CHAPTER 129
Irritant Contact Dermatitis
Jonathan M. L. White
Department of Cutaneous Allergy, St John's Institute of Dermatology, Guy's and St Thomas’ NHS Foundation Trust, UK
Irritant contact dermatitis
Definition and nomenclature
Irritant contact dermatitis is the cutaneous response to the physical/toxic effects of a wide range of environmental exposures. This may be an acute (toxic) irritant contact dermatitis or a cumulative irritant/insult dermatitis.
Introduction and general description
Reversible cellular injury may cause contact urticaria or dermatitis dependent on the nature of the insult. Where there is no apparent cellular injury, various sensory symptoms such as stinging, smarting and burning, may occur. The following types of irritant contact reaction may be distinguished:
- Burns.
- Irritant contact dermatitis:
- Acute (toxic) irritant contact dermatitis.
- Cumulative irritant/insult contact dermatitis.
- Transient or immediate-type, non-immune, contact urticaria.
- Symptomatic (subjective) irritant responses.
- Other: pigmentary and granulomatous responses and those localized to appendageal structures (Table 129.1).
Table 129.1 Other irritant contact responses of the skin and their causes.
| Folliculitis | Tar and oils, arsenic trioxide, fibreglass |
| Acne | Halogenated aromatic hydrocarbons |
| Miliaria | Aluminium chloride, occlusion |
| Pigmentary | |
|
Phototoxic agents, metals (arsenic, silver, gold, mercury, bismuth) Substituted phenols and catechols |
| Granulomatous | Silica, talc, beryllium |
| Alopecia | Borax, chloroprene dimmers |
There is considerable variability in responses between individuals, as well as within the same patient due to various endogenous and exogenous variables (Table 129.2). Various chemicals have irritant properties, including plants (Boxes 129.1 and 129.2).
Table 129.2 Factors influencing irritancy potential of substances on human skin.
| Exogenous | Endogenous | Co-factors |
| Chemical characteristics | Individual susceptibility | Mechanical |
| Molecular structure | Atopy | Thermal |
| pH | Ethnicity/skin colour/phototype | Climatic |
| pKa | Age | |
| Hydrophobicity (log P) | Hormonal | |
| Inherent toxicity | Barrier function | |
| Concentration/dose | Repair capacity | |
| Penetration characteristics | Eczema elsewhere | |
| Vehicle | Other skin diseases | |
| Solubility | Other unknown | |
| Duration of contact | Site of exposure | |
| Type of contact |
Epidemiology
Incidence and prevalence
Many studies on the epidemiology of irritant contact dermatitis are diagnostically vague or open to considerable selection bias. A questionnaire study of 20 000 persons in an industrial town in the south of Sweden revealed a point prevalence of hand eczema of 5.4% (with a 1-year period prevalence of 11%), and in 35% the hand eczema was thought to be irritant in nature. Atopic hand eczema accounted for 22% of cases; the presence of childhood eczema increasing the prevalence of hand dermatitis threefold compared with non-atopic individuals. Allergic contact dermatitis accounted for 19%. The most frequent sources of exposure were ‘unspecified’ chemicals, water, detergents, dust and dirt [1]. Occupationally, soaps (22.0% of cases), wet work (19.8%), petroleum products (8.7%), and cutting oils and coolants (7.8%) are the most frequently cited causes. Individuals involved in mining/manufacturing, hairdressing, agriculture and medical and nursing occupations had the highest frequency of dermatitis [2] (Box 129.3). In one large study of adverse reactions to cosmetics [3], 16% were thought to be irritant. In a large cohort, up to 98.6 females per 10 000 workers in high-risk occupations suffered with irritant contact dermatitis. They were younger, on average, than those with allergic contact dermatitis [4].
Sex
Females may be twice as commonly affected as males [1].
Ethnicity
In North America, there has been shown to be no ethnic difference in the prevalence of sensitive skin, although there are racial differences in how it is perceived. Euro-Americans experience greater reactivity to wind and less to cosmetics; African Americans have reduced reactivity to most environmental factors; Asians have greater reactivity to spices, change in temperature and wind, and itch more frequently; Hispanics react less to alcohol. Overall, however, there were more similarities than differences [5]. This was confirmed by a study on populations from South Asia with differing skin pigmentation [6].
Associated diseases
- Allergic contact dermatitis.
- Atopic eczema.
Pathophysiology
Certain chemicals have intrinsic irritant properties to varying degrees, but external factors may also influence this, including temperature (of the chemical, environment or individual), air flow (chapping), low humidity (by itself responsible for the common condition ‘low humidity occupational dermatosis’, which generally affects the face) and occlusion. Skin barrier dysfunction is a key reason for irritation.
The skin provides the first and most important line of defence against exogenous noxious agents, and this is one of its primary physiological functions [7]. This defence is far from perfect, as many substances penetrate readily into and through the epidermis, even when it is intact.
The principal epidermal barrier resides almost entirely in the stratum corneum. This is normally renewed every 17–27 days, but barrier function can be restored in 2–5 days following stripping or superficial injury. The stratum corneum appears to function as a homogeneous unit, the largest amount of penetrant always being found in the outermost layers. Damage to the stratum corneum is normally followed by an increase in percutaneous absorption and in transepidermal water loss (TEWL), the increase in TEWL being proportional to the decrease in thickness of the stratum corneum.
Solvent extraction studies indicate that epidermal lipid is a main contributor to the barrier, consisting of ceramides (45–50%), cholesterol (25%), free fatty acids (10–15%) and other lipids including cholesterol sulphate. The lipids are arranged as stacked membrane sheets in the intercellular space and are produced from lamellar granules in the cells of the granular cell layer of the epidermis. It follows that inherited abnormalities of the pathway might result in impaired barrier function usually associated with ichthyosis.
In addition to the lipid barrier, tight junctions between epidermal cells have also been shown to provide a block to water loss. Claudin-1 (an essential protein of tight junctions) knock-out mice developed wrinkly skin and greatly increased TEWL. They died within 1 day of birth [8].
For certain materials, there may be a second barrier at or near the dermal–epidermal junction or basement membrane [9] but, for most substances, the horny layer remains the principal barrier.
Emerging data suggest that filaggrin mutations may predispose to irritant contact dermatitis [10].
Mechanism of action of irritants
An irritant is any agent, physical or chemical, that is capable of producing cellular perturbation if applied for sufficient time and in sufficient concentration. Immunological memory is not involved and dermatitis occurs without prior sensitization. Many chemicals penetrate the skin, and many substances will alter or damage skin cells. Dermatitis arises when the defence or repair capacity of the skin is exhausted, or when the penetration of chemicals excites an inflammatory response. Strong irritants will induce a clinical reaction in almost all individuals, whereas with less potent irritants the response may be physiological rather than apparent, with dermatitis developing only in the most susceptible or in situations where there is repeated contact with irritants [11].
The relationship between physicochemical structure and cytotoxic activity remains to be fully elucidated, but it would appear that hydrophobicity (log P) and dissociation constant (pKa) are among the factors that contribute to irritation potential [12, 13] (see Table 129.2). For sodium lauryl sulphate, concentration has been shown to be a more important determinant of subsequent dermatitis than exposure time [14]. The nature of the response is in part determined by the irritant [15].
In the laboratory, barrier disruption has been shown to induce interleukin 1α (IL-1α) release from a preformed pool in mouse epidermis [16] and upregulation of tumour necrosis factor α (TNF-α) and granulocyte–macrophage colony-stimulating factor (GM-CSF). There is then a rise in Langerhans cell-derived IL-1α stimulated by GM-CSF and IL-1α production. Concurrently, the loss of the normal extracellular calcium gradient stimulates lamellar body secretion and barrier repair [17]. Oxidative stress also contributes to the development of inflammation with various irritants [18].
Detergents at lower levels of exposure predominantly affect the horny layer, causing dryness and scaling by destroying lysosomal enzymes in the horny layer [19], whereas at higher concentrations they will dissolve cell membranes and damage lysosomes [20]. With repeated exposure, there will be signs of chronic inflammation, with increased DNA synthesis, acanthosis and changes in cellular metabolism.
Detergents and other irritants such as croton oil and phenol esters are chemotactic for polymorphonuclear leukocytes at non-toxic concentrations [21], and may cause pustular reactions. Organic solvents such as methanol or chloroform will damage blood vessels, causing hyperaemia [22], and dimethyl sulfoxide (DMSO) is a very effective degranulator of mast cells [23].
Irritants affect everyone, although individual susceptibility with regard to the development of dermatitis varies greatly. The body's immune response is important in generating dermatitis, and this has been shown in the attenuated response to irritants in CD4-deficient mice [24].
Whereas allergic contact dermatitis reactions are histologically almost always eczematous and rather monomorphic, those elicited by irritants show much greater pleomorphism. Histological changes vary according to the chemical nature and concentration of the irritant, the type and duration of exposure, the severity of the response and the time of sampling. Some irritant reactions may be histologically indistinguishable from allergic contact dermatitis, whereas others may possess morphological features characteristic of a certain type of chemical. More than one pattern of response may be induced by the same irritant.
Clinical features
History and presentation
Irritants produce a wide range of responses on the skin which are not necessarily eczematous. These may range from purely subjective sensations, such as stinging, smarting, burning, or sensations of dryness and tightness, through delayed stinging or transient urticarial reactions to more persistent irritant reactions or irritant contact dermatitis. Irritant contact dermatitis has a spectrum of clinical features, ranging from a little dryness, redness or chapping through various types of eczematous dermatitis to an acute caustic burn. Irritants may also penetrate skin via appendageal structures and cause folliculitis and other types of reaction (see Table 129.1).
The same chemical may cause different irritant reactions depending on concentration; DMSO, for instance, is able to induce both conventional irritant dermatitis and immediate, non-immunological, contact urticarial reactions. The response may also vary according to the site and mode of application, vehicle, environmental conditions (mechanical, thermal, climatic) and there is considerable variability between individuals (see Table 129.2).
Irritant contact dermatitis frequently presents on the hand. However, constitutional, irritant and allergic factors frequently coexist. Although hand eczema is more common in women [39], this seems to be the result of increased irritant exposure rather than an inherent susceptibility [40]. Allergy can never be completely excluded, nor is any pattern of hand dermatitis pathognomonic for a single causation. In spite of this, there are certain types of hand dermatitis that are at least suggestive of irritant contact dermatitis. These include a patchy ‘housewife’-type eczema affecting principally the dorsa, sides and webs of the fingers, or a ‘ring’ eczema – both are patterns associated with wet work and exposure to detergent. What may start as dryness can develop into patchy or diffuse erythema with scaling, fissuring and even vesiculation.
However, vesicles are less commonly seen in irritant than allergic or constitutional eczema, and the principal clinical features are usually dryness or chapping. The wrists and distal arms may also be affected. With increased mechanization in the house and at work, with more widespread use of protective gloves and hand creams, this pattern of hand eczema is less common than previously.
Another common pattern of irritant hand eczema is the ‘apron’ or extended fingertip eczema, with dryness, redness and fissuring affecting principally the palmar aspects of the fingers and distal palm. This pattern of dermatitis commonly occurs in those who frequently hold wet cloths containing detergent or household chemicals in the unprotected hand. Friction, irritants and repetitive wetting/desiccation all play a part. A similar pattern may be seen in occupations where employees are repeatedly exposed to solvents, friction or irritating food components. Discoid or nummular hand eczema is another rarer pattern of irritant contact dermatitis, affecting especially the dorsa of the hands or fingers.
The differential diagnosis of hand dermatitis includes fungal infection, which simulates a unilateral palmar dermatitis (Figure 129.1) and can resemble eczema on the dorsum of the hand (Figure 129.2). Skin scrapings are important to exclude tinea as the cause of a hard to treat ‘dermatitis’. Psoriasis frequently affects the palms, resulting in a hyperkeratotic appearance. This can be difficult to distinguish from dermatitis when there are no lesions elsewhere. Further, there may be a history of exacerbation when the disease leads to the Koebner phenomenon on the hands as a result of manual work. The presence of scaling erythema over the interphalangeal joints is often a helpful clue to the diagnosis (Figure 129.3). Scabies in the interdigital spaces can simulate an irritant dermatitis (Figure 129.4). Rarely, the presence of milia may lead to a diagnosis of porphyria cutanea tarda (Figure 129.5).
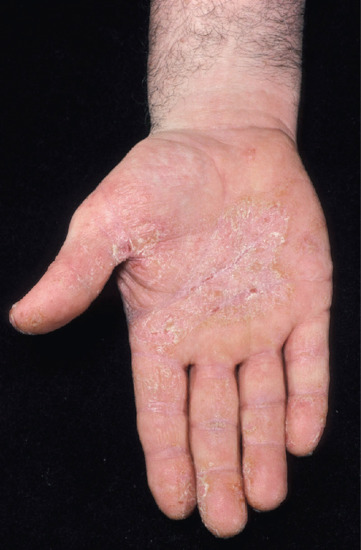
Figure 129.1 Mechanical irritation causing a psoriasiform irritant contact dermatitis of the palmar surface. (Courtesy of St John's Institute of Dermatology, London, UK.)
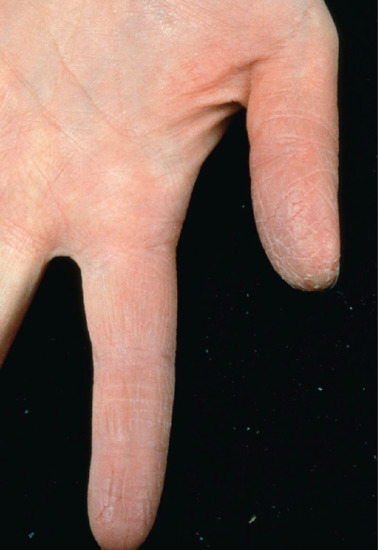
Figure 129.2 Pulpitis is usually caused by wet work. (Courtesy of St John's Institute of Dermatology, London, UK.)
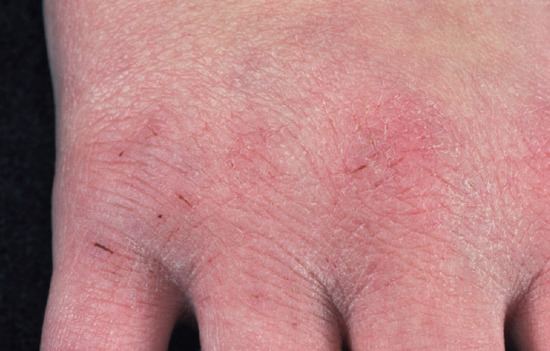
Figure 129.3 Fissuring on the dorsum occurs most commonly as a result of frequent hand washing and outdoor exposure. (Courtesy of St John's Institute of Dermatology, London, UK.)
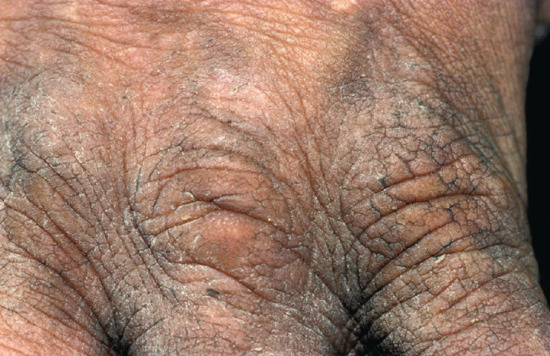
Figure 129.4 Lichenification from chronic irritation. Erythema is less visible in darker skin types. (Courtesy of St John's Institute of Dermatology, London, UK.)
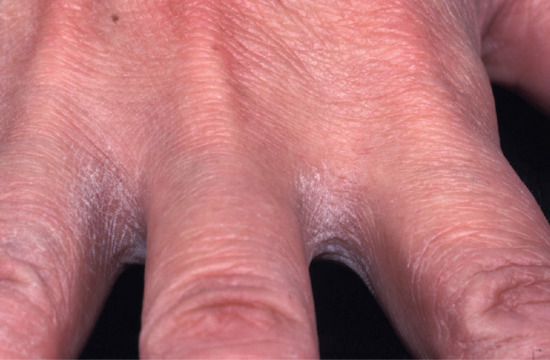
Figure 129.5 Interdigital irritant contact dermatitis is frequently caused by inadequate drying of the hand after washing. It is commonly seen in hairdressers on the non-dominant hand. (Courtesy of St John's Institute of Dermatology, London, UK.)
Cosmetics, irritant contact dermatitis due to
Cosmetics, toiletries and skincare products, including sunscreens, quite frequently cause adverse irritant reactions [41]. In most cases, these are only mild or transient, and most consumers simply change to an alternative product. In a minority, reactions may be more severe, with redness, oedema, dryness and scaling. The eyelids are particularly susceptible to irritants [42], as are atopic individuals and those with very fair, rosaceous or seborrhoeic skins. It is of interest that irritant reactions are commoner in younger (premenopausal) women. Those using many products are at risk of ‘cosmetic exhaustion’, a form of cumulative cosmetic irritant contact dermatitis. Allergy is excluded only by comprehensive patch testing including both products and ingredients.
Volatile/airborne irritants, contact dermatitis due to
Irritants, as well as allergens, may cause volatile contact dermatitis [43]. Volatile irritants are a not infrequent cause of eyelid dermatitis. In any exposed-site dermatitis, one should consider the possibility of irritant volatile fumes or airborne particles. The fumes can be from acids, alkalis, solvents, resins or any other irritant chemical, such as ammonia or formaldehyde. Irritant dusts include those of some (mostly tropical) woods, cement, fibreglass or rockwool, some metals and metal salts, and powdered chemicals.
Cheilitis, irritant contact
Cheilitis is a common problem, often of multifactorial aetiology. Atopic eczema frequently predisposes to its development [44]. The most common identifiable causes of cheilitis are irritant dermatitis, due to lip licking, cosmetics and medication, and allergic contact dermatitis, of many different causes.
Napkin (diaper), peristomal and perianal dermatitis
Irritant dermatitis will develop in situations of prolonged or too frequent contact with degraded urine or faeces/faecal residues [45]. Sweat, occlusion, irritant cleansers, secondary infection and secondary medicament allergy are all additional complicating factors. It occurs most frequently in very young, or in elderly individuals in situations of urinary or faecal incontinence. Measures to improve continence in the elderly and sufficiently frequent changes of absorbent [46] napkins in infants are important, as are mild cleansers and protective pastes or silicone-based creams. Any dermatitis or secondary infection should be controlled with appropriate-strength topical steroids or steroid–antimicrobial combinations. In napkin dermatitis, secondary candidal infection is sufficiently common that routine treatment with an imidazole antifungal is of benefit [47].
A similar situation appertains to perianal dermatitis, where mucus or faecal leakage may occur in association with haemorrhoids and/or poor sphincter function. A bidet, or ‘wet’ cleansing routine using aqueous cream or equivalent, is of benefit.
With peristomal dermatitis, there is the additional complication of the need to maintain a protective seal between the stoma bag and skin. The use of corticosteroid-containing lotions, either aqueous or alcoholic, has been shown to be effective without interfering with stoma adhesion [48] (see Chapter 114). Dermatitis, as well as being caused by leakage, may also be due to continuous occlusion and repeated stripping of the skin. Where there is erosive disease, the use of topical sucralfate has been shown to promote healing in peristomal disease but not erosions from other causes [49]. The sucralfate acts as both a physical barrier and, it is suggested, by binding to basic fibroblast growth factor preventing its degradation, as a stimulus to healing.
Clinical features
Differential diagnosis
- Atopic eczema.
- Allergic contact dermatitis.
- Contact urticaria.
- Thermal injury.
- Non-accidental injury in children.
Classification of severity
In clinical practice, there are no objective ways of classifying severity and the response may be assessed by the degree of erythema and surface change. Non-specific assessment tools such as the Physician Global Assessment or the Dermatology Life Quality Index (DLQI; a patient-centred questionnaire) may be used. A number of research tools exist which may quantify the irritant response by measuring erythema, TEWL, hydration and skin thickness. The optimal method to be used varies with the nature of the irritant [50].
Erythema
Among the most overt clinical features of irritant reactions is erythema, which may be quantified using a number of different approaches. Laser Doppler flowmetry (LDF) provides a measure of superficial blood flow by transmitting monochromatic light from a helium–neon laser through optical fibres to the skin surface. The light is Doppler-shifted by moving blood cells in the upper dermis, remaining unchanged in the surrounding stationary tissue. By means of a differential signal detector and signal processing arrangement, the back-scattered or reflected light is interpreted. The final output, which is linearly related to the product of the number of blood cells and their average velocity in the measured volume, is expressed in relative and dimensionless units. Studies by a number of investigators have shown that LDF generally correlates well with visually assessed erythema, and is capable of discriminating between negative and weakly positive irritant reactions [51].
Alternative methods for objectively quantifying erythema rely upon the generalized increase in red blood cells resulting from both increased blood flow and blood vessel dilatation. Those which are based upon remittance spectroscopy emit red and green light from a tungsten halogen lamp or LED source. Oxyhaemoglobin in the blood vessels absorbs a proportion of the green light, and largely reflects the red light. Changes in the quantity of oxyhaemoglobin significantly alter the amount of green light absorbed, but have very little influence on the red light. An erythema index can therefore be calculated from the ratio between the reflected green and red light, such that the greater the erythema, the higher the value of the erythema index [52].
Erythema may also be quantified using tristimulus colorimeters, virtually all of which employ a system for colour definition known as the Commission Internationale de l'Eclairage (CIE) L*a*b* colour system. This provides a three-dimensional coordinate system where L* represents an axis for brightness, a* represents a green–red axis and b* represents a yellow–blue axis.
Transepidermal water loss
In addition to inducing erythema, irritants commonly affect barrier function, leading to alterations in TEWL. Measuring instruments employ open chambers, through which, when applied to the surface of the skin, water vapour evaporates, creating a water pressure gradient from which the evaporative TEWL, expressed in g/m2/h, is calculated. Many variables influence TEWL measurements. Some relate to the environment and to instrument operation, necessitating a careful adherence to ‘good laboratory practice’, as outlined in a report from the Standardization Group of the European Society of Contact Dermatitis [53]. Others relate directly to the individual; age and anatomical site are among the most important variables. Measurements of TEWL have proved valuable in predicting susceptibility to skin irritation, assessing the protective effects of barrier creams, and evaluating the irritancy potential of different chemicals.
Hydration
Changes in the hydration state of the skin also commonly occur in irritant contact dermatitis and, again, this parameter may be objectively measured. Several different devices are available, based on differing biophysical approaches. Using the principle of capacitance, hydration of the stratum corneum can be measured to a depth of approximately 0.1 mm. In contrast, skin conductance has also been used as a measure of hydration. Studies suggest that capacitance may be more effective in the assessment of dry skin, whereas conductance is better suited for studies of water accumulation in the stratum corneum [54]. A third method uses the principle of impedance-based capacitance to assess hydration levels.
Skin thickness
Although not extensively applied, high-frequency ultrasound has also proved valuable for the assessment of another aspect of the irritant response, namely changes in skin thickness [55].
Investigations
Irritant contact dermatitis is a clinical diagnosis based on knowledge of the chemical(s) involved and exposure factors. However, some chemicals may have both irritant and allergic potential, or the patient may be exposed to several chemicals with irritant or allergic potential. Consequently, patch testing should be undertaken to exclude an allergic component to the skin rash. Patch testing should never be undertaken with unknown chemicals supplied by the patient, and the testing clinician should either avoid testing known irritants, or carefully test with serial dilutions and be vigilant for false positive reactions.
Management
The successful management [56] of irritant contact dermatitis requires both prevention and subsequently treatment if dermatitis develops. The most important aspect of treatment is avoidance of the cause (Box 129.4). In an occupational setting, automation of the production process may avoid exposure but can be expensive. A cost-effective compromise is the use of personal protective equipment and/or substitution of a chemical. It should be remembered that natural rubber latex gloves provide protection against water-miscible substances but may be inappropriate for other exposures (Table 129.3). With organic solvents and chemicals the choice of glove material may vary [57]; advice may be found on the material safety data sheet of each product.
Table 129.3 Recommended glove materials for chemical protection.
| Glove materials | Nitrile | Butyl | Neoprene | Fluorocarbon | PVC | PVA | Notes |
| Aliphatic hydrocarbons | + | + | +* | Except cyclohexane* | |||
| Aromatic hydrocarbons | + | + | +* | Except ethylbenzene* | |||
| Halogenated hydrocarbons | + | +* | Except methyl chloride* and halothane* | ||||
| Aldehydes, amines, amides | +* | Except butylamine* and triethylamine* | |||||
| Esters | +* | +† | Except butylacrylate* and octylphthalate† | ||||
| Alkalis | + | + | + | ||||
| Organic acids | +* | + | +† | Except acrylic*†, methacrylic*† and acetic* acids | |||
| Inorganic acids | +* | +† | +‡ | Except chromic†, hydrofluoric*, nitric*‡ and sulphuric*‡ acids |
From Berardinelli 1988 [57].
*†‡, These gloves do not provide adequate protection with the listed chemical.
PVA, polyvinyl alcohol; PVC, polyvinyl chloride.
Once present, dermatitis requires palliation of symptoms with topical steroids and emollients. The efficacy of topical corticosteroids in irritant contact dermatitis has been questioned [58, 59], although another study has shown benefit [60]. Retinoids and vitamin D analogues are not of any value [58].
Experimentally, an emollient alone has been shown to improve barrier repair. The choice of emollient may be important, with lipid-rich preparations being more effective. Studies have shown that barrier repair may be impaired or accelerated according to the constituents of a physiological lipid mixture [61]. It has been suggested that conditions in which the lamellar body secretory system is impaired or immature (radiation dermatitis, sunburn, irritant dermatitis due to some surfactants and retinoids, and premature infants of less than 33 weeks’ gestation) should be treated with non-physiological lipids, e.g. petrolatum, whereas most other causes of irritant dermatitis, where lipid metabolism has not been deranged (e.g. diaper dermatitis), should be treated with a mixture of cholesterol : ceramides : free fatty acids, in a 3 : 1 : 1 ratio, to achieve most rapid return to normal barrier function [62].
In severe cases, phototherapy or systemic drugs such as corticosteroids, azathioprine and ciclosporin may be required. Where there is secondary infection, topical or systemic antimicrobial agents may be necessary. Regulation of occupational practice is essential in primary prevention (Box 129.5), and this is especially important as some patients may have persistent skin problems even after exposure has ceased [63] (see Chapter 130).
Non-immune contact urticaria
Definition and nomenclature
A localized erythema or weal and flare reaction caused by contact with a substance where there is no substance-specific immune response
Introduction and general description
Immediate contact reactions may be either allergic or toxic. They are transient, developing within minutes, and fade quickly (usually within hours). They may appear on both normal and damaged/eczematous skin, and may present as only a transient, symptomatic erythema or as a contact urticaria (see also Chapter 42). For some agents that cause immediate-type reactions, it is still unclear as to whether or not the mechanism is immunological. Non-immunological, immediate contact reactions occur without prior sensitization. The reactions remain localized, and may present as a transient erythema or as an urticarial weal and flare, depending on concentration, area of contact, mode of exposure and agent involved [1, 2]. Substances reported to cause non-immunological contact urticaria are listed in Box 129.6. The most potent of these, such as benzoic acid, sorbic acid, cinnamic acid, cinnamal and nicotine acid esters, may induce a local reaction within 45 min in more than 50% of those tested. Reactions may occur at concentrations as low as 0.1% for benzoic acid, sorbic acid and sodium benzoate, and as low as 0.01% for cinnamal. Reactions are not enhanced by occlusion, but may affect mucosal surfaces; low concentrations of cinnamal are sometimes added to toothpaste and mouthwashes to impart a sensation of ‘freshness’. Studies on an unselected population have shown that reactions to urticants are not predictable; an individual who reacted strongly to one urticant did not necessarily react to another. In addition, there was no significant correlation between age or sex and urticant response [3].
Epidemiology
Incidence and prevalence
This is unknown, but many patients with mild disease will not seek medical advice, hence there is likely to be considerable underreporting.
Pathophysiology
The mechanism of action of non-immunological contact reactions is not known, but is presumed to be via direct release of inflammatory mediators, including prostaglandins and leukotrienes [4]; the reaction is blocked by non-steroidal anti-inflammatory drugs and UV light, or by pretreatment with capsaicin, but not by antihistamines [5–7].
Clinical features
Differential diagnosis
- Immunological contact urticaria (type I hypersensitivity).
- Chronic ordinary urticaria.
Investigations
Twenty-minute patch tests or skin prick tests may confirm the diagnosis but will not necessarily differentiate this from immunological contact urticaria. Control subjects may also be positive on testing.
Management
Phototoxic contact dermatitis
Definition and nomenclature
Phototoxic contact dermatitis occurs when light is absorbed by a chemical which then is modified and becomes an irritant stimulus.
Introduction and general description
Chemicals applied either topically or systemically may absorb photons and be chemically modified to cause irritation. Clinically, the reaction resembles a sunburn and only occurs in sun-exposed areas of skin. Primary prevention is critical. An initial screen of potential phototoxic chemicals involves the measurement of its absorption spectrum. If this indicates the chemical may be photoreactive then the 3T3 neutral red uptake phototoxicity test (3T3 NRU PT) can be performed, which has been validated and accepted into legislation. This test compares the cytotoxicity of a chemical when tested with and without exposure to a non-cytotoxic dose of simulated solar light (UVA/visible spectrum). Mouse 3T3 fibroblasts are incubated with the chemical and the concentration-dependent reduction of the uptake of the vital dye neutral red is measured 24 h after treatment. Substances identified are likely to be phototoxic following systemic administration and distribution to the skin or following topical application. The test has been found to detect photoallergens in addition to irritants, and the use of further photobinding studies to human albumin may distinguish between the two. In general, photoallergy requires protein binding as a prelude to antigen presentation.
The use of ethical tests in human volunteers may be considered where testing in vivo is considered essential [1]. Strong irritants may cause dermatitis on first exposure, whereas weak irritants may be detected only by repeated application [2]. Internal standards are necessary to allow comparison with other established irritants [3].
Clinical features
History and presentation
Tetracycline-related antibiotics may cause phototoxicity, as can psoralens, porphyrins, amiodarone, phenothiazines and non-steroidal anti-inflammatory drugs. Plant sap (e.g. from the giant hogweed) may cause a phototoxic reaction known as phytophotodermatitis. Such reactions can be flagellate or show linear vesicles. They often resolve with persistent hyperpigmentation.
Differential diagnosis
- Photoallergy.
- Allergic contact dermatitis.
- (Non-photo) irritant contact dermatitis.
Investigations
Photopatch testing is important to exclude a photoallergy in some cases, otherwise, the diagnosis is clinical.
Management
Subjective sensory irritation
Definition and nomenclature
Subjective sensory irritation is a subjective feeling of skin discomfort when in contact with a substance in the absence of any visible skin change.
Introduction and general description
With some irritants, there is little or nothing to see, but individuals complain of a subjective sensation of stinging, burning or smarting. These sensations most commonly affect the head and neck, may present as one form of cosmetic intolerance and are frequently termed ‘sensitive skin’ [1]. Although stinging potential is often assessed by the application of lactic acid to the nasolabial fold, the results do not necessarily correlate with the sensation perceived at other facial sites [2]. With soaps and detergents, the perceived sensory symptoms correlate with, and predict the development of, clinical signs of irritant dermatitis [3]. However, more typically the sensation does not correlate with a predisposition to irritant dermatitis or non-immune contact urticaria. Little is known of the mechanisms involved in subjective irritant reactions [1]. It is presumed that penetration of the irritant is primarily via sweat ducts and hair follicles, is not related to pH and that the reaction involves stimulation of sensory nerve endings. The reaction is substantially reduced in the absence of sweating and is commoner in the summer. Factors thought to predispose to sensitive skin include female sex and hormonal status, young age, fair skin, susceptibility to blushing/flushing, thin stratum corneum, increased number of sweat glands and innervation and impairment of epidermal barrier function. The presence of sensitive skin also affects quality-of-life scores, with affected individuals having an impaired psychological component, although there was no significant relationship to depressive symptoms [4]. Triggers are not necessarily chemical, and include environmental factors such as wind. Sensory irritation from woollen garments is also well known among patients with atopic eczema. Experimental studies have shown this to be due to stimulation of nerve fibres which transmit pain. It required a 100-mg force on the end of a 40-μm diameter textile fibre to trigger the nerve receptor. Thus, garments that induce the sensation will have protruding fibre ends that can withstand 100-mg pressure without buckling. Prickle was not experienced if the fabric was rubbed over the skin, if the skin was cold or if the area of contact was less than 1 cm2. Moisture increased the severity of the sensation [5].
Immediate-type stinging
Some chemicals will cause painful sensations within seconds of contact [6]. These include acids, where the stinging may be a prodrome to the development of more severe cutaneous damage. Other chemicals, however, will cause stinging without any significant cutaneous damage. The best known of these are chloroform and methanol (1 : 1), and 95% ethanol. Responses vary according to site and individual susceptibility, and probably relate indirectly to stratum corneum thickness. The sensation abates quickly following removal of the irritant substance.
Delayed-type stinging
Delayed-type stinging [6] may occur following contact with a number of substances (Box 129.7). Typically, there is no immediate stinging, but discomfort develops within 1–2 min, reaches a maximum in 5–10 min and fades slowly over the next half hour. The reaction normally affects only the face, especially in association with heat and humidity or sweating. The sensation is not alleviated by washing off the offending chemical. It is an idiosyncratic response, and only a proportion of the population will be affected.
Individuals can be screened to ascertain whether they are stingers or not by the application of 5% aqueous lactic acid to the nasolabial fold after induction of sweating. Preparations can be screened for stinging potential by testing a predetermined panel of ‘stingers and smarters’.
Epidemiology
Incidence and prevalence
‘Sensitive skin’ might be considered a marker of a form of skin irritancy. In a questionnaire-based study of 3300 women and 500 men, 51.4% of women and 38.2% of men considered that they were susceptible [7]. Fifty-seven per cent of women and 31.4% of men had had an adverse reaction to a personal care product during their lives. Among women, symptoms of subjective skin irritation (burning, stinging, etc.) occurred more frequently in those who considered that they had a sensitive skin (53%) than in those who did not (17%). Dry skin and a predisposition to blushing/flushing were factors associated with a sensitive skin. An atopic background was a predictive factor for the presence of sensitive skin, as the incidence of atopy was higher among those with sensitive skin (49%) than among those in the non-sensitive group (27%). However, equal numbers of atopics and non-atopics constituted the sensitive skin group, indicating that other variables were involved.
Clinical features
Differential diagnosis
- Irritant contact dermatitis.
- Allergic contact dermatitis.
- Atopic eczema.
- Psychological illness.
Investigations
Sometimes patch testing can be helpful to eliminate other differential diagnoses.
Management
Treatment is largely that of avoidance, although strontium salts have been shown experimentally to inhibit the sensation [8]. Currently, this seems of little clinical value.
Chemical burns
Definition
A chemical burn results when tissue is exposed to a corrosive chemical, such as a strong acid or alkali. Irreversible cell damage and necrosis occurs (see also Chapter 126).
Introduction and general description
There is usually rapid onset of painful erythema, often within minutes, at the site of exposure, followed by blistering and the development of necrotic ulcers. Weals may also be seen. Symptoms coincide with the exposure, but with some chemicals, including phenols and weak hydrofluoric acid, the onset may be delayed. Damage continues to occur until all of the agent has chemically reacted or has been neutralized as a result of treatment. Occupational settings are still common for chemical burns but the frequency of domestic settings is increasing [1]. The burn is classified according to depth (1 – superficial to 4 – extending deep to the skin).
Epidemiology
Incidence and prevalence
Scanty data exist but some studies suggest an incidence of 2–10% [1] Chemical burns are relatively common, with an American survey showing that 119 hospitals treated 11 759 patients during a 1-year period. The majority of chemical burns involved the upper limb, with most occurring in young adults and infants. Occupational chemical burns are also relatively common, with 29% of chemical burns requiring admission being work related, with a rate of 26.4/10 000 employees. In women, chemical burns of the wrist and hand were most frequent, whereas in men eye involvement was more common. Welders, labourers, cooks and mechanics were most at risk [2].
Age
Limited data suggest that those of working age suffer most from chemical burns [1].
Sex
Men are more frequently affected than women (6 : 1 in some series [1]).
Associated diseases
Sometimes scars following burns may lead to functional disability, such as joint contractures.
Clinical features
History and presentation
Pain and erythema are usually present within minutes of exposure, but presentation may be delayed. Most acids (e.g. sulphuric, nitric, hydrochloric, chromic) coagulate skin proteins, and as a result form a barrier that impedes further penetration. Some acids can discolour (e.g. nitric acid turns the skin yellow). Hydrofluoric acid [3] differs in that it causes a liquefactive necrosis, and penetration can continue for several days after exposure, even down to bone. Pain, which can last several days, is typical of burns due to hydrofluoric acid and other fluorides. It is related to the ability of the fluoride ion to bind calcium and disrupt neural function. If more than 1% of the body surface area is affected, systemic toxicity can develop.
Alkalis (e.g. sodium, calcium, potassium hydroxides; wet concrete [4]; sodium and potassium cyanides) degrade lipids, and saponification of the resulting fatty acids forms soaps which aid penetration deeper into the skin. As a consequence, damage is more severe than with most acids, and pain is also a feature. The dead skin turns brown and later black, usually without blistering, and forms a hard eschar. Phenols [5] and unhardened phenolic resins penetrate the skin easily and rarely can cause nerve damage in the absence of visible skin change. Vasoconstriction may contribute to the necrosis that develops, and in the case of systemic absorption can lead to shock and renal damage.
Differential diagnosis
- Irritant contact dermatitis.
- Allergic contact dermatitis.
- Contact urticaria.
- Thermal injury.
- Non-accidental injury in children.
Classification of severity
Burns are classified according to the depth of cutaneous involvement:
- Superficial partial-thickness burns extend to the level of the dermal papillae. As the papillary blood vessels remain intact, the skin blanches on pressure and vasodilatation of the vessels results in the skin appearing shiny pink to red and wet as a result of capillary leakage. Blisters may be present, and sensation is preserved. The burn typically heals within 10–14 days without scarring.
- Deep partial-thickness burns extend into the dermis but the appendages are spared. The burn appears white or pale pink and oedematous. Some sensation may be retained but often only that of deep pressure. Re-epithelialization begins from the residual adnexal structures, taking up to 6 weeks to occur, with scarring.
- Full-thickness burns extend into the subcutaneous tissue. The burn appears brown/black or pale white, with a leathery eschar. Sensation is completely lost and healing occurs slowly from the margins. Healing occurs with scarring and a risk of contracture.
- Fourth-degree burns extend into tendon, muscle, bone or joint.
Management
Knowledge of the chemical causing the burn is vital, as decontamination or other specific neutralization may be required. Initial treatment of chemical burns [6, 7] requires irrigation with large volumes of lukewarm water and removal of contaminated clothing. Where the chemical is insoluble in water, a soap solution or solvent may be used instead. High pressures should not be used, to avoid splashing other areas of the body or bystanders with the corrosive material.
Although neutralizing solutions offer an alternative to irrigation, theoretically an exothermic reaction and potential delay in obtaining the treatment might result in increased tissue damage, and they are not generally recommended [8]. Specific antidotes that have been suggested include the use of milk or egg whites for oxidizing agents such as chromic acid and potassium permanganate. Reducing agents such as hydrochloric and nitric acids can be neutralized with soap, or sodium and magnesium hydroxides.
Consideration should be given to referral to a burns unit in the following circumstances:
- Partial-thickness burns with >10% surface area involvement.
- Burns of the face, hands, feet, genitalia or over joints where contractures may affect function.
- Full-thickness burns.
- Chemical and inhalational injury where there is a risk of systemic involvement.
- Burns in individuals with co-morbidities that may complicate management.
On arrival in hospital, initial assessment involves providing systemic support and fluid replacement. The fluid requirement varies depending on body weight and surface area involved. Jewellery should be removed to prevent it acting as a tourniquet as oedema develops, and tetanus status reviewed.
For some chemicals such as hydrofluoric acid, specific antidotes should be used subsequently, for example 2.5% calcium gluconate gel. Application should be repeated 4-hourly and disappearance of pain is a sign of successful treatment [9]. If the pain fails to resolve, infiltration or regional infusion have been used. If treatment is delayed, the fluoride ion disassociates and complexes with calcium and magnesium forming insoluble salts in the tissues, with destruction of soft and bony tissue. Hypocalcaemia leads to cardiac arrhythmia. When there is a risk of toxicity from systemic absorption, as with chromic acid [10], early debridement of necrotic areas reduces blood levels, and consideration should be given to the use of dialysis to remove circulating chromium. Ulcerated areas should be managed with antibacterial creams to prevent secondary infection whilst re-epithelialization occurs. If there is a surrounding inflammatory reaction, a moderately potent topical corticosteroid can be applied. Vapour-permeable dressings are recommended in view of the role of TEWL in stimulating barrier repair [11].
Frequent review is required because the ulcers can progress over several days. Subsequent management with excision/debridement and/or grafting may speed the healing process. Where the ulcer extends into the dermis, healing frequently results in a scar, and pigmentary change is common. Several chemicals (e.g. hydrofluoric acid, phenolic compounds, chromic acid, gasoline) carry a significant risk of systemic toxicity even when cutaneous involvement is small (∼1%). In these instances, regular monitoring of blood, liver and kidney function, with appropriate supportive treatment, is required [12].
When the chemical is a sensitizer, allergic contact dermatitis may subsequently occur on re-exposure to non-irritant concentrations, as burns and irritant dermatitis appear to promote sensitization [13].
References
Irritant contact dermatitis
- Meding B, Jarvholm B. Incidence of hand eczema – a population based retrospective study. J Invest Dermatol 2004;122:873–7.
- Meyer JD, Chen Y, Holt DL, et al. Occupational contact dermatitis in the UK: a surveillance report from EPIDERM and OPRA. Occup Med 2000;50:265–73.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977–80. J Am Acad Dermatol 1982;6:909–17.
- Schwensen JF, Friis UF, Menné T, et al. One thousand cases of occupational contact dermatitis. Contact Dermatitis 2013;68:259-68.
- Jourdain R, De Lacharrière O, Bastien P, Maibach HI. Ethnic variations in self-perceived sensitive skin: epidemiological survey. Contact Dermatitis 2002;46:162–9.
- Peters L, Marriott M, Mukerji B, et al. The effect of population diversity on skin irritation. Contact Dermatitis 2006:55:357–63.
- Madison KC. Barrier function of the skin: ‘La raison d’être' of the epidermis. J Invest Dermatol 2003;121:231–41.
- Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian permeability barrier: a lesson from claudin-1 deficient mice. J Cell Biol 2002;156:1099–111.
- Van der Valk PGM, Maibach HI. A functional study of the skin barrier to evaporative water loss by means of repeated cellophane-tape stripping. Clin Exp Dermatol 1990;15:180–2.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol 2013;168:326–32.
- Lisby S, Baadsgaard O. Mechanisms of irritant contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:69–82.
- Barratt MD. Quantitative structure–activity relationships for skin irritation and corrosivity of neutral and electrophilic organic chemicals. Toxicol Vitro 1996;10:247–56.
- Nangia A, Andersen PH, Berner B, Maibach HI. High dissociation constants (pKa) of basic permeants are associated with in vivo skin irritation in man. Contact Dermatitis 1996;34:237–42.
- Aramaki J, Loffler C, Kawana S, et al. Irritant patch testing with sodium lauryl sulphate: interrelation between concentration and exposure time. Br J Dermatol 2001;145:704–8.
- Grangsjo A, Leijon-Kuligowski A, Torma H, et al. Different pathways in irritant contact eczema? Early differences in the epidermal elemental content and expression of cytokines after application of 2 different irritants. Contact Dermatitis 1996;35:355–60.
- Wood LC, Elias PM, Calhoun C, et al. Barrier disruption stimulates interleukin-1 alpha expression and release from a preformed pool in murine epidermis. J Invest Dermatol 1996;106:397–403.
- Elias PM, LaDonna C, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermatitis 1999;10:119–26.
- Willis CM, Reiche L, Wilkinson JD. Immunocytochemical demonstration of reduced Cu,Zn-superoxide dismutase levels following topical application of dithranol and sodium lauryl sulphate: an indication of the role of oxidative stress in acute irritant dermatitis. Eur J Dermatol 1998;8:8–12.
- Prottey C, Oliver D, Coxon AC. Prediction and measurement of surfactant action upon human skin under realistic conditions. Int J Cosmet Sci 1984;6:263–73.
- Fulmer AW, Kramer GJ. Stratum corneum lipid abnormalities in surfactant induced dry scaly skin. J Invest Dermatol 1986;86:598–602.
- Frosch P, Czarnetzki BM. Surfactants cause in vitro chemotaxis and chemokinesis of human neutrophils. J Invest Dermatol 1987;88:52s–5s.
- Steele RH, Wilhelm DL. The inflammatory reaction in chemical injury, 3: leucocytosis and other histological changes induced by superficial injury. Br J Exp Pathol 1970;51:265–79.
- Sjogren F, Anderson C. The spectrum of inflammatory cell response to dimethyl sulfoxide. Contact Dermatitis 2000;42:216–21.
- Kondo S, Beissert S, Wang B, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J Invest Dermatol 1997;108:811–12.
- Lisby S, Baadsgaard O. Mechanisms of irritant contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P,eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:69–82.
- Barratt MD. Quantitative structure–activity relationships for skin irritation and corrosivity of neutral and electrophilic organic chemicals. Toxicol Vitro 1996;10:247–56.
- Nangia A, Andersen PH, Berner B, Maibach HI. High dissociation constants (pKa) of basic permeants are associated with in vivo skin irritation in man. Contact Dermatitis 1996;34:237–42.
- Aramaki J, Loffler C, Kawana S, et al. Irritant patch testing with sodium lauryl sulphate: interrelation between concentration and exposure time. Br J Dermatol 2001;145:704–8.
- Grangsjo A, Leijon-Kuligowski A, Torma H, et al. Different pathways in irritant contact eczema? Early differences in the epidermal elemental content and expression of cytokines after application of 2 different irritants. Contact Dermatitis 1996;35:355–60.
- Wood LC, Elias PM, Calhoun C, et al. Barrier disruption stimulates interleukin-1 alpha expression and release from a preformed pool in murine epidermis. J Invest Dermatol 1996;106:397–403.
- Elias PM, LaDonna C, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermatitis 1999;10:119–26.
- Willis CM, Reiche L, Wilkinson JD. Immunocytochemical demonstration of reduced Cu,Zn-superoxide dismutase levels following topical application of dithranol and sodium lauryl sulphate: an indication of the role of oxidative stress in acute irritant dermatitis. Eur J Dermatol 1998;8:8–12.
- Prottey C, Oliver D, Coxon AC. Prediction and measurement of surfactant action upon human skin under realistic conditions. Int J Cosmet Sci 1984;6:263–73.
- Fulmer AW, Kramer GJ. Stratum corneum lipid abnormalities in surfactant induced dry scaly skin. J Invest Dermatol 1986;86:598–602.
- Frosch P, Czarnetzki BM. Surfactants cause in vitro chemotaxis and chemokinesis of human neutrophils. J Invest Dermatol 1987;88:52s–5s.
- Steele RH, Wilhelm DL. The inflammatory reaction in chemical injury, 3: leucocytosis and other histological changes induced by superficial injury. Br J Exp Pathol 1970;51:265–79.
- Sjogren F, Anderson C. The spectrum of inflammatory cell response to dimethyl sulfoxide. Contact Dermatitis 2000;42:216–21.
- Kondo S, Beissert S, Wang B, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J Invest Dermatol 1997;108:811–12.
- Lantinga H, Nater JP, Coenraads PJ. Prevalence, incidence and course of eczema on the hand and forearm in a sample of the general population. Contact Dermatitis 1984;18:135–9.
- Lammintausta K, Maibach HI, Wilson D. Irritant reactivity in males and females. Contact Dermatitis 1987;17:276–80.
- Foley P, Nixon R, Marks R, et al. The frequency of reactions to sunscreens: results of a longitudinal population based study on the regular use of sunscreen in Australia. Br J Dermatol 1993;128:512–18.
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis 1992;27:143–7.
- Dooms Gossens A, Debusschere KM, Gevers DM, et al. Contact dermatitis caused by airborne agents. J Am Acad Dermatol 1986;15:1–10.
- Freeman S, Stephens R. Cheilitis: an analysis of 75 cases referred to a contact dermatitis clinic. Am J Contact Dermatitis 1999;10:198–200.
- Scardillo J, Aronovitch SA. Successfully managing incontinence-related irritant dermatitis across the lifespan. Ostomy Wound Manage 1999;45:36–40.
- Akin F, Spraker M, Aly R, et al. Effects of breathable disposable diapers: reduced prevalence of Candida and common diaper dermatitis. Pediatr Dermatol 2001;18:282–90.
- Concannon P, Gisoldi E, Phillips S, Grossman R. Diaper dermatitis – a therapeutic dilemma: results of a double-blind placebo-controlled trial of miconazole nitrate 0.25%.Pediatr Dermatol 2001;18:149–55.
- Lyon CC, Smith AJ, Griffiths CE, Beck MH. Peristomal dermatoses: a novel indication for topical steroid lotions. J Am Acad Dermatol 2000;43:679–82.
- Lyon CC, Stapleton M, Smith AJ, et al. Topical sucralfate in the management of peristomal skin disease: an open study. Clin Exp Dermatol 2000;25:584–8.
- Fluhr JW, Kuss O, Diepgen T, et al. Testing for irritation with a multifactorial approach: comparison of eight non-invasive measuring techniques on five different irritation types. Br J Dermatol 2001;145:696–703.
- Willis CM, Stephens CJM, Wilkinson JD. Assessment of erythema in irritant contact dermatitis: comparison between visual scoring and laser Doppler flowmetry. Contact Dermatitis 1988;18:138–42.
- Babulak SW, Rhein LD, Scala DD, et al. Quantification of erythema in a soap chamber test using the Minolta Chroma (reflectance) meter: comparison of instrumental results with visual assessments. J Soc Cosmet Chem 1986;37:475–7.
- Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement: a report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990;22:164–78.
- Agner T, Serup J. Comparison of two electrical methods for measurement of skin hydration: an experimental study on irritant patch test reactions. Bioeng Skin 1988;4:263–9.
- Seidenari S, Di Nardo A. B scanning evaluation of irritant reactions with binary transformation and image analysis. Acta Derm Venereol Suppl (Stockh) 1992;175:3–7.
- Saary J, Qureshi R, Palda V, et al. A systematic review of contact dermatitis treatment and prevention. J Am Acad Dermatol 2005;53:845–55.
- Berardinelli SP. Prevention of occupational skin disease through use of chemical protective gloves. Dermatol Clin 1988;6:115–19.
- Le TK, de Mon P, Schalkwijk J, van der Valk PG. Effect of a topical steroid, a retinoid, and a vitamin D3 derivative on sodium dodecyl sulphate induced skin irritation. Contact Dermatitis 1997;37:19–26.
- Levin C, Zhai H, Bashir S, et al. Efficacy of corticosteroids in acute experimental irritant contact dermatitis? Skin Res Technol 2001;7:214–18.
- Ramsing DW, Agner T. Efficacy of topical corticosteroids on irritant skin reactions. Contact Dermatitis 1995;32:293–7.
- Yokota M, Maibach HI. Moisturizer effect on irritant dermatitis: an overview. Contact Dermatitis 2006;55:65–72.
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol 2001;137:1079–81.
- Nicholson PJ, Llewellyn D and English JS. Evidence-based guidelines for the prevention, identification and management of occupational contact dermatitis and urticaria. Contact Dermatitis 2010;63:177–86.
- Holness DL, Nethercott JR. Is a worker's understanding of their diagnosis an important determinant of outcome in occupational contact dermatitis? Contact Dermatitis 1991;25:296–301.
Non-immune contact urticaria
- Wakelin SH. Contact urticaria. Clin Exp Dermatol 2000;26:132–6.
- Lahti A, Basketter D. Immediate contact reactions. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn Berlin: Springer, 2006:83–96.
- Basketter DA, Wilhelm KP. Studies on non-immune immediate contact reactions in an unselected population. Contact Dermatitis 1996;35:237–40.
- Lahti A, Maibach HI. Immediate contact reactions: contact urticarial syndrome. Semin Dermatol 1987;6:313–20.
- Johansson J, Lahti A. Topical non-steroidal anti-inflammatory drugs inhibit non-immunologic immediate contact reactions. Contact Dermatitis 1988;19:161–5.
- Larmi E, Lahti A, Hannuksela M. Ultraviolet light inhibits non-immunologic contact reactions to benzoic acid. Arch Dermatol Res 1988;280:420–3.
- Lahti A, Vaaneucu A, Kokkoneu EL, Hannuksela M. Effects of capsaicin and topical anesthesia on non-immunologic immediate contact reactions to benzoic acid and methyl nicotinate. In: Frosch PJ, Dooms-Goossens A, La Chapelle JM et al., eds. Current Topics in Contact Dermatitis. Berlin: Springer, 1989:441–7.
Phototoxic contact dermatitis
- Cooper K, Marriott M, Peters L, Basketter DA. Stinging and irritating substances: their identification and assessment. In: Lóden M, Maibach HI, eds. Dry Skin and Moisturisers, 2nd edn. Boca Raton, FL: CRC Taylor & Francis, 2005:501–14.
- Kligman AM, Wooding WM. A method for the measurement and evaluation of irritants on human skin. J Invest Dermatol 1967;49:78–94.
- Basketter DA, Griffiths HA, Wang XM, et al. Individual, ethnic and seasonal variability in irritant susceptibility of skin: the implications for a predictive human patch test. Contact Dermatitis 1996;35:208–13.
Subjective sensory irritation
- Farage M, Katsarou A, Maibach HI. Sensory, clinical and physiological factors in sensitive skin: a review. Contact Dermatitis 2006;55:1–14.
- Marriott M, Whittle E, Basketter DA. Facial variations in sensory responses. Contact Dermatitis 2003;49:227–31.
- Simion FA, Rhein LD, Morrison BM, et al. Self-perceived sensory responses to soap and synthetic detergent bars correlate with clinical signs of irritation. J Am Acad Dermatol 1995;32:205–11.
- Misery L, Myon E, Martin N, et al. Sensitive skin: psychological effects and seasonal changes. J Eur Acad Dermatol 2007;21:620–8.
- Hatch KL, Maibach HI. Textile dermatitis: an update, 1: resins, additives and fibers. Contact Dermatitis 1995;32:319–26.
- Frosch PJ, John SM. Clinical aspects of irritant contact dermatitis. In: Frosch PJ, Menné T, Lepoittevin J-P, eds. Contact Dermatitis, 4th edn. Berlin: Springer, 2006:255–94.
- Willis CM, Shaw S, De Lacharrière O, et al. Sensitive skin: an epidemiological study. Br J Dermatol 2001;145:258–63.
- Zhai H, Hannon W, Hahn GS, et al. Strontium nitrate suppresses chemically-induced sensory irritation in humans. Contact Dermatitis 2000;42:98–100.
Chemical burns
- Hardwicke J, Hunter T, Staruch R, Moiemen N. Chemical burns. An historical comparison and review of the literature. Burns 2012;38:383–7.
- Pruitt VM. Work-related burns. Clin Occup Environ Med 2006;5:423–33.
- Kirkpatrick JJ, Enion DS, Burd DA. Hydrofluoric acid burns: a review. Burns 1995;21:483–93.
- Spoo J, Elsner P. Cement burns: a review 1960–2000. Contact Dermatitis 2001;45:68–71.
- Horch R, Spilker G, Stark GB. Phenol burns and intoxications. Burns 1994;20:45–50.
- Bruze M, Fregert S, Gruvberger B. Chemical skin burns. In: Kanerva L, Elsner P, Wahlberg JE, Maibach HI, eds. Handbook of Occupational Dermatology. Berlin: Springer, 2000:325–32.
- Pruitt VM. Work-related burns. Clin Occup Environ Med 2006;5:423–33.
- Flamminger A, Maibach HI. Sulfuric acid burns (corrosion and acute irritation): evidence based overview to management. Cutan Ocular Toxicol 2006;25:55–61.
- Matsuno K. The treatment of hydrofluoric acid burns. Occup Med 1996;46:313–17.
- Matey P, Allison KP, Sheehan TMT, et al. Chromic acid burns: early aggressive excision is the best method to prevent systemic toxicity. J Burn Care Rehabil 2000;21:241–5.
- Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res 1989;30:323–33.
- Chan TC, Williams SR, Clark RF. Formic acid skin burns resulting in systemic toxicity. Ann Emerg Med 1995;26:383–6.
- McFadden JP, Basketter DA. Contact allergy, irritancy and danger. Contact Dermatitis 2000;42:123–7.