9
HIDDEN STRANDS
IT'S HUMBLING TO STAND in the belly of an ancient redwood, to be enveloped by one of the largest living things on Earth. The thick trunk surrounding me on this particular day was heavily charred, its base hollowed out by some long-ago fire, leaving behind an expansive interior cavity. Fires don’t ravage this forest often, at least not in the human sense. But measured in redwood lifetimes, sometimes well in excess of 1,000 years, fire is as inevitable as death. Fortunately, evolution has endowed these majestic trees with flame-retardant bark and other survival strategies, even allowing them to benefit from the periodic cataclysms.
Exiting the tree, I shifted my gaze skyward, feeling vertigo as my eyes followed the trunk to dizzying heights. Although the uppermost branches were obscured by a sea of green foliage, I knew that the topmost needles were processing sunlight about 90 meters (300 feet) above me. This was a coast redwood, Sequoia sempevirens, a species that includes the tallest organisms on Earth. The largest known examples achieve heights of about 115 meters (375 feet), taller than a 35-story building. Today, only three kinds of sequoia remain, but their legacy, like their trunks and life spans, is long. Sequoias not so very different from these graced the world of dinosaurs, so a visit to a redwood forest is like stepping back into deep time. As such, these forests provide another excellent place to contemplate the dinosaur odyssey.
This particular hollowed-out tree lives in Muir Woods National Monument, a stand of old-growth redwoods named after the famous tree lover and environmentalist John Muir. It is also the closest patch of old-growth forest to the city of San Francisco. As a result, the Monument receives almost a million visitors each year, a fact that would have pleased Muir greatly. Although I had visited Muir Woods many times, on this day I had come not to dwell on the majestic trees but to contemplate some of the less obvious, hidden aspects of the forest.
Muir Woods is home to several varieties of nonredwood trees. Meandering farther down the loamy path, my attention was drawn to a large overhanging leaf from a California bay laurel. Several holes were visible within it, and a few chunks were missing from the leaf margin. Still closer inspection revealed that these imperfections were edged by characteristic patterns left by the jaws of feeding insects. Though I could not see or hear them, I knew that the world around me was full of tiny herbivores.
A butterfly flitted by chaotically, looking for a meal of flower nectar, totally unaware that its activities were part of the plant’s sexual dance. My next encounter was with a massive fallen redwood paralleling the path. After inhaling carbon dioxide and exhaling oxygen for more than a millennium, and after providing sustenance, shade, and safe haven to countless shrubs, microbes, fungi, and animals, this monstrous plant fell to earth, hammering several smaller trees on the way out. Yet the shattering event was not so much an end as a beginning.
When a tree dies, it is occupied by a slow parade of decomposer organisms, all of which, in one way or another, break down and consume its carbon, returning its nutrients from whence they came. The process often begins with beetles, which tunnel into the bark in order to generate larval nurseries. The battery of holes in the tree bark before me revealed that beetle activity was already well underway, providing access points for water and a host of other decomposer organisms—bacteria, mites and worms, among others. Beetles carry fungi on their bodies, which transfer to the wood and begin to consume and further decompose the tree. When the beetle larvae emerge in the tunnels, they feed on the newly arrived fungi before boring still deeper into the fallen tree. About the same time, more kinds of beetles arrive, as well as ants; together these insects penetrate the heart of the dead log. Wasps enter many of the tunnels, locate the beetle larvae, and lay eggs nearby, so that the newly hatched wasps have a plentiful food supply. After a year or so, different kinds of fungi capable of processing the highly fibrous redwood interior follow the insect highways, spreading throughout the log’s interior. They are followed closely by termites and their gut bacteria, which together process the extremely coarse cellulose through a fermentation process. Bacteria in the termite guts also capture nitrogen and return it to the environment as their hosts die.
This fallen giant may take three or four centuries to fully decompose. Throughout the lengthy process, the redwood will provide a constant and vital source of nutrients to the environment. In fact, the tree will become a complex microecosystem unto itself, home to a succession of tiny life-forms. Without this complex organic cavalcade, the tree would take much longer to decompose and the valuable chemicals locked within its body would be unavailable for use by the larger system. Paradoxically, this redwood will become more alive in death than it was in life. During its lifetime, a tree is composed of about 5 percent living tissue; other than a thin layer near the surface that transmits water and nutrients up and down, the massive trunk is made up of dead matter. (This is why a tree with a fire-hollowed trunk can still thrive for many years.) A fallen tree in an advanced state of decay may be 20 percent living tissue by weight, thanks largely to occupation by decomposers. In a sense, then, trees have two lives, the first dominated by the capture of solar energy and the second by breakdown of that energy into a bounty of nutrients. The rotting log also underlines an important ecological truism: every species has a unique role in maintaining the larger ecosystem of which it is a part.
Stooping down, I picked up a handful of loose earth. There in my hand was the heart and soul of decomposition, capable of transforming any fallen leaf, tree, or animal into the chemical building blocks of future generations. Running the rich, soft substance through my fingers revealed a moisture-laden mixture of plant detritus, fungal strands, and tiny organisms. I reminded myself for the umpteenth time that microbes make the living world go round, as they always have. A random fistful of forest soil like this one contains billions of microscopic life-forms, mostly bacteria, exceeding the number of humans currently living on the planet—a staggering thought.
Of course, microbes are not restricted to the soil. They exist virtually everywhere in every ecosystem, so small and so firmly embedded in all aspects of life that we barely notice them. To give an example close to home, the human mouth contains more than seven hundred different kinds of symbiotic bacteria, each of which has carved out a unique existence amid a complex topography of tongue, teeth, and gums. Before you run off and brush every square millimeter of your mouth raw, remember that, by combating disease-causing bacteria, this phalanx of microbes is vital to your health. The same is true for many of the bacteria in your gut, on your eyelashes, in your nose, and indeed throughout your body. What you regard as your physical self includes on the order of 10 trillion cells, yet nine out of ten of these are not human cells. Put another way, your body is home to many more life-forms than there are people on Earth or stars in the Milky Way galaxy. Like it or not, you are a walking colony—or, better still, an ecosystem—living in unwitting harmony with these smidgens of life. This phenomenon held true for dinosaurs as well; it is awe inspiring to contemplate the long-necked sauropods, largest animals ever to walk the Earth, as gargantuan, four-legged bacterial colonies.
The previous pair of chapters followed the flow of energy from plants to herbivores to carnivores. Yet the changing web of life includes additional paths of energy flow involving the microscopic extremes of life’s spectrum. Although human attention is naturally diverted toward dinosaurs and other creatures at the macro end of the spectrum, this bias should in no way lead us to conclude that absolute size is somehow proportional to ecological importance. Indeed, most ecosystems would likely persist in some form if the large vertebrate contingent were suddenly removed. But so critical are the strands of energy flow maintained by insects and microbes that expulsion of either would lead to immediate systemic collapse. Although Tyrannosaurus never stalked a fly or a bacterium, the complex interlinking of ecosystem subparts means that these Lilliputians of the Mesozoic were every bit as important to the Tyrant King’s survival as was Triceratops. Therefore, any discussion of the dinosaurian web of life would be grossly incomplete without some consideration of the Mesozoic microworld.
Unfortunately, the fossil record of Mesozoic insects, fungi, and bacteria is grossly incomplete, not surprising given their small sizes and lack of hard parts. By necessity, then, much of the following discussion is derived from the living realm. Yet even our understanding of modern microbes is woefully inadequate. Despite sending manned and unmanned vehicles to other worlds, we have the barest acquaintance with the surface of our own planet. Our gross ignorance of Earth’s biota is revealed by the fact that we can only make the roughest of guesses as to the total number of species currently living on Earth. Estimates vary wildly, ranging from about 4 million to greater than 100 million, with 15 million an oft-cited ballpark figure. Of that astounding total, less than 2 million kinds of life-forms have been recognized as distinct and given Latinized genus and species names. Of those formally named species, only a tiny fraction is known much beyond this label. For most of nature’s burgeoning diversity, then, we lack even basic information regarding life span, diet, and reproduction, let alone details of interactions within and between species.
On the plus side, the fossil record is far from silent with regard to these hidden strands. The past two decades have witnessed an abundance of insights relating to Mesozoic plants and insects in particular, and several exciting discoveries even point to a key role for bacteria in the process of fossilization. The scientific strategies used to find and unravel these fossil strands closely resemble those portrayed nightly in the highly successful crime scene investigation (CSI) genre of television dramas. A diverse range of forensic-style tools and techniques tease out bits of evidence preserved at prehistoric death scenes. In the current media-driven lingo, these investigations into ancient processes amount to the ultimate in “cold cases.”
Our record of Mesozoic plants comes from fossilized tree parts—particularly trunks, branches, leaves, and flowers—as well as from microscopic pollen grains. Unlike dinosaurs and other vertebrates, for which mostly complete skeletons are not infrequently recovered, complete trees are virtually absent from the fossil record. Like several blind people touching different parts of an elephant, paleobotanists do their best to connect the pieces and describe entire plant species. Despite this uncertainty, we can be sure that Mesozoic plants faced the same kinds of challenges as their modern counterparts. And, as discussed in chapter 7, they also developed various kinds of mechanical, chemical, and biological defenses to ward off herbivores. Many defenses observed in living plants appear to have arisen during the Mesozoic, when both insects and dinosaurs were proliferating. Like the herbivorous dinosaurs, insects undoubtedly responded by evolving specialized jaw mechanisms and digestive strategies.
Given that insects represent a major, even dominant herbivorous impact on plant communities, it would be very useful to know about the insects that coexisted and coevolved with dinosaurs. Despite their relative lack of hard parts, we have an extensive fossil record of insects. Much of this evidence comes in the form of insects trapped in tree sap and subsequently turned to amber. The vast majority of the insect groups alive today lived alongside dinosaurs from the Jurassic onward. Insect species tend to be long-lived, persisting on average about 10 million years, and many Mesozoic insects appear closely related to forms alive today. This remarkable longevity stands in stark contrast to mammals and other vertebrates, whose species typically persist for about one million years. Most mammal groups, not to mention the species within them, evolved well after the major dinosaur extinction 65.5 million years ago.
Although Mesozoic insects are plentiful at a growing number of amber localities around the world, much more abundant are fossil leaves that preserve distinctive insect-feeding traces. Go outside to the nearest tree, and chances are that many leaves will show insect feeding traces akin to those that I saw in Muir Woods. Spend some time examining those traces under a microscope, and you will soon find that there are several distinct types in any particular place. Studies of living insects demonstrate that these variations in feeding traces correspond to specialized mouth parts and feeding behaviors that represent alternative strategies to counter plant defenses. As evolution adjusts plant composition or leaf shape, it also counters with insect-feeding innovations.

FIGURE 9.1
Photographs of fossilized insects preserved in amber. Clockwise from top right: a tick, a leafcutter ant, a bee, and an unidentified insect.
In an exceptional piece of detective work, paleontologists Conrad Labandeira and Peter Wilf of the Smithsonian Institution discovered that many distinctive feeding traces left by insects on modern plants can be found on fossil leaves. This deep time correlation has provided an amazing diagnostic tool. Just as human footprints provide conclusive evidence of people, insect-feeding traces from the Mesozoic can be matched to modern groups to demonstrate the presence of those insects (or near relatives) in the fossil record. One example involves a group of leaf beetles known as hispines, which today feed on ginger and leave behind long, narrow feeding marks about 2.5–3.0 millimeters (about 0.1 inch) wide. The researchers found the same traces on fossil ginger leaves dating to the end of the Cretaceous, pushing back the known fossil record of leaf beetles more than 20 million years. The presence of these specialized beetles feeding on Mesozoic gingers implies that this relationship is ancient, dating back at least to the Cretaceous. Together, the evidence of insect body fossils and feeding traces provides a surprisingly detailed picture of ancient insect diversity for certain places and times, an understanding that is certain to grow in the coming years.
How important were insect pollinators during the Mesozoic? Some Cretaceous insects, particularly flies, have elongate noses, or probosces, that likely served as straws to sip flower nectar. Direct (and remarkable) evidence of nectar feeding includes nectar preserved within the guts of Mesozoic insects. Bees, the most important group of modern pollinators, appear to have evolved and spread during the Cretaceous alongside many other insect groups. In all, considering evidence for both herbivory and pollination, it’s likely that insects, perhaps much more so than dinosaurs, underwent extensive coevolution with plants (particularly flowering plants) during the latter half of the Cretaceous.

FIGURE 9.2
Photograph of Late Cretaceous angiosperm leaf fossil (Leepierceia perartocarpoides) showing two kinds of insect damage.

PLATE 1 The Berivotra field area, northwestern Madagascar.

PLATE 2 Site 93-18 in the Berivotra field area, Madagascar, which has produced numerous fossils of Late Cretaceous dinosaurs.

PLATE 3 Wildebeest herd crossing the Mara River, Kenya.

PLATE 4 Three Diplodocus sauropods—two adults and one juvenile—saunter across a Late Jurassic floodplain.

PLATE 5 The Late Jurassic plated wonder Stegosaurus.

PLATE 6 While a Late Cretaceous tyrannosaur, Albertosaurus, feeds on a rotting carcass, the small maniraptor theropod Bambiraptor must be content with scraps.

PLATE 7 Exposures of the Late Triassic Chinle Formation, Utah.
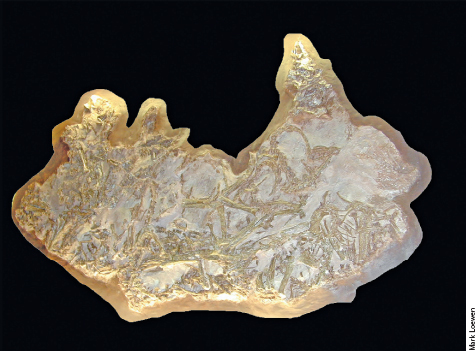
PLATE 8 Photograph of a Coelophysis fossil block collected at Ghost Ranch, showing a jumble of skeletons of this Late Triassic coelophysoid theropod.

PLATE 9 A Late Jurassic Morrison scene. A large theropod, Allosaurus, attempts to chase down a small ornithopod, Dryosaurus.

PLATE 10 Exposures of the Late Jurassic Morrison Formation, Utah.

PLATE 11 Achelousaurus, a short-frilled (centrosaurine) horned dinosaur from the Late Cretaceous of Montana that was named by the author.

PLATE 12 Corythosaurus, a crested (lambeosaurine) hadrosaur from the Late Cretaceous of Alberta.

PLATE 13 Predator and prey in Late Cretaceous Montana. A large tyrannosaur theropod, Daspletosaurus, attacks a herd of horned dinosaurs, Einiosaurus.

PLATE 14 Exposures of the Late Cretaceous Kaiparowits Formation, Grand Staircase–Escalante National Monument, Utah.
So insects clearly played important roles guiding energy flow within Mesozoic ecosystems. Through their efforts as pollinators, some participated in plant reproduction and energy production. As major plant consumers, others participated in the flow of energy through food webs. And just as plant toxins kept insect populations in check, insects must have returned the favor, limiting the spread of plants and helping maintain ecosystem balance. As we shall discover, insects were also important decomposers, converting organic materials into nutrients for future generations of plants.
Waste is an empty concept as applied to ecosystems. Imagine a forest in which dead plant and animal matter—branches, leaves, bones, trunks, and desiccated flesh— collected on the forest floor rather than decomposing. In addition to being a treacherous and smelly place to walk, such an ecosystem would not long survive, because organisms of successive generations are made up largely from the reconstituted remains of previous generations.
Ecology and evolution have worked in tandem millions upon millions of years to all but perfect the recycling of organic matter, whatever its source. Just as for plants, the death of any animal—be it a beetle or a buffalo—triggers a succession of decomposing organisms. For up to a century after a whale dies and sinks to the bottom of the sea, its body becomes the focal point—indeed, the foundation—of a richly diverse ecosystem. These “whale falls,” which often descend to depths in excess of 2,000 meters (6,500 feet), inject a tremendous amount of food—up to 145 tons (320,000 pounds) of organic material—into an otherwise resource-poor setting. The animals that congregate around cetacean carcasses include scavengers such as sharks, hagfish, and crabs, which devour the whale’s soft tissues. Others organisms exploit the sulfide-rich habitat generated by bacterial decomposition of blubber. Many of the greater than four hundred species that inhabit these deep sea menageries appear to have evolved specifically to take advantage of these transient islands of resources. Perhaps most bizarre are bone-burrowing worms lacking eyes, mouth, or stomach yet sporting feathery plumes that behave like gills. The worms also carry symbiotic bacteria specialized for digesting oils and fats within whale bone.
It is interesting to contemplate a parallel situation involving “dinosaur falls.” A dead sauropod would also have yielded a multiton island of resources, attracting a spectrum of terrestrial decomposers to the feast. And, as we shall see, some of these Mesozoic waste- recyclers were also specialized to exploit bone.
In the case of large, land-living vertebrates, the distinctly disgusting sequence of carcass decay has been well studied and can be summarized as follows. During the first two days following death, the carcass remains fresh looking, though it is beset upon by a variety of bacteria, protists, and nematode worms. Various vertebrate carnivores are also most likely to devour their share of the remains during the first day or so. Days 2 to 12 comprise the period of putrefaction. Blowflies and wasps arrive, laying their larvae on the carcass. Within the body, the activities of gut-living bacteria have continued despite the death of their host, causing the buildup of gases. Unable to escape the GI tract, these internal gases lead to bloating and distortion. From days 12 to 20, the degrading flesh changes texture, becoming “creamy,” and the greatly distended gut finally ruptures, generating a foul odor. The collapsed carcass then becomes the target of still more wasps. From about days 20 to 40, the carcass dries out and dermestid beetles arrive in great numbers, rapidly devouring any remaining flesh. Mites and fungi also get in on the act, now able to infiltrate almost any region of the carcass. For about a year following day 40, a more gradual transformation occurs, marked by further drying and decay. Dermestids, mites, and fungi continue their work unabated until only the bones remain. In some instances, the bones are targeted by boring beetles or other insects, which often leave larvae within the excavated tubes. In some ecosystems, specialized vertebrates like porcupines or hyenas assist in the bone decomposition. Ultimately, left exposed to the elements, the bones break down and disintegrate, disappearing within a few years.
In recent decades, paleontologists have begun to look more closely at dinosaur fossils, with an eye toward identifying other life-forms that interacted with the decaying carcasses. On the macro side are such modifications as trampling breaks and tooth marks, some left behind by dinosaurian carnivores that scavenged the carcass or perhaps made the kill. On the micro side are bone modifications made by other groups of organisms, providing clues to broader ecological interactions. Just as with leaves, ancient insects left distinctive traces on bones. Most consist either of borings that penetrate the bone’s interior or meandering trails on the surface. The former have been interpreted as pupal chambers excavated by carrion beetles, whereas the latter are thought to be feeding traces. In some instances, the same osteophilic (bone-loving) beetles, or at least close relatives, may have been responsible for both kinds of traces, the first associated with survival (surface traces), and the second with reproduction (borings).
Occasionally, residues found around dinosaur skeletons appear to be the fossilized remnants of bacteria and perhaps fungi. Several recent studies indicate that bacteria are integral to fossilization, particularly in the rare instances of soft tissue preservation. This might seem counterintuitive, because many bacteria are excellent decomposers, notorious for breaking down and recycling organic matter rather than preserving it. Yet decay and fossilization may sometimes go hand in hand. Particularly under watery conditions, bacterial colonies can generate gel-like secretions that generate a biofilm halo around a carcass. Through a process called BIOMINERALIZATION, these bacteria can also secrete minerals, either within their cell walls or directly into the surrounding environment, and thereby play a critical role in fossilization. This topic is likely to see a lot of attention in the coming years, and my suspicion is that bacteria will turn out to be critical in the preservation of fossils generally.
I once saw a sewer-and-drain plumbing van emblazoned with the cartoon of a proud, uniform-clad man bearing a broad smile and a Roto-Rooter-style cleaning hose. Beneath was the slogan “Your waste is my bread and butter.” Although unappetizing, this motto is apt for many poop-loving organisms. After all, dead animals are not the only organic debris in need of recycling within ecosystems. Throughout their lifetimes, animals generate excrement, cumulatively depositing many tons of it each day in almost every ecosystem. And the “no waste” rule imposed for carcasses applies equally to fecal matter. Dung provides a rich source of nutrients for organisms that have evolved to consume it. Regardless of their metabolic strategies, giant dinosaurs undoubtedly produced whopping masses of dung. Surely we would expect important clues to be hidden inside ancient poop, were we to find any.
Coprolite is the term scientists use to refer to fossilized dung, and Karen Chin of the University of Colorado, Boulder, is a world expert. Fortunately, her chosen subject matter is matched with a wry sense of humor. So it’s not surprising that Chin has variously been dubbed “Queen of Coprolites,” “Duchess of Dung,” and “Princess of Poop,” among other unmentionable titles. She has identified a variety of different kinds of fossilized scat ascribed to dinosaurs, including both herbivores and carnivores. She was lead author of the study that described the king-sized T. rex coprolite mentioned in the previous chapter. Perhaps contrary to your first squeamish impressions, “paleoscatology” (as the field is sometimes labeled) is neither dirty nor disgusting. Feces that survive the rigors of fossilization are fully lithified and thus bereft of squishiness and foul smells. Like their modern counterparts, fossilized dung comes in a variety of shapes and sizes. Some appear classically scatlike, complete with pinched ends and striations left behind by the anal sphincter, whereas others are nondescript masses whose identification requires an expert eye.
To tease out secrets hidden within coprolites, Chin uses many of the same technologies employed by dinosaur histologists. A diamond-studded saw blade cuts thin slices from rock-hard dung specimens. These sections are then ground down to thicknesses on the order of a few thousandths of an inch before being mounted on glass slides. Viewed through a high-powered microscope, the slices reveal a complex amalgam of organic flotsam and jetsam. Carnivorous dinosaur scat may preserve bits of bone, teeth, and even muscle, whereas herbivore coprolites often include a mash of leaf parts, stems, branches, seeds, pollen, and/or spores. Interestingly, Chin thinks that the preservation of dinosaur dung is also due, at least in part, to the mineralization-promoting metabolic activities of bacteria.
Poop spotting, at least when it involves the lithified scat of dinosaurs, is as much art as science. One has to get the right search image. To complicate matters, coprolites, like other kinds of fossils, vary in color and texture, as well as shape. I remember a cool summer morning in the late 1980s, walking with Chin through scenic Late Cretaceous aged bad-lands in northern Montana. The region is known as Egg Mountain because of the spectacular discoveries of dinosaur nests and eggs found there by Jack Horner and his crews. Egg Mountain had also yielded several large mass death assemblages of a hadrosaur that Horner named Maiasaura, the “good mother lizard,” because its fossils were associated with nests of juveniles that appear to have benefited from parental care. Chin told me that she had identified several large coprolites from the same area that were attributable to a large herbivore, perhaps even Maiasaura. I was skeptical, as I had walked this small chunk of terrain daily for several weeks and seen no sign of any fossilized dung. However, Chin walked purposefully to several irregular broken masses of rock, the largest being more than a foot across. Kneeling down, she pointed out numerous plant fragments within the rock. She told me the woody fibers, mostly derived from conifers, had traveled through the intestinal tract of an animal (a big one, given the magnitude of the dung blocks). She also noted what appeared to be small tunnels through the specimen and conveyed her suspicion that these might be fossilized insect burrows, perhaps left behind by dung beetles.
This suspicion was later confirmed when Chin contacted dung beetle specialist Bruce Gill in Ontario, Canada. Although the ancient scat contained no remains of the dung beetles themselves, many of the burrows were backfilled with organic matter from the dung pat. Plenty of invertebrates can dig through a pile of dung, but only dung beetles (formally known as paracoprids) backfill their tunnels. The beetles burrow through fresh dung in order to create nutrient-filled brood chambers for their young. Chin’s subsequent studies of these same dung masses revealed two additional hidden web insights. First, the coprolites contain dozens of snails of four different species; the large, moist, microbe-laden maiasaur pies apparently offered excellent food sources and roosting sites for the snails. Second, the chunks of conifer wood in the dung are not mixed with leaves, stems, or branches, suggesting that the maiasaurs deliberately ate wood from the core of the tree. Living wood is typically not nutritious, but the partially digested chunks in the dung were degraded by fungus. Chin’s idea is that the hadrosaurs fed on trees that were not only dead but in an advanced state of rot, loaded with fungus and other detritus feeders. If so, the giant dinosaurs may have, at least on occasion, consumed the rotten wood not so much for the wood itself but for the calories and carbon in the decomposers.
Chin’s discoveries, made with a series of colleagues, are significant on several fronts. They provide direct evidence of an odd food item, rotting wood, in the diet of a Cretaceous dinosaur. They push back the known fossil record of dung beetles millions of years into the Mesozoic, demonstrating that these poop-loving insects evolved alongside dinosaurs instead of mammals, as previously thought. And, perhaps most intriguing, this research illuminates multiple ecological links in a Cretaceous food web—from conifer producers to dinosaur consumers to insect, snail, and fungal decomposers—a rare and marvelous look at a 75-million-year-old ecosystem.
Another example comes from India, where researchers studying a collection of Late Cretaceous coprolites recovered not only plant remains within the fossilized dung but several kinds of fungi as well. Remarkably, these prehistoric fungi appear to belong to forms still alive today. Some of the fungi are plant parasites that attach to leaves. One of these, Colletotrichum, causes leaf spot and red rot diseases, whereas others, exemplified by Ersiphe and Uncinula, produce powdery mildew on leaves (something I see in my own garden). The study also revealed examples of a mycorrhizal soil fungus named Glomus. Here, then, we glimpse another ancient food web. The herbivorous dinosaurs (perhaps the titanosaur sauropods recovered from the same deposits) consumed leaves inhabited by parasitic fungi; after passing through the mouths and GI tracts of the dinosaurs, undigested portions of these meals were defecated and later inhabited by a different kind of fungus, which removed nutrients from the fecal matter and perhaps distributed them via symbiotic alliances to other plants.
Discoveries of Mesozoic fungi provide an appropriate segue from aboveground habitats to subterranean realms, where the real heavy lifting of ecosystem recycling takes place. Fungi also propel us back to the present day.
Soil is Mother Nature’s mistreated stepchild, either overlooked or treated with disdain as mere “dirt.” Walking through field, forest, or desert, we tend to think of the substrate beneath (if we think of it at all) as merely a physical substance. Yet this view is entirely wrongheaded. Yes, soil contains plenty of minerals, air, and water. But, as noted earlier, it is also brimming with life-forms, living and dead—plants, fungi, earthworms, insects, mites, millipedes, centipedes, nematodes, and sow bugs, to name a few. While we are being entranced by soaring trees, colorful birds, and majestic predators, a greater mass of life-forms is living within the soil than above it.
Many of the animal denizens inhabiting soils are smaller than the period at the end of this sentence. One entomologist calculated that, with every step taken in a temperate forest, your foot is supported on the backs of 16,000 invertebrates, in turn held up by an average total of 120,000 legs!1 To focus on just two groups, the total weight of all ants in the world is roughly equal to the cumulative mass of all 6.8 billion humans, and the tiny nematode worms, virtually ubiquitous in soils worldwide, are estimated to comprise four-fifths of all animals on Earth. A single cubic inch (16 cubic centimeters) of soil can contain more than a mile of interwoven mycelia, the fungal mats that pervade the first few inches of soil. As if these figures weren’t sufficiently mind-boggling, a single gram of fertile soil held between your fingers will contain on the order of ten billion bacterial individuals and perhaps six thousand bacterial kinds, or species. To date, biologists have formally named just over six thousand bacterial species, about the same number found in that gram of soil.2
This means that every handful of forest soil is a burgeoning microecosystem— complete with a stunning array of plants (or at least plant parts), herbivores, carnivores, and decomposers—about which we have only the faintest inkling. Beneath our feet are tiny wildernesses in which the dramas continually playing out are every bit as compelling as lions stalking zebra or tyrannosaurs preying on horned dinosaurs. In this alien microrealm, in the darkness amid tangled roots of trees and shrubs, vast inter-linked networks of diaphanous fungal strands transfer nutrients between plants, ants are frequently the dominant predators and scavengers, and a succession of ever-smaller organisms continually grind up and recycle the dead.
Soil, then, is the epitome of the hidden web. We know that soils are essential to the health of terrestrial ecosystems, that they provide, to use a recent phrase, vital “ecosystem services.” Primary among these is the recycling of several essential elements, including nitrogen, phosphorus, and sulfur. Yet our ignorance of this fertile, belowground realm is such that we don’t really understand why there are so many species packed into such small spaces. Larger, burrowing animals, including many mammals, mix air, water, minerals, and organic materials, a churning task taken to another level by smaller organisms such as ants and earthworms. Still-smaller life-forms break down and recycle organic remains, the feces of one becoming the food of another. As one researcher noted, soil is perhaps best thought of as “bug poop.” The activities of these myriad composters, all supported by specialized bacteria within their guts, replenish available nutrients and enable plant growth, thereby allowing the system to continue capturing solar energy. In addition to sustaining the terrestrial food supply, soil is also the primary water filter, cleansing and storing water for ecosystem use. Moreover, together with climate, soil determines which life-forms can live in a given area.
Although numerous examples exist of aboveground symbioses (e.g., the aforementioned ants and bull’s-horn acacia), such partnerships are even more plentiful below-ground. One of the most important is mycorrhizae (“fat roots”), the multispecies unions that partner plants with either bacteria or fungi. All the nitrogen used by plants is derived from the decomposition of previous generations of plants and animals. However, because plants are unable to absorb nitrogen directly from the soil, an intermediary is required. Plant roots are generally accompanied by stringy bacteria, seen as swellings on the roots. These plant-bacteria symbioses are the mycorrhizae. The bacterial side of this partnership takes up nitrogen from the soil and transforms it into amino acids that can be used by the plant for growth. The bacteria in turn receive nourishment from the plant roots.
Mycorrhizae also come in fungal varieties, which absorb phosphorus for plants and enable them to deal with harsh environmental conditions such as drought, high temperatures, and toxins. It turns out that fungi are remarkable organisms, far more critical to the health and survival of terrestrial ecosystems than ever imagined just a few decades ago. Together with bacteria, fungi are the most important organic recyclers. A mushroom is merely the small fruiting body of a fungus, the proverbial tip of the iceberg. The great bulk of fungal biomass is hidden belowground, forming dense mats of interwoven mycelia. Virtually ubiquitous in the first few inches of soil all over the planet, mycelia rank among the most important soil builders. Today, and likely for most of the past half billion years, the largest organisms on Earth have not been dinosaurs or sequoias but fungi, in the guise of these mycelial mats. The largest example known, a 2,400-year-old honey mushroom (Armillaria ostoyae), runs only 1 meter (3 feet) deep but spans about 9square kilometers (3.5 square miles, or 2,200 acres) and weighs an estimated 550,000 kilograms (1,200,000 pounds)!
Like all parts of an ecosystem, soil is always changing. Forming millimeter by millimeter, soil is constructed from a mixture of rock below and organic debris above, a process dependent on rock weathering. Physical or mechanical weathering—driven by wind, rain, and freezing—causes rocks to break into smaller fragments. Water percolating into cracks may freeze and thaw, leading to expansion, contraction, and, ultimately, splitting of rocks. Chemical weathering, often the result of rain and water flow, alters the chemical structure of rock. Over large spans of time, water with dissolved carbon dioxide literally erodes rock surfaces. A third factor, biological weathering, was overlooked until recently. Roots penetrate rocks in search of nutrients and lead to fracturing. Fungi in lichen produce more acids that corrode rock faces. And many kinds of bacteria secrete compounds that cause swelling inside fissures and cracks within the rock, leading to further fragmentation. In all, it is estimated that rates of rock weathering and erosion are a thousand times greater than they would be on a lifeless planet. Over geologic time, entire mountain ranges are flattened by this cadre of forces.
But what about Mesozoic soils? Can we be sure that soils comparable to modern examples, with such a bewildering array of tiny and microscopic diversity, were present beneath the feet of dinosaurs? After all, it’s not like we can sample the diversity of, say, a Late Cretaceous soil horizon. The truth is, we can actually dig into ancient soil horizons and find evidence of life, often including abundant root traces, burrows, and even signs of bacterial activity. Beyond these clues, however, scientists are extremely confident that Mesozoic terrestrial ecosystems, like their modern counterparts, were nourished and dependent on a highly diverse, organic-rich interface between earth, water, and air.
The early Earth lacked soils altogether. Prior to life’s migration onto land, the unconsolidated material derived from rock was a barren substance that we refer to as “regolith,” the kind of stuff we find on the moon’s surface. The spread of life into the terrestrial realm was initiated by bacteria, whose metabolic activities began to dissolve exposed rocks. It’s likely that fungi were the next major group to follow, ultimately forming their dense mats of mycelia. Later came the plants, establishing a terrestrial beachhead more than 350 million years ago. Through their inputs into rock weathering, countless generations of these varied life-forms generated the first true soils, disintegrating boulders while alive and contributing their own remains to the organic mix postmortem. This earthy matrix provided a rich substrate for land-based evolution, including the invertebrates and vertebrates that subsequently migrated inland from the water’s edge. Over millions of years, an increasing diversity of organisms occupied more and more land, with additional incursions likely dependent in large part on the generation of soils. This thin veneer of organic matter began to envelope the continents, carrying with it a suite of complex molecules that enabled plants to flourish, together with the consumers and decomposers dependent on them. Dinosaurs, latecomers on the terrestrial scene, arrived into a world of complex soils replete with mycelial mats, mycorrhizal symbionts, and numerous other specialized soil orgranisms. Importantly, without this previous, lengthy occupation of land by soil-dependent life-forms, there would have been no dinosaurs—and no us, for that matter.
So life has depended on soils virtually from the time it first ventured beyond its aquatic confines. Whether or not the numbers of Mesozoic invertebrate and bacterial soil species approximated the astonishing modern levels of diversity is an open question. But, without doubt, that all-important brown, crumbly, poop-filled matter was there, providing the substrate for the dinosaur odyssey. The take-home message here is that, even in a world graced by such biological splendors as dinosaurs, redwoods, and humans, we are forced to concede that microbes rule.
Bizarre headgear in short-frilled (centrosaurine) horned dinosaurs. Whereas Styracosaurus albertensis (top middle) displays one of the more flamboyant expressions of frill ornamentation, others possess equally unusual skulls: (from left to right) Centrosaurus brinkmani, Einiosaurus procurvicornis, Pachyrhinosaurus lakustai, Achelousaurus horneri, and Albertoceratops nesmoi.