11.1 Introduction
Traumatic brain injury (TBI) is the most common cause of trauma-related death and disability in industrialized countries [1]. Following primary injury subsequent mechanisms like cerebral inflammation, apoptosis and malperfusion lead to progression of secondary brain damage [2]. After initial treatment in the emergency room and operating theater, severely injured patients may require intensive care treatment. Therefore, TBI patients should be transferred directly to a neurosurgical center to avoid delay and to prevent secondary transfer associated with worsening of outcome [3]. Depending on the initial presentation (Glasgow Coma Scale (GCS), severity of TBI, and concomitating injuries), typical indications for admission to the intensive care unit (ICU) are severe TBI (initial GCS <9, focal lesion in CT scan), need for ventilation, sedation, or invasive ICP monitoring.
11.2 Causes of Poor Outcome
Treatment has improved substantially in the last 60 years and resulted in a reduction in TBI-associated mortality [4]. This is mostly related to advances in general ICU treatment algorithms and special treatment guidelines, e.g., by the Brain Trauma Foundation (see update 2017 [5]), who defined management standards for TBI patients to limit secondary brain damage. Most recent analyses of patient outcomes failed to demonstrate substantial improvement in outcome in level I trauma centers [6], which indicates that new and more effort has to be put into improvement of TBI care. Although underlying mechanisms of secondary brain damage are well studied and treated in experimental studies, this knowledge has not yet been successfully transferred to clinical settings [7]. This is in part related to the highly variable clinical presentation of TBI with differences in trauma mechanism, type and extent of brain lesions (e.g., focal, diffuse), and presence of additional organ injuries. To achieve optimal outcome, key principles of care are therefore focusing on the individual normal physiology (“5 normos”): normotension, normoxia, normocapnia, normothermia, and normoglycemia. In TBI patients special attention needs to be put on cerebral perfusion and cerebral oxygenation. To maintain and monitor cerebral perfusion, the cerebral perfusion pressure (CPP) was defined as surrogate parameter, which is calculated as the difference between mean arterial pressure and intracranial pressure. Until recently, the focus was to maintain CPP between 60 and 70 mmHg and to keep intracranial pressure (ICP) below 20 mmHg. Unfortunately, measures to reduce ICP may have deleterious side effects, because they can cause cerebral vasoconstriction or arterial hypotension thereby reducing brain perfusion and putting brain tissue at risk for insufficient oxygenation. Focusing on ICP only by, e.g., craniectomy failed to improve outcome [8]. Recently, a phase II randomized controlled trial (RCT) with a combined strategy focusing on both ICP and brain tissue oxygen levels indicated reduced mortality and better outcome compared to ICP control only [9].
Non-neurological and neurological complication after TBI
Non-neurological complication | Neurological complication |
|---|---|
Pulmonary infections, ARDS | Seizures |
Delirium | Ischemic stroke |
Decubitus | Hydrocephalus |
Thrombosis, pulmonary embolism | Intracranial bleeding |
Acute kidney failure or dysregulation (diabetes insipidus, SIADH) | |
Wound infections | |
Myocardial infarction | |
Coagulopathy |
11.3 Intracranial Pressure and Regulation of Cerebral Perfusion
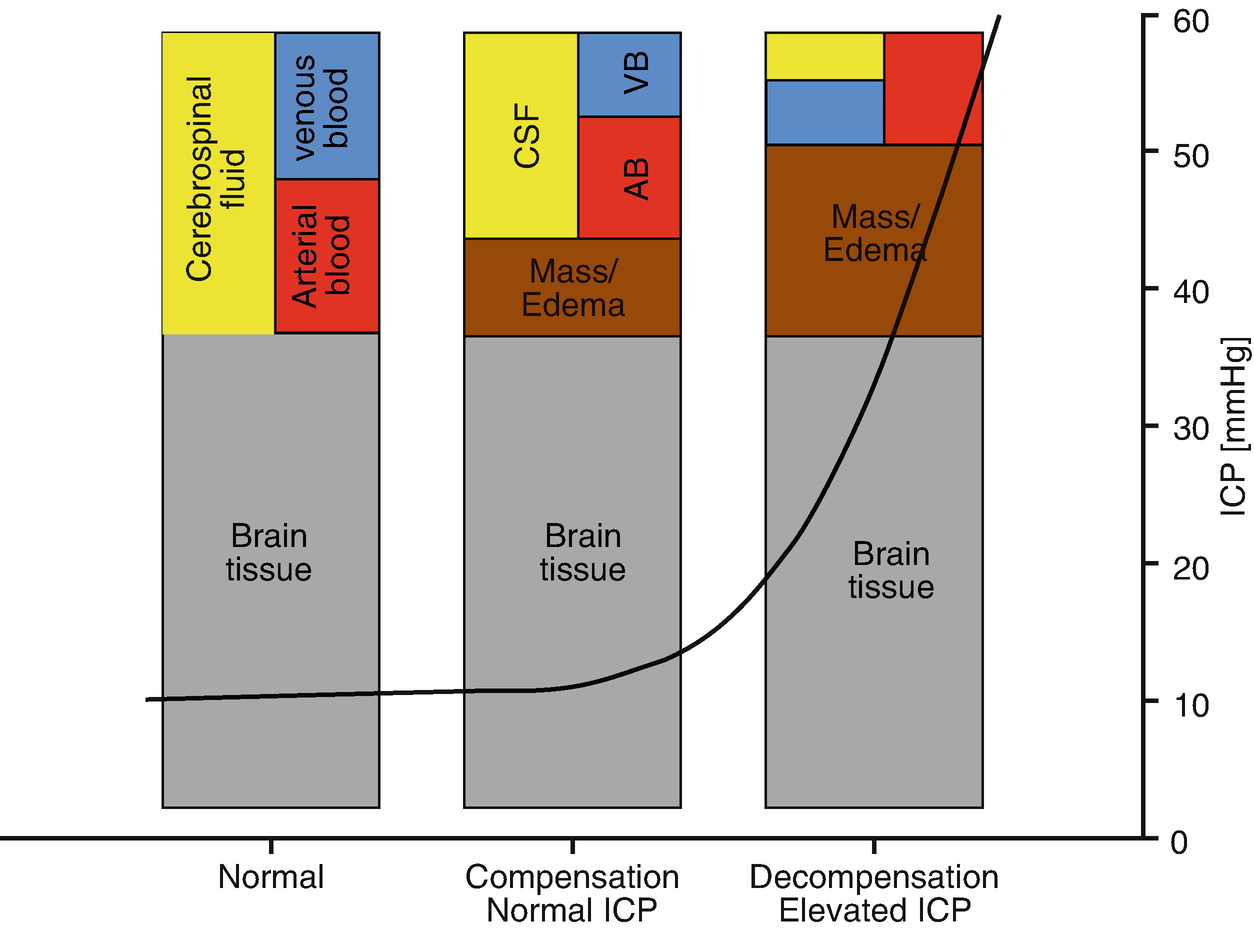
Monro–Kellie doctrine
Factors influencing intracranial pressure (ICP)
Factors reducing ICP | Factors increasing ICP |
|---|---|
• Reduction of intracranial liquor volume (external ventricular drainage) • Reduction of brain tissue volume Osmodiuretics Corticosteroids Hypertonic saline • Reduction of intracranial blood volume Position of the head (elevation) Reduction of cerebral perfusion (arterial pCO2⇓, pO2⇓, hypnotics) Evacuation of intracranial hematoma Hypothermia • Decompressive craniectomy | • Drugs causing: Histamine liberation, cerebral vasodilation, cerebral metabolism⇑ • Central venous pressure Limitation of venous return Transmission of central venous pressure to intracranial veins • Positive end expiratory pressure (PEEP) No clear data, generally believed to be save: 10–15 cm H2O • Fever (increase of metabolism and CBF) |
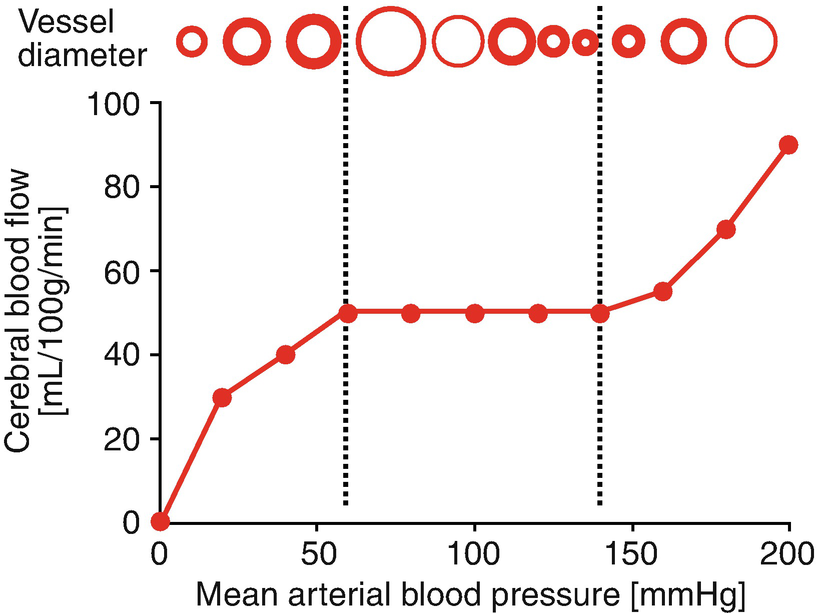
Cerebral autoregulation
11.4 Critical Care Treatment of the Head-Injured Patient
11.4.1 Cerebral Hemodynamics and Intracranial Hypertension
The primary goal of the treatment is to maintain a sufficient oxygen supply for the brain. To maintain best cerebral oxygen supply, target CPP limits are defined in most ICUs. To determine CPP it is required to measure both mean arterial blood pressure (MAP) and ICP.
Side effects of ICP control
Side effects of interventions |
• Sedation and ventilation Ventilator associated pneumonia, delirium • ICP probe/external ventricular drainage Infections • Hyperosmolar therapy Volume overload, electrolyte disbalance • Hyperventilation, hypocarbia Cerebral vasoconstriction, ischemia • Decompressive craniectomy Secondary hematoma, bleeding, hydrocephalus • Hypothermia/metabolic suppression Arterial hypotension, coagulopathy, pneumonia • Barbiturate sedation Arterial hypotension, pneumonia |
Measures to increase cerebral oxygenation
Factors increasing brain oxygen levels |
• Correct mechanical cause Head position |
• Increase supply (oxygen delivery) Increase blood pressure Normalize pCO2 to physiologic level Increase FiO2 Increase hematocrit Increase cardiac output |
• Decrease demand (cerebral metabolism) Increase anesthetic depth Decrease body temperature |
The second parameter of CPP, the MAP, is also by itself an important factor for survival and represents the available pressure to allow perfusion of all organs. Current trauma guidelines therefore recommend to maintaining systolic blood pressure (SBP) at ≥100 mmHg for patients 50–69 years of age or at ≥110 mm Hg or above for patients with the age of 15–49 or over 70 to decrease mortality and improve outcomes [5].
Due to individual differences and limited clinical data, the lower and upper limits of CCP are in constant debate. In the last years, the view on CPP has changed. The current 4th guideline of the Brain Trauma Foundation (BTF) recommends a range between 60 and 70 mmHg based on two clinical studies suggesting a favorable outcome [14, 15]. However, a study from 1999 demonstrated more complications with CCP target above 70 mmHg [16]. Interestingly, determining a general optimal CCP by means of autoregulation analysis in TBI patients by different institutions resulted in highly different numbers [11]. It is therefore debatable if CPP targets should be individualized by personalized analysis of the cerebral autoregulation to determine the optimal CPP level in single patients at different time points. The principal idea is to avoid hypoperfusion by adjusting the CPP to the optimal autoregulated level and to avoid hyperperfusion or vasogenic brain edema formation by preventing high CPP levels in patients with abrogated autoregulation. To determine a tailored blood pressure and CPP range based on individualized limits of autoregulation, MAP needs to be correlated online with intracranial pressure, cerebral perfusion, or measures of cerebral oxygenation using, e.g., cerebral oximetry (NIRS, rSO2), brain tissue O2 levels (ptO2), or jugular venous oxygen saturation. So far, no prospective data is available demonstrating the superiority of an individualized CPP-based treatment compared to maintaining generalized CPP targets.
11.4.2 Interventions to Prevent, Treat, and Aggressively Treat ICP Increase
The basis of intervention in most neuroICUs is the continuous monitoring of the patients to guide treatment decisions. The most prominent parameters are the intracranial pressure and blood pressure. Unfortunately, there is no clear evidence from literature to give a level 1 recommendation on when and whom to place an ICP probe or intraventricular drainage. The current 4th BTF guideline gives only a level 2B recommendation to place an ICP monitoring based on data demonstrating reduced in-house or 2-week mortality [17]. Originally (3rd BTF guideline) and current clinical practice is to monitor ICP in patients with severe TBI and abnormal CT findings (hematomas, contusions, swelling, herniation, or compressed basal cisterns) or in patients with severe TBI, normal CT scan, and two or more features including age above 40, unilateral or bilateral motor posturing, or systolic blood pressure (BP) <90 mmHg. In general, ICP monitoring is placed for better care and to guide basic interventions to prevent ICP increase in all TBI patients requiring sedation and therefore not allowing continuous clinical examination of neurological function. The principal steps in patients with severe TBI are [13] (I) basic treatment to prevent rise in ICP by sedation, intubation with normocapnic ventilation, and avoidance of pyrexia; (II) hyperosmolar therapy and cerebrospinal fluid (CSF) drainage to treat rise in ICP; and (III) metabolic suppression, hypothermia, decompressive craniectomy, and hypocapnia to treat persistent and severe rise in ICP. Importantly, all these strategies have side effects and put the patients at beforehand described risks (Table 11.3).
11.4.3 Level I: Basic Treatment
In addition to the three basic interventions (sedation, normocapnic ventilation, avoidance of pyrexia) to control ICP, 25° to 30° elevation of the head is routinely performed based on clinical experience. However, a recent Cochrane analysis did not show strong evidence for the efficacy of this intervention [18]. Future studies are required to determine if and when different backrest positions affect outcome. Prophylactic cooling has been investigated in several experimental and clinical studies to reduce cerebral metabolism and to protect the brain from secondary brain damage. Unfortunately, prophylactic use within the first 48 h after injury showed conflicting results in clinical trials. The body of evidence for beneficial effects is rather low due to various methodological limitations, low sample size, missing impact on mortality, and no clear influence on neurofunctional outcome. Due to side effects of hypothermia, the use of cooling is limited to therapy-refractory ICP increase.
11.4.4 Level II: Hyperosmolar Therapy and CSF Drainage
ICP levels of 22 mmHg and above are level 2B trigger thresholds for therapeutic interventions. However, it remains unclear which extend and duration in ICP increase, e.g., during wake-up phases, should be tolerated. Also, the speed required to successfully limit brain damage by escalating measures to treat rise in ICP is not clear. First-line intervention is the intravenous hyperosmolar therapy, which has become routine in the management of intracranial hypertension and herniation syndromes. Mannitol and hypertonic saline are routinely employed as hyperosmolar agents for ICP reduction. The selection of a specific substance is mostly dependent on the patients’ needs, as both agents reduce intracranial pressure to a great extent by increasing plasma osmolarity, increasing the osmotic gradient between the brain and blood, shifting water from the brain tissue to the bloodstream, expansion of plasma volume and reduction of blood viscosity, and increasing MAP and thereby improving microcirculation. The use of mannitol, however, is limited by the decreased efficacy after repeated administration and rebound phenomenon (for review see [19]). Recent meta-analyses have identified hypertonic saline as the favorable substance compared with mannitol due to a reduced rebound rate and less kidney injury [20–22]. To improve the effect of hypertonic saline in patients with intracranial hypertension, bolus 20% NaCl and continuous infusion with serum Na+ target increase by 5 mmol/l up to 155 mmol/l for a minimum duration of 24 h were investigated in a retrospective analysis and in pooled data from the Corti-TC, BI-VILI, ATLANREA trials [23–25]. In the pooled data set, continuous NaCl treatment was associated with improved 90-day survival [25].
External ventricular drainage (EVD) in a closed position allows for monitoring of ICP, while the open EVD allows the drainage of cerebrospinal fluid. In severely traumatized patients, a continuous drainage of CSF can be used to prevent sudden increases in ICP [26, 27]. The current 4th BTF guideline gives a level 3 recommendation for a continuous drainage zeroed at the midbrain for more effective drainage than intermittent use. Furthermore, they give a level III recommendation to lower ICP in patients with an initial GCS <6 during the first 12 h after injury [28]. However, continuous drainage and monitoring ICP via frequently performed catheter closures for ICP assessment may mask a significant amount of ICP increases above thresholds. Therefore, the trend goes to an EVD with an integrated ICP probe, e.g., an air-pouch-based ICP probe, for simultaneous CSF drainage and ICP assessment [29] or new systems using an integrated system with a roller pump and pressure probe to allow continuous ICP measurement and CSF drainage (defined levels and amount).
11.4.5 Level III: Metabolic Suppression, Hypothermia, Decompressive Craniectomy, and Hypocapnia
Basic technique to facilitate intensive care treatment but also to limit mobility and cerebral metabolism is the proper sedation and analgesia. Despite several experimental data on neuroprotective and neurotoxic effects of different sedation strategies in TBI models, limited data is available from clinical trials. Most data are on the impact of sedation on intracranial pressure and cerebral perfusion, demonstrating the safety of most anesthetics in patients with normal ICP. In clinical settings it is well-known that patients with TBI, especially young male patients, require multimodal sedation paradigms with multiple substances and that standard sedation strategies are often insufficient (e.g., propofol and sufentanil only). The effects of a mixture combining propofol with benzodiazepines, α-agonist, and ketamine on the damaged brain tissue remain unclear. Sedation with volatile anesthetics may present as an alternative. One reason for the lack of outcome studies on sedation with volatile anesthetics in brain-injured patients may be the concern that volatile anesthetics may raise intracranial pressure and reduce cerebral perfusion pressure by their vasodilative properties. However, clinical studies investigating this topic demonstrated sufficient sedation depth without relevant increase in ICP [30] and even improved regional cerebral blood flow in comparison to propofol [31]. A recent study demonstrated cases of marked ICP increases and MAP drops with sevoflurane sedation [32]. However, this may be attributed to a marked reduction in mean arterial blood pressure leading to malperfusion and consecutive vasodilation. Important for the use of volatile anesthetics in TBI patients is therefore the continuous control of arterial pressure and pCO2 monitoring.
A quite popular strategy to quickly reduce cerebral metabolism and consecutively ICP is the use of barbiturates. Unfortunately, the use of barbiturates is associated with drop in blood pressure and an increased risk for the early onset of pneumonia, which again is associated with a worsening of secondary brain injuries [33]. Moreover, barbiturate coma therapy for ICP treatment has been associated with refractory hypokalemia [34]. The current 4th BTF guideline gives a level 3 recommendation for a high-dose barbiturate administration only to control elevated ICP refractory to maximum standard medical and surgical treatment and only in combination with control of hemodynamic stability. Propofol may present as a new alternative to classical barbiturates, because a comparison of thiopental sodium (bolus 2 mg/kg and maintenance 2 mg/kg/h) with propofol (bolus 0.5 mg/kg and maintenance 20 μg/kg/h) found comparable effects on ICP reduction and no significant differences in mean CPP, SpO2, and arterial blood pressure [35].
As mentioned above, the use of cooling is limited to therapy refractory ICP increase. Still, there is uncertainty about the proper timing and duration as well as the lack of evidence of benefit in long-term clinical outcomes. Although a recent retrospective study demonstrated that therapeutic hypothermia was effective for lowering ICP after decompressive craniectomy and reduced mortality in the ICU [36], the Eurotherm Trial using titrated hypothermia as the primary intervention to reduce elevated ICP demonstrated harmful effects in patients with a lower severity of injury and no clear benefits in patients with a higher severity of injury [37]. The investigators conclude that therapeutic hypothermia should not be used after TBI, for neuroprotection or to reduce ICP. Again a combination of hypothermia with monitoring of brain tissue partial pressure of oxygen can help guide the course of therapeutic hypothermia by detecting local malperfusion [38].
The current 4th BTF guideline gives a level 2A recommendation for a large frontotemporoparietal decompressive craniectomy for reduced mortality and improved neurologic outcomes in patients with severe TBI. The DECRA trial demonstrated that early large bifrontotemporoparietal decompressive craniectomy failed to improve outcome and was associated with more unfavorable outcomes although it decreased ICP and the length of stay in the ICU [8]. In the recently released RESCUEicp trial, decompressive craniectomy was performed in patients with refractory elevated intracranial pressure and compared with ongoing medical care [39]. After 6 months the effects of craniotomy were lower mortality and higher rates of vegetative state, lower severe disability, and upper severe disability than medical care. The rates of moderate disability and good recovery were similar in the two groups. A recent retrospective study showed that early decompressive craniectomy for CT evidence of intracranial hypertension decreased abnormal ICP and CPP time and improved ICP and CPP thresholds but had no obvious effect on the outcome [40]. Focusing on ICP only may not be sufficient to improve patient outcome. Recent phase II RCT data indicate that a combined strategy to simultaneously measure and reduce ICP and improve brain tissue oxygen levels could reduce mortality and show better outcome compared to ICP control only [9].
The PaCO2 is a powerful vasoconstrictor of cerebral arteries, and ICP can therefore be reduced by controlled hyperventilation by the prize of reduction CBF. The influence of cerebral perfusion was shown in several studies demonstrating, e.g., substantial increase in amount of critically low perfusion brain tissue when switching from normoventilation to hyperventilation [41]. The current 4th BTF guideline gives a level 2B recommendation that prophylactic hyperventilation with PaCO2 of 25 mmHg or less is not recommended. However, hyperventilation may be considered as a temporizing measure for reduction of elevated ICP although it should be avoided in the first 24 h after injury when CBF is often critically reduced. Again, monitoring of brain tissue oxygenation is recommended for detection of local malperfusion induced by the hypocapnia [42]. Only one relatively old study evaluated the effects of hyperventilation in a prospective randomized controlled trial, which demonstrated worse clinical outcome when hyperventilation (PaCO2 25 ± 2 mmHg) was used compared with normoventilation (PaCO2 35 ± 2 mmHg) [43]. To the prompt lowering effect on cerebral perfusion, hyperventilation should be avoided and used as ultima ratio only.
11.5 Conclusion
In addition to modern concept of intensive care treatment, TBI patients require special attention to limit secondary brain injury. The basic concept is to maintain the individual normal physiology (“5 normos”: normotension, normoxia, normocapnia, normothermia, and normoglycemia), to promptly treat elevated intracranial pressure, and to improve drop in cerebral oxygenation.
Key Points
The basic concept is to maintain the individual normal physiology.
Treatment of elevated intracranial pressure may have side effects that lower cerebral oxygenation level.
Measurement of intracranial pressure and tissue oxygenation could allow treatment of both high ICP and tissue hypoxia.