3 : Stimulating Effect of the Plant Extract Complex “AdMax” on Expression Level of the Gene Involved in Energy Metabolism in Cultured Human Fibroblasts
Abstract
Ethanol/water extracts from roots of Leuzea carthamoides, Rhodiola rosea, Eleutherococcus senticosus and fruits of Schizandra chinensis are known as adaptogenic remedies, which enhance physical endurance and counteract fatigue. The purpose of this study was examination of a complex of these four dry extracts (AdMaxTM, Nulab, FL, USA) for its ability to influence expression level of the genes involved in processes of energy metabolism in cultured human fibroblasts. Screening of gene expression level of whole genome was carried out by using Affymetrix oligonucleotide microarrays. We found that AdMax treatment results in significant (2.3 fold, p < 0.05) enhancing expression of the PANK2 gene. PANK2 encodes a mitochondrial enzyme pantothenate kinase 2, which activates coenzyme A (CoA) biosynthesis and thus plays a key role in energy metabolism.
Key words: Gene expression, Gene PANK2, Energy metabolism, Leuzea carthamoides, Eleutherococcus senticosus, Schizandra chinensis
1. Genext Research, Inc., Clearwater, Florida 33765, USA.
2. Adaptogenic Medical Center, Clearwater, Florida 33755, USA.
3. Central Clinical Hospital, Moscow 121359, Russian Federation.
4. N.N. Blokhin Cancer Research, Center of Russian Academy of Medical Sciences, Moscow 115478, Russian Federation.
* Corresponding author : E-mail: antoshechkin@earthlink.net
Introduction
Leuzea carthamoides (Iljin, Asteraceae), Rhodiola rosea (L., Crassulaceae), Eleutherococcus senticosus (Maxim, Araliaceae) and Schizandra chinensis (Baill, Magnoliaceae) are known as adaptogenic plants, which promote adaptation of the body to intensive and regular exercises (Walker, 1994; Antoshechkin, 2005). Ethanol/water extracts from these plants restore physical power and improve endurance exercise performance (Asano et al., 1986; Azizov & Sefulla, 1998; De Bock et al., 2004).
Biologically active compounds of these plants possess lower effectiveness when isolated from the plant extract in comparison with the effectiveness of the total extract indicating synergistic action of the extract’s components (Steinmann et al., 2001; Antoshechkin et al., 2007). Probably, some constituents of the plant extract act as adjuvant compounds, which enhance the activity of the compounds actually responsible for the biological effect (Gilbert & Alves, 2003).
Molecular mechanisms underling stimulating effects of the four plants are poorly understood. It has been suggested that active compounds of an extract may interact with cell membrane-localized members of various signaling pathways resulting in specific changes of gene expression (Lafont & Dinan, 2003). To test this directly, we examined the ability of the complex of dry ethanol/water extracts from the four adaptogenic plants to influence expression levels of the genes involved in processes of energy metabolism in cultured human fibroblasts.
Materials and Methods
The extract complex “AdMax”
AdMaxTM is a combination of lyophilized ethanol/water extracts from dry roots of Leuzea carthamoides, Rhodiola rosea, Eleutherococcus senticosus, and from dry berries of Schizandra chinensis. The preparation is manufactured in the form of encapsulated powder by Nulab, Inc., Clearwater, FL 33765, USA. For AdMax standardization 70% ethanol/water extract of AdMax was analyzed by means of reverse phase gradient C 18 HPLC (Agilent 1100, Santa Clara, CA, USA) coupled with ESI-MS (ThermoFinnigan LCQ, Clayton, NC, USA). The obtained chromatogram was used as AdMax fingerprint.
Cell culture and total RNA isolation
Human fibroblast cell line MRC-5 (Cornell Cell Repositories, Camden, NJ, USA) was grown under standard conditions in Minimum Essential Medium (MEM, Gibco, Carlsbad, CA, USA) supplemented with 10% of fetal bovine serum (Gibco) at 37 oC. When cells were close to confluence, culture medium was replaced with the medium containing AdMax at concentration of 3 µg/ ml and cells were grown for additional 16 hours. Control cells were grown in parallel in identical medium without AdMax. Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following manufacturer’s instructions. Control and experimental cell cultures were grown in triplicates and processed independently producing six RNA samples, each of which was analyzed on an individual microarray chip.
Microarray analysis and data processing
Microarray analysis was performed in accordance with standard procedures recommended by Affymetrix, Inc (Santa Clara, CA, USA) using Affymetrix Gene Chip Human Genome U133 Plus 2.0 arrays. These microarray chips allow to register expression level of more than 38,500 genes. Following hybridization and scanning, raw data in the form of image files were converted to gene expression values using Affymetrix Gene Chip Operating Software (GCOS), which utilizes MAS 5.0 algorithm for data normalization, background subtraction, estimation of nonspecific binding, calculation of detection p-values, and generation of “presence” calls. Two-tailed Student’s t-test assuming unequal sample variance was used to identify genes that displayed significant changes in the mean expression levels between control and treated samples of at least 2 fold with the t-test p-value less then 0.05. Only probes that were called “present” in at least two samples were considered in the analysis.
Results and Discussion
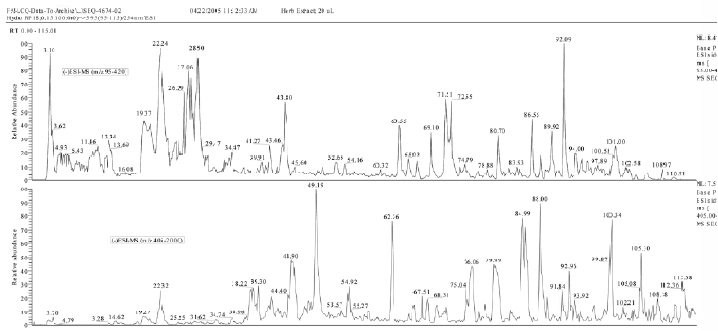
Results of HPLC/ESI-MS analysis of AdMax composition are presented in Fig 1. The chromatogram contains more than 80 prominent peaks, each of which characterizes the amount of corresponding compound in AdMax. The chromatogram represents AdMax composition fingerprint (Fig 1).
Microarray analysis identified 12 genes that increased more than 2 fold (p < 0.05) their expression levels upon treatment of the fibroblasts with AdMax vs. control cells. Symbols of these genes are: IGHG1, CCDC15, COG6, MYST3, SNF8, PANK2, PAN3, HMCN1, PRCP, FLJ13236, FLJ32786 and LOC646590. Of the genes listed above, PANK2 is especially interesting for the understanding of mechanisms of the stimulating effects of AdMax since the other 11 genes are not directly involved in the energy supply of cellular metabolism.
Expression of PANK2 increases 2.3 fold (P=0.004) upon treatment with AdMax. PANK2 encodes a mitochondrial enzyme pantothenate kinase 2, which activates coenzyme A (CoA) biosynthesis. CoA and its acylated form (acetyl-CoA) play a key role in energy metabolism. Decrease in acetyl-CoA formation in the cells of the body leads to fatigue and decline of physical performance. Activation of CoA biosynthesis by AdMax is likely to counteract these manifestations of energy insufficiency. Deficiency of pantothenate kinase activity is also associated with neurodegeneration (Westaway et al., 2006), which is probably caused by energy insufficiency in neurons as well.
Gene expression analyses generate important data that can be used to elucidate biological activity of pharmaceutical drugs and promote our understanding of molecular mechanisms of their action (Breitling, 2006). The use of microarray technology is especially important for objective registration of bioactivity of herbal preparations and evaluation of their effects on health. It has recently been suggested that gene expression analyses can be used for development of a screening system to determine the value of natural medicinal products (Katz et al., 2006).

Fig 1. HPLC/ESI-MS registered base peak ion chromatogram of AdMax composition upon negative (-) ionization with two scan ranges(m/z 95-420 and 405-2000)
Acknowledgements
The work was carried out in cooperation with Microarray Facility of Pennsylvania University, PA, USA.
References
Antoshechkin, A. 2005. Adaptogens and Health Care. Authorhouse, Bloomington, IN, USA. p. 81.
Antoshechkin, A., Olalde, J., Magarici, M., Muhammad, A., Salom, A., Suares, J. and Amendola, F. 2007. Analysis of effects of the herbal preparation circulat on gene expression levels in cultured human fibroblasts. Phytother. Res. 21(8): 777-789.
Asano, K., Takahashi, T., Miyashita, M., Matsuzaka, A., Muramatsu, S., Kuboyama, M., Kugo, H. and Imai, J. 1986. Effect of Eleutherococcus senticosus extract on human physical working capacity. Planta Med. 52(3): 175-177.
Azizov, A.P. and Sefulla, R.D. 1998. The effect of elton, leveton, fitoton and adapton on the work capacity of experimental animals. Eksp. Klin. Farmacol. 61(3): 61-63.
Breitling, R. 2006. Biological microarray interpretation: the rules of engagement, Biochim. Biophys. Acta 1759(7): 319-327.
De Bock, K., Eijnde, B.O., Ramaekers, M. and Hespel, P. 2004. Acute Rhodiola rosea intake can improve endurance exercise performance. Int. J. Sport Nutr. Exerc. Metab. 14(3): 298-307.
Gilbert, B. and Alves, L.F. 2003. Synergy in plant medicines. Curr. Med. Chem. 10(1): 13-20.
Katz, S., Harris, R., Lau, J.T. and Chau, A. 2006. The use of gene expression analysis and proteomic database in the development of a screening system to determine the value of natural medical products. Evid. Based Complement. Alternat. Med. 3(1): 65-70.
Lafont, R. and Dinan, L. 2003. Practical uses for ecdysteroids in mammals including humans: an update. J. Insect Sci. 3(3): 7-35.
Steinmann, G.G., Esperester, A. and Joller, P. 2001. Immunopharmacological in vitro effects of Eleutherococcus senticosus extracts. Arzneimittelforschung 51(1): 76-83.
Walker, M. 1994. Adaptogens: nature’s answer to stress. Townsend Lett. Doctors July: 751-755.
Westaway, S.K., Ching, K.H., Levinson, B., Gitschier, J. and Hayflick, S.J. 2006. Gene symbol: PANK2. Disease: pantothenate kinase-associated neurodegeneration. Hum. Genet. 119(6): 671-672.