CHAPTER 146
Skin Cancer in the Immunocompromised Patient
Catherine A. Harwood1, Jane M. McGregor1 and Charlotte M. Proby2
1Department of Dermatology, The Royal London Hospital, London; Centre for Cutaneous Research, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK
2Skin Tumour Laboratory, Division of Cancer Research, Medical Research Institute, Jacqui Wood Cancer Centre, Dundee, UK
Introduction
The immune system plays a critical role in skin cancer development, progression and destruction. Skin cancers in individuals with compromised immune systems represent a growing challenge in terms of their frequency and diversity as well as their atypical and often aggressive nature. Mortality and morbidity associated with skin tumours in this clinical context are often considerable, their pathogenesis is multifactorial and an evidence base to guide management is lacking in many key areas.
Epidemiology
Certain primary immunodeficiencies predispose to skin cancer but the greatest burden of disease is associated with acquired immunodeficiency, including immunosuppressive drug therapy (e.g. following solid-organ and haematopoietic transplantation and for immune-mediated inflammatory disorders), non-Hodgkin lymphoma/chronic lymphocytic leukaemia (NHL/CLL) and HIV infection (Table 146.1).
Table 146.1 Primary and acquired immunodeficiency conditions and associated skin cancers
| Immunodeficiency | Condition | cSCC/CIS | BCC | Melanoma | MCC | PCL | KS | Appendageal | DFSP | AFX |
| Primary | EV | ++ | + | |||||||
| SCID | + | |||||||||
| WHIM | + | + | + | |||||||
| DOCK8 | + | + | + | |||||||
| CVID | + | |||||||||
| WAS | + | + | ||||||||
| DC | + | |||||||||
| Netherton syndrome | + | + | ||||||||
| Acquired | HIV | ++ | +++ | + | ++ | ++ | +++ | + | + | + |
| NHL/CLL | ++ | + | + | + | + | + | ||||
| Solid OTR | ++++ | ++ | + | + | + | ++ | + | + | + | |
| HCT | + | + | + | + | ||||||
| IMID (IBD, RA) | + | + | + | + | + | + |
Signs + to ++++ gives an approximate frequency of reports in the literature.
AFX, atypical fibroxanthoma; BCC, basal cell carcinoma; cSCC/CIS, cutaneous squamous cell carcinoma/carcinoma in situ; CVID, common variable immunodeficiency; DC, dyskeratosis congenita; DFSP, dermatofibrosarcoma protuberans; DOCK8, dedicator of cytokinesis 8; EV, epidermodysplasia verruciformis; HCT, haematopoietic cell transplant; HIV, human immunodeficiency virus; IMID, immune-mediated inflammatory disorder (IBD, inflammatory bowel disease; RA, rheumatoid arthritis); KS, Kaposi sarcoma; MCC, Merkel cell carcinoma; NHL/CLL, non-Hodgkin lymphoma/chronic lymphocytic leukaemia; OTR, organ transplant recipient; PCL, primary cutaneous lymphoma; SCID, severe combined immunodeficiency; WAS, Wiskott–Aldrich syndrome; WHIM, warts, hypogammaglobulinaemia, infections and myelokathexis.
Primary immunodeficiency
The range of primary defects of the immune system associated with skin cancer underscores the complexity of the immune repertoire associated with skin cancer development and may provide important insights into its pathogenesis in the general population [1]. The following examples are illustrative of this spectrum of underlying immunological abnormalities.
Epidermodysplasia verruciformis (see Chapter 25). Epidermodysplasia verruciformis (EV) is the most distinctive condition in which skin cancer is associated with a primary immunological deficit. This rare autosomal recessive genodermatosis is characterized by predisposition to persistent infection with a specific genus of human papillomavirus (HPV), namely EV or β-HPV types [2]. Disseminated plane warts, multiple common warts and pityriasis versicolor-like lesions start to appear in early childhood and cutaneous squamous cell carcinomas (cSCCs) develop on UV-exposed sites in up to 60% of patients from the third decade onwards, with no other abnormalities in most patients [2]. Invasive cSCCs are located mainly on the forehead and associated with sun exposure, but onset may be exacerbated by irradiation; they develop slowly, may be locally destructive, but are only rarely metastatic [3]. EV is caused in most cases by mutations in EVER1 (TMC6) and EVER2 (TMC8), adjacent genes located on 17q25 which are involved in the regulation of intracellular zinc distribution, activity of zinc-dependent transcription factors and proliferation [2]. EVER deficiency may alleviate inhibition of expression of the whole genome of HPVs in keratinocytes, and/or impair T-cell immunity against HPVs [3, 4]. EVER2-deficient patients also display mild T-cell abnormalities [5]. Primary T-cell disorders due to deficiency in RHOH and MST1 may cause an EV phenotype [6], as may secondary T-cell defects in conditions such as HIV [7–10].
Severe combined immunodeficiency (SCID) is caused by deficiency and impaired function of T cells and, in some forms, additional reduction or dysfunction of natural killer (NK) cells and/or B cells. It results from diverse molecular defects, e.g. in IL-7R, CD45, IL-2Rγ, JAK3, RAG1, RAG2, ARTEMIS and ADA [1]. Increased cutaneous viral infections are a common feature and include HPV infection [11]. EV-like lesions occur in JAK3- and IL-2-Rγ-deficient patients after bone marrow transplantation [12, 13]. A similar association is reported in other SCID-like immunodeficiences such as WHIM syndrome (warts, hypogammaglobulinaemia, infections and myelokathexis), resulting from mutations of the CXCR4 chemokine receptor and in which vulvar SCC, basal cell carcinoma (BCC) and cutaneous T-cell lymphoma (CTCL) are reported [14]. Autosomal recessive mutations in the DOCK8 (dedicator of cytokinesis 8) gene, cause a combined immunodeficiency syndrome characterized by low T, B and NK cells, elevated serum immunoglobulin E (IgE) levels, depressed IgM levels, eosinophilia, sinopulmonary infections, cutaneous viral infections (HPV, herpes simplex virus and molluscum contagiosum) and malignancy, particularly SCC [15]. Mucocutaneous SCCs occur in 19%, with a young age of onset and are often associated with viral warts. Aggressive CTCL, diffuse large B-cell lymphoma and cutaneous microcystic adnexal carcinoma have also been reported [15, 16].
Common variable immunodeficiency (CVID) is the most prevalent of the primary immunodeficiencies in adults [17]. CVID encompasses a group of genetic disorders characterized by failure of B-cell maturation; the principle defect is in antibody formation, but there are related defects of both humoral and cell-mediated immune responses. It is characterized by hypogammaglobulinaemia, recurrent bacterial infections, autoimmune diseases and malignancy. Particularly lymphoma and gastric cancer, with rare reports of cSCC [18, 19].
Wiskott–Aldrich syndrome is a severe X-linked immunodeficiency caused by mutations in the WASP gene, which encodes key regulators of signalling in haematopoietic cells. Clinical manifestations include immunodeficiency, eczema, autoimmunity and tumour susceptibility, including head and neck SCC and Kaposi sarcoma (KS) [20].
Dyskeratosis congenita is an inherited bone marrow failure syndrome caused by abnormal telomere maintenance resulting from germline mutations in one of nine genes present in approximately 60% of patients. Nail dystrophy, abnormal skin pigmentation and oral leukoplakia are characteristic and patients are at high risk of bone marrow failure, pulmonary fibrosis, liver disease and mucocutaneous SCC [21].
Netherton syndrome due to SPINK5 mutation is an autosomal recessive disorder characterized by congenital ichthyosiform erythroderma, trichorrhexis invaginata, atopy, food allergies and asthma [11]. HPV infection with β-HPV types also occurs and has been associated with keratinocyte skin cancers (KC) in some cases [11, 22].
Acquired immunodeficiency
The total burden of skin cancer associated with acquired immunodeficiency is far greater than that of primary disorders and is predicted to represent an increasingly significant proportion of total skin cancer burden in the future [23]. Although there is no doubting this risk, the exact magnitude has been difficult to quantify because accurate data in the general population on incidence rates of many skin cancers, particularly with non-melanoma skin cancers (NMSC), are incomplete compared with most other tumour types [24] (Table 146.2).
Table 146.2 Reported ranges of skin cancer risk in immunodeficiencies
| Skin cancer | OTR | NHL/CLL | HIV | IBD | RA |
| SCC | 65–480 | 5–8 | 2.6–4 | 1.23–5.4 | 1.5–1.72 |
| BCC | 4.5–10 | 5–8 | 2.1 | 1.2 | – |
| Melanoma | 1.35–4 | 1.92–7.74 | 0.81–12.6 | 1.09 | 1.16–2.3 |
| Kaposi sarcoma | 40–208 | 3.37–5 | 449–3640 | 2.89 | 2.52 |
| Merkel cell carcinoma | 24–182 | 3.4–10.4 | 11.95–13.4 | 4.02 | 1.39–2.42 |
| Appendageal | 20–100 | – | 3.26–8.1 | – | – |
| Vulva/vagina | 6.9–23.91 | – | 4.41–6.79 | – | – |
| Penis | 4.5–25 | – | 3.9–8 | – | – |
| Anal | 2.7–14 | 2.44 | 19.63–50 | – | – |
| Oral cavity | 2.75–7.5 | 0.9–2.33 | 1.1–2.93 | – | – |
The figures shown are the risk ranges cited in publications which include: Harwood et al. 2013 [50]; Brewer et al. 2013 [33]; Deeken et al. 2012 [27]; Long et al. 2010 [106]; Grulich et al. 2007 [25]; Mercer et al. 2012 [123]; Krynitz et al. 2013 [47]; Jensen et al. 2010 [79]; Lanoy et al. 2009 [26]; Hisada et al. 2001 [43]; Royle et al. 2011 [36]; Shiels et al. 2012 [397]; Clarke et al. 2015 [375]; Hemminki et al. 2012 [373]; Olsen et al. 2014 [518]; Morton et al. 2010 [35]; Mansfield et al. 2014 [399]; Singh et al. 2011 [110]; Crum-Cianflone et al. 2009 [31]; Silverberg et al. 2013 [32].
BCC, basal cell carcinoma; IBD, inflammatory bowel disease; NHL/CLL, non-Hodgkin lymphoma/chronic lymphocytic leukaemia; OTR, organ transplant recipient; RA, rheumatoid arthritis; SCC, squamous cell carcinoma.
HIV infection (see Chapter 31)
HIV/AIDS is associated with a 1.5–2-fold elevated risk of malignancy [25–27]. KS is increased more than 3000-fold compared to the immunocompetent population and, together with NHL and cervical cancer, is an AIDS-defining malignancy [27]. Since the introduction of highly active antiretroviral therapy (HAART), the incidence of AIDS-defining malignancies has fallen threefold and that of non-AIDS-defining malignancies has risen threefold; cSCC/BCC and melanoma are among the most common non-AIDS-defining malignancies, with reported standardized incidence ratios (SIR) of 2.1–4 and 0.81–12.6, respectively [7, 28–30] and BCC is now more common than KS in white skin [31]. In contrast to organ transplant recipients (OTRs) in whom the BCC : cSCC ratio of approximately 4 : 1 in the general population is reversed, this ratio is maintained in HIV with CD4 counts >500 cells/μL, but cSCC predominate when CD4 falls below 200 cells/μL [32]. cSCC arising in the context of an EV-like phenotype has been associated with the immune reconstitution syndrome [9]. Unlike AIDS-defining malignancies, duration of HIV infection rather than degree of immunosuppression, as measured by CD4 count, is the main risk factor for skin cancer [27, 31]. HIV may have direct cellular and molecular effects that contribute to the development of skin cancer, including activation of proto-oncogenes, alterations in cell cycle regulation, inhibition of tumour suppressor genes, induction of microsatellite gene instability and promotion of pro-angiogenesis signaling [7]. HIV-infected patients also have an increased risk of exposure and subsequent infection with other skin cancer associated viruses, including HHV-8 and Epstein–Barr virus (EBV) [25].
Non-Hodgkin lymphoma/chronic lymphocytic leukaemia
NHL is an increasingly common lymphoproliferative malignancy and includes CLL, a clonal B-cell disorder, which accounts for 25% of all leukaemias [33]. These malignancies are associated with innate immunosuppression and defects in both cell and humoral-mediated immune responses, which may be exacerbated by therapy [33, 34]. The overall risk for second malignancies in patients with NHL/CLL is more than doubled compared with the general population [35, 36] and a fivefold to eightfold increase risk of skin cancer has been documented [33, 37], with higher risk in CLL compared with non-CLL NHL [38]. This risk association is reciprocal: the increased risk of subsequent NHL/CLL in patients with melanoma is increased up to 2.7-fold [28] and a similar increased risk for CLL occurs in patients with previous cSCC (SIR 2.3; 95% CI 1.9–2.7) [39]. In CLL, the cumulative skin cancer incidence by 20 years in a US cohort was 43.2% for cSCC and 30.6% for BCC [38]. These skin cancers also behave more aggressively [40, 41, 45]. Mortality from skin cancer was as high as from CLL in one recent study and more advanced CLL stage was predictive of worse skin cancer prognosis [42]. This is particularly evident in Australia where death from skin cancer had the highest standardized mortality ratio (SMR) of all causes in patients with CLL [36]. KS is fivefold more common [43] and melanoma approximately three- to sixfold more common [43, 44]; the melanoma SMR for patients with CLL is 2.8 in the US rising to almost 5 in Australia, where the overall SMR for KC is 17 [36]. Patients with CLL who develop Merkel cell carcinona (MCC) are almost four times more likely to develop metastases [44].
Immunosuppressive drug therapy
Skin cancer is a well-recognized complication of immunosuppressive drug treatment. Solid-organ transplant recipients, in whom long-term immunosuppressive therapy is required to prevent allograft rejection, represent the largest and most comprehensively studied group. However, the risk after allogenic haematopoetic cell transplantation, in which immunosuppressive drugs are used to prevent graft-versus-host disease (GVHD), and that associated with immunosuppression for immune-mediated inflammatory disorders has been the focus of more recent research.
Solid-organ transplantation
Organ transplantation is a highly successful treatment for end-stage organ failure, with more than 114 000 organ transplants perfomed worldwide in 2012 45. Survival continues to steadily increase, as does the risk of malignancy [25]. Overall risk for any cancer is twofold to sixfold greater than that of the general population [25, 46–49] with a disproportionate increase in four tumour types: KC, post-transplant lymphoproliferative disorders (PTLD), anogenital malignancy and KS, with smaller but significant increases in hepatocellular and renal cancers and some sarcomas [46].
Spectrum of skin cancers post-transplant KC accounts for more than 95% of all post-transplant skin cancer; cSCCs predominate with an overall SIR ranging from 45 to 480 [47, 50, 51] (see Table 146.2) which is significantly greater for those under 50 years [23, 50] and increases with time post-transplant, reaching more than 200 and 300 in renal and cardiac OTRs, respectively, at 10–20 years post-transplant [47]. BCC are the second most common skin cancer and incidence is approximately fivefold to 10-fold increased, with consequent reversal in the 3–4 : 1 BCC : cSCC ratio usually seen in the general population. Region-specific differences in the frequency of post-transplant BCC alter the extent of this reversal, with lower SCC : BCC ratios reported in Spain and Italy in some studies [52–58], but not all [59]. The BCC : cSCC ratio is also influenced by time from transplantation, since BCC show a more linear increase compared with the exponential rise in SCC [57, 60, 61]. Up to 50% of OTR with cSCC also have BCC [62] and a threefold increased risk of developing internal malignancies [63]. Melanoma incidence is increased 2.1–8-fold [28, 47], KS 40–200-fold [47], appendageal tumours 20–100-fold [47, 395] and MCC up to 60-fold [50, 54, 62, 377, 384], cutaneous lymphoma (PCL) and sarcomas such as dermatofibrosarcoma protuberans (DFSP) and atypical fibroxanthoma (AFX) are also overrepresented, although population-based studies quantifying this risk are few [64, 66, 387, 403].
Tumour burden and accrual The high incidence of skin cancers in OTRs is compounded by their multiplicity, which increases with duration post-transplantation [50, 68–71]. In a UK OTR cohort, almost 30% of all OTRs who had been transplanted longer than 6 months (median 10 years) had developed skin cancer, rising from 10% at 5 years to almost 75% at 30 years. Two-thirds of affected individuals had more than one skin cancer, with an average of six tumours per patient (2–106); a minority of OTRs contributed disproportionately to the total cohort tumour burden, with more than 50% of the total number of cSCCs arising in just 3.4% of individuals [50]. Once the first KC has developed, more than s30% will develop a further KC by 1 year and almost 75% by 5 years [50, 69–71] compared with 14.5% and 40.7%, respectively, in the general population [72]. In an Australian cohort, OTRs developed an average of 3.35 ± 4.29 tumours per year at 20 years post-transplant [73]. In a UK cohort, the time interval between subsequent KC shortened progressively from 24 months to second cancer, 14.7 and 8.4 months to third and fourth, respectively; patients with 10 or more tumours developed a new cancer every 3 months and were at increased risk for metastastic disease. Time to first cancer was reduced from 104.9 to 71 months if there was a history of a pre-transplant KC [50]. The impact of skin cancers on measurements of quality of life in OTRs is unclear: in one US study, the number of skin cancers correlated with higher levels of anxiety although this did not quite reach significance [74], whilst in a cohort from Ireland, skin cancer impacted less on quality of life than certain benign dermatoses associated with transplantation [75].
Geographical or ethnic influences Comparison between studies from different geographical regions is complicated by the diversity in population characteristics, notably skin phototype, as well as environmental factors, including latitude and level of sun exposure [76]. White people living in Australia have the highest incidence of post-transplant KC, affecting more than 80% of those transplanted for 20 years [73, 77, 78], although the SIR may be equivalent in more temperate countries because of their relatively low frequency in the general population [68, 79]. KS is most common in HHV-8 endemic areas such as the Mediterranean and is, for example, more common in the south compared with the north of Italy, reflecting HHV-8 prevalence [49]. It is even more common in sub-Saharan Africa [80] and is the most common post-transplant malignancy in Saudi Arabia [81, 82]. In South Africa, KC was seen only in patients of European origin [80] whereas KS was the most common cancer in non-whites [83]. Japan and Taiwan report a low incidence of KC and KS [84, 85], and although also low in Korea, incidence of KC is still significantly greater than in the general population [86].
Paediatric transplantation The spectum of malignancies developing after paediatric organ transplantation differs to that seen in adult OTR populations; skin cancer is rare [87] but can develop in early adulthood [88]. KC is the most frequent malignancy following renal transplantation and in other organ transplants is the second most common after PTLD [89]. Melanoma is proportionately more common in paediatric OTRs and accounts for 15% of all skin cancers [28].
Type of solid-organ transplant In a population-based study of more than 10 000 OTRs over 20 years, cSCC risk appears to be greatest after cardiac and/or lung transplantation, followed by renal transplantation with risk lowest in liver transplant recipients [47]. Most, although not all, other studies also confirm the risk to be significantly lower in liver compared with renal transplant recipients [60, 90, 91]. Incidence is particularly high after simultaneous pancreas and kidney transplants, with cSCC reported to be 6.2-fold higher than age- and sex-matched renal transplant recipients [92]. However, the number of transplantations does not appear to increase risk [93]. The reasons for the differences according to organ type are not entirely clear, but may relate in part to intensity of immunosuppression [92].
Cause of end-stage organ disease End-stage organ failure itself is associated with a small increased risk of cancer: in one study, SIR for malignancy was 3.27 after renal transplant, 1.35 during dialysis and 1.16 before renal replacement therapy [46]. A Danish registry study also reported an SIR of 4.8 for cSCC among patients with renal failure, but not for cardiac, lung or liver failure [79]. There is some evidence that the cause of end-stage renal disease may have an impact on skin cancer risk with, for example, polycystic kidney disease conferring a higher risk than diabetic renal disease [94], but no association was identified with causes for end-stage liver disease in a French series [60]. Kidney and liver transplantation for HIV-related organ failure may be predicted to significantly increase risk, but early evidence indicates that rates of KS and skin cancer are relatively low, although HPV-related anal neoplasia may be at an increased risk of progression [95]. Pre-transplant immunosuppression did not increase risk of melanoma or KS in an Australian study, although KC was not included in this analysis [96].
Haematopoetic cell transplantation
Survival after haematopoietic cell transplantation (HCT) for haematological malignancy has increased steadily over the past two decades and secondary solid cancers, including skin cancers are an increasingly important late complication of both conventional myeloablative and non-myeloablative transplants [97–103]. Cumulative incidence estimates in one large study for BCC and SCC at 20 years were 6.5% and 3.4%, respectively [101], and skin cancers occur in both adult and paediatric populations [104].
Immune-mediated inflammatory disorders
Inflammatory bowel disease (IBD: Crohn disease and ulcerative colitis), rheumatoid arthritis (RA), psoriasis and systemic lupus erythematosus are associated with an increased risk of skin cancer [105]. Whilst this may be partly due to intrinsic immune dysregulation, most studies have focused on the role of iatrogenic immunosuppression, principally non-biological immunomodulatory drugs (e.g. azathioprine, ciclosporin) and biological response modifiers (BRMs), particularly the anti-tumour necrosis factor (TNF) agents (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab).
In IBD, several observational studies document an increased risk of skin cancer. A large retrospective, nested case–control study of KC in patients with IBD showed a 60% excess risk compared with controls; thiopurine use was associated with an adjusted odds ratio (OR) of 3.56, rising to 4.27 for use for more than 1 year and an OR of 2.18 for persistent BRM use in Crohn disease [106]. A meta-analysis also confirmed an increased risk of KC with thiopurine use in IBD, with a hazards ratio (HR) of 2.28 [107] and a retrospective cohort study of more than 14 000 patients with IBD showed a similar HR of 2.1 for KC with thiopurine use, but no increase for melanoma; KC risk increased with duration of thiopurine exposure and returned to pre-exposure levels when thiopurines were stopped [108]. A prospective observational cohort study in France confirmed this increased risk with HR 5.9 for ongoing thiopurine exposure, which was almost doubled for patients >65 years of age compared with those <65 years of age, but in this study the increased risk continued even after stopping thiopurines [109]. A smaller cohort study showed an equally high risk of cSCC with thiopurines (HR 5.4), with an increased risk of BCC (HR 1.2) in those not on thiopurines [110, 111]. A study from South Africa found the KC risk was highest in white patients [112]. Not all studies confirm an association and the smaller risk of KC in patients with IBD compared with OTRs probably reflects the use of intermittent monotherapy in IBD, in contrast to the two- or three-drug regimens used to prevent graft rejection [113]. In an observational study, IBD was also associated with an excess risk of melanoma with BRM therapy, particularly in Crohn disease, with an OR of 1.88 [114]. In contrast, a meta-analysis of 22 randomized controlled trials (RCTs) failed to provide conclusive evidence of increased skin cancer risk with BRMs in IBD, although as these trials did not extend beyond 12 months, a longer term risk cannot be excluded [115].
In RA, observational cohort studies have also shown an increased risk of cSCC in patients treated with azathioprine for more than 1 year [116] and a significant increase in both KC and melanoma (ORs, 1.5 and 2.3, respectively) with BRM use [117], but no increased risk of BRMs over other therapies [118]. However, systematic reviews and meta-analyses of skin cancer risk associated with BRMs have provided conflicting results. A twofold increased risk of KC was identified with adalimumab, infliximab and etanercept [119]; a similar excess risk for KC (OR, 1.45) and melanoma (OR1.79) was also found in a separate study [120]; a trend for increased KC was identified in another meta-analysis [121]; but no increased risk was seen with the newer agents certolizumab and golimimab [122]. Data from the British Society of Rheumatologists Biologics Register identified an increased risk of KC of approximately 1.72 and 1.83 in patients, respectively, on disease-modifying antirheumatic drugs and BRMs compared with the general population, but no evidence of differing excess risk between these drug groups [123]; an observation confirmed in another meta-analysis of 63 RCTs with 9 BRMs [124]. Similarly, no statistically significant association with anti-TNF therapy was identified in a meta-analysis of psoriasis trials [125]. It is possible that discrepancies between studies may be due to methodological differences and duration of follow-up is likely to be an important confounding factor; the lack of statistical association in some of these large trials contrast with the case reports and cohort studies in which rapid development of KC and recurrence of melanoma are described [126].
Pathophysiology
The pathogenesis of skin cancer arising in the context of immunosuppression is likely to be multifactorial and current evidence suggests a complex interplay primarily between UV radiation (UVR), altered immune surveillance, drugs and oncogenic viruses, with likely additional roles for host genetic susceptibility factors, chronic inflammation and donor-derived cells.
UV radiation and genetic changes
As in the general population, UVR is an important risk factor (see Chapter 9); most immunosuppression-associated KC is more prevalent in regions of high ambient solar radiation, 75% occur on photoexposed body sites, are more common in those with fair skin phototype and a history of chronic UV exposure and, in particular, childhood sunburn [127, 128, 129]. UVR increases skin cancer risk by local reduction of tumour immune surveillance and is mutagenic; targeted gene sequencing of p53 in OTR SCCs, PTCH in OTR BCCs and whole exome sequencing studies of cSCC from OTRs have shown a high prevalence of characteristic UV-induced mutations [130–132]. Although there are no clear differences in the genetic changes present in tumours from immunocompromised individuals compared with the general population [133, 134], some of the drugs used in these patients, e.g. ciclosporin, azathioprine and voriconazole, may also interact with UVR and directly or indirectly enhance its carcinogenic effects, as may HPV (see below).
Reduced tumour immune surveillance
The parallels between malignancy in OTRs and patients with HIV/AIDS support the likely importance of immunosuppression and reduced tumour immune surveillance or ‘immunoediting’ per se as an important contributory factor to the increased skin cancer risk [25, 126, 135]. In general, the incidence of OTR KC is proportional to the level of immunosuppression [136, 137] and associated with lower peripheral CD4 counts [138]. Intensity of immunosuppression also influences risk of post-transplant melanoma and KS [46, 139]. Reduction of immunosuppression in OTRs reduces the rate of subsequent accrual of skin cancers [140, 141], particularly those such as KS, which are virus related [142]. Synergy between viral oncogenesis and immune dysregulation, e.g. by enhanced viral replication or integration, may provide additional or alternative mechanisms in immunosuppression-related skin carcinogenesis [143]. The immunophenotype also differs in OTR cSCC, with higher numbers of circulating regulatory T cells predictive for new cSCC development [144]. In HIV, the risk of KS but not KC was proportionate to the absolute CD4 count in one study [31], although the risk of cSCC but not BCC may increase with lower CD4 counts [32]. NHL/CLL is associated with innate immune dysregulation involving complex defects of both humoral and cell-mediated immunity, which, independent of treatment-related risk factors, may be sufficient to account for the increased skin cancer risk [33, 38].
In addition to systemic immune dysregulation, the local tumour microenvironment also plays a critical role in carcinogenesis [145] and there is evidence for a unique immune microenvironment in OTR cSCC. The density of inflammatory infiltrate appears to be reduced [146, 147] and the combination of reduced CD4+ T-cell infiltration [148], decreased cytotoxic CD8+ T cells [144, 147, 149] and increased regulatory T cells described in some [144, 149] but not all [147, 148] studies, is predicted to lead to a ‘permissive’ tumour microenvironment with decreased immune surveillance. In addition, impaired antigen presentation though reduced CD123+ plasmacytoid dendritic cells [147] and increased exposure to IL-22, may accelerate tumour growth [149] and potentially contribute to the aggressive nature of some OTR cSCC.
Drugs
Non-biological immunosuppressive drugs
Current immunosuppressive drug regimens usually use a combination of agents with differing modes of action at specific sites of the T-cell activation cascade [150]. In transplantation, immunosuppressive protocols consist of two phases: a perioperative induction phase (using for example OKT3, antithymocyte globulin, basiliximab, daclizumab) is followed by a long-term maintenance phase (e.g. ciclosporin, tacrolimus, azathioprine, mycophenolate mofetil (MMF), sirolimus, everolimus) [51]. Characteristics of the immunosuppressive regimen including duration, use of induction therapy and type of maintenance therapy may all be important risk factors for skin cancer, but establishing the degree of risk conferred by individual drugs is challenging, given the large number of potential confounding factors, including variations in individual dosage and regimens depending on the type of transplant and tolerability [50, 150, 151]. There is some evidence that azathioprine may pose an increased risk over ciclosporin and corticosteroids [152, 153], but this has not been confirmed in all studies [58, 154]. Similarly, some studies [59, 155–157] but not all [158] have shown a reduced risk with MMF compared with azathioprine. MMF, however, has been linked to BCC risk in cardiac transplant recipients [159]. Tacrolimus has a relative protective effect compared with ciclosporin in some studies [58] but not all [59, 155, 158]. There is clear evidence, however, that mammalian target of rapamycin (mTOR) inhibitors (rapamycin/sirolimus, everolimus) confer reduced skin cancer risk [160] and this is discussed in more detail later.
The overall level of immune suppression may be more important than the effects of specific drugs. Both duration and dose intensity of immunosuppressive drug therapy appear to be relevant: triple versus dual versus monotherapy and higher versus lower dose ciclosporin regimens are associated with increased risk [136, 137, 161, 162], whereas less intensive immunosuppression in liver transplant recipients may account for their significantly lower rates of skin cancer compared with other OTRs [91]. Similarly, the lower skin cancer risk in HCT recipients reflects the generally shorter duration of immunosuppressive drug use post-transplant compared with OTRs [163]. However, even prolonged use of single agent oral corticosteroids and azathioprine is associated with a twofold to fourfold increased risk of KC [30, 106, 164, 165].
In addition to induction of decreased immunosurveillance, certain immunosuppressants have direct effects on carcinogenesis and tumour progression [150]. Thiopurines (e.g. azathioprine) and calcineurin inhibitors (CNIs, e.g. ciclosporin, tacrolimus) demonstrate synergistic interactions with UVB and UVA, which may, for example, promote UV-induced DNA damage and/or inhibit DNA repair. In contrast, mTOR inhibitors have direct anticarcinogenic properties including suppression of angiogenesis, autophagy-mediated DNA repair and promotion of memory T-cell function [150, 166–171]. Examples of direct pro- and anticarcinogenic mechanisms are summarized in Table 146.3 [150, 166, 169, 172–197].
Table 146.3 Direct pro- and antitumour effects of immunosuppressive drugs
| Drug | Mechanism | Reference |
| Ciclosporin | Reduced repair of UV-induced DNA damage | Heman et al. [172] Sugie et al. [173] Yarosh et al. [174] Thoms et al. [175] |
| Increased TGF-β production | Hojo et al. [176] Maluccio et al. [177] |
|
| Enhanced UVB-induced inflammation and angiogenesis | Duncan et al. [178] | |
| Induction of oncogene ATF3 and suppression of p53-dependent senescence | Wu et al. [179] | |
| Reduced apoptotic response to UV by MPTP inhibition | Norman et al. [180] | |
| Activation of AKT by PTEN suppression | Han et al. [181] | |
| Activation of TAK1/TAB1 signalling | Xu et al. [182] | |
| Augmented EMT by TGF-β1 signalling | Walsh et al. [183] | |
| Reduced NER by downregulation of XPA and XPG | Kuschal et al. [184] | |
| Potentiation of oncogenic ATF3 by UVA | Dziunycz et al. [185] | |
| Azathioprine | Reduced repair of UV DNA damage | Kelly et al. [186] de Graaf et al. [187] |
| Incorporation of metabolite 6-thioguanine into DNA and generation of mutagenic oxidative DNA damage with UVA | O'Donovan et al. [188] | |
| Photosensitizes skin to UVA in vivo | Perrett et al. [189] Hofbauer [190] |
|
| Protein oxidation and damage to the DNA repair proteome by 6-thioguanine and UVA with impaired NER | Gueranger et al. [191] | |
| mTOR inhibitors | Inhibits rather than promotes cancer | Campistol et al. [192] Kauffman et al. [193] Mathew et al. [194] |
| Suppression of angiogenesis by reduction of VEGF | Guba et al. [166] | |
| Antiproliferative | Aissat et al. [195] | |
| Increased autophagy-mediated DNA repair | Saha et al. [196] | |
| Increased AKT1 and reduced AKT2 | Sully [169] | |
| Reduced EGFR expression | Liu et al. [197] | |
| Promotion of memory T-cell function | Jung et al. [150] |
AKT, Ak strain transforming; ATF3, activating transcription factor 3; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; MPTP, mitochondrial permeability transition pore; NER, nucleotide excision repair; PTEN, phosphatase and tensin homologue; TAK1/TAB1, transforming growth factor β-activated kinase 1/TAK1 binding protein 1; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; NER XPA/XPG, nuclelotide excision repair genes xeroderma pigmentosa-A/-G.
Biological response modifiers
The effects of BRMs, including anti-TNF agents, are discussed earlier.
Antiretroviral drugs
There are conflicting data on whether antiretroviral drugs affect the risk of non-AIDS-defining cancers, including skin cancers [198], with no clear patterns emerging [27].
Chemotherapeutic drugs
Data are conflicting on whether chemotherapy for CLL/NHL affects the incidence of skin cancers [38]. Although it has been suggested in CLL that cytotoxic chemotherapy contributes to skin cancer risk in CLL [199], more recent studies have shown that chemotherapeutic regimens probably do not influence the development of secondary malignancy, indicating a relationship with skin cancer that is unlikely to be primarily iatrogenic [35, 200, 201].
Other drugs
Certain drugs commonly used in immunocompromised individuals may also affect skin cancer risk. Voriconazole is a triazole antifungal often used in the treatment and prophylaxis of invasive fungal infections such as aspergillosis in solid-organ (particularly lung) and HCT recipients. There are numerous case reports and series of its association with cSCC, which may be multiple and aggressive [202, 203]. Retrospective studies have identifed it as an independent risk factor for cSCC in lung transplant recipients [204–207]. In a French series of 19 cases, the majority of patients affected were immunosuppressed and cSCC developed after a mean of 35 months [208]. A multistep photo-induced process was observed in most cases, with acute phototoxicity in the first year, actinic keratosis (AK) in the second/third year and cSCC by the third year onwards, suggesting that voriconazole phototoxicity, possibly enhanced by immunodeficiency, was responsible for a carcinogenic effect [203, 208]. Statins have immunomodulatory effects, which have led to concerns that they may increase the risk of KC [209]. However, no effect on risk was identified in a recent meta-analysis [210]. Thiazide diuretics were associated with a modestly increased risk of KC in a population-based case–control study [211]. Non-steroidal anti-inflammatory drugs have been proposed to have a possible protective effect against skin cancer, confirmed in one recent systematic review [212], but not in a large meta-analysis [213].
Oncogenic viruses
The most common immunosuppression-associated malignancies are those due to known or suspected oncogenic viruses [25]. In the skin, these include KS (HHV-8) (see Chapter 139), post-transplant lymphoproliferative disorders (EBV) and, most recently, Merkel cell carcinoma (Merkel cell polyomavirus, MCPyV) (see Chapter 145) [252]. These pathogens and their multiple mechanistic pathways of viral oncogenesis are discussed in more detail elsewhere, but these mechanisms may be further complicated in the setting of immuncompromise, for example by interactions with immunosuppressive drugs [143]. This section focuses on the role played by HPV in immunosuppression-related skin malignancy (see Chapter 25).
HPV has long been proposed to contribute to the pathogenesis of cSCC, but its role remains controversial [215, 216]. HPV is a double-stranded DNA virus and more than 170 types are recognized [216, 217]. High-risk mucosal HPV types (principally α-HPV types 16, 18) are the main carcinogens responsible for anogenital SCC [218] and recognition of this has culminated in preventative vaccination against HPV-16 and -18 [219]. α-HPVs also cause a proportion of head and neck SCC (HNSCC) [220] and periungual SCC [221]. Anogenital dysplasia, head and neck SCC and periungual SCC are all more frequent in long-term immunosuppressed patients. In EV, β-HPVs are detected in over 90% of cSCC and appear to act as co-carcinogens with UVR [3]. In contrast to high-risk α-HPVs, β-HPVs rarely integrate into the host genome and most β E6 and E7 proteins do not target p53 or Rb [222]. However, research by many groups has provided evidence of alternative mechanisms by which β-HPV types in the skin may have pro-carcinogenic effects in cooperation with UVR (Table 146.4) [223–247]. In particular, functional studies have shown that specific β-HPV oncoproteins abrogate UV-induced apoptosis, delay DNA repair and overcome cell cycle arrest [248], interfere with NOTCH tumour suppression [242], enhance dermal invasion [241] and β-HPV types 8 and 38 E2, E6 and E7 proteins are oncogenic in transgenic mice [245–247].
Table 146.4 Possible roles for β papillomaviruses in skin carcinogenesis
| Mechanism | Cellular target | β papillomavirus type |
| Abrogation of cell cycle check points | pRB p16 p53 p53, pRb |
HPV-38 [223] HPV-5, -8 [224] HPV-38 [225] HPV-49 [226] |
| Prolong cell lifespan | Telomerase | MHPV-38 [227] |
| Inhibition of DNA repair | XRCC1 Unknown P300, ATR |
HPV-8 [228] HPV-5 [229] HPV-5, -8 [230] |
| Genome destabilization (aberrant mitosis and dysregulated centrosome duplication) | P300, p53 | HPV-5, -8, -38 [231] |
| Inhibition of apoptosis | Bak | HPV-5, -8, -20, -38, -76, -92, -96 [232, 233] HPV-8, -20 [234] |
| Bax | HPV-5, -8, -38 [235, 236] | |
| P300, p53 | HPV-38 [237] HPV-8 [238] |
|
NF-kB TIP60 HIPK2, p53 |
HPV-23 [239] | |
| Evasion of host immune surveillance | TAP-1 IL-8 |
HPV-8 [240] HPV-5, -8 [241] |
| Interfere with NOTCH tumour suppression | NOTCH signalling in epidermis and mesenchyme | β papillomaviruses [242] |
| Repression of TGF-β signalling pathway | SMAD3 | HPV-5 [243] |
| Enhanced dermal invasion | MMP-1, MMP-8, MT-1-MMP | HPV-8 E7 protein [244] |
| Oncogenic in transgenic mice | – | HPV-8 E2, E6 and E7 proteins expressed from a keratin promoter [245–247] |
HPV, human papillomavirus; IL, interleukin; MMP: matrix metalloproteinase; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; TGF, transforming growth factor.
A role for HPV in non-EV immunosuppression-related cSCC is plausible, given the association with oncogenic viruses of the most overrepresented malignancies in immunosuppressed individuals, the widespread cutaneous HPV infection seen in many immunodeficiencies and the HPV-related histological features in some cSCCs [146]. However, an oncogenic role for HPV remains unproven [215, 216, 249–253]. More than 100 studies have investigated the epidemiological relationship between HPV and cSCC, particularly in OTRs, and most show a significant correlation between the presence of the virus and cSCC development. However, interpreting this literature is complicated by the heterogeneity of studies in terms of patient populations, sampling and HPV detection methods (which vary markedly in sensitivity and ability to detect the large number and diversity of potentially relevant HPV types) [215, 216, 249–253]. In addition, β-HPVs are ubiquitous in normal skin, with a probable reservoir in hair follicles; individuals are colonized with a specific and persistent profile of multiple β-HPV types early in life [254] and β-HPV seroprevalence increases with age [255] but not UV exposure [256]. Against this background, epidemiological studies have confirmed the presence of β-HPV DNA in >90% of OTR SCC, a higher prevalence than in immunocompetent tumours and significantly higher than in normal skin [257]. In situ hybridization has also identified viral gene expression in tumours [258] although viral load is usually <1 viral copy per cell and higher in AK compared with cSCC [259]. There is an association between cSCC and seroprevalence for β-HPV types in both OTR and immunocompetent populations [260–262], although serology does not always correlate with HPV DNA presence in skin [263]. Concordant detection of HPV DNA in hair follicles and antibodies for the same virus type may be more relevant, with an overall OR of 1.6 for SCC in one large study [262]. In addition, a positive seroresponse to β-HPVs at the time of transplantation was predictive for subsequent KC risk, with a hazard ration of 2.9 [264]. In contrast to anogenital cancer, no clear hierarchy of specific HPV types has been defined, but there is a trend towards β-HPV types oncogenic in EV, notably HPV-5, -8, -36 and -38, which fits well with functional studies [262].
More recently, whole transcriptome sequencing has revealed low transcriptional activity of HPV in tumours, evidence that perhaps HPV is not involved in cutaneous oncogenesis and is merely a marker of immunosuppression [214, 265]. Alternatively, HPV may be acting through a ‘hit and run’ mechanism, involved in initiation rather than promotion or maintenance of oncogenesis, with HPV-induced perturbation of cellular DNA repair or apoptosis predisposing keratinocyte stem cells to UV-induced damage [215, 216, 248, 253]. Clarification of the part played by HPV may provide future directions for more targeted therapy. HPV vaccination is one such strategy and has been suggested as a possible approach to prevention of post-transplant HPV-associated epithelial malignancies, although as current vaccines protect only against mucosal HPV types, there is a less convincing rationale for their use in cSCC compared with anogenital and HNSCC [253, 266]. However, preliminary experimental data from mouse models have supported vaccination against cutaneous HPV types as a viable approach to cSCC prevention, even in the setting of immunosuppression [267, 268] although immunogenicity of HPV vaccines may be suboptimal in immunocompromised patients [269].
Host genetic predisposition
Germline single nucleotide polymorphisms (SNPs) may be associated with increased risk of skin cancer. Of those investigated in relation to immunosuppression-associated skin cancer, pigmentation genes are the most extensively studied [270]. In Norwegian OTRs, variation in the key signalling regulator MC1R but not other pigmentation-associated genes (the MC1R antagonist ASIP, and downstream melanization regulatory genes TYR and TYRP1) had a significant impact on cSCC risk, independent of conventional risk phenotypes including hair colour and skin phototype [271]. A common polymorphism of p53 results in either a proline or arginine at residue 72 of exon 4 and these polymorphic alleles have distinct biochemical and functional properties, including their ability to signal apoptosis following DNA damage. A significant association was identified between p53 codon 72 arginine homozygosity in cSCC from OTR but not immunocompetent individuals [272]. A correlation has been identified between polymorphisms in detoxifying enzymes glutathione-S-transferase [273, 274] and IL-10 polymorphisms and cSCC risk in OTR [275]. COX2 gene regulatory region variants appear to be associated with risk of OTR KC, although differ in cSCC compared with BCC [276]. Association of folate pathway-related methylenetetrahydrofolate reductase MTHFR:C677T gene polymorphism and risk of OTR cSCC has been observed [277] and MTHFR polymorphisms also associate with aberrant OTR cSCC methylation [278]. Haplotypes containing T(1686)-T(3944) alleles but not polymorphisms in the proximal 5′ regulatory region of the PTCH1 gene were shown to be associated with an increased BCC risk in Italian OTRs [279]. Vitamin D receptor (Intron8G/T, [280]), EGFR +61 A-G [281] and Toll-like receptor 4, 7 and 8 polymorphisms [280] are not associated with transplant KC. Polymorphisms in DNA repair genes have been investigated, particularly in relation to azathioprine exposure; MSH2 and MLH1 protein expression was not altered in OTR cSCCs and there was no difference in expression between cSCCs from OTRs and immunocompetent patients and no association between MSH2 polymorphism genotype frequency and OTR skin cancer status [282]. Despite these data, none of the known biomarkers are yet sufficiently robust to use as part of a skin cancer prediction algorithm in OTRs.
Ionizing radiation
Radiation therapy may contribute to skin carcinogenesis in immunocompromised individuals. Increased melanoma risk persists for more than 20 years after radiotherapy for Hodgkin lymphoma and, as not all tumours arise within the irradiated field, radiation may be having an additional systemic effect [283]. In HCT, total-body irradiation conditioning regimens increase BCC risk; those exposed to radiation at age less than 10 years show significantly greater risk than older individuals [101].
Graft-versus-host disease
Acute GVHD after HCT is an independent risk factor for cSCC and chronic GVHD increases the risk of both SCC and BCC [101–103, 163], with a RR of 5.8 in one study [103]. A large case–control analysis demonstrated that the severity and duration of chronic GVHD was a significant risk factor as was use of azathioprine, particularly in combination with ciclosporin and prednisolone [163].
Donor-derived cells
The presence of donor-derived cells has been reported as potentially pathogenic in HCT-related oral malignancies [284] and in OTR KC [285, 286] and KS [287], although the mechanisms involved remain unclear.
Clinicopathological features of specific skin cancers
Skin tumours in the setting of immunosuppression may have atypical presentations and altered clinical courses [33, 50, 128, 288].
Clinical risk factors
Epidemiological research, including prospective cohort studies, have identified clinical features predicting OTRs at greatest risk for developing skin cancer which are clinically robust, allowing stratification of patients at highest risk of future skin malignancies (Table 146.5) [50, 51, 127, 262, 289–291].
- Duration of immunosuppression is a major risk factor; 50% of OTRs overall will have developed a skin cancer by 20 years post-transplant in the UK, rising to more than 80% in Australia [50, 292]. Similarly, in CLL the cumulative incidence by 20 years was 43.2% for cSCC and 30.6% for BCC in the US [38].
- Age at transplant is a significant predictor of both time to first skin cancer and cumulative skin cancer burden; risk is increased 12-fold if transplanted >55 years compared with <34 years, with median time to diagnosis of 8, 12 and 19 years for those >55, 45–54 and 35–44 years of age at transplant, respectively [50].
- In a UK cohort, skin phototype and sunburn pre-transplant, particularly sunburn in childhood, were associated with time to first skin cancer; these factors together with male sex and chronic UV exposure were associated with cumulative skin cancer burden [50, 289].
- AKs are an important marker of skin cancer risk: cSCC risk was increased more than 30-fold by the presence of AK in one study [289]. Warts and AK may be difficult to distinguish clinically at non-palmoplantar sites and were therefore grouped as ‘keratotic lesions’ in a large multicentre European study; the association between total number of keratotic lesions and cSCC was significant with a 12-fold increased risk of cSCC for 50 or more lesions and a fourfold increase for BCC. In the same study, common palmoplantar warts were associated with an odds ratio for cSCC of 1.6, but were not associated with BCC [127]. In a French study, cSCC was associated with verrucokeratotic lesions and not common warts [290].
- The effects of other factors including smoking and alcohol are not consistently significant [127].
Table 146.5 Risk factors for skin cancer development in solid-organ transplant recipients
| Risk factors for time to first skin cancer | Risk factors for total number of skin cancers | Other patient-related risk factors | Transplant-related risk factors |
Duration of immunosuppression Age at transplant Sunburn pre-transplant Ethnicity (white versus non-white) Skin cancer pre-transplant |
Duration of immunosuppression Age at transplant Sunburn pre-transplant Chronic UV exposure Skin phototype Male Number of keratotic lesions (actinic keratoses and verrucokeratotic lesions) |
Smoking – inconsistent Alcohol – inconsistent Genetic polymorphisms CD4 count β human papillomavirus DNA/serology concordance |
Allograft type Cause of end-stage organ disease |
Based on references cited in this chapter, including the following studies: Bouwes Bavinck et al. [127], Casabonne et al. [289], Joly et al. [290], Bouwes Bavinck et al. [291], Harwood et al. [50] and Proby et al. [262].
Squamous cell carcinoma
Cutaneous SCC are more than 150-fold more common in OTRs, with lower levels of increased risk, ranging from 1.5 to 8, observed in other immunocompromised groups (see Table 146.2). They are predominantly located on UV-exposed sites [50], but are more common on non-head and neck sites in immunocompromised compared with immunocompetent individuals [146, 293]. Although diagnosis is usually made clinically, appearances may be atypical [40, 146, 294] diagnostic accuracy may be relatively low and a high index of suspicion is required [294]. Pain is a useful symptom of invasive malignancy in this context [67, 295]. Differential diagnoses include Bowen disease, AK and other rare skin tumours such as appendageal malignancies. Infections may also simulate cSCC, in particular viral warts (which may be clinically and histologically atypical), chronic herpes simplex and atypical mycobacterial infections. Keratoacanthomas (see Chapter 142) are regarded as spontaneously resolving, well-differentiated cSCC-like lesions [296]. They appear karyotypically simpler than cSCC [297, 298] and have other genetic differences [299–302]. However, clinicopathological differentiation from cSCC is not straightforward and they tend to be managed as well-differentiated cSCC in the setting of immunosuppression.
In terms of histology, differentiation status of OTR cSCC is not significantly different to immunocompetent populations in most studies [146, 303], but there are reports of increased frequency of spindle cell morphology, reduced inflammatory infiltrate, evidence of HPV infection [146], increased perineural and lymphatic invasion [303], increased acantholysis and increased depth of invasion [293]. However, these features are not consistent across all studies, and while they may account for the observed increase in local recurrence and metastasis [303], it is plausible that differences in overall prognosis may instead reflect the significantly greater overall tumour burden in individual OTRs, rather than increased aggression of most individual tumours [146]. In a retrospective study, the cumulative incidence of local recurrence was 19% for stage T1 SCC at 5 years, and 54% for stage T2 SCC at 3 years [304]. There is an estimated metastatic risk of up to 7% for cSCC in OTRs, more than twice that in the general population [50, 62, 303–305]; prognosis for metastatic disease is worse in immunocompromised individuals [306] with an overall 5-year survival of 14–39% and median 3-year survival of 56% in OTRs [307]. In transit metastases are more common in OTRs [308], with scalp representing a particularly high-risk site [309]. OTRs from countries with a high incidence of KC, such as Australia, experience greater overall morbidity and mortality compared with recipients from more temperate climates [310]. However, although the SMR for KC was almost 50 in one study of Australian liver and heart/lung transplant recipients [311], an even higher SMR for cSCC has been reported in the UK [50].
Cutaneous SCC in patients with HIV and CLL are also more aggressive; rates of local recurrence after Mohs micrographic surgery are higher than expected and may be partly the result of factors such as dense lymphocytic infiltrate obscuring tumour margins and perineural invasion [312, 313]. Locoregional recurrences are sevenfold more common than in the general population and metastases occur in 18% [33, 38, 314–316].
Actinic keratoses, Bowen disease, field carcinogenesis and porokeratosis
The true SIR of AKs and Bowen disease in immunocompromised individual OTRs is not known, although prevalence was approximately 50% in one French OTR series [317]. Whether individual dysplastic lesions progress more frequently or rapidly to cSCC compared with the general population is also unclear, although ‘field cancerization’ is a common problem in OTRs and other immunocompromised individuals [296, 318] (Figure 146.1) and refers to the presence of multiple genetic abnormalities in a tissue as a result of exposure to a carcinogen – in the case of the skin, UVR. It manifests clinically as areas of confluent AK and Bowen disease, sites at which cSCC preferentially develop [318]. In one study, cSCC risk was significantly increased in OTR with AK and field cancerization and higher than the corresponding risk of cSCC in OTR with AK but no field cancerization [319].
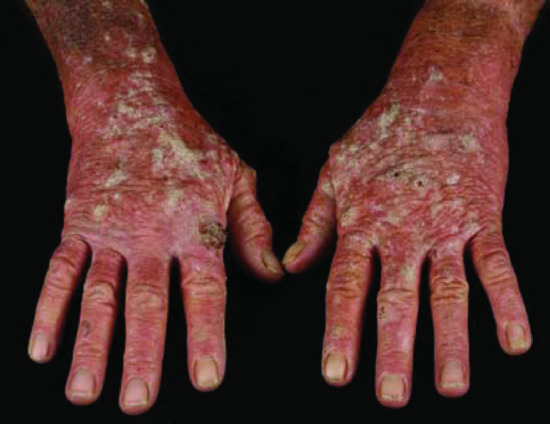
Figure 146.1 Field cancerization in a patient with Crohn disease who was taking azathioprine for many years. Confluent actinic keratoses and Bowen disease (field cancerization/field change) are present and a squamous cell carcinoma has developed on the dorsum of the right hand.
AK may be contiguous with multiple plane warts in areas such as the dorsum of the hand and forehead, and it may be difficult to distinguish viral and dysplastic lesions from each other without diagnostic biopsy (Figure 146.2). Certain histological features are more common in OTR compared with immunocompetent AK, including mitotic activity, parakeratosis and verrucous change [320].
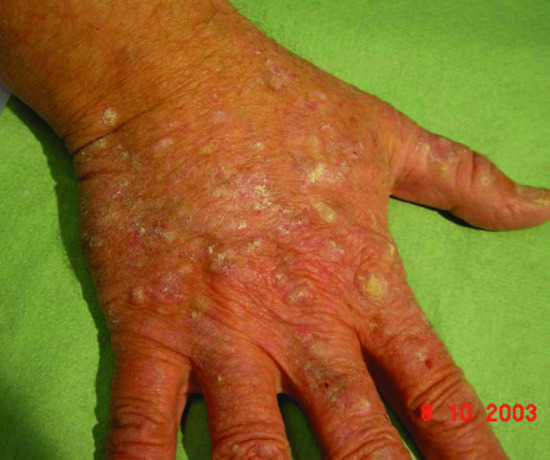
Figure 146.2 Multiple actinic keratoses, viral warts and verrucokeratotic papules on the hand on an organ transplant recipient: ‘transplant hands’.
Porokeratosis is considered to have pre-malignant potential and is well documented in association with immunosuppression [321]. Up to 10% of OTRs are affected [322, 323]. It may cause diagnostic confusion [296] and may rarely progress to cSCC, including metastatic disease [324, 325].
Basal cell carcinoma (see also Chapter 141)
BCC are up to 10-fold more common in the setting of immunocompromise. Anatomical location differs between BCC and cSCC in OTRs: in one UK cohort study, 22.2% BCC versus 8.5% cSCC were truncal whereas 5.2% BCC versus 36.3% cSCC occurred on the hands/forearms [50]. Superficial BCCs on the upper trunk are also observed more frequently in OTRs and HIV [146, 326], and whilst this may indicate pathogenetic differences, it may also be partly the result of closer surveillance of these patients compared with the general population [327]. High-risk BCC (infiltrative/morphoeic, micronodular and basosquamous BCC) do not appear to be more common in OTRs [146], but are more frequent in HIV [328]. Histologically, in addition to an overrepresentation of superficial morphology, inflammatory infiltrate is reduced, squamous differentiation is increased and HPV changes are more frequent in OTRs but tumour depth, perineural and vascular invasion are similar to control tumours [146, 326]. Common differential diagnoses include molluscum contagiosum, particularly in HIV, and sebaceous gland hyperplasia, which is more common in OTRs, often in association with CNI use [329]. Recurrence rates for BCC are significantly increased after conventional and Mohs surgery in both HIV and CLL, although this is not reported in OTRs [38, 146, 303, 315, 316, 330, 331].
Melanoma (see also Chapter 143)
Given the strong influence of the immune system on melanoma pathogenesis and progression, a high incidence of melanoma in immunocompromised individuals might be expected; although risks of up to 12 times that of the general population have been reported, most studies report SIRs of 2–5 which is significantly lower than for cSCC [28, 51, 128, 332, 333]. Melanoma arises in three clinical scenarios in OTRs: as recurrence of pre-transplant melanoma, by transmission from the organ donor and, most commonly, de novo post-transplant [28]. In a multicentre European study of 100 patients, only post-transplant melanomas >2 mm (T3 and T4) Breslow thickness had a significantly worse prognosis compared with matched American Joint Committee on Cancer melanoma database controls [334]. However, a study from North America comparing outcomes of 638 OTRs with those expected in control subjects showed worse overall survival, regardless of Breslow thickness [335], as did an Australian study [336]. Despite historic data suggesting recurrence rates approaching 20% in patients with pre-transplant melanoma [337], most recent studies have not shown a worse outcome, although numbers are small [334, 335, 338]. Melanoma is the second most common donor-derived malignancy after renal cancer [339], but the prognosis is much worse, with rapid development of metastatic disease in almost 80% and death in 66% of cases reported to date, including a melanoma resected 32 years previously [340–346]. A past history of melanoma is usually regarded, therefore, as an absolute contraindication to organ donation in most cases [342]. In HIV, melanoma is approximately 2.6 times more common [25, 29] and has a more aggressive clinical course [348]. Melanoma risk is increased approximately 2–7 times in CLL and overall survival is worse, with a SMR of more than 7 in Australia [28, 35, 36, 201, 335, 349].
Risk factors for post-transplant melanoma in OTRs appear to be similar to those in the general population [62, 334]. A particularly important risk factor may be increased numbers of melanocytic naevi. This has been observed in paediatric organ transplant recipients [350, 351] and in individuals with HIV [352]. Also reported is the entity of eruptive melanocytic naevi (EMN), which describes the rapid simultaneous appearance of multiple melanocytic naevi, often hundreds in number, on previously uninvolved sun-exposed skin. Although also reported in otherwise healthy individuals, it arises most often in association with bullous dermatoses, BRAF inhibitors, α-melanocyte-stimulating hormone (MSH) agonists and, in particular, with immunosuppression. It has been described in OTRs [353, 354], HIV [355, 356], in patients immunosuppressed with thiopurines or biological agents (infliximab, etanercept, and alefacept) for conditions such as IBD, psoriasis and myasthenia gravis [357–359] and post-chemotherapy [360]. EMN have been observed to regress upon withdrawal of immunosuppression [361]; generalized eruptive lentiginosis has also been reported in similar groups of patients, although less frequently [358, 519]. BRAF V600E gene mutations have been detected in EMN in the setting of immunosuppression [362]. However, no clear progression of EMN to melanoma has been described [363].
Kaposi sarcoma (see also Chapter 139)
KS is an AIDS-defining disease in HIV and was the most common skin malignancy in the pre-HAART era, and although its incidence has now dramatically reduced, it remains a significant problem in areas where access to HAART is limited [364, 365]. KS is also seen in the context of iatrogenic immunosuppression, particularly in patients from geographical areas of high HHV-8 seroprevalence [366]. In OTRs, it is usually due to reactivation of latent virus [367, 368], although post-transplant acquisition, for example through blood transfusion or via the donor organ (particularly after liver transplantation), is also recognized [369]. In African patients, presentation may be similar to African endemic KS with oedema of the legs (usually unilateral) preceding the appearance of typical purple/red papules, plaques and nodules on the skin. Upper gastrointestinal endoscopy and lung imaging/bronchoscopy may be required to exclude visceral disease. In one series from the UK, almost 15% of African OTRs were affected [50]. The role of HHV-8 serological screening of both recipients and donors is not yet established [370]. However, in a large prospective French series, 13% of recipients who were HHV-8 positive pre-transplant went on to develop KS, as did 3.1% of recipients who received an organ from an HHV-8 positive donor, with no differences in survival and allograft loss compared to HHV-8 negative donors and recipients [367, 369].
Merkel cell carcinoma (see also Chapter 145)
Immunocompromised individuals represent approximately 10% of all Merkel cell carcinoma (MCC) patients [371] and have an early age of onset and a more aggressive course [44, 143, 372–377], with a MCC-specific survival of 40% at 3 years compared with 74% in the general population in one large series [371]. In OTRs, the SIR is up to 60, incidence increases with age and time post-transplant, the majority of tumours are on UV-exposed sites and affected patients frequently have multiple other skin cancers [128, 375]. Epidemiological evidence from a US registry-based cohort study suggested a possible synergistic effect of ciclosporin or azathioprine with UV exposure [375] and the poorer prognosis is stage independent [378, 379]. In HIV, SIR was 11.95 in a registry linkage study from the US; the age of onset was significantly younger than in the general population and increased with age and UV exposure [380], with an average survival of 18 months [381]. Compared with healthy controls, MCPyV prevalence and viral load is increased in both OTR and HIV-infected individuals, which could partly explain the increased MCC risk [382]. It is difficult to predict how HAART will affect MCC incidence rates; prolonged life expectancy could lead to a rise in MCC incidence rates, whereas immune restoration due to HIV suppression could help to control cutaneous MCPyV replication and thus lower the risk of MCC development [382]. In a Scandanavian registry study, the SIR for MCC in NHL/CLL was 17.9 and, conversely, that for CLL after a diagnosis of MCC was 15.7 [377] and this reciprocal relationship was also observed in a cohort from Israel [383]. Prognosis for MCC appears to be worse in patients with CLL with an SMR of 3.8 in one population-based study [44]. Although MCPyV has some lymphotropism, it is unlikely that it is directly involved in CLL genesis, as has previously been speculated [384].
Primary cutaneous lymphoma (see also Chapter 140)
Systemic lymphomas are more common in immunocompromised individuals and may present with cutaneous involvement but PCLs are rare. In OTRs, primary CTCLs and cutaneous B-cell lymphomas (CBCL) without nodal or visceral disease at presentation are an uncommon manifestation of PTLD, but their incidence is increased compared with the general population [62, 385–388]. In a multicentre European study of 35 OTRs with PCL, the spectrum of disease was similar to that seen in the general population with 69% T-cell and 31% B-cell lymphomas, the majority of the latter being EBV positive [387]. This contrasts to earlier studies in which B-cell lymphomas were reported to be more common [388]. Prognosis of CD30-positive CTCL was worse than post-transplant mycosis fungoides and its counterpart in the immunocompetent population [387]. Lymphoma is the most common malignancy in HIV [365] and PCLs are also more common in HIV, often of T-cell origin and include CD30-positive anaplastic T-cell lymphoma, which may have a worse prognosis than in the general population [25, 45, 389, 390]. CTCL is rare in HIV but appears to have a conventional clinicopathological presentation. However, a non-clonal lymphoproliferative disorder closely simulating CTCL which is frequently CD8-positive but rarely progresses to true lymphoma (pseudo-CTCL or atypical cutaneous lymphoproliferative disorder) is also recognized [390–392]. PCLs in NHL/CLL are also overrepresented [35, 393].
Rare skin cancers
Skin appendage tumours (see also Chapter 138)
Tumours of eccrine, apocrine, follicular and sebaceous appendages, particularly sebaceous carcinoma and eccrine porocarcinoma, are up to 100-fold more common in OTRs [378, 394, 395] and are also reported in HIV [396, 397] and NHL/CLL [398, 399]. Sebaceous carcinoma has been linked to dysregulated DNA mismatch repair similar to that observed in Muir–Torre syndrome (MTS): this may be as a result of chronic azathioprine exposure in OTRs [395] and HIV infection has been described to exacerbate skin tumour development in known MTS [400]. Establishing the diagnosis of appendageal tumours prospectively may be challenging: these tumours often arise in individuals who have many other skin cancers and may closely simulate more common skin cancers [395].
Skin sarcomas
Sarcomas other than KS also appear to be overrepresented [401]; the true extent of the increased incidence is difficult to assess given the rarity of these tumours in the general population [378]. AFX and its deeper variant, undifferentiated pleomorphic sarcoma, have been reported in OTRs, HIV and NHL/CLL, with increased rates of recurrence and metastasis in some but not all studies [66, 113, 402–407]. DFSP has been reported in OTRs [64, 378, 408, 409]. Cutaneous leiomyosarcomas have been reported in HIV and OTRs and are frequently EBV positive [401]. Angiosarcomas also occur in both HIV and OTRs and in the latter appear to have a particular predilection for arteriovenous fistula sites [378, 401].
Management
There are few RCTs and limited non-randomized prospective and retrospective studies to guide decision-making in the management of skin cancers arising in immunocompromised individuals. In the setting of organ transplantation, efforts have been made to obtain consensus expert opinion in particularly important skin cancer management areas [141, 410, 411] and a multidisciplinary approach with close dialogue between dermatologists, transplant clinicians, oncologists, surgeons and other relevant health care professionals plays a key part in delivering comprehensive care [412–415]. Many of these same principles apply to the management of skin cancer in other immunocompromised patient groups including CLL [399] and HIV [288].
Table 146.6 Suggested management protocol for primary skin cancers in organ transplant recipients based upon published literature summarized in the text
| Stage | Therapeutic considerations | Surveillance |
| Pre-transplant | Risk assessment Education Photoprotection Treatment of pre-cancerous lesions |
|
| Post-transplant | Baseline risk assessment Education Photoprotection |
Within 6–12 months of transplantation (risk stratified by age at transplantation, skin phototype, number of sunburns – see Figure 146.3) |
| No lesions | Education, photoprotection | Surveillance according to risk levels 1–5 (see Figure 146.3) |
| AK/Bowen disease/FC | Photoprotection Lesion-directed therapy (e.g. cryotherapy, surgery) Field-directed therapy (e.g. topical 5-FU, imiquimod; diclofenac gel; ingenol mebutate gel; PDT) |
12 months |
| Early skin cancer | ||
| cSCC | Low risk: surgery (excision, curettage/cautery) Treatment of AK/Bowen disease/FC High risk: surgery (excision, Mohs micrographic surgery); consider sentinel node biopsy, adjuvant radiotherapy, revision of immunosuppression in appropriate circumstances (evidence that mTOR inhibitors more effective if introduced after first cSCC) |
4 months (see Figure 146.3) |
| BCC | Infiltrative, nodular: surgery (excision, Mohs’ micrographic surgery) Superficial: surgery (excision, curettage/cautery); non-surgical (cryotherapy; 5-FU; imiquimod; PDT) |
6 months (see Figure 146.3) |
| Moderate risk skin cancer (>3–5 KC; early KS) | Treatment of individual lesions as indicated above Rigorous treatment of AK/Bowen disease/FC Consider revision of immunosuppression (reduction or switch to mTOR inhibitor) with transplant clinicians Consider systemic retinoids |
3 months (see Figure 146.3) |
| High risk skin cancer (>10 SCC; melanoma; extensive KS; Merkel cell carcinoma; certain appendageal tumours) | Aggressive treatment of individual lesions as indicated above Strong indication to revise immunosuppression (reduction or switch to mTOR inhibitor) with transplant clinicians Strong indication for systemic retinoids |
2 months (see Figure 146.3) |
Adapted from Harwood et al. [50].
AK, actinic keratosis; BCC, basal cell carcinoma; PDT, photodynamic therapy; FC, field cancerization; KC, keratinocyte cancer; KS, Kaposi sarcoma; cSCC, cutaneous squamous cell carcinoma; 5-FU, 5-fluorouracil.
Low-risk primary tumours (Table 146.6) [50]
There is no evidence to suggest that low-risk invasive skin tumours and premalignancies (e.g. superficial and nodular BCC, Bowen disease, low-risk SCC) require significantly different management approaches to the general population although the index of suspicion for possible malignancy should be high and the threshold for biopsy correspondingly low, particularly in areas of field cancerization [128, 414, 415].
Surgery
Excision is the most appropriate option for the majority of tumours although rates of recurrence may be higher even after Mohs micrographic surgery [33, 410] and optimal excision margins have not been defined. Curettage and electrocautery may offer satisfactory clearance rates for selected low-risk tumours, including well-differentiated SCC, and may be considered in certain clinical situations for reasons of cosmesis and convenience [416].
Non-surgical modalities
Non-surgical approaches including cryotherapy, photodynamic therapy (PDT), imiquimod cream and 5-fluorouracil cream (see later) may have a therapeutic role in selected cases, particularly in patients with multiple low-risk malignancies in whom repeated surgery is otherwise required. Although response rates may be lower, e.g. for PDT [417, 418], there is no evidence that these agents carry additional significant risk.
High-risk primary tumours (see Table 146.6)
Melanoma, MCC, certain appendageal tumours and high-risk SCC and BCC are a particular management challenge in immunocompromised individuals, but there is little evidence to guide optimal treatment, which currently largely parallels that in the general population.
Staging
There is also limited evidence on the role of staging investigations such as sentinel lymph node biopsy [419, 420]. Sentinel node biopsy as a staging procedure has been reported in selected OTR with melanomas [411] and may provide prognostic information in other OTR skin cancers, including high-risk cSCC and MCC [410], although there are few data to guide selection of appropriate patients [421]. There are some data to suggest that positron emission tomography–computed tomography (PET-CT) may be promising in nodal staging of cSCC in patients with CLL [41].
Surgery
It is usually recommended that high-risk OTR tumours require ‘more aggressive’ surgery [410], but this has not been clearly defined. For example, in high-risk cSCC, optimal excision margins are not established and the circumstances in which micrographic surgery is preferable to conventional surgery have not been rigorously evaluated [410, 421]. For melanoma, there is better evidence for recommended surgical excision margins in the general population, but no specific validation in OTRs [411].
Radiotherapy
In the general population, radiotherapy may be indicated for selected inoperable primary cSCC and BCCs, positive SCC excision margins not amenable to further surgery or as adjuvant therapy for extensive perineural invasion in cSCC and for MCC, although evidence is limited [422]. Radiotherapy may have a similar role in OTRs, although is not the first option for most primary tumours given the high incidence of multiple primary tumours and the long-term risk of second skin malignancies [410, 423].
Revision of immunosuppression
Consensus expert opinion recommends reduction of immunosuppression should be considered for high-risk tumours in OTRs, but when and how this should be performed remain uncertain. The International Transplant Skin Cancer Collaborative-Skin Care in Organ Transplant Patients, Europe (ITSCC-SCOPE) collaborative has published recommendations for reductions in immunosuppression based on the type of high-risk tumour as well as the type of donor organ [141, 411]. In KS, long-term tumour remissions can be achieved by the reduction (or discontinuation) of immunosuppression [150]. In addition, conversion from CNIs to mTOR inhibitors has been reported to induce dramatic KS regression together with recovery of HHV-8-specific T cells and has been proposed as a first line intervention for patients with KS [368, 424]. However, this may not always prove successful and some patients may be refractory or progress after partial response suggesting that overall reduction in immunosuppression is important in addition to the antitumour effects of mTOR inhibitors in mediating reduction of KS [425, 426].
Locally advanced and metastatic disease
Current management for advanced and metastatic skin tumours in immunosuppression is similar to the general population in the absence of specific clinical studies, although with the additional strategy of immunosuppression reduction, conversion to mTOR inhibitors or, in some cases, complete withdrawal of immunosuppression [141, 411, 427, 428]. Thresholds for considering each approach and their sequencing have not been validated and tend to be on a case-by-case basis.
Squamous cell carcinoma
There are currently no standards of care for the management of regionally advanced and metastatic cSCC in OTRs [428, 429]. Surgery and radiotherapy approaches are currently broadly similar to those used in the general population [423, 427]. Chemotherapy in this setting has included use of systemic 5-fluorouracil (capecitabine), cisplatin, paclitaxel and retinoids, with transplant-directed dosage adjustment and close monitoring of allograft function [427, 428, 430]. Epidermal growth factor receptor (EGFR) is overexpressed and amplified in SCC; EGFR inhibitors are showing promise in metastatic head and neck SCC [431] and there are case reports of benefit from cetuximab, a chimeric human–mouse monoclonal IgG1 anti-EGFR antibody, in OTR cSCC [432], although fatal pulmonary toxicity has occurred in lung transplant recipients [433]. EGFR tyrosine kinase inhibitors such as gefitinib and erlotinib have also been used [434, 435]. Although use of these targeted therapies in immunocompromised individuals has not been specifically evaluated in clinical trials, some ongoing studies allow their recruitment, e.g. OTRs with aggressive and/or metastatic cSCC are eligible for inclusion in phase II trials of erlotinib prior to surgery or radiation and a phase II study of dasatinib, a multi-kinase inhibitor that targets BCR/Abl and Src family tyrosine kinases 436. Systemic retinoids may also play a role in the management of advanced cSCC, as may revision of immunosuppression and/or switch to mTOR inhibitors (see later).
Other advanced skin cancers
Targeted BRAF and MEK inhibitors and immunomodulatory therapy with checkpoint inhibitory anti-CTLA4 and anti-PD-1/PD-L1 agents have had a significant impact on the management of melanoma in the general population in recent years, but have not been specifically evaluated in immunocompromised individuals. The checkpoint inhibitors pose a theoretical risk of precipitating organ rejection or GVHD, but ipilimumab has been used safely to treat melanoma after solid-organ and haematopoietic cell transplantation without doing so [437–439] and has also been used in HIV [440]. Use of BRAF inhibitors requires the presence of a targetable BRAF mutation and one study has suggested that their rate in OTR melanomas is less frequent than in control lesions [441]. In terms of conventional chemotherapies for advanced skin cancers, concerns arise when combining with HAART and in OTRs, as there is the possibility of overlapping toxicities, chemotherapy effects on immune status and potential drug–drug interactions [427, 428].
Pre-transplant skin cancer
A patient with a past history of skin cancer being considered for transplantation or other iatrogenic immunosuppression is an increasingly common clinical scenario. For most, the benefits will outweigh the risks associated with further skin cancers or possible recurrence and metastasis, but decisions should be made in consultation with a transplant clinician on a case-by-case basis [94, 442]. Patients with pre-transplant KC develop their first post-transplant KC in the same time interval as between first and second cancers in those without pre-transplant KC [50]. The role of mTOR immunosuppression and systemic retinoids in delaying this onset are not established, but close surveillance is warranted [50]. A past history of primary melanoma is perhaps the most challenging situation and the evidence base to guide decision making is limited [28, 334, 335]; expert consensus has proposed that in situ melanoma should not be considered a contraindication to transplantation; for melanoma Breslow <2 mm transplantation is generally deemed an acceptable risk after 2 years and after 5 years for Breslow >2 mm [411]. In cases of metastatic KC or more advanced primary melanoma, transplantation is almost always contraindicated [411].
Prevention of skin cancer
Possible strategies to prevent immunosuppression-related skin cancer include primary prevention of de novo malignancies, preventing progression of pre-malignant lesions and preventing second and subsequent primary tumours.
Primary prevention of de novo pre-invasive and invasive malignancies
European, North American and other expert consensus guidelines recommend advice for strict photoprotection post-transplant including avoidance of sunburn, intentional tanning and unnecessary UVR exposure, especially between 1100 h and 1500 h, April to October in the UK. Sunscreens with broad spectrum, high factor UVB and UVA protection are recommended, together with use of broad-brimmed hats, sunglasses and protective clothing [410, 443, 444]. There is convincing evidence that sunscreens reduce AK, SCC and melanoma but not BCC in the general population [436, 445–447], but it is less clear whether their use post-transplant has the same effect. A prospective open non-randomized trial of liposomal sunscreen in OTRs showed significant reduction at 24 months in AK and cSCC but not BCC [448]. Results from a European RCT are awaited (www.clinicaltrials.gov; identifier NCT01532453), but these data do provide some support for the adoption of photoprotective measures, at least in OTRs at high risk for developing skin cancer. One possible adverse effect of adhering to photoprotective measures is reduction in vitamin D levels, which may already be compromised in OTRs [450]. In the liposomal sunscreen study, 25-hydroxyvitamin D levels were lower in the sunscreen group [448] and monitoring of vitamin D levels in OTRs may be advisable when rigorous photoprotection is being advocated [450].
Preventing progression of pre-malignant lesions to invasive tumours
It is assumed that AK and Bowen disease are precursors of cSCC, providing a rationale for treating AK in order to prevent cSCC, although this remains unproven. In the general population estimated rates of progression of up to 0.075% per year per lesion rising to 0.53% with a past history of skin cancer [451] 65% of SCC arise in AK [452] and 7.7 AK predict a 10% risk of transformation to cSCC within 10 years [453]. No equivalent data exist for immunocompromised individuals, although it is likely that rates of progression, particularly in areas of field cancerization, may be accelerated compared with the general population.
Cryotherapy and surgery are used as lesion-directed treatments for individual AKs/Bowen lesions. In one study, excision and grafting of areas of severe field carcinogenesis within which cSCC were developing was reported as a successful strategy for OTR cSCC prevention [454]. More often, areas of field cancerization are treated with ‘field-directed’ therapies including topical agents (5-fluorouracil cream, imiquimod cream, diclofenac gel and ingenol mebutate gel) and PDT. It is not clear how frequently such field treatments should be used to maintain clearance and whether treatment reduces cSCC risk. Field-directed treatments are often combined with lesion-directed therapies, with a low threshold for biopsy of any persistent lesions to exclude invasive malignancy [128, 415].
5-Fluorouracil (5% and 0.5%) an inhibitor of thymidylate synthetase, is cytostatic to proliferating cells. As a 5% cream it has been used widely for many years, although there are few controlled studies of its efficacy in treating AK in either the general population or OTRs [455]. It is usually applied once or twice daily for 3–4 weeks. In two studies in the general population, it achieved 70% clearance of AK at 6 months [456] and 78% clearance sustained for 12 months [457]. Lower clearance rates have been reported in OTRs [458].
Imiquimod (5% and 3.75%) is an immunomodulator belonging to the family of imidazoquinolines and is an agonist for toll-like receptor 7, activates the innate immune system and generates a Th1 cytotoxic T-cell response. RCTs in the general population demonstrated 64–84% clearance of AK [459, 460]. In a multicentre RCT recruiting 43 OTRs in six countries, complete response was observed in 62.1% and partial response in 80% [461]. In a smaller RCT of 20 OTRs, 50% clinical and 37% histological improvement were obtained and there were fewer cSCC in treated skin over 12 months, although this did not reach statistical significance [462]. Although there is a theoretical risk of immunostimulation and allograft rejection [415], limited use appears to be safe in OTRs and most clinicians limit imiquimod in OTRs to 25–50 mg/day [461, 462].
Diclofenac 3% in 2.5% hyaluronic acid gel inhibits cyclo-oxygenase-2 pathways. In an RCT of 32 OTR with multiple AK treated twice daily for 16 weeks, complete clearance was obtained in 41% but recurrent disease was noted at 24 months in 55% [448].
Ingenol mebutate is a biologically active macrocyclic diterpene ester extracted from the sap of the Euphorbia peplus plant. It has recently been licensed for treatment of AK and has the advantage of requiring only two to three applications. There are currently no clinical trials reported of its use in immunocompromised individuals.
Photodynamic therapy has been assessed in a number of studies in OTRs. In an RCT of aminolaevulinic acid (ALA) PDT with red light, 88% of OTRs responded at 4 weeks, reducing to 48% at 48 weeks, compared with 94% reducing to 72% response, respectively, in immunocompetent patients (P <0.05). This difference possibly reflects either more persistent lesions in OTRs or increased recurrence rates. Response was lowest on the hands/arms, a feature also noted with use of topical agents [463]. In a separate RCT in 17 OTRs using methyl aminolevulinate (MAL) PDT, 76% complete clearance was observed at 16 weeks [464], with similar rates observed for facial AKs in another study, but with significantly lower rates of clearance (72% versus 40%) on acral sites [465]. In a small within-patient RCT comparing MAL-PDT with topical 5-fluorouracil, PDT was found to be significantly more effective, with superior cosmesis and patient satisfaction, but also more pain [458]. Several RCTs have directly investigated PDT in cSCC prevention [417, 418, 466] including one study in OTRs [467]. Although all have shown reductions in AK, this is not always sustained and only one has shown a possible activity in cSCC prevention [466].
Prevention of subsequent primary tumours
Several approaches to secondary prevention have been reported, including revision of immunosuppression (both reduction and conversion to mTOR inhibitors) and use of systemic retinoids. However, evidence regarding the threshold at which to initiate these approaches and how to sequence them is lacking and currently undertaken on an individualized basis.
Revision of immunosuppression Most skin cancers are significantly less frequent when immunosuppression is reduced or stopped following graft failure [139], but there is limited evidence regarding when, and to what extent, immunosuppression should be reduced whilst ensuring allograft survival. An expert consensus survey reported by the ITSCC and SCOPE proposed guidelines for thresholds at which alteration of immunosuppression should be considered which take into account the number of tumours and the type of transplanted organ [141]. Possible approaches include reduction of immunosuppressive drug dosage or discontinuing of one drug in a multidrug regimen, often azathioprine [128]. In the absence of controlled trials, this is ultimately a multidisciplinary decision made on a case-by-case basis.
Conversion to mTOR inhibitors An alternative strategy for revision of immunosuppression is conversion from CNIs to mTOR inhibitors, which have both immunosuppressive and antitumour properties. Retrospective registry data analyses, observational series and post hoc analyses of immunosuppression RCTs have demonstrated a reduced incidence of malignancy in OTRs receiving mTOR inhibitors as part of their immunosuppressive drug regimen [193, 194, 468, 469]. Conversion to mTOR inhibitors specifically to reduce or prevent skin cancer was first reported in OTRs with KS [192, 424, 470, 471] and subsequently for other skin cancers [472], with reduction in the thickness and peritumoural vascularization of cSCC [473]. Four RCTs and a retrospective case series have now confirmed such a switch significantly reduces the incidence of post-transplant cSCC [474–478], and an RCT of everolimus in cardiac transplant recipients is ongoing (www.clinicaltrials.gov; identifier NCT0079918). The published data suggest that conversion to mTOR inhibitors should be undertaken early: reduction in cSCC accrual was most significant for OTRs with only one previous cSCC, with a non-significant reduction of subsequent cSCCs in patients who had multiple cSCCs before conversion to sirolimus [475, 477, 480]. A phase II randomized study to evaluate the effectiveness of converting from CNIs to sirolimus in treating existing cSCC in OTRs is in progress (www.clinicaltrials.gov; identifier NCT01764607). The effect on BCC reduction is less significant, possibly because of differential expression of phosph-mTOR in BCC compared with cSCC [482] and MCC was actually increased in one large registry-based study [375]. The adverse event profile of these agents means that this strategy is not appropriate for all OTRs [475]. A recent systematic review of 21 RCTs confirmed a 56% reduction in KC and 40% reduction in malignancies overall in patients converted to mTOR inhibitors, but the authors also identified an increased risk of death (HR 1.4) with no reduction in malignancy-associated death, bringing into question the role for their use in reducing or preventing malignancy [483].
Retinoids Experimental data suggest that systemic retinoids exert chemopreventive effects during the promotion and progression stages of carcinogenesis [484, 485]. The mechanism is not fully characterized but retinoids alter gene transcription through their actions on cellular retinoid receptors and are antiproliferative, antiapoptotic, immunomodulatory, promote keratinocyte differentiation and arrest growth and replication of HPV [486]. Topical retinoids may have some activity in OTRs [487]. Systemic retinoids have resulted in reductions in cSCC in OTRs in case series [65, 488–491] and three RCTs have confirmed significant reduction in AK and/or cSCC [492–494]. In most studies, the main adverse effects have been cheilitis, xerosis and hyperlipidaemia and may be dose limiting [65, 492, 493, 495]. Since rebound flares of cSCC may occur upon their discontinuation, systemic retinoid chemoprevention should be viewed as long term [65, 485, 492, 494, 495]. Currently, there are no US Food and Drug Administration (FDA) or European Medicines Agency (EMA) approvals for their use in skin cancer chemoprevention, so no formal prescribing guidelines exist. There is a theoretical risk of allograft rejection given their immunomodulatory properties [485], and consensus opinion recommends starting at low dose (e.g.10 mg/day acitretin) and escalating as tolerated to an effective maintenance dose (e.g. up to 30 mg/day acitretin) [65]. Further research is needed to clarify indications for their initiation, as well as the tolerability and efficacy of optimal dosing regimens [485].
Future prospects for prevention
T4 endonuclease V (T4N5) is an enzyme involved in the repair of DNA damage after exposure to UVR. Clinical efficacy of a topical liposomal formulation in reducing AKs has been demonstrated in patients with xeroderma pigmentosum [496]. Results of a phase II study of T4N5 lotion looking at safety and efficacy in preventing KC in OTRs are awaited (www.clinicaltrials.gov; identifier NCT00089180).
Capecitabine is an oral prodrug of 5-fluorouracil. In two observational case series of OTRs with multiple and advanced KC in whom it was used at low dose, it significantly reduced incidence rates of cSCC, BCC and AK and associated toxicity, although resulting in discontinuation in 60%, was deemed to be manageable [497, 498].
Afamelanotide is a chemical analogue of α-MSH, a peptide hormone produced by keratinocytes and melanocytes in response to UVB and induces synthesis of melanin in melanocytes as part of the tanning response. Afamelanotide is more potent and longer acting than natural α-MSH and is administered by subcutaneous pellet. Results of a multicentre randomized double-blind placebo-controlled phase II trial to investigate its efficacy in reducing AKs and SCC in OTRs are awaited (www.clinicaltrials.gov; identifier NCT00829192).
Screening and surveillance
Preliminary data exploring the cost–benefits of screening for skin cancer in the general population are available [499], but there is no equivalent information relating to immunocompromised populations [500]. Despite this, regular skin cancer surveillance in OTRs is increasingly undertaken: in 2006, at least annual surveillance was provided by 66% of UK centres and 97% of centres in Australia, with most examinations being undertaken by non-dermatologists in the UK [501]. Given the limited evidence base, the following is a proposed approach to OTR skin cancer surveillance and draws upon expert consensus opinion and prospective cohort data [50, 70, 308, 410, 414, 444, 502–504].
Baseline post-transplant assessment and educational programmes
Following transplantation, baseline assessment of individual skin cancer risk and education focusing on photoprotection, self-skin examination and early detection of suspicious lesions is justified for all patients based on current evidence [51, 502], although the timing of initial screening may be stratified according to risk [504]. Such health promotion advice is better recalled and implemented and skin cancer awareness is improved if provided in the setting of a specialist skin clinic [505] and may be further optimised by strategies such as use of written and audiovisual material [449, 506, 507]. Subsequent repeated reinforcement of educational messages both improves recall and adherence to advice [479, 508, 509]. Optimal timing of delivery of this information is unclear; in one study patients expressed a preference for receiving information on malignancy pre-transplant which was then regularly repeated post-transplant [510] whilst in another, post-transplant intervention was preferred [344, 520].
Subsequent post-transplant surveillance
Many consensus guidelines recommend that all OTRs should have a full skin examination by a specialist at least every 12 months [410, 444, 502, 503, 511]. There are, for example, more than 140 000 OTRs in the US [128], 21 000 in the UK, 7000 in Australia and, if extended to other immunocompromised patient groups, the challenge for health care resources would be considerable. Recent data suggest that such surveillance may be better optimised and deployed if targeted by risk stratification, with age at transplant, skin type and previous history of sunburn being the most significant predictive risk factors (Figure 146.3) [73, 504, 512]. Based on accrual of skin cancers established in a UK cohort, five risk categories were defined and follow-up intervals assigned aimed at ensuring cumulative KC remained below 5% (Figure 146.3) [50]. Once pre-malignancy or skin cancer has developed, closer surveillance is justified [50, 69, 70, 481] and in the UK, the National Institute for Health and Care Excellence (NICE) has recommended that immunosuppressed individuals with pre-cancerous lesions or invasive skin cancer should be seen in dedicated clinics for this purpose [513], an approach also being adopted in other countries [128, 502]. Those with low-risk lesions (e.g. AK, Bowen disease) are likely to benefit from at least annual review and following a first skin cancer, surveillance should be more frequent: based on ensuring a cumulative KC risk below 15%, initial intervals of 4–6 months depending on skin cancer type are justified [50], reducing to 3–4 monthly or more frequently in those with multiple or very high-risk tumours (Table 146.7) [50, 69, 70, 308, 410, 414, 415, 502, 503].
Table 146.7 Suggested surveillance protocol in organ transplant recipients with skin cancer
| Risk level | Surveillance intervals |
| First squamous cell carcinoma | 4, 8, 12 months; then annually if no further cancers |
| First basal cell carcinoma | 6, 12 months; then annually if no further cancers |
| 2nd/3rd cancer | 3, 6, 9, 12 months; then annually if no further cancers |
| 10th cancer | 2 monthly |
Based on Harwood et al. 2013 [50].
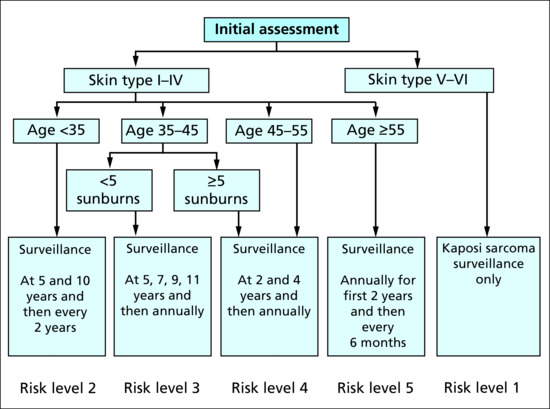
Figure 146.3 Screening and surveillance post-transplant until first skin cancer.
Pre-transplantation screening
Potential risk reduction strategies such as photoprotection and treatment of AK may be most effective if initiated in the pre-transplant period but have yet to be validated [410, 442, 503]. Risk stratification including, for example, determination of HHV-8 status, may inform appropriate tailoring of transplant immunosuppressive drug regimens.
Surveillance in other immunocompromised patient cohorts
It has been recommended that patients with CLL be referred for full-body skin evaluation within 6 months of diagnosis but, as for OTRs, there are few data demonstrating the clinical utility of such a screening approach. Nonetheless, 22% of those screened in one cohort had developed skin cancer within 6 months of CLL diagnosis, which the authors cited as evidence to support routine screening [399]. Whether or not routine surveillance is justifiable in CLL and other patient groups such as those with IBD [109, 111, 514], it is likely that heightened awareness of skin cancer risk amongst both patients and health care providers through targeted education initiatives may ultimately improve early detection and treatment of immunosuppression-associated skin cancer.
Organizations for patients and health care professionals
Several special interest groups have formed in recent years and focus on education (for patients, carers and health care providers), prevention and treatment. In Europe, SCOPE [515] has coordinated a number of research initiatives and, with ITSCC [516], has produced expert consensus guideline documents for the management of post-transplant skin cancer. In the UK, BSSCII (British Society for Skin Care in Immunosuppressed Individuals) [517], a group affiliated to the British Association of Dermatologists, aims to fulfil a similar role with a broad remit across all immunocompromised patient groups. The AT-RISC Alliance (After Transplantation – Reduce Incidence of Skin Cancer) [516] is linked to ITSCC and has developed educational resources for both patients and health care providers.
Conclusion
Skin cancers in immunocompromised patients represent a significant burden of disease for affected individuals and an escalating challenge for health care providers and resources. Risk stratification together with appropriate counselling, surveillance, access to rapid diagnosis and treatment and preventative strategies may reduce the incidence and impact of these skin cancers in the future. However, the evidence base in many of these key areas is limited and further research is urgently required. Such high-risk patients present a model of accelerated skin carcinogenesis and are a relevant population in whom to further investigate pathogenesis, therapeutic and preventative approaches. Indeed, it is likely that many future advances in improving patient outcomes for skin cancer, particularly cSCC, will come from research in immunocompromised patient populations.
References
- Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2014;5:162.
- Orth G. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr Top Microbiol Immunol 2008;321:59–83.
- Orth G. Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Semin Immunol 2006 Dec;18(6):362–74.
- Lazarczyk M, Cassonnet P, Pons C, Jacob Y, Favre M. The EVER proteins as a natural barrier against papillomaviruses: a new insight into the pathogenesis of human papillomavirus infections. Microbiol Mol Biol Rev 2009 Jun;73(2):348–70.
- Crequer A, Picard C, Pedergnana V, et al. EVER2 deficiency is associated with mild T-cell abnormalities. J Clin Immunol 2013 Jan;33(1):14–21.
- Crequer A, Picard C, Patin E, et al. Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLOS One 2012;7(8):e44010.
- Davison SC, Francis N, McLean K, Bunker CB. Epidermodysplasia verruciformis-like eruption associated with HIV infection. Clin Exp Dermatol 2004 May;29(3):311–12.
- Hohenstein E, Rady PL, Hergersberg M, et al. Epidermodysplasia verruciformis in a HIV-positive patient homozygous for the c917A–>T polymorphism in the TMC8/EVER2 gene. Dermatology 2009;218(2):114–18.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol 2011 May;147(5):590–6.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol 2009 Feb;60(2):315–20.
- Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol 2012 Nov;130(5):1030–48.
- Laffort C, Le Deist F, Favre M, et al. Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common gammac cytokine receptor subunit or JAK-3 deficiency. Lancet 2004 Jun 19;363(9426):2051–4.
- Gaspar HB, Harwood C, Leigh I, Thrasher AJ. Severe cutaneous papillomavirus disease after haematopoietic stem-cell transplantation in patients with severe combined immunodeficiency. Br J Haematol 2004 Oct;127(2):232–3.
- Beaussant Cohen S, Fenneteau O, Plouvier E, et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis 2012;7:71.
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009 Nov 19;361(21):2046–55.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol 2012 Jan;148(1):79–84.
- Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS. Common variable immunodeficiency: a new look at an old disease. Lancet 2008 Aug 9;372(9637):489–502.
- Green HA, Moschella S. Multiple invasive squamous cell carcinomas and common variable immunodeficiency. Arch Dermatol 1992 Mar;128(3):412–13.
- Vu J, Wallace GR, Singh R, et al. Common variable immunodeficiency syndrome associated with epidermodysplasia verruciformis. Am J Clin Dermatol 2007;8(5):307–10.
- Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M. Recent advances in understanding the pathophysiology of Wiskott–Aldrich syndrome. Blood 2009 Jun 18;113(25):6288–95.
- Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med 2010 Dec;12(12):753–64.
- Weber F, Fuchs PG, Pfister HJ, Hintner H, Fritsch P, Hoepfl R. Human papillomavirus infection in Netherton's syndrome. Br J Dermatol 2001 May;144(5):1044–9.
- Moloney FJ, Comber H, O'Lorcain P, O'Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol 2006 Mar;154(3):498–504.
- Leigh IM. Progress in skin cancer: the U.K. experience. Br J Dermatol 2014 Sep;171(3):443–5.
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007 Jul 7;370(9581):59–67.
- Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS (London) 2009 Jan 28;23(3):385–93.
- Deeken JF, Tjen ALA, Rudek MA, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 2012 Nov;55(9):1228–35.
- Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012 Oct;87(10):991–1003.
- Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Int Med 2008 May 20;148(10):728–36.
- Sorensen HT, Mellemkjaer L, Nielsen GL, Baron JA, Olsen JH, Karagas MR. Skin cancers and non-hodgkin lymphoma among users of systemic glucocorticoids: a p6opulation-based cohort study. J Natl Cancer Inst 2004 May 5;96(9):709–11.
- Crum-Cianflone N, Hullsiek KH, Satter E, et al. Cutaneous malignancies among HIV-infected persons. Arch Intern Med 2009 Jun 22;169(12):1130–8.
- Silverberg MJ, Leyden W, Warton EM, Quesenberry CP Jr, Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst 2013 Mar 6;105(5):350–60.
- Brewer JD, Habermann TM, Shanafelt TD. Lymphoma-associated skin cancer: incidence, natural history, and clinical management. Int J Dermatol 2014 Mar;53(3):267–74.
- Lens MB, Newton-Bishop JA. An association between cutaneous melanoma and non-Hodgkin's lymphoma: pooled analysis of published data with a review. Ann Oncol 2005 Mar;16(3):460–5.
- Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 2010 Nov 20;28(33):4935–44.
- Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer 2011 Sep 27;105(7):1076–81.
- Onajin O, Brewer JD. Skin cancer in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. Clin Adv Hematol Oncol 2012 Sep;10(9):571–6.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol 2015 Feb;72(2):302–9.
- van den Broek EC, Liu L, Posthuma EF, Janssen-Heijnen ML, Coebergh JW, Soerjomataram I. Increased risk of chronic lymphocytic leukaemia among cancer survivors in the Netherlands: increased detection, causal factors or both? Ann Hematol 2014 Jan;93(1):157–62.
- Agnew KL, Ruchlemer R, Catovsky D, Matutes E, Bunker CB. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol 2004 Jun;150(6):1129–35.
- Tomaszewski JM, Lau E, Corry J. Utility of positron emission tomography/computed tomography for nodal staging of cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. Am J Otolaryngol 2014 Jan–Feb;35(1):66–9.
- Velez NF, Karia PS, Vartanov AR, Davids MS, Brown JR, Schmults CD. Association of advanced leukemic stage and skin cancer tumor stage with poor skin cancer outcomes in patients with chronic lymphocytic leukemia. JAMA Dermatol 2014 Mar;150(3):280–7.
- Hisada M, Biggar RJ, Greene MH, Fraumeni JF Jr, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood 2001 Sep 15;98(6):1979–81.
- Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol 2012 Mar 10;30(8):843–9.
- Saggini A, Anemona L, Chimenti S, et al. HIV-associated primary cutaneous anaplastic large cell lymphoma: a clinicopathological subset with more aggressive behavior? Case report and review of the literature. J Cutan Pathol 2012 Dec;39(12):1100–9.
- Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA 2006 Dec 20;296(23):2823–31.
- Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008-A Swedish population-based study. Int J Cancer 2013 Mar 15;132(6):1429–38.
- Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant 2007 Apr;7(4):941–8.
- Piselli P, Serraino D, Segoloni GP, et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. Eur J Cancer 2013 Jan;49(2):336–44.
- Harwood CA, Mesher D, McGregor JM, et al. A surveillance model for skin cancer in organ transplant recipients: a 22-year prospective study in an ethnically diverse population. Am J Transplant 2013 Jan;13(1):119–29.
- Mudigonda T, Levender MM, O'Neill JL, West CE, Pearce DJ, Feldman SR. Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: a review of single- and multicenter retrospective studies from 2006 to 2010. Dermatol Surg 2013 Mar;39(3):345–64.
- Caforio AL, Fortina AB, Piaserico S, et al. Skin cancer in heart transplant recipients: risk factor analysis and relevance of immunosuppressive therapy. Circulation 2000 Nov 7;102(19 Suppl. 3):III222–7.
- Bernat Garcia J, Morales Suarez-Varela M, Vilata JJ, Marquina A, Pallardo L, Crespo J. Risk factors for non-melanoma skin cancer in kidney transplant patients in a Spanish population in the Mediterranean region. Acta Dermato-Venereol 2013;93(4):422–7.
- Espana A, Redondo P, Fernandez AL, et al. Skin cancer in heart transplant recipients. J Am Acad Dermatol 1995 Mar;32(3):458–65.
- Ferrandiz C, Fuente MJ, Ribera M, et al. Epidermal dysplasia and neoplasia in kidney transplant recipients. J Am Acad Dermatol 1995 Oct;33(4):590–6.
- Montagnino G, Lorca E, Tarantino A, et al. Cancer incidence in 854 kidney transplant recipients from a single institution: comparison with normal population and with patients under dialytic treatment. Clin Transplant 1996 Oct;10(5):461–9.
- Fuente MJ, Sabat M, Roca J, Lauzurica R, Fernandez-Figueras MT, Ferrandiz C. A prospective study of the incidence of skin cancer and its risk factors in a Spanish Mediterranean population of kidney transplant recipients. Br J Dermatol 2003 Dec;149(6):1221–6.
- Marcen R, Pascual J, Tato AM, et al. Influence of immunosuppression on the prevalence of cancer after kidney transplantation. Transplant Proc 2003 Aug;35(5):1714–16.
- Molina BD, Leiro MG, Pulpon LA, et al. Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplant Proc 2010 Oct;42(8):3001–5.
- Ducroux E, Boillot O, Ocampo MA, et al. Skin cancers after liver transplantation: retrospective single-center study on 371 recipients. Transplantation 2014 Aug 15;98(3):335–40.
- Webb MC, Compton F, Andrews PA, Koffman CG. Skin tumours posttransplantation: a retrospective analysis of 28 years' experience at a single centre. Transplant Proc 1997 Feb–Mar;29(1–2):828–30.
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003 Apr 24;348(17):1681–91.
- Wisgerhof HC, Wolterbeek R, de Fijter JW, Willemze R, Bouwes Bavinck JN. Kidney transplant recipients with cutaneous squamous cell carcinoma have an increased risk of internal malignancy. J Invest Dermatol 2012 Sep;132(9):2176–83.
- Brown VL, Proby CM, Harwood CA, Cerio R. Dermatofibrosarcoma protruberans in a renal transplant recipient. Histopathology 2003 Feb;42(2):198–200.
- Harwood CA, Leedham-Green M, Leigh IM, Proby CM. Low-dose retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients: a 16-year retrospective study. Arch Dermatol 2005 Apr;141(4):456–64.
- McCoppin HH, Christiansen D, Stasko T, et al. Clinical spectrum of atypical fibroxanthoma and undifferentiated pleomorphic sarcoma in solid organ transplant recipients: a collective experience. Dermatol Surg 2012 Feb;38(2):230–9.
- Bouwes Bavinck JN, Harwood CA, Genders RE, et al. Pain identifies squamous cell carcinoma in organ transplant recipients: the SCOPE-ITSCC PAIN study. Am J Transplant 2014 Mar;14(3):668–76.
- Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation 2004 Feb 27;77(4):574–9.
- Euvrard S, Kanitakis J, Decullier E, et al. Subsequent skin cancers in kidney and heart transplant recipients after the first squamous cell carcinoma. Transplantation 2006 Apr 27;81(8):1093–100.
- Wisgerhof HC, Edelbroek JR, de Fijter JW, et al. Subsequent squamous- and basal-cell carcinomas in kidney-transplant recipients after the first skin cancer: cumulative incidence and risk factors. Transplantation 2010 May 27;89(10):1231–8.
- Tessari G, Naldi L, Boschiero L, et al. Incidence and clinical predictors of a subsequent nonmelanoma skin cancer in solid organ transplant recipients with a first nonmelanoma skin cancer: a multicenter cohort study. Arch Dermatol 2010 Mar;146(3):294–9.
- Wehner MR, Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Chren MM. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol 2015 Apr;151(4):382–8.
- Carroll RP, Ramsay HM, Fryer AA, Hawley CM, Nicol DL, Harden PN. Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: a prospective study in Queensland, Australia. Am J Kidney Dis 2003 Mar;41(3):676–83.
- O'Reilly F, Traywick C, Pennie ML, Foster JK, Chen SC. Baseline quality of life and anxiety in solid organ transplant recipients: a pilot study. Dermatol Surg 2006 Dec;32(12):1480–5.
- Moloney FJ, Keane S, O'Kelly P, Conlon PJ, Murphy GM. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol 2005 Sep;153(3):574–8.
- Proby CM, Wisgerhof HC, Casabonne D, Green AC, Harwood CA, Bouwes Bavinck JN. The epidemiology of transplant-associated keratinocyte cancers in different geographical regions. Cancer Treat Res 2009;146:75–95.
- Ong CS, Keogh AM, Kossard S, Macdonald PS, Spratt PM. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol 1999 Jan;40(1):27–34.
- Bouwes Bavinck JN, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation 1996 Mar 15;61(5):715–21.
- Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sorensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol 2010 Sep;90(5):474–9.
- Moosa MR. Racial and ethnic variations in incidence and pattern of malignancies after kidney transplantation. Medicine 2005 Jan;84(1):12–22.
- Qunibi W, Akhtar M, Sheth K, et al. Kaposi's sarcoma: the most common tumor after renal transplantation in Saudi Arabia. Am J Med 1988 Feb;84(2):225–32.
- Qunibi WY, Barri Y, Alfurayh O, et al. Kaposi's sarcoma in renal transplant recipients: a report on 26 cases from a single institution. Transplant Proc 1993 Feb;25(1 Pt 2):1402–5.
- Moosa MR. Kaposi's sarcoma in kidney transplant recipients: a 23-year experience. Q J Med 2005 Mar;98(3):205–14.
- Hoshida Y, Aozasa K. Malignancies in organ transplant recipients. Pathol Int 2004 Sep;54(9):649–58.
- Hsu RB, Chen RJ, Chou NK, Ko WJ, Wang SS, Chu SH. Low incidence of malignancy after transplantation in Chinese heart allograft recipients. Transplant Int 2005 Mar;18(3):283–8.
- Park GH, Chang SE, Won CH, et al. Incidence of primary skin cancer after organ transplantation: An 18-year single-center experience in Korea. J Am Acad Dermatol 2014 Mar;70(3):465–72.
- Thomson MA, Suggett NR, Nightingale PG, et al. Skin surveillance of a U.K. paediatric transplant population. Br J Dermatol 2007 Jan;156(1):45–50.
- Koukourgianni F, Harambat J, Ranchin B, et al. Malignancy incidence after renal transplantation in children: a 20-year single-centre experience. Nephrol Dial Transplant 2010 Feb;25(2):611–16.
- Euvrard S, Kanitakis J, Cochat P, Claudy A. Skin cancers following pediatric organ transplantation. Dermatol Surg 2004 Apr;30(4 Pt 2):616–21.
- Euvrard S, Claudy A. Post-transplant skin cancer: the influence of organ and pre-transplant disease. Cancer Treat Res 2009;146:65–74.
- Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant 2010 Aug;10(8):1889–96.
- Wisgerhof HC, van der Boog PJ, de Fijter JW, et al. Increased risk of squamous-cell carcinoma in simultaneous pancreas kidney transplant recipients compared with kidney transplant recipients. J Invest Dermatol 2009 Dec;129(12):2886–94.
- Wisgerhof HC, Wolterbeek R, Haasnoot GW, et al. The risk of cancer is not increased in patients with multiple kidney transplantations. Transplant Immunol 2012 Dec;27(4):189–94.
- Otley CC, Cherikh WS, Salasche SJ, McBride MA, Christenson LJ, Kauffman HM. Skin cancer in organ transplant recipients: effect of pretransplant end-organ disease. J Am Acad Dermatol 2005 Nov;53(5):783–90.
- Nissen NN, Barin B, Stock PG. Malignancy in the HIV-infected patients undergoing liver and kidney transplantation. Curr Opin Oncol 2012 Sep;24(5):517–21.
- Hibberd AD, Trevillian PR, Wlodarczyk JH, et al. Effect of immunosuppression for primary renal disease on the risk of cancer in subsequent renal transplantation: a population-based retrospective cohort study. Transplantation 2013 Jan 15;95(1):122–7.
- Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997 Mar 27;336(13):897–904.
- Hasegawa W, Pond GR, Rifkind JT, et al. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant 2005 Jan;35(1):51–5.
- Cavalier M, Shmalo JA, Yu M, Billings SD, Abonour R, Nelson RP Jr. Skin cancer after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant 2006 Jun;37(12):1103–8.
- Lowe T, Bhatia S, Somlo G. Second malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2007 Oct;13(10):1121–34.
- Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol 2006 Mar 1;24(7):1119–26.
- Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009 Jan 29;113(5):1175–83.
- Atsuta Y, Suzuki R, Yamashita T, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol 2014 Feb;25(2):435–41.
- Rork JF, Margossian SP, Nambudiri VE, Huang JT. Nonmelanoma skin cancer in childhood after hematopoietic stem cell transplant: a report of 4 cases. J Pediatr Haematol/Oncol 2014 Apr;36(3):224–7.
- Beyaert R, Beaugerie L, Van Assche G, et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer 2013;12(1):98.
- Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2010 Mar;8(3):268–74.
- Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 2014 Feb;109(2):163–9.
- Abbas AM, Almukhtar RM, Loftus EV Jr, Lichtenstein GR, Khan N. Risk of melanoma and non-melanoma skin cancer in ulcerative colitis patients treated with thiopurines: a nationwide retrospective cohort. Am J Gastroenterol 2014 Nov;109(11):1781–93.
- Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011 Nov;141(5):1621–28 e1–5.
- Singh H, Nugent Z, Demers AA, Bernstein CN. Increased risk of nonmelanoma skin cancers among individuals with inflammatory bowel disease. Gastroenterology 2011 Nov;141(5):1612–20.
- Ramiscal JA, Brewer JD. Thiopurines and risk of nonmelanoma skin cancer in inflammatory bowel disease. JAMA Dermatol 2013 Jan;149(1):92–4.
- Setshedi M, Epstein D, Winter TA, Myer L, Watermeyer G, Hift R. Use of thiopurines in the treatment of inflammatory bowel disease is associated with an increased risk of non-melanoma skin cancer in an at-risk population: a cohort study. J Gastroenterol Hepatol 2012 Feb;27(2):385–9.
- Perrett CM, Macedo C, Francis N, Ion L, Bunker CB. Atypical fibroxanthoma in an HIV-infected individual. J Cutan Pathol 2011 Apr;38(4):357–9.
- Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012 Aug;143(2):390–9 e1.
- Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-alpha therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2014 Mar;39(5):447–58.
- van den Reek JM, van Lumig PP, Janssen M, et al. Increased incidence of squamous cell carcinoma of the skin after long-term treatment with azathioprine in patients with auto-immune inflammatory rheumatic diseases. J Eur Acad Dermatol Venereol 2014 Jan;28(1):27–33.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthr Rheum 2007 Sep;56(9):2886–95.
- Dreyer L, Mellemkjaer L, Andersen AR, et al. Incidences of overall and site specific cancers in TNFalpha inhibitor treated patients with rheumatoid arthritis and other arthritides – a follow-up study from the DANBIO Registry. Ann Rheum Dis 2013 Jan;72(1):79–82.
- Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf 2011 Feb;20(2):119–30.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis 2011 Nov;70(11):1895–904.
- Moulis G, Sommet A, Bene J, et al. Cancer risk of anti-TNF-alpha at recommended doses in adult rheumatoid arthritis: a meta-analysis with intention to treat and per protocol analyses. PLOS One 2012;7(11):e48991.
- PLEB, Mouterde G, Barnetche T, Morel J, Combe B. Short-term risk of total malignancy and nonmelanoma skin cancers with certolizumab and golimumab in patients with rheumatoid arthritis: metaanalysis of randomized controlled trials. J Rheumatol 2012 Apr;39(4):712–15.
- Mercer LK, Green AC, Galloway JB, et al. The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2012 Jun;71(6):869–74.
- Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA 2012 Sep 5;308(9):898–908.
- Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol 2011 Jun;64(6):1035–50.
- Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol 2011 Nov;165(5):953–65.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol 2007 Jul;127(7):1647–56.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol 2011 Aug;65(2):263–79; quiz 80.
- Atkar R, Ocampo M, Euvrard S, McGregor J, Kanitakis J, Harwood C. Ultraviolet radiation exposure through window glass may be associated with localization of nonmelanoma skin cancer in organ transplant recipients: a study in France and the UK. Br J Dermatol 2013 Aug;169(2):484–5.
- McGregor JM, Berkhout RJM, Rozycka M, et al. p53 mutations implicate sunlight in post-transplant skin cancer irrespective of human papillomavirus status. Oncogene 1997 Oct 2;15(14):1737–40.
- Harwood CA, Attard NR, O'Donovan P, et al. PTCH mutations in basal cell carcinomas from azathioprine-treated organ transplant recipients. Br J Cancer 2008 Oct 21;99(8):1276–84.
- South AP, Purdie KJ, Watt SA, et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol 2014 Oct;134(10):2630–8.
- Lambert SR, Mladkova N, Gulati A, et al. Key differences identified between actinic keratosis and cutaneous squamous cell carcinoma by transcriptome profiling. Br J Cancer 2014 Jan 21;110(2):520–9.
- Purdie KJ, Harwood CA, Gulati A, et al. Single nucleotide polymorphism array analysis defines a specific genetic fingerprint for well-differentiated cutaneous SCCs. J Invest Dermatol 2009 Jun;129(6):1562–8.
- Martinez OM, de Gruijl FR. Molecular and immunologic mechanisms of cancer pathogenesis in solid organ transplant recipients. Am J Transplant 2008 Nov;8(11):2205–11.
- Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 1998 Feb 28;351(9103):623–8.
- Glover MT, Deeks JJ, Raftery MJ, Cunningham J, Leigh IM. Immunosuppression and risk of non-melanoma skin cancer in renal transplant recipients. Lancet 1997 Feb 8;349(9049):398.
- Ducloux D, Carron PL, Rebibou JM, et al. CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation 1998 May 15;65(9):1270–2.
- van Leeuwen MT, Webster AC, McCredie MR, et al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ 2010;340:c570.
- Otley CC, Coldiron BM, Stasko T, Goldman GD. Decreased skin cancer after cessation of therapy with transplant-associated immunosuppressants. Arch Dermatol 2001 Apr;137(4):459–63.
- Otley CC, Berg D, Ulrich C, et al. Reduction of immunosuppression for transplant-associated skin cancer: expert consensus survey. Br J Dermatol 2006 Mar;154(3):395–400.
- Nagy S, Gyulai R, Kemeny L, Szenohradszky P, Dobozy A. Iatrogenic Kaposi's sarcoma: HHV8 positivity persists but the tumors regress almost completely without immunosuppressive therapy. Transplantation 2000 May 27;69(10):2230–1.
- Locke FL, Rollison DE, Sondak VK. Merkel cell carcinoma and immunosuppression: what we still need to know. J Natl Cancer Inst 2015 Feb;107(2).
- Carroll RP, Segundo DS, Hollowood K, et al. Immune phenotype predicts risk for posttransplantation squamous cell carcinoma. J Am Soc Nephrol 2010 Apr;21(4):713–22.
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012 Dec 1;125(23):5591–6.
- Harwood CA, Proby CM, McGregor JM, Sheaff MT, Leigh IM, Cerio R. Clinicopathologic features of skin cancer in organ transplant recipients: a retrospective case-control series. J Am Acad Dermatol 2006 Feb;54(2):290–300.
- Muhleisen B, Petrov I, Gachter T, et al. Progression of cutaneous squamous cell carcinoma in immunosuppressed patients is associated with reduced CD123+ and FOXP3+ cells in the perineoplastic inflammatory infiltrate. Histopathology 2009 Jul;55(1):67–76.
- Kosmidis M, Dziunycz P, Suarez-Farinas M, et al. Immunosuppression affects CD4(+) mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J Immunother 2010 Jun;33(5):538–46.
- Zhang SL, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLOS One 2013 May 7;8(5).
- Jung JW, Overgaard NH, Burke MT, et al. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients? Int J Cancer 2015 Jan 22.
- Geusau A, Dunkler D, Messeritsch E, et al. Non-melanoma skin cancer and its risk factors in an Austrian population of heart transplant recipients receiving induction therapy. Int J Dermatol 2008 Sep;47(9):918–25.
- Ingvar A, Smedby KE, Lindelof B, et al. Immunosuppressive treatment after solid organ transplantation and risk of post-transplant cutaneous squamous cell carcinoma. Nephrol Dial Transplant 2010 Aug;25(8):2764–71.
- Doesch AO, Muller S, Konstandin M, et al. Malignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawal. Transplant Proc 2010 Nov;42(9):3694–9.
- Gallagher MP, Kelly PJ, Jardine M, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol 2010 May;21(5):852–8.
- O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl 2006 Oct;25(10):1186–91.
- Einollahi B, Nemati E, Lessan-Pezeshki M, et al. Skin cancer after renal transplantation: Results of a multicenter study in Iran. Ann Transpl 2010 Jul–Sep;15(3):44–50.
- Braconnier P, Del Marmol V, Broeders N, et al. Combined introduction of anti-IL2 receptor antibodies, mycophenolic acid and tacrolimus: effect on malignancies after renal transplantation in a single-centre retrospective cohort study. Nephrol Dial Transplant 2012 Jun;27(6):2547–53.
- Bichari W, Bartiromo M, Mohey H, et al. Significant risk factors for occurrence of cancer after renal transplantation: a single center cohort study of 1265 cases. Transplant Proc 2009 Mar;41(2):672–3.
- Brewer JD, Colegio OR, Phillips PK, et al. Incidence of and risk factors for skin cancer after heart transplant. Arch Dermatol 2009 Dec;145(12):1391–6.
- Klintmalm GB, Saab S, Hong JC, Nashan B. The role of mammalian target of rapamycin inhibitors in the management of post-transplant malignancy. Clin Transplant 2014 Jun;28(6):635–48.
- Abou Ayache R, Thierry A, Bridoux F, et al. Long-term maintenance of calcineurin inhibitor monotherapy reduces the risk for squamous cell carcinomas after kidney transplantation compared with bi- or tritherapy. Transplant Proc 2007 Oct;39(8):2592–4.
- Rodriguez-Peralvarez M, Germani G, Papastergiou V, et al. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol 2013 Feb;58(2):262–70.
- Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood 2005 May 15;105(10):3802–11.
- Karagas MR, Cushing GL Jr, Greenberg ER, Mott LA, Spencer SK, Nierenberg DW. Non-melanoma skin cancers and glucocorticoid therapy. Br J Cancer 2001 Sep 1;85(5):683–6.
- Jensen AO, Thomsen HF, Engebjerg MC, et al. Use of oral glucocorticoids and risk of skin cancer and non-Hodgkin's lymphoma: a population-based case–control study. Br J Cancer 2009 Jan 13;100(1):200–5.
- Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Medicine 2002 Feb;8(2):128–35.
- de Gruijl FR, Koehl GE, Voskamp P, et al. Early and late effects of the immunosuppressants rapamycin and mycophenolate mofetil on UV carcinogenesis. Int J Cancer 2010 Aug 15;127(4):796–804.
- Voskamp P, Bodmann CA, Rebel HG, et al. Rapamycin impairs UV induction of mutant-p53 overexpressing cell clusters without affecting tumor onset. Int J Cancer 2012 Sep 15;131(6):1267–76.
- Sully K, Akinduro O, Philpott MP, et al. The mTOR inhibitor rapamycin opposes carcinogenic changes to epidermal Akt1/PKBalpha isoform signaling. Oncogene 2013 Jul 4;32(27):3254–62.
- Kuschal C, Thoms KM, Schubert S, et al. Skin cancer in organ transplant recipients: effects of immunosuppressive medications on DNA repair. Exp Dermatol 2012 Jan;21(1):2–6.
- Carroll RP, Hester J, Wood KJ, Harden PN. Conversion to sirolimus in kidney transplant recipients with squamous cell cancer and changes in immune phenotype. Nephrol Dialysis Transpl 2013 Feb;28(2):462–5.
- Herman M, Weinstein T, Korzets A, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med 2001 Jan;137(1):14–20.
- Sugie N, Fujii N, Danno K. Cyclosporin-A suppresses p53-dependent repair DNA synthesis and apoptosis following ultraviolet-B irradiation. Photodermatol Photoimmunol Photomed 2002 Aug;18(4):163–8.
- Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol 2005 Nov;125(5):1020–5.
- Thoms KM, Kuschal C, Oetjen E, et al. Cyclosporin A, but not everolimus, inhibits DNA repair mediated by calcineurin: implications for tumorigenesis under immunosuppression. Exp Dermatol 2011 Mar;20(3):232–6.
- Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999 Feb 11;397(6719):530–4.
- Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation 2003 Aug 15;76(3):597–602.
- Duncan FJ, Wulff BC, Tober KL, et al. Clinically relevant immunosuppressants influence UVB-induced tumor size through effects on inflammation and angiogenesis. Am J Transplant 2007 Dec;7(12):2693–703.
- Wu X, Nguyen BC, Dziunycz P, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 2010 May 20;465(7296):368–72.
- Norman KG, Canter JA, Shi M, Milne GL, Morrow JD, Sligh JE. Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients. Mitochondrion 2010 Mar;10(2):94–101.
- Han W, Ming M, He TC, He YY. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem 2010 Apr 9;285(15):11369–77.
- Xu J, Walsh SB, Verney ZM, Kopelovich L, Elmets CA, Athar M. Procarcinogenic effects of cyclosporine A are mediated through the activation of TAK1/TAB1 signaling pathway. Biochem Biophys Res Commun 2011 May 13;408(3):363–8.
- Walsh SB, Xu J, Xu H, et al. Cyclosporine a mediates pathogenesis of aggressive cutaneous squamous cell carcinoma by augmenting epithelial-mesenchymal transition: role of TGFbeta signaling pathway. Mol Carcinogen 2011 Jul;50(7):516–27.
- Kuschal C, Thoms KM, Boeckmann L, et al. Cyclosporin A inhibits nucleotide excision repair via downregulation of the xeroderma pigmentosum group A and G proteins, which is mediated by calcineurin inhibition. Exp Dermatol 2011 Oct;20(10):795–9.
- Dziunycz PJ, Lefort K, Wu X, et al. The oncogene ATF3 is potentiated by cyclosporine A and ultraviolet light A. J Invest Dermatol 2014 Jul;134(7):1998–2004.
- Kelly GE, Meikle W, Sheil AG. Scheduled and unscheduled DNA synthesis in epidermal cells of hairless mice treated with immunosuppressive drugs and UVB-UVA irradiation. Br J Dermatol 1987 Oct;117(4):429–40.
- de Graaf YG, Rebel H, Elghalbzouri A, et al. More epidermal p53 patches adjacent to skin carcinomas in renal transplant recipients than in immunocompetent patients: the role of azathioprine. Exp Dermatol 2008 Apr;17(4):349–55.
- O'Donovan P, Perrett CM, Zhang X, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 2005 Sep 16;309(5742):1871–4.
- Perrett CM, Walker SL, O'Donovan P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol 2008 Jul;159(1):198–204.
- Hofbauer GF, Attard NR, Harwood CA, et al. Reversal of UVA skin photosensitivity and DNA damage in kidney transplant recipients by replacing azathioprine. Am J Transplant 2012 Jan;12(1):218–25.
- Gueranger Q, Li F, Peacock M, et al. Protein oxidation and DNA repair inhibition by 6-thioguanine and UVA radiation. J Invest Dermatol 2014 May;134(5):1408–17.
- Campistol JM, Schena FP. Kaposi's sarcoma in renal transplant recipients–the impact of proliferation signal inhibitors. Nephrol Dial Transplant 2007 May;22 Suppl. 1:i17–22.
- Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation 2005 Oct 15;80(7):883–9.
- Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004 Aug;18(4):446–9.
- Aissat N, Le Tourneau C, Ghoul A, et al. Antiproliferative effects of rapamycin as a single agent and in combination with carboplatin and paclitaxel in head and neck cancer cell lines. Cancer Chemother Pharmacol 2008 Jul;62(2):305–13.
- Saha B, Cypro A, Martin GM, Oshima J. Rapamycin decreases DNA damage accumulation and enhances cell growth of WRN-deficient human fibroblasts. Aging Cell 2014 Jun;13(3):573–5.
- Liu J, Fan HJ, Ma YY, et al. Notch1 Is a 5-fluorouracil resistant and poor survival marker in human esophagus squamous cell carcinomas. PLOS One 2013 Feb 7;8(2).
- Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009 Feb 20;27(6):884–90.
- Davidovitz Y, Ballin A, Meytes D. Flare-up of squamous cell carcinoma of the skin following fludarabine therapy for chronic lymphocytic leukemia. Acta Haematol 1997;98(1):44–6.
- Callea V, Brugiatelli M, Stelitano C, Gentile M, Nobile F, Morabito F. Incidence of second neoplasia in patients with B-cell chronic lymphocytic leukemia treated with chlorambucil maintenance chemotherapy. Leuk Lymph 2006 Nov;47(11):2314–20.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma editorial comment. J Urol 2009 Jul;182(1):149–50.
- Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010 Jan;62(1):31–7.
- Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis 2014 Apr;58(7):997–1002.
- Vadnerkar A, Nguyen MH, Mitsani D, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transpl 2010 Nov;29(11):1240–4.
- Zwald FO, Spratt M, Lemos BD, et al. Duration of voriconazole exposure: an independent risk factor for skin cancer after lung transplantation. Dermatol Surg 2012 Aug;38(8):1369–74.
- Singer JP, Boker A, Metchnikoff C, et al. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transpl 2012 Jul;31(7):694–9.
- Feist A, Lee R, Osborne S, Lane J, Yung G. Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transpl 2012 Nov;31(11):1177–81.
- Epaulard O, Villier C, Ravaud P, et al. A multistep voriconazole-related phototoxic pathway may lead to skin carcinoma: results from a French nationwide study. Clin Infect Dis 2013 Dec;57(12):e182–8.
- Mascitelli L, Pezzetta F, Goldstein MR. Why statin therapy may increase the risk for posttransplantation squamous cell carcinoma. Transplant Immunol 2010 Aug;23(4):224–5.
- Li X, Wu XB, Chen Q. Statin use is not associated with reduced risk of skin cancer: a meta-analysis. Br J Cancer 2014 Feb 4;110(3):802–7.
- Robinson SN, Zens MS, Perry AE, Spencer SK, Duell EJ, Karagas MR. Photosensitizing agents and the risk of non-melanoma skin cancer: a population-based case–control study. J Invest Dermatol 2013 Aug;133(8):1950–5.
- Muranushi C, Olsen CM, Pandeya N, Green AC. Aspirin and nonsteroidal anti-inflammatory drugs can prevent cutaneous squamous cell carcinoma: a systematic review and meta-analysis. J Invest Dermatol 2015 Apr;135(4):975–83.
- Zhang B, Liang X, Ye L, Wang Y. No chemopreventive effect of nonsteroidal anti-inflammatory drugs on nonmelanoma skin cancer: evidence from meta-analysis. PLOS One 2014;9(5):e96887.
- Arron ST, Ruby JG, Dybbro E, Ganem D, Derisi JL. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. J Invest Dermatol 2011 Aug;131(8):1745–53.
- Aldabagh B, Angeles JGC, Cardones AR, Arron ST. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg 2013 Jan;39(1):1–23.
- Quint KD, Genders RE, de Koning MN, et al. Human beta-papillomavirus infection and keratinocyte carcinomas. J Pathol 2015 Jan;235(2):342–54.
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010 May 25;401(1):70–9.
- Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol 2014 Dec;234(4):431–5.
- Dochez C, Bogers JJ, Verhelst R, Rees H. HPV vaccines to prevent cervical cancer and genital warts: an update. Vaccine 2014 Mar 20;32(14):1595–601.
- Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010 Aug;11(8):781–9.
- Kreuter A, Gambichler T, Pfister H, Wieland U. Diversity of human papillomavirus types in periungual squamous cell carcinoma. Br J Dermatol 2009 Dec;161(6):1262–9.
- Schulz TF. Cancer and viral infections in immunocompromised individuals. Int J Cancer 2009 Oct 15;125(8):1755–63.
- Caldeira S, Zehbe I, Accardi R, et al. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol 2003 Feb;77(3):2195–206.
- Westphal K, Akgul B, Storey A, Nindl I. Cutaneous human papillomavirus E7 type-specific effects on differentiation and proliferation of organotypic skin cultures. Cell Oncol 2009;31(3):213–26.
- Accardi R, Dong W, Smet A, et al. Skin human papillomavirus type 38 alters p53 functions by accumulation of deltaNp73. EMBO Rep 2006 Mar;7(3):334–40.
- Cornet I, Bouvard V, Campo MS, et al. Comparative analysis of transforming properties of E6 and E7 from different beta human papillomavirus types. J Virol 2012 Feb;86(4):2366–70.
- Bedard KM, Underbrink MP, Howie HL, Galloway DA. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J Virol 2008 Apr;82(8):3894–902.
- Iftner T, Elbel M, Schopp B, et al. Interference of papillomavirus E6 protein with single–strand break repair by interaction with XRCC1. EMBO J 2002 Sep 2;21(17):4741–8.
- Giampieri S, Storey A. Repair of UV-induced thymine dimers is compromised in cells expressing the E6 protein from human papillomaviruses types 5 and 18. Br J Cancer 2004 Jun 1;90(11):2203–9.
- Wallace NA, Robinson K, Howie HL, Galloway DA. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLOS Pathogens 2012;8(7):e1002807.
- Wallace NA, Robinson K, Galloway DA. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J Virol 2014 Jun;88(11):6112–27.
- Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev 2000 Dec 1;14(23):3065–73.
- Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol 2008 Nov;82(21):10408–17.
- Struijk L, van der Meijden E, Kazem S, et al. Specific betapapillomaviruses associated with squamous cell carcinoma of the skin inhibit UVB-induced apoptosis of primary human keratinocytes. J Gen Virol 2008 Sep;89(Pt 9):2303–14.
- Muench P, Probst S, Schuetz J, et al. Cutaneous papillomavirus E6 proteins must interact with p300 and block p53-mediated apoptosis for cellular immortalization and tumorigenesis. Cancer Res 2010 Sep 1;70(17):6913–24.
- Howie HL, Koop JI, Weese J, et al. Beta-HPV 5 and 8 E6 promote p300 degradation by blocking AKT/p300 association. PLOS Pathogens 2011 Aug;7(8):e1002211.
- Hussain I, Fathallah I, Accardi R, et al. NF-kappaB protects human papillomavirus type 38 E6/E7-immortalized human keratinocytes against tumor necrosis factor alpha and UV-mediated apoptosis. J Virol 2011 Sep;85(17):9013–22.
- Jha S, Vande Pol S, Banerjee NS, Dutta AB, Chow LT, Dutta A. Destabilization of TIP60 by human papillomavirus E6 results in attenuation of TIP60-dependent transcriptional regulation and apoptotic pathway. Mol Cell 2010 Jun 11;38(5):700–11.
- Muschik D, Braspenning-Wesch I, Stockfleth E, Rosl F, Hofmann TG, Nindl I. Cutaneous HPV23 E6 prevents p53 phosphorylation through interaction with HIPK2. PLOS One 2011;6(11):e27655.
- Cordano P, Gillan V, Bratlie S, et al. The E6E7 oncoproteins of cutaneous human papillomavirus type 38 interfere with the interferon pathway. Virology 2008 Aug 1;377(2):408–18.
- Akgul B, Garcia-Escudero R, Ghali L, et al. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res 2005 Mar 15;65(6):2216–23.
- Connolly K, Manders P, Earls P, Epstein RJ. Papillomavirus-associated squamous skin cancers following transplant immunosuppression: one Notch closer to control. Cancer Treatment Rev 2014 Mar;40(2):205–14.
- Mendoza JA, Jacob Y, Cassonnet P, Favre M. Human papillomavirus type 5 E6 oncoprotein represses the transforming growth factor beta signaling pathway by binding to SMAD3. J Virol 2006 Dec;80(24):12420–4.
- Akgul B, Bostanci N, Westphal K, et al. Human papillomavirus 5 and 8 E6 downregulate interleukin-8 secretion in primary human keratinocytes. J Gen Virol 2010 Apr;91(Pt 4):888–92.
- Schaper ID, Marcuzzi GP, Weissenborn SJ, et al. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res 2005 Feb 15;65(4):1394–400.
- Pfefferle R, Marcuzzi GP, Akgul B, et al. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J Invest Dermatol 2008 Sep;128(9):2310–15.
- Marcuzzi GP, Hufbauer M, Kasper HU, Weissenborn SJ, Smola S, Pfister H. Spontaneous tumour development in human papillomavirus type 8 E6 transgenic mice and rapid induction by UV-light exposure and wounding. J Gen Virol 2009 Dec;90(Pt 12):2855–64.
- Storey A, Simmonds M. Interaction between ultraviolet radiation and human papillomavirus. Cancer Treat Res 2009;146:159–67.
- Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J Clin Virol 2008 Dec;43(4):353–60.
- Wang SJ, Gu W. To be, or not to be: functional dilemma of p53 metabolic regulation. Curr Opin Oncol 2014 Jan;26(1):78–85.
- Harwood CA, Proby CM. Human papillomaviruses and non-melanoma skin cancer. Curr Opin Infect Dis 2002 Apr;15(2):101–14.
- Arron ST, Jennings L, Nindl I, et al. Viral oncogenesis and its role in nonmelanoma skin cancer. Br J Dermatol 2011 Jun;164(6):1201–13.
- Frazer IH. The actinic keratosis virome: can we prevent squamous cell carcinoma with a vaccine? Curr Prob Dermatol 2015;46:28–35.
- de Koning MN, Weissenborn SJ, Abeni D, et al. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol 2009 Jul;90(Pt 7):1611–21.
- Michael KM, Waterboer T, Sehr P, et al. Seroprevalence of 34 human papillomavirus types in the German general population. PLOS Pathogens 2008 Jun;4(6):e1000091.
- Waterboer T, Neale R, Michael KM, et al. Antibody responses to 26 skin human papillomavirus types in the Netherlands, Italy and Australia. J Gen Virol 2009 Aug;90(Pt 8):1986–98.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol 2000 Jul;61(3):289–97.
- Purdie KJ, Surentheran T, Sterling JC, et al. Human papillomavirus gene expression in cutaneous squamous cell carcinomas from immunosuppressed and immunocompetent individuals. J Invest Dermatol 2005 Jul;125(1):98–107.
- Weissenborn SJ, Nindl I, Purdie K, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol 2005 Jul;125(1):93–7.
- Waterboer T, Abeni D, Sampogna F, et al. Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. Br J Dermatol 2008 Aug;159(2):457–9.
- Karagas MR, Waterboer T, Li Z, et al. Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case–control study. BMJ 2010;341:c2986.
- Proby CM, Harwood CA, Neale RE, et al. A case–control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am J Transplant 2011 Jul;11(7):1498–508.
- Andersson K, Waterboer T, Kirnbauer R, et al. Seroreactivity to cutaneous human papillomaviruses among patients with nonmelanoma skin cancer or benign skin lesions. Cancer Epidemiol Biomarkers Prev 2008 Jan;17(1):189–95.
- Genders RE, Mazlom H, Michel A, et al. The presence of betapapillomavirus antibodies around transplantation predicts the development of keratinocyte carcinoma in organ transplant recipients: a cohort study. J Invest Dermatol 2015 May;135(5):1275–82.
- Ganzenmueller T, Yakushko Y, Kluba J, Henke-Gendo C, Gutzmer R, Schulz TF. Next-generation sequencing fails to identify human virus sequences in cutaneous squamous cell carcinoma. Int J Cancer 2012 Oct 1;131(7):E1173–9.
- Savani BN, Goodman S, Barrett AJ. Can routine posttransplant HPV vaccination prevent commonly occurring epithelial cancers after allogeneic stem cell transplantation? Clin Cancer Res 2009 Apr 1;15(7):2219–21.
- Vinzon SE, Braspenning-Wesch I, Muller M, et al. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: a preclinical study using a natural outbred animal model. PLOS Pathogens 2014 Feb;10(2):e1003924.
- Marcuzzi GP, Awerkiew S, Hufbauer M, et al. Tumor prevention in HPV8 transgenic mice by HPV8-E6 DNA vaccination. Med Microbiol Immunol 2014 Jun;203(3):155–63.
- Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant 2013 Sep;13(9):2411–17.
- Binstock M, Hafeez F, Metchnikoff C, Arron ST. Single-nucleotide polymorphisms in pigment genes and nonmelanoma skin cancer predisposition: a systematic review. Br J Dermatol 2014 Oct;171(4):713–21.
- Andresen PA, Nymoen DA, Kjaerheim K, Leivestad T, Helsing P. Susceptibility to cutaneous squamous cell carcinoma in renal transplant recipients associates with genes regulating melanogenesis independent of their role in pigmentation. Biomark Cancer 2013;5:41–7.
- McGregor JM, Harwood CA, Brooks L, et al. Relationship between p53 codon 72 polymorphism and susceptibility to sunburn and skin cancer. J Invest Dermatol 2002 Jul;119(1):84–90.
- Marshall SE, Bordea C, Haldar NA, et al. Glutathione S-transferase polymorphisms and skin cancer after renal transplantation. Kidney Int 2000 Nov;58(5):2186–93.
- Ramsay HM, Harden PN, Reece S, et al. Polymorphisms in glutathione S-transferases are associated with altered risk of nonmelanoma skin cancer in renal transplant recipients: a preliminary analysis. J Invest Dermatol 2001 Aug;117(2):251–5.
- Alamartine E, Berthoux P, Mariat C, Cambazard F, Berthoux F. Interleukin-10 promoter polymorphisms and susceptibility to skin squamous cell carcinoma after renal transplantation. J Invest Dermatol 2003 Jan;120(1):99–103.
- Lira MG, Mazzola S, Tessari G, et al. Association of functional gene variants in the regulatory regions of COX-2 gene (PTGS2) with nonmelanoma skin cancer after organ transplantation. Br J Dermatol 2007 Jul;157(1):49–57.
- Laing ME, Dicker P, Moloney FJ, et al. Association of methylenetetrahydrofolate reductase polymorphism and the risk of squamous cell carcinoma in renal transplant patients. Transplantation 2007 Jul 15;84(1):113–16.
- Laing ME, Cummins R, O'Grady A, O'Kelly P, Kay EW, Murphy GM. Aberrant DNA methylation associated with MTHFR C677T genetic polymorphism in cutaneous squamous cell carcinoma in renal transplant patients. Br J Dermatol 2010 Aug;163(2):345–52.
- Begnini A, Tessari G, Turco A, et al. PTCH1 gene haplotype association with basal cell carcinoma after transplantation. Br J Dermatol 2010 Aug;163(2):364–70.
- Laing ME, Kay E, Conlon P, Murphy GM. Genetic factors associated with skin cancer in renal transplant patients. Photodermatol Photoimmunol Photomed 2007 Apr–Jun;23(2–3):62–7.
- Tartaglia S, Belloni-Fortina A, Stefano P, et al. The +61 A-G polymorphism of the epidermal growth factor gene is not associated with occurrence of non-melanocytic skin tumors in transplant recipients. J Dermatol Sci 2007 May;46(2):147–9.
- Perrett CM, Harwood CA, McGregor JM, Warwick J, Cerio R, Karran P. Expression of DNA mismatch repair proteins and MSH2 polymorphisms in nonmelanoma skin cancers of organ transplant recipients. Br J Dermatol 2010 Apr;162(4):732–42.
- Foss Abrahamsen A, Andersen A, Nome O, et al. Long-term risk of second malignancy after treatment of Hodgkin's disease: the influence of treatment, age and follow-up time. Ann Oncol 2002 Nov;13(11):1786–91.
- Janin A, Murata H, Leboeuf C, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplant. Blood 2009 Feb 19;113(8):1834–40.
- Aractingi S, Kanitakis J, Euvrard S, et al. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res 2005 Mar 1;65(5):1755–60.
- Verneuil L, Varna M, Ratajczak P, et al. Human skin carcinoma arising from kidney transplant-derived tumor cells. J Clin Invest 2013 Sep;123(9):3797–801.
- Barozzi P, Luppi M, Facchetti F, et al. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nature Med 2003 May;9(5):554–61.
- Wilkins K, Dolev JC, Turner R, LeBoit PE, Berger TG, Maurer TA. Approach to the treatment of cutaneous malignancy in HIV-infected patients. Dermatol Ther 2005 Jan–Feb;18(1):77–86.
- Casabonne D, Lally A, Mitchell L, et al. A case–control study of cutaneous squamous cell carcinoma among Caucasian organ transplant recipients: the role of antibodies against human papillomavirus and other risk factors. Int J Cancer 2009 Oct 15;125(8):1935–45.
- Joly P, Bastuji-Garin S, Frances C, et al. Squamous cell carcinomas are associated with verrucokeratotic cutaneous lesions but not with common warts in organ-transplant patients. A case–control study. Transplantation 2010 May 27;89(10):1224–30.
- Bouwes Bavinck JN, Neale RE, Abeni D, et al. Multicenter study of the association between betapapillomavirus infection and cutaneous squamous cell carcinoma. Cancer Res 2010 Dec 1;70(23):9777–86.
- Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN. Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol 2002 Nov;147(5):950–6.
- Smith L, Dahler AL, Cavanagh LL, et al. Modulation of proliferation-specific and differentiation-specific markers in human keratinocytes by SMAD7. Exp Cell Res 2004 Apr 1;294(2):356–65.
- Cooper SM, Wojnarowska F. The accuracy of clinical diagnosis of suspected premalignant and malignant skin lesions in renal transplant recipients. Clin Exp Dermatol 2002 Sep;27(6):436–8.
- Kwatra SG, Mills KC, Zeitany A, et al. Pain and nonmelanoma skin cancer in transplant patients. J Am Acad Dermatol 2012 Dec;67(6):1387–8.
- Stoff B, Salisbury C, Parker D, O'Reilly Zwald F. Dermatopathology of skin cancer in solid organ transplant recipients. Transpl Rev (Orlando) 2010 Oct;24(4):172–89.
- Clausen OP, Aass HC, Beigi M, et al. Are keratoacanthomas variants of squamous cell carcinomas? A comparison of chromosomal aberrations by comparative genomic hybridization. J Invest Dermatol 2006 Oct;126(10):2308–15.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis 2005 Oct;26(10):1657–67.
- Jacobs MS, Persons DL, Fraga GR. EGFR and MYC gene copy number aberrations are more common in squamous cell carcinoma than keratoacanthoma: a FISH study. J Cutan Pathol 2013 May;40(5):447–54.
- Poligone B, Hayden MS, Chen LJ, Pentland AP, Jimi E, Ghosh S. A role for NF-kappa B activity in skin hyperplasia and the development of keratoacanthomata in mice. PLOS One 2013 Aug 19;8(8).
- Goudie DR, D'Alessandro M, Merriman B, et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat Genet 2011 Apr;43(4):365–9.
- Hua HK, Jin C, Yang LJ, Tao SQ, Zhu XH. Expression of cyclooxygenase-2 in squamous cell carcinoma and keratoacanthoma and its clinical significance. Cell Biochem Biophys 2015 Jan 10.
- Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation 2010 Sep 27;90(6):683–7.
- Metchnikoff C, Mully T, Singer JP, Golden JA, Arron ST. The 7th edition AJCC staging system for cutaneous squamous cell carcinoma accurately predicts risk of recurrence for heart and lung transplant recipients. J Am Acad Dermatol 2012 Nov;67(5):829–35.
- Buell JF, Hanaway MJ, Thomas M, Alloway RR, Woodle ES. Skin cancer following transplantation: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc 2005 Mar;37(2):962–3.
- Southwell KE, Chaplin JM, Eisenberg RL, McIvor NP, Morton RP. Effect of immunocompromise on metastatic cutaneous squamous cell carcinoma in the parotid and neck. Head Neck J 2006 Mar;28(3):244–8.
- Martinez JC, Otley CC, Stasko T, et al. Defining the clinical course of metastatic skin cancer in organ transplant recipients: a multicenter collaborative study. Arch Dermatol 2003 Mar;139(3):301–6.
- Carucci JA, Martinez JC, Zeitouni NC, et al. In-transit metastasis from primary cutaneous squamous cell carcinoma in organ transplant recipients and nonimmunosuppressed patients: clinical characteristics, management, and outcome in a series of 21 patients. Dermatol Surg 2004 Apr;30(4 Pt 2):651–5.
- Cooper JZ, Brown MD. Special concern about squamous cell carcinoma of the scalp in organ transplant recipients. Arch Dermatol 2006 Jun;142(6):755–8.
- Veness MJ, Quinn DI, Ong CS, et al. Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer 1999 Apr 15;85(8):1758–64.
- Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am J Transplant 2013 May;13(5):1296–304.
- Wilson ML, Elston DM, Tyler WB, Marks VJ, Ferringer T. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J 2010;16(3):4.
- Verburg M, Lang M, Muhlstadt M, et al. Cutaneous squamous cell carcinoma with perineural invasion: report on eight cases and review of the literature. Dermatology 2015;230(2):135–42.
- Nguyen P, Vin-Christian K, Ming ME, Berger T. Aggressive squamous cell carcinomas in persons infected with the human immunodeficiency virus. Arch Dermatol 2002 Jun;138(6):758–63.
- Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of Basal cell carcinoma after mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol 2004 Aug;140(8):985–8.
- Chen CH, Chung CY, Wang LH, Lin C, Lin HL, Lin HC. Risk of cancer among HIV-infected patients from a population-based nested case–control study: implications for cancer prevention. BMC Cancer 2015;15:133.
- Euvrard S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol 1995 Aug;33(2 Pt 1):222–9.
- Ulrich C. Topical treatment of field cancerization. Cancer Treat Res 2009;146:439–46.
- Wallingford SC, Russell SA, Vail A, Proby CM, Lear JT, Green AC. Actinic keratoses, actinic field change and associations with squamous cell carcinoma in renal transplant recipients in Manchester, UK. Acta Derm Venereol 2015 Mar 18.
- Boyd AS, Stasko T, Cameron GS, Russell M, King LE Jr. Histologic features of actinic keratoses in solid organ transplant recipients and healthy controls. J Am Acad Dermatol 2001 Aug;45(2):217–21.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol 2014 Sep–Oct;24(5):533–44.
- Herranz P, Pizarro A, De Lucas R, et al. High incidence of porokeratosis in renal transplant recipients. Br J Dermatol 1997 Feb;136(2):176–9.
- Proby C, Harwood C. Porokeratosis in organ transplantation recipients. In: Otley CC, Stasko T, eds. Skin Disease in Organ Transplantation. Cambridge: Cambridge University Press, 2008:119–21.
- Silver SG, Crawford RI. Fatal squamous cell carcinoma arising from transplant-associated porokeratosis. J Am Acad Dermatol 2003 Nov;49(5):931–3.
- Anzai S, Takeo N, Yamaguchi T, et al. Squamous cell carcinoma in a renal transplant recipient with linear porokeratosis. J Dermatol 1999 Apr;26(4):244–7.
- Kanitakis J, Alhaj-Ibrahim L, Euvrard S, Claudy A. Basal cell carcinomas developing in solid organ transplant recipients: clinicopathologic study of 176 cases. Arch Dermatol 2003 Sep;139(9):1133–7.
- Valery PC, Neale R, Williams G, Pandeya N, Siller G, Green A. The effect of skin examination surveys on the incidence of basal cell carcinoma in a Queensland community sample: a 10-year longitudinal study. J Investig Dermatol Symp Proc 2004 Mar;9(2):148–51.
- Oram Y, Orengo I, Griego RD, Rosen T, Thornby J. Histologic patterns of basal cell carcinoma based upon patient immunostatus. Dermatol Surg 1995 Jul;21(7):611–14.
- de Berker DA, Taylor AE, Quinn AG, Simpson NB. Sebaceous hyperplasia in organ transplant recipients: shared aspects of hyperplastic and dysplastic processes? J Am Acad Dermatol 1996 Nov;35(5 Pt 1):696–9.
- Gordon Spratt EA, Fischer M, Kamino H. Eruptive basal-cell carcinomas in the setting of human immunodeficiency virus infection. Dermatol Online J 2012 Dec;18(12):1.
- Hausauer AK, Maurer T, Leslie KS, Parvataneni R, Stuart SE, Chren MM. Recurrence after treatment of cutaneous basal cell and squamous cell carcinomas in patients infected with human immunodeficiency virus. JAMA Dermatol 2013 Feb;149(2):239–41.
- Hollenbeak CS, Todd MM, Billingsley EM, Harper G, Dyer AM, Lengerich EJ. Increased incidence of melanoma in renal transplantation recipients. Cancer 2005 Nov 1;104(9):1962–7.
- Dahlke E, Murray CA, Kitchen J, Chan AW. Systematic review of melanoma incidence and prognosis in solid organ transplant recipients. Transpl Res 2014;3:10.
- Matin RN, Mesher D, Proby CM, et al. Melanoma in organ transplant recipients: clinicopathological features and outcome in 100 cases. Am J Transplant 2008 Sep;8(9):1891–900.
- Brewer JD, Christenson LJ, Weaver AL, et al. Malignant melanoma in solid transplant recipients: collection of database cases and comparison with surveillance, epidemiology, and end results data for outcome analysis. Arch Dermatol 2011 Jul;147(7):790–6.
- Vajdic CM, Chong AH, Kelly PJ, et al. Survival after cutaneous melanoma in kidney transplant recipients: a population-based matched cohort study. Am J Transplant 2014 Jun;14(6):1368–75.
- Penn I. Malignant melanoma in organ allograft recipients. Transplantation 1996 Jan 27;61(2):274–8.
- Dapprich DC, Weenig RH, Rohlinger AL, et al. Outcomes of melanoma in recipients of solid organ transplant. J Am Acad Dermatol 2008 Sep;59(3):405–17.
- Xiao D, Craig JC, Chapman JR, Dominguez-Gil B, Tong A, Wong G. Donor cancer transmission in kidney transplantation: a systematic review. Am J Transplant 2013 Oct;13(10):2645–52.
- Penn I. Transmission of cancer from organ donors. Ann Transpl 1997;2(4):7–12.
- Morris-Stiff G, Steel A, Savage P, et al. Transmission of donor melanoma to multiple organ transplant recipients. Am J Transplant 2004 Mar;4(3):444–6.
- Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol 2010 Aug;11(8):790–6.
- MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Engl J Med 2003 Feb 6;348(6):567–8.
- Kim JK, Carmody IC, Cohen AJ, Loss GE. Donor transmission of malignant melanoma to a liver graft recipient: case report and literature review. Clin Transplant 2009 Aug–Sep;23(4):571–4.
- Bajaj NS, Watt C, Hadjiliadis D, et al. Donor transmission of malignant melanoma in a lung transplant recipient 32 years after curative resection. Transplant Int 2010 Jul;23(7):e26–31.
- Ceribelli M, Kelly PN, Shaffer AL, et al. Blockade of oncogenic I kappa B kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A 2014 Aug 5;111(31):11365–70.
- Ulrich C, Jurgensen JS, Degen A, et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br J Dermatol 2009 Nov;161 Suppl. 3:78–84.
- Burgi A, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer 2005 Oct 1;104(7):1505–11.
- Famenini S, Martires KJ, Zhou H, Xavier MF, Wu JJ. Melanoma in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. J Am Acad Dermatol 2015 Jan;72(1):78–84.
- McGregor JM, Barker JN, MacDonald DM. The development of excess numbers of melanocytic naevi in an immunosuppressed identical twin. Clin Exp Dermatol 1991 Mar;16(2):131–2.
- Smith CH, McGregor JM, Barker JN, Morris RW, Rigden SP, MacDonald DM. Excess melanocytic nevi in children with renal allografts. J Am Acad Dermatol 1993 Jan;28(1):51–5.
- Grob JJ, Bastuji-Garin S, Vaillant L, et al. Excess of nevi related to immunodeficiency: a study in HIV-infected patients and renal transplant recipients. J Invest Dermatol 1996 Nov;107(5):694–7.
- Barker JN, MacDonald DM. Eruptive dysplastic naevi following renal transplantation. Clin Exp Dermatol 1988 Mar;13(2):123–5.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol 2003 Dec;49(6):1020–2.
- Duvic M, Lowe L, Rapini RP, Rodriguez S, Levy ML. Eruptive dysplastic nevi associated with human immunodeficiency virus infection. Arch Dermatol 1989 Mar;125(3):397–401.
- Betlloch I, Amador C, Chiner E, Pasquau F, Calpe JL, Vilar A. Eruptive melanocytic nevi in human immunodeficiency virus infection. Int J Dermatol 1991 Apr;30(4):303.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol 2006 May;154(5):880–4.
- de Boer NK, Kuyvenhoven JP. Eruptive benign melanocytic naevi during immunosuppressive therapy in a Crohn's disease patient. Inflamm Bowel Dis 2011 Jun;17(6):E26.
- Braun SA, Helbig D, Frank J, Hanneken S. [Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis.] Hautarzt 2012 Oct;63(10):756–9.
- Martin Hernandez JM, Donat Colomer J, Monteagudo Castro C, et al. [Acral eruptive nevi after chemotherapy in children with acute lymphoblastic leukemia.] Anales Pediatr (Barcelona) 2006 Sep;65(3):260–2.
- Piaserico S, Alaibac M, Fortina AB, Peserico A. Clinical and dermatoscopic fading of post-transplant eruptive melanocytic nevi after suspension of immunosuppressive therapy. J Am Acad Dermatol 2006 Feb;54(2):338–40.
- Sekulic A, Colgan MB, Davis MD, DiCaudo DJ, Pittelkow MR. Activating BRAF mutations in eruptive melanocytic naevi. Br J Dermatol 2010 Nov;163(5):1095–8.
- John JK, Smalley KS. Identification of BRAF mutations in eruptive melanocytic nevi: new insights into melanomagenesis? Exp Rev Anticancer Ther 2011 May;11(5):711–14.
- Ameen M. The impact of human immunodeficiency virus-related diseases on pigmented skin types. Br J Dermatol 2013 Oct;169 Suppl. 3:11–18.
- Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nature Rev Clin Oncol 2014 Apr;11(4):223–38.
- Gramolelli S, Schulz TF. The role of Kaposi sarcoma-associated herpesvirus in the pathogenesis of Kaposi sarcoma. J Pathol 2015 Jan;235(2):368–80.
- Frances C, Marcelin AG, Legendre C, et al. The impact of preexisting or acquired Kaposi sarcoma herpesvirus infection in kidney transplant recipients on morbidity and survival. Am J Transplant 2009 Nov;9(11):2580–6.
- Razonable RR. Human herpesviruses 6, 7 and 8 in solid organ transplant recipients. Am J Transplant 2013 Feb;13 Suppl. 3:67–77; quiz -8.
- Lebbe C, Porcher R, Marcelin AG, et al. Human herpesvirus 8 (HHV8) transmission and related morbidity in organ recipients. Am J Transplant 2013 Jan;13(1):207–13.
- Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood 2012 Nov 15;120(20):4150–9.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol 2013 Mar;133(3):642–6.
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet 2002 Feb 9;359(9305):497–8.
- Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Kaposi sarcoma and Merkel cell carcinoma after autoimmune disease. Int J Cancer 2012 Aug 1;131(3):E326–8.
- Asgari MM, Sokil MM, Warton EM, Iyer J, Paulson KG, Nghiem P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol 2014 Jul;150(7):716–23.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst 2015 Feb;107(2).
- Ma JE, Brewer JD. Merkel cell carcinoma in immunosuppressed patients. Cancers 2014;6(3):1328–50.
- Koljonen V, Kukko H, Pukkala E, et al. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer 2009 Oct 20;101(8):1444–7.
- Kanitakis J. Rare skin cancers. Cancer Treat Res 2009;146:323–8.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation. J Am Acad Dermatol 2014 Oct;71(4):684–90.
- Lanoy E, Costagliola D, Engels EA. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer 2010 Apr 1;126(7):1724–31.
- Izikson L, Nornhold E, Iyer JG, Nghiem P, Zeitouni NC. Merkel cell carcinoma associated with HIV: review of 14 patients. AIDS (London) 2011 Jan 2;25(1):119–21.
- Wieland U, Kreuter A. Merkel cell polyomavirus infection and Merkel cell carcinoma in HIV-positive individuals. Curr Opin Oncol 2011 Sep;23(5):488–93.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol 2011 Feb;22(2):250–6.
- Koljonen V, Kukko H, Tukiainen E, et al. Second cancers following the diagnosis of Merkel cell carcinoma: a nationwide cohort study. Cancer Epidemiol 2010 Feb;34(1):62–5.
- Lok C, Viseux V, Denoeux JP, Bagot M. Post-transplant cutaneous T-cell lymphomas. Crit Rev Oncol/Hematol 2005 Oct;56(1):137–45.
- Pomerantz RG, Campbell LS, Jukic DM, Geskin LJ. Posttransplant cutaneous T-cell lymphoma: case reports and review of the association of calcineurin inhibitor use with posttransplant lymphoproliferative disease risk. Arch Dermatol 2010 May;146(5):513–16.
- Seckin D, Barete S, Euvrard S, et al. Primary cutaneous posttransplant lymphoproliferative disorders in solid organ transplant recipients: a multicenter European case series. Am J Transplant 2013 Aug;13(8):2146–53.
- Ravat FE, Spittle MF, Russell-Jones R. Primary cutaneous T-cell lymphoma occurring after organ transplantation. J Am Acad Dermatol 2006 Apr;54(4):668–75.
- Beylot-Barry M, Vergier B, Masquelier B, et al. The spectrum of cutaneous lymphomas in HIV infection: a study of 21 cases. Am J Surg Pathol 1999 Oct;23(10):1208–16.
- Wilkins K, Turner R, Dolev JC, LeBoit PE, Berger TG, Maurer TA. Cutaneous malignancy and human immunodeficiency virus disease. J Am Acad Dermatol 2006 Feb;54(2):189–206; quiz 7–10.
- Zhang P, Chiriboga L, Jacobson M, et al. Mycosis fungoides like T-cell cutaneous lymphoid infiltrates in patients with HIV infection. Am J Dermatopathol 1995 Feb;17(1):29–35.
- Bachelez H, Hadida F, Parizot C, et al. Oligoclonal expansion of HIV-specific cytotoxic CD8 T lymphocytes in the skin of HIV-1-infected patients with cutaneous pseudolymphoma. J Clin Invest 1998 Jun 1;101(11):2506–16.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol 2014 Aug;53(8):966–70.
- Harwood CA, Swale VJ, Bataille VA, et al. An association between sebaceous carcinoma and microsatellite instability in immunosuppressed organ transplant recipients. J Invest Dermatol 2001 Feb;116(2):246–53.
- Harwood CA, McGregor JM, Swale VJ, et al. High frequency and diversity of cutaneous appendageal tumors in organ transplant recipients. J Am Acad Dermatol 2003 Mar;48(3):401–8.
- Mahomed F, Blok J, Grayson W. The squamous variant of eccrine porocarcinoma: a clinicopathological study of 21 cases. J Clin Pathol 2008 Mar;61(3):361–5.
- Shiels MS, Engels EA. Increased risk of histologically defined cancer subtypes in human immunodeficiency virus-infected individuals: clues for possible immunosuppression-related or infectious etiology. Cancer 2012 Oct 1;118(19):4869–76.
- Dewan P, Jawad A, Goldsmith P, Harwood C, Cerio R. Melanoma in patients with rheumatoid arthritis treated with antitumour necrosis factor: cause or coincidence? Report of two cases. Br J Dermatol 2009 Dec;161(6):1412–14.
- Mansfield AS, Rabe KG, Slager SL, et al. Skin cancer surveillance and malignancies of the skin in a community-dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia. J Oncol Pract 2014 Jan;10(1):e1–4.
- Warschaw KE, Eble JN, Hood AF, Wolverton SE, Halling KC. The Muir–Torre syndrome in a black patient with AIDS: histopathology and molecular genetic studies. J Cutan Pathol 1997 Sep;24(8):511–18.
- Bhatia K, Shiels MS, Berg A, Engels EA. Sarcomas other than Kaposi sarcoma occurring in immunodeficiency: interpretations from a systematic literature review. Curr Opin Oncol 2012 Sep;24(5):537–46.
- Kemp JD, Stenn KS, Arons M, Fischer J. Metastasizing atypical fibroxanthoma. Coexistence with chronic lymphocytic leukemia. Arch Dermatol 1978 Oct;114(10):1533–5.
- Perrett CM, Cerio R, Proby CM, Harwood CA. Atypical fibroxanthoma in a renal transplant recipient. Histopathology 2005 Sep;47(3):326–7.
- Cooper JZ, Newman SR, Scott GA, Brown MD. Metastasizing atypical fibroxanthoma (cutaneous malignant histiocytoma): report of five cases. Dermatol Surg 2005 Feb;31(2):221–5; discussion 5.
- Colgan MB, Brewer JD, Weaver AL, Roenigk RK, Otley CC. Atypical fibroxanthoma in the setting of chronic lymphocytic leukemia and other non-Hodgkin lymphomas. Dermatol Surg 2011 May;37(5):671–6.
- Kubica AW, Rose PS, Weaver AL, Brewer JD. Increased metastasis of malignant fibrous histiocytoma in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. Mayo Clin Proc 2011 Aug;86(8):738–43.
- Paquet P, Pierard GE. Invasive atypical fibroxanthoma and eruptive actinic keratoses in a heart transplant patient. Dermatology 1996;192(4):411–13.
- Lai KN, Lai FM, King WW, et al. Dermatofibrosarcoma protuberans in a renal transplant patient. Australian New Zealand J Surg 1995 Dec;65(12):900–2.
- Picciotto F, Basolo B, Massara C, et al. Dermatofibrosarcoma protuberans at the site of arteriovenous fistula in a renal transplant recipient. Transplantation 1999 Oct 15;68(7):1074–5.
- Stasko T, Brown MD, Carucci JA, et al. Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Dermatol Surg 2004 Apr;30(4 Pt 2):642–50.
- Zwald FO, Christenson LJ, Billingsley EM, et al. Melanoma in solid organ transplant recipients. Am J Transplant 2010 May;10(5):1297–304.
- Geusau A, Pohanka E. Aftercare – a multi-disciplinary approach. Cancer Treat Res 2009;146:405–15.
- Morelon E, Mahe E, Touraine J-L. The role of the transplant physician in the management of skin cancers after organ transplantation. Skin cancer after organ transplantation. Cancer Treatment Res 2009;146:377–90.
- Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol 2002 Jul;47(1):1–17; quiz 8–20.
- Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treatment Options Oncol 2012 Sep;13(3):354–76 (review).
- de Graaf YG, Basdew VR, van Zwan-Kralt N, Willemze R, Bavinck JN. The occurrence of residual or recurrent squamous cell carcinomas in organ transplant recipients after curettage and electrodesiccation. Br J Dermatol 2006 Mar;154(3):493–7.
- Wennberg AM, Stenquist B, Stockfleth E, et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation 2008 Aug 15;86(3):423–9.
- Wulf HC, Pavel S, Stender I, Bakker-Wensveen CA. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol 2006;86(1):25–8.
- Kwon S, Dong ZM, Wu PC. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma: clinical experience and review of literature. World J Surg Oncol 2011;9:80.
- Allen JE, Stolle LB. Utility of sentinel node biopsy in patients with high-risk cutaneous squamous cell carcinoma. Eur J Surg Oncol 2015 Feb;41(2):197–200.
- Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthetic Dermatol 2010 Apr;3(4):39–48.
- Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg 2009 Apr;35(4):574–85.
- Veness MJ, Harris D. Role of radiotherapy in the management of organ transplant recipients diagnosed with non-melanoma skin cancers. Australasian Radiol 2007 Feb;51(1):12–20.
- Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 2005 Mar 31;352(13):1317–23.
- Boratynska M, Zmonarski SC, Klinger M. Reccurence of Kaposi's sarcoma after increased exposure to sirolimus. Int Immunopharmacol 2006 Dec 20;6(13–14):2018–22.
- Lebbe C, Euvrard S, Barrou B, et al. Sirolimus conversion for patients with posttransplant Kaposi's sarcoma. Am J Transplant 2006 Sep;6(9):2164–8.
- Nicholson S. Management of metastatic skin cancers in organ transplant recipients. Cancer Treat Res 2009;146:467–81.
- Wells JL, 3rd, Shirai K. Systemic therapy for squamous cell carcinoma of the skin in organ transplant recipients. Am J Clin Oncol 2012 Oct;35(5):498–503.
- Bejar C, Maubec E. Therapy of advanced squamous cell carcinoma of the skin. Curr Treatment Options Oncol 2014 Jun;15(2):302–20.
- Martinez JC, Otley CC, Okuno SH, Foote RL, Kasperbauer JL. Chemotherapy in the management of advanced cutaneous squamous cell carcinoma in organ transplant recipients: theoretical and practical considerations. Dermatol Surg 2004 Apr;30(4 Pt 2):679–86.
- Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: Molecular bases for EGFR-targeted therapy. Pathol Res Pract 2011;207(6):337–42.
- Suen JK, Bressler L, Shord SS, Warso M, Villano JL. Cutaneous squamous cell carcinoma responding serially to single-agent cetuximab. Anti-Cancer Drugs 2007 Aug;18(7):827–9.
- Leard LE, Cho BK, Jones KD, et al. Fatal diffuse alveolar damage in two lung transplant patients treated with cetuximab. J Heart Lung Transpl 2007 Dec;26(12):1340–4.
- Lewis CM, Glisson BS, Feng L, et al. A Phase II Study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res 2012 Mar 1;18(5):1435–46.
- Heath CH, Deep NL, Nabell L, et al. Phase 1 study of erlotinib plus radiation therapy in patients with advanced cutaneous squamous cell carcinoma. Int J Radiation Oncol Biol Phys 2013 Apr 1;85(5):1275–81.
- Gordon LG, Scuffham PA, van der Pols JC, McBride P, Williams GM, Green AC. Regular sunscreen use is a cost-effective approach to skin cancer prevention in subtropical settings. J Invest Dermatol 2009 Dec;129(12):2766–71.
- Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007 Oct 15;179(8):5204–10.
- Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009 Feb 12;113(7):1581–8.
- Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol 2014 Jul 1;32(19):e69–71.
- Burke MM, Kluger HM, Golden M, Heller KN, Hoos A, Sznol M. Case Report: response to ipilimumab in a patient with HIV with metastatic melanoma. J Clin Oncol 2011 Nov 10;29(32):e792–4.
- Kanitakis J, Baldassini S, Lora V, Euvrard S. BRAF mutations in melanocytic tumors (nevi and melanomas) from organ transplant recipients. Eur J Dermatol 2010 Mar–Apr;20(2):167–71.
- Otley CC. Pre-transplantation dermatologic screening and prophylaxis. The SCOPE Collaborative Group In: Rosen T, ed. Cancer Treatment and Research, vol. 146. London: Springer, 2009.
- European Best Practice Guidelines for Renal Transplantation. Section IV: Long-term management of the transplant recipient. Nephrol Dial Transplant 2002;17 Suppl. 4:1–67.
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009 Nov;9 Suppl. 3:S1–155.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999 Aug 28;354(9180):723–9.
- Green A, Harwood C, Lear J, Proby C, Sinnya S, Soyer H. Skin cancer prevention: recent evidence from randomized controlled trials. Curr Dermatol Rep 2012;1(3):123–30.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993 Oct 14;329(16):1147–51.
- Ulrich C, Johannsen A, Rowert-Huber J, Ulrich M, Sterry W, Stockfleth E. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol 2010 Jul–Aug;20(4):482–8.
- Loescher LJ, Hansen C, Hepworth JT, Quale L, Sligh J. A preliminary study of a video intervention to inform solid organ transplant recipients about skin cancer. Transplant Proc 2013 Nov;45(9):3187–9.
- Reichrath J. Dermatologic management, sun avoidance and vitamin D status in organ transplant recipients (OTR). J Photochem Photobiol B 2010 Nov 3;101(2):150–9.
- Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013 Sep;169(3):502–18.
- Criscione VD, Weinstock MA, et al. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009 Jun 1;115(11):2523–30.
- Dodson JM, DeSpain J, Hewett JE, Clark DP. Malignant potential of actinic keratoses and the controversy over treatment. A patient-oriented perspective. Arch Dermatol 1991 Jul;127(7):1029–31.
- van Zuuren EJ, Posma AN, Scholtens RE, Vermeer BJ, van der Woude FJ, Bouwes Bavinck JN. Resurfacing the back of the hand as treatment and prevention of multiple skin cancers in kidney transplant recipients. J Am Acad Dermatol 1994 Nov;31(5 Pt 1):760–4.
- Amini S, Viera MH, Valins W, Berman B. Nonsurgical innovations in the treatment of nonmelanoma skin cancer. J Clin Aesthetic Dermatol 2010 Jun;3(6):20–34.
- Kurwa HA, Yong-Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol 1999 Sep;41(3 Pt 1):414–18.
- Witheiler DD, Lawrence N, Cox SE, Cruz C, Cockerell CJ, Freemen RG. Long-term efficacy and safety of Jessner's solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Dermatol Surg 1997 Mar;23(3):191–6.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol 2007 Feb;156(2):320–8.
- Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol 2004 May;50(5):714–21.
- Stockfleth E, Sterry W, Carey-Yard M, Bichel J. Multicentre, open-label study using imiquimod 5% cream in one or two 4-week courses of treatment for multiple actinic keratoses on the head. Br J Dermatol 2007 Dec;157 Suppl 2:41–6.
- Ulrich C, Bichel J, Euvrard S, et al. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol 2007 Dec;157 Suppl. 2:25–31.
- Brown VL, Atkins CL, Ghali L, Cerio R, Harwood CA, Proby CM. Safety and efficacy of 5% imiquimod cream for the treatment of skin dysplasia in high-risk renal transplant recipients: randomized, double-blind, placebo-controlled trial. Arch Dermatol 2005 Aug;141(8):985–93.
- Dragieva G, Prinz BM, Hafner J, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients. Br J Dermatol 2004 Jul;151(1):196–200.
- Dragieva G, Hafner J, Dummer R, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen's disease in transplant recipients. Transplantation 2004 Jan 15;77(1):115–21.
- Piaserico S, Belloni Fortina A, Rigotti P, et al. Topical photodynamic therapy of actinic keratosis in renal transplant recipients. Transplant Proc 2007 Jul–Aug;39(6):1847–50.
- Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg 2010 May;36(5):652–8.
- de Graaf YG, Kennedy C, Wolterbeek R, Collen AF, Willemze R, Bouwes Bavinck JN. Photodynamic therapy does not prevent cutaneous squamous-cell carcinoma in organ-transplant recipients: results of a randomized-controlled trial. J Invest Dermatol 2006 Mar;126(3):569–74.
- Schena FP, Pascoe MD, Alberu J, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 2009 Jan 27;87(2):233–42.
- Guba M, Pratschke J, Hugo C, et al. Early conversion to a sirolimus-based, calcineurin-inhibitor-free immunosuppression in the SMART trial: observational results at 24 and 36months after transplantation. Transplant Int 2012 Apr;25(4):416–23.
- Gutierrez-Dalmau A, Sanchez-Fructuoso A, Sanz-Guajardo A, et al. Efficacy of conversion to sirolimus in posttransplantation Kaposi's sarcoma. Transplant Proc 2005 Nov;37(9):3836–8.
- Yaich S, Charfeddine K, Zaghdane S, et al. Sirolimus for the treatment of Kaposi sarcoma after renal transplantation: a series of 10 cases. Transplant Proc 2012 Nov;44(9):2824–6.
- de Fijter JW. Use of proliferation signal inhibitors in non-melanoma skin cancer following renal transplantation. Nephrol Dial Transplant 2007 May;22 Suppl. 1:i23–6.
- Rival-Tringali AL, Euvrard S, Decullier E, Claudy A, Faure M, Kanitakis J. Conversion from calcineurin inhibitors to sirolimus reduces vascularization and thickness of post-transplant cutaneous squamous cell carcinomas. Anticancer Res 2009 Jun;29(6):1927–32.
- Salgo R, Gossmann J, Schofer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-) malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant 2010 Jun;10(6):1385–93.
- Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 2012 Jul 26;367(4):329–39.
- Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 2012 May;12(5):1146–56.
- Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol 2013 Apr 1;31(10):1317–23.
- Alter M, Satzger I, Schrem H, Kaltenborn A, Kapp A, Gutzmer R. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Deutschen Dermatol Gesellsch 2014 Jun;12(6):480–8.
- Tavadia S, Dawn G, Payne C, Ramrakha-Jones V, Murday A, Holmes S. Skin-cancer awareness in Scottish cardiac transplant recipients. Clin Exp Dermatol 2006 May;31(3):354–7.
- Colegio OR, Hanlon A, Olasz EB, Carucci JA. Sirolimus reduces cutaneous squamous cell carcinomas in transplantation recipients. J Clin Oncol 2013 Sep 10;31(26):3297–8.
- Brewer JD. Skin cancer in patients with non-Hodgkin's lymphoma. Exp Rev Dermatol 2010;5:525–33.
- Karayannopoulou G, Euvrard S, Kanitakis J. Differential expression of p-mTOR in cutaneous basal and squamous cell carcinomas likely explains their different response to mTOR inhibitors in organ-transplant recipients. Anticancer Res 2013 Sep;33(9):3711–14.
- Knoll GA, Koko MB, Lick RM, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014 Nov 24;349.
- Cheepala SB, Syed Z, Trutschl M, Cvek U, Clifford JL. Retinoids and skin: microarrays shed new light on chemopreventive action of all-trans retinoic acid. Mol Carcinogen 2007 Aug;46(8):634–9.
- Hardin J, Mydlarski PR. Systemic retinoids: chemoprevention of skin cancer in transplant recipients. Skin Ther Lett 2010 Jul–Aug;15(7):1–4.
- Lien MH, Fenske NA, Glass LF. Advances in the chemoprevention of non-melanoma skin cancer in high-risk organ transplant recipients. Semin Oncol 2012 Apr;39(2):134–8.
- De Graaf YG, Euvrard S, Bouwes Bavinck JN. Systemic and topical retinoids in the management of skin cancer in organ transplant recipients. Dermatol Surg 2004 Apr;30(4 Pt 2):656–61.
- Shuttleworth D, Marks R, Griffin PJ, Salaman JR. Treatment of cutaneous neoplasia with etretinate in renal transplant recipients. Q J Med 1988 Sep;68(257):717–25.
- Kelly JW, Sabto J, Gurr FW, Bruce F. Retinoids to prevent skin cancer in organ transplant recipients. Lancet 1991 Nov 30;338(8779):1407.
- Gibson GE, O'Grady A, Kay EW, Murphy GM. Low-dose retinoid therapy for chemoprophylaxis of skin cancer in renal transplant recipients. J Eur Acad Dermatol Venereol 1998 Jan;10(1):42–7.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol 1999 Apr;140(4):656–60.
- George R, Weightman W, Russ GR, Bannister KM, Mathew TH. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol 2002 Nov;43(4):269–73.
- de Sevaux RG, Smit JV, de Jong EM, van de Kerkhof PC, Hoitsma AJ. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol 2003 Sep;49(3):407–12.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol 1995 Aug;13(8):1933–8.
- Martinez JC, Otley CC, Euvrard S, Arpey CJ, Stasko T. Complications of systemic retinoid therapy in organ transplant recipients with squamous cell carcinoma. Dermatol Surg 2004 Apr;30(4 Pt 2):662–6.
- Yarosh D, Klein J, O'Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet 2001 Mar 24;357(9260):926–9.
- Jirakulaporn T, Endrizzi B, Lindgren B, Mathew J, Lee PK, Dudek AZ. Capecitabine for skin cancer prevention in solid organ transplant recipients. Clin Transplant 2011 Jul–Aug;25(4):541–8.
- Endrizzi B, Ahmed RL, Ray T, Dudek A, Lee P. Capecitabine to reduce nonmelanoma skin carcinoma burden in solid organ transplant recipients. Dermatol Surg 2013 Apr;39(4):634–45.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer 2012 Nov 1;118(21):5395–402.
- Webster AC, Wong G, Craig JC, Chapman JR. Managing cancer risk and decision making after kidney transplantation. Am J Transplant 2008 Nov;8(11):2185–91.
- Garg S, Carroll RP, Walker RG, Ramsay HM, Harden PN. Skin cancer surveillance in renal transplant recipients: re-evaluation of U.K. practice and comparison with Australian experience. Br J Dermatol 2009 Jan;160(1):177–9.
- Christenson LJ, Geusau A, Ferrandiz C, et al. Specialty clinics for the dermatologic care of solid-organ transplant recipients. Dermatol Surg 2004 Apr;30(4 Pt 2):598–603.
- Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients – where do we stand today? Am J Transplant 2008 Nov;8(11):2192–8.
- Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation 2009 Jun 15;87(11):1667–71.
- Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol 2006 Nov;155(5):916–25.
- Robinson JK, Turrisi R, Mallett KA, et al. Efficacy of an educational intervention with kidney transplant recipients to promote skin self-examination for squamous cell carcinoma detection. Arch Dermatol 2011 Jun;147(6):689–95.
- Trinh N, Novice K, Lekakh O, Means A, Tung R. Use of a brief educational video administered by a portable video device to improve skin cancer knowledge in the outpatient transplant population. Dermatol Surg 2014 Nov;40(11):1233–9.
- Clowers-Webb HE, Christenson LJ, Phillips PK, et al. Educational outcomes regarding skin cancer in organ transplant recipients: Randomized intervention of intensive vs standard education. Arch Dermatol 2006 Jun;142(6):712–18.
- Thomas BR, Barnabas A, Agarwal K, et al. Patient perception of skin-cancer prevention and risk after liver transplantation. Clin Exp Dermatol 2013 Dec;38(8):851–6.
- Kauffman HM, Woodle ES, Cole EH, Paykin C. Transplant recipient's knowledge of posttransplant malignancy risk: implications for educational programs. Transplantation 2008 Apr 15;85(7):928–33.
- Kasiske BL, Vazquez MA, Harmon WE, et al. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol 2000 Oct;11 Suppl. 15:S1–86.
- Gogia R, Binstock M, Hirose R, Boscardin WJ, Chren MM, Arron ST. Fitzpatrick skin phototype is an independent predictor of squamous cell carcinoma risk after solid organ transplantation. J Am Acad Dermatol 2013 Apr;68(4):585–91.
- National Institute for Health and Care Excellence (NICE). NICE The Guidelines Manual. London: NICE, 2006.
- Magro F, Peyrin-Biroulet L, Sokol H, et al. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohn Colitis 2014 Jan;8(1):31–44.
- Skin Care in Organ Transplant Patients (SCOPE). Skin Care in Organ Transplant Patients (cited 2015 30/07/2015) http://www.scopenetwork.org (last accessed September 2015).
- International Transplant Skin Cancer Collaborative (ITSCC) (cited 2015 30/07/2015) http://www.itscc.org/ (last accessed September 2015).
- British Society for Skin Care in Immunosuppressed Individuals (BSSCII) (cited 2015 30/07/2015) www.bsscii.org.uk (last accessed September 2015).