CASCADES FROG
Rana cascadae Slater 1939

Cascades frog, Trinity County, California. Courtesy of Adam Clause.

Status Summary
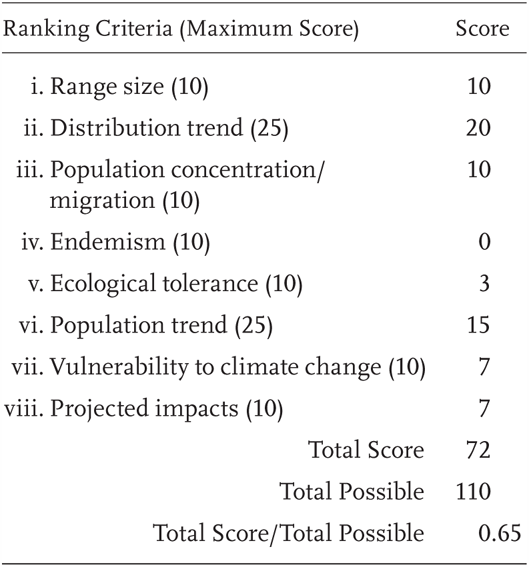
Rana cascadae is a Priority 2 Species of Special Concern, receiving a Total Score/Total Possible of 65% (72/110). During the previous evaluation, it was also considered a Species of Special Concern (Jennings and Hayes 1994a).
Cascades Frog: Risk Factors
Identification
Rana cascadae is a medium-sized (2.0–8.0 cm SVL) frog with drab-green, tan, or brown dorsal coloration and well-defined black blotches scattered across the back (Slater 1939, Stebbins 2003). The number of blotches varies from very few to about 50 (Slater 1939), and unmarked individuals occur rarely (Jennings and Hayes 1994a). Blotches appear to be on the surface of the frog’s skin and are reminiscent of spattered ink (Stebbins 2003). The species has a prominent light stripe above the jaw and strong dorsolateral folds. The venter is cream or buff, usually with yellowish (sometimes reddish) areas posteriorly and on the undersides of the legs. Laterally, the sides are mottled and fade into the ventral coloration (Slater 1939). The male advertisement call is a series of low chucks given in rapid succession, usually ending with one slightly drawn out chuck (Elliott et al. 2009).
In California, this species could be confused with the California or northern red-legged frogs (R. draytonii and R. aurora), both of which it resembles in overall body shape. In adults, R. aurora/R. draytonii have extensive mottling on the venter with red pigmentation on the ventral thighs and groin, rather than the yellow that often characterizes R. cascadae (Dunlap 1955). However, the color of the thighs is variable in R. cascadae and may not be a reliable character to separate these taxa (S. Barry, pers. comm.).
Taxonomic Relationships
This species is closely related to Rana aurora and R. draytonii (Shaffer et al. 2004, Hillis and Wilcox 2005). It was proposed as a distinct species based on morphology (Slater 1939), and this interpretation has been repeatedly confirmed with additional morphological and genetic data (Dunlap 1955, Case 1978, Shaffer et al. 2004).
Based on genetic data, the species appears to show considerable differentiation among local populations that is consistent with an overall isolation-by-distance model of gene flow (Monsen and Blouin 2003, Monsen and Blouin 2004). In addition, the California populations appear to be strongly divergent in both mitochondrial and nuclear DNA from the remainder of the species’ range in the Cascade and Olympic mountain ranges (Monsen and Blouin 2003). Populations of this species appear to have consistently small effective population sizes (<50; Phillipsen et al. 2011). The available data are primarily from outside of California, although the observed pattern is likely consistent throughout the range. Preliminary results based on mitochondrial DNA, nuclear DNA, and microsatellites suggested little divergence between Lassen and Klamath populations in California (Chang and Shaffer 2010). However, more extensive work with larger range-wide sampling is needed.
Life History
Rana cascadae breeds in the spring, soon after emerging from hibernation and the spring thaw that opens breeding pools (Nussbaum et al. 1983, Stebbins 2003). First-time breeders frequently disperse to new areas of suitable breeding habitat (51% of first-time breeders relative to only 7% of experienced breeders in Echo Lake Basin; Garwood 2009), which may help to connect local subpopulations into larger more stable metapopulations. Breeding occurs at the margins of waterbodies, with oviposition often occurring in large aggregations (Sype 1975, Nussbaum et al. 1983, Garwood 2009). Oviposition behavior appears to be variable throughout the species’ range, with some authors reporting diurnal oviposition of largely unattached egg masses (Briggs 1987), and others noting that most egg masses are deposited at night and are attached to vegetation (Nussbaum et al. 1983; K. Pope, pers. comm.). Breeding at individual sites is relatively synchronous and occurs over a few days, although the timing of breeding across the range can vary widely with local weather conditions and elevation (Briggs 1987, Garwood 2009). Embryo development can occur at temperatures ranging from 6°C to 27°C (Sype 1975, Nussbaum et al. 1983). After hatching, larvae sometimes aggregate into dense clusters (generally fewer than 40 individuals) composed primarily of siblings (O’Hara and Blaustein 1981, O’Hara and Blaustein 1985, Blaustein and O’Hara 1987) and choose higher water temperature than those required during embryo development (up to ∼28°C; Wollmuth et al. 1987, Bancroft et al. 2008). After metamorphosis, lower water temperatures are again preferred.
Rana cascadae appears to be largely diurnal. The diet of adult frogs is generalized and includes a wide variety of arthropods, as is the case for most other California ranids (Joseph et al. 2011). An analysis of stomach contents for 275 frogs documented the presence of 110 invertebrate taxa (Larson 2012). Frogs across all size classes generally avoided small prey items (<4 mm), and larger frogs more strongly preferred large prey items (Larson 2012).
Habitat Requirements
Rana cascadae utilizes a wide variety of aquatic habitats, including temporary and permanent ponds, lakes, marshes, and streams, as well as adjacent vegetated terrestrial habitat (Nussbaum et al. 1983, Jennings and Hayes 1994a, Stebbins 2003, Pearl and Adams 2005, Garwood 2009). The species will also use wet meadows (often those that have formed from old sphagnum bogs) and can occasionally be found a large distance from water (Nussbaum et al. 1983). They require water year-round at all life stages and cannot tolerate habitats that freeze solid in the winter (K. Pope, pers. comm.). Montane lentic habitat is required for breeding and overwintering, with small, shallow, spring-fed ponds serving as the primary breeding habitat (Garwood 2009). Populations appear to be sustained by a matrix of varying habitat types that individual frogs disperse among throughout the year (Garwood and Welsh 2007, Garwood 2009), suggesting that habitat conservation needs to consider spatial scales larger than single lakes (or other patches of habitat). The presence of predaceous fish may limit their distribution (Welsh et al. 2006, Pope et al. 2008), although this alone cannot explain the broadscale pattern of population declines in this species (Fellers et al. 2008; also see the “Nature and Degree of Threat” section below).
Distribution (Past and Present)
In California, Rana cascadae occurs in two population segments. One is in the Lassen area and the extreme northern end of the Sierra Nevada (Lassen, Plumas, Shasta, and Tehama Counties) and is now nearly extirpated. The other occurs in the Trinity Alps and Siskiyou Mountains region. The species’ range in California extends from Siskiyou County south to the northern end of Butte County. Outside of California, the range of R. cascadae follows the Cascade Range nearly to the United States– Canadian border, with another disjunct population at high elevations on the Olympic Peninsula (Stebbins 2003).
Trends in Abundance
Populations of this frog have declined strongly in the Lassen area, where nearly all known populations have disappeared in the last 30 years (Fellers and Drost 1993, Jennings and Hayes 1994a). More recent surveys in the Lassen region further confirm these declines. Rana cascadae was found at only 6 of 856 sites surveyed over 14 years, population sizes were small, and breeding was limited at these 6 sites (Fellers et al. 2008). Populations elsewhere, including the Klamath Mountains region in Siskiyou and Trinity Counties, are also fragmented, generally small, and at risk, although they are more intact overall than in the Lassen area (K. Pope, pers. comm.). Localized declines have also been detected elsewhere in the range (Pearl and Adams 2005, Fellers et al. 2008, Piovia-Scott et al. 2011). Welsh et al. (2006) found R. cascadae to be the most common anuran in the Klamath region. By contrast, more recent and ongoing surveys of eight populations in the Trinity Alps within the Klamath region find that only one of the populations is large and robust and that some of the threats present in the Lassen region are likely also operating there (K. Pope, pers. comm.). Pope and Larson (2013) report 11 remaining populations in the Lassen area and find that the number of young frogs was low at all sites that they surveyed.
Nature and Degree of Threat
Threats to this species appear to be complex and derived from multiple stressors. The largest factor contributing to declines in the Lassen region appears to be overall low recruitment due to changing hydrological conditions that lead to detrimentally high water temperatures and desiccation of egg masses and tadpoles, as well as impacts from Bd among subadult frogs (Pope et al. 2011). Extensive mark-recapture surveys in the Lassen region between 2008 and 2010 indicate widespread desiccation of egg masses and tadpoles and a lack of metamorphs relative to more stable populations in the Klamath area (Pope et al. 2011, Pope and Larson 2013). In comparisons between two of the remaining Lassen populations, the population with higher Bd prevalence and load in adult and subadult frogs had lower survivorship for these two age classes. In both Lassen and the Klamath Ranges, subadult frogs had higher Bd prevalence and load than adult frogs, and the prevalence of Bd increased throughout the active season for subadult frogs but not for adult frogs. These results are consistent with previous studies of Bd in this species that suggest the pathogen has differential impacts depending on age class. Blaustein et al. (2005) examined the effect of Bd on larvae and found an increased incidence of mouthpart abnormalities but no effect on mortality or behavior. Garcia et al. (2006), however, found significant mortality in new metamorphs of Rana cascadae due to Bd.
Interestingly, Bd also appears to be widespread in the Klamath region where this species is currently much more stable than in the Lassen region (Piovia-Scott et al. 2011), suggesting more than one factor is playing a role in the declines. Ongoing characterization of Bd prevalence in these populations could help determine what factors are involved, although one hypothesis is that Bd achieves higher loads on frogs in declining populations than stable populations (J. Piovia-Scott, pers. comm.). Infection by the water mold Saprolegnia has also been implicated in R. cascadae declines. This pathogen is known to increase mortality in embryos, larvae, and metamorphs (Kiesecker and Blaustein 1999, Romansic et al. 2009a) and may have strong impacts on the outcome of competition between R. cascadae and sympatric Pseudacris regilla (Kiesecker and Blaustein 1999).
Habitat loss and modification is also a threat to continued persistence of populations in both the Klamath and Lassen regions. The species is highly associated with meadows, which have been impacted by cattle grazing, tree encroachment due to lack of wildfire, and changing hydrology associated with changes in the snow-pack (K. Pope, pers. comm.; Pope et al. 2014).
Other possible contributors to R. cascadae declines that have been proposed include introduced fishes, environmental contaminants, pathogens, and UV-B radiation. The presence of introduced trout appears to be inversely related to the distribution of R. cascadae (Welsh et al. 2006) and almost certainly impacts some populations. Aside from direct predation, introduced trout may affect R. cascadae indirectly by supporting higher populations of the aquatic garter snake (T. atratus), a predator on both trout and R. cascadae (Garwood and Welsh 2007, Pope et al. 2008), and by preemptive competition for aquatic prey (Joseph et al. 2011). However, trout have been present in the Lassen region for nearly a century and are also widely distributed in other areas where R. cascadae persists, making it unlikely that they alone can explain the declines over the last 30 years (Fellers et al. 2008). Nevertheless, populations appear to respond favorably to trout removal, showing marked increases in population size and recruitment following fish removal (Pope 2008).
Pesticide use is inversely correlated with the presence of R. cascadae (Davidson 2004). In particular, downwind transport of pesticides from intensively farmed areas in the Central Valley appears to be correlated with declines in several species of ranid frogs, including R. cascadae (Davidson et al. 2002, Davidson 2004). This hypothesis is attractive in that it explains the differential declines between the Trinity Alps region and the Lassen region because the Lassen region is directly downwind of areas that experience heavy agricultural use, whereas the Trinity Alps are not (Davidson et al. 2002). However, recent field measurements of contaminant residues in sediment and in R. cascadae and P. regilla tissue do not indicate higher levels in the Lassen compared to the Trinity Alps region, at least for the handful of different chemicals that have been analyzed to date, calling this hypothesis into question (Davidson et al. 2012). In addition, Sparling et al. (2001) measured the presence of cholinesterase levels in the non-declining P. regilla as a measure of the extent of pesticides that are locally deposited in an area and found strong effects in the Sierra Nevada but not in the Lassen area; these results seem to indicate that pesticides may not be a major factor in the Lassen R. cascadae declines. Environmental contaminants at sub-lethal levels have also been shown to induce behavioral and morphological changes in R. cascadae (Marco and Blaustein 1999), suggesting that low-level agricultural residues may have important biological consequences. In summary, it appears that pesticides may be playing some role in R. cascadae declines in the Lassen region, but they are certainly not the entire story.
Finally, UV-B radiation may play a role, possibly in combination with other factors, in causing declines. Some studies have documented larval mortality and retinal damage due to UV-B, although the effect depends strongly on the intensity of UV-B, the duration of exposure, and possibly other factors including the presence of competitors, predators, or supplementary food (Fite et al. 1998, Hatch and Blaustein 2000, Belden et al. 2003, Garcia et al. 2006, Romansic et al. 2009b). The importance of these results has not yet been demonstrated in natural settings, however. Palen et al. (2002) found that dissolved organic matter in natural environments provided protection from UV-B at 89% of the sites examined for R. cascadae. Thus, it remains possible that UV-B is having an effect, although its importance in nature remains unclear.
Ultimately, it is likely that no one factor is solely responsible for the precipitous declines in Lassen region R. cascadae populations. Further, the causes of the initial range-wide declines may be distinct from the local factors that threaten the continued persistence of the few remaining populations. The most recent work suggests that the major factors playing a role in the range-wide declines are the presence of introduced fishes and Bd, while continued local persistence of the remaining populations is also threatened by low recruitment stemming from desiccation and detrimentally high water temperatures. Pope et al. (2014) present a recent and comprehensive review of both regional and local-scale threats to R. cascadae throughout the range. The evidence that synergistic effects occur between several alternative mechanisms of decline is now widespread for a variety of amphibian species (Fellers et al. 2008). As declines have occurred, whatever the cause, it is likely that a breakdown of metapopulation dynamics will contribute to further declines as existing populations become more and more fragmented, decreasing the opportunity for population rescue via recolonization.
Status Determination
The catastrophic declines in the Lassen area are the primary reason for the SSC designation. Rana cascadae is nearly extirpated in the Lassen region, is undergoing local population declines elsewhere in its range, and appears to be susceptible to a wide range of threats. However, this frog is a moderate ecological specialist that appears to be relatively stable through much of its range, including a significant fraction of its range in California. The factors that caused declines in the Lassen area appear to not have operated in the Klamath area to date, leading us to project moderate future impacts on extant populations and a Priority 2 status. If strong declines begin to occur in the Klamath area, then a higher priority status will rapidly become justifiable.
Management Recommendations
Fellers et al. (2008), Pope et al. (2011, 2014), and Pope and Larson (2013) provide thorough reviews of threats to, and management recommendations for, Rana cascadae, and our recommendations largely follow those of these authors.
Habitat that supports this species in the Lassen area should be protected from modification that negatively impacts hydrology while further research is carried out. Pope et al. (2011) began some habitat restoration measures, and these efforts should be continued (coupled with ongoing monitoring to determine their effects). Fish removal in key populations has also been documented to increase recruitment and should be considered as a management strategy, particularly in the Klamath where a larger number of existing populations might be stabilized before declines can occur. Pope et al. (2011) also proposed experimental treatment for Bd in newly metamorphosed frogs. Effective treatments for Bd may be essential for the long-term survival of many amphibian species, so these efforts should be further explored and potentially implemented if they are successful. At the same time, a captive colony of Lassen-area R. cascadae should be established, as the prospects for long-term survival in the wild appears to be low. If additional research can determine the causes of the declines and effective mitigation measures can be enacted, this captive population could eventually form the basis of a reintroduction program.
Monitoring, Research, and Survey Needs
Monitoring efforts should focus on the few remaining Lassen populations, with additional monitoring of stable populations elsewhere in the range as reference populations. Areas that have undergone habitat restoration or experimental treatments for Bd infection will require ongoing monitoring to quantify the long-term effects of these efforts and to inform further work aimed at controlling the impact of these threats. If additional declines occur, this monitoring will facilitate early detection and, hopefully, provide the background data needed to understand the causes of declines.
As a reintroduction effort may eventually become necessary, it is important to further characterize the extent of intraspecific variation within this taxon now, before additional declines occur. Preliminary genetic work has been initiated, and it should form the basis of additional work that examines fine-scale population differentiation and structure. The obvious initial focus of such genetic work should be to assess the validity of the Lassen and Klamath regions as separate evolutionary units requiring their own management strategies. This work will also help to identify any potential population segments within either region that may qualify for independent management. Finally, additional studies that quantify the interactive effects among different causes of declines would be useful in providing a more complete picture of conservation threats in this taxon.