SONORAN DESERT TOAD
Bufo alvarius Girard 1859

Sonoran Desert toad, Cochise County, Arizona. Courtesy of Rob Schell Photography.

Status Summary
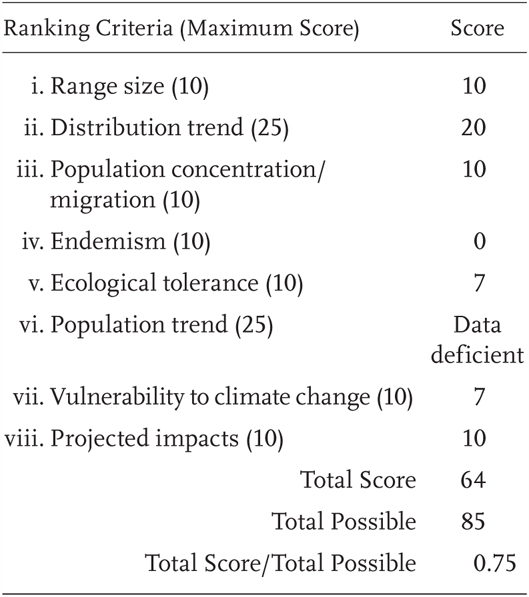
Bufo alvarius is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 75% (64/85). During the previous evaluation, it was also designated as a Species of Special Concern (Jennings and Hayes 1994a). The species has not been found in California since 1955 (but see the “Distribution” section).
Sonoran Desert Toad: Risk Factors
Identification
Bufo alvarius is a large (10.1–19.0 cm SVL) olive, brown, or gray toad with prominent cranial crests and large elongate paratoid glands (Stebbins 2003). The skin is smoother than in other North American toads, with few warts along the dorsum. Bufo alvarius has large warts on the hind limbs and prominent white warts at the corners of the mouth (Stebbins 2003). The call is a low-pitched bleat or screech (Elliott et al. 2009).
This species is unlikely to be confused with any other anuran within its California range. All other true toads (family Bufonidae) in the region have extensive warts over the entire dorsal surface and lack large warts on the hind legs. The spadefoots (Scaphiopus and Spea, family Scaphiopodidae) are much smaller as adults and have a conspicuous black keratinized spade on the ventral surface of the rear feet.
Taxonomic Relationships
The validity of this taxon has never been questioned, although confusion about the type specimen and locality has been discussed (Fouquette 1968). Osteological and genetic data, as well as call characteristics, suggest that it is related to Central American bufonids (Tihen 1962, Martin 1972, Sullivan and Malmos 1994, Pauly et al. 2004, Frost et al. 2006a).
Frost et al. (2006a) suggested a taxonomic revision that placed this species in the genus Cranopsis. Based on subsequent discoveries of older available names, the genus name for this taxon was later revised to Ollotis (Frost et al. 2006b) and then Incilius (Frost et al. 2009b, Pauly et al. 2009). We retain the older taxonomy both for taxonomic stability and because the analyses supporting the original rearrangement are controversial (Crother 2009, Frost et al. 2009a, Pauly et al. 2009).
Life History
The life history of this species in California is unknown, and we base the following discussion on observations from other areas. Bufo alvarius spends much of the year underground, presumably in rodent burrows (Degenhardt et al. 1996). Bufo alvarius is primarily nocturnal and becomes active before summer rains. It is more strongly aquatic than most North American toads (Stebbins 1951). Breeding behavior appears to be generally associated with summer rains (Sullivan and Malmos 1994), although amplexus has been reported in stock ponds before rains have occurred (Degenhardt et al. 1996). Several years may pass between breeding events depending on the presence of sufficient rainfall (Sullivan and Fernandez 1999). The species sometimes congregates in large numbers for breeding, with nearly all reproduction of a local breeding population occurring in a single night (Degenhardt et al. 1996). The time required for hatching and metamorphosis is unknown but may be less than a month (notes of Thornber, reported in Ruthven 1907 and Storer 1925). This species appears to be a dietary generalist, feeding on any live arthropod or small vertebrate prey that it can successfully capture (Stebbins 1951, Cole 1962). Poison secreted by the skin and paratoid glands is particularly toxic and has caused death and paralysis in dogs and is a potent hallucinogen in humans (Musgrave 1930, Stebbins 1951, Stebbins 2003).
Habitat Requirements
The habitat requirements for Bufo alvarius in California are unknown. In arid habitats of Arizona and New Mexico, the species can be found in and around a variety of water sources used for breeding, including springs, stock ponds, washes, river bottoms, and irrigation ditches (Stebbins 1951, Stebbins 1972), though it is occasionally found at great distances (>1 mi) from water (Slevin 1928). Upland habitat surrounding known aquatic breeding localities elsewhere in the range include mesquite-creosote desert lowland, arid grassland, rocky riparian zones, oak–sycamore–walnut assemblages in mountain canyons, and montane pine–oak–juniper plant communities (Stebbins 2003, Fouquette et al. 2005).
Distribution (Past and Present)
There are no known extant populations in California. Historically, the species ranged in California along the Lower Colorado River and into the Imperial Valley (Grinnell and Camp 1917, Stebbins 1951, Jennings and Hayes 1994a), likely ranging as far north as the southern tip of Nevada (Cooper 1869). It is not known if records in the Imperial Valley are a natural part of the historic range or whether they represent recent range expansion following the development of irrigation (Stebbins 1951).
The last verified record (LACM 87044) from California dates to 31 July 1955, 7 km north of Winterhaven. More recent surveys have failed to detect the species (King and Robbins 1991, Jennings and Hayes 1994b). Sporadic records continue to be reported on the Arizona side of the Colorado River, however. Several individuals were found near the Cibola National Wildlife Refuge in 1980 (Anderson and Ohmart 1982; B. Anderson, pers. comm.), and a single individual was found at the refuge itself in 1986 (J. Rorabaugh, pers. comm.). On 1 July 2004, a large individual was found “by the golf course on the Parker Strip,” La Paz County, Arizona (J. Rorabaugh, pers. comm.). On 29 July 2009, an amplexing pair was found along the Bill Williams River at Planet Ranch, Mohave County, Arizona, and the species is reportedly “fairly common” 24–32 km above the confluence of the Gila and Colorado Rivers, Yuma County, Arizona (J. Rorabaugh, pers. comm.). A single, unverified record of a calling Bufo alvarius was reported near Bard, California, in the spring of 2007 or 2008, though the time of year was unexpected and the observer was inexperienced with the species (J. Rorabaugh, pers. comm.).
Outside of California, B. alvarius ranges across southern Arizona to the southwestern corner of New Mexico and south into Sonora and the northern edge of Sinaloa, Mexico. The known elevational range extends from near sea level to 1615 m (Cole 1962).
Trends in Abundance
Though the paucity of records from California makes assessing former abundance difficult, Bufo alvarius was apparently common at Yuma, Arizona, on the California border, along the Lower Colorado River, and in parts of the Imperial Valley (Slevin 1928, Klauber 1934). As no populations are currently known in these areas, declines leading to probable population extirpations or extremely low population sizes have clearly occurred. The species is also known to be declining in New Mexico (Degenhardt et al. 1996). Throughout the rest of the range the species appears to be stable and abundant at many localities (Fouquette et al. 2005, Lazaroff et al. 2006), though some have suggested that declines are occurring throughout the range (B. Brattstrom, R. Ruibal, and C. Schwalbe, pers. comms., reported in Jennings and Hayes 1994a)
Nature and Degree of Threat
The causes of declines, and therefore the threats to this species, are poorly understood. Declines occurred before any studies were carried out in California, though it is likely that landscape modification and pesticide applications that occurred with the growth of agriculture in the Imperial Valley contributed to declines (Ohmart et al. 1988, Jennings and Hayes 1994a). Bufonids are generally very susceptible to amphibian declines (Stuart et al. 2004). In California, toad declines have been linked to habitat loss and pesticide use (Davidson et al. 2002) and pathogenic fungi (Green and Kagarise Sherman 2001).
Status Determination
The declines and possible extirpation of Bufo alvarius in California are the primary concerns for this taxon. The species may require permanent aquatic environments making it a moderate ecological specialist, given the arid environments that characterize its range. This also makes the taxon sensitive to the effects of climate change, particularly changes in hydrology and the increasing year-to-year variation in precipitation that have been projected (Cayan et al. 2008b). Finally, because little understanding of the causes of declines in California exists, we are poorly positioned to protect any remaining populations should they be found in future surveys.
Management Recommendations
The development of an effective management strategy for Bufo alvarius in California is not possible without further distributional and ecological information. As no populations are currently known, the first management priority should be to undertake comprehensive surveys, as described below, aimed at identifying remaining fragmentary California populations. Habitat protection and enhancement would then become the critical management tools to build these populations to larger and viable sizes. Simultaneous ecological research is also needed on habitat use, home range size, life history, and population connectivity before more complex management programs focused on reestablishing the species are considered.
Monitoring, Research, and Survey Needs
A critical first step toward developing a comprehensive management plan for this species is to undertake comprehensive surveys of remaining potential habitat in southeastern California. These surveys should take place during the summer rains and should involve biologists who are familiar with Bufo alvarius’ breeding behavior. If any remaining populations are found, a population-monitoring program should rapidly be established to determine both geographical extent and population size. As little is known about this species in California, this monitoring program should take place in conjunction with a study of the species’ life history and habitat use, in California and/or adjacent Arizona. These surveys should specifically target the remaining moist areas of the southwest California deserts that are known to support other water-dependant vertebrate species, such as the desert mule deer (Odocoileus hemionus crooki). Using existing survey data from other, and better known, species may help to guide toad survey efforts toward the wettest areas or most consistent water supplies, thereby increasing odds of detection.
A second critical priority is to work with wildlife managers in Arizona to survey for and study the nearest remaining populations on the Arizona side of the Lower Colorado River. These populations are likely the most ecologically similar to the former California populations and should therefore provide information valuable to the eventual development of management programs in California. Genetic samples from both California and Arizona should be collected to help inform managers about levels of genetic differentiation, and therefore the appropriateness of possible reintroduction of Arizona animals to California.
As any populations that remain in California are likely isolated, study of these populations is unlikely to yield information on the metapopulation dynamics that we presume are key in sustaining this species elsewhere. Reestablishing these dynamics would form an important part of a comprehensive management program in California, and research focused on better understanding these dynamics will also need to take place outside of California, preferably in adjacent Arizona.