12 : Effect of Gossypol Tetra-acetic Acid on the Reproductive Organs in Male Mice
Abstract
The effect of oral administration (10, 20, and 40 mg/kg body weight/day for 28 and 42 days) of gossypol tetra-acetic acid on the male reproductive organs of the Parkes (P) strain mice was investigated. In gossypol-treated mice, testes showed non-uniform degenerative changes in the seminiferous tubules as both affected and normal tubules were observed in the same section; in a few cases, however, the response of the testes to the treatment was uniform. The affected tubules showed intraepithelial vacuolation, occurrence of giant cells, exfoliation of germ cells, depletion of germ cells, and degenerated appearance of germ cells in the epithelium. Also, the frequency of affected tubules in testes of gossypol-treated mice was significantly higher than the controls. Gossypol treatment for 28 days did not cause appreciable histologic alterations in the epididymis, while 42 days treatment did cause alterations in the organ. The treatment also had adverse effects on sperm parameters in the epididymis. Forty two days after cessation of treatment, the alterations induced in the reproductive organs recovered to control levels, though the sperm morphology still remained adversely affected. Our results thus advocate cautious use of gossypol in fertility related studies.
Key words: Gossypol, Mice, Testes, Seminiferous tubules, Epididymes, Spermatozoa
1. Department of Zoology, Banaras Hindu University, Varanasi, India.
* Corresponding author : E-mail: shioks@rediffmail.com/ shio@bhu.ac.in
Introduction
Several plant-derived substances have been tested in the hope of developing an antifertility agent for control of fertility in the male. Such studies have led to the discovery of gossypol, which is a yellow polyphenol compound present in the seed of the cotton plant genus Gossypium. The discovery of gossypol as a male fertility regulating agent by Chinese scientists is regarded as a great success in the search of a male contraceptive (National Co-ordinating Group, 1978; Prasad & Diczfalusy, 1982). This prompted several investigators around the world to confirm and extend the above information in a number of species. However, reports of the effects of gossypol on the male reproductive organs are not uniform. For example, in rat some authors (Chang et al., 1982; Giridharan et al., 1987) have reported no alterations in the histoarchitecture of the testis after gossypol treatment, while there are others like Hoffer (1983) and Srivastava et al. (1989) who have shown non-uniform degenerative changes in the seminiferous tubules in the testis. Likewise, Hadley et al. (1981) and Hoffer (1982) did not observe any change in the histologic appearance of rat epididymis after gossypol treatment. By contrast, Kaur et al. (1988) and Udoh et al. (1992) reported marked degenerative changes in epididymis of the treated rats.
In a previous study (Singh & Rath, 1990) in P mice, we have reported effects of administration of 20 and 40 mg/kg body weight of gossypol tetra-acetic acid (henceforth referred to as gossypol) for 21 days on histologic changes in the testis. In the present study, we have investigated, in detail, the effect of 10, 20, and 40 mg/kg body weight of gossypol for 28 and 42 days on the testis, epididymis and accessory sex glands of P mice. Recovery studies were also performed to assess the reversibility of the effects induced by gossypol in the reproductive organs.
Materials and Methods
Animals and treatments
Adult (age: 12-14 weeks) male laboratory mice belonging to the Parkes (P) strain, weighing 30-40 g, were used in the experiments. Animals were housed under strict hygienic conditions in well-ventilated rooms at 23 ± 2 °C with a 12 h photoperiod, and maintained on pelleted food (Lipton India Ltd.) and drinking water ad libitum. Mice were randomly allocated into three main groups (Groups I, II, and III), and each group was then further divided into five subgroups (Ia–Ie; IIa-IIe; and IIIa–IIIe). Mice (five animals per subgroup) were housed in separate polypropylene cages (430 x 270 x 150 mm) and treated as follows: subgroups: Ia, IIa, and IIIa – untreated controls; subgroups Ib, IIb, and IIIb – administration of vehicle, 0.15 ml/40g body weight/day, for 28 (Ib) and 42 (IIb and IIIb) days; subgroups Ic, IIc, and IIIc – administration of gossypol, 10 mg/kg body weight/day, for 28 (Ic) and 42 (IIc and IIIc) days; subgroups Id, IId, and IIId – administration of gossypol, 20 mg/kg body weight/day, for 28 (Id) and 42 (IId and IIId) days; and subgroups Ie, IIe, and IIIe – administration of gossypol, 40 mg/kg body weight/day, for 28 (Ie) and 42 (IIe and IIIe) days. Animals in groups I (Ib - Ie) and II (IIb - IIe) were sacrificed 24h after the last treatment, while those in group III (IIIb – IIIe) were sacrificed 42 days after cessation of the treatment. The experimental design is summarized in Table 1.
Table 1. Experimental design
| Group | Subgroup | Treatment | Duration of treatment (days) | Day(s) of autopsy after drug withdrawal |
| I | Ia | Untreated control | - | - |
| Ib | Vehicle (0.15 ml/40g body weight/day) | 28 | 1 | |
| Ic | Drug (10 mg/kg body weight/day) | 28 | 1 | |
| Id | Drug (20 mg/kg body weight/day) | 28 | 1 | |
| Ie | Drug (40 mg/kg body weight/day) | 28 | 1 | |
| II | IIa | Untreated control | - | - |
| IIb | Vehicle (0.15 ml/40g body weight/day) | 42 | 1 | |
| IIc | Drug (10 mg/kg body weight/day) | 42 | 1 | |
| IId | Drug (20 mg/kg body weight/day) | 42 | 1 | |
| IIe | Drug (40 mg/kg body weight/day) | 42 | 1 | |
| III | IIIa | Untreated control | - | - |
| IIIb | Vehicle (0.15 ml/40g body weight/day) | 42 | 42 | |
| IIIc | Drug (10 mg/kg body weight/day) | 42 | 42 | |
| IIId | Drug (20 mg/kg body weight/day) | 42 | 42 | |
| IIIe | Drug (40 mg/kg body weight/day) | 42 | 42 |
Gossypol was dissolved in absolute ethanol and diluted with nine volumes of sunflower seed oil (Hunt & Mittwoch, 1984) and administered orally with the help of an oral dosing needle. Control groups (Ib, IIb and IIIb) received the vehicle only in a similar manner. Body weights of mice were monitored regularly. Animals were sacrificed by dislocation of cervical vertebrae, and testes, epididymes, and seminal vesicles were excised, blotted free of blood and weighed. Testis and epididymis were then fixed for histological studies in freshly prepared Bouin’s fluid, dehydrated in graded ethanol series, cleared in benzene and embedded in paraffin wax. Tissues were sectioned at 6 mm and the sections were stained with periodic acid–Schiff (PAS) and counterstained with Harris haematoxylin. For determination of percentage of affected seminiferous tubules, all the tubules in a randomly selected section of the testis from each mouse in each group were counted, and then the percentage of affected tubules was obtained (Singh & Rath, 1990). The seminiferous tubules which were considered as affected were as follows: tubules lined with mainly Sertoli cells and spermatogonia; tubules lined with Sertoli cells, spermatogonia, and spermatocytes; tubules lined with Sertoli cells, spermatogonia, few spermatocytes and early spermatids; tubules showing giant cells; tubules showing exfoliation of germ cells; tubules showing intraepithelial vacuolization; and tubules showing pycnotic nuclei of germ cells.
Assessment of sperm parameters
At autopsy, spermatozoa were obtained from cauda epididymidis of each mouse in physiological saline maintained at 37 0C, and their motility, viability, and number were assessed according to WHO laboratory manual (1992). The preparations which were used for assessing sperm viability were also used for assessing morphological abnormalities in sperm as per the criteria of Wyrobek and Bruce (1975) and Zaneveld and Polakoski (1977).
Analysis of fructose content
The concentration of fructose in the seminal vesicle was determined according to the method of Lindner and Mann (1960).
Statistical analysis
Data were analysed by the Student t-test. Values were considered significant atp < 0.05.
Results
Body and organ weight
No significant differences were found between the initial and final body weight of gossypol-treated mice and controls, except that a marked reduction was noted in body weight of mice treated with 40 mg dose of the drug for 42 days (IIe, initial vs final). The treatment, however, had no effect on weight of the testis. In mice treated with gossypol (10, 20, and 40 mg/kg body weight) for 28 days (Ic-Ie) and in those with 10 mg dose for 42 days (IIc), weight of the epididymis remained unaffected by the treatment; however, in mice treated with 20 and 40 mg doses of gossypol for 42 days (IId and IIe), significant reduction was noted in epididymal weight compared to controls. Treatment with 10 mg dose of gossypol for 28 and 42 days (Ic and IIc) had no effect on weight of the seminal vesicle, but 20 and 40 mg doses of the drug caused significant reductions in gland weight in treated mice compared to controls (Table 2).
Histological studies
Testis: The testes of untreated controls (Fig 1) and vehicle-treated controls showed normal histologic features in nearly all the seminiferous tubules, except in a few in which the epithelium consisted of mainly Sertoli cells and spermatogonia. By contrast, marked alterations were observed in the histoarchitecture of the testis in gossypol-treated mice (Figs 2-7). Individual differences were, however, observed in response of the testis to the treatment with some mice showing more alterations in the seminiferous tubules than others. Within the same sections, some tubules were more affected than others. In one of five mice treated with 10 mg, and also in one of five mice treated with 40 mg doses of gossypol for 42 days, the seminiferous tubules in the testis appeared unaffected by the treatment, while in one of five mice treated with 40 mg dose of gossypol for 28 days, and in all five mice treated with 20 mg dose for 42 days, all the seminiferous tubules in the testis were affected (Figs 6-7).
In testes of mice treated with 10, 20, and 40 mg/kg body weight of gossypol for 28 and 42 days, the histologic changes noticed in the seminiferous tubules were not much different, and they are, therefore, described together. The frequency of affected tubules was neither dose - nor duration - dependent (Table 2). In general, the affected seminiferous tubules showed depletion of germ cells and were lined with Sertoli cells, spermatogonia, spermatocytes and early spermatids (Fig 2). Intraepithelial vacuolation, occurrence of giant cells, exfoliation of germ cells, depletion of germ cells, and degenerated appearance of germ cells in the epithelium were common features in the affected seminiferous tubules (Figs 2-7); giant cells contained two to five nuclei of early spermatids (Figs 3-4). In severe cases, the epithelium in affected seminiferous tubules consisted of only a layer of Sertoli cells and spermatogonia. Forty two days after cessation of treatment, the alterations caused in the seminiferous tubules recovered to control levels and the tubules presented a normal appearance in the testis (Fig 8).
Epididymis: The epididymis of untreated controls (Figs 9-13) and vehicle–treated controls exhibited normal histologic features. In the present study, mice (Parkes strain) epididymis was divided into five segments (I-V) segments I-III (Figs 9-11) constituted the head; segment IV (Fig 12), the body; and segment V (Fig 13), the tail of the epididymis (Takano, 1980; Abe et al., 1982; Mishra & Singh, 2005). Individual differences, as seen in testis, were also noticed in the epididymis with some mice showing more alterations in the epididymal segments than others. Mice that showed more testicular alterations also showed more of the epididymal alterations.
In mice treated with 10, 20, and 40 mg/kg body weight of gossypol for 28 days, almost normal features were observed in all segments of the epididymis, except that there was a little increase in the amount of stroma and lumen of the tubules appeared narrow and contained less spermatozoa; the epithelial cells showed almost normal appearance throughout the duct in treated animals (Figs 14-18). In one of five mice treated with 40 mg dose of gossypol for 28 days (subgroup Ie), PAS-positive inclusions were noticed in the epithelium in segment IV (Figs 19-20). The lumen of the epididymal duct in this mouse was either empty or contained only PAS-positive materials. By contrast, in mice treated with 10, 20, and 40 mg/kg body weight of gossypol for 42 days, histologic alterations were observed in the epididymis (Figs 21-24), and that the alterations were not different in different dosage groups (IIc-IIe) and they are described together. The epithelium of the epididymal duct sometimes showed loss of stainability, and vacuolated appearance of the cells; the nuclei of epithelial cells were condensed and appeared pycnotic; the lumen contained PAS-positive materials and sperm debris. The clear cells in segment V were distended (Fig 24). However, forty two days after cessation of treatment, epididymis presented histologic features which were similar to those seen in controls.
Table 2. Effect of gossypol administration on body weight and weights of the testis, epididymis and seminal vesicle and on the percentage of affected seminiferous tubules in the testis

Sperm parameters
Significant reductions were found in motility, viability, and in the number of spermatozoa in the caudae epididymidis of gossypol–treated mice compared to controls, except that the sperm motility in mice treated with 10 mg dose of gossypol for 28 days (Ic), and sperm number in mice treated with 10, 20, and 40 mg/kg body weight of the drug for 28 days (Ic-Ie) remained unaffected by the treatment (Table 3). There was a significant increase in the number of morphologically abnormal spermatozoa in drug-treated mice than in controls (Table 3). Head- and tail-less spermatozoa were often observed in drug-treated mice. By forty two days after cessation of treatment, sperm parameters such as motility, viability, and number recovered to control levels, but there was still a significant increase in the number of morphologically abnormal spermatozoa in gossypol-treated mice compared to controls (Table 3).
Analysis of fructose content
Gossypol at a dose of 10 mg/kg body weight administered for 28 and 42 days had no effect on the level of fructose in the seminal vesicle. By contrast, significant reductions were noted in fructose level in mice that were treated with 20 and 40 mg/kg body weight of the drug for 28 and 42 days compared to controls. By forty two days of drug withdrawal, however, fructose level in treated mice recovered to control levels (Table 3).
Discussion
The results in P mice indicated that gossypol treatment had no effect on body weight, except that a marked reduction was noted in body weight in mice treated with 40 mg/kg body weight of the drug for 42 days. In rat, Jensen et al. (1982) have also reported a decrease in body weight after treatment with 40 mg/kg body weight of gossypol for 56 days. As reported in other strains of mice (Coulson et al., 1980; Hunt & Mittwoch, 1984) and other mammalian species including rat, hamster and rabbit (Rath, 1995), gossypol treatment in P mice in the present study had no effect on weight of the testis. In testes of P mice, gossypol treatment caused non-uniform degenerative changes in the seminiferous tubules as both normal and affected tubules were observed in the same section; this is consistent with the findings reported in rat by Hoffer (1983) and Srivastava et al. (1989). However, in one of five mice treated with 40 mg/kg body weight of gossypol for 28 days and in those treated with 20 mg dose of the drug for 42 days, almost all the seminiferous tubules in the testis were affected; this indicated that the frequency of affected tubules in the testis was neither dose- nor duration-related. The affected tubules showed depletion of germ cells, intraepithelial vacuolation, giant cells, exfoliation of germ cells, and degenerated appearance of germ cells in the epithelium. Similar histologic changes have also been observed in testes of P mice after treatment with aqueous leaf extract of Azadirachta indica and several antispermatogenic agents such as nitrofurazone, STS 557, SC 12937, DICA, and AF 1312/TS (Singh, 1999, 2006; Mishra & Singh, 2005). Multinucleated giant cells as described in the present study have also been noticed in mouse testis after efferent duct ligation (Singh & Abe, 1987), vasectomy (Singh & Chakravarty, 2000), and treatment with aqueous leaf extract of Azadirachta indica (Mishra & Singh, 2005) and several antifertility agents including PGF2α, DICA, nitrofurazone and SC12937 (Singh, 1999, 2006). It appears that these cells are formed by fusion of spermatids because the haploid spermatids are not normally known to divide.
Table 3. Effect of gossypol administration on motility, viability, morphology, and number of spermatozoa in the cauda epididymidis and on fructose concentration in the seminal vesicle

The results in P mice also showed that gossypol treatment (10, 20 or 40 mg/kg body weight) for 28 days did not cause appreciable changes in the histologic appearance of the epididymis, while the same treatment for 42 days caused detectable changes in the duct; this suggests that gossypol treatment had duration-related effect on the histoarchitecture of the epididymis. Degenerative changes have also been reported in rat epididymis after gossypol treatment (Kaur et al., 1988; Udoh et al., 1992). In only one of five mice examined after treatment with 40 mg/kg body weight of gossypol for 28 days, PAS-positive inclusions were observed in the epithelial cells of the corpus epididymidis. It is difficult to explain the significance of this observation in view of the fact that only one mouse demonstrated this phenomenon. Such inclusions have also been observed in the epithelial cells of the corpus epididymidis in mice after testicular irradiation (Abe et al., 1983) and cryptorchidism (Abe & Takano, 1987); Abe et al. (1983, 1987) have described the significance of such observations. Distension of clear cells in cauda epididymidis of the gossypol-treated mice as noticed in the present study suggests that these cells might be involved in the clearance of the luminal material (Flickinger, 1977; Sinha Hikim, 1986).
Gossypol treatment had also adverse effects on motility, viability, morphology and on the number of spermatozoa in the cauda epididymidis, though sperm motility in mice treated with 10 mg/kg body weight of the drug for 28 days and number of spermatozoa in those treated with 10-40 mg doses for 28 days remained unaffected. The reduction in the number of spermatozoa in cauda epididymidis of mice treated with 10-40 mg/kg body weight of gossypol for 42 days in the present study appeared to be due to the sup-pressive effect of the drug on spermatogenesis, while the alterations in sperm motility, viability and morphology might have resulted from disturbances in epididymal function (Rajalakshmi, 1992; de Andrade et al., 2006). The observation that there was a marked reduction in the level of fructose in the seminal vesicle accompanied by a significant reduction in the weight of the gland in mice treated with 20 and 40 mg/kg body weight of gossypol for 28 and 42 days, but not in those treated with 10 mg/kg body weight of the drug for 28 or 42 days suggested that higher doses of gossypol had an adverse effect on the secretory function of the gland.
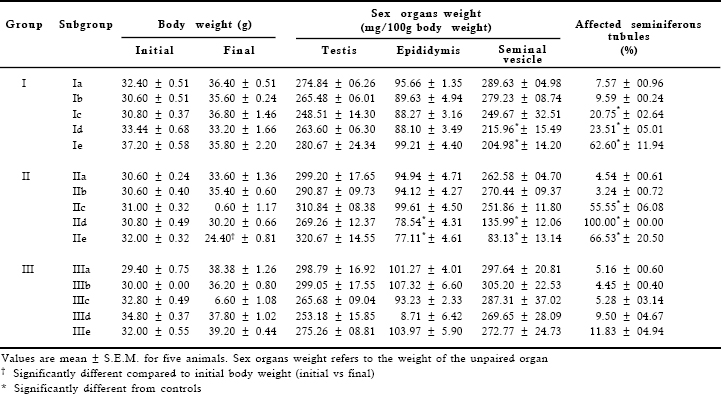
Figs are PAS-haematoxylin-stained sections of mouse testis
Fig 1. Testis of a control (Subgroup Ia). Note the normal appearance of the seminiferous tubules. X 175.
Fig 2. After treatment with gossypol, 10mg/kg body weight/day for 28 days and sacrificed 24 hr after the last treatment (Subgroup Ic). Note marked depletion of germ cells in the seminiferous tubules; the tubules are lined with Sertoli cells, spermatogonia, spermatocytes and early spermatids. X 175.
Fig 3. After the same treatment as shown in Fig 2. Note the degenerated appearance of the seminiferous tubules. A multinucleated giant cell (broken arrow) is seen in the tubular lumen, and intraepithelial vacuoles (arrows) are also seen. X 700.
Fig 4. After the same treatment as shown in Fig 2. A portion of seminiferous tubule enlarged to show the presence of giant cell (broken arrow) and vacuoles (arrows) in the epithelium. X 700.
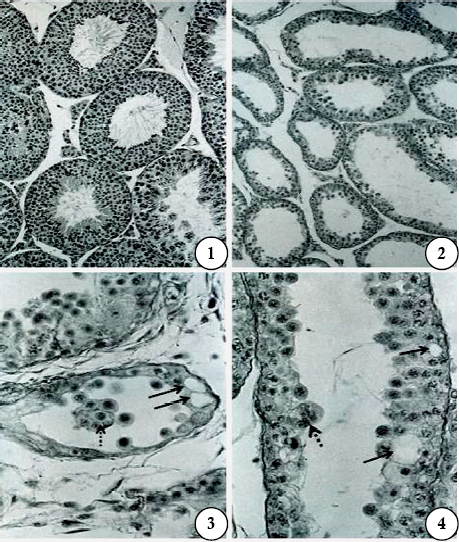
Figs are PAS-haematoxylin-stained sections of mouse testis. X175
Fig 5. After treatment with gossypol, 40mg/kg body weight/day for 28 days and sacrificed 24 hr after the last treatment (Subgroup Ie). Note the presence of affected seminiferous tubules and a normal tubule.
Fig 6. After the same treatment as shown in Fig 5. Note the complete degeneration of the seminiferous tubules.
Fig 7. After treatment with gossypol, 20mg/kg body weight/day for 42 days and sacrificed 24 hr after the last treatment (Subgroup IId). Germ cells have a degenerated appearance in the epithelium.
Fig 8. After treatment with gossypol, 40mg/kg body weight/day for 42 days and sacrificed 42 days after cessation of treatment (Subgroup IIIe). Note the normal appearance of the seminiferous tubules.
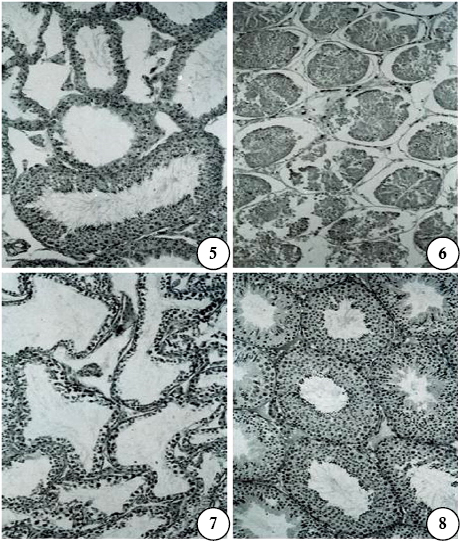
Figs are PAS-haematoxylin-stained sections of mouse epididymis. X 175
Figs 9-11. Segment I (Fig 9), II (Fig 10), and III (Fig 11) of a control (Subgroup Ia) to show normal features; segments I-III constitute the head of the epididymis. In segments II and III, spermatozoa and PAS-positive materials are seen in the lumen.
Fig 12. Segment IV of a control (Subgroup Ia) to show normal features; the lumen contains abundant spermatozoa and PAS-positive materials.

Figs are PAS-haematoxylin-stained sections of mouse epididymis. X 175
Fig 13. Segment V of a control (Subgroup Ia) to show normal features; the lumen contains abundant spermatozoa and PAS-positive materials.
Figs 14-16. Segment I (Fig 14), II (Fig 15), and III (Fig 16) after treatment with gossypol, 10mg/kg body weight/day for 28 days and sacrificed 24 hr after the last treatment (Subgroup Ic). A normal appearance of the epithelium in the segments is seen; there is a slight increase in stroma in segments II and III.
The mechanism by which gossypol treatment causes suppression of spermatogenesis is not properly understood though it has been suggested that it may cause disruption of testosterone secretion (Lin et al., 1981),inhibition of germ cell differentiation (National Coordinating Group, 1978; Hadley et al., 1981; Xue, 1981), or breakdown of the blood testis barrier (Pelletier & Friend, 1980). Studies of Ahluwalia et al. (1986) indicated that antispermatogenic effect of gossypol is mediated locally within the germinal epithelium and that it does not involve hypothalamic-hypophyseal-gonadal axis. In P mice in the present study, intraepithelial vacuoles were often observed in the affected seminiferous tubules in the testis. Hoffer (1983) has also reported occurrence of intraepithelial vacuoles in affected seminiferous tubules in rat testis after gossypol treatment, and that these vacuoles occurred primarily in the Sertoli cells. It is, therefore, probable that in P mice gossypol treatment causes suppression of spermatogenesis by acting through Sertoli cells. However, it is important to remember that intraepithelial vacuoles are also reported to occur in Sertoli cells after testicular injuries, and they have often been interpreted as a non-specific reaction of these cells (Fawcett, 1975). Thus, the mechanism of the antispermatogenic action of gossypol in P mice remains unclear and needs further study.
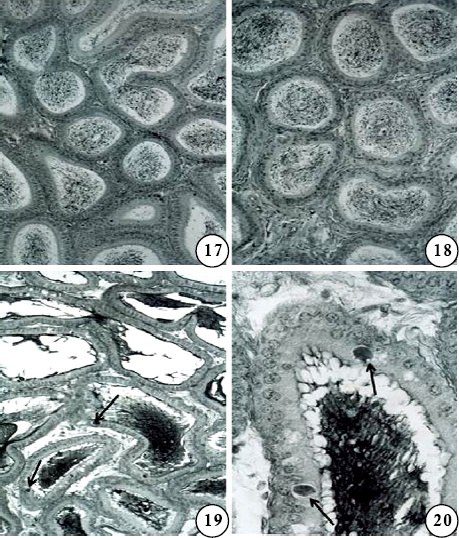
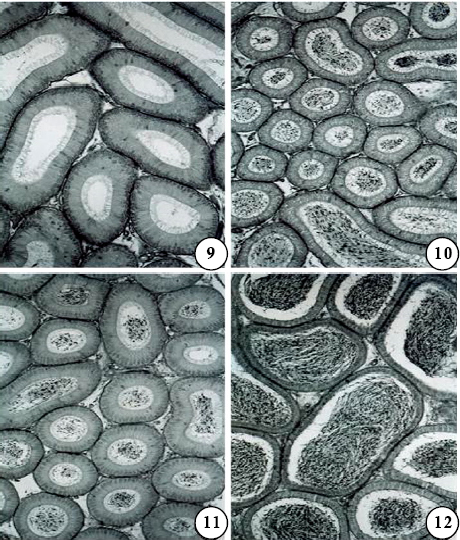
Figs are PAS-haematoxylin-stained sections of mouse epididymis.
Figs 17-18. Segment IV (Fig 17) and V (Fig 18) of the same mouse as shown in Figs 14-16. Note the almost normal appearance of the segments; in segment V, the stroma is slightly increased. X 175.
Fig 19. Segment IV after treatment with gossypol, 40mg/kg body weight/day for 28 days and sacrificed 24 hr after the last treatment (Subgroup Ie). Note the accumulation of PAS-positive inclusions (arrows) in the epithelial cells. The lumen shows PAS-positive materials, but no spermatozoa. X 175.
Fig 20. A portion of Fig 19 enlarged to show accumulation of PAS-positive inclusions(arrows) in the epithelium, and abundant PAS-positive materials in the lumen.X700.
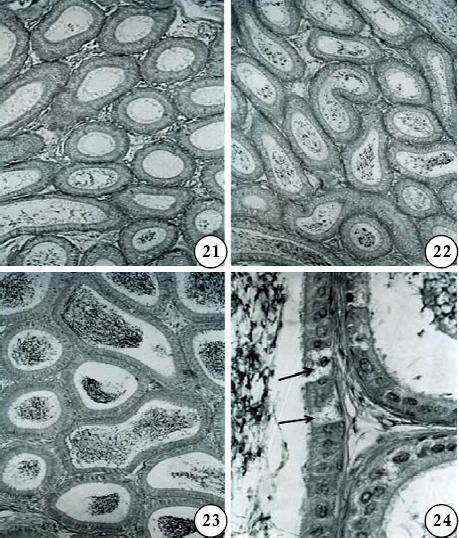
Figs are PAS-haematoxylin-stained sections of mouse epididymis.
Figs 21-23. Segment I (Fig 21), II (Fig 22), and IV (Fig 23) after treatment with gossypol, 40mg/kg body weight/day for 42 days and sacrificed 24 hr after the last treatment (Subgroup IIe). Note the vacuolated appearance of the epithelium and the nuclei of epithelial cells appear pycnotic; the lumen is empty or contains sperm debris and PAS-positive materials.
Fig 24. Segment V of the same mouse as shown in Figs 21-23 to show distension of clear cells (arrows) in the epithelium. X 700.
In conclusion, our results in P mice suggest that gossypol treatment causes marked alterations in the male reproductive organs, and that the alterations, except for those in sperm morphology, are reversible by 42 days after cessation of treatment. The sperm morphology, on the other hand, however, still remained affected as there was a significant increase in the number of morphologically abnormal spermatozoa in treated mice up to 42 days of cessation of treatment compared to controls. This advocates cautious use of gossypol in fertility related studies.
Acknowledgements
This work was supported by funds from the University Grants Commission through CAS in Zoology, Banaras Hindu University. Srikanta Kumar Rath was recipient of a Senior Research Fellowship of the Council of Scientific and Industrial Research, New Delhi.
References
Abe, K. and Takano, H. 1987. Response of the mouse epididymal duct to the disappearance and reappearance of spermatozoa induced by temporal cryptorchidism. Arch. Histol. Jap. 50: 315-324.
Abe, K., Takano, H. and Ito, T. 1982. Response of the epididymal duct in the corpus epididymidis to efferent or epididymal duct ligation in the mouse. J. Reprod. Fert. 64: 69-72.
Abe, K., Takano, H. and Ito, T. 1983. Response of epididymal duct to the temporary depletion of spermatozoa induced by testicular irradiation in mice. Anat. Rec. 207: 17-24.
Ahluwalia, B., Hypolite, F. and Anderson, W. 1986. Morphologic and endocrine changes in the reproductive organs in rats implanted with gossypol acetate pellet in the testis. J. Androl. 7: 254-263.
Chang, C. C., Gu, Z. and Tsong, Y. Y. 1982. Studies on gossypol. I. Toxicity, antifertility and endocrine analyses in male rats. Int. J. Fertil. 27: 213-218.
Coulson, P. B., Snell, R. L. and Parise, C. 1980. Short term metabolic effects of the anti-fertility agent, gossypol, on various reproductive organs of male mice. Int. J. Androl., 3: 507-518. de Andrade, S. F., Oliva, S.U., Klinefelter, G.R. and Kempinas, W.G. 2006.
Epididymis-specific pathologic disorders in rats exposed to gossypol from weaning through puberty. Toxicol. Pathol. 34: 730-737.
Fawcett, D. W. 1975. Ultrastructure and function of the Sertoli cell. In: Hamilton, D. W. and R. O. Greep, eds., Handbook of Physiology, American Physiological Society, Washington DC, pp. 21-55.
Flickinger, C. J. 1977. The influence of progestin and androgen on the fine structure of the male reproductive tract of the rat. II Epididymis and sex accessory glands. Anat. Rec. 187: 431-462.
Giridharan, N., Sesikeran, B., Bamji, M. S. and Madhyastha, M. N. 1987. Dose and time related changes in LDH-X activity, epididymal carnitine levels and fertility in gossypol-treated male rats. Contraception 35: 89-100.
Hadley, M. A., Lin. Y. C. and Dym, M. 1981. Effects of gossypol on the reproductive system of male rats. J. Androl. 2: 190-199.
Hoffer, A. P. 1982. Ultrastructural studies on spermatozoa and the epithelial lining of the epididymis and vas deferens in rats treated with gossypol. Arch. Androl. 8: 233-246. Hoffer, A. P. 1983.
Effects of gossypol on the seminiferous epithelium in the rat: a light and electron microscope study. Biol. Reprod. 28: 1007-1020.
Hunt, S. and Mittwoch, V. 1984. Effects of gossypol on sperm counts in two inbred strains of mice. J. Reprod. Fert. 70: 341-345.
Jensen, D. R., Tone, J. N., Sorensen, R. H. and Bozek, S. A. 1982. Deposition pattern of the antifertility agent, gossypol, in selected organs of male rats. Toxicology 24: 65-72.
Kaur, P., Khullar, M., Mittal, S. and Rai, U. C. 1988. Effect of gossypol on testes & epididymis of albino rats. Indian J. Med. Res. 87: 368-376.
Lin, T., Murono, E. P., Osterman, J., Nankin, H. R. and coulson, P. B. 1981. Gossypol inhibits testicular steroidogenesis. Fertil. Steril. 35:563-566.
Lindner, H. R. and Mann, T. 1960. Relationship between the content of androgenic steroids in the testes and the secretory activity of the seminal vesicles in the bull. J. Endocrinol. 21: 341-360.
Mishra, R. K. and Singh, S. K. 2005. Effect of aqueous leaf extract of Azadirachta indica on the reproductive organs in male mice. Indian J. Exp. Biol. 43: 1093-1103.
National Coordinating Group on Male Antifertility Agents. 1978. Gossypol - a new antifertility agent for males. Chinese. Med. J. 4: 417-428.
Pelletier, R. M. and Friend, D. S. 1980. Effects of the experimental contraceptive agent gossypol on guinea pig Sertoli-Sertoli cell junctions. J. Cell Biol. 87: 151a. Prasad, M. R. N. and Diczfalusy, E. 1982.
Gossypol. Int. J. Androl. Suppl. 5: 53-70. Rajalakshmi, M. 1992. Regulation of male fertility: epididymis as a potential extragonadal site. In: Ghosh, D. and J. Sengupta, eds., Frontiers in Reproductive Physiology, Wiley Eastern Limited, New Delhi, pp. 63-66.
Rath, S. K. 1995. Effects of certain antifertility agents on the reproductive organs of the male laboratory mouse. Ph. D. Thesis, Banaras Hindu University, Varanasi. Singh, S. K. 1999. Antispermatogenic agents. In: Joy, K. P., A. Krishna, and C. Haldar, eds., Comparative Endocrinology and Reproduction, Narosa Publishing House, New Delhi, pp. 285-295.
Singh, S. K. 2006. Testicular toxicity of some chemical agents. In: Joshi, S. C. and A. S Ansari, eds., Advances in Reproductive Toxicology, Pointer International Publishers, Jaipur, pp. 138-145.
Singh, S. K. and Abe, K. 1987. Light and electron microscopic observations of giant cells in the mouse testis after efferent duct ligation. Arch. Histol. Jap.50: 579-585. Singh, S.K. and Chakravarty, S. 2000.
Histologic changes in the mouse testis after bilateral vasectomy. Asian J. Androl. 2: 115-120.
Singh, S. K. and Rath, S. K. 1990. Histologic changes in the mouse testis after treatment with gossypol tetra-acetic acid. Arch. Histol. Cytol. 53: 393-396.
Sinha Hikkim, A. P. 1986. Effect of DL-6-(N-á-pipecolinomethyl)-5-hydroxyindane maleate (PMHI) on the reproductive system of a wild rat, Bandicota bengalensis. II. Epididymis and accessory sex glands. Exp. Mol. Pathol. 44: 190-196.
Srivastava, A., Gupta, G. and Setty, B. S. 1989. Studies on mechanism (s) of antifertility action of gossypol in rat and hamster. Contraception 39: 337-355.
Takano, H. 1980. Qualitative and quantitative histology and histogenesis of the mouse epididymis with special emphasis on the regional difference. Acta Anat. Nippon. 55: 573-587.
Udoh, P., Patil, D. R. and Deshpande, M. K. 1992. Histopathological and biochemical effects of gossypol acetate on pituitary-gonadal axis of male albino rats. Contraception 45: 493-509.
World Health Organization. 1992. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge University Press, Cambridge, U. K. Wyrobek, A. J. and Bruce, W. R. 1975.
Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci., USA. 72: 4425-4429.
Xue, S. P. 1981. Studies on the antifertility effect of gossypol: a new contraceptive for males. In: Chang, C. F., D. Griffin and A. Woolman, eds., Recent Advances in Fertility Regulation, Atar, Geneva, pp. 122-147.
Zaneveld, L. and Polakoski, K. L. 1977. Collection and physical examination of the ejaculate. In: Hafez, E.S.E., ed., Techniques of Human Andrology, Elsevier/North Holland Biomedical Press, New York, pp. 147-172.