51
Renal and urinary tract disorders
Electrolyte problems
Sodium
Normal range 135–145 mmol/L.
Hyponatremia – Na <130 mmol/L
Results from excess water relative to sodium or insufficient sodium relative to water.
Key is to assess fluid status overall. If detailed assessment required, measure paired urinary and serum sodium, osmolality and creatinine to establish fractional excretion of sodium. (Fractional excretion of sodium, FENa = UNa/PNa × PCr/UCr × 100, where UNa is urinary sodium, PNa plasma sodium, PCr is plasma creatinine, UCr urinary creatinine).
Term infants, hyponatremia usually from giving excessive volume of IV dextrose containing no or insufficient sodium. Especially in first 48 hours of life and when there is intravascular volume depletion with water reabsorption. Also excess IV fluids to mother during labor; oliguric renal failure (renal impairment with little urine output and fluid overload).
Preterm – marked Na loss in urine, poor at conserving sodium as tubular reabsorptive capacity not fully developed. This results in hyponatremia of prematurity (see Fig. 51.1).
Other causes include:
- insufficient Na supplementation (low FENa)
- gastrointestinal losses – diarrhea, vomiting (low FENa)
- renal losses – diuretics (high FENa), renal dysplasia, congenital adrenal hyperplasia, renal tubulopathies
- SIADH, syndrome of inappropriate antidiuretic hormone (high FENa, low POsm and high UOsm) – probably rare in newborn infants.
Hypernatremia – Na >150 mmol/L
Result of excessive water loss over sodium or excess sodium intake over water.
If detailed assessment required, assess fluid status and measure FENa.
Hypernatraemic dehydration may be from:
- insufficient input of fluids e.g. insufficient breast milk
- excessive water losses, e.g. evaporative through skin in extremely preterm, phototherapy, radiant heater or gastrointestinal losses
- excess sodium intake, e.g. sodium containing flushes of lines, sodium bicarbonate, sodium phosphate, etc (high UOsm and high FENa)
Rare causes – diabetes insipidus (central, e.g. septo-optic dysplasia or nephrogenic, no ADH or ADH effect) (low UOsm, low FENa), deliberate salt poisoning.
Potassium (normal range 3.5–5.5 mmol/L)
Hyperkalemia – K >6.0 mmol/L
Serious condition as can result in arrhythmias and death. But most common reason is hemolyzed blood sample.
Other causes – renal impairment (transient is relatively common in extremely preterm infants), excess K supplementation, congenital adrenal hyperplasia.
Neonates tolerate hyperkalemia better than older children, so only treat if K >6.5 mmol/L. ECG monitoring is required.
Treatment involves giving calcium gluconate to stabilize myocardium, salbutamol IV or nebulized, correcting acidosis, stopping all K, changing to low K feed, infusion of glucose and insulin, calcium resonium orally or rectally but can cause gastrointestinal obstruction.
Hypokalaemia – K <3.0 mmol/L
Causes include insufficient supplementations, diuretics, diarrhea, vomiting, renal tubular losses (e.g. Bartter syndrome), drugs, e.g. amphotericin.
Calcium and phosphate
Hypocalcemia
Relatively common problem and can lead to seizures.
Causes: birth trauma/asphyxia, infants of diabetic mothers, exchange transfusion with blood reconstituted in citrate, maternal hyperparathyroidism, Di George syndrome, associated with hypomagnesemia, maternal vitamin D deficiency.
Hypophosphatemia
Usually results from insufficient supplementation in feeds or parenteral nutrition.
Urinary tract infection (UTI)
- Commoner in boys than in girls – the reverse of older children.
- Should be suspected in any infant who is non-specifically unwell.
Presentation
- Fever or sometimes low temperature or temperature instability
- Poor feeding
- Vomiting
- Prolonged jaundice
- Diarrhea
- Failure to thrive
Investigations
Urine – collecting urine samples:
- clean catch specimen
- urethral catheterization
- suprapubic aspiration (see Chapter 76).
Blood culture and sepsis work-up (with or without lumbar puncture) should be performed as urinary tract infection is often accompanied by septicemia in neonates.
Diagnosis
Is made by culture of a single strain of any organism on a catheter sample or suprapubic aspirate. However, may get false-positive suprapubic aspirate result from skin commensal or bowel perforation.
White cells may or may not be present on microscopy or urinalysis.
E. coli is the commonest organism (>75%); remainder caused by Klebsiella, Proteus, Enterobacter.
Management
Intravenous antibiotics – start immediately whilst awaiting the result of the urine culture. Subsequent choice of antibiotics will depend on the sensitivities of the cultured organism. Should be continued at full dosage until the infant has been well for 2–3 days and a negative follow-up urine culture obtained. Following treatment of culture positive UTI, oral prophylactic antibiotics, e.g. trimethoprim or cefalexin, should be given until the results of imaging of the kidneys and urinary tract are known.
Imaging – if culture is positive, ultrasound of the kidneys and urinary tract is performed to detect renal tract abnormalities. A VCUG (voiding cystourethrogram, micturating cystourethrogram) is performed to identify bladder outflow obstruction, e.g. from posterior urethral valves or vesicoureteral reflux (Fig. 51.2). A radionuclide scan (DMSA, dimercaptosuccinic acid) is performed 3 months later to identify renal scarring (Fig. 51.3).
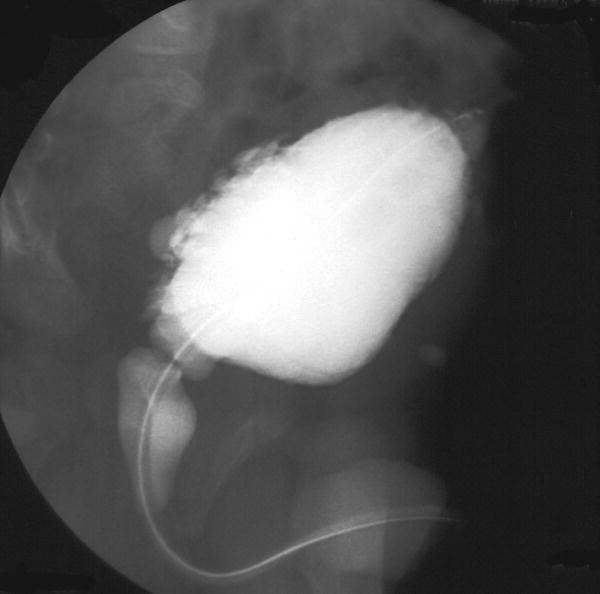
Fig. 51.2 VCUG (voiding cystourethrogram, micturating cystourethrogram) showing trabeculation of the bladder wall, hypertrophy of the bladder and dilated posterior urethra from posterior urethral valves.
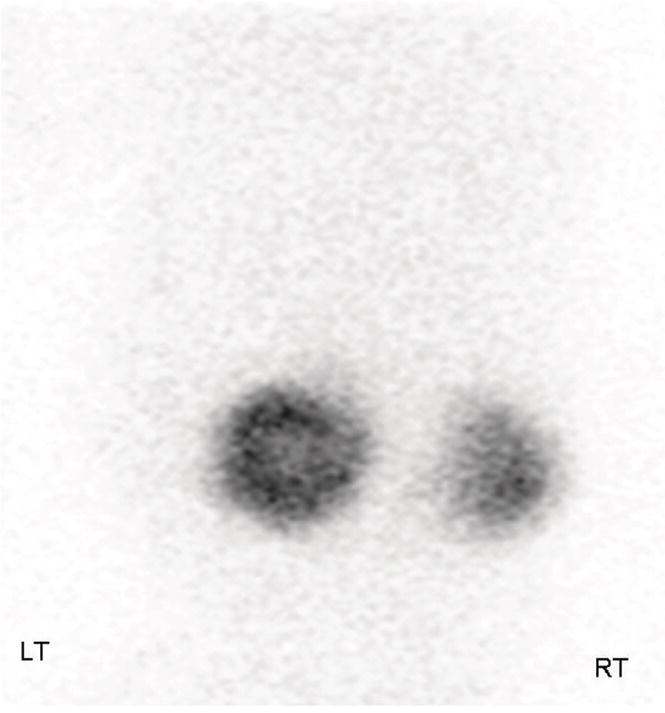
Fig. 51.3 Bilateral renal scarring, more severe on right, on DMSA radionuclide scan on investigation following a urinary tract infection.
Acute kidney injury, AKI (acute renal failure)
In acute kidney injury (acute renal failure) there is sudden impairment in renal function leading to inability of the kidney to excrete nitrogenous waste and electrolytes. It is defined as a rise in the plasma creatinine concentration to twice the upper limit of normal, i.e. 1.5 mg/dL (130 mmol/L) accompanied by a reduction in urine flow rate to <1 mL/kg/hour. However, renal failure can occur without oliguria. It results from a significant fall in glomerular filtration rate with failure of tubular reabsorption of salt and water.
Causes
Different in neonates from children and adults as usually prerenal (Table 51.1). Mild renal impairment is not uncommon in the first few days of life, particularly in preterm infants, and is usually transient.
Table 51.1 Causes of acute kidney injury (acute renal failure) in neonates.
| Prerenal | Renal | Postrenal |
| Hypovolemia | Acute tubular necrosis secondary to an uncorrected prerenal cause | Congenital obstructive uropathy – posterior urethral valves, etc. |
| Dehydration, sepsis, necrotizing enterocolitis | Congenital renal abnormality, e.g. polycystic kidney disease, renal agenesis, renal hypodysplasia | |
| Blood loss: antepartum, neonatal | ||
| Heart failure | Vascular insult – renal vein thrombosis, renal artery thrombosis (associated with use of umbilical arterial lines) | Neurogenic bladder |
| Hypoxia including birth asphyxia | ||
| Nephrotoxins, e.g. aminoglycosides | ||
| Infection – pyelonephritis |
Clinical features
- Creatinine concentration raised – at birth it reflects maternal creatinine, falls over the next 4-6 weeks; a rising creatinine after day 1 suggests acute kidney injury
- Urinary features – oliguria, hematuria, proteinuria
- Clinical features – edema, dehydration, vomiting, lethargy, seizures, hypertension
- Other biochemical features – hyperkalemia, acidosis, hyperphosphatemia and hypocalcemia
Investigations
Ultrasound of kidneys and urinary tract – identifies if there are abnormal kidneys, outflow obstruction, abnormal blood flow in renal arteries and veins.
Management
Prevention
- Monitor the creatinine, blood urea nitrogen (urea) and electrolytes of newborn infants who have been exposed to risk factors for acute kidney injury, such as birth asphyxia or sepsis
- Early treatment of hypovolemia
- Relief of obstruction
- Avoid nephrotoxic agents if possible
Electrolyte and fluid management
- Restrict sodium, potassium and phosphate. Use calcium carbonate as phosphate binder. Correct metabolic acidosis.
- High-dose furosemide 2–5 mg/kg to convert oliguric into non-oliguric renal failure.
- Nutritional support.
- Renal replacement therapy – rarely needed, only if fluid and metabolic abnormalities cannot be corrected. Peritoneal dialysis is preferable but may not be possible (e.g. abdominal wall defects or necrotizing enterocolitis). Hemodialysis is difficult due to vascular access and risks associated with anticoagulation. Continuous veno-venous hemofiltration is more gentle and better tolerated, especially in the sick neonate unable to tolerate intermittent hemodialysis.
Prognosis
Infants who develop acute kidney injury in the neonatal period have increased mortality, highest in extremely preterm.
Infants who have chronic kidney disease and need to start renal replacement therapy in the neonatal period have a 2-year survival rate of 81%, with infection being the most common cause of death. The 5-year survival rate is 76%. However, there is significant comorbidity in the survivors, with growth problems, anemia and hypertension.