7
THE GREEN GRADIENT
WILDEBEEST CROWDED THE RIVER'S EDGE, snorting loudly and jostling nervously for position. The tension in the air was palpable. It was clear that, at any moment, the herd would leap into the murky water and swim to the opposite shore. It was equally evident from their agitated behavior that no one wanted to be first.
In 2004, following a successful field season hunting dinosaurs (or at least their bones) in the remote Turkana region of northwest Kenya, a few of the crew members decided to travel to the Masai Mara National Reserve to experience the famed Mara ecosystem firsthand. Situated in the southwest corner of the country, Masai Mara comprises the northernmost portion of a vast, contiguous protected area, the Serengeti-Mara Ecosystem, the bulk of which is located in Tanzania. This is a land of shimmering grasses and breathtaking skies interrupted only by an occasional rocky escarpment or verdant riverine forest. Encompassing 25,000 square kilometers (9,700 square miles), this ecosystem is also one of the last great refuges on Earth for free-roaming large animals, predator and prey alike.
That makes Masai Mara a fine place to think about dinosaurs.
For most of the year, a vast assemblage of ungulates—dominated by wildebeest, zebra, and gazelle—inhabit the southern portion of this reserve. They live off the abundant short-grass plains, feeding, rutting, giving birth, and earnestly avoiding large predators. During June or July, with the onset of the dry season, the animals begin a long, northward trek of about 800 kilometers (500 miles). Many wind up in Kenya’s Mara region, which remains wetter at this time and thus offers more food. This magnificent event, referred to simply as “the migration,” culminates with the arrival of the wet season several months later, which triggers a return en masse southward.
We had arrived in August, near the migration’s peak. At one point, our guide, a Kenyan man named Ken Kamau, drove the Toyota Land Cruiser slowly over the crest of a hill into the midst of a vast mixed throng of zebras, wildebeest, and gazelles. Ken turned off the engine, and we found ourselves immersed in a sea of hoofed animals stretching to the horizon in all directions. I squinted, and the entire landscape seemed to move. We marveled at the cacophony of hundreds of jaws consuming solar energy masquerading as grass. I felt like a voyeur in another world and couldn’t help but imagine similar scenes played out with dinosaurs many millions of years ago.
In order to reach the lush promised land of tall waving grass in the north, the wildebeest must first ford the Mara River. Months later, they will do so again on the return trip to Tanzania. These brief water crossings, each requiring less than a minute for most animals, nevertheless comprise some of the most frightening moments in a wildebeest year. In terms of distance—perhaps 50 meters (160 feet)—the swim seems trivial, though you need to imagine dog-paddling in a strong current while being pushed and bumped by dozens of panicked companions.
Why the mayhem, fear, and tension? In a word…crocodiles. Mara crocodiles gorge themselves annually during the wildebeest migration, consuming enough meat to satisfy their cold-blooded metabolic needs for months to come. In addition to those taken by predators, some of the hoofed herbivores drown in the frenzied crossing.
As we stood in the openair truck on the far side of the river, the herd of several hundred wildebeest ambled slowly toward us, munching on grass along the way. We asked Ken whether they might be preparing for a river crossing. He expressed doubt, stating that he had conducted game drives in Masai Mara for several years and had yet to witness this famous event. However, he happily agreed to stop and wait, just in case. Over the next 15 minutes the herd moved ever closer to the short, steep slope that descended into the muddy waters of the Mara. Suddenly, one adult wildebeest stepped slowly, hesitatingly, off the grassy plain and headed down the slope toward the water. Before reaching the river, however, it turned and ran back up to rejoin the herd. Then another came down the hill, followed by another. Each time the nervous animals ventured closer to the water, and each time they returned to the growing mass of herbivores above. This anxious sequence continued a while, until crowding from the rear of the herd literally forced the animals up front to descend the slope. Now there was no longer any option to return to the safety of the plains above; a wildebeest wall blocked the way. The herd’s front guard continued to advance until several adults teetered at the muddy water’s edge.
Meanwhile, in the river, several massive hippos lounged in the shallows, doing what hippos do during the day—pretty much nothing. Nearby, a large crocodile perhaps four meters (12 feet) long sat motionless, partially submerged, while the herbivores gathered. After a few minutes, the croc casually roused itself and, with long powerful strokes of its armored tail, set off slowly downstream. Perhaps, we speculated, the oversized reptile sensed what was about to happen and was preparing for an underwater attack.
Finally, suddenly, the first wildebeest leapt high into the air, splashed down midstream in the river, and made for the opposite shore. Other animals quickly followed until there was a frenzy of ungulates leaping and swimming for their lives. The resulting commotion was staggering. Soon a long line several wildebeest wide stretched from one side of the river to the other. The reaction of the hippos lounging nearby seemed to be a combination of boredom and disgust that their quiet day had been so rudely interrupted. In less than ten minutes, the entire wildebeest herd had negotiated the crossing. One frightened youngster made the trip almost three times, paddling most of the way across, heading back in confusion, and then performing the swim again. In the end, all made it safely to the south side of the river. Perhaps the crocodiles had fed recently enough so as not to be tempted by this particular glut of meat fording their realm.
Wildebeest are one of many varieties of plant-eating ungulates that inhabit the Serengeti’s grasslands and woodlands. Smaller-bodied examples include impala, as well as Thompson’s and Grant’s gazelles. Also present are several similarly sized herbivores— zebra, topi, and hartebeest—and a handful of larger forms—buffalo, eland, giraffe, and elephant. Rhinos venture into the open savannah on occasion, though they prefer the cover of bushland, also home to diminutive antelope like dikdik and bushbuck. Although one occasionally glimpses the long, curving horns of a waterbuck poking above the grass, these majestic ungulates favor the lush riverine forest that snakes through this landscape. Given such a bounty of prey species, it’s not surprising that the Serengeti is also predator central, with lion, leopard, cheetah, hyena, wild dogs, jackals, and foxes, among others. Of course, all of these glamorous members of the fauna are accompanied by a spectrum of smaller animals such as rodents, turtles, fishes, birds, lizards, and insects. Finally, as in any ecosystem, most of the biodiversity is tied up in microbes that remain hidden from view.
People often think of the Age of Dinosaurs as a strange and unique period in Earth history when giant animals were common worldwide. Yet for the vast majority of time since the dinosaurs first diversified in the Early Jurassic—that is, for the better part of 200 million years—terrestrial ecosystems over most of the globe have typically included numerous bigbodied animals. Although post-Mesozoic faunas may have lacked animals approaching body sizes of the largest dinosaurs, various mammals such as elephants and huge rhino relatives regularly filled the role of land-dwelling giants. The few exceptions to the persistent presence of bigness have occurred in the wake of mass extinctions, including the one that wiped out the dinosaurs. Today, due primarily to human-induced extinctions, we are in the midst of another of these exceptional intervals, one in which giant animals are increasingly restricted to small, protected reserves. With the exception of Africa, the cradle of our bipedal ancestors, the arrival of humans on virtually every other landmass has precipitated the rapid disappearance of megafaunal species. Consequently, today you must travel to places such as Kenya to glimpse habitats full of glorious big beasts.
The highly diverse Serengeti ecosystem is fueled largely by grass. The dominant varieties here are red oat grass, thatch grass, and sweet pitted grass. With so many herbivore species feeding on this seemingly homogenous plant community, you might wonder how the grasslands manage to survive at all. The answer involves a mixture of specialized anatomy, partitioned resources, and a division of labor. Zebra feed on the coarse, highfiber, and generally unpalatable portions of the grass, mostly seed heads and stems. They are the first wave of herbivores, exposing the greener, high-protein grass leaves that are the preferred fodder of the second wave—wildebeest and several other large ungulates. The feeding activities of wildebeest in turn expose fresh shoots and herbs favored by a third wave of smaller ungulates such as Thompson’s gazelles. Other players in this sequence include topi and hartebeest, with long pointed faces and narrow mouths ideally suited for selectively picking out the most nutritious grasses. African buffalo, with their broad mouths and massive molars, adopt the opposite strategy, munching unselectively through grasslands like a battalion of horned John Deere mowers. Finally, a few animals such as elephant, eland, and Grant’s gazelle are even more generalized in their feeding habits, not only grazing on grass but also browsing on a variety of broad-leaved plants.
Surprisingly, it appears that the grasses thrive in response to this multipronged, coordinated assault. Studies have shown that the succession of grazers—first zebra, then wildebeest, then smaller ungulates like Thompson’s gazelle—passing through a grassland actually stimulates growth of grass species and produces a greater diversity of low, herbaceous plants palatable to a range of herbivores. This is not to say that the system is impervious to overgrazing. But, in general, the spectrum of carnivores helps to keep the total number of plant consumers in check. Similar types of relationships interlinking plants, herbivores, and carnivores almost certainly existed in dinosaur-rich Mesozoic ecosystems.
The Mara woodlands, as well as the grasslands, take a thrashing from the activities of herbivores. While driving around, we noticed that the larger acacia trees were bare throughout most of their heights yet were topped by neat, broad, bell-shaped canopies of vegetation. One could almost imagine large teams of Serengeti gardeners trimming the vast woodlands, a vision not far from the truth. Ken explained that the acacias are regularly pruned by giraffes, which use their long tongues and prehensile upper lips to great effect, stripping leaves to a height of about 6 meters (18 feet). This activity produces a “browse line,” above which the tree is able to spread beyond the reach of even these long-necked herbivores.
Much more devastating are the elephants. These behemoths frequently topple mature trees and indiscriminately trample thickets of bush. It’s amazing to watch elephants tear off large tree limbs with their muscular trunks or literally snap tree trunks merely by leaning on them. In a short time, a few rambunctious proboscidians can decimate large expanses of woodlands. Sauropods and other giant dinosaurian herbivores may well have generated giraffe-like browse lines. And, given that many dinosaurs such as hadrosaurs and ceratopsians approached elephantine proportions, and that the sauropods exceeded this limit by many times, it’s difficult to conceive that dinosaurs were not ancient precursors to elephants in their treatment of woodlands. It was likely trivial for sauropods to knock down small to medium-sized trees.
The end result of these varied herbivore-plant interactions in the Serengeti is a continuous, abundant flow of energy and cycling of nutrients, with each species having a unique role that perpetuates the ecosystem as a whole. The energy gradient generated by the sun is transformed by photosynthetic plants into a green gradient, which herbivorous animals attempt to dismantle. Transformed into the flesh of herbivores, the green gradient then feeds a variety of carnivores and, ultimately, decomposers.
Animal diversity, then, is inextricably tied to plant diversity. The green gradient defines the capacity of an ecosystem to support consumers of varying sizes and trophic levels. Of the millions of species currently living on Earth, it’s estimated that about 50 percent, and perhaps as much as 90 percent, of that diversity occurs in the exceptionally productive tropical rain forests. As one moves away from the equator north or south, there is a progressive drop in biodiversity, with the frigid polar regions supporting the fewest number of species.
In the Mesozoic, things were very different. Remember, this was a hothouse world lacking polar ice caps, with elevated sea levels and greatly reduced temperature differences between high and low latitudes. The poles supported a range of plants, dinosaurs, and other animals; however, just as today, dramatic seasonal variations in solar energy would have limited the potential for life at the highest latitudes. In contrast to the present day, the aridity associated with Mesozoic global warming appears to have severely restricted plant productivity in the equatorial regions as well. The world of the dinosaurs lacked tropical rain forests and all of their associated biomass, with peak levels of biodiversity perhaps occurring instead at the wetter midlatitudes. Thus far, the fossil record bears out this prediction, with diversity and abundance very high at the midlatitudes (e.g., North America and Asia) and much more limited in the polar and equatorial regions (Alaska and North Africa, respectively). However, we still have relatively few fossil localities from outside temperate latitudes with which to test this idea.
Herbivores come in all shapes and sizes, from gargantuan elephants and sauropods to tiny beetles and caterpillars. Despite their diminutive nature, by virtue of sheer numbers, insects usually have a much greater overall impact on plant communities than do vertebrates. Even in savannah grasslands like the Serengeti—replete with vast herds of wildebeest, zebra, and impala—vertebrates are responsible for less than one-third of the plant matter consumed, whereas plant-eating insects carry out closer to two-thirds of the herbivory. Like mammals and dinosaurs, insects evolved a variety of strategies to help them process plants, including a wondrous array of jaw structures and vast numbers of symbiotic gut microbes. Just as in us, those gut microbes participate in digestion by breaking down food and allowing nutrients to be absorbed into the body.
Among vertebrates, food quality and herbivore body size are inextricably interwoven. Any kid knows that big animals eat more food than small animals. No surprise there. However, plant foods are extremely variable in quality, even within the same plant. And plants, like animals, vary greatly in their occurrence within ecosystems. Small animals, with their lesser dietary requirements, can afford to search out high-quality, easily digestible items, such as fruits, seeds, and fresh shoots, which typically have patchy distributions. Consequently, small herbivores tend to have more specialized diets, consuming not the entire plant but the most nutritious parts. Think about squirrels storing nuts for the winter. Conversely, because highly nutritious dietary items such as seeds and fruits tend to occur in smaller quantities with more limited distributions, they simply cannot support populations of bigger animals. Instead, large herbivores must consume not only greater quantities of food but also food of poorer quality, high in fiber and low in fat and protein. Serengeti wildebeest must migrate hundreds of miles annually because they can’t make a living off stored nuts or other highly nutritious plant parts.
This intimate relationship between body size and food quality results in highly divergent strategies for large and small herbivores. Unable to find sufficient amounts of easily digestible fruits and seeds, larger herbivores tend to be “whole-plant predators,” consuming substantial portions of plants rather than select parts. Yet by themselves, herbivores generally lack the enzymes to break down the cell wall compounds of plants, such as cellulose and lignin. So they unknowingly enlist the help of microorganisms, which reside in fermentation vats within the hind gut. With big appetites, big populations, and unselective diets, these animals tend to have a major impact on their native habitats. Here, then, is a pattern with clear implications for a Mesozoic world of giants.
Yet, past or present, plants do not take abuse from herbivores lightly. Despite their relative immobility and seemingly passive approach to life, plants have evolved an array of effective defenses to thwart the best efforts of herbivores. Most obvious is a range of tough, nonnutritious outer coverings such as bark and spines, though leaves, too, can be highly fibrous or covered in a waxy, unpalatable coating. Faced with such tough, lownutrition (not to mention bad-tasting) fodder, digestibility is typically a major obstacle for herbivores. In response, plant consumers tend to adopt one of two strategies. The first is mechanical breakdown, using such “front-end” tools as jaws, teeth, and stomach stones. The second strategy involves back-end modifications, such as large, elongate guts and prolonged passage times of food; these features in turn enable bacteria living within the gut to ferment the plants and thereby gain access to nutrients. Over time, plants and herbivores engage in evolutionary “arms races,” each attempting to achieve ecological one-upmanship over the other.
To extend the military metaphor, some of these arms races escalate into chemical “warfare,” with plants producing biotoxins, even cancer-causing agents, as a means of combating herbivores. Much of this biochemical production is aimed at herbivorous insects, which often have a greater overall impact on plant communities than vertebrates, despite dramatic size differences. Some of these chemicals are merely distasteful, whereas others can be downright deadly, resulting in damage to the reproductive cycle or even the demise of the consumer.1 Remarkably, plant-generated insecticides may be the most potent force limiting the numbers of herbivorous insects.2
Some plants, rather than resorting to intimidating armor or poisonous cocktails, adopt an alternative defense strategy, fending off voracious herbivores through symbioses with other species. For example, the bull’s-horn acacia, which inhabits the lowlands of Mexico and Central America, teams up with ants. Like most acacia, the bull’s-horn is thorny, but the thorns are swollen and hollow, providing a home for the ants; in addition to shelter, the plant provides sugars, fats, and proteins to its ant partners. In return, the ants swarm the surface of the acacia, biting, stinging, and in general deterring animals of all sizes that come into contact with the plant.3
The last and perhaps most “ingenious” strategy that plants have evolved for dealing with herbivores is to welcome them to the dinner table, even going so far as to put out a sign advertising a free meal and then put the unwitting animals to work. Flowering plants in particular take advantage of consumers’ need to feed, producing a variety of irresistible delicacies. Less than two centuries ago, it was thought that plants made beautiful flowers because the Creator had put them there to please humans. Later it became clear that flowers serve an important ecological function that has nothing to do with bipedal primates (no surprise in retrospect, given that the first flowering plants preceded humans by over 125 million years!). Flowers turn out to be the siren song of the plant world, the means of signaling pollinators. Drawn like flies to dung, these pollinators— from butterflies and bees to bats and birds—spread pollen from flower to flower and, in doing so, facilitate the proliferation of angiosperms. Today, the great majority of angiosperms (up to 98 percent in the highly diverse lowland tropical rain forests) are pollinated by animals. Flowering plants literally depend on these coevolved symbioses in order to disperse and reproduce.
Pollination partnerships are the result of millions of years of coevolution, and many are finely tuned. For example, interspecies relationships are often highly specific, with a single animal pollinator linked to a single plant. Moreover, both plant and pollinator typically have specialized structures that facilitate their relationship. Thus, bees possess color vision, sensitivity to certain odors, and specialized pollen- and nectar-carrying structures, all linked to the flowers they pollinate. Insect-pollinated flowers tend toward hues of blue and yellow, because most insects cannot see red. Red flowers, in contrast, are largely the domain of hummingbirds. Paleontologists often assume that many herbivorous dinosaurs during the Cretaceous fed on angiosperms. It’s important to remember, however, that these giant herbivores depended on insects to aid in the reproduction of their food supply, just as we humans do today.
Mesozoic plants and herbivores coevolved for almost 200 million years, with dinosaurs making up the dominant large-bodied plant eaters for most of that era. As with modern examples, Mesozoic plants and animals evolved a range of shifting strategies for dealing with each other. Here I briefly describe innovations on both sides of this coevolutionary dance, and then I address some best guesses as to how this dance progressed through time. This endeavor requires that we try to envision plants from the point of view of herbivorous dinosaurs and contemplate herbivorous dinosaurs from a plant perspective. As we proceed, I invite you to keep the Serengeti in mind. Although we can currently reconstruct Mesozoic ecosystems with only the broadest of brushes, like the African example, they also were undoubtedly complex and tightly interwoven.
Chapter 5 introduced eight major groups of plants that dominated the world of dinosaurs. First were three clans of spore-bearing pteridophytes—ferns, horsetails, and lycopods—most of which were low-growing, water-dependent herbaceous plants. Next came four groups of seed-bearing gymnosperms that were widespread from the Triassic through the Early Cretaceous: cycads, ginkgos, conifers, and bennettites. Finally, angiosperms, or flowering plants, were the latest-appearing group of seed plants, originating in the Early Cretaceous and diversifying globally by the end of that period. These Mesozoic plants faced the same kinds of challenges as their modern counterparts. And they, too, evolved a variety of mechanical and chemical defenses to ward off herbivores, from insects to dinosaurs. Indeed, paleobotanists think that many of the defenses we see in living plants arose during the Mesozoic, when both insects and dinosaurs were proliferating.
Plants respond to herbivores in a variety of ways, with the preferred “strategy” dependent in large part on the evolutionary raw materials inherited from ancestral forms. The foliage of spore-bearing pteridophytes tends to be succulent and lacking in any major mechanical defenses such as spines or tough bark. However, living examples like ferns and horsetails frequently produce poisonous chemicals. It’s feasible, and perhaps likely, that Mesozoic spore bearers also possessed chemical defenses with similar effects on dinosaurs. From the perspective of a dinosaur herbivore, pteridophytes may have offered a potentially fast-growing, renewable resource. Yet, this resource would have been restricted in abundance by the need for aquatic reproduction, and chemical defenses may have generated considerable obstacles for digestibility.
Among nonangiosperm seed plants, we find a good news/bad news situation for the dinosaurs. Cycads, gingkos, conifers, and bennettites were able to spread widely and somewhat independently of bodies of water, creating diverse habitats and providing an abundant food source. Yet many living representatives of these groups possess tough outer coverings and/or resistant foliage, sometimes with spines. In addition, the cells often have thick walls, making them difficult to break down and digest. Finally, a large proportion of seed plants are rich in indigestible chemicals and resins. Despite these many obstacles, members of all five of these gymnosperm families may well have been consumed by dinosaurs during most of their tenure.
In contrast to the spore bearers and other seed bearers, angiosperms may have offered a welcome alternative for plant-eating dinosaurs. If modern flowering plants are any indication, Mesozoic examples had succulent foliage and fewer chemical defenses. Like other seed plants, angiosperms grow in a wide range of habitats largely independent of standing water. Unlike most seed plants, a damaged angiosperm can often recover quickly by regrowing from underground buds. Thus, they had the potential to spread widely, exploiting (and cocreating) a diverse range of habitats. In short, flowering plants may have been a dinosaur herbivore’s dream: abundant, succulent, and fast growing, with fewer indigestible chemicals, tolerance to intensive herbivory, and the ability to recover quickly following cropping. Keep in mind, however, the fact that angiosperms were Mesozoic latecomers, first appearing in the Early Cretaceous. Jurassic herbivores such as Brachiosaurusand Stegosaurusnever saw a flower. Some paleobotanists have argued that the terrestrial world became much more vegetated after the appearance of flowering plants, allowing animals to disperse much more widely as well.
The most important thing to remember about plant-eating dinosaurs is that most were really big. The largest sauropods were on the order of 70 tons (150,000 pounds), greater than ten times the weight of the biggest elephants. Even the smallest known dinosaur herbivores were still pretty large; Lesothosaurus, a primitive form from South Africa, was about 1 meter long and would have weighed somewhere in the range of 4–7 kilograms (9–15 pounds). This is still many times larger than the smallest examples among birds and mammals.
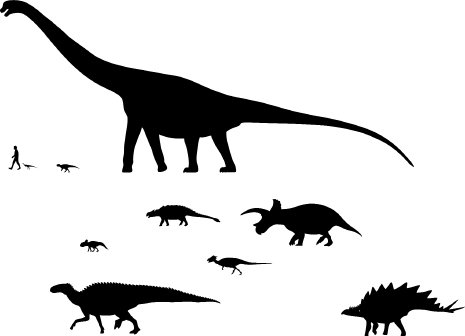
FIGURE 7.1
Size variation in dinosaurian herbivores. Top row from left to right: Lesothosaurus (an Early Jurassic primitive ornithischian), Psittacosaurus (an Early Cretaceous ceratopsian), and Argentinosaurus (a Late Cretaceous sauropod). Second row from left to right: Protoceratops (a Late Cretaceous ceratopsian), Euoplocephalus (a Late Cretaceous ankylosaur), Pachycephalosaurus (a Late Cretaceous pachycephalosaur), and Triceratops (a Late Cretaceous ceratopsian). Bottom row from left to right: Edmontosaurus (a Late Cretaceous ornithopod) and Stegosaurus (a Late Jurassic stegosaur). Human in top row added for scale.
If you’re interested in dinosaur feeding behavior, teeth are the best place to start searching for clues. These resistant bits of enamel and dentine yield valuable indicators as to their owner’s diet. When it comes to teeth, dinosaurs were much like sharks. Sharks replace their teeth continuously and are constantly generating new teeth. Employing a marvelous, conveyer belt–like system, sharks eject old teeth and rotate fresh replacements into position. Break a few teeth attacking a sea lion? No worries— there’s more on the way. Although the jaw mechanisms of sharks and dinosaurs are radically different in many respects, both groups generate(d) new teeth and replace(d) old teeth continuously.
In contrast, humans and most other mammals have just two sets of teeth, commonly referred to as “baby” and “adult.” When the adult teeth wear out, humans (at least those who can afford them) turn to dentures. Old individuals of other mammal species, like elephants and horses, have been known to grind their teeth smooth; unable to feed, these animals quickly succumb to starvation. Why would evolution curse mammals with a mere two sets of teeth, whereas the remainder of the vertebrate world happily pumps out replacements indefinitely? The answer is specialization. Because their teeth do not continuously fall out, mammals are able to have precise and varied kinds of contacts between upper and lower dentitions. Look in the mouth of your average mammal (or simply look in the mirror and open your own mouth), and you’ll find a variety of tooth types, each crafted over millions of years to serve a particular function. Depending on the mammal in question, there may be pointed teeth for stabbing, bladelike teeth for slicing, and broad molar teeth for grinding and pulping.
Now check out the mouth of a dinosaur (or, say, a shark, lizard, or frog), and what you’ll see is sameness. The teeth at the front and back of the jaws tend simply to be larger or smaller variations on a single theme. Importantly, however, the specific characteristics of each tooth type vary considerably, depending on the group in question and on the animal’s diet. In other words, though dinosaur teeth tend to be homogenous from back to front within a single species, evolution crafted a diverse set of solutions to the challenge of feeding. In addition, much of the plant processing undertaken by dinosaurs occurred farther along the gastrointestinal tract.
The closely related prosauropods and sauropods—together known as the sauropodomorphs—represent one the most successful “experiments” in the history of land animals. They also gave rise to the earliest radiation of dinosaur herbivores. First appearing in the late Triassic, these dinosaurs smashed all size records set by previous large-bodied land animals, and few animals have come close since the Mesozoic. They were the dominant terrestrial herbivores globally during the Jurassic, and they persisted in this role over much of the planet—particularly in the Southern Hemisphere—during the Cretaceous as well. People have long marveled at the incredible sizes of sauropods and wondered how such animals could have swallowed sufficient food to keep their internal furnaces stoked. Regardless of metabolic rate, animals of such great mass could not have been overly selective in their dietary preferences.
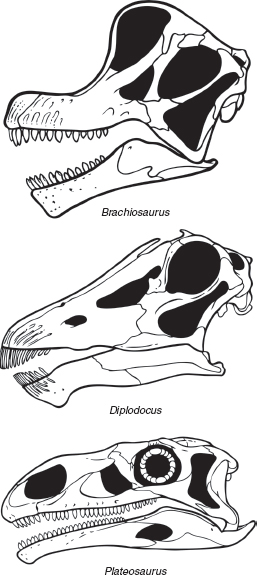
FIGURE 7.2
Variation in the skulls of sauropodomorph dinosaurs: Brachiosaurus (a sauropod), Diplodocus (a sauropod), and Plateosaurus (a prosauropod). Not to scale.
Sauropod skulls come in two general types, with plenty of odd variations. The first skull type, epitomized by Camarasaurus and Brachiosaurus, is short front to back, but tall, with thick spoon-shaped teeth and the bony external nostril positioned up front. The second type, characteristic of Diplodocus and a number of titanosaurs, is longer and lower with narrow, pencil-like teeth restricted to the front of the jaws. It is generally thought that sauropods fed by raking their teeth across a branch to remove the foliage. Many forms had tooth-to-tooth occlusion, with upper and lower tooth crowns meeting in a relatively precise fashion when the jaws were closed. Yet sauropods had little if any ability to chew as mammals do. Some researchers speculate that, like giraffes, they relied on powerful tongues to manipulate food items for slicing. Much has been written about how such giant animals were able to pass enough food down their gullets to sustain their enormous bulks, and we will return to this fascinating problem in chapter 13.
Sauropods are typically regarded as high browsers with graceful, upright, swanlike necks. With the neck held nearly vertical, the largest of these animals would have been able to peer, periscope-like, into a fourth-story window. More extreme reconstructions depict these animals rearing up, using a tripodal stance composed of tail and hind legs in order to fight, mate, or feed. In recent years, various arguments have been presented against these behaviors. Some say that the articulation of bones within sauropod necks prohibited upright, giraffe-like neck positions. Others point to a lack of muscular leverage to lift the head and neck. Still others argue that these animals would have been unable to generate sufficient blood pressure to pump blood from the chest to the head. At present, there is little consensus on this problem. Nevertheless, the evidence at hand indicates that most sauropods tended to hold their necks in a more horizontal posture and that different species and groups varied in their ability to raise the neck to an erect position. Some, like Brachiosaurus, may have habitually positioned the neck as giraffes do; others, like Dicraeosaurus, likely lacked the ability to raise the head much at all; and still others, like Diplodocus, probably possessed some abilities for horizontal and vertical movements. As for rearing up onto the hind legs, this behavior seems intuitively unlikely for such giants. Yet the act of copulation demanded that at least some males engaged in this behavior on occasion. Like elephants living in the wild (as opposed to the circus variety), I imagine that sauropod rearing, though possible, was not a common sight on Mesozoic landscapes.
Other than sauropodomorphs, all major groups of dinosaur herbivores fall within the bird-hipped clan, Ornithischia. Members of this group possessed toothless beaks presumably covered in life with a horny sheath. Feeding strategies, however, apparently varied greatly among ornithischians. The simplest teeth—mostly spoon- or leaf-shaped structures topped with coarse ridges—are found in animals such as ankylosaurs, stegosaurs, and pachycephalosaurs. Whereas the former two groups were low-browsing quadrupeds, the dome-headed pachycephalosaurs were bipeds, capable of feeding some-what higher in the canopy. The largest examples within these herbivorous clans were on the order of 10 meters (30 feet) long and weighed as muchas 6,000 kilograms (13,000 pounds). Thus, here also we must wonder how the animals managed to ingest sufficient foliage to maintain such massive bodies, particularly given their wimpy teeth.
The most specialized teeth and jaws among ornithischians are found in the duck-billed dinosaurs (hadrosaurs) and horned dinosaurs (ceratopsids). These two groups independently evolved powerful dental batteries, each composed of closely packed columns of teeth that together formed a continuous cutting edge. With up to forty rows of teeth on each side of both upper and lower dentitions, and multiple replacement teeth in each column, the jaws of a single adult Triceratops or Edmontosaurus contained literally hundreds of teeth. In carnivores, the jaw joint of the lower jaw is generally located at the same level as the tooth row, causing the jaws to close in a simple, scissor-like fashion. However, the scissor design is less effective for processing plants. In ceratopsids and hadrosaurs, the jaw joint is located below the level of the tooth row, a pattern seen in other herbivores before and since. The offset arrangement allows the entire upper and lower dentitions to contact each other almost simultaneously, a great advantage when dealing with a mouth full of plants. The lower teeth passed inside the uppers, allowing the upper and lower tooth rows to come into contact and slide past each other like two elongate blades.
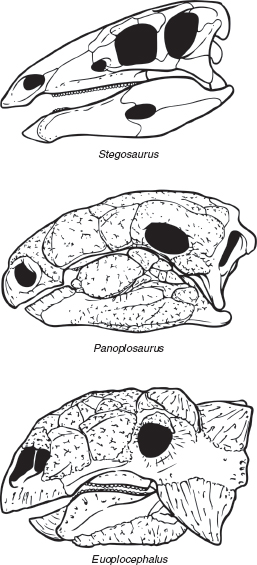
FIGURE 7.3
Variation in the skulls of thyreophoran (armored) dinosaurs: Stegosaurus (a stegosaur), Panoplosaurus (a nodosaurid ankylosaur), and Euoplocephalus (an ankylosaurid ankylosaur). Not to scale.
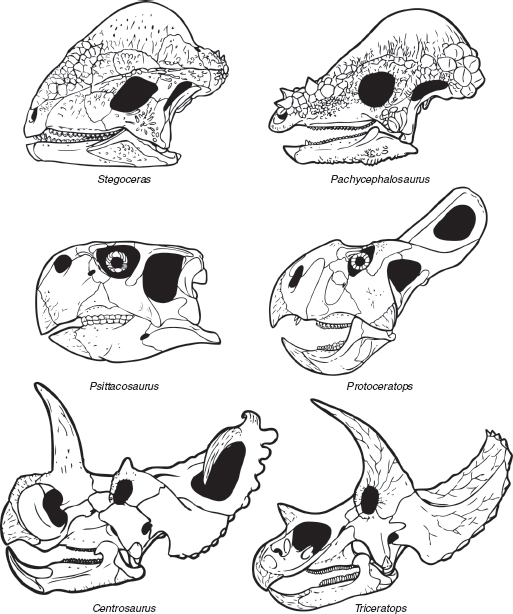
FIGURE 7.4
Variation in the skulls of margin-headed (marginocephalian) dinosaurs: top row, the pachycephalosaurs Stegoceras and Pachycephalosaurus; middle row, the ceratopsians Psittacosaurus and Protoceratops; bottom row, the ceratopsid ceratopsians Centrosaurus and Triceratops. Not to scale.
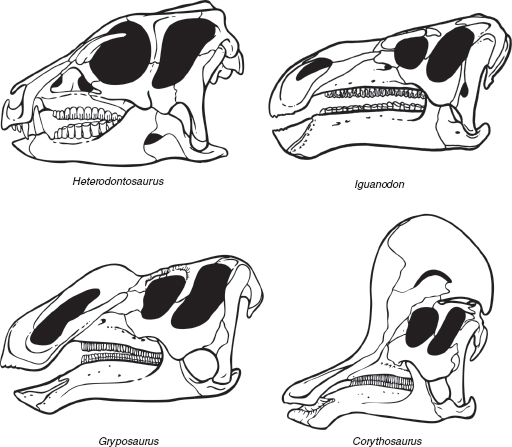
FIGURE 7.5
Variation in the skulls of ornithopod dinosaurs: Heterodontosaurus (a heterodontosaur); Iguanodon (an iguanodont); Gryposaurus (a noncrested hadrosaur); Corythosaurus (a crested hadrosaur).
These elaborate food-processing systems, which evolved independently in hadrosaurs and ceratopsids, were not well suited for chewing, as in mammals. Ceratopsids jaws simply sliced food items up into smaller chunks, whereas an oblique contact between upper and lower dentitions in hadrosaurs suggests some ability to crush and grind as well as slice. In both groups, the tooth-ridden jaws were augmented up front by an extremely thick, reinforced beak, narrow and parrot-like in ceratopsids, broader and more ungulate-like in hadrosaurs. Presumably the beak was used to break off plant matter, which was then sliced by the teeth prior to swallowing. With a keratin-covered beak, a formidable set of dental batteries, a lowered jaw joint, and plenty of muscle power, these advanced food-processing systems were the Cuisinarts of the Cretaceous, equipping horned and duck-billed dinosaurs with finely tuned feeding apparatuses as intricate as any that large herbivorous mammals would evolve more than 30 million years later. Based on their capacious gut regions, we can be quite sure that all of these giant herbivores also possessed elongate intestines full of fermenting microbes.
When it comes to pairing up particular kinds of herbivorous dinosaurs with their preferred plant diets, we remain entrenched in the dark. This unfortunate situation represents a major gap in our knowledge that future research may be able to fill. Studies of microscopic wear patterns on teeth could help, because different plants can leave varying traces of wear. Tooth enamel can also preserve isotopes, allowing researchers to investigate at least large-scale questions regarding diet—for example, meat versus plants, and open versus closed canopy forest settings. At the back end of the gastrointestinal (GI) tract, exceptionally rare instances of preservation sometimes include gut contents with processed plant materials. Similarly, there are a growing number of fossilized dung specimens (COPROLITES) sufficient in size to implicate dinosaurs as the poop makers. Examples of the latter two lines of evidence—gut contents and dung—suggest that hadrosaurs consumed conifers at least on occasion. Conversely, a few other coprolite specimens attributed to dinosaurs appear to include partially digested bits of angiosperms. Mostly, however, we are currently limited to sweeping generalizations and bald speculations.
Yet, given that plants were the dominant source of energy on land, and that dinosaurs were the dominant large-bodied herbivores in terrestrial ecosystems, we can be quite confident that coevolution between these groups occurred. Plants must have evolved various techniques for curtailing dinosaur herbivory, and plant-eating dinosaurs undoubtedly countered with a number of strategies to thwart the evolutionary innovations of plants. We must also keep in mind that there were many other kinds of smaller herbivores in these ecosystems, including mammals, birds, and insects. As noted earlier, insects in particular are known to have major effects on plant communities.
In lieu of direct evidence linking specific kinds of dinosaurs and plants, we must turn to indirect sources of information. One of the strategies most commonly employed by paleontologists is to search for patterns of evolutionary change in two coexisting groups, and then see whether these patterns coincide in meaningful ways. So, let’s take a look at the entire Mesozoic tenure of dinosaurs and plants and consider some possible examples of coevolution. Although the details of these plant-herbivore interactions remain obscured by millions of years of deep time, we can begin to construct testable hypotheses. And the known evolutionary patterns on both sides of the plant-dinosaur equation lead to some provocative scenarios. Many of the hypotheses summarized here come from the work of two paleobotanists, Scott Wing of the Smithsonian Institution and Bruce Tiffney of the University of California, Santa Barbara.
When dinosaurs first appeared, during the Late Triassic, the plant world was dominated by a range of seed plants. Remember that terrestrial ecosystems had undergone a dramatic transformation after the formation of Pangaea, with the water-loving spore bearers displaced in large part by arid-adapted seed bearers. Some of these, particularly among the conifers, reached heights far beyond those of any previous land plants, concentrating much of the foliage high above the ground. In contrast to their predecessors, Late Triassic plants also tended to have tough or thorny outer coverings, which meant that big herbivores now had to get by on relatively low-quality diets.
As previously noted, the Late Triassic rise of conifer trees, in both an evolutionary and an absolute height sense, may have been linked to coevolution with herbivorous dinosaurs. Conifers reached new heights at approximately the same time as the prosauropods. Long-necked prosauropods such as Plateosaurus in Europe and Riojasaurus in South America emerged as major players in Late Triassic ecosystems, greatly exceeding the sizes of all previous land-dwelling herbivores. They were accompanied by primitive sauropods and, whereas the prosauropods went extinct in the Early Jurassic, the sauropods remained the dominant large herbivores globally through the Jurassic. So it’s possible that the Late Triassic shift in defensive tactics seen in plant communities may have occurred partially as a response to dinosaurian consumers. Sauropodomorphs, with their newly evolved giant sizes and elongate necks, would have been able to browse at greater heights. With high and low browsers present, Triassic ecosystems appear to have been the first to include partitioning of plant resources by herbivores on the basis of height. This multitiered approach to herbivory is a pattern that evolved again and again, becoming a standard feature of terrestrial ecosystems during and after the Mesozoic.
The sauropod-conifer connection is thought to have persisted through the Jurassic and, at least in some areas, into the Cretaceous, with both groups diversifying into a broad range of forms around the globe. The largest Jurassic sauropods were capable of reaching more than 10 meters (30 feet) into the canopy. If certain kinds of sauropods were able to rear up on their hind legs, these animals would have been able to access even greater heights. Although some sauropods were likely unable to lift their heads high above their bodies, others probably used their large bodies and elongate necks to browse the crowns of tall trees. So long necks and tall trees could have evolved in tandem. Yet, if we are to be scientific in this investigation, we must consider all other possible reasons why trees became tall. For example, there may have been increased competition among the plants themselves for access to sunlight, with the advantage going to species that achieved greater heights.
Herbivore height, if it was a factor at all, was only part of the equation. Another important evolutionary factor was body mass—sheer bulk. That is, herbivores with greater body masses may have been more successful because Late Triassic floras were tougher and thus offered a lower-quality diet. In a world dominated by increasingly tough, fibrous vegetation, bigger animals with more elongate guts may have had a distinct advantage because they would have been better able to digest these problematic plants. Some have even argued that the feedback loop exemplified by this coevolutionary race provides the solution to an enduring mystery. Why were dinosaurs so big? Perhaps because herbivorous dinosaurs evolved increasingly large sizes in order to feed on taller, poorer-quality plants. Carnivorous dinosaurs, in order to be effective predators in a world of giants, simply followed suit, forced to mimic this prevalent pattern in their herbivorous prey. Although reality was undoubtedly more complex and less reminiscent of a “Just-So” story, this simplistic scenario may encapsulate an important kernel of truth, revealing a set of linked events that forever changed the face of terrestrial ecosystems.
Let’s now turn to the Cretaceous, during which the biggest event in the floral realm by far was the origin and spread of angiosperms. Flowering plants first appear in the fossil record in the Early Cretaceous, about 125 million years ago, and became the dominant land plants by the close of the Late Cretaceous, 65.5 million years ago. The Cretaceous also witnessed the diversification of many groups of dinosaur herbivores. This list includes duck-billed hadrosaurs (a subgroup of ornithopods) and the horned ceratopsids, with their highly specialized, food-processing jaws. Recognition of these patterns led to the hypothesis, first suggested in the mid-1980s by Robert Bakker and independently by Scott Wing and Bruce Tiffney, that herbivorous dinosaurs triggered the evolution of flowering plants. Bakker, in characteristically provocative style, went so far as to claim that “dinosaurs invented flowers.” According to this idea, during the Late Jurassic and Early Cretaceous the pervasive feeding activities of plant-eating dinosaurs placed great pressure on communities of seed bearers like cycads, gingkos, and conifers. This profound disturbance, they argued, resulted in greater evolutionary success (thanks to natural selection and adaptation) for plants with certain characteristics: rapid growth, tolerance to distur-bance, and the ability to regrow from underground buds. With spore bearers and most seed bearers unable to respond to this pressure, the stage was set for the spread of a new group of seed plants that possessed all of these features—angiosperms.
This hypothesis has received a pretty thorough thrashing from other paleobiologists, who note several contradictory points. First, there is minimal evidence of angiospermdinosaur interactions in the Early Cretaceous (and in the Late Cretaceous, for that matter). Second, while flowering plants became widespread by the Early Cretaceous, the fossil record suggests that they were minor ecosystem players, and that conifers and other nonangiosperm seed plants remained the dominant plants in terms of actual biomass. Third, many of the complex jaw mechanisms and other major feeding innovations in herbivorous dinosaurs appeared well before or long after the origin of angiosperms, suggesting that these features had little impact on the origin of flowering plants. In other words, the key evolutionary patterns in angiosperms and dinosaurs were not coincident, suggesting that other factors must have been involved in the “invention” of flowers.
Nevertheless, it is certainly conceivable that angiosperms effectively “underwrote” the Late Cretaceous radiations of herbivores such as hadrosaurs and ceratopsids. About half of all dinosaurs are known from the last 20 million years of the Mesozoic—thus, on the order of 50 percent of all dinosaur species are restricted to the final 12 percent of their 160-million-year Mesozoic tenure. Although this observation may result, at least in part, from problems of preservation and sampling, it has prompted some to argue that latest Cretaceous ecosystems were truly distinct from anything that had come before. Flowering plants provided an abundant, accessible energy source for plant-eating dinosaurs, and the capacity of these plants to exploit a wide range of environments meant that the dinosaurs could follow along into these novel settings. Angiosperms lacked many of the deterrents—particularly nonnutritious tissues and poisons—that made pteridophytes and earlier seed plants unappetizing by comparison.
This is not to say that flowers “invented” Cretaceous dinosaurs, any more than the opposite. Rather, plants and dinosaurs, together with the many other life-forms, coevolved—they cocreated each other; that is, the diverse parts of the ecosystem responded to one another via evolution. The danger with this kind of an explanation is that one is simply left with “everything caused everything”—not an enviable position for a scientist because, at least stated in this broadest sense, it precludes testing. Nevertheless, this kind of dependent co-arising of forms is likely a much more accurate description of the events and, indeed, of the way evolution works in general.
Although evolution’s complexity can be daunting, the situation paleontologists face today is exciting rather than bleak. Now that we are better able to identify patterns and their timing, we can begin to refine our ideas. And while we are still a long way from understanding Mesozoic ecosystems like we do the Serengeti, it’s important to remember that paleontologists have only begun to seek answers at the higher, more integrative level of ecosystems. Once this new, more integrative, coevolutionary perspective really takes hold, numerous unexpected discoveries are likely to follow. Of course, the dinosaur odyssey included carnivores as well as herbivores, and it is to the meat eaters that we now direct our attention.
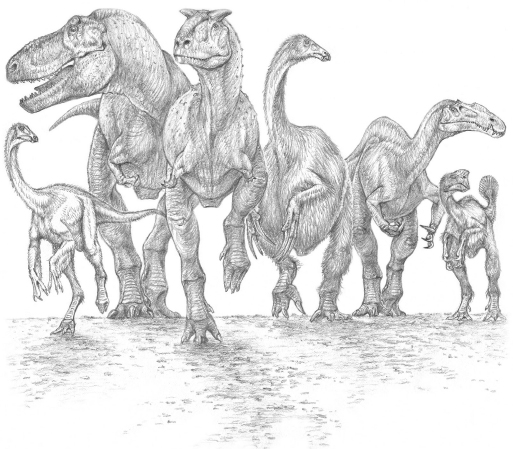
Representative theropod dinosaurs, including probable omnivores and herbivores, as well as carnivores. From left to right: the ornithomimosaur Ornithomimus (omnivore?); the tyrannosaur Tyrannosaurus (carnivore); the abelisaur Carnotaurus (carnivore); the therizinosaur Therizinosaurus (herbivore); the spinosaur Suchomimus (carnivore); the oviraptorosaur Khaan (omnivore?).