LOWLAND LEOPARD FROG
Rana yavapaiensis Platz and Frost 1984

Lowland leopard frog, Cochise County, Arizona. Courtesy of Brian Freiermuth.

Status Summary
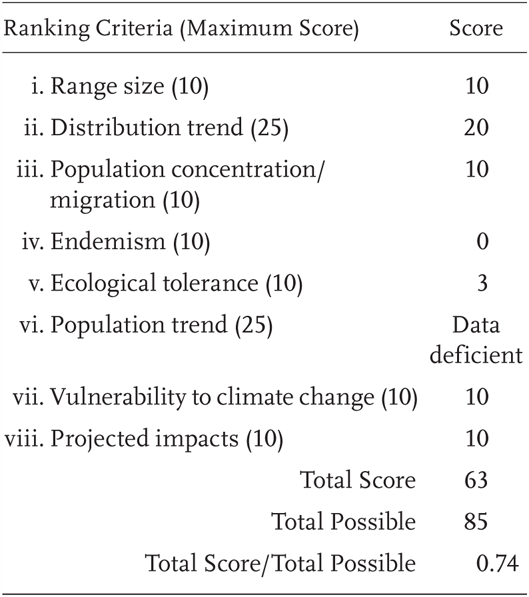
Rana yavapaiensis is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 74% (63/85). During the previous evaluation, it was also considered a Species of Special Concern (Jennings and Hayes 1994a). Rana yavapaiensis has not been confirmed to occur in California since 1965 (Jennings and Hays 1994a).
Lowland Leopard Frog: Risk Factors
Identification
Rana yavapaiensis is a medium-sized ranid frog (4.6–8.7 cm SVL) with prominent dorsolateral folds that are discontinuous and angle inward posteriorly (Platz and Frost 1984). The coloration is variable, but is generally gray green, gray brown, or tan with irregular blotches above and cream or white on the venter. The ventral pelvic region is yellow, and this sometimes extends onto the legs. In older individuals, there is also dark mottling on the chin (Jennings and Hayes 1994a; Stebbins 2003). A cream-colored supralabial stripe is present that fades anteriorly in front of the eye (Platz and Frost 1984).
In California, this frog is most likely to be confused with the closely related, nonnative Rio Grande leopard frog (R. berlandieri). The distinguishing characters for the two species widely overlap, and positive identification is therefore difficult. Rana berlandieri attains larger body sizes (up to 11.4 cm SVL) and has proportionately larger eyes than R. yavapaiensis. Coloration of the two species is similar, but R. yavapaiensis generally has more extensive reticulation between the blotches on the hind legs, and its ventral coloration is often less dusky than R. berlandieri (Stebbins 2003). Rana berlandieri‘s call consists of a low trill often followed by grunts, whereas R. yavapaiensis calls with higher-pitched notes that are given in rapid succession, often followed by lower-pitched chucks (Stebbins 2003, Elliott et al. 2009). Given that there are no known extant R. yavapaiensis localities remaining in California and that it is similar in appearance to the nonnative species R. berlandieri, positive identifications should be made cautiously. The species are readily distinguishable using molecular data (Hillis and Wilcox 2005, Frost et al. 2006a), which should be used to confirm any potential R. yavapaiensis specimens from California.
Taxonomic Relationships
Rana yavapaiensis was recognized as a distinct species in the leopard frog complex primarily on the basis of morphology, reproductive isolation, and allozyme variation (Platz and Platz 1973, Platz 1976, Platz and Frost 1984). The species is morphologically similar to other species of leopard frogs in the southwest. Jaeger et al. (2001) distinguished relict leopard frogs (R. onca) from R. yavapaiensis using genetic and morphological data. Based on a mitochondrial DNA dataset, Hillis and Wilcox (2005) confirmed a close relationship between these two species to the exclusion of other leopard frog taxa, including several geographically nearby members of the complex.
Frost et al. (2006a) recommended placing this species and many other North American ranids in the genus Lithobates, although this proposal and the analyses that support it are controversial (Crother 2009, Frost et al. 2009a, Pauly et al. 2009). We retain the traditional taxonomy here to maintain stability and pending further analyses.
Life History
Life history characteristics of California populations of Rana yavapaiensis are poorly known. The species apparently breeds opportunistically during winter rains (Stebbins 1972), and breeding has been documented to occur from late December through March in California (Storer 1925, Ruibal 1959). Elsewhere in the range, breeding has been documented from October to April (Platz and Platz 1973, Collins and Lewis 1979, Frost and Platz 1983, Sartorius and Rosen 2000). The reproductive biology of R. yavapaiensis has only been studied in Arizona. There, the species is known to experience at least two reproductive peaks within a year (once in the fall, once in the winter or spring), and tadpoles may overwinter (Collins and Lewis 1979, Sartorius and Rosen 2000). However, some authors have observed among-population variation in the occurrence of multiple breeding peaks, and it is unknown whether California populations had one or two breeding peaks per year.
Rana yavapaiensis undergoes marked year-to-year fluctuations in population size throughout its range (Clarkson and Rorabaugh 1989, Sredl et al. 1997, Sartorius and Rosen 2000), which renders isolated populations susceptible to extirpation. This also makes it difficult to confirm the absence or extirpation of populations with single-year surveys, emphasizing the importance of multiyear surveys for this species.
Habitat Requirements
Habitat requirements for Rana yavapaiensis are poorly understood, particularly in California. The species was historically found in slow-moving water along the San Felipe Creek drainage and the Lower Colorado River (Storer 1925, Stebbins 1972). The species has been found predominantly in marshy areas with bulrushes, cattails, and grasses with a willow overstory (Storer 1925, Jennings and Hayes 1994a, Jennings and Hayes 1994b), but it is unknown whether this vegetation type is required for population persistence. The species also expanded into artificial canals and ditches in the Imperial Valley as agriculture developed in the region (Storer 1925, Klauber 1934), as is the case currently for R. berlandieri in Imperial County. It is unknown whether R. yavapaiensis can persist in these artificial habitats or whether they represent non-sustaining sink habitat requiring immigrants from nearby source populations.
Aquatic dissolved salt levels probably limit the distribution of this species, at least in some situations. Ruibal (1959) examined salt tolerance in adults and eggs from the San Felipe Creek drainage and found that salinities observed throughout most of the drainage were lethal to eggs (though not to adults) and that suitable areas for breeding were limited to the springs and seeps that fed the drainage. Whether salt concentration was always a limiting factor in California, or agricultural practices led to unnaturally high salt levels in some water bodies, is unknown.
Distribution (Past and Present)
No extant populations are presently known in California (Jennings and Fuller 2004). The distribution of Rana yavapaiensis was historically patchy, even before recent declines. In California, the species was historically present in suitable habitat along the Lower Colorado River, the Imperial Valley, and the San Felipe Creek drainage (Platz 1988, Stebbins 2003). Outside of California, the species historically ranged along the Lower Colorado River from northern Mexico to Arizona, from near sea level to 1700 m (Platz and Frost 1984, Platz 1988, Jennings and Hayes 1994a, Jennings and Hayes 1994b, Stebbins 2003). The last confirmed record in California is from 1965 in an irrigation ditch east of Calexico, Imperial County (Jennings and Hayes 1994a).
Trends in Abundance
Severe declines have occurred throughout the known California range of Rana yavapaiensis, and currently there are no known extant populations. Repeated surveys since 1965 have failed to locate this species (Vitt and Ohmart 1978, Clarkson and Rorabaugh 1989, Jennings and Hayes 1994b). In addition, in 1976 Hurricane Kathleen apparently modified the surface drainage patterns around San Sebastian Marsh, Imperial County, eliminating the wetland habitat that supported the species previously (E. Ervin, pers. comm.). Rana yavapaiensis also appears to be declining through parts of its range outside of California (Clarkson and Rorabaugh 1989, Stebbins 2003).
Nature and Degree of Threat
The declines in Rana yavapaiensis occurred before extensive collections were made or studies were carried out. As a consequence, threats to this species in California are poorly understood, with few actual data supporting any of the potential threats considered here. Possible threats that contributed to its decline include direct impacts from agricultural runoff, which has been shown to be highly detrimental to other species in the leopard frog complex (Relyea 2008), habitat alteration, including water availability and/or flow regimes (Hayes and Jennings 1986), and predation by or competition with introduced bullfrogs, predaceous fishes, and invertebrates (Clarkson and Rorabaugh 1989). Some recent declines in the closely related R. onca appear to be linked to encroachment of dense emergent vegetation into open water habitats (Bradford et al. 2004), and this process could plausibly also affect R. yavapaiensis. All of these factors were occurring simultaneously within the range of R. yavapaiensis along with declines, making it difficult to disentangle their effects (Hayes and Jennings 1986). In addition, over 13,000 km of ditches in the Imperial Valley were burned and subsequently sprayed with oil during this time, and this presumably adversely affected these frogs (Twining and Hensley 1943).
Chytridiomycosis has been documented as contributing to declines in R. yavapaiensis populations in Arizona (Bradley et al. 2002), and this disease is a concern for any remaining California populations. An additional concern is the possibility of competition or hybridization with R. berlandieri in California. Rana berlandieri was introduced into California well after R. yavapaiensis declined (Platz et al. 1990), so it is presumably not involved in the initial decline of the species. However, as it continues to expand its range in southern California, R. berlandieri may pose a risk to any remaining R. yavapaiensis populations (Rorabaugh et al. 2002). Hybridization has been documented between other species pairs of the leopard frog complex, including rare natural hybridization between R. yavapaiensis and the Chiricahua leopard frog (R. chiricahuensis) (Platz and Frost 1984). Molecular phylogenic analyses suggest that R. berlandieri is more closely related to R. yavapaiensis than to R. chiricahuensis, implying that natural hybridization between R. berlandieri and R. yavapaiensis may be possible. Because R. berlandieri is now far more common in California than R. yavapaiensis, ongoing hybridization, should it occur, may result in genetic swamping of any remaining populations.
Status Determination
Rana yavapaiensis has undergone severe declines and has not been documented in California in over 40 years, and there is a strong possibility that the species is already extirpated statewide. However, it remains possible that the frog is present in scattered isolated localities that have not been surveyed, or that frogs have gone undetected despite surveys.
If any populations persist, it is likely that they are vulnerable to the causes of initial decline throughout most of the California range of this species. Such populations, which are almost certainly small and/or isolated, would also be vulnerable to the natural fluctuations in population size that occur in this species. This natural vulnerability could be exacerbated by changing precipitation regimes in the southeastern part of California, where increasing temperatures, declines in precipitation, and greater year-to-year variation in rainfall are expected to occur due to climate change (Cayan et al. 2008b, PRBO 2011).
Management Recommendations
If new surveys locate remaining populations of this species, the habitat supporting these frogs should be protected while further study is carried out. Without a better understanding of this species’ life history in California, establishing an effective management program will be difficult. If native California populations are not found, Rana yavapaiensis is a potential candidate for assisted reintroductions from nearby populations in Arizona, particularly in areas where introduced R. berlandieri are not present or have been eliminated. More generally, such future introductions should be attempted in habitats that are as pristine as possible, and are free of introduced anurans of any species, introduced predatory fishes (including mosquitofish), and pathogenic fungi.
Monitoring, Research, and Survey Needs
Survey efforts need to be renewed along the San Felipe Creek drainage, the Imperial Valley, and the Lower Colorado River. Although the most likely areas for remnant populations are those that have been the least impacted by agriculture and development, even degraded agricultural habitat can be utilized by Rana yavapaiensis, and therefore should be surveyed. Because populations are prone to large yearly fluctuations, surveys should be repeated over multiple years in both the wet and dry seasons. Surveys for larvae should also be undertaken since tadpoles are often more reliably detected than adults. If any remaining populations are located, the habitat surrounding these areas should be protected, and researchers should begin a monitoring program to quantify and track population sizes. Any suspected R. yavapaiensis populations should be confirmed using a set of molecular markers, both to firmly establish species identity and to check for hybridization between R. yavapaiensis and R. berlandieri. Because hybridization is a concern, both mitochondrial and nuclear markers should be used. Given the difficulty in distinguishing the two species, we recommend that populations of presumptive R. berlandieri be sampled for genetic material using nonlethal means (such as toe clips) and checked for diagnostic molecular markers to confirm that no native R. yavapaiensis DNA is present.
Should surveys discover extant populations of R. yavapaiensis, research into the basic life history and the causes of decline in California will be a prerequisite to developing an effective management program. Life history studies with a particular focus on habitat suitability should be undertaken on any populations that are located or reestablished.