14 : Multi-tier Analysis for Screening Neuroprotective Extracts from Chinese Herbs
Abstract
To multi-tier evaluation of extracts from Chinese herbs that have neuroprotective effects in stroke, microdialysis-HPLC to examine time-course changes of infarct size and amino acid transmitter including glutamate (Glu), GABA and the ratio of Glu/GABA in the rat ischemia/reperfusion after transient middle cerebral arterial occlusion (MCAO), combined flow cytometric analysis and confocal laser scanning microscope to observe neuronal cell death, the level of [Ca2+]i and NO concentration during oxygen/ glucose deprivation (OGD) in vitro. Furthermore, we detected the apoptotic gene caspase3 expression was established. The series of methods were validated by extracts from Chinese herbs including Puerarin, Gastrodin, which demonstrated that these herbal extracts have potential neuroprotective effects in brain injury resulting from ischemia and reperfusion. The present results suggest that this multi-tier analytic method is all-round and efficient for screening and evaluating the neuroprotective extracts from herbs.
Key words: Cerebral ischemia/reperfusion, Neuroprotective, Chinese herbs, Oxygen/glucose deprivation, Microdialysis-HPLC, Flow cytometer, Confocal laser scanning microscope
1. Dept. of Biomedical Engineering, Key Laboratory of Biomedical Engineering of Ministry of Education, Zhejiang University, Zheda Road 38, Hangzhou 310027, China.
* Corresponding author : E-mail: zxx@gmail.hz.zj.cn
Introduction
Cerebral ischemia is known to produce severe histopathological damage and result in stroke. The middle cerebral artery perfused brain areas such as the parietal cortex, hippocampus, and striatum are mainly affected after cerebral ischemia. During ischemia, the lack of energy to the brain may depolarize neurons, producing a large increase in neurotransmitters such as glutamate (Glu) and GABA1-3. The overactivation of glutamate receptors causes an increased influx of Ca2+ and then resulted in neuronal Ca2+overload, which is the key mechanism of neuronal death in cerebral ischemia4. Furthermore, Ca2+overload in ischemic neurons leads to continuous production of nitric oxide (NO)5, a free radical that acts as a signaling molecule and a neurotoxin, has been shown to be involved in the mechanisms of cerebral ischemia6. Gamma-aminobutyric acid (GABA), the inhibitory amino acid neurotransmitter, has been implied to be involved directly and/or indirectly in the pathogenesis of neurological and psychiatric disorders7. Hence, the infarct size and the levels of amino acid transmitter, intracellular [Ca2+], NO concentration and neuronal apoptosis can be used as sensitive indicators of a drug’s potential effect on cerebral ischemia. Based on these principles, we established multi-tier analytical methods for quantitative assessing neuroprotective drugs from Chinese herbs.
Materials and Methods
Middle Cerebral Artery Occlusion (MCAO)
Male Sprague–Dawley rats (weighing 300~350g, Zhejiang Academy of Medical Science, Hangzhou, China) were anesthetized with an intraperitoneal injection of chloral hydrate (400 mg/kg). A polyethylene tube was inserted into the right femoral artery for continuously monitoring blood pressure with a computer-assisted system (Medlab-U, Nanjing Medease Science and Technology Co., China). The arterial blood gases and hemoglobin (Hb) were monitored using blood gas analyzer (CIBA850, Corning, USA) before the surgery, during MCAO and 30 min after reperfusion, respectively. Blood glucose was measured at the same time with one-touch basic blood glucose monitoring system (Lifescan Co., USA). The rectal temperature was kept at 37 ± 0.5 °C with a heating pad and light during the entire stroke procedure. The MCAO was induced using the intraluminal filament method, as described by Zea-Longa et al.8. Briefly, a midline incision was made and the right common carotid artery (CCA), the external carotid artery (ECA) and the internal carotid artery (ICA) were exposed. The distal ECA was coagulated completely. The CCA and the ICA were temporarily clamped with microvascular clips. A monofilament nylon filament (diameter 0.234 mm) with a blunted tip was introduced into the ECA lumen. The filament was then gently advanced to the distal ICA until it reached the clipped position. After removing the microvascular clip, the filament was inserted until resistance was felt, which ensured the occlusion of the origin of the middle cerebral artery. The distance between the CCA bifurcation and the resistive point was about 18.5~19.5 mm. The filament was withdrawn from the ICA after 50 min to allow MCA reperfusion. Control rats received sham surgery, in which an identical procedure was followed but inserting the filament 5 mm. All animal handling and surgery were performed in accordance with the Care Standards of the Laboratory Animal (China Ministry of Health publication, 1998).
Measurement of neurological function, edema radio and infarct size
Neurological deficits were evaluated on all sham-operated and MCAO animals at 24h after reperfusion. Rats were scored on a four-point scale (0– 4) described by Zea-Longa et al.8 (0=no deficit, 1=failure to extend left forepaw fully, 2=circling to the left, 3=falling to the left, 4=no spontaneous walking with a depressed level of consciousness).
In this study, ischemia was induced by occlusion of the MCAO for 50 min followed by reperfusion for 24 h. This duration of ischemia produces infarct in the ipsilateral hemisphere, affecting large portions of both striatum and cortex (infarct core)9. The surrounding cortex (dorsolateral cortex) was moderately hypoperfused (border of infarct). For infarct volume measurement, brains were isolated 24 h after MCAO, and sectioned into six coronal sections of 2 mm thick from the frontal tip. They were stained with 2% 2, 3, 5-triphenyltetrazolium chloride (TTC), then fixed with 10% phosphate-buffered formalin. Infarct areas of all the sections were calculated using image analysis software (provided by Dept. of Biomedical Engineering, Zhejiang University, China). The total infarct area was multiplied by thickness of brain sections to get infarct volume. Volumes of ipsilateral and contralateral hemispheres were calculated. Edema volume was obtained by subtracting the contralateral volume from the ipsilateral volume; edema ratio was then obtained by dividing the edema volume by the contralateral volume. Edema correction for infarct volume was done using the formula, corrected infarct volume = (infarct volume×contralateral hemisphere volume)/ipsilateral hemisphere volume. The percentage of corrected infarct (infarct ratio) was calculated by dividing the corrected infarct volume by the total volume of the bilateral hemisphere. The percentage of infarct in striatum or cortex was calculated by dividing the infarct volume in striatum or cortex by the total volume of the ispilateral striatum or cortex.
Microdialysis procedure
The sham-operated and MCAO animals were anaesthetized with chloral hydrate (400 mg/kg i.p.) and placed on a stereotaxic frame (ASI SAS-4100, USA). An intracerebral guide cannula (BAS MD-2251, USA) was implanted in the hippocampus (coordinates: P=-5.8, L=+5.0 H=-3.0 from Bregma) to guide and secure the probe. At least five days were allowed for recovery from surgery before the ischemia experiments were conducted. The microdialysis probes (BAS MD2204, 4mm membrane, USA) were perfused with artificial cerebrospinal fluid (ACSF: 125 mmol/l NaCl, 2.5 mmol/l KCl, 0.9 mmol/l NaH2PO4·H2O, 5 mmol/l Na2HPO4, 1 mmol/l MgCl2·6 H2O, 1.2 mmol/l CaCl2·2 H2O, and pH7.4~7.6). The recoveries of glutamate and GABA in the probes were approximately 19 and 23% respectively. After a 2 h equilibrium period at a flow rate of 2 µl/min with an infusion micropump (BAS MD-2262, USA), dialysates were collected in polyethylene vials every 20 or 10 min throughout the experiments. The first sample obtained from every animal before ischemic surgery was used for the baseline. The second sample was collected at the onset of the occlusion of the middle cerebral artery. Seven more samples were collected 130 min afterwards.
Amino acids detection
The extracellular concentrations of amino acids glutamate and GABA were measured by OPA-b-mercaptoethanol precolumn derivatization, reversed-phase gradient elution and fluorescence detection10. The amino acid in dialysate was first derivated to its fluorescent isoindoles. 20 µL dialysate and 10 µl OPA derivating fluid were allowed to react for 1 min at room temperature. The HPLC employed buffer A: 0.1 mol/l KH2PO4 buffer (adjusted to pH 6.6): methanol=65:35, v/v; and buffer B: 0.1 mol/LKH2PO4 buffer (adjusted to pH 6.6): methanol=10:90, v/v. Buffer A was ultrasonically degassed, buffer B was filtered and degassed through a 0.2 µm nitrocellulose membrane. The above two-buffer HPLC system (Shimadzu-10AVP, Japan) was coupled to a fluorescent detector (RF-10AXL, Shimadzu, Japan). Separation was achieved on a C18 column (Hypersil, BDS, 5µm). 20 µl of the reaction mixture was injected into the column and separated with a gradient from A: B (100:0) to 40% B within 12min; then eluted with 100% B 5 min to elute other components. The flow rate was set to 1 ml/min. Excitation wavelength: 357 nm; Emission wavelength: 455 nm.
Primary hippocampal cell culture and OGD treatment
Primary hippocampal cultures were prepared from 2 day-old Sprague-Dawley rats as described by Isaev et al. with modifications11. Briefly, the tissue was digested with trypsin (0.25% in phosphate-buffered saline (PBS); 20 min; 37 °C) followed by mechanical dissociation. Hippocampal cells were seeded in poly-L-lysine-coated plates (1.2×105/cm2) and grown in neurobasal medium with B-27 serum-free supplement (Gibco), 100U/ml penicillin, 100 µg/ml streptomycin, and 2mmol/l L-glutamine. The cultures were maintained at 36.5 °C in a CO2-incubator (5% CO2, 95% air). The medium was changed starting from day in vitro by replacing half of the medium twice a week. Serum-free primary hippocampal cultures were utilized for the experiments after day in vitro.
For oxygen/glucose deprivation (OGD) the cultures were washed twice in glucose-free balanced salt solution (BSS) of the following content (in mmol/ l): NaCl 130, KCl 5.5, CaCl2 1.8, MgCl2 1.0, HEPES 20, pH7.4, and incubated in oxygen- and glucose-free BSS (pre-treated by gassing 95% N2/5% CO2) in a humidified chamber filled with 95% N2/5% CO2 (36.5 °C) for 4 h. Control cultures were incubated in BSS with glucose (5.6 mmol/l) under normal conditions (in a CO2-incubator). After OGD the cultures were replaced into neurobasal medium and maintained in a CO2-incubator for 24 h.
Assay for apoptosis by the flow cytometry
For flow cytometric analysis, cells were harvested and washed with Ca2+- and Mg2+-free PBS. After centrifugation at 1500 rpm for 5 min, the pellet was resuspended in 1×Binding Buffer at a density of 1×106 cell/ml. Cells (100 µl) were transferred to a culture tube, and 5 µl of Annexin V-FITC and 10 µl of PI were added. Following gentle vortex, the tube was incubated for 15 min at room temperature (20~25 °C) in the dark, 400 µl of 1×Binding Buffer was then added into it. The sample was analyzed using flow cytometer (FAC Sort, Becton Dickinson) as soon as possible (within 1h). The percentages of apoptotic and necrotic cell of each sample were estimated.
Measurement of intracellular free Ca2+ and NO concentrations
A confocal laser scanning microscope (Zeiss LSM510, Germany) was used to evaluate relative changes in intracellular Ca2+ and NO concentrations by monitoring Fluo-3 and DAF-2 fluorescence after intracellular cleavage of superfused Fluo-3 AM (5×10-6mol/l; Molecular Probes) and DAF-2 DA (4,5-diaminofluorescein diacetate, 10-5mol/l; Calbiochem) with excitation at 488 nm and emission at >510 nm respectively12,13. After added glucose- and oxygen-free BSS into dish, laser scanning began to obtain time series of images. Acquisition rate is 1 frame every 30 seconds. The obtained images were quantitatively analyzed for changes in fluorescence intensities within a cell using Zeiss LSM software. Data were obtained by evaluating the fluorescence (F) after OGD within a cell, with following subtraction of background fluorescence, and division by the fluorescence intensity before OGD (F0), expressed as F/F0. Control cultures were added BSS with glucose.
Caspase-3 activity assay
Caspase-3 activity was analyzed as Lee et al. reported with modification14. Briefly, each dish (35mm) of cell layers was rinsed with cold Ca2+- and Mg2+-free PBS and harvested with a rubber policeman into lysis buffer (5 mmol/l Tris–HCl, pH7.4, 20mmol/l EDTA and 0.5% Triton-X 100) at 4°C for 15 min. The lysates were then centrifuged at 1200 rpm for 10 min at 4 °C, and supernatants were centrifuged at 17000 rpm for 20 min at 4 °C. Protein concentrations were determined using the Bradford method (Bio-Rad, Richmond, CA, USA). Ac-DEVD–AMC [N-acetyl–Asp– Glu–Val–Asp–AMP (7-amino-4-methylcoumarin)] (Pharmingen, San Diego, CA, USA) was used for the caspase-3 activity assays, and 200µg of protein, 200µl of reaction buffer (20 mmol/l HEPES, pH7.5, 10% glycerol, 4 mmol/l DTT), and 5 µl (1 µg/µl) of reconstituted Ac-DEVD–AMC were added into each well of a 96-well plate. The reaction mixture was incubated for 1h at 37 °C. The amount of AMC released was measured using a fluorescence spectrophotometer (Shimadzu, Japan) at 380 nm excitation and 460 nm emission.
Statistical analysis
All results were expressed as mean ± SD. Statistical comparisons between different treatments were performed by one-way ANOVA with Dunnett’s multiple comparison post test. Differences with p value less than 0.05 were considered statistically significant.
Results
Infarct size, edema ratio and neurological score
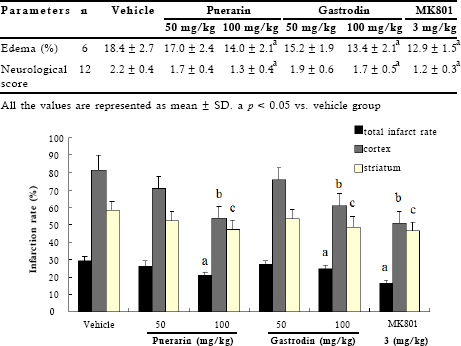
Sham-operated rats did not show any lesions in either hemisphere. 50 min of MCAO and 24h of reperfusion resulted in significant infarct (25.9%) as shown in TTC-stained coronal brain sections (Fig 1). Neurological deficit score of 2.2 ± 0.3 was observed in vehicle-treated MCAO rats (Table 1). We used MK801 as the positive control in vivo experiment. MK801, a noncompetitive NMDA receptor antagonist, has effectiveness in focal models of cerebral ischemia15. We then screened 20 compounds extracted from Chinese herbs (collected by our lab) and identified two active compounds: puerarin (Zhejiang Conba Pharmaceutical Co., Ltd, China, purity above 98%) and gastrodin (Kunming Pharmaceutical Co.; Kunming, China, purity 99.6%), namely. Puerarin and gastrodin at 100 mg/kg significantly decreased total infarct volume and were prominent in cerebral cortex as it decreased the infarct volume of striatum (Fig 1). Meanwhile, the higher dose of them produced obvious improvement in neurological functions and reduction in edema volume (Table 1).
Table 1. Edema and neurological scores of the rats after transient focal ischemia

Fig 1. Evaluation of total infarct rate, infarct rate of cortex or striatum after transient focal ischemia. The percentage of infarct (infarct ratio) was calculated by dividing the corrected infarct volume by the total volume of the bilateral hemisphere. The percentage of infarct in striatum or cortex was calculated by dividing the infarct volume in striatum or cortex by the total volume of the ispilateral striatum or cortex. Data represent means ± SD (n=6). a, b, cp < 0.05 vs. vehicle group
Neurochemical changes in the ischemic/reperfused rat cerebral hippocampus
Glutamate accumulation in the hippocampus during transient ischemia is widely regarded to play an important role in inducing neuronal cell death during ischemia4,16. Dialysis-HPLC analysis showed neither the values of glutamate nor those of GABA in the sham group had altered through the ischemic/reperfused periods. For glutamate and GABA, basal concentrations of dialysate were 2.4 µmol/l and 0.67 µmol/l respectively. As shown in Figs 2A and 2B, during ischemia the concentrations of glutamate and GABA in the vehicle group peaked almost 13 and 8 times higher than the initial values, respectively. And thereafter they began to decline after reperfusion. The glutamate/GABA ratios at the baseline were approximately 3.5 in preischemic conditions (Fig 2C). In the ischemic period, glutamate/GABA ratios of the vehicle group significantly increased by about 2 fold, then declined modestly after reperfusion. Then, the effects of the two compounds on the concentrations of these animo acids during ischemia were assayed. Administration of puerarin and gastrodin significantly reduced the ischemia-induced elevation levels of glutamate and increased GABA concentrations (Figs 2A and 2B), which regulated the imbalances of glutamate/GABA to normal levels (Fig 2C).
Percentages of apoptosis and necrosis
In our culture system, apoptotic cells in hippocampus were estimated by flow cytometric analysis of Annexin-V and PI labeled cells. Four hours of OGD induced cell death, and the percentage of apoptosis increased from 5.4 to 27.9% (Fig 3A), confirmed by activation of caspase-3 (Fig 3B), which is a major executioner of the apoptotic signals. In the presence of Puerarin and Gastrodin, cell death induced by OGD was dramatically suppressed, and the percentages of apoptosis were reduced by 33% compared with OGD-treated group. In addition, the compounds remarkably blocked OGD-induced activation of caspase-3. Likewise, MK801 also significantly decreased the percentages of apoptosis in OGD-treated hippocampal cultures.
Confocal microscopic study of the neuronal [Ca2+]i and NO content
Because increasing intracellular Ca2+ and eNOS were toxic to brain cells in the early stages of a focal ischemia, we evaluated real-time of Ca2+ homeostasis and NO synthesis with Fluo-3 and DAF-2 labeling in cultured hippocampal neurons. During 30 min exposure to OGD, marked changes of [Ca2+]i and NO content were observed with obvious increase rapidly about 2-15 min after the beginning of OGD and then gradually during the later time course of OGD. The addition of puerarin (40, 100 µmol/l) and gastrodin (50, 100 µmol/l) 10 min before OGD slowed down the elevation of [Ca2+] and NO synthase, and lowered their peak (Fig 4), which was similar to the effects of MK80115 and L-NAME (a non-specific NO synthase inhibitor)17. These results indicated that the two compounds inhibited accumulation [Ca2+] and NO concentration and prevented hippocampal neurons from injury i during OGD.
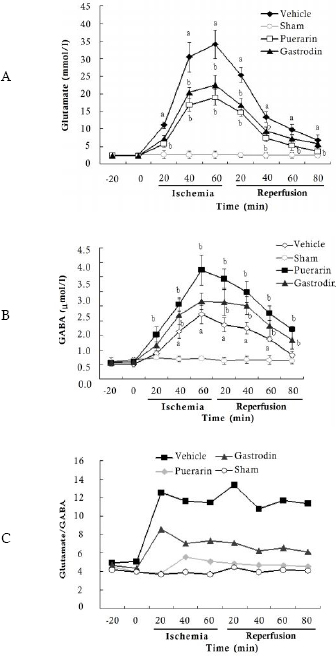
Fig 2. Time course of the concentrations of glutamate and GABA, the ratios of glutamate/ GABA before and after cerebral ischemia/reperfusion. All the values are expressed as mean ± SD (n=6). Onset of ischemia is designated as time zero. a p < 0.05 vs. sham group; b p < 0.05 vs. vehicle group
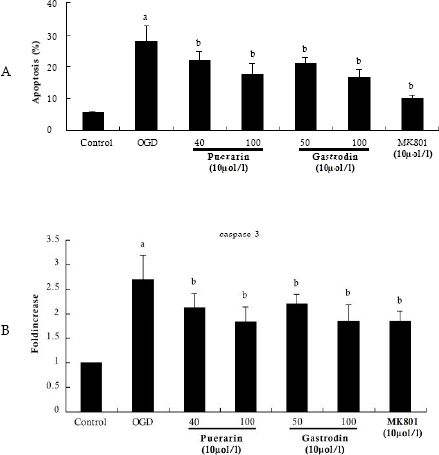
Fig 3. Apoptosis and caspase-3 activity in cultured hippocampual neurons under oxygen/ glucose deprivation (OGD) (n=8). Data represent mean ± SD. a p < 0.05 vs. control group; bp< 0.05 vs. OGD-treated group
Discussion
Based on the findings that the infarct size and the levels of amino acid transmitter, Ca2+ and NO, together with neuronal apoptosis can be used as sensitive indicators of a drug’s potential effect on cerebral ischemia, we established multi-tier analytical methods. These methods were used to evaluate the known drug MK801 and 20 compounds extracted from Chinese herbs and successfully identified two non-cytotoxic compounds, puerarin and gastrodin. Puerarin and gastrodin have been reported to attenuate cerebral or spinal cord injury resulting from ischemia and reperfusion in rats or rabbits 18-22, and our results are in accordance with the previous findings.
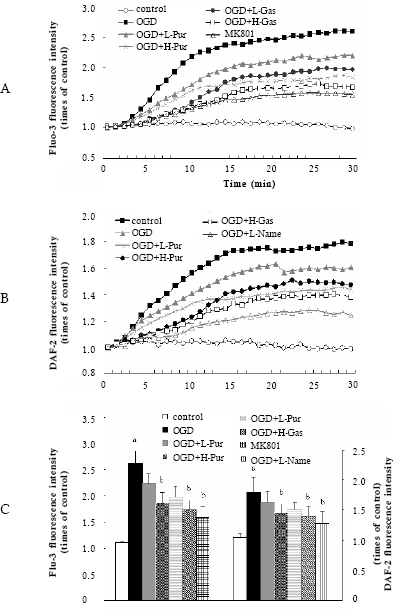
Fig 4. Time course of intracellular Ca2+ and NO levels and peaks of [Ca2+]i and NO in cultured hippocampal cells under oxygen/glucose deprivation (OGD) (n=25-28 cells). Puerarin, gastrodin were added 10 min before OGD. (A) Time course of intracel-lular Ca2+ level; (B) Time course of intracellular NO level; (C) The peaks of intra-cellular Ca2+ and NO. Data represent mean ± SD. a p < 0.05 vs. control group; b p < 0.05 vs. OGD-treated group.
We examined the prevention of puerarin and gastrodin from cerebral infarct after transient focal ischemia. The two compounds both significantly decreased the infarct volumes of total, cerebral cortex and striatum, together with obvious improvement in neurological functions and reduction in edema volume. Both of them obviously reduced the ischemia-induced elevation levels of glutamate and GABA in time-dependent manner, and resulting in alteration of the imbalances of glutamate/GABA, which plays an important role in the ischemia and early reperfusion period. The results may be convenient to observe their dynamic modulation of neurotransmitters. The experiment in cultured hippocampal neurons demonstrated the compounds decreased OGD-stimulated the elevation of [Ca2+]i and NO concentration, which is toxic to brain cells in the early stages of a focal ischemia, and prevented hippocampal neurons from apoptosis. The experiment in vitro is helpful for elucidation of their mechanisms. In addition, we randomly selected 4 compounds derived from Chinese herbs that had no detectable phenotype in vivo and in vitro experiments. No significant bioactivity was found in any assay. With these experiments, we proved the validity of the methods and applicability of the assay to screening of plant extracts.
A possible drawback of our methods is that the screened compounds may exert broad effects that are not specific. However, the etiology of cerebral ischemia is so complex that, even if the drugs have no effect on a particular drug target, they may affect other targets through some unknown mechanisms. The method presented in this publication is adaptable to high throughput technology and is therefore useful for the primary screening of drug candidates from herbal extracts.
Acknowledgements
This work was supported by National Nature Science Foundation of China (NO. 30470463) and the Key Laboratory for Biomedical Engineering of Ministry of China, the Economic a & Trade commission of Zhejiang Province, & the Key Laboratory of Chinese Medicine Screening, Exploitation & Medicinal Effectiveness Appraise for Cardio-cerebral Vascular and Nervous System of Zhejiang Province.
References
1. Siesjo, B.K. 1992a. Pathophysiology and treatment of focal cerebral ischaemia: Part I: Pathophysiology, J. Neurosurg 77(2): 169–184.
2. Siesjo, B.K. 1992b. Pathophysiology and treatment of focal cerebral ischaemia: Part II: Mechanisms and drug treatment. J. Neurosurg 77(3): 337–354.
3. Globus, M.Y., Busto, R., Dietrich, W.D., Martinez, E., Valdes, I. and Ginsberg. M.D. 1988. Effect of ischaemia on the in vivo release of striatal DA, Glu and gamma-aminobutyric acid studied by intracerebral microdialysis. J. Neurochem. 51(5): 1455– 1464.
4. Bouchelouche, P., Belhage, B., Frandsen, A., Drejer, J. and Schousboe. A. 1989. Glutamate receptor activation in cultured cerebellar granule cells increases cytosolic free Ca2+ by mobilization of cellular Ca2+ and activation of Ca2+ influx. Exp. Brain. Res. 76(2): 281-291.
5. Jiang, M.H., Kaku, T., Hada, J. and Hayashi, Y. 2002. Different effects of eNOS and nNOS inhibition on transient forebrain ischemia. Brain Res. 946(1): 139–147.
6. Iadecola C. 1997. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 20(3): 132–139.
7. Sherif, F.M. and Ahmed, S.S. 1995. Basic aspects of GABA-transaminase in neuropsychiatric disorders. Clin. Biochem. 28(1): 145–154.
8. Zea-Longa, E., Weinstein, P.R., Carlson, S. and Cummins, R. 1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1): 84–91.
9. Prakasa, B.P., Yoshida, Y., Su, M., Segura, M., Kawamura, S. and Yasui, N. 2000. Immunohistochemical expression of Bcl-2, Bax and cytochrome c following focal cerebral ischemia and effect of hypothermia in rat. Neurosci. Lett. 291(3): 196–200.
10. Begley, D.J., Reichel, A. and Ermisch, A. 1994. Simple high-performance liquid chromatographic analysis of free primary amino acid concentrations in rat plasma and cisternal cerebrospinal fluid. J. Chromatogr. B. Biomed. 657(1): 185-191.
11. Isaev, N.K., Stelmashook, E.V., Dirnagl, U., Andreeva, N.A., Manuhova, L., Vorobjev, V.S., Sharonova, I.N., Skrebitsky, G., Victorov, I.V., Katchanov, J., Weih, M. and Zorov, D.B. 2002. Neuroprotective effects of the antifungal drug clotrimazole. Neuroscience 113(1): 47–53.
12. Al-Mehdi, A.B., Song, C., Tozawa, K. and Fisher, A.B. 2000. Ca2+ and phosphatidylinositol 3-kinase-dependent nitric oxide generation in lung endothelial cells in situ with ischemia. J. Biol. Chem. 275(51): 39807–39810.
13. Chen X.C. and Zheng X.X. 2001. Direct nitric oxide imaging in cultured hippocampal neurons with diaminoanthraquinone and confocal microscopy. Cell Biol. Int. 25(7): 593–598.
14. Lee, S.H., Kim, M., Kim, Y.J., Kim, Y.A., Chi, J.G., Roh, J.K. and Yoon, B.W. 2002. Ischemic intensity influences the distribution of delayed infarction and apoptotic cell death following transient focal cerebral ischemia in rats. Brain Res. 956(1): 14– 23.
15. Hatfield, R.H., Gill, R. and Brazell, C. 1992. The dose-response relationship and therapeutic window for dizocilpine (MK-801) in a rat focal ischemia model. Eur. J. Pharmacol. 216(1): 1–7.
16. Benveniste, H., Drejer, J., Schousboe, A. and Diemer, N.H. 1984. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43(5): 1369–1374.
17. Wainwright, M.S., Grundhoefer, D., Sharma, S. and Black, S.M. 2007. A nitric oxide donor reduces brain injury and enhances recovery of cerebral blood flow after hypoxia-ischemia in the newborn rat. Neurosci. Lett. 415(2): 124-129.
18. Wang, L., Zhao, A., Wang, F., Chai, Q. and Chai, X. 1997. Protective effect of puerarin on acute cerebral ischemia in rats. Chin. Materia. Med. J. 22(12): 752-754.
19. Sang, H.F., Zhang, Y.M., Xu, L.X., Wang, Q., Ji, G.L. and Wu, M.C. 2001. Protective effect of puerarin on spinal cord injury resulting from ischemia and reperfusion in rabbits. J. Fourth Mil. Med. Univ. 22: 414-417.
20. Xu, X.H., Zheng, X.X., Zhou, Q. and Li, H. 2007.Inhibition of excitatory amino acid efflux contributes to protective effects of puerarin against cerebral ischemia in rats. Biomed. Environ. Sci. 20(4): 336-342.
21. Zeng, X.H., Zhang, Y., Zhang, S.M. and Zheng, X.X. 2007. A microdialysis study of effects of gastrodin on neurochemical changes in the ischemic/reperfused rat cerebral hippocampus. Biol. Pharm. Bull. 30(4): 801-804.
22. Zeng, X.H., Zhang, S.M., Zhang, L., Zhang K.P. and Zheng, X.X. 2006. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 72(15): 1359-1365.