CHAPTER 149
The Skin and Endocrine Disorders
Ralf Paus
Centre for Dermatology Research, Institute of Inflammation and Repair, University of Manchester, Manchester, UK; and Department of Dermatology, University of Münster, Germany
Introduction and overview
When classical endocrine glands and their systemically secreted products (hormones) were originally recognized, it did not take long to realize that excessive or insufficient circulating levels of these hormones could affect the skin. Only much later, when the specific receptors for these steroid and peptide hormones were identified and the molecular basis of interactions between hormonal ligands and their receptors were better understood, was it realized that cutaneous responses to endocrine abnormalities reflect the fact that all constituent cell populations of human skin express multiple cognate receptors not just for these hormones but also for many other neuromediators. Though the mechanisms involved are still insufficiently understood, the visible effects of abnormal ligand–receptor interactions on the skin certainly provide important diagnostic clues to underlying endocrine disease. Yet, the importance of hormones in dermatology goes well beyond this.
The field of dermatoendocrinology has undergone a major revolution over the past two decades: human skin and its appendages are now recognized not only as prominent hormone target tissues, but also as major endocrine organs themselves. Human scalp hair follicles have provided a model for exploring this relatively recent research frontier in investigative dermatology and for identifying ‘novel’ functions of classical neurohormones, particularly as they represent exquisitely hormone-sensitive mini-organs [1, 2, 3]. Therefore, where appropriate, hair follicles will be used as models for illustrating and exploring general principles.
This chapter reviews the key elements of the biological basis of dermatoendocrinology to help clinicians understand how general skin signs and symptoms such as pruritus, skin dryness, hair loss, hypertrichosis, hirsutism, hyperhidrosis and/or hyperpigmentation result from defined endocrine disorders. Also discussed are the characteristic skin manifestations of underlying endocrine disease and a practical approach to skin patients with suspected underlying hormonal problems, with special emphasis on thyroid and pituitary disorders and mention of dermatologically relevant drugs which may cause endocrine abnormalities that affect the skin. Diabetes (see Chapter 64) and hormonally active vitamins (hyper- or hypovitaminosis A or D) (see Chapter 63) are discussed elsewhere.
This chapter concludes by examining why the fact that skin operates as a complex (neuro-) endocrine organ has important practical implications for future dermatological therapy: the clinical significance and biomedical fascination of dermatoendocrinology extend beyond providing diagnostic clues to the identification of underlying endocrine disease.
Biological basis of dermatoendocrinology
Principles of endocrinology
Enshrined as a concept by Bayliss’ and Starling's famous Croonian Lecture [4], ‘hormones’ have generally come to be understood as relatively stable secreted molecules that are released into the bloodstream to reach and modulate the function of distant tissue targets. This ‘endocrine’ secretory activity is distinguished from ‘paracrine’ hormone secretion that targets adjacent cells, ‘autocrine’ signalling which describes the autostimulation of a cell with a hormone released by that cell and ‘intracrine’ signalling whereby the hormone in question does not leave the cell that has produced it and acts intracellularly [5].
Classical endocrine signalling operates along several central axes through which the brain controls key functions of ‘professional’ endocrine glands. The most familiar of these are the hypothalamopituitary–adrenal (HPA) and the hypothalamopituitary–thyroid (HPT) axes; central nervous system (CNS) controlled signalling axes dominated by prolactin, catecholamines or acetylcholine are also well known (Figure 149.1). If hormone imbalances within these central axes lead to excessive or insufficient serum levels of key hormones, peripheral tissues such as the skin will be affected provided that they express cognate hormone receptors. Such imbalances can result from a wide range of disorders including hormone-secreting tumours, autoimmune attack directed at endocrine glands resulting in either failure of or excessive hormone secretion, biochemical abnormalities, environmental or nutritional signals, drugs, psychological and emotional stress, or the physiological consequences of puberty, menopause or ageing on systemic hormone levels.

Figure 149.1 Schematic representation of three hypothalamopituitary axes that impact on human skin: key elements of the central hypothalamopituitary–adrenal (HPA) and the hypothalamopituitary–thyroid (HPT) axes, and the central control of prolactin (PRL) release from the pituitary gland. 5-HT, 5-hydroxytryptamine; ACTH, adrenocorticotrophic hormone; CRH, corticotropin-releasing hormone; E2, oestrogen; EGF, epidermal growth factor; FGF, fibroblast growth factor; TSH, thyrotropin; TRH, thyrotropin-releasing hormone; VIP, vasoactive intestinal peptide. (Redrawn from Paus et al. Trends Mol Med 2014 [3].
Reproduced with permission from Elsevier.)
The term endocrine was originally coined to distinguish the control of organ function by secreted hormones from those exerted more directly by the nervous system. With increased knowledge, however, the borders between endocrinology, neuroendocrinology, neuropharmacology and other neuroscience disciplines have become indistinct. Hormones can act both locally and at far distant body sites as well as on the nervous system, and the distribution of high-affinity hormone receptors is far more widespread than previously thought. Unsurprisingly, all hormones and neuromediators exert many more biological activities than had been apparent during the early years following their discovery. For example, classical steroid hormones generated outside the CNS or administered therapeutically (e.g. glucocorticoids, retinoids, androgens, oestrogens) exert profound neurochemical effects in the brain, while classical neurohormones, neuropeptides and neurotransmitters have an impact not only on the growth, regeneration and other specific functions of epithelial and mesenchymal tissues but are also produced by them. Essentially, all tissues and organs, including human skin and its appendages, can generate a wide range of hormones and neuromediators.
Human epidermal and hair follicle keratinocytes, sebocytes and subcutaneous adipocytes are recognized as potent sources of steroid and peptide hormones as well as of a steadily growing list of neuromediators (Table 149.1 and see later). In situ, many of these were first discovered in human scalp hair follicles [3] (Figure 149.2).
Table 149.1 Key components in the human cutaneous (neuro-) endocrine signalling mechanism. For a comprehensive review and additional relevant ligands and receptors see [3, 8, 9, 15].
| Ligand | Intracutaneous receptor | Selected activities | References |
| Generated within human skin and/or its appendages | |||
| CRH | CRHR1, CRHR2 | Stress response coordinator, controls skin HPA axis equivalent and both mast cell activation and local maturation | [8, 9, 26] |
| ACTH | MC2R (MC1R) | Stimulates intracutanous cortisol synthesis, activates mast cells | [9, 26] |
| α-MSH | MC1R | Stimulates pigmentation, maintains hair follicle immune privilege, multiple immune-inhibitory, anti-allergic, tolerogenic and oxidative damage-protective functions, regulates collagen synthesis; possibly also has antimicrobial activities | [42-46] |
| β-endorphin | μ-opioid receptor | Key role in itch modulation, mast cell secretagogue, stimulates pigmentation | [6, 8, 47, 125] |
| Cortisol | GR | Regulates cell metabolism, epidermal barrier function, skin and pilosebaceous immune homeostasis (maintenance of relative immunoinhibition?) | [3, 8, 9, 26, 57] |
| Androgens (DHT) | AR | DTH synthesis controlled by 5-α-reductase, AR stimulation of hair follicles results in either TGF-β or IGF-1 secretion (location dependent); stimulate sebaceous gland | [40, 50, 51, 52] |
| Oestrogens (17-β-oestradiol) | ER | 17-β ER synthesis controlled by aromatase, large gender-dependent differences in response to ER stimulation, promote wound healing; inhibit sebaceous gland | [53-56] |
| Vitamin D3 and its metabolites | VDR | Pleiotropic effects (e.g. inhibit keratinocyte proliferation, stimulate keratin expression, anti-inflammatory) | [39, 41, 57] |
| Retinoids | RAR, RXR | Pleiotropic effects | |
| PPARγ ligands (small lipids) | PPARγ | Inhibit keratinocyte proliferation and hair growth, but maintain epithelial stem cells; anti-inflammatory; promote melanogenesis | [59, 60, 85] |
| Endocannabinoids (e.g. anandamide, 2-AG) | CB1, CB2 | CB1 stimulation inhibits hair growth and excessive mast cell degranulation/maturation, but stimulate sebogenesis | [61, 62, 63, 64, 65] |
| Catecholamines (also derived from intracutaneous nerve fibres) | α-/β-adrenergic receptors | Regulate skin perfusion and sweating; inhibit mast cell degranulation, retard keratinocyte migration and wound healing; regulate melanocyte functions | [30, 33, 34, 36, 66-71] |
| Acetylcholine receptor ligands (also derived from intracutaneous nerve fibres) | Nicotinic and muscarinic acetylcholine receptors | Regulate keratinocyte proliferation, differentiation, migration, adhesion, and apoptosis; activate/attract neurotrophils; stimulate sweating; regulate sebocyte lipid production | [29-30, 72, 73] |
| Melatonin | MT-1, MT-2 | Oxidative damage control, DNA damage repair | [74-80] |
| TRH | TRH-R | Promotes hair growth and keratinocyte mitochondrial function, stimulates hair follicle pigmentation; regulates keratin expression; stimulates epidermal TSH expression; regulates intracutaneous prolactin expression | [81, 82, 83, 84, 87, 88] |
| TSH | TSH-R | Stimulates keratinocyte mitochondrial activity and biogenesis; regulates keratin expression | [3, 27, 28, 84, 366] |
| Prolactin | PRL-R | Inhibits or promotes hair growth (species and gender dependent), stimulates sebum production; immunomodulatory; regulates keratin expression | [3, 87, 88-91] |
| PTH/PTHrp | PTH/PTHrp receptor | Regulates keratinocyte proliferation and differentiation; modulates hair growth (mice); regulate skin angiogenesis | [92-96] |
| Generated (mainly) outside the skin (transported via nerve fibres or blood vessels) | |||
| Substance P | NK1 | Activates mast cells; pro-inflammatory; induces collapse of hair follicle immune privilege | [18, 97, 98] |
| CGRP | CGRP-R | Anti-inflammatory and tolerogenic; guardian of hair follicle immune privilege | [99, 100] |
| Dopamine | DR 1 | Hair growth inhibitory | [101] |
| Thyroid hormones (T3, T4) | TRα, TRβ | Regulate keratin expression; prolong anagen; promote wound healing; stimulate keratinocyte mitochondrial activity and heat production; regulates epithelial stem cell functions | [84, 102-104, 195] |
For additional relevant (neuro-) endocrine ligands and their receptors in human skin, see these papers and references cited therein: proenkephalin [105], dynorphin [106], galanin [107], somatostatin [108, 109, 110], oxytocin [111], neuropeptide Y [112, 113], serotonin [74, 114], leptin [22, 115], endovanilloids [49, 116, 117, 118, 119, 120], erythropoietin [121, 122, 123] and growth hormone (GH) [124].
ACTH, adrenocorticotropic hormone; AR, androgen receptor; CB1/2, cannabinoid receptors 1/2; CGRP/CGRP-R, calcitonin gene related protein/CGRP receptor; CRH, corticotropin-releasing hormone (synonym: CRF); CRHR1/R2, CRH receptors 1/2; DR1, dopamine receptor type 1; ER, oestrogen receptor; GR, glucocorticoid receptor; MC1R/2R, melanocortin receptors 1/2; α-MSH, α-melanocyte-stimulating hormone (synonym: melanotropin); MT1/2, melatonin receptors 1/2; NK1, neurokinin receptor 1; PPARγ, peroxisome proliferator activator receptor γ; PRLR, prolactin receptor; RAR/RORα/RXR, retinoid receptors; PTH, parathyroid hormone; TRs, thyroid hormone receptors; TRPV1/3/4, transient receptor potential ion channels of vanilloid type 1/3/4; VDR, vitamin D receptor.
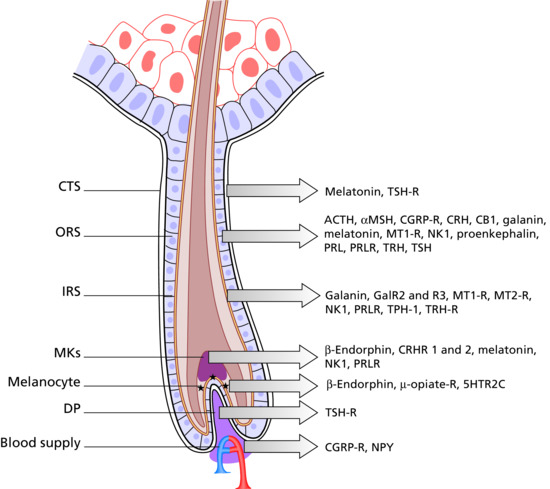
Figure 149.2 The human hair follicle as a (neuro-) endocrine microcosm. This schematic drawing of a human anagen VI scalp hair follicle indicates the main intrafollicular expression sites for a few selected neuroendocrine mediators and their receptors. Note that they are all located within the hair follicle epithelium, namely the outer root sheath (ORS). Just as is seen in the interactions between the hypothalamus, the pituitary and the adrenal glands, the ORS produces and secretes CRH, which stimulates ACTH production and thereby local cortisol synthesis; cortisol in turn down-regulates intrafollicular CRH production. This complex neuroendocrine activity, complete with positive and negative feedback loops, occurs alongside and often within the same hair follicle tissue compartments, as does the metabolism and/or synthesis of steroid hormones such as retinoids, androgens, oestrogens, progesterones and vitamin D derivatives. These hormones modulate the synthesis of numerous other signalling molecules such as cytokines, growth factors, leukotrienes and antimicrobial peptides, and in turn are modulated by additional signals received from the hair follicle's vasculature and innervation. It remains one of the great enigmas of skin biology how all this signalling activity is coordinated and controlled within a tiny mini-organ. CB1, cannabinoid 1 receptor; CGRP-R, calcitonin gene related peptide receptor; CRHR1 and -2, corticotropin-releasing hormone 1 and 2 receptor; CTS, connective tissue sheath; DP, dermal papillae; GalR2 and -3, galanin 2 and 3 receptor; IRS, inner root sheath; MKs, matrix keratinocytes; MT-1R, MT-2R, melatonin 1 and 2 receptor; NK 1, neurokinin 1 receptor; NP-Y, neuropeptide Y; ORS, outer root sheath; PRL, prolactin; PRLR, prolactin receptor; TPH-1, tryptophan hydroxylase; TRH, thyrotropin-releasing hormone; TRH-R, thyrotropin-releasing hormone receptor; TSH-R, thyroid-stimulating hormone receptor. (Redrawn from Paus et al. Trends Mol Med 2014 [3].
Reproduced with permission from Elsevier.)
Current evidence suggests that inflammation, oxidative and psycho-emotional stress, UV irradiation, microbiomal signals, certain nutritional and sensory stimuli and some drugs can influence intracutaneous hormone and neuromediator production, often in a similar though not necessarily identical manner to the regulation of central hormone production [3, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. Thus, endocrine organs, the CNS, the peripheral nervous system and peripheral tissues such as the skin share and generate a comparable spectrum of hormonal and neuromediator ligands and express a very similar repertoire of cognate receptors which together facilitate intersystem communication, even though the subtype and preferred intracellular signal transduction pathways may differ (e.g. between the brain and the skin). Hormones such as prolactin and leptin also exert potent cytokine-like effects in addition to their classical endocrine activities [20, 21, 22, 23, 24], while steroid hormones, which regulate the function of multiple non-neuronal cells outside the nervous system, double as profound neuromodulators and neurotransmitters [25].
Key elements of the three major central endocrine signalling axes which regulate the body's overall metabolism, its response to environmental stressors and many aspects of specific organ function, i.e. the HPA axis, the HPT axis and the regulatory system of pituitary prolactin release, have been identified in other peripheral tissues together with negative and positive feedback regulatory loops. In conclusion, the traditional distinctions between hormones, neuropeptides, neurotransmitters and cytokines have ceased to be meaningful.
Skin as a (neuro-) endocrine organ
This explicitly includes human skin, which is now known to contain integral functional equivalents of the HPA axis (CRH → ACTH → cortisol) [3, 6, 8, 9, 26] and key elements of the HPT axis (TRH→TSH) [3, 27, 28]. In addition, human skin displays complete cholinergic and adrenergic signalling systems [29, 30, 31, 32, 33] with potentially important functions in the control of wound healing [34, 35, 36], epidermal immunity [37] and skin cancer [38]. Human skin also metabolizes numerous hormones and neuromediators, including glucocorticoids, androgens, oestrogens, neuropeptides such as substance P, and lipid neuromediators such as endocannabinoids [3, 8, 9, 10, 39].
Human skin and its pilosebaceous units and sweat glands also produce a bewildering range of steroid and peptide hormones, ranging from vitamin D, androgens, oestrogens and retinoids to erythropoietin, and classical neurohormones such as corticotrophin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), thyroid-stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH), α-MSH, β-endorphin and other opioids, melatonin and prolactin, along with multiple other neuromediators; this is complemented by lipid mediators such as endocannabinoids and endovanilloids, which exert neuromediator and many other functions (see Tables 149.1 and 149.2 for details and references; see also [3, 8, 40, 41]). Intriguingly, human scalp hair follicles have been shown to be an important component of the neuroendocrine biology of human skin and show prominent expression/production of and/or sensitivity to a surprisingly wide range of neuromediators (Table 149.3), some of which are indicated in Figure 149.2.
Table 149.2 Examples of (neuro-) endocrine contributions to cutaneous pathogenesis.
| Mediator | Condition | Action | References |
| Androgens +, Insulin – | Wound healing | Impairment | [55, 104, 158, 159, 160, 207-209] |
| T3/T4 – | Wound healing | Impairment | [104] |
| T3/T4 + | Telogen effluvium | Promotion | [50, 210, 327] |
| Oestrogens + | Melasma | Promotion | [211, 212] |
| Chemotherapy-induced alopecia. | Promotion of hair regrowth? | [213] | |
| DHT + | Androgenetic alopecia | Essential for pathogenesis | [207] |
| Seborrhoea | Promotion | [50, 52] | |
| Acne vulgaris | Promotion | [214] | |
| Prolactin + | Psoriasis | Aggravation (see text) | [215] |
| Psoriatic arthritis | [20, 216-218] | ||
| Seborrhoea | Promotion | ||
| Systemic lupus erythematosus | Aggravation (see text) | [219, 220] | |
| Female pattern androgenetic alopecia | Aggravation | [221] | |
| Substance P + | Atopic eczema | Triggering/aggravation via induction of neurogenic inflammation | [16] |
| Psoriasis | [3, 97] | ||
| Urticaria | [172] | ||
| ‘Stress’-induced telogen effluvium | [49] | ||
| Alopecia areata | [169] | ||
| Prurigo nodularis | [174] | ||
| Chronic pruritus | [98, 222] | ||
| Rosacea | [222, 223] | ||
| Cortisol + | Cushing syndrome | Induction | [211] |
| Acne | Induction, aggravation | ||
| ACTH + | Hyperpigmentation | Induction | [211] |
| Hypertrichosis | |||
| α-MSH + | Hyperpigmentation | Induction | [211] |
| Melanoma | Potential suppression of anti-tumour immunity | [42] | |
| Autocrine secretion as survival factor for MM cells? | [224] | ||
| Increased risk after Melanotan® therapy? | [225-227] | ||
| Psoriasis | Potential induction of tolerogenic dendritic cells and T-regs | [193] | |
| Chemotherapy-induced alopecia | Protective effect? | [44] | |
| Melanocytic/dysplastic naevi | Increased growth after Melanotan® or α-MSH therapy | [228, 229] | |
| α-MSH – | Alopecia areata | Insufficient maintenance of hair bulb immune privilege | [46, 189] |
| Lichen planopilaris | Insufficient maintenance of bulge immune privilege | [190] | |
| Acne vulgaris | α-MSH analogue may be therapeutically beneficial | [230] | |
| Scleroderma | Insufficient α-MSH-mediated signalling and therapeutic effect of α-MSH postulated | [192] | |
| MC1R variants | Melanoma | Increased risk | [231-234] |
| Antagonizing MC1 signalling as antimelanoma strategy? | [233] | ||
| Vitiligo | (association discussed) | [235, 236] | |
| CRH + | Atopic eczema | Stress-related triggering/aggravation via induction of neurogenic inflammation (see text) | [9, 237] |
| Alopecia areata | [238] | ||
| Acne vulgaris | Promotion (see text) | [99, 239] | |
| CGRP + | Atopic eczema | Increased IL-13-secretion (see text) | [99] |
| AIDS | Inhibits HIV transmission from Langerhans to T cells | ||
| UV-associated immunosuppression | Mediated by CGRP (mice) | [241] | |
| Allergic contact dermatitis | Induction of hapten-specific tolerance (mice) | [240, 241] | |
| GH + | Acromegaly | Induction | [243] |
| Seborrhoea | Promotion | [244, 245] | |
| Melanocytic naevi | Increased growth | [246] | |
| Somatostatin | Merkel cell carcinoma | Growth inhibition by therapy with somatostatin analogue | [247] |
| Alopecia areata | Important for hair follicle immune privilege? | [110] | |
| β-endorphin | Atopic eczema | Increased serum level | [248, 249] |
| Psoriasis | [250] | ||
| Bradykinin | Hereditary angio-oedema | Receptor antagonist may be therapeutically beneficial | [251] |
| β2-adrenergic receptor (BAR) – | Atopic eczema | Reduced signalling due to BAR point mutation | [69] |
| Reduced catecholamine synthesis and increased catecholamine degradation in atopic epidermis | [68] | ||
| Vitiligo, psoriasis | Insufficient BAR signalling (see text) | ||
| TRPVs | Rosacea | TRPVs overexpressed | [252] |
| Can be stimulated by recognized rosacea trigger factors | [222] | ||
| Pruritus | Stimulation can promote itch | [49, 118, 253, 254] |
+, Increased; –, reduced. Abbreviations: see Table 149.1.
Table 149.3 When to suspect a hormonal basis for a skin disease: general signs and symptoms.
| Sign/symptom | Underlying (neuro-) endocrine causes |
| Anaemia | Anaemia caused by insufficient renal production of erythropoietin (tumour anaemia, diabetic nephropathy and other renal diseases) |
| Body odour (unpleasant) | Acromegaly (due to enlarged apocrine glands) |
| Dryness | Thyroid dysfunction (mainly hypothyroidism) |
| Exophthalmus | Hyperthyroidism (Graves disease) |
| Extremities enlarged (notably fingers and toes, ‘spade-like’ hands) | Acromegaly |
| Facial features (overall change) | Coarse features: acromegaly (look also for other signs of acromegaly (see Tables 149.4 and 149.5) and bone and cartilage abnormalities (e.g. prognathism, frontal bossing, enlarged hands/feet)‘Moon facies’: hypercortisolism (Cushing syndrome) |
| Hyperhidrosis (see Chapter 94) | Hyperthyroidism; acromegaly (enlarged eccrine glands) |
| Hair, dry/brittle (see Chapter 89) | Hypothyroidism |
| Hair, loss or gain of (effluvium, alopecia, hirsutism, hypertrichosis) (see Chapter 89) | Virilizing tumour, adrenogenital syndrome, polycystic ovary syndrome Insufficient oestrogen serum level Hypo- or hyperthyroidism, hyperprolactinaemia ACTH-secreting tumour (e.g. pituitary tumour or small cell bronchial carcinoma) |
| Joints, swollen | Sometimes associated with acromegaly (note thickening of phalangeal joints!), hyper- or hypoparathyroidism, hypothyroidism |
| Libido, loss of/impotence | Hypopituitarism |
| Menorrhoea | Hyperprolactinaemia, hypogonadism |
| Pigmentary abnormalities (see Chapter 88) | Hypo-/hyperpigmentation: Addison disease, ACTH-secreting tumour, hypopituitarism Yellow tint of skin: hypercarotenaemia in association with hypothyroidism or diabetes Pallor with yellow tint: hypopituitarism |
| Pruritus (see Chapter 83) | Diabetes, thyroid dysfunction, anaemia due to insufficient EPO production (see later) |
| Psychological and neuropsychiatric disturbances (see Chapter 86) | Hypo- and hyperthyroidism, hypercortisolism |
| Skin texture thickened | Acromegaly |
| Skin thinning/atrophy(increased skin vulnerablility to minor trauma) | Cushing disease |
| Weight loss or gain | Thyroid dysfunction, Cushing syndrome |
| Wound healing impaired | Androgen excess or relative lack of oestrogens Diabetes, hypothyroidism, hypercortisolism |
Human keratinocytes not only express functional adrenergic and cholinergic receptors but also synthesize and metabolize corresponding ligands (catecholamines, acetylcholine). Additionally, the skin displays a complex endocannabinoid and endovanilloid neuroendocrine signalling system, complete with intracutaneously expressed cannabinoid and vanilloid receptors, locally produced (typically lipid-based) ligands and a refined enzymatic machinery for synthesizing and degrading the latter. This machinery controls the intracutaneous level of these versatile neuromediators. The list of human skin functions known to be modulated by these systems is growing steadily: to name a few, it currently includes the regulation of keratinocyte and sebocyte proliferation, migration, apoptosis, differentiation, cytokine secretion and lipid production; mast cell differentiation from resident precursors and mast cell degranulation; control of epidermal and/or hair pigmentation; and the modulation of immune responses (see Tables 149.1 and 149.2). This list is bound to grow.
These multipurpose neuromediators are complemented by an array of intraepithelially generated classical (neuro-)hormones such as melatonin [75, 77], the pro-opiomelanocortin (POMC) products, α-MSH, ACTH and β-endorphin [46, 76, 125], as well as CRH [26, 45, 48, 126, 127], erythropoietin [121, 122, 123], neuropeptides released by sensory nerve fibres (mainly substance P, calcitonin gene related peptide (CGRP) and vasoactive intestinal peptide (VIP) [18, 128]) and cytokine-like hormones released by skin adipocytes (adipokines, e.g. leptin) [22], with important differences in the secretory profile of what is now called ‘dermal adipose tissue’ and adipocytes of the deeper subcutis [129, 130]. These neuromediators both exert long-distance effects (e.g. regulation of the hypothalamic controls of feeding behaviour) and regulate skin physiology and repair in a para- and autocrine manner via the stimulation of locally expressed specific receptors.
In addition, human skin and its appendages synthesize opioids (e.g. β-endorphin, a psychotropic, analgesic, and pigmentation-stimulatory POMC product) and enkephalins and express corresponding receptors. Apart from the long-recognized role of these neuromediators in the modulation of itch and pain (via the stimulation of cannabinoid, vanilloid, opioid or enkephalin receptors expressed by intracutaneous sensory nerve fibres and the corresponding neuons in dorsal root ganglia) they are now recognized as regulating numerous other aspects of skin biology. Examples of these are listed in Table 149.1.
Since Merkel cells engage in substantial neuroendocrine secretory activities (e.g. CGRP, VIP, neuropeptide Y, neurokinin A, galanin, substance P and somatostatin) [109, 113, 131, 132], their local functions within skin epithelium are likely to extend well beyond their long-recognized role as mechanosensory cells [132, 133, 134, 135].
This complex hormone and neuromediator-based dermatoendocrinological signalling mechanism intimately links specialized (neuro-)endocrine glands, the nervous system and peripheral tissue physiology, and is further complicated by the fact that the same hormones and neuromediators also regulate immune responses. Many of these substances are generated and secreted by immunocytes such as mast cells, T cells and macrophages [136, 137]. These immunocytes in turn, are now understood to alter peripheral tissue function in the skin and elsewhere, not only under conditions of infection, inflammation or tumour growth but also essentially ‘around the clock’, thereby contributing substantially to skin homeostasis [8, 13, 18, 138].
To illustrate this, skin mast cells are highly sensitive to neuroendocrine stimuli (e.g. by CRH, ACTH, endocannabinoids, substance P and catecholamines) and are often in intimate physical contact with sensory nerve fibres that release neuropeptides such as substance P or CGRP [16, 18, 64, 97, 127, 139, 140]. At least in mice, mast cells regulate T-cell function [141, 142], angiogenesis [143, 144], connective tissue turnover (including ‘mast cell-directed collagenolysis’) [145], wound healing [146] and hair growth; they also detoxify venoms [147, 148]. Thus, the neuroendocrine controls that these key protagonists of innate immunity are subjected to must have a profound impact on skin immune reponses and other aspects of skin function in health and disease. We can assume that every resident and transient cell found in human skin can respond to multiple different hormones and neuromediators, can generate, secrete and/or metabolize many of these, and can engage in very complex endocrinologically and neuroimmunologically relevant signalling interactions with its neighbours. Some of these signals can reach distant organs via the bloodstream or even the CNS by manipulating the firing sequence of action potentials generated by sensory skin nerve fibres.
Skin as a hormone target
Insight into the complexities of the skin's endocrine and neuroendocrine pathways can provide the practising dermatologist not only with a better understanding of how hormones such as glucocorticoids, retinoids and calcitriols exert their therapeutic action but also with valuable assistance in recognizing classical endocrine and neuroendocrine diseases that can affect the skin (see later).
The classical signalling scenarios consist in either stimulation of specific cell surface receptors (e.g. by peptide hormones, neuropeptides, neurotransmitters or lipid neuromediators), resulting in rapid cell responses or binding to nuclear hormone receptors, which produce slightly less immediate responses (as seen with all steroid hormones) [5, 149, 150]. While the effects of hormones and other mediators are commonly ascribed to their interaction (or lack of interaction) with cognate intracutaneously expressed receptors, it is unclear how exactly a given hormone or neuromediator induces any of the defined skin phenomena discussed later. Tables 149.1 and 149.2 list examples of important receptors for which there either is already persuasive clinical or preclinical evidence of a significant impact on human skin physiology or pathology, or for which studies with mutant mice have provided compelling evidence that signalling events mediated by these receptors regulate important mammalian skin functions in vivo.
When examining the skin as a hormone target, one needs to consider the many different means by which a given hormone or neuromediator can signal. Some steroid hormones (e.g. glucocorticoids, calcitriols and oestradiol) can exert very rapid and transient signalling effects via cell surface receptors as well as slower responses via classical interaction with hormone response elements in nuclear DNA. In addition, steroid hormones typically show dose-dependent ‘promiscuous’ effects, because they can also bind to receptors other than their chief signalling partner and can then influence the binding properties of these receptors to their main ligands [5, 25]. Such receptor promiscuity and cross-regulation has also been shown to occur with some peptide hormones and lipid-based neuromediators [25, 149, 150, 151].
Many of the biological effects of hormones depend on the extent to which a cell stimulated by a given hormone generates secondary mediators, such as the production of insulin-like growth factor 1 (IGF-1) by insulin and growth hormone (GH) (synonym: somatotropin (STH)), or whether the stimulation of a hair follicle with the potent androgen dihydrotestosterone (DTH) results predominantly in the production of hair growth inhibitory growth factors (e.g. TGFβ1, TGFβ2) or the hair growth promoting factor IGF-1 [50]. Thus, to understand the role of GH in human epidermal physiology [152] or of androgens in scalp hair follicle biology [153] it will be necessary to separate the direct from the indirect and perhaps clinically even more important growth factor-mediated effects of these hormones.
Another factor to be taken into account is the extent to which a given target tissue in the skin is capable of transforming (typically enzymatically) a prohormone into active metabolites [156]. This can make a significant difference to the biological outcome of hormone stimulation of a defined skin cell population or structure and has been extensively studied for intracutaneous steroid hormone metabolism. Examples include the intracutaneous conversion of the relatively weak androgen testosterone into the highly active androgen DTH by 5-α-reductase and of androgens into oestrogens (e.g. of testosterone to 17-β-oestradiol by aromatase) as well as vitamin D metabolism [10, 41, 154, 155]. Key examples for intracutaneous neurohormone metabolism are the conversion of POMC by prohormone convertases to either ACTH, α-MSH or β-endorphin [8, 10]. In addition, ligands produced in the skin (e.g. sex steroids, glucocorticoids, retinoids and β-endorphin) can pass through the blood–brain barrier to the CNS, where they may be further metabolized, producing complex neuropsychological and neuroendocrine responses with potential effects directed back at the skin.
Other ligands can exert additional, receptor-independent effects, such as the direct, intra- and extracellular scavenging of reactive oxygen species by melatonin [75], or the modulation of tyrosinase activity by α-MSH [156]. Finally, the specific biological activities exerted by most hormones or neuromediators in a given skin territory depend on how quickly they are degraded by local enzymes (e.g. rapid degradation of substance P by neutral endopeptidase and angiotensin-converting enzyme, or of endocannabinoids by fatty acid amide hydrolase (FAAH)), the activity of which may vary from one specific skin territory to another. All these signalling variations are again influenced by the simultaneous presence of other ligands, co-factors, decoy receptors, binding proteins, the overall cytokine signalling milieu (e.g. whether or not the skin area in question is inflamed) and numerous other variables.
Matters are further complicated by substantial gender and/or regional differences in the response of human skin and its appendages to a given hormone or neuromediator. While this has long been appreciated for androgens, as for example reflected in the ‘paradoxical’ response to DTH of beard versus temporofrontal scalp hair follicles [50, 153], it also applies to key hormones such as 17-β-oestradiol [53, 157] and prolactin [20]. Another example where sex steroid controlled gender differences have been demonstrated is cutaneous wound healing, which is promoted by oestrogens but inhibited by androgens [158, 159, 160, 161].
This level of (neuro-)endocrine signalling complexity explains why it is often extremely difficult to pinpoint mechanistically exactly how a given hormone or neuromediator has induced the human skin phenomena we observe in an individual patient. Conversely, it also explains why the same dose of the same agent used in hormonal dermatotherapy (e.g. in the management of acne or psoriasis) can produce such distinct clinical results in different individuals, despite belonging to the same gender and age group and having a comparable medical background. Obviously, dermatologists need to be aware of these hidden (neuro-)endocrine dimensions of their daily work.
Additional reasons why dermatoendocrinological considerations are inescapable in routine dermatological practice are listed below:
- Key dermatological symptoms and signs such as pruritus, flushing, erythema, eczema and wealing, and complex skin parameters such as skin barrier function, wound healing, hair growth, pigmentation and skin immune status, are greatly influenced by the characteristics of this intracutaneous (neuro-)endocrine signalling mechanism.
- The intraepithelial endocrine and neuroendocrine signalling milieu that is created under physiological circumstances in human epidermis and hair follicles is probably predominantly immunoinhibitory [3, 8, 9, 57], and this may well be a fundamental prerequisite for skin homeostasis: increasing evidence suggests that maintenance of this signalling milieu plays a key role in preventing excessive skin inflammation and itch that would otherwise result from the continuous onslaught of environmental stressors such as microbial pathogens, excessive colonization with skin microflora, UV irradiation, physical and chemical skin trauma, or metabolic, oxidative and psychoemotional stressors. Imbalances in this neuroendocrine signalling system may influence dermatoses where neurogenic skin inflammation plays a significant role and which are known to be triggered or aggravated by environmental stressors (e.g. psoriasis, atopic eczema, alopecia areata, urticaria, pruritus).
- The skin's response to excessive or deficient systemic hormone levels is greatly influenced by underlying systemic and local factors, ranging from UV exposure, humidity, barrier function via inflammation and microbiological colonization of the skin to a patient's psychoemotional status, medication and concomitant chemical or physical skin trauma. All these factors impact on the endocrine and neuroendocrine signalling concert of human skin.
- Emerging experimental evidence suggests that important stress-associated neuropeptides such as substance P may even modulate the composition of the skin microbiome [162].
- Ageing of the human organism is associated with significant changes in systemic hormone levels, particularly in circulating androgens and oestrogens. These changes, such as the declining 17-β-oestradiol serum levels during and after the menopause, affect human skin on many more levels than were previously recognized, ranging from changes in overall skin architecture, skin immune responses and cutaneous microbiology via altered skin barrier function, cutaneous drug absorption and metabolism to hormone-dependent effects on hair growth, sebum production and wound healing [163, 164, 165, 166, 167, 168].
Neuroendocrine stress response systems in human skin and the brain–skin axis
The importance of hormones in dermatology is further underscored when considering the role of psychoemotional stress as a triggering or aggravating factor in common dermatoses such as psoriasis, atopic eczema, urticaria, nodular prurigo, lichen planopilaris or alopecia areata (see corresponding chapters, and Chapter 86). Rather than reliance on questionable psychoanalytical explanations, solid experimental and clinical data can now explain how major neuroendocrine stress mediators such as CRH, ACTH, prolactin and substance P may trigger or aggravate skin disease [9, 13, 17, 18, 49, 151, 169, 170, 171]. Stress-induced neurogenic skin inflammation represents the best-defined neuroendocrine explanation for how psychoemotional stress influences dermatoses such as psoriasis, atopic eczema or urticaria [13, 16, 18, 172, 173, 174, 175].
Pro-inflammatory activities of skin mast cells assume a ‘central switchboard’ role in neurogenic skin inflammation. Mast cells undergo enhanced degranulation after direct stimulation by increased serum and tissue levels of stress-associated mediators such as CRH, ACTH, nerve growth factor (NGF), substance P and NGF, all of which act as secretagogues for human skin mast cells. The latter are often found in close proximity to sensory nerve fibres and it has been shown that neuropeptide-releasing sensory neurons are stimulated by NGF to synthesize substance P for transport via sensory nerve fibres to the skin, where it is able to provoke or exacerbate skin inflammation [13, 49, 98]. Simultaneously, psychoemotional stress can up-regulate the intracutanous generation of stress-response hormones, such as CRH and ACTH [16, 18, 49, 169, 175, 176].
Certain stress-associated hormones are an important element of what has been termed ‘fetal programming’, a process during which maternal stress impacts on the offspring's stress responses in later life [177, 178]. For example, an inverse association between the maternal serum progesterone level and the risk of girls subsequently developing atopic eczema has been described. Since progesterone is thought to operate as an endocrine feto-maternal ‘stress sentinel’ and to promote fetal tolerance it is, thus, conceivable that a lowered maternal progesterone level may predispose the fetus to the development of atopic eczema [179].
These interactions along the brain–skin axis [13, 17, 98, 140, 176, 180, 181, 182] can recruit a cascade of secondary inflammatory events, thus conspiring to trigger or aggravate inflammatory, pruritic and/or hyperproliferative dermatoses. Conversely, most recent neuroimaging evidence suggests that chronic skin inflammation can exert profound retrograde pro-inflammatory effects on the human brain, for example in patients with psoriasis [183]. This may even negatively affect their cognitive performance [184]. If confirmed in follow-up studies, the presence of central neuroinflammation and cognitive impairment in association with chronic inflammatory dermatoses will add major new diagnostic and therapeutic challenges to the management of these patients.
Human skin and hair research models as discovery tools for general neuroendocrinology
It should be emphasized that a stringent (neuro-)endocrinological approach to the investigation of skin disease has already contributed to major translationally relevant progress in general endocrine and neuroendocrinological research, e.g. by organ-culturing intact human skin and scalp hair follicles and by studying human keratinocyte and sebocytes cell cultures.
Apart from the discovery that the skin and its pilosebaceous units rank among the most endocrinologically active organs of the human body and display regulatory neuroendocrine signalling loops that parallel in complexity those found in the central HPA and HPT axes [3, 6, 7, 8, 9, 39, 57], this line of research has identified novel neuroendocrine controls of pigmentation [15], e.g. the discovery of β-endorphin as melanotropin [125], and of TRH as a promoter not only of human hair pigmentation [82] but also of human epidermal re-epithelialization after wounding [161], suggesting that TRH is involved in the control of wound healing.
Human skin and hair research has helped to show that α-MSH, apart from its long-known key role as a pigmentation stimulatory melanocortin, exerts multiple anti-inflammatory activities [185] and acts as a powerful immune privilege guardian in human skin [46, 186, 187, 188, 189, 190]. Intriguing leads from mouse research suggest that α-MSH may also act as a potential antifibrotic neurohormone. This may be of relevance to the pathogenesis of systemic sclerosis [191, 192] and studies on human skin in situ are awaited to see whether α-MSH might promote the development of tolerogenic dendritic cells and/or regulatory T cells [193]. Preclinical evidence already suggests that α-MSH may exert local anti-allergic activity by inhibiting human basophils both in vitro and in situ [43] and that it may promote systems that limit UV-induced oxidative damage in human skin [194].
Prolactin and thyroid hormones have also been shown to act as important, previously unknown, stimulators of human hair follicle epithelial stem cells in situ [91, 103, 195]. Through the use of human skin and hair follicle organ culture assays, TRH, TSH, prolactin and the cannabinoid system have all recently surfaced as powerful novel neuroendocrine regulators of keratin gene and protein expression (e.g. TRH, TSH, prolactin and endocannabinoids potently regulate intraepidermal and intrafollicular expression of selected keratins, including stem cell and hair shaft associated keratins in situ; see [58, 87, 196, 197]). This has revealed an entire new level of keratin expression control.
The systematic dissection of vitamin D metabolism in human skin and keratinocytes [41, 198] has also identified an entire new line of secosteroids with intriguing therapeutic potential [39, 57]. The analysis of PPARγ-mediated signalling in human skin in health and disease has shown that these nuclear hormone receptors and their agonists operate as important ‘guardians’ of human epithelial (hair follicle) stem cells [59, 85, 199] and as molecular curbs on excessive skin inflammation [59, 200]. PPARγ stimulation may also help to reduce skin ageing induced by photoxidative damage [201].
Finally, recent research in healthy organ-cultured human epidermis and hair follicles has revealed that TRH and TSH act as previously unsuspected potent neuroendocrine stimulators of human mitochondrial activity and even mitochondrial biogenesis in situ [83, 84, 86]. This important and novel insight into the neuroendocrine control of mitochondria had been missed by mainstream mitochondrial and neuroendocrinology research, which has traditionally focused on the role of thyroid and steroid hormones as stimulators of mitochondrial function in tissues other than skin. Given the central role of mitochondrial dysfunction in ageing and in many degenerative diseases including maternally inherited mitochondrial DNA disorders [202, 203, 204, 205], this discovery opens up interesting new avenues for clinical research (see later).
These few examples demonstrate that the study of skin from a (neuro-)endocrinological perspective promises benefits well beyond the integument. They also show that human skin and pilosebaceous research models provide excellent discovery tools for exploring the full range of physiological activities in which neurohormones and other neuromediators play a part in human biology [3, 8, 87]. In this respect, important clues may be drawn from the study of evolutionarily much older vertebrate skin, namely that of frogs, which generates a striking variety and quantity of neurohormones and neuropeptides. An understanding of these may help to reveal evolutionarily conserved but as yet unappreciated functions common to both frog and human skin [161, 206].
(Neuro-)endocrine contributions to cutaneous pathogenesis
The example of stress-induced neurogenic skin inflammation (see earlier) has already illustrated how neuroendocrine and neuroimmunological mechanisms can contribute to the pathogenesis and course of stress-triggered or aggravated human skin diseases. Table 149.2 lists selected dermatoses for which a neuroendocrine contribution to disease pathogenesis has been postulated. For example, there is increasing evidence that the modulation of immune responses by a wide range of neurohormones, neuropeptides and neurotransmitters may contribute to the development and/or clinical course of psoriasis, atopic eczema, urticaria, pruritus, alopecia areata, systemic lupus erythematosus, systemic sclerosis, retarded wound healing and melanoma (see Table 149.3 for details and references).
However, conclusive proof that neuroendocrine mechanisms contribute fundamentally, rather than peripherally, to the primary pathogenesis of the most common human skin diseases such as atopic eczema and psoriasis, as opposed to playing a role in triggering or aggravating them, is still missing. Regrettably, a stringent neuroendocrine approach is rarely adopted when investigating human skin diseases. Mainstream neuroendocrinology research has been very slow to recognize and adopt human skin and its appendages as instructive research objects and experimental models.
One notable exception is the long line of research that has shed light on a significant β-adrenergic signalling defect in atopic eczema. Starting from Szentivanyi's β-adrenergic theory of atopy [255], this has arguably come very close to demonstrating primary relevance for the pathogenesis and neuropharmacological management of atopic diseases, including atopic eczema [68, 69, 256, 257, 258]. While the role of β-adrenergic receptors in haemangioma pathogenesis remains ill understood, the often impressive therapeutic response seen with β-blocker administration in haemangioma [259] certainly serves as an additional encouragement to undertake a systematic re-exploration of the role of catecholamines and their receptors in human skin physiology and pathology [30, 33, 66, 67, 6870, 71, 77260, 261, 262, 263, 264, 265]. It opens up the possibility that adrenergic receptor agonists and antagonists, which have already been used for decades in clinical medicine, may yet find new roles in the management of skin disease.
A second important exception is the melanocortin receptor type 1 (MC1R), the chief receptor for α-MSH, whose loss-of-function polymorphisms (found in red-haired individuals who fail to tan) is associated with a significantly increased risk of developing melanoma [231, 232, 233, 234, 266] (see Chapter 143). α-MSH also operates as a powerful immune privilege guardian in human skin [46, 186, 189]. A relative insufficiency of α-MSH/MC1R-mediated signalling may contribute to the collapse of immune privilege in the anagen hair bulb, a key element in the pathogenesis of alopecia areata [46, 189, 267]. Likewise, it has been speculated that insufficient α-MSH/MC1R-mediated signalling at the hair follicle bulge, the repository of follicle stem cells, may contribute to the pathogenesis of lichen planopilaris, facilitating the collapse of immune privilege at that site [190].
A third area where endocrine research has contributed to an understanding of skin disease pathogenesis is the study of endogenous or exogenously administered vitamin D and vitamin A derivatives in a wide range of dermatoses. It would not be surprising to find that abnormalities in intracutaneous calcitriol and retinoid synthesis and metabolism have an impact on an individual's susceptibility to skin diseases such as skin cancer, alopecia, retarded wound healing and acne, or on that individual's response to therapy with steroid hormones. The first intriguing examples of the potential importance of this new endocrine dimension in dermatology have already emerged [19, 41, 268, 269, 270, 271, 272, 273].
It is in this context of widely underappreciated progress in cutaneous (neuro-)endocrinology that traditional clinical dermatoendocrinology is still unfolding.
Basics of clinical dermatoendocrinology
How to evaluate a patient for a suspected (neuro-)endocrine disorder
Hormones may control or influence general body characteristics such as height, weight, body contour and posture, mood, agility, nervousness, hair phenotype, and also food and fluid intake. Being alert to changes in such general characteristics will therefore greatly help to identify a potential endocrinological dimension in patients presenting with a skin complaint. At the very least, when a patient presents with any of the lead signs or symptoms in Table 149.3 a hormonal basis for the dermatological problem should be considered and systematically confirmed or excluded.
Some characteristic skin signs provide invaluable indicators of specific endocrine diseases (Table 149.4). Additional ‘diagnostic pearls’ that will greatly help in identifying a potential underlying endocrine pathology are summarized in Table 149.5.
Table 149.4 Characteristic skin signs indicating specific endocrine diseases.
| Sign | Figure | Associated endocrine disease/condition |
| Acanthosis nigricans (see Chapter 87) | Figure 149.3 | Puberty, diabetes, other causes of insulin resistance including HAIR-AN syndrome of young black females (hyperandrogenism, insulin resistance, aconthosis nigricans; often associated with polycystic ovary syndrome, hirsutism and others signs of androgen excess), acromegaly |
| Acne (see Chapter 91) | Figure 149.4 | Cushing syndrome, glucocorticoid therapy, ACTH-secreting tumours (e.g. small cell bronchial carcinoma, acromegaly) |
| Cutis verticis gyrata (see Chapter 107) | Figure 149.5 | Acromegaly |
| Flushing (see Chapter 106) | Figure 149.6 | Menopause-associated hot flushes Phaeochromocytoma, carcinoid |
| Galactorrhoea | Hyperprolactinaemia (e.g. due to prolactinoma, tumour-associated ectopic prolactin production or medication with neuroleptic drugs, oral contraceptives, tricyclic antidepressants) | |
| Granuloma annulare (see Chapter 97) | Diabetes (see Chapter 64) | |
| Gynaecomastia (usually, but not always, symmetrical) | Figures 149.7 and 149.8 | Puberty, hypogonadism (e.g. Klinefelter syndrome), pituitary and gonadal tumours (e.g. prolactinoma), testis carcinoma), excessive endogenous oestrogen production (e.g. oestrogen-secreting tumours), insufficient oestrogen metabolism (e.g. liver cirrhosis), drugs (e.g. oestrogens, spironolactone, isoniazid, resumption of normal hypophyseal gonadotropin secretion after longer period of hunger, extreme diet, or major consumptive disease), hypopituitarism |
| Hyperpigmentation | Figure 149.9 | Addison disease |
| Hypertrichosis (see Chapter 89) | Excess ACTH production (tumour, Cushing disease), hyperthyroidism (often in association with myxoedema in Graves disease) | |
| Melasma (see Chapter 88) | Figure 149.10 | Pregnancy, oral contraceptives, oestrogen-secreting tumour |
| ‘Moon facies’ | Cushing syndrome | |
| Myxoedema, pretibial (see Chapter 59) | Figure 149.11 | Hypo- or hyperthyroidism (notably in Graves disease) |
| Necrobiosis lipoidica (see Chapter 97) | Diabetes (search for additional cutaneous complications of diabetes in Box 149.1 and in Chapter 64) | |
| Necrolytic migratory erythema (see Chapter 147) | Figure 149.12 | Glucagonoma |
| Palmar erythema | Hyperthyroidism | |
| Scleredema adultorum (Buschke) (see Chapter 59) | Diabetes | |
| Stretch marks (striae distensae) (see Chapter 96) | Figure 149.13 | Cushing disease, glucocorticoid therapy, ACTH-secreting tumours (e.g. small cell bronchial carcinoma); pregnancy, contraceptive therapy, puberty (growth spurt, namely in adipose individuals) |
Adapted from Braverman 1998 [211], Du Vivier 2002 [274] and Luger and Böhm [275] © John Wiley.
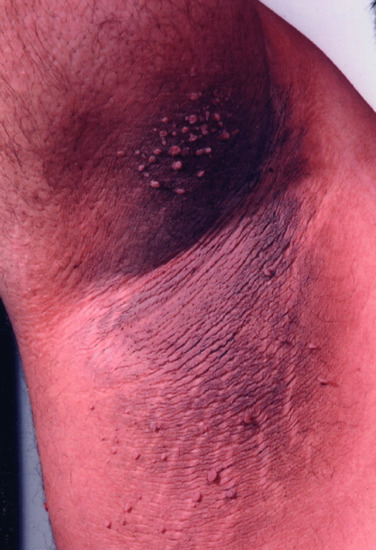
Figure 149.3 Acanthosis nigricans, skin tags and striae in a 41-year-old obese male with type 2 diabetes.
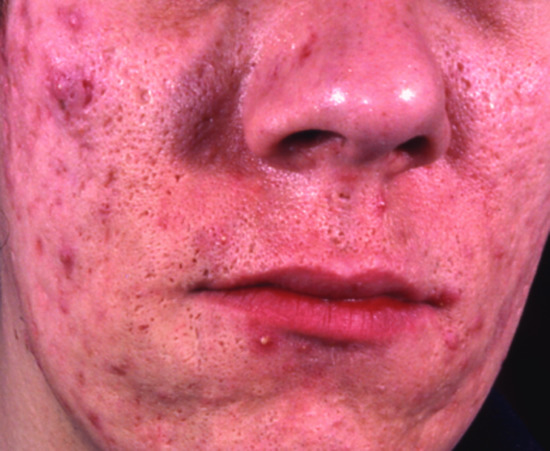
Figure 149.4 Acromegaly: note the coarse features, severe acne and seborrhoea.

Figure 149.5 Cutis verticis gyrata.
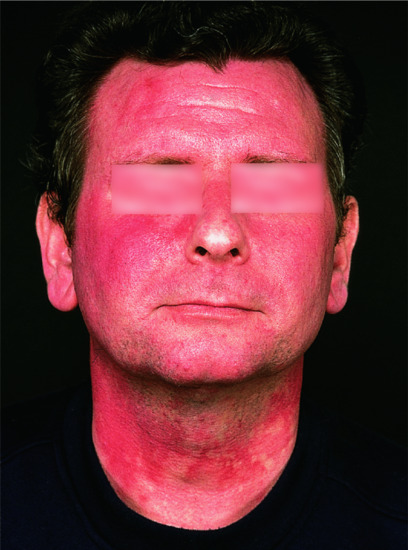
Figure 149.6 Histamine-evoked ‘geographical’ pattern of flushing due to foregut carcinoid tumour.
(Courtesy of Professor M. Greaves, London, UK.)
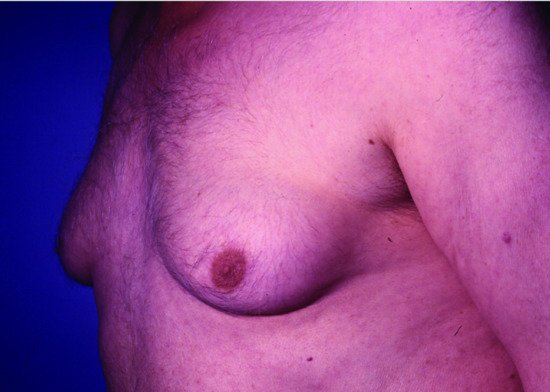
Figure 149.7 Gynaecomastia due to long-term spironolactone therapy given for hypertension.
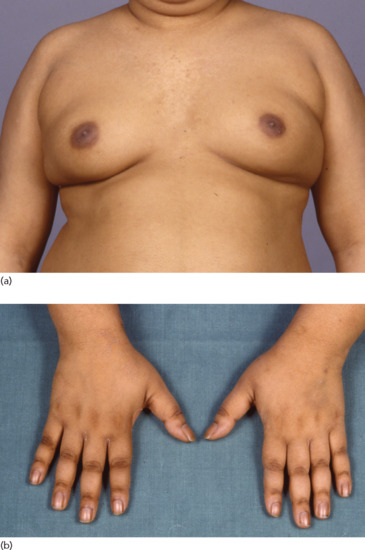
Figure 149.8 An 18-year-old male with pituitary Cushing disease followed by hypopituitarism. (a) Obesity and gynaecomastia. (b) Insulin resistance with acanthosis nigricans of the knuckles, an unusual site in common causes of acanthosis nigricans.
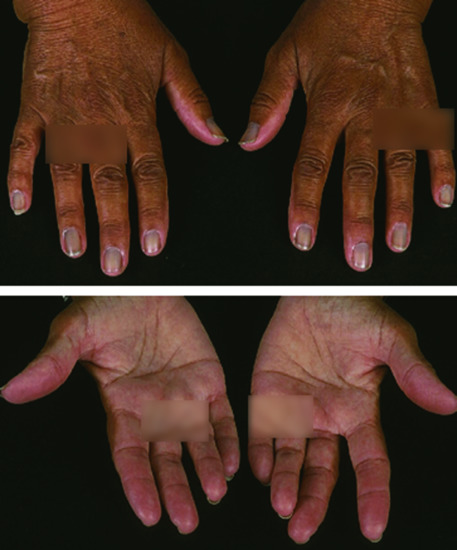
Figure 149.9 Addisonian pigmentation of the palmar creases 37 years after bilateral adrenalectomy for Cushing disease from ACTH-producing pituitary adenoma (Nelson syndrome).
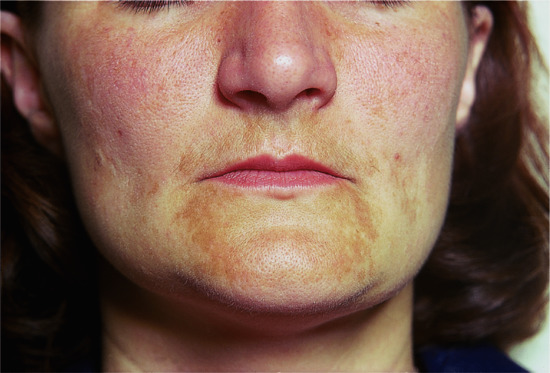
Figure 149.10 Melasma.
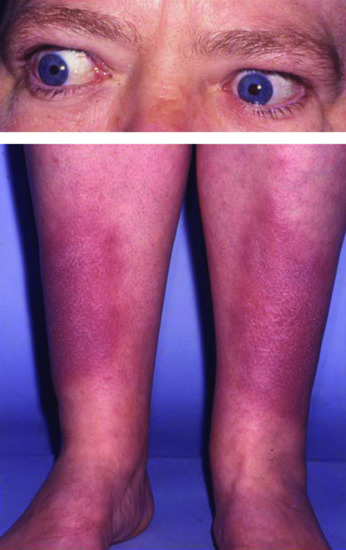
Figure 149.11 A patient with Graves disease with pretibial myxoedema and exophthalmos.
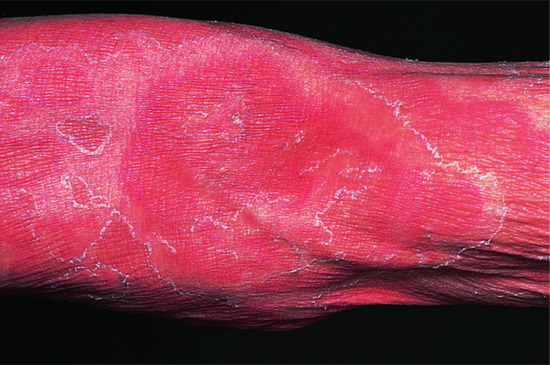
Figure 149.12 Necrolytic migratory erythema.
(Courtesy of Dr Kristian Thomsen, Finsen Institute, Copenhagen, Denmark.)
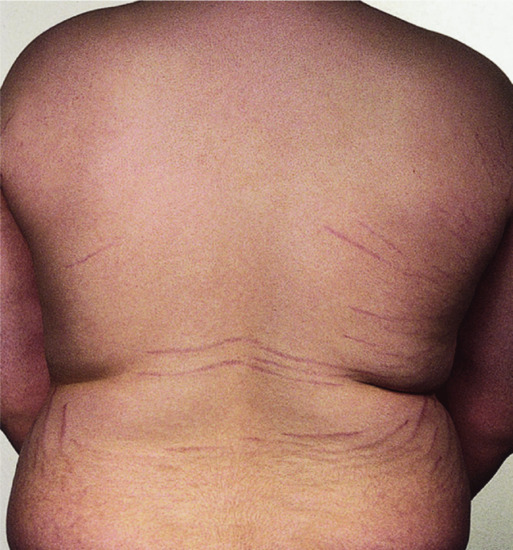
Figure 149.13 Striae due to obesity in a young man.
Table 149.5 Additional ‘diagnostic pearls’ in clinical dermatoendocrinology.
| Sign/symptom | Figure | Consider presence/role of… |
| Alopecia areata (see Chapter 89) | Thyroid autoimmune disease | |
| Alopecia, rapidly progressing pattern balding (especially in female patients) | Virilizing tumour, adrenogenital syndrome; pregnancy or discontinuation of oral contraceptive; thyroid dysfunction, hyperprolactinaemia | |
| Axillary hair, diminished growth | Panhypopituitarism | |
| ‘Buffalo hump’ | Cushing syndrome | |
| Digital clubbing (see Chapter 95) | Hyperthyroidism (thyroid acropachy), hyper- or hypoparathyroidism (periosteal formation of new bone) | |
| Epidermoid cysts | Acromegaly | |
| Eyelids, thickened/oedematous | Acromegaly | |
| Gingival or oral hyperpigmentation | Figure 149.14 | Addison disease (gingival hyperpigmentation: consider also hyperthyroidism and Cushing disease; dintinguish from ephelide-like hyperpigmentation of Peutz–Jeghers syndrome) |
| Hair, repigmentation of grey hair | ACTH-producting tumour (e.g. pituitary, or ectopic ACTH production by small cell bronchial carcinoma) | |
| Macroglossia (tongue often also fissured) | Figure 149.15 | Acromegaly; hypothyroidism (usually less pronounced than in acromegaly) |
| Mastitis, neonatal | Temporary stimulation of mammary gland by maternal hormones | |
| Nails, thickened and hardened | Acromegaly | |
| Sebaceous gland hypertrophy (see Chapter 93) | Acromegaly | |
| Seborrhoea | Virilizing tumour, hyperprolactinaemia Parkinson disease (→ insufficient dopamine production, associated with hyperprolactinaemia) |
|
| Skin tags (acrochordons) | Acromegaly | |
| Telogen effluvium (see Chapter 89) | Thyroid dysfunction, hyperprolactinaemia, virilizing tumour, adrenogenital syndrome; polycystic ovary syndrome; pregnancy or discontinuation of oral contraceptive | |
| Ulcer, gangrene | Diabetes | |
| Urticaria (see Chapter 42) | Chronic urticaria associated with thyroid hormone abnormalities (due to Hashimoto thyroiditis or Graves disease) | |
| Vitiligo (see Chapter 88) | Figure 149.16 | Can be associated with thyroid autoimmune disease and Addison disease |
Adapted from Braverman 1998 [211], Du Vivier 2002 [274], Luger and Böhm [275], Jabbour 2003 [276] and Jabbour 2010 [277] © John Wiley.
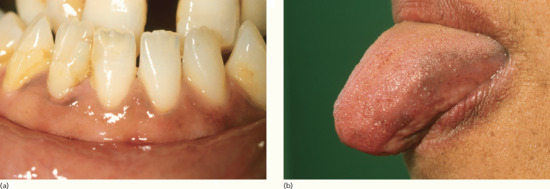
Figure 149.14 Pigmentation of (a) the gingivae and (b) the tongue in a woman who presented with darkening skin due to Addison disease.
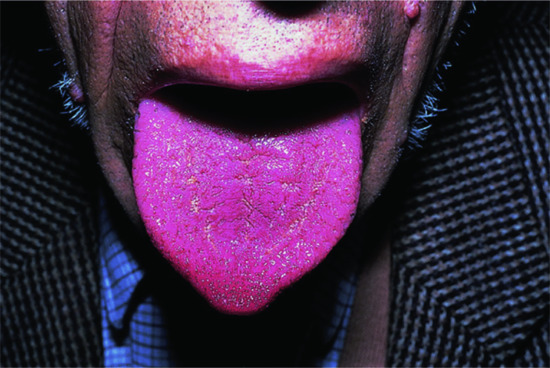
Figure 149.15 Acromegalic macroglossia.
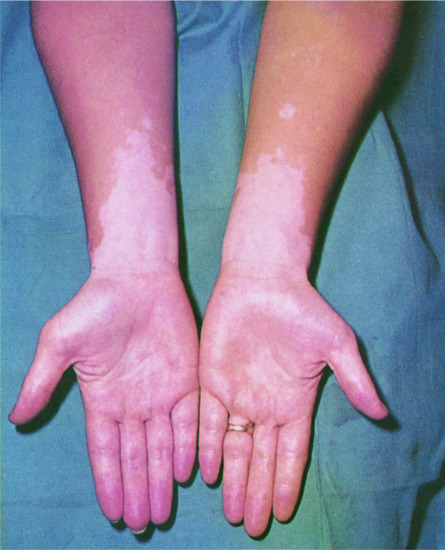
Figure 149.16 Vitiligo.
Comparing a patient's current facial appearance with an older photograph, e.g. on a driver's licence or stored on a mobile phone, can offer important, rapidly collectable information about changes in overall facial features, as may occur from acromegaly or Cushing disease.
Given the increasing incidence and prevalence of diabetes in most societies, it is important to consider this as a factor. The cutaneous features of diabetes (Box 149.1) are discussed elsewhere (see Chapter 64).
There are also the cutaneous consequences of hypo- or hyperthyroidism to be considered. Finally, given that many tumours can be hormonally active, if still in doubt diagnostically, it is possible that the observed skin phenomena may have been caused by a hormone-secreting tumour, such as skin hyperpigmentation, acneform lesions and/or cushingoid features in ACTH-secreting small cell bronchial carcinoma [279], sudden attacks of hyperhidrosis (typically along with headache, tachycardia and hypertension) in catecholamine-secreting phaeochromocytoma, or necrolytic migratory erythema in glucagonoma syndrome [280, 281] (see Tables 149.4 and 149.5). (For details on paraneoplastic skin disease, see [277, 282] and Chapter 147.)
Endocrinological considerations in skin therapy
Once an endocrinological diagnosis has been made or is suspected, patients should be referred to an appropriate specialist, such as an endocrinologist or neurosurgeon, to undergo further diagnostic procedures and appropriate management. However, even replacement or suppressive therapy (as appropriate) may not always result in a rapid return of the skin to its premorbid state. The skin's response to corrective therapeutic intervention, for example with thyroxine, removal of a pituitary tumour or treatment of diabetes, can be very slow and protracted or may be impossible to achieve.
Some skin changes that occur in association with endocrine disease (e.g. necrobiosis lipoidica, lipoatrophy, striae distensae, ulcers, gangrene; see Tables 149.4 and 149.5) routinely result in irreversible skin damage, where progressive loss of function can only be halted if the underlying endocrine abnormality is aggressively sought and eliminated.
If a patient is known to have an endocrine disorder, whether that has come to light from its cutaneous manifestations or not, it should not be forgotten that the endocrine abnormality may also aggravate, increase susceptibility to or alter response to therapy of other skin disorders, including psoriasis, atopic eczema, alopecia areata and acne, and that it may affect wound healing (see Table 149.3). Accelerated skin ageing may also result from endocrine disease. The possibility that neuroendocrine abnormalities associated with psychoemotional stress may have triggered or aggravated a dermatosis via the induction of neurogenic skin inflammation (see earlier) should always be carefully considered and discussed with the patient.
Application of exogenous glucocorticosteroid hormone to human skin is likely to have a significant impact not only on the metabolism and synthesis of endogenous steroid hormones in human skin but also on intracutaneous neuroendocrine signalling axes, which affect the secretion of potent growth factors/cytokines or the signalling of receptors that are not classical targets of the administered therapeutic hormone. Glucocorticoid application may also synchronize or reset peripheral clock gene activity in human skin and its appendages. Given the increasing insight into the regulation of such diverse aspects of skin physiology as hair growth and pigmentation by changes in clock gene activity [283, 284, 285, 286, 287], this chronobiological dimension of glucocorticoid therapy could be more important than previously appreciated.
Whilst this field of dermatoendocrinology is still in its infancy and its practical implications for clinical disease management are still unclear, the dermatologist should be aware of the complex interplay between hormones and the skin. For example, it is known that cortisol administration reduces the intrafollicular expression of CRH in human scalp hair follicles ex vivo [26]. Although it remains to be formally demonstrated that this also happens in vivo, it is eminently conceivable that chronic glucocorticoid administration reduces the skin's CRH-dependent constitutive cortisol synthesis as well as that in the adrenal gland [9, 26]. This iatrogenic disruption of the brain–skin axis may contribute to the classical rebound phenomena typically seen after withdrawal of glucocorticoid therapy [3, 7, 9]. Moreover, all-trans-retinoic acid modulates the intrafollicular expression of key growth factors; that is, it up-regulates TGFβ1 and TGFβ2, thereby switching scalp hair follicles from anagen to catagen; this may explain why patients under retinoid therapy can experience a telogen effluvium [288]. Instead, the frequently observed retinoid-associated skin irritation results partly from sensory hypersensitivity induced by retinoid activation of a vanilloid receptor (TRPV1) [289].
Dermatologists should remember that abuse or misuse of hormonally active substances (e.g. anabolic hormones or glucocorticosteroids) may manifest in the skin; a patient's medication may also result in hormonally-mediated adverse cutaneous effects. These may be obvious when they result from systemic or high potency topical glucocorticosteroids or systemic retinoids but less so when due to anabolic agents or hormonal contraceptives. Adverse cutaneous effects may also manifest when a patient has taken novel neuroendocrine agents, such as the synthetic α-MSH analogue, Melanotan, which is increasingly (ab-)used for tanning purposes. This agent may also stimulate the appearance of multiple melanocytic naevi and/or dysplastic changes in existing naevi (see Table 149.3).
Other medications have less obvious (neuro-)endocrinological effects on the skin. For example, dopamine exerts direct hair growth-inhibitory properties on human scalp hair follicles [101]. This may explain why bromocriptine, the dopaminergic inhibitor of pituitary prolactin secretion used for treating prolactinoma patients, can cause effluvium in female patients [290]. Neuroleptics and other antipsychotic agents frequently cause hyperprolactinaemia [291]. Therefore, it is reasonable to consider whether skin abnormalities in which excessive prolactin signalling may play a part (see Table 149.3) might have been aggravated by such a medication, or whether standard therapy may have been less effective than expected in patients treated with these drugs. Where medically justifiable, it may be worth temporarily discontinuing or at least reducing such medication to observe whether this improves the dermatological complaint.
Long-term ciclosporin therapy not only frequently results in hypertrichosis, but rarely can also lead to reversible gynaecomastia associated with hyperprolactinaemia [292]. In murine skin, ciclosporin controls hair follicle stem cell activation and thus hair growth in a prolactin receptor-dependent manner [293], while it prolongs the duration of anagen in human scalp hair follicles [294]. It has been suggested, somewhat controversially, that the efficacy of ciclosporin therapy might be enhanced by co-administering bromocriptine, which lowers the serum prolactin level [295], while neuroleptics which cause hyperprolactinaemia may reduce the effectiveness of immunosuppression by ciclosporin [296].
Angiotensin-converting enzyme inhibitors are the leading cause of drug-induced angio-oedema and account for up to one third of cases of angio-oedema presenting to emergency departments [297, 298]. These agents inhibit the intracutaneous degradation of substance P, bradykinin and other pro-inflammatory, vasoactive and mast cell activating neuropeptides and as a result can aggravate neurogenic skin inflammation, vasodilatation and extravasation [16, 25, 49, 299, 300].
These considerations illustrate the value of a (neuro-)endocrinological perspective when approaching and managing patients with skin disease. Traditionally, however, it is the diagnostic benefits of such a perspective that secure the place of hormones in the dermatologist's mind.
Systematic review of clinical dermatoendocrinology
All the central endocrine signalling axes (see Figure 149.1) and (neuro-)endocrine systems described earlier can show abnormalities that result in either excessive or insufficient hormone levels. In the following sections, the skin signs or symptoms summarized in Tables 149.4 and 149.5 are briefly discussed. Further clinical information can be obtained from relevant review articles [98, 207, 276, 277, 282, 301, 302] and Braverman's classical monograph [211]. For clinical illustrations, follow the indicators listed in Tables 149.4 and 149.5.
As the diagnostic confirmation and therapeutic management of the endocrinological disease states listed later typically lie in the hands of endocrinologists, paediatricians, gynaecologists or neurosurgeons, investigation and management are not discussed here: see [5, 151] for details on diagnostic procedures and disease management.
Hypopituitarism
Inherited or acquired insufficiency of pituitary hormone production can result from many different causes. These range from congenital defects in POMC synthesis [303] or processing, via traumatic brain injury, ionizing irradiation, tumours (e.g. metastases, craniopharyngioma, adenomata, Langerhans cell histiocytosis) and infection (e.g. tuberculosis, syphilis) to sarcoidosis and postpartum pituitary necrosis (Sheehan syndrome) [211, 276]. As all of these may impair pituitary hormone production, the associated skin phenotype is dominated by the main neurohormones that are produced in insufficient amounts.
Given the usually non-hormone-selective nature of hypopituitarism, the levels of several pituitary neurohormones are reduced simultaneously, and the serum levels of other key hormones (e.g. thyroxine, cortisol) and growth factors (e.g. IGF-1) regulated by the former are typically reduced as well. Thus, it is often impossible to discern exactly which hormonal imbalance has caused the observed skin phenotype in a given patient. Once hypopituitarism is clinically suspected, it is diagnostically important to identify which pituitary hormone(s) is/are deficient [276, 304]. Classically, the absence of melanotropic neurohormones (e.g. ACTH, α-MSH) results in pale hypopigmented skin, not uncommonly with a yellowish tint. Instead, GH (STH) and/or prolactin deficiency gives rise to structural skin changes, e.g. overall skin thinning and reduced sebum and sweat secretion. Rarely, gynaecomastia can be a result of secondary hypopituitarism [305], as in the sarcoidosis patient shown in Figure 149.17.
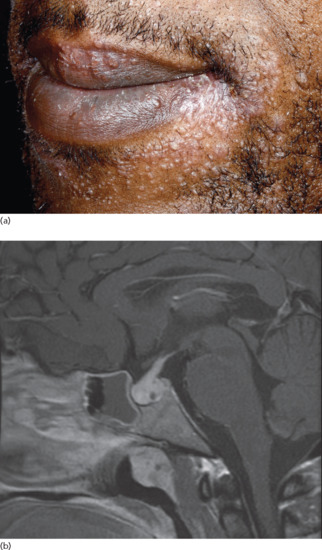
Figure 149.17 Neurosarcoidosis with hypopituitarism presenting as a rash. A diagnosis of cutaneous and pulmonary sarcoidosis was readily established in a 35-year-old man who presented with a widespread undiagnosed papular rash (a) associated with fatigue and shortness of breath. He was, however, incidentally noted to have gynaecomastia and, on further questioning, reduced libido. Endocrinological assessment and pituitary magnetic resonance imaging demonstrated, respectively, hypogonadotrophic hypogonadism and pituitary enlargement (b) in keeping with sarcoid infiltration.
(Courtesy of Dr S. Walsh, King's College Hospital, London, UK.)
In a small cohort of children and adolescents with isolated GH deficiency or multiple pituitary hormone deficiencies, elastin fibres were abnormal: besides being reduced in number, they were shorter and slimmer, suggesting that GH and other pituitary hormones regulate elastogenesis in human skin [306]. Reduced collagen synthesis has also been seen in patients with hypopituitarism [307]. In GH-deficient patients with Sheehan syndrome, skin capacitance and sebum content were found to be decreased compared with control subjects [308]. Patients affected by congenital POMC deficiency [303] are often red-haired and show a pale skin and early onset of obesity.
Hyperpituitarism
The major disease states resulting from hyperpituitarism (i.e. acromegaly, hyperprolactinaemia, and Cushing disease) each display very characteristic skin signs that aid diagnosis.
In acromegaly, a hormonally active adenoma of the anterior pituitary secretes excessive GH, leading to a secondary rise in IGF-1 serum and tissue levels. The clinical consequences depend upon whether this condition starts during childhood, adolescence or adulthood. It is characterized by excessive growth of acral cartilage and skin with prominent epidermal hyperplasia and dermal glycosaminoglycan accumulation (hence the name). External manifestations include progressive enlargement of earlobes and fingers (‘My gloves don't fit any more’), increasingly coarse facial features with a prominent chin (Figure 149.4) and large frontal skin folds (‘thinker's folds’), which may be associated with cutis verticis gyrata (Figure 149.5). Other characteristic changes include hypermelanosis, hypertrichosis, seborrhoea, thickened finger- and toenails, acanthosis nigricans, hyperhidrosis and macroglossia (Figure 149.15; Tables 149.4 and 149.5) [5, 309]. Decreased transepidermal water loss, reduced skin surface temperature and an increase in skin pH and elasticity have also been reported in patients with acromegaly [242, 310].
The treatment of acromegaly by neurosurgery, radiotherapy, with GH antagonists (e.g. pegvisomant), somatostatin analogues (e.g. octreotide) or dopaminergic agents (e.g. bromocriptine, cabergoline) may partially reverse the cutaneous phenomena induced by long-term exposure to excessive GH serum levels. Unfortunately, the opportunities to investigate how these surgical and medical therapies affect human skin have been largely overlooked as study of this might shed new light on the still fairly obscure role of GH receptor mediated signalling in human skin physiology. It has, however, recently been shown that dopamine actually inhibits human scalp hair growth in vitro [101] and may thus be a functional antagonist to GH in terms of hair growth control.
Conversely, GH therapy (e.g. in patients with Turner syndrome) can enhance the growth of melanocytic naevi, whose melanocytes appear to be activated as deduced from their increased HMB-45 and Ki-67 immunoreactivity [243, 244]. Indeed, in murine wound healing studies, treatment with GH-releasing hormone (GHRH) or a GH agonist increased the density of fibroblasts during the early stages of wound healing and accelerated re-epithelialization later on. Therefore, the undesired dermatological phenomena seen in acromegaly may well indicate novel strategies for promoting cutaneous wound healing [152]. It has also been proposed that replacement therapy with GH or the key growth factor stimulated by it (IGF-1) represents a potential anti-skin ageing strategy that is worth systematically exploring [166].
Although prolonged hyperprolactinaemia can have many causes, it most frequently results either from a prolactin-secreting micro- or macroadenoma of the anterior pituitary gland or from drugs (e.g. phenothiazine, dopamine antagonists, oestrogens, morphine derivates, H2-antagonists and neuroleptic agents) [5, 311].
As with GH, a sustained elevation of prolactin serum levels results in increased serum and tissue IGF-1 levels [20]. Thus, there is some limited overlap in the cutaneous phenomena seen in acromegaly and hyperprolactinaemia, namely seborrhoea, though less than expected if increased IGF-1 secretion were the primary mode of prolactin action on human skin. Instead, hyperprolactinaemia in women is typically associated with seborrhoea, acne, hirsutism and androgenetic alopecia, a combination for which the term SAHA syndrome has been coined. SAHA can also be associated with ovarian and adrenal dysfunction (see Chapter 90). Almost pathognomonically, hyperprolactinaemia can induce galactorrhoea in both men and women, usually along with gynaecomastia in men.
However, recent research suggests that the prominent effects on the pilosebaceous unit and the mammary gland seen in patients with hyperprolactinaemia represent only a small part of prolactin's full range of actions on human skin in health and disease. These range from the modulation of keratinocyte and sebocyte proliferation, differentiation and apoptosis to the regulation of cytokine secretion and keratin expression, including the promotion of keratin 15 expression, a key epithelial stem cell marker, indicating a wider role for prolactin in epithelial stem cell biology; moreover, prolactin actions may differ between the genders [3, 88, 89, 91, 312]. If it is confirmed that prolactin also modulates peripheral androgen metabolism [313] and plays a significant role in cutaneous autoimmunity [314], this only serves as additional motivation to explore fully the impact of excessive or insufficient prolactin production by the pituitary gland, human skin or its appendages [88, 90, 312] on skin physiology and function.
Most patients affected by Cushing disease suffer from an ACTH-producing tumour, either in the anterior pituitary or in an extrapituitary location where the tumour (most frequently small cell bronchial carcinoma) engages in ectopic ACTH production. The associated skin phenomena are dominated by the pigmentary effects of ACTH and the hypercortisolism induced by the chronically elevated serum cortisol level [275] (see later for description of cutaneous phenomena). The observation that a patient shows proximal repigmentation of previously grey/white hair and/or hyperpigmented palmar lines (in an individual of Caucasian ethnicity) should immediately indicate screening for an ACTH-secreting tumour or Addison disease (see later).
Adrenal hyperfunction
There are three forms of adrenal hyperfunction.
Hypercortisolism (Cushing syndrome) This is most frequently iatrogenic and results from systemic or occasionally extensive topical glucocorticoid therapy. Less common are ACTH-producing tumours (Cushing disease) and, very rarely, CRH-producing hypothalamic tumours [277] or autoimmune stimulation of adrenal ACTH receptors (MC2R) by autoantibodies (Carney syndrome) [315]; the latter is to be distinguished from the Carney complex, an exceptionally rare, dominantly inherited syndrome associated with multiple endocrine neoplasias, endocrine overactivity, and spotty skin hyperpigmentation that also shows ACTH-independent Cushing features resulting from a mutation in the PRKAR1A gene (see Chapter 150) [316, 317, 318].
The non-pigmentary skin manifestations of both Cushing disease and Cushing syndrome are thought to result primarily from excessive adrenal cortisol production (hypercortisolism), even though it is now well documented that ACTH also stimulates steroidogenesis in human skin and its appendages [26, 57]. Therefore, it remains to be explored whether some of the steroid hormone-dependent cutaneous phenomena in Cushing disease are actually due at least in part to excessive compensatory intracutaneous steroidogenesis.
The dermatological manifestations of hypercortisolism (including that due to Cushing disease) include facial and neck changes due to altered subcutaneous fat distribution (‘moon face’, ‘buffalo hump’) (see Tables 149.4 and 149.5), generalized hypertrichosis, acne and the consequences of increased collagen breakdown, namely skin fragility, stretch marks, vascular fragility with resulting purpura and impaired wound healing. Hypercortisolism can also induce diabetes, thus adding to the spectrum of diabetes-associated skin phenomena (see Box 149.1).
Hyperaldosteronism Several indications from the experimental literature (in the skin of mineralocorticoid receptor overexpressing mice) would lead one to expect that hyperaldosteronism (Conn syndrome) should also affect human skin, which expresses the aldosterone receptor [319, 320]. However, prominent skin signs or symptoms have not yet been described in individuals with hyperaldosteronism.
Congenital adrenal hyperplasia (CAH, formerly known as adrenogenital syndrome, MIM201910) is the third form of adrenal hyperfunction and is in the majority of cases due to an inherited deficiency of 21-hydroxylase due to mutations in the CYP21 gene. This means that the cortisol precursor 17-hydroxyprogesterone cannot be metabolized to cortisol but is shunted towards enhanced androgen production instead. This results in more or less pronounced virilization with associated skin signs including acne and hirsutism. ACTH levels are increased due to the body's attempts to compensate for insufficient cortisol production and this may result in hypermelanosis, especially of the buccal mucosa, lips and genitalia. In women, the clinical features of CAH may be difficult to distinguish from those of polycystic ovary syndrome, idiopathic hirsutism or hyperinsulinaemia and the possibility of underlying CAH in women presenting with these signs should not be overlooked: short stature may be a helpful clue [321].
Adrenal insufficiency (Addison disease)
Insufficient adrenal cortisol production is most commonly the consequence of autoimmune disease (autoimmune adrenalitis) or of suppression of ACTH secretion following long-term glucocorticoid therapy. Infectious diseases (tuberculosis, systemic fungal infection) are also important causes. For a full list of the many rarer causes endocrinology textbooks should be consulted [5]. For the practising dermatologist, it is important to remember that recovery of endogenous ACTH production after long-term suppression by exogenous glucocorticoids may be delayed and give rise to the same clinical picture.
Vitamin B12 deficiency may rarely result in epidermal hyperpigmentation similar to Addison disease but is easily distinguishable by its haematological abnormalities (megaloblastic anaemia) [322, 323]. In early-onset obesity that is not otherwise explained, adrenal insufficiency should be considered as a potential cause [324], as this may indicate a rare loss-of-function mutation in POMC, the precursor hormone from which ACTH is produced enzymatically [303].
Apart from general symptoms (see Table 149.1) hypermelanosis due to compensatory pituitary (and possibly intracutaneous) oversecretion of ACTH or its enzymatic cleavage product, α-MSH, is a prominent component of Addison disease. This is often first and best visible as hyperpigmented palmar skin creases but also affects intertriginous skin, external genitalia, nail beds, oral mucosa and lips [325]. The biological reason for this apparently UV-independent peculiarly distributed but characteristic pigmentation phenotype is not yet clear; however, one may speculate that melanocytes in the preferentially affected skin regions are particularly sensitive to ACTH or α-MSH stimulation, perhaps as a result of higher levels of MC1R or MC2R expression.
If Addisonian pigmentation presents in association with vitiligo (see Chapter 88) or, rarely, alopecia areata (see Chapter 89) it is reasonable to assume that it is due to autoimmune adrenalitis; this possibility should then be excluded or confirmed by a specialist to guide subsequent therapy. It has been argued that patients presenting with generalized hyperpigmentation of unclear origin should have serum ACTH and mineralocorticoid function checked without delay for undiagnosed primary adrenal insufficiency [326].
Hyperandrogenism
While the testes (and to a minimal extent ovaries) are the primary site of testosterone synthesis, androgens are also produced in the adrenal glands. The main adrenal androgens are dehydroepiandrosterone (DHEA) and androstendione. Androgens are further metabolized in their target tissues. The biologically most important enzymatic processes involved are the conversion of testosterone to DHT by 5-α-reductase and the metabolic conversion of androgens to 17-β-oestradiol [50, 52]. All relevant enzyme activities and androgen receptors are present in human skin, most prominently in its pilosebaceous units. It is thought that the higher androgen content of male skin partly explains why it is thicker, more hairy and slightly more pigmented than female skin [275, 327]. This is in accordance with the skin phenotype seen in hyperandrogenism.
Apart from exogenous androgen use or abuse (e.g. anabolic steroids) and CAH, polycystic ovary syndrome with the production of androgens by ovarian theca cells is the most frequent cause of hyperandrogenism in women [328, 329, 330]. Acromegaly, Cushing disease, androgen-secreting (virilizing) tumours of the ovary or adrenal gland, and hyperprolactinaemia represent other less common causes. Amongst the latter, hyperprolactinaemia is probably the most common.
The cutaneous hallmarks of hyperandrogenism are seborrhoea, severe acne, hirsutism, telogen effluvium and female or male pattern balding [214, 331, 332]. Hyperandrogenism should be considered as a potential underlying cause of late-onet acne in women. Other signs of virilization (e.g. male habitus in women, deepening of the voice, clitoral hypertrophy) may make the clinical diagnosis of hyperandrogenism very straightforward.
A full endocrinological assessment should however be undertaken without delay. Polycystic ovary syndrome is often coupled with hyperprolactinaemia and/or increased luteinizing hormone levels [328, 329]. An important variant also shows insulin resistance, diabetes and acanthosis nigricans, but normal luteinizing hormone levels (HAIR-AN type: hyperandrogenism, insulin resistance, acanthosis nigricans; see Chapter 90).
Whether or not antiandrogenic therapy is indicated in patients with hyperandrogenism requires careful consideration, with the available pharmacological options differing greatly between countries [333]. Androgen suppression, e.g. via administration of a gonadotropin-releasing hormone antagonist, may also improve microvascular dilation in polycystic ovary syndrome, possibly via an endothelin receptor-dependent mechanism [51].
Hypoandrogenism
Whether it is caused by castration, tumours, antiandrogen therapy, genetically deficient androgen production (e.g. Klinefelter syndrome) or defective androgen receptor-mediated signalling, the dermatological picture of hypoandrogenism is mainly determined by the timing of androgen deficiency. If uncorrected hypoandrogenism occurs before puberty, it will manifest as eunuchoid habitus, with undeveloped male body habitus, minimal or absent male secondary hair and no acne during puberty; if it occurs after puberty, there is a slow progressive loss of male secondary sexual characteristics and androgenetic alopecia does not progress [5, 52, 150, 275].
As androgen deficiency is usually corrected in male patients, one dermatologically important point is that replacement therapy is not infrequently carried out too aggressively, so that features of hyperandrogenism such as seborrhoea, acne and rapidly progressive male pattern balding can develop.
One cause of infertility in hypoandrogenized males, typically with reduced beard and body hair growth, is a function-diminishing polymorphism of the androgen receptor [334].
Hyperoestrogenism
Besides oral contraceptives and excessive consumption of phyto-oestrogens (a possibility not to be ignored in the current climate of ‘health food’ frenzy), oestrogen-producing tumours are the most common and most serious causes of hyperoestrogenism. The classical skin signs are melasma, spider telangiectases and increased susceptibility to Candida vulvovaginitis. While girls can undergo precocious puberty, boys develop gynaecomastia. Much more rarely erythema nodosum, porphyria cutanea tarda, pemphigoid gestationis and systemic lupus erythematosus can be seen [211, 275, 276].
Hypo-oestrogenism
Hypo-oestrogenism as the natural consequence of the menopause or due to ovariectomy may result in female pattern balding. Sometimes this female variant of androgenetic alopecia may follow the male pattern or can also show a mixed phenotype, i.e. with features of both male and female pattern balding (see Chapter 89). The latter very common hair phenotype is associated with postmenopausal hypo-oestrogenism [54] and supports the hypothesis that, in contrast to androgenetic alopecia in males, a relative deficiency in the stimulation of scalp hair follicles by 17-β-oestradiol is a major factor in female pattern balding; this might well be as important an aetiological factor in female pattern balding as the hair growth inhibitory effects of DHT [53, 221, 335, 336, 337, 338, 339].
The significance of adequate oestrogen stimulation of female scalp hair follicles is further supported by observations in iatrogenic hypo-oestrogenism: aromatase inhibitors such as anastrazole and letrozole administered for management of breast cancer frequently cause a telogen effluvium [340] and sometimes may induce male pattern hair loss [338]. Reportedly, skin with higher aromatase expression (expected to be associated with elevated local oestrogen levels) also shows thicker elastic fibres than skin where aromatase expression is lower [40].
Given the recognized key role of 17-oestradiol as a promoter of wound healing [54, 159, 160, 161, 167], the observation that impaired wound healing can be associated with hypo-oestrogenism [158] and that 17-β-oestradiol may be capable of slowing or even partially reversing skin ageing [54, 55, 164, 167, 245], hypo-oestrogenism is a state that justifies consideration of hormone replacement, even by dermatologists. The potential benefits of this have to be carefully balanced against the long-debated, potentially serious adverse effects of oestrogen therapy [341].
In contrast to androgens, 17-β-oestradiol restricts sebocyte proliferation and sebum production [56]. Therefore, in addition to genital pruritus and burning mouth syndrome, seborrhoea is another dermatological manifestation of hypo-oestrogenism. If these occur concomitantly with osteoporosis, typical menopausal mood swings, paroxysmal hyperhidrosis and flushing, a clinical working diagnosis of hypo-oestrogenism is easily reached and can be confirmed by measuring serum hormone levels.
Phaeochromocytoma
Phaeochromocytoma is a potentially life-threating catecholamine-secreting endocrine neoplasm of the adrenal medulla. It can occur bilaterally or may be ectopic; in 10–15% of cases it is malignant. In comparison with its dominant clinical presentations (hypertension, paroxysmal hypertensive crises, headaches, tremor, palpitations, anxiety), its skin manifestations may not be obvious: hyperhidrosis, paroxysmal facial pallor, and sometimes aggravation of pre-existing Raynaud phenomenon [211, 276, 277, 342]. Dermatologists should be alert to the significance of such systemic signs in patients with these cutaneous features, particularly if they have neurofibromatosis type I, which conveys an increased risk of developing phaeochromocytoma [276, 342, 343].
Carcinoid
Carcinoid is a malignant neoplasm of specialized serotonin-producing epithelial cells of the small intestine and classically presents as paroxysmal flushing of the face and upper torso. These flushes, which may be triggered by psychoemotional stress, hot drinks or spicy food, often generate a sensation of rapidly ascending heat, which may be accompanied by wheezing and/or diarrhoea [277, 302, 342, 344]. It is assumed that not only the neurotransmitter serotonin but also neuropeptides such as substance P and bradykinin are involved in inducing these signs and symptoms.
However, as flushing reportedly occurs in less than 25% of patients with carcinoid [275], there are often no associated dermatological phenomena: the absence of flushing does not rule out the diagnosis. This must be kept in mind, as over the past 30 years the incidence of gastrointestinal carcinoid has been rising faster than that of any other cancer and the 5-year survival rate of patients with it is only 28.5% [345].
As an extreme rarity, human skin can also produce primary carcinoid tumours [346], while visceral neuroendocrine tumours may metastasize to the skin [347].
Glucagon and glucagonoma
Necrolytic migratory erythema (see Chapter 147) is the pathognomonic dermatological presentation of glucagonoma [280, 281, 348, 349], a neoplasm which typically arises from α-cells of the pancreas and secretes excessive amounts of glucagon. The associated cutaneous histopathological findings (intercellular epidermal oedema together with hydropic degeneration of epidermal keratinocytes) are shared with pellagra, zinc deficiency and acrodermatitis enteropathica, even though patients with glucagonoma syndrome have normal zinc serum levels [211].
Besides surgery, somatostatin analogues usually improve the skin eruption [350]. Little is known about how somatostatin or glucagon itself affects normal skin physiology or how excessive glucagon levels induce the pathognomonic clinical phenotype in the skin. Interestingly, some frog species generate substantial amounts of glucagon as well as many other neuropeptides in the skin [206]. Somatostatin may play a role in maintaining the physiological immune privilege of the hair follicle [110]. It has been postulated that it may not be glucagon itself but another as yet unidentified tumour-secreted peptide that is responsible for necrolytic migratory erythema [211]. Yet, it has been reported that necrolytic migratory erythema can be induced by glucagon therapy alone [351], suggesting that tumour-derived glucagon may well be capable of inducing this unique erythema phenotype.
Glucagon-like peptide-1 (GLP-1) is a gut hormone derived from proglucagon which has affinity for specific GLP-1 receptors present particularly in the pancreas and the brain. Amongst other actions, GLP-1 inhibits secretion of glucagon, enhances glucose-dependent insulin secretion and induces a feeling of satiety. Synthetic GLP-1 receptor agonists are now employed for treating type 2 diabetes. The observation that these drugs appear to be of some benefit for psoriasis in diabetic patients with insulin resistance before significant metabolic improvement can be detected [352, 353] provides interesting new clues to the possible role of GLP-1 in human skin, especially since psoriatic lesions can show increased expression of GLP-1 receptors [352, 354].
Polyendocrine disease
Multiple endocrine neoplasia (MEN-1 and MEN-2) and the autoimmune polyglandular syndromes (e.g. APS-1, due to a defective AIRE gene) can generate several of the skin lesions and symptoms described in this chapter [211, 355, 356, 357, 358, 359]. They are discussed in more detail in Chapter 147.
Diabetes
The dermatological dimensions of diabetes are described in Chapter 64 and summarized in Box 149.1.
Hyperthyroidism and hypothyroidism
Along with diabetes, hyper- and hypothyroidism are the endocrine disorders that most frequently cause skin abnormalities [210, 360]. If they occur together, pretibial myxoedema, weight loss, hyperhidrosis and an increased state of nervousness quickly indicate hyperthyroidism. By contrast, the combination of dry skin, telogen effluvium, brittle hair, pallor, weight gain, increased tiredness and decreased alertness is suggestive of hypothyroidism [211, 275]. Textbooks and reviews therefore traditionally deal with the dermatological aspects of hyper- and hypothyroidism in separate chapters. However, it may actually be more helpful to consider these conditions jointly, as we do here.
The dermatologist will identify the skin signs and symptoms (see Tables 149.3, 149.4 and 149.5) which point to the possibility of thyroid dysfunction; the dermatologist will then request routine thyroid function tests (TSH, free triiodothyronine (T3) and thyroxine (T4)) leaving the establishment of the finer details to the thyroid specialist.
Thyroid hormone receptors are abundantly expressed in human skin and hair follicles. Organ-cultured human skin and hair follicles respond directly to thyroid hormone stimulation in multiple ways with effects on keratinocyte proliferation and keratin expression, intracutaneous regulation of neurohormone production and hair follicle epithelial stem cell functions, stimulation of hair follicle pigmentation, keratinocyte energy metabolism and wound healing [28, 84, 102, 104, 195, 361, 362, 363]. Recently, it has been found that thyroxine strongly up-regulates expression of core clock genes in human skin [364].
Given increasing insight into the importance of peripheral clock activity for many different aspects of skin physiology ranging from cell cycle control and cell metabolism via pigmentation to hair growth control [283, 284, 285, 286, 287], this newly identified link between skin endocrinology and chronobiology only underscores the importance of hyper- or hypothyroidism from a dermatological perspective. Therefore it is not surprising that both excessive and insufficient serum levels of T3 or thyroxine T4 can generate a plethora of dermatological signs and symptoms. However, none of these mechanisms are yet fully understood.
A hyperthyroid state most frequently results from Graves disease, the most prevalent endocrinopathy after diabetes. It primarily affects women or occurs during the initial phase of autoimmune thyroiditis (Hashimoto type) [5, 150]. While the latter eventually results in hypothyroidism, its first dermatological presentation can be due to hyperthyroidism. Postpartum thyroiditis also manifests initially as hyperthyroidism, which is then followed by hypothyroidism – two more arguments for considering all thyroid disease associated skin and hair changes as one complex, at least for now. Excessive T4 medication or exposure to iodine (in particular in neonates or patients with impaired epidermal barrier function), hormone-secreting thyroid adenomas, multinodular goitre and, very rarely, a TSH-secreting pituitary adenoma are other important causes of hyperthyroidism. All these can induce the dermatological phenomena summarized earlier. An additional, thyroid automimmunity-related skin sign may be chronic urticaria, as it has been postulated to be inducible by IgE autoantibodies against thyroid peroxidase [365].
How stimulatory anti-TSH receptor autoantibodies are generated in Graves disease is still debated: one hypothesis holds that intradermal TSH receptors may provide the main inciting autoantigen [14, 366]. In any case, the stimulation of TSH receptors expressed by skin fibroblasts may induce the characteristic increased production and deposition of glycosaminoglycans responsible for pretibial and eyelid myxoedema as well as exophthalmos. Interestingly, excessive cutaneous glycosaminoglycans can induce both hypertrichosis and alopecia, possibly depending on whether the deposition of these extracellular matrix components act on relatively ‘quiescent’ telogen or maximally proliferating anagen hair follicles [215]. Likewise, both excessive and insufficient thyroid hormone serum levels can be associated with effluvium and alterations in hair shaft structure, elasticity or sheen [14, 102, 210]. The cutaneous microcirculation also appears to be affected in patients with Graves disease [367].
Hashimoto thyroiditis, the main cause of hypothyroidism apart from dietary iodine deficiency, can be associated with a few additional characteristic signs, namely lateral sparseness of the eyebrows (the sign of Hertoghe; key differential diagnoses include atopic eczema, trichotillomania and ulerythema ophryogenes) and a yellowish hue of the skin due to β-carotene accumulation (aurantiasis cutis) [368]. If these are accompanied by carpal tunnel syndrome, ptosis and brittle, slow-growing nails, this further indicates the presence of hypothyroidism [211, 276]. Apart from pretibial myxoedema (see Table 149.4) a more generalized myxoedema may also be present, often most prominently on the face, giving it a waxen appearance. With these characteristic signs, the diagnosis of hypothyroidism should not be challenging.
Iatrogenic hypothyroidism used to be largely restricted to patients following thyroid surgery, thyroid radiation or thyrostatic drug therapy. More recently, however, another iatrogenic form of hypothyroidism must also be considered: bexarotene, a second line therapy for cutaneous T-cell lymphoma, can induce significant hypothyroidism [369, 370], accompanied by the corresponding skin signs and symptoms (see Chapter 19).
Hyperparathyroidism
Most frequently, the typical hypercalcaemia that is associated with hyperparathyroidism causes only mild pruritus and/or skin dryness or no dermatological signs and symptoms at all. It is therefore easily overlooked by dermatologists.
Primary hyperparathyroidism most commonly results from an autonomous parathormone-secreting adenoma; secondary hyperparathyroidism may arise from vitamin D deficiency or from disturbances of the calcium-phosphate balance, especially in patients with chronic kidney disease. This predisposes to calciphylaxis (calcific uraemic arteriolopathy), though not all patients with this frequently fatal condition have either abnormal parathormone levels or high calcium-phosphate products [5, 120, 121]. Calciphylaxis is characterized by widespread vascular calcification and thrombotic occlusion of cutaneous blood vessels with resultant extensive tissue necrosis. Calciphylaxis is a frequent complication of end-stage renal insufficiency [371, 372]. Given the very high mortality of calciphylaxis, its development requires rapid diagnostic action so as to ascertain its origin and treatability [373].
Rarely, hyperparathyroidism may result in metastatic calcification in the dermis and subcutis: this does not specifically affect blood vessels and, if treated promptly, is reversible. It again is commoner in patients with renal failure and typically presents as firm papules, nodules or plaques, particularly around large joints or flexural sites. Unlike calciphylaxis it does not lead to tissue necrosis [374, 375]. These conditions are described in more detail in Chapter 61.
Primary hyperparathyroidism can also occur as a component of the MEN-1 syndrome (see earlier) [376].
Interestingly, both autosomal recessive congenital ichthyosis and epidermolytic ichthyosis in children were recently found to be associated with significantly increased parathyroid hormone (PTH) serum levels as well as with a higher radiological rickets score compared with common ichthyosis [377]. This raises the intriguing question whether some chronic dermatoses, namely those characterized by abnormalities of keratinization, are associated with increased intracutaneous production of PTH/PTH-related peptide (PTHrp). As associated endocrinological abnormalities (i.e. hyperparathyroidism and hypercalcaemia) could have major clinical consequences for distant organs such as the skeletal and gastrointestinal systems, this possibility deserves systematic investigation.
Hypoparathyroidism
Hypoparathyroidsm used to arise predominantly as a surgical complication following thyroidectomy. It is clinically most obvious from the characteristic resultant tetanic muscle cramps. However, hypoparathyroidism can also be seen in polyglandular autoimmune endocrinopathies or rare genetic defects, where its onset and progression can be far more insidious. In patients with pseudohypoparathyroidism the target tissues show greatly decreased sensitivity to stimulation with PTH.
In both cases, hair and nail growth disorders and skin dryness are the most frequently associated dermatological phenomena. ‘Dry, rough skin; coarse, brittle hair; and lustreless distally split nails’ [378] are frequently encountered skin signs in patients with idiopathic hypoparathyroidism. In a small Indian cohort of such patients, loss of axillary and pubic hair, coarsening of body hair, brittle and ridged nails and xerosis cutis were the most common skin signs observed [379]. Another study involved patients exposed to ionizing radiation following the nuclear reactor accident in Chernobyl who had had to undergo thyroidectomy and who developed permanent hypoparathyroidism as a surgical complication. Apart from frequently recurring paraesthesiae and joint pains, hair loss and progressively dry skin were the chief dermatological abnormalities [380]. A study in Brazilian women found hypoparathyroidism to be associated with a significantly reduced hair shaft content of calcium and phosphate, while these minerals were increased in patients with hyperparathyroidism or hyperthyroidism [381].
This hair phenotype corresponds well with the knowledge that both PTH/PTHrp agonists and antagonists profoundly modulate rodent hair follicle cycling in vivo as well as the hair follicle response to chemotherapy-induced damage [92, 93, 94, 382]. It has been proposed that this may in part be related to the effects of PTH/PTHrp-mediated signalling on angiogenesis in murine skin [95]. While the fine details of these murine studies do not concern us here, what really matters from a clinical dermatoendocrinological perspective are three concepts:
- The interactions between PTH/PTHrp with its receptor constitute a functionally important, intracutaneous hormonal signalling system, the epithelial–mesenchymal interactions of which have a profound impact on rodent hair follicle growth and skin vasculature.
- That patients with hypoparathyroidism also show hair growth abnormalities strongly suggests that this signalling system also operates in human skin, is clinically important and might be targeted therapeutically (e.g. in the management of telogen effluvium and chemotherapy-induced alopecia) [92, 383].
- Any hormonal signalling system that modulates both the activities of an entire mini-organ (here, the hair follicle) and a process as complex as angiogenesis is almost guaranteed to have effects on multiple other aspects of skin physiology and pathology that await discovery. The finding that PTHrp regulates proliferation and differentiation in a human keratinocyte cell line in vitro [384] supports this notion.
Thus, hypoparathyroidism illustrates the principle that the skin phenotype seen in patients with a defined hormone deficiency, if interpreted in conjunction with the experimental literature, can highlight clinically relevant novel research frontiers in investigative dermatoendocrinology.
Future perspectives
For now, recognizing the skin signs and symptoms that indicate underlying endocrine abnormalities or conditions remains the core endocrinological challenge for the practising dermatologist. However, owing to the discovery that human skin is a major endocrine organ and metabolizes numerous hormones and neuromediators, the future scope and practice of dermatoendocrinology will change. With increasing insight into previously unsuspected (neuro-)endocrine dimensions of common skin diseases (see Table 149.2) and of standard therapeutic agents used to treat them, clinical dermatology cannot escape or ignore these important recent developments.
A few examples for future perspectives of dermatoendocrinology underscore this point:
- All routinely administered hormone-based agents used for treating skin disease (glucocorticoids, retinoids, vitamin D derivatives) are likely to have a major impact on the physiological (neuro-)endocrine signalling milieu of a given patient's skin. A better understanding of these interactions should help to reduce adverse effects and enhance therapeutic efficacy.
- In all endocrine disorders that affect the skin, there are likely to be alterations in the ways that relevant (neuro-)hormones and neuromediators are expressed or metabolized in the skin; and receptor-mediated signalling must be expected to be altered as a secondary consequence of systemic endocrine abnormalities.
- It remains a matter of speculation to what extent secondary intracutaneous (neuro-)endocrine abnormalities contribute to discernible skin phenotypes associated with classical endocrine disease (see Tables 149.4 and 149.5 and Box 149.1). This deserves further systematic study.
- Better understanding of how the extremely complex signalling interactions between the individual (neuro-)hormones and neuromediators present in the skin affect its functioning in health and disease should help to develop new strategies for improving skin function and retarding or even partially reversing skin ageing [54, 166].
- Understanding in greater detail which specific (neuro-)endocrine abnormalities associated with inflamed, hyperproliferating and/or fibrosing skin diseases significantly contribute to the triggering, aggravation or pathogenesis of common dermatoses will identify novel therapeutic targets and corresponding management strategies.
- The ‘epidemic’ of allergic diseases throughout Western civilization [385] calls for the development of innovative and effective anti-allergic strategies. Neuroendocrine strategies are particularly promising in this context. Examples include studies in human skin and hair follicle organ culture which have shown that: (i) endogenous and exogenous agonists of cannabinoid receptor 1 have a potent suppressive effect on mast cell degranulation and the maturation of functional mast cells from resident progenitor cells in human skin and airway mucosa [64, 139], while CRH promotes both [3, 127, 169]; (ii) SP stimulates human skin mast cell degranulation and neurogenic inflammation [13, 18, 97]; and (iii) the α-MSH-derived peptide, K(D)PT, inhibits human mast cell degranulation in situ [187]. Thus, in addition to neuropeptides, CB1 agonists and CRH and SP receptor (NK1) antagonists are promising candidates for anti-allergy therapeutics.
- Antagonizing CRH [127, 237] or substance P [98, 143, 169, 386], or stimulating CGRP receptors [99], holds promise for the future management of inflammatory skin diseases such as atopic eczema by counteracting SP- and/or CRH-driven neurogenic skin inflammation. Although pharmacological antagonists of substance P or CRH have been shown to abrogate or significantly mitigate neurogenic skin inflammation and its sequelae (e.g. hair growth inhibition, itch, eczema) in animal experiments, this remains to be fully explored in human skin.
Finally, it has long been suspected that the modern penchant for sun exposure, despite its much publicised effects on skin ageing and skin cancer risk, has a habit-forming, if not addictive, neuroendocrine component. A recent animal study supports this notion: UV-seeking addictive behaviour in mice was influenced greatly by the UV-induced intracutaneous synthesis of the endogenous opioid, β-endorphin, leading to elevated plasma levels even after low-dose UV irradiation in vivo [387]. This is exciting because it shows that endorphins synthesized peripherally in mammalian skin can, in principle, reach both the circulation and the brain, and can cause complex behavioural changes. Interestingly, an increase of enkephalin plasma levels has already been reported in patients undergoing UVA phototherapy [388]. Therefore, it has become at least conceivable that future dermatological therapy will be capable of modulating this brain–skin axis in ways that may help dermatological patients whose skin disease has a major psychological component [389].
In addition to its paramount importance for the identification of underlying endocrine disorders, it is for these reasons that dermatoendocrinology lies at the very heart of modern dermatological practice.
References
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev 2001 Jan;81(1):449–94.
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol 2009 Feb 10;19(3):R132–42.
- Paus R, Langan EA, Vidali S, Ramot Y, Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med 2014 20(10):559–70.
- Bayliss HM, Starling EH. The chemical regulation of the secretory process. Proc R Soc Lond 1904 73:310–22.
- Melmed S, Polonsky KS, Polonsky KS, Kronenberg HM, Larsen PR, eds. Williams Textbook of Endiocrinology, 12th edn. Amsterdam: Elsevier, 2012.
- Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol 2007 Feb;265–266:143–9.
- Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech 2008 Jun 1;5(2):137–44.
- Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol 2012;212:v, vii, 1–115.
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev 2013 Dec;34(6):827–84.
- Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol 2014 Jun;23(6):369–74.
- Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene 2013 Nov 1;530(1):1–7.
- Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol 2013 Mar;168(3):595–601.
- Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain–skin connection’. Trends Immunol 2006 Jan;27(1):32–9.
- Paus R. Exploring the “thyroid–skin connection”: concepts, questions, and clinical relevance. J Invest Dermatol 2010 Jan;130(1):7–10.
- Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res 2011 Feb;24(1):89–106.
- Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 2006 Aug;126(8):1697–704.
- Arck P, Handjiski B, Hagen E, et al. Is there a ‘gut–brain–skin axis’? Exp Dermatol 2010 May;19(5):401–5.
- Peters EM. The neuroendocrine-immune connection regulates chronic inflammatory disease in allergy. Chem Immunol Allergy 2012;98:240–52.
- Agak GW, Qin M, Nobe J, et al. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J Invest Dermatol 2014 Feb;134(2):366–73.
- Langan EA, Foitzik-Lau K, Goffin V, Ramot Y, Paus R. Prolactin: an emerging force along the cutaneous-endocrine axis. Trends Endocrinol Metab 2010 Sep;21(9):569–77.
- Brooks CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev 2012 Aug;33(4):504–25.
- Poeggeler B, Schulz C, Pappolla MA, et al. Leptin and the skin: a new frontier. Exp Dermatol 2010 Jan;19(1):12–18.
- Procaccini C, Pucino V, Mantzoros CS, Matarese G. Leptin in autoimmune diseases. Metabolism 2015 Jan;64(1):92–104.
- Watabe R, Yamaguchi T, Kabashima-Kubo R, Yoshioka M, Nishio D, Nakamura M. Leptin controls hair follicle cycling. Exp Dermatol 2014 Apr;23(4):228–9.
- Nester EJ, Hyman SE, Holtzman DM, Malenka RC. Molecular Neuropharmacology – A Foundation for Clinical Neuroscience, 3rd edn. New York: McGrawHill, 2015.
- Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal axis and synthesize cortisol. FASEB J 2005 Aug;19(10):1332–4.
- Slominski A, Wortsman J, Kohn L, et al. Expression of hypothalamic–pituitary–thyroid axis related genes in the human skin. J Invest Dermatol 2002 Dec;119(6):1449–55.
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology 2010 Apr;151(4):1633–42.
- Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol 2006 Apr;15(4):265–82.
- Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol 2006 Sep;126(9):1948–65.
- Grando SA. Muscarinic receptor agonists and antagonists: effects on keratinocyte functions. Handb Exp Pharmacol 2012;208:429–50.
- Grando SA, Kawashima K, Kirkpatrick CJ, Meurs H, Wessler I. The non-neuronal cholinergic system: basic science, therapeutic implications and new perspectives. Life Sci 2012 Nov 27;91(21–22):969–72.
- Schallreuter KU, Lemke KR, Pittelkow MR, Wood JM, Körner C, Malik R. Catecholamines in human keratinocyte differentiation. J Invest Dermatol 1995 Jun;104(6):953–7.
- Pullar CE, Le Provost GS, O'Leary AP, Evans SE, Baier BS, Isseroff RR. β2AR antagonists and β2AR gene deletion both promote skin wound repair processes. J Invest Dermatol 2012 Aug;132(8):2076–84.
- Chernyavsky AI, Marchenko S, Phillips C, Grando SA. Auto/paracrine nicotinergic peptides participate in cutaneous stress response to wounding. Dermatoendocrinology 2012 Jul 1;4(3):324–30.
- Kim MH, Gorouhi F, Ramirez S, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol 2014 Mar;134(3):809–17.
- Curtis BJ, Radek KA. Cholinergic regulation of keratinocyte innate immunity and permeability barrier integrity: new perspectives in epidermal immunity and disease. J Invest Dermatol 2012 Jan;132(1):28–42.
- Grando SA. Connections of nicotine to cancer. Nat Rev Cancer 2014 Jun;14(6):419–29.
- Slominski A, Kim TK, Zmijewski MA, et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology 2013 Jan 1;5(1):7–19.
- Inoue T, Miki Y, Abe K, et al. Sex steroid synthesis in human skin in situ: the roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol Cell Endocrinol 2012 Oct 15;362(1–2):19–28.
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014 Mar 20;21(3):319–29.
- Böhm M, Luger TA, Tobin DJ, García-Borrón JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol 2006 Sep;126(9):1966–75.
- Böhm M, Apel M, Sugawara K, et al. Modulation of basophil activity: a novel function of the neuropeptide α-melanocyte-stimulating hormone. J Allergy Clin Immunol 2012 Apr;129(4):1085–93.
- Böhm M, Bodó E, Funk W, Paus R. α-Melanocyte-stimulating hormone: a protective peptide against chemotherapy-induced hair follicle damage? Br J Dermatol 2014 Apr;170(4):956–60.
- Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol 2004 Jan;122(1):235–7.
- Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol 2004 Feb;164(2):623–34.
- Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia 1992 Jan 15;48(1):50–4.
- Kauser S, Slominski A, Wei ET, Tobin DJ. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J 2006 May;20(7):882–95.
- Paus R, Schmelz M, Bíró T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 2006 May;116(5):1174–86.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol 2013 Mar;22(3):168–71.
- Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 2013 Oct 1;305(7):E818–25.
- Batrinos ML. The endocrinology of baldness. Hormones (Athens) 2014 Apr–Jun;13(2):197–212.
- Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev 2006 Oct;27(6):677–706.
- Thornton MJ. Estrogens and aging skin. Dermatoendocrinology 2013 Apr 1;5(2):264–70.
- Shu YY, Maibach HI. Estrogen and skin: therapeutic options. Am J Clin Dermatol 2011 Oct 1;12(5):297–311.
- Hinde E, Haslam IS, Schneider MR, et al. A practical guide for the study of human and murine sebaceous glands in situ. Exp Dermatol 2013 Oct;22(10):631–7.
- Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol 2013 Sep;137:107–23.
- Ramot Y, Zhang G, Bíró T, et al. TSH is a novel neuroendocrine regulator of selected keratins in the human hair follicle. J Dermatol Sci 2011 Oct;64(1):67–70.
- Ramot Y, Mastrofrancesco A, Camera E, Desreumaux P, Paus R, Picardo M. The role of PPARγ-mediated signalling in skin biology and pathology: New targets and opportunities for clinical dermatology. Exp Dermatol 2015;24(4):245–51.
- Flori E, Mastrofrancesco A, Kovacs D, et al. 2,4,6-Octatrienoic acid is a novel promoter of melanogenesis and antioxidant defence in normal human melanocytes via PPAR-γ activation. Pigment Cell Melanoma Res 2011 Aug;24(4):618–30.
- Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci 2009 Aug;30(8):411–20.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest 2014 Jul 25; pii: 64628.
- Telek A, Bíró T, Bodó E, et al. Inhibition of human hair follicle growth by endo- and exocannabinoids. FASEB J 2007 Nov;21(13):3534–41.
- Sugawara K, Bíró T, Tsuruta D, et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J Allergy Clin Immunol 2012 Mar;129(3):726–738.e8.
- Tóth BI, Dobrosi N, Dajnoki A, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol 2011 May;131(5):1095–104.
- Schallreuter KU, Wood JM, Lemke R, et al. Production of catecholamines in the human epidermis. Biochem Biophys Res Commun 1992 Nov 30;189(1):72–8.
- Schallreuter KU, Körner C, Pittelkow MR, Swanson NN, Gardner ML. The induction of the alpha-1-adrenoceptor signal transduction system on human melanocytes. Exp Dermatol 1996 Feb;5(1):20–3.
- Schallreuter KU, Pittelkow MR, Swanson NN, et al. Altered catecholamine synthesis and degradation in the epidermis of patients with atopic eczema. Arch Dermatol Res 1997 Nov;289(12):663–6.
- Schallreuter KU, Wei Y, Pittelkow MR, Swanson NN, Gibbons NC, Wood JM. Structural and functional alterations in the beta2-adrenoceptor are caused by a point mutation in patients with atopic eczema. Exp Dermatol 2007 Oct;16(10):807–13.
- Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol 2004 Aug;123(2):346–53.
- Sivamani RK, Shi B, Griffiths E, et al. Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes. J Invest Dermatol 2014 Aug;134(8):2258–66.
- Matsui S, Murota H, Takahashi A, et al. Dynamic analysis of histamine-mediated attenuation of acetylcholine-induced sweating via GSK3β activation. J Invest Dermatol 2014 Feb;134(2):326–34.
- Li ZJ, Park SB, Sohn KC, et al. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J Dermatol Sci 2013 Nov;72(2):116–22.
- Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J 2002 Jun;16(8):896–8.
- Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab 2008 Jan;19(1):17–24.
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 2012 Apr 4;351(2):152–66.
- Kobayashi H, Kromminga A, Dunlop TW, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J 2005 Oct;19(12):1710–12.
- Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol 2008 Sep;17(9):713–30.
- Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res 2008 Jan;44(1):1–15.
- Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J 2013 Jul;27(7):2742–55.
- Gáspár E, Hardenbicker C, Bodó E, et al. Thyrotropin releasing hormone (TRH): a new player in human hair-growth control. FASEB J 2010 Feb;24(2):393–403.
- Gáspár E, Nguyen-Thi KT, Hardenbicker C, et al. Thyrotropin-releasing hormone selectively stimulates human hair follicle pigmentation. J Invest Dermatol 2011 Dec;131(12):2368–7.
- Knuever J, Poeggeler B, Gáspár E, et al. Thyrotropin-releasing hormone controls mitochondrial biology in human epidermis. J Clin Endocrinol Metab 2012 Mar;97(3):978–86.
- Vidali S, Knuever J, Lerchner J, et al. Hypothalamic–pituitary–thyroid axis hormones stimulate mitochondrial function and biogenesis in human hair follicles. J Invest Dermatol 2014 Jan;134(1):33–42.
- Ramot Y, Mastrofrancesco A, Herczeg-Lisztes E, et al. Advanced inhibition of undesired human hair growth by PPARγ modulation? J Invest Dermatol 2014 Apr;134(4):1128–31.
- Poeggeler B, Knuever J, Gáspár E, et al. Thyrotropin powers human mitochondria. FASEB J 2010 May;24(5):1525–31.
- Ramot Y, Paus R. Harnessing neuroendocrine controls of keratin expression: a new therapeutic strategy for skin diseases? Bioessays 2014 Jul;36(7):672–86.
- Langan EA, Ramot Y, Hanning A, et al. Thyrotropin-releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro. Br J Dermatol 2010 May;162(5):1127–31.
- Langan EA, Ramot Y, Goffin V, Griffiths CE, Foitzik K, Paus R. Mind the (gender) gap: does prolactin exert gender and/or site-specific effects on the human hair follicle? J Invest Dermatol 2010 Mar;130(3):886–91.
- Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression. Am J Pathol 2006 Mar;168(3):748–56.
- Ramot Y, Bíró T, Tiede S, et al. Prolactin – a novel neuroendocrine regulator of human keratin expression in situ. FASEB J 2010 Jun;24(6):1768–79.
- Peters EM, Foitzik K, Paus R, Ray S, Holick MF. A new strategy for modulating chemotherapy-induced alopecia, using PTH/PTHrP receptor agonist and antagonist. J Invest Dermatol 2001 Aug;117(2):173–8.
- Safer JD, Ray S, Holick MF. A topical parathyroid hormone/parathyroid hormone-related peptide receptor antagonist stimulates hair growth in mice. Endocrinology 2007 Mar;148(3):1167–70.
- Katikaneni R, Ponnapakkam T, Seymour A, Sakon J, Gensure R. Parathyroid hormone linked to a collagen binding domain promotes hair growth in a mouse model of chemotherapy-induced alopecia in a dose-dependent manner. Anticancer Drugs 2014 Aug;25(7):819–25.
- Diamond AG, Gonterman RM, Anderson AL, et al. Parathyroid hormone hormone-related protein and the PTH receptor regulate angiogenesis of the skin. J Invest Dermatol 2006 Sep;126(9):2127–34.
- Cho YM, Woodard GL, Dunbar M, Gocken T, Jimènez JA, Foley J. Hair-cycle-dependent expression of parathyroid hormone-related protein and its type I receptor: evidence for regulation at the anagen to catagen transition. J Invest Dermatol 2003 May;120(5):715–27.
- Peters EM, Liotiri S, Bodó E, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol 2007 Dec;171(6):1872–86.
- Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 2014 Jan;94(1):265–301.
- Granstein RD, Wagner JA, Stohl LL, Ding W. Calcitonin gene-related peptide: key regulator of cutaneous immunity. Acta Physiol (Oxf) 2015 Mar;213(3):586–94.
- Kinori M, Bertolini M, Funk W, et al. Calcitonin gene-related peptide (CGRP) may award relative protection from interferon-γ-induced collapse of human hair follicle immune privilege. Exp Dermatol 2012 Mar;21(3):223–6.
- Langan EA, Lisztes E, Bíró T, et al. Dopamine is a novel, direct inducer of catagen in human scalp hair follicles in vitro. Br J Dermatol 2013 Mar;168(3):520–5.
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab 2008 Nov;93(11):4381–8.
- Contreras-Jurado C, Lorz C, García-Serrano L, Paramio JM, Aranda A. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell 2015 Feb 5. pii: mbc.E14-07-1251.
- Safer JD. Thyroid hormone and wound healing. J Thyroid Res 2013;2013:124538.
- Slominski AT, Zmijewski MA, Zbytek B, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol 2011 Mar;131(3):613–22.
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A 2012 Nov 27;109(48):19846–51.
- Holub BS, Kloepper JE, Tóth BI, Bíro T, Kofler B, Paus R. The neuropeptide galanin is a novel inhibitor of human hair growth. Br J Dermatol 2012 Jul;167(1):10–16.
- Hagforsen E, Michaëlsson G, Stridsberg M. Somatostatin receptors are strongly expressed in palmoplantar sweat glands and ducts: studies of normal and palmoplantar pustulosis skin. Clin Exp Dermatol 2011 Jul;36(5):521–7.
- Vockel M, Pollok S, Breitenbach U, Ridderbusch I, Kreienkamp HJ, Brandner JM. Somatostatin inhibits cell migration and reduces cell counts of human keratinocytes and delays epidermal wound healing in an ex vivo wound model. PLOS One 2011 May 11;6(5):e19740.
- Breitkopf T, Lo BK, Leung G, et al. Somatostatin expression in human hair follicles and its potential role in immune privilege. J Invest Dermatol 2013 Jul;133(7):1722–30.
- Deing V, Roggenkamp D, Kühnl J, et al. Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol 2013 Jun;22(6):399–405.
- Gilaberte Y, Roca MJ, Garcia-Prats MD, Coscojuela C, Arbues MD, Vera-Alvarez JJ. Neuropeptide Y expression in cutaneous melanoma. J Am Acad Dermatol 2012 Jun;66(6):e201–8.
- Hodges GJ, Sparks PA. Noradrenaline and neuropeptide Y contribute to initial, but not sustained, vasodilatation in response to local skin warming in humans. Exp Physiol 2014 Feb;99(2):381–92.
- Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol 2008 Apr;17(4):301–11.
- Törőcsik D, Kovács D, Camera E, et al. Leptin promotes a pro-inflammatory lipid profile and induces inflammatory pathways in human SZ95 sebocytes. Br J Dermatol 2014;171(6):1326–35.
- Bodó E, Bíró T, Telek A, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signalling in human hair growth control. Am J Pathol 2005 Apr;166(4):985–98.
- Bíró T, Bodó E, Telek A, et al. Hair cycle control by vanilloid receptor-1 (TRPV1): evidence from TRPV1 knockout mice. J Invest Dermatol 2006 Aug;126(8):1909–12.
- Bíró T, Tóth BI, Marincsák R, Dobrosi N, Géczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta 2007 Aug;1772(8):1004–21.
- Borbíró I, Lisztes E, Tóth BI, et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Invest Dermatol 2011 Aug;131(8):1605–14.
- Nilius B, Bíró T. TRPV3: a ‘more than skinny’ channel. Exp Dermatol 2013 Jul;22(7):447–52.
- Bodó E, Kromminga A, Funk W, Laugsch M, Duske U, Jelkmann W, Paus R. Human hair follicles are an extrarenal source and a nonhematopoietic target of erythropoietin. FASEB J 2007 Oct;21(12):3346–54.
- Bodó E, Wiersma F, Funk W, Kromminga A, Jelkmann W, Paus R. Does erythropoietin modulate human hair follicle melanocyte activities in situ? Exp Dermatol 2010 Jan;19(1):65–7.
- Paus R, Bodó E, Kromminga A, Jelkmann W. Erythropoietin and the skin: a role for epidermal oxygen sensing? Bioessays 2009 Mar;31(3):344–8.
- Pérez-Ibave DC, Rodríguez-Sánchez IP, Garza-Rodríguez Mde L, Barrera-Saldaña HA. Extrapituitary growth hormone synthesis in humans. Growth Horm IGF Res 2014 Apr–Jun;24(2–3):47–53.
- Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. Beta-endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol 2004 Jul;123(1):184–95.
- Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology 2004 Feb;145(2):941–50.
- Ito N, Sugawara K, Bodó E, et al. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol 2010 Apr;130(4):995–1004.
- Peters EM, Ericson ME, Hosoi J, et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol 2006 Sep;126(9):1937–47.
- Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol 2014 Sep;23(9):629–31.
- Yang CC, Sheu HM, Chung PL, et al. Leptin of dermal adipose tissue is differentially expressed during the hair cycle and contributes to adipocyte-mediated growth inhibition of anagen-phase vibrissa hair. Exp Dermatol 2015 Jan;24(1):57–60.
- Fantini F, Johansson O. Neurochemical markers in human cutaneous Merkel cells. An immunohistochemical investigation. Exp Dermatol 1995 Dec;4(6):365–71.
- Maksimovic S, Baba Y, Lumpkin EA. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann N Y Acad Sci 2013 Mar;1279:13–21.
- Xiao Y, Williams JS, Brownell I. Merkel cells and touch domes: more than mechanosensory functions? Exp Dermatol 2014 Oct;23(10):692–5.
- Maksimovic S, Nakatani M, Baba Y, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014 May 29;509(7502):617–21.
- Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends Cell Biol 2015 Feb;25(2):74–81.
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol 2002;20:125–63.
- Chavan SS, Tracey KJ. Neurophysiological reflex mechanisms in immunology. In: Paul WE, ed. Fundamental Immunology. Philadelphia: Wolters Kluwer/Lippincott, 2013: 850–62.
- Liezmann C, Stock D, Peters EM. Stress induced neuroendocrine-immune plasticity: A role for the spleen in peripheral inflammatory disease and inflammaging? Dermatoendocrinology 2012 Jul 1;4(3):271–9.
- Sugawara K, Zákány N, Hundt T, et al. Cannabinoid receptor 1 controls human mucosal-type mast cell degranulation and maturation in situ. J Allergy Clin Immunol 2013 Jul;132(1):182–93.
- Arck PC, Handjiski B, Kuhlmei A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl) 2005 May;83(5):386–96.
- Stelekati E, Bahri R, D'Orlando O, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 2009 Oct 16;31(4):665–76.
- Brown MA, Hatfield JK. Mast cells are important modifiers of autoimmune disease: with so much evidence, why is there still controversy? Front Immunol 2012 Jun 7;3:147.
- Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A 2010 Mar 2;107(9):4448–53.
- Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res 2013 Nov;1(5):269–79.
- Krejci-Papa NC, Paus R. A novel in-situ-zymography technique localizes gelatinolytic activity in human skin to mast cells. Exp Dermatol 1998 Dec;7(6):321–6.
- Wulff BC, Wilgus TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol 2013 Aug;22(8):507–10.
- Metz M, Piliponsky AM, Chen CC, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science 2006 Jul 28;313(5786):526–30.
- Akahoshi M, Song CH, Piliponsky AM, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest 2011 Oct;121(10):4180–91.
- Moline PE. Endocrine Physiology, 4th edn. New York: McGrawHill, 2013.
- Jameson JL. Harrison's Endocrinology, 3rd edn. New York: McGraw Hill, 2013.
- Fink G, Pfaff D, Levine J, eds. Handbook of Neuroendocrinology. Amsterdam: Elsevier/Academic Press, 2012.
- Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev 2003 Dec;24(6):737–64.
- Randall VA. Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol 2007 Apr;18(2):274–85.
- Nikolakis G, Zouboulis CC. Skin and glucocorticoids: effects of local skin glucocorticoid impairment on skin homeostasis. Exp Dermatol 2014 Nov;23(11):807–8.
- Azmahani A, Nakamura Y, Felizola SJ, et al. Steroidogenic enzymes, their related transcription factors and nuclear receptors in human sebaceous glands under normal and pathological conditions. J Steroid Biochem Mol Biol 2014 Oct;144 Pt B:268–79.
- Wood JM, Schallreuter KU. A plaidoyer for cutaneous enzymology: our view of some important unanswered questions on the contributions of selected key enzymes to epidermal homeostasis. Exp Dermatol 2008 Jul;17(7):569–78.
- Conrad F, Ohnemus U, Bodo E, Bettermann A, Paus R. Estrogens and human scalp hair growth–still more questions than answers. J Invest Dermatol 2004 Mar;122(3):840–2.
- Gilliver SC, Ashworth JJ, Ashcroft GS. The hormonal regulation of cutaneous wound healing. Clin Dermatol 2007 Jan–Feb;25(1):56–62.
- Campbell L, Emmerson E, Davies F, et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J Exp Med 2010 Aug 30;207(9):1825–33.
- Campbell L, Emmerson E, Williams H, et al. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J Invest Dermatol 2014 Sep;134(9):2447–57.
- Meier NT, Haslam IS, Pattwell DM, et al. Thyrotropin-releasing hormone (TRH) promotes wound re-epithelialisation in frog and human skin. PLOS One 2013 Sep 2;8(9):e73596.
- Mijouin L, Hillion M, Ramdani Y, et al. Effects of a skin neuropeptide (substance P) on cutaneous microflora. PLOS One 2013 Nov 8;8(11):e78773.
- Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas 2011 Jul;69(3):249–56.
- Jackson RL, Greiwe JS, Schwen RJ. Ageing skin: oestrogen receptor β agonists offer an approach to change the outcome. Exp Dermatol 2011 Nov;20(11):879–82.
- Trüeb RM, Tobin DJ, eds. Aging Hair. Berlin: Springer/Heidelberg, 2010.
- Zouboulis CC, Makrantonaki E. Hormonal therapy of intrinsic aging. Rejuvenation Res 2012 Jun;15(3):302–12.
- Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv Wound Care (New Rochelle) 2013 Feb;2(1):5–10.
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 2014 May;14(5):289–301.
- Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: does stress play a role? J Invest Dermatol 2009 Jun;129(6):1324–6.
- Hunter HJ, Griffiths CE, Kleyn CE. Does psychosocial stress play a role in the exacerbation of psoriasis? Br J Dermatol 2013 Nov;169(5):965–74.
- Slominski AT, Botchkarev V, Choudhry M et al. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?” Ann N Y Acad Sci 1999 Oct 20;885:287–311.
- Siebenhaar F, Sharov AA, Peters EM, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata). J Invest Dermatol 2007 Jun;127(6):1489–97.
- Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol 2008 Apr;121(4):955–61.
- Asadi S, Alysandratos KD, Angelidou A, et al. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J Invest Dermatol 2012 Feb;132(2):324–9.
- Chen Y, Lyga J. Brain–skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets 2014;13(3):177–90.
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A 2012 Oct 16;109 Suppl. 2:17180–5.
- Fink G, ed. Stress Science – Neuroendocrinology. Amsterdam: Academic Press, 2010.
- Del Giudice M. Fetal programming by maternal stress: Insights from a conflict perspective. Psychoneuroendocrinology 2012 Oct;37(10):1614–29.
- Pincus M, Keil T, Rücke M, et al. Fetal origin of atopic dermatitis. J Allergy Clin Immunol 2010 Jan;125(1):273-5.e1-4.
- Joachim RA, Kuhlmei A, Dinh QT, et al. Neuronal plasticity of the “brain–skin connection”: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J Mol Med (Berl) 2007 Dec;85(12):1369–78.
- Pavlovic S, Daniltchenko M, Tobin DJ, et al. Further exploring the brain–skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol 2008 Feb;128(2):434–46.
- Denda M, Takei K, Denda S. How does epidermal pathology interact with mental state? Med Hypotheses 2013 Feb;80(2):194–6.
- Hunter HJ, Hinz R, Gerhard A, et al. Brain inflammation and psoriasis: a [11C]-(R)-PK11195 positron emission tomography study. Br J Dermatol 2015 Mar 27 doi: 10.1111/bjd.13788. Epub ahead of print.
- Gisondi P, Sala F, Alessandrini F, Avesani V, et al. Mild cognitive impairment in patients with moderate to severe chronic plaque psoriasis. Dermatology 2014;228(1):78–85.
- Brzoska T, Luger TA, Maaser C, Abels C, Böhm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev 2008 Aug;29(5):581–602.
- Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol 2005 Jan;26(1):32–40.
- Meyer KC, Bodó E, Brzoska T, Abels C, Paus R. Immunomodulatory effects of the alpha-melanocyte-stimulating hormone-related tripeptide K(D)PT on human scalp hair follicles under proinflammatory conditions. Br J Dermatol 2009 Dec;161(6):1400–3.
- Meyer KC, Brzoska T, Abels C, Paus R. The alpha-melanocyte stimulating hormone-related tripeptide K(D)PT stimulates human hair follicle pigmentation in situ under proinflammatory conditions. Br J Dermatol 2009 Feb;160(2):433–7.
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med 2012 Apr 19;366(16):1515–25.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol 2013 Oct;231(2):236–47.
- Kokot A, Sindrilaru A, Schiller M, et al. alpha-melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma: melanocortin peptides as a novel treatment strategy for scleroderma? Arthritis Rheum 2009 Feb;60(2):592–603.
- Böhm M, Stegemann A. Bleomycin-induced fibrosis in MC1 signalling-deficient C57BL/6J-Mc1r(e/e) mice further supports a modulating role for melanocortins in collagen synthesis of the skin. Exp Dermatol 2014 Jun;23(6):431–3.
- Auriemma M, Brzoska T, Klenner L, et al. α-MSH-stimulated tolerogenic dendritic cells induce functional regulatory T cells and ameliorate ongoing skin inflammation. J Invest Dermatol 2012 Jul;132(7):1814–24.
- Kokot A, Metze D, Mouchet N, et al. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology 2009 Jul;150(7):3197–206.
- Tiede S, Bohm K, Meier N, Funk W, Paus R. Endocrine controls of primary adult human stem cell biology: thyroid hormones stimulate keratin 15 expression, apoptosis, and differentiation in human hair follicle epithelial stem cells in situ and in vitro. Eur J Cell Biol 2010 Oct;89(10):769–77.
- Ramot Y, Sugawara K, Zákány N, Tóth BI, Bíró T, Paus R. A novel control of human keratin expression: cannabinoid receptor 1-mediated signalling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ 2013 Feb 19;1:e40.
- Ramot Y, Zhang G, Bíró T, Langbein L, Paus R. Is thyrotropin-releasing hormone a novel neuroendocrine modulator of keratin expression in human skin? Br J Dermatol 2013 Jul;169(1):146–51.
- Chen J, Wang J, Kim TK, et al. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res 2014 May;34(5):2153–63.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol 2009 May;129(5):1243–57.
- Mastrofrancesco A, Kovacs D, Sarra M, et al. Preclinical studies of a specific PPARγ modulator in the control of skin inflammation. J Invest Dermatol 2014 Apr;134(4):1001–11.
- Briganti S, Flori E, Bellei B, Picardo M. Modulation of PPARγ provides new insights in a stress induced premature senescence model. PLOS One 2014 Aug 7;9(8):e104045.
- Wallace DC. Bioenergetics in human evolution and disease: implications for the origins of biological complexity and the missing genetic variation of common diseases. Philos Trans R Soc Lond B Biol Sci 2013 Jun 10;368(1622):20120267.
- Wallace DC. A mitochondrial bioenergetic etiology of disease. J Clin Invest 2013 Apr 1;123(4):1405–12.
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen 2010 Jun;51(5):440–50.
- Boulton SJ, Bowman A, Koohgoli R, Birch-Machin MA. Skin manifestations of mitochondrial dysfunction: more important than previously thought. Exp Dermatol 2015 Jan;24(1):12–13.
- Haslam IS, Roubos EW, Mangoni ML, et al. From frog integument to human skin: dermatological perspectives from frog skin biology. Biol Rev Camb Philos Soc 2014 Aug;89(3):618–55.
- Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res 2012 Sep;304(7):499–510.
- Wright CS, Berends RF, Flint DJ, Martin PE. Cell motility in models of wounded human skin is improved by Gap27 despite raised glucose, insulin and IGFBP-5. Exp Cell Res 2013 Feb 15;319(4):390–401.
- Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012 Nov;61(11):2937–47.
- Messenger AG. Thyroid hormone and hair growth. Br J Dermatol 2000 Apr;142(4):633–4.
- Braverman IM. Skin Signs of Systemic Disease, 3rd edn. Philadelphia: Saunders, 1998.
- Kim NH, Cheong KA, Lee TR, Lee AY. PDZK1 upregulation in estrogen-related hyperpigmentation in melasma. J Invest Dermatol 2012 Nov;132(11):2622–31.
- Bodó E, van Beek N, Naumann V, Ohnemus U, Brzoska T, Abels C, Paus R. Modulation of chemotherapy-induced human hair follicle damage by 17-beta estradiol and prednisolone: potential stimulators of normal hair regrowth by “dystrophic catagen” promotion? J Invest Dermatol 2009 Feb;129(2):506–9.
- Zouboulis CC. Acne as a chronic systemic disease. Clin Dermatol 2014 May–Jun;32(3):389–96.
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol 1991 May;124(5):415–22.
- Buskila D, Sukenik S, Holcberg G, Horowitz J. Improvement of psoriatic arthritis in a patient treated with bromocriptine for hyperprolactinemia. J Rheumatol 1991 Apr;18(4):611–12.
- Langan EA, Griffiths CE, Paus R. Utilizing the hair follicle to dissect the regulation and autocrine/paracrine activities of prolactin in humans. Am J Physiol Endocrinol Metab 2012 May 1;302(10):E1311–12.
- Langan EA, Griffiths CE, Paus R. Exploring the role of prolactin in psoriasis. Arch Dermatol Res 2012 Mar;304(2):115–18.
- Langan EA, Griffiths CE, Paus R. In search of the source of hyperprolactinaemia in systemic lupus erythematosus. Clin Exp Rheumatol 2011 Nov–Dec;29(6):1060.
- Czuwara-Ladykowska J, Sicinska J, Olszewska M, Uhrynowska-Tyszkiewicz I, Rudnicka L. Prolactin synthesis by lymphocytes from patients with systemic sclerosis. Biomed Pharmacother 2006 May;60(4):152–5.
- Langan EA, Paus R. Female pattern hair loss: beyond an androgenic aetiology? Br J Dermatol 2010 Nov;163(5):1140–1; author reply 1141–2.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol 2013 Dec;69(6 Suppl. 1):S15–26.
- Helfrich YR, Maier LE, Cui Y, et al. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol 2015 151(8):825–36.
- Eves PC, MacNeil S, Haycock JW. Melanocyte stimulating hormone, inflammation and human melanoma. Peptides 2006 Feb;27(2):444–52.
- Paurobally D, Jason F, Dezfoulian B, Nikkels AF. Melanotan-associated melanoma. Br J Dermatol 2011 Jun;164(6):1403–5.
- Ong S, Bowling J. Melanotan-associated melanoma in situ. Australas J Dermatol 2012 Nov;53(4):301–2.
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology 2014;228(1):34–6.
- Cardones AR, Grichnik JM. Alpha-melanocyte-stimulating hormone-induced eruptive nevi. Arch Dermatol 2009 Apr;145(4):441–4.
- Cousen P, Colver G, Helbling I. Eruptive melanocytic naevi following melanotan injection. Br J Dermatol 2009 Sep;161(3):707–8.
- Böhm M, Ehrchen J, Luger TA. Beneficial effects of the melanocortin analogue Nle4-D-Phe7-α-MSH in acne vulgaris. J Eur Acad Dermatol Venereol 2014 Jan;28(1):108–11.
- Mitra D, Luo X, Morgan A, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 2012 Nov 15;491(7424):449–53.
- Bassoli S, Maurichi A, Rodolfo M, et al. CDKN2A and MC1R variants influence dermoscopic and confocal features of benign melanocytic lesions in multiple melanoma patients. Exp Dermatol 2013 Jun;22(6):411–16.
- Abdel-Malek ZA, Swope VB, Starner RJ, Koikov L, Cassidy P, Leachman S. Melanocortins and the melanocortin 1 receptor, moving translationally towards melanoma prevention. Arch Biochem Biophys 2014 Dec 1;563:4–12.
- Pasquali E, García-Borrón JC, Fargnoli MC, et al. for the M-SKIP Study Group. MC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: A pooled-analysis from the M-SKIP project. Int J Cancer 2014 136(3):618–31.
- Swope VB, Jameson JA, McFarland KL, et al. Defining MC1R regulation in human melanocytes by its agonist α-melanocortin and antagonists agouti signalling protein and β-defensin 3. J Invest Dermatol 2012 Sep;132(9):2255–62.
- Haddadeen C, Lai C, Cho SY, Healy E. Variants of the melanocortin-1 receptor: do they matter clinically? Exp Dermatol 2015 Jan;24(1):5–9.
- Vasiadi M, Therianou A, Sideri K, et al. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J Allergy Clin Immunol 2012 May;129(5):1410–13.
- Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol 2009 Feb;160(2):345–52.
- Antúnez C, Torres MJ, López S, et al. Calcitonin gene-related peptide modulates interleukin-13 in circulating cutaneous lymphocyte-associated antigen-positive T cells in patients with atopic dermatitis. Br J Dermatol 2009 Sep;161(3):547–53.
- Legat FJ, Jaiani LT, Wolf P, et al. The role of calcitonin gene-related peptide in cutaneous immunosuppression induced by repeated subinflammatory ultraviolet irradiation exposure. Exp Dermatol 2004 Apr;13(4):242–50.
- Kitazawa T, Streilein JW. Hapten-specific tolerance promoted by calcitonin gene-related peptide. J Invest Dermatol 2000 Dec;115(6):942–8.
- Borlu M, Karaca Z, Yildiz H, et al. Acromegaly is associated with decreased skin transepidermal water loss and temperature, and increased skin pH and sebum secretion partially reversible after treatment. Growth Horm IGF Res 2012 Apr;22(2):82–6.
- Bourguignon JP, Piérard GE, Ernould C, et al. Effects of human growth hormone therapy on melanocytic naevi. Lancet 1993 Jun 12;341(8859):1505–6.
- Piérard GE, Piérard-Franchimont C, Nikkels A, Nikkels-Tassoudji N, Arrese JE, Bourguignon JP. Naevocyte triggering by recombinant human growth hormone. J Pathol 1996 Sep;180(1):74–9.
- Piérard GE, Humbert P, Berardesca E, Gaspard U, Hermanns-Lê T, Piérard-Franchimont C. Revisiting the cutaneous impact of oral hormone replacement therapy. Biomed Res Int 2013;2013:971760.
- Slovacek L. Somatostatin analog and mTOR inhibitor treatment of Merkel cell tumor. J Cancer Res Ther 2012 Jul–Sep;8(3):460.
- Fakiha M, Letertre P, Vuillez JP, Lebeau J. Remission of Merkel cell tumor after somatostatin analog treatment. J Cancer Res Ther 2010 Jul–Sep;6(3):382–4.
- Glinski W, Brodecka H, Glinska-Ferenz M, Kowalski D. Increased concentration of beta-endorphin in sera of patients with psoriasis and other inflammatory dermatoses. Br J Dermatol 1994 Aug;131(2):260–4.
- Glinski W, Brodecka H, Glinska-Ferenz M, Kowalski D. Increased concentration of beta-endorphin in the sera of patients with severe atopic dermatitis. Acta Derm Venereol 1995 Jan;75(1):9–11.
- Georgala S, Schulpis KH, Papaconstantinou ED, Stratigos J. Raised beta-endorphin serum levels in children with atopic dermatitis and pruritus. J Dermatol Sci 1994 Oct;8(2):125–8.
- Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 2010 Aug 5;363(6):532–41.
- Sulk M, Seeliger S, Aubert J, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol 2012 Apr;132(4):1253–62.
- Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013 Apr 25;368(17):1625–34.
- Gibson RA, Robertson J, Mistry H, et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLOS One 2014 Jul 21;9(7):e100610.
- Townley RG, Trapani IL, Szentivanyi A. Sensitization to anaphylaxis and to some of its pharmacological mediators by blockade of the beta adrenergic receptors. J Allergy 1967 Mar;39(3):177–97.
- Reed CE, Busse WW, Lee TP. Adrenergic mechanisms and the adenyl cyclase system in atopic dermatitis. J Invest Dermatol 1976 Sep;67(3):333–8.
- Roguedas AM, Audrezet MP, Scotet V, Dupré-Goetghebeur D, Ferec C, Misery L. Intrinsic atopic dermatitis is associated with a beta-2 adrenergic receptor polymorphism. Acta Derm Venereol 2006;86(5):447–8.
- Szentivanyi A. The radioligand binding approach in the study of lymphocytic adrenoceptors and the constitutional basis of atopy. J Allergy Clin Immunol 1980 Jan;65(1):5–11.
- Hermans DJ, Bauland CG, Zweegers J, van Beynum IM, van der Vleuten CJ. Propranolol in a case series of 174 patients with complicated infantile haemangioma: indications, safety and future directions. Br J Dermatol 2013 Apr;168(4):837–43.
- Stüttgen G, Schön HJ, Ollig D. [On the determination of biogenic amines in the human skin and its tumors by thin layer chromatographic method (according to Seiler). II. Catecholamines (Dopamine, norepinephrine, epinephrine) and beta-phenylethylamine.] Arch Klin Exp Dermatol 1968;233(1):33–43.
- Schallreuter KU. Epidermal adrenergic signal transduction as part of the neuronal network in the human epidermis. J Investig Dermatol Symp Proc 1997 Aug;2(1):37–40.
- Romana-Souza B, Otranto M, Almeida TF, Porto LC, Monte-Alto-Costa A. Stress-induced epinephrine levels compromise murine dermal fibroblast activity through β-adrenoceptors. Exp Dermatol 2011 May;20(5):413–19.
- Yang EV, Eubank TD. The impact of adrenergic signaling in skin cancer progression: possible repurposing of β-blockers for treatment of skin cancer. Cancer Biomark 2013;13(3):155–60.
- Moretti S, Massi D, Farini V, et al. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest 2013 Mar;93(3):279–90.
- Hogan SR, Mandrell J, Eilers D. Adrenergic urticaria: review of the literature and proposed mechanism. J Am Acad Dermatol 2014 Apr;70(4):763–6.
- Yin K, Sturm RA, Smith AG. MC1R and NR4A receptors in cellular stress and DNA repair: implications for UVR protection. Exp Dermatol 2014 Jul;23(7):449–52.
- McElwee KJ, Gilhar A, Tobin DJ, et al. What causes alopecia areata? Exp Dermatol 2013 Sep;22(9):609–26.
- Cheung BB, Koach J, Tan O, et al. The retinoid signalling molecule, TRIM16, is repressed during squamous cell carcinoma skin carcinogenesis in vivo and reduces skin cancer cell migration in vitro. J Pathol 2012 Feb;226(3):451–62.
- Jiang YJ, Bikle DD. LncRNA: a new player in 1α, 25(OH)(2) vitamin D(3)/VDR protection against skin cancer formation. Exp Dermatol 2014 Mar;23(3):147–50.
- Everts HB, Silva KA, Montgomery S, et al. Retinoid metabolism is altered in human and mouse cicatricial alopecia. J Invest Dermatol 2013 Feb;133(2):325–33.
- Duncan FJ, Silva KA, Johnson CJ, et al. Endogenous retinoids in the pathogenesis of alopecia areata. J Invest Dermatol 2013 Feb;133(2):334–43.
- Okano J, Levy C, Lichti U, et al. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. J Biol Chem 2012 Nov 16;287(47):39304–15.
- Skazik C, Amann PM, Heise R, et al. Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models. J Invest Dermatol 2014 Jun;134(6):1579–88.
- Du Vivier A. Atlas of Clinical Dermatology, 3rd edn. London: Churchill Livingstone, 2002.
- Luger TA, Böhm M. Endokrinologische Erkrankungen. In: Plewig G, et al., eds. Braun Falco's Dermatologie, Venerologie und Allergologie, 6th edn. Berlin: Springer/Heidelberg, 2012:1591–605.
- Jabbour SA. Cutaneous manifestations of endocrine disorders: a guide for dermatologists. Am J Clin Dermatol 2003;4(5):315–31.
- Jabbour SA. Skin manifestations of hormone-secreting tumors. Dermatol Ther 2010 Nov–Dec;23(6):643–50.
- Huntley AC. Finger pebbles: a common finding in diabetes mellitus. J Am Acad Dermatol 1986 Apr;14(4):612–17.
- Doi M, Sugiyama T, Izumiyama H, Yoshimoto T, Hirata Y. Clinical features and management of ectopic ACTH syndrome at a single institute in Japan. Endocr J 2010;57(12):1061–9.
- Wilkinson DS. Necrolytic migratory erythema wuth pancreatic carcinoma. Proc R Soc Med 1971;64:1197.
- Fang S, Li S, Cai T. Glucagonoma syndrome: a case report with focus on skin disorders. Onco Targets Ther 2014 Aug 14;7:1449–53.
- Abreu Velez AM, Howard MS. Diagnosis and treatment of cutaneous paraneoplastic disorders. Dermatol Ther 2010 Nov–Dec;23(6):662–75.
- Geyfman M, Kumar V, Liu Q, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A 2012 Jul 17;109(29):11758–63.
- Al-Nuaimi Y, Hardman JA, Bíró T, et al. A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J Invest Dermatol 2014 Mar;134(3):610–19.
- Luber AJ, Ensanyat SH, Zeichner JA. Therapeutic implications of the circadian clock on skin function. J Drugs Dermatol 2014 Feb;13(2):130–4.
- Plikus MV, Van Spyk EN, Pham K, et al. The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms 2015;30(3):163–82.
- Hardman JA, Tobin DJ, Haslam IS, et al. The peripheral clock regulates human pigmentation. J Invest Dermatol 2015 Apr;135(4):1053–64.
- Foitzik K, Spexard T, Nakamura M, Halsner U, Paus R. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol 2005 Jun;124(6):1119–26.
- Yin S, Luo J, Qian A, et al. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest 2013 Sep 3;123(9):3941–51.
- Blum I, Leiba S. Increased hair loss as a side effect of bromocriptine treatment. N Engl J Med 1980 Dec 11;303(24):1418.
- Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 2014 May;28(5):421–53.
- Iaria G, Urbani L, Catalano G, et al. Switch to tacrolimus for cyclosporine-induced gynecomastia in liver transplant recipients. Transplant Proc 2005 Jul–Aug;37(6):2632–3.
- Goldstein J, Fletcher S, Roth E, Wu C, Chun A, Horsley V. Calcineurin/Nfatc1 signalling links skin stem cell quiescence to hormonal signalling during pregnancy and lactation. Genes Dev 2014; 28(9):983–94.
- Hawkshaw N, Haslam IS, Ansell D, Shamalak A, Paus R. Re-evaluating cyclosporine a as a hair growth-promoting agent in human scalp hair follicles. J Invest Dermatol 2015;135(8):2129–32.
- Carrier M, Wild J, Pelletier LC, Copeland JG. Bromocriptine as an adjuvant to cyclosporine immunosuppression after heart transplantation. Ann Thorac Surg 1990 Jan;49(1):129–32.
- Kast R. Blocking of cyclosporine immunosuppression by neuroleptics. Transplantation 1989 Jun;47(6):1095–6.
- Bouillet L, Boccon-Gibod I, Massot C. Les angiœdèmes bradykiniques héréditaires ou acquis (Bradykinin mediated angioedema). Rev Med Int 2011;32:225–31.
- Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med 2015 Jan 29;372(5):418–25.
- Steckelings UM, Artuc M, Wollschläger T, Wiehstutz S, Henz BM. Angiotensin-converting enzyme inhibitors as inducers of adverse cutaneous reactions. Acta Derm Venereol 2001 Oct–Nov;81(5):321–5.
- Holgate ST, Church MK, Broide DH, Martinez FD. Allergy, 4th edn. Philadelphia: Saunders, 2012.
- Davidovici BB, Orion E, Wolf R. Cutaneous manifestations of pituitary gland diseases. Clin Dermatol 2008 May–Jun;26(3):288–95.
- Shah KR, Boland CR, Patel M, Thrash B, Menter A. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol 2013 Feb;68(2):189.e1–21.
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 1998 Jun;19(2):155–7.
- Geller JL, Braunstein GD. Dermatologic manifestations of hypopituitarism. Clin Dermatol 2006 Jul–Aug;24(4):266–75.
- Kapoor S. Cutaneous manifestations of systemic conditions associated with gynecomastia. Skinmed 2010 Mar–Apr;8(2):87–92.
- Abramovici A, Josefsberg Z, Mimouni M, Liban E, Laron Z. Histopathological features of the skin in hypopituitarism and Laron-type dwarfism. Isr J Med Sci 1983 Jun;19(6):515–19.
- Black MM, Shuster S, Bottoms E. Skin collagen and thickness in acromegaly and hypopituitarism. Clin Endocrinol (Oxf) 1972 Jul;1(3):259–63.
- Borlu M, Tanriverdi F, Koc CA, Unluhizarci K, Utas S, Kelestimur F. The effects of severe growth hormone deficiency on the skin of patients with Sheehan's syndrome. J Eur Acad Dermatol Venereol 2007 Feb;21(2):199–204.
- Melmed S. Medical progress: Acromegaly. N Engl J Med 2006 Dec 14;355(24):2558–73.
- Braham C, Betea D, Piérard-Franchimont C, Beckers A, Piérard GE. Skin tensile properties in patients treated for acromegaly. Dermatology 2002;204(4):325–9.
- Fliers E, Korbonitis M, Romijn JA, eds. Clinical Neuroendocrinology, Handbook of Clinical Neurology, 124:1–432. Edinburgh: Elsevier, 2014.
- Langan EA, Vidali S, Pigat N, et al. Tumour necrosis factor alpha, interferon gamma and substance P are novel modulators of extrapituitary prolactin expression in human skin. PLOS One 2013 Apr 23;8(4):e60819.
- Serafini P, Lobo RA. Prolactin modulates peripheral androgen metabolism. Fertil Steril 1986 Jan;45(1):41–6.
- Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev 2012 May;11(6–7):A465–70.
- Lau D, Rutledge C, Aghi MK. Cushing's disease: current medical therapies and molecular insights guiding future therapies. Neurosurg Focus 2015 Feb;38(2):E11.
- Bertherat J. Carney complex (CNC). Orphanet J Rare Dis 2006 Jun 6;1:21.
- Bourdeau I, Lampron A, Costa MH, Tadjine M, Lacroix A. Adrenocorticotropic hormone-independent Cushing's syndrome. Curr Opin Endocrinol Diabetes Obes 2007 Jun;14(3):219–25.
- Tung SC, Hwang DY, Yang JW, Chen WJ, Lee CT. An unusual presentation of Carney complex with diffuse primary pigmented nodular adrenocortical disease on one adrenal gland and a nonpigmented adrenocortical adenoma and focal primary pigmented nodular adrenocortical disease on the other. Endocr J 2012;59(9):823–30.
- Sainte Marie Y, Toulon A, Paus R, et al. Targeted skin overexpression of the mineralocorticoid receptor in mice causes epidermal atrophy, premature skin barrier formation, eye abnormalities, and alopecia. Am J Pathol 2007 Sep;171(3):846–60.
- Farman N, Maubec E, Poeggeler B, Klatte JE, Jaisser F, Paus R. The mineralocorticoid receptor as a novel player in skin biology: beyond the renal horizon? Exp Dermatol 2010 Feb;19(2):100–7.
- Trakakis E, Loghis C, Kassanos D. Congenital adrenal hyperplasia because of 21-hydroxylase deficiency. A genetic disorder of interest to obstetricians and gynecologists. Obstet Gynecol Surv 2009 Mar;64(3):177–89.
- Cherqaoui R, Husain M, Madduri S, Okolie P, Nunlee-Bland G, Williams J. A reversible cause of skin hyperpigmentation and postural hypotension. Case Rep Hematol 2013;2013:680459.
- Agrawala RK, Sahoo SK, Choudhury AK, Mohanty BK, Baliarsinha AK. Pigmentation in vitamin B12 deficiency masquerading Addison's pigmentation: A rare presentation. Indian J Endocrinol Metab 2013 Oct;17(Suppl. 1):S254–6.
- Shipman AR, Millington GW. Obesity and the skin. Br J Dermatol 2011 Oct;165(4):743–50.
- Nieman LK, Chanco Turner ML. Addison's disease. Clin Dermatol 2006 Jul–Aug;24(4):276–80.
- Hinz LE, Kline GA, Dias VC. Addison's disease in evolution: an illustrative case and literature review. Endocr Pract 2014 Sep;20(9):e176–9.
- Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res 2007 Feb;39(2):85–95.
- Lee AT, Zane LT. Dermatologic manifestations of polycystic ovary syndrome. Am J Clin Dermatol 2007;8(4):201–19.
- Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010 Sep;203(3):201.e1–5.
- McAllister JM, Legro RS, Modi BP, Strauss JF 3rd. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab 2015 Mar;26(3):118–24.
- Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part I. History and clinical examination. J Am Acad Dermatol 2014 Sep;71(3):415.e1–415.e15.
- Clark CM, Rudolph J, Gerber DA, Glick S, Shalita AR, Lowenstein EJ. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed 2014 Mar–Apr;12(2):84–8.
- Krausz A, Friedman AJ. Cutaneous hyperandrogenism: role of antiandrogen therapy in acne, hirsutism, and androgenetic alopecia. J Drugs Dermatol 2013 Nov;12(11):1297–300.
- Canale D, Caglieresi C, Moschini C, et al. Androgen receptor polymorphism (CAG repeats) and androgenicity. Clin Endocrinol (Oxf) 2005 Sep;63(3):356–61; erratum in: 2005 Oct;63(4):482.
- Conrad F, Ohnemus U, Bodo E, et al. Substantial sex-dependent differences in the response of human scalp hair follicles to estrogen stimulation in vitro advocate gender-tailored management of female versus male pattern balding. J Invest Dermatol Symp Proc 2005 Dec;10(3):243–6.
- Orme S, Cullen DR, Messenger AG. Diffuse female hair loss: are androgens necessary? Br J Dermatol 1999 Sep;141(3):521–3.
- Messenger AG. Hair through the female life cycle. Br J Dermatol 2011 Dec;165 Suppl. 3:2–6.
- Rossi A, Iorio A, Scali E, et al. Aromatase inhibitors induce ‘male pattern hair loss’ in women? Ann Oncol 2013 Jun;24(6):1710–11.
- Redler S, Birch P, Drichel D, et al. The oestrogen receptor 2 (ESR2) gene in female-pattern hair loss: replication of association with rs10137185 in German patients. Br J Dermatol 2014 Apr;170(4):982–5.
- Gallicchio L, Calhoun C, Helzlsouer KJ. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast Cancer Res Treat 2013 Nov;142(2):435–43.
- Cavalieri E, Rogan E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol Aspects Med 2014 Apr;36:1–55.
- Feingold KR, Elias PM. Endocrine–skin interactions. Cutaneous manifestations of adrenal disease, pheochromocytomas, carcinoid syndrome, sex hormone excess and deficiency, polyglandular autoimmune syndromes, multiple endocrine neoplasia syndromes, and other miscellaneous disorders. J Am Acad Dermatol 1988 Jul;19(1 Pt 1):1–20.
- Shinall MC, Solórzano CC. Pheochromocytoma in neurofibromatosis type 1: when should it be suspected? Endocr Pract 2014 Aug;20(8):792–6.
- Bell HK, Poston GJ, Vora J, Wilson NJ. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol 2005 Jan;152(1):71–5.
- Mocellin S, Nitti D. Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25 531). Ann Oncol 2013 Dec;24(12):3040–4.
- Jedrych J, Pulitzer M. Primary carcinoid tumor of the skin: a literature review. Int J Surg Pathol 2014 Apr;22(2):129–35.
- Jedrych J, Busam K, Klimstra DS, Pulitzer M. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol 2014 Feb;41(2):113–22.
- Stacpoole PW. The glucagonoma syndrome: clinical features, diagnosis, and treatment. Endocr Rev 1981;2(3):347–61.
- Esdaile BA, Hollowood K, Burge S. A serpiginous eruption. Arch Dermatol 2012 Mar;148(3):385–90.
- Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ. Glucagonoma and the glucagonoma syndrome – cumulative experience with an elusive endocrine tumour. Clin Endocrinol (Oxf) 2011 May;74(5):593–8.
- Wald M, Lawrenz K, Luckner D, Seimann R, Mohnike K, Schober E. Glucagon therapy as a possible cause of erythema necrolyticum migrans in two neonates with persistent hyperinsulinaemic hypoglycaemia. Eur J Pediatr 2002 Nov;161(11):600–3.
- Hogan AE, Tobin AM, Ahern T, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia 2011 Nov;54(11):2745–54.
- Al-Badri MR, Azar ST. Effect of glucagon-like peptide-1 receptor agonists in patients with psoriasis. Ther Adv Endocrinol Metab 2014 Apr;5(2):34–8.
- Faurschou A, Pedersen J, Gyldenløve M, et al. Increased expression of glucagon-like peptide-1 receptors in psoriasis plaques. Exp Dermatol 2013 Feb;22(2):150–2.
- Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol 2010 May;6(5):270–7.
- Kumar V, Pedroza LA, Mace EM, et al. The autoimmune regulator (AIRE), which is defective in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients, is expressed in human epidermal and follicular keratinocytes and associates with the intermediate filament protein cytokeratin 17. Am J Pathol 2011 Mar;178(3):983–8.
- Maródi L, Cypowyj S, Tóth B, Chernyshova L, Puel A, Casanova JL. Molecular mechanisms of mucocutaneous immunity against Candida and Staphylococcus species. J Allergy Clin Immunol 2012 Nov;130(5):1019–27.
- Puzenat E, Bellaud G, Saugier-Veber P, et al. [The challenge for dermatologists of early APECED diagnosis.] Ann Dermatol Venereol 2014 Apr;141(4):290–4.
- Laakso SM, Kekäläinen E, Heikkilä N, et al. In vivo analysis of helper T cell responses in patients with autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy provides evidence in support of an IL-22 defect. Autoimmunity 2014 Dec;47(8):556–62.
- Brent GA. Clinical practice. Graves' disease. N Engl J Med 2008;358:2594–605.
- Ahsan MK, Urano Y, Kato S, Oura H, Arase S. Immunohistochemical localization of thyroid hormone nuclear receptors in human hair follicles and in vitro effect of L-triiodothyronine on cultured cells of hair follicles and skin. J Med Invest 1998 Feb;44(3–4):179–84.
- Antonini D, Sibilio A, Dentice M, Missero C. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne) 2013 Aug 22;4:104.
- Safer JD. Thyroid hormone action on skin. Curr Opin Endocrinol Diabetes Obes 2012 Oct;19(5):388–93.
- Hardman JA, Haslam IS, Farjo N, Farjo B, Paus R. Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle. PLOS One 2015 Mar 30;10(3):e0121878.
- Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase – a novel pathomechanism of chronic spontaneous urticaria? PLOS One 2011 Apr 12;6(4):e14794.
- Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol 2009 May;129(5):1126–39.
- Bajuk NB, Zaletel K, Gaberšček S, Lenasi H. Hyperthyroidism induced by Graves' disease reversibly affects skin microvascular reactivity. Clin Hemorheol Microcirc 2014 Nov 5. Epub ahead of print.
- Cowley NC, McLelland J. Aurantiasis cutis. Arch Dermatol 1992 Mar;128(3):417.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol 2013 Jan;168(1):192–200.
- Graeppi-Dulac J, Vlaeminck-Guillem V, Perier-Muzet M, Dalle S, Orgiazzi J. Endocrine side-effects of anti-cancer drugs: the impact of retinoids on the thyroid axis. Eur J Endocrinol 2014 Jun;170(6):R253–62.
- Sprague SM. Painful skin ulcers in a hemodialysis patient. Clin J Am Soc Nephrol 2014 Jan;9(1):166–73.
- Wangen T, Anderson S, Fencl K, Mangan S. Calciphylaxis: an unusual case with an unusual outcome. Am J Nurs 2014 Oct;114(10):24–31.
- Ong S, Coulson IH. Diagnosis and treatment of calciphylaxis. Skinmed 2012 May–Jun;10(3):166–70.
- Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol 2011 Jul;65(1):1–12.
- Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part II. Treatment options. J Am Acad Dermatol 2011 Jul;65(1):15–22.
- Schernthaner-Reiter MH, Trivellin G, Stratakis CA. MEN1, MEN4, and Carney complex: pathology and molecular genetics. Neuroendocrinology 2015 Jan 9. Epub ahead of print.
- Sethuraman G, Sreenivas V, Yenamandra VK, et al. Threshold levels of 25-hydroxyvitamin D and parathyroid hormone for impaired bone health in children with congenital ichthyosis and type IV and V skin. Br J Dermatol 2015 Jan;172(1):208–14.
- Kalb RE, Grossman ME. Ectodermal defects and chronic mucocutaneous candidiasis in idiopathic hypoparathyroidism. J Am Acad Dermatol 1986 Aug;15(2 Pt 2):353–6.
- Sarkar S, Mondal M, Das K, Shrimal A. Mucocutaneous manifestations of acquired hypoparathyroidism: An observational study. Indian J Endocrinol Metab 2012 Sep;16(5):819–20.
- Bohrer T, Pasteur I, Lyutkevych O, Fleischmann P, Tronko M. [Permanent hypoparathyroidism due to thyroid cancer surgical procedures in patients exposed to radiation in the Chernobyl, Ukraine, nuclear reactor accident.] Dtsch Med Wochenschr 2005 Nov 4;130(44):2501–6, in German.
- Miekeley N, de Fortes Carvalho LM, Porto da Silveira CL, Lima MB. Elemental anomalies in hair as indicators of endocrinologic pathologies and deficiencies in calcium and bone metabolism. J Trace Elem Med Biol 2001;15(1):46–55.
- Schilli MB, Ray S, Paus R, Obi-Tabot E, Holick MF. Control of hair growth with parathyroid hormone (7-34). J Invest Dermatol 1997 Jun;108(6):928–32.
- Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol 2013 Feb;14(2):e50–9.
- Burrell HE, Simpson AW, Mehat S, et al. Potentiation of ATP- and bradykinin-induced [Ca2+]c responses by PTHrP peptides in the HaCaT cell line. J Invest Dermatol 2008 May;128(5):1107–15.
- Krämer U, Schmitz R, Ring J, Behrendt H. What can reunification of East and West Germany tell us about the cause of the allergy epidemic? Clin Exp Allergy 2015 Jan;45(1):94–107.
- Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLOS One 2010 Jun 4;5(6):e10968.
- Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates addiction to UV light. Cell 2014 Jun 19;157(7):1527–34.
- Nissen JB, Avrach WW, Hansen ES, Stengaard-Pedersen K, Kragballe K. Increased levels of enkephalin following natural sunlight (combined with salt water bathing at the Dead Sea) and ultraviolet A irradiation. Br J Dermatol 1998 Dec;139(6):1012–19.
- Senra MS, Wollenberg A. Psychodermatological aspects of atopic dermatitis. Br J Dermatol 2014 Jul;170 Suppl. 1:38–43.