COASTAL TAILED FROG
Ascaphus truei Stejneger 1899

Coastal tailed frog, Del Norte County, California. Courtesy of Rob Schell Photography.

Status Summary
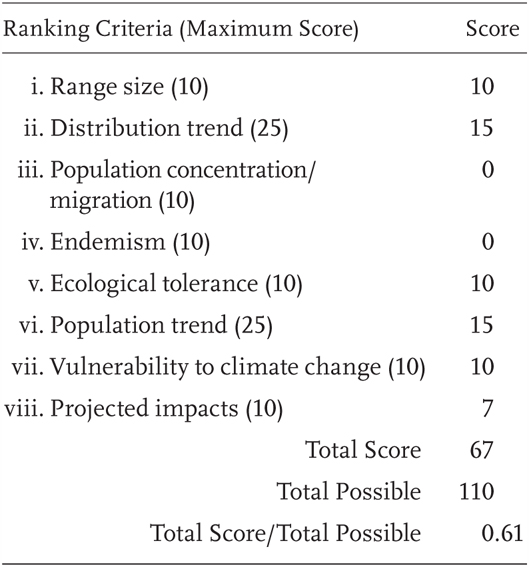
Ascaphus truei is a Priority 2 Species of Special Concern, receiving a Total Score/Total Possible of 61% (67/110). During the previous evaluation, it was also considered a Species of Special Concern (Jennings and Hayes 1994a).
Coastal Tailed Frog: Risk Factors
Identification
Ascaphus truei is a small (2.5–5.0 cm SVL) dark frog with an olive, brown, gray, or reddish dorsum and lighter colored ventral surface. Other color characters include a pale triangular blotch on the snout and a dark eye stripe. This species has rough, granular skin, and the outermost toes on the hind feet are broad. Males have a unique tail-like copulatory organ that is unmistakable. This frog is nocturnal and adults have vertical pupils (Stebbins 2003).
Larvae grow up to 6.0 cm in TL and are adapted to life in fast-flowing streams. They have dorsoventrally flattened bodies and large sucking mouthparts that extend nearly halfway down their head-body on the ventral surface. These morphological traits allow larvae to attach to rock substrates (Altig and Brodie 1972, Nussbaum et al. 1983, Welsh and Hodgson 2011). Larvae often have a light-colored tail tip with a proximal dark band (Stebbins 2003).
In California, metamorphosed A. truei may be confused with co-occurring foothill yellow-legged frogs (Rana boylii). Rana boylii have horizontal pupils, more robust hind legs, and males lack enlarged toes and “tails” (Stebbins 2003). In addition, the enlarged mouthparts of A. truei tadpoles are distinctive.
Taxonomic Relationships
The formerly monotypic genus Ascaphus was recently split into a coastal (A. truei) and an inland species (A. montanus), but California populations remain A. truei (Nielson et al. 2001, Nielson et al. 2006). The two species of Ascaphus comprise the family Ascaphidae. This family forms the sister group to all other anurans either alone or in combination with the New Zealand endemic Leiopelmatidae (Roelants et al. 2007). In either case, it is from one of the oldest and most phylogenetically distinctive extant anuran lineages.
Life History
Ascaphus truei exhibits substantial geographic variation in life history. Here, we focus on data from California populations where possible. Breeding occurs primarily in the spring and summer in coastal populations (Sever et al. 2001, Burkholder and Diller 2007), but there are reports from Trinity County of animals found in breeding condition in the fall (J. Garwood, pers. comm., in Burkholder and Diller 2007). Females likely breed in alternate years (Burkholder and Diller 2007) and can store viable sperm for up to a year (Nussbaum et al. 1983, Sever et al. 2001). Eggs begin developing in the fall, and oviposition occurs the following summer between July and September in California populations (Sever et al. 2001, Karraker et al. 2006). Egg diameter is 4 mm on average (Brown 1977), and clutch size averages around 40 for the species with a range of 28–89 eggs per clutch documented in California populations (Karraker et al. 2006). Egg masses can be difficult to find in the field (Karraker et al. 2006). Recent surveys in coastal California have found single and multiple clutches, with the timing of the surveys (late August–early September) likely the most important factor for detecting eggs (R. Bourque, pers. comm.). Clutches are pearl-like strings of eggs and have been found attached to the underside of cobble or boulder substrates in riffles and pools (Karraker et al. 2006).
Time to metamorphosis in lowland coastal California populations (elevation <200 m) is 1–2 years (Wallace and Diller 1998, Bury and Adams 1999). Longer developmental times have been observed in montane populations (e.g., 4 years to metamorphose in a Washington population at ∼1500 m elevation; Brown 1990). In a population in Humboldt County, California, females reached sexual maturity 2.5–3 years after metamorphosis, while males were sexually mature 1.5–2 years after metamorphosis (Burkholder and Diller 2007). Post-metamorphic frogs grow year-round, with growth rates fastest in the summer (Burkholder and Diller 2007).
Adults and post-metamorphic juveniles are generalist invertebrate predators (Bury 1970b). Larvae are generalist grazers and scrapers, consuming diatoms and other periphyton (observations from A. montanus; Metter 1964).
Landscape genetic studies have detected different patterns of connectivity among populations in California and Washington. In four watersheds in Mendocino County at the southern range limit of the species, high population structure among watersheds suggested limited long-distance gene flow, and movements within watersheds were inferred to occur along waterways (Aguilar et al. 2013). By contrast, a study in Washington concluded that some animals engage in long-distance dispersal through terrestrial habitats, and these movements do not rely on stream connectivity (Spear and Storfer 2008). These differences may be due to regional variation in climate and forest type, though additional studies are needed.
Habitat Requirements
Ascaphus truei requires cold, permanent, swift-flowing streams with coarse (e.g., cobble, boulder, bedrock) substrates. Some populations may persist in streams that occasionally dry depending on the length of the larval period (Wallace and Diller 1998). Ascaphus truei tends to be more common in mature and old-growth forest relative to younger stands, in terms of both presence and abundance (Bury and Corn 1988, Corn and Bury 1989, Welsh 1990, Gomez and Anthony 1996, Welsh and Lind 2002, Welsh et al. 2005, Ashton et al. 2006).
Several studies have examined the relationship between A. truei presence and abundance and environmental variables at different scales. Larvae are positively associated with low stream temperatures, high water velocity, steep gradients, and the presence of riffles, waterfalls, and cobble and boulder substrates (Hawkins et al. 1988, Bury et al. 1991, Welsh and Ollivier 1998, Diller and Wallace 1999, Adams and Bury 2002, Welsh and Lind 2002, Wahbe and Bunnell 2003). Larvae are negatively associated with fine sediment load (i.e., embeddedness), pools, and slow-flowing stream habitat (Hawkins et al. 1988, Corn and Bury 1989, Welsh and Ollivier 1998, Diller and Wallace 1999, Welsh and Hodgson 2008). Steep gradients allow for flushing of fine sediments, although gradient effects may be more pronounced in harvested compared to primary forest habitat (Corn and Bury 1989). Adults are positively associated with high rainfall, moist forest habitats, and pool habitat, and negatively associated with fine sediment loads (Welsh and Lind 2002, Ashton et al. 2006). Adults and larvae in the Mattole Watershed were restricted to headwater channels, and canopy closure was the best single predictor of A. truei presence (Welsh and Hodgson 2011). Ascaphus truei were never detected in streams where canopy closure was less than 83% (Welsh and Hodgson 2011).
Some researchers have suggested a positive association between A. truei and the presence of harder, more consolidated parent geologies because they produce less sediment (Diller and Wallace 1999, Dupuis et al. 2000, Wilkins and Peterson 2000). However, A. truei does occur in streams with unconsolidated geologies, such as those derived from marine sediments, particularly in areas not subjected to recent or historical anthropogenic disturbance (e.g., Adams and Bury 2002, Welsh and Lind 2002, Ashton et al. 2006). The absence of A. truei from some streams with unconsolidated geologies may be because the presence of easily erodable substrates exacerbates the impacts of habitat disturbance, which can have long-lasting effects (Adams and Bury 2002, Welsh and Lind 2002, Ashton et al. 2006).
Ascaphus truei is extremely sensitive to warm temperatures at all life stages. Eggs have a temperature tolerance range from 5°C to 18.5°C (Brown 1975a). The critical thermal maximum range for larvae is 28.9–30.1°C, and larvae avoided temperatures above 22°C in laboratory trials (de Vlaming and Bury 1970). First-year larvae collected from Del Norte County selected temperatures below 10°C along a thermal gradient in the laboratory, while second-year larvae selected temperatures closer to 15°C (de Vlaming and Bury 1970). The critical thermal maxima for adults ranged on average from 27.6°C to 29.6°C (data from A. montanus; Claussen 1973). Field temperatures at occupied sites are usually well below these limits, with larvae occurring in streams with a mean of 11.6°C (range 5.7–15.8°C; Welsh and Hodgson 2008).
In addition to narrow thermal tolerances, A. truei is also extremely sensitive to desiccation (Brattstrom 1963), which may limit adult use of upland habitat to periods of wet weather conditions (Nussbaum et al. 1983). One mark-recapture study in Humboldt County documented movements of only 0–30 m along the stream channel over a two-year period (Burkholder and Diller 2007). However, recapture probabilities were low, and some animals may have moved beyond the study area. Longer distance movements have been documented from populations outside of California, from tens of meters up to 400 m into upland habitat (McComb et al. 1993, Gomez and Anthony 1996, Vesely 1996, Wahbe et al. 2004, Matsuda and Richardson 2005). Seasonal variation in adult location in managed forests in Washington was hypothesized to be a localized breeding migration, with downstream movements for oviposition and a return upstream in late summer (Hayes et al. 2006). It is unknown whether similar movements also occur in older, less disturbed forests in the area. In an A. montanus population in Montana, seasonal movements may be due to behavioral thermoregulation (Adams and Frissell 2001).
Distribution (Past and Present)
Ascaphus truei ranges from British Columbia to northern California, mostly west of the Cascades Mountains (Stebbins 2003). California is the southern limit of the range, with A. truei occurring south from the Oregon border along the coast to Mendocino County and east to Shasta County (Grinnell and Camp 1917, Mittleman and Myers 1949, Salt 1952, Bury et al. 1969, Welsh 1985). Ascaphus truei ranges from near sea level in Humboldt County up to 2150 m in the Trinity Alps (J. Garwood, pers. comm.).
Random sampling of streams has documented higher occupancy rates for A. truei in unmanaged or older forests compared to managed or younger stands (Welsh 1990). We therefore assume that some historically occupied localities are no longer occupied due to disturbance. In one study in the Mattole Watershed in Mendocino and Humboldt counties, A. truei was present in 71% of streams in old and mature forests, but was not found in second growth forests (Welsh et al. 2005). Further studies in the Mattole Watershed have found A. truei in 67% (14/21) of streams in unmanaged forests, but only in 4% (1/28) of streams in managed stands (H. Welsh and G. Hodgson, unpublished data). Streams with mixed harvest histories in the South Fork of the Trinity River had an intermediate level of occupancy, with 28% of streams occupied (17/60; Welsh et al. 2010). Studies from outside of California also indicate that A. truei is present in a greater proportion of streams in unmanaged forests (Bury and Corn 1988, Corn and Bury 1989, Hayes et al. 2006). A survey of streams in private, managed timber lands all less than 80 years old along the northern California coast found stream occupancy rates of 37% (18/49) at the level of 30 m sampling reaches and 76% (54/72) at the level of entire stream reaches (Diller and Wallace 1999). The relatively high occupancy rates in these young forests are thought to be due to the ameliorating effect of maritime climate, as most sites were within 30 km of the coast (Bury 1968, Diller and Wallace 1999).
Trends in Abundance
Ascaphus truei tends to be lower in abundance in managed compared to unmanaged forest stands (Bury and Corn 1988, Corn and Bury 1989, Welsh 1990, Gomez and Anthony 1996, Welsh and Lind 2002, Ashton et al. 2006). Clear-cuts can have immediate effects on abundance. Larval densities were higher in late-succession and old-growth forests compared to adjacent clear-cuts lacking streamside buffers in Oregon and British Columbia (Dupuis and Steventon 1999, Biek et al. 2002). Upland pitfall trapping in clear-cuts and mature forests in British Columbia found similar total numbers of A. truei in both forest types, but very few adults in clear-cuts, suggesting that immature frogs in clear-cuts are transients or incur high mortality rates (Matsuda and Richardson 2005). Several researchers have predicted declines or continuing declines if anthropogenic disturbances continue (e.g., Corn and Bury 1989, Dupuis and Steventon 1999, Welsh and Lind 2002, Ashton et al. 2006, Olson et al. 2007).
Nature and Degree of Threat
Declines and local extirpations to date are largely due to land management including timber harvesting and road construction (Welsh and Ollivier 1998, Welsh et al. 2005). Marijuana cultivation and climate change are also emerging as potential threats to this taxon.
The mechanisms underlying declines and extirpations due to timber harvesting and road construction are primarily increased sedimentation, increased stream temperatures, and fragmentation. While the initial impacts of road construction may be relatively short-lived, longer-term impacts are caused by sedimentation due to runoff from poorly maintained dirt and gravel roads (L. Diller, pers. comm.). Reduced canopy cover does not seem to increase temperatures as much at high-elevation sites, and Ascaphus truei may be more resilient to timber harvesting in areas where stream temperature is cooler due to overall climate (e.g., Diller and Wallace 1999, Wahbe and Bunnell 2003). Reductions in canopy or riparian vegetation that result in increased light levels may cause shifts in the algal community (i.e., from diatoms to filamentous green algae) that negatively affect the quality and abundance of larval food (L. Diller, pers. comm.). Landscape genetic studies in Washington suggest that significant overland dispersal occurs through terrestrial habitat, with gene flow detected between populations on a scale of 25–30 km (Spear and Storfer 2008). While timber harvests have some initial effect on gene flow, it may take multiple generations before the effects of fragmentation on population genetic structure can be detected.
An emerging threat to A. truei is large-scale marijuana cultivation, though little data is currently available due to limited accessibility of private lands. Similar to timber harvesting, marijuana cultivation requires clearing land and building roads which can increase sedimentation. Contamination from pesticides used on marijuana grows has been documented to negatively affect mammals in the field (Thompson et al. 2014), and amphibians are likely to be susceptible as well because of their permeable skin. Of particular concern for headwater amphibians like A. truei is the dewatering of waterways that are diverted for irrigation (CDFG 2013).
Climate change poses potential risks to A. truei through increased temperatures, changes in hydrology, changes in fire regime, and vegetation shifts. Mean annual temperatures are expected to increase throughout northwestern California (reviewed in PRBO 2011). The frequency of extremely hot days is projected to increase, with roughly nine additional days over 32.2°C (Bell et al. 2004). Such temperatures exceed the critical thermal maxima for all life stages of A. truei, though water temperatures, microhabitat structure, and behavioral thermoregulation may ameliorate these effects. For coastal populations, upwelling is expected to intensify, which may increase fog development and contribute to cooler, moister conditions (Snyder et al. 2003, Lebassi et al. 2009). Coastal areas may therefore continue to provide more favorable climatic conditions than areas farther inland. Potential changes in precipitation are less clear, with some models predicting modest increases, some modest decreases, and some reductions in rainfall of up to 28% (reviewed in PRBO 2011). Warmer temperatures will result in less precipitation stored as snow, and reductions of 30–80% are predicted for snowpack accumulation in northwestern California (Snyder et al. 2004, Cayan et al. 2008b). The timing of spring snowmelt has shifted later in the spring in this region over the last 50 years (Stewart et al. 2005), though the timing of future shifts is unknown. Reductions in water availability due to reduced snowpack and possibly reduced precipitation will affect the timing and magnitude of stream flows and may lead to a mismatch between the timing of breeding and appropriate stream conditions. How fire regime will be affected by climate change in northwestern California is not well understood. Some models predict little change in fire regime or even decreases in area burned along the northern coast (Fried et al. 2004, Lenihan et al. 2008). Increases in area burned have been predicted for the southern coast of northwestern California (Lenihan et al. 2008). Westerling et al. (2011) projected a 100% increase in area burned in northwestern California under some scenarios. Direct mortality of adults and larvae due to fire has been documented in A. montanus populations (P. Van Eimeren, pers. comm., in Pilliod et al. 2003, Hossack et al. 2006). Short-term impacts of fire may be due to warmer temperatures and/or increased ammonia levels or other changes to water chemistry (Pilliod et al. 2003), but long-term impacts are understudied. Vegetation communities are expected to shift from moist conifer to drier mixed evergreen forest, with reductions in Douglas fir and redwood forest in particular (Lenihan et al. 2008, PRBO 2011). It is unclear what effect these shifts may have on A. truei because stream conditions and forest age seem to be more important indicators of habitat quality than forest type.
Status Determination
Ascaphus truei is a specialist of cold, headwater stream habitats in old and mature forests, a habitat type that incurs substantial disturbance from land management activities. Declines in distribution and abundance have been documented in response to anthropogenic disturbances, and climate change has the potential to further negatively impact this species. These factors all contribute to a Priority 2 designation for this species.
Management Recommendations
Remaining old and mature forest habitats should be protected, with a focus on managing the entire stream network (Olson et al. 2007, Welsh 2011). Retaining streamside buffers on managed lands can help mitigate the effects of logging and roadbuilding, but more research is needed to determine buffer prescriptions, particularly how to preserve stream network processes (Olson et al. 2007). One model recommends riparian management zones 40–150 m wide and patch reserves along headwater streams to accommodate upland habitat use and promote connectivity among drainages (Olson et al. 2007). The ecological effects of buffer protections may vary across habitat types, and narrower buffers may be effective in more mesic coastal habitat compared to more xeric inland sites in the California range of Ascaphus truei.
Construction of new roads should be minimized or avoided in areas where protecting A. truei is a high conservation priority. To reduce the sedimentation impacts of runoff from roads, forest roads should be disconnected from stream systems (e.g., through the use of ditch-relief culverts). Use of heavy equipment should be avoided or restricted on forest roads when larvae are present in nearby aquatic habitat. Road management strategies should be applied to all forest roads, not just those used for timber harvest.
Ascaphus truei management would benefit from greater legal clarity regarding state and federal law on marijuana cultivation in California. Currently, some cultivation is legal under state law but prohibited under federal law, which may be hampering regulation of cultivation sites. Greater enforcement of existing environmental and land use laws is needed, and development of additional regulations should consider environmental impacts on A. truei.
Monitoring, Research, and Survey Needs
The presence of uncut streamside buffers on the entire channel network can ameliorate the impacts of land management on Ascaphus truei populations, but more research is needed into optimum buffer widths as they relate to different life history requirements and different portions of the catchment network. Studies from A. truei populations in British Columbia and Oregon have found positive effects of buffers 5–60 m wide (Bull and Carter 1996, Dupuis and Steventon 1999, Stoddard and Hayes 2005, Pollett et al. 2010). Experiments to determine optimal buffer widths in California habitats are needed. We recommend, at a minimum, that comparative data from coastal Mendocino County (the southern limit of the species range), coastal Humboldt/Del Norte Counties (the northern limit of the species range in California), and inland Trinity County are needed to assess the minimum forest buffer on industrial timber lands to retain key temperature and stream clarity conditions for A. truei.
Much of the research on A. truei has focused on stream-breeding habitat and presence/absence studies. While more difficult, monitoring efforts to document abundance and population dynamics are needed to gain insight into declines that cannot be inferred from presence/absence surveys (Welsh 2011). Such studies could also determine which life history stages limit population growth in this species. When possible, population estimates in managed forests should be compared to A. truei abundance in nearby undisturbed mature forest stands (i.e., reference populations) to assess the impacts of disturbance (Welsh 2011).
More studies are needed on use of upland habitats by adults and dispersing animals. Such studies should be targeted at identifying terrestrial habitat corridors, if present, which can then be protected to maintain connectivity among populations (Olson et al. 2007, Olson and Burnett 2009). Landscape genetic analyses from replicate California populations may be particularly informative, given that recent studies from different parts of the range reach different conclusions about population connectivity (Spear and Storfer 2008, Spear and Storfer 2010, Aguilar et al. 2013).
Field research on impacts of marijuana cultivation on amphibian populations would contribute to development of environmental regulations for this growing industry and inform management strategies in cultivated areas.