CHAPTER 124
Pressure Injury and Pressure Ulcers
Robyn Evans1, Carol Ott2 and Madhuri Reddy3
1Wound Healing Clinic, Women's College Hospital, Toronto; and University of Toronto, Toronto, Ontario, Canada
2Wound Healing Clinic, Women's College Hospital, Toronto; Geriatrics and Wound Care Clinics, Baycrest Hospital, Toronto, Ontario, Canada
3Wound Healing Program, Department of Medicine, Harvard Medical School, Boston, MA, USA
Definition and nomenclature
A pressure ulcer is an area of localized tissue injury which occurs as a result of compression between a bony prominence and the external surface [1]. The most important force is pressure, but other forces such as friction, shear, moisture, patient co-morbidities and mechanical factors of the bone, skin, fat and muscle also contribute to tissue strain [1, 2].
The most common anatomical sites for pressure ulcer development are the sacrum and heel [3]. Other sites are represented in Figure 124.1 [3].
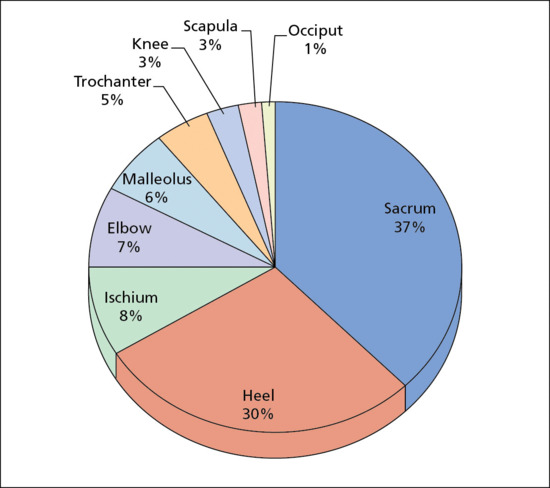
Figure 124.1 Anatomical locations of pressure ulcer.
Introduction and general description
Pressure ulcers represent a significant health concern for patients, families and funding agencies. The majority of pressure ulcers occur in patients over 65 years of age [4]. Patients with spinal cord injuries represent a high-risk group with the prevalence of a pressure ulcer being 20–30% during the first 5 years after the injury [5]. The presence of ulcers is often considered an indicator of quality of care in health institutions and this is increasingly the subject of (successful) litigation in the long-term care setting [6]. The prevention and management of pressure ulcers are thus best served by a multidisciplinary team. Pressure ulcers are known to increase the length of stay in acute care hospitals and may contribute to premature death [5].
Pressure ulcers are largely preventable with the appropriate assessment and management of the various intrinsic (patient-related) and extrinsic (pressure, shear, friction, skin microclimate) factors. They are classified by the extent of damage to the underlying tissue using the National Pressure Ulcer Advisory Panel staging system (NPUAP) [1].
There are many choices of support surfaces for the prevention and treatment of pressure ulcers; however, the challenge is to use the most cost-effective surface given the available evidence.
Infection in a pressure ulcer is one of the leading causes of infections in nursing homes and significantly delays healing [7]. Detection of infection in pressure ulcers is challenging as the classic signs and symptoms may be absent in this chronic type of wound.
Epidemiology
In the US, over 2.5 million pressure ulcers are treated each year in acute care hospitals [8]. Estimates of the incidence and prevalence of pressure ulcers varies greatly from study to study.
In the US, a large study of over 90 000 patients followed for 2 years (2008–2009) in acute care facilities showed a prevalence of 12.3–13.5% and an incidence of 5–6% [9]. Incident rates were highest (8.8–12.1%) in the adult intensive care and general cardiac units. In 2007, a pilot pressure prevalence survey was conducted at 25 hospitals with 5947 patients in Belgium, Italy, Portugal, Sweden and the UK. The overall prevalence of pressure ulcers was found to be 18.1% (1078). Most of these pressure ulcers were stage I (42%, 454) or stage II (26%, 282), with stage III (18%, 199) and IV (13%, 143) being less common [10].
In long-term care, the incidence of pressure ulcers in the US has been reported between 2.2 and 23.9% [11] and prevalence from 2.3 to 28% [12].
The cost of treating a pressure ulcer depends on its stage. A UK study found it to vary from £1064 for a stage I ulcer to £10 551 for a stage IV ulcer [13]. The total cost was estimated at £1.4–2.1 billion annually or 4% of total NHS expenditure, with most of this being nursing time [13].
Pathophysiology
Offloading pressure alone (i.e. using a pressure relief mattress) is often inadequate in pressure ulcer prevention and management because of the complex interplay between intrinsic and extrinsic factors.
Intrinsic risk factors
Assessing each patient's intrinsic risk for the development of ulcers permits the proper targeting of resources.
A recent systematic review of 54 studies and over 34 000 patients was undertaken to understand the risk factors that predict pressure ulcer development [14]. The three major risk factors and examples are indicated in Table 124.1. All studies indicated that the poorer the mobility, the increased risk of pressure ulcers. Skin/pressure ulcer status is related to factors which may make the skin more vulnerable to pressure ulcers [14]. Other factors considered important were: age, haematological measures, nutrition and co-morbidities.
Table 124.1 Major intrinsic risk factor for pressure ulcer development.
| Mobility challenges | Vascular perfusion | Skin/pressure ulcer status |
Chair bound Bed bound Walking with limitations |
Diabetes Hypertension Smoking Oedema Vascular disease |
Redness Blanching erythema Dryness Stage l skin changes Previous pressure ulcers |
Coleman et al. 2013 [14]. Reproduced with permission of Elsevier.
Extrinsic risk factors
The extrinsic factors associated with tissue damage are pressure, shearing, friction and skin microclimate.
Pressure
The most important of the extrinsic factors is pressure, which is defined as a perpendicularly applied force. In the case of pressure ulcers, this is usually over a bony prominence (Figure 124.2) [15]. If sufficient pressure exists, flow in capillaries and lymphatics is occluded, resulting in oedema and tissue ischaemia [5]. The amount of tissue damage is related to the intensity and duration of the pressure applied.
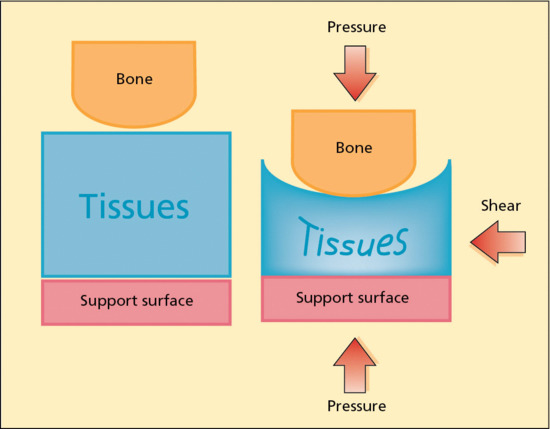
Figure 124.2 Representation of pressure. Pressure is a perpendicular force applied to the tissues whereas shear is a tangential force. The result is compression and distortion of the tissues beneath the bone. In this diagram the support surface is represented as solid not supplying any pressure redistribution. (Adapted from Takahashi et al. 2010 [15].)
Friction
Friction refers to the action of two objects rubbing against each other. Friction is not usually a direct cause of a pressure ulcer but induces a number of changes to the skin making it more susceptible to ulceration.
Shear
Shear results from a parallel (tangential) force to a fixed object [16]. Examples include a patient sliding down in bed with the sacral skin and subcutaneous tissue moving relative to the fixed bone; patients with involuntary muscle contractions or spasms; and when skin elasticity and turgor are reduced, as typically occurs with ageing. These situations cause compression of blood vessels, distortion of tissues and pinching of vessels.
Friction and shearing forces frequently occur during patient transfer (i.e. from bed to commode or bed to chair) in contrast to pressure. Pressure ulcers that have significant shearing forces may result in narrow, deep tunnelling wounds.
Skin microclimate
Skin microclimate is a relatively new concept that relates to the temperature and humidity as well as the moisture at the interface between the skin and the support surface [17]. Tissue metabolism increases by 10% for each 1°C rise in skin temperature [17, 18]. Tissue damage thus occurs faster as body temperature increases with the same applied local pressure. Excessive moisture as a result of perspiration, urine, faeces or draining fistulas can result in maceration of the skin making it more vulnerable to tissue breakdown and less resilient to pressure, shearing and frictional forces [17]. Excessive drying of the skin can result in a similar outcome.
Clinical features and classification
The NPUAP staging system and the European Pressure Ulcer Advisory Panel (EPUAP) classify pressure ulcers based on the extent of the damage to the skin and underlying tissues [1]. The NPUAP's most recent pressure ulcer staging system (2007) has defined six stages (Table 124.2). Pressure ulcers are the only type of wounds that are staged using this system; other chronic wounds (e.g. venous stasis ulcers, diabetic foot ulcers) have their own classification systems.
Table 124.2 National Pressure Ulcer Advisory Panel (NPUAP) staging system for pressure ulcers.
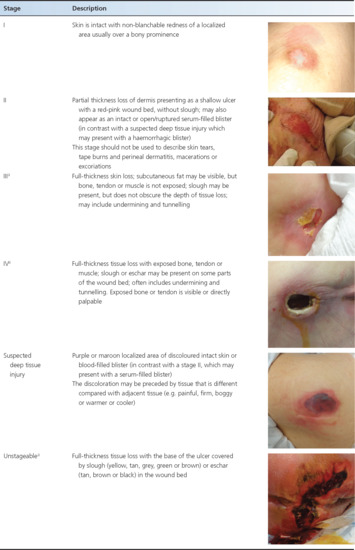
One of the stages included in the most recent NPUAP staging system is suspected deep tissue injury (sDTI) [1]. sDTI presents as a purple or bruised appearance to the skin that is frequently boggy or warm compared with adjacent tissue. It may also present as a haemorrhagic blister (in contrast with a stage II pressure ulcer, which may present as a serum-filled blister). It is an insult at the bone–muscle interface that occurs before skin compromise is seen so may be mistaken for a stage I pressure ulcer, particularly in persons with dark skin colour. It is important to consider sDTI in the differential diagnosis of a stage I or stage II pressure ulcer because sDTI can progress much more rapidly to a stage III or IV, i.e. not following the usual progression from stage I through to IV. sDTI (see Table 124.2) is believed to result from excessive pressure at the bone–muscle interface with the damage starting from the inside and moving towards the outside [18].
It can be difficult to differentiate a stage I or II pressure ulcer from a skin lesion caused by moisture-associated skin damage (MASD) due to incontinence of urine and/or faecal material. MASD treatment may involve different management options than pressure ulcer treatment, so it is important to consider it in the differential diagnosis [19].
It is not accurate to stage in reverse order as a pressure ulcer heals. For example, a superficial wound that was once a stage IV is referred to a healing stage IV pressure ulcer. Once a full-thickness pressure ulcer (stage III or higher) has healed, it remains at risk for further ulceration as the tensile strength is decreased after healing.
Investigations: histopathology
The most complete study of the histopathology of pressure ulcer and its antecedents in humans is given by Witkowski and Parish [20]. In the earliest clinically recognizable stage of blanchable erythema, there is dilatation of superficial dermal venules and papillary capillaries with a mild perivascular inflammatory infiltrate and degenerative changes in occasional sweat coils and ducts. At the stage of non-blanchable erythema, there is marked red cell engorgement of superficial vessels, platelet thrombi in many of them, and extravasation of red cells. More eccrine units are degenerate and there is evident fat necrosis. Before ulceration occurs, various additional changes are recognized, including epidermal atrophy, subepidermal blister formation, tissue eosinophilia and necrosis of hair follicles. Some other studies have emphasized the presence of intra- and extravascular fibrin [21, 22]. In early ulceration, there is loss of the epidermis, and at the stage of a black eschar there is full-thickness destruction of the skin. The antecedent for black eschar formation was not seen in the biopsies studied, but was presumed to be vascular disruption at a deeper plane. In a chronic ulcer, there was fibrosis with isolated collections of capillaries and no residual appendages.
Management
Complications
Pressure ulcers can contribute to significant complications.
Hospital stay
Pressure ulcers are known to prolong hospital stays but more importantly are associated with increased mortality. There is a fivefold increased risk of death in elderly patients with pressure ulcer and in this group, in acute care, mortality is 25–30% [23].
Pain
Pain needs to be considered in all patients with pressure ulcers. It is recommended that pain assessment tools be used to assess pain. The prevalence of pain was assessed in two large studies that included patients from hospital as well as community and palliative care with estimates of pain prevalence being 37% and 66%, respectively [24]. Pain may be chronically present, related to dressing changes or occur during debridement. Each of these aspects needs to be managed, which often requires a multidisciplinary approach. Pain can be either nociceptive (e.g. gnawing, aching, throbbing) or neuropathic (e.g. burning, stinging). Medication needs to be directed to the type of pain. Pain is often associated with infection and this needs to be fully evaluated.
Infection
Pressure ulcers are subject to infection, which may be local or deep, causing osteomyelitis, or systemic, causing sepsis or necrotizing fasciitis. Since ulceration frequently occurs over a bony prominence, osteomyelitis is not uncommon particularly if the ulcer probes to bone. Plain X-rays may be helpful in evaluating this but magnetic resonance imaging is superior [25]. Bone scan with labelled white blood cells is an alternative but is not as sensitive [25].
Marjolin ulcer
This usually refers to a squamous cell carcinoma developing in a burn or scar although it can also occur in any chronic ulcer including pressure ulcers [26]. This is a rare complication with an incidence of less than 0.5% [27].
Systemic amyloidosis
This is a rare complication of pressure ulcers.
Prevention
Pressure ulcers can develop quickly and are challenging to heal. It is therefore preferable to prevent rather than to treat pressure ulcers once they have occurred because treatment is usually much more costly and less efficacious.
Risk assessment
Risk assessment tools can help to identify high-risk individuals as an aid to pressure ulcer prevention.
A systematic review of pressure ulcer risk assessment scales found that three studies reported on the clinical effectiveness of the scales and 30 studies reported on their validation [28].
- The Braden scale showed a sensitivity of 57.1% and specificity of 67.5%. It predicted pressure ulcers with an odds ratio of 4.08 (95% CI 2.56–6.48).
- The Norton scale had a sensitivity of 46.8% and specificity of 61.8% and predicted risk with odds ratio of 2.16 (95% CI 1.03–4.54).
- The Waterlow scale showed the highest sensitivity at 82.5% but low specificity of 27.4% and an odds ratio of 2.05 (95% CI 1.11–3.76).
This study concluded that there is no decrease in actual pressure ulcer incidence that could be attributed to the use of a scale, but that the Braden and Norton scales predict risk better than clinical judgement by nurses. The Braden scale showed the best sensitivity/specificity balance [28]. Other reviews did not find that available studies were adequate enough to recommend one risk assessment scale over another or even over clinical judgement [29, 30].
Repositioning
Repositioning is one of the most important interventions to reduce pressure to susceptible areas. There is insufficient evidence regarding how often patients should be repositioned [6]; however, most pressure ulcer prevention strategies recommend every 2 h. Use of advanced support surfaces is not a substitute for repositioning patients.
Other considerations in repositioning include:
- Maintaining the head of the bed at less than 30 degrees to minimize shearing.
- Lifting rather than dragging/pulling patients over surfaces to prevent shear and friction, which may include the use of lifts.
- Employing heel lifts to reduce pressure to this vulnerable area; however, there is insufficient evidence regarding the optimum device [31]. There are issues related to the ability to keep the device in place and not cause further friction or pressure to other areas of the foot.
- Correctly using and positioning medical devices, such as drainage tubes, catheters and braces. In one study of 2079 hospitalized patients, the most common sites for medical device associated pressure ulcers were the ear, leg and heels. Patients with medical devices were twice as likely to develop pressure ulcers [32].
Support surfaces (Table 124.3)
Table 124.3 Pressure offloading mattresses.
| Method | Comments | Classification |
| Foams | Specialized foam-convoluted or cubed foam. Foam mattresses are used in most long-term care facilities | Non-powered |
| Air of gel filled systems | Air or gel filled columns or compartments. Air filled surfaces are often referred to as low air loss surfaces. May be useful to manage skin temperature and the microclimate | Non-powered |
| Air fluidized | Silicone or glass beads with air forced through making the system take on the characteristics of a fluid | Powered |
| Alternating pressure | Cells inflate and deflate in a cyclical manner and can be adjusted for frequency and inflation pressures.Expensive systems | Powered |
To address the issue of pressure over a bony prominence, many support surfaces or pressure redistributing systems have been developed. Support surfaces include specialized beds, mattresses, mattress overlays and wheelchair cushions.
Support surfaces are generally classified as ‘powered or non-powered’ depending on whether electricity is required.
Despite the plethora of studies evaluating the effectiveness of support surfaces, the quality of these studies is limited. More advanced support surfaces (specialized foam and denser sheepskin) are more effective than standard mattresses in high-risk patients for reducing the incidence of pressure ulcers [6, 33]. Comparing powered to non-powered support surfaces in a randomized control trial of 447 patients, there was no difference in pressure ulcer incidence [34]. There is evidence that overlays on the operating table may reduce the incidence of pressure ulcers [35].
Nutrition
Good nutritional intake may affect the risk of developing ulcers and most patients should have their nutritional status assessed by a dietitian.
Current trials show little evidence to support the use of oral or enteral nutritional supplementation for the prevention of ulcers. Most trials are of poor quality with inadequate randomization, allocation concealment methods and failure to blind outcome assessors [6]. One trial of 672 critically ill in-patients over the age of 65 compared a standard diet alone to a standard diet plus two oral nutritional supplements per day showed a decreased incidence of pressure ulcers at 15 days [36].
Skin care
Attention to the skin is important to prevent dryness and protect against surface moisture; however, there is no specific topical agent found to be beneficial in preventing pressure ulcer [6].
Treatment
Once a pressure ulcer has developed in a patient, a complete assessment needs to be undertaken to address the specific intrinsic and extrinsic factors involved. A patient-centred approach should tackle concerns such as pain, mobility and personal factors. These problems should be determined by taking an appropriate history and by understanding the patient's circumstances.
Both preventative and treatment measures need to take place simultaneously for optimum management of these complex patients. An algorithm for the prevention and treatment identifies the categories that should be considered in managing the whole patient (Figure 124.3).
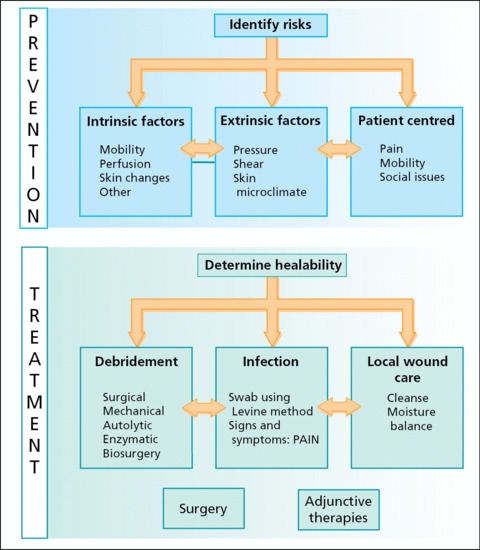
Figure 124.3 Algorithm for the prevention and treatment of pressure ulcers. (Adapted from Sibbald et al. 2011 [40].)
Support surfaces
Pressure relief to the vulnerable area should be provided.
Moderate evidence was found that wound improvement was superior with air fluidized beds compared to standard hospital beds [37, 38].
There are inadequate studies comparing other advanced care surfaces. In particular, the evaluation of powered versus non-powered support surfaces show inconsistent results in treating pressure ulcers [39].
Determination of the ‘healability’ of the wound also needs to be undertaken (Table 124.4) [40]. This will give the patient, family and other health care professionals realistic expectations. A wound is considered healable when the underlying issues contributing to the wound can be corrected. A maintenance wound is a wound that could heal; however, there are health care delivery issues or patient-related factors preventing the wound from healing. Examples of this include the lack of pressure offloading devices or expected patient difficulties in adhering to the treatment plan. A non-healing wound is one in which the underlying conditions cannot be corrected. In a pressure ulcer this may be due to an irreversible vascular problem, an underlying malignancy or co-morbidities.
Table 124.4 Categories of ‘healability’.
| Healability | Definition | Comments |
| Healable wound | The underlying issues leading to the wound can be addressed and corrected | Proceed on algorithm |
| Maintenance wound | The wound would be healable except that there are underlying causes that are not correctable |
Examples:
|
| Non-healable wound | The wound is unlikely to heal. Underlying issues cannot be treated | Examples:
|
Riet 1995 [41]. Reproduced with permission of Elsevier.
Nutrition
There has been interest in the effects of nutritional supplementation in the promotion of pressure ulcer healing. In 12 studies, protein supplementation was found to be helpful in the reduction in ulcer size [37]. However, due to the lack of appropriate comparison trials, it is not clear if there is a specific protein supplementation regimen which is most beneficial.
Vitamin C and zinc supplementation have been studied. There is insufficient data to recommend zinc at this time [30]. Similarly, vitamin C in a study of 500 mg twice daily and 10 mg twice daily over 12 weeks did not show improvement in wound closure rates or mean change in ulcer size [41]. A study from 1974 showed that vitamin C 500 mg twice daily did reduce the size of pressure ulcers compared to placebo [42]. There may be a role for vitamin C supplementation, but at present unambiguous evidence for benefit is lacking [44].
Local wound care
Cleansing the wound
The wound should be cleaned with normal saline, sterile water or wound cleanser. Superficial Pseudomonas infection can often be managed by cleaning the wound with diluted acetic acid. Acetic acid solutions of 0.5–1% are antimicrostatic especially to Pseudomonas and other Gram-negative bacteria [43].
Debridement
Necrotic tissues (dead cells and debris) along with slough (fibrin, cells and proteinaceous material) can form on the surface of wounds, promoting the growth of bacteria and affecting the moisture balance. These in turn lead to slower and/or delayed healing of the wound. Through debridement, this material is removed decreasing the microbes and toxins. There are five main types of debridement.
- Surgical/sharp: this is the quickest way to remove the dead tissue. It may cause pain and bleeding; however, this method can encourage a stalled wound to begin healing as a reaction to the now acute wound.
- Autolytic: endogenous enzymes will eventually break down necrotic tissues. It is painless but takes a significant amount of time. Occlusive dressings and hydrogels can help in the process.
- Enzymatic: enzymatic treatments can be applied to debride wounds. The available enzymes include bacterial collagens, papain/urea, fibrinolysin/DNAase, trypsin, a streptokinase/streptodornase combination, and subtilisin.
- Mechanical: physical methods can be used to remove the necrotic tissue and debris from a wound. Employing wet-to-dry dressings is the most common method. It involves applying a saline-soaked gauze to the wound, allowing it to dry, and then removing it and pulling off the dead tissue. Other mechanical methods include pressurized irrigation, whirlpool therapy, ultrasound and negative pressure wound therapy (NPWT). However, these methods can cause damage to the healthy granulation tissue and are often painful for the patient.
- Biosurgery: sterilized fly maggots can be applied to the wounds as a means of debridement; however, this method is not commonly used [23].
Dressings
The dressing should be chosen to optimize moisture at the wound bed, to prevent damage to the surrounding skin and to treat superficial infection when present. There are no specific recommendations or evidence for selecting a dressing type [40]. If packing is required, the dressing must be of sufficient strength to be removed intact, preventing strands being lost in the ulcer or parts of the dressing being retained in the ulcer [41]. If the pressure ulcer is considered non-healable or a maintenance wound, it is appropriate to use antiseptic agents. There is some controversy regarding agents such as poviodine-iodine; however, evidence from non-randomized controlled trials has supported their use for these specific types of ulcers (Table 124.5) [44]. It is unknown if biological agents such as platelet-derived growth factor are cost-effective compared to standard wound care. [45]
Table 124.5 Classes of dressing and their use in pressure ulcers.
| Dressing type | Uses in pressure ulcers |
| Films |
|
| Hydrogels |
|
| Hydrocolloids |
|
| Calcium alginates |
|
| Foams |
|
| Hydrofibres |
|
| Cadexomer iodine |
|
| Gauze |
|
Infection
When a wound is not healing, infection should be suspected. The diagnosis of infection in a chronic wound can be challenging, as the classic signs of infection may not be present. The diagnosis relies on symptoms/signs and information from the wound swab. Other considerations include underlying co-morbidities. Patients with diabetes have a 10-fold increased risk of being hospitalized for soft-tissue infection compared to persons without diabetes [47].
High-quality studies evaluating the quantitative swab using the Levine method (i.e. rotating the swab over 1 cm2 over 5 s applying sufficient pressure to cause fluid to be released from the wound) compared to the standard of a deep tissue biopsy was found to be useful in predicting wound infection (likelihood ratio (LR) 6.3; 95% CI, 2.5–15) [48]. A negative swab using this method makes the presence of infection less likely (LR 0.47; 95% CI, 0.31–0.73). The reference standard for infection using a deep tissue biopsy is 105 microbes per gram of wound tissue or any level of β-haemolytic Streptococcus [49]. Deep tissue biopsy is appropriate in some clinical situations; however, it is invasive and not practical for general use. Z-technique swabbing (i.e. the swab is applied over the entire wound area in a zigzag manner) does not predict or exclude infection when compared to deep tissue biopsy (LR 1.3, 95% CI 0.91–2.0) [50].
In evaluating the symptoms often associated with wound infection, including odour, pain, red granulation tissue, exudate, delayed healing, heat, purulent discharge and pocketing; only pain was predictive of infection [51]. Two studies using deep tissue biopsy as the reference showed pain having an LR of 11–20 (95% CI) [51]. The absence of pain, however, is not prognostic as there is still an LR of 0.64–0.88 (95% CI) [51].
In combining symptoms and signs against deep tissue biopsy, the sensitivity and specificity was only 52% and 46%, respectively, with an LR of 0.96 (95% CI 0.60–1.6) [51]. Other studies not using a reference standard and combining at least three of five signs (non-healing, exudate, friable tissue, debris and smell) showed a sensitivity of 73% and specificity of 80% for superficial infection [50]. For deeper infection, combining three of seven signs (increasing size, elevated local temperature, probing to bone, new tissue breakdown, exudate, oedema or odour) resulted in a sensitivity and specificity of 90% and 69%, respectively [52]. The Infectious Disease Society of America defines infected diabetic foot ulcers as those producing purulent discharge or bearing two or more indicators of inflammation (pain, erythema, induration, heat or oedema) [51].
When there is clinical suspicion of infection, a quantitative swab should be done using the Levine method. If pain is increased, it should alert the clinician to consider infection as the cause [51]. However, the absence of pain does not rule out infection and this needs to be especially considered in the case of patients who lack sensation. Diagnosis of infection hence remains challenging and the subject of further investigation.
Adjunctive therapies
Negative pressure wound therapy involves the application of controlled suction to the wound bed via a computerized unit attached to an open-cell foam dressing placed in the wound and held in place by an adhesive clear dressing [52].
Seven trials compared NPWT with either moistened gauze dressings or other topical agents and found no difference in effects [53]. Only one small, low-quality trial (seven wounds) showed a reduction in wound volume and depth in favour of NPWT [55]. There is no valid or reliable evidence that topical negative pressure increases chronic wound healing [55].
Similarly, reviews of trials of other adjuvant therapies (electromagnetic, ultrasound and hyperbaric oxygen treatments) have not shown strong evidence that they help or hinder the healing of pressure ulcers [54–56].
Surgery
Surgical management may be an option for certain patients. The two most common reconstructive procedures are the musculocutaneous and the fasciocutaneous flap. These provide vascularized tissue for closure of deep pressure ulcers [57]. The patient selection criteria are not well defined in the literature; however, it is clear that the patient must be medically well and be able to participate in the rehabilitation programme (Box 124.1) [58].
There is a high recurrence rate with surgery and patients should be carefully screened.
Are pressure ulcers avoidable?
Despite studies and efforts to reduce pressure ulcers, the incidence and prevalence of pressure injury and ulcers have not changed dramatically over the years [17]. While the consensus is that pressure ulcers are largely preventable, there may be individuals for whom pressure ulcers are unavoidable [59]. There are few high-qualitystudies that evaluate pressure ulcer development in patients with advanced disease [60]. Two consensus documents concluded that in cases of critical illness in which nutrition/hydration and pressure redistribution cannot be provided, pressure ulcers may be unavoidable [61, 62]. The potential unavoidability of pressure ulcers can only be determined after preventative care has been fully implemented.
It is also clear that skin failure in the palliative care setting is a separate entity from pressure ulcers. The Kennedy terminal ulcer illustrates this concept. This pear or butterfly shaped ulcer usually at the sacrum develops quickly and frequently indicates death is imminent [63]. Skin changes at the end of life can develop despite optimum care [64]. With advanced illness, physiological changes occur in the skin as part of multiorgan failure. The prevalence of pressure ulcers in the palliative care setting is 11–18% [18]. The goal for both unavoidable and end-of-life skin changes is to provide these fragile patients comfort measures specific to their needs.
References
- National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: clinical practice guidelines. Washington DC, USA: National Pressure Ulcer Advisory Panel, 2009 http://www.npuap.org (last accessed August 2014).
- Oomens CWJ, Loerakker S, Bader DL. The importance of internal strain as opposed to interface pressure in the prevention of pressure related deep tissue injury. J Tissue Viability 2010;19:35–42.
- Amlung SR, Miller WL, Bosley LM. The 1999 National Pressure Ulcer Prevalence Survey: a benchmarking approach. Adv Skin and Wound Care 2001;14(6):297–301.
- Whittington K, Patrick M, Roberts JL. A national study of pressure ulcer prevalence and incidence in acute care hospitals. JWOCN 2000;27:209–15.
- Grey J, Harding K, Enoch S. Pressure ulcers. BMJ. 2006;332:472–5.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA 2006;296(8):974–84.
- Smith PW, Black JM, Black SB. Infected pressure ulcers in the long-term-care facility. Infect Control Hosp Epidemiol 1999;20(5):358–61.
- Staas Jr WE, Cioschi HM. Pressure sores – a multifaceted approach to prevention and treatment. West J Med 1991;154:539–44.
- Van Gilder C, Amlung S, Harrison P, Meyer S. Results of the 2008–2009 International Pressure Ulcer Prevalence Survey and a 3-year, acute care, unit specific analysis. Ostomy Wound Manag 2009;55(11):39–45.
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract 2007;13(2):227–35.
- Cuddigan J, Ayello EA, Sussman C, eds. Pressure Ulcers in America. Prevalence, incidence, and implications for the future. Reston, VA: National Pressure Ulcer Advisory Panel, 2001.
- Coleman EA, Martau JM, Lin MK, et al. Pressure ulcer prevalence in long-term nursing home residents since the implementation of OBRA ‘87. Omnibus Budget Reconciliation Act. J Am Geriatr Soc 2002;50:728–32.
- Bennett G, Dealey C, Posnett. The cost of pressure ulcers in the UK. Age and Ageing 2004;33(3):230–5.
- Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nursing Studies 2013;50:974–1003 doi:10.1016/j.ijnurstu.2012.11.019 epub 2013 Feb 1.
- Takahashi M, Black J, Dealey C, Gefen A. Pressure in context. In: International Review: pressure ulcer prevention; pressure, shear, friction and microclimate. A consensus document. London: Wounds International, 2010.
- Reger SI, Ranganathan VK, Orsted HL, Ohura T, Gefen A. Shear and friction in context. In: International Review: pressure ulcer prevention: pressure, shear, friction and microclimate. A consensus document. London: Wounds International, 2010.
- Dealey C, Brindle CT, Black J, Alves P, Santamaria N, Call E, Clark M. Challenges in pressure ulcer prevention. Int Wound J 2013; Jun 20 doi:10.1111/iwj.12107 (epub ahead of print).
- Thomas DR. Does pressure cause pressure ulcers? An inquiry into the etiology of pressure ulcers. J Am Med Dir Assoc 2010;11:397–405.
- Defloor T, Schoohoven L, Fletcher J, et al. Statement of the European Pressure Ulcer Advisory Panel – Pressure Ulcer Classification. JWOCN 2005;32(5):302–6.
- Witkowski JA, Parish LC. Histopathology of the decubitus ulcer. J Am Acad Dermatol 1982;6:1014–21.
- Seiler WO, Stahelin HB. Recent findings on decubitus ulcer pathology: implications for care. Geriatrics 1986;41:47–60.
- Vande Berg JS, Rudolph R. Pressure (decubitus) ulcer: variation in histopathology – a light and electron microscope study. Hum Pathol 1995;26:195–200.
- Enoch S, Harding K. Wound bed preparation: the science behind the removal of the barriers to healing. Wounds. 2003;15(7):213–29.
- Briggs M, Collinson M, Wilson L, et al. The prevalence of pain at pressure area and pressure ulcers in hospitalized patients. BMC Nursing 2013;12:19.
- Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54(12):e132–73.
- Fairbairn NG, Hamilton SA. Management of Marjolin's ulcer in a chronic pressure sore secondary to paraplegia: a radical surgical solution. Int Wound J 2011;8:533–6.
- Mustoe T, Upton J, Marcellino V, Tun CJ, Rossier AB, Hachend HJ. Carcinoma in chronic pressure sores: a fulminant disease process. Plast Reconstr Surg 1986;77:116–21.
- Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs 2006;54(1):94–110.
- Smith MB, Totten A, Hickam DH, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Ann Intern Med 2013;159:39–50.
- Moore ZEH, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev 2014;Issue 2:CD006471, doi:10.1002/14651858.CD006471.pub3.
- Junkin J, Gray M. Are pressure redistribution surfaces or heel protection devices effective for preventing heel pressure ulcers? JWOCN 2009;36:602–8.
- Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device related pressure ulcers in hospitalized patients. Int Wound J 2010;7:358–65.
- Chou R, Dana T, Bougatsos C, et al. Pressure ulcer risk assessment and prevention. Ann Intern Med 2013;159:28–38.
- Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age Ageing 2005;34: 261–7.
- McInnes E, Dumville JC, Jammali-Blasi A, Bell-Syer SEM. Support surfaces for treating pressure ulcers. Cochrane Database Syst Rev 2011;Issue 12;Art. No.: CD009490 doi: 10.1002/14651858.CD009490.
- Bourdel-Marchasson I, Barateau M, Rondeau V, et al. A multi-center trial of the effects of oral nutritional supplementation in critically ill older inpatients. GAGE Group. Groupe Aquitain Geriatrique d'Evaluation. Nutrition 2000;16:1–5.
- Smith MEB, Totten A, Hickam DH, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Ann Intern Med 2013;159:39–50.
- Reddy M. Pressure ulcers. Clin Evidence 2011;5:1901.
- Reddy M, Gill S, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300(22):2647–62.
- Sibbald RG, Goodman L, Woo KY, et al. Special considerations in wound bed preparation 2011: an update. Adv Skin Wound Care 2011;24:415–36.
- ter Riet G, Kessels AG, Knipschild PG. Randomized clinical trial of ascorbic acid in the treatment of pressure ulcers. J Clin Epidemiol 1995;48(12):1453–60.
- Taylor TV, Rimmer S, Day B, Butcher J, Dymock IW. Ascorbic acid supplementation in the treatment of pressure sores. Lancet 1974;2(7880):544–6.
- Dolynchuk K, Keast D, Campbell K, Houghton P. Best practices for the prevention and treatment of pressure ulcers. Ostomy Wound Manag 2000;46(11):38–52.
- Banwell H. What is the evidence for tissue regeneration impairment wen using a formulation of PVP-1 antiseptic on open wounds? Dermatology 2006;212(Suppl. 1):66–76.
- Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo controlled study. Wound Repair Regen 1999;7(3):141–7.
- Sayag J, Meaume S, Bohbot S. Healing properties of calcium alginate dressings. J Wound Care 1996;5(8)357–62.
- Boykoe EJ, Lipsky BA. Infection and diabetes mellitus. In: Harris MI, ed. Diabetes in America, 2nd edn. Bethesda, MD: National Institutes of Health, 1995: 485–96.
- Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs 2009;11(2):119–28.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA 2012;307(6):605–11.
- Woo KY, Sibbald RG. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manag 2009; 55(8):40–8.
- Lipsky BA, Berendt AR, Deery HG, et al. Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39(7):885–910.
- Gupta S, Baharestani M, Baranoski S, et al. Guidelines for managing pressure ulcers with negative pressure wound therapy. Adv Skin Wound Care 2004;Nov–Dec;17(Suppl. 2):1–16.
- Ubbink DT, Westerbos SJ, Evans D, Land L, Vermeulen H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev 2008;Issue 3:CD001898, doi: 10.1002/14651858.CD001898.pub2.
- Baba-Akbari Sari A, Flemming K, Cullum NA, Wollina U. Therapeutic ultrasound for pressure ulcers. Cochrane Database Syst Rev 2000;Issue 4:CD001275, doi: 10.1002/14651858.CD001275.
- Aziz Z, Flemming K. Electromagnetic therapy for treating pressure ulcers. Cochrane Database Syst Rev 2012;Issue 12:CD002930, doi: 10.1002/14651858.CD002930.pub5.
- Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2012;Issue 4:CD004123, doi: 10.1002/14651858.CD004123.pub3.
- Levine SM, Sinno S, Levine J P, Saadeh PB. An evidence-based approach to the surgical management of pressure ulcers. Ann Plast Surg 2012;69:482–4.
- Lefemine V, Enoch S, Boyce DE. Surgical and reconstructive management of pressure ulcer. Eur J Plast Surg 2009;32:63–75.
- Black JM, Edsberg LE, Baharestani MM, et al. Pressure ulcers: avoidable and or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manag 2011;52:24–37.
- White-Chu E, Reddy M. Pressure ulcer prevention in patients with advanced illness. Curr Opin Support Palliat Care 2013;7:111–15.
- Black JM, Edsberg LE, Baharestani MM, et al. National Pressure Ulcer Advisory Panel. Pressure ulcers: avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manag 2011;52:24–37.
- Sibbald RG, Krasner DL, Lutz JB, et al. The SCALE Expert Panel: skin changes at life's end. Final Consensus Document. October 1, 2009.
- Kennedy KL. The prevalence of pressure ulcers in an intermediate care facility. Decubitus 1989;2(2):44–5.