15.1 Introduction
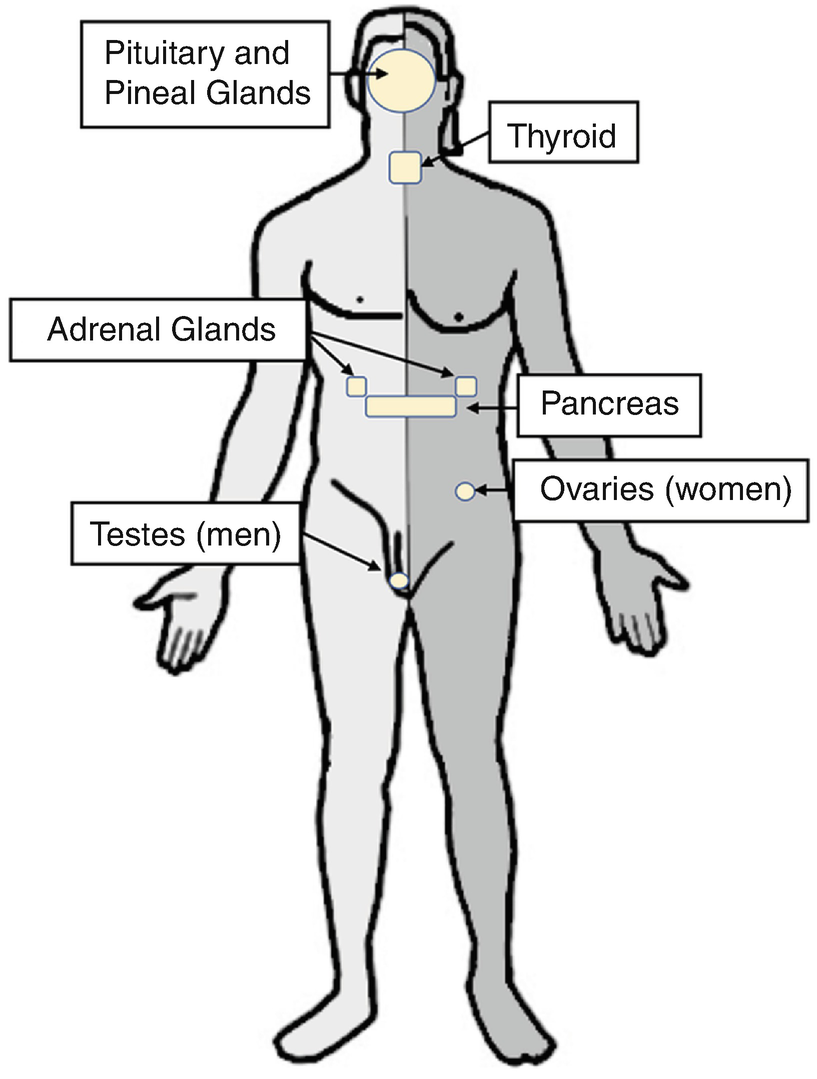
Anatomy of the endocrine system
15.2 Basic Anatomy and Physiology
The anatomy and physiology of the endocrine system is generally classified according to the principal location of the endocrine organ: intracranial and extracranial. The hypothalamus, pituitary, and pineal glands make up the intracranial endocrine organs, whereas the extracranial endocrine glands include the parathyroid, thyroid, thymus, and adrenal glands. Extracranial endocrine glands are present in many organs throughout the human body, including the pancreas, kidneys, testes in men, and ovaries in women.
The primary intracranial endocrine organs, the hypothalamus and pituitary, form the hypothalamic–pituitary axis. The hypothalamic–pituitary axis governs the endocrine output from a wide range of extracranial glands. The hypothalamus, which is situated superiorly to the pituitary gland in the sella turcica, is composed of neuronal cell bodies and connects to both the anterior and posterior pituitary to transmit regulatory signals. The axonal projections of hypothalamic cell bodies compose the posterior pituitary. Antidiuretic hormone (ADH) and oxytocin are secreted via exocytosis from the axonal terminals in the posterior pituitary to regulate water reabsorption by the kidney and total peripheral resistance, as well as milk ejection from the female milk ducts in response to infant suckling and uterine contraction during labor, respectively. The hypothalamus secretes hormones into the hypophysical portal vessel network, which links the hypothalamus and anterior pituitary and ensures delivery of high concentrations of hypothalamic hormones to the anterior pituitary. The hormonal control by the hypothalamus regulates the secretion of multiple distinct anterior pituitary hormones: thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), adrenocorticotropic hormone (ACTH), melanocyte-stimulating hormone (MSH), prolactin, and growth hormone (GH). This collection of anterior pituitary hormones governs many disparate functions. For example, FSH and LH contribute to gonadal development and function in males and females, whereas prolactin contributes to breast development and lactation in females, GH in linear as well as organ growth, TSH in thyroid gland output, and ACTH in adrenal gland output. Consequently, the hypothalamic–pituitary axis has numerous and significant effects on human physiology.
The extracranial endocrine organs are the principle effectors of endocrine physiology. The parathyroid glands secrete parathyroid hormone (PTH), which is essential for calcium homeostasis. Enveloping the four parathyroid glands in the anterior neck, the thyroid gland secretes the thyroid hormones triiodothyronine (T3) and thyroxine (T4). Decreased or increased levels of T3 and/or T4 may lead to hypo- or hyperthyroidism, respectively. Hypothyroidism typically results in fatigue, weight gain, cold intolerance, constipation, hair loss, and dry skin, while hyperthyroidism frequently demonstrates as anxiety, hyperactivity, weight loss, heat intolerance, diarrhea, weakness, and polydipsia as well as polyuria. Subclinical, or asymptomatic, hyperthyroidism is also common. The systemic nature of both hypo- and hyperthyroidism symptoms reflects the broad functions of T3 and T4. The adrenal glands, otherwise known as the suprarenal glands due to their anatomical location, also secrete several hormones with systemic effects, including the corticosteroids such as aldosterone and cortisol, epinephrine, and the sex hormones testosterone and estrogen.
The pancreas has both exocrine and endocrine functions. The endocrine pancreas secretes insulin and glucagon, which are made by the β- and α-cells, respectively, which function to regulate blood glucose concentrations. To achieve this homeostatic balance, insulin and glucagon act antagonistically by driving glucose either into or out of cells, respectively. Diabetes mellitus (DM) results when either insulin is not secreted by β-cells (i.e., type 1 DM) or insulin does not elicit its desired effect on the target cell (i.e., type 2 DM) [1, 2].
15.3 Considerations in Pituitary Adenoma Resection
Perturbations of the hypothalamic–pituitary axis often result in symptoms for which neurosurgical intervention is warranted [3]. Clinically, nonfunctioning pituitary adenomas can often grow to large sizes before the onset of symptoms, and the presenting symptoms typically reflect mechanical compression of surrounding structures. For example, patients with nonfunctioning pituitary adenomas can present with headache and bitemporal hemianopsia due to optic chiasm compression, cranial nerve palsies (e.g., III, IV, and VI), or symptoms related to increased intracranial pressure (ICP) from mass effect or compression of the third ventricle, including nausea, vomiting, and papilledema.
By contrast, patients with functional pituitary adenomas typically present with associated endocrinopathies and are often diagnosed before the tumor has grown to a size that is large enough to increase the ICP. The associated endocrinopathies can include acromegaly or growth failure, hypo- or hyperthyroidism, sexual dysfunction, inappropriate lactation, and/or irregular menstruation in females. Surgical resection is the gold standard in the treatment of most pituitary adenomas. A notable exception is for prolactinomas, a type of pituitary tumor that results in inappropriate lactation due to excess prolactin secretion, in which surgical resection is typically considered second-line after conservative (i.e., medical) management has failed [4].
15.3.1 Preoperative Evaluation
The preoperative assessment of a patient presenting for pituitary adenoma resection should include a thorough history, physical examination, and workup as indicated by their physiological reserve and comorbid conditions [5]. In addition to the syndromes associated with functional pituitary adenomas, hypopituitarism is an important consideration for all patients presenting for resection of a pituitary tumor. Mass effect exerted on the anterior pituitary most commonly causes secondary hypothyroidism and adrenal insufficiency, whereas compression of the posterior pituitary can cause diabetes insipidus. Mass effect can also cause prolactinemia by disrupting the tonic inhibition of pituitary lactotrophs.
Standard workup that should be considered in this patient population includes a complete blood count, a basic metabolic panel, and an endocrine panel. Hyperglycemia is a common finding, which may be due to comorbid DM (which might or might not have been previously recognized), or excessive secretion of gluconeogenic–glycolytic hormones such as cortisol and growth hormone. Hyponatremia suggests preoperative diabetes insipidus, whereas hypercalcemia is associated with multiple endocrine neoplasia type 1. A comprehensive endocrine panel should include TSH, free T3 and T4, cortisol, ACTH, IGF-1, testosterone, LH, FSH, and prolactin. Lastly, women presenting with secondary amenorrhea must be tested for pregnancy.
Hormone replacement therapy with corticosteroids forms the cornerstone of preoperative optimization for patients with hypopituitarism [5]. Hydrocortisone is often administered in the days leading to surgery, followed by perioperative “stress-dose” steroids to account for the physiological challenge of surgery. Because oral bioavailability may be reduced in hypopituitarism, parenteral routes are preferred in these patients. Importantly, it should be noted that dexamethasone is the agent of choice for patients with Cushing’s disease because it does not interfere with postoperative cortisol assays. Thyroxine should also be replaced in patients with hypothyroidism.
15.3.2 Acromegaly
In addition to the standard perioperative considerations for an intracranial mass, acromegalic patients present unique cardiovascular and respiratory challenges for the perioperative physician. All acromegalic patients should have high suspicion for having a difficult airway, and induction of general anesthesia and endotracheal intubation should proceed with great caution. Studies have shown that in nearly two-thirds of acromegalic patients, endotracheal intubation proves difficult even in the setting of a normal preoperative airway examination [6]. Interestingly, IGF-1 levels were found to be an independent risk factor for difficult intubation in this cohort. Hypertrophy of upper airway structures such as the mandible, nose, lips, and tongue is often, but not always, apparent on clinical exam. A hoarse voice suggests laryngeal stenosis, which may be due to soft tissue thickening, laryngeal calcinosis, or injury of the recurrent laryngeal nerve. Cervical spine abnormalities are also common. Importantly, because the preoperative airway assessment poorly predicts the difficult airway in these patients, advanced airway management techniques should always be immediately available [7]. Obstructive sleep apnea (OSA) affects up to 70% of acromegalic patients [8]. Therefore, benzodiazepines and narcotics in the perioperative period should be used sparingly and cautiously.
Acromegalic cardiomyopathy is the most common cause of death in untreated acromegaly and should be considered in all patients [9, 10]. Elevated plasma levels of GH cause concentric ventricular hypertrophy, which ultimately leads to diastolic dysfunction, most commonly affecting the left ventricle. Hypertension is also a common finding, and electrocardiogram changes such as bundle branch blocks, ST segment depression, and T wave abnormalities are present in more than 50% of patients [11]. These patients are also at increased risk of developing supraventricular and ventricular ectopy in the face of physiological stress. We recommend the use of invasive arterial blood pressure monitoring during pituitary adenoma resections due to the risk of sudden hypertensive episodes. Patients with acromegalic cardiomyopathy are particularly vulnerable to these episodes. However, impaired blood flow in the ulnar artery is common in these patients, especially in the presence of carpel tunnel syndrome [12]. Therefore, ulnar blood flow should always be assessed prior to radial artery catheterization, and other sites should be considered (e.g., femoral artery).
15.3.3 Cushing’s Disease
Glucocorticoid receptors are expressed by almost every human cell. As a result, patients with Cushing’s disease present with diverse perioperative challenges. One of the most vital functions of cortisol is to increase vascular smooth muscle sensitivity to endogenous vasoconstrictors such as catecholamines and angiotensin [5, 13]. These and other mechanisms contribute to the observation that most patients with Cushing’s disease will present with systemic hypertension and left ventricular hypertrophy. Up to 40% of patients will also have evidence of diastolic dysfunction. The increased vascular sensitivity to catecholamines should also be considered whenever administering vasoactive drugs.
Common comorbid conditions that should be considered include OSA, DM, and osteoporosis [5, 14]. Due to thinning of the skin, difficult intravenous access and easy bruising should be anticipated [15]. Appropriate eye protection should be used to mitigate the increased risk of corneal abrasions in patients with exophthalmos. Although myopathies of the shoulder girdle and proximal lower limbs are often presented, there is no data to indicate that these patients have increased sensitivity to neuromuscular blockade. Therefore, normal weight-based dosing is recommended.
15.3.4 Perioperative Course
Transsphenoidal pituitary surgery has traditionally been guided by fluoroscopy, with the patient in the semi-seated position [5]. However, the endoscopic endonasal approach has recently become more widespread. Nasal intubations are contraindicated in either approach, and neuromuscular blockade is important to mitigate the risks of cerebrospinal fluid (CSF) leak, visual tract injury, and vascular damage secondary to patient movement during surgery. Visual-evoked potentials have been described to monitor for injury to visual pathways in proximity to the pituitary gland, but these evoked potentials are very sensitive to anesthetics and are not routinely recommended [16].
As discussed above, invasive arterial blood pressure monitoring should be considered in patients undergoing pituitary adenoma resection due to the high risk of sudden hypertensive episodes. Local infiltration of the nasal mucosa with epinephrine and lidocaine may also induce dysrhythmias and myocardial ischemia. The ability to quickly diagnose and treat sudden hypertensive episodes is especially important for patients with known cardiomyopathy, congestive heart failure, poor exercise tolerance, and poorly controlled systemic hypertension. Patients under perioperative beta-blockade are particularly susceptible to unopposed alpha adrenergic stimulation by epinephrine. The resulting hypertension should be treated immediately by administering phentolamine, direct vasodilators, or by increasing the concentration of volatile anesthetic.
Lumbar intrathecal drains are commonly used to improve tumor visualization by injecting saline or draining cerebrospinal fluid. The surgeon may also inject air into the lumbar drain to outline the tumor on fluoroscopy, in which case nitrous oxide should be avoided. The presence of intracranial hypertension and risk of herniation should always be considered before placing a lumbar drain. A Valsalva maneuver can be used to detect CSF leaks once the resection is complete. If positive, the surgeon will typically pack the sella turcica with autologous fat before reconstruction.
Once the surgical field is closed, meticulous suctioning of the oropharynx is essential. Unless a nasopharyngeal airway was placed by the surgeon before nasal packing, nose breathing will not be possible in the early postoperative period. Therefore, placement of an oral airway should be considered in patients with confirmed or suspected OSA. A rapid emergence from general anesthesia is highly desirable, which will facilitate an optimal early postoperative neurological assessment. To achieve a fast emergence, we recommend the use of rapidly cleared analgesic agents such as remifentanil combined with a low-dose intravenous or volatile anesthetic agent.
15.4 Management of Chronic Extracranial Endocrinopathies in the Neurosurgical Patient
15.4.1 Hyperthyroidism
Thyroid hormones have important cardiovascular effects that should be considered in the perioperative period [17, 18]. The underlying principle is that T3 promotes beta adrenergic tone. This causes a decrease in systemic vascular resistance (SVR) via beta-2 receptors, which subsequently activates the renin–angiotensin–aldosterone system (RAAS), increasing circulating blood volume and cardiac output to maintain perfusion pressures. Inotropic and chronotropic effects of beta-1 receptors on cardiac myocytes also contribute to an increase in cardiac output by 50–300%. Through these mechanisms, chronic hyperthyroidism recruits much of a patient’s physiological reserve, rendering them vulnerable to cardiovascular collapse in the perioperative period.
Pharmacological agents and their desired effects in the patient with uncontrolled hyperthyroidism undergoing emergency surgery
Desired effect | Pharmacological agents |
|---|---|
Inhibit sympathetic effects of T3/T4 | Beta blockers |
Inhibit peripheral conversion of T4 to T3 | Beta blockers, and propylthiouracil (PTU) |
Inhibit production of T3/T4 in the thyroid gland | PTU, methimazole, and inorganic iodide |
15.4.2 Hypothyroidism
In contrast to the hyperthyroid state, hypothyroid patients suffer from a low beta adrenergic tone on their cardiac myocytes and unopposed alpha adrenergic tone in their vasculature. The resulting increase in SVR and decrease in cardiac inotropy and chronotropy leads to inhibition of RAAS and intravascular volume depletion. Of note, hypothyroid patients also tend to have depressed respiratory responses to hypoxemia and hypercapnia [21]. Severe cases may also present with decreased lung diffusion capacity.
Like hyperthyroidism, hypothyroidism should be pharmacologically optimized prior to elective procedures, typically with levothyroxine (T4). Because the half-life of T4 is approximately 1 week, a missed dose on the morning of surgery should not have significant physiological effects [20]. However, we still recommend patients continue taking their usual dose of thyroxine throughout the perioperative period. Preoperative sedation with benzodiazepines or narcotics is best avoided in this patient population due to increased sensitivity to their effects. Signs of hypothyroidism that may be observed postoperatively include delirium, prolonged ileus, infection without fever, and myxedema coma.
If a patient with uncontrolled or newly diagnosed hypothyroidism presents for urgent or emergent surgery, perioperative management should focus on avoiding cardiovascular collapse. Severe hypothyroidism can be managed with a 200–500 μg infusion of levothyroxine over 30 min, followed by 50–100 μg IV daily [22]. These patients often have concomitant adrenal insufficiency, and 50 mg of hydrocortisone administered four times daily mitigates the risk of adrenal crisis following thyroid replacement. Intravascular volume should also be maintained with normal saline and dextrose.
15.4.3 Adrenal Insufficiency
The hypothalamic–pituitary–adrenal (HPA) axis plays an essential role in generating the surgical stress response, primarily due to the central role of glucocorticoids in modulating the sensitivity of vascular smooth muscle. Therefore, the timely diagnosis and management of adrenal insufficiency (AI) are essential skills for the perioperative physician.
The classic signs of adrenal insufficiency are hypotension, hyponatremia, and hyperkalemia. However, these signs may only become apparent intraoperatively when AI is unmasked by surgical stress. Therefore, preoperative risk assessment is important. Causes of primary AI include autoimmune adrenalitis, adrenalectomy, sepsis, and tuberculosis. Secondary AI, caused by suppression of the HPA axis by exogenous glucocorticoids, is much more common [23]. In general, any patient who has received at least 20 mg of prednisone (or its dose equivalent) daily for more than 5 days should be considered at risk of developing AI [24]. Furthermore, the HPA axis can remain suppressed for 6–12 months after discontinuing chronic (>1 month) corticosteroid therapy. Conversely, a maximum daily dose of 5 mg of prednisone (or equivalent) for any length of time is very unlikely to cause secondary AI [23].
Approach to intraoperative steroid use in the patient with adrenal insufficiency
Stress-dose steroids: drug of choice | Intravenous hydrocortisone. Adjust doses accordingly if using another steroid |
Frequency and duration of treatment | Every 8 h, for 48 h |
Individual dose—minor procedure | 25 mg |
Individual dose—moderate procedure | 50 mg |
Individual dose—major procedure | 100 mg |
In the postoperative period, the perioperative physician should consider measuring a random plasma cortisol, TSH, and T4 in patients exhibiting signs of AI, such as hypotension, orthostasis, altered mental status, nausea, vomiting, hyponatremia, and hyperkalemia. Empiric stress-dose steroids can also be considered.
15.4.4 Diabetes Mellitus
DM is associated with numerous comorbid conditions that impact a wide range of organ systems. For example, DM is associated with an increased incidence of obesity, hyperlipidemia, hypertension, atherosclerosis, coronary artery disease, cerebrovascular disease, depression, an increased risk of cancer, and chronic kidney disease and retinopathy secondary to vasculitis [27]. As many of these comorbidities also connote increased perioperative risk, the presence of DM and its associated comorbidities should be considered in all neurosurgical patients. An understanding of the effects of DM on endocrine regulation of human physiology and organ system dysfunction is essential in optimizing surgical outcomes in these patients.
Patients with insulin resistance or insufficiency are more susceptible to the gluconeogenic and glycolytic hormones released in response to surgical stress (e.g., cortisol, epinephrine, glucagon, and growth hormone), which are usually countered by a parallel increase in insulin. Perioperative hyperglycemia is therefore common in these patients, which increases the risk of infection, diabetic ketoacidosis, and hyperglycemic hyperosmolar coma. Diabetic patients are also at risk of developing perioperative hypoglycemia due to prolonged fasting.
Perioperative considerations and complications in the diabetic patient
Diabetic complication | Perioperative consideration |
|---|---|
Nephropathy | Adjust dosage of drugs cleared by kidneys and suspect hyporenin–hypoaldosterone state |
Autonomic neuropathy | Rapid identification and treatment of hypotension |
Gastroparesis | Avoid medications that prolong gastric-emptying time and consider a rapid sequence intubation |
Cystopathy | Consider straight or Foley catheterization |
Peripheral neuropathy | Carefully document preoperative somatosensory and motor function |
Retinopathy | Carefully document preoperative visual function, especially for procedures with a risk of ischemic retinopathy |
Peripheral vascular insufficiency | Monitor closely for signs of infection and poor wound healing |
Effective glucose control is a major perioperative goal for diabetic patients. However, the optimal target glucose concentration that confers the best clinical outcomes in neurosurgical patients is currently unknown. Moreover, there is little evidence to support that any one approach to glycemic management is advantageous over another in improving perioperative outcomes. We recommend that, when possible, diabetic patients are best scheduled for surgery early in the day to minimize fasting time. Because diabetic patients undergoing intracranial neurosurgery are especially prone to hyperglycemia, we recommend frequent monitoring of blood glucose concentration and maintaining a target plasma glucose concentration of 140–180 mg/dL. Maintaining tight blood glucose concentrations between 80 and 120 mg/dL management is not recommended in these patients because of the potential risk of hypoglycemia.
Short-acting oral hypoglycemic agents, such as sulfonylureas, thiazolidinediones, and DPP-4 inhibitors, should be held on the morning of surgery and restarted postoperatively once the patient is tolerating an oral diet [28]. Due to the risk of lactic acidosis, metformin should be held 24 h before surgery and restarted 24–48 h postoperatively, once baseline renal function is documented. Incretins can be continued perioperatively.
Insulin-dependent diabetics should continue their usual regiment up until the eve of surgery and be instructed to not skip dinner the night before their scheduled surgery [29]. Basal insulin is essential to decrease the risk of perioperative diabetic ketoacidosis and hyperglycemic hyperosmolar coma. Therefore, full-dose long-acting basal insulin analogues should be continued the morning of surgery. For patients who use an insulin pump, we recommend maintaining their basal rate perioperatively and titrating a dextrose infusion to stay in the target glucose concentration range. For patients on NPH or other mixtures, we recommend administering half the dose of intermediate-acting insulin on the morning of surgery. Short-acting insulins should be avoided preoperatively.
Patients with DM or glucose intolerance spend on average 50% more time in the hospital postoperatively and have worse outcomes than patients with normal insulin function. These patients are particularly at increased risk of hypoglycemia, dehydration, acute kidney injury, electrolyte imbalances, cerebrovascular accidents, myocardial infarction, and postoperative wound complications. Special attention should be paid in decreasing the risk of these complications in the postoperative period.
15.5 Acute Complications of Endocrine Dysfunction in the Neurosurgical Patient
15.5.1 Pituitary Adenoma Resection
The most common postoperative complaint after transsphenoidal surgery is headache [5]. Nonsteroidal anti-inflammatory drugs (e.g., ketorolac) or acetaminophen are usually effective at controlling the pain. Opioids may be also used but should be cautioned in the elderly and in patients with diagnosed or suspected OSA due to their sedative effects. Routine pharmacological prophylaxis for nausea and vomiting is recommended due to their high incidence in this patient population. There are many classes of antiemetic agents that have been successfully used, with no one agent conferring the highest efficacy in all patients [30].
Due to their proximity to the transsphenoidal surgical approach, cranial nerves II-VI should be systematically assessed after pituitary adenoma resection. A new cranial nerve deficit should be further investigated with imaging (i.e., CT or MRI). Depending on the nature of the deficit, an unexpected focal change in neurological status might warrant emergent surgical intervention. Another potential postoperative complication is CSF leak. Minor nasal drainage is expected postoperatively, but continuous fluid leakage that is exacerbated by leaning forward or associated with headache should be further investigated. Beta2-transferrin is a specific marker for CSF; if the draining fluid tests positively for this marker, autologous fat packing of the defect is indicated [5].
Up to one-third of patients will develop diabetes insipidus (DI) after pituitary adenoma resection, with most cases resolving spontaneously within the first postoperative week [31]. Polyuria and polydipsia are the hallmarks of DI and may appear abruptly in the postoperative period. Because most patients’ thirst mechanisms are intact and they have access to water, intravascular volume contraction with hypernatremia and hyperosmolarity are rare. Postoperative DI can be distinguished from other causes of polyuria by measuring the urine specific gravity (SG). In DI, the urine SG is usually <1.005, in contrast to acromegalic diuresis and iatrogenic perioperative fluid administration, which usually cause a diuresis with urine SG >1.005. Glycosuria can be distinguished from these other causes of postoperative diuresis by measuring urine glucose concentration. Indications to treat postoperative DI are volume depletion (i.e., the patient cannot increase their fluid intake to compensate their diuresis) and sleep disruption secondary to polyuria [5]. Treatment typically consists of 0.1 mg of oral desmopressin (DDAVP), which is usually sufficient to control this mostly self-limited postoperative complication. If the patient cannot tolerate oral medications, 1 μg DDAVP can be administered subcutaneously. Once treatment is initiated, monitoring the patient’s oral hydration, urine output, and electrolytes is important to avoid inducing hyponatremia.
Patients may also develop a transient syndrome of inappropriate antidiuretic hormone (SIADH) after resection of a pituitary adenoma [32]. SIADH is characterized by low serum osmolarity and sodium, in association with high urine osmolarity and sodium. These patients are usually asymptomatic and euvolemic, distinguishing them from hypovolemic hyponatremic patients suffering from cerebral salt wasting [33]. In severe (sodium <120 mEq/L) or rapidly developing hyponatremia, patients can present with nausea, vomiting, altered mental status, and seizures. The first line of treatment for patients with SIADH is fluid restriction. For symptomatic or severe cases, a slow infusion of hypertonic saline or intravenous urea should be considered [5, 34]. The goal for hyponatremia correction should be about 1–2 mEq/L/h for the first 3–4 h until the symptoms have resolved or the serum sodium is above 120 mEq/L. To avoid the rare but serious complication of osmotic demyelination syndrome, the rate of sodium correction should not exceed 6–12 mEq/L over the first 24 h or 18 mEq/L over 48 h.
All patients should be screened for hypopituitarism after pituitary adenoma resection. The most common manifestations of hypopituitarism are adrenal insufficiency and hypothyroidism [35]. Therefore, as discussed above, the cornerstone of treatment includes corticosteroids and thyroxine, respectively, as indicated. Patients who received perioperative stress-dose steroids due to hypopituitarism may be discharged on low-dose steroids, with subsequent evaluation of their hypothalamic–pituitary–adrenal axis on an outpatient basis. Alternatively, exogenous steroids can be held 24 h postoperatively to measure morning cortisol levels and be titrated accordingly. This strategy is only effective in institutions with clinical laboratories capable of reporting cortisol assays in a timely fashion.
15.5.2 Pituitary Apoplexy
Pituitary apoplexy, i.e., hemorrhage of the pituitary gland, often results from an underlying pituitary tumor that has hemorrhaged after exposure to an external stressor, such as traumatic brain injury, pregnancy, or surgical stress [36]. Pituitary apoplexy will usually lead to pituitary failure with subsequent endocrine dysfunction due to the central role of the pituitary hormones on orchestrating endocrine output. If pituitary apoplexy results in impaired vision, transsphenoidal surgery is recommended in an attempt to preserve vision [37]. As previously discussed, surgical intervention for pituitary apoplexy carries similar inherent risk to the pituitary gland as surgery for pituitary adenoma or craniopharyngioma, and postoperative endocrine dysfunction must therefore be considered in the postoperative period.
15.5.3 Perioperative Hyperglycemia
Perioperative hyperglycemia is common in neurosurgical patients and might result from perioperative stress or administration of exogenous corticosteroids. Blood glucose monitoring and management is essential because chronic hyperglycemia is an independent marker of poorer clinical outcomes in neurosurgical patients [38]. Perioperative hyperglycemia is associated with poor wound healing, which is particularly concerning after craniotomy. Moreover, severe intraoperative hyperglycemia (blood glucose concentration > 180 mg/dL) was recently shown to be an independent risk factor for postoperative infection within the first 7 days after craniotomy [39]. Importantly, the association between severe intraoperative hyperglycemia and postoperative infection includes both intracranial and extracranial (e.g., lung and blood) infections. Diabetic patients with hyperglycemia may be at particularly high perioperative risk because of the presence of comorbid atherosclerotic disease of the coronary arteries, cerebrovascular network, and renal arteries and arterioles. This results in increased risk of cerebral ischemia, cardiac ischemic events, hypertensive crises, and electrolyte abnormalities during the perioperative period.
Although the optimal blood glucose concentration that confers the least risk of perioperative complications is unknown, we recommend that the blood glucose concentration be maintained between 140 and 180 mg/dL. Importantly, it should be noted that perioperative hypoglycemia can also occur in the neurosurgical patient, most often due to aggressive insulin therapy [40]. The risk of insulin-induced hypoglycemia is thought to be higher in insulin-naïve patients, although evidence-based data on this is lacking. The optimal timing, dose, and method of insulin delivery in neurosurgical patients are unknown and are largely institution-dependent or at the discretion of the treating clinician. Moreover, it is currently unknown whether any one insulin regimen increases the risk of hypoglycemia more than another regimen.
15.5.4 Endocrine Stress Response
Contrary to the generalized endocrine dysfunction that can occur with surgical intervention on the pituitary, increases in endocrine output may also arise in the perioperative period resulting from surgical stress or underlying pathology. As detailed above, hyperglycemia may result due to excesses of glucagon, cortisol, and catecholamines in the setting of surgical stress. In addition to hyperglycemia, cortisol and catecholamine excess as part of the stress response can cause perioperative hypertension, which carries risks for hypertensive hemorrhage, coronary artery ischemia, aortic dissection, and subsequent cardiovascular collapse [41]. In contrast to endocrine dysfunction, hormonal excess also results in a unique set of perioperative complications and considerations for the neurosurgical patient.
15.5.5 Electrolyte Imbalance
Electrolyte imbalances are associated with several endocrinopathies and should be considered in the differential diagnosis in any neurosurgical procedure that is complicated by a perioperative seizure. The fact that seizure is a significant and relatively frequent complication of neurosurgical procedures [42], and the recognition of the associated risk in these patients have led to the development of effective guidelines for seizure prophylaxis [43]. However, electrolyte imbalances should be considered in the underlying etiology because they are often easily preventable and treatable. Perturbations in serum calcium concentrations might reflect changes in PTH levels or underlying parathyroid pathology, whereas sodium disturbances might be due to changes in either cortisol or aldosterone levels or by renal vascular changes as seen in the setting of DM.
15.5.6 Thyroid Storm
Hyperthyroid patients are most likely to develop thyroid storm intraoperatively or within 48 h postoperatively [19]. The cardinal signs of this rare complication include hyperpyrexia (up to 41 °C), tachycardia, and delirium. The differential diagnoses included malignant hyperthermia, neuroleptic malignant syndrome, and pheochromocytoma. Due to the high associated mortality (10–75%), it is often necessary to initiate empiric treatment before confirming the diagnosis with thyroid hormone assays [44].
Managing thyroid storm requires critical care resources. Intravenous beta blockade should be titrated for a heart rate goal of <90 beats per minute, and volume resuscitation should be supplemented with dextrose to replete glycogen reserves [19]. As in thyrotoxicosis, the thionamides methimazole and PTU are used to inhibit the peripheral conversion of T4 to T3 and decrease secretion from the thyroid gland. Acetaminophen is the antipyretic of choice because salicylates decrease thyroid protein binding, therefore increasing free T3 and T4 [28]. Although infection and sepsis are the most common underlying cause of thyroid storm, empiric antibiotics are not recommended unless there is evidence of infection. Blood, urine, and sputum cultures however should be ordered as soon as possible.
15.5.7 Myxedema Coma
Patients with poorly controlled hypothyroidism can rarely develop myxedema coma, a state of decompensated hypothyroidism. With a reported mortality reaching as high as 80% [45], timely diagnosis and treatment of myxedema coma are paramount. The most common perioperative precipitants are infection, cold exposure, sedatives, analgesics, and other medications. Signs and symptoms include a severely depressed mental status which can progress to coma and seizures, hypothermia, hypopnea, bradycardia, and heart failure. Management of myxedema is similar to the management of hypothyroid patients presenting for emergency surgery, and includes aggressive volume resuscitation with normal saline and dextrose, as well as intravenous administration of steroids and levothyroxine or liothyronine [22]. Rapid rewarming may result in widespread vasodilation and cardiovascular collapse for patients with heart failure and volume depletion. Therefore, correction of hypothermia should be gradual and in parallel with volume resuscitation [46].
15.6 Conclusions
Endocrine disorders are common and can affect numerous physiological processes throughout the body. Neurosurgical patients can present with intracranial or extracranial endocrinopathies that result in hormone hypo- or hypersecretion, each with unique clinical implications. Providing perioperative care for the patient with endocrine dysfunction can often be challenging, and successful management is dependent on an in-depth understanding of how endocrine dysfunction affects neurophysiology and postoperative outcomes. In this chapter, we reviewed the relevant principles in endocrine anatomy and physiology as well as a practical and evidence-based approach to the neurosurgical patient with endocrine dysfunction. We also highlighted some areas in which studies on optimal management strategies are lacking, and in which management is currently guided by expert consensus and applied theory.
Key Points
Endocrine disorders are common in neurosurgical patients, and can include disorders of endocrine gland hyposecretion, hypersecretion, and tumors of the endocrine glands.
The perioperative care of the neurosurgical patient with comorbid endocrine dysfunction can be challenging, and requires an in-depth understanding of how the endocrine dysfunction affects neurophysiology and postoperative outcomes.
A thorough preoperative workup and optimization of all comorbid conditions is essential to minimize the risk of perioperative complications.
Perioperative complications in the patient with endocrine dysfunction (e.g., electrolyte imbalances, thyroid storm or myxedema coma, and blood glucose derangements) can be severe and even life threatening, and must be promptly diagnosed and treated.