
Each day, the human body is exposed to numerous bacteria, viruses, fungi, and even parasites. Yet our bodies are able to protect us from infection most of the time. Even when people do get sick, the immune system is usually able to contain and eliminate the infection.
To fight infection, the human body has 2 different divisions of the immune system: innate and adaptive immunity. Innate immunity is composed of defenses that are always active against infection, but they lack the ability to target specific invaders over others; for this reason, it is also called nonspecific immunity. Adaptive or specific immunity refers to the defenses that target a specific pathogen. This system is slower to act but can maintain immunological memory of an infection to be able to mount a faster attack in subsequent infections.
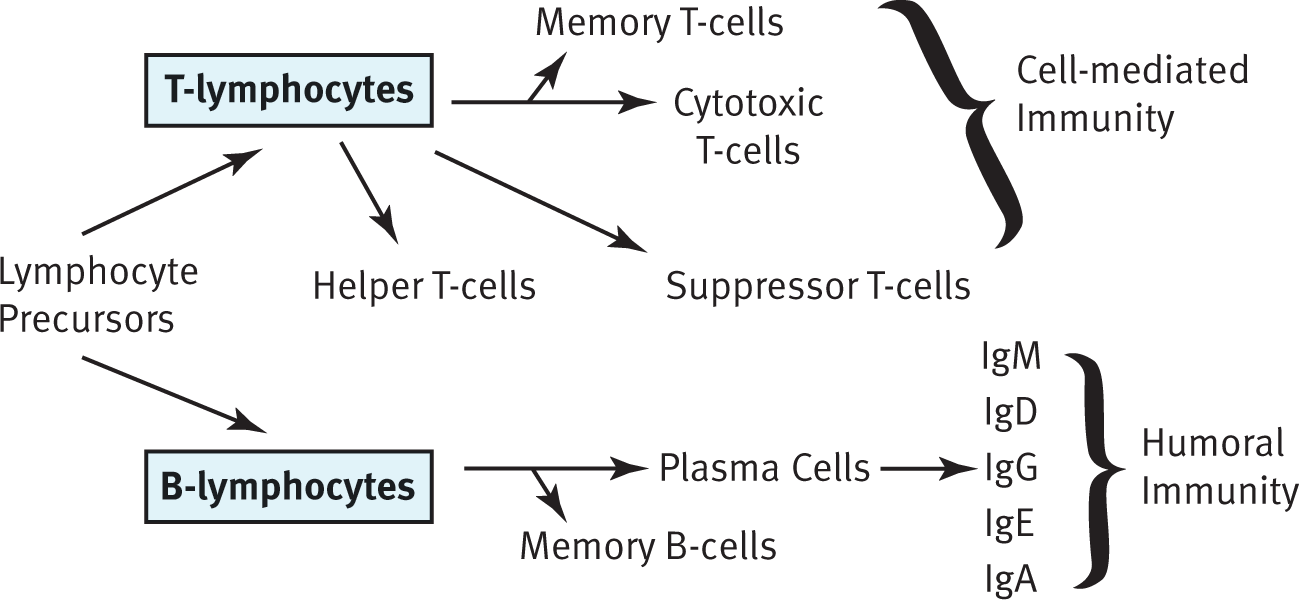
The immune system is not housed in a single organ. The structure and components that serve as nonspecific defenses often serve functions in other organ systems. The bone marrow produces all the leukocytes (white blood cells) that participate in the immune system through the process of hematopoiesis. The spleen functions as blood storage and activation of B-cells, which turn into plasma cells to produce antibodies as part of adaptive immunity. When B-cells leave the bone marrow, they are considered mature but naïve (because they have not yet been exposed to an antigen). Because these antibodies dissolve and act in the blood (rather than within cells), this division of adaptive immunity is called humoral immunity. T-cells, another class of adaptive immune cells, mature in the thymus, a small gland just in front of the pericardium, the sac that protects the heart. T-cells are the agents of cell-mediated immunity because they coordinate the immune system and directly kill virally infected cells. Finally, lymph nodes, a major component of the lymphatic system, provide a place for immune cells to communicate and mount an attack; B-cells can be activated here as well. Other immune tissue is found in close proximity to the digestive system, which is a site of potential invasion by pathogens. These tissues are commonly called gut-associated lymphoid tissue and include the tonsils and adenoids in the head, Peyer’s patches in the small intestine, and lymphoid aggregates in the appendix.
Leukocytes are produced in the bone marrow through hematopoiesis. Leukocytes are divided into 2 groups of cells: granulocytes and agranulocytes. These names refer to the presence or absence of granules in the cytoplasm. These granules contain toxic enzymes and chemicals, which can be released by exocytosis, and are particularly effective against bacterial, fungal, and parasitic pathogens. Both granulocytes and agranulocytes come from a common precursor: hematopoietic stem cells. Hematopoietic stem cells also are the cell type that gives rise to red blood cells and platelets. Granulocytes include cells such as neutrophils, eosinophils, and basophils. The names of these cells actually refer to the way that the cells appear after staining with certain chemicals. Agranulocytes include the lymphocytes, which are responsible for antibody production, immune system modulation, and the targeted killing of infected cells. Monocytes, which are phagocytic cells in the bloodstream, are considered agranulocytes. They become macrophages in tissues; many tissues have resident populations of macrophages with specific names (such as microglia in the central nervous system, Langerhans cells in the skin, and osteoclasts in bone).

Innate immunity refers to the responses that cells can carry out without learning; for this reason, it is also known as the nonspecific immune response. Conversely, adaptive immunity is developed as immune cells learn to recognize and respond to particular antigens and often is aptly referred to as the specific immune response. The specific immune system can be divided into humoral immunity (driven by B-cells and antibodies) and cell-mediated immunity (provided by T-cells).
The innate immune system consists of cells and structures that offer nonspecific protection. Our first line of defense is the skin (integument). The skin provides a physical barrier between the outside world and the body’s internal organs, excluding most bacteria, viruses, fungi, and parasites from entering the body. In addition, antibacterial enzymes called defensins can be found on the skin. Sweat also has antimicrobial properties. The skin is an important first line of defense: a cut or an abrasion on the skin provides an entry point for pathogens into the body. Deeper wounds allow pathogens to penetrate deeper into the body.
The respiratory system also has mechanisms to prevent pathogens from entering the body. The respiratory passages are mucous membranes, lined with cilia to trap particulate matter and push it up toward the oropharynx, where it can be swallowed or expelled. Although mucus helps to trap particulates such as smoke and dirt, it also helps prevent bacteria and viruses from gaining access to the lung tissue below. Several other mucous membranes, including around the eye and in the oral cavity, produce a nonspecific bacterial enzyme called lysozyme, which is secreted in tears and saliva, respectively.
The gastrointestinal (GI) tract also plays a role in nonspecific immunity. First, the stomach secretes acid, resulting in the elimination of most pathogens. In addition, the gut also is colonized by bacteria. Most of these bacteria lack the necessary characteristics to cause infection. Because there is already such a large bacterial population in the gut, many potential invaders are not able to compete and are thus kept at bay. Many antibiotics reduce the population of gut flora, thus providing an opportunity for the growth of pathogens resistant to that antibiotic.
The complement system consists of a number of proteins in the blood that act as a nonspecific defense against bacteria. Complement can be activated through a classical pathway (which requires the binding of an antibody to a pathogen) or an alternative pathway (which does not require antibodies). The complement proteins punch holes in the cell walls of bacteria, making them osmotically unstable. Despite the association with antibodies, complement is considered a nonspecific defense because it cannot be modified to target a specific organism over others.
To protect against viruses, cells that have been infected with viruses produce interferons, proteins that prevent viral replication and dispersion. Interferons cause nearby cells to decrease the production of both viral and cellular proteins. They also decrease the permeability of these cells, making it harder for a virus to infect them. In addition, interferons upregulate MHC (major histocompatibility complex) class I and class II molecules, resulting in increased antigen presentation and better detection of the infected cells by the immune system, as described in the next section. Interferons are responsible for many flu-like symptoms that occur during viral infection, including malaise, tiredness, muscle soreness, and fever.
So, what happens when bacteria, viruses, fungi, or parasites breach these defenses? The cells of the innate immune system are always poised and ready to attack.
Macrophages, a type of agranulocyte, reside within the tissues. These cells derive from blood-borne monocytes and can become a resident population within a tissue (becoming a permanent, rather than transient, cell group in the tissue). When a bacterial invader enters a tissue, the macrophages become activated. The activated macrophage does 3 things. First, it phagocytizes the invader through endocytosis. Then, it digests the invader using enzymes. Finally, it presents little pieces of the invader (mostly peptides) to other cells using a protein called MHC. MHC binds to a pathogenic peptide (also called an antigen) and carries it to the cell surface, where it can be recognized by cells of the adaptive immune system. In addition, macrophages release cytokines—chemical substances that stimulate inflammation and recruit additional immune cells to the area.
MHC molecules come in 2 main classes: class I and class II. All nucleated cells in the body display MHC class I molecules. Any protein produced within a cell can be loaded onto MHC-I and presented on the surface of the cell. This allows the immune system to monitor the health of these cells and to detect if the cells have been infected with a virus or another intracellular pathogen; only those cells that are infected would be expected to present an unfamiliar (nonself) protein on their surface. Therefore, the MHC-I pathway often is called the endogenous pathway because it binds antigens from inside the cell. Cells that have been invaded by intracellular pathogens can then be killed by a certain group of T-cells (cytotoxic T-lymphocytes) to prevent infection of other cells.

MHC class II molecules are mainly displayed by professional antigen-presenting cells such as macrophages. Remember that these phagocytic cells pick up pathogens from the environment, process them, and then present them on MHC-II. An antigen is a substance (usually a pathogenic protein) that can be targeted by an antibody. Although antibody production is the domain of the adaptive immune system, it is important to understand that cells of the innate immune system also present antigens. Because these antigens originate outside the cell, this pathway often is called the exogenous pathway. The presentation of an antigen by an immune cell may result in the activation of both the innate and adaptive immune systems. Professional antigen-presenting cells include macrophages, dendritic cells in the skin, some B-cells, and certain activated epithelial cells.

Macrophages and dendritic cells also have special receptors known as pattern recognition receptors (PRRs), the best described of which are toll-like receptors (TLRs). PRRs are able to recognize the category of the invader (bacterium, virus, fungus, or parasite). This allows for the production of appropriate cytokines to recruit the right type of immune cells; each immune cell has different weapons that can target particular groups of pathogens.
In the arms race between the human immune system and pathogens, some pathogens have found ways to avoid certain defenses. For example, some viruses cause downregulation of MHC molecules, making it harder for T-cells to recognize the presence of an infection. Natural killer (NK) cells, a type of nonspecific lymphocyte, are able to detect the downregulation of MHC and induce apoptosis in these virally infected cells. Cancer cells also may downregulate MHC production, so NK cells offer protection from the growth of cancer as well.
In addition to macrophages, the granulocytes, which include neutrophils, eosinophils, and basophils (and closely related mast cells) also are involved in nonspecific defense. Neutrophils are the most populous leukocyte in blood and are very short lived (a bit more than 5 days). These cells are phagocytic, like macrophages, and target bacteria. Neutrophils can literally follow bacteria using chemotaxis—the sensing of certain products given off by bacteria and the migration of neutrophils to follow these products back to the source (the bacterium itself). Neutrophils also can detect bacteria once they have been opsonized (marked with an antibody from a B-cell). Other cells, such NK cells, macrophages, monocytes, and eosinophils, also contain receptors for antibodies and can attack opsonized bacteria. Dead neutrophil collections are responsible for the formation of pus during an infection.
Eosinophils contain bright red-orange granules and are primarily involved in allergic reactions and invasive parasitic infections. Upon activation, eosinophils release large amounts of histamine, an inflammatory mediator. This results in vasodilation and increased leakiness of the blood vessels, allowing additional immune cells (especially macrophages and neutrophils) to move out of the bloodstream and into the tissue. Inflammation is particularly useful against extracellular pathogens, including bacteria, fungi, and parasites.
Finally, basophils contain large purple granules and are involved in allergic responses. Under normal conditions, they are the least populous leukocyte in the bloodstream. Mast cells are closely related to basophils but have smaller granules and exist in the tissues, mucosa, and epithelium. Both basophils and mast cells release large amounts of histamine in response to allergens, leading to inflammatory responses.
The adaptive immune system can identify specific invaders and mount an attack against that pathogen. The response is variable and depends on the identity of the pathogen. The adaptive immune system can be divided into 2 divisions: humoral immunity and cell-mediated (cytotoxic) immunity. Each involves the identification of the specific pathogen and organization of an appropriate immune response.
The adaptive immune system consists mainly of 2 types of lymphocytes: B-cells and T-cells. B-cells govern the humoral response, whereas T-cells mount the cell-mediated response. All cells of the immune system are created in the bone marrow, but B- and T-cells mature in different locations. B-cells mature in the bone marrow (although the B in their name originally stood for the bursa of Fabricius, an organ found in birds), and T-cells mature in the thymus. When exposed to a pathogen, it may take a few days for the physical symptoms to be relieved. This occurs because the adaptive immune response takes time to form specific defenses against the pathogen.
Humoral immunity, which involves the production of antibodies, may take as long as a week to become fully effective after the initial infection. These antibodies are specific to the antigens of the invading microbe. Antibodies are produced by B-cells, which are lymphocytes that originate and mature in the bone marrow and are activated in the spleen and lymph nodes.
Antibodies (also called immunoglobulins [Igs]) can carry out many different jobs in the body. Just as antigens can be displayed on the surface of cells or float freely in blood, chyle (lymphatic fluid), or air, so too can antibodies be present on the surface of a cell or secreted into body fluids. When an antibody binds to an antigen, the response will depend on the location. For antibodies secreted into body fluids, there are 3 main possibilities. First, once bound to a specific antigen, antibodies may attract other leukocytes to phagocytize those antigens immediately. This is called opsonization, as described earlier. Second, antibodies may cause pathogens to clump together or agglutinate, forming large insoluble complexes that can be phagocytized. Third, antibodies can block the ability of a pathogen to invade tissues, essentially neutralizing it. For cell-surface antibodies, the binding of antigen to a B-cell causes activation of that cell, resulting in its proliferation and the formation of plasma and memory cells, as described later in this chapter. In contrast, when antigen binds to antibodies on the surface of a mast cell, it causes degranulation (exocytosis of the granule contents), allowing the release of histamine and causing an inflammatory allergic reaction.
Antibodies are Y-shaped molecules that are composed of 2 identical heavy chains and 2 identical light chains. Each antibody has an antigen-binding region at the end of what is called the variable region (domain), at the tips of the Y. Within this region, specific polypeptide sequences will bind 1, and only 1, specific antigenic sequence. Part of the reason it takes so long to initiate the antibody response is that each B-cell undergoes hypermutation of its antigen-binding region, trying to find the best match for the antigen. Only those B-cells that can bind the antigen effectively survive. The remaining part of the antibody molecule is known as the constant region (domain). It is this region that cells such as NK cells, macrophages, monocytes, and eosinophils have receptors for and that can initiate the complement cascade.
Each B-cell makes only 1 type of antibody; because the human body has many B-cells, the body’s immune system can recognize many antigens. Further, antibodies come in 5 different isotypes (IgM, IgD, IgG, IgE, and IgA). Although knowing the specific purposes of each antibody is not necessary for the paramedic, you should know that the different types can be used at different times during the adaptive immune response, for different types of pathogens, or in different locations in the body. The antibody IgE is the antibody involved in allergic reactions. Cells can change which isotype of antibody they produce when stimulated by specific cytokines in a process called isotype switching.
Not all B-cells that are generated actively or constantly produce antibodies. Antibody production is an energetically expensive process, and there is no reason to expend energy producing antibodies that are not needed. Instead, naïve B-cells (those that have not yet been exposed to an antigen) wait in the lymph nodes for their particular antigen to come along. After exposure to the correct antigen, a B-cell will proliferate and produce 2 types of daughter cells. Plasma cells produce large amounts of antibodies, whereas memory B-cells stay in the lymph node, awaiting reexposure to the same antigen. This initial activation takes approximately 7–10 days and is known as the primary response. The plasma cells will eventually die, but the memory cells may last the lifetime of the organism. If the same microbe is ever encountered again, the memory cells jump into action and produce the antibodies specific to that pathogen. This immune response, called the secondary response, will be more rapid and robust. The development of these lasting memory cells is the basis of the efficacy of vaccinations.
Whereas humoral immunity is based on the activity of B-cells, cell-mediated immunity involves the T-cells. T-cells mature in the thymus, where they undergo both positive and negative selection. Positive selection refers to maturing only those cells that can respond to the presentation of antigen on MHC (cells that cannot respond to MHC undergo apoptosis because they will not be able to respond in the periphery). Negative selection refers to causing apoptosis in cells that are self-reactive (activated by proteins produced by the organism itself). The maturation of T-cells is facilitated by thymosin, a peptide hormone secreted by thymic cells. Once the T-cell has left the thymus, it is mature but naïve. After exposure to an antigen, T-cells also will undergo clonal selection so that only those with the highest affinity for a given antigen proliferate.
The 3 major types of T-cells are helper T-cells, suppressor T-cells, and killer (cytotoxic) T-cells. Helper T-cells (Th), also called CD4+ T-cells, coordinate the immune response by secreting chemicals known as lymphokines. These molecules are capable of recruiting other immune cells (such as plasma cells, cytotoxic T-cells, and macrophages) and increasing their activity. The loss of these cells, which occurs in a human immunodeficiency virus (HIV) infection, prevents the immune system from mounting an adequate response to infection; in advanced HIV infections, also called acquired immunodeficiency syndrome (AIDS), even weak pathogens can cause devastating consequences as opportunistic infections. CD4+ T-cells respond to antigens presented on MHC-II molecules. Because MHC-II presents exogenous antigens, CD4+ T-cells are most effective against bacterial, fungal, and parasitic infections.
Cytotoxic T-cells (Tc or CTL, for cytotoxic T-lymphocytes), also called CD8+ T-cells, are capable of directly killing virally infected cells by injecting toxic chemicals that promote apoptosis into the infected cell. CD8+ T-cells respond to antigens presented on MHC-I molecules. Because MHC-I presents endogenous antigens, CD8+ T-cells are most effective against viral (and intracellular bacterial or fungal) infections.
Suppressor or regulatory T-cells (Treg) also express CD4 but can be differentiated from helper T-cells because they also express a protein called Foxp3. These cells help to tone down the immune response once an infection has been adequately contained. These cells also turn off self-reactive lymphocytes to prevent autoimmune diseases, this is termed self-tolerance.
Finally, memory T-cells can be generated. Similar to memory B-cells, these cells lie in wait until the next exposure to the same antigen. When activated, they result in a more robust and rapid response.
When the human body encounters an antigen, the immune system must be able to respond. It is important to note that the innate and adaptive immune systems are not really disparate entities that function separately. The proper functioning of the entire immune system depends on the interactions between these 2 systems. There are 5 types of infectious pathogens: bacteria, viruses, fungi, parasites (including protozoa, worms, and insects), and prions (for which there are no immune defenses). Although the immune system’s response depends on the specific identity of the pathogen, 2 classic examples are presented: a bacterial (extracellular pathogen) infection and a viral (intracellular pathogen) infection. Keep in mind that this categorization is imperfect; for example, some bacteria, such as Mycobacterium tuberculosis and Listeria monocytogenes, actually live intracellularly.
Macrophages are like the sentinels of the human body, always on the lookout for potential invaders. Let’s say a person suffers a laceration, and bacteria are introduced into the body via this laceration. First, macrophages (and other antigen-presenting cells) engulf the bacteria and subsequently release inflammatory mediators. These cells also digest the bacteria and present antigens from the pathogen on their surfaces in conjunction with MHC-II. The cytokines attract inflammatory cells, including neutrophils and additional macrophages. Mast cells are activated by the inflammation and degranulate, resulting in histamine release and increased leakiness of the capillaries. This augments the ability of the immune cells to leave the bloodstream to travel to the affected tissue. The dendritic cell then leaves the affected tissue and travels to the nearest lymph node, where it presents the antigen to B-cells. B-cells that produce the correct antibody proliferate through clonal selection to create plasma cells and memory cells. Antibodies then travel through the bloodstream to the affected tissue, where they tag the bacteria for destruction.
At the same time, dendritic cells also are presenting the antigen to T-cells, activating a T-cell response. In particular, CD4+ T-cells are activated. These cells come in 2 types: Th1 and Th2. Th1 cells release interferon gamma (IFN-γ), which activates macrophages and increases their ability to kill bacteria. Th2 cells help activate B-cells and are more common in parasitic infections.
After the pathogen has been eliminated, the plasma cells die, but the memory B- and T-cells remain. These memory cells allow for a much faster secondary response during reexposure to the pathogen at a later time.
In a viral infection, the virally infected cell will begin to produce interferons, which reduce the permeability of nearby cells (decreasing the ability of the virus to infect these cells), reduce the rate of transcription and translation in these cells (decreasing the ability of the virus to multiply), and cause systemic symptoms (malaise, muscle aching, fever, and so on). These infected cells also present intracellular proteins on their surface in conjunction with MHC-I; in a virally infected cell, at least some of these intracellular proteins will be viral proteins.
CD8+ T-cells will recognize the MHC-I and antigen complex as foreign and will inject toxins into the cell to promote apoptosis. In this way, the infection can be shut down before it is able to spread to nearby cells. In the event that the virus downregulates the production and presentation of MHC-I molecules, NK cells will recognize the absence of MHC-I and will accordingly cause apoptosis of this cell.
Again, once the pathogen has been cleared, the memory T-cells will be retained to allow a much faster response to be mounted during a 2nd exposure.
Self-antigens are the proteins and carbohydrates present on the surface of every cell of the body. Under normal circumstances, these self-antigens signal to immune cells that the cell is not threatening and should not be attacked. However, when the immune system fails to make the distinction between self and foreign, it may attack cells expressing particular self-antigens, a condition known as autoimmunity. Note that autoimmunity is only 1 potential problem with immune functioning: another problem arises when the immune system misidentifies a foreign antigen as dangerous when, in fact, it is not. Pet dander, pollen, and peanuts are not inherently threatening to human life, yet some people’s immune systems are hypersensitive to these antigens and become overactivated when these antigens are encountered, in what is called an allergic reaction. Allergies and autoimmunity are part of a family of immune reactions classified as hypersensitivity reactions.
The human body strives to prevent autoimmune reactions very early in T-cell and B-cell maturation processes. T-cells are educated in the thymus. Part of this education involves the elimination of T-cells that respond to self-antigens, called negative selection. Immature B-cells that respond to self-antigens are eliminated before they leave the bone marrow. However, this process is not perfect, and occasionally a cell that responds to self-antigens is allowed to survive. Most autoimmune diseases can be treated with a number of therapies; a common example is the administration of glucocorticoids (modified versions of cortisol), which have potent immunosuppressive qualities.
Often, diseases can have significant, long-term consequences. Infection with the poliovirus, for example, can leave a person disabled for the remainder of his or her life. Polio used to be a widespread illness; however, today, polio is rarely heard about beyond the Indian subcontinent because of a highly effective vaccination program, which led to the elimination of polio from the Western hemisphere.
Immunization can be achieved in an active or a passive fashion. In active immunity, the immune system is stimulated to produce antibodies against a specific pathogen. The means by which people are exposed to this pathogen may either be natural or artificial. Through natural exposure, antibodies are generated by B-cells once an individual becomes infected. Artificial exposure (through vaccines) also results in the production of antibodies; however, the individual never experiences a true infection. Instead, he or she receives an injection or intranasal spray containing an antigen that will activate B-cells to produce antibodies to fight the specific infection. The antigen may be a weakened or killed form of the microbe, or it may be a part of the microbe’s protein structure.
Immunization also may be achieved passively. Passive immunity results from the transfer of antibodies to an individual. The immunity is transient because only the antibodies, not the plasma cells that produce them, are given to the individual. Natural examples are the transfer of antibodies across the placenta during pregnancy to protect the fetus and the transfer of antibodies from a mother to her nursing infant through breast milk. In some cases of exposure, such as the rabies virus or tetanus, intravenous immunoglobulin may be given to prevent the pathogen from spreading.
The immune system and the lymphatic system are intimately related. B-cells proliferate and develop within the lymphatic system, especially the lymph nodes. This system also serves other necessary functions for the body.
The lymphatic system, along with the cardiovascular system, is a type of circulatory system. It is made up of 1-way vessels that become larger as they move toward the center of the body. These vessels carry lymphatic fluid (lymph), and most join to comprise a large thoracic duct in the posterior chest, which then delivers the fluid into the left subclavian vein (near the heart).
Lymph nodes are small, bean-shaped structures along the lymphatic vessels. Lymph nodes contain a lymphatic channel, as well as an artery and a vein. The lymph nodes provide a space for the cells of the immune system to be exposed to possible pathogens.
The lymphatic system serves many different purposes in the body by providing a secondary system for circulation.
At the capillaries, fluid leaves the bloodstream and goes into the tissues. The quantity of fluid that leaves the tissues at the arterial end of the capillary bed depends on both hydrostatic and oncotic pressures (Starling forces). Hydrostatic pressure exists at the arteriole end of the capillary bed and is caused entirely by the pressure of the blood on the arterial walls, essentially the blood pressure. The oncotic pressure is pressure that is caused primarily by the presence of proteins (instead of ions or sugars) and exists at the venule end of the capillary bed. Remember that the oncotic pressure of the blood draws water back into the vessel at the venule end, once hydrostatic pressure has decreased. Because the net pressure drawing fluid in at the venule end is slightly less than the net pressure pushing fluid out at the arterial end, a small amount of fluid remains in the tissues. Lymphatic vessels drain these tissues and subsequently return the fluid to the bloodstream.
The lymphatics offer some protection against pathology. For example, if the blood has a low concentration of albumin (a key plasma protein), the oncotic pressure of the blood is decreased, and less water is driven back into the bloodstream at the venule end. Thus, this fluid will collect in the tissues. Provided that the lymphatic channels are not blocked, much of this fluid may eventually return to the bloodstream via the lymphatics. Only when the lymphatics are overwhelmed does edema occur—swelling caused by fluid collecting in tissue.
The lymphatic system also transports fats from the digestive system into the bloodstream. Lacteals, small lymphatic vessels, are located at the center of each villus in the small intestine. Fats, packaged into chylomicrons by intestinal mucosal cells, enter the lacteal for transport. Lymphatic fluid carrying many chylomicrons takes on a milky white appearance and is called chyle.
As stated previously in this chapter, lymph nodes are a place for antigen-presenting cells and lymphocytes to interact. B-cells proliferate and mature in the lymph nodes in collections called germinal centers.
Anaphylaxis is a result of the body’s overreaction to an otherwise harmless invader. At some time prior, the patient was exposed to the allergen, which, for the duration of this section, will be a peanut. So, the patient ate a few peanuts a while back and had no problem with them, but inside, the body was sensitized to this allergen so that the next time the body had this allergen introduced into it, it was prepared. Now, the patient has a peanut again and the body’s defense system—the immune system—short circuits.
First, the mast cells remember this invader from before and begin to release large quantities of histamine, which is a chemical mediator the body uses to signal a problem and begin the inflammatory response. The histamine release causes immediate swelling in the area of the security breach, which, in times of bacteria introduced through the skin in a laceration, is a very good thing; in the case of a peanut, however, it is now a system-wide problem. With systemic histamine overproduction in anaphylaxis, the capillaries dilate and become leaky, leading to edema. This swelling is seen as urticaria (hives) on the skin and swelling of the tongue, lips, and eyelids on the face. Histamine also causes constriction of smooth muscles, which is seen as laryngospasm, bronchospasm, and abdominal cramping, in addition to the edema of the upper and lower airways. Histamine also has a negative inotropic effect on the heart, which in the presence of dilated, leaky capillaries will lead to potentially profound hypotension.
The presence of histamine causes white blood cells to produce leukotrienes, which compound the effects of the histamine, worsening edema and capillary leakiness, bronchoconstriction, vasodilation, and smooth muscle contraction. Leukotrienes also can cause coronary vasoconstriction, possibly leading to myocardial ischemia or MI. This entire process can happen in minutes to hours depending on the body’s sensitivity to the allergen.
Anaphylaxis leaves no body system completely unaffected; in some way, every body system bears the effects of the histamine flood in anaphylaxis. The following represents the signs and symptoms of anaphylaxis, and the physiological reason for it is in parentheses:
Anaphylaxis can be lethal very shortly after symptoms start to appear, sometimes resulting in full airway closure in >30 minutes. To be considered in anaphylaxis, a patient should have signs of airway compromise or swelling, such as stridor or wheezing and hypotension. Many things need to be accomplished in a fairly short amount of time for the patient to achieve an optimal outcome. What follows is a list of treatments in the order in which they should be completed.
In addition to the above, some treatments to consider include aggressive airway management, inhaled beta-agonists, beta-blockade reversal agents, and blood pressure support. Patients may go into anaphylaxis quickly, so be prepared to manage an airway that is closing, either with early intubation or later with a cricothyrotomy. For patients who are on beta-blockers, consider administering 1–2 mg glucagon as an intravenous piggyback every 5 minutes. This can help minimize beta receptor antagonism, resulting in increased cardiac contractility and heart rate. Treat refractory bronchoconstriction with inhaled beta-agonists such as albuterol. Albuterol can be essentially continuous. Intramuscular or subcutaneous epinephrine can be repeated if necessary. In the event that the transport time is extended, and the patient is not improving after at least 2 L of NSS or lactated Ringer solution has been administered (some case studies indicate fluid resuscitation exceeding 4 L), the paramedic should consider administering a pressor. Mix 1 mg of 1:1,000 epinephrine in a 1,000 mL bag of NSS and run this mix at 0.1 mcg/kg/min. Most likely, pharmacologic pressure support will be used only during interfacility transports because it should take a couple hours to get 4 L of fluid into the patient.
Systemic lupus erythematosus (lupus or SLE) is a multisystem autoimmune disease that affects the entire body. It should be suspected in women of child-bearing age who complain of joint pain, rash, and a fever. The assessment and treatment of these patients focuses on addressing life threats and other acute problems.
Paramedics will, at some point in time, likely encounter a patient who has had an organ transplanted. The most common organs to be transplanted are the heart, liver, kidney, lung, and pancreas. Treatment for any problem with an organ transplant patient should be completed as per normal. The only major difference in treatment for the paramedic would be in a heart transplant patient. Because the new heart is not connected to the vagus nerve, atropine will not work to increase the heart rate. In the case of a bradycardic heart transplant patient, consult medical control.
If the patient’s body begins to reject these organs, the initial signs are subtle and can be confused for the flu—general malaise, weakness, and fever. Treatment for patients suspected of organ transplant rejection should be transported to the nearest facility. Consider contacting their organ transplant facility for guidance.
Leukemia literally means “white blood” and is a form of cancer that causes white blood cells to develop abnormally and excessively. Leukemia can cause anemia and thrombocytopenia (low platelet count), and the chemotherapy used to cure it often will often cause leukocytopenia (low white blood cell count). If caught early, leukemia is one of the more curable cancers.
Assessment of the patient with leukemia often is related to a problem other than the leukemia itself. For example, paramedics may be called to treat a patient with leukemia who has had excessive vomiting after chemotherapy; or perhaps the patient is short of breath owing to the anemia. How the patient presents will be wholly the result of the complaint and the overall state of the cancer. Therefore, management of this patient will generally be based on the presenting symptoms. Some overarching guidelines would be to provide supplemental O2 in the event the patient is having a bout with anemia and to treat the patient as positively as possible. The patient may not have the most positive outlook on life at the moment the paramedic arrives, so even a smile can go a long way to brighten the patient’s day. If it is possible to know, try to find out if the patient is leukocytopenic at the time. If so, consider putting a surgical mask on the patient to minimize the chances that he or she gets an infection on top of everything else.
Lymphoma is any of a group of cancers that involves the lymphatic cells. The most common lymphoma is called non-Hodgkin’s lymphoma. Other common types of lymphomas include Hodgkin’s lymphoma and multiple myeloma. These cancers are treatable depending on how early they are found and based on how aggressive they are. More aggressive cancers are generally found later, have already moved to other organs or body systems, and require a more complicated course of cytotoxic chemotherapy to treat. This makes successfully battling the cancer highly difficult. With any of these cancers, the initial signs are insidious, including vague complaints such as night sweats, chills, and a persistent cough. Some patients may note swelling of the neck lymph nodes, loss of appetite, and weight loss. Any treatment a paramedic will render for these patients is largely supportive and aimed at relieving symptoms.