FOOTHILL YELLOW-LEGGED FROG
Rana boylii Baird 1854

Foothill yellow-legged frog, Del Norte County, California. Courtesy of Rob Schell Photography.

Status Summary
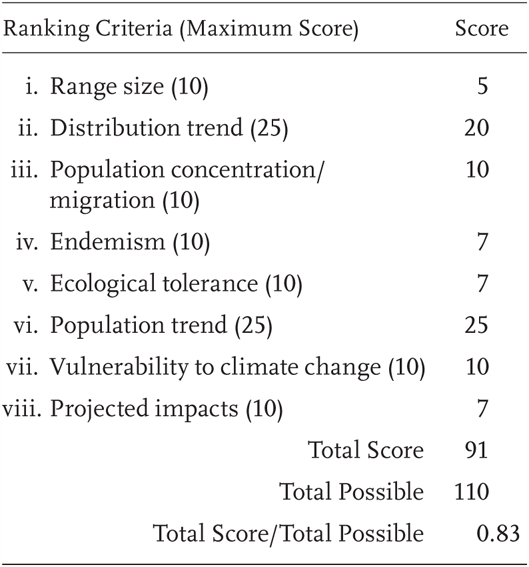
Rana boylii is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 83% (91/110). During the previous evaluation, it was also considered a Species of Special Concern, with varying levels of threat in different parts of the range (Jennings and Hayes 1994a).
Foothill Yellow-Legged Frog: Risk Factors
Identification
Rana boylii is a small to medium-sized frog (up to 81 mm SVL) (Stebbins 2003). The skin usually appears rough and granular, with many tiny tubercles on the surface, including on the tympanum (Nussbaum et al. 1983). The dorsal coloration is variable and can be gray, brown, reddish, or olive, sometimes with extensive brick-red coloration around the weak dorsolateral folds (Nussbaum et al. 1983). Individuals can also change their overall coloration from relatively light to dark (Wheeler et al. 2005). An inverted triangle-shaped patch of buff coloration is usually present on the snout, but its distinctiveness varies (Stebbins 2003). The ventral coloration is typically yellow on the hind legs and posterior abdomen, with the rest of the venter mostly white with dark mottling on the throat and chest (Nussbaum et al. 1983). Jennings and Hayes (2005) documented orange or red coloration on the ventral surfaces of the hind limbs in post-metamorphic animals from Glenn, Tehama, and Stanislaus Counties. Tadpoles reach a maximum size of 55 mm and are usually olive dorsally with dark spots or mottling that matches the stream substrate and a silvery venter (Nussbaum et al. 1983). Males call primarily underwater but will also call above (MacTague and Northen 1993).
Other species that R. boylii could potentially be confused with in California include the California and northern red-legged frogs (R. draytonii and R. aurora), the mountain and Sierra Nevada yellow-legged frogs (R. muscosa and R. sierrae), and juvenile bullfrogs. Rana draytonii and R. aurora have smooth skin, a prominent jaw stripe, distinct dorsolateral folds, and usually have red coloration under the hind limbs (although R. boylii can also have red ventral coloration, and young R. draytonii and R. aurora often have yellowish thighs) (Stebbins 2003, Jennings and Hayes 2005). Rana muscosa and R. sierrae have smoother skin, smooth tympana, and tend to lack the light patch on the snout (Stebbins 2003). Bullfrogs occasionally co-occur with R. boylii but tend to be greenish in color, with smoother skin, and large, smooth tympana (Stebbins 2003).
Taxonomic Relationships
Rana boylii has been recognized as a distinct species for a long period of time, although its phylogenetic placement among other North American ranids has been revised repeatedly (Baird 1854, Macey et al. 2001, Hillis and Wilcox 2005). Zweifel (1955) documented variation in color and morphology among California R. boylii populations. Recent phylogeographic studies have found that genetic variation among R. boylii populations is structured along hydrologic boundaries (Dever 2007, Peek 2010, Lind et al. 2011). In a range-wide phylogeographic study, Lind et al. (2011) identified some peripheral populations that are deeply divergent from populations within the core of the range. In California, populations in southern-most Monterey County west and south of the Salinas River Valley and populations from the southern Sierra Nevada were found to be phylogenetically distinct from the rest of R. boylii, suggesting a long history of isolation. While extreme southern populations from Los Angeles County are now extirpated, Lind et al. (2011) hypothesized that animals from those localities may also have been genetically distinct.
Life History
As a stream-dwelling frog, the life history of Rana boylii coincides with seasonal patterns in river flows associated with California’s Mediterranean climate. The most sensitive life stages (eggs and larvae) develop during relatively stable conditions when streams are at their lower stages (Kupferberg et al. 2009b). Breeding and oviposition occur in spring after flood waters recede, and tadpoles metamorphose in late summer through early autumn before winter rains (reviewed in Lind 2005, Haggarty 2006, Wheeler and Welsh 2008). Southern populations breed earlier than northern populations (Zweifel 1955), and the onset and duration of breeding can be influenced by water temperature, cessation of rainfall, water velocity and depth, and day length (Zweifel 1955, Kupferberg 1996a, Lind et al. 1996). Between 2002 and 2007 at a site in Del Norte County, breeding activity was initiated in early April and lasted for 19–52 days, with earlier breeding occurring in low-f low years (Wheeler and Welsh 2008). Breeding activity ceased briefly during rain events that increased f lows (Wheeler and Welsh 2008).
Females lay a single cluster of up to 2000 eggs (Zweifel 1955) attached to pebble or cobble substrates (Fuller and Lind 1992) or to bedrock (M. van Hattem, pers. comm.). Eggs take 2–3 weeks to hatch, depending primarily on water temperature (Kupferberg 1996a). Major sources of natural egg mortality are desiccation through stranding in dry years, and scour from floods in wet years (Kupferberg et al. 2009b). Adults breed at 2 or 3 years of age depending on the geographic location, and this translates into fluctuations in adult populations being determined by environmental conditions during recruitment 2–3 years prior (Kupferberg et al. 2009b). Metamorphosed animals captured in Tehama County were 1.2–7.2 years old based on skeletochronology (Bourque 2008), suggesting that they can be relatively long-lived.
Radiotelemetry studies are beginning to offer more insight into terrestrial movements. Adults aggregate at pools in the spring but become more difficult to find in the summer (Van Wagner 1996, Haggarty 2006, Wheeler and Welsh 2008). In one study in Tehama County, frogs used watercourses for movement and were rarely more than 12 m from the stream channel (Bourque 2008). Females tended to move upstream during spring and downstream during the fall and winter. Travel rates in this population were up to 1386 m/day, faster than previously thought. In other studies, the longest distances traveled have been closer to 500 m at rates of tens to a few hundred meters per day (Van Wagner 1996, Drennan et al. 2006, Wheeler et al. 2006). Females tend to move farther distances than males, with female movements up to 7 km documented in one study (Bourque 2008, Gonsolin 2010). At one locality in Del Norte County, 68% of males remained in one breeding site during the reproductive season, with average home range sizes of 0.58 m2 (Wheeler and Welsh 2008). At a site where the availability of permanent water is a limiting factor in Santa Clara County, resident tributary frogs moved to the main stem to breed and moved greater distances than resident main stem frogs (Gonsolin 2010). Greater than 90% of movements were associated with movements to or from breeding sites, and all movements outside of the breeding season were made in response to the channel drying back or to rainfall (Gonsolin 2010).
Larvae appear to be herbivorous, while metamorphs and adults consume terrestrial and aquatic insects. Algae with epiphytic diatoms are a preferred food for larvae, and the abundance of floating algae indicates the quality of larval food resources (Kupferberg 1996b, Kupferberg 1997). Metamorphosed animals primarily forage terrestrially (Zeiner et al. 1988, Van Wagner 1996, Haggarty 2006, Hothem et al. 2009). Spiders, beetles, and flies are common prey items (Haggarty 2006, Wiseman and Bettaso 2007, Hothem et al. 2009). Gut content analyses of adults collected from 22 sites in the Cache Creek watershed found that 98% of individuals contained terrestrial prey, 28% contained aquatic prey, and one animal contained mammal hair and bone fragments (Hothem et al. 2009). Two occurrences of adults cannibalizing juvenile conspecifics have been documented (Wiseman and Bettaso 2007).
Habitat Requirements
Rana boylii is primarily stream dwelling and requires shallow, flowing water in streams and rivers with at least some cobble-sized substrate (Hayes and Jennings 1988). Different life stages use different habitat types for development, foraging, and overwintering.
Breeding and oviposition occur at the margins of relatively wide and shallow channel sections, habitats that experience reduced flow variation (Storer 1925, Fitch 1936, Kupferberg 1996b, Lind et al. 1996). Breeding sites are often located near tributary confluences (Kupferberg 1996a, Bourque 2008). Egg masses are attached in low-f low locations behind and sometimes under rocks. The most commonly used substrates for breeding sites are cobble, boulders, and gravel (Fuller and Lind 1992, Kupferberg 1996a). Eggs have been found at water depths up to 87 cm (C. Bondi, S. Yarnell, and A. Lind, pers. comm.), in water velocities of 0–0.21 m/s, and up to 12.5 m from shore (Kupferberg 1996a, reviewed in Lind 2005). The critical thermal maximum for embryos is 26°C, and eggs have been found in water ranging from 9°C to 21.5°C (Zweifel 1955). Density of egg masses was highest in Eel River reaches when July mean temperatures were between 17.5°C and 19°C (Catenazzi and Kupferberg 2013). Egg mass surveys from 1991 to 2002 across 11 small and large streams in the Northern Coast Ranges and the Sierra Nevada found that oviposition sites occurred in a very narrow range of microhabitat conditions that were different from randomly selected habitats, strongly suggesting active habitat selection by frogs (Lind 2005). High-quality breeding areas are often used over multiple years (Lind 2005). Larvae tend to stay in natal habitats until they metamorphose (Van Wagner 1996). Surveys in the Mattole Watershed in northern coastal California across different channel types found that tadpole presence was best predicted by relatively warmer water temperatures (Welsh and Hodgson 2011). Tadpoles were never found in water colder than 13°C, and tadpole abundance increased with water temperature (Welsh and Hodgson 2011). In choice experiments, tadpoles selected temperatures between 16.5°C and 22.2°C (Catenazzi and Kupferberg 2013).
Metamorphosed animals use a variety of aquatic habitats, including riffles, pools, and glides (reaches intermediate between riffles and pools) depending on the life stage and season (Van Wagner 1996, Yarnell 2000, Lind 2005, Yarnell 2005, Haggarty 2006). At Red Creek in Tehama County, post-breeding season adults and subadults preferred pool and riffle habitats, while young of the year metamorphs selected slower-moving glides and runs (Haggarty 2006). In Nevada County, all age classes used riffles after the breeding season (Van Wagner 1996). In the Sierra Nevada foothills, subadults chose fast-flowing sections of stream, while adults used slower-moving pool habitats (Yarnell 2000, Yarnell 2005). In the Mattole Watershed, the best predictor of adult presence in streams was canopy openness (Welsh and Hodgson 2011). Abundance of adults and larvae was positively associated with larger basin areas and finer substrates, conditions more typical of alluvial channels than other channel types (Welsh and Hodgson 2011).
Less is known about terrestrial habitat use. Adults typically occur along waterways with some degree of shading (Fitch 1938, Zweifel 1955, Moyle 1973, Hayes and Jennings 1988, Van Wagner 1996), although they also occur in open habitats (Welsh et al. 2005, Haggarty 2006, Welsh and Hodgson 2011). During the spring, radio-tracked males and females in Tehama County were often found on land near water (38% and 66% of the time, respectively; Bourque 2008). The average distance from water was less than 3 m in all seasons, although adults occasionally used habitat up to 40 m distant from streams (Bourque 2008). Adults move to tributaries or upland habitats to avoid floods following large rain events (Kupferberg 1996b, Van Wagner 1996, Yarnell 2000, Bourque 2008). Tributaries are also used for overwintering in early spring before adults are abundant on the principal channels (Kupferberg 1996b, Yarnell 2000). Juveniles will also move into tributaries, with maximum movements of 860 m from hatching site to upstream tributaries observed in Santa Clara County (Gonsolin 2010). Adults may aggregate above ground in terrestrial microhabitats on tributaries post-breeding (Leidy et al. 2009).
Distribution (Past and Present)
Historically, Rana boylii occurred in foothill and mountain streams from the San Gabriel River in Los Angeles County to southern Oregon west of the Sierra-Cascade crest (Nussbaum et al. 1983, Stebbins 2003), from sea level to 1940 m (Hemphill 1952). There is an isolated, unverified record from northern Baja California, Mexico, at ∼2000 m (Loomis 1965).
Jennings and Hayes (1994a) considered R. boylii endangered in central and southern California south of the Salinas River, threatened in the west slope drainages of the Sierra Nevada and Cascades, and of special concern in the Coast Ranges north of the Salinas River. They estimated that R. boylii were extirpated from 45% of their historical localities in California, and 66% of historical localities from the Sierra Nevada. Building on that mapping effort, Lind (2005) looked at 394 historic localities in California and Oregon, and found that 201 localities (51%) were no longer occupied, with extirpations largely in southern California and northern Oregon. Kupferberg et al. (2012) determined current occupancy of 310 randomly selected sites that were occupied prior to 1975. They found that half of the sites still had R. boylii populations, with frogs more likely to be present in sites without large dams.
Extirpations likely began in the second half of the twentieth century. Grinnell and Storer (1924) noted several sites in the Sierra Nevada foothills around Yosemite where R. boylii were common. In resurveys of those sites and surveys of additional sites in the early 1990s, Drost and Fellers (1996) did not find any R. boylii. Surveys by Moyle (1973) in the 1970s found R. boylii at only 30/95 sites in the southern and central Sierra Nevada foothills. Field surveys since 1993 have found at least one frog at only 213/804 sites in 28/40 California counties (Fellers 2005a). Fellers (2005a) estimated that extant populations occur in 40% of streams in the Pacific Northwest, 30% of streams in the Cascade Mountains, 30% of streams in the south Coast Range (south of San Francisco), and 12% of streams in the Sierra Nevada.
Trends in Abundance
Kupferberg et al. (2012) compiled egg mass density data from multiple sources on 27 Sierran and coastal populations in northern California between 1991 and 2010. The range of densities reported was between 1.9 and 105.7 clutches/km of reach sampled. Average density was higher in free-flowing rivers (31.1 clutches/km) than in rivers with dams (5.5 clutches/km), but no differences were detected between abundances in coastal versus montane watersheds (Kupferberg et al. 2012). Fellers (2005a) reported that only 30 of 213 occupied California sites had population sizes greater than 20 adults. In the Coast Ranges, population sizes of greater than 100 adult frogs occurred at six sites, and populations greater than 50 adult frogs occurred at nine sites (Fellers 2005a). Small population sizes are presumably due to population declines, leading to predictions that populations in the southern Sierra Nevada will not be viable for more than another decade (Fellers 2005a). Minimum viable population sizes are unknown, however, and may vary across the range.
Nature and Degree of Threat
The main threats to and likely causes of Rana boylii decline are human activities that alter natural hydrologic regimes of streams and rivers, such as dams for hydroelectric power generation, water storage, and water delivery. Other potential stressors include land use changes that degrade or destroy riparian habitat (particularly urban and agricultural development), pesticides, disease, and invasive species.
Alterations to the natural flow regime, for example, through dam releases, can have direct mortality effects and indirect negative effects on R. boylii by altering habitat availability and quality. Kupferberg et al. (2009b) reviewed published literature and Federal Energy Regulatory Commission hydroelectric dam relicensing reports to assess the effects of pulsed flow releases on R. boylii. The data spanned 1997–2007 and included seven major river basins in California. Pulsed flows from dam releases after oviposition resulted in scouring of egg masses, while flow changes during oviposition led to stranding when water levels subsequently dropped and exposed egg masses. Similarly, tadpoles can be scoured and stranded due to pulsed-flow releases. The effect of releases on post-metamorphic animals is less clear, and the impact of flow changes on habitat availability is highly site specific. Reservoirs and dams may also disrupt patterns of connectivity among R. boylii populations. Comparisons of genetic structure within and among R. boylii populations in three pairs of regulated versus unregulated Sierran rivers found that regulated rivers exhibited lower genetic diversity and greater genetic drift compared to unregulated river populations (Peek 2010).
Kupferberg et al. (2009c) modeled R. boylii population growth under different flow scenarios. A major result was that populations in regulated rivers had 4–13-fold greater extinction risk than populations in unregulated rivers due to smaller population sizes. Kupferberg et al. (2009c) simulated how an unregulated population would be affected by flows more typical of regulated rivers. When subjected to aseasonal flow conditions, modeled populations showed a doubling of extinction risk. Many different kinds of hydrologic changes can contribute to these negative effects, and when different stressors are combined, the impact on frog populations is greater than expected from simply adding up the effects of individual stressors.
Field and laboratory experiments conducted by Kupferberg et al. (2011) showed that tadpoles suffered negative effects including death at or below water velocities experienced during aseasonal pulsed flows. For example, most tadpoles could no longer swim or seek refuge at velocities of ∼20 cm/s or greater, and in the absence of refugia tadpoles reached exhaustion in ∼7 min in a 5 cm/s current. Rates of flow in regulated reaches can be much higher than this. For example, in the North Fork Feather River, surface velocity measured in larval rearing habitat near channel edges can reach over 30 cm/s after releases for recreational purposes (Garcia and Associates 2005).
Smaller-scale hydrologic modification and loss or degradation of riparian habitat due to urban and agricultural use is also a threat to R. boylii. Analyses correlating R. boylii distribution with landscape characteristics demonstrated negative effects of urban and agricultural land use change and pesticides on R. boylii presence (Davidson et al. 2002, Davidson 2004, Lind 2005). Vineyard conversion can have impacts on small creeks, and the establishment of permanent ponds used for irrigation and frost protection can create habitat for bullfrogs (S. Kupferberg, pers. comm.). Marijuana cultivation practices that divert water from small creeks can lead to premature drying. Growers have been observed to construct plastic-lined impoundments in creeks and add fertilizers directly to creek water, as well as use pesticides and herbicides in and around frog habitat (Gonsolin 2010). These practices are suspected to have contributed to declines in some populations near Gilroy (Gonsolin 2010). Similar impacts are likely in Humboldt, Mendocino, and Trinity Counties (CDFG 2013). The large-scale effects of such illegal operations are unknown, and potentially dangerous to study. While in-stream gravel and suction dredge gold mining may have been more of a concern in the past, current regulations protecting salmonids have likely largely reduced the direct impact of such activities on R. boylii. For example, in Humboldt County in-stream gravel mining occurs above (in elevation) and outside the wetted channel, and relatively high egg mass density has been documented in reaches where gravel mining occurs in the Mad River (M. van Hattem, pers. comm.).
The current distribution of R. boylii is strongly correlated with climate variables, which suggests that this species may be sensitive to future climate changes, particularly those that affect stream hydrology (reviewed in PRBO 2011). Comparisons of occupied and extirpated historic localities found that sites where R. boylii persists have higher mean annual precipitation, less variability in precipitation, and fewer dry years than extirpated sites (Davidson et al. 2002, Lind 2005). Within the range of R. boylii, warming temperatures are predicted to result in more precipitation falling as rain instead of snow, and consequently less storage of water as snowpack. Reductions of 30–80% in snowpack accumulation are predicted within the northwestern range of R. boylii, and up to 90% reduction in snowpack is predicted for the south coast hydrologic region (Snyder et al. 2004, Cayan et al. 2008b). In the Sierra Nevada, snowpack losses of 50–90% are predicted by the end of the twenty-first century, with greatest losses at low to mid-elevations (Knowles and Cayan 2002, Hayhoe et al. 2004, Knowles and Cayan 2004, Maurer 2007, Cayan et al. 2008b). Loss of snowpack is likely to result in earlier runoff and reduced spring and summer streamflows. Timing of spring snowmelt is predicted to shift earlier in the spring in the Sierra Nevada (Snyder and Sloan 2005), while in northwestern California the opposite has occurred over the last 50 years (Stewart et al. 2005). How frogs will respond to these changes in hydrology is unknown, but negative effects due to anthropogenic changes in hydrology are well documented. Reduction in water availability may also lead to more conflict with human use of water and affect how regulated reaches are managed (reviewed in Franco et al. 2011). It is important to note, however, that predictions of changes in precipitation are much less certain than predictions for temperature (Franco et al. 2011, PRBO 2011). In addition, climate change may also affect disease dynamics. Outbreaks of nonnative parasitic copepods occurred during two recent warm years at a long-term study site, resulting in morphological abnormalities and smaller sizes at metamorphosis (Kupferberg et al. 2009a). The outbreak was likely caused by increased summer water temperature, decreased daily discharge, or a combination of these factors. These conditions may increase under a changing climate, but could also occur as a result of marijuana cultivation.
No declines to date have been associated with Bd, but the disease does infect R. boylii in the field. Padgett-Flohr and Hopkins (2009) examined museum specimens from 1890 to 2000, and found that Bd first appeared in R. boylii samples from the 1960s, with 10% of specimens infected. In all, 0–40% of specimens were infected with Bd in the following decades. In laboratory trials, R. boylii appeared to be protected by skin peptides against Bd and therefore may not be very susceptible to chytridiomycosis (Davidson et al. 2007). Chytrid infection did not affect survival, even in the presence of a co-applied pesticide, but did suppress growth of recently metamorphosed individuals by approximately 40% (Davidson et al. 2007).
Observational data and surveys have found that R. boylii is rare or absent in habitats with introduced fishes and bullfrogs (Hayes and Jennings 1986, Hayes and Jennings 1988, Kupferberg 1997, Lind et al. 2003, Fuller 2008). Breeding populations of R. boylii can be an order of magnitude smaller when bullfrogs are present compared to uninvaded reaches (Kupferberg 1997). In field experiments in outdoor enclosures, bullfrog tadpoles caused a 48% reduction in survivorship of R. boylii tadpoles, and a 24% decline in mass at metamorphosis. The mechanism behind the negative impacts of bullfrogs was competition for food (Kupferberg 1997). Metamorphosed bullfrogs prey on R. boylii, including post-metamorphic individuals (Crayon 1988, Hothem et al. 2009), but the population-level consequences of this predation are unclear. Another nonnative predator, the signal crayfish (Pacifastacus leniusculus), has been introduced into several Sierra Nevada drainages from farther north where the two species co-occur (Wiseman et al. 2005). Signal crayfish have been observed eating and dislodging egg masses and attacking larvae (Wiseman et al. 2005). Within R. boylii’s range, signal crayfish have been documented at 30–40 sites, with ∼25 invaded sites occurring in the Sierran foothills (G. Fellers, pers. comm.).
Status Determination
Documented declines and extirpations of Rana boylii populations combined with continuing threats to remaining populations result in a Priority 1 designation for this species.
Management Recommendations
Several aspects of the biology of Rana boylii can help inform management efforts. Rana boylii use a variety of stream and streamside habitats during different life stages; therefore, protected habitat needs to provide adequate habitat diversity. The timing and pattern of releases of water from dams during April through June should be managed to minimize egg scouring and stranding. For example, dam releases can be staggered to better mimic the natural spring recession in snowmelt-fed streams. Further recommendations for hydrologic management can be found in Kupferberg et al. (2009b, 2009c). Dam removal should be explored where appropriate and is likely to benefit R. boylii and other native taxa. River management for other taxa needs to take R. boylii into account. For example, in-stream structures to improve habitat for fish such as steelhead can negatively impact R. boylii (Fuller and Lind 1992). Habitat restoration and possibly repatriation of southern Sierra Nevada populations should be considered. Southern populations in general should be priorities for conservation because of the degree of losses and distinctive genetic diversity represented in this part of the range (Lind et al. 2011). Removal or management of nonnative predators such as fish and bullfrogs may help restore R. boylii habitat. For example, projects that remove artificial pools (e.g., relict mine tailing ponds) by restoring linkages to main river channels would result in more natural hydrologic conditions and reduce breeding habitat for bullfrogs (Fuller et al. 2010). Finally, Lind et al. (2011) suggested that an approach using genetic analyses of R. boylii and co-distributed riverine taxa would help in prioritizing drainages for protection based on levels of diversity.
Monitoring, Research, and Survey Needs
Modeling of population dynamics and hydrology are highly site specific and limited by available demographic data, and acquiring those additional data should be a high priority for Rana boylii. More research is needed on survivorship of tadpoles and juveniles, especially during overwintering. The mechanisms underlying hydrological effects are currently best understood for egg masses, and we need to develop a better functional understanding of how hydrology affects different life stages (Kupferberg et al. 2009b). More research is also needed on post-metamorphic stages. Post-metamorphic stages may be less at risk from aseasonal pulses in river flow because they are more mobile, but in regulated rivers the timing of pulsed flow events can be decoupled from climatic cues (such as the first appreciable fall rains) that would normally trigger movement to safer refuges (Kupferberg et al. 2009b). Caution should be taken in using radio telemetry to study post-metamorphic animals, as 62% of frogs in one study suffered skin injuries from transmitters (Bourque 2008). Modeling efforts would also be improved by monitoring a Sierra Nevada population in an unregulated reach for comparison with more regulated sites (Kupferberg et al. 2009b). Egg mass counts are commonly used to monitor R. boylii populations. Females only lay one mass/year, so egg mass counts accurately reflect the number of reproductive females. However, operational sex ratios are female biased; therefore, accurate population size estimates cannot be made based on egg counts alone (Wheeler and Welsh 2008). Lind et al. (2011) provided important range-wide phylogeographic data, but their study was limited by very low nuclear genetic diversity and relied primarily on mitochondrial data. Additional work could provide valuable additional data on levels of variation and genetic isolation among local hydrologic basins, as might be predicted for this stream-restricted anuran. Finally, efforts to find remnant R. boylii populations in the San Gabriel Mountains and upper Piru Creek in southern California should continue.