NEUROIMAGING
Tips for Reviewing Scans
Review all images & sequences. Reconstructed images screen for abnl findings, but always review raw data. Be systematic: eval gyral-sulcal pattern, gray & white structures, ventricles & CSF spaces, vessels, bones, sinuses, & soft tissues. Look for patterns of abnormalities, asym., mass effect vs. atrophy, shift of midline structures. Note whether lesion involves gray matter, WM, or both.
Computed Tomography (CT)
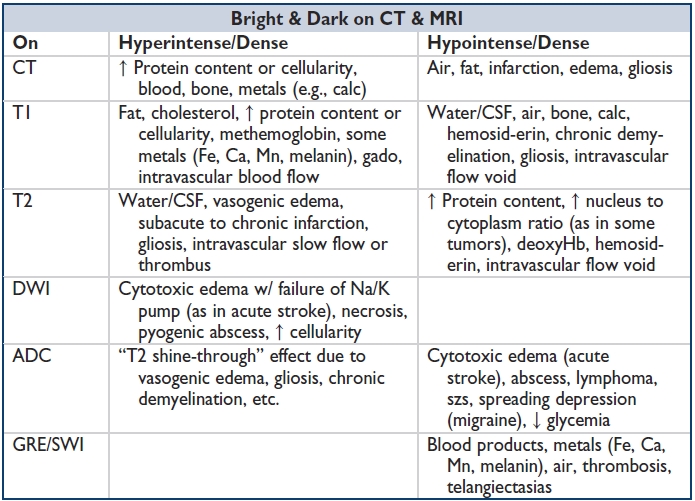
Signal measured in Hounsfield units (HU). From black (hypodense) to white (hyperdense): air −1,000; fat −30–70; water 0; CSF +15; WM 20–30; GM 35–45; acute hemorrhage/thrombus +60–100; bone +1,000. HU values can complement visual inspection in identifying tissues/lesions. CT w/ contrast (iodine based) enhances vessels & areas of BBB breakdown (necrosis, infxn, acute demyelination, many tumors). Delayed postcontrast images to assess temporal dynamics of enhancement (e.g., slow flow, vascular malformations). CT angiography (CTA) uses timed boluses of IV contrast to enhance arteries. CT venography (CTV) uses timed IV contrast to enhance veins. CT perfusion (CTP) measures cerebral blood flow (CBF), cerebral blood volume (CBV), & mean transit time (MTT), used to define an area of tissue w/ infarction or ↓ perfusion at risk of infarction (i.e., ischemic penumbra surrounding an infarct). Can also be used to demonstrate ↑ perfusion to a tumor or other highly vascular or hypermetabolic lesion. CBF = CBV/MTT.
Magnetic Resonance Imaging (MRI)
Signal from applying magnetic field & measuring relaxation times of hydrogen nuclei.
Gadolinium: MRI paramagnetic contrast, causes “T1 shortening” (hyperintensity). Enhances vessels & areas of BBB breakdown (necrosis, infxn, acute demyelination, many tumors). Delayed postcontrast images to assess temporal dynamics of enhancement. Lesions that enhance usually neoplastic, pustulent, or bloody.
MRI sequences: T1: GM darker than WM, CSF dark. T2: WM darker than GM, CSF bright. FLAIR (fluid-attenuated inversion recovery); T2-weighted sequence, but CSF signal suppressed (dark). “Fat saturation” or STIR: Helps differentiate diff tissue densities by suppressing bright signal from fat. DWI/ADC (diffusion-weighted imaging/apparent diffusion coefficient): Assesses for acute ischemia or cytotoxic injury (restricted diffusion is DWI bright, ADC dark); several processes besides acute infarction show restricted diffusion (abscess & hypercellular tumors). GRE (gradient echo) & SWI (susceptibility-weighted images): Dark signal corresponds to heavy metals (Fe, Ca, Mn, melanin), including iron-containing blood products; useful for identifying microhemorrhages (hemosiderin). Diffusion tensor imaging (DTI): Useful for WM tractography, emerging clinical applications. MR angiography (MRA): Uses timed boluses of gado to enhance arteries. “Time of flight” (TOF) MR angios are noncontrast-based vessel reconstructions of flow void signal, demonstrate flow rather than vessel structure, & can overestimate stenoses. MR venogram (MRV): Venous study, w/o contrast (TOF). MR perfusion (MRP): Timed contrast bolus to measure perfusion parameters (e.g., for “ischemic penumbra”): CBF, CBV, MTT. MR spectroscopy (MRS): Compares measures of neuronal integrity (N-acetyl aspartate, NAA), cellular metabolism (creatinine, Cr), cell membrane synthesis (choline, Cho) w/in selected foci. In dzs w/ ↑ cell turnover, Cho is ↑'d. In neurodegenerative dzs, NAA is ↓'d. Functional MRI (fMRI): Blood oxygen level–dependent (BOLD) T2-based measurements of oxy- & deoxy-Hb. OxyHb is hyperintense compared to deoxyHb on T2-weighted images. W/ high perfusion to active brain tissue, oxyHb levels rise & deoxyHb fall. Net effect is hyperintense signal in metabolically active tissue. Used in surgical planning to localize eloquent cortex adjacent to infiltrating tumors.
Things That Enhance
- Patent vessels, breakdown of BBB (e.g., w/in cytotoxic processes: infarction, necrosis, infxn, acute demyelination, expanding tumors).
- Rim enhance: Fungal or parasitic infxn, abscess, acute demyelinating plaques, granuloma, infarction, lymphoma (in immunocompromised host), radiation necrosis, GBM, subacute ICH. (Mnemonic: “MAGIC DR”: Metastases, Abscess, Glioma (& lymphoma), Infarction, Contusion, Demyelination, Resolving hematoma/Radiation necrosis.)
- Pachymeninges (dura & outer arachnoid): CSF leak or intracranial hypotension, SAH, infxn, inflammation, mets.
- Leptomeninges (inner arachnoid & pia): Acute stroke, infxn, inflammation, mets.
- Cauda equina or roots: GBS, disc herniation, Charcot-Marie-Tooth, mets, neurofibromas, schwannomas, arachnoiditis, granulomatous dz, Lyme, CMV, schistosomiasis.
Other Imaging Techniques
Conventional angiography: Intra-arterial (IA) contrast to study indiv vessels; allows focal Rx (coiling, embolization, IA tPA). Resolution ~0.5 mm.
Ultrasound: Helpful to evaluate arterial anatomy, stenosis, & flow (direction & quality). Carotid Doppler: Extracranial carotids & verts; transcranial Doppler: Intracranial vessels.
Positron emission tomography (PET): Indirectly measures metabolism by uptake of a radioactively labeled biologically active compounds, e.g., glucose (tumors & abscesses are hypermetabolic; atrophy & gliosis are hypometabolic). Can be combined w/ CT or MRI to improve anatomical localization of lesions.
Single-photon emission computed tomography (SPECT): Eval distribution of a radiologically active compound by emission of gamma rays to study perfusion & metabolism.
Myelography: Intrathecal injection of contrast to assess cord & nerve root anatomy (e.g., protruding discs & other masses).
Imaging Protocols, Indications, & Cautions
- Acute focal neurologic deficit: Acute head/neck trauma, concern for stroke/SAH: I-CT.
- Hyperacute stroke: ? Thrombolysis/IA intervention: I-CT (w/ CTA).
- Acute/subacute stroke: MRI w/ DWI & GRE/SWI, w/head/neck MRA.
- Multiple sclerosis: MRI brain, cervical +/− thoracic spine, w/ DWI & gado.
- Neoplasm: MRI brain w/ DWI, GRE/SWI, gado.
- Cranial nerve or brainstem lesion: MRI brain w/ SSFP sequence (FIESTA or CISS): thin cuts through brainstem & detailed views of CNs.
- Optic nerve lesion: Brain & orbital MRI w/ gado coronal slices through orbits.
- Aneurysm: CTA if acutely symptomatic, MRA for surveillance.
- Dissection: CTA or MRA w/ T1 fat-saturated (“fat-sat”) images (to visualize intramural hematoma).
- Conventional angiogram: Gold standard for vascular imaging & dissections, residual lumen, vasculitis, vasospasm, moyamoya. Invasive, risk of stroke 0.5%–1%.
NEURORADIOLOGY OF SPECIFIC DISEASES
Hemorrhage
CT: Hyperdense (bright) & surrounded by hypodensity (edema, extruded serum).
Note: Hyperacute/chronic subdural hematomas & hygromas can be isodense to CSF.
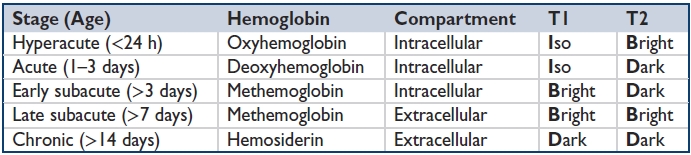
Blood on MRI: T1/T2 appearance depends on “age” of blood (see table). Blood, hemosiderin, & other substances containing metal (Fe, Ca, Mn, melanin) hypointense on GRE/SWI.
ABC/2 formula for estimating hematoma volume: (A × B × C)/2, where A = max hematoma transverse diameter, B = max hematoma AP diameter, C = no. of axial slices containing hematoma x slice thickness (usually 0.5 cm).
Mnemonic for determining age of intraparenchymal hematoma on MRI: “i be iddy biddy baby doodoo” (or “i bleed, i die, bleed die, bleed bleed, die die”).
Epidural: Biconvex shape; cannot spread past suture lines (dura adherent to skull).
Subdural: Concave shape; cannot spread past dural reflections (falx, tentorium).
SAH: Aneurysms most often located at branch points around circle of Willis (ICA-PCom, PCA-PCom, ICA-ophth, etc.); depending on artery & extent of bleed, blood can track into parenchyma, ventricles, cisterns, & along tentorium. If SAH seen, CTA indicated.
ICH: HTN: Most commonly basal ganglia, thalamus, pons, & cerebellum. Cerebral amyloid angiopathy (CAA): Typically lobar. Mets that commonly hemorrhage include breast & lung (by incidence) Melanoma, Renal cell ca, Choriocarcinoma, Thyroid papillary ca (“MR/CT”) (by propensity).
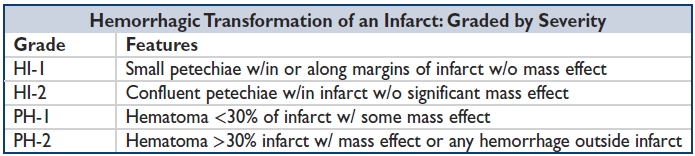
Venous infarct: Does not respect arterial territory, extensive edema, hemorrhagic transf.
Intraventricular: Typically seen w/ SAH & hypertensive hemorrhage.
Clues to secondary hemorrhage: Aneurysm rupture: ICH&SAH; Coagulopathy: Fluid-fluid level (=nonclotting blood) w/in hematoma; TBI: Soft-tissue edema of scalp, fractures, or other injury overlying SAH or ICH. Look for coup & contracoup effects.
Infarction
CT: Noncontrast: use high-contrast center/window values (30/30) to assess for early infarction: loss of gray-white differentiation, parenchymal hypodensity, sulcal effacement. Hyperdensity w/in a vessel may represent acute thrombus. Soft thrombus appears hypodense compared to calcified atherosclerotic plaque (hyperdense). CTA: Vessel cutoff/stenosis; flame-shaped tapering suggests dissection. CTP: defines ischemic penumbra.
MRI: DWI-bright & ADC-dark acutely. MRA/MRP: Interpreted like corresponding CT studies. Wallerian degeneration can be seen following infarctions involving parent neurons; output tracks appear DWI hyperintense acutely, T2 hyperintense chronically.
Other Vascular Diseases
Microvascular WM disease: Aka leukoaraiosis: 2/2 lipohyalinosis & arteriosclerosis of small vessels. Subcortical sym T2-hyperintense lesions, usu punctate but confluent w/ more advanced dz. Binswanger disease: SC WM process a/w HTN & lacunes; spares U-fibers 2/2 to collaterals from cortical arteries.
Developmental venous anomaly (DVA or venous angioma): Dilated veins that converge radially (like a caput medusa) to a draining vein. W/ contrast, early venous filling, persistence of venous phase. Low-risk hemorrhage.
Capillary telangiectasias: Capillaries surrounded by nl brain, predilection for pons. Most never hemorrhage. Enhance on CT/MRI, GRE/SWI hypointensity.
Cavernous angiomas/hemangiomas/malformations: Congenital vasc hamartoma of vessels w/o interspersed nl brain parenchyma. Can have assoc DVA. Hyperdense on CT w/ calc. T1 hyperdense & T2 heterogeneous “Popcorn” appearance; GRE/SWI: dark rim (hemosiderin). Angiographically occult if no DVA.
Arteriovenous malformation (AVM): Arterialization of veins, large feeding arteries, absent or abnl capillaries, & enlarged draining veins. Often has aneurysms of feeding vessels. CT: Hyperdense, enhances. MR: Irregular serpentine flow voids on T2, enhances. Conventional angiogram: Early venous filling 2/2 absence of capillary phase.
Dural arteriovenous fistula (AVF): Dural-based AVM a/w venous hypertension. Can occur anywhere in CNS. In spine, mostly in thoracolumbar area. Cord infarction w/ necrotizing myelopathy can occur causing paraparesis (Foix-Alajouanine syndrome, spinal cord appears T2 hyperintense, tangle of T2 flow voids on surface of spinal cord).
Aneurysms: Focal arterial dilations typically at branch points; fusiform (atherosclerotic dilation), saccular/berry (branch points), mycotic (infectious), neoplastic, pseudoaneurysm (traumatic, dissection).
Dissections: Flame-shaped tapering of vessel lumen, sometimes in corkscrew or spiral orientation on CTA, MRA, or conventional angiography. T1 fat-saturated images may demonstrate thrombus w/in false lumen overall similar sensitivity to CTA (AJR Am J Roentgenol 2009;193:1167–1174). Note whether dissection extracranial or intracranial, whether there is intradural extension (risk for SAH). Carotid: Tend to occur near C2-C3 vertebral level, 2–3 cm superior to bifurcation. Vertebral: Tend to occur where artery is nearest bone, at C1 & through transverse foramina.
CAA: Lobar ICHs & evidence of prior microhemorrhages in SC WM, T2 hyperintense & GRE/SWI hypointense, superficial siderosis sometimes seen as well.
Moyamoya: Stenosis or occlusion of ICAs → development of abnl network of collateral capillary circulation arising from ACA, MCA, or PCA branches, lenticulostriates, or ECA transdural anastamoses. Angiography: ICA stenosis, proximal ACA/MCA occlusion w/ extensive collaterals, & dilation of perforating lenticulostriate arteries (“puff of smoke”).
CADASIL (cerebral autosomal dominant arteriopathy w/ subcortical infarcts & leukoencephalopathy): Sym T1 hypointense & T2 hyperintense lesions in anterior temporal lobe WM, external capsules, & throughout SC WM.
Fibromuscular dysplasia (FMD): Large vessel (ICA & renal artery) “string of beads” appearance; diameter of beading greater than diameter of artery; commonly spares first few cm of ICA (unlike atherosclerosis).
Vasculitis: Segments of circumferential vessel narrowing (atherosclerotic lesions usu. more eccentric & focal c/w vasculitic lesions); multiple areas of cortical & SC infarction, hemorrhage, & nonspecific WM T2 hyperintensities. Differentiate from reversible cerebral vasoconstriction syndrome & vasospasm via clinical presentation.
Migraine: Nonspecific SC T2 hyperintensities, “UBOs” (“unidentified bright objects”).
Transient global amnesia (TGA): Punctate foci of restricted diffusion w/in limbic & paralimbic structures, reversible.
Susac syndrome (retinocochleocerebral vasculopathy): “Punched out” punctate T1 hypointense & T2 hyperintense lesions in the corpus callosum & deep gray nuclei.
Demyelinating & Other White Matter Diseases
Multiple sclerosis: Focal, ovoid WM lesions of PV > SC (perpendicular “Dawson fingers”) hemispheric WM, corpus callosum, optic nerves, brainstem (esp medial longitudinal fasciculus), cerebellum (esp middle cerebellar peduncle), optic nerves, spinal cord (<3 spinal segments, incomplete transverse lesion); old lesions T1 dark; acute lesions enhance w/ gado (rim or incomplete rim), may restrict diffusion. Rare cortical & deep gray matter lesions.
ADEM: Multiple lesions of same age; round, enhancing SC T2 bright lesions in hemispheric WM (& brainstem & cerebellum). More mass effect than typical MS lesions.
Acute MS or ADEM variants: Acute hemorrhagic leukoencephalitis (Weston-Hurst dz): confluent T2 bright WM signal w/ mass effect & minimal enhance; hemorrhage on GRE/SWI or CT. Marburg variant of MS: Large region of T2 bright signal in hemispheric WM w/ mass effect & periph enhancement. Balo concentric sclerosis: Alternating concentric rings of T2 bright & dark signal (& alternating rings of enhancement) in hemispheric WM w/ var mass effect. NMO (Devic dz): Longitudinal cord T2 bright lesion (>3 spinal seg, central, or occupying entire cross section) w/ optic nerves & chiasm T2 bright lesions; all lesions expansile w/ var enhance; var PV WM T2 bright lesions.


Adapted from Schiffman & van der Knapp. Neurology 2009;72:750–759.
Other Inflammatory Diseases
SLE/APLA: SC WM (posterior predilection) T2 bright lesions; may be in vascular distribution & ischemic infarct may occur (DWI bright); relative PV sparing; cortical lesions & diffuse atrophy also seen; T2 bright signal in cord (transverse myopathy).
Sjögren: SC & PV WM T2 bright lesions; also w/ basal ganglia T2 bright lesions; corpus callosum involvement less common.
Behcet: Multifocal or confluent hemispheric WM T2 bright lesions w/ var enhancement; usually w/ concurrent diencephalon & upper brainstem lesions (often edema & enhancement); occasional cortical vein thrombosis/infarctions (var hemorrhage).
Sarcoidosis: Focal, multifocal, or confluent T2 bright lesions of WM w/ var enhancement; also enhancement & thickening of pachymeninges w/ parenchymal infiltration (pituitary-hypothalamus, optic nerve, optic chiasm).
Primary CNS vasculitis: Acute lesions DWI bright in hemispheric white (& gray) matter (may follow clear vascular territory), a/w stenoses & aneurysms; var lesional, meningeal & perivascular enhancement; chronic lesions w/ T2 bright hemispheric WM.
Immune reconstitution inflammatory syndrome (IRIS): Confluent, multifocal T2 bright diffuse WM lesions w/ areas of focal enhancement & mass effect.
Inflammatory CAA: Predominantly cortical & SC microhemorrhages (on GRE/SWI) w/ extensive asym & confluent ↑ T2 & ↓ T1 signal of SC WM w/ minimal enhancement (Neurology 2007;68:1411).
Tolosa-Hunt: Idiopathic granulomatous dz typically involving cavernous sinus, orbital apex & adjacent structures; lesions are T1/T2 isointense & strongly enhance.
Paraneoplastic disease: a/w multiple systemic ca's incl SCLC, testicular germ cell, ovarian, etc. Sym T2 hyperintensity w/ edema.
PRES & hypertensive encephalopathy: b/l, sym T2 bright WM lesions in posterior hemispheres at MCA-PCA border zones; other regions may be affected (frontal & temporal, brainstem, cerebellum, gray structures); var restricted diffusion & peripheral enhancement.
Metabolic diseases: B12 deficiency: Scattered PV T2 bright WM lesions; ↑ T2 signal in posterior cord. Marchiafava-Bignami syndrome: Acutely, central T2 & DWI bright lesions of corpus callosum; chronically, central cavitation; var mass effect & enhancement. Osmotic demyelination: b/l, sym T2 bright lesions of central pontine WM (var sparing of corticospinal tracts), & var SC WM, midbrain, & deep gray lesions w/o enhance or mass effect. Hypoxia-ischemia: b/l, sym diffuse DWI & T2 bright confluent lesions after resp or cardiac arrest; predilection for SC WM (involves U-fibers), corpus callosum, capsules (internal & external), globus pallidi hippocampus, cerebellum. High-altitude encephalopathy: b/l, sym posterior deep WM > SC WM & corpus callosum T2 bright lesions; var DWI bright lesions. Acute intermittent porphyria (AIP): Similar to PRES (above).
Toxic diseases: Chemotherapy (cyclosporine, tacrolimus, methotrexate): (1) Acutely, PRES (see above); (2) chronically, b/l, sym, confluent, diffuse, deep T2 bright WM lesions (spares U-fibers). Radiation: (1) w/ focal exposure, T2 bright WM lesion (often spares corpus callosum) w/ var mass effect & ring enhancement. Ddx: Tumor recurrence (choline peak on MRS, may involve corpus callosum); (2) w/ diffuse exposure, b/l, sym, confluent T2 bright deep & PV lesions w/o enhancement or mass effect. Illicits: b/l, sym lesions; (1) IV or inhaled heroin: diffuse WM high T2 signal (sparing U-fibers), high convexities; (2) cocaine: scattered T2 bright WM lesions; stroke; ICH; vasculitis; (3) MDMA: globus pallidi & diffuse WM high T2 signal. Organic solvents: b/l, sym, confluent, diffuse T2 bright lesions; (1) toluene: corpus callosum & cerebellum; (2) methanol: SC WM, putamen, & optic nerve; (3) ethylene glycol: thalamus & pons. Mercury: b/l, sym, postcentral gyrus, occipital lobe & cerebellar T2 bright WM lesions w/ cortical atrophy in chronic phase. Carbon monoxide (CO) poisoning: Similar to hypoxic-ischemic injury (above), but sparing SC region & T2 bright globus pallidi lesions typical.
Infectious diseases: PML/JC virus: Often begins asym, usually evolves into b/l sym dz; parietal & occip (but may occur anywhere) SC (involves U-fibers) T2 bright confluent nonenhancing WM dz w/ var pontocerebellar WM lesions; no mass effect. HIV/AIDS: b/l, sym PV T2 bright lesions, nonenhancing; diff atrophy. CMV: (1) AIDS related: Ventriculitis w/ PV T2 bright lesions & subependymal enhance; assoc lumbosacral meningeal & nerve root thickening & enhancement, (2) Congenital CMV: Temporal SC & PV T2 bright lesions w/ cystic regions. VZV: (1) Immunocompromised pts (small vessel vasculitis): Diffuse, patchy T2 bright multifocal WM lesions; angiographic abnormalities (proximal MCA-ACA); (2) Immunocompetent pts (large vessel vasculitis): Acute stroke from VZV vasculitis w/ DWI bright lesions in deep WM & cortex; var enhancement. HTLV-1 & HTLV-2: Small, multifocal T2 bright SC WM lesions; in early stages of spinal dz, multifocal enhancing T2 bright thoracic lesions w/ var mass effect. Lyme: Scant T2 bright PV WM lesions; meningitis, polyradiculitis, & cranial neuritis (w/ enhancement of corresp structures). SSPE (rubeola/measles): Early stages, posterior SC (spares U-fibers) T2 patchy bright lesions w/ adjacent gray matter involvement; var enhancement & mass effect; midstages w/ PV & putamen & pontocerebellar T2 bright lesions w/ regression of SC lesions; late stages w/ atrophy & worsening PV T2 bright lesions.
Hereditary diseases: Metachromic leukodystrophy: Diffuse, sym T2 bright signal of cerebral (+corpus callosum, −U-fibers) & cerebellar WM; tigroid WM pattern; predom frontal involvement in adult-onset forms. Pelizaeus-Merzbacher: Diffuse, sym T2 bright signal of WM w/ var T2 dark signal in deep gray nuclei, midbrain, & cerebellum; “eye of the tiger” sign. X-linked ALD: Sym SC & deep WM T2 bright signal (U-fiber sparing); begins parietal & occip w/ enhancement at leading edge & var mass effect; involves corpus callosum; progresses anteriorly & posteriorly. Krabbe (globoid cell): Parietal & cerebellar WM T2 bright signal; var bright lesions in deep gray (thalami), WM & cortex. Alexander dz: (1) Childhood forms: begins w/ T2 bright signal in frontal lobes w/ enhancing edge, followed by cystic changes; macrocephaly; var bright lesions in caudate on CT; (2) adult form—var PV T2 bright lesions; upper cervical cord & medulla atrophy. Canavan dz: SC (involves U-fibers) & deep gray T2 bright signal; macrocephaly; ↑ NAA peak on MRS. Vanishing WM (VWM) dz: Sym, diffuse T2 bright signal in cerebral & cerebellar hemispheres (loss of WM; rel temporal lobe & U-fiber sparing); nl head size. Megaloencephalitic leukoencephalopathy: Temporal T2 bright signal w/ cysts; macrocephaly. Aicardi-Goutiere: Diffuse, T2 bright signal in hemispheric WM; basal ganglia calc on CT. Tuberous sclerosis: Cortical tubers & areas of dysmyelination: T2 bright SC lesions (in adults); subependymal enhancing lesions (small hamartomas, large giant cell astrocytomas [SEGA]).
Noninfectious Immune/Inflammatory Disease
Infectious Diseases
Bacterial meningitis: See table; FLAIR bright signal (pus) in subarachnoid spaces; concurrent ventriculitis (subependymal enhance, ventriculomegaly, & PV T2 bright signal) & empyema (DWI & T2 bright; T1 dark; enhances); listeria w/ predilection for brainstem & cerebellum (rhombencephalitis).
Bacterial abscess: Solitary or multiple; MCA territory (frontoparietal) gray-white junction
1. Early cerebritis (days): ill-defined T1 dark, T2 bright region
2. Late cerebritis (weeks): similar to early, but increasing enhancement
3. Early capsular (1–2 wk): T2 bright, T1 dark lesion w/ surrounding T2 bright signal (edema) & early rim enhancement
4. Late capsular (>2 wk): less surrounding edema (T2 bright signal) & increasing capsule (rim enhancement); DWI bright core; concurrent paranasal or mastoid sinus dz on CT
Bartonella henselae (cat scratch dz): Var thalamic & deep WM T2 bright lesions, optic disc edema w/ macular star (ODEMS) on funduscopy/retinography.
Treponema pallidum (syphilis)
1. Pachymeningitis: see table.
2. Meningovascular syphilis: anterior > posterior circulation infarcts w/ meningitis.
3. Gummas: enhancing mass lesions of dura w/in cerebral hemispheres or CNs.
4. Tabes dorsalis: high T2 signal of posterior cord.
5. Parenchymal syphilis: diffuse atrophy.
Mycobacterium tuberculosis
1. Meningitis: pus in basal cisterns (T2 bright); skull base dural thickening & enhancement; basal ganglia & internal capsular infarcts.
2. Tuberculoma: solitary or multiple T2 bright masses adjacent to dura (may occur anywhere) w/ enhancement (rim or diffuse).
Borrelia burgdorferi (Lyme): See WMD.
Tropheryma whipplei (Whipple dz): Diencephalic & thalamic T2 bright lesions w/ enhancement.
HSV-1 (adult): Early DWI gray & white matter changes (medial temporal, orbitofrontal, insular); later T2 bright signal (cingulate, capsules, & beyond); var hemorrhage (by CT or GRE/SWI), mass effect, & enhancement; pseudo-hyperdense MCA sign; spares deep gray nuclei; occasional pontine & CN V involvement.
HSV-2 (neonate): Diffuse hemispheric gray & WM T2 bright & T1 dark changes—loss of gray-white differentiation, var enhancement, occ cerebellar & brainstem involvement; WMD difficult to apprec in neonate brain; no predilection for temporal lobes; calc (after weeks).
VZV: Zoster paresis: Dorsal root enhancement on T1 & dorsal cord T2 bright signal (short segment) w/ enhancement; immunocompetent (large-vessel vasculitis): see WMD; immunocompromised (small-vessel vasculitis): see WMD; may evolve into diffuse encephalitis (gyriform enhancement, hemorrhages) & ventriculitis (subependymal enhancement).
EBV: (1) ADEM: likely represents most brain cases; see WMD; (2) meningitis: see table.
CMV: Congenital: PV calc; anterior temporal & subependymal cysts, ventriculomegaly w/ vol loss, neuronal migration abnormalities (e.g., polymicrogyria, pachygyria, lissencephaly), cerebellar hypoplasia, & PV WM T2 bright signal; adult (immunocompromised): ventriculitis w/ deep WM & PV T2 bright signal + subependymal enhancement (owl eyes); lumbosacral nerve root enhancement & conus enlargement w/ ↑ T2 signal & enhancement; rarer brainstem & cerebellar lesions.
HIV/AIDS: see WMD; basal ganglia calc & proximal vasc ectasia in women/children.
WNV (West Nile virus encephalitis): variable T2 hyperintense lesions & restricted diffusion.
EEE (Eastern equine encephalitis): Thalamic & basal ganglia T2 bright lesions; var upper brainstem lesions; lack of enhancement.
JE (Japanese encephalitis): Thalamic, basal ganglia, upper brainstem, hippocampal & deep WM T2 bright lesions; ±hemorrhage (by CT or GRE) in thalami; no enhancement.
SSPE: See WMD.
Aspergillosis: Parenchymal T2 bright lesions (often poorly defined capsule) w/ surrounding T2 bright signal (edema), var enhancement; var adjacent hemorrhage (by GRE) & infarction (tends to be angioinvasive).
Mucormycosis: Paranasal bony sinus & soft tissue changes w/ var bony changes on CT; meningeal thickening & enhancement; stenoses & dilation by angio (vasculitis & aneurysms); anterior > posterior DWI bright lesions (infarctions).
Cryptococcus: (1) Meningitis: dilated Virchow-Robin spaces, enhancing nodules w/in leptomeninges, choroid plexus, parenchyma; lack of diffuse meningeal thickening & enhancement. (2) Cryptococcomas: concurrent meningitis; T2 bright lesions of midbrain & basal ganglia.
Candidiasis: Small, scattered parenchymal T2 bright lesions; parenchymal & meningeal enhancing nodules; meningeal thickening & enhancement; abscesses (similar to bacterial above).
Coccidioidomycosis: Meningitis: base of skull predominant meningeal changes; granulomatous lesions, similar to TB; cerebellar predilection.
Cysticercosis: (1a) Early stages (w/o encephalitis): T2 bright, T1 dark cysts (w/ central scolex) w/ thin capsule & minimal surrounding edema (T2 bright signal) or enhancement; cysts in parenchyma, ventricles & subarachnoid space; (1b) early stages (w/ encephalitis): Diffuse nodular enhancing lesions w/ marked surrounding edema (T2 bright signal); hydrocephalus; (2) Middle stages: Diffuse T1 bright & T2 dark or isointense lesions w/ enhancement of thickened capsule & var surrounding edema; encephalitic changes may occur; (3) Late stages: Lesion calc (bright on CT or T1, dark on GRE).
Toxoplasmosis: Deep GM nuclei T2 bright, enhancing lesions w/ surrounding edema (T2 bright signal); rim enhancement; var hemorrhage (by CT or GRE/SWI).
Echinococcus: Parietal > other lobar cystic lesions (T2 bright, T1 dark core) w/ central parasitic contents visualized.
Malaria: Loss of gray-white differentiation, diffuse cerebral edema & WM changes including corpus callosum (T2 bright signal), & cortical stroke (by DWI).
CJD (Creutzfeldt-Jakob dz): Cortical ribbon, putamen, caudate, thalamic DWI bright lesions; T2 bright lesions follow.
Variant CJD: Bithalamic (pulvinar) DWI bright lesions; T2 bright lesions follow.
GSS (Gerstmann-Sträussler-Scheinker): Similar to CJD.
FFI (fatal familial insomnia): Limited data, but T2/FLAIR & DWI may be normal; possible ↑ ADC values & MRS abnormalities w/in thalami.
Intracranial Neoplasia & Other Mass Lesions
Determine compartment containing the lesion
- Extra-axial lesions: Intradural (e.g., meningioma, w/ dura between lesion & underlying brain) or extradural (e.g., bone metastasis). Inward buckling of underlying parenchyma, expansion of subarachnoid space adjacent to lesion, inward displacement of meningeal vessels.
- Intra-axial lesions: E.g., primary brain tumors or metastases. Absence of expansion of subarachnoid space, outward displacement of meningeal vessels, intraparenchymal mass effect or blurring of normal anatomic boundaries.
Extra-axial tumors
- Meningioma: Looks like egg w/ dural tail, hyperdense on CT, isointense to GM on T1-weighted MRI, w/ homogenously bright enhancement due to high vascularity, T2 hypo- or hyperintense depending on tumor density. Typical locations: parasagittal dura, convexities, sphenoid wings, & olfactory groove. Can encroach & surround vessels; commonly see adjacent overlying bony Δs including hyperostosis. Typically makes obtuse angle w/ dura or bone. Less common features: Calc, cysts, necrosis, hemorrhage. Multiple meningiomas can be seen in NF2.
- Nerve sheath tumors (schwannomas, neuromas, neurofibromas): T2 hypo- or hyperintense depending on tumor density. Vestibular schwannomas often remodel/expand internal auditory canal & can have intracanalicular &/or cisternal components. Typically makes acute angle w/ bone. Assoc arachnoid cysts occur w/ larger tumors. Less common features include cysts & hemorrhage. Schwannomas, if b/l, suggest NF2. Plexiform neurofibromas most often affect skin & subcutaneous tissue & are a/w NF1. Neuromas are posttraumatic neuronal prolif.
- Choroid plexus tumors: Papilloma: WHO grade I, typically in atria of lateral ventricles, less commonly in third ventricle or cistern; p/w obstructive hydrocephalus (due to block of CSF outflow) or communicating hydrocephalus (due to overproduction of CSF). CT hyperdense, T1 hypointense, T2 mixed intensity, enhances, can hemorrhage. Carcinoma: WHO grade III, also in lateral ventricles, appears similar to papilloma, metastasizes via CSF.
- Chordoma: Malignant tumor of notochord remnants, destructive bone lesion usually in clivus or sacrum/coccyx, differential includes chondrosarcoma
- Primary or metastatic dz: Can involve meninges & subarachnoid space. On MRI, nodular or diffuse pachy- &/or leptomeningeal thickening & enhancement (carcinomatous meningitis). Primary CNS tumor: Think glioma & lymphoma. Metastatic dz: Think melanoma, lung, breast, GI, prostate cancer, hematologic malignancies (leukemia, lymphoma), chloroma (granulocytic sarcoma a/w myelogenous leukemias).
- Nonneoplastic mass lesions: Lipoma: Congenital developmental abnormality from neural crest, composed of lipid, CT hypodense, T1 hyperintense, T2 hyperintense. Epidermoid: Desquamated ectodermal remnants that appear homogenous, commonly found near cerebellopontine angle & not midline. Slow growing. CT hypodense (brighter than CSF), MRI nonenhancing, T2 hyperintense w/ restricted diffusion. Can have calc & can scallop adjacent bone. Resemble arachnoid cysts but arachnoid cysts do not restrict diffusion. Dermoid: Ectodermal/mesodermal remnants contain lipid & calc, heterogeneous appearance, commonly found at or near midline. Male predominance. Look for fat intensity. Can rupture. Teratoma: Ectodermal/mesodermal/endodermal congenital tumors, endodermal component forms cysts, commonly in midline near pineal or suprasellar areas. Heterogeneous appearance w/ enhancement.
Intra-axial tumors
- Pituitary adenoma: T1 hypointense, homogenous w/ delayed enhancement, & mass effect on neighboring structures, microadenoma <10 mm, macroadenoma >10 mm diameter.
- Craniopharyngioma: Typically suprasellar; cysts, calc, & homogenous enhancement in a T2-hyperintense lesion. Benign Rathke cleft cysts do not commonly calc or enhance.
- Pilocytic astrocytoma: WHO grade I, most common juvenile infratentorial primary tumor. Well-defined margins, CSF intensity cyst w/ enhancing vascular mural nodule.
- Brain stem astrocytoma: WHO grade II, more common in children than adults, var enhancing T2-hyperintense lesion.
- Pleomorphic xanthoastrocytoma (PXA): WHO grade II, most common juvenile supratentorial primary tumor, typically in temporal lobe, often w/ clear borders, enhancing w/ meningeal attachment.
- Primitive neuroectodermal tumor (PNET): Aka medulloblastoma, WHO grade IV, common juvenile infratentorial lesion, M > F, midline vermis lesion that extends into superior & inferior vela of fourth ventricle. CT hyperdense, heterogeneous enhance; also include ependymoblastoma, medulloepithelioma, neuroblastoma.
- Ependymoma: WHO grades I–II depending on site; fourth ventricle > spine, supratentorial.
- Subependymomas: WHO grade I, present in adulthood, mostly located along lateral ventricle wall, CT isodense, T1 isointense, T2 hyperintense, var enhancement.
- Ganglioglioma: Mixed neuronal/glial, low-grade tumor, young & female predominance. Well-defined margins, calcified, cystic mass w/ minimal edema, var enhancement.
- L'Hermitte-Duclos: Dysplastic gangliocytoma (WHO grade I), T2 hyperintense nonenhancing cerebellar “tigroid” lesion affecting gray & WM.
- Hemangioblastoma: Most common infratentorial intraparenchymal primary tumor in adults. Cystic mass w/ mural nodule w/ flow voids from feeding vessels.
- Gliomas: Infiltrating lesions, grading is based pathologically on presence of necrosis, vascular endothelial proliferation, mitoses, nuclear pleomorphism, & cellular density.
- Astrocytoma: Grade II, most common supratentorial intraparenchymal primary tumor in adult. CT hypodense, non- or low-enhancing, T1 hypointense, T2 hyperintense, lower CBV on perfusion imaging.
- Oligodendroglioma: Grade II, often calc, T1 hypointense, T2 hyperintense, minimal edema, var enhancing.
- Anaplastic astrocytoma: Grade III, poorly defined borders w/ extensive edema & enhancement w/o necrosis.
- Glioblastoma (GBM): Grade IV, mass effect & extensive edema, follows WM tracts such as corpus callosum, irregular poorly defined borders, irregular rim enhancement w/ internal necrosis (restricted diffusion), variably elevated CBV on perfusion imaging.
- Gliomatosis cerebri: Neuroepithelial tumor, diffuse infiltration of at least two lobes of cerebral hemispheres w/o mass effect, T2 hyperintense gray & WM throughout, min enhancement.
- Germinoma: Germ cell origin, also known as seminoma, typically in pineal or suprasellar regions, male & Asian predominance (female predominance when tumor is suprasellar), sometimes multifocal, CT hyperdense & enhancing, T2 hypointense.
- Choriocarcinoma: Typically hemorrhagic, male predominance, subarachnoid seeding common, can occur w/ retinoblastoma.
- Neurocytoma (aka neuroepithelioma): neural mass typically extending into the lateral ventricle & adjacent to septum pellucidum, “bubbly” appearance, heterogeneous enhancement, var vascularity.
- Pineal tumors: More common in childhood, pineocytoma (WHO grade II), pineoblastoma (WHO grade IV), calc, enhance, hemorrhage in pineoblastoma. Important to distinguish from benign pineal cysts, which, unlike cystic tumors, should not have a solid component.
- Primary CNS lymphoma: More common in immunocompromised host, lesions usually supratentorial w/in deep gray nuclei or PV WM. Spread across corpus callosum is common. May coat ventricles. Lesions are heterogeneous (T2 iso- to hypointense w/ marked enhancement & internal restricted diffusion); rim enhancement seen in immunocompromised but not immunocompetent hosts. May radiographically disappear following treatment w/ steroids.
- Intravascular lymphoma (IVL): Proliferation of malignant large B-cell lymphoma w/in lumen of small blood vessels, w/o extravascular mass. Lesions resemble small infarcts w/ focal parenchymal or meningeal enhancement; multifocal WM dz common.
- Intraparenchymal metastatic dz: Multifocal T1 hypointense, T2 var intensity (depending on presence of cysts, necrosis, calc, etc.), enhancing lesions w/ clear borders & marked surrounding edema most commonly at or near gray-white junction, more commonly in anterior circulation (especially MCA), often a/w skull (bony) mets. Mets that hemorrhage: Breast, lung, melanoma, renal cell, choriocarcinoma, retinoblastoma, thyroid carcinoma.
- Non-neoplastic mass lesions: Colloid cyst: Near foramen of Monro in anterior third ventricle. Highly proteinaceous (T1 hyperintense), T2 hyperintense, well-defined borders.
Toxic & Metabolic Diseases & Conditions
Acquired metabolic diseases
(Note: see WMD section for CNS dz caused by B12 deficiency, Marchiafava-Bignami syndrome, osmotic demyelination, hypoxia-ischemia, high-altitude encephalopathy.)
- Hepatic encephalopathy: Acute, variable SC WM T2 bright lesions; chronic, sym T1 hyperintense nonenhancing lesions in basal ganglia (manganese deposition).
- Hypoglycemia: b/l & sym DWI & FLAIR bright signal of cortex, hippocampus, & basal ganglia; var DWI & FLAIR bright signal of WM incl splenium.
- Thiamine (vitamin B1) deficiency (Wernicke encephalopathy): Sym, b/l T2 bright in medial thalami, mam bodies, reticular formation, & periaqueductal gray matter; var microhemorrhages in affected structures (GRE/SWI dark, T1 bright).
Hereditary metabolic diseases
- Wilson dz: Sym caudate, putamen, thalamus, sup cerebellar peduncle T2 bright, T1 dark signal; central pontine T1 dark region (central metallic dep); globus, red nuclei T2 dark.
- Pantothenate kinase deficiency (formerly Hallervorden-Spatz syndrome): b/l globus, red nucleus, substantia nigra T2 dark lesions; globus w/ central T2 bright portion (eye of tiger); var cortical atrophy.
- MELAS: Acutely DWI bright (ADC typically isointense) cortical & SC lesions; often posterior; lesions do not respect vascular territories.
- Leigh syndrome: b/l, sym putamen > thalamus, caudate, globus, brainstem, WM T2 bright lesions.
- Kearns-Sayre syndrome: b/l, sym basal ganglia T2 bright signal; CT bright & GRE dark basal ganglia lesions (calcs); diffuse atrophy.
- Mucopolysaccharidosis: Diffuse atrophy, WM T2 bright lesions (cystic changes), dural thickening w/ var mass effect on brain stem, var macrocephaly, & thickened skull.
- Amino acidopathies: Early diffuse swelling; delayed/absent myelination; later diffuse atrophy; var neuronal migrational abnormalities.
Acquired toxic diseases
- EtOH: Chronically, midline cerebellum > diffuse cerebral atrophy. Diffuse WM dz.
- Manganese: b/l globus pallidus T2 bright lesions.
- Lead: Chronically, b/l basal ganglia calcs (CT hyperdense, GRE hypointense).
- Arsenic: B/l T2 bright lesions around cerebral aqueduct & midbrain tegmentum.
- Kernicterus: Early, b/l globus pallidus T2 & T1 bright lesions; later, T2/FLAIR bright & T1 dark; b/l subthalamic nucleus ↑ T2 bright lesions; var ↑ T2 signal in WM & gen atrophy.
- Cyanide: Acutely, b/l putamen DWI bright lesions & diffuse cerebral swelling.
Neurodegenerative Dz & Hydrocephalus
- Alzheimer dz: Hippocampal, amygdala, & temporal lobe atrophy, ↓ NAA on MRS. W/ hippocampal atrophy, temporal horns of lateral ventricles enlarge ex vacuo. Also, ↓ metabolism in posterior parietal areas on FDG-PET, & ↑ Pittsburgh compound B (PiB, binds to amyloid protein). PiB to distinguish from frontotemporal lobar degeneration (FTLD), which has no abnl PiB signal. 18F-florbetapir also binds to amyloid, approved for clinical diagnostic use, has longer half life than PiB.
- Lewy body dementia: Substantia nigra, posterior cortical, & brainstem atrophy.
- Parkinson dz: Atrophy of substantia nigra.
- Parkinson plus syndromes:
Progressive supranuclear palsy (PSP): Midbrain tectum atrophy, abnl periaqueductal signal, ↑ iron in putamen & red nucl (T2/SWI hypointense), ↓ NAA on MRS.Cortical-basal ganglionic degeneration (CBGD): “Knife-blade” atrophy of structures near central sulcus & superior parietal lobe.Multiple systems atrophy (MSA): Olivopontocerebellar atrophy (OPCA): “Hot cross bun” sign on axial images of pons.Striatonigral degeneration: Caudate, putamen, & substantia nigra atrophy.
- FTLD:
Frontal variant: Asym frontal & anterior temporal atrophy.Semantic variant: Asym temporal lobe/pole & parahippocampal atrophy.Progressive nonfluent aphasia: Perisylvian, insular, & superior temporal atrophy.
- Huntington dz: Head of caudate atrophy (loss of convex shape) w/ ex vacuo “rounding” of anterior lateral ventricles, best seen on axial images.
- Multi-infarct “vascular” dementia: White & deep gray matter lacunes, multiple strokes of different ages, ↓ NAA on MRS.
- HIV/AIDS: Diffuse atrophy, hyperintense basal ganglia structures, superimposed PML, lymphoma, toxoplasma, IRIS, etc.
- CJD: Hyperintense basal ganglia structures & diffuse cortical restricted diffusion.
- Amyotrophic lateral sclerosis (ALS): Anterior horn cell atrophy & corticospinal myelomalacia (T2 bright w/ atrophy).
Ventriculomegaly & ICP Abnormalities
Ventriculomegaly
- Hydrocephalus (obstructive & communicating): Flattened cortical sulci, ↑ PV & periaqueductal T2 signal (transependymal flow), elevated & thinned corpus callosum, dispropor enlarged temporal horns, convex third ventricle w/ enlarged anterior recess, var fourth vent enlargement.
- Cerebral atrophy (ex vacuo): Enlarged sulci, sym involvement of ventricles (depending on underlying dz; AD also has temporal horn enlargement); concave third ventricle w/ nl anterior recess, fourth ventricle nl size (unless concurrent, marked cerebellar atrophy).
- Normal pressure hydrocephalus (NPH): Diffuse ventricular enlargement out of proportion to enlarged cortical sulci, minimal ↑ PV & periaqueductal T2 signal (transependymal flow).
- Pseudotumor cerebri: nl or slightly ↓ ventricles, ↑ T2 signal (CSF) in enlarged optic sheaths, flat pituitary gland/empty sella & posterior sclera, compressed venous sinuses, reverse cupping of optic discs (papilledema).
Neurodevelopmental & Genetic Diseases
Migrational disorders
- Heterotopia: Diffuse, subcortical, or PV region isointense to cortex (area of ectopic gray matter).
- Cortical dysplasia: An area of ectopic gray matter.
- Pachygyria: Abnormally thick cortical mantle.
- Polymicrogyria: Excessive or redundant abnl folding of cortical mantle.
- Lissencephaly: Abnl “smoothness” (lack of nl gyral pattern) of cortex.
- Porencephaly: Cyst, cleft, or cavity lined by WM, disrupts nl cortical architecture.
- Schizencephaly: Cyst, cleft, or cavity lined by heterotopic GM, disrupts nl cortical architecture.
- Holoprosencephaly: Failure of forebrain development.
Other developmental disorders
- Dandy-Walker malformation: Usually obstructive hydrocephalus, cerebellar vermis agenesis, cystic enlargement of fourth ventricle w/ enlargement of posterior cranial fossa.
- Chiari malformation: (1) Type 1: Low-lying cerebellar tonsils (>5 mm below foramen magnum); var assoc hydrocephalus, high T2 signal in central cord (syringomyelia); (2) type 2: Hydrocephalus, small posterior fossa w/ compression of cerebellar structures, elongated cerebellar tonsils & fourth ventricle, beak-shaped midbrain tectum, kinking of medullocervical region; assoc protrusion of lumbar spinal cord through meningeal defect (lumbar myelomeningocele) & syringomyelia; (3) type 3: As in type 2 + occipital encephalocele or cervical myelomeningocele.
- Joubert syndrome: Dysgenesis of cerebellar vermis, “molar tooth” sign on axial MRI corresponding to lateral displacement of superior cerebellar peduncles & lack of decussation of these fibers.
- Vermian hypoplasia: Can be incidental or a/w Dandy-Walker malformation.
- Septooptic dysplasia: Partial or complete absence of the septum pellucidum and/or optic nerves or chiasm.
Other disorders
(Note: see WMD section for CNS dz caused by TS.)
- NF-1: (1) Neurofibromas: T1 isointense (to brain) & T2 bright skin, soft tissue, bone, & nerve lesions w/ enhancement; (2) schwannomas: T1 isointense (to brain) nerve lesions w/ enhancement; (3) anterior visual pathway gliomas: Enlargement of optic nerve, chiasm, or tract w/ minimal enhancement; (4) Tectal glioma: Enlargement of midbrain tectum w/ minimal enhancement; var hydrocephalus; (5) myelin vacuolization (children): T2 bright lesions of WM; regress in teenage years; (6) Bony abnormalities: Macrocephaly; sphenoid wing hypoplasia, creates defect for temporal lobe to protrude; (7) Other: Dural calcs (by CT or GRE); aneurysms.
- NF-2: (1) schwannomas: T1 isointense (to brain) nerve lesions w/ enhance; b/l or u/l CN VIII lesions; other cranial nerves, may be multiple; (2) meningiomas: T1 isointense (to brain) dural lesions w/ enhancement; often multiple.
- Sturge-Weber syndrome (SWS): Cortical & meningeal calcs (by CT or GRE/SWI) w/ hemispheric volume loss & cranial thickening; region of meningeal thickening & enhancement (hemangioma).
- Von-Hippel Lindau (VHL): Cerebellar hemangioblastoma: Cystic-appearing (T2 hyperintense cyst) w/ nodular enhancement; var regions of hemorrhage; may be multiple.