NORTHERN LEOPARD FROG
Rana pipiens Schreber 1782

Northern leopard frog, Washington County, Utah. Courtesy of William Flaxington.

Status Summary
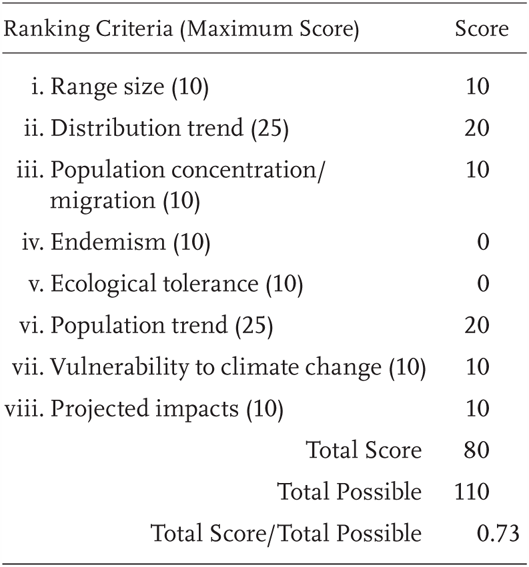
Rana pipiens is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 73% (80/110). During the previous evaluation, it was also designated as a Species of Special Concern (Jennings and Hayes 1994a).
Northern Leopard Frog: Risk Factors
Identification
Rana pipiens is a medium-sized ranid frog with strong, continuous dorsolateral folds that do not angle inward posteriorly. Its dorsal coloration is green to brown with large well-defined black or dark-brown oval or round spots. Each spot is ringed with a narrow band of white or cream. The ventral coloration is white or cream with no mottling or other dark markings (Stebbins 2003). The call is a low, snore-like trill, often followed by low chuckling and/or grunts (Stebbins 2003, Elliott et al. 2009).
Within its range in California, this species can potentially be confused with the Oregon spotted frog (R. pretiosa). However, R. pretiosa has much smaller, more irregular spots, which often have diffuse borders and are not ringed in white. It also has conspicuous red or salmon markings on the underside, which R. pipiens lacks. Other members of the leopard frog complex in California, the lowland leopard frog (R. yavapaiensis) and the Rio Grande leopard frog (R. berlandieri), have dorsolateral folds that are discontinuous and angle inward posteriorly. In addition, both are yellow ventrally. The Cascades frog (R. cascadae) has more numerous, small, irregular black dots that are not ringed in white.
Taxonomic Relationships
The taxonomic history of the leopard frog species complex, and Rana pipiens in particular, is complicated (Hillis 1988) and remains incompletely understood. The name R. pipiens previously included all members of the leopard frog complex from Canada south to Panama, including R. yavapaiensis, also native in California, and the introduced R. berlandieri. However, this concept of a single wide-ranging leopard frog species changed in the last several decades, and over a dozen species are recognized at present. The current taxonomy of the R. pipiens complex was initially based on variation in morphology and vocalizations (Pace 1974). Subsequent work including molecular analyses recognized several additional taxa and clarified relationships among the contained species (Platz and Mecham 1979, Hillis et al. 1983, Platz and Frost 1984, reviewed by Hillis 1988).
Frost et al. (2006a) recommended placing this species and many other North American ranids in the genus Lithobates, although this proposal and the analyses that support it are controversial (Crother 2009, Frost et al. 2009a, Pauly et al. 2009). We retain the traditional taxonomy here to maintain stability pending further analyses.
Life History
No life history data for California populations have been published. Because Rana pipiens in California are a mixture of introduced and presumably native populations (see the “Distribution” section) and live on the extreme western edge of the species’ range, we are reluctant to use information from more easterly populations as a proxy for those that occur in California. In Colorado, breeding occurs during the first spring nights that have relatively “mild” temperatures near or above freezing (Corn and Livo 1989), and this presumably is also the case in California. Tadpoles are present through the summer months and are not known to over-winter, suggesting a late summer or fall metamorphosis. Further east, adults and juveniles are known to range far from water and breeding sites (Dole 1971), although it is unknown if this also characterizes California populations. Range-wide, R. pipiens is a generalist predator, feeding on a wide variety of arthropods and small vertebrates (Knowlton 1944, Linzey 1967, Harding 1997), and this presumably also characterizes the species in California.
Habitat Requirements
Despite the paucity of records from California, this species is known from a variety of habitats, including small streams, rivers, and lakes (Storer 1925, Stebbins 1951, Jennings and Hayes 1994a). Rana pipiens occupies a wide variety of habitat types throughout its range, so we are hesitant to speculate on microhabitat requirements in California. Generally, the species hibernates underwater and requires aquatic habitats that do not freeze solid during winter (Emery et al. 1972, Licht 1991), and this presumably is also the case for California populations. Nearby damp upland habitat is utilized for foraging during the active season (Dole 1967). The species has been found in a variety of open grassy areas and meadows, although heavily grazed areas and cultivated fields do not appear to be suitable (Pope et al. 2000). In the Midwestern United States, the presence of quality upland foraging habitat seems to affect the abundance of this species. When grasslands were restored around suitable pond-breeding habitat, the density of frogs increased markedly (K. Mierzwa, pers. comm., in Pope et al. 2000).
Distribution (Past and Present)
Outside of California, Rana pipiens ranges widely across North America, from Nova Scotia and Newfoundland, Canada, west to Washington and Nevada. In California, R. pipiens populations that may be native are known from Modoc and Siskiyou Counties, the Lake Tahoe basin, and the upper Owens Valley (Jennings and Fuller 2004), although some workers question whether the latter two regions constitute natural, as opposed to purely introduced, populations (S. Barry, pers. comm.). Numerous introductions have occurred throughout the state, including some within the putative native range. The vicinity of Fallen Leaf Lake in the Lake Tahoe Basin is one such example (Bryant 1917). It is also possible that putatively native populations of this frog are all the result of human introductions, and determining their status is an important research priority. The upper Owens Valley supports tiger salamander populations that were recently shown to be introduced (Johnson et al. 2010), demonstrating that similarly distributed nonnative species have been established in this region. The tiger salamander introductions occurred as a consequence of the fishbait industry (Riley et al. 2003), which also sometimes sells leopard frog tadpoles and adults.
We are not aware of any additional recent records in California beyond those reported by Jennings and Hayes (1994a), though an unverified sight record of a “spotted frog” in Surprise Valley, Modoc County, California, could have been R. pipiens. However, the circumstances and description of this frog make it more likely that it was R. pretiosa, another California Species of Special Concern (see that species account for additional information).
Trends in Abundance
Trends in abundance for California populations of Rana pipiens are difficult to interpret because of the uncertainty regarding which populations are native or introduced. However, assuming that historical California populations are native, severe declines have clearly occurred. We are aware of only scattered sight records for the species over the last two decades. Jennings and Hayes (1994a) reported two relatively recent sight records in the early 1990s from Siskiyou and Inyo Counties. Macey and Papenfuss (1991a) reported that leopard frogs occurred on the east side of the White Mountains below Boundary Peak, though they failed to detect the species in follow-up surveys (T. Papenfuss, pers. comm.). More recent surveys of historical localities in the Owens River also did not detect this species and found that much of the habitat currently appears to be unsuitable (Becker and Henderson 2010). We are not aware of any presumed-native populations of this species occurring in the state since these records. Elsewhere in its range, R. pipiens has undergone severe declines and localized extirpations, particularly in the western parts of the United States (reviewed by Rorabaugh 2005).
Nature and Degree of Threat
Habitat modification is probably the most important threat for Rana pipiens in California. Rana pipiens forages in upland habitat having moderately tall vegetation with a moist substrate. Livestock grazing in these habitats tends to reduce vegetation height, which leads to drying of the substrate, apparently rendering this habitat unsuitable for the frog. It is likely that this process contributed to the declines observed in both the Owens Valley and the Modoc Plateau areas where most California records for R. pipiens are concentrated. Changing hydrology elsewhere in the range has led to the extirpation of some local populations (Corn and Fogleman 1984). Given that California populations are at the western range limit of the species, projected climate changes may have a strong effect in the state. Current models project warmer summer and winter temperatures, decreases of 8–21% of annual precipitation, and a 34% decrease in snowpack (PRBO 2011). Taken together, these climate projections indicate that the moist soil and wetland complexes favored by this species will probably decrease in the Great Basin of California, further reducing the already sparse habitat for this species.
Some studies have detected significant negative impacts from pesticides on R. pipiens, although the importance of this threat in nature is not well understood. In other parts of their range, R. pipiens are known to be sensitive to herbicides and pesticides used in agriculture (Relyea 2008, Relyea and Jones 2009), and mixtures of these chemicals can result in 99% mortality rates (Relyea 2008). However, the evidence on this topic is complex and dependent on the specific chemicals tested. A popular herbicide consisting of a mixture of glyphosate and POEA (commonly marketed under the commercial name Roundup®) is one such example. Some studies have found limited impacts from these chemicals and concluded that direct mortality in wild populations from this herbicide is unlikely (e.g., Wojtaszek et al. 2004), while other studies have found very strong direct lethal effects (e.g., Relyea 2005b). When direct lethal effects were not found, several studies demonstrated that chemical contaminants can have lethal impacts when combined with other stressors (e.g., predator cues; Relyea 2005a) or sublethal detrimental effects such as decreased immune system functionality (Christin et al. 2003, Gilbertson et al. 2003, Rohr et al. 2008). These seemingly unpredictable effects of agrochemicals may depend on specific populations and conditions in a local area (Relyea 2005b). Although these results are both complex and sometimes contradictory, substantial evidence exists that environmental contaminants are likely to have significant impacts on R. pipiens and other amphibians in California (e.g., Davidson et al. 2002, Davidson 2004).
Other potential threats to R. pipiens include introduced exotic bullfrogs and predatory fishes, and extensive habitat modification associated with agriculture (Hayes and Jennings 1986).
Status Determination
Rana pipiens’ small range in California coupled with severe declines drives the high score for this species. None of these threats are currently being reversed, so it is reasonable to expect additional declines in the future, assuming that native populations still exist in California. Rana pipiens is sensitive to localized extirpation due to drought (Corn and Folgeman 1984), and the expected increase in temperature and decrease in precipitation due to climate change are likely to have additional negative impacts. The combination of these factors justifies a Priority 1 status.
Management Recommendations
The development of an effective management strategy will largely depend on finding remnant populations in the state, carrying out research on the life history of those specific populations to determine their habitat needs, and then taking a proactive management and habitat restoration approach to recover it in its native range. A key first step with any remnant population is to determine whether it is native or introduced. Researchers can most easily accomplish this using DNA markers, and we recommend that larval tail tips be collected for any population that is discovered. A considerable amount of phylogenetic work, particularly using mitochondrial DNA markers, has been published for this species, and straightforward DNA sequencing of California animals should allow them to be placed into a phylogenetic context with other Rana pipiens from across the species’ range. This approach was used by Johnson et al. (2010) and demonstrated that potentially native populations of tiger salamanders (Ambystoma tigrinum) were in fact nonnative introductions. If native populations of R. pipiens are found, the habitat supporting them should be protected in order to reduce potential threats such as nonnative predators, agricultural disturbance, grazing, off-highway vehicle use, pesticide applications, and changes to local hydrology. If nonnative populations are found, managers should evaluate their potential to spread and pose a threat to other native taxa. In certain cases, removal programs could be effective at mitigating threats posed by nonnative R. pipiens.
Monitoring, Research, and Survey Needs
Comprehensive surveys of historical localities as well as the Modoc Plateau area, including the Goose Lake Basin and the Warner Mountains, should be conducted to determine whether any viable populations persist in California and to identify areas of potential habitat for ongoing surveys. The most recent records for this species come from the vicinity of Owens Valley, and all drainages flowing into the valley should be carefully surveyed. It is critically important that tissue samples be collected from any extant populations that are found so that frogs can be genetically characterized with respect to their introduced or native status.
Given our current lack of information about the life history of this species in California, basic ecological research is a key priority for any native populations that remain in the state. Information about habitat preferences and requirements, demography, and timing of key life history events would all improve our ability to conserve remnant populations of Rana pipiens.
Finally, if remnant populations are found, multi-locus microsatellite or single nucleotide polymorphism DNA data should be analyzed to estimate the effective population size and potential connectivity with other remaining populations. If populations are determined to be native, small, and genetically isolated, R. pipiens could be a prime candidate for human-mediated translocations to establish new populations in currently unoccupied habitat patches.