6
THE RIVER OF LIFE
AS THE FIRST ORANGE-YELLOW HUES OF DAYLIGHT illuminated the California coast, a great horned owl flapped hurriedly past the window, presumably headed to daytime sanctuary. Stars were still plentiful. Off in the east, Venus sat perched like a beacon just above the horizon, looking more like an oncoming 747 than a planetary member of our solar system. A short time later, the sun climbed above the Marin headlands, revealing the Pacific surf pounding the rocky coastline. Above this grand juxtaposition of land and sea, six turkey vultures soared in broad, sweeping arcs, seemingly in silent celebration of the new day. Two coal-black ravens squawked at each other from a nearby Monterey pine. From the corner of my eye, I detected movement and shifted focus to just beyond the window. An Anna’s hummingbird, replete with emerald green back and scarlet head and throat, had arrived to sip from the feeder. After consuming its fill, at least for the moment, this 3-inch ball of feathers and energy lifted off and hovered, its tiny wings beating something like 50 times per second. Suddenly, the iridescent apparition was gone. Were I to venture down to the nearby estuary on this day, I would see many other birds, potentially dozens of species, with evocative names like black-necked stilt, Caspian tern, belted kingfisher, marbled godwit, and long-billed dowitcher.
The remarkable truth of the matter is that all of these creatures are flying dinosaurs. To the best of our knowledge, every bird species past and present can be traced to a single common ancestor that evolved perhaps 160 million years ago from dinosaurian stock among small, bipedal carnivores akin to Velociraptor. Contrary to popular belief, then, dinosaurs didn’t go extinct 65.5 million years ago. Represented by about ten thousand living species, avian dinosaurs still rank among the most successful groups of vertebrates. Despite millions of years of separation, today we are directly connected to the world of dinosaurs through these wondrous volant creatures. I still find this idea stirring, not just intellectually but on a deeper level of awareness. And it makes me chuckle to contemplate all of those binocular-toting members of the Audubon Society as dinosaur lovers.
But how do we know? How can we paleontologists be so sure that birds are linked through direct evolutionary ties to a particular group of animals that lived millions of years ago?
Archaeopteryx, the oldest and most primitive bird, was discovered in 1860, just one year after the publication of Darwin’s famous treatise on evolution, On the Origin of Species. Archaeopteryx is presently known from ten specimens, including several nearly complete skeletons, all recovered from the Solnhofen limestones in Germany, which date to the Late Jurassic (about 155 million year ago). These same deposits have revealed a diverse variety of animals, among them a small theropod dinosaur named Compsognathus. Archaeopteryx and Compsognathus are so similar that specimens of each have been confused for the other multiple times over the years. Particularly given the timing of its discovery, Archaeopteryx made an ideal transitional fossil, providing strong support for Darwin’s revolutionary theory that all life is related. On the one hand, this Jurassic bird has several primitive, reptilian features, including teeth, three separate clawed fingers, and a long bony tail. On the other, it possesses specialized bird features such as feathers, fused clavicles, and an elongate forelimb modified into a wing. Various nineteenth-century scientists picked up on the transitional nature of this ancient avian and argued that birds are closely related to dinosaurs, perhaps even their direct descendants. Chief among these people was the famed biologist T. H. Huxley.
Yet for most of the twentieth century, an alternative view of bird origins prevailed. In 1926, Danish artist and paleontologist Gerhard Heilmann published a landmark work titled “The Origin of Birds.” Heilmann agreed that Archaeopteryx and other birds were clearly similar to theropod dinosaurs, but he dismissed the notion of bird ancestry from dinosaurs because dinosaurs apparently lacked clavicles (the collarbones of humans). In birds, the clavicles fuse into a single element, the FURCULA or wishbone. In keeping with the prevalent idea that evolution could not retrace its steps, Heilmann argued that, once lost, these elements could not have reevolved. He concluded that bird origins were to be found among more primitive members of Archosauria—the larger, more encompassing group that includes dinosaurs, crocodiles, and birds. It was presumed that fossil representatives of the specific group that gave rise to birds had simply not yet been discovered.
Enter John Ostrom, whose work in the 1960s and 1970s provided the catalyst for the dinosaur renaissance described in chapter 1. Following his description of the “raptor” dinosaur Deinonychus, Ostrom reignited the bird origins debate through several papers that detailed specialized anatomical features shared between birds and certain raptorlike carnivorous dinosaurs. Subsequent workers have added many more characteristics to this list, such that the total number today exceeds one hundred. Indeed, we now know that many features traditionally associated with birds evolved in the prebird theropod ancestor. For example, like birds, carnivorous dinosaurs have thin-walled limb bones and air-filled bones within the vertebral column. Additional evidence supporting the dinosaur-bird connection includes growth patterns, nesting behavior, and even sleeping behavior.
But what of Heilmann’s objection regarding the absence of clavicles in dinosaurs? Over the past few decades, clavicles have been found in a broad range of theropod specimens, including those thought to be closest to the origin of birds. So this objection has been removed.
By far the most convincing evidence supporting the notion that birds are directly descended from dinosaurs are the numerous transitional fossil forms, the so-called dino-birds, discovered over the past 15 years. Most spectacular of the recent discoveries are numerous forms unearthed from Cretaceous-aged rocks in Liaoning Province in northeastern China. Like the specimens found in the Solnhofen quarries of Germany, the Chinese fossils show exquisite preservation, not only hard tissues like bones and teeth but also soft tissues such as skin impressions and feathers.
Biology has few absolutes, but even kids know that fishes have gills, mammals have fur, and birds have feathers—at least so the story goes. In a world where all species are created independently of one another, this might be true. But in a Darwinian world, we expect transitional forms that blur the lines. Many of the Liaoning fossilized feathers occur on exquisitely preserved birds such as Confuciusornis. Yet many others are associated with the skeletons of nonbird dinosaurs. To date, more than a dozen different kinds of feathered nonavian dinosaurs have been found in China, and that number increases with every passing year. One of the most exciting examples is Microraptor gui, a diminutive dromaeosaur theropod bearing feathers not only on its forelimbs, but on its hindlimbs as well. In the initial scientific paper describing Microraptor, Xu Xing, a paleontologist at the Institute of Vertebrate Paleontology and Paleoanthropology in Beijing, and his colleagues resurrected an old idea, hypothesizing that the origin of flight in birds included a four-winged gliding stage before evolution honed the system to a pair of wings up front. So even feathers, previously the quintessential avian feature, now cloud the boundaries between two major groups of organisms.1
Evolution can’t make something from nothing. Instead, it modifies existing structures. For example, both mammal hair and bird feathers are highly modified scales. The Liaoning dino-bird discoveries supply important insights into the evolution of feathers, from very simple, hairlike structures to complex flight feathers. Moreover, the discovery of feathered, ground-dwelling dinosaurs indicates that feathers originally evolved to serve some function other than flight. The most likely alternatives are control of body temperature and courtship/display. Together with the evolution of feathers, the origins of flight in birds entailed wholesale transformation of the forelimb into an elongate wing capable of flapping flight. Related changes included loss of the bony tail, reorganization of the hindlimb muscles, and modification of the foot for perching instead of running.
Archaeopteryx and the many recent dino-bird discoveries are often referred to as “missing links.” This unfortunate term is a double misnomer. Most trivially, because they are now in our possession, these finds are clearly no longer missing. More fundamentally, few if any of these fossils qualify as links in the sense of being part of the ancestor-descendant lineage leading from dinosaurs to modern birds. In the previous chapter, we found that the term food chain was a poor descriptor of the flow of energy through an ecosystem; rather, the degree of life’s interconnectedness means that the web metaphor is much more useful for understanding ecological dynamics. The term missing link similarly invokes a linear chain—in this case, a chain of relationships, with primitive ancestral forms evolving through a ladderlike progression into more advanced descendants. Yet reality is once again much more messy and interesting. The history of virtually any major group, and for life as a whole, is better conceived as a densely growing bush than a series of step-by-step, linear progressions. The new dino-bird discoveries are filling in some of the branches near the base of the avian family tree, showing that this particular part of the foliage was bushy indeed.
The dinosaur-to-bird story is one of thousands of narratives relating to the history of life on Earth. All life on this planet belongs to a single extended family, descended from a common ancestor that lived between 3.5 billion and 4 billion years ago. Darwin’s great contribution was to firmly establish these evolutionary ties in a reasoned, well-supported theory, resulting in a fundamental shift in our perception of the world and the human place within it. The underlying tenet of the theory of evolution is that species are not fixed or immutable. All species have ancestors and relatives, and many pass on descendant forms. For example, domestic cats and panthers come from a common stock of feline mammals, and among their close relatives are extinct forms like saber-toothed cats. I examine this idea in some detail later because knowledge of a few evolutionary basics is prerequisite to understanding the dinosaurian odyssey.
Darwinian evolution forms the conceptual bedrock of the life sciences and is resoundingly accepted by the scientific community. However, outside the realm of science, considerable doubt and misunderstanding persist about this revolutionary idea. A number of recent polls indicate that only about a third of the U.S. population regards the theory of evolution to be well supported by the evidence. These same polls show that about two-thirds of respondents favor teaching alternatives to evolution—for example, intelligent design—along with evolution in science classrooms. Yet no scientific alternatives account for the diversity of life through time. Evolution is often maligned as “just a theory,” as if to say that it is no more than our current best guess. In contrast to its vernacular usage, however, the word theory as applied in science does not imply mere speculation; it refers instead to an interconnected set of hypotheses that have withstood numerous attempts at falsification. Descent with modification—the notion that all living and extinct species share a common ancestry—is one such theory, accepted with the same degree of confidence as the theories of gravity and relativity.
Why are scientists so confident about the evolutionary ties that link all life on Earth? Because over the past one and a half centuries, biologists have amassed a veritable mountain of evidence in support of Darwin’s “dangerous idea.” In brief, this evidence includes the following:
Biological structures, from genes to gross morphology, support the same major evolutionary groupings. With few exceptions, organisms thought to be close relatives on the basis of gross anatomy are also closely similar in their genes (DNA sequences)—for example, horses and zebras, or lions and tigers. Sometimes these genetic similarities are even closer than first anticipated; for example, chimpanzees and humans share about 95 percent of their genes, which means that we are more closely related to each other than either of us is to gorillas! The reverse pattern is typically true, too; the more dissimilar the body types and presumed relationships, the more dissimilar their DNA (genomes). Occasional exceptions to this genetics-anatomy linkage do not disprove evolution; on the contrary, they point out interesting and unexpected relationships that further our understanding.
Organisms within a given group have been modified from a single ancestral form. All bodily structures represent the culmination of deep time interactions between organisms and environment. Evolutionary theory predicts that related organisms will share features derived from common ancestors. Such shared characteristics passed on from an ancestor to multiple descendants are known as HOMOLOGIES. For example, frogs, birds, rabbits, and lizards all have different forelimbs, each reflective of a unique lifestyle. But those different forelimbs all share the same set of elements—a single upper arm bone (the humerus) and a pair of lower arm bones (radius and ulna)—inherited from a common ancestor.2 This same trio of arm bones is present in the vast majority of all living and extinct land-living vertebrates, providing further evidence of common ancestry. The few exceptions are groups like snakes that secondarily lost their limbs.
Organisms have numerous features that make no sense in terms of independent creation or functional design. Because evolution proceeds by modifying preexisting forms, adult organisms possess traits that reflect their evolutionary history. These include vestigial structures, or evolutionary “leftovers,” such as hip bones in whales, nonfunctioning eyes in cave-dwelling creatures, and the appendix in humans. The history of life is replete with examples of evolutionary jury-rigging, in which evolution made the best of a bad situation by transforming raw materials present in ancestral organisms. A classic example is the panda’s thumb. These Asian bears lack a true thumb, which is found only in a few primates. However, in response to a need for stripping shoots from bamboo stalks, evolution modified one of the panda’s wrist bones into a thumb substitute.
Geographic distributions of organisms are best explained through a combination of evolution and physical events, such as continental movements. Perhaps the best known example of this pattern is the distribution of marsupials, with the bulk of modern forms restricted to Australia. The island of Madagascar, featured in chapter 1, is another example, with a largely unique flora and fauna resulting from its ancient isolation in the southern Indian Ocean. In groups of creatures with distributions that encompass two or more continents, such as the primate and deer families, the isolated subgroupings possess many unique, shared features, indicating that ancestral forms migrated long ago and the descendants then evolved along independent paths. On the flip side, comparable ecosystems in different parts of the world often include ecologically similar species that appear physically alike, suggesting that evolution has occurred in parallel within entirely distinct evolutionary lineages. A classic fossil example of such CONVERGENT EVOLUTION relates to a pair of distantly related saber-toothed carnivores among mammals—a placental saber-toothed cat from North America and a saber-toothed marsupial from Australia.
Key processes of evolution have been extensively documented by experiment and observation. These processes include mutation, natural selection, and even the origin of new species. Substantial evolutionary changes have now been documented in a variety of organisms. One of the best studies of natural selection in action relates to Darwin’s finches on the Galápagos Islands. Within a given finch species, there is considerable variation in beak sizes. When a drought alters the availability of certain kinds of seeds, individuals with beaks best able to feed on the remaining seeds are able to outcompete and outreproduce their peers in a single generation. In times of plenty, the fortunes of beak types can be reversed. In both instances, the result is a significant shift in average beak size within the population. Equivalent kinds of changes have been observed in a variety of groups, including bacteria, moths, fruit flies, and fishes.
Transitional forms abound in the fossil record. Antievolutionists often decry the lack of transitional fossils. Yet the truth of the matter is that many sequences of transitional, or intermediate, fossils are known, among them the dino-birds described earlier. As noted, although very few of these fossils contributed to the direct line of descent leading to modern forms (remember, the history of life is a bush, not a ladder), they do record key stages in the evolution of their particular groups. Finely preserved examples of such transitional creatures have multiplied rapidly in the past two decades and are now scattered throughout the range of life’s diversity, from plankton and clams to horses and horned dinosaurs. Key exemplars among vertebrates include intermediate forms linking fishes to amphibians, land-dwelling ungulates to whales, and apelike ancestors to upright human bipeds. Not surprisingly, it is this last transition that most troubles antievolutionists, despite the fact that we now have on the order of two dozen species of bipedal hominids that include a wonderful array of forms intermediate between chimpanzee-type apes and modern humans. Today our planet is home to only one species of upright hominid, Homo sapiens. Over most of the past 5 million years, however, at least two, and sometimes four or five, bipedal, humanlike forms existed at any one time. Like the dino-bird example, the human family tree is surprisingly bushy.
The order of appearance of fossils in the geologic record is comprehensible only from an evolutionary perspective. The fossil record reveals a progression from simple to complex—from single-celled bacteria all the way to whales, redwoods, and humans. This pattern cannot be explained by a single creation event or catastrophe, such as a flood. Instead, the appearance and disappearance of species in the fossil record supports the idea of ongoing origins and extinctions. That is, only the simplest organisms are found in the oldest rocks and the most complex life-forms occur only in much younger rocks. Yet simpler forms have not simply disappeared. Instead, representatives of all of life’s major groupings (bacteria, protists, fungi, plants, and animals) have persisted to contribute to the presentday biosphere.
In short, overwhelming evidence confirms that all organisms on Earth—starfish, dinosaurs, mushrooms, petunias, and humans alike—trace their origins to a humble single-celled bacterium perhaps one ten-millionth of a meter in diameter. This fact is astounding and difficult to grasp. Recall, however, that you and I began our lives as a single cell. If nine months is sufficient to transform one cell into a highly complex 7-pound baby, surely it’s conceivable that life’s diversity could arise from a single cell in several billion years!
Charles Darwin was by no means the first evolutionist. Others before Darwin, including his grandfather Erasmus, argued for the changeability of species. Most famous of these early evolutionists was Jean-Baptiste de Lamarck who, in the early 1800s, proposed that species could take on new forms in response to their needs. The textbook example of this hypothesis relates to the giraffe neck. This is a book about dinosaurs, however, so let’s use a more appropriate example—sauropods. As the story goes, primitive, short-necked sauropods were unable to reach succulent leaves in tall trees. Driven by the need for food, sauropods stretched upward to higher and higher branches, producing slightly longer necks during their lifetimes. Those elongate necks would have been passed on to off-spring, which continued the stretching trend in an effort to reach ever higher into the canopy. Over many generations, sauropod necks would become progressively longer until they reached the exaggerated form we are familiar with in animals like Brachiosaurus.
This hypothesis, known as “the inheritance of acquired characteristics,” might seem at first to be an attractive solution. Yet it has a fatal flaw. To use a human example, imagine a man and a woman working out regularly at a gym, lifting weights for years on end. Both decide to become professional bodybuilders, ultimately bulking out to massive proportions. Now suppose that the muscle-bound couple marry and have children. Do you think that their offspring would emerge from the womb looking like mini–Arnold Schwarzeneggers? Would their kids even inherit a greater affinity toward “pumping up” later in life? No, no such simple correlation exists between one’s actions in life and the inherited characteristics of one’s children. The chief lesson here is that evolution operates only on heritable features—in modern parlance, this means features coded in DNA.
Charles Darwin provided the first convincing mechanism for evolution, a process he termed NATURAL SELECTION. Darwin compared selection in nature with artificial selection conducted by breeders of pigeons, plants, and livestock. Breeders preferentially combine varieties that have characteristics of particular interest—for example, larger size in pigs, more elaborate plumage in pigeons, or bigger seeds in cereals. Over time, the breed changes in direct response to this selective force. Darwin argued that nature does the same thing, demonstrating an unconscious bias toward animals best able to compete for limited resources. Offspring vary in characteristics that relate to survival and reproduction, and those variations that are most successful are the ones most likely to be passed on to offspring. Over many generations, numerous features of that species will change in response to this unrelenting external pressure. So, in contrast to the Lamarckian view, common ancestry plays a pivotal role in evolution.
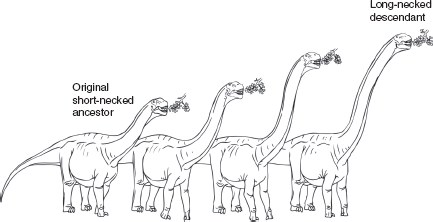
FIGURE 6.1
The evolution of long necks in sauropod dinosaurs. Lamarck hypothesized that evolution of this sort accrued through the inheritance of features acquired in the lifetimes of individuals. In contrast, Darwin recognized that populations rather than individuals evolve. In this example, sauropod dinosaurs with slightly longer necks proved to be more successful than individuals with shorter necks, tending to live longer and pass on more offspring. Over time, this process of change in populations resulted in increasing neck length for species within this lineage.
Darwin’s theory involves two major themes, and it’s important to distinguish between them. The first is a pattern, descent with modification, or the common ancestry of all life on Earth. The second major theme, natural selection, is a process—an explanation for how evolutionary change occurs over time. The great bulk of the scientific community embraced descent with modification within a few decades after the publication of Darwin’s Origin, and today virtually no professional biologists question the veracity of evolution as a guiding principle of their field. (Ironically, descent with modification is the most controversial part of the theory of evolution among the general public, largely because of its implications for the origin of humans.) What scientists continue to examine is Darwin’s second theme, natural selection. Although there is strong agreement that natural selection is a key factor directing evolutionary change, lively debate persists about its efficacy and the possible roles of other factors.
Darwin’s second theme, natural selection, is founded on three fundamental premises. First, individuals within a population vary in their expressions of numerous heritable traits. Variation within species has long been known to breeders of plants and animals who establish new varieties—of cats, pigeons, wheat, cotton, corn, and so on—based on breeding particular variants. You can easily observe this pattern yourself. Go to a park and check out the dogs, or turn your attention to their human companions. Or look closely at a particular species of plant in the forest or in your own garden. If you observe a bunch of individuals closely, you will find plenty of variability within species. Paleontologists see this kind of variation as well. Every dinosaur skeleton is different in some way from every other member of its own species. Variation, it turns out, is the sine qua non of evolution, the fodder that natural selection feeds on.
Second, organisms produce more offspring than the environment is able to support. One oak tree drops thousands of acorns each year. A female salmon produces about 30,000 eggs during each spawning. One oyster can generate 114,000,000 eggs in a single reproductive event. Even among elephants, one of the slowest-breeding animals known, if all the young of a single female survived and reproduced at the maximal rate, after 750 years the descendants of this single mother would number 19,000,000. Clearly, only a tiny fraction of all these individuals grows to adulthood. And this is a good thing, because otherwise the world would soon be overrun by oak trees, salmon, oysters, and elephants!
Third, organisms must compete to survive and reproduce, and individuals possessing variations that best fit them to their current environments are the ones most likely to survive, reproduce, and pass along those desirable variations to the next generation. This process is called ADAPTATION. Almost all biological activities can come under the scrutiny of natural selection and adaptation, from catching and evading predators to acquiring mates, avoiding disease, and forming symbiotic alliances with other species. Adaptation tends to reward successful traits and eliminate less successful variants. For example, if a desert plant inherited some feature that favored the retention of water, such a trait would likely be passed on to future generations, and individuals lacking this feature might not be able to compete. Over time, such favorable traits accumulate. If the lineage spawns new species, these successful traits are likely to be passed on. This three-step process is evolution by natural selection.
Returning to our sauropod example, let’s suppose that the ancestral forms had short necks. Although all had short necks, neck length would have varied within the population, and those with slightly longer necks might have been able to browse on higher foliage. As a result, these fortunate animals would be more likely to live longer lives and produce more offspring. Succeeding generations would then inherit slightly longer necks, and so on, until, after many thousands of years, sauropod necks became very long. In truth, the actual course of events was much more complex and interesting. Neck elongation likely occurred independently in several groups of sauropod dinosaurs, with change occurring in fits and starts over millions of years across numerous species. In some forms, neck lengthening involved adding more neck (cervical) vertebrae, whereas other forms paralleled the giraffe solution, increasing the lengths of existing vertebrae. Nevertheless, the Darwinian scenario based on natural selection gives a relatively accurate account of neck elongation within this group.
As envisioned by Darwin, natural selection modifies the traits of plants and animals by making use of heritable variation that arises naturally within all populations. These variations are neither inherently good nor bad. They are simply there, and the rigors of life ensure that only the fitter combinations of variations are inherited by future generations. An inevitable consequence is a change in the genetic information stored within the population.
You can think of natural selection as a sieve. In each generation, all members of a given population are tossed into the sieve. Those that are less fit tend to fall through the holes and make no contribution to the next generation. Those that are most fit for that environment remain on the mesh—that is, they survive and pass on offspring. The off-spring then grow up to comprise the next generation, which receives the same harsh sieve treatment, resulting in more evolutionary winners and losers. Over time, the genetic makeup of the population shifts, rewarding the features that are most successful. Because environments change periodically, a set of features that confers success, or fitness, at one ecological moment may send an organism tumbling through the sieve at some later time.3 In short, individuals do not adapt or evolve—only populations do. Let me restate this fundamental and frequently misunderstood point. Populations, not individuals, evolve. Evolution occurs because certain kinds of individuals have greater success than others in survival and reproduction, resulting in shifts in the heritable variation of the population as a whole.
In a nutshell, natural selection is driven by two kinds of events. First, nature randomly alters the content of genetic information through such processes as mutation and recombination, resulting in variations at the level of organisms. Second, organisms interact with their environment, and those best able to survive and reproduce tend to pass on more offspring to the next generation. Over time, the information content of the entire population shifts. Evolution happens.
While Darwin was contemplating the workings of natural selection, he soon recognized a problem. He noted that many characteristics of animals did not seem to confer any advantage in the fight for survival. The famous evolutionist soon realized, however, that these same features increased an organism’s odds of winning the reproductive lottery. In particular, males with elaborate signaling structures had a greater success rate when it came to competing for mates. Darwin’s answer was to label a new selective process: SEXUAL SELECTION.
Like natural selection, sexual selection weeds out less successful traits in favor of those that are more successful. In this case, however, success is measured on the basis of reproductive success rather than survival. Sexual selection often favors the evolution of elaborate weapons and mating signals—horns, crests, colors, calls, and the like—that increase the chances of acquiring mates and passing on genes to the next generation. Sometimes, the bizarre features that prove most successful in reproduction actually decrease the odds of survival, working contrary to natural selection. Peacock feathers and deer antlers, for instance, are costly to build and maintain. Other features, like the calls of many frogs and insects, make these animals more vulnerable to predators.
Darwin reasoned that if such elaborate signaling structures resulted in improved mating success, they would be passed on to subsequent generations, even if these same features were detrimental to survival. In short, when it comes to evolution, sex trumps death, at least to a point. If female preference results in the evolution of more elaborate horns or more intricate calls, sexual selection might then lead to a runaway effect, causing succeeding generations to become increasingly extreme and bizarre. Numerous examples of bizarre structures among dinosaurs may have evolved at least in part under the pressure of sexual selection. Examples include ceratopsian horns and frills, hadrosaur crests, pachycephalosaur domes, stegosaur plates and spikes, and theropod crests and horns. The origin and functions(s) of these strange bony appendages have spurred a long and ongoing debate, but that’s a topic for a later chapter.
If energy is the currency of ecology, information is the currency of evolution. Life can be likened to a vast river of information flowing through time. Information arises, flows from generation to generation within the bodies of organisms, and branches into myriad tributaries. Over time, as environments shift—for example, becoming hot or cold, dry or wet—some of these waterways dry up (extinction), while others become raging torrents that top their banks and spawn new sets of tributaries (speciation).
Darwin’s view of evolution was founded on unending gradual change. According to this perspective known as GRADUALISM, organisms are forever under the close and merciless scrutiny of natural selection. Consequently, variable species are continually shifting from one form to another as they become ever-more adapted to their (frequently unstable) ecological surroundings. Over geologic time, these progressive, generation-by-generation heritable changes accumulate until they result in major evolutionary transformations. Following this view, species boundaries must be arbitrarily defined, because no discernible breaks occur in the ongoing flow of change. In keeping with the Darwinian perspective, if we could trace the evolutionary lineage of, say, bald eagles thorough an unbroken chain of fossil ancestors back to some small Jurassic theropod dinosaur, the boundaries between species would be arbitrary, a matter of personal preference because one form would grade seamlessly into another.
Placing gradualism into the context of our river metaphor, the information content of the flow is in constant flux; a sample of water taken at one point in the river would differ from other samples taken farther downstream, with the degree of difference proportional to the distance between sampling sites and the rate of flow. If you were to take a series of samples between these two well-separated sites, there would be no spot where you could pinpoint a major transition. The river would fork occasionally, producing new tributaries, but transitions in the composition of the flow would remain gradual.
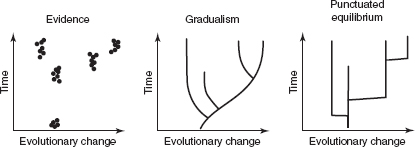
FIGURE 6.2
Two modes of evolution compared: gradualism, or evolutionary change within an unbranching lineage; and punctuated equilibrium, or long periods of minimal change within species followed by brief bursts of evolutionary change associated with the origin of new species. The graph on the left depicts a series of data points that represent fossil samples. The remaining two graphs show how these data might be interpreted to represent either gradual evolution (continuous change) or punctuated equilibrium (long periods of stability punctuated by short bursts of change). In all three graphs, evolutionary change is shown on the horizontal axis and time is shown on the vertical axis. See text for discussion.
Over the past few decades, the gradualist view has come under attack. Today, many, perhaps the majority of, evolutionary biologists consider species to have individual histories, or “lifetimes,” bounded by discrete births (SPECIATION) and deaths (EXTINCTION). As early as the 1930s, the population geneticist Sewall Wright presented a view of the natural world that should have made all biologists pause. He described species in terms of semiisolated populations whose members periodically interbreed and thereby exchange genetic material. If these populations are spread over a large area, every population would tend to undergo minor adaptive changes in response to local conditions. Given this structure, it’s difficult to envision how significant, directional change could accumulate across an entire species, as Darwin thought, because any evolutionary modification in one population would tend to be swamped by periodic interbreeding with individuals from different populations. One could imagine an occasional mutation—leading to better eyesight, improved digestion, or faster running ability—that might spread widely because it proved beneficial to members of all of the populations. However, in general, beneficial changes would likely improve fitness only locally, and distinct populations might even evolve in opposite directions.
The gradualist view of Darwin has also been challenged by direct observation of patterns in the fossil record. When a paleontologist walks up a hillside littered with fossils, she is moving through time. Because strata are deposited in layer-cake fashion, fossils eroding out at the bottom of the hill tend to be older than those at the top. In some cases, millions of years separate the deaths of organisms preserved in adjacent layers. Paleontologists have long observed that fossils of a given species tend to persist with minimal change through long time intervals, sometimes millions of years, only to be replaced abruptly by some distinct but related form. This pattern, which clearly conflicts with the Darwinian notion of constant gradual change, was traditionally attributed to gaps in the fossil record rather than to evidence of evolutionary process. It was thought that a more complete record would surely reveal continuous changes consistent with gradualism.
Then, in 1972, Niles Eldredge of the American Museum of Natural History and Stephen J. Gould of Harvard University turned the paleontological world on its head. Suppose, they said, that the fossil record is more complete than we thought, that observations of long-term stasis and relatively brief bursts of change reflect the real, dominant pattern of evolution rather than deficiencies of the fossil record. Suppose that evolutionary change is generally concentrated around the origin of new species and that species then persist largely unchanged until their eventual extinction. Eldredge and Gould cited abundant evidence in support of their idea. They gave the hypothesis the name PUNCTUATED EQUILIBRIUM, because it entailed punctuations of change layered on a background of equilibrium, or stasis. Punctuated equilibrium allows for small-scale evolutionary change within species and within populations, but it predicts that directional change in the sense of evolving entirely new forms requires evolutionary branching events—that is, the origin of new species.
Over the past several decades, this hypothesis has been the subject of intense testing and debate. Paleontologists have scoured old data sets and generated new ones in a concerted effort to reveal patterns and check them against contrasting sets of predictions. Today punctuated equilibrium is supported in one form or another by most paleobiologists. However, its profound ramifications have yet to be appreciated by biologists generally, who still cling to a gradualist view of evolution. Several exceptions to punctuated equilibrium have been documented, yet species stasis (i.e., minimal change within species over deep time) has emerged as an overwhelming pattern in the fossil record. This finding marks a dramatic departure from Darwinian gradualism. In general, directional change does not accumulate continually within local populations. Local modifications to the information flow occur, but these are generally swamped by interbreeding, which results in the exchange of information between population tributaries.
Most of the time, then, it seems that species don’t do much of anything, at least from an evolutionary standpoint. Contrary to the gradualism perspective, they appear to persist relatively unchanged for most of their histories. But if so, how do evolutionary changes build up over time to lead to new forms? The answer is to be found in the origin of new species, or speciation. Speciation is pivotal because it sets in place a reproductive barrier that prevents interbreeding and genetic mixing. For most sexually reproducing organisms, once that reproductive barrier is in place between two populations, the flow of information thereafter remains isolated, whether the populations-turned-species persist for one year or millions of years.4
Returning to the river metaphor, populations within a species act like criss-crossing tributaries. The information flow is separated into distinct channels some of the time but is reunited at other times by the act of interbreeding. Within regional ecosystems, this pattern of branching and reconnecting enables a limited amount of information change (evolution) to occur, but little in the way of directed, transformational change that affects the entire species. A portion of this new information cascades through the entire river (via matings between members of different populations), but many other bits are swamped by exchange between and among the various tributaries. However, once two tributaries (populations) become isolated from each other (i.e., the members of each no longer interbreed), the resulting (reproductive) barrier generally ensures that the flows travel in separate channels, and the result is at least one new species. In the process, new information—the evolutionary changes accumulated in the local population—is injected into the long-term flow of evolutionary lineage. And the longer those streams are isolated, the less likely it is that they will commingle in the future, because evolutionary changes in information content accrued along the way increase the likelihood that the two streams will be unable to intermix. Speciation, then, is a fork in the river, a permanent branching in the flow of information. It promotes the origin and capture of new information, leading to new forms, which in turn can produce dramatic changes to the overall information flow of an ecosystem. Given such a revised perspective, speciation takes on heightened importance. In particular, any factors that influence the pace of species births and deaths are likely to have a major effect on patterns of diversity.
How do ecology and evolution interact? As we’ve seen, ecological processes deal with the day-to-day running of ecosystems—capturing and distributing solar energy on the one hand, and cycling key nutrients (think CHNOPS) on the other. Conversely, through natural selection and a host of other processes, evolution ensures that life is able to adjust to the inevitable changes that occur over deep time. Whereas ecology can be thought of as nature’s short-term memory, maintaining the flow of energy to handle the immediate tasks at hand, evolution, tapping into vast stores of genetic information, provides the long-term memory, making midstream course corrections as necessary.
Life’s episodic pattern of lengthy stasis and brief bursts of change demonstrates that there is much more to evolution than the generation-by-generation battle for genetic dominance. Indeed, evolution in the sense of generating new species is perhaps better regarded as a “last resort” than an ongoing survival strategy. When faced with changing environmental circumstances—for example, the onset of an ice age or hothouse conditions—species and their populations do not merely stay put, grimly determined to change their form to meet the new conditions. Instead, where possible, organisms track their preferred habitats. Often it is only when habitat tracking is no longer an option that we see much evidence of extinction and speciation, the removal and addition of ecosystem players.
Several recent paleontological studies, from both the marine and terrestrial realms, suggest that the origins and extinctions of species are not arrayed haphazardly throughout Earth history; rather, these events tend to be clumped and cross-genealogical, impacting many distantly related groups of organisms over short durations. In one of the best-documented examples, Carlton Brett (University of Cincinnati) and Gordon Baird (State University of New York at Brockport) documented a sequence of eight assemblages of invertebrates (e.g., trilobites, clams, and corals) from the Middle Devonian (about 380 million years ago) of North America. Brett and Baird found that each assemblage formed a community that could be characterized by its own unique composition of organisms. And while there was an occasional isolated appearance or disappearance of a species, the great bulk of species changes were concentrated in brief turnover events that completely restructured the ecosystem.
The bursts of species turnovers documented in this and other studies have been linked to major environmental shifts. The apparent synchronicity between evolutionary and environmental change (although questioned by some investigators) suggests that evolution is frequently triggered by external factors that affect entire ecosystems. So not only do single species remain in stasis over geologic time; entire groups of species within regional ecosystems may do so as well. This is not to say that species originate and go extinct only in large-scale turnover events; a regular background of such events continues during even the most stable intervals. At present, debate continues as to how such ecological widespread stability might be maintained over deep time. Nevertheless, the dominant pattern appears to be periods of long-term stability dominated by the forces of ecology, paired with brief episodes or pulses of upheaval, dominated by the forces of evolution. Entire ecosystems become established over (geologically) short durations, and their constituent species then persist relatively unchanged for extended periods (up to millions of years). Ultimately, some environmental change forces species turnover and a major reorganization, resulting in the formation of a new ecosystem.
This is an exciting time in the history of evolutionary biology. Whereas Darwin’s first theme—descent with modification—has been confirmed beyond all reasonable doubt, his proposed mechanism for change—natural selection resulting in continual gradual change—no longer explains all of the patterns that biologists (and particularly paleontologists) observe in nature. The next decade or so is likely to yield important new insights regarding the integration of ecology and evolution. I’m quite certain that Darwin would have appreciated the discussion.
Whether evolution occurs gradually or in fits and starts, the central message of Darwin’s legacy is that change is ubiquitous. Observed on the scale of a human lifetime, the world may sometimes appear permanent and unwavering (although less so in an age of rapid global warming). And, as we’ve seen, species can persist relatively unchanged for hundreds of thousands, even millions, of years. Yet viewed from the perspective of deep time, change is a constant, and nothing escapes its touch. Armed with the dual perspective of ecology and evolution, let’s return to the Mesozoic and unravel some of the threads that linked dinosaurs to one another and to their shifting environments.
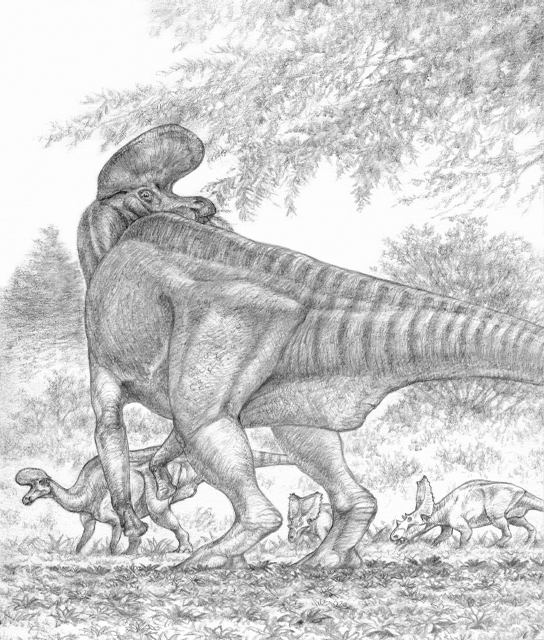
Contrasting feeding strategies in a pair of Cretaceous herbivores. The crested, bipedal duck-billed dinosaur Lambeosaurus feeds on overhead browse, while the four-footed horned dinosaur Chasmosaurus concentrates on cropping low plants. Mixed-species associations like these may have occurred as a means of avoiding predators. At present, however both the behavioral differences and the mixing of species are based on speculation.