
Once it leaves the kidneys, urine moves through the ureters to be stored in the urinary bladder until it is excreted through the urethra.
Much of this section will center on the kidneys and problems associated with their failure. It has been said that if the kidneys die or fail, so does the organism. With dialysis, the rate of kidney failure has been slowed; however, without dialysis, a patient would not be able to survive more than a week or so because of the buildup of toxins in the bloodstream.
The excretory system serves many functions, including the regulation of blood pressure, blood osmolarity, acid–base balance, and the removal of nitrogenous wastes. The kidneys play an essential role in these functions.
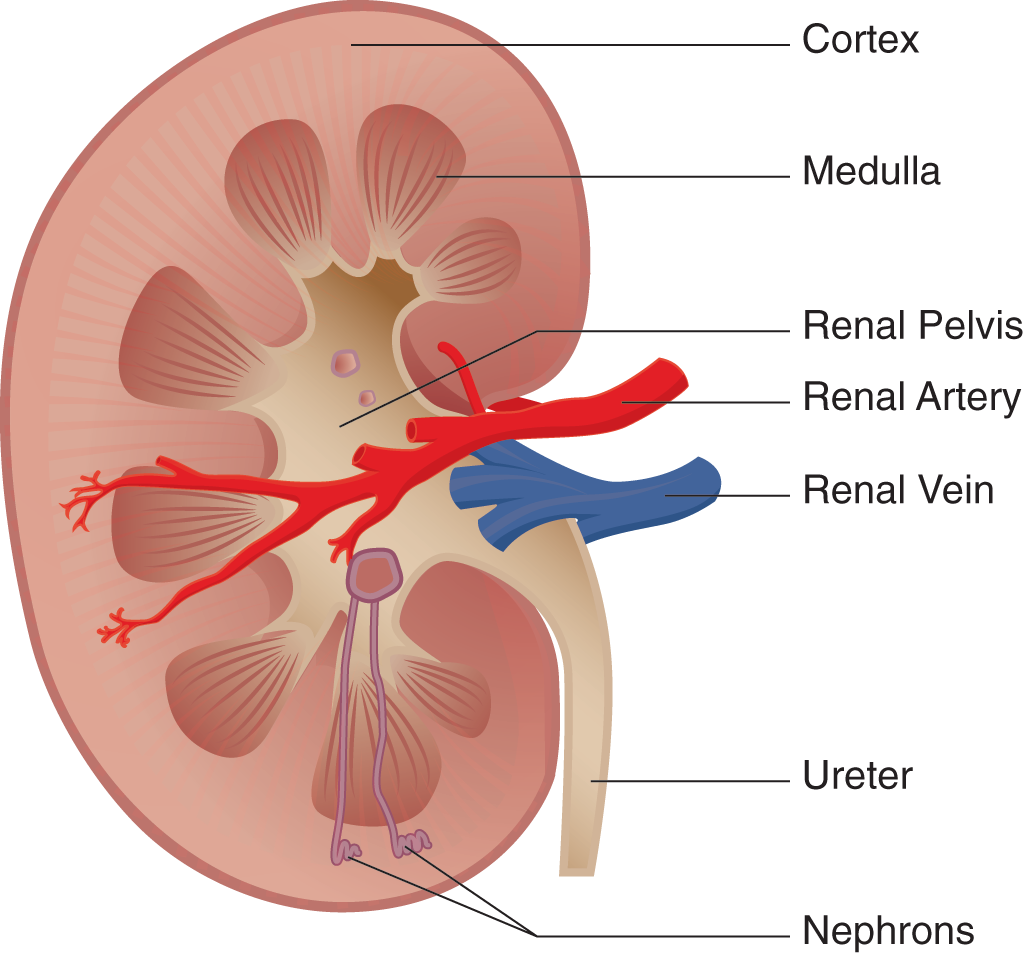
The excretory system consists of the kidneys, ureters, bladder, and urethra. The kidneys are 2 bean-shaped structures located behind the digestive organs at the level of the bottom rib. The functional unit of the kidney is the nephron; each kidney has approximately 1 million nephrons. All the nephrons eventually empty into the renal pelvis, which narrows to form the ureter. Urine travels through the ureter to the bladder. From the bladder, urine is transported through the urethra to exit the body.

Each kidney is subdivided into a cortex and a medulla. The cortex is the kidney’s outermost layer, and the medulla of the kidney sits within the cortex. Each kidney also has a renal hilum, which is a deep slit in the center of its medial surface. The widest part of the ureter, the renal pelvis, spans almost the entire width of the renal hilum. The renal artery, renal vein, and ureter enter and exit through the renal hilum.
The kidney has 1 of the few portal systems in the body. A portal system consists of 2 capillary beds in series through which blood must travel before returning to the heart. The renal artery branches out, passes through the medulla, and enters the cortex as afferent arterioles. The highly convoluted capillary tufts derived from these afferent arterioles are known as glomeruli. After blood passes through a glomerulus, the efferent arterioles then form a 2nd capillary bed. These capillaries surround the loop of Henle and are known as vasa recta.

Around the glomerulus is a cup-like structure known as the Bowman capsule. The Bowman capsule leads to a long tubule with many distinct areas; in order, these are the proximal convoluted tubule, the descending and ascending limbs of the Loop of Henle, the distal convoluted tubule, and the collecting duct. The kidney’s ability to excrete waste is intricately tied to the specific placement of these structures and their physiology.
The bladder has a muscular lining known as the detrusor muscle. Parasympathetic activity causes the detrusor muscle to contract. However, to leave the body, urine must pass through 2 sphincters: the internal and external urethral sphincters. The internal urethral sphincter, consisting of smooth muscle, is contracted in its normal state. Because the internal sphincter is made of smooth muscle, it is under involuntary control. The external urethral sphincter consists of skeletal muscle and is under voluntary control. When the bladder is full, stretch receptors convey to the nervous system that the bladder requires emptying. This causes parasympathetic neurons to fire, and the detrusor muscle contracts. This contraction also causes the internal sphincter to relax. This reflex is known as the micturition reflex. The next step is up to the individual. The person can choose to relax the external sphincter to urinate or can maintain the tone of the external sphincter to prevent urination. This can cause a few moments of discomfort, but the reflex usually dissipates in a few minutes. However, if the bladder is not emptied, then the process will begin anew shortly thereafter. Urination itself is facilitated by contraction of the abdominal musculature, which increases pressure within the abdominal cavity, resulting in compression of the bladder and an increased urine flow rate.
The kidney filters the blood to form urine. The composition and quantity of urine is determined by the present state of the body. For example, if blood volume is low and blood osmolarity is high, then it is most beneficial to the body to maximally retain water. This results in low-volume, highly concentrated urine. Likewise, a patient receiving large amounts of intravenous fluids is likely to produce a larger volume of less concentrated urine. Thus, the primary job of the kidneys is to regulate blood volume and osmolarity. To do this, kidney function may be divided into 3 different processes: filtration, secretion, and reabsorption.
The nephron’s first function is filtration. In the kidneys, approximately 20% of the blood that passes through the glomerulus is filtered as fluid into the Bowman capsule. The collected fluid is known as the filtrate. The movement of fluid into the Bowman capsule is governed by Starling forces, which account for the pressure differentials in both hydrostatic and oncotic pressures between the blood and the Bowman capsule. The hydrostatic pressure in the glomerulus is significantly higher than that in the Bowman capsule, which causes fluid to move into the nephron. On the other hand, the osmolarity of blood is higher than that of the Bowman capsule, resulting in pressure opposing the movement of fluid into the nephron. However, hydrostatic pressure is much larger than oncotic pressure, so the net flow is still from blood into the nephron.
Under most circumstances, fluid will flow from the glomerulus into the Bowman capsule. However, various pathologies can cause derangements of this flow. Consider what might happen if the ureter was obstructed by a kidney stone. An obstruction would result in a buildup of urine behind the stone. Eventually, enough fluid will build up and cause distention of the renal pelvis and the nephrons. What will happen to filtration in this case? The hydrostatic pressure in the Bowman capsule would increase to the point that filtration could no longer occur because there would be excessive pressure opposing movement of fluid into the nephron.
The filtrate is similar in composition to blood but does not contain cells or proteins because of the filter’s ability to select based on size. In other words, molecules or cells that are larger than glomerular pores will remain in the blood. As described earlier, the blood remaining in the glomerulus then travels into the efferent arterioles, which empty into the vasa recta. The filtrate is isotonic to blood so that neither the capsule nor the capillaries swell. Our kidneys filter about 180 L per day, which is approximately 36 times our blood volume. This means that the entire volume of a person’s blood is filtered about every 40 minutes.
In addition to filtering blood, the nephrons are able to secrete salts, acids, bases, and urea directly into the tubule by either active or passive transport. The quantity and identity of the substances secreted into the nephron are directly related to the needs of the body at that time. For example, a diet heavy in meat results in the intake of large amounts of protein, which contains a significant amount of nitrogen. Ammonia is a byproduct of the metabolism of nitrogen-containing compounds and, as a base, can disturb the pH of blood and cells. The liver converts the ammonia to urea, a neutral compound, which travels to the kidney and is secreted into the nephron for excretion with the urine. The kidneys are capable of eliminating ions or other substances when present in relative excess in the blood, such as potassium cations, hydrogen ions, or metabolites of medications. Secretion also is a mechanism for excreting wastes that are simply too large to pass through glomerular pores.
Some compounds that are filtered or secreted may be taken back up for use via reabsorption. Certain substances are almost always reabsorbed, such as glucose, amino acids, and vitamins. In addition, hormones such as ADH (vasopressin) and aldosterone can alter the quantity of water reabsorbed within the kidney to maintain blood pressure.
The kidney uses mechanisms such as filtration, secretion, and reabsorption to produce urine and regulate the blood volume and osmolarity. However, the function of the nephron isn’t quite that simple. In fact, renal physiology often is considered one of the most difficult topics covered in medical school.
To simplify this topic, it is important to understand that the kidney has 2 main goals: (1) keep what the body needs and lose what it doesn’t and (2) concentrate the urine to conserve water. The kidney allows the human body to reabsorb certain materials for reuse while also selectively eliminating waste. For example, glucose and amino acids are not usually present in the urine because the kidney is able to reabsorb these substances for later use. On the contrary, waste products such as hydrogen and potassium ions, ammonia, and urea remain in the filtrate and are excreted. Finally, water is reabsorbed in large quantities to maintain blood pressure and adequate hydration.
To understand this complex organ, the nephron will be studied piece by piece, which will include a discussion of exactly what is occurring in each segment. Follow along with the nephron diagram. As a theme, note that segments that are horizontal in the diagram (the Bowman capsule, the proximal convoluted tubule, and the distal convoluted tubule) are primarily focused on the identity of the particles in the urine (keep what the body needs and lose what it doesn’t). In contrast, the segments that are vertical in the diagram (the loop of Henle and collecting duct) are primarily focused on the volume and concentration of the urine (concentrate the urine to conserve water).

The filtrate first enters the proximal convoluted tubule (PCT). In this region, amino acids, glucose, water-soluble vitamins, and the majority of salts are reabsorbed along with water. Almost 70% of filtered sodium will be reabsorbed here, but the filtrate remains isotonic to the interstitium (connective tissue surrounding the nephron), as other solutes and a large volume of water also are reabsorbed. Solutes that enter the interstitium are picked up by the vasa recta to be returned to the bloodstream for reuse within the body. The PCT also is the site of secretion for a number of waste products, including hydrogen ions, potassium ions, ammonia, and urea.
Filtrate from the proximal convoluted tubule then enters the descending limb of the loop of Henle, which dives deep into the medulla before turning around to become the ascending limb of the loop of Henle. The descending limb is permeable only to water, and the medulla has an ever-increasing osmolarity as the descending limb travels deeper into it. Think for a moment how this would affect the flow of water. As the descending limb traverses deeper into the medulla, the increasing interstitial concentration favors the outflow of water from the descending limb, which is reabsorbed into the vasa recta.
The kidney is capable of altering the osmolarity of the interstitium. This creates a gradient that, coupled with the selective permeability of the nephron, allows maximal reabsorption and conservation of water. In the normal physiological state, the osmolarity in the cortex is approximately the same as that of the blood and remains at that level. Deeper in the medulla, the osmolarity in the interstitium can range from isotonic with blood (when trying to excrete water) to 4 times as concentrated (when trying to conserve water). Water will move out of the tubule, into the interstitium, and eventually back into the blood. If the concentration is the same in the tubule and the interstitium, there is no driving force (gradient), and the water will be lost in urine.
Together, the vasa recta and the nephron create a countercurrent multiplier system: The flow of filtrate through the loop of Henle is in the opposite direction from the flow of blood through the vasa recta. If the 2 flowed in the same direction, they would quickly reach equilibrium, and the kidney would be unable to reabsorb as much water. By making the 2 flow in opposite directions, the filtrate is constantly being exposed to hypertonic blood, which allows maximal reabsorption of water.
As the descending limb transitions to become the ascending limb of the loop of Henle, a change in permeability occurs. The ascending limb is permeable only to salts and is impermeable to water. Here, the opposite occurs: At the deeper parts of the medulla, salt concentrations are high but decrease as the ascending limb rises. Thus, increasing amounts of salt are removed from the filtrate as it travels up the loop of Henle.
At the transition from the inner to outer medulla, the loop of Henle becomes thicker, in what is termed the diluting segment. This is not because the lumen within the tube has enlarged; the cells lining the tube are larger. These cells contain large amounts of mitochondria, which allow the reabsorption of sodium and chloride by active transport. Indeed, because so much salt is reabsorbed while water is stuck in the nephron, the filtrate actually becomes hypotonic compared with the interstitium. Although tending to focus on the concentrating abilities of the nephron, this segment is noteworthy because it is the only portion of the nephron that can produce urine that is more dilute than the blood. This is important during periods of overhydration and provides a mechanism for eliminating excess water.
At the beginning of the loop of Henle, the filtrate is isotonic to the interstitium. Thus, from the beginning of the loop of Henle to the end, there is a slight degree of dilution. Far more important, however, is the fact that the volume of the filtrate has been significantly reduced, demonstrating a net reabsorption of a large volume of water.
Next, the filtrate enters the distal convoluted tubule (DCT). The DCT responds to aldosterone, which promotes sodium reabsorption. Because sodium ions are osmotically active particles, water will follow the sodium, concentrating the urine and decreasing its volume. The DCT also is the site of waste product secretion, like the PCT.
The final concentration of the urine will depend largely on the permeability of the collecting duct, which is responsive to both aldosterone and ADH (vasopressin). As the permeability of the collecting duct increases, so too does water reabsorption, resulting in further concentration of the urine. The reabsorbed water enters the interstitium and makes its way to the vasa recta, where it reenters the bloodstream to once again become part of the plasma. The collecting duct almost always reabsorbs water, but the amount is variable. When the body is very well hydrated, the collecting duct will be fairly impermeable to salt and water. When in conservation mode, ADH and aldosterone will each act to increase reabsorption of water in the collecting duct, allowing for greater water retention and more concentrated urine output.
Ultimately, anything that does not leave the tubule by the end of the collecting duct will be excreted; the collecting duct is the point of no return. After that, there are no further opportunities for reabsorption. As the filtrate leaves the tubule, it collects in the renal pelvis. The fluid, which carries mostly urea, uric acid, and excess ions (sodium, potassium, magnesium, and calcium), flows through the ureter to the bladder, where it is stored until voiding.
The kidneys use osmolarity gradients and selective permeability to filter, secrete, and reabsorb materials in the process of making urine. However, these processes have larger implications for the human body has a whole. The selective elimination of water and solutes allows the kidneys, in conjunction with the endocrine, cardiovascular, and respiratory systems, to control blood pressure, blood osmolarity, and the acid–base balance.
The hormones aldosterone and ADH (vasopressin) are very important for maintaining proper blood pressure.
Aldosterone is a steroid hormone that is secreted by the adrenal cortex in response to decreased blood pressure. Decreased blood pressure stimulates the release of renin from juxtaglomerular cells in the kidney. Renin then cleaves angiotensinogen, a liver protein, to form angiotensin I. This peptide is then metabolized by angiotensin-converting enzyme in the lungs to form angiotensin II, which promotes the release of aldosterone from the adrenal cortex.
Aldosterone works by altering the ability of the DCT and collecting duct to reabsorb sodium. Remember that water does not move on its own but rather travels down an osmolarity gradient. Thus, if more sodium is reabsorbed, water will flow with it. This reabsorption of isotonic fluid has the net effect of increasing blood volume and therefore blood pressure. Aldosterone also will increase potassium and hydrogen ion excretion.
ADH (also known as vasopressin) is a peptide hormone synthesized by the hypothalamus and released by the posterior pituitary gland in response to high blood osmolarity. It directly alters the permeability of the collecting duct, allowing more water to be reabsorbed by making the cell junctions of the duct leaky. Increased concentration in the interstitium (hypertonic to the filtrate) will then cause reabsorption of water from the tubule. Alcohol and caffeine both inhibit ADH release and lead to the frequent excretion of dilute urine.
In addition to the kidneys, the cardiovascular system also can vasoconstrict or vasodilate to maintain blood pressure. Constriction of the afferent arteriole will lead to a lower pressure of blood reaching the glomeruli, which are adjacent to the juxtaglomerular cells. Therefore, this vasoconstriction will secondarily lead to renin release, which also will help raise blood pressure.
The osmolarity of the blood must be tightly controlled to ensure correct oncotic pressures within the vasculature. A note on terminology: osmotic pressure is the “sucking” pressure that draws water into the vasculature caused by all dissolved particles. Oncotic pressure, on the other hand, is the osmotic pressure that is attributable to dissolved proteins specifically. Blood osmolarity is usually maintained at approximately 290 milliosmoles (mOsm) per liter. As described earlier, the kidneys control osmolarity by modulating the reabsorption of water and filtering and secreting dissolved particles. When blood osmolarity is low, excess water will be excreted, whereas solutes will be reabsorbed in higher concentrations. In contrast, when blood osmolarity is high, water reabsorption increases and solute excretion increases.
The bicarbonate buffer system is the major regulator of blood pH. Remind yourself of the buffer equation:

The respiratory system can contribute to acid–base balance by
increasing or decreasing the respiratory rate. If the blood pH is too low, then increasing
the respiratory rate blows off more CO2 and favors the conversion of H+ and
 to water and CO2, increasing the pH. If the blood pH is too high, then
decreasing the respiratory rate causes the opposite effects. The respiratory system
can react to derangements of pH quickly. What can the excretory system do to contribute?
The kidneys are able to selectively increase or decrease the secretion of both hydrogen
ions and bicarbonate. When blood pH is too low, the kidneys excrete more hydrogen
ions and increase reabsorption of bicarbonate, resulting in a higher pH. Likewise,
when blood pH is too high, the kidneys can excrete more bicarbonate and increase reabsorption
of hydrogen ions. This is slower than the respiratory response, but it is a highly
effective way for the body to maintain its acid–base balance.
to water and CO2, increasing the pH. If the blood pH is too high, then
decreasing the respiratory rate causes the opposite effects. The respiratory system
can react to derangements of pH quickly. What can the excretory system do to contribute?
The kidneys are able to selectively increase or decrease the secretion of both hydrogen
ions and bicarbonate. When blood pH is too low, the kidneys excrete more hydrogen
ions and increase reabsorption of bicarbonate, resulting in a higher pH. Likewise,
when blood pH is too high, the kidneys can excrete more bicarbonate and increase reabsorption
of hydrogen ions. This is slower than the respiratory response, but it is a highly
effective way for the body to maintain its acid–base balance.
Acute renal failure (ARF) is a sudden decrease of filtration of blood entering the kidneys and can be fatal in up to about 80% of cases. ARF could present as oliguria, which is a reduction in urine output to <500 mL per day, or anuria, which literally means without urine and is a complete lack of urine production. ARF can be further characterized into prerenal, intrarenal or postrenal causes.
Prerenal causes are those that prevent blood from entering the glomeruli within the kidneys or significantly reduce the pressure in the glomeruli to the point where filtrate cannot be collected in the capsule. Such causes include profound hypotension from hemorrhage, dehydration, trauma, shock of any kind, or sepsis. This is typically the easiest ARF cause to treat because it should resolve itself once kidney perfusion is restored.
Intrarenal ARF is far more complicated and is ultimately caused by damage to any of the glomerular capillaries and arterioles, the renal tissue itself or the cells of the nephron. These issues can be brought on by such widely varying conditions as diabetes mellitus (blood vessel damage), heavy metals (nephron damage), or certain medications or street drugs (renal cells surrounding nephrons and vasculature).
Postrenal ARF is caused by inhibition of urinary outflow from the renal pelvis. If urinary outflow fails, urine will back up through the collecting ducts and eventually all the way back to the capsule, thus preventing filtrate from entering the capsule from the glomerulus. Failure of the kidneys in this case to filter the blood will quickly lead to the retention of toxins, potassium ions, and hydrogen ions, any of which can alter the blood chemistry and lead to altered mental status and death relatively quickly.
Although all the ARF causes can be reversed with time and appropriate treatments, chronic renal failure (CRF) is a progressive and irreversible failure of the kidneys caused by destruction of the nephrons. This situation usually takes years to develop and often is found in late stages of uncontrolled or poorly controlled hypertension and diabetes. Nephron destruction has something of a domino effect on the kidney as a whole. First, 1 or several nephrons are damaged and cease to function and are replaced by scar tissue. This scarring then causes surrounding tissue to waste away and shrink, damaging other neighboring nephrons. This leads to renal mass loss and eventually irreversible kidney failure.
ARF and CRF can present with similar findings, including fatigue, weight loss, and sleep disturbances, ARF just occurs over a shorter time frame. ARF likely also will be a result of a bigger problem, such as hemorrhage, sepsis, and trauma to the mid-back or flanks (such as what might result from a football player being tackled from behind). Evaluation of the patient’s recent urinary output will be helpful information. Sudden oliguria or anuria likely indicates ARF. Patients in both cases may present with altered mental status caused by toxin buildup in the blood. Patients also frequently have cardiac dysrhythmias, including tachycardias, ectopic beats, VT, and indications of hyperkalemia (peaked T waves and absent P waves).
Treatment for ARF is primarily related to restoring kidney perfusion and removing anything that may be causing the kidney failure. For example, if the patient is hypotensive, restoring renal blood flow can be accomplished with aggressive fluid resuscitation or pressor administration. If a pressor is needed, dopamine is preferred to epinephrine. The removal of offending agents could mean stopping certain antibiotics or clearing the body of street drugs. Patients may need dialysis to accomplish this; however, for ARF, many patients will not need that aggressive of a treatment. For CRF, on the other hand, dialysis is almost always needed. Once patients start on dialysis for CRF or end stage renal disease, they will remain on that for life, unless a kidney becomes available and they receive a transplant. For patients in any of these conditions, it is recommended to not give any medications based solely on protocol. Seek advice from medical control before administering any medications to a patient on dialysis because with the kidneys unable to remove the medication, it may build up to overdose or even toxic levels.
Two types of dialysis are performed on a routine basis in an outpatient setting: peritoneal dialysis and hemodialysis. Peritoneal dialysis involves bathing the entire abdominal cavity with special dialysis fluid by injecting it through the abdominal wall, usually near the umbilicus. The fluid is allowed to remain in the abdominal cavity for about 2 hours, and then it is allowed to drain back out of the body. During the time it spent in the body, waste products diffuse into the fluid as they would in the kidneys. This is as effective as hemodialysis.
Hemodialysis is what is commonly thought of when thinking of dialysis. It involves the patient being connected to a machine that draws blood out of the body and functions much the same way as the kidneys. The patient is connected to the machine 3 days a week for about 4 hours per session. To be on dialysis, the patient has a shunt somewhere in the body that connects an artery and a vein that not only allows for repeated needle punctures but also can handle the pressures of the machine removing and returning the blood. Commonly, the shunt is placed near the anterior surface of the elbow and can be seen under the skin as a bump that looks like a large vessel. If there is a shunt in the arm, blood pressure cannot be measured in that arm. Alternatively, the shunt may be located in the anterior thigh or in the lateral anterior chest.
Patients undergoing dialysis are more vulnerable to conditions the general population also experience. They are more likely to experience hypertension and CHF as a result of the hypertension. Patients undergoing dialysis also are more likely to have an MI and life-threatening dysrhythmias caused in large part by potassium not being removed from the body consistently. They also may get a condition known as uremic pericarditis, which is caused by high levels of blood urea nitrogen (BUN) in the blood, although exactly how it causes pericarditis is not well understood. Patients on dialysis also experience several issues directly related to the act of receiving dialysis to which paramedics are frequently called.
Kidney stones form when insoluble salts or uric acid precipitate out of solution in the renal pelvis and form large stones, sometimes filling the entire renal pelvis. These stones can be composed of various chemicals and most often are attributable to an insufficient intake of water. As long as the stone stays in the renal pelvis and does not move around, it generally is not a problem, unless it blocks urinary outflow, leading to ARF. Once the stone begins to move, it will quickly enter the ureter. Here, the diameter narrows considerably and the stone gets stuck. Urine then backs up behind it, forcing it further and further down the ureter, sometimes lacerating the ureter as it travels. This is where the excruciating pain originates. This pain will continue until the kidney stone is “passed” or removed from the body through the urethra.
Patients will complain of extreme pain on 1 flank. Tapping the back lightly with a closed fist will elicit a spike in pain as the kidney is briefly squeezed like a sponge, sending a surge of urine down the ureter, jarring the lodged stone. The patient will not be able to find a comfortable position and may vomit from the sheer amount of pain. Treatment with 0.1 mcg/kg fentanyl via an intravenous piggyback would bring welcome relief to the patient with a kidney stone. Most kidney stones do not require surgery.
Urinary tract infections (UTIs) are common infections throughout the life span. UTIs can be classified as either an upper UTI or a lower UTI. Upper UTIs can become a much more serious infection called pyelonephritis, which can lead to kidney failure and sepsis. Lower UTIs are typically isolated to the urethra and bladder. If left untreated, the infection can move up the 1 or both ureters and become an upper UTI.
A patient will be at heightened risk of UTI if he or she has an indwelling urinary catheter for the treatment of chronic urinary retention, incontinence, or being in a bed-ridden state. An indwelling catheter can harbor bacteria that can be delivered directly to the bladder if urine is permitted to back flow from the Foley bag or tubing and into the body. To prevent this, it is essential to keep the bag, and as much of the tubing as possible, at a height lower than the patient’s pelvis.
Patients will complain of pain on urination and possibly an increase in frequency and urgency of urination. There also could be lower midline pelvic pain or discomfort in both men and women. Patients may note that the urine has a strong, foul odor. Depending on the degree to which the infection has progressed, the patient may not display any other physical symptoms. On the other hand, the patient may be in septic shock, where he or she will be either warm or cool to the touch, have dry skin, and have an altered mental status. Patients should be treated based on their level of consciousness. If the patient has altered mental status, check blood sugar level and treat if hypoglycemic, initiate an intravenous line and cardiac monitoring, give fluids at least at a KVO rate and consider a 200 mL fluid bolus.
Loss of voluntary control over urination is known as incontinence. There are 3 types of incontinence, the first 2 of which are clinically significant: urge incontinence, overflow incontinence, and stress incontinence. Urge incontinence is when the patient gets the urge to urinate, as all people do, but the patient is unable to hold it beyond a few seconds, resulting in involuntary urine loss. Urge incontinence can be a sign of UTI, Parkinson disease, Alzheimer disease, stroke, or neuropathies. Overflow incontinence is a continuous trickle of urine out the urethra. It is associated with diabetic neuropathy and prostate problems. Stress incontinence is a common condition where a person involuntarily allows some urine to dribble out when there is an increase in intra-abdominal pressure, such as during a cough, a sneeze, or hearty laughing.
The 3 conditions listed here are for your information and will seldom require anything more for the patient beyond courteous transport to the hospital. On certain occasions, analgesia would make you that man’s favorite person on Earth.