Dermatol 1988;119:684.
- Tosti A, Peluso AM, Zucchelli V. Clinical features and long-term follow-up of 20 cases of parakeratosis pustulosa. Pediatr Dermatol 1998;15:259–63.
- McGonagle D, Tan AL, Benjamin M. The nail as a musculoskeletal appendage: implications for an improved understanding of the link between psoriasis and arthritis. Dermatology 2009;218:97–102.
- Tan AL, Tanner SF, Waller ML, et al. High-resolution [18F]fluoride positron emission tomography of the distal interphalangeal joint in psoriatic arthritis: a bone–enthesis–nail complex. Rheumatology (Oxford) 2013;52:898–904.
- Brazzelli V, Carugno A, Alborghetti A, et al. Prevalence, severity and clinical features of psoriasis in fingernails and toenails in adult patients: Italian experience. J Eur Acad Dermatol Venereol 2012;26:1354–9.
- Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003;49:206–12.
- Baran R. A nail psoriasis severity index. Br J Dermatol 2004;150:568–9.
- Chruściel A, Karlinska-Jachowska M, Młynek A, Sysa-Jędrzejowska A, Zalewska-Janowska A. Application of R.L. Baran scale in nail plates evaluation in psoriasis. Dermatol Klin 2007;9:84–8.
- Zaias N. Psoriasis of the nail unit. Dermatol Clin 1984;2:493–505.
- Grammer-West NY, Corvette DM, Giandoni MB, Fitzpatrick JE. Clinical pearl: nail plate biopsy for the diagnosis of psoriatic nails. J Am Acad Dermatol 1998;38:260–2.
- Sonnex TS, Griffiths WAD, Nicol WJ. The nature and significance of the transverse white band of human nails. Semin Dermatol 1991;10:12–16.
- Gupta AK, Lynde CW, Jain HC, et al. A higher prevalence of onychomycosis in psoriatics compared with non-psoriatics: a multicentre study. Br J Dermatol 1997;136:786–9.
- Cohen PR, Prystowsky JH. PRP: a view of diagnosis and treatment. J Am Acad Dermatol 1989;20:801–7.
- Macaulay WL. Transverse ridging of the thumbnails. Arch Dermatol 1966;93:421–3.
- Calvert HT, Smith MA, Wells RS. Psoriasis and the nails. Br J Dermatol 1963;75:415–18.
- Ganor S. Chronic paronychia and psoriasis. Br J Dermatol 1975;92:685–8.
- Burden AD, Kemmett D. The spectrum of nail involvement in palmoplantar pustulosis. Br J Dermatol 1996;134:1079–82.
- Dulanto P, Armijo-Morens M, Camacho-Martinez F. Histological finding in parakeratosis pustulosa. Acta Derm Venereol (Stockh) 1974;54:365–7.
- Hjorth N, Thomsen K. Parakeratosis pustulosa. Br J Dermatol 1967;79:527–32.
- Miller JL, Soltani K, Toutellotte CD. Psoriatic acro-osteolysis without arthritis. J Bone Joint Surg Am 1971;53:371–4.
- Mahowald ML, Parrish RM. Severe osteolytic arthritis mutilans pustular psoriasis. Arch Dermatol 1982;118:434–7.
- Pirracini BM, Fanti PA, Morelli R, Tosti A. Hallopeau's acrodermatitis continua of the nail apparatus: a clinical and pathological study of 20 patients. Acta Derm Venereol (Stockh) 1994;74:65–7.
- Lovy M, Bluhm G, Morales A. The occurrence of nail pitting in Reiter's syndrome. J Am Acad Dermatol 1980;2:66–8.
- Sonnex TS, Dawber RPR, Zachary CB, et al. The nails in adult type I pityriasis rubra pilaris. A comparison with Sézary syndrome and psoriasis. J Am Acad Dermatol 1986;15:956–60.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica. A new cutaneous marker of malignancy. Br J Dermatol 1980;103:301–6.
- Richard M, Giroux JM. Acrokeratosis paraneoplastica (Bazex syndrome). J Am Acad Dermatol 1987;16:178–83.
- Handfield-Jones S, Matthews CNA, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med 1992;85:548–50.
- Wright V, Roberts MC, Hill AGS. Dermatological manifestations in psoriatic arthritis. A follow up study. Acta Derm Venereol (Stockh) 1979;59:235–40.
- Baker H, Golding DN, Thompson M. The nails in psoriatic arthritis. Br J Dermatol 1964;76:549–54.
- Jones SM, Armas JB, Cohen MG, et al. Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Br J Rheumatol 1994;33:834–9.
- Boisseau-Garsaud AM, Beylot-Barry M, Doutre MS, et al. Psoriatic onycho-pachydermo-periostitis. Arch Dermatol 1996;132:176–80.
- Lewin K, Dewit S, Ferrington RA. Pathology of the fingernail in psoriasis. Br J Dermatol 1972;86:555–63.
- Samman PD. The ventral nail. Arch Dermatol 1961;84:192–5.
- de Berker D, Westgate G, Leigh I. Patterns of hard keratin (Ha-1) expression in nail matrix correspond to nail plate morphology [Abstract]. Br J Dermatol 1996;134:584–5.
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol 2000;25:357–62.
- Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol 2007;57:1–27.
- Cassell S, Kavanaugh AF. Therapies for psoriatic nail disease. A systematic review. J Rheumatol 2006;33:1452–6.
- de Berker DA, Lawrence CM. A simplified protocol of steroid injection for psoriatic nail dystrophy. Br J Dermatol 1998;138:90–5.
- Tosti A, Piraccini BM, Cameli N, et al. Calcipotriol ointment in nail psoriasis: a controlled double-blind comparison with betamethasone dipropionate and salicylic acid. Br J Dermatol 1998;139:655–9.
- Rigopoulos D, Ioannides D, Prastitis N, Katsambas A. Nail psoriasis: a combined treatment using calcipotriol cream and clobetasol propionate cream. Acta Derm Venereol (Stockh) 2002;82:140.
- Piraccini BM, Tosti A, Iorizzo M, Misciali C. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol 2001;144:1000–5.
- Marx JL, Scher RK. The response of psoriatic nails to photochemotherapy. Arch Dermatol 1980;116:1023–4.
- Handfield-Jones SE, Boyle J, Harman RRM. Local PUVA treatment for nail psoriasis. Br J Dermatol 1987;116:280–1.
- Baran R. Retinoids and the nails. J Dermatol Treat 1990;1:151–4.
- Tosti A, Ricotti C, Romanelli P, Cameli N, Piraccini BM. Evaluation of the efficacy of acitretin therapy for nail psoriasis. Arch Dermatol 2009;145:269–71.
- Scher RK, Stiller M, Zhu YI. Tazarotene 0.1% gel in the treatment of fingernail psoriasis: a double blind, randomized, vehicle-controlled study. Cutis 2001;68:355–8.
- Sánchez-Regaña M, Sola-Ortigosa J, Alsina-Gibert M, Vidal-Fernández M, Umbert-Millet P. Nail psoriasis: a retrospective study on the effectiveness of systemic treatments (classical and biological therapy). J Eur Acad Dermatol Venereol 2011;25:579–86.
- de Vries AC, Bogaards NA, Hooft L, et al. Interventions for nail psoriasis. Cochrane Database Syst Rev 2013;(1):CD007633.
- Gümüşel M, Özdemir M, Mevlitoğlu I, Bodur S. Evaluation of the efficacy of methotrexate and cyclosporine therapies on psoriatic nails: a one-blind, randomized study. J Eur Acad Dermatol Venereol 2011;25:1080–4.
- Lawry M. Biological therapy and nail psoriasis. Dermatol Ther 2007;20:60–7.
- de Berker D. Biologics in nail psoriasis. Br J Dermatol 2014;170:236–7.
- Jemec GB, Ibler KS. Treatment of nail psoriasis with TNF-α or IL12/23 inhibitors. J Drugs Dermatol 2012;11:939–42.
- Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum 2005;52:2513–18.
- Bauza A, Redondo P, Aquerreta D. Psoriatic onycho-pachydermo periostitis: treatment with methotrexate. Br J Dermatol 2000;143:901–2.
- Tosti A, Guerra L, Bardazzi F, Lanzarini M. Topical cyclosporin in nail psoriasis. Dermatologica 1990;180:110.
- Fritz K. Psoriasis of the nail. Successful topical treatment with 5-fluorouracil. Z Hautkr 1988;64:1083–8.
- Fredriksson T. Topically applied fluorouracil in the treatment of psoriatic nails. Arch Dermatol 1974;110:735–6.
- Yu RCH, King CM. A double blind study of superficial radiotherapy in psoriatic nail dystrophy. Acta Derm Venereol (Stockh) 1992;72:134–6.
- Kwang TY, Nee TS, Seng KTH. A therapeutic study of nail psoriasis using electron beams. Acta Derm Venereol (Stockh) 1995;75:90.
Darier disease of the nails
- Bingham EA, Burrows D. Darier's disease. Br J Dermatol 1984;111(Suppl. 26):88–9.
- Burge SM, Wilkinson JD. Darier–White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol 1992;27:40–50.
- Ronchese F. The nail in Darier's disease. Arch Dermatol 1965;91:617–18.
- Schubert H. Darier's disease. Z Haut Geschlechskr 1966;41:239–44.
- Zaias N, Ackerman AB. The nail in Darier–White disease. Arch Dermatol 1973;107:193–9.
- Downs AM, Ward KA, Peachey RD. Subungual squamous cell carcinoma in Darier's disease. Clin Exp Dermatol 1997;22:277–9.
Eczema involving the nails
- Yu M, Kim SW, Kim MS, Han TY, Lee JH, Son SJ. Clinical study of patients with hand eczema accompanied by nail dystrophy. J Dermatol 2013;40:406–7.
- Maio P, Carvalho R, Amaro C, Santos R, Cardoso J. Letter: Allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J 2012;18(2):13.
- Marren P, de Berker DAR, Powell S. Occupational contact dermatitis due to Quaternium 15 presenting as nail dystrophy. Contact Dermatitis 1991;25:253–5.
- Boiko S, Kaufman RA, Lucky AW. Osteomyelitis of the distal phalanges in three children with severe atopic dermatitis. Arch Dermatol 1988;124:418–23.
Lichen planus of the nails
- Samman PD. The nails in lichen planus. Br J Dermatol 1961;73:288–92.
- Tosti A, Peluso AM, Fanti PA, et al. Nail lichen planus. Clinical and pathological study of 24 patients. J Am Acad Dermatol 1993;28:724–30.
- Tosti A, Fanti PA, Morelli R, et al. Trachyonychia associated with alopecia areata. A clinical and pathological study. J Am Acad Dermatol 1991;25:266–70.
- de Berker D, Dawber RPR. Childhood lichen planus. Clin Exp Dermatol 1991;16:233.
- Milligan A, Graham-Brown RAC. Lichen planus in childhood: a review of six cases. Clin Exp Dermatol 1990;15:340–2.
- Baran R, Jancovici E, Sayag J, Dawber RPR. Longitudinal melanonychia in lichen planus. Br J Dermatol 1985;113:369–74.
- Baran R. Lichen planus of the nails mimicking the yellow nail syndrome. Br J Dermatol 2000;143:1117–18.
- Tosti A, Piraccini BM, Cameli N. Nail changes in lichen planus may resemble those of yellow nail syndrome. Br J Dermatol 2000;142:848–9.
- Cornelius CE, Shelley WB. Permanent anonychia due to lichen planus. Arch Dermatol 1967;96:434–5.
- Samman PD. Idiopathic atrophy of the nails. Br J Dermatol 1985;81:746–9.
- de Berker D, Dalziel K, Dawber RPR, Wojnarowska F. Pemphigus associated with nail dystrophy. Br J Dermatol 1993;129:461–4.
- Tosti A, Piraccini BM, Cambiaghi S, Jorizzo M. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol 2001;137:1027–32.
- Munro CS, Cox NH, Marks JM, et al. Lichen nitidus presenting as palmoplantar hyperkeratosis and nail dystrophy. Clin Exp Dermatol 1993;18:381–3.
- Saurat JH, Gluckman E. Lichen planus-like eruption following bone marow transplantation: a manifestation of the graft-versus-host disease. Clin Exp Dermatol 1977;2:335–44.
- Ramrakha-Jones VS, Paul M, McHenry P, Burden AD. Nail dystrophy due to lichen sclerosus? Clin Exp Dermatol 2001;26:507–9.
- Tosti A, Peluso AM, Misciali C, Cameli N. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol 1997;36:908–13.
- Barth JH, Millard PR, Dawber RPR. Idiopathic atrophy of the nails. A clinicopathological study. Am J Dermatopathol 1988;10:514–17.
- Zaias N. The nail in lichen planus. Arch Dermatol 1970;101:264–71.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol 2014;31:59–67.
- Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol 1996;21:56–7.
- Manousaridis I, Manousaridis K, Peitsch WK, Schneider SW. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges 2013;11:981–91.
- Pinter A, Pätzold S, Kaufmann R. Lichen planus of nails: successful treatment with alitretinoin. J Dtsch Dermatol Ges 2011;9:1033–4.
Nails in childhood and old age
- Baran R, Barth J, Dawber RPR, eds. Nail Disorders. London: Dunitz, 1991:78–101.
- de Berker D. Childhood nail diseases. Dermatol Clin 2006;24:355–63.
- Hammerton MD, Shrank AB. Congenital hypertrophy of the lateral nail folds of the hallux. Pediatr Dermatol 1988;5:243–5.
- Piraccini BM, Parente GL, Varotti E, Tosti A. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol 2000;17:348–51.
- Baran R. Congenital malalignment of the toe nail. Arch Dermatol 1980;116:1346.
- Gupta AK, Skinner AR, Baran R. Onychomycosis in children: an overview. J Drugs Dermatol 2003;2:31–4.
- Gupta AK, Skinner AR. Onychomycosis in children: a brief overview with treatment strategies. Pediatr Dermatol 2004;21:74–9.
- Turano AF. Beau's lines in infancy. Pediatrics 1968;41:996–4.
- Parry EJ, Morley WN, Dawber RPR. Herringbone nails: an uncommon variant of nail growth in childhood. Br J Dermatol 1995;132:1021–2.
- Singh G, Haneef NS, Uday A. Nail changes and disorders among the elderly. Indian J Dermatol Venereol Leprol 2005;71:386–92.
- Baran R. Nail care in the ‘golden years’ of life. Curr Med Res Opin 1982;7:96–101.
- Brauer E, Baran R. Cosmetics: the care and adornment of the nail. In: Baran R, Dawber RPR, de Berker DAR, Haneke E, Tosti A, eds. Diseases of the Nails and Their Management, 3rd edn. Oxford: Blackwell Science, 2001:366–8.
- Woodrow P, Dickson N, Wright P. Foot care for non-diabetic older people. Nurs Older People 2005;17:31–2.
- Helfand AE. Assessing onychial disorders in the older patient. Clin Podiatr Med Surg 2003;20:431–42.
- Loo DS. Onychomycosis in the elderly: drug treatment options. Drugs Aging 2007;24:293–302.
- Tavakkol A, Fellman S, Kianifard F. Safety and efficacy of oral terbinafine in the treatment of onychomycosis: analysis of the elderly subgroup in Improving Results in Onychomycosis Concomitant Lamisil and Debridement (IRON-CLAD), an open-label, randomized trial. Am J Geriatr Pharmacother 2006;4:1–13.
Imaging of the nail
- Destouet JM, Murphy WA. Acquired osteolysis and acronecrosis. Arthritis Rheum 1983;26:1150–4.
- El Komy MHM, Baran R. Acroosteolysis presenting with brachyonychia following exposure to cold. J Eur Acad Dermatol Venereol 2014; doi: 10.1111/jdv.12826.
- Maroteaux P. Five cases of microgeodic disease of phalanges of unknown etiology in infants. Ann Radiol (Paris) 1970;13:229–36.
- Van Acker T, Eykens A, Wouters C, Toelen J. The phalangeal microgeodic syndrome in childhood: awareness leads to diagnosis. Eur J Pediatr 2013;172:763–7.
- Baran R, Tosti A. Acroosteolysis in a guitar player. Acta Derm Venereol 1993;73:64–5.
- Baran R, Turkmani MG, Mubki T. Acquired racquet nails: a useful sign of hyperparathyroidism. J Eur Acad Dermatol Venereol 2014;28:257–9.
- Mathis WH Jr, Schulz MD. Roentgen diagnosis of glomus tumors. Radiology 1948;51:71–6.
- Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. Retrospective Study of 51 cases. J Hand Surg 1996;21B:257–60.
- Lovett JA, Haines TA, Bentz ML. Subungual kerato-acanthoma masquerading as a chronic paronychia. Ann Plast Surg 1995;38:84–7.
- Meng Q, Watt I. Phalangeal osteoid osteoma. Br J Radiol 1989;62:321–5.
- Schajowicz F, Aiello CA, Slullitel I. Cystic and pseudo-cystic lesions of the terminal phalanx with special reference to epidermoid cyst. Clin Orthop Relat Res 1970;68:84–92.
- Baran R. Nail tumours: Clinical overview. In: Wortsman X, Jemec GBE, eds. Dermatologic Ultrasound with Clinical and Histologic Correlations. Heidelberg: Springer, 2013:409–18.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res 2011;303:457–73.
- Aydin SZ, Castillo-Hallego C, Ash ZR, et al. Potential use of optical coherence tomography and high-frequency ultrasound for the assessment of nail disease in psoriasis and psoriatic arthritis. Dermatology 2013;227:45–51.
- Hongcharu W, Dwyer P, Anderson RR. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol 2000;42:214–16.
- Pharaon M, Garri-Toussaint M, Khemis A, et al. Diagnosis and treatment monitoring of toenail onychomycosis by reflectance confocal microscopy: prospective cohort study in 58 patients. J Am Acad Dermatol 2014;71:56–61.
- Hirata SH, Yalada S, Enokihara MY, et al. Patterns of nail matrix and bed of longitudinal melanonychia by intraoperative dermatoscopy. J Am Acad Dermatol 2011;65:297–303.
- Debarbieux S, Hospod V, Depaepe L, Balme B, Poulalhon N, Thomas L. Perioperative confocal microscopy of the nail matrix in the management of in situ or minimally invasive subungual melanomas. Br J Dermatol 2012;167:828–36.
- Drapé JL. Magnetic resonance imaging. In: Baran R, De Berker DAR, Holzberg M, Thomas L, eds. Baran and Dawber Diseases of the Nails and Their Management, 4th edn. Oxford: Wiley-Blackwell, 2012:154–82.
- Rossi D, Russo A, Manna E, et al. The role of nail videocapillaroscopy in early diagnosis of scleroderma. Autoimmun Rev 2013;12:821–5.
- Ribeiro CF, Siqueira EB, Holler AP, Fabricio L, Skare TL. Periungual capillaroscopy in psoriasis. An Bras Dermatol 2012;87:550–3.
Nail surgery
- Richert B. Basic nail surgery. Dermatol Clin 2006;24:313–22.
- Abimelec P, Dumontier C. Basic and advanced nail surgery. In: Scher RK, Daniel CR, eds. Nails: Diagnosis, Therapy, Surgery, 3rd edn. Philadelphia: Elsevier Saunders, 2005:265–89.
- Serour F, Ben-Yehuda Y, Boaz M. EMLA cream prior to digital nerve block for ingrown nail surgery does not reduce pain at injection of anesthetic solution. Acta Anaesthesiol Scand 2002;46:203–6.
- Cornelius P, Kendall J, Meek S, Rajan R. Alkalinisation of lignocaine to reduce the pain of digital nerve blockade. J Accid Emerg Med 1996;13:339–40.
- Davidson JA, Boom SJ. Warming lignocaine to reduce pain associated with injection. BMJ 1992;305:617–18.
- Andrades PR, Olguin FA, Calderón W. Digital blocks with or without epinephrine. Plast Reconstr Surg 2003;111:1769–70.
- Katis PG. Epinephrine in digital blocks: refuting dogma. CJEM 2003;5:245–6.
- Krunic AL, Wang LC, Soltani K, Weitzul S, Taylor RS. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol 2004;51:755–9.
- Chowdhry S, Seidenstricker L, Cooney DS, Hazani R, Wilhelmi BJ. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg 2010;126:2031–4.
- Reichl M, Quinton D. Comparison of 1% lignocaine with 0.5% bupivacaine in digital ring blocks. J Hand Surg 1987;12B:375–6.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, di Chiacchio N, Haneke E, eds. Nail Surgery. London: Informa, 2010:24–30.
- Casati A, Vinciguerra F, Scarioni M, et al. Lidocaine versus ropivacaine for continuous interscalene brachial plexus block after open shoulder surgery. Acta Anaesthesiol Scand 2003;47:355–60.
- Peng PWH, Coleman MM, McCartney CJL, et al. Comparison of anesthetic effect between 0.375% ropivacaine versus 0.5% lidocaine in forearm intravenous regional anesthesia. Reg Anesth Pain Med 2002;27:595–9.
- Moffitt DL, De Berker DA, Kennedy CT, Shutt LE. Assessment of ropivacaine as a local anesthetic for skin infiltration in skin surgery. Dermatol Surg 2001;27:437–40.
- Fayman M, Beeton A, Potgieter E, Becker PJ. Comparative analysis of bupivacaine and ropivacaine for infiltration analgesia for bilateral breast surgery. Aesthetic Plast Surg 2003;27:100–3.
- Gherardini G, Samuelson U, Jernbeck J, Aberg B, Sjöstrand N. Comparison of vascular effects of ropivacaine and lidocaine on isolated rings of human arteries. Acta Anaesthesiol Scand 1995;39:765–8.
- Flarity-Reed K. Methods of digital block. J Emerg Nurs 2002;28:351–4.
- Schnitzler L, Baran R, Civatte J, Schubert B, Verret JL, Hurez D. Biopsy of the proximal nail fold in collagen diseases. J Dermatol Surg 1976;2:313–15.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989;21:1165–75.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001;27:580–4.
- Di Chiacchio, Richert B, Haneke E. Surgery of the matrix. In: Richert B, di Chiacchio N, HanekeE, eds. Nail Surgery. London: Informa, 2010:103–32.
- Richert B, Theunis A, Norrenberg S, André J. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol 2013;69:96–104.
- De Berker DA, Baran R. Acquired malalignment: a complication of lateral longitudinal nail biopsy. Acta Derm Venereol 1998;78:468–70.
- de Berker DA. Lateral longitudinal nail biopsy. Australas J Dermatol 2001;42:142–4.
- Richert B. Surgery of the nail plate. In: Richert B, di Chiacchio N, Haneke E, eds. Nail Surgery. London: Informa, 2010:31–41.
- Baran R, Richert B. The management of onychomycosis. Ann Dermatol Vénéréol 2003;130:1260–71.
- Keyser JJ, Littler JW, Eaton RG. Surgical treatment of infections and lesions of the perionychium. Hand Clin 1990;6:137–53; discussion 155–7.
- Baran R, Bureau H. Surgical treatment of recalcitrant chronic paronychias of the fingers. J Dermatol Surg Oncol 1981;7:106–7.
- Haneke E, Richert B, di Chiacchio N. Surgery of the proximal nail fold. In: Richert B, di Chiacchio N, Haneke E, eds. Nail Surgery. London: Informa, 2010:42–54.
- Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev 2005;(2):CD001541.
- Eekhof JAH, Van Wijk B, Knuistingh Neven A, van der Wouden JC. Interventions for ingrowing toenails. Cochrane Database Syst Rev 2012;(4):CD001541.
- Becerro de Bengoa Vallejo R, Losa Iglesias ME, Viejo Tirado F, Serrano Pardo R. Cauterization of the germinal nail matrix using phenol applications of differing durations: a histologic study. J Am Acad Dermatol 2012;67:706–11.
- Cordoba Diaz D, Losa Iglesias ME, Cordoba Diaz M, Becerro de Bengoa Vallejo R. Evidence of the efficacy of alcohol lavage in the phenolization treatment of ingrown toenails. J Eur Acad Dermatol Venereol 2011;25:794–8.
- Ozdemir E, Bostanci S, Ekmekci P, Gurgey E. Chemical matricectomy with 10% sodium hydroxide for the treatment of ingrowing toenails. Dermatol Surg 2004;30:26–31.
- Kim S-H, Ko H-C, Oh C-K, Kwon K-S, Kim M-B. Trichloroacetic acid matricectomy in the treatment of ingrowing toenails. Dermatol Surg 2009;35:973–9.
The nail and cosmetics
- Schoon D, Baran R. The care and adornment of the nail. In: Baran R, De Berker DA, Holzberg M, Thomas L, eds. Nail Diseases and Their Management. Oxford: Wiley-Blackwell, 2012:471–83.
- Baran R, André J. Side-effects of nail cosmetics. J Cosmet Dermatol 2005;4:204–9.
- Fisher AA, Baran R. Adverse reactions to acrylate sculptured nails with particular reference to prolonged paresthesia. Am J Contact Dermatitis 1991;2:38–42.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis in artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol 1996;35:377–80.