Chapter 8
Funding the epidemic
The response to HIV and AIDS has to be funded, whether the emphasis is on prevention or treatment, or both. This disease is unique in part because of the complex financing. It also requires long-term commitment for those increasing numbers on relatively expensive lifesaving treatment. There are, of course, other diseases that require long-term care too such as diabetes and hypertension. The numbers requiring ART, their relative youth at initiation, and the cost of treatment, make AIDS different.
The history of funding
Initially AIDS was viewed primarily as a health concern for rich countries. Even after the acknowledgement that HIV was present in Africa and Asia, and that it spread through heterosexual transmission, the scale and potential impact was grossly underestimated. There was consequently little money allocated to the disease. In defence of early responses, it was neither clear where funds should be spent nor how much was needed. One of the early WHO interventions was to send out teams to countries to put in place standard short-term plans (STPs), which were, as the scale of the epidemic became apparent, replaced by medium-term plans (MTPs). These were limited, health-based, and relatively cheap.
The exceptions to this lack of funding were research and drug development costs incurred, mainly in the US, and to a lesser extent in France (Institute Pasteur in Paris) and in the UK (Wellcome Trust). In 1982, the US Congress allocated US$5 million to the Centers for Disease Control (CDC) for surveillance and another US$10 million to the National Institutes of Health (NIH) for research into the disease. In 1983, the first bill that sanctioned funding (US$12 million for the DHHS) specifically for AIDS research and treatment was passed. By 1985, US$70 million had been allocated for research, excluding resources mobilized in the private sector who, again, were primarily Western and American.
Why were money and people (especially scientists) allocated to this new problem so quickly in the West? The answer lies in the rapid mobilization of the gay community who set up AIDS-specific organizations even before the virus had been identified. In January 1982, the Gay Men’s Health Crisis was established in New York City as a non-profit, volunteer-supported, and community-based AIDS service organization. When, in 1985, American actor Rock Hudson died of AIDS, he left US$250,000 to set up the American Foundation for AIDS Research (amfAR).
Lack of attention to the disease that was killing so many of their number, and slow progress in finding treatments, led the gay community to channel their anger and frustration in extraordinary and effective activism. ACT-UP, established in 1987, became an international advocacy group working for legislation, medical research, treatment, and policies to end AIDS.
The effectiveness of these articulate, organized activists, many from creative careers, in putting pressure on governments, scientists, and the pharmaceutical industry, cannot be overstated. The same level of political engagement around financial mobilization and drug prices was not seen in the resource-poor world, with the exception of the South African Treatment Action Campaign (TAC) and in Brazil. The numerous grassroots and civil society organizations were more geared to providing support to patients, their families, and orphans.
In 1986, the US National Academy of Sciences issued a report critical of the government response, and called for a US$2 billion investment. In 1989, the Health Resources and Services Administration (HRSA) was granted US$20 million for HIV care and treatment through the Home-Based and Community-Based Care State Grant programme. In 1990, Congress enacted the Ryan White Comprehensive AIDS Resources Emergency (CARE) Act, providing US$220.5 million for HIV community-based care and treatment services in its first year alone. Ryan White was an Indiana teenager with haemophilia who acquired AIDS through contaminated blood products. Excluded from school, his plight became a focus for activism.
This pattern of expenditure on research, development, and support for those affected by AIDS was mirrored across the developed world, frequently with the impetus of activist engagement. It was not until the mid-1990s that the potential global impact of AIDS was acknowledged. Indeed there was active opposition to dealing with the disease. Epidemiologist Jonathan Mann founded the WHO’s Global Programme for AIDS in 1986. By March 1990 he had resigned on principle to protest about the lack of UN response, in particular from the WHO Director-General Hiroshi Nakajima. That the WHO should be so short-sightedly unresponsive was unforgivable: tragically they were not alone in this failure.
In 1994, the UN finally acknowledged ‘the magnitude and impact [of HIV/AIDS were] greatest in developing countries.’ In 1996 UNAIDS was established under the leadership of Peter Piot in Geneva, with the mandate of coordinating the response of the UN agency co-sponsors and mobilizing the global response. Of especial significance for funding in the low- and middle-income countries were the January 2000 UN Security Council meeting, the MDGs, and the 2001 UNGASS Declaration.
Mobilizing international money
There was a time of plenty for the epidemic. The establishment of the Global Fund (GF) in 2002 began this trend, which is also shown in Figure 12. The first round of GF grants—worth US$378 million—were awarded to thirty-one countries in April 2002. The GF was established to respond to AIDS, TB, and malaria, and the distribution was 50 per cent for AIDS, 18 per cent for TB, and 32 per cent for malaria. This was maintained up to and during the 2014-16 allocation periods, and the split was reconfirmed at the GF board meeting in Geneva in November 2015.
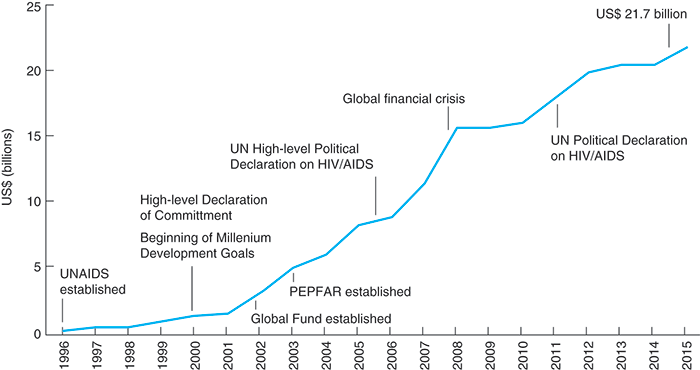
12. Total resources for HIV/AIDS in low- and middle-income countries.
GF funding was allocated to countries on the basis of detailed applications made in ‘rounds’, with deadlines for submissions. These were evaluated by independent panels. In 2014, the allocation method changed, and countries were given notional amounts they could apply for. There was some ‘incentive’ money for which countries could compete. The new funding model focused on countries with the highest burden and least ability to pay; offered predictable funding through the ‘allocation amount’; actively supported applications to improve the success rates; and put in place a requirement for some level of domestic funding. The GF has mobilized large sums, but is a technical solution to a complex problem. The one area where success has been limited is in health systems strengthening.
The World Trade Organization’s (WTO) Doha Declaration in November 2002, affirmed the rights of developing countries to buy or manufacture generic medications for public health crises, and AIDS was determined to be such a crisis. In the same year, the WHO issued its first guidelines for the use of ART in low-income settings.
The subsequent agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), provided an opportunity for poorer countries to expand access to low cost ART. This allowed production and importation of drugs in cheaper, generic forms. There is pressure on countries to limit use of TRIPS flexibility clauses, known as TRIPS-plus provisions, and adapt their laws to provide greater intellectual property protection and enforcement. This could cause prices to escalate.
The year 2002 also saw the establishment of the Clinton HIV/AIDS Initiative as part of the Clinton Foundation. This was a global organization committed to expanding access to care and treatment for HIV/AIDS, malaria, and TB, and for strengthening health systems. In 2010, the AIDS Initiative became a separate non-profit organization: the Clinton Health Access Initiative (CHAI).
The next major boost to funding was President Bush’s creation of PEPFAR in 2003. This began as a US$15 billion, five-year plan to combat AIDS, primarily in countries with a high burden of infections. In March, the Bill and Melinda Gates Foundation (BMGF) awarded a US$60 million grant to the International Partnership for Microbicides to support research and development of microbicides to prevent transmission of HIV.
In 2006, UNITAID, a global health initiative, was established by Brazil, Chile, France, Norway, and the United Kingdom. It is hosted by the WHO in Geneva. Over twenty-five countries, and the BMGF, were UNITAID members by 2016. UNITAID looks for innovative interventions, as well as tackling inefficiencies in markets for medicines, diagnostics, and prevention for HIV and AIDS, malaria, and TB. Between 2007 and 2014, US$2.4 billion was raised of which 62 per cent came from a ‘solidarity levy’ on airline tickets (see the section on ‘Domestic and innovative funding’).
Global HIV and AIDS spending in the developing world and Eastern Europe increased from US$4.6 billion in 2003 to US$14.6 billion in 2008. At the end of that year, President Bush signed legislation reauthorizing PEPFAR for an additional five years for up to US$48 billion. The US contribution meant that, with the 2008 financial crash, AIDS funding flatlined rather than fell.
From 2008 onward, major shifts occurred in the global HIV and AIDS response. The financial crisis created uncertainty. The status of HIV and AIDS as a premier health threat was eroded. There was a shift from an emergency state to a normalization of the response, helped by the fact that, due to new drugs, HIV-infected people were living longer, more productive lives.
The United States accounts for the majority of bilateral and multilateral funding for global HIV and AIDS responses (64.5 per cent from the United States, followed by 12.9 per cent from the UK, 3.7 per cent from France, 3.2 per cent from Germany, and 2.5 per cent from the Netherlands). Since 2006, these countries have accounted for roughly 80 per cent of all HIV funding from donor governments. Bilateral funding provided over 70 per cent of HIV funding from donors in 2014.
In 2014, 27 per cent of international HIV assistance was provided through multilateral organizations such as the Global Fund, UNITAID, and other United Nations agencies. Six donor governments provided the majority of their HIV funding through the Global Fund (Canada, the European Commission, France, Germany, Italy, and Japan). Private philanthropies provided US$592 million for global HIV and AIDS programmes in 2013. The Bill and Melinda Gates Foundation is the leading philanthropic funder of international HIV efforts. To date, the foundation has provided more than US$2.5 billion towards tackling the global HIV epidemic and has given an additional US$1.4 billion to the Global Fund.
Taking stock
At the end of 2015, total investments in the AIDS response in low- and middle-income countries totalled US$21.7 billion. This almost met the investment target set in the 2011 Declaration: mobilizing between US$22 billion and US$24 billion by 2015. It is quite remarkable so much money was raised from such a variety of sources.
The financial mobilization has not been unproblematic. AIDS was, correctly, treated as an exceptional global challenge. Its champions in the early years were articulate and outraged. The argument that this exceptional disease needed an exceptional response was accepted in the West. The result was that funding came from international sources. In some countries, over 95 per cent of funding was external. According to a 2015 Lancet article, in Tanzania in 2011 only 1.2 per cent of the AIDS spending came from government, external expenditure on AIDS was US$432 million with government spending just US$8 million. In Zambia two years later in 2013, only 10.6 per cent came from government.
The international contribution led to a lack of local ownership and a dependency mind-set. A review of twelve PEPFAR countries by Results for Development noted: ‘deeply ingrained perceptions by finance and other senior government officials that donors will take care of the AIDS programme’, as indeed donors have done over the past decade. This gave rise to low domestic allocations, little local involvement, and an abnegation of responsibility in some countries. Donor-determined strategies and priorities might not reflect local needs or conditions. A prime example was the abstinence ‘earmark’ by the US government, where a proportion of US money had to be spent on promoting abstinence.
Since international funding went to marginal groups like IDUs in Russia, MSMs in a number of African countries, and support services for sex workers in many locations, governments ignored these populations, allowing donors to take care of their needs. Doubtless support went to key populations who otherwise would have been neglected, but the question is: where does government responsibility begin and end?
The influx of money led to vertical programmes or ‘stovepipes’. The funding was for specific diseases as opposed to the broader health system. The advantage of this for the donors was twofold: firstly, the money was easier to track; secondly, the results were more easily attributed to their interventions. Unfortunately, it was not always what the recipient countries needed or would have prioritized and led to waste and duplication.
In 2016 health funding in most low- and middle-income countries was through government budgets fed from general tax revenues. HIV and AIDS have been the exception. The global trend is towards universal health care (UHC) in various forms. This is more appropriate for mainstreaming and normalizing AIDS. UHC would be expected to include ART and possibly prevention activities. In Thailand in 2006, the ART programme was transferred to the UHC scheme which covered 98 per cent of its population.
Looking forward
AIDS resource needs are projected to increase at least until 2020. A 2015 modelling study by Atun and others suggests that, in sub-Saharan Africa, the total resources required for HIV prevention and treatment from 2015 to 2050 range from US$110 billion to US$293 billion, depending on treatment and prevention scenarios. For example, in order to maintain current coverage levels for treatment and prevention, with eligibility for treatment initiation at CD4 count of < 500/mm3, the total cost to 2050 is US$110 billion. If treatment is extended to all HIV+ individuals and prevention scaled up, the total cost to 2050 would be around US$293 billion.
The UNAIDS ‘Fast-Track Approach’ argues scaling the response up by 2020 would prevent infections, save lives, and ultimately require less money. The view from Geneva is that the world has limited time to make these investments. They estimated current investments were close to US$22 billion in 2015. Another US$8 to US$12 billion would allow the fast-track goals to be met. Their strategy will require US$31 billion in 2020. The argument that frontloading would work is undoubtedly sensible, especially for successful prevention. The unspoken assumption is that the interventions will work as well as projected.
Some innovative thinking came from the RUSH Foundation’s RethinkHIV consortium of researchers who looked at evidence related to the costs, benefits, effects, fiscal implications, and developmental impacts of HIV interventions in sub-Saharan Africa. The title of the 2015 roundtable event in Boston, ‘From a Death Sentence to a Debt Sentence: Meeting the Challenge of Long-term Liabilities of HIV Funding’, succinctly encapsulates the essential issue. Providing ART is an irreversible commitment across the lifetime of the person living with HIV. Almost all of the costs lie in the future: putting someone on treatment incurs a liability similar to a debt. These debts are not currently ‘on the books’ and remain unacknowledged by governments and donors. ‘There is a disturbing disconnect between short-term funding cycles and long-term financial liabilities, as well as accountability for these between donors and affected governments.’
The global economic downturn and a climate of austerity mean Development Assistance for Health (DAH) has plateaued and is unlikely to increase in the near future. Traditional donor countries face their own challenges. There is concern over the ever-present threat of new diseases (or the re-emergence of old diseases—e.g. Ebola in 2014). The reality is that global development assistance will remain static, since any new money will go to environmental issues, which are now at the top of the global agenda. While health exponents advocate for increased assistance, it won’t materialize from traditional sources unless convincing new arguments are found.
Domestic and innovative funding
While the total global spending on HIV and AIDS grew significantly, the share of external funding declined from 60 per cent in 2008 to 43 per cent in 2014. The GF and PEPFAR are looking to see increased local ownership—in the case of PEPFAR they plan to transition out of countries. Some countries have made significant progress; South Africa has the largest resource need for HIV and AIDS and contributes approximately 82 per cent from the domestic budget.
Between 2009 and 2014, eighty-four out of 121 low- and middle-income countries increased domestic AIDS spending. Of these countries, forty-six reported an increase of more than 50 per cent, including thirty-five countries that increased in domestic spending by more than 100 per cent. High increases in the percentage may be the result of low initial levels. If a country allocated US$100 to HIV/AIDS in 2009 but US$200 by 2014, that would be a 100 per cent increase. Indeed in sub-Saharan Africa, the Democratic Republic of the Congo, Gambia, Liberia, and Zimbabwe, reported more than 100 per cent increases between 2009 and 2014, off very low bases. Economic growth and new revenue sources will increase the fiscal space for health spending. As countries progress and incomes grow, HIV will be concentrated in middle-income countries, which will receive less donor support and may face higher prices.
The question is what to do in poor countries with a low GDP per capita and high HIV prevalence. Malawi, Mozambique, Uganda, Zimbabwe, Lesotho, and Zambia all have prevalence over 10 per cent and per capita GDPs of less than US$3,000. Malawi is the worst case: the GDP per capita is US$255 and the government health expenditure per capita is only US$26. Clearly the AIDS programmes will remain dependent on donor input for the foreseeable future.
Innovative funding may generate potential new finance. From 2002 to 2012, more than US$6 billion was raised internationally by innovative financing instruments. For example, Ghana’s National Health Insurance Levy added 2.5 per cent to VAT. The revenue generated by this tax was used to fund approximately 70 per cent of the National Health Insurance Scheme, a fund designed to reduce financial barriers to health care. In Thailand, a consumption tax on alcohol and tobacco earmarked 2 per cent of the revenue generated to go to the Thai Health Promotion Foundation. Almost US$60 million has been generated and spent on health promotion. Zimbabwe’s National AIDS Trust was set up in 1999 to fund the National AIDS Council (NAC) and reduce its reliance on external funding. This 3 per cent levy generated US$2.6 billion between 2000, when it began operating, and 2006, and in 2011, US$26 million was collected.
Kenya established a High Level Steering Committee for Sustainable HIV Financing which pools additional public and private resources. They have allocated between 0.5 and 1 per cent of government revenues to a Trust Fund which will also be financed by other sources (such as an airline levy). It has been estimated this will fill 70 per cent of the HIV funding gap between 2010 and 2020, and 159 per cent between 2020 and 2030.
A number of earmarked special taxes have been used and others are being considered. These include financial and currency transactions, which are levies placed on specific monetary transactions. There is currently no example of these taxes being used for health services. Versions of employment levies based on Zimbabwe’s levy are being considered in other settings but there is no evidence yet of them being implemented. Air ticket taxes have raised money for UNITAID. Nine countries have since implemented these: Cameroon, Chile, Congo, France, Madagascar, Mali, Mauritius, Niger, and the Republic of Korea. The ticket levy is described on the UNITAID web site as: ‘A painless addition to the cost of a ticket, the levy is a leading example of globalisation working for the poor.’
Mobile phone levies are reported from Rwanda and Uganda: a consumption tax. Earmarked taxes on alcohol and tobacco (sin taxes), have a great deal of potential in mobilizing and sustaining resources to health and changing behaviours. The question is whether HIV, and even health, is the priority of the ministry of finance once the money is collected.
Other funding sources suggested include concessionary loans for HIV and AIDS programmes; debt conversion (known also ‘debt2health’); AIDS lotteries; utilizing dormant funds (e.g. property which is unclaimed); social impact bonds; social development bonds; diaspora bonds; a micro-levy on extractive industries; sovereign bonds securitized against future revenue streams; and special fundraising such as the existing GF Product Red, a brand where partner companies such as Nike, Apple Inc., Coca-Cola Company, and Starbucks brand a product with the Product Red logo and 50 per cent of the profit generated is donated to the GF.
Innovative funding is notoriously unreliable. Schemes may not work, be as lucrative as expected, or be allocated to intended targets. Innovative financing mechanisms are not the ‘magic bullet’ that will end development’s funding woes. Most are still in their infancy, and success has not yet been seen. There is concern that innovative financing mechanisms may be used to justify replacing (or decreasing) DAH and domestic budgetary commitments.
Efficiency savings in health systems could theoretically create substantial fiscal space, achieving higher HIV treatment and prevention coverage without requiring budgetary increases. The International Monetary Fund (IMF) estimates that as much as 50 per cent of health expenditures in low- and middle-income countries may be wasted or inefficiently used. Better health care system design and financing may improve the efficiency of global resource use. In South Africa in 2010, bound by the terms of its existing tender for ARTs, the government only bought one-third of its products at internationally competitive prices. Over the following two years there was a 53 per cent reduction in the cost of ART drugs, with savings of US$640 million.
Security is crucial
In AIDS we can plot the trajectory of epidemic. This includes the demographic and social impact, the epidemiology, and resource requirements needed to control, maintain, and ultimately end this disease. What is required is an upfront commitment led by governments where the epidemic is an issue. Funding from international organizations and NGOs will be useful in subsidizing the costs of the HIV/AIDS response, but they are finite resources shared by many needy countries and other causes.
A report funded by USAID on financing ART in low- and middle-income countries articulated the issue. The ability of a country to finance HIV and AIDS depends on the wealth of the country and the distribution of the wealth; the cost of the ART programme; and the willingness of the country (government, private sector, and people) to allocate funds to ART. As the authors noted the wealth of a country is hard to affect, but economies are growing. The distribution of wealth can be influenced but is a general political decision. The cost of an ART programme depends on prioritization, cost-effectiveness, and efficiency. The crucial question is the degree to which a country will allocate resources. However, if people are put on ART, then this is a long-term commitment that requires timely and predictable funding flows.