Chapter 7
Psychological Trauma and the Brain: Toward a Neurobiological Treatment Model
Ruth Lanius, Ulrich Lanius, Janina Fisher,1 and Pat Ogden
THE PREVIOUS CHAPTERS HAVE REVIEWED NUMEROUS ways that traumatic experience impacts both the mind and body of a developing child by affecting hierarchical information processing, attachment and social engagement systems, autonomic arousal and self-regulatory ability, and action tendencies related to systems of defense and of daily living. An understanding of the neurobiology of trauma may further enhance our conceptualization of the long-term sequelae of trauma and help to guide our therapeutic efforts toward increasing the accuracy and specificity of clinical interventions. In this chapter we review relevant brain areas that have been identified in recent neuroimaging research as being involved in traumatic stress syndromes, focusing specifically on the heterogeneity of response to traumatic reminders and on how different brain regions may relate to the biphasic response to trauma. Finally, we use examples from the neuroscience literature to discuss how experience is processed on cognitive, emotional, and sensorimotor levels, as well as to examine the impact of unresolved sensorimotor responses on all levels of information processing.
TRAUMA, LEVELS OF INFORMATION PROCESSING, AND THE TRIUNE BRAIN
As discussed in Chapter 1, the concept of hierarchical information processing (Wilber, 1996) proposes that there are intertwined, functional relationships among different levels of information processing. To work with this hierarchy in clinical practice, we must attend to all three levels: cognitive processing (thoughts, beliefs, interpretations, and other cognitions), emotional processing (emotion and affect), and sensorimotor processing (physical and sensory responses, sensations, and movement). Similarly, MacLean(1985) has conceptualized a “brain within a brain within a brain.” The reptilian brain, first to develop from an evolutionary perspective, governs arousal, homeostasis of the organism, and reproductive drives. The “paleomammalian brain” or “limbic brain,” found in all mammals, surrounds the reptilian brain and is concerned with emotion, memory, some social behavior, and learning (Cozolino, 2002). Last to develop phylogenetically is the neocortex, which enables self-awareness and conscious thought and includes large portions of the corpus callosum, which bridges the right and left hemispheres of the brain (MacLean, 1985).
Each of the three levels of the brain has its own perception of the environment and responds accordingly, such that a particular level may override the others, depending on the environmental conditions. Even when one level supercedes the others, however, cognitive, emotional, and sensorimotor processing are functionally mutually dependent and intertwined (Damasio, 1999; LeDoux, 1996; Schore, 1994); the three levels of the brain and the corresponding information processing interact and affect each other simultaneously, functioning as a cohesive whole, with the degree of integration of each level of processing affecting the efficacy of other levels.
THALAMUS
The interactive process among the three levels of the brain is likely facilitated by the thalamus, a structure that plays a key role in relaying sensory information to the limbic system and neocortex, thus eventually leading to the integration of sensory information (Lanius et al., 2005). All sensory information, except for olfaction, is routed through the thalamus to the cerebral cortex, and thus the thalamus is often referred to as the sensory gateway to the cortex. It has also been suggested that the thalamus might be involved in mediating the interaction between attention and arousal (Portas et al., 1998), both of which are clearly relevant to the integration of information and the phenomenology of traumatic stress syndromes. The thalamus is located at the “intersection” of the reptilian and mammalian brains on top of the brainstem, connecting the latter with the limbic system and the neocortex. Disruptions in thalamic functioning are likely to interfere with the relay of sensory information to the limbic system and neocortex, as well as with the integration of such information. In that the thalamus serves as a gateway that directly or indirectly modulates the access of sensory information to the cortex, amygdala, and hippocampus, Krystal et al. (1998) proposed that the thalamus facilitates transmission of sensory information to these brain areas. We therefore hypothesize that the thalamus has a crucial function in the interaction of the three brain layers and may be important for the interaction of cognitions, emotions, and behaviors.
Our research group has recently reported thalamic dysfunction in subjects with PTSD, as have some other laboratories (Bremner, Narayan, et al., 1999a; Liberzon, Taylor, Fig, & Koeppe, 1996). Disruptions in thalamic functioning would be likely to interfere with the relay of sensory information to the limbic system and neocortex, as well as with the integration of such information. Such a process could potentially account for the ongoing experience of sensory fragments in PTSD. That is, sensory input from the lower brain structures would remain unintegrated into normal consciousness because the information cannot reach either the limbic system or neocortex in the context of thalamic dysfunction. Thoughts, emotions, and physical sensations initially pertaining to a single event would remain split into separate representations, which are not recalled as an integrated whole. Thalamic dysfunction may therefore underlie PTSD flashbacks—traumatic memories that are often experienced as timeless, vivid sensory fragments of the original experience. To further complicate the clinical picture, these dissociated memory fragments are often associated with marked emotional liability, unexplained somatic symptoms, or negative self-evaluations and self-defeating behaviors (Brewin et al., 1996; van der Kolk et al., 1996).
Thus thalamic dysfunction may be one factor accounting for the inability to integrate traumatic memories into the present context so often observed in trauma-related disorders, a phenomenon that may be related to disruptions in thalamus-mediated temporal binding. Temporal binding refers to the 40 Hz oscillations of the thalamus that results in synchronous activity of “reentrant thalamocortical loops.” In other words, when mental activity is clearly present in the alert mental state, nerve cells in the thalamus oscillate at a frequency of 40 Hz. The connections of thalamic nerve cells with nerve cells in the cortex have been proposed to lead to similar frequencies of cortical nerve cell oscillations, thereby creating reentrant thalamocortical feedback loops. Temporal binding has been suggested to be “a temporally coherent event that binds, in the time domain, the fractured components of external and internal reality into a single construct…the ‘self’” (Joliot, Ribary, & Llinas, see also Llinas, 2001). Such a lack of temporal binding and the resulting lack of thalamocortical dialogue may be a process that accounts, among others, for flashback experiences in PTSD (Lanius, Bluhm, Lanius, & Pain, 2005). In the absence of temporal binding, then, individuals experience an inability to integrate the totality of what is happening into personal memory and identity, such that these fragments of memory remain isolated from ordinary consciousness. Such a conceptualization raises the question of whether dynamic state changes in the corticothalamic system may account for the fragmented nature of memory observed in people with PTSD and whether PTSD is a neuropsychiatric disorder that can be characterized by thalamocortical dysrhythmia (Llinas, Ribary, Contreras, & Pedroarena, 1998). If PTSD results in interference with the relay function of the thalamus, that interference could partly account for the predominantly sensory nature of the intrusive phenomena noted in trauma-related disorders: Thalamic dysfunction would disrupt the integration of sensory information by interfering with its relay to the limbic system and neocortex. That is, the upper brain structures would become temporarily disconnected from lower brain structures each time the traumatic memory is accessed, simultaneously interfering with effective bottom-up, as well as top-down, processing.
TRAUMA AND LATERALIZATION
In addition to what may appear to be a horizontal disconnection between lower and upper brain structures, neuroimaging studies in PTSD also offer evidence of differences in lateralization secondary to trauma, with increased brain activity during recall of traumatic memories in the right hemisphere and decreased brain activity in the left hemisphere. Such differential activation may be attributable to the different nature and quality of a traumatic memory (Lanius et al., 2004), and to the involvement of different neural networks implicated in the recall of memories. For example, differences in brain networks engaged in traumatic memory recall have been observed in subjects with PTSD who experienced flashbacks versus subjects without PTSD who recalled the traumatic events as ordinary autobiographical memories. These differences suggest differences in episodic memory retrieval between the two groups (Figure 7.1).
Reexperiencing traumatic events in the form of flashbacks is very different from the recall of events as ordinary autobiographical memories (Brewin et al., 1996; van der Kolk & Fisler, 1995). Flashbacks most often occur spontaneously, triggered by internal or external events, and their occurrence usually cannot be controlled. They involve a subjective distortion in time and are much more vivid than ordinary recall; the event is often experienced as though it were happening again in the present. Flashbacks are experienced as fragments of the sensory components of the event, such as visual images or olfactory, auditory, or kinesthetic sensations (van der Kolk, McFarlane, & Weisaeth, 1996) and as unchanging over time (Brewin et al., 1996)—which differs from ordinary memories, which are altered by repeated recall (Brewin et al., 1996). Overall, flashbacks are vivid sensory experiences, whereas ordinary autobiographical memories are personal narratives that describe sensory elements of the experience (Brewin et al., 1996; van der Kolk, McFarlane, & Weisaeth, 1996) and are recalled rather than reexperienced.
Our laboratory has shown that subjects who did not suffer from PTSD showed brain activation patterns consistent with verbal episodic memory retrieval (Lanius et al., 2004). Comparison of the brain networks of the PTSD subjects and the non-PTSD subjects showed that the non-PTSD subjects had greater levels of brain activation in left prefrontal areas, whereas the PTSD patients showed more activation in right posterior areas. Activation of the left prefrontal areas of the brain has been proposed to play a role in more verbal forms of memory recall (Figures 7.2a and 7.2b), whereas the brain networks activated in PTSD patients showed patterns of neural activation associated with much more nonverbal patterns of memory retrieval (reviewed by Cabeza & Nyberg, 2000, 2003). These distinctly different neuronal network activations observed in PTSD versus non-PTSD subjects may help to explain the neuronal underpinnings of sensory-based, nonverbal flashback phenomena in PTSD. Based on these results, PTSD-related symptoms appear to be associated with differences in laterality, specifically right-hemispheric dominance.
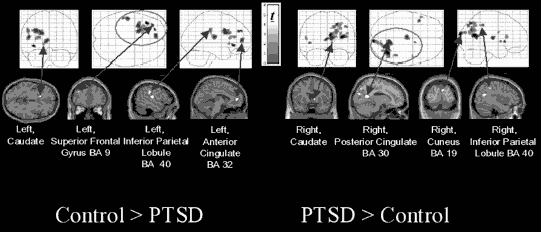
Figure 7.1. Brain regions with activation showing significantly greater connectivity/covariation with activation in the right anterior cingulate in traumatized subjects with PTSD than in traumatized subjects without PTSD during recall of a traumatic event.
The importance of lateralized responses in PTSD has been previously examined by using electroencephalography (EEG) and auditory probe-evoked potential attenuation. Schiffer, Teicher, and Papanicolaou (1995), for example, reported that subjects who had experienced early trauma displayed significant left-dominant asymmetry during neutral memory recall and relative right dominance during traumatic memory recall. In addition, psychological abuse has been shown to be associated with an increased prevalence of left-sided EEG abnormalities and an increased prevalence of right–left hemispheric asymmetries (Teicher, Ito, Glod, Anderson, & Ackerman, 1997). EEG coherence studies of abused versus nonabused children have also reported that abused children had greater average left-hemispheric coherence than did nonabused children but that the two groups had a comparable degree of right-hemispheric coherence. Teicher et al. (1997) suggested that these findings may be related to diminished left-hemispheric differentiation in the abused group and thus may provide evidence that childhood abuse has a significant effect on cortical development. Previous neuroimaging studies in PTSD, using the script-driven imagery symptom provocation paradigm, have also suggested lateralized responses. Using traumatic script-driven imagery, Rauch et al. (1996) found that in the traumatic condition, relative to the neutral condition, regional cerebral blood flow increases occurred in the right medial orbitofrontal cortex, insula, amygdala, and anterior temporal pole.
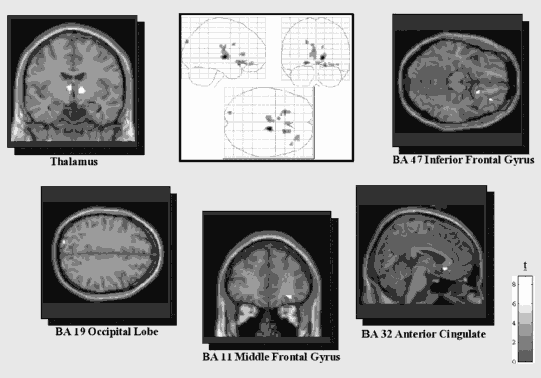
Figure 7.2a. Regions of activation during traumatic memory recall versus implicit baseline, where the comparison group (n = 10) shows greater activation than the flashback/reliving PTSD group (n =11), k > 10.

Figure 7.2b. Regions of activation during traumatic memory recall versus implicit baseline, where the dissociated PTSD group (n = 10) shows greater activation than the comparison group (n = 10), k> 10.
We have discussed what may appear to be a horizontal disconnection between lower and upper brain structures in PTSD as well as evidence supporting relative right dominance during traumatic memory recall. In treatment, the client and therapist may also be able to capitalize on the fact that the brain appears to have another alternate connection between the hemispheres, sometimes referred to as the “subcortical bridge,” across which information can be exchanged (Austin, 1998). Despite the fact that the cortical structures are bifurcated into left and right hemispheres, the brain remains undivided at the level of the lower reptilian brain structures (Sperry, Zaidel, & Zaidel, 1979). Indeed, the existence of these subcortical connections may account for the fact that split-brain patients (whose corpus calossum has been severed) still behave in a unified manner during their everyday activities.
Generally, it appears that language-based information does not exchange readily across the subcortical bridge (Gazzaniga, Holtzman, & Smylie, 1987), whereas nonverbal information, including “unconscious or preconscious codes, nuances we can never attach a name to,” cross most readily (Austin). For example, the subcortical bridge can easily transmit emotionally linked subcortical messages that convey impressions of danger, sudden movements, and stimuli indicating potential violence. When these messages cross hemispheres from the right to the left side, they tend to engage a kind of “response readiness” for adaptive action, such as facilitating potential speech responses in the left hemisphere that reflect action tendencies necessary to invoke the social engagement system as a first response in ensuring survival.
Using somatic experience as an entry point in therapy and maintaining mindful awareness of the body may facilitate information processing by enhancing information transfer between the hemispheres. Traumatized clients frequently have difficulty regulating affect and arousal within the window of tolerance, and working at the body level enables fixed action tendencies to be evoked without unmanageable autonomic activation; thus “bottom-up hijacking” occurs less frequently and readily. It stands to reason that awareness of the body may also promote the exchange of somatosensory and nonverbal information between hemispheres; however, such a hypothesis is speculative at this point.
NEURAL CORRELATES OF PTSD
The study of the neural mechanisms underlying traumatic experiences was primarily a matter of conjecture until the advent of neuroimaging technology and the burgeoning interest in neuroscience research that took place in the late 1990s and early 2000s and served to transform the trauma treatment field. Neuroimaging has become an important technique in understanding the neurochemistry, as well as the functional changes, underlying various psychiatric disorders. Studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) in subjects with PTSD have attempted to elucidate which areas of the brain might be involved in the recall of traumatic events. The goal of these studies has been to examine regional cerebral blood flow (rCBF) changes during exposure to reminders of the traumatic event, using one of two paradigms: traumatic script-driven imagery symptom provocation, which involves remembering the traumatic memory after hearing a script of the experience, or exposure to photographs or sounds reminiscent of the traumatic experience. In addition, several studies have implemented pharmacological challenges, cognitive tasks, or emotional stimuli such as masked faces in order to examine the neuronal circuitry underlying trauma-related disorders. Using these paradigms, neuroimaging research has identified a number of brain areas that appear to be involved in traumatic stress syndromes: amygdala, medial prefrontal cortex, anterior cingulate gyrus, hippocampus, insula, and orbitofrontal cortex (Bremner, 2002; Hull, 2002; Lanius, Bluhm, Lanius, & Pain, 2006; Liberzon & Phan, 2003; Pitman, Shin, & Rauch, 2001; Tanev, 2003).
Amygdala
Located in the right and left temporal lobes, the amygdalae are considered to be part of the brain’s emotional processing system (often referred to as the limbic system). The amygdala plays a profound role in fear conditioning (LeDoux, 2002) because of its function in “sounding the alarm” when a stimulus is perceived as threatening, and it may be involved in initiating sympathetic nervous system responses. Several neuroimaging studies have shown increased activation of the amygdala in PTSD (Bremner, 2002; Hull, 2002; Lanius, Bluhm, Lanius, & Pain, 2006; Liberzon & Phan, 2003; Pitman et al., 2001; Tanev, 2003). It has been suggested that an overactive amygdala in PTSD may result in generalization of the fear response, leading to an overall increase in fearful behavior.
However, the finding of increased amygdala activation in PTSD has not always been consistent. For instance, Lanius’s findings, in script-driven symptom provocation studies with multiply traumatized individuals, suggest a lack of amygdala activation during traumatic recall (reviewed in Lanius, Bluhm, Lanius, & Pain, 2006). Similarly, Britton, Phan, Taylor, Fig, and Liberzon (2005) and Bremner (Bremner, Narayan, et al., 1999; Bremner, Staib, et al., 1999) have also observed a lack of amygdala activation during recall of traumatic events in PTSD subjects. Indeed, Perry et al. (1995) suggest that some traumatized individuals may develop “limbic irritability,” with a tendency toward overactive amygdala responses to traumatic stimuli, whereas others may develop a propensity toward hypoactive amygdala responses. In support of this hypothesis, Chugani et al. (2001) reported an association between a history of neglect and a lack of amygdala activation.
The hypothesis that a lack of amygdala activation may indeed be adaptive under certain circumstances is born out by the animal literature, which suggests that a lack of amygdala activation may allow continued functioning in situations characterized by ongoing threat. For instance, it was observed that, when a rat intruded into another rat’s territory and was defeated by that occupant, it remained quiet and frozen in a corner, not leaving, approaching, or challenging the victorious rat (Austin, 1998). Note the similarity with the immobilizing defenses described in Chapter 5 and the loss of ability to explore observed in human beings under threat conditions. Just like the child in a traumatogenic environment, who must adapt to a threat occurring in a context in which caregivers have dominance, the rat relies on the immobilizing defenses of avoidance, freeze, and submission to facilitate survival and adaptation in another rat’s territory. In behavior that may resemble that of children with disorganized attachment, the rat maintains proximity to its cage-mate but uses autoregulatory, immobilizing strategies to avoid danger and increase safety. In contrast, rats whose corticomedial amygdala has been lesioned behave strikingly different after they have been defeated by another rat: They move freely about their cage and thrust out their muzzles, sniffing incautiously toward the victorious rat. These rats “seem oblivious of the proprieties, of their expected social boundaries. They have not learned their lesson” (Austin). Monkeys have also been shown to become “strikingly fearless” after destruction of their amydalae; they have been described as “so naïve, that they will approach and handle a snake!” No normal monkey comes near a snake (Austin, 1998; Horel, Keating, & Misantone, 1975).
These animal behaviors bear striking resemblance to some of the behaviors often observable clinically in individuals with chronic trauma histories and may explain such phenomena as traumatic reenactments or the Stockholm syndrome. It is not uncommon for clients with chronic trauma histories to continue seeking abusive relationships without being aware of the dangers involved in such relationships. Future research therefore needs to address the clinical implications of altered amygdala activation.
Medial Prefrontal Cortex
The medial prefrontal cortex is considered part of the cognitive processing system and has been hypothesized to play a role in the extinction of conditioned fear responses (Morgan, Romanski, & LeDoux, 1993). By exerting inhibitory influences over the limbic system, including the amygdala, the medial prefrontal cortex thereby regulates the generalization of fear and overall increase in fearful behavior mediated by the amygdala. For example, PET studies have shown negative correlations between blood flow in the left prefrontal cortex and the amygdala (reviewed in Lanius, Bluhm, Lanius, & Pain, 2006; Pitman et al., 2001).
Medial prefrontal cortex dysfunction has been consistently described in the majority of PTSD neuroimaging studies and has been hypothesized to be associated with attentional and frontal deficits sometimes associated with a quasi dementia-like syndrome in PTSD (Markowitsch et al., 2000). The medial prefrontal cortex has also been shown to suppress the stress response mediated by the hypothalamic–pituitary–adrenal axis and thus plays a role in the regulation of cortisol, the stress hormone (reviewed in Lanius, Bluhm, Lanius, & Pain, 2006). Finally, a role of the medial prefrontal cortex in emotion regulation has been identified (Lane & McRae, 2004).
In addition, this region is thought to play an important role in the retrieval of episodic memory (Tulving, Kapur, Craik, Moscovitch, & Houle, 1994) and may also be involved in the temporal segregation of memories (Schnider, Ptak, von Daniken, & Remonda, 2000). The brain function of temporal segregation ensures that “currently relevant memories can be differentiated from memories that may have been relevant once but are no longer” (Moscovitch & Winocur, 2002). Thus, altered levels of medial prefrontal cortex activation may be partly responsible for the “timeless” nature of the traumatic memories experienced by many PTSD patients.
Neuroimaging studies have also investigated the neural correlates of self-referential processing and have identified a network of brain regions, including the medial prefrontal cortex (Johns, Baxter, et al., 2002). This self-referential function is particularly relevant to the aspects of sensorimotor therapy that focus on awareness of present experience; for example, when the client is asked to mindfully track (a top-down, cognitive process) the physical sensations and impulses (sensorimotor process) as they progress through the body, and to temporarily disregard emotions and thoughts that arise, until the bodily sensations and impulses resolve to a point of rest and stabilization in the body. Generally, mindfulness (the ability to self-witness or to employ “observing ego”) is thought to engage the medial prefrontal cortex.
Anterior Cingulate Gyrus
The anterior cingulate gyrus is a complex structure with multiple functions; it has been shown to play a key role in the representation of subjective experience, in the integration of bodily responses with behavioral demands (Vogt & Gabriel, 1993), and in emotional awareness. Lane, Fink, Chau, and Dolan (1997) have reported positive correlations between scores on the Levels of Emotional Awareness Scale and cerebral blood flow in BA 24 of the anterior cingulate gyrus during film-and recall-induced emotion. These results indicate that the anterior cingulate cortex may also play a role in the experiential aspects of emotion as well as in the integration of emotion and cognition.
Animal research (Vogt, 2005) has suggested that the anterior cingulate gyrus has extensive connections with multiple brain structures, including the amygdala, hypothalamus, nucleus accumbens, ventral tegmental area, substantia nigra, raphe, locus coeruleus, periaqueductal grey, and brainstem autonomic nuclei. The anterior cingulate gyrus is thus part of a system that orchestrates the autonomic, neuroendocrine, and behavioral expression of emotion and may play a key role in the visceral aspects of emotion (reviewed in Lanius, Bluhm, Lanius, & Pain, 2006). On the basis of the key involvement of the anterior cingulate gyrus in the regulation of the autonomic as well as the experiential and/or expressive aspects of emotion, it is possible that disruption in its functioning, as observed in PTSD, may provide a neural basis of emotion dysregulation, including extremes of reexperiencing and avoidance of emotionally distressing memories, as well as generalized problems with physiological hyperarousal and emotional numbing. In addition to these functions, the anterior cingulate gyrus also plays significant roles in other responses crucial to preventing or surviving trauma, including pain, response selection, maternal behavior, vocalization, and skeletomotor control.
It is interesting to note that psychological trauma, including attachment trauma, in the first through third quarters of the first year of life has been observed to negatively impact the experience-dependent maturation of the anterior cingulate limbic circuits (Schore, 2001). Because the functions of the anterior cingulate gyrus are crucial to the optimal engagement of a number of action systems, its experience-dependent maturation may increase the likelihood that the action systems of daily life will be negatively impacted as a result of chronic childhood trauma and neglect. For example, the action system of exploration depends upon attentional focusing, response selection, autonomic regulation, and skeletomotor movement. The social engagement system also requires movement and response selection, along with vocalization. Thus both of these important systems, upon which other systems are dependent for their optimal development, may be compromised in the context of altered anterior cingulate development.
Helping clients to voluntarily orient and focus attention, as well as address cognitive, emotional, and sensorimotor elements, may help to optimize anterior cingulate functioning. Additionally, the use of movement may normalize anterior cingulate activation by evoking skeletomotor responses that would previously have been inhibited by trauma-related tendencies. Lisa, who reported that she wanted to “get away” but froze during childhood sexual abuse, repeated the tendency to freeze during subsequent unwanted sexual encounters. When working with the traumatic memory in therapy, she reported a freezing sensation but also the impulse to run. Her therapist asked her to stand and walk around the office, feeling the capacity of her legs to move—action that Lisa experienced as empowering. In this way, the motor responses (movement in her legs) were executed when she experienced a freezing sensation, whereas these responses were previously inhibited by the trauma-dominated tendency. It should be noted, however, that the hypothesis that such action may affect anterior cingulate functioning is highly speculative and warrants further research.
Hippocampus
The hippocampus, most commonly associated with memory function, is part of the temporal lobe and receives inputs from, and sends efferents to, both the amygdala and the cortex. The hippocampus plays an important role in declarative memory and may thus be involved in mediating learned responses to a constellation of cues. Furthermore, preclinical studies demonstrate death of hippocampal neurons and hippocampal shrinkage after exposure of animals to chronic stress. This reaction may be mediated, in part, by cortisol through action on hippocampal glucocorticoid receptors (Charney et al., 1993).
Given the multitude of trauma effects that include both vivid reexperiencing of traumatic memories as well as amnesia, it is not surprising that the hippocampus has been implicated in traumatic stress syndromes. A number of studies has demonstrated hippocampal involvement in PTSD (reviewed by Geuze in Bremner, Vermetten, Afzal, & Vythilingam, 2004; Geuze, Vermetten, & Bremner, 2005; Shin et al., 2004).
Magnetic resonance imaging (MRI) studies have also shown that both male combat veterans and women survivors of childhood sexual abuse with PTSD have shrunken hippocampal volumes (reviewed in Geuze et al., 2005). In some of these studies, decreased hippocampal volume correlated with trauma exposure or memory deficit. It is believed that this reaction is related to the effects of cortisol on hippocampal glucocorticoid receptors, leading to cell degeneration. At the same time, it should be noted that some researchers have also argued that small hippocampal volumes may not be a result of chronic stress exposure but rather represent a preexisting risk factor for the development of PTSD (reviwed in Gueze et al., 2005).
Whether a preexisting condition or the result of traumatic exposure, or both, there are some beginning suggestions that, given the ability of the hippocampus to generate new cells, reduced hippocampal volume may be reversible through treatment. In one study hippocampal shrinkage was reversed through administration of the antidepressant paroxetine (reviewed in Geuze et al., 2005).
Insula
In previous chapters we have shown that traumatic experiences affect all three levels of information processing. Not only do autonomic responses drive habitual tendencies, but trauma-related cognitive distortions, perception, and emotion also play a role in perpetuating procedural learning once adaptive for traumatic situations. The insula, located within the cerebral cortex, “appears to be preferentially involved in the emotional response to potentially distressing cognitive stimuli, interoceptive sensory stimuli, and body sensations” (Reiman, Lane, Ahern, Schwartz, & Davidson, 2000). Thus the insula plays a role in all three levels of information processing: It (1) mediates responses to cognitive stimuli; (2) plays a key role in body perception (interoception); and (3) it also affects perception of emotions. In fact, Craig (2003) hypothesized that the insula of the nondominant (right) hemisphere provides a neuronal basis for the subjective evaluation of one’s condition, that is, for knowing “how one feels.” The insula has also been shown to receive signals related to pain states, body temperature, and visceral sensations, as well as signals regarding the state of the smooth musculature in blood vessels and other viscera (described in Craig, 2003). Reiman et al . (2000) hypothesized that the insula may play a role in the evaluation of potentially distressing body sensations for emotional content and, in that role, may serve as an “internal alarm center” (Nijenhuis et al., 2002) via its input–output relationship with the amygdala.
Damasio (1999) has also emphasized the role of the insula and the somatosensory cortices in processing signals regarding bodily state, suggesting that these signals form the basis for human emotions. In a PET study investigating brain activity during self-generated emotion, Damasio et al. (2000) found insula activation across a range of emotions. Bilateral activation of the insula was observed to be associated with recall of memories that caused feelings of sadness and anger, whereas right hemispheric activation was observed in the context of happiness and fear. Similar findings have been reported in conjunction with recall of traumatic memories: Rauch et al.’s 1996 study of subjects with PTSD demonstrated increased metabolism in the right insula when subjects were exposed to trauma memory scripts and became autonomically aroused. In a brain-imaging study comparing individuals with and without PTSD, Lanius, Bluhm et al. (2006) observed activation of different neuronal networks involving the insula in subjects with PTSD who experienced a dissociative response to the recall of traumatic memories, as compared to subjects who were not suffering from PTSD. Patients with PTSD in this study reported feeling removed from their own bodies as well as from the emotional content of the traumatic memory. This research has important implications for treatment: As traumatized clients learn to slowly increase awareness of body sensation, movement, and impulses and to tolerate sensation and emotional arousal, changes in activation of the insula and medial prefrontal cortex may take place, thus increasing their ability for self-referential processing of bodily states and emotions. Clinically, we have observed that this ability to mindfully observe present-moment internal experience, in most instances, allows for down-regulation of defensive action systems and increased engagement of action systems related to daily life, especially the attachment, exploration, and sociability systems.
Orbitofrontal Cortex
In the field of neuroscience, particularly in research related to trauma and/or attachment, the role of the orbitofrontal cortex has stimulated increasing interest. The orbitofrontal cortex is the part of the frontal lobe that lies just above the orbit of the eyes, in a position that facilitates direct inputs from a number of adjoining cortical and subcortical brain areas: the dorsome-dial thalamus, temporal cortex, ventral tegmental area, olfactory system, and the amygdala. Its outputs also extend to both cortical and subcortical brain regions, including the cingulate cortex, hippocampal formation, temporal cortex, lateral hypothalamus, and amygdala. Its complex system of inputs provide the orbitofrontal cortex with a wealth of information about what is happening in the environment and what plans are being organized by other cortical areas. In turn, its communication outputs affect a variety of behaviors and physiological responses, including emotional and autonomic responses organized by the amygdala. The orbitofrontal cortex also plays a role in mediating autonomic and behavioral responses via its connections with the cingulate cortex. In sum, the orbitofrontal cortex is uniquely situated to mediate communication between subcortical and cortical systems.
The orbitofrontal system may also be involved in “the regulation of the body state and in the reflection of changes in that state” (Luria, 1980, Schore, 2003a) and thus may play an important role in the regulation of arousal within the window of tolerance. In its function as part of the attachment action system, the orbitofrontal cortex is believed to enable cortically processed information concerning the environment (e.g., visual and auditory stimuli emanating from a facial expression) to be integrated with subcortically processed information in the internal visceral environment, thus facilitating the association of incoming information from the external environment with motivational and emotional states. In the mother–infant dyadic interaction, these experiences, in which the perception of facial expression is integrated with the sensations associated with optimal body states, become the basis for secure attachment and, in turn, facilitate the optimal development of the orbitofrontal cortex (Schore, 2003a).
It has been suggested that abuse and/or neglect over the first 2 years of life negatively impacts the maturation of the orbital prefrontolimbic system (Schore, 1994, 2003a, 2003b). Different theorists propose different mechanisms of action for this process. Martin, Spicer, Lewis, Gluck, and Cork(1991) suggested that in early postnatal life, maintaining critical levels of tactile input of specific quality and emotional content is important for normal brain maturation, whereas Greenough and Black (1992) proposed that multiple sensory inputs, derived from contact with the mother during feeding/ nursing, are crucial in shaping the development of the orbitofrontal cortex. Schore (2003a) argued that sensory inputs are just one aspect of a larger process of dyadic regulation of arousal and affect, within the context of secure attachment, that contributes to optimal synaptic pruning:
The early social environment, mediated by the primary caregiver, directly influences the final wiring of the circuits in the infant brain that are responsible for the future social and emotional coping capacities of the individual. The ultimate product of this social–emotional development is a particular system in the [orbitofrontal cortex] of the right brain that is dominant for the unconscious processing of socioemotional information, the regulation of bodily states, the capacity to cope with emotional stress, and the corporeal and emotional self.
Thus the orbitofrontal cortex is thought to play a central role in capacities that contribute to the expansion of self-regulatory ability, sophistication of the social engagement system, development of the attachment system, and as a result, maturation of the exploration system. An interference in development or activation of this part of the brain may therefore contribute to the autonomic, emotional, and cognitive dysregulation observed in our traumatized clients.
EMOTION AND THE IMPORTANCE OF SUBCORTICAL PROCESSES
Panksepp (1998) and Damasio et al. (2000) have cogently argued that affect is largely a subcortical process. In a brain-imaging study of the phenomenology of emotion, subjects were asked to narrate personal reminiscences that evoked deep, existentially experienced feeling states of anger, fear, sadness, and happiness (Damasio et al., 2000). When subjects were judged by investigators to be experiencing those feelings, radioactive water was infused to obtain PET-scan images. The results demonstrated markedly increased arousal in subcortical brain regions, accompanied by substantial reductions of blood flow in many higher brain areas, suggesting a decrease in information processing in neocortical systems during intense emotional states along with an increase in subcortical activity. Specifically with regard to traumatic stress syndromes, Pissiota et al. (2002) suggested that traumatic symptom provocation in PTSD is associated with an emotionally determined motor preparatory response that may be subcortically initiated rather than cortically controlled.
Trauma treatment techniques that focus on increasing emotional arousal run the risk of escalating subcortically mediated autonomic activation, thus leading to hyper-or hypoarousal. In sensorimotor psychotherapy, integrative capacity is facilitated by separately attending to each level of information processing and tracking signs of dysregulation that could impede integration. By increasing mindfulness and focusing exclusively on body sensation, clients work first with the sensate precursors to emotion and are often able to expand their window of tolerance. As the window of tolerance increases, clients are often gradually able to reestablish integrative emotional and cognitive processing. That is, the initial focus on sensation, in the absence of accessing the full range of the traumatic experience, may paradoxically facilitate more integrated brain functioning and help to ensure that clients do not suffer the discomfort of activation with little integration or transformation of that distress.
THE HETEROGENEITY RESPONSE TO TRAUMATIC REMINDERS
In trauma-related disorders fragments of traumatic memory take on a life of their own, able to intrude at any moment, thereby fueling hyper-and hypoarousal responses that are beyond cognitive control. In the context of trauma-related dysregulation, emotional experiences, which also activate the amygdala and insula, can become so overwhelming that the individual is further challenged to maintain arousal within the window of tolerance.
The preponderance of neuroimaging research has shown that subjects experiencing flashback/reliving responses when asked to recall a traumatic memory exhibit brain activity that is strikingly different from those who do not have PTSD and who can recall their traumatic memory as a memory of the past, a normal autobiographical memory (Lanius, Bluhm, et al., 2006). PTSD subjects typically demonstrate brain connection patterns that are consistent with a nonverbal pattern of memory recall (i.e., activation of the occipital lobes, right parietal lobe, and posterior cingulate gyrus), as compared to control subjects who activate neural networks more consistent with verbal patterns of memory retrieval (i.e., left prefrontal cortex and anterior cingulate) (Figure 7.3). These neuroimaging findings are consistent with what we often observe clinically: PTSD patients experience their traumatic memories as timeless, intrusive, sensory fragments that often cannot be expressed as a narrative, whereas people who have suffered a trauma but do not suffer from PTSD usually recall traumatic memories as an integrated whole that can easily be expressed as a narrative. This observation calls into question the benefit of purely verbal therapies as modalities for processing information that is experienced primarily at a sensory level and suggests the need to explore body-centered methods. Sensorimotor psychotherapy engages clients in the processing of such sensory fragments by encouraging careful tracking of sensations associated with the traumatic memories until such sensations are no longer fragmented and can be experienced as an integrated whole. Because the fragmentation of memory occurs on all three levels of information processing, the sensorimotor psychotherapist is alert to all the building blocks of present experience: cognitions, emotions, perceptual and sensory input, inner body sensations, and movement or movement impulses. For example, fragmented sensory experiences can be associated with the urge to engage in certain movements, particularly movements in which the individual was not able to engage or which could not be completed as a result of the traumatic experience.
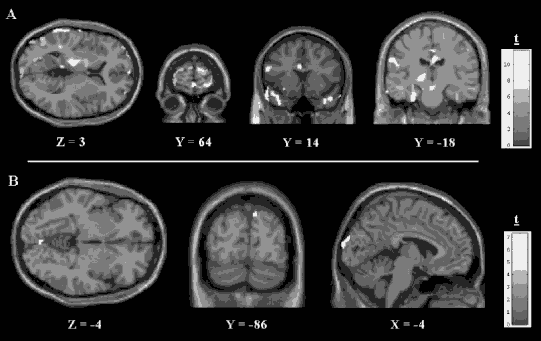
Figure 7.3. Regions of significantly increased brain activation during the traumatic memory recall. A = Male; B = Female.
A woman abused in the context of domestic violence might have wanted to push away her abusive partner but instinctively relied on immobilizing defenses in a situation from which there was no immediate escape or hope of successful self-defense. As she and the therapist observe what happens in her body as she recalls being trapped with her enraged partner, she may find herself experiencing a particular sensory fragment associated with the urge to push away, such as a tightening in her jaw or upper arm or the curling of her hand into a fist. In a sensorimotor psychotherapy session, the client would be supported in studying the action tendency of the mobilizing defense until she could complete the movement of pushing away and reinstate a somatic sense of self reinforced by the ability to defend and protect the body. With the completion of the mobilizing defensive action, the sensory fragments related to both the immobilizing and mobilizing responses can be integrated; emotions such as elation or triumph can be tolerated; and cognitive meaning making can emerge, such as a new belief “I can protect myself now,” to counter the physical experience of being overpowered. We would speculate that, in this type of body-oriented processing, the brain activation pattern associated with the recall of memory would become modified from a predominantly right, posterior activation pattern to a more left, prefrontal pattern.
Most symptom provocation studies have focused attention on patients who experience a hyperarousal/reliving response (reviewed in Lanius, Bluhm, et al., 2006). More recently, however, our laboratory has also begun to study a group of trauma patients with dissociative response patterns, to compare their brain activity in response to symptom provocation with that of the hyperarousal/reliving group. For example, Lanius et al. (2002) found that a small but significant percentage of patients responded to traumatic cues by showing classic symptoms of dissociation sometimes coupled with autonomic hypoarousal. In response to script-driven imagery provocation of traumatic memory, these patients reported feelings of numbness, of leaving their body, or of experiencing the traumatic memory “at a distance.” Moreover, this subjective pattern of response to script-driven imagery was associated with patterns of brain activation distinctly different from those associated with the flashback/reliving response (see Figures 7.2a and 7.2b).
Sensorimotor-informed techniques can be equally effective with clients who demonstrate primarily dissociative responses to traumatic reminders. In the context of dissociative responses that can be coupled with hypoarousal, the goal of psychotherapy becomes to increase somatic awareness as a vehicle for bringing arousal into the window of tolerance; for example, teaching clients to slowly track the physical sensations connected to the numbing or depersonalization symptoms. Becoming mindfully aware of such sensations and feelings, heightening curiosity about how they are organized in the body, may lead to increased activation of higher brain areas, such as the prefrontal cortex, and thereby increase clients’ ability to maintain optimal arousal and orient adaptively to both external and internal environments. Utilizing somatic resources that counteract numbing (such as movement, standing in a grounded, supported posture, or lengthening the spine) can also increase integrative capacity in the face of automatic tendencies toward dissociation or hypoarousal.
HETEROGENEITY OF RESPONSE: A CASE EXAMPLE
To appreciate the different challenges created by different action tendencies and activation patterns, we describe the case of a husband and wife who shared in the same traumatic event and evidenced two very different response strategies. In these two cases Lanius, Hopper, and Menon (2003) observed widely different subjective, heart rate, brain activation responses to traumatic script-driven imagery. The husband and wife were traveling together when they were involved in a serious motor vehicle accident—an accident that affected over 100 vehicles and caused multiple deaths and serious injuries. After crashing into the car in front of them, both subjects were trapped for several minutes, during which they witnessed a child in a nearby car burn to death and feared that they too would die. Neither sustained physical injuries.
Both subjects were assessed 4 weeks after the accident. The husband, a 48-year-old professional, reported having been completely healthy until the accident. During the accident, he recalled feeling extremely aroused and then becoming actively involved, both cognitively and behaviorally, in rescuing himself and his wife, ultimately breaking the windshield to allow their escape. The next day, however, he began experiencing flashbacks and nightmares, and these reexperiencing symptoms often included feeling as if the accident were recurring. He also became psychologically and physiologically hyperaroused when thinking or talking about the accident. Subsequently, he avoided driving on the highway where the accident had occurred, as well as avoiding thoughts and conversations about it. His sleep was very poor and his concentration severely impaired, rendering him unable to function at work. Other hyperarousal symptoms included irritability and startle reactions. He reported no complicating factors: no past or present substance abuse, past psychiatric history or current medical problems, no prescribed medications, and no family psychiatric history. He described his childhood as uneventful, stated that he had a good relationship with his parents, and reported no history of neglect or emotional, physical, or sexual abuse. He was sociable as a child and adolescent, completed an undergraduate accounting degree, and has since worked as an accountant.
His wife, a 55-year-old professional, was also healthy until the traumatic event. She described being “in shock” during the accident and, though trapped but not pinned in the car, reported, “I could hardly move because I was completely frozen.” It was her husband’s action of breaking the windshield and pulling her from the car that allowed their escape from the vehicle. Like her husband, she began experiencing flashbacks and nightmares the following day, often feeling “numb and frozen,” as if the event were recurring. She avoided driving or reading newspaper stories about the accident. Her sleep was extremely poor, her concentration significantly impaired, and she was highly irritable and easily angered. Worst of all, her work functioning was completely impaired (she sold her business several months after the accident). She denied any past or present substance abuse but reported a postpartum depression after the birth of her first child and a past history of mild panic disorder. She had no medical problems and was not taking any medications. She reported no family psychiatric history but described her childhood as quite “traumatic.” That is, though she denied any history of physical or sexual abuse, she reported that her father had died when she was 9 years old, leaving her to be raised by a mother described as a very “cold” and “distant” woman with whom she did not feel safe. Nonetheless, she was sociable while growing up, and her school performance was above average. She graduated from business school and had run a business for several years before the accident.
In response to script-driven imagery of the accident, the husband reported a vivid memory that included thoughts about how to escape, the physical urge to break the windshield, and feeling very anxious and “jumpy.” His heart rate increased 13 beats per minute from baseline, and this experience corresponded with brain activation of areas that include the prefrontal cortex and the amygdala (see Figure 7.3). Planning how to get himself and his wife out of the car may have led to activation of the prefrontal cortex to mediate the functions of planning and problem solving. Activation of the amygdala in response to the script-driven imagery may have contributed to his hyperarousal symptoms (feeling “anxious and jumpy”), as well as his symptoms of PTSD, given the key role of the amygdala in both fear conditioning and PTSD.
In marked contrast, but consistent with her original peritraumatic response, the woman reported feeling extremely “numb” and “frozen” while recalling the traumatic memory, and her heart rate did not change from baseline. Increases in brain activation were found only in occipital regions, the brain areas involved with the processing of visual information, and may be a mechanism underlying the vivid visual images of the accident reported by the client during the brain-imaging procedure (see Figure 7.3).
In terms of subjective experiences, heart rate responses, and patterns of neural activation, these two survivors of the same traumatic event exhibited two distinct yet internally coherent peritraumatic and subsequent pathological responses to traumatic reminders. The husband’s reports of cognitive and behavioral activation, along with physiological arousal, were consistent with the increases in activation observed in heart rate and in the prefrontal cortex and amygdala. In contrast, the wife’s “numb” and “frozen” tendency, lack of heart rate increase, and very different pattern of neural activations, despite having a severe case of acute and subsequent PTSD, attest to the fact that hypoarousal responses are equally a reaction to trauma, and that the common denominator in trauma may be autonomic dysregulation outside of the window of tolerance.
CONCLUSION
Sensorimotor psychotherapy was developed entirely from clinical practice, and at this point in time the mechanisms underlying its interventions are unknown. These mechanisms will be an exciting area to explore in the future. However, anecdotal reports from both clients and therapists attest to the efficacy of utilizing somatic interventions. Professionals who have learned sensorimotor psychotherapy report that clients often experience a reduction in symptoms such as nightmares, panic attacks, aggressive outbursts, and general hyperarousal, and that the new ability to track body sensations helps clients experience present reality rather than reacting as if the trauma were still occurring. Sensorimotor psychotherapy provides clients with tools to deal with disturbing bodily reactions. They frequently report feeling increasingly able to remain present in the here-and-now as they begin to learn how to limit the amount of information they must process at any given moment by focusing their attention on movement and sensation. Clients also report that their feeling of safety is enhanced when they practice protective and defensive actions such as pushing away.
Sensorimotor psychotherapy techniques may facilitate the integration of traumatic material sequestered in subcortical or right brain areas by working bottom-up, deepening mindfulness (which may increase cortical activity), evoking and studying trauma-related fixed action tendencies, and then experimenting with the practice of new actions. Because of its emphasis on regulating arousal and expanding the window of tolerance, sensorimotor psychotherapy attempts to avoid excessive autonomic or emotional arousal that might interfere with the integration of information. The pronounced focus on the body often facilitates the experience of emotion within a window of tolerance. When the client is encouraged to track even minimal physiological sensations and movements, mindfulness of present-moment experience is enhanced. Orienting to the here-and-now, the body, and the self may increase prefrontal and medial prefrontal activity, which may mediate perceptions that the traumatic event is not occurring in present time. By allocating attention to both traumatic activation and somatic resources, tracking and modulating levels of arousal in response to internal and external stimuli, and teaching new, more adaptive actions, improved functioning and integrative capacity of both brain and body are facilitated. This improvement enables the traumatic events to be processed and a renewed experience of self to emerge.
However, accessing too much sensation too quickly, particularly before clients are able to observe their experience and put aside content and emotional states, may actually increase dissociation and exacerbate symptoms. Therefore, therapists must proceed in accordance with each client’s pace and ability to integrate. Additionally, therapists using sensorimotor psychotherapy report that some clients are not interested in working with the body. Over time, such clients may slowly and painstakingly learn to be aware of their somatic experience and find value in that awareness. An occasional client may remain unable or unwilling to work with somatic interventions, finding body sensations and movements too overwhelming and distressing, or otherwise finding a somatic approach uninteresting, unappealing, or ineffective. In such cases, sensorimotor psychotherapy is contraindicated, and the therapist must employ other techniques.
Along with understanding the particular needs and capacities of our clients, awareness of the brain regions and systems involved in both top-down and bottom-up processing can contribute to our ability to select optimal approaches and techniques to help traumatized clients achieve mastery over symptoms and satisfaction in daily life pursuits. In this chapter we have given an overview of brain areas hypothesized or known to be implicated in trauma-related disorders with the aim of expanding understanding of the role of the brain in trauma-related disorders and providing food for thought in working toward a neurobiologically informed treatment model.