NORTHERN RED-LEGGED FROG
Rana aurora Baird and Girard 1852

Northern red-legged frog, Humboldt County, California. Courtesy of William Flaxington.

Status Summary
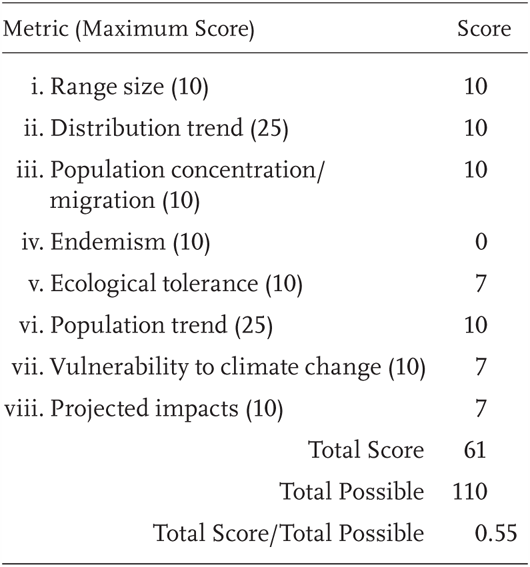
Rana aurora is a Priority 2 Species of Special Concern, receiving a score of 55% (61/110). Previously this species was included as a Species of Special Concern by Jennings and Hayes (1994a).
Northern Red-Legged Frog: Risk Factors
Identification
Rana aurora is a medium-sized (70–100 mm SVL) brown, red, gray, or tan frog with dorsolateral folds (Dumas 1966, Nussbaum et al. 1983). The dorsum varies from having indistinct, irregular black spots 2–3 mm in diameter with many tiny flecks to an allover network pattern of black lines (Dunlap 1955, Dumas 1966, Nussbaum et al. 1983). The dorsum can also be largely unmarked, though this is less common. A light lip line from eye to shoulder is usually present, often with a dark mask above (Nussbaum et al. 1983). Ventrally, the chest and abdomen are often marbled gray, with the groin area heavily and darkly mottled (Dunlap 1955). Red coloration on the venter and underneath the hind legs is typical but varies in intensity and extent (Dunlap 1955). Yellow coloration is common in the groin, as well as red and sometimes green coloration (Dunlap 1955). Larvae are dark brown from above, with scattered small clumps of metallic flecks and are 50–75 mm in TL (Nussbaum et al. 1983). In southern Mendocino County, this species could be confused with R. draytonii (Shaffer et al. 2004). Rana draytonii is a larger frog (up to 138 mm SVL; Stebbins 2003), and typically its dark dorsal markings have light centers (Stebbins 2003).
Taxonomic Relationships
Although initially described as a distinct species (Baird and Girard 1852), for much of the twentieth century northern red-legged frogs were regarded as the subspecies Rana aurora aurora (Camp 1917). Studies over the last few decades have supported the current taxonomic arrangement, with R. aurora and R. draytonii both recognized as distinct species. While they look superficially similar, these two species differ in morphology (vocal sacs, body size) and breeding behavior, and are genetically distinct (Hayes and Miyamoto 1984, Green 1985, Green 1986a, Green 1986b, Hayes and Kremples 1986, Shaffer et al. 2004). A narrow contact zone occurs between R. aurora and R. draytonii in southern Mendocino County (Shaffer et al. 2004).
Life History
Limited information is available on Rana aurora life history, with most studies occurring outside of its California range. Adults migrate to wetlands to breed for a few weeks between December and April when temperatures range from 4°C to 18°C (Storm and Pimentel 1954, Storm 1960, Dumas 1966, Licht 1969, Calef 1973). Males call beneath the water (Licht 1969, Brown 1975b). During one breeding season in Humboldt Bay National Wildlife Refuge, two breeding events more than a month apart produced over half of the egg masses (J. Betasso et al., unpublished data). Egg masses are 15–25 cm in diameter and contain approximately 500–600 eggs on average (Calef 1973, Licht 1974, Brown 1975b). Eggs are attached to emergent and floating vegetation, branches, or logs up to 150 cm below the water surface (Brown 1975b, Storm 1960, Calef 1973, Cary 2010). Surveys in Humboldt Bay National Wildlife Refuge found that most egg masses occurred between 30 and 60 cm elevation in the stream channel, at an average height of 37 cm above the bottom and 8 cm below the water surface (J. Betasso et al., unpublished data). In southwestern British Columbia, eggs were found at least a meter away from the pond edge or river bank (Licht 1969). Water temperatures near developing eggs in a Washington pond were 6.2°C on average (Brown 1975b), and embryos tolerate temperatures from 4°C to 21°C (Licht 1971). Dumas (1966) reared embryos at 11°C, 15°C, and 20°C, and observed the greatest embryo mortality at 20°C.
Embryonic development (from laying to hatching) takes 34–49 days (Storm 1960, Licht 1971, Brown 1975b). In Humboldt Bay Wildlife Refuge, most egg masses (103/232) persisted for 4 weeks before completely hatching out (J. Betasso et al., unpublished data). Larvae hatch at 8–12 mm long (Storer 1925, Storm 1960, Brown 1975b) and grow to up to 80 mm TL (Brown 1975b). Metamorphosis occurs after 3–7 months (Brown 1975b, Storer 1925) and metamorphs are 18–29 mm (Brown 1975b, Storm 1960, Calef 1973). Larger metamorphs are more likely to survive and to emigrate farther (Chelgren et al. 2006). In a Washington population, eggs were laid in February and March, the first larvae hatched in April, and metamorphosis was completed in late July (Brown 1975b).
Rana aurora juveniles disperse from breeding sites within days or weeks after transformation (Licht 1974, Licht 1986a). While daily movements of adults may be on average only a few meters per day, movements of several hundred meters to 4.8 kilometers have been documented over longer periods (Haggard 2000, Hayes et al. 2001, Chan-McLeod and Wheeldon 2004, Hayes et al. 2007).
Larvae are algal grazers (Dickman 1968). Metamorphs and adults are generalist predators of insects, spiders, and mollusks (Licht 1986b).
Habitat Requirements
Rana aurora occurs in mesic forests and riparian areas, which in its northern California range are primarily steep coniferous forests, coastal terraces, and floodplains (Nussbaum et al. 1983, Stebbins 2003). Rana aurora is relatively terrestrial for a ranid frog. Adults can occur hundreds of meters from water, and are often found in dense vegetated or downed log cover (Dunlap 1955, Dumas 1966). Adult frogs radio-tracked from March to July in Humboldt County were detected on land 90% of the time and usually within 5 m of water, though animals were found up to 80 m away from water (Haggard 2000). In habitat choice experiments, juvenile frogs spent most of their time out of the water (Pearl et al. 2004).
Both permanent and temporary breeding habitats are used, such as ponds, freshwater lagoons, lakes, and slow-moving streams (Licht 1969, Cary 2010, Sun 2012). Artificial habitats such as drainage ditches are also used (T. Fuller, J. Garwood, and M. van Hattem, pers. comm.). Coastal streams may be important dispersal corridors to inland populations. For example, R. aurora have been found outside of the breeding season in coastal streams in Humboldt and Del Norte Counties, and egg masses have been found in backwaters and alcoves of the Smith River where surrounding areas have been diked, drained, and converted (J. Garwood, pers. comm.). Both aquatic and terrestrial vegetation are important determinants of breeding habitat quality. In Humboldt County, egg mass presence was positively correlated with low canopy cover (ponds with less than ∼40% canopy cover are more likely to have egg masses present; Cary 2010). Egg mass density was higher in smaller ponds ( 2000 m2) and in ponds where the percentage of floating and emergent vegetation cover was at least ∼40% (Cary 2010). Surveys in Oregon also found support for the importance of emergent vegetation, as wetlands used for breeding had 27% open water on average compared to 50% open water in unused wetlands (Pearl et al. 2005a). Occupancy models fit to 5 years of survey data in Oregon predicted that local extinction probability decreased as the percentage of trees along the shoreline increased and surface area of emergent vegetation increased (Adams et al. 2011).
2000 m2) and in ponds where the percentage of floating and emergent vegetation cover was at least ∼40% (Cary 2010). Surveys in Oregon also found support for the importance of emergent vegetation, as wetlands used for breeding had 27% open water on average compared to 50% open water in unused wetlands (Pearl et al. 2005a). Occupancy models fit to 5 years of survey data in Oregon predicted that local extinction probability decreased as the percentage of trees along the shoreline increased and surface area of emergent vegetation increased (Adams et al. 2011).
Distribution (Past and Present)
Rana aurora occurs from Mendocino County, California, north along the west side of the Cascade Crest up through Vancouver Island and the adjacent mainland coast of British Columbia (Stebbins 2003). Populations also occur on Graham Island, British Columbia (Ovaska et al. 2002), and on Chichagof Island, Alaska (Hodge 2004). The elevational range extends from near sea level to 1160 m in Lane County, Oregon (Dunlap 1955), with populations in California occurring up to approximately 300 m (Jennings and Hayes 1994a). Two localities included on our map possibly extend the eastern edge and elevation range in California, and are in need of further investigation. A specimen collected by Camp in 1913 from eastern Mendocino County is in the UC Berkeley collection (MVZ 5068), photographs of which were reviewed by several experts. It is possible that the specimen is a misidentified R. draytonii, or it may be that R. aurora was historically more widespread. Despite the presence of potentially suitable habitat, contemporary CDFW biologists working in this region have not observed any R. aurora east of Highway 101 or in Mendocino National Forest (T. Fuller, pers. comm.). At another site, two individuals were found recently in eastern Humboldt County at around 800 m elevation (M. van Hattem, pers. comm.).
In California, surveys have found R. aurora to be mostly absent from the river bottom lands of the Eel, Mad, and Smith Rivers. These areas have undergone extensive habitat conversion to beef, dairy, and bulb farming, though populations may persist on inaccessible private lands (M. van Hattem, unpublished data). Surveys in Oregon’s Willamette valley found R. aurora at 50% of sites, with highest occupancy probability observed in seasonal sites without fish (Rowe and Garcia 2013).
Trends in Abundance
Population declines have been suspected for Rana aurora, particularly in Oregon’s Willamette Valley (e.g., Nussbaum et al. 1983; Hayes and Jennings 1986). However, systematic surveys are lacking. Data on R. aurora abundance in California are limited, particularly with regard to documenting trends over time. Mean density of egg masses in breeding ponds in Humboldt County during one breeding season was 0.2/m2, with densities up to 0.7/m2 observed (Cary 2010). In Del Norte County, 382 egg masses were found in a 40 × 40 m area of a pond near the confluence of East Fork Mill Creek and West Branch Mill Creek (J. Garwood, unpublished data). Surveys in California have found more egg masses in areas where natural vegetation buffers the breeding habitat compared to developed areas (M. van Hattem, unpublished data).
Nature and Degree of Threat
The major threat to Rana aurora is development and forest conversion leading to habitat loss and degradation. Other threats include introduced predators, disease, and climate change, though more data are needed on each of these stressors.
Due to issues such as low capture rates, it is unclear whether R. aurora abundance varies consistently with stand age in harvested forests (reviewed in Pearl 2005). For example, terrestrial (Welsh et al. 2007) and aquatic (Ashton et al. 2006) amphibian surveys in northwestern California forests have documented only a handful of R. aurora. In Washington, breeding sites with high primary forest cover within 2 km had higher egg mass counts, as did breeding sites greater than 0.25 km away from roads (Holcomb 2012). On Vancouver Island, radio-tracked frogs tended to move toward old-growth stands and away from clear-cuts <12 years old, suggesting that recolonization of impacted sites may require several years (Chan-McLeod 2003). In an Oregon study, the highest capture rates of R. aurora were in mature, mixed large sawtimber forest (Martin and McComb 2003).
Agricultural and residential development has likely contributed to habitat loss and degradation for R. aurora, and is projected to continue to increase in the future. For example, much of the Smith River coastal plain in Del Norte County has been converted to lily bulb production (J. Garwood, pers. comm.). In addition to habitat loss, such agricultural conversion can further degrade habitat through use of chemicals such as pesticides, herbicides, and fungicides. Similarly, the emerging issue of largely unregulated marijuana cultivation can degrade watersheds through grading and roadbuilding (which both destroy habitat and create runoff into aquatic habitats), application of pesticides and herbicides, and through dewatering of springs, streams, and wetlands used for irrigation (e.g., Thompson et al. 2014). Residential and commercial development is likely to increase in northern California, potentially leading to losses of breeding habitat or loss of access to remaining habitat. For example, the Humboldt County General Plan is currently being updated, with some proposals considering a doubling or tripling of rural development. However, R. aurora does use artificial habitat for breeding, and amount of urban cover was not a strong predictor of frog occurrence in surveys in Oregon (Rowe and Garcia 2013), suggesting some tolerance for certain kinds of habitat modification.
Introduced predatory fish and bullfrogs are widespread throughout R. aurora habitat in California, including sites near the coast (T. Fuller, J. Garwood, and M. van Hattem, pers. comm.). Negative impacts have been documented in mesocosm experiments, but field observations have yielded both negative and neutral effects of fish and bullfrogs on R. aurora distribution and abundance. Field-enclosure experiments in Oregon have shown reduced survivorship, shifts in microhabitat use, slower development, and smaller size at metamorphosis of R. aurora in the presence of fish and bullfrogs (Kiesecker and Blaustein 1998). Surveys in Oregon and Washington have found evidence for negative associations between R. aurora presence or abundance and the presence of nonnative fish but weak or no evidence for an effect of bullfrogs (Adams 1999, Pearl et al. 2005a, Rowe and Garcia 2013). Other studies in Oregon and Washington have not detected any effects of fish or bullfrogs on R. aurora presence (Richter and Azous 1995, Adams et al. 1998, Adams et al. 2011). Little data are available from California. Freshwater Lagoon and Big Lagoon in Humboldt County both have a long history of fish stocking, and surveys of suitable habitat during the 2010 and 2011 breeding seasons never found more than 1 egg mass in either lagoon (M. van Hattem, unpublished data). While introduced fish and bullfrogs can prey upon R. aurora, the population-level impacts of such predation are unknown. Gut content analysis of 5075 bullfrogs collected over 5 years on Vancouver Island found R. aurora in only 0.2% of stomachs (Jancowski and Orchard 2013.
Expected climate changes within the California range of R. aurora over the next 100 years include increased temperatures, sea-level rise, changes in hydrology, changes in fire regime, and vegetation shifts (reviewed in PRBO 2011). The frequency of extremely hot days is projected to increase, with roughly nine additional days over 32.2°C (Bell et al. 2004), though the effects of increased temperature are difficult to predict. A mesocosm experiment on larval R. aurora found that the combined effects of warming and drying can offset each other: warmer conditions result in more algal resources, allowing larvae to develop faster and escape costs of drying (O’Regan et al. 2014). Sea-level rises as high as 72 cm above 1990 levels are predicted under some models for California (reviewed in PRBO 2011), which may cause saltwater intrusion into estuarine habitat used for breeding. Upwelling is expected to intensify, which may increase fog development and contribute to cooler, moister conditions (Snyder et al. 2003, Lebassi et al. 2009), possibly facilitating terrestrial habitat use by this species along the coast. Potential changes in precipitation are less clear, some models predict either modest increases or decreases in rainfall, while others predict sharp reductions of up to 28%. (reviewed in PRBO 2011). Reductions in water availability due to reduced snowpack and possibly reduced precipitation will affect the timing and magnitude of stream flows, which may negatively affect habitat (Snyder et al. 2004, Stewart et al. 2005, Cayan et al. 2008b). How fire regime will be affected by climate change in northwestern California is not well understood. Some models predict little change in fire regime or even decreases in area burned along the northern coast (Fried et al. 2004, Lenihan et al. 2008), while increases in area burned have been predicted for the southern coast of northwestern California (Lenihan et al. 2008). Westerling et al. (2011) projected a 100% increase in area burned in northwestern California under some scenarios. How R. aurora responds to wildfire is unknown. Vegetation communities are expected to shift from moist conifer to drier mixed evergreen forest, with reductions in Douglas fir and redwood forest in particular (Lenihan et al. 2008, PRBO 2011). Loss of moist forest habitat would likely be detrimental to R. aurora; however, most of the predicted vegetation changes occur farther inland from its range.
Disease has been repeatedly implicated in amphibian declines, but to date there is little evidence that disease has played a major role in determining R. aurora abundance. While Bd has been documented from a high proportion of sites examined in Humboldt County (11/13; Nieto 2004, Sun 2012), the prevalence of infected individuals is relatively low ( 15%; Nieto 2004, Sun 2012). Water mold infection of egg masses has been observed in the field (Cary 2010, M. van Hattem, unpublished data) but population consequences of infection are unknown. Terrestrial versus aquatic life stages may respond differently to fungal infection. Juvenile metamorphs infected with Saproglenia in the lab did not have significantly higher mortality than uninfected individuals (Romansic et al. 2007), while two weeks of exposure was lethal to R. aurora larvae (Romansic et al. 2009a).
15%; Nieto 2004, Sun 2012). Water mold infection of egg masses has been observed in the field (Cary 2010, M. van Hattem, unpublished data) but population consequences of infection are unknown. Terrestrial versus aquatic life stages may respond differently to fungal infection. Juvenile metamorphs infected with Saproglenia in the lab did not have significantly higher mortality than uninfected individuals (Romansic et al. 2007), while two weeks of exposure was lethal to R. aurora larvae (Romansic et al. 2009a).
Status Determination
Rana aurora has a small range in California in a region that is undergoing continuing development, agricultural use, and timber harvest, making it a Priority 2 Species of Special Concern.
Management Recommendations
Management of Rana aurora should focus on addressing habitat degradation and loss due to development, timber harvest, and agriculture (including marijuana cultivation), introduction and spread of nonnative predatory fish and bullfrogs, and on minimizing unintended negative impacts due to salmonid restoration. Observations of higher abundance in breeding habitat with intact terrestrial vegetation nearby (though not excessively shading ponds; Cary 2010, Adams et al. 2011, Holcomb 2012) coupled with the terrestrial habitat use and long distances traveled by adults (Hayes et al. 2007) support the idea of maintaining vegetation buffers around breeding habitat in forested areas and setbacks between wetlands and development. Current regulations for development setbacks under the California Coastal Act of 1976 give distances from breeding wetlands of up to 30 m depending on land use. However, these setbacks are reducible upon request and we recommend that consistent, biologically based setbacks be developed. Rana aurora may experience less impact from timber harvesting methods that leave residual tree patches, particularly if multiple trees are included in patches between 0.8 and 1.5 ha in size and are near streams (Chan-McLeod and Moy 2007). Marijuana cultivation appears to pose a growing threat to maintenance of high-quality habitat for this species. Enforcement and regulation of marijuana cultivation is an ongoing issue in California and we suggest that the environmental impact of such activities be considered. Populations of introduced fish and bullfrogs should be prevented from invading R. aurora breeding habitat. While bullfrogs may already be widespread, intentional fish stocking should be restricted to avoid R. aurora habitat. Restoration projects for native salmonids should also take into consideration potential impacts to R. aurora that may be caused by converting freshwater wetlands to estuarine habitats and salt marshes.
Monitoring, Research, and Survey Needs
Monitoring of Rana aurora egg mass counts should continue in order to provide baseline data on distribution and abundance and to detect declines. Rana aurora management would benefit from additional study of movement and habitat use, life history, effects of marijuana cultivation, and impacts of introduced species in the field. Particularly as habitat becomes increasingly fragmented, data on connectivity among habitat patches, effects of road density, and use of terrestrial habitat away from breeding ponds can help inform appropriate setback distances and buffer configurations. Genetic studies may also be helpful for understanding patterns of frog movement across the landscape. Basic life history information overall and from the California range in particular is also lacking. Understanding saltwater tolerance of different life stages would be useful for predicting the extent of sea-level-rise effects on coastal populations. Field research on impacts of marijuana cultivation on amphibian populations would contribute to developing environmental regulations for this growing industry. Much of the concern for bullfrog impacts on R. aurora is from experimental mesocosm studies. Additional research that addresses the effects of bullfrogs and fish on R. aurora in the field is necessary to understand the community context of impacts, as the effects of bullfrogs in combination with fish may be greater than either singly (Kiesecker and Blaustein 1998), and fish may be facilitating bullfrog survival (Adams et al. 2003). Under the assumption that eradication of well-established introduced species is unlikely to be feasible at a large scale, a main goal of this work should be identifying factors that can potentially be manipulated to promote coexistence between R. aurora and nonnative predators, such as managing terrestrial and aquatic vegetation cover and hydroperiod.