Geoff H. Palmer
CONTENTS
5.3.1 Drying, Storage, and Handling
5.3.2 Steeping, Germination, Kilning, and Malt Quality
5.4 Special Barley Varieties: Maris Otter, a Descendant of Proctor Barley
5.1 INTRODUCTION
Malt is made from germinated cereals such as barley, wheat, oats, rye, sorghum, millet, rice, maize (corn), and pseudo-cereals such as buckwheat and quinoa. However, barley remains the main cereal used for the production of beverages such as standard beer, craft beer, and malt whiskey.1–7
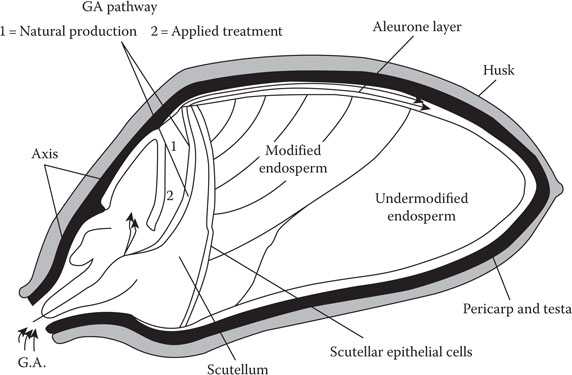
Barley and other cereal grains are fruits because they contain a pericarp. The pericarp is the fruit wall (Figure 5.1). All cereals are members of the family Graminea and are monocotyledons. Plants such as beans and peas are dicotyledons because their seeds contain two cotyledons. In barley, and other cereals, the term monocotyledon refers to a single cotyledon, which is called the scutellum.

Figure 5.1 Relationship between transport of gibberellic acid (GA), aleurone activity, and enzymic modification of the endosperm.
Barley is grown in many parts of the world, but it grows best in temperate climates. Malting barley is divided into two main species Hordeum vulgare and Hordeum distichon.8 Hordeum distichon is two-rowed barley and Hordeum vulgare is six-rowed barley. The ear or inflorescence of barley contains six rows of grains. In six-rowed barleys, all six rows of grains develop. In two-rowed barleys, only two of the six rows of grains develop; the developed rows of grains face each other and are subtended on each side by two rows of undeveloped grains. Although there are more grains per year in six-rowed varieties, most of these grains are too small for malting purposes. In two-rowed barleys, most of the grains are of suitable size for malting. In this regard, two-rowed barleys produce more brewers’ (starch) extract per hectare than six-rowed barleys.8
Six-rowed and two-rowed barleys may be winter barleys or spring barleys.8 Winter barleys are usually planted in about September and harvested in about June to July. In the Northern Hemisphere, although spring barleys are usually planted in about March and harvested in July to August, in highland areas, harvesting can be as late as September. Late harvesting can cause pregermination. Winter barleys are usually harvested before spring barleys, which is of economic benefit to the maltster in terms of availability of new grain. Although modern winter barleys are of equivalent quality to spring barleys, there is a perception in some quarters that winter barleys are of poorer quality. This is clearly not the case. For example, Maris Otter is a winter barley, and its celebrated malting quality is equivalent to or better than the best spring barleys.
Barley is a very old crop. Images of the ear (inflorescence) of barley are depicted on ancient Egyptian relics. Recent evidence9 suggests that barley was malted in ancient Egypt and was likely to have been used to make beer and bread. Historically, although barley has retained its dominance as the preferred cereal for making malt, wheat also has an important position in malt production. Wheat malt is mainly used to make Weiss beer, which has grown in popularity.4, 10 Sorghum malt can be used to make European clear beer,4, 6–8 but it is the primary raw material of traditional (African) opaque beer. Millet malt is also an important raw material of some traditional foods and African beers. Wheat grain proteins have significant foam inducing properties and, like sorghum and millet, wheat has high levels of soluble pentosans.8
Barley malt is used in different quantities to make Scotch, Irish, Japanese, American, and Canadian whiskeys.11 It is also used to produce vinegar and various kinds of foods such as breads, biscuits, confectionary, and malt drinks. Malt extract not only provides different colors and flavors to food products and beverages, it is also used as a growth medium for microorganisms.
Barley has been described as feed barley or malting barley. Malting barleys are usually “recommended.” In the United Kingdom, malting barleys usually have grades that extend from a low grade to a high grade.8, 12 Grades are given for yield, disease resistance, and malting quality. These properties of the grain are under genetic control and are distinct in different barley varieties. It takes about ten years to produce a new barley variety by traditional breeding methods. This time period can be reduced to five years, if two crops are achieved each year, by planting and harvesting in two countries each year. New varieties are tested for malting and brewing quality and must be distinct and uniform before they can be recommended by official bodies such as the National Institute of Agricultural Botany (NIAB), Cambridge, England.12
A significant amount of research interest is being directed at using new gene transformation techniques to breed improved barley varieties. Transformation may involve substitution of genetic (chromosome) fragments (restriction fragment length polymorphism, or RFLP) with markers called quality trait loci (QTL) or the insertion of specific genes into the genomes of present barley varieties.13–17, 18 The insertion of new genes in plant tissues is followed by cell division and the development of new plants containing the new genes. Although genome fragments techniques have been used to identify barley varieties,17 no genetically transformed barley plant has been recommended officially for malting. Indeed, many transformations have not been successful because transformed genes have failed to be inherited over succeeding generations. The quality parameters of malting barleys are well known and any genetic change that affects the usual structure and function of malting barley varieties negatively will not be accepted by the industry. The possible use of genetically modified barley in the industry remains a controversial matter.19 However, the problem the industry faces in this kind of research is not uncommon. For example, important techniques such as polymerase chain reaction (PCR)15 are producing published results but are not providing the analytical or technological outcomes required to improve the performance of malting barleys in the industry.
However, acceptance of genetically modified malting barley will have to overcome limitations such as product image and customer concerns regarding the use of genetically transformed barley to make malt. Other aspects of genetic transformations relate to the replacement of the genes of heat-labile enzymes such as β-amylase and endo-β-glucanase with genes from microorganisms that produce heat-stable versions of these enzymes. Potential improvements in malting barley quality also relate to the possibility of increasing disease resistance and crop yield.
Crop yield of malting barley is a very important factor in the economics of malt production worldwide. World production of barley is about 135 million metric tons per annum, but only about 25 million metric tons are considered suitable for producing the 20 million metric tons of malt required by the industry worldwide. The world’s malting capacity is about 27 million metric tons per annum. Regarding the latter tonnage of malt, about 94% is required to produce 2.0 billion hL of beer, with about 4% being required for distilling and about 2% for food and vinegar production. Europe plays a significant role in the production of barley and malt in the world. About 60% of malting barley and about 50% of malt in the world are produced in Europe, and 60% of the malt produced is traded worldwide.
The large quantity of barley that is regarded as unsuitable for malting makes it necessary that malting barley is selected, handled, and malted effectively. Various degrees of manual and mechanical skills are used in the production of modern malts and beers in the craft and main sector of the brewing industry. However, irrespective of the type of malt being produced, malting depends on optimal induction of physiological processes such as the modification of the endosperm by enzymes during malting.2, 8, 20 A better understanding of these functions is an essential feature of modern malting science and technology.
In terms of malting practice, purchase specification should include known barley variety and necessary analytical results. These parameters should ensure that processing and end product expectations are met.21, 22
5.2 BARLEY
5.2.1 Structure and Function
Barley grains (Figure 5.1), except for naked (huskless) barley, contain: husk, pericarp, testa, aleurone layer, starchy endosperm, and embryo.2, 8, 20 In terms of total dry weight of the barley grain: the husk is 10% to 12%; the pericarp and testa, 2% to 3%; the aleurone layer, 4% to 5%; the starchy endosperm, 77% to 82%; and the embryo 2% to 3%.
5.2.2 The Husk
The husk is composed of two leaf-like structures. The dorsal half is called the lemma; the ventral half is the palea. The husk protects the underlying structures of the grain, especially the embryo. Husk damage is regarded as unacceptable, and barley samples are rejected if husk damage is beyond specification requirements. Husk damage implies embryo damage, uncontrollable embryo growth, and loss of filtration potential in conventional mash tun operations. The husk carries background levels of microorganisms such as fungi and bacteria. These microorganisms may have invaded the grains in the field or during storage. For example, Alternaria, Cladosporium, and Fusarium tend to invade the grain in the field, whereas Aspergillus, Penicillium, and Rhizopus can develop significantly during grain storage. Although microbial activity can be high during malting, grain drying and kilning reduce microbial levels on barley and malt, respectively.
Fungi (e.g., Fusarium) can release mycotoxins such as trichothecenes (e.g., deoxynivalenol, DON) and zearalenone. DON is associated with Fusarium infection,21, 23 and its presence is linked with “beer gushing.” Aspergillus produces aflatoxins and ochratoxins. Mycotoxins are associated more with maize than barley and can be dangerous to the health and life of animals; aflatoxins from Aspergillus are potentially hazardous. In animals, the mycotoxin zearalenone is an estrogenic and tumor-producing toxin. Although zearalenone (less than 2.0 ppb) has been found in fermenting worts and would have been extracted from mashed cereals, it is changed to α-zearalenol during fermentation. The latter compound is more of a threat to human health than zearalenone. The levels of harmful fungi on malting barley are normally very low and should not be harmful to humans that drink beer. In contrast, spent grains from the mash tun can have concentrated levels of mycotoxins, especially if stored wet. However, spent grains can be detoxified using specified levels of formaldehyde or by ammoniation, before being fed to farm animals. Toxins from Ergot infections are dangerous to human health and can cause nervous diseases. In general, sprouted, infected grain should not be used as animal feed. The control of microbial infection of cereal grains is of vital importance to the industry. Although rainfall and warm temperatures can promote the development of Fusarium, management techniques such as judicious crop rotation, removal of plant debris, appropriate varietal selection, and selective fungicide spraying can help to reduce the risk levels of the disease.
Excessive levels of microorganisms in the husk can produce uneven germination. This type of germination failure is called water sensitivity.8, 21 Air-resting during steeping can reduce water sensitivity significantly. Barley husks contain significant quantities of lignin and cellulose, which are constituents of cell walls. Lignin is a complex noncarbohydrate polymer and is produced from phenolic compounds such as coniferyl alcohol. Residual phenolic compounds range from simple phenolic acids (e.g., ferulic acid and coumaric acid) to more complex phenols (polyphenols) such as the dimer, procyanidin B3. Phenols such as procyanidin B3 have oxidative properties and can react with proteins to form hazes in beer. Phenolic substances are leached from the husk during steeping.4, 8, 21 Insufficient leaching during submersion steeping (see following) causes phenols to be extracted later during mashing, and the resultant beer may have an astringent taste. Steeping with alkaline (lime) steep water reduces the phenol levels of malt.
Color pigments (anthocyanidins) in the husk may assist barley variety identification. Structural features of the husk, such as the shape of attachment points of the grain to the flower (ear) stalk, can also be used to identify barley varieties. The husk releases a significant quantity of dust during handling, storage, malting, and kilning. Dust can carry microorganisms such as Aspergillus, which together can produce lung diseases, if health and safety regulations are not followed.
5.2.3 The Pericarp
The pericarp is the fruit wall of the grain. Therefore, cereal grains are fruits and, strictly speaking, should not be referred to as seeds. Like the husk, it contains a waxy cuticle. Below this waxy layer is a compressed structure of cells. The pericarp is semipermeable. Certain chemicals will pass through it, other chemicals will not—for example, gibberellic acid. Water can pass through the pericarp. Damage to the pericarp during the abrasion process8 allows gibberellic acid to enter the aleurone layer directly, rather than via the germinated embryo, thereby improving modification of the underlying starchy endosperm by improving the efficiency of the aleurone layer to produce endosperm-degrading enzymes.2, 8, 20
5.2.4 Testa
Dehusking of barley grains using cold 50% sulfuric acid will remove the husk and pericarp but not the testa. The testa comprises two lipid layers that enclose cellular material. In contrast, dehusking by hand leaves both the pericarp and testa on the grain. Submersion of the distal (nonembryo) end of hand-dehusked barley into water causes hydration of the starchy endosperm, suggesting that hydration of the endosperm can take place in the absence of embryo activity and that water can pass directly through the pericarp and testa during steeping.8
The testa is permeable to gibberellic acid. Phenolic color compounds such as anthocyanogens (proanthocyanidins) are associated with the aleurone layer and testa and can be seen clearly in some varieties of barley and sorghum. The small area of the pericarp-testa that lies over the coleorhiza (chit) is called the micropyle. The latter may facilitate the uptake of water and salts into the embryo during germination.
5.2.5 Aleurone Layer
The aleurone layer is about two to three cells deep over the starchy endosperm (Figure 5.2a). It extends over the embryo as a single cell layer. Therefore, excised embryos or scutella contain aleurone cells that can produce endosperm-degrading enzymes,8, 20 which could be assessed wrongly as originating from the epithelial cells of the scutellum. During malting, gibberellic acid, from germinated embryos, can induce aleurone cells to produce endosperm-degrading enzymes such as α-amylase, endo-β-1,3:1,4-glucanases, limit dextrinases, endoproteases, and xylanases (pentosanases). Biochemical evidence has established that α-amylase, endoprotease, and limit dextrinase are produced de novo in aleurone layers stimulated by gibberellic acid. The aleurone layers of some barley varieties are blue in color, which can be seen through the husk, assisting the identification of these varieties.
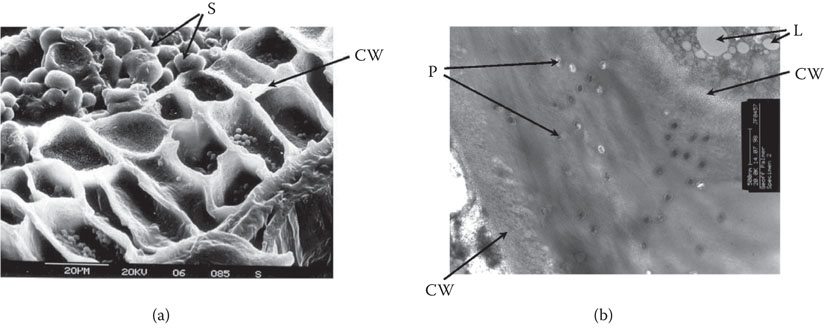
Figure 5.2 Cell walls of the aleurone layer at low magnification (a) and at high magnification (b); S = starch granules of barley endosperm; CW = cell wall surface; P = plasmodesmata; L = lipid body.
Aleurone layers (Figure 5.2a) have thick cell walls (3 µm) that contain mainly pentosans (about 60%) and β-glucans (about 30%), which are degraded in localized areas during malting. This degradation process may facilitate the release of endosperm-degrading enzymes into the starchy endosperm during malting.2, 8, 20 Details of the biochemistry and physiology of this enzyme-producing function of the aleurone cell have been published,2, 8, 20, 24 and from a technical viewpoint, it has been confirmed25 that the viability of the aleurone layer is an important feature of the malting quality potential of barley. In contrast, in sorghum, the enzyme-producing function resides in the embryo not the aleurone.8
Gibberellic acid is produced in the germinated embryo but must be transported to the distal end of the grain (Figure 5.1) to produce a progression of enzymes, which during malting (Figure 5.3) will convert the hard, hydrated, starchy endosperm of barley into friable malt during germination (Figure 5.4a and b). Transport of gibberellic acid through aleurone cells may occur through plasmodesmata, which are present in aleurone cells (Figure 5.2b).8, 20 The aleurone layer is therefore a large interconnected jacket of living cells that, except for the surface of the scutellum, encloses the starchy endosperm (Figure 5.1). Therefore, plasmodesmata in aleurone cells facilitate the transport of gibberellic acid from the embryo to aleurone layer. Research has shown that factors that limit the rate of transport of gibberellic acid in the aleurone layer during malting can reduce the evenness of enzymic modification of the starchy endosperm.8, 20, 26 For example, reduced transport of gibberellic acid, caused by understeeping, can retard modification of the distal end of the malting grain (Figures 5.1 and 5.4a). Aleurone layers of different barleys can produce different levels of endosperm-degrading enzymes with similar doses of gibberellic acid. This is usually a varietal characteristic. In addition, it is worth noting that endosperm-degrading enzymes, such as endo-β-glucanase, require higher concentrations of gibberellic acid than α-amylase to develop optimally.2, 8
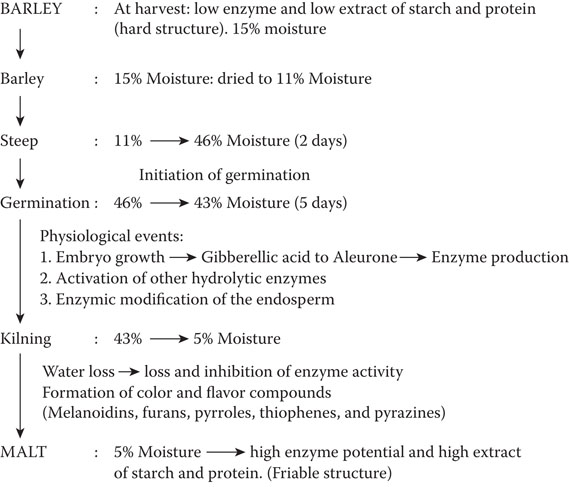
Figure 5.3 Basic steps of malt production.
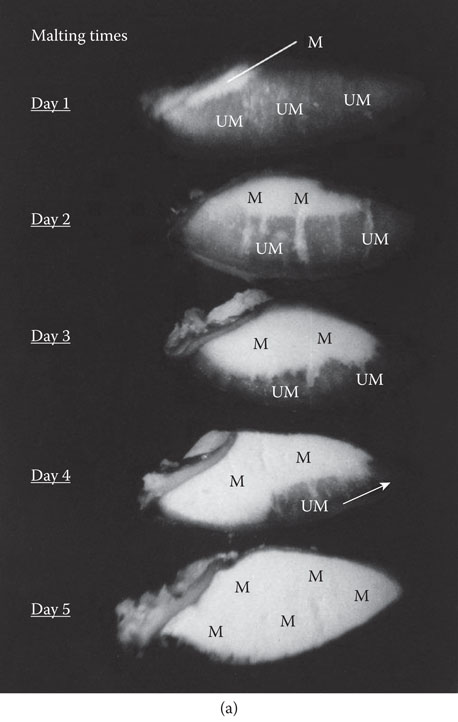
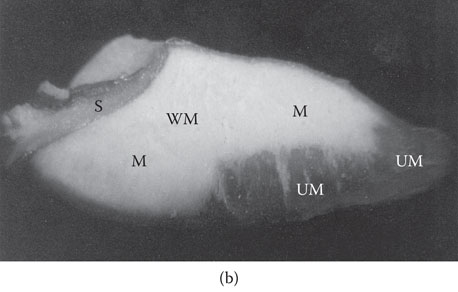
Figure 5.4 (a) Longitudinally hand-cut sections of the endosperms of malting barley grains at malting times: Day 1, Day 2, Day 3, Day 4, and Day 5. Note progressive asymmetric pattern of enzymic modification of the endosperm from Day 1 to Day 5. UM = under-modified endosperm; M = modified endosperm. Husk, shoot, and roots (axis) removed. (b) Asymmetric pattern of modification of the starchy endosperm of malting barley (see Figure 5.4a, Day 4 malting time). Note under-modified distal (nonembryo) end of the grain. Shoot and roots removed. WM = well-modified endosperm; M = modified endosperm; UM = under-modified (barley-like) endosperm; S = scutellum.
The levels of aleurone enzymes such as α-amylase, endo-β-1,3:1,4-glucanase, and protease that are produced during malting are not always related to the nitrogen (protein) content of the grain because the protein content of the aleurone layer can be different from the total protein content of the grain.27, 28 The nitrogen (protein) content of aleurone layers of steely grains in a barley sample tend to be higher than the nitrogen contents of the aleurone layers of corresponding mealy grains. The nitrogen level of different barley varieties can be the same, but they will produce different levels of endosperm-degrading enzymes.27, 28 However, it should be noted that the relationship between nitrogen content and endosperm enzymes can be altered by the malting process. For example, a low nitrogen barley sample (e.g., 1.5%) can produce more β-amylase than a corresponding sample of barley at higher nitrogen content (e.g., 1.7%) if endosperm modification was better in the low nitrogen barley sample.28 The action of proteolytic enzymes during endosperm modification appears to activate enzymes such as β-amylase and limit dextrinase.8, 29
The aleurone layer is a major constituent of bran. The aleurone layer contains high levels of lipid, proteins, and phytic acid. Phytic acid acts as a chelating agent and reduces the levels of available metal ions in the diets of humans and animals. The hydrolysis of phytic acid (inositol hexaphosphate) by phytase in the aleurone layers of malting grains produces phosphate products, which range from initial levels of phytic acid (inositol hexaphosphate), through intermediates, to final levels of inositol and phosphoric acids. During the mashing process, calcium reacts with phosphoric acids to produce calcium phosphate and hydrogen ions, which help to create the acid conditions of the mash from which a wort of about pH 5.2 is derived. Vitamin B and the nonreducing sugar sucrose are also present in the aleurone layer. Sucrose is the major fermentable sugar of malt, but it disappears quickly during fermentation.
5.2.6 Starchy Endosperm
The term endosperm comprises the pericarp, testa, aleurone, and the starchy endosperm tissue. The starchy endosperm is the largest structure of the grain and is made up of thousands of cells. The walls of these cell are about 2-µm thick and contain about 70% β-D-glucans, 20% pentosans, and about 5% protein. The inner walls of these cell walls mainly contain β-D-glucans. The outer walls are composed of β-D-glucans and pentosans.8, 30 The β-D-glucans have about 70% β-1,4 links and 30% β-1,3 links. Approximately 50% of the cell wall is soluble in hot water (65°C). The cell wall is degraded by proteases, endo-β-1,3:1,4-glucanases, endo-β-1,3-glucanases, exo-β-glucanases, arabinosidases, and xylanases to produce mainly glucose, cellulose products, laminaribiose, arabinose, xylose, and small fragments of soluble arabinoxylans. Although there is a view that the cell walls of the endosperm do not contain consecutive β-1,3 links, it has been reported that this feature of β-glucans is associated with high molecular weight β-glucans and is varietal—the β-glucans of poor-quality barleys such as Julia were more susceptible to the action of endo-β-1,3-glucanase than the corresponding high molecular size β-glucans of high-quality barleys such as Maris Otter and Proctor.30
The next major storage compound of starchy endosperm cells is protein. Hordein and glutelin are soluble in dilute alkali solutions and are the major protein compounds (about 40% and 30%, respectively) of the protein matrix in which the large and small starch granules of the endosperm are embedded.8 Albumins and globulins are soluble in salt solution and are present (about 20% and 10%, respectively) in the embryo, aleurone, and starchy endosperm. Barley and barley malt contain gluten. Gluten is a protein that is insoluble in water, such as barley hordein. Wheat malts contain high levels of soluble proteins, which promote beer foam development.10, 17 Wheat malts such as barley malts contain gluten, which can cause celiac disease. Cereals such as sorghum, millet, and pseudo-cereals such as buckwheat, amaranth, and quinoa are regarded as gluten-free.5 The detection of gluten relates to the method used. Therefore, failure to detect gluten does not mean “gluten-free.” Also, excessive hydrolysis of proteins that contain celiac-producing properties may produce a hydrolysate that may still cause celiac disease.
Enzymes such as β-amylase and carboxypeptidases are part of the salt (sodium chloride)-soluble albumin and globulin fractions of the total protein of the grain. Hordein and glutelin are similar in structure and contain high levels of proline and glutamine. Hordein is soluble in hot (70%) ethanol or hot (60%) isopropanol. The main enzymes causing degradation of protein in the starchy endosperm are endo-proteases (endopeptidases)—some are thiol-dependent, others are metallo-dependent. These enzymes convert hordein proteins into soluble proteins during malting. Carboxypeptidases release amino acids from solubilized proteins. The pH of the endosperm of malting barley varies between pH 5 and pH 6 and facilitates the activities of the proteases enzymes. New analytical techniques known as proteomics,15 which detect all proteins in a mix of soluble proteins, could be used to improve our understanding of protein modification during malting and mashing. Proteases, in combination with enzymes such as endo-β-1,3-glucanase and cellobiase, may initiate the release of β-D-glucans from the cell walls of the endosperm during malting. High protein levels and inadequate hydration (wetting) of the starchy endosperm will limit protein degradation, which facilitates the action of enzymes that degrade endosperm cell walls during malting.8, 20 The development, movement, and progressive action of proteolytic enzymes in the endosperm of the malting grain appear to be a primary factor in the relationship among protein modification, cell wall breakdown, and the optimal development of trouble-free, modified, brewers’ extract during malting. Although the total soluble nitrogen/total nitrogen ratio of the malted grain is used as an index of malt (protein) modification, it gives no indication of the distribution of protein breakdown in the malting grain. However, hordein (and possibly glutelin) degradation and proline release may provide additional information that should improve our understanding of the mechanism of malt modification.8, 20, 31
Glutelin, like all the other protein fractions, is soluble in 4% sodium hydroxide. During malting, proteases develop rapidly, and the hordein fraction of stored protein is hydrolyzed extensively to produce the soluble proteins of brewers’ hot water extract, which contain polypeptides, peptides, and amino acids. Structurally, the starchy endosperm may be mealy, steely, or mealy/steely. These features of the grain are controlled to a great degree by field-growth conditions such as temperature, moisture, and fertilizer application. However, some varieties tend to maintain a high degree for mealiness or steeliness in different environmental conditions, suggesting this structural feature of the grain is under heritable genetic control. Steely grains contain more proteins than mealy grains of the same sample.8, 27, 32 Steely grains also contain higher percentages of hordein than corresponding mealy grains. The starchy endosperms of steely grains are compact (hard) whereas the starchy endosperms of mealy grains are looser (softer) in structure. Mealy grains have a higher starch content than steely grains and will yield a higher starch extract.8 Mealy grains of Proctor and Julia barleys have about 10% lower levels of β-glucan than corresponding steely grains.30
In barley, the starchy endosperm contains two types of starch granules, large (10–25 µm) and small (1–5 µm).8 Large starch granules are referred to as A-type, and small starch granules are referred to as B-type. Barley grains that develop during a drought tend to have reduced grain size and higher proteins and may contain starch that has reduced enzyme digestibility.33 The digestibility of other carbohydrate food reserves of the starchy endosperm, such as cell walls, may also be reduced. This could cause a reduction in the modification rate, which could limit extract development. Both large and small starch granules are associated with lipoprotein, which may limit their digestibility. About 10% of the starch content of the starchy endosperm is degraded during malting. Because the small starch granules comprise 10% of the weight of the starch, and because about half of the small starch granules are degraded during malting,8 similar weights of starch are degraded from the large and small starch granules during malting.
The β-amylase of the starchy endosperm is associated with the endosperm protein. During malting, this enzyme is activated by reducing conditions and by the action of proteolytic enzymes in the endosperm of the malting grain. Although, β-amylase activity is supposed to be linked to the nitrogen (protein) levels of the grain, recent research suggests that β-amylase activity may, inter alia, be associated with the hordein content of the grain.27 The β-amylase enzyme is a very important during mashing because it attacks gelatinized starch to produce maltose. Maltose is the main sugar (45%) of brewers’ worts. About 90% of the diastatic power (DP) of malted barley is β-amylase activity. Although the malting grain has a high β-amylase activity (DP), the 10% of raw starch that is degraded during malting is degraded by α-amylase. Limit dextrinase is another starch-degrading enzyme.3, 29 It hydrolyses the α-1,6-links of the amylopectin found in gelatinized starch. During the conversion of gelatinized starch during mashing, α-amylase and to a lesser extent limit dextrinase accelerate the maltose-producing action of β-amylase. Limit dextrinase and β-amylase are more heat labile than α-amylase. Both limit dextrinase and β-amylase undergo significant destruction during kilning and mashing. However, the activities of limit dextrinase and β-amylase are increased by proteolytic activity, reducing conditions and acid pH.8, 29 Both β-amylase and limit dextrinase are more active in distillers’ fermentations than in brewers’ fermentations. Because distillers’ worts (washes) are not boiled, both of these heat labile enzymes continue to act during distillers’ fermentations. As a consequence of this, brewers’ worts are about 75% fermentable, while distiller worts are about 86% fermentable, which results in high fermentable extracts and high spirit yields.
Hot water extract is derived mainly from hydrolyzed starch and protein. The weight of the large starch granules is an important index of malt quality. An increase in the protein content of the grain reduces the sizes and numbers of large starch granules.2, 8 Although the actions of cell wall-degrading and protein-degrading enzymes are important for extract development during malting and mashing, the sizes and numbers of the large starch granules determine sugar extract yield during mashing and alcohol production during fermentation. Brewers’ wort is about 75% fermentable and has about 10% glucose, 45% maltose, 15% maltotriose, 10% maltotetraose, 15% dextrins, and 5% sucrose. The soluble proteins that are released during malting constitute the other major component of brewers’ worts. Soluble proteins include proteins, polypeptides, peptides, and amino acids. Of the protein fractions, lipid transfer proteins of 9,000 Da and hordein-type proteins of 40 kDa are regarded as playing important roles in foam development and stability.8, 23 These proteins have hydrophobic properties. However, although the biochemistry of foam is important, it is worth bearing in mind that commercial malts will produce foam if their hordein proteins, some of which have molecular weights of 40 kDa, are not degraded excessively during malting.8 Excessive degradation of lipid transfer proteins in over-modified malts limits foam development. Some proteins may combine with polyphenols to form beer haze. Although large quantities of soluble proteins are lost during beer production, functional quantities remain as part of the physicochemical properties of the beer. Amino acids are about one-quarter or one-third of the soluble proteins of the wort and help to promote yeast growth and flavor (e.g., ester) development during the fermentation processes of both the brewer and the distiller. In general, during malting, over-modification or under-modification of proteins is unacceptable because such malts can produce unexpected processing problems and undesirable beer qualities.
5.2.7 The Embryo
The embryo contains about 30% to 35% protein in contrast to the endosperm, which only contains about 10% to 12%. However, the protein level of the embryo is very small because the embryo is about 3% of the weight of the grain and the endosperm is about 87%. The lipid content of the embryo is about 15%, while that of the entire grain is about 3%. The embryo absorbs large quantities of water during steeping and, for a grain with 45% moisture, the moisture content of the embryo is about 60%.
Structurally (Figure 5.1), the embryo comprises two major tissues: the axis (shoot, node, and roots) and the scutellum (a single cotyledon). During malting, gibberellic acid is synthesized at the nodal region of the axis and transported mainly through dorsally oriented vascular stands to the dorsally placed aleurone cells. This irregular distribution of gibberellic acid initiates a lopsided (symmetric) pattern of enzyme production and endosperm modification (Figure 5.4a and b).8 Although the aleurone layer is the dominant enzyme-producing tissue of the grain,8 it has been confirmed recently25 that the aleurone layer has the dominant enzyme producing cells in malting barley. This is in keeping with the low polyphenol barley variety (Galant), which had dysfunctional aleurone layers8 and failed to modify its endosperm successfully.8
Because gibberellic acid is produced by germinating barley, germination potential is a very important index of malting quality. Notwithstanding, if the level of gibberellic acid is not optimal and transport to the aleurone layer is limited, malting performance will be suboptimal.8, 20, 21 In some maltings, small quantities of gibberellic acid (0.2–0.25 ppm) are sometimes added to the chitted (germinated) grain to optimize the levels of gibberellic acid in the grain (Figure 5.1). Optimal levels of gibberellic acid are required at the beginning of the germination process (Figures 5.3 and 5.4a—Day 1) because a delay in the production and secretion of endosperm-degrading enzymes by the aleurone layer in different grains will produce unevenly modified malts (Figure 5.4a and b).
During germination, sucrose and raffinose are degraded in the embryo but are resynthesized when the sugar products of the modifying endosperm pass from the starchy endosperm to the embryo.8 The embryo contains significant quantities of lipid.8 Hydrolysis by lipases produces substrates that the embryo can use for energy or for starch synthesis in the scutellum. Of the 3% of lipid that is present in malted barley, only about 0.01% of lipid materials (mainly fatty acids) are extracted from malts into worts. Lipases such as lipoxygenases (LOX-1 or LOX-2) are found in malt. LOX-2 is present in barley. LOX-1 develops in the embryo during malting and can cause lipid oxidation, producing beer staling compounds such as trans-2-nonenal. Lipoxygenases survive kilning21 and act quickly during mashing because of the availability of oxygen and the presence of high temperatures.
5.3 MALT PRODUCTION
5.3.1 Drying, Storage, and Handling
The drying, storage, and handling of barley must be properly managed in order to avoid damage to the living tissues of the grain—the embryo and aleurone layer. Barley should not be dried above an air-on temperature of 50°C to 60°C. The lower end of this temperature range is recommended for high moisture barleys (20%–25%). It is important that grains above 15% to 16% moisture are not stored for long periods. Irrespective of air-on temperature, the internal temperature of the grain driers should be significantly lower than the air-on and air-off temperatures. Only driers that are officially recognized as malting barley driers should be used to dry malting barley. Grains at 15% to 16% moisture should be malted or dried, soon after harvest. Air-flow conditions in the drier should be optimal for the grain load being dried. Suboptimal air-flow can cause stewing and will damage germination potential. The embryos of stewed grains may give positive tetrazolium (viability) results. However, such damaged grains are unsuitable for malt production because embryo function is impaired. Long-term storage of high moisture grain can also damage germination potential, especially in warm–hot climatic conditions. However, very low storage temperatures (less than 5°C) can damage germination especially of undried grains.
Dried barley (less than 12% moisture) can be warm stored to remove dormancy. Warm storage can be carried out in carefully controlled storage conditions at 20°C to 40°C for up to six weeks depending on the depth of dormancy. Germination potential should be monitored regularly. During warm storage, the moisture anywhere within the grain bulk should be about 11.0%. Moisture should be below 12%. To ensure aeration, the grain bulk should be turned or air passed through the bulk of the grains while maintaining warm storage temperatures. Failure to germinate in optimal (usual) conditions is described as natural dormancy.2, 8 Some barley varieties tend to show high levels of natural dormancy or a type of dormancy called water sensitivity. Climatic conditions can induce natural dormancy in barley grains leaving higher than normal levels of growth inhibitors such as abscisic acid. Structural immaturity of the pericarp-testa of the grain may restrict germination (root emergence) by limiting oxygen uptake and or by restricting root emergence physically.8 Nevertheless, dormant embryos are metabolically active and can be damaged during normal storage or warm storage. Physiologically, warm storage may induce germination by promoting “maturation” of the pericarp-testa, which may facilitate the leaching out of inhibitors and/or may promote the ingress of oxygen into the embryo. Absorbed oxygen may induce accelerated respiration and destroy dormancy inhibitors such as abscisic acid.
During normal storage, air should be passed through the bulk of the grain, and the germination potential of the grain should be monitored during storage using appropriate tests. Pests and extraneous materials should be eliminated and the development of fungi and bacteria avoided. Stored grains should be checked regularly for “hot-spots” or localized condensation. There should be a scheme for tracing barleys as regards variety, total nitrogen, and germination potential. If there is concern about the authenticity of a barley variety, it should be checked visually and genetically. Grain damage (husk loss, broken grains) should be avoided. On the management of malting barley, preventive hygiene is preferable to chemical treatment.21, 22
5.3.2 Steeping, Germination, Kilning, and Malt Quality
Steeping is designed8, 34 to increase grain moisture (water) level from the moisture of the stored grain to out-of-steep moisture (Figure 5.3). Air resting procedures break up the submersion periods in different steeping regimes. The duration of water submersion and air rest periods reflect sample requirements, water availability, and the production cycle. Each submersion steep can require about 900 liters (200 gallons) of water per metric ton of barley. The disposal of such large quantities of water of high biological oxygen demand (BOD) is expensive.21 However, short washing or limited steeping, supplemented by spray steeping, may reduce water usage and solve effluent problems but may limit the development and action of cell wall-degrading enzymes such as endo-β1,3:1,4-glucanase. Under-steeping can limit endosperm modification and reduce malt quality.7, 8 Spray steeping, after submersion steeping, should be a “top-up” exercise where about 5% of water is added to obtain optimal moisture levels prior to the commencement of the germination process.
Submersion steeping8, 21 washes out of the grain a wide range of materials such as phenols, amino acids, sugars, minerals, microorganisms, and polymers such as pentosans, β-glucans, and proteins. Therefore, poststeep water is active biologically. The reuse of steep water without prior treatment to reduce contaminating material is unwise because used steep water can inhibit germination. If permitted, various additives can be used during steeping, depending upon acceptability. For example, calcium hydroxide (minimal level 0.5%) and sodium hydroxide (minimal level 0.05%), followed by alkali removal, may be used to assist phenol extraction. Formaldehyde (minimal level 0.05%) can reduce microbial infection, and hydrogen peroxide (minimal level 0.1%) may assist oxygenation. Improved oxygenation supplied by aeration during steeping can improve germination. Hypochlorites can reduce microbial infection but impart a disinfectant taint to the malt and the derived beer. Additives should be acceptable to the customer. Additives can inhibit germination. Therefore, where germination inhibition is not intended, the impact of additives on the germination process should be assessed appropriately. Concentrated levels of detergent residues can inhibit germination. Germination can be slower in the conical region of deep steeps than at the middle or top of such steeps. High-pressure pumping of slurries of fully steeped actively respiring (germinated) barley samples can damage the embryo and limit the subsequent potential of the grain to malt effectively.
The loss of oxygen from the steep is very rapid; therefore, aeration of the steep can improve germination. Air-resting (air/oxygen exposure) reduces water sensitivity that prevents germination but may not break natural dormancy. Usual extraction (displacement) of carbon dioxide may also improve germination. Steeping temperature varies, but 16°C seems to be a good average temperature. In general, steeping at higher temperatures (e.g., 20°C) tends to reduce proteolysis but increases friability of the endosperm, indicating in-homogeneity of malt modification.35,36 About 70% to 75% of the soluble protein in wort is produced during malting. Proteolysis is more effective at a malting temperature of 15°C to 16°C. Optimal starting moisture of the germination process is about 45% to 46% (Figure 5.3), and moistures below 40% will retard the modification process, even in high-quality barleys. Optimal moisture levels over the first two days of the germination process are required for optimal initiation of the process of endosperm modification. This is the period when endosperm-degrading enzymes are produced in, and released rapidly from, the aleurone layer. At this early stage of malting, these enzymes are not only required to release extract, they are required to reduce the high viscosity of solubilized β–glucan materials. Short grown, Day 2 and Day 3 malts (Figure 5.4a) tend to contain β–glucans that can cause brewhouse problems.8, 35, 36
Steely (compact, high protein) endosperms absorb water more slowly than mealy (loose structure, low protein) endosperms. As a consequence of differences in hydration (wetting), mealy grains tend to malt faster than steely grains. Because of the importance of mealiness and steeliness as quality parameters, methods have been developed to detect the degrees of mealiness and steeliness of barley samples.8, 32 However, endosperm structure is not the only quality factor that must be considered in the complex process of malting.
Enzyme development in the aleurone and variations in β-glucanase development in the starchy endosperms of single grains37 are important features of the malting process. It is known that out-of-steep moisture influences the extent to which the aleurone layer is activated during malting.8, 31 These studies showed that limited steeping will retard aleurone activity and the usually slow-modifying distal (nonembryo) end of the grain will remain under-modified (Figure 5.4a and b). This reduction in aleurone function was worse in low-grade barley varieties. Under-steeping appears to delay the transport of gibberellic acid through the aleurone layer. Therefore, three of the most important aspects of steeping are: First, to initiate germination and the development of gibberellic acid in the germinated embryo; second, to facilitate the transport and action of gibberellic acid in the aleurone layer; and third, to hydrate the starchy endosperm to a level that facilitates enzymic modification of the endosperm.
Limitations to endosperm modification caused by suboptimal hydration during the first one to two days of germination are difficult to correct later in the germination (malting) process (Figure 5.3). Spraying during the later stages of germination can cause localized over-modification of the embryo end of the endosperm (Figures 5.4a). Premature yeast flocculation (PYF) during fermentation is undesirable and has been subjected to detailed study.38 Personal observations in commercial practice suggest that spraying of water during germination to compensate for limited submersion steeping can result in the production of malt that can cause premature flocculation of yeast. The reasons for this are not clear, but limited submersion steeping can limit extraction of husk compounds such as pentosans and phenols, which could contribute to factors that promote the premature flocculation of yeast during fermentation.
Spray steeping, as a result of limited submersion steeping, applied late in the germination process can cause localized over-modification of proteins, which can result in high levels of amino acids. Such malts can have high colors after kilning but are nevertheless under-modified. For normal malts, high malt colors are usually associated with general over-modification of the malted grain. A germination temperature of 16°C produces better proteolysis than a higher temperature of 20°C that encourages enzymic breakdown of the cell walls of the endosperm during malting.36 The latter temperature tends to give better friability but greater in-homogeneity of malt modification.20, 35, 39 In contrast, 16°C malts had better amylase development and better fermentabilities than malts produced at 20°C.
An essential feature of the germination process is that the relative humidity (RH) of the airflow through the grain bed should be as close to 100% as possible. This avoids water loss that reduces the rate of modification of the endosperm. In commerce, gibberellic acid is produced by the fungus Gibberella fujikuroi in fermentation systems and then, processed, packaged, and sold to the industry. Gibberellic acid can be used to suppress seed development in the production of seedless grapes. Maltsters have been using gibberellic acid since 1959. This was long before definitive physiological research was carried out on the role that this hormone had on enzyme synthesis in aleurone cells.2, 8, 20, 24 Gibberellic acid (0.2–0.25 ppm) is usually applied early during the first day of the malting process (Figure 5.4a—Day 1). The grain should have chitted (germinated) before gibberellic acid is applied as this facilitates the uptake of the hormone. When potassium bromate (50–100 ppm) and gibberellic acid are applied together, the increased proteolysis caused by the gibberellic acid is reduced by potassium bromate.8 Potassium bromate also reduces extract loss by reducing root growth of the malting grain. The levels of gibberellic acid produced naturally by some grains are insufficient to produce the malting rates required by many maltsters. However, although added gibberellic acid can increase the malting rate, the asymmetric pattern of enzymic modification of the endosperm does not change (Figures 5.1 and 5.4a and b).31
The abrasion process was developed to accelerate the modification process by improving enzyme distribution in the endosperm.8 Because the embryo end of the endosperm modified faster than the distal end of the grain (Figure 5.4a), the abrasion process was developed to accelerate modification of the distal (nonembryo end) of the malting grain. Abrading machines8, 40, 41 selectively damage the pericarp layer, especially at the distal ends (regions) of the grain. During the malting of abraded grains, gibberellic acid enters the embryo as well as the distal end of the grain simultaneously. Therefore, abraded grains are malted from both ends and enzyme development, endosperm modification, and extract development occurs quickly. Malting time is reduced. Physiologically, the abrasion process showed that the rate of distribution of endosperm-degrading enzymes in the starchy endosperm was a primary factor controlling the malting rates of barley grains.8, 40, 41 In this regard, gibberellic acid/aleurone efficiency and the speed of penetration and actions of endosperm degrading enzymes are key aspects of the malting quality of barley samples.
In a well-modified malt, at least 90% of the β-glucan is broken down. Although there is a positive correlation between endo-β-glucanase activity and β-glucan breakdown during malting, the endo-β-glucanase levels of the individual grains of a barley sample do not correlate with β-glucan breakdown, suggesting that other enzymes may complement the action of endo-β-glucanases during malting.20, 37 The actions of proteases, pentosanases,8, 30 and xylanases39 may be important because it has been suggested8 that the walls of cells of the endosperm are a complex of outer middle lamella protein, associated with an outer complex of mainly pentosan and β-glucan, associated with a distinct inner complex of mainly β-glucan.8, 20, 30, 39 Therefore, the degradation of β-glucan during enzymic modification of the endosperm during malting is likely to be controlled by the structure of endosperm cell walls, which will regulate the actions of proteases, pentosanases, and endo-β-glucanases during malting.31
In terms of what has been said about β-glucan breakdown, in the malting grain, β-glucan breakdown is very rapid at Day 2 and Day 3 of malting. However, such malts (Figure 5.4a and b) still appear to contain under-modified barley-like areas. Brewing with such short-grown malts can cause β-glucan-related problems. Therefore, malt samples containing short grown malts may cause β-glucan-related problems even though they meet analytical specifications. It has been reported that the β-glucans from short grown malt can be more troublesome during brewing than corresponding β-glucans from raw (un-malted) barley.8
Studies of factors that influence the homogeneity of malt modification (Figure 5.4a) suggest that the under-modified grains of a malt sample always contain higher levels of high molecular weight β-glucans and nitrogen (protein) than well-modified grains.19, 36 The blending of high protein (steely) barleys with low protein (mealy) barleys is likely to produce malts whose modification is not homogeneous.20, 42 However, it should be noted that different levels of soil nitrogen can result in different levels of nitrogen in the grains of a sample of malting barley. This natural difference in grain nitrogen can widen the in-homogeneity of modification of the derived malt. The blending of malts of different modifications to arrive at specifications should not conceal under-modification. The in-homogeneity of modification in grains in samples of malts is not always revealed by standard malt analyses.20, 35, 42 However, processing of unevenly modified malts in the brewhouse can cause unexpected problems such as slow wort separation and slow beer filtration. Haze development may also be a feature of uneven malt modification. The addition of extraneous commercial enzymes to the mash tun can remove problems caused by the in-homogeneity (unevenness) of malt modification.43, 44 Beer containing low levels of malt (less than 67%) is called Happoshu beer in Japan. Malts used at low addition should be well-modified and homogeneous. Although Happoshu beers provide tax benefits, beers brewed at lower costs from un-malted barley such as the Kenyan beer Senator (personal experience) are brewed to widen affordability and to reduce illicit and unregulated production of alcoholic drinks.
Kilning of malt inactivates many microorganisms and reduces the moisture content of the undried green malt from about 43% to about 5%. This reduction in moisture stabilizes the grain and permits long-term storage. During the early stages of kilning, rapid water loss reduces malt temperatures, and malt modification continues to a limited degree until excessive water loss stops enzymic action. Malt types are determined, in the main, by kilning or roasting procedures (Tables 5.1 and 5.2). During kilning, there is development of color, a reduction in enzyme activity, and an increase in acceptable flavors. Color development results from reactions between sugars and amino acids of the malt to form melanoidins (Maillard reaction).8, 21 Some of these products of roasting treatment are described as reductones and have antioxidant properties, which are important with regard to improving beer stability and as a source of antioxidants in the diets of beer drinkers. Minerals and vitamin B from malt are also important additions to the diets of those who drink beer. During kilning, the activities of endo-β-1,3:1,4-glucanase, β-amylase, limit dextrinase, and endoproteases are reduced to greater degrees than the activities of enzymes such as α-amylase and carboxypeptidase. The activities of troublesome lipoxygenases, which can promote oxidation and the staling of beer, are also reduced.
Table 5.1 Average Analysis of Colored and Roasted Malts and Barley
|
Extract (L/kg) |
Moisture (%) |
Color (°EBC) |
Final Kilning Temperature (°C) |
|---|---|---|---|---|
Ale |
305 |
4.0 |
5.0 |
100 |
Lager |
300 |
4.5 |
2.0 |
80 |
Light crystal a |
265 |
7.0 |
25–35 |
75 |
Crystal malta |
268 |
4.0 |
100–300 |
75 |
Amber/brown malta |
280 |
2.0 |
100–140 |
150 |
Chocolate malta |
268 |
1.5 |
900–1100 |
220 |
Roasted malta |
265 |
1.5 |
1100–1400 |
230 |
Roasted barleya |
270 |
1.5 |
1000–1550 |
230 |
a These malts and barleys do not contain enzymes.
Table 5.2 Average Malt Analysis of Different Kinds of Malts
Analyses |
Analytica EBC Analysesa |
Analysesa |
Analyses |
IOB Analysesb |
||||
|---|---|---|---|---|---|---|---|---|
Pilsner |
Lager |
Munich |
Wheat |
Lager |
Ale |
Distillers’ |
||
Moisture, % |
4.0 |
4.0 |
4.0 |
5.0 |
Moisture % |
4.5 |
4.0 |
4.0 |
Extract, % |
80.0 |
81.0 |
80.0 |
84.0 |
Extract (l°/kg) |
300 |
305 |
310.0 |
|
|
|
|
|
pH |
5.9 |
5.6 |
5.9 |
Fine/coarse difference, % |
2.0 |
1.5 |
1.0 |
1.0 |
Fine/coarse difference (L°/kg) |
5.0 |
2.5 |
1.0 |
Color, °EBC |
2.0 |
2.0 |
15.0 |
3.0 |
Color, °EBC |
2.0 |
5.0 |
2.0 |
α-Amylase (DU) |
35.0 |
35.0 |
28.0 |
45.0 |
α-Amylase (DU) |
35.0 |
30.0 |
38.0 |
Diastatic power |
250.0 |
250.0 |
100.0 |
300.0 |
Diastatic power |
70.0 |
65.0 |
75.0 |
Windisch–Kolbach |
76.0 |
76.0 |
33.0 |
90.0 |
β-Glucanase (IRV units) |
700 |
500 |
700 |
Total protein, % |
11.0 |
10.5 |
11.0 |
13.0 |
Total nitrogen, % |
1.7 |
1.6 |
1.5 |
Kolbach Index |
40.0 |
42.0 |
45.0 |
42.0 |
Index of modification |
38.0 |
40.0 |
39.0 |
Friability, % |
87.0 |
87.0 |
88.0 |
- |
Friability, % |
88.0 |
92.0 |
90.0 |
Homogeneity, % |
98.0 |
98.0 |
98.0 |
- |
Anthocyanogens (ppm) |
55.0 |
50.0 |
60.0 |
Whole grain, % |
2.0 |
2.0 |
2.0 |
- |
Polyphenols (ppm) |
150.0–410.0 |
140.0– |
150.0 |
Note: α-Amino nitrogen generally >160 mg/L at 1.6% nitrogen. Grain size ≥2.5 mm.
TSN, total soluble nitrogen (%); TN, total nitrogen (%).
a Programmed mashing (European and North American malts).
b 65°C mashing.
The dimethyl sulfide (DMS) levels in beer (e.g., 50–100 ppb) are related to the levels of its precursor, S-methylmethionine (SMM), in the malt. DMS is an important flavor compound of lagers but is not usually found in ales. Malts kilned below 65°C can develop high levels of DMS in hot worts. Malts kilned between 80°C and 82°C develop DMS, from dimethyl sulfoxide (DMSO, 5.0 ppm) during the fermentation process. N-Nitrosodimethylamine (NDMA) is a carcinogenic compound that can develop during malting when hordenine in the embryo reacts with nitrogen oxides in kilns fired directly by sulfur-free natural gas.8,21 Sulfur dioxide from burnt sulfur and organic fuels reduces nitrosamine development. Indirect kilning can do the same. Commercial malts tend to have less than 1.0 ppb of NDMA. Ethyl carbamate is another carcinogenic compound that originates from malted barley but develops during the distillation process. Its control can be effected through the use of barley varieties that have low levels of natural cyanide precursors. Some carcinogens are regarded as safe for human consumption at low levels. However, with some carcinogens, low levels means low risk. Acrylamide is also a carcinogen. It can be produced in grain products such as adjuncts, malts, and roasted grains that are heated during production. Kilning and roasting of malts and grain above 120°C can cause the production of acrylamide. Acrylamide is produced from the reaction between the amino acid asparagine and reducing sugars that occur in un-malted and malted grains. Asparagine tends to be associated with the globulin proteins of the aleurone layer and the embryo.45 Because the level of asparagine is reduced during kilning,46 acrylamide levels in kilned malts may be associated with this decline in asparagine.
5.3.3 Malt Types
Different kinds of malts are available to the industry. Distilling malts fall into two main categories: malts used for malt whiskey production and malts used for grain whiskey production.11 Both malts are lightly kilned and have high diastatic power (β-amylase activity). Another type of distilling malt is unkilned and is known as green malt. Green malt has a higher enzyme content than kilned malt and is used to produce grain whiskey. The amylase potential of grain distilling malts tends to be higher than that of malt whiskey malts because grain distilling malts are required to convert about 90% of cooked, but unmalted, cereals such as wheat or maize. Distilling malts used for the production of malt whiskey can be peated by passing peat smoke through them during kilning.8, 11, 21 Peat levels reflect the quantities of phenols (about 1–55 ppm) present on the malt. A primary feature of distilling malts is that they should have high levels of fermentable extracts, which give high spirit yields.
The large varieties of colors and types of beers have helped beer to remain the most popular alcoholic drink in the world. Beer colors are related to kilning and roasting of different malt and un-malted cereals. Lager and pilsner malts, like distilling malts, have low colors of about 2 °EBC (European Brewery Convention) units and have high enzyme activities. Ale malts can have colors of about 5 °EBC units. Mild ale malts have colors of about 7 °EBC units. The diastatic power of lager (DP°L) and distillers’ malts are usually higher than those of ale malts. Malt worts (10% solid materials—1040 specific gravity) contain about 91% carbohydrate and about 6% protein. The high levels of the disaccharide maltose found in worts (45%–50%) reflect the dominant actions α-amylase, limit dextrinase, and mainly β-amylase during mashing.
Munich malts have colors of about 15 to 20 °EBC units, and their enzyme levels are much lower than those of lager and ale malts. Vinegar or food malts, similar to lager malts, also have low colors and high enzyme potential. Special malts are made from kilned ale type (white) malts (color about 3 °EBC). These are heated to different temperatures to produce the required types of malts. For example,8, 21 amber/brown malts (nutty, biscuit flavors) have colors of about 40 to 100 °EBC units; chocolate malts (treacle, chocolate flavors) colors range from about 900 to 1200 °EBC units; roasted (black) malts (smoky, coffee flavors) have colors that range from about 1200 to 1500 °EBC. Malts such as: light crystal (sweet, nutty, toffee flavors); crystal (sweet, malty, caramel flavors); and dark crystal (burnt, caramel flavors) have colors (straw, blonde, golden, red, amber-brown, and dark) that range from 20 to 60, 100 to 250, and 300 to 400 °EBC units, respectively. These can be produced from green (unkilned) malts. Roasted barley (burnt, smoky flavors) is an important ingredient of stouts and some ales, is produced from barley, and will have colors of about 1100 to 1500 °EBC. The colors produced in these malts relate to the kilning temperatures used (Table 5.1). Because of the higher kilning temperatures used to produce them, special dark-black malts and roasted barley contain no enzyme activity. Malts are used to provide extracts that are used by yeast to produce alcohol and flavor compounds. Some specialty malts provide mainly color and flavor compounds. The significant development of extracts from different beers by Pure Malt Products, Haddington, Scotland, is providing brewers and the food industry with a range of extracts that widen the colors and range of flavors of commercial beers.
In general, hot water extract is one of the most important parameters in malt specifications. To achieve the potential extract of a malt in the brewhouse is a vital part of the economics of the industrial processing of malt. However, recent studies show that although extract yield may be optimal, in-homogeneity of endosperm modification may conceal serious processing problems.20, 42, 47 For example, it was observed that a malt sample with 0.3% total β-glucans had 0.2% β-glucans in 80% of its grains but 0.7% β-glucans in the remaining 20% of its grains. Irrespective of the details of the malt specification set by the brewer or the distiller, such unevenly modified malts can cause processing problems. Therefore, one of the major problems facing the industry is that traditional analyses of malt do not always reflect potential brewhouse performance.20, 31, 42 For example, the soluble nitrogen ratio (total soluble nitrogen divided by total nitrogen, or TSN/TN) does not reflect the demographics of protein breakdown in the malted grain. The friability test is a very useful addition to other modification methods, but a partly modified fragmented endosperm can have similar friability results to that of a modified endosperm. In addition, the friable flours of different malt samples can have different levels of β-glucans. This should not be the case because friable flours are assumed to come from modified areas of the endosperm of the malted grain.8 Distillers deal with the problem of modification by specifying spirit yield. At usual grain size (about 2.5 mm), lower than expected spirit yield reflects “under-modification.” In general, optimal malt modification is likely to be achieved when progressive solubilization of cell wall matrix protein and storage proteins in the endosperm during malting are associated with the breakdown of endosperm cell walls and the release of modified brewers’ extract from the entire grain.8, 20 In this regard, factors that control the distribution of protein breakdown play roles in determining malt modification and other aspects of malt quality.
5.4 SPECIAL BARLEY VARIETIES: MARIS OTTER, A DESCENDANT OF PROCTOR BARLEY
There are many special malting barley varieties from the past that are being used to make specialty malts today, such as Klages, Golden Promise, and Maris Otter. With regard to the quality of Maris Otter, it is a two-rowed winter barley that was bred from Proctor, a high-quality spring barley, by G. D. H. Bell.48 This variety has been available since 1965 and has been in use since 1966. Other high-quality malting barleys bred from Proctor are Clipper and Chariot. Maris Otter is famous for its consistent quality in terms of ease of modification and high expected extract development. Research work on the malting potential of Maris Otter30,31 suggested that the endosperm of Maris Otter, unlike the corresponding endosperms of other high-quality malting barleys, retained its potential to modify its endosperm readily over a nitrogen range of 1.5% to 1.8% (personal observation). In a barley quality sedimentation test,8, 30 the loosely packed (mealy-like) structure of the endosperm of Maris Otter and Proctor produced very high (cloudy) sedimentation scores (see Figure 5.5). Barley varieties such as Proctor, Maris Otter, and Mazurka, which had high sedimentation scores, malted faster and more uniformly than low-quality varieties with low sedimentation scores such as Lofa Abed, Julia, and Maris Mink. Low sedimentation scores were linked to high starch-protein compaction (similar to steeliness). In contrast, the endosperms of barley varieties such as Maris Otter and Proctor had endosperms that had looser starch-protein compaction than other varieties of malting barleys at similar nitrogen (protein) levels.
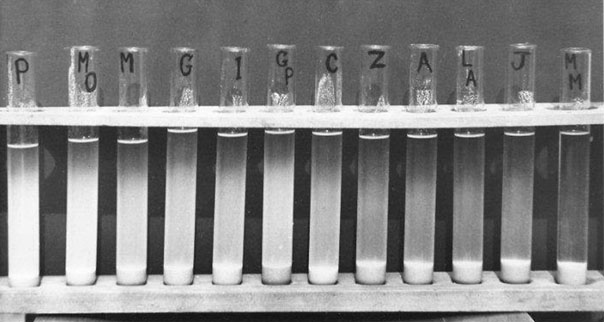
Figure 5.5 Sedimentation behavior of the milled endosperms of barley varieties.8, 30 Milled flours were allowed to sediment in alcohol solution. Note that the looser endosperm structures of the high-quality barleys, Proctor and Maris Otter, gave the highest degrees of cloudiness (released starch). A = Atem; C = Clipper; G = Gerkra; GP = Golden Promise; I = Imber; J = Julia; LA = Lofa Abed; M = Mazurka; MM = Maris Mink;MO = Maris Otter; P = Proctor; Z = Zepher.
Comparative research showed that the inner β-glucan cell walls of isolated cells of Maris Otter expanded to a greater degree than those of the low-quality barley Julia, suggesting that a better hydration (wetting) potential may exist in Maris Otter, which would facilitate enzymic modification of the endosperm.8, 30 The isolated β-glucans of Maris Otter were less viscous than those of Julia. An important quality parameter of Maris Otter as a malt is that, at a soluble nitrogen ratio of 40%, it can produce a higher extract and friability than other high-quality barley varieties at similar soluble nitrogen ratios. The levels of proteases and endo-β-glucanase enzymes in Maris Otter are not significantly greater than other varieties; therefore, the structure of its starchy endosperm8, 30 must be an important factor in its ability to malt well consistently. Despite the special malting quality of barleys such as Maris Otter and Proctor, malts of excellent malting quality can be produced from other high-quality malting barleys.
A high-quality product is one that meets the expectations of the customer. If traditional malt analyses do not indicate the processing quality of purchased malt, then a radical improvement in the precision of the analyses that are used to define malt quality is required.8, 20–23 Accuracy relates to the repeatability of analyses of a malt sample; analytical precision reflects the true state of modification of grains of a malt sample and the overall potential of these gains to process well or poorly in the brewhouse. This kind of practical precision in analysis is essential if brewers or distillers are to produce high-quality products economically and efficiently. Incidentally, in terms of commercial practice, it was informed recently by a large brewing company that despite carrying out routine analyses, the company routinely hand-sections the distil (nonembryo) ends20 of the grains of purchased malts to gain additional information on the homogeneity of malt modification (Figure 5.4a and b). Therefore, basic manual skills are not only important in the craft sector of the industry, they are also important in the entire industry!
5.5 RESEARCH AND DEVELOPMENT
The development of precision analyses requires scientific information that is usually derived from research work on the structure, function, and processing of cereal grains and malts. For example, research is in progress to develop a new test for pregermination using single grain sections and amylase analysis. Results showed that although 2% of barley grains had high levels of amylase, they showed no growth but caused a reduction in starch viscosity similar to that caused by a grain sample that had 5% pregermination, assessed as grain growth. Also, wheat or barley grains that had similar levels of growth had different levels of amylase. These results suggest that embryo growth or viscosity does not provide the best possible assessment of pregermination. To arrive at an improved assessment of pregermination, the amylase activity of single grains should be determined. Here, preliminary work relating to amylase activity, single grain analysis, and grain sectioning are being combined to improve precision of analysis and better knowledge of pregermination.
In order to gain more information on malt modification, a two-temperature mashing process was developed.36, 47 Mashes were carried out at 40°C and then at 75°C. The worts from the first mash of 40°C were discarded because past research studies8, 30, 35, 47 showed that 40°C extracted β-glucans are likely to be less troublesome in the brewing process than β-glucans extracted subsequently at 75°C. It was found35 that when β-glucans from the worts of the 75°C mashes were precipitated using ammonium sulfate, worts made from malts containing 0.2% to 0.27% total β-glucan released less than 100 mg/L of precipitated β-glucan. In contrast, corresponding worts made from malts that contained 0.3% to 0.36% total β-glucan released more than 100 mg/L and as much as 250 mg/L of precipitated β-glucan. This preliminary study suggests that malt samples that contain more than about 0.3% β-glucans could contain high levels of hot water-extracted β-glucans. These β-glucans can have high molecular weight properties8, 47 and could cause processing problems in the brewhouse. In this regard, malts with less than 0.3% β-glucan should not have β-glucan-related problems. However, if this happens, the homogeneity of modification should be checked. The Palmer-Wang36 wort β-glucan precipitation method could also be used to determine if such malts contained significant levels of 75°C hot water-extracted β-glucans.
Past practices,1, 34 and a recent study of the history of the malting industry in the United Kingdom,49 suggest that developments in processing are linked to innovative research that has the potential to meet commercial objectives and develop the industry. Future developments in the industry should take into account the wide range of malts required by modern industry. In this regard, craft malting requirements1, 34, 48 should be included in the scientific work required to sustain the economy and technology that will ensure that raw materials and products continue to meet the expectations of customers.3, 8, 20, 21, 30, 36
ACKNOWLEDGMENTS
The author thanks Miss C. Brown for help with preparation of this manuscript. This work is dedicated to Samuel Whitbread II (1764–1815) and Sir Thomas Fowell Buxton (1786–1845), who were both brewers and slavery abolitionists.
REFERENCES
1. Morris, A., Common Brewer, A Treatise on Brewing; The Art and Mystery of Brewing, Sherwood, Neely and Jones, London, pp. 1–179, 1815.
2. Palmer, G. H., Malting, in The Encyclopaedia of Seeds: Science, Technology and Uses, Black M., Bewley, D.T. and Halmer, P., Eds., CAB International, Wallingford pp. 396–404, 2006.
3. Bringhurst, T. A., 125th anniversary review: Barley research in relation to Scotch Whisky production: A journey to new frontiers, J. Inst. Brew., 121: 1–18, 2015.
4. Goode, D. L. and Arendt, E. K., Developments in the supply of adjuncts materials for Brewing, in Brewing—New Technology, Bamforth, C. W., Ed., Woodhead Publishing, Cambridge, pp. 31–37, 2006.
5. Dezelak, M., Zarnkow, M., Becker, T. and Kosur I.J., Proceedings of bottom fermenting gluten-free beer-like beverages based on buckwheat and quinoa malts made with chemical and sensory characterisation, J. Inst. Brew., 120: 360–370, 2014.
6. Schnitzenbaumer, B. and Arendt, E. K., Brewing with up to 40% unmalted oats (Avena sativa) and sorghum (Sorghum bicola): A review, J. Inst. Brew., 120: 315–330, 2013.
7. Agu, R. C. and Palmer, G. H., A reassessment of sorghum for lager beer brewing, Bioresour. Technol., 66: 253–261, 1998.
8. Palmer, G. H., Cereals in malting and brewing, in Cereal Science and Technology, Palmer, G. H., Ed., Aberdeen University Press, Aberdeen, pp. 61–242, 1989.
9. Palmer, G. H., Structure of ancient cereal grains, J. Inst. Brew., 101: 103–112, 1995.
10. Faltermaier, A., Waters, D., Becker, T., Arendt, E. K., and Gastl, M., Common wheat (Triticum aestivum), J. Inst. Brew., 120: 1–15, 2014.
11. Palmer, G. H., Beverages: Distilled, in Encyclopedia of Food Grains, Vol. 3, 2nd ed., Academic Press, Cambridge, pp. 193–205, 2016.
12. Sage, G. C. M., Cereal breeding and quality, in Cereal Science and Technology, Palmer G. H., Ed., Aberdeen University Press, Aberdeen, pp. 37–60, 1989.
13. Jacks, P. L., Application of DNA probes (RFLPs) in barley breeding, Eur. Brew. Conv., Monograph XV—EBC Symposium, Plant Biotechnology, Helsinki, Finland, pp. 130–136, 1989.
14. Ritala, A., Aspegen, K., Kurtin, U., Salmentallio-Nattila, M., Mannonen, L., Hannus, R., Dauppinen, V., Teeri, T. H., and Enari, T.-N., Fertile transgenic barley by particle size bombardment of immature embryos, Plant Mol. Biol., 24: 317–325, 1994.
15. Fincher, G. B. and Langridge, P., Genomics, in Encyclopaedia of Grain Science, Vol. 2, Wrigley, C., Cooke, H. and Walker, C. E., Eds., Academic Press, Cambridge, pp. 16–24, 2004.
16. Faris, J. D., Friebe, B. and Gill B. S., Genome mapping, in Encyclopaedia of Grain Science, Vol. 2, Wrigley, C., Cooke, H. and Walker, C. E., Eds., pp. 7–16, 2004.
17. Dorocicz, W. M. and Kasha, K. J., Use of microsatellite DNA to distinguish malting and non-malting barley cultivars, J. Am. Soc. Brew. Chem., 55: 107–111, 1997.
18. Duke, S. H., Comparisons of amylolytic enzyme activities and β-amylase with differing Bmyl Intron 111 allies to osmolyte concentration and malt extract during congress mashing with North American barley cultivars (1), J. Am. Soc. Brew. Chem., 71: 193–207, 2013.
19. Jones, J. M. and Jones, C. I. M., Genetically modified grains and consumer, in Encyclopedia of Grain Science, Vol. 2, Wrigley, C., Cooke H. and Walker, Eds., Academic Press Cambridge, pp. 1–7, 2004.
20. Palmer, G. H., Achieving homogeneity in malting, Proc. Eur. Brew. Conv. Congr., Cannes, IRL Press, Oxford, pp. 323–363, 1999.
21. Briggs, D. E., Sole, S. M. and Latham P., Tetrazolium staining, mitochondria and barley quality, J. Inst. Brew., 115: 41–48, 2009.
22. Brissart, R., Brauminger, U., Haydon, S., Morand, R., Palmer, G., Sanvage, R. and Seward, B., Eur. Brew. Conv. Manual of Good Practice, Malting Technology, Fachverlang Hans Carl, Nürenberg, 2000.
23. Ryder, D. S. and Vogelsang, F., Adopting malt quality to modern brewing techniques, Eur. Brew. Conv. Symp.—Malting Technology, Andernach, Germany, pp. 180–193, 1994.
24. Black, M., Bewely, J. D., and Halmer, P., Mobilisation of reserves—Cereals, in The Encyclopaedia of Seeds: Science, Technology and Uses, CAB International, Wallingford, pp. 421–426, 2006.
25. Davies, N., Malt and malt products, in Brewing—New Technologies, Bamforth, C.W., Ed., Woodland Publishing, Cambridge, pp. 68–100, 2006.
26. Palmer, G. H. and Sattler, R., Different ratios of development of α-amylase in the distal endosperm ends of germinated/malted Chariot and Tipper barley varieties, J. Inst. Brew., 102: 11–17, 1996.
27. Broadbent, R. E. and Palmer, G. H., Relationship between β-amylase activity, steeliness, mealiness, nitrogen content and nitrogen fraction of barley grains, J. Inst. Brew., 107: 349–354, 2001.
28. Agu, R. C. and Palmer, G. H., Some relationships between the protein nitrogen of barley and the production of amylolytic enzymes during malting, J. Inst. Brew., 104: 273276, 1998.
29. Bryce, J. H., McCafferty, C. H., Cooper, C. S., and Brosman, J. N., Optimizing the fermentability of wort in a distillery—The role of limit dextrinase, in Distilled Spirits: Tradition and Innovation, Bryce, J. H. and Stewart, G. G., Eds., Nottingham University Press, Nottingham, pp. 69–74, 2004.
30. Palmer, G. H., The influence of endosperm structure on extract development, J. Am. Soc. Brew. Chem., 33: 174–180, 1975.
31. Palmer, G. H., Jamaica, Modification, Justice, and Industry. J. Am. Soc. Brew. Chem., 59: 75(2): 59–84, 2017.
32. Koliatsou, M. and Palmer, G. H., A new method to assess mealiness and steeliness of barley varieties and relationship of mealiness with malting parameters, J. Am. Soc. Brew. Chem., 61: 114–118, 2003.
33. Gous, P. W., Gilbert, R. G. and Fox G. P., Drought proofing barley (Hordeum vulgare) and its impact on grain quality. A review, J. Inst. Brew., 121: 19–27, 2015.
34. Reports on Scotch Barley Malt & Bigg (rate of duty payable on Malt from England grown barley and Scotland grown barley and Bigg), 1804–1806.
35. Letters, R., Carbohydrate gels: A problem with newer brewing techniques, Proc. 5th Scient. and Tech. Convention, Institute of Brewing (Central and South African Section) pp. 115–121, 1995.
36. Palmer, G. H. and Wang, Y., A new β-glucan method which measures malt modification, Brewer Distiller, September, pp. 50–53, 2005.
37. Marins De Sa R. and Palmer, G. H., Assessment of enzymic modification of malting barley using individual grain analysis, malt modification by single grain analysis, J. Inst. Brew., 110: 43–50, 2004.
38. Patel, J. K., Speers, R. and Lake, J. C., Colloidal examination of worts associated with premature yeast flocculation, J. Am. Soc. Brew., 69: 81–89, 2011.
39. Kanauchi, M., Chijimi A., Ohnishi – Kamejama, M., and Bamforth, C.W., An investigation of two xylan-degrading enzymes and a novel xylanase inhibitor in malting, J. Inst. Brew., 119: 32–40, 2013.
40. Palmer G. H., An introduction to barley abrading; SIMON barley abrader, Selling Agents for Henry Simon Ltd., Norfolk, 1972.
41. Northam, P. C. and Button, A. H., The commercial advantages of malting abraded barley and brewing with abraded malt, Proc. Eur. Brew. Conv. Congr., Salzberg, Elsevier, Amsterdam, pp. 99–110, 1973.
42. Palmer, G. H., Malt performance is more related to in-homogeneity of protein and β-glucan breakdown than to standard analyses, J. Inst. Brew., 106: 189–192, 2000.
43. Lalor, E., Creating the future in brewing, Version One, Quest International, Cork, Ireland, pp. 2–53, 2002.
44. O’Rouke, T., Enzymes in brewing, Brewing Distilling Int., 11: 20–34, 2015.
45. Robert, F., Vuataz, G., Pollien, P., Saucy, F., Alonso, M.I., Bauwens, I. and Blank, I., Acrylamide formation from asparagines under low-moisture Maillard reaction condition. 1. Physical and chemical aspects in crystalline model systems, J. Agric. Food Chem., 52: 6837–6842, 2004.
46. Harris, G., The Structural Chemistry of Barley and Malt in The Structural Chemistry of Barley and Malt, Cook, A. H., Ed., Academic Press, New York , pp. 431–582, 1962.
47. Palmer, G. H. and Agu, R. C., Effect of mashing temperatures and endo-β-glucanase on β-glucan content of malt worts, J. Inst. Brew., 105: 233–235, 1999.
48. Thomas, D., The Craft Maltsters Handbook, White Mule Press, Hayward, CA, p. 198, 2014.
49. Clark, C., The British Malting Industry Since 1830, The Hambledon Press, London, 1998.