26
Respiratory support
Forms of respiratory support
Respiratory support includes:
- supplemental oxygen
- CPAP – continuous positive airway pressure
- high-flow nasal therapy (HFNT)/high-flow nasal cannulae (HFNC)
- non-invasive mechanical ventilation (NIMV/NIPPV)
- positive-pressure ventilation (PPV)
- HFOV – high-frequency oscillatory ventilation
- iNO – inhaled nitric oxide
- ECMO – extracorporeal membrane oxygenation.
The approach to respiratory support is continually evolving and new strategies and modes of support are being introduced. Whilst aiming to maintain adequate gas exchange, there is an emphasis on minimizing the risk of ventilator-induced lung injury. Hyperinflation from high pressures (volutrauma) and repeated opening and closing of alveoli (atelectotrauma) may cause lung damage. Non-invasive respiratory support, which includes CPAP, high-flow nasal therapy and non-invasive mechanical ventilation with or without surfactant therapy in preterm infants, are used increasingly either as the primary mode of support or following extubation to avoid reintubation (Fig 26.1). Positive-pressure ventilation is required for infants with significant respiratory distress that cannot be managed with non-invasive respiratory support; some of the newer approaches are described in this chapter.
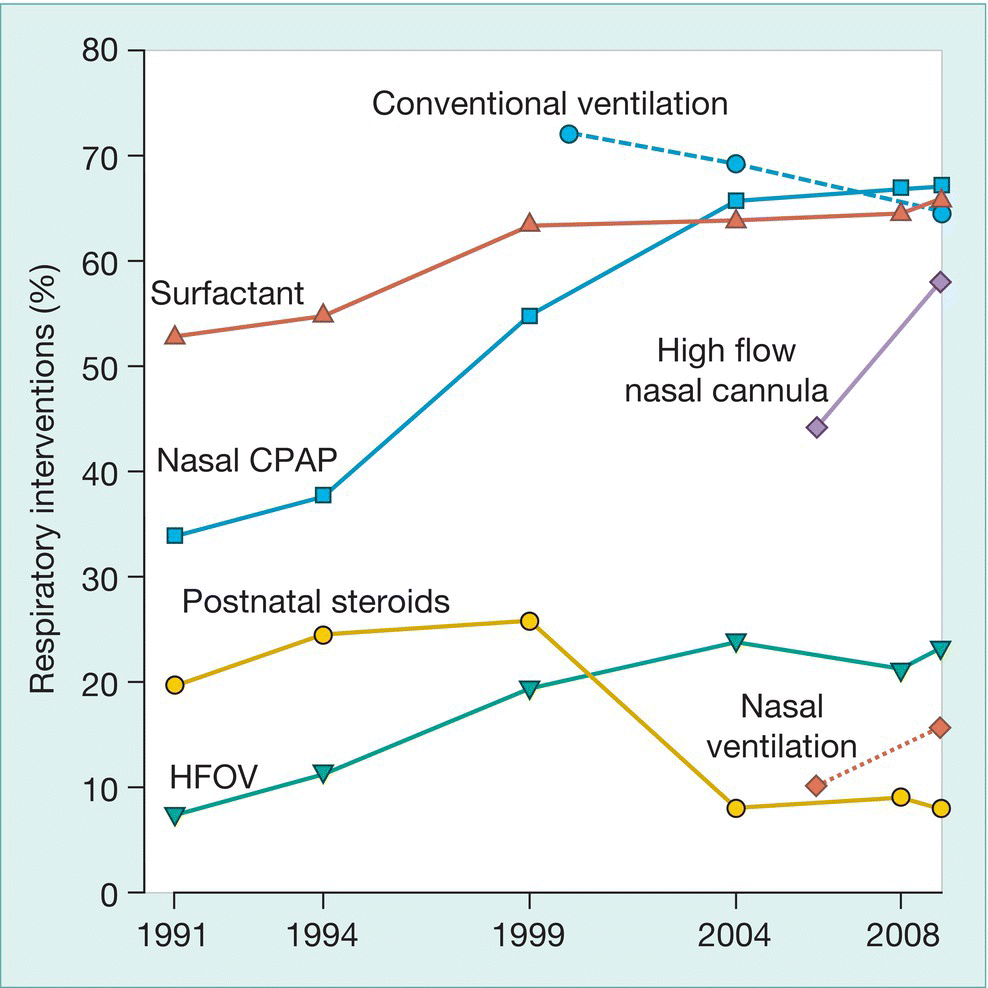
Fig. 26.1 Changes in use of respiratory interventions in VLBW (very low birthweight) infants with time.
(Vermont−Oxford Network; data from Soll R.F. et al. Obstetric and neonatal care practices for infants 501−1500 g from 2000 to 2009. Pediatrics 2013; 132: 222−228 and Dr Jeff Horbar.)
Supplemental oxygen therapy
Oxygen is given to prevent hypoxia (Fig. 26.2), which may cause ischemic damage to the brain and other organs, apnea and pulmonary hypertension. Hyperoxia should also be avoided as elevated levels of oxygen in the blood may cause tissue damage due to the release of oxygen free radicals and retinopathy of prematurity in preterm infants.
The optimal values for arterial oxygen tension and saturation have not been established, but in practice:
- In preterm infants, arterial oxygen tension (PaO2) is maintained at 45–80 mmHg (6.0–10.5 kPa) and oxygen saturation at 91–95%. Lower saturations are associated with less retinopathy of prematurity but increased mortality. Saturation above 95% in infants receiving supplemental oxygen may represent dangerously high oxygen tensions (Fig. 26.3).
- Term infants – maintain oxygen saturation at 95−99% and PaO2 between 60–80 mmHg (8–10.5 kPa).
Fig. 26.2 Oxygen delivered via nasal cannula.
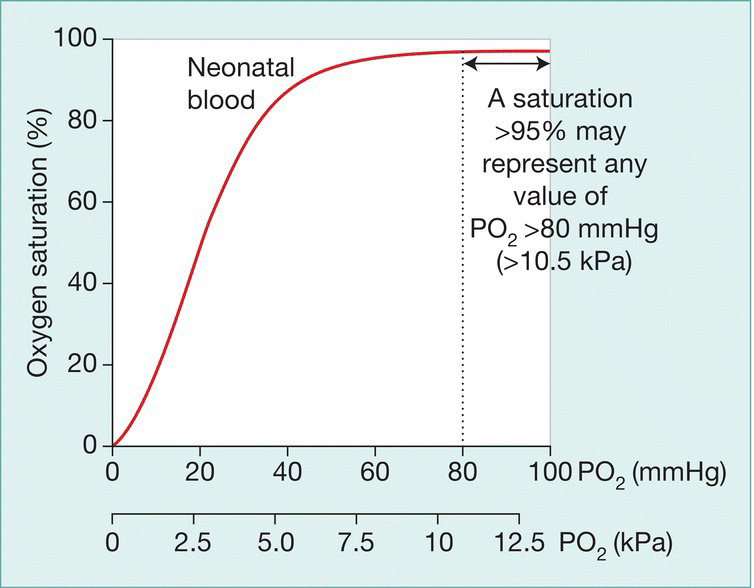
Fig. 26.3 Saturation measurements above 95% in preterm infants receiving supplemental oxygen may represent dangerously high oxygen tensions.
Continuous positive airway pressure (CPAP)
Distending pressure is usually applied via nasal prongs (Fig. 26.4) in the nasal airway or by a close-fitting nasal mask. CPAP aims to prevent alveolar collapse at end expiration, stabilize the chest wall and reduce the work of breathing. It also allows supplemental oxygen to be delivered continuously. It is used for infants with moderate respiratory distress and for recurrent apnea. There is increasing use of early CPAP as respiratory support immediately after birth even for very preterm infants, with mechanical ventilation used only as rescue. CPAP may also facilitate weaning from mechanical ventilation. Larger infants may not tolerate CPAP well.
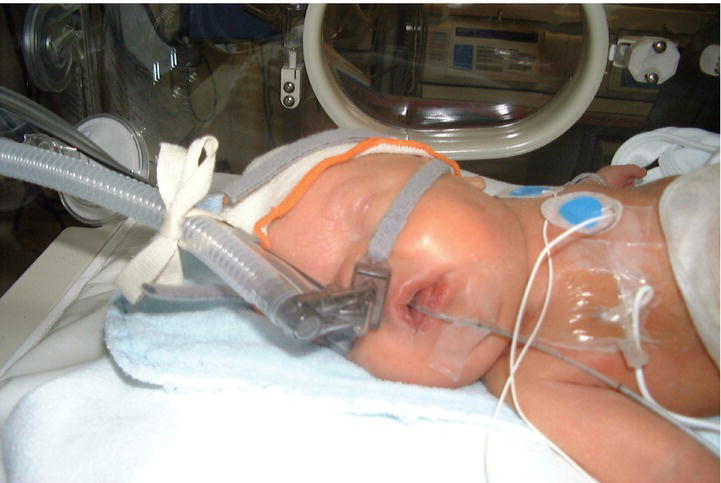
Fig. 26.4 Nasal CPAP (continuous positive airway pressure) with flow driver that maintains a constant pressure by changing the direction of gas flow during expiration (fluidic flip).
CPAP may be delivered as:
- bubble CPAP – the pressure is determined using a water manometer
- flow-driver CPAP – the flow driver provides a constant stream of air/oxygen; special nasal prongs maintain a constant pressure throughout the infant’s respiratory cycle by changing the direction of gas flow during expiration (fluidic flip).
A pressure of 6−8 cmH2O is used and there needs to be minimal air leak around the nasal prongs or mask.
Complications of CPAP are:
- pneumothorax
- feeding difficulties due to gaseous distension of the stomach
- nasal trauma causing nasal septum breakdown or erosion and nasal deformity; minimized by correct size and fixation of prongs or mask.
If respiratory failure develops, mechanical ventilation is required. CPAP can be stopped abruptly or weaned by gradually reducing the PEEP or increasing the period of time off CPAP. The limited evidence suggests that gradually weaning pressure is superior and reduces length of stay. If weaned too quickly, there may respiratory deterioration or poor growth.
Some infants with bronchopulmonary dysplasia require nasal CPAP for many weeks.
High-flow nasal therapy
Increasingly used as an alternative for CPAP or to wean off ventilators or CPAP. Warmed, humidified oxygen/air is delivered at a high flow rate (>2 L/min) via nasal cannulae. It probably generates some distending pressure to the lungs but works mainly by flushing CO2 from the nasopharynx. Babies, especially when more mature, tolerate it better than CPAP. In contrast to CPAP, there is leakage of gases around loose- fitting cannulae. Its efficacy as a mode of support compared with CPAP is being evaluated; for weaning infants off mechanical ventilation it appears to have similar efficacy to CPAP with less nasal trauma.
Non-Invasive Mechanical Ventilation (NIMV)
The terms NIMV (non-invasive mechanical ventilation or nasal intermittent mandatory ventilation) and NIPPV (nasal intermittent positive pressure ventilation) are used interchangeably for positive-pressure respiratory support without the use of a tracheal tube. It is gaining popularity as a primary means of respiratory support and also after extubation to prevent reintubation.
Whereas CPAP provides only continuous positive airway pressure, NIMV also delivers intermittent peak inspiratory pressure, either mandatory or triggered, via rigid nasal prongs. This is postulated to provide better oxygenation and CO2 removal than CPAP. There is currently no convincing evidence that it is better than CPAP in preventing reintubation or bronchopulmonary dysplasia in preterm infants.
Positive-pressure ventilation (PPV)
Indications
- Increasing oxygen requirement or work of breathing or increasing PaCO2 while on nasal CPAP/HFNT.
- Respiratory failure – inadequate oxygenation (hypoxia) and/or CO2 elimination (hypercarbia).
- Apnea – prolonged/recurrent.
- Upper airway obstruction or vocal cord paralysis.
- Congenital diaphragmatic hernia.
- Perioperative respiratory support for anesthesia.
- Circulatory failure.
Intermittent positive-pressure ventilation (IPPV)
Respiratory support is administered using a mechanical ventilator through a tracheal tube. With conventional ventilation, intermittent positive-pressure ventilator breaths are given on a background of continuous distending pressure (positive end-expiratory pressure, PEEP) (Fig. 26.5). Alveolar ventilation (CO2 clearance) is determined by the difference between peak inspiratory pressure (PIP) and PEEP and the respiratory rate. Oxygenation is determined by the mean airway pressure (area under the curve) and administered oxygen concentration (FiO2).
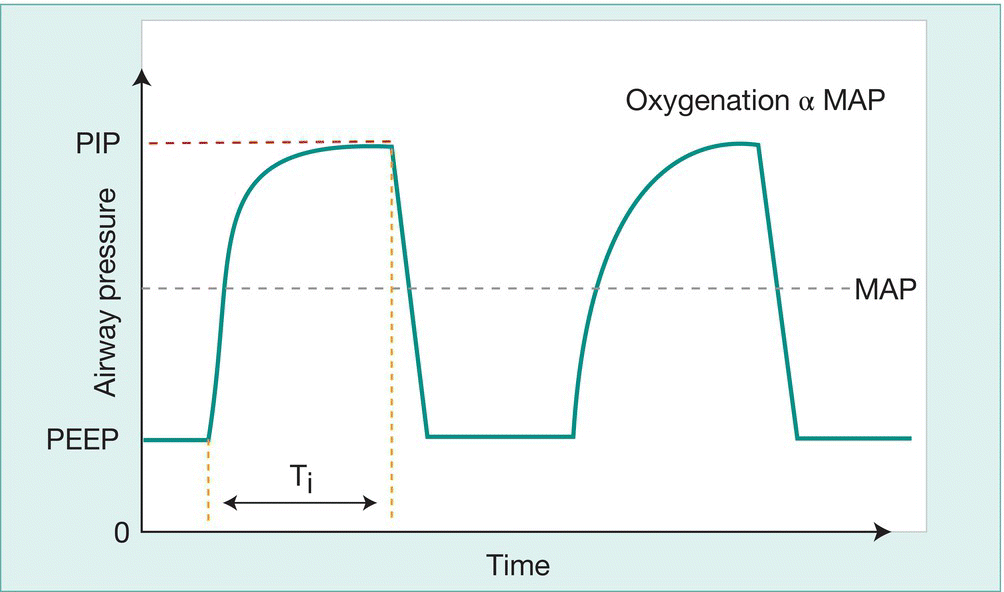
Fig. 26.5 Intermittent positive-pressure ventilation (IPPV). Diagram of change in airway pressure with time. (PIP – peak inspiratory pressure; PEEP – positive end-expiratory pressure; Ti – inspiratory time; MAP – mean airway pressure.)
Conventional ventilation is pressure limited and time cycled. Peak inspiratory pressure is set by the operator and tidal volume varies from breath to breath depending upon lung compliance and airway resistance. This may increase the risk of volutrauma and atelectotrauma.
Volume-limited or targeted ventilation is increasingly used. In volume-limited ventilation, instead of setting the desired peak inspiratory pressure, a maximum tidal volume is set (limited) by the operator so that the peak inspiratory pressure generated will only rise to meet the desired volume. In volume-targeted ventilation, a desired tidal volume is set (targeted) and the peak inspiratory pressure will automatically adjust to achieve the desired tidal volume. Tidal volumes are usually 4−6 mL/kg, the normal tidal volume of neonates, but higher volumes may be required by infants with bronchopulmonary dysplasia and lower volumes with lung hypoplasia. This mode of ventilation is particularly useful when lung compliance is changing rapidly, e.g. rapid improvement of compliance after surfactant administration, when the ventilator will be able to deliver the desired tidal volume using lower pressures, reducing the risk of an air leak. It is less useful if there is a large leak around the tracheal tube (>40%). Advances in ventilator technology allow the measurement and delivery of small tidal volumes, which was not previously possible. Studies suggest that there may be a decreased risk of pneumothorax and bronchopulmonary dysplasia compared with pressure-limited ventilation.
Patient-triggered ventilation (PTV) and synchronous intermittent mandatory ventilation (SIMV)
Two forms of synchronized mechanical ventilation are available to promote synchrony between the ventilator and an infant’s own respiratory efforts – patient-triggered ventilation (PTV) and synchronous intermittent mandatory ventilation (SIMV). Both methods use the infant’s own spontaneous respiration to trigger the ventilator to deliver a breath, usually from the change in airway pressure or flow measured in the ventilator circuit, or from a recording of the infant’s respiration. In patient-triggered ventilation, also called assist control (AC) or spontaneous intermittent positive-pressure ventilation (SIPPV), each breath is supported by the ventilator; in SIMV, only a preset number of breaths in a given time is supported by the ventilator and other breaths are unsupported. In both, there is a backup ventilation rate if the infant does not breathe.
High-frequency oscillatory ventilation (HFOV)
High-frequency ventilators operate at frequencies approximately 10 times greater than conventional ventilators and can achieve good gas exchange despite using tidal volumes smaller than dead space (Fig. 26.11). The rationale for using high-frequency ventilation is to recruit collapsed alveoli (‘open’ the lung) and minimize ventilator-induced lung damage. The mechanism of gas exchange is unclear but may include facilitated diffusion and turbulence. Rescue treatment with high-frequency ventilation in term and preterm infants with severe respiratory failure is associated with short-term improvement in gas exchange, especially when used in combination with inhaled nitric oxide. Some units choose to ventilate all their extremely preterm infants by HFOV to minimize barotrauma. Studies have failed to show a decrease in duration of ventilation, incidence of bronchopulmonary dysplasia, mortality or need for extracorporeal membrane oxygenation (ECMO) compared with conventional ventilation.
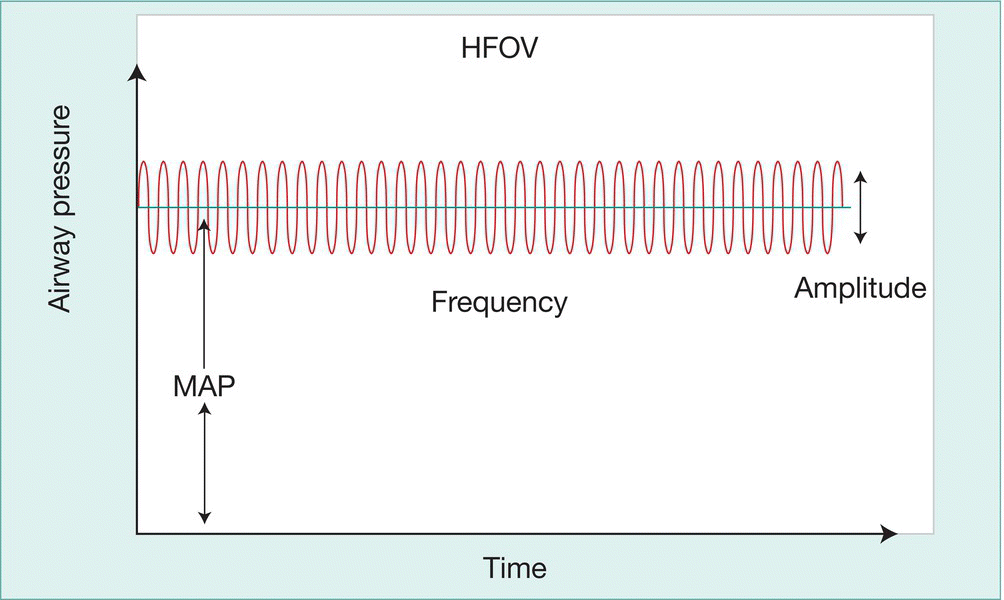
Fig. 26.11 High - frequency oscillatory ventilation (HFOV). Diagram showing changes in airway pressure with time. (MAP – mean airway pressure.)
Inhaled nitric oxide (iNO)
Inhaled nitric oxide causes selective pulmonary vasodilation. It is used in infants with hypoxemic respiratory failure with or without persistent pulmonary hypertension of the newborn (PPHN) to improve oxygenation (Fig. 26.12). It reduces the need for ECMO in term and near-term infants with severe respiratory failure. There is increasing evidence that sildenafil is as effective with reduced expense and complexity, but is not currently approved in the US for this purpose. The efficacy of inhaled nitric oxide in preterm infants remains to be established.
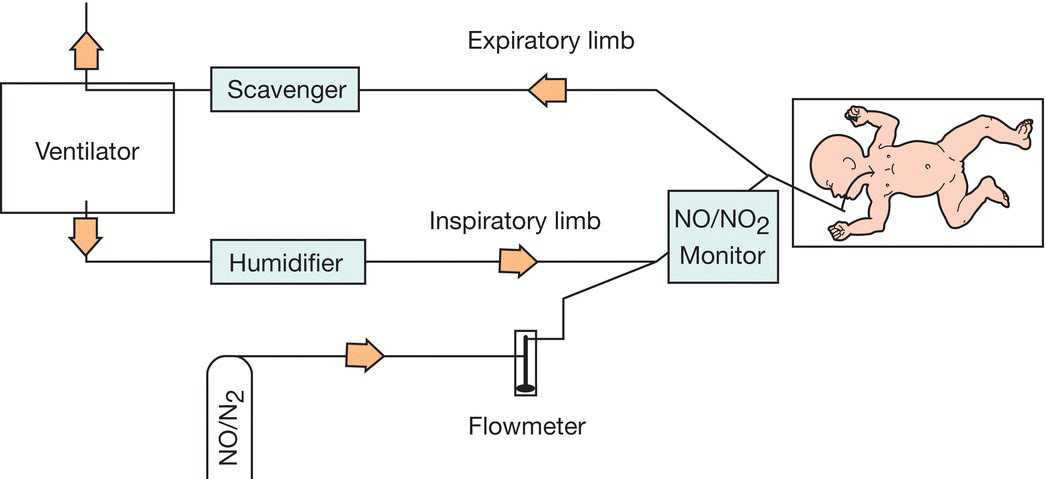
Fig. 26.12 Components in delivery of inhaled nitric oxide. There is a scavenger for removing nitric oxide released into the atmosphere. The blood concentration of methemoglobin, a potentially toxic by-product, is checked periodically. Inspired nitrogen dioxide (NO2) levels (a by-product of mixing nitric oxide and oxygen) are monitored continuously.
Respiratory failure
The severity of hypoxemic respiratory failure can be assessed by calculating the oxygenation index (OI):
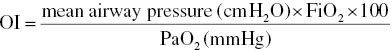
In term infants, OI ≥40 is associated with a 40% risk of mortality.
In preterm infants, OI ≥20 is associated with a 50% risk of mortality.
Therapeutic options for respiratory failure if on conventional mechanical ventilation with high pressures and in high concentration of oxygen are:
- extra rescue doses of surfactant
- high-frequency ventilation
- nitric oxide or sildenafil therapy (although sildenafil is not currently approved in the US for this purpose)
- ECMO for infants of ≥34 weeks’ gestation.
Extracorporeal membrane oxygenation (ECMO)
Infants are placed on heart–lung bypass for up to 2−3 weeks to allow the lungs to recover (Figs 26.13 and 3.3). Blood is removed from the circulation, oxygenated and then returned to the circulation. It is performed in relatively few specialized centers. Because of the need for anticoagulation and large surgical catheters, there is a risk of intraventricular hemorrhage in preterm infants and it is therefore reserved for infants of ≥34 weeks’ gestation and birthweight >=2 kg. Conditions that cause recoverable respiratory failure which may respond to ECMO are listed in Table 26.1. Indication is an oxygenation index of ≥40 in spite of optimal mechanical ventilation and circulatory support. Other requirements are <10−14 days’ mechanical ventilation, no lethal congenital abnormalities and no significant intracranial hemorrhage. The need for ECMO has declined markedly since the introduction of inhaled nitric oxide, surfactant and HFOV. The most common indication is now congenital diaphragmatic hernia.
Table 26.1 Conditions that may require ECMO.
|
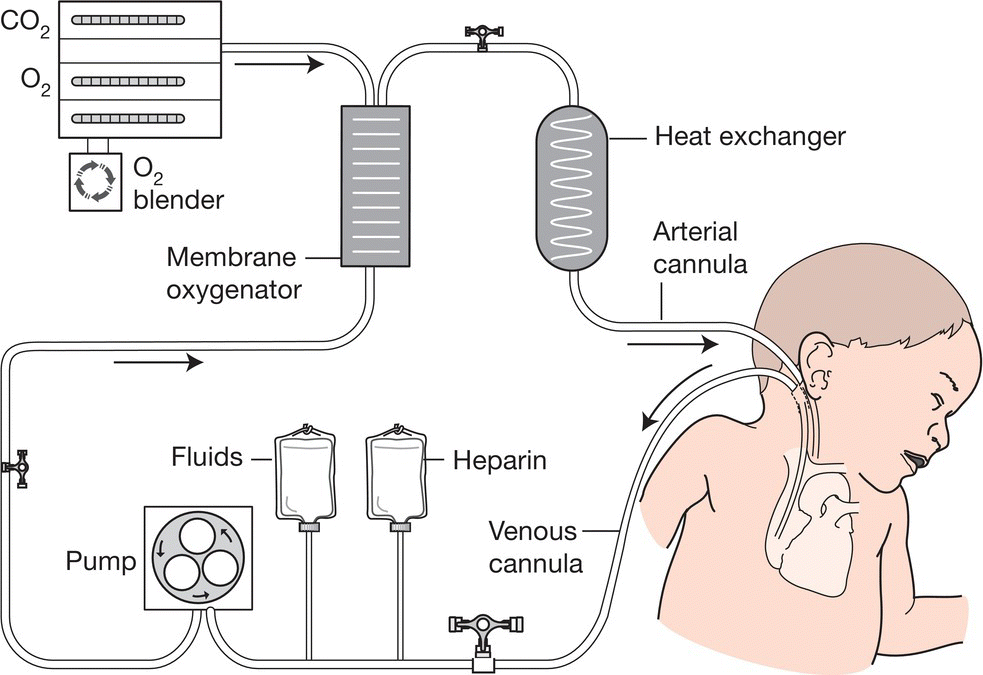
Fig. 26.13 ECMO (extracorporeal membrane oxygenation) circuit. The infant’s venous blood is pumped through a membrane oxygenator (an artificial lung), which extracts carbon dioxide and adds oxygen. The blood is returned to the baby into the right carotid artery (veno-arterial ECMO, as shown in the diagram). In veno-venous ECMO, blood is removed and returned into the right atrium through a double-lumen catheter. The lungs continue to be ventilated but at a low resting level.