80
Amplitude-integrated electroencephalography (aEEG)
Amplitude-integrated electroencephalography (aEEG) is a bedside tool for continuous monitoring of changes in the amplitude of the electroencephalogram using a cerebral function monitor (CFM). It compares well with standard EEG when used to assess the severity of neonatal encephalopathy, but a standard EEG is still required to provide additional important information about changes in frequency and in the synchrony, distribution and other characteristics of cerebral cortical activity.
Use of aEEG in neonates
Term infants
- To assess the severity of hypoxic–ischemic encephalopathy (HIE).
- For prediction of neurologic outcome following HIE.
- For seizure detection.
- To monitor response to anticonvulsant therapies.
- To select infants for clinical trials of neuroprotection.
- To monitor and assess etiology of neonatal encephalopathy.
Preterm infants
- To detect complications such as intraventricular hemorrhage and posthemorrhagic ventricular dilatation.
- To predict neurodevelopmental outcome following preterm delivery.
Cerebral function monitor
The CFM records one or two channels of EEG from scalp electrodes; the signal is filtered and the signal amplitude is displayed. Frequencies <2 and >15 Hz are selectively filtered to reduce artifacts caused by movement, ECG and other electronic equipment. The speed is usually set at 6 cm/hour, making every major division equal to 10 minutes.
Interpretation
The standard CFM display appears as a band of activity moving slowly across the display screen. The lower edge of the band indicates the lowest peak-to-peak amplitude reached by the filtered EEG over a period of time, whereas the upper edge is related to the highest levels. The width of the band indicates the variability of the EEG amplitude. In term infants the aEEG trace can be classified according to voltage or pattern of trace (Fig. 80.1).

Fig. 80.1 Classification of aEEG. This is based on voltage (upper and lower margins of the trace), shown on the left, or on pattern of the trace, shown on the right. They overlap.
Normal
The upper margin of the trace is above 10 μV and the lower margin is greater than 5 μV. The width of the band fluctuates between 10 and 50 μV, changing with the sleep–awake state of the infant (sleep–awake cycling), and is called a continuous pattern.
Moderately abnormal
The upper margin is > 10 μV and the lower margin is < 5 μV. Hence the band appears wider and is called a discontinuous pattern. This is seen in infants with moderately severe encephalopathy. It may also be seen immediately after administration of anticonvulsants and sedatives. The aEEG should therefore not be used for assessing severity of encephalopathy during the first 30–60 minutes after therapy with these medications. A discontinuous pattern may be normal in preterm infants.
Severely abnormal
The upper margin is < 10 μV and lower margin is usually < 5 μV. Hence the band appears narrow and is called a low-voltage pattern. Rarely, the lower margin may be raised above 5 μV because of interference from ECG.
This low-voltage pattern may be accompanied by brief bursts of higher voltage spikes, which appear as single spikes above the background activity. This is called ‘burst suppression’. A severely abnormal trace is usually seen with severe encephalopathy and is often accompanied by seizure activity.
Isoelectric EEG
Absent cerebral electrical activity is seen as a flat line or narrow band of activity with very low voltage.
Seizure detection and response to anticonvulsants
- Seizures are characterized by a sudden rise and narrowing of the trace, reflecting the increase in EEG voltage. The trace returns to the previous appearance or is depressed when the seizure activity stops (Fig. 80.2).
- Very frequent or continuous seizure activity (status epilepticus) may result in a ‘sawtooth’ appearance or in an elevated narrow band of activity.
- Inspection of the underlying EEG helps to confirm seizures suspected from the CFM trace.
- Since the aEEG is usually displayed at 6 cm/hour, brief seizures less than 1–2 minutes will not be seen.
- A standard EEG is needed to document the electrographic characteristics and distribution of seizures.
- aEEG is often used to monitor the response to treatment with anticonvulsants (Fig. 80.3).
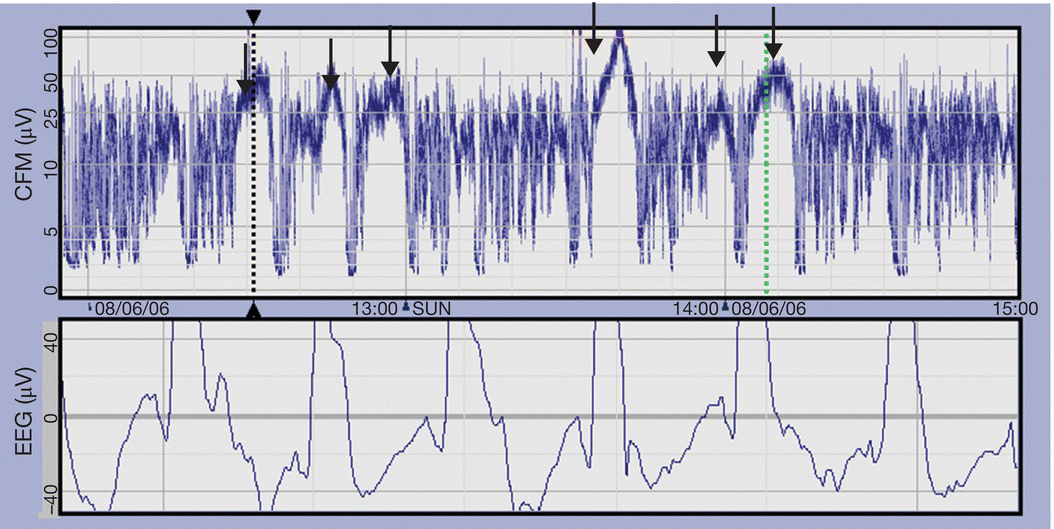
Fig. 80.2 aEEG showing seizures (arrows), with rise in baseline and lack of variability in voltage. The corresponding raw EEG with high amplitude spikes is shown in the lower panel.

Fig. 80.3 Seizures and response to anticonvulsants. Point A: phenobarbitone was given followed by a second dose at point B, resulting in termination of seizures. Seizure activity is seen again at point C.
Artifacts on an aEEG trace
- Poor contact with electrodes causes high amplitude artifact.
- An artifact from electrical activity of the heart may result in elevation of the band of activity even in the absence of cerebral activity.
- Muscle or movement artifact can result in an artificially broad band or sudden changes in the band of activity.
- The underlying raw EEG should always be inspected if artifact is suspected.
aEEG as a prognostic tool in HIE
- The aEEG, in conjunction with other neurophysiologic investigations and imaging is helpful in predicting neurodevelopmental outcome following neonatal HIE. Combining clinical assessment with the aEEG trace further improves prognostic accuracy.
- Hypothermia improves neurologic outcomes when started within the first 6 hours of age in infants with HIE with a moderately or severely abnormal aEEG trace. As normalization of the aEEG occurs later in cooled infants, it should not be relied upon as the sole prognostic factor before 48h of age.
- In cooled infants, normal background amplitude or recovery of background amplitude within 6 hours of birth is associated with a likely normal outcome.
- Many infants with recovery to normal EEG activity within 36–48 hours of birth go on to make a good clinical recovery.
- A burst suppression pattern or depressed trace persisting beyond 48 hours or absence of sleep–awake cycling by 96 h indicates a high probability of abnormal neurodevelopmental outcome
- It is not clear whether the occurrence of seizures in HIE alters the prognosis in infants with a suppressed EEG.
Use of aEEG in preterm infants
- There are well-characterized developmental changes in the EEG of preterm infants; the EEG is discontinuous at 24–25 weeks’ gestation and gradually becomes less discontinuous with increasing maturity.
- Sleep–awake cycles begin to emerge from about 30 weeks, but remain incomplete until about 37 weeks’ gestation.
- A semiquantitative scoring system of maturity based on sleep–awake cycling pattern, continuity and bandwidth has been described and shown to correlate with subsequent neurologic outcome, although its reliability is not proven.