Noncardiac Causes of ECG Abnormalities
The remaining ECG abnormalities to be discussed are noncardiac causes, including genetic disorders that affect the size or function of the heart.
Pulmonary Embolism A pulmonary embolism may also be identified on a 12-lead ECG. The criteria for suspecting this include the presence of an S1Q3T3 patterrr, new RBBB, and ST-segment depression in leads V1 to V3 Figure 115. The pattern refers to a deep S wave in lead I, a deep, narrow Q wave in lead III, and T-wave inversion in lead III. This is also sometimes written as S1Q3T3, with the T upside-down, to indicate T-wave inversion. It is important to note that only approximately 12% of pulmonary embolisms have these ECG changes. Unfortunately, these changes are almost exclusively seen in cases of large pulmonary embolism. The absence of S1Q3I3 and RBBB on the surface ECG does not rule out a pulmonary embolism. Pulmonary embolism remains one of the most frequently missed conditions. It is critical to collect pertinent information about the patient’s medical history, including any surgeries or medications, and current health status, including a family history, and to perform a thorough physical exam.
Figure 113 A 12-lead ECG showing benign early repolarization.
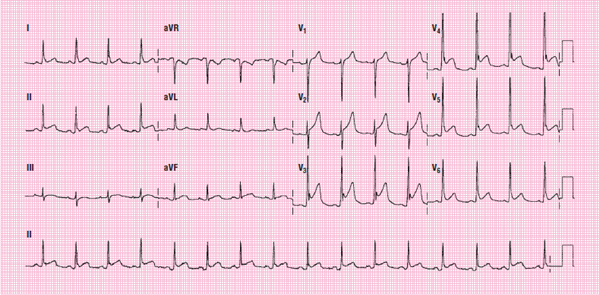
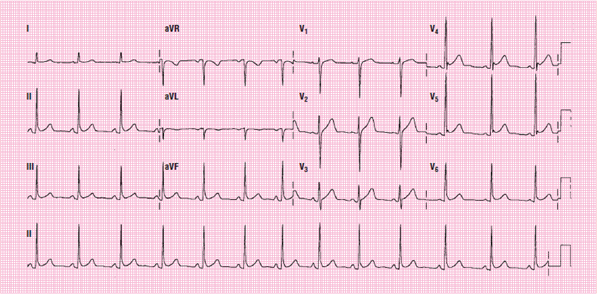
Figure 114 A 12-lead ECG showing pericarditis.
Figure 115 A 12-lead ECG showing pulmonary embolism.
Patients experiencing severe hypothermia may develop J waves (Osborne) on the ECG. The J wave is often a large, upright wave that occurs on the terminal wave of the QRS complex. The ECG also typically appears as a bradycardic rhythm and baseline containing artifact from shivering and poor electrode adhesion to wet or cold skin Figure 116. The J wave may also be accompanied by ST-segment depression and T-wave inversion. Generally, the more serious the hypothermia, the larger the J wave. Evidence of a J wave should be considered only an indication of hypothermia; it is not enough to make a definitive diagnosis.
Electrolyte imbalances can also cause changes on the ECG. The two most common electrolyte imbalances involve potassium and calcium. Hyperkalemia causes specific ECG changes. First, tall, peaked, asymmetric T waves develop and the P waves can become flattened and eventually disappear from the tracing. In hyperkalemia, the T wave may be tall and sharply peaked. In more severe cases, wide QRS complexes appear on the tracing Figure 117. By contrast, hypokalemia usually presents with flat or apparently absent T waves along with the development of a U wave. The U wave is a small wave (smaller even than a P wave) that occurs after a T wave but before the next P wave. U waves are uncommon and may often be mistaken for extra P waves or another unknown abnormality Figure 118.
Hypercalcemia may cause a shortened QT interval, for example, whereas hypocalcemia may slightly lengthen the QT interval. It is important to note that the overall shortening or lengthening of the QT interval in hypercalcemia or hypocalcemia, respectively, is specifically due to the change in length of the ST segment. The T wave itself is unaffected by changes in calcium concentrations. The isolated ST-segment change is attributed to the fact that the ST segment represents phase 2 of the myocardial action potential.
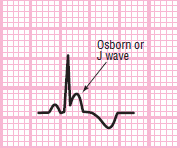
Figure 116 Osborne (J) wave.
Hypertrophic cardiomyopathy is a condition in which the myocardial walls become very thick. Patients often experience shortness of breath, chest pain, or syncope, which is often associated with physical exercise. Patients are often diagnosed in their 30s or 40s. Hypertrophic cardiomyopathy is characterized by deep, narrow Q waves in the inferior leads and high lateral leads, and very tall R waves in the left precordial leads, similar to the changes seen in LVH Figure 119.
Brugada syndrome is a genetic disorder involving sodium channels in the heart.

Figure 117 Hyperkalemia
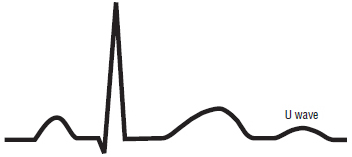
Figure 118 Hypokalemia.
Figure 119 A 12-lead ECG showing hypertrophic cardiomyopathy.
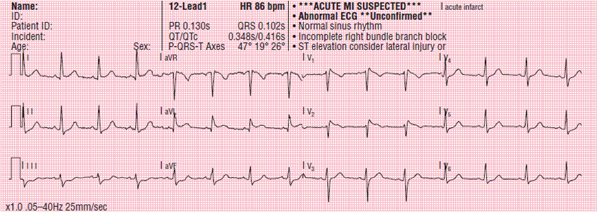
Figure 120 A 12-lead ECG showing Brugada syndrome.
Brugada syndrome is believed to be responsible for approximately 4% of all sudden cardiac arrest. It is most common in men of Southeast Asian origin. The disease is often diagnosed in a person’s 40s or 50s, and often, patients are unaware of the condition until a sudden onset, in the form of syncope or cardiac arrest, prompts them to seek medical care. Brugada syndrome is characterized by incomplete RBBB (rSR pattern in lead V1, QRS duration of shorter than 140 ms), and ST-segment elevation that aggressively returns to baseline Figure 120. These changes are seen exclusively in leads V1 to V2 (and possibly in V3).
Long QT syndrome is a condition characterized by a QT interval exceeding approximately 450 ms Figure 121. A prolonged QT interval (long QT syndrome) indicates that the heart is experiencing an extended refractory period, making the ventricle more vulnerable to dysrhythmias. LQTS is a result of genetic mutation of several genes. LQTS syndrome may also occur with administration of certain drugs (such as amiodarone), and can result from certain conditions such as hypocalcemia, AMI, and pericarditis. Conversely, the QT interval may be shortened in hypercalcemia and in patients taking digitalis.
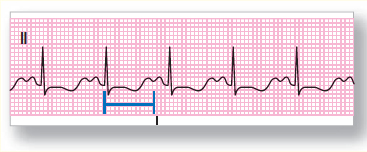
Figure 121 A 12-lead ECG showing long QT syndrome.
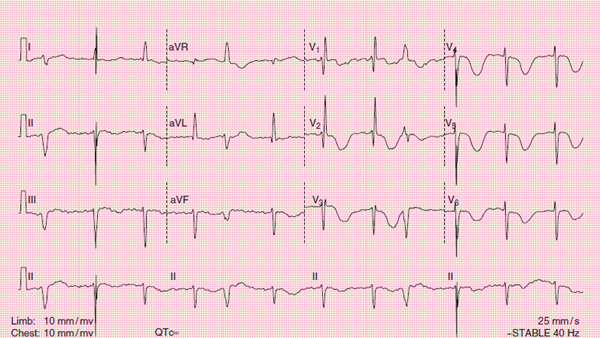
Figure 122 A 12-lead ECG showing intracranial hemorrhage.
The QT interval is age-specific and gender-specific, so there is no single value for all patients. Long QT syndrome predisposes the patient to ventricular dysrhythmias, which can result in syncope and sudden cardiac arrest and can be a result of medication administration, myocardial ischemia, intracranial hemorrhage, or congenital disorders. Patients with LQTS are at increased risk for ventricular dysrhythmias, including torsade de pointes and ventricular fibrillation. EMS is often called to treat patient who experienced syncope, palpitations, or sudden death. It is critical to record an ECG on all patients experiencing syncope including younger patients.
Intracranial hemorrhage can also cause ECG changes. The mechanism by which the changes appear is not fully understood. Intracranial hemorrhage may cause deeply inverted, symmetric T waves in the precordial leads, along with a prolonged QT interval Figure 122. As a general rule, patients with these ECG changes resulting from intracranial hemorrhage almost always have neurologic symptoms including unresponsiveness.
The 12-lead ECG is an amazing tool that can provide insight about the function of the cardiac conduction system and dysrhythmias. The QRS axis can also be measured from the ECG. Chamber size and ischemic changes can also be seen on the surface tracing. The ECG can also provide information about many noncardiac causes such as disorders of the lungs, kidneys, or brain.
The following analogy by the late Dr. Nancy Caroline helps to illustrate the function of the electrical conduction system in the context of the three types of heart blocks.
Cast
Sidney Sinus. Sidney, the SA node, is boss of the heart. He ordinarily dispatches messengers 70 to 80 times per minute; the messengers are supposed to dash down the atria, slip through the AV junction, and depolarize the ventricles. Sidney is not terribly bright but is usually conscientious and reliable.
Albert and Alice Atria. Albert and Alice are the right and left atria. These somewhat temperamental little pouches normally contract in response to the messages sent by Sidney and squeeze their blood into the ventricles, providing the ventricles with an atrial kick. Their contraction is represented on the ECG by the P wave.
AV Abe. Abe, the AV node, is a lower-level pacemaker who secretly yearns to be boss of the heart. Unfortunately, because of his lower intrinsic rate, he rarely gets the opportunity to run the show. Abe stands at the threshold of the ventricles and checks out every messenger sent by Sidney Sinus. Normally, Abe lets the messengers pass into the ventricles after a brief security check (PR interval). However, as the node in charge of traffic control into the ventricles, Abe does regulate the flow of messengers and occasionally closes a few southbound lanes, especially when the traffic gets heavy or when he’s not feeling well.
Vance and Virginia Ventricle. Vance and Virginia are big, tough, muscular types, also not very bright, who are charged with the enormous responsibility of pumping blood to the whole body. Normally, they take their orders from the messengers sent by Sidney Sinus, but sometimes the Ventricles grow irritable and contract without orders, especially when they run a little short on oxygen. They also tend to be impatient when they don’t hear from Sidney on time; under that circumstance, they sometimes contract on their own.
Montgomery, Mimi, Mortimer, Millicent, et al. These messengers consist of tiny electric impulses. Earnest and dedicated, their job is to carry the orders for depolarization from Sidney’s headquarters all the way to the ventricles.
First-Degree AV Block, or “The Little Messenger That Could” One fine day, Sidney Sinus dispatched Mortimer Messenger with the usual order: “Depolarize the ventricles.” Mortimer scampered down the atria without difficulty but arrived at the AV node to find a pile of debris blocking the entrance to the ventricles. “Sorry,” said AV Abe, “we’re closed for repairs.”
“But I have to get through,” said Mortimer.
“Impossible,” said Abe.
But Mortimer was brave and determined. “I think I can. I think I can. I know I can,” he said, gathering his few milliamps of strength. Finally, after a long struggle (prolonged PR interval), Mortimer crashed through the AV junction into the ventricles and breathlessly issued the order to contract Figure 123. The ventricles were depolarized, and everyone lived happily ever after, until…
Second-Degree AV Block, Type I
When Montgomery Messenger left for work that day, there was no sign there would be trouble. He took his first set of orders from Sidney Sinus, whistled down the atria, zipped through the AV node, and smartly ordered the ventricles to depolarize.
On the next trip down, however, Montgomery felt just a bit tired and slowed slightly as he crossed the AV junction. “Why break my neck?” he thought. “So, the PR interval will be a tiny bit prolonged. Who’ll notice, anyway?”
On his third trip, Montgomery encountered several roadblocks in the region of the AV junction and had to pick his way around them. Glancing at his watch as he reached the ventricles, he scowled. “Nuts,” he said, “240 ms. Boy, is Sidney going to be mad.”
Making his fourth trip from the SA node, Montgomery found the gates to the ventricles closed and locked. Frantically, he banged on the gates. “Come on, Abe, I know you’re around somewhere. Let me through.” To no avail. The gates remained tightly shut. Defeated, Montgomery returned to the SA node, leaving a lonely P wave to chronicle his struggle Figure 124.
“What do you mean, you couldn’t get through?” Sidney Sinus demanded.
“I couldn’t get through,” Montgomery said. “I’m telling you the gates were locked tight.”
“Okay,” said Sid, “off to the showers. You’ve had it for the day.” So Sidney called Mimi Messenger. “Now look,” he told her, “I want you to go straight down to the ventricles and give them this message, and no fooling around at the AV junction, understand?”
“Oh, yes sir,” said Mimi, always eager to please. So off Mimi went, sailing down the atria, through the AV junction, and into the ventricles. “Hmm, 140 ms,” she noted to herself. “Sid can’t complain about that.” On her second run, however, Mimi tripped over a shoelace and barely made it in under 200 ms. On the third trip, some highway construction held her up for 240 ms. But the fourth trip south was the worst, for she arrived at the AV junction to find that once again Abe had locked the gates. Mimi banged and banged on the gates. “Come on, Abe, open up. I’m going to lose my job.” No response. Crestfallen, Mimi returned to the SA node.
“And what happened to you?” Sid demanded.
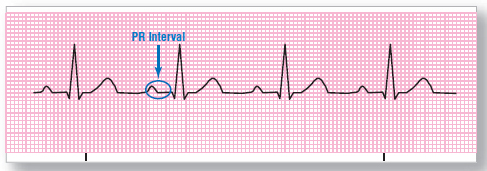
Figure 123 First-degree AV block. When there is trouble at the AV junction, it may take the messengers from the SA node longer to get through.
“I couldn’t get through to the ventricles this time.”
“Couldn’t get through? Did you get lost, maybe?”
“But at least I made a nice P wave,” Mimi ventured.
“A nice P wave! A nice P wave, she says. What good’s a P wave without a QRS complex? Do you think the atria are going to supply blood to the whole body? They’re strictly small-time. The big guns are in the ventricles. That’s why I sent you to depolarize them. Now you get to the showers.”
And so it went. Messenger after messenger faltered at the AV junction, but the worst was yet to come.
Second-Degree AV Block, Type II
It just wasn’t Montgomery’s week. Reporting for work the next day, he received the usual order from Sidney to depolarize the ventricles. Montgomery set out full of confidence and vigor, traversing the atria without difficulty. But when he arrived at the threshold of the ventricles, he found his path blocked by AV Abe.
“Let me through,” Montgomery said. “I have an important message for the ventricles.”
“Get lost,” said Abe, who was feeling rather dyspeptic that day.
“But I have to get through. I’ve already used up 190 ms.”
“Beat it, sonny. I’m the boss around here.”
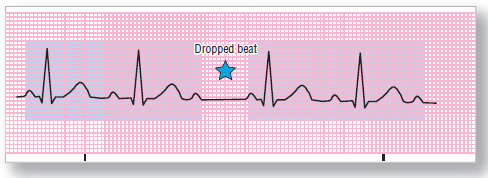
Figure 124 Second-degree AV block, type I (Wenckebach). Each transit through the AV junction is a bit slower, until finally the messenger cannot get through, and a beat is dropped.
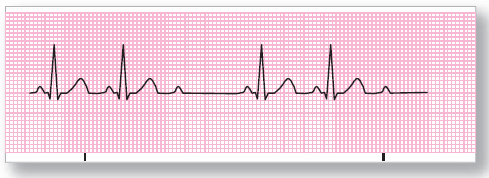
Figure 125 Second-degree AV block, type II. Every second impulse from the SA node is blocked at the AV junction.
Montgomery returned to Sidney Sinus disgraced. “What happened to you?” Sidney wanted to know. “You were supposed to order the ventricles to contract.”
“I couldn’t get past Abe,” Montgomery replied.
“What do you mean, you couldn’t get past Abe? I just sent your friend, Mimi Messenger down there, and she got through without any problem.”
“But he wouldn’t let me pass,” whimpered Montgomery.
“I don’t want to hear any excuses. You just go right back down there and deliver your message to the ventricles. I can’t tolerate weaklings on my staff.”
So Montgomery squared his shoulders, sailed down the atria again, and arrived once more at the gate of the ventricles.
“Are you here again?” said Abe. “I thought I told you to beat it.”
“Please,” said Montgomery, “I have to get through. You don’t know what Sid is like when he gets upset.”
“Sorry, sonny, I’m closed for lunch.”
Montgomery returned to Sidney Sinus. “I couldn’t make it,” he said.
“Look, Montgomery,” said Sidney, “Millicent Messenger just breezed by Abe right after you left. Now you march back down there and do your job.”
“Yes, sir,” said Montgomery.
Arriving again at the threshold of the ventricles, Montgomery once more found Abe blocking his path.
“Listen, Abe, I’m not kidding this time. If you don’t let me through, I’m going to use some atropine and blast the gate open.”
“Those are big words, sonny,” said Abe, “but I’m not scared of a little atropine.”
“The last time they used atropine, you were zonked for hours,” Montgomery reminded him.
“I’ll take my chances.”
And so it went. Each time Montgomery reached the gate to the ventricle, AV Abe barred his path. Yet the messenger coming right after Montgomery kept getting through (2:1 block) Figure 125.
“Montgomery,” cautioned Sidney, “if this keeps up, they’re going to put in a pacemaker, and we’ll all be out of a job. Shape up.”
But the worst was yet to come.
Complete Heart Block (Third-Degree AV Block)
The next day was even worse for Sidney’s operation. It was bad enough, Montgomery not getting through. “Every second P wave not followed by a QRS complex,” wailed Sidney. “My reputation is being ruined!” But then, suddenly, the situation became even worse. Sidney had just sent Mildred Messenger down to the ventricles, and she arrived at the AV junction to find the gate shut and bolted. A sign tacked to the gate read: “Closed until further notice.”
“That’s impossible,” said Sidney when he heard the story. “Abe can’t do that to me.” So he sent another messenger, Marvin, to depolarize the ventricles. Marvin charged down the atria and ran smack into the closed gate. He banged and shouted, but there was no response.
“Impossible,” said Sidney. “Abe must be sleeping.” So he dispatched Melvin Messenger. Again the door was bolted tight.
“Oh, what I’d give for a bolus of atropine,” sighed Sidney.
Meanwhile, the ventricles were starting to get nervous, and Vance, the right ventricle, said to Virginia, the left ventricle, “Have you heard anything from the atria lately?”
“Not a thing.”
“Funny. Those messengers are usually pretty prompt.”
“Must have run into some problems with Abe.”
“Yeah. Every time that guy has a little too much digitalis, he gets delusions of grandeur and starts hassling the messengers.”
“How long do you suppose we ought to wait?”
“I don’t know. It’s already been more than a second, and the brain is starting to complain about not getting enough oxygen.”
“The brain is always complaining about something.”
“Yeah, but the kidneys don’t sound happy either.”
“Okay, okay. Let’s go ahead and contract. I hate to do it without authorization from above. The last time we decided to go ahead and fire on our own, some of that disgusting lidocaine came barreling down the pipes. I was sick for a week.”
So Vance and Virginia set off on their own, contracting slowly (about 30 times per minute) so as not to attract much attention, little appreciating that back in the atria Sidney was frantically sending messenger after messenger, all in vain, to assault the closed gate Figure 126.
“What’s happened to Sidney?” Virginia said to Vance, as they plodded along slowly.
“I wish I knew,” said Vance.
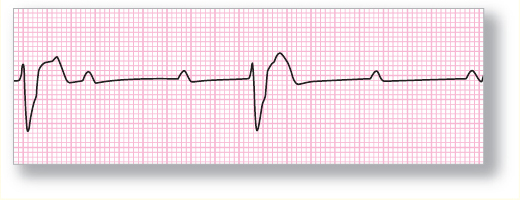
Figure 126 Third-degree AV block. The atria and ventricles are marching to the beat of different drummers.
Words of Wisdom
Impulse blockage within the SA node is referred to as sinoatrial block. Electrocardiographic changes associated with sinoatrial block are often very subtle or invisible on the surface ECG and require electrophysiologic study to diagnose.
This section profiles some of the devices and methods used in the treatment of patients with cardiac emergencies. Not all of the techniques or devices described are used in every EMS system, and not all are required for certification as a paramedic. Direct your attention to the material that is relevant to your local practice.
The 2010 AHA guidelines suggest that a checklist be used to assist in the triage of patients with ACS Figure 127.
Defibrillation
Defibrillation is the process by which a surge of electric energy is delivered to the heart. Recall that when the heart fibrillates, its individual muscle fibers get “out of synch” with one another and begin contracting individually. As a result, the heart as a whole ceases any useful movement. Indeed, if you were to look at a fibrillating heart, you would see movement resembling that of a bag of energetic worms. The idea behind defibrillation is to deliver a current to the heart that is powerful enough to depolarize all of its component muscle cells; ideally, when those cells repolarize after the shock, they will respond to an impulse from the SA node and begin organized depolarization, leading to cardiac contraction.
Defibrillation needs to be carried out as soon as possible in two rhythms—ventricular fibrillation and pulseless ventricular tachycardia—because the likelihood of its success declines rapidly with time (in seconds, not minutes!). If the arrest is not witnessed and CPR is not in progress, immediately start CPR and continue for 2 minutes before delivering the first shock. If the patient’s rhythm converts to ventricular fibrillation or pulseless ventricular tachycardia and the defibrillator is already attached, perform CPR only long enough to charge the defibrillator and then defibrillate. Defibrillation is not useful in asystole because there is no evidence that the myocardial cells are spontaneously depolarizing. Defibrillation of asystole is unlikely to be beneficial and is harmful (due to the unnecessary interruption of compressions). Thus, if you are unsure about asystole after checking more than one lead, resume CPR and follow the asystole pathway in the pulseless arrest algorithm until the next pulse and rhythm check.
Figure 127 Chest pain checklist.
Manual Defibrillation Some defibrillators are combination units that can perform either manual or automated defibrillation. An automated external defibrillator (AED) interprets the cardiac rhythm and determines if defibrillation is needed. In manual defibrillation, the paramedic interprets the cardiac rhythm and determines if defibrillation is needed. Automated external defibrillation is used by personnel who are not trained in ECG rhythm interpretation. The AED mode will recommend a shock and walk the responders through the procedure.
Remember to immediately note the patient’s age. Use pediatric defibrillation pads when appropriate.
Paramedics may arrive to a scene at which EMTs have brought an AED that is set in AED mode. In such cases, the paramedics use the AED, but switch it to manual mode, allowing all electrical therapy functions to work (ie, transcutaneous pacing and synchronized cardioversion), as well as the multiple lead cardiac monitoring and 12-lead ECG acquisition.
Paramedics may also arrive at a scene where an AED is not in use, but the patient then goes into cardiac arrest. In that case, manual mode should be selected on the defibrillator unit. The paramedic can then look at the monitor and determine if the rhythm is shockable. If so, he or she can then proceed with charging the unit and shocking the patient. This saves time since CPR can continue until the moment the monitor is ready to shock; the paramedic does not need to wait for the AED mode to analyze the rhythm and make a recommendation.
Remember, with patients in cardiac arrest, it is essential to minimize any interruptions in chest compressions! A well-trained paramedic can interpret a cardiac rhythm more quickly than an AED can.
Follow the same safety measures when performing manual defibrillation as you would when using an AED. Ensure that no one is touching the patient. Do not defibrillate a patient who is in pooled water. There will be some danger to you if you are in the water, and also, the electricity will diffuse into the water instead of traveling between the defibrillation pads and through the patient’s heart. Therefore, the heart will not receive enough electricity to cause defibrillation. You can defibrillate a soaking wet patient, but try first to dry the patient’s chest. Do not defibrillate someone who is touching metal that others are touching.
If the patient has an implanted pacemaker or internal defibrillator, place the defibrillation pad below the pacemaker or defibrillator, or place the defibrillation pads in anterior and posterior positions.
To perform manual defibrillation, attach the adhesive defibrillation pads to the patient’s chest as instructed on the package. As with ECG electrode placement, you may have to dry the skin before placing the defibrillation pads. Depending on the device, you may place the defibrillation pads before turning on the main power switch, or may turn the switch on once the pads are in place. After the pads are placed and the main power switch is turned on, set the energy level to 200 J (for biphasic devices), or follow the defibrillator manufacturer’s recommendations regarding the appropriate energy level. Monophasic defibrillators should be set to 360 J for the first and all successive shocks. Charge the defibrillator.
Today, most EMS agencies use combination pacing/defibrillation pads, which allow the paramedic to quickly assess the patient’s cardiac rhythm and deliver an electrical shock, if indicated. Some devices still require the use of hand-held paddles to analyze the rhythm and deliver a shock. Paddles consist of a large metal surface that contacts the patient’s skin, and require application of a conductive gel on the paddle surface to ensure maximum contact with the skin. Failure to use conductive gel on the paddles often results in burns to the skin and ineffective energy delivery to the heart. Use electrode paste or saline gel pads to make good electric contact between the paddles and the skin. Apply about 25 lb of pressure to hold the paddles in contact with the chest.
Whether using the combination pads or the older style, hand-held paddles, it is critical to follow manufacturer’s recommended placement on the chest to avoid electrical arcing between the two contact points. Paramedics must also ensure that the devices are not placed over metal objects such as jewelry and internal pacemakers or medication patches as burns to the patient may result. From here on, we will use the term defibrillation pads to refer to pads, paddles, and combination pads.
Position the defibrillation pads so that the negative (sternum) pad is just to the right of the upper part of the sternum below the right clavicle and the positive (apex) pad is just below and to the left of the left nipple Figure 128. If using paddles, exert firm pressure (20 to 25 lb) on each paddle to make good skin contact.
When the defibrillator is charged, clear the area so that no one—including the operator—is in contact with the patient or stretcher. The operator should then announce, “All clear!” At this point, discharge the defibrillator by pressing the button on each handle simultaneously or pressing the button on the machine if using a hands-free system. If current has reached the patient, contraction of the chest and other muscles will be evident. If you do not see contraction, check the defibrillator to be certain the synchronizing switch is off and the battery is charged.
Immediately after delivering the defibrillating current, resume CPR. Continue CPR for 2 minutes or five cycles, and then pause to check for a pulse and reevaluate the rhythm. If at any point you see an organized rhythm on the monitor, check for a pulse (maximum of 10 seconds).
If you determine that the rhythm requires an additional shock, deliver one shock followed immediately by CPR, beginning with chest compressions. Repeat these steps if needed.
If you determine that the rhythm does not require a shock, but the patient has no pulse, perform five cycles (approximately 2 minutes) of CPR beginning with chest compressions. After five cycles (2 minutes) of CPR, reanalyze the patient’s cardiac rhythm. If the rhythm is still not shockable, continue CPR. Transport the patient, and contact medical control as needed.
If you determine that the rhythm does not require a shock, and the patient has a pulse, check the patient’s breathing. If the patient is breathing, but his or her SpO2 is less than 94%, administer oxygen and transport.
Figure 128 Position the pads for defibrillation. A. Anterior-anterior placement. B. Anterior-posterior placement.
Words of Wisdom
Manual defibrillation can be performed more quickly than defibrillation with an AED; when using an AED, you must wait while the machine analyzes rhythm. Therefore, manual defibrillation is the defibrillation method of choice for paramedics.
An implanted artificial pacemaker—which you may detect from the pacemaker-produced spikes on the ECG or the bulge where its battery pack has been implanted under the patient’s skin—is not a contraindication to defibrillation. Just make certain that you do not place the defibrillation pads directly over the pacemaker battery.
The defibrillator should be inspected at the beginning of each shift, using a checklist to cover all aspects of the apparatus and its gear. Inspection should include the defibrillation pads, cables and connectors, power supply, monitor, ECG recorder, and any ancillary supplies (such as electrode gel, pads, spare battery). The US Food and Drug Administration has developed an Operator’s Shift Checklist for inspecting defibrillators. Conscientious use of the checklist should significantly reduce the incidence of defibrillator failures.
Skill Drill 3 summarizes the procedures for manual defibrillation:
Skill Drill 3
1. Take standard precautions.
2. Prepare the skin for placement of the defibrillation pads if needed. Attach the adhesive defibrillation pads to the patient’s chest as instructed on the package Step 1. If using paddles, lubricate them with a conductive gel.
3. Turn on the main power switch.
4. Set the energy level to 200 J (for biphasic devices), or follow the defibrillator manufacturer’s recommendations regarding the appropriate energy level Step 2. Monophasic defibrillators should be set to 360 J for the first and all successive shocks.
5. Charge the defibrillator.
6. If using paddles, exert firm pressure (20 to 25 lb) on each paddle to make good skin contact.
7. Ensure that no one is touching the patient. Remember not to defibrillate a patient who is in pooled water. Ensure that the patient is not touching metal.
8. Clear the area. Announce, “All clear!”
9. Press the button on the machine if using a hands-free system; if not, discharge the defibrillator by pressing the button on each handle simultaneously Step 3.
10. Observe for contraction of the patient’s chest muscles. If you do not see contraction, check the defibrillator to be certain the synchronizing switch is off and the battery is charged.
11. Resume CPR immediately. Continue CPR for 2 minutes or five cycles, and then pause to check for a pulse and reevaluate the rhythm. If at any point you see an organized rhythm on the monitor, check for a pulse (maximum of 10 seconds).
Performing Manual Defibrillation
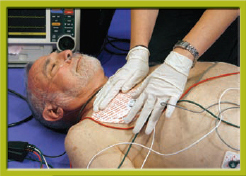
Step 1 Take standard precautions. Prepare the skin. Attach the adhesive defibrillation pads to the patient’s chest as instructed on the package. If using paddles, lubricate them with a conductive gel.

Step 2 Turn on the main power switch. Set the defibrillator to the proper energy setting. Charge the defibrillator. If using paddles, exert firm pressure to make good skin contact. Ensure that no one is touching the patient.
Step 3 Clear the area. Announce, “All clear!” Press the button on the machine if using a hands-free system; if not, discharge the defibrillator by pressing the button on each handle simultaneously. Observe for contraction of the patient’s chest muscles. Resume CPR immediately. Continue CPR for 2 minutes or five cycles, and then pause to check for a pulse and reevaluate the rhythm. If at any point you see an organized rhythm on the monitor, check for a pulse (maximum of 10 seconds).
Patients who do not regain a pulse on the scene of the cardiac arrest usually do not survive. What you do with these patients depends on your EMS system. Whether you should transport the patient should be dictated by the local protocols established by medical control.
Administration of CPR while patients are being moved or transported is usually not effective. The best chance for patient survival occurs when the patient is resuscitated where found, unless the location is unsafe.
If your local protocols agree, you should begin transport when one of the following occurs:
 The patient regains a pulse.
The patient regains a pulse.
 Six to nine shocks have been delivered (or as directed by local protocol).
Six to nine shocks have been delivered (or as directed by local protocol).
 The machine gives three consecutive messages (separated by 2 minutes of CPR) that no shock is advised (or as directed by local protocol).
The machine gives three consecutive messages (separated by 2 minutes of CPR) that no shock is advised (or as directed by local protocol).
If you transport a patient while performing CPR, you need a plan for managing the patient in the ambulance. Ideally, you will have two EMS providers in the patient compartment while a third drives. You may deliver additional shocks at the scene or en route with the approval of medical control. It is not as safe to defibrillate in a moving ambulance. Therefore, you should come to a complete stop if an additional shock is needed. Be sure you know and follow the protocol of your EMS service. The algorithm for cardiac arrest is shown later in this chapter, in the section, Treatment for Ventricular Fibrillation or Pulseless Ventricular Tachycardia.
Automated External Defibrillator As mentioned, paramedics usually perform manual defibrillation, but as a paramedic you may encounter AEDs when responding to a scene where law enforcement or other EMS providers have already attached an AED to the patient; therefore, you must know how to use AEDs.
The AED can analyze the patient’s ECG rhythm and determine whether a defibrillating shock is needed. They assess the patient’s rhythm and—if ventricular fibrillation or ventricular tachycardia is present—charge the pads and deliver countershocks, without any intervention by the rescuer. Some AEDs may be fully automated, though these are now rare. A semiautomated AED, on the other hand, detects ventricular fibrillation and rapid ventricular tachycardia, a voice prompt may say, “Shock advised. Press to shock.” The rescuer must then depress the shock button to defibrillate the patient.
Remember to observe safety measures. Distance yourself from the patient. Do not defibrillate a patient who is in pooled water. Do not defibrillate a patient who is touching metal. Remove a nitroglycerin patch from a patient’s chest and wipe the area with a dry towel before defibrillation to prevent ignition of the patch.
If you witness a patient’s cardiac arrest, begin CPR starting with chest compressions and attach the AED as soon as it is available. However, if the patient’s cardiac arrest was not witnessed, especially if the call-to-arrival time is longer than 4 minutes, you should perform five cycles (about 2 minutes) of CPR before applying the AED. The rationale for this is that the heart is more likely to respond to defibrillation within the first few minutes of the onset of ventricular fibrillation. If the arrest interval is prolonged, however, metabolic waste products accumulate within the heart, energy stores are rapidly depleted, and the chance of successful defibrillation is reduced. Therefore, a 2-minute period of CPR before applying the AED to patients with prolonged cardiac arrest (greater than 4 to 5 minutes) can “prime the pump,” thus restoring oxygen to the heart, removing metabolic waste products, and increasing the chance of successful defibrillation.
The steps for using the AED are listed here and shown in Skill Drill 4:
Skill Drill 4
1. If CPR is in progress, assess the effectiveness of chest compressions by palpating for a carotid or femoral pulse. It is important to limit the amount of time compressions are interrupted. If the patient is responsive, do not apply the AED.
2. If the patient is unresponsive and CPR has not been started yet, begin providing chest compressions and rescue breaths at a ratio of 30 compressions to 2 breaths (beginning with compressions), continuing until an AED arrives and is ready for use Step 1. It is important to start chest compressions and use the AED as soon as possible. Compressions provide vital blood flow to the heart and brain, improving the patient’s chance of survival.
3. Turn on the AED now, or after pad placement, depending on manufacturer recommendations for the device you are using. Remove clothing from the patient’s chest area. Apply the pads to the chest: one just to the right of the breastbone (sternum) just below the collarbone (clavicle), the other on the left lower chest area with the top of the pad 2′ to 3′ below the armpit Step 2. Do not place the pads on top of breast tissue. If necessary, lift the breast out of the way and place the pad underneath. Ensure that the pads are attached to the patient cables (and that they are attached to the AED in some models). Plug in the pads connector to the AED.
4. Stop CPR.
5. State aloud, “Clear the patient,” and ensure that no one is touching the patient.
6. Push the Analyze button, if there is one, and wait for the AED to determine whether a shockable rhythm is present.
7. If a shock is not advised, perform five cycles (about 2 minutes) of CPR beginning with chest compressions and then reanalyze the cardiac rhythm. If a shock is advised, reconfirm that no one is touching the patient and push the Shock button.
8. After the shock is delivered, immediately resume CPR, beginning with chest compressions Step 3.
9. After five cycles (about 2 minutes) of CPR, reanalyze the patient’s cardiac rhythm Step 4. Do not interrupt chest compressions for more than 10 seconds.
10. If the AED advises a shock, clear the patient, push the Shock button, and after the shock is delivered immediately resume CPR compressions. If no shock is advised, immediately resume CPR, beginning with chest compressions.
11. Gather additional information about the arrest event.
12. After five cycles (2 minutes) of CPR, reassess the patient.
13. Repeat the cycle of 2 minutes of CPR, one shock (if indicated), and 2 minutes of CPR.
14. Begin to transport (if ALS has not yet arrived), and contact medical control as needed.
The care of the patient after the AED delivers a shock depends on your location and EMS system; therefore, you should follow your local protocols. After the AED protocol is completed, one of the following is likely:
 Pulse is regained.
Pulse is regained.
 No pulse is regained, and the AED indicates that no shock is advised.
No pulse is regained, and the AED indicates that no shock is advised.
 No pulse is regained, and the AED indicates that a shock is advised.
No pulse is regained, and the AED indicates that a shock is advised.
For each of these scenarios, the sequence of compressions and defibrillation is the same as described in the earlier section on manual defibrillation, with the only difference being that the AED determines whether the cardiac rhythm is shockable.
Cardiac Arrest During Transport If you are traveling to the hospital with an unresponsive patient, closely monitor the patient and watch for an ECG rhythm change as well as a pulse change. If a pulse is not present, take the following steps:
1. Stop the vehicle.
2. If the defibrillator is not immediately ready, perform CPR, beginning with chest compressions, until it is available.
3. Analyze the rhythm.
4. Deliver one shock, if indicated, and immediately resume CPR.
5. Continue resuscitation according to your local protocol.
Performing Defibrillation With an AED
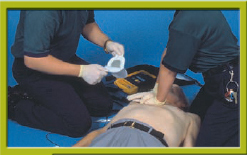
Step 1 Assess compression effectiveness if CPR is already in progress. If the patient is unresponsive and CPR has not been started yet, begin providing chest compressions and rescue breaths at a ratio of 30 compressions to 2 breaths, continuing until an AED arrives and is ready for use.
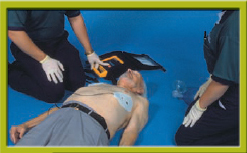
Step 2 Turn on the AED. Apply the AED pads to the chest and attach the pads to the AED. Stop CPR.
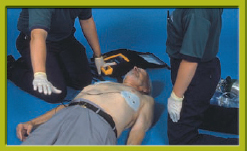
Step 3 Verbally and visually clear the patient. Push the Analyze button, if there is one. Wait for the AED to analyze the cardiac rhythm. If no shock is advised, perform five cycles (2 minutes) of CPR and then reanalyze the cardiac rhythm. If a shock is advised, recheck that all are clear, and push the Shock button. After the shock is delivered, immediately resume CPR beginning with chest compressions.
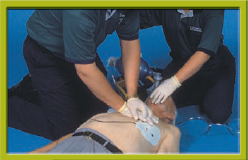
Step 4 After five cycles (2 minutes) of CPR, reanalyze the cardiac rhythm. Do not interrupt chest compressions for more than 10 seconds. If shock is advised, clear the patient, push the Shock button, and immediately resume CPR compressions. If no shock is advised, immediately resume CPR compressions. After five cycles (2 minutes) of CPR, reanalyze the cardiac rhythm. Repeat the cycle of five cycles (2 minutes) of CPR, one shock (if indicated), and 2 minutes of CPR. Begin transport (if ALS has not yet arrived), and contact medical control as needed.
If you are en route with a conscious adult patient who is having chest pain and becomes unconscious, take the following steps:
1. Check for a pulse.
2. Stop the vehicle.
3. If the defibrillator is not immediately ready, perform CPR, beginning with chest compressions, until it is ready.
4. Analyze the rhythm.
5. Deliver one shock, if indicated, and immediately resume CPR.
6. Begin compressions, and continue resuscitation according to your local protocol, including transporting the patient.
Synchronized cardioversion is the use of the defibrillator to terminate hemodynamically unstable tachydysrhythmias. Unlike defibrillation where energy is delivered at any time during the cardiac cycle, synchronized cardioversion involves a “timed” energy delivery. The device identifies R waves on the ECG and will only deliver energy at the peak of the R wave. Recall from the electrophysiology section that the R wave indicates ventricular depolarization. The peak of the R wave is significant because the majority of myocardial tissue is already depolarized and refractory to outside stimulus. Delivering energy during this time period increases the probability of depolarizing any myocytes that are polarized, allowing the SA to resume the primary pacemaker function. Synchronized cardioversion is performed just as defibrillation except that the synchronize setting on the defibrillator is selected first.
Emergency cardioversion is indicated for rapid ventricular and supraventricular rhythms that are associated with severely compromised CO—such as rapid ventricular tachycardia or SVT.
In the field, cardioversion is carried out only for patients whose CO is severely impaired. These patients are usually unconscious, so premedication is not necessary. When cardioversion is performed electively on a conscious patient, the patient must be sedated first; cardioversion is a painful and terrifying experience for a patient who is awake. Medications commonly used for sedation in these circumstances include benzodiazepines such as diazepam (Valium) or midazolam (Versed) (follow your protocol).
The procedure for cardioversion is listed here and shown in Skill Drill 5. Make sure the patient is placed supine; be prepared for the possibility that the patient could go into cardiac arrest.
Skill Drill 5
1. Take standard precautions Step 1.
2. Prepare the equipment Step 2.
3. Place ECG electrodes and lead wires in the same position as you would if you were performing cardiac monitoring or acquiring a 12-lead ECG (either way is fine). The best lead to use is lead II, since the R wave is tallest in this lead.
 White electrode: Right arm/shoulder
White electrode: Right arm/shoulder
 Black electrode: Left arm/shoulder
Black electrode: Left arm/shoulder
 Red electrode: Left leg/lower chest
Red electrode: Left leg/lower chest
 Green electrode: Right leg/lower chest
Green electrode: Right leg/lower chest
4. Place the multipurpose quick-connect pads in the proper positions. Turn the main power on, and assess the patient’s rhythm Step 3.
5. Turn the synchronize switch on the machine to the on position (unlike for defibrillation) Step 4. Note that the limb leads and defibrillation pads must be in place in order to perform synchronized cardioversion as the device is unable to sense electrical activity and delivery electricity through the same cable.
6. Assess the pulse Step 5. If pulse is absent, reevaluate the ECG rhythm and the need for other interventions. If a pulse is present, cardiovert.
7. Connect the pads to the monitor Step 6.
8. Check the patient’s blood pressure. Consider basic care, such as oxygen, if time permits. Sedate the patient Step 7.
9. Confirm the rhythm by looking at the monitor Step 8. It is best that this confirmation be made verbally so other caregivers are aware of the patient’s situation.
10. Prepare and apply the pads or paddles as described for defibrillation.
11. Set the energy level as ordered by the physician. Note that the energy level could be set by the physician or by protocol. Energy levels required for cardioversion vary depending on the type of dysrhythmia and the type of defibrillator. Supraventricular tachycardia, for example, can often be converted with energy levels as low as 50 J; by contrast, ventricular tachycardia will usually require at least 100 J. In emergencies, if an initial attempt to convert a rapid rhythm with a low energy level fails, immediately turn the setting up (stepwise to 100, 200, 300, and then 360 J) and repeat the shock as needed.
12. Charge the pads.
13. Clear the area by announcing, “All clear!” Step 9.
14. Reconfirm the rhythm by looking at the monitor.
15. Depress the shock buttons, and keep them depressed until the defibrillator discharges Step 10. That may take a few seconds because the charge is synchronized to fire about 10 ms after the peak of the R wave.
16. Reassess the patient’s condition (ECG rhythm and pulse) Step 11. Repeat the cardioversion if necessary.
17. If a cardioversion shock produces ventricular fibrillation, immediately take the following steps:
 Recharge the defibrillator to the setting for defibrillation.
Recharge the defibrillator to the setting for defibrillation.
 Turn the synchronizer circuit to the off position if it has not defaulted to that position.
Turn the synchronizer circuit to the off position if it has not defaulted to that position.
 Deliver the defibrillation and then immediately begin chest compressions.
Deliver the defibrillation and then immediately begin chest compressions.
Transcutaneous Cardiac Pacing
Artificial pacemakers deliver repetitive electric currents to the heart. Like the tiny electric currents generated by natural pacemakers, the current from an artificial pacemaker can cause the myocardial tissue to depolarize. In this way, the artificial pacemaker can substitute for a natural pacemaker that has become blocked or nonfunctional.
The artificial pacemakers first developed for emergency use consisted of a small battery pack and a wire that had to be threaded through a vein into the right ventricle of the heart. Insertion of one of those transvenous pacemakers was a tricky and often time-consuming job, usually best undertaken in a coronary care unit. Recently, however, effective transcutaneous pacemakers—that is, pacemakers that deliver their current through the skin of the chest—have been developed and have come into widespread use. Indeed, most prehospital monitor-defibrillators now come equipped with TCP capability.
Performing Cardioversion

Step 1 Take standard precautions.
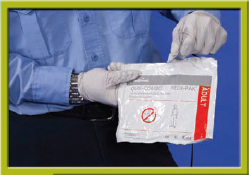
Step 2 Prepare the equipment. Place the electrodes in the same position as you would when performing cardiac monitoring or acquiring a 12-lead ECG.
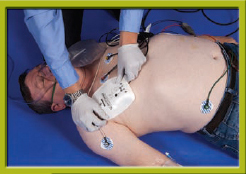
Step 3 Place the multipurpose quick-connect pads in the proper positions. Turn the main power on, and assess the patient’s rhythm.

Step 4 Turn the synchronize switch on the machine to the on position (unlike for defibrillation).
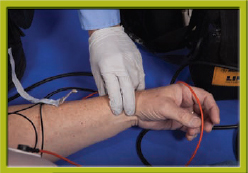
Step 5 Assess the pulse. If a pulse is present, prepare to cardiovert.

Step 6 Connect the pads to the monitor.
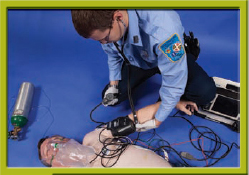
Step 7 Check the patient’s blood pressure. Sedate the patient.

Step 8 Confirm the rhythm. Prepare and apply the pads or paddles as described for defibrillation. Set the energy level as ordered by the physician. Charge the pads. Note that the energy level could be set by the physician or by protocol.
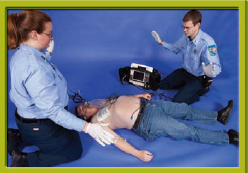
Step 9 Clear the area by announcing, “All clear!”
Step 10 Reconfirm the rhythm by looking at the monitor. Depress the shock buttons, and keep them depressed until the defibrillator discharges.
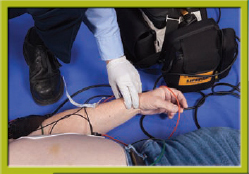
Step 11 Reassess the patient’s condition (ECG rhythm and pulse).
In TCP, a small electrical charge is passed through the patient’s skin across the heart between one externally placed pacing pad and another. The pacer is set for a specific rate, and the energy is increased until the heart just begins to respond to the stimulus. This phenomenon, which is termed “capture,” is usually associated with depolarization of the ventricles, which appears as a wide QRS complex on the ECG and results in a corresponding pulse.
TCP may have several useful applications in prehospital care:
 Interhospital transfer of patients needing pacemaker implantation (for example, a patient with complete heart block admitted to a small community hospital that does not have the facilities to implant a permanent pacemaker)
Interhospital transfer of patients needing pacemaker implantation (for example, a patient with complete heart block admitted to a small community hospital that does not have the facilities to implant a permanent pacemaker)
 Symptomatic patients with artificial pacemaker failure
Symptomatic patients with artificial pacemaker failure
 Patients with bradydysrhythmias or blocks associated with severely reduced CO and that are unresponsive to atropine, before cardiac arrest
Patients with bradydysrhythmias or blocks associated with severely reduced CO and that are unresponsive to atropine, before cardiac arrest
In any of those circumstances, TCP may buy time for the patient and enable him or her to reach the hospital in a state of optimal perfusion rather than in or near cardiac arrest.
Many brands of transcutaneous external cardiac pacemakers are available, and you must become familiar with the particular pacemaker used in your local EMS system. In general, the steps in initiating TCP are listed here and shown in Skill Drill 6:
Skill Drill 6
1. Take standard precautions Step 1.
2. Recognize the need for pacing. After connecting the patient to the ECG monitor and assessing the initial vital signs, determine the need for transcutaneous pacing based on the indications previously listed.
3. Obtain a precapture strip of the ECG tracing for documentation Step 2.
4. Explain the need for transcutaneous pacing to the patient and the family.
5. If you need to sedate the patient or pretreat the patient with IV medication, obtain IV access. Do not delay initiation of TCP to place an intravenous catheter!
6. Apply pacing electrodes Step 3. Often the defibrillation position is used when the same pads can be used for defibrillation and pacing. The alternative is to place one pad anteriorly left of the lower sternum and the other pad posteriorly just below the left scapula.
7. Attach the cables to the electrodes if not done previously.
8. Switch the pacer power on Step 4.
9. Set the pacing rate (70 to 80 beats/min is commonly chosen) Step 5.
10. Start increasing the current Step 6. Raise the current by 10 to 20 milliamps every few seconds.
11. Check for capture Step 7; that is, look for every pacemaker spike being followed by a (usually wide) QRS complex Figure 129. If the QRS is not present, the pacemaker current is not depolarizing the ventricles. Increase the current gradually until there is consistent capture.
12. Once capture is achieved, briefly lower the current until capture is lost, and then increase it by the smallest amount possible to restore capture Step 8. The purpose of this action is to find the lowest energy setting that achieves consistent capture.
13. Obtain rhythm strips for documentation.
14. Immediately transport the patient.
Transcutaneous pacemakers depolarize not only cardiac muscle, but also muscles in the chest wall beneath the pacing electrode. As a result, patients who are conscious when TCP is initiated (or who regain consciousness during pacing) usually experience chest discomfort and sometimes severe pain from the procedure. Some form of analgesia and sedation (such as diazepam [Valium] or morphine [Astramorph PF]) should be given to conscious patients when transcutaneous pacemakers are used.
 Management of Symptomatic Bradycardia
Management of Symptomatic Bradycardia
A patient who presents with or develops symptomatic bradycardia needs to be treated in a manner that will increase the heart rate and improve CO. Symptoms such as altered mental status and hypotension are common indications for treatment of bradycardic patients. Assuming that airway and breathing have been supported:
Performing Transcutaneous Pacing

Step 1 Take standard precautions.
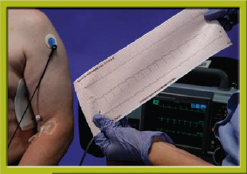
Step 2 Obtain a precapture strip.
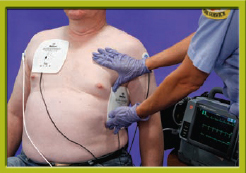
Step 3 Explain the need for transcutaneous pacing to the patient and the family. Apply the pacing electrodes.

Step 4 Switch the pacer power on.

Step 5 Set the pacing rate.
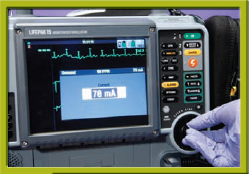
Step 6 Start increasing the current.
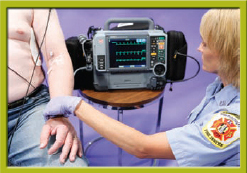
Step 7 Check for mechanical capture.

Step 8 Once capture is achieved, briefly lower the current until capture is lost, and then increase it by the smallest amount possible to restore capture. Obtain rhythm strips for documentation.
Figure 129 Pacemaker spike followed by QRS complex.
1. Establish an IV line of normal saline.
2. Administer atropine, 0.5-mg IV bolus. You may repeat this dose every 3 to 5 minutes until the heart reaches the desired rate (usually 60 beats/min or faster) or until the maximum total dose of 0.04 mg/kg has been reached.
3. If the patient is in severely compromised condition or does not respond to the administration of atropine, establish TCP as quickly as possible. If the patient is in a second-degree type II or third-degree heart block, TCP is the firstline treatment.
4. If atropine and TCP are unsuccessful (or if TCP is unavailable), consider the administration of a sympathomimetic drug—most commonly, dopamine (Intropin) or epinephrine, albeit only as a drip in this situation. Dopamine, which is the milder of the two, is administered at a dose of 2 to 10 μg/kg/min. The epinephrine drip rate is 2 to 10 μg/min. To mix an epinephrine drip, put 1 mg of epinephrine into a 250-mL bag of normal saline, start the drip at 30 drops/min with a microdrip administration set, and titrate it to the desired heart rate.
5. Transport the patient to a hospital capable of transvenous pacing and surgical implantation of pacemakers.
Patients who are symptomatic and require TCP in the field often require the surgical implantation of a pacemaker in the hospital Figure 130. Early identification and hospital notification can often speed this process.
A patient who presents with or develops tachycardia presents a more complicated situation than one in bradycardia. Tachycardia can have a supraventricular pacemaker site or may be ventricular in origin. In addition, the patient may be mildly or severely symptomatic owing to the tachycardia or another condition. Because of the many possible variations in tachycardic patients, several judgments must be made before treatment is begun.
The first decision relates to the seriousness of the signs or symptoms the patient is exhibiting. Patients who present with serious signs and symptoms such as chest pain, dyspnea, hypotension, or altered mental status should be considered in unstable condition and may need immediate treatment. First, however, you must determine whether these signs and symptoms are the result of the tachycardia or whether the tachycardia and signs and symptoms are the response to another condition.
Tachycardias with rates of less than 150 beats/min are rarely fast enough to cause serious signs and symptoms. For example, a patient who is experiencing an MI is likely to be mildly tachycardic, but obviously the MI—not the tachycardia—is causing the signs and symptoms. Conversely, a patient who was previously asymptomatic but becomes symptomatic only after the onset of the tachycardia is more likely presenting with symptoms resulting from the tachycardia. This brings to mind the adage: “Treat the patient, not the monitor.” It is critical to make this distinction before beginning treatment, because slowing the heart rate of a patient whose heart is compensating for a medical condition may be a fatal mistake.
A patient in unstable condition whose signs and symptoms are determined to be the result of tachycardia needs cardioversion. Electrical cardioversion is similar to defibrillation and, as such, is a serious intervention. For this reason, it is limited to patients whose condition is so serious as to make them likely to arrest if the treatment is not administered quickly. Most of these patients will be unconscious. In the unlikely case of a conscious patient who needs cardioversion, sedation (usually with diazepam [Valium] or midazolam [Versed]) is a necessity. Wait an appropriate amount of time for the drugs to take effect before cardioversion. Should the patient become unconscious, sedation is no longer a concern.
When a patient in tachycardia has limited or mild signs and symptoms, a slower but safer treatment regimen is recommended. In these cases, it becomes necessary to determine the origin of the tachycardia or the pacemaker site of the rhythm. Generally speaking, wide QRS complexes are presumed to be ventricular in origin, whereas narrow QRS complexes (< 0.12 s) are presumed to be supraventricular in origin. SVTs may originate in the SA node, elsewhere in the atria, or in the AV node (junctional rhythms). The differentiation among these three pacemaker sites requires examining the P wave. In tachycardias with rates exceeding 150 beats/min, however, the P waves (if present) are usually “buried” within the T wave of the preceding beat. The inability to see P waves limits you to labeling these tachycardias as supraventricular rather than giving a specific site of origin.
Figure 130 Algorithm for bradycardia.
Occasionally, aberrant conduction of a supraventricularly originated beat will make it difficult to identify a tachycardia as truly ventricular or supraventricular. In most cases of uncertainty, the rhythm is ventricular rather than supraventricular and should be treated as such. In either case, you should administer oxygen and establish an IV line for normal saline.
In SVTs, you should attempt to stimulate the patient’s vagus nerve. Many vagal stimulation techniques exist, including carotid sinus massage, which is shown in Figure 131, but the most common technique is having the patient bear down against a closed glottis. The patient is instructed to perform this technique as if attempting to have a bowel movement. The stimulation of the vagal nerve in turn stimulates the parasympathetic nervous system to slow the heart. Never massage both carotid arteries simultaneously as significant bradycardia or asystole may result Figure 132. One factor to consider when deciding whether to perform carotid massage is the patient’s history. If the patient’s condition involves risks that would override the potential rewards, do not perform the technique. For example, a patient with advanced age, coronary artery disease, and high cholesterol would not be a good candidate for carotid massage because of the high risk of thromboembolism. If carotid massage is successful, the patient should still be transported for hospital evaluation because the condition is likely to recur. If it reappears, instruct the patient to repeat the vagal maneuver. If at any time the vagal stimulation proves unsuccessful, pharmacologic treatment should be attempted.
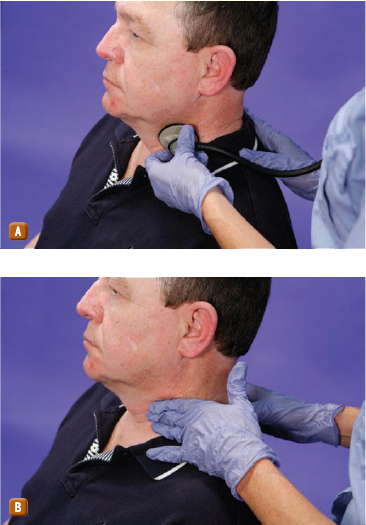
Figure 131 Carotid sinus massage. A. Listen for bruits. B. Massage the carotid artery.
Next, administer adenosine (Adenocard), 6 mg, by rapid IV push. Adenosine is in the class of drugs called purine nucleosides. In EMS, adenosine is used to transiently induce AV nodal blockade in order to interrupt tachydysrhythmias involving the AV node. Before you begin this treatment, you should always recheck the history for allergies and advise the patient of the possible adverse effects of adenosine administration. To administer the medication, choose the closest IV site to the patient and insert the syringe of adenosine. In the same site, insert another syringe containing at least 20 mL of normal saline solution. After clamping off the IV line above the site, push the adenosine as rapidly as possible and then push the saline as soon as the adenosine plunger hits bottom. Be prepared to see a short run of asystole with the administration of adenosine (although this response does not always occur). If the first dose of adenosine is unsuccessful, you may administer it again in 1 to 2 minutes up to two times at 12 mg each. If the adenosine is unsuccessful in converting the patient’s rhythm, transport expeditiously to the hospital without further treatment as long at the patient remains in stable condition.
If at any time the condition of a patient with SVT becomes unstable, you should move to the “unstable” or cardioversion algorithm. Remember that when cardioversion of SVT is required, you should start at a lower energy setting than with a ventricular rhythm.
If the patient is in stable condition but the rhythm is ventricular in origin, the patient should be transported to the hospital while you watch carefully for the development of serious signs and symptoms. If they appear, the patient should undergo cardioversion according to the unstable tachycardia algorithm. If your transport time to the hospital is long, medical control may order the administration of a ventricular antidysrhythmic medication such as amiodarone (Cordarone, Pacerone) or lidocaine (Xylocaine).
Any patient with a tachycardic rhythm should be monitored carefully Figure 133. A heart that is stressed by the requirements of excessive tachycardia is likely to become ischemic and is at high risk for arrest.
Nothing gets the adrenaline pumping more furiously—in paramedics, even if not in the patient—than a “code,” or cardiopulmonary arrest. Most cardiac arrest victims have evidence of atherosclerosis or other underlying cardiac disease. However, cardiac arrest can also occur after electrocution, drowning, and other types of trauma. Indeed, many cardiac arrest victims have no warning before the event occurs. No matter what the cause, cardiac arrest is a stressful event for all involved. The best way to reduce the stress in providers and increase the potential for return of spontaneous circulation is to practice, practice, and practice so your team works like a pit crew. This concept is discussed further in the chapter, Responding to the Field Code.
Figure 132 Never massage both the internal and external carotid arteries at the same time.
Words of Wisdom
The calcium channel blocker verapamil is often used by paramedics to achieve rate control of tachdysrhythmias. Verapamil’s mechanism of action includes blocking conduction of electrical impulses through the AV node. AV nodal blockade is a safe and effective way to protect the ventricles from atrial tachydysrhythmias and to slow the overall heart rate. Wide QRS complexes on the ECG may be signs of bundle branch block, ventricular dysrhythmia, or preexcitation. Administration of verapamil to a patient with preexcitation can lead to VF or VT and sudden death. Therefore, administration of verapamil must be reserved for patients exhibiting narrow QRS complex tachydysrhythmias and should never be administered in wide complex tachycardias.
Management of cardiac arrest requires you to deploy a great many of the advanced life support (ALS) skills that you have learned and to do so under urgent circumstances in which minutes may mean the difference between life and death. It is difficult to think clearly in such stressful circumstances, especially when there are likely to be other stressed and panicky people at the scene (the patient’s family, for example). For these reasons, it is absolutely essential for you to follow an orderly, systematic approach to cardiac arrest emergencies. That approach needs to be rehearsed repeatedly, in a team setting, until it is nearly automatic, and must include the steps of BLS and ALS.
BLS: A Review
The techniques and sequences of BLS should be familiar to all paramedic students. Remember, good ALS builds on good BLS, and good BLS builds on prompt bystander action. This section reviews the guidelines for ensuring maximally effective (and minimally damaging) CPR to adults in cardiac arrest. In the 2010 AHA Guidelines, there was a change from the “ABC” routine to “CAB.” That is, CPR should now be initiated prior to the assessment of the airway and breathing in the unresponsive patient.
 Concentrate on high-quality compressions (deep enough–more than 2″, fast enough–100 times a minute, and with full chest recoil) with a minimum of interruptions.
Concentrate on high-quality compressions (deep enough–more than 2″, fast enough–100 times a minute, and with full chest recoil) with a minimum of interruptions.
 Avoid excessive volume and inflation pressure in artificial ventilation. Inflate just enough to observe visible chest rise.
Avoid excessive volume and inflation pressure in artificial ventilation. Inflate just enough to observe visible chest rise.
 Keep your compressions smooth, regular, and uninterrupted.
Keep your compressions smooth, regular, and uninterrupted.
1. Maintain each compression for at least half the compression-release cycle.
2. Avoid bouncing or jerky compressions,
3. Keep your shoulders directly over the patient’s sternum, and keep your elbows straight.
4. Maintain proper hand position: fingers off the chest, and hands coming up off the sternum slightly between compressions to allow for complete chest recoil.
5. Rotate fresh compressors every 2 minutes when help is available.
 As a single rescuer for adults, give 30 compressions to 2 ventilations at a rate of 100 compressions per minute. Once an advanced airway is placed, compressions continue at a rate of 100/min uninterrupted with 8 to 10 ventilations given with 100% supplemental oxygen.
As a single rescuer for adults, give 30 compressions to 2 ventilations at a rate of 100 compressions per minute. Once an advanced airway is placed, compressions continue at a rate of 100/min uninterrupted with 8 to 10 ventilations given with 100% supplemental oxygen.
 Do not interrupt CPR compressions except for advanced airway placement, defibrillation, or moving the patient. In all cases, minimize the duration of the interruption to 10 seconds or less. Any stop in compressions also stops perfusion—and perfusion is what it is all about!
Do not interrupt CPR compressions except for advanced airway placement, defibrillation, or moving the patient. In all cases, minimize the duration of the interruption to 10 seconds or less. Any stop in compressions also stops perfusion—and perfusion is what it is all about!
Now you will learn how to integrate these well-rehearsed steps of BLS into the sequences of ACLS.
Advanced Cardiac Life Support
BLS is defined as maintenance of circulation and the airway and breathing without adjunctive equipment. Early quality compressions and defibrillation, both of which are BLS, are the measures that have been scientifically proven to have the greatest success for patients in ventricular fibrillation. In addition to high-quality BLS, you will be called on to deliver more definitive therapy as well, so the skills of ACLS must also become second nature, to be deployed swiftly and systematically in the event of cardiac arrest.
Figure 133 Algorithm for tachycardia.
The AHA has defined ACLS for a patient in cardiac arrest (or a patient at immediate risk of cardiac arrest) as consisting of the following elements:
 Effective and minimally interrupted chest compression (for cardiac arrest)
Effective and minimally interrupted chest compression (for cardiac arrest)
 Use of adjunctive equipment for ventilation and circulation
Use of adjunctive equipment for ventilation and circulation
 Cardiac monitoring for dysrhythmia recognition and control
Cardiac monitoring for dysrhythmia recognition and control
 Establishment and maintenance of an IV infusion line
Establishment and maintenance of an IV infusion line
 Use of definitive therapy, including defibrillation and drug administration, to:
Use of definitive therapy, including defibrillation and drug administration, to:
2. Aid in establishing an effective cardiac rhythm and circulation when cardiac arrest occurs
3. Stabilize the patient’s condition
 Administration of hypothermia therapy for patients who are in a coma after return of spontaneous circulation
Administration of hypothermia therapy for patients who are in a coma after return of spontaneous circulation
 Transport to an appropriate facility—one that is prepared to provide successful resuscitation treatment
Transport to an appropriate facility—one that is prepared to provide successful resuscitation treatment
 Transport with continuous monitoring.
Transport with continuous monitoring.
The use of airway adjuncts and equipment for artificial ventilation has already been discussed. In this section, the focus is on the sequence of actions in ACLS. Some of the specific techniques—such as defibrillation—for restoring an effective cardiac rhythm were discussed earlier in this chapter.
The Universal Algorithm The approach to every patient in cardiac arrest will start with the same steps, which the AHA calls the BLS Healthcare Provider Algorithm Figure 134. These basic steps are always deployed as soon as a person is found unresponsive and possibly in cardiac arrest. The BLS health care provider algorithm includes measures that bystanders should take before your arrival (such as “phone 9-1-1 or emergency number”), so you need to modify the universal algorithm a bit to make it applicable to emergency medical services personnel.
As always, you should bring your defibrillator with you when you initially approach the scene, as well as a portable oxygen cylinder and a “jump kit” that contains equipment for managing the airway. Also take the intubation kit, the IV equipment, and the drug box. If you are shorthanded, do not spend time carrying every piece of equipment from the ambulance to the patient; you can send someone to the ambulance for other equipment, such as the backboard and stretcher, later.
As soon as you reach the patient, one paramedic should ready the monitor-defibrillator while the other carries out the following steps:
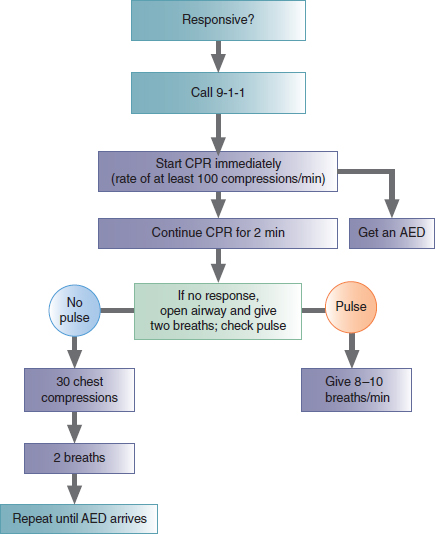
Figure 134 Algorithm for BLS Healthcare Providers.
1. Assess the circulation. If there is no pulse, start CPR. CPR should continue for 2 minutes or five cycles of 30 compressions and 2 ventilations. As CPR continues, the second paramedic should attach the monitor-defibrillator. At the end of 2 minutes, pause CPR and proceed with the next steps.
2. Assess responsiveness. If the patient is not responsive:
 Open the airway and assess for breathing. If the patient is not breathing:
Open the airway and assess for breathing. If the patient is not breathing:
 Give two slow breaths. Use the bag-mask device or a barrier device.
Give two slow breaths. Use the bag-mask device or a barrier device.
3. Check for a pulse, and check the rhythm on the monitor. At this point, all you want to know is the answer to one question: Is ventricular fibrillation or ventricular tachycardia present?
 If ventricular fibrillation or ventricular tachycardia is present on the monitor-defibrillator, follow the ventricular fibrillation/ventricular tachycardia arm of the algorithm.
If ventricular fibrillation or ventricular tachycardia is present on the monitor-defibrillator, follow the ventricular fibrillation/ventricular tachycardia arm of the algorithm.
 If ventricular fibrillation or ventricular tachycardia is not present on the monitor-defibrillator, resume CPR immediately.
If ventricular fibrillation or ventricular tachycardia is not present on the monitor-defibrillator, resume CPR immediately.
What you see on the monitor at this point will determine which side of the algorithm you will now follow. If the patient is still in cardiac arrest, he or she may be in any of the following situations:
 Ventricular fibrillation or pulseless ventricular tachycardia
Ventricular fibrillation or pulseless ventricular tachycardia
 PEA (that is, you can see an organized rhythm on the monitor, but there is no detectable pulse)
PEA (that is, you can see an organized rhythm on the monitor, but there is no detectable pulse)
 Asystole
Asystole
Each of these situations requires a different, specific approach (a different pathway down the pulseless arrest algorithm).
Treatment for Ventricular Fibrillation or Pulseless Ventricular Tachycardia Managing ventricular fibrillation or pulseless ventricular tachycardia is probably the most important algorithm for you to know because patients found in ventricular fibrillation or ventricular tachycardia are the most likely to be successfully resuscitated—if they receive timely and appropriate treatment. The steps of the ventricular fibrillation/ventricular tachycardia pathway down the pulseless arrest algorithm are presented schematically in Figure 135. Additionally, Table 23 lists possible causes and treatment of Cardiac arrest rhythms.
The steps in managing ventricular fibrillation and pulseless ventricular tachycardia are as follows:
1. Address CAB issues.
2. Begin CPR immediately and attach the defibrillator simultaneously, if sufficient personnel are available. Continue CPR for 2 minutes if you did not witness the cardiac arrest.
3. Confirm ventricular fibrillation or ventricular tachycardia on the monitor-defibrillator.
4. Confirm absence of a pulse (in a maximum of 10 seconds). Other things besides ventricular fibrillation and ventricular tachycardia can make squiggly lines on a monitor, such as loose ECG leads or muscle tremor. Remember: Treat the patient, not the monitor.
5. Resume CPR while charging the defibrillator.
6. Clear the patient and then defibrillate the ventricular fibrillation or ventricular tachycardia:
 If using a biphasic defibrillator, set it to 120 to 200 joules (J). This energy level depends on the manufacturer’s recommendation. If the recommendation is unknown and the defibrillator is biphasic, use 200 J as the default energy dose.
If using a biphasic defibrillator, set it to 120 to 200 joules (J). This energy level depends on the manufacturer’s recommendation. If the recommendation is unknown and the defibrillator is biphasic, use 200 J as the default energy dose.
 If using a monophasic defibrillator, set it to 360 J.
If using a monophasic defibrillator, set it to 360 J.
As soon as the defibrillator discharges, resume CPR. It is important not to delay resuming CPR at this time to determine the rhythm. Continue CPR for 2 minutes or five cycles. Recent research indicates that even if an organized rhythm appears in the post-resuscitation period, the presence of an immediate pulse is unlikely. It has also been shown that 2 minutes of post-resuscitation CPR is unlikely to cause a return of ventricular fibrillation. After 2 minutes or five cycles, stop CPR, and assess the patient’s circulation and check the rhythm on the monitor.
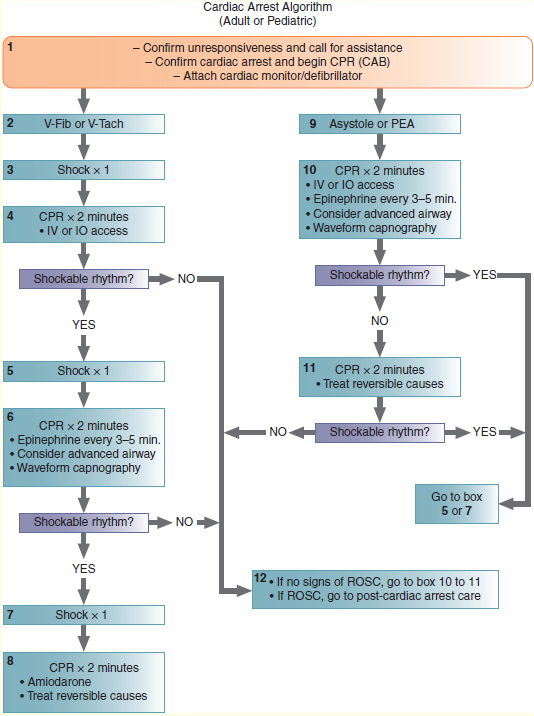
Figure 135 ACLS algorithm for cardiac arrest. Reversible causes in adults that can be treated include Hs and Ts: hypovolemia, hypoxia, hydrogen ion (acidosis), hypokalemia, hyperkalemia, hypothermia, tension pneumothorax, toxins, cardiac tamponade, and pulmonary and coronary thrombosis.
Table 23 Possible Causes and Treatment of rdiac Arrest Rhythms
Possible Cause of PEA to Consider During Arrest |
Clues to Cause |
Treatment (Beyond Managing the Cardiac Arrest) |
Hypovolemia |
Patient history |
Volume infusion |
Hypoxemia |
Cyanosis, airway problem |
Intubation and ventilation with 100% oxygen |
Hypoglycemia |
Blood glucose level < 60 mg/dL |
Dextrose 50% in water, 25 g |
Hypothermia |
History of exposure to cold |
See hypothermia algorithm in the chapter, Environmental Emergencies |
Hyperkalemia, hypokalemia, hydrogen ions (acidosis) |
History, ECG changes |
Immediate transport Consider sodium bicarbonate if certain of acidosis |
Tension pneumothorax |
History, no pulse with CPR, unequal breath sounds with hyperresonance to percussion on affected side |
Needle decompression of the affected side of the chest |
Cardiac tamponade |
History, no pulse with CPR, jugular venous distention |
Pericardiocentesis (immediate transport) |
Others: Drug overdose, trauma, massive MI, pulmonary embolism |
History |
Consider need for immediate transport. Naloxone (Narcan) for opioid or narcotic overdose. |
7. If a rhythm other than ventricular fibrillation or ventricular tachycardia appears on the monitor screen:
 Identify the new rhythm.
Identify the new rhythm.
 If there is no pulse, move to the asystole-PEA pathway down the algorithm and resume CPR immediately.
If there is no pulse, move to the asystole-PEA pathway down the algorithm and resume CPR immediately.
 If there is a pulse, move to the appropriate algorithm for the new rhythm.
If there is a pulse, move to the appropriate algorithm for the new rhythm.
8. If the rhythm continues to be ventricular fibrillation or ventricular tachycardia, resume CPR while charging the defibrillator.
9. Clear the patient and then defibrillate the ventricular fibrillation or ventricular tachycardia:
 Use the same energy setting as for the initial shock.
Use the same energy setting as for the initial shock.
 Resume CPR immediately, and continue for 2 minutes after the shock. The CPR compressor and ventilator should change positions at the end of each 2-minute session of CPR (while the rhythm and pulse are being checked) to avoid fatigue, which can reduce the effectiveness of chest compressions.
Resume CPR immediately, and continue for 2 minutes after the shock. The CPR compressor and ventilator should change positions at the end of each 2-minute session of CPR (while the rhythm and pulse are being checked) to avoid fatigue, which can reduce the effectiveness of chest compressions.
 During these 2 minutes of CPR, you should insert an advanced airway only if the BLS airway is not adequate. Advanced airways include intraglottic and extraglottic devices including the endotracheal tube, King LT airway, laryngeal mask airway, and Combitube or EasyTube. After intubation, verify placement using multiple methods include waveform capnography, and secure the tube. Once the patient has been intubated, it is no longer necessary to pause CPR compressions for ventilation to be administered. Ventilations should be administered at a rate of 8 to 10 breaths per minute (one breath every 6 to 8 seconds or one breath after each 10 to 12 compressions). The rate of compressions is 100/min.
During these 2 minutes of CPR, you should insert an advanced airway only if the BLS airway is not adequate. Advanced airways include intraglottic and extraglottic devices including the endotracheal tube, King LT airway, laryngeal mask airway, and Combitube or EasyTube. After intubation, verify placement using multiple methods include waveform capnography, and secure the tube. Once the patient has been intubated, it is no longer necessary to pause CPR compressions for ventilation to be administered. Ventilations should be administered at a rate of 8 to 10 breaths per minute (one breath every 6 to 8 seconds or one breath after each 10 to 12 compressions). The rate of compressions is 100/min.
 Start an IV line with normal saline.
Start an IV line with normal saline.
 If unable to establish IV access, establish intraosseous (IO) access via an adult IO access system. If IV access is not obtained but IO access is, all drugs and fluids that would normally be administered via IV should be administered via IO until IV access is established.
If unable to establish IV access, establish intraosseous (IO) access via an adult IO access system. If IV access is not obtained but IO access is, all drugs and fluids that would normally be administered via IV should be administered via IO until IV access is established.
 As soon as IV or IO access has been established, administer a vasopressor drug. The two recommended vasopressor drugs are epinephrine and vasopressin (Pitressin synthetic). Epinephrine (1:10,000) is given as 1 mg IV push; this dose should be repeated every 3 to 5 minutes as long as a pulse is absent. Vasopressin is given as 40 units IV push, one time only. Vasopressin can be given in place of the first or second dose of epinephrine (but not both). Whenever you give a medication through a peripheral IV line during CPR, follow it immediately with a 20- to 30-mL bolus of IV fluid and then elevate the extremity to facilitate delivery of the medication to the central circulation (which may take 1 to 2 minutes). Note that IO administration has the same effect of central circulation.
As soon as IV or IO access has been established, administer a vasopressor drug. The two recommended vasopressor drugs are epinephrine and vasopressin (Pitressin synthetic). Epinephrine (1:10,000) is given as 1 mg IV push; this dose should be repeated every 3 to 5 minutes as long as a pulse is absent. Vasopressin is given as 40 units IV push, one time only. Vasopressin can be given in place of the first or second dose of epinephrine (but not both). Whenever you give a medication through a peripheral IV line during CPR, follow it immediately with a 20- to 30-mL bolus of IV fluid and then elevate the extremity to facilitate delivery of the medication to the central circulation (which may take 1 to 2 minutes). Note that IO administration has the same effect of central circulation.
10. At the end of 2 minutes of CPR, pause compressions to check for circulation and check the rhythm on the monitor.
11. If ventricular fibrillation or ventricular tachycardia is still present, resume CPR while charging the defibrillator.
12. Clear the patient and then defibrillate the ventricular fibrillation or ventricular tachycardia:
 Use the same energy setting as before.
Use the same energy setting as before.
 Resume CPR immediately, and continue for 2 minutes after the shock. Remember to change CPR compressors after each rhythm check.
Resume CPR immediately, and continue for 2 minutes after the shock. Remember to change CPR compressors after each rhythm check.
 During these 2 minutes of CPR, you should consider the administration of an antidysrhythmic medication. The preferred antidysrhythmic medication is amiodarone (Cordarone, Pacerone), which is given as a 300-mg bolus during CPR. Amiodarone may be repeated once at 150 mg in 3 to 5 minutes after the initial dose. If amiodarone is unavailable, you may administer lidocaine, 1 to 1.5 mg/kg IV push. Lidocaine (Xylocaine) can be repeated at 0.5 to 0.75 mg/kg every 5 to 10 minutes until the maximum dose of 3 mg/kg has been reached. It is important not to combine these two antidysrhythmic medications because this practice can actually cause dysrhythmias.
During these 2 minutes of CPR, you should consider the administration of an antidysrhythmic medication. The preferred antidysrhythmic medication is amiodarone (Cordarone, Pacerone), which is given as a 300-mg bolus during CPR. Amiodarone may be repeated once at 150 mg in 3 to 5 minutes after the initial dose. If amiodarone is unavailable, you may administer lidocaine, 1 to 1.5 mg/kg IV push. Lidocaine (Xylocaine) can be repeated at 0.5 to 0.75 mg/kg every 5 to 10 minutes until the maximum dose of 3 mg/kg has been reached. It is important not to combine these two antidysrhythmic medications because this practice can actually cause dysrhythmias.
13. At the end of 2 minutes of CPR, pause compressions to check for circulation and check the rhythm on the monitor.
14. If ventricular fibrillation or ventricular tachycardia is still present, resume CPR while charging the defibrillator.
15. Clear the patient and then defibrillate the ventricular fibrillation or ventricular tachycardia:
 Use the same energy setting as before.
Use the same energy setting as before.
 Resume CPR immediately, and continue for 2 minutes after the shock. Remember to change CPR compressors after each rhythm check.
Resume CPR immediately, and continue for 2 minutes after the shock. Remember to change CPR compressors after each rhythm check.
 If ventricular fibrillation or ventricular tachycardia is still present, consider making a transport decision with the advice of medical control. Continue the cycle of defibrillation followed by immediate CPR for 2 minutes while administering repeated doses of medications.
If ventricular fibrillation or ventricular tachycardia is still present, consider making a transport decision with the advice of medical control. Continue the cycle of defibrillation followed by immediate CPR for 2 minutes while administering repeated doses of medications.
16. If at any point during this sequence there is a return of spontaneous circulation:
 Assess the patient’s vital signs.
Assess the patient’s vital signs.
 Support the airway and breathing, as required.
Support the airway and breathing, as required.
 Provide medications as indicated for regulating the heart rate, controlling cardiac dysrhythmias, and maintaining the blood pressure.
Provide medications as indicated for regulating the heart rate, controlling cardiac dysrhythmias, and maintaining the blood pressure.
17. Consider implementation of hypothermia protocol and transport to appropriate cardiac care center.
Treatment for Pulseless Electrical Activity The term pulseless electrical activity (PEA) refers to an organized cardiac rhythm (other than ventricular tachycardia) on the monitor that is not accompanied by any detectable pulse. This category includes what was once called electromechanical dissociation and conditions in which the heart beats so weakly that it cannot produce a palpable pulse, which may occur, for example, in cardiogenic or hypovolemic shock, cardiac tamponade, massive pulmonary embolism, disturbances of electrolyte imbalance, including hyperkalemia in renal failure, or drug overdose. Providing the appropriate treatment depends on identifying the cause of PEA in a specific case.
When the monitor is applied to a pulseless patient and a rhythm (other than ventricular fibrillation, ventricular tachycardia, or asystole) is seen:
1. Immediately resume CPR.
2. Insert an advanced airway if the BLS airway is not adequate.
3. Start an IV line with normal saline.
4. If you are unable to establish IV access, consider establishing IO access.
5. As soon as IV or IO access has been established, administer a vasopressor drug. The two recommended vasopressor drugs are epinephrine and vasopressin. Epinephrine (1:10,000) is given as 1 mg IV push; this dose should be repeated every 3 to 5 minutes for as long as a pulse is absent. Vasopressin (Pitressin synthetic) is given as 40 units IV push, one time only. Vasopressin can be given in place of the first or second dose of epinephrine (but not both). Whenever you give a medication through a peripheral IV line during CPR, follow it immediately with a 20- to 30-mL bolus of IV fluid and then elevate the extremity to facilitate delivery of the medication to the central circulation (which may take 1 to 2 minutes).
6. At the end of 2 minutes of CPR, pause the compressions to check for circulation and check the rhythm on the monitor.
7. If PEA is still present:
 Continue CPR immediately.
Continue CPR immediately.
 Search for and treat the possible causes (“Hs and Ts,” discussed in Table 23).
Search for and treat the possible causes (“Hs and Ts,” discussed in Table 23).
Words of Wisdom
Sodium bicarbonate is in the class of drugs called alkalinizing agents. These drugs are used to increase the pH of blood and urine (alkalinize), for example in cases of acidosis.
Asystole Treatment A flat line on an ECG monitor may or may not be asystole. Thus, one of the first things to do when you see a flat-line ECG is to rule out causes other than asystole. Possible causes of a flat-line ECG include leads that are not connected to the patient, loose leads, leads that are not connected to the monitor-defibrillator, an incorrect monitor setting, very-low-voltage ventricular fibrillation, and true asystole.
When the monitor is applied to a pulseless patient and asystole is seen:
1. Immediately resume CPR, assuming you are not presented with a valid advance directive indicating not to perform CPR (“do not resuscitate” orders).
2. Confirm asystole by checking for other causes of the flat line. Make sure that all monitoring electrodes are firmly fastened to the patient and that the cables are hooked into the monitor. Switch to another lead to detect low-voltage ventricular fibrillation. If the rhythm is asystole, you need to be aware that the prognosis is grim and the chances for successful resuscitation are poor.
3. Insert an advanced airway if the BLS airway is not adequate.
4. Start an IV line with normal saline.
5. If unable to establish IV access, consider establishing IO access.
6. As soon as IV or IO access has been established, administer a vasopressor drug. The two recommended vasopressor drugs are epinephrine and vasopressin. Epinephrine (1:10,000) is given as 1 mg IV push; this dose should be repeated every 3 to 5 minutes for as long as a pulse is absent. Vasopressin (Pitressin synthetic) is given as 40 units IV push, one time only. Vasopressin can be given in place of the first or second dose of epinephrine (but not both). Whenever you give a medication through a peripheral IV line during CPR, follow it immediately with a 20- to 30-mL bolus of IV fluid and then elevate the extremity to facilitate delivery of the medication to the central circulation (which may take 1 to 2 minutes).
7. At the end of every 2 minutes of CPR, pause the compressions to check for circulation and to check the rhythm on the monitor.
8. If asystole is still present:
 Immediately resume CPR.
Immediately resume CPR.
 Search for and treat possible causes. Possible causes are the same as for PEA and are listed in Table 23.
Search for and treat possible causes. Possible causes are the same as for PEA and are listed in Table 23.
 Seriously consider termination of the resuscitation with advisement from medical control and then focus on the survivors.
Seriously consider termination of the resuscitation with advisement from medical control and then focus on the survivors.
Postresuscitative Care
If an effective cardiac rhythm is restored in the field, your next task is to make sure that the rhythm stays restored and that optimal conditions are provided to promote recovery of the patient’s brain from the hypoxic insult of cardiac arrest.
First, the heart rate should be stabilized. If the rhythm is bradycardic or tachycardic in the postresuscitation period, the bradycardia or tachycardia algorithms should be followed.
Next, cardiac rhythm should be stabilized to the degree possible. If the arrest rhythm was ventricular fibrillation or ventricular tachycardia, consider administering a bolus of an anti-dysrhythmic drug, followed by an infusion of the same drug. Historically, lidocaine (Xylocaine) has been given in this situation, but if amiodarone (Cordarone, Pacerone) was given to the patient in arrest, then an infusion of amiodarone should be started and lidocaine should not be used. If severe bradycardia is present in the postarrest period, atropine or TCP may be required, and the hospital should be alerted to prepare a transcutaneous pacemaker.
Once the cardiac rhythm is stable, attention turns to the patient’s brain and to ameliorating the effects of cardiac arrest on it. Marked hypotension needs to be corrected rapidly because the brain will not be adequately perfused if the blood pressure is very low. If the patient has marked hypotension and the transport time to the hospital will be prolonged, the physician may order an infusion of dopamine (Intropin). In an intubated patient, avoid tracheal suctioning unless absolutely necessary; suctioning tends to increase intracranial pressure. Finally, consider elevating the patient’s head to about 30° to increase cerebral venous drainage.
Words of Wisdom
BiPAP increases intrathoracic pressure, which in turn stimulates baroreceptors in the aorta and causes a decrease in venous return and blood pressure. BiPAP must be used with extreme caution or withheld in patients who are borderline normotensive or hypotensive, respectively. Conversely, deep inspiration causes an increase in venous return because of the negative pressure in the thoracic cavity.
Postresuscitative care is an important component of caring for cardiac arrest patients. If an effective cardiac rhythm is restored in the field, transport immediately; the patient needs careful monitoring and titrated therapy that can most effectively be given in an intensive care unit. However, if the patient is comatose after return of spontaneous circulation, begin hypothermia treatment immediately.
The following is a summary of postresuscitative care:
1. Stabilize the cardiac rhythm (give an antidysrhythmic drug for post–ventricular fibrillation or post–ventricular tachycardia; give atropine or use a transcutaneous pacemaker for symptomatic bradycardia).
2. Normalize the blood pressure (give a dopamine [Intropin] or norepinephrine infusion to raise the systolic pressure to at least 100 mm Hg).
3. Elevate the patient’s head to 30° if the blood pressure allows.
When to Stop CPR
Since the dawn of paramedic-staffed ambulances in the early 1970s, many communities have not permitted the termination of CPR in the field. That policy was established because, in most jurisdictions, only a physician is authorized to pronounce a person dead (stopping CPR is considered equivalent to pronouncing a person dead). Cardiac arrest patients who were not successfully resuscitated at the scene were invariably transported urgently to the hospital, with some semblance of CPR occurring en route.
With the accumulation of vast experience from EMS systems throughout the United States, it soon became clear that transport to the ED of adults who did not respond to an adequate trial of prehospital ACLS was an exercise in futility: Fewer than 1% of patients ultimately survived. This policy was also given as the example of an unethical practice in the AHA’s 2010 guidelines because it instills false hope in the family. Furthermore, rapid transport of patients in cardiac arrest, with CPR en route, involves considerable hazards to EMS personnel: The risks of vehicular crashes or of injuries while working in a moving ambulance are greatly increased during urgent transport.
AHA criteria for terminating CPR in the prehospital setting are listed in Table 24.
In some jurisdictions, state legislation will be required to permit pronouncement of death at the scene by a paramedic. Once such legislation is enacted, each EMS system will have to formulate its own criteria for the termination of CPR in the prehospital setting.
Gaining permission to stop CPR in the field will not necessarily make your life easier. Delicate issues are involved, such as the expectations of the patient’s family or the disposition of the body. You may face enormous pressure from bystanders to continue resuscitative efforts long after there is any medical justification for doing so. Stopping CPR may also be difficult for you; you may not be accustomed to having to tell a family that the person is dead and nothing more can be done. It is much easier to provide transport to the hospital with red lights flashing and sirens blaring and leave the emergency department staff with the “expectation of providing a miracle” and the unpleasant task of breaking bad news, as well as a very expensive ED bill.
Table 24 AHA Criteria for Terminating CPR in the Field
|
1. The arrest was not witnessed by anyone. 2. No bystander CPR was provided. 3. Spontaneous circulation did not return after complete ALS care in the field. 4. No shocks were administered. |
If and when it becomes legal in your EMS system to terminate CPR in the field, it will be a good idea to meet with your medical director and “walk through” some of the scenes you may have to face. Role-play exercises can be particularly useful in helping you to pinpoint situations in which you feel uncomfortable and develop strategies in advance for dealing with those situations. Many jurisdictions have adopted protocols to assist providers in deciding when resuscitation attempts are futile and should be terminated.
 Pathophysiology, Assessment, and Management of Specific Cardiovascular Problems
Pathophysiology, Assessment, and Management of Specific Cardiovascular Problems
 Coronary Artery Disease and Angina
Coronary Artery Disease and Angina
Coronary artery disease (CAD) is the most common form of heart disease and the leading cause of death in US adults. The coronary arteries supply oxygen and nutrients to the myocardium. If one of these blood vessels becomes blocked, the muscle it supplies will be deprived of oxygen (ischemia). If this oxygen supply is not quickly restored, the ischemic area of heart muscle will eventually die (undergo infarction).
Atherosclerosis is of particular concern because it affects the inner lining of the aorta and cerebral and coronary blood vessels, leading to the narrowing of those vessels and reduction of blood flow through them. The atherosclerotic process begins, probably in childhood, when small amounts of fatty material are deposited along the inner wall (intima) of arteries, usually at points of turbulent blood flow (such as where the arteries bifurcate or where the arterial wall has been damaged). As the streak of fat enlarges, it becomes a mass of fatty tissue, an atheroma, which gradually calcifies and hardens into a plaque. The atheromatous plaque infiltrates the arterial wall and decreases its elasticity. At the same time, it narrows the arterial lumen and interferes with blood flow through the lumen. The narrowed, roughened area of the arterial intima provides a locus for the formation of a fixed blood clot, or thrombus, which may then obstruct the artery altogether (when in a coronary artery is known as a coronary thrombosis). In addition, calcium may precipitate from the bloodstream into the arterial walls, causing arteriosclerosis, which greatly reduces the elasticity of the arteries.
Risk Factors for Atherosclerosis
Although atherosclerosis is widespread in industrialized countries, certain factors increase the risk of developing atherosclerosis and CAD: hypertension (high blood pressure), cigarette smoking, diabetes, high serum cholesterol levels (which may be related to a high dietary intake of saturated fats and calories), lack of exercise, obesity, family history of heart disease or stroke, and male gender. Clearly, these risk factors include some things a person cannot do anything about. You cannot, for example, select your parents and grandparents or choose to be born female. Nevertheless, something can be done for about nearly half the risk factors for CAD, which are, therefore, called modifiable risk factors:
 Cigarette smoking is the most significant cause of preventable death in the United States, and a smoker’s chances of sudden death are several times greater than those of a nonsmoker. The good news is that smokers who quit return rapidly to the same risk level as nonsmokers.
Cigarette smoking is the most significant cause of preventable death in the United States, and a smoker’s chances of sudden death are several times greater than those of a nonsmoker. The good news is that smokers who quit return rapidly to the same risk level as nonsmokers.
 Hypertension cannot be prevented or cured, but it can be controlled with changes in diet and with medications. A person with uncontrolled hypertension has two to three times the risk of CAD as a person with normal blood pressure.
Hypertension cannot be prevented or cured, but it can be controlled with changes in diet and with medications. A person with uncontrolled hypertension has two to three times the risk of CAD as a person with normal blood pressure.
 The levels of serum cholesterol are at least in part a consequence of dietary intake of saturated fats. In populations with low fat intake, the incidence of CAD is also low. Furthermore, lowering the serum cholesterol levels has been shown to reduce the incidence of heart attacks and other dangerous cardiac events. Cholesterol may also be controlled with medications, if necessary.
The levels of serum cholesterol are at least in part a consequence of dietary intake of saturated fats. In populations with low fat intake, the incidence of CAD is also low. Furthermore, lowering the serum cholesterol levels has been shown to reduce the incidence of heart attacks and other dangerous cardiac events. Cholesterol may also be controlled with medications, if necessary.
 One behavior that may have a role in elevating serum cholesterol is lack of exercise, which also has a variety of other untoward effects on the body. Exercise improves overall fitness, cardiac reserve, and collateral coronary circulation.
One behavior that may have a role in elevating serum cholesterol is lack of exercise, which also has a variety of other untoward effects on the body. Exercise improves overall fitness, cardiac reserve, and collateral coronary circulation.
 Obesity may go hand in hand with several other risk factors (such as diabetes and hypertension). But obesity by itself also may contribute to an increased risk of CAD. Weight reduction, through consumption of a sensible diet and increased physical exercise, can reap several lifelong and life-extending benefits. Normalizing body weight will lower elevated blood pressure, elevated serum cholesterol levels, elevated blood glucose levels, and the risk of CAD.
Obesity may go hand in hand with several other risk factors (such as diabetes and hypertension). But obesity by itself also may contribute to an increased risk of CAD. Weight reduction, through consumption of a sensible diet and increased physical exercise, can reap several lifelong and life-extending benefits. Normalizing body weight will lower elevated blood pressure, elevated serum cholesterol levels, elevated blood glucose levels, and the risk of CAD.
Data suggest that risk factor modification can make a difference in the impact of CAD. According to the AHA, from 1993 to 2003, mortality from CAD declined 22% in the United States Figure 136. From 2006 to 2007, mortality from diseases of the heart decreased by 4.6%. According to the CDC, in 2007, approximately 25% of deaths resulted from diseases of the heart. Although experts cannot say precisely what caused that decline (it would be rewarding to think that paramedic-staffed ambulances had a significant role!), reduction in smoking, better control of hypertension, changes in dietary habits, and an upsurge of interest in fitness undoubtedly made substantial contributions to this trend. Unfortunately, the incidence of obesity and type 2 diabetes is increasing, which will likely overshadow steps that have been taken to help decrease CAD.
Peripheral Vascular Disorders
Although atherosclerosis is rarely the primary cause of medical emergencies, it is a major contributor to other conditions that may become medical emergencies. For example, arterial bruits or “swishing” sounds (heard with a stethoscope placed over the carotid arteries) signal the presence of atherosclerosis and contraindicate the use of carotid sinus massage. Atherosclerosis can also contribute to claudication, a severe pain in the calf muscle caused by narrowing of the arteries in this muscle and leading to a painful limp. Finally, atherosclerosis may be associated with phlebitis—swelling and pain along the veins that can lead to the formation of blood clots called deep vein thrombosis (DVT). If dislodged, these thrombi become emboli that could travel to the heart and through its right side, lodging in the pulmonary arterial tree and causing a pulmonary embolism.
Figure 136 Crude and age-adjusted death rates, United States, 1960–2007.
An estimated 5 to 20 million Americans are affected by significant peripheral vascular disorders annually. The most dangerous complication of these disorders is pulmonary embolism, which causes approximately 200,000 deaths each year. Risk factors for peripheral vascular disorders include age, oral contraceptive use, smoking, recent surgery, recreational IV drug use, trauma, and extended immobilization. Identification of these risk factors has a significant role in diagnosing peripheral vascular occlusions. Signs of peripheral vascular occlusion may include pain, redness, swelling, warmth, and tenderness in the extremity; these signs are present in only about half of all cases, however. The presence of claudication indicates a significant narrowing of the peripheral arteries associated with peripheral vascular disorders. Arterial bruits are another sign of vascular narrowing that can contribute to ischemia or stroke.
Because peripheral vascular disorders can have serious consequences, such as pulmonary embolism or loss of a limb through arterial occlusions, you must be familiar with the signs, symptoms, and risk factors for these conditions. Unfortunately, prehospital treatment of peripheral vascular conditions is limited. Beyond providing supplementary oxygen, obtaining IV access, and, possibly, aspirin administration, little can be done in the field if you suspect a peripheral vascular disorder.
Thromboembolism The development of a clot, or thrombus, either in the arteries or in the veins can lead to decreased blood flow to certain areas within the body. Furthermore, if the clot were to become dislodged, at which point it is termed a thromboembolism, it could produce a complete blockage of the vessel, resulting in a stoppage of the flow of oxygen-rich blood if the blockage occurs on the arterial side and a decreased venous return if it occurs in a vein. Prehospital treatment of thromboembolism includes supportive care. For example, if a pulmonary embolism is suspected, therapy is centered on oxygenation and ventilation to maintain appropriate oxygen saturation levels.
Angina Pectoris
The principal symptom of CAD is angina pectoris (literally “choking in the chest”). Angina occurs when the supply of oxygen to the myocardium is insufficient to meet the demand. As a result, the cardiac muscle becomes ischemic, and a switch to anaerobic metabolism leads to the accumulation of lactic acid and carbon dioxide. The concept of “supply and demand” is critical here. When at rest, a person with heart disease may have an adequate supply of oxygen to the heart to meet these sedentary needs, despite some narrowing of the coronary arteries. When the same person exercises or experiences some other stress, however, the blood flow to the myocardium may not be able to satisfy the heart’s increased demand for oxygen; in that case, angina will result. Clearly, the patient who experiences angina at rest, when oxygen needs are minimal, has more severe CAD than a person who experiences angina only with vigorous exercise.
Prinzmetal angina results in chest pain at rest and is caused by coronary artery vasospasm. Although men and women can experience Prinzmetal angina, it is more common in women in their 50s. People with Prinzmetal angina have an increased risk of experiencing ventricular dysrhythmias, myocardial infarction, heart block, or sudden death. This condition can present itself with little adjustment in vital signs. Although most patients with this type of angina have significant CAD, some patients may have none.
When you are obtaining the history from a patient with chest pain, it is important to distinguish between stable angina and unstable angina. Stable angina follows a recurrent pattern: A person with stable angina experiences pain after a certain, predictable amount of exertion, such as climbing one flight of stairs or walking for three blocks. The pain also has a predictable location, intensity, and duration. The patient may report, for example, “Every time I walk up the hill to the bus stop, I get a squeezing pain under my breast bone, and I have to sit down for 2 or 3 minutes until it goes away.”
Words of Wisdom
Other names for unstable angina include preinfarction angina, crescendo angina, and ACS.
Patients with chronic, stable angina often take nitroglycerin or some other form of “nitrate” for relief of anginal pain. In its usual formulation, nitroglycerin is supplied as a white tablet, which is placed under the tongue (sublingual) and allowed to dissolve there, or in a spray form that is sprayed under the tongue. It may also be given as sustained-release capsules taken two or three times a day, as a cream rubbed into the skin (topical), or as a patch worn on the skin. Regardless of which form is used, nitroglycerin will have a predictable effect in stable angina, producing relief of symptoms within a few minutes.
Unstable angina is much more serious than stable angina and indicates a greater degree of obstruction of the coronary arteries. It is characterized by noticeable changes in the frequency, severity, and duration of pain and often occurs without predictable stress. The patient may report that the anginal attacks have grown more frequent and severe during the past several days or weeks or that they awaken him or her from sleep or occur when otherwise at rest. It may or may not be relieved by rest or medications. Such attacks are often warning signs of an impending MI.
Acute Coronary Syndrome
Acute coronary syndrome (ACS) is the term used to describe any group of clinical symptoms consistent with acute myocardial ischemia. Acute myocardial ischemia typically presents as chest pain due to insufficient blood supply to the heart muscle, which itself is a result of CAD. The life-threatening ACS disorders are responsible for much of the emergency medical care and hospitalization in the United States.
Patients experiencing symptomatic, acute myocardial ischemia should receive a 12-lead ECG to determine whether they have an ST-segment elevation. Most patients whose ECG displays ST-segment elevation will ultimately develop a “Q-wave AMI” (heart attack), also known as STEMI (ST-elevation myocardial infarction). Patients who have ischemic discomfort (chest pain) without an ST-segment elevation are having unstable angina or a non–ST-segment elevation MI that usually leads to a non–Q-wave MI; these conditions are collectively known as UA/NSTEMI (unstable angina/non–ST-elevation myocardial infarction). Patients who experience angina may also present with ST-segment depression. Finally, some patients experiencing angina or MI may have no changes indicated by the ECG.
Management
Of course, not all chest pain is caused by cardiac ischemia or injury. Many other conditions—such as pulmonary embolism, pneumothorax, pneumonia, pericarditis, aortic dissection, indigestion, and peptic ulcer—may cause chest pain that can be mistaken for angina or an MI. It is important for you to perform a thorough physical exam, including history taking, to determine whether the cause of the complaint is likely cardiac in origin.
Words of Wisdom
When a patient with chest pain calls for an ambulance, it means that the patient never had chest pain before or that his or her chronic chest pain has changed. Take all chest pain complaints seriously.
As a general rule, it is safe to assume that any patient who has called for an ambulance because of chest pain has, at the least, unstable angina and perhaps an evolving AMI. Patients with chronic, stable angina rarely call for help unless something has changed—often dramatically—for the worse. Because it is difficult and sometimes impossible to differentiate between angina and an MI in the field, the treatment of angina should be the same as for an MI. It is far better for you to overtreat angina as an MI than to undertreat an MI by assuming it is angina.
Pathophysiology
An acute myocardial infarction (AMI), or heart attack, occurs when a portion of the cardiac muscle is deprived of coronary blood flow long enough that portions of the muscle die (undergo necrosis, or infarct). Several things can diminish flow through coronary vessels, especially if the vessels are already narrowed by atherosclerotic disease: occlusion of a coronary artery by a blood clot (thrombus), spasm of a coronary artery, or reduction of overall blood flow from any cause (such as shock, dysrhythmias, or pulmonary embolism).
The location and size of a myocardial infarct depend on which coronary artery is blocked and where along its course the blockage occurred. Most infarcts involve the left ventricle. When the anterior, lateral, or septal walls of the left ventricle are infarcted, the source is usually occlusion of the left coronary artery or one of its branches. Inferior wall infarcts are usually the result of RCA occlusion. When the ischemic process affects only the inner layer of muscle, the infarct is referred to as a subendocardial myocardial infarction. When the infarct extends through the entire wall of the ventricle, it is a transmural myocardial infarction. The infarcted tissue is invariably surrounded by a ring of ischemic tissue—an area that is relatively deprived of oxygen but still viable. That ischemic tissue tends to be electrically unstable and is often the source of cardiac dysrhythmias.
Words of Wisdom
For purposes of treatment outside the hospital, the patient with chest pain must be assumed to be having an acute myocardial infarction until proven otherwise and should therefore be treated as any other patient with a suspected AMI.
Cardiovascular disease accounted for more than 191,000 deaths in 2005. Of deaths related to cardiovascular disease, AMI is the leading cause of death in the United States; 60% to 70% of AMIs occur outside the hospital, during the first 2 to 3 hours after the onset of symptoms. Of all deaths from AMI, 90% are due to dysrhythmias, usually ventricular fibrillation, which typically occur during the early hours of the infarct. Dysrhythmias can be prevented or treated, so most deaths from AMI are preventable.
Symptoms of AMI Although there is no “typical AMI patient,” when most Americans think about the symptoms of an AMI, they envision the classic pain presentation usually associated with men. In fact, AMIs can occur in younger and older people and in men and women. The patient may be slightly overweight and may have recently overindulged at the dinner table or perhaps on the tennis court. Nevertheless, many heart attacks occur at rest or just after arising in the morning.
The most common symptom of AMI is chest pain. This pain is similar to that of angina but can be much more severe and last more than 15 minutes. A patient with chronic angina will be aware that something different from previous anginal attacks is happening. The pain of AMI is typically felt just beneath the sternum and is variously described as heavy, squeezing, crushing, or tight. Often the patient unconsciously clenches a fist when describing the pain (Levine sign) to convey in body language the squeezing nature of the pain. In 25% of patients, the pain radiates to the arms (most often the left arm) and into the fingers; it may also radiate to the neck, jaw, upper back, or epigastrium. Occasionally, a patient will mistake the pain of AMI for indigestion and may take antacids in an attempt to relieve the discomfort. The pain of AMI is not influenced by coughing, deep breathing, or other body movements, and may or may not be relieved by nitrates or rest.
Not every AMI patient has chest pain, however. In fact, 10% to 20% of patients with AMI do not experience any chest pain. Women, diabetics, the elderly, and heart transplant patients, for example, generally do not present with chest pain, a condition referred to as “silent MI.” Instead, these patients may present with symptoms related to a drop in CO. It is not unusual for them to develop sudden dyspnea, progressing rapidly to pulmonary edema, a sudden loss of consciousness, an unexplained drop in blood pressure, an apparent stroke, or simply confusion.
Women with an AMI may present differently from men with the same condition. Women may experience nausea, lightheadedness, epigastric burning, or sudden onset of weakness or unexplained tiredness. Women may also have pain radiating down the right arm instead of the classic left arm pain. Because they are not experiencing the typical chest pain expected with an AMI, many women ignore their symptoms. Unfortunately, cardiovascular disease is the number one cause of death for US women.
Words of Wisdom
More men have heart disease, but more women die of heart disease, in part because their symptoms are less clear-cut.
When you are obtaining the history from a patient whose chief complaint is chest pain, ask the usual OPQRST questions to elaborate on the chief complaint, but also ask whether the patient has taken anything for the pain and, if so, whether it helped. If the patient reports having taken nitroglycerin without relief, it is important to establish why the patient did not obtain relief.
Two reasons might explain this failure. One possibility is that the patient is, indeed, having an AMI, for which nitroglycerin would not provide complete pain relief. The other possibility is that the nitroglycerin has simply gone stale. To retain its potency, nitroglycerin must be stored in a dark, airtight container; if it is left out in the open for any period (for example, if the patient stores the medicine on the window sill above the kitchen sink), it loses its therapeutic effectiveness. To distinguish between the two explanations, ask the patient whether he or she noticed the usual effects of the nitroglycerin. Nitroglycerin tablets that are therapeutically active cause a slight burning under the tongue, may make the patient feel flushed, or may give the patient a transient throbbing headache. If the patient confirms that he or she felt one of those effects but the chest pain still would not go away, then you know there was nothing wrong with the nitroglycerin but there may be something very wrong with the patient.
Words of Wisdom
Start treatment immediately for any patient with chest pain.
As soon as you have elicited a chief complaint of a cardiac nature, you will need to start treatment of the patient; the more in-depth history taking and the secondary assessment can wait. For purposes of discussion, though, this section will continue to proceed through the history and secondary assessment. Besides pain (or, sometimes, instead of pain), a number of other symptoms are associated with AMI:
 Diaphoresis (sweating), often profuse, is principally the result of massive discharge by the autonomic nervous system. The patient may soak through his or her clothing and report a cold sweat.
Diaphoresis (sweating), often profuse, is principally the result of massive discharge by the autonomic nervous system. The patient may soak through his or her clothing and report a cold sweat.
 Dyspnea may be a warning of impending left-sided heart failure.
Dyspnea may be a warning of impending left-sided heart failure.
 Anorexia (loss of appetite), nausea, vomiting, or belching frequently accompanies MI. Hiccups may occasionally occur as well, due to irritation of the diaphragm by an inferior wall MI.
Anorexia (loss of appetite), nausea, vomiting, or belching frequently accompanies MI. Hiccups may occasionally occur as well, due to irritation of the diaphragm by an inferior wall MI.
 Weakness may be profound, and the patient may describe this feeling with phrases such as “a limp rag.”
Weakness may be profound, and the patient may describe this feeling with phrases such as “a limp rag.”
 If CO is significantly diminished, dizziness may reflect the reduced circulation to the brain.
If CO is significantly diminished, dizziness may reflect the reduced circulation to the brain.
 Palpitations are sometimes experienced by patients with cardiac dysrhythmias as a sensation that the heart has skipped a beat.
Palpitations are sometimes experienced by patients with cardiac dysrhythmias as a sensation that the heart has skipped a beat.
 A feeling of impending doom is common among patients having an MI. The patient is frightened, looks frightened, and expresses his or her fear to other people—all of which adds to a general atmosphere of panic and dread.
A feeling of impending doom is common among patients having an MI. The patient is frightened, looks frightened, and expresses his or her fear to other people—all of which adds to a general atmosphere of panic and dread.
Signs of AMI Although patients with an AMI often have abnormalities in the physical exam, many have relatively normal physical exam findings, and the diagnosis in the field (and in the emergency department) depends chiefly on the history. Nevertheless, it is important for you to take note of a few specific things during the physical exam to detect the development of complications to AMI, such as heart failure or cardiogenic shock.
 Pay attention to the patient’s general appearance. Does the patient appear anxious? Frightened? In obvious pain? What is the patient’s state of consciousness? Is he or she fully alert? Confused? Remember: Poor perfusion creates confusion. If the patient does not seem “all there,” it may be because the heart is giving out and not enough oxygenated blood is reaching the brain.
Pay attention to the patient’s general appearance. Does the patient appear anxious? Frightened? In obvious pain? What is the patient’s state of consciousness? Is he or she fully alert? Confused? Remember: Poor perfusion creates confusion. If the patient does not seem “all there,” it may be because the heart is giving out and not enough oxygenated blood is reaching the brain.
 Is the skin pale, cold, and clammy?
Is the skin pale, cold, and clammy?
 Assess the patient’s vital signs. Is the pulse strong or weak? Regular or irregular? Is the respiratory rate abnormally rapid? Is the blood pressure abnormally high or low?
Assess the patient’s vital signs. Is the pulse strong or weak? Regular or irregular? Is the respiratory rate abnormally rapid? Is the blood pressure abnormally high or low?
 Are there signs of left-sided heart failure (wheezes or crackles)? Signs of right-sided heart failure (distended neck veins, pedal or presacral edema)?
Are there signs of left-sided heart failure (wheezes or crackles)? Signs of right-sided heart failure (distended neck veins, pedal or presacral edema)?
A typical patient with an AMI is apprehensive, with ashengray pallor and cold, wet skin. He or she looks scared. The pulse rate may be rapid unless heart block has occurred. The blood pressure may be decreased, reflecting decreased CO from the damaged heart, or it may be elevated from pain and anxiety.
Management of AMI and Suspected AMI in the Field
On your arrival at the scene, start treatment at once for any middle-aged or older patient with chest pain, even before you complete the history and secondary assessment. One of the most important components in the rapid identification and treatment of a cardiac event is the rapid acquisition of the 12-lead ECG. A 12-lead ECG should always be performed before the administration of any medication (except possibly aspirin and oxygen; in some regions, these are administered prior to taking a 12-lead ECG); data suggest that rapid acquisition of a 12-lead ECG in the prehospital environment decreases the door-to-balloon time by an average of 18 minutes. Therefore, a 12-lead ECG should be acquired immediately, then treatment can begin. If a medication is administered prior to the acquisition of the 12-lead ECG, certain conditions may be resolved by this treatment, so there may be no documentation of the initial condition that can assist in further treatment of the patient. For example, suppose a patient is experiencing chest pain and ST depression is apparent on the 12-lead ECG. If nitroglycerin was administered to that same patient prior to the 12-lead ECG, the ST elevation may not be present at the time of the monitoring.
The longest delay in treatment seems to be the phase from onset of symptoms to patient recognition, so your care must begin immediately. The goals of treatment are to limit the size of the infarct, to decrease the patient’s fear and pain, and to prevent the development of serious cardiac dysrhythmias.
Place the Patient at Physical and Emotional Rest The stress response causes the adrenal glands to squeeze out a surge of catecholamines (epinephrine and norepinephrine), which in turn can send the damaged heart racing. At the same time, the massive discharge throughout the fight-or-flight system puts the peripheral circulation in a state of severe vasoconstriction; thus, not only is the heart being pushed to go faster and faster, but it also has to work harder and harder against the increased afterload. The heart’s need for oxygen, therefore, soars precisely when it is already in a state of marked oxygen deprivation. This cycle can lead quickly to dysrhythmias and death. Prehospital deaths are related to dysrhythmias (often ventricular fibrillation), and most occur during the first 4 hours after onset of symptoms. Nevertheless, this deadly cycle can be interrupted by community education programs designed to assist citizens in early recognition of symptoms, early activation of EMS, and, if needed, CPR and early access to an AED.
To begin your treatment, put the patient physically at ease. Recall that one goal of treatment is to try to limit the size of the infarct; one way to do so is to decrease the amount of work that the heart must do, which will begin to decrease the patient’s myocardial oxygen requirements immediately. The position in which cardiac work is minimal is the semi-Fowler position—that is, reclining on the stretcher with the back of the stretcher raised about 30°. Of course, the patient has to get to the stretcher and must not be permitted to do so alone. From the time you arrive, the patient must not do anything, including walking to the ambulance.
Administer Oxygen and Aspirin The mnemonic MONA is used to help you remember the supportive treatments of Morphine, Oxygen, Nitroglycerin, and Aspirin for a patient with an ACS—but these treatments are not to be given in that order. MONA is administered in the following order, provided these measures are not contraindicated by hypotension: (1) oxygen, (2) aspirin, (3) nitroglycerin, and (4) morphine.
Oxygen may limit ischemic myocardial injury and reduce the amount of ST-segment elevation. Its effects on morbidity and mortality in acute infarction are unknown. Treatment with oxygen should be individualized and titrated to maintain the SpO2 level above 94%.
In most EMS systems, as long as the patient has no aspirin allergy or gastrointestinal bleeding, dispatchers may advise patients to chew baby aspirin (160 mg to 325 mg). If this has not been done before your arrival or the patient has not already taken aspirin on his or her own, then give the patient 160 mg to 325 mg of non–enteric-coated aspirin to chew.
Provide Pain Relief Some form of pain relief must be provided because the pain of AMI is severe and places enormous stress on the patient’s autonomic nervous system—stress that may contribute to complications. Nitroglycerin is a good place to start, but make sure the patient’s blood pressure is adequate before its administration. In particular, before giving this medication, it is imperative that you ascertain whether the patient is taking phosphodiesterase-5 (PDE-5) inhibitors for erectile dysfunction Table 25. These drugs may worsen certain medical conditions and interact with a number of drugs, especially nitrate medications (such as nitroglycerin) prescribed to prevent or treat acute angina. Both types of medication dilate blood vessels, and their combined effects can cause dizziness, low blood pressure, and loss of consciousness.
Place a 0.4-mg tablet (or spray) of nitroglycerin under the patient’s tongue. If the patient is experiencing an AMI and not simply angina, this medication is unlikely to relieve his or her pain, but it may help to reduce the size of the infarction. Do not give nitroglycerin if there is hypotension or bradycardia, and do not give it to patients having epigastric symptoms (“indigestion”) or hiccups. Nitroglycerin may be repeated every 3 to 5 minutes, up to a total of three doses as long as the patient’s condition remains stable.
Brand Name |
Generic Name |
Duration of Effect |
Viagra |
Sildenafil citrate |
Up to 4 h |
Levitra |
Vardenafil |
Up to 4 h |
Cialis |
Tadalafil |
24 to 36 h |
If nitroglycerin provides no relief of pain and if authorized by medical command, morphine sulfate may be titrated in IV doses according to local protocols. Give this medication in 2- to 4-mg IV doses as needed for pain, being sure to reassess the patient’s blood pressure, pulse, and respiratory rate after each dose, until the patient experiences relief of pain or experiences a drop in pulse or blood pressure. If bradycardia occurs, notify the physician immediately. Remember that morphine should not be given to patients with low blood pressure (less than about 100 mm Hg systolic or according to local protocol), dehydrated patients, or patients suspected of having an AMI involving the inferior wall of the heart. At least half of all patients with inferior wall MI will also experience a right ventricular infarction; as a consequence, they may already be hypotensive or the administration of nitroglycerin and morphine may cause hypotension.
In some EMS medical protocols, fentanyl (Sublimaze) is favored over morphine for pain not relieved with nitroglycerin because of its rapid onset and relatively short duration. Fentanyl also has fewer side effects than morphine.
Perform Cardiac Monitoring Apply the ECG monitor, and run a strip to document the initial rhythm. As long as you are applying electrodes to the chest, also place your anterior chest leads in anticipation of obtaining a 12-lead ECG. Ideally, your monitor should have an audible tone that beeps with each QRS complex (also called systole beep), so that you can keep track of the patient’s cardiac rhythm even when you have to take your gaze from the monitor to do other things. The ear, in any case, is far more sensitive than the eye to slight irregularities in rhythm, so the chances are that you will hear the beginning of a cardiac dysrhythmia much sooner than you will see it on the monitor. Keep the other cardiac drugs that you carry close at hand so you can reach them quickly if a cardiac dysrhythmia develops.
Words of Wisdom
Patients may not be forthcoming about taking medications. They may omit something from their list of home medications if they do not take the medicine daily. Be sure to ask.
Record the Vital Signs Obtain vital signs, including pulse, respirations, blood pressure, and oxygen saturation. Measure the blood pressure, and repeat that measurement at least every 5 minutes. Measure the pulse rate. The ECG monitor provides information only about the electrical activity of the heart; it gives no information about the strength of the heartbeat (muscular activity) or even about whether the heart is beating at all! The ECG does not record mechanical function of the heart. It is, therefore, necessary to monitor the patient’s pulse to assess peripheral blood flow, especially during transport, when blood pressure measurements are difficult to obtain and unreliable.
Take a History and Perform the Secondary Assessment After you have completed the preceding steps (as appropriate), you should obtain a more detailed history and perform the secondary assessment. Find out if the patient has a history of cardiac disease; takes any heart medications, such as beta blockers, angiotensin-converting enzyme inhibitors, diuretics, or nitroglycerin (nitrates); or has had a previous heart attack or any heart surgery (such as coronary artery bypass graft). Also obtain a more complete description of the present symptoms, especially regarding their onset. It is also important to make sure to find out all past medical history that may be relevant to the existing problem—that is, differential diagnoses. Some examples can include but are not limited to cholecystitis, acute viral pericarditis, aneurysm, hiatal hernia, esophageal disease, gastric reflux, pulmonary embolism, peptic ulcer disease, pancreatitis, chest wall syndrome, costochondritis, acromioclavicular disease, pleural irritation, respiratory infection, aortic dissection, pneumothorax, dyspepsia, herpes zoster, chest wall tumors, and chest wall trauma.
Gathering that information should not delay transport to the hospital. Once you have taken the necessary precautions to stabilize the patient’s condition (aspirin, oxygen, IV saline lock, monitor/12-lead ECG, analgesia), there is no reason to remain any longer at the scene unless a cardiac arrest or dysrhythmia requires immediate treatment. Obtain the rest of the history en route to the hospital. Remember that “time is muscle.” Heart cells are being destroyed during the infarction before reperfusion is started in the hospital.
Transport the Patient Once the patient is in stable condition, transport him or her to an appropriate hospital in a semi-Fowler position (unless the patient is in shock, in which case he or she should be supine). Do all you can to ensure that the patient is as relaxed and as comfortable as possible. En route, some additional treatment measures may be worthwhile, especially when transport time will be lengthy.
Safe and appropriate transport is what you need to strive for. Do not rush and do not use sirens when you are transporting the patient to the hospital. High speed and sirens send two clear messages to the patient: (1) Something is terribly wrong. (2) The personnel on the ambulance do not feel capable of dealing with the situation. Those are not the messages you want to convey to a frightened patient with a damaged heart! The patient needs to feel confident that those caring for him or her are in control of the situation.
If a serious dysrhythmia occurs during transport, consider stopping the vehicle, institute treatment immediately, and notify medical control. Except under unusual circumstances, treatment of life-threatening situations should not be attempted in a moving ambulance. Whenever possible, the driver should pull over to the side of the road and go to the back of the vehicle to help the other provider.
Reperfusion Techniques for AMI and Suspected AMI
Most AMIs occur as a result of thrombus (fixed blood clot) formation at the site of a preexisting atherosclerotic plaque. The thrombus occludes the coronary artery, preventing further blood flow through it. Thus, it seems reasonable to try to restore circulation through the occluded coronary artery, thereby restoring perfusion to the ischemic myocardium. Simply put, that is reperfusion.
The most immediate forms of reperfusion are fibrinolytic therapy and percutaneous intervention (PCI). All paramedics should be alert for patients who are good candidates for reperfusion, should know which hospitals in their area carry out fibrinolytic therapy and/or PCI, and should provide early notification (along with 12-lead ECG results) to the emergency department that a candidate for such therapy is en route.
Fibrinolysis One way in which to reperfuse the blocked coronary artery is to try to dissolve the occluding blood clot, thereby restoring circulation to the ischemic heart. That idea is the essence of fibrinolytic therapy.
In fact, this concept is not altogether new. Attempts to use fibrinolytic agents in the treatment of AMI were reported over 50 years ago, albeit without success. In retrospect, one reason the early attempts failed was that fibrinolytic therapy was started too late, after irreversible damage to the myocardium had already occurred. With that realization came the concept that “time is myocardium”: The longer a segment of myocardium remains unperfused, the smaller the chances of salvaging that tissue and restoring its normal function. The obvious corollary is that the sooner fibrinolytic therapy can begin with respect to the onset of the blockage, the better the chances for saving the affected distal myocardium. Indeed, fibrinolytic treatment given within 30 to 60 minutes of the onset of symptoms can sometimes abort the MI altogether.
In the 1980s, providers began to start fibrinolytic treatment as soon as possible after the patient with an AMI reached the emergency department, rather than waiting until he or she was admitted to the coronary care unit. Inevitably, applying the doctrine that time is myocardium led to the idea of starting fibrinolytic treatment even earlier, in the prehospital phase of care.
Recent clinical trials have shown the benefit of starting fibrinolysis as soon as possible after the onset of ischemic-type chest pain in patients with STEMI or new or presumably new left bundle branch block. Several prospective studies have also documented reduced time to administration of fibrinolytics and decreased mortality rates when prehospital fibrinolytics were given to patients with STEMI and no contraindications to fibrinolytics. Some EMS systems may opt to start fibrinolytic treatment in the field, and in rural areas with long transport times, prehospital initiation of fibrinolytic therapy may make a lot of sense. Even in EMS systems in which paramedics do not give fibrinolytic therapy, their ability to identify candidates for such therapy has a decisive role in helping emergency department personnel administer fibrinolytic therapy early enough to make a difference. For these reasons, all paramedics should thoroughly understand the principles of fibrinolytic therapy for AMI.
Words of Wisdom
Time is muscle (myocardium)!
Fibrinolytic therapy seeks to administer, during the early hours of AMI, an agent that will activate the body’s own internal system for dissolving clots, the fibrinolytic system. Once activated, that system can begin to dissolve the clot that has formed within the coronary artery, thereby reopening the artery (recanalization) and allowing the resumption of blood flow through it (reperfusion). Unfortunately, if an agent capable of promoting clot dissolution is given intravenously, its effects cannot be limited to the clot in the coronary artery; it can also act anywhere else in the body where clots are being formed and, therefore, may lead to bleeding. Thus, the benefit of fibrinolytic therapy—the possible salvage of myocardium—must always be weighed against its risks—principally, the risk of bleeding.
To determine the appropriate candidates for fibrinolytic agents, you need to be as certain as possible that your patient is experiencing an AMI. A patient having chest pain from another source would receive no potential benefit from fibrinolytic therapy—so he or she would be subjected to this therapy’s risks for no reason. Although it is difficult in the early hours of an AMI to be certain of the diagnosis, inclusion criteria have been established to help select patients most likely to be having an AMI. At the same time, exclusion criteria are used to identify patients for whom the risk of fibrinolytic therapy is unacceptably high—for example, patients most likely to experience hemorrhagic complications. Table 26 summarizes the inclusion and exclusion criteria for fibrinolytic therapy.
Most treatment regimens for fibrinolysis include one of three agents: alteplase (Activase; a tissue plasminogen activator), streptokinase (Streptase), or reteplase (Retavase; recombinant tissue). All of them work by converting, in one way or another, the body’s own clot-dissolving enzyme from its inactive form, plasminogen, to its active form, plasmin.
According to the AHA’s 2010 guidelines, the focus continues to be on the early recognition and notification of those patients who may benefit from fibrinolysis therapy early on in the process. A prehospital fibrinolytic program is recommended only in systems with well-established protocols, checklists, experience in ACLS, ability to communicate with the receiving institution, and a medical director with training and experience in the management of STEMI.
Percutaneous Intervention As an alternative to fibrinolysis, many institutions perform a percutaneous coronary intervention (PCI). Patients with complex, multivessel disease or ACSs may benefit from PCI. In this therapy, balloons, stents, or other devices are passed through a 2-mm-diameter catheter via a peripheral artery to recanalize and keep the blocked coronary artery open. The success rate is high, and the risks are low. PCI is often used for patients who are not candidates for fibrinolytic therapy.
Table 26 ST-Segment Elevation or New or Presumably New LBBB: Evaluation for Reperfusion
Step 1: Assess time and risk | |
| |
Step 2: Select reperfusion (fibrinolysis or invasive) strategy | |
Note: If presentation < 3 hours and no delay for PCI, then PCI is preferred. | |
Fibrinolysis is generally preferred if: |
An invasive strategy is generally preferred if: |
– Medical contact-to-balloon or door-to-balloon > 90 min – (Door-to-balloon) minus (door-to-needle) is > 1 hour
|
|
Source: Modified from the 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. | |
Congestive heart failure (CHF) (also known as chronic heart failure) occurs when the heart is unable, for any reason, to pump powerfully enough or fast enough to empty its chambers; as a result, blood backs up into the systemic circuit, the pulmonary circuit, or both. Although CHF may develop in situations other than AMI—for example, in a patient with chronic high blood pressure—the basic principles of diagnosis and treatment are similar, whatever the precipitating factors.
More than 2 million people in the United States have CHF, and an additional 500,000 cases are diagnosed each year. Nearly half of the patients with CHF classified as severe die within 1 year of diagnosis.
Left-Sided Heart Failure
Pathophysiology The left ventricle is most commonly damaged during an AMI. Likewise, in chronic hypertension, the left ventricle tends to suffer the long-term effects of having to pump against an increased afterload (constricted peripheral arteries). In both cases, the right side of the heart continues to pump relatively normally and to deliver normal volumes of blood to the pulmonary circulation. By comparison, the left side of the heart may no longer be able to pump the blood being delivered from the pulmonary vessels. As a result, blood backs up behind the left ventricle, and the pressure in the left atrium and pulmonary veins increases. As the pulmonary veins become engorged with blood, serum is forced out of the pulmonary capillaries and into the alveoli. The serum mixes with air in the alveoli to produce foam (pulmonary edema). This is left-sided heart failure.
When fluid occupies the alveoli, oxygenation is impaired. The patient experiences that impairment as shortness of breath (dyspnea), particularly in the recumbent position (orthopnea). If left ventricular failure is the result of chronic overload (as opposed to AMI), the patient is likely to give a history of a week or two of paroxymal nocturnal dyspnea (PND). To compensate for the impairment in oxygenation, the patient’s respiratory rate increases (tachypnea); even so, if the patient’s condition is advanced enough, cyanosis may become evident. In some patients with pulmonary edema, especially elderly patients, Cheyne-Stokes respirations may be present.
Fluid from the pulmonary vessels also leaks into the interstitial spaces in the lungs, and increasing interstitial pressure causes narrowing of the bronchioles. Air passing through the narrowed bronchioles creates wheezing noises, whereas air bubbling through the fluid-filled alveoli produces crackles. Furthermore, the patient may cough up the edema fluid in the form of foamy, blood-tinged sputum. As the airways narrow and the lungs grow heavier from the accumulation of fluid, the work of breathing increases, which puts an even greater strain on the already floundering heart. Dyspnea and hypoxemia produce a state of panic, which induces the release of epinephrine from the adrenal glands. The heart is pushed even harder, and its oxygen demand is increased precisely when fluid in the alveoli is reducing the amount of oxygen available.
To make matters worse, the sympathetic nervous system response produces peripheral vasoconstriction: Peripheral resistance (afterload) increases, and the weakened, hypoxic heart now has to push blood out into smaller and smaller vessels. Clinically, peripheral vasoconstriction is apparent as pallor and elevated blood pressure. The massive sympathetic discharge also produces sweating of the pale, cold skin.
It is not unusual for a patient with left-sided heart failure to become frantic from air hunger. He or she may pace or thrash about or may even be combative and struggle with the rescue team. Furthermore, hypoxemia results in inadequate oxygen supply to the brain, often manifested as confusion or disorientation. If hypoxemia is severe, cardiac arrest may follow quickly.
Assessment The signs and symptoms of left-sided heart failure include extreme restlessness and agitation, confusion, severe dyspnea and tachypnea, tachycardia, elevated blood pressure, crackles and possibly wheezes, and frothy, pink sputum. Sometimes, it may be difficult to distinguish the wheezing of asthma from that of left-sided heart failure. Table 27 presents some of the features that can help you differentiate the two conditions.
The ECG may show cannon atrial waves. Also called cannon A waves, these are pulsations seen in the jugular veins. When the atria and ventricles contract simultaneously, there is a large surge in pressure back through the venous system resulting in jugular vein pulsations. Cannon A waves are often seen in patients with fluid overload and heart failure.
Management Prehospital treatment of left-sided heart failure is aimed at improving oxygenation and decreasing the workload of the heart, chiefly by reducing the volume of venous blood returned to the heart (the preload), so that the left ventricle is less overburdened.
Administer 100% supplemental oxygen, preferably by demand valve or bag-mask device with positive end-expiratory pressure, because positive pressure is helpful in driving fluid out of the alveoli. Continuous positive airway pressure (CPAP) has been showed to be an effective tool in the management of pulmonary edema as it pertains to CHF. CPAP provides a continuous increase of pressure in the lower airway passages. This in turn helps to improve gas exchange in the alveoli by reinflating the collapsed alveoli. CPAP will also increase the surface area of the alveoli, thereby providing a larger area over which gas exchange can take place. If the patient will not tolerate those modalities, use the nonrebreathing mask. The patient’s respiratory status should be monitored via waveform capnography; if it is unavailable, the use of pulse oximetry will suffice.
Table 27 Differentiation and Treatment of Asthma and Left-Sided Heart Failure
Asthma |
Left-Sided Heart Failure | |
History |
Often a younger patient May have allergic history or family history of allergy Previous attacks of acute, episodic dyspnea May have had recent respiratory infection Unproductive cough Medications may include:
|
Often an older patient May have history of heart problems, hypertension Dyspnea worse when lying down (orthopnea) Recent rapid weight gain Cough with watery or foamy sputum Medications may include:
|
Possible physical findings |
Wheezing Chest hyperinflated and hyperresonant Use of accessory muscles to breathe If bronchospasm severe, chest may be silent |
Wheezing Crackles S3 gallop Distended neck veins Pedal or presacral edema |
Treatment |
Oxygen (humidified) Intermittent positive-pressure breathing Monitor IV: normal saline Selective beta-2 adrenergic medications Sometimes bicarbonate (morphine and diuretics contraindicated) |
Oxygen Intermittent positive-pressure breathing Monitor IV: normal saline to keep open or saline lock (adrenergics and bicarbonate usually contraindicated) Morphine Diuretics (furosemide) Nitroglycerin |
Sit the patient up, with the feet dangling. That position encourages venous pooling in the legs, thereby reducing venous return to the heart. The sitting position also makes breathing easier for a patient in respiratory distress.
Start a saline lock or an IV line with normal saline at a keep-vein-open rate. Also, attach monitoring electrodes because patients in CHF are prone to dysrhythmias.
Pharmacologic therapy of left-sided heart failure may vary slightly from place to place, but the mainstays of drug therapy include the drugs mentioned below (in order of the author’s preference). Refer to your usual protocols, and have the appropriate medications drawn up and ready, pending the physician’s order to administer them. Remember to constantly monitor the blood pressure because many of these medications lower it.
Nitroglycerin, 0.4 mg sublingually, may be ordered as a vasodilator to create venous pooling, thereby reducing the volume of blood returned from the periphery to the heart. Before ordering this medication, the physician will want to know how much, if any, nitroglycerin the patient has already taken. The initial dose of 0.4 mg may be repeated at 5-minute intervals up to a total of 1.2 mg (three doses).
Furosemide (Lasix) is a diuretic that has two positive effects in left-sided heart failure. Initially (within the first 5 to 10 minutes), it has a venodilating effect, increasing peripheral pooling of blood. Subsequently, it removes excess fluid from the body by promoting its excretion by the kidneys. If ordered, furosemide is given in a dose of 20 to 40 mg or 0.5 to 1 mg/kg by IV bolus. If the patient already takes furosemide, the higher dose should be used. The use of furosemide in the prehospital setting remains a controversial topic. Several studies have found poor clinical outcomes in patients receiving prehospital diuretics.
Morphine sulfate has long been part of the standard treatment of cardiogenic pulmonary edema. Like nitroglycerin, morphine works as a vasodilator, increasing the pooling of blood in the periphery, but it also has a substantial calming effect on a frantic patient. If morphine is ordered, first check the patient’s blood pressure (do not give morphine if the patient is hypotensive). Then administer approximately 3 mg slowly by IV bolus, and recheck the blood pressure. If the blood pressure remains stable, another 3 mg may be given. Proceed in that manner until the total dose ordered by the physician has been administered.
The presence of wheezing indicates that bronchoconstriction has developed from the excessive fluid. In such a case, bronchodilator drugs such as albuterol (Proventil, Ventolin, Volmax), metaproterenol sulfate (Alupent), or ipratropium (Atrovent) may be ordered.
Under special circumstances, when transport will be prolonged and the patient’s blood pressure is low, the physician may order a pressor such as dopamine (Intropin), which will increase blood pressure and/or CO. The dose is based on protocol, and ranges from 2 to 20 μg/kg/min by IV drip titrated to the desired blood pressure.
Transport the patient to the hospital in a sitting position, with the legs dangling down.
Some patients who present with left-sided heart failure may receive a pacemaker in an effort to address an electrical conduction problem within the heart as well as the synchronization of the heart muscle. Heart synchronization therapy uses a special pacemaker that helps to stimulate or pace both of the heart’s ventricles. This treatment improves the pumping function of the left ventricle so that it can pump more volume, which may help in alleviating some of the symptoms of left-sided heart failure, such as dysrhythmias or pulmonary edema.
A left ventricular assist device (LVAD) may be used in those patients who have sustained significant damage to their left ventricle such that it cannot meet the demands of the body. These patients require a heart transplant to meet these demands, but may have to wait for some time to find a compatible heart. This is where the LVAD comes into use: It can support the body while the patient is waiting for a donor heart to become available. With the LVAD, a tube is placed in the left ventricle that draws blood from the left ventricle into the device’s pump. The pump then sends this oxygen-rich blood through another tube to the aorta.
The LVAD is typically inserted into the abdominal cavity of the patient. A tube from the pump goes through the abdominal wall to the outside, where it connects to the battery pack for the unit. EMS providers should be aware that if there is power to the battery pack, the issue is probably not the LVAD itself, but rather another underlying problem, such as infection.
Right-Sided Heart Failure
Right-sided heart failure most commonly occurs as a result of left-sided heart failure. As blood backs up from the left side of the heart into the lungs, the right side has to work increasingly harder to pump blood into the engorged pulmonary vessels. Eventually, the right side of the heart is unable to keep up with the increased workload, and it, too, fails. Right-sided heart failure may also occur as a result of pulmonary embolism or long-standing chronic obstructive pulmonary disease (COPD), especially chronic bronchitis.
When right-sided heart failure occurs, blood backs up behind the right ventricle and increases the pressure in the systemic veins, causing them to become engorged. Distention can be seen in the veins visible on the surface of the body, such as the external jugular veins, which should be assessed in the semi-Fowler position. Over time, as the pressure within the systemic veins increases, serum is forced out of the veins and into the surrounding tissues, producing edema. Edema is most likely to be visible in dependent parts of the body, such as the feet in a person who is sitting or standing or the lower back in a bedridden patient. Edema is also present in parts of the body that are not visible; a painful liver easily palpable in the right upper quadrant, for example, signals engorgement and swelling within that organ (hepatomegaly). Right-sided heart failure also causes fluid to leak into the peritoneal cavity causing abdominal distention. Ascites is the medical term used to describe the accumulation of fluid in the abdominal cavity.
The development of right-sided heart failure can actually improve left-sided heart failure because the failing right side of the heart can no longer pump as much blood into the lungs. The decrease in output from the right side, in essence, amounts to a decrease in preload for the left side of the heart and may lessen pulmonary congestion.
Right-sided heart failure, by itself, is seldom a life-threatening emergency. Usually it develops gradually over days to weeks; likewise, it requires days to weeks to reverse the process by slowly ridding the body of excess salt and water. Therefore, treatment in the field of a patient with right-sided heart failure is simply to make the patient comfortable, preferably in the semi-Fowler position. Monitoring is always indicated in any patient with significant cardiac disease. If signs of associated left-sided heart failure are present, treat them as outlined in the previous section.
Pathophysiology
The pericardium is a tough, fibrous membrane with the ability to stretch only up to a point. Normally, a small amount of pericardial fluid separates the pericardium and the outer surface of the heart. Cardiac tamponade occurs when excessive fluid accumulates within the pericardium, limiting the heart’s ability to expand fully after each contraction and resulting in reduced CO. If unrecognized and untreated, this condition will reduce cardiac filling to the point that the heart is unable to circulate the blood.
Assessment
Cardiac tamponade can occur as a result of tumors, pericarditis, or trauma to the chest. Rarely, cardiac tamponade can also occur after myocardial infarction as a result of cardiac rupture. Pericarditis, for example, can cause excessive amounts of fluid to accumulate in the pericardial space. Blunt or penetrating trauma can cause bleeding from blood vessels on the surface of the heart, allowing accumulation of blood in the pericardial space.
Signs and symptoms of cardiac tamponade vary depending on its cause. If the onset is gradual (as with pericarditis), the patient may initially report dyspnea and weakness. If the cause is traumatic, the chief complaint might be chest pain. As the volume of fluid increases in the pericardium, the SV decreases, causing an initial drop in the systolic blood pressure. Eventually, the diastolic pressure will slowly rise, resulting in the classic symptom of narrowing pulse pressure. The initial drop in blood pressure is usually followed by an increase in the heart rate, which leads to tachycardia. The heart sounds may be muffled or quieter than usual owing to the buildup of fluid, although this sign may be difficult to identify in the field. The patient may experience jugular vein distention as well, owing to the backup of blood from the right side of the heart. The combination of narrowing pulse pressure (hypotension) along with jugular vein distention and muffled heart sounds is commonly known as Beck’s triad.
The ECG is of limited value to you in identifying cardiac tamponade. Aside from tachycardia, you might see electrical alternans (alternating small- and large-amplitude QRS complexes). In addition, you might identify pulsus alternans (alternating strong and weak pulses). Pulsus paradoxus—a drop in systolic blood pressure of more than 10 mm Hg with the patient’s inhalation that may be associated with a weakening pulse during inhalation—may also be present.
Identification of cardiac tamponade requires a thorough assessment. Changes in blood pressure can be recognized only after at least three values have been obtained, usually 5 to 10 minutes apart. Muffled heart sounds, pulsus alternans, electrical alternans, and pulsus paradoxus are not common signs and so may be easily overlooked. Occasionally, you may have difficulty distinguishing between cardiac tamponade and tension pneumothorax. One way to differentiate between the two is to remember that in cardiac tamponade, the breath sounds will be equal and the trachea will be midline because the lungs are not affected.
Management
The ultimate treatment for cardiac tamponade is pericardiocentesis, which involves inserting a needle attached to a syringe into the chest far enough to penetrate the pericardium and then withdrawing fluid. Often, withdrawal of as little as 50 mL of fluid will result in significant improvement in the patient’s condition. This technique is risky, however, and medical direction rarely allows it to be performed by paramedics. If pericardiocentesis is not allowed, you should provide rapid transport to a facility that can perform this procedure.
Supporting the patient’s airway, breathing, and oxygenation during transport is essential. An IV fluid bolus of 500 mL of saline might be ordered by medical direction. When you are reporting to medical control, make sure that you identify all signs and symptoms that led you to believe the patient has cardiac tamponade so that the receiving hospital will be prepared to perform the pericardiocentesis.
Pathophysiology
Cardiogenic shock occurs when the heart is so severely damaged that it can no longer pump a volume of blood sufficient to maintain tissue perfusion. An AMI nearly always produces some impairment of left ventricular function. When 25% of the left ventricular myocardium is involved in the AMI, left-sided heart failure usually develops. When 40% or more of the left ventricle has been infarcted, cardiogenic shock occurs. Thus, cardiogenic shock indicates extensive injury to the myocardium; accordingly, it is a high priority in the field. Transient cardiogenic shock can occur after resuscitation. Patients recovering from defibrillation for ventricular fibrillation, for example, often have signs of cardiogenic shock. Symptoms of cardiogenic shock include difficulty breathing, extreme fatigue, and general malaise.
The signs and symptoms of cardiogenic shock are similar to those of most other kinds of shock. Because of the reduced cerebral perfusion, the patient is often confused or even comatose; if awake, he or she is likely to be restless and anxious. Massive peripheral vasoconstriction results in pale, cold skin, and poor renal perfusion is reflected in minimal or absent urine output. Respirations are rapid and shallow, with a possibility of adventitious breath sounds, and the pulse is racing and thready. The patient’s ECG may be normal or reveal fast or slow dysrhythmias. The diagnosis of cardiogenic shock is made clinically, not by ECG changes.
As these compensatory mechanisms begin to fail, the blood pressure will fall, sometimes to less than 90 mm Hg systolic. However, this vital sign may be deceptive. In patients with preexisting hypertension, systolic pressures higher than 90 mm Hg may still be associated with cardiogenic shock. The goal in treatment of cardiogenic shock is to identify and support the patient before the blood pressure drops to the point where the shock becomes irreversible.
Management
Treatment of cardiogenic shock focuses on improving oxygenation and peripheral perfusion without adding to the work of the heart. Secure the patient’s airway, and administer 100% supplemental oxygen by mask or bag-mask device. An advanced airway (endotracheal tube, laryngeal mask airway, King LT, or Combitube) will be necessary if the patient is unresponsive. Place the patient in a supine position unless pulmonary edema is present; in that case, the patient should be placed in the semi-Fowler position.
Start an IV line with normal saline at a keep-vein-open rate. The physician may order a trial of fluids to determine whether the shock includes a hypovolemic component. If so, rapidly infuse 100 to 200 mL of saline, and closely monitor the patient’s pulse, blood pressure, and level of consciousness (LOC). Report those observations to the physician.
Apply monitoring electrodes and obtain a 12-lead ECG. Dysrhythmias may bring about hypotension by causing severe disturbances in CO; thus, until major dysrhythmias are corrected, you cannot be certain that the patient’s hypotension is due to cardiogenic shock.
Depending on the distance to the hospital and local protocols, you may be asked to administer a vasopressor drug, such as one of those listed in Table 28. Dopamine might be preferred because, at beta doses, it maintains renal perfusion better than the other agents listed. To prepare a dopamine infusion, add 400 mg of dopamine to a 250-mL bag of normal saline, to yield a concentration of 1,600 μg/mL. The infusion rate will depend on the patient’s weight and response, but it is usually initiated at 5 μg/kg/min. The administration of dopamine (Intropin) or any other vasopressor drug requires careful titration and frequent monitoring of the blood pressure. Measure the blood pressure at least every 5 minutes. Slow the infusion if the systolic pressure rises to more than 90 or 100 mm Hg; speed up the infusion if the systolic pressure falls below 70 mm Hg.
Except for the correction of life-threatening dysrhythmias, there are no measures that you can perform to stabilize the condition of a patient in cardiogenic shock in the field. Therefore, transport the patient expeditiously to the hospital.
The word aneurysm comes from a Greek word meaning a widening; it refers to the dilation or outpouching of a blood vessel. The aneurysms of greatest concern to you are those that involve the aorta, particularly acute dissecting aneurysms of the thoracic aorta and expanding or ruptured aneurysms of the abdominal aorta.
Acute Dissecting Aneurysm of the Aorta
Pathophysiology The proximal aorta is subject to enormous hemodynamic forces. Anywhere from 60 to 100 times a minute, 60 minutes an hour, 24 hours a day—that is, around 40 million times a year—pulsatile waves of blood come pounding out of the left ventricle against the aortic walls. Over the years, that pounding takes its toll, producing degenerative changes in the media (the middle layer) of the aorta, especially the ascending aorta (the part of the aorta that rises from the heart toward the aortic arch). The degenerative changes are more pronounced with advancing age and in people with chronic high blood pressure, and their effect is to “unglue” the layers of the aortic wall from one another.
Eventually, the degenerative changes in the aortic media may lead to a disruption of the underlying intima (innermost layer of the artery). Tearing of the intima is most likely to occur in the portions of the thoracic aorta that are under the greatest stress—specifically, the ascending aorta just distal to the aortic valve (approximately 65% of cases) and the descending aorta just beyond the takeoff point of the left subclavian artery.
Once the intima is torn, the process of dissection, or separation of the arterial wall, often begins. With each ventricular systole, a jet of blood is forced into the torn arterial wall, creating a false channel between the intimal and medial layers of the wall. This channel is propagated distally and sometimes proximally along the length of the wall. If the dissection progresses back into the aortic valve, it may prevent the valve from closing, so that blood regurgitates back from the aorta into the left ventricle during systole. Recall that the coronary arteries branch off from the aorta just above the leaflets of the aortic valve; thus, if the valve is affected, coronary blood flow will likely be affected as well. If the dissection involves the takeoff point of the innominate, left common carotid, or left subclavian artery, blood flow through the affected artery or arteries will be compromised.
Table 28 Vasopressor Agents
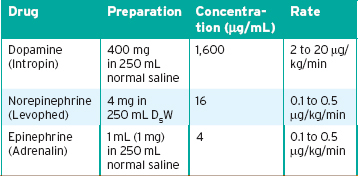
Two classification systems are used for determining the significance of an aneurysm. The Stanford classification distinguishes two types of aortic dissection: Type A, which involves the ascending aorta, and Type B, which does not. Type A will require surgical intervention while Type B can be managed medically. The DeBakey classification places aortic dissections into three categories: Type 1 involves the ascending aorta, aortic arch, and descending aorta; Type II is localized to the ascending aorta; and Type III focuses on dissections involving the descending aorta that is distal to the left subclavian artery. Type III is further broken down into Type IIIa and Type IIIb. Type IIIa dissections start at the left subclavian artery but extend both proximal and distally mostly above the diaphragm. Type IIIb dissections start distally to the left subclavian artery but extend only distally and may be below the diaphragm.
Assessment The typical patient with a dissecting aneurysm is a middle-aged or older man with chronic hypertension, although dissection may occur during pregnancy and in younger patients with Marfan syndrome. In Marfan syndrome, the walls of the major arteries—including the aorta—are weakened. When blood flow leaks through the tears of the weakened aortic wall, it results in an aortic dissection. By far, the most common chief complaint is chest pain, which is usually described as “the worst pain I have ever experienced,” or as “ripping,” “tearing,” “sharp,” or “like a knife.” This pain comes on suddenly and is located in the anterior part of the chest or in the back between the shoulder blades.
On the basis of the patient’s description, it may be difficult to differentiate the chest pain of a dissecting aneurysm from that of an AMI, but a number of distinctive features may help. The pain of an AMI is often preceded by other symptoms—nausea, “indigestion,” weakness, and sweating—and tends to come on gradually, getting more severe with time and often being described as “pressure” rather than “stabbing.” By contrast, the pain of a dissecting aneurysm usually comes on full force from one minute to the next, without prodromal symptoms. Table 29 summarizes the differences in the clinical presentations of AMI and dissecting aortic aneurysm.
Other signs and symptoms of dissecting aneurysm will depend on the site of the intimal tear and the extent of the dissection. In dissections of the ascending aorta, which tend to occur in younger patients previously in good health, one or more of the vessels of the aortic arch are usually compromised. Disruption of flow through the innominate artery, for example, is likely to produce a difference in blood pressure between the two arms. (If you do not routinely check the blood pressure in both arms of a patient, you will never pick up that sign!) You may also find that one femoral or carotid pulse is missing or weak. Disruption of blood flow into the left common carotid artery may produce signs and symptoms of a stroke. When the dissection extends proximally to the ostia of the coronary arteries, coronary blood flow is likely to be compromised, and ECG changes of myocardial ischemia are likely. Death from dissection of the ascending aorta is nearly always a result of aortic rupture into the pericardium and resultant cardiac tamponade. In such a case, you will see the characteristic signs of cardiac tamponade: distended neck veins, hypotension, narrow pulse pressure, and muffled heart sounds.
Dissection of the descending aorta occurs more commonly in older patients, especially those with a history of hypertension. The pain is likely to be somewhat less severe when the descending aorta is involved; indeed, the patient may wait a few days before seeking help. The dissection usually proceeds distally, so the aortic arch is spared, which means that blood pressure discrepancies between the two arms are not part of the picture. The pulses in the lower extremities, however, may be affected.
Management The goal of prehospital management in a suspected dissecting aneurysm is primarily to provide adequate pain relief. In the hospital setting, medications will be given to lower the patient’s blood pressure and reduce myocardial contractility to take some of the hemodynamic load off the aorta. Only in unusual circumstances would such therapy be started in the field because it requires careful monitoring of intra-arterial pressure.
The steps of prehospital management in suspected dissecting aneurysm are as follows:
 Calm and reassure the patient.
Calm and reassure the patient.
 Administer 100% supplemental oxygen by nonrebreathing mask.
Administer 100% supplemental oxygen by nonrebreathing mask.
 Insert an IV line, and give a crystalloid solution.
Insert an IV line, and give a crystalloid solution.
 Apply monitoring electrodes and obtain an ECG rhythm strip.
Apply monitoring electrodes and obtain an ECG rhythm strip.
 If the patient is not hypotensive, administer IV morphine sulfate, 2 mg at a time, up to a total dose of 10 mg during 10 to 15 minutes.
If the patient is not hypotensive, administer IV morphine sulfate, 2 mg at a time, up to a total dose of 10 mg during 10 to 15 minutes.
 Transport without delay. Nothing can be done to stabilize the patient’s condition in the field. The patient will need aggressive therapy in the intensive care unit and possibly surgery.
Transport without delay. Nothing can be done to stabilize the patient’s condition in the field. The patient will need aggressive therapy in the intensive care unit and possibly surgery.
Table 29 AMI Versus Dissecting Aortic Aneurysm
|
AMI |
Dissecting Aneurysm |
Onset of pain |
Gradual, with prodromal symptoms |
Abrupt, without prodromal symptoms |
Severity of pain |
Increases with time |
Maximal from the outset |
Timing of pain |
May wax and wane |
Does not abate once it has started |
Location of pain |
Substernal; back is rarely involved |
Back is often involved, between the shoulder blades |
Clinical signs |
Peripheral pulses equal |
Blood pressure discrepancy between arms or decrease in a femoral or carotid pulse |
Expanding and Ruptured Abdominal Aortic Aneurysms
Pathophysiology Abdominal aortic aneurysms (AAAs) affect approximately 2% of the US population older than 50 years and account for 15,000 deaths each year. Most commonly, the aneurysm is located just distal to the renal arteries. An expanding aneurysm is, as the name implies, an aneurysm that is getting larger and producing symptoms by compressing on adjacent structures, although the aortic wall remains intact. When an aneurysm starts expanding and producing symptoms, it can be assumed that rupture is imminent.
Assessment The typical patient with an AAA is a man in his late 50s or 60s. As long as the aneurysm remains stable, the patient usually will be asymptomatic. When the aneurysm starts to expand, however, the patient becomes symptomatic, with the sudden onset of abdominal or back pain. When the pain is principally in the abdomen, it tends to center on the umbilicus. Often, the pain may be located solely in the lower back, leading the patient to think he or she has “pulled a muscle” or otherwise injured the back. The pain is constant and moderate to severe; it cannot be relieved by changes in position. It tends to radiate into the thigh and groin. If the aneurysm is leaking blood into the retroperitoneal space, the patient may report an urge to defecate. In some patients, an episode of syncope heralds the onset of symptoms.
The most characteristic physical finding in a patient with an AAA is a pulsatile mass palpable in the abdomen. The patient is likely to be normotensive when first seen, but signs of shock, with or without hypotension, may develop rapidly if the aneurysm has ruptured.
Management Prehospital management of an expanding or ruptured aortic aneurysm is aimed at getting the patient to the hospital as expeditiously as possible because the definitive treatment requires urgent surgery. The key is to maintain a high index of suspicion whenever a middle-aged or older man presents with sudden back pain and a pulsatile abdominal mass. The more likely problem in the field in a conscious patient is a leaking aneurysm that has yet to rupture.
The steps of prehospital management of patients with an expanding or ruptured aortic aneurysm are as follows:
 Administer supplemental oxygen.
Administer supplemental oxygen.
 Consider applying (but do not inflate) the pneumatic antishock garment (PASG)/military antishock trousers (MAST) if available.
Consider applying (but do not inflate) the pneumatic antishock garment (PASG)/military antishock trousers (MAST) if available.
 Transport without delay.
Transport without delay.
 Insert an IV line en route, and give normal saline or lactated Ringer’s. Use a large-gauge catheter, but maintain the flow to keep the vein open unless signs of shock appear. If there are signs of shock, treat as for any other case of shock, with IV fluids.
Insert an IV line en route, and give normal saline or lactated Ringer’s. Use a large-gauge catheter, but maintain the flow to keep the vein open unless signs of shock appear. If there are signs of shock, treat as for any other case of shock, with IV fluids.
Pathophysiology
Hypertension (high blood pressure) affects nearly 60 million Americans and is directly responsible for more than 30,000 deaths per year. In addition, it is a major contributing cause in many cases of MI, CHF, and stroke. Most hypertension is the result of advanced atherosclerosis or arteriosclerosis, which decreases the lumen of the arteries and reduces their elasticity. The resulting high afterload on the heart leads to an increase in filling volume and stimulates the Frank-Starling reflex, which raises the pressure behind the blood leaving the heart.
Hypertension is present when the blood pressure at rest is consistently greater than about 140/90 mm Hg. Many conditions, such as anxiety or pain, can transiently elevate a person’s blood pressure (especially the systolic blood pressure), so a single blood pressure measurement taken during an emergency scarcely constitutes adequate grounds for telling a patient that he or she is hypertensive. Instead, you may say something like this: “Sir, your blood pressure is a little high right now. That may be because of the stress you are under and may not have any real significance. To be safe, you should have your blood pressure rechecked a couple of times in the next few months under less stressful circumstances.”
Persistent elevation of the diastolic pressure, by contrast, is indicative of hypertensive disease. If left untreated, hypertension significantly shortens a person’s life span and predisposes the person to a variety of other medical problems. The most common complications of hypertension include renal damage, stroke, and heart failure—the last a result of the left ventricle having to pump for years against a markedly increased afterload.
Assessment
In the majority of cases, hypertension is entirely asymptomatic and is detected by chance during a routine examination. By the time symptoms start to occur, hypertension is already in a more advanced stage and has probably produced at least some damage to organs such as the heart, kidneys, and brain.
The symptoms that occur in advanced hypertensive disease may be related to the elevated blood pressure or to secondary complications. Headache is the most common symptom directly related to blood pressure elevation; hypertensive headache is usually localized to the occipital region of the head and occurs when the patient first awakens in the morning, then subsides gradually over the next few hours. Other symptoms of moderately severe hypertension include dizziness, weakness, epistaxis, tinnitus, and blurring of vision. Rarely, seizures can result from severe hypertension. Often a patient with these hypertension-related signs and symptoms has already been prescribed medication for hypertension but is not taking it as prescribed.
Management
Hypertensive emergencies occur in about 1% of all hypertensive patients. A hypertensive emergency is defined as an acute elevation of blood pressure with evidence of end-organ damage. That last phrase is important, because it is the evidence of end-organ dysfunction that determines the urgency of the situation, not the reading on the sphygmomanometer. Two end-organ emergencies that may result from uncontrolled hypertension were discussed earlier in this chapter: left-sided heart failure and dissecting aortic aneurysm. A rare but much more devastating complication of hypertension is hypertensive encephalopathy.
Hypertensive encephalopathy (also known as acute hypertensive crisis) may complicate any form of hypertension. Hypertensive crisis is usually signaled by a sudden, marked rise in blood pressure to levels of greater than 200/130 mm Hg. The determining factor for hypertensive encephalopathy is usually the mean arterial pressure (MAP). The MAP is calculated by adding one third of the difference between the systolic blood pressure (SBP) and diastolic blood pressure (DBP) to the diastolic blood pressure.
MAP = DBP + 1/3 (SBP − DBP)
When the MAP exceeds 150 mm Hg, the pressure breaches the blood-brain barrier and fluid leaks out, increasing intracranial pressure. Usually the first symptoms noticed are severe headache, nausea, and vomiting. They are followed by seizures and alternations in mental status (that is, confusion to unresponsiveness). Sometimes patients may show focal neurologic signs, such as sudden blindness, aphasia (disturbances in speech production or comprehension), or hemiparesis. Widespread neuromuscular irritability may be signaled by muscle twitching.
The goal of treatment in hypertensive encephalopathy is to lower the blood pressure in a gradual, controlled manner during a 30- to 60-minute period so that cerebral blood flow is restored to normal. This is best accomplished under controlled conditions in a hospital. Thus, if you are within 20 to 30 minutes of the nearest hospital, provide supportive treatment only:
 Secure the airway, and administer supplemental oxygen by nasal cannula or nonrebreathing mask.
Secure the airway, and administer supplemental oxygen by nasal cannula or nonrebreathing mask.
 Establish an IV line with normal saline at a keep-vein-open rate.
Establish an IV line with normal saline at a keep-vein-open rate.
 Apply monitoring electrodes, and run an ECG rhythm strip (consider running a 12-lead ECG en route to the emergency department).
Apply monitoring electrodes, and run an ECG rhythm strip (consider running a 12-lead ECG en route to the emergency department).
 Transport without delay.
Transport without delay.
Paramedics working in rural areas or other circumstances where long transport times to the hospital are unavoidable may have to initiate drug therapy for hypertensive encephalopathy in the field. One widely accepted drug for this purpose is labetalol (Normodyne, Trandate), which has alpha- and beta-blocking properties. As an alpha blocker, it prevents vasoconstriction, thereby decreasing the overall peripheral vascular resistance. Meanwhile, its beta-blocking actions prevent the reflex tachycardia that would otherwise occur in response to a drop in blood pressure. As a beta blocker, however, labetalol is relatively contraindicated in patients with asthma and COPD.
Labetalol can be given initially by slow IV push at 20 mg, repeated in 10 minutes as necessary, or an IV drip can be started. To administer a labetalol drip, add 250 mg to 250 mL of normal saline, yielding a concentration of 1 mg/mL. Start the infusion at a rate of 2 mg/min (2 mL/min), and watch the infusion carefully! A runaway IV could prove disastrous. Monitor the patient’s blood pressure every 2 to 3 minutes. When the blood pressure has fallen to the target level specified by the physician, stop the infusion.
The other drug that may be ordered to lower a dangerously high blood pressure is nitroglycerin, 0.4 mg sublingual. This drug is not the first choice for this indication, but its use is acceptable if labetalol is not available.
Whenever you give a drug to lower a patient’s blood pressure, keep the patient supine, and measure his or her blood pressure at least every 3 to 5 minutes. Record each measurement on a flowchart.
 Infectious Diseases of the Heart
Infectious Diseases of the Heart
Endocarditis is an infection of the inner lining of the heart, characterized by inflammation of the endocardium (the inside lining of the heart chambers, including the heart valves). It is almost exclusively caused by staphylococcal or streptococcal infection. Patients with prosthetic heart valves and intravenous drug abusers top the list of those at highest risk for developing endocarditis. Typically this infection comes from another location of the body such as the mouth; it is spread through the bloodstream, ultimately reaching the heart. If left untreated, large growths on the valve leaflets can develop as a result of the infection, and can damage or even destroy the heart valves, which in turn can lead to life-threatening complications such as regurgitation and hemodynamic instability. Treatment for endocarditis typically consists of administration of antibiotics, but severe cases can require surgery.
Pericarditis is an acute inflammation of the pericardium that can last anywhere from several weeks to several months. The pericardial sac becomes red and swollen, and occasionally a buildup of fluid in the pericardial sac is noted. Patients report chest pain that is sharp and stabbing that can increase with coughing, swallowing, deep breathing, or lying flat. Patients may also indicate that the pain decreases if they are in the sitting position while leaning forward. Treatment for these patients consists of administration of nonsteroidal anti-inflammatory drugs (NSAIDs) or antibiotics.
Myocarditis is defined as inflammation of the myocardium. It can be caused by viral, bacterial, and fungal infections, and also from traumatic injury. Myocarditis can cause chest pain, dysrhythmias, heart failure, and sudden cardiac arrest.
Rheumatic fever is an inflammatory disease that is caused by streptococcal bacteria strains. This disease can cause a stenosis of the mitral valve or aortic valve, leading to heart complications.
Scarlet fever is a disease caused by the bacterium Streptococcus pyogenes. This is the same bacterium responsible for causing strep throat. The disease is characterized by a sore throat, fever, rash, and “strawberry tongue” (a white-colored tongue with red speckles). Patients younger than 1 year are at greatest risk for developing the infection. Scarlet fever is treated with antibiotics and prehospital care is entirely supportive.
In conclusion, there are a few other cardiac conditions that may lead to emergencies. These include congenital heart disease and cardiomyopathy. Congenital heart disease is covered in the chapter, Neonatal Care. Cardiomyopathy is discussed in the chapter, Pediatric Emergencies.
YOU are the Medic SUMMARY
1. What is your initial impression of this patient’s condition?
Right now, the patient’s condition is uncertain. There could be a number of causes for the pain in the patient’s jaw. These range from a dental problem to recent trauma to referred pain from another condition. The patient looks sick with pale, moist skin, which is concerning. This could be related to the patient’s diabetes, or may be unrelated to that. As always, you will need to do a thorough assessment to determine what is happening with this patient.
2. What initial history do you need for your assessment of this patient?
You should obtain baseline vital signs and OPQRST findings to assess the pain as soon as possible. Not every patient with an AMI has chest pain, however. People with diabetes, older people, and heart transplant patients, for example, generally do not present with chest pain. Because this patient has diabetes, her perception of pain will be altered.
3. Having ruled out a diabetes-related problem, what is your next step?
The blood glucose reading of 152 mg/dL is slightly elevated. Unexplained jaw pain in any patient should raise your index of suspicion for a possible cardiac event. Acquire a 12-lead ECG early and perform a focused cardiac examination.
4. What are the differences between the presentations of cardiac problems in men and women?
Women may experience different symptoms during AMI than men. Women may experience nausea, light-headedness, epigastric burning, or sudden onset of weakness or unexplained fatigue. Women may also have pain radiating down the right arm instead of the classic left arm pain. Because they may not experience the typical chest pain expected with an AMI, many women ignore their symptoms. Unfortunately, cardiovascular disease is the number one cause of death in US women.
5. What are PVCs and what does “multifocal” mean?
Premature ventricular complexes (PVCs) are ventricular beats that occur early in the cardiac cycle. Premature ventricular complexes are also known as ectopic complexes, meaning that they occur outside of the normal conduction pathway. Premature ventricular complexes can be categorized into two groups: unifocal and multifocal. Unifocal PVCs originate from the same spot or “focus” within the ventricle and will have the same shape and direction on the ECG. Multifocal premature ventricular complexes originate from two or more irritated foci in the ventricles and will have differing morphologic features on the ECG.
6. What is your first-line treatment for PVCs?
If the SpO2 is below 94%, the PVCs may be due to hypoxia. Administer oxygen and titrate to an SpO2 of 98% to 100%. If the SpO2 is above 94%, consider other causes of PVCs including myocardial infarction, cocaine and other drug use, alcohol, mitral valve prolapse, and caffeine.
7. What does the acronym MONA stand for?
The mnemonic MONA is used to help us remember treatments for acute coronary syndromes. MONA stands for Morphine, Oxygen, Nitroglycerin, and Aspirin. The mnemonic MONA is easy to remember, but it is important to note that the order of administration does not follow the order of letters in the acronym. The order of treatment can be controversial. Generally, the order should be: Oxygen (if the SpO2 is 94% or lower), Aspirin, Nitroglycerin, and Morphine; however, always follow your local protocol.
8. Of the steps in MONA, which should you perform next?
You have already seen results from the oxygen administration: PVCs dropped from 8 to 10 per minute, to 2 per minute, and they appear to be unifocal. Therefore, the next step in treatment should be administration of aspirin. In most EMS systems, as long as the patient has no aspirin allergy or gastrointestinal bleeding, dispatchers may advise patients to chew baby aspirin (160 to 325 mg). If this has not been done before your arrival or the patient has not already taken aspirin on his or her own, then give the patient 160 to 325 mg of non–enteric-coated aspirin to chew. Aspirin prevents platelets from sticking to one another, minimizing the growth of clots. Morphine at 2 mg slow IV push is also appropriate in this patient.
 Ready for Review
Ready for Review
 Cardiovascular disease has been the number one killer in the United States almost every year since 1900.
Cardiovascular disease has been the number one killer in the United States almost every year since 1900.
 The cardiovascular system is composed of the heart and blood vessels. Its primary function is to deliver oxygenated blood and nutrients to every cell.
The cardiovascular system is composed of the heart and blood vessels. Its primary function is to deliver oxygenated blood and nutrients to every cell.
 The body attempts to maintain a fairly constant blood pressure to ensure perfusion of vital organs.
The body attempts to maintain a fairly constant blood pressure to ensure perfusion of vital organs.
 Patients experience a variety of symptoms when they have a cardiovascular problem, the most common of which are chest pain, dyspnea, fainting, palpitations, and fatigue.
Patients experience a variety of symptoms when they have a cardiovascular problem, the most common of which are chest pain, dyspnea, fainting, palpitations, and fatigue.
 Patients with cardiovascular disease are often prescribed several medications. Patients with advanced age typically experience more side effects from medications than do younger patients. Adverse drug events account for approximately 10% of all ED visits in the elderly.
Patients with cardiovascular disease are often prescribed several medications. Patients with advanced age typically experience more side effects from medications than do younger patients. Adverse drug events account for approximately 10% of all ED visits in the elderly.
 Cardiac rhythm disturbances (dysrhythmias) may arise from a variety of causes, including AMI.
Cardiac rhythm disturbances (dysrhythmias) may arise from a variety of causes, including AMI.
 One of the most important tasks in the prehospital care of a patient with an AMI is to anticipate, recognize, and treat life-threatening dysrhythmias. In fact, ECG analysis is indicated in any patient who might have a cardiac-related condition.
One of the most important tasks in the prehospital care of a patient with an AMI is to anticipate, recognize, and treat life-threatening dysrhythmias. In fact, ECG analysis is indicated in any patient who might have a cardiac-related condition.
 Most deaths from AMI are due to dysrhythmias, which typically occur during the early hours of the infarct.
Most deaths from AMI are due to dysrhythmias, which typically occur during the early hours of the infarct.
 Cardiac monitors consist of leads that are connected to electrodes, which are placed on the patient. Each lead offers an electrical snapshot of a certain part of the heart. The cardiac monitor records the electrical activity acquired by each lead used, resulting in a 12-lead ECG tracing.
Cardiac monitors consist of leads that are connected to electrodes, which are placed on the patient. Each lead offers an electrical snapshot of a certain part of the heart. The cardiac monitor records the electrical activity acquired by each lead used, resulting in a 12-lead ECG tracing.
 Cardiac monitoring and ECG analysis are indicated in any patient who might have a cardiac-related condition. Any patient with chest pain or a history of heart problems should undergo ECG analysis.
Cardiac monitoring and ECG analysis are indicated in any patient who might have a cardiac-related condition. Any patient with chest pain or a history of heart problems should undergo ECG analysis.
 Cardiac monitoring usually involves using four limb leads to create an ECG printout showing lead I, II, and III to monitor the electrical activity in the patient’s heart.
Cardiac monitoring usually involves using four limb leads to create an ECG printout showing lead I, II, and III to monitor the electrical activity in the patient’s heart.
 Components of a rhythm strip produced by an ECG include the P wave, PR interval, QRS complex, J point, ST segment, T wave, and QT interval.
Components of a rhythm strip produced by an ECG include the P wave, PR interval, QRS complex, J point, ST segment, T wave, and QT interval.
 Each component of the rhythm strip represents a phase of electrical activity within the heart. One whole complex on a rhythm strip represents one complete cycle of electrical conduction.
Each component of the rhythm strip represents a phase of electrical activity within the heart. One whole complex on a rhythm strip represents one complete cycle of electrical conduction.
 Items to assess when you are analyzing a rhythm strip or ECG include the following: P waves, the PR interval, QRS duration, rhythm, and rate.
Items to assess when you are analyzing a rhythm strip or ECG include the following: P waves, the PR interval, QRS duration, rhythm, and rate.
 The 12-lead ECG enables localization of cardiac ischemia to specific areas of the heart. The 12 leads include three limb leads (I, II, and III), three augmented limb leads (aVR, aVL, and aVF), and six precordial leads (V1 to V6).
The 12-lead ECG enables localization of cardiac ischemia to specific areas of the heart. The 12 leads include three limb leads (I, II, and III), three augmented limb leads (aVR, aVL, and aVF), and six precordial leads (V1 to V6).
 Leads that look at the same general area of the heart are called contiguous leads.
Leads that look at the same general area of the heart are called contiguous leads.
 Transmission of 12-lead ECG findings to the receiving facility is an important step that can lead to a faster diagnosis, decrease the time from emergency onset to definitive therapy (door-to-balloon time and door-to-needle time), and decrease mortality.
Transmission of 12-lead ECG findings to the receiving facility is an important step that can lead to a faster diagnosis, decrease the time from emergency onset to definitive therapy (door-to-balloon time and door-to-needle time), and decrease mortality.
 Management of cardiac arrest involves BLS and ALS skills, including CPR, defibrillation, cardiac monitoring, IV fluid or medication infusion, post-resuscitative care, and cooling.
Management of cardiac arrest involves BLS and ALS skills, including CPR, defibrillation, cardiac monitoring, IV fluid or medication infusion, post-resuscitative care, and cooling.
 Defibrillation is an intervention used to interrupt rapid chaotic rhythms such as ventricular tachycardia and ventricular fibrillation. Defibrillation simultaneously depolarizes all cardiac tissue in hopes of allowing the SA node to resume the function of primary pacemaker. Paramedics most often perform manual defibrillation rather than automated external defibrillation, because they are trained in interpreting cardiac rhythms.
Defibrillation is an intervention used to interrupt rapid chaotic rhythms such as ventricular tachycardia and ventricular fibrillation. Defibrillation simultaneously depolarizes all cardiac tissue in hopes of allowing the SA node to resume the function of primary pacemaker. Paramedics most often perform manual defibrillation rather than automated external defibrillation, because they are trained in interpreting cardiac rhythms.
 Synchronized cardioversion is an intervention used to interrupt rapid, organized, and hemodynamically unstable rhythms such as supraventricular tachycardia and atrial fibrillation. Unlike defibrillation, synchronized cardioversion is timed or “synchronized” to the patient’s cardiac rhythm. Energy is delivered precisely at the peak of the R wave to capitalize on the already depolarized state of the ventricles. The energy delivered during synchronized cardioversion is aimed at depolarizing any tissue that remains polarized in hopes that the SA node resumes the primary pacemaking function.
Synchronized cardioversion is an intervention used to interrupt rapid, organized, and hemodynamically unstable rhythms such as supraventricular tachycardia and atrial fibrillation. Unlike defibrillation, synchronized cardioversion is timed or “synchronized” to the patient’s cardiac rhythm. Energy is delivered precisely at the peak of the R wave to capitalize on the already depolarized state of the ventricles. The energy delivered during synchronized cardioversion is aimed at depolarizing any tissue that remains polarized in hopes that the SA node resumes the primary pacemaking function.
 Transcutaneous pacing is an intervention used to depolarize heart muscle using an external stimulus. Pads placed on the patient’s chest deliver electrical energy to the heart, causing muscle contraction. The external pacemaker serves as a bridge to permanent internal pacemaker implantation. Transcutaneous pacing is used to treat patients with hemodynamically unstable bradycardias.
Transcutaneous pacing is an intervention used to depolarize heart muscle using an external stimulus. Pads placed on the patient’s chest deliver electrical energy to the heart, causing muscle contraction. The external pacemaker serves as a bridge to permanent internal pacemaker implantation. Transcutaneous pacing is used to treat patients with hemodynamically unstable bradycardias.
 Treatment of hemodynamically unstable dysrhythmias should be centered on rate control of the dysrhythmia using electrical therapies.
Treatment of hemodynamically unstable dysrhythmias should be centered on rate control of the dysrhythmia using electrical therapies.
 Most cardiac arrest victims have evidence of atherosclerosis or other underlying cardiac disease. However, cardiac arrest can also occur secondary to electrocution, submersion, and other types of trauma. Indeed, many cardiac arrest victims have no warning before the event occurs.
Most cardiac arrest victims have evidence of atherosclerosis or other underlying cardiac disease. However, cardiac arrest can also occur secondary to electrocution, submersion, and other types of trauma. Indeed, many cardiac arrest victims have no warning before the event occurs.
 Coronary artery disease is the most common form of heart disease and the leading cause of death in adults in the United States.
Coronary artery disease is the most common form of heart disease and the leading cause of death in adults in the United States.
 Cardiac tamponade occurs when excessive fluid accumulates within the pericardium, limiting the heart’s ability to fully expand. This can reduce cardiac filling to the point that the heart is unable to circulate blood. Emergent pericardiocentesis is lifesaving in patients with cardiac tamponade and hemodynamic instability. Transport the patient to the closest emergency facility.
Cardiac tamponade occurs when excessive fluid accumulates within the pericardium, limiting the heart’s ability to fully expand. This can reduce cardiac filling to the point that the heart is unable to circulate blood. Emergent pericardiocentesis is lifesaving in patients with cardiac tamponade and hemodynamic instability. Transport the patient to the closest emergency facility.
 Cardiogenic shock results from extensive injury to the myocardium and carries a high mortality rate. Transport the patient expeditiously to the hospital. Except for the correction of life-threatening dysrhythmias, there are no measures that can stabilize the condition of a patient in cardiogenic shock in the field.
Cardiogenic shock results from extensive injury to the myocardium and carries a high mortality rate. Transport the patient expeditiously to the hospital. Except for the correction of life-threatening dysrhythmias, there are no measures that can stabilize the condition of a patient in cardiogenic shock in the field.
 Aortic aneurysms—particularly acute dissecting aneurysms of the thoracic aorta and expanding or ruptured aneurysms of the abdominal aorta—are of greatest concern to the EMS responder. The most common chief complaint is sudden severe ripping or tearing pain in the chest or abdomen.
Aortic aneurysms—particularly acute dissecting aneurysms of the thoracic aorta and expanding or ruptured aneurysms of the abdominal aorta—are of greatest concern to the EMS responder. The most common chief complaint is sudden severe ripping or tearing pain in the chest or abdomen.
 Treatment of hypertensive emergencies includes slow, controlled lowering of the patient’s blood pressure. Management of elevated blood pressure should be reserved for the emergency department physician. Remember that systemic hypertension can be a result of increased intracranial pressure and is essential to maintain cerebral perfusion pressure.
Treatment of hypertensive emergencies includes slow, controlled lowering of the patient’s blood pressure. Management of elevated blood pressure should be reserved for the emergency department physician. Remember that systemic hypertension can be a result of increased intracranial pressure and is essential to maintain cerebral perfusion pressure.
 Vital Vocabulary
Vital Vocabulary
abdominal aortic aneurysm (AAA) A sac or bulge in the wall of the abdominal portion of the aorta, resulting in weakening of that wall; it is considered life threatening if it ruptures.
aberration A term used to describe the shape of the QRS complex in aberrantly conducted beats.
absolute refractory period The early phase of cardiac repolarization, wherein the heart muscle cannot be stimulated to depolarize.
acetylcholine (ACh) A chemical mediator used in both the sympathetic and parasympathetic nervous systems.
acute coronary syndrome (ACS) Term used to describe any group of clinical symptoms consistent with acute myocardial ischemia.
acute myocardial infarction (AMI) A condition present when a period of cardiac ischemia caused by sudden narrowing or complete occlusion of a coronary artery leads to death (necrosis) of myocardial tissue.
adrenaline The hormone produced by the adrenal gland with alpha and beta sympathomimetic properties.
afterload The resistance against which the ventricle contracts.
agonal Pertaining to the period of dying.
agonal rhythm A cardiac dysrhythmia seen just before the heart stops altogether; essentially asystole with occasional QRS complexes that are not associated with cardiac output.
aneurysm A sac or bulge resulting from the weakening of the wall of a blood vessel or ventricle.
angina pectoris The sudden pain from myocardial ischemia, caused by diminished circulation to the cardiac muscle. The pain is usually substernal and often radiates to the arms, jaw, or abdomen and usually lasts 3 to 5 minutes and disappears with rest.
aorta The largest artery in the body, originating from the left ventricle.
aortic semilunar valve The valve between the left ventricle and the aorta; also called the aortic valve.
arrhythmia The lack of a cardiac rhythm; asystole.
arteries The muscular, thick-walled blood vessels that carry blood away from the heart.
arteriole A small blood vessel that carries oxygenated blood, branching into yet smaller vessels called capillaries.
arteriosclerosis A pathologic condition in which the arterial walls become thickened and inelastic.
artifact An artificial product; in cardiology, is used to refer to noise or interference in an ECG tracing.
asystole The absence of ventricular contractions; a “straight-line ECG.”
atheroma A mass of fatty tissue.
atherosclerosis An accumulation of fat inside blood vessels resulting in narrowing of the lumen diameter.
atrial kick The volume (percentage) of blood pumped into the ventricles by the atria.
atrioventricular (AV) node A specialized structure located in the AV junction that slows conduction through the AV junction.
atrioventricular (AV) valves The mitral and tricuspid valves.
atropine A parasympathetic blocker; opposes the action of acetylcholine on the heart and elsewhere, causing an increase in heart rate.
augmented unipolar leads On an ECG, leads that only contain one true pole; the other is a combination of information from other leads; includes leads aVR, aVL, and aVF.
automated external defibrillator (AED) A “smart” defibrillator that can analyze the patient’s ECG rhythm and determine whether a defibrillating shock is needed.
automaticity Spontaneous initiation of depolarizing electric impulses by pacemaker sites within the electric conduction system of the heart.
autonomic nervous system A subdivision of the nervous system that controls primarily involuntary body functions; comprised of the sympathetic and parasympathetic nervous systems.
AV junction The atrioventricular junction; the portion of the electric conduction system of the heart located in the upper part of the interventricular septum that conducts the excitation impulse from the atria to the bundle of His.
axis deviation A component of an ECG that looks at the direction of travel for the electricity going through the heart as it depolarizes.
Beck’s triad The combination of narrowed pulse pressure, muffled heart tones, and jugular vein distention associated with cardiac tamponade.
benign early repolarization Early repolarization that is thought to be a normal variant; characterized by ST-segment elevation (or J-point elevation), a J or fishhook appearance at the J point, and concave ST-segment morphology.
bifascicular block Blockage of any combination of two of the fascicles or conduction pathways: a right bundle branch block (RBBB) and anterior hemiblock, a RBBB and posterior hemiblock, or an anterior hemiblock and posterior hemiblock.
bigeminy A dysrhythmia in which every other heartbeat is a premature contraction; can be atrial or ventricular.
bipolar leads On an ECG, leads that contain a positive and a negative pole; includes leads I, II, and III.
blood pressure The pressure exerted by the pulsatile flow of blood against the arterial walls.
bronchoconstriction Narrowing of the bronchial tubes.
bronchodilation Widening of the bronchial tubes.
Brugada syndrome A genetic disorder involving sodium channels in the heart; characterized by incomplete RBBB and ST-segment elevation that aggressively returns to baseline.
bruits Abnormal whooshing sounds indicating turbulent blood flow within a blood vessel.
bundle branch block A disturbance in electric conduction through the right or left bundle branch from the bundle of His.
bundle of His The portion of the electric conduction system in the interventricular septum that conducts the depolarizing impulse from the atrioventricular junction to the right and left bundle branches.
capillaries Extremely narrow blood vessels composed of a single layer of cells through which oxygen and nutrients pass to the tissues; these form a network between arterioles and venules.
cardiac cycle The period from one cardiac contraction to the next. Each cardiac cycle consists of ventricular contraction (systole) and relaxation (diastole).
cardiac output (CO) Amount of blood pumped by the heart per minute, calculated by multiplying the stroke volume by the heart rate per minute.
cardiac tamponade Restriction of cardiac contraction, failing cardiac output, and shock, caused by the accumulation of fluid or blood in the pericardium.
cardiopulmonary arrest The sudden and often unexpected cessation of adequate cardiac output.
chordae tendineae Fibrous strands shaped like umbrella stays that attach the free edges of the leaflets, or cusps, of the atrioventricular valves to the papillary muscles.
chronotropic effect The effect on the rate of contraction of the heart.
circumflex coronary artery One of the two branches of the left main coronary artery.
claudication A severe pain in the calf muscle that is caused by narrowing of the arteries in this muscle and that leads to a painful limp.
collateral circulation The mesh of arteries and capillaries that supplies blood to a segment of tissue whose original arterial supply has been obstructed.
concordant precordial pattern A pattern in which the QRS complexes are all in the same direction in the precordial leads.
conductivity The property that enables cardiac cells to pass an electrical impulse from one cell to another.
congestive heart failure (CHF) A condition that occurs when the heart is unable to pump powerfully enough or fast enough to empty its chambers; as a result, blood backs up into the systemic circuit, the pulmonary circuit, or both.
contiguous leads Leads that view geographically similar areas of the myocardium; useful for localizing areas of ischemia.
contractility The ability to shrink, shorten, or contract.
coronary arteries The blood vessels of the heart that supply blood to its walls.
coronary artery disease (CAD) A pathologic process caused by atherosclerosis that leads to progressive narrowing and eventual obstruction of the coronary arteries.
coronary sinus A large vessel in the posterior part of the coronary sulcus into which the coronary veins empty.
coronary sulcus The groove along the exterior surface of the heart that separates the atria from the ventricles.
couplet Two premature ventricular contractions occurring sequentially.
DeBakey classification A classification system for aortic dissections that includes three categories.
defibrillation The use of an unsynchronized direct current (DC) electric shock to terminate ventricular fibrillation.
delta wave The slurring of the upstroke of the first part of the QRS complex that occurs in Wolff-Parkinson-White syndrome.
depolarization The process of discharging resting cardiac muscle fibers by an electric impulse that causes them to contract.
diastole The period of ventricular relaxation during which the ventricles passively fill with blood.
digitalis preparations The drugs used in the treatment of congestive heart failure and certain atrial dysrhythmias.
dissection In reference to blood vessels, an aneurysm, or bulge, formed by the separation of the layers of an arterial wall.
dromotropic effect The effect on the velocity of conduction.
dysrhythmias Disturbances in the cardiac rhythm.
ejection click A high-pitched heart sound that occurs just after the S1 sound.
ejection fraction (EF) The percentage of blood that leaves the heart each time it contracts.
electrical conduction system In the heart, the specialized cardiac tissue that initiates and conducts electric impulses; includes the SA node, internodal conduction pathways, atrioventricular junction, atrioventricular node, bundle of His, and the Purkinje network.
endocarditis Inflammation of the endocardium.
endocardium The thin membrane lining the inside of the heart.
epicardium The thin membrane lining the outside of the heart.
excitability The property that allows cells to respond to an electrical impulse.
fascicular block Disease or ischemia of either of the anterior and posterior fascicles of the electrical conduction system of the heart; also called hemiblock.
fibrinolysis The process of dissolving blood clots.
fibrinolytic therapy The therapy that uses medications that act to dissolve blood clots.
first-degree heart block A partial disruption of the conduction of the depolarizing impulse from the atria to the ventricles, causing prolongation of the PR interval.
Frank-Starling mechanism A characteristic of cardiac muscle that enables it, when stretched, to contract with greater force; the more cardiac muscle stretches, the greater the force of its contraction, the more completely it empties, and the greater the stroke volume.
heart rate (HR) The number of heart contractions per minute.
hyperkalemia An excessive amount of potassium in the blood.
hypertension High blood pressure, usually a diastolic pressure of greater than 90 mm Hg.
hypertensive emergency An acute elevation of blood pressure with evidence of end-organ damage.
hypertensive encephalopathy A condition that may complicate any form of hypertension, and which is usually signaled by a sudden, marked rise in blood pressure to levels greater than 200/130 mm Hg; also known as acute hypertensive crisis.
hypertrophic cardiomyopathy A condition in which the heart muscle is unusually thick, which means that the heart has to pump harder to get blood to leave.
hypocalcemia A low level of calcium in the blood.
hypokalemia A low concentration of potassium in the blood.
idioventricular Related to only the ventricles; produced by the ventricles.
infarction Death (necrosis) of a localized area of tissue caused by the cutting off of its blood supply.
inferior wall MI Death of myocardial tissue involving the lower (inferior) portion of the heart.
inotropic effect The effect on the contractility of muscle tissue, especially cardiac muscle.
internodal pathways The three pathways of the electrical conduction system found in the atria that transmit the impulse from the SA node to the AV node.
interventricular septum A thick wall that separates the right and left ventricles.
ischemia Tissue anoxia from diminished blood flow to tissue, usually caused by narrowing or occlusion of an artery.
isoelectric When referring to a wave, the wave is neither positive nor negative.
isoelectric line The baseline of the ECG.
junctional rhythm A dysrhythmia arising from ectopic foci in the area of the atrioventricular junction; often shows an absence of the P wave, P-wave inversion, a short PR interval, or a P wave appearing after the QRS complex.
leads The electrical cable attaching the electrode to the ECG monitor; the voltage difference between two points. For example, lead I is the voltage difference between the right and left arm electrodes.
left atrial enlargement Dilation of the left atrium that results either from systemic hypertension, mitral or aortic valve stenosis, or an athletic heart.
left atrium The upper left chamber of the heart; receives blood from the pulmonary veins.
left-sided heart failure A condition in which the left ventricle cannot effectively pump; this leads to a backup of blood behind the left ventricle, and eventually serum is forced out of the pulmonary capillaries and into the alveoli.
left ventricle The thick-walled, muscular, lower left chamber of the heart; receives blood from the left atrium and pumps it out through the aorta into the systemic arteries.
left ventricular hypertrophy (LVH) A cardiac condition in which the left ventricle becomes enlarged, most commonly due to hypertension.
limb leads The ECG leads attached to the limbs and that form the hexaxial system, along the frontal plane.
long QT syndrome A condition characterized by a QT interval exceeding approximately 450 ms.
Lown-Ganong-Levine syndrome A disorder that causes preexcitation of ventricular tissue and which is characterized on the ECG by a short PR interval and a normal QRS duration.
lumen The inside of an artery or other hollow structure.
manual defibrillation A mode available on automated external defibrillators, allowing the paramedic to interpret the cardiac rhythm and determine if defibrillation is needed (rather than the monitor making the determination).
mitral valve The valve located between the left atrium and the left ventricle of the heart.
monomorphic Having one common shape.
multifocal Arising from or pertaining to many foci or locations.
murmur An ambiguous heart sound that is associated with turbulent blood flow through the heart valves.
myocarditis Inflammation of the myocardium.
myocardium The cardiac muscle.
necrosis The death of tissue, usually caused by a cessation of its blood supply.
norepinephrine A neurotransmitter and drug sometimes used in the treatment of shock; produces vasoconstriction through its alpha stimulator properties.
normal sinus rhythm The normal rhythm of the heart, wherein the excitation impulse arises in the SA node, travels through the internodal pathways to the atrioventricular junction, down the bundle of His, through the bundle branches, and into the Purkinje network without interference.
opening snap A heart sound indicative of a noncompliant valve.
orthopnea Severe dyspnea experienced when lying down and relieved by sitting up.
orthostatic hypotension A fall in blood pressure when changing to an erect position.
P wave The first wave of the ECG complex, representing depolarization of the ventricles.
pacemaker The specialized tissue within the heart that initiates excitation impulses; an electronic device used to stimulate cardiac contraction when the electric conduction system of the heart is malfunctioning, especially in complete heart block; consists of a battery-powered pulse generator and a wire that transmits the electric impulse to the ventricles.
palpitations A sensation felt under the left breast of the heart “skipping a beat,” usually caused by a premature ventricular contraction.
papillary muscles Protrusions of the myocardium into the ventricular cavities to which the chordae tendineae are attached.
parasympathetic nervous system A subdivision of the autonomic nervous system that is involved in control of involuntary, vegetative functions, mediated largely by the vagus nerve through the chemical acetylcholine.
paroxysmal nocturnal dyspnea (PND) Severe shortness of breath occurring at night after several hours of recumbency, during which fluid pools in the lungs; the person is forced to sit up to breathe; caused by left heart failure or decompensation of chronic obstructive pulmonary disease.
pericardial friction rub A to-and-fro sound that is an abnormal heart sound and which can be heard in systole and diastole; heard in patients who have pericarditis.
pericardial knock A high-pitched heart sound heard during diastole.
pericarditis Inflammation of the pericardium.
pericardium The double-layered sac containing the heart and the origins of the superior vena cava, inferior vena cava, pulmonary artery, and aorta.
percutaneous coronary intervention (PCI) A therapy in which balloons, stents, or other devices are passed through a catheter via a peripheral artery to recanalize and keep a blocked coronary artery open.
phlebitis Inflammation of the wall of a vein, sometimes caused by an IV line, manifested by tenderness, redness, and slight edema along part of the length of the vein.
plaque In cardiology, the white to yellow lesion found in atherosclerosis that is made up of lipids, cell debris, and smooth muscles cells; in older people, may also include calcium.
plasmin A naturally occurring clot-dissolving enzyme, usually present in the body in its inactive form, plasminogen.
point of maximal impulse (PMI) The palpable beat of the apex of the heart against the chest wall during ventricular contraction; normally palpated in the fifth left intercostal space in the midclavicular line.
PR interval The period between the beginning of the P wave (atrial depolarization) and the onset of the QRS complex (ventricular depolarization), signifying the time required for atrial depolarization and passage of the excitation impulse through the atrioventricular junction.
precordial leads Another term used to describe the chest leads in an ECG.
preexcitation Early depolarization of ventricular tissue due to the presence of an accessory pathway between the atria and ventricles.
preload The pressure under which the ventricle fills.
Prinzmetal angina A type of chest pain that occurs when a person is at rest, when oxygen needs are minimal.
pulmonary artery One of two arteries that carry deoxygenated blood from the right ventricle to the lungs.
pulmonary circulation The flow of blood from the right ventricle through the pulmonary arteries and all of their branches and capillaries in the lungs and back to the left atrium through the venules and pulmonary veins; also called the lesser circulation.
pulmonary edema Congestion of the pulmonary air spaces with exudate and foam, often secondary to left heart failure.
pulmonary embolism Obstruction of a pulmonary artery or arteries by solid, liquid, or gaseous material swept through the right side of the heart into the lungs.
pulmonary semilunar valve The valve between the right ventricle and the pulmonary artery; also called the pulmonic valve.
pulmonary veins The vessels that carry oxygenated blood from the lungs to the left atrium.
pulse deficit A situation in which the palpated radial pulse rate is less than the apical pulse rate; reported numerically as the difference between the two.
pulseless electrical activity (PEA) An organized cardiac rhythm (other than ventricular tachycardia) on an ECG monitor that is not accompanied by any detectable pulse.
pulsus alternans A pulse that alternates between strong and weak beats, characteristic of left ventricular systolic damage.
pulsus paradoxus A weakening or loss of a palpable pulse during inhalation, characteristic of cardiac tamponade and severe asthma.
Purkinje fibers A system of fibers in the ventricles that conducts the excitation impulse from the bundle branches to the myocardium.
QRS complex Deflections of the ECG produced by ventricular depolarization.
recanalization The opening up of new channels through a blocked artery.
receptors Specialized cells that respond to stimuli such as pressure, light, or chemicals.
refractory period A short period immediately after depolarization in which the myocytes are not yet repolarized and are unable to fire or conduct an impulse.
relative refractory period That period in the cell-firing cycle at which it is possible but difficult to restimulate the cell to contract.
reperfusion The resumption of blood flow through an artery.
retrosternal Situated or occurring behind the sternum.
rheumatic fever An inflammatory disease caused by streptococcal bacteria strains that can cause a stenosis of the mitral valve or aortic valve.
right atrial enlargement Dilation of the right atrium that results when returning venous pressure is elevated or pulmonary pressures are high.
right atrium The upper right chamber of the heart; receives blood from the venae cavae and supplies blood to the right ventricle.
right-sided heart failure A condition in which the right side has to work increasingly harder to pump blood into engorged pulmonary vessels, which eventually leads to an inability to keep up with the increased workload.
right ventricle The lower right chamber of the heart; receives blood from the right atrium and pumps blood out through the pulmonic valve into the pulmonary artery.
right ventricular hypertrophy (RVH) A cardiac condition in which the right ventricle becomes enlarged, most commonly due to pulmonary hypertension.
R-R interval The period between the onset of one QRS complex and the onset of the next QRS complex.
scarlet fever A disease caused by the bacterium Streptococcus pyogenes, and characterized by a sore throat, fever, rash, and “strawberry tongue.”
semilunar valves The two valves, the aortic and pulmonic, that divide the heart from the aorta and pulmonary arteries.
sinoatrial (SA) node The dominant pacemaker of the heart, located at the junction of the superior vena cava and the right atrium.
sinus bradycardia A sinus rhythm with a heart rate of less than 60 beats/min.
sinus dysrhythmia A slight irregularity of the heart rate caused by changes in parasympathetic tone during breathing.
sinus tachycardia A sinus rhythm with a heart rate of greater than 100 beats/min.
ST segment The interval between the end of the QRS complex and the beginning of the T wave; often elevated or depressed with respect to the isoelectric line when there is significant myocardial ischemia.
stable angina Angina pectoris characterized by periodic pain with a predictable pattern.
Stanford classification A classification system for aortic dissections that includes two categories.
stroke volume (SV) The volume of blood pumped forward with each ventricular contraction.
subendocardial myocardial infarction A type of acute myocardial infarction in which the ischemic process affects only the inner layer of muscle.
sympathetic nervous system A subdivision of the autonomic nervous system that governs the body’s fight-or-flight reactions, stimulating cardiac activity.
synchronized cardioversion The use of a synchronized direct current (DC) electric shock to convert tachydysrhythmias (such as atrial fibrillation) to normal sinus rhythm.
syncope Fainting; brief loss of consciousness caused by transiently inadequate blood flow to the brain.
systemic circulation The flow of blood from the left ventricle through the aorta, to all of its branches and capillaries in the tissues, and back to the right atrium through the venules, veins, and venae cavae; also called the greater circulation.
systole The period of time when the atria or ventricles are contracting; also called atrial systole.
T waves The upright, flat, or inverted wave following the QRS complex of the ECG, representing ventricular repolarization.
thrill A vibration heart sound that occurs frequently and remains constant.
thromboembolism A blood clot that has formed within a blood vessel and is floating within the bloodstream.
thrombus A fixed blood clot.
transcutaneous pacing The act of depolarizing myocardial tissue with a small electrical charge delivered by a device that sends a small electrical charge through the skin of the chest between one externally placed pacing pad and another.
transmural myocardial infarction A type of acute myocardial infarction in which the infarct extends through the entire wall of the ventricle.
tricuspid valve The valve between the right atrium and right ventricle of the heart.
trifascicular block A blockage or impairment of all three components of the ventricular conduction system, with one working occasionally to provide AV conduction.
trigeminy A premature complex in every third heartbeat.
tunica adventitia The outer layer of tissue of a blood vessel wall, composed of elastic and fibrous connective tissue.
tunica intima The smooth, thin, inner lining of a blood vessel.
tunica media The middle and thickest layer of tissue of a blood vessel wall, composed of elastic tissue and smooth muscle cells that allow the vessel to expand or contract in response to changes in blood pressure and tissue demand.
U wave A small flat wave sometimes seen after the T wave and before the next P wave.
unifocal Arising from a single site.
unstable angina Angina pectoris characterized by a changing, unpredictable pattern of pain, which may signal an impending acute myocardial infarction.
vagus nerve One of 12 nerves that comprise the parasympathetic nervous system and is responsible for decreasing heart rate.
Valsalva maneuver Forced exhalation against a closed glottis, the effect of which is to stimulate the vagus nerve and, thereby, slow the heart rate.
vasoconstriction Narrowing of the diameter of a blood vessel.
vasodilation Widening of the diameter of a blood vessel.
veins The blood vessels that carry blood to the heart.
vena cava The largest vein of the body; there are two, of which each return blood to the right atrium.
venules Very small veins.
Wolff-Parkinson-White syndrome A syndrome characterized by short PR intervals, delta waves, nonspecific ST-T wave changes, indicating the presence of an accessory pathway.
Your unit is dispatched to a single-family residence for a person with chest pain. The dispatcher tells you the patient is a 66-year-old man who has an extensive cardiac history. On arrival inside the residence, you see the patient is lying on the sofa in his living room. He tells you he has a history of two prior heart attacks and has had bypass surgery. He states he has pain that feels like previous heart attacks.
1. Cardiac output is defined as:
A. the number of cardiac contractions (heartbeats) per minute.
B. the amount of blood pumped out by either ventricle in a single contraction.
C. the stroke volume times the heart rate.
D. the percentage of blood that is leaving the heart each time it contracts.
2. What is a normal ejection fraction?
A. 30% to 55%
B. 55% to 70%
C. 75% to 100%
D. 80% to 90%
3. The pressure under which a ventricle fills is called:
A. vascular resistance.
B. preload.
C. afterload.
D. contractility.
4. You have obtained vital signs on this patient, which are: BP, 96/58 mm Hg; pulse, 82 beats/min; respirations, 16 breaths/min; and SpO2, 96%. The patient’s skin is pale, cool, and diaphoretic. What is the next step in the assessment of this patient?
A. Obtain a list of current medications.
B. Obtain an ECG strip of lead aVL.
C. Obtain a 12-lead ECG.
D. Obtain a blood draw for blood chemistry.
5. The 12-lead ECG assessment of this patient shows ST changes in leads II, III, and aVF. Changes in these leads reflect a problem with which anatomic region of the heart?
A. Anterior
B. Lateral
C. Septal
D. Inferior
6. The patient describes his pain as an 8 on a 1:10 scale. You have followed your protocol and administered nitroglycerin for pain relief. When you reassess after administration, the patient’s pain level did not decrease. What is your next choice of medication for pain relief?
A. Ativan
B. Morphine
C. Haldol
D. Demerol
7. The patient now becomes unresponsive, stops breathing, and has no pulse. Your partner has already applied defibrillation pads on the patient’s chest. What is your first course of action to treat this patient?
A. Insert an endotracheal tube and ventilate the patient with 100% oxygen.
B. Start an IV line of normal saline.
C. Start CPR.
D. Leave the patient and retrieve the AED.
8. The primary pacemaker of the heart is the:
A. sinoatrial node.
B. atrioventricular node.
C. atrioventricular junction.
D. bundle of Kent.
9. The process by which muscle fibers are stimulated to contract is called:
A. inotrope.
B. depolarization.
C. repolarization.
D. chronotrope.
10. What electrolytes are primarily responsible for depolarization?
A. Sodium and potassium
B. Sodium and calcium
C. Potassium and calcium
D. Potassium and magnesium
Additional Questions
11. Which drug is considered a vagolytic? What part of the nervous system does this drug act on?
12. What part of the nervous system does norepinephrine affect?