CHAPTER 128
Allergic Contact Dermatitis
Mark Wilkinson1 and David Orton2
1Leeds Teaching Hospitals NHS Trust, Leeds, UK
2Hillingdon Hospitals NHS Foundation Trust, Uxbridge; and Royal Free London NHS Foundation Trust, London, UK
History
The term ‘allergie’ was first coined by the scientist von Pirquet in 1906 [1, 2]. The word was derived from the Greek allos and ergon, meaning other or different work [3]. However, idiosyncratic reactions to various substances had been recognized since the 17th century [2]. In 1829, Dakin observed the selectivity of Rhus dermatitis [4] and Fuchs suggested that ‘dermatitis venenata’ was an expression of constitutional idiosyncrasy in 1840 [5]. The word ‘idiosyncrasy’ was again applied by Neisser in his descriptions of iodoform dermatitis in 1884 [6].
Allergic sensitization of the skin was first proved experimentally by Bloch and Steiner-Woerlich using Primula extract on humans [7]. Thereafter, research on the pathogenesis of allergic dermatitis has largely involved animal experiments using guinea pigs. Landsteiner and Jacobs [8] performed a basic experiment that showed that a simple chemical capable of causing contact dermatitis must be combined with proteins in order to sensitize. Up to 1940 it was not known whether sensitization depended on a factor localized in the skin, but in 1942 Landsteiner and Chase [9] succeeded in transmitting sensitivity from one guinea pig to another by the use of a mainly mononuclear peritoneal exudate from sensitized guinea pigs. In the same year, Haxthausen's transplantation experiments [10] finally proved that allergy was due to a factor supplied to the skin from within.
Patch testing is the diagnostic tool for allergic dermatitis and it is Josef Jadassohn who is generally accepted as the founder of this technique in 1895 while working at Breslau University, publication taking place the following year [11]. Nevertheless, anecdotal observations of a similar nature had been made prior to this, usually by applying the suspected causative agent to intact skin [12]. By 1847 Stadeler had developed a rudimentary patch test using blotting paper to reproduce lesions provoked by Anacardium occidentale [13].
Bruno Bloch was a dermatological pioneer who was able to expand and enhance Jadassohn's technique while working in Basel in 1911, when he also produced a grading system for patch test reactions [14]. He then moved to Zurich where he introduced the concept of a standard series of allergens [15]. He furthermore conceived important ideas about both cross-sensitization and systemic allergic contact dermatitis [1]. Marion Sulzberger had been an assistant to both Bloch and Jadassohn before returning to New York where he introduced the patch test technique and was a strong advocate and promoter of its use in the New World. Another former assistant of Bloch's, Paul Bonnevie, Professor of Occupational Medicine in Copenhagen, expanded the standard series to what could be considered the prototype of our present-day series.
By the early 1960s, Scandinavian dermatologists were developing a standardized protocol for patch testing and their group was expanded to involve, initially, other European members before it finally evolved into the International Contact Dermatitis Research Group (ICDRG) [1]. Further national and international research groups have proliferated in the last 20 years, a fitting recognition of the significance of the findings and researches of these earlier pioneers.
Definition and nomenclature
Allergic contact dermatitis is an eczematous reaction that occurs as an immunological response following exposure to a substance to which the immune system has previously been sensitized.
Introduction and general description
Contact allergy is caused by skin contact with low molecular weight haptens and may evolve into allergic contact dermatitis if exposure exceeds the individual threshold. A substantial number of studies have investigated the prevalence of contact allergy in the general population and in unselected subgroups of the general population. A review of published research mainly originating from North America and western Europe in the general population identified that the median prevalence of contact allergy to at least one allergen was 21.2% (range 12.5–40.6%), and the weighted average prevalence was 19.5%, based on data collected on all age groups and all countries between 1966 and 2007. The overall prevalence estimates do not seem to depend much on age, race or geographical origin of the study group. The most prevalent contact allergens were nickel, thimerosal and fragrance mix. In addition, cobalt, chromium, p-phenylenediamine (PPD) and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI) were prevalent allergens in several studies.
Most other epidemiological studies have been based on patients already attending dermatology clinics, or have involved either specific occupational or other population groups such as those with atopic eczema, which can of course skew the data and are discussed more fully in the chapter. Niels Hjorth and Siegfried Fregert – two eminent contact dermatitis investigators – are quoted as saying that ‘in a particular clinic the incidence of allergic contact dermatitis is determined by the interest the dermatologist takes in allergic contact dermatitis’.
The diagnosis of allergic contact dermatitis can only be confirmed by patch testing and should always be used to exclude contact allergy as a complicating factor in stubborn cases of eczematous diseases, as well as cases where allergic contact dermatitis is suspected from the pattern or distribution of the eczema. It is particularly important in chronic cases of dermatitis that are unresponsive to traditional treatments. Nevertheless, one of the UK's most well-respected dermatologists and proponent of patch testing, Etain Cronin, sums up the problem succinctly: ‘Ideally every patient with eczema should be patch tested and the importance of this investigation is now universally accepted. The simplicity of the technique belies its many pitfalls, the greatest being to lack the knowledge required to select the correct allergens and to interpret the results’ [1]. Calnan wrote in 1982 that ‘the greatest abuse of patch testing is failure to use it and that a number of dermatologists have never patch tested a patient’ [2]. Equally or more serious than this may be to patch test incorrectly and thus provide erroneous conclusions [3].
Patch testing, which is actually a bioassay, remains, at present, the only practical scientific procedure for the diagnosis of allergic contact dermatitis. During the last few decades much effort has been put into the standardization of allergens, vehicles, concentrations, tapes and the scoring of test reactions. Despite this, both the investigator's skill and experience are crucial factors in providing accurate information to patients. Kligman argued that ‘Anyone can do a patch test. Few do it well. Fewer still can properly interpret patch tests’ [4]. Some authors argue that patch testing should only ever be done by dermatologists [5].
Despite all these potential difficulties, it is imperative to collect patch test data over time and across many centres, including in different countries. Constant surveillance allows for trends to be determined and for epidemics to be recognized, which in turn have shaped the response of regulatory authorities – for example, the epidemics of biocide contact dermatitis caused by methyl dibromoglutaronitrile and methylisothiazolinone resulting in the withdrawal and the recommendation of withdrawal, respectively, of such chemicals from cosmetics.
Allergens exist in the home environment and the occupational setting. Prevention strategies include primary, secondary and tertiary prevention. In primary prevention the focus is on minimizing the risk of inducing sensitization in workers and consumers. At the workplace, primary prevention includes pre-employment screening, minimizing contact between allergens and the skin, and education to employees in at-risk occupations. These strategies have all been reported as effective.
The quantitative risk assessment (QRA) procedure currently developed by the cosmetic industry for fragrances (primary prevention) has a major flaw as it is not able to predict the elicitation risk of chemicals. Hence the ‘acceptable exposure level’ does not protect those already sensitized. About a third of all allergies against cosmetic products are caused by fragrance allergies.
However, in recent years Europe has successfully implemented a whole set of regulations aimed at reducing the exposure of the workforce and consumers to contact allergens. Examples are the ‘Nickel Directive’, limiting the release of nickel in contact with skin to 0.5 μg/cm2 per week [6], and the ‘Chromium Directive’, limiting chromium (CrVI) to 2 ppm in the total dry weight of cement [7]. The directive on detergents requires the listing of preservatives and certain fragrances if their content in detergents and similar household products exceeds 100 ppm [8]. Detergents are thus treated as rinse-off cosmetics. Furthermore, details of the product formulation have to be released when necessary, such as to investigate adverse reactions. As a result nickel allergy among young patients showed a decline in several countries such as Germany [9], Sweden [10] and Denmark [11]. In Denmark, the frequency of nickel allergies dropped from 26.9% before the European Union (EU) directive to 12.4% thereafter [11].
Contact dermatitis against CrVI has been a recognized problem in the occupational setting, with a prevalence of, for example, 17% in cement workers during the construction of the Channel tunnel connecting continental Europe with Britain [12]. The EU therefore regulated the content of CrVI in cement in 2005 and sensitization to chromate in construction workers has since declined [13, 14]. However, this regulation does not include leather products such as shoes, where an increasing incidence has been recognized [15].
Product labelling may be one method of handling this problem but since a part of the sensitized population might not be diagnosed, and the main part of those who know they are sensitized find it difficult to read the labelling and identify the allergens to which they are allergic, this way of preventing allergic contact dermatitis may not be efficient.
Secondary and tertiary prevention aims at reducing the risk of elicitation and the morbidity amongst those with dermatitis.
Although it is not the remit in this chapter to go into very much detail, several in vivo systems have been established that are able to identify potential allergens reliably and to assess their potency. At the same time in vitro tests are being developed because of public demand to replace in vivo tests, animal welfare and costs. Their regulatory acceptance of these tests will depend on thorough validation, not only against other methods but also against human observational data from clinical epidemiological surveillance systems. Such validation is the gold standard for any predictive safety assessment.
In this context clinical data and the epicutaneous patch test, as described by Jadassohn over 100 years ago [16], remain invaluable, as they continue to highlight substances and problems missed by other approaches.
Epidemiology [1]
Incidence and prevalence
Definition of these terms is important as they mean two different things. Prevalence relates to the number or proportion of individuals who are identified with the condition being studied (e.g. contact dermatitis, nickel allergy) at a given point in time, or over a certain period of time. Incidence relates to the number of new cases developing over a defined period of time and is expressed as number of cases per unit of time. Knowledge of the sample size is also essential to give an indication of the reliability of the data.
Methodologies
Epidemiological studies may be undertaken on the general population or on selected groups, for example those referred for patch testing or those with a specific occupation.
Follow-up studies select individuals on the basis of the presence or absence of a defined risk factor such as ‘wet work’. The relative risk of developing hand dermatitis can then be calculated as the ratio of those developing dermatitis in the exposed population compared with the unexposed population. The attributable risk is the difference in incidence rates between the two populations.
Case–control studies select individuals based on the presence or absence of a particular disease. By comparing the frequency of exposure to a factor such as ‘wet work’ in the two populations, an odds ratio can be calculated that expresses the relative contribution of the exposure to the development of the disease. In such studies the choice of controls is critical if the results are not to be biased.
In cross-sectional studies, all individuals are studied irrespective of exposure or disease status (in contrast with the above).
Data collection. The method of data collection in studies on the general population, which need to be large to gain useful information and are challenging to perform, can significantly influence the results. For reasons of expediency, questionnaires have been used, but when performed alone will underestimate those suffering from dermatitis because accuracy of recall fades with time. The validity of the results also depends on the extent to which those who respond to the questionnaire differ from those who do not. As a rough guide, studies in which the response rate is below 70% may be unrepresentative.
Population assessments made on individuals attending a general practitioner or referred to a dermatologist may be unreliable, particularly in the UK where prompt access to a dermatologist is achieved only by the fortunate few. In a UK survey, only 21% of those with skin disease thought to justify medical care had seen their general practitioner about it in the previous 6 months [2]. In another large-scale study of a Swedish population of over 107 000, only 50% of the patients with dermatitis had seen a doctor within the previous year [3].
The reporting of contact dermatitis also varies according to the method of collection and the type of person collecting the data. Results from the UK EPIDERM occupational dermatoses surveillance study show how reports of occupational dermatoses differ according to whether the returns are made by dermatologists or by occupational health physicians (Table 128.1) [4]. The differences probably reflect the different types of occupational population accessed by the two groups. Occupational physicians will relate to large industries and collective working groups, whereas dermatologists will mainly receive individual referrals, accounting for the comparatively high representation of, for example, hairdressers, florists and beauticians seen by them.
Table 128.1 Occupational skin disease: estimated rate per 100000 workers as reported by dermatologists and occupational doctors to EPIDERM (Occupational Dermatoses Surveillance Scheme, University of Manchester).
| Reporting group | Rate per 100000 |
Dermatologists Hairdressers and barbers |
116.3 |
| Printers | 85.8 |
| Beauticians | 76.8 |
| Other chemical operatives | 69.1 |
| Window dressers, floral arrangers | 68.1 |
| Occupational physicians | |
| Other chemical operatives | 183.8 |
| Glass product and ceramic makers | 101.2 |
| Vehicle and metal assemblers | 94.8 |
| Engineering labourers | 82.4 |
| Machine tool operatives | 67.9 |
Case definition. Studies of the epidemiology of dermatitis may be further confounded by the fact that it is commonly multifactorial in origin. It is therefore difficult to analyse the relative prevalence of irritant versus allergic contact dermatitis because the two commonly coexist, and constitutional eczema may also be involved. Ideally, all those studied should be examined and patch tested, but this is not always a practical proposition when large numbers of an unselected group are being assessed.
Standardization Apparent differences in overall sensitization frequencies may be due to differences in population structure, especially in relation to age and sex. This can be compensated for, either by using standardized populations or by reporting results within specified age bands, and by reporting results for each sex separately.
However, in a particular clinic the incidence of allergic contact dermatitis is reflected not only by the sex and age of the patients but also by the industrial development in the area and the degree of interest dermatologists take in the various facets of contact dermatitis (e.g. occupational dermatitis, medicament allergy, leg ulcers). Furthermore, local prescribing habits can influence patch test results. It has been suggested that all comparative patch test data should include an analysis of patient details, the MOAHL index (where M is the percentage of males tested, O is percentage occupational, A is percentage of atopics, H is percentage of patients with hand eczema, and L is percentage of patients with leg ulcers or stasis eczema). The percentage of atopics is important, particularly in relation to irritant contact dermatitis. Certain body sites, especially the lower legs in those with stasis eczema or leg ulcers and the ears, eyelids and perineum, have a particularly high level of allergic contact dermatitis from medicaments. Inclusion of a significant number of any such cases in a patch test series will affect the overall sensitivity rates for various allergens. Further enhancement to the index has been suggested by adding F, the proportion of those with facial dermatitis, and A, those above the age of 40, to generate the MOAHLFA index [5]. Guidelines for the presentation of contact allergy data have been produced [6].
General population studies
Contact dermatitis accounts for 4–7% of all dermatological consultations [1]. Skin disease, chiefly dermatitis, accounts for almost half of all reported cases of occupational disease. Over 20% of females will suffer from hand eczema at some stage in their lives. In one population study in southern Sweden, hand eczema was shown to affect 11.8% of the population aged 20–65 years over a 12-month period. A recent follow-up study indicates that this frequency has dropped to 9.8% despite a rise in the level of childhood atopic eczema [7].
A number of other studies on the prevalence of contact dermatitis in the unselected general population have been undertaken, but those that include clinical assessments and patch tests are rare, making it more difficult to ascertain the prevalence of allergic contact dermatitis. A study of 1500 children aged 12–16 years in Denmark, which involved an initial questionnaire followed by interview, clinical examination and patch testing, found a lifetime prevalence of hand dermatitis of 9.2%, with a 1-year prevalence of 7.3% and point prevalence of 3.2%. Patch testing showed a point prevalence of contact sensitivity of 15.2%. There was a clear sex difference, with 19.4% of girls and 10.3% of boys being patch test positive. Following interview and examination, a lifetime prevalence of allergic contact dermatitis of 7.2% and point prevalence of 0.7% was demonstrated. Of the girls, 11.3% gave a history of relevant contact allergy, compared with 2.5% of boys. Allergens responsible included nickel 8.6%, fragrance 1.8%, colophony 1% and para-tertiary butyl phenol (PTBP) formaldehyde resin 0.9%. The study showed a significant association between contact allergy and hand eczema but no relation between contact allergy and either atopic eczema or inhalant allergy [8]. A smaller study in adults, with participation rates of 69% in 1990 and 51% in 1998, showed a point prevalence of contact sensitivity of 15.9% in 1990 rising to 18.6% in 1998. Risk factors for nickel allergy included female sex, young age and ear piercing (before 1990). With the introduction of controls on nickel release from jewellery, the association of ear piercing with nickel allergy was lost [9]. A recent Norwegian study with a response rate of 60%, that included 1236 adults patch tested to the TRUE® (thin layer, rapid use, epicutaneous) test, found contact sensitivity in 35.4% of women and 14.8% of men. Positive allergens included nickel 17.6%, cobalt 2.8%, thiomersal 1.9%, fragrance mix 1.8% and colophony 1.2%. Smoking and atopic eczema were thought to be risk factors [10].
Selected population studies
Most other epidemiological studies have been based on patients already attending dermatology clinics, or have involved either specific occupational or other population groups. The selective nature of patients patch tested in dermatological clinics for the investigation of contact allergy is not necessarily representative of the general population. Nevertheless, the findings may reflect the relative frequency of the causes of allergic contact dermatitis in that population. Patch testing can be used to generate information on individuals, groups of patients and allergens, and also to assess risk factors in groups of workers and particular subgroups of the population.
Dermatology patients
The percentage of patients with positive reactions to many standard test substances remains largely constant [11], and although some allergens such as colophony, thiuram mix and nickel in women have become less common, this has been balanced by an increase in other sensitizers such as Myroxylon pereirae and paraphenylenediamine. The prevalence of allergy to specific allergens in patch-tested patients is discussed later in the chapter. It should be remembered that the presence of sensitization does not imply the presence of dermatitis. In addition, variations in the reading or interpretation of patch test results will affect the perceived prevalence of allergic contact dermatitis.
In general, the commoner allergens are similar from one country to another, although there are differences in rank order [12, 13, 14, 15]. Some environmental allergens are widely dispersed and the level of sensitivity remains fairly constant, but cosmetics and fragrance materials are becoming increasingly important sources of sensitivity. Medicament allergens, such as benzocaine, neomycin and lanolin, are common in all countries. However, there may be differences in prescribing habits even within the same country, which can be reflected by the pattern of medicament sensitization. Corticosteroid allergy has been shown to have a very different profile in Oxford compared with Manchester, UK, by virtue of differences in prescribing habits leading to a greater usage of non-fluorinated corticosteroids in the latter catchment [16].
Young females tend to have more cosmetic and occupational sensitivities. In older people, many sensitivities will be of past relevance only, and there will be a higher prevalence of medicament sensitivity. Nickel sensitivity is common in women and, unless allowance is made for this, false occupational associations may be inferred. Allergens can come and go [17], and the prevalence of sensitivity to an individual substance will depend on many variables, including the selection of individuals to be tested, exposure levels, fashion, environment, introduction of new materials and loss of others, maximum permitted concentrations and usage.
The incidence and prevalence of allergic reactions will therefore parallel the extent of such exposure, and occasionally this may lead to localized ‘epidemics’ of sensitivity to a particular allergen. Cosmetic and preservative exposure varies from country to country and from region to region, according to the degree of usage. This principle may extend to other allergenic sources, so there is a rationale for each centre and country developing its own epidemiological base.
Patterns also change with fashion, as shown by the virtual disappearance of suspender dermatitis from nickel, which was replaced by an increase in dermatitis from earrings, watches and jeans studs. The sensitizers found vary with the patients' social backgrounds, and these may change over the years.
Differences in environmental exposures influence the nature of sensitizers; for instance Toxicodendron species dermatitis is extremely common in the USA but virtually absent in Europe, whereas Primula dermatitis was well recognized in the UK but practically non-existent in many other countries. The introduction of new potential sensitizers, such as methylisothiazolinone will increase the incidence of contact dermatitis due to them in the exposed population [18]; at the same time, allergens that were previously common may disappear.
Technological advances have led to new and more widespread exposures to allergens, such as epoxy and acrylic resins in the occupational setting [19], although the potential for contact allergy may be reduced by improved personal protective equipment, better containment of sensitizing chemicals and allergen substitution. Similarly, in the domestic environment, phosphorus sesquisulphide allergy in the UK has disappeared because the production of ‘strike anywhere’ matches has diminished in this country.
Occupational studies
The incidence of occupational dermatitis in most western European countries is in the range of 0.5–1.9 cases per 1000 workers per year; skin diseases account for 13–34% of all occupational diseases and contact dermatitis constitutes 90–95% of this [1]. Risk reflects both constitutional susceptibility (atopy) and exposure. Skin disease is a significant occupational problem, accounting for 5.73 claims per 100000 workers, with 47% having job tenure of less than 1 year. The average disability time in this American study was 23.9 days at a cost of $3552 [20]. Twenty-two per cent of occupational skin disease may be attributable to atopy [21].
Occupational disease surveillance and compensation registries identify occupations at high risk of dermatitis (see Table 128.1). Most are unable to distinguish between irritant and allergic dermatitis. Some countries have mandatory reporting. In the UK, EPIDERM is a scheme accepting reports made on a voluntary basis from dermatologists and occupational physicians [4]. In a recent study covering the years 1993–99, 52% of dermatitis cases reported by dermatologists and 30% of those reported by occupational physicians had allergic contact dermatitis as the primary cause or as a contributory factor [19]. The higher rate reported by dermatologists might be a reflection of their more frequent use of patch testing. The commonest allergens were rubber chemicals (including those in gloves), nickel and resins. The numbers and proportion of cases of contact dermatitis within occupations remained fairly constant over the 6-year reporting period, although nursing personnel showed an increase, perhaps as a result of increased exposure to agents required to reduce infectious disease transmission. In northern Bavaria there is a mandatory reporting and follow-up investigation scheme [22]. In a survey of occupations at higher risk of dermatitis, positive patch tests of occupational and clinical relevance occurred in 52% of those with occupational skin disease, including 73% of construction workers, and 72% of hairdressers and barbers, but only 20% of food industry workers.
The pattern of employment has a significant effect on the incidence of skin disease, but most common allergens are widely dispersed and, except within small occupational groups, the pattern of sensitivity in a population mainly reflects environmental rather than occupational allergens. Chromate, however, remains a predominantly occupational allergen, the incidence of sensitivity in the normal population being low.
Age
Age has little influence on capacity for sensitization [1]. Children are sensitized as easily as adults, and both infants and elderly people can be sensitized to poison ivy (Toxicodendron spp.). Toxicodendron dermatitis is very common in American children [2]. This suggests that the paucity of other types of contact dermatitis may be due to the simpler environment of childhood and, being younger, they have had less time to develop sensitivities. Susceptibility to sensitization with dinitrochlorobenzene (DNCB) declines after the age of 70 years but is otherwise constant, and sensitivities may fade with time [3]. However, the number of positive patch test reactions tends to increase with age [4], due to the accumulation of allergies acquired over a lifetime. Young adults are more likely to have occupational or cosmetic allergies, whereas elderly people are more liable to medicament [5] and ‘historic’ sensitivities. Age is an important factor in any patch test study [6].
Contact dermatitis in children seems to be increasing, either because a child's environment is now more complex or dermatologists have been underestimating the frequency of childhood allergic contact dermatitis, possibly because of their reluctance to patch test younger children. There have now been several series of results of patch testing in children, summarized by Goossens and Morren [7]. The increased prevalence of sensitivity in children has been associated with increased exposure to nickel-containing objects and an earlier age of ear piercing [8]. The commonest allergens are nickel (especially in girls), fragrance, thimerosal, medicaments, rubber chemicals, chromate and resins in footwear [9]. The relevance of the unexpectedly high number of reactions to thimerosal remains obscure, but the increased level of reactivity to it has been blamed on vaccines and inoculations [10]. The use of PPD-contaminated henna tattoos on children is resulting in increasing numbers of cases of contact allergy from this source [11].
Small children pose practical problems with patch testing. There is a limited area to which a series of patch tests can be applied and they may become restless once the tests are applied, creating problems with adhesion. It is advised that more than one session of patch tests should be undertaken if necessary and a stronger adhesive used to keep the test units in place [12]. It has also been suggested that children are more susceptible to irritant patch test reactions than adults [13]. This is not our experience, except for nickel and cobalt, and although positive patch test reactions are less common than in adults, most reactions appear to be relevant apart from thimerosal. The use of lower concentrations for certain allergens has been suggested, but most published reports have advocated no change.
Attempts have been made to identify clinical patterns to indicate which children should be patch tested, but recent studies suggest that any child with persistent eczema should be considered for patch testing [14, 15]. Although an abbreviated standard series based on previous published results has been suggested for children [16], we endeavour to perform a full adult standard series, plus relevant extra tests whenever possible.
Sex
Women have stronger cell-mediated immune responses than men [1, 2] and yet, at least experimentally, women do not appear to be more susceptible to sensitization [3]. However, sensitization is accomplished more easily with some allergens, for example lanolin, fragrance and PPD, perhaps as a result of prior ‘conditioning’ exposure and subclinical sensitization [4]. In one study, women were found to have greater reactivity to DNCB than men [5] whereas, in another, men were more susceptible to DNCB sensitization than women [6]. The reason for the female preponderance in clinical patch test studies is mainly explained by exposure [1] – for instance the large number of metal-sensitive women, largely the result of ear piercing [7] and the greater exposure to fragrances, cosmetics and hair dyes. Multiple allergies are found most frequently in elderly women [8]. It is of interest that nickel sensitivity seems to be less common in men even if they wear earrings [9].
Hormones have some effect on contact dermatitis [10]. In one study the response to DNCB was enhanced in women taking an oral contraceptive [11]. Pregnancy and the use of progestogens may, unpredictably, either improve or aggravate contact dermatitis [12, 13]. Contact dermatitis may flare premenstrually, and cutaneous reactivity to patch testing may vary according to the stage of the menstrual cycle [14], with patch tests to nickel being less intense during the ovulatory than the progestagenic phase [15]. Premenstrual exacerbation of nickel allergy has been described [16].
Ethnicity
Racial differences appear to exist, judging from experimental sensitization to poison ivy and DNCB, where Afro-Caribbeans are generally more resistant than white people [1], although weak reactions to the eliciting dose are difficult to discern on Afro-Caribbean skin. Differences in the prevalence of sensitization to individual allergens among racial groups is felt to be a reflection of exposure rather than predisposition [2, 3].
Associated diseases
Patients with acute or debilitating diseases, such as cancer, Hodgkin disease or mycosis fungoides, have impaired capacity for contact sensitization [1, 2, 3]. This may also apply to patients who for other reasons have impaired T-lymphocyte function.
Drugs
Drug influences on skin-test reactivity have been reviewed by Schopf [1]. Antihistamines and sodium cromoglicate (disodium cromoglycate) appear to have little effect, whereas both prednisolone (dose >15 mg/day) [2] and potent topical steroids [3] suppress allergic patch test reactions. Similarly, other immunomodulators such as ciclosporin and azathioprine may reduce the intensity of allergic contact reactions [4]. Therapeutic UVB or psoralen and UVA (PUVA) therapy may also temporarily reduce contact allergic reactions [5, 6]. Experimentally, in rats, opiates have been found to enhance allergic, but not irritant, contact dermatitis. This effect was greater in female rats, and was found to be mediated through central mechanisms enhanced by peripheral hormonal effects [7].
Pathophysiology
Sensitization and elicitation
The immunology of skin disease is discussed in detail in Chapter 8. There are two main processes involved in allergic contact dermatitis: (i) sensitization (induction or afferent limb of sensitivity); and (ii) elicitation (or efferent limb) of contact dermatitis. Four different types of delayed-type hypersensitivity reactions to exogenous chemicals, of which allergic contact dermatitis is a form, have been proposed [1]:
- Th1-mediated, with the release of interferon γ (IFN-γ) and tumour necrosis factor α (TNF-α), and the activation of monocytes and macrophages in allergic contact dermatitis, bullous exanthema and the tuberculin skin test.
- Th2-mediated, with the release of interleukin 5 (IL-5), IL-4, IL-13 and eotaxin, resulting in eosinophilic inflammation seen in maculopapular and bullous exanthema.
- Mediated by cytotoxic CD4+ and CD8+ T cells, with the release of perforin, granzyme and Fas ligand, resulting in allergic contact dermatitis and maculopapular, pustular and bullous exanthema.
- Release of CXCL-8 and granulocyte–macrophage colony-stimulating factor (GM-CSF) by T cells, resulting in the recruitment of neutrophils in pustular exanthema.
It is clear that dendritic cells and the local tissue microenvironment are crucial factors in the development of allergic contact dermatitis. Within the immune system, dendritic cells are the cell type that primes naive T cells and thus forms a crucial link between the innate and adaptive immune system. The precise role of dendritic cells in allergic contact dermatitis is still under investigation; in particular, the contributions of the respective cellular pools are still disputed.
Newer studies have identified that allergic contact dermatitis has been associated with defective Treg cells and indeed it has become clear that Treg cells influence sensitization as well as elicitation. Originally, Treg cells were defined as CD4+ CD25+ T cells and were mainly associated with self-tolerance. We now know that this definition comprises a heterogeneous cell population that includes natural Treg and inducible Treg cells. The skin contains predominantly inducible Treg cells, which can be triggered by Langerhans cells as well as dermal dendritic cells. However, the precise phenotypes of Treg cells involved in allergic contact dermatitis are still not known.
Finally, Treg cells are involved in the control and eventual termination of the inflammatory response [2].
Sensitization
The induction of sensitivity is the primary event, which has to take place before the clinical expression of dermatitis can occur. The main events are described below.
Binding of allergen to skin components. Broadly speaking, the chemicals that result in allergic contact dermatitis are too small to be recognized by the immune system. Allergens penetrating the skin may be sufficiently chemically reactive that they bind covalently with skin peptides directly or, alternatively, metabolism may result in a reaction product that is able to bind. The products formed associate with major histocompatibility complex (MHC) class II molecules [3]. Interference in the process of protein binding to thiol and amino groups in cysteine and lysine residues has been shown to interfere with the process of sensitization [4]. Chemicals may also bind directly to MHC class II molecules, inducing a sensitization reaction. MHC class II molecules are coded on the human leukocyte antigen (HLA) D region genes, and are present on epidermal dendritic cells and Langerhans cells. Epicutaneously applied allergen associates with these antigen-presenting cells within 6 h.
Recognition of ‘complete’ or conjugated antigen. The ‘danger model’ supposes that sensitization to MHC-bound antigen does not occur unless other co-stimulatory factors are also present and that it is produced as a consequence of cell ‘stress’. IL-1β, TNF-α and GM-CSF are all required for the activation, maturation and migration of Langerhans cells [5]. The production of these cytokines by injured keratinocytes may lead to Langerhans cell migration and subsequent sensitization. The danger hypothesis has been adapted to contact hypersensitivity, and evidence produced to support a role for irritant dermatitis in the generation of contact hypersensitivity [6]. In the absence of these co-factors it is assumed that tolerance would develop.
Sensitization is possible only if the connection to the regional lymph nodes is intact [7]. The allergen-carrying Langerhans cells travel via the afferent lymphatics to the paracortical areas of the regional lymph nodes, where they become apposed to T lymphocytes. The binding is assisted not only by physical factors – the ruffled membrane and dendritic nature of the Langerhans cells and the intricate structure of the paracortical areas – but also by specialist cellular adhesion molecules (CAMs). These CAMs act at different loci to encourage binding. For example, leukocyte functional antigen-1 (LFA-1) on CD4 helper cells interacts with intercellular adhesion molecule-1 (ICAM-1) on Langerhans cells, and CD2 on T cells binds to LFA-3 in plasma membranes on most nucleated cells. With recognition of the antigen, many mediators or cytokines are released by this apposition, for example IL-1 by antigen-presenting cells and IL-2 by T lymphocytes.
Proliferation and dissemination of sensitized T lymphocytes. The cytokines cause blast formation in the lymph nodes and the proliferation of antigen-specific cytotoxic CD8+ (Tc1) and CD4+ (Th1) lymphocytes [8]. The type of T-cell response generated is dependent on the pathway by which the antigen is processed: small lipid-soluble molecules such as urushiol enter the cytoplasm and are presented on MHC class I as an endogenous antigen; polar haptens are more likely to be presented on MHC class II as an exogenous antigen [9].
The T cells disseminate via the efferent lymphatics throughout the body and interact with Langerhans cells and residual antigen in the skin. Contact hypersensitivity is mediated through a subset of T cells that express cutaneous lymphocyte-associated antigen (CLA). Localization to areas of inflammation occurs via the production of the chemokine CCL27 by basal keratinocytes, which binds to dermal glycoprotein. CLA-positive lymphocytes also express CCR10, the receptor for CCL27 [10]. The cytotoxic T cells induce keratinocyte death through the release of Fas ligand and perforin-mediated pathways [11].
Elicitation
On first exposure to a strong sensitizer such as DNCB, most subjects develop a local reaction after 5–25 days. During this period, sensitization has been accomplished, and the residues of the allergen in the skin react with the newly formed, sensitized T lymphocytes. Such a response has been termed a ‘late’ reaction. There is evidence to suggest that allergen-specific T lymphocytes persist at the site of original contact for some months following an initial sensitization exposure, and this may explain the ‘re-test’ or ‘flare-up’ reactions that are sometimes observed during patch testing, following re-exposure at a distant site [6].
If a sensitized person is re-exposed to a specific allergen in sufficient concentration, the clinical reaction subsequently develops much more quickly, usually within 24–48 h. However, depending on the degree of sensitivity, penetration and other factors, this may vary from a few hours to many days. Antigen may be presented not only by antigen-presenting Langerhans cells but also by IL-1-secreting keratinocytes that acquire Ia/HLA-DR status, augmenting the cascade of cytokine, immune cell and inflammatory response. This cascade is autoregulating, and although the mechanism of this is not well understood it probably involves CD4+ T cells.
A delayed reaction time (sometimes also referred to as a ‘late’ reaction) describes a delayed elicitation response following antigenic challenge in persons who are already sensitized. There has been confusion over the use of this term, as it has been used not only to describe reactions that have taken more than the usual 4 days to develop, but also acute primary sensitization reactions which, in normal clinical practice, often present as more sudden and florid reactions around 21 days after challenge. A delayed reaction time is found with low degrees of sensitivity (when there are very few memory T cells), following exposures to small amounts of allergen (when it takes longer to augment the T-cell response) and in situations of delayed penetration of allergens (e.g. neomycin in petrolatum).
Predisposing factors
It is convenient to categorize eczemas as endogenous or exogenous, and the latter can be divided into contact irritant and allergic. It is common to see combinations of these disorders, particularly on the hands. Pre-existing or concomitant constitutional and/or irritant contact dermatitis damages the skin, affecting its barrier function and producing increased opportunities for allergen absorption and secondary sensitization. It is known that hand eczema predisposes to nickel sensitivity and vice versa [1], and that the prevalence of chromate, cobalt and balsam sensitivity is increased in men with hand eczema [2]. The longer the duration of the eczema, the greater is the chance of sensitization. Occlusion greatly promotes percutaneous absorption and probably contributes to the high incidence of medicament dermatitis in stasis eczema, otitis externa and perianal dermatitis, and is also a factor in dermatitis from shoes and rubber gloves.
As sensitivity is more easily acquired if an allergen is applied to damaged skin, concomitant irritant contact dermatitis will promote sensitization and lower the threshold for the elicitation of an allergic contact dermatitis in those exposed to associated allergens [3]. In experimental sensitization, skin damage may be produced by a previous application of sodium lauryl sulphate. The enhanced risk of sensitization may be due to: (i) increased absorption of the allergen as a result of skin barrier disruption; (ii) priming of the immunological response with prior recruitment of immunocompetent cells, cytokines, etc.; or (iii) accumulation of mononuclear cells. Furthermore, by adapting Matzinger's ‘danger model’ concept for sensitization [4], it has been suggested that contact allergy can only develop in the presence of cytokine release from non-immune skin cells (principally keratinocytes) provoked by a coexisting irritant (often the same as the allergen) or trauma [5, 6]. If there is no concomitant irritancy, then tolerance rather than allergy will follow.
In guinea pigs, sensitization is facilitated by acanthosis induced by detergents or paraffins, even in the absence of dermatitis [7]. Although the mechanism for this promotion of sensitization by acanthosis is unknown, it may be relevant to burns and other types of skin damage known to increase the chance of sensitization [8].
Once allergy is established, it seems reasonable to suppose that an allergen may be able to reactivate or maintain dermatitis at a low concentration. However, even when such exposure seems to have ceased, a hand eczema that started as a contact dermatitis may continue as an apparently ‘constitutional’ post-insult form of dermatitis [9].
Chemical factors
Skin cells, especially their nucleic acids and proteins, are composed of molecules that contain nucleophilic atoms that are negatively charged and electron rich. Most allergens (haptens) are ‘simple’ chemicals of low molecular weight (less than 500–1000 Da) that contain electrophilic atoms [10, 11] that are positively charged and electron deficient. Interaction between these two types of atoms leads to strong covalent bonding to form a hapten–protein complex or ‘complete antigen’. Metal and metal salts can bond to electron-rich atoms (ligands) by taking some of the electrons and forming coordinate bonds. Haptens can be grouped according to their chemical reactivity in relation to putative carrier proteins or according to functional groups (Table 128.2).
Table 128.2 Classification of haptens based on functional grouping.
| Hapten group | Example |
| 1 Acids | Maleic acid |
| 2 Aldehydes | Formaldehyde |
| 3 Amines | Ethylenediamine, p-phenylenediamine |
| 4 Diazo compounds | Bismark brown, Congo red |
| 5 Esters | Benzocaine |
| 6 Ethers | Benzyl ether |
| 7 Epoxides | Epoxy resin |
| 8 Halogenated compounds | Dinitrochlorobenzene, picryl chloride |
| 9 Quinones | Primin, hydroquinone |
| 10 Metals | Ni2+, Co2+, Cr3+, Hg2+, etc. |
| 11 Unsaturated compounds | Δ3-carene (turpentine) |
Thus, the potential of a low-molecular-weight compound to become a hapten is determined by its chemical reactivity towards skin proteins. Some compounds react directly (e.g. nickel), while others require activation, either metabolically inside the skin or externally. The latter are classified either as pro- or prehaptens, depending on the mode of activation. Non-sensitizing compounds that require metabolic activation are prohaptens, while prehaptens are compounds with no or low sensitizing potential that are activated externally.
Prohaptens are metabolically activated in the skin and thus activation could vary depending on the individuals’ enzymatic expression patterns. Well-known examples of prohaptens are cinnamyl alcohol (3-phenyl-2-propen-1-ol) and urushiols.
Examples of prehaptens are the common fragrance terpenes, the diterpenes in colophony and ethoxylated surfactans. Patch tests revealed some of these substances to be potent skin sensitizers following their activation by auto-oxidation. Auto-oxidation of limonene (from citrus) and linalool (from lavender), two frequently used fragrances, results in the formation of the corresponding hydroperoxides. Multicentre studies imply that oxidized limonene and oxidized linalool are among the most common causes for allergic contact dermatitis, while the compounds themselves rarely cause sensitization [12].
Cutaneous enzymatic transformation of a chemical into many different metabolites, depending on the pathway taken, makes determination of the allergenicity of the original chemical more difficult. It also explains the difficulty in deciding if multiple sensitivities are cross-reactions or concomitant sensitization.
Enzymatic systems may also play a preventative role, as with glutathione in some drug-induced reactions [13].
Assessment of sensitization potential
The sensitization potential is the relative capacity of a given agent to induce sensitization in a group of humans or animals [14]. Both in guinea pigs and humans, an estimate of the sensitizing index requires patch test exposures modified to increase the sensitizing impact. Such predictive patch tests are used to compare the sensitizing properties of new products or chemicals with those of known substances. Many test procedures have been developed over the last 40 years to evaluate the sensitizing properties of new chemicals. Kligman and Basketter [15] have critically evaluated the various methods of predictive testing. Most previous methods could not reveal even potent sensitizers. Kligman and Epstein have described a ‘maximization test’, based on the application of a high concentration of the chemical to be studied on a skin area previously irritated by sodium lauryl sulphate [14]. This method was later modified by Marzulli and Maibach, who used repeated patch tests with high concentrations of the allergen to be studied. Jordan and Kinghave have shown that some substances giving negative reactions in maximization tests in males sometimes sensitize females. This may reflect previous subliminal exposure to substances such as the ingredients of cosmetics.
Ethical considerations may prevent experimental sensitization in humans. The guinea pig maximization test described by Magnusson and Kligman gives results that compare favourably with predictive patch tests in humans. To enhance sensitization, the guinea pig maximization test employs a combination of patch testing and intradermal injection of allergen in a simple solution of Freund's adjuvant. Other tests, such as the Buehler test and the open epicutaneous test, use the epicutaneous route only, whereas the Draize test and Freund's complete adjuvant test use a purely intradermal method of sensitization. There is, however, no absolute conformity in the sensitizing potential of a substance in mouse, guinea pig or human.
The 6th Amendment of the EC Cosmetic Directive, which came into effect in January 1997, is committed to banning all animal testing. The local lymph node assay is a mouse model that has gained regulatory approval. A logarithmic scale is used to classify the potential of chemicals to induce sensitization, from strong to non-sensitizing, depending on the dose needed to induce lymphocyte proliferation [16]. The mouse ear swelling test [17] avoids postmortem examination of tested animals.
The theoretical allergenicity [11, 18] of a compound may be studied by reference to databases of cases of reported sensitivity and the results of previously performed guinea pig maximization tests. By comparing the structure of known allergens and reactive groups with that of any new compound, an expert system can be developed to predict a compound's likely sensitization potential. Molecular modelling using structure–activity relationships has been used with the sesquiterpene lactones and primin and a relative alkylation index for sultones to test such a model.
Sensitization risk
The risk of sensitization depends not only on the sensitization potential of the substance applied, but prior to stimulating the immune system a chemical must penetrate the epidermis [19]. Subsequently, the log dose applied per unit area, where the area of application is greater than 1 cm2, appears to be the most important determinant of the risk of sensitization, with the reactivity showing a sigmoid dose–response curve [20]. In practice, the conditions of exposure are also important: the duration of exposure (rinse off or leave on product), if the exposure is repeated [21], and the condition of the skin (the presence of pre-existing dermatitis predisposing to the presence of accessory signals in the sensitization process).
There are also individual factors; studies in individuals already sensitized indicate that those with more contact allergies have a greater susceptibility to sensitization by other allergens compared with those who do not demonstrate any pre-existing contact allergy. With high concentrations of a strong allergen such as DNCB, individual susceptibility is of little importance; nearly everyone is capable of being sensitized.
In personal care products the concentration of any allergen is adjusted so that the risk of inducing sensitization is small, although there may still be sufficient to induce dermatitis in an individual already sensitized. An approach to sensitization risk assessment for such products has been described [22]. This involves an assessment of both exposure (including knowledge of skin absorption) and sensitization potential, based on literature review and known structure–activity relationships. If in vivo testing is needed, various animal tests or human repeat-insult patch tests would then be performed. Legislative measures have been introduced in an attempt to reduce the prevalence of contact dermatitis [23].
Development of dermatitis
Some persons sensitive to a substance may tolerate normal contact with it, and are said to have a latent sensitivity. There is no immunological difference between latent and expressed sensitivity. Whether sensitivity is manifest or latent is determined partly by the threshold of sensitivity, which is the lowest concentration of allergen giving a positive patch test response. The dose at induction determines, in part, the strength of response at challenge – higher induction doses resulting in greater reactions at challenge [20]. Persons who are clinically sensitive to poison ivy invariably have a positive reaction to 1 : 10000 pentadecylcatechol (PDC), but many who react only to 1 : 100 PDC are clinically immune. Patch test sensitivity and clinical sensitivity are not necessarily proportional. The threshold determined by patch tests depends on a number of technical factors, such as the base used and the region where the tests are applied. It also varies from time to time in the same person. The threshold may fall after repeated contact with an allergen, and positive test reactions in latent allergy may reveal candidates for future allergic contact dermatitis.
Patch testing with a new substance may reveal that some people are already sensitive to it, either from contact with related substances or from exposure to the compound in other forms. Negative reactions in 200 people do not exclude the possible occurrence of sensitivity in one of 38 consumers (99.5% level). This frequency would immediately preclude any practical use of the substance. It has been calculated that negative patch tests in 5300 subjects indicate that sensitivity would be liable to occur in less than one of 1000 consumers.
Immunological tolerance
The sensitization reaction induces effector T cells and suppressor T cells, the latter curtailing the immune response so that the epidermal reaction regresses and does not continue indefinitely [24]. Theoretically, therefore, preferential stimulation of suppressor cells could lead to antigen unresponsiveness. This can be achieved by administering the allergen (in previously unsensitized individuals) by non-cutaneous routes, such as intravenously, orally or peritoneally [25], thereby bypassing epidermal Langerhans cells. This tolerance is also achieved by applying the allergen to skin with no Langerhans cells, for example mouse tails, or skin in which the Langerhans cells have been inhibited by UV radiation or depleted by glucocorticoids. Suppressor T cells, or their precursors, are sensitive to cytostatic drugs, so that the administration of cyclophosphamide can reverse a tolerant state.
Pathology
Biopsies are of limited help in contact dermatitis. Most types of eczema show identical pathological changes, and allergic and primary irritant contact dermatitis cannot be distinguished with certainty. It would appear that the only sure way to distinguish irritant from allergic contact dermatitis is by study of the very early events of the inflammatory process, because the remainder of the inflammatory cascade is similar in the two processes [1].
Causative organisms
Fauna are not a major cause of contact allergy, although European fishermen are liable to contact dermatitis of exposed skin during the summer when handling nets containing marine organisms known as bryozoans. The disorder is known as ‘Dogger Bank itch’ in the UK [1]. The allergen has been identified as the (2-hydroxyethyl)dimethylsulfoxonium ion [2].
Genetics
Genetic risk factors are based on variations in genes (e.g. polymorphisms) involved in relevant steps for the development of contact dermatitis. Genetically influenced steps are the antigen uptake through the skin barrier, the antigen-specific response by immune cells and the metabolism of antigens by cutaneous enzymes [1]. An example for the latter is the metabolism and possible activation of antigens by epidermal N-acetyltransferases (NATs). Studies found a relationship between the genetic polymorphism for these phase II enzymes and the risk for contact dermatitis. Patients with contact dermatitis tended to have NATs with a higher than average enzymatic activity [2]. Other studies link the allele for a rapid acetylating NAT-1 to a lower susceptibility for PPD sensitization [3].
Cytokine gene polymorphisms represent possible genetic risk factors at the level of an immunological response [4]. Other gene polymorphisms increasing the risk for allergic contact dermatitis have been observed in the coding regions of enzymes, for example angiotensin-converting enzyme [5].
Sensitization presupposes individual susceptibility. This has been investigated using epidemiological, family and twin studies [6]. In humans, susceptibility does not seem to follow Mendelian inheritance and, in some cases, may occur by non-antigen-specific amplification of the immune response [7]. Nearly everyone can be sensitized with Primula extract, and most with 2,4-DNCB. However, experiments with the latter indicate that nearly all susceptible subjects will be sensitized after one or two applications of the allergen in a suitable concentration; repeated applications increase the number of persons sensitized only marginally. Some individuals are thus resistant to sensitization. This resistance may have been acquired by repeated exposure to subsensitizing doses of the allergen [8] or as a result of exposure through the oral route with the development of tolerance [9].
The capacity for sensitization varies from person to person, but certain individuals are more prone to developing sensitivity to a particular substance, for example nickel. The ‘heritability’ of nickel sensitivity has been calculated to be about 60% [10]. This may be a genetically determined trait but, if so, it is not known whether the property inherited is an increased capacity for conjugation to form an effective antigen, for sensitization or for facilitation of percutaneous absorption. In guinea pigs, the capacity for sensitization, both in general and to particular substances, has been shown to be inherited [11, 12]. In humans, such studies are less likely to be conclusive because of the difficulty in distinguishing between genetic and environmental factors. One experiment compared the susceptibility of parents and their children to contact sensitization with DNCB and p-nitrodimethylaniline. Children whose parents became sensitized were sensitized more commonly than were children whose parents were not sensitized [13]. Siblings and children of patients suffering from allergic contact dermatitis have an increased incidence of positive patch tests, and first-degree relatives of nickel-allergic subjects have increased prevalence of the same disorder [14]. Conversely, studies on twins with hand eczema and nickel allergy indicate environmental rather than genetic factors are the more important [15], and a previous study also failed to show any difference in capacity for sensitization to DNCB between monozygotic and dizygotic twins [16]. Studies of HLA types and blood groups have not proved very helpful to date [17, 18]. However, a statistically significant increased proportion of rapid acetylators has been found in contact allergic patients [19]. The authors were unable to say whether this state was contributory or was a genetic marker for the ability to become sensitized. IL-16-295 promoter [20] and TNF-α-308 polymorphism in polysensitized subjects suggest that these may also influence susceptibility to contact allergy [21].
The relationship of atopy, particularly atopic eczema, to predispose to allergic contact dermatitis has prompted much debate. Atopics are known to exhibit down-regulation of Th1 cells [22, 23], which should mean a decreased tendency to develop allergic contact dermatitis; indeed, patients with severe atopic eczema may have a diminished capacity for DNCB sensitization [24]. However, clinical studies are conflicting, some showing an increase in the prevalence of contact allergy, especially to medicaments [25], others the same [26] and others a decrease [27, 28]. In a study of 101 sets of twins, no correlation was found between positive patch tests and atopy and the prevalence of allergic contact dermatitis in atopics was found to be similar to that in patients suffering from discoid or seborrhoeic eczema. An increased level of nickel sensitization noted in one study [29] contrasts with another where there was no increase [30]. Confounding factors include the fact that in many cases of chronic atopic eczema there has been considerable exposure, both in extent and time, to medicaments and emollients applied to broken skin, which might explain the increased rate of allergy to medicament components noted in some studies. False positive patch test reactions to nickel, chromate and cobalt, and probably other marginally irritant allergens, are frequently seen in patients with atopic eczema and can be difficult to interpret. At present no certain conclusion can be made about the relative risk of contact sensitization in atopic patients.
Environmental factors
By definition, the environment will influence exposure to potential allergens, which in turn will affect liability to contact allergy. For the individual, certain immediate environments, including those encountered in the home, at work and during spare-time activities, are particularly relevant. However, more general influences are important, including climatic, geographical, ecological, socio-economic and cultural factors. Some of these may also affect the individual's response to allergen exposure. Climate, geography and ecology are often interrelated.
Climate
Climate, by virtue of varying UV exposure, heat and relative humidity, may play a part in liability to contact allergy. UVB exposure has been shown to diminish the skin's immune response to contact allergens [1, 2]. UVA exposure, however, does not appear to have the same effect, and there is evidence that the reduction in immune responsiveness is transient, perhaps due to an adaptive mechanism [2]. UVB exposure from the sun may therefore temporarily reduce contact allergic reactions, although there is conflicting evidence about the effect of sunshine on patch test reactions [3, 4]. Conversely, chapping of the skin during winter predisposes to irritant contact dermatitis and also increases the incidence of false positive patch test reactions to substances such as formaldehyde [4] and propylene glycol [5]. Occlusion and increased sweating may increase allergy from shoes and clothing. Exposure to UV-absorbing chemical filters increases where there is a higher exposure to sunshine, with a consequent increase in contact and photocontact allergy from this source during the summer months, when photoallergy from other causes would also be anticipated to be more of a problem. These varying observations indicate that several factors must influence the seasonal liability to contact dermatitis.
Flora and fauna
Plant dermatitis commonly shows a distinct seasonal pattern, the allergenicity of some plants such as Primula obconica varying considerably with light and season [6]. Many allergenic plants, especially those belonging to the Compositae (Asteraceae) family, are destroyed by cold and frosty weather but return during the warmer spring and summer months. Global warming is also felt to be an issue, with evidence that Toxicodendron species may become more abundant and allergenic as a result [7]. Distribution of allergenic plant material will be facilitated by dry and windy climates. Similarly, geographical location is a very important influence. Exposure to Toxicodendron species is mainly confined to North America. Compositae allergy is seen in many parts of the world but the plants responsible vary: in the USA ragweed is the main cause, in Europe it is chrysanthemums and garden weeds, in India the weed Parthenium, and in Australia a number of wild Compositae found in the ‘bush’. Occupational contact allergy from plants is often seasonal, for instance in lichen pickers [8] and from plant and vegetable cultivation [9, 10].
Socio-economic and cultural factos
The relationship of contact dermatitis to socio-economic groups has not been studied in detail, but exposure to cheap (nickel-releasing) metals used as jewellery might be expected to be relatively increased in those with less disposable income. Similarly, the pattern of perfume and cosmetic use and exposure might vary according to social class.
Cultural factors are important, and not always fully appreciated as a predisposing cause for contact allergy, particularly the use of sensitizing traditional herbal medicines and balms to treat skin disorders in the Middle and Far East [13, 14]. Furthermore, ingested herbal folk remedies containing Toxicodendron have caused outbreaks of systemic allergic contact dermatitis in Korea [15].
Hair dyes are used much more commonly by men in the Middle East and the Indian subcontinent, including use on the beard [16]. Indian women may become sensitized to dyes and adhesives used in kumkum and bindi applied to the forehead [17]. Western culture, in contrast, is associated with higher cosmetic use and leisure pursuits, including lying in the sun and seaside holidays requiring the application of sunscreens. The frequency of tattooing and body piercing has increased in recent years, especially amongst young adults, thereby increasing their risk of contact with potential allergens including nickel and PPD.
Clinical features
History
Contact dermatitis can mimic or be associated with any type of eczematous eruption. The diagnosis is based on a careful history combined with a sound knowledge of common allergens and irritants in the environment. A comprehensive history is essential to identify contact with allergens, and some knowledge of chemistry and industrial processes is of value. Sensitization and subsequent contact dermatitis may result from a single exposure [1], although usually several or many exposures are necessary before sensitization and dermatitis occur.
Primary site
This must be ascertained by questioning the patient carefully. By definition, contact dermatitis must begin in sites where contact has taken place with the responsible agent(s), and the sites of origin are an important clue to the cause. Patients are frequently assessed at a stage when there has been worsening and secondary spread of the dermatitis, obscuring the original pattern.
Duration and behaviour
Once the date of onset and the primary site(s) have been identified, it is necessary to establish the subsequent behaviour of the disorder. In particular, did the condition spread and if so where? Has the problem been persistent or intermittent? Repeated sudden exacerbations may point to an allergic contact dermatitis. Are there any obvious exacerbating factors?
Improvement of dermatitis during weekends or holidays may indicate an occupational origin. Relapse at weekends may favour a hobby or non-occupational allergen. Seasonal variation (worsening when light intensity is greatest) may suggest a plant allergen, perhaps with photoaggravation, or photoallergy. Plant dermatitis may recur in atypical patterns. Dermatitis around a wound, especially leg ulcers, suggests sensitization to medicaments (Figure 128.1) and exacerbations and recurrences induced by particular medicaments or cosmetics suggest contact allergy from these sources.
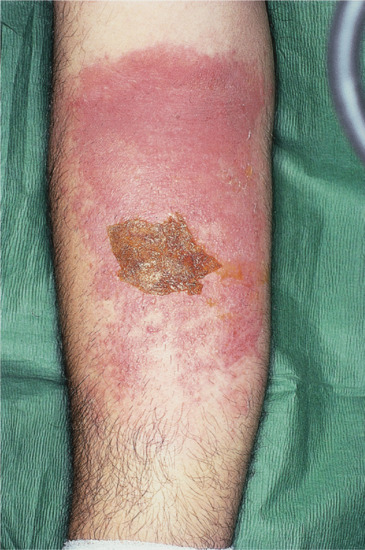
Figure 128.1 Medicament contact dermatitis. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Previous history
A history of previous dermatitis may provide a clue to the origin of a relapse. For example, earring dermatitis may precede nickel dermatitis of the hands by several years. Previous dermatitis, especially if localized to the lower legs, may have been caused or complicated by the repeated use of applications containing sensitizers. It is useful to ask specifically about skin reactions to costume jewellery, perfume and adhesive plasters.
A history of infantile or childhood flexural eczema, asthma or seasonal allergic rhino-conjunctivitis, may point to an atopic diathesis. Atopic eczema also predisposes to irritant contact dermatitis of the hands, and in such cases constitutional factors may be a major, but not necessarily the sole, cause.
Sources of allergy
A search for possible sources of allergic contact dermatitis should include a review of all the patient's activities, but initially should concentrate on: (i) occupation, present and past; (ii) hobbies; (iii) cosmetics, clothing and personal objects; (iv) home environment; and (v) current and previous topically applied medicaments both prescribed and over-the-counter. Most patients believe that newly encountered items are the cause of dermatitis, whereas in fact those that have been in use for a long time are commonly responsible.
Occupational. A precise history backed up by a thorough knowledge of the materials handled at work, the machinery operated and the personal protection employed will all be necessary when occupational dermatitis is suspected (Figure 128.2). However, no dermatologist can rely entirely on his or her knowledge of industrial processes, and a factory visit may be required to become familiar with the process described, especially if it is new. Health and safety data sheets must be examined as these may give the chemical names of materials used, as well as an indication of their irritancy or allergenicity. A telephone number or email address may be given for further enquiries, if required. The presence of other similar cases will alert one to an increased probability of occupational dermatitis. There are increasing reports of allergy to components of cleansers, barrier creams and conditioners supplied at work, and workers must be asked about the use of these too [2].
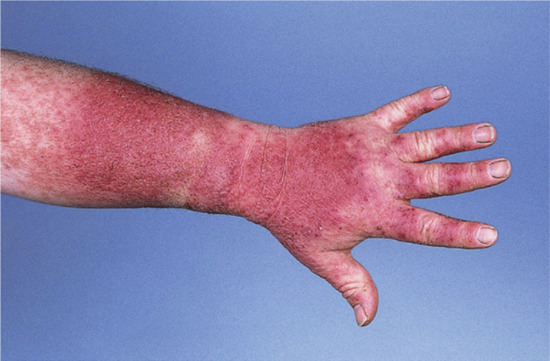
Figure 128.2 Acute allergic contact dermatitis in a patient allergic to acrylates used in the printing industry. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Problems associated with housework should not be overlooked. The amount of housework performed and methods employed are extremely variable. The number and age of children and availability of labour-saving devices should also be determined. Few people volunteer information about domestic work outside their own home, and all must be directly questioned about this.
Patients who are unemployed or students may, in fact, be engaged in casual work, and even employed persons should be asked about second jobs.
Hobbies. Common sensitizers, well known as industrial allergens, are introduced into most homes for do-it-yourself work. Cement, glues, paint, wood and wood preservatives are handled by many householders. Another important source of hobby dermatitis is gardening. Other pursuits, such as car maintenance, sports and cookery should also be considered.
Personal objects and medicines. These are items either worn in context with or applied to the skin, and include textiles, footwear, protective clothing and gloves, jewellery, spectacles, hearing aids, medical appliances, cosmetics, toiletries, fragrances and medicaments. Untoward reactions to cosmetics, toiletries and topical medicaments are among the commonest reasons for hospital referral with suspected allergic contact dermatitis. The number of products used may be large, and some may be used only intermittently. Often, only prescribed therapies are declared, and repeated specific enquiry must be made about over-the-counter preparations, including cosmetics used as moisturizers, herbal treatments and borrowed medicaments. Often patients will not mention ‘hypoallergenic’ products in the mistaken belief that they could not be responsible. Applied cosmetics may be removed from the skin by employing creams, lotions or wipes, the use of which may easily be overlooked. Patients should be specifically asked about the use of nail varnish, artificial nails and hair dyes. Skin cleansing and hair products, which are ‘rinse off’ as opposed to ‘leave on’, may also be responsible. Many patients have a poor recollection of products used, and most forget some items. They should be invited to bring all their topically applied items when they attend for patch testing and these can be specifically tested if indicated, and also examined for ingredient listings when appropriate.
Presentation
Eczematous responses (dermatitis)
The severity of the dermatitis is determined by the intensity of exposure and the level of sensitivity. The clinical picture is also to some extent dependent upon the site of dermatitis and on the causative agent. The distribution of the dermatitis may suggest a cause, for example that due to nickel or textiles.
The primary signs in acute contact dermatitis are erythema, swelling, papules and papulovesicles, which reflect the sequence of inflammatory changes in the dermis and the intracellular and intercellular oedema in the epidermis. In more acute and severe cases this may progress to disruption of the intercellular bridges and the development of larger vesicles or blisters; if they burst, a weeping dermatitis results. The dominant symptom is itching.
If contact dermatitis persists, it may be due to continued or repeated exposure to the allergen or to secondary irritants or allergens. The skin becomes dry, scaly and thicker as a result of acanthosis, hyperkeratosis, oedema and cellular infiltration in the dermis. Lichenification and fissuring may develop later (Figure 128.3). These clinical features of chronic allergic contact dermatitis cannot always be distinguished from constitutional (Figure 128.4) or irritant contact dermatitis, and the aetiology is indeed often mixed.
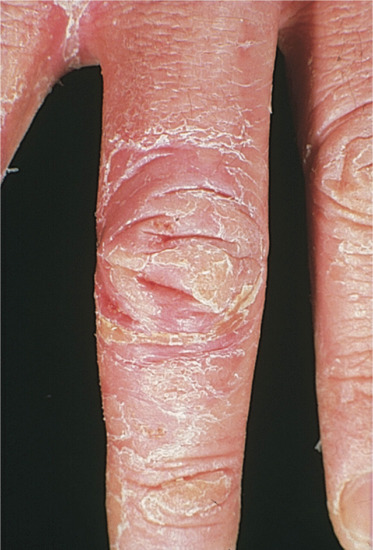
Figure 128.3 Dry, scaling, thickened skin with fissuring due to chronic contact dermatitis.
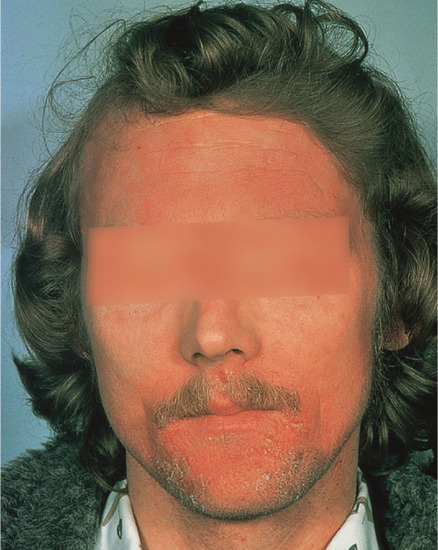
Figure 128.4 A seborrhoeic dermatitis-like pattern of allergic contact dermatitis due to phosphorus sesquisulphide (‘strike anywhere’ matches). (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
The distribution of the dermatitis is of diagnostic importance but its morphology is usually of no help in tracing the cause, with some exceptions, for example exceptionally strong allergens may provoke a bullous eruption even after brief contact and dermatitis from plant leaves may provoke a linear pattern of dermatitis.
Primary patterns
Anatomical patterns of dermatitis often suggest a specific cause, but in other cases the pattern merely indicates a range of possible allergens, such as in shoe dermatitis. Sometimes, the dermatitis is sharply limited to the usual site of contact, but because the area of contact with most objects varies, the distribution may be more erratic. Some allergens may be spread locally by the fingers or be carried to distant body regions. Even when there is no eruption on the hands, allergens on the fingertips may cause dermatitis elsewhere, for example the genital area, eyes, or face and neck.
Once the primary site has been established, questioning should focus on those allergens that are particularly frequent causes of dermatitis in that region.
Hands and arms. Hand dermatitis is usually multifactorial. About two-thirds of all cases of contact dermatitis involve the hands, which are the most important site for both irritant and allergic contact dermatitis [1, 2]. No pattern of hand eczema is characteristic of a particular aetiology, and it is important to emphasize that allergic contact dermatitis may mimic constitutional patterns. Housewives’ dermatitis and most occupational dermatitis remain confined to the hands. Although the majority of cases are of primary irritant nature, the yield of relevant positive reactions to patch tests is high [1]. Allergens may be traced by relating the shape and site of the eczematous patches to the items handled. Rubber gloves may induce a clear pattern of dermatitis over the sites where they are worn, particularly involving the dorsa of the hands with a sharp line of demarcation at the wrists.
Vesicular palmar contact dermatitis may mimic constitutional eczema and may result not only from contact with, but also from ingestion of, an allergen to which the person is already sensitized. Chromate in cement, N-isopropyl-N'-phenyl-p-phenylenediamine (IPPD) and 1,2-benzisothiazolin-3-one (Figure 128.5) are three allergens particularly liable to induce a palmar pattern of allergic dermatitis. Discoid patterns of eczema may be seen with chromate allergy.
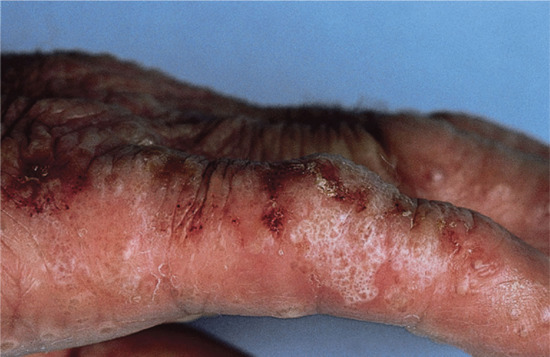
Figure 128.5 Acute vesicular eczema in a patient allergic to 1,2-benzisothiazolin-3-one mimicking constitutional pompholyx. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Streaky dermatitis on the fingers, dorsa of the hands and forearms is typically caused by plants (Figure 128.6), and may be allergic (e.g. Primula obconica and poison ivy), irritant (e.g. Dieffenbachia and spurge) or phototoxic (e.g. giant hogweed and rue).
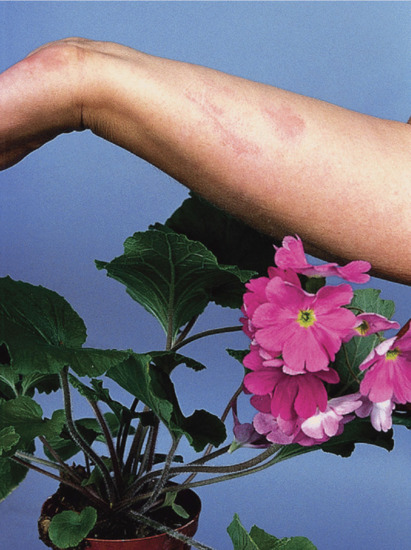
Figure 128.6 Characteristic ‘streaky’ contact dermatitis on the wrists in a patient allergic to Primula obconica. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Dermatitis of the hands in those involved with agriculture, animals and food preparation may be associated with immediate-type hypersensitivity to animal and plant proteins [3, 4]. Allergic contact dermatitis of the fingertips is seen with plant allergens such as tulipalins in horticulturists and florists (‘tulip fingers’) [5]. Garlic allergy in chefs typically affects the non-dominant thumb and fore and middle fingers (Figure 128.7) [6].
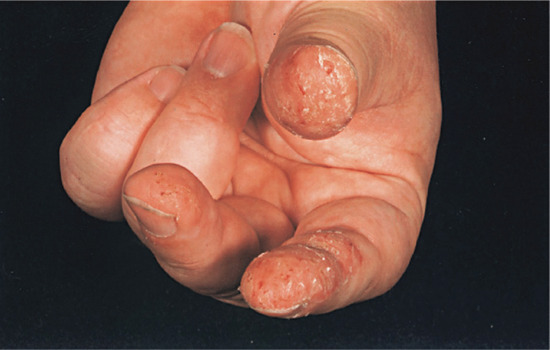
Figure 128.7 Fingertip pattern of allergic contact dermatitis from garlic affecting the non-dominant thumb, forefinger and middle fingers.
The arms are affected by the same allergens as the hands, but usually later. If the hands have been protected by gloves at work, the forearms may be the major sites of occupational dermatitis (Figure 128.8). Allergy to nickel, chromate and p-tertiary-butylphenol formaldehyde resin may develop at the wrists from sensitivity to the metal, leather and glue, respectively, in watchstraps containing these allergens. Dust (exotic woods, cement), nickel and textiles produce dermatitis in the elbow flexures, and this must be distinguished from atopic eczema.
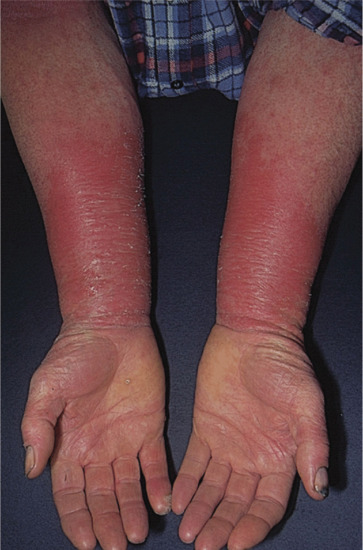
Figure 128.8 Contact allergy to epoxy resin and hardener affecting unprotected forearms.
Face. Dermatitis of the face may occur alone or in association with eczema elsewhere. Facial allergic contact dermatitis from fragrances, hair dyes, preservatives and other constituents of skin-care products and cosmetics, including nail varnish, is common.
Dermatitis due to a cosmetic may start with dryness, tightness and itching. Most women change to another brand at this stage and are never assessed by a dermatologist. They are referred only if symptoms persist or are severe. Some allergens, for example hair dyes, may provoke acute oedema (Figure 128.9) and intense pruritus – but not eczema – followed by desquamation. Nail varnish allergy often affects the face in well-localized patches, and may be associated with eyelid dermatitis and more extensive involvement of the neck, chest and even further afield [7]. It is important to note that these nail varnish allergens may also be present in other nail cosmetics such as nail strengtheners and hardeners. The clinical presentation can suggest artefact because the affected sites are so well demarcated [8]. A similar distribution may be seen from allergy to acrylic-based artificial nails and rubber sponge applicators [9, 10]. The forehead is affected by allergy to anything applied to the hair and also to chromate in leather hatbands. Spectacle frames containing nickel or plastics may cause dermatitis on areas of contact with the cheeks, nose, eyelids and ears.
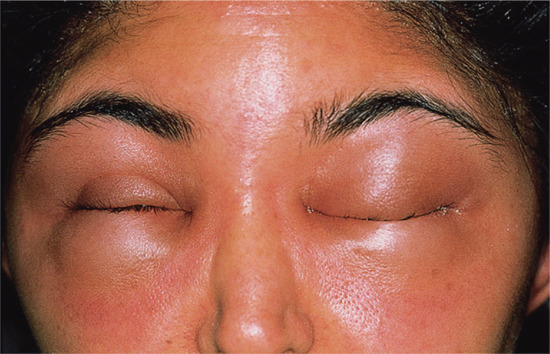
Figure 128.9 Contact dermatitis presenting as acute oedema, as seen in patients sensitive to Primula and p-phenylenediamine-type hair dyes and in those with volatile patterns of contact dermatitis. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
The patterns of dermatitis caused by airborne or volatile allergens [11] and photosensitizers can often be distinguished by involvement of the eyelids in the former, and by triangles of relatively spared skin below the chin and behind and below the ear lobes in the latter.
Facial allergic contact dermatitis must be distinguished from cosmetic intolerance, irritant contact dermatitis and constitutional eczemas, but it is sometimes multifactorial.
Eyelids. Allergens affecting the face may initially produce eyelid dermatitis [12, 13], as the skin of the eyelids is thin, sensitive and may be contaminated by the fingers (e.g. nail varnish [7]), airborne droplets (e.g. fragrance sprays) or volatile substances (e.g. epoxy resin). Eye creams, eye shadows, mascara and eye make-up removers may be responsible, often for irritant dermatitis, but patch testing may reveal relevant allergens. Eyelash curlers and make-up applicators may contain nickel and/or rubber and cause contact allergy at this site [14, 15].
Primula obconica and poison ivy allergy may involve the eyelids, usually associated with a streaky pattern of dermatitis at contact sites, which may be haemorrhagic.
Dermatitis is frequently caused by remedies for ocular disorders. Sensitizers in eye drops and ointments include neomycin, framycetin, gentamicin, tobramycin, chloramphenicol, fucidin, sulphonamides, local anaesthetics, antihistamines, β-blockers, anticholinergics and sympathomimetics. Eye drops and contact lens solutions often contain preservatives (e.g. benzalkonium chloride, ethylenediamine tetracetate (EDTA), mercurials), which may also sensitize.
Lips or perioral area. Sensitivity may occur from lipsticks and salves, nickel, medicaments, flavourings, garlic [16], shellac [17] and cosmetic excipients. Lipstick dermatitis may be limited to the vermilion border, which appears dry, scaling or cracked; but the perioral area may also be affected. Eosin was a common sensitizer in lipsticks before 1960 [18] but since its allergenicity was found to be due to impurities there have been no further reports of adverse effects, and lipstick dermatitis is less common.
Allergy to toothpaste is a recognized cause of cheilitis and perioral eczema. Flavours (fragrances) are the usual cause, such as cinnamic aldehyde [19], spearmint oil, peppermint oil, anethole and l-carvone [20]. Colophonium and derivatives may be found in chewing gum and cause allergic contact cheilitis [21]. Allergy to other food additives such as sodium metabisulphite, preservatives, colours and antioxidants may have a potential to cause cheilitis. In Europe, their presence can be determined by identifying the relevant E number on the ingredient label of the foodstuff packaging. Lip cosmetics with SPFs may also contain sunscreen agents and even photoallergic contact cheilitis has been described [22].
Allergic reactions to dentures and fillings are considered in the section on mucous membranes later in this chapter. Angular cheilitis is usually due to badly fitting dentures, but cheilitis may exceptionally be caused by sensitizers habitually carried to the mouth, such as nail varnish or nickel-plated objects (e.g. keys, pins, musical instruments) [23].
Ears. External otitis has a complex aetiology (see Chapter 108) and usually runs a chronic relapsing course. Neurodermatitis (lichen simplex chronicus) is also common, and may be superimposed on seborrhoeic eczema. Secondary medicament contact dermatitis, which is often unsuspected, is particularly common in the ear [24]. Dermatitis can also be both caused and maintained by habitual scratching with hairpins (nickel) or fingertips (nail varnish).
Although dermatitis from hearing aids occurs it is often a non-specific consequence of occlusion [25]. Hearing aids may contain acrylates and plasticizing and stabilizing chemicals. Headsets may contain urea and phenol-formaldehyde resins, or rubber in earphones.
Spectacle-frame dermatitis may be of irritant origin, especially behind and over the ears. Metals, particularly nickel and palladium, may cause allergy, and some frames responsible for dermatitis have been wrongly described as being nickel-free or made of titanium [26, 27]. Plastic components, including epoxy resins, acrylates, plasticizers, UV inhibitors and dyes, have also been identified as the cause of the dermatitis. Earplugs for noise protection may contain antiseptics, dyes, rubber and plastic chemicals, and finishes including formaldehyde resins. Elastic on shower caps and hair dye caps can cause dermatitis in the retro-auricular area.
Earrings and clips commonly cause dermatitis on the ear lobes as a result of the presence of nickel or, less commonly, gold. Piercing of the ear lobe may be the sensitizing event in nickel dermatitis, leading to a chronic contact dermatitis. Granulomatous contact allergy to nickel, palladium and gold has been seen after ear piercing [28].
Scalp. The scalp tends to be relatively spared from involvement of allergic contact dermatitis. Dermatitis caused by fragrances, preservatives and amphoteric detergents in hair cosmetics is usually limited to the ears, neck and face, but may be preceded by persistent itching of the scalp. Permanent hair dye allergens, PPD and related semipermanent dyes still remain an important source of dermatitis. Correctly used, permanent hair dyes are applied to the hair and not the scalp, followed by oxidation and rinsing. Bleaches contain ammonium persulphate, which can cause peculiar urticarial eruptions as well as contact dermatitis. Glyceryl monothioglycollate, used for acid or cold perms, is a significant sensitizer in hairdressers but only occasionally causes problems in their clients. Hair-styling products such as mousses, gels, waxes and holding sprays often contain fragrances and preservatives that may be allergenic, but they also contain conditioning quaternary ammonium compounds, which are often irritant. Medicated shampoos may contain tar extracts, zinc pyrithione or other agents, and many shampoos contain formaldehyde, formaldehyde releasers or isothiazolinones, added as preservatives. Cocamidopropylbetaine is also found in many shampoos. All these materials may potentially sensitize, although allergic subjects may tolerate them because of the short duration of contact, provided the hair is thoroughly rinsed after washing. Topical minoxidil lotion, prescribed to promote hair growth, will sensitize occasionally [29]. Scalp dermatitis from allergy to azo disperse dyes in a nylon wig has been reported [30].
Neck. Nickel in the clasps of necklaces or zip fasteners produces a small area of dermatitis on the nape of the neck. Nail varnish (Figure 128.10) or primin from fingertips may be the cause of a patchy allergic dermatitis, sometimes simulating lichen simplex. Textiles (finishes in collars, dyes) and necklaces (nickel, exotic wood) may cause a collar-like dermatitis, or eruptions on the sides of the neck. Dermatitis from airborne allergens and photosensitizers is sharply limited by the collar to the ‘V’ of the neck if blouses or open-necked shirts are worn. Perfume may cause both allergic contact dermatitis and phototoxic dermatitis (Berloque dermatitis) on the neck, especially the sides.

Figure 128.10 A patch of lichen simplex-like eczema on the nape of the neck associated with allergy to tosylamide formaldehyde resin (nail varnish). (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Axillae. Many cases of dermatitis are due to irritation from sweating, occlusion and the use of antiperspirants, which often contain aluminium salts to block the sweat glands. Allergic sensitivity may occur to fragrances used to mask odour, and to antiseptics intended to reduce the bacterial flora. The dermatitis produced by textiles tends to be periaxillary.
Trunk. The distribution of a clothing dermatitis may provide a clue to the responsible garment. In both sexes, nickel buttons and zip fasteners cause dermatitis localized to where they are worn, but a more widespread secondary spread rash may often occur. This is less usually seen in Europe due to the ‘Nickel Directive’. Chromate sensitivity from leather, and rubber allergy from elastic, may present as truncal eczema. Dermatitis from dresses, blouses and sweaters predominantly affects the neck and folds of the axilla, and spare areas of skin covered by undergarments. The allergens are usually textile dyes or finishes.
Outdoor workers sensitized to Compositae (Asteraceae) plants may have a diffuse dermatitis or dermatitis of an airborne pattern, which affects all the exposed areas, including the trunk if they remove their clothing to work.
Detergents and fabric conditioners are commonly blamed for truncal skin eruptions but objective confirmation is usually lacking [31]. Diffuse papular eczema may be a feature of medicament sensitivity with secondary spread.
Ano-genital. The ano-genital region is a common site for medicament sensitization. There is often experimentation with a wide range of prescribed and over-the-counter medicaments for pruritus, skin eruptions and haemorrhoids, and many of these contain sensitizers, most commonly fragrance, local anaesthetics and balsam of Peru (Myroxylon pereirae). Other sensitizers prescribed by the medical profession include neomycin, hydroxyquinolines, ethylenediamine, corticosteroids and topical antifungals. Moist toilet tissues and wipes may contain unnecessarily high levels of preservative, which have been associated with an increased prevalence of allergic sensitivity and in particular methylisothazolinone [32]. Ectopic contact dermatitis from nail varnish may also affect this site [33]. Nylon dyes, especially in tights, may also produce allergic contact dermatitis largely confined to this area.
The ingestion of contact allergens may cause pruritus ani, particularly if they are excreted unchanged. Spices and medicaments may occasionally be suspected. Cashew nut oil in butter was found to induce perianal dermatitis in an individual allergic to the cross-sensitizing allergenic urushiol found in poison ivy [34].
Many studies in women have not distinguished an ano-genital pattern from dermatitis confined to the perianal or vulval area, but allergic contact dermatitis confined to the vulva appears to be less common [35]. Medicaments used for vaginitis rarely provoke allergic reactions on the mucosa but sometimes produce a rash on the adjacent skin, and may cause connubial dermatitis in sexual partners. Medicament allergy has frequently been associated with vulval dermatitis [36, 37], and in another study investigating pruritus vulvae without associated inflammation, 49% had one or more relevant allergic reactions on patch testing, as did seven of 16 patients with lichen sclerosus. In over 50% of these patients, symptoms improved significantly or resolved when avoidance measures were taken [38]. Perfumes or antiseptics in soaps, sprays or sanitary pads are rare causes of genital dermatitis, although feminine hygiene sprays may cause both irritant and allergic reactions. Vulvodynia does not appear to be frequently associated with contact allergy [39].
Rubber accelerators in condoms can be a cause of genital eczema or pruritus vulvae. Delayed hypersensitivity to semen has also been reported [40]. Genital dermatitis from the transfer of material carried on the hands may occur in carpenters and cabinet makers and those who work with resins.
Thighs. Dermatitis from the nickel and rubber in suspenders is now rarely seen. Textile dermatitis starts at the edge of the underwear, and is usually more pronounced in the popliteal spaces or gluteal folds. Finishes in the material of the pockets or objects kept in the pockets (e.g. nickel coins or boxes of matches) may provoke a patch of dermatitis on the underlying skin (Figure 128.11). Allergens may penetrate working clothes.
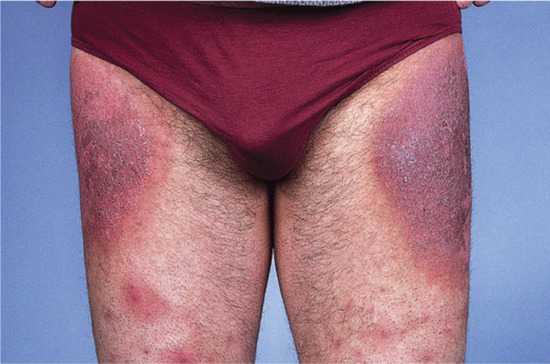
Figure 128.11 Allergic contact dermatitis due to items kept in trouser pockets. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Lower legs. The lower leg is particularly prone to contact allergy. Allergic contact dermatitis from medicaments and dressings predominate, especially in those with varicose eczema and ulcers. The common medicament allergens are topical antibiotics and components of creams and paste bandages, such as lanolin, cetostearyl alcohol and preservatives [41]. Allergy seems to occur readily to materials that are rarely problematic in other sites (e.g. parabens). Rubber allergy may be associated with compression bandaging and elastic hosiery. Allergy to colophony and derivatives may occur from dressing adhesives, and nylon dye allergy may be seen from hosiery. Rubber boots provoke dermatitis either at their upper edge or on the calf in areas of greatest friction.
Feet. Dermatitis may result from shoe materials including leather, rubber, glues and nickel, stockings, topical medicaments, antiseptics and antiperspirants.
Generalized. Generalized erythroderma may be the result of a chronic contact dermatitis maintained by continued exposure to a wide range of allergens, including components of topical medicaments. Patch testing is not possible until the skin has cleared.
Exposed sites
Contact dermatitis from dust, sprays, pollens or volatile chemicals is typically confined to the exposed surfaces of the hands, arms, face and neck. The first attack often originates from direct handling of an allergen, but recurrences may be seen despite avoidance of direct contact. There is great diversity in the nature of airborne reactions, which may be irritant, allergic, phototoxic, photoallergic or contact urticarial. Some agents may cause more than one type of reaction.
Lists of airborne allergens have been published and updated by Huygens and Goossens [1]. Plants, natural resins and woods are among the commoner causes of this distribution of contact allergy. In the USA, the oleoresins of ragweed commonly cause allergic contact dermatitis. A similar pattern in the UK during the summer months is caused by other Compositae (Asteraceae) weeds which, when they occur in other parts of the world, also produce an ‘airborne’ pattern of dermatitis. In India and Pakistan, Parthenium has been associated with widespread epidemics of severe dermatitis and even deaths [2].
Dermatitis from wood dust is common in carpenters and cabinet makers. It normally starts on the eyelids or the lower half of the face, and is often preceded by a period of itching. Swelling and redness spread to the neck, hands and forearms. By the time the patient attends for treatment, a diffuse dermatitis may have developed, distinctly limited at the margins of the sleeves and collar. Because of the accumulation of dust and sweat, the elbow flexures and the skin under a tight collar are often lichenified. Cabinet makers frequently develop a genital dermatitis from the accumulation of sawdust on the clothes during sawing and planing, and by hand contact. Swelling and redness of the eyelids may be the only signs of recurrence. Exotic woods are more likely to sensitize than fir or spruce, although the latter may cause dermatitis in patients sensitive to colophony and turpentine. Dermatitis in woodworkers may additionally be caused by liverworts and lichens on the bark of trees. Colophony can also give an exposure pattern of dermatitis from its presence in solder fluxes, paper dust, polish and linoleum flooring [3, 4].
Resin systems, particularly epoxy resins, including the more volatile amine hardeners, may induce an airborne pattern of allergy, especially in the occupational setting. Other causes of this pattern include perfumes, metals, many industrial and pharmaceutical chemicals, pesticides, fungicides, animal feed additives, textile dyes and matches [1]. Equivalent patterns of airborne dermatitis may be seen with type I allergens, such as house-dust mite antigens in atopics [5].
Photocontact allergy causes a similar distribution, and is discussed in a separate section in this chapter.
Mucous membranes
The application of DNCB to the oral mucosa may, in some cases, induce a low degree of contact sensitivity, but more often an immunological tolerance to later sensitization [1]. Similarly, prior exposure to nickel and chromate in orthodontic appliances seems to reduce the risk of later sensitization [2, 3].
Contact inflammation of the mucous membranes is uncommon, and is often secondary to skin sensitization with the same substances. Reactions may be allergic or irritant in nature. Both immune and non-immune immediate-type contact urticarial reactions may occur.
The skin and mucous membranes differ in both anatomy and environment. In the mouth, except on the gums and hard palate, there is no horny layer with a barrier function and storage capacity. There is no lipid secretion, but instead a continuous flow of saliva, which washes away foreign substances. The penetration of water-soluble substances is rapid. It is not known whether these differences are relevant to the paucity of allergic contact reactions in mucous membranes. The oral mucosa of sensitized animals has been shown to have an identical cellular phenotype and cytokine expression as the skin when challenged by an allergen [4].
Allergic reactions in the mouth show erythema and swelling, but vesicles are rarely seen, except on the vermilion border. Intraoral blistering has been seen from cinnamon allergy [5]. The symptoms are soreness and burning, and itching is uncommon. Eczematous reactions of the adjacent skin may occur, and these may be the only signs of an allergic contact dermatitis.
The burning mouth syndrome is an entity in which psychological factors are important (see also Chapter 84). Sometimes, allergens such as metals, rubber, food additives and flavourings have been identified and the symptoms relieved by contact avoidance [6], but the return from investigations is often disappointing [7].
Oro-facial granulomatosis has been associated with contact allergy to food additives and some of these individuals may obtain a favourable response, often only partial, to dietary elimination of the identified allergens [8] (see also Chapter 110).
Dentures are frequently incriminated as the cause of oral symptoms and lesions. Allergic reactions to denture materials have been found in some cases [9], due to traces of residual acrylic monomer following the wearing of new dental appliances or following their repair with cold-curing resins. Most cases are the result of irritation from ill-fitting dentures. Candidal infection may also play a role [10], and is often present in angular cheilitis. Acrylate allergy has also been seen rarely after dental restorative work [11], but may be seen secondary to primary sensitization from their use in artificial nails.
Mercury from amalgam fillings may cause local mucosal [12] or lichenoid [13, 14] reactions; perioral dermatitis after dental filling may also occur, as may generalized skin eruptions [15]. Contact reactions to other metals have also been reported, especially gold used in dental restorative materials, and nickel and palladium in orthodontic appliances [16, 17]. Gingivitis is reported from eugenol in dental cement [18]. Toothpaste flavours can cause stomatitis, glossitis, gingivitis, cheilitis and perioral eczema [19].
The nasal mucosa may react to medications containing antibacterial agents and antihistamines. Corticosteroid allergy from nasal sprays has been reported, with one case of associated perforation of the nasal septum [20]. In the conjunctivae, various drugs are reported to have elicited allergic contact reactions, for example β-blocking compounds for the treatment of glaucoma, antibiotics, and preservatives in both drugs and contact lens solutions [21].
Genital mucous membranes may be affected by allergens, particularly medicaments, causing dermatitis on the surrounding skin [22].
There remains debate as to whether materials sensitize via the mucosal route or whether there is only elicitation of pre-existing sensitivity (in those already sensitized) and the induction of tolerance (in those who have not already been sensitized) [3, 4].
Specific allergens
Metals
Chemistry. In common with cobalt, but unlike chromium, the metal itself sensitizes and is, in practice, the most frequent source of sensitization [2]. Most salts, for example nickel chloride (NiCl2) and nickel sulphate (NiSO4), are readily soluble in water and sweat and have strong sensitizing properties. Some oxides (e.g. Ni2O3) and the hydroxide (Ni(OH)2) can elicit contact dermatitis, but heated NiO does not. This compound is insoluble even in hydrochloric acid.
Incidence and prevalence. Nickel is the most frequent contact allergen, and sensitivity is more common in women than in men. The prevalence of nickel sensitivity recorded in a patch test clinic is between 15% and 30%, and is influenced by the relative number of females tested. Nickel is the most usual cause of contact dermatitis in women. All age groups are affected, but the prevalence of nickel allergy among females tends to rise from 10 years of age onwards [4]. The incidence of nickel dermatitis in Denmark varied with the total imports of nickel during and after the Second World War [5], which suggests that the level of sensitivity is determined by the total nickel exposure in the environment. In Denmark in the early 1990s it was found that the prevalence of nickel dermatitis in the general population was 11.1% in women and 2.2% in men. Sensitization to nickel was found in 14.8% of those with pierced ears and 1.4% of those who had not had their ears pierced, confirming that ear piercing is a significant risk factor for the development of nickel sensitivity [6]. A more recent study from northern Norway showed the prevalence of nickel allergy in women was 27.5% and in men 5.1%, with a clear relationship in women to the number of earlobe piercings [7].
This female predominance is not universal. In Kuwait, nickel allergy was commoner in males [8], and in Nigeria [9] and Japan [10] the prevalence was similar in both sexes in patch-tested patients.
The prevalence may be higher in some occupational groups, for example hairdressers, in whom studies have shown that 27–38% are nickel allergic [11, 12].
Legislation was introduced in Denmark in 1990 with the intention of controlling the use of nickel-releasing objects in contact with the skin. In 1994 the EU passed regulations based on the Danish legislation (Box 128.1). These laws only relate to metallic items in prolonged and direct skin contact, for example jewellery, clothing items and spectacle frames. Other metallic materials with which there is relatively short-term contact (e.g. coins, keys, cutlery) were excluded from the legislation. In a large pan-European retrospective study of patch test data from 1985 to 2010, a statistically significant decrease of nickel allergy was observed in Danish, German and Italian women below the age of 30 years. In British female patients, this was observed between 2004 and 2010. In young men, a statistically significant decrease of nickel allergy was observed in Germany and the UK, whereas a non-significant increase was observed in Italy. The reduction in the prevalence of nickel allergy in young women was contemporaneous with the introduction of the nickel regulations. A reduction is also suggested in German and British men [13].
The prevalence of allergy to nickel in under-18s attending for patch tests in Denmark has decreased considerably, from 24.8% in 1985–86 to 9.2% in 1997–98 [14], and in Germany there has also been a significant fall in frequency of nickel allergy in those under 30 [15].
Occurrence [16]. The commonest sources of metallic nickel are alloys and plated objects. Sensitization is chiefly the result of frequent skin contact with corroded objects containing nickel. A high rate of corrosion has been documented from nickel-plated items, nickel-iron, German silver, coin and several other alloys [17]. Chromium-plated metal is often first nickel-plated, and after long use the nickel may reach the surface, for example on water taps. Stainless steels contain nickel but most are incapable of releasing sufficient quantities to elicit allergic contact dermatitis. Quantitative studies indicate that repeated exposure to occluded metal items releasing nickel at a rate greater than 0.5 μg/cm2/week involves a significant risk of nickel sensitization [18], but thereafter very small amounts of nickel are sufficient to elicit dermatitis in sensitized persons.
Jewellery and metal components of clothing are the usual sources of nickel in prolonged contact with the skin. Transient but potentially frequent and repeated exposure may occur from handling coins, keys, scissors, knitting needles, thimbles, scouring pads and other metallic tools and utensils. Most silver coins, including the new UK 1 and 2 pound sterling coins, contain nickel, and there is debate as to whether exposure might be a risk factor in those already sensitized to nickel [19]. Platers and some metal machinists are necessarily at risk of occupational nickel allergy. Other sources include pigments in glass, pottery and enamel, electrocautery plates [20], mobile phones [21], laptop computers [22], bindi [23], intravenous cannulae [24], tattoo pigment [25],orthodontic appliances [26], metal scouring pads and even soaps [27] and detergents [16]. Nickel has been identified in some eye cosmetics [28, 29].
Systemic exposure may take place from the diet. Certain foods and plants contain much higher concentrations than others, as can particular sources of domestic water [30], and nickel may also be a contaminant in fertilizers [31] and fungicides. Stainless steel saucepans release negligible nickel, but cooking acid fruit in them, particularly when new, has the potential to contribute to dietary intake [32].
Systemic exposure from implanted metals is considered in the section ‘Cutaneous reactions to implanted metals’.
Clinical features. Classic nickel allergy is identified by patches of dermatitis at sites of contact with metal objects, most commonly the ears from earrings, the wrists from watches and bracelets, the neck from necklaces and their clasps, the central back and upper chest from bra components, the central abdomen from studs and zips in trousers, especially jeans (Figure 128.12), and the dorsa of the feet from shoe buckles. Lesions on the upper cheeks and sides of the nose and face may relate to metal-framed spectacles, and on the eyelids to eyelash curlers. A discoid pattern around the lower trunk and thighs from metal studs in clothing is quite frequent, although the involvement of the thighs from metal suspenders has all but disappeared following the advent of tights (pantyhose).
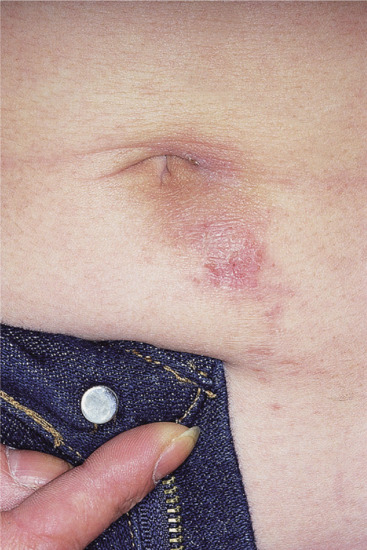
Figure 128.12 Allergic contact dermatitis to nickel in metal studs on jeans. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
The eruption may be papular, nummular, diffuse or consist only of excoriated papules on almost normal-looking skin. Some patients are referred to dermatologists because of the spread of dermatitis to distant regions. These secondary eruptions used to be a characteristic feature of nickel dermatitis [33], but now seem to be less common. The secondary rash normally starts shortly after, or at the same time as, the primary eruption. It affects the neck, face (especially the eyelids; Figure 128.13), the elbow flexures and the flexor surfaces of the arms; the ano-genital area may also be affected, and the rash may be generalized. Flexural lesions may resemble textile dermatitis or atopic eczema.
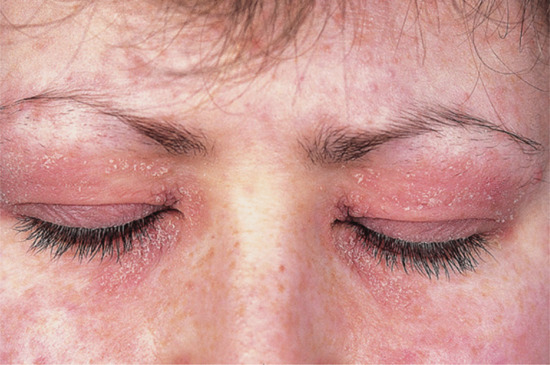
Figure 128.13 Secondary eyelid dermatitis in a patient sensitive to nickel. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
The relationship between hand eczema and nickel sensitivity remains complex [34]. However, well-controlled statistical studies support a connection between hand eczema and nickel allergy [35, 36, 37], and nickel-sensitive women do appear to have a predilection for hand eczema [34, 38]. However, nickel allergy in childhood did not seem to predispose to hand dermatitis later in life in one study [39]. Hand eczema is often multifactorial, and is particularly common in women who have a heavy burden of housework or who are employed in occupations that expose the skin regularly to trauma or wet work. There may be a vesicular palmar (dyshidrotic) pattern, but other distributions occur without being diagnostic. Wet work, atopy and nickel sensitivity are associated with an increased risk of hand dermatitis [39], although atopy is probably the most important factor [40]. Analytical data demonstrate that consumer products such as personal care items, detergents and cleaning products do not contain sufficient nickel, cobalt or chromium to pose a risk to those who do wet work [16].
Sometimes, nickel allergy is directly of occupational origin, and in more than half of these cases it starts on the hands. It is normally associated with the metal and nickel-plating industries. The pattern of dermatitis on the hands is rarely diagnostic. Spread occurs to the elbow flexures, eyelids and face in the same manner as described above.
A recurrent vesicular palmar (dyshidrotic) pattern of eczema has been related to dietary intake of nickel. Ingestion of nickel sulphate caused a flare of vesicular hand eczema in nine of 12 patients studied by Christensen and Möller [41]. The significance of this has been disputed, as similar results have been demonstrated in non-sensitized patients and the challenge dose was artificially high [42, 43, 44, 45]. However, a subsequent placebo-controlled oral challenge did show a flare of dermatitis, including the original site, in four of 10 nickel-allergic subjects receiving the nickel equivalent to a normal dietary intake [46]. A further meta-analysis of 17 relevant studies has concluded that 1% of those exposed to a normal dietary nickel intake will develop systemic contact dermatitis [47].
Oral symptoms and systemically reactivated dermatitis have also resulted from nickel in orthodontic appliances, in those already sensitized [26, 48, 49]. Paradoxically, tolerance to nickel exposure may develop as a result of nickel release from orthodontic appliances in those who are not already sensitized [50, 51].
Avoidance. Most people think of their environment in terms of objects rather than materials, and it is important to realize that they find it difficult to identify nickel, unless the possible causes of contact are specifically listed. The importance of minor items, such as eyelash curlers, zip fasteners and jeans studs, may not otherwise be appreciated. Many dermatologists provide all nickel-sensitive patients with a list of possible contact items. A dimethylglyoxime test kit (see separate section later in this chapter) may also be of use in identifying nickel-containing objects among a patient's personal items at work or in the home [17].
Nickel cannot be entirely avoided in daily life, but the elimination of nickel from clothing and avoidance of nickel-containing jewellery may be sufficient to clear dermatitis. In our experience, initial compliance with avoidance advice is poor, particularly with clothing, and repeated explanations may be necessary. Waterproof tape and metal lacquer can be used to cover nickel-plated objects that cannot be replaced, although nickel can leach out if the contact site is sweaty and prone to friction. Contact may be difficult to avoid in certain occupations. Protection with rubber gloves may be insufficient, as nickel solutions may penetrate them [52]. Heavy-duty vinyl gloves have been suggested as an alternative.
Prognosis. The prognosis of dermatitis due to nickel in jewellery and clothing is excellent if further use of nickel-plated objects is avoided. Once the hands are involved, the eczema may remain chronic, persistent or intermittent. Ingestion of nickel is a possible cause of chronicity [53].
Specific therapies. Barrier creams and cleansers containing chelating agents may have potential benefit, and a number have shown promise under experimental conditions [54, 55, 56]. Clioquinol is known to chelate nickel [57], and a topical clioquinol–steroid combination can be considered as a treatment.
Dietary reduction of nickel intake is recommended, by some, for those nickel-allergic subjects with recurrent palmar vesicular eczema. Knowledge of the nickel content of foods is at present imprecise, and the prescription of a low-nickel diet [58] is not always practical [45]. Nevertheless, there are strong advocates for this approach and a trial of dietary reduction may be worthwhile, although this is frequently disappointing in our experience.
Treatment with tetraethylthiuramdisulphide (disulfiram; Antabuse), which chelates nickel, has been reported as helpful [59], but has a significant prevalence of side effects [60]. Liver enzymes should be carefully monitored. An alternative chelating agent, trientine, gave disappointing results in a small, open trial [61].
Patch tests. Nickel sulphate 5% in petrolatum is used for patch tests. False negative reactions are common with 2.5% nickel in petrolatum or 2% aqueous nickel [62]. False negative reactions may also occur with 5% nickel sulphate in petrolatum because nickel ions penetrate the skin only very slowly [63]. Testing with nickel sulphate may produce irritant false positive reactions with a deep erythema and pustulation, especially in atopics. Some follicular reactions are irritant, but those with raised papules are often truly allergic in our experience.
Cobalt [1]
Chemistry. Cobalt metal and its oxides (e.g. Co2O3 and CoO) and salts (e.g. CoCl2 and CoSO4) are sensitizers. Also, heated CoO elicits positive patch test reactions (unlike NiO).
Prevalence. Little is known of the prevalence of allergy in the general population but one study showed that 1.1% of an unselected Danish population of 567 individuals were patch test positive [2]. A more recent Norwegian study found 4.3% of women and 0.9% of men to be allergic [3]. Of patients with dermatitis, 4.6–9% were patch test positive, with females predominating [4].
Occurrence. Metallic cobalt is present in ‘hard metal’ used for metal cutting and drilling. It is used in magnets and jewellery. It is always present as a contaminant in nickel [5]. It occurs in alloys, for example vitallium used in dentures and in nails for pinning fractures.
Cobalt oxides, present as traces in cement, are sensitizers; however, isolated cobalt allergy from cement is much rarer than its occurrence in association with chromium allergy. The cobalt content of cement is about the same as that of chromium [6]. Cobalt compounds are found in paints, glass, china, pottery, ceramics, enamel (blue), coloured crayons and animal feed additives [7], multivitamin pills, textile dyes [8], tattoos [9], soaps [10], cosmetic pigments, hair dye and detergents [11]. Recently, leather has also been shown to be a frequent source of sensitization [12].
The salts are seldom used for plating, unlike nickel salts, although cobalt chloride has sensitized in a metal-etching solution [13]. Organic compounds (e.g. cobalt naphthenate, resinate, stearate) are used as driers in paints and varnishes, bonders of rubber to metal [13] and accelerators for unsaturated polyester resins [14]. They may also be present as additives in lubricating oils.
Clinical features. As cobalt is an invariable contaminant of nickel, the clinical features of cobalt allergy can be identical to those of nickel allergy. Cobalt sensitivity might explain why some women with dermatitis typical of that provoked by nickel have a negative patch test reaction to the latter. Furthermore, its presence in cement may induce a clinical pattern identical to allergy from chromate in this source. Isolated cobalt allergy is seen in hard-metal workers and in the pottery and glass industries, when it is usually associated with hand dermatitis. Stomatitis has been reported from dentures [15]. Allergic granulomatous reactions to tattoo pigment are recognized, but are rare in our experience. Animal feed may induce contact allergy [16], and photocontact dermatitis has been reported from this source, as well as from cement [17]. Vitamin B12 is a cobalt-containing compound and cheilitis has been reported from oral vitamin B12 ingestion [15], and dermatitis from its parenteral use [18]. An oral lichenoid eruption to a chrome/cobalt prosthesis has been reported [19]. It can be difficult to identify the source of allergy when there is an isolated positive cobalt patch test.
The relationship of cobalt allergy to metal implants is discussed in the section ‘Cutaneous reactions to implanted metals’.
Avoidance. This will depend on identifying a relevant cause and eliminating contact. In those with a nickel allergic pattern, the advice is the same as for nickel-allergic subjects; similarly, for those with cement allergy, the advice is the same as for chromate. Reduction of the dietary intake of cobalt (monitoring plasma vitamin B12 if prolonged) may benefit some cobalt-sensitive patients [20]. A cobalt spot test has also been developed and is now commercially available [21].
Prognosis. Concomitant cobalt and chromate sensitivity is associated with more troublesome dermatitis than that which occurs with chromate allergy alone [22]. Possibly the same applies to a combined nickel and cobalt sensitivity because of the increased number of contact sources, which may cause recurrence of the dermatitis.
Patch tests. Cobalt chloride 1% in petrolatum is reliable for testing [11]. False positive, irritant, purpuric reactions are common, especially in atopics.
Chromium [1, 2]
Chemistry. The metal itself, if not dissolved in oil [3] or acids or as a salt, seems to be non-sensitizing, unlike nickel and cobalt. This is probably due to the insoluble monomolecular layer of chromium (III) oxide (Cr2O3) on the surface [4].
Hexavalent chromate (occurring as an anion), for example in chromic acid or chromium (VI) trioxide (CrO3) and in chromates and dichromates of potassium, sodium and ammonium, is the commonest sensitizer. It occurs in alkaline solution as chromate (K2CrO4) and in acid solution as dichromate (K2Cr2O7). The less soluble lead chromate, barium chromate and zinc chromate (ZnCrO4) are also allergenic.
The trivalent chromium compounds (occurring as cations), for example chromium trichloride (CrCl3), are sensitizers but, being less readily absorbed into the skin, they have been considered to be of less clinical importance [5]. However, recent work challenges this view and combined Cr III and Cr VI allergy may be equally important [6].
Incidence and prevalence. In Europe, chromate was for many years a frequent cause of occupational allergic contact dermatitis and chronic incapacity [5]. The prevalence of sensitivity is commoner in men than in women and is higher in clinics where men with occupational dermatitis predominate. A study of construction workers attending occupational contact dermatitis clinics in Germany showed that potassium dichromate was the commonest allergen, at 31.9% [7], whereas chromate sensitivity was found in less than 2% of patients attending the general patch test clinic. In some countries (including the UK) chromate sensitivity is less common [8]. Nevertheless, nearly 25% of UK-based grouters assessed during the construction of the Channel tunnel gave a history of occupational dermatitis and 17% were allergic to chromate [9]. Review of EPIDERM data in the UK found that 6% of cases of occupational dermatitis reported by dermatologists were from chromate, often with an onset later in life [10].
In Scandinavian countries, the addition of ferrous sulphate to cement to convert the more sensitizing hexavalent chromate to the less sensitizing trivalent chromate (because it is less easily absorbed) appears to have decreased the risk of sensitization in construction workers [11], although other changes in cement manufacture and increased mechanization may also be contributory factors [12]. In 2005, the EU directed that levels of chromate in cement should be restricted to 2 ppm hexavalent chromium (EU Directive 2003/53/EC) [13], and the use or supply of other sources of cement was prohibited in the UK. Data concerning work-related allergic contact dermatitis to chromate has subsequently shown a significant decline [14].
Occurrence [1, 2]. The main source of hexavalent chromium is cement [9], although the amount varies widely [15, 16]. Other important sources are antirust paints (lead chromate and zinc chromate) [17], including dust liberated by drilling, cutting or sandpapering of painted metals which may cause contact dermatitis on the hands, arms and face. Further sources are plating salts, metal alloys, lithography/offset printing materials, anticorrosive oil, cutting oils, cooling water [18], foundry sand, polysulphide sealants [19], matches [20], photographic chemicals, chemicals for fat determination in milk, welding fumes [21], wood preservatives, wood ashes, wood pulp [22], mordant in wool dyeing, stains in glass, glazing enamels [23], catgut, violin strings [24], coating on zinc-galvanized iron sheets [25], textiles [26], glass polishing [27], flour [28], tyre-fitting solution [29], colour television manufacture [30], soaps and detergents [31] and dental prostheses [32]. Chromate sensitivity in some European women was found to be related to chromate in household bleach [33], which was subsequently removed.
Among trivalent compounds, basic chromium sulphate used as a tanning agent for leather is the most important [5]. Exposure to chromate in leather occurs occupationally in tanners, and in the general population from clothing, especially shoes, and furniture. It is the most important source of non-occupational allergic contact dermatitis to chromium.
Clinical features. Acute weeping dermatitis is unusual in patients allergic to chromate in cement; more commonly there is a dry insidious eruption, which tends to fissure, particularly on the hands. Secondary lichenification is often a feature. There is frequently a concomitant irritant element, because cement is alkaline, hygroscopic and abrasive. Primary irritant dermatitis and discoid and atopic eczema may be mimicked, and a palmar distribution may be difficult to distinguish from chronic tinea manuum. Widespread eruptions may occur from cement dust, with flexural accentuation and involvement of the ankles and dorsa of the feet. Palmar vesicular eruptions have been blamed on traces of chromate in the diet [34]. Contact with leather footwear, gloves, belts and other clothing, or even handbags and purses, may produce dermatitis in those areas in contact with the material. Exposure to leather furniture has induced eczematous flares on the back, calves, arms and feet in sensitized subjects [35]. An oral lichenoid eruption to a chrome/cobalt prosthesis has been reported [36].
Prognosis. Chromate sensitivity tends to persist [37], and the prognosis of occupational dermatitis is poor as a result of its persistence and the associated social and financial handicap [38]. Fewer than 20% of cases were clear of dermatitis when reviewed after 10 years [39]. In men, allergy to chromate carries a worse prognosis than does sensitization to other allergens [40]. Chronicity and frequent relapses are the rule; the latter are more frequent than in any other industrial dermatosis [41] and affected individuals have been labelled as ‘chrome cripples’ [39]. Once established, hand dermatitis tends to continue, and superimposed shoe dermatitis may prevent any improvement unless chromate-free shoes can be acquired. Few of those affected give up their work despite the chronicity of the condition [42], and in one study only 8% of chromate-sensitized cement workers were clear of dermatitis 10–13 years after the initial eruption. Changing work to avoid contact with cement does not seem to improve the prognosis [43]. These findings contrast with a Swiss study in which occupational chromate dermatitis resolved in 72% of individuals as a result of strictly enforced avoidance measures and financial support given by their regulatory authorities [44]. Many chromate-sensitized cement workers develop hardening and are able to continue at work, albeit with ongoing but manageable dermatitis. Positive patch tests have been reported in cement workers with no dermatitis [45]. Insufficient knowledge of the occurrence of chromate in the environment may account for the poor prognosis, and it is suggested that tiny amounts and oral ingestion may maintain the dermatitis [46].
Avoidance. Avoidance of contact with sources of chromate, including leather footwear and gloves, will be necessary, although those cement workers with hardening may be able to stay at their work, remembering that there is a poor prognosis. Ferrous sulphate added to cement converts soluble hexavalent chromate to insoluble trivalent chromate, thus potentially preventing chromium sensitization by cement. Various reducing agents [47], chelating compounds and ion exchangers have been recommended as components of hand creams to prevent dermatitis in chromate-sensitive individuals [48, 49], and these may have value; however, long-term studies are lacking.
It is not yet known whether reduction of the dietary intake of chromate might benefit chromate-sensitive patients [50]. Dapsone has been suggested as a treatment, but no controlled trial has been undertaken [51]. In patients sensitized to chromium from leather footwear it is possible to source ‘vegetarian shoes’ and replace leather inners with cork insoles; it is usually necessary to replace contaminated hosiery.
Patch tests. Sensitivity is demonstrated by a closed patch test with potassium dichromate 0.5% in petrolatum. At this concentration, weak irritant reactions are quite common, especially in atopics, but lower concentrations will miss relevant positives [51]. Nevertheless, in the USA, a concentration of 0.25% is recommended because of the potential for false positive results. A compromise (0.375%) has been suggested, although there may still be a risk of false positive and false negative reactions [52]. Dilutions can be tested to assist in distinguishing allergic from irritant reactions.
Palladium [1]
Chemistry. Palladium is a relatively inexpensive metal of the platinum group of elements.
Prevalence. Of patients undergoing routine patch testing to palladium chloride, 3–8% were shown to be allergic [2]. The sensitization rate is reported to be increasing [3]. Nearly always there is concomitant sensitivity to nickel, and guinea pig and clinical studies have suggested this may be a true cross-reaction [4, 5]. There are, however, mixed views as to whether this association is concomitant sensitivity, cross-reactivity or contamination of palladium chloride by nickel sulphate [6, 7, 8].
Occurrence. Palladium is increasingly used in dental alloys, prostheses and industry [3]. It can be used as a whitener in white gold. Occupationally, its main uses are in electrical components and as a catalyst.
Clinical features. The clinical relevance of a positive palladium chloride patch test reaction is questionable in many instances, and may just be a reflection of nickel allergy. Stomatitis and lichen planus have nevertheless been related to palladium in dental materials [9, 10]. The removal of prostheses or dental alloys containing palladium may need to be considered in these instances. A granulomatous reaction after ear piercing has also been seen with palladium allergy [11].
Patch tests. Palladium chloride is normally tested at 1% in petrolatum.
Gold [1]
Chemistry. Metallic gold is soft, malleable and ductile. It is stable and resistant to corrosion. Its strength is increased when alloyed with other metals. Gold salts, such as gold trichloride and potassium dicyanoaurate, are recognized as sensitizing as well as irritant.
Occurrence. Metallic gold is mainly encountered in jewellery, stents and dental materials. Gold salts are used in the plating, electronics, photographic, glass and porcelain industries [1].
Prevalence. Metallic gold has, until recently, been regarded as safe and very unlikely to sensitize. However, when gold sodium thiosulphate was added to the standard patch test series, positive reactions were obtained in 8.6% of a series of Swedish patients [2], and subsequent surveys of various selected subgroups recorded a frequency of positive reactions ranging from 1% to 23% [1]. There is a female predominance, and where relevance has been found it has usually been in the context of jewellery or gold dental work [3]. However, the allergic mechanism behind the positive patch tests, and their relevance, have been questioned [3, 4]. There is a relationship between the amount of dental gold and frequency of allergy [5]. There is also evidence of an increased rate of allergy to gold after the use of gold-plated cardiac stents (see the section on cutaneous reactions to implanted metals later in this chapter) [6].
Clinical features. In our experience a relevance for a gold sodium thiosulphate positive patch test is found infrequently, and generally these patients can wear jewellery and have gold dental fillings without problems [2, 3]. Nevertheless, analysis of the involved anatomical sites has been undertaken by others who have found that involvement of fingers, ear lobes and eyes by dermatitis predominates [3]. A seborrhoeic eczema pattern has been described [7], as have persistent papules and nodules on the ear lobes, with lymphomatoid or granulomatous histology [8, 9]. Reported oral manifestations of allergy have included erythema, burning mouth, erosions, ulceration, oro-facial granulomatosis and lichen planus-like lesions [10, 11, 12, 13].
Sodium aurothiomalate injections for rheumatoid arthritis have induced systemic contact dermatitis and ‘fever’ in those previously sensitized to gold [14].
Acral dermatitis has been described from allergy to gold salts in the gilding industry [15].
Patch tests. Many gold salts have been used for patch testing, but most centres now use gold sodium thiosulphate 0.5% in petrolatum. Late reactions are common and an additional 7-day or even 2- or 3-week reading has been advised [16]. The appearance of a positive patch test may be ‘dermal’, with erythema and oedema but no vesiculation, and persistent patch test reactions are well recognized [16]. The controversy over the debatable relevance has led many to advise against routine standard-series screening for gold allergy [4].
Mercury [1]
Chemistry. The metal and its inorganic salts, for example corrosive sublimate (HgCl2), calomel (HgCl), fulminate (Hg(CNO)2) and ammoniated mercury (HgCl·2NH4Cl), as well as organic compounds (e.g. mercurochrome, thimerosal and phenylmercuric salts), may all sensitize.
Occurrence. The metal is used in instruments and amalgam (alloy of silver or copper and mercury) for filling teeth. Mercury and inorganic mercurials may be used in disinfectants, fungicides, herbicides, insecticides, detonators, emulsion paints and jewellery, as well as in the production of caustic soda and chlorine. Red mercuric sulphide (cinnabar, HgS) is used in red tattoos and in artists’ paints.
Organic mercurials may be found in topical and parenteral medicaments (see the section on organic mercurial later in this chapter). Ammoniated mercury has been used in the topical treatment of psoriasis, although concerns about toxicity have led to its withdrawal in the UK. Mercury and ammoniated mercury have been used in skin-lightening creams.
Clinical features. Contact dermatitis is only rarely seen on the skin from mercury and inorganic mercurials in the UK but amalgam fillings in patients already sensitized to mercury have caused local mucosal reactions and stomatitis, which settled when they were removed [2]. Hypertrophic amalgam dermatitis simulating carcinoma of the tongue has been described in one patient [3]. Perioral dermatitis after dental filling may also occur [2].
There are many reports of oral lichen planus in association with amalgam fillings [4, 5] but the relationship is not consistent [6, 7]. Patch tests to mercury are frequently positive when lichen planus is adjacent to amalgam fillings, but less so when there is not a close anatomical relationship [5]. The disorder is more apparent when there is corrosion [8]. In many sensitized subjects, the condition will improve or settle when the amalgam is removed [5, 9]. The causes of lichen planus are nevertheless likely to be multifactorial [5].
The relationship between oral inflammation, burning mouth syndrome and mercury allergy is contentious [6, 10, 11], but some individuals with mercury allergy have responded to amalgam removal. Oro-facial granulomatosis has also been seen in association with mercury allergy and has resolved after removal of amalgam fillings [6, 12].
Generalized exanthems and erythema multiforme have been reported from mercury exposure, including inhalation, dental fillings, following the breakage of thermometers in the mouth and also the use of an antiparasitic powder for the treatment of crab lice [13, 14]. Recalcitrant eczemas in mercury-sensitized individuals are recorded as clearing after the removal of mercury amalgam fillings [15], although in most cases systemic reactions from amalgam seem to develop a few hours after insertion or removal and settle after 10–14 days [16]. In our view, malaise and general ill health are not related to allergy to mercury in amalgams.
Red mercuric sulphide (cinnabar) in a tattoo may induce granulomatous reactions in allergic subjects [17]. We have seen several granulomatous and lichenoid reactions confined to the red parts of tattoos but none of our patients has been allergic to mercurials.
Patch tests. Mercury is normally tested at 0.5% in petrolatum, mercurochrome 2% in petrolatum or aqueous, mercuric chloride 0.1% in petrolatum and ammoniated mercury 2% in petrolatum (pet.). However, mercury compounds can be irritant, and aqueous solutions of mercury salts may react with aluminium in Finn chambers to cause false positive reactions [2]. Patch testing with both mercury and ammoniated mercury is suggested if allergy is suspected [2]. Patch testing to amalgam is also possible and is available commercially at 5% pet. and as amalgam alloying metals at 20% pet.
Aluminium
Occurrence and clinical features. Aluminium is widely used but contact allergy is very rare. Most reported cases are from aluminium-adsorbed vaccines and parenteral solutions used for hyposensitization, with granulomatous reactions at the injection site [1, 2]. It is found in antiperspirants, and axillary dermatitis (usually irritant) may occur. Allergy in a child with chronic otitis externa treated with aluminium acetate ear drops has been seen [3].
Patch tests. As Finn chambers are aluminium, a positive patch test, often annular in configuration, may develop under every single test site in sensitized persons. Patch testing is best undertaken with plastic chambers if this diagnosis is suspected. Pure aluminium metal or salts, for example aluminium acetate 10% aqueous or aluminium chloride 2% aqueous, can be used for testing.
Other metals
Copper is a ubiquitous metal found especially in coinage, jewellery, pipes, electrical equipment and wiring. Its salts are used in insecticides, fungicides, wood preservatives, food processing, fertilizers and fur dyes. Contact allergy is very rare. Dermatitis has been reported from copper intrauterine contraceptive devices and proven by patch testing and resolution of the dermatitis after removal [1, 2].
Other metals used in dentistry may have the potential to cause contact allergy, including platinum, rhodium, indium and iridium [3, 4, 5].
Fragrances, balsams, flavouring agents and spices
Perfumes are blends of fragrance chemicals producing an odour intended to be aesthetically pleasant or to mask other less pleasant odours. The components are either of natural origin or produced synthetically. Natural sources include extracts from plants, trees, lichens and animals (e.g. musk, civet) [1, 2]. Commercially available perfumes are mixtures of essential oils from these sources and synthetic compounds, with usually at least 10, and up to several hundred, ingredients [3]. The scent or ‘note’ is determined by the mixture of volatile substances. ‘Fixatives’ are added to delay evaporation, influencing the quality and persistence of the perfume. Common ‘fixatives’ are balsams, benzyl benzoate, benzyl salicylate and synthetic musks.
Tree balsams contain many different fragrance and flavouring components. Balsam of Peru is one such material that has been studied in depth [4]. It comes from a tree, Myroxylon pereirae, that grows in Central America (not Peru!). The balsam was given its name because it was packed in, and shipped from, Peru to Europe [5]. It was widely used earlier this century for treating wounds, and also for scabies [4]. Its composition is still not completely known, but the balsam does contain benzyl benzoate, benzyl cinnamate, cinnamic acid alcohol and aldehyde, benzoic acid, vanillin, farnesol and nerolidol [6]. It may cross-sensitize with resorcinol monobenzoate used in cellulose ester plastics [7]. Other related balsams include balsam of Tolu, balsam of spruce, gum benzoin and storax.
Flavours may similarly be of natural or synthetic origin. Examples of natural flavours include citrus fruit peel, peppermint oil, spearmint and vanilla. Natural spices include nutmeg, mustard, cinnamon, cloves and oil of juniper. In the modern food industry a large number of synthetic flavouring agents are used. As with perfumes, flavours may be complicated mixtures.
A Euorpean Scientific Committee on Consumer Safety (SCCS) review of fragrances in 2011 (SCCS/1459/11) listed 82 substances that can be classified as established fragrance contact allergens. Of these, 54 are single chemicals and 28 are natural extracts, which in turn include all the ones that are required to be listed on consumer products. In addition 12 of the chemicals and eight of the natural extracts were found to pose a high risk of sensitization to the consumer, when considering the high number of reports with at least 100 such cases.
Prevalence. In general, as measured by the frequency of allergic reactions in routinely patch-tested patients, fragrances are the second most common allergen (after nickel). Studies have indicated that fragrance allergy affects in the region of 1% of the adult population [8], whereas children and adolescents have shown rates of 1.8% [9]. In those clinics investigating allergic contact dermatitis, the rates have varied between 5.7% and 17.4%, with roughly 10% being an average for European investigation clinics [1]. Sex incidence has generally only shown a slight preponderance of females, and in some instances it has been equal [10]. There is evidence from some centres that perfume allergy is increasing quite significantly [1].
The pattern of allergy may change. In one UK centre, although the level of allergy to the test allergen Fragrance mix I remained stable, a significant reduction of cinnamic aldehyde and cinnamic alcohol allergy occurred when components of the mix were tested [10]. This is thought to reflect a decreasing concentration of these materials in cosmetics in the last 17 years.
Routine patch testing with balsam of Peru as a marker of allergy to perfume and certain flavours has shown a positive rate of allergy of around 4% [11]. It was thought to be a decreasingly relevant marker of perfume allergy but a recent study from Finland has revealed a sharp rise in the frequency of allergy, perhaps in parallel with increased exposure to relevant components [12].
Fragrance labelling regulations have now been extended in the EU. One or more of 26 named fragrance allergens will be found on the ingredient label if present at 10 ppm or more in leave-on products and 100 ppm or more in rinse-off products. This should improve evaluation and surveillance of fragrance allergy within the population in the future.
Occurrence. Fragrances are ubiquitous. Perfumes, cosmetics, moisturizers, deodorants, aftershaves, soaps, bath additives, aromatherapy oils and toilet tissues and wipes are typical sources. Medicaments and work creams and cleansers often contain perfume. In the domestic environment cleansers, fabric conditioners, candles, pot pourri, air fresheners and polishes may all be perfumed. At work some materials (e.g. coolant oils) may contain a masking perfume [13]. Limonene is used in industrial and histology solvents and degreasing agents. d-limonene and other terpenes have been shown to act as allergens when they become oxidized and may therefore only be allergenic with prolonged exposure to air [14]. Auto-oxidation of terpenes may make a significant contribution to the allergenicity of perfumes [15].
Flavours and spices are found in foods, beverages, lipsalves and dental products, including toothpastes, which will often use the term ‘aroma’ to denote their presence.
Clinical features. The analysis of common patterns of perfume dermatitis has shown a tendency to involve the hands, face and neck in women (Figure 128.14); the hands, face and lower legs in men; and the axillae in both sexes [16]. A streaky pattern may be observed. There is evidence that allergy to more than one perfume component may result in a synergistic effect [1].
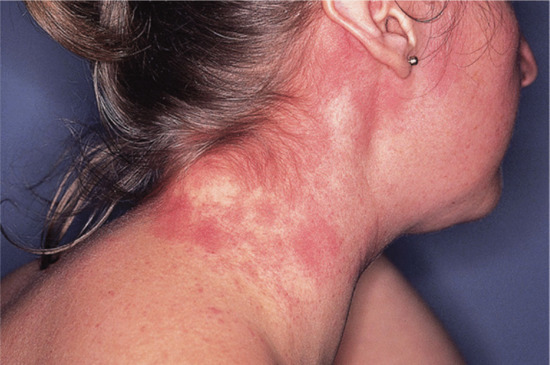
Figure 128.14 An urticated contact dermatitis in a patient allergic to fragrance. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Connubial (consort) allergy is well recognized [17], and allergy to lavender applied to a pillow has been described [18]. Many affected subjects suspect their allergy, but a substantial number do not. Furthermore, those who are aware of their allergy may continue to suffer dermatitis by failing to take appropriate avoidance measures, for example by unwittingly applying perfumed medicaments and cosmetics to their skin. Aromatherapists and their clients are liable to sensitization in sites where there is contact with the essential oils [19, 20].
d-limonene in its oxidized state may cause allergic occupational hand dermatitis [14], which can also develop in bakers and chefs as a result of contact with sensitizing flavouring agents [21]. Peeling of citrus fruit in the domestic environment may also induce allergic hand dermatitis.
Cheilitis may be a reflection of allergy to flavouring agents in toothpastes [22, 23], lipsalves [24] and food and drink [25]. Gingivitis has occurred from allergy to eugenol in dental cement [26], and cinnamon has induced oral blisters, erosions and lichen planus [27, 28].
Balsam of Peru is still used as a medicament, particularly in haemorrhoid preparations, and allergy is therefore relevant to perianal problems. Sensitizing balsams are used in medicaments and balms for wounds, sprains and joint pains, particularly in the Far East [29]. Tincture of benzoin is used in a similar way, and may also be used under orthopaedic plaster casts [30]. Vesicular hand dermatitis has been related to dietary intake of flavours related to balsam of Peru [31].
Musk ambrette is a synthetic perfume component responsible for photoallergy, and although its use has been stopped in the western world it may still be present in perfumed materials from other parts of the world.
Fragrance-allergic subjects appear to be at an increased risk of more frequent and more severe eye and respiratory symptoms than would be expected by chance [32].
Avoidance. Perfumes are marketed as concentrated liquids and in more diluted forms such as eau de toilette, or as sprays, and all should be avoided. The application of perfume to clothing may still cause problems in allergic subjects, who often believe they will only react if perfume is applied directly to the skin. Occasionally, affected subjects are able to use a specific perfume without any problem. Other perfumed skin products to be avoided include deodorants, aftershaves, talcum powders, soaps and bath additives.
The presence of perfume in a cosmetic or wet wipe within the EU will be denoted by the International Nomenclature of Cosmetic Ingredients (INCI) term ‘parfum’, and the presence of balsam of Peru by the INCI term ‘Myroxylon pereirae’; fragrance-allergic patients should avoid these products. Some cosmetics’ labels may suggest they are fragrance-free, yet the products are found to contain perfume when the full ingredient label is studied, reinforcing the need to avoid unlabelled products. Some plant extracts may potentially be a hidden source of fragrance in cosmetics as the INCI nomenclature may use the plant's Linnaean name rather than the word parfum. Some extracts, however, may only contain traces of fragrance chemicals. Within some personal care products the term ‘fragrance’ may also be replaced by the word ‘aroma’. Patients need to be made aware that the ever-increasingly popular range of products containing ‘botanicals’ can also act as a source of fragrance chemical exposure and might be best avoided.
Surprisingly, some prescribable moisturizers, emollients, bath additives and corticosteroids, as well as over-the-counter medicaments, contain perfume. Menthol, lavender oil and peppermint oil are the top allergens in patients reacting to a cream [33]. Fragrance-allergic patients with ongoing problems should also be counselled carefully about avoidance of these sources in medicaments.
In the domestic situation, perfume-containing sprays such as air fresheners, insect repellents and hairsprays should be avoided, as should skin contact with perfumed household cleansing products and polishes. Unperfumed soaps or soap substitutes are required for washing the skin. The levels of perfume residues from washing powders and fabric conditioners for clothes are probably too low to cause clinical problems [34], but in those with a clothing pattern of eczema, extra rinsing and avoidance of fabric conditioners can be considered. Peeling citrus fruit with the bare hands should be avoided by those with hand eczema and an allergy to balsam of Peru.
Furthermore, if a fragrance material has a second function in a formulation, such as a preservative (benzyl alcohol) or emollient (methyl benzoate), it can still be included in ‘fragrance-free’ products by unscrupulous manufacturers. Therefore, a review of the patient's own personal care products may be very useful.
In the occupational environment many cleansers, conditioning creams and barrier creams are perfumed, and similar avoidance measures are needed. Some work materials, including cutting oils and paints, may contain masking perfume and enquiries may be necessary to establish their components.
Dietary measures may be helpful not only for oral and perioral allergy from flavours but also in those with vesicular palmar eczema associated with balsam of Peru allergy. However, the response can be disappointing [35, 36].
Patch tests. The complexity of perfumes is such that there is not a perfect screening patch test for perfume allergy. Before 1977, the main recommended marker for perfume allergy was balsam of Peru, which is still advised. It is tested at 25% in petrolatum, but was thought to identify only 50% of perfume-allergic subjects [37]. Screening for perfume allergy was significantly advanced by the development of Fragrance mix as a result of Larsen's studies [38, 39]. He advised a mix of eight substances (Table 128.3). The original mix has been modified so that now each component is present at 1% concentration [40]. The mix now known as Fragrance mix I (FMI) contains an emulsifier, sorbitan sesquioleate at 5%, which is reported to have improved the return of identified perfume allergies [41]. Fragrance mix I will identify approximately 75% of perfume-allergic subjects, although this figure was reported to increase to 90% if balsam of Peru is also tested [42, 43]. However, more recently efforts have been made to improve the identification of allergic subjects following studies on a range of fragrances [44, 45]. In particular there was evidence from Europe that 4-(4-hydroxy-4-methylpentyl)3-cyclohexene carboxyaldehyde (Lyral®) was a perfume sensitizer that might be missed if reliance was placed on Fragrance mix I and balsam of Peru testing [42, 46]. This and five other fragrances (citral, farnesol, coumarin, citronellal and α-hexyl-cinnamaldehyde) were combined to make a further mix for patch testing known as Fragrance mix II (FMII) as a result of these studies (Table 128.4). Since 2008 the European Society of Contact Dermatitis (ESCD) has recommended using FMII mix in the baseline series and also that Lyral is tested separately at 5% pet, since it is such an important allergen to consider, with rates of allergy not seemingly declining from 2.5% in certain European centres [47].
Table 128.3 Ingredients of Fragrance mix I.a
| Substance | Concentration (%)b |
| Cinnamaldehyde | 1 |
| Cinnamyl alcohol | 1 |
| Eugenol | 1 |
| Amyl cinnamaldehyde | 1 |
| Hydroxycitronnellol | 1 |
| Geraniol | 1 |
| Isoeugenol | 1 |
| Oak moss absolute (Evernia prunastri) | 1 |
aFragrance mix allergens contain sorbitan sesquioleate (5% in petrolatum) as an emulsifier.
bAll ingredients are diluted in petrolatum.
Table 128.4 Ingredients of Fragrance mix II.
| Substance | Concentration (%)a |
| Alpha-hexyl cinnamldehyde | 5 |
| Citral | 1 |
| Citronellal | 0.5 |
| Farnesol | 2.5 |
| Coumarin | 2.5 |
| Hydroxy-methylpentyl-cyclohexene carboxyaldehyde (Lyral) | 2.5 |
aAll ingredients are diluted in petrolatum.
The pattern of fragrance allergy has changed over time because of industry developments, changing fashion trends and regulatory interventions. In a large Belgian cross sectional study of 13 332 patients between 1990 and 2011, 9.6% showed allergic reactions to FMI, with individual ingredients showing a fluctuating trend but Evernia prunastri being the most frequent allergen. In this study FMII identified 6% of patients with fragrance allergy and 99 fragrance-allergic patients would have been missed if using FMI alone. The commonest sensitizers were Lyral and farnesol [48].
Other potentially missed fragrance allergens include jasmine, sandalwood, spearmint oil, lemon grass oil, narcissus and ylang-ylang oil [43, 49]. These five commercially available allergens may be worth testing for as some important relevant allergies may otherwise be missed, most particularly in suspected cases of occupationally induced hand dermatitis (e.g. beauty therapists and masseuses working with essential oils).
Oxidized terpenes, especially hydroperoxides of d-limonene and linalool, have also emerged as leading causes of fragrance allergy and can now be tested for using commercially available allergens. Linalool is found in more than 200 natural oils including lavender, ylang ylang, bergamot, jasmine and geranium oils. Studies show that it might also be present in 90–95% of prestige perfumes. A multicentre patch test study evaluating oxidized linalool 6% pet (linalool hydroperoxides 1%) showed that it was a useful tool for investigating fragrance contact allergy with an overall prevalence of contact allergy of 6.9% [50]. Limonene is also a prehapten and, following oxidation, the main hydroperoxide sensitizers are formed. It is found in many oils including rosemary, eucalyptus, lavender, lemongrass and peppermint. A multicentre study determined that r-limonene 3.0% pet, with a specified concentration of limonene hydroperoxides at 0.33%, was also shown to be a useful tool in identifying fragrance allergy in an international setting with a mean prevalence of 5.2% allergic patch test reactions [51].
Both FMI and FMII may give false positive irritant reactions, and testing the ingredients separately may help to avoid these. However, when individual materials are mixed they may combine in such a way as to produce a compound allergy, or other synergistic effects inducing a true allergic reaction, despite the components themselves being negative. The reverse situation (quenching) – that is the mix is negative and one or more of the components positive – has been reported, but also questioned [52]. Nevertheless, it is worthwhile testing with the breakdown in addition to the fragrance mix when perfume allergy is strongly suspected.
An extended additional flavours series of patch tests can be developed for those with cheilitis or oral problems.
There has also been some recent work on the stability of various fragrance patch test allergens. These chemicals are volatile, and the petrolatum preparations may not be stable over time. One group looked at the stability of the seven ingredients of FMI applied in patch test chambers stored at room temperature versus those stored in a refrigerator for between 4 and 144 h. A deviation of ± 20% of the stated concentration in a test preparation is considered acceptable by commercial manufacturers. Using liquid chromatographic methods on FMI constituent ingredients, the concentration of four of the seven test fragrance preparations decreased ≥20% within 8 h at room temperature. When stored in a refrigerator only cinnamal had decreased ≥20% within 24 h. In fact, cinnamyl and cinnamyl alcohol were found to be more stable when analysed as ingredients in FMI than in individual preparations. This shows that within a few hours several fragrance chemicals evaporate from the patch test chambers to an extent that may affect the outcome of the patch test [53]. Therefore, the general consensus is that the patch test chambers should be used as quickly as possible, and that storage in a refrigerator is recommended. Also, it is essential practice to keep syringes capped and refrigerated, and for volatile allergens to be replaced regularly.
Applied medicaments [1, 2]
Prevalence and incidence. Of all allergic contact dermatitis, about 15% is caused, or complicated, by sensitivity to medicaments, although this may be higher in susceptible patient populations. The literature on contact dermatitis abounds with reports of reactions to medicaments, and it is not possible to review all of these. It is doubtful whether the incidence has changed significantly, although the incidence of sensitivity to a particular allergen varies from country to country and from decade to decade, according to both local prescribing habits and the number of patients who are at high risk, for example with leg ulcers and stasis eczema, included in any series. Contact allergy to medicaments is also more common in an elderly population, particularly to fragrance, lanolin, local anaesthetics, neomycin and corticosteroids [3]. Cases are missed unless patch tests are routinely performed and if locally used medicaments are not included in a medicament series. Owing to the increased use of antiseptic hand washes both in the occupational setting and home setting, testing for sensitization to benzalkonium chloride and chlorhexidine should also be considered. Meaningful sensitization indices for the various medicaments can be calculated only if the prevalence of sensitivity is correlated with the usage.
Clinical features. Certain sites appear to be prone to the development of allergic contact dermatitis from medicaments. This is probably the result of frequent medicament usage at these sites, occlusive skin conditions and pre-existing skin damage. Sensitization to medicaments is particularly common in patients with leg ulcers or eczema of the lower legs (Figure 128.15), and is found in about half of those with chronic stasis eczema. Even weak allergens appear to sensitize if used on the lower leg. Contact dermatitis is also common in patients with chronic perianal inflammatory disorders (Figure 128.16), pressure sores, chronic otitis externa [4, 5] and in those who frequently use ocular medicaments [6].
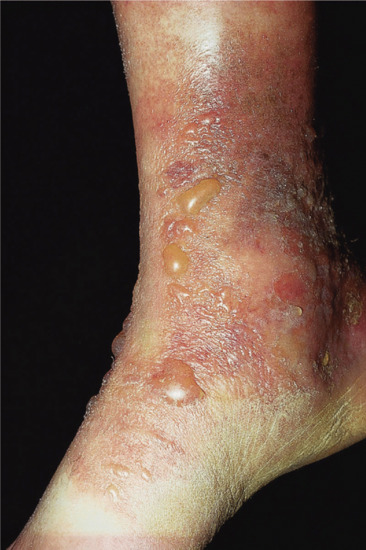
Figure 128.15 Medicament allergic contact dermatitis superimposed on stasis eczema. Topical antibiotics/antibacterials, preservatives, lanolin and other constituents of the medicament base are often to blame. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
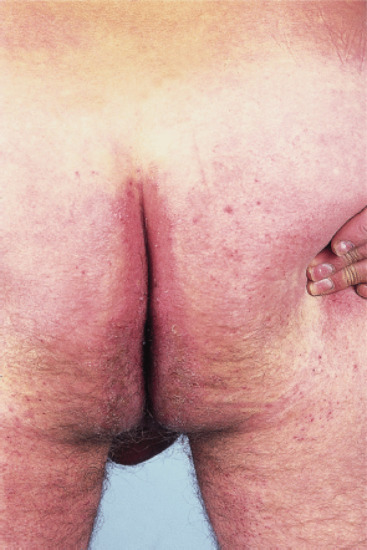
Figure 128.16 Pruritus ani is often complicated by secondary contact dermatitis to local anaesthetics or other medicaments. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
However, dermatitis from applied medicaments can develop anywhere. Sometimes, the sensitivity is obvious but often it is occult and easily overlooked, and it will then only be detected by patch testing. In burns, the damaged skin may be incapable of reacting, and dermatitis may only be apparent at the periphery of the burn site.
Sensitivity to a topically applied medicament may result in several types of reaction.
- Local aggravation, with increased itching and redness.
- Spread to other regions, in most cases preceded by local aggravation. This is especially common in patients with stasis eczema or leg ulcers.
- A local reaction may not develop, and dissemination may be the only sign of sensitivity. This typically occurs with creams and ointments containing a potent steroid capable of suppressing the reaction locally, but not in other regions.
- Sensitization can also manifest merely as failure to respond to treatment. The original condition may worsen or fail to improve, without there being any acute flares or spread to arouse suspicion. This is seen mainly when there is a low degree of sensitivity and low concentration of allergens, typically with parabens and lanolin, or where the contact allergen is a corticosteroid.
- Persistent generalized erythroderma is a rare manifestation of allergic contact sensitization to medicaments.
- Contact urticarial reactions have also been reported.
Systemic reactions. Patients sensitized by the topical use of a drug may develop systemic reactions if that drug, or one that is closely related, is then given systemically. A pattern of dermatitis with erythema of the buttocks and flexural involvement elsewhere has been termed the ‘baboon syndrome’. Widespread dermatitis or generalized exfoliative dermatitis has been reported following challenge with a systemic drug to which the patient already has contact allergy.
Other patients may develop a systemic reaction after topical application of a medicament. Anaphylactic reactions have been reported, for example following the topical use of bacitracin, cephalosporins, rifamycin and chlorhexidine. Erythema multiforme-like reactions to topical medicaments have also been reported. Some patients have positive patch test reactions to a topically applied drug, having previously been sensitized by its systemic use.
Patients who have been sensitized by the topical use of promethazine hydrochloride may develop serious photosensitivity if the drug is given systemically. Care must always be taken in prescribing an antihistamine systemically if the patient is known to have been exposed to the same or a chemically similar drug topically. Patients who are known to be allergic to ethylenediamine should not be given antazoline hydrochloride, piperazine or several other antihistamines.
Avoidance and prognosis. Sensitization from a single constituent may lead to recurrent dermatitis due to its inclusion in several proprietary formulations. In only a few countries are the contents of a proprietary medicament stated on the package or listed on the data sheet and, even then, the information is often insufficient, constituents sometimes being given as trade names or only ‘active’ ingredients listed. In order to reduce the risk of relapse, the ingredients of all topical medicaments should be established. Ideally, all topical medicaments, whether prescribed or purchased without a prescription, would exhibit full ingredient labelling.
It is also necessary to consider cross-sensitivity to other, untested medicaments. This has received particular study in relation to contact sensitivity to the aminoglycoside group of antibiotics. A similar situation may develop in patients sensitive to the ‘para’ group of chemicals, with cross-sensitization between local anaesthetics, dyes, sulphonamides, UV filters, etc.
Patch tests. Patients with suspected contact dermatitis should be tested with all the medicaments they have applied. The information obtained in the history may be incomplete, and commonly used medicaments should also be routinely tested. It is often helpful to have a vehicle and medicament series, or several ‘site’ series with the ingredients of the most commonly used topical preparations in that geographical location. Testing to the medicament (‘as is’) may miss allergens, because they may be present in insufficient concentration. Where there is a high index of suspicion, the individual components should be obtained and appropriately diluted for patch testing.
It is important not to forget self-prescription of over-the-counter preparations. Popular habits of self-treatment vary from country to country and region to region. Knowledge of these habits is obtained by experience, but local pharmacists can often supply information. Certain remedies may be popular in one country but almost unknown in another. ‘Natural’ or herbal treatments are increasing in popularity. Some of these are irritant, and others, such as Chinese herbal remedies [7] and tea tree oil [8], contain allergens.
Various pharmaceutical developments have been associated with successive waves of dermatitis from applied medicaments; for example sulphonamides from 1945 to 1950, then antihistamine creams, and later neomycin and ethylenediamine. Sensitivity to neomycin, gentamicin, lanolin, balsams, mercurials and preservatives is still common today. Newer sensitizers include the imidazoles, topical non-steroidal anti-inflammatory drugs (NSAIDs), transdermal delivery systems and topical steroids. Photosensitivity also occurs with some topical NSAIDs. Less common medicament allergens are listed in Box 128.2.
Sensitivity may be confirmed in some patients exposed to drugs, particularly the penicillins, by in vitro lymphocyte transformation tests and, for type I reactions, by specific IgE assay. Skin tests may also sometimes be positive in those who have had eczematous or urticarial reactions to ingested drugs. Care must be taken in re-challenging anyone who has had a type I reaction because anaphylactic reactions may be induced. Guidelines for investigating potential drug reactions have been produced [9].
Medicament allergens included in the European Environmental and Contact Dermatitis Research Group recommended European standard series include the following [10].
- Neomycin 20% in petrolatum. Neomycin has two active components, neomycin B and neomycin C, which are stereoisomers. It cross-reacts with other aminoglycoside antibiotics. The pattern of cross-sensitivity has been studied in guinea pigs. Clinically, neomycin is known to cross-react frequently with kanamycin and framycetin (Soframycin®), which would be anticipated with the latter as it consists almost entirely (99%) of neomycin B. Cross-sensitivity also occurs to a varying degree with gentamicin and tobramycin. Neomycin classically produces late reactions (beyond 96 h readings).
- Clioquinol 3% in petrolatum. In areas where there is high use of chlorquinaldol, the retention of the quinoline mix may be of value due to the lack of cross-reactions between it and clioquinol.
- Benzocaine 5% in petrolatum. Experience suggests that, in the UK at least, the replacement of benzocaine with a mixture containing cinchocaine (dibucaine) 2.5% and amethocaine (tetracaine) 2.5% will double the yield of allergic-positive reactions. Local anaesthetics are either of the ester or amide type, and cross-reactions can occur within groups. Although cinchocaine is an aminoalkylamide and lidocaine (lignocaine) is an aminoacylamide, most individuals do not cross-react. Ideally, any reaction to the mix should be followed up by testing to the constituents and to lidocaine. Not all reactions to local anaesthetics are detected by the mix and patch tests should always be dictated by the particular exposure of the patient.
- Corticosteroids (tixocortol pivalate 1.0% in petrolatum and budesonide 0.1% in petrolatum) [11, 12]. When testing with corticosteroids it is not unusual to see reflex vasodilatation following the steroid-induced vasoconstriction and this should not be interpreted as a positive reaction. Conversely, an annular response is frequently allergic, as a result of central suppression of the reaction by the corticosteroid.
A reaction to tixocortol pivalate almost invariably means that the patient is allergic to hydrocortisone. The British Society of Cutaneous Allergy (BSCA) recommends that tixocortol pivalate is tested at 1.0% [12]. In the UK, a reaction to budesonide almost certainly represents a cross-reaction to another corticosteroid, most likely an ‘ester’ such as hydrocortisone 17-butyrate or an ‘acetonide’ such as triamcinolone acetonide. A reaction to either of these steroids in the standard series should prompt further testing to an extended steroid series, as 50% of tixocortol pivalate-positive and 90% of budesonide-positive individuals react to other corticosteroids. Our experience suggests that although intradermal testing may have a role, testing other corticosteroids at 1% in ethanol is more sensitive although these test preparations are not available commercially at present. Together with knowledge of cross-reaction patterns [13], this helps in deciding what topical steroid to use as an alternative. Empirically, fluocinolone acetonide (Synalar®) preparations react least frequently, and are available in a range of potencies. In view of the potential cross-reactivity between these two corticosteroids and prednisolone and its derivatives, it seems prudent to advise the use of either betamethasone or dexamethasone if a systemic steroid is needed, in order to reduce the risk of inducing a generalized dermatitis.
Although steroids are also applied topically to mucosal surfaces in the treatment of respiratory diseases, reports of contact allergy are rare. Indeed, individuals challenged with inhaled steroid to which they are sensitized, typically develop a cutaneous but not a respiratory response. In contrast, there are reports of both immediate and delayed-type allergic reactions following various other systemic routes of administration.
Cosmetics [1, 2, 3]
Cosmetics have been defined as any preparation applied to the skin, mouth, hair or nails for the purpose of cleansing, enhancing appearance, giving a pleasant smell or providing protection [1]. There is consequently a considerable range of products that can be included within this definition, for example perfumes, deodorants, aftershaves, hairsprays, lipsticks, nail varnishes and artificial nails, moisturizers, cleansers and wipes, mascara, eye shadow, make-up, make-up removers, sunscreens, hair colours and styling agents, depilatories, soaps, shampoos, shower gels, bath oils and toothpastes.
Good manufacturers aim to eliminate known sensitizers and irritants. However, because all cosmetics and toiletries have to be protected against bacteriological contamination and decomposition, and as most consumers require their cosmetics to have a pleasing smell, there are potentially sensitizing preservatives and fragrances in most cosmetic products. The substitution of one allergen by an alternative component may lead to the introduction of perhaps an even more sensitizing substance [4]. When entirely new products or ingredients are used on a large number of consumers, unexpected allergic or irritant reactions may occur, and it may be some time before the cause is identified.
The range of cosmetic allergens is potentially considerable. The more frequently detected allergens are discussed in the specific sections relating to fragrances, preservatives, vehicles and excipients, p-phenylenenediamine and related dyes and UV filters.
Allergy to tosylamide formaldehyde resin in nail varnish has been shown to be relatively frequent in those who wear it, and to have important adverse consequences [5, 6]. Allergy to acrylates used in artificial nail glues and sculptured nails can produce similar results [7, 8].
There is an increasing vogue for including natural plant-based products in cosmetics, for example tea tree oil (Melaleuca alternifolia), and these may be potentially allergenic [9, 10, 11].
Hairdressers (and their clients) are exposed to a wide range of allergens in dyes, bleaches (ammonium persulphate), permanent wave solutions (thioglycollates), shampoos and hairsprays, etc. [12, 13]. Depilatory waxes may contain colophony derivatives which can induce allergic dermatitis [14].
Incidence and prevalence. Contact dermatitis to ingredients of cosmetics and toiletries is common in patients attending patch test clinics; approximately 10% of patients investigated for contact dermatitis in a multicentre European study were allergic to cosmetic products [15]. The exact incidence and prevalence of sensitivity in the population is difficult to establish. In a UK study of 1022 persons, 8.3% had experienced some sort of adverse reaction to a cosmetic or toiletry in the preceding year; most reactions were irritant rather than allergic in nature [16]. In one American survey comprising 30 000 consumers, 700 reactions had occurred during 1 year [17]. Some reactions are transient, such as stinging and smarting, and contact urticarial. Most people simply change brand and do not report adverse reactions to the manufacturer. It is nevertheless estimated that 1–3% of the population is allergic to a cosmetic or cosmetic ingredient [18], with a female predominance.
The commonest allergens are fragrances and preservatives. Also of importance are PPD, UV filters, tosylamide formaldehyde resin in nail varnish, lanolin and derivatives and cocamidopropyl betaine, but there are potentially many others [1]. As many as 6.6% of women habitually wearing nail varnish are allergic to it, and 1.6% of patients routinely tested to the usual allergen, tosylamide formaldehyde resin, are patch test positive [5, 19].
Clinical features [1].
Cosmetic allergy is unsuspected in about half of those in whom it is subsequently diagnosed [20]. Apart from hair dye allergy, acute weeping and oedematous reactions are unusual. More commonly, there are erythematous scaling patches or a more diffuse erythema. Differentiation from atopic and seborrhoeic eczema and lupus erythematosus may be difficult, especially on the face.
Sites of involvement are very varied, and depend on the type of product containing the allergen(s) and where it has been applied. Patterns of perfume allergy are described in the section on fragrances, and hair dye allergy in the section on p-phenylenediamine and related dyes. The eyelids, face (Figure 128.17) and neck (see Figure 128.14) are sites commonly involved in cosmetic allergy, but hand involvement and more widespread dermatitis are seen. It is not always appreciated how often cosmetics, particularly moisturizers, are applied not only to dry skin but also to pre-existing eczemas, including constitutional forms. Flares may wrongly be blamed on the underlying disorder, and cosmetic allergy may go undetected unless appropriate patch testing is undertaken. In general, leave-on products are more likely to sensitize than wash-off cosmetics, although dermatitis may be maintained from the latter source in allergic subjects [1].
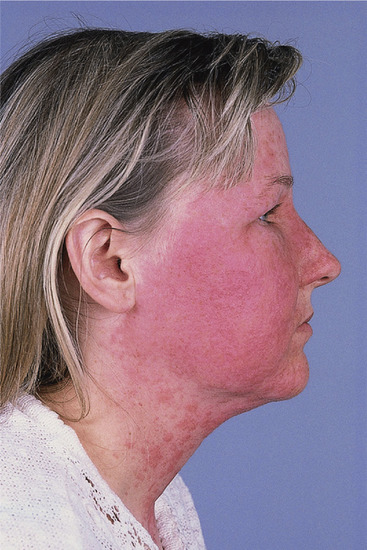
Figure 128.17 Facial allergic contact dermatitis, often due to fragrance, preservatives or other ingredients of cosmetics. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Cheilitis is seen from lipstick, lipsalve and toothpaste allergy. Hair cosmetic allergy may cause a scalp margin pattern as well as periorbital swelling. A similar distribution is seen in hairdressers’ clients allergic to permanent wave chemicals (usually glyceryl monothioglycollate).
Nail varnish allergy is often ectopic, with patches and streaks on the face, neck (see Figure 128.10) and behind the ears, and episodic periorbital swelling. The face and neck are involved in about 80% of cases, the eyes in about 50%, and periungual dermatitis, although often absent or minimal, occurs in 60% [6]. The allergen is usually tosylamide formaldehyde resin, but other agents are becoming increasingly important, such as phthalic anhydrides [21]. More widespread involvement of, for example, the chest and ano-genital regions may be seen [4, 22]. Onycholysis may occur [23], but this is more likely with allergy from acrylates in artificial nails, which may also cause dystrophy and paronychia [24]. A similar, potentially widespread, ectopic pattern of contact allergy may occur from acrylic nail cosmetics [8]. The socio-economic and medical consequences of nail varnish allergy have been investigated and shown to be potentially severe, with sick leave, work loss and even hospitalization [6].
Passive transfer of allergy from cosmetics used by partners (connubial or consort allergy) and relatives [25, 26, 27] should not be forgotten.
Occupational allergic hand dermatitis associated with hairdressing materials is described in (see Chapter 130).
Avoidance. Full ingredient labelling of cosmetics in Europe has made a major contribution to avoidance measures. It is important to give the patient the INCI name of the material to which they are allergic, as this is the nomenclature used on cosmetic ingredient labels. There is still potential for confusion, particularly with plants which, when used in cosmetics, are identified by their Latin name in the Linnaean system. Some plant extracts may potentially contain, or cross-react with, fragrances, and it may be difficult for the patient and the dermatologist to be absolutely sure if a product containing natural plant extracts is safe for fragrance-allergic subjects. Unlabelled products should not be used. The reader is referred to the sections on individual cosmetic allergens for more details.
A complete list of INCI names for substances can be obtained from http://ec.europa.eu/consumers/cosmetics/cosing/ (last accessed February 2015).
Patch testing. The ESCD baseline series contains a number of cosmetic allergens, including FMI and FMII, balsam of Peru (Myroxylon pereirae), Lyral, parabens mix, quaternium-15, MCI/MI, methylisothiazolinone, formaldehyde, PPD and colophonium (colophony). The BSCA include additional cosmetic chemicals in their recommended baseline series: imidazolidinyl urea, diazolidinyl urea, methyldibromo glutaronitrile, amerchol L101, chloroxylenol and 2-bromo-2-nitropropane-1,3-diol (see http://www.cutaneousallergy.org/ (last accessed February 2015)).
However, a wider screen of allergens is advised when investigating cosmetic allergy, in particular, tosylamide formaldehyde resin (10% in petrolatum) and, if relevant, an acrylic nail chemical series when investigating facial and patchy disseminated eczemas and periungual problems. Patch testing with a series of UV filters may also be advised. In addition, the main allergen suppliers have a range of other potential allergens, including more preservatives, antioxidants, surfactants, emulsifiers and other cosmetic excipients.
It is also important to consider patch testing with the cosmetics used by the patient. As a general rule, leave-on products and perfumes can be tested ‘as is’ but, because of irritancy, soaps and shampoos should be diluted to 1% aqueous. There is still a risk of false positive reactions and also, because of the dilution, false negatives. We test toothpastes at 25% in petrolatum. Mascara and nail varnish are often irritant, and should be applied to a chamber and the solvents left to evaporate before applying them as a patch test.
Allergy cannot be totally ruled out unless all ingredients of all cosmetics have been tested individually at appropriate concentration and in a suitable vehicle. In practice, however, most cosmetics are initially tested ‘as is’, but false negative reactions and marginal irritant reactions are common. Ideally, each component of a suspect cosmetic should be tested individually and where there is a high index of suspicion the individual components should be obtained from the manufacturer if they are willing to provide them. Ideally, the raw material should be the same as that used in the suspect product, because batch differences, source and purity may all be important. Sometimes the allergy is to the substance itself, and sometimes to an impurity. The concentration necessary to test an individual substance is often greater than its concentration in the product. Manufacturers’ patch test kits, which may contain ingredients at the concentration in which they are present in the product, are likely to be misleading and should not be used.
Testing with hair dyes is discussed in the section on p-phenylenediamine and related dyes.
Other tests. If cosmetic allergy is still suspected despite negative patch test reactions to a cosmetic containing sunscreens, then the possibility of photoallergy should also be considered and, if clinically indicated, photopatch tests should be undertaken [28].
Repeat open application tests (ROATs; see later in this chapter) may also be worthwhile to try and identify the offending cosmetic, although these will not necessarily differentiate between irritant and allergic reactions. Finally, after discussion with the patient, a useage test can be considered, with reintroduction of the suspected products, one at a time, and using each for up to 3 days.
Antimicrobial agents and preservatives
One of the greatest challenges facing the cosmetic industry formulators at present is the choice of preservative(s) to use in their products. The palette of available chemicals is rapidly diminishing following regulatory control, not predominantly for the reasons of contact allergy, although there have been recent such examples. Therefore, on the basis of possible regulatory led withdrawal of commoner preservatives in Europe it will be important to monitor the use of the less commonly used ones.
Formaldehyde [1]
Chemistry. Formaldehyde (HCHO) is a gas, and formalin is a solution of the gas in water (about 38%). Methylol groups can be combined with other compounds to form formaldehyde releasers, which are widely used as preservatives. Formaldehyde may combine with other chemicals to produce resins, which may sensitize (see the section on resins and plastics).
Prevalence. In individuals routinely patch tested for the investigation of contact dermatitis, the frequency of allergic-positive reactions to formaldehyde is generally 2–3%, although the North American Contact Dermatitis Group (NACDG) found 8.4% were positive in 2001–2002 [2, 3].
Occurrence. Formaldehyde is a ubiquitous allergen and Box 128.3 gives an idea of the wide variety of potential sources. Threshold concentrations for the elicitation of contact dermatitis from formaldehyde are as low as 30 ppm in the axillae [4], and as low as 250 ppm under an occluded patch test [5].
It can often be difficult to find relevance for a positive patch test, but more commonly identified causes are cosmetic ingredients [1] and less commonly now clothing resins. Shampoos may contain formaldehyde, although this is more likely to be of relevance in the context of hairdressers’ hand dermatitis than in relation to transient use on the hair. Some textile resins will release formaldehyde, and free formaldehyde may be found in treated cotton clothing and rayons. Paints/lacquers, printing inks and cleaning products, filling agents and glues were the most frequently registered products containing formaldehyde marketed in Denmark [6]. Formaldehyde is used for the preservation of anatomical and pathological specimens, and those working with such specimens, for example histopathologists and embalmers, are at risk of allergy from free formaldehyde. It is used medically in renal dialysis and may be found in orthopaedic casts. It is also used as a treatment for warts and hyperhidrosis, especially of the feet, where powders containing paraformaldehyde may also be used. Formaldehyde has been found in reusable protective gloves [7]. The very widely used surfactant sodium lauryl sulphate may be preserved with formaldehyde at a level of 0.1% [8]. It is used in detergents, shampoos, shower gels and bubble baths.
In addition, formaldehyde-releasing chemicals must be considered, including certain preservatives and biocides widely used in industry (e.g. in cutting oils). Many preservatives used in cosmetics, and to a lesser degree in topical medicaments, may release formaldehyde (Table 128.5). Many of these releasers not only sensitize simultaneously with, but also independently of, formaldehyde [9, 10, 11, 12]. It is common practice in Sweden to prescribe corticosteroid ointments in formaldehyde-allergic patients because some cream-based products have been shown to release small quantities of formaldehyde [13].
Table 128.5 Formaldehyde-releasing preservatives in cosmetics and medicaments.
| Substance | Patch test concentration |
| Quaternium-15 | 1% in petrolatum |
| Imidazolidinyl urea | 2% in petrolatum (or 2% aqueous) |
| Diazolidinyl urea | 2% in petrolatum (or 2% aqueous) |
| 2-Bromo-2-nitropropane-1,3-diol | 0.25% in petrolatum (or 0.5% in petrolatum) |
| DMDM hydantoin | 2% aqueous |
DMDM, 1,3-dimethylol-5,5-dimethylhydantoin.
Clinical features. The presenting dermatitis will depend on the source of contact, for instance a clothing pattern, a cosmetic pattern or involvement of the hands in occupational dermatitis. Formaldehyde allergy is often only diagnosed retrospectively by finding a positive patch test, and relating this to the distribution of the problem by identifying formaldehyde or formaldehyde-releasing chemicals that come into contact with the affected site.
Avoidance. Avoidance may be difficult, bearing in mind the wide exposure possibilities, but it is important to recognize that avoidance steps are only required if the individual has skin problems that are relevant to the exposure. For those with a clothing pattern, avoidance advice should be given (see the section on clothing later in this chapter). If cosmetics, medicaments and moisturizers come into contact with the affected sites, their ingredient labels should be carefully assessed in order that those containing not only formaldehyde but also the formaldehyde-releasing preservatives listed in Table 128.5 are avoided. Some allergic patients may find this difficult [14]. It may also be necessary to contact manufacturers or check the material safety data sheet to establish the presence of formaldehyde in their products, particularly cutting oils. The difficulties faced by patients in identifying formaldehyde in products is highlighted by the fact that in one study of sensitized persons with persistent dermatitis, all were still using at least one product containing formaldehyde. Only by detailed enquiries and access to product databases could the presence of formaldehyde be demonstrated [15]. A particular problem that may be encountered is the addition of formaldehyde to detergents at source by suppliers, which are then used in formulations and since no further formaldehyde may be added to the product its presence goes unlabelled.
A number of tests can be used to detect the presence of formaldehyde. The chromotropic acid test may give false positive reactions, and the alternative acetylacetone method may be more sensitive and specific (see ‘Spot tests’ later in this chapter). A closed container diffusion method for quantification of formaldehyde has also been devised [16]. High-performance liquid chromatography is an alternative method that is more accurate than the chromatropic acid test [13].
Prognosis. In a follow-up study of 57 patients with formaldehyde dermatitis, 29 (51%) still had frequent or persistent dermatitis several years later. Formaldehyde was identified in cosmetics, toiletries, household cleaners and other materials still being used by 38 of these patients. The authors concluded that patients who paid attention to their allergy had statistically significantly fewer eruptions than those who did not [17].
Patch tests. Patch testing is now recommended with formaldehyde 2% aqueous [18]. It is a generally recommended baseline test allergen.
Formaldehyde-releasing preservatives/biocides [1]
Quaternium-15
Quaternium-15 is also known as Dowicil 75, 100 or 200, chlorallyl methenamine chloride, N-(3-chlorallyl)-hexaminium chloride and 1-(3-chlorallyl)-3,5,7-triaza-1-azoniondamantane. It is water soluble, odourless and colourless. Its broad antimicrobial activity is independent of the pH of the product.
Prevalence. Quaternium-15 can sensitize either independently or via formaldehyde release, or both. The prevalence of positive patch tests in those attending for routine testing in North America is high, with 9.3% positive in a NACDG survey of 4910 patients [2]. Equivalent returns in Europe have generally shown lower levels, although the prevalence is dependent on the amount of usage in a given country. Pan European studies have shown levels of contact allergy of between 1% and 1.5% which have been fairly constant over the last two decades [3, 4].
Occurrence. Quaternium-15 is found in cosmetic products and hand creams, including barrier and other creams used at work. It is found in a small number of medicaments in the UK [5] and in electroencephalography skin preparation gel [6].
Clinical features. These are discussed in the sections on allergy to cosmetics and medicaments.
Avoidance. The INCI name is quaternium-15. Only ingredient-labelled products should be used, and any product shown to contain it should be avoided. Knowledge of its synonyms is helpful, particularly as non-cosmetic products, including medicaments, may not adhere to INCI terminology.
Patch tests. For quaternium-15, 1% in petrolatum is the generally recommended concentration and vehicle. It is recommended as a standard test allergen in Europe and North America.
Diazolidinyl urea
Diazolidinyl urea is also known as Germall II. It is a broad-spectrum biocide, soluble in water and effective at various pH levels [1].
Prevalence. Studies in the UK on routinely patch-tested individuals showed that 0.7% of 3062 patients were patch test positive [7], similar to the finding of 0.79% in a multicentre Spanish study [8], whereas in North America 3.1% were positive [3].
Occurrence. Diazolidinyl urea has been used since 1982, predominantly in cosmetics, shampoos and creams, including barrier and other work creams.
Clinical features. These are discussed in the section on allergy to cosmetics.
Avoidance. The INCI name is diazolidinyl urea. Only ingredient-labelled cosmetics and creams should be used, and any product shown to contain it should be avoided. ROATs confirm that formaldehyde-allergic subjects should also avoid creams preserved with diazolidinyl urea [9].
Patch tests. Patch testing at 1% and 2% aqueous has been advised [10], but it is generally supplied at 2% in petrolatum, which we have found satisfactory. Although not a frequent sensitizer in the UK, the BSCA has recommended its inclusion in the baseline series [11].
Imidazolidinyl urea
Imidazolidinyl urea is also known as Germall 115. It has broad-spectrum antimicrobial activity and is colourless, water soluble and not pH dependent. It acts synergistically with other preservatives and will kill Pseudomonas aeruginosa [1]. It releases only small amounts of formaldehyde, and may therefore possibly be less of a problem than other formaldehyde releasers for formaldehyde-sensitive subjects [12].
Prevalence. It is not a common allergen in most European studies, for example positive reactions occur in 0.7% of routinely patch-tested persons in the UK [2] with a slightly higher rate in Spain of 1.05% [8]. In North America the NACDG has reported a figure of 3.0% [3].
Occurrence. Imidazolidinyl urea is used in cosmetics, shampoos and hand creams, including barrier and other work creams. It is found in a cream containing the corticosteroid fluticasone, marketed in the UK as Cutivate®, where its presence is denoted by the word ‘imidurea’.
Clinical features. These are discussed in the section on allergy to cosmetics.
Avoidance. Imidazolidinyl urea is the INCI name. Only ingredient-labelled cosmetics should be used, and any product shown to contain it should be avoided. Cutivate® cream should also be avoided and this also applies to those allergic to formaldehyde [13]. In topical medicaments it may be labelled as imidurea.
Patch tests. Although patch testing with 2% aqueous has been advised [14], 2% in petrolatum is generally used. Its inclusion in the baseline series is recommended by the BSCA in the UK [11].
2-Bromo-2-nitropropane-1,3-diol
2-Bromo-2-nitropropane-1,3-diol is also known as bronopol and BNPD. It has broad-spectrum antimicrobial activity and is particularly effective against P. aeruginosa. It is soluble in water, alcohols, glycols and, to a lesser degree, oils [1].
Prevalence. The reported prevalence of positive reactions to BNPD in routinely patch-tested individuals in North America in 2001–2002 was 3.3% [3]. In the UK, 0.8% were positive in a 2003 study [11] and 0.16% in a Spanish study [8].
Occurrence. BNPD is present in a wide range of cosmetics, moisturizers, shampoos, medicaments and hand creams. It is used as a preservative when testing milk samples, and outbreaks of dermatitis have been reported from this source [15]. In the USA, allergic problems have arisen from Eucerin® cream [16], and in the UK from metronidazole gel used to treat rosacea [17]. It is also found in a number of chemical products, especially paints, lacquers and cleaning agents [18]. We have seen it sensitize printers when used as a biocide with isothiazolinones in fountain solution.
Clinical features. These are discussed in the section on allergy to cosmetics. In the occupational setting the usual site of involvement is the hands.
Avoidance. The INCI name is 2-bromo-2-nitropropane-1,3-diol. The simpler name of bronopol may be used in other products. Only ingredient-labelled cosmetics should be used, and any product shown to contain it should be avoided.
Patch tests. The two recommended concentrations are 0.5% and 0.25% in petrolatum; 0.5% may occasionally give false positive reactions. It is recommended by the BSCA for inclusion in the baseline series at 0.5% (pet) [11].
DMDM hydantoin
DMDM hydantoin is also known as Glydant and is a colourless liquid that contains 0.5–2% free formaldehyde and over 17% combined formaldehyde [1].
Prevalence. In the Netherlands, 1.2% of patients routinely patch tested to DMDM hydantoin showed allergic reactions [4], and the NACDG reported 2.8% positivity [3]. Testing with formaldehyde demonstrated concomitant sensitivity in eight of 15 (57%) individuals [19], but a much lower rate was seen in Germany [20] and a rate of 0.85% in a Spanish study [8].
Occurrence. DMDM hydantoin is used in a wide range of cosmetics. Surprisingly, there are no specific reports in the literature of allergy from this source [12], but we have seen occasional allergies relevant to cosmetics.
Clinical features. These are discussed in the section on allergy to cosmetics.
Avoidance. DMDM hydantoin is the INCI name, and it can be identified in a product provided this is fully labelled with ingredients. There is evidence from ROATs that formaldehyde-allergic patients should avoid products containing DMDM hydantoin [19].
Patch tests. Patch tests have been undertaken at 1–3% aqueous and 1% in petrolatum. We have found 2% aqueous satisfactory.
Other biocides
The above formaldehyde releasers are encountered particularly in cosmetics, including shampoos and other hair care products. A much broader series of formaldehyde releasers is to be found in materials such as industrial and household cleaning agents, colouring agents, paints and lacquers, polishes and especially metalworking fluids [21, 22, 23]. A number of formaldehyde-releasing biocides in these fluids will sensitize, and a special series of allergens should be used for testing in those exposed, as well as the material itself [24].
Isothiazolinones [1]
Isothiazolinone preservative systems have effective broad-spectrum activity against both bacteria and fungi. A number of different formulations have been shown to be sensitizing to the skin.
- Mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one in a 3 : 1 ratio by weight. The INCI name is methylchloroisothiazolinone and methylisothiazolinone (MCI/MI). This mixture has various other names, including Kathon CG, Kathon WT, Euxyl K 100 and Acticide. MI by itself has also been introduced as a cosmetic preservative at a permitted usage level of 100 ppm in Europe since 2005. At the time of writing we are in the midst of an unprecedented epidemic of MI allergy across Europe and Australia, predominantly from its use in cosmetic products.
- 1,2-Benzisothiazolin-3-one (BIT). This is used under the commercial name Proxel in a range of biocides.
- 2-n-octyl-4-isothiazolin-3-one. This is also known as Kathon 893, Kathon LP and Skane M-8.
There seems to be little in the way of cross-sensitization between these compounds [2]. A further isothiazolinone, 2-methyl-4,5-trimethylene-4-isothiazolin-3-one (MTI), has also sensitized, but only in the laboratory setting [3].
Methylchloroisothiazolinone and methylisothiazolinone [4, 5]
Prevalence. Since first marketed in 1980, there have been many reports of MCI/MI allergy, particularly from Europe, with a prevalence of positive reactions as high as 8.3% in routinely tested patients in the first few years after its introduction [6]. This level had stabilized to around 2.5% until recently [7]. However, there has been considerable variability in the prevalence of allergy from country to country. In the USA, rates of 2–3% have generally been the rule [8].
There has been much discussion about the reason for differing prevalence rates worldwide. It has been suggested that in some countries there has been lack of control over the amount of this biocide added to products that come in contact with the skin [8]. Furthermore, patch testing with concentrations as high as 300 ppm in some centres may have produced false positive reactions, whereas other centres have tested with 100 ppm [8].
Levels below 15 ppm are considered unlikely to induce sensitization [9], and in those already sensitized this concentration has been shown to be insufficient to elicit a dermatitis in many instances [9]. A decrease in, and stabilization of, the frequency of allergy was felt to reflect tighter regulation of concentrations used. The maximum allowable concentration in the EU for both rinse-off and leave-on cosmetics is 15 ppm (i.e MI concentration of 3.75 ppm), with a lower recommended concentration of 7.5 ppm for leave-on products in the USA [7]. Due to the high rates of allergy to MI across Europe, the rates of cutaneous allergy to the mix of MCI/MI are now similarly increasing [10].
Occurrence. MCI/MI is used in cosmetics, mainly in rinse-off products, including liquid soaps and cleansers, shower gels, bubble baths and shampoos. Nevertheless, leave-on cosmetics may also contain it. It may be present in medicated wipes and moist toilet paper [11]. In 1990, a study showed its presence in 48% of rinse-off and 31% of leave-on cosmetic products used in Denmark [12]. In 1988, it was reported as being present in 25% of all cosmetic products in the Netherlands [4].
However, this biocide can be found in other situations, most notably soluble cutting oils, paints, wallpaper pastes, glues, spin finishes, military fuel, household cleansers, printing inks and fountain solution, latex emulsions, water cooling systems and as a slimicide in paper mills.
Clinical features. These are discussed in the section on allergy to cosmetics. Shampoos do not usually cause problems from washing hair, but allergy may be associated with hairdressers’ hand dermatitis. A positive patch test to MCI/MI associated with perianal dermatitis suggests the possibility of moist toilet paper or wipes as a cause [11]. However, this source of MCI/MI can also provoke allergic dermatitis of the hands and elsewhere [11, 13].
An airborne exposure pattern of dermatitis has been seen in sensitized subjects staying in newly painted rooms, where the source of the problem may easily be overlooked [14].
Hands are the usual sites for occupational allergic dermatitis, although spread to other parts of the body, including an airborne exposure pattern, may occur [15]. A chemical burn from a spillage of concentrated MCI/MI on to any part of the skin may be followed by a secondary delayed dermatitis from active sensitization [16, 17].
Patch tests. The recommended patch test concentration and vehicle is now 200 ppm in water as there is evidence that this may identify sensitized subjects missed by the 100 ppm patch test [18, 19]. MCI/MI is generally recommended as a standard allergen.
Methylisothiazolinone
The sensitizing properties of MCI/MI were attributed to methylchloroisothiazolinone, whereas methyisothiazolinone was considered a weak sensitizer, unable to sensitize individuals in concentrations below 1000 ppm [20, 21]. After 2000, MI was introduced in industrial products (paints, glues, lacquers, varnishes, cooling fluids) due to its weaker biocide effects – at higher concentrations.
Prevalence. The first case report of occupational allergic contact dermatitis to MI appeared in 2004 [16] and in 2005 MI was allowed to be used independently of MCI as a cosmetic preservative in Europe, at a maximum permitted level of 100 ppm (Cosmetic Directive 2005/42/EC). Several cases of occupational allergic contact dermatitis to MI were then observed from paints, followed in 2010 by the first case reports following cosmetic exposure [22]. Further case reports then emerged due to its presence in wet wipes, hair cosmetics, facial cosmetics, deodorants and sunscreens. These showed a wide range of presentations according to the causative products and including ano-genital eczema, facial eczema and hand eczema. Furthermore, cases of airborne exposure to MI causing severe airborne and generalized dermatitis from recently painted walls and even from toilet cleaners were described [10].
In the UK, France, Denmark, Portugal, Germany and Spain there have been steeply rising rates of allergy to MI recorded over the past few years [23, 24, 25] with a steep rise in prevalence amongst 14 UK patch test centres from 1.7% to 11.1% [26] The relevant exposures identified in the patients were predominantly from cosmetic exposure. Both the increase in the number of European cosmetics containing MI and a shift toward using it more in leave-on products have given great cause for concern. At the time of writing, Europe and Australia are facing an epidemic of contact allergy to MI with the ESCD calling for the European regulatory authorities to act quickly to avert the crisis.
Patch tests. The recommended patch test concentration and vehicle is now 2000 ppm in water in the European baseline series [27].
1,2-Benzisothiazolin-3-one
Occurrence. Sensitization normally occurs from manufacturing or handling the raw material, for example paint manufacture, water treatment or in the laboratory. Painters and decorators may be exposed not only from paints but also wallpaper pastes. Allergy has been reported in the pottery industry from its presence in mould-release agents. Other potential sources include soluble cutting oils, laundry detergents and fabric softeners, varnishes, dyes, printing materials, water softener and air-freshener manufacture. Allergy to polyvinyl chloride gloves has been traced to BIT used in their manufacture [28].
Clinical patterns. Classically, with hand dermatitis, a low-grade palmar psoriasiform or pompholyx pattern occurs (see Figure 128.5). In more severe cases an exposed-site pattern develops [29]. Sensitized workers involved in manufacture may complain of a burning sensation of the eyes and face within the factory environment without there being observable dermatitis.
Patch tests. A number of patch test concentrations in petrolatum have been suggested, varying from 0.05% to 1%. False positive reactions have been reported with 0.1% in petrolatum [30], and as our experience is that false positive reactions occur above 0.05% in petrolatum we advocate the use of this concentration.
2-n-octyl-4-isothiazolin-3-one
Occurrence. 2-n-octyl-4-isothiazolin-3-one may occur in leather, textiles, soluble cutting oils, paints and polishes, adhesives, cleaning agents, wood preservatives and plastic manufacture.
Clinical features. Reports of contact allergy tend to be sporadic and anecdotal, and these include hand dermatitis associated with its presence in paints [31]. Contact allergy to leather in footwear has been reported [32] and we have found a small number of positive reactions in individuals with possible shoe dermatitis, but of unproven relevance.
Patch tests. 2-n-octyl-4-isothiazolin-3-one is usually patch tested at 0.1% in petrolatum.
Parabens (hydroxybenzoates) [1, 2]
Parabens are esters of p-hydroxybenzoic acid. The four main esters used are methyl-, ethyl-, propyl- and butyl-paraben (hydroxybenzoate). They may have a synergistic effect when used in combination. They are more active against Gram-positive than Gram-negative bacteria (including poor activity against Pseudomonas). They are also active against moulds and yeasts. They are stable, colourless, odourless and poorly soluble in water [1].
Prevalence. There is a relatively low prevalence of positive reactions in routinely patch-tested patients, and rates between 0.5% and 1.7% are typical [3–5].
Occurrence. Parabens are very widely used preservatives in topical and parenteral medicaments, paste bandages, ultrasound gels and foods. However, due to other health concerns, notwithstanding their relatively low risk for causing allergic sensitization, they are no longer widely used as preservatives in European cosmetics.
Clinical features. The striking feature of allergy to parabens is its relative infrequency compared with the degree of usage and exposure in the general population. Relevant allergies are mainly from sensitization to medicaments (including paste bandages) used on venous ulcers and eczema, but contact allergy may be superimposed on other inflammatory dermatoses, particularly on high-risk sites such as the ano-genital region. Relevant problems from parabens in cosmetics are rare [1]. Interestingly, many individuals allergic to parabens in medicaments can use cosmetics containing them on normal skin without any problem, the so-called ‘paraben paradox’ [6]. However, there are exceptions, and sometimes cosmetics containing parabens have to be abandoned [7]. Flares from parabens in food and medicaments [8] have been reported in sensitized subjects, but a low-paraben diet did not help two patients whose eczema flared with oral challenge [9].
Avoidance. The INCI name for this group of preservatives ends in ‘-paraben’ according to the ester used. In individuals in whom cosmetic allergy may be relevant, the full ingredient label must be examined in order that they may be avoided. Terminology for medicaments may be different, and the name may end in ‘-hydroxybenzoate’. Furthermore, the commercial names may sometimes be used, in particular Nipagin, Nipsasol and Nipabutyl, but there are many others. Only medicaments and paste bandages whose ingredients are known in full should be used, avoiding those containing parabens or agents whose names are synonyms of parabens. It is advisable to avoid all parabens even if only one or two are positive in breakdown testing.
Patch tests. Parabens are normally tested as a mix of the four esters, each at 3% in petrolatum. The mix is marginally irritant, and testing with each ester individually will help to confirm whether the patch test reaction is truly allergic. Often more than one ester will react, which may be a marker of both concomitant sensitization and cross-sensitization. Parabens mix 12% in petrolatum is generally advised for the baseline series.
Methyldibromo glutaronitrile [1]
Methyldibromo glutaronitrile (MDBGN), also known as dibromocyanobutane, is to be found in the preservative system Euxyl K 400 (also called Tektamer 38), which is a mix of MDBGN and phenoxyethanol in a ratio of 1 : 4. Euxyl K 400 is a broad-spectrum preservative with activity against fungi and bacteria. MDBGN is nearly always the allergen when sensitization to Euxyl K 400 occurs.
Prevalence. Until the European ban, increasing rates of sensitization were reported throughout Europe and the USA. Of particular significance was the finding of a multicentre European study monitoring rates of preservative allergy, where the frequency of MDBGN allergy between 1991 and 2000 rose from 0.7% to 3.5%, whereas the level of all other cosmetic preservative allergies had remained stable [2]. In consequence, EC countries have banned the use of this preservative, initially in leave-on, and later in wash-off, cosmetics. It is therefore anticipated that sensitization rates will fall very significantly. At the same time, high rates have been reported in the USA, with 11.7% being allergic to MDBGN in one small study [3]. Rates reported by the NACDG have varied from 2.7% to 7.6% according to the test concentration used [4]. This has caused concern about the correct test concentration [5].
Occurrence. MDBGN was historically widely used in cosmetics, sunscreens, shampoos, liquid soaps, and barrier and moisturizing creams used at work. Other sources include moistened toilet tissues, ultrasound gel, adhesives, soluble cutting oils, latex paints, vaginal examination gel and sanitary pads. The EU ban has reduced these exposures very significantly. Nevertheless, its inclusion in products used in the workplace such as barrier creams and after-work creams makes its inclusion in the European and BSCA baseline series still relevant at the current time.
Clinical features. These are discussed in the section on allergy to cosmetics. However, hand dermatitis is a frequent finding as a result of exposure to hand creams and liquid soaps [6]. Irritant hand dermatitis may be suspected prior to patch testing but the demonstration of allergy and withdrawal of the incriminated products has resolved the dermatitis in many instances [7, 8].
Allergy to MDBGN in wipes and moistened toilet tissues, vaginal examination gels and sanitary pads is a potential cause of ano-genital dermatitis [8, 9, 10].
Avoidance. Methyldibromo glutaronitrile is the INCI name that should be sought on the full ingredient label. In some instances its presence may be identified by its synonym 1,2-dibromo-2,4-dicyanobutane or Euxyl K400 (Tektamer 38). Many producers of work cleansers and creams now give a full ingredient list on the health and safety data sheet. Skin products whose ingredients are not known should not be used.
Other potentially allergenic sources may require specific enquiry as to the nature of the biocide/preservative used.
Patch tests. There has been much discussion about the optimal patch test concentration. We prefer to test MDBGN at 0.3% in petrolatum, although this has been found by some [2] and ourselves to be marginally irritant. Others have advised 0.2% as the optimum concentration [11], and 0.1% has been used, but may give false negative reactions [12]. Conversely, positive ROAT findings and some relevant allergies have only been confirmed by testing with 0.5% or 1% concentrations [13]. Euxyl K 400 is also available at 0.5% and 1% (containing 0.1% and 0.2% MDBGN) in petrolatum. A 2.5% concentration may increase the return, but with the risk of false positive reactions [4]. At present it is still recommended for the BSCA baseline series at a concentration of 0.3% [14].
Chloroxylenol [1]
Chloroxylenol (parachlorometaxylenol, PCMX) is a halogenated aromatic compound used not only as a preservative but also as an active disinfectant. It is water and oil soluble, and active against Gram-positive and Gram-negative bacteria.
Prevalence. Generally, reports of chloroxylenol allergy have been sporadic, with few large-scale studies. In one UK study 1.8% of 951 routinely tested persons were patch test positive, with a high level of current or previous relevance [1]. A more recent British study yielded a lower prevalence rate of 0.4% [2]. Reports from the USA document seven patients sensitized by medicated Vaseline or electrocardiogram paste and two with allergy to soap and hand cream [3, 4].
Occurrence. Chloroxylenol is a potential allergen for the UK as it is found in Dettol®, a widely used household disinfectant. This may also be used in diluted form as a wound cleanser. However, it is important to know that Dettol is used in many ways that may not always be predictable. Some people use the product to ‘decontaminate’ themselves or their environment. It is not uncommon for them to add Dettol to bath water, and if they have a skin disorder that they consider represents an infection, they may add extra in the hope of its eradication. Persons with perineal inflammatory disorders are particularly liable to do this. Some may apply neat Dettol to their skin in the hope of eradicating a genuine or imagined infection; the residual smell may be a helpful diagnostic indicator of use. Clothes and bedding may be washed in Dettol and inadequately rinsed, and then worn or used.
Chloroxylenol may also be found in a number of over-the-counter pharmaceutical preparations for cuts, grazes and infections [1]. Other sources include foot and talcum powders, soaps and cleansers, work creams, coolant oils, electrocardiograph pastes and, rarely, cosmetics.
Clinical features. In many cases there is a localized skin eruption at the site where a product containing chloroxylenol has been applied, or allergy may present as an unexpected exacerbation of pre-existing dermatitis.
Hand dermatitis is a potential problem for cleaners coming in contact with disinfectants when their hands are unprotected. Allergy to chloroxylenol in other work materials (e.g. coolant oils) may give a similar distribution of rash.
More widespread eruptions may be associated with its use for washing and bathing (Figure 128.18), and also when applied to clothing. Recently, widespread hypopigmentation following contact allergy to chloroxylenol added to bath water has been reported [5].
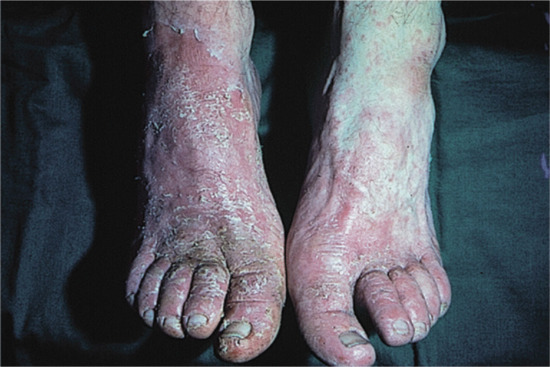
Figure 128.18 Allergy to chloroxylenol from washing with Dettol. (Courtesy of Geoffrey Auckland Collection, Hope Hospital, Manchester, UK.)
Often, the source is only identified retrospectively after finding a positive patch test.
Avoidance. Chloroxylenol is the INCI name. Cosmetics and work creams that contain it can usually be identified from the full ingredient label or data sheet. Labels on medicated foot powders and talcs generally acknowledge it as an ingredient, but specific enquiries may be necessary to establish its presence in some topical medicaments and disinfectants.
Patch tests. Chloroxylenol is generally patch tested at 1% in petrolatum. It may cross-sensitize with chlorocresol [6]. It has been recommended as a baseline allergen for the UK [1, 4].
Chlorocresol
Chlorocresol (parachlorometacresol, PCMC) is identical with chloroxylenol, except for the absence of a methyl group on the benzene ring. It is active against Gram-positive and Gram-negative bacteria, and is water and oil soluble.
Prevalence. Chlorocresol is a rare allergen. A multicentre UK survey of routinely patch-tested patients confirmed a low rate of 0.6% [2].
Occurrence. The major source is corticosteroid creams. In the past, aqueous cream BP was preserved with chlorocresol, but this is generally no longer the case. We have only seen it as a sensitizer from topical medicaments, although it may be used in hand cleaners, metal-working fluids and occasionally cosmetics.
Clinical features. These are discussed in the section on medicament allergy. Erythroderma has occurred in a diabetic patient known to be allergic to chlororesol when given insulin preserved with m-cresol, and this improved on changing to a parabens-preserved insulin [7].
Avoidance. It is helpful to give a sensitized patient a list of corticosteroid creams that indicates their constituents and which are free from chlorocresol. Moisturizers should not be used unless they are fully ingredient labelled or known to be free from this preservative. The INCI name is chlorocresol.
Patch tests. The recommended test concentration and vehicle is 1% chlorocresol in petrolatum. Although contact allergy is rare, its use in many popular corticosteroid creams available in the UK has prompted the BSCA to recommend its inclusion in the baseline series [8]. Cross-sensitivity with chloroxylenol is well recognized [6].
Organic mercurials
Sensitizing compounds include phenylmercuric salts and thimerosal (thiomersal; merthiolate). Thimerosal is composed of an organic mercurial and thiosalicylate. Allergy may occur to either moiety [1].
Occurrence. Organic mercurials are used as preservatives in vaccines and antigen extracts, eye drops, contact lens solutions, oils, bindi and eye make-up and remover products. Their use in all other cosmetics is banned by European legislation. Phenylmercuric salts have been used in contraceptive jelly, antifungal treatments, shoe linings and emulsion paints.
Prevalence. Positive reactions occur in 4–5% of individuals routinely patch tested with thimerosal; higher rates have been reported in North America (10.9%) and Japan (9.5%) [2, 3]. Even higher levels are found in children and adolescents [4].
Clinical features. Allergy to organic mercurials in eye medicaments and contact lens preservatives will induce a localized dermatitis affecting the eyelids, with periorbital extension. Hand eczema has been reported in a sensitized nurse undertaking vaccinations [5]. Palmar and fingertip dermatitis, isolated conjunctivitis, and even corneal neovascularization from contact lens solutions, are described [6, 7, 8].
Many people have a positive patch test to thimerosal of no demonstrable relevance [9, 10]. Consequently, it was ironically entitled ‘contact (non) allergen of the year’ in 2002 in North America [11]. A much higher return of relevant allergy, mainly inducing hand dermatitis, was seen in Israel, particularly in mechanics. It was blamed on contact with oil containing thimerosal [12]. Most sensitization is thought to develop from parenteral vaccinations and immunotherapeutic agents preserved with thimerosal [13, 14, 15]. Localized reactions from injections are rare but have been observed, and generalized dermatitis is very rare [16, 17]. Of 57 patients with demonstrable thimerosal allergy only five reacted with a mild localized erythema following intramuscular challenge, and this led the authors to state that vaccines preserved with thimerosal were relatively safe [18].
Allergy to the thiosalicylic acid component may be associated with photoallergy to piroxicam [19].
Patch tests. Phenylmercuric salts may be tested at 0.01% and 0.05% in petrolatum and water. There is evidence that 0.05% gives frequent false positive irritant reactions [20]. Thimerosal is normally tested at 0.1% in petrolatum.
Other preservatives/biocides
Many other antimicrobial agents have been used as preservatives and biocides, and have been reported to sensitize. These include chloracetamide, triclosan (Irgasan DP300) [1], benzalkonium chloride [2], glutaraldehyde, sorbic acid, benzyl alcohol, chlorphenesin [3], glyoxal, captan, chlorhexidine [4], EDTA, dichlorophene, iodopropynyl butyl carbamate [5] and many more [6, 7]. In particular, sodium metabisulphite as a marker of sulphite allergy and in its own right has been reported as a frequent and often relevant allergen in medicaments as well as cosmetics (especially hair dyes) and whose relevance may easily be overlooked [8].
In our own experience within the UK it may be relevant particularly in certain medicaments including Trimovate cream®. Sources where antimicrobial protection is required are legion, but include in particular medicaments, cosmetics, cleansing agents, paints and soluble coolant oils. The possibility of allergy to preservatives/biocides in this group of materials must always be considered as a cause of dermatitis.
Vehicles and other cosmetic and medicament excipients
Lanolin [1]
Lanolin is a natural product obtained from sheep fleece. It is a complex and variable mixture of sterols, fatty alcohols, fatty acids and their esters. Wool wax alcohols are obtained by hydrolysis of the oily wax fraction of the fleece. Although they are not all known, it is thought the allergens are in this fraction [2].
Attempts to reduce allergenicity include modification by acetylation, hydrogenation, ethylenation, transesterification and removal of the allergenic fractions by a purification process [1]. Allergenicity has been shown virtually to disappear by removing detergent residues and reducing the level of alcohols to below 3% (w/w) [3].
Prevalence. The prevalence of lanolin allergy in the general population is thought to be low [4]. Contact allergy is normally detected by patch testing with wool alcohols, and enhanced identification of allergic subjects has been attempted by testing with a wider range of lanolin derivatives and lanolin itself. Most surveys of patients routinely patch tested to wool alcohols report positive reactions in 1.7–3.3% [5, 6, 7]. However, other studies, in which patch testing with lanolin derivatives including Amerchol L 101 (mineral oil and lanolin alcohol) was undertaken, have shown a much higher rate of positive reactions than those using wool alcohols alone [8, 9].
The belief that lanolin is a frequent sensitizer has been questioned by Kligman [10], and there are grounds for this because experimental sensitization of animals and humans has not been achieved [11]. Furthermore, patch testing with wool alcohols at 30% in petrolatum (as generally recommended) and with Amerchol L 101, particularly if patch tested at 100%, may give false positive results [5, 10]. In addition, retesting showed that the allergy ‘disappeared’ in up to 40% of those originally considered to have positive reactions [12]. Nevertheless, there is good evidence of a high prevalence of allergy to lanolin in medicaments applied to varicose eczema [13, 14]. The use of lanolin-containing medicaments on other chronic eczemas, particularly in elderly women, may be associated with the development of lanolin sensitivity. However, usage on normal skin rarely seems to be associated with significant problems [15].
Occurrence. Lanolin is most commonly encountered in medicaments, emollients, bath additives and cosmetics. Other sources include polishes, waxes, inks, adhesive tapes and bandages, anticorrosive coatings, sealants and cutting oil emulsions.
Clinical features. These are discussed in the sections on allergy to cosmetics and medicaments.
Avoidance. Lanolin alcohols is the INCI name for lanolin, and its presence in cosmetics can be established by examining the full ingredient label. However, prescribed and over-the-counter medicaments are not always fully ingredient labelled in the UK, and examination of the data sheet or contacting the manufacturer may be necessary to ascertain whether it is in a medicament. This also applies to other potential domestic and work exposures such as polishes, waxes, coatings and oils.
Patch tests. Many patients state that they are allergic to lanolin but patch testing does not substantiate this. Conversely, allergy may be unsuspected, particularly when there is an associated eczema being treated with a lanolin-containing medicament.
Standard testing with wool alcohols 30% in petrolatum is advised, but where medicament sensitivity is suspected or to be excluded, extra lanolin allergens should be tested. We use Amerchol L 101 50% in petrolatum and lanolin ‘as is’. Weak positive reactions may be false positives, but can be exceedingly difficult to distinguish from weak allergic reactions.
Cetearyl alcohol
Cetearyl alcohol has emulsifying and stabilizing properties, and is also known as cetylstearyl alcohol and Lanette O. It is essentially a mixture of two long-chained stereoisomers, cetyl and stearyl alcohol. These alcohols are components of lanolin.
Prevalence. Reports of allergy are often anecdotal, although there is evidence of it being a significant allergen complicating varicose eczema and ulcers, with up to 16% positive reactions in patients with these conditions attending for patch testing [13, 16]. In the UK, a level of 0.8% was seen in routinely patch-tested patients [17].
Occurrence. Cetearyl alcohol is widely used in steroid creams, emollients and cosmetics. Sometimes only one of the stereoisomers is used. It is a component of emulsifying wax and therefore found in emulsifying ointment and aqueous cream BP.
Clinical features. These are discussed in the sections on allergy to cosmetics and medicaments.
Avoidance. Cetearyl alcohol is the INCI name, but cosmetics labelled as containing cetyl or stearyl alcohol should also be avoided. Avoidance of medicaments, including emollients, is more difficult, as they are not always fully ingredient labelled. Even when they are, they may not follow the rules for cosmetics. Emulsifying wax is an ingredient that may be listed without it being clear that the preparation contains cetearyl alcohol. The designations cetylstearyl alcohol or Lanette O may be used instead of the INCI name. It may be preferable to provide a sensitized individual with a list of products free of cetearyl alcohol.
Patch tests. Although it is an uncommon allergen, its ubiquitous presence in dermatological therapies means that identification of allergy is important. The BSCA has therefore recommended its inclusion in the standard series for the UK [17]. Patients suffering from varicose eczema should always be patch tested with it. It is normally tested at 20% in petrolatum.
Ethylenediamine dihydrochloride
Ethylenediamine is a low-molecular-weight aliphatic amine. Some antihistamines are chemically related, which may be of significance to the sensitized patient.
Prevalence. Allergy to ethylenediamine is becoming less common, and sufficiently so for the European Environmental and Contact Dermatitis Research Group (EECDRG) to recommend omitting it from their recommended baseline series [19]. In general, the prevalence of allergy reflects the amount of nystatin/neomycin sulphate/gramicidin/triamcinolone acetonide cream being used in the catchment area of those being tested, as this is the usual source of sensitization. This preparation has been reformulated in the USA, but the original formula may still occur in generic creams. It is still used in the UK. Of routinely patch-tested patients, 1.3% were shown to be allergic, so the BSCA recommend the continued inclusion of ethylenediamine in the baseline series [17, 18].
Occurrence. Ethylenediamine was used as a stabilizer in a combined preparation that contains nystatin, neomycin sulphate, gramicidin and triamcinolone acetonide, marketed in the UK as Tri-Adcortyl® cream and in the USA and other countries as Mycolog® cream. The equivalent ointment does not contain ethylenediamine. It is a component of oral and parenteral aminophylline preparations, which may also come in contact with the hands. Other systemic and topical medicaments are also related to ethylenediamine, most notably hydroxyzine and probably cetirizine and levocetirizine [19, 20], as well as piperazines [21], which include the antihistamines meclozine and cyclizine [22]. Industrial exposure is potentially wide, as it and related amines are used as epoxy hardeners and in coolant oils, wire-drawing lubricants, floor polish remover, antifreeze, synthetic waxes, anticorrosive paints and dye manufacture. It is used as a rubber stabilizer, but we have not seen sensitization occurring from rubber garments.
Clinical features. These are discussed in the sections on medicament and systemic contact allergy. Occupational patterns will depend on the source of the exposure, but the hands are the most likely site to be affected. In those sensitized to epoxy systems, there is often concomitant sensitization to epoxy resin, with an associated exposed-site pattern of dermatitis.
Avoidance. The avoidance of topical exposure to the creams containing nystatin, neomycin sulphate, gramicidin and triamcinolone acetonide is essential. It may also be necessary to avoid topical antihistamine creams and eye drops. Once sensitized, individuals may be at risk of systemic contact dermatitis. In the UK, avoidance of systemic hydroxyzine, piperazine, cetirizine and levocetirizine is advisable [20, 21, 24]. Sensitized patients must also avoid oral and parenteral aminophylline [25, 26].
Avoidance of occupational exposure depends on identification of the source.
Patch tests. Ethylenediamine is tested at 1% in petrolatum. It is still recommended in the BSCA baseline series, but since Tri-Adcortyl® cream is no longer prescribable it is likely to be omitted in the future.
Other excipients
There is potential for virtually any vehicular component of a cosmetic or medicament to cause sensitization. If allergy is suspected, it may be necessary to widen the range of allergens tested. Examples include antioxidants (butylated hydroxyanisole, butylated hydroxytoluene, t-butylhydroquinone and gallates), surfactants (e.g. cocamidopropyl betaine), which may cause hand dermatitis in hairdressers from shampoos, alkyl glucosides including decyl glucoside in particular [27], cocamide DEA (coconut diethanolamide) and humectants (e.g. propylene glycol).
This list is by no means exhaustive. Many excipient allergens are available from the main allergen suppliers, suitably prepared for patch testing, but others may need to be sought from the material's manufacturer and diluted appropriately for patch testing.
p-Phenylenediamine and related dyes [1, 2]
p-Phenylenediamine (PPD) and toluene-2,5-diamine (PTD) are aniline derivatives, whose main use is for dyeing hair. These chemicals are colourless until oxidized by hydrogen peroxide in the presence of ammonia, and polymerized by a coupler, often in the presence of other intermediates, to produce a variety of shades of colour that stay fast within the hair shaft [1]. Once oxidized and polymerized, PPD is no longer allergenic [3]. Semipermanent hair dyeing may be undertaken with related dyes, for example o-nitro-p-phenylenediamine (ONPPD).
There is structural similarity to some azo dyes (e.g. p-aminoazobenzene). Many disperse dyes used to dye synthetic clothing and fibres are azo dyes and these are discussed in the secton on clothing.
Prevalence. The use of hair colours has increased greatly in recent years, with 75% of US and Danish women reported to dye their hair [4]; in Japan the prevalence of hair dyeing increased during the period 1991–2001 amongst female high school students from 14% to 41%, in women in their twenties from 6% to 85% and in men in their twenties from 2% to33% [5]. There is clear evidence of increasing frequency of allergy in the UK, and the frequency of allergy in one clinic almost doubled to 7.1% over a 6-year period [6]. Of those patch tested in the early 2000s by the NACDG, 4.8% were allergic to PPD [7]. Similarly, 4.8% have been shown to be allergic to PPD in Germany and Austria, where there was considerable geographical variation in frequency (2.8–7.1%) [8]. In a large Belgian study of over 5000 routinely tested patients, 7.2% were allergic to PPD, 1.6% were allergic to PTD and 1.8% were allergic to ONPPD [9]. In a recent publication review, the median prevalence of PPD allergy amongst dermatitis patients was 4.3% in Asia, 4% in Europe and 6.2% in North America. Relevant positive PPD reactions are most often associated with hair dye exposure, although some positive reactions may be the result of cross-sensitization [10]. PPD is also the second most common allergen of relevance for hairdressers in Europe [11].
Occurrence. PPD and PTD are found in permanent hair dyes, and ONPPD in semipermanent hair dyes whose colour will persist for 5–10 shampooings. In the EU, PPD is allowed in hair dyes up to a maximum concentration of 6% free base [12]. PPD has also been used to dye fur. PPD may be mixed with henna and used on the skin as a temporary tattoo (black henna tattoo) and the actual concentration of PPD may vary greatly with some containing concentrations >10%. The use of these has become very popular amongst children, adolescents and young adults in western Europe, usually being applied in holiday resorts. A proportion of these patients will become actively sensitized to PPD through the application of the tattoo and characteristically will develop severe allergic reactions to hair dyes containing PPD or related chemicals [13].
Allergy to PPD has also been reported from a violin chin-rest and cello bowstring stain [14, 15]. PPD derivatives are used as rubber antioxidants, particularly in heavy-duty black rubber.
Azo dyes are mainly encountered as disperse dyes for synthetic clothing. Allergy to azo dyes in maggots used for fishing bait has been reported [16].
Clinical features. PPD and related hair dye allergy can result in extremely severe skin reactions. The scalp is often relatively spared, but severe oedema and weeping of the scalp margin, ears and eyes, with more extensive secondary-spread eruptions, may be seen. However, there can be lower grade reactions, usually around the scalp margin. The patient does not always recognize the relationship of the skin eruption to dyeing the hair. Allergic contact dermatitis from hair, including connubial allergy from a partner's dyed hair, has been described. This is probably the result of the presence of unoxidized residue when there is poor dyeing technique, which is more likely with self-application of the dye [17, 18].
Although banned in the EU, permanent hair dye products are readily available for use on eyelashes and eyebrows. Severe reactions including blindness have been reported [19].
The possibility of contact allergy from the use of permanent hair dyes on the beard, particularly in Arabic or Asian men, should not be overlooked [20]. Lichen planus-like presentations of hair dye allergy have been reported from the Indian subcontinent, and we have seen similar patterns in Asian patients in the UK [21]. Furthermore, in our experience, hair dye allergy is relatively common in both sexes in this ethnic group. An equivalent overrepresentation has been identified in African Americans [22]. Lymphomatoid eruptions are also described [23].
Hairdressers can become sensitized by the dyeing process, resulting in hand dermatitis. A pre-existing irritant hand dermatitis may predispose to this. Styling of dyed hair should theoretically not present a problem in view of the reported non-allergenicity of the oxidized dye.
As previously stated, allergy to PPD contaminating black henna tattoos has been reported frequently in recent years [24]. Reactions in the site of application of temporary tattoos may be delayed for about 2 weeks while sensitization takes place, but the subsequent reaction can be severe and persistent. Erythema multiforme-like and lichenoid eruptions are described, and both post-inflammatory hypopigmentation and hyperpigmentation can be a feature [25, 26, 27]. If affected persons dye their hair at a later date then there may be a major recrudescence of allergic contact dermatitis [28]. Subsequent clothing dye allergy is also possible [29].
Immediate-type hypersensitivity presenting as an urticarial reaction to PPD is also recognized [30], and contact anaphylaxis is described [31].
Clinical presentation of clothing dye allergy is described in the section on clothing.
Avoidance. Permanent hair dyes should be clearly marked ‘contains phenylenediamines’, and should advise that consumer led patch testing be undertaken on each occasion prior to dyeing the hair. Open testing on retroauricular skin, with a 2-day reading, is thought to be an accurate method of identifying sensitized persons [32], but this is rarely done by the individual or the hairdressing salon. Once PPD allergy is diagnosed, the hair should not be permanently dyed. Semipermanent dyes might be tolerated, but about 25% of PPD-allergic subjects are likely to have problems due to cross-sensitivity [1]. An open patch test with these dyes is also advised before use. Other alternatives include pure henna and colour rinses with temporary (non-PPD related) dyes.
A new ‘less sensitizing’ variant of PPD has recently been developed and marketed (2-methoxymethyl-p-phenylenediamine). At present it is aimed at the market to reduce the number of consumers who might become sensitized. As yet there are no published data on the degree of cross-reactivity with PPD and it is not recommended for use in those already sensitized to PPD.
Disperse azo clothing dye avoidance is discussed in the section on clothing.
Cross-sensitivity. Molecules with a similar structure may cross-sensitize with PPD, for example benzocaine, procaine, sulphonamides, mesalazine, diaminodiphenylmethane, para-aminobenzoic acid (PABA) UV filters and certain azo dyes, and patients should be counselled about this possibility [1, 2, 29].
Patch tests. A significant drop in the frequency of positive allergic reactions was noted when the PPD base 1% in petrolatum was changed to PPD dihydrochloride by the allergen suppliers, and relevant positive cases were missed [33]. PPD base is again the preferred baseline test allergen.
Concern has arisen over the risk of active sensitization on routine patch testing with PPD [34, 35]. Most data on this issue have been reassuring [36, 37]. It is our and others’ view that it should continue to be tested as a standard allergen [38], especially as relevant allergy is not always suspected.
In some individuals, close examination of the patch test site is required, as a positive reaction may be obscured by the black colour left by the patch test. Fierce ‘+++’ reactions to PPD are seen on occasions. In those with a recent severe presumed hair dye allergic dermatitis, and particularly those with a temporary tattoo reaction, we generally undertake an initial test concentration of 0.5% in petrolatum, but a more conservative stepwise increase starting at 0.01%, and increasing to 0.1% before progressing to 1% has been advocated [39]. Other strategies include not applying the recommended 1% petrolatum patch test for the full 48 h.
Related hair dye chemicals are also usually tested at 1% in petrolatum. Where permanent hair dye allergy is suspected it is important to test also with PTD, as many dyes contain this and not PPD. It has been shown that about 9% of PTD-allergic subjects were patch test negative to PPD [40].
Azo dyes will normally be incorporated into a larger series of allergens for the investigation of textile dermatitis (see the section on clothing). They are also tested at 1% in petrolatum.
Ultraviolet filters
Epidemiology. UV filters work by absorbing light chemically or by acting as a physical block. The latter agents are usually based on titanium or zinc oxide, which are not sensitizers. However, some chemical UV filters may be contact allergens, photocontact allergens or both. The pattern of usage of UV filters varies, and prevalence figures will reflect this. Current data are provided in the photoallergy segment.
Clinical features. Allergy and photoallergy from UV filters may coexist or occur separately. Clinical features are discussed under photoallergic contact dermatitis and in the cosmetics section. It is important to appreciate that other photodermatoses can be complicated by photoallergy to UV filters being used in treatment, and this may easily go unrecognized. The possibility of allergy and photoallergy to UV filters must be considered before individuals are diagnosed as having an idiopathic photodermatosis such as polymorphic light eruption.
Investigations. These chemicals are not confined to sunscreens. They may be added to cosmetics in small quantities to prevent photodegradation and also as an ‘antiageing’ agent. UV light absorbers may be added to plastics and spandex. Allergy from this source is unusual but has been reported, most notably from 2-(2-hydroxy-5-methylphenyl)-benzotriazole (Tinuvin P) [1, 2]. UV filters are generally tested at 10% in petrolatum, although 5% has also been advocated. We have found that benzophenone-4 tested at 10% gives many weak reactions that we have interpreted as false positive, particularly as they have been of questionable relevance. A lower concentration may be a better test concentration. Sunscreens and cosmetics containing UV filters should also be patch tested, and if necessary photopatch tested at the same time.
Management. Once allergy or photoallergy has been demonstrated, patients should be apprised of the need to avoid sunscreens and cosmetics containing the allergen, which will be identified by its INCI name. They should only use fully ingredient-labelled cosmetics (including hairsprays). In addition, a list of synonyms should be given to the patients as they may encounter sunscreens labelled differently in other countries.
Rubber
Epidemiology. The term ‘latex’ defines an aqueous dispersion of a rubber. The rubber obtained from latex by drying or coagulation is called latex rubber. Natural latex is derived from the sap of the tree Hevea brasiliensis. During the world wars the availability of natural latex was limited and proved a stimulus to the development of various synthetic rubbers such as nitrile and neoprene. Rubber dermatitis is usually caused by accelerators, antioxidants and other chemicals used in its manufacture. More than 1000 substances are employed for these purposes.
The incidence of sensitivity is of the order of 5–10% of patients tested in most patch test series [1]. Rubber dermatitis occurs with equal frequency in both sexes, but the actual sensitizers differ, depending on exposure, the antioxidants in black rubber more frequently causing problems in men from occupational exposure.
Potential sources of exposure include the following:
- Rubber industries and revulcanization shops: both non-vulcanized and vulcanized rubber-containing additives are sensitizers; in rubber tyre factories, much dermatitis is irritant rather than allergic.
- Other workplaces: rubber gloves, other protection for hands and fingers, electric cords, tubes, handles (e.g. on hammers), packings, masks, rubber bands, etc.
- Daily life: shoes, gloves, clothing, condoms and many other articles.
Clinical features. Rubber sensitivity may be the primary cause of a dermatitis or it may become superimposed on an existing dermatitis, as sometimes occurs following the use of protective rubber gloves (Figure 128.19). It is not always obvious, and many cases will be missed if patients are not routinely patch tested with a series of the more common rubber chemicals. A positive patch test reaction to a rubber chemical is usually relevant.
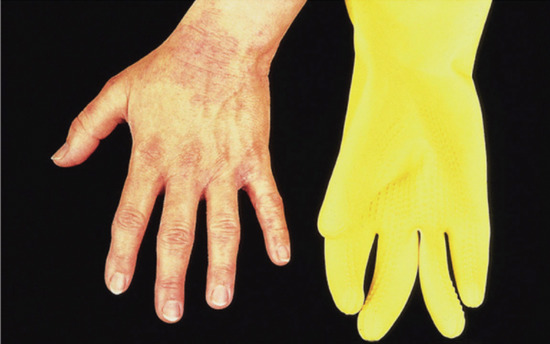
Figure 128.19 Contact dermatitis from rubber gloves. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Dermatitis from rubber gloves [2] may be diffuse, but is more often localized to the dorsa of the hands, especially over the knuckles and the wrist, where a sharp proximal margin is often evident. The eyelids and face may also be involved from touching the face while wearing gloves. Dermatitis may be caused by objects touched only briefly during a daily routine. A digitate and patchy dermatitis has occurred in previously sensitized patients following examination with surgeons’ gloves.
Shoe dermatitis occurs in both adults and children, and in the latter group needs to be differentiated from juvenile plantar dermatosis [3]. Rubber chemicals may occur in almost any part of the shoe, and a rubber adhesive is commonly used to glue parts together. The dermatitis may occur on the dorsum of the foot, soles or toes, usually with sparing of the web spaces and instep. A secondary dermatitis, especially of the hands, is not uncommon. The outer soles rarely cause shoe dermatitis. Primary sensitization from all-rubber boots and rubber shoes is common, especially in agricultural workers.
Antioxidants related to PPD are used in car tyres and wear-resistant rubber products. They often impart a dark or black colour to the rubber materials vulcanized with them. Not all cases have an occupational origin. Black rubber flexes or cables, hoses, grips and even scuba masks or squash balls may be responsible. The ensuing dermatitis may sometimes be purpuric, and an erythema multiforme-like presentation has also been reported.
In some cases, the site of dermatitis may provide a clue as, for instance, when the dermatitis is due to a rubber finger-stall used when counting money or rubber bands under a wrist watch. Rubber dermatitis may also occur at the site of contact with rubber in clothing (Figure 128.20) or dressings, on the face from swimming goggles, between the thighs or on the abdomen from hot water bottles, and on the knees from kneeling mats. Genital dermatitis or pruritus vulvae may occur following the use of condoms and diaphragms, and may also result from rubber catheters, when the dermatitis also spreads down the thighs. An apparent worsening of venous eczema may be related to allergy to rubber in elastic bandaging, and such patients are prone to develop a secondary generalized eczema. A generalized dermatitis may occur after sleeping on a rubber mattress or using rubber pillows, or the dermatitis may be predominantly on the side on which the patient sleeps.
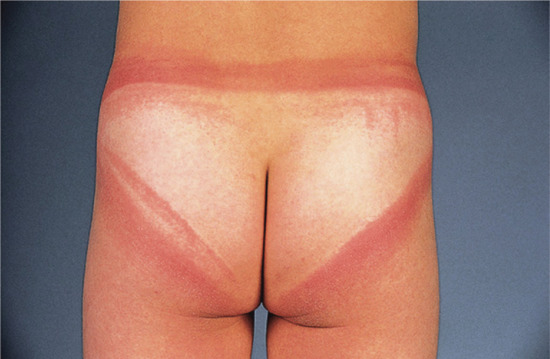
Figure 128.20 Allergic contact dermatitis to elastic in clothing. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Investigation. Most types of rubber contain up to 5% of potentially allergenic additives. Dermatitis from the smoked sheets of rubber used as raw material is rarely reported. The sensitizers in rubber change in accordance with industrial development, technical requirements and market prices. Unless the choice of substances kept for patch testing is constantly supplemented, rubber dermatitis will be missed. A list of potential sensitizers in rubber is shown in Box 128.4.
Most standard series contain rubber chemicals, both mixes and individual chemicals, as a screen for rubber-induced contact hypersensitivity. The mixes of rubber chemicals are useful because they allow an allergy to be detected with fewer patch tests. In order to avoid patch test sensitization, the concentration of the individual chemicals must be reduced, and this involves the risk of false negative reactions. This is especially so for mercaptobenzothiazole (MBT), and therefore MBT and MBT mixes are usually both included in most patch test series [4]. The concentrations of the individual chemicals in these mixes are therefore selected as a compromise. Too high a concentration will carry the risk of active sensitization, whereas too low a concentration of any of the individual ingredients may lead to false negative reactions and missed sensitivities. PPD derivatives are sensitizing when tested at 2% in petrolatum, and should be tested at 0.25% and 0.1%. Simultaneous sensitivity to PPD and to the PPD derivatives in rubber is uncommon. Black rubber mix is no longer included in the European standard series, having been replaced by IPPD. Testing with IPPD alone will potentially miss 10% of individuals allergic to this group of antioxidants [5]. Carba mix and the diphenylguanidine in the carba mix often produce marginal irritant reactions. Although carba mix has been deleted from the European standard series, the predominant use of carbamates as accelerators in rubber gloves [6] argues for retention of the mix, as in the North American and British series, rather than relying on a cross-reaction with thiurams to detect the allergy.
Where rubber allergy is suspected, additional testing with the ingredients of the mixes and additional rubber-related allergens may reveal what would otherwise have been a missed contact allergy [7, 8]. Cyclohexylthiophthalimide, a common rubber antidegradant, frequently causes reactions, but the relevance is often uncertain, particularly where there is no ingredient labelling [9]. Dithiodimorpholine [10] is also reported as a rubber allergen in footwear; sensitivity to which is potentially missed by not testing with an extended series. It is also essential to test with a sample of the suspect rubber in case sensitization has occurred to a chemical not present in any standard or rubber series. Delayed-type hypersensitivity reactions to natural rubber latex itself have been reported [11].
Synthetic rubbers such as styrene–butadiene, polybutadiene, polychloroprene (neoprene) and polyurethane (spandex) may contain similar accelerators and antioxidants. In particular, neoprene items such as gloves, shoes and wet suits frequently contain thioureas [12]. Elastane (Lycra) does not contain rubber accelerators.
Sensitivity to a certain rubber chemical does not necessarily indicate any specific source, although sensitivity to carbamates and thiurams suggests rubber gloves, mercapto compounds suggests shoes, and the PPD group is mainly associated with black rubber products such as tyres.
Management. Although it may be impossible to avoid contact with rubber entirely, many patients remain clear of dermatitis, and others may only have intermittent symptoms if they take simple precautions to avoid contact with rubber material. In many cases, hand eczema improves or clears if patients can be persuaded to change from rubber gloves to cotton-lined vinyl gloves.
Clothing
Epidemiology. Textile fibres may be natural, for example cotton, wool, silk, linen and rubber, or they may be synthetic, for example cellulose derivatives (rayon), polyamides such as nylon, polyesters, acrylics and elastomers. Apart from rubber, they rarely sensitize in their own right.
Commoner allergens in clothing include nickel, chromate (in leather and as a dyeing mordant), rubber, glues, textile dyes, formaldehyde and resins. Since 1970, textile dermatitis has probably become rarer, mainly due to changed methods of production, although accurate information on the incidence and prevalence of clothing allergy is lacking. In those undergoing patch testing, the frequency of allergy to textile dyes has varied from 1.1% to 5.8% and for resins from 1.2% to 2.3%, either from formaldehyde or the resin, or both [1].
Disperse dyes are the class of dye most likely to sensitize [2], particularly as it is not possible to make them completely fast to the fibres. They are principally anthroquinone and azo dyes. Disperse dyes may contain more than one fraction, as well as impurities, all of which can sensitize [3]. A mixture of several different dyes may be responsible for the final colour.
Fibre-reactive dyes are covalently bound to the fibre and unlikely to cause problems from clothing, but may sensitize those handling the dye powder [4]. However, if the clothing is not adequately rinsed during the production process, reactive dye residue in new unwashed garments may then cause allergic dermatitis [5]. Clothing dermatitis has also been reported from acid, basic, direct, vat and solvent dyes, as well as coupling agents.
Many clothing resins will release formaldehyde [6], and free formaldehyde may be found on treated garments and will induce dermatitis in its own right. Most, but not all, patients with formaldehyde textile resin dermatitis are also sensitive to formaldehyde. Between 1950 and 1965, formaldehyde resins used for crease-resistant finishes caused numerous cases of textile dermatitis. Finishes are used on textiles to give ‘body’ to inexpensive materials, and provide crease-resistant and stain-repellent properties. Urea and melamine formaldehyde resin finishes are now being superseded by others, such as dimethylolalkyl carbamate, dimethylolethylene urea, dihydroxydimethylethylene urea and other similar reactive cyclic urea resins. These new resins may be less sensitizing [7], particularly as they release less formaldehyde and remain relatively fast.
Clinical features. The distribution of contact dermatitis, in areas of sweating and friction, is the same for dyes and finishes (Figure 128.21). The eruption typically starts in the axillae, sparing the hairy part of the vault, and forms a crescentic patch on the anterior chest wall sharply limited by the underwear (Figure 128.22). The anterior and posterior folds are also affected. The dermatitis is often sheeted, and the inner posterior thighs, popliteal fossae and lower leg may be involved when trousers or tights (pantyhose) are the responsible garments. Allergy to dyes in socks, stockings and tights often starts on the dorsa of the feet, where they are occluded by footwear. The typical pattern for allergy to tights and stockings is shown in Figure 128.23. Long sleeves cause eruptions in the elbow flexures, and collars provoke a rash around the neck. Later in the course of the disease, the chest and upper back may be involved, sparing areas protected by shoulder straps and underwear. In the flexures and round the neck, the lesions are diffuse and oedematous; on the shoulders and chest they are usually papular. Papular dermatitis may cover the whole body.
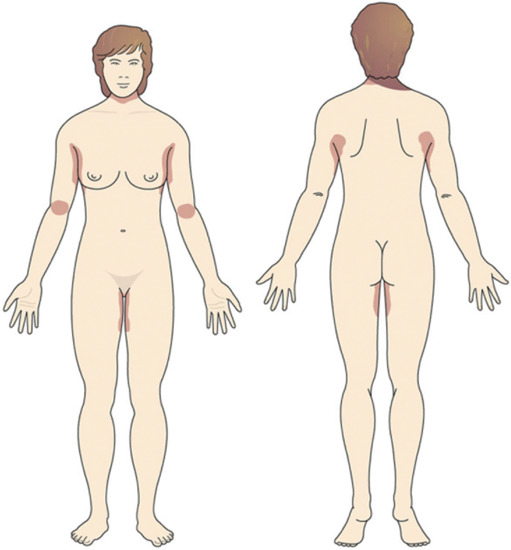
Figure 128.21 Pattern of textile dermatitis.
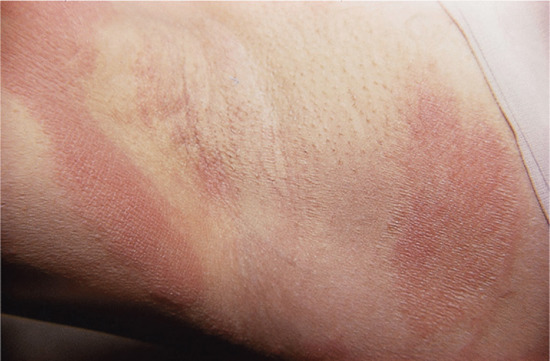
Figure 128.22 Axillary dermatitis (sparing the axillary vault). The characteristic pattern of eczema seen in patients allergic to textile dyes and finishes. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
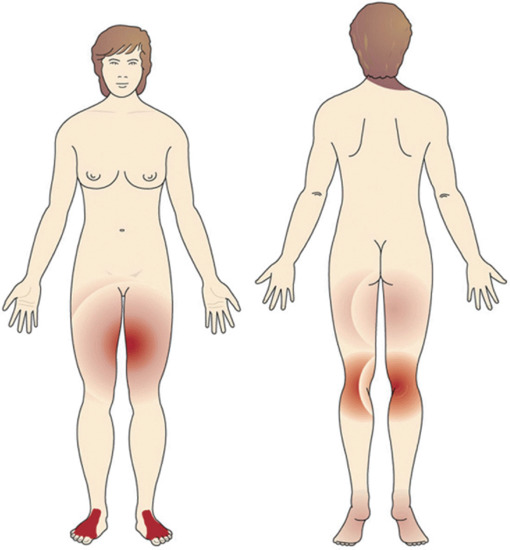
Figure 128.23 Pattern of dermatitis from nylon stockings.
In the early 1970s permanent-press resins were the source of an outbreak of follicular dermatitis due to a range of coloured Canadian bed sheets [8]. Patients developed a widespread itchy, follicular rash especially affecting the arms and legs, but also with eczema of the face and ears due to contact with pillowcases. Dyes have also been described as sensitizers in bed linen [9].
Some fabrics provoke a purpuric, sometimes lichenoid, dermatitis in areas of contact, as seen with uniforms in the Second World War (khaki dermatitis) [10]. Textile dyes and resins will occasionally cause purpuric eruptions associated with contact allergy [11]. In the early 1970s, a pigmented dermatitis was described that was caused by allergy to optical whiteners in detergents. It started on the inner aspects of the upper arms, from where it spread to the trunk, with indistinct coalescing macules as the major lesions [12]. In some cases, the dermatitis left persistent hyperpigmentation.
Hand dermatitis may be a feature of textile allergy from occupational exposure [13].
Patterns of allergy from nickel, chromate (in leather) and rubber are described in the sections discussing these allergens.
Investigations. Patch testing with the suspected clothing can be undertaken, although there is a high risk of obtaining a false negative reaction. Soaking 1 cm2 of the fabric in water for 10 min before testing might increase the return, and extraction techniques have also been suggested [14].
Formaldehyde is a standard-series allergen but not sufficient to detect contact allergy to formaldehyde-based resins, which should be tested in addition when clothing allergy is suspected. The most frequently identified resins causing allergy in recent studies are melamine formaldehyde, ethyleneurea melamine formaldehyde, dimethylol dihydroxyethyleneurea, dimethylol propylene urea and urea formaldehyde resins [15, 16].
A PPD-positive patch test may alert one to clothing dye allergy, but it is an inadequate screen. Standard-series screening with four textile disperse dye allergens has been advocated, and the possibility of using a textile dye mix explored, but further data are required [17]. As various azo dyes and PPD are structurally similar there is a risk of multiple strong positive reactions in allergic patients screened for clothing dye allergy. Patients investigated with a series of textile azo dyes should be warned of the risk, although low, of multiple positive reactions, particularly if there is a previous history of hair dye or temporary tattoo allergy [18].
Recent surveys [19, 20, 21, 22] confirm that the most commonly identified azo disperse dye allergens are Disperse Blue 124 and the very closely related Disperse Blue 106, followed by Disperse Orange 3, Red 1, Yellow 3 and Red 17. In particular, Disperse Blue dyes 106 and 124 have been reported as causing frequent problems in Canada, especially from blue/black polyester or acetate garment liners. In southern Sweden, however, Disperse Orange 1 was the most common textile dye allergen. The most frequently reported sensitizing anthroquinone dyes are Disperse Red 11, Blue 3 and Blue 35. Basic Red 46 in acrylic blend socks has caused an outbreak of foot dermatitis, but the problem so far seems to have been confined to Australia [23].
Rarely, other chemicals such as naphthol AS (a coupling agent for cotton dyeing) [24] and fire retardants in clothing have been reported to cause sensitivity [25]. Isocyanates in an antipill finish have caused dermatitis occupationally [26]. Optical whiteners are frequently added to clothes, and although in the past they were associated with contact dermatitis, there are no recent reports of allergy.
Management. Patients sensitive to formaldehyde or one of the formaldehyde resins should be advised to avoid treated fabrics, for example drip-dry, crease-resistant or durable-press cotton, cotton mix and rayon clothes. Satisfactory alternatives include wool, silk and 100% nylon, polyester or acrylic fabric, as these rarely contain significant amounts of formaldehyde or formaldehyde resins. The washing of new clothes at least twice before wearing may also be useful as this will help to remove free formaldehyde [27].
Disperse dyes are used to colour artificial fibres such as polyester, acetate, acrylic and nylon. Both azo and anthraquinone dyes may cause dermatitis from modern artificial fibres, and non-disperse azo dyes from natural fibres. Reactive dyes will combine with protein and cellulose in natural fibres and polyamides. A precise knowledge of the dye responsible for an individual's allergy is not helpful as cross-sensitivity is common and the finished colour is commonly a mix of several dyes. Strongly coloured synthetic clothing should be avoided, but lightly coloured garments may sometimes be tolerated. Pure natural fibres, such as cotton, wool, linen and silk of any colour, can generally be worn.
Patients with disperse dye stocking dermatitis can buy undyed nylon stockings and dye them by soaking in a potassium permanganate solution (0.3–0.6%) for 30–60 min. Stockings and tights without azo dyes may be commercially available, but manufacturers have to be contacted directly. Grey stockings and tights do not usually contain Disperse Yellow 3 or Orange 3. Lycra products are normally dyed with acid dyes rather than azo dyes.
The wearing of undergarments may protect the skin from allergy to outer clothing; although there is still the possibility that sweating could leach out allergens.
Shoes
Epidemiology. The prevalence of shoe allergy has ranged between 3% and 11% in patients referred for routine patch testing. The highest frequencies of dermatitis have been reported from hot climates, which will promote sweating and leaching out of allergenic shoe chemicals [1]. A wide age range is seen, with young children prominently represented in many studies. In one study on 55 patients the breakdown of the most frequent positive reactions was as follows: rubber 43.1%, chromate 23.6%, PTB formaldehyde resin (PTBPFR) 20.0%, colophony 9.0% and PPD 3.6%. However, a further 14.5% reacted to pieces of their shoes but not to the shoe allergens tested. Most patients were noted to have hyperhidrosis and 43% were atopic. More recent European studies had a similar range of allergens responsible but at a generally lower frequency. Chromate was commoner than rubber allergy in these reports [2]. Cobalt was also a common allergen but the source for this is not always clear.
Clinical features. Sweating causes allergens in shoes to leach out and migrate, and as a result of this the pattern of dermatitis is often not distinctive. It may be patchy or superimposed on a pre-existing constitutional eczema. Nevertheless, in many instances the distribution will reflect whether the sensitizer is present in the upper or sole of the shoe [1].
Dermatitis from the upper commonly starts over the dorsal surface of the big toes and spreads to the dorsa of the feet and the other toes. Outbreaks of dermatitis from tanning agents (chromate and vegetable), dyes and adhesives have often followed this pattern. The interdigital spaces are normally spared. The heels may be involved, but less frequently than the toes. On the heels, patches of dermatitis may correspond to the heel cap, and on the dorsum of the foot they may correspond to the tongue of the shoe. Adhesives and rubber components may cause localized areas of dermatitis limited to the toe cap [3]. Nickel allergy from shoe buckles and eyelets may cause a localized dermatitis on the adjacent skin. Indian sandal dermatitis has a characteristic pattern, is often severe, and affects mainly the first toe web and adjacent toes and the dorsum of the foot [4].
Rubber chemical allergy has an increased tendency to affect the soles. Involvement of the sole usually affects only the weight-bearing areas – the instep is frequently spared. In sports shoes, the sole is usually moulded to fit into the instep and the dermatitis may affect the whole sole [5]. Sometimes, only the forefoot is involved (Figure 128.24), and in children the condition must be differentiated from juvenile plantar dermatosis by patch testing.
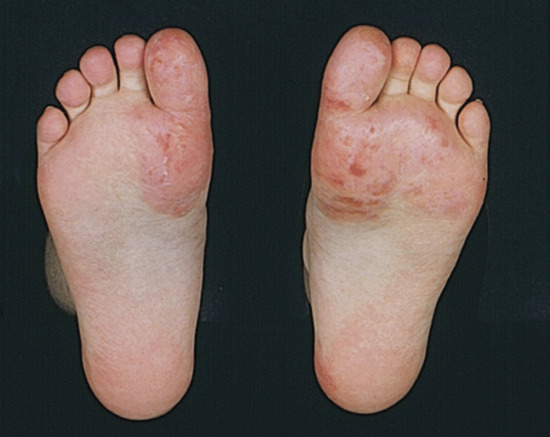
Figure 128.24 Forefoot dermatitis from shoe allergy.
Surprisingly, not all cases are bilateral [6], but the great majority are. Patients with shoe dermatitis often have evidence of dermatitis elsewhere, especially on the hands.
Boots produce a pattern similar to shoe dermatitis, sometimes with an additional eruption on the calves. An eczematous and purpuric allergic contact dermatitis has been reported in a patient sensitive to thiourea and IPPD [7].
Allergy to socks and stockings, agents such as perfumed sprays, talcs and antifungal powders used in shoes, and medicaments applied to the skin may simulate footwear dermatitis [8]. Shoe fabrics may become reservoirs for materials applied to the skin of the feet and act as a source for continued exposure even though the items are no longer being actively applied.
Investigations. Many of the commoner shoe allergens are found in the standard series, including dichromate, certain rubber accelerators and antioxidants, PTBPFR, colophony and nickel. In addition, a special shoe series should be used, and commercial allergen suppliers have such a series of allergens. Non-standard allergens of particular importance include nylon dyes, dodecyl mercaptan, 2-thiocyanomethyl benzothiazole (TCMTB), thioureas, dithiomorpholine, isocyanates, dimethylaminoethyl ether, 2-n-octyl-4-isothiazolin-3-one and p-tert-butylcatechol (PTBC) [9]. More extensive series have been advocated as a screen, but even these may miss some shoe allergens, including biocides, vegetable tans, hydroquinone and polyurethane agents. Occasional cases of allegy to dimethyl fumarate during the epidemic from 2008 to 2009 were associated with footwear due to its use as an anitfungal in the manufacture of leather sofas and shoes [10].
The investigation of possible shoe allergy can be frustrating, as even with special screening series 10–20% of shoe allergies have only been identified by testing with pieces of the shoe itself. Ideally, pieces for patch testing should be taken from the parts of the shoe in contact with the dermatitic area. They should be thin and 1 cm2 or larger. Some have suggested testing the pieces under a special large Finn chamber, whereas others recommend occlusive tape. Further suggestions have been to soak the pieces in water for 15 min before they are applied, and to leave the test pieces in place for 4–5 days [11]. False positive reactions may be seen from pressure, particularly around the edge, and false negative reactions are common. Furthermore, positive reactions may develop as a result of contamination by a non-shoe allergen, for example a medicament or perfume to which the patient is allergic.
Management. A typical shoe will be formed from an upper, a sole, an insole, and heel and toe counters to stiffen the shoe and give it shape. Adhesives may be required throughout the shoe.
Uppers tend to be made of leather, rubber or synthetic material. Leather is tanned, usually with chromate, but other tanning agents, including vegetable tans, colophony, formaldehyde and glutaraldehyde, may be used. Formaldehyde is associated with the tanning of white or water-resistant leather and is tightly bound, making sensitization less likely. After tanning, the leather may be oiled, dyed and finished. Biocides such as 2-n-octyl-4-isothiazolin-3-one may be added to the oils and finishes. Uppers may be made from, or be lined with, dyed fabric. Polyurethane, rubber and neoprene foams are used in the uppers, particularly of sports footwear. Neoprene is a synthetic rubber to which phenolic resins, most notably PTBPFR, thioureas, carbamates and other accelerators and additives, may be added.
Shoe soles are made from similar materials and more solid forms of rubber. Insoles can also be made of a similar range of materials. Fibreboard used for insoles is a composite of fibres, usually paper but occasionally wood or leather in a glue matrix, which may contain biocides. Insoles may also contain allergenic deodorizers, including formaldehyde and fragrances.
Counters may be made from many different potentially allergenic materials, including natural rubber, formaldehyde resins, biocides and pine oil.
The main adhesives are hot melt, urethane, neoprene and natural rubber. Hot melt adhesives do not tend to cause allergy but the others may, particularly rubber accelerators and PTBPFR. Additives include isocyanates, epoxy resins and biocides. Tackifiers may contain formaldehyde resins and colophony.
Individuals who are allergic to leather tanning agents and additives can be advised to wear synthetic fabric or rubber footwear. Some specialized outlets sell ‘vegetarian’ shoes that should not be leather.
However, with other allergens avoidance is often difficult. Manufacturers and distributors will not generally guarantee their shoes are free of rubber chemicals and PTBPFR in adhesives, and may know even less about other sensitizers. Patients allergic to rubber, PTBPFR and colophony should consider all-leather stitched footwear with no insoles, or injection-moulded plastic shoes, moccasins or wooden shoes. Certain manufacturers will produce bespoke shoes free of the allergen(s) but these are expensive. Sometimes, orthotists advising hospital orthopaedic departments are helpful in making special shoes.
Those allergic to dyes will need to avoid dyed fabric and nylon-lined footwear, as well as coloured nylon socks and stockings. Old socks may act as a reservoir of allergen and should be discarded with the incriminated shoes, medicaments, etc.
Hyperhidrosis is common in shoe dermatitis, and the dermatitis may be helped by treating the sweating with iontophoresis, or by other means, and by wearing cotton socks to absorb the sweat.
Follow-up (average 3 years) of 48 patients after they had employed a number of strategies to avoid contact with the allergens responsible for their dermatitis revealed that 87.5% were clear or significantly better, 10% the same and only one person was worse [12].
Resins and plastics
Resins are intermediate synthetic substances that are polymerized, often using a hardener and other additives to produce a plastic end-product. Many low-molecular-weight materials, including the monomers and oligomers used in resins, are allergenic, but a fully polymerized resin should not be sensitizing. Many additives, fillers and hardeners also have allergenic potential. The systems most commonly associated with contact allergy include epoxy, acrylic and formaldehyde resins.
Epoxy resins
Epidemiology. In terms of usage, 75–90% of epoxy resins comprise diglycidyl ether of bisphenol A (DGEBA). They are reaction products of epichlorhydrin and bisphenol A. The monomer (molecular weight 340) is the main sensitizer, and oligomers with molecular weights above 900 do not sensitize [1]. Epoxy resin of the bisphenol A type is a standard allergen. However, figures for the prevalence of allergy in patch-tested patients with dermatitis will reflect the degree of occupational interest of a particular clinic, and also the local industry. Series of relatively unselected patch-tested patients have reported rates of 0.4–3% positive reactions, with a male preponderance. However, higher rates are recorded for occupational referrals. Annually, approximately 1% of exposed workers are believed to develop an epoxy resin allergy [2].
Allergy to other components of epoxy systems is commonly concomitant with resin sensitization but may also occur by itself [3, 4]. Detailed analysis of 182 cases in Finland showed that 80% were allergic to DGEBA epoxy resins, 23% to polyamine hardeners, 16% to reactive diluents and 9% to non-DGEBA epoxy resins.
A high incidence of allergy among exposed individuals can occur in factory outbreaks, for example 56% in an aircraft construction factory, 45% in marble workers, 27% in ski factory workers and 21% in paint factory workers [5, 6, 7]. The epoxy resins are among the most sensitizing substances that have been introduced to industrial work in recent years. Coatings, including paints, varnishes and metals, account for roughly 45% of all epoxy resin use. They are widely used in the construction industry, in cement to make it waterproof, and in floorings, grouts and filling materials, including those for marble and window frames. They are commonly used as binders and coatings for fibreglass and carbon fibre, for example in car body repairs, wind turbine rotor blades and aircraft construction. In the electronics industry they are used for insulation and in printed-circuit boards. They are efficient glues for metals, rubber, polyester resins and ceramics. Cardiac pacemakers and hypodermic needles may contain them. Dental personnel and their patients sensitized by epoxy acrylates in filling materials often also react to epoxy resin. In the laboratory they have been found as sensitizers in microscopy immersion oil.
High-molecular-weight resins, which may contain residual low-molecular-weight resin, are used for coating metal or wood. Occasionally, uncured epoxy resins are used as stabilizers and plasticizers in, for example, polyvinyl chloride plastic and spectacle frames. Thus, contact dermatitis may be elicited in consumers as well as occupationally.
Non-DGEBA epoxy resins have found increasing use in electron microscopy, electronics and carbon and glass fibre composite materials, especially in the aerospace construction industry. Cycloaliphatic epoxy resin in hydraulic fluid and neat metal-working oil has also sensitized. Triglycidyl isocyanurate is used, mainly as a hardener, in thermosetting one-component polyester powder paints.
Clinical features. Dermatitis is predominantly occupational. It usually affects the hands and arms (see Figure 128.8), and often also the face and eyelids. Facial and periorbital involvement may be indicators of associated or isolated allergy to the more volatile epoxy diluents and hardeners [8]. Partially cured epoxy resin dusts from sanding and drilling may induce dermatitis with a similar distribution.
Severe oedematous and weeping eruptions are not uncommon, and widespread generalized eruptions can develop if exposure continues. Erythema multiforme-like reactions have been described [9].
Localized dermatitis can sometimes be attributed to traces of free epoxy monomer found in a wide range of products, such as twist-off caps, coated door knobs, tool handles, microscopy immersion oil, stoma bags, clothing labels, portable infusion pumps, spectacle frames, plastic tubing in medical devices and gloves. A flare-up of hand eczema has been reported from an implanted epoxy resin-containing needle [10]. Other body sites, especially the genitals, may be affected following hand contact. Gingivitis and stomatitis may result from the use of epoxy acrylates in dental materials [11].
Contact allergy to epoxy components may rarely cause vitiligo [12].
Investigations. Epoxy resin of the bisphenol A type is included in the standard series at 1% in petrolatum; other components of epoxy resin systems are not, apart from ethylenediamine. Extra patch test reagents, which incorporate the commoner amine hardeners and reactive diluents, are available from the commercial allergen suppliers, although these are not all-inclusive. Allergy to non-DGEBA epoxy resins and other components may still be missed unless the worker's own materials are tested.
Epoxy resins may be reacted with other resins, for example acrylates and formaldehyde resins, to produce new resins that may have an allergic profile different from their parent resins [13].
Higher-molecular-weight resins may contain small amounts of the oligomers or monomer but rarely sufficient for the induction of sensitivity. However, they can elicit clinical and patch test reactions in those already sensitized.
Sometimes, ‘reactive diluents’ in the resins used to reduce viscosity are responsible for their sensitizing capacity. These diluents are usually glycidyl ethers or, occasionally, glycidyl esters, and are thought to be present in over 50% of epoxy resin products. Bisphenol A and epichlorhydrin themselves are seldom responsible for allergy from epoxy resin.
The commonest sensitizers among the hardeners are amines, for example the aliphatic amines ethylenediamine, diethylenetetramine, triethylenetetramine, dipropylenetriamine and dimethylaminopropylamine. Triethylenetetramine is a particularly strong sensitizer [14]. There are also sensitizing cycloaliphatic amines (e.g. isophoronediamine) and aromatic amines, such as m-xylenediamine, diaminodiphenylmethane (methylene dianiline) and 2,4,6-tris-(dimethylaminomethyl)phenol.
Hardeners of the polyaminoamide type are much less likely to sensitize, and so are anhydrides (e.g. phthalic anhydride). They are used for thermal hardening.
A rough guide to patch test concentrations (all in petrolatum) is 0.5% for non-DGEBA epoxy resins, 0.25% for reactive diluents and 1% for most polyamine hardeners, but a literature search should also be undertaken, and lower concentrations considered initially if in doubt.
Additives include colours, fillers, UV light absorbers, flame retardants and plasticizers.
Management. Redeployment away from contact with epoxy resin is usually required for occupational dermatitis but even so the prognosis is not always favourable [15]. The use of epoxy (usually two-part) adhesives and fillers in domestic and spare-time activities (e.g. car body repairs) should be avoided. The identification of epoxy chemicals in suspect materials by chromatography techniques may be helpful in confirming a suspected source of epoxy resin [16].
The prevention of epoxy dermatitis is important, and includes education, instructions and warning notices for the workforce, stressing the need for ‘good housekeeping’. High-molecular-weight epoxy resins and diluents are less sensitizing and, where possible and appropriate, are to be preferred. Aliphatic and aromatic amines may be replaced by polyaminoamides or amine-epoxy adducts. If feasible, automation or a ‘two in one’ mixing package is advised or, if not, mixing should be done in disposable containers. Protective impermeable, preferably disposable, clothing and gloves should be worn. Epoxy resin will nevertheless penetrate plastic and rubber gloves [17]. Heavy-duty vinyl gloves or multilayered gloves of folio type (4H-Glove; Safety 4, Denmark) provide the best protection [18].
Acrylate resins
Epidemiology. Acrylate allergy is not routinely sought, and levels of allergy will reflect the referral pattern to a particular clinic. A UK study examined the records of approximately 14 000 unselected patients, and identified 440 who had been tested for possible (meth)acrylate allergy: 67 had one or more positive reactions; 47 cases were occupational, with dental personnel and printers being the most frequent, followed by gas workers, gearbox fitters and beauty therapists. Sixteen cases were sensitized by wearing acrylic nail cosmetics. A similar survey in the USA on 56 allergic patients showed the commonest sources of allergy were nail cosmetics (25 cases), dental materials (14 cases) and adhesives (seven cases) [2]. Most other series have concentrated on occupational exposure.
Clinical features. The commonest sites of occupational allergy are the fingertips and hands (see Figure 128.2), but the face, arms and eyelids may also be involved. Manicurists may develop hand dermatitis, and sometimes a more extensive exposure pattern dermatitis from dust generated by nail filing. Rhinitis has been seen in association [3], and acrylates may also induce occupational asthma. Workers with fingertip dermatitis should always be asked about contact with screwlocks and glues, as this is a typical distribution of allergic dermatitis from this source. A similar distribution may be seen in dentists and dental technicians. Localized dermatitis is seen from limb prostheses, the use of diathermy plates during surgery and incontinence pads. There may also be secondary spread of the eruption.
Dermatitis from artificial nails may be associated with painful onycholysis, nail dystrophy, periungual dermatitis, paraesthesiae and an ectopic dermatitis of the face and neck, and sometimes other parts of the body. Paraesthesiae can persist for some months after patients stop wearing the nails [4].
Stomatitis has been blamed on incompletely cured acrylate in newly made or repaired dentures, and gingivo-stomatitis on acrylates in a temporary crown. Oral problems may arise in those previously sensitized to acrylic nails [5]. An extensive skin eruption has been seen from allergy to a dental composite resin used to fix an orthodontic prosthesis [6].
Investigations. Allergen suppliers produce three (meth)acrylate series aimed at the main sources of exposure: adhesive, nail and printing. In general, methacrylated monomers are tested at 2% in petrolatum and acrylated monomers at 0.1% in petrolatum. The lower 0.1% concentrations have reduced the incidence of the previously noted problem of active sensitization [7]. Multiple positives may be seen if the full series is tested as a consequence of complex cross-reaction patterns. As a result of retrospective analyses shorter screening series have been suggested [8]. Late and persistent patch test reactions may occur [9]. Contact leukoderma has also been described following testing [10].
Monomeric acrylates and methacrylates may be used to produce transparent plastics (e.g. Perspex), dentures, hearing aids, limb prostheses, spectacle frames, nail cosmetics and bone cement for orthopaedic surgery. Other commoner exposures include coatings, varnishes, paints, inks and adhesives (including those for stick-on nails). UV-cured monomers are also encountered in coatings, printing plates, printing inks and dentistry.
Multifunctional acrylates, which have at least two reactive acrylic groupings, are also used in UV-cured resins, as well as printed circuit boards, artificial nails, adhesives, dental materials and anaerobic sealants used in screwlocks and gas pipes. Epoxy acrylates [11] are used in dental restorative materials, UV curable printing inks and anaerobic glues. Acrylamide and derivatives have sensitized from printing plates and paint manufacture.
Cyanoacrylates are known as ‘superglues’ and are used extensively to bond artificial nails, metal, glass, rubber, plastics and textiles. They are also used by surgeons to bind tissues and seal wounds, and by dermatologists to treat painful fissures of the hands and feet. Sensitization to cyanoacrylates is rare, as a result of almost immediate polymerization, but it has been reported more recently from its use as false eyelash glue [12]. Cyanoacrylates are tested at 10% in petrolatum.
Sometimes, additives such as dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, p-methoxyphenol, pyrogallol, resorcinol or pentaerythritol tetrakis 3-mercaptopropionate may elicit contact dermatitis.
Management. Once identified, avoidance should be possible by removal of the cause, redeployment, adequate protection or altered work practice. Acrylates penetrate latex and vinyl gloves [13]. The 4H multilayer glove (Safety 4, Denmark) [14] is the best protection, but may be impractical for some activities. In those with finger problems (especially dentists), it may be possible to remove the 4H glove fingers and wear these under another more pliable glove, although ideally dental personnel should use a no-touch technique. Double gloving, polyethylene gloves and nitrile gloves are possible, but potentially less effective, alternatives.
Education, instructions on handling, printed warning notices and ‘good housekeeping’ are important preventative measures.
Formaldehyde resins
Epidemiology. Apart from allergy associated with clothing, shoes and nail varnish, formaldehyde resin allergy is uncommon. Many cases of phenol formaldehyde resin (PFR) allergy are sporadic. Laminate manufacturers in Sweden were found to have a high frequency of allergy [1]. In a recent review, 17 cases of PFR allergy were seen in one clinic over a 15-year period. Commoner occupational associations were friction material (e.g. brake linings) production, work with fibreglass and contact with foundry sand [2]. Of routinely patch-tested patients, 0.3–2.6% are allergic to PTBPFR [3]. In many instances it is difficult to find relevance for a positive patch test.
Clinical features. Dermatitis from formaldehyde resins in clothing, shoes and nail varnish is discussed in the relevant sections. In most other cases of PTBPFR allergy, dermatitis is localized under leather watchstraps and limb prostheses, although the hands may be affected by contact with glues in the working and domestic environments.
Most cases of PFR allergy have occupational dermatitis of the hands, with occasional manually transmitted spread to the face and genitals.
Usually, sensitization occurs when the resins are handled in ‘half-condensed’ form. Coexistent formaldehyde sensitivity is rare with PTBPFR and PFRs, but is quite common with urea and melamine formaldehyde resins [4].
Investigations. PTBPFR patch testing is discussed in the section on shoes, amine formaldehyde resin in the section on clothing, and tosylamide formaldehyde resin in the section on cosmetics. PFRs are variable in composition and allergenicity [5].
‘Phenoplastics’ are condensation products of formaldehyde and phenolic compounds, for example phenol, cresol, p-tertiary-butylphenol and resorcinol. There are two main types of PFR: resol (phenol reacted with excess formaldehyde in alkaline conditions) and novolac (formaldehyde reacted with excess phenol in acid conditions). Fourteen different sensitizers have been isolated from PFRs [6]. The two types of PFR do not necessarily cross-sensitize and neither of them seems to cross-sensitize to any significant degree with PTBPFR.
There are a number of commercially available allergens: PFR-2 1% in petrolatum and monomethylol phenol 1% in petrolatum from Chemotechnique, and a novolac and resol resin, each at 5% in petrolatum, from TROLAB. PFR-2 is the most successful in the identification of allergic subjects. However, testing with the patient's own resin at 1% and 5% in petrolatum, followed by testing controls if positive, is probably the most reliable method.
Management. Once identified, avoidance should be possible by removal of the cause, redeployment, adequate protection or altered work practice.
PFRs have electrical resistance and binding properties, resulting in their widespread use in electrical appliances, glues, laminated floorboards, plywood, fibreglass (including insulation), brake linings, clutch facings, grinding wheels, foundry sand moulds, abrasive cloths and papers, plastic moulds, telephones and steering wheels. Finished plastics are often brown or black and of the Bakelite type. Cashew nut shell oil has been used to modify the PFRs incorporated into brake linings, and this has sensitized [7].
PTBPFR is used as an adhesive and is found in sealants and neoprene glues. Contact may occur directly following its use as a glue, particularly in shoemakers and cobblers, or when it is used to attach artificial nails and electrocardiogram monitoring electrodes [8], and indirectly from its use in shoes, watch straps, wet suits and limb prostheses. Other sources include furniture and upholstery glue and marking pen ink.
Amino formaldehyde resins occur in textiles, plywood and waterproof paper. They are also used for finishing parquet floors, gluing wood and in orthopaedic casts. Tosylamide formaldehyde resin is extensively used in nail varnish. Formaldehyde, which is continuously liberated from formaldehyde resins in floors and walls, may elicit contact dermatitis in very sensitive people. ‘Amino-plastics’ are condensation products of formaldehyde or hexamethylenetetramine, and carbamide (= urea), thiourea, melamine, sulphonamide or anilide. They are often white or transparent. Formaldehyde, hexamethylenetetramine or low-molecular-weight condensation products can sensitize separately or simultaneously.
Other plastics
Other plastics are rarely the cause of allergic contact dermatitis outside industry. Other resin systems, most notably unsaturated polyesters and their hardeners, and isocyanates in polyurethanes may sensitize. Co-polymers are emerging as sensitizers in cosmetics, sunscreens and nail varnish [1]. Additives in cellulose acetate spectacle frames have caused dermatitis. The literature contains many case reports of allergens traced to specific products, for example spectacle frames [2], hearing aids, ballpoint pens and other plastic items. Other plastic additives such as plasticizers, antioxidants, UV light absorbers, initiators, cross-linking agents, flame retardants and pigments may sometimes sensitize during the manufacturing process or during use.
Plants [13]
Epidemiology. Plant life is exceedingly diverse, with much geographical and seasonal variation, and consequently the range of reported allergens is huge, with considerable differences worldwide in the incidence and prevalence of allergy. We concentrate only on those plant families frequently associated with contact allergy. Accurate statistics for the prevalence and incidence of plant allergy as a whole are not available. These parameters vary from country to country and depend on the local flora and the population's way of life. Numerous investigations have shown that 25–60% of North Americans are sensitive to poison ivy and other members of the Anacardiaceae family. Occupational dermatitis to plants is common in gardeners, florists and undertakers. English ivy (Hedera helix) is not related but it is becoming increasingly recognized as a cause of plant allergy [4].
It has been estimated that 5–10% of all cases of contact allergy seen in European dermatology clinics are caused by plants or their products. Primin, found in Primula obconica, is recommended as a standard-series allergen in Europe. Of 3075 patients patch tested in Denmark, positive reactions were recorded in 1.8%, about 95% of the positive reactors being female. In the UK, positive reactions were recorded in 1% of 3462 patients routinely tested. There is evidence of a statistically significant decreasing frequency of allergy in the UK and Europe [5] due to the development of allergen-free plants. In the UK, this allergen has now been removed from the standard series due to the low frequency of reactions and lack of relevance.
Sesquiterpene lactone mix in the standard series is used to identify Compositae (Asteraceae) allergy. A multicentre European study showed varying frequencies of allergy in patch-tested patients, ranging from 0.1% to 2.7% according to the centre, with clinical relevance found in approximately three-quarters of cases [6]. However, it has been suggested that the sesquiterpene lactone mix might only detect about one-third of those allergic to Compositae [7] and a Compositae mix has been developed to test in conjunction.
Garlic allergy is frequent in Spain, with 2% of patients attending for investigation of dermatitis being sensitized, mainly housewives [8]. Other countries where garlic is used frequently in cooking might have a similar prevalence. In the UK, curry chefs are an at-risk group.
Tea tree oil (Melaleuca alternifolia) is being increasingly used in cosmetics and medicaments and has caused allergic reactions from these sources [9].
Clinical features
- Anacardiaceae. Toxicodendron spp. dermatitis occurs after contact with the sap of the plant. Classically the rash is streaky, with erythema, papules and vesiculo-bullous lesions on exposed sites. The hardened sap may leave a black spot on the skin in the areas of dermatitis and this may be helpful diagnostically [10]. Distant spread is common, particularly facial and genital involvement from contaminated hands. More profound erythema multiforme-like, exanthematous and urticarial eruptions, and even renal damage, may occur from systemic absorption. Stomatitis and proctitis have occurred after chewing the leaves, and with hyposensitization. Contamination of clothing, animals, garden tools, firewood, fishing rods and golf clubs may also act as sources of contact. Phytophotodermatitis (see Chapter 127) and allergy to Primula and other plants has to be considered in the differential diagnosis. Plants from this family have caused more contact allergy than all other plants combined. Much of this sensitization relates to poison ivy, sumac and oak, which are species of Toxicodendron found extensively in North America. The plants are generally found outdoors and recognized by their three-leafed configuration. Their diverse morphology and various habitats have been described by Guin et al. [11].
- Compositae (Asteraceae). Eight patterns of dermatitis are described, which are generally worse during the summer months in temperate climates [12].
- Pseudophotodermatitis. Exposed sites are involved, including both eyelids, and photoprotected areas under the chin and behind the ears. In hot regions, during summer months, dry dead plant material contributes to the airborne pattern of dermatitis. In the USA many Compositae weeds, including ragweed (Ambrosia spp.), induce this pattern of dermatitis, almost exclusively in males. A similar pattern is seen in Europe from Compositae flowers and weeds, in India from Parthenium hysterophorus and in Australia where it is known as bush dermatitis. Chronic cases may produce a marked thickening of the facial skin – a leonine facies. Photosensitivity quite commonly coexists with Compositae allergy (Figure 128.25). In one UK study, 22% of the contact-allergic patients were also photosensitive. True photoallergy to Compositae is, however, generally not a feature.
- Atopic eczema-like. Compositae allergy may mimic late-onset atopic eczema, with a flexural accentuation of involvement, which may include the groins and genital area.
- Erythrodermatous exfoliative. This pattern is classically seen from the weed Parthenium hysterophorus, which was transported to India from the USA in contaminated seed wheat. Unfortunately, the weed has spread over much of the subcontinent, including urban areas. It has become markedly allergenic in these environmental conditions, which also enhance the spread of dry plant dust and pollen. There is increased opportunity for skin exposure because many of the indigenous male population wear relatively scanty clothing. Severe incapacity and even fatalities have resulted from Parthenium dermatitis.
- Hand eczema. This pattern is seen particularly in gardeners after contact with weeds. A palmar distribution often predominates. Dermatitis of the hands is also associated with handling lettuce.
- Localized dermatitis. Dermatitis may be confined to one or more localized areas, although this pattern is unusual in our experience. Facial dermatitis has occurred from steaming chamomile tea, and hand and arm dermatitis from herbal compresses.
- Oral. Oral swelling and soreness after eating lettuce has been reported in sensitized persons [13].
- Erythema multiforme. This has been reported, when it recurred after patch testing [14].
-
Systemic. Oral swelling, perianal pruritus and dermatitis of the trunk and arms have been reported after a sensitized subject drank chamomile tea [15].
Compositae plants are also known as Asteraceae. There are over 25 000 species found throughout the world and more than 200 have been reported to cause allergic contact dermatitis. They may be decorative plants (e.g. chrysanthemums, dahlias, sunflowers), weeds (e.g. ragweed, dandelion, tansy, marsh elder, feverfew, chamomile, yarrow, arnica, Parthenium) or foods (e.g. lettuce, endive, artichoke). Herbal teas, medicines and cosmetics may all contain Compositae plants or extracts. As might be expected, there is considerable but variable cross-reactivity among Compositae plants. Cross-sensitivity with Frullania liverworts has been described and has also occurred with members of other plant families, most notably Lauraceae and Magnoliaceae. Furthermore, there is some evidence of cross-sensitivity to colophonium and a number of essential oils that may be found in fragrances [16].
- Primulaceae. The classic appearance of Primula allergy is linear papulovesicles, oedema and blisters, which may be haemorrhagic, on the palms, dorsa of the hands and forearms (see Figures 128.6 and 128.26). Transfer of the allergen via the fingers to the face, or more widely, is common. In some patients palpebral oedema is the presenting feature, but half of the cases have other patterns, and the diagnosis is easily missed unless the possibility of Primula dermatitis is kept in mind. Misdiagnoses include constitutional pompholyx, urticaria or recurrent angio-oedema, and disseminated herpes simplex. Erythema multiforme, a lichen planus-like eruption and toxic erythema as a result of Primula allergy can also cause diagnostic difficulty. Primula obconica is the most important allergenic plant, although other Primula species may also cause allergic contact dermatitis [17]. P. obconica is a decorative indoor plant. Contact occurs particularly when dead leaves and plant heads are removed manually. Primin levels are at their highest between April and August. The allergen content of the plant also varies with sun exposure, temperature and feeding. The pattern of dermatitis is determined by both the allergen content of the plant and the patient's degree of sensitivity and exposure. Primin-free strains have now been developed [18] and there is evidence of a decreased frequency of allergy [5].
- Alstroemeriaceae and Liliaceae. Dermatitis from tulip bulbs may cause a painful, dry, fissured and hyperkeratotic allergic dermatitis, at first underneath the free margins of the nails and then on the fingertips. Tulips are members of the Liliaceae family, and dermatitis is a particular risk for bulb collectors, sorters and packers, as well as florists. A similar pattern of dermatitis is seen in florists sensitized to Alstroemeria, and this may be followed by depigmentation. Alstroemeria (Peruvian lily) is a highly decorative plant commonly displayed as a spray with other flowers. The damaged plant's sap is allergenic to florists when the stems are wired and leaves stripped, in preparation for making the spray.
- Alliaceae. Garlic and onion are both members of this family and may sensitize, but do not seem commonly to cross-sensitize mutually. Classically there is fingertip involvement in those allergic to garlic (see Figure 128.7) and onion. This may preferentially affect the non-dominant hand, as this is the one that holds the vegetable while it is being cut with an implement held by the dominant hand. Cheilitis and photoallergic contact dermatitis have also been reported [19]. Systemic contact allergy, including pompholyx, caused by ingestion of garlic has been described [20].
- Lichens and liverworts. A pattern similar to pseudophotodermatitis from Compositae has been seen in woodcutters’ dermatitis caused by sensitivity to lichens and liverworts. Erythroderma may ensue in severe cases. Even walking through a forest may cause an exposed-site pattern of dermatitis in sensitized individuals [21]. Lichens consist of a fungus and an alga. They are found on trees, rocks, roofs and walls. Forestry workers, gardeners, lichen pickers and woodcutters are particularly liable to come in contact with them. Liverworts (Frullania) are small red-brown plants often growing with lichens and mosses.
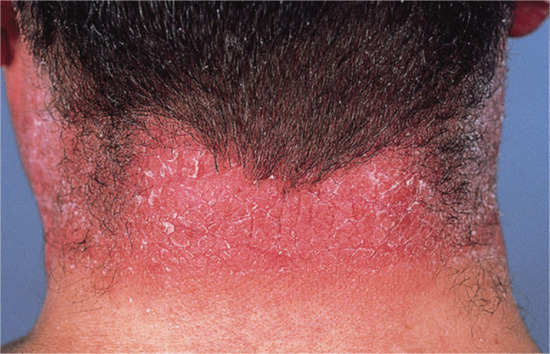
Figure 128.25 Photosensitive eczema in a patient also allergic to Compositae (sesquiterpene lactones). A similar pattern may be seen in woodcutters sensitive to lichens, and in others with photosensitive eczema, including photocontact allergy. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
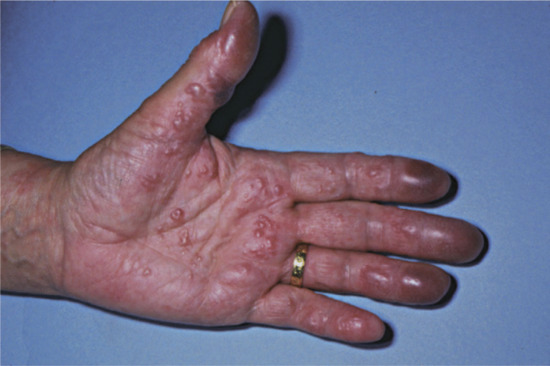
Figure 128.26 Haemorrhagic blisters on the palm from Primula allergy.
Investigations. There are two plant allergens recommended for the European baseline series: primin is tested at 0.01% in petrolatum; sesquiterpene lactone mix contains alantolactone 0.033%, dehydrocostus lactone 0.033% and costunolide 0.033% emulsified with sorbitan sesquioleate.
Primin is the major allergen in P. obconica, a quinone found in the tiny breakable hairs on the leaves, stem and flowers of the plant; however, it may occasionally fail to detect Primula allergy [22] as another potential allergen is miconidin [23]. Other Primula species may contain primin. The diminishing number of cases of allergy has brought into question the need for its inclusion in the standard series.
Sesquiterpene lactone mix does not identify all persons with Compositae allergy [24]. The allergens are sesquiterpene lactones, and more than 1350 have been described, including dehydrocostus lactone, alantolactone, costunolide and parthenolide. In lettuce and chicory, lactucin and lactucopicrin have also been identified as sensitizers [25]. Monoterpenes may also contribute to Compositae allergy [26].
An alternative screen consisting of a mix of arnica, yarrow, tansy, German chamomile and feverfew extracts has been developed [27]. In one study, it identified twice the number of sensitized persons detected by sesquiterpene lactone mix, and by testing with both allergens 76% of all allergic subjects were identified. However, this Compositae mix gave frequent false positive reactions and was felt to be sensitizing. Further preparations with 6% and 5% concentrations have been developed to avoid these problems [28]. Dandelion allergy, in particular, may be missed by the sesquiterpene lactone mix patch test, and supplementary testing with extract has increased the number of Compositae-allergic subjects identified [29]. Other commercially available plant allergens comprise a number of Compositae extracts, including dandelion [30].
Diallyl disulphide, the main allergen in garlic, is tested at 1% in petrolatum, and α-methylene-γ-butyrolactone (tulipalin A), the allergen in tulips and Alstroemeria, is tested at 0.01% in petrolatum.
Oak moss (Evernia prunastri) and other tree mosses are perfume ingredients derived from lichens. Chloroatranol and atranol are the main allergens [31]. They are common components of perfumed materials. Other allergenic components include atranornin, usnic acid and evernic acid. Lichen acid mix consists of atranornin, usnic acid and evernic acid, each at 0.1% in petrolatum. The allergens in liverworts, in common with Compositae with which they commonly cross-react, are sesquiterpene lactones. Oxidized tea tree oil is available at 5% but recent work suggests 10% may be the concentration of choice [9].
Plant extracts, preferably of known concentration, can be used for patch testing. Dipping the plant in diethyl ether for 60–90 s, evaporating to dryness and resuspending in petrolatum (1–10%) is a suggested simple method, although there are many alternative approaches [32].
Patch testing for Toxicodendron, if considered necessary, can be undertaken by diluting the oleoresin 1 : 10 in acetone. The main allergens found in the oleoresin (or urushiol) are derivatives of catechol, particularly pentadecylcatechols, phenol, resorcinol and salicylic acid. Cross-reactions occur with cashew nut oil, which may be used industrially in resins, mucilages, printers’ inks and electrical insulation. Haitian voodoo dolls and swizzle sticks made from cashew nut shells have also sensitized. Further cross-sensitivity is found with mangoes, gingko tree fruit, indelible laundry marking ink from the marking nut tree in India, furniture lacquer from the Japanese lacquer tree, Lithraea trees in South America, and plants and trees from the genus Grevillea found in Australia. Although poison ivy is not a native European plant it may be brought back from North America and planted. Isolated cases of contact sensitization have been seen from this source. Localized outbreaks of dermatitis from contact with the Japanese lacquer tree have occurred in the UK.
Patch testing with the plants themselves may be undertaken but carries the risk of false positive irritant reactions and active sensitization. If multiple tests with plants and plant allergens are carried out, there is a possibility that many strong positives may occur, leading to an ‘angry back’ and inducing false positive reactions to other allergens. Ideally, before patch testing with a plant, it should be identified and if it is a known irritant then testing may not be advisable. A textbook on plant dermatitis is a useful reference source. Several parts of one plant may contain the same allergen, and if this is the case 1 cm2 of leaf bruised gently with an orange stick is sufficient for patch testing. Sometimes, however, the allergen is concentrated in one organ of the plant (orange peel, cinnamon bark) or the concentration of the allergen varies from one part to another. When testing with unknown plants, several parts should be tested. Half of the material should be kept in a refrigerator for later botanical identification. Any plant that has given positive allergic reactions should be properly identified by its Linnaean name.
In order to prevent registration of irritant tests, it is important to employ controls when testing with the plants and their extracts.
Management. Patients who know of their sensitivity may manage to avoid further contact if taught to recognize the plants to which they are allergic. This is fairly straightforward for Primula obconica, Alstroemeria, tulips, Alliaceae, lichens and liverworts. Tulipalin A, in tulips and Alstroemeria, penetrates vinyl gloves. Nitrile gloves are more satisfactory for handling bulbs and the plants [33]. Diallyl disulphide penetrates most glove materials [34].
The recognition of Toxicodendron spp. is particularly important. Although the classic three-lobed leaves are a helpful feature, clusters of five or more leaves can occur. As there is considerable regional variability in the morphology of these species, it is preferable that sensitized persons become familiar with the appearance of Toxicodendron spp. in their own region. Toxicodendron oleoresin may remain under the fingernails and on the clothes, resulting in continuing problems. Detergents, soap and water will inactivate the residual unreacted allergen. After exposure, thorough washing of the hands, fingers and the rest of the body should be carried out as soon as possible, ideally within 10 min. Clothes should be changed. Contaminated tools and clothing, including shoes, should be washed in detergent. Specific creams, containing quaternium-18 bentonite and other barriers, have been developed and these may help prophylactically to a varying but incomplete extent [35, 36]. Heavy-duty vinyl gloves afford better protection than rubber gloves.
Seasonal Compositae exposure may be difficult to avoid. Severe Compositae allergy may necessitate changing occupation (e.g. florists, gardeners) or avoiding pastimes such as flower arranging and gardening. It may be necessary to avoid handling lettuce, chicory, artichokes and endives in food preparation. Those with associated photosensitivity may have significant problems from this, especially over the summer months. A high-protection broad-spectrum sunscreen is required for this subgroup. Where contact with Toxicodendron spp. and certain Compositae such as ragweed is unavoidable (e.g. outdoor workers), hyposensitization has been attempted, with limited success. There is a risk of unpleasant side effects, including extensive skin eruptions and perianal dermatitis [37]. This treatment does not have the approval of the Food and Drug Administration in the USA.
Those sensitized to lichens may also be allergic to certain perfumes, particularly those containing oak moss (Evernia prunastri). Perfume avoidance advice may also have to be followed.
Woods, colophony, turpentine and propolis [1, 2, 3]
Epidemiology. The incidence and prevalence of occupational wood allergies are unknown. Colophony is a standard allergen, with a 2–6% prevalence of allergy in patch-tested populations. Turpentine was removed from the ICDRG's recommended standard series because of infrequent allergy. It has been excluded from many industrial products, especially solvents, and replaced by petroleum products such as white spirit, although a high prevalence of sensitization continues to occur in Spain and Portugal [4]. An unexplained rise in frequency of allergy (from 1.7% to 3.1%) was reported in patch-tested patients from Germany in the late 1990s [5]. The frequency of allergy to propolis in patch-tested patients in various centres has ranged from 1.2% to 6.6% [6].
Clinical features. Most cases of wood allergy present in the occupational setting and are related to contact with airborne sawdust [7]. The pattern of dermatitis therefore affects the exposed sites, with the scalp of bald men being typically involved. Differentiation from a photosensitive eczema may be difficult, but light-protected sites (e.g. under the chin, behind the ears) are more likely to be equally affected in wood dermatitis. However, sawdust can gain access inside clothing to produce dermatitis predominating in the flexures. Genital involvement is a particular feature, in part from transfer of the allergen during urination. Severe erythema multiforme-like eruptions have been described, particularly from Machaerium scleroxylon allergy [8]. Localized dermatitis may occur under exotic hardwoods, for example from a violin chin-rest or wooden adornments and utensils.
Colophony allergy may present in many different ways because colophony is ubiquitous. Over 300 potential allergenic sources have been identified. An exposed-site pattern may be seen after machining pine and cutting down branches when gardening. Sensitivity to Cupressocyparis leylandii trees has been associated with concomitant colophony allergy. Allergy to colophony in solder fumes can give a similar distribution, but dermatitis may be confined to the face. Unsuspected sources for an exposed-site pattern have included linoleum flooring, paper dust and floor polish [9].
Facial and eye dermatitis can develop from contact with colophony-containing cosmetics, particularly mascara [10]. Reactions to sticky tapes and plasters, and colophony-containing medicaments, are often confined to the site of application, but secondary spread may be a feature from allergy to both colophony and colophony derivatives (e.g. ester gum resin) used as adhesives for lower leg dressings. This may be confused with varicose eczema and secondary medicament sensitization [11]. Adhesive plasters are often used to cover painful fissures on the hands and feet. These may have been caused by a pre-existing eczema or psoriasis, which may consequently be perpetuated or exacerbated by colophony allergy. Adhesive depilatory strips may contain colophony or derivatives and cause localized dermatitis [12], as may topical colophony-containing medicaments, including wart treatments.
Colophony may induce hand dermatitis due to contact with a diverse range of colophony-containing materials such as glues, polishes, paper, rosin, antislip powders, topical medicaments, waxes and tall oils in metal-machining coolants.
Perioral dermatitis and cheilitis have been related to colophony in chewing gum. Dental materials, including floss, fluoride varnish, dressings and impression materials, may contain colophony, but rarely sensitize in the mouth. A case of widespread dermatitis has been recorded after dental treatment in an allergic individual.
Colophony can also be present in adhesives in footwear. It has also been incorporated, in a modified form, in footwear in an impregnated cloth.
Turpentine allergy is usually associated with hand dermatitis or a localized pattern of dermatitis.
Propolis is a resinous material collected by bees and used as glue in beehives. It is derived mainly from poplar. Sensitized beekeepers may develop problems on the face and around the eyes. Allergy to propolis in cosmetics and medicaments is manifest at their sites of application. Allergy to chewed propolis in gum may induce a perioral distribution of dermatitis. Fixed drug eruption from ingested allergen has also been reported [13].
Investigations. Woods are normally of two types, hard and soft. The same woods may have many different names, and sometimes an incorrect name is mistakenly or deliberately applied. The situation is complicated further by the occasional introduction of ‘rogue’ timbers into batches of hardwoods. The commonest allergenic woods are listed in Table 128.6.
Table 128.6 Principal timbers causing dermatitis.a
| Botanical name | Common nameb | Origin | Uses |
| Apocynaceae | |||
| Dyera costulata | Jelutong | South-East Asia | Model making |
| Woodwork teaching | |||
| Boraginaceae | |||
| Cordia goeldiana Huber | Freijo | Brazil | Boat building |
| Frei jorge | Furniture | ||
| Interior construction | |||
| Joinery | |||
| Cordia gerascanthus R. Br. | Canalete | Venezuela | Furniture |
| Interior construction | |||
| Joinery | |||
| Cordia millenii Baker | Cordia | West Africa | Furniture |
| Interior construction | |||
| Joinery | |||
| Cordia platythyrsa Baker | Cordia | West Africa | Furniture |
| Interior construction | |||
| Joinery | |||
| Cupressaceae | |||
| Calocedrus decurrens (Torrey) Florin | Incense cedar | USA | Pencils |
| Fence posts | |||
| Furniture | |||
| Interior construction | |||
| Cupressocyparis leylandii | Leyland cypress | Temperate | Garden shrub |
| Hedges | |||
| Thuja plicata Donn ex D. Don | Western red cedar | USA | Construction |
| Arbor vitae | Boat building | ||
| Ebenaceae | |||
| Diospyros celebica Bakh. | Macassar | Indonesia | Cabinet and inlay work |
| Musical instruments | |||
| Rulers | |||
| Diospyros crassifolia Hiern | African ebony | Africa | Cabinet and inlay work |
| Musical instruments | |||
| Diospyros ebenum Koenig | Ceylon ebony | Sri Lanka, India, Indonesia | Cabinet and inlay work |
| East Indian ebony | Musical instruments | ||
| Diospyros melanoxylon Roxb. | Coromandel | Sri Lanka, India, Indonesia | Cabinet and inlay work |
| Musical instruments | |||
| Leguminosae | |||
| Caesalpiniaceae | |||
| Distemonanthus benthamianus Baillon | Ayan | West Africa | Coffins |
| Movingui | Furniture | ||
| Nigerian satinwood | Floors | ||
| Window frames | |||
| Mimosaceae | |||
| Acacia melanoxylon R. Br. | Australian blackwood | Western Australia | Boat building |
| Construction | |||
| Furniture | |||
| Musical instruments | |||
| Papilionaceae | |||
| Bowdichia nitida Spruce ex Benth. | Sucupira | Brazil | Construction |
| Floors | |||
| Furniture | |||
| Brya ebenus | Cocus | West Indies | Musical instruments |
| Jamaica ebony | Handles | ||
| Plates | |||
| Dalbergia Iatifolia Roxb. | East Indian rosewood | India, Indonesia | Veneers |
| Bombay blackwood | Furniture | ||
| Sissoo | Musical instruments | ||
| Handles | |||
| Wooden jewellery | |||
| Dalbergia melanoxylon Guillemin & Perrottet | African blackwood | Africa | Musical instruments |
| Grenadil | Handles | ||
| Dalbergia nigra All. | Brazilian rosewood | Brazil | Veneers |
| Rio-Palisander | Furniture | ||
| Grenadilla | Musical instruments | ||
| Jacaranda | Handles | ||
| Wooden jewellery | |||
| Dalbergia retusa Hemsley | Cocobolo | Central America | Handles |
| Scientific instruments | |||
| Wooden jewellery | |||
| Machaerium scleroxylon Tul. | Pao ferro | Brazil | Veneers |
| Santos palisander | Furniture | ||
| Caviuna vermelha | Handles | ||
| Pterocarpus soyauxii Taub. | Red African padauk | West Africa | Veneers |
| Handles | |||
| Musical Instruments | |||
| Furniture | |||
| Malvaceae (L.) Sol. | |||
| Thespesia populnea (L.) Sol. | Milowood | USA | Carved utensils |
| Bracelets | |||
| Furniture | |||
| Meliaceae | |||
| Khaya anthotheca C. DC | African mahogany | West Africa | Furniture |
| Krala | |||
| Khaya grandiflora DC | Big leaf mahogany | West Africa | Furniture |
| Khaya ivorensis A. Chev. | Khaya mahogany | West Africa | Furniture |
| Khaya senegalensis (Desr.) A. Juss. | Dry zone mahogany | West Africa | Furniture |
| Moraceae | |||
| Chlorophora excelsa Benth. & Hook. | Iroko | West Africa | Construction |
| Kambala | Shipbuilding | ||
| African teak | Laboratory benches | ||
| Pinaceae | |||
| Pinus spp. | Pine | Northern temperate areas | Construction |
| Furniture | |||
| General | |||
| Picea spp. | Spruce | Northern temperate areas | Construction |
| Fir | Furniture | ||
| General | |||
| Proteaceae | |||
| Grevillea robusta Cunn. ex R. Br. | Australian silky oak | Australia (planted elsewhere) | Floors |
| Furniture | |||
| Plywood | |||
| Telegraph poles | |||
| Sterculiaceae | |||
| Mansonia altissima A. Chev. | Mansonia | West Africa | Furniture |
| African black walnut | Walnut substitute | ||
| Bété | |||
| Verbenaceae | |||
| Tectona grandis L. | Teak | India, South-East Asia | Furniture |
| Floors | |||
| Construction | |||
| Shipbuilding |
Adapted from Hausen [4].
aLichens on the wood may also sensitize.
bThere is no accepted international nomenclature.
Patch testing with freshly made, uncontaminated sawdust 10% in petrolatum is recommended but may carry the risk of false positive and false negative patch tests, and active sensitization. Apparent allergic positive reactions should only be confirmed after testing on controls. It is advisable to ask the patient to bring a piece of unmachined wood at the same time as the sawdust. If a positive allergic reaction develops, the piece can be sent to a wood anatomist who will confirm the correct name for the wood. If the allergen for that wood is known, it is sometimes possible to patch test with it at the appropriate concentration. However, this may be difficult as most wood allergens are not commercially available.
Pine trees are the source of two significant allergenic materials, colophony and turpentine. Turpentine is the balsam from species of Pinus. Oil of turpentine is the volatile oil distilled from this balsam. The term ‘turpentine’ is commonly used to designate oil of turpentine. Colophony is the non-volatile part of the balsam and is known as gum rosin. Swedish and Finnish turpentine is made in the processing of paper pulp from wood. Venice turpentine is the balsam from larch trees.
Colophony is also extracted as a distillate from pine tree stumps, when it is known as wood rosin, and as a by-product of pulping pine wood, when it is called tall oil rosin. The chemical composition varies according to geographical source, production method and storage conditions. It is composed of approximately 90% resin acids and 10% neutral substances. Auto-oxidation products of abietic and dehydroabietic acids, including peroxides, hydroperoxides, epoxides and ketones, have been proposed as allergens. The most potent allergen has been shown to be 15-hydroperoxyabietic acid. Colophony may be modified, thereby altering its allergenicity with the development of new allergens [14, 15]. Maleopimaric acid and glyceryl monoabietate have been identified as allergens in modified rosins.
Colophony is a standard allergen tested at 20% in petrolatum. A mixture of Chinese and Portuguese gum rosin is presently used in commercially available patch test allergens. The allergen profile may differ according to the source of the colophony; particularly if it has been modified, and consequently false negative reactions can occur when patch testing with the unmodified standard-series allergen. Where modified colophony allergy is suspected a wider series of patch tests should be considered, for example ester gum resin, Granuflex®, as well as the suspected product [16].
The major sensitizer in turpentine is the hydroperoxide of Δ3-carene, which is also an auto-oxidation product. Swedish and Finnish turpentine contains more of this substance than, for example, French and American turpentine. Turpentine oil is tested at 10% in petrolatum. Oxidized limonene (d or l) and pinene (α or β) can also sensitize [17]. The term ‘mineral turpentine’ is used for the non-sensitizing, but irritant, white spirit that is a petroleum product.
Propolis is patch tested at 10% in petrolatum. It may be found also in beeswax [18]. The allergens include caffeates and benzyl isoferulate [19].
Lichens, liverworts and sensitizing plants may cause allergic sensitization by virtue of their coexistence with trees. Additives to wood such as varnishes, dyes, glues or preservatives may also sensitize at work.
Management. A demonstration of allergy to a wood should be followed by anatomical confirmation of its botanical name and, ideally, by testing with the known allergen(s) for that wood. Subsequent avoidance of the wood and related timbers may be necessary. Occupational allergic contact dermatitis is more frequently associated with hardwoods, especially among cabinet makers, carpenters, instrument makers, and so on. Some tropical hardwoods are especially allergenic. In many instances the precise allergens are not known, but some have been identified. Chemically, these include quinones (including dalbergiones and lapachol), phenols, terpenes, stilbenes and anthothecol. Softwoods, apart from pines and other conifers, are not commonly associated with contact allergy. Jelutong (Dyera costulata) is a South-East Asian tree whose timber has sensitized woodwork teachers [8].
Detection of the origin of colophony allergy requires careful appraisal of potential sources. The commoner sources of colophony and its modifications are identified in Box 128.5. The use of traditional sticking plasters should be replaced by ‘hypoallergenic’ tapes. Insulating tapes may also contain colophony, as may certain adhesive leg ulcer dressings. Contact with pine and other coniferous trees, and probably Cupressocyparis leylandii, should be avoided; in those with extreme sensitivity, felling and removing the offending trees may be necessary. Occupationally, it may be possible to change the allergenic product to an alternative.
Colophony and derivatives can be identified in fully ingredient-labelled cosmetics. The INCI term colophonium is used. Derivatives that may be used in cosmetics include abietic acid, hydroabietic acid and hydroabietyl alcohol. Transparent colophony-containing soap should be avoided for washing. Wart paints incorporating collodion should also be avoided, along with colophony-containing topical medicaments and balms. Colophony allergy from paper has been implicated in hand dermatitis, and the use of cotton gloves is suggested if this is a possibility [21]. However, the list of potential exposures is so extensive that it will often be a case of establishing whether any of the sources identified in Box 128.5 are relevant and tailoring avoidance advice accordingly.
Turpentine substitutes are now readily available for sensitized subjects. Turpentine is present in balsams and sawdust from pine and spruce. It was used as an industrial solvent but has now largely been replaced by petroleum derivatives and d-limonene. It is still used by artists and in ceramic decoration. In certain producing countries such as Spain and Portugal, turpentine is still more widely used than elsewhere, and it remains a common allergen there. In the USA it is still commonly used as a paint remover.
Propolis is encountered not only by beekeepers but also in both systemic and topically applied agents used in ‘natural’ products from health food stores and mainstream cosmetic outlets. Solid propolis can be chewed. Increasing self-medication may mean increased contact allergy from this source. It may also be found in beeswax used in cosmetics and topical medicaments.
Clinical variants
Systemically reactivated contact dermatitis
Systemically reactivated allergic contact dermatitis, where ingestion or other systemic exposure to a contact allergen takes place in an already sensitized person, may result in a number of different patterns of skin eruption. The threshold of reaction varies in each individual case and depends on the dose given and the level of sensitivity. Reactions may occur not only after systemic exposure to the primary allergen but also to closely related allergens.
The most frequent types of reaction are focal flares of previous patch tests and sites of previous dermatitis, vesicular hand eczema, or more widespread eczema and erythema, sometimes with additional urticarial features. In severe cases vasculitis [1], erythema multiforme [2] and systemic upset may occur. Involvement of the eyelids, body folds and buttocks induced by oral challenge with nickel in allergic subjects led to this particular reaction being labelled the ‘baboon syndrome’ [3]. This is often also the pattern seen in patients with a mercury exanthem [4]. In some patients following widespread reactions, and in others following attempts at ‘desensitization’, the level of patch test reactivity appears to be reduced [5].
Probably all contact allergens can cause systemic reactions, provided the patient has a sufficient degree of pre-existing sensitivity and the dose administered is sufficiently large. The causes are many, and include medicaments that may have been given not only by mouth but also parenterally, rectally, intravesically or as an inhalant. Dietary causes include metals, plants and spices.
Systemic contact dermatitis from medicaments [6] has decreased as a result of reduced use of topical sensitizers such as the antibiotics streptomycin, sulphonamides and penicillin, and topical antihistamines such as promethazine. Nevertheless, exposure to other topical and systemic medicament sensitizers continues to give problems. Standard allergens responsible for these reactions include neomycin, quinolines, local anaesthetics, ethylenediamine and corticosteroids. In subjects with contact allergy to ethylenediamine, parenteral and oral administration of aminophylline (which contains ethylenediamine) has resulted in widespread eczematous eruptions. Ethylenediamine is structurally related to some antihistamines (e.g. hydroxyzine, cetirizine, levocetirizine) and may therefore also trigger a systemic flare [7]. A positive patch test to tixocortol pivalate is an indication of hydrocortisone allergy, and the systemic administration of hydrocortisone has induced the recurrence and extension of dermatitis [8]. Furthermore, the administration of parenteral adrenocorticotrophic hormone, thereby raising endogenous hydrocortisone, has resulted in flares in hydrocortisone-allergic individuals. Other systemic steroids have also induced systemic contact dermatitis. Inhalation of budesonide has been associated with reactivation of positive patch tests, and continued exposure to budesonide from this source may therefore maintain dermatitis in sensitized subjects [9].
The persistence of dermatitis, especially vesicular hand eczema in metal-allergic subjects, has been blamed on dietary intake, particularly of nickel. Traces of metal dissolved by cooking acid or salty food in stainless steel may be of consequence in the persistence of dermatitis due to metals, such as chromium, nickel and cobalt [10]. In one study, the dietary restriction of nickel helped about one-quarter of selected nickel-sensitive patients with resistant dermatitis. However, the role of ingested or dietary nickel in hand dermatitis remains controversial, especially as a percentage of patch test-negative patients also appear to have flares of vesicular hand eczema following oral metal challenge, and the challenge dosage has been artificially high. A recent meta-analysis suggests that 1% of nickel-allergic subjects will develop systemic allergic contact dermatitis from normal daily exposure to nickel in drinking water and the diet. Systemic nickel dermatitis is reported also to have been induced by peripheral intravenous catheters and orthodontic appliances [11, 12].
Balsam of Peru, garlic, certain ingested food colours, preservatives and antioxidants have also been reported as causing flares of vesicular hand eczema [13, 14, 15].
Flares of dermatitis and perianal pruritus may occur in patients undergoing desensitization to Toxicodendron species, and systemic contact dermatitis may be induced following the ingestion of cashew nuts, whose shells contain an oleoresin closely related to that found in poison ivy [16]. Similar problems may also result after eating the fruit of the Ginkgo tree and using herbal medicines [17].
Cutaneous reactions to implanted metals
Orthopaedic metallic prosthetic implants [18] are made from a variety of metals, often alloys and especially stainless steel. Stainless steels contain up to 25% nickel, but for orthopaedic use generally contain 13–16% nickel and a minimum of 17% chromium. However, nickel release from stainless steels, apart from those containing sulphur, is very low [19]. Nickel, cobalt and chromate may also be used in wrought and cast alloys. Vitallium, a cast cobalt/chromium alloy, and titanium may also be used for implants.
Orthopaedic implants may be static (e.g. plate and screws) or dynamic (e.g. artificial hips). There are two potential concerns in relation to metal allergy and these implants: namely allergic skin disorders and loosening. There is little doubt that static implants can be associated with localized eczema over the site of implantation and more extensive skin eruptions in sensitized subjects, sometimes only resolving after removal [20]. The delay between the insertion of the prosthesis and the onset of dermatitis may be days or years. Similar eruptions were reported in the early days of hip replacements when metal heads articulated with metal cups. This problem seemed to have largely disappeared following the introduction of plastic joint surfaces, and prospective studies of hip joint replacements in known metal-allergic subjects are reassuring.
There is evidence of increased metal sensitization associated with the loosening and failure of joints, particularly when these joints involve metal–metal contact. It is suggested that the increased allergy is caused by, rather than being responsible for, the loosening.
In recent years there has been a return to metal on metal hip prostheses because it is felt that they produce less wear debris. Consequently, the issue of metal allergy in relation to these procedures has resurfaced [18, 21]. As allergy is common in the general population, prospective controlled studies will be required to assess whether there is a genuine risk of dermatitis and loosening as a result. It is suggested that preoperatively, patch testing is undertaken where requested to guide the choice of a suitable prosthesis, and that postoperatively, where a problem has occurred, removal of an implant in a senstized patient results in a resolution of symptoms in the majority [22].
Titanium allergy is virtually unknown, and thus titanium is an alternative for patients with extreme sensitivity to other metals. The patient may need to be appraised that the metal is not thought to be as long lasting as stainless steel.
Reactions have also been reported to sternotomy wires, shrapnel, mitral valve prostheses, dental prostheses and fillings, pacemakers, atrial septal occluders, infusion and acupuncture needles and implanted gynaecological devices [23]. Whilst the release of metal ions from stents has been shown, the clinical relevance in restenosis of the coronary artery after stenting has yet to be definitively proven [24]. However, there is evidence of an increased rate of restenosis and allergy to gold after the stenting procedure when using gold-plated stents [25].
A diagnostic algorithm for use where a reaction to a metal implant is suspected has been proposed [26].
Non-eczematous responses
Erythema multiforme-like reactions
The characteristic presentation is that of a spreading eruption from the primary site, which may also involve distant sites. The rash has features of erythema multiforme, in that single lesions appear target-like, but the distribution is not necessarily acral as in classic erythema multiforme nor is the histology characteristic. There is sometimes a vasculitic purpuric element to the rash and, although the mechanism is unknown, it appears to represent an immune complex (type III) reaction as well as a delayed hypersensitivity (type IV) reaction. Many of these patients will give a very strong patch test response to the causative allergen, often accompanied by a flare of their dermatosis.
It is often precipitated by strong allergens, such as quinones in exotic woods [1], and Primula. Contact with other plant materials may cause this reaction, including poison ivy (Toxicodendron spp.), Compositae (Asteraceae) and tea tree oil. Ingestion of herbal remedies containing Toxicodendron and sesquiterpene lactones by sensitized persons has also induced erythema multiforme-like eruptions.
Topical medicaments, especially antimicrobials, corticosteroids and anti-inflammatories, have all caused erythema multiforme-like eruptions. A nitroglycerin patch has also induced erythema multiforme at the applied site, with a secondary spread eruption. Medicaments applied to mucosal surfaces may sensitize and may also be absorbed, causing systemic erythema multiforme-like reactions, for example sulphonamide in vaginal creams and ocular preparations.
PPD in hair dye and temporary tattoos, rubber chemicals and clothing dyes are also recognized causes of this reaction pattern.
Erythema annulare centrifugum of the trunk has been linked to contact allergy to nickel and cobalt in clothing hooks and studs.
Purpuric reactions
Originally described in association with khaki uniforms, although the precise cause was not established, pigmented purpuric reactions are uncommon and have mostly been described recently from textile azo dyes [2] and textile resins. The presence of the rubber chemical IPPD in footwear, diving suits, bandages and bras is also reported as a cause of allergic contact purpura [3]. Purpuric reactions have been described with allergy to diphenylthiourea in heat retainers, PPD in black hats and as a secondary spread eruption from balsam of Peru.
Lichen planus and lichenoid reactions
These have been described following contact with colour developers used in the photographic industry [4]; the developers are PPD derivatives. New chemicals have been introduced to replace older, more sensitizing ones, not always successfully. PPD-induced allergic lichenoid contact reactions from hair dye have been reported from India [5]. Primula obconica allergy has also produced a lichen planus-like eruption of the hands [6].
Lichen planus-like reactions of the buccal mucosa may represent allergy to metals [7], other materials used in dental treatments, cinnamal and spearmint. Some patients have had improvement in their lichen planus following the removal of some or all of their fillings [8]. Oral lichen planus is more apparent where there is evidence of corrosion and the aetiology of lichenoid lesions is likely to be multifactorial. The histology may show features compatible with lichen planus or a non-specific, chronic, superficial perivascular dermatitis.
Lichenoid reactions to tattoo pigments are discussed in the section on granulomatous reactions.
Lymphomatoid eruptions
Occasionally, contact dermatitis presents with cutaneous lymphoma-like plaques and histopathology suggestive of mycosis fungoides [9]. These have been seen at the site of ear piercing in those sensitized to gold. The reaction tends to persist for months, even when contact with metallic gold is avoided. The patch test reaction to gold sodium thiosulphate in these patients is papular and very strongly positive. The histology of both the papular eruption and the patch test reaction shows a dense T-cell infiltrate. Other reported causes include matches, nickel, dental amalgam, medicament components, PPD, isopropyl-diphenylenediamine and PTBPFR.
Pigmented dermatitis
Contact dermatitis may induce post-inflammatory hyperpigmentation, and distinctive patterns of pigmented dermatitis without a lichenoid appearance or histopathology are recognized. These patterns are much more commonly seen in the Far East [10] but have been seen in Europe. The hyperpigmentation occurred mainly on covered areas, with or without dermatitis, and was traced to an optical whitener in washing powder, Tinopal CH 3566. Another outbreak was described in textile workers and traced to contact with naphthol AS, an azo dye coupling agent. Cases also occurred on covered sites from garments in Japan, and this led to a systematic search for other causes of textile dermatitis. Chemicals implicated included the fungicide Biochek 60, an impurity of colour index blue CI Blue 19, and textile finishes.
Pigmented cosmetic dermatitis is seen mainly in Asian women [11]. Slight dermatitis may precede or coexist with the hyperpigmentation, which occurs mainly on the cheeks. The allergens associated with this have been found to be fragrances and pigments, especially D and C Red 31 (from an impurity), and Yellow 11 in cosmetics and soaps. Pigmented cheilitis has occurred from allergy to ricinoleic acid in castor oil used in lipsticks [12]. Components of kumkum, applied as a cosmetic to the forehead, commonly cause a pigmented dermatitis [13].
Oral ingestion of flavourings allied to the fragrances, such as cinnamon, may cause not only a focal flare but also diffuse hyperpigmentation on the body [10].
Environmental agents, possibly pesticides and fungicides, have sometimes been thought to be a factor. In one study, a majority of pigmented dermatitis cases were found to have positive patch tests to chlorothalonil used as a fungicide in banana plantations. It is used in other parts of the world as a preservative for wood and paint [14].
Depigmentation
Irritant and allergic contact dermatitis can induce hypopigmentation as a post-inflammatory effect or by koebnerization of vitiligo. This has to be distinguished from the direct melanocytotoxic effect of certain quinones and substituted phenols, which most commonly present as an occupational vitiligo (see Chapter 130). This effect occurs independently of their sensitizing potential.
A number of cases of persistent leukoderma following allergic contact dermatitis have been reported. In some it has been difficult to be certain whether the cause was post-inflammatory or melanocytotoxic. In particular, it is reported from hair dyes and is commonly seen from temporary tattoos; persistence of hypopigmentation for longer than 2 years has been seen [15].
Epoxy resin components, methacrylates, perfumes, Alstroemeria and chloroxylenol are also reported as causes. Primula allergy has resulted in the extension of pre-existing vitiligo to sites affected by the dermatitis (Figure 128.27). The allergic reaction to primin was followed by vitiligo at the positive patch test sites [16].
Figure 128.27 Koebnerization of vitiligo as a result of previous Primula obconica allergy.
Granulomatous reactions
Some topically applied metal salts produce non-allergic granulomatous skin reactions, for example zirconium in deodorants. Granulomas occurring at the site of previous immunization with aluminium-adsorbed vaccines, or following the use of parenteral hyposensitization preparations, are often due to aluminium allergy [17]. Patch tests are positive either to aluminium chloride 2% aqueous or an empty Finn chamber. Granulomatous reactions have also been found in association with allergy to gold and palladium in earrings [18].
Pigments in tattoos may cause allergic granulomatous and lichenoid reactions. Historically, metal salts have been identified as culprits: mercury (red colour), chromium (green colour), cobalt (blue colour) and cadmium (yellow colour). However, in our experience most reactions are in the red areas, and patch testing with mercurials is negative, suggesting another cause. Unfortunately, tattooists are extremely secretive about the nature of the pigments they use but an analysis has been undertaken of a series of pigments obtained from 20 tattoo parlours [19]. This has shown that a wide range of metallic salts, pigments and dyes may be used, and a suitable patch test series for tattoo reactions has been suggested as a result of this investigation.
Oro-facial granulomatosis has been associated with allergy to gold crowns and mercury fillings [20]. In one study 22% of patients with this condition had allergy to one or more food additives on patch testing, and most of these improved with an elimination diet. The authors concluded that the cause of the disorder was multifactorial and that patch testing had been helpful for a subgroup of those affected [21].
Onycholysis
Onycholysis may be the only presenting feature in contact dermatitis to hairdressing chemicals. It can occur as an isolated finding in nail varnish allergy. More commonly, it is found in subjects sensitized to multifunctional acrylates in anaerobic sealants and false nails, when there may be concomitant dystrophy and persistent paraesthesiae.
Systemic non-eczematous reactions
Extensive allergic contact dermatitis is not uncommonly associated with systemic upset from the metabolic effects of the disorder itself and secondary infection, particularly in those who are erythrodermic. Sultones occurring as impurities in lauryl ethyl sulphate have in the past caused several outbreaks of contact dermatitis characterized by intense oedema accompanied by general malaise [22].
Differential diagnosis
It should always be remembered that allergic contact dermatitis can mimic or complicate other types of eczema and other dermatoses. Sensitization to topical applications may be a complication of almost any dermatosis that leads to specialist referral. The diagnostic problem differs according to the site of the dermatitis. Patch testing will often be required before confirming the cause in its entirety.
Head. Allergic and photoallergic conditions of the face must be distinguished from a number of disorders. Atopic eczema may be confined to the face, especially around the eyes and particularly the medial aspects. A previous or family history of infantile or childhood flexural eczema, asthma, allergic conjunctivitis, hay fever or immediate skin reactivity to animals and certain foods may point to the patient's atopic status. Associated flexural eczema, ichthyosis or xeroderma may be features. Multiple positive radioallergosorbent or prick tests to common environmental allergens are used by some to confirm an atopic diathesis.
Seborrhoeic eczema, which commonly starts around the alae nasi, is usually accompanied by dandruff or seborrhoeic eczema in the scalp and eyebrows, and by blepharitis. Involvement of the presternal region, the external auditory meati and the retro-auricular areas is common. Older patients frequently develop a flexural pattern of seborrhoeic eczema. Allergic contact dermatitis may imitate seborrhoeic eczema [1].
Psoriasis is normally easy to distinguish as there is evidence elsewhere on the body, although psoriasis in and around the ears and scalp margins may mimic a dry scaly contact dermatitis.
Photosensitivity, including reactions to ingested drugs, cannot always be distinguished from photoallergic contact dermatitis, and may also simulate contact dermatitis from airborne sensitizers. Lupus erythematosus may be confused both clinically and histologically with contact allergy [2]. Dermatomyositis may initially appear identical to allergic contact dermatitis. The purple/mauve hue of the eyelids is a major clue, and the hands, fingers and nail folds must be carefully examined. The characteristic associated muscle pains and weakness may not always be present.
Angio-oedema, especially of the eyelids, is notoriously difficult to differentiate from contact allergy. The swelling would be expected to resolve within 24–48 h. Patch tests are helpful in reaching a conclusion.
Cellulitis and erysipelas may be difficult to distinguish from an acute allergic contact dermatitis, but the former are usually accompanied by pyrexia and systemic symptoms.
Herpes simplex may be simulated by Primula obconica dermatitis affecting the face and elsewhere. Haemorrhage into the blisters seems to be more common with the latter.
Basal cell carcinoma has been simulated by nickel allergy from spectacle frames [3].
Hands and arms. Allergic and irritant contact dermatitis and constitutional eczema of the hands may only be distinguishable by a careful history and patch testing. They commonly coexist. Superimposed irritant contact dermatitis from home and work exposures is common.
Indicators of atopic eczema are discussed earlier in the chapter. The eczema may be confined to the hands, especially in later life. A nummular pattern of eczema is commonly constitutional but may be a feature of irritant and allergic contact dermatitis, particularly from chromate in cement.
Recurrent vesicular eczema of the palms may indicate constitutional pompholyx, although contact allergy can produce an identical appearance. Some contact allergies, for example from IPPD and BIT, seem to induce a palmar pattern of dermatitis preferentially. Primula allergy often induces a haemorrhagic vesicular dermatitis of the palmar surfaces and fingertips. The relationship of pompholyx to the ingestion of contact allergens, especially nickel, by sensitized subjects is controversial [4]. There is evidence that oral intake of balsams and garlic can induce palmar vesicular eczema in patch test-positive subjects [5, 6]. Tinea pedis can induce palmar pompholyx as an id eruption. Papules and vesicles on the hands and fingers are a feature of scabies, and this disorder must always be excluded by a careful history and examination for diagnostic burrows. The condition is normally associated with a more generalized pruritus and rash.
Psoriasis of the palms and hyperkeratotic eczema are often confused. Differentiation is sometimes somewhat arbitrary. Often, hyperkeratotic plaques are localized at points of contact, for example with tools, but not all frictional hyperkeratosis is necessarily an expression of psoriasis. The possibility of psoriasis koebnerizing into areas of contact dermatitis should not be forgotten [7].
Tinea manuum is classically unilateral or asymmetrical. An inflammatory edge may be seen extending on to the dorsum of the hand. Nail dystrophy may be an association. Scaling palms should be scraped for mycology. These appearances may be complicated by a vesicular id eruption.
Lichen planus confined to the palms can be difficult to distinguish from a palmar dermatitis, but usually there are more typical changes elsewhere on the skin or in the mouth. Lichen planus-like contact reactions from colour developers may occur on the hands and forearms [8].
Porphyria cutanea tarda may simulate a bullous contact dermatitis such as plant dermatitis. The formation of bullae after minor trauma and the presence of white atrophic scars and milia suggest the diagnosis, which can be confirmed by porphyrin assays.
Flexures and ano-genital region. Seborrhoeic eczema and psoriasis may preferentially involve the flexures and be difficult to distinguish from allergic contact dermatitis, but there is often evidence of these conditions elsewhere. Tinea is usually asymmetrical or unilateral and has an inflammatory edge. Scrapings for mycology should be taken from inflammatory flexural rashes. The typical coral-pink fluorescence under Wood's light will help to distinguish erythrasma from other flexural rashes.
Legs and feet. Persistent varicose eczema is an indication for patch testing as it is often complicated by sensitivity to topical medicaments and dressings, including rubber in support bandages and stockings. Vesicular and vesiculobullous areas may occur in tinea pedis, and mycological specimens should be taken if this is suspected. In common with the hands, scabies affecting the feet may induce a papulovesicular eruption and must be considered in the differential diagnosis.
Trunk. Papular drug eruptions or scabies may sometimes be difficult to distinguish from nickel or textile dermatitis. Dermatomyositis and mycosis fungoides sometimes show eczematous features.
Exposed sites. Photosensitive dermatoses and drug eruptions must be distinguished from contact allergy to volatile and airborne materials. Although not always reliable, sparing in certain sites – behind the ears and under the chin – might indicate a photosensitive eruption. Nevertheless, some patients will require thorough investigation with phototesting, patch and photopatch testing before a diagnosis can be made.
Generalized. Erythroderma is rarely primarily due to contact allergy, and other causes such as drug eruptions, constitutional eczema and psoriasis should be considered; the possibility of secondary contact allergy from topical medicaments must not be forgotten. Skin biopsy may be helpful in these cases. Scabies can easily be overlooked as a cause of a widespread pruritic rash, especially as skin lesions may look classically eczematous. Careful examination of the hands, feet and genitals for diagnostic lesions is required.
Complications and co-morbidities
Complications of contact allergic dermatitis include those of eczema in general. Contact dermatitis may start at one site, but commonly other sites are subsequently involved, and sometimes several regions simultaneously. By the time the patient has been sensitized, many body regions may have been in contact with the allergen, some indirectly by contamination from the fingertips. Heavily contaminated areas, or those that were exposed last, tend to be the ones to react first, other sites flaring later. This has been shown experimentally with poison ivy, and is an obvious clinical feature in Primula obconica dermatitis. Regions close to the primary site of allergic contact dermatitis are easily contaminated by the allergen.
Such a simple explanation cannot account for the frequent spread of dermatitis from the feet to the hands and vice versa. This occurs primarily in constitutional eczema, but may occur in contact dermatitis. Sometimes, a common allergen is found that could explain occurrence at both sites, yet often it is a pattern of secondary spread. No precise explanation exists. Because of the similarity to Darier's trichophytids and eczematids, dissemination to distant regions has been termed an ‘id-like’ spread. Local aggravation may precede secondary spread by several days.
The pattern of spread is largely determined by the primary site. Dermatitis of the hands commonly spreads to the arms and face; dermatitis of the feet tends to spread to the legs and hands. Many patients with stasis dermatitis have secondary eruptions by the time they are seen by a dermatologist, and are referred because of the alarming dissemination. This may be due to an ‘id’ reaction or secondary contact dermatitis. Dissemination from leg eczema commonly involves the arms and shoulders in a patchy fashion before becoming generalized, often beginning with pruritus and sometimes progressing to generalized erythroderma. On the face, diffuse redness and oedema are common. Eyelid dermatitis or a diffuse dry dermatitis of seborrhoeic type may be seen.
Severe nickel allergy may induce extensive patchy eczema, which is slow to respond to treatment, and this will not settle unless strict nickel avoidance measures are undertaken.
Contact allergy to components of topical treatments presents special difficulties. The allergen may be an active ingredient or an excipient. If the dermatitis spreads further in spite of treatment, it may wrongly be assumed to be an endogenous process. In contact dermatitis caused by topical steroid preparations, the action of the steroid may partially suppress the local reaction. The sensitivity becomes clinically manifest only as ‘failure to heal’ of the original eczema, or with the development of a secondary eruption.
Constant alertness is therefore a prerequisite for the diagnosis of allergic contact dermatitis in which dissemination is a dominant feature. Patch tests should be delayed until the acute eruption has settled.
Disease course and prognosis
The prognosis of allergic contact dermatitis depends on its cause and the feasibility of avoiding repeated or continued exposure to the causative allergen. Associated irritant dermatitis and constitutional factors are also important. Some studies suggest age of onset is not important prognostically for occupational dermatitis but others suggest a poorer outlook in older age [1, 2]. In the USA, patients with allergic contact dermatitis tended to have a poorer quality of life if they were non-white, younger or industrial workers, but gender was not important [3]. In Sweden, skin atopy was the factor that carried the worst outlook, followed by contact allergy, especially to nickel, and female sex [2].
The prognosis is generally relatively poor for those allergic to nickel [4] and chromate [5], probably as a result of their ubiquity in the environment, even though most chromate studies have involved those with occupational dermatitis, which is a selective group. It has been suggested that dietary nickel [6] and chromate [7] exposure might be responsible for the chronicity, but this is disputed [8].
There is a better outlook for those allergic to materials that are easy to identify and avoid, and often the dermatitis will resolve within a few weeks if conscientious avoidance measures are taken. This was exemplified by a European joint study where the sources of contact with allergens could be traced in only 35% of those who reacted to colophony, but in 85% of those sensitive to tetramethylthiuram disulphide. The reason for the limited success with colophony was probably lack of knowledge of the sources of this sensitizer. However, in a small Australian study, workers with epoxy resin allergy had a worse outlook than might be expected in spite of avoidance [9]. Greater age, associated atopy, and longer duration and severity of dermatitis at diagnosis correlated with poorer prognosis in this survey. It is clear from a number of other studies that poor compliance and understanding results in a higher rate of ongoing exposure to the causative allergen, and is associated with a worse prognosis [10, 11, 12].
As the skin integrity is compromised, there are enhanced opportunities for new sensitivities to medicaments or other substances to develop during the course of dermatitis. Sensitivity to rubber gloves may complicate pre-existing dermatitis of the hands. Such allergies are revealed only by repeat patch tests. During a long course of relapsing dermatitis, sensitivity to various allergens may accumulate, and this increases the risk of recurrence or persistence [13].
Contact dermatitis of the hands is often of mixed origin, with alternating or simultaneous exposure to allergens and irritants. In one study of the prevalence of dermatitis of the hands, half the patients had suffered from their dermatitis for more than 5 years. Follow up after 6–22 months revealed one-quarter had healed completely, half had improved and one-quarter were unchanged or worse. There was no difference in prognosis between irritant and allergic dermatitis. A change of occupation does not necessarily alter the prognosis of occupational hand dermatitis, particularly if the change is inappropriate [14]. The concept of persistent post-occupational dermatitis despite avoidance of the original cause(s) is now well established, and may occur following both irritant and allergic contact dermatitis [15].
Once acquired, contact sensitivity tends to persist [16]. The degree of sensitivity may decline unless boosted by repeated exposure, but with a high initial level of sensitivity it often remains demonstrable even several years later [17]. Sensitivity to ubiquitous allergens, such as nickel and chromate, and to strong allergens, such as primin and PPD, is reported to persist, whereas sensitivity to other weaker and avoidable allergens may disappear. Patterns of cross-sensitization tend to persist.
Relapse or chronicity is due not only to unavoidable or unrecognized re-exposure to allergens and irritants but also to other contributory mechanisms.
- The barrier function of the skin is impaired for months after an attack of dermatitis. Recovery is prevented by exposure to allergens or irritants in concentrations that might well be tolerated by normal skin.
- Inappropriate treatment, including the overzealous use of cleansers and antiseptics, and the use of sensitizing popular or herbal remedies may also prolong the course of dermatitis.
- Ingestion of allergens.
- Secondary infection, especially with dermatitis of the hands. Microbial allergy may also be a factor in some eczemas.
- Contact sensitivity has been thought in some cases to involve sensitization to the protein moiety (‘protigen’) of the hapten–protein conjugate. On this assumption, autosensitization might account for chronicity [18].
- Stress is common in chronic dermatitis and may be both a consequence of, and a trigger for, eczema.
- Constitutional factors predispose to chronicity.
- There appears to be an ‘inherent tendency’ in almost any eczema to become continuous and chronic, but the factors causing this are unknown.
Investigations
Patch testing
Background
The diagnosis of allergic contact dermatitis is made by patch testing. The techniques have evolved into a generally standardized methodology worldwide, although there are some variations, particularly with regard to reading times and test units. Patch testing relies on the observation that primed antigen-specific T lymphocytes will be present throughout the body, and hence allergen in the patch test can be applied to normal skin, usually on the upper back where the tests are least likely to be disturbed. Other sites may be considered when this is not practicable, for example when there is pre-existing inflammation or other skin changes are on the back.
The test relies on the allergen being absorbed in sufficient quantity to induce a reproducible inflammation of the skin at the site of application in sensitized subjects. A positive reaction to a correctly prepared and applied patch test confirms the person has an allergic contact sensitivity, although this does not necessarily mean that the substance is the cause of the presenting clinical dermatitis, and its relevance should always be carefully considered.
Indications
It is well established that aimed patch testing with a few suspected allergens is suboptimal. The reason is that even experienced dermatologists are poor predictors of the outcome of patch tests; 17% of patients with allergies were missed on a prepatch test assessment in one large clinic [1]. This parallels our own experience, with 20% of allergic patients regarded as definitely not allergic prior to patch tests and, conversely, 16% of patients thought to have contact allergy who were negative when patch tested.
An audit of patch testing has suggested that the investigation is underused, and consequently important opportunities to improve or resolve potentially disabling and wrongly classified eczema/dermatitis are lost [2]. The audit concluded that facilities should be available to patch test at least 142 per 100 000 population annually and that patient with the indications listed in Box 128.6 should be patch tested where practicable. Dermatology-specific quality of life has been shown to improve significantly more in those patients who are patch tested, because of more accurate diagnosis and earlier intervention [3, 4, 5]. Furthermore, the investigation has been shown to be cost-effective and to reduce the cost of therapy in patients with severe allergic contact dermatitis [3, 6].
Methods
The basis of testing is to elicit an immune response by challenging already sensitized persons to defined amounts of allergen and assessing the degree of response. The amount of allergen is defined by its concentration in the vehicle and the amount applied. By testing the same allergens in parallel, the technique has been confirmed to be generally reproducible [7, 8].
Chambers or discs are used to ensure occluded contact with the skin. The fixing tape should be non-occlusive, non-allergenic and non-irritant. If the adhesive tapes peel off, the test should be repeated. Ideally, patch testing should not be carried out in patients with active eczema because it may reduce the threshold of activity and cause non-specific reactions, although in practice this is commonly not possible. The procedure ideally should be delayed until the test site has been clear of eczema for at least a fortnight. Patch testing should be delayed for 4 weeks following sunbathing, and the patches should not be exposed to the sun or other sources of UV light. This information should be given to the patients before they book their appointments. Corticosteroids and other immunosuppressive drugs should be stopped (if this is feasible) before patch testing as they may reduce or extinguish positive patch tests in sensitized subjects. Nevertheless, this is unlikely at doses below 15 mg prednisolone daily [9], and we have identified relevant positive patch tests in patients who could only be investigated while they were taking other immunomodulators.
We prefer not to patch test pregnant patients in case an adverse event is blamed on the test, although we are unaware of any proven problem. Young children, even infants, can be patch tested when indicated, but the number of allergens tested may have to be reduced because of lack of space [10].
Test materials
Allergens are obtainable from the following manufacturers or from their local distributors.
- SmartPractice Europe (http://www.trolab.com/, last accessed May 2015), which markets TROLAB® allergens.
- Chemotechnique Diagnostics AB, Modemgatan 9, S-235 39 Vellinge, Sweden (http://www.chemotechnique.se/; last accessed March 2015).
- SmartPractice® Canada, 2150 29 Street NE, Unit 30, Calgary, AB T1Y 7G4 Canada (http://allergeaze.com/; last accessed March 2015), which markets allergEAZE®.
The commonest system used to apply allergens is the Finn chamber (Epitest Ltd, Oy., Rannankoukku 22, FIN-04300 Tuusula, Finland) on Scanpor tape (Norgesplaster, Granliveien 21, N-4702 Vennesia, Norway). These are also available from local distributors. The chambers are supplied in strips of five or 10 (two rows of five), and consist of small, occlusive aluminium discs. They are mounted on non-occlusive tape with an acrylic-based adhesive backing that has been chosen for its hypoallergenicity.
The older AL Test system (a filter paper disc mounted on aluminized paper) is now rarely used. Other systems consist of square plastic chambers (Van der Bend chambers), and oval plastic chambers (Epicheck). Systems continue to be developed (e.g. IQ Ultra) to improve adhesion and comfort and also to impart water resistance to enable brief showering during the tests (e.g. Curatest F).
There is also a prepackaged, ready-to-use patch test system, TRUE test, based on a dispersion of allergen in a hydrophilic polymer. This was available as a series of 24 allergens but has been extended to 36. This system has been tested in parallel with the established Finn chamber system and there was close correlation of results [11]. It is a consistent, convenient, portable method for those wishing to test only a few allergens, but supplementary tests are necessary to achieve a comprehensive range of investigations [12]. It has been estimated that by using preprepared tests alone between 60% and 70% of relevant allergic reactions may be missed.
Patch test vehicles
Few substances can be applied to the skin as they are. In order to avoid an irritant effect, they must be mixed or dissolved in a vehicle to achieve a suitable test concentration. The test substance should, if possible, be soluble in the vehicle. If a dispersion of allergen in petrolatum is used, contact with the skin depends on the size of the particles and on their solubility or dispersion in petrolatum. Uniform dispersion and particle size are important. Many substances can also be dissolved in water, alcohol, acetone, methylethylketone or olive oil, as appropriate. Irritant solvents such as chloroform and benzene must not be employed. False positive or false negative reactions may occur when inappropriate vehicles are used. Petrolatum is generally more reliable, and has the added advantage of being occlusive, which helps to prevent oxidation and prolongs shelf-life. Allergic reactions to petrolatum itself are very rare [13]. In hot climates, petrolatum may not be ideal, as it melts too quickly between preparation and application of the patch test. A series in modified Plastibase has been devised for the Indian Contact Dermatitis Group [14].
Patch test concentrations
The choice of a suitable concentration is of fundamental importance. Excessive concentrations result in false positive reactions, because of their irritant effect, and may even sensitize patients; insufficient concentrations produce false negative results. The concentration of allergen routinely employed for patch tests may, under some conditions and in some individuals, give rise to false negative or false positive reactions. The choice of concentration is thus a compromise, but most have been chosen by long experience with commonly used allergens. The concentrations used for patch testing are usually much higher than those encountered during the development of dermatitis. To demonstrate the existence of nickel dermatitis produced by the minute amounts dissolved from nickel-plated objects, a 5% concentration of nickel sulphate in petrolatum is necessary. Chromate 0.25–0.5% is required to prove sensitivity to cement containing 0.0005–0.002% of chromate. Neomycin should be tested at 20% despite only being at 0.5% concentration in many topical medicaments.
Lists of suitable concentrations and vehicles are provided in the text by De Groot (see Resources list). Metal salts in particular are tested at the margins of irritancy and may give false positive, irritant patch test reactions, especially in atopic individuals. Weak reactions may not be allergic. In important cases of doubt the patient can be retested later with serial dilutions. Other standard allergens such as fragrance mix, parabens mix and wool alcohols may also be marginally irritant. On rare occasions, active sensitization may still occur even at the concentrations recommended. Irritant reactions are rarer with other substances used in the baseline series. They are potentially much more common with materials brought to the clinic for testing. Many industrial or domestic chemicals, if undiluted, will give irritant false positive reactions which may be severe. No chemical or substance should be applied to the skin until full details of its composition and potential irritancy or toxicity are known.
If substances from work or other materials are brought, a number of factors should be considered before they are used for patch testing. Is it appropriate to test with the material? Some materials are strong irritants and not allergens (e.g. strong acids and alkalis), and others may be contaminated or of uncertain or mixed composition (e.g. dust or grime from a working environment). The product may be intrinsically dangerous (e.g. explosive or produces toxic fumes) and require special handling. Some patients bring foods in the mistaken belief that the test will diagnose ingested food allergy.
The precise nature of the material should be ascertained by questioning the patient and examining the product label. In the UK, employers are required by law to have Health and Safety data sheets for all materials handled at work. These provide key information and a contact point with the manufacturing company. They must be scrutinized carefully, particularly with regard to irritancy, allergenicity, stability and solubility of the product and its components. Named chemical substances may be recognized as irritants or allergens, and their concentrations documented. Substances can be checked for pH, and neutralized if necessary [15]. It should be remembered that a data sheet is only as good as the individual writing it and consequently may not be comprehensive or completely accurate. Consequently a degree of scepticism is helpful in their interpretation.
An initial patch test concentration can often be selected either by reference to standard texts or by contacting the manufacturer for details of toxicological testing data. A range of differing test concentrations is advised where there is no literature on the material. It is advisable to start low (0.01% or less), and increase the concentration gradually if there is doubt about the optimum level for testing. It may be advisable to perform open tests before proceeding to closed patch tests because the effect of irritants is enhanced by occlusion. Materials intended to be left on the skin, such as medicaments and cosmetics, can be tested ‘as is’, rinse-off products at 5%, and soaps, shampoos and detergents at 1% or less. However, the dermatologist administering a patch test will need to refer to standard references for guidance on dilutions and vehicles when testing finished products or specific chemicals.
If a positive reaction to an unknown substance occurs, it should not be immediately accepted as allergic. Volunteers, who are not suffering from dermatitis related to the same agent, should be tested at the same concentration and using the same methods. If any reaction occurs among 50 controls, the substance should be regarded as a primary irritant at that concentration, and subsequent tests should be performed with decreasing concentrations.
Patch test dose
If petrolatum is used as the vehicle and disposable syringes are the containers, a length of 5 mm of test substance in a vehicle will suffice. For a Finn chamber, 20 mg of allergen as a petrolatum dispersion has been shown to be the optimum dose [16]. If the vehicle is a fluid, a digital pipette should be used to deliver 15 μL to a filter paper in the chamber. Dropper bottles supplied by the allergen manufacturers tend to overfill the chambers. A surplus should be avoided, as it may contaminate neighbouring test sites. With the TRUE test, the patches are preprepared. The risk of patch test sensitization increases with the concentration and amount of test substance applied.
Storage of allergens
Shelf-life’ is prolonged if test substances not in daily use are stored in the dark in a refrigerator at 4°C. Many substances are unstable if exposed to light. Commercially available allergens are labelled with an expiry date. With potentially volatile allergens, the allergen may be lost over time even when refrigerated so substances should be regularly replaced. For some materials such as isocyanates, freezing is essential to prevent the loss of allergen [17].
Storage in small jars has the drawbacks of oxidation, drying and evaporation of volatile test substances. Rubber pipette caps contaminate the solutions and may cause false positive reactions in persons sensitive to rubber. Homogeneity of patch test allergens may be lost, especially in hot climates, if they are not refrigerated.
Many centres preprepare their allergens prior to application. However, care must be taken, particuarly with volatile allergens where it has been shown that following application, even with refrigeration, the allergen is rapidly lost from the test chamber [18]. It is preferable to prepare on the day and apply immediately to avoid consequent false negative reactions.
Test site
Most dermatologists prefer to apply patch tests to the back. The region used for testing will affect the results of the investigation: both allergic and irritant reactions are most easily provoked on the upper back (Table 128.7) [19]. Reactions on the lateral aspect of the upper arm are stronger than on the medial aspect. Sites other than the back and lateral aspect of the upper arm are generally less suitable as test areas, but when necessary we have used the abdomen or even the thighs rather than abandoning the investigation.
Table 128.7 Reactivity of various test sites.
| Type of reaction | ||
| Test site | Irritant (%) | Allergic (%) |
| Upper back | 100 | 100 |
| Lower back | 50 | 95 |
| Upper arm | 52 | 72 |
| Forearm | 38 | 74 |
| Thigh | 36 | 50 |
Marking
Test sites must be marked with indelible ink or stratum corneum stains, or fluorescent markers on dark skins. Marking materials can be obtained from allergen suppliers. It is necessary to repeat marking before removal of the patches, because their positions cannot be distinguished once the pressure effects have subsided. The patient should be instructed not to bathe or shower for the duration of the tests, and to avoid exercise or other activity likely to dislodge the patches.
Exposure time
The mere touch of a Primula leaf may provoke a subsequent bullous response in a sensitive person, but with some materials (e.g. textiles) even 5 days of occlusive patch tests may lead to false negative reactions. However, few formal studies on the relationship of exposure time, dose and elicitation have been undertaken. Recent work with PPD and isoeugenol has shown that elicitation is dependent not only on exposure time but also concentration and number of applications of the allergen [20, 21].
Well-established allergens, however, are conventionally tested in such concentrations that a 48 h exposure under an occlusive patch will generally allow penetration of an amount sufficient to provoke a reaction. With low sensitivity, low concentration of allergen or poor absorption of a particular agent, there may be a long period of latency. A typical regimen is a 48 h application time, with readings taken 1 h after removal and again 48 h later (that is day 2 and day 4), with preferably the same observer performing each reading. Others have suggested that a second reading on day 5 is better. A single day 2 reading is not advised as it may lead to the labelling of some marginal irritants as allergens, and positive reactions to more poorly absorbed allergens may be missed. Variations to this schedule are made for expediency, to fit in with clinic times, and for the convenience of patients travelling long distances. If only one patch test reading is possible, a day 4 reading has been recommended [22] although, according to some authors, a single day 4 reading is also associated with the risk of missing some significant positive reactions. A third reading on day 5–7 seems to identify a small proportion of additional relevant positive allergies where sensitivity is weak or partially ‘forgotten’, or where there is poor absorption of the allergen [23]. Neomycin (Figure 128.28) and corticosteroids are particularly liable to give late reactions.
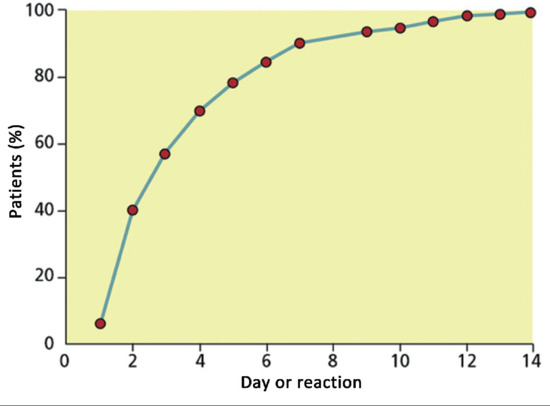
Figure 128.28 Patch tests with neomycin 20% in petrolatum: positive reaction times after application.
Immediately after removal of the patch tests, there may be erythema from the stripping action of the tape, especially in dermographic subjects, and this must be allowed to settle. Furthermore, some reactions may take up to 1 h to develop once the pressure of the strips has been released, and the infiltration allowed to swell the dermis.
Readings and interpretation
It is important that patch test readings are scored according to the reactions seen and not according to the interpretation placed on the reaction by the reader. As the strength of a reaction is not always reproducible, an over-detailed quantification should be avoided. The scoring system devised by the ICDRG in 1970 is shown in Table 128.8 [24]. There are drawbacks to the ICDRG system in that it confuses morphology with interpretation. The ideal system is to record what is seen at 2 and 4 days, and then to decide if this represents an allergic or irritant response. This is done by assessing morphology and skin type, combined with knowledge and experience of the substance and the patient‘s history. Patch test results should be recorded objectively, and the interpretation of the results should be recorded separately. In this way raw data remain available for re-examination.
Table 128.8 Recording of patch-test reactions according to the International Contact Dermatitis Research Group scoring system.
| Score | Description |
| – | Negative |
| ?+ | Doubtful reaction; faint erythema only |
| + | Weak positive reaction; palpable erythema, infiltration, possibly papules |
| ++ | Strong positive reaction; erythema, infiltration, papules, vesicles |
| +++ | Extreme positive reaction; intense erythema and infiltration and coalescing vesicles |
| IR | Irritant reaction of different types |
| NT | Not tested |
Once they have developed, positive allergic reactions often persist for several days. The strength of the reaction depends on barrier function, the presence or absence of sweating, the atmospheric humidity, test material, technique and the reactivity of the individual.
Strong reactions of an allergic nature are erythematous and infiltrated, commonly with minute papules or vesicles (Figure 128.29), which in severe reactions coalesce into bullae. The infiltration causes a thickening in the dermis, which is palpable and can be distinguished from surface changes in the epidermis. The reaction may extend beyond the margins of the patch, and there is often some itching. Nevertheless, sometimes true allergic reactions can be weaker than this, making interpretation more difficult.
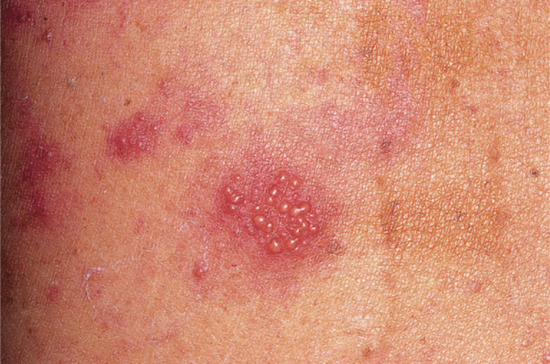
Figure 128.29 A positive allergic (++) patch test response in a patient sensitive to neomycin. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
In most instances there is little difficulty for the experienced clinician in identifying true positive allergic patch test reactions. Nevertheless, there are occasions when distinguishing an allergic from a false positive non-allergic irritant reaction can be difficult, or even impossible. There may be clues: no infiltration, lack of itching, deep redness or a brown hue, and sharp delineation corresponding to the margins of the patch test all point to an irritant reaction. Some irritants provoke a ‘soap effect’, with a well-localized, glistening, finely wrinkled surface. Patch tests with nickel may cause pustular reactions that are often false positive (Figure 128.30), although some progress to more typical allergic reactions. Cobalt also produces a distinctive false positive purpuric reaction which may result from poor dispersion of the allergen in the petrolatum base. In our experience these tend to occur much more in atopic individuals, and need to be distinguished from allergic follicular reactions consisting of papules without pustules or purpura.
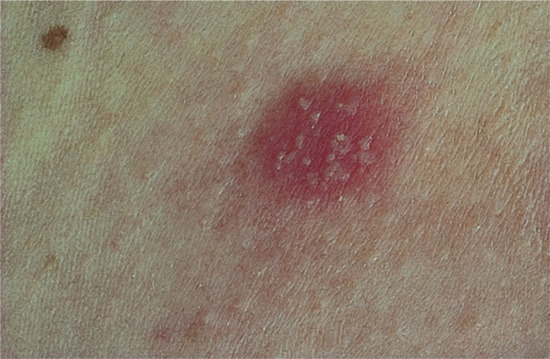
Figure 128.30 Pustular patch test reactions to metals are common in patients with atopy and are often irritant in type.
False positive irritant reactions are liable to induce stronger reactions at day 2 than at day 4, the so-called crescendo–decrescendo effect. However, this is not always the case and the reverse can happen in our experience. Difficulties in evaluation are particularly common with substances brought in by the patient for testing. It may be necessary to apply several concentrations at the first visit; controls should be performed with any substances giving positive reactions.
There is no substitute for a thorough knowledge and experience of the allergens used for patch testing. Even some standard allergens may be liable to induce weak false positive reactions (e.g. metal salts, fragrance mix, parabens mix, wool alcohols, carba mix). Repeat patch testing may be helpful, especially with a breakdown of the mixes.
Relevance of patch tests
Once a decision has been reached that a patient has an allergic positive patch test, it is important to establish relevance by carefully re-examining the patient‘s history, distribution of the rash and materials with which there has been contact. In many cases relevance can be clearly established and avoidance advice given. In some instances the relevance may be in the past, and may no longer apply. In other cases the relevance may be uncertain or impossible to ascertain. Patients will need to be advised on the potential sources of all their allergies for future reference, and if their problem is ongoing it may be necessary to reassess their exposures. A person may react to a patch test but still tolerate contact with the allergen. However, confusion can occur if they are given extensive avoidance advice for materials of no relevance to their dermatitis, and therefore advice should be targeted.
Non-invasive measurement techniques
There are several non-invasive techniques that can be used to quantify and delineate the effects of patch tests, including measurements of changes in skin surface, epidermal hydration and water barrier function, and parameters of inflammation. At present, these are more useful to the investigator than to the clinician. They include replica techniques, transepidermal water loss, skin reflectance, laser Doppler flowmetry, thermography and high-frequency ultrasound. Attempts have been made to use some of these techniques to differentiate irritant from allergic patch test reactions but they have not superseded the combination of human brain, eye and hand in the assessments of patch tests.
Sources of error
False positive reactions (Box 128.7). Irritant reactions occur if a chemical is tested at excessive concentration. Incorrectly interpreted false positive reactions may lead to the wrong conclusions about the cause of a dermatitis, and this may result in inappropriate career or medicolegal advice. Recent or active dermatitis in the test area lowers the threshold for irritant reactions, as does dermatitis in other areas; non-specific reactions can also occur. Secondary non-specific reactions close to genuine positive ones have been termed ‘angry back’ or the ‘excited skin syndrome’ [25], and this may be an important cause of false positive patch test reactions. The phenomenon has been extensively investigated by Bruynzeel [26]. However, multiple positive reactions to nickel did not cause ‘angry back’ in a study by Andersen et al. [27].
If there is any doubt, the patch tests should be repeated some weeks later, preferably with individual agents and at various dilutions, as false positive irritant reactions tend to stop abruptly below a certain concentration whereas allergic responses tend to persist, albeit proportionally weaker, at lower concentrations. Testing the same substance on a panel of controls, using the ROAT on the elbow flexure or usage tests, may help to differentiate allergic from irritant responses. Controls should be tested at the lowest concentration of a positive test to avoid interpreting a false positive irritant reaction as allergic.
False negative reactions (Box 128.8). Sometimes a patch test fails to provoke a positive reaction in a person who is sensitive to the substance tested. The dermatitis therefore persists because of continued exposure to the allergen. The most common cause of false negative reactions is insufficient penetration through the skin. A low degree of sensitivity or poor penetration sometimes results in a long period of latency before a positive reaction develops, so that up to 7.5% of allergic positive patch tests do not become apparent until after 4 days, and may go unnoticed unless read 7–14 days after application. This particularly applies to neomycin and corticosteroids.
The apparent discrepancy between the concentration of allergen needed to elicit clinical dermatitis and the occasional failure of a patch test to elicit a reaction can be explained by many factors. In particular, a single exposure on normal skin is probably not representative of the accumulation of the allergen during repeated exposure conditions and chronic usage on already primed skin.
False negative reactions are common when testing with textiles, cosmetics, medicaments, leather and rubber, as some ingredients are present in very low concentrations. False negative reactions also occur when allergens are present in irritant products. Because of irritancy, a product may have to be diluted to such an extent before it can be safely tested, that the allergen is present in insufficient concentration to elicit a response. Such products include cutting oils and washing materials. Sensitivity to finished products and topically applied preparations is best confirmed and revealed by testing with the individual components.
An allergy may be missed on patch testing if the test material has been wrongly diluted in a material in which it is immiscible or insoluble. Furthermore, an incorrect diluent may change the allergen into another substance altogether. Partition coefficients are also important, because oil/water solubility may be a significant factor in skin penetration and allergenic potential.
Local treatment with topical corticosteroids, and systemic treatment with immunomodulators including ciclosporin, azathioprine and corticosteroids such as prednisolone (at a dose above 15–20 mg/day), may diminish or abolish reactions [28], as does preceding sunbathing [29]. Negative reactions, in spite of clinical sensitivity, also occur in photocontact dermatitis if appropriate allergens are not photopatch tested.
Compound allergy [30]. Compound allergy occurs when a positive allergic patch test reaction is seen to a finished product but tests with the ingredients are negative. Hence, the product and the constituents should be patch tested when allergy is suspected.
New compounds may be formed within a product, and their presence can be confirmed by the finding of incongruous peaks on spectrometry. This was elegantly demonstrated in Hirudoid cream, where a new allergen was formed as a reaction product of two preservatives in the medicament [31]. The additive effect of multiple weak sensitizers [32], or the additive effect of weak allergens and irritants, should be considered [33].
There are several possible alternative explanations. The reaction to the finished product may be irritant. A product‘s irritancy is not merely the sum of the irritancy of the ingredients, but an expression of the hydrophilic–hydrophobic balance of its ingredients. This can change with varying manufacturing techniques, for example changing the temperature or manipulating the proportion of one of the ingredients. A constituent allergen may be an undeclared ingredient or there may be batch/source differences between the original compound and the subsequently provided components. The allergen may be in the container, for example a rubber stopper, and not in the product. The allergen may not have been tested in the correct vehicle or at the correct concentration, and testing it in its own base may reveal the allergy.
Quenching. Theoretically, just as there may be potentiation of allergic and irritant responses, so a combination of chemicals may lead to a quenching effect. This phenomenon has been investigated mostly in fragrance material aldehydes. It might be explained by the combined compounds changing available bonding sites for class II molecules or forming a compound that does not follow the same detoxification pathway. However, some authors have been unable to demonstrate any physicochemical interaction, and its existence has been questioned [34].
Other observed quenching effects may be due to one of the compounds having anti-inflammatory properties [35], such as triclosan having a ‘quenching’ effect on nickel allergic contact dermatitis.
Other factors. The interpretation of patch test reactions can be affected by the presence or absence of impurities or degradation products, hidden additives, batch differences and the fact that some chemicals may undergo reactive metabolic changes in the skin. Natural products vary according to source, season and method of extraction. Storage or ‘ageing’ of a product may also affect its allergenicity and irritancy; d-limonene has been shown to be allergenic only in its old and oxidized state [36]. Patients should therefore always be tested with their own product. Season may also influence patch test results, but whether this is due to UV radiation suppression of test reactions in summer or an enhancement of irritant-type reactions in winter remains uncertain [37].
Errors may occur in the registration of the relative sites of the tests. It is therefore advisable to repeat the test if in doubt.
Selection of test substances
The decision about what to test is dependent on a sound knowledge of the common sensitizers, in conjunction with a thorough history of exposure. Fortunately, a high proportion of cases of contact dermatitis are caused by sensitivity to a small number of contactants, although there are potentially thousands. In relatively few cases of contact dermatitis are the clinical appearances and history so typical that an allergen can be incriminated readily.
It is therefore essential to test with a standard series of common contact allergens. Many investigation clinics have extra allergens and some of these may be grouped into additional special test series (e.g. for certain occupations or affected sites). Furthermore, it may be necessary to test with materials encountered in patients‘ working and domestic environments, and with any medicaments and cosmetics applied to affected areas.
Baseline series
The principle of screening all patients with a series of allergens commonly encountered in their environment is now well established. Aimed patch testing is ill-advised. The decision as to what should be in the standard series has now generally devolved from the ICDRG to other national and international groups. The baseline series recommended by the EECDRG contains 28 allergens [38]. The BSCA presently has 39 allergens for their standard series (Table 128.9) [39]. The most recent standard screening results published by the NACDG includes 65 allergens [40]. As some allergens disappear from a given environment and others attain significance, it is important that a baseline series evolves.
Table 128.9 Comparative lists of allergens in European and British standard series.
| ESCD/EECDRGa | BSCAa | |
| Potassium dichromate | 0.5 | 0.5 |
| Neomycin sulphate (fradiomycin) | 20 | 20 |
| Thiuram mix | 1 | 1 |
| p-Phenylenediamine (PPD) base | 1 | 1 |
| Cobalt chloride (CoCl2·6H2O) | 1 | 1 |
| Benzocaine | 5 | – |
| Formaldehyde | 1 (aq.) | 2 (aq.) |
| Colophony (colophonium) | 20 | 20 |
| Clioquinol | 5 | – |
| Balsam of Peru (Myroxylon pereirae) | 25 | 25 |
| N-isopropyl-N′-phenyl-p-phenylenediamine | 0.1 | 0.1 |
| Wool (lanolin) alcohols | 30 | 30 |
| Mercapto mix | 2 | 2 |
| Epoxy resin | 1 | 1 |
| Parabens mix | 12 | 12 |
| p-Tertiary-butylphenol formaldehyde resin | 1 | 1 |
| Fragrance mix I | 8 | 8 |
| Quaternium-15 | 1 | 1 |
| Nickel sulphate (NiSO4·6H2O) | 5 | 5 |
| Methylchloroisothiazolinone/methylisothiazolinone | 0.01 (aq.) | 0.02 (aq.) |
| Mercaptobenzothiazole | 2 | 2 |
| Primin | 0.01 | – |
| Sesquiterpene lactone mix | 0.1 | 0.1 |
| Tixocortol pivalate | 0.1 | 1 |
| Budesonide | 0.01 | 0.1 |
| Methyldibromo glutaronitrile | 0.5 | 0.3 |
| Fragrance mix II | 14 | 14 |
| Hydroxyisohexyl 3-cyclohexene carboxaldehyde | 5 | 5 |
| Compositae mix | – | 2.5 |
| Quinoline mix | – | 6 |
| Methylisothiazolinone | – | 0.2 |
| Imidazolidinyl urea | – | 2 |
| Diazolidinyl urea | – | 2 |
| 2-Bromo-2-nitropropane-1,3-diol | – | 0.5 |
| Sodium metabisulphite | – | 1 |
| Amerchol L101 | – | 50 |
| Chloroxylenol | – | 0.5 |
| Chlorocresol | – | 1 |
| Carba mix | – | 3 |
| Ethylenediamine dihydrochloride | – | 1 |
| Caine mix | – | 10 |
| Cetearyl alcohol | – | 20 |
| Fusidic acid | – | 2 |
| Chlorocresol | – | 1 |
| Disperse Blue mix | – | 1 |
aConcentrations are quoted as percentages in petrolatum except where otherwise stated.
aq, aqueous; BSCA, British Society of Cutaneous Allergy; EECDRG, European Environmental and Contact Dermatitis Research Group; ESCD, European Society of Contact Dermatitis.
In the past, several common sources of contact dermatitis were overlooked until they were included in a baseline series. Nowadays, fragrance materials are familiar contact allergens but were virtually unknown 40 years ago [41]. Conversely, others (e.g. wood tars, turpentine) were removed from the baseline series some years ago. Newer baseline allergens recommended for Europe include fragrance mix II and the preservative methylisothiazolinone, which is currently causing an epidemic and should be tested on its own in addition to the MCI/MI mix. The European standard series identified 75–80% of all allergies diagnosed in one multicentre study [42].
In some studies as many as half of the relevant positive reactions were unexpected. Obviously, if patch testing is carried out for very wide indications, the percentage of negative reactions will increase, but at the same time unexpected positive reactions will correct misdiagnoses of constitutional or irritant dermatitis.
The selection of substances for a baseline patch test series must be based on local experience, but several substances are universally recognized allergens. Unless a permanent record is kept, a number of substances will continue to be included despite a low yield of positive reactions. In general, a substance should be included in the standard battery if it gives positive reactions in more than 1% of those tested, or if without it a significant number of unsuspected allergic reactions would be missed. This is true of ubiquitous allergens such as rubber chemicals, nickel and chromate, fragrance materials and common therapeutic and cosmetic allergens such as lanolin, neomycin and preservatives. However, less common allergens may be included if they are potentially easily overlooked and are important. The results of testing to a standard series of allergens vary from one part of a country to another, and from one country to another (Table 128.10) (B. N. Statham, personal communication; [40, 43, 44, 45]). In most countries, additions to the international standards are required. In order to reduce the number of tests, defined groups of substances can be made up as ‘mixes’.
Table 128.10 Comparative results of patch test series (expressed as percentage positive).
| UK (BSCA)2007 | Europe1996–2000 | USA2007–2008 | Japan1994 | |
| Metals | ||||
| Nickel sulphate | 21.0 | 17.9 | 19.5 | 13.5 |
| Cobalt chloride | 6.3 | 5.9 | 8.2 | 17.3 |
| Potassium dichromate | 2.5 | 4.6 | 4.1 | 9.2 |
| Rubber chemicals | ||||
| Thiuram mix | 2.6 | 3.2 | 3.4 | 2.6 |
| Carba mix | 2.2 | NT | 4.5 | 0.5 |
| Mercapto mix | 0.7 | 1.0 | 0.6 | 0.6 |
| IPPD/black rubber mix | 0.3 | 0.9 | 2.0 | 1.2 |
| Pharmaceuticals | ||||
| Caine mix/benzocaine | 1.1 | 1.6 | 1.3 | 1.8 |
| Neomycin sulphate | 2.5 | 3.0 | 10.1 | 4.0 |
| Quinoline mix/chinoform | 0.5 | 0.8 | NT | NT |
| Ethylenediamine dihydrochloride | 1.1 | NT | 1.5 | 0.3 |
| Parabens | 0.7 | 0.6 | 1.1 | 1.8 |
| Lanolin alcohols | 1.8 | 2.9 | 2.1 | 2.8 |
| Tixocortol pivalate | 1.5 | 1.3 | 2.5 | – |
| Budesonide | 0.7 | 1.6 | 0.9 | – |
| Cosmetic ingredients | ||||
| Balsam of Peru (Myroxylon pereirae) | 6.6 | 6.0 | 11.0 | 5.2 |
| Fragrance mix | 8.1 | 9.7 | 9.4 | 5.8 |
| Formaldehyde | 2.3 | 2.3 | 7.7 | 1.2 |
| Quaternium-15 | 2.1 | 1.3 | 8.6 | NT |
| Methylchloroisothiazolinone/methylisothiazolinone | 1.9 | 2.2 | 3.6 | 1.3 |
| Methyldibrom glutaronitrile | 1.2 | NT | 5.5 | – |
| Plants | ||||
| Sesquiterpene lactone mix | 1.1 | 0.7 | 0.6 | NT |
| Primin | 0.2 | 1.1 | NT | 0.7 |
| Miscellaneous | ||||
| p-Tertiary-butylphenol formaldehyde resin | 0.7 | 1.3 | 1.2 | 1.7 |
| Epoxy resin | 1.0 | 1.3 | 1.7 | NT |
| Colophony | 3.8 | 4.0 | 2.8 | 2.3 |
| p-Phenylenediamine | 3.8 | 3.9 | 5.3 | 6.1 |
BSCA, British Society of Cutaneous Allergy; IPPD, N-isopropyl-N'-phenyl-p-phenylenediamine; NT, not tested.
Additional series
There are many situations in which additional series of allergens are useful, for example in the investigation of dermatitis occurring in certain sites liable to medicament allergy (eyes, ears, perineum and venous ulcers/eczema) or sensitization from components of shoes or clothing. Some occupational groups, for example hairdressers, florists, dentists and metal machinists, are exposed at work to a variety of potential allergens not found in the standard series. Others may handle a specific group of allergenic chemicals, for example epoxy or acrylic resins. The main patch test allergen producers now market extra series, although these may have to be further adapted to local habits or occupational exposures. Allergens provided by commercial allergen manufacturers tend to be of pharmaceutical grade, and may be negative when the actual sensitizer is an impurity in a commercial-grade product.
Other materials
Commercially produced patch test allergens, either singly or in small numbers, may be applied where relevant. Patients may bring a wide variety of materials from their own from home or work for testing and, as mentioned previously, these must be thoroughly assessed and diluted appropriately before being tested. In those units with access to thin layer chromatography there is the opportunity to patch test with extracted components of textiles, plastics, plants and other materials [46].
Concentrations and vehicles for patch testing
Recommended patch test concentrations and vehicles for many different materials, including specific chemicals, chemical groups and substances, and finished products, have been collated in a number of standard contact dermatitis references given in the resources list at the end of this section. Most (but not necessarily all) of these lists are reliable, in that the stated concentrations do not usually give an irritant effect. Before patch testing with any unfamiliar material, the appropriate vehicle and concentration should be sought from one or more of these databases.
Complications of patch and photopatch tests
Generally, the risks of patch testing when it is performed correctly are minimal, but there are a number of potential complications (Box 128.9).
Positive reactions may spread locally and cause a flare of contact dermatitis at the original site or more generally. The long strips of adhesive semiocclusive tape, which preclude bathing for several days, may lead to eczema, itching or folliculitis, especially with high temperatures and humidity. In warm weather there may be leakage of the test materials on to clothing, and patients should be advised to wear an old shirt or blouse during the test. Irritants at excessive concentrations may induce caustic burns and scarring, and even a strong allergic reaction that might leave a scar on extremely rare occasions. Secondary infection of a positive reaction is virtually never a problem.
Short-term post-inflammatory hypopigmentation does occur occasionally following positive patch tests, but more permanent hypopigmentation may develop, including koebnerization of vitiligo (Figure 128.31). Post-inflammatory hyperpigmentation may also develop, although this is usually temporary. Phototoxic substances may cause pigmentation if exposed to UV light from photopatch tests or natural sunlight.
Figure 128.31 Persistent hypopigmentation after patch tests.
Significant epidermal detachment has been seen in patients with blistering disorders including pemphigus foliaceus and Hailey–Hailey disease after removal of the patch test units [47, 48].
Short-lived, non-immunological, urticarial reactions are common, particularly from cinnamates and sorbic acid. More importantly, anaphylactic reactions are a potential risk when patch testing with some materials, especially natural rubber latex [49] and penicillin. A history of immediate hypersensitivity to rubber should be sought before patch testing with latex.
Active sensitization
Patch testing involves a small risk of sensitization. A reaction appearing 7 or more days after the application may indicate either delayed expression of a pre-existing sensitivity or sensitization from the patch test. However, some late reactions, occurring up to 14 days after the application of patch tests, are weak sensitivities from poorly penetrating allergens. Active sensitization usually presents as a strong positive patch test occurring at around 3 weeks. Few clinics observe their patients long enough to note such reactions, but patients report them. The true incidence of sensitization is therefore difficult to establish, because even re-examination of a random sample of the patients tested cannot differentiate between those sensitized by patch testing and those whose pre-existing subliminal sensitivity has been boosted by further exposure from patch testing. Patch test sensitization from most routinely tested substances is very uncommon, and occurs more frequently when new substances are being investigated to ascertain the correct patch test concentration. Sensitization is more common when testing with unrefined wood or plant extracts or with material provided by the patient.
The possibility of active sensitization to PPD has caused the German Contact Dermatitis Research Group to remove it from their standard series pending further evaluation. However, an in-depth study looking at patients who were repeat patch tested over a 10-year period has shown that the maximal sensitization rate was at the most 0.3% for PPD [50]. The authors therefore support its continued retention in the standard series.
Testing itself may cause a reawakening of sensitivity. However, the practical consequences of this are uncertain. Patients who can be resensitized by patch tests must also be easily resensitized by contact with the allergen under everyday conditions.
Such induced sensitivity tends to fade relatively quickly, and the patient‘s clinical course does not appear to be adversely affected. These rare adverse events are usually of no long-term consequence and must be balanced against the benefits of finding one or more relevant allergens. Rarely, persistence of patch test reactions may continue for several weeks unless treated. This has been especially noted with gold sodium thiosulphate [51].
Multiple patch test reactions
The finding of multiple positive patch tests is common, and it is important to consider the reasons for this so that the correct advice can be given to the patient. The main explanations are:
- Non-specific hyperreactivity.
- Multiple primary hypersensitivities.
- Cross-reactions (true and false).
Non-specific hyperreactivity
Ideally, patch tests should be applied at a concentration that always identifies the allergen and never induces false positive reactions. Unfortunately, some allergens have to be applied at a concentration that is marginally irritant in some subjects in order that allergic positive reactions are not missed. The threshold at which a false positive irritant reaction develops differs from individual to individual and may even be variable in the same subject. During active dermatitis, uninvolved skin, even at distant body sites, exhibits increased susceptibility to irritant reactions. This ‘status eczematicus’ may lead to false positive patch test results. It has become an established tenet that ‘eczema creates eczema’, and that a strongly positive patch test reaction may induce other non-specific false positive patch test reactions [52]. Such reactions occur more readily with marginally irritant chemicals. When this affects adjacent patch test sites it is often referred to as ‘spillover’, ‘excited skin’ or ‘angry back’. Rietschel [53] has proposed that ‘stochastic resonance’ may be involved. This suggests that there is signal amplification of immune-mediated events by neurological influences. The incidence has been variously assessed as 8.6% to 63.5% [54]. In view of these findings, it has been proposed that repeat patch tests should be undertaken in all individuals with three or more strong positive allergic reactions, with exclusion of the strongest reactants. However, some studies have not found evidence to support a concept of non-specific hypersensitivity [55].
The occurrence of weak false positive patch test reactions can be reduced by delaying patch testing until all active eczema has settled. As skin hyperirritability may persist for some weeks or months, even when the dermatitis has resolved, this is often impractical.
Multiple primary hypersensitivities
Multiple primary specific (or concomitant) sensitivities to substances that are unrelated chemically are frequent among patients with contact dermatitis. Among 5000 Scandinavian patients, they occurred in 20% of all persons tested. The reason why some patients develop multiple sensitivities and others do not is not clear. Patients with a long history of dermatitis are those most likely to accumulate several primary sensitivities, because of the opportunities to encounter new allergens under conditions favourable for sensitization. Patients with leg ulcers are especially prone to developing multiple allergies, as are patients with chronic actinic dermatitis. One sensitivity may predispose to the acquisition of another, and there may be a genetic or constitutional predisposition to acquire sensitivities [56].
Sensitization is facilitated if an allergen is applied on injured (e.g. eczematous) skin [57], particularly if it is occluded, and such local factors may be sufficient to explain the frequency of sensitivity to topical medicaments and simultaneous sensitivity to several constituents. In dermatitis from applied medicaments, concomitant sensitivity to both an antibiotic and an unrelated component of the vehicle is quite common. Similarly, in rubber dermatitis, sensitivity to unrelated vulcanizing agents is not unusual.
In dermatitis of the feet, concomitant sensitivity to chromate, rubber and dyes in shoes or stockings presents a particularly difficult clinical problem; one allergen may be primarily responsible but others are important in maintaining the eczematous state. The inflammatory response to allergens has been shown to be additive [58], as has the response to an allergen and an irritant [59].
Cross-reactions
Cross-sensitization is defined as the phenomenon where sensitization engendered by one compound, the primary allergen, extends to one or more other compounds, the secondary allergens, as a result of structural similarity [60]. The proposal is that the primary and secondary allergens are so closely related that sensitized T cells are unable to distinguish between them, and therefore react as if the compounds were identical. Aromatic compounds with a ‘para’ group, for example PPD, benzocaine, procaine, sulphonamides, mesalazine, diaminodiphenylmethane and PABA UV filters, may all cross-sensitize. Aminoglycosides may do the same to a varying degree. Other examples include chlorocresol and chloroxylenol, as well as corticosteroids of a similar structure. Enantiospecificity or stereospecificity may lead to cross-reactivity with some isomers and not others. Examples include usnic acid, 4-methoxydalbergiones and frullanolides. A computerized resource has been used for the systematic evaluation of structure–activity relationships.
Contaminants may cause ‘false’ cross-sensitivity as one substance may contain traces of another. In studies of cross-sensitivity, absolutely pure test substances must be used. Few investigations in the past have fulfilled these requirements, and most should be repeated using modern methods of separation.
In simultaneous sensitivity to natural products such as perfumes, balsams and wood tars, it is impossible to decide whether reactions to several of the substances may be due to related or identical chemicals. Cinnamic aldehydes, for example, may occur in them all. The same applies to plants such as Compositae (Asteraceae), Frullania and Toxicodendron species.
Other tests
Occlusive patch testing has stood the test of time. Although it is an artificial procedure, it has not been superseded. Nevertheless, alternatives continue to be sought and some of these may be useful adjunctive investigations.
Open tests
Patch testing is usually performed with the test site occluded, in order to increase percutaneous absorption. This is an artificial procedure, and clinical exposure might be more closely simulated by simple application of the sensitizer to uninvolved skin. However, few allergens provoke dermatitis with a single exposure on normal skin. It is seen in Primula dermatitis, some patients having positive, even bullous, reactions to an open patch test to the leaf. In most cases of clinical contact dermatitis, however, the allergen gradually accumulates in the epidermis, and irritants and mechanical injury promote its absorption. These conditions are not readily reproduced in a test procedure.
In highly sensitive individuals, allergens with good penetration can produce positive reactions in an open test, although the concentrations used for testing must be much higher than those used in a closed test. Thus, potassium dichromate 5% in water and nickel chloride 10–20% in alcohol provoke a positive reaction in many chromate- or nickel-sensitive persons [1]. Positive reactions often develop in a few hours.
The technique is simple. The liquid test substance is dropped on an area of skin measuring about 1 cm in diameter and the solution is allowed to dry. The time for reading and the characteristics of the reaction are the same as for closed patch testing. The reaction can be followed from the start and may develop sooner than with a closed patch test reaction. It is often weaker, and a positive reaction, especially in the initial phase, may consist of isolated papules only.
One situation where open testing has been widely used and advocated is prior to dyeing hair. Application of the dye to the retro-auricular area and examination of the site 2 days later was shown to be an accurate method of detecting sensitized subjects [2]. However, hairdressers and individual users tend to do this only once and not each time the hair is tinted, and often they mistakenly undertake a 30 min reading. They may therefore miss the allergy if it develops subsequently.
With irritants, the reactions are also usually fewer and weaker in open than in closed patch testing because of reduced absorption. Open tests are therefore sometimes used as a preliminary screening procedure with less well-known substances to reduce the risk of severe reactions. However, experience with open tests is limited and the risk of sensitization cannot always be estimated.
Usage tests
In cases of doubt, when either a closed patch test or open test is negative yet the history suggests a contact dermatitis, the patient can be asked to use the preparation again. This is especially helpful with cosmetic and clothing dermatitis. Because it reproduces all the other factors associated with the original dermatitis, for example sweating, friction and application of allergen on damaged or pre-sensitized skin, it is sometimes positive when conventional patch tests fail to reveal sensitivity. However, it is not always possible to differentiate between an allergic and a non-specific or irritant response. With cosmetic preparations or medicaments, a repeat ‘dab’ test may be performed on previously affected skin.
Repeat open application tests
In this test, substances are applied twice daily for up to 4 weeks or until an eczematous reaction develops [3]. The most appropriate site is the upper arm or flexor surface of the forearm, as patients can perform the test and observe any developing reaction. They should be told to discontinue the application if eczema occurs. An area of at least 5 cm2 should be employed. The test may be used to determine the relevance of doubtful positive patch test reactions to preparations in which the putative allergen is present in a low concentration, although false negative results may occur. It may also establish the clinical relevance of such products and confirm the source of the allergy. A scale for recording ROAT reactions has been proposed and advocated [4].
Intradermal tests
Intracutaneous tests, as used in tuberculin sensitivity, have also been performed with simple chemicals, although mainly for investigative purposes. Within 1 day, erythema and swelling appear at the site of injection. Later, usually after 2–4 days, papules or vesicles may develop. Sometimes, a flare may be seen shortly after the injection, and this lasts a few hours. Over the next few days, erythema and infiltration are sometimes seen along the lymphatics leading from the test site.
Technical pitfalls with intracutaneous testing are numerous, as is known from extensive studies with tuberculin. Too deep an injection will result in negative reactions. An immediate reaction, which is not a rarity with metal salts, may result in dilution and removal of the test substance. The concentrations employed should be at least 10–100 times lower than those used for epicutaneous testing [5]. In cases of extreme sensitivity, the concentration may need to be 1000–10 000 times lower.
The technique has proved reliable for nickel and may identify allergy in patients with false negative patch tests. It has proven particularly helpful in the investigation of corticosteroid allergy [6].
In vitro tests
The principle of diagnosing contact allergy by in vitro testing is attractive, although the use of peripheral blood as a routine investigation for contact dermatitis may not be viable, not only from the budgetary point of view but also for logistical and practical reasons. Nevertheless, attempts continue to be made to achieve this, albeit with single or small numbers of allergens. A number of different techniques have been tried and these are described below.
As yet, none of these tests is a substitute for the in vivo system of the challenge patch test. However, they may be helpful in elucidation of the immune cascade as they are based on measurements of products from T-cell activation. They may also have a role in looking at cross-sensitivity patterns.
Migration inhibition test. Migration inhibition factor is a soluble factor released by sensitized lymphocytes following stimulation. It inhibits the migration of monocytes and macrophages but not polymorphonuclear leukocytes. A direct and indirect assay is performed and the results expressed as a migration index. The test has been employed in nickel and chromate allergy, but is not completely reliable as there is overlap between sensitized and non-sensitized patients. Interference may be caused by a cytotoxic action of the allergens, and concentrations have to be optimum at just below non-toxic levels. The technique was not reliable as an investigation for medicament contact allergy [7], but a capillary tube assay has been demonstrated to be of practical clinical value for diagnosing chromium allergy [8]. The method requires further investigation and refinement.
Lymphocyte transformation tests. Antigens are able to induce specific transformation of lymphocytes to large lymphoblasts, culminating in mitosis. Most work has been carried out on nickel allergy [9, 10], although there are conflicting accounts of the optimum stimulatory concentration of nickel and the method of preparation of cells following culture for radioactive thymidine uptake assays. Nickel may induce non-specific transformation in non-allergic subjects, but several groups of investigators have reported a significant difference between lymphocyte transformation in nickel-sensitive patients and controls. The assay may be enhanced by adding a combination of cytokines to the culture [11]. Other standard-series allergens investigated, with potentially promising results, include fragrance, chromate, PPD, neomycin sulphate and thiuram [12, 13], but testing with a range of medicament allergens failed to reach statistical significance [7].
Measurement of cytokine levels after culture may also improve the accuracy of the in vitro test and, depending on the study, raised levels of IL-2, IL-4, IL-5, IL-10, IL-13 and IFN-γ have all been noted [14, 15]. Unfortunately, many haptens are toxic to lymphocytes even in low concentrations [16].
Leukocyte procoagulant activity. When stimulated by an antigen, leukocytes produce a significant level of procoagulant that activates the extrinsic cascade of blood clotting. Its activity is measured as a ratio of the clotting time of plasma incubated with cells, with and without antigen. It has been used in nickel-sensitive patients [17], when the procoagulant activity increased as the stimulatory nickel concentration increased.
Spot tests
Two spot tests are of particular practical value in the patch test clinic as the materials are easy to handle and store.
Dimethylglyoxime test for nickel [18]. Dimethylglyoxime 1% (alcoholic solution) and ammonium hydroxide (aqueous solution) are stored in separate bottles. A few drops of each are put in separate clean white saucers, a cotton bud is then dipped in each of these and rubbed on the surface of the test object. A pink coloration on the cotton bud denotes the presence of nickel (Figure 128.32). This test is accurate to 10 ppm of nickel, but the immune system may be able to detect lower levels than this. It is a very useful test, and patients can be given kits to test items in the home and at work.
Figure 128.32 Dimethylglyoxime test: a pink colour is detected when metals release a significant amount of nickel. (Courtesy of Dr J. D. Wilkinson, Amersham General Hospital, Amersham, UK.)
Acetylacetone method for formaldehyde [19]. The reagent is prepared by dissolving 15 g of ammonium acetate, 0.2 mL of acetylacetone and 0.3 mL of glacial acetic acid in 100 mL of distilled water. It can then be stored in a refrigerator. A sample (1 mL or 1 mg) of the product to be tested is put in a disposable glass test tube and 2.5 mL of the reagent is added. The mixture is shaken and stoppered and then placed in a water bath at 60°C for 10 min. A yellow colour is produced in the presence of formaldehyde, due to the formation of 3,5-diacetyl-1,4-dihydrolutidine. The alternative chromotropic acid method is less specific.
Other analyses. There are other spot tests for chromate and epoxy resin [20], but these are not simple to perform during a clinic. More sophisticated tests such as chromatography, spectrophotometry, mass spectrometry and nuclear magnetic resonance spectroscopy require specialized equipment and expertise.
Management
General principles
Avoidance advice
A diagnosis of allergic contact dermatitis is reached on the basis of a detailed history and examination followed by patch tests, with an assessment of the relevance of any positive reactions. Once a diagnosis has been made, possible sources of exposure to the causative allergen(s) should be identified, and avoidance advice given. In some instances, particularly of work-related problems, appropriate protective clothing or changes in handling technique may be advised. Materials used for protection, especially gloves, should not allow penetration of the allergen responsible for the dermatitis.
The first principle of management is to give advice on avoidance tailored to an individual. Examples of specific avoidance measures include plastic instead of rubber gloves, cosmetics and medicaments free of an identified allergen, and clothing free of nickel-containing studs, zips, etc. More general written information on the allergen sources may be helpful, but may also be confusing if many are not relevant to that person.
Ideally, the result of this advice will be a resolution of the dermatitis, but this does not always occur, and other factors, such as the possible contribution of irritant or constitutional eczemas to persistence of the problem, should be considered and discussed with the patient. The possibility of non-compliance with avoidance advice should be considered. Factors affecting compliance include social and educational status of the individual and lack of resources [1]. Reassessment and reinforcement of avoidance measures is often required, sometimes repeatedly, in order that patients are fully aware of what action they should take. In some patients continued exposure is unavoidable, but can be reduced to a sufficient degree to keep the dermatitis at an acceptable level. It is advisable to stress that allergy does not disappear when the dermatitis clears but that the risk of relapse after further contact with the allergen persists throughout life.
Active treatment
The mainstay of treatment of allergic contact dermatitis is avoidance of the causative factor(s), although topical corticosteroids will be required in most instances to control the disorder. The manner in which they are used will vary, and optimum regimens have yet to be established [2]. Potent steroids are typically used on the hands [3]. The general principles of treatment are the same as for other forms of eczema. In particular, the regular and liberal use of hydrating emollients and soap substitutes is recommended. Fissures of the fingers, palms and soles can be covered with hypoallergenic tape. Alternatively, zinc and salicylic acid paste BPC twice daily may be helpful, and cyanoacrylates (superglues) have been used with benefit.
Recalcitrant, disabling cases may require consideration of systemic therapy such as alitretinoin, azathioprine and ciclosporin. There is evidence in animals that ciclosporin suppresses allergic contact dermatitis [4], but most reported clinical studies have been undertaken on chronic hand eczemas of mixed aetiology. Superficial X-rays and Grenz rays, which have been shown to suppress experimental contact dermatitis [5], can be safely used for localized dermatitis, although facilities for this treatment are gradually dwindling in the UK. Phototherapy, both PUVA and UVB, is helpful in some subjects, including Compositae-allergic individuals with photosensitivity [6].
The use of barrier creams as preventatives in already sensitized persons is generally unsatisfactory. However, there is documented evidence of the value of products containing quaternium-18 bentonite in the prevention of Toxicodendron spp. dermatitis [7]. Other barrier creams containing active agents (e.g. chelating agents) against specific allergens may have future potential [8, 9]. In one study clioquinol was the most effective agent at preventing nickel dermatitis [10].
It has been reported that certain patterns, especially vesicular palmar eczema, have benefited from dietary avoidance or reduction in the intake of allergen, most notably nickel and balsams, in sensitized subjects [11, 12]. The effects of a low-nickel diet have been disappointing in our experience; nevertheless, there are strong advocates of these measures. Dietary chelation of nickel has also been attempted [13], but is not widely used in practice because of side effects.
Dietary manoeuvres have also been reported to be helpful for cheilitis and oral symptoms, particularly in those with positive patch tests to balsam of Peru, cinnamates, eugenol, colophony, flavours and antioxidants [14]. Diets for other food-related preservative allergens have been developed [15]. However, the relationship between ‘burning mouth’ and contact allergy is questionable [16].
Hyposensitization [17]
Many attempts have been made to down-regulate the immune response to allergens in an already sensitized individual. This has proved difficult to realize in practice. The degree of hyposensitization achieved by oral doses of allergens is limited and transient, for example DNCB and chromate in guinea pigs, and poison ivy in humans. Although it has been attempted for Toxicodendron spp. allergy [18], oral hyposensitization is not routinely recommended. Some success has nevertheless been claimed in India for hyposensitization against Parthenium hysterophorus [19].
Prevention
Many statutory bodies have a role in the prevention of contact dermatitis, including medical personnel, legislative bodies, central and local government, corporate industry, the media, surveillance and consumer bodies, and patient support groups. Principles of prevention can be related to two categories, individual and collective, and further divided into primary, secondary and tertiary [20]. Primary prevention focuses on the induction of contact sensitization and control of exposure. Secondary prevention relates to elicitation, and tertiary to measures for established and continuing dermatitis. Some of the more important elements of prevention are discussed below.
Allergen containment and replacement [21]
Potent allergens encountered in industry can be kept in closed systems, thereby avoiding the potential for direct skin contact. In other instances products can be kept in special containers, which allow a no-touch technique when using the contents. Replacement and elimination of potential allergenic hazards can be helpful in both the domestic and working environments, for example perfume-free cosmetics and medicaments, non-latex gloves, high-molecular-weight epoxy resins, and white spirit instead of turpentine.
Legal and other regulatory measures [22]
Regulatory measures can influence the incidence of dermatitis [23]. They may be legally or voluntarily enforced. The EU has passed a number of directives relating to contact dermatitis, particularly in relation to nickel, chromate and cosmetics.
As most consumers are primarily sensitized to nickel either following ear piercing or by prolonged close contact with nickel-releasing alloys, it was proposed that such items should not release more than 0.5 μg/cm2/week of nickel. Ten per cent of the female population of Europe and the USA are sensitive to nickel, and this has significant implications with regard to hand eczema and employment; hence, nickel sensitivity is an issue where legal restraints could prove effective in improving the health of the population. Following the lead of Denmark and Sweden, the ‘Nickel Directive’ was introduced in 1994 with the aim of primary and secondary prevention of the high levels of nickel allergy in the EU [24]. The recommendations are summarized in Box 128.1. A Danish follow-up study comparing 1985–86 with 1997–98 patterns of nickel sensitization has already shown a decrease in allergy from 24.8% to 9.2% in the tested populations aged under 19, and a significant drop in the frequency of females with nickel allergy under the age of 30 has been seen in Germany since 1994 [23].
Dermatitis accounts for a significant proportion of occupational disease. Allergy to chromate in cement is a significant problem in the construction industry. Since the early 1980s some Scandinavian countries have restricted hexavalent chromium in cement to below 2 ppm, which is achieved by adding ferrous sulphate. Subsequently, there has been a reduction of chromate allergy amongst construction workers [25]. In 2005, the EU followed suit by directing that levels of hexavalent chromate in cement should be restricted to this level.
Latex contact urticaria also resulted in significant occupational and non-occupational disease that led to local, national and European glove-related legislation resulting in a reduction in sensitization [26].
The EU Cosmetics Directive lists materials as allowed, not allowed and restricted. For instance, the preservative MCI/MI is not permitted above 15 ppm and formaldehyde above 0.2% in cosmetic products. Methyldibromo glutaronitrile is a preservative that came into prominence in the 1990s when the frequency of allergy amongst patch-tested patients rose by a factor of 5 over the 10-year period [27]. As a result, steps were taken to ban its use, firstly in leave-on cosmetics, but later in wash-off products as well. Enforced ingredient labelling on the packaging of cosmetics, which is also a requirement in the USA, has been a major factor in enabling the avoidance of cosmetic allergens by sensitized customers. Since 2005, in the EU, labelling of certain fragrance substances (26 in all), at levels of >10 ppm for leave-on products and >100 ppm for wash-off products, has also been required.
The Directives on Dangerous Substances and Dangerous Preparations list 360 skin sensitizers and their concentration limits (e.g. formaldehyde 0.2%, acrylates 0.5–2%). A chemical product containing a classified skin sensitizer above 1% concentration must be labelled with the risk phrase ‘R43 – may cause sensitization by skin contact’. For many substances 1% is above the level of sensitization and elicitation of contact dermatitis. The usefulness of labelling in this unselective quantitative way has been questioned [28].
Some legislation may apply only to one or a few countries. For instance, the use of PPD in hair dyes is forbidden in some countries and controlled in many others. Formaldehyde in clothing is limited in Finland. Prohibition of persulphate improvers in flour in Denmark (1938) and Germany (1956) led to a striking decline in bakers‘ dermatitis in both countries. In Germany, the use of turpentine for paint is strictly limited [29]. In the UK, the assessment and monitoring of hazards and risks at work has improved following the introduction of the Control of Substances Hazardous to Health (COSHH) legislation, but attention to dermatitis checks and risks is still suboptimal [30]. In addition, the Health and Safety Executive have a statutory right to investigate skin problems at work through the Employment and Medical Advisory Service, provided they are reported [31].
Corporate responsibility
Although legal measures can influence the incidence of dermatitis, few have been introduced. In many instances governments will not intervene with legislation, relying on self-regulation, and this includes the cosmetic and pharmaceutical industries. The withdrawal of musk ambrette is an example of cosmetic industry self-regulation. Manufacturers of all goods should ensure that their products are safe to use, including the performance of pre- and post-marketing risk assessments. A risk assessment programme involves hazard identification, dose–response assessment, exposure assessment and risk characterization, including any potential for allergenicity [32, 33]. A product must be clearly labelled to ensure that it is handled safely.
Dermatologists and consumers have a pivotal role by alerting authorities to the emergence of both new and existing allergens within communities. National groups, surveillance systems, particularly of occupational dermatitis, or more comprehensive data networks such as the European Surveillance System of Contact Allergens, can feedback their findings to responsible agencies who can then respond to any concerns [34]. Rapid computerized analysis of epidemiological information, with feedback to interested parties, can provide early warning of new allergens and sources of work-related dermatoses.
Work
The preventative aspects of occupational contact dermatitis are discussed in detail in Chapter 130.
Domestic
The availability of modern domestic equipment should significantly reduce skin contact with irritants and potential sensitizers in the home; however, housewives are still one of the greatest ‘at-risk’ groups as far as the development of hand dermatitis is concerned. Cotton-lined gloves should be worn when the hands are in contact with irritants, including food, cleaning agents and polishes. Plastic gloves are less allergenic than rubber but are less pliable and malleable.
Education
Education of the community and workforces through the media, courses, lectures and wall charts in public places (including medical waiting areas) and at work will help to promote awareness of the problem of contact dermatitis. Skin protection courses and education have been shown to reduce occupational dermatitis [35]. Patient support groups have played an increasing role in education of the general public as well as those suffering from dermatitis.
Resources
Further information
De Groot AC. Patch Testing, 3rd edn. Wapserveen: Acdegroot Publishing, 2008 (and update at http://www.escd.org/education/Patch_testing_update_2008-2012.pdf; last accessed March 2015).
Johansen JD, Frosch PJ, Lepoittevin J-P, eds. Contact Dermatitis, 5th Edn. Berlin: Springer, 2010.
Johansen JD, Thyssen J, Lepoittevin JP. Quick Guide to Contact Dermatitis. Berlin: Springer, in press.
Lachapelle JM, Maibach HI. Patch Testing and Prick Testing: A Practical Guide. Official Publication of the ICDRG. Berlin: Springer, 2012.
Lovell CR. Plants and the Skin. Oxford: Blackwell Science, 1993.
Rietschel RL, Fowler JF. Fisher's Contact Dermatitis, 6th edn. Hamilton: BC Decker, 2008.
Rustemeyer T, Elsner P, John SM, Maibach HI, eds. Kanerva's Occupational Dermatology, 2nd edn. Berlin: Springer, 2012.
Photoallergic contact dermatitis
Definition and nomenclature
Photoallergic contact dermatitis (PACD) is a classic type IV cell-mediated hypersensitivity reaction of the skin in response to a hapten in a person who has been previously been sensitized to the same chemical or one that cross-reacts with it. The hapten is produced following UV exposure – resulting in a photoactivated chemical or a photoproduct.
Introduction and general description
Photoallergic contact dermatitis is a delayed type IV hypersensitivity reaction and has a similar presentation to other forms of allergic contact dermatitis but with a predilection for photoexposed sites, particularly during the initial stages. However, the dermatitis may subsequently involve covered sites due to the presence of circulating activated T lymphocytes, which then masks the underlying clues for considering diagnostic patch testing.
Both topically applied and systemically administered substances can produce photoallergic reactions. Systemic reactions are reviewed elsewhere and this section will focus on photoallergic reactions induced by topical agents on the skin in the presence of, or followed by, exposure to UV or visible light. It is much rarer than phototoxicity.
Epidemiology
Incidence and prevalence
Photoallergy is a relatively rare condition and adequate population studies have not been conducted. Exposure is essential for sensitization to develop.
Age
With increasing use of sunscreens, contact allergy is developing in children as a consequence of appropriate preventative measures to reduce sun-induced skin damage [1]. Contact allergy to octocrylene has been particularly associated with childhood use.
Ethnicity
Typically, sunscreens are used predominantly by those with a propensity to burn in the sun, that is with FitzPatrick type I and II skin.
Associated diseases
Photosensitive disorders require the use of sunscreen in the mitigation of symptoms. The presence of a photosensitive disorder is associated with the development of photoallergy with a reported wide incidence varying from 1% to 40%. Investigation by photopatch testing is essential in those with photosensitivity disorders. This variation in the reported incidence of photoallergy may result from differences in patient selection, photopatch testing methodology, the test battery that is used and interpretation of test results.
A European multicentre photopatch test study of 1031 patients provided information on the incidence of PACD and common photoallergens in Europe [2]. A total of 346 photoallergic reactions occurred in 200 (19.4%) subjects. PACD was most commonly caused by the topical NSAIDs ketoprofen (128 subjects) and etofenomate (59 subjects). Of the organic sunscreen absorbers, octocrylene, bemzophenone-3 and butylmethoxydibenzoylmethane most frequently elicited PACD. These results contradict previous literature and suggest that PACD might not be as infrequent as once thought, at least compared with allergic contact dermatitis to sunscreen ingredients.
Pathophysiology
Certain substances are transformed into sensitizers (photosensitizers) after irradiation with UV or short-wave visible radiation (280–600 nm). The wavelength required is usually, but not always, the same as the absorption spectrum of the substance [3].
The basic mechanisms of photosensitization have been reviewed by Thune [4]. The initial phase of all photoreactions is dependent upon absorption of photons by light-sensitive chemicals. Following absorption, a higher state of energy (excited state) is induced in the molecule (photoactivation). Some of the energy may be released as fluorescence – that is emission of radiation at a longer wavelength. Alternatively, there may be phosphorescence, heat or other energy transfer to another molecule, or photochemical alteration of the molecule [5].
Photoactivation is a physical phenomenon and may occur in vitro. When it occurs in vivo the activation may have a phototoxic (non-immunological) or photoallergic (immunological) action. The photoactivated molecules may be transformed into new substances capable of acting as irritants or haptens. Photoallergic reactions are based on immunological mechanisms, and can be provoked by UV radiation only in a small number of individuals who have been sensitized by previous exposure to the photosensitizer. The reaction to a photoallergen is based on the same immunological mechanism as contact allergic reactions. In guinea pigs the sensitivity can be transferred with mononuclear cells [6]. The action spectrum for photoallergy is generally in the UVA range but some elicit reactions in both the UVA and UVB range, as is the case with diphenhydramine hydrochloride and NSAIDs [7].
Newly formed haptens may, by virtue of the excited state and free-radical formation, be able to combine chemically with other substances, for example protein, to produce a complete antigen. The photoallergen tribromosalicylanilide has been shown to change into dibromosalicylanilide and monobromosalicylanilide [8], and with sulphonamides it has been suggested that an oxidation product is formed [9]. Some photosensitizers may, in the presence of UV radiation, produce only short-lived reactive molecules [10]. The duration of the response to light irradiation after stopping the application of a known photoallergen is variable and depends on the photoallergen. For sunscreens, for example, it is probably less than 4–6 days [11]. However, NSAIDs such as ketoprofen may cause subjects to react to sunlight up to several weeks after having stopped its local application. This is most probably due to retention of the molecule in the epidermis [12].
Several photoallergic substances simultaneously produce phototoxic reactions when applied in high concentrations and with a sufficient amount and type of radiation. Thus, in an individual case, the two reactions may be clinically indistinguishable.
Pathology
The histological changes are identical to those of other forms of contact dermatitis.
Environmental factors
Photoallergic contact dermatitis will only occur in the event of exposure to potential photoallergens in the environment. In the present era, UV-absorbing chemical filters and topical NSAIDs are the main substances causing clinical photoallergic problems. Exposure to UV filters is becoming more common as a result of their increasing use not only in sun products but also in cosmetics, including hairsprays. Their presence in cosmetics may be to prolong shelf-life and to support ‘antiageing’ claims for the products. These UV filters can also induce conventional contact allergy including in children. The other main group of photosensitizers are topical non-steroidal anti-inflammatory agents, especially ketoprofen. Ketoprofen may cross-sensitize with the UV filter benzophenone-3 and octocrylene (Figure 128.33) [13].
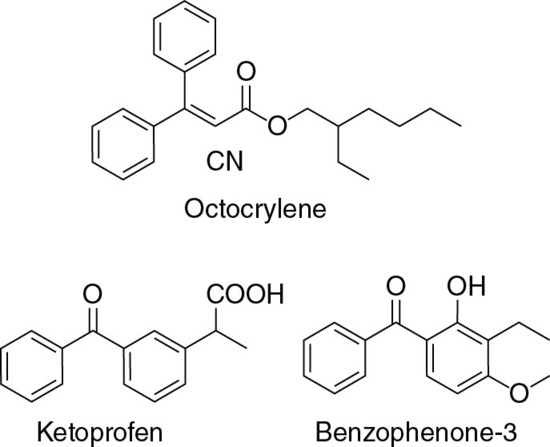
Figure 128.33 Structures of cross-reacting photoallergens. (Adapted from Karlsson et al. [12].)
Nevertheless, other photocontact allergens have been identified with varying degrees of confirmatory evidence, and these are summarized below.
- Perfumes: musk ambrette and 6-methyl coumarin, although now prohibited in Europe and the USA, caused significant problems in the 1980s. These products have now virtually disappeared.
- Halogenated salicylanilides: tribromosalicylanilide and tetrachlorsalicylanilide, used as antibacterials in soaps and detergents, caused many outbreaks of photosensitive eczema in the 1960s [10]. Fentichlor (bis(2-hydroxy-5-chlorphenyl)sulphide and bromosalicylchloranilide) is used as a topical antifungal agent in Australia and is used domestically in Sweden. It is a known photosensitizer.
- Phenothiazines: tranquillizers causing occupational dermatitis in hospital personnel, topical antihistamines and insecticides.
- Sulphonamides: used for topical treatment.
- Bithionol and hexachlorophene: used in toilet soaps, shampoos and deodorants.
- N-butyl-4-chlorsalicylamide: for example the antifungal Jadit.
- Quinines: hair tonic, quinidine, quindoxin and olaquindox used in animal feeds [14].
Cross-reactions between chemically related substances have been frequently reported.
Clinical features
History
The first significant recognized problems from photoallergy were related to the use of chlorinated salicylanilides in germicidal soaps in the early 1960s, with many thousands being affected. Regulatory elimination of these photoallergens resulted in the disappearance of the allergy, but some affected individuals became persistent light reactors [15]. By the mid-1980s the most important photoallergen was the fragrance musk ambrette whose use is now prohibited [16]. Musk ambrette, used in men‘s aftershave lotions and colognes, was historically the cause of a distinctive patchy pattern of photosensitivity on the face.
Presentation
Photoallergic reactions can resemble sunburn, but usually show the same spectrum of features seen with allergic contact dermatitis. The dermatitis is localized to exposed areas of the skin, usually with well-demarcated margins where the skin is covered by clothing, for example at the collar and ‘V’ of the neck, below the end of sleeves on the backs of the hands and on the ankles below the trouser legs. The area below the chin is usually spared. The most distinctive sign is the exempt ‘Wilkinson‘s triangle’ behind the earlobe. There may nevertheless be some spread to covered sites. Asymmetry may result from increased UV exposure to one side of the body, for example those who drive with the vehicle windows open. The photoallergen may be transferred from one body site to another, for example, to the contralateral areas, or may be due to a cross-leg effect or to transfer by the hands.
Photoallergy to UV filters in cosmetics may be clinically identical to that seen from conventional allergy to cosmetics. It may be widespread when related to liberal use of sunscreen agents. Furthermore, it may simulate sunburn and other causes of photosensitivity.
The intensity of the response to photoallergens depends upon a number of factors:
- The nature and concentration of the substance applied.
- The duration of exposure to the substance.
- Percutaneous absorption.
- The intensity and wavelength of the radiation.
- The duration of radiation exposure.
- Radiation absorption in the skin, depending on the thickness of the stratum corneum as well as the amount and distribution of melanin.
- Extraneous matter and secretions on the skin.
Differential diagnosis
Photoallergy may simulate other photosensitive dermatoses and airborne contact allergy, and vice versa. Furthermore, there may be a combination of these disorders in the same person. It is also important to recognize that photoallergy may sometimes fail to follow the typical pattern of sparing of light-protected sites, and airborne contact allergy may paradoxically induce the classic photosensitivity distribution. Combined airborne and photoaggravated contact allergy is seen particularly with Compositae (Asteraceae) (see Figure 128.25) [17] and lichens [18]. A similar pattern of dermatitis may also be seen in patients sensitive to colophonium, pine and spruce. It is therefore important to identify every potential component of these clinical presentations by screening for contact allergy using patch tests (especially to plants), for photoallergy with photopatch tests, and also for photosensitivity using phototesting.
Complications and co-morbidities
In some individuals, photoallergic reactions may progress to produce a light sensitivity that can persist a long time after the elimination of the sensitizer. This is known as a persistent light reaction [19]. The phenomenon has been reported with many different substances, including chlorpromazine [20], halogenated salicylanilides, musk ambrette, promethazine hydrochloride, ketoprofen [21], quindoxin [22] and olaquindox. This chronic photosensitive dermatitis presents as chronic eczematous changes on light-exposed areas with or without spread elsewhere. On monochromator testing, the patients have abnormal responses to UV radiation, with a shift to UVB sensitivity [23].
Patients with established photosensitivity who have a flare of their dermatitis may have reacted to an increase in light levels or re-exposure to their primary allergen or a cross-reacting allergen by airborne contact. Alternatively, they may have developed a secondary allergic or photoallergic contact sensitivity to their sunscreen or to one of their other medicaments.
The disorder of chronic actinic dermatitis (see Chapter 127) may be associated with contact allergy, particularly to Compositae. Often, there are multiple contact allergies. Phototesting reveals abnormal results but photopatch tests to Compositae and other allergens are generally normal. Nevertheless, persistent light reactivity following photoallergy may progress to chronic actinic dermatitis.
Disease course and prognosis
Typically, with avoidance of the cause the dermatitis will resolve; rarely, complications supervene.
Investigations
Photo-patch testing
Indications. The main clinical indications for photopatch tests include the investigation of patients with eczematous rashes predominantly affecting light-exposed sites and a history of worsening following sun exposure. A history of a reaction to sunscreens is a further indication. Grounds may be extended to testing anyone with an exposed-site distribution of dermatitis. Some patients have coexisting photosensitive disorders, causing practical problems in performing and interpreting the investigation.
Method A British Photodermatology Group (BPG) workshop has achieved a consensus on the protocol for photopatch testing in the UK and Ireland [24], but the technique may vary slightly in other parts of the world. The BPG stated that photopatch testing is an evolving technique with a need for more research.
A UVA source is required, which in most centres will be the UVA lamps used for PUVA therapy, commonly a hand/foot treatment unit. In photobiology centres, the more sophisticated irradiation monochromator may be used as an alternative. Other UVA sources include UVA blacklights, and filtered metal halide and xenon arc lamps. In all cases irradiance should be measured with a calibrated UVA meter. The energy source must be monitored regularly, as the tubes deteriorate with time.
Historically, administered dosages of UVA to the photopatch test site have generally ranged from 5 to 10 J/cm2. However, the higher doses have the disadvantage of being more likely to induce false positive phototoxic responses without an increased detection of photoallergic subjects, and therefore a dose of 5 J/cm2 is recommended. Modification of the dose may be necessary in UVA-photosensitive individuals, in which case 50% of the UVA minimal erythema dose is suggested. However, UVA phototesting to establish the minimal erythema dose is not always feasible or practicable, but is nevertheless advised before photopatch testing known photosensitive individuals. Application of the allergens is performed in an identical fashion to conventional patch tests, except that they must be applied in duplicate – one set is irradiated and the other (the control) is not. Usually, the two sets of tests are applied on either side of the vertebral column at the same level. It is suggested that the patient‘s back is positioned 15 cm from the front panel of the lamps. Steps must be taken to avoid any incidental irradiation by natural light of both the irradiated and the control set of allergens. The control site and the rest of the skin must be covered with opaque material during irradiation of the photopatch test site. Three protocols have been used and these are described in Table 128.11. There is evidence to show that a 48 h application is superior to a 24 h one [25], and one study has failed to show an improved return with UVB irradiation (Figure 128.34) or with a 7-day reading [26].
Table 128.11 Photopatch test protocols.
| Day | |||||
| Protocol | 0 | 1 | 2 | 3 | 4 |
| Protocol 1 | Phototest | Read phototest results | |||
| Apply allergens | Remove patches and irradiate allergens | Read results | |||
| Protocol 2 | Apply allergens | Remove patches, read results and irradiate allergens | Read results | ||
| Protocol 3 | Apply allergens | Phototest | Read phototest results | Read results | |
| Remove patches, read results and irradiate allergens | |||||
From British Photodermatology Group [23].
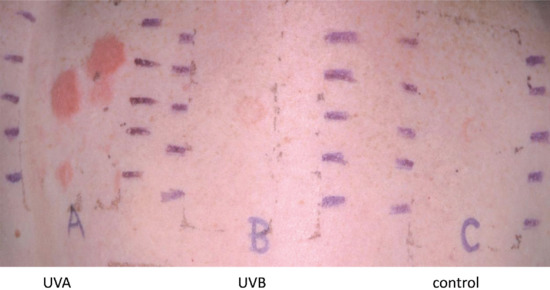
Figure 128.34 Phototesting demonstrating photo allergy after irradiation with UVA (A) but no response to the control (C) or after irradiation with UVB (B).
Test materials. The principle of a baseline series also applies to photopatch tests. The European Multicentre Photopatch Test Study Taskforce has recommended such a series based on a recent European study (Table 128.12). However, for some centres more UV filters and other materials may be advisable as new exposures and photoallergens are identified.
Table 128.12 Photopatch test baseline series.
| Type of agent | Name of agent (INCI name for UV absorbers) | Concentration and vehicle | Comment |
| ‘Older’ organic UV absorbers | Butyl methoxydibenzoylmethane | 10% pet. | Among UV absorbers, the third highest cause of PPT reactions in the EMCPPTS. A commonly used UVA absorber |
| Benzophenone-3 | 10% pet. | Among UV absorbers, the second highest cause of PPT reactions in the EMCPPTS. Many published reports of PACD over time | |
| Benzophenone-4 | 2% pet. | Permitted in sunscreens but mainly used in other cosmetics to prevent photodegradation. Few PPT reactions in the EMCPPTS | |
| Octocrylene | 10% pet. | The UV absorber most frequently leading to PPT reactions in the EMCPPTS. Seems to have potential for cross-reacting with ketoprofen and benzophenone-3 | |
| 4-methylbenzylidene camphor | 10% pet. | These agents led to relatively few PPT reactions in the EMCPPTS, but continue to be used in sunscreens | |
| Ethylhexyl methoxycinnamate | 10% pet. | ||
| Isoamyl-p-methoxycinnamate | 10% pet. | ||
| PABA | 10% pet. | Sunscreens in Europe are mostly PABA-free but this classic photoallergen may still be found in sunscreens bought elsewhere | |
| ‘Newer’ organic UV absorbers | Methylene bis-benzotriazolyl tetramethylbutylphenol | 10% pet. | The agent most frequently leading to positive patch test reactions in the EMCPPTS. The role of the added surfactant decyl glucoside requires further elucidation |
| Bis-ethylhexyloxyphenol methoxyphenyl triazine | 10% pet. | These agents led to few PPT reactions in the EMCPPTS. However, owing to the relatively short time for which they have been present in the marketplace, further useful information on their photoallergenic potential will be gained by their inclusion in this European baseline photopatch test series | |
| Drometrizole trisiloxane | 10% pet. | ||
| Terephthalylidene dicamphor sulfonic acid | 10% aqua | ||
| Diethylamino hydroxybenzoyl hexyl benzoate | 10% pet. | ||
| Ethylhexyl triazone | 10% pet. | ||
| Diethylhexyl butamido triazone | 10% pet. | ||
| Topical NSAIDs | Ketoprofen | 1% pet. | The agent most frequently leading to PPT reactions in the EMCPPTS, as well as in other studies in several countries |
| Etofenamate | 2% pet. | The second highest cause of PPT reactions in the EMCPPTS, often with unknown relevance | |
| Piroxicam | 1% pet. | An NSAID class of its own responsible for PACD and systemic photosensitivity | |
| Benzydamine | 2% pet. | Frequently responsible for PACD presenting as lip or hand dermatitis in Portugal and Spain. Also used in other European countries | |
| Topical antihistamine | Promethazine | 0.1% pet. | Widely used in several southern European countries as a topical antihistamine |
Adapted from Gonçalo et al. [27].
EMCPPTS, European Multicentre Photopatch Test Study; INCI, International Nomenclature of Cosmetic Ingredients; NSAID, non-steroidal anti-inflammatory drug; PABA, p-aminobenzoic acid; PACD, photoallergic contact dermatitis; pet., petrolatum; PPT, photopatch test; UV, ultraviolet.
Readings. A positive reaction on the irradiated side only is an indication of photoallergy. There are occasional difficulties distinguishing a false positive phototoxic reaction from photoallergy, but this is less likely with a dose of 5 J/cm2. Readings are scored identically to conventional patch tests, but the positive symbol is preceded by the prefix Ph, for example Ph++ is a strong positive photoallergic reaction. If the same allergen provokes an equally strong reaction on both sides, it is an indication of contact allergy alone; if it is significantly stronger on the irradiated side, then combined allergy and photocontact allergy may be occurring. Doubtful and slight amplification of photoallergic reactions may be the result of phototoxicity.
Management
Photoallergy to UV filters should be straightforward to eliminate. Once photoallergy has been demonstrated to a UV filter, the patient should be informed of the INCI name and synonyms of the material to which they are sensitive. UV filters relying totally on opaque/reflectant micronized titanium dioxide and zinc oxide will be free of chemical UV-filtering agents, and can be used for coexistent photodermatoses in those allergic to chemical UV filters. Where topical NSAIDs are commonly used, once the photoallergy has been confirmed avoidance should be straightforward.
Resources
Further information
De Groot AC. Patch Testing, 3rd edn. Wapserveen: Acdegroot Publishing, 2008 (and update at http://www.escd.org/education/Patch_testing_update_2008-2012.pdf; last accessed March 2015).
European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multi-centre photopatch test study. Br J Dermatol 2012;166:1002–9.
Gonçalo M, Ferguson J, Bonevalle A, et al. Photopatch testing: recommendations for a European photopatch test baseline series. Contact Dermatitis 2013;68:239–43
Johansen JD, Frosch PJ, Lepoittevin J-P, eds. Contact Dermatitis, 5th edn. Berlin: Springer, 2010.
Rietschel RL, Fowler JF. Fisher's Contact Dermatitis, 6th edn. Hamilton: BC Decker, 2008.
Rustemeyer T, Elsner P, John SM, Maibach HI, eds. Kanerva's Occupational Dermatology, 2nd edn. Berlin: Springer, 2012.
Wong T, Orton D. Sunscreen allergy and its investigation. Clin Dermatol 2011;29:306–10.
Allergic contact urticaria
Definition and nomenclature
The term was introduced by Fisher in 1973 [1] and defined as a weal and flare reaction following contact with an external substance, usually appearing within 30 min and completely clearing within hours, without residual signs [2].
Introduction and general description
Contact urticaria may be non-immune, or immune due to IgE antibodies against protein peptides. Rarely, low-molecular-weight chemicals may induce an allergic reaction. Immune contact urticaria is commoner in, but not exclusive to, atopic individuals. It belongs to a heterogenous group of immediate contact inflammatory responses that appear within minutes following contact with eliciting substances.
Contact uticaria, in association with protein contact dermatitis, comprises the contact urticaria syndrome. The heterogeneity of this syndrome includes both mechanistic and clinical presentations that can present either locally or generally, and involve organs other than the skin including the respiratory, gastrointestinal and vascular systems, leading to anaphylaxis.
Epidemiology
Most of the epidemiological data concerning contact urticaria comes from occupationally based studies, which in turn may skew the reported aetiologies. General population-based data are limited.
Incidence and prevalence
In Finland, contact urticaria has been classified as a distinct occupational skin disease since 1989. Kanerva et al. studied the Finnish Register of Occupational Diseases (1990–94) and identified that contact urticaria (29.5%) was the second commonest occupationally related allergic contact dermatosis after allergic contact dermatitis (70.5%) [3]. The most common causes (in decreasing order of frequency) were cow dander, natural rubber latex and flour/grains/feed. These causal agents accounted for 79% of all cases and the most affected occupations (per 100 000 workers), in decreasing order of frequency, were bakers, preparers of processed food and dental assistants [3].
A retrospective Australian study carried out in a tertiary-level occupational dermatology clinic identified a prevalence of 8.3%. Atopy was a significant risk factor for natural rubber latex allergy and contact urticaria induced by foodstuffs or ammonium persulphate. In this study, the three most common occupations were healthcare workers, food handlers and hairdressers [4].
Pathophysiology
The mechanisms underlying contact urticaria are divided into two main types – immunological and non-immunological.
Non-immunological contact urticaria is the more common form of the condition and is secondary to the release of vasogenic mediators without the involvement of immunological processes. The clinical reaction is often erythema without oedema rather than a true weal and flare response. Substances capable of producing non-immunological contact urticaria are usually low-molecular-weight chemicals that easily cross the barrier of the skin, such as plants, animals or chemical substances. Many of these chemicals are used as flavourings, fragrances and preservatives in the cosmetic, pharmaceutical and food industries [5].
The pathogenesis of immunological (allergic) contact urticaria involves the classic type 1 hypersensitivity reaction mediated by specific immunoglobulin E in a presensitized individual. IgE binding on mast cells, its subsequent degranulation and release of histamine and other vasoactive mediators is described in Chapter 8.
A third category of uncertain mechanisms also exists where the clinical symptoms resemble an immunological contact urticarial reaction yet no specific IgE can be demonstrated in the patient's serum or in the affected tissues, and passive transfer tests are negative. An example of this includes reactions to ammonium persulphate.
Predisposing factors
In addition to atopy, the presence of any pre-existing dermatitis predisposes to the development of allergic contact urticaria due to the presence of ‘danger signals’ that promote sensitization. It has been reported that in patients with atopic eczema and raised IgE levels, the IgE may attach to the high-affinity IgE receptors on the surface of Langerhans cells or other antigen-presenting cells. This in turn may present the protein allergens to T-helper 2 cells, inducing a delayed-type hypersensitivity reaction resulting in eczematous lesions. This does not occur in atopic individuals with normal IgE levels or non-atopic controls and may explain why patients with atopic eczema have delayed hypersensitivity on patch testing to aeroallergens and develop a vesicular response to handling food proteins (protein contact dermatitis) [6, 7].
Pathology
The histology of contact urticaria is identical to that of urticaria from other causes and is not further discussed here.
Genetics
There is a strong association of allergic contact urticaria with inherited atopic disease of all forms. There is also a reported association between the development of immediate-type hypersensitivity reactions, specifically to peanut, with filaggrin mutations in these individuals [8].
Environmental factors
Contact sensitization develops following exposure, and changes in the exposure to an allergen in the environment will influence the development of the allergy. This has been demonstrated by the control of latex allergy by environmental measures and the use of low-protein-powder-free gloves [9].
Clinical features
Presentation
The symptoms usually occur within 1 h and fade by 3 h. The spectrum of associated symptoms is wide. Local symptoms include itching and burning, and the development of erythema and the characteristic weal and flare reaction. Early symptoms are commonly missed by physicians although well recognized by patients. In ‘invisible’ contact urticaria, only subjective symptoms (itching, tingling, burning) occur without any objective change or only mild erythema. These reactions are often seen from cosmetics, fruits or vegetables.
Exposure to allergens in those who are highly sensitized, topically or via the oral or respiratory route, may result in widespread urticaria and swelling of the mucous membranes, resulting in conjunctivitis, rhinitis, oro-pharyngeal swelling, bronchoconstriction and rarely anaphylaxis. Gimenez-Arnau et al. have compiled a comprehensive list of causative substances [10].
Contact urticaria to foodstuffs. The commonest causes of contact urticaria are foodstuffs, which can provoke oro-pharyngeal symptoms (the oral allergy syndrome) following ingestion, or cutaneous hand symptoms in food handlers. The oral allergy syndrome (pollen–fruit syndrome) results from eating raw/uncooked/unprocessed fruits, vegetables and nuts. Symptoms are usually confined to the localized mucosal surfaces with irritation or tingling. Clinical signs are usually confined to localized mucosal swelling, although anaphylaxis is rarely reported.
In the birch-rich areas of northern and central Europe almost all birch pollen-allergic patients are sensitized to Bet v1, the major allergen component of pollen from Betula verucosa. Fifty to 90% of birch pollen-allergic patients have been reported to have some pollen-related food allergy – in particular to the Bet v1 homologous proteins (PR-10 proteins) in the Rosaceae family such as apple, peach and cherry [11]. Other clinically important food allergies commonly associated with Bet v1 are reactions to the Apiaceae family (e.g. celery, carrot) and/or the Fabaceae family (e.g. peanut, soybean). The Bet v1 homologous proteins in the Rosaceae family are very sensitive to heat and proteases and the clinical reactions are often aborted when eating cooked or tinned forms of the fruit.
The most important molecular basis of the latex–fruit syndrome is the homology between the hevein (Hev b 6.02) of the latex with the hevein-like N-terminal domain of the class I chitinases of plants.
This immunological phenomenon of cross-reactivity has consequences for the diagnosis and treatment of certain food allergies (Table 128.13) [12]. It is important to determine the clinical relevance of these cross-reactions, and whether they represent sensitization only or actual clinical reactivity (allergy). The dermatologist may be called upon to determine the risk of reactions to related foods and a variety of other plant-derived foods that may share proteins with pollens, latex and each other.
Table 128.13 Examples of cross-reactions between foods, foods and pollens and foods and latex that may also cause contact urticaria.
| Food type | Risk (%), if known | Cross-reaction | Other cross-reacting allergens |
| Fin fish, e.g. cod | 5 | Other fish, e.g. haddock, salmon | |
| Crustacea | 75 | Other crustacea | |
| Grain, e.g. wheat | 20 | Other grains, e.g. barley, rye | Grass pollens |
| Apple | 55 | Other Rosaceae fruit, e.g. peach, pear, cherry, plum | Birch tree pollen |
| Peanuts | Other legumes | Tree nuts, grass pollen | |
| Cow's milk | 92 | Goat's milk | |
| Latex (latex food syndrome) | 35 | Banana, avocado, kiwi, chesnut, potato, papaya | |
| Pollen type | |||
| Birch | Raw apple, raw carrot, cherry, pear, peach, plum, fennel, walnut, potato, spinach, wheat, peanut, kiwi, hazelnut, fennel, coriander, cumin | ||
| Mugwort | Celery, carrot, melon, watermelon, hazelnut | ||
| Grass | Potato, melon, tomato, watermelon, orange, cherry, peanut | ||
| Ragweed | Melon, chamomile, banana |
Adapted From Sicherer [25].
Clinical evaluation requires a careful history, skin prick tests and IgE-mediated blood tests. The pitfalls in evaluation are interpreting the clinical relevance of a positive skin prick test or radioallergosorbent test and deciding when it is a false positive result. The use of component-resolved diagnostics that detects IgE antibodies to single allergen components can be used to help in this process.
In an occupational setting contact urticaria frequently develops in those who are exposed to animals, such as agricultural workers, fish processors and slaughterhouse workers, or are involved in food production such as bakers and chefs [5, 13]. In Scandinavia, the commonest cause of occupational contact urticaria is from cow dander in farmers [14]. Of those workers exposed to contact allergic urticants, bakers, butchers and agricultural workers appear to be most at risk of becoming sensitized, more so than health care workers.
Contact urticaria to natural rubber latex. Allergy to natural rubber latex was first recognized by Nutter in 1979 [15]. The allergens are present in the water-soluble protein moiety of the sap collected from the rubber-bearing tree Hevea braziliensis, harvested mainly in Malayasia and South-East Asia. The problem has been associated primarily with dipped rubber items, including gloves (Figure 128.35), condoms, balloons, catheters and medical tubing. These items are vulcanized at a lower temperature than solid rubber products such as tyres, seals and gaskets. With the advent of AIDS and the huge increase in the use of latex examination gloves among health care personnel, the production of inexpensive, disposable, natural rubber latex gloves escalated. During the production process, the natural rubber latex was not left to stand in holding tanks as long, the process was shortened by lower vulcanization temperatures, and there was less thorough washing of the final product [16]. All these measures led to an increase in the protein content of the gloves and this, coupled with their increasing use, resulted in an increase in the incidence of allergy to natural rubber latex.
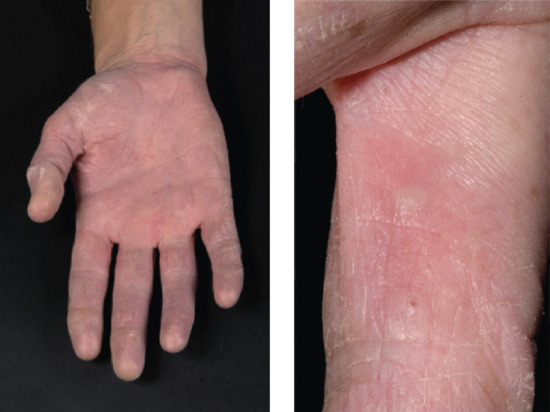
Figure 128.35 Open testing to a solution of chapatti flour causing an immune allergic contact urticarial response within 15 min. C, chapatti flour; H, histamine. (From Davies and Orton [26].)
Anaphylaxis can occur in any sensitized patient, and seems to be particularly prevalent when challenge is via the mucosal surfaces, as in dental and vaginal examinations, intraperitoneal operations, catheter changing (especially in spina bifida patients who have frequent surgery and catheter changes) and barium enemas. The allergenic proteins are multiple. Many of the allergenic peptides in natural rubber latex cross-react with those found in other plants [17], such as banana, lychees, chestnuts and avocado, and patients allergic to latex may exhibit sensitivity to such foods.
Due to several interventional measures including an increased use of powder-free latex gloves, and non-latex gloves and medical equipment, the frequency of natural rubber latex allergy in health care workers seems to have peaked [9].
Protein contact dermatitis. Hjorth and Roed-Peterson (1976) defined this as an immediate dermatitis induced by contact with proteins. They described 33 caterers suffering from itch within 10–30 min following contact with meat, fish and vegetables, which was followed by erythema and vesicle formation. Application of the relevant food to the affected skin resulted in either urticaria or eczema [18]. Patients who have repeated exposure of the hands, especially the fingertips, to contact urticants, such as food proteins, may develop protein contact dermatitis. Characteristically, the condition involves skin sites that have been affected previously by dermatitis. Damaged skin probably facilitates penetration of the allergens, and inflammatory cells already present in the dermis may explain the accelerated clinical response. An association between atopy and protein contact dermatitis occurs in approximately 50% cases. It is common in dairy workers and veterinarians, slaughterhouse workers, chefs and sandwich makers, who become sensitized to the proteins they touch during work [5].
Differential diagnosis
The differential diagnosis of allergic contact urticaria is that of other forms of urticaria and is discussed in Chapter 42. In the identification of an exogenous cause, consideration of its possibility and referral for specialist investigation is essential. Localized symptomatic dermographism is a relatively common cause of urticaria to gloves in the absence of latex allergy [19], contact urticaria to rubber chemicals being extremely rare [20].
Classification of severity
The following staging system for contact urticaria syndrome has been described by Amin and Maibach [21].
- Stage 1: Localized urticaria (redness and swelling), dermatitis (eczema) and non-specific symptoms (e.g. itching, tingling, burning sensation).
- Stage 2: Generalized urticaria.
- Stage 3: Bronchial asthma (wheezing), rhinitis, conjunctivitis (e.g. runny nose, watery eyes), oro-laryngeal symptoms (e.g. lip swelling, hoarseness, difficulty in swallowing) and gastrointestinal symptoms (e.g. nausea, vomiting, diarrhea, cramps).
- Stage 4: Anaphylactoid reactions (shock).
Disease course and prognosis
Provided the allergen is identified and eliminated, even though sensitization may persist, symptoms will resolve.
Investigations
The diagnosis of contact urticaria is based on a full medical history and skin testing with suspected substances. In particular, the study of chronic hand eczema in professional food handlers should include both immediate and delayed tests. A detailed history concerning the occurrence of immediate symptoms – whether confined to the skin or not – and their association, particularly with occupational exposure, should always be considered. With an unknown allergen, exposure should be graded with an initial application test (open and subsequently occluded) followed by a prick test and, if appropriate, an intradermal test. NSAIDs, antihistamines and exposure to UV light may all cause false negative results.
Von Krogh and Maibach produced guidelines for evaluating immediate types of cutaneous response [22]. The simplest test for contact urticaria is the open test, whereby 0.1 mL of the suspected causative agent is rubbed firstly on a 3 × 3 cm area of normal skin (upper back or extensor aspect of upper arm or forearm (Figure 128.36). If this is negative, the test is then repeated on slightly affected skin or previously affected skin. The use of alcohol vehicles or the addition of propylene glycol to the vehicle enhances the sensitivity of the test [23]. A positive result occurs when oedema and/or erythema is observed, usually within 15 min. The test sites are usually read at 20, 40 and 60 min to see the maximal response. Immunological contact urticaria reactions typically appear within 15-20 min, whereas non-immunological contact urticaria reactions can be delayed up to 45–60 min following application. When performing tests with ‘whole’ foods at least six control subjects should also be tested.

Figure 128.36 Allergic contact urticarial weals developing within 20 min following a powdered latex glove challenge in a natural rubber latex-allergic patient.
When the open test is negative, prick testing of suspected agents with appropriate histamine and saline controls should be used. Although commercial allergen extracts are available, it should be remembered that unless standardized they may not contain the relevant protein allergens, and the gold standard should always be test and challenge with a sample of fresh material. Skin tests should only be performed where resuscitation facilities are available.
If the patient has experienced an anaphylactic reaction and a specific IgE test is available, the blood test may confirm the diagnosis and thus avoid the risk of anaphylaxis as a result of skin tests.
Scratch tests and scratch patch tests (contact with a small aluminium chamber for 15 min) are less standardized tests that should also be performed with appropriate controls. A small piece of the substance is applied on a closed patch test (Finn chamber, Epitest; Hyryla, Finland) to an area of skin. The skin may be lightly scarified with a needle or degreased with 96% alcohol [24]. After 20 min the area is examined for erythema or weal and flare. Occasionally, there is no reaction on normal skin and the substance has to be applied to previously affected skin, for example the fingertips. The scratch patch test is particularly useful when testing non-standardized materials, when a delayed reading is mandatory.
Management
Management is achieved by avoiding the causative substance or the use of appropriate personal protective equipment (e.g. gloves made of an appropriate material), since desensitization for the majority of relevant allergens is not available. The general management of the symptoms of urticaria and its complications is discussed in Chapter 42. Treatment of the acute episode includes the use of systemic antihistamines and adrenaline in rare cases of anaphylaxis.
Resources
Further information
De Groot AC. Patch Testing, 3rd edn. Wapserveen: Acdegroot Publishing 2008 (and update at http://www.escd.org/education/Patch_testing_update_2008-2012.pdf; last accessed March 2015).
Gimenez-Arnau A, Maurer M, de la Cuadra J, Maibach H. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis – “a never ending story”. Eur J Dermatol 2010; 20: 552–62.
Johansen JD, Frosch PJ, Lepoittevin J-P, eds. Contact Dermatitis, 5th edn. Berlin: Springer, 2010.
Rietschel RL, Fowler JF. Fisher's Contact Dermatitis, 6th edn. Hamilton: BC Decker, 2008.
Rustemeyer T, Elsner P, John SM, Maibach HI Eds. Kanerva's Occupational Dermatology, 2nd edn. Berlin: Springer, 2012.
Patient resources
DermNetz, NZ: http://dermnetnz.org/reactions/contact-urticaria.html (last accessed March 2015).
References
History
- Lachapelle JM. Giant Steps in Patch Testing: A Historical Memoir. Phoenix, AZ: Smart Practice, 2010.
- Adams RM. Diagnostic patch testing. In: Occupational Skin Disease. New York: Grune and Stratton, 1983:136.
- Ayto J. Dictionary of Word Origins. London: Bloomsbury, 1990:18.
- Dakin R. Remarks on a cutaneous affection produced by certain poisonous vegetables. Am J Med Sci 1829;4:98–100.
- Fuchs CH. Die Krankhaften Veränderungen der Haut – und ihre Anhänge. Göttingen: Dieterichs'sche Buch-handlung, 1840.
- Neisser A. Ueber Iodoform-Exantheme. Dtsch Med Wochenschr 1884;10:467–8.
- Bloch B, Steiner-Woerlich A. Die willkürliche Erzeugung der Primelüberempfindlichkeit bein Menschen und ihre Bedeutung für das Idiosynkrasieproblem. Arch Dermatol Syphilol 1926;152:283–303.
- Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J Exp Med 1936;64:629–39.
- Landsteiner K, Chase MW. Experiments on transfer of cutaneous sensitivity to simple compounds. Proc Soc Exp Biol Med 1942;49:688–90.
- Haxthausen H. The pathogenesis of allergic eczema elucidated by transplantation experiments on identical twins. Acta Derm Venereol (Stockh) 1942;23:438–57.
- Jadassohn J. Zur Kenntnis der medikamentosen Dermatosen. In: Verhandlungen der Deutsch Dermatoligischen Gesellschaft, V Congress, Graz (1895). Vienna: Braumuller, 1896:103–29.
- Foussereau J. History of epicutaneous testing: the blotting-paper and other methods. Contact Dermatitis 1984;11:219–23.
- Stadeler J. Uber die eigenthumthumlichen Bestandteile der Anacardium Fruchte. Ann Chemie Pharmacie 1847;63:117–65.
- Bloch B. Experimentelle Studien über das Wesen der Iodoformidiosynkrasie. Z Exp Pathol Ther 1911;9:509–38.
- Bloch B. The role of idiosyncrasy and allergy in dermatology. Arch Dermatol Syphilis 1929;19:175–97.
Introduction and general description
- Cronin E. Cronin E. Contact Dermatitis. Edinburgh: Churchill Livingstone, 1980.
- Calnan CC. The use and abuse of patch tests. In: Maibach HI, ed. Occupational and Industrial Dermatology 2nd edn. Chicago: Year Book Medical Pubhshers, 1987:28–31.
- Fisher AA. The use and abuse of patch testing. Cutis 1985:36:451.
- Kligman AM. Lanolin allergy: crisis or comedy. Contact Dermatitis 1983:9;99–107.
- Aberer W, Andersen KE, White IR. Should patch testing be restricted to dermatologists only. Contact Dermatitis 1993;28:1–2.
- European Parliament and Council Directive 94/27/EC (1994). Off J Eur Union L 1994;188:1–2.
- European Parliament and Council Directive 2003/53/EC (2003). Off J Eur Union L 2003;178:24–7.
- European Parliament and Council Directive 648/2004/EC (2004). Off J Eur Union L 2004;104:1–35.
- Schnuch A, Uter W. Decrease in nickel allergy in Germany and regulatory interventions. Contact Dermatitis 2003;49(2):107–8.
- Lindberg M, Edman B, Fischer T, Stenberg B. Time trends in Swedish patch test data from 1992 to 2000. A multi-centre study based on age- and sex-adjusted results of the Swedish standard series. Contact Dermatitis 2007;56(4):205–10.
- Jensen CS, Lisby S, Baadsgaard O, Vølund A, Menné T. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol 2002;146(4):636–42.
- Irvine C, Pugh CE, Hansen EJ, Rycroft RJG. Cement dermatitis in underground workers during construction of the Channel Tunnel. Occup Med 1994;44(1):17–23.
- European Parliament and Council Directive 2003/53/EC (2003). Off J Eur Union L 2003;178:24–7.
- Geier J, Krautheim A, Uter W, Lessmann H, Schnuch A. Occupational contact allergy in the building trade in Germany: influence of preventive measures and changing exposure. Int Arch Occup Environ Health 2011;84(4):403–11.
- Geier J, Schnuch A, Frosch PJ. Contact allergy to dichromate in women. Dermatol Beruf Umwelt 2000;48(1):4–10.
- Jadassohn J. Zur Kenntnis der medikamentösen Dermatosen. In: Braunmüller W, ed. Verhandlungen der Deutschen Dermatologischen Gesellschaft, Fünfter Kongreß, Graz, 1895. Wien and Leipzig: Wilhelm Braunmüller, 1896:103–29.
Epidemiology
Incidence and prevalence
- Coenraads P-J, Diepgen T, Uter W, et al. Epidemiology. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:135–63.
- Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med 1976;30:107–14.
- Agrup G. Hand eczema and other hand dermatoses in South Sweden. Acta Derm Venereol Suppl (Stockh) 1969;61.
- Cherry N, Meyer JD, Adisesh A, et al. Surveillance of occupational skin disease: EPIDERM and OPRA. Br J Dermatol 2000;142:1128–34.
- Schnuch A, Geier J, Uter W, et al. National rates and regional differences in sensitization to allergens of the standard series. Population-adjusted frequencies of sensitization (PAFS) in 40,000 patients from a multicenter study (IVDK). Contact Dermatitis 1997;37:200–9.
- Uter W, Schnuch A, Gefeller O. Guidelines for the descriptive presentation and statistical analysis of contact allergy data. Contact Dermatitis 2004;51:47–56.
- Meding B, Jarvholm B. Hand eczema in Swedish adults: changes in prevalence between 1983 and 1996. J Invest Dermatol 2002;118:719–23.
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, Andersen KE. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. Br J Dermatol 2001;144:523–32.
- Nielsen NM, Linneberg A, Menné T, et al. Incidence of allergic contact sensitization in Danish adults between 1990 and 1998; the Copenhagen Allergy Study, Denmark. Br J Dermatol 2002;147:487–92.
- Dotterud LK, Smith-Sivertsen T. Allergic contact sensitization in the general adult population: a population-based study from Northern Norway. Contact Dermatitis 2007;56:10–15.
- Lindberg M, Edman B, Fischer T, Stenberg B. Time trends in Swedish patch test data from 1992 to 2000. A multi-centre study based on age- and sex-adjusted results of the Swedish standard series. Contact Dermatitis 2007;56:205–10.
- Pratt M, Belsito DV, DeLeo VA, et al. North American contact dermatitis group patch-test results, 2001–2002 study period. Dermatitis 2004;15:176–83.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/3—first results of the European surveillance system on contact allergies. Contact Dermatitis 2005;53:136–45.
- Britton JER, Wilkinson SM, English JSC, et al. The British standard series of contact allergens: validation in clinical practice and value for clinical governance. Br J Dermatol 2003;148:259–64.
- Lim JTE, Goh CL, Ng SK, et al. Changing trends in the epidemiology of contact dermatitis in Singapore. Contact Dermatitis 1992;26:321–6.
- Thomson KF, Wilkinson SM, Powell S, Beck MH. The prevalence of corticosteroid allergy in two UK centres: prescribing implications. Br J Dermatol 1999;141:863–6.
- Ayala F, Balato N, Lembo G, et al. Statistical evaluation of the persistence of acquired hypersensitivity by standardized patch tests. Contact Dermatitis 1996; 34:354–8.
- Wilkinson JD, Shaw S, Andersen KE, et al. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991–2000). Contact Dermatitis 2002;46:207–10.
- Meyer JD, Chen Y, Holt DL, et al. Occupational contact dermatitis in the UK: a surveillance report from EPIDERM and OPRA. Occup Med 2000;50:265–73.
- McCall BP, Horwitz IB, Feldman SR, Balkrishnan R. Incidence rates, costs, severity, and work-related factors of occupational dermatitis: a workers' compensation analysis of Oregon, 1990–1997. Arch Dermatol 2005;141:713–18.
- Dickel H, Bruckner TM, Schmidt A, Diepgen TL. Impact of atopic skin diathesis on occupational skin disease incidence in a working population. J Invest Dermatol 2003;121:37–40.
- Dickel H, Kuss O, Blesius CR, et al. Occupational skin diseases in Northern Bavaria between 1990 and 1999: a population-based study. Br J Dermatol 2001;145:453–62.
Age
- Kwangsukstith C, Maibach HI. Effects of age and sex on the induction and elicitation of allergic contact dermatitis. Contact Dermatitis 1995;33:289–98.
- Epstein E. Contact dermatitis in children. Pediatr Clin North Am 1971;18:839–52.
- Schwartz M. Eczematous sensitization in various age groups. J Allergy 1953;24:143–8.
- Berit CC, Menné T, Johansen JD. 20 years of standard patch testing in an eczema population with focus on patients with multiple contact allergies. Contact Dermatitis 2007;57:76–83.
- Green CM, Holden CR, Gawkrodger DJ. Contact allergy to topical medicaments becomes more common with advancing age: an age-stratified study. Contact Dermatitis 2007;56:229–31.
- Christopherson J, Menné T, Tanghof P, et al. Clinical patch test data evaluated by multivariate analysis. Contact Dermatitis 1989;21:291–9.
- Goossens A, Morren M. Contact allergy in children. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:811–30.
- Larsson-Stymne B, Widström L. Ear piercing: a cause of nickel allergy in schoolgirls? Contact Dermatitis 1985;13:289–93.
- Möller H. All these positive tests to thiomersal. Contact Dermatitis 1994;31:209–14.
- Osawa J, Kitamura K, Izekawa Z, et al. A probable role for vaccines containing thiomersal in thiomersal sensitivity. Contact Dermatitis 1991;24:183–7.
- Sosted H, Johansen JD, Andersen KE, et al. Severe allergic hair dye reactions in 8 children. Contact Dermatitis 2006; 54: 87–91.
- Mallory SB. The pediatric patient. In: Guin JD, ed. Practical Contact Dermatitis. New York: McGraw-Hill, 1995:603–16.
- Marcussen PV. Primary irritant patch test reactions in children. Arch Dermatol 1963;87:378–82.
- Clayton TH, Wilkinson SM, Rawcliffe C, et al. Allergic contact dermatitis in children: should pattern of dermatitis determine referral? A retrospective study of 500 children tested between 1995 and 2004 in one UK centre. Br J Dermatol 2006;154:114–17.
- Beattie PE, Green C, Lowe G, Lewis-Jones MS. Which children should we patch test? Clin Exp Dermatol 2007;32:6–11.
- Vigan M, Sauvage C, Adessi B, et al. Pourquoi et comment réaliser une batterie standard chez les enfants? Nouv Dermatol 1994;13:12–15.
Sex
- Modjtahedi BS, Modjtahedi SP, Maibach HI. The sex of the individual as a factor in allergic contact dermatitis. Contact Dermatitis 2004;50:53–9.
- Ansar Ahmed S, Penhale WJ, Talat N. Sex hormones, immune responses and autoimmune diseases. Am J Pathol 1985;121:531–51.
- Leyden JJ, Kligman AM. Allergic contact dermatitis. Sex differences. Contact Dermatitis 1977;3:333–6.
- Jordan WP, King SE. Delayed hypersensitivity in females. The development of allergic contact dermatitis in females during the comparison of two predictive patch tests. Contact Dermatitis 1977;3:19–26.
- Rees JL, Friedmann PS, Matthews JNS. Sex difference in susceptibility to development of contact hypersensitivity to dinitrochlorobenzene (DNCB). Br J Dermatol 1989;120:371–4.
- Walker FB, Smitt PD, Maibach HI. Genetic factors in human allergic contact dermatitis. Int Arch Allergy Appl Immunol 1987;32:453–62.
- Peltonen L, Terho P. Nickel sensitivity in schoolchildren in Finland. In: Frosch P, Dooms-Goossens A, LaChapelle J-M, et al., eds. Current Topics in Contact Dermatitis. Heidelberg: Springer, 1989:184–7.
- Carlsen BC, Menné T, Johansen JD. 20 years of standard patch testing in an eczema population with focus on patients with multiple contact allergies. Contact Dermatitis 2007;57:76–83.
- Meijer C, Bredberg M, Fischer T, et al. Ear piercing, and nickel and cobalt sensitization, in 520 young Swedish men doing compulsory military service. Contact Dermatitis 1995;32:147–9.
- Fabris N. Hormones and ageing. In: Makinodan T, Yunis EJ, eds. Immunology and Ageing. New York: Plenum Press, 1977:73–89.
- Rea TH. Quantitative enhancement of dinitrochlorobenzene responsivity in women receiving oral contraceptives. Arch Dermatol 1979;115:361–2.
- Denman AM. Pregnancy and immunological disorders. BMJ 1982;284:999–1000.
- Hawes CS, Kemp AS, Jones WR, et al. A longitudinal study of cell-mediated immunity in human pregnancy. J Reprod Immunol 1981;3:165–73.
- Alexander S. Patch testing and menstruation. Lancet 1988;ii:751.
- Bonamonte D, Foti C, Antelmi AR, Biscozzi AM, et al. Nickel contact allergy and menstrual cycle. Contact Dermatitis 2005;52:309–13.
- McLelland J, Lawrence CM. Premenstrual exacerbation of nickel allergy. Br J Dermatol 1991;125:83.
Ethnicity
- Anderson KE, Maibach HI. Black and white human skin differences. J Am Acad Dermatol 1979;1:276–82.
- Goh CL. Prevalence of contact allergy by sex, race and age. Contact Dermatitis 1986;14:237–40.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol 2002;46:S107–12.
Associated diseases
- Grossman J, Baum J, Gluckman J, et al. The effect of aging and acute illness on delayed hypersensitivity. J Allergy Clin Immunol 1975;55:262–75.
- Johnson MW, Maibach HI, Salmon SE. Quantitative impairment of primary inflammatory response in patients with cancer. J Natl Cancer Inst 1973;51:1075–6.
- Van der Harst-Oostven CJGR, van Vloten WA. Delayed-type hypersensitivity in patients with mycosis fungoides. Dermatologica 1978;157:129–35.
Drugs
- Schopf E. Drug influences upon skin test reactivity. In: Ring J, Burg G, eds. New Trends in Allergy. Berlin: Springer, 1981:108–14.
- Feuerman E, Levy A. A study of the effect of prednisolone and an antihistamine on patch test reactions. Br J Dermatol 1972;86:68–71.
- Moed H, Stoof TJ, Boorsma DM, et al. Identification of anti-inflammatory drugs according to their capacity to suppress type-1 and type-2 T cell profiles. Clin Exp Allergy 2004;34:1868–75.
- Sukanto H, Nater JP, Bleumink E. Influence of topically applied corticosteroids on patch test reactions. Contact Dermatitis 1981;7:180–5.
- Thorvaldsen J, Volden G. PUVA-induced diminution of contact allergic and irritant skin reactions. Clin Exp Dermatol 1980;5:43–6.
- Skov L, Hansen H, Barker JN, et al. Contrasting effects of ultraviolet-A and ultraviolet-B exposure on induction of contact sensitivity in human skin. Clin Exp Immunol 1997;107:585–8.
- Elliott JC, Picker MJ, Nelson CJ, et al. Sex differences in opioid induced enhancement of contact hypersensitivity. J Invest Dermatol 2003;121:1053–9.
Pathophysiology
Sensitization and elicitation
- Posadas SJ, Pichler WJ. Delayed drug hypersensitivity reactions – new concepts. Clin Exp Allergy 2007;37:989–99.
- Tomura M, Honda T, Tanizaki H, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest 2010;120(3):883-93.
- Lepoittevin J-P. Molecular aspects of allergic contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:45–68.
- Becker D, Valk E, Zahn S, et al. Coupling of contact sensitizers to thiol groups is a key event for the activation of monocytes and monocyte-derived dendritic cells. J Invest Dermatol 2003;120:233–8.
- Cumberbatch M, Dearman R, Kimber I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology 1997;92:388–95.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol 2002;27:138–46.
- Rustemeyer T, van Hoogstraten IMW, von Blomberg BME, Scheper R. Mechanisms in allergic contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:45–68.
- Kimber I, Dearman RJ. Allergic contact dermatitis: the cellular effects. Contact Dermatitis 2002;46:1–5.
- Gruchalla RS. Drug metabolism, danger signals, and drug-induced hypersensitivity. J Allergy Clin Immunol 2001;108:475–88.
- Homey B, Alenius H, Müller A, et al. CCL27–CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 2002;8:157–65.
- Trautmann A, Akdis M, Kleemann D, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000;106:25–35.
Predisposing factors
- Menné T, Borgan O, Green A. Nickel allergy and hand dermatitis in a stratified sample of the Danish female population. Acta Derm Venereol (Stockh) 1982;62:35–41.
- Wilkinson DS, Bandmann H-J, Calnan CD, et al. The role of contact allergy in hand eczema. Trans St John's Hosp Dermatol Soc 1970;56:15–19.
- Pedersen LK, Johansen JD, Held E, Agner T. Augmentation of skin response by exposure to a combination of allergens and irritants – a review. Contact Dermatitis 2004;50:265–73.
- Matzinger P. An innate sense of danger. Semin Immunol 1998;10:399–415.
- McFadden JP, Basketter DA. Contact allergy, irritancy and ‘danger’. Contact Dermatitis 2000;42:123–7.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol 2002;27:138–46.
- Hunziker N. Experimental Studies on Guinea-pig's Eczema. Berlin: Springer, 1969.
- Meneghini CL. Sensitization in traumatised skin. Am J Ind Med 1985;8:319–21.
- Sajjachareonpong P, Cahill J, Cahill T, et al. Persistent post-occupational dermatitis. Contact Dermatitis 2004;51:278–83.
- Basketter D, Dooms-Goossens A, Karlberg AT, LePoittevin J-P. The chemistry of contact allergy: why is a molecule allergenic? Contact Dermatitis 1995;32:65–73.
- Lepoittevin JP. Molecular aspects of allergic contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:45–68.
- Matura M, Sköld M, Börje A, et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermatitis 2005;52(6):320–8.
- Gruchalla RS. Drug metabolism, danger signals, and drug-induced hypersensitivity. J Allergy Clin Immunol 2001;108:475–88.
- Klecak G. Test methods for allergic contact dermatitis in animals. In: Marzulli FN, Maibach HI, eds. Dermatotoxicology, 5th edn. Washington, DC: Hemisphere, 1996:437–59.
- Kligman AM, Basketter DA. A critical commentary and updating of the guinea pig maximisation test. Contact Dermatitis 1995;32:129–34.
- Basketter DA, Gerberick F, Kimber I. The local lymph node assay and the assessment of relative potency: status of validation. Contact Dermatitis 2007;57:70–5.
- Gad SC, Dunn BJ, Dobbs DW, et al. Development and validation of an alternative dermal sensitisation test: the mouse ear swelling test (MEST). Toxicol Appl Pharmacol 1986;84:93–114.
- Barratt MD, Basketter DA, Chamberlain M, et al. Development of an expert system rulebase for identifying contact allergens. Toxicol In Vitro 1994;8:1053–60.
- Schaefer H, Redelmeier TE. Skin penetration. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:167–78.
- Friedmann PS. The relationships between exposure dose and response in induction and elicitation of contact hypersensitivity in humans. Br J Dermatol 2007;157:1093–102.
- Basketter DA, Jefferies D, Safford BJ, et al. The impact of exposure variables on the induction of skin sensitization. Contact Dermatitis 2006;55:178–85.
- Gerberick GF, Robinson MK. A skin sensitization risk assessment for evaluation of new ingredients and products. Am J Contact Dermatitis 2000;11:65–73.
- Lidén C. Legislative and preventive measures related to contact dermatitis. Contact Dermatitis 2001;44:65–9.
- Rustemeyer T, van Hoogstraten IMW, von Blomberg BME, Scheper R. Mechanisms in allergic contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:45–68.
- White JML, Goon A, Jowsey IR, et al. Oral tolerance to contact allergens: a common occurrence? A review. Contact Dermatitis 2007;56:247–54.
Pathology
- Meller S, Lauerma AI, Kopp FM, et al. Chemokine responses distinguish chemical-induced allergic from irritant skin inflammation: memory T cells make the difference. J Allergy Clin Immunol 2007;119:1470–80.
Causative organisms
- Pathmanaban ON, Porter JS, White IR. Dogger Bank itch in the eastern English Channel: a newly described geographical distribution of an old problem. Clin Exp Dermatol 2005;30:622–6.
- Carle JS, Christophersen C. Dogger bank itch. 4. An eczema-causing sulfoxonium ion from the marine animal, Alcyonidium gelatinosum [Bryozoa]. Toxicon 1982;20:307–10.
Genetics
- Schnuch A, Westphal G, Mossner R, Uter W, Reich K. Genetic factors in contact allergy – review and future goals. Contact Dermatitis. 2011;64(1):2–23.
- Schnuch A, Westphal GA, Müller MM, et al. Genotype and phenotype of N-acetyltransferase 2 (NAT2) polymorphism in patients with contact allergy. Contact Dermatitis 1998;38(4):209–11.
- Blömeke B, Brans R, Coenraads PJ, et al. para-Phenylenediamine and allergic sensitization: risk modification by N-acetyltransferase 1 and 2 genotypes. Br J Dermatol 2009;161(5):1130–5.
- Westphal GA, Schnuch A, Moessner R, et al. Cytokine gene polymorphisms in allergic contact dermatitis. Contact Dermatitis 2003;48(2):93–8.
- Nacak M, Erbagci Z, Buyukafsar K, Yurtsever AS, Tiftik RN. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with allergic contact dermatitis. Basic Clin Pharmacol Toxicol 2007;101(2):101–3.
- Menné T, Holm V. Genetic susceptibility in human allergic sensitization. Semin Dermatol 1986;5:301–6.
- Moss C, Friedmann PS, Shuster S, et al. Susceptibility and amplification of sensitivity in contact dermatitis. Clin Exp Immunol 1985;61:232–41.
- Lowney ED. Attenuation of contact sensitization in man. J Invest Dermatol 1968;50:241–9.
- White JML, Goon A, Jowsey IR, et al. Oral tolerance to contact allergens: a common occurrence? A review. Contact Dermatitis 2007;56:247–54.
- Menné T, Holm NV. Nickel allergy in a female twin population. Int J Dermatol 1983;22:22–8.
- Parker D, Sommer G, Turk JL. Variations in guinea pig responsiveness. Cell Immunol 1975;18:233–8.
- Polak L, Barnes JM, Turk JL. The genetic control of contact sensitization to inorganic metal compounds in guinea-pigs. Immunology 1968;14:707–11.
- Walker FB, Smith PD, Maibach HI. Genetic factors in human allergic contact dermatitis. Int Arch Allergy Appl Immunol 1967;32:453–62.
- Fleming CJ, Burden AD, Forsyth A. The genetics of allergic contact hypersensitivity to nickel. Contact Dermatitis 1999;41:251–3
- Bryld LE, Hindsberger C, Kyvik KO, et al. Genetic factors in nickel allergy evaluated in a population-based female twin sample. J Invest Dermatol 2004;123:1025–9.
- Forsbeck M, Skog E, Ytterborn KH. Delayed type of allergy and atopic disease among twins. Acta Derm Venereol (Stockh) 1968;48:192–7.
- Lidén S, Beckman C, Groth O, et al. Lack of association between allergic contact dermatitis and HLA antigens of the A and B series. Acta Derm Venereol (Stockh) 1980;61:155–7.
- Valsecchi R, Bontempelli M, Vicari O, et al. HLA antigens and contact sensitivities. Arch Dermatol 1982;118:533–4.
- Schnuch A, Westphal GA, Muller MM, et al. Genotype and phenotype of N-acetyltransferase 2 (NAT2) polymorphism in patients with contact allergy. Contact Dermatitis 1998;38:209–11.
- Reich K, Westphal G, König IR, et al. Association of allergic contact dermatitis with a promoter polymorphism in the IL16 gene. J Allergy Clin Immunol 2003;112:1191–4.
- Westphal GA, Schnuch A, Mössner R, et al. Cytokine gene polymorphisms in allergic contact dermatitis. Contact Dermatitis 2003;48:93–8.
- Clark RAF. Cell-mediated and IgE-mediated responses in atopic dermatitis. Arch Dermatol 1989;125:413–16.
- Bos JO, Wierenga EA, Smitt JHS, et al. Immune dysregulation in atopic eczema. Arch Dermatol 1992;128:1509–12.
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol 1989;125:366–8.
- Bandmann H-J, Breit R, Leutgeb C. Kontakallergie und Dermatitis atopica. Arch Dermatol Forsch 1972;244:332–4.
- Cronin E, Bandmann H-J, Calnan CD, et al. Contact dermatitis in the atopic. Acta Derm Venereol (Stockh) 1970;50:183–7.
- De Groot AC. The frequency of contact allergy in atopic patients with dermatitis. Contact Dermatitis 1990;22:273–7.
- Hanifin JH. Atopic dermatitis. J Am Acad Dermatol 1982;6:1–13.
- Diepgen TL, Fartasch M, Hornstein OP. Evaluation and relevance of atopic basic and minor features in patients with atopic dermatitis and in the general population. Acta Derm Venereol Suppl (Stockh) 1989;144:50–4.
- McDonagh AJ, Wright AL, Cork MJ, et al. Nickel sensitivity: the influence of ear piercing and atopy. Br J Dermatol 1992;126:16–18.
Environmental factors
- Skov L, Hansen H, Barker JN, et al. Contrasting effects of ultraviolet-A and ultraviolet-B exposure on induction of contact sensitivity in human skin. Clin Exp Immunol 1997;107:585–8.
- Damian DL, Barnetson RS, Halliday GM. Low-dose UVA and UVB have different time courses for suppression of contact hypersensitivity to a recall antigen in humans. J Invest Dermatol 1999;112:939–44.
- Dooms-Goossens A, Lesaffre E, Heidbuchel M, et al. UV sunlight and patch test reactions in humans. Contact Dermatitis 1988;19:36–42.
- Uter W, Geier J, Land M, et al. Another look at seasonal variation in patch test results. A multifactorial analysis of surveillance data of the IVDK. Information Network of Departments of Dermatology. Contact Dermatitis 2001;44:146–52.
- Hannuksela M, Pirilä V, Salo OP. Skin reactions to propylene glycol. Contact Dermatitis 1975;1:112–16.
- Hjorth N. Seasonal variations in contact dermatitis. Acta Derm Venereol (Stockh) 1967;47:409–18.
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci USA 2006;103:9086–9.
- Salo H, Hannuksela M, Hausen B. Lichen picker's dermatitis (Cladonia alpestris (L.) Rab.). Contact Dermatitis 1981;7:9–13.
- Paulsen E, Andersen KE. Compositae dermatitis in a Danish dermatology department in 1 year (II). Clinical features in patients with Compositae contact allergy. Contact Dermatitis 1993;29:195–201.
- Malten KE. Chicory dermatitis from September to April. Contact Dermatitis 1983;9:232.
- Li LF. A clinical and patch test study of contact dermatitis from traditional Chinese medicinal materials. Contact Dermatitis 1995;33:392–5.
- Goh CL. The need for epidemiological studies. Am J Contact Dermatitis 1997;8:135–6.
- Park SD, Lee SW, Chun JH, et al. Clinical features of 31 patients with systemic contact dermatitis due to the ingestion of Rhus (lacquer). Br J Dermatol 2000;142:937–42.
- Hsu TS, Davis MD, el-Azhary R, et al. Beard dermatitis due to para-phenylenediamine use in Arabic men. J Am Acad Dermatol 2001;44:867–9.
- Dwyer CM, Forsyth A. Allergic contact dermatitis from bindi. Contact Dermatitis 1994;30:174.
Clinical features
History
- Kanerva L, Tarvainen K, Pinola A, et al. A single accidental exposure may result in a chemical burn, primary sensitization and allergic contact dermatitis. Contact Dermatitis 1994;31:229–36.
- Wong CS, Beck MH. Occupational contact allergy to methyldibromoglutaronitrile in abrasive cleansers and work creams. Contact Dermatitis 2001;44:311–12.
Presentation
Primary patterns
- Wilkinson DS, Bandmann H-J, Calnan CD, et al. The role of contact allergy in hand eczema. Trans St John's Hosp Dermatol Soc 1970;56:19–25.
- Agrup G. Hand eczema and other hand dermatoses in South Sweden. Acta Derm Venereol Suppl (Stockh) 1969;61:54.
- Hjorth N, Roed-Petersen J. Occupational protein contact in food handlers dermatitis. Contact Dermatitis 1976;2:28–42.
- Kanerva L, Toikkanen J, Jolanki R, Estlander T. Statistical data on occupational contact urticaria. Contact Dermatitis 1996;35:299–33.
- Bruynzeel DP, De Boer EM, Brouwer EJ, et al. Dermatitis in bulb growers. Contact Dermatitis 1993;29:11–15.
- Burks JW. Classic aspects of onion and garlic dermatitis in housewives. Ann Allergy 1954;12:592–6.
- Lidén C, Berg M, Farm G, et al. Nail varnish allergy with far-reaching consequences. Br J Dermatol 1993;128:57–62.
- Calnan CD, Sarkany I. Studies in contact dermatitis. III. Nail varnish. Trans St John's Hosp Dermatol Soc 1958;40:1–11.
- Tucker SC, Beck MH. A 15-year study of patch testing to (meth)acrylates. Contact Dermatitis 1999;40:278–9.
- Helbling I, Beck MH. Rubber sponge applicator responsible for ‘cosmetic’ facial dermatitis. Contact Dermatitis 1998;39:43.
- Dooms-Goossens AE, Debusschere KM, Cevers DM, et al. Contact dermatitis caused by airborne agents. J Am Acad Dermatol 1986;15:1–10.
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis 1992;27:143–7.
- Guin JD. Eyelid dermatitis: a report of 215 patients. Contact Dermatitis 2004;50:87–90.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis 1991;25:77.
- Vestey JP, Buxton PK, Savin JA. Eyelash curler dermatitis. Contact Dermatitis 1985;13:274–5.
- Ekeowa-Anderson AL, Shergill B, Goldsmith P. Allergic contact cheilitis to garlic. Contact Dermatitis 2007;56:174–5.
- Orton DI, Salim A, Shaw S. Allergic contact cheilitis due to shellac. Contact Dermatitis 2001;44:250.
- Calnan CD, Sarkany I. Studies in contact dermatitis. II. Lipstick cheilitis. Trans St John's Hosp Dermatol Soc 1957;39:28–36.
- Magnusson B, Wilkinson DS. Cinnamic aldehyde in a toothpaste. Contact Dermatitis 1975;1:70–6.
- Andersen KE. Contact allergy to toothpaste flavors. Contact Dermatitis 1978;4:195–8.
- Gupta G, Forsyth A. Allergic contact reactions to colophony presenting as oral disease. Contact Dermatitis 1999;40:332–3.
- Veysey EC, Orton DI. Photoallergic contact cheilitis due to oxybenzone in a lip cosmetic. Contact Dermatitis 2006;55:54.
- Fisher AA. Perlèche (angular cheilitis) due to contactants. Cutis 1974;14:499–501.
- J Millard TP, Orton DI. Changing patterns of contact allergy in chronic inflammatory ear disease. Contact Dermatitis 2004;50:83–6.
- Lear JT, Sandhu G, English JS. Hearing aid dermatitis: a study in 20 consecutive patients. Contact Dermatitis 1998;38:212.
- Glas B, Egelrud T. Nickel in ‘nickel-free’ spectacle frames. Contact Dermatitis 1999;40:217.
- Bircher AJ, Stern WB. Allergic contact dermatitis from ‘titanium’ spectacle frames. Contact Dermatitis 2001;45:244–5.
- Casper C, Groth W, Hunzelmann N. Sarcoidal-type allergic contact granuloma: a rare complication of ear piercing. Am J Dermatopathol 2004;26:59–62.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol 2002;46:309–12.
- Shehade SA, Beck MH. Contact dermatitis from disperse dyes in synthetic wigs. Contact Dermatitis 1990;23:124–5.
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis 2006;55:48–53.
- Kazandjieva J, Gergovska M, Darlenski R. Contact dermatitis in a child from methlychloroisothiazolinone and methylisothiazolinone in moist wipes. Pediatr Dermatol 2014;31:225–7.
- Lazarov A. Perianal contact dermatitis caused by nail lacquer allergy. Am J Contact Dermatitis 1999;10:43–4.
- Rosen T, Fordice DB. Cashew nut dermatitis. South Med J 1994;87:543–6.
- Goldsmith PC, Rycroft RJ, White IR, et al. Contact sensitivity in women with anogenital dermatoses. Contact Dermatitis 1997;36:174–5.
- Nardelli A, Degreef H, Goossens A. Contact allergic reactions of the vulva: a 14-year review. Contact Dermatitis 2004;15:131–6.
- Batchelor RJ, Young HS, Beck MH. The role of patch testing in perineal disorders affecting women. Exog Dermatol 2003;2:178–83.
- Lewis FM, Shah M, Gawkrodger DJ. Contact sensitivity in pruritus vulvae: patch test results and clinical outcome. Am J Contact Dermatitis 1997;8:137–40.
- Nunns D, Ferguson J, Beck M, et al. Is patch testing necessary in vulval vestibulitis? Contact Dermatitis 1997;37:87–9.
- Guillet G, Dagregorio G, Guillet MH. Vulvar contact dermatitis due to seminal allergy: 3 cases. Ann Dermatol Venereol 2005;132:123–5.
- Wilson CL, Cameron J, Powell SM, et al. High incidence of contact dermatitis in leg-ulcer patients: implications for management. Clin Exp Dermatol 1991;16:250–3.
Exposed sites
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis 2001;44:1–6.
- Mitchell JC, Calnan CD. Scourge of India: Parthenium dermatitis. Int J Dermatol 1978;17:303–4.
- Sadhra S, Foulds IS, Gray CN, et al. Colophony: uses, health effects, airborne measurement and analysis. Ann Occup Hyg 1994;38:385–96.
- Karlberg AT, Gäfvert E, Meding B, et al. Airborne contact dermatitis from unexpected exposure to rosin (colophony). Rosin sources revealed with chemical analyses. Contact Dermatitis 1996;35:272–8.
- De Groot AC, Young E. The role of contact allergy to aeroallergens in atopic dermatitis. Contact Dermatitis 1989;21:209–14.
Mucous membranes
- Lowney ED. Immunologic unresponsiveness to a contact sensitizer in man. J Invest Dermatol 1968;51:411–17.
- Van Hoogstraten MW, Andersen KE, Von Blomberg BME, et al. Preliminary results of a multicenter study on the incidence of nickel allergy in relationship to previous oral and cutaneous contacts. In: Frosch PJ, Dooms-Goossens A, LaChapelle JM, et al., eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:178–83.
- Vreeburg KJJ, De Groot K, Von Blomberg BME, et al. Induction of immunological tolerance by oral administration of nickel and chromium. J Dent Res 1984;63:124–8.
- Ahlfors EE, Lyberg T. Contact sensitivity reactions in the oral mucosa. Acta Odontol Scand 2001;59:248–54.
- Nadiminti H, Ehrlich A, Udey MC. Oral erosions as a manifestation of allergic contact sensitivity to cinnamon mints. Contact Dermatitis 2005;52:46–7.
- Shah M, Lewis F, Gawkrodger DJ. Contact allergy in patients with oral symptoms: a study of 47 patients. Am J Contact Dermatitis 1996;7:146–51.
- Helton J, Storrs F. The burning mouth syndrome: lack of a role for contact urticaria and contact dermatitis. J Am Acad Dermatol 1994;31:201–5.
- Armstrong DK, Biagioni P, Lamey PJ, et al. Contact hypersensitivity in patients with orofacial granulomatosis. Am J Contact Dermatitis 1997;8:35–8.
- Koutis D, Freeman S. Allergic contact stomatitis caused by acrylic monomer in a denture. Australas J Dermatol 2001;42:203–6.
- Renner RP, Lee M, Andors L, et al. The role of C. albicans in denture stomatitis. Oral Surg Oral Med Oral Pathol 1979;47:323–8.
- Alanko K, Kanerva L, Jolanki R, et al. Oral mucosal diseases investigated by patch testing with a dental screening series. Contact Dermatitis 1996;34:263–7.
- Jolly M, Moule AJ, Freeman S. Amalgam related chronic ulceration of oral mucosa. Br Dent J 1986;160:434–7.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury sensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis 1995;33:323–9.
- Scalf LA, Fowler JF, Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermatitis 2001;12:146–50.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis 1983;9:411–17.
- Van Loon Heidelberg LAJ, van Elzas PW, van Joost Th, et al. Contact stomatitis and dermatitis to nickel and palladium. Contact Dermatitis 1984;11:294–7.
- Laeijendecker R, van Joost Th. Oral manifestations of gold allergy. J Am Acad Dermatol 1994;30:205–9.
- Silvestre JF, Albares MP, Blanes M, et al. Allergic contact gingivitis due to eugenol present in a restorative dental material. Contact Dermatitis 2005;52:341.
- Sainio E-L, Kanerva L. Contact allergens in toothpastes and a review of their hypersensitivity. Contact Dermatitis 1995;33:100–6.
- Isaksson M, Bruze M, Wihl JA. Contact allergy to budesonide and perforation of the nasal septum. Contact Dermatitis 1997;37:133.
- Herbst RA, Maibach HI. Contact dermatitis caused by allergy to ophthalmic drugs and contact lens solutions. Contact Dermatitis 1991;25:305–12.
- Goldsmith PC, Rycroft RJ, White IR, et al. Contact sensitivity in women with anogenital dermatoses. Contact Dermatitis 1997;36:174–5.
Specific allergens
Nickel
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:455–9.
- Maibach HI, Menné T, eds. Nickel and the Skin: Immunology and Toxicology. Boca Raton, FL: CRC Press, 1989.
- Menné T. Nickel Allergy. Copenhagen: Marselis tryk a-s, 1983.
- Cronin E. Contact Dermatitis. Edinburgh: Churchill Livingstone, 1980:344–5, 353–7.
- Marcussen PV. The rise in prevalence of nickel sensitivity. Br J Dermatol 1959;71:97–101.
- Nielsen NH, Menné T. Nickel sensitisation and ear piercing in an unselected Danish population. Contact Dermatitis 1993;29:16–21.
- Kåre Dotterud L, Smith-Sivertsen T. Allergic contact sensitization in the general adult population: a population-based study from northern Norway. Contact Dermatitis 2007;56:10–15.
- Kanan MW. Contact dermatitis in Kuwait. J Kuwait Med Assoc 1968;3:129–44.
- Olumide YM. Contact dermatitis in Nigeria. Contact Dermatitis 1985;12:241–6.
- Sugai T, Takagi T, Yamamoto S, et al. Age distribution of the prevalence of contact sensitivity to standard allergens. Contact Dermatitis 1979;5:383–8.
- Van der Burg CKH, Brunyzeel DP, Vreeburg KHH, et al. Hand eczema in hairdressers and nurses: a prospective study. I. Evaluation of atopy and nickel hypersensitivity at the start of apprenticeship. Contact Dermatitis 1986;14:275–9.
- Van der Walle HB, Brunsveld VM. Dermatitis in hairdressers. (1). The experience of the last 4 years. Contact Dermatitis 1994;30:217–21.
- Garg S, Thyssen JP, Uter W, et al. Nickel allergy following EU regulation in Denmark, Germany, Italy and the United Kingdom. Br J Dermatol 2013;169:854–8.
- Johansen J, Menné T, Christophersen J, et al. Changes in the pattern of sensitization to common contact allergens in Denmark between 1985–86 and 1997–98, with a special view to the effect of preventive strategies. Br J Dermatol 2000;142:490–5.
- Schnuch A, Uter W. Decrease in nickel allergy in Germany and regulatory interventions. Contact Dermatitis 2003;49:107–8.
- Basketter DA, Briatico-Vangosa G, Kaestner W, et al. Nickel, cobalt and chromium in consumer products: a role in allergic contact dermatitis? Contact Dermatitis 1993;28:15–25.
- Menné T, Andersen KE, Kaaber K, et al. Evaluation of the dimethylglyoxime test for detection of nickel. Berufsdermatosen 1987;35:128–30.
- Menné T, Christophersen J, Green A. Epidemiology of nickel dermatitis. In: Maibach HI, Menné T, eds. Nickel and the Skin: Immunology and Toxicology. Boca Raton, FL: CRC Press, 1989:109–15.
- Jellesen MS, Hilbert LR, Menné T, Möller H. Nickel-containing coins: a health risk for nickel-sensitive individuals? Br J Dermatol 2006;155:1301–2.
- Trevisan G, Kokelj F. Allergic contact dermatitis from nickel in an electrocautery plate. Contact Dermatitis 1992;26:267.
- Wöhrl S, Jandl T, Stingl G, Kinaciyan T. Mobile telephone as new source for nickel dermatitis. Contact Dermatitis 2007;56:113.
- Jensen P, Jellesen MS, Møller P, Johansen JD, Lidén C, Menné T, Thyssen JP. Nickel may be released from laptop computers. Contact Dermatitis. 2012;67(6):384–5
- Baxter KF, Wilkinson SM. Contact dermatitis from a nickel-containing bindi. Contact Dermatitis 2002;47:55.
- Raison-Peyron N, Guillard O, Khalil Z, et al Nickel-elicited systemic contact dermatitis from a peripheral intravenous catheter. Contact Dermatitis 2005;53:222–5.
- Morales-Callaghan AM, Aguilar-Bernier M, Martínez-García G, Miranda-Romero E. Sarcoid granuloma on black tattoo. J Am Acad Dermatol 2006;55:S71–3.
- Genelhu MC, Marigo M, Alves-Oliveira LF, et al. Characterization of nickel-induced allergic contact stomatitis associated with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop 2005;128:378–81.
- Kokelj F, Daris F, Lutmann A, et al. Nickel, chromate and cobalt in toilet soaps analysed by inductively coupled plasma mass spectrometry. Contact Dermatitis 1994;31:270.
- Van Ketel WG, Bruynzeel DP. Allergic contact dermatitis from nickel in eyeshadow [Letter]. Contact Dermatitis 1989;21:355.
- Berne B, Boström Å, Grahnén AF, Tammela M. Adverse effects of cosmetics and toiletries reported to the Swedish Medical Protection Agency 1989–94. Contact Dermatitis 1996;34:359–62.
- Lee AY, Lee YS. A case of allergic contact dermatitis due to nickel in underground water. Contact Dermatitis 1990;22:141–3.
- Pecegueiro M. Contact dermatitis due to nickel in fertilizers. Contact Dermatitis 1990;22:114–15.
- Flint GN, Packirisamy S. Systemic nickel: the contribution made by stainless-steel cooking utensils. Contact Dermatitis 1995;32:218–24.
- Marcussen PV. Spread of nickel dermatitis. Dermatologica 1957;115:596–607.
- Wilkinson DS, Wilkinson JD. Nickel allergy and hand eczema. In: Maibach HI, Menné T, eds. Nickel and the Skin: Immunology and Toxicology. Boca Raton, FL: CRC Press, 1989:133–63.
- Christophersen J, Menné TM, Tanghof P, et al. Clinical patch test data evaluated by multivariate analysis. Contact Dermatitis 1989;21:291–9.
- Edman B. Sites of contact dermatitis in relationship to particular allergens. Contact Dermatitis 1985;13:129–35.
- Meding B, Swanbeck G. Predictive factors for hand eczema. Contact Dermatitis 1990;23:154–62.
- Menné T, Borgan Ø, Green A. Nickel allergy and hand dermatitis in a stratified sample of the Danish female population. Acta Derm Venereol (Stockh) 1982;62:35–41.
- Josefson A, Färm G, Stymne B, Meding B. Nickel allergy and hand eczema – a 20-year follow up. Contact Dermatitis 2006;55:286–90.
- Nilsson EJ, Bäck O. The importance of anamnestic information of atopy, metal dermatitis and earlier hand eczema for the development of hand dermatitis in women in wet hospital work. Acta Derm Venereol (Stockh) 1986;66:45–50.
- Nilsson EJ, Knutson A. Atopic dermatitis, nickel sensitivity and xerosis as risk factors for hand eczema in women. Contact Dermatitis 1995;33:401–6.
- Christensen OB, Möller H. Nickel allergy and hand eczema. Contact Dermatitis 1975;1:129–35.
- Burrows D. Mischievous metals: chromate, cobalt, nickel and mercury. Clin Exp Dermatol 1989;14:266–72.
- Gawkrodger DJ, Cook SW, Fell GS, et al. Nickel dermatitis: the reaction to oral nickel challenge. Br J Dermatol 1986;115:33–8.
- Jordan WP, King SE. Nickel feeding in nickel-sensitive patients with hand eczema. J Am Acad Dermatol 1979;1:506–8.
- Roduner J, Haudenschilde-Falb E, Kunz E, et al. Oral nickel challenge in non-pompholyx and pompholyx-type nickel eczema. Hautarzt 1987;38:262–6.
- Jensen CS, Menné T, Lisby S, et al. Experimental systemic contact dermatitis from nickel: a dose–response study. Contact Dermatitis 2003;49:124–32.
- Jensen CS, Menné T, Johansen JD. Systemic contact dermatitis after oral exposure to nickel: a review with a modified meta-analysis. Contact Dermatitis 2006;54:79–86.
- Pigatto PD, Guzzi G. Systemic contact dermatitis from nickel associated with orthodontic appliances. Contact Dermatitis 2004;50:100–1.
- Schultz JC, Connelly E, Glesne L, Warshaw EM. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis 2004;15:154–7.
- Van Hoogstraten IMW, Andersen KE, von Blomberg BME, et al. Preliminary results of a multicentre study on the prevalence of nickel allergy in relationship to previous oral and cutaneous contacts. In: Frosch P, Dooms-Goossens A, LaChapelle J-M, et al., eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:178–83.
- White JM, Goon AT, Jowsey IR, et al. Oral tolerance to contact allergens: a common occurrence? A review. Contact Dermatitis 2007;56:247–54.
- Wall LM. Nickel penetration through rubber gloves. Contact Dermatitis 1980;6:461–3.
- Veien NK. Nickel dermatitis: its relationship to food and experimental oral challenge. In: Maibach HI, Menné T, eds. Nickel and the Skin: Immunology and Toxicology. Boca Raton, FL: CRC Press, 1989:165–97.
- Gawkrodger DJ, Healy J, Howe AM. The prevention of nickel contact dermatitis. A review of the use of binding agents and barrier creams. Contact Dermatitis 1995;32:257–65.
- Wohrl S, Kriechbaumer N, Hemmer W, et al. A cream containing the chelator DTPA (diethylenetriaminepenta-acetic acid) can prevent contact allergic reactions to metals. Contact Dermatitis 2001;44:224–8.
- Healy J, Johnson S, Little MC, et al. An in vitro study of the use of chelating agents in cleaning nickel-contaminated human skin: an alternative approach to preventing nickel allergic contact dermatitis. Contact Dermatitis 1998;39:171–81.
- Memon AA, Molokhia MM, Friedmann PS. The inhibitory effects of topical chelating agents and antioxidants on nickel-induced hypersensitivity reactions. J Am Acad Dermatol 1994;30:560–5.
- Kaaber K, Veien NK, Tjell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol 1978;98:197–201.
- Sharma AD. Disulfiram and low nickel diet in the management of hand eczema: a clinical study. Indian J Dermatol Venereol Leprol 2006;72:113–18.
- Christensen OB, Kristensen M. Treatment with disulfiram in chronic nickel hand dermatitis. Contact Dermatitis 1982;8:59–63.
- Burrows D, Rogers S, Beck M, et al. Treatment of nickel dermatitis with trientine. Contact Dermatitis 1986;15:55–7.
- Cronin E. Patch testing with nickel. Contact Dermatitis 1975;1:56–7.
- Fullerton A, Anderson JR, Hoelgaard A, et al. Permeation of nickel salts through human skin in vitro. Contact Dermatitis 1986;15:173–7.
Cobalt
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:461–2.
- Nielsen NH, Linneberg A, Menné T, et al. Incidence of allergic contact sensitization in Danish adults between 1990 and 1998; the Copenhagen Allergy Study, Denmark. Br J Dermatol 2002;147:487–92.
- Dotterud LK, Smith-Sivertsen T. Allergic contact sensitization in the general adult population: a population-based study from northern Norway. Contact Dermatitis 2007;56:10–15.
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Philadelphia: Lippincott, Williams and Wilkins, 2001:605–62.
- Basketter DA, Briatico-Vangosa G, Kaestner W, et al. Nickel, cobalt and chromium in consumer products: a role in allergic contact dermatitis? Contact Dermatitis 1993;28:15–25.
- Tandon R, Aarts B. Chromium, nickel and cobalt contents of some Australian cements. Contact Dermatitis 1993;28:201–5.
- Tuomi M-L, Räsänen L. Contact allergy to tylosin and cobalt in a pig-farmer. Contact Dermatitis 1995;33:285.
- Laing ME, Hackett CB, Murphy GM. Unusual allergen in nurse uniform trousers. Contact Dermatitis 2005;52:293.
- Björnberg A. Allergic reaction to cobalt in light blue tattoo markings. Acta Derm Venereol (Stockh) 1961;41:259–63.
- Kokelj F, Daris F, Lutmann A, et al. Nickel, chromate and cobalt in toilet soaps analysed by inductively coupled plasma mass spectrometry. Contact Dermatitis 1994;31:270.
- Allenby CF, Basketter DA. Minimum eliciting patch test concentrations of cobalt. Contact Dermatitis 1989;20:185–90.
- Thyssen JP, Johansen JD, Jellesen MS, Møller P, Sloth JJ, Zachariae C, Menné T. Consumer leather exposure: an unrecognized cause of cobalt sensitization. Contact Dermatitis 2013;69:276–9.
- Gawkrodger DJ, Lewis FM. Isolated cobalt sensitivity in an etcher. Contact Dermatitis 1993;29:46.
- Foussereau J, Cavelier C. Allergic contact dermatitis from cobalt in the rubber industry. Contact Dermatitis 1988;19:217.
- Tarvainen K, Jolanki R, Forsman-Grönholm L, et al. Exposure, skin protection and occupational skin diseases in the glass-fibre-reinforced plastics industry. Contact Dermatitis 1993;29:119–27.
- Price ML, MacDonald DM. Cheilitis and cobalt allergy related to ingestion of vitamin B12. Contact Dermatitis 1981;7:352.
- Ratcliffe J, English JS. Allergic contact dermatitis from cobalt in animal feed. Contact Dermatitis 1998;39:201–2.
- Romaguera C, Lecha M, Grimalt F, et al. Photocontact dermatitis to cobalt salts. Contact Dermatitis 1982;8:383–8.
- Fisher AA. Contact dermatitis at home and abroad. Cutis 1972;10:719–23.
- Sockanathan S, Setterfield J, Wakelin S. Oral lichenoid reaction due to chromate/cobalt in dental prosthesis. Contact Dermatitis 2003;48:342–3.
- Veien N, Hattel T, Laurberg G. Placebo-controlled oral challenge with cobalt in patients with positive patch tests to cobalt. Contact Dermatitis 1995;33:54–5.
- Midander K, Julander A, Skare L, Thyssen JP, Lidén C. The cobalt spot test – further insights into its performance and use. Contact Dermatitis 2013;69:280–7.
- Förström L, Pirilä V, Huju P. Rehabilitation of workers with cement eczema due to hypersensitivity to bichromate. Scand J Rehab Med 1969;1:95–100.
Chromium
- Burrows D, ed. Chromium: Metabolism and Toxicity. Boca Raton, FL: CRC Press, 1983.
- Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:459–61, 548–54.
- Wahlberg JE, Lindstedt G, Einarsson Ö. Chromium, cobalt and nickel in Swedish cement, detergents, mould and cutting oils. Berufsdermatosen 1977;25:220–8.
- Fregert S. Chromium valencies and cement dermatitis. Br J Dermatol 1981;105(Suppl. 21):7–9.
- Fregert S, Rorsman H. Allergic reactions to trivalent chromium compounds. Arch Dermatol 1966;93:711–13.
- Hansen MB, Johansen JD, Menné T. Chromium allergy: significance of both Cr(III) and Cr(VI). Contact Dermatitis 2003;49:206–12.
- Geier J, Schnuck A. A comparison of contact allergens among construction and non-construction workers attending contact dermatitis clinics in Germany: results of the Information Network of Departments of Dermatology from November 1989 to July 1993. Am J Contact Dermatitis 1995;6:86–94.
- Wilkinson DS, Wilkinson JD. Comparison of patch test results in two adjacent areas of England. I. Industrial allergens. Acta Derm Venereol Suppl (Stockh) 1979;89:189–92.
- Irvine C, Pugh CE, Hansen E, Rycroft RJ. Cement dermatitis in underground workers during construction of the Channel Tunnel. Occup Med 1994;44:17–23.
- Athavale P, Shum KW, Chen Y, et al. Occupational dermatitis related to chromium and cobalt: experience of dermatologists (EPIDERM) and occupational physicians (OPRA) in the UK over an 11-year period (1993–2004). Br J Dermatol 2007;157:518–22.
- Zachariae COC, Agner T, Menné T. Chromium allergy in consecutive patients in a country where ferrous sulphate has been added to cement since 1981. Contact Dermatitis 1996;35:83–6.
- Goh CL, Gan SL. Change in cement manufacturing process, a cause for decline in chromate allergy? Contact Dermatitis 1996;34:51–4.
- Thyssen JP, Johansen JD, Menné T. Contact allergy epidemics and their controls. Contact Dermatitis 2007;56:185–95.
- Stocks SJ, McNamee R, Turner S, Carder M, Agius RM. Has European Union legislation to reduce exposure to chromate in cement been effective in reducing the incidence of allergic contact dermatitis attributed to chromate in the UK? Occup Environ Med 2012;69(2):150–2.
- Fregert S, Gruvberger B. Chemical aspects on chromate in cement. Dermatosen 1982;30:76–8.
- Tandon R, Aarts B. Chromium, nickel and cobalt contents of some Australian cements. Contact Dermatitis 1993;28:201–5.
- Engel HO, Calnan CD. Chromate dermatitis from paint. Br J Ind Med 1963;20:192–8.
- Calnan CD, Harman RRM. Studies in contact dermatitis XIII. Diesel coolant chromate dermatitis. Trans St John's Hosp Dermatol Soc 1961;46:13–21.
- Handley J, Burrows D. Dermatitis from hexavalent chromate in the accelerator of an epoxy sealant (PR1422) used in the aircraft industry. Contact Dermatitis 1994;30:193–6.
- Fregert S. Chromate eczema and matches. Acta Derm Venereol (Stockh) 1961;41:433–42.
- Fregert S, Övrum P. Chromate in welding fumes with special reference to contact dermatitis. Acta Derm Venereol (Stockh) 1963;43:119–24.
- Fregert S, Gruvberger B, Heijer A. Sensitization to chromium and cobalt in processing of sulphate pulp. Acta Derm Venereol (Stockh) 1972;52:221–4.
- Wilkinson SM, Heagerty AHM, English JSC. Hand dermatitis in the pottery industry. Contact Dermatitis 1992;26:91–4.
- Buckley DA, Rogers S. ‘Fiddler's fingers’: violin-string dermatitis. Contact Dermatitis 1995;32:46–7.
- Fregert S, Gruvberger B. Chromate dermatitis from oil emulsion contaminated from zinc-galvanized sheet. Contact Dermatitis 1976;2:121.
- Fregert S, Gruvberger B, Göransson K, et al. Allergic contact dermatitis from chromate in military textiles. Contact Dermatitis 1978;4:223–4.
- Richter G, Heidelbach U. Chromatekzem nach Glasmattierung mit einem Korund. Berufsdermatosen 1969;17:8–12.
- Heine A, Fox G. Bäckerekzem durch Chromverbindung in Mehlen. Dermatosen 1980;28:113–15.
- Burrows D. Chromium dermatitis in a tyre fitter. Contact Dermatitis 1981;7:55–6.
- Stevenson CJ, Morgan PR. Investigation and prevention of chromate dermatitis in colour television manufacture. J Soc Occup Med 1983;33:19–20.
- Basketter DA, Briatico-Vangosa G, Kaestner W, et al. Nickel, cobalt and chromium in consumer products: a role in allergic contact dermatitis? Contact Dermatitis 1993;28:15–25.
- Veien NK, Borchorst E, Hattel T, Laurberg G. Stomatitis or systemically-induced contact dermatitis from metal wire in orthodontic materials. Contact Dermatitis 1994;30:210–13.
- LaChapelle J-M, Lauwerys R, Tennstedt D, et al. Eau de Javel and chromate allergy in France. Contact Dermatitis 1980;6:107–10.
- Kaaber K, Veien NK. The significance of chromate ingestion in patients allergic to chromate. Acta Derm Venereol (Stockh) 1977;57:321–3.
- Patel TG, Kleyn E, King CM, Wilson NJ. Chromate allergy from contact with leather furnishings. Contact Dermatitis 2006;54:171–2.
- Sockanathan S, Setterfield J, Wakelin S. Oral lichenoid reaction due to chromate/cobalt in dental prosthesis. Contact Dermatitis 2003;48:342–3.
- Thormann J, Jespersen NB, Joensen HD. Persistence of contact allergy to chromium. Contact Dermatitis 1979;5:261–4.
- Breit R, Türk RBM. The medical and social fate of the dichromate allergic patient. Br J Dermatol 1976;94:349–51.
- Burry JN, Kirk J. Environmental dermatitis: chrome cripples. Med J Aust 1975;2:720–1.
- Czarnecki N. Die Persistenz der Chromatallergie beim Zementekze. Hautartz 1979;30:80–3.
- Dooms-Goossens A, Ceuterick A, Vanhaele N, et al. Follow-up study of patients with contact dermatitis caused by chromates, nickel and cobalt. Dermatologica 1980;160:249–60.
- Fregert S. Occupational dermatitis in 10-year material. Contact Dermatitis 1975;1:96–107.
- Burrows D. Prognosis in industrial dermatitis. Br J Dermatol 1972;87:145–8.
- Lips R, Rast H, Elsner P. Outcome of job change in patients with occupational chromate dermatitis. Contact Dermatitis 1996;34:268–71.
- Burrows D, Calnan CD. Cement dermatitis. II. Clinical aspects. Trans St John's Hosp Dermatol Soc 1965;51:27–39.
- Kaaber K, Veien NK. The significance of chromate ingestion in patients allergic to chromate. Acta Derm Venereol 1977;57:321–3.
- Valsecchi R, Caineti T. Chromium dermatitis and ascorbic acid. Contact Dermatitis 1984;10:252–97.
- Samitz NH, Katz S. A study of the chemical reactions between chromium and skin. J Invest Dermatol 1964;43:35–43.
- Schuppli R. Uber einen neuen Typus von Schutzalben gegen Chromatekzeme. Berufsdermatosen 1970;18:350–5.
- Veien NK, Hattel T, Laurberg G. Chromate-allergic patients challenged orally with potassium dichromate. Contact Dermatitis 1994;31:137–9.
- Miyachi Y, Uchida K, Komura J, et al. Auto-oxidative damage in cement dermatitis. Arch Dermatol Res 1985;277:288–92.
- Burrows D, Andersen KE, Camarasa JG, et al. Trial of 0.5% versus 0.375% potassium dichromate. Contact Dermatitis 1989;21:351.
Palladium
- Lidén C. Metals. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:566–7.
- Aberer W, Holub H, Strohal R, et al. Palladium in dental alloys: the dermatologists' responsibility to warn? Contact Dermatitis 1993;28:163–5.
- Larese Filon F, Uderzo D, Bagnato E. Sensitization to palladium chloride: a 10-year evaluation. Am J Contact Dermatitis 2003;14:78–81.
- Wahlberg JE, Boman AS. Cross-reactivity to palladium and nickel studied in the guinea pig. Acta Derm Venereol (Stockh) 1992;72:95–7.
- Hindsén M, Spirén A, Bruze M. Cross-reactivity between nickel and palladium demonstrated by systemic administration of nickel. Contact Dermatitis 2005;53:2–8.
- Kanerva L, Kerosuo H, Kullaa A, et al. Allergic patch test reactions to palladium chloride in schoolchildren. Contact Dermatitis 1996;34:39–42.
- Kranke B, Aberer W. Multiple sensitivities to metals. Contact Dermatitis 1996;34:225.
- Todd DJ, Burrows D. Patch testing with pure palladium metal in patients with sensitivity to palladium chloride. Contact Dermatitis 1992;26:327–31.
- Downey D. Contact mucositis due to palladium. Contact Dermatitis 1989;21:54.
- Murao Y, Yamada S, Kameyoshi Y, Yamamoto S. A case of generalized lichen planus due to palladium in dental metals. Environ Dermatol 1995;2:197–203.
- Goossens A, De Swerdt A, De Coninck K, et al. Allergic contact granuloma due to palladium following ear piercing. Contact Dermatitis 2006;55:338–41.
Gold
- Lidén C. Metals. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:563–5.
- Björkner B, Bruze M, Möller H. High frequency of contact allergy to gold sodium thiosulfate: an indication of gold allergy? Contact Dermatitis 1994;30:144–51.
- Bruze M, Edman B, Björkner B, et al. Clinical relevance of contact allergy to gold sodium thiosulfate. J Am Acad Dermatol 1994;31:579–83.
- Bruze M, Andersen KE. Gold: a controversial sensitizer. European Environmental and Contact Dermatitis Research Group. Contact Dermatitis 1999;40:295–9.
- Ahlgren C, Ahnlide I, Björkner B, et al. Contact allergy to gold is correlated to dental gold. Acta Derm Venereol 2002;82:41–4.
- Svedman C, Tillman C, Gustavsson CG, et al. Contact allergy to gold in patients with gold-plated intracoronary stents. Contact Dermatitis 2005;52:192–6.
- McKenna KE, Dolan O, Walsh MY, et al. Contact allergy to gold sodium thiosulfate. Contact Dermatitis 1995;32:143–6.
- Fleming C, Burden D, Fallowfield M, et al. Lymphomatoid contact reaction to gold earrings. Contact Dermatitis 1997;37:298–9.
- Armstrong DK, Walsh MY, Dawson JF. Granulomatous contact dermatitis due to gold earrings. Br J Dermatol 1997;136:776–8.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol 1994;30:205–9.
- Alanko K, Kanerva L, Jolanki R, et al. Oral mucosal diseases investigated by patch testing with a dental screening series. Contact Dermatitis 1996;34:263–7.
- Torgerson RR, Davis MD, Bruce AJ, et al. Contact allergy in oral disease. J Am Acad Dermatol 2007;57:315–21.
- Lazarov A, Kidron D, Tulchinsky Z, Minkow B. Contact orofacial granulomatosis caused by delayed hypersensitivity to gold and mercury. J Am Acad Dermatol 2003;49:1117–20.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis 1999;40:200–4.
- Nava C, Briatico Vangosa G. Allergy to gold salts. Med Lavoro (Milano) 1971;62:572–5.
- Bruze M, Hedman H, Björkner B, Möller H. The development and course of test reactions to gold sodium thiosulfate. Contact Dermatitis 1995;33:386–91.
Mercury
- Burrows D. Mischievous metals: chrome, cobalt, nickel and mercury. Clin Exp Dermatol 1989;14:266–72.
- Handley J, Todd D, Burrows D. Mercury allergy in a contact dermatitis clinic in Northern Ireland. Contact Dermatitis 1993;29:258–61.
- Zenarola P, Lomuto M, Bisceglia M. Hypertrophic amalgam dermatitis of the tongue simulating carcinoma. Contact Dermatitis 1993;29:157–8.
- Koch P, Baumer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis 1995;33:323–9.
- Thornhill MH, Pemberton MN, Simmons RK, Theaker ED. Amalgam-contact hypersensitivity lesions and oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:291–9.
- Khamaysi Z, Bergman R, Weltfriend S. Positive patch test reactions to allergens of the dental series and the relation to the clinical presentations. Contact Dermatitis 2006;55:216–18.
- Hietanen J, Pihlman K, Forstrom L, et al. No evidence of hypersensitivity to dental restorative metals in oral lichen planus. Scand J Dent Res 1987;95:320–7.
- Martin MD, Broughton S, Drangsholt M. Oral lichen planus and dental materials: a case–control study. Contact Dermatitis 2003;48:331–6.
- Henriksson E, Mattsson U, Håkansson J. Healing of lichenoid reactions following removal of amalgam. A clinical follow-up. J Clin Periodontol 1995;22:287–94.
- Pigatto PD, Guzzi G, Persichini P, Barbadillo S. Recovery from mercury-induced burning mouth syndrome due to mercury allergy. Dermatitis 2004;15:75–7.
- Bergdahl BJ, Anneroth G, Anneroth I. Clinical study of patients with burning mouth. Scand J Dent Res 1994;102:299–305.
- Skoglund A, Egelrud T, Guttman-Yassky E, et al. Resolution of orofacial granulomatosis with amalgam removal. J Eur Acad Dermatol Venereol 2003;17:344–7.
- Vena GA, Foti C, Grandolfo M, Angelini G. Mercury exanthem. Contact Dermatitis 1994;31:214–16.
- Lerch M, Bircher AJ. Systemically induced allergic exanthem from mercury. Contact Dermatitis 2004;50:349–53.
- Johnson HH, Schonberg IL, Bach NF. Chronic atopic eczema with pronounced mercury sensitivity: partial clearing after extraction of teeth containing mercury amalgam. Arch Derm Syph 1951;63:279–80.
- Thomson J, Russell JA. Dermatitis due to mercury following amalgam dental restorations. Br J Dermatol 1970;82:292–7.
- Levy J, Sewell M, Goldstein N. A short history of tattooing. J Dermatol Surg Oncol 1979;5:851–3.
Aluminium
- Bergfors E, Björkelund C, Trollfors B. Nineteen cases of persistent pruritic nodules and contact allergy to aluminium after injection of commonly used aluminium-adsorbed vaccines. Eur J Pediatr 2005;164:691–7.
- Hindsén M. Contact allergy to aluminium in patients hyposensitized with aluminium-containing hyposensitizing extracts. Contact Dermatitis 2005;53:301–2.
- O'Driscoll JB, Beck MH, Kesseler ME, Ford G. Contact sensitivity to aluminium acetate eardrops. Contact Dermatitis 1991;24:156–7.
Other metals
- Barranco VP. Eczematous dermatitis caused by internal exposure to copper. Arch Dermatol 1972;106:386–7.
- Romaguera C, Grimalt F. Contact dermatitis from a copper-containing intrauterine contraceptive device. Contact Dermatitis 1981;7:163–4.
- Koch P, Baum HP. Contact stomatitis due to palladium and platinum in dental alloys. Contact Dermatitis 1996;34:253–7.
- Vilaplana J, Romaguera C, Cornellana F. Contact dermatitis and adverse oral mucous membrane reactions related to the use of dental prostheses. Contact Dermatitis 1994;30:80–4.
- Marcusson JA, Cederbrant K, Heilborn J. Indium and iridium allergy in patients exposed to dental alloys. Contact Dermatitis 1998;38:297–8.
Fragrances, balsams, flavouring agents and spices
- Johansen JD. Contact allergy to fragrances. Clinical and experimental investigations of the fragrance mix and its ingredients. Contact Dermatitis 2002;46(Suppl. 3):1–31.
- Beck MH. Fragrance allergy. Br J Dermatol 2000;142:203–4.
- Harder U. The art of creating a perfume. In: Frosch PJ, Johansen JD, White IR, eds. Fragrances: Beneficial and Adverse Effects. Berlin: Springer, 1998:3–5.
- Hjorth N. Eczematous allergy to balsams, allied perfumes and flavouring agents: with special reference to balsam of Peru [Thesis]. Copenhagen: University of Copenhagen, 1961.
- Amado A, Taylor JS. Balsam of Peru or balsam of El Salvador? Contact Dermatitis 2006;55:119.
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:462–6.
- Jordan WP, Jr. Resorcinol monobenzoate, steering wheels, Peruvian balsam. Arch Dermatol 1973;108:278.
- Nielsen NH, Menné T. Allergic contact sensitization in an unselected Danish population. The Glostrup Allergy Study, Denmark. Acta Derm Venereol (Stockh) 1992;72:456–60.
- Mørtz CG. The prevalence of atopic dermatitis, hand eczema, allergic contact dermatitis, type IV and type I sensitisation in 8th grade school children in Odense [PhD thesis]. Faculty of Health Sciences, University of Southern Denmark, 1999.
- Buckley DA, Wakelin SH, Seed PT, et al. The frequency of fragrance allergy in a patch-test population over a 17-year period. Br J Dermatol 2000;142:279–83.
- Christophersen J, Menné T, Tanghoj P, et al. Clinical patch test data evaluated by multivariate analysis. Danish Contact Dermatitis Group. Contact Dermatitis 1989;21:291–9.
- Hasan T, Rantanen T, Alanko K, et al. Patch test reactions to cosmetic allergens in 1995–1997 and 2000–2002 in Finland – a multicentre study. Contact Dermatitis 2005;53:40–5.
- Owen CM, August PJ, Beck MH. Contact allergy to oak moss resin in a soluble oil. Contact Dermatitis 2000;43:112.
- Karlberg AT, Dooms-Goossens A. Contact allergy to oxidized d-limonene among dermatitis patients. Contact Dermatitis 1997;36:201–6.
- Matura M, Sköld M, Börje A, et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermatitis 2005;52:320–8.
- Edman B. Sites of contact dermatitis in relationship to particular allergens. Contact Dermatitis 1985;13:129–35.
- Held JL, Ruszkowski AM, Deleo VA. Consort contact dermatitis due to oak moss. Arch Dermatol 1988;124:261–2.
- Coulson IH, Khan AS. Facial ‘pillow’ dermatitis due to lavender oil allergy. Contact Dermatitis 1999;41:111.
- Keane FM, Smith HR, White IR, Rycroft RJ. Occupational allergic contact dermatitis in two aromatherapists. Contact Dermatitis 2000;43:49–51.
- Boonchai W, Iamtharachai P, Sunthonpalin P. Occupational allergic contact dermatitis from essential oils in aromatherapists. Contact Dermatitis 2007;56:181–2.
- Malten KE. Four bakers showing positive patch-tests to a number of fragrance materials, which can also be used as flavors. Acta Derm Venereol Suppl (Stockh) 1979;59:117–21.
- Sainio EL, Kanerva L. Contact allergens in toothpastes and a review of their hypersensitivity. Contact Dermatitis 1995;33:100–5.
- Francalanci S, Sertoli A, Giorgini S, et al. Multicentre study of allergic contact cheilitis from toothpastes. Contact Dermatitis 2000;43:216–22.
- Ferguson JE, Beck MH. Contact sensitivity to vanilla in a lip salve. Contact Dermatitis 1995;33:352.
- Guin JD. Rosemary cheilitis: one to remember. Contact Dermatitis 2001;45:63.
- Silvestre JF, Albares MP, Blanes M, et al. Allergic contact gingivitis due to eugenol present in a restorative dental material. Contact Dermatitis 2005;52:341.
- Hoskyn J, Guin JD. Contact allergy to cinnamal in a patient with oral lichen planus. Contact Dermatitis 2005;52:160–1.
- Nadiminti H, Ehrlich A, Udey MC. Oral erosions as a manifestation of allergic contact sensitivity to cinnamon mints. Contact Dermatitis 2005;52:46–7.
- Leow YH, Ng SK, Wong WK, Goh CL. Contact allergic potential of topical traditional Chinese medicaments in Singapore. Am J Contact Dermatitis 1995;6:4–8.
- James WD, White SW, Yanklowitz B. Allergic contact dermatitis to compound tincture of benzoin. J Am Acad Dermatol 1984;11:847–50.
- Veien NK, Hattel T, Justesen O, et al. Reduction of intake of balsams in patients sensitive to balsam of Peru. Contact Dermatitis 1985;12:270–3.
- Elberling J, Linneberg A, Mosbech H, et al. A link between skin and airways regarding sensitivity to fragrance products? Br J Dermatol 2004;151:1197–203.
- Nardelli A, Carbonez A, Ottoy W, Drieghe J, Goossens A. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis 2009:60:303–13.
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis 2006;55:48–53.
- Veien NK, Hattel T, Laurberg G. Can oral challenge with balsam of Peru predict possible benefit from a low-balsam diet? Am J Contact Dermatitis 1996;7:84–7.
- Niinimaki A. Double-blind placebo-controlled peroral challenges in patients with delayed-type allergy to balsam of Peru. Contact Dermatitis 1995;33:78–83.
- Larsen WG. Perfumes. In: Baran R, Maibach HI, eds. Cosmetic Dermatology. London: Martin Dunitz, 1994:21–6.
- Larsen WG. Perfume dermatitis: a study of 20 patients. Arch Dermatol 1977;113:623–6.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol 1985;12:1–9.
- Larsen WG. Detection of allergic dermatitis to fragrances. Acta Derm Venereol Suppl (Stockh) 1987;134:83–6.
- Frosch PJ, Pilz B, Burrows D, et al. Testing with fragrance mix. Is the addition of sorbitan sesquioleate to the constituents useful? Contact Dermatitis 1995;32:266–72.
- Frosch PJ, Pilz B, Andersen KE, et al. Patch testing with fragrances: results of a multicenter study of the European Environmental and Contact Dermatitis Research Group with 48 frequently used constituents of perfumes. Contact Dermatitis 1995;33:333–42.
- Larsen W, Nakayama H, Lindberg M, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (Part I). Am J Contact Dermatitis 1996;7:77–83.
- Frosch PJ, Pirker C, Rastogi SC, et al. Patch testing with a new fragrance mix detects additional patients sensitive to perfumes and missed by the current fragrance mix. Contact Dermatitis 2005;52:207–15.
- Frosch PJ, Rastogi SC, Pirker C, et al. Patch testing with a new fragrance mix – reactivity to the individual constituents and chemical detection in relevant cosmetic products. Contact Dermatitis 2005;52:216–25.
- Frosch PJ, Johansen JD, Menné T, et al. Lyral is an important sensitizer in patients sensitive to fragrances. Br J Dermatol 1999;141:1076–83.
- Heisterberg MV, Laurberg G, Veien NK, et al. Prevalence of allergic contact dermatitis caused by hydroxyisohexyl 3-cuclohexene carboxaldehyde has not changed in Denmark. Contact Dermatitis 2012;67:49–51.
- Nardelli A, Carbonez A, Drieghe J, et al. Results of patch testing with fragrance mix I, fragrance mix II, and their ingredients, and Myroxylon pereirae and colophonium, over a 21-year period. Contact Dermatitis 2013;68:307–13.
- Frosch PJ, Johansen JD, Menné T, et al. Further important sensitizers in patients sensitive to fragrances. Contact Dermatitis 2002;47:279–87.
- Christensson JB, Andersen KE, Bruze M, et al. Air-oxidised linalool – a frequent cause of fragrance contact allergy. Contact Dermatitis 2012;67:247–59.
- Christensson JB, Andersen KE, Bruze M, et al. An international multicentere study on the allergenic activity of air-oxidised R-limonene. Contact Dermatitis 2013;68:214–23.
- Basketter D. Quenching: fact or fiction? Contact Dermatitis 2000;43:253–8.
- Mowitz M, Zimerson E, Svedman C, Bruze M. Stability of fragrance patch test preparations applied in test chambers. Br J Dermatol. 2012;167(4):822–7.
Applied medicaments
- De Groot AC, Weyland JW, Nater JP. Unwanted Effects of Cosmetics and Drugs Used in Dermatology, 3rd edn. New York: Elsevier, 1994.
- Brandao FM, Goossens A, Tosti A. Topical drugs. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:623–52.
- Green CM, Holden CR, Gawkrodger DJ. Contact allergy to topical medicaments becomes more common with age: an age-stratified study. Contact Dermatitis 2007;56:229–31.
- Yariktas M, Yildirim M, Doner F, et al. Allergic contact dermatitis prevalence in patients with eczematous external otitis. Asian Pac J Allergy Immunol 2004;22:7–10.
- Millard TP, Orton DI. Changing patterns of contact allergy in chronic inflammatory ear disease. Contact Dermatitis 2004;50:83–6.
- Jappe U, Uter W, Menezes de Padua CA, et al. Allergic contact dermatitis due to beta-blockers in eye drops: a retrospective analysis of multicentre surveillance data 1993–2004. Acta Derm Venereol 2006;86:509–14.
- Leow Y-H, Ng S-K, Wong W-K, et al. Contact allergic potential of topical traditional Chinese medicaments in Singapore. Am J Contact Dermatitis 1995;6:4–8.
- Simpson EL, Law SV, Storrs FJ. Prevalence of botanical extract allergy in patients with contact dermatitis. Dermatitis 2004;15:67–72.
- Barbaud A, Goncalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis 2001;45:321–8.
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:453–92.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol 2000;25:261–4.
- Kalavala M, Statham BN, Green CM, King C, Ormerod AD, Sansom J, English JS, Wilkinson MS, Horne H, Gawkrodger D. Tixocortol pivalate: what is the right concentration? Contact Dermatitis 2007;57:44–6.
- Wilkinson SM. Corticosteroid cross-reactions: an alternative view. Contact Dermatitis 2000;42:59–63.
Cosmetics
- White IR, De Groot AC. Cosmetics and skin care products. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:493–506.
- Rietschel RL, Fowler JF,, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:211–61.
- De Groot AC, Weyland JW, Nater JP. Unwanted Effects of Cosmetics and Drugs Used in Dermatology, 3rd edn. Amsterdam: Elsevier, 1994.
- Thyssen JP, Johansen JD, Menné T. Contact allergy epidemics and their controls. Contact Dermatitis 2007;56:185–95.
- Tosti A, Guerra L, Vincenzi C, et al. Contact sensitization caused by toluene sulfonamide-formaldehyde resin in women who use nail cosmetics. Am J Contact Dermatitis 1993;4:150–3.
- Lidén C, Berg M, Farm G, et al. Nail varnish allergy with far-reaching consequences. Br J Dermatol 1993;128:57–62.
- Freeman S, Lee MS, Gudmundsen K. Adverse contact reactions to sculptured acrylic nails: 4 case reports and a literature review. Contact Dermatitis 1995;33:381–5.
- Lazarov A. Sensitization to acrylates is a common adverse reaction to artificial fingernails. J Eur Acad Dermatol Venereol 2007;21:169–74.
- Thomson KF, Wilkinson SM. Allergic contact dermatitis to plant extracts in patients with cosmetic dermatitis. Br J Dermatol 2000;142:84–8.
- Rutherford T, Nixon R, Tam M, Tate B. Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4.5 years. Australas J Dermatol 2007;48:83–7.
- Simpson EL, Law SV, Storrs FJ. Prevalence of botanical extract allergy in patients with contact dermatitis. Dermatitis 2004;15:67–72.
- Katugampola RP, Statham BN, English JS, et al. A multicentre review of the hairdressing allergens tested in the UK. Contact Dermatitis 2005;53:130–2.
- Uter W, Lessmann H, Geier J, Schnuch A. Contact allergy to ingredients of hair cosmetics in female hairdressers and clients – an 8-year analysis of IVDK data. Contact Dermatitis 2003;49:236–40.
- Goossens A, Armingaud P, Avenel-Audran M, et al. An epidemic of allergic contact dermatitis due to epilating products. Contact Dermatitis 2002;47:67–70.
- De Groot AC. Labelling cosmetics with their ingredients. BMJ 1990;300:1636–8.
- Consumer Association. Consumer Association Report on Reactions of the Skin to Cosmetics and Toiletries. London: Consumer Association, 1979.
- Greif M, Maibach HI. United States cosmetic ingredient labelling. Contact Dermatitis 1977;3:94–8.
- De Groot AC, Beverdam E, Tjong Ayong C, et al. The role of contact allergy in the spectrum of adverse effects caused by cosmetics and toiletries. Contact Dermatitis 1988;19:195–201.
- Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol 1998;38:911–18.
- Adams RM, Maibach HI. A five-year study of cosmetic reactions. J Am Acad Dermatol 1985;13:1062–9.
- Gach JE, Stone NM, Finch TM. A series of four cases of allergic contact dermatitis to phthalic anhydride/trimellitic anhydride/glycols copolymer in nail varnish. Contact Dermatitis 2005;53:63–4.
- Lazarov A. Perianal contact dermatitis caused by nail lacquer allergy. Am J Contact Dermatitis 1999;10:43–4.
- Guin JD, Wilson P. Onycholysis from nail lacquer: a complication of nail enhancement? Am J Contact Dermatitis 1999;10:34–6.
- Kanerva L, Estlander T. Allergic onycholysis and paronychia caused by cyanoacrylate nail glue, but not by photobonded methacrylate nails. Eur J Dermatol 2000;10:223–5.
- Veysey EC, Burge S, Cooper S. Consort contact dermatitis to paraphenylenediamine, with an unusual clinical presentation of tumid plaques. Contact Dermatitis 2007;56:366–7.
- Swinyer LJ. Connubial contact dermatitis from perfumes. Contact Dermatitis 1980;6:226.
- Madan V, Beck MH. Contact allergy to octocrylene in sunscreen with recurrence from passive transfer of a cosmetic. Contact Dermatitis 2005;53:241–2.
- Veysey EC, Orton DI. Photoallergic contact cheilitis due to oxybenzone in a lip cosmetic. Contact Dermatitis 2006;55:54.
Formaldehyde
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:473–5.
- Schnuch A, Geier J, Uter W, et al. National rates and regional differences in sensitization to allergens of the standard series. Population-adjusted frequencies of sensitization (PAFS) in 40,000 patients from a multicenter study (IVDK). Contact Dermatitis 1997;37:200–9.
- Pratt MD, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch-test results, 2001–2002 study period. Dermatitis 2004;15:176–83.
- Jordan WP, Jr, Sherman WT, King SE. Threshold responses in formaldehyde-sensitive subjects. J Am Acad Dermatol 1979;1:44–8.
- Flyvholm MA, Hall BM, Agner T, et al. Threshold for occluded formaldehyde patch test in formaldehyde-sensitive patients. Relationship to repeated open application test with a product containing formaldehyde releaser. Contact Dermatitis 1997;36:26–33.
- Flyvholm MA. Preservatives in registered chemical products. Contact Dermatitis 2005;53:27–32.
- Pontén A. Formaldehyde in reusable protective gloves. Contact Dermatitis 2005;54:268–71.
- Fisher AA. Dermatitis due to the presence of formaldehyde in certain sodium lauryl sulfate (SLS) solutions. Cutis 1981;27:360–2.
- Jacobs MC, White IR, Rycroft RJ, et al. Patch testing with preservatives at St John's from 1982 to 1993. Contact Dermatitis 1995;33:247–54.
- Ford GP, Beck MH. Reactions to Quaternium 15, Bronopol and Germall 115 in a standard series. Contact Dermatitis 1986;14:271–4.
- Kranke B, Szolar-Platzer C, Aberer W. Reactions to formaldehyde and formaldehyde releasers in a standard series. Contact Dermatitis 1996;35:192–3.
- Anderson BE, Tan TC, Marks JG. Patch-test reactions to formaldehydes, bioban, and other formaldehyde releasers. Dermatitis 2007;18:92–5.
- Goon AT, Gruvberger B, Persson L, et al. Presence of formaldehyde in topical corticosteroid preparations available on the Swedish market. Contact Dermatitis 2003;48:199–203.
- Noiesen E, Munk MD, Larsen K, et al. Difficulties in avoiding exposure to allergens in cosmetics. Contact Dermatitis 2007;57:105–9.
- Flyvholm MA, Menné T. Allergic contact dermatitis from formaldehyde. A case study focussing on sources of formaldehyde exposure. Contact Dermatitis 1992;27:27–36.
- Karlberg AT, Skare L, Lindberg I, et al. A method for quantification of formaldehyde in the presence of formaldehyde donors in skin-care products. Contact Dermatitis 1998;38:20–8.
- Agner T, Flyvholm MA, Menné T. Formaldehyde allergy: a follow-up study. Am J Contact Dermatitis 1999;10:12–17.
- Pontén A, Aalto-Korte K, Agner T, et al.. Patch testing with 2.0% (0.60 mg/cm2) formaldehyde instead of 1.0% (0.30 mg/cm2) detects significantly more contact allergy. Contact Dermatitis. 2013;68:50–3.
Formaldehyde-releasing preservatives/biocides
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:211–61.
- Pratt MD, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch-test results, 2001–2002 study period. Dermatitis 2004;15176–83.
- Wilkinson JD, Shaw S, Andersen KE, et al. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991–2000). Contact Dermatitis 2002;46:207–10.
- Svedman C, Andersen KE, Brandao FM, et al. Follow-up of the monitored levels of preservative sensitivity in Europe. Overview of the years 2001–2008. Contact Dermatitis 2012;67:312–14.
- Boffa MJ, Beck MH. Allergic contact dermatitis from quaternium 15 in Oilatum cream. Contact Dermatitis 1996;35:45–6.
- Finch TM, Prais L, Foulds IS. Occupational allergic contact dermatitis from quaternium-15 in an electroencephalography skin preparation gel. Contact Dermatitis 2001;44:44–5.
- Britton JE, Wilkinson SM, English JS, et al. The British standard series of contact dermatitis allergens: validation in clinical practice and value for clinical governance. Br J Dermatol 2003;148:259–64.
- Latorre N, Borrego L, Fernandez-Rodendo V, Garcia-Bravo B, Gimenez- Arnau A, Sanchez J, Silvestre JF. Patch testing with formaldehyde and formaldehyde-releasers: multicentre study in Spain (2205–2009). Contact Dermatitis 2011;65:286–92.
- Zachariae C, Hall B, Cupferman S, et al. ROAT: morphology of ROAT on arm, neck and face in formaldehyde and diazolidinyl urea sensitive individuals. Contact Dermatitis 2006;54:21–4.
- De Groot AC, Bruynzeel DP, Jagtman BA, et al. Contact allergy to diazolidinyl urea (Germall II). Contact Dermatitis 1988;18:202–5.
- Bourke J, Coulson I, English J. Guidelines for care of contact dermatitis. Br J Dermatol 2001;145:877–85.
- White IR, De Groot AC. Cosmetics and skin care products. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:498.
- Isaksson M, Gruvberger B, Goon AT, Bruze M. Can an imidazolidinyl urea-preserved corticosteroid cream be safely used in individuals hypersensitive to formaldehyde? Contact Dermatitis 2006;54:29–34.
- Van Neer PA, van der Kley AM. Imidazolidinyl urea (Germall 115) should be patch tested in water. Contact Dermatitis 1991;24:302.
- Grattan CE, Harman RR, Tan RS. Milk recorder dermatitis. Contact Dermatitis 1986;14:217–20.
- Storrs FJ, Bell DE. Allergic contact dermatitis to 2-bromo-2-nitropropane-1,3-diol in a hydrophilic ointment. J Am Acad Dermatol 1983;8:157–70.
- Choudry K, Beck MH, Muston HL. Allergic contact dermatitis from 2-bromo-2-nitropropane-1,3-diol in Metrogel. Contact Dermatitis 2002;46:60–1.
- Flyvholm MA. Preservatives in registered chemical products. Contact Dermatitis 2005;53:27–32.
- De Groot AC, van Joost T, Bos JD, et al. Patch test reactivity to DMDM hydantoin. Relationship to formaldehyde allergy. Contact Dermatitis 1988;18:197–201.
- Uter W, Frosch PJ. Contact allergy from DMDM hydantoin, 1994–2000. Contact Dermatitis 2002;47:57–8.
- Flyvholm M-A. Formaldehyde and formaldehyde releasers. In: Kanerva L, Elsner P, Wahlberg JE, Maibach HI, eds. Handbook of Occupational Dermatology. Berlin: Springer, 2000:474–8.
- Madan V, Beck MH. Occupational allergic contact dermatitis from N, N-methylene-bis-5-methyl-oxazolidine in coolant oils. Contact Dermatitis 2006;55:39–41.
- Geier J, Lessmann H, Dickel H, et al. Patch test results with the metalworking fluid series of the German Contact Dermatitis Research Group (DKG). Contact Dermatitis 2004;51:118–30.
- Geier J, Uter W, Lessmann H, Frosch PJ. Patch testing with metalworking fluids from the patient's workplace. Contact Dermatitis 2004;51:172–9.
Isothiazolinones
- Lepoittevin J-P, Le Coz CJ. Dictionary of occupational allergens. In: Kanerva L, Elsner P, Wahlberg JE, Maibach HI, eds. Handbook of Occupational Dermatology. Berlin: Springer, 2000:1183–5.
- Geier J, Schnuch A. No cross-sensitization between MCI/MI, benzisothiazolinone and octylisothiazolinone. Contact Dermatitis 1996;34:148–9.
- Burden AD, O'Driscoll JB, Page FC, Beck MH. Contact hypersensitivity to a new isothiazolinone. Contact Dermatitis 1994;30:179–80.
- De Groot AC, Weyland JW. Kathon CG: a review. J Am Acad Dermatol 1988;18:350–8.
- De Groot AC. Methylisothiazolinone/methylchloroisothiazolinone (Kathon CG) allergy: an updated review. Am J Contact Dermatitis 1990;1:151–6.
- De Groot AC, Herxheimer A. Isothiazolinone preservative: cause of a continuing epidemic of cosmetic dermatitis. Lancet 1989;i:314–16.
- Wilkinson JD, Shaw S, Andersen KE, et al. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991–2000). Contact Dermatitis 2002;46:207–10.
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:220–2.
- Fewings J, Menné T. An update of the risk assessment for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI) with focus on rinse-off products. Contact Dermatitis 1999;41:1–13.
- Goncalo M, Goossens A. Whilst Rome burns: the epidemic of contact allergy to methylisothiazolinone. Contact Dermatitis 2013;68:257–8.
- De Groot AC. Vesicular dermatitis of the hands secondary to perianal allergic contact dermatitis caused by preservatives in moistened toilet tissues. Contact Dermatitis 1997;36:173–4.
- Rastogi SC. Kathon CG and cosmetic products. Contact Dermatitis 1990;22:155–60.
- Timmermans A, Hertog SD, Gladys K, et al. ‘Dermatologically tested’ baby toilet tissues: a cause of allergic contact dermatitis in adults. Contact Dermatitis 2007;57:97–9.
- Bohn S, Niederer M, Brehm K, Bircher AJ. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. Abolition of symptoms by chemical allergen inactivation. Contact Derm atitis 2000;42:196–201.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, Menné T. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis 2006;54:322–4.
- Kanerva L, Tarvainen K, Pinola A, et al. A single accidental exposure may result in a chemical burn, primary sensitization and allergic contact dermatitis. Contact Dermatitis 1994;31:229–35.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis 2004;15:201–5.
- Farm G, Wahlberg JE. Isothiazolinones (MCI/MI): 200 ppm versus 100 ppm in the standard series. Contact Dermatitis 1991;25:104–7.
- Davies E, Orton D. Identifying the optimal patch test concentration for methylchloroisothiazolinone and methylisothiazolinone. Contact Dermatitis 2009;60:288–9.
- Basketter DA, Gilmour NJ, Wright Z, Walters T, Boman A, Liden C. Biocides: characterization of the allergenic hazard of methylisothiazolinone. Cutaneous Ocul Toxicol 2003:22:187–99.
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final report of the safety assessment of methylisothiazolinone. Int J Toxicol 2010:29:S187–213.
- Garcia-Gavin J, Vansina S, Kerre S, Naert A, Goossens A. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis 2010:63:96–101.
- Geier J, Lessmann H, Schnuch A, Uter W. Recent increase in allergic reactions to methylchloroisothiazolinone/methylisothiazolinone: is methylisothiazolinone the culprit? Contact Dermatitis 2012;67:334–41.
- Urwin R, Wilkinson M. Methylchloroisothiazolinone and methylisothiazolinone contact allergy: a new “epidemic”. Contact Dermatitis 2013;68:253–5.
- Lundov MD, Opstrup MS, Johansen JD. Methylisothiazolinone contact allergy – a growing epidemic. Contact Dermatitis 2013;69:271–5.
- Johnston GA; contributing members of the British Society for Cutaneous Allergy (BSCA). The rise in prevalence of contact allergy to methylisothiazolinone in the British Isles. Contact Dermatitis 2014;70(4):238–40.
- Bruze M, Engfeldt M, Goncalo M, Goossens A. Reccomendation to include methylisothiazolinone in the European baseline patch test series – on behalf of the European Society of Contact Dermatitis and the European Environmental and Contact Dermatitis Research Group. Contact Dermatitis 2013;69:263–70.
- Aalto-Korte K, Alanko K, Henriks-Eckerman ML, Jolanki R. Antimicrobial allergy from polyvinyl chloride gloves. Arch Dermatol 2006;142:1326–30.
- Hardcastle NJ, Gawkrodger DJ. Occupational contact dermatitis to 1,2-benzisothiazolin-3-one and 5-chloro-2-methylisothiazolin-3-one/2-methylisothiazolin-3-one in paint manufacturers. Contact Dermatitis 2005;53:115–16.
- Chew AL, Maibach HI. 1,2-Benzisothiazolin-3-one (Proxel): irritant or allergen? A clinical study and literature review. Contact Dermatitis 1997;36:131–6.
- Mathias CG, Andersen KE, Hamann K. Allergic contact dermatitis from 2-n-octyl-4-isothiazolin-3-one, a paint mildewcide. Contact Dermatitis 1983;9:507–9.
- Oleaga JM, Aguirre A, Landa N, et al. Allergic contact dermatitis from Kathon 893. Contact Dermatitis 1992;27:345–6.
Parabens (hydroxybenzoates)
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:218–20.
- Cashman AL, Warshaw EM. Parabens: a review of epidemiology, structure, allergenicity, and hormonal properties. Dermatitis 2005;16:57–66.
- Wilkinson JD, Shaw S, Andersen KE, et al. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991–2000). Contact Dermatitis 2002;46:207–10.
- Schnuch A, Geier J, Uter W, et al. Patch testing with preservatives, antimicrobials and industrial biocides. Results from a multicentre study. Br J Dermatol 1998;138:467–76.
- Marks JG, Jr, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch-test results, 1996–1998. Arch Dermatol 2000;136:272–3.
- Fisher AA. The paraben paradox. Cutis 1973;12:830–2.
- Simpson JR. Dermatitis due to parabens in cosmetic creams. Contact Dermatitis 1978;4:311–12.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis 2006;54:117–18.
- Veien NK, Hattel T, Laurberg G. Oral challenge with parabens in paraben-sensitive patients. Contact Dermatitis 1996;34:433.
Methyldibromo glutaronitrile
- De Groot AC, van Ginkel CJ, Weijland JW. Methyldibromoglutaronitrile (Euxyl K 400): an important ‘new’ allergen in cosmetics. J Am Acad Dermatol 1996;35:743–7.
- Wilkinson JD, Shaw S, Andersen KE, et al. Monitoring levels of preservative sensitivity in Europe. Contact Dermatitis 2002;46:207–10.
- Jackson JM, Fowler JF. Methyldibromoglutaronitrile (Euxyl K400): a new and important sensitizer in the United States? J Am Acad Dermatol 1998;38:934–7.
- Marks JG, Jr, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch-test results, 1996–1998. Arch Dermatol 2000;136:272–3.
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:222.
- Tosti A, Guerra L, Bardazzi F, et al. Euxyl K 400: a new sensitizer in cosmetics. Contact Dermatitis 1991;25:89–93.
- Wong CS, Beck MH. Occupational contact allergy to methyldibromo glutaronitrile in abrasive cleansers and work creams. Contact Dermatitis 2001;44:311–12.
- Zachariae C, Johansen JD, Rastogi SC, Menné T. Allergic contact dermatitis from methyldibromo glutaronitrile – clinical cases from 2003. Contact Dermatitis 2005;52:6–8.
- De Groot AC, de Cock PAJJM, Coenraads PJ, et al. Methyldibromoglutaronitrile is an important contact allergen in the Netherlands. Contact Dermatitis 1996;34:118–20.
- Williams JD, Frowen KE, Nixon RL. Allergic contact dermatitis from methyldibromo glutaronitrile in a sanitary pad and review of Australian clinic data. Contact Dermatitis 2007;56:164–7.
- Schnuch A, Kelterer D, Bauer A, et al. Quantitative patch and repeated open application testing in methyldibromo glutaronitrile-sensitive patients. Contact Dermatitis 2005;52:197–206.
- De Groot AC, van Ginkel CJ, Weyland JW. How to detect sensitization to Euxyl K 400. Contact Dermatitis 1996;34:373–4.
- Gruvberger B, Andersen KE, Brandão FM, et al. Repeated open application test with methyldibromo glutaronitrile, a multicentre study within the EECDRG. Contact Dermatitis 2005;52:19–23.
- Britton JE, Wilkinson SM, English JS, et al. The British standard series of contact dermatitis allergens: validation in clinical practice and value for clinical governance. Br J Dermatol 2003;148:259–64.
Chloroxylenol
- Myatt AE, Beck MH. Contact sensitivity to parachlorometaxylenol (PCMX). Clin Exp Dermatol 1985;10:491–4.
- Britton JE, Wilkinson SM, English JS, et al. The British standard series of contact dermatitis allergens: validation in clinical practice and value for clinical governance. Br J Dermatol 2003;148:259–64.
- Storrs FJ. Para-chloro-meta-xylenol allergic contact dermatitis in seven individuals. Contact Dermatitis 1975;1:211–13.
- Berthelot C, Zirwas MJ. Allergic contact dermatitis to chloroxylenol. Dermatitis 2006;17:156–9.
- Malakar S, Panda S. Post-inflammatory depigmentation following allergic contact dermatitis to chloroxylenol. Br J Dermatol 2001;144:1275–6.
- Burry JN, Kirk J, Reid J, et al. Chlorocresol sensitivity. Contact Dermatitis 1975;1:41–2.
- Rajpar SF, Foulds IS, Abdullah A, Maheshwari M. Severe adverse cutaneous reaction to insulin due to cresol sensitivity. Contact Dermatitis 2006;55:119–20.
- Bourke J, Coulson I, English J. Guidelines for care of contact dermatitis. Br J Dermatol 2001;145:877–85.
Organic mercurials
- Goncalo M, Figueiredo A, Goncalo S. Hypersensitivity to thimerosal: the sensitizing moiety. Contact Dermatitis 1996;34:201–3.
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001.
- Fransway AF. The problem of preservation in the 1990s. III. Agents with preservative function independent of formaldehyde release. Am J Contact Dermatitis 1991;2:145–74.
- Heine G, Schnuch A, Uter W, Worm M. Frequency of contact allergy in German children and adolescents patch tested between 1995 and 2002: results from the Information Network Departments of Dermatology and the German Contact Dermatitis Research Group. Contact Dermatitis 2004;51:111–17.
- Kiec-Swierczynska M, Krecisz B, Swierczynska-Machura D. Occupational allergic contact dermatitis due to thimerosal. Contact Dermatitis 2003;48:337–8.
- Sertoli A, Di Fonzo E, Spallanzani P, et al. Allergic contact dermatitis from thimerosol in a soft contact lens wearer. Contact Dermatitis 1980;6:292–3.
- Van Ketel WG, Melzer-van Riemsdijk FA. Conjunctivitis due to soft lens solutions. Contact Dermatitis 1980;6:321–4.
- Pedersen NB. Allergic contact conjunctivitis from merthiolate in soft contact lenses. Contact Dermatitis 1978;4:165.
- Möller H. All these positive tests to thimerosal. Contact Dermatitis 1994;31:209–13.
- Suneja T, Belsito DV. Thimerosal in the detection of clinically relevant allergic contact reactions. J Am Acad Dermatol 2001;45:23–7.
- Belsito DV. Thimerosal: contact (non)allergen of the year. Am J Contact Dermatitis 2002;13:1–2.
- Slodownik D, Ingber A. Thimerosal – is it really irrelevant? Contact Dermatitis 2005;53:324–6.
- Möller H. Merthiolate allergy: a nationwide iatrogenic sensitization. Acta Derm Venereol (Stockh) 1977;57:509–17.
- Tosti A, Guerra L, Bardazzi F. Hyposensitizing therapy with standard antigenic extracts: an important source of thimerosal sensitization. Contact Dermatitis 1989;20:173–6.
- Osawa J, Kitamura K, Ikezawa Z, et al. A probable role for vaccines containing thimerosal in thimerosal hypersensitivity. Contact Dermatitis 1991;24:178–82.
- Cox NH, Forsyth A. Thiomersal allergy and vaccination reactions. Contact Dermatitis 1988;18:229–33.
- Lee-Wong M, Resnick D, Chong K. A generalized reaction to thimerosal from an influenza vaccine. Ann Allergy Asthma Immunol 2005;94:90–4.
- Audicana MT, Munoz D, del Pozo MD, et al. Allergic contact dermatitis from mercury antiseptics and derivatives: study protocol of tolerance to intramuscular injections of thimerosal. Am J Contact Dermatitis 2002;13:3–9.
- De Castro JL, Freitas JP, Brandao FM, et al. Sensitivity to thimerosal and photosensitivity to piroxicam. Contact Dermatitis 1991;24:187–92.
- Geier J, Lessmann H, Uter W, Schnuch A. Patch testing with phenylmercuric acetate. Contact Dermatitis 2005;53:117–18.
Other preservatives/biocides
- Schena D, Papagrigoraki A, Girolomoni G. Sensitizing potential of triclosan and triclosan-based skin care products in patients with chronic eczema. Dermatol Ther 2008:21:S35–8.
- Chowdhury MM, Statham BN. Allergic contact dermatitis from dibutyl phthalate and benzalkonium chloride in Timodine cream. Contact Dermatitis 2002:46:57.
- Brown VL, Orton DI. Two cases of facial dermatitis due to chlorphenesin in cosmetics.Contact Dermatitis 2005:52:48–9
- Toholka R, Nixon R. Allergic contact dermatitis to chlorhexidine. Australas J Dermatol 2013;54:303–6.
- Martin-Gorgojo A, Johansen JD. Contact dermatitis caused by iodopropynyl butylcarbamate in Denmark. Contact Dermatitis 2013;69:78–85.
- Timmer C. Antimicrobials and disinfectants. In: Kanerva L, Elsner P, Wahlberg JE, Maibach HI, eds. Handbook of Occupational Dermatology. Berlin: Springer, 2000:462–73.
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:211–59.
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen: a case series and literature review. Contact Dermatitis 2012;67:260–9.
Vehicles and other cosmetic and medicament excipients
- Hoppe U, ed. The Lanolin Book. Hamburg: Beiersdorf AG, 1999.
- Schlossman ML, McCarthy JP. Lanolin and derivatives chemistry: relationship to allergic contact dermatitis. Contact Dermatitis 1979;5:65–72.
- Clark EW, Blondeel A, Cronin E, Oleffe JA. Lanolin of reduced sensitizing potential. Contact Dermatitis 1981;7:80–3.
- Clark EW. Estimation of the general incidence of specific lanolin allergy. J Soc Cosmet Chem 1975;26:323–5.
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol 2001;145:28–31.
- Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol 1998;38:911–18.
- Schnuch A, Geier J, Uter W, et al. National rates and regional differences in sensitization to allergens of the standard series. Population-adjusted frequencies of sensitization (PAFS) in 40,000 patients from a multicenter study (IVDK). Contact Dermatitis 1997;37:200–9.
- Matthieu L, Dockx P. Discrepancy in patch test results with wool wax alcohols and Amerchol L-101. Contact Dermatitis 1997;36:150–1.
- Miest RY, Yiannias JA, Chang YH, Singh N. Diagnosis and prevalence of lanolin allergy. Dermatitis 2013:24:119–23.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis 1998;39:103–7.
- Kligman AM. Lanolin allergy: crisis or comedy. Contact Dermatitis 1983;9:99–107.
- Carmichael AJ, Foulds IS, Bransbury DS. Loss of lanolin patch test positivity. Br J Dermatol 1991;125:573–6.
- Wilson CL, Cameron J, Powell SM, et al. High incidence of contact dermatitis in leg-ulcer patients: implications for management. Clin Exp Dermatol 1991;16:250–3.
- Machet L, Couhé C, Perrinaud A, et al. A high prevalence of sensitization still persists in leg ulcer patients: a retrospective series of 106 patients tested between 2001 and 2002 and a meta-analysis of 1975–2003 data. Br J Dermatol 2004;150:929–35.
- Wolf R. The lanolin paradox. Dermatology 1996;192:198–202.
- Pasche-Koo F, Piletta PA, Hunziker N, et al. High sensitization rate to emulsifiers in patients with chronic leg ulcers. Contact Dermatitis 1994;31:226–8.
- Britton JE, Wilkinson SM, English JS, et al. The British standard series of contact dermatitis allergens: validation in clinical practice and value for clinical governance. Br J Dermatol 2003; 148:259–64.
- Bourke J, Coulson I, English J. Guidelines for care of contact dermatitis. Br J Dermatol 2001;145:877–85.
- Bruynzeel DP, Andersen KE, Camarasa JG, et al. The European standard series. European Environmental and Contact Dermatitis Research Group (EECDRG). Contact Dermatitis 1995;33:145–8.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermatitis 1997;8:2–5.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology 2006;213:353–5.
- Calnan CD. Occupational piperazine dermatitis. Contact Dermatitis 1975;1:126.
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:186–7.
- Burry JN. Ethylenediamine sensitivity with a systemic reaction to piperazine citrate. Contact Dermatitis 1978;4:380.
- Walker SL, Ferguson JE. Systemic allergic contact dermatitis due to ethylenediamine following administration of oral aminophylline. Br J Dermatol 2004;150:594.
- Thompson PJ, Gibb WR, Cole P, et al. Generalised allergic reactions to aminophylline. Thorax 1984;39:600–3.
- Andrade P, Gonçalo M, Figueiredo A. Allergic contact dermatitis to decyl glucoside in Tinosorb M. Contact Dermatitis 2010;62:119–20.
p-Phenylenediamine and related dyes
- Rietschel RL, Fowler JF, Jr. Fisher's Contact Dermatitis, 5th edn. Baltimore: Lippincott, Williams and Wilkins, 2001:248–9.
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:478–80.
- Reiss F, Fisher AA. Is hair dyed with para-phenylenediamine allergenic? Arch Dermatol 1974;109:221–2.
- Thyssen JP, Johansen JD, Menné T. Contact allergy epidemics and their control. Contact Dermatitis 2007;56:185–95.
- McFadden JP, White IR, Frosch PJ, Sosted H, Johansen JD, Menne T. Allergy to hair dye. BMJ 2007:334:220.
- Patel S, Basketter DA, Jefferies D, et al. Patch test frequency to p-phenylenediamine: follow up over the last 6 years. Contact Dermatitis 2007;56:35–7.
- Pratt MD, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch-test results, 2001–2002 study period. Dermatitis 2004;15:176–83.
- Schnuch A, Geier J, Uter W, et al. National rates and regional differences in sensitization to allergens of the standard series. Population-adjusted frequencies of sensitization (PAFS) in 40,000 patients from a multicenter study (IVDK). Contact Dermatitis 1997;37:200–9.
- Broeckx W, Blondeel A, Dooms-Goossens A, Achten G. Cosmetic intolerance. Contact Dermatitis 1987;16:189–94.
- Thyssen JP, White JML. Epidemiological data on consumer allergy to p-paraphenylenediamine. Contact Dermatitis 2008:59:327–43.
- Frosch PJ, Burrows D, Camarasa JG, et al. Allergic reactions to a hairdressers' series: results from 9 European centres. The European Environmental and Contact Dermatitis Research Group (EECDRG). Contact Dermatitis 1993;28:180–3.
- White IR, De Groot AC. Cosmetics and skin care products. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:499.
- De Groot A. Side-effects of henna and semi-permanent “black henna” tattoos: a full review. Contact Dermatitis 2013;69:1–25.
- Bork K. Allergic contact dermatitis on a violinist's neck from para-phenylenediamine in a chin rest stain. Contact Dermatitis 1993;28:250–1.
- O'Hagan AH, Bingham EA. Cellist's finger dermatitis. Contact Dermatitis 2001;45:319.
- Warren LJ, Marren P. Textile dermatitis and dyed maggot exposure. Contact Dermatitis 1997;36:106.
- Veysey C, Burge S, Cooper S. Consort contact dermatitis to paraphenylenediamine, with an unusual clinical presentation of tumid plaques. Contact Dermatitis 2007;56:366–7.
- Rastogi SC, Søsted H, Johansen JD, et al. Unconsumed precursors and couplers after formation of oxidative hair dyes. Contact Dermatitis 2006;55:95–100.
- Teixeira M, de Wachter L, Ronsyn E, Goossens A. Contact allergy to para-phenylenediamine in a permanent eyelash dye. Contact Dermatitis 2006;55:92–4.
- Hsu TS, Davis MD, el-Azhary R, et al. Beard dermatitis due to para-phenylenediamine use in Arabic men. J Am Acad Dermatol 2001;44:867–9.
- Sharma VK, Mandal SK, Sethuraman G, Bakshi NA. Para-phenylenediamine-induced lichenoid eruptions. Contact Dermatitis 1999;41:40–1.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol 2002;46:S107–12.
- Calzavara-Pinton P, Capezzera R, Zane C, et al. Lymphomatoid allergic contact dermatitis from para-phenylenediamine. Contact Dermatitis 2002;47:173–4.
- Wolf R, Wolf D, Matz H, Orion E. Cutaneous reactions to temporary tattoos. Dermatol Online J 2003;9:3.
- Jappe U, Hausen BM, Petzoldt D. Erythema-multiforme-like eruption and depigmentation following allergic contact dermatitis from a paint-on henna tattoo, due to para-phenylenediamine contact hypersensitivity. Contact Dermatitis 2001;45:249–50.
- Rubegni P, Fimiani M, de Aloe G, et al. Lichenoid reaction to temporary tattoo. Contact Dermatitis 2000;42:117–18.
- Valsecchi R, Leghissa P, Di Landro A, et al. Persistent leukoderma after henna tattoo. Contact Dermatitis 2007;56:108–9.
- Redlick F, DeKoven J. Allergic contact dermatitis to paraphenylendiamine in hair dye after sensitization from black henna tattoos: a report of 6 cases. Can Med Assoc J 2007;176:445–6.
- Saunders H, O'Brien T, Nixon R. Textile dye allergic contact dermatitis following paraphenylenediamine sensitization from a temporary tattoo. Australas J Dermatol 2004;45:229–31.
- Birnie AJ, English JS. Immediate hypersensitivity to paraphenylenediamine. Contact Dermatitis 2007;56:240.
- Pasche-Koo F, French L, Piletta-Zanin P. Contact urticaria and shock due to hair dye. Allergy 1998;53:904–5.
- Krasteva M, Cristaudo A, Hall B, et al. Contact sensitivity to hair dyes can be detected by the consumer open test. Eur J Dermatol 2002;12:322–6.
- Andersen KE, Burrows D, Cronin E, et al. Recommended changes to standard series. Contact Dermatitis 1988;19:389–90.
- Hillen U, Jappe U, Frosch PJ, et al. Late reactions to the patch-test preparations para-phenylenediamine and epoxy resin: a prospective multicentre investigation of the German Contact Dermatitis Research Group. Br J Dermatol 2006:154:665–70.
- Devos SA, Van der Valk PGM. The risk of active sensitisation to PPD. Contact Dermatitis 2001:44:273–5.
- Dawe SA, White IR, Rycroft RJ, et al. Active sensitization to para-phenylenediamine and its relevance: a 10-year review. Contact Dermatitis 2004:51:96–7.
- Thyssen JP, Menné T, Nielsen NH, Linneberg A. Is there a risk of active sensitization to PPD by patch testing the general population? Contact Dermatitis 2007;57:133–4.
- Mcfadden JP, White IR, Johansen J, Bruze M. Should para-phenylenediamine (PPD) 1% pet. be part of commercially available standard series? Contact Dermatitis 2005;53:183–4.
- Ho SG, White IR, Rycroft RJ, McFadden JP. A new approach to patch testing patients with para-phenylenediamine allergy secondary to temporary black henna tattoos. Contact Dermatitis 2004;51:213–14.
- Winhoven SM, Rutter KJ, Beck MH. Toluene-2,5-diamine may be an isolated allergy in individuals sensitized by permanent hair dye. Contact Dermatitis 2007;57:193.
Ultraviolet filters
- Niklasson B, Björkner B. Contact allergy to the UV-absorber Tinuvin P in plastics. Contact Dermatitis 1989;21:330–4.
- Arisu K, Hayakawa R, Ogino Y, et al. Tinuvin P in a spandex tape as a cause of clothing dermatitis. Contact Dermatitis 1992;26:311–16.
Rubber
- Geier J, Gefeller O. Sensitivity of patch test with rubber mixes: results of the information network of departments of dermatology from 1990 to 1993. Am J Contact Dermat 1995;6:143–9.
- Rose RF, Lyons P, Horne H, Wilkinson SM. A review of the materials and allergens in protective gloves. Contact Dermatitis 2009;61:129–37.
- Cockayne SE, Shah M, Messenger AG, Gawkrodger DJ. Foot dermatitis in children: causative allergens and follow-up. Contact Dermatitis 1998;38:203–6.
- Adams AK, Warshaw EM. Allergic contact dermatitis from mercapto compounds. Dermatitis 2006;17:56–70.
- Andersen KE, White IR, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:623–52.
- Bergendorff O, Persson C, Hansson C. High performance liquid chromatography analysis of rubber allergens in protective gloves used in health care. Contact Dermatitis 2006;55:210–15.
- Holness DL, Nethercott JR. Results of patch testing with a special series of rubber allergens. Contact Dermatitis 1997;36:207–11.
- Bendewald MJ, Farmer SA, Davis MP. An 8-year retrospective review of patch testing with rubber allergens. Dermatitis 2010;21:33–40.
- Huygens S, Barbaud A, Goossens A. Frequency and relevance of positive patch tests to cyclohexylthiophthalimide, a new rubber allergen. Eur J Dermatol 2001;11:443–5.
- Belsito DV. Common shoe allergens undetected by commercial patch-testing kits: dithiodimorpholine and isocyanates. Am J Contact Dermat 2003;14:95–6.
- Sommer S, Wilkinson SM, Beck MH et al. Type IV hypersensitivity reactions to natural rubber latex: results of a multicentre study. Br J Dermatol 2002;146:114–17.
- Dall AB-H, Andersen KE, Mortz CG. Targeted testing with diethylthiourea often reveals clinically relevant allergic contact dermatitis caused by neoprene rubber. Contact Dermatitis 2012;67:89–93.
Clothing
- Reich HC, Warshaw EM. Allergic contact dermatitis from formaldehyde textile resins. Dermatitis 2010;21:65–76.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis 2000;42:187–95.
- Malianauskiene L, Bruze M, Ryberg K, et al. Contact allergy from disperse dyes in textiles – a review. Contact Dermatitis 2013;68:65–75.
- Wilkinson SM, McGechaen K. Occupational allergic contact dermatitis from reactive dyes. Contact Dermatitis 1996;35:376.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis 2005;53:150–4.
- De Groot AC, Le Coz CJ, Lensen GJ, et al. Formaldehyde releasers: relationship to formaldehyde contact allergy. Formaldehyde releasers in clothes: durable press chemical finishes. Part 1. Contact Dermatitis 2010;62:259–71.
- De Groot AC, Le Coz CJ, Lensen GJ, et al. Formaldehyde releasers: relationship to formaldehyde contact allergy. Part 2. Formaldehyde releasers in clothes: durable press chemical finishes. Contact Dermatitis 2010;63:1–9.
- Rycroft RJG, Cronin E, Calnan CD. Canadian sheet dermatitis. BMJ 1976;ii:1175.
- Brown R. Allergy to dyes in permanent press bed linen. Contact Dermatitis 1990;20:303–4.
- Hodgson GA, Hellier FF. Dermatitis in shirts in B.L.A. J R Army Med Corps 1946;87:110–17.
- Komericki P, Aberer W, Arbab E, et al. Pigmented purpuric contact dermatitis from Disperse Blue 106 and 124 dyes. J Am Acad Dermatol 2001;45:456–8.
- Osmundsen PE, Alani MD. Contact allergy to an optical whitener, ‘CPY’, in washing powders. Br J Dermatol 1971;85:61–6.
- Giusti F, Mantovani L, Martella A, Seidenari S. Hand dermatitis as an unsuspected presentation of textile dye contact sensitivity. Contact Dermatitis 2002;47:91–5.
- Fregert S. Extractions of allergens for patch tests. Acta Derm Venereol (Stockh) 1964;44:107–9.
- Carlson RM, Smith MC, Nedorost ST. Diagnosis and treatment of dermatitis due to formaldehyde resins in clothing. Dermatitis 2004;15:169–75.
- Metzler-Brenckle L, Rietschel RL. Patch testing for permanent-press allergic contact dermatitis. Contact Dermatitis 2002;46:33–7.
- Sertoli A, Francalanci S, Giorgini S. Sensitization to textile disperse dyes: validity of reduced-concentration patch tests and a new mix. Contact Dermatitis 1994;31:47–8.
- Winhoven SM, Rutter KJ, Beck MH. Multiple positive allergic reactions from patch testing to p-phenylenediamine and azo dyes. Is this a frequent risk and can it be reduced? Contact Dermatitis 2008;58:182–3.
- Seidenari S, Giusti F, Massone F, Mantovani L. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermatitis 2002;13:101–7.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Am J Contact Dermatitis 2000;11:30–41.
- Lazarov A, Trattner A, David M, et al. Textile dermatitis in Israel: a retrospective study. Am J Contact Dermatitis 2000;11:26–9.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis 2006;54:313–21.
- Slodownik D, Williams J, Tate B, et al. Textile allergy – the Melbourne experience. Contact Dermatitis 2011;65:38–42.
- Roed-Petersen J, Batsberg W, Larsen E. Contact dermatitis from naphthol AS. Contact Dermatitis 1990;22:161–3.
- Andersen KE. Sensitivity to flame retardant tris (2,3-dibromopropyl) phosphate (Firemaster LVT 23P). Contact Dermatitis 1977;3:297–300.
- Wilkinson SM, Cartwright PH, Armitage JD, English JS. Allergic contact dermatitis from 1,6-diisocyanatohexane in an anti-pill finish. Contact Dermatitis 1991;25:94–6.
- Hatch KL, Maibach HI. Textile chemical finish dermatitis. Contact Dermatitis 1986;14:1–13.
Shoes
- Nardelli A, Taveirne M, Drieghe J, et al. The relation between the localization of foot dermatitis and the causative allergens in shoes: a 13-year retrospective study. Contact Dermatitis 2005;53:201–6.
- Holden CR, Gawkrodger DJ. 10 years' experience of patch testing with a shoe series in 230 patients: which allergens are important? Contact Dermatitis 2005;53:37–9.
- Weston JA, Hawkins K, Weston WL. Foot dermatitis in children. Pediatrics 1983;72:824–7.
- Adams RM. Shoe dermatitis. Calif Med 1972;117:12–16.
- Roberts JL, Hanifin JM. Athletic shoe dermatitis. JAMA 1979;241:275–6.
- Angelini G, Vena GA, Meneghini CL. Shoe contact dermatitis. Contact Dermatitis 1980;6:279–83.
- Romaguera C, Grimalt F, Vilaplana J. Eczematous and purpuric allergic contact dermatitis from boots. Contact Dermatitis 1989;21:269.
- Landeck L, Uter W, John SM. Patch test characteristics of patients referred for suspected contact allergy of the feet – retrospective 10-year cross-sectional study of the IVDK data. Contact Dermatitis 2012;66:271–8.
- Belsito DV. Common shoe allergens undetected by commercial patch-testing kits: dithiodimorpholine and isocyanates. Am J Contact Dermatitis 2003;14:95–6.
- Gimenez Arnau A. Dimethyl fumarate: a human health hazard. Dermatitis 2011;22:47–9.
- Jordan WP Jr. Clothing and shoe dermatitis. Recognition and management. Postgrad Med 1972;52:143–8.
- Freeman S. Shoe dermatitis. Contact Dermatitis 1997;36:247–51.
Epoxy resins
- Thorgeirsson A, Fregert S. Allergenicity of epoxy resins in the guinea pig. Acta Derm Venereol (Stockh) 1977;57:253–6.
- Holness DL, Nethercott JR. Results of testing with epoxy resin in an occupational health clinic population. Am J Contact Dermatitis 1992;3:169–74.
- Chu CY, Pontén A, Sun CC, Jee SH. Concomitant contact allergy to the resins, reactive diluents and hardener of a bisphenol A/F-based epoxy resin in subway construction workers. Contact Dermatitis 2006;54:131–9.
- Geier J, Lessmann H, Hillen U, et al. An attempt to improve diagnostics of contact allergy due to epoxy resin systems. First results of the multicentre study EPOX 2002. Contact Dermatitis 2004;51:263–72.
- Angelini G, Rigano L, Foti C, et al. Occupational sensitization to epoxy resin and reactive diluents in marble workers. Contact Dermatitis 1996;35:11–16.
- Jolanki R, Tarvainen K, Tatar T, et al. Occupational dermatoses from exposure to epoxy resin compounds in a ski factory. Contact Dermatitis 1996;34:390–6.
- Omer SA, al-Tawil NG. Contact sensitivity among workers in a paint factory. Contact Dermatitis 1994;30:55–7.
- Dahlquist I, Fregert S. Allergic contact dermatitis from volatile epoxy hardeners and reactive diluents. Contact Dermatitis 1979;5:406–7.
- Whitfield MJ, Rivers JK. Erythema multiforme after contact dermatitis in response to an epoxy sealant. J Am Acad Dermatol 1991;25:386–8.
- Geldof BA, Oranje AP, van Joost Th. Hand eczema associated with continuous subcutaneous insulin infusion. Contact Dermatitis 1989;20:384–5.
- Koch P. Allergic contact stomatitis from BIS-GMA and epoxy resins in dental bonding agents. Contact Dermatitis 2003;49:104–5.
- Silvestre JF, Albares MP, Escutia B, et al. Contact vitiligo appearing after allergic contact dermatitis from aromatic reactive diluents in an epoxy resin system. Contact Dermatitis 2003;49:113–14.
- Kanerva L, Estlander T, Jolanki R. Allergic contact dermatitis from dental composite resins due to aromatic epoxy acrylates and aliphatic acrylates. Contact Dermatitis 1989;20:201–11.
- Thorgeirsson A. Sensitization capacity of epoxy resin hardeners in the guinea pig. Acta Derm Venereol (Stockh) 1978;58:332–6.
- Cahill J, Keegel T, Dharmage S, et al. Prognosis of contact dermatitis in epoxy resin workers. Contact Dermatitis 2005;52:147–53.
- Beck MH, Burrows D, Fregert S, et al. Allergic contact dermatitis to epoxy resin in ostomy bags. Br J Surg 1985;72:202–3.
- Pegum JS. Penetration of protective gloves by epoxy resin. Contact Dermatitis 1979;5:281–3.
- Roed-Petersen J. A new glove material protective against epoxy and acrylate monomer. In: Frosch PJ, Dooms-Goossens A, LaChapelle J-M, et al., eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:569–78.
Acrylate resins
- Tucker SC, Beck MH. A 15-year study of patch testing to (meth)acrylates. Contact Dermatitis 1999;40:278–9.
- Sood A, Taylor JS. Acrylic reactions: a review of 56 cases. Contact Dermatitis 2003;48:346–7.
- Torres MC, Linares T, Hernandez MD. Acrylates induced rhinitis and contact dermatitis. Contact Dermatitis 2005;53:114.
- Slodownik D, Williams JD, Tate BJ. Prolonged paresthesia due to sculptured acrylic nails. Contact Dermatitis 2007;56:298–9.
- Jung HP, Jarisch R, Hemmer W. Hypersensitivity from dental acrylates in a patient previously sensitized to artificial nails. Contact Dermatitis 2005;53:119–20.
- Menni A, Lodi A, Coassini D, et al. Unusual widespread vesicular eruption related to dental composite resin sensitization. Contact Dermatitis 2003;48:174.
- Kanerva L, Estlander T, Jolanki R. Sensitization to patch test acrylates. Contact Dermatitis 1988;18:10–15.
- Goon AT, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis 2007;57:21–7.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis 2005;53:292–7.
- Kwok C, Wilkinson SM, Sommer S. A rare case of acquired leukoderma following patch testing with an acrylate series. Contact Dermatitis 2011;64:292–4.
- Aalto-Korte K, Jungewelter S, Henriks-Eckerman ML, et al. Contact allergy to epoxy (meth)acrylates. Contact Dermatitis 2009;61:9–21.
- Shanmugam S, Wilkinson SM. Allergic contact dermatitis caused by a cyanoacrylate containing false eyelash glue. Contact Dermatitis 2012;67:309–10.
- Munksgaard EC. Permeability of protective gloves to (di)methacrylates in resinous dental materials. Scand J Dent Res 1992;100:189–92.
- Roed-Petersen J. A new glove material protective against epoxy and acrylate monomer. In: Frosch PJ, Dooms-Goossens A, LaChapelle J-M, et al., eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:569–78.
Formaldehyde resins
- Bruze M, Almgren G. Occupational dermatoses in workers exposed to resins based on phenol and formaldehyde. Contact Dermatitis 1988;19:272–7.
- Owen CM, Beck MH. Occupational allergic contact dermatitis from phenol-formaldehyde resins. Contact Dermatitis 2001;45:294–5.
- Tarvainen K. Analysis of patients with allergic patch test reactions to a plastics and glues series. Contact Dermatitis 1995;32:346–51.
- Bruze M, Fregert S, Zimerson E. Contact allergy to phenol-formaldehyde resins. Contact Dermatitis 1985;12:81–6.
- Bruze M. Contact sensitizers in resins based on phenol and formaldehyde. Acta Derm Venereol Suppl (Stockh) 1985;119:1–83.
- Bruze M, Persson L, Trulsson L, et al. Demonstration of contact sensitizers in resins and products based on phenol-formaldehyde. Contact Dermatitis 1986;14:146–54.
- Beck MH. Experiences of contact dermatitis with phenol formaldehyde resins. In: Frosch PJ, Dooms-Goossens A, LaChapelle J-M, Rycroft RJG, Scheper RJ, eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:374–6.
- Avenel-Audran M, Goossens A, Zimerson E, Bruze M. Contact dermatitis from electrocardiograph-monitoring electrodes: role of p-tert-butylphenol-formaldehyde resin. Contact Dermatitis 2003;48:108–11.
Other plastics
- Quartier S, Garmyn M, Becart S, Goossens A. Allergic contact dermatitis to copolymers in cosmetics – case report and review of the literature. Contact Dermatitis 2006;55:257–67.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis 2006;55:130–9.
Plants
- Mitchell JC, Rook A. Botanical Dermatology. Philadelphia: Lea and Febiger, 1979.
- Schmidt RJ, ed. The Botanical Dermatology Database. http://www.botanical-dermatology-database.info/ (last accessed March 2015).
- Lovell CR. Plants and the Skin. Oxford: Blackwell Scientific Publications, 1993.
- Paulsen E, Christensen LP, Andersen KE. Dermatitis from common ivy (Hedera helix L. subsp helix) in Europe: past, present and future. Contact Dermatitis 2010;62:201–9.
- Zachariae C, Engkilde K, Johansen JD, Menné T. Primin in the European standard patch test series for 20 years. Contact Dermatitis 2007;56:344–6.
- Paulsen E, Andersen KE, Brandao FM, et al. Routine patch testing with the sesquiterpene lactone mix in Europe: a 2-year experience. A multicentre study of the EECDRG. Contact Dermatitis 1999;40:72–6.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for compositae allergy. Contact Dermatitis 1994;31:151–3.
- Cabanillas M, Fernández-Redondo V, Toribio J. Allergic contact dermatitis to plants in a Spanish dermatology department: a 7-year review. Contact Dermatitis 2006;55:84–91.
- Rutherford T, Nixon R, Tam M, Tate B. Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4.5 years. Australas J Dermatol 2007;48:83–7.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol 1980;2:332–3.
- Guin JD, Gillis WT, Beaman JH. Recognizing the toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol 1981;4:99–114.
- Guin JD. Occupational contact dermatitis to plants. In: Kanerva L, Elsner P, Wahlberg JE, Maibach HI, eds. Handbook of Occupational Dermatology. Berlin: Springer, 2000:730–66.
- Oliwiecki S, Beck MH, Hausen BM. Compositae dermatitis aggravated by eating lettuce. Contact Dermatitis 1991;24:318–19.
- Jovanovic' M, Mimica-Dukic' N, Poljacki M, Boza P. Erythema multiforme due to contact with weeds: a recurrence after patch testing. Contact Dermatitis 2003;48:17–25.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, Aliaga A. Allergic and systemic contact dermatitis from matricaria chamomilla tea. Contact Dermatitis 1998;39:192–3.
- Paulsen E, Andersen KE. Colophonium and Compositae mix as markers of fragrance allergy: cross-reactivity between fragrance terpenes, colophonium and compositae plant extracts. Contact Dermatitis 2005;53:285–91.
- Aplin CG, Lovell CR. Contact dermatitis due to hardy primula species and their cultivars. Contact Dermatitis 2001;44:23–9.
- Christensen LP, Larsen E. Primin-free Primula obconica plants available. Contact Dermatitis 2000;43:45–6.
- Ekeowa-Anderson AL, Shergill B, Goldsmith P. Allergic contact cheilitis to garlic. Contact Dermatitis 2007;56:174–5.
- Pereira F, Hatia M, Cardoso J. Systemic contact dermatitis from diallyl disulfide. Contact Dermatitis 2002;46:124.
- Salo H, Hannuksela M, Hausen B. Lichen pickers dermatitis (Cladonia alpestris (L) Rab.). Contact Dermatitis 1981;7:9–13.
- Dooms-Goossens A, Biesemans G, Vandaele M, et al. Primula dermatitis: more than one allergen? Contact Dermatitis 1989;21:122–4.
- Krebs M, Christensen LP. 2-Methoxy-6-pentyl-1,4-dihydroxybenzene (miconidin) from Primula obconica: a possible allergen? Contact Dermatitis 1995;33:90–3.
- Lepoittevin JP, Tomb R. Sesquiterpene lactone mix is not an adequate screen for compositae allergy. Contact Dermatitis 1995;32:254.
- Jacob M, Brinkmann J, Schmidt TJ. Sesquiterpene lactone mix as a diagnostic tool for asteraceae allergic contact dermatitis: chemical explanation for its poor performance and sesquiterpene lactone mix II as a proposed improvement. Contact Dermatitis 2012;66:233–40.
- Paulsen E, Christensen LP, Andersen KE. Do monoterpenes released from feverfew (Tanacetum parthenium) plants cause airborne compositae dermatitis? Contact Dermatitis 2002;47:14–18.
- Hausen BM. A 6-year experience with compositae mix. Am J Contact Dermat 1996;7:94–9.
- Paulsen E, Andersen KE. Patch testing with constituents of compositae mixes. Contact Dermatitis 2012;66:241–6.
- Goulden V, Wilkinson SM Patch testing for compositae allergy. Br J Dermatol 1998;138:1018–21.
- Jovanovic' M, Poljacki M, Mimica-Dukic' N, et al. Sesquiterpene lactone mix patch testing supplemented with dandelion extract in patients with allergic contact dermatitis, atopic dermatitis and non-allergic chronic inflammatory skin diseases. Contact Dermatitis 2004;51:101–10.
- Rastogi SC, Bossi R, Johansen JD, et al. Content of oak moss allergens atranol and chloroatranol in perfumes and similar products. Contact Dermatitis 2004;50:367–70.
- Guin J. Patch testing to plants: some practical aspects of what has become an esoteric area of contact dermatitis. Am J Contact Dermatitis 1995;6:232–5.
- Marks JG, Jr. Allergic contact dermatitis to alstroemeria. Arch Dermatol 1988;124:914–16.
- Moyle M, Frowen K, Nixon R. Use of gloves in protection from diallyl disulphide allergy. Australas J Dermatol 2004;45:223–5.
- Marks JG, Jr, Fowler JF, Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol 1995;33:212–16.
- Grevelink SA, Murrell DF, Olsen EA. Effectiveness of various barrier preparations in preventing and/or ameliorating experimentally produced toxicodendron dermatitis. J Am Acad Dermatol 1992;27:182–8.
- Marks JG, Jr, Trautlein JJ, Epstein WL, et al. Oral hyposensitization to poison ivy and poison oak. Arch Dermatol 1987;123:476–8.
Woods, colophony, turpentine and propolis
- Hausen BM. Woods Injurious to Human Health. A Manual. Berlin: Walter de Gruyter, 1981.
- Mitchell JC, Rook AJ. Botanical Dermatology. Vancouver: Greengrass, 1979.
- Woods B, Calnan CD. Toxic woods. Br J Dermatol 1976;94(Suppl. 13):1–97.
- Cachao P, Menezes Brandao F, Carmo M, et al. Allergy to oil of turpentine in Portugal. Contact Dermatitis 1986;14:205–8.
- Treudler R, Richter G, Geier J, et al. Increase in sensitization to oil of turpentine: recent data from a multicenter study on 45 005 patients from the German Austrian Information Network of Departments of Dermatology (IVDK). Contact Dermatitis 2000;42:68–73.
- Walgrave SE, Warshaw EM, Glesne LA. Allergic contact dermatitis from propolis. Dermatitis 2005;16:209–15.
- Rojas-Hijazo B, Lezaun A, Hausen BM, et al. Airborne contact dermatitis in gaitas (flageolets) constructors after exposure to sawdust of caviuna. Contact Dermatitis 2007;56:274–7.
- Holst R, Kirby J, Magnusson B. Sensitization to tropical woods giving erythema multiforme-like eruptions. Contact Dermatitis 1976;2:295–6.
- Karlberg AT, Gäfvert E, Meding B, et al. Airborne contact dermatitis from unexpected exposure to rosin (colophony). Rosin sources revealed with chemical analyses. Contact Dermatitis 1996;35:272–8.
- Karlberg AT, Lidén C, Ehrin E. Colophony in mascara as a cause of eyelid dermatitis. Chemical analyses and patch testing. Acta Derm Venereol (Stockh) 1991;71:445–7.
- Salim A, Shaw S. Recommendation to include ester gum resin when patch testing patients with leg ulcers. Contact Dermatitis 2001;44:34.
- Goossens A, Armingaud P, Avenel-Audran M, et al. An epidemic of allergic contact dermatitis due to epilating products. Contact Dermatitis 2002;47:67–70.
- Ramien ML, Pratt MD. Fixed drug eruption to ingested propolis. Dermatitis 2013;23:173–5.
- Hausen BM, Mohnert J. Contact allergy due to colophony (V). Patch test results with different types of colophony and modified-colophony products. Contact Dermatitis 1989;20:295–301.
- Karlberg AT, Bråred-Christensson J, Börje A, et al. Methyl esterification of 15-hydroperoxyabietic acid does not affect the patch-test result in colophonium allergic patients. Contact Dermatitis 2007;56:355–6.
- Pereira TM, Flour M, Goossens A. Allergic contact dermatitis from modified colophonium in wound dressings. Contact Dermatitis 2007;56:5–9.
- Romaguera C, Alomar A, Condé Salazar L, et al. Turpentine sensitization. Contact Dermatitis 1986;14:197.
- Jensen CD, Andersen KE. Allergic contact dermatitis from cera alba (purified propolis) in a lip balm and candy. Contact Dermatitis 2006;55:312–13.
- Hausen BM, Evers P, Stuwe HT, et al. Propolis allergy (IV). Studies with further sensitizers from propolis and constituents common to propolis, poplar buds and balsam of Peru. Contact Dermatitis 1992;26:34–44.
- Meding B, Karlberg AT, Ahman M. Wood dust from jelutong (Dyera costulata) causes contact allergy. Contact Dermatitis 1996;34:349–53.
- Karlberg AT, Lidén C. Colophony (rosin) in newspapers may contribute to hand eczema. Br J Dermatol 1992;126:161–5.
Clinical variants
Systemically reactivated contact dermatitis
- Hjorth N. Nickel dermatitis. Contact Dermatitis 1976;2:356–7.
- Le Coz CJ, Lepoittevin JP. Occupational erythema-multiforme-like dermatitis from sensitization to costus resinoid, followed by flare-up and systemic contact dermatitis from beta-cyclocostunolide in a chemistry student. Contact Dermatitis 2001;44:310–11.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome. Systemically induced allergic contact dermatitis. Contact Dermatitis 1984;10:97–100.
- Nakayama H, Shono M, Hada S. Mercury exanthem. J Am Acad Dermatol 1989;11:137–9.
- Ekelund AG, Moller H. Oral provocation in eczematous contact allergy to neomycin and hydroxy-quinolines. Acta Derm Venereol (Stockh) 1969;49:422–6.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis 2008;19:9–15.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology 2006;213:353–5.
- Lauerma AI, Reitamo S, Maibach HI. Systemic hydrocortisone/cortisol induces allergic skin reactions in presensitized subjects. J Am Acad Dermatol 1991;24:182–5.
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol 2002;46:880–5.
- Jensen CS, Menné T, Johansen JD. Systemic contact dermatitis after oral exposure to nickel: a review with a modified meta-analysis. Contact Dermatitis 2006;56:79–86.
- Raison-Peyron N, Guillard O, Khalil Z, et al. Nickel-elicited systemic contact dermatitis from a peripheral intravenous catheter. Contact Dermatitis 2005;53:222–5.
- Pigatto PD, Guzzi G. Systemic contact dermatitis from nickel associated with orthodontic appliances. Contact Dermatitis 2004;50:100–1.
- Veien NK, Hattel T, Justensen O, et al. Oral challenge with balsam of Peru. Contact Dermatitis 1985;12:104–7.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis 1994;30:299–300.
- Baer RL, Leider M. The effects of feeding certified food azo dyes in paraphenylenediamine-hypersensitive subjects. J Invest Dermatol 1949;13:223–32.
- Marks JG, Demelfi E, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol 1984;10:627–31.
- Park SD, Lee SW, Chun JH, et al. Clinical features of 31 patients with systemic contact dermatitis due to the ingestion of Rhus (lacquer). Br J Dermatol 2000;142:937–42.
- Gawkrodger DJ. Metal sensitivities and orthopaedic implants revisited: the potential for metal allergy with the new metal-on-metal joint prostheses. Br J Dermatol 2003;148:1089–93.
- Haudrechy P, Mantout B, Frappaz A, et al. Nickel release from stainless steels. Contact Dermatitis 1997;37:113–17.
- Gawkrodger DJ. Nickel sensitivity and the implantation of orthopaedic prostheses. Contact Dermatitis 1993;28:257–9.
- Antony W, Holden CA. Metal allergy resurfaces in failed hip endoprostheses. Contact Dermatitis 2003;48:45–55.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol 2012;148:687–93
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis 2011;22:65–79.
- Svedman C, Möller H, Gruvberger B, et al. Implants and contact allergy: are sensitizing metals released as haptens from coronary stents? Contact Dermatitis 2014;71: 92–7.
- Svedman C, Ekqvist S, Möller H, et al. A correlation found between contact allergy to stent material and restenosis of the coronary arteries. Contact Dermatitis 2009;60:158–64.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants - diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis 2012;66:4–19.
Non-eczematous responses
- Shimizu S, Chen KR, Pratchyapruit WO, Shimizu H. Tropical-wood-induced bullous erythema multiforme. Dermatology 2000;200(1):59–62
- Lazarov A, Cordoba M. Purpuric contact dermatitis in patients with allergic reaction to textile dyes and resins. J Eur Acad Dermatol Venereol 2000;14:101–5.
- Verma K, Sharma NL, Mahajan VK, et al. Purpuric contact dermatitis from footwear. Contact Dermatitis 2007;56:362–4.
- Lidén C, Brehmer-Andersson E. Occupational dermatoses from colour developing agents. Clinical and histopathological observations. Acta Derm Venereol (Stockh) 1988;68:514–22.
- Sharma VK, Mandal SK, Sethuraman G, et al. Para-phenylenediamine-induced lichenoid eruptions. Contact Dermatitis 1999;41:40–1.
- Lapiere K, Matthieu L, Meuleman L, et al. Primula dermatitis mimicking lichen planus. Contact Dermatitis 2001;44:199.
- Athavale PN, Shum KW, Yeoman CM, Gawkrodger DJ. Oral lichenoid lesions and contact allergy to dental mercury and gold. Contact Dermatitis 2003;49:264–5.
- Martin MD, Broughton S, Drangsholt M. Oral lichen planus and dental materials: a case–control study. Contact Dermatitis 2003;48:331–6.
- Orbaneja JG, Diez LI, Lozano JL, et al. Lymphomatoid contact dermatitis: a syndrome produced by epicutaneous hypersensitivity with clinical features and a histopathologic picture similar to that of mycosis fungoides. Contact Dermatitis 1976;2:139–43.
- Osmundsen PE. Pigmented contact dermatitis. Br J Dermatol 1976;81:799–803.
- Nakayama H, Matsuo S, Hayakawa K, et al. Pigmented cosmetic dermatitis. Int J Dermatol 1984;23:299–305.
- Leow YH, Tan SH, Ng SK. Pigmented contact cheilitis from ricinoleic acid in lipsticks. Contact Dermatitis 2003;49:48–9.
- Nath AK, Thappa DM. Kumkum-induced dermatitis: an analysis of 46 cases. Clin Exp Dermatol 2007;32:385–7.
- Penegos H, Jiminez V, Fallas V, et al. Chlorothalonil, a possible cause of erythema dyschromicum perstans (ashy dermatosis). Contact Dermatitis 1996;35:214–18.
- Valsecchi R, Leghissa P, Di Landro A, et al. Persistent leukoderma after henna tattoo. Contact Dermatitis 2007;56:108–9.
- Bhushan M, Beck MH. Allergic contact dermatitis from primula presenting as vitiligo. Contact Dermatitis 1999;41:292–3.
- Kaaber K, Nielsen AO, Veien NK. Vaccination granulomas and aluminium allergy, course and prognostic factors. Contact Dermatitis 1992;26:304–6.
- Goossens A, De Swerdt A, De Coninck K, et al. Allergic contact granuloma due to palladium following ear piercing. Contact Dermatitis 2006;55:338–41.
- Vilaplana J, Chimenos JM, Fernández AI, et al. Problems in the diagnosis of contact dermatitis by tattooing. Exog Dermatol 2002;1:307–12.
- Lazarov A, Kidron D, Tulchinsky Z, Minkow B. Contact orofacial granulomatosis caused by delayed hypersensitivity to gold and mercury. J Am Acad Dermatol 2003;49:1117–20.
- Armstrong DK, Biagioni P, Lamey PJ, et al. Contact hypersensitivity in patients with orofacial granulomatosis. Am J Contact Dermatitis 1997;8:35–8.
- Lindup WE, Nowell PT. Role of sultone contaminants in an outbreak of allergic contact dermatitis caused by alkyl ethoxysulphates: a review. Food Cosmet Toxicol 1978;16:59–62.
Differential diagnosis
- Kuznetsov AV, Erlenkeuser-Uebelhoer I, Thomas P. Contact allergy to propylene glycol and dodecyl gallate mimicking seborrheic dermatitis. Contact Dermatitis 2006;55:307–8.
- Sánchez-Pérez J, Gala SP, Jiménez YD, et al. Allergic contact dermatitis to prednicarbate presenting as lupus erythematosus. Contact Dermatitis 2006;55:247–9.
- Hague J, Ilchyshyn A. Nickel allergy mimicking basal cell carcinoma. Contact Dermatitis 2006;54:344–5.
- Wilkinson DS, Wilkinson JD. Nickel allergy and hand eczema. In: Maibach HI, Menné T, eds. Nickel and the Skin. Immunology and Toxicology. Boca Raton, FL: CRC Press, 1989:133–63.
- Veien NK, Hattel T, Justensen O, et al. Oral challenge with balsam of Peru. Contact Dermatitis 1985;12:104–7.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis 1994;30:299–300.
- Spiewak R. Köbnerizing occupational contact allergy to thiuram in a farmer with psoriasis. Contact Dermatitis 2004;51:214–15.
- Lidén C, Brehmer-Andersson E. Occupational dermatoses from colour developing agents. Clinical and histopathological observations. Acta Derm Venereol (Stockh) 1988;68:514–22.
Disease course and prognosis
- Cahill J, Keegel T, Nixon R. The prognosis of occupational contact dermatitis in 2004. Contact Dermatitis 2004;51:219–26.
- Meding B, Lantto R, Lindahl G, et al. Occupational skin disease in Sweden – a 12-year follow-up. Contact Dermatitis 2005;53:308–13.
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis 2004;15:117–24.
- Christensen OB. Prognosis in nickel allergy and hand eczema. Contact Dermatitis 1982;8:7–15.
- Halbert AR, Gebauer KA, Wall LM. Prognosis of occupational chromate dermatitis. Contact Dermatitis 1992;27:214–19.
- Kaaber K, Veien NK, Tjell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol 1978;98:197–201.
- Kaaber K, Veien NK. The significance of chromate ingestion in patients allergic to chromate. Acta Derm Venereol (Stockh) 1977;57:321–3.
- Burrows D. The Prosser White oration 1988. Mischievous metals: chromate, cobalt, nickel and mercury. Clin Exp Dermatol 1989;14:266–72.
- Cahill J, Keegel T, Dharmage S. Prognosis of contact dermatitis in epoxy resin workers. Contact Dermatitis 2005;57:147–53.
- Holness DL, Nethercott JR. Is a worker's understanding of their diagnosis an important determinant of outcome in occupational contact dermatitis? Contact Dermatitis 1991;25:296–301.
- Kalimo K, Lammintausta K, Jalava J, et al. Is it possible to improve the prognosis in nickel contact dermatitis? Contact Dermatitis 1997;37:121–4.
- Agner T, Flyvholm MA, Menné T. Formaldehyde allergy: a follow-up study. Am J Contact Dermatitis 1999;10:12–17.
- Moss C, Friedmann PS, Shuster S, Simson JM. Susceptibility and amplification of sensitivity in contact dermatitis. Clin Exp Immunol 1985;61:232–41.
- Wall LM, Gebauer KA. A follow-up study of occupational skin disease in Western Australia. Contact Dermatitis 1991;24:241–3.
- Sajjachareonpong P, Cahill J, Keegel T. Persistent post-occupational dermatitis. Contact Dermatitis 2004;51:278–83.
- Ayala F, Balato N, Lembo G, et al. Statistical evaluation of the persistence of acquired hypersensitivity by standardized patch tests. Contact Dermatitis 1996;34:354–8.
- Valsecchi R, Ross A, Bigardi A, Pigatto PD. The loss of contact sensitization in man. Contact Dermatitis 1991;24:183–6.
- Williams J, Cahill J, Nixon R. Occupational autoeczematization or atopic eczema precipitated by occupational contact dermatitis? Contact Dermatitis 2007;56:21–6.
Investigations
Patch testing, Concentrations and vehicles for patch testing, Complications of patch and photopatch tests, Multiple patch test reactions
- Podmore P, Burrows D, Bingham EA. Prediction of patch test results. Contact Dermatitis 1984;11:283–4.
- Bhushan M, Beck MH. An audit to identify the optimum referral rate to a contact dermatitis investigation unit. Br J Dermatol 1999;141:570–2.
- Rajagopalan R, Anderson RT, Sarma S, et al. An economic evaluation of patch testing in the diagnosis and management of allergic contact dermatitis. Am J Contact Dermatitis 1998;9:149–54.
- Thomson KF, Wilkinson SM, Sommer S, et al. Eczema: quality of life by body site and the effect of patch testing. Br J Dermatol 2002;146:627–30.
- Woo PN, Hay JC, Ormerod AS. An audit of the value of patch testing and its effect on the quality of life. Contact Dermatitis 2003;48:244–7.
- Rietschel RL. Is patch testing cost-effective? J Am Acad Dermatol 1989;21:885–7.
- Belsito DV, Storrs FJ, Taylor SJ, et al. Reproducibility of patch tests: a United States multicenter study. Am J Contact Dermatitis 1992;3:193–200.
- Brasch J, Henseler T, Aberer W, et al. Reproducibility of patch tests. A multi-center study of synchronous left- versus right-sided patch tests by the German Contact Dermatitis Research Group. J Am Acad Dermatol 1994;31:584–91.
- Feuerman E, Levy A. A study of the effect of prednisone and an antihistamine on patch test reactions. Br J Dermatol 1972;86:68–71.
- Vigan M, Sauvage C, Adessi B, et al. Pourquoi et comment réaliser une batterie standard chez les enfants? Nouv Dermatol 1994;13:12–15.
- Wilkinson JD, Bruynzeel DP, Ducombs G, et al. European multicentre study of True Test. Panel 2. Contact Dermatitis 1990;22:218–25.
- Patel D, Belsito DV. The detection of clinically relevant contact allergens with a standard screening tray of 28 allergens. Contact Dermatitis 2012;66:154–8.
- Dooms-Goossens A, Degreff H. Contact allergy to petrolatums. I. Sensitizing capacity of different brands of yellow and white petrolatums. Contact Dermatitis 1983;9:175–85.
- Bajaj AK, Gupta SC, Chatterjee A. Suitable bases for patch testing in tropical countries. Int J Dermatol 1986;25:379–80.
- Bruze M. Use of buffer solutions for patch testing. Contact Dermatitis 1984;10:267–9.
- Bruze M, Isaksson M, Gruvberger B, Frick-Engfeldt M. Recommendation of appropriate amounts of petrolatum preparation to be applied at patch testing. Contact Dermatitis 2007;56:281–5.
- Goon AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol 2011;164:116–24.
- Mowitz M, Zimerson E, Svedman C, Bruze M. Stability of fragrance patch test preparations in test chambers. Br J Dermatol 2012;167;822–7.
- Magnusson B, Hersle K. Patch test methods: II. Regional variations of patch test responses. Acta Derm Venereol (Stockh) 1965;45:257–61.
- Hextall JM, Alagaratnam NJ, Glendinning AK, et al. Dose–time relationships for elicitation of contact allergy to para-phenylenediamine. Contact Dermatitis 2002;47:96–9.
- Andersen KE, Johansen JD, Bruze M, et al. The time–dose–response relationship for elicitation of contact dermatitis in isoeugenol allergic individuals. Toxicol Appl Pharmacol 2001;170:166–71.
- Todd DJ, Handley J, Metwali M, et al. Day 4 is better than day 3 for a single patch test reading. Contact Dermatitis 1996;34:402–4.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of TRUE tests on D6/7. Contact Dermatitis 2013;68:94–7.
- Wilkinson DS, Fregert S, Magnusson B, et al. Terminology of contact dermatitis. Acta Derm Venereol (Stockh) 1970;50:287–92.
- Maibach HI, Fregert S, Magnusson B, et al. Quantification of the excited skin syndrome (the ‘angry back’). Retesting one patch at a time. Contact Dermatitis 1982;8:78–9.
- Bruynzeel DP. Angry back or excited skin syndrome [Thesis]. Free Universiteit te Amsterdam, the Netherlands, 1983.
- Andersen KE, Lidén C, Hansen J, Vølund A. Dose–response testing with nickel sulphate using the TRUE test in nickel sensitive subjects. Multiple nickel sulphate patch test reactions do not cause an ‘angry back’. Br J Dermatol 1993;129:50–6.
- Anveden I, Lindberg, Andersen KE, et al. Oral prednisone suppresses allergic but not irritant patch test reactions in individuals hypersensitive to nickel. Contact Dermatitis 2004;50:298–303.
- Sjovall P. Ultraviolet radiation and allergic contact dermatitis. an experimental and clinical study [Thesis]. University of Lund, Sweden, 1988.
- Bashir SJ, Maibach HI. Compound allergy. An overview. Contact Dermatitis 1997;36:179–83.
- Smeenk G, Kerckhoffs HP, Schreurs PH. Contact allergy to a reaction product in Hirudoid cream: an example of compound allergy. Br J Dermatol 1987;116:223–31.
- McLelland J, Shuster S. Contact dermatitis with negative patch tests: the additive effect of allergens in combination. Br J Dermatol 1990;122:623–30.
- Seidenari S, Motolese A, Bettetti B. Pre-treatment of nickel test areas with sodium lauryl sulphate detects nickel sensitivity in subjects reacting negatively to routinely performed patch tests. Contact Dermatitis 1996;34:88–92.
- Basketter D. Quenching: fact or fiction? Contact Dermatitis 2000;43:253–8.
- Barkroll P, Rolla G. Triclosan reduces the clinical symptoms of the allergic patch test reaction (APR) elicited with 1% nickel sulphate in sensitised patients. J Clin Periodontol 1995;22:485.
- Karlberg AT, Dooms-Goossens A. Contact allergy to oxidized d-limonene among dermatitis patients. Contact Dermatitis 1997;36:201–6.
- Edman B. Seasonal influence on patch test results. Contact Dermatitis 1989;20:206.
- Bruze M, Andersen KE, Goossens A. Recommendation to include fragrance mix 2 and hydroxyisohexyl 3-cyclohexene carboxaldehyde (Lyral) in the European baseline patch test series. Contact Dermatitis 2008;58:129–33.
- British Society of Cutaneous Allergy. BSCA baseline series: January 2012. http://www.cutaneousallergy.org/downloads/BSCA_baseline_series_2012.pdf (last accessed March 2015).
- Fransway AF, Zug KA, Belsito DV, et al. North American Contact Dermatitsi Group Patch test results for 2007–8. Dermatitis 2013;24:10–21.
- Magnusson B, Fregert S, Hjorth N, et al. Routine patch testing: V. Correlations of reactions to the site of dermatitis and the history of the patient. Acta Derm Venereol (Stockh) 1969;49:556–63.
- Menné T, Dooms-Goossens A, Wahlberg JE, et al. How large a proportion of contact sensitivities are diagnosed with the European standard series? Contact Dermatitis 1992;26:201–2.
- Adachi A. JSCD Research Group study. Results of patch tests with standard allergen series of the Research Group of the Japanese Society for Contact Dermatitis in 1994 and annual variations of patients with pigmented contact dermatitis of lichenoid type in 1993. Environ Dermatol 1996;3:140–50.
- Bruynzeel DP, Diepgen TL, Andersen KE, et al. Monitoring the European standard series in 10 centres 1996-2000. Contact Dermatitis 2005;53:146–9.
- Uter W, Aberer W, Armario-Hita JC, et al. Current patch test results with the European baseline series and extensions to it from the ‘European Surveillance System on Contact Allergy’ network, 2007–2008. Contact Dermatitis 2012;67:9–19.
- Bruze M, Frick M, Persson L. Patch testing with thin-layer chromatograms. Contact Dermatitis 2003;48:278–9.
- Cocklin CL, Shackelford K, Wolverton SE, Fett DD. Pemphigus foliaceus with epidermal detachment: adverse events from patch testing. Dermatitis 2006;17:32–5.
- Walker SL, Beck MH. Undiagnosed Hailey-Hailey disease causing painful erosive skin changes during patch testing. Br J Dermatol 2005;153:233–4.
- Parry EJ, Beck MH. Acute anaphylaxis resulting from routine patch testing with latex. Contact Dermatitis 1999;41:236–7.
- Dawe SA, White IR, Rycroft RJ, et al. Active sensitization to para-phenylenediamine and its relevance: a 10-year review. Contact Dermatitis 2004;51:96–7.
- Bruze M, Hedman H, Björkner B, Möller H. The development and course of test reactions to gold sodium thiosulfate. Contact Dermatitis 1995;33:386–91.
- Bruynzeel DP. Angry back or excited skin syndrome [Thesis]. Vrie Universiteit te Amsterdam, the Netherlands, 1983.
- Rietschel R. Stochastic resonance and angry back syndrome: noisy skin. Am J Contact Dermatitis 1996;7:152–4.
- Duarte I, Almeida FA, Proenca NG. Excited skin syndrome. Am J Contact Dermatitis 1996;7:24–34.
- Memon AA, Friedmann PS. The angry back syndrome: a non-reproducible phenomenon. Br J Dermatol 1997;135:924–30.
- Carlesen BC, Andersen KE, Menne T, Johansen JD. Patients with multiple contact allergies: a review. Contact Dermatitis 2008;58:1–8.
- Pedersen LK, Johansen JD, Held E, Agner T. Augmentation of skin response by exposure to a combination of allergens and irritants – a review. Contact Dermatitis 2004;50:265–73.
- McLelland J, Shuster S. Contact dermatitis with negative patch tests: the additive effect of allergens in combination. Br J Dermatol 1990;122:623–30.
- Seidenari S, Molotese A, Belletti B. Pre-treatment of nickel test areas with sodium lauryl sulfate detects nickel sensitivity in subjects reacting negatively to routinely performed patch tests. Contact Dermatitis 1996;34:88–93.
- Benezra C, Maibach HI. True cross-sensitization, false cross-sensitization and otherwise. Contact Dermatitis 1984;11:65–9.
Other tests
- Christensen OB, Wall LM. Open, closed and intradermal testing in nickel allergy. Contact Dermatitis 1987;16:21–6.
- Krasteva M, Cristaudo A, Hall B, et al. Contact sensitivity to hair dyes can be detected by the consumer open test. Eur J Dermatol 2002;12:322–6.
- Hannuksela M, Salo H. The repeated open application test (ROAT). Contact Dermatitis 1986;14:221–7.
- Johansen JD, Bruze M, Andersen KE, et al. The repeated open application test: suggestions for a scale of evaluation. Contact Dermatitis 1998;39:95–6.
- Meneghini C, Angelini G. Intradermal test in contact allergy to metals. Acta Derm Venereol Suppl (Stockh) 1979;85:123–4.
- Seukeran DC, Wilkinson SM, Beck MH. Patch testing to detect corticosteroid allergy: is it adequate? Contact Dermatitis 1997;36:127–30.
- Jovanovic M, Poljacki M, Milakov J, et al. [Skin and laboratory tests: comparison of the epicutaneous patch test with the TTL and LIF tests in the diagnosis of medicamentous allergic contact dermatitis.] Med Pregl 1992;45:365–8.
- Tio D. A study on the clinical application of a direct leukocyte migration test in chromium contact allergy. Br J Dermatol 1976;94:65–70.
- Everness KM, Gawkrodger DJ, Botham PA, et al. The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermatol 1990;122:293–8.
- Kimber I, Quirke S, Beck MH. Attempts to identify the causative allergen in cases of contact dermatitis using an in vitro lymphocyte transformation test. Toxicol In Vitro 1990;4:302–6.
- Spiewak R, Moed H, von Blomberg BM, et al. Allergic contact dermatitis to nickel: modified in vitro test protocols for better detection of allergen-specific response. Contact Dermatitis 2007;56:63–9.
- Sieben S, Hertl M, Al Masaoudi T, et al. Characterization of T cell responses to fragrances. Toxicol Appl Pharmacol 2000;172:172–8.
- Kimber I, Quirke S, Cumberbatch M, et al. Lymphocyte transformation and thiuram sensitization. Contact Dermatitis 1991;24:164–71.
- Minang JT, Areström I, Troye-Blomberg M, et al. Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin Exp Immunol 2006;146:417–26.
- Cederbrant K, Anderson C, Andersson T, et al. Cytokine production, lymphocyte proliferation and T-cell receptor Vbeta expression in primary peripheral blood mononuclear cell cultures from nickel-allergic individuals. Int Arch Allergy Immunol 2003;132:373–9.
- Wolf R, Davidovici B, Marcos B, Orion E. Lymphocyte transformation test in patients with allergic contact dermatitis. Contact Dermatitis 2005;53:245.
- Aldridge RD, Milton JI, Thompson AW. Leucocyte procoagulant activity as an in vitro index of nickel hypersensitivity. Int Arch Allergy Appl Immunol 1985;76:350–3.
- Shore RN. Dimethylglyoxime stick test for easier detection of nickel. Arch Dermatol 1977;113:1734.
- Dahlquist I, Fregert S, Gruvberger B. A simple method for the detection of formaldehyde. Contact Dermatitis 1982;8:301–3.
- Fregert S, Trulsson L. A simple method for the detection of epoxy resins of bisphenol A type. Contact Dermatitis 1978;4:111–19.
Management
- Noiesen E, Larsen K, Agner T. Compliance in contact allergy with focus on cosmetic labelling: a qualitative research project. Contact Dermatitis 2004;51:189–95.
- Levin C, Maibach HI. An overview of the efficacy of topical corticosteroids in experimental human nickel contact dermatitis. Contact Dermatitis 2000;43:317–21.
- Veien NK, Olholm Larsen P, Thestrup-Pedersen K, et al. Long-term, intermittent treatment of chronic hand eczema with mometasone furoate. Br J Dermatol 1999;140:882–6.
- Anderson C, Groth O. Suppression of the allergic contact reaction in the guinea pig by cyclosporin A. Int Arch Allergy Appl Immunol 1985;78:396–400.
- Lindelof B, Wrangso K, Lidén S. A double-blind study of Grenz ray therapy in chronic eczema of the hands. Br J Dermatol 1987;117:77–80.
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermatitis 1996;7:171–6.
- Fowler JF, Jr. A skin moisturizing cream containing quaternium-18-bentonite effectively improves chronic hand dermatitis. J Cutan Med Surg 2001;5:201–5.
- Wohrl S, Kriechbaumer N, Hemmer W, et al. A cream containing the chelator DTPA (diethylenetriaminepenta-acetic acid) can prevent contact allergic reactions to metals. Contact Dermatitis 2001;44:224–8.
- Gruvberger B, Bruze M. Can glutathione-containing emollients inactivate methylchloroisothiazolinone/methylisothiazolinone? Contact Dermatitis 1998;38:261–5.
- Memon AA, Molokhia MM, Friedmann PS. The inhibitory effects of topical chelating agents and antioxidants on nickel-induced hypersensitivity reactions. J Am Acad Dermatol 1994;30:560–5.
- Veien NK, Hattel T, Laurberg G. Low nickel diet: an open, prospective trial. J Am Acad Dermatol 1993;29:1002–7.
- Veien NK, Hattel T, Laurberg G. Can oral challenge with balsam of Peru predict possible benefit from a low-balsam diet? Am J Contact Dermatitis 1996;7:84–7.
- Menné T, Kaaber K, Tjell JC. Treatment of nickel dermatitis. The influence of tetraethylthiuramdisulfide (Antabuse) on nickel metabolism. Ann Clin Lab Sci 1980;10:160–4.
- Armstrong DK, Biagioni P, Lamey PJ, et al. Contact hypersensitivity in patients with orofacial granulomatosis. Am J Contact Dermatitis 1997;8:35–8.
- Scheman A, Cha C, Jacob SE, Nedorost S. Food avoidance diets for systemic lip and oral contact allergy. Dermatitis 2013;23:248–47.
- Helton J, Storrs F. The burning mouth syndrome: lack of a role for contact urticaria and contact dermatitis. J Am Acad Dermatol 1994;31:201–5.
- Sjovall P, Christensen OB. Oral hyposensitization in allergic contact dermatitis. Semin Dermatol 1990;9:206–9.
- Watson ES. Toxicodendron hyposensitization programs. Clin Dermatol 1986;4:160–70.
- Handa S, Sahoo B, Sharma VK. Oral hyposensitization in patients with contact dermatitis from Parthenium hysterophorus. Contact Dermatitis 2001;44:279–82.
- Thyssen JP, Johansen JD, Menné T. Contact allergy epidemics and their control. Contact Dermatitis 2007;56:185–95.
- Calnan CD. Studies in contact dermatitis. XXIII. Allergen replacement. Trans St John's Hosp Dermatol Soc 1970;56:131–8.
- Lidén C. Legislative and preventative measures related to contact dermatitis. Contact Dermatitis 2001;44:65–9.
- Johansen J, Menné T, Christophersen J, et al. Changes in the pattern of sensitization to common contact allergens in Denmark between 1985–86 and 1997–98, with a special view to the effect of preventive strategies. Br J Dermatol 2000;142:490–5.
- Thyssen JP. Uter W, McFadden J, et al. The EU Nickel directive revisited – future steps towards better protection against nickel allergy. Contact Dermatitis 2011;64:121–5.
- Zachariae COC, Agner T, Menné T. Chromium allergy in consecutive patients in a country where ferrous sulphate has been added to cement since 1981. Contact Dermatitis 1996;35:83–6.
- Wrangsjo K, Boman A, Liden C, Meding B Primary prevention of latex allergy in healthcare – spectrum of strategies including European glove standardisation. Contact Dermatitis 2012;66:165–71
- Wilkinson JD, Shaw S, Andersen KE, et al. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991–2000). Contact Dermatitis 2002;46:207–10.
- Roggeband R, Basketter DA, De Groot AC, et al. Labelling of skin sensitizers: the new European Dangerous Preparations Directive. Contact Dermatitis 2001;44:321–4.
- Behrbohm P. Legislation on prevention of occupational dermatoses. Contact Dermatitis 1975;1:207–10.
- Douglas E, Rushton L, Williams HC. Is occupational dermatitis being taken seriously by UK industries? Occup Med (Lond) 1999;49:85–91.
- Dornan JD. The work of the Employment Medical Advisory Service. Br J Dermatol 1981;105(Suppl. 21):79–83.
- Felter SP, Ryan CA, Basketter DA, et al. Application of the risk assessment paradigm to the induction of allergic contact dermatitis. Regul Toxicol Pharmacol 2003:37:1–10.
- Jowsey IR. Proactive surveillance of contact allergies: an important component of the risk management strategy for skin sensitizers. Contact Dermatitis 2007;56:305–10.
- Uter W, Aberer W, Armario-Hita JC, et al. Current patch test results with the European baseline series and extensions to it from the ‘European Surveillance System on Contact Allergy’ network, 2007–2008. Contact Dermatitis 2012;67:9–19.
- Löffler H, Bruckner T, Diepgen T, Effendy I. Primary prevention in health care employees: a prospective intervention study with a 3-year training period. Contact Dermatitis 2006;54:202–9.
Photoallergic contact dermatitis
- Avenel-Audran M, Dutartre H, Goossens A, et al. Octocrylene, an emerging photoallergen. Arch Dermatol 2010;146:753–7.
- The European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European photopatch test study. Br J Dermatol 2012;166:1002–9.
- Frain-Bell W. Photodermatoses. In: Rook A, ed. Recent Advances in Dermatology. Edinburgh: Churchill Livingstone, 1973:101–33.
- Thune P. Basic mechanisms of photosensitization. In: Frosch PJ, Dooms-Goossens A, LaChappelle LM, et al., eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:473–9.
- Kornhauser A, Wamer W, Giles A. Light-induced dermal toxicity: effects on the cellular and molecular level. In: Marzulli FN, Maibach HI, eds. Dermatoxicology, 3rd edn. Washington, DC: Hemisphere, 1987:377–412.
- Harber LC, Targovnik SE, Baer RL. Studies on contact photosensitivity to hexachlorophene and trichlorocarbanilide in guinea pigs and man. J Invest Dermatol 1968;51:373–84.
- Adamski H, Benkalfate L, Delaval Y, et al. Photodermatitis from non-steroidal anti-inflammatory drugs. Contact Dermatitis 1998;38:171–4.
- Osmundsen PE. Contact photo-allergy to tribromosalicylanilide. Br J Dermatol 1968;31:429–34.
- Salser H. Photochemische Kupplung des Sulfanilamids und aromatischer Amine an Eiweib und andere hochmolekulare Verbindungen. Arch Klin Exp Dermatol 1962;215:266–78.
- Epling GA, Wells JL, Ungchan Yoon. Photochemical transformations in salicylanilide photoallergy. Photochem Photobiol 1988;47:167–71.
- Buckley DA, Wayte J, O'Sullivan D, Murphy GM. The duration of response to UVA irradiation after application of a known photoallergen. Contact Dermatitis 1995;33:138–9.
- Le Coz CJ, El Aboubi S, Lefèbvre C, et al. Photoallergy from topical ketoprofen: a clinical, allergological and photobiological study. Contact Dermatitis 2000;42(Suppl.):47.
- Karlsson I, Vanden Broecke K, Mårtensson J, et al. Clinical and experimental studies of octocrylene's allergenic potency. Contact Dermatitis 2011;64:343–52.
- Schauder S, Schroder W, Geier J. Olaquindox-induced airborne photoallergic contact dermatitis followed by transient or persistent light reactions in 15 pig breeders. Contact Dermatitis 1996;35:344–54.
- Wilkinson DS. Patch test reactions to certain halogenated salicylanilides. Br J Dermatol 1962;74:302–6.
- Wojnarowska F, Calnan CD. Allergy to musk ambrette. Br J Dermatol 1986;114:667–75.
- Murphy GH, White IR, Hawk JL. Allergic airborne contact dermatitis to Compositae with photosensitivity: chronic actinic dermatitis in evolution. Photodermatol Photoimmunol Photomed 1990;7:38–9.
- Thune P. Contact allergy due to lichens in patients with a history of photosensitivity. Contact Dermatitis 1977;3:267–72.
- Thune P, Eeg-Larsen T. Contact and photocontact allergy in persistent light reactivity. Contact Dermatitis 1984;11:98–107.
- Baer RL, Harber LC. Photosensitivity induced by drugs. JAMA 1965;192:989–90.
- Albes B, Marguery MC, Schwarze HP, et al. Prolonged photosensitivity following contact photoallergy to ketoprofen. Dermatology 2000;201:171–4.
- Zaynoun S, Johnson BE, Frain-Bell W. The investigation of quindoxin photosensitivity. Contact Dermatitis 1976;2:343–52.
- Wolf C, Honigsmann H. [The syndrome of chronic actinic dermatitis. Persistent light reaction – actinic reticuloid.] Hautarzt 1988;39:635–41.
- British Photodermatology Group. Photopatch testing: methods and indications. Br J Dermatol 1997;136:371–6.
- Batchelor RJ, Wilkinson SM. Photopatch testing – a retrospective review using the 1 day and 2 day irradiation protocols. Contact Dermatitis 2006;54:75–8.
- Pollock B, Wilkinson SM. Photopatch test method: influence of type of irradiation and value of day-7 reading. Contact Dermatitis 2001;44:270–2.
- Gonçalo M, Ferguson J, Bonevalle A, et al. Photopatch testing: recommendations for a European photopatch test baseline series. Contact Dermatitis 2013;68:239–43.
Allergic contact urticaria
- Fisher AA. Contact Dermatitis. Philadelphia: Lea and Febiger, 1973.
- Lahti A, Basketter D. Immediate contact reactions. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:83–96.
- Kanerva L, Toikkanen J, Jolanki R, Estlander T. Statistical data on occupational contact urticaria. Contact Dermatitis 1996;35:229–33.
- Williams JD, Lee AY, Matheson MC, Frowen KE, Noonan AM, Nixon RL. Occupational contact urticaria: Australian data. Br J Dermatol 2008;159:125–31.
- Ale SI, Maibach HI. Occupational contact urticaria. In Kanerva L, Wahlberg JE, Maibach HI, eds. Handbook of Occupational Dermatology. Berlin: Springer-Verlag, 2000:200–16.
- Reitamo S, Visa K, Kahonen K, et al. Eczematous reactions in atopic patients caused by epicutaneous testing with inhalant allergens. Br J Dermatol 1986;114:303–9.
- Barker JNWN, Alegre VA, McDonald DM. Surface bound immunoglobulin E on antigen-presenting cells in cutaneous tissue of atopics. J Invest Dermatol 1988;90:117–21.
- Brown SJ, Asai Y, Cordell HJ, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol 2011;127:661–7.
- Allmers H, Schmengler J, John SM. Decreasing incidence of occupational contact urticaria caused by natural rubber latex allergy in German health care workers. J Allergy Clin Immunol 2004;114:347–51.
- Gimenez-Arnau A, Maurer M, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis – “a never ending story”. Eur J Dermatol 2010;20:552–62.
- Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann NY Acad Sci 2002;964:47–68.
- Oranje AP, Van Gysel D, Mulder PGH, et al. Food-induced contact urticaria syndrome (CUS) in atopic dermatitis: reproducibility of repeated and duplicate testing with a skin provocation test, the skin application food test (SAFT). Contact Dermatitis 1994;31:314–18.
- Jeebhay MF, Robbins TG, Lehrer SB, Lopata AL. Occupational seafood allergy: a review. Occup Environ Med 2001;58:553–62.
- Kanerva L, Susitaival P. Cow dander: the most common cause of occupational contact urticaria in Finland. Contact Dermatitis 1996;35:309–10.
- Nutter AF. Contact urticarial to rubber. Br J Dermatol 1979;101:597–8.
- Dalrymple SJ, Audley BG. Allergenic proteins in dipped rubber products: factors influencing extractable protein levels. Rubber Dev 1992;45:51–60.
- Blanco C. Latex–fruit syndrome. Curr Allergy Asthma Rep 2003;3:47–53.
- Hjorth N, Roed-Peterson J. Occupational protein contact dermatitis in food handlers. Contact Dermatitis 1976;2:28–42.
- Thomson KF, Wilkinson SM. Localised dermographism: a differential diagnosis of latex glove allergy. Contact Dermatitis 1999;41:103–4.
- Brehler R, Sedlmayr S. Contact urticaria due to rubber chemicals? Contact Dermatitis 1997;37:125–7.
- Amin S, Maibach HI. Immunologic contact urticaria definition. In: Amin S, Lahti A, Maibach HI, eds. Contact Urticaria Syndrome. New York: Informa Healthcare USA, 1997:11–26.
- Von Krogh G, Maibach HI. The contact urticarial syndrome. Semin Dermatol 1982;1:59–66.
- Ylipieti S, Lahti A. Effect of the vehicle on non-immunologic immediate contact reactions. Contact Dermatitis 1989;21:105–6.
- Oranje AP, Van Gysel D, Mulder PGH, et al. Food-induced contact urticaria syndrome (CUS) in atopic dermatitis: reproducibility of repeated and duplicate testing with a skin provocation test, the skin application food test (SAFT). Contact Dermatitis 1994;31:314–18.
- Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol 2001;108:881–90.
- Davies E, Orton D. Contact urticarial and protein contact dermatitis to chapatti flour. Contact Dermatitis 2009;60:113.