15 : Phytochemical Screening and In vitro Antiamoebic Activity of Extracts from Some Antidiarrhoeal Medicinal Plants Used in Kinshasa, Democratic Republic of Congo
Abstract
Crude aqueous extracts (traditional preparations) from 31 medicinal plants used as antidiarrhoeal remedies and, their n-butanol soluble fractions rich in polyphenolic compounds, crude saponin and total alkaloid extracts were investigated in vitro for their potential antiamoebic activity. Results indicated that 4 crude aqueous extracts from Carica papaya mature seeds, Mangifera indica stem bark, Paropsia brazzeana root bark and Psidium guajava stem bark exhibited good antiamoebic activity with minimum amoebicidal concentrations (MAC) values £ 7.8 µg/ml. Among the n-butanol soluble fractions, those from Alchornea cordifolia leaves, Euphorbia hirta whole plant, Harungana madagascariensis stem bark, Morinda morindoides leaves, Nauclea latifolia root bark, P. guajava stem bark and Tithonia diversifolia exhibited pronounced antiamoebic activity with MAC £ 5 µg/ml. Those from Crossopteryx febrifuga leaves, M. indica stem bark, P. brazzeana root bark, Pentaclethra macrophylla stem bark, Quassia africana root bark and Rauwolfia obscura root bark displayed good antiamoebic activity with 5 < MAC £ 10 µg/ ml. Only crude saponin extract from P. guajava stem bark showed good activity against Entamoeba histolytica growth with MAC value of 8.3 µg/ml. The total alkaloid extracts from Sida rhombifolia leaves, Rauwolfia obscura root bark and Voacanga africana were also found to display very pronounced antiamoebic activity with MAC values £ 5 µg/ml.
Key words : Medicinal plants,Traditional preparations, Diarrhoea, Antiamoebic activity, Polyphenols, Saponins, Alkaloids
1. Faculty of Pharmaceutical Sciences, University of Kinshasa, P.O. Box 212, Kinshasa XI, Democratic Republic of Congo.
2. Department of Pharmaceutical Sciences, University of Antwerp, Universiteitsplein 1, B-2610, Antwerp, Belgium Antwerp, Belgium.
* Corresponding author : E-mail: kanyanga.cimanga@ua.ac.be
Introduction
Amoebiasis is an infection of human gastrointestinal disorders caused by the protozoa Entamoeba histolytica leading to the significant morbidity and mortalitity in developing countries (Calzada, 2005).
Metronidazole is a cheaper drug widely used for the treatment of amoebiasis, but the drug is less effective in the tissue than in the gut lumen. It produces some adverse effects which are dose-related. The common are gastrointestinal disturbances, especially nausea and an unpleasant metalic taste. Vomiting and diarrhoea or constipation may also occur (Sweetman, 2002). Thus, people turn to traditional preparations mainly based on different medicinal plant species claimed to be effective against gastrointestinal disorders and especially amoebic dysentery by traditional healers in their daily practices for the treatment of the disease and find some relief. Nowadays, these medicinal plant species were investigated in vitro and/or in vivo models to prove their effectiveness against the protozoa E. histolytica. Results reported from these studies revealed that some medicinal plant species have been shown to possess an antiamoebic activity at different extents as reviewed by Sharma and Sharma (2001) and recently reported in other studies (Calzada et al., 2003, 2005, 2007; Moundipa et al., 2005; Cimanga et al., 2006). Extensive bioassay-guided isolation have also resulted in the characterization of active antiamoebic principles belonging to different phytochemical groups such as alkaloids (Wright et al., 2000; Di Stasi, 1995), flavonoids and terpenes (Calzada et al., 2003, 2005, 2006, 2007; Cimanga et al., 2006), and flavan-3-ol (Calzada et al., 1999, 2005).
In Kinshasa, capital of Democratic Republic of Congo (DR-Congo), an ethnopharmacological and ethnobotanical inventory was conducted based only on a healer’s knowledge for the treatment of diarrhoea in its daily practices using different medicinal plant species. Some of them were selected and collected on the basis of their high frequency use from which aqueous extracts were prepared by a traditional healer according to its daily practices. They were included in a vast biological programme aiming the evaluation of their potential antibacterial, anti-spasmodic and anti-amoebic activities which can at least in part justify their use as antidiarrhoeal agents. Preliminary results obtained were interesting and encouraging because most of these tested plant extracts were found to act as antidiarrhoeal remedies by the three or two biological properties cited above according to the case (Tona et al., 1998, 1999).
Taking account of the level of the antiamoebic activity displayed by these traditional preparations, some of them were selected here and investigated in the aim to locate active fraction containing at least one specific active phytochemical group such as polyphenols, saponins or alkaloids as the major constituents.
Materials and Methods Plant materials
All selected plants were collected in Kinshasa, capital of DR-Congo in Augustus 2005. They were identified at the Institut National d’Etudes et de Recherches en Agronomie (INERA) of the University of Kinshasa. A voucher specimen of each plant was deposited at the herbarium of this institute. All plant parts were used in a fresh state.
Preparation of extracts
All crude extracts are aqueous preparations obtained from a traditional healer according to its daily practices. The amount of each part used per 100 ml water, and the method of preparation are given in Table 1. Briefly, except for leaves, an amount of other plant materials (root bark, stem bark) cut in small pieces was macerated with water for 24 hours. The mixture was filtered and the filtrate evaporated in vacuo to give corresponding dried extracts. On the other hand, the decoction was obtained by boiling an amount of plant material for 15 or 30 minutes according to the case. The mixture was cooled, filtered and the filtrate treated as described above yielding dried extract. All aqueous dried extracts were denoted as extract A. In this step, it is important to point out that the only scientific contributions were the weighing plant materials, to measure volume of water used to prepare a macerate and a decoction, the final volume of the preparation and the time required to obtain a decoction.
To obtain extract containing a specific phytochemical group, 5 g of each crude extract were dissolved in 50 ml distilled water and filtered. The filtrate was exhaustively extracted with n-butanol. The organic phase was treated as described above yielding dried extracts containing total polyphenols denoted as extract B. Their presence was confirmed by a positive test with FeCl3 5%. For saponins, an other batch of 5 g of crude extract was dissolved in 50 ml methanol and heated under reflux for 1 hour. The mixture was cooled and filtered. An excess of diethyl ether (200 ml) was added to the filtrate giving an abundant precipitate. After filtration, the precipitate was dried at room temperature and denoted as extract C. The presence of saponins was detected in this extract by the frothing test. Total alkaloid extracts were obtained by the conventional procedure acid/base and identified with Dragendorff’s and Mayer’s reagents. All dried total alkaloids were denoted as extract D.
Phytochemical screening
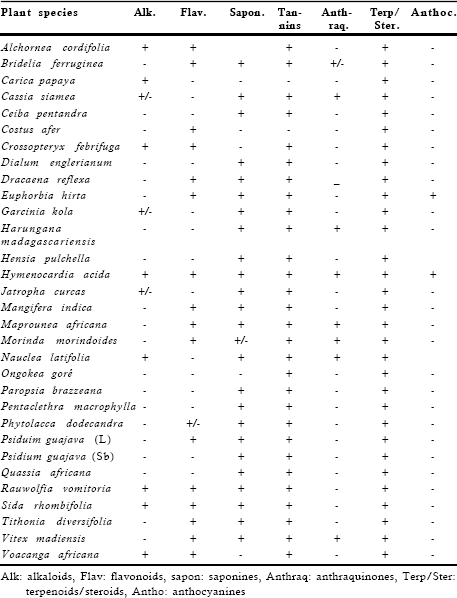
Different phytochemical groups in selected plant extracts were identified by TLC using silica gel plates 60 F254 (thikness layer 0.25 mm, Merck) and various appropriated mobile phases described in the literature. Alkaloids were detected with Dragendorff”s reagent, flavonoids with Neu’s reagent, anthraquinones with Borntranger’reagent and magnesium acetate 5% (methanol solution), terpenes and steroids with Lieberman Buchard’s reagent. Saponins were detected in aqueous extracts by the frothing test while tannins were revealed in aqueous extracts with gelatinous salt block and lead sub-acetate tests. Anthocyanidins were characterized in aqueous extracts by their red color developed in the presence of hydrochlorid acid after heating and extracted with n-butanol. The presence of flavonoids was also established in aqueous extracts with Shinoda’s test (Harborne, 1984; Wagner & Bladt, 1996).
Table 1. Obtention of crude aqueous extracts (traditional preparations)
| Plant names and familly | G.F | P.U | Quantity (g) | Yield (%) * |
| Alchornea cordifolia Müll. Arg. | dec. | L | 16.5 | 5.2 |
| (Euphorbiaceae) | ||||
| Bridelia ferruginea Benth. (Rubiaceae) | mac. | L | 32.8 | 4.7 |
| Carica papaya L. (Caricaceae) | dec. | msd | 20 | 4.2 |
| Cassia siamea Lam. (Caesalpiniaceae) | dec. | Sb | 40 | 6.3 |
| Ceiba pentandra (L) Gaertn. (Bombaceae) | dec. | Sb | 22 | 6.1 |
| Costus afer Ker Gawel. (Zingiberaceae) | - | J | - | 3.2 |
| Crossopteryx febrifuga Benth. | dec. | L | 20 | 3.2 |
| (Rubiaceae) | ||||
| Dialum englerianum Henriq. | dec. | Sb | 20 | 6.4 |
| (Caesalpiniaceae) | ||||
| Dracaena reflexa var. Nittens | dec. | L | 30 | 5.1 |
| Welw ex. Back. (Agavacaceae) | ||||
| Euphorbia hirta L. (Euphorbiaceae) | dec. | Wp | 16 | 4.3 |
| Garcinia kola Haeckel (Clusiaceae) | dec. | Sb | 50 | 8.3 |
| Harungana madagascariensis | dec. | Sb | 50 | 7.6 |
| Lam. ex. Poir. (Hypericaceae) | ||||
| Heinsia pulchella K. Schum. (Rubiaceae) | dec. | .Rb | 20 | 4.2 |
| Hymenocardia acida Tull. (Euphorbiaceae) | dec. | Sb. | 21 | 5.2 |
| Jatropha curcas L. (Euphorbiaceae) | ||||
| Mangifera indica L. (Anacardiaceae) | dec. | Sb | 66 | 10.5 |
| Maprounea africana Müll. Arg. | dec. | Sb | 25 | 4.7 |
| (Euphorbiaceae) | ||||
| Morinda morindoides (Baker) | dec. | L | 20 | 5.7 |
| Milne-Redhead (Rubiaceae) | ||||
| Nauclea latifolia Smith. (Rubiaceae) | dec. | Rb | 20 | 4.6 |
| Ongokea goré (Hua) Pierre (Olacaceae) | dec. | Sb | 16.3 | 3.2 |
| Paropsia brazzeana Baill. (Passifloraceae) | dec. | Rb | 10 | 3.7 |
| Pentaclethra macrophylla Benth. | dec. | .Sb | 45.3 | 7.2 |
| (Mimosaceae) | ||||
| Phytolacca dodecandra H. (Phytolaccaceae) | dec. | L | 30.5 | 5.8 |
| Psidium guajava L. (Myrtaceae) | dec. | L | 54 | 7.6 |
| dec. | Sb | 37.3 | 5.3 | |
| Quassia africana Baill. (Simaroubaceae) | dec. | Sb | 32.1 | 4.8 |
| Rauwolfia obscura K. Schum. (Apocynaceae) dec. | Rb | 20 | 4.1 | |
| Sida rhombifolia L. (Malvaceae) | dec. | L | 25.3 | 3.7 |
| Tithonia diversifolia (Hasmel) | dec. | L | 32.2 | 5.6 |
| A. Gray (Asteraceae) | ||||
| Vitex madiensis Oliv. (Verbenaceae) | dec. | L | 22.3 | 4.7 |
| Voacanga africana Stapf. (Apocynaceae) | mac. | Rb | 57.8 | 9.1 |
G.F: galenic form, L: leaves; Sb: stem bark, Rb: root bark, , msd: mature seeds, J: juice dec.: decoction, Wp.: whole plant, mac.: macerate, *yield of dried extract
In vitro antiamoebic testing
Entamoeba histolytica strain used in this investigation is a laboratory strain isolated from patients with acute amoebic dysentery, and kindly provided by the Institute of Tropical Medicine, Faculty of Medicine, University of Kinshasa. The maintenance of Entamoeba histolytica cultures on Dobbel and Laidlaw’s medium as well as the evaluation of the antiamoebic activity of plant extracts were performed according to the procedure previously described (Tona et al., 1999). To test samples, 2 mg of each was dissolved in 2 ml EtOH to give stock solutions with a concentration of 1 mg/ml. They were diluted in 2-fold dilution with culture medium to give the required range of test concentrations from 500 to 1.5 µg/ml. Intermediate test concentrations were also prepared. In a series of test tubes containing 8 ml of culture medium and an average number of amoebae 2.5 x 106/ml of culture medium, 1 ml of test sample was added. The tubes were filled up with sterile cotton and the mixture stirred. Each test was performed in duplicate. Two sets of controls were used. One control was the Entamoeba histolytica control cultured in medium without test sample, and the second consisted of test sample in cultured medium for the sterility control. All tubes were plugged with sterile cotton and incubated at 37 oC for one week. A daily counting of dead and alive amoebae was performed through a microscope with the aid of Neubauer’s cell. The minimal amoebicidal concentration (MAC) was determined and defined as the minimal concentration of the test sample at which no vegetative or cystic forms were microscopically observed. Metronidazole was used as a reference antiamoebic product.
Results
Table 3 presents the minimum amoebicidal concentrations (MAC) of 31 crude aqueous extracts, their 32 n-butanol soluble fractions, 22 crude saponin extracts and 9 total alkaloid extracts. Results indicated that 4 crude aqueous extracts (12.90%) from Carica papaya mature seeds, Mangifera indica stem bark, Paropsia brazzeana root bark and Psidium guajava stem bark were found to exhibit good antiamoebic activity with MAC values < 10 µg/ml. 2 extracts (6.45%) from Heinsia pulchella root bark and Morinda morindoides leaves were moderately active (10 < MAC £ 30 µg/ml), 12 extracts (38.71%) showed low activity ( 30 < MAC £ 62.5 µg/ml), 9 extracts (29.03%) displayed very low activity (62.5 < MAC £ 250 µg/ml and 4 extracts (12.90%) were inactive at the highest tested concentration of 250 µg/ml.
Of the 32 n-butanol soluble fractions rich in polyphenolic compounds tested, 7 (21.88%) of them exhibited a very pronounced antiamoebic activity with MAC values £ 5 µg/ml. It concerns n-butanol soluble fractions from Alchornea cordifolia leaves, Euphorbia hirta whole plant, Harungana madagascariensis stem bark, M. morindoides leaves, Psidium guayava stem bark, Nauclea latifolia root bark and Tithonia diversifolia leaves. 6 other n-butanol soluble fractions (18.75%) showed good activity with MAC values between 5 and 10 µg/ml. This group includes n-butanol fractions from Crossopteryx febrifuga leaves, M. indica stem bark, P. brazzeana root bark, Pentaclethra macrophylla stem bark, Quassia africana root bark and Rauwolfia vomitoria root bark. The last 19 n-butanol soluble fractions (59.37%) were either moderately active (10 < MAC £ 30 µg/ml) or showed low activity (30 < MAC £ 62.5 µg/ml) (Table 3). Among 22 crude saponin extracts, only 1 extract (4.55%) from P. guavaja stem bark exhibited good antiamoebic activity (MAC < 10 µg/ml). 8 extracts (36.36%) showed moderate activity (10 < MAC £ 30 µg/ml), 5 extracts (22.73%) displayed low activity (30 < MAC £ 62.5 µg/ml) and 8 extracts (36.36%) did not show an activity when tested at a concentration of 50 µg/ml (Table 3). For the total alkaloid extracts, it was found that 3 extracts (33.33%) from S. rhombifolia leaves, Rauwolfia obscura root bark and Voacanga africana root bark of 9 tested exhibited very pronounced antiamoebic activity with MAC values ranging from 4 to 5 µg/ml. 3 extracts (33.3%) from A. cordifolia, C. febrifuga and N. latifolia were moderately active (10 < MAC £ 30 µg/ml) and 3 other (33.3%) from C. papaya, D. reflexa and H. madagascariensis were lowly active (30 < MAC £ 62.5 µg/ml).
Discussion
The present study reports the antiamoebic activity of 31 aqueous extracts from some medicinal plant species used in Kinshasa for the treatment of diarrhoea and that of their different soluble fractions containing one phytochemical group as major constituent such as polyphenols, saponins or alkaloids. Results from the phytochemical screening of selected plant species are listed in Table 2. The minimal amoebicidal concentrations (MAC) of these tested samples are presented in Table 3. The level of the antiamoebic activity displayed by each tested sample was appreciated according to the following criteria: MAC £ 5 µg/ml: very pronounced activity, 5 < MAC £ 10 µg/ml: good activity, 10 < MAC £ 30 µg/ml: moderate activity, 30 < MAC £ 62.5 µg/ml low activity, 62.5 < MAC £ 250 µg/ml: very low activity and MAC > 250 µg/ml: inactive.
From these results, it was observed that four crude aqueous extracts and the n-butanol soluble fractions rich in polyphenols from 3 of them exhibited good antiamoebic activity with MAC values ranging from 5 to 10 µg/ml. Their crude saponin extracts showed good or moderate activity with MACvalues ranging from 8 to 21 µg/ml according to the case. It concerns samples from Carica papaya mature seeds, Mangifera indica stem bark, Psidium guajava stem bark and Paropsia brazzeana root bark . The total alkaloid extracts from Rauwolfia obscura stem bark, Sida rhombifolia leaves and Voacanga africana root bark exhibited very pronounced antiamoebic activity with MAC values ranging from 1 to 5 µg/ml (Table 3).
Table 2. Phyochemical screening of the plant part used

| Plant species | Alk. | Flav. | Sapon. | Tannins | Anth-raq. | Terp/ Ster. | Anthoc. |
| Alchornea cordifolia | + | + | + | - | + | - | |
| Bridelia ferruginea | - | + | + | + | +/- | + | - |
| Carica papaya | + | - | - | - | - | + | - |
| Cassia siamea | +/- | - | + | + | + | + | - |
| Ceiba pentandra | - | - | + | + | - | + | - |
| Costus afer | - | + | - | - | - | + | - |
| Crossopteryx febrifuga | + | + | - | + | - | + | - |
| Dialum englerianum | - | - | + | + | - | + | - |
| Dracaena reflexa | - | + | + | + | _ | + | - |
| Euphorbia hirta | - | + | + | + | - | + | + |
| Garcinia kola | +/- | - | + | + | - | + | - |
| Harungana | - | - | + | + | + | + | - |
| madagascariensis | |||||||
| Hensia pulchella | - | - | + | + | - | + | |
| Hymenocardia acida | + | + | + | + | + | + | + |
| Jatropha curcas | +/- | - | + | + | - | + | - |
| Mangifera indica | - | + | + | + | - | + | - |
| Maprounea africana | - | + | + | + | + | + | - |
| Morinda morindoides | - | + | +/- | + | + | + | - |
| Nauclea latifolia | + | - | + | + | + | + | |
| Ongokea goré | - | - | - | + | - | + | - |
| Paropsia brazzeana | - | - | + | + | - | + | - |
| Pentaclethra macrophylla - | - | + | + | - | + | - | |
| Phytolacca dodecandra | - | +/- | + | + | - | + | - |
| Psiduim guajava (L) | - | + | + | + | - | + | - |
| Psidium guajava (Sb) | - | - | + | + | - | + | - |
| Quassia africana | - | - | + | + | - | + | - |
| Rauwolfia vomitoria | + | + | + | + | - | + | - |
| Sida rhombifolia | + | + | + | + | - | + | - |
| Tithonia diversifolia | - | + | + | + | - | + | - |
| Vitex madiensis | - | + | + | + | + | + | - |
| Voacanga africana | + | + | - | + | - | + | - |
Table 3. Amoebicidal activity of extracts from traditional preparations (MAC, µg/ml)
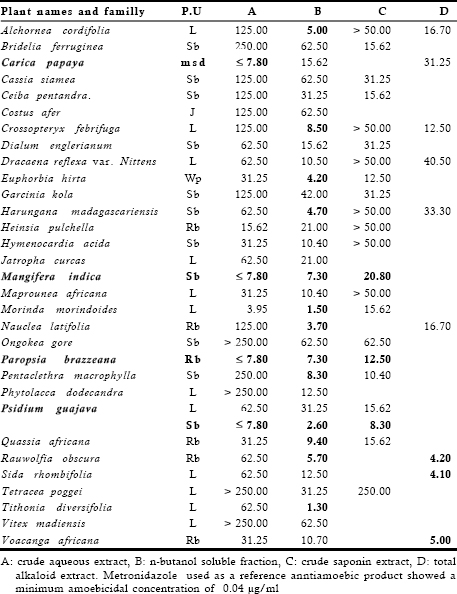
Carica papaya L. (Caricaceae) is a medicinal plant originating from tropical America and is extensively grown for its fruit. Its mature seeds are used in traditional medicine to treat intestinal worms and amoebiasis while other plant parts known various medical indications (Kambu, 1990; Newinger, 2000). In the present study, an aqueous decoction from papaya mature seeds was found to exhibit good antiamoebic activity with MAC < 7.8 µg/ml. The n-butanol soluble fraction from the partition of the decoction and the total alkaloid extract showed moderate and low antiamoebic activity with MAC values of 15.62 and 31.25 µg/ml respectively. Our results are in good agreement with Okeniyi et al. (2007) who have recently reported the effectiveness of an elixir from air-dried C. papaya seeds in the treatment of human parasites (intestinal worms and amoeba) without significant side effects. In addition, a petroleum-ether extract from C. papaya seeds has been reported to produce 100% mortality of Ischthyophthirins multifillis (a fish protozoan) in vitro tests at a concentration of 200 mg/L (Ekanen et al., 2004). Other previous interesting reported biological activities of crude extracts from papaya seeds include the antifertility of a chloroform extract in male albino rats producing a suppression of cauda epidimyal sperm motility without influencing toxicological profile and libido of the animals (Lohiya & Goya, 1992; Lohiya et al., 1999), the reversible azoospermia in langur monkey caused by the administration of the same extract (Lohiya et al., 2002), the antifertility of an aqueous extract in male albino rats characterized by a significant reduced sperm motility associated with morphological effects which were found to return to normal after treatment cessation (Lohiya et al., 1994), but these observations were in disagreement with those reported by the same authors in another study (Lohiya et al., 2000). The aqueous extract was found to alter cauda epididymal microenvironment (Verma & Chinoy, 2001), to produce a reversible contraction response of causa epididymal tubules (Verma & Chinoy, 2002), and a contraception in albino rats characterized by the absence of spermatozoa or disruption of spermatozoa clusters in the lumen (Lohiya et al., 2005). The low dose of aqueous extract of C. papaya seeds does not alter the normal development of the foetus in female rats (Oderinde et al., 2002). The extract significantly suppresses cauda epididymal sperm motility coincinding with a decrease in sperm count and viability (Verma et al., 2006). Furthermore, an ethanol extract was reported to produce a dose-dependent inhibition of jejunal contractions which was significanlty irreversible (Adebyi et al., 2005) while an 80% ethanol extract caused a concentration-dependent tocolysis in uterine strips of female rats (Adebiyi et al., 2003). In general, the bioactive ingredient of C. papaya seeds is benzyl isothiocyanate (Kermanshal et al., 2001; Wilson et al., 2002; Adebiyi et al., 2003, 2005; Nakamura et al., 2007).
Mangifera indica L. (Anacardiaceae) is a plant growing in tropical and subtropical regions. Its parts (fruit, leaf, root bark and stem bark) are commonly used in folk medicine for a wide variety of remedies, especially the stem bark is used as an aqueous decoction to treat diarrhoea and amoebic dysentery (Kambu, 1990; Neuwinger, 2000). In the present study, the crude aqueous extract (decoction) and its n-butanol soluble fraction containing polyphenolic compounds as major constituents were found to be more effective against Entamoeba histolytica growth with MAC values of < 7.8 and 7.3 µg/ml respectively. The crude saponin extract exhibited moderate antiamoebic activity with MAC value of 20.8 µg/ml. Previous biological activities on the M. indica stem bark revealed that an aqueous decoction was reported to possess anti-inflammatory, analgesic and hypoglycemic effects (Ojewole, 2005). The xanthone mangiferin, a predominant constituent in the extract has been reported to display a significant antidiabetic, antilipidemic and antiatherogenic properties suggesting its beneficial effect in the treatment of diabetes mellitus associated with hyperlipidemia and related cardiovascular complications (Muruganandom et al., 2005). A soxhlet ethanolic extract was found to exhibit a very low cytotoxic effect against Brin schrimp with LC50 value of 596.9 µ/ml after 24 h of incubation (Padmaja et al., 2002). Interestingly, a standardized aqueous extract named “Vimang” from M. indica stem bark has been reported to possess various pharmacological activities such as antioxidant (Martinez et al., 2000), modulatory of the humoral response in different immunological disorders, (Garcia et al., 2003a), anthelminthic and antiallergic (Garcia et al., 2003b). These pharmacological activities were attributed to mangiferin by these authors. The extract also possessed vascular effects (Beltan et al., 2004), in vitro and in vivo anti-inflammatory activities (Garido et al., 2004), antiallergic properties indicating its beneficial effects in the treatment of allergic disorders for which the contribution of mangiferin was appreciated (Rivera et al., 2006). Vimang has also been reported to have a capacity of protection against serum oxidative stress in elderly human (Pardo-Andreu et al., 2006). Phytochemically, studies performed on M. indica stem bark reported the presence of tannins and flavonoids (Kambu, 1990; Tona et al., 1998), triterpenoids (Anjaneyulu et al., 1994, 1999; Tona et al., 1998), a biflavone amenthoflavone and saponins (Khan et al., 1993; Tona et al., 1998), phenolic acids such as gallic acid, 3,4-dihydroxybenzoic acid; phenolic esters such as gallic acid methylester, gallic acid propylester, benzoic acid propyl ester; flavon-3-ols such as catechin and epicathechin; and the xanthone mangiferin as the major compound (Garrido et al., 2004). On the basis of this chemical composition, the observed antiamoebic activity in the aqueous extract from M. indica stem bark would be due to the presence of epicathechin which was previously reported to exhibit a very pronounced antiamoebic activity in vitro (Calzada et al., 1999, 2005). The contribution of flavonoids, terpenes and other flavan-3-ols could not be neglected because some compounds belonging to these phytochemical groups have been reported to exhibit an in vitro antiamoebic activity at different extents (Calzada et al., 1999, 2001, 2003, 2005; Cimanga et al., 2006).
The genus Paropsia also called Hounea (Passifloraceae) is an old world genus of eleven species among which one is found in Asia and ten in Africa including four on the mainland and six in Madagascar. Of the four mainland species, three occur in Central Africa and the fourth in East-Africa (Brutelet et al, 2003). Paropsia brazzeana Baill. is a medicinal plant for which the root is used to treat gonorrhea, toothache or as a mouthwash agent. The fruit is used against headache and nasal troubles. The root bark is employed as an aqueous decoction for the treatment of diarrhoea and amoebic dysentery (Neuwinger, 2000). Results reported here show that the crude aqueous extract and its n-butanol soluble fraction exhibited good antiamoebic activity with MAC values of £ 7.8 and 7.3 µg/ml respectively. The crude saponin extract was found to be inactive against E histolytica growth when tested at a concentration of 50 µg/ml. Previous phytochemical studies of the root bark revealed the presence of steroids and/or triterpenoids, tannins and saponins (Tona et al, 1998). The antiprotozoal activity in the crude extract may be due to the presence of some polyphenol compounds since the total crude polyphenol soluble fraction showed a higher activity than the crude saponin extracts. But, the influence of terpenes for the manifestation of the activity could not be neglected for the same reasons evoked above.
The leaves and stem of Psidium guajava L. (Myrtaceae) are widely used in traditional medicine to treat various diseases. These two plant parts are especially used as aqueous decoction to treat diarrhea and amoebic dysentery, other plant parts also known various medical indications in traditional medicine (Kambu, 1990; Neuwinger, 2000). In the present biological investigation, the crude aqueous extract from the leaves and its n-butanol soluble fraction were found to exhibit low antimoebic activity (30 < MAC < 62.5) while its saponin extract showed moderate activity (10 < MAC < 30 mg/ml). On the other hand, the crude aqueous extract from the stem bark, its n-butanol soluble fraction and crude saponin extract were more effective against E histolytica growth with MAC values of < 7.8, 2.6 and 8.3 µg/ml respectively. Some reported biological activities of the stem bark of guajava revealed that an aqueous decoction from this plant part has been found to exhibit an antiplasmo dial activity in vitro against the chloroquine-sensitive D10 strain of Plasmodium falciparum with IC50 values from 10 to 20 µg/ml (Nundkumar & Ojewole, 2002). An ethanol extract has been reported to possess an antidiabetic activity (Muktar et al, 2006). Previous chemical analysis of the stem bark indicated the presence of tannins, terpenes and/or steroids, saponins (Tona et al, 1998; Nundkumar & Ojewole, 2002) and various phenolic compounds (Kambu, 1990). The observed antiprotozoal activity in the crude aqueous extract may be due to the presence of polyphenolic and terpenic compounds because some compounds belonging to these phytochemical groups have been reported to possess a potential capacity of inhibition E histolytica growth at different extents (Calzada et al, 1999, 2005; Nundkumar & Oljewole, 2002; Cimanga et al, 2006). Anyway, the antidiarrhoeal activity of P. guajava leaves is well documented and is attributed to the spasmolytic properties of quercetin and its glycoside derivatives (Luzoya et al, 1994; Morales et al, 1994).
Beside this group, an other group of selected and tested plant species include those for which one of the soluble fractions from the partition of the parent extract showed a higher activity than the initial extract. It concerns firstly the n-butanol soluble fractions from Alchornea cordifolia leaves, Crossopteryx febrifuga leaves, Euphorbia hirta whole plant, Harungana madagascariensis stem bark, Morinda morindoides leaves, Nauclea latifolia root bark, Pentaclethra macrophylla stem bark, Quassia africana root bark and Rauwolfia obsura root bark found to exhibit very pronounced or good antiamoebic activity with MAC values ranging from 1 to 9 µg/ml. Secondly, crude saponin extracts from Bridelia ferruginea stem bark, Cassia siamea stem bark, Ceiba pentandra stem bark, Dialum englerianum stem bark, Euphorbia hirta whole plant, Garcinia kola stem bark, Ongokea goré stem bark, Pentaclethra macrophylla stem bark, Psidium guajava leaves and Quassia africana root bark displaying moderate or low antiamoebic activity with MAC values ranging from 10 to 32 µg/ml. Thirdly, the total alkaloid extracts of R. obscura, Sida rhombifolia leaves and Voacanga africana displayed very pronounced antiamobeic with MAC values ranging from 4 to 5 µg/ml than their respective parent extracts. Except those which were found inactive, other n-butanol soluble fractions, crude saponin and total alkaloid extracts showed moderate or low activity with MAC values ranging from 10 to 62.5 mg/ml, but higher than that of their respective parent extracts according to the case (Table 3). In general, our results are in good agreement with those previously reported by Tona et al. (1999) for the antiamoebic activity of some selected plant extracts suggesting that the collection time has not a significant influence for the manifestation of the activity or with Moundipa et al. (2005) for the same biological activity of crude extracts from some selected plant species collected in other African countries and studied here.
Among these plant species, an extensive investigation was previously carried out on the n-butanol soluble fraction from the partition of the 80% MeOH extract of M. morindoides leaves leading to the isolation and identification of a series of flavonoids and iridoids with an appreciable antiamoebic activity in vitro at different extents. The most active constituents were found to be iridoids exhibiting an in vitro antiamoebic activity with MAC values < 10 µg/ml (Cimanga et al., 2006).
Interestingly, it is important to point out that all selected medicinal plant species studied in the present investigation were previously reported to possess a wide antibacterial spectrum against various microorganisms including enteropathogenic bacteria and spasmolytic properties at different extents (Tona et al., 1999). These biological activities (antibacterial, antiamoebic and spasmolytic) can at least in part justify and support their use as antidiarrhoeal remedies since the cause of diarrhoea is often unknown because of the lacking of an adequate diagnosis in traditional medicine.
However, the previously reported antiprotozoal properties (antiplasmodial, antitrypanosomal and antileishmanial) of some selected medicinal plants such A. cordifolia, C. febrifuga, E. hirta, H. acida, M. indica, N. latifolia and Q. africana highlight their potentiality as potential sources of antiprotozoal agents according to the case (Banzouzi et al., 2002; Le Tran et al., 2003; Atindehou et al., 2004; Eufroye et al., 2004; Hoet et al., 2004; Tona et al., 2004; Zirihi et al., 2005; Mbatchi et al., 2006; Ménan et al., 2006).
Conclusions
On the basis of the present results obtained in our experimental conditions, it was speculated that polyphenolic and alkaloids constituents may be considered as responsible for the observed antiamoebic activity in some crude aqueous extracts tested in the present study since different compounds belonging to these phytochemical groups and isolated from other plant species have been reported to exhibit an antiamoebic activity at different extents (Calzada et al., 1999, 2001, 2005; Wright et al., 1991, 2000; Cimanga et al., 2006). These results can partly justify and support the use of these medicinal plant species for the treatment of amoebiasis and particularly diarrhoea in traditional medicine since the cause of this disease is often unknown. The four promising medicinal plants C. papaya seeds, M. indica stem bark, P. brazzeana root bark and P. guajava stem bark, other more active n-butanol soluble fractions and total alkaloid extracts from other selected plant species were selected for the isolation and structure elucidation of active principles in further studies.
References
Adebiyi, A., Ganesan Adaikan, P. and Prasad, R. 2003. Tocolytic and toxic activity of papaya seed extract on isolated rat uterus. Life Sci. 74(5): 581-592.
Adebiyi, A. and Adaikan, P.G. 2005. Modulation of jejumal contractions by extract of Carica papaya L. seeds. Phytother. Res. 15(7): 628-632.
Anjaneyulu, V., Suresh Badu, J. and Counolly, J.D. 1994. 29-Hydroxymangiferone acid from Mangifera indica. Phytochemistry 35(5): 1301-1303.
Anjaneyulu, V., Satyanarayana, P., Vieswanadham, K.N., Jyothi, V.G., Nageswara Rao, K. and Radhika, P. 1999.
Triterpenoids from Mangifera indica. Phytochemistry 50(7): 1229-1236.
Atindehou, K.K., Sshimid, C., Brun, R., Kone, M.W. and Traor, D. 2004. Antitrypanosomal and antiplasmodial activity of medicinal plants from Cote d’Ivoire. J. Ethnopharmacol. 90(2-3): 221-227.
Banzouzi, J.T., Prado, R., Menan, H., Valentin, A., Roumestan, C., Mallie, M., Pelissier, Y. and Blache, Y. 2002. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent : Ellargic acid. J. Ethnopharmacol. 81(3): 399-401.
Beltan, A.E., Alvarez, Y., Xavier, F.E., Hermanz, R., Rodriguez, J., Nunez, A.J., Alonso, M.J. and Salaices, M. 2004. Vascular effects of the Mangifera indica L. extract (Vimang), Eur. J. Pharmacol. 499(3): 297-305.
Brettler, F.J. 2003. Novitales Gabonenses 48. A new species of Paropsia (Passifloraceae) from Gabon, Adansonia Ser. 3(25): 1-7.
Calzada, F., Meckes, M. and Cedillo-Rivera, R. 1999. Antiamoebic and antigiardial activity of plant flavonoids. Planta Med. 65(1): 78-80.
Calzada, F., Cedilla-Rivera, K. and Mata, R. 2001. Antiprotozoal activity of the constituents of Conyza filaginoides. J. Nat. Prod. 64(5): 671-673.
Calzada, F., Velazquez, C., Cedillo-Rivera, R. and Esquivet, B. 2003. Antiprotozoal activity of the constituents of Teloxys graveolens. Phytother. Res. 17(7): 731-732.
Calzada, F., Cevantes-Martine, J.A. and Yepez-Mulia, L. 2005. In vitro antiprotozoal activity from the roots of Geranium mexicanum and its constituents on Entamoeba histolytica and Giardia lamblia. J. Ethnopharmacol. 98(1-2): 191-193.
Calzada, F. 2005. Additional antiprotozoal constituents from Cuphes pinetorum, a plant used in Mayan traditional medicine to treat diarrhoea. Phytother. Res. 19(8): 725-727.
Calzada , F., Yépez-Mulia, L. and Aguilar, A. 2006. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J. Ethnopharmacol. 108(3): 367-370.
Calzada, F. and Alanis, A.D. 2007. Additional antiprotozoal flavonol glycosides of the aerial parts of Helianthemum glomeratum. Phytother. Res. 21(1): 78-80.
Cimanga, K.R., Kambu, K., Tona, L., Hermans, N., Apers, S., Totte, J., Pieters, L. and Vlientick, A.J. 2006. Cytotoxic and in vitro susceptibility of Entamoeba histolytica to Morinda morindoides leaf extracts and its isolated constituents. J. Ethnopharmacol. 107(1): 83-90.
Di Stasi., L.C. 1995: Amoebicidal compounds from medicinal plants, Parasitologia 37(1): 29-39.
Ekamen, A.P. et al., 2004. Effect of crude extracts of Macuna purian (Fabaceae) and Carica papaya (Caricaceae) against the protozoan fish parasite Ichthlophthirius multifiliis. Parasitol. Res. 92(5): 361-366.
Eufioye, T.O. and Agebahunsi, J.M. 2004. Antiplasmodial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J. Ethnopharmacol. 93(1): 167-171.
Garcia, D., Leiro, J., Delgado, R., Sanmartin, M.L. and Ubeira, F.M. 2003a. Mangifera indica L. extract (Vimang) and mangiferin modulate mouse humoral immune response. Phytother. Res. 17(10): 1182-1187.
Garcia, D., Escalante, M., Delgado, R., Ubeira, F.M. and Leiro, J. 2003b: Anthelminthic and antiallergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother. Res. 17(10): 1203-1208.
Garrido, G., Gonzalez, D., Lemus, Y., Garcia, D., Lodeiro, L., Quintero, G., Delporte C., Nunez-Selles, A.J. and Delgado, R. 2004. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (Vimang). Pharmacol. Res. 50 (2): 143-149.
Harborne, J.B. 1984. Phytochemical Methods. A Guide to Modern techniques of Plant Analysis. Second Edition. Chapman and Hall, p. 192.
Hoet, S., Oppredoes, F., Brun, R., Adjakidje, K. and Quetin-Leclerq, J. 2004. In vitro antitrypanosomal activity of ethnobotanically selected Beninese plants. J. Ethnopharmacol. 91(1): 37-42.
Kambu, K. 1990. Eléments de Phytothérapie Comparée. Plantes Médicinales Africaines. CRP-Kinshasa, pp. 53-55; 74-76.
Kemanshal, R. et al., 2001. Benzyl isothiocyanate is the chief or sole anthelmintic in papaya seed extracts. Phytochemistry 57(3): 427-435.
Khan, M.A., Nizami, S.S., Khan, I.M.N. and Azeem, S.W. 1992. Biflavone from Mangifera indica. Pask. J. Pharm. Sci. 5(2): 155-159.
Khan, M.N., Nizami, S.S., Khan, M.A. and Ahmed, Z. 1993. New saponins from Mangifera indica. J. Nat. Prod. 56(5): 767-770.
Le Tran, Q., Tezuka, Y., Ueda, J.Y., Ngunyen, N.T., Muruyama, Y., Begum, K., Kim, H.S., Wataya, Y., Tran, Q.K. and Kadoto, S. 2003. In vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. J. Ethnopharmacol. 86 (2-3): 249-252.
Lohiya, N.K. and Goyal, R.B. 1992. Antifertility on the crude chloroform extract of Carica papaya Linn. seeds in male albino rats. Indian J. Exp. Biol. 30(11): 1051-1055.
Lohiya, N.K., Goyal, R.B., Jayaprakash, D., Ansari, A.S. and Sharma, S. 1994. Antifertility effects of aqueous extract of Carica papaya seeds in male rats. Planta Med. 60(5): 400-404.
Lohiya, N.K., Pathak, N., Mishra, P.K. and Manivannan, B. 1999. Reversible contraction with chloroform extract of Carica papaya Linn. seeds in male rabbits. Reprod. Toxicol. 13(1): 59-66.
Lohiya, N.K., Pathak, N., Mishra, P.K. and Manivannan, B. 2000. Contraceptive evaluation and toxicological study of aqueous extract of the seeds of Carica papaya in male rabits. J. Ethnopharmacol. 70(1): 17-27.
Lohiya, N.K., Manivannan, B., Mishra, P.K., Pathak, N., Sriram, S., Shande, S.S. and Panneerdoss, S. 2002. Chloroform extract of Carica papaya seeds induces long-term reversible azoospermi in langur monkey. Asian J. Androl. 4(1): 17-26.
Lohiya, N.K. et al., 2005. Efficacy trial of the purified compounds of the seeds of Carica papaya for male contraception in albino rat. Reprod. Toxicol. 20(1): 135-148.
Luzoya, X., Meckes, M., Abou-Zaid, M., Tortoriello, J., Nozzolillo, C. and Arnason, J.J. 1994. Quercetin glycosides in Psidium guajava L. leaves and determination of spasmolytic principles. Arch. Med. Res. 25(1): 11-15.
Martinez, G., Delgado, R., Perez, G., Garrido, G., Nunez Selles, A.J. and Leon, O.S. 2000. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother. Res. 14(6): 424-427 Mbatchi, S.F., Mbatchi, B., Banzouzi, J.T., Bansimba, T., Nsomde Ntandou, G.F., Ouamba, J.M., Berry, A. and Benoit-Vical, F. 2006. In vitro antiplasmodial activity of 18 plants used in Congo Brazzaville traditional medicine. J. Ethnopharmacol. 104(1-2): 168-174.
Menan, H., Banzouzi, J.T., Hoequette, A., Pélissier, Y., Blache, Y., Koné, M., Mallié, M., Ake, Assi, L. and Valentin, A. 2006. Antiplasmodial and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J. Ethnopharmacol. 105(1-2): 131-136.
Morales, M.A. et al., 1994. Calcium-antagonist effect of quercetin and its relation with the spasmolytic properties of Psidium guajava L. Arch. Med. Res. 25(1): 17-21.
Moundipa, P.F., Flore, K.G.M., Bilong Bilong, C.F. and Bruchhaus, I. 2005. In vitro amoebicidal activity of some medicinal plants of the Bamun region (Cameroon). Afr. J. Traditional 2(2): 113-121.
Mukhtar, H.M., Ansari, S.H., Bhat, Z.A., Naved, T. and Slunhg, P. 2006. Antidiabetic activity of an ethanol extract obtained from the stem bark of Psidium guajava (Myrtaceae). Pharmazie 61(8): 725-727.
Murugamandan, S., Srinivasan, K., Gupta, S., Gupta, P.K. and Lal, J. 2005. Effect of mangiferin on hypoglycemia and atherogenicity in streptozoccin diabetic rats. J. Ethnopharmacol. 97(3): 497-501.
Nakamura, Y., Yoshimoto, M., Shimoishi, Y., Asai, Y., Park, E.Y., Salo, K. and Nakamura, Y. 2007. Papaya seed represents a rich source of biologically active isothiocyanate. J. Agric. Food Chem. 55(11): 4407-4413.
Neuwinger, H.D. 2002. African Traditional Medicine. A Dictionary of Plant Uses and Application. Medipharm Scientific Publishers, Stuttgart, p. 318, 379, 421-422.
Nundkumar, N. and Ojewole, J.A. 2002. Studies on the antiplasmodial properties of some South African medidinal plants used as antimalarial remedies in Zulu folk medicine. Methods Find. Exp. Clin. Pharmacol. 24(7): 397-401.
Oderinde, O., Noronha, C., Oremosu, A., Kusemiju, T. and Okanlawon, O.A. 2002. Abortifacient properties of aqueous extract of Carica papaya (Linn) seeds on female Sprague-Dawley rats. Niger. Postgrad. Med. J. 9(2): 95-98. 224 RPMP Vol. 25 - Chemistry and Medicinal Value Okeniyi, J.A. et al., 2007. Effectiveness of dried Carica papaya seeds against human intestinal parasitosis: a pilot study. J. Nat. Prod. 10(1): 194-196.
Ojewole, J.A. 2005. Antiinflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anacardiaceae) stem bark aqueous extract. Methods Find. Exp. Clin. Pharmacol. 27(8): 547-554.
Padmaja, R., Arun, P.C., Prashonth, D., Deepak, M., Arnit, A. and Anjana, M. 2002. Brine shrimp lethality bioassay of selected Indian medicinal plants. Fitoterapia 73(6): 508-510.
Pardo-Andreu, G.L. et al., 2006. Mangifera indica L (Vimang) protection against serum oxidative stress in elderly humans. Arch. Med. Res. 37(1): 158-164.
Rivera, D.G., Balmaseda, I.H., Leon, A.A., Hernandez, B.G., Montiel, L.M., Garrido, G.G., Guzzoeres, S. and Hernandez, R.H. 2006. Anti-allergic properties of Mangifera indica L. extract (Vimang) and contribution of its glucosylxanthone mangiferin. J. Pharm. Pharmacol. 58(3): 385-392.
Sharma, P. and Sharma, J.D. 2001 A review of plant species assessed in vitro for antiamoebic activity or both antiamoebic and antiplasmodial properties. Phytother. Res.15(1): 1-17.
Swetetman, S.C. 2002. Martindale. The complete drug reference. Pharmaceutical Press. London, pp. 594-597.
Tona, L., Kambu, K., Ngimbi, N., Cimanga, K. and Vlietinck, A.J. 1998. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 61(1): 57-65.
Tona, L., Kambu, K., Mesia, K., Cimanga, K., Apers, S., De Bruyne, T., Pieters, L., Totté, J. and Vlietinck, A.J. 1999. Biological screening of traditional preparations from some medicinal plants used as antidiarrhoeal in Kinshasa, Congo. Phytomedicine 6(1): 59-66.
Tona, L., Cimanga, K.R., Mesia, K.G., Musuamba, T.C., De Bruyne, T., Apers, S., Hermans, N., Van Miert, S., Pieters, L., Totté, J. and Vlietinck, A.J. 2004. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J. Ethnopharmacol. 92(1): 27-32.
Verma, R.J. and Chinoy, N.J. 2001. Effect of papaya seed on microenvironment of cauda epididymis. Asian J. Androl. 3(2): 143-146.
Verma, R.J. et al., 2002. Effect of papaya seed extract on contractile response of cauda epididymal tubules. Asian J. Androl. 4(1): 77-78.
Wagner, B. and Bladt, S. 1996. Plant Drugs Analaysis: A Thin Layer Chromatography Atlas. Second Edition, Springer. Berlin. pp. 306-364.
Wilson, R.K. et al., 2002. Effect of papaya seed extract and benzyl isocyanate on vascular contraction. Life Sci. 71(5): 497-507.
Wright, C.W., Marshall, S.J., Russell, P.P., Anderson, M.M., Phillipson, J.D., Kirby, G.C., Warhurst, D.C. and Schiff, P.L. 2000. In vitro antiplasmodial, antiamoebic and cytotoxic activities of some monomeric isoquinoline alkaloids. J. Nat. Prod. 63(12): 1638-1640.
Wright, C.W., Bray, D.H., O’Neil, J.D., Quetin-Leclercq, J. and Angenot, L. 1991. Antiamoebic and antiplasmodial activities of alkaloids isolated from Stychnos usambarensis. Planta Med. 57(3): 337-340.
Zirihi, G.N., Mambu, L., Guéde-Guina, F., Bodo, B. and Grellier, P. 2005. In vitro antilpasmodial activity and cytotoxicity of 33 West African plants used for the treatment of malaria. J. Ethnopharmacol. 98(3): 281-285.