Chapter 40
Infrared Sensory Organs
Chapter Outline
III. Nature of the Stimulus: What is Infrared (IR) Radiation?
I Summary
Certain snakes, such as pythons and rattlesnakes, utilize infrared radiation as well as visible light to detect prey. Infrared radiation emitted from warm objects is captured mainly by specialized sensory structures in the snake head called pit organs. The anatomy of pit organs is such that a crude image of the infrared-emitting object is cast upon a thin membrane within the organ. This infrared image locally warms the thin tissue which is innervated by heat-sensitive axon terminals of trigeminal sensory ganglion cells. Molecular profiling of snake trigeminal ganglion neurons indicates that a unique variant of the heat-sensitive transient receptor potential cation channel, TRPA1, is the heat-transducing protein found in infrared sensory neurons.
II Introduction
Infrared radiation is an important and ubiquitous component of the diverse physical environments in which animals live. All warm objects radiate infrared energy. Naturally-occurring infrared sources include terrestrial, atmospheric and astronomical bodies. Animals, especially ectothermic species, use radiant infrared energy to assist in maintaining their body temperature and must avoid excess infrared radiation to avoid overheating. In a few species, infrared radiation is also used in a novel manner – to form spatial images of the environment and to hunt prey. These animals possess specialized infrared-receptive sensory organs, the topic of this chapter.
Infrared-receptive organs occur in both vertebrate and invertebrate animals. Buprestid beetles, including the European fire beetle Melanophila acuminata, have specialized infrared receptive organs on the ventral surfaces of their abdomens (Evans, 1964). Melanophila and similar beetles congregate in large numbers at forest fire sites to deposit eggs in recently burned wood. They likely use chemoreceptors and mechanoreceptors (to detect wind direction) to guide them to sites of fires, and their infrared detectors to identify potential egg-laying sites of appropriate temperature. The deep-sea shrimp Rimicaris exoculata also possesses an elaborate organ capable of detecting 350°C “black smoker” thermal vents in the deep Atlantic Ocean (Pelli and Chamberlain, 1989). Their infrared sensory detection system may help the shrimp maintain close proximity to life-sustaining thermal vents in the deep sea without directly contacting the steep thermocline (350°C to 2°C over a 2–3 cm lateral distance) adjacent to the thermal vent. The common vampire bat (Desmodus rotundus) may possess infrared radiation detectors, but its infrared sensory system has been little investigated. Behavioral experiments show that vampire bats can detect infrared radiation as low as 50 μW/cm2 and thus should be able to detect mammalian prey at distances of up to 16 cm (also see Campbell et al., 2002). Vampire bats may use their infrared detection system to locate suitable prey.
However, by far the best-studied infrared sensory detectors are those of snakes. As stated previously, ectothermic animals, such as snakes, rely on infrared radiation to regulate their body temperature. In some snakes (Boidae, which include boa constrictors and pythons, and Crotalinae, which include rattlesnakes and water moccasins), infrared radiation is also used for accurate and precise targeting of prey. These snakes preferentially feed on endothermic (warm-blooded) prey. In addition to mediating predatory behavior, the infrared imaging system also mediates defensive behavior (Van Dyke and Grace, 2010). Unlike other infrared-sensing animals, boid and crotaline snakes are the only animals known to form spatial images of their environments using extremely sensitive infrared-receptive organs.
Boid and crotaline snakes are efficient predators. While vision may be useful for some aspects of their predatory behaviors, infrared imaging alone can allow accurate prey targeting. Snakes experimentally blinded by occluding both eyes and snakes born without eyes are capable of accurately placing strikes at mammalian prey (Kardong and Mackessey, 1991). Infrared-imaging snakes can target based upon vision alone but when one eye is occluded, strike-targeting performance is indistinguishable from that of normally sighted snakes (Grace and Woodward, 2001). Moreover, snakes readily strike their prey regardless of visual contrast (black versus white mice against a black background) (Grace et al., 2001) and they preferentially target the warm aspect of thermal differentials (Van Dyke and Grace, 2010). Thus, vision and infrared imaging appear to be redundant targeting systems and the infrared imaging system can compensate for loss of visual information.
III Nature of the Stimulus: What is Infrared (IR) Radiation?
Radiant infrared energy is a region of the spectrum of electromagnetic radiation that lies between visible light (wavelengths of 400–750 nm) and microwaves (wavelengths of ≈1 mm to 1 cm). The English astronomer Sir William Herschel discovered infrared radiation at the beginning of the 19th century. Herschel was exploring the ability of light passing through a prism to heat a thermometer that he positioned at different points in the color spectrum. Herschel noticed that visible light was capable of heating the thermometer to some extent but that this effect was much more pronounced when the thermometer was placed beyond the red end of the spectrum. Electromagnetic radiation in the infrared region is absorbed as the radiation passes through objects in its pathway. Most of this absorbed infrared energy is dissipated by the resulting increase in molecular collisions (i.e. heat). Infrared radiation penetrates body tissues deeper than does visible light, and thus IR generators produce the so-called deep heat used in physical therapy. Any object above absolute zero radiates electromagnetic waves in the infrared, the more so as it is warmed. With increasing heat, an object radiates increasingly shorter wavelengths. Objects at room temperature radiate entirely in the IR and not at all in the visible light spectrum. At 500°C, objects begin to radiate also in the visible spectrum and they appear dull red. Endothermic (warm-blooded) animals, such as rats and mice, radiate maximally in the infrared region at a wavelength of approximately 10 μm. IR radiation can thus be used to detect objects in the absence of visible light (i.e. in the dark) with an object’s detection being enhanced by having a temperature that contrasts with its background temperature.
Water vapor and carbon dioxide in the atmosphere absorb and severely attenuate the transmission of certain wavelengths of the IR spectrum and allow other wavelengths to pass. That is, there are windows for IR transmission in the atmosphere. One of these windows is in a region where certain IR-sensitive photographic emulsions absorb well, thereby allowing IR images of landscapes and cityscapes to be collected by satellite cameras. Another atmospheric transmission window includes the region around 10 μm, i.e. the region of the IR spectrum in which endothermic animals maximally radiate. IR sensory organs in snakes are believed to have a maximum sensitivity in this region (Grace et al., 1999) and thus are well suited to detect endothermic prey.
IV Infrared-Sensitive Pit Organs in Snakes
Infrared sensory organs in snakes consist of pit organs. Pit organs are invaginations within or between scales in the head. These invaginations are up to 3–4 mm wide and 3–4 mm deep. In boid snakes (e.g. pythons), the pit organs are arrayed in rows within labial scales of both the upper and lower jaws.1 In crotaline snakes (e.g. rattlesnakes), there is a single facial pit organ on each side of the face between the nostril and eye (Fig. 40.1). There may be additional infrared-sensitive receptor terminals in the oral cavity (especially the palate) that may be important for guiding the predatory strike when the snake’s mouth is open and fangs extended (Dickman et al., 1987) (Fig. 40.2).
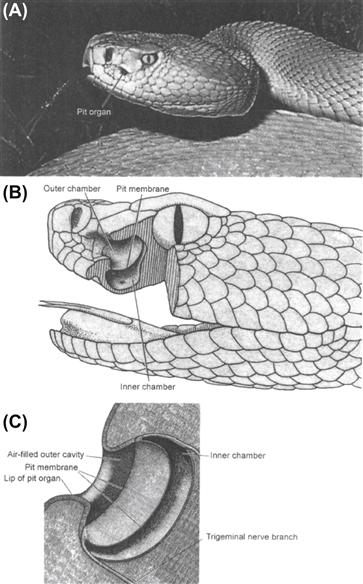
FIGURE 40.1 Infrared pit organ in the rattlesnake. (A) Photograph of the head of a rattlesnake (Crotalus viridis) showing the distinctive pit organ between the nostrils and the eye. (Photograph by Bill Love from The World’s Most Spectacular Reptiles and Amphibians, 1997. World Publications, Tampa, FL.) (B) Schematic drawing of the internal structure of the infrared sensory organ in rattlesnake (Crotalus viridis). (Reproduced fromGamow and Harris, 1973 .) (C) Higher magnification schematic view of the rattlesnake pit organ. (Reproduced from Newman and Hartline, 1982.)

FIGURE 40.2 Photograph of a rattlesnake striking at prey, demonstrating how infrared sensing receptors in the oral cavity may play a role in guiding the strike. (Photograph by Pete Carmichael from Florida’s Fabulous Reptiles and Amphibians, 1991. World Publications, Tampa, FL.)
A dense plexus of infrared-receptive primary sensory afferent nerve terminals is present in pit organs. In boid snakes, these terminals lie in the epidermis just under the surface of the pit organ. Immediately below the layer of infrared-receptive neuronal terminals is a thick capillary network. In crotaline snakes, a thin (15 μm) membrane stretches across the cavity of the pit (see Fig. 40.1B). Infrared-receptive nerve terminals are embedded within this membrane, also over a thick capillary bed. Infrared receptor terminals and the dense capillary plexus are mainly confined to the pit organs.2
The anatomical configuration of the pit organ, namely that the receptive tissue is stretched across the bottom of a cavity that has a narrowed opening to the outside environment, provides the snake with a means to direct its pit organs towards prey. Thus, the pit organs can provide information about the location of an infrared-emitting source in front of the snake. A distinct image, per se, such as a pin-hole camera might afford, is not formed in the pit organ, but the shadows cast by the infrared radiation passing through the narrowed opening into the pit project a crude approximation of the infrared source onto the sensory terminals. This “image” is conveyed to the snake’s brain, much like visual information, to establish a spatiotopic map on the optic tectum (see Section IVA).
Infrared receptor terminals are embedded within the thin membrane that is stretched across the bottom of the pit organ. These terminals form large, highly-branched, entwined clusters surrounding an aggregate of Schwann cells. Collectively, this structure is called a terminal nerve mass (≈30–100 μm diameter) (Fig. 40.3). Each terminal branch is densely packed with mitochondria. Clusters of electron-lucent vesicles are present just beneath the plasma membrane. These specialized endings are the sites where infrared transduction takes place (see Section IVB). A complex and extensive bed of capillary loops embraces the terminal nerve masses. This capillary bed is believed to have a dual function: (1) to provide a robust oxygen supply for the mitochondria in the receptor terminals; and (2) to act as a thermal exchanger to carry away excess heat and prevent the epithelial tissues from retaining temperature increases produced by infrared radiation (Amemiya et al., 1999). The thermal exchange function of the capillary bed, combined with the low heat capacity of the thin epithelial tissues in the pit organs, may ensure that the sensory receptors are capable of responding to the small transient temperature changes that are expected to occur when an infrared source (e.g. prey) passes in front of the pit organ (discussed in Section IVB).
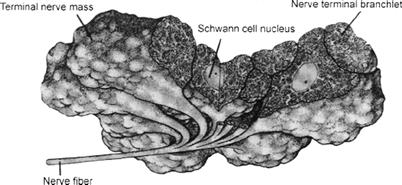
FIGURE 40.3 Reconstruction of the nerve terminals for infrared sensing trigeminal sensory axons. The branches form nerve terminal masses that include Schwann cells and receptor terminals. The nerve terminals are filled with numerous mitochondria. (Reproduced from Terashima et al., 1970.)
IVA Innervation and Central Nervous System Pathways of Pit Organs
Pit organs are innervated by branches of the trigeminal nerve (reviewed by Molenaar, 1992). These same nerve branches also subserve mechanoreception, nociception and thermoreception in the facial region of reptiles, mammals and other vertebrates. Cell bodies for the infrared-sensitive nerve terminals are located in sensory ganglia of the trigeminal nerve.
In infrared-sensitive snakes, sensory neurons in the trigeminal ganglia synapse with neurons in two distinct nuclei located in the hindbrain – the nucleus of the solitary tract (NST) and a unique group of cells termed the nucleus of the lateral descending trigeminal tract (nLTTD) (see Molenaar, 1992). Neurons in the NST subserve such functions as mechanoreception, thermoreception and nociception, as in other vertebrates. However, the nLTTD is found only in infrared-sensing snakes and is responsible for processing information specifically from the pit organs (Stanford et al., 1981). This nucleus is present in all infrared-sensitive snakes studied thus far, and is absent from animals that are not infrared-sensitive.
Ultimately, infrared information from the nLTTD reaches the optic tectum. Spatial relationships among the axons and synapses of the infrared receptor pathway are preserved such that a systematic topographical map of the receptive membrane in the pit organ is projected onto the tectum, much like the orderly retinotopic mapping of visual information that also occurs in the optic tectum. Indeed, the infrared sensory map overlies and is aligned with the retinotopic map. Individual neurons in the optic tectum may be excited by visual and infrared stimuli arising from approximately the same point in space3 (Hartline et al., 1978). Thus, snakes possessing infrared-sensitive pit organs view their environments simultaneously using two distinct regions of the electromagnetic spectrum.
IVB Infrared Sensory Transduction
Infrared-detecting pit organs in snakes are exquisitely sensitive and selective (Bullock and Diecke, 1956; Gamow and Harris, 1973; de Cock Buning et al., 1981a,b; Newman and Hartline, 1982; Hartline, 1999). The adequate stimulus is a sharp thermal gradient, or contrast, in the surrounding environment, such as an endothermic animal (e.g. a mouse) against thermoneutral vegetation, or cool prey (e.g. a frog) against a cooler background (e.g. a pond). The temperature of the snake’s body itself is not a significant factor, nor is the average temperature of the surroundings. Air temperature is irrelevant; the pit organ responds only to radiant energy in the infrared, not conductive energy transferred by warm air. Thus, a snake is an effective hunter in the heat of midday as well as in the cool of the night. The sensitivity of pit organs is such that a pit viper can use its infrared detector to orient to and accurately strike a rat at a distance of about 0.5 m. At this distance, a rat with a surface temperature 10°C above background emits infrared radiation (≈10 μm wavelength) that would be expected to warm the pit organ tissues by only 0.001°C (Bullock and Diecke, 1956). This low threshold for temperature detection was also observed experimentally. Researchers infused water of different temperatures into pit organs and measured threshold nerve responses to temperature changes as small as 0.003°C in 0.06 s (Bullock and Diecke, 1956). Rapid temperature increases as small as 0.1°C in the pit organ elicit robust responses (i.e. well above threshold detection) in the receptors (de Cock Buning et al., 1981a, b).4
Recent studies at the molecular level have tremendously illuminated how infrared radiation can be transduced into neuronal excitation. Gracheva et al. (2010) reported that rattlesnake trigeminal sensory ganglia express a unique variant of the heat-activated cation channel, TRPA1 (Fig. 40.4). When expressed in heterologous systems, rattlesnake TRPA1 channels are exceptionally sensitive to heat and begin conducting at or above 28°C. In contrast, TRPA1 channels from snakes lacking pit organs are not activated until temperatures reach 37°C.
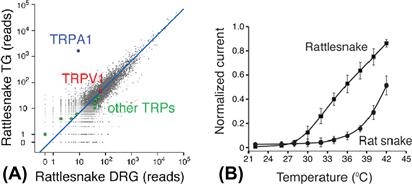
FIGURE 40.4 Rattlesnake TRPA1 channels are highly expressed in sensory neurons innervating the pit organ and are activated by temperatures above 28°C. (A) Quantification of genes expressed in rattlesnake ganglia that innervate the pit organ (trigeminal, TG) relative to rattlesnake dorsal root ganglia (i.e. non-pit organ sensory neurons). Axes show number of mRNA-Seq reads from snake ganglia that align to the chicken proteome. TRPA1 and another heat-sensitive channel, TRPV1, are highlighted, as are other TRP channels. Blue line indicates similar expression levels in the two samples. (B) Functional analysis of snake TRPA1 channels. The graph shows the relative heat response profiles of infrared-sensitive rattlesnake and infrared-sensitive rat snake channels expressed in oocytes (response at each temperature was normalized to maximal response at 45°C). (Modified from Gracheva et al., 2010.)
Gracheva et al. (2010) concluded that rattlesnake TRPA1 is the pit organ receptor activated by heat that is generated in the pit membrane during absorption of infrared radiation.5
Interestingly, the pit organs in pythons are exquisitely sensitive to gentle touch in addition to infrared radiation (de Cock Buning et al., 1981b). This observation may be explained by the expression of TRPA1 in trigeminal ganglion cells innervating the pit organ. TRPA1 has been identified with mechanotransduction in other species, including mice (Vilceanu and Stucky, 2010).
BIBLIOGRAPHY
1. Amemiya E, Nakano M, Goris RC, et al. Microvasculature of crotaline snake pit organs: possible function as a heat exchange mechanism. Anat Rec. 1999;254:107–115.
2. Bullock TH, Diecke EPJ. Properties of an infra-red receptor. J Physiol. 1956;134:47–87.
3. Campbell AL, Naik RR, Sowards L, Stone MO. Biological infrared imaging and sensing. Micron. 2002;33:211–225.
4. de Cock Buning T, Terashima S, Goris RC. Crotaline pit organs analyzed as warm receptors. Cell Mol Neurobiol. 1981a;1:69–85.
5. de Cock Buning T, Terashima S, Goris R. Python pit organs analyzed as warm receptors. Cell Mol Neurobiol. 1981b;1:271–278.
6. Dickman JD, Colton JS, Chiszar D, Colton CA. Trigeminal responses to thermal stimulation of the oral cavity in rattlesnakes (Crotalus viridis) before and after bilateral anesthetization of the facial pit organs. Brain Res. 1987;400:365–370.
7. Evans WG. Infra-red receptors in Melanophila acuminata DeGeer. Nature. 1964;202:211.
8. Gamow RI, Harris JS. The infrared receptors of snakes. Sci Am. 1973;228:94–100.
9. Grace MS, Church DR, Kelley C, Cooper TM. The python pit organ: immunocytochemical and imaging analysis of a sensitive natural infrared detector. Biosens Bioelectron. 1999a;14:53–59.
10. Grace MS, Woodward OM. Altered visual experience and acute visual deprivation affect predatory targeting by infrared-imaging Boid snakes. Brain Research. 2001;919:250–258.
11. Grace MS, Woodward OM, Church DR, Calisch G. Prey targeting by the infrared-imaging snake python: effects of experimental and congenital visual deprivation. Behav Brain Res. 2001;119:23–31.
12. Gracheva EO, Ingolia NT, Kelly YM, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011.
13. Hartline PH. Infrared sense. In: Adelman G, Smith BH, eds. Encyclopedia of Neuroscience. New York: Elsevier; 1999;:957–959.
14. Hartline PH, Kass L, Loop MS. Merging of modalities in the optic tectum: infrared and visual integration in rattlesnakes. Science. 1978;199:1225–1229.
15. Kardong K, Mackessey G. The strike behavior of a congenitally blind rattlesnake. J Herpetol. 1991;25:208–211.
16. Molenaar GJ. Anatomy and physiology of infrared sensitivity of snakes. In: Gans C, Ulinski ES, eds. Biology of the Reptilia. Chicago: University of Chicago Press; 1992;:367–453.
17. Newman EA, Hartline EH. The infrared “vision” of snakes. Sci Am. 1982;246:116–127.
18. Pelli DG, Chamberlain. SC. The visibility of 350°C black-body radiation by the shrimp Rimicaris exoculata and man. Nature. 1989;337:460–461.
19. Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336.
20. Stanford LR, Schroeder DM, Hartline EH. The ascending projection of the nucleus of the lateral descending trigeminal tract: a nucleus in the infrared system of the rattlesnake (Crotalus viridis). J Comp Neurol. 1981;201:161–174.
21. Terashima S, Goris RC, Katsuki Y. Structure of warm fiber terminals in the pit membrane of vipers. J Ultrastruct Res. 1970;31:494–506.
22. Van Dyke JU, Grace MS. The role of thermal contrast in infrared-based predatory targeting behavior by the copperhead, Agkistrodon contortrix. Anim Behav. 2010;79:993–999.
23. Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One. 2010;5:e12177.
1 Boid snakes that do not have obvious pit organs possess clusters of infrared-receptive neuronal terminals in these same labial areas.
2 The oral cavity of rattlesnakes is also sensitive to infrared and possesses dense ramifications of infrared-receptive sensory nerve terminals, similar to those in pit organs (Dickman et al., 1987).
3 The two maps – visual and infrared – do not appear to overlap well in the peripheral fields of view. The overlap is more precise in the central field, i.e. directly in front of the snake.
4 Bullock and Diecke (1956) and de Cock Buning et al. (1981a, b) alike point out that when factors such as the relative thickness of the overlying tissues, the low heat capacity of the thin pit organ membrane and the density of innervation are taken into account, the threshold for snake pit organs is similar to that of other warm receptors, including human cutaneous thermoreceptors. One reason for the apparent higher sensitivity to small temperature increases in snakes is that the density of innervation in pit organs is very high and the epithelial tissue overlying the sensory nerve terminals is extremely thin. Thus, infrared radiation can readily and rapidly increase the temperature around a dense plexus of sensory nerve terminals.
5 Despite these impressive findings, the identification of rattlesnake TRPA1 as the infrared sensor is not entirely without question. There is a discrepancy between the operating range for rattlesnake TRPA1 channels and the temperature response of the pit organ. Rattlesnake TRPA1 channels expressed in HEK293 cells or Xenopus oocytes show a monotonic increase in activity with a threshold ≈28° and continuing to respond ≥42°, roughly comparable to the temperature sensitivity of trigeminal ganglion cells isolated from rattlesnakes (Gracheva et al., 2010). In marked contrast, the effective temperature range for the pit organ membrane, measured by the impulse activity of axons innervating the pit organ, is an inverted “U” shape with a threshold of ≈14°C, a peak at ≈25°C and a return to inactivity at ≈33°C (de Cock Buning et al., 1981a). This discrepancy may be explained by technical differences such as properties of TRPA1 in native versus heterologous expression systems, or species differences, or it may indicate that additional molecular mechanisms are involved in infrared detection. In this light, it may be pertinent that a light-sensitive G protein-coupled receptor, rhodopsin, was recently shown to be co-expressed with TRPA1 in temperature-sensitive neurons of Drosophila larvae (Shen et al., 2011). Remarkably, the two proteins appear to function in tandem as a temperature sensor, in the vicinity of 18°C.