5 : Modulation in the Hepatocytes by Terminalia belerica Roxb. against Acetaminophen Intoxication
Abstract
Hepatoprotective potential of Terminalia belerica Roxb. against acetaminophen induced acute toxicity in rats was evaluated during this study. Experimental design was used to evaluate the four different doses of T. belerica fruit extract (100, 200, 400 and 800 mg/kg, p.o.) against acetaminophen (APAP, 2.0 g/kg, p.o. once only) induced liver injury in female albino rats. APAP administration caused significant increase in the activities of serum transaminases, alkaline phosphatase and lactate dehydrogenase (p < 0.05). Toxicant caused injury in the parenchymal cells thereby affecting triglycerides, cholesterol, total and direct bilirubin level. A significant decline was observed in total protein content, blood sugar level and glycogen content whereas level of albumin increased during toxicity. Hepatic lipid peroxidation increased significantly on the contrary significant depletion was observed in reduced glutathione level after acetaminophen administration (p < 0.05). Activities of hepatic microsomal drug metabolizing enzymes i.e. aniline hydroxylase and amidopyrine-N-demethylase were inhibited significantly after APAP intoxication. Single administration of extract after toxicant administration significantly reversed the APAP induced alterations in the liver function tests and drug metabolizing enzymes. All biochemical results indicated that 400 and 800 mg/kg dose of extract were equally effective when analyzed statistically. These findings were further strengthened by recovery seen in the hepatocytes through light and electron microscopic studies. Thus T. belerica extract possessed considerable hepatoprotective efficacy.
Key words: Acetaminophen, Terminalia belerica Roxb., Liver function tests, Drug metabolizing enzymes
1. UNESCO Satellite center of Trace Element Research & Reproductive Biology and Toxicology Laboratory, School of Studies in Zoology, Jiwaji University, Gwalior 474011, M.P., India.
* Corresponding author : E-mail : dr_sshukla@hotmail.com
Introduction
Liver diseases still remain as one of the serious health problems in spite of tremendous strides in the modern medicine. At least 80% of the world’s population in developing countries uses plant materials for primary health care, they are also sources of very potent and powerful drugs (Cordell & Colvard, 2005). Terminalia belerica Roxb. (Family: Combretaceae) commonly known as Bahera in Hindi is a plant, which is not explored extensively. It is used traditionally for the relief of piles, dropsy, dysentery, diarrhoea, leprosy and dyspepsia (Wealth of Asia, 1996). It is present in a significant proportion in an Ayurvedic herbal preparation, Triphala, and is consumed by majority of Indian population for digestive disorders. The fruits are bitter, astringent, tonic, laxative and have antipyretic activity (Chopra et al., 1996). They contain high quantity of polyphenolic compounds, which are effective antioxidants, and also defenses against stresses and tissue injuries. Preliminary investigation clearly reveals hepatoprotective effects against CCl4 induced toxicity (Jadon et al., 2005). So far no scientific data have been published against acetaminophen poisoning which involves phase II conjugation reactions and cytochrome P450 system. Thus we have evaluated the efficacy of Terminalia belerica (TB) at different dose levels against acetaminophen induced liver injury.
Materials and Methods
Preparation of the extract
Fruits of Terminalia belerica (TB) were procured from authenticated Ayurvedic dealer and were identified (voucher specimen no. 336 Acronym JUG) by the taxonomist, Botany Department, Jiwaji University, Gwalior. Fruits were dried in the shade, chopped and series of extraction was conducted to obtain ethanolic extract (17.6% yield w/w). An aqueous suspension was prepared in 2% gum acacia and different doses of extract (100, 200, 400 and 800 mg/kg, p.o.) were given to the animals. Silybum marianum (Silymarin, 50 mg/kg, p.o.) was used as a reference drug in this experiment.
Experimental animals and chemicals
Female albino rats of Sprague Dawley strain (8-10 weeks old having 130 + 10 g b.w.) were used for this study. They were maintained under standard husbandry conditions (24 + 2 °C temperature, 60-70% relative humidity and 12 h photoperiod) and were fed on pellet diet (Pranav Agro Industries, New Delhi, India) having metal contents in ppm dry weight Cu 10; Mn 33; Zn 45; Co 5 and water ad libitum. Animals were treated and cared for in accordance with the guidelines recommended by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, Ministry of Culture, Chennai, India. All chemicals used in this study were of analytical grade and were obtained from Sigma-Aldrich, E-Merck and Loba Chemicals Pvt. Ltd. etc. All diagnostic kits were procured from E-Merck.
Hepatotoxicant
Acetaminophen (2.0 g/kg, p.o. once only) (Chattopadhyay et al., 1992).
Study design
Animals were divided into seven groups of six animals each and were treated as follows:
| Group 1 | : | Normal (vehicle only) |
| Group 2 | : | Experimental control (APAP: 2.0 g/kg, p.o. once only) |
| Groups 3-6 | : | APAP + TB Extract (100, 200, 400 & 800 mg/kg, p.o.) |
| Group 7 | : | APAP + Silymarin (50 mg/kg, p.o.) |
Animals were sacrificed 24 h after therapy of extract. Blood samples were withdrawn from each animal just before the necropsy by puncturing the retro-orbital venous sinus, centrifuged and serum was separated for the estimation of serum transaminases (Reitman & Frankel, 1957), serum alkaline phosphatase (Fiske & Subbarow, 1925), lactate dehydrogenase (Wroblewski & Due, 1955), total protein (Lowry et al., 1952) and blood sugar (Asatoor & King, 1954). Liver of each rat were promptly excised to determine glycogen (Seifter et al., 1950), drug metabolizing enzymes i.e. aniline hydroxylase (Kato & Gillette, 1965) and amidopyrine-N-demethylase (Chochin & Axelord, 1959) were estimated. Hepatic reduced glutathione was estimated using dithionitrobenzoic acid (DTNB) (Brehe & Bruch, 1976). The quantitative measurement of hepatic lipid peroxidation was done by measuring the concentration of thiobarbituric acid reactive substances (TBARS) (Sharma & Krishnamurthy, 1968). Total and direct bilirubin, triglycerides, cholesterol and albumin were also determined (Kit methods, E-Merck) using Autoanalyzer (Microlab, 200).
Histopathological studies
Liver tissues were collected from the large lobe for histopathological study. They were fixed in Bouin’s solution and H&E stained slides were used for photomicroscopic observations. For transmission electron microscopic studies, small pieces (1 mm3) of liver were fixed in 3.2% glutaraldehyde prepared in 0.1 M phosphate buffer and they were kept at 4°C for 18 h. This was followed by washing the tissue with phosphate buffer. Post fixation of the tissue was done with 1% osmium tetraoxide solution. Then the tissues were dehydrated in acetone series and subsequently embedded in epon resin and were polymerised for 20 h at 70°C. Ultrathin sections were cut on a Reichert jung Ultracut-E Microtome, using glass knives. The sections were placed on uncoated grids and stained with uranyl acetate and lead citrate (Hayat & Arif, 1970). They were examined in a JEOL JEM 1200 EX transmission electron microscope at 80 KV.
Statistical analysis
Statistical calculations were carried out with the Graph Pad software package (Statistica, San Jose, USA). Results are expressed as the mean ± S.E.M followed by Student’s ‘t’-test. P value at 5% level was considered as significant. One-way analysis of variance (ANOVA) was done to compare the different experimental groups (Snedecor & Cochran, 1994).
Results
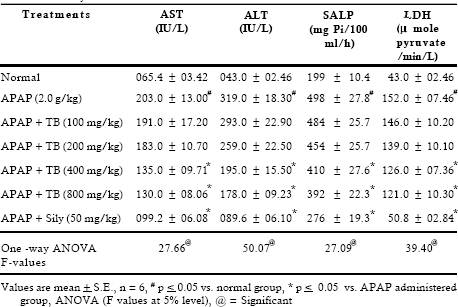
Results of this study represented that four different doses of extract (100, 200, 400 an d 800 m g/kg, p.o.) reversed in varying degree the changes induced by acetaminophen (2.0 g/kg, p.o.). AST, ALT, SALP and LDH activities were increased 3.1, 7.4, 2.5 and 3.5 fold, respectively in the toxicant exposed rats. Treatment with extract at different doses showed effectiveness in a dose dependent manner. It was interesting to note that both the doses 400 and 800 mg/kg showed almost same degree of recoupment (Table 1). Hypercholesterolaemia, hyperbilirubineamia and steatosis often arised in biliary obstruction and reached a very high level in APAP induced toxicity. Treatment with 400 and 800 mg/kg doses of extract showed significant (p < 0.05) inhibition in cholesterol, triglycerides and direct bilirubin level whereas 100 and 200 mg/kg dose of extract exhibited insignificant effect (Table 2).
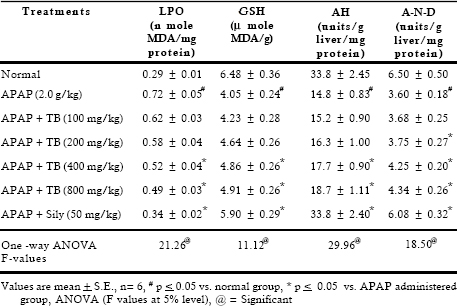
Significant decrease was observed in total protein content, on the other hand albumin level increased after toxicant exposure. Extract therapy (400 and 800 mg/kg) restored depletion in protein synthesis. High bolus dose of acetaminophen caused significant inhibition in blood sugar level (1.48 folds) and glycogen content (2.44 folds). Considerable recovery was seen at 400 and 800 mg/kg doses whereas 100 and 200 mg/kg doses of extract were found to be less effective. Lipid peroxidation is a highly destructive process that induces a plethora of alterations in the structure and function of cellular membrane. Results indicated that APAP accentuated the amount of malonyldialdehyde and decreased the reduced glutathione level. Treatment with extract reversed these parameters at higher doses. Results revealed that APAP caused significant (p < 0.05) inhibition in the activities of drug metabolizing enzymes such as aniline hydroxylase and amidopyrine-N-demethylase. Treatment with extract at 400 and 800 mg/kg after APAP administration showed significant recoupment in these enzymatic activities (Table 4).
Discussion
Liver is the main site of biotransformation of xenobiotic substances, it usually occurs in two phases. It is also one of the primary organs tested in drug safety evaluation. The major target organ of paracetamol poisoning is the liver and the primary lesion is acute centrilobular hepatic necrosis. As acetaminophen poisoning involves Phase II reactions i.e. conjugation of certain endogenous molecules to the products of phase I reaction, it requires bioactivation by cytochrome P450 in order to initiate the events that result in hepatic injury (WHO, 1999). Thus the effectiveness of this therapeutic agent was estimated by various liver function tests, markers of oxidative stress and drug metabolizing enzymes.
Table 1. Effect of TB extract on liver function tests against acetaminophen induced acute toxicity in rats

Table 2. Effect of TB extract on liver function tests against acetaminophen induced acute toxicity in rats
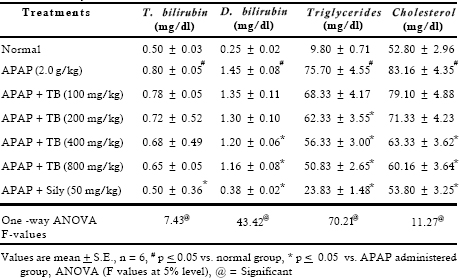
Table 3. Effect of TB extract on liver function tests against acetaminophen induced acute toxicity in rats
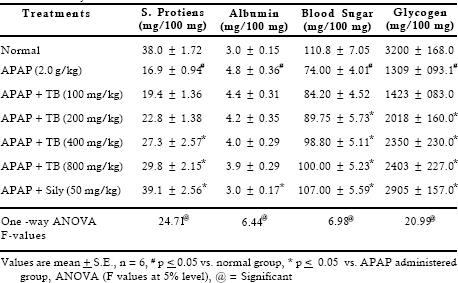
Table 4. Effect of TB extract on liver function tests against acetaminophen induced acutetoxicity in rats
The acute toxicity produced by acetaminophen was associated with a variety of biochemical abnormalities. It caused severe hepatotoxicity as indicated by sharp elevation in the activities of serum transaminases, alkaline phosphatase and lactate dehydrogenase. The elevated activities of AST and ALT in serum are indicative of cellular leakage and loss of the functional integrity of cell membranes in liver. Alkaline phosphatase mainly arises from the lining of the canaliculi and also from the sinusoidal surface of hepatocytes. It is excreted normally via bile by the liver and is involved in active transport across the capillary wall. The increased level of alkaline phosphatase is reliable marker of liver damage, which occurs due to the de novo synthesis by the liver cells (Muriel & Escobar, 2003). The stabilization of these enzymes by the extract is a clear indication of the improvement of the functional status of hepatocytes. Activity of this enzyme rises in many types of liver diseases, highest levels are seen with obstruction to the bile flow, either intrahepatic or extrahepatic. Other plant extracts viz. Phyllanthus niruri (Bhattacharjee & Sil, 2006) and Hemidesmus indicus Rbr. (Baheti, 2006) also help to replenish APAP toxicity. These findings are further corroborated with the histopathological examination by recouping centrilobular necrosis.
The vulnerable effect of APAP on carbohydrate metabolism was observed by decreasing glycogen and blood sugar level. This might be attributed to failure of liver to produce glucose. Liver glycogen phosphorylase acts as the glucose sensor of liver, showing the breakdown of glycogen whenever the level of blood glucose is low. Therapy with extract showed dose dependent recovery in blood sugar and glycogen content which indicated hepatoprotective activity of extract. A high bolus dose of paracetamol results significant loss of the total protein content. It may be due to lipid peroxidation of cell membranes, which results in loss of cytosolic proteins (Yeh et al., 2003). Recovery may be due to promotion of ribosomal assembly to facilitate uninterrupted protein biosynthesis. The cytoplasm of the cell is full of lipid vacuoles that are almost exclusively triglycerides and serve as an energy reservoir. Acetaminophen caused injury in the parenchymal cells by affecting the fat accumulation, particularly triglycerides. Blockage secretion of hepatic triglycerides into the plasma may result in steatosis. Increase in the total and direct bilirubin concentration might be attributed to the failure of normal uptake, conjugation and excretion by the damaged hepatic cells. Thus hyperbilirubinaemia reflects pathophysiology of liver (Klaassen & Watkin, 1984). Treatment with extract also resulted in a decrease of serum bilirubin level. This study also corroborated the earlier reports of the hepatoprotective activity of Trianthema portalacastrum L. (Kumar et al., 2004) and Nymphaea stellata Willd. (Bhandarkar & Khan, 2004).
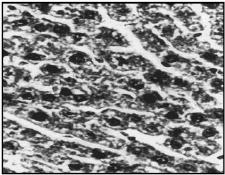
Fig 1. Section of normal rat liver showing normal appearance (X 400)
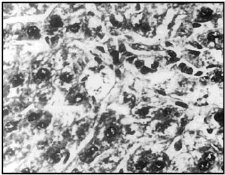
Fig 2. APAP caused swollen hepatocytes, necrosis and fatty accumulation along with hyperchromatic nuclei (X 400)
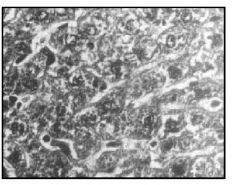
Fig 3. Treatment with TB (400 mg/kg, p.o.) showing cuboidal hepatocytes and clear sinusoidal spaces (cf. 2) (X 400)
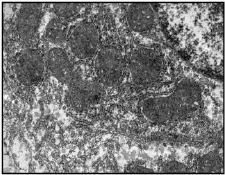
Fig 4. EM of normal rat liver showing well formed nucleus, mitochondria, ER and glycogen particles (X 1100)
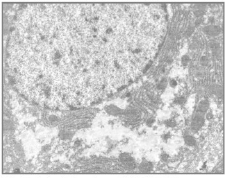
Fig 5. Administration of APAP. Note severe vacuolation and loss of cytoplasmic matrix (X 1100)
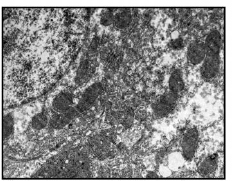
Fig 6. Treatment with TB showing well formed mitochondria along with ER and glycogen particles (X 1100)
LPO mainly arises from damaged kupffer cells. It is a highly destructive process that induces a plethora of alterations in the structure and function of cellular membranes and involves oxidation of fatty acids (Balasubramaniyan et al., 2003). The increased TBARS as seen in the present study is due to the tissue injury and failure of antioxidant defense mechanism, thus the increased accumulation of lipid peroxidation products might well be the consequences of a progressive degradation of necrotic tissue. In addition, toxicants directly inhibit cellular proliferation, to induce oxidative stress and alter Ca 2+ homeostasis (Bosch et al., 2006). APAP induced depletion of hepatic glutathione has been constructed as evidence supporting the hypothesis that reactive oxygen intermediates generated leads to glutathione oxidation. Studies suggest that there exists an inverse relationship between peroxidative decomposition of membrane polyunsaturated fatty acid (PUFA) and GSH levels (Singh et al., 2003). Here the role of TB extract in reversing the elimination of hydrogen peroxide free radicals may be visualized as a form of adaptation on the part of GSH dependent defense system against lipid peroxidation. The antioxidant nature of the extract may also hinder the reactive oxygen species, which are produced by the toxicant.
Liver microsomal enzymes such as amidopyrine-N-demethylase and aniline hydroxylase are cytochrome P450 enzymes. Activities of these enzymes are generally determined in liver microsomes to assess the metabolic potential of drug metabolizing enzymes (DMEs). In the present study the damage inflicted by APAP causes a loss of drug metabolizing capacity of the liver, the microsomes originating from the ER are the most susceptible site. This is substantiated by the earlier findings, which may be due to the damage to the microsomal oxidation system (Singh et al., 2003). Significant recoupment by the therapy was seen. The histopathological examination reveals centrilobular necrosis, damaged hepatic cells, central vein and portal triad were almost normal by the therapy of extract. This suggests modulation in the functional status and accelerated regeneration of parenchyma cells, thus protecting against membrane fragility and decrease of leakage of the markers enzymes into the circulation (Rajesh & Latha, 2004). Electron microscopic study clearly demonstrates degeneration in mitochondria of the hepatocytes. Recoupment may be ascribed to the antioxidative activity of the extract. The mitochondrial damage is prevented when oxidative stress is suppressed. This study is supported by the administration of picroliv (Dwivedi et al., 1990).
In conclusion, the possible mechanisms of hepatoprotective action of TB extract may be due to its antioxidant activity as indicated by decrease in LPO and increase of glutathione contents. Rest of the biochemical and histopathological parameters studied indicate the structural and functional integrity of the cells and provide further support to proposed mechanism of action. Results obtained strongly suggest that chemical ingredient of the extract have something in common that interferes with the generation of reactive metabolites consequent to the inhibition of specific CYP involved in APAP bioactivation, that some components of the extract have membrane stabilizing effects or it may also directly combine with free radicals to form free radical adduct. Thus Terminalia belerica extract showed remarkable recovery as hepatoprotective agent.
Acknowledgements
Authors are thankful to Jiwaji University, Gwalior (M.P.) and ICMR (45/8/ 2003/BMS/TRM) New Delhi, for financial assistance.
References
Asatoor, A.M. and King, E. 1954. Simplified colorimetric blood sugar method. Biochem. J. 44: 56.
Baheti, J.R., Goyal, R.K. and Shah, G.B. 2006. Hepatoprotective activity of Hemidesmus indicus Rbr. in rats. Indian. J. Exp. Biol. 44(5): 399-402.
Balasubramaniyan, V. Sailja, K.J. Nalini, 2003. Role of leptin on alcohol induced oxidative stress in swiss mice. Pharmacol Res. 47: 211-216.
Bhandarkar, M.R. and Khan, A. 2004. Antihepatotoxic effect of Nymphaea stellata Wild. against carbon tetrachloride induced hepatic damage in albino rats. J. Ethnopharmacol. 91: 61-74.
Bhattacharjee, R. and Sil, P.C. 2006. The protein fraction of Phyllanthus niruri plays a protective role against acetaminophen induced hepatic disorder via its antioxidant properties. Phytotherapy Res. 20(7): 595-601.
Bosch, M.E., Sanchez, A.J., Rojas, F.S. and Ojeba, C.B. 2006. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. Anal. 42(3): 291-321.
Brehe, J.E. and Bruch, H.B. 1976. Enzymatic assay for glutathione. Anal Biochem. 74: 189.
Chattopadhyay, R.R., Sarkar, S.K., Ganguly, S., Madda, C. and Baru, T.K. 1992. Hepatoprotective activity of Ocimum sanctum leaf extract against paracetamol induced hepatic damage in rats. Indian J. Pharmacol. 24: 163-165.
Chochin, J. and Axelord, J. 1959. Biochemical and pharmacological changes in the rat following chronic administration of morphine and non-morphine. J. Pharmacol. Exp. Ther. 125: 105-110.
Chopra, R.N., Nayar, S.L. and Chopra, I.C. 1996. Glossary of India Medicinal Plants. CSIR Publication, New Delhi, p. 241.
Cordell, G.A. and Colvard, M.D. 2005. Some thoughts on the future of Ethnopharmacology. J. Ethnopharmacol. 100: 5-14.
Dwivedi, Y., Rastogi, R., Chander, R., Sharma, S.K., Kapoor, N.K., Garg, N.K. and Dhawan, B.N. 1990. Hepatoprotective activity of picroliv against carbon tetrachloride liver damage in rat. Indian J. Medical Res. 92: 195.
Fiske, C.H. and Subbarow, Y. 1925. The colorimetric determination of phosphates. J. Biol. Chem. 66: 375-380.
Hayat, A. and Arif, M. 1970. Biological Applications. In: Principle and Techniques of Electron Microscopy. Van Nostrard Reinhold Company, New York. Jadon, A., Bhadauria, M. and Shukla, S. 2005. 3,4,5-trihydroxybenzoic acid and Terminalia belerica: A hepatoprotective study. Indian Drugs 42 (3): 136-143.
Kato, R. and Gillette, J.R. 1965. Effect of starvation on NADPH dependent enzymes in liver microsomes of male and female rats. J. Pharmacol. Exp. Ther. 150: 279-284.
Klaassen, C.D. and Watkin, J.B. 1984. Mechanism of formation, hepatic uptake and biliary excretion. Pharmacol. Review. 36: 1-67.
Kumar, G., Sharmila, B.G., Vanita, P.P., Sundararajan, M. and Rajsekara, P.M. 2004. Hepatoprotective activity of Trianthema portuacastrum L. against paracetamol and thioacetamide intoxication in albino rats. J. Ethnopharmacol. 92(1): 37-40.
Lowry, O.H., Rosenbrough, N.J., Farr, A.L. and Randall, R.J. 1951. Protein measurement with Folin’s phenol reagent. J. Biol. Chem. 193: 265-269.
Muriel, P. and Escobar, Y. 2003. Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J. Appl. Toxicol. 23: 103-108.
Rajesh, M.G. and Latha, M.S. 2004. Preliminary evaluation of the antihepatotoxic effect of Kamilari, a polyherbal formulation. J. Ethnopharmacol. 91: 99-104.
Reitman, S. and Frankel, S. 1957. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28: 56-63.
Seifter, S., Dayton, S., Novic, B. and Muintwyler, E. 1950. The estimation of glycogen with anthrone reagent. Arch. Biochem. 25: 191-193.
Sharma, S.K. and Krishnamurthy, C.R. 1968. Production of lipid peroxides of brain. J. Neurochem. 15: 147-149.
Singh, R.P., Khanna, R., Kaw, J.L., Khanna, S.K. and Das, M. 2003. Comparative effect of benzanthrone and 3-bromobenzanthrone on hepatic xenobiotics metabolism and antioxidative defense system in guinea pigs. Molecular Toxicol. 77: 94-99.
Snedecor, G.W. and Cochran, W.G. 1994. Statistical Method. Published by Affiliated East West Press Pvt. Ltd., Iowa State University Press, Amsterdam, Iowa (8th edn). pp. 217-236.
Wealth of Asia. 1996. CD-ROM (D-2.3), New Delhi, NISCOM. World Health Organization. 1999. Environmental Health Criteria for carbon tetrachloride (EHC: 208). pp. 54-55.
Wroblewski and Due, L. 1955. Colorimetric method for LDH. In: Micro-analysis in Medical Biochemistry. Wootton, I.D.P. (eds), J & A. Churchill Ltd., 104 Gloucester Place, (4th edn). pp. 115-118.
Yeh, M.L, Liu., C.F, Huang, C.L. and Huang, T.C. 2003. Hepatoprotective effect of Angelica archangelica in chronically ethanol treated mice. Pharmacol. 68: 70-73.