3 Viruses and the Emergence of Pandemics
When Pasteur was trying to make a vaccine for rabies, he was unable to find the causative agent. Filters were often used to trap and isolate bacteria that might be contained in fluids. These filters were unable to trap the causative agent of rabies. Pasteur reasoned that the microbe that caused rabies must be very small. Rabies is caused by a virus, and indeed it is very tiny. The virus that causes the COVID-19 disease is a member of a family of viruses called coronaviruses. These viruses are roughly spherical in shape and have a diameter of roughly 100 nanometers, which is a thousand times smaller than the diameter of a human hair. The influenza virus, which causes the seasonal flu, is also of similar size. In comparison, the bacterium that causes tuberculosis is rod-shaped, and its length is 20–40 times larger. Pasteur also failed when he attempted to culture the rabies virus using the procedure that Koch developed to grow bacteria. It was only in the twentieth century that methods to visualize and study viruses were developed.
In this chapter, we will describe why viruses need us, and plants and animals, to survive. We will also describe the different types of viruses that exist and how they function, as well as how pandemic-causing viruses emerge to wreak havoc. We begin, however, with a bit of history about our long war with viruses.

Our Eternal War with Viruses
Viruses are very simple ancient organisms that have probably existed since life began. For reasons that will become clear in the next section, viruses cannot reproduce on their own. They have to colonize bacteria, plants, and animals (including humans) in order to replicate and propagate their species. Therefore, viruses have specialized skills that let them invade other species and replicate inside them. When a virus invades the human body and replicates, it can damage our cells and tissues. The immune system, about which we will learn in the next chapter, tries to kill viruses that invade us to prevent and combat viral infections. This war between viruses and our immune system has raged since time immemorial.
Pre-agrarian humans contended with fewer types of viruses than we do. These ancient humans were also afflicted by viruses, but they were different in nature from the highly contagious ones that circulate today like those that cause flu, measles, or COVID-19. This is largely because of differences in the lifestyles of modern and ancient humans.
Many viruses that circulate today spread by casual contact between people. Infected people exhibit symptoms of disease and some people die because of the acuity of disease. Our immune system usually succeeds in completely eradicating the virus from our bodies, and we are cured. Remarkably, for a period of time, our immune system “remembers” that a particular virus had previously infected us. If the same virus reinfects us, the immune system can swat it away. For some viruses, this shield of immunity can last as long as a person’s lifetime.
Contagious viruses that kill some infected people, but which are normally eradicated from our bodies by the immune system, were unlikely to survive as a species in a pre-agrarian world. Our ancestors at that time lived as small groups of loosely connected people who occupied a large area. So, people encountered very few others in their daily lives. If a virus like that which causes COVID-19 infected a person, that individual would therefore infect very few others. The infected people would either die or recover and be immune to the virus. In a small population, over time, most would become immune, and a newly infected person would be very unlikely to encounter a susceptible individual during the course of disease. Therefore, the virus could not be transmitted to new people in whom it could replicate. Thus, over time, the virus would become extinct. If the virus caused a very lethal disease, it would kill everyone in a small community and become extinct because again there would be no one to infect.
This is why most viruses that circulated in pre-agrarian humans were probably not terribly contagious or deadly, and were not eradicated from the body by the immune system. These viruses adapted to coexist for the lifetime of the infected person, hiding out quietly most of the time. Periodically, they would rear their heads and infect new cells, causing recurrence of disease symptoms. The immune system would then suppress this recurrence, and the cycle would continue. Herpesviruses are an example of such an ancient type of virus that circulates in modern human populations.
Viruses like those that cause the flu, measles, and COVID-19 that were unable to thrive in pre-agrarian times became viable once human ingenuity led our ancestors to learn how to grow crops. In the agrarian society that emerged, people started living together in larger communities concentrated in smaller areas of land. In a dense population, a contagious virus can potentially be transmitted to many others by an infected person. So, the virus can replicate in many people. If the population is large, many individuals have to be infected and then recover to reach a point where a sufficiently large proportion of the population is immune. As this takes a long time, the virus can keep spreading to new people. Furthermore, in a large and dense population, new births provide a constant stream of new susceptible people. This is why highly contagious viruses that cause diseases that our immune system can usually clear from our bodies began to thrive in the agrarian era. Farming also led humans to domesticate animals and live closely with them. Viruses that infected animals and could also replicate in humans began to spread in the human population. Thus, it came to be that a great diversity of viruses began to circulate among humans.
The exchange of individuals between communities increased as travel became easier. These “immigrants” could bring diseases caused by viruses in their communities to others. For example, as mentioned in chapter 1, European immigrants brought a disease caused by a virus, smallpox, to the Americas. Immigration is also a source of new susceptible people for a virus that already exists in a community. When the industrial revolution began, people started to live in cities with even higher population densities than farming communities. Highly contagious viruses flourished even more. The human race is connected today by our shared history of battling the same contagious viruses.
The transition from hunter-gatherer societies to agrarian ones, the industrial revolution, and the many technologies and innovations that followed have improved the quality of human life as measured by a myriad of metrics. For example, we live longer now and childhood mortality is much lower. But the accompanying changes to the way we live also made us more susceptible to infection by a greater diversity of contagious viruses that can cause acute disease. Yet in spite of how the changes in our way of life have favored viruses, we have been winning the war against them. This is because of human ingenuity. We learned to develop vaccines that protect us from many disease-causing viruses. But vaccines take time to develop. So, anytime a new virus emerges, we remain vulnerable to devastating pandemics. The COVID-19 pandemic, caused by the SARS-CoV-2 virus, is only the most recent example.
Let us now dig into how viruses work, how they replicate, and why they cannot do so without us. But first we need a primer on the basic machinery that enables living organisms to function and replicate.
DNA, RNA, and Proteins
All living organisms try to replicate and propagate their species into the future. Since ancient times, people noticed that children share some traits with their parents, and the origin of heredity was hotly debated. But it was only in the nineteenth century that the Catholic monk and botanist Gregor Mendel’s careful studies while breeding peas provided the first rigorous basis for heredity. His work led to the concept of genes, which are inherited from one’s parents. But Mendel did not know what a gene really was. The discovery of genes had to wait until 1953 when James Watson and Francis Crick, two young scientists working at the University of Cambridge in the United Kingdom, first described what a gene really is. Informed by the studies of many others, including Rosalind Franklin, they had a flash of insight that has transformed how we think of ourselves as individuals and as a species, and indeed our understanding of all living things. Their discovery of how a molecule called DNA stores all our genetic information and faithfully reproduces it in our progeny also laid the foundation for modern medicine.
A DNA molecule is made up of two long strands, each comprised of four types of units that are connected together. The four types of units are called bases, and are labeled A, T, G, and C. The two strands of DNA wind around each other to form a double helix. This is made possible by the fact that A on one strand pairs only with T on the other strand, and G pairs only with C. Therefore, each DNA strand in the helix has a sequence of bases that is the complement of the sequence of the other. The sequence in which these four types of bases are connected in a DNA molecule encodes information about the organism. This is the information that is passed on to progeny.
Complex organisms, like us, are made up of many cells. Each cell contains a copy of our DNA within an enclosure inside the cell called the nucleus. The cells in an organism need to be continually replenished with new cells. This is accomplished by a process by which one cell divides into two identical daughter cells. During this replication process, the DNA double helix in the original cell is copied into two identical DNA molecules. First, the two strands of DNA are separated. Each original strand now serves as a template for the synthesis of a new complementary strand. This is accomplished by a cellular molecule called DNA polymerase, which joins the right complementary bases one by one to the growing new strand. The structure of DNA provides a mechanism to “proofread” the growing complementary strand of DNA. If the wrong base is added, it will not pair with the template strand, and so it is excised and the correct base is then added. In the end, we have the two old strands of DNA, each paired with a new complementary one. Each of the two new DNA helices becomes the DNA molecule in each daughter cell. Of course, errors do occur sometimes, and the errors are called mutations. The error rate for copying DNA in higher organisms is small—during each replication cycle, the probability that an erroneous base will be inserted into the new growing strand is roughly one in a billion.

Our cells work together to allow us to perform all our functions. The functions of a cell are carried out by proteins. Proteins make life work. If we imagine that a cell is like a car, proteins make up all the parts that allow the car to function. Proteins are long strands of units called amino acids. There are 20 types of amino acids. So, an enormous diversity of protein sequences can be generated by connecting these amino acids in different ways. For example, as there are 20 choices for amino acids at each position, a string of just three amino acids could be arranged in 20 × 20 × 20 = 8,000 different sequences. Proteins are much longer, with an average length of about 400 amino acids. The number of possible proteins, each with a different sequence, that can be created is therefore immense. The sequence of amino acids in a particular protein determines its function. Proteins with different sequences have different functions. Information about all the proteins we can have in our cells is encoded in our DNA.
Ingenious experiments carried out after the structure of DNA was discovered showed how DNA encodes information about the sequence of amino acids in proteins. Different sequences of three contiguous bases in a DNA molecule correspond to different amino acids. For example, AGC corresponds to one particular amino acid, while GCC corresponds to another, and so on. Given that there are four types of bases, there are 4 × 4 × 4 = 64 combinations of three bases. So, our DNA can encode information on 64 types of amino acids. In reality, there are only 20 amino acids. So, multiple types of three-letter strings of bases correspond to the same amino acid. The sequence of three-letter strings of bases in a stretch of DNA (a gene) corresponds to a particular amino acid sequence, and hence encodes information on a specific protein. So, you see that DNA, using only a four-letter alphabet, is a compact and ingenious way to encode complex information.
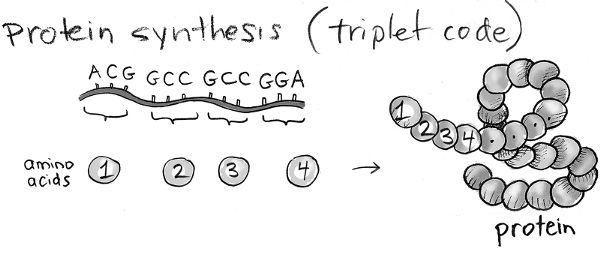
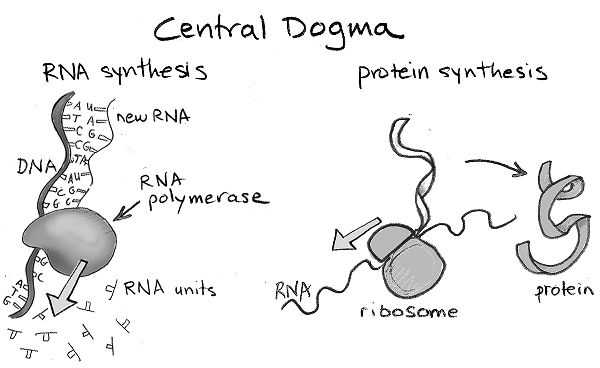
How does the information encoded in DNA get translated into making proteins in a cell? Just as a car needs many bolts to function, cells usually need to make many copies of a protein in order to enable their functions. The gene that encodes information on a particular protein’s sequence is first converted into a molecule related to DNA called RNA. RNA is a very ancient molecule, and almost certainly existed before DNA or proteins. An RNA molecule is usually composed of a single strand of connected bases, and looks very similar to a single strand of DNA. The difference is that RNA’s four-letter alphabet of bases is not, A, T, G, and C, but A, U, G, and C. So, U replaces T. A cellular molecule, called RNA polymerase, transcribes the DNA sequence that comprises a gene into many RNA molecules that have the complementary sequence. Each RNA molecule now contains the information on the sequence of amino acids in the corresponding protein. A large and complex machine in cells, called the ribosome, then takes each RNA molecule and translates its sequence of bases into the sequence of amino acids in the corresponding protein. The way in which the information encoded in genes in our DNA is first transcribed into RNA and then translated into the synthesis of corresponding proteins is called the “central dogma of molecular biology.”
Viruses Need Us to Replicate
Just like animals and us, viruses have to replicate in order to propagate their species. In fact, their principal function is to make copies of themselves. To carry out its functions, just like our cells, a virus needs proteins. Just like our cells, each virus particle also contains its genetic information. But, unlike each one of our cells, a virus particle does not have all the machinery needed to translate its genes to corresponding proteins. Viruses enter a person, animal, or plant and invade their cells. A virus then hijacks the machinery in the cell they have invaded (e.g., the ribosome) to translate its own genetic information into many copies of its proteins. This enables the assembly of many new virus particles that can go on to infect other cells. In a sense, viruses are parasites.
How Viruses Enter Our Cells
Cells respond to changes in their environment in order to carry out their functions. Each type of cell has specific proteins, called receptors, that stick out on their surface to sense the environment. A receptor on a particular cell binds only to a specific substance in the environment. If the receptor binds to this substance, the cell detects its presence and responds accordingly. Viruses have spikes on their surface made up of viral proteins. To cause infection, a virus’s spike must bind to receptors on the surface of cells in the tissues that it invades. Once a virus’s spike binds to a receptor, it can force its way in through the cell wall and then hijack its machinery to replicate.
The SARS-CoV-2 virus, which causes the COVID-19 disease, binds to a specific human receptor, called ACE2, that helps regulate blood pressure. ACE2 is abundantly present on the surface of cells in the lung. So, an airborne virus can enter through our respiratory tract and bind to ACE2 on our lung cells. This is why SARS-CoV-2 spreads through the air and infects our lungs. But ACE2 is also expressed on cells in the heart, intestine, and kidney, which may explain why other medical problems arise in COVID-19 patients.
Types of Viruses
There are two broad classes of viruses, DNA viruses and RNA viruses. DNA viruses carry their genomic information in the form of DNA, just like we do. The herpesviruses are examples of DNA viruses. RNA viruses carry their genomic information in the form of RNA. Viruses that cause common childhood diseases like measles, mumps, and rubella are RNA viruses, as are the ones that cause influenza, AIDS, and the COVID-19 disease. RNA and DNA viruses hijack the host cell’s machinery in different ways to replicate.

Once a virus enters a cell, its DNA or RNA genome is released into the cell. The DNA genome of DNA viruses enters the cell’s nucleus. Now, new viral proteins are made using the host cell’s machinery in the same way that our own DNA is translated into our own proteins. First, some viral genes are transcribed to the corresponding RNA molecules, and then these are translated into some “early” viral proteins. Along with the host cell’s machinery, these early proteins help translate the rest of the virus’s DNA into a complete set of viral proteins. These proteins are then assembled into new virus particles, which then exit the cell and search for new cells to infect. Sometimes, the host cell dies during this process.
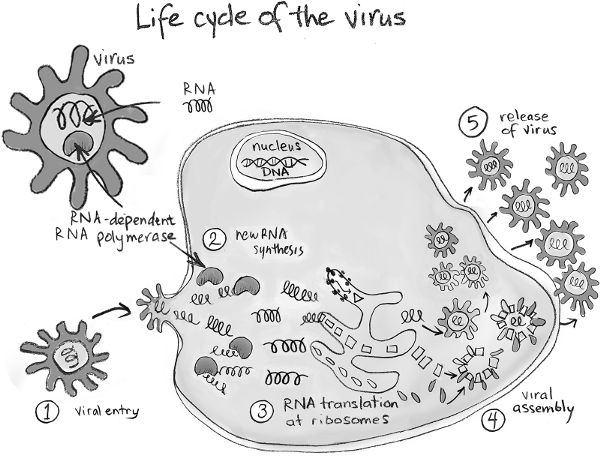
Once inside the cell, RNA viruses need to make many copies of their RNA genome, which can then be translated into many viral proteins, which are assembled into new virus particles. Most RNA viruses carry a molecule that makes copies of their own RNA. This molecule is called an RNA-dependent RNA polymerase. Once many copies of the virus’s RNA are available, the host cell’s protein-making machine, the ribosome, is used to translate the information in the RNA to the virus’s proteins. New virus particles are then assembled and they bud out of the cell.
While most RNA viruses replicate as described above, one type of RNA viruses does it differently. These viruses, for example, HIV, are called retroviruses. Retroviruses first convert their RNA into the corresponding DNA sequence. This is accomplished by a protein that the virus carries with it, called reverse transcriptase. The viral DNA then enters the cell’s nucleus, and a viral protein called integrase makes a nick in the host cell’s DNA and inserts the virus’s DNA there. Now, the host cell’s genome is altered forever, as it contains this piece of viral DNA. Using the host cell’s machinery, the virus’s DNA is then translated into its proteins, enabling the assembly of many new virus particles that go on to infect new cells. Until the mechanism by which retroviruses replicate was discovered, it was believed that the only way that genomic information was translated into proteins was by following the central dogma—DNA to RNA to proteins. Retroviruses translate their genomic information to proteins by the route, RNA to DNA to RNA to protein. For their paradigm-shifting discovery of how retroviruses replicate, David Baltimore and Howard Temin were awarded a Nobel Prize in 1975.
Retroviruses were first identified as tumor-causing viruses in animals. Whether they played any role in human cancer or any other human disease was not clear until the 1980s when a retrovirus was identified as the cause of a rare leukemia. Subsequently, HIV, a retrovirus, was found to be the causative agent of AIDS. When the human genome was first sequenced, a big surprise was that a large percentage of our DNA genome was comprised of retrovirus genes. These genes are usually not translated into proteins. This tells us that we have waged war with retroviruses for a long time, and they are hiding in our DNA. Understanding whether the retrovirus genes in our genome are implicated in cancer and how they impacted evolution of the human species is an active area of research.

RNA Viruses Can Change Guise Rapidly
When our cells translate the information in our DNA genomes into corresponding RNA and then into proteins, very few errors are made. RNA-dependent RNA polymerase, however, makes mistakes at much higher rates when it copies an RNA virus’s RNA. Similarly, reverse transcriptase makes many errors when it creates a DNA molecule from a retrovirus’s RNA genome. So, the proteins of RNA viruses that are made in a host cell often have a variety of amino acid sequences that are different from the proteins of the virus that originally infected the cell. Most such mutations result in defective proteins that prevent the new virus from functioning. But some mutations allow the virus to function just as well, and some even better. Thus, RNA viruses have an ability to change guise quickly and still function. Sometimes, they can even evolve new functions.
Using Genomic Information to Test for Viral Infection
During an ongoing epidemic or pandemic, it is very important to be able to rapidly identify infected people. These are the people who need to be isolated from others. To detect the virus, we need to know its RNA or DNA sequence. Once we know the sequence, a standard method called polymerase chain reaction (PCR) can be used to detect whether a sample of human fluid contains the virus that is the causative agent of a disease. PCR is a simple, ingenious idea for which its inventor, Kary Mullis, won a Nobel Prize. It takes advantage of a small synthetic DNA fragment called a primer that is designed to bind to the specific viral DNA or RNA sequence. If the RNA or DNA sequence specific to the primer is present, addition of a DNA polymerase allows a double-stranded fragment of DNA to be made. Doing this repeatedly many times amplifies this DNA fragment, and when there are many copies of DNA, it is easy to detect. This enables highly sensitive detection of rare DNA or RNA sequences. A few days after the RNA sequence of the virus that causes COVID-19 was published, Christian Drosten in Germany published a PCR method for detecting this virus. Implementing this method only required ordering a set of primers, and it was rapidly adopted by most countries around the world. However, the Centers for Disease Control and Prevention (CDC) in the United States decided to design its own test, which delayed the introduction of testing in that country.
Examples of RNA Viruses and Why They Cause Pandemics
In this section, we describe three examples of RNA viruses that have caused pandemics in the last century, and how the pandemic-causing viruses emerged.
SARS-CoV-2
Coronaviruses are a family of RNA viruses, and for years, four different types of these viruses have circulated in the human population. Some of them are among the many types of viruses that cause the common cold. No one pays much attention to them because these viruses cause mild disease symptoms in the vast majority of people whom they infect. They are basically just a nuisance.

In 2003, many patients in China were found to be suffering from acute respiratory distress syndrome that was caused by severe lung damage. Soon it was determined that the disease was due to a new type of coronavirus. The virus was called severe acute respiratory syndrome coronavirus, or SARS-CoV. The virus started spreading across East Asia to Hong Kong and Singapore, and then to Canada. SARS-CoV was deadly, resulting in the deaths of about 10 percent of those infected. Strong public health measures in China and other countries were ultimately able to control the SARS epidemic. About a decade later, in 2012, a virus emerged from Saudi Arabia that spread around the world, but mainly to South Korea. It too caused a severe respiratory illness, which was called Middle East respiratory syndrome (MERS). The causative agent was again a coronavirus. This virus was even more lethal than SARS, killing about 35 percent of those that it infected. Again, strict public health measures were able to extinguish MERS.
In late December 2019, physicians in Wuhan, China, began to suspect that a new virus was responsible for a flu-like respiratory disease. On January 10, 2020, Chinese officials announced that a new coronavirus caused this disease, and published its RNA sequence. The sequence was more similar to the virus that caused SARS than the one that caused MERS. In late January, almost on the same day, South Korea and the United States both detected patients who tested positive for the novel coronavirus. Within months, the virus would spread around the world, resulting in the death of hundreds of thousands of people. In response to the fast-spreading pandemic, countries around the world shut down their economies to keep people apart. The cost of this worldwide economic catastrophe is measured in many trillions of dollars, and millions have lost their jobs. The novel coronavirus that devastated the world came to be called SARS-CoV-2, and the disease it causes, coronavirus disease of 2019 (COVID-19).
Why do new viruses cause pandemics or epidemics? How do these new pandemic- or epidemic-causing viruses emerge? Why did SARS and MERS not spread around the world, while COVID-19 became a global pandemic? Let us consider these questions in turn.
When a virus has been circulating in the human population for a while, most people have some level of immunity to it. So, they can fight the virus adequately, and only a few people become ill. When a totally new virus emerges, no one has immunity to this virus. So, the virus can infect anyone, and if the virus is easily transmitted, infected people can spread the virus to many others they encounter. If the virus also causes a lethal disease, a frightening pandemic can result. The coronaviruses that caused SARS, MERS, and COVID-19 were such new viruses to which humans were not immune.
How did these new coronaviruses arise? As we discussed earlier, RNA viruses mutate, and this provides them with a mechanism to change guise and evade human immunity. Did new coronaviruses, like SARS-CoV-2, evolve from mutations in the coronaviruses that circulate in humans and cause mild diseases, like the common cold?
To illustrate a point pertinent to this question, let us consider an RNA genome with 10 genes. Suppose that, due to errors made by RNA-dependent RNA polymerase, the chance of mutations arising in a gene when it is copied is one in five. On average, there will be mutations in two genes every time this RNA is replicated. If the RNA genome had 20 genes, there would be 4 mutations, if it had 80 genes, there would be 16 mutations, and so on. So, an RNA virus with a longer genome should have mutations in more genes. Compared with other RNA viruses, coronaviruses have a very long RNA genome. But when we peer at RNA sequences of these viruses, we do not see many mutations. Indeed, SARS-CoV-2 also has not mutated very much since it was first identified. Why is this?
Genes encode information about proteins that have to work together to enable a virus to function. Mutations make a protein different, which is likely to make it less compatible for working with other proteins. The larger the number of proteins with mutations, the more difficult it will be for viral proteins to work together, increasing the chance that the mutant viruses will not be viable. So, coronaviruses, with their long RNA genomes, would produce many progeny that would likely not function because of replication errors made by RNA-dependent RNA polymerase in many of its proteins. This is why coronaviruses have a protein that serves as a proofreader, like the one our cells have for DNA replication. So, if a wrong base is inserted as a new RNA strand is being created, the proofreader can excise it and the error is corrected. This is why coronaviruses mutate less than expected. The spikes of most coronaviruses that circulate in humans and cause mild disease do not bind to ACE2 (the receptor for SARS-CoV and SARS-CoV-2). They enter cells by binding to a completely different receptor. In order to bind to ACE2, many new mutations would have to arise in their spike proteins. Thus, since coronaviruses have a proofreader, it is unlikely that mutations in the common human coronaviruses led to the emergence of SARS-CoV or SARS-CoV-2.
Some families of viruses not only infect humans but can also infect and propagate in animals. For example, coronaviruses also infect rodents and bats. Although these viruses share some features with the human coronaviruses, their proteins are different in important ways. These differences usually make it difficult for a member of a virus family that thrives in a particular animal to do so in humans. Why this is so is explained by Darwin’s theory of how species evolve to adapt to their surroundings. Darwin’s studies showed that organisms mutate randomly. Most mutants are likely to be less adapted to their environment than their parent. If by chance a mutation arises that is better adapted to the environment than the parent, it slowly outcompetes the older species and becomes dominant in the population. In an analogous manner, mutations arise over time in coronaviruses that infect bats, for example, and they become better adapted for multiplying in bats. But these changes make the coronaviruses that infect bats less suitable for thriving in humans because we are a different environment.
However, every now and then, changes can occur in a virus that allow it to jump from being a virus that thrives in an animal to one that can productively infect humans. Often, this jump occurs in stages. For example, a bat coronavirus through a few mutations could become capable of infecting another animal. Also, bats can harbor many types of coronaviruses at the same time, and so two different coronaviruses could coexist in an infected cell. The two viruses could swap pieces of their genomes with each other to create a new hybrid virus that can infect another animal or a human. This process wherein the genomes of two viruses mix is called recombination. If the new hybrid bat virus infects an intermediate animal, it could acquire a couple of additional mutations therein that makes it thrive better in humans. This is especially likely to be true if the intermediate animal shares some traits with humans. SARS, MERS, and COVID-19 are diseases that were almost surely caused by viruses that jumped from bats to us, perhaps through intermediate animals.
During the first SARS epidemic, it was first thought that SARS-CoV was passed to humans by civets. These are small, cat-like animals that were being sold at live animal markets in southern China. There were documented examples of humans being infected by civets. But later it became clear that the SARS virus did not originate in civets. This initiated a worldwide search for the origin of the animal from which SARS-CoV jumped to humans. In 2005, working with an international consortium, Shi Zhengli, a Chinese virologist from the Wuhan Institute of Virology, identified coronaviruses in bats in China that were closely related to the SARS virus. This suggested that bats were the original source. Over the next 12 years, Shi traveled throughout China exploring bat caves. She collected and cataloged the coronaviruses that she found in bats throughout China. In 2017, she finally discovered what she was looking for. In a cave in southern China, she found a bat colony with viruses that were almost identical to the SARS virus, and were therefore its original source. Given her unusual devotion, the popular press dubbed her China’s “Bat Woman.”

When COVID-19 first emerged in China, Shi was urgently summoned home to Wuhan and she was the first to sequence and analyze the new virus. She reported that the new virus was related to the SARS virus, but, remarkably, it was almost identical to a bat virus that she had collected earlier. The major difference was in the proteins that made up the virus’s spike. The spike protein was very similar to that of a virus isolated from a pangolin, a small mammal with an armadillo-like shell. This suggested that the virus had passed from the bat to the pangolin before making the jump to humans. But this picture is uncertain, and will be clarified as more data become available. Whether this virus further adapted to thrive in humans after directly infecting humans, or whether it passed through an intermediate animal like a pangolin, is unclear.
SARS and MERS were lethal viruses, which rapidly killed many of those that it infected. But neither of these viruses caused global pandemics. Humans infected with these viruses quickly felt very sick with cough, fever, and malaise, and sought medical help. A virus that immobilizes infected people early in this way cannot spread too widely. This is because infected people largely come in contact only with close family members and healthcare professionals. So, it is relatively easy to contain the virus by isolating healthcare workers and close family members who came in contact with infected persons. This is how SARS was rather quickly eradicated. In the case of MERS, the virus jumped from bats to camels and then to humans. MERS infections occurred mainly in people with close contact with camels. All of these factors served to limit the spread of these two coronaviruses.
Comparing SARS-CoV and MERS with SARS-CoV-2 (the virus that causes COVID-19) reveals the kinds of features a virus needs to acquire in order to cause a worldwide pandemic. A person infected with SARS-CoV-2 can spread the virus to others before feeling any symptoms. Many infected people have mild or even no symptoms at all. So, infected people can move around and infect many others before realizing that they are infected. This allows the virus to spread rapidly through populations. Although SARS and MERS have a higher fatality rate than COVID-19, the latter is deadly and kills about 1 percent of infected people. Because it infects many people and is quite lethal, SARS-CoV-2 has features that make it almost perfect for causing a deadly pandemic. The saving grace is that it does not mutate much, which would greatly complicate efforts to design a vaccine that can protect us from it (you will have to wait until later chapters to see why).
Influenza
Influenza is an RNA virus that is not a member of the coronavirus family of viruses. We are very familiar with influenza because it causes the seasonal flu, which like COVID-19 is a respiratory illness. Influenza has a relatively low rate of infectivity, but it can also be transmitted a few days before symptoms appear. Also, like COVID-19, a significant fraction of those infected do not feel very ill, which facilitates the spread of infection. Influenza clearly has a seasonal preference because cases peak in the Northern and Southern Hemispheres during their respective winters. Various factors are responsible for the seasonal preference. Some important factors are that people spend more time with each other in close proximity indoors when the weather is colder, and the virus survives better in cold, dry weather than in warm, humid weather.
The protein that makes up the viral spike of the influenza virus has a long name that is usually abbreviated as HA. Another important protein that is displayed on the surface of the virus is a protein whose name is abbreviated as NA. There are 18 different types of HA and 11 different types of NA. The different families of influenza viruses are classified by the specific combination of HA and NA that they have.
Influenza is an RNA virus that, unlike coronaviruses, does not have a proofreader. So, its proteins, including HA and NA, mutate continuously. For reasons that will become clear in the next chapter, humans often mount strong immune responses against influenza that target HA and prevent it from binding to its receptor. This prevents the virus from infecting new cells, and the infection is controlled. Vaccines also elicit immune responses designed to target HA and thus prevent infection. As the winter progresses, many people develop immune responses to the HA proteins in the circulating strains of the influenza virus, due either to natural infection or to vaccination. Thus, they become immune to these viruses. The virus mutates, and the mutant strains that are both functional and able to evade this shield of immunity established in past years then prevail among the circulating strains the following year. Thus, the war between influenza and our immune systems continues every year.
The World Health Organization has a network of people and countries who surveil the world, sequencing RNA from influenza viruses. Based on this data, and which viruses have circulated among humans in past years, a group of experts make educated guesses about which strains of influenza are likely to be prevalent in the next year. Next year’s vaccine is then designed. It usually takes several months to manufacture millions of doses of next year’s flu shot, and so this decision is made well before the flu season begins. Even if the educated guess that leads to the design of the vaccine is not correct in some years, since the prevailing mutant strains of influenza that circulate in a given year are usually not too different from ones in past years, most people have partial immunity to them. So, illnesses and deaths are mostly confined to vulnerable groups, such as the elderly and the immunocompromised. But influenza nevertheless kills anywhere between 15,000 and 60,000 people every year in the United States.
The number of deaths and hospitalized patients changes dramatically when influenza pandemics arise. There have been four such pandemics in the last century, in 1918, 1957, 1968, and 2009. Twenty to fifty million people died during the 1918 pandemic, when the world population is estimated to be almost four times smaller than today. How do influenza pandemics arise?
The RNA genome of the influenza virus is made up of eight discrete segments, each of which encodes information about one or two of its proteins. Influenza viruses can also infect animals, such as birds and pigs. Of course, these viruses are different because they have adapted to live in their host animals. However, each of the eight segments of the influenza genome is like a cassette that can be taken out and replaced by another variant of the same gene segment. For example, one of the eight segments encodes information about HA, which is the spike protein. A particular variant of this segment could be swapped for another variant. If the new spike protein is compatible with the proteins encoded by the other gene segments, you have a viable virus. But now the virus has a completely new HA spike to which humans have no immunity, and such a novel influenza virus could cause a pandemic. Indeed, influenza pandemics occur when gene segments of the virus circulating in humans are swapped with those of a bird or a pig.
The 1957 pandemic occurred when three gene segments in the influenza virus that was circulating in humans at the time were swapped with those from a bird influenza virus. The swapped segments included the one corresponding to HA. The 1968 pandemic arose when two gene segments of the strain then circulating in humans, including the one for HA, were swapped for those from birds. The bird viruses are not well adapted to thrive in humans, but since the majority of gene segments in the pandemic causing viruses that emerged in 1957 and 1968 were from viruses that were already circulating in humans, they thrived in the human population. Swaps of gene segments from different influenza viruses usually occur during coinfections. A person who works closely with birds that harbor influenza viruses could be infected by a bird virus and a human virus at the same time, and the viruses might swap their gene segments by a process called gene reassortment. These swaps likely do not produce viable viruses most of the time, but sometimes, as in 1957 and 1968, they do so with devastating consequences.
The 2009 pandemic, however, was caused by a virus that emerged in pigs and directly infected humans. This was likely because multiple viruses derived from humans, pigs, and birds were circulating in pigs at that time. It is thought that a number of reassortments occurred within pigs until the pandemic-causing virus that thrived in humans emerged. We are much more similar to pigs than to birds, which may have helped the direct jump of the virus from pigs to humans in 2009.
Human Immunodeficiency Virus (HIV)
In Africa, a relative of the HIV retrovirus circulates in many species of primates like monkeys and apes. It is believed that HIV, the form of this virus that can infect humans, jumped from a certain kind of chimpanzee to us. Hunters who captured chimpanzees for meat (bush meat) came in contact with blood from these animals and ate the meat. This sort of contact with a large amount of the animal virus likely allowed a few mutant forms of the virus that could multiply in humans to emerge in a few individuals. Using computational approaches based on viral sequences and rates of mutation, scientists now believe that HIV started circulating in humans perhaps as far back as the 1920s, primarily around the Republic of Congo in Africa. It was only in the early 1980s, however, that a number of unusual cases of lung and mouth infections and cancer, all among young men in California and New York, raised the alarm that a new type of disease might be spreading. This is because these conditions usually arise in people whose immune systems are compromised, and young healthy people are not normally immunocompromised. In 1981, Dr. Michael Gottlieb, an immunologist at the University of California Los Angeles Medical Center, published a report of the cases in California among young gay men in the CDC’s Morbidity and Mortality Weekly Report. This marked the beginning of the pandemic disease that we call acquired immunodeficiency syndrome (AIDS).
In 1983, Françoise Barré-Sinoussi and Luc Montagnier at the Pasteur Institute in Paris announced that they had identified the virus that is the causative agent of AIDS. In 1984, Dr. Robert Gallo at the National Institute of Health in the United States, who had previously discovered the first human retrovirus, reported that his laboratory had also identified the retrovirus that caused the disease. Soon it was realized that the two viruses were identical. A test was then developed, which is used to this day. There was a bitter patent rights dispute between the United States and France, which was ultimately resolved in 1987 when Presidents Reagan and Chirac agreed to share the profits, and donate the bulk of it for AIDS-related research and treatments. Barré-Sinoussi and Montagnier shared the Nobel Prize in 2008 for their discovery of HIV.
HIV is transmitted to others by exchange of bodily fluids, such as blood, semen, and milk from lactating mothers. Before tests were available, tainted supplies in blood banks infected hemophiliacs. Initially, it was thought that the disease infects only gay men or intravenous drug users, but this is not true. Heterosexual sex is the principal cause of HIV infections in sub-Saharan Africa, the epicenter of the disease today. To date, HIV has infected almost 75 million people, and as many as 40 million people have likely died from complications associated with AIDS. In South Africa, there are still approximately 1,000 new infections every day.
Upon initial infection, HIV causes flu-like symptoms that then go away. The virus infects a cell that plays a key role in coordinating our immune responses, resulting in a decline in the number of these cells. This is why patients have a compromised immune system, which results in vulnerability to infections that our immune system normally controls with ease. Without treatment, ultimately, the numbers of the immune cells decline to very low levels, resulting in death. Today, innovations in HIV drug treatment (described in chapter 6) keep the virus under control in treated individuals. But the virus is not eradicated in these people, and it comes right back if treatment is interrupted. The search for a cure for HIV is an active area of research, as is the search for a vaccine. After over 30 years of effort and enormous expense, we still do not have a vaccine that can protect against HIV infection. As we will see in chapter 7, this is because HIV mutates at a very high rate. Fortunately, this is not the case for the virus that caused the COVID-19 pandemic.
Why Do RNA Viruses Cause So Many Pandemics?
As we have described, most RNA viruses mutate quite a bit. Also, their genomes are relatively malleable, which makes possible reassortment of genes as occurred during influenza pandemics, or recombination of genes as that which might have resulted in COVID-19. This malleability and the circulation of related forms of RNA viruses in animals make the emergence of new RNA viruses a perpetual existential threat to humanity. For example, an influenza pandemic is always waiting to happen. Various forms of influenza viruses circulate among birds and pigs. Humans interacting closely with pigs and poultry in farms and markets can facilitate exchanges of the influenza virus between them and these animals. The global population will have no immunity to the new virus, and if, like SARS-CoV-2, it is easily transmitted by casual human contact and is quite lethal, a pandemic will result. As the COVID-19 pandemic has made vivid, this threat is not localized to particular nations or peoples.
Examples of DNA Viruses
DNA viruses are a different beast. Most have a double-stranded helical DNA genome, just like us. As we described earlier, double-stranded DNA can be copied with very high fidelity. So, mutations are rare, which allows DNA viruses to have much longer genomes than RNA viruses. For example, the herpesvirus genome has around 80–100 genes, and smallpox has around 200. Most RNA viruses contain about 10 genes or fewer. With many more genes, DNA viruses are more complex machines and do not change guise as rapidly as RNA viruses.
DNA viruses are very familiar to us, especially those that belong to the herpes family. The herpesvirus family includes the viruses that cause chicken pox and shingles (varicella zoster), cold sores and contagious genital rashes, and mononucleosis (Epstein–Barr virus). Another herpesvirus known as cytomegalovirus (CMV) is also prevalent. Most people are infected by one or more herpesviruses by the teenage years. These viruses are passed from human to human in different ways, but close human contact is usually necessary.
Herpes is an ancient virus, which inserts its genome into the nucleus of the host cell. So, after infection, the cell is permanently infected. For most of us, the virus stays silent after one recovers from the initial infection, and never bothers us again. But in some cases, the virus can reawaken and make new virus particles. This is what happens during a shingles outbreak. After exposure to chicken pox in childhood, the virus hibernates in cells of the nervous system. Usually in older adults, the virus can reawaken and cause a reddened, painful, skin condition, which we call shingles. We really do not understand why the virus gets activated again. It is likely that the immune system normally keeps the virus under control. But in stressful circumstances, or when the immune system is suppressed, herpesviruses are reactivated. Reactivation can occur with all herpesviruses, not just the one that causes chicken pox.
With this understanding of the enemy, viruses, let us turn to our immune system, which combats these scourges on the planet.