CHAPTER 151
The Skin and Disorders of the Respiratory System
Sonja Molin and Thomas Ruzicka
Department of Dermatology and Allergology, Ludwig Maximilian University, Munich, Germany
INTRODUCTION
There are many disorders that affect both the skin and the respiratory system, ranging from common infections and allergies to complex multisystem diseases. This chapter examines the relationship between the two organ systems and discusses selected disorders from amongst the diverse groups of diseases that may affect both (Table 151.1). After a brief discussion of allergic disorders affecting the upper respiratory tract, the chapter focuses on pulmonary involvement in autoimmune disorders, vasculitides, infections and hereditary syndromes. Many of these often complex diseases are described in more detail elsewhere in this book.
Table 151.1 Conditions that affect the skin and respiratory system.
| Disease | Respiratory system features or comment |
| Inflammatory | |
| Sarcoidosis | Pulmonary fibrosis, hilar lymphadenopathy, laryngeal involvement, necrotizing sarcoid granulomatosis |
| Pulmonary vasculitides | See text (this section) |
| Systemic sclerosis | Interstitial fibrosis, pneumothorax, pulmonary hypertension |
| Sjögren syndrome | Decreased secretions, sinusitis, bronchoalveolitis, interstitial lung disease |
| Lupus erythematosus | Pleuritis, pleural effusion, shrinking lungs syndrome |
| Mixed connective tissue disease | Fibrosing alveolitis (especially U1 ribonucleoprotein antibody positive) |
| Antiphospholipid syndrome | Pulmonary embolism, infarction, thrombosis, haemorrhage |
| Dermatomyositis | Muscular weakness, pharyngeal dysfunction (aspiration pneumonia), interstitial lung disease, bronchiolitis obliterans |
| Relapsing polychondritis | Tracheal collapse |
| Multicentric reticulohistiocytosis | May be associated with bronchial neoplasia, also lung infiltration, pleural effusion |
| Bullous diseases (epidermolysis bullosa, pemphigus, Stevens–Johnson syndrome, toxic epidermal necrolysis) | Upper respiratory tract involvement; paraneoplastic pemphigus is associated with intrathoracic disease, especially Castleman disease, thymoma |
| Graft-versus-host disease | Restrictive defect, fibrosis |
| Pyoderma gangrenosum | Neutrophilic nodules in lung, tracheal involvement |
| Familial Mediterranean fever | Pleurisy |
| Infections and infestations | |
| Tuberculosis | Specific skin lesions, erythema nodosum |
| Mycobacterium avium–intracellulare infection | May disseminate to skin (usually in HIV infection) |
| Leprosy | Laryngeal involvement |
| Mycoplasma infection | Causes erythema multiforme (often mucosal) |
| Dissemination of pulmonary fungal infections | Blastomycosis, coccidioidomycosis, cryptococcosis, aspergillosis, histoplasmosis, melioidosis |
| Scrub typhus | Pneumonia (common) |
| Varicella | Pneumonia |
| Measles | Pneumonia |
| Larva migrans | Asthma/bronchitis with eosinophilia |
| Chronic mucocutaneous candidiasis | Bronchiectasis |
| Whipple disease | Cough, pleural effusion, pulmonary infiltrate, hilar lymphadenopathy |
| Congenital/inherited | |
| Atopic disease | Asthma, hay fever |
| Cutis laxa | Emphysema, cor pulmonale |
| Tuberous sclerosis | Rhabdomyomas |
| Neurofibromatosis | Kyphoscoliosis, intrathoracic neuromas, lung fibrosis, bullae |
| Ataxia–telangiectasia | Pneumonia, bronchiectasis, pulmonary fibrosis |
| Hereditary haemorrhagic telangiectasia | Haemoptysis, dyspnoea, cyanosis due to arteriovenous shunting |
| α1-antitrypsin deficiency | Emphysema |
| Darier disease | Lower lobe fibrosis, laryngeal involvement |
| Dyskeratosis congenita | Interstitial pneumonia, fibrosis |
| Lipoid proteinosis | Laryngeal involvement |
| Riley–Day syndrome | Lung infiltrate, pneumonia |
| Birt–Hogg–Dubé syndrome | Lung cysts, pneumothorax |
| Hyper-IgE syndrome | Abscesses, pneumonia |
| Infiltrations and metabolic | |
| Histiocytoses (Langerhans cell histiocytosis, Rosai–Dorfman disease, haematophagocytic syndrome, necrobiotic xanthogranuloma, sea-blue histiocytosis, others) | Pulmonary nodules and fibrosis, upper respiratory tract infiltration disease, xanthoma disseminatum |
| Amyloidosis | Cutaneous amyloid deposition secondary to chronic lung disease, lung infiltration in primary amyloidosis |
| POEMS syndrome | Pleural effusion, bronchospasm |
| Carcinoid syndrome | Bronchospasm |
| Hypothyroidism | Laryngeal involvement |
| Myxoma | Pleurisy |
| Drugs | |
| Used in respiratory disease, causing rash, phototoxicity | For example co-trimoxazole (drug eruptions), pirfenidone |
| May cause skin eruptions and respiratory tract disease | For example antibiotic-induced toxic epidermal necrolysis, cisplatin (bronchospasm, pigmentation) |
| Used for skin disease, respiratory side effects | For example isotretinoin (bronchospasm) |
| Miscellaneous | |
| Angio-oedema | Upper airway obstruction |
| Anaphylaxis | Bronchospasm |
| Pancreatitis | Basal pleural reaction, cutaneous fat necrosis |
| Yellow nail syndrome | Pleural effusion, bronchiectasis |
| Mastocytosis | Rhinorrhoea, laryngeal oedema, bronchospasm |
| Tumours | Metastatic disease, Kaposi sarcoma, lymphomatoid granulomatosis, extensive mycosis fungoides/Sézary syndrome, others |
HIV, human immunodeficiency virus; POEMS, polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes.
The mucosa of the upper respiratory tract may be affected by the same processes as the skin in a range of infective and allergic disorders.
Pulmonary disease rarely occurs as a direct consequence of a primary skin disease, except for instances such as metastasis from a primary skin tumour (e.g. melanoma). Similarly, there are relatively few instances in which skin abnormalities occur as a direct consequence of respiratory pathology. Examples include cyanosis due to severe pulmonary disease or intrapulmonary right-to-left shunts, and finger clubbing due to chronic cyanotic lung disease or neoplasm.
Some drugs used for the treatment of pulmonary disease may cause side effects on the skin and vice versa. A very few drugs may cause skin symptoms and respiratory tract disease at the same time (e.g. cisplatin).
ALLERGIC DISORDERS
The respiratory system is frequently involved in allergic reactions, particularly those due to type I allergic sensitization. Amongst the atopic disorders, allergic rhinoconjunctivitis and allergic asthma affect the respiratory tract and contribute significantly to the burden of disease in many individuals who also have atopic eczema. Angio-oedema is a component part of urticaria (Figure 151.1) and can cause life-threatening pharyngeal and laryngeal oedema, most commonly in the context of IgE-mediated anaphylaxis. These conditions are addressed in Chapters 41, 42 and 43.

Figure 151.1 Acute angio-oedema and giant urticaria.
AUTOIMMUNE DISORDERS
Amongst autoimmune disorders it is the connective tissue diseases in particular that may affect both organs.
Respiratory disease is the second most common clinical manifestation of systemic sclerosis (see Chapter 56), with a prevalence of between 25% and 90% [1]. Systemic sclerosis is associated with interstitial lung disease and pulmonary vascular disease leading to pulmonary hypertension. In addition, bronchiectases are found in up to 68% of patients on high-resolution computer tomography. Pneumothorax, pleural effusion, respiratory muscle involvement and ‘splinting’ of the chest by sclerotic skin may all occur.
The proportion of patients with systemic lupus erythematosus (SLE) (see Chapter 51) who develop pulmonary disease is similar in reported series (20–90%) [2]. The clinical manifestations involve all compartments of the respiratory tract (pleura, parenchyma, vessels and airways) and can follow an acute or chronic course. Pleuritis, pulmonary hypertension or interstitial lung disease may be present in mixed connective tissue disease [3]. Involvement of the respiratory muscles may lead to diaphragmatic elevation and the ‘shrinking lungs’ syndrome [4]. Pulmonary embolism, haemorrhage, infarction and hypertension as well as thrombosis of the lung vessels may occur in patients with the antiphospholipid syndrome (see Chapter 52) [5].
Relapsing polychondritis (see Chapter 154) [6] is due to autoantibodies against type II collagen, and is a potentially fatal disease. Dyspnoea and inspiratory stridor occur in over 50% of patients as a result of oedema of the respiratory tract mucosa and collapse of the cartilaginous support of the larynx and trachea. Inflamed nasal and auricular cartilages are present in most patients, with nasal obstruction, arthropathy and a high erythrocyte sedimentation rate. Costal cartilage is involved in one-third of cases. The non-cartilaginous lobe of the ear is classically spared in relapsing polychondritis.
In dermatomyositis (see Chapter 53), there are three main mechanisms that provide a link with respiratory disease [7]. Firstly, dermatomyositis may occur as a consequence of bronchial carcinoma. Secondly, muscular weakness due to myositis may affect either the intercostal and thoracic musculature, or the larynx and pharynx – involvement of the latter may lead to aspiration pneumonia as a complication. Finally, interstitial lung disease or bronchiolitis obliterans may occur in dermatomyositis. The lung disease is typically associated with the presence of antiaminoacyl transfer RNA (tRNA) synthetase antibodies, such as the histidyl tRNA synthetase (Jo-1) antibody. The antisynthetase syndrome consists of dermatomyositis (or polymyositis) with interstitial lung disease, arthritis and Raynaud phenomenon [8]. The presence of antisynthetase antibodies is also associated with the clinical entity of ‘mechanics’ hands’ in dermatomyositis, characterized by hyperkeratotic skin on the lateral aspects of the fingers. Dermatomyositis may also indirectly be associated with lung disease as a consequence of treatment – either infection due to immunosuppression, or rarely drug-induced pneumonitis (methotrexate), which may cause diagnostic confusion with pulmonary involvement by the disease itself.
In Sjögren syndrome (see Chapter 55), the frequency of lung involvement varies between 9% and 75%. It can affect the small airways but may also present with interstitial lung disease [9]. Alveolitis can be demonstrated in about 50% of patients but is often asymptomatic.
VASCULITIS
Vasculitis of many types may affect the lung (see Chapter 10). The major pulmonary vasculitides belong either to the group of small-vessel vasculitis or variable-vessel vasculitis [10].
small-vessel vasculitis
Any form of systemic small-vessel vasculitis (SVV) can affect the lung. According to current classifications [10], SVV can be divided into antineutrophil cytoplasmic antibody (ANCA) associated vasculitis and immune complex SVV. The ANCA-associated forms are: granulomatosis with polyangiitis (GPA), eosinophilic GPA (EGPA, formerly the Churg–Strauss syndrome) and microscopic polyangiitis (MPA).
Granulomatosis with polyangiitis [11, 12, 13] This rare disease is characterized by necrotizing granulomatous vasculitis of the upper and lower respiratory tracts, necrotizing glomerulonephritis, and disseminated vasculitis of various organs. ANCAs directed against proteinase 3 are found in 40–95% of cases. Skin lesions are present in 30–60% of patients, including vasculitis with purpura, subcutaneous nodules and ulcers (see Chapter 102). Upper airway disease causes nasal discharge, ulceration and bleeding, and may be associated with oral ulceration. Pulmonary changes are typical with bilateral infiltrates, nodules or cavities. Subglottic or tracheobronchostenosis may also occur. Prognosis is poor in patients with lung or renal disease. Chronic nasal staphylococcal carriage is more frequent in GPA than in healthy controls, and is associated with a poorer prognosis and a higher relapse rate [11]. Diffuse alveolar haemorrhage, due to extensive pulmonary capillaritis, is a life-threatening complication of GPA; it also occurs in MPA, and more rarely in SLE, antiphospholipid syndrome, Behçet disease and secondary to drugs such as D-penicillamine [14].
Eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) [12, 13] This rare condition comprises rhinitis, asthma, pneumonitis, fever, malaise, eosinophilia (usually over 10%) and widespread vasculitis, which may cause skin lesions, neuropathy and cardiac or, less commonly, renal disease. Cutaneous lesions include palpable purpura and nodular lesions and are present in up to 70% of patients (Figure 151.2). ANCAs are not present in all cases of EGPA. They may be helpful in differentiating the disease into two subgroups: one with more vasculitic and one with more eosinophilic manifestations. The latter ANCA-negative subgroup is reported to have endomyocardial involvement and lung infltrates more often [15]. The ANCAs detected in EGPA are directed against myeloperoxidase in 32–92% of cases [11].
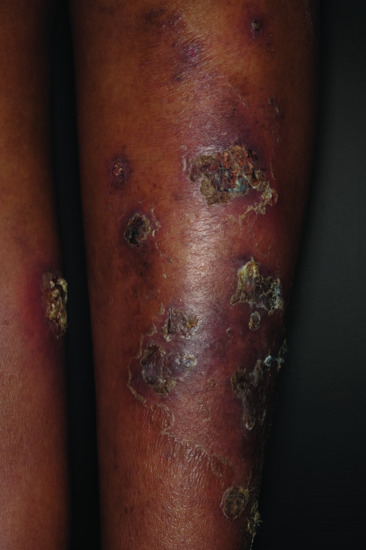
Figure 151.2 Eosinophilic granulomatosis with polyangiitis.
Microscopic polyangiitis ANCAs against myeloperoxidase (MPO-ANCA) can be found in up to 80% of patients with MPA [11]. It is a necrotizing form of vasculitis that predominantly affects small vessels: arterioles, venules and capillaries may all be affected. Lung involvement is reported in 20–60% and skin involvement in 40–70% of cases [11]. Common pulmonary clinical symptoms are dyspnoea, cough and haemoptysis. Alveolar haemorrhage can occur in up to a third of MPA patients [16].
Immune complex small-vessel vasculitis Pathognomonic are vessel wall deposits of immunoglobulin and complement factors that predominantly affect the small vessels. Urticarial vasculitis, cryoglobulinaemic vasculitis and IgA vasculitis (Henoch–Schönlein purpura) belong to this subgroup [10]. In urticarial vasculitis, pulmonary involvement is common and comprises pleuritis and obstructive lung disease [17]. In one large series, over 20% of patients with urticarial vasculitis had lung involvement, either chronic obstructive pulmonary disease or asthma. Although it is not clear that these were always causally related to the vasculitis, obstructive pulmonary disease was more frequent in the group of patients with hypocomplementaemia [18], and pulmonary vasculitis was demonstrated in over 50% of patients with lung disease.
Variable-vessel vasculitis
Behçet disease. Behçet disease has been classified amongst the variable-vessel vasculitis group [10], although it also has many features of an autoinflammatory disease (Figure 151.3) [19]. The frequency of lung involvement in Behçet disease shows a wide variation, from <1% to 18%. Pulmonary involvement can be classified into three groups: pulmonary artery aneurysm, parenchymal changes and a subgroup of diverse pulmonary disorders including pleural effusion. Thrombophlebitis may be a marker for an increased risk of pulmonary artery aneurysm as about 80% of patients with this complication also have thrombophlebitis [20].
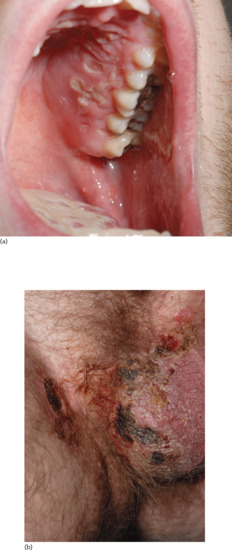
Figure 151.3 Behçet disease with ulceration of (a) the hard palate and (b) the scrotum and perigenital skin.
INFECTIONS
Numerous infections may involve both the respiratory tract and the skin: a selection of these have been listed in Table 151.1. Many viral infections, for example, may cause upper and sometimes lower respiratory tract symptoms in association with either a non-specific exanthem or with erythema multiforme. Mycoplasma infection is particularly associated with Stevens–Johnson syndrome. Psittacosis (ornithosis) may be accompanied by erythema nodosum and erythema multiforme.
Associations between tuberculosis and the skin include non-specific reactions such as erythema nodosum or erythema multiforme, as well as several patterns of specific skin lesions such as lichen scrofulosorum, Bazin disease and papulonecrotic tuberculide (see Chapter 27). Several systemic mycoses (see Chapter 32) are caused by inhalation but may subsequently cause skin lesions, either non-specific reactions including erythema multiforme and erythema nodosum, or specific lesions caused by haematogenous dissemination. Other mycoses may have primary cutaneous lesions with occasional spread to internal organs, including the lung (e.g. sporotrichosis).
CONGENITAL AND INHERITED DISORDERS/GENETIC SYNDROMES
Examples are listed in Table 151.1.
Neurofibromatosis 1 (see Chapter 80). This is not uncommonly associated with the development of lung disease as a result of kyphoscoliosis. Intrathoracic, intra-abdominal or retroperitoneal diffuse plexiform neurofibromas can also compromise pulmonary function. Fibrosis affects mainly the lower lobes whereas bullous changes may occur in the upper lobes [21].
Tuberous sclerosis. Pleural effusions have been reported in tuberous sclerosis [22] (see Chapter 80). Pulmonary involvement is uncommon but, especially in adult female patients, there may be numerous small cysts, that represent lymphangioleiomyomatosis [23]. These may be mistaken for tuberculosis or sarcoidosis radiologically.
α1-antitrypsin deficiency. α1-antitrypsin deficiency, particularly the ZZ genotype, links cutaneous panniculitis with emphysema and hepatic cirrhosis [24].
Familial dysautonomia. In familial dysautonomia (Riley–Day syndrome), there are acute episodes of bronchopneumonia with profuse mucous secretion causing dyspnoea. Skin changes include multiple excoriations, and erythematous mottling associated with fever and sweating [25]. Radiological features of lung disease [26] may be accompanied by abdominal distension as the ‘chest–abdomen’ sign [27].
Ataxia–telangiectasia. Ataxia–telangiectasia (Louis–Bar syndrome) may be associated with pulmonary problems, including recurrent pneumonia, bronchiectasis and pulmonary fibrosis [28].
Birt–Hogg–Dubé syndrome. Lung cysts and pneumothorax can occur in Birt–Hogg–Dubé syndrome (see Chapters 80 and 153).
NEUTROPHILIC DERMATOSES
Pulmonary and major airway involvement has been described with pyoderma gangrenosum and with neutrophilic dermatoses [29, 30], usually comprising focal, dense neutrophilic infiltrates with scattered radiological opacities. In Sweet syndrome, the principal clinical symptoms of lung involvement are dyspnoea, cough and general malaise. Endobronchial involvement may also occur in eosinophilic states such as the hypereosinophilic syndrome, although cardiac disease is more important in this condition. See also Chapter 49.
OTHER SYSTEMIC DISEASES
Sarcoidosis (see Chapter 98) Hilar lymphadenopathy occurs with erythema nodosum in acute sarcoidosis (Löfgren syndrome). Pulmonary involvement is the major feature in chronic sarcoidosis [31].
Necrotizing sarcoid granulomatosis. This is a very rare disorder of unknown aetiology [32]. In the respiratory system, a nodular pulmonary infiltrate is typical as well as local pleural thickening or effusion. Extrapulmonary involvement may be ophthalmological (uveitis) but other organs including the skin [33] may be involved, and erythema nodosum or necrosis due to arteritic vascular occlusion may be a feature.
Multicentric reticulohistiocytosis (see Chapter 136). Multicentric reticulohistiocytosis is associated with pleural effusion [34]; it may also occur as a paraneoplastic phenomenon.
Scleromyxoedema (see Chapter 59). Scleromyxoedema has been associated with lung disease, mainly causing dyspnoea (Figure 151.4) [35].
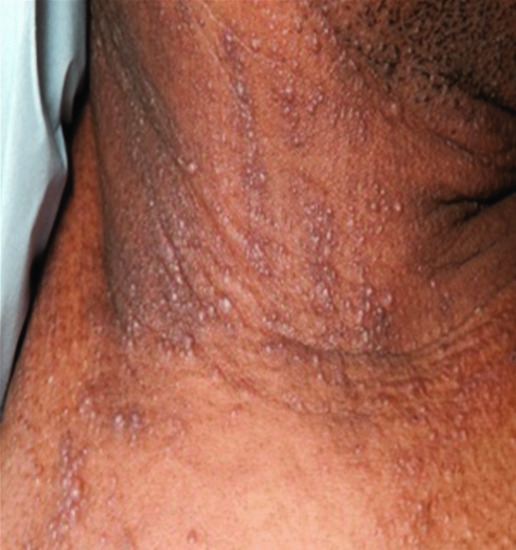
Figure 151.4 Scleromyxoedema with typical linear arrays of dermal papules on the neck.
Amyloidosis (see Chapter 58). Involvement of the respiratory tract is common in primary amyloidosis. It may cause dyspnoea but is often asymptomatic.
Lymphomatoid granulomatosis (see Chapter 140). This is a rare Epstein–Barr virus infection-driven lymphoproliferative disease in which the lung is involved in more than 90% of patients [36]. Radiology shows multiple small nodules that affect predominantly the periphery of the lower lung fields. Cutaneous lesions are present in 25–50% of cases, typically on the extremities, and consist of infiltrated flat or nodular lesions that may become necrotic and ulcerated.
Yellow nail syndrome (see Chapter 95). Yellow nail syndrome (Figure 151.5) is associated with a diverse array of respiratory abnormalities including dyspnoea or cough, pleural effusion, bronchiectasis and chronic sinus and lung infections [37].
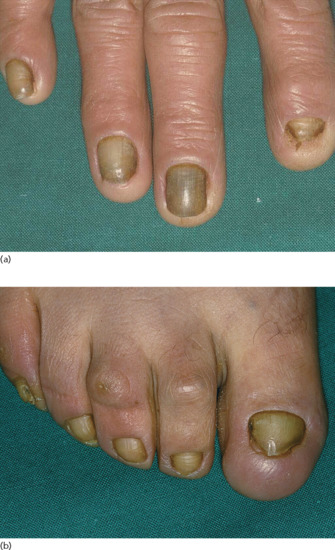
Figure 151.5 Yellow nail syndrome affecting the nails of (a) the fingers and (b) the toes.
MISCELLANEOUS DISORDERS
Paraneoplastic pemphigus (see Chapter 147). Respiratory failure in paraneoplastic pemphigus (PNP) is particularly important as it might be the cause of death in patients with this disorder. Pathologically, there is a diffuse, segmental, constrictive bronchiolitis of the small bronchioles causing a bronchiolitis obliterans clinical picture. The mechanism may be due to autoantibody-mediated damage, as direct immunofluorescence of bronchial mucosal biopsies may demonstrate linear deposition of IgG and complement in the lamina propria [38]. CD8+ T-lymphocytes may also play a key role in this process [39]. An ectopic expression of desmoglein 3 and other epidermal antigens might explain the pulmonary involvement in PNP [40]. The underlying neoplasm, toxic effects of immunosuppressive therapy and secondary infection may all contribute to the pulmonary morbidity.
Hoarseness as a sign of systemic disease. Hoarseness from laryngeal or tracheal involvement is an important audible sign of certain systemic diseases with skin involvement (Box 151.1) [41]. In particular, a range of inherited or acquired bullous diseases may affect the pharyngeal or laryngeal mucosa, for example mucous membrane pemphigoid, in which chronic inflammation may predispose to carcinoma of the larynx.
References
- Gómez Carrera L, Bonilla Hernan G. Pulmonary manifestations of collagen diseases. Arch Bronconeumol 2013;49:249–60.
- Carmier D, Marchand-Adam S, Diot P, et al. Respiratory involvement in systemic lupus erythematosus. Rev Mal Respir 2010;27:e66–78.
- Gunnarsson R, Aaløkken TM, Molberg Ø, et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: a nationwide, cross-sectional study. Ann Rheum Dis 2012;71:1966–72.
- Cavallasca JA, Dubinsky D, Nasswetter GG. Shrinking lungs syndrome, a rare manifestation of systemic lupus erythematosus. Int J Dermatol 2006;60:1683–6.
- Stojanovich L. Pulmonary manifestations in antiphospholipid syndrome. Autoimmun Rev 2006;5:344–8.
- Braverman IM. Skin Signs of Systemic Disease, 3rd edn. Philadelphia: Saunders, 1998:501–2.
- Ungprasert P, Bethina NK, Jones CH. Malignancy and idiopathic inflammatory myopathies. N Am J Med Sci 2013;5:569–72.
- Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 2013;143:814–24.
- Parambil JG, Myers JL, Lindell RM, et al. Interstitial lung disease in primary Sjögren syndrome. Chest 2006;130:1489–95.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 2013;17:603–6.
- Millet A, Pederzoli-Ribeil M, Guillevin L, et al. Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group? Ann Rheum Dis 2013;72:1273–9.
- Gross WL. Systemic necrotizing vasculitis. Baillière's Clin Rheumatol 1997;11:259–84.
- Mouthon L, Lhote F, Guillevin L. Pulmonary vasculitides. In: Ansell BM, Bacon PA, Lie JT, Yazici H, eds. The Vasculitides: Science and Practice. London: Chapman and Hall Medical, 1996:222–45.
- Specks U. Diffuse alveolar haemorrhage syndromes. Curr Opin Rheumatol 2001;13:12–17.
- Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): state of the art. Allergy 2013;68:261–73.
- Villiger PM, Guillevin L. Microscopic polyangiitis: clinical presentation. Autoimmun Rev 2010;9:812–19.
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc 2007;28:97–100.
- Mehregan DR, Hall MJ, Gibson LE. Urticarial vasculitis: a histopathologic and clinical review of 72 cases. J Am Acad Dermatol 1992;26:441–8.
- Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behcet's disease. Autoimmun Rev 2012;11:687–98.
- Uzun O, Akpolat T, Erkan L. Pulmonary vasculitis in Behcet disease: a cumulative analysis. Chest 2005;12:2243–53.
- Zamora AC, Collard HR, Wolters PJ, et al. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007;29:210–14.
- Broughton RBK. Pulmonary tuberous sclerosis presenting with pleural effusion. BMJ 1970;i:477–8.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000;75:591–4.
- Ortiz PG, Skov BG, Benfeldt E. Alpha1-antitrypsin deficiency-associated panniculitis: case report and review of treatment options. J Eur Acad Dermatol Venereol 2005;19:487–90.
- Fellner MJ. Manifestations of familial autonomic dysautonomia: report of a case with an analysis of 125 cases in the literature. Arch Dermatol 1964;89:190–5.
- Fishbein D, Grossman RF. Pulmonary manifestations of familial dysautonomia in an adult. Am J Med 1986;80:709–13.
- Grunebaum M. The ‘chest–abdomen sign’ in familial dysautonomia. Br J Radiol 1975;48:23–7.
- Canny GJ, Roifman C, Weitzman S, et al. A pulmonary infiltrate in a child with ataxia telangiectasia. Ann Allergy 1988;61:466–8.
- Batalla A,Pérez-Pedrosa A, García-Doval I, et al. Lung Involvement in pyoderma gangrenosum: a case report and review of the literature. Actas Dermosifiliogr 2011;102:373–7.
- Astudillo L, Sailler L, Launay F, et al. Pulmonary involvement in Sweet's syndrome: a case report and review of the literature. Int J Dermatol 2006;45:677–80.
- Judson MA. Sarcoidosis: clinical presentation, diagnosis, and approach to treatment. Am J Med Sci 2008;335:26–33.
- Huang H, Li C, Bai C, et al. Necrotizing sarcoid granulomatosis with hemoptysis: a case report and literature review. Diagn Pathol 2013;8:79.
- Shirodaria CC, Nicholson AG, Hansell DM, et al. Lesson of the month: necrotising sarcoid granulomatosis with skin involvement. Histopathology 2003;43:91–3.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol 2006;24:486–92.
- Morales P, Martinez MA, Vera F, et al. Severe lung involvement in systemic scleromyxoedema: a highly unusual finding. Eur Respir J 2002;19:976–9.
- Roschewski M, Wilson WH. Lymphomatoid granulomatosis. Cancer J 2012;18:469–74.
- Maldonado F, Ryu JH. Yellow nail syndrome. Curr Opin Pulm Med 2009;15:371–5.
- Nousari HC, Deterding R, Wotjczack H, et al. The mechanism of respiratory failure in paraneoplastic pemphigus. New Engl J Med 1999;340:1406–10.
- Hoffman MA, Qiao X, Anhalt GJ. CD8+ T lymphocytes in bronchiolitis obliterans, paraneoplastic pemphigus, and solitary Castleman's disease. New Engl J Med 2003;349:407–8.
- Hata T, Nishimoto S, Nagao K, et al. Ectopic expression of epidermal antigens renders the lung a target organ in paraneoplastic pemphigus. J Immunol 2013;191:83–90.
- Bernhard JD. Non-rashes, 4: audible signs of cutaneous disease. Cutis 1983;31:189–90.