7 : In vitro Antisickling Activity of Anthocyanins Extracts of Vigna unguiculata (L.) Walp.
Abstract
In the present work, aqueous and ethanolic extracts from a Congolese plant Vigna unguiculata (L.) Walp. were evaluated for their antisickling activity using microscopic technique. Ethanolic extract was found to be more active than the aqueous extract. The antisickling activity was found to be dose dependent. Anthocyanins extracts was found to be responsible for the inhibition of the sickling process, thus, justifying the claims of the traditional medical practitioners and suggesting a possible correlation between the chemical composition of this plant and its use in traditional medicine.
Key words: Vigna unguiculata , Aqueous extract, Ethanolic extract, Emmel’s test, Antisickling activity, Anthocyanins extract
1. Departement de Chimie, Faculte des Sciences B.P. 190, Universite de Kinshasa, Kinshasa XI, R.D. Congo.
2. Departement de Biologie, Faculte des Sciences B.P. 190, Universite de Kinshasa, Kinshasa XI, R.D. Congo.
3. Departement de Chimie, Faculté des Sciences, Université de Lubumbashi, R.D. Congo.
* Corresponding author : E-mail : ptmpiana@yahoo.fr
Introduction
The use of medicinal plants for the treatment and prevention of human diseases including sickle cell disease (SCD), is an age-old indigenous technology in Africa (Neuwinger, 2000; Cordeiro et al., 2004; Elujoba et al., 2005a). SCD is a hereditary blood disorder (Mehanna, 2002). Despite the fact that the cause of SCD has been known for many years, little progress has been made in suitable treatment of the disease. Presently, the major treatment for the painful crises is medication relief of pain, which merely treats the immediate symptoms.
Several attempts have been made in the past to treat and manage SCD chemotherapeutically. In this regard, some agents were developed by rational drug design to inhibit the red blood cells (RBCs) sickling process (Ekong et al., 1975; Lubin et al., 1975; Rifai et al., 1995; Mehanna, 2002; Eliot et al., 2006).
Most of these agents, unfortunately, did not show promising success in terms of clinical use.
Alternative strategy in the management of SCD is now focusing on the identification of the novel antisickling agents. Traditional medicine continues to play a very significant role in the medical primary health care implementation in developing countries. Almost 80% of the African population use mineral, animal or plant material for healing purposes.
Plants elaborate a remarkably diverse array of natural products. Among them, phenolic compounds have been recently shown to have multiple biological functions. Benzoic acid derivatives and divanilloyl quinic acids were cited to have promising antisickling activity in vitro (Elujoba et Sofowora, 1977b; Adesanya et Sofowora, 1983; Osoba et al.,1989; Akojie & Fung, 1992; Ouatara et al., 2004).
While, searching for antisickling activity of some Congolese plants, we found that, human blood from homozygous SCD patients (SS Blood) exposed in vitro to ethanolic and/or aqueous extracts, reverse the pathological events leading to sickling of RBCs under low oxygen tension (Mpiana et al., 2007a).
In our searches for antisickling compounds from plants, we discovered that anthocyanins would be the basis of antidrepanocytary activity in vitro (Mpiana et al., 2007b; Mpiana et al., 2007c). These vacuolar flavonoid pigments change their coloration according to pH. Like several other flavonoids, anthocyanins are powerful free radical scavengers (Kahkonen et al., 2003a). They also show antioxidant activity in lipid environments (Satué-Gracia et al., 1997).
The present study was performed with the aim of evaluation of the antisickling activity of Vigna unguiculata (L.) Walp., a Congolese edible plant also used in the management of various SCD-associated disorders in Kinshasa, Democratic Republic of Congo (DRC). The antisickling activity of anthocyanins extracted from this plant will be evaluated in vitro on SS blood using Emmel’s test (Courtejoie & Hartaing, 1992). This activity is expressed as the normalization of sickled cells. The minimal concentration of normalization (MCN) of sickle cell erythrocytes will be determined.
Materials and Methods
Plant material
The leaves of Vigna unguiculata (L.) Walp. were collected from plants growing in Kinshasa, DRC and were authenticated by Mr. B.L. Nlandu of the INERA (Institut National d’Etudes et Recherches Agronomiques). Voucher specimen (n°) is on deposit at the INERA Herbarium of the Faculty of Science (Université de Kinshasa).
Extraction
The dried and powdered plant material (leaves, 10 g) was repeatedly extracted by cold percolation with 95% EtOH and water (100 ml x 1) for 48 hrs. Fractions were filtered and concentrated to dryness under reduced pressure using a rotary evaporator. Extraction of anthocyanins was then done using 100 g of dried powdered plant material with distillated water and diethyl ether following an established protocol as previously reported (Mpiana et al., 2007b; Mpiana et al., 2007c).
Biological material
The sodium citrate suspension of blood samples used to evaluate the antisickling activity of the plant extracts in this study were taken from known sickle cell adolescent patients attending the “Centre de Médecine Mixte et d’Anémie SS” and “Centre Hospitalier Monkole”, both located in Kinshasa area, DRC. None of the patients had been transfused recently with Hb AA blood. All antisickling experiments were carried out with freshly collected blood. In order to confirm their SS nature, the above-mentioned blood samples were first characterized by haemoglobin electrophoresis on cellulose acetate gel at pH 8.5. They were found to be SS blood and were then stored at ± 4 °C in a refrigerator.
Antisickling assay
In order to evaluate the antisickling activity of our plant extract samples, an in vitro antisickling assay was performed, in which blood sample is put in contact with plants extracts at different concentrations, according to Emmel’s test procedure as previously reported (Mpiana et al., 2007a; Mpiana et al., 2007b; Mpiana et al., 2007c ).
The RBCs were analysed by measuring various parameters including the area, perimeter, and the radius of each RBC using a computer assisted image analysis system (Motic Images, 2000, version 1.3) and statistical data analysis were done using Microcal Origin 6.1 package software. The graph was obtained by computer simulation using the same software. The in vitro bioassay was performed in triplicate and the number of observed erythrocytes was determined using Thomas’ cell. All results presented in this study are mean ± S.D.
Results and Discussion
Two extracts from a plant used in Congolese traditional medicine were tested for their sickling reversal activities. Both aqueous and ethanolic extracts of V. unguiculata have shown a sickling reversal activity, thus, justifying the claims of the traditional medical practitioners and suggesting a possible correlation between the chemical composition of this plant and its use in traditional medicine. V. unguiculata ethanolic extract was found to exhibit the highest antisickling activity. The phytochemical screening revealed the presence of anthocyanins, tannins, alkaloids and steroids. Figs 1 and 2 illustrate the morphology of SS blood erythrocytes (control) and that of SS blood erythrocytes in the presence of Vigna unguiculata ethanolic extract.

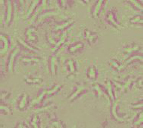
Fig 1. Morphology of drepanocytes of untreated SS blood (control) (X500)
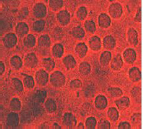
Fig 1. Morphology of drepanocytes treated with of 39.06 µg/mL ethanolic extract of V. unguiculata (X500) unguiculata (X500)
As it can be seen from the above images, the normalization of erythrocytes of SS blood sample treated with Vigna unguiculata extract indicated the influence of the extract on the sickliness of cells.
Fig 3 shows the dose dependent antisickling activity of ethanolic extract of Vigna unguiculata.
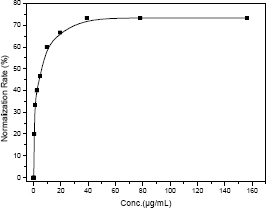
Fig 3. Concentration-dependent antisickling effect of ethanolic extract of Vigna unguiculata
The normalization of sickled cells with the ethanolic extract concentration increased with the extract concentration and reached a maximum and constant value at 39.06 µg/mL (MCN). This corresponds to a normalization rate of 74%. So, the antisickling activity of V. unguiculata ethanolic extract is dose dependent.
Taking into consideration both the chemical screening results and the solubility of different chemical groups, and according to our previous finding, we suppose that the antisickling activity of Vigna unguiculata ethanolic extract as well as of some other medicinal Congolese plants would be due to the presence of anthocyanins, which are present in this plants (Mpiana et al., 2007b; Mpiana et al., 2007c). We then decided to focus on the continuation of our investigation on this biologically active compounds group.
Fig 4 illustrates the morphology of SS blood erythrocytes treated with anthocyanins extracts of Vigna unguiculata.
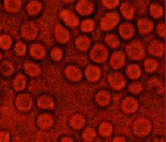
Fig 4. Morphology of SS blood erythrocytes treated with anthocyanins extract of V. unguiculata (X500)
These morphological SS blood cells were observed in hypoxic conditions, i.e. after deoxygenation of haemoglobin. As it can be seen from the above images, a normalization of erythrocytes of SS blood sample treated with ethanolic extract (EtOHE) and anthocyanins extract (ACE) of Vigna unguiculata indicated the influence of the extract on the sickliness of RBCs.
The computer program used in this study did not give the average radius for the erythrocytes (Table 1), as sickled cells of untreated SS blood was not circular. The average radius appeared after treatment of SS blood cells with V. unguiculata ethanolic and anthocyanins extracts, indicating the re-appearance of the normal and classical biconcave form of RBCs.
Table 1. Average values of radius, perimeter and surface of erythrocytes before and after treatment with ethanolic extract and anthocyanins extract of V. unguiculata
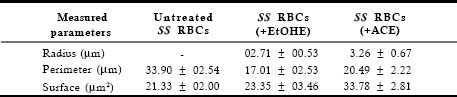
Statistical treatments according to Student’s t-test (Tomassone, 1993), applied with a probability threshold of both 0.01 and 0.05 for 18 degrees of freedom, enabled the determination of a significant difference between the average values of both the perimeter and the surface of the untreated and treated erythrocytes on the images, thus confirming the modification of the RBCs form in the presence of anthocyanins extract. No significant difference was found between the average values of the surface of the untreated and ethanolic extract treated RBCs. This indicates that the in vitro antisickling activity of anthocyanins is efficient and reliable. Indeed, the RBCs were observed to change from the sickled shape to normal biconcave cells. These values were in agreement with previously reported values (Mpiana et al., 2007b; Mpiana et al., 2007c). The treated SS RBCs demonstrated a remarkable similarity to normal blood values.
Therefore, from the above results, anthocyanins might be potential antisickling drug candidate for sickle cell patients.
Anthocyanins are found in large quantities in the plant tissues, and they have been shown to act as powerful antioxidants helping to protect the cells from free radicals formed during metabolic processes. There is considerable current interest in the possible health effects of anthocyanins in humans owing to their reported positive effects on blood vessel walls (Kahkonen et al., 2003a; Kahkonen et al., 2003b; Mian et al., 1977; Wang et al., 1997). Our results in this study suggest that the effectiveness of the antisickling activity of Vigna unguiculata (L.) Walp. leaves extract is related to the anthocyanins, and the observed bioactivity may be probably due to the anthocyanins behaviour consisting in a non covalent binding reaction to proteins (Charpentier et al., 1998; Wagner et al., 1983 ). A similar finding has been previously reported by our research team in the plant extracts of Ocimum basilicum L. (Mpiana et al., 2007b).
The ability of the anthocyanin extracts, in this study, to reverse the pathological events leading to sickling of RBCs under low oxygen tension may represent a rational explanation for the use of this plant in managing sickle cell disease by Congolese traditional medical practitioners. However, anthocyanins have not yet been reported to exhibit antisickling effects, so far.
Further studies, involving the structure elucidation of anthocyanins from Vigna unguiculata and other Congolese plants using a combination of chromatographic and spectroscopic techniques are in progress.
Acknowledgements
The authors are indebted to Third World Academy of Science (TWAS) (Grant No. 07-077 LDC/CHE/AF/AC-UNESCO FR-3 144 804) for providing financial assistance.
References
Adesanya, S.A. and Sofowora, A. 1983. Biological standardisation of zanthoxylum roots for antisickling activity. Planta Med. 48: 27-33.
Akojie, F.O. and Fung, L.W. 1992. Antisickling activity of hydroxybenzoic acids in Cajanus cajan. Planta Med. 58(4): 317-320.
Charpentier, B., Hamon, F.L., Harlay, A., Huardar, A. and Ridoux, L. 1998. Guide du préparateur en pharmacie, Masson, Paris. Cordeiro, J.V. and Oniyangi, O. 2004. Phytomedicines (medicines derived from plants) for sickle cell disease. The Cochrane Database of Systematic Reviews 3: CD004448.pub2.
Ekong, D.E.U., Okogun, J.I., Enyenihi, V.U., Baloghnair, V. and Natta, C. 1975. New antisicking agent 3,4-dihydro-2,-2-dimethyl-2H-1benzopyran-6-butyric acid. Nature 258: 743-746.
Eliot, R., Davies, M. and Harmse, D.J. 2006. Dermatomyositis-like eruption with long-term hydrourea. Dermatolog. Treat. 17(1): 56-60.
Iyamu, E.W., Turner, E.A. and Asakura, T. 2002. Niprisan (NIX-0699) improves the survival rates of the transgenic sickle cell mice under accurate severe hypoxic conditions. British Journal of Haematology. 118: 337-343.
Elujoba, A.A., Odeleye, O.M. and Ogunyemi, C.M. 2005a. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr.J.Trad.CAM 2(1): 46 – 61.
Elujoba, A.A. and Sofowora, E. 1977b. Detection and estimation of total acid in the antisickling fraction of Fagara species. Planta Med. 32: 54–59.
Kahkoonen, M.P., Heinamaki, J. and Heinonen, M. 2003a. Antioxidant activity of anthocyanins and their aglycons. J. Agric Food Chem. 52: 628–633.
Kahkoonen, M.P., Heinamaki, J. and Heinonen, M. 2003b. Berry anthocyanins: isolation, identification and antioxidant activities. J. Agric Food Chem. 83: 1403–1411.
Lubin, B.H., Pena, V., Mentzer, W.C., Edwin, B., Bradley, T.B. and Lester, P. 1975. Dimethyl Adipimidate. A New Antisickling Agent. Nat’l Acad. Sci. 72: 43.
Mehanna, A.S. 2002. Sickle cell anaemia and antisickling agents: Then and now. Curr. Med. Chem. 8(2): 79-88.
Mian, E., Curri, S.B., Lietti, A. and Borbardelli, E. 1977. Anthocyanosides and the walls of microvessels: further aspects of the mechanism of action of their protective effect in syndromes due to abnormal capillary fragility. Minerva Med. 68: 3565-3581.
Mpiana, P.T., Tshibangu, D.S.T., Shetonde, O.M. and Ngbolua, K.N. 2007a. In vitro antidrepanocytary activity (anti-sickle cell anaemia) of some Congolese plants. Phytomedicine 14: 192-195.
Mpiana, P.T., Mudogo, V., Ngbolua, K.N., Tshibangu, D.S.T., Shetonde, O.M. and Mbala, B.M. 2007b. In vitro antisickling activity of anthocyanins from Ocimum basilicum L. (Lamiaceae). Int. J. Pharmacol. 3(4): 371-374.
Mpiana, P.T., Mudogo, V., Tshibangu, D.S.T., Ngbolua, K.N., Shetonde, O.M., Mangwala, P.K. and Mavakala, B.K., 2007. In vitro antisickling activity of anthocyanins extracts of a Congolese Plant: Alchornea cordifolia M. Arg. J. Med. Sci. 7(7): 1182-1186.
Neuwinger, M.D. 2000. African Traditional Medicine. Medpharm Scientific Publisher. Stuttgart, p. 590.
Osoba, O., Adesanya, S. and Durosimi, M. 1989. Effect of zanthoxylum xanthoyloides and some substituted benzoic acids on glucose-6-phosphate and 6-phosphogluconate dehydrogenases in Hb SS red blood cells, J. Ethnopharmacology 27: 177-183.
Ouattara, B., Angenot, L., Guissou, P., Fondu, P., Dubois, J., Frederich, M., Jansen, O., van Heugen, J.C., Wauters, J.N. and Tits, M. 2004. LC/MS/NMR analysis of isomeric divanilloylquinic acids from the root bark of Fagara zanthoxyloides Lam. Phytochemistry 65: 1145 – 1151.
Rifai, N., Sakamoto, M., Law, T., Platt, O., Mikati, M., Armsby, C.C. and Brugnara, C. 1995. Measurement by HPLC, blood distribution and pharmacokinetics of oral clotrimazole, a new potential antisickling agent. Clin. Chem. 41(3): 387-391.
Satué Gracia, M.T., Heinonen, M. and Frankel, E.N. 1997. Anthocyanins as antioxidants on human low–density lipoprotein and lecithin – liposome systems. J. Agric Food Chem. 45: 3362 – 3367.
Tomassone, R., Dervin, C. and Masson, J.P. 1993. Biométrie: Modélisation des phénomènes biologiques, Masson, Paris, p. 553.
Wagner, H., Bladt, S. and Zgainski, E.M. 1985. Plant drug analysis. Springer-Verlag, Berlin. Wang, H., Cao, G. and Prior, R.L. 1997.
Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 45: 304-309.