YOSEMITE TOAD
Bufo canorus Camp 1916a

Yosemite toad, Mono County, California. Courtesy of Rob Grasso.

Status Summary
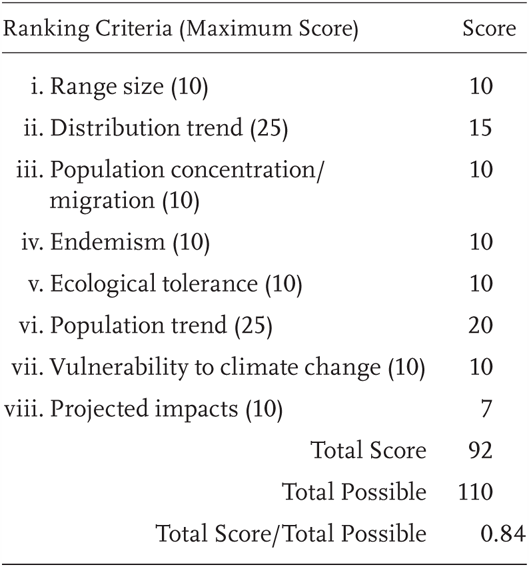
Bufo canorus is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 84% (92/110). During the previous evaluation, it was also considered a Species of Special Concern (Jennings and Hayes 1994a). This species is also listed as Threatened under the US Endangered Species Act.
Yosemite Toad: Risk Factors
Identification
Bufo canorus is a moderately sized (1.0–7 cm SVL) sexually dichromatic toad (Camp 1916a, Grinnell and Storer 1924, Kagarise Sherman 1980, Stebbins 2003). Females and juveniles have tan or brown dorsal coloration with extensive dark blotches over the dorsal surface and legs that are edged with white or cream. Males are pale green-yellow or olive green above without, or with only minimal, dark blotching or flecking (Camp 1916a, Stebbins 2003). A pale, very thin middorsal line is often present in juveniles and young females, but is usually lost in males and older females (Camp 1916a). The paratoid glands are large, flat, and circular, and are separated by a space less than or equal to their diameter (Camp 1916a). The advertisement call of this species is a musical trill lasting 3–9 s (Elliott et al. 2009). The specific epithet “canorus” (Latin for “tuneful”) refers to the melodic quality of the call (Camp 1916a, Karlstrom 1962).
This species may be confused with high-elevation populations of the western toad (B. boreas). Bufo boreas often has a conspicuous light middorsal stripe and smaller, more widely spaced paratoid glands that are separated by a space approximately twice their diameter (Stebbins 2003). Bufo boreas also has more extensive webbing on the hind feet than B. canorus (Camp 1917). Populations of B. boreas that occur in the Sierra Nevada do not produce advertisement calls; thus, toad breeding choruses are diagnostic for B. canorus (Mullally 1956). Juveniles of the two species (<20–30 mm) are very similar to one another, and identifications of this size class should be made with caution (Karlstrom 1962).
Taxonomic Relationships
Bufo canorus was initially described on the basis of coloration and morphology (Camp 1916a). Its status as a distinct species has gone largely unquestioned since this time. Subsequent genetic analyses based on mitochondrial data suggest that this taxon is a close relative of B. boreas (Shaffer et al. 2000, Pauly et al. 2004). In addition, mitochondrial DNA data suggest that B. canorus may be paraphyletic with respect to the black toad, B. exsul, and some lineages of B. boreas (Graybeal 1993, Shaffer et al. 2000, Goebel et al. 2009). These relationships have not been corroborated with nuclear sequence data, and thus it is unclear whether cryptic diversity exists within the taxon or if this is a case of mitochondrial introgression. Unpublished genetic data suggest that mitochondrial introgression associated with past or ongoing hybridization may explain these results (G. Pauly, unpublished data). Some morphological variation has been observed in size and degree of melanism in eggs and larvae of this taxon (Karlstrom and Livezey 1955, Karlstrom 1962). Whether this is plasticity in response to elevation and/or the local environment or genetic differentiation has not been investigated further. Mitochondrial data also indicate that some intraspecific variation and isolation by distance may exist within the taxon (Shaffer et al. 2000, Pauly et al. 2004, Goebel et al. 2009), although sample sizes in these studies were small. Wang (2012) collected data from 10 microsatellites for toads from 24 populations in Yosemite National Park and found significant variation in the amount of genetic distance between populations. This analysis concluded that environmental factors such as slope and precipitation were associated with genetic structure.
Frost et al. (2006a) recommended placing this species and many other North American bufonids in the genus Anaxyrus, although this proposal and the analyses that support it are controversial (Crother 2009, Frost et al. 2009a, Pauly et al. 2009).
Life History
Bufo canorus is primarily a diurnal toad that occasionally exhibits crepuscular or nocturnal activity on warm days (Mullally 1956, Martin 2008). Males emerge from hibernation as soon as snowmelt pools form along the margins of preferred high-elevation meadow habitat and quickly form breeding choruses (Karlstrom 1962). As in many high-elevation amphibians, the timing of emergence is correlated with elevation, and generally occurs in May and June (Karlstrom 1962, Kagarise Sherman 1980). Males are territorial and often maintain interindividual spacing of 7–14 m (Karlstrom 1962, Kagarise Sherman 1980). Fighting occurs between males that encroach on one another’s territory (Kagarise Sherman and Morton 1984). Breeding activity and egg-laying commence soon after males begin calling, with females depositing eggs along shallow edges of pools and streams in meadows (Karlstrom 1962, Kagarise Sherman 1980). Hatching occurs in as few as 3–4 days at relatively high water temperatures (20–23°C) or up to 10–14 days at lower temperatures (16–17°C) (Karlstrom 1962, Kagarise Sherman 1980). Metamorphosis occurs approximately 40–60 days after oviposition, again depending on temperature and elevation (Karlstrom 1962, Kagarise Sherman 1980, Kagarise Sherman and Morton 1984). The seasonal activity period extends into late September and early October, after which toads hibernate in rodent burrows, crevices under rocks, and root tangles (Kagarise Sherman 1980). Adult toads do not begin to breed until they are 3–6 years old, after which females may only breed every few years (Kagarise Sherman 1980, Kagarise Sherman and Morton 1984). Adults grow slowly, averaging only 2.5 mm per year at Tioga Pass, Tuolumne County, California (Kagarise Sherman and Morton 1984). The post-metamorphic diet consists of a variety of small arthropods including ants, bees, flies, wasps, beetles, millipedes, and spiders (Grinnell and Storer 1924, Mullally 1953, Kagarise Sherman and Morton 1984). The slow growth rate and lack of breeding every year is likely attributable to low metabolic rates associated with low caloric intake and relatively cold temperatures (Kagarise Sherman and Morton 1984). In the wild, adults appear to be able to tolerate a relatively wide range of temperatures (from 2°–30°C) (Karlstrom 1962), although they prefer temperatures higher in this range (Cunningham 1963). The estimated critical thermal maximum is 37–40°C for adults and 36–38°C for larvae (Karlstrom 1962).
Bufo canorus is known to occur sympatrically with B. boreas in two areas (see the “Distribution” section) and may occasionally hybridize. At the Frog Lakes locality, individuals that are morphologically intermediate in paratoid gland width and the extent of webbing on the hind feet occur and may represent natural hybrids (Morton and Sokolski 1978). No putative hybrids have been described from the Blue Lakes locality (Karlstrom 1962), although some authors suggest that hybridization may also occur there (Stebbins 2003). Artificial crosses in the laboratory readily produce hybrids (Karlstrom 1962).
Habitat Requirements
Bufo canorus prefers relatively open high-elevation meadows vegetated with grasses, sedges, rushes, and/or willow stands (Karlstrom 1962). This species can be found in the margins of water bodies that form from snowmelt runoff, as well as in moist meadows. During the early part of the active season, individuals are often localized along meadow margins within approximately 30 m of the forest edge. This behavior may allow them to easily retreat to forest cover at night to avoid freezing temperatures (Karlstrom 1962). As the active season progresses and nights become warmer, the toads tend to move toward the center of meadows and become less restricted to the margins (Karlstrom 1962). This species prefers shallow (probably <7.5 cm) snowmelt pools on the margins of meadows or very slow moving runoff streams in which to breed, although they have also been found in deeper (>3 m) permanent pools (G. Fellers, pers. comm.). These need to be deep enough to avoid premature desiccation—a significant cause of mortality for larvae—but shallow enough to achieve the temperatures needed for rapid development (Karlstrom 1962). This species may prefer to oviposit in dark-bottomed pools, particularly at high elevations, as these may provide warmer water temperatures and more rapid larval development (Karlstrom 1962). The presence of pocket gopher, mouse, and vole burrows may provide additional beneficial cover and protection from predation (Grinnell and Storer 1924, Karlstrom 1962).
Distribution (Past and Present)
Bufo canorus is restricted to a relatively small area approximately 240 km (north–south) by 60 km (east–west) in higher elevation areas of the Sierra Nevada (Karlstrom 1962, Kagarise Sherman and Morton 1993). It ranges from the vicinity of Blue Lakes, Alpine County, California, south past Kaiser Pass to the Evolution Lakes area, Fresno County, California (Grinnell and Storer 1924, Livezey 1955, Karlstrom 1962, Jennings and Hayes 1994a, Stebbins 2003, Davidson and Fellers 2005). The known elevational range extends from 1950 to 3599 m, with most localities between 2590 and 3048 m (Karlstrom 1962).
Between 1915 and 1992, this species exhibited declines throughout some areas of its range. Drost and Fellers (1996) resurveyed localities from Grinnell and Storer (1924) and found that this species had disappeared from 6 of 13 sites in the Yosemite area. Jennings and Hayes (1994a) also estimated that the species has disappeared from low-elevation areas on the western edge of the range, as well as at the northern edge of the range.
Trends in Abundance
In areas where Bufo canorus persists, marked declines in abundance have also been documented. In the Drost and Fellers (1996) resurveys, B. canorus was present in lower densities than in 1915 at three sites where it was still present. Between 1976 and 1982, the number of male toads entering breeding pools at Tioga Pass meadow declined from a maximum of 342 individuals to a low of 28, a ninefold decrease from the 1974–1978 mean (Kagarise Sherman and Morton 1993). However, the number of females entering breeding pools showed no obvious changes during this time period (Kagarise Sherman and Morton 1993). The average number of toads encountered in daily surveys also declined in the vicinity of Tioga Pass meadow between the early 1970s and 1990. In addition, these surveys documented declines in female toads, although they were not as severe as those documented in males (Kagarise Sherman and Morton 1993). Similar declines in abundance have also been documented at six additional localities in this region (Kagarise Sherman and Morton 1993).
Nature and Degree of Threat
The causes of decline in Bufo canorus require additional study. The declines have occurred in seemingly undisturbed areas and do not appear to be localized, suggesting that they are being driven by general changes to the environment, rather than localized causes such as habitat destruction. Several possible causes have been advanced, and more than one factor may be playing a role. These causes include environmental contamination, disease, drought and/or climate change, habitat modification due to grazing or other activities, human disturbance of breeding choruses, increased predation pressure from birds and fish, and pesticides. Based on current data it is not possible to understand in detail which, if any, of these factors are most important in B. canorus declines.
Snowmelt pools have extremely low acid neutralizing capacity, leading to the hypothesis that acidification of aquatic breeding habitat due to atmospheric deposition may be contributing to declines. Bradford et al. (1992) examined the effect of increasing acidification and the associated increase in dissolved aluminum on embryos and hatchlings of B. canorus. Embryos and hatchlings exposed to decreasing pH (and increasing aluminum solute) showed no increase in mortality at levels found in nature. However, these factors did cause earlier hatching and smaller body size at metamorphosis. Bradford et al. (1994) attempted to correlate the distribution of declining Sierran amphibians with these environmental factors and found no relationship, concluding that acid deposition was an unlikely source of amphibian declines in the Sierra Nevada.
Disease has also been considered as a factor in declines, though there is little evidence to date. Green and Kagarise Sherman (2001) examined the cause of death in 12 adult B. canorus that were found during a die-off that immediately preceded the population declines documented at Tioga Pass meadow by Kagarise Sherman and Morton (1993). They found that a variety of diseases and parasites were present in the population, and chytridiomycosis and septicemia, alone or in combination, caused the death of at least four individuals. However, no single infectious disease was present in more than 25% of the samples, which is far below the proportion typically observed in other die-offs caused by these diseases (Worthylake and Hovingh 1989, Berger et al. 1998, Vredenburg et al. 2010).
California experienced a relatively severe drought between 1987 and 1992, a time when B. canorus population declines were occurring (Roos 1992, Drost and Fellers 1996). Although it may have played an exacerbating role, drought alone seems unlikely to be responsible for declines. California experiences drought with some regularity, including during the time period of the Grinnell and Storer (1924) survey, which occurred before any major declines in B. canorus were observed (Drost and Fellers 1996). Drought does affect year-to-year reproductive success for this species, and prolonged drought may have a cumulative effect on populations (Kagarise Sherman and Morton 1993). Because climate change is expected to impact the amount of snow present in the Sierra Nevada and the speed and timing of snowmelt (Cayan et al. 2008b), drought might play an increasing role in declines of this species in the future.
Habitat modification is a leading cause of decline in many species throughout California and has been suggested as a factor for B. canorus. However, B. canorus is found largely within the boundaries of Yosemite National Park and other public (mostly National Forest) lands that have experienced varying impacts over the last 100 years. Drost and Fellers (1996) compared photos of habitat from the Grinnell and Storer (1924) surveys with current habitat and saw no apparent differences. Over the course of their 20-year study, Kagarise Sherman and Morton (1993) were also unable to detect significant habitat changes. That said, local impacts from changing habitat remain a potential driver of declines. All-terrain vehicle and snowmobile use in some localized areas may degrade habitat quality (D. Emery, pers. comm.). Some workers have postulated that livestock grazing in alpine meadows of National Forest land causes changes to hydrology, which may affect the suitability of breeding habitat and increase sedimentation in pools. Two recent, relatively short-term studies (5 years) have addressed this hypothesis: one that used experimental fencing treatments to exclude livestock from B. canorus breeding meadows and a second that included occupancy surveys across gradients of meadow moisture and livestock use levels (K. Tate and A. Lind, pers. comm.). Both of these studies demonstrated that meadow wetness was more influential in determining the current distribution and abundance of B. canorus than the level of livestock use. Sean Barry (pers. comm.) documented that toads seem to persist and even concentrate in areas that had been disturbed by cattle in the Kaiser Meadow population. It is also possible that the presence of cattle feces increases insect food supply for adult toads, although this remains untested. Martin (2008) suggests that the practice of fencing individual breeding pools to prevent grazing might actually lead to stronger habitat disturbance from cattle grazing in the terrestrial foraging habitats, potentially increasing the overall impact from grazing. These local-scale influences of livestock grazing along with more detailed and longer-term investigations of livestock use in the context of B. canorus metapopulation dynamics require further study.
Some researchers have suggested that increasing predation pressure could be causing declines. A variety of avian predators are known to feed on adult and larval toads, and increasing densities of common raven (Corvus corax) have been postulated as a possible cause of decline (Kagarise Sherman and Morton 1993). Ravens are known to prey upon other toad species in the B. boreas complex and likely also take B. canorus. Evidence suggests that declines in other amphibian species have occurred in areas where fish have been introduced (Drost and Fellers 1996). However, fish alone are unlikely to explain the declines in B. canorus. Most B. canorus reproduction takes place in ephemeral water bodies that do not contain fish (Drost and Fellers 1996). Knapp (2005) found no evidence for an effect of introduced trout on B. canorus presence and absence. Further, Grasso (2005) and Grasso et al. (2010) examined the palatability of early life stages of B. canorus to introduced brook trout and found that all life stages were highly unpalatable, suggesting that introduced trout may have little direct impact on populations.
Some authors have noted that breeding choruses of B. canorus are sensitive to human disturbance. Grinnell and Storer (1924) documented that choruses would abruptly stop calling when humans entered a meadow. They specifically noted that B. canorus seemed to be more sensitive to this disturbance than the sympatric Pacific treefrog (Pseudacris regilla). Karlstrom (1962) as well as several biologists presently working on this species disagree that this species’ calling behavior is impacted more strongly by human disturbance than other toad species. Karlstrom (1962) did notice wariness at night and that cars moving through the area even at 0.8 km distance would cause choruses to cease calling and that “the almost continual daytime traffic in [Yosemite National Park] might help to explain the paucity of roadside populations” of B. canorus. It has also been suggested that the relatively frequent handling and study experienced by some populations could induce stress and immunosuppression, which may also be playing a role in declines (Green and Kagarise Sherman 2001). To our knowledge, this possibility has not been investigated.
Davidson et al. (2002) found that areas where toads had disappeared were downwind from disproportionately large areas of agricultural land (primarily the low-elevation populations on the western side of the Sierra Nevada), suggesting that wind-borne agrochemicals may be a factor in declines. However, this relationship was not statistically significant.
When this evidence is taken together, it is clear that the causes of decline for B. canorus are still poorly understood. It is possible that several factors act in combination, perhaps interacting with variation in life history or metapopulation dynamics. Individual populations may be susceptible to localized extirpation due to small population sizes and the species’ slow maturation rate. Increasing frequency of localized extirpations could cause a breakdown of broader-scale metapopulation dynamics, leading to additional declines as recolonization ceases to counteract local population extirpations. Landscape genetic data suggest that migration rates between local populations are already low in several areas and this situation would likely be exacerbated by additional localized population declines and extirpations (Wang 2012). These inferences are still speculative, however, and further research is needed on many aspects of B. canorus population biology to better understand ongoing declines.
Status Determination
Declines in both distribution and abundance, coupled with a poor understanding of the factors leading to decline, are the major factors justifying a Priority 1 Species of Special Concern status.
Management Recommendations
An effective management program for this species will depend on identifying and prioritizing the factors leading to observed, ongoing declines. Until this is accomplished, protecting breeding meadows from disturbance of natural hydrologic regimes and water table dynamics and limiting human disturbance to meadows during the breeding season may be helpful in safeguarding populations. In addition, upland wintering habitats adjacent to breeding areas should also be protected from grazing and other disturbances.
Monitoring, Research, and Survey Needs
Ongoing monitoring and study of this species is required with a particular aim of identifying the major factors leading to decline. It is possible that some populations are relatively stable, and comparisons with declining sites could lead to important insights into reasons for declines and potential management solutions. Experimental work, going beyond the primarily correlational studies that have been carried out thus far, could also be helpful in identifying the most important factors. In particular, experimental studies of human disturbance, susceptibility to disease, and the potential role of reduced snowpack on hibernation and breeding biology would all be useful. Populations should also be monitored for disease outbreaks.
Further genetic work also needs to be completed to characterize genetic diversity within the species. Several studies have already been carried out, although they rely primarily on mitochondrial data alone, which is unable to distinguish true population substructure (or multiple lineages) from introgression from nearby B. boreas populations. Wang (2012) adds important information from the nuclear genome, but focuses on Yosemite National Park rather than the species’ range as a whole. Future studies should utilize multiple unlinked nuclear markers to clarify the diversity present in the species, gene flow among meadows, and effective population sizes.
Finally, the majority of survey efforts to date have focused on populations within the boundaries of Yosemite National Park. A committed survey effort is needed to better understand the location of populations, their trends in distribution and abundance, and their disease status and level of infection (or lack of) in areas outside of the park itself.