Chapter 20
Patch-Clamp Techniques
Chapter Outline
II. Applications of the Patch-Clamp Technique
V. Current Recordings and Analysis
I Introduction
The patch-clamp technique allows the electrophysiological measurements of currents through ion channels in the cell membrane. Developed in 1976, it has been the technique of choice for measurements of ion-channel activities in cells with resolution up to a single channel (Neher and Sakmann, 1976; Sakmann and Neher, 1984). This technique has wide applications ranging from the recording of the activity of native channels in their natural environment to recombinant channels expressed in heterologous cells.
Historically the patch-clamp technique was based on the work of Alan Hodgkin and Andrew Huxley who, in the 1950s, conducted a series of elegant voltage-clamp experiments that allowed the recording of macroscopic currents in the squid giant axon by controlling the voltage of the membrane (Hodgkin and Huxley, 1952). These studies established the physiological importance of ion fluxes through ion channels in the activity of cells and set the foundation for much of the subsequent work in electrophysiology. In 1963, Hodgkin and Huxley were awarded the Nobel Prize in Physiology or Medicine for this work. A limitation of the technique developed by Hodgkin and Huxley was that it did not have the resolution to measure the current through a single channel. This was made possible in 1976 by Neher and Sackman, who developed the patch-clamp technique. Using this new technique, they succeeded in recording from a tiny area (a patch) of the plasma membrane of frog skeletal muscle the first acetylcholine (ACh)-activated single channel (Neher and Sakmann, 1976). For their discoveries concerning the function of single ion channels in cells, Neher and Sackman were awarded the Nobel Prize in 1991. Since then the patch-clamp technique remains the technique of choice for measuring the activity of ion channels.
This technique and its further refinements represent a major advance in the ability to monitor cell membrane function (Hamill et al., 1981). Sealing a small pipette tip to a clean cell membrane allows recording of the ionic currents through single channels contained in small patches of cell membranes. Together with the method of whole-cell recording, patch-clamp techniques permit the investigation of ion channel conductance and kinetic behavior. This information has led to the discovery of new classes of ion channels and their physiological role in cells too small to be amenable to the standard voltage-clamp techniques and to cells that are not electrically excitable.
In this chapter, we describe the well-established techniques of patch-clamp recording with an extension to newly developed high-throughput applications of this technique. This chapter is divided into five parts: the first part focuses on the applications of the patch-clamp technique. The second section focuses on the basis of the patch-clamp technique with an emphasis on the technical aspects of the recording process including equipment and procedural steps. The third part describes the applications of the different recording configurations to measure single-channel activity and whole-cell currents. The fourth section is on data acquisition and analysis of single-channel and whole-cell data. The last part of this chapter presents the automated patch-clamp system, a newly developed multiwell plate format of the patch-clamp technique that allows high-throughput recording of ion-channel activities.
II Applications of the Patch-Clamp Technique
The patch-clamp technique provides the ability to observe, in real time, the changes in the activity of a single channel including changes in conductance (the rate of ions going through the channel) and kinetic properties (the speed with which a channel opens and closes) in response to a pre-set stimulus. Furthermore, it allows determination of the characteristic sensitivities of specific ion channels to voltage, ions and ligands. Patch-clamp studies have led to the discovery of a variety of ion-channel types and their classification. In general, ion channels are functionally classified based on their ion selectivity and their modulation by voltage, ligands, second messengers, phosphorylation and mechanical deformations. Voltage-gated (or voltage-sensitive) channels are activated by changes in membrane potential. To this family belong the well characterized Na+, K+ and Ca2+ channels of nerve and muscle (Caterall, 2010). The extracellular ligand-activated channels are regulated by ligands such as transmitters. These channels are often named according to the ligand to which they bind, e.g. ACh-sensitive channels of the neuromuscular junction and the GABAA (γ-aminobutyric acid) channels of the inhibitory synapses (Collingridge et al., 2009). The intracellular ligand-gated ion channels include channels that are activated indirectly by G-protein-coupled receptors (GPCRs), such as cystic fibrosis transmembrane conductance regulator (CFTR) and other ion channels that are activated by intracellular ligands such as Ca2+, ATP, cyclic AMP and cyclic GMP as well as phosphoinositides (Gadsby et al., 2006). The mechanosensory and volume-regulated channels form a rather ubiquitous class of channels that can be activated by mechanical tension of the membrane. To this class belong channels such as transient receptor potential (TRP) and volume-activated Cl− channels that are important in a variety of key physiological functions ranging from touch sensitivity to regulation of cell volume (Nilius et al., 1996; Sharif-Naeini et al., 2008; Wu et al., 2010). A fifth group includes ion channels that do not have any of the distinct characteristics of the above groups. This group includes the GAP junctions and peptide ion channels like gramicidin (Saez et al., 2003; Kelkar and Chattopadhyay, 2007). There are additional systems of nomenclature which have joined the extracellular- and intracellular-gated channel groups into the “chemically-activated” or just simply “ligand-gated” ion channels. It has been shown by sequence comparison that ion channels within each of the distinct functional groups described above also show the greatest sequence similarity, indicating that they are most likely all descending from a common ancestor. Therefore, within these functional families, ion channels are classified based on the genes encoding their main pore-forming subunits. For the classification of ion channels please refer to the IUPHAR (International Union of Basic and Clinical Pharmacology) database (DB) which provides a cohesive nomenclature and nomenclature guidelines, together with detailed peer-reviewed pharmacological, chemical, genetic, functional and anatomical information on G-protein-coupled receptors, voltage-gated ion channels and ligand-gated ion channels (Harmar et al., 2009). The IUPHAR-DB provides a comprehensive description of the genes encoding these channels and their functions, with information on protein structure and interactions, ligands, expression patterns, signaling mechanisms, functional assays and biologically important channel variants (e.g. single-nucleotide polymorphisms and splice variants). The IUPHAR-DB is freely available at http://www.iuphar-db.org.
Through the patch-clamp technique, the detailed electrical and kinetic properties of a multitude of ion-channel types have been described, giving insight into the physiological modulation of these channels and also their alterations in disease states. Many diseases have been associated with defects in ion channels (Ashcroft, 2000). Ion-channel-associated diseases, i.e. channelopathies, often result from a mutation or mutations in the genes encoding the ion channel subunits or associated regulatory subunits. A recent special issue of Pflügers Archiv European Journal of Physiology was dedicated to this topic (for relevant websites refer to Nilius, 2010). The patch-clamp technique has been fundamental in discovering the functional implications of mutations in channel protein sequences. Through structure–function studies, it is possible to mutate even a single amino acid in a channel protein, express the mutated channel using a heterologous expression system, measure its electrophysiological properties and compare those properties with those of wild-type (native) channels. These studies provide information on how a specific mutation changes the physiological activity of a channel and are thus instrumental in discovering the functional implications of disease-associated mutations. Furthermore, they allow the elucidation of the amino acids associated with specific functional properties of the channels, such as gating, as well as sensitivity to ligands and second messengers.
Although patch-clamping finds wide application, it still remains a technically challenging method that requires a carefully controlled experimental setting and a skillful experimentalist.
III Patch-Clamp Techniques
IIIA Patch-Clamp Set-up
Conventional patch clamping, now also referred to as “manual patch-clamp” to distinguish it from the recently developed “automated patch-clamp” described below (see Section VI), is accomplished by sealing the small tip of a pipette to the surface of the cell membrane in such a way that is possible to isolate a tiny membrane area (patch) from the rest of the membrane and to control its voltage while simultaneously recording the currents through the ion channels in the patch. A typical electrophysiology rig consists of a Faraday cage (which isolates the equipment from electrical noise), a vibration isolation table, a microscope for imaging cells, micromanipulators for moving and positioning the electrodes, low-noise amplifiers, a computer (for generating the stimulus waveform and data acquisition) and a perfusion line. The technique of patch-clamp requires a very steady platform where vibrations are minimized to allow maintaining a stable contact between the pipette and the cell. The first step in reducing vibrations is to find a quiet spot with floors less subject to vibrations. A good anti-vibration table is also used to reduce the problem of vibrations. This table consists of a very heavy tabletop suspended on air cushions. The other main pieces of equipment that are on the table are the microscope and the micromanipulators. The microscope has the optics necessary for the visualization of single cells (ten to one hundred microns in size) and the tip of the patch pipette (a few microns). Usually, phase-contrast and Nomarski (or interference contrast) microscopy allow satisfactory visualization of the membrane. Furthermore, the microscope is an inverted microscope; the objectives are located under the stage. A perfusion (bath) chamber, where the cells are placed for recording, is located on the stage of the microscope. By using an inverted microscope there is sufficient space between the stage and the condenser to place the patch pipette. The schematic of the bath chamber placed on the stage of the microscope is shown in Fig. 20.1. The bath chamber is where the cells are seeded and where the actual patch-clamping procedures occur. The ground electrode in the bath is usually a silver/silver chloride (Ag/AgCl) electrode which has a low junction potential, thus minimizing the development of a solid–liquid junction potential between the electrode and the bath solution (see Section V for description of junction potentials). The patch pipette is usually a borosilicate glass pipette with tip diameter of 1–5 μm and is filled with a salt solution. Quartz glass is instead recommended when patch clamping is combined with fluorescence techniques, such as detection of intracellular Ca2+ with a fluorescent ion sensitive dye, as this material has better fluorescence properties. For patch pipettes and their fabrication refer to the publications of Rae and Levis (Levis and Rae, 1998; Rae and Levis, 2001). Patch pipettes, especially for single-channel recordings, may be coated with a hydrophobic material to reduce capacitative currents. The patch pipettes are filled with a conducting salt solution (known as pipette solution) in contact with a recording electrode (Ag/AgCl wire) which feeds the signal to a low-noise amplifier. The amplifier, which contains the appropriate measuring and clamping circuits and controls, processes the experimental commands, such as the voltage imposed to the cell membrane, and receives the data from the membrane. Furthermore, the patch-clamp amplifier is designed to minimize the background electrical noise that obscures very small currents in the single-channel experiments.
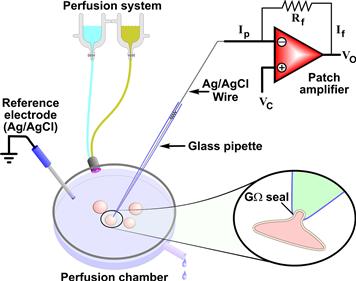
FIGURE 20.1 Schematic of a patch-clamp setting. The cells are plated in the perfusion chamber where solutions of different compositions can be rapidly switched via a perfusion system. The ground (or reference electrode) is made of Ag/AgCl and placed in the perfusion chamber either directly or through an agar bridge (1–3% agar in 0.9% saline) to reduce junction potential. One cell at the time is patched using a glass pipette which comes in contact with the cell membrane and forms the gigaseal (shown in detail in the inset). The glass pipette, filled with a salt solution, is connected to the amplifier via an Ag/AgCl wire. During a voltage-clamp experiment a current is injected through the amplifier so that the recorded voltage (V) (negative junction of the amplifier) is maintained equal to the command V (Vc) in such a way that the output V (Vo) is equal to −Vc. In this condition, If × Rf = −Ip × Rf, where If is the feedback current, Ip is the pipette current and Rf is the feedback resistance.
Once the appropriate equipment is in place, a series of specific steps are followed to make an actual patch-clamp experiment.
IIIB Patch-Clamp Experiments
The patch-clamp recording is generally performed to measure the electrical properties of the membrane (or portions of it) attached to the pipette. The sequence of events leading to the patch-clamp recording of channel activity can be described as follows. Isolated cells are placed in the perfusion chamber and bathed in the recording solution (see Fig. 20.1). The patch pipette is then moved towards the cell surface while a low-voltage square pulse is applied to the pipette. The current amplitude of this pulse is monitored to follow the formation of the seal. As soon as the pipette touches the membrane there is an increase in resistance which produces, in accordance with Ohm’s law (I = V/R where I = current, V = voltage and R = resistance), a decrease in current. Upon gentle suction, a tight seal is formed, allowing the recording of the current through the channels in the patch with very high resolution. The tight seal that is formed between the membrane and the pipette is called a “gigaseal” because it has a resistance greater than one gigaohm (109 Ω). This high-resistance seal allows the reduction of background noise and makes possible high-resolution recordings of single-channel currents of less than 1 pA. The successful achievement of a gigaseal depends on the enzymatic cleaning of the cell membrane, the pipette configuration and solution composition, among other factors. The tight gigaseal makes the connection between the pipette and the membrane mechanically stable and amenable to manipulations that allow the establishment of different recording configurations. Recording configurations are specific architectures of the membrane patch in relationship to the bath and pipette solutions that allow different degrees of control over the channels under investigation. The recording configuration obtained at this point is the cell-attached configuration and, while it is used to measure single channels in their most physiological setting, it is also the starting point to reach other recording configurations (Fig. 20.2).
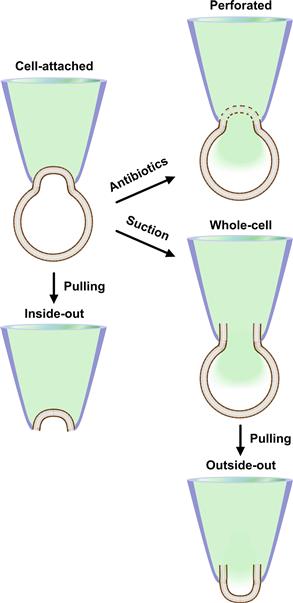
FIGURE 20.2 Patch-clamp recording configurations. The schematic of the recording configurations possible in patch-clamping and the maneuvers necessary to achieve them are shown. Each configuration is described in detail in the text. The patch pipette is labeled in blue and the pipette solution is shown in green.
IIIC Recording Configurations
Various recording configurations have been used to study ion channels, each with its advantages and disadvantages. These configurations can all be achieved by mechanically manipulating the cell-attached configuration described above. The cell-attached configuration itself is ideal to measure the electrophysiological properties of single channels within the patch. The greatest advantage of this configuration is that it is relatively simpler to achieve than other configurations. Moreover, it allows maintaining the most physiological conditions at which to study the channel behavior, as the channel in the patch is in the cell membrane surrounded by the intact membrane microenvironment and intact cytoplasm. Unfortunately, this could be also considered a limitation of this configuration as there is no way to access and control the intracellular environment. Another drawback of this configuration is that the membrane potential is intact, but it is not possible to determine accurately its value. This information is necessary to set the patch potential. Theoretically, it is possible to use another electrode to measure the actual membrane potential, but this is technically quite unpractical. Commonly, it is chosen to use extracellular solutions with high K+ concentration to “zero” the voltage (the intracellular and extracellular K+ are made equal so that the equilibrium potential for K+ (EK) is set around 0 mV), after which the patch voltage is easily set. These limitations can be overcome by using other recording configurations described below.
When a cell-attached mode is established, the tight seal makes a very strong bond between the membrane and the pipette and the patch of membrane attached to the pipette can be excised from the cell by a variety of methods (see Fig. 20.2). By mechanically pulling the pipette away from the cell, a vesicle is detached. Once exposed to the air the vesicle is destroyed leaving a patch of membrane within the pipette (estimated area of the patch 1–10 μm2). The patch is detached from the rest of the cell and the intracellular side of the membrane contacts the bath solution. This is known as the inside-out configuration. The cytoplasmic side of the membrane is easily accessible because it is immersed in the bath solution, which can be changed to deliver the desired compounds. This is an ideal configuration to study signaling of channels, providing that the lack of the physiological cytoplasmic environment does not modify the activity of the channel and the mechanical breaking of the membrane does not loosen the gigaseal. Other variations of the inside-out configuration are excised inside-out macro and giant patches in Xenopus oocytes.
The oocytes of the African clawed frog, Xenopus laevis, are widely used to study the behavior of ion channels of known molecular identity and have been the expression method of choice for studies of the structure-function relationships of ion channels (Stuhmer, 1998). Xenopus oocytes are large cells (ca. 1 mm in diameter) with low expression of endogenous channels. They also have very efficient translation machinery and are easy to use, making them an ideal heterologous expression system. A few days after injection with the messenger RNA of a specific channel, these cells express great quantities of the corresponding channels. Macropatches can be obtained in oocytes using pipettes with an opening 3–8 μm in diameter (Stuhmer et al., 1987). Macropatches allow the recording of macroscopic currents (sum of multiple channels) from a large membrane area, but in an excised configuration that is largely independent of cytosolic factors. Macropatches enable low-noise, fast-clamp patch-recordings of many channels and can be used to study channels with fast kinetics. Furthermore, excised giant membrane patches have also been successfully obtained in Xenopus oocytes (Hilgemann, 1995). These giant patches of diameter 12–40 μm allow recordings of transporter currents, charge movements and single-channel recordings of low-density channels. Interestingly, the large size of the oocytes also allows a variation of the inside-out patch that is very useful when studying the regulation of ion channels by cytoplasmic factors. The oocytes offer the unique possibility of reinserting the inside-out patch into the same cell from which the patch was obtained (so-called “patch cramming”) (Kramer, 1990). It is also possible to introduce the excised patch into another oocyte that has been exposed to different conditions with a technique called “cross-cramming” (Parekh et al., 1993).
From the cell-attached configuration it is possible to obtain a second excised patch configuration, the outside-out configuration (see Fig. 20.2). First, the gigaseal is broken by applying suction through the glass pipette, reaching the whole-cell configuration that will be described in detail below. From this configuration the outside-out configuration is achieved by slowly withdrawing the pipette from the cell. The membrane stretches, eventually breaks and folds back in itself. The electrode thus contains a portion of the membrane with the outside surface facing the bath. This configuration allows making single-channel recordings with easy manipulation of the extracellular milieu while controlling the environment at the intracellular site (with a pipette solution of known composition). Since the external portion of the channel is immersed in the bath solution, it is easily accessible and amenable to manipulations such as exposure to drugs. Still, this remains a difficult configuration to accomplish.
The configurations described so far, with the exception of macro and giant patches in oocytes, allow measurements of the activities of single channels. There are other configurations that are instead used to measure simultaneously the currents through multiple channels on the entire cell membrane: whole-cell and perforated-patch configurations. The whole-cell configuration is achieved from a cell-attached configuration by breaking the patch with suction. This allows continuity between the pipette solution and the cytoplasm with dialysis of the cell’s content. This can be viewed as an advantage because it provides control over the intracellular ionic concentration and allows delivery of chemicals and peptides to the interior of the cell. However, it is also a disadvantage as it dilutes the cytoplasm. Thus, any response that depends on soluble intracellular second messengers or ionic gradients may be altered. Furthermore, loss of cytoplasmic components essential for maintaining the channel activity can result in progressive decrease or “rundown” of the recorded currents. The perforated-patch configuration, like the whole-cell configuration, allows measurement of total currents, but the dialysis of the cytoplasm is reduced. This configuration can be reached from the cell-attached configuration providing that the pipette contains a small amount of an antibiotic, such as amphothericin-B or nystatin (both antifungal drugs) (Horn and Marty, 1988). These compounds form pores in the membrane, providing electrical access to the cell interior. These pores are permeable to ions but do not allow the passage of large molecules, thus reducing the loss of intracellular components. Practically, the pipette tip is filled with antibiotic-free solution and back filled with a solution of the same composition but containing the antibiotic. After the formation of a gigaohm seal, with time, the antibiotic contacts the cell membrane and forms pores. This can take 5 to 15 min, depending on the antibiotic concentration and the amount of antibiotic-free solution in the pipette. One of the disadvantages of this configuration is that the access resistance (Raccess) is usually higher (>20 MΩ) than in the whole-cell configuration due to the perforated membrane at the electrode. This decreases voltage-control and current resolution while increasing recording noise. A further disadvantage is that the membrane under the electrode tip is damaged by the perforations and could rupture, in which case the recording will occur in whole-cell mode with antibiotics leaking into the cytoplasm.
Once the desired configuration is achieved, the recording of the activity of a single or multiple channels can begin.
IV Data Acquisition
Cells express a variety of ion channels, so experimental interventions are made to make sure that recording occurs only from a specific type of channel. The manipulations used are chemical and electrical.
IVA Chemical Isolation of Specific Channels
When establishing recording configurations, the chemical composition of the bath and the pipette solutions can be controlled. By strategically placing certain ions in the pipette and bath solutions or by replacing certain ions with impermeable ions of equivalent charges, it is possible to isolate specific ionic currents and/or suppress others. Furthermore, it is possible to add drugs that affect the activity of channels such as specific blockers (e.g. intracellular tetraethylammonium to block some potassium channel currents) or drugs that enhance the activity (e.g. Bay K8644 to increase the open probability of L-type calcium channels).
IVB Voltage-Clamp
The activity of many channels also depends on the membrane voltage and this feature can be used to isolate specific currents from others. The technique used to control the voltage of the membrane is called voltage-clamp and is the most widely used method of recording ion-channel activity associated with the patch-clamp technique. This technique takes advantage of a speedy, low-noise differential patch-clamp amplifier which allows maintaining (clamping), through a feedback circuit, a specified membrane voltage and measuring, at the same time, the current across the membrane. The glass pipette containing a conductive solution is the electrode through which the voltage-clamp is maintained and currents are recorded. In practice, during a voltage-clamp experiment, the electronic feedback system of the amplifier measures the membrane voltage and compares it to a pre-set voltage defined by the experimenter. When a current is activated, the voltage of the membrane changes. To compensate for this change and bring the voltage to the preset value, a current of equivalent magnitude (but opposite direction) is injected through the pipette. In voltage-clamp, different command voltages are applied depending on the channel property under investigation. These command voltages vary in duration and waveform from simple steps to ramps and other complicated waveforms. Most patch-clamp experiments are voltage-clamp experiments. However, it is also possible not to clamp the voltage and to inject a fixed amount of current. This recording technique is called current-clamp.
The concept of voltage-clamp applies to many recording configurations. The electrical parameters involved during a patch-clamp experiment can be described by equivalent electric circuits. These help in understanding the complexity as well as the associated advantages and limitations of each configuration.
IVC Equivalent Circuits
In every recording mode, the patch pipette and the cell or membrane patch form a complex circuit. The equivalent circuits for the cell-attached, excised-patch and whole-cell configurations are shown in Fig. 20.3.
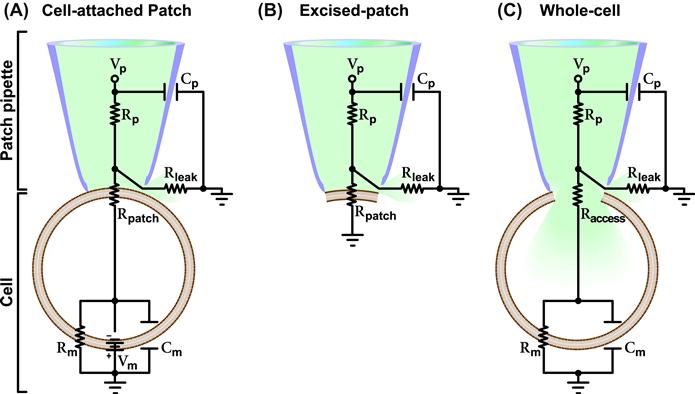
FIGURE 20.3 Recording configuration equivalent circuits. Reported are the equivalent circuitries for the cell-attached patch (A), excised-patch (B) and whole-cell (C) configurations. The patch pipette and cell (or membrane patch) are marked on the left. Vp, Rp and Cp denote the voltage, axial resistance and capacitance of the pipette. Vm, Rm and Cm denote the voltage, resistance and capacitance of the membrane. Rpatch is the resistance of the patch of membrane which is replaced by the Raccess in the whole-cell configuration where the patch of membrane is disrupted. Rleak denotes the leak resistance which depends on the tightness of the seal between the glass of the pipette and the membrane. Each circuit is described in detail in the text.
IVC1 Cell-attached
In this configuration, a patch-pipette is sealed to an intact cell. This configuration allows measuring the current through the channel(s) in the membrane patch within the pipette tip. This is possible because of the high resistance (R) of the patch (Rpatch). To understand how this technique makes the recording of the patch current possible, we should refer to the associated equivalent circuit. The overall electrical circuit of the cell-attached patch can be viewed as two circuits in series: one at the pipette level and the other at the cell level. The circuit of the intact cell originates from the fact that a cell has a membrane potential (Vm, i.e. the potential difference between the inside and the outside of the cell), a resistance (Rm, the lipid bilayer of the membrane, which acts as a barrier for the movement of charges through the membrane, and any open channels) and a capacitance (Cm, the capacity of the membrane to store charges at a given potential). In the cell-attached configuration, other resistors are present; one at the patch level and another at the pipette level. The latter depends on the opening and length of the pipette and the composition of the pipette solution. Overall, in this recording configuration there are three resistors in series. Since the highest resistance in a series of resistors is the one which dictates the current flow and Rpatch is higher than Rm and the axial resistance of the pipette (Rp, usually between 1 and 3 MΩ), then, if a voltage (Vp) is applied across the pipette, the circuit effectively monitors current flowing through the ion channels in the patch. In this circuit there is another resistor in parallel that could drain current away, the leak resistance (Rleak), which depends on the quality of the seal between the glass of the pipette and the membrane. A very high Rleak means that no significant current will leak away and that the noise is reduced. This explains why the key requirement for a patch-clamp recording is to maintain a high seal resistance, which is the resistance between the pipette solution and the surrounding bath solution. In a low-resistance scenario, there will be a current through the seal that is as high, or higher, than the ionic current to be measured, making the measurement of the actual current impossible. Thus, a gigaohm (109 Ω) seal is necessary for the resolution of ionic currents in the order of picoamperes (10−12 A). Three capacitances are present in this circuit: the pipette capacitance (Cp), the whole-cell capacitance (Cm) and the capacitance of the patch of membrane. The latter is very small and thus neglected in the equivalent circuit drawn in Fig. 20.3. The Cp (which depends on the size, shape and material of the patch pipette and also on the height of the bath solution) influences the time-course and the size of the signal, especially when small currents are measured. Patch-clamp amplifiers are equipped with electronics that permit the cancellation of Cp. The whole-cell capacitance Cm is also usually cancelled through the amplifier and/or with the application of a voltage-pulse protocol called positive/negative (P/N) subtraction present in most patch-clamp software. A complication unique to the cell-attached configuration is that the cell is intact; thus the Vm should be factored in unless it is set to 0 mV with a high K+ extracellular solution as described before (see Section III).
IVC2 Excised-Patch
An excised-patch consists of a small portion of membrane sealed to the patch pipette. This translates into the simplest equivalent circuit (see Fig. 20.3). This circuit is comparable to the one described for the cell-attached configuration without Cm (which is very small for a patch), Rm and Vm. Rpatch is the dominant resistor and the circuit monitors the current through this resistor. As discussed above for the cell-attached configuration, Rleak should be very high to reduce current loss. The Rp is generally very low, but it may vary depending on the size of the pipette tip needed. In some instances, such as when the channels are highly expressed, recording must be done from a small membrane patch. Thus, the pipette has a smaller tip and therefore a higher Rp which must be compensated for. Since the cell is absent in the excised-patch configuration, voltage-clamping is simple. The patch under investigation is clamped at the same voltage as Vp, apart from a very small voltage drop over the Rp. As for cell-attached recordings, since single-channel currents are very small, on the order of 1 pA or lower, a requisite of this technique is to minimize the background electrical noise that will otherwise obscure the small single-channel current fluctuations. For the detailed analysis of the various components of the equivalent circuits on the minimization of background noise and the features of the patch-clamp amplifiers built in to control for background noise, the reader is referred to other publications (Levis and Rae, 1998).
IVC3 Whole-Cell Recordings
Whole-cell recording allows the measurement of the overall electrical properties of a cell membrane and, specifically, either the total current through all the channels on the membrane or the membrane potential. This configuration is achieved from the cell-attached configuration by breaking the membrane patch within the pipette tip. The equivalent circuit for the whole-cell configuration is simplified compared to that for the cell-attached patch configuration. More importantly, the Vm is disrupted and it can now be controlled by the experimenter. Furthermore, a very small access resistance (Raccess) replaces the Rpatch of the cell-attached configuration leaving Rp, Raccess and Rm as the relevant resistors in series of this circuit. Rm is the largest resistor and thus the one through which the current is monitored. The same considerations described for the other recording configurations should be given to Rleak, which must be as high as possible to reduce current loss.
Examples of current measurements through the patch and whole-cell configurations are shown in Fig. 20.4.
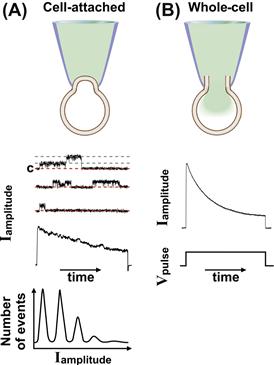
FIGURE 20.4 Current recordings during patch-clamp and whole-cell experiments. (A) Examples of recordings in cell-attached configuration. The cell-attached configuration is reported in the top panel. Middle panel: Single-channel recordings of voltage-dependent outward currents activated by a depolarization step pulse. The voltage pulse is shown in the bottom of panel (B). Three separate recordings of the single-channel fluctuations over time and, below, their corresponding ensemble average current are shown. The closed state (c) and two open states are marked in the upper trace with red and gray dash lines, respectively. Two levels of opening are detectable. Bottom: Amplitude histograms. (B) Example of total current recorded in whole-cell configuration and induced by step depolarization. The whole-cell configuration is reported in the top panel, the outward current in the middle and the pulse protocol in the bottom panel.
V Current Recordings and Analysis
VA Single-Channel Currents
When the appropriate experimental conditions are established, the spontaneous activity of a channel in the patch can be measured. Furthermore, channels can be activated with the appropriate stimulus such as a voltage step/ramp for voltage-gated channels or application of ligands (extracellular or intracellular) for ligand-gated channels. The actual recordings of single-channel activity consist of measurements of the current flowing through an ion channel or a small number of channels over time. Examples of current recordings from a cell-attached patch configuration are shown in Fig. 20.4A. Similar recordings and similar considerations can be applied to excised patches. Basically, recordings of single-channel currents in a patch appear like sudden jumps between current levels and they are generally of small amplitudes (1 pA or less). The jumps correspond to the actual openings and closings of channels which are very rapid stochastic processes due to the protein switching between different conformational states. The small amplitudes and fast kinetics of these events require minimization of the capacitive transients and adequate amplification and filtering to increase the current-to-noise ratio. The noise is reduced using a low-pass filter since the amplitude of noise increases with frequency (Colquhoun and Sigworth, 1995).
Analysis of single-channel data is critical since very little can be concluded from looking at raw recordings due to the random nature of the events. In-depth analyses of single-channel data have been elegantly described elsewhere (Magleby, 1992; Colquhoun and Hawkes, 1995; Colquhoun and Sigworth, 1995). Here we provide a brief description of these methods. The information gained with the analysis of the single-channel recordings are the amplitude(s) of the single-channel currents (Iamplitude), the durations of the close and open periods (dwell time) and the order in which these events occur. The Iamplitude is defined by the difference between two current levels, the close and open states and it is, at least in the simplest cases, nearly constant from one opening to the next. The durations of the open and close events and the order in which they occur are random. Since the number of channels in a patch can be (and often is) more than one, observation and analysis of the current levels provides information not only of the Iamplitude but also of the number and type of channels in the patch. Figure 20.4A shows representative recordings of single currents (middle). Close observation of the uppermost trace indicates that there are at least two channels in the patch. This can be deduced by the two sudden steps from the closed state (C, 0 current) to two open states (indicated by the gray dashed lines) of equal current amplitude. The ensemble average current obtained by averaging a large number of single-channel recordings is shown in the bottom of the traces. A way to display the single-channel data is to report the distribution of amplitudes of each opening separately as amplitude histograms (see Fig. 20.4A, bottom). Current levels can be identified by peaks in the histogram: a peak at the closed level and other peaks at each of the open levels. Generally, the number of peaks at the open levels is indicative of the number of channels in the patch (providing that the probability of channel opening allows multiple channel openings to occur within the time of the experiment), but it could also indicate the subconductance states of a channel as some channels undergo multiple conformational changes which are associated with different current levels. In practice, the peaks in the amplitude histograms are fitted by a Gaussian curve using interactive computer algorithms provided in the patch-clamp analysis software. In case of the recording of a single channel in the patch, the area under the open peak is proportional to the fraction of time spent at the open level or open probability (Po). Po is one of the parameters that can be measured through the dwell time analysis which is applied to study the event transition patterns in single-channel recordings. The Po is the time for which the channel is in the open state relative to the total recording time. The Po is a quick indicator of the activity of the channel and changes in Po are used as measures of the effects of channel activators and blockers. Still, although it can provide such powerful information, the Po does not give any indication of the channel open/closing kinetics. This can be achieved through the analysis of dwell time distributions. Ion channels transition through multiple closed and open states. The times a channel dwells (sojourns) in an open or closed state are of different lengths and they can be grouped in dwell time histograms for each current state. This distribution provides information on the stochastic behavior of a channel. Fitting of the distribution of channel open or close dwell times can be done with exponential equations. The exponential terms and the associated time constants are related to the kinetics of the channel transitioning from the open to the closed state and vice versa. Based on these data, it is possible to build mathematical kinetic models that best describe the possible sequence of opening and closing events that lead to the channel behavior observed in response to a certain stimulus (Avdonin and Hoshi, 1998). Because it is very difficult experimentally to determine the behavior of a single channel, even if the single-channel current can be measured with a good degree of resolution, computer modeling is thus an invaluable tool in understanding ion-channel behaviors.
VB Whole-Cell Currents
While single-channel data can provide a detailed description of the kinetic behavior of a single protein, the sum of the single-channel recordings (the ensemble current) reflects the total current flowing across the channels on the entire membrane surface. The ensemble average current from a series of single-channel recordings is shown in Fig. 20.4A. This current is devoid of contamination of currents from channels other than those in the patch or any current that develops because of the lack of voltage control (described later in this section). The ensemble current is equivalent to the whole-cell currents that can be recorded in whole-cell or perforated-patch configurations (see Fig. 20.4B). Because these configurations measure the currents through all the channels in the membrane, precautions should be taken to isolate the specific channels of interest. This could be achieved through either chemical or electrical interventions as described above in Section IV. Although whole-cell configuration is the configuration of choice for recording macroscopic currents (in the tens to thousands of pA), it presents with a series of limitations that should be considered when establishing and analyzing these types of recordings including rundown, voltage and space control and junction potential.
VB1 Rundown
Rundown is the spontaneous progressive decline of current amplitude over time. In the whole-cell configuration this phenomenon is most likely due to the dilution of regulatory components associated with the channel that occurs with the dialysis of the cytoplasm through the pipette solution. If these factors are known, it is possible to add them to the pipette or bath solution thus maintaining the channel activity. For example, ATP supplementation in the pipette was shown to reduce the rundown rate of Ca2+ currents. Similarly, addition of BAYK8644 to the bath prolongs the activity of voltage-dependent Ca2+ channels recorded in inside-out patches (Ohya and Sperelakis, 1989). The perforated-patch method, which limits the diffusion of large molecules, is used to minimize rundown (see Section III). This method has been also applied to reduce current rundown in single-channel outside-out recordings. Basically, once the perforated-patch configuration is achieved, the membrane patch is excised with a maneuver similar to that used to generate outside-out patches (see Fig. 20.2). The only difference is that when the outside-out configuration is achieved the membrane is separated from the pipette via a perforated membrane and contains an intact cytoplasm where the ion composition can be controlled via diffusion of ions through the pores (Levitan and Kramer, 1990).
VB2 Voltage Control and Space-Clamp
The requirement for a successful whole-cell recording under voltage-clamp conditions is that adequate and homogeneous control of the voltage throughout the whole membrane is achieved and maintained. Poor voltage control distorts the kinetics of the current and will give a wrong current-voltage relationship. An inadequate voltage control occurs when the cell membrane is not “space-clamped”. According to cable theory, in a linear cable the degree of charge dissipation depends on the electrical properties of the cable. Thus, in the whole-cell circuit (see Fig. 20.3C), when voltage is applied, the actual voltage measured at a point distant from the source (in this case at the membrane) is lower because of the dissipation of charges over the distance. Normally, in round cells the resistance of the cytoplasm is small compared to the Rm. In large cells with long and thin branches, the long volumes of the cytoplasm in the branches can be considered like multiple resistances in series which will add to a significant overall cytoplasmic resistance. Since the voltage is clamped over the dominant resistance in a circuit, poor clamping of the membrane portion far from the pipette will occur. Furthermore, in these large and convoluted cells, the overall Cm is much larger, further delaying the changes in clamp voltage. Inadequate space-clamp is a limitation that should be taken into account when deciding if a cell is suitable for whole-cell experiments. In general, small and electrically tight cells will have a better voltage control than cells with membrane folding and low input Rm, while large cells such as neurons that have formed a dendritic network are unsuitable. Such cells can still be patch-clamped via whole-cell current-clamp recording, which is not subject to space clamping issues. A further consideration to be made regards the type of current recorded: some currents such as voltage-activated Na+ currents that have a steep voltage-dependent activation and/or inactivation are more prone to space-clamp distortion than others.
VB3 Junction potential
Junction potentials present a problem in voltage-clamp as they can introduce an error in potential, ultimately shifting the actual voltage at which the cell membrane is clamped. Two types of junction potential should be considered during a voltage-clamp experiment. The solid–liquid junction potential is the potential between the surface of the recording electrode and the surface of the ground. Minimization of this potential can be achieved as described in Section III (Neher, 1992). Furthermore, any residual solid–liquid potential can be neutralized after positioning the patch pipette in the bath through the voltage offset compensation of the amplifier. Another source of junction potential for which there is no analog compensation is the liquid–liquid junction potential which occurs when the pipette solution meets the bath solution. Liquid junction potential in general occurs when two solutions of different composition and concentrations are in contact with each other and it is generated by the different mobility of the ions in the two solutions. The rate of diffusion of the ions depends on their size and charge, temperature, hydration, etc. Normally, the pipette and bath solutions include ions with similar mobilities in which case the junction potential is quite small (2–3 mV) and can be ignored. However, when ions are replaced by ions of corresponding charges but of low mobility (such as Na+ with Tris+ and Cl− with aspartate− or glutamate−), the junction potential is fairly large (in the tens of mV) and cannot be ignored. This potential can be assessed experimentally and the actual voltage imposed to the cell should be corrected for this value. Detailed methods for making corrections have been reported (Barry and Lynch, 1991; Neher, 1992).
Overall, manual patch-clamp has been instrumental in understanding the physiology of ion channels. This technique presents with a high degree of flexibility in the recording configuration which provides control over the recording conditions. Furthermore, a highly detailed analysis of channel behavior can be attained. Unfortunately, at the same time, this is a technically challenging method with low throughput. Recently automated high-throughput versions of the patch-clamp technique have been developed.
VI Automated Patch-clamp
Conventional patch-clamp has been very effective in measuring, with a great deal of confidence, ionic currents and has supplied invaluable information on ion channels. Unfortunately, this technique is a time-consuming technique of limited throughput. It requires a highly trained patch clamper and, because of the technically challenging steps involved in achieving recording configurations, it only allows recording from a limited number (8–10) of cells during a working day. Multicell-based screening strategies have been developed to increase output. The need for high-throughput is particularly felt in the drug discovery field where screening of a large library of compounds is required. This screening is routinely performed to determine the safety profile of a new compound. It is based on the measurement of its effects on the activity of hERG (human ether-a-go-go-related gene) potassium channels which is indicative of the potential cardiac toxicity of the compound in question (cardiac liability screening). The hERG channels have been identified as the molecular targets of drugs associated with an increased risk of cardiac arrhythmias (Pollard et al., 2008). Furthermore, it is used to screen libraries of compounds to find new ion-channel blockers/activators. Ion channels are, in fact, arising as attractive targets for new therapeutic agents. Approximately 13% of all marketed drugs have their mechanism of action attributed to modulation of the activity of either ligand- or voltage-gated channels and two out of 18 new compounds approved by the US Food and Drug Administration in 2006 target ion channels (Overington et al., 2006; Dunlop et al., 2008).
Initially, the high-throughput ion-channel assays used were binding, fluorescence and radioactive assays that allowed monitoring ion fluxes and membrane potential simultaneously in a large number of cells. Unfortunately, these assays present with various limitations including a high degree of false-positive and false-negative hits thus requiring verification using conventional patch-clamping. Because of these limitations, several companies have developed automated patch-clamp systems that allow testing of hundreds of cells each day. Some of these systems utilize conventional electrodes while others are based on planar arrays (Zhao et al., 2008). The latter have transformed the concept of patch-clamping by switching from the “top-down” access to the cell of conventional patch-clamp technique to a “bottom-up” access in plates or chips (Fig. 20.5). In the manual patch-clamp, the glass electrode approaches cells plated on a dish from above, one cell at the time, and forms a gigaseal with the portion of the membrane that does not make contact with the dish. In the automated system, the cells are suspended on a low-loss dielectric surface partitioning two solutions. This surface is etched with micrometer holes that mimic the conventional glass pipette openings. Application of negative pressure captures the cells on these holes and allows formation of the gigaseals. After this, either through the suction system itself or pore-forming agents, the patch of membrane in the hole is disrupted, allowing access to the interior of the cell and current recording either in whole-cell or in perforated-patch configurations. These new automated systems have integrated features such as fluidic control, suction mechanisms and sophisticated acquisition software that allow the serial execution of the experiment.
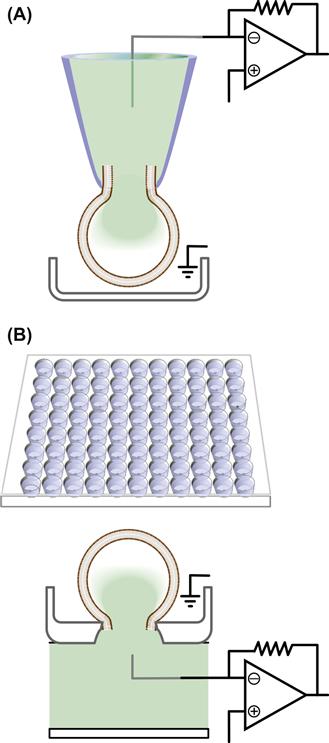
FIGURE 20.5 Comparison of the automated patch and conventional manual patch-clamp settings. (A) Schematic of the “top-down” approach in the conventional patch-clamp. The cell is plated in a dish and contacted by a patch pipette from the top to form the gigaseal and then the whole-cell configuration. (B). Schematic of the automated patch-clamp system performed on a multiwell chip (top). The plates have multiple wells with either single or multiple micrometer holes per well. The cells are seeded in the wells and, upon application of negative pressure, they are sucked in the hole. After this, the portion of the membrane in the hole is disrupted either by further suction or through antibiotics, and the whole-cell configuration is achieved.
The IonWorks platform from Molecular Devices (currently MDS) was the first commercially available automated patch-clamp system, but it used pore-forming agents and did not fully mirror the conventional patch-clamp as it did not form gigaseals (Schroeder et al., 2003). Although this system did not have the low-noise capability of manual patch-clamping and produced lower quality recordings, it was quite effective in reaching high throughput and it has revolutionized the process of screening small compound libraries for ion-channel targeting (John et al., 2007). After this, a second generation of automated patch-clamp systems followed, including PatchXpress, QPatch, Patchliner and Nanion’s Port-a-patch. From single-cell recordings some of these systems have been now optimized for recording from up to 384 cells. A detailed description of these different platforms and their comparison is beside the scope of this chapter (for this please refer to the review of Dunlop et al., 2008) as this field is rapidly changing and new technologies are quickly developing. Our goal here was to introduce the reader to a shift in patch-clamp paradigm that has impacted the way patch-clamping is performed, at least in the pharmaceutical setting. Limitations still exist with these systems that reduce their application in an academic research laboratory.
The major disadvantage of these systems is that they require homogeneous, healthy cells with high expression of the ion channel under investigation which, in reality, only applies to stably transfected cell lines expressing high levels of the ion channels of interest. Primary cell cultures, such as neuronal or cardiac preparations, do not fit these criteria, although robotic multiwell planar patch-clamp has begun to be applied successfully to record native currents from primary cell cultures (Milligan et al., 2009). There are other issues associated with the automated systems that limit their use in an academic research setting where more technically demanding experiments are usually performed. Importantly, since the automated systems only allow limited flexibility, single-channel recordings are still limited to manual patch-clamp and require skilled electrophysiologists. Last, but not least, the costs associated with the platform itself and related consumables such as chips and plates have an impact on the choice of patch-clamp equipment by academic researchers. Future platforms are expected to address these limitations and be useful in both academic and industry settings with a relatively low cost per data point.
Overall, because of these limitations drug screening and cardiac safety profiling are currently the principal applications of these new systems, while conventional patch-clamp still remains the technique of choice for the academic research setting.
Acknowledgments
My thanks go to Dr Steven Kleene who has critically revised and edited this chapter.
BIBLIOGRAPHY
1. Ashcroft F. Ion Channels and Disease: Channelopathies. Boston: Academic Press; 2000.
2. Avdonin V, Hoshi T. Kinetic models and simulation: practical approaches and implementation notes. In: Conn PM, ed. Ion Channels part B, Vol 293. New York: Academic Press; 1998;:724–740.
3. Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117.
4. Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928.
5. Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5.
6. Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion channel mechanisms. In: Sackman B, Neher E, eds. Single-channel Recordings. New York: Plenum Press; 1995;:397–482.
7. Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sackman B, Neher E, eds. Single-channel Recordings. New York: Plenum Press; 1995;:483–587.
8. Dunlop J, Bowlby M, Peri R, Vasilyev D, Arias R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nat Rev Drug Discov. 2008;7:358–368.
9. Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483.
10. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100.
11. Harmar AJ, Hills RA, Rosser EM, et al. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucl Acids Res. 2009;37:D680–D685.
12. Hilgemann DW. The giant membrane patch. In: Sakmann B, Neher E, eds. Single-channel Recording. New York: Plenum Press; 1995;:307–327.
13. Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544.
14. Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159.
15. John VH, Dale TJ, Hollands EC, et al. Novel 384-well population patch clamp electrophysiology assays for Ca2+-activated K+ channels. J Biomolec Screen. 2007;12:50–60.
16. Kelkar DA, Chattopadhyay A. The gramicidin ion channel: a model membrane protein. Biochim Biophys Acta. 2007;1768:2011–2025.
17. Kramer RH. Patch cramming: monitoring intracellular messengers in intact cells with membrane patches containing detector ion channels. Neuron. 1990;4:335–341.
18. Levis RA, Rae JL. Low-noise patch-clamp techniques. Meth Enzymol. 1998;293:218–266.
19. Levitan ES, Kramer RH. Neuropeptide modulation of single calcium and potassium channels detected with a new patch clamp configuration. Nature. 1990;348:545–547.
20. Magleby KL. Preventing artifacts and reducing errors in single-channel analysis. In: Rudy B, Iverson LE, eds. Ion Channels, Vol 207. New York: Academic Press; 1992.
21. Milligan CJ, Li J, Sukumar P, et al. Robotic multiwell planar patch-clamp for native and primary mammalian cells. Nat Protocols. 2009;4:244–255.
22. Neher E. Correction for liquid junction potentials in patch clamp experiments. Meth Enzymol. 1992;207:123–131.
23. Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976;260:799–802.
24. Nilius B. A special issue on channelopathies. Pflügers Arch Eur J Physiol. 2010;460:221–222.
25. Nilius B, Eggermont J, Voets T, Droogmans G. Volume-activated Cl- channels. Gen Pharmacol Vasc Syst. 1996;27:1131–1140.
26. Ohya Y, Sperelakis N. Modulation of single slow (L-type) calcium channels by intracellular ATP in vascular smooth muscle cells. Pflügers Arch. 1989;414:257–264.
27. Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there?. Nat Rev Drug Discov. 2006;5:993–996.
28. Parekh AB, Terlau H, Stuhmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993;364:814–818.
29. Pollard CE, Valentin JP, Hammond TG. Strategies to reduce the risk of drug-induced QT interval prolongation: a pharmaceutical company perspective. Br J Pharmacol. 2008;154:1538–1543.
30. Rae JL, Levis RA. Fabrication of patch pipets. Curr Prot Neurosci, John Wiley & Sons, Inc. 2004;:6.3.1–6.3.32.
31. Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400.
32. Sakmann B, Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol. 1984;46:455–472.
33. Schroeder K, Neagle B, Trezise DJ, Worley J. IonWorks™ HT: a new high-throughput electrophysiology measurement platform. J Biomolec Screen. 2003;8:50–64.
34. Sharif-Naeini R, Dedman A, Folgering J, et al. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflügers Arch Eur J Physiol. 2008;456:529–540.
35. Stuhmer W. Electrophysiologic recordings from Xenopus oocytes. Meth Enzymol. 1998;293:280–300.
36. Stuhmer W, Methfessel C, Sakmann B, Noda M, Numa S. Patch clamp characterization of sodium channels expressed from rat brain cDNA. Eur Biophys J. 1987;14:131–138.
37. Wu L-J, Sweet T-B, Clapham DE. International Union of Basic and Clinical Pharmacology LXXVI Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404.
38. Zhao Y, Inayat S, Dikin DA, Singer JH, Ruoff RS, Troy JB. Patch clamp technique: review of the current state of the art and potential contributions from nanoengineering. Proc Inst Mech Eng J Nanoeng Nanosyst. 2008;222:1–11.